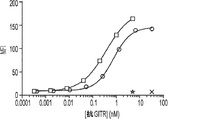
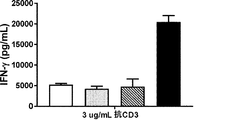
JP2019528683A - 多量体gitr結合分子及びその使用 - Google Patents
多量体gitr結合分子及びその使用 Download PDFInfo
- Publication number
- JP2019528683A JP2019528683A JP2019502565A JP2019502565A JP2019528683A JP 2019528683 A JP2019528683 A JP 2019528683A JP 2019502565 A JP2019502565 A JP 2019502565A JP 2019502565 A JP2019502565 A JP 2019502565A JP 2019528683 A JP2019528683 A JP 2019528683A
- Authority
- JP
- Japan
- Prior art keywords
- seq
- sequence number
- binding molecule
- gitr
- binding
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2878—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30, CD40, CD95
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/46—Hybrid immunoglobulins
- C07K16/468—Immunoglobulins having two or more different antigen binding sites, e.g. multifunctional antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/33—Crossreactivity, e.g. for species or epitope, or lack of said crossreactivity
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/35—Valency
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/52—Constant or Fc region; Isotype
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/75—Agonist effect on antigen
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Animal Behavior & Ethology (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Peptides Or Proteins (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662364762P | 2016-07-20 | 2016-07-20 | |
| US62/364,762 | 2016-07-20 | ||
| PCT/US2017/043166 WO2018017889A1 (en) | 2016-07-20 | 2017-07-20 | Multimeric gitr binding molecules and uses thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2019528683A true JP2019528683A (ja) | 2019-10-17 |
| JP2019528683A5 JP2019528683A5 (OSRAM) | 2020-08-13 |
Family
ID=60992910
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019502565A Withdrawn JP2019528683A (ja) | 2016-07-20 | 2017-07-20 | 多量体gitr結合分子及びその使用 |
Country Status (9)
| Country | Link |
|---|---|
| US (2) | US20190330360A1 (OSRAM) |
| EP (1) | EP3487299A4 (OSRAM) |
| JP (1) | JP2019528683A (OSRAM) |
| CN (1) | CN109561681A (OSRAM) |
| AU (1) | AU2017300647A1 (OSRAM) |
| CA (1) | CA3030659A1 (OSRAM) |
| IL (1) | IL263800A (OSRAM) |
| MX (1) | MX2019000799A (OSRAM) |
| WO (1) | WO2018017889A1 (OSRAM) |
Families Citing this family (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2014332458B2 (en) | 2013-09-05 | 2020-03-12 | Igm Biosciences, Inc. | Constant chain modified bispecific, penta- and hexavalent Ig-M antibodies |
| BR112016018313A8 (pt) | 2014-02-10 | 2018-06-12 | Igm Biosciences Inc | Moléculas de ligação multiespecíficas iga |
| CN112759650B (zh) | 2014-04-03 | 2024-10-01 | Igm生物科学股份有限公司 | 修饰的j链 |
| KR102435324B1 (ko) | 2015-01-20 | 2022-08-23 | 아이쥐엠 바이오사이언스 인코포레이티드 | 종양 괴사 인자(tnf) 수퍼패밀리 수용체 결합 분자 및 그의 용도 |
| SI3265575T1 (sl) | 2015-03-04 | 2021-08-31 | Igm Biosciences, Inc. | Vezne molekule CD20 in njihove uporabe |
| WO2016154593A1 (en) | 2015-03-25 | 2016-09-29 | Igm Biosciences, Inc. | Multi-valent hepatitis b virus antigen binding molecules and uses thereof |
| AU2016249404B2 (en) | 2015-04-17 | 2021-01-21 | Igm Biosciences, Inc. | Multi-valent human immunodeficiency virus antigen binding molecules and uses thereof |
| ES2996834T3 (en) | 2015-09-30 | 2025-02-13 | Igm Biosciences Inc | Binding molecules with modified j-chain |
| EP3356401B1 (en) | 2015-09-30 | 2020-06-24 | IGM Biosciences, Inc. | Binding molecules with modified j-chain |
| CA3021669A1 (en) | 2016-05-09 | 2017-11-16 | Igm Biosciences, Inc. | Anti-pd-l1 antibodies |
| WO2018187702A2 (en) | 2017-04-07 | 2018-10-11 | Igm Biosciences A/S | Modified human igm constant regions for modulation of complement-dependent cytolysis effector function |
| BR112020017296A2 (pt) | 2018-03-01 | 2020-12-29 | Igm Biosciences, Inc. | Mutações de igm fc e de cadeia j que afetam a meia-vida sérica de igm |
| JP2022505663A (ja) * | 2018-10-23 | 2022-01-14 | アイジーエム バイオサイエンシズ インコーポレイテッド | IgM-Fcベース及びIgA-Fcベースの多価結合分子 |
| CN114269785A (zh) | 2019-08-15 | 2022-04-01 | Igm生物科学股份有限公司 | 免疫刺激性多聚体结合分子 |
| CN115485299A (zh) * | 2020-04-22 | 2022-12-16 | Igm生物科学股份有限公司 | Pd-1激动剂多聚结合分子 |
| US20240002526A1 (en) * | 2020-11-17 | 2024-01-04 | Igm Biosciences, Inc. | Uses of effector cell engaging molecules with moieties of differing potencies |
| CN113151186B (zh) * | 2021-02-04 | 2022-02-18 | 上海交通大学 | 抗人cd271的单克隆抗体及用途 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20140294825A1 (en) * | 2011-09-26 | 2014-10-02 | Jn Biosciences Llc | Hybrid constant regions |
| US20150064204A1 (en) * | 2013-08-30 | 2015-03-05 | Amgen Inc. | Gitr antigen binding proteins |
| US9228016B2 (en) * | 2014-06-06 | 2016-01-05 | Bristol-Myers Squibb Company | Antibodies against glucocorticoid-induced tumor necrosis factor receptor (GITR) and uses thereof |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU6290090A (en) * | 1989-08-29 | 1991-04-08 | University Of Southampton | Bi-or trispecific (fab)3 or (fab)4 conjugates |
| US8658159B2 (en) * | 2008-06-30 | 2014-02-25 | Versitech Limited | Method to induce and expand therapeutic alloantigen-specific human regulatory T cells in large-scale |
| NZ590667A (en) * | 2008-07-02 | 2013-01-25 | Emergent Product Dev Seattle | Tgf-b antagonist multi-target binding proteins |
| EP2760891B1 (en) * | 2011-09-26 | 2018-11-07 | JN Biosciences LLC | Hybrid constant regions |
| US20150038682A1 (en) * | 2013-08-02 | 2015-02-05 | Jn Biosciences Llc | Antibodies or fusion proteins multimerized via homomultimerizing peptide |
| PL3148579T3 (pl) * | 2014-05-28 | 2021-07-19 | Agenus Inc. | Przeciwciała anty-gitr i sposoby ich zastosowania |
| KR102435324B1 (ko) * | 2015-01-20 | 2022-08-23 | 아이쥐엠 바이오사이언스 인코포레이티드 | 종양 괴사 인자(tnf) 수퍼패밀리 수용체 결합 분자 및 그의 용도 |
| EP3356401B1 (en) * | 2015-09-30 | 2020-06-24 | IGM Biosciences, Inc. | Binding molecules with modified j-chain |
-
2017
- 2017-07-20 AU AU2017300647A patent/AU2017300647A1/en not_active Abandoned
- 2017-07-20 WO PCT/US2017/043166 patent/WO2018017889A1/en not_active Ceased
- 2017-07-20 MX MX2019000799A patent/MX2019000799A/es unknown
- 2017-07-20 CN CN201780045191.5A patent/CN109561681A/zh active Pending
- 2017-07-20 EP EP17831909.1A patent/EP3487299A4/en not_active Withdrawn
- 2017-07-20 JP JP2019502565A patent/JP2019528683A/ja not_active Withdrawn
- 2017-07-20 US US16/317,816 patent/US20190330360A1/en not_active Abandoned
- 2017-07-20 CA CA3030659A patent/CA3030659A1/en not_active Abandoned
-
2018
- 2018-12-18 IL IL263800A patent/IL263800A/en unknown
-
2022
- 2022-02-24 US US17/680,026 patent/US20220177595A1/en not_active Abandoned
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20140294825A1 (en) * | 2011-09-26 | 2014-10-02 | Jn Biosciences Llc | Hybrid constant regions |
| US20150064204A1 (en) * | 2013-08-30 | 2015-03-05 | Amgen Inc. | Gitr antigen binding proteins |
| US9228016B2 (en) * | 2014-06-06 | 2016-01-05 | Bristol-Myers Squibb Company | Antibodies against glucocorticoid-induced tumor necrosis factor receptor (GITR) and uses thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3487299A1 (en) | 2019-05-29 |
| EP3487299A4 (en) | 2020-03-11 |
| CN109561681A (zh) | 2019-04-02 |
| MX2019000799A (es) | 2019-06-03 |
| AU2017300647A1 (en) | 2019-02-07 |
| CA3030659A1 (en) | 2018-01-25 |
| US20190330360A1 (en) | 2019-10-31 |
| WO2018017889A1 (en) | 2018-01-25 |
| US20220177595A1 (en) | 2022-06-09 |
| IL263800A (en) | 2019-02-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20220177595A1 (en) | Multimeric gitr binding molecules and uses thereof | |
| US20220169751A1 (en) | Multimeric ox40 binding molecules and uses thereof | |
| US20230203173A1 (en) | Tumor necrosis factor (tnf) superfamily receptor igm antibodies and uses thereof | |
| US20210388098A1 (en) | Multimeric cd137/4-1bb binding molecules and uses thereof | |
| US20220106399A1 (en) | Multimeric cd40 binding molecules and uses thereof | |
| WO2020163646A1 (en) | Anti-gitr antigen-binding domains and uses thereof | |
| HK40043005A (en) | Tumor necrosis factor (tnf) superfamily receptor binding molecules and uses thereof | |
| HK1242348B (en) | Tumor necrosis factor (tnf) superfamily receptor binding molecules and uses thereof | |
| HK1242348A1 (en) | Tumor necrosis factor (tnf) superfamily receptor binding molecules and uses thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20190118 Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190319 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200629 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20200629 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20210514 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20210721 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20210827 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20220314 |
|
| A761 | Written withdrawal of application |
Free format text: JAPANESE INTERMEDIATE CODE: A761 Effective date: 20220627 |