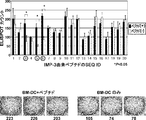
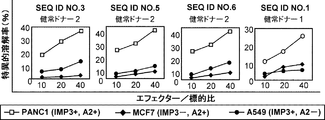
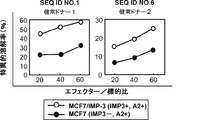
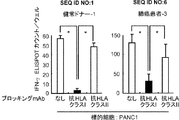
JP2013511958A - Imp−3オリゴペプチドおよびそれを含むワクチン - Google Patents
Imp−3オリゴペプチドおよびそれを含むワクチン Download PDFInfo
- Publication number
- JP2013511958A JP2013511958A JP2012524961A JP2012524961A JP2013511958A JP 2013511958 A JP2013511958 A JP 2013511958A JP 2012524961 A JP2012524961 A JP 2012524961A JP 2012524961 A JP2012524961 A JP 2012524961A JP 2013511958 A JP2013511958 A JP 2013511958A
- Authority
- JP
- Japan
- Prior art keywords
- hla
- antigen
- oligopeptide
- imp
- ctl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 108010038807 Oligopeptides Proteins 0.000 title claims abstract description 93
- 102000015636 Oligopeptides Human genes 0.000 title claims abstract description 93
- 229960005486 vaccine Drugs 0.000 title claims description 19
- 102100030403 Signal peptide peptidase-like 2A Human genes 0.000 title 1
- 108050005900 Signal peptide peptidase-like 2a Proteins 0.000 title 1
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 claims abstract description 173
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 150
- 201000011510 cancer Diseases 0.000 claims abstract description 115
- 150000001413 amino acids Chemical class 0.000 claims abstract description 58
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 48
- 230000002265 prevention Effects 0.000 claims abstract description 34
- 238000011282 treatment Methods 0.000 claims abstract description 33
- 239000003814 drug Substances 0.000 claims abstract description 19
- 229940079593 drug Drugs 0.000 claims abstract description 14
- 230000002980 postoperative effect Effects 0.000 claims abstract description 12
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 358
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 168
- 210000004027 cell Anatomy 0.000 claims description 150
- 238000000034 method Methods 0.000 claims description 124
- 210000000612 antigen-presenting cell Anatomy 0.000 claims description 97
- 108010074032 HLA-A2 Antigen Proteins 0.000 claims description 78
- 102000025850 HLA-A2 Antigen Human genes 0.000 claims description 76
- 239000000427 antigen Substances 0.000 claims description 72
- 108091007433 antigens Proteins 0.000 claims description 72
- 102000036639 antigens Human genes 0.000 claims description 72
- 108091033319 polynucleotide Proteins 0.000 claims description 65
- 102000040430 polynucleotide Human genes 0.000 claims description 65
- 239000002157 polynucleotide Substances 0.000 claims description 65
- 230000001939 inductive effect Effects 0.000 claims description 45
- 239000008177 pharmaceutical agent Substances 0.000 claims description 44
- 210000001744 T-lymphocyte Anatomy 0.000 claims description 33
- 239000004480 active ingredient Substances 0.000 claims description 31
- 108091008874 T cell receptors Proteins 0.000 claims description 29
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 claims description 27
- 210000001808 exosome Anatomy 0.000 claims description 22
- 239000012634 fragment Substances 0.000 claims description 20
- 229920001184 polypeptide Polymers 0.000 claims description 20
- 238000004519 manufacturing process Methods 0.000 claims description 19
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 claims description 15
- 230000028993 immune response Effects 0.000 claims description 15
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 claims description 14
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 claims description 12
- 229930182817 methionine Natural products 0.000 claims description 12
- 238000006467 substitution reaction Methods 0.000 claims description 12
- 239000003795 chemical substances by application Substances 0.000 claims description 10
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 claims description 8
- 239000003937 drug carrier Substances 0.000 claims description 8
- 239000004474 valine Substances 0.000 claims description 8
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 claims description 7
- 238000003780 insertion Methods 0.000 claims description 7
- 230000037431 insertion Effects 0.000 claims description 7
- 125000001433 C-terminal amino-acid group Chemical group 0.000 claims description 6
- 238000012217 deletion Methods 0.000 claims description 6
- 230000037430 deletion Effects 0.000 claims description 6
- 230000030741 antigen processing and presentation Effects 0.000 claims description 2
- 125000003275 alpha amino acid group Chemical group 0.000 abstract description 31
- 229940022399 cancer vaccine Drugs 0.000 abstract description 4
- 238000009566 cancer vaccine Methods 0.000 abstract description 4
- 238000002619 cancer immunotherapy Methods 0.000 abstract description 2
- 108090000623 proteins and genes Proteins 0.000 description 73
- 235000001014 amino acid Nutrition 0.000 description 52
- 229940024606 amino acid Drugs 0.000 description 49
- 230000014509 gene expression Effects 0.000 description 43
- 235000018102 proteins Nutrition 0.000 description 35
- 102000004169 proteins and genes Human genes 0.000 description 35
- 102100037850 Interferon gamma Human genes 0.000 description 29
- 108010074328 Interferon-gamma Proteins 0.000 description 29
- 239000000523 sample Substances 0.000 description 28
- 208000020816 lung neoplasm Diseases 0.000 description 27
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 26
- 201000005202 lung cancer Diseases 0.000 description 26
- 150000007523 nucleic acids Chemical class 0.000 description 26
- 230000001472 cytotoxic effect Effects 0.000 description 24
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 24
- 230000000638 stimulation Effects 0.000 description 24
- 150000003839 salts Chemical class 0.000 description 23
- 239000013598 vector Substances 0.000 description 23
- 102000039446 nucleic acids Human genes 0.000 description 22
- 108020004707 nucleic acids Proteins 0.000 description 22
- 239000000203 mixture Substances 0.000 description 20
- 210000001519 tissue Anatomy 0.000 description 19
- 239000003153 chemical reaction reagent Substances 0.000 description 17
- 239000000463 material Substances 0.000 description 17
- 210000004443 dendritic cell Anatomy 0.000 description 16
- 230000000694 effects Effects 0.000 description 16
- 230000004048 modification Effects 0.000 description 16
- 238000012986 modification Methods 0.000 description 16
- 125000003729 nucleotide group Chemical group 0.000 description 16
- 230000004044 response Effects 0.000 description 15
- 238000003556 assay Methods 0.000 description 14
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 14
- 230000006698 induction Effects 0.000 description 14
- 239000002773 nucleotide Substances 0.000 description 14
- 239000000126 substance Substances 0.000 description 14
- 239000011651 chromium Substances 0.000 description 13
- 201000010099 disease Diseases 0.000 description 13
- 238000000338 in vitro Methods 0.000 description 13
- 208000000461 Esophageal Neoplasms Diseases 0.000 description 12
- 108020004999 messenger RNA Proteins 0.000 description 12
- 206010030155 Oesophageal carcinoma Diseases 0.000 description 11
- 238000003114 enzyme-linked immunosorbent spot assay Methods 0.000 description 11
- 201000004101 esophageal cancer Diseases 0.000 description 11
- 238000001727 in vivo Methods 0.000 description 11
- 230000005764 inhibitory process Effects 0.000 description 11
- 238000011510 Elispot assay Methods 0.000 description 10
- 102100035133 Lysosome-associated membrane glycoprotein 1 Human genes 0.000 description 10
- 230000006870 function Effects 0.000 description 10
- 241000699670 Mus sp. Species 0.000 description 9
- 238000004458 analytical method Methods 0.000 description 9
- 230000003013 cytotoxicity Effects 0.000 description 9
- 231100000135 cytotoxicity Toxicity 0.000 description 9
- 101001023379 Homo sapiens Lysosome-associated membrane glycoprotein 1 Proteins 0.000 description 8
- 210000004369 blood Anatomy 0.000 description 8
- 239000008280 blood Substances 0.000 description 8
- 238000012360 testing method Methods 0.000 description 8
- 108020004705 Codon Proteins 0.000 description 7
- 238000007792 addition Methods 0.000 description 7
- 125000000539 amino acid group Chemical group 0.000 description 7
- 150000001875 compounds Chemical class 0.000 description 7
- 238000001514 detection method Methods 0.000 description 7
- 238000009169 immunotherapy Methods 0.000 description 7
- 238000002156 mixing Methods 0.000 description 7
- 230000008685 targeting Effects 0.000 description 7
- 210000004881 tumor cell Anatomy 0.000 description 7
- 230000008901 benefit Effects 0.000 description 6
- 238000011161 development Methods 0.000 description 6
- 230000002163 immunogen Effects 0.000 description 6
- 108091005601 modified peptides Proteins 0.000 description 6
- 238000010647 peptide synthesis reaction Methods 0.000 description 6
- 230000009467 reduction Effects 0.000 description 6
- 125000006850 spacer group Chemical group 0.000 description 6
- 241000124008 Mammalia Species 0.000 description 5
- 108091028043 Nucleic acid sequence Proteins 0.000 description 5
- 239000002671 adjuvant Substances 0.000 description 5
- 230000004071 biological effect Effects 0.000 description 5
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 5
- 230000001404 mediated effect Effects 0.000 description 5
- 210000000056 organ Anatomy 0.000 description 5
- 210000005259 peripheral blood Anatomy 0.000 description 5
- 239000011886 peripheral blood Substances 0.000 description 5
- 230000008569 process Effects 0.000 description 5
- 238000011321 prophylaxis Methods 0.000 description 5
- 208000024891 symptom Diseases 0.000 description 5
- 238000013519 translation Methods 0.000 description 5
- 108020004414 DNA Proteins 0.000 description 4
- 102100037920 Insulin-like growth factor 2 mRNA-binding protein 3 Human genes 0.000 description 4
- 241001465754 Metazoa Species 0.000 description 4
- 241000699666 Mus <mouse, genus> Species 0.000 description 4
- 239000002585 base Substances 0.000 description 4
- 239000012472 biological sample Substances 0.000 description 4
- 231100000433 cytotoxic Toxicity 0.000 description 4
- 239000003085 diluting agent Substances 0.000 description 4
- 238000009472 formulation Methods 0.000 description 4
- 238000009396 hybridization Methods 0.000 description 4
- 229910052739 hydrogen Inorganic materials 0.000 description 4
- 150000002632 lipids Chemical class 0.000 description 4
- 229910052751 metal Inorganic materials 0.000 description 4
- 239000002184 metal Substances 0.000 description 4
- 150000007522 mineralic acids Chemical class 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 239000013641 positive control Substances 0.000 description 4
- 230000003389 potentiating effect Effects 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- 230000001105 regulatory effect Effects 0.000 description 4
- 238000010186 staining Methods 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 3
- 108010013476 HLA-A24 Antigen Proteins 0.000 description 3
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 3
- 108700018351 Major Histocompatibility Complex Proteins 0.000 description 3
- 241000699660 Mus musculus Species 0.000 description 3
- 108091034117 Oligonucleotide Proteins 0.000 description 3
- 108091005804 Peptidases Proteins 0.000 description 3
- 102000035195 Peptidases Human genes 0.000 description 3
- 241000700618 Vaccinia virus Species 0.000 description 3
- 235000004279 alanine Nutrition 0.000 description 3
- 229910052783 alkali metal Inorganic materials 0.000 description 3
- 150000001340 alkali metals Chemical class 0.000 description 3
- 230000005809 anti-tumor immunity Effects 0.000 description 3
- 230000005975 antitumor immune response Effects 0.000 description 3
- 238000013459 approach Methods 0.000 description 3
- -1 cationic lipid Chemical class 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 230000034994 death Effects 0.000 description 3
- 210000002919 epithelial cell Anatomy 0.000 description 3
- 230000001900 immune effect Effects 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 210000004698 lymphocyte Anatomy 0.000 description 3
- 210000002433 mononuclear leukocyte Anatomy 0.000 description 3
- 230000035772 mutation Effects 0.000 description 3
- 239000013642 negative control Substances 0.000 description 3
- 150000007524 organic acids Chemical class 0.000 description 3
- 150000007530 organic bases Chemical class 0.000 description 3
- 239000002831 pharmacologic agent Substances 0.000 description 3
- 239000008196 pharmacological composition Substances 0.000 description 3
- 239000004033 plastic Substances 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 210000004989 spleen cell Anatomy 0.000 description 3
- 238000007619 statistical method Methods 0.000 description 3
- 230000004936 stimulating effect Effects 0.000 description 3
- 230000020382 suppression by virus of host antigen processing and presentation of peptide antigen via MHC class I Effects 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- 230000009261 transgenic effect Effects 0.000 description 3
- 238000011830 transgenic mouse model Methods 0.000 description 3
- 241000283690 Bos taurus Species 0.000 description 2
- 241000282472 Canis lupus familiaris Species 0.000 description 2
- 150000008574 D-amino acids Chemical class 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- 241000283086 Equidae Species 0.000 description 2
- 241000282326 Felis catus Species 0.000 description 2
- ZHNUHDYFZUAESO-UHFFFAOYSA-N Formamide Chemical compound NC=O ZHNUHDYFZUAESO-UHFFFAOYSA-N 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 102000011786 HLA-A Antigens Human genes 0.000 description 2
- 108010075704 HLA-A Antigens Proteins 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 101100452137 Homo sapiens IGF2BP3 gene Proteins 0.000 description 2
- 101000599782 Homo sapiens Insulin-like growth factor 2 mRNA-binding protein 3 Proteins 0.000 description 2
- 101000702394 Homo sapiens Signal peptide peptidase-like 2A Proteins 0.000 description 2
- 101000960621 Homo sapiens U3 small nucleolar ribonucleoprotein protein IMP3 Proteins 0.000 description 2
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 2
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 2
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 2
- 101710116782 Lysosome-associated membrane glycoprotein 1 Proteins 0.000 description 2
- 206010027476 Metastases Diseases 0.000 description 2
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 2
- 108010046117 N-palmitoyl-5,6-dipalmitoyl-S-glycerylcysteinyl-seryl-serine Proteins 0.000 description 2
- 108700020796 Oncogene Proteins 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- 108020004511 Recombinant DNA Proteins 0.000 description 2
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 2
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 239000008186 active pharmaceutical agent Substances 0.000 description 2
- 125000003277 amino group Chemical group 0.000 description 2
- 230000000890 antigenic effect Effects 0.000 description 2
- 210000003719 b-lymphocyte Anatomy 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 210000001124 body fluid Anatomy 0.000 description 2
- 239000010839 body fluid Substances 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- 230000005880 cancer cell killing Effects 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 230000010261 cell growth Effects 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 238000003776 cleavage reaction Methods 0.000 description 2
- 230000000295 complement effect Effects 0.000 description 2
- 239000002299 complementary DNA Substances 0.000 description 2
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 2
- 230000009089 cytolysis Effects 0.000 description 2
- 239000000539 dimer Substances 0.000 description 2
- 210000003038 endothelium Anatomy 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 239000013604 expression vector Substances 0.000 description 2
- 229910052731 fluorine Inorganic materials 0.000 description 2
- 239000011888 foil Substances 0.000 description 2
- 238000001415 gene therapy Methods 0.000 description 2
- 230000002068 genetic effect Effects 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 230000013595 glycosylation Effects 0.000 description 2
- 238000006206 glycosylation reaction Methods 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 230000003053 immunization Effects 0.000 description 2
- 238000002649 immunization Methods 0.000 description 2
- 238000010253 intravenous injection Methods 0.000 description 2
- 238000002372 labelling Methods 0.000 description 2
- 125000001909 leucine group Chemical group [H]N(*)C(C(*)=O)C([H])([H])C(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 2
- 210000002540 macrophage Anatomy 0.000 description 2
- 239000003550 marker Substances 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- 230000009401 metastasis Effects 0.000 description 2
- 238000002493 microarray Methods 0.000 description 2
- 210000001616 monocyte Anatomy 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 229910052698 phosphorus Inorganic materials 0.000 description 2
- 230000026731 phosphorylation Effects 0.000 description 2
- 238000006366 phosphorylation reaction Methods 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 230000035755 proliferation Effects 0.000 description 2
- 235000019833 protease Nutrition 0.000 description 2
- 238000010188 recombinant method Methods 0.000 description 2
- 230000001177 retroviral effect Effects 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 230000007017 scission Effects 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 239000007790 solid phase Substances 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- 238000007910 systemic administration Methods 0.000 description 2
- 230000009258 tissue cross reactivity Effects 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- 239000003053 toxin Substances 0.000 description 2
- 231100000765 toxin Toxicity 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- XVQKZSLOGHBCET-INVHGPFASA-N tripalmitoyl-S-glyceryl-cysteinyl-seryl-serine Chemical compound CCCCCCCCCCCCCCCC(=O)N[C@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O)CSCC(OC(=O)CCCCCCCCCCCCCCC)COC(=O)CCCCCCCCCCCCCCC XVQKZSLOGHBCET-INVHGPFASA-N 0.000 description 2
- 229910052721 tungsten Inorganic materials 0.000 description 2
- 230000003612 virological effect Effects 0.000 description 2
- 229910052727 yttrium Inorganic materials 0.000 description 2
- UKAUYVFTDYCKQA-UHFFFAOYSA-N -2-Amino-4-hydroxybutanoic acid Natural products OC(=O)C(N)CCO UKAUYVFTDYCKQA-UHFFFAOYSA-N 0.000 description 1
- LEBVLXFERQHONN-UHFFFAOYSA-N 1-butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide Chemical compound CCCCN1CCCCC1C(=O)NC1=C(C)C=CC=C1C LEBVLXFERQHONN-UHFFFAOYSA-N 0.000 description 1
- 108010085238 Actins Proteins 0.000 description 1
- 102000007469 Actins Human genes 0.000 description 1
- 102100027241 Adenylyl cyclase-associated protein 1 Human genes 0.000 description 1
- 108700028369 Alleles Proteins 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 1
- 208000023275 Autoimmune disease Diseases 0.000 description 1
- 241000193738 Bacillus anthracis Species 0.000 description 1
- 108010077333 CAP1-6D Proteins 0.000 description 1
- 101100314454 Caenorhabditis elegans tra-1 gene Proteins 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 241000700198 Cavia Species 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- 102000009016 Cholera Toxin Human genes 0.000 description 1
- 108010049048 Cholera Toxin Proteins 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- 108091026890 Coding region Proteins 0.000 description 1
- 241000702421 Dependoparvovirus Species 0.000 description 1
- 206010061818 Disease progression Diseases 0.000 description 1
- 102000004533 Endonucleases Human genes 0.000 description 1
- 108010042407 Endonucleases Proteins 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 108700039887 Essential Genes Proteins 0.000 description 1
- 241000700662 Fowlpox virus Species 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- PMMYEEVYMWASQN-DMTCNVIQSA-N Hydroxyproline Chemical compound O[C@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-DMTCNVIQSA-N 0.000 description 1
- 108060003951 Immunoglobulin Proteins 0.000 description 1
- 102000048143 Insulin-Like Growth Factor II Human genes 0.000 description 1
- 108090001117 Insulin-Like Growth Factor II Proteins 0.000 description 1
- 108010002350 Interleukin-2 Proteins 0.000 description 1
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- UKAUYVFTDYCKQA-VKHMYHEASA-N L-homoserine Chemical compound OC(=O)[C@@H](N)CCO UKAUYVFTDYCKQA-VKHMYHEASA-N 0.000 description 1
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 1
- LRQKBLKVPFOOQJ-YFKPBYRVSA-N L-norleucine Chemical compound CCCC[C@H]([NH3+])C([O-])=O LRQKBLKVPFOOQJ-YFKPBYRVSA-N 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- 108090001030 Lipoproteins Proteins 0.000 description 1
- 102000004895 Lipoproteins Human genes 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 102000043129 MHC class I family Human genes 0.000 description 1
- 108091054437 MHC class I family Proteins 0.000 description 1
- 108091061960 Naked DNA Proteins 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 102100035593 POU domain, class 2, transcription factor 1 Human genes 0.000 description 1
- 101710084414 POU domain, class 2, transcription factor 1 Proteins 0.000 description 1
- 241000282579 Pan Species 0.000 description 1
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 1
- 241000282520 Papio Species 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 206010036790 Productive cough Diseases 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 238000012228 RNA interference-mediated gene silencing Methods 0.000 description 1
- 238000010240 RT-PCR analysis Methods 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- 241000607142 Salmonella Species 0.000 description 1
- 241000293871 Salmonella enterica subsp. enterica serovar Typhi Species 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 1
- 239000004473 Threonine Substances 0.000 description 1
- 206010046865 Vaccinia virus infection Diseases 0.000 description 1
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 230000000172 allergic effect Effects 0.000 description 1
- 229940037003 alum Drugs 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- ILRRQNADMUWWFW-UHFFFAOYSA-K aluminium phosphate Chemical compound O1[Al]2OP1(=O)O2 ILRRQNADMUWWFW-UHFFFAOYSA-K 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 229940035676 analgesics Drugs 0.000 description 1
- 239000000730 antalgic agent Substances 0.000 description 1
- 230000001093 anti-cancer Effects 0.000 description 1
- 229940124599 anti-inflammatory drug Drugs 0.000 description 1
- 230000000259 anti-tumor effect Effects 0.000 description 1
- 230000002155 anti-virotic effect Effects 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 235000009582 asparagine Nutrition 0.000 description 1
- 229960001230 asparagine Drugs 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 208000010668 atopic eczema Diseases 0.000 description 1
- 230000002238 attenuated effect Effects 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 102000015736 beta 2-Microglobulin Human genes 0.000 description 1
- 108010081355 beta 2-Microglobulin Proteins 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 239000003124 biologic agent Substances 0.000 description 1
- 238000001815 biotherapy Methods 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 210000001185 bone marrow Anatomy 0.000 description 1
- 239000004067 bulking agent Substances 0.000 description 1
- 229960003150 bupivacaine Drugs 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 230000005779 cell damage Effects 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 230000007969 cellular immunity Effects 0.000 description 1
- 229940044683 chemotherapy drug Drugs 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 238000003501 co-culture Methods 0.000 description 1
- 230000009918 complex formation Effects 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 238000012136 culture method Methods 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 230000001461 cytolytic effect Effects 0.000 description 1
- 210000000805 cytoplasm Anatomy 0.000 description 1
- 230000000254 damaging effect Effects 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 230000001934 delay Effects 0.000 description 1
- 238000000432 density-gradient centrifugation Methods 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000000368 destabilizing effect Effects 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 229910003460 diamond Inorganic materials 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- 230000005750 disease progression Effects 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- PMMYEEVYMWASQN-UHFFFAOYSA-N dl-hydroxyproline Natural products OC1C[NH2+]C(C([O-])=O)C1 PMMYEEVYMWASQN-UHFFFAOYSA-N 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- 238000009509 drug development Methods 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- UHBYWPGGCSDKFX-VKHMYHEASA-N gamma-carboxy-L-glutamic acid Chemical compound OC(=O)[C@@H](N)CC(C(O)=O)C(O)=O UHBYWPGGCSDKFX-VKHMYHEASA-N 0.000 description 1
- 230000009368 gene silencing by RNA Effects 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 102000006602 glyceraldehyde-3-phosphate dehydrogenase Human genes 0.000 description 1
- 108020004445 glyceraldehyde-3-phosphate dehydrogenase Proteins 0.000 description 1
- 230000006801 homologous recombination Effects 0.000 description 1
- 238000002744 homologous recombination Methods 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 125000004435 hydrogen atom Chemical class [H]* 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 229960002591 hydroxyproline Drugs 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 230000002998 immunogenetic effect Effects 0.000 description 1
- 102000018358 immunoglobulin Human genes 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 238000002991 immunohistochemical analysis Methods 0.000 description 1
- 230000001976 improved effect Effects 0.000 description 1
- 238000000126 in silico method Methods 0.000 description 1
- 239000005414 inactive ingredient Substances 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 238000011813 knockout mouse model Methods 0.000 description 1
- 210000001821 langerhans cell Anatomy 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 238000001638 lipofection Methods 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 102000033952 mRNA binding proteins Human genes 0.000 description 1
- 108091000373 mRNA binding proteins Proteins 0.000 description 1
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 230000001394 metastastic effect Effects 0.000 description 1
- 206010061289 metastatic neoplasm Diseases 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- LSDPWZHWYPCBBB-UHFFFAOYSA-O methylsulfide anion Chemical compound [SH2+]C LSDPWZHWYPCBBB-UHFFFAOYSA-O 0.000 description 1
- 239000000693 micelle Substances 0.000 description 1
- 102000035118 modified proteins Human genes 0.000 description 1
- 108091005573 modified proteins Proteins 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 229920005615 natural polymer Polymers 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- IPCSVZSSVZVIGE-UHFFFAOYSA-N palmitic acid group Chemical group C(CCCCCCCCCCCCCCC)(=O)O IPCSVZSSVZVIGE-UHFFFAOYSA-N 0.000 description 1
- 201000002528 pancreatic cancer Diseases 0.000 description 1
- 208000008443 pancreatic carcinoma Diseases 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 229940023041 peptide vaccine Drugs 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- BZQFBWGGLXLEPQ-REOHCLBHSA-N phosphoserine Chemical compound OC(=O)[C@@H](N)COP(O)(O)=O BZQFBWGGLXLEPQ-REOHCLBHSA-N 0.000 description 1
- 230000001766 physiological effect Effects 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 210000002826 placenta Anatomy 0.000 description 1
- 239000013612 plasmid Substances 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000009862 primary prevention Effects 0.000 description 1
- 239000013615 primer Substances 0.000 description 1
- 239000002987 primer (paints) Substances 0.000 description 1
- 238000004393 prognosis Methods 0.000 description 1
- 108010031970 prostasin Proteins 0.000 description 1
- 238000002331 protein detection Methods 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 239000013074 reference sample Substances 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000003757 reverse transcription PCR Methods 0.000 description 1
- 108010008790 ribosomal phosphoprotein P1 Proteins 0.000 description 1
- 230000000630 rising effect Effects 0.000 description 1
- 238000011309 routine diagnosis Methods 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 230000037432 silent mutation Effects 0.000 description 1
- 229910001415 sodium ion Inorganic materials 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 210000004988 splenocyte Anatomy 0.000 description 1
- 210000003802 sputum Anatomy 0.000 description 1
- 208000024794 sputum Diseases 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 150000003462 sulfoxides Chemical class 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- 210000001550 testis Anatomy 0.000 description 1
- FGMPLJWBKKVCDB-UHFFFAOYSA-N trans-L-hydroxy-proline Natural products ON1CCCC1C(O)=O FGMPLJWBKKVCDB-UHFFFAOYSA-N 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 238000010361 transduction Methods 0.000 description 1
- 230000026683 transduction Effects 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 230000004614 tumor growth Effects 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 241000701161 unidentified adenovirus Species 0.000 description 1
- 241001430294 unidentified retrovirus Species 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 238000002255 vaccination Methods 0.000 description 1
- 208000007089 vaccinia Diseases 0.000 description 1
- 125000002987 valine group Chemical group [H]N([H])C([H])(C(*)=O)C([H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 229910052720 vanadium Inorganic materials 0.000 description 1
- 239000013603 viral vector Substances 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K7/00—Peptides having 5 to 20 amino acids in a fully defined sequence; Derivatives thereof
- C07K7/04—Linear peptides containing only normal peptide links
- C07K7/06—Linear peptides containing only normal peptide links having 5 to 11 amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/14—Blood; Artificial blood
- A61K35/17—Lymphocytes; B-cells; T-cells; Natural killer cells; Interferon-activated or cytokine-activated lymphocytes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/08—Peptides having 5 to 11 amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/11—T-cells, e.g. tumour infiltrating lymphocytes [TIL] or regulatory T [Treg] cells; Lymphokine-activated killer [LAK] cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/19—Dendritic cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/20—Cellular immunotherapy characterised by the effect or the function of the cells
- A61K40/24—Antigen-presenting cells [APC]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4202—Receptors, cell surface antigens or cell surface determinants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0636—T lymphocytes
- C12N5/0638—Cytotoxic T lymphocytes [CTL] or lymphokine activated killer cells [LAK]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/515—Animal cells
- A61K2039/5154—Antigen presenting cells [APCs], e.g. dendritic cells or macrophages
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/515—Animal cells
- A61K2039/5156—Animal cells expressing foreign proteins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/57—Medicinal preparations containing antigens or antibodies characterised by the type of response, e.g. Th1, Th2
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/38—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterised by the dose, timing or administration schedule
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/46—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterised by the cancer treated
- A61K2239/55—Lung
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/998—Proteins not provided for elsewhere
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2510/00—Genetically modified cells
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Biomedical Technology (AREA)
- Organic Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Zoology (AREA)
- Epidemiology (AREA)
- Genetics & Genomics (AREA)
- Biotechnology (AREA)
- Medicinal Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Cell Biology (AREA)
- Wood Science & Technology (AREA)
- Pharmacology & Pharmacy (AREA)
- Hematology (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- General Engineering & Computer Science (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Developmental Biology & Embryology (AREA)
- Biophysics (AREA)
- Virology (AREA)
- Mycology (AREA)
- Oncology (AREA)
- Molecular Biology (AREA)
- Toxicology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Peptides Or Proteins (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US26565709P | 2009-12-01 | 2009-12-01 | |
| US61/265,657 | 2009-12-01 | ||
| US37143410P | 2010-08-06 | 2010-08-06 | |
| US61/371,434 | 2010-08-06 | ||
| PCT/JP2010/006966 WO2011067920A1 (en) | 2009-12-01 | 2010-11-30 | Imp-3 oligopeptides and vaccines including the same |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2013511958A true JP2013511958A (ja) | 2013-04-11 |
| JP2013511958A5 JP2013511958A5 (enExample) | 2014-01-23 |
Family
ID=44114782
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012524961A Pending JP2013511958A (ja) | 2009-12-01 | 2010-11-30 | Imp−3オリゴペプチドおよびそれを含むワクチン |
Country Status (14)
| Country | Link |
|---|---|
| US (1) | US20120308590A1 (enExample) |
| EP (1) | EP2507256A4 (enExample) |
| JP (1) | JP2013511958A (enExample) |
| KR (1) | KR20120099106A (enExample) |
| CN (1) | CN102741271B (enExample) |
| AU (1) | AU2010327878B2 (enExample) |
| BR (1) | BR112012013139A2 (enExample) |
| CA (1) | CA2782271A1 (enExample) |
| IL (1) | IL219976A0 (enExample) |
| MX (1) | MX2012006126A (enExample) |
| RU (1) | RU2550695C2 (enExample) |
| SG (2) | SG10201407944TA (enExample) |
| TW (1) | TW201124530A (enExample) |
| WO (1) | WO2011067920A1 (enExample) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016021506A1 (ja) * | 2014-08-04 | 2016-02-11 | オンコセラピー・サイエンス株式会社 | Koc1由来ペプチドおよびそれを含むワクチン |
| JP2016525498A (ja) * | 2013-05-24 | 2016-08-25 | オンコセラピー・サイエンス株式会社 | Th1細胞のIMP−3エピトープペプチドおよびこれを含有するワクチン |
| JP2019512242A (ja) * | 2016-03-16 | 2019-05-16 | イマティクス バイオテクノロジーズ ゲーエムベーハー | がんに対する免疫療法で使用するための形質移入t細胞およびt細胞受容体 |
| JP2019515653A (ja) * | 2016-03-16 | 2019-06-13 | イマティクス バイオテクノロジーズ ゲーエムベーハー | がんに対する免疫療法で使用されるペプチドおよびペプチドの組み合わせ |
| US11730796B2 (en) | 2016-03-16 | 2023-08-22 | Immatics Biotechnologies Gmbh | Transfected t-cells and t-cell receptors for use in immunotherapy against cancers |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20150359864A1 (en) | 2012-03-09 | 2015-12-17 | Oncotherapy Science, Inc. | Pharmaceutical composition containing peptides |
| GB201603568D0 (en) * | 2016-03-01 | 2016-04-13 | Immatics Biotechnologies Gmbh | Efficient treatment options including peptides and combination of peptide and cell based medicaments for use in immunotherapy against urinary bladder cancer |
| LT3573600T (lt) | 2017-01-25 | 2022-06-27 | Ose Immunotherapeutics | Peptidų pristatymui skirtos stabilios emulsijos gamybos būdas |
| SG11202005203UA (en) * | 2017-12-23 | 2020-07-29 | Rubius Therapeutics Inc | Artificial antigen presenting cells and methods of use |
| CN114853847B (zh) * | 2022-06-29 | 2022-09-27 | 中国农业大学 | 辣椒籽分离的寡肽ftle及其在预防或治疗癌症中的应用 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002020036A1 (de) * | 2000-09-06 | 2002-03-14 | Mueller Friederike | Arzneimittel mit einer für das rna-bindende koc-protein kodierenden dna-sequenz, einem koc-protein oder einer dna-sequenz des koc-promotors |
| JP2008510464A (ja) * | 2004-08-19 | 2008-04-10 | コリクサ コーポレイション | 肺癌の治療および診断のための組成物および方法 |
| JP2008530975A (ja) * | 2005-02-25 | 2008-08-14 | オンコセラピー・サイエンス株式会社 | Ttk、urlc10、またはkoc1ポリペプチドを発現する肺癌に対するペプチドワクチン |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10611818B2 (en) * | 2007-09-27 | 2020-04-07 | Agilent Technologies, Inc. | MHC multimers in tuberculosis diagnostics, vaccine and therapeutics |
| DK2172211T3 (en) * | 2008-10-01 | 2015-02-16 | Immatics Biotechnologies Gmbh | Composition of tumor-associated peptides and related anti-cancer vaccine for the treatment of glioblastoma (GBM) and other cancers |
-
2010
- 2010-11-26 TW TW099140933A patent/TW201124530A/zh unknown
- 2010-11-30 SG SG10201407944TA patent/SG10201407944TA/en unknown
- 2010-11-30 MX MX2012006126A patent/MX2012006126A/es active IP Right Grant
- 2010-11-30 SG SG2012039277A patent/SG181107A1/en unknown
- 2010-11-30 CA CA2782271A patent/CA2782271A1/en not_active Abandoned
- 2010-11-30 BR BR112012013139A patent/BR112012013139A2/pt not_active IP Right Cessation
- 2010-11-30 JP JP2012524961A patent/JP2013511958A/ja active Pending
- 2010-11-30 CN CN201080062874.XA patent/CN102741271B/zh not_active Expired - Fee Related
- 2010-11-30 WO PCT/JP2010/006966 patent/WO2011067920A1/en not_active Ceased
- 2010-11-30 AU AU2010327878A patent/AU2010327878B2/en not_active Ceased
- 2010-11-30 US US13/513,120 patent/US20120308590A1/en not_active Abandoned
- 2010-11-30 KR KR1020127017018A patent/KR20120099106A/ko not_active Withdrawn
- 2010-11-30 EP EP10834374.0A patent/EP2507256A4/en not_active Withdrawn
- 2010-11-30 RU RU2012127358/04A patent/RU2550695C2/ru not_active IP Right Cessation
-
2012
- 2012-05-24 IL IL219976A patent/IL219976A0/en unknown
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002020036A1 (de) * | 2000-09-06 | 2002-03-14 | Mueller Friederike | Arzneimittel mit einer für das rna-bindende koc-protein kodierenden dna-sequenz, einem koc-protein oder einer dna-sequenz des koc-promotors |
| JP2008510464A (ja) * | 2004-08-19 | 2008-04-10 | コリクサ コーポレイション | 肺癌の治療および診断のための組成物および方法 |
| JP2008530975A (ja) * | 2005-02-25 | 2008-08-14 | オンコセラピー・サイエンス株式会社 | Ttk、urlc10、またはkoc1ポリペプチドを発現する肺癌に対するペプチドワクチン |
Non-Patent Citations (2)
| Title |
|---|
| JPN6015007974; Cancer Sci. 98 (11), 2007, p.1803-1808 * |
| JPN7015000540; J. Immunol. 152, 1994, p.163-175 * |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016525498A (ja) * | 2013-05-24 | 2016-08-25 | オンコセラピー・サイエンス株式会社 | Th1細胞のIMP−3エピトープペプチドおよびこれを含有するワクチン |
| JP7070945B2 (ja) | 2014-08-04 | 2022-05-18 | オンコセラピー・サイエンス株式会社 | Koc1由来ペプチドおよびそれを含むワクチン |
| JPWO2016021506A1 (ja) * | 2014-08-04 | 2017-05-18 | オンコセラピー・サイエンス株式会社 | Koc1由来ペプチドおよびそれを含むワクチン |
| AU2015300256B2 (en) * | 2014-08-04 | 2020-01-30 | Oncotherapy Science, Inc. | KOC1-Derived peptide and vaccine including same |
| US10576102B2 (en) | 2014-08-04 | 2020-03-03 | Oncotherapy Science, Inc. | KOC1-derived peptide and vaccine including same |
| JP2020182487A (ja) * | 2014-08-04 | 2020-11-12 | オンコセラピー・サイエンス株式会社 | Koc1由来ペプチドおよびそれを含むワクチン |
| IL280417A (en) * | 2014-08-04 | 2021-03-01 | Oncotherapy Science Inc | A peptide derived from KOC1 and a vaccine containing it |
| WO2016021506A1 (ja) * | 2014-08-04 | 2016-02-11 | オンコセラピー・サイエンス株式会社 | Koc1由来ペプチドおよびそれを含むワクチン |
| US11547723B2 (en) | 2014-08-04 | 2023-01-10 | Oncotherapy Science, Inc. | KOC1-derived peptide and vaccine including same |
| JP2019512242A (ja) * | 2016-03-16 | 2019-05-16 | イマティクス バイオテクノロジーズ ゲーエムベーハー | がんに対する免疫療法で使用するための形質移入t細胞およびt細胞受容体 |
| JP2019515653A (ja) * | 2016-03-16 | 2019-06-13 | イマティクス バイオテクノロジーズ ゲーエムベーハー | がんに対する免疫療法で使用されるペプチドおよびペプチドの組み合わせ |
| JP2022130472A (ja) * | 2016-03-16 | 2022-09-06 | イマティクス バイオテクノロジーズ ゲーエムベーハー | がんに対する免疫療法で使用されるペプチドおよびペプチドの組み合わせ |
| US11730796B2 (en) | 2016-03-16 | 2023-08-22 | Immatics Biotechnologies Gmbh | Transfected t-cells and t-cell receptors for use in immunotherapy against cancers |
| US12466878B2 (en) | 2016-03-16 | 2025-11-11 | Immatics Biotechnologies Gmbh | Peptides and combination of peptides for use in immunotherapy against cancers |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2010327878A1 (en) | 2012-06-21 |
| SG181107A1 (en) | 2012-07-30 |
| WO2011067920A1 (en) | 2011-06-09 |
| TW201124530A (en) | 2011-07-16 |
| AU2010327878B2 (en) | 2014-11-20 |
| IL219976A0 (en) | 2012-07-31 |
| US20120308590A1 (en) | 2012-12-06 |
| EP2507256A1 (en) | 2012-10-10 |
| CA2782271A1 (en) | 2011-06-09 |
| RU2550695C2 (ru) | 2015-05-10 |
| CN102741271B (zh) | 2014-11-05 |
| KR20120099106A (ko) | 2012-09-06 |
| RU2012127358A (ru) | 2014-01-10 |
| EP2507256A4 (en) | 2013-10-16 |
| BR112012013139A2 (pt) | 2016-10-11 |
| SG10201407944TA (en) | 2015-01-29 |
| CN102741271A (zh) | 2012-10-17 |
| MX2012006126A (es) | 2012-06-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5728717B2 (ja) | Foxm1ペプチドおよびそれを含むワクチン | |
| RU2550695C2 (ru) | Олигопептиды imp-3 и содержащие их вакцины | |
| JP5663790B2 (ja) | Rab6kifl/kif20aエピトープペプチドおよびそれを含むワクチン | |
| JP5728716B2 (ja) | C1orf59ペプチドおよびそれを含むワクチン | |
| US9745343B2 (en) | Method of inducing an immune response by administering WDRPUH epitope peptides | |
| WO2010070877A1 (en) | Elovl7 epitope peptides and vaccines containing the same | |
| AU2010216980B2 (en) | FOXM1 peptides and vaccines containing the same | |
| HK1240953A1 (en) | Wdrpuh epitope peptides and vaccines containing the same | |
| HK1165460A (en) | Foxm1 peptides and vaccines containing the same | |
| HK1165460B (en) | Foxm1 peptides and vaccines containing the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20131127 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20131127 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20150302 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20150317 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20150706 |