定義
本発明がより容易に理解され得るように、所定の用語を最初に定義する。追加の定義は、発明を実施するための形態全体にわたって示される。
本明細書で使用されるところの「抗体」という用語は、広範な意味で使用され、モノクローナル抗体(本明細書に記載される全長モノクローナル抗体及び抗体変異体を含む)、ポリクローナル抗体、多特異的な抗体(例えば、二重特異性抗体)、及び抗体断片を、それらが望ましい生物活性を呈し、本明細書で定義されるようなFcドメインを含む限り、特異的に網羅する。
「CNTO 860抗体」、「CNTO 860」、又は「CNTO 860モノクローナル抗体(Mab)」は、ヒト組織因子特異抗体を意味し、FR1、CDR1、FR2、CDR2、FR3、CDR3、及びFR4を含む結合ドメインが、それぞれ重鎖及び軽鎖可変ドメインのための配列番号:2、及び4の、特定領域によって、米国特許出願第11/010、797号において開示されるように表わされる。本明細書で使用されるところの、「CNTO 860変異体」という用語は、CNTO 860抗体を含み、重鎖(配列番号:2)の1つ以上のアミノ酸が、本明細書で開示されるように置換、削除、又は追加されている。
「ADCC活性」という用語は、抗体依存性細胞媒介細胞毒性を表わし、非感光性エフェクター細胞による、抗体媒介標的細胞の破壊現象を意味する。標的細胞の同一性は異なるが、Fc部分が正常である結合表面免疫グロブリンGを有する必要がある。エフェクター細胞は、Fc受容体を有する「キラー」細胞である。標的細胞の同一性に基づいて、従来のB又はT細胞マーカーを欠くリンパ球、又は単核細胞、マクロファージ、又は多核リンパ球であり得る。反応は、補体独立性である。本発明の抗体のADCC活性は、ADCC媒介細胞死滅を示すその能力が、本明細書で記載されるアッセイ等の細胞死滅の標準生体内又は生体外アッセイにおいて決定されるように、非修飾抗体、例えば、抗TF IgG1の能力を超える場合に「増強される」。好ましくは、増強されたADCC活性を有する抗TFは、参照IgG1抗体よりも低用量及び/又は短時間で、同一の効果(腫瘍細胞増殖の予防又は阻害)を得る。好ましくは、本発明の範囲内の抗体の効力と参照抗体との間の差は、選択された標準クロミウム放出ADCCアッセイにおける隣り合わせの比較によって決定されるように、少なくとも約1倍、より好ましくは少なくとも約2倍、更に好ましくは少なくとも約3倍、最も好ましくは少なくとも約5倍である。
抗体又は抗体類似体の「エフェクター機能」は、本明細書で使用されるように、病原体又は異常細胞、例えば、腫瘍細胞が破壊され、体から除去されるプロセスである。内因性及び適応免疫応答は、同一のエフェクター機構の大部分を使用して病原体を排除し、ADCC、CA(補体活性化)、C1q結合、及びオプソニン化を含む。
本明細書で使用されるところの「Fc」、「Fc含有タンパク質」、又は「Fc含有分子」という用語は、少なくとも免疫グロブリンCH2及びCH3ドメインを有する、単量体、二量体、又はヘテロ二量体タンパク質を意味する。CH2及びCH3ドメインは、抗体ヒンジドメイン等の二量体化又は多量体化ドメインに機能的に連結される場合、タンパク質分子(例えば、抗体)の二量体領域の少なくとも一部を形成することができる。抗体分子のFc部分(結晶化可能な断片又は相補体結合断片)は、様々なペプチダーゼ、典型的にパパインをでの抗体の消化によって産生される、十分に特徴付けられた断片の1つを示す。様々な抗体断片が、正常な抗体の消化に関して定義されるが、当業者は、かかるFc断片が化学的に又は組み換えDNA方法論、ペプチド表示等のいずれかによって新たに合成され得ることを理解するであろう。抗体の定領域は、カバット(Kabat)ら著、「免疫学的関心のタンパク質の配列(Sequence of Proteins of Immunological Interest)」(米国保健社会福祉省(U.S. Department of Health and Human Services)、1983)により提案された可変領域以外の領域を意味する。Fc部分は、抗原との結合に関与しない領域、及びタンパク質分解酵素、パパインで分割される断片の中で、エフェクター機能を主に担う領域を意味する。一態様においては、本発明のFc含有タンパク質は、Fcポリペプチド配列の複合体化(多量体化)を通して形成される。「Fcポリペプチド配列」は、上で定義されるようなFcを典型的に含むドメインを意味する。二量体構造の各ポリペプチドは、それらが二量体化して(本明細書に定義されるように)Fc領域を形成する能力を有する場合、同一の配列及び/又はドメインを有しても、又は有さなくてもよい。
「Fc受容体」又は「FcR」は、抗体又は融合タンパク質のFc領域に結合する受容体を説明する。十分に知られているFcRは、IgG抗体(γ受容体)に結合するものであり、FcγRI、FcγRII、及びFcγRIIIサブクラスの受容体を含み、これらの受容体の対立遺伝子多型及びオルターナティブにスプライスされる形態を含む。FcγRII受容体は、その細胞質ドメインにおいて主に異なる類似アミノ酸配列を有する、FcγRIIA(「活性化受容体」)及びFcγRIIB(「阻害受容体」)を含む。活性化受容体FcγRIIAは、その細胞質ドメインに免疫受容活性化チロシンモチーフ(ITAM)を含有する。阻害受容体FcγRIIBは、その細胞質ドメインに免疫受容抑制性チロシンモチーフ(ITIM)を含有する(M. in Daeron、「(Annu.Rev.Immunol)」15:203〜234、1997を参照)。FcRは、ラヴェッチ(Ravetch)及びキネット(Kinet)著、「(Annu.Rev.Immunol)」9:457〜92、1991、カペル(Capel)ら著、「免疫方法(Immunomethods)」4:25〜34、1994、及びデハース(de Haas)ら著、「(J.Lab.Clin.Med.)」126.330〜41、1995において検討されている。将来同定されるものを含む他のFcRは、本明細書において「FcR」という用語によって包含される。かかる用語は、母体IgGの胎児への移動を担う新生児受容体、FcRnも含む(ガイヤー(Guyer)ら著、「免疫学ジャーナル(J.Immunol.)117:587、1976及びキム(Kim)ら著、「免疫学ジャーナル(J.Immunol.)」24:249、1994)。
「ヒトエフェクター細胞」は、1つ以上のFcRを発現し、エフェクター機能を実行する白血球である。好ましくは、かかる細胞は、少なくともFcγRIIIを発現し、ADCCエフェクター機能を実行する。ADCCを媒介するヒト白血球の実施例は、末梢血単核細胞(PBMC)、ナチュラルキラー(NK)細胞、単核球、細胞毒性T細胞、及び好中球を含み、PBMC及びNK細胞が好ましい。エフェクター細胞は、天然源、例えば血液から単離され得る。
「補体依存性細胞傷害」又は「CDC」は、補体の存在下での標的細胞の溶解を意味する。古典的な補体経路の活性化は、補体システム(Clq)の第1構成要素を、同種抗原に結合する(適切なサブクラスの)抗体に結合することによって開始される。補体活性を評価するために、CDCアッセイ、例えば、ガッツァーノ−サントロ(Gazzano-Santoro)ら著、「免疫学ジャーナル(J.Immunol.)方法(Methods)」202:163、1996において説明されるようなCDCアッセイを実行してもよい。
本明細書で使用されるところの「モノクローナル抗体」という用語は、動物抗体の少なくとも1つの種の重鎖又は軽鎖抗体可変ドメインのうちの少なくとも1つに対して実質的に相同性を保持し、結合ドメインは、抗原又は該抗原のエピトープに対して特異的かつ定義された親和性を有する少なくとも1つのリガンド結合ドメインを含む、Fc含有タンパク質の特定の形態である。本明細書で使用されるところの「モノクローナル抗体」又は「モノクローナル抗体組成物」という用語は、単一分子組成物の抗体分子の調製を意味する。モノクローナル抗体組成物は、特定エピトープの単一の結合特異性及び親和性を示す。更に、本明細書で使用されるところのモノクローナル抗体は、単離され、したがって異なる抗原特異性を有する他の抗体を実質的に含んでいない抗体、例えば、その既知の組成又は特異性に基づいて単離され得る抗体を意味することが意図される。
「組織因子タンパク質」、「TF」、及び「哺乳類組織因子タンパク質」という用語は、自然に発生する哺乳類組織因子又は、凝固因子III及びCD142としても既知の組み換え組織因子に対応するアミノ酸配列を有するポリペプチドを意味するように使用される。自然発生するTFは、ヒト種(NCBI受入番号NP_001984)並びに他の動物種、例えばウサギ、ラット、ブタ、非ヒト霊長類、ウマ、ネズミ、及びヒツジ組織因子を含む。他の哺乳類組織因子タンパク質のアミノ酸配列は、一般に知られているか、又は従来技術を通じて取得できる。
本発明に従う「TF媒介又は関連プロセス又は活性」若しくは同等にあるいは「TF活性」は、TFの存在によって媒介される、任意の生物活性である。「TF関連疾患又は疾病」は、TFの阻害、特に組織因子発現細胞上の腫瘍増殖の阻害を通じて影響され得る疾患又は疾病を意味するが、他の組織因子媒介疾患及びプロセスも含む。
「エピトープ」という用語は、抗体に対する特異的結合が可能なタンパク質決定因子を意味する。エピトープは、通常、アミノ酸又は糖側鎖等の、化学的に活性な表面分子の分類からなり、通常、特異的な三次元構造特性並びに特異的な電荷特性を有する。構造的及び非構造的エピトープは、後者ではなく前者に対する結合が、変性溶媒の存在下で損失するという点で区別される。「ネイティブ構造的エピトープ」又は「ネイティブタンパク質エピトープ」という用語は、本明細書において同義的に使用され、インテグリン分子の直線配列の異なる部分からのアミノ酸が、三次元空間において近接近して集まるときに生じるインテグリン分子の構造的折り畳みの結果生じるタンパク質エピトープを含む。かかる構造的エピトープは、細胞膜の細胞外側上で分散される。
本明細書で使用されるところの「特異的結合」は、事前設定された抗原への抗体結合を意味する。典型的に、抗体は、10-7M以下の解離定数(KD)で結合し、事前設定された抗原又は密接に関連する抗原以外の非特異的抗原(例えば、BSA、カゼイン)に結合するためのそのKDの少なくとも2倍未満であるKDを有する事前設定された抗原に結合する。抗原を「認識する抗体」及び抗原、例えば、TF「に特異的な抗体」という表現は、本明細書において、抗原に「特異的に結合する抗体」という用語と同義的に使用される。抗体は、その二量体化又は多量体化構造のために、典型的には、抗原結合のために二価又は多価である。当該技術分野において知られる標準技術を通じて、抗原結合ドメインは、二重特異性又は多重特異性抗体と交換され得ることを理解されたい。
本明細書において使用されるところのIgG抗体の「高親和性」という用語は、10-8M以下、より好ましくは10-9M以下、更に好ましくは10-10M以下のKDを有する抗体を意味する。しかしながら、「高親和性」結合は、他の抗体アイソタイプに対して異なり得る。例えば、IgMアイソタイプの「高親和性」結合は、10-7M以下、より好ましくは10-8M以下のKDを有する抗体を意味する。本明細書で使用されるところの「Kassoc」又は「Ka」という用語は、特定の抗体抗原相互作用の会合速度を意味することが意図されるが、本明細書で使用されるところの「Kdis」又は「Kd」という用語は、特定の抗体抗原相互作用の解離速度を意味することが意図される。本明細書で使用されるところの「KD」という用語は、Kaに対するKdの比(すなわち、Kd/Ka)から得られ、モル濃度(M)として表わされる、解離定数を意味することが意図される。KDは、抗原との抗体会合の「on」(kon)及び「off」(koff)速度の測定からも算出又は決定され得、KDはkon/koffである。
本明細書で使用されるところの抗体「アイソタイプ」又は「クラス」は、重鎖定常領域遺伝子によってコード化される、IgA、IgD、IgE、IgG、又はIgM指定を意味する。ヒトIgGアイソタイプの中には、血清中の天然存在度の最高位から最低位の順にIgG1、IgG2、IgG3、及びIgG4と命名された4つのサブクラスがある。IgA抗体は、2つのサブクラスIgA1及びIgA2として見出されている。本明細書で使用されるところの「アイソタイプスイッチング」は、IgGサブクラス間又はサブタイプ間の変更も意味する。
本明細書で使用されるところの「核酸分子」という用語は、DNA分子及びRNA分子を含むことが意図される。核酸分子は、一本鎖又は二本鎖であり得るが、好ましくは、二本鎖DNAである。組織因子に結合する抗体又は抗体部分をコード化する核酸(例えば、VH、VL、CDR3)を参照して、本明細書で使用されるところの核酸分子は、抗体又は抗体部分をコード化するヌクレオチド配列が、単離され得、及び組織因子以外の抗原を結合する抗体又は抗体部分をコード化する、他のヌクレオチド配列を含まない、核酸分子の参照であることが意図される。一実施形態においては、抗組織因子抗体又はその部分は、CNTO 860抗体変異体の単離ヌクレオチド又はアミノ酸配列を含む。
本明細書で使用されるところの「患者」という用語は、任意のヒト又は非ヒト動物を含む。「非ヒト動物」という用語は、全ての脊椎動物、例えば、非ヒト霊長類、ヒツジ、イヌ、ウシ、鶏、両生類、爬虫類等の哺乳類及び非哺乳類を含む。
引用文献
本明細書で引用される全ての発行物又は特許は、本発明の時点の当該技術分野の状態を示すため、参考として本明細書に全体が組み入れられ、及び/又は本発明の説明及び使用可能性を提供する。発行物とは、任意の科学的又は特許公開、又は全ての記録、電子、又は印刷形式を含む任意のメディア形式で入手可能な任意の他の情報を意味する。以下の参考文献は、参照することによって、その全体が本明細書に組み込まれる。デービッド・クロッティ(主幹編集)「コールド・スプリング・ハーバー・プロトコル(Cold Spring Harbor Protocols)」2007、(コールド・スプリング・ハーバー・ラボラトリー・プレス(Cold Spring Harbor Laboratory Press)オンラインISSN:1559〜6095トピック別に検索可能、オースベル(Ausubel)ら(編)「分子生物学における現行プロトコル(Current Protocols in Molecular Biology)」ニューヨーク:ジョン・ウィリー・アンド・サン(John Wiley & Sons, Inc.)、1987〜2007、コリガン(Coligan)ら(編)、「免疫学における現行プロトコル(Current Protocols in Immunology)」ニューヨーク:ジョン・ウィリー・アンド・サン(John Wiley & Sons, Inc.)、1994〜2007、コリガン(Coligan)ら(編)、「タンパク質化学における現行プロトコル(Current Protocols in Protein Science)」ニューヨーク:ジョン・ウィリー・アンド・サン(John Wiley & Sons, Inc.)、1997〜2007、エンナ(Enna)ら(編)「薬理学における現行プロトコル(Current Protocols in Pharmacology)」ニューヨーク:ジョン・ウィリー・アンド・サン(John Wiley & Sons, Inc.)、1994〜2006、及びワン(Wang)ら(編)「薬物送達(Drug Delivery)」ジョン・ウィリー・アンド・サン(John Wiley & Sons, Inc.)2005、特に10〜19章。
本明細書及び請求項全体を通して、免疫グロブリン重鎖における残基の付番は、カバット(Kabat)ら著「免疫学的関心のタンパク質の配列(Sequences of Proteins of Immunological Interest)」第5版、メリーランド州ベセスダ:国立衛生研究所公衆衛生局、1991において見られるようなEUインデックスのそれと同様であり、参照することによって本明細書に明示的に組み込まれる。「Kabatと同様のEUインデックス」は、ヒトIgG1 EU抗体の残基付番を意味する。
1.抗体の生成、スクリーニング、及び産生
本発明は、増強されたADCC活性を有する単離、組み換え、及び/又は合成抗組織因子モノクローナル抗体、並びにかかる抗体をコード化する少なくとも1つのポリヌクレオチドを含む組成物及びコード化核酸分子を提供する。
ますます多くのAbが開発されており、それらの治療活性に影響するように、治療薬としての使用、その標的に対する抗体の固有の特異性、更に抗体依存性細胞傷害(ADCC)等の抗体の非抗原結合機能が意図されている。Mabの非抗原結合機能は、免疫細胞上のFc受容体への結合を伴い、重鎖の定常ドメイン(Fcドメイン)によって形成される構造に属する。
治療Mabの非抗原結合活性が臨床結果に及ぼす影響は、変異体FcγIIIaを有する抗CD20 Abリツキサンで治療される非ホジキンリンパ腫患者において、通常のIgGFc親和性よりも高い水準で認められた(カールトン(Cartron)ら著、「血液(Blood)」98:754、2002)。結果として、治療Mab候補の非抗原結合機能を制御及び最適化するための手段が注目されている。考えられる利点には、より良い臨床応答、より多くの患者応答、又は同程度の応答を得るために必要とされる用量レベルの低下、副作用及び費用削減の可能性が含まれる。
Fc受容体に対して増強された親和性を有する変異体、特にFcγ受容体(FcγR)と指定される変異体を同定する方策は、ADCC活性を増強するために論理的なアプローチであると思われる。しかしながら、FcγR型の数及び多様な機能のために、受容体結合プロファイルを最適化して治療活性を増強する作業課題は複雑である。治療上有利なFc受容体結合プロファイルは、マウス主要モデル系において示されるようなFc受容体への最適化された異なる結合の1つであり得る(ニメルジャン(Nimmerjahn)及びラヴェッチ(Ravetch)、「科学(Science)」310:1510、2005)。表1は、当該技術分野において知られる主要なクラスのヒト及びマウスFcγRの一覧を提供し、活性化及び阻害化受容体への重要な分類を示す。同一列における受容体は、互いに機能的相同分子種であると考えられる。同一細胞上の活性受容体と同時に阻害受容体を通じたシグナルは、活性化受容体を起源とするシグナル伝達カスケードを遮断し得ることが理解されるであろう。
出願人は、CNTO 860として知られる抗組織因子抗体の変異体、それぞれ配列番号:2及び4の、成熟配列を有する重鎖及び軽鎖ポリペプチドで構成される野生型抗体を調製し、Fc受容体種への結合に関して変異体を評価するとともに、生物活性、生体外ADCC活性を試験して、有利な非抗原結合機能特性を有する治療抗体候補を産生する抗体工学の方法を同定した。特に、CNTO 860変異体は、変異体が増強されたADCC活性、つまり、標的腫瘍細胞死滅活性を産生する場合に、不変の(親又は野生型)抗体と比較して、有利な非抗原結合特性を有することが考慮される。
本発明の抗組織因子抗体は、従来のモノクローナル抗体技術、例えば、コーラー(Kohler)及びミルスタイン(Milstein)、「自然(Nature)」256:495、1975の標準ハイブリドーマ技術を含む、多様な技術によって生成され得る。単離組織因子タンパク質又はその部分(合成ペプチド等の合成分子を含む)等の免疫原性抗原の調製、及びモノクローナル抗体生成、選択、単離、及びクローン化は、任意の適切な技術を使用して行うことができる。
本発明のモノクローナル抗体の産生には、当該技術分野でよく知られるように、多様な細胞株、混合細胞株、不死化細胞、又は不死化細胞のクローン集団を使用することができる。一アプローチにおいては、ハイブリドーマは、適切な不死化細胞株(例えば、骨髄腫細胞株)を融合することによって産生され、それらに限定されないが、チャイニーズハムスター卵巣(CHO)由来細胞株、NS0及びそこから派生した細胞株、NS0、NS1、NS2、Sp2/0及びそこから派生した細胞株、Sp2 SA3、Sp2 MA1、Sp2 SS1、Sp2 SA5、AE−1、L.5、P3X63Ag8.653、U937、MLA144、ACT IV、MOLT4、DA−1、JURKAT、WEHI、K−562、BHK、HEK−293、COS、RAJI、NIH 3T3、HL−60、MLA 144、NAMAIWA、NEURO 2A、ヒト網膜由来PerC.6、Y3−Ag1.2.3及び誘導体、YB2/0他、又はヘテロ骨髄腫、その融合産物、又はそこから派生する任意の細胞又は融合細胞、又は当該技術分野において知られる任意の他の適切な細胞株(例えば、www.atcc.orgを参照)等を、抗体産生細胞との融合パートナーとして使用してもよく、それらに限定されないが、単離又はクローン化脾臓、末梢血、リンパ、扁桃腺、又は他の免疫若しくはB細胞含有細胞、あるいは任意の他の細胞発現重鎖又は軽鎖の一定若しくは可変又はフレームワーク又はCDR配列を内因性又は異種性核酸のいずれかとして使用してもよい。抗体産生細胞は、関心の抗原で免疫付与されたヒト若しくは他の適切な動物の末梢血液、又は好ましくは脾臓若しくはリンパ節から取得することもできる。
ハイブリドーマ、トランスフェクトーマ、その誘導体又はクローンの産生前又は後に、細胞株によって発現された抗体について、結合の特異性及び親和性を試験することができる。結合の特異性及び親和性(強度)はいずれも、ELISA等によって、液相又は固相形式で試験することができる。類似タンパク質又は断片への特異的結合に関する抗体のスクリーニングは、ペプチド表示ライブラリーを使用して、便宜的に達成することもできる。ペプチドのライブラリーは、化学合成及び組み換え方法のいずれか、特にバクテリオファージ表示方法を使用することによって生成され得る。
ウイルス、細菌、藻類、原核生物、植物、両生類、昆虫、爬虫類、魚類、哺乳類、齧歯類、ウマ、ヒツジ、ヤギ、ヒツジ、霊長類、又はヒトから選択される本発明の抗体、その特定の断片又は変異体をコード化する異種性又は内因性核酸を発現するために、任意の適切な宿主細胞を使用することもできる。上に列挙される哺乳類細胞に加えて、特にCHO及びNSO由来宿主細胞株、遺伝子操作された大腸菌、メタノール資化酵母、又はキイロショウジョウバエ、タバコ、トウモロコシ、大豆、米、又は麦等の遺伝子組み換え植物、及びヤギ又はマウス等の遺伝子組み換え動物を使用して、試験又は商業販売用の量で抗体を産生してもよい。本発明の組み換え抗体を発現するための好適な哺乳類宿主細胞は、チャイニーズハムスター卵巣(CHO細胞)(ウルラウブ(Urlaub)及びチェイシン(Chasin)著、「(Proc.Natl.Acad.Sci.USA)」77:42164220、1980に記載されるdhfrCHO細胞、例えば、カウフマン(Kaufman)R.J.及びシャープ(Sharp)P.A.、「分子生物学(Mol.Biol.)」)159:601〜621、1982において説明されるように、DHFR選択可能マーカーとともに使用される)、NSO骨髄腫細胞、COS細胞、及びSP2細胞を含む。特に、NSO骨髄腫細胞と使用する場合、別の好適な発現系は、WO 87/04462、WO 89/01036、及びEP 338,841等のGS遺伝子発現系である。抗体遺伝子をコード化する組み換え発現ベクターが哺乳類宿主細胞に導入される場合、抗体は、宿主細胞における抗体の発現、又はより好ましくは、宿主細胞が増殖する培養培地への抗体の分泌を可能にするために十分な期間宿主細胞を培養することによって産生される。抗体は、標準タンパク質精製方法を使用して、培地から回収することができる。
本発明のヒト抗体は、例えば、当該技術分野においてよく知られるように、組み換えDNA技術と遺伝子トランスフェクション方法の組み合わせを使用して、宿主細胞トランスフェクトーマにおいて産生することもできる(例えば、モリソン(Morrison)S.、「サイエンス(Science)」229:1202、1985)。
例えば、抗体又はその抗体断片を発現するために、標準的な分子生物学技術(例えば、PCR増幅、部位特異的突然変異誘発)によって、部分又は完全長軽鎖及び重鎖をコード化するDNAを得ることができ、及び遺伝子を転写及び翻訳制御配列に操作可能に連結されるように、発現ベクターに挿入することができる。この文脈において、「操作可能に連結される」という用語は、抗体遺伝子がベクターに連結されて、ベクター内の転写及び翻訳制御配列が、抗体遺伝子の転写及び翻訳を調節するというそれらの意図される機能を果たすことを意味することが意図される。発現ベクター及び発現制御配列は、使用される発現宿主細胞と適合するように選択される。抗体軽鎖遺伝子及び抗体重鎖遺伝子は、個別のベクターに挿入することができるが、又はより典型的には、両遺伝子は、同一の発現ベクターに挿入される。抗体遺伝子は、標準的な方法(例えば、抗体遺伝子断片及びベクター上の相捕的制限部位の連結、又は制限部位が存在しない場合には平滑末端連結)によって発現ベクターに挿入される。本明細書に記載される抗体の軽鎖及び重鎖可変領域を使用し、望ましいアイソタイプの重鎖定常部及び軽鎖定常部を既にコード化している発現ベクターにそれらを挿入することによって、任意の抗体アイソタイプの全長抗体遺伝子を形成して、VHセグメントが、ベクター内のCHセグメントに操作可能に連結され、VIセグメントがベクター内のCLセグメントに操作可能に連結されるようにする。追加的又は代替えとして、組み換え発現ベクターは、宿主細胞からの抗体鎖の分泌を促進する、シグナルペプチドをコード化することができる。抗体鎖遺伝子は、ベクターにクローン化されて、シグナルペプチドが抗体鎖遺伝子のアミノ末端にインフレームで連結されるようにすることができる。シグナルぺプチドは、免疫グロブリンシグナルペプチドであるか、又は異種シグナルぺプチド(すなわち、非免疫グロブリンタンパク質からのシグナルペプチド)であり得る。
軽鎖及び重鎖の発現の場合、重鎖及び軽鎖をコード化する発現ベクターは、標準的な技術によって宿主細胞にトランスフェクトされる。様々な形態の「トランスフェクション」という用語は、外因性DNAを原核又は真核宿主細胞に導入するために一般に使用される多様な技術、例えば、電気穿孔、カルシウム−リン酸沈殿、DEAE−デキストラントランスフェクション等を包含することが意図される。原核又は真核宿主細胞のいずれかにおいて本発明の抗体を発現することは理論的に可能であるが、真核細胞及び最も好ましくは哺乳類宿主細胞における抗体の発現は、原核細胞よりも、適切に折り畳まれ免疫学的に活性である抗体を、組み立てて分泌する可能性が高いため、かかる真核細胞、及び特に哺乳類細胞が、最も好適である。抗体遺伝子の、原核生物による発現は、高収率の活性型抗体の産生には無効であることが報告されている(ボス(Boss)M.A.及びウッド(Wood)C.R.「今日の免疫学(Immunology Today)」6:12〜13、1985)。
本発明の抗体は、核酸をコード化する少なくとも1つの組織因子抗体を使用して調製し、かかる抗体を乳汁中に産生するヤギ、ウシ、ウマ、ヒツジ等のトランスジェニック動物又は哺乳類に提供することもできる。かかる動物は、周知の方法を使用して提供され得る。例えば、限定するものではないが、米国特許第5,827,690号、同第5,849,992号、同第4,873,316号、同第5,849,992号、同第5,994,616号、同第5,565,362号、同第5,304,489号等を参照されたい。それぞれは参照することによってその全体が本明細書に組み込まれる。
宿主細胞
正常なグリコシル化タンパク質を発現可能な多数の適切な宿主細胞株が当該技術分野において開発されており、COS−1(例えば、ATCC CRL 1650)、COS−7(例えば、ATCC CRL−1651)、HEK293、BHK21(例えば、ATCC CRL−10)、CHO(例えば、ATCC CRL 1610)及びBSC−1(例えば、ATCC CRL−26)細胞株、Cos−7細胞、PerC.6細胞、hepG2細胞、P3X63Ag8.653、SP2/0−Ag14、293細胞、HeLa細胞等を含み、それらは、例えば、アメリカ型培養コレクション、バージニア州マナサス( HYPERLINK "http://www.atcc.org" www.atcc.org)から容易に入手できる。好適な宿主細胞には、骨髄腫及びリンパ腫細胞等のリンパ系起源の細胞を含む。
本発明の抗体は、核酸をコード化する少なくとも1つのCNTO860抗体変異体を使用して、追加的に調製し、植物の一部又はそこから培養される細胞においてかかる抗体、特定部分又は変異体を産生する、トランスジェニック植物及び培養植物細胞(例えば、限定するものではないが、タバコ、トウモロコシ、菜種、及びウキクサ)に提供することができる。非限定例として、組み換えタンパク質を発現するトランスジェニックタバコ葉は、例えば、誘導プロモーターを使用して、大量の組み換えタンパク質を提供するために良好に使用されている。例えば、クラメール(Cramer)ら著、「(Curr.Top.Microbol.Immunol.)」240:95〜118、1999及びそこで引用されている参考文献を参照されたい。またトランスジェニックトウモロコシは、他の組み換え系において産生されるか、又は天然源から精製されるものと同等の生物活性で、商業生産レベルで哺乳類タンパク質を発現するために使用されている。例えば、フッド(Hood)ら著、「(Adv.Exp.Med.Biol.)」464:127〜147、1999、及びそこで引用されている参考文献を参照されたい。また単鎖抗体(scFv)等の抗体断片を含む抗体は、タバコの種及びジャガイモ塊茎等のトランスジェニック植物の種から大量に産生されている。例えば、コンラッド(Conrad)ら著、「植物分子生物学(Plant Mol. Biol.)」38:101〜109、1998及びそこで引用されている参考文献を参照されたい。したがって、本発明の抗体は、周知の方法に基づいて、トランスジェニック植物を使用して産生することもできる。例えば、フィッシャー(Fischer)ら著、「バイオテクノロジー応用生物化学(Biotechnol. Appl. Biochem.)」30:99〜108、Oct.1999、マ(Ma)ら著、「バイオテクノロジーの傾向(Trends Biotechnol)」13:522〜7、1995、マ(Ma)ら著、「植物生理学(Plant Physiol.)」109:341〜6、1995、ホワイトラム(Whitelam)ら著、「(Biochem.Soc.Trans.)」22:940〜944、1994、及びそこで引用されている参考文献を参照されたい。上記の参考文献はそれぞれ、参照することによってその全体が本明細書に組み込まれる。
本明細書で開示及び請求されるように、配列番号2及び4において説明される配列は、「保存的配列修飾」、すなわち、ヌクレオチド配列によってコード化される抗体の結合特異性、又は含有するアミノ酸配列に有意に影響又は改変しないアミノ酸配列修飾を含む。かかる保存的配列修飾は、アミノ酸の置換、追加、及び削除を含む。保存的なアミノ酸置換は、アミノ酸残基が、類似の側鎖を有するアミノ酸残基と置換されるものを含む。類似の側鎖を有するアミノ酸残基のファミリーは、当該技術分野において定義されている。これらのファミリーは、塩基性側鎖(例えば、リシン、アルギニン、ヒスチジン)、酸性側鎖(例えば、アスパラギン酸、グルタミン酸)、極性無電荷側鎖(例えば、グリシン、アスパラギン、グルタミン、セリン、トレオニン、チロシン、システイン、トリプトファン)、非極性側鎖(例えば、アラニン、バリン、ロイシン、イソロイシン、プロリン、フェニルアラニン、メチオニン)、β分岐側鎖(例えば、トレオニン、バリン、イソロイシン)、及び芳香族側鎖(例えば、チロシン、フェニルアラニン、トリプトファン、ヒスチジン)を持つアミノ酸を含む。したがって、組織因子抗体において予測される非必須アミノ酸残基は、好ましくは、同一の側鎖ファミリーからの他のアミノ酸残基と置換される。
2.核酸分子及び産生細胞株
配列番号:1の、隣接アミノ酸の少なくとも70〜100%をコード化するヌクレオチド配列等の、本明細書で提供される情報を使用して、特定断片、その変異体又はコンセンサス配列、あるいはこれらの配列の少なくとも1つを含むベクター、CNTO 860抗体Fc変異体である少なくとも1つの抗組織因子抗体をコード化する本発明の核酸分子は、本明細書に記載の方法又は当該技術分野で知られている方法を用いて取得することができる。
本発明の単離核酸分子は、限定されるものではないが、配列番号:1の、少なくとも1つの重鎖の、又は配列番号:3の、軽鎖の、CDR1、CDR2、及び/又はCDR3のような少なくとも1つのCDRの、少なくとも1つの特定部分に対するコード化配列を含む核酸分子を含むことができ、遺伝子コードの変性のために、核酸分子は上記のものとは実質的に異なるヌクレオチド配列を含むが、本明細書で記載されるように、及び/又は当該技術分野で知られるように、少なくとも1つの抗組織因子抗体を依然としてコード化する核酸分子に対するコード配列を含むことができる。当然のことながら、遺伝子コードは、当該技術分野においてよく知られている。したがって、当業者には、本発明の特異的な抗組織因子抗体をコードするかかる変性核酸変異体を生成することは、日常的であるだろう。例えば上記のオースベル(Ausubel)らを参照されたく、かかる核酸変異体は、本発明に含まれる。
修飾は、部位特異的変異誘発及びPCR媒介変異誘発等の当該技術分野において知られる標準的な技術によって、配列番号:1及び3に導入することができる。コード化されたタンパク質の配列を改変しない配列番号:1及び3におけるコドン置換も本発明に含まれる。コード配列のコドン置換は、例えば、ネズミ細胞系から大腸菌系に改変するなど、抗体の発現系が改変される場合に望ましい場合が多い。代替えとして、別の実施形態においては、飽和変異誘発等によって、配列をコードする抗組織因子抗体の全て又は一部に沿って、ランダムに変異を導入することができ、結果として生じる修飾抗組織因子抗体は、結合活性についてスクリーニングすることができる。
したがって、本明細書で開示されるヌクレオチド配列によってコード化される抗体及び/又は本明細書で開示されるアミノ酸配列を含有する抗体(すなわち、配列番号:2及び4)は、保存的に修飾された類似配列によってコード化されるか、又はそれを含有する実質的に類似する抗体を含む。2つの配列間の同一性パーセントは、配列によって共有される、同一位置の数の関数であり(すなわち、%相同性=同一位置の数/位置の総数×100)、2つの配列を最適に整列させるために導入する必要があるギャップの数及びそれぞれのギャップの長さを考慮に入れる。2つの配列間の配列の比較及び同一性パーセントの決定は、以下の非限定例において記載されるように、数学的アルゴリズムを使用して達成することができる。
2つのヌクレオチド配列間の同一性パーセントは、NWSgapdna.CMPマトリクス及びギャップ重40、50、60、70、又は80及び長さ重1、2、3、4、5、又は6を使用し、GCGソフトウェアパッケージ(www.gcg.comで入手可能)におけるGAPプログラムを使用して決定することができる。2つのヌクレオチド又はアミノ酸配列間の同一性パーセントは、PAM 1 20重残基表、ギャップ長ペナルティ12及びギャップペナルティ4を使用し、ALIGNプログラム(バージョン2.0)に組み込まれているマイヤーE.及びミラーW.「コンピュータ応用生物科学(Comput. AppL Biosci.)」4:11〜17、1988)のアルゴリズムを使用して決定することもできる。更に、2つのアミノ酸配列間の同一性パーセントは、Blossum 62マトリクス又はPAM2 50マトリクスのいずれか、及びギャップ重16、14、12、10、8、6、又は4及び長さ重1、2、3、4、5、又は6を使用し、GCGソフトウェアパッケージ(www.gcg.com)のGAPプログラムに組み込まれている、ニードルマン(Needleman)及びウンシュ(Wunsch)「分子生物学ジャーナル(J. Mol. Biol.)」48:444〜453、1970)アルゴリズムを使用して決定することができる。本発明の核酸及びタンパク質配列は、「クエリ配列」として更に使用し、公的なデータベースに対して検索を実行して、例えば、関連配列を同定することができる。かかる検索は、オルトシュル(Altschul)ら著、「分子生物学ジャーナル(J. Mol. Biol.)」215.403〜10、1990のNBLAST及びXBLASTプログラム(バージョン2.0)を使用して、実行することができる。BLASTヌクレオチド検索は、NBLASTプログラム、スコア=100、ワード長=12を実行し、本発明の核酸分子に相同するヌクレオチド配列を取得することができる。BLASTタンパク質検索は、XBLASTプログラム、スコア=50、ワード長=3を実行し、本発明のタンパク質分子に相同するアミノ酸配列を取得することができる。比較目的のギャップ付整列を取得するために、オルトシュル(Altschul)ら著、「核酸応答(Nucleic Acid Res.)」25(17):3389、1997に記載されるように、GappedBLASTを利用することができる。BLAST及びGappedBLASTプログラムを利用する場合、それぞれのプログラムのデフォルトパラメータ(例えば、XBLAST及びNBLAST)を使用することができる。http://www.ncbi.nlm.nih.govを参照されたい。
本明細書で使用されるところの「ベクター」という用語は、連結されている別の核酸を輸送できる核酸分子を意味することが意図される。ベクターの一種である「プラスミド」は、環状二本鎖DNAループを意味し、追加のDNAセグメントが連結され得る。別の種類のベクターは、ウイルスベクターであり、追加のDNAセグメントは、ウイルスゲノムに連結され得る。所定のベクターは、それらが導入される宿主細胞(例えば、複製の細菌性起源を有する細菌ベクター及びエピソーム性哺乳類ベクター)において自己複製できる。他のベクター(例えば、非エピソーム性哺乳類ベクター)は、宿主細胞への導入時に、宿主細胞のゲノムに組み込むことができ、それによって、宿主ゲノムに沿って複製される。更に、所定のベクターは、それらが操作可能に連結される遺伝子の発現を指向することができる。かかるベクターは、本明細書において「組み換え発現ベクター」(又は単に「発現ベクター」)と称される。一般に、組み換えDNA技術において実用的な発現ベクターは、プラスミドの形態である場合が多い。プラスミドは最も一般的に使用される形態のベクターであるため、本明細書においては、「プラスミド」及び「ベクター」は同義的に使用され得る。しかしながら、本発明は、同等の機能を果たすウイルスベクター(例えば、複製欠損のレトロウイルス、アデノウイルス及びアデノ関連ウイルス)等のかかる他の形態の発現ベクターを含むことが意図される。
本明細書で使用されるところの「組み換え宿主細胞」(又は単に「宿主細胞」)という用語は、組み換え発現ベクターが導入された細胞を意味することが意図される。かかる用語は、特定の対象細胞だけでなく、かかる細胞の子孫を意味することが意図されることを理解されたい。所定の修飾は、変異又は環境の影響のいずれかのために連続する世代で生じ得るため、かかる子孫は、実際は、親細胞と同一でない場合があるが、それでも本明細書で使用されるところの「宿主細胞」という用語の範囲内に含まれる。組み換え宿主細胞は、例えば、CHO細胞及びリンパ球細胞を含む。
別の態様においては、本発明は、プラスミド指定クローンp2401に含有される核酸によってコード化されるようなアミノ酸配列を有する、抗組織因子、CNT O860抗体変異体をコード化する単離核酸分子を提供する。
本明細書で示されるように、CNTO 860抗体Fc変異体をコード化する核酸を含む本発明の核酸分子は、追加の機能を提供するもの等の追加アミノ酸をコードする追加コード配列を含むことができる。したがって、抗体をコード化する配列は、抗体断片又は部分を含む融合された抗体の精製を促進するペプチドをコード化する配列等のマーカー配列に融合させることができる。代替えとしては、本発明のCNTO 860抗体Fc変異体は、追加の生物活性又は治療活性を抗体に付与する、サイトカイン部分又は第2の結合ドメイン等の別のポリペプチドに、融合され得る。かかる融合構造は知られており、それらを形成する方法は、当該技術分野において記載されている。
本発明は、本明細書で開示されるポリヌクレオチドに対して、選択的なハイブリダイゼーション条件下で、ハイブリダイズする単離核酸を提供する。したがって、本実施形態のポリヌクレオチドは、かかるポリヌクレオチドを含む核酸を単離、検出、修飾、又は定量するために使用することができる。典型的な核酸は、配列番号:5〜16を含む。例えば、本発明のポリヌクレオチドを使用して、蓄積されたライブラリーにおける部分又は全長クローンを同定、単離、又は増幅することができる。一部の実施形態においては、ポリヌクレオチドは、単離された、又はそうでなければヒト又は哺乳類核酸ライブラリーからのcDNAに相補的なゲノム配列又はcDNA配列である。
本発明の単離核酸は、当該技術分野においてよく知られているように、(a)組み換え方法、(b)合成技術、(c)精製技術、又はそれらの組み合わせを使用して形成することができる。追加の配列をかかるクローン化及び/又は発現配列に追加して、クローン化及び/又は発現におけるそれらの機能を最適化して、ポリヌクレオチドの単離を助けることができるか、又は細胞へのポリヌクレオチドの導入を改善することができる。クローン化ベクター、発現ベクター、アダプター、及びリンカーの使用は、当該技術分野においてよく知られている。(例えば、上記オースベル(Ausubel)又は上記クロッティ(Crotty)を参照されたい)。化学合成は、一般に、相補的配列とのハイブリダイゼーション、又は単鎖をテンプレートとして使用するDNAポリメラーゼでの重合によって、二本鎖DNAに変換可能な単鎖オリゴヌクレオチドを産生する。当業者であれば、DNAの化学合成が約100以上の塩基の配列に限定され得る一方で、より長い配列は、より短い配列の連結によって得ることができることを認識するであろう。機能的dsDNA分子を構成するかかる方法は、US6521427及びWO 02081490において教示される。
本明細書に記載されるように、本発明は、本発明の核酸を含む組み換え発現プラスミドを更に提供する。組み換え発現プラスミド又はカセットは、典型的には、意図される宿主細胞においてポリヌクレオチドの転写を指向する、転写開始調節配列に操作可能に連結される、本発明のポリヌクレオチドを含む。相同性及び非相同性(すなわち、外因性)プロモーターの両方を採用して、本発明の核酸の発現を指向することができる。
抗体鎖遺伝子に加えて、本発明の組み換え発現ベクターは、宿主細胞における抗体鎖遺伝子の発現を制御する調節配列を担持する。「調節配列」という用語は、プロモーター、エンハンサー、及び抗体鎖遺伝子の転写又は翻訳を制御する他の制御要素(例えば、ポリアデニル化シグナル)を含むことが意図される。哺乳類宿主細胞発現に好適な調節配列は、哺乳類細胞において高レベルのタンパク質発現を指向するウイルス要素、サイトメガロウイルス(CMV)に由来するプロモーター及び/又はエンハンサー、シミアンウイルス40(SV40)、アデノウイルス(例えば、アデノウイルス主要後期プロモーター(AdMLP))及びポリオーマを含む。代替えとして、ユビキチンプロモーター又はPグロビンプロモーター等の非ウイルス調節配列を使用してもよい。これらの細胞の発現ベクターは、以下の発現制御配列の1つ以上を含み、複製起点、プロモーター(例えば、後期又は早期SV40プロモーター、CMVプロモーター(米国特許第5,168,062号、同第5,385,839号)、HSVtkプロモーター、pgk(ホスホグリセリン酸キナーゼ)プロモーター、EF−1αプロモーター(米国特許第5,266,491号)、少なくとも1つのヒト免疫グロブリンプロモーター、エンハンサー、及び/又はリボソーム結合部位、RNAスプライス部位、ポリアデニル化部位(例えば、SV40大型TAgポリA追加部位)、及び転写終了配列等の、処理情報部位を含むが、それらに限定されない。
様々なcis作用DNA要素は、ベクターに組み込まれている。ベクター工学の場合、かかるDNA要素は、比較的小さいサイズ(>6kb)、不変的機能、及び望ましくは、複製数依存をもたらす能力(発現がゲノムに組み込まれるベクターの複製数に直接相関することで、増幅手順において関連する利点を有する能力)を有することが必要である。遺伝子座調節領域及びインスレーター等の要素は、これらに含まれる。広範な他の要素、哺乳類発現ベクターにおける隣接する導入遺伝子に使用される抗リプレッサー又はSTAR(安定化及び抗リプレッサー)要素は、周囲ゲノムから組み換えDNAへのメチル化及びヒストン脱アセチル化パターンの拡散に影響し、核マトリクスに結合する足場/マトリクス関連領域(S/MAR)は、これらに含まれる。偏在性クロマチンオープニング要素(UCOE)は、ハウスキーピング遺伝子のプロモーターに由来する要素である。ハウスキーピング遺伝子は、通常、著しい程度のヒストンアセチル化のために転写的に活性であり、発現ベクターにおけるUCOEの包含は、CHO細胞におけるトランス遺伝子発現の産生及び安定性を増加させることができる。伸長因子1α遺伝子等の高度に発現したハウスキーピング遺伝子からの5’及び3’配列を有する導入遺伝子の隣接は、哺乳類細胞株の範囲で導入遺伝子からの著しい産生の増大に至り得ることも報告されている。バーンズ(Barnes)及びディクソン(Dickson)、「(現在のバイオテクノロジーの見解(Current Opinion Biotechnol.))」17(4):381〜386、2006を参照して検討されたい。
発現ベクター内の調節エレメントの包含に対する代替えとして、第2のアプローチに、導入遺伝子の挿入部位を囲むクロマチンの、一般的にエピジェネティックである環境の改変が注目されている。一般に増強された転写に関連づけられるヒストンアセチル化は、ヒストンアセチルトランスフェラーゼ(HAT)とヒストンデアセチラーゼ(HDAC)活性とのバランスから生じる。
抗体鎖遺伝子及び調節配列に加えて、本発明の組み換え発現ベクターは、追加の選択可能なマーカー遺伝子を担持し得る。選択可能なマーカー遺伝子は、ベクターが導入された宿主細胞の選択を促進する(例えば、米国特許第4,399,216号、同第4,634,665号、及び同第5,179,017号、全てAxelらによるを参照されたい)。選択可能なマーカー遺伝子は、ジヒドロ葉酸還元酵素(DHFR)遺伝子(メトトレキサート選択/増幅を用いてdhfr宿主細胞において使用するため)及びネオ遺伝子(G418選択用)を含む。発現ベクターは、好ましくは少なくとも1つの選択可能なマーカーを含むが、これは任意である。かかるマーカーは、例えば、メトトレキサート(MTX)、ジヒドロ葉酸還元酵素(DHFR、米国特許第4、399、216号、同第4、634、665号、同第4、656、134号、同第4、956、288号、同第5、149、636号、同第5、179、017号)、アンピシリン、ネオマイシン(G418)、ミコフェノール酸、又は真核細胞培養に耐性のあるグルタミン合成酵素(GS、米国特許第5、122、464号、同第5、770、359号、同第5、827、739号)、及び大腸菌及び他の細菌又は原核生物において培養するためのテトラサイクリン又はアンピシリン耐性遺伝子を含むが、これらに限定されない(上記の特許は、参照することによってその全体が本明細書に組み込まれる)。上記の宿主細胞に対して適切な培養培地及び条件は、当該技術分野において知られている。
選択可能なマーカーの使用は、組み換え遺伝子数の増幅を可能にし、導入遺伝子からの増強された転写効率をもたらし得る。代替えとして、それぞれの増幅工程後の生産的な細胞株のクローン化は、結果として、細胞株プールの反復増幅及び増幅の最終段階におけるクローン化よりも生産的なクローンを生じ得る。したがって、本発明のCNTO860抗体は、抗体をコードする核酸配列が導入された細胞株の選択された誘導体クローンである細胞株において産生され得る。
本発明の少なくとも1つの抗体は、融合タンパク質等の修飾された形態で発現され得、分泌シグナルだけでなく、追加の相同性機能領域も含み得る。例えば、追加アミノ酸の領域、特に荷電アミノ酸を抗体のN末端に追加して、精製中又は後次の処理及び保存中に、宿主細胞における安定性及び持続性を改善することができる。また、ペプチド部分を本発明の抗体に追加して、精製を促進することもできる。抗体又は少なくとも1つのその断片の最終調製前に、かかる領域を除去することができる。かかる方法は、上記クロッティ(Crotty)の例えば、「PCRによるタンパク質のタグ付け又はクローン化(Tagging Proteins or Cloning by PCR)」、上記オースベル(Ausubel)16章、17章、及び18章等の多くの標準的な研究室マニュアルに記載されている。
当業者であれば、本発明のタンパク質をコード化する核酸の発現に使用できる多数の発現系について精通している。更に、ベクター内で本発明の抗体ポリペプチドをコードする核酸のレシピエントとして適切な多くの宿主細胞株があり、宿主細胞株は、コード化された抗体配列の発現を、促進、増強、指向、調節するか、又はその他の方法で生じる核酸配列に、操作可能に連結される。
3.抗体の精製
本発明のCNTO860抗体変異体は、典型的には、ろ過段階の後に様々な種類の材料上でのクロマトグラフィーを含む周知の方法によって、組み換え細胞培養液から回収及び精製でき、タンパク質A精製、硫酸アンモニウム又はエタノール沈殿、酸抽出、陰又は陽イオン交換クロマトグラフィー、ホスホセルロースクロマトグラフィー、疎水性相互作用クロマトグラフィー、親和性クロマトグラフィー、ヒドロキシアパタイトクロマトグラフィー、及びレクチンクロマトグラフィーを含むが、これらに限定されない。高速液体クロマトグラフィー(「HPLC」を精製に採用することもできる。例えば、コリガン(Coligan)著、「免疫学における現行プロトコル(Current Protocols in Immunology)」又は「タンパク質科学における現行プロトコル(Current Protocols in Protein Science)」、ニューヨーク:ジョン・ウィリー・アンド・サン、2007、例えば第1章、4章、6章、8章、9章、10章を参照し、それぞれ参照することによってその全体が本明細書に組み込まれる。
本発明の抗体は、化学合成手順の産生物、及び例えば、イースト、高等植物、昆虫、及び哺乳類細胞を含む真核宿主から、組み換え技術によって産生された産生物を含む。組み換え産生手順において選択される宿主に応じて、本発明の抗体は、グリコシル化又は非グリコシル化され得るが、グリコシル化が好ましい。かかる方法は、上記オースベル(Ausubel)著の第10章、12章、13章、16章、18章及び20章、コリガン(Coligan)著、上記「タンパク質科学(Protein Science)」第12章〜14章等の多くの標準的な研究室マニュアルに記載され、全て参照することによってその全体が本明細書に組み込まれる。
4.本発明の抗体組織因子抗体
抗体重鎖及び軽鎖CDRドメインが、抗原に対する抗体の結合特異性/親和性を付与することは、当該技術分野においてよく知られているため、上記のように調製される本発明の組み換え抗体は、好ましくは、それぞれ配列番号:2及び4の、重鎖及び軽鎖可変領域の配列内の特定残基として記載されるCNTO 860の重鎖及び軽鎖CDRを含む。重鎖及び軽鎖フレームワーク領域の可変領域内の非CDR領域は、フレームワーク領域(FR1、FR2、FR3、及びFR4)として知られるものを含み、完全な可変ドメインは、FR1−CDR1−FR2−CDR2−FR3−CDR3−FR4)で構成される。好適な実施形態においては、抗体又は抗原結合断片の3つの重鎖CDR及び3つの軽鎖CDRは、本明細書で記載されるように、対応するCNTO 860のCDRのアミノ酸配列を有する。かかる抗体は、従来技術を使用して、組み換えDNA技術の従来技術を使用することで抗体をコード化する(すなわち、1つ以上の)核酸分子を調製及び発現することによって、又は任意の他の適切な方法を使用することによって、抗体の様々な部分(例えば、CDR及びフレームワーク部分、FR1、FR2、FR3、及びFR4)を一緒に化学的に結合することにより調製することができる。
好ましくは、上述の人工抗体のCDR1、2、及び/又は3は、本明細書で開示されるCNTO 860のもののように、正確なアミノ酸配列を含む。しかしながら、当業者であれば、ヒト組織因子を有効に結合する抗体の能力(例えば、保存的置換)を依然として保持しながらも、CNTO 860の正確なCDR配列からの一部逸脱が可能であり得ることを理解するであろう。したがって、別の実施形態においては、人工抗体は、例えば、CNTO 860の1つ以上のCDRに対して90%、95%、98%、又は99.5%同一である、1つ以上のCDRから構成され得る。
結合組織因子に加えて、本発明のCNTO 860抗体変異体は、FcγRI、FcγRII、FcγRIII、FcγRIV等のFc受容体を結合する。上記のような人工抗体は、以下の本発明の抗体の他の機能的特性を保持するために選択され得る:
1)ヒト組織因子を発現する生細胞に結合する;
2)10-8M以下(例えば、10-9M又は10-10M以下)のKDを有するヒト組織因子に結合する;
3)TF8−5G9抗体によって認識される組織因子上の固有エピトープに結合する;
4)腫瘍細胞の生体内での増殖を阻害する;
5)免疫エフェクター細胞の表面上のFc受容体に結合する。
選択したヒト抗組織因子モノクローナル抗体が固有のエピトープに結合するかどうかを決定するための典型的な方法は、商業的に入手可能な試薬(Pierce,Rockford,IL)を使用するビオチニル化によって試験される抗体の標識化を含む。非標識化モノクローナル抗体及びビオチニル化モノクローナル抗体を使用する競合作用研究は、組織因子でコーティングされたELISAプレートを使用して行うことができる。ビオチニル化mAb結合は、ストレプトアビジン−アルカリホスファターゼプローブを用いて検出することができる。精製された抗体のアイソタイプを決定するために、アイソタイプELISAを実行することができる。組織因子を発現している生細胞に対するモノクローナル抗体の結合を示すために、フローサイトメトリーを使用することができる。抗組織因子ヒトIgGは、ウェスタンブロットによって組織因子抗原との反応性について更に試験することができる。
本発明の抗体は、任意のクラス(IgG、IgA、IgM、IgE、IgD等)であり得るか、又はFc受容体結合ドメインを含有し、したがってそのアイソタイプ及びサブクラスによって付与される望ましい範囲のエフェクター機能を有するものであり得、κ又はλ軽鎖を含み得る。抗体アイソタイプ及びサブクラスの定量可能な特性は、ADCC、CA等の生体内での活性を付与すると考えられ、オプソニン化を以下(表2)に示し、及び例えば、ジェンウェイ(Janeway)ら編、「免疫生物学(Immunobiology):健康及び疾患における免疫系(The immune system in health and disease)」ニューヨーク:ガーランド・パブリッシング(Garland Publishing)、2001、第4章及び9章において説明される。一実施形態においては、抗体は、IgG重鎖又は定義された断片、例えば、IgG1、IgG2、IgG3、又はIgG4の、少なくとも1つのアイソタイプ、好ましくはIgG1クラスを含む。別の実施形態においては、抗ヒト組織因子ヒト抗体は、IgG1重鎖及びIgGκ軽鎖を含む。
異なるIgGサブクラスは、典型的に、IgG2及びIgG4よりも実質的に良好に受容体に結合するIgG1及びIgG3を有するFcγRに対して異なる親和性を有する(ジェファリス(Jefferis)ら著、「(Immunol Lett)」82:57〜65、2002)。全てのFcγRは、IgGFc上の同一領域を結合するが、異なる親和性を有し、高い親和性のバインダーFcγR1は、108M-1のKdをIgG1に有するが、低親和性受容体FcγRII及びFcγRIIIは、一般にそれぞれ106で結合する。FcγRIIIa及びFcγRIIIbの細胞外ドメインは、96%同一であるが、FcγRIIIbは、細胞内シグナル伝達ドメインを有さない。上記のように、FcγRI、FcγRIIa/c、及びFcγRIIIaは、免疫複合体トリガー活性の正の調節であるが、FcγRIIbは阻害である。したがって、前者は活性化受容体と称され、FcγRIIbは阻害受容体と称される。受容体は、異なる免疫細胞上の発現パターン及びレベルにおいても異なる。更に別のレベルの複雑性は、ヒトプロテオームにおける多数のFcγRポリ多型の存在である。臨床上の重要性と特に関連するポリ多型は、VI 58/FI 58 FcγRIIIaである。ヒトIgGIは、FI 58アロタイプよりもVI58アロタイプに対してより高い親和性で結合する。親和性におけるこの差と、及び想定されるそのADCC及び/又はADCPに対する影響は、抗CD20抗体リツキシマブの有効性の重要な決定因子となることが示されている(Rituxan(登録商標)、IDECファーマスーティカルズ・コーポレーションの登録商標)。VI 58アロタイプを有する患者は、リツキシマブ治療に良好に応答するが、より低い親和性のFI 58アロタイプを有する患者は、応答が乏しい(Cartronら著、2002年、血液(Blood)99:754〜758、参照することによって明示的に組み込まれる)。ヒトの約10〜20%は、VI 58N1 58ホモ接合型であり、45%はVI 58/FI 58ヘテロ接合型であり、ヒトの35〜45%はFI 58/FI58ホモ接合である(Lehrnbecherら著、1999年、血液(Blood)94:4220〜4232;Cartronら著、2002年(上記))。したがって、ヒトの80〜90%は応答に乏しく、つまり、少なくとも1つのFI58 FcγRIIIaの対立遺伝子を有する。
Fc上の、重複するが個別の部位は、補体タンパク質CIqのインターフェースとして機能する。Fc/FcγR結合がADCCを媒介するのと同様の方法で、Fc/CIq結合は、補体依存細胞傷害(CDC)を媒介する。CH2とCH3との間のドメインFc上の部位は、新生児受容体、FcRnとの相互作用を媒介し、その結合はエンドソームから取り込まれた抗体を再利用して血流に戻す(Raghavanら著、1996年、Annu Rev Cell Dev Biol 12:181〜220;Ghetieら著、2000年、Annu Rev Immunol 18:739〜766)。腎ろ過の防止と結合されるこのプロセスは、全長分子のサイズが大きいため、1〜3週間の良好な抗体血清半減期をもたらす。FcRnに対するFcの結合は、抗体輸送においても重要な役割を果たす。Fc上のFcRnに対する結合部位は、細菌タンパク質A及びGが結合する部位でもある。これらのタンパク質による強力な結合は、タンパク質精製中にタンパク質A又はタンパク質G親和性クロマトグラフィーによって抗体を精製する手段として、典型的に利用される。
本発明の別の態様においては、本発明のヒト抗組織抗体の構造的特徴、CNTO 860を使用して、本発明の抗体の機能的特性、すなわち、ヒト組織因子への結合及びFc受容体への結合を保持する、構造的に関連するヒト抗組織因子抗体を形成する。
本発明の抗体は、広範な親和性(KD)を持つヒト組織因子を結合することができる。好適な実施形態においては、本発明の少なくとも1つのヒトmAbは、ヒト組織因子と高い親和性で任意に結合することができる。例えば、ヒトmAbは、ヒト組織因子を、約10―7M以下のKDで結合することができ、例えば、0.1〜9.9(又はそこでの任意の範囲若しくは値)X10-7、10-8、10-9、10-10、10-11、10-12、10-13M又はそこでの任意の範囲又は値であるが、これらに限定されない。
本発明の抗組織因子抗体は、本明細書で特定されるように、自然変異又はヒトによる操作のいずれかから、1つ以上のアミノ酸の置換、削除、又は追加を含むことができる。
本発明の抗組織因子抗体は、配列番号:2及び4の、少なくとも1つの、5から全ての隣接アミノ酸から選択される少なくとも1つの部分、配列、又は組み合わせを含み得るが、これらに限定されない。抗組織因子抗体は配列番号:2及び4、並びにFc部分の、少なくとも1つの隣接アミノ酸の70〜100%の少なくとも1つのポリペプチドを、任意で更に含み得る。
典型的な重鎖及び軽鎖可変領域配列は、配列番号:2の、残基1〜117、及び配列番号:4の、残基1〜108として提供される。本発明の抗体、又はその特定の変異体は、本発明の抗体から任意の数の隣接アミノ酸残基を含み得、その数は、抗TF抗体における隣接残基数の10〜100%からなる整数の群から選択される。任意で、隣接アミノ酸のこの部分列は、少なくとも約10、20、30、40、50、60、70、80、90、100、110、120、又はそれ以上のアミノ酸長であるか、あるいはそこでの任意の範囲又は値である。更に、かかる部分列の数は、少なくとも2、3、4、又は5等の、1〜20からなる群から選択される任意の整数であり得る。
重鎖定常部における保存位置における全ての自然に産生される抗体の糖鎖構造は、アイソタイプによって異なる。それぞれのアイソタイプは、明確な一連のN結合型オリゴ糖構造を有し、タンパク質アセンブリ、分泌、又は機能活性に可変的に影響する(Wright,A.及びMorrison,S.L.,Trends Biotech.15:26〜32(1997))。付着したN結合型オリゴ糖構造は著しく変化し、分泌前及び分泌後プロセスで異なり、二分岐GlcNac及びコアフコース残基の有無に関わらず、複雑な二分岐オリゴ糖構造であり得る(Wright,A.及びMorrison,S.L.、(上記))。典型的に、均一なモノクローナル抗体が複数の糖型として存在するように、特定のグリコシル化部位に付着するコアオリゴ糖構造は不均一な処理である。同様に、抗体グリコシル化における主要な差は、抗体産生細胞株間で生じ、異なる培養条件下で増殖した所定の細胞株にさえ、わずかな差が見られることが示されている。
Fc領域に存在するN結合型オリゴ糖(ヒンジ、CH2及びCH3ドメインの二量体化によって形成される)は、エフェクター機能に影響する。共有結合されたオリゴ糖は、複雑な二分岐型構造であり、高度に不均一である。全てのIgGサブタイプのCH2ドメインは、固有の保存されたNグリコシル化部位を残基297に含有する(図1、配列番号:2)。成熟した抗体においては、Asn297に付着した2つの複雑な二分岐オリゴ糖は、CH2ドメインの間に埋め込まれ、ポリペプチド骨格との広範な接触を形成する。それらの存在は、抗体がADCC等のエフェクター機能を媒介するために必須であることが判明した(Lifely,M.R.ら著、Glycobiology 5:813〜822(1995);Jefferis,R.ら著、Immunol Rev.163:59〜76(1998);Wright,A.及びMorrison,S.L.,1997(上記))。
Fc含有分子におけるグリカンの存在又は欠損は、FcγRI、FcγRIIA、及びFcγRIIIA受容体の1つ以上に対する親和性、ADCC活性、マクロファージ又はモノサイト活性化、及び血清半減期に影響する(Lifelyら著、Jeffreis,及びWright and Morrison、1997年(上記))。真核細胞による抗体及びMIMETIBODY(商標)組成物の組み換え産生は、宿主細胞の典型的なグリカン構造を用いた最終組成物の装飾に影響し、グリカン構造は、細胞培養条件によって更に影響を受け得る。これらの異種オリゴ糖は、主にシアル酸、フコース、ガラクトース、及びGlcNAc残基を末端糖としてを含有する(Raju,T.S.ら著、Glycobiology 2000年、10(5):477〜86)。これらの末端糖の一部、露出したガラクトース、コアフコース、及び二分岐GlcNac残基等は、ADCC活性、CDC活性に影響し、及びClq補体タンパク質を含む様々なリガンドに対する抗体結合にも影響することが示された(Presta L.2003.Curr Opin Struct Biol.13(4):519〜25)。
別の態様においては、本発明は、本明細書で説明されるように、抗体及び変異体に関連し、有機部分の共有結合連結によって修飾される。かかる修飾は、改善された薬物動態特性(例えば、増大した、生体内での血清半減期)を持つ抗体又は抗原結合断片を産生することができる。有機部分は、直線状又は分岐した親水性ポリマー基、脂肪酸基、又は脂肪酸エステル基であり得る。特定の実施形態においては、親水性ポリマー基は、約800〜約120,000ダルトンの分子重量を有し、ポリアルカングリコール(例えば、ポリエチレングリコール(PEG)、ポリプロピレングリコール(PPG))、糖質ポリマー、アミノ酸ポリマー又はポリビニルピロリドンであり得、脂肪酸又は脂肪酸エステル基は、約8〜約40の炭素原子を含み得る。連結したグリカンに沿ったヒンジコア鎖間ジスルフィド結合によって影響される三次構造に関して、Fc含有タンパク質は、抗体の非抗原結合特性がFcに残留するため、PEG化に対する固有の暴露を表す。したがって、生体内で最終組成物の生物活性を保持するために、グリカン構造に著しく影響しない接合方法、又は重鎖ポリペプチドの、鎖間ジスルフィド結合を形成する能力は、Fc含有タンパク質のPEG化において重要である。
本発明の修飾された抗体及び抗原結合断片は、直接又は間接的に抗体に共有結合される、1つ以上の有機部分を含み得る。本発明の抗体又は抗原結合断片に結合されるそれぞれの有機部分は、独立して、親水性ポリマー基、脂肪酸基、又は脂肪酸エステル基であり得る。本明細書で使用されるところの「脂肪酸」という用語は、モノカルボン酸及びジカルボン酸を包含する。本明細書で使用されるところの「親水性ポリマー基」という用語は、オクタンよりも水への可溶性の高い有機ポリマーを意味する。本発明の抗体を修飾するために適切な親水性ポリマーは、直線状又は分岐状であり得、例えば、ポリアルカングリコール(例えば、PEG、モノメトキシ−ポリエチレングリコール(mPEG)、PPG等)、糖質(例えば、デキストラン、セルロース、オリゴ糖、多糖等)、親水性アミノ酸のポリマー(例えば、ポリリシン、ポリアルギニン、ポリアスパラギン酸等)、ポリアルカンオキシド(例えば、ポリエチレンオキシド、ポリプロピレンオキシド等)、及びポリビニルピロリドンを含む。好ましくは、本発明の抗体を修飾する親水性ポリマーは、個別の分子体として、約800〜約150,000ダルトンの分子量を有する。例えば、PEG5000及びPEG20,000を使用することができ、下付き文字は、ポリマーの平均分子量(ダルトン)である。親水性ポリマー基は、1〜約6アルキル、脂肪酸、又は脂肪酸エステル基と置換され得る。脂肪酸又は脂肪酸エステル基と置換される親水性ポリマーは、適切な方法を採用することによって調製することができる。
本発明の抗体を修飾するために適切な脂肪酸及び脂肪酸エステルは、飽和され得るか、又は1つ以上の不飽和単位を含有し得る。本発明の抗体を修飾するために適切な脂肪酸は、例えば、n−ドデカン酸(C12、ラウリン酸)、n−テトラデカン酸(C14、ミリスチン酸)、n−オクタデカン酸(C18、ステアリン酸)、n−エイコ酸(C20、アラキジン酸)、n−ドコサン酸(C22、ベヘン酸)、n−トリアコンタノエート(C30)、n−テトラコンタノエート(C40)、cis−Δ9−オクタデカノエート(C18、オレイン酸)、全てのcis−Δ5、8、11、14−エイコサテトラエノエート(C20、アラキドン酸)、オクタンジオン酸、テトラデカンジオン酸、オクタデカンジオン酸、ドコサンジオン酸等を含む。適切な脂肪酸エステルは、直線状又は分岐状の低級アルキル基を含む、ジカルボキシル酸のモノエステルを含む。低アルキル基は、1〜約12、好ましくは1〜約6の炭素原子を含み得る。
修飾したヒト抗体及び抗原結合断片は、1つ以上の変性剤との反応等、適切な方法を使用して調製することができる。本明細書で使用されるところの「変性剤」という用語は、活性化基を含む。「活性化基」は、適切な条件下で反応して、変性剤と抗体、又は連結部分等の第2の有機分子との間の共有結合を形成できる、化学的な部分又は官能基である。例えば、アミン反応性活性化基は、トシル酸、メシル酸、ハロ(クロロ、ブロモ、フルオロ、ヨード)等の求電子性基、Nヒドロキシスクシニミジルエステル(NHS)等を含む。チオールと反応できる活性化基は、例えば、マレイミド、ヨードアセチル、アクリロリル、ピリジルジスルフィド、5−チオール−2−ニトロベンゾ酸チオール(TNB−チオール)等を含む。アルデヒド官能基は、アミン又はヒドラジド含有分子と結合することができ、アジド基は、三価リン基と反応して、ホスホルアミド化又はホスホルイミド結合を形成することができる。分子に活性化基を導入するための適切な方法は、当該技術分野において知られている(例えば、ハーマンソン(Hermanson),G.T.Bioconjugate Techniques、Academic Press:カリフォルニア州サンディエゴ(1996)を参照されたい)。リンカー部分は、例えば、二価のC1〜C12基であり得、1つ以上の炭素原子を、酸素、窒素、又は硫黄等のヘテロ原子によって置換することができる。適切なリンカー部分は、例えば、柔軟なペプチド(GGGSn)、−(CH2)3−、−NH−(CH2)6−NH−、−(CH2)2−NH−、及び−CH2−O−CH2−CH2−O−CH2−CH2−O−CH−NH−を含む。
本発明の修飾抗体は、抗体又は抗原結合断片を変性剤と反応させることによって産生することができる。例えば、有機部分は、アミン反応性変性剤、例えば、PEGのNHSエステルを採用することによって、非部位特異的方法で抗体に結合させることができる。
2つの物質の複合体の調製において、その少なくとも1つは、タンパク質又はポリペプチドを含み、二官能性剤を使用して、複合体の構成要素を共役的に通常複合反応に利用されている複合される分子におけるアミノ基に連結することが知られている。二官能性タンパク質連結剤には、N−スクシニミジル−(2−ピリジルジチオ)プロピオン酸(SPDP)、スクシニミジル−4−(N−マレイミドメチル)シクロヘキサン−1−カルボキシル酸、イミノチオラン(IT)、ジメチルアジプイミド酸/HCl等のイミドエステルの二官能性誘導体、ジスクシニミジルスベル酸等の活性エステル、グルタルアルデヒド等のアルデヒド、ビス(p−アキシドベンゾイル)ヘキサンジアミン等のビスアジド化合物、ビス−(p−ジアゾニウムベンゾイル)−エチレンジアミン等のビスジアゾニウム誘導体、ジイソシアネート(トリエン2,6−ジイソシアン酸)、及び1,5−ジフルオロ−2,4−ジニトロベンゼン)等のビス活性フルオリン化合物、を含む。SPDPは、この目的で最も頻繁に使用される試薬であり、多くの他のN−スクシニミジル−(2−ピリジルジチオ)−、N−スクシニミジル−(5−ニトロ−2−ピリジルジチオ)−又はN−スクシニミジル−(4−ピリジルジチオ)−短鎖アルカン酸が、有用であることが証明された。
抗体は、更に共役的に修飾、複合されることで活性になり、それによって免疫複合体を形成し得る。免疫複合体は、当該技術分野において知られ、説明されている。実施例は、ドキソルビチン結合Mab BR96(Braslawskyら著、Cancer Immunol Immunother 33:367〜374、1991)及び抗増殖因子抗体又は断片に融合される、緑膿菌外毒素(Kreitmentら著、Internat.J.Immunopharm.14(3):465〜72、1992)である。標的部位における細胞が破壊されることが望ましい場合に、極めて高い毒性を抗体標的治療のために選択することは特に重要である。癌細胞表面上の腫瘍関連抗原の数が105分子/細胞であると推定される場合、これらの複合体において有効に使用できる細胞毒性剤は、標的癌細胞に対して10-10〜10-11MのIC50値を有する必要がある(Chari,R.V.J.Adv.Drug Delivery Rev.1998,31,89〜104)。第2に、薬剤は、標的に結合する際に放出されて細胞を貫通する必要があるか、又は作成物全体が細胞内に輸送され、そこで毒素が開裂するか又は他の方法で活性化される必要がある。細胞外で徐々に変性するジスルフィド結合によって連結される高毒性マイタンシンの抗体複合体は、これらの特性を有する典型的な型の作成物である(Chariら著、Cancer Res.52:127〜131,1992;Liuら著、米国科学アカデミー紀要(Proc.Natl.Acad.Sci USA)93:8618〜8623,1996;米国特許第5,208,020号)。
5.抗体の試験
抗原に対する抗体の親和性又は結合活性は、任意の適切な方法を使用して実験的に決定することができる。特定の抗体抗原相互作用の測定される親和性は、異なる条件(例えば、塩濃度、pH)下で測定される場合に異なり得る。したがって、親和性及び他の抗原結合パラメータ(例えば、KD、Ka、Kd)の測定値は、好ましくは、抗体及び抗原の標準化溶液、及び本明細書で記載される緩衝液等の標準化緩衝液で形成される。
好ましくは、本発明の抗体又は抗原結合断片は、ヒト組織因子を結合し、それによって、部分的又は実質的にタンパク質の少なくとも1つの生物活性を中和する。部分的又は好ましくは実質的に、少なくとも1つの組織因子タンパク質又は断片の少なくとも1つの生物学的活性を中和する、抗体又はその特定部分又は変異体は、タンパク質又は断片を結合することができ、それによって、組織因子の結合を通じてそのリガンドに媒介される、又は他の組織因子依存又は媒介機構を通じて媒介される活性を阻害することができる。本明細書で使用されるように、「中和化抗体」という用語は、アッセイに応じて約20〜120%、好ましくは少なくとも約10、20、30、40、50、55、60、65、70、75、80、85、90、91、92、93、94、95、96、97、98、99、100%又はそれ以上、組織因子依存活性を阻害できる抗体を意味する。抗組織因子抗体が組織因子依存活性を阻害する能力は、好ましくは、本明細書に記載される、及び/又は当該技術分野において知られるように、少なくとも1つの適切な組織因子タンパク質又は受容体アッセイによって評価される。
表1は、ヒト及びマウスFcγRの主要クラスの一覧を提供し、活性化及び阻害化受容体への重要な分類を示す(同一細胞上の活性化受容体と同時に阻害化受容体を通じたシグナル伝達は、活性化受容体を起源とするシグナル伝達カスケードを遮断し得る)。これらの受容体のこれらのアミノ酸配列は知られている。
本発明の抗体変異体及び他のFc含有タンパク質は、いくつかのよく知られた生体外アッセイによって機能性を比較することができる。特に、Fcγ受容体のFcγRI、FcγRII、及びFcγRIIIファミリーのメンバーに対する親和性は、関心の対象である。これらの測定値は、受容体の組み換え可溶型又は受容体の細胞細胞結合型を使用して形成することができる。更に、FcRnに対する親和性、IgGの延長された循環半減期に応答可能な受容体は、組み換え可溶型FcRnを使用して測定することができる。これらのアッセイは、例えば、ELISA又は表面プラズモン共鳴(BIAコア)によって固体支持体を使用する、直接又は間接的検出方法を使用して、便宜的に実行され得る。ADCCアッセイ及びCDCアッセイ等の細胞ベースの機能性アッセイは、特定の変異体構造の可能な機能性の結果に関する見識を提供する。一実施形態においては、ADCCアッセイは、主要なエフェクター細胞であるNK細胞を有するように構成され、それによって、FcγRIIIA受容体上で機能的効果を反映させる。過酸化又は炎症媒介放出等の細胞応答を測定できるため、食作用アッセイを使用して、異なる変異体の免疫エフェクター機能を比較することもできる。例えば、マウスにおけるT細胞活性化の測定、Fcγ受容体等の特定のリガンドと会合しているFcドメインに依存する活性の測定、あるいは腫瘍移植などの疾患モデルの使用などの、生体モデルの使用も、腫瘍細胞の退縮(腫瘍体積の減少)又は腫瘍増殖の鈍化のいずれかで測定される様な、腫瘍細胞の破壊の増強を測定することができる。
6.抗組織因子抗体組成物
本発明は、非自然発生組成物、混合物又は形態で提供される、本明細書で記載されるような少なくとも1つのCNTO860抗体変異体を含む、少なくとも1つのCNTO860抗体変異体組成物も提供する。本発明のCNTO860抗体変異体組成物又は組み合わせは、任意の適切な助剤の少なくとも1つを更に含み得、希釈剤、結合剤、安定剤、緩衝剤、塩、親油性溶媒、保存剤、アジュバント等を含むが、これらに限定されない。製薬上許容できる助剤が好ましい。かかる滅菌溶液を調製する非限定例及び方法は、当該技術分野においてよく知られており、例えば、Gennaro,Ed.,Remington’s Pharmaceutical Sciences,18th Edition,Mack Publishing Co.(Easton,PA)1990等であるが、それに限定されない。当該技術分野においてよく知られているような、又は本明細書に記載されようなCNTO860抗体変異体組成物の投与モード、溶解性及び/又は安定性が、投与方法に適する製薬上許容できる担体は、日常的に選択できる。
他の賦形剤、例えば、等張剤、緩衝剤、抗酸化剤、保存剤エンハンサーは、任意にかつ好適に希釈剤に添加することができる。トレハロース等のポリペプチド安定剤は、周知の濃度で使用してもよい。好ましくは、生理的に許容のある緩衝剤を添加して、改善されたpH制御を提供する。製剤は、約pH4〜約pH10の広範なpH、及び好ましくは約pH5〜約pH9の範囲、最も好ましくは、約6.0〜約8.0の範囲を網羅することができる。好適な緩衝液には、ヒスチジン及びリン酸緩衝液を含み、最も好ましくは、リン酸ナトリウム、特にリン酸緩衝生理食塩水(PBS)を含む。
他の添加材、例えばTween20(ポリオキシエチレン(20)ソルビタンモノラウレート)、Tween40(ポリオキシエチレン(20)ソルビタンモノパルミテート)、Tween80(ポリオキシエチレン(20)ソルビタンモノオレエート)、Pluronic F68(ポリオキシエチレンポリオキシプロピレンブロックコポリマー)、並びにPEG(ポリエチレングリコール)あるいはポリソルベート20若しくは80又はポロキサマー184若しくは188等の非イオン性界面活性剤、Pluronic(登録商標)ポリル、他のブロックコポリマー、並びにEDTA及びEGTA等のキレート剤等の、製薬上許容できる可溶化剤を、任意に製剤又は組成物に添加することができる。
本組成物において有用な医薬賦形剤及び添加剤には、タンパク質、ペプチド、アミノ酸、脂質、及び糖質(例えば、糖には、単糖、ジ−、トリ−、テトラ−、及びオリゴ糖等の糖、アルジトール、アルドン酸、エステル化糖等の誘導体化糖等、及び多糖又は糖ポリマーが挙げられる)を含むが、これらに限定されず、単独又は組み合わせで存在し得、1〜99.99%重量又は体積での、単独又は組み合わせを含む。典型的なタンパク質賦形剤には、ヒト血清アルブミン(HSA)等の血清アルブミン、組み換えヒトアルブミン(rHA)、ゼラチン、カゼイン等を含む。緩衝能においても機能し得る代表的なアミノ酸/抗体構成要素には、アラニン、グリシン、アルギニン、ベタイン、ヒスチジン、グルタミン酸、アスパラギン酸、システイン、リシン、ロイシン、イソロイシン、バリン、メチオニン、フェニルアラニン、アスパルテーム等を含む。1つの好適なアミノ酸はヒスチジンである。
本発明における使用に適した糖質賦形剤は、例えば、フルクトース、マルトース、ガラクトース、グルコース、D−マンノース、ソルボース等の単糖、ラクトース、スクロース、トレハロース、セロビオース等の二糖、ラフィノース、メレジトース、マルトデキストリン、デキストラン、スターチ等の多糖、及びマンニトール、キシリトール、マルチトール、ラクチトール、キシリトールソルビトール(グルシトール)、ミオイノシトール等のアルジトールを含む。本発明における使用に適した糖質賦形剤は、マンニトール、トレハロース、及びラフィノースである。
CNTO 860抗体Fc変異体組成物は、緩衝剤又はpH調節剤を任意に含むことができ、典型的には、緩衝剤は、有機酸又は塩基から調製される塩である。代表的な緩衝剤は、クエン酸、アスコルビン酸、グルコン酸、カルボン酸、酒石酸、コハク酸、酢酸、又はフタル酸の塩等の有機酸塩、トリス、塩酸トロメタミン、又はリン酸緩衝剤を含む。本組成物における使用に適した緩衝剤は、クエン酸等の有機酸塩である。
追加として、本発明のCNTO 860抗体Fc変異体組成物は、ポリビニルピロリドン、フィコール(ポリマー糖)、デキストレート(dextrates)(例えば、2−ヒドロキシプロピル−α−シクロデキストリン等のシクロデキストリン)、ポリエチレングリコール、着香剤、抗菌剤、甘味料、抗酸化剤、帯電防止剤、界面活性剤(例えば、「TWEEN20」及び「TWEEN80」等のポリソルベート)、脂質(例えば、リン脂質、脂肪酸)、ステロイド(例えば、コレステロール)、及びキレート剤(例えば、EDTA)等のポリマー賦形剤/添加剤を含むことができる。
本発明に従うCNTO 860抗体Fc変異体組成物における使用に適するこれら及び追加の周知の医薬賦形剤及び/又は添加剤は、例えば、「Remington:The Science & Practice of Pharmacy」19th ed.,Williams & Williams,(1995)及び「Physician’s Desk Reference」52nd ed.,Medical Economics,Montvale,NJ(1998)において一覧表示されるように、当該技術分野において知られており、これらの開示は、参照することによって本明細書に組み込まれる。好適な担体又は賦形剤材料は、糖質(例えば、単糖類及びアルジトール)及び緩衝剤(例えば、クエン酸)又はポリマー剤である。
本発明のCNTO 860抗体Fc変異体組成物は、任意で更に抗リウマチ剤(例えば、メトトレキサート、オーラノフィン、アウロチオグルコース、アザチオプリン、エタネルセプト(etanercept)、金チオリンゴ酸ナトリウム、硫酸ヒドロキシクロロキン、レフルノミド、スルファサラジン)、筋弛緩剤、睡眠薬、非ステロイド抗炎症薬(NSAID、セレコキシブ)、鎮痛剤、麻酔薬、鎮静剤、局所麻酔、神経筋遮断薬、抗菌剤(例えば、アミノグリコシド、抗真菌剤、駆虫薬、抗ウイルス薬、カルバペネム、セファロスポリン、フルオロキノロン、マクロリド、ペニシリン、スルホアミド、テトラサイクリン、別の抗菌薬)、乾癬治療薬、コルチコステロイド(デキサメタゾン)、アナボリックステロイド(テストステロン)、高血糖を緩和できる薬剤、ミネラル、栄養素、甲状腺剤、ビタミン、カルシウム関連ホルモン、下痢止め薬、鎮咳薬、制吐薬(5−HT3阻害剤等、ドラステロン、グラニセトロン、オンダセトロン、パロノセトロン)、抗潰瘍薬、便秘薬、抗凝固剤、エリスロポエチン(例えば、エポエチンα)、フィルグラスチム(例えば、G−CSF、ニューポゲン)、サルグラモスチム(GM−CSF、ロイキン)、免疫付与、免疫グロブリン(リツキシマブ)、免疫抑制薬(例えば、バシリキシマブ、シクロスポリン、ダクリズマブ)、成長ホルモン、ホルモン拮抗薬、生殖ホルモン拮抗剤(フルタミド、ニルタミド)、ホルモン放出調節因子(ロイプロリド、ゴセレリン)、ホルモン置換薬、エストロゲン受容体調節因子(タモキシフェン)、レチノイド(トレチノイン)、トポイソメラーゼ阻害剤(エトポシド、イリノテカン)サイトキシン(ドキソルビチン、ダカルバジン)、散瞳薬、毛様筋調節薬、アルキル化剤(シクロホスファミド、クロラムブチル)、プラチナ化合物(シスプラチン、カルボプラチン、オキサリプラチン、サトラプラチン)、ナイトロジェンマスタード(メルファレン、クロラブチル)、ニトロソウレア(カルムスチン、エストラムスチン、ロムスチン)、代謝拮抗物質(メトトレキサート、シタラビン、フルオロウラシル、ゲムシタビン、カプシタビン)、ミオチン阻害剤(ビンクリスチン、タキソル、タキソテル、ドセタキソル)、アポトーシスを刺激できる薬剤(三酸化ヒ素)、シグナル形質導入阻害剤(ゲフィチニブ、エルロチニブ、ヨランジス、スニチブ、メシル化イマチニブ)放射性医薬品(ヨウ素131−トシツモマブ)、放射線感受性増強物質(ミソニダゾル、チラパザミン)、抗うつ剤、抗躁薬、抗精神病薬、抗不安薬、睡眠薬、交感神経興奮剤、興奮剤、ドネペジル、タクリン、ぜんそく薬、β作用薬、吸入ステロイド、ロイコトリエン阻害剤、メチルキサンチン、クロモリン、エピネフリン又は類似体、ドルナーゼα(Pulmozyme)サイトカイン(インターフェロンα−2、IL2)、又はサイトカイン拮抗薬(インフリキシマブ)の少なくとも1つから選択される、少なくとも1つの添加剤の投与を含むか、又は組み合され得、引用される特定の薬剤及び産生物は、作用のクラス又は機構を代表する非限定例である。適切な薬剤及び用量は、当該技術分野においてよく知られている。例えば、Bruntonら(編)Goodman and Gilman’s The Pharmacological Basis of Therapeutics,11th Edition(2006)McGraw−Hill,NY,NY及び利用可能なオンライン、Wellsら編、Pharmacotherapy Handbook,2nd Edition,Appleton and Lange,Stamford,CT(2000)、PDR Pharmacopoeia,Tarascon Pocket Pharmacopoeia 2000,Deluxe Edition,Tarascon Publishing,Loma Linda,CA(2000)を参照されたく、それぞれは参照することによってその全体が本明細書に組み込まれる。
方法は、本発明のCNTO860抗体変異体の投与を、抗体腫瘍効果又は生体内の腫瘍増殖を阻害する異種機構を有する1つ以上の他の薬剤と組み合わせることによって実行することができ、化学療法薬を含むが、これに限定されない。
更に、本発明のCNTO860抗体変異体は、抗ビトロネクチン受容体抗体、例えば、エタラシズマブ又はCNTO95、又は米国特許第5,985,278号及び同第6,160,099号、米国特許第5,766,591号及びWO 0078815、並びにWO 02012501として公開された出願人の同時係属出願で開示されるもの等のような1つ以上の血管形成剤、抗VEGF又は抗VEGFR抗体、例えば、ベバシズマブ(AVSTIN)、又は非生物的薬剤、例えばサリドマイド等と組み合わせることができる。血管新生は、腫瘍転移、固形癌成長(異常増殖)、骨粗鬆症、パジェット疾患、悪性腫瘍の液性高カルシウム血症、腫瘍血管新生を含む血管新生、黄斑変性を含む網膜症、関節リウマチを含む関節炎、歯周病、乾癬及び平滑筋細胞遊走(例えば、再狭窄)を含む様々な状態又は疾患状態において役割を果たすことが知られている。
本発明は、化学療法剤、毒素(例えば、細菌、菌類、植物、又は動物起源の酵素的に活性な毒素、又はその断片)、又は放射性同位体(すなわち、放射性複合体)等の細胞毒性剤に共役される、本明細書に記載の抗体を含む免疫複合体にも関する。
かかる抗癌剤は、本発明の少なくとも1つの抗体と関連、結合、共製剤、又は共投与される毒性分子も含み得る。毒素は任意に作用して、病原性細胞又は組織を選択的に死滅させることができる。病原細胞は、癌細胞又は他の細胞であり得る。かかる毒素は、限定されないが、例えば、リシン、ジフテリア毒、ヘビ毒、又は細菌毒の少なくとも1つから選択される、毒素の少なくとも1つの機能的細胞毒性ドメインを含む、精製又は組み換え毒素又は毒素断片であり得る。毒素という用語は、ヒト及び他の哺乳類において、死に至り得る毒素性ショックを含む、任意の病原状態をもたらし得る任意の自然発生する突然変異若しくは組み換え細菌又はウイルスによって産生される内毒素及び外毒素のいずれも含む。かかる毒素には、腸毒素産生の大腸菌熱に不安定なエンテロトキシン(LT)、熱安定エンテロトキシン(ST)、赤痢菌細胞毒素、アエロモナス属エンテロトキシン、中毒性ショック症候群毒素−1(TSST−1)、ブドウ球菌エンテロトキシンA(SEA)、B(SEB)、又はC(SEC)、連鎖球菌エンテロトキシン等を含み得るが、それらに限定されない。かかる細菌は、エンテロトキシン大腸菌(ETEC)種、腸管出血性大腸菌(例えば、セロタイプ0157:H7の株)、ブドウ球菌種(例えば、黄色ブドウ球菌、化膿ブドウ球菌)、赤痢菌種(例えば、シゲラ赤痢、シゲラflexneri、シゲラboydii、及びシゲラsonnei)、サルモネラ種(例えば、チフス菌、Salmonella cholera−suis、サルモネラ腸炎)、クロストリジウム種(例えば、Clostridium perfringen、Clostridium dificile、ボツリヌス菌)、カンプロバクター種(例えば、Camphlobacter jejuni、Camphlobacter fetus)、ヘリコバクター種(例えば、ヘリコバクターピロリ)、アエロモナス種(例えば、アエロモナス、アエロモナス細菌、アエロモナスキャビア)、Pleisomonas shigelloide、エルシニア腸コリティカ、ビブリオ種(例えば、コレラ菌、ビブリオ属)、クレブシエラ種、緑膿菌、及び連鎖球菌の株を含むが、それらに限定されない。例えば、Stein,ed.,INTERNAL MEDICINE,3rd ed.,pp 1〜13,Little,Brown and Co.,Boston,(1990);Evansら編、Bacterial Infections of Humans:Epidemiology and Control,2d.Ed.,pp 239〜254,Plenum Medical Book Co.,New York(1991);Mandellら著、Principles and Practice of Infectious Diseases,3d.Ed.,Churchill Livingstone, New York(1990);Berkowら編、The Merck Manual,16th edition,Merck and Co.,Rahway,N.J.,1992;Woodら著、FEMS Microbiology Immunology,76:121〜134(1991);Marrackら著、Science,248:705〜711(1990)を参照し、これらの参考文献の内容は、参照することによってその全体が本明細書に組み込まれる。
抗体及び細胞毒素剤の複合は、N−スクシニミジル(2−ピリジルジチオール)プロピオン酸(SPDP)、イミノチオラン(IT)、イミドエステルの二官能性誘導体(ジメチルアジピミデートHCL等)、活性エステル(ジスクシニミジルスベリン酸等)、アルデヒド(グルタルアルデヒド等)、ビスアジド化合物(ビス(p−アジドベンゾイル)ヘキサンジアミン等)、ビスジアゾニウム誘導体(ビス−(p−ジアゾニウムベンゾイル)−エチレンジアミン)、ジイソシアネート(トリエン2,6ジイソシアネート等)、及びビス−活性フルオリン化合物(1,5−ジフルオロ−2,4−ジニトロベンゼン)等の多様な二官能性タンパク質連結剤を使用して形成される。例えば、リシン免疫毒素は、Vitettaら編、Science 238:1098(1987)において記載されるように調製することができる。炭素標識化I−イソチオシアネートベンジルメチルジエチレントリアミン五酢酸(MX−DTPA)は、放射性ヌクレオチドを抗体に複合するための典型的なキレート剤である。WO 94/11026を参照されたい。
7.製造準備及び製品
上記のとおり、本発明は、好ましくは、生理食塩水又は選択された塩溶液である安定した製剤、並びに保存剤を含有する保存溶液及び製剤、並びに製薬上許容できる製剤中の少なくとも1つの抗組織因子抗体を含む医薬又は獣医薬使用に適した多用途保存製剤、を提供する。生体内投与に使用される抗体又はそれらの結合断片は、滅菌される必要がある。これは、凍結乾燥及び再構成の前又は後に、滅菌ろ過膜を通すろ過によって容易に達成される。抗体又はその結合断片は、通常凍結乾燥形態又は溶液中で保管される。
包装材、並びに、上記の緩衝液及び/又は保存剤、任意には水溶性希釈剤と共に、少なくとも1つの抗組織因子サブユニット抗体の溶液を含む、少なくとも1つのバイアルを含む製造製品を提供し、前記包装材は、かかる溶液が一定の時間又はそれ以上の間にわたって保持され得ることを示す標識を含む。本発明は、包装材、凍結乾燥された少なくとも1つの抗組織因子抗体を含む第1のバイアル、及び処方された緩衝剤又は保存剤の水性希釈剤を含む第2のバイアルを含む、製造製品を更に含み、前記包装材は、水溶性希釈剤中の少なくとも1つの抗組織因子抗体を再構成して、24時間以上の期間にわたって保持できる溶液を形成するように患者に指示するラベルを含む。
本発明の産生物における組織因子抗体の範囲は、再構成時に産生する量、湿/乾システムの場合には、約1.0μg/ml〜約1000mg/mlの濃度を含むが、より低い濃度及び高い濃度が実施可能であり、及び意図される送達担体に依存し、例えば、溶液製剤は、経皮パッチ、肺、経粘膜、又は浸透圧性若しくはミクロポンプ方法とは異なる。
より具体的には、抗体の治療製剤、又はその結合断片は、望ましい程度の純度を有する抗体又はそれらの結合断片を、任意に、生理的に許容できる担体、賦形剤、又は安定剤と混合することによって、凍結乾燥形態又は水溶性溶液の形態で保管用に調製される(Remington’s Pharmaceutical Sciences,17th edition,(Ed.)A.Osol,Mack Publishing Company,Easton,Pa.,1985;Gennaro,Ed.,Remington’s Pharmaceutical Sciences,18th Edition,Mack Publishing Co.(Easton,PA)1990)。許容できる担体、賦形剤、又は安定剤は、採用される用量及び濃度でレシピエントに対して非毒性であり、リン酸、クエン酸、及び他の有機酸等の緩衝液、アスコルビン酸を含む抗酸化剤、低分子量(約10アミノ酸残基未満)のポリペプチド、血清アルブミン、ゼラチン、又は免疫グロブリン等のタンパク質、ポリビニルピロリドン等の親水性ポリマー、グリシン、グルタミン、アスパラギン、アルギニン、又はリシン等のアミノ酸、単糖、二糖、及びグルコース、マンノース又はデキストリンを含む他の糖質、EDTA等のキレート剤、マンニトール又はソルビトール等の糖アルコール、ナトリウム等の塩形成対イオン、及び/又はTween、Pruronics又はポリエチレングリコール(PEG)等の非イオン性界面活性剤等を含む。
抗体、又はその結合断片は、例えば、コアセルベート技術又は界面重合(例えば、それぞれヒドロキシメチルセルロース、又はゼラチンマイクロカプセル及びポリ−[メチルメタクリレート]マイクロカプセル)によって調製されたマイクロカプセル中に、コロイド性薬剤送達系(例えば、リポソーム、アルブミンミクロスフェア、マイクロエマルジョン、ナノ粒子及びナノカプセル)中に、又はマクロエマルション中に封入されてもよい。かかる技術は、上記のレミントン製剤化学(Remington’s Pharmaceutical Sciences)において開示されている。
治療抗体組成物は、一般に、滅菌アクセス口、例えば、静脈内輸液バッグ、又は皮下注射針によって穿孔可能なストッパーを有するバイアル、を有する容器に配置される。本発明に基づく、抗体又はその結合断片の投与経路は、周知方法、例えば、静脈内、腹腔内、筋肉内、動脈内、皮下、病巣内経路に基づく、エアロゾル又は鼻腔内経路に基づく、あるいは以下に記載されるような徐放性系に基づく、注射又は注入と合致する。抗体又はその結合断片は、注入又はボーラス注射によって連続的に投与される。徐放性製剤の適切な実施例は、タンパク質を含有する固形疎水性ポリマーの半透性マトリクスを含み、このマトリクスは、造形品の形態、例えば、フィルム又はマイクロカプセルである。徐放性マトリクスの実施例は、Langerら著、1981,J.Biomed.Mater.Res.,15:167〜277 and Langer,1982,Chem.Tech.,12:98〜105)によって説明されるようなポリエステル、ヒドロゲル(例えば、ポリ(2−ヒドロキシエチル−メタクリレート)、又はポリ(ビニルアルコール)、ポリラクチド(米国特許第3,773,919号、欧州特許第58,481号)、L−グルタミン酸及びγエチル−L−グルタミン酸のコポリマー(Sidmanら著、1983,Biopolymers,22:547〜556)、分解不可能なエチレン−ビニル酢酸(ランゲル(Langer)ら、上記)、LUPRON DEPOT(登録商標)(乳酸−グリコール酸コポリマー及び酢酸ロイプロリドからなる注射可能なミクロスフェア)等の分解可能な乳酸−グリコール酸コポリマー、及びポリ−D−(−)−3−ヒドロキシブチル酸(EP 133,988)を含む。
エチレン−酢酸ビニル等のポリマー及び乳酸−グリコール酸は、100日以上にわたって分子の放出を可能にするが、所定のヒドロゲルは、より短期間タンパク質を放出する。カプセル化された抗体が体内に長時間残留する場合、それらは、37℃で湿気に露出した結果として、変性又は凝集し、生物活性の損失と、及び有効性が変化する可能をもたらす。関与する機構に応じて、抗体安定化のために合理的な方策を考案することができる。例えば、凝集機構が、チオ−ジスルフィド交換を通じた分子間S−S結合形成であることが発見される場合、安定化は、スルフヒドリル残基の修飾、酸性溶媒からの凍結乾燥、水分含有量の制御、適切な添加剤を使用、特定のポリマーマトリクス組成物の開発、によって取得され得る。
徐放性抗体組成物は、抗体又はそれらの結合断片の、リポソーム封入体も含む。抗体を含有するリポソームは、周知の方法で調整され、例えば、DE 3,218,121;Epsteinら著、1985、米国科学アカデミー紀要(Proc.Natl.Acad.Sci.USA)82:3688〜3692;Hwangら著、1980、米国科学アカデミー紀要(Proc.Natl.Acad.Sci.USA)77:4030〜4034;EP 52,322;EP 36,676;EP 88,046;EP 143,949;EP 142,641;日本国特許出願第83−118008号;米国特許第4,485,045号及び同第4,544,545号;並びにEP 102,324を参照されたい。通常、リポソームは、小(約200〜800オングストローム)単層型であり、脂質含有量は、約30モル%コレステロールよりも高く、選択される割合は、最適な抗体治療のために調節される。
治療上採用される有効量の抗体は、例えば、療法及び治療目的、投与経路、治療又は療法を受ける患者の年齢、状態、及び体重、及び患者に提供される助剤又は補助治療に依存する。したがって、施術者は、最適な治療効果を得るために、用量を滴定し、投与経路を必要に応じて変更する必要があり、所定の役割となる。典型的な日用量は、上記の要因に応じて、約1mg/kg〜最大約100mg/kgまで、好ましくは、1日当たり約1〜約10mg/kgの範囲であり得る。典型的に、医師は、望ましい効果を得る用量に到達するまで、抗体を投与する。この治療の過程は、従来のアッセイによって容易に監視される。
請求される製剤は、透明な溶液として、あるいは水、保存剤、及び/又は賦形剤、好ましくは、リン酸緩衝液及び/又は生理食塩水及び選択された塩を水溶性希釈剤に含有する第2のバイアルで再構成される凍結乾燥した組織因子抗体のバイアルを含む二重バイアルとして、患者に提供することができる。単一溶液バイアル又は再構成を必要とする二重バイアルのいずれかを複数回再利用することができ、及び患者治療の単一又は複数サイクルを満たすことができ、したがって、現在使用可能であるよりも多くの便宜的な治療レジメンを提供することができる。
請求される本製造品は、即時から、24時間以上の期間にわたる投与に有用である。したがって、今回請求される製造品は、患者に著しい利益を提供する。本発明の製剤は、約2度〜約40度の温度で、任意で安全に保管し、長期間タンパク質の生物活性を保持することができ、したがってパッケージラベルは、溶液が6、12、18、24、36、48、72、又は96時間以上にわたって保持及び/又は使用され得ることを示すことができる。
請求される製品は、薬局、クリニック、又は他のかかる機関及び施設に提供することによって、少なくとも1つの抗組織因子抗体を、乾燥粉末として、事前に測定された量の抗体を含有する単一バイアルとして、又は抗体の滅菌溶液として、間接的に患者に提供することができる。少なくとも1つの抗体は、より小さいバイアルに移すために1回又は複数回抽出できる溶液として調製し、薬局又はクリニックから顧客及び/又は患者に提供することができる。
単一バイアルシステムを含む認知された装置には、当該技術分野において知られる類似物、例えば、ベクトン・ディケンセン(Becton Dickensen)(Franklin Lakes、NJ、www.bectondickenson.com)、Disetronic(Burgdorf,Switzerland,www.disetronic.com;Bioject,Portland,Oregon(www.bioject.com)、National Medical Products,Weston Medical(Peterborough,UK,www.weston−medical.com)、Medi−Ject Corp(Minneapolis,MN,www.mediject.com)によって製造又は開発されるような、BD Pens、BD Autojector(登録商標)、Biojector(登録商標)、Needle−Free Injector(登録商標)、Intraject(登録商標)、Medi−Ject(登録商標)、等の溶液を送達するための「ペン注射器」装置等の自己注射器を含む。二重バイアルを含む認知された装置は、乾燥凍結された薬物を、HumatronPen(登録商標)等の再構成溶液の送達用カートリッジ中に再構成するためのそれらのペン−注射器システムを含む。
今回請求される製品は、包装材を含む。包装材は、規制当局によって必要とされる情報に加えて、製品を使用できる条件を提供する。本発明の包装材は、患者に、水溶性希釈剤中の少なくとも1つの組織因子抗体を再構成し溶液を形成する、及び2つのバイアル湿/乾製品の場合には、2〜24時間にわたって溶液を使用する、という指示を提供する。単一バイアルの溶液製品の場合、ラベルは、かかる溶媒を2〜24時間以上にわたって使用できることを示す。今回請求される製品は、ヒト医薬製品使用に有用である。
本明細書に記載される、安定製剤又は保存製剤、あるいは溶液の、いずれかにおける組織因子抗体は、本発明に従って、多様な送達経路及びSC又はIM注射、経皮、肺、経粘膜、移植、浸透圧ポンプ、カートリッジ、マイクロポンプあるいは当業者に理解される他の手段を含む方法を介して、当該技術分野においてよく知られるように、患者に投与することができる。
8.治療適用
TF拮抗体である、本発明のCNTO 860抗体Fc変異体は、TF活性に関連する疾患の阻害及び予防に有用である。TF及びTFを含む複合体に関連した1つ以上の生物活性の阻害を通じた本発明の方法においてTF拮抗体を用いる治療によって、多数の病態が改善される。したがって、本発明の抗体又はその特定の変異体を使用して、細胞、組織、器官、又は動物(哺乳類及びヒトを含む)に作用し、TFによって媒介、影響、又は調節される少なくとも1つの状態の、診断、監視、制御、治療、緩和、発症予防の援助、又は症状の低減、を行うことができる。
「治療上有効な量」という用語は、哺乳類における疾患又は疾病の治療に有効な薬物の量を意味する。癌の場合においては、治療上有効量の薬物は、癌細胞の数を低減し、腫瘍サイズを減少させ、がん細胞の抹消器官への浸潤を阻害し(すなわち、ある程度遅延させ及び好ましくは停止させる)、腫瘍転移を阻害し(すなわち、ある程度遅延させ及び好ましくは停止させる)、ある程度腫瘍の成長を抑え、又は疾患に関連した1つ以上の症状をある程度緩和し得る。「治療」という用語は、治療上の処置、及び予防又は回避手段の両方を意味する。治療を必要とする者は、既に疾患を持つ者並びに疾患を予防する予定の者を含む。
TF関連病理には、様々な形態の固形初期癌、血管新生に関連する疾患、及び慢性血栓塞栓症又は深部静脈血栓症等の血管疾患を含むフィブリン形成に関連する疾患等の凝血に関連する疾患、糖尿病、動脈血栓症、脳卒中、腫瘍転移、移植臓器、組織、又は細胞の拒絶反応、及び炎症、敗血性ショック、敗血症、低血圧症、成人呼吸窮迫症候群(ARDS)、播種性血管内凝固症候群(DIC)及び他の疾患等の急性及び慢性兆候に続く血栓溶解、動脈硬化、及び再狭窄がある。
凝固カスケードの最も強力なトリガーとなる組織因子は、血糖管理が乏しい糖尿病患者において増加し、循環する組織因子微粒子はまた、プラークマクロファージのアポトーシスと関連するため、糖尿病患者においては、炎症、プラーク破裂、及び血液血栓形成の間に、並びに糖尿病とアテローム性動脈硬化との間に、因果関係を形成する。
関節リウマチ(RA)のある患者は、余命を5〜10年短縮する若年性心血管疾患を発症するリスクが2倍から5倍高い。アテローム性動脈硬化の発病における炎症と、RAの発病におけるよく確立された炎症の機構との間には類似点が存在する。増加したレベルのTF、ウィルブランド因子及びプラスミノゲン活性剤阻害因子(PAI−)1等の凝固因子は、RA及び冠動脈疾患の両方において重要である。最近の研究は、既に疾患の早期段階にあるRA患者における内皮機能不全を示した。全身性紅斑性狼瘡(SLE)の患者は、同様に炎症自体が血管機能不全を引き起こすという指摘を示す。したがって、RA及びSLEを持つ対象の治療は、抗凝固治療、並びに本発明のCNTO 860抗体Fc変異型による細胞表面におけるTFの活性の低減、という点から有利であり得る。
血栓症と悪性疾患との間の関連は、数世紀にわたって知られている(Trousseauら著、Lectures on clinical medicine,R Hardwicke,London(1867))。中でも、良性及び悪性腫瘍にはいずれも、子宮頚部、肛門癌及び口腔癌、胃、結腸、膀胱、直腸、肝臓、膵臓、肺、胸、至急子宮頚、子宮体、卵巣、前立腺、睾丸、腎臓、脳/cns(例えば、グリオーマ)、頭及び首、目又は接眼レンズ、喉、皮膚黒色腫、急性リンパ性白血病、急性骨髄性白血病、ユーイング肉腫、カポジ肉腫、基底細胞癌、及び扁平細胞癌、小細胞肺癌、絨毛癌、横紋筋肉腫、血管肉腫、hemangioendothelioma、ウィルムス腫瘍、神経芽細胞腫、口/咽頭、食道、甲状腺、腎臓及びリンパ腫などの多様な癌が挙げられ、これらは本発明の抗TF抗体を使用して治療され得る。癌における血栓塞栓症の臨床症状は、深い静脈塞栓、静脈血栓、肺塞栓、散在性血管内凝固、門脈塞栓、及び動脈血栓塞栓症を含む。
したがって、本発明は、細胞、組織、臓器、動物、又は患者において、少なくとも1つの悪性疾患、又は悪性疾患に関連する病態(例えば、血栓塞栓性合併症)を制御又は治療するための方法を提供し、急性前骨髄球性白血病、急性骨髄性白血病(AML)、多発性骨髄腫、及びワルデンストロームマクログロブリン血症、乳癌、直腸結腸癌、腎細胞癌、膵臓癌、前立腺癌、鼻咽頭癌、悪性組織球増殖症、腫瘍随伴症候群/悪性腫瘍に伴う高カルシウム血症、固形腫瘍、腺癌、肉腫、悪性黒色腫、血管腫、転移性疾患等の少なくとも1つを含むが、これらに限定されない。かかる方法は、任意に、かかるTF拮抗剤の投与前に、同時に、又は後に投与することによって、組み合わせて使用することができ、外部光線による、内部に配置された光源による、若しくは組成物を含有する放射性同位体としての投与による放射線治療、光線力学療法、又はTF拮抗薬を、追加治療薬、又は治療の補助形態を示す薬剤と併せて投与してもよい。癌を治療するための抗新生物組成物において適した治療薬は、化学療法剤、放射性同位体、毒素、インターフェロン等のサイトカイン、ホルモン及びホルモン拮抗薬、並びにサイトカインを標的とする拮抗薬、腫瘍細胞に関連するサイトカイン受容体又は抗原を含むが、これらに限定されない。
免疫関連疾患
本発明は、細胞、組織、器官、動物、又は患者において少なくとも1つの免疫関連疾患を制御又は治療するための方法も提供し、関節リウマチ、若年性関節リウマチ、全身性発症若年性関節リウマチ、乾癬性関節炎、屈曲脊椎炎、胃潰瘍、血清反応陰性関節症、変形性関節症、炎症性腸疾患、潰瘍性大腸炎、全身性狼瘡紅斑、抗リン脂質症候群、虹彩水晶体嚢炎/ブドウ膜炎/視神経炎、特発性肺線維症、全身性脈管炎/ウェゲナー肉芽腫、サルコイドーシス、精巣炎/精管切除術、アレルギー性/アトピー性疾患、ぜんそく、アレルギー性鼻炎、湿疹、アレルギー性接触皮膚炎、アレルギー性結膜炎、過敏性肺炎、移植、臓器移植拒絶反応、移植変対宿主疾患、全身性炎症応答症候群、敗血症候群、グラム陽性敗血症、培養陰性敗血症、真菌敗血症、好中球減少熱、尿貯留、髄膜meningococcemia、外傷/出血、火傷、電離放射線露出、急性膵炎、成人呼吸困難症候群、関節リウマチ、アルコール誘発肝炎、慢性炎症性病理、サルコイドーシス、クローン病理、鎌状赤血球貧血、糖尿病、ネフローゼ、アトピー性疾患、過敏反応、アレルギー性鼻炎、花粉症、永続性鼻炎、結膜炎、子宮内膜症、喘息、蕁麻疹、全身性アナフィラキシー、皮膚炎、悪性貧血、溶血性疾患、血小板減少、任意の臓器又は組織の移植変拒絶反応、腎臓移植拒絶反応、心臓移植拒絶反応、肝臓移植拒絶反応、膵臓移植拒絶反応、肺移植拒絶反応、骨髄移植拒絶反応(BMT)、皮膚同種移植拒絶反応、軟骨移植拒絶反応、骨移植拒絶反応、小腸移植拒絶反応、胎児胸腺移植拒絶反応、副甲状腺移植拒絶反応、任意の臓器又は組織の異種移植拒絶反応、異種移植拒絶反応、抗受容体過敏性反応、バセドウ病、レイノー病、B型インシュリン耐性糖尿病、喘息、重症筋無力症、抗体媒介細胞毒性、III型超過敏反応、全身紅斑性狼瘡、POEMS症候群(ポリニューロパシー、臓器肥大症、内分泌障害、単クローン性免疫グロブリン血、及び皮膚変化症候群)、多発ニューロパシー、臓器肥大症、内分泌障害、単クローン性免疫グロブリン血、皮膚変化症候群、抗リン脂質症候群、天疱瘡、強皮症、混合結合組織疾患、特発のアジソン疾患、糖尿病、慢性活性肝炎、原発性肝硬変、白斑、脈管炎、MI心臓切開術後症候群、IV型超過敏反応、接触皮膚炎、過敏性肺炎、同種移植拒絶反応、細胞内有機体による肉芽腫、薬物過敏、代謝/特発性ウィルソン病、ヘマクロマトーシス、α−1−抗トリプシン不全、糖尿病性網膜症、橋本甲状腺炎、骨粗鬆症、視床下部−下垂体−副腎軸評価、原発性肝硬変、甲状腺炎、脳脊髄炎、悪液質、嚢胞性線維症、新生児の慢性肺疾患、慢性閉塞性疾患(COPD)、familial hematophagocyticlymphohistiocytosis、皮膚病変、乾癬、脱毛、ネフローゼ症候群、腎炎、糸球体腎炎、急性腎不全、血液透析、尿毒症、毒性、子癇前症、OKT3治療、抗CD3治療、サイトカイン治療、化学療法、放射線治療(例えば、衰弱、貧血、悪液質等を含むが、それらに限定されない)、慢性サリチル酸中毒等の少なくとも1つを含むが、これらに限定されない。例えば、Merck Manual,12th〜17th Editions,Merck & Company,Rahway,NJ(1972,1977,1982,1987,1992,1999),Pharmacotherapy Handbook,Wellsら編、Second Edition,Appleton and Lange,Stamford,Conn.(1998,2000)を参照し、それぞれ参照することによってその全体が組み込まれる。
心臓血管疾患
本発明は、細胞、組織、器官、動物、又は患者において、少なくとも1つの心血管疾患を制御又は治療するための方法も提供し、心臓気絶症候群(cardiac stun syndrome)、心筋梗塞、鬱血心不全、脳卒中、虚血性脳卒中、出血、動脈硬化、アテローム性動脈硬化、再狭窄、糖尿病性動脈硬化性疾患、高血圧、動脈性高血圧、腎血管性高血圧、失神、ショック、心臓血管系の梅毒、心不全、肺性心、原発性肺高血圧、心不整脈、心房異所性拍動、心房粗動、心房細動(持続性又は発作性)、潅流後症候群、心肺バイパス炎症応答、無秩序又は多源性心房性頻脈、規則的狭QRS頻脈、特異的不整脈、心室細動、ヒスバンドル不整脈、房室ブロック、脚ブロック、心筋虚血性疾患、冠動脈疾患、狭心症、心筋梗塞、心筋ミオパチー、拡張型鬱血性心筋症、拘束型心筋症、心臓弁膜症、心内膜炎、心膜疾患、心臓腫瘍、大動脈及び抹消血管動脈瘤、大動脈解離、大動脈の炎症、腹大動脈及びその分岐の閉塞、抹消血管疾患、閉塞性動脈疾患、抹消アテローム性動脈硬化症、閉塞性血栓血管炎、機能的抹消動脈疾患、レノー現象及び疾患、肢端チアノーゼ、肢端紅痛症、静脈疾患、静脈血栓症、静脈瘤、動静脈瘻、リンパ水腫、脂肪性浮腫、不安定狭心症、再潅流傷害、ポンプ後症候群、虚血−再潅流傷害等の少なくとも1つを含むが、それらに限定されない。かかる方法は、少なくとも1つのCNTO 860抗体Fc変異体を含む有効量の組成物又は医薬組成物を、かかる制御、治療又は療法を必要とする、細胞、組織、器官、動物、又は患者に対して投与する工程を、任意に含み得る。
感染症
本発明は、細胞、組織、器官、動物、又は患者において少なくとも1つの感染症を制御又は治療するための方法も提供し、急性又は慢性細菌感染、細菌、ウイルス、及び菌感染を含む急性及び慢性寄生又は感染プロセス、HIV感染/HIV神経障害、髄膜炎、肝炎(A、B、又はC等)、敗血症性関節炎、腹膜炎、肺炎、喉頭蓋炎、大腸菌0157:h7、溶血性尿毒症症候群/塞栓性血小板減少性紫斑病(thrombolytic thrombocytopenic purpura)、マラリア、デング出血熱、リーシュマニア症、ハンセン病、中毒性ショック症候群、連鎖球菌筋炎、ガス壊疽、ヒト型結核菌、トリ型結核菌、ニューモシスティス・カリニ肺炎、骨盤感染症、精巣炎/精巣上体炎(epidydimitis)、レジオネラ、ライム病、A型インフルエンザ、エプスタイン・バーウイルス、ウイルス関連血球貪食症候群(vital associated hemophagocytic syndrome)、ウイルス性脳炎(vital encephalitis)/無菌性髄膜炎等の少なくとも1つを含むが、それらに限定されない。
本発明の任意の方法は、本発明のTF拮抗剤の少なくとも1つを含む、有効量の組成物又は医薬組成物を投与する工程を含み得、腫瘍成長の阻害及び予防に有用である。さまざまな形態の固形一次腫瘍を伴う多数の病態は、本発明の方法でTF拮抗剤で治療することによって改善される。
用量
典型的に、病態治療は、合計が平均で、少なくとも1つの組織因子抗体が用量につき患者の体重1キログラム当たり少なくとも約0.01〜500mgの範囲である、好ましくは、単一又は複数の投与当たり、少なくとも約0.1〜100mgの抗体/患者の体重1キログラムの範囲である、少なくとも1つのCNTO 860抗体Fc変異体組成物の有効量又は用量を、組成物に含有される特異的活性に応じて投与することによって、もたらされる。代替として、有効な血清濃度は、単一又は複数投与当たり0.1〜5000μg/ml血清濃度を含み得る。適切な用量は、医療実践者には周知であり、当然のことながら、特定の疾患状態、投与される組成物の特異的活性、及び治療を受けている特定の患者に依存する。一部の例においては、望ましい治療量に達するために、反復投与、すなわち特定の監視された量又は計量された量の反復個別投与を提供することが必要となり得、個別投与は、望ましい日用量又は効果が得られるまで繰り返される。
代替として、投与される用量は、特定薬剤の薬力学的特性等の周知の要素、及びその投与方法及び投与経路、レシピエントの年齢、健康、及び体重、症状の性質及び程度、現行治療の種類、治療の頻度、及び所望の効果に応じて異なり得る。活性成分の用量は、通常、体重1キログラム当たり約0.1〜100ミリグラムであり得る。通常、0.1〜50、好ましくは投与につき1キログラム当たり0.1〜10ミリグラムが、又は徐放性形態が、望ましい結果を得るために有効である。
体内投与に適した投薬形態(組成物)は、一般に、1単位又は容器当たり約0.1ミリグラム〜約500ミリグラムの活性成分を含む。これらの医薬組成物において、活性成分は、組成物の総重量に基づいて、通常、約0.5〜99.999重量%で存在する。
非経口投与の場合、抗体は、製薬上許容できる非経口担体と共に溶液、懸濁液、エマルション、又は凍結乾燥された粉末として製剤されるか、又は個別に提供され得る。かかる担体の実施例は、水、生理食塩水、リンガー溶液、デキストロース溶液、及び1〜10%ヒト血清アルブミンである。リポソーム及び固定油等の非水性担体を使用することもできる。担体又は凍結乾燥粉末は、等張性(例えば、塩化ナトリウム、マンニトール)及び化学安定性(例えば、緩衝剤及び保存剤)を維持する添加剤を含有することができる。製剤は、周知又は適切な技術によって滅菌される。
記載されるように、本発明のCNTO 860抗体Fc変異体は、疾患の副作用を治療、予防、又は緩和する異なる活性の投与前に、同時に、又は後に投与され得る。一態様においては、本発明のCNTO 860抗体Fc変異体の投与は、TNF拮抗薬の投与と併用され得る(例えば、インフリキシマブ、ゴリムマブ(golimulmab)、又はアダリムマブ、又はセルトリズマブペゴール等の断片、可溶性TNF受容体又は断片、エタネルセプト(enteracept)等のその融合タンパク質、又は小分子TNF拮抗剤等のTNF抗体であるが、これらに限定されない)。本発明の実施の別の態様においては、CNTO 860抗体Fc変異体は、別のモノクローナル抗体治療薬と併せて投与され得、アレムツズマブ、ゲムツズマブ、オゾガマイシン、リツキシマブ、セツキシマブ、ニモツズマブ、マツズマブ、ベバシズマブ、アブシキシマブ、ダクリズマブ、バシリキシマブ、トラスツズマブ、アレムツズマブ、オマリズマブ、エファリズマブ、パリビズマブ、デノスマブ、トシリズマブ(MRA、R1569)、又はこの特定断片、複合体、又は変異型を含むが、それらに限定されない。
本発明は一般的な用語で説明したが、本発明の実施形態は、以下の実施例において更に開示される。
実施例1:アイソタイプスイッチ型抗体の調製
腫瘍適応症に関しては、概して、IgG4ではなく、ヒトIgG1アイソタイプサブクラス抗体を使用して、腫瘍細胞死滅のADCC及びCDC機構を最大にすることが好ましい。CNTO 859のIgG1型であり、可変領域がTF8−5G9(EP0833911(B1)に開示するCDR移植抗体TF8HCDR20×TF8LCDR3)として周知の抗体に由来する抗体は、CNTO860と指定され(公開特許出願第US20050220793(A1)号)、これらの内容は、参照することにより、完全に組み込まれる。
CNTO860重鎖発現プラスミドを、プラスミドpEe6TF8HCCDR20(EP0833911(B1))からCNTO859重鎖可変領域のポリメラーゼ連鎖反応(PCR)増幅により調製した。参照用に、CNTO859(IgG4)及びCNTO860(IgG1)の両方の重鎖完全長配列を並べて図1に示し、残基の差異は太字で示す。得られたPCR産物をNcoI及びHindIIIで消化し、p1340と指定されたプラスミドの同一の制限部位にクローン化した。得られたベクターは、マウス免疫グロブリンプロモーターの一部のCNTO859 HC可変領域下流を含有していた。このベクターをXba Iで消化し、ベクターp730にクローン化した。得られた発現プラスミドであるp2401は、無傷のマウス免疫グロブリンプロモーター、CNTO859 HC可変領域、ヒトG1定常領域のエクソン、及び大腸菌グアニン・ホスホリボシルトランスフェラーゼの遺伝子を含有していた。p2401のHC可変領域を配列決定し、PCR又はクローニングエラーを含まないことが発見された。
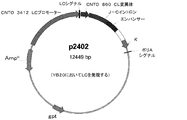
CNTO860軽鎖発現プラスミドをプラスミドpEe12TF8LCDR3(EP0833911(B1))からCNTO859軽鎖可変領域のポリメラーゼ連鎖反応増幅により調製した。得られたPCR産物をBglII及びSalIで消化し、p2287の同一の制限部位にクローン化した。得られたベクターは、マウスκプロモーターのCNTO859LC可変領域下流を含有していた。このベクターをHindIIIで消化し、ベクターp95にクローン化した。得られた発現プラスミドであるp2402(図4)は、マウスκプロモーター、CNTO859LC可変領域、ヒトκ軽鎖定常部、及び大腸菌グアニン・ホスホリボシルトランスフェラーゼの遺伝子を含有していた。p2402のLC可変領域を配列決定し、PCR又はクローニングエラーを含まないことが発見された。
CNTO860発現プラスミドであるp2401及びp2402を安定した発現のためにNSO細胞にトランスフェクションした。
実施例2:変異型の産生
抗組織因子AbであるCNTO 860のヒトIgGlAbsのFcγR結合及びADCC活性を増強するための、特異的なFcアミノ酸置換の効果が評価されており、その置換は、Xencor社に付与された米国特許出願第20060483250号に開示されている。本明細書に具体的に記載するFc置換に加え、本発明は、上記の特許出願並びに他の資料に記載される、A330L、S298A/E333A/K334A、及びS239D/I332E/A330L等の他のFc置換の使用を企図する。
CNTO 860重鎖(配列番号:2)の3つの単一突然変異体(I332E、A330Y、A330I)及び3つの二重突然変異体(A330I/I332E、V264I/I332E、S239D/I332E)を、CNTO 860(配列番号:1)をコード化するDNAを変異させて調製した。残基226から始まるCNTO 860のヒンジ及びFc領域のアミノ酸配列(非置換の配列は、野生型又はWTと称される)が図1に見られる。変異重鎖遺伝子は、一過性トランスフェクションを経たCHO細胞又は安定トランスフェクションを経たラットYB2/0細胞のいずれにおいても、哺乳類細胞内の正常なCNTO 860軽鎖(配列番号:4)とともに発現した。一過性トランスフェクションにより生成物のより適切な産生が可能になるが、効果的な一過性トランスフェクションプロトコルは、YB2/0細胞株に対しては確立されていない。
両CHO及びYB2/0細胞における各CNTO 860 Fc−変異体の発現により、得られた抗体は宿主細胞に特有のグリカン(N結合型糖鎖付加)により装飾される。典型的に、CHO細胞は、95%フコシル化されたAbを産生し、YB2/0細胞は、典型的に、40〜60%フコシル化されたAbを産生する。Fcグリカンにおけるコアフコース含量の低下は、ADCC効力を増強することが分かっている。したがって、異なる宿主細胞からの抗体の対を評価して、アミノ酸変化並びに異なるグリカン構造により生産された、Fcを媒介した生物活性への影響がサブ添加的、添加的、又は相乗的かどうか測定することができる。
発現プラスミドの調製
DNA変異誘発を実行する前に、より便利な制限部位を有するシャトルベクターを、事前に調製したセントコー(Centocor)社のプラスミドであるp1483から、cDNAフォーマットでヒトIgG1定常部コード配列の全てを含む2.4kbのSpeI−HindIII断片を、pBC(ストラタジーン社)の3.4kbのSpeI−HindIIIベクター骨格に移入して調製した。得られたプラスミドをp4114と称した。次いで、6つのCNTO 860変異体のそれぞれの重鎖をコード化する発現プラスミドを2段階プロセスで構築した。第1に、クイックチェンジII部位特異的変異誘導(QuikChange II Site-Directed Mutagenesis)キット(ストラタジーン社)、表3に記載したプライマー、及び鋳型としてプラスミドp4114を使用して、所望の変異をp4114に導入した。
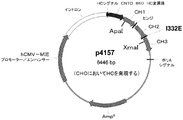
変異誘発の成功と及び偶発性変異の非存在は、それぞれの変異の全体の定常部コード配列のDNA配列決定により確認された。第2に、変異誘発されたp4114シャトルベクター内の変更された配列にまたがる制限断片を、最終発現ベクターに導入した。CHO細胞発現のためのプラスミドの場合では、これは、CMVプロモーターの後ろのCNTO860重鎖の野生型をコード化する発現プラスミドであるp4145内の対応する断片の代わりに、変異誘発されたp4114プラスミドから0.7kbのApaI−XmaI断片の導入を要した。得られたプラスミドの1つである、I332E変異体をコード化するp4157を、CNTO 860軽鎖をコード化する、事前に調製されたプラスミドであるp4146と共に図2に概略的に示す。CHO発現のための他の変異体をコード化する重鎖プラスミドは図4に記載する。
YB2/0細胞内のCMV駆動Ab遺伝子の非常に低い発現の報告により、YB2/0細胞発現では、CHO細胞で使用した同一のプラスミドは使用しなかった。その代わり、高発現のマウス/ヒトキメラ抗ヒトCD4抗体である、抗体CNTO3412の重鎖遺伝子プロモーターの後ろのCNTO 860重鎖をコード化する、事前に調製された発現プラスミドである、p2401内の対応する断片の代わりに変異誘発されたp4114プラスミドからの2.3kbのSpeI−SalI断片をクローン化した(ルーニー(Looney)JEら著、1992年、Hum Antibod Hybrid、3(4):191)。得られたプラスミドの1つである、I332E変異体をコード化するp4148を、CNTO 860軽鎖をコード化する、事前に調製されたプラスミドであるp2402(自然の免疫グロブリン軽鎖遺伝子プロモーターにより発現が駆動される)と共に、図3に概略的に示す。YB2/0発現のために他の変異体をコード化する重鎖プラスミドを表4に記載する。
CNTO 860変異体の発現及び精製
抗体は、一過性トランスフェクションされたCHO細胞及び安定トランスフェクションされたYB2/0細胞の単離したクローンから発現した。野生型トランスフェクションを、表4に示す野生型重鎖及び野生型軽鎖発現プラスミドをそれぞれの適切な宿主細胞に同時トランスフェクションすることで行った。宿主細胞に適切な野生型軽鎖プラスミドで同時トランスフェクションしたそれぞれの異なる重鎖プラスミドを使用して、変異を移入した。
標準的なプロトコルにより、LipofectAMINE試薬(インビトロジェン社)を使用して、CHO細胞の一過性トランスフェクションを実施した。安定したYB2/0トランスフェクタントを975uFD、0.2kVでBioRadモデルを使用したエレクトロポレーションにより作製した。細胞を限界希釈で蒔き、抗ヒトIgG(Fc−特異的)ELISAによって単一コロニーをスクリーニングした。ラージスケールの培養後培地から細胞上清を得て、分泌Absを標準的なプロトコルを使用してタンパク質Aにより精製した。
実施例3.変異体の活動の評価
精製したCNTO 860変異体を末梢血単核細胞(PBMC)による抗原発現標的細胞の細胞死誘導(ADCC)の相対活性で評価した。標的細胞である、HCT116ヒト結腸直腸癌細胞をATCCから得、DMEM−10%加熱不活性化FBS+2mM L−グルタミン、1mMのピルビン酸ナトリウム、及び0.1mMの非必須アミノ酸中で培養した。細胞を2週間継代し、対数増殖期で維持した。培養培地及びサプリメントをギブコ(Gibco)(インビトロジェン(InVitrogen)社)から購入した。実験日に、細胞をトリプシン処理により除去し、2度洗浄した。細胞を培養培地で1×106細胞/mlに調整し、15ulのBATDA蛍光標識試薬(デルフィア(Delfia)EuTDA サイトトキシティ(Cytotoxicity)キット内、パーキンエルマーライフサイエンス社(Perkin-Elmer Life Sciences))を5mlの細胞に添加した(ブロムバーグ(Blomberg)Kら著、1996年、免疫学ジャーナル(J.Immunol.)方法(Methods)193:199〜206)。時々振とうして、細胞を37℃で30分間インキュベートし、次いで、培地で2度洗浄した。エフェクター細胞と混合する直前に、標的細胞を遠心分離し、培養培地中の2×105細胞/mlで再懸濁した。エフェクター細胞であるPBMCを健康な提供者のヘパリン添加血液から単離した。血液試料をリン酸緩衝食塩水(PBS)で希釈し、PBMCをFicoll−Hypaque(アマシャム社)の密度勾配遠心分離法により単離した。遠心分離後、PBMCを収集し、2度洗浄し、5%のCO2で37℃の培養培地中に一晩保持した。翌日、PBMCを回収し、洗浄し、1×107細胞/mlの培地中に再懸濁した。100μlの培養培地中の抗体希釈物を丸底の96−ウェルプレートに添加した。50μlのエフェクター細胞及びユウロピウム標識標的細胞を、50:1のエフェクター細胞対標的細胞の比率でAb希釈物に添加した。エフェクター細胞及び標的細胞が互いに接触するようにプレートを短期間遠心し、次いで、5%のCO2の環境で37℃で2時間インキュベートした。インキュベート後、20μlの上清を平底の96ウェルプレートのウェルに移し、200μlアリコートのユウロピウム増強試薬(デルフィア・サイトトキシティ(Delfia Cytotoxicity)キット内)をそれぞれのウェルに添加した。プレートを10分間振とうした後、蛍光を時間分解蛍光光度計(エンビジョンインスツルメント(EnVision instrument)、パーキン−エルマー(Perkin-Elmer)社)内で測定した。特定の細胞毒性の割合を(実験的放出−自然放出)/(最大放出−自然放出)×100で算出した。標的細胞をエフェクター細胞の代わりに培地でインキュベートして自然放出を決定し、10μlのジギトニン(デルフィア(Delfia)EuTDA サイトトキシティ(Cytotoxicity)キット内)含有溶解液で標的細胞をインキュベートして最大放出(100%溶解)を決定した。試料は三つ組みで試験し、示す結果は、2つ又は3つの独立したの実験の代表である。
図4は、実施したADCCアッセイデータの代表的な一連の曲線を示す。CHO細胞内で産生された抗体では、CHO細胞内に発現した単一変異体CNTO 860のI332Eは、ADDCアッセイにおいて約7倍以上強力であり、つまり、3ng/mlで存在するCHO−CNTO 860 WTは25%の特異的溶解をもたらし、CHO−CNTO 860のI332Eは、ほんの約0.4ng/mlで25%の溶解をもたらした(図4)。同様に、WTで0.65ng/mlと比較して、0.08ng/mlが25%の溶解をもたらしたため、YB2/0細胞内に発現した単一変異体CNTO 860のI332Eは、約8倍以上強力であった。
全ての変異体を同一条件下で比較した別の実験において、試験した変異体又はWT抗体の全てが100%の溶解をもたらさなかったため、25%の溶解をもたらすために必要な抗体の濃度を比較基準(表5)として使用した。
表5に示すデータは、YB2/0細胞内に発現した抗体の変異体が、概して、インビトロADCCアッセイにおいて、CHO細胞内に発現した同一の変異体よりも約10倍より強力であることを裏付けた。全体的に見て、I332Eの変異が最も大きい効果を有し、同一の宿主株内に発現したWTに関して、変異が単独である又は他の変異と存在しているそれぞれの変異体が、3〜10倍増加した効果を示した。他のCHO細胞内で発現させた変異体の中で、A330Y単一変異体は、ADCC活性がほとんどないか、又は全く増強していないようであり、及びA330Iは、ADCC活性を減少しているようであった。二重変異体は、I332E単一変異体よりも強い効力を呈しなかったが、CHO細胞内に発現させた3つの二重変異体は、全てCNTO 860 WTよりも強い活性を呈したが、二重変異体はI332E単一変異体以上に強い効果は示さなかった。
YB2/0細胞発現により発現させたI332E変異体も、CHOに由来する試料で見られたものほどではないが、この宿主細胞により発現させた3つの単一変異体の中で最も効力が強いと思われた。I332E、A330Y、及びA330I変異体は、それぞれ、YB2/0細胞からのWTコントロールよりも数倍強い効力を呈した。YB2/0細胞内に発現させた二重変異体は、全てYB2/0−CNTO 860 WTよりも5〜8倍強い活性を呈した。
これらのデータは、CHO及びYB2/0宿主細胞株を使用して産生された、それぞれの変異体へのFcグリカン構造の効果を、単独及びFc領域置換と組み合わせて示す。酵素的に脱グリコシル化したCNTO 860(Gno)は、脱グリコシル抗体ですでに報告されているとおり、全ての濃度において基底値での特定の溶解のみを産生した。重要なことは、I332E変異体及び低フコースグリカン構造(YB2/0産生材料)の組み合わせは、25%の溶解を達成するのに必要とされる濃度がYB2/0−CNTO 860 I332Eで著しく減少し(0.08ng/ml)、CHO宿主細胞からのWT CNTO860からの効力においてほぼ100倍減少したため、相加効果又は相乗効果さえ有するように思われた。溶解の特定の程度を達成するのに必要なAbの量を減少する以外に、I332E変異体は、例えば、YB2/0−CNTO 860のI332Eの70%の溶解に対し、YB2/0−CNTO 860 WTは50%の溶解であるなど、より高い最大溶解をもたらした(図4)。
実施例4.変異体によるヒト受容体結合
実施例2で産生した重鎖CNTO 860抗体のFc変異体を使用して、FcγRI、FcγRII、FcγRIII、及びFcγRIVを含む、Fc受容体ガンマタイプ(FcγR)と総称され周知のFcドメイン結合受容体における変化を評価した。上記のとおり、受容体を細胞媒介抗体機能の活性化型又は抑制型として分類することができる。
FcγRI(CD64)ELISA−ELISA緩衝液(PBS、pH7.4、4mg/ml BSA、0.01%Tween−20)中の50μlの1μg/mlの組み換え型ヒトHisタグFCγRI(細胞外ドメイン、R&Dシステムズ(R&D Systems)社、#1257−FC−050)の溶液をHisGrab 96ウェルプレート(ピアス社(Pierce)#15142)のそれぞれのウェルに添加した(パワーズ(Powers)G.、ノートブック(Notebook)9006、166〜168ページ、180〜181ページ)。プレートを振とうして3時間インキュベートした。プレートをプレート洗浄器上で300μlの洗浄緩衝液(PBS、0.01%Tween−20)で3回洗浄した。CNTO860抗体Fc−変異体試料の連続希釈をELISA緩衝液で作製し、ウェル当たり50μlのそれぞれの滴定をFCγRI被覆プレートに2回添加した。プレートを振とうしながら1時間インキュベートした。プレートをプレート洗浄器上で300μlの洗浄緩衝液で3回洗浄した。ELISA緩衝液中のHRP標識ヤギF(ab’)2抗ヒトIgGF(ab’)2(ジャクソン・イムノリサーチ(Jackson ImmunoResearch)社、#109−036−097)の1:10,000の希釈物50μlをそれぞれのウェルに添加した。プレートを振とうしながら30分間インキュベートした。プレートをプレート洗浄器上で300μlの洗浄緩衝液で3回洗浄した。ウェル当たり50μlのTMB基質(RDI社、#RDI−TMBSU−1L)をそれぞれのウェルに添加し、5分間展開させた。反応は、100μlの0.2N硫酸を添加して停止した。プレートを、OD450でエンビジョン(EnVision)社のプレートリーダーで解析した。
FcγRIIa(CD32a)アルファスクリーン−ニッケルアクセプタービーズ(パーキンエルマー(PerkinElmer)社、#6760619)を分析緩衝液内(PBS、pH7.4、4mg/mlBSA、0.01%Tween−20)で1:50に希釈し、27.5μlをNuncV底ポリプロピレンプレート(VWR社、#62409−108)のそれぞれのウェルに添加した(Powers G.,Notebook 9006,pp.169〜175,182〜185)。2.0μg/mlの組み換え型ヒトHisタグFcγRIIa(R&Dシステムズ(R&D Systems)社、#1330−CD−050)の溶液を分析緩衝液内で調製し、27.5μlを個別のNuncV底ポリプロピレンプレートのぞれぞれのウェルに添加した。試験するAbをNuncV底ポリプロピレンプレート内で調製し、滴定を30μg/mlで開始した。2μg/mlのビオチン化ヤギF(ab’)2抗ヒトIgGF(ab’)2(ジャクソン・イムノリサーチ(Jackson ImmunoResearch)社、#109−066−097)の溶液を分析緩衝液で調製し、27.5μlを別のNuncV底ポリプロピレンプレートのそれぞれのウェルに添加した。ストレプトアビジンドナービーズ(パーキンエルマー(PerkinElmer)社、#6760002)の1:50の希釈溶液を分析緩衝液で調製し、27.5μlを別のNuncV底ポリプロピレンプレートのそれぞれのウェルに添加した。5μlの量のそれぞれの試薬を、低容量で非結合性の白い384−ウェルプレート(コーニング(Corning)社、#3673)の対応する四分円に、アクセプタービーズ、FcγRIIa、試験Ab、ビオチン化した抗ヒトIgGF(ab’)2、及びストレプトアビジンドナービーズの順序で添加した。プレートを振とうしながら30分間インキュベートし、エンビジョン(EnVision)社のプレートリーダーを使用してODを決定した。
FcγRIIb(CD32b)アルファスクリーン−FcγRIIaをFcγRIIb(R&Dシステムズ(R&D Systems)社、#1875−CD−050)で置換した以外、上記のとおり、FcγRIIaに対する分析を実施した。
異なる変異体のFcγRIIbへの結合は、他の2つのヒト受容体との結合よりも高い変動を呈した(表6)。より高い親和結合は、S239D/I332Eの二重変異体によるもであり、WTよりも約6倍の増強であった。最も弱い結合は、A330Iの単一変異によるものであり、親和結合において、500倍の減少であった。A330I/I332Eの二重突然変異体は、WTと同一の親和性でFcγRIに結合したが、FcγRIIa受容体をWTより30〜100倍弱く結合した。他のFcγRsへ高い結合の一方での、FcγRIIbへの結合の低下は、免疫エフェクター細胞が両方の受容体型を発現する場合(例えば、マクロファージ)等に、より高い治療効果を提供し得るため、そのような受容体結合プロファイルは、特に興味深いものであり得る。
FcγRIIIa(CD16a)ELISA−ヒトFcγRIIIa結合アッセイを、上記のFcγRIのアッセイに類似したELISAフォーマットで行った。ELISA緩衝液(PBS、4mg/mlBSA、0.01%Tween−20)中の組み換え型ヒトHisタグFcγRIIIa(セントコー(Centocor)社で作製した細胞外領域)の2μg/mlの溶液50μlを、HisGrab 96ウェルプレート(ピアス(Pierce)社#15142)のそれぞれのウェルに添加した(パワーズ(Powers)G.、ノートブック(Notebook)10378、19−23)。プレートを室温で3時間インキュベートした。プレートを200μlのスターティングブロック(Starting Block)(ピアス(Pierce)社、#15142)で30分間ブロック処理し、次いで、プレート洗浄器上で300μlの洗浄緩衝液(PBS、0.01%Tween−20)で3回洗浄した。CNTO 860テスト試料の連続希釈溶液をスターティングブロック(Starting Block)緩衝液で作製し、ウェル当たり50μlのそれぞれの滴定を、FcγRIIIaでコーティングされたプレートに2回添加した。プレートを1時間インキュベートした。プレートをプレート洗浄器上で300μlの洗浄緩衝液で3回洗浄した。スターティングブロック(Starting Block)中のHRP標識ヤギF(ab’)2抗ヒトIgGF(ab’)(ジャクソン・イムノリサーチ(Jackson ImmunoResearch)社#109−036−097)の1:10,000の希釈物50μlを、それぞれのウェルに添加した。プレートを30分間インキュベートした。プレート洗浄器上で300μlの洗浄緩衝液で3回洗浄した。ウェル当たり50μlのTMB基質(RDI社#RDI−TMBSU−1L)をそれぞれのウェルに添加し、3〜5分間展開させた。反応は、100μlの0.2N硫酸を添加して停止した。プレートをエンビジョン(EnVision)社のプレートリーダーを使用して、OD450で解析した。抗体濃度対OD450値をそれぞれのCNT O860変異体でグラフ化し、0.14のOD450読み取りを達成するために必要な抗体濃度をそれぞれのデータ曲線内を補間することで確定した。
ヒトFcγRIの場合では、結果は、抗体がCHO細胞又はYB2/0細胞内に発現したかに関わらず、重鎖の単一及び二重変異体は、抗体の親和結合に対し比較的わずかな影響を生み出したことを示した。YB2/0に由来する変異体の結合は、概して、CHOに由来する変異体による結合に類似し、FcγRIがAbフコースレベルに敏感ではないという以前の所見に一致する。I332E変異体は、2倍結合が増強したことを示し、A330I変異体は、2〜3倍の結合の減少を示しているように思われ、いくつかの変異体は、CNTO 860 WTと同一の結合を示した。ヒトFcγRI結合で分析した全ての変異体からのデータの概要を、アッセイにおいて1.0のOD値を得るために必要とされるAbの量として示し、表Xに示す。示した値は、ELISAアッセイにおいて1.0のOD値を得るために必要とされるCNTO 860変異体の濃度(ng/ml)である。
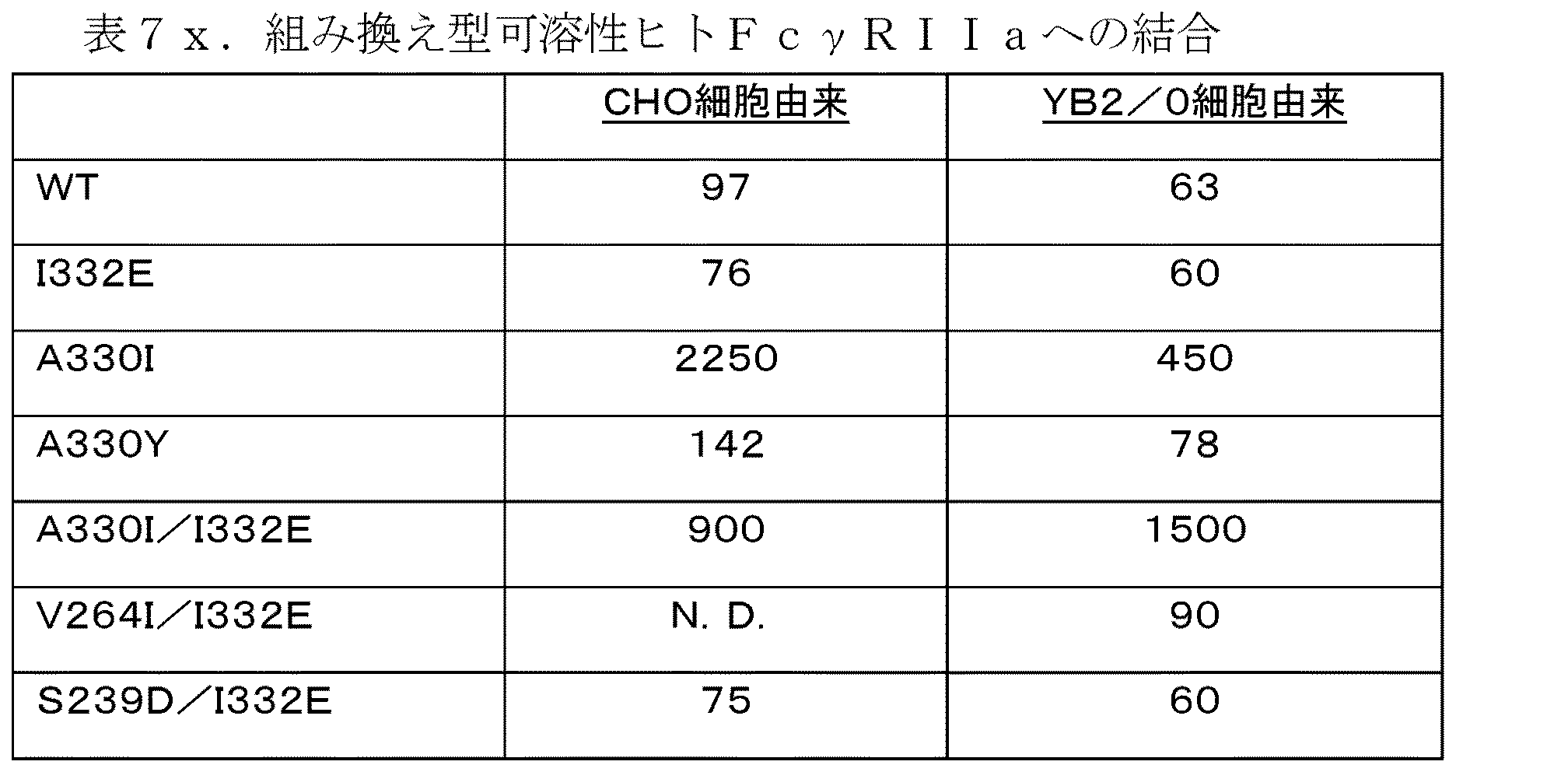
ヒトFcγRIIa結合分析(表7)からの結果は、WTと同様の結合を呈するI332E単一変異体及びS239D/I332E二重変異体を除き、変異体は、CNTO 860 WTよりも可溶性の組み換え型ヒトFcγRIIaへより弱く結合したことを示した。しかしながら、互いに関連する異なる変異体による結合は、Xencorの出版で報告されているものと同様のパターンに従い、これらの実験におけるCNTO 860 WTにより認められた結合が異常に高いという可能性を少なくとも高めた。興味深いことに、A330I/I332Eの二重変異体は、これらの2つの試料によるFCγRIへの結合において相違はなかったが、200,000のODシグナルを送るためにWTよりも約20倍高い濃度で存在する必要があった。FcγRIIa受容体は、単核球及びマクロファージに加え、血小板で発現するため、それへのAb結合は、Ab最適化効果の間に考慮されなければならない。示した値は、ELISAアッセイにおける1.0のOD値を得るために必要とされるCNTO 860変異体の濃度(ng/ml)である。
FcγRIIbへの異なる変異体の結合は、他の2つのヒト受容体との結合よりも高い変動を示した(表8)。最も強力な結合は、S239D/I332E二重変異体により約6倍増強され、最も弱い結合は、A330I単一変異体により結合が500倍減少された。FcγRIIa受容体で言及したのと同様に、A330I/I332E二重変異体は、WTと同一の親和性でFcγRIに結合したが、WTよりも30〜100倍弱く結合した。他のFcγRsへ高い結合の一方での、FcγRIIbへの結合の低下は、免疫エフェクター細胞が両方の受容体型を発現する場合(例えば、マクロファージ)等に、より高い治療効果を提供し得るため、そのような受容体結合プロファイルは、特に興味深いものであり得る。アルファスクリーン(AlphaScreen)アッセイにおいて200,000のO.D値を得るために必要とされるCNTO 860変異体の濃度(ng/ml)の値を、表Xにおいて示す。
結果は、A330I単一変異体を除き、それぞれの変異体がCNTO 860 WTよりもFcγRIIaにより強く結合したことを示す。材料の供給が限定されているため、一部の試料では完全な曲線は得られなかったが、CHO細胞からのI332E変異体は、CHO細胞からのWT(表9)よりも6倍以上結合するように思われ、YB2/0細胞からのI332Eは、YB2/0細胞からのWT(表9)より約10倍以上結合した。CHO細胞からのA330I/I332E変異体は、CHO細胞からのWTよりも約8倍以上結合し、YB2/0細胞からのA330I/I332Eは、YB2/0細胞からのWTよりも6倍以上結合した。一般的に、YB2/0細胞内に発現したAbは、それらのCHO由来の対照物よりも約10倍以上結合すると思われる。FcγRIIaは、ADCCアッセイに使用したPBMC群における一次エフェクター細胞である、NK細胞上の唯一のFcγRであるため、これらの結合の結果は、確定したADCCデータとも相関して示さなければならない。更に、FcγRIIa結合データは、アミノ酸置換をYB2/0細胞からの低フコースグリカン構造と組み合わせるときの相加/相乗効果を示して、ADCCデータを裏付ける。例えば、CHO細胞からのI332Eは、CHO細胞からのWTよりも6倍以上結合し、YB2/0からのWTは、CHOからのWTよりも約3倍以上結合し、YB2/0細胞からのI332Eは、CHOからのWTよりも約30以上倍結合する。ヒトFcγRIIa結合で分析した全ての試料の概要を表9に示す。示した値は、ELISAアッセイにおいて、0.14のO.D値を得るために必要とされるCNTO 860変異体の濃度(ng/ml)である。
実施例5:変異体によるマウス受容体結合
組み換え型マウスFcγRを使用して、実施例2で産生されたCNTO860抗体Fc変異体の結合親和性において変異体を評価した。
市販される組み換え型ポリヒスチジンタグ化マウスFcγR、FcγRII、FcγRIII、及びFcγRIV(R&Dシステムズ(R&D Systems)社)をPBSで1μl/mlに希釈し、100μl/ウェルを銅でコーティングしたプレート(ピアス社(Pierce))に4℃で一晩捕捉した。プレートを洗浄緩衝液(0.15M NaCl、0.02%Tween−20)で3回洗浄した。200μl/ウェルのSuperBlack(ピアス社(Pierce))を使用して、非特異性結合を室温で15分間ブロックした。プレートを上記のとおり3回洗浄した。PBS中に連続希釈されたCNTO 860野生型抗体及び変異抗体は、室温で1時間100μl/ウェルの量で結合させた。結合していないmAbを除去するために、上記のとおりプレートを3回洗浄した。室温で1時間インキュベートしたPBS中で1:5,000に希釈したのHRP標識ヤギF(ab’)2抗ヒトIgGF(ab’)2(ジャクソン・イムノリサーチ社(Jackson ImmunoResearch))を100μl/ウェル使用して、プレートに結合させた受容体に結合したCNTO 860変異体を検出した。プレートを上記のとおり洗浄緩衝液を使用して5回洗浄し、TMB Stable Stop基質(フィツジェラルド・インダストリーズ社(Fitzgerald Industries))を100μl/ウェル使用して展開し、0.5M HClで停止させた。吸光度を450nmで検出した。
CNTO 860変異体は、5〜10倍弱い結合のCNTO 860 WTより5〜10倍高い親和性結合の範囲でマウスFcγR結合における変化を示した。OD450(結合材料)対濃度としてプロットされたとき、4つのマウスFc受容体のそれぞれに結合したCNTO 860 WT、CNTO 860I332E、及びCNTO 860のA330I/I332Eのデータは、典型的なS字型結合曲線を生じた(データは表示せず)。
それぞれの変異体の相対的結合親和性を要約し比較すると、1.0のOD読み取り値(「ECOD1」)を得るために必要とされる濃度は、結合曲線から補間された(表10に示す)。全ての結合アッセイは、コーティングしたEIAプレート上で捕獲した組み換え型可溶性受容体を用いた、ELISAフォーマットであった。示した値は、1.0のO.D.値をもたらすために必要とされるCNTO 860変異体の濃度(ng/ml)である。1.0のODは、全ての試料の反応曲線の線形部分に対応する濃度として選択された。
概して、異なる変異体による相対結合は、ヒト受容体で認められたパターンに従う傾向があり、例えば、I332E変異体はFcγRI及びFcγRIIの両方に2〜4倍更に結合し(それぞれ、ヒトFcγRI及びFcγRIIb)、A330Yは、FcRII並びにWTに結合するが、A330Iはより弱い結合である。注目すべき例外の1つは、抑制型マウス受容体FcγIIにWTと同様に強く、又はそれよりも強く結合するのに対し、抑制型ヒト受容体であるFcγRIIbに実質的にWTよりも弱く結合する、A330I/I332E変異体である。WTに相対するI332Eによる抑制型マウス受容体FcRIIへの増強した結合は、マウスFcγRIII及びFcγRIVで認められた増強の程度(3〜6倍)に類似し、後に活性化する受容体への増強した結合の有益な効果は、免疫細胞が両方の受容体型を発現するときに、抑制型受容体により増強した結合によって相殺し得る可能性を高めるという見解は興味深い。
実施例2で産生した変異体を使用した実施例3〜5における実験的所見の概要:
CNTO 860に対し、本明細書に記載する特定のCNTO 860配列変異は、FcγRIIb抑制型受容体の親和性の減少を示し、抑制受容体への結合は、Abの有効性を減少することが認められているため、それは、生体内のより高い有効性をもたらし得る。特に、YB2/0細胞内に発現したA330I変異体は、FcγRIIaへの結合をほんの適度に減少(8×)させ、FcγRIへの結合をほんのわずかに減少(2×)させ、NK媒介のADCCにおける活性をわずかに増加(2〜3×)させながら、FcγRIIbへの結合を著しく減少(500×)させた。したがって、低フコースグリカンと結合したアミノ酸変異体の相加効果は、修飾のみで同一のAbよりも強力にAb変異体を産出した。
A330I変異体及びI332E変異体を混合する効果は、Fcエンジニアリングにおいて新しい方法であり、FcγRIの高い親和性を維持するAb変異体を産出し、記載したADCC分析において活性を約10倍増強させたが(FcγRIIIa親和性に関連して)、実質的に、抑制受容体FcγRIIbの親和性を減少させたことを示した。そのようなFcγR結合プロファイルは、マクロファージ様細胞が生体内の関連する免疫エフェクター細胞であるときに、明白な利点を提供し得る。