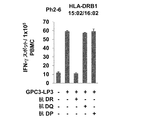
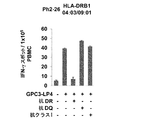
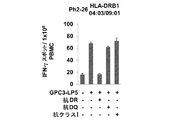
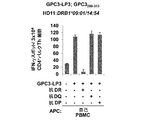
JP2018508181A - Th1細胞のためのGPC3エピトープペプチドおよびこれを含有するワクチン - Google Patents
Th1細胞のためのGPC3エピトープペプチドおよびこれを含有するワクチン Download PDFInfo
- Publication number
- JP2018508181A JP2018508181A JP2017518379A JP2017518379A JP2018508181A JP 2018508181 A JP2018508181 A JP 2018508181A JP 2017518379 A JP2017518379 A JP 2017518379A JP 2017518379 A JP2017518379 A JP 2017518379A JP 2018508181 A JP2018508181 A JP 2018508181A
- Authority
- JP
- Japan
- Prior art keywords
- hla
- cells
- peptide
- gpc3
- peptides
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 108090000765 processed proteins & peptides Proteins 0.000 title claims abstract description 614
- 210000000447 Th1 cell Anatomy 0.000 title claims abstract description 209
- 101001014668 Homo sapiens Glypican-3 Proteins 0.000 title claims abstract description 15
- 102100032530 Glypican-3 Human genes 0.000 title claims abstract 4
- 229960005486 vaccine Drugs 0.000 title description 19
- 102000004196 processed proteins & peptides Human genes 0.000 claims abstract description 271
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 206
- 238000000034 method Methods 0.000 claims abstract description 161
- 201000011510 cancer Diseases 0.000 claims abstract description 149
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 claims abstract description 114
- 108091033319 polynucleotide Proteins 0.000 claims abstract description 94
- 102000040430 polynucleotide Human genes 0.000 claims abstract description 94
- 239000002157 polynucleotide Substances 0.000 claims abstract description 94
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 58
- 108091054438 MHC class II family Proteins 0.000 claims abstract description 43
- 230000028993 immune response Effects 0.000 claims abstract description 43
- 230000027455 binding Effects 0.000 claims abstract description 42
- 238000011282 treatment Methods 0.000 claims abstract description 34
- 239000004480 active ingredient Substances 0.000 claims abstract description 33
- 230000002265 prevention Effects 0.000 claims abstract description 31
- 102000043131 MHC class II family Human genes 0.000 claims abstract description 15
- 230000002708 enhancing effect Effects 0.000 claims abstract description 13
- 210000004027 cell Anatomy 0.000 claims description 243
- 210000000612 antigen-presenting cell Anatomy 0.000 claims description 182
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 113
- 210000001744 T-lymphocyte Anatomy 0.000 claims description 89
- 239000012634 fragment Substances 0.000 claims description 75
- 102000018713 Histocompatibility Antigens Class II Human genes 0.000 claims description 67
- 150000001413 amino acids Chemical class 0.000 claims description 62
- 230000001939 inductive effect Effects 0.000 claims description 59
- 108010029172 HLA-DR9 antigen Proteins 0.000 claims description 55
- 239000000203 mixture Substances 0.000 claims description 52
- 108700037339 HLA-DR13 antigen Proteins 0.000 claims description 45
- 108091008874 T cell receptors Proteins 0.000 claims description 43
- 108010086066 HLA-DR8 antigen Proteins 0.000 claims description 41
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 claims description 41
- 108010027412 Histocompatibility Antigens Class II Proteins 0.000 claims description 39
- 239000013598 vector Substances 0.000 claims description 36
- 238000000338 in vitro Methods 0.000 claims description 35
- 108010064366 HLA-DPw2 antigen Proteins 0.000 claims description 32
- 230000001404 mediated effect Effects 0.000 claims description 31
- 108010063970 HLA-DR15 antigen Proteins 0.000 claims description 30
- 238000001727 in vivo Methods 0.000 claims description 30
- 108010055807 HLA-DR14 Proteins 0.000 claims description 28
- -1 HLA-DR52b Proteins 0.000 claims description 24
- 239000013604 expression vector Substances 0.000 claims description 15
- 125000003729 nucleotide group Chemical group 0.000 claims description 12
- 238000012258 culturing Methods 0.000 claims description 11
- 239000002773 nucleotide Substances 0.000 claims description 10
- 230000002980 postoperative effect Effects 0.000 claims description 8
- 238000009007 Diagnostic Kit Methods 0.000 claims description 2
- 230000006698 induction Effects 0.000 abstract description 38
- 206010073071 hepatocellular carcinoma Diseases 0.000 abstract description 33
- 231100000844 hepatocellular carcinoma Toxicity 0.000 abstract description 33
- 201000001441 melanoma Diseases 0.000 abstract description 14
- 238000002619 cancer immunotherapy Methods 0.000 abstract description 12
- 229940022399 cancer vaccine Drugs 0.000 abstract description 4
- 238000009566 cancer vaccine Methods 0.000 abstract description 4
- 102000010956 Glypican Human genes 0.000 description 215
- 108050001154 Glypican Proteins 0.000 description 215
- 108050007237 Glypican-3 Proteins 0.000 description 215
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 63
- 102100040485 HLA class II histocompatibility antigen, DRB1 beta chain Human genes 0.000 description 61
- 108010039343 HLA-DRB1 Chains Proteins 0.000 description 61
- 235000001014 amino acid Nutrition 0.000 description 60
- 108090000623 proteins and genes Proteins 0.000 description 59
- 229940024606 amino acid Drugs 0.000 description 58
- 239000000427 antigen Substances 0.000 description 58
- 102000036639 antigens Human genes 0.000 description 58
- 108091007433 antigens Proteins 0.000 description 58
- 101001100327 Homo sapiens RNA-binding protein 45 Proteins 0.000 description 54
- 102100038823 RNA-binding protein 45 Human genes 0.000 description 54
- 102100037850 Interferon gamma Human genes 0.000 description 47
- 108010074328 Interferon-gamma Proteins 0.000 description 47
- 230000004044 response Effects 0.000 description 47
- 101000691214 Haloarcula marismortui (strain ATCC 43049 / DSM 3752 / JCM 8966 / VKM B-1809) 50S ribosomal protein L44e Proteins 0.000 description 40
- 239000003814 drug Substances 0.000 description 39
- 235000018102 proteins Nutrition 0.000 description 35
- 102000004169 proteins and genes Human genes 0.000 description 35
- 210000004443 dendritic cell Anatomy 0.000 description 33
- 241000282414 Homo sapiens Species 0.000 description 31
- 239000000523 sample Substances 0.000 description 31
- 108010074032 HLA-A2 Antigen Proteins 0.000 description 30
- 102000025850 HLA-A2 Antigen Human genes 0.000 description 30
- 108010058597 HLA-DR Antigens Proteins 0.000 description 30
- 102000006354 HLA-DR Antigens Human genes 0.000 description 30
- 239000003795 chemical substances by application Substances 0.000 description 25
- 102000011786 HLA-A Antigens Human genes 0.000 description 24
- 108010075704 HLA-A Antigens Proteins 0.000 description 24
- 230000036755 cellular response Effects 0.000 description 24
- 239000002502 liposome Substances 0.000 description 24
- 229940079593 drug Drugs 0.000 description 22
- 210000001519 tissue Anatomy 0.000 description 22
- 239000000463 material Substances 0.000 description 21
- 229920001184 polypeptide Polymers 0.000 description 21
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 19
- 101150039713 gpc3 gene Proteins 0.000 description 19
- 239000003153 chemical reaction reagent Substances 0.000 description 18
- 201000010099 disease Diseases 0.000 description 18
- 210000002865 immune cell Anatomy 0.000 description 18
- 230000003053 immunization Effects 0.000 description 18
- 230000004048 modification Effects 0.000 description 18
- 238000012986 modification Methods 0.000 description 18
- 150000007523 nucleic acids Chemical class 0.000 description 18
- 108700028369 Alleles Proteins 0.000 description 17
- 230000000638 stimulation Effects 0.000 description 17
- 239000000126 substance Substances 0.000 description 17
- 230000001419 dependent effect Effects 0.000 description 16
- 102000039446 nucleic acids Human genes 0.000 description 16
- 108020004707 nucleic acids Proteins 0.000 description 16
- 239000000047 product Substances 0.000 description 16
- 238000003114 enzyme-linked immunosorbent spot assay Methods 0.000 description 15
- 102000004127 Cytokines Human genes 0.000 description 14
- 108090000695 Cytokines Proteins 0.000 description 14
- 238000011510 Elispot assay Methods 0.000 description 14
- 241000699670 Mus sp. Species 0.000 description 14
- 125000000539 amino acid group Chemical group 0.000 description 14
- 238000003556 assay Methods 0.000 description 14
- 238000002649 immunization Methods 0.000 description 14
- 230000001965 increasing effect Effects 0.000 description 14
- 241000699666 Mus <mouse, genus> Species 0.000 description 13
- 238000012360 testing method Methods 0.000 description 13
- 239000002671 adjuvant Substances 0.000 description 12
- 238000002360 preparation method Methods 0.000 description 12
- 230000006870 function Effects 0.000 description 11
- 102000048373 human GPC3 Human genes 0.000 description 11
- 238000009169 immunotherapy Methods 0.000 description 11
- 238000004519 manufacturing process Methods 0.000 description 11
- 108020004999 messenger RNA Proteins 0.000 description 11
- 241000588724 Escherichia coli Species 0.000 description 10
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 10
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 10
- 230000014102 antigen processing and presentation of exogenous peptide antigen via MHC class I Effects 0.000 description 10
- 230000000694 effects Effects 0.000 description 10
- 238000002474 experimental method Methods 0.000 description 10
- 150000003839 salts Chemical class 0.000 description 10
- 230000004936 stimulating effect Effects 0.000 description 10
- 108010013476 HLA-A24 Antigen Proteins 0.000 description 9
- 108091054437 MHC class I family Proteins 0.000 description 9
- 241000124008 Mammalia Species 0.000 description 9
- 238000001514 detection method Methods 0.000 description 9
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 8
- 108010002350 Interleukin-2 Proteins 0.000 description 8
- 108700018351 Major Histocompatibility Complex Proteins 0.000 description 8
- 241001465754 Metazoa Species 0.000 description 8
- 238000007792 addition Methods 0.000 description 8
- 230000000735 allogeneic effect Effects 0.000 description 8
- 239000002953 phosphate buffered saline Substances 0.000 description 8
- 210000002966 serum Anatomy 0.000 description 8
- 230000020382 suppression by virus of host antigen processing and presentation of peptide antigen via MHC class I Effects 0.000 description 8
- 108020004705 Codon Proteins 0.000 description 7
- 230000005867 T cell response Effects 0.000 description 7
- 230000007969 cellular immunity Effects 0.000 description 7
- 210000004408 hybridoma Anatomy 0.000 description 7
- 230000005847 immunogenicity Effects 0.000 description 7
- 150000002632 lipids Chemical class 0.000 description 7
- 210000002540 macrophage Anatomy 0.000 description 7
- 239000002609 medium Substances 0.000 description 7
- 230000008569 process Effects 0.000 description 7
- 238000011321 prophylaxis Methods 0.000 description 7
- 230000001603 reducing effect Effects 0.000 description 7
- 238000006467 substitution reaction Methods 0.000 description 7
- 238000003786 synthesis reaction Methods 0.000 description 7
- 108020004414 DNA Proteins 0.000 description 6
- 102000004190 Enzymes Human genes 0.000 description 6
- 108090000790 Enzymes Proteins 0.000 description 6
- 102000004457 Granulocyte-Macrophage Colony-Stimulating Factor Human genes 0.000 description 6
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 6
- 102000043129 MHC class I family Human genes 0.000 description 6
- 230000005809 anti-tumor immunity Effects 0.000 description 6
- 230000008901 benefit Effects 0.000 description 6
- 230000004071 biological effect Effects 0.000 description 6
- 238000004422 calculation algorithm Methods 0.000 description 6
- 150000001875 compounds Chemical class 0.000 description 6
- 238000011161 development Methods 0.000 description 6
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 6
- 229940088598 enzyme Drugs 0.000 description 6
- 108091005601 modified peptides Proteins 0.000 description 6
- 238000010647 peptide synthesis reaction Methods 0.000 description 6
- 230000001225 therapeutic effect Effects 0.000 description 6
- 238000013519 translation Methods 0.000 description 6
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 5
- 238000002965 ELISA Methods 0.000 description 5
- 102100031618 HLA class II histocompatibility antigen, DP beta 1 chain Human genes 0.000 description 5
- 102100040482 HLA class II histocompatibility antigen, DR beta 3 chain Human genes 0.000 description 5
- 108010010378 HLA-DP Antigens Proteins 0.000 description 5
- 102000015789 HLA-DP Antigens Human genes 0.000 description 5
- 108010045483 HLA-DPB1 antigen Proteins 0.000 description 5
- 102000000588 Interleukin-2 Human genes 0.000 description 5
- 241000283973 Oryctolagus cuniculus Species 0.000 description 5
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 5
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 5
- 230000000259 anti-tumor effect Effects 0.000 description 5
- 239000002585 base Substances 0.000 description 5
- 230000001413 cellular effect Effects 0.000 description 5
- 239000003085 diluting agent Substances 0.000 description 5
- 239000003937 drug carrier Substances 0.000 description 5
- 238000009472 formulation Methods 0.000 description 5
- 230000036039 immunity Effects 0.000 description 5
- 230000002163 immunogen Effects 0.000 description 5
- 210000004698 lymphocyte Anatomy 0.000 description 5
- 238000002156 mixing Methods 0.000 description 5
- 210000005259 peripheral blood Anatomy 0.000 description 5
- 239000011886 peripheral blood Substances 0.000 description 5
- 239000013641 positive control Substances 0.000 description 5
- 238000012545 processing Methods 0.000 description 5
- 239000000725 suspension Substances 0.000 description 5
- 208000024891 symptom Diseases 0.000 description 5
- 230000008685 targeting Effects 0.000 description 5
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 4
- 108010093013 HLA-DR1 Antigen Proteins 0.000 description 4
- 108010061311 HLA-DRB3 Chains Proteins 0.000 description 4
- 102000008949 Histocompatibility Antigens Class I Human genes 0.000 description 4
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 4
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 4
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 4
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 4
- 206010035226 Plasma cell myeloma Diseases 0.000 description 4
- 241000700159 Rattus Species 0.000 description 4
- 102100030403 Signal peptide peptidase-like 2A Human genes 0.000 description 4
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 4
- 238000004458 analytical method Methods 0.000 description 4
- 238000013459 approach Methods 0.000 description 4
- 239000011324 bead Substances 0.000 description 4
- 239000012620 biological material Substances 0.000 description 4
- 239000012472 biological sample Substances 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 210000004369 blood Anatomy 0.000 description 4
- 239000008280 blood Substances 0.000 description 4
- 230000007910 cell fusion Effects 0.000 description 4
- 238000012217 deletion Methods 0.000 description 4
- 230000037430 deletion Effects 0.000 description 4
- 230000013595 glycosylation Effects 0.000 description 4
- 238000006206 glycosylation reaction Methods 0.000 description 4
- 238000009396 hybridization Methods 0.000 description 4
- 229910052739 hydrogen Inorganic materials 0.000 description 4
- 230000001900 immune effect Effects 0.000 description 4
- 239000004615 ingredient Substances 0.000 description 4
- 238000003780 insertion Methods 0.000 description 4
- 230000037431 insertion Effects 0.000 description 4
- 210000001165 lymph node Anatomy 0.000 description 4
- 229930182817 methionine Natural products 0.000 description 4
- 210000002433 mononuclear leukocyte Anatomy 0.000 description 4
- 201000000050 myeloid neoplasm Diseases 0.000 description 4
- 239000013642 negative control Substances 0.000 description 4
- 229940023041 peptide vaccine Drugs 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 230000001737 promoting effect Effects 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 230000001105 regulatory effect Effects 0.000 description 4
- 238000010561 standard procedure Methods 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 3
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 3
- 241000282693 Cercopithecidae Species 0.000 description 3
- 108010001041 HLA-DR7 Antigen Proteins 0.000 description 3
- 101000599782 Homo sapiens Insulin-like growth factor 2 mRNA-binding protein 3 Proteins 0.000 description 3
- 101000946889 Homo sapiens Monocyte differentiation antigen CD14 Proteins 0.000 description 3
- 101000702394 Homo sapiens Signal peptide peptidase-like 2A Proteins 0.000 description 3
- 101000960621 Homo sapiens U3 small nucleolar ribonucleoprotein protein IMP3 Proteins 0.000 description 3
- 108010002586 Interleukin-7 Proteins 0.000 description 3
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 3
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 3
- 102100035877 Monocyte differentiation antigen CD14 Human genes 0.000 description 3
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 3
- 108091028043 Nucleic acid sequence Proteins 0.000 description 3
- 108091034117 Oligonucleotide Proteins 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- 108010076504 Protein Sorting Signals Proteins 0.000 description 3
- 241000283984 Rodentia Species 0.000 description 3
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 3
- 101710120037 Toxin CcdB Proteins 0.000 description 3
- 241000700618 Vaccinia virus Species 0.000 description 3
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 3
- 230000009471 action Effects 0.000 description 3
- 235000004279 alanine Nutrition 0.000 description 3
- 230000005975 antitumor immune response Effects 0.000 description 3
- 235000009582 asparagine Nutrition 0.000 description 3
- 229960001230 asparagine Drugs 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- 239000012707 chemical precursor Substances 0.000 description 3
- 238000004587 chromatography analysis Methods 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 239000002299 complementary DNA Substances 0.000 description 3
- 239000012228 culture supernatant Substances 0.000 description 3
- 230000001472 cytotoxic effect Effects 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 210000002919 epithelial cell Anatomy 0.000 description 3
- 238000001415 gene therapy Methods 0.000 description 3
- 238000003384 imaging method Methods 0.000 description 3
- 230000002998 immunogenetic effect Effects 0.000 description 3
- 238000010324 immunological assay Methods 0.000 description 3
- 230000005764 inhibitory process Effects 0.000 description 3
- 108010045069 keyhole-limpet hemocyanin Proteins 0.000 description 3
- 230000000670 limiting effect Effects 0.000 description 3
- 230000007774 longterm Effects 0.000 description 3
- 239000003550 marker Substances 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 238000011275 oncology therapy Methods 0.000 description 3
- 230000036961 partial effect Effects 0.000 description 3
- 239000000546 pharmaceutical excipient Substances 0.000 description 3
- 239000002831 pharmacologic agent Substances 0.000 description 3
- 239000004033 plastic Substances 0.000 description 3
- 229920003023 plastic Polymers 0.000 description 3
- 238000004393 prognosis Methods 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- 238000003127 radioimmunoassay Methods 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 238000010186 staining Methods 0.000 description 3
- 230000004083 survival effect Effects 0.000 description 3
- 238000013518 transcription Methods 0.000 description 3
- 230000035897 transcription Effects 0.000 description 3
- 230000003612 virological effect Effects 0.000 description 3
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 2
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 2
- 241000283690 Bos taurus Species 0.000 description 2
- 241000282472 Canis lupus familiaris Species 0.000 description 2
- 150000008574 D-amino acids Chemical class 0.000 description 2
- 101150034979 DRB3 gene Proteins 0.000 description 2
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 2
- 206010059866 Drug resistance Diseases 0.000 description 2
- 241000283086 Equidae Species 0.000 description 2
- 241000282326 Felis catus Species 0.000 description 2
- ZHNUHDYFZUAESO-UHFFFAOYSA-N Formamide Chemical compound NC=O ZHNUHDYFZUAESO-UHFFFAOYSA-N 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 101000590482 Homo sapiens Kinetochore protein Nuf2 Proteins 0.000 description 2
- 241000725303 Human immunodeficiency virus Species 0.000 description 2
- 108060003951 Immunoglobulin Proteins 0.000 description 2
- 102100032431 Kinetochore protein Nuf2 Human genes 0.000 description 2
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 2
- 102000004083 Lymphotoxin-alpha Human genes 0.000 description 2
- 108090000542 Lymphotoxin-alpha Proteins 0.000 description 2
- 241000282567 Macaca fascicularis Species 0.000 description 2
- 206010027476 Metastases Diseases 0.000 description 2
- 241000699660 Mus musculus Species 0.000 description 2
- 108010046117 N-palmitoyl-5,6-dipalmitoyl-S-glycerylcysteinyl-seryl-serine Proteins 0.000 description 2
- 108010038807 Oligopeptides Proteins 0.000 description 2
- 102000015636 Oligopeptides Human genes 0.000 description 2
- 101100278514 Oryza sativa subsp. japonica DRB2 gene Proteins 0.000 description 2
- 108010058846 Ovalbumin Proteins 0.000 description 2
- 241000282579 Pan Species 0.000 description 2
- 241000282520 Papio Species 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- 108020004511 Recombinant DNA Proteins 0.000 description 2
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 2
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 2
- FKNQFGJONOIPTF-UHFFFAOYSA-N Sodium cation Chemical compound [Na+] FKNQFGJONOIPTF-UHFFFAOYSA-N 0.000 description 2
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 2
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 2
- 241000700605 Viruses Species 0.000 description 2
- 239000008186 active pharmaceutical agent Substances 0.000 description 2
- 238000001042 affinity chromatography Methods 0.000 description 2
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 2
- 230000003321 amplification Effects 0.000 description 2
- 230000030741 antigen processing and presentation Effects 0.000 description 2
- 210000003719 b-lymphocyte Anatomy 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 238000004113 cell culture Methods 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 238000003501 co-culture Methods 0.000 description 2
- 230000000295 complement effect Effects 0.000 description 2
- 238000012790 confirmation Methods 0.000 description 2
- 230000016396 cytokine production Effects 0.000 description 2
- 231100000433 cytotoxic Toxicity 0.000 description 2
- 238000003745 diagnosis Methods 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- 238000004520 electroporation Methods 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 230000004927 fusion Effects 0.000 description 2
- 230000002068 genetic effect Effects 0.000 description 2
- 238000010353 genetic engineering Methods 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 2
- 210000002443 helper t lymphocyte Anatomy 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- FDGQSTZJBFJUBT-UHFFFAOYSA-N hypoxanthine Chemical compound O=C1NC=NC2=C1NC=N2 FDGQSTZJBFJUBT-UHFFFAOYSA-N 0.000 description 2
- 238000003018 immunoassay Methods 0.000 description 2
- 238000010166 immunofluorescence Methods 0.000 description 2
- 102000018358 immunoglobulin Human genes 0.000 description 2
- 230000001976 improved effect Effects 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 239000007928 intraperitoneal injection Substances 0.000 description 2
- 230000009545 invasion Effects 0.000 description 2
- 238000004255 ion exchange chromatography Methods 0.000 description 2
- 238000002372 labelling Methods 0.000 description 2
- 239000006166 lysate Substances 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- 230000009401 metastasis Effects 0.000 description 2
- 230000001394 metastastic effect Effects 0.000 description 2
- 206010061289 metastatic neoplasm Diseases 0.000 description 2
- 229940098779 methanesulfonic acid Drugs 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- 238000002493 microarray Methods 0.000 description 2
- 150000007522 mineralic acids Chemical class 0.000 description 2
- 238000012544 monitoring process Methods 0.000 description 2
- 238000003199 nucleic acid amplification method Methods 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 229940092253 ovalbumin Drugs 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 239000008177 pharmaceutical agent Substances 0.000 description 2
- 238000009520 phase I clinical trial Methods 0.000 description 2
- 229910052698 phosphorus Inorganic materials 0.000 description 2
- 230000026731 phosphorylation Effects 0.000 description 2
- 238000006366 phosphorylation reaction Methods 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 230000003389 potentiating effect Effects 0.000 description 2
- 230000037452 priming Effects 0.000 description 2
- 238000002331 protein detection Methods 0.000 description 2
- 230000006798 recombination Effects 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 2
- 229910001415 sodium ion Inorganic materials 0.000 description 2
- DAEPDZWVDSPTHF-UHFFFAOYSA-M sodium pyruvate Chemical compound [Na+].CC(=O)C([O-])=O DAEPDZWVDSPTHF-UHFFFAOYSA-M 0.000 description 2
- 239000007790 solid phase Substances 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 238000007619 statistical method Methods 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- 238000010257 thawing Methods 0.000 description 2
- 230000009258 tissue cross reactivity Effects 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- 239000003053 toxin Substances 0.000 description 2
- 231100000765 toxin Toxicity 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- 238000010361 transduction Methods 0.000 description 2
- 230000026683 transduction Effects 0.000 description 2
- 238000011830 transgenic mouse model Methods 0.000 description 2
- XVQKZSLOGHBCET-INVHGPFASA-N tripalmitoyl-S-glyceryl-cysteinyl-seryl-serine Chemical compound CCCCCCCCCCCCCCCC(=O)N[C@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O)CSCC(OC(=O)CCCCCCCCCCCCCCC)COC(=O)CCCCCCCCCCCCCCC XVQKZSLOGHBCET-INVHGPFASA-N 0.000 description 2
- 241001430294 unidentified retrovirus Species 0.000 description 2
- UHEPSJJJMTWUCP-DHDYTCSHSA-N (2r,3r,4r,5r)-2-[(1s,2s,3r,4s,6r)-4,6-diamino-3-[(2s,3r,4r,5s,6r)-3-amino-4,5-dihydroxy-6-[(1r)-1-hydroxyethyl]oxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-5-methyl-4-(methylamino)oxane-3,5-diol;sulfuric acid Chemical compound OS(O)(=O)=O.OS(O)(=O)=O.O1C[C@@](O)(C)[C@H](NC)[C@@H](O)[C@H]1O[C@@H]1[C@@H](O)[C@H](O[C@@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H]([C@@H](C)O)O2)N)[C@@H](N)C[C@H]1N UHEPSJJJMTWUCP-DHDYTCSHSA-N 0.000 description 1
- UKAUYVFTDYCKQA-UHFFFAOYSA-N -2-Amino-4-hydroxybutanoic acid Natural products OC(=O)C(N)CCO UKAUYVFTDYCKQA-UHFFFAOYSA-N 0.000 description 1
- LEBVLXFERQHONN-UHFFFAOYSA-N 1-butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide Chemical compound CCCCN1CCCCC1C(=O)NC1=C(C)C=CC=C1C LEBVLXFERQHONN-UHFFFAOYSA-N 0.000 description 1
- BFSVOASYOCHEOV-UHFFFAOYSA-N 2-diethylaminoethanol Chemical compound CCN(CC)CCO BFSVOASYOCHEOV-UHFFFAOYSA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- XZKIHKMTEMTJQX-UHFFFAOYSA-N 4-Nitrophenyl Phosphate Chemical compound OP(O)(=O)OC1=CC=C([N+]([O-])=O)C=C1 XZKIHKMTEMTJQX-UHFFFAOYSA-N 0.000 description 1
- TVZGACDUOSZQKY-LBPRGKRZSA-N 4-aminofolic acid Chemical compound C1=NC2=NC(N)=NC(N)=C2N=C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 TVZGACDUOSZQKY-LBPRGKRZSA-N 0.000 description 1
- 102100022900 Actin, cytoplasmic 1 Human genes 0.000 description 1
- 108010085238 Actins Proteins 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 206010003445 Ascites Diseases 0.000 description 1
- 101100136076 Aspergillus oryzae (strain ATCC 42149 / RIB 40) pel1 gene Proteins 0.000 description 1
- 208000023275 Autoimmune disease Diseases 0.000 description 1
- 238000000035 BCA protein assay Methods 0.000 description 1
- 241000304886 Bacilli Species 0.000 description 1
- 241000193738 Bacillus anthracis Species 0.000 description 1
- 244000063299 Bacillus subtilis Species 0.000 description 1
- 235000014469 Bacillus subtilis Nutrition 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 108010022366 Carcinoembryonic Antigen Proteins 0.000 description 1
- 102100025475 Carcinoembryonic antigen-related cell adhesion molecule 5 Human genes 0.000 description 1
- 241000700198 Cavia Species 0.000 description 1
- 102000009016 Cholera Toxin Human genes 0.000 description 1
- 108010049048 Cholera Toxin Proteins 0.000 description 1
- 108091026890 Coding region Proteins 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 241000702421 Dependoparvovirus Species 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 206010061818 Disease progression Diseases 0.000 description 1
- 206010013457 Dissociation Diseases 0.000 description 1
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 1
- 108010000912 Egg Proteins Proteins 0.000 description 1
- 102000002322 Egg Proteins Human genes 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 102000004533 Endonucleases Human genes 0.000 description 1
- 108010042407 Endonucleases Proteins 0.000 description 1
- 108700039887 Essential Genes Proteins 0.000 description 1
- 239000004606 Fillers/Extenders Substances 0.000 description 1
- 241000700662 Fowlpox virus Species 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 102100036242 HLA class II histocompatibility antigen, DQ alpha 2 chain Human genes 0.000 description 1
- 241000238631 Hexapoda Species 0.000 description 1
- 108010088652 Histocompatibility Antigens Class I Proteins 0.000 description 1
- 101000864089 Homo sapiens HLA class II histocompatibility antigen, DP alpha 1 chain Proteins 0.000 description 1
- 101000930802 Homo sapiens HLA class II histocompatibility antigen, DQ alpha 1 chain Proteins 0.000 description 1
- 101000930801 Homo sapiens HLA class II histocompatibility antigen, DQ alpha 2 chain Proteins 0.000 description 1
- 101000968032 Homo sapiens HLA class II histocompatibility antigen, DR beta 3 chain Proteins 0.000 description 1
- PMMYEEVYMWASQN-DMTCNVIQSA-N Hydroxyproline Chemical compound O[C@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-DMTCNVIQSA-N 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- UGQMRVRMYYASKQ-UHFFFAOYSA-N Hypoxanthine nucleoside Natural products OC1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 UGQMRVRMYYASKQ-UHFFFAOYSA-N 0.000 description 1
- 108700005091 Immunoglobulin Genes Proteins 0.000 description 1
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 1
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 1
- 108090000978 Interleukin-4 Proteins 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- UKAUYVFTDYCKQA-VKHMYHEASA-N L-homoserine Chemical compound OC(=O)[C@@H](N)CCO UKAUYVFTDYCKQA-VKHMYHEASA-N 0.000 description 1
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- LRQKBLKVPFOOQJ-YFKPBYRVSA-N L-norleucine Chemical compound CCCC[C@H]([NH3+])C([O-])=O LRQKBLKVPFOOQJ-YFKPBYRVSA-N 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 108090001030 Lipoproteins Proteins 0.000 description 1
- 102000004895 Lipoproteins Human genes 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 241000282560 Macaca mulatta Species 0.000 description 1
- 125000001429 N-terminal alpha-amino-acid group Chemical group 0.000 description 1
- 108091061960 Naked DNA Proteins 0.000 description 1
- 229930193140 Neomycin Natural products 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 102000043276 Oncogene Human genes 0.000 description 1
- 108700020796 Oncogene Proteins 0.000 description 1
- 108090000526 Papain Proteins 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 241000235648 Pichia Species 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 241000607142 Salmonella Species 0.000 description 1
- 241000293871 Salmonella enterica subsp. enterica serovar Typhi Species 0.000 description 1
- 229920002684 Sepharose Polymers 0.000 description 1
- 108050005900 Signal peptide peptidase-like 2a Proteins 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 101710137500 T7 RNA polymerase Proteins 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 239000004098 Tetracycline Substances 0.000 description 1
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 1
- 239000004473 Threonine Substances 0.000 description 1
- 206010046865 Vaccinia virus infection Diseases 0.000 description 1
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 1
- 210000000683 abdominal cavity Anatomy 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 238000011481 absorbance measurement Methods 0.000 description 1
- 235000011054 acetic acid Nutrition 0.000 description 1
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 238000005377 adsorption chromatography Methods 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 239000000556 agonist Substances 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- 230000000172 allergic effect Effects 0.000 description 1
- 230000007815 allergy Effects 0.000 description 1
- 125000000746 allylic group Chemical group 0.000 description 1
- 229940037003 alum Drugs 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- ILRRQNADMUWWFW-UHFFFAOYSA-K aluminium phosphate Chemical compound O1[Al]2OP1(=O)O2 ILRRQNADMUWWFW-UHFFFAOYSA-K 0.000 description 1
- 230000001668 ameliorated effect Effects 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 229960003896 aminopterin Drugs 0.000 description 1
- 238000012870 ammonium sulfate precipitation Methods 0.000 description 1
- 229960000723 ampicillin Drugs 0.000 description 1
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 1
- 229940035676 analgesics Drugs 0.000 description 1
- 230000033115 angiogenesis Effects 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 239000000730 antalgic agent Substances 0.000 description 1
- 230000001772 anti-angiogenic effect Effects 0.000 description 1
- 230000001093 anti-cancer Effects 0.000 description 1
- 239000002260 anti-inflammatory agent Substances 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 210000000628 antibody-producing cell Anatomy 0.000 description 1
- 230000000890 antigenic effect Effects 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 208000010668 atopic eczema Diseases 0.000 description 1
- 230000002238 attenuated effect Effects 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 238000001815 biotherapy Methods 0.000 description 1
- 229930189065 blasticidin Natural products 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 239000004067 bulking agent Substances 0.000 description 1
- 229960003150 bupivacaine Drugs 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 210000004900 c-terminal fragment Anatomy 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 230000005880 cancer cell killing Effects 0.000 description 1
- 230000009702 cancer cell proliferation Effects 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- UHBYWPGGCSDKFX-UHFFFAOYSA-N carboxyglutamic acid Chemical compound OC(=O)C(N)CC(C(O)=O)C(O)=O UHBYWPGGCSDKFX-UHFFFAOYSA-N 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 230000020411 cell activation Effects 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 229960005091 chloramphenicol Drugs 0.000 description 1
- WIIZWVCIJKGZOK-RKDXNWHRSA-N chloramphenicol Chemical compound ClC(Cl)C(=O)N[C@H](CO)[C@H](O)C1=CC=C([N+]([O-])=O)C=C1 WIIZWVCIJKGZOK-RKDXNWHRSA-N 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 238000010668 complexation reaction Methods 0.000 description 1
- 230000001268 conjugating effect Effects 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- NKLPQNGYXWVELD-UHFFFAOYSA-M coomassie brilliant blue Chemical compound [Na+].C1=CC(OCC)=CC=C1NC1=CC=C(C(=C2C=CC(C=C2)=[N+](CC)CC=2C=C(C=CC=2)S([O-])(=O)=O)C=2C=CC(=CC=2)N(CC)CC=2C=C(C=CC=2)S([O-])(=O)=O)C=C1 NKLPQNGYXWVELD-UHFFFAOYSA-M 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 239000012531 culture fluid Substances 0.000 description 1
- 238000012136 culture method Methods 0.000 description 1
- 238000011498 curative surgery Methods 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 210000000172 cytosol Anatomy 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- 230000000254 damaging effect Effects 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 230000000368 destabilizing effect Effects 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 230000005750 disease progression Effects 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 208000018459 dissociative disease Diseases 0.000 description 1
- PMMYEEVYMWASQN-UHFFFAOYSA-N dl-hydroxyproline Natural products OC1C[NH2+]C(C([O-])=O)C1 PMMYEEVYMWASQN-UHFFFAOYSA-N 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 235000013345 egg yolk Nutrition 0.000 description 1
- 210000002969 egg yolk Anatomy 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 210000003038 endothelium Anatomy 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- 230000017188 evasion or tolerance of host immune response Effects 0.000 description 1
- 239000012091 fetal bovine serum Substances 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 239000011888 foil Substances 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 230000008014 freezing Effects 0.000 description 1
- 238000007710 freezing Methods 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 235000011087 fumaric acid Nutrition 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 238000002523 gelfiltration Methods 0.000 description 1
- 238000003205 genotyping method Methods 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 102000006602 glyceraldehyde-3-phosphate dehydrogenase Human genes 0.000 description 1
- 108020004445 glyceraldehyde-3-phosphate dehydrogenase Proteins 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 230000006801 homologous recombination Effects 0.000 description 1
- 238000002744 homologous recombination Methods 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 125000004435 hydrogen atom Chemical class [H]* 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 229960002591 hydroxyproline Drugs 0.000 description 1
- 229940027941 immunoglobulin g Drugs 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 238000002991 immunohistochemical analysis Methods 0.000 description 1
- 230000006054 immunological memory Effects 0.000 description 1
- 238000000126 in silico method Methods 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 229960000318 kanamycin Drugs 0.000 description 1
- 229930027917 kanamycin Natural products 0.000 description 1
- SBUJHOSQTJFQJX-NOAMYHISSA-N kanamycin Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N SBUJHOSQTJFQJX-NOAMYHISSA-N 0.000 description 1
- 229930182823 kanamycin A Natural products 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 101150066555 lacZ gene Proteins 0.000 description 1
- 210000001821 langerhans cell Anatomy 0.000 description 1
- 238000001638 lipofection Methods 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000007791 liquid phase Substances 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 230000036210 malignancy Effects 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 230000015654 memory Effects 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 239000000693 micelle Substances 0.000 description 1
- 238000010208 microarray analysis Methods 0.000 description 1
- 239000011325 microbead Substances 0.000 description 1
- 238000002715 modification method Methods 0.000 description 1
- 102000035118 modified proteins Human genes 0.000 description 1
- 108091005573 modified proteins Proteins 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 210000004898 n-terminal fragment Anatomy 0.000 description 1
- 229920005615 natural polymer Polymers 0.000 description 1
- 230000017066 negative regulation of growth Effects 0.000 description 1
- 229960004927 neomycin Drugs 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 238000007474 nonparametric Mann- Whitney U test Methods 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- IPCSVZSSVZVIGE-UHFFFAOYSA-N palmitic acid group Chemical group C(CCCCCCCCCCCCCCC)(=O)O IPCSVZSSVZVIGE-UHFFFAOYSA-N 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 229940055729 papain Drugs 0.000 description 1
- 235000019834 papain Nutrition 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 101150040383 pel2 gene Proteins 0.000 description 1
- 101150050446 pelB gene Proteins 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 210000001322 periplasm Anatomy 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 238000009521 phase II clinical trial Methods 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- BZQFBWGGLXLEPQ-REOHCLBHSA-N phosphoserine Chemical compound OC(=O)[C@@H](N)COP(O)(O)=O BZQFBWGGLXLEPQ-REOHCLBHSA-N 0.000 description 1
- 230000001766 physiological effect Effects 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 239000013612 plasmid Substances 0.000 description 1
- 230000010287 polarization Effects 0.000 description 1
- 238000007694 polyacrylamide gel isoelectric focusing Methods 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 230000009862 primary prevention Effects 0.000 description 1
- 230000000644 propagated effect Effects 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- 235000019833 protease Nutrition 0.000 description 1
- 238000000159 protein binding assay Methods 0.000 description 1
- 238000012514 protein characterization Methods 0.000 description 1
- 238000001742 protein purification Methods 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 239000013074 reference sample Substances 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000004366 reverse phase liquid chromatography Methods 0.000 description 1
- 238000003757 reverse transcription PCR Methods 0.000 description 1
- 108010008790 ribosomal phosphoprotein P1 Proteins 0.000 description 1
- YDBYJHTYSHBBAU-UHFFFAOYSA-O s-methylmethionine Chemical compound C[S+](C)CCC(N)C(O)=O YDBYJHTYSHBBAU-UHFFFAOYSA-O 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 210000003296 saliva Anatomy 0.000 description 1
- 238000005185 salting out Methods 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 229940054269 sodium pyruvate Drugs 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 150000003462 sulfoxides Chemical class 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- 238000007910 systemic administration Methods 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 229960002180 tetracycline Drugs 0.000 description 1
- 229930101283 tetracycline Natural products 0.000 description 1
- 235000019364 tetracycline Nutrition 0.000 description 1
- 150000003522 tetracyclines Chemical class 0.000 description 1
- 229940126622 therapeutic monoclonal antibody Drugs 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 229940104230 thymidine Drugs 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- FGMPLJWBKKVCDB-UHFFFAOYSA-N trans-L-hydroxy-proline Natural products ON1CCCC1C(O)=O FGMPLJWBKKVCDB-UHFFFAOYSA-N 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 230000009261 transgenic effect Effects 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 230000004614 tumor growth Effects 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 238000000108 ultra-filtration Methods 0.000 description 1
- 241000701161 unidentified adenovirus Species 0.000 description 1
- 241000701447 unidentified baculovirus Species 0.000 description 1
- 241001515965 unidentified phage Species 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 238000002255 vaccination Methods 0.000 description 1
- 208000007089 vaccinia Diseases 0.000 description 1
- 239000004474 valine Substances 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 239000013603 viral vector Substances 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
- C07K14/4701—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals not used
- C07K14/4725—Proteoglycans, e.g. aggreccan
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/11—T-cells, e.g. tumour infiltrating lymphocytes [TIL] or regulatory T [Treg] cells; Lymphokine-activated killer [LAK] cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4261—Proteoglycans, e.g. glypican, brevican or CSPG4
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K7/00—Peptides having 5 to 20 amino acids in a fully defined sequence; Derivatives thereof
- C07K7/04—Linear peptides containing only normal peptide links
- C07K7/06—Linear peptides containing only normal peptide links having 5 to 11 amino acids
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57484—Immunoassay; Biospecific binding assay; Materials therefor for cancer involving compounds serving as markers for tumor, cancer, neoplasia, e.g. cellular determinants, receptors, heat shock/stress proteins, A-protein, oligosaccharides, metabolites
- G01N33/57492—Immunoassay; Biospecific binding assay; Materials therefor for cancer involving compounds serving as markers for tumor, cancer, neoplasia, e.g. cellular determinants, receptors, heat shock/stress proteins, A-protein, oligosaccharides, metabolites involving compounds localized on the membrane of tumor or cancer cells
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/80—Vaccine for a specifically defined cancer
- A61K2039/844—Liver
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/80—Vaccine for a specifically defined cancer
- A61K2039/876—Skin, melanoma
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2510/00—Genetically modified cells
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Medicinal Chemistry (AREA)
- Biochemistry (AREA)
- Genetics & Genomics (AREA)
- Biomedical Technology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Biotechnology (AREA)
- Biophysics (AREA)
- Cell Biology (AREA)
- Zoology (AREA)
- Microbiology (AREA)
- Physics & Mathematics (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Analytical Chemistry (AREA)
- Epidemiology (AREA)
- Oncology (AREA)
- Pathology (AREA)
- Food Science & Technology (AREA)
- Wood Science & Technology (AREA)
- General Physics & Mathematics (AREA)
- Pharmacology & Pharmacy (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- Gastroenterology & Hepatology (AREA)
- Hospice & Palliative Care (AREA)
- Toxicology (AREA)
- Plant Pathology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014248759 | 2014-12-09 | ||
| JP2014248759 | 2014-12-09 | ||
| PCT/JP2015/006029 WO2016092787A1 (en) | 2014-12-09 | 2015-12-04 | Gpc3 epitope peptides for th1 cells and vaccines containing the same |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2018508181A true JP2018508181A (ja) | 2018-03-29 |
| JP2018508181A5 JP2018508181A5 (enExample) | 2019-01-17 |
Family
ID=56107012
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2017518379A Withdrawn JP2018508181A (ja) | 2014-12-09 | 2015-12-04 | Th1細胞のためのGPC3エピトープペプチドおよびこれを含有するワクチン |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US20170362287A1 (enExample) |
| EP (1) | EP3230301A4 (enExample) |
| JP (1) | JP2018508181A (enExample) |
| TW (1) | TW201636358A (enExample) |
| WO (1) | WO2016092787A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2020510698A (ja) * | 2017-03-03 | 2020-04-09 | トレオス バイオ ゼットアールティー | 個別化された免疫原性ペプチドの同定のプラットフォーム |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018143454A1 (ja) * | 2017-02-06 | 2018-08-09 | 国立研究開発法人国立がん研究センター | 新規t細胞受容体 |
| WO2025110187A1 (ja) * | 2023-11-21 | 2025-05-30 | 国立研究開発法人国立がん研究センター | Hla-a02拘束性グリピカン3由来ペプチド特異的tcr |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2001011033A2 (en) * | 1999-08-04 | 2001-02-15 | Abbott Laboratories | Identification of genes essential for the survival of haemophilus influenzae through genome scanning by transposition mutagenesis |
| AU2003254950A1 (en) * | 2002-08-26 | 2004-03-11 | Kirin Beer Kabushiki Kaisha | Peptides and drugs containing the same |
| KR101304243B1 (ko) * | 2005-08-09 | 2013-09-05 | 온코세라피 사이언스 가부시키가이샤 | HLA-A2 양성 환자용 글리피칸-3(glypican-3)유래의 암 거절항원 펩티드 및 그를 포함하는 의약 |
| JP5025159B2 (ja) * | 2006-04-28 | 2012-09-12 | シスメックス株式会社 | 生体成分測定装置 |
| TW201425333A (zh) * | 2007-04-11 | 2014-07-01 | Oncotherapy Science Inc | 腫瘤血管內皮標誌8胜肽及包含此胜肽之疫苗 |
| US8088574B2 (en) * | 2007-07-31 | 2012-01-03 | Wisconsin Alumni Research Foundation | Poly(A) polymerase |
| TW200932260A (en) * | 2007-11-28 | 2009-08-01 | Oncotherapy Science Inc | STAT3 epitope peptides |
| JP2013523084A (ja) * | 2010-04-09 | 2013-06-17 | オンコセラピー・サイエンス株式会社 | Cdca5ペプチドおよびそれを含むワクチン |
| US8928735B2 (en) * | 2011-06-14 | 2015-01-06 | Microsoft Corporation | Combined lighting, projection, and image capture without video feedback |
| US9091651B2 (en) * | 2011-12-21 | 2015-07-28 | Integrated Diagnostics, Inc. | Selected reaction monitoring assays |
| US20130217122A1 (en) * | 2012-02-21 | 2013-08-22 | The Trustees Of The University Of Pennsylvania | Expansion of Interferon-Gamma-Producing T-Cells Using Glypican-3 Peptide Library |
| US9016790B2 (en) * | 2012-10-04 | 2015-04-28 | William E. Voyce, IV | Convertible seating reclining chair |
| TWI693073B (zh) * | 2012-12-21 | 2020-05-11 | 日商中外製藥股份有限公司 | 對gpc3標的治療劑療法為有效之患者投與的gpc3標的治療劑 |
-
2015
- 2015-12-01 TW TW104140060A patent/TW201636358A/zh unknown
- 2015-12-04 WO PCT/JP2015/006029 patent/WO2016092787A1/en not_active Ceased
- 2015-12-04 EP EP15867784.9A patent/EP3230301A4/en not_active Withdrawn
- 2015-12-04 US US15/534,311 patent/US20170362287A1/en not_active Abandoned
- 2015-12-04 JP JP2017518379A patent/JP2018508181A/ja not_active Withdrawn
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2020510698A (ja) * | 2017-03-03 | 2020-04-09 | トレオス バイオ ゼットアールティー | 個別化された免疫原性ペプチドの同定のプラットフォーム |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3230301A1 (en) | 2017-10-18 |
| TW201636358A (zh) | 2016-10-16 |
| WO2016092787A1 (en) | 2016-06-16 |
| EP3230301A4 (en) | 2018-08-01 |
| US20170362287A1 (en) | 2017-12-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5799394B2 (ja) | Neil3ペプチドおよびそれを含むワクチン | |
| JP6259983B2 (ja) | Th1細胞のKIF20Aエピトープペプチドおよびこれを含有するワクチン | |
| JP6124149B2 (ja) | Topkペプチドおよびそれを含むワクチン | |
| JP5884104B2 (ja) | 改変melkペプチドおよびそれを含むワクチン | |
| JP6255593B2 (ja) | Th1細胞のCDCA1エピトープペプチドおよびこれを含有するワクチン | |
| JP5564730B2 (ja) | Mphosph1ペプチドおよびそれを含むワクチン | |
| JP2014519311A (ja) | Sema5bペプチドおよびそれを含むワクチン | |
| JP2013523084A (ja) | Cdca5ペプチドおよびそれを含むワクチン | |
| JP6518921B2 (ja) | Th1細胞のIMP−3エピトープペプチドおよびこれを含有するワクチン | |
| JP6255594B2 (ja) | Th1細胞のLY6Kエピトープペプチドおよびこれを含有するワクチン | |
| JP2016222676A (ja) | Tomm34ペプチドおよびそれを含むワクチン | |
| JP2018508181A (ja) | Th1細胞のためのGPC3エピトープペプチドおよびこれを含有するワクチン | |
| JP5838482B2 (ja) | Hjurpペプチドおよびそれを含むワクチン | |
| JP2012519470A (ja) | Vangl1ペプチドおよびそれを含むワクチン | |
| US8697631B2 (en) | TMEM22 peptides and vaccines including the same | |
| JP2013512659A (ja) | Mybl2ペプチドおよびそれを含むワクチン | |
| JP2013540419A (ja) | Ttll4ペプチドおよびそれを含むワクチン | |
| US20150322112A1 (en) | Sema5b peptides and vaccines containing the same | |
| JP2014500001A (ja) | C18orf54ペプチドおよびそれを含むワクチン | |
| JP2014504146A (ja) | Wdhd1ペプチドおよびそれを含むワクチン | |
| JP2019535270A (ja) | Th1細胞のためのDEPDC1エピトープペプチドおよびこれを含有するワクチン | |
| JP2020501524A (ja) | Th1細胞のためのMPHOSPH1エピトープペプチドおよびこれを含有するワクチン | |
| JP2013523083A (ja) | Cluap1ペプチドおよびそれを含むワクチン |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20181122 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20181122 |
|
| A761 | Written withdrawal of application |
Free format text: JAPANESE INTERMEDIATE CODE: A761 Effective date: 20190708 |