JP2014508527A - 免疫調節活性を有する細胞集団、単離方法および使用 - Google Patents
免疫調節活性を有する細胞集団、単離方法および使用 Download PDFInfo
- Publication number
- JP2014508527A JP2014508527A JP2013557133A JP2013557133A JP2014508527A JP 2014508527 A JP2014508527 A JP 2014508527A JP 2013557133 A JP2013557133 A JP 2013557133A JP 2013557133 A JP2013557133 A JP 2013557133A JP 2014508527 A JP2014508527 A JP 2014508527A
- Authority
- JP
- Japan
- Prior art keywords
- cells
- cell
- cell population
- disease
- present
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000002955 isolation Methods 0.000 title description 8
- 230000002519 immonomodulatory effect Effects 0.000 title description 7
- 210000004027 cell Anatomy 0.000 claims abstract description 561
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 98
- 208000023275 Autoimmune disease Diseases 0.000 claims abstract description 61
- 238000011282 treatment Methods 0.000 claims abstract description 59
- 230000001404 mediated effect Effects 0.000 claims abstract description 54
- 201000010099 disease Diseases 0.000 claims abstract description 53
- 208000024891 symptom Diseases 0.000 claims abstract description 48
- 208000035475 disorder Diseases 0.000 claims abstract description 45
- 102100029740 Poliovirus receptor Human genes 0.000 claims abstract description 29
- 208000037979 autoimmune inflammatory disease Diseases 0.000 claims abstract description 29
- 102100035488 Nectin-2 Human genes 0.000 claims abstract description 28
- 210000000056 organ Anatomy 0.000 claims abstract description 28
- 238000000034 method Methods 0.000 claims description 88
- 210000001519 tissue Anatomy 0.000 claims description 55
- 208000027866 inflammatory disease Diseases 0.000 claims description 53
- 239000000203 mixture Substances 0.000 claims description 36
- 210000002901 mesenchymal stem cell Anatomy 0.000 claims description 32
- 239000008194 pharmaceutical composition Substances 0.000 claims description 32
- 206010039073 rheumatoid arthritis Diseases 0.000 claims description 23
- 208000022559 Inflammatory bowel disease Diseases 0.000 claims description 19
- 210000000577 adipose tissue Anatomy 0.000 claims description 19
- 239000007787 solid Substances 0.000 claims description 16
- 208000037976 chronic inflammation Diseases 0.000 claims description 14
- 239000002609 medium Substances 0.000 claims description 13
- 208000037893 chronic inflammatory disorder Diseases 0.000 claims description 11
- -1 CD11c Proteins 0.000 claims description 10
- 239000006143 cell culture medium Substances 0.000 claims description 10
- 239000003937 drug carrier Substances 0.000 claims description 10
- 210000000265 leukocyte Anatomy 0.000 claims description 9
- 210000005259 peripheral blood Anatomy 0.000 claims description 9
- 239000011886 peripheral blood Substances 0.000 claims description 9
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 claims description 7
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 claims description 7
- 102100031573 Hematopoietic progenitor cell antigen CD34 Human genes 0.000 claims description 5
- 101000777663 Homo sapiens Hematopoietic progenitor cell antigen CD34 Proteins 0.000 claims description 5
- 230000001464 adherent effect Effects 0.000 claims description 5
- 101000946889 Homo sapiens Monocyte differentiation antigen CD14 Proteins 0.000 claims description 4
- 102100035877 Monocyte differentiation antigen CD14 Human genes 0.000 claims description 4
- 102100024616 Platelet endothelial cell adhesion molecule Human genes 0.000 claims description 4
- 239000006285 cell suspension Substances 0.000 claims description 4
- 101001046686 Homo sapiens Integrin alpha-M Proteins 0.000 claims description 3
- 102100022338 Integrin alpha-M Human genes 0.000 claims description 3
- 102100022297 Integrin alpha-X Human genes 0.000 claims description 3
- 230000014509 gene expression Effects 0.000 abstract description 45
- 210000000987 immune system Anatomy 0.000 abstract description 23
- 239000003446 ligand Substances 0.000 abstract description 21
- 102000005962 receptors Human genes 0.000 abstract description 19
- 108020003175 receptors Proteins 0.000 abstract description 19
- 230000009286 beneficial effect Effects 0.000 abstract description 17
- 102100029647 Apoptosis-associated speck-like protein containing a CARD Human genes 0.000 abstract description 11
- 230000002265 prevention Effects 0.000 abstract description 10
- 101000728679 Homo sapiens Apoptosis-associated speck-like protein containing a CARD Proteins 0.000 abstract description 3
- 238000011467 adoptive cell therapy Methods 0.000 abstract description 2
- 230000003247 decreasing effect Effects 0.000 abstract description 2
- 108010074328 Interferon-gamma Proteins 0.000 description 107
- 102100037850 Interferon gamma Human genes 0.000 description 103
- 210000000822 natural killer cell Anatomy 0.000 description 80
- 239000000427 antigen Substances 0.000 description 78
- 102000036639 antigens Human genes 0.000 description 78
- 108091007433 antigens Proteins 0.000 description 78
- 239000003814 drug Substances 0.000 description 71
- 210000003289 regulatory T cell Anatomy 0.000 description 25
- 210000002966 serum Anatomy 0.000 description 22
- 208000015943 Coeliac disease Diseases 0.000 description 20
- 238000002054 transplantation Methods 0.000 description 20
- 230000001939 inductive effect Effects 0.000 description 18
- 241001465754 Metazoa Species 0.000 description 17
- 102100040061 Indoleamine 2,3-dioxygenase 1 Human genes 0.000 description 16
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 15
- 108010068370 Glutens Proteins 0.000 description 15
- 238000003501 co-culture Methods 0.000 description 15
- 238000002474 experimental method Methods 0.000 description 15
- 238000000684 flow cytometry Methods 0.000 description 13
- 201000006417 multiple sclerosis Diseases 0.000 description 13
- 238000002360 preparation method Methods 0.000 description 13
- 230000001225 therapeutic effect Effects 0.000 description 13
- 102000006386 Myelin Proteins Human genes 0.000 description 12
- 108010083674 Myelin Proteins Proteins 0.000 description 12
- 238000003556 assay Methods 0.000 description 12
- 239000003795 chemical substances by application Substances 0.000 description 12
- 230000000694 effects Effects 0.000 description 12
- 210000003743 erythrocyte Anatomy 0.000 description 12
- 210000005012 myelin Anatomy 0.000 description 12
- 230000003478 prestimulatory effect Effects 0.000 description 12
- 239000006228 supernatant Substances 0.000 description 12
- 102000004190 Enzymes Human genes 0.000 description 11
- 108090000790 Enzymes Proteins 0.000 description 11
- 230000003213 activating effect Effects 0.000 description 11
- 238000007796 conventional method Methods 0.000 description 11
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 11
- 229940088598 enzyme Drugs 0.000 description 11
- 239000012091 fetal bovine serum Substances 0.000 description 11
- 235000021312 gluten Nutrition 0.000 description 11
- 239000002953 phosphate buffered saline Substances 0.000 description 11
- 230000000735 allogeneic effect Effects 0.000 description 10
- 210000004271 bone marrow stromal cell Anatomy 0.000 description 10
- 230000002757 inflammatory effect Effects 0.000 description 10
- 230000000638 stimulation Effects 0.000 description 10
- 230000004069 differentiation Effects 0.000 description 9
- 238000004519 manufacturing process Methods 0.000 description 9
- 102100038077 CD226 antigen Human genes 0.000 description 8
- 101000884298 Homo sapiens CD226 antigen Proteins 0.000 description 8
- 101001023379 Homo sapiens Lysosome-associated membrane glycoprotein 1 Proteins 0.000 description 8
- 102100035133 Lysosome-associated membrane glycoprotein 1 Human genes 0.000 description 8
- 206010003246 arthritis Diseases 0.000 description 8
- 230000028993 immune response Effects 0.000 description 8
- 208000026278 immune system disease Diseases 0.000 description 8
- 230000000670 limiting effect Effects 0.000 description 8
- 239000000463 material Substances 0.000 description 8
- 239000013642 negative control Substances 0.000 description 8
- 150000001413 amino acids Chemical group 0.000 description 7
- 238000004458 analytical method Methods 0.000 description 7
- 239000012634 fragment Substances 0.000 description 7
- 238000000338 in vitro Methods 0.000 description 7
- 238000007443 liposuction Methods 0.000 description 7
- 239000003550 marker Substances 0.000 description 7
- 108090000765 processed proteins & peptides Proteins 0.000 description 7
- 108090000623 proteins and genes Proteins 0.000 description 7
- 230000004044 response Effects 0.000 description 7
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 6
- 102000008186 Collagen Human genes 0.000 description 6
- 108010035532 Collagen Proteins 0.000 description 6
- 102100037241 Endoglin Human genes 0.000 description 6
- 101000881679 Homo sapiens Endoglin Proteins 0.000 description 6
- 101000581981 Homo sapiens Neural cell adhesion molecule 1 Proteins 0.000 description 6
- 101000800116 Homo sapiens Thy-1 membrane glycoprotein Proteins 0.000 description 6
- 102100027347 Neural cell adhesion molecule 1 Human genes 0.000 description 6
- 102100033523 Thy-1 membrane glycoprotein Human genes 0.000 description 6
- 210000001185 bone marrow Anatomy 0.000 description 6
- 238000004113 cell culture Methods 0.000 description 6
- 239000002458 cell surface marker Substances 0.000 description 6
- 229920001436 collagen Polymers 0.000 description 6
- 238000002648 combination therapy Methods 0.000 description 6
- 230000009089 cytolysis Effects 0.000 description 6
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 6
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 6
- YGPSJZOEDVAXAB-UHFFFAOYSA-N kynurenine Chemical compound OC(=O)C(N)CC(=O)C1=CC=CC=C1N YGPSJZOEDVAXAB-UHFFFAOYSA-N 0.000 description 6
- 102100022002 CD59 glycoprotein Human genes 0.000 description 5
- 208000011231 Crohn disease Diseases 0.000 description 5
- 241000282412 Homo Species 0.000 description 5
- 101000897400 Homo sapiens CD59 glycoprotein Proteins 0.000 description 5
- 101000935043 Homo sapiens Integrin beta-1 Proteins 0.000 description 5
- 102100025304 Integrin beta-1 Human genes 0.000 description 5
- 210000001744 T-lymphocyte Anatomy 0.000 description 5
- 230000004913 activation Effects 0.000 description 5
- 230000001363 autoimmune Effects 0.000 description 5
- 238000010367 cloning Methods 0.000 description 5
- 238000006731 degradation reaction Methods 0.000 description 5
- 208000037765 diseases and disorders Diseases 0.000 description 5
- 238000001727 in vivo Methods 0.000 description 5
- 238000002347 injection Methods 0.000 description 5
- 239000007924 injection Substances 0.000 description 5
- 230000003993 interaction Effects 0.000 description 5
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 5
- 229920001184 polypeptide Polymers 0.000 description 5
- 230000008569 process Effects 0.000 description 5
- 102000004196 processed proteins & peptides Human genes 0.000 description 5
- 230000005855 radiation Effects 0.000 description 5
- 210000000130 stem cell Anatomy 0.000 description 5
- 229940124597 therapeutic agent Drugs 0.000 description 5
- 102100032912 CD44 antigen Human genes 0.000 description 4
- 102100037904 CD9 antigen Human genes 0.000 description 4
- 206010009900 Colitis ulcerative Diseases 0.000 description 4
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 4
- 208000007465 Giant cell arteritis Diseases 0.000 description 4
- 108010061711 Gliadin Proteins 0.000 description 4
- 102000001398 Granzyme Human genes 0.000 description 4
- 108060005986 Granzyme Proteins 0.000 description 4
- 101000868273 Homo sapiens CD44 antigen Proteins 0.000 description 4
- 101000738354 Homo sapiens CD9 antigen Proteins 0.000 description 4
- 101000599852 Homo sapiens Intercellular adhesion molecule 1 Proteins 0.000 description 4
- 102100037877 Intercellular adhesion molecule 1 Human genes 0.000 description 4
- 108010000123 Myelin-Oligodendrocyte Glycoprotein Proteins 0.000 description 4
- 102100023302 Myelin-oligodendrocyte glycoprotein Human genes 0.000 description 4
- 108010004729 Phycoerythrin Proteins 0.000 description 4
- 201000004681 Psoriasis Diseases 0.000 description 4
- 102000004142 Trypsin Human genes 0.000 description 4
- 108090000631 Trypsin Proteins 0.000 description 4
- 201000006704 Ulcerative Colitis Diseases 0.000 description 4
- 210000001789 adipocyte Anatomy 0.000 description 4
- 238000013019 agitation Methods 0.000 description 4
- 229940024606 amino acid Drugs 0.000 description 4
- 235000001014 amino acid Nutrition 0.000 description 4
- 230000003321 amplification Effects 0.000 description 4
- 229940121363 anti-inflammatory agent Drugs 0.000 description 4
- 239000002260 anti-inflammatory agent Substances 0.000 description 4
- 231100000433 cytotoxic Toxicity 0.000 description 4
- 230000001472 cytotoxic effect Effects 0.000 description 4
- 230000006378 damage Effects 0.000 description 4
- 239000012636 effector Substances 0.000 description 4
- 239000012894 fetal calf serum Substances 0.000 description 4
- 230000006870 function Effects 0.000 description 4
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 4
- 229940121354 immunomodulator Drugs 0.000 description 4
- 230000001506 immunosuppresive effect Effects 0.000 description 4
- 238000011534 incubation Methods 0.000 description 4
- 230000002401 inhibitory effect Effects 0.000 description 4
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 230000007246 mechanism Effects 0.000 description 4
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 4
- 238000003199 nucleic acid amplification method Methods 0.000 description 4
- 239000008188 pellet Substances 0.000 description 4
- 239000004033 plastic Substances 0.000 description 4
- 230000000069 prophylactic effect Effects 0.000 description 4
- 235000018102 proteins Nutrition 0.000 description 4
- 102000004169 proteins and genes Human genes 0.000 description 4
- 230000002829 reductive effect Effects 0.000 description 4
- 230000001105 regulatory effect Effects 0.000 description 4
- 230000008439 repair process Effects 0.000 description 4
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 4
- 208000011580 syndromic disease Diseases 0.000 description 4
- 206010043207 temporal arteritis Diseases 0.000 description 4
- 239000012588 trypsin Substances 0.000 description 4
- 230000000381 tumorigenic effect Effects 0.000 description 4
- 230000035899 viability Effects 0.000 description 4
- 238000005406 washing Methods 0.000 description 4
- 102000029816 Collagenase Human genes 0.000 description 3
- 108060005980 Collagenase Proteins 0.000 description 3
- 208000024869 Goodpasture syndrome Diseases 0.000 description 3
- 102000008070 Interferon-gamma Human genes 0.000 description 3
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 3
- 229930182555 Penicillin Natural products 0.000 description 3
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 3
- 241000288906 Primates Species 0.000 description 3
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 3
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 3
- 208000031981 Thrombocytopenic Idiopathic Purpura Diseases 0.000 description 3
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 3
- 206010047115 Vasculitis Diseases 0.000 description 3
- 239000002671 adjuvant Substances 0.000 description 3
- 235000019270 ammonium chloride Nutrition 0.000 description 3
- 239000003242 anti bacterial agent Substances 0.000 description 3
- 230000033228 biological regulation Effects 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 230000015556 catabolic process Effects 0.000 description 3
- 230000001413 cellular effect Effects 0.000 description 3
- 238000005119 centrifugation Methods 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 230000006020 chronic inflammation Effects 0.000 description 3
- 229960002424 collagenase Drugs 0.000 description 3
- 206010012601 diabetes mellitus Diseases 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 239000002552 dosage form Substances 0.000 description 3
- 210000002889 endothelial cell Anatomy 0.000 description 3
- 210000002950 fibroblast Anatomy 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 238000004128 high performance liquid chromatography Methods 0.000 description 3
- 239000002955 immunomodulating agent Substances 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 239000003978 infusion fluid Substances 0.000 description 3
- 229960003130 interferon gamma Drugs 0.000 description 3
- 238000010212 intracellular staining Methods 0.000 description 3
- 230000001678 irradiating effect Effects 0.000 description 3
- 239000008297 liquid dosage form Substances 0.000 description 3
- 239000006193 liquid solution Substances 0.000 description 3
- 239000006194 liquid suspension Substances 0.000 description 3
- 206010025135 lupus erythematosus Diseases 0.000 description 3
- 229910052618 mica group Inorganic materials 0.000 description 3
- 206010028417 myasthenia gravis Diseases 0.000 description 3
- 239000006179 pH buffering agent Substances 0.000 description 3
- 229940049954 penicillin Drugs 0.000 description 3
- 239000000546 pharmaceutical excipient Substances 0.000 description 3
- 239000008299 semisolid dosage form Substances 0.000 description 3
- 230000003637 steroidlike Effects 0.000 description 3
- 210000002536 stromal cell Anatomy 0.000 description 3
- 238000011277 treatment modality Methods 0.000 description 3
- 239000003981 vehicle Substances 0.000 description 3
- KISWVXRQTGLFGD-UHFFFAOYSA-N 2-[[2-[[6-amino-2-[[2-[[2-[[5-amino-2-[[2-[[1-[2-[[6-amino-2-[(2,5-diamino-5-oxopentanoyl)amino]hexanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]pyrrolidine-2-carbonyl]amino]-3-hydroxypropanoyl]amino]-5-oxopentanoyl]amino]-5-(diaminomethylideneamino)p Chemical compound C1CCN(C(=O)C(CCCN=C(N)N)NC(=O)C(CCCCN)NC(=O)C(N)CCC(N)=O)C1C(=O)NC(CO)C(=O)NC(CCC(N)=O)C(=O)NC(CCCN=C(N)N)C(=O)NC(CO)C(=O)NC(CCCCN)C(=O)NC(C(=O)NC(CC(C)C)C(O)=O)CC1=CC=C(O)C=C1 KISWVXRQTGLFGD-UHFFFAOYSA-N 0.000 description 2
- 208000026872 Addison Disease Diseases 0.000 description 2
- 206010002556 Ankylosing Spondylitis Diseases 0.000 description 2
- 208000003343 Antiphospholipid Syndrome Diseases 0.000 description 2
- 208000023328 Basedow disease Diseases 0.000 description 2
- 102100036008 CD48 antigen Human genes 0.000 description 2
- 241000283707 Capra Species 0.000 description 2
- 208000031229 Cardiomyopathies Diseases 0.000 description 2
- 102000004127 Cytokines Human genes 0.000 description 2
- 108090000695 Cytokines Proteins 0.000 description 2
- 201000004624 Dermatitis Diseases 0.000 description 2
- 208000001640 Fibromyalgia Diseases 0.000 description 2
- 206010018364 Glomerulonephritis Diseases 0.000 description 2
- 229930186217 Glycolipid Natural products 0.000 description 2
- 208000009329 Graft vs Host Disease Diseases 0.000 description 2
- 208000015023 Graves' disease Diseases 0.000 description 2
- 208000030836 Hashimoto thyroiditis Diseases 0.000 description 2
- 208000035186 Hemolytic Autoimmune Anemia Diseases 0.000 description 2
- 101001109501 Homo sapiens NKG2-D type II integral membrane protein Proteins 0.000 description 2
- 102000002265 Human Growth Hormone Human genes 0.000 description 2
- 108010000521 Human Growth Hormone Proteins 0.000 description 2
- 239000000854 Human Growth Hormone Substances 0.000 description 2
- 206010020751 Hypersensitivity Diseases 0.000 description 2
- 201000009794 Idiopathic Pulmonary Fibrosis Diseases 0.000 description 2
- 206010021245 Idiopathic thrombocytopenic purpura Diseases 0.000 description 2
- 206010061218 Inflammation Diseases 0.000 description 2
- 102000004877 Insulin Human genes 0.000 description 2
- 108090001061 Insulin Proteins 0.000 description 2
- 108010002352 Interleukin-1 Proteins 0.000 description 2
- 102000000589 Interleukin-1 Human genes 0.000 description 2
- 108010002350 Interleukin-2 Proteins 0.000 description 2
- 108020003285 Isocitrate lyase Proteins 0.000 description 2
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 2
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 2
- 102100030301 MHC class I polypeptide-related sequence A Human genes 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- 102100022680 NKG2-D type II integral membrane protein Human genes 0.000 description 2
- 108010004217 Natural Cytotoxicity Triggering Receptor 1 Proteins 0.000 description 2
- 102100032870 Natural cytotoxicity triggering receptor 1 Human genes 0.000 description 2
- 208000003076 Osteolysis Diseases 0.000 description 2
- 206010034277 Pemphigoid Diseases 0.000 description 2
- KHGNFPUMBJSZSM-UHFFFAOYSA-N Perforine Natural products COC1=C2CCC(O)C(CCC(C)(C)O)(OC)C2=NC2=C1C=CO2 KHGNFPUMBJSZSM-UHFFFAOYSA-N 0.000 description 2
- 208000031845 Pernicious anaemia Diseases 0.000 description 2
- 102000010780 Platelet-Derived Growth Factor Human genes 0.000 description 2
- 108010038512 Platelet-Derived Growth Factor Proteins 0.000 description 2
- RJKFOVLPORLFTN-LEKSSAKUSA-N Progesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)C)[C@@]1(C)CC2 RJKFOVLPORLFTN-LEKSSAKUSA-N 0.000 description 2
- 102000016202 Proteolipids Human genes 0.000 description 2
- 108010010974 Proteolipids Proteins 0.000 description 2
- 201000001263 Psoriatic Arthritis Diseases 0.000 description 2
- 208000036824 Psoriatic arthropathy Diseases 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- 208000021386 Sjogren Syndrome Diseases 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 108091008874 T cell receptors Proteins 0.000 description 2
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 2
- 208000001106 Takayasu Arteritis Diseases 0.000 description 2
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 2
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 2
- 206010053613 Type IV hypersensitivity reaction Diseases 0.000 description 2
- 102100023543 Vascular cell adhesion protein 1 Human genes 0.000 description 2
- 208000027418 Wounds and injury Diseases 0.000 description 2
- LEBBDRXHHNYZIA-LDUWYPJVSA-N [(2s,3r,4s,5r,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] n-[(z)-1,3-dihydroxyoctadec-4-en-2-yl]carbamate Chemical compound CCCCCCCCCCCCC\C=C/C(O)C(CO)NC(=O)O[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O LEBBDRXHHNYZIA-LDUWYPJVSA-N 0.000 description 2
- 230000035508 accumulation Effects 0.000 description 2
- 238000009825 accumulation Methods 0.000 description 2
- 208000026935 allergic disease Diseases 0.000 description 2
- 230000007815 allergy Effects 0.000 description 2
- APKFDSVGJQXUKY-INPOYWNPSA-N amphotericin B Chemical compound O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1/C=C/C=C/C=C/C=C/C=C/C=C/C=C/[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)OC(=O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 APKFDSVGJQXUKY-INPOYWNPSA-N 0.000 description 2
- 229940124599 anti-inflammatory drug Drugs 0.000 description 2
- 229940088710 antibiotic agent Drugs 0.000 description 2
- 208000006673 asthma Diseases 0.000 description 2
- 201000000448 autoimmune hemolytic anemia Diseases 0.000 description 2
- 201000003710 autoimmune thrombocytopenic purpura Diseases 0.000 description 2
- 230000005784 autoimmunity Effects 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 239000012888 bovine serum Substances 0.000 description 2
- 244000309466 calf Species 0.000 description 2
- 230000005779 cell damage Effects 0.000 description 2
- 230000003915 cell function Effects 0.000 description 2
- 208000037887 cell injury Diseases 0.000 description 2
- 210000001612 chondrocyte Anatomy 0.000 description 2
- 208000025302 chronic primary adrenal insufficiency Diseases 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 210000001608 connective tissue cell Anatomy 0.000 description 2
- 238000004163 cytometry Methods 0.000 description 2
- 231100000135 cytotoxicity Toxicity 0.000 description 2
- 230000003013 cytotoxicity Effects 0.000 description 2
- 210000003074 dental pulp Anatomy 0.000 description 2
- 229960003957 dexamethasone Drugs 0.000 description 2
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 2
- 108010007093 dispase Proteins 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 238000006911 enzymatic reaction Methods 0.000 description 2
- 210000004700 fetal blood Anatomy 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 238000005194 fractionation Methods 0.000 description 2
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 2
- 208000024908 graft versus host disease Diseases 0.000 description 2
- 230000012010 growth Effects 0.000 description 2
- 230000009931 harmful effect Effects 0.000 description 2
- 229960000890 hydrocortisone Drugs 0.000 description 2
- 230000002584 immunomodulator Effects 0.000 description 2
- 230000004957 immunoregulator effect Effects 0.000 description 2
- CGIGDMFJXJATDK-UHFFFAOYSA-N indomethacin Chemical compound CC1=C(CC(O)=O)C2=CC(OC)=CC=C2N1C(=O)C1=CC=C(Cl)C=C1 CGIGDMFJXJATDK-UHFFFAOYSA-N 0.000 description 2
- 230000004054 inflammatory process Effects 0.000 description 2
- 208000014674 injury Diseases 0.000 description 2
- 229940125396 insulin Drugs 0.000 description 2
- 208000036971 interstitial lung disease 2 Diseases 0.000 description 2
- 230000005865 ionizing radiation Effects 0.000 description 2
- 210000004698 lymphocyte Anatomy 0.000 description 2
- 239000006166 lysate Substances 0.000 description 2
- 230000002934 lysing effect Effects 0.000 description 2
- 208000029791 lytic metastatic bone lesion Diseases 0.000 description 2
- 239000010445 mica Substances 0.000 description 2
- 108091005601 modified peptides Proteins 0.000 description 2
- 210000000663 muscle cell Anatomy 0.000 description 2
- 102000039446 nucleic acids Human genes 0.000 description 2
- 108020004707 nucleic acids Proteins 0.000 description 2
- 150000007523 nucleic acids Chemical class 0.000 description 2
- 230000001575 pathological effect Effects 0.000 description 2
- 229930192851 perforin Natural products 0.000 description 2
- 230000008823 permeabilization Effects 0.000 description 2
- 238000007747 plating Methods 0.000 description 2
- 239000013641 positive control Substances 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 108060006613 prolamin Proteins 0.000 description 2
- 230000035755 proliferation Effects 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 238000004062 sedimentation Methods 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 239000002002 slurry Substances 0.000 description 2
- 210000000329 smooth muscle myocyte Anatomy 0.000 description 2
- DAEPDZWVDSPTHF-UHFFFAOYSA-M sodium pyruvate Chemical compound [Na+].CC(=O)C([O-])=O DAEPDZWVDSPTHF-UHFFFAOYSA-M 0.000 description 2
- 239000007909 solid dosage form Substances 0.000 description 2
- 238000007619 statistical method Methods 0.000 description 2
- 229960005322 streptomycin Drugs 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 230000009469 supplementation Effects 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 208000037816 tissue injury Diseases 0.000 description 2
- 102000035160 transmembrane proteins Human genes 0.000 description 2
- 108091005703 transmembrane proteins Proteins 0.000 description 2
- 208000027930 type IV hypersensitivity disease Diseases 0.000 description 2
- 230000002792 vascular Effects 0.000 description 2
- RDJGLLICXDHJDY-NSHDSACASA-N (2s)-2-(3-phenoxyphenyl)propanoic acid Chemical compound OC(=O)[C@@H](C)C1=CC=CC(OC=2C=CC=CC=2)=C1 RDJGLLICXDHJDY-NSHDSACASA-N 0.000 description 1
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 1
- FUFLCEKSBBHCMO-UHFFFAOYSA-N 11-dehydrocorticosterone Natural products O=C1CCC2(C)C3C(=O)CC(C)(C(CC4)C(=O)CO)C4C3CCC2=C1 FUFLCEKSBBHCMO-UHFFFAOYSA-N 0.000 description 1
- PWKSKIMOESPYIA-UHFFFAOYSA-N 2-acetamido-3-sulfanylpropanoic acid Chemical compound CC(=O)NC(CS)C(O)=O PWKSKIMOESPYIA-UHFFFAOYSA-N 0.000 description 1
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 1
- 102100022464 5'-nucleotidase Human genes 0.000 description 1
- RTAPDZBZLSXHQQ-UHFFFAOYSA-N 8-methyl-3,7-dihydropurine-2,6-dione Chemical class N1C(=O)NC(=O)C2=C1N=C(C)N2 RTAPDZBZLSXHQQ-UHFFFAOYSA-N 0.000 description 1
- 208000008190 Agammaglobulinemia Diseases 0.000 description 1
- PQSUYGKTWSAVDQ-ZVIOFETBSA-N Aldosterone Chemical compound C([C@@]1([C@@H](C(=O)CO)CC[C@H]1[C@@H]1CC2)C=O)[C@H](O)[C@@H]1[C@]1(C)C2=CC(=O)CC1 PQSUYGKTWSAVDQ-ZVIOFETBSA-N 0.000 description 1
- 208000032671 Allergic granulomatous angiitis Diseases 0.000 description 1
- 229930183010 Amphotericin Natural products 0.000 description 1
- QGGFZZLFKABGNL-UHFFFAOYSA-N Amphotericin A Natural products OC1C(N)C(O)C(C)OC1OC1C=CC=CC=CC=CCCC=CC=CC(C)C(O)C(C)C(C)OC(=O)CC(O)CC(O)CCC(O)C(O)CC(O)CC(O)(CC(O)C2C(O)=O)OC2C1 QGGFZZLFKABGNL-UHFFFAOYSA-N 0.000 description 1
- 206010002412 Angiocentric lymphomas Diseases 0.000 description 1
- 208000032467 Aplastic anaemia Diseases 0.000 description 1
- 206010003210 Arteriosclerosis Diseases 0.000 description 1
- 206010003645 Atopy Diseases 0.000 description 1
- 206010003827 Autoimmune hepatitis Diseases 0.000 description 1
- 206010050245 Autoimmune thrombocytopenia Diseases 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 208000035143 Bacterial infection Diseases 0.000 description 1
- 208000009137 Behcet syndrome Diseases 0.000 description 1
- 208000008439 Biliary Liver Cirrhosis Diseases 0.000 description 1
- 208000033222 Biliary cirrhosis primary Diseases 0.000 description 1
- 108010006654 Bleomycin Proteins 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 101150013553 CD40 gene Proteins 0.000 description 1
- 108010038940 CD48 Antigen Proteins 0.000 description 1
- 201000002829 CREST Syndrome Diseases 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 241001631457 Cannula Species 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- 206010008909 Chronic Hepatitis Diseases 0.000 description 1
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 description 1
- 208000030939 Chronic inflammatory demyelinating polyneuropathy Diseases 0.000 description 1
- 208000006344 Churg-Strauss Syndrome Diseases 0.000 description 1
- MFYSYFVPBJMHGN-ZPOLXVRWSA-N Cortisone Chemical compound O=C1CC[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 MFYSYFVPBJMHGN-ZPOLXVRWSA-N 0.000 description 1
- MFYSYFVPBJMHGN-UHFFFAOYSA-N Cortisone Natural products O=C1CCC2(C)C3C(=O)CC(C)(C(CC4)(O)C(=O)CO)C4C3CCC2=C1 MFYSYFVPBJMHGN-UHFFFAOYSA-N 0.000 description 1
- 108010091326 Cryoglobulins Proteins 0.000 description 1
- CKLJMWTZIZZHCS-UHFFFAOYSA-N D-OH-Asp Natural products OC(=O)C(N)CC(O)=O CKLJMWTZIZZHCS-UHFFFAOYSA-N 0.000 description 1
- QNAYBMKLOCPYGJ-UHFFFAOYSA-N D-alpha-Ala Natural products CC([NH3+])C([O-])=O QNAYBMKLOCPYGJ-UHFFFAOYSA-N 0.000 description 1
- KDXKERNSBIXSRK-RXMQYKEDSA-N D-lysine Chemical compound NCCCC[C@@H](N)C(O)=O KDXKERNSBIXSRK-RXMQYKEDSA-N 0.000 description 1
- 206010048768 Dermatosis Diseases 0.000 description 1
- 201000003066 Diffuse Scleroderma Diseases 0.000 description 1
- 102100025137 Early activation antigen CD69 Human genes 0.000 description 1
- 208000017701 Endocrine disease Diseases 0.000 description 1
- 208000018428 Eosinophilic granulomatosis with polyangiitis Diseases 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 206010016207 Familial Mediterranean fever Diseases 0.000 description 1
- 108010087819 Fc receptors Proteins 0.000 description 1
- 102000009109 Fc receptors Human genes 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 102100024785 Fibroblast growth factor 2 Human genes 0.000 description 1
- 108090000379 Fibroblast growth factor 2 Proteins 0.000 description 1
- 206010016717 Fistula Diseases 0.000 description 1
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 101000766307 Gallus gallus Ovotransferrin Proteins 0.000 description 1
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 1
- 229930182566 Gentamicin Natural products 0.000 description 1
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 1
- 206010072579 Granulomatosis with polyangiitis Diseases 0.000 description 1
- 208000035895 Guillain-Barré syndrome Diseases 0.000 description 1
- 208000001204 Hashimoto Disease Diseases 0.000 description 1
- 101000678236 Homo sapiens 5'-nucleotidase Proteins 0.000 description 1
- 101000716130 Homo sapiens CD48 antigen Proteins 0.000 description 1
- 101000934374 Homo sapiens Early activation antigen CD69 Proteins 0.000 description 1
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 description 1
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 1
- 101001055145 Homo sapiens Interleukin-2 receptor subunit beta Proteins 0.000 description 1
- 101000917858 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-A Proteins 0.000 description 1
- 101000917839 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-B Proteins 0.000 description 1
- 101000971513 Homo sapiens Natural killer cells antigen CD94 Proteins 0.000 description 1
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 description 1
- 102000003839 Human Proteins Human genes 0.000 description 1
- 108090000144 Human Proteins Proteins 0.000 description 1
- 208000023105 Huntington disease Diseases 0.000 description 1
- 206010020983 Hypogammaglobulinaemia Diseases 0.000 description 1
- 206010021074 Hypoplastic anaemia Diseases 0.000 description 1
- HEFNNWSXXWATRW-UHFFFAOYSA-N Ibuprofen Chemical compound CC(C)CC1=CC=C(C(C)C(O)=O)C=C1 HEFNNWSXXWATRW-UHFFFAOYSA-N 0.000 description 1
- 206010062016 Immunosuppression Diseases 0.000 description 1
- 102100027268 Interferon-stimulated gene 20 kDa protein Human genes 0.000 description 1
- 102100026879 Interleukin-2 receptor subunit beta Human genes 0.000 description 1
- 208000003456 Juvenile Arthritis Diseases 0.000 description 1
- 206010059176 Juvenile idiopathic arthritis Diseases 0.000 description 1
- 229930194542 Keto Natural products 0.000 description 1
- QNAYBMKLOCPYGJ-UWTATZPHSA-N L-Alanine Natural products C[C@@H](N)C(O)=O QNAYBMKLOCPYGJ-UWTATZPHSA-N 0.000 description 1
- CKLJMWTZIZZHCS-UWTATZPHSA-N L-Aspartic acid Natural products OC(=O)[C@H](N)CC(O)=O CKLJMWTZIZZHCS-UWTATZPHSA-N 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-N L-arginine Chemical compound OC(=O)[C@@H](N)CCCN=C(N)N ODKSFYDXXFIFQN-BYPYZUCNSA-N 0.000 description 1
- 229930064664 L-arginine Natural products 0.000 description 1
- 235000014852 L-arginine Nutrition 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- LEVWYRKDKASIDU-IMJSIDKUSA-N L-cystine Chemical compound [O-]C(=O)[C@@H]([NH3+])CSSC[C@H]([NH3+])C([O-])=O LEVWYRKDKASIDU-IMJSIDKUSA-N 0.000 description 1
- 235000019393 L-cystine Nutrition 0.000 description 1
- 239000004158 L-cystine Substances 0.000 description 1
- 229930182816 L-glutamine Natural products 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- BFVQTKQTUCQRPI-YYEZTRBPSA-N LPS with O-antigen Chemical compound O([C@@H]1[C@@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O[C@@H]4[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO[C@@H]5[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O5)O)O4)O)[C@@H](O)[C@@H](CO)O3)NC(C)=O)[C@@H](O)[C@@H](CO[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O3)NC(C)=O)O2)NC(C)=O)[C@H](O)[C@@H](CO)OC1O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@H]1[C@H](O)[C@@H](CO[C@@H]2[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O2)O)OC([C@@H]1O)O[C@H]1[C@H](O)[C@@H]([C@@H](O)COC2[C@H]([C@@H](O)[C@H](OP(O)(O)=O)[C@@H]([C@@H](O)CO)O2)O)OC([C@H]1O)O[C@H]1[C@H](OP(O)(=O)OP(O)(=O)OCCN)[C@@H]([C@@H](O)CO)OC([C@H]1O)O[C@H]1[C@H](O[C@]2(O[C@@H]([C@@H](O)[C@H](O[C@]3(O[C@@H]([C@@H](O)[C@H](OP(O)(=O)OCCN)C3)[C@@H](O)CO)C(O)=O)C2)[C@@H](O)CO)C(O)=O)C[C@](O[C@@H]1[C@@H](O)CO)(OC[C@H]1O[C@@H](OC[C@@H]2[C@H]([C@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](NC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](OP(O)(O)=O)O2)O)[C@H](NC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCCCCCCCC)[C@H]([C@@H]1OP(O)(O)=O)OC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCCCCCCCCCC)C(O)=O)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1NC(C)=O BFVQTKQTUCQRPI-YYEZTRBPSA-N 0.000 description 1
- 102100029185 Low affinity immunoglobulin gamma Fc region receptor III-B Human genes 0.000 description 1
- 108700018351 Major Histocompatibility Complex Proteins 0.000 description 1
- 208000027530 Meniere disease Diseases 0.000 description 1
- 206010049567 Miller Fisher syndrome Diseases 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- 208000003250 Mixed connective tissue disease Diseases 0.000 description 1
- 241001430197 Mollicutes Species 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 229940121948 Muscarinic receptor antagonist Drugs 0.000 description 1
- 206010052904 Musculoskeletal stiffness Diseases 0.000 description 1
- 208000009525 Myocarditis Diseases 0.000 description 1
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 1
- 230000006051 NK cell activation Effects 0.000 description 1
- 102000027581 NK cell receptors Human genes 0.000 description 1
- 108091008877 NK cell receptors Proteins 0.000 description 1
- CMWTZPSULFXXJA-UHFFFAOYSA-N Naproxen Natural products C1=C(C(C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-UHFFFAOYSA-N 0.000 description 1
- 108010004222 Natural Cytotoxicity Triggering Receptor 3 Proteins 0.000 description 1
- 108010077854 Natural Killer Cell Receptors Proteins 0.000 description 1
- 102000010648 Natural Killer Cell Receptors Human genes 0.000 description 1
- 102100032852 Natural cytotoxicity triggering receptor 3 Human genes 0.000 description 1
- 102100021462 Natural killer cells antigen CD94 Human genes 0.000 description 1
- 102000002356 Nectin Human genes 0.000 description 1
- 108060005251 Nectin Proteins 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 101710160107 Outer membrane protein A Proteins 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 241000721454 Pemphigus Species 0.000 description 1
- 102000004503 Perforin Human genes 0.000 description 1
- 108010056995 Perforin Proteins 0.000 description 1
- 206010065159 Polychondritis Diseases 0.000 description 1
- 208000007048 Polymyalgia Rheumatica Diseases 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 208000012654 Primary biliary cholangitis Diseases 0.000 description 1
- 102100024819 Prolactin Human genes 0.000 description 1
- 108010057464 Prolactin Proteins 0.000 description 1
- 102100038277 Prostaglandin G/H synthase 1 Human genes 0.000 description 1
- 108050003243 Prostaglandin G/H synthase 1 Proteins 0.000 description 1
- 102100038280 Prostaglandin G/H synthase 2 Human genes 0.000 description 1
- 108050003267 Prostaglandin G/H synthase 2 Proteins 0.000 description 1
- 102000004005 Prostaglandin-endoperoxide synthases Human genes 0.000 description 1
- 108090000459 Prostaglandin-endoperoxide synthases Proteins 0.000 description 1
- 102000001708 Protein Isoforms Human genes 0.000 description 1
- 108010029485 Protein Isoforms Proteins 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 238000010240 RT-PCR analysis Methods 0.000 description 1
- 208000012322 Raynaud phenomenon Diseases 0.000 description 1
- 208000033464 Reiter syndrome Diseases 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 206010039710 Scleroderma Diseases 0.000 description 1
- BUGBHKTXTAQXES-UHFFFAOYSA-N Selenium Chemical compound [Se] BUGBHKTXTAQXES-UHFFFAOYSA-N 0.000 description 1
- 206010070834 Sensitisation Diseases 0.000 description 1
- 206010040070 Septic Shock Diseases 0.000 description 1
- 108010071390 Serum Albumin Proteins 0.000 description 1
- 102000007562 Serum Albumin Human genes 0.000 description 1
- 102000005157 Somatostatin Human genes 0.000 description 1
- 108010056088 Somatostatin Proteins 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 206010042742 Sympathetic ophthalmia Diseases 0.000 description 1
- 201000009594 Systemic Scleroderma Diseases 0.000 description 1
- 206010042953 Systemic sclerosis Diseases 0.000 description 1
- 102100027222 T-lymphocyte activation antigen CD80 Human genes 0.000 description 1
- 210000000662 T-lymphocyte subset Anatomy 0.000 description 1
- 206010043561 Thrombocytopenic purpura Diseases 0.000 description 1
- GLNADSQYFUSGOU-GPTZEZBUSA-J Trypan blue Chemical compound [Na+].[Na+].[Na+].[Na+].C1=C(S([O-])(=O)=O)C=C2C=C(S([O-])(=O)=O)C(/N=N/C3=CC=C(C=C3C)C=3C=C(C(=CC=3)\N=N\C=3C(=CC4=CC(=CC(N)=C4C=3O)S([O-])(=O)=O)S([O-])(=O)=O)C)=C(O)C2=C1N GLNADSQYFUSGOU-GPTZEZBUSA-J 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 102100040245 Tumor necrosis factor receptor superfamily member 5 Human genes 0.000 description 1
- 101150042088 UL16 gene Proteins 0.000 description 1
- 206010046543 Urinary incontinence Diseases 0.000 description 1
- 206010046851 Uveitis Diseases 0.000 description 1
- 108010000134 Vascular Cell Adhesion Molecule-1 Proteins 0.000 description 1
- 208000036142 Viral infection Diseases 0.000 description 1
- 206010047642 Vitiligo Diseases 0.000 description 1
- UYRDHEJRPVSJFM-VSWVFQEASA-N [(1s,3r)-3-hydroxy-4-[(3e,5e,7e,9e,11z)-11-[4-[(e)-2-[(1r,3s,6s)-3-hydroxy-1,5,5-trimethyl-7-oxabicyclo[4.1.0]heptan-6-yl]ethenyl]-5-oxofuran-2-ylidene]-3,10-dimethylundeca-1,3,5,7,9-pentaenylidene]-3,5,5-trimethylcyclohexyl] acetate Chemical compound C[C@@]1(O)C[C@@H](OC(=O)C)CC(C)(C)C1=C=C\C(C)=C\C=C\C=C\C=C(/C)\C=C/1C=C(\C=C\[C@]23[C@@](O2)(C)C[C@@H](O)CC3(C)C)C(=O)O\1 UYRDHEJRPVSJFM-VSWVFQEASA-N 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 208000038016 acute inflammation Diseases 0.000 description 1
- 230000006022 acute inflammation Effects 0.000 description 1
- 210000005006 adaptive immune system Anatomy 0.000 description 1
- 230000001919 adrenal effect Effects 0.000 description 1
- 210000004504 adult stem cell Anatomy 0.000 description 1
- 229960003767 alanine Drugs 0.000 description 1
- 230000000172 allergic effect Effects 0.000 description 1
- 208000004631 alopecia areata Diseases 0.000 description 1
- 210000004381 amniotic fluid Anatomy 0.000 description 1
- 229940009444 amphotericin Drugs 0.000 description 1
- 229960000723 ampicillin Drugs 0.000 description 1
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 1
- 238000000540 analysis of variance Methods 0.000 description 1
- 230000033115 angiogenesis Effects 0.000 description 1
- 238000002399 angioplasty Methods 0.000 description 1
- 230000000843 anti-fungal effect Effects 0.000 description 1
- 230000003110 anti-inflammatory effect Effects 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 230000030741 antigen processing and presentation Effects 0.000 description 1
- 230000000890 antigenic effect Effects 0.000 description 1
- 208000011775 arteriosclerosis disease Diseases 0.000 description 1
- 229960001230 asparagine Drugs 0.000 description 1
- 229960005261 aspartic acid Drugs 0.000 description 1
- 210000001130 astrocyte Anatomy 0.000 description 1
- 208000010668 atopic eczema Diseases 0.000 description 1
- 208000035362 autoimmune disorder of the nervous system Diseases 0.000 description 1
- 208000036923 autoimmune primary adrenal insufficiency Diseases 0.000 description 1
- 208000022362 bacterial infectious disease Diseases 0.000 description 1
- 230000003542 behavioural effect Effects 0.000 description 1
- 229940125388 beta agonist Drugs 0.000 description 1
- 102000023732 binding proteins Human genes 0.000 description 1
- 108091008324 binding proteins Proteins 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 229960001561 bleomycin Drugs 0.000 description 1
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 210000002449 bone cell Anatomy 0.000 description 1
- 208000000594 bullous pemphigoid Diseases 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 210000004413 cardiac myocyte Anatomy 0.000 description 1
- 230000001925 catabolic effect Effects 0.000 description 1
- 229960000590 celecoxib Drugs 0.000 description 1
- RZEKVGVHFLEQIL-UHFFFAOYSA-N celecoxib Chemical compound C1=CC(C)=CC=C1C1=CC(C(F)(F)F)=NN1C1=CC=C(S(N)(=O)=O)C=C1 RZEKVGVHFLEQIL-UHFFFAOYSA-N 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 238000002659 cell therapy Methods 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 229930002875 chlorophyll Natural products 0.000 description 1
- 235000019804 chlorophyll Nutrition 0.000 description 1
- ATNHDLDRLWWWCB-AENOIHSZSA-M chlorophyll a Chemical compound C1([C@@H](C(=O)OC)C(=O)C2=C3C)=C2N2C3=CC(C(CC)=C3C)=[N+]4C3=CC3=C(C=C)C(C)=C5N3[Mg-2]42[N+]2=C1[C@@H](CCC(=O)OC\C=C(/C)CCC[C@H](C)CCC[C@H](C)CCCC(C)C)[C@H](C)C2=C5 ATNHDLDRLWWWCB-AENOIHSZSA-M 0.000 description 1
- 239000000812 cholinergic antagonist Substances 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 201000005795 chronic inflammatory demyelinating polyneuritis Diseases 0.000 description 1
- 210000001728 clone cell Anatomy 0.000 description 1
- 206010009887 colitis Diseases 0.000 description 1
- 230000024203 complement activation Effects 0.000 description 1
- 230000001143 conditioned effect Effects 0.000 description 1
- 210000002808 connective tissue Anatomy 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 239000003246 corticosteroid Substances 0.000 description 1
- 229960001334 corticosteroids Drugs 0.000 description 1
- 229960004544 cortisone Drugs 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 229960003067 cystine Drugs 0.000 description 1
- 230000016396 cytokine production Effects 0.000 description 1
- 231100000050 cytotoxic potential Toxicity 0.000 description 1
- 210000004443 dendritic cell Anatomy 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 201000001981 dermatomyositis Diseases 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 229960001259 diclofenac Drugs 0.000 description 1
- DCOPUUMXTXDBNB-UHFFFAOYSA-N diclofenac Chemical compound OC(=O)CC1=CC=CC=C1NC1=C(Cl)C=CC=C1Cl DCOPUUMXTXDBNB-UHFFFAOYSA-N 0.000 description 1
- RGLYKWWBQGJZGM-ISLYRVAYSA-N diethylstilbestrol Chemical compound C=1C=C(O)C=CC=1C(/CC)=C(\CC)C1=CC=C(O)C=C1 RGLYKWWBQGJZGM-ISLYRVAYSA-N 0.000 description 1
- XEYBRNLFEZDVAW-ARSRFYASSA-N dinoprostone Chemical compound CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O XEYBRNLFEZDVAW-ARSRFYASSA-N 0.000 description 1
- 229960002986 dinoprostone Drugs 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 230000008482 dysregulation Effects 0.000 description 1
- 150000002066 eicosanoids Chemical class 0.000 description 1
- 206010014599 encephalitis Diseases 0.000 description 1
- 239000002158 endotoxin Substances 0.000 description 1
- 239000003797 essential amino acid Substances 0.000 description 1
- 235000020776 essential amino acid Nutrition 0.000 description 1
- 229960005309 estradiol Drugs 0.000 description 1
- 229960005293 etodolac Drugs 0.000 description 1
- XFBVBWWRPKNWHW-UHFFFAOYSA-N etodolac Chemical compound C1COC(CC)(CC(O)=O)C2=N[C]3C(CC)=CC=CC3=C21 XFBVBWWRPKNWHW-UHFFFAOYSA-N 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 230000028023 exocytosis Effects 0.000 description 1
- 206010016256 fatigue Diseases 0.000 description 1
- 229960001419 fenoprofen Drugs 0.000 description 1
- 230000001605 fetal effect Effects 0.000 description 1
- 230000003890 fistula Effects 0.000 description 1
- ZZUFCTLCJUWOSV-UHFFFAOYSA-N furosemide Chemical compound C1=C(Cl)C(S(=O)(=O)N)=CC(C(O)=O)=C1NCC1=CC=CO1 ZZUFCTLCJUWOSV-UHFFFAOYSA-N 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 229960002518 gentamicin Drugs 0.000 description 1
- 208000005017 glioblastoma Diseases 0.000 description 1
- 239000003862 glucocorticoid Substances 0.000 description 1
- 229960002989 glutamic acid Drugs 0.000 description 1
- 229960002743 glutamine Drugs 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 230000003394 haemopoietic effect Effects 0.000 description 1
- 208000006454 hepatitis Diseases 0.000 description 1
- 210000003494 hepatocyte Anatomy 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 230000007236 host immunity Effects 0.000 description 1
- 229960001680 ibuprofen Drugs 0.000 description 1
- 230000005934 immune activation Effects 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 239000012642 immune effector Substances 0.000 description 1
- 230000036737 immune function Effects 0.000 description 1
- 230000008629 immune suppression Effects 0.000 description 1
- 230000006058 immune tolerance Effects 0.000 description 1
- 238000010166 immunofluorescence Methods 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 229960000905 indomethacin Drugs 0.000 description 1
- 239000000411 inducer Substances 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 208000000509 infertility Diseases 0.000 description 1
- 230000036512 infertility Effects 0.000 description 1
- 231100000535 infertility Toxicity 0.000 description 1
- 229960000598 infliximab Drugs 0.000 description 1
- 108091008042 inhibitory receptors Proteins 0.000 description 1
- 230000014828 interferon-gamma production Effects 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 208000028867 ischemia Diseases 0.000 description 1
- 229960000318 kanamycin Drugs 0.000 description 1
- 229930027917 kanamycin Natural products 0.000 description 1
- SBUJHOSQTJFQJX-NOAMYHISSA-N kanamycin Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N SBUJHOSQTJFQJX-NOAMYHISSA-N 0.000 description 1
- 229930182823 kanamycin A Natural products 0.000 description 1
- 125000000468 ketone group Chemical group 0.000 description 1
- OZWKMVRBQXNZKK-UHFFFAOYSA-N ketorolac Chemical compound OC(=O)C1CCN2C1=CC=C2C(=O)C1=CC=CC=C1 OZWKMVRBQXNZKK-UHFFFAOYSA-N 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 238000002386 leaching Methods 0.000 description 1
- 150000002617 leukotrienes Chemical class 0.000 description 1
- 201000011486 lichen planus Diseases 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 210000003563 lymphoid tissue Anatomy 0.000 description 1
- 208000006116 lymphomatoid granulomatosis Diseases 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 230000000873 masking effect Effects 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000012577 media supplement Substances 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 229960004857 mitomycin Drugs 0.000 description 1
- 210000002894 multi-fate stem cell Anatomy 0.000 description 1
- 229960002009 naproxen Drugs 0.000 description 1
- CMWTZPSULFXXJA-VIFPVBQESA-N naproxen Chemical compound C1=C([C@H](C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-VIFPVBQESA-N 0.000 description 1
- 210000000581 natural killer T-cell Anatomy 0.000 description 1
- 210000002569 neuron Anatomy 0.000 description 1
- 201000001119 neuropathy Diseases 0.000 description 1
- 230000007823 neuropathy Effects 0.000 description 1
- 238000010606 normalization Methods 0.000 description 1
- 238000010899 nucleation Methods 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- 210000000963 osteoblast Anatomy 0.000 description 1
- 229960002739 oxaprozin Drugs 0.000 description 1
- OFPXSFXSNFPTHF-UHFFFAOYSA-N oxaprozin Chemical compound O1C(CCC(=O)O)=NC(C=2C=CC=CC=2)=C1C1=CC=CC=C1 OFPXSFXSNFPTHF-UHFFFAOYSA-N 0.000 description 1
- UTIQDNPUHSAVDN-UHFFFAOYSA-N peridinin Natural products CC(=O)OC1CC(C)(C)C(=C=CC(=CC=CC=CC=C2/OC(=O)C(=C2)C=CC34OC3(C)CC(O)CC4(C)C)C)C(C)(O)C1 UTIQDNPUHSAVDN-UHFFFAOYSA-N 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 208000033808 peripheral neuropathy Diseases 0.000 description 1
- 230000002688 persistence Effects 0.000 description 1
- 201000006292 polyarteritis nodosa Diseases 0.000 description 1
- 208000005987 polymyositis Diseases 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 238000011533 pre-incubation Methods 0.000 description 1
- 229960005205 prednisolone Drugs 0.000 description 1
- OIGNJSKKLXVSLS-VWUMJDOOSA-N prednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OIGNJSKKLXVSLS-VWUMJDOOSA-N 0.000 description 1
- 229960004618 prednisone Drugs 0.000 description 1
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 230000037452 priming Effects 0.000 description 1
- 229960003387 progesterone Drugs 0.000 description 1
- 239000000186 progesterone Substances 0.000 description 1
- 230000000770 proinflammatory effect Effects 0.000 description 1
- 229940097325 prolactin Drugs 0.000 description 1
- 230000000644 propagated effect Effects 0.000 description 1
- XEYBRNLFEZDVAW-UHFFFAOYSA-N prostaglandin E2 Natural products CCCCCC(O)C=CC1C(O)CC(=O)C1CC=CCCCC(O)=O XEYBRNLFEZDVAW-UHFFFAOYSA-N 0.000 description 1
- 208000005069 pulmonary fibrosis Diseases 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 208000002574 reactive arthritis Diseases 0.000 description 1
- 239000003087 receptor blocking agent Substances 0.000 description 1
- 208000037803 restenosis Diseases 0.000 description 1
- 229960000371 rofecoxib Drugs 0.000 description 1
- RZJQGNCSTQAWON-UHFFFAOYSA-N rofecoxib Chemical compound C1=CC(S(=O)(=O)C)=CC=C1C1=C(C=2C=CC=CC=2)C(=O)OC1 RZJQGNCSTQAWON-UHFFFAOYSA-N 0.000 description 1
- 201000000306 sarcoidosis Diseases 0.000 description 1
- 230000037390 scarring Effects 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 229910052711 selenium Inorganic materials 0.000 description 1
- 239000011669 selenium Substances 0.000 description 1
- 230000008313 sensitization Effects 0.000 description 1
- 230000036303 septic shock Effects 0.000 description 1
- 230000001568 sexual effect Effects 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 210000002027 skeletal muscle Anatomy 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 208000017520 skin disease Diseases 0.000 description 1
- 230000036560 skin regeneration Effects 0.000 description 1
- 229940054269 sodium pyruvate Drugs 0.000 description 1
- 229960000553 somatostatin Drugs 0.000 description 1
- NHXLMOGPVYXJNR-ATOGVRKGSA-N somatostatin Chemical compound C([C@H]1C(=O)N[C@H](C(N[C@@H](CO)C(=O)N[C@@H](CSSC[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CC=2C3=CC=CC=C3NC=2)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(=O)N1)[C@@H](C)O)NC(=O)CNC(=O)[C@H](C)N)C(O)=O)=O)[C@H](O)C)C1=CC=CC=C1 NHXLMOGPVYXJNR-ATOGVRKGSA-N 0.000 description 1
- 208000020431 spinal cord injury Diseases 0.000 description 1
- 201000005671 spondyloarthropathy Diseases 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 229960000894 sulindac Drugs 0.000 description 1
- MLKXDPUZXIRXEP-MFOYZWKCSA-N sulindac Chemical compound CC1=C(CC(O)=O)C2=CC(F)=CC=C2\C1=C/C1=CC=C(S(C)=O)C=C1 MLKXDPUZXIRXEP-MFOYZWKCSA-N 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 230000020382 suppression by virus of host antigen processing and presentation of peptide antigen via MHC class I Effects 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 210000001258 synovial membrane Anatomy 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 229940037128 systemic glucocorticoids Drugs 0.000 description 1
- 210000002435 tendon Anatomy 0.000 description 1
- RZWIIPASKMUIAC-VQTJNVASSA-N thromboxane Chemical compound CCCCCCCC[C@H]1OCCC[C@@H]1CCCCCCC RZWIIPASKMUIAC-VQTJNVASSA-N 0.000 description 1
- 230000017423 tissue regeneration Effects 0.000 description 1
- 229960005294 triamcinolone Drugs 0.000 description 1
- GFNANZIMVAIWHM-OBYCQNJPSA-N triamcinolone Chemical compound O=C1C=C[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@]([C@H](O)C4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 GFNANZIMVAIWHM-OBYCQNJPSA-N 0.000 description 1
- YNJBWRMUSHSURL-UHFFFAOYSA-N trichloroacetic acid Chemical compound OC(=O)C(Cl)(Cl)Cl YNJBWRMUSHSURL-UHFFFAOYSA-N 0.000 description 1
- 230000001228 trophic effect Effects 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 230000005740 tumor formation Effects 0.000 description 1
- 238000007492 two-way ANOVA Methods 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 239000011534 wash buffer Substances 0.000 description 1
- 239000003643 water by type Substances 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
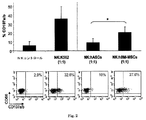
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0652—Cells of skeletal and connective tissues; Mesenchyme
- C12N5/0662—Stem cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0652—Cells of skeletal and connective tissues; Mesenchyme
- C12N5/0662—Stem cells
- C12N5/0667—Adipose-derived stem cells [ADSC]; Adipose stromal stem cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/15—Natural-killer [NK] cells; Natural-killer T [NKT] cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/20—Cellular immunotherapy characterised by the effect or the function of the cells
- A61K40/22—Immunosuppressive or immunotolerising
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/416—Antigens related to auto-immune diseases; Preparations to induce self-tolerance
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0636—T lymphocytes
- C12N5/0637—Immunosuppressive T lymphocytes, e.g. regulatory T cells or Treg
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0646—Natural killers cells [NK], NKT cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0652—Cells of skeletal and connective tissues; Mesenchyme
- C12N5/0662—Stem cells
- C12N5/0663—Bone marrow mesenchymal stem cells (BM-MSC)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K2035/122—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells for inducing tolerance or supression of immune responses
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2502/00—Coculture with; Conditioned medium produced by
- C12N2502/13—Coculture with; Conditioned medium produced by connective tissue cells; generic mesenchyme cells, e.g. so-called "embryonic fibroblasts"
- C12N2502/1352—Mesenchymal stem cells
- C12N2502/1358—Bone marrow mesenchymal stem cells (BM-MSC)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2502/00—Coculture with; Conditioned medium produced by
- C12N2502/13—Coculture with; Conditioned medium produced by connective tissue cells; generic mesenchyme cells, e.g. so-called "embryonic fibroblasts"
- C12N2502/1352—Mesenchymal stem cells
- C12N2502/1382—Adipose-derived stem cells [ADSC], adipose stromal stem cells
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- General Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Zoology (AREA)
- Biotechnology (AREA)
- Wood Science & Technology (AREA)
- Genetics & Genomics (AREA)
- Immunology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Epidemiology (AREA)
- Cell Biology (AREA)
- Microbiology (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- Developmental Biology & Embryology (AREA)
- Hematology (AREA)
- Rheumatology (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pain & Pain Management (AREA)
- Transplantation (AREA)
- Virology (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP11157930.6 | 2011-03-11 | ||
| EP11157930 | 2011-03-11 | ||
| PCT/EP2012/054251 WO2012123401A1 (en) | 2011-03-11 | 2012-03-12 | Cell populations having immunoregulatory activity, method for isolation and uses |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2014508527A true JP2014508527A (ja) | 2014-04-10 |
| JP2014508527A5 JP2014508527A5 (enExample) | 2015-04-23 |
Family
ID=45812789
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2013557133A Pending JP2014508527A (ja) | 2011-03-11 | 2012-03-12 | 免疫調節活性を有する細胞集団、単離方法および使用 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20140154227A1 (enExample) |
| EP (1) | EP2683813A1 (enExample) |
| JP (1) | JP2014508527A (enExample) |
| KR (1) | KR20140016933A (enExample) |
| ES (1) | ES2479565T1 (enExample) |
| WO (1) | WO2012123401A1 (enExample) |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060045872A1 (en) | 2004-08-25 | 2006-03-02 | Universidad Autonoma De Madrid Ciudad Universitaria de Cantoblanco | Use of adipose tissue-derived stromal stem cells in treating fistula |
| MX342474B (es) | 2005-09-23 | 2016-09-30 | Cellerix Sl | Poblaciones celulares con actividad inmunoreguladora, método de aislamiento y usos. |
| US20160120910A1 (en) * | 2013-06-25 | 2016-05-05 | Tigenix S.A.U. | Cell populations having immunoregulatory activity, methods for the preparation and uses thereof |
| US20160161504A1 (en) * | 2013-08-01 | 2016-06-09 | Fundación Pública Andaluza Progreso Y Salud | Method for predicting treatment response and test for safe use of mesenchymal stem cells on inflammatory diseases |
| WO2015166845A1 (ja) * | 2014-05-01 | 2015-11-05 | 株式会社島津製作所 | 細胞の分化状態の評価方法 |
| JP6722598B2 (ja) * | 2014-06-30 | 2020-07-15 | ティジェニクス エス.エー.ユー. | 関節リウマチ治療のための間葉系間質細胞 |
| GB201604304D0 (en) | 2016-03-14 | 2016-04-27 | Tigenix S A U | Adipose tissue-derived stromal stem cells for use in treating refractory complex perianal fistulas in crohn's disease |
| CN110592002B (zh) * | 2019-10-10 | 2021-04-02 | 山东大学齐鲁医院 | 一种用于单细胞测序的膀胱尿路上皮单细胞悬液的制备方法及应用 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005056777A1 (ja) * | 2003-12-10 | 2005-06-23 | Hitachi Medical Corporation | 接着性幹細胞分画並びにその分離方法、品質管理方法及び品質管理装置 |
| JP2007536915A (ja) * | 2004-05-12 | 2007-12-20 | ザ ウォルター アンド エリザ ホール インスティテュート オブ メディカル リサーチ | 細胞単離方法 |
| JP2009508507A (ja) * | 2005-09-23 | 2009-03-05 | セリェリクス、ソシエダッド、リミターダ | 免疫調節活性を有する細胞集団、単離方法および使用 |
| WO2010052313A1 (en) * | 2008-11-07 | 2010-05-14 | Cellerix S.A. | Cells, nucleic acid constructs, cells comprising said constructs and methods utilizing said cells in the treatment of diseases |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060045872A1 (en) * | 2004-08-25 | 2006-03-02 | Universidad Autonoma De Madrid Ciudad Universitaria de Cantoblanco | Use of adipose tissue-derived stromal stem cells in treating fistula |
-
2012
- 2012-03-12 KR KR1020137026776A patent/KR20140016933A/ko not_active Withdrawn
- 2012-03-12 ES ES12708032.3T patent/ES2479565T1/es active Pending
- 2012-03-12 US US14/004,004 patent/US20140154227A1/en not_active Abandoned
- 2012-03-12 EP EP12708032.3A patent/EP2683813A1/en not_active Withdrawn
- 2012-03-12 JP JP2013557133A patent/JP2014508527A/ja active Pending
- 2012-03-12 WO PCT/EP2012/054251 patent/WO2012123401A1/en not_active Ceased
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005056777A1 (ja) * | 2003-12-10 | 2005-06-23 | Hitachi Medical Corporation | 接着性幹細胞分画並びにその分離方法、品質管理方法及び品質管理装置 |
| JP2007536915A (ja) * | 2004-05-12 | 2007-12-20 | ザ ウォルター アンド エリザ ホール インスティテュート オブ メディカル リサーチ | 細胞単離方法 |
| JP2009508507A (ja) * | 2005-09-23 | 2009-03-05 | セリェリクス、ソシエダッド、リミターダ | 免疫調節活性を有する細胞集団、単離方法および使用 |
| WO2010052313A1 (en) * | 2008-11-07 | 2010-05-14 | Cellerix S.A. | Cells, nucleic acid constructs, cells comprising said constructs and methods utilizing said cells in the treatment of diseases |
Also Published As
| Publication number | Publication date |
|---|---|
| ES2479565T1 (es) | 2014-08-20 |
| WO2012123401A1 (en) | 2012-09-20 |
| KR20140016933A (ko) | 2014-02-10 |
| EP2683813A1 (en) | 2014-01-15 |
| US20140154227A1 (en) | 2014-06-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6089004B2 (ja) | 免疫調節活性を有する細胞集団、単離方法および使用 | |
| JP5892931B2 (ja) | 細胞治療において使用するための方法および組成物 | |
| JP5683264B2 (ja) | 移植片対宿主病の治療 | |
| JP2014508527A (ja) | 免疫調節活性を有する細胞集団、単離方法および使用 | |
| JP2018134086A (ja) | 免疫調節活性を有する細胞集団、その調製方法、及び、その使用 | |
| JP2012510279A (ja) | 脂肪由来幹細胞の産生方法および疾患の治療における当該細胞の使用 | |
| JP2013507945A (ja) | 免疫調節活性を有する細胞集団、その製造方法及び使用 | |
| JP5745419B2 (ja) | 細胞、核酸構築物、前記構築物を含む細胞、及び疾患の治療において前記細胞を利用する方法 | |
| AU2012268272B2 (en) | Cell populations having immunoregulatory activity, method for isolation and uses | |
| HK1239744A1 (en) | Cell populations having immunoregulatory activity, method for isolation and uses | |
| AU2016269436A1 (en) | Cell populations having immunoregulatory activity, method for isolation and uses | |
| HK1124884A (en) | Cell populations having immunoregulatory activity, method for isolation and uses | |
| MX2008003881A (en) | Cell populations having immunoregulatory activity, method for isolation and uses | |
| HK1158979A (en) | Cell populations having immunoregulatory activity, method for isolation and uses |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150305 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20150305 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20160119 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20160906 |