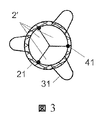
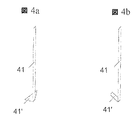
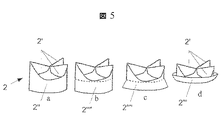
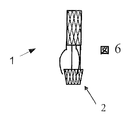
JP2010535554A - 内部人工弁 - Google Patents
内部人工弁 Download PDFInfo
- Publication number
- JP2010535554A JP2010535554A JP2010519496A JP2010519496A JP2010535554A JP 2010535554 A JP2010535554 A JP 2010535554A JP 2010519496 A JP2010519496 A JP 2010519496A JP 2010519496 A JP2010519496 A JP 2010519496A JP 2010535554 A JP2010535554 A JP 2010535554A
- Authority
- JP
- Japan
- Prior art keywords
- fact
- valve
- stent
- lower support
- support part
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000000463 material Substances 0.000 claims description 20
- 239000000835 fiber Substances 0.000 claims description 17
- 239000002184 metal Substances 0.000 claims description 13
- 229910052751 metal Inorganic materials 0.000 claims description 13
- 238000000465 moulding Methods 0.000 claims description 12
- 238000000034 method Methods 0.000 claims description 10
- 239000012528 membrane Substances 0.000 claims description 9
- 230000000694 effects Effects 0.000 claims description 7
- 239000002826 coolant Substances 0.000 claims description 5
- 239000011248 coating agent Substances 0.000 claims description 4
- 238000000576 coating method Methods 0.000 claims description 4
- 238000009940 knitting Methods 0.000 claims description 4
- 239000012781 shape memory material Substances 0.000 claims description 4
- 238000009941 weaving Methods 0.000 claims description 4
- 238000005520 cutting process Methods 0.000 claims description 3
- 229920002994 synthetic fiber Polymers 0.000 claims description 3
- 239000000956 alloy Substances 0.000 claims description 2
- 229910045601 alloy Inorganic materials 0.000 claims description 2
- 206010061592 cardiac fibrillation Diseases 0.000 claims description 2
- 239000002131 composite material Substances 0.000 claims description 2
- 230000002600 fibrillogenic effect Effects 0.000 claims description 2
- 238000004519 manufacturing process Methods 0.000 claims description 2
- 239000007769 metal material Substances 0.000 claims description 2
- 238000007493 shaping process Methods 0.000 claims 1
- 210000002216 heart Anatomy 0.000 abstract description 6
- 230000002829 reductive effect Effects 0.000 abstract description 4
- 238000007675 cardiac surgery Methods 0.000 abstract description 2
- 238000002316 cosmetic surgery Methods 0.000 abstract description 2
- 230000035882 stress Effects 0.000 description 25
- 238000002513 implantation Methods 0.000 description 10
- 210000000709 aorta Anatomy 0.000 description 9
- 230000006378 damage Effects 0.000 description 9
- 210000003291 sinus of valsalva Anatomy 0.000 description 7
- 230000017531 blood circulation Effects 0.000 description 6
- 230000006870 function Effects 0.000 description 6
- 210000003709 heart valve Anatomy 0.000 description 6
- 230000033001 locomotion Effects 0.000 description 6
- 208000007536 Thrombosis Diseases 0.000 description 5
- 210000001367 artery Anatomy 0.000 description 5
- 230000007850 degeneration Effects 0.000 description 5
- 210000004115 mitral valve Anatomy 0.000 description 5
- 210000004165 myocardium Anatomy 0.000 description 5
- 239000003146 anticoagulant agent Substances 0.000 description 4
- 229940127219 anticoagulant drug Drugs 0.000 description 4
- 210000001765 aortic valve Anatomy 0.000 description 4
- 230000000747 cardiac effect Effects 0.000 description 4
- 238000013459 approach Methods 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 238000009954 braiding Methods 0.000 description 3
- 230000005012 migration Effects 0.000 description 3
- 238000013508 migration Methods 0.000 description 3
- 230000036961 partial effect Effects 0.000 description 3
- 238000005086 pumping Methods 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 238000001356 surgical procedure Methods 0.000 description 3
- 230000000472 traumatic effect Effects 0.000 description 3
- 230000008901 benefit Effects 0.000 description 2
- 230000002146 bilateral effect Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 230000002308 calcification Effects 0.000 description 2
- 230000006835 compression Effects 0.000 description 2
- 238000007906 compression Methods 0.000 description 2
- 210000004351 coronary vessel Anatomy 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 230000007547 defect Effects 0.000 description 2
- 230000002950 deficient Effects 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 239000002657 fibrous material Substances 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 230000035479 physiological effects, processes and functions Effects 0.000 description 2
- 238000007789 sealing Methods 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 239000004753 textile Substances 0.000 description 2
- 238000002054 transplantation Methods 0.000 description 2
- 238000003466 welding Methods 0.000 description 2
- 206010002329 Aneurysm Diseases 0.000 description 1
- 102000008186 Collagen Human genes 0.000 description 1
- 108010035532 Collagen Proteins 0.000 description 1
- 208000033999 Device damage Diseases 0.000 description 1
- 102000009123 Fibrin Human genes 0.000 description 1
- 108010073385 Fibrin Proteins 0.000 description 1
- BWGVNKXGVNDBDI-UHFFFAOYSA-N Fibrin monomer Chemical compound CNC(=O)CNC(=O)CN BWGVNKXGVNDBDI-UHFFFAOYSA-N 0.000 description 1
- 206010019280 Heart failures Diseases 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 208000031481 Pathologic Constriction Diseases 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 239000000560 biocompatible material Substances 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 229920001436 collagen Polymers 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 230000003412 degenerative effect Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 230000003205 diastolic effect Effects 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 229950003499 fibrin Drugs 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 230000004217 heart function Effects 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 238000005470 impregnation Methods 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 210000005240 left ventricle Anatomy 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 238000003754 machining Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000004660 morphological change Effects 0.000 description 1
- HLXZNVUGXRDIFK-UHFFFAOYSA-N nickel titanium Chemical compound [Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni] HLXZNVUGXRDIFK-UHFFFAOYSA-N 0.000 description 1
- 229910001000 nickel titanium Inorganic materials 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 230000006461 physiological response Effects 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 230000000284 resting effect Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 230000007480 spreading Effects 0.000 description 1
- 238000003892 spreading Methods 0.000 description 1
- 230000003068 static effect Effects 0.000 description 1
- 230000036262 stenosis Effects 0.000 description 1
- 208000037804 stenosis Diseases 0.000 description 1
- 230000008354 tissue degradation Effects 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 230000002861 ventricular Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/24—Heart valves ; Vascular valves, e.g. venous valves; Heart implants, e.g. passive devices for improving the function of the native valve or the heart muscle; Transmyocardial revascularisation [TMR] devices; Valves implantable in the body
- A61F2/2412—Heart valves ; Vascular valves, e.g. venous valves; Heart implants, e.g. passive devices for improving the function of the native valve or the heart muscle; Transmyocardial revascularisation [TMR] devices; Valves implantable in the body with soft flexible valve members, e.g. tissue valves shaped like natural valves
- A61F2/2418—Scaffolds therefor, e.g. support stents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/24—Heart valves ; Vascular valves, e.g. venous valves; Heart implants, e.g. passive devices for improving the function of the native valve or the heart muscle; Transmyocardial revascularisation [TMR] devices; Valves implantable in the body
- A61F2/2412—Heart valves ; Vascular valves, e.g. venous valves; Heart implants, e.g. passive devices for improving the function of the native valve or the heart muscle; Transmyocardial revascularisation [TMR] devices; Valves implantable in the body with soft flexible valve members, e.g. tissue valves shaped like natural valves
- A61F2/2415—Manufacturing methods
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2220/00—Fixations or connections for prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2220/0025—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements
- A61F2220/0066—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements stapled
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Prostheses (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR0757012A FR2919798B1 (fr) | 2007-08-09 | 2007-08-09 | Endoprothese valvulaire |
| PCT/FR2008/051456 WO2009024716A2 (fr) | 2007-08-09 | 2008-08-04 | Endoprothese valvulaire |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2010535554A true JP2010535554A (ja) | 2010-11-25 |
| JP2010535554A5 JP2010535554A5 (enExample) | 2011-09-22 |
Family
ID=39204638
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010519496A Pending JP2010535554A (ja) | 2007-08-09 | 2008-08-04 | 内部人工弁 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20110153008A1 (enExample) |
| EP (1) | EP2185106A2 (enExample) |
| JP (1) | JP2010535554A (enExample) |
| CA (1) | CA2695873A1 (enExample) |
| FR (1) | FR2919798B1 (enExample) |
| WO (1) | WO2009024716A2 (enExample) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016506794A (ja) * | 2013-02-04 | 2016-03-07 | エドワーズ ライフサイエンシーズ コーポレイションEdwards Lifesciences Corporation | 僧帽弁置換のための人工弁 |
| JP2016047481A (ja) * | 2011-06-21 | 2016-04-07 | トゥエルヴ, インコーポレイテッド | 人工治療装置 |
| JP2017124265A (ja) * | 2011-10-19 | 2017-07-20 | トゥエルヴ, インコーポレイテッド | 人工心臓弁デバイス |
| JP2021000479A (ja) * | 2011-08-11 | 2021-01-07 | テンダイン ホールディングス, インコーポレイテッド | 人工心臓弁 |
| US11202704B2 (en) | 2011-10-19 | 2021-12-21 | Twelve, Inc. | Prosthetic heart valve devices, prosthetic mitral valves and associated systems and methods |
Families Citing this family (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ITTO20040135A1 (it) | 2004-03-03 | 2004-06-03 | Sorin Biomedica Cardio Spa | Protesi valvolare cardiaca |
| ITTO20050074A1 (it) | 2005-02-10 | 2006-08-11 | Sorin Biomedica Cardio Srl | Protesi valvola cardiaca |
| US9848981B2 (en) | 2007-10-12 | 2017-12-26 | Mayo Foundation For Medical Education And Research | Expandable valve prosthesis with sealing mechanism |
| ES2386239T3 (es) | 2008-05-16 | 2012-08-14 | Sorin Biomedica Cardio S.R.L. | Prótesis cardiovalvular atraumática |
| ES2551694T3 (es) | 2008-12-23 | 2015-11-23 | Sorin Group Italia S.R.L. | Válvula protésica expansible dotada de apéndices de anclaje |
| BRPI1008902A2 (pt) | 2009-02-27 | 2016-03-15 | St Jude Medical | válvula cardíaca protética. |
| EP2628465A1 (en) | 2009-04-27 | 2013-08-21 | Sorin Group Italia S.r.l. | Prosthetic vascular conduit |
| IT1400327B1 (it) | 2010-05-21 | 2013-05-24 | Sorin Biomedica Cardio Srl | Dispositivo di supporto per protesi valvolari e corrispondente corredo. |
| EP2654624B1 (en) | 2010-12-23 | 2023-10-04 | Twelve, Inc. | System for mitral valve repair and replacement |
| EP2486894B1 (en) | 2011-02-14 | 2021-06-09 | Sorin Group Italia S.r.l. | Sutureless anchoring device for cardiac valve prostheses |
| EP2486893B1 (en) | 2011-02-14 | 2017-07-05 | Sorin Group Italia S.r.l. | Sutureless anchoring device for cardiac valve prostheses |
| AU2012325809B2 (en) | 2011-10-19 | 2016-01-21 | Twelve, Inc. | Devices, systems and methods for heart valve replacement |
| US9763780B2 (en) | 2011-10-19 | 2017-09-19 | Twelve, Inc. | Devices, systems and methods for heart valve replacement |
| US9039757B2 (en) | 2011-10-19 | 2015-05-26 | Twelve, Inc. | Prosthetic heart valve devices, prosthetic mitral valves and associated systems and methods |
| US9655722B2 (en) | 2011-10-19 | 2017-05-23 | Twelve, Inc. | Prosthetic heart valve devices, prosthetic mitral valves and associated systems and methods |
| FR2982763B1 (fr) * | 2011-11-17 | 2015-07-17 | Ct Hospitalier Regional Universitaire D Amiens | Implant destine a etre place dans un passage de circulation du sang et dispositif de traitement associe |
| EP2842517A1 (en) | 2011-12-29 | 2015-03-04 | Sorin Group Italia S.r.l. | A kit for implanting prosthetic vascular conduits |
| US9579198B2 (en) | 2012-03-01 | 2017-02-28 | Twelve, Inc. | Hydraulic delivery systems for prosthetic heart valve devices and associated methods |
| CN105246431B (zh) | 2013-05-20 | 2018-04-06 | 托尔福公司 | 可植入心脏瓣膜装置、二尖瓣修复装置以及相关系统和方法 |
| US9974650B2 (en) * | 2015-07-14 | 2018-05-22 | Edwards Lifesciences Corporation | Prosthetic heart valve |
| EP3337428B1 (en) | 2015-08-21 | 2024-09-25 | Twelve Inc. | Mitral valve repair devices |
| EP3448316B1 (en) | 2016-04-29 | 2023-03-29 | Medtronic Vascular Inc. | Prosthetic heart valve devices with tethered anchors |
| US10575950B2 (en) | 2017-04-18 | 2020-03-03 | Twelve, Inc. | Hydraulic systems for delivering prosthetic heart valve devices and associated methods |
| US10433961B2 (en) | 2017-04-18 | 2019-10-08 | Twelve, Inc. | Delivery systems with tethers for prosthetic heart valve devices and associated methods |
| US10702378B2 (en) | 2017-04-18 | 2020-07-07 | Twelve, Inc. | Prosthetic heart valve device and associated systems and methods |
| US10792151B2 (en) | 2017-05-11 | 2020-10-06 | Twelve, Inc. | Delivery systems for delivering prosthetic heart valve devices and associated methods |
| US10646338B2 (en) | 2017-06-02 | 2020-05-12 | Twelve, Inc. | Delivery systems with telescoping capsules for deploying prosthetic heart valve devices and associated methods |
| US10709591B2 (en) | 2017-06-06 | 2020-07-14 | Twelve, Inc. | Crimping device and method for loading stents and prosthetic heart valves |
| US10786352B2 (en) | 2017-07-06 | 2020-09-29 | Twelve, Inc. | Prosthetic heart valve devices and associated systems and methods |
| US10729541B2 (en) | 2017-07-06 | 2020-08-04 | Twelve, Inc. | Prosthetic heart valve devices and associated systems and methods |
| CN112384174B (zh) | 2018-05-23 | 2024-02-27 | 恪心有限责任公司 | 原位递送心脏瓣膜假体的装置 |
| CA3101165A1 (en) | 2018-05-23 | 2019-11-28 | Sorin Group Italia S.R.L. | A cardiac valve prosthesis |
| DE102020127884A1 (de) | 2020-10-22 | 2022-04-28 | Rheinisch-Westfälische Technische Hochschule (Rwth) Aachen | Textil funktionalisierte implantierbare Klappenprothesen |
| DE102021134525A1 (de) | 2021-12-23 | 2023-06-29 | Rheinisch-Westfälische Technische Hochschule (RWTH) Aachen, Körperschaft des öffentlichen Rechts | Implantierbare Klappenprothesen mit gewebten Klappensegeln |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006070372A2 (en) * | 2004-12-30 | 2006-07-06 | Ventor Technologies, Ltd. | Fluid flow prosthetic device |
| WO2006086135A2 (en) * | 2005-01-21 | 2006-08-17 | Innovia, Llc | Stent-valve and deployment catheter for use therewith |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2815844B1 (fr) * | 2000-10-31 | 2003-01-17 | Jacques Seguin | Support tubulaire de mise en place, par voie percutanee, d'une valve cardiaque de remplacement |
| NL1017275C2 (nl) * | 2001-02-02 | 2002-08-05 | Univ Eindhoven Tech | Hartklep. |
| FR2826863B1 (fr) * | 2001-07-04 | 2003-09-26 | Jacques Seguin | Ensemble permettant la mise en place d'une valve prothetique dans un conduit corporel |
| EP3821852A3 (en) * | 2003-10-06 | 2021-09-22 | Medtronic 3F Therapeutics, Inc. | Minimally invasive valve replacement system |
| ITTO20040135A1 (it) * | 2004-03-03 | 2004-06-03 | Sorin Biomedica Cardio Spa | Protesi valvolare cardiaca |
| ITTO20050074A1 (it) * | 2005-02-10 | 2006-08-11 | Sorin Biomedica Cardio Srl | Protesi valvola cardiaca |
-
2007
- 2007-08-09 FR FR0757012A patent/FR2919798B1/fr not_active Expired - Fee Related
-
2008
- 2008-08-04 EP EP08827747A patent/EP2185106A2/fr not_active Withdrawn
- 2008-08-04 US US12/733,155 patent/US20110153008A1/en not_active Abandoned
- 2008-08-04 CA CA2695873A patent/CA2695873A1/fr not_active Abandoned
- 2008-08-04 WO PCT/FR2008/051456 patent/WO2009024716A2/fr not_active Ceased
- 2008-08-04 JP JP2010519496A patent/JP2010535554A/ja active Pending
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006070372A2 (en) * | 2004-12-30 | 2006-07-06 | Ventor Technologies, Ltd. | Fluid flow prosthetic device |
| WO2006086135A2 (en) * | 2005-01-21 | 2006-08-17 | Innovia, Llc | Stent-valve and deployment catheter for use therewith |
Cited By (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016047481A (ja) * | 2011-06-21 | 2016-04-07 | トゥエルヴ, インコーポレイテッド | 人工治療装置 |
| JP2018020246A (ja) * | 2011-06-21 | 2018-02-08 | トゥエルヴ, インコーポレイテッド | 人工治療装置 |
| US11382737B2 (en) | 2011-08-11 | 2022-07-12 | Tendyne Holdings, Inc. | Prosthetic valves and related inventions |
| US12121434B2 (en) | 2011-08-11 | 2024-10-22 | Tendyne Holdings, Inc. | Prosthetic valves and related inventions |
| US12059343B2 (en) | 2011-08-11 | 2024-08-13 | Tendyne Holdings, Inc. | Prosthetic valves and related inventions |
| JP2021000479A (ja) * | 2011-08-11 | 2021-01-07 | テンダイン ホールディングス, インコーポレイテッド | 人工心臓弁 |
| US11484404B2 (en) | 2011-08-11 | 2022-11-01 | Tendyne Holdings, Inc. | Prosthetic valves and related inventions |
| JP6999005B2 (ja) | 2011-08-11 | 2022-01-18 | テンダイン ホールディングス, インコーポレイテッド | 人工心臓弁 |
| US11311374B2 (en) | 2011-08-11 | 2022-04-26 | Tendyne Holdings, Inc. | Prosthetic valves and related inventions |
| US11364116B2 (en) | 2011-08-11 | 2022-06-21 | Tendyne Holdings, Inc. | Prosthetic valves and related inventions |
| JP2018130567A (ja) * | 2011-10-19 | 2018-08-23 | トゥエルヴ, インコーポレイテッド | 人工心臓弁デバイス |
| US11202704B2 (en) | 2011-10-19 | 2021-12-21 | Twelve, Inc. | Prosthetic heart valve devices, prosthetic mitral valves and associated systems and methods |
| US11497603B2 (en) | 2011-10-19 | 2022-11-15 | Twelve, Inc. | Prosthetic heart valve devices, prosthetic mitral valves and associated systems and methods |
| US11617648B2 (en) | 2011-10-19 | 2023-04-04 | Twelve, Inc. | Prosthetic heart valve devices, prosthetic mitral valves and associated systems and methods |
| US11628063B2 (en) | 2011-10-19 | 2023-04-18 | Twelve, Inc. | Prosthetic heart valve devices, prosthetic mitral valves and associated systems and methods |
| JP2017124265A (ja) * | 2011-10-19 | 2017-07-20 | トゥエルヴ, インコーポレイテッド | 人工心臓弁デバイス |
| JP2016506794A (ja) * | 2013-02-04 | 2016-03-07 | エドワーズ ライフサイエンシーズ コーポレイションEdwards Lifesciences Corporation | 僧帽弁置換のための人工弁 |
Also Published As
| Publication number | Publication date |
|---|---|
| FR2919798B1 (fr) | 2010-08-27 |
| WO2009024716A2 (fr) | 2009-02-26 |
| FR2919798A1 (fr) | 2009-02-13 |
| EP2185106A2 (fr) | 2010-05-19 |
| WO2009024716A3 (fr) | 2009-05-22 |
| US20110153008A1 (en) | 2011-06-23 |
| CA2695873A1 (fr) | 2009-02-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2010535554A (ja) | 内部人工弁 | |
| JP6006218B2 (ja) | 大動脈弁装置 | |
| JP6594289B2 (ja) | 僧帽弁接合強化用補綴デバイス | |
| CN112336498B (zh) | 二尖瓣瓣膜组件 | |
| US9895220B2 (en) | Mitral bileaflet valve | |
| JP2023184533A (ja) | 人工弁尖器具 | |
| US5376112A (en) | Valveless conduit with sigmoid valve annuloplasty ring | |
| JP6105196B2 (ja) | 弁交換において使用するためのステント構造 | |
| CN102119013B (zh) | 心脏瓣膜假体系统 | |
| US20150005874A1 (en) | Atrial Thrombogenic Sealing Pockets for Prosthetic Mitral Valves | |
| JP2019048176A (ja) | 人工心臓弁の血栓管理及び構造コンプライアンス特徴 | |
| US20140379076A1 (en) | Halo Wire Fluid Seal Device for Prosthetic Mitral Valves | |
| EP3388027A1 (en) | Structural members for prosthetic mitral valves | |
| JP2019193874A (ja) | 僧帽弁逆流症処置用のデバイスおよび方法 | |
| US20200060814A1 (en) | Engineered tissue prosthesis | |
| KR102563467B1 (ko) | 카테터경유 폐의 볼 판막 조립체 | |
| US20050222675A1 (en) | Implantable prosthetic heart valve comprising a valve body and a tubular vascular graft | |
| JP2021506536A (ja) | 移植可能な人工心臓弁装置用の拡張可能なフレームおよび弁周囲リーク軽減システム | |
| CN109561961A (zh) | 一种人工瓣膜及人工瓣膜植入方法 | |
| JP2012101062A (ja) | 大動脈弁人工器官 | |
| CN209377809U (zh) | 一种人工心脏瓣膜假体及其支架 | |
| JP2013542761A (ja) | 大動脈弁の修復のための弁輪内装着フレーム | |
| WO2019062366A1 (zh) | 心脏瓣膜假体 | |
| WO2022007384A1 (zh) | 用于人工心脏瓣膜的复合型裙边及人工心脏瓣膜 | |
| RU2737577C1 (ru) | Протез клапана сердца (варианты) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110804 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20110804 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20130130 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130205 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20130709 |