JP2011182658A - Method for reducing nitric acid concentration - Google Patents
Method for reducing nitric acid concentration Download PDFInfo
- Publication number
- JP2011182658A JP2011182658A JP2010048467A JP2010048467A JP2011182658A JP 2011182658 A JP2011182658 A JP 2011182658A JP 2010048467 A JP2010048467 A JP 2010048467A JP 2010048467 A JP2010048467 A JP 2010048467A JP 2011182658 A JP2011182658 A JP 2011182658A
- Authority
- JP
- Japan
- Prior art keywords
- nitric acid
- concentration
- acid concentration
- nutrient solution
- organic nutrient
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Abstract
Description
本発明は、食用植物中に存在する硝酸の低減に関するものである。 The present invention relates to the reduction of nitric acid present in edible plants.
現在、市販されている野菜には、多量の硝酸態窒素がふくまれていることが報告されている。野菜の中でもレタス、キュウリ、サラダ用ホウレンソウや玉葱、貝割れダイコンやモヤシなどのスプラウトは生食材のため、硝酸はそのまま体内に取り込まれる。本来、土壌では硝酸は多量には含まれていないが、肥料として収穫前に多量に施用することで野菜が萎れないため、店頭に並んだときに新鮮な印象を与えることができる。またスプラウトでも水のみより無機肥料を与えたときのほうが生育や品質が良いため一般に施用されている。 Currently, it is reported that a large amount of nitrate nitrogen is contained in commercially available vegetables. Among vegetables, sprouts such as lettuce, cucumber, salad spinach and onion, shellfish radish and sprout are raw ingredients, so nitric acid is taken into the body. Originally, it does not contain a large amount of nitric acid in the soil, but it can give a fresh impression when it is lined up at the store because it does not wither the vegetables by applying it as a fertilizer before harvesting. Also, sprout is generally applied when it is fed with inorganic fertilizer rather than water alone because it has better growth and quality.
硝酸は多量でなければ人体に取り込まれても害はないとされている。体内で亜硝酸に変る場合は問題となる。硝酸を過剰に摂取した場合は、血液中のヘモグロビンと亜硝酸とが結合されてメトヘモグロビンが作られ、酸素欠乏を生じるという中毒症(例えば、乳幼児に生ずるブルーベビー症)を引き起こす原因にもなる。欧米では、野菜の種類にもよるが硝酸は2000−3000ppm以下でなければならないとされている。 It is said that nitric acid is not harmful if taken in the human body unless it is a large amount. It becomes a problem if it changes to nitrous acid in the body. Excessive intake of nitrate can cause hemoglobin and nitrite to combine to produce methemoglobin, which can lead to addictions that cause oxygen deficiency (eg, blue baby disease in infants) . In Europe and the United States, nitric acid must be 2000-3000 ppm or less, depending on the type of vegetable.
このため、食用植物中に含まれる硝酸(特に、硝酸態窒素)を減少させる方法が検討されている(例えば、特許文献1参照)。 For this reason, the method of reducing nitric acid (especially nitrate nitrogen) contained in an edible plant is examined (for example, refer patent document 1).
なお、本明細書中で、硝酸というときは、一般的な硝酸に限られず、硝酸態窒素を意味する。 In the present specification, nitric acid means not only general nitric acid but also nitrate nitrogen.
しかし、上記文献に記載の方法でも、十分に硝酸濃度を減少させていない。このため、更に硝酸濃度を減少させる方法が要求される。
また、食用植物は、ヒトなどが摂取するものである。このため、硝酸濃度を減少させる方法に用いられるものは、より安全なものが要求される。
However, even the method described in the above document does not sufficiently reduce the nitric acid concentration. For this reason, a method for further reducing the nitric acid concentration is required.
In addition, edible plants are ingested by humans and the like. For this reason, what is used for the method of reducing a nitric acid concentration is required to be safer.
本発明者らは、通常の無機肥料を与えて培養・生育させた食用植物に有機栄養液を灌水および/または塗布することにより、連続族無機培養法に比較して硝酸濃度を削減できることを見出し、本発明を完成させた。
また、本発明は、食用植物中の硝酸濃度を低減する有機栄養剤であってもよい。
The present inventors have found that the concentration of nitric acid can be reduced by irrigating and / or applying an organic nutrient solution to an edible plant cultured and grown with a normal inorganic fertilizer as compared with a continuous group inorganic culture method. The present invention has been completed.
The present invention may also be an organic nutrient that reduces the concentration of nitric acid in edible plants.
本発明では、ヒトなどの動物が日常摂取する有機栄養液を食用植物に与えることで、硝酸濃度を効果的に削減することができる。
また、有機栄養液は、ヒトなどの動物が日常摂取するので、残留しても問題にならない。さらに、有機栄養液に用いられる有機物は、極めて少量で有効である。このため、安価で、安全な硝酸濃度低減方法を提供することができる。
In the present invention, the concentration of nitric acid can be effectively reduced by providing an edible plant with an organic nutrient solution that is consumed daily by animals such as humans.
In addition, since organic nutrient solutions are ingested daily by animals such as humans, there is no problem even if they remain. Furthermore, the organic substance used for the organic nutrient solution is effective in a very small amount. For this reason, an inexpensive and safe method for reducing nitric acid concentration can be provided.
本発明では、食用植物に有機栄養液を灌水および/または塗布することにより、硝酸濃度を低減させる。 In the present invention, the nitric acid concentration is reduced by irrigating and / or applying an organic nutrient solution to the edible plant.
本発明の硝酸濃度を低減させる対象となる植物は、ヒトや動物が食用する植物であれば、特に制限はない。たとえば、レタス、キュウリ、サラダ用ホウレンソウや玉葱、貝割れダイコンやモヤシなどの生食材が例示される。 If the plant used as the object which reduces the nitric acid concentration of this invention is a plant which humans and animals eat, there will be no restriction | limiting in particular. For example, raw materials such as lettuce, cucumber, spinach for salad, onion, shellfish radish and sprout.
有機栄養液は、有機酸、アミノ酸、糖などを含む水溶液である。有機酸は、特に制限はないが、クエン酸、酢酸、乳酸、コハク酸などが例示される。アミノ酸は、特に制限はないが、グリシン、グルタミン酸、アスパラギン酸などが例示される。糖は、特に制限はないが、スクロース、グルコースなどが例示される。これらは、単独であっても、2種以上を組み合わせて使用してもよい。 An organic nutrient solution is an aqueous solution containing organic acids, amino acids, sugars and the like. The organic acid is not particularly limited, and citric acid, acetic acid, lactic acid, succinic acid and the like are exemplified. The amino acid is not particularly limited, and glycine, glutamic acid, aspartic acid and the like are exemplified. The sugar is not particularly limited, and examples thereof include sucrose and glucose. These may be used alone or in combination of two or more.
有機栄養液中の有機酸、アミノ酸の濃度は、2〜10mM、好ましくは4〜6mM、更に好ましくは、4.5〜5.5mMである。例えば、5mMの濃度のものが好適である。また、有機栄養液中の糖の濃度は、2〜4重量%である。 The concentration of the organic acid and amino acid in the organic nutrient solution is 2 to 10 mM, preferably 4 to 6 mM, and more preferably 4.5 to 5.5 mM. For example, a concentration of 5 mM is suitable. Moreover, the density | concentration of the saccharide | sugar in an organic nutrient solution is 2 to 4 weight%.
有機栄養液は、食用植物に灌水および/または塗布することで供与することができる。灌水することで、根茎または切断した茎から植物内に吸収される。また、塗布することで葉部から吸収される。塗布には、散布を含む。 The organic nutrient solution can be provided by irrigating and / or applying to edible plants. By irrigation, it is absorbed into the plant from the rhizome or cut stem. Moreover, it is absorbed from a leaf part by apply | coating. Application includes spraying.
有機栄養液は、例えば、食用に付す24時間程度前から食用植物に与える。具体的には、出荷前の24時間程度前、流通過程、あるいは保存中などに行えばよい。 The organic nutrient solution is given to edible plants from about 24 hours before being edible, for example. Specifically, it may be performed about 24 hours before shipment, during the distribution process, or during storage.
なお、本発明で使用する、有機栄養液である有機栄養剤は、等倍の液体に限られず、粉剤、錠剤、濃縮液、あるいはゲル状の形で供給されてもよい。使用に際し、希釈・溶解等により等倍液を作製する、あるいはゲル状のまま使用することとすればよい。 In addition, the organic nutrient which is an organic nutrient liquid used by this invention is not restricted to a liquid of equal magnification, You may supply in the form of a powder agent, a tablet, a concentrated liquid, or a gel form. At the time of use, it is sufficient to prepare an equal-magnification solution by dilution / dissolution or the like or to use it as a gel.
以下、実施例により本発明を説明するが、本発明はかかる実施例に限定されるものではない。 EXAMPLES Hereinafter, although an Example demonstrates this invention, this invention is not limited to this Example.
以下の実験は、貝割れ大根を用いて行った。貝割れ大根は、25℃の条件下で播種後1日間暗期におき、その後2〜4日は、14時間明期(40μmol photon.m−2・s−1)、10時間暗期に置いた。1〜4日間は、公知の無機栄養液を与えた。5日目は明期におき、本発明の有機栄養液を与え、24時間後に下胚軸を破砕し、細胞内物を抽出した。 The following experiment was performed using a radish shell. Shellfish radishes are placed in the dark period for 1 day after seeding at 25 ° C., and then placed in the dark period for 14 hours (40 μmol photon.m −2 · s −1 ) for 2 to 4 days. It was. A known inorganic nutrient solution was given for 1 to 4 days. On the fifth day, in the light period, the organic nutrient solution of the present invention was given, and after 24 hours, the hypocotyl was crushed and the intracellular matter was extracted.
なお、貝割れ大根では、通常の生産中では、播種から4日目まで連続的に硝酸含量が増加し(約3800ppm)、その後この濃度のまま維持され、出荷されることを確認した。 In addition, during normal production, the cracked radish was confirmed to increase in nitric acid content continuously from sowing to the fourth day (about 3800 ppm), and then maintained at this concentration and shipped.
糖の濃度は、HPLCにより測定した。NO3 −濃度は、硝酸テスト紙により測定した。また、硝酸還元酵素活性は、NADHにより測定した。 The sugar concentration was measured by HPLC. NO 3 - concentration were measured by nitrate test paper. The nitrate reductase activity was measured by NADH.
(実施例1)
有機栄養液中のスクロースの濃度を変えて、貝割れ大根下胚軸中の硝酸濃度を測定した。結果を図1に示す。図1から、スクロース濃度が、2.5〜3.5重量%の場合は、硝酸濃度が効果的に減少することがわかる。
Example 1
The concentration of sucrose in the organic nutrient solution was changed, and the nitric acid concentration in the hypocotyl hypocotyl was measured. The results are shown in FIG. FIG. 1 shows that the nitric acid concentration is effectively reduced when the sucrose concentration is 2.5 to 3.5% by weight.
(実施例2)
有機栄養液の成分としてスクロース、グルコース、クエン酸、スクロース+クエン酸、グルコース+クエン酸として、貝割れ大根下胚軸中の硝酸濃度を測定した。結果を図2に示す。図2から、クエン酸単独の場合は、硝酸濃度が最も効果的に減少することがわかる。
(Example 2)
Nitric acid concentrations in the hypocotyl hypodermis hypocotyl were measured as sucrose, glucose, citric acid, sucrose + citric acid, glucose + citric acid as components of the organic nutrient solution. The results are shown in FIG. FIG. 2 shows that the concentration of nitric acid decreases most effectively when citric acid alone is used.
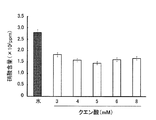
(実施例3)
有機栄養液の成分としてクエン酸を用い、濃度を変えて、貝割れ大根下胚軸中の硝酸濃度を測定した。結果を図3に示す。図3から、クエン酸濃度が3〜8mMの場合は、硝酸濃度を効果的に減少することがわかる。
(Example 3)
Citric acid was used as a component of the organic nutrient solution, and the concentration of nitric acid in the shell radish hypocotyl was measured at various concentrations. The results are shown in FIG. FIG. 3 shows that when the citric acid concentration is 3 to 8 mM, the nitric acid concentration is effectively reduced.
(実施例4)
有機栄養液の成分として有機酸の種類を変え、濃度を変えて、貝割れ大根下胚軸中の硝酸濃度を測定した。結果を図4に示す。図4から、有機酸は、硝酸濃度を減少することができることがわかり、特にクエン酸が好ましいことがわかる。
Example 4
The concentration of nitric acid in the hypocotyl hypocotyl was measured by changing the concentration of organic acid as a component of the organic nutrient solution. The results are shown in FIG. FIG. 4 shows that the organic acid can reduce the nitric acid concentration, and citric acid is particularly preferable.
(実施例5)
有機栄養液の成分としてアミノ酸の種類を変え、濃度を変えて、貝割れ大根下胚軸中の硝酸濃度を測定した。結果を図5に示す。図5から、アミノ酸は、硝酸濃度を減少することができることがわかり、特にグリシンが好ましいことがわかる。
(Example 5)
The concentration of nitric acid in the hypocotyl hypocotyl was measured by changing the concentration of amino acids as components of the organic nutrient solution. The results are shown in FIG. FIG. 5 shows that amino acids can reduce the nitric acid concentration, and that glycine is particularly preferred.
(実施例6)
有機栄養液の成分としてグルタミン酸、グルタミン酸+クエン酸、クエン酸を用いて、貝割れ大根下胚軸中の硝酸濃度を測定した。結果を図6に示す。図6から、両者を併用しても、硝酸濃度の減少に相乗作用はないことがわかる。
(Example 6)
Using glutamic acid, glutamic acid + citric acid, and citric acid as components of the organic nutrient solution, the nitric acid concentration in the hypocotyl hypocotyl hypocotyl was measured. The results are shown in FIG. From FIG. 6, it can be seen that there is no synergistic effect on the decrease in nitric acid concentration even when both are used together.
(実施例7)
有機栄養液の成分としてクエン酸を用いて、処理時間を変えて、貝割れ大根下胚軸中の硝酸濃度を測定した。結果を図7に示す。図7から、クエン酸による処理は、処理時間が24時間を経過した後は硝酸濃度の減少は変わらないことがわかる。
(Example 7)
Using citric acid as a component of the organic nutrient solution, the nitric acid concentration in the hypocotyl hypodermis hypocotyl was measured while changing the treatment time. The results are shown in FIG. FIG. 7 shows that the treatment with citric acid does not change the decrease in nitric acid concentration after the treatment time of 24 hours has elapsed.
(実施例8)
有機栄養液の成分としてクエン酸、酢酸、グルタミン酸、グリシンを用いて、貝割れ大根中の硝酸還元酵素の酵素活性を調べた。結果を図8に示す。図6から、クエン酸において、硝酸還元酵素の酵素活性が向上していることがわかる。一方、グルタミン酸、グリシンでは、硝酸還元酵素の酵素活性は余り向上していないことがわかる。このことから、本発明の有機栄養液の効果は、単に硝酸還元酵素の酵素活性のみではないことがわかる。
(Example 8)
Using citric acid, acetic acid, glutamic acid, and glycine as components of the organic nutrient solution, the enzyme activity of nitrate reductase in the cracked radish was examined. The results are shown in FIG. FIG. 6 shows that the enzyme activity of nitrate reductase is improved in citric acid. On the other hand, in glutamic acid and glycine, it can be seen that the enzyme activity of nitrate reductase is not so improved. From this, it can be seen that the effect of the organic nutrient solution of the present invention is not only the enzyme activity of nitrate reductase.
食用として安全な食用植物を提供する。 Provide an edible plant that is safe for consumption.
Claims (2)
An organic nutrient that reduces the concentration of nitric acid in edible plants.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010048467A JP2011182658A (en) | 2010-03-04 | 2010-03-04 | Method for reducing nitric acid concentration |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010048467A JP2011182658A (en) | 2010-03-04 | 2010-03-04 | Method for reducing nitric acid concentration |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2011182658A true JP2011182658A (en) | 2011-09-22 |
| JP2011182658A5 JP2011182658A5 (en) | 2013-02-28 |
Family
ID=44789692
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010048467A Pending JP2011182658A (en) | 2010-03-04 | 2010-03-04 | Method for reducing nitric acid concentration |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP2011182658A (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103348863A (en) * | 2013-07-22 | 2013-10-16 | 镇江瑞繁农艺有限公司 | Method for seed reproduction of ground cucumbers |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0753312A (en) * | 1993-08-19 | 1995-02-28 | Cosmo Sogo Kenkyusho:Kk | Reducing agent for nitrate nitrogen content in plant |
| JP2001190154A (en) * | 2000-01-11 | 2001-07-17 | Yoshizawa Lime Industry | Method for culturing crop and agent for improving quality of crop |
| JP2001288010A (en) * | 2000-04-10 | 2001-10-16 | Kao Corp | Agent for vitalizing plant |
-
2010
- 2010-03-04 JP JP2010048467A patent/JP2011182658A/en active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0753312A (en) * | 1993-08-19 | 1995-02-28 | Cosmo Sogo Kenkyusho:Kk | Reducing agent for nitrate nitrogen content in plant |
| JP2001190154A (en) * | 2000-01-11 | 2001-07-17 | Yoshizawa Lime Industry | Method for culturing crop and agent for improving quality of crop |
| JP2001288010A (en) * | 2000-04-10 | 2001-10-16 | Kao Corp | Agent for vitalizing plant |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103348863A (en) * | 2013-07-22 | 2013-10-16 | 镇江瑞繁农艺有限公司 | Method for seed reproduction of ground cucumbers |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101812553B1 (en) | Composite peptide selenoprotein nutrient solution, preparation method and application thereof | |
| TWI642357B (en) | Utilization method of growth supplement for efficient production of agricultural products | |
| KR100918106B1 (en) | Cultivation method for farm produce contianing selenium | |
| SI20615A (en) | Environmentally compatible processes compositions and materials treated thereby | |
| KR20150103994A (en) | Nutrients for plants | |
| CN103030466B (en) | Dendrobium nutrient solution formula for facilitating fast growth and high quality of dendrobium transplanting test-tube plantlets and application of dendrobium nutrient solution formula | |
| WO2013091291A1 (en) | Selenium-rich cardamine hupingshanensis plant material and cultivation method and use thereof | |
| JP2001190154A (en) | Method for culturing crop and agent for improving quality of crop | |
| WO2015103902A1 (en) | Pollution-free organic pesticide and preparation method therefor | |
| JP2009017860A (en) | Method for increasing organic selenium compound content in crop, utilizing sulfur deficiency | |
| JP2001192310A (en) | Method for promoting absorption of calcium ion from surface of plant | |
| JP5478029B2 (en) | Fertilizer suitable for the production of edible plants with reduced nitrate content | |
| JP2011182658A (en) | Method for reducing nitric acid concentration | |
| JP2008136944A (en) | Method of dissolving metal compound, method for producing fertilizer, and method for producing mineral water for ingestion in human body | |
| Tikhomirova et al. | Possibility of Salicornia europaea use for the human liquid wastes inclusion into BLSS intrasystem mass exchange | |
| KR20000053863A (en) | Method for preparing soil improver by using germanium ore-charcoal powder | |
| JP2005261319A (en) | Method for cultivating plant of genus allium having high selenium content | |
| JP5372368B2 (en) | Method for cultivating plants with improved antioxidant function | |
| JP2011088802A (en) | Spraying agent for leaf surface of leaf vegetables or the like | |
| JP4066240B2 (en) | Shellfish freshness maintenance method and transportation method | |
| JP2007215462A (en) | Edible plant body containing mineral absorption-enhancing substance, and method for producing the same | |
| KR20170132486A (en) | The organic farming natural fertilizer | |
| JP4310441B2 (en) | Method for producing a plant containing vitamin B12 | |
| KR100523759B1 (en) | A method for preparing green tea with high amount of organic selenium and use thereof | |
| JP2008178372A (en) | Technique for cultivating succulent plant (cam plant) for foods and ingredient-holding technique |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130111 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20130111 |
|
| RD03 | Notification of appointment of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7423 Effective date: 20130111 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20130815 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130820 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20131001 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20140617 |