JP2012515548A - 単一細胞の細胞傷害性を査定するための組成物および方法 - Google Patents
単一細胞の細胞傷害性を査定するための組成物および方法 Download PDFInfo
- Publication number
- JP2012515548A JP2012515548A JP2011547913A JP2011547913A JP2012515548A JP 2012515548 A JP2012515548 A JP 2012515548A JP 2011547913 A JP2011547913 A JP 2011547913A JP 2011547913 A JP2011547913 A JP 2011547913A JP 2012515548 A JP2012515548 A JP 2012515548A
- Authority
- JP
- Japan
- Prior art keywords
- cells
- cell
- microwell
- hiv
- effector
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 238000000034 method Methods 0.000 title claims abstract description 109
- 230000003013 cytotoxicity Effects 0.000 title claims description 11
- 231100000135 cytotoxicity Toxicity 0.000 title claims description 11
- 239000000203 mixture Substances 0.000 title description 4
- 239000012636 effector Substances 0.000 claims abstract description 94
- 210000004027 cell Anatomy 0.000 claims description 300
- 210000000822 natural killer cell Anatomy 0.000 claims description 108
- 210000003719 b-lymphocyte Anatomy 0.000 claims description 96
- 210000001744 T-lymphocyte Anatomy 0.000 claims description 60
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 claims description 49
- 102000004127 Cytokines Human genes 0.000 claims description 42
- 108090000695 Cytokines Proteins 0.000 claims description 42
- 239000000758 substrate Substances 0.000 claims description 39
- 239000003795 chemical substances by application Substances 0.000 claims description 28
- 238000001514 detection method Methods 0.000 claims description 27
- 230000009089 cytolysis Effects 0.000 claims description 25
- 208000015181 infectious disease Diseases 0.000 claims description 22
- 208000031886 HIV Infections Diseases 0.000 claims description 18
- 108090000623 proteins and genes Proteins 0.000 claims description 18
- 239000000725 suspension Substances 0.000 claims description 18
- 210000002845 virion Anatomy 0.000 claims description 18
- 108010074328 Interferon-gamma Proteins 0.000 claims description 14
- 102100037850 Interferon gamma Human genes 0.000 claims description 13
- 238000012258 culturing Methods 0.000 claims description 13
- 108010029697 CD40 Ligand Proteins 0.000 claims description 12
- 102100032937 CD40 ligand Human genes 0.000 claims description 12
- 230000001154 acute effect Effects 0.000 claims description 12
- BQRGNLJZBFXNCZ-UHFFFAOYSA-N calcein am Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC(CN(CC(=O)OCOC(C)=O)CC(=O)OCOC(C)=O)=C(OC(C)=O)C=C1OC1=C2C=C(CN(CC(=O)OCOC(C)=O)CC(=O)OCOC(=O)C)C(OC(C)=O)=C1 BQRGNLJZBFXNCZ-UHFFFAOYSA-N 0.000 claims description 12
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical group OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 claims description 11
- 108060008682 Tumor Necrosis Factor Proteins 0.000 claims description 11
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 claims description 11
- 230000014509 gene expression Effects 0.000 claims description 11
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 claims description 10
- 108010002350 Interleukin-2 Proteins 0.000 claims description 10
- 102000000588 Interleukin-2 Human genes 0.000 claims description 10
- 229910052791 calcium Inorganic materials 0.000 claims description 10
- 239000011575 calcium Substances 0.000 claims description 10
- 238000000151 deposition Methods 0.000 claims description 10
- 230000002101 lytic effect Effects 0.000 claims description 8
- 239000000463 material Substances 0.000 claims description 8
- 102000001398 Granzyme Human genes 0.000 claims description 7
- 108060005986 Granzyme Proteins 0.000 claims description 7
- 101000917858 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-A Proteins 0.000 claims description 7
- 101000917839 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-B Proteins 0.000 claims description 7
- 102100029185 Low affinity immunoglobulin gamma Fc region receptor III-B Human genes 0.000 claims description 7
- 239000000975 dye Substances 0.000 claims description 7
- 239000006166 lysate Substances 0.000 claims description 7
- 238000012544 monitoring process Methods 0.000 claims description 7
- 239000000126 substance Substances 0.000 claims description 7
- KHGNFPUMBJSZSM-UHFFFAOYSA-N Perforine Natural products COC1=C2CCC(O)C(CCC(C)(C)O)(OC)C2=NC2=C1C=CO2 KHGNFPUMBJSZSM-UHFFFAOYSA-N 0.000 claims description 6
- 230000015788 innate immune response Effects 0.000 claims description 6
- 239000003550 marker Substances 0.000 claims description 6
- 229930192851 perforin Natural products 0.000 claims description 6
- 102000004169 proteins and genes Human genes 0.000 claims description 6
- 208000037357 HIV infectious disease Diseases 0.000 claims description 5
- 102000003814 Interleukin-10 Human genes 0.000 claims description 5
- 108090000174 Interleukin-10 Proteins 0.000 claims description 5
- 230000005875 antibody response Effects 0.000 claims description 5
- 230000006037 cell lysis Effects 0.000 claims description 5
- 239000007850 fluorescent dye Substances 0.000 claims description 5
- 208000033519 human immunodeficiency virus infectious disease Diseases 0.000 claims description 5
- 230000003834 intracellular effect Effects 0.000 claims description 5
- 238000002372 labelling Methods 0.000 claims description 5
- 230000002934 lysing effect Effects 0.000 claims description 5
- 238000011225 antiretroviral therapy Methods 0.000 claims description 4
- 101710149863 C-C chemokine receptor type 4 Proteins 0.000 claims description 3
- 102100036301 C-C chemokine receptor type 7 Human genes 0.000 claims description 3
- 102100028990 C-X-C chemokine receptor type 3 Human genes 0.000 claims description 3
- 102100032976 CCR4-NOT transcription complex subunit 6 Human genes 0.000 claims description 3
- 102100025137 Early activation antigen CD69 Human genes 0.000 claims description 3
- 101000716065 Homo sapiens C-C chemokine receptor type 7 Proteins 0.000 claims description 3
- 101000916050 Homo sapiens C-X-C chemokine receptor type 3 Proteins 0.000 claims description 3
- 101000934374 Homo sapiens Early activation antigen CD69 Proteins 0.000 claims description 3
- 101001018097 Homo sapiens L-selectin Proteins 0.000 claims description 3
- 108090000978 Interleukin-4 Proteins 0.000 claims description 3
- 102000004388 Interleukin-4 Human genes 0.000 claims description 3
- 102100033467 L-selectin Human genes 0.000 claims description 3
- 230000003321 amplification Effects 0.000 claims description 3
- DZNKOAWEHDKBEP-UHFFFAOYSA-N methyl 2-[6-[bis(2-methoxy-2-oxoethyl)amino]-5-[2-[2-[bis(2-methoxy-2-oxoethyl)amino]-5-methylphenoxy]ethoxy]-1-benzofuran-2-yl]-1,3-oxazole-5-carboxylate Chemical group COC(=O)CN(CC(=O)OC)C1=CC=C(C)C=C1OCCOC(C(=C1)N(CC(=O)OC)CC(=O)OC)=CC2=C1OC(C=1OC(=CN=1)C(=O)OC)=C2 DZNKOAWEHDKBEP-UHFFFAOYSA-N 0.000 claims description 3
- 238000003199 nucleic acid amplification method Methods 0.000 claims description 3
- 230000009260 cross reactivity Effects 0.000 claims description 2
- 230000016784 immunoglobulin production Effects 0.000 claims description 2
- 238000002493 microarray Methods 0.000 abstract description 15
- 230000003993 interaction Effects 0.000 abstract description 7
- 238000013537 high throughput screening Methods 0.000 abstract description 3
- 241000725303 Human immunodeficiency virus Species 0.000 description 67
- 238000003556 assay Methods 0.000 description 45
- 102100036011 T-cell surface glycoprotein CD4 Human genes 0.000 description 25
- 230000006870 function Effects 0.000 description 19
- 230000001461 cytolytic effect Effects 0.000 description 18
- 238000013459 approach Methods 0.000 description 16
- 108090000765 processed proteins & peptides Proteins 0.000 description 15
- 241000700605 Viruses Species 0.000 description 13
- 230000004044 response Effects 0.000 description 13
- 238000011534 incubation Methods 0.000 description 11
- 108010043610 KIR Receptors Proteins 0.000 description 9
- 102000002698 KIR Receptors Human genes 0.000 description 9
- 238000002474 experimental method Methods 0.000 description 9
- 230000002147 killing effect Effects 0.000 description 9
- 210000004698 lymphocyte Anatomy 0.000 description 9
- 230000001404 mediated effect Effects 0.000 description 9
- 102000005962 receptors Human genes 0.000 description 9
- 108020003175 receptors Proteins 0.000 description 9
- 241000713772 Human immunodeficiency virus 1 Species 0.000 description 8
- 238000004458 analytical method Methods 0.000 description 8
- 239000011521 glass Substances 0.000 description 8
- 238000011160 research Methods 0.000 description 8
- 108700028369 Alleles Proteins 0.000 description 7
- 230000000840 anti-viral effect Effects 0.000 description 7
- 230000010056 antibody-dependent cellular cytotoxicity Effects 0.000 description 7
- 230000007774 longterm Effects 0.000 description 7
- 230000007246 mechanism Effects 0.000 description 7
- 238000003757 reverse transcription PCR Methods 0.000 description 7
- 229960005486 vaccine Drugs 0.000 description 7
- 229940033330 HIV vaccine Drugs 0.000 description 6
- 230000004913 activation Effects 0.000 description 6
- 238000003491 array Methods 0.000 description 6
- 238000012512 characterization method Methods 0.000 description 6
- 231100000433 cytotoxic Toxicity 0.000 description 6
- 230000001472 cytotoxic effect Effects 0.000 description 6
- 238000005516 engineering process Methods 0.000 description 6
- 210000000987 immune system Anatomy 0.000 description 6
- 238000005259 measurement Methods 0.000 description 6
- 244000052769 pathogen Species 0.000 description 6
- 230000035755 proliferation Effects 0.000 description 6
- 230000001681 protective effect Effects 0.000 description 6
- 230000029812 viral genome replication Effects 0.000 description 6
- 230000003612 virological effect Effects 0.000 description 6
- 102000004503 Perforin Human genes 0.000 description 5
- 108010056995 Perforin Proteins 0.000 description 5
- 108091008874 T cell receptors Proteins 0.000 description 5
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 5
- 239000000427 antigen Substances 0.000 description 5
- 108091007433 antigens Proteins 0.000 description 5
- 102000036639 antigens Human genes 0.000 description 5
- 230000008901 benefit Effects 0.000 description 5
- DEGAKNSWVGKMLS-UHFFFAOYSA-N calcein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC(CN(CC(O)=O)CC(O)=O)=C(O)C=C1OC1=C2C=C(CN(CC(O)=O)CC(=O)O)C(O)=C1 DEGAKNSWVGKMLS-UHFFFAOYSA-N 0.000 description 5
- 230000003915 cell function Effects 0.000 description 5
- 238000000684 flow cytometry Methods 0.000 description 5
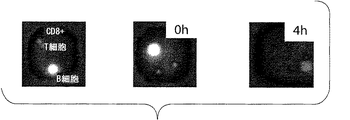
- 238000002073 fluorescence micrograph Methods 0.000 description 5
- 238000011068 loading method Methods 0.000 description 5
- 230000003472 neutralizing effect Effects 0.000 description 5
- 229960002378 oftasceine Drugs 0.000 description 5
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 5
- 230000010076 replication Effects 0.000 description 5
- 238000012216 screening Methods 0.000 description 5
- 206010061818 Disease progression Diseases 0.000 description 4
- 101000581981 Homo sapiens Neural cell adhesion molecule 1 Proteins 0.000 description 4
- 102100027347 Neural cell adhesion molecule 1 Human genes 0.000 description 4
- 230000001684 chronic effect Effects 0.000 description 4
- 238000013461 design Methods 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 230000005750 disease progression Effects 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 238000012252 genetic analysis Methods 0.000 description 4
- 238000007639 printing Methods 0.000 description 4
- 102000004196 processed proteins & peptides Human genes 0.000 description 4
- 230000009385 viral infection Effects 0.000 description 4
- 208000030507 AIDS Diseases 0.000 description 3
- 229940124718 AIDS vaccine Drugs 0.000 description 3
- 206010000807 Acute HIV infection Diseases 0.000 description 3
- 102000001326 Chemokine CCL4 Human genes 0.000 description 3
- 108010055165 Chemokine CCL4 Proteins 0.000 description 3
- 101000945492 Homo sapiens Killer cell immunoglobulin-like receptor 3DS1 Proteins 0.000 description 3
- 102100034833 Killer cell immunoglobulin-like receptor 3DS1 Human genes 0.000 description 3
- 102000043129 MHC class I family Human genes 0.000 description 3
- 108091054437 MHC class I family Proteins 0.000 description 3
- 108091008877 NK cell receptors Proteins 0.000 description 3
- 102000010648 Natural Killer Cell Receptors Human genes 0.000 description 3
- 241000713311 Simian immunodeficiency virus Species 0.000 description 3
- 230000005867 T cell response Effects 0.000 description 3
- 206010058874 Viraemia Diseases 0.000 description 3
- 208000036142 Viral infection Diseases 0.000 description 3
- 230000006399 behavior Effects 0.000 description 3
- 238000003501 co-culture Methods 0.000 description 3
- 230000000875 corresponding effect Effects 0.000 description 3
- 230000034994 death Effects 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 3
- 230000002068 genetic effect Effects 0.000 description 3
- 238000012203 high throughput assay Methods 0.000 description 3
- 230000001900 immune effect Effects 0.000 description 3
- 230000028993 immune response Effects 0.000 description 3
- 230000036039 immunity Effects 0.000 description 3
- 230000002163 immunogen Effects 0.000 description 3
- 230000004957 immunoregulator effect Effects 0.000 description 3
- 238000002955 isolation Methods 0.000 description 3
- 230000000670 limiting effect Effects 0.000 description 3
- 230000001717 pathogenic effect Effects 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 238000010791 quenching Methods 0.000 description 3
- 230000000171 quenching effect Effects 0.000 description 3
- 238000007423 screening assay Methods 0.000 description 3
- 230000028327 secretion Effects 0.000 description 3
- 210000002966 serum Anatomy 0.000 description 3
- 238000010186 staining Methods 0.000 description 3
- 238000002255 vaccination Methods 0.000 description 3
- 102000011727 Caspases Human genes 0.000 description 2
- 108010076667 Caspases Proteins 0.000 description 2
- 208000035473 Communicable disease Diseases 0.000 description 2
- 238000002965 ELISA Methods 0.000 description 2
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 2
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 2
- 102000006390 HLA-B Antigens Human genes 0.000 description 2
- 108010058607 HLA-B Antigens Proteins 0.000 description 2
- 102000012153 HLA-B27 Antigen Human genes 0.000 description 2
- 108010061486 HLA-B27 Antigen Proteins 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 101000945351 Homo sapiens Killer cell immunoglobulin-like receptor 3DL1 Proteins 0.000 description 2
- -1 IFNγ Proteins 0.000 description 2
- 102000013691 Interleukin-17 Human genes 0.000 description 2
- 108050003558 Interleukin-17 Proteins 0.000 description 2
- 102100033627 Killer cell immunoglobulin-like receptor 3DL1 Human genes 0.000 description 2
- 241000282553 Macaca Species 0.000 description 2
- 230000006051 NK cell activation Effects 0.000 description 2
- 108010004217 Natural Cytotoxicity Triggering Receptor 1 Proteins 0.000 description 2
- 102100032870 Natural cytotoxicity triggering receptor 1 Human genes 0.000 description 2
- 108010047620 Phytohemagglutinins Proteins 0.000 description 2
- 238000011529 RT qPCR Methods 0.000 description 2
- 230000024932 T cell mediated immunity Effects 0.000 description 2
- 230000006052 T cell proliferation Effects 0.000 description 2
- 238000009825 accumulation Methods 0.000 description 2
- 230000033289 adaptive immune response Effects 0.000 description 2
- 210000000612 antigen-presenting cell Anatomy 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 210000004970 cd4 cell Anatomy 0.000 description 2
- 230000001413 cellular effect Effects 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 230000001086 cytosolic effect Effects 0.000 description 2
- 230000007123 defense Effects 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 230000003111 delayed effect Effects 0.000 description 2
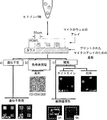
- 238000010586 diagram Methods 0.000 description 2
- 239000004205 dimethyl polysiloxane Substances 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 230000001771 impaired effect Effects 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 210000005007 innate immune system Anatomy 0.000 description 2
- 238000007689 inspection Methods 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 238000005457 optimization Methods 0.000 description 2
- 230000001885 phytohemagglutinin Effects 0.000 description 2
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 description 2
- 239000013641 positive control Substances 0.000 description 2
- 238000000513 principal component analysis Methods 0.000 description 2
- 230000000750 progressive effect Effects 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 238000012207 quantitative assay Methods 0.000 description 2
- 238000003762 quantitative reverse transcription PCR Methods 0.000 description 2
- 108010043277 recombinant soluble CD4 Proteins 0.000 description 2
- 238000011084 recovery Methods 0.000 description 2
- 230000003248 secreting effect Effects 0.000 description 2
- 230000000638 stimulation Effects 0.000 description 2
- 230000002459 sustained effect Effects 0.000 description 2
- 210000000225 synapse Anatomy 0.000 description 2
- 238000011282 treatment Methods 0.000 description 2
- 238000012800 visualization Methods 0.000 description 2
- RJPSHDMGSVVHFA-UHFFFAOYSA-N 2-[carboxymethyl-[(7-hydroxy-4-methyl-2-oxochromen-8-yl)methyl]amino]acetic acid Chemical compound OC(=O)CN(CC(O)=O)CC1=C(O)C=CC2=C1OC(=O)C=C2C RJPSHDMGSVVHFA-UHFFFAOYSA-N 0.000 description 1
- 206010069754 Acquired gene mutation Diseases 0.000 description 1
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 description 1
- BHPQYMZQTOCNFJ-UHFFFAOYSA-N Calcium cation Chemical compound [Ca+2] BHPQYMZQTOCNFJ-UHFFFAOYSA-N 0.000 description 1
- 208000024172 Cardiovascular disease Diseases 0.000 description 1
- 206010057248 Cell death Diseases 0.000 description 1
- 241000193163 Clostridioides difficile Species 0.000 description 1
- 201000007336 Cryptococcosis Diseases 0.000 description 1
- 241000221204 Cryptococcus neoformans Species 0.000 description 1
- 238000011510 Elispot assay Methods 0.000 description 1
- 108090000371 Esterases Proteins 0.000 description 1
- 108010067306 Fibronectins Proteins 0.000 description 1
- 102000016359 Fibronectins Human genes 0.000 description 1
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 1
- 229940033332 HIV-1 vaccine Drugs 0.000 description 1
- 102100028972 HLA class I histocompatibility antigen, A alpha chain Human genes 0.000 description 1
- 108010075704 HLA-A Antigens Proteins 0.000 description 1
- 108010088652 Histocompatibility Antigens Class I Proteins 0.000 description 1
- 102000008949 Histocompatibility Antigens Class I Human genes 0.000 description 1
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 description 1
- 101001023379 Homo sapiens Lysosome-associated membrane glycoprotein 1 Proteins 0.000 description 1
- 108010073807 IgG Receptors Proteins 0.000 description 1
- 102000009490 IgG Receptors Human genes 0.000 description 1
- 108060003951 Immunoglobulin Proteins 0.000 description 1
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 1
- 102000008070 Interferon-gamma Human genes 0.000 description 1
- 108090001060 Lipase Proteins 0.000 description 1
- 102000004882 Lipase Human genes 0.000 description 1
- 239000004367 Lipase Substances 0.000 description 1
- 102100035133 Lysosome-associated membrane glycoprotein 1 Human genes 0.000 description 1
- 206010065764 Mucosal infection Diseases 0.000 description 1
- 241000187479 Mycobacterium tuberculosis Species 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 108010039918 Polylysine Proteins 0.000 description 1
- 238000002123 RNA extraction Methods 0.000 description 1
- 241000193998 Streptococcus pneumoniae Species 0.000 description 1
- 230000006044 T cell activation Effects 0.000 description 1
- 230000010782 T cell mediated cytotoxicity Effects 0.000 description 1
- 230000008649 adaptation response Effects 0.000 description 1
- 230000003044 adaptive effect Effects 0.000 description 1
- 210000005006 adaptive immune system Anatomy 0.000 description 1
- 230000001640 apoptogenic effect Effects 0.000 description 1
- 238000003782 apoptosis assay Methods 0.000 description 1
- 238000011948 assay development Methods 0.000 description 1
- 230000002238 attenuated effect Effects 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 229910001424 calcium ion Inorganic materials 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 238000010370 cell cloning Methods 0.000 description 1
- 230000006727 cell loss Effects 0.000 description 1
- 239000002458 cell surface marker Substances 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 230000007969 cellular immunity Effects 0.000 description 1
- 230000008614 cellular interaction Effects 0.000 description 1
- 230000036755 cellular response Effects 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 238000012875 competitive assay Methods 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 230000001010 compromised effect Effects 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 238000011266 cytolytic assay Methods 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- 239000002254 cytotoxic agent Substances 0.000 description 1
- 231100000599 cytotoxic agent Toxicity 0.000 description 1
- 238000007405 data analysis Methods 0.000 description 1
- 230000008260 defense mechanism Effects 0.000 description 1
- 230000005860 defense response to virus Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 238000003114 enzyme-linked immunosorbent spot assay Methods 0.000 description 1
- 238000013401 experimental design Methods 0.000 description 1
- 239000011536 extraction buffer Substances 0.000 description 1
- 238000011223 gene expression profiling Methods 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 210000004602 germ cell Anatomy 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 238000007417 hierarchical cluster analysis Methods 0.000 description 1
- 230000005745 host immune response Effects 0.000 description 1
- 230000028996 humoral immune response Effects 0.000 description 1
- 210000004408 hybridoma Anatomy 0.000 description 1
- 238000010191 image analysis Methods 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 230000003100 immobilizing effect Effects 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 230000036737 immune function Effects 0.000 description 1
- 238000010166 immunofluorescence Methods 0.000 description 1
- 102000018358 immunoglobulin Human genes 0.000 description 1
- 230000006054 immunological memory Effects 0.000 description 1
- 239000003547 immunosorbent Substances 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 239000012678 infectious agent Substances 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 230000008611 intercellular interaction Effects 0.000 description 1
- 229960003130 interferon gamma Drugs 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- 125000000741 isoleucyl group Chemical group [H]N([H])C(C(C([H])([H])[H])C([H])([H])C([H])([H])[H])C(=O)O* 0.000 description 1
- 238000009533 lab test Methods 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 235000019421 lipase Nutrition 0.000 description 1
- 239000012139 lysis buffer Substances 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 238000013507 mapping Methods 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 230000015654 memory Effects 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 210000004877 mucosa Anatomy 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- VYNDHICBIRRPFP-UHFFFAOYSA-N pacific blue Chemical compound FC1=C(O)C(F)=C2OC(=O)C(C(=O)O)=CC2=C1 VYNDHICBIRRPFP-UHFFFAOYSA-N 0.000 description 1
- 238000004091 panning Methods 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 230000007110 pathogen host interaction Effects 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- 230000008823 permeabilization Effects 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 238000002823 phage display Methods 0.000 description 1
- 238000000206 photolithography Methods 0.000 description 1
- 238000009832 plasma treatment Methods 0.000 description 1
- 229920000656 polylysine Polymers 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000004321 preservation Methods 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 230000005522 programmed cell death Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 125000001500 prolyl group Chemical group [H]N1C([H])(C(=O)[*])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 238000004445 quantitative analysis Methods 0.000 description 1
- 238000003259 recombinant expression Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000000820 replica moulding Methods 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 239000000523 sample Substances 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 230000037439 somatic mutation Effects 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 238000012289 standard assay Methods 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 229940031000 streptococcus pneumoniae Drugs 0.000 description 1
- 208000010648 susceptibility to HIV infection Diseases 0.000 description 1
- 230000031068 symbiosis, encompassing mutualism through parasitism Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 239000013638 trimer Substances 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/02—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving viable microorganisms
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/569—Immunoassay; Biospecific binding assay; Materials therefor for microorganisms, e.g. protozoa, bacteria, viruses
- G01N33/56966—Animal cells
- G01N33/56972—White blood cells
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/5005—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells
- G01N33/5008—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics
- G01N33/5044—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics involving specific cell types
- G01N33/5047—Cells of the immune system
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/52—Use of compounds or compositions for colorimetric, spectrophotometric or fluorometric investigation, e.g. use of reagent paper and including single- and multilayer analytical elements
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/563—Immunoassay; Biospecific binding assay; Materials therefor involving antibody fragments
-
- C—CHEMISTRY; METALLURGY
- C40—COMBINATORIAL TECHNOLOGY
- C40B—COMBINATORIAL CHEMISTRY; LIBRARIES, e.g. CHEMICAL LIBRARIES
- C40B30/00—Methods of screening libraries
- C40B30/06—Methods of screening libraries by measuring effects on living organisms, tissues or cells
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/005—Assays involving biological materials from specific organisms or of a specific nature from viruses
- G01N2333/08—RNA viruses
- G01N2333/15—Retroviridae, e.g. bovine leukaemia virus, feline leukaemia virus, feline leukaemia virus, human T-cell leukaemia-lymphoma virus
- G01N2333/155—Lentiviridae, e.g. visna-maedi virus, equine infectious virus, FIV, SIV
- G01N2333/16—HIV-1, HIV-2
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Hematology (AREA)
- Biomedical Technology (AREA)
- Molecular Biology (AREA)
- Urology & Nephrology (AREA)
- Cell Biology (AREA)
- Analytical Chemistry (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- Physics & Mathematics (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- Pathology (AREA)
- Food Science & Technology (AREA)
- Medicinal Chemistry (AREA)
- General Physics & Mathematics (AREA)
- Tropical Medicine & Parasitology (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Organic Chemistry (AREA)
- Toxicology (AREA)
- Virology (AREA)
- Wood Science & Technology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- General Engineering & Computer Science (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Peptides Or Proteins (AREA)
- Investigating, Analyzing Materials By Fluorescence Or Luminescence (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14610609P | 2009-01-21 | 2009-01-21 | |
| US61/146,106 | 2009-01-21 | ||
| PCT/US2009/050411 WO2010085275A1 (en) | 2009-01-21 | 2009-07-13 | Compositions and methods for assessing cytotoxicity of single cells |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015094509A Division JP6017620B2 (ja) | 2009-01-21 | 2015-05-05 | 単一細胞の細胞傷害性を査定するための組成物および方法 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2012515548A true JP2012515548A (ja) | 2012-07-12 |
| JP2012515548A5 JP2012515548A5 (cg-RX-API-DMAC7.html) | 2013-03-21 |
Family
ID=42356144
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011547913A Withdrawn JP2012515548A (ja) | 2009-01-21 | 2009-07-13 | 単一細胞の細胞傷害性を査定するための組成物および方法 |
| JP2015094509A Active JP6017620B2 (ja) | 2009-01-21 | 2015-05-05 | 単一細胞の細胞傷害性を査定するための組成物および方法 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015094509A Active JP6017620B2 (ja) | 2009-01-21 | 2015-05-05 | 単一細胞の細胞傷害性を査定するための組成物および方法 |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US9244071B2 (cg-RX-API-DMAC7.html) |
| EP (1) | EP2389446B1 (cg-RX-API-DMAC7.html) |
| JP (2) | JP2012515548A (cg-RX-API-DMAC7.html) |
| KR (1) | KR20110134386A (cg-RX-API-DMAC7.html) |
| CN (2) | CN102388145A (cg-RX-API-DMAC7.html) |
| AU (1) | AU2009338127B2 (cg-RX-API-DMAC7.html) |
| CA (1) | CA2689681C (cg-RX-API-DMAC7.html) |
| IL (1) | IL213938A0 (cg-RX-API-DMAC7.html) |
| RU (1) | RU2532228C2 (cg-RX-API-DMAC7.html) |
| SG (2) | SG10201401643PA (cg-RX-API-DMAC7.html) |
| WO (1) | WO2010085275A1 (cg-RX-API-DMAC7.html) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017169267A1 (ja) * | 2016-03-30 | 2017-10-05 | ソニー株式会社 | 細胞観察装置、免疫細胞の活性度の評価方法及び免疫細胞の品質管理方法 |
| JPWO2020171020A1 (ja) * | 2019-02-18 | 2021-03-11 | 株式会社エヌビィー健康研究所 | 細胞の選抜方法、核酸の製造方法、組換え細胞の製造方法、目的物質の製造方法、医薬組成物の製造方法、及び試薬 |
| JP2022087206A (ja) * | 2016-11-11 | 2022-06-09 | アイソプレキシス コーポレイション | 単一細胞のゲノム、トランスクリプトームおよびプロテオームの同時解析のための組成物および方法 |
| US11518977B2 (en) | 2014-01-31 | 2022-12-06 | AgBiome, Inc. | Modified biological control agents and their uses |
| US11760971B2 (en) | 2014-01-31 | 2023-09-19 | AgBiome, Inc. | Modified biological control agents and their uses |
Families Citing this family (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9404924B2 (en) | 2008-12-04 | 2016-08-02 | Massachusetts Institute Of Technology | Method of performing one-step, single cell RT-PCR |
| EP2370814B1 (en) | 2008-12-04 | 2015-04-29 | Massachusetts Institute of Technology | Method for diagnosing allergic reactions |
| EP2389446B1 (en) | 2009-01-21 | 2017-07-05 | Massachusetts Institute of Technology | Compositions and methods for assessing cytotoxicity of single cells |
| WO2012072823A1 (en) * | 2010-12-03 | 2012-06-07 | Mindseeds Laboratories Srl | Rapid screening of monoclonal antibodies |
| CN103392124B (zh) | 2010-12-03 | 2016-04-20 | 塞普莱有限公司 | 细胞功能的微分析 |
| US9944983B2 (en) | 2011-12-13 | 2018-04-17 | Single Cell Technology, Inc. | Method of screening a plurality of single secreting cells for functional activity |
| US20150191772A1 (en) | 2012-06-29 | 2015-07-09 | Danmarks Tekniske Universitet | Method of charging a test carrier and a test carrier |
| CN104884605B (zh) * | 2012-08-24 | 2018-05-18 | 耶鲁大学 | 用于高通量多重检测的系统、装置和方法 |
| WO2015031190A1 (en) * | 2013-08-26 | 2015-03-05 | President And Fellows Of Harvard College | Determination of immune cells and other cells |
| EP3227684B1 (en) | 2014-12-03 | 2019-10-02 | Isoplexis Corporation | Analysis and screening of cell secretion profiles |
| US10488321B2 (en) | 2015-03-19 | 2019-11-26 | The Board Of Trustees Of The Leland Stanford Junior University | Devices and methods for high-throughput single cell and biomolecule analysis and retrieval in a microfluidic chip |
| JP6855382B2 (ja) | 2015-03-26 | 2021-04-14 | ユニバーシティー オブ ヒューストン システム | 細胞の統合された機能性および分子のプロファイリング |
| EP3402902B1 (en) | 2016-01-15 | 2021-10-27 | Massachusetts Institute Of Technology | Semi-permeable arrays for analyzing biological systems and methods of using same |
| WO2017165791A1 (en) | 2016-03-25 | 2017-09-28 | President And Fellows Of Harvard College | Microfluidic determination of immune and other cells |
| US12138625B2 (en) | 2016-06-14 | 2024-11-12 | Cellply S.R.L | Screening kit and method |
| EP3472351B1 (de) | 2016-06-15 | 2020-10-28 | Ludwig-Maximilians-Universität München | Einzelmolekülnachweis bzw. -quantifizierung durch dna-nanotechnologie |
| CN106086171A (zh) * | 2016-06-15 | 2016-11-09 | 广州中大南沙科技创新产业园有限公司 | 一种抑制肿瘤细胞增殖的药物筛选方法 |
| CN109964126B (zh) | 2016-09-12 | 2022-12-27 | 伊索普莱克西斯公司 | 用于细胞治疗法和其他免疫治疗法的多重分析的系统和方法 |
| AU2017345402A1 (en) * | 2016-10-19 | 2019-05-23 | General Automation Lab Technologies Inc. | High resolution systems, kits, apparatus, and methods for screening microorganisms and other high throughput microbiology applications |
| US11085912B2 (en) * | 2016-12-14 | 2021-08-10 | University Of Cincinnati | Methods of diagnosing Clostridium difficile infection or recurrence in a subject |
| SE543211C2 (en) * | 2017-06-29 | 2020-10-27 | Mabtech Production Ab | Method and system for analyzing Fluorospot assays |
| JP2021505157A (ja) | 2017-12-07 | 2021-02-18 | マサチューセッツ インスティテュート オブ テクノロジー | 単一細胞分析 |
| CN108728356B (zh) * | 2018-05-23 | 2022-02-22 | 北京科技大学 | 用于不同三维细胞团配对的装置及共培养方法 |
| CN113614532A (zh) * | 2019-01-16 | 2021-11-05 | 烟台澳斯邦生物工程有限公司 | 沉积用于显微镜检查的生物样品的自动液体处理系统和方法 |
| US12064768B2 (en) | 2020-02-21 | 2024-08-20 | Northeastern University | Single cell isolation and processing system with reversible well shape |
| US20240182849A1 (en) | 2022-11-07 | 2024-06-06 | Shennon Biotechnologies Inc. | Microfluidic Devices for High Throughput Screening of Cell-Cell Interactions |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007035633A2 (en) * | 2005-09-16 | 2007-03-29 | President & Fellows Of Harvard College | Screening assays and methods |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IL68507A (en) | 1982-05-10 | 1986-01-31 | Univ Bar Ilan | System and methods for cell selection |
| SU1650094A1 (ru) * | 1987-12-29 | 1991-05-23 | Научно-исследовательский институт проктологии | Способ диагностики прот женности активного воспалени в толстой кишке при неспецифическом звенном колите |
| US6410252B1 (en) | 1995-12-22 | 2002-06-25 | Case Western Reserve University | Methods for measuring T cell cytokines |
| US6210910B1 (en) | 1998-03-02 | 2001-04-03 | Trustees Of Tufts College | Optical fiber biosensor array comprising cell populations confined to microcavities |
| JP2003501011A (ja) * | 1999-04-09 | 2003-01-14 | ヒューマン ジノーム サイエンシーズ, インコーポレイテッド | 49個のヒト分泌タンパク質 |
| AU5591200A (en) * | 1999-06-11 | 2001-01-02 | Human Genome Sciences, Inc. | 49 human secreted proteins |
| US7332286B2 (en) * | 2001-02-02 | 2008-02-19 | University Of Pennsylvania | Peptide or protein microassay method and apparatus |
| US20020141906A1 (en) | 2001-03-30 | 2002-10-03 | American Type Culture Collection | Microtiter plate sealing cover with well identifiers |
| DK2297333T3 (en) | 2008-05-30 | 2015-04-07 | Massachusetts Inst Technology | Method for spatial separation and for screening cells |
| US9404924B2 (en) | 2008-12-04 | 2016-08-02 | Massachusetts Institute Of Technology | Method of performing one-step, single cell RT-PCR |
| EP2370814B1 (en) | 2008-12-04 | 2015-04-29 | Massachusetts Institute of Technology | Method for diagnosing allergic reactions |
| EP2389446B1 (en) | 2009-01-21 | 2017-07-05 | Massachusetts Institute of Technology | Compositions and methods for assessing cytotoxicity of single cells |
| US8569046B2 (en) | 2009-02-20 | 2013-10-29 | Massachusetts Institute Of Technology | Microarray with microchannels |
-
2009
- 2009-07-13 EP EP09839012.3A patent/EP2389446B1/en not_active Not-in-force
- 2009-07-13 JP JP2011547913A patent/JP2012515548A/ja not_active Withdrawn
- 2009-07-13 AU AU2009338127A patent/AU2009338127B2/en not_active Ceased
- 2009-07-13 WO PCT/US2009/050411 patent/WO2010085275A1/en not_active Ceased
- 2009-07-13 US US13/145,300 patent/US9244071B2/en active Active
- 2009-07-13 SG SG10201401643PA patent/SG10201401643PA/en unknown
- 2009-07-13 RU RU2011134898/10A patent/RU2532228C2/ru not_active IP Right Cessation
- 2009-07-13 SG SG2011049459A patent/SG172889A1/en unknown
- 2009-07-13 CN CN2009801551526A patent/CN102388145A/zh active Pending
- 2009-07-13 CN CN201510140107.0A patent/CN104745669A/zh active Pending
- 2009-07-13 KR KR1020117018895A patent/KR20110134386A/ko not_active Ceased
- 2009-07-13 CA CA2689681A patent/CA2689681C/en not_active Expired - Fee Related
-
2011
- 2011-07-05 IL IL213938A patent/IL213938A0/en unknown
-
2015
- 2015-05-05 JP JP2015094509A patent/JP6017620B2/ja active Active
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007035633A2 (en) * | 2005-09-16 | 2007-03-29 | President & Fellows Of Harvard College | Screening assays and methods |
Non-Patent Citations (3)
| Title |
|---|
| JPN6014005771; Proceedings of the National Academy of Sciences of the United States of America Vol.104, No.16, 2007, p.6776-6781 * |
| JPN6014005772; Nature Biotechnology Vol.24, No.6, 2006, p.703-707 * |
| JPN6014005773; Clinical Immunology Vol.129, 2008, p.10-18 * |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11518977B2 (en) | 2014-01-31 | 2022-12-06 | AgBiome, Inc. | Modified biological control agents and their uses |
| US12398364B2 (en) | 2014-01-31 | 2025-08-26 | Certis U.S.A. L.L.C. | Modified biological control agents and their uses |
| US11760971B2 (en) | 2014-01-31 | 2023-09-19 | AgBiome, Inc. | Modified biological control agents and their uses |
| CN108779427A (zh) * | 2016-03-30 | 2018-11-09 | 索尼公司 | 细胞观察装置、用于评估免疫细胞活性水平的方法以及用于控制免疫细胞品质的方法 |
| JPWO2017169267A1 (ja) * | 2016-03-30 | 2019-03-14 | ソニー株式会社 | 細胞観察装置、免疫細胞の活性度の評価方法及び免疫細胞の品質管理方法 |
| JP7021633B2 (ja) | 2016-03-30 | 2022-02-17 | ソニーグループ株式会社 | 細胞観察装置及び免疫細胞の品質管理方法 |
| WO2017169267A1 (ja) * | 2016-03-30 | 2017-10-05 | ソニー株式会社 | 細胞観察装置、免疫細胞の活性度の評価方法及び免疫細胞の品質管理方法 |
| JP2022087206A (ja) * | 2016-11-11 | 2022-06-09 | アイソプレキシス コーポレイション | 単一細胞のゲノム、トランスクリプトームおよびプロテオームの同時解析のための組成物および方法 |
| JP7568672B2 (ja) | 2016-11-11 | 2024-10-16 | アイソプレキシス コーポレイション | 単一細胞のゲノム、トランスクリプトームおよびプロテオームの同時解析のための組成物および方法 |
| US11357787B2 (en) | 2019-02-18 | 2022-06-14 | Nb Health Laboratory Co., Ltd. | Method for selecting cells, method for producing nucleic acid, method for producing recombinant cells, method for producing target substance, method for producing pharmaceutical composition, and reagent |
| JP2021118722A (ja) * | 2019-02-18 | 2021-08-12 | 株式会社エヌビィー健康研究所 | 細胞の選抜方法、核酸の製造方法、組換え細胞の製造方法、目的物質の製造方法、医薬組成物の製造方法、及び試薬 |
| JP7456627B2 (ja) | 2019-02-18 | 2024-03-27 | 株式会社エヌビィー健康研究所 | 細胞の選抜方法、核酸の製造方法、組換え細胞の製造方法、目的物質の製造方法、医薬組成物の製造方法、及び試薬 |
| US12208113B2 (en) | 2019-02-18 | 2025-01-28 | Nb Health Laboratory Co., Ltd. | Method for selecting cells, method for producing nucleic acid, method for producing recombinant cells, method for producing target substance, method for producing pharmaceutical composition, and reagent |
| JPWO2020171020A1 (ja) * | 2019-02-18 | 2021-03-11 | 株式会社エヌビィー健康研究所 | 細胞の選抜方法、核酸の製造方法、組換え細胞の製造方法、目的物質の製造方法、医薬組成物の製造方法、及び試薬 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2389446A1 (en) | 2011-11-30 |
| AU2009338127B2 (en) | 2015-11-26 |
| KR20110134386A (ko) | 2011-12-14 |
| CA2689681A1 (en) | 2010-07-21 |
| RU2011134898A (ru) | 2013-02-27 |
| SG172889A1 (en) | 2011-08-29 |
| CN102388145A (zh) | 2012-03-21 |
| US20120149592A1 (en) | 2012-06-14 |
| EP2389446B1 (en) | 2017-07-05 |
| CA2689681C (en) | 2017-08-29 |
| US9244071B2 (en) | 2016-01-26 |
| CN104745669A (zh) | 2015-07-01 |
| RU2532228C2 (ru) | 2014-10-27 |
| WO2010085275A1 (en) | 2010-07-29 |
| AU2009338127A1 (en) | 2011-07-28 |
| JP2015144617A (ja) | 2015-08-13 |
| EP2389446A4 (en) | 2012-10-03 |
| IL213938A0 (en) | 2011-07-31 |
| SG10201401643PA (en) | 2014-08-28 |
| JP6017620B2 (ja) | 2016-11-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6017620B2 (ja) | 単一細胞の細胞傷害性を査定するための組成物および方法 | |
| JP7711049B2 (ja) | 初代ヒト細胞における、同族t細胞及びエピトープ反応性をスクリーニングするハイスループット法 | |
| US8975069B2 (en) | Method for identifying antigen-specific regulatory T cells | |
| CA2968543C (en) | Characterization of adaptive immune response to vaccination or infection using immune repertoire sequencing | |
| Richard | Toll-like receptor (TLR) 7 and TLR8 stimulation of mucosal-associated invariant T cells and gamma delta T cells: a role in HIV susceptibility | |
| van Dijk | Single-cell based assessment of the human T cell response against Plasmodium falciparum circumsporozoite protein | |
| Perez | Cd151+ T Cell Frequencies As An Immunological Clock That Identifies Premature Immunological Aging In People With Hiv | |
| Rojas-Cordero et al. | Viral loads in transplant patients and cytomegalovirus genotyping | |
| Williams | Role of TCR affinity in function, proliferation, memory generation, and exhaustion during human viral infection | |
| Wehr et al. | Proinflammatory impact of putative viral-specific CD8+ T cells infiltrating new-onset seropositive rheumatoid arthritis synovium | |
| Tavares | HIV-2 ability to infect Human Follicular T helper cells. | |
| Phetsouphanh | Novel approaches to the characterization of antigen-specific CD4+ T cells in HIV-1 infection | |
| Brock et al. | Role of TCR affinity in function, proliferation, memory generation, and exhaustion during human viral infection | |
| Clutton | HIV-specific interleukin-10 responses and immune modulation | |
| Nogueira | HIV-2 Ability to Infect Human Follicular T Helper Cells | |
| Parsons | Lessons learned: natural killer cell education as a determinant of the anti-viral functional potential of natural killer cells | |
| Aguilera-Sandoval | The Dynamic Interplay between HIV-1 and T cells | |
| Moodley-Govender | Cellular Immunity, Immune Activation and Regulation in HIV-1 Infected Mother-child Pairs: What are the Determinants of Protective Immunity | |
| Wragg et al. | High CD26 and Low CD94 Expression Identifies an IL-23 Responsive Vd2 T Cell Subset with a MAIT Cell-like Transcriptional Profile | |
| van Dijk | obtaining the doctoral degree |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120710 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20120710 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130129 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140210 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140509 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140516 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140624 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20150107 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150227 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20150408 |
|
| RD02 | Notification of acceptance of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7422 Effective date: 20150408 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20150408 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20150410 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150505 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20150513 |
|
| A761 | Written withdrawal of application |
Free format text: JAPANESE INTERMEDIATE CODE: A761 Effective date: 20150515 |