JP2009535162A - 緑内障治療用二重排出経路シャントデバイス及び方法 - Google Patents
緑内障治療用二重排出経路シャントデバイス及び方法 Download PDFInfo
- Publication number
- JP2009535162A JP2009535162A JP2009509656A JP2009509656A JP2009535162A JP 2009535162 A JP2009535162 A JP 2009535162A JP 2009509656 A JP2009509656 A JP 2009509656A JP 2009509656 A JP2009509656 A JP 2009509656A JP 2009535162 A JP2009535162 A JP 2009535162A
- Authority
- JP
- Japan
- Prior art keywords
- anterior chamber
- schlemm
- canal
- aqueous humor
- eye
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000000034 method Methods 0.000 title claims description 45
- 208000010412 Glaucoma Diseases 0.000 title description 26
- 230000037361 pathway Effects 0.000 title description 13
- 230000009977 dual effect Effects 0.000 title 1
- 210000002159 anterior chamber Anatomy 0.000 claims abstract description 120
- 210000001742 aqueous humor Anatomy 0.000 claims abstract description 90
- 230000004410 intraocular pressure Effects 0.000 claims description 32
- 239000007943 implant Substances 0.000 claims description 31
- 210000001519 tissue Anatomy 0.000 claims description 23
- 210000001585 trabecular meshwork Anatomy 0.000 claims description 20
- 208000024304 Choroidal Effusions Diseases 0.000 claims description 16
- 230000010261 cell growth Effects 0.000 claims description 5
- 210000003161 choroid Anatomy 0.000 claims description 4
- 230000002401 inhibitory effect Effects 0.000 claims description 3
- 210000001508 eye Anatomy 0.000 description 71
- 210000003786 sclera Anatomy 0.000 description 32
- 239000012530 fluid Substances 0.000 description 29
- 210000000554 iris Anatomy 0.000 description 27
- 239000000463 material Substances 0.000 description 21
- 238000004891 communication Methods 0.000 description 16
- 238000001356 surgical procedure Methods 0.000 description 14
- 239000007787 solid Substances 0.000 description 13
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 11
- 208000002352 blister Diseases 0.000 description 11
- 210000000795 conjunctiva Anatomy 0.000 description 11
- 210000004087 cornea Anatomy 0.000 description 11
- 239000003814 drug Substances 0.000 description 9
- 206010052428 Wound Diseases 0.000 description 8
- 208000027418 Wounds and injury Diseases 0.000 description 8
- 238000002513 implantation Methods 0.000 description 8
- 239000003795 chemical substances by application Substances 0.000 description 7
- 229940079593 drug Drugs 0.000 description 7
- 239000004677 Nylon Substances 0.000 description 6
- 210000004027 cell Anatomy 0.000 description 6
- 210000004240 ciliary body Anatomy 0.000 description 6
- 229960004857 mitomycin Drugs 0.000 description 6
- 229920001778 nylon Polymers 0.000 description 6
- 210000001328 optic nerve Anatomy 0.000 description 6
- 239000004033 plastic Substances 0.000 description 6
- 229920003023 plastic Polymers 0.000 description 6
- 229920001296 polysiloxane Polymers 0.000 description 6
- 201000004569 Blindness Diseases 0.000 description 5
- 230000007423 decrease Effects 0.000 description 5
- 206010014801 endophthalmitis Diseases 0.000 description 5
- 210000002950 fibroblast Anatomy 0.000 description 5
- 238000001914 filtration Methods 0.000 description 5
- 208000015181 infectious disease Diseases 0.000 description 5
- 210000005239 tubule Anatomy 0.000 description 5
- 241000894006 Bacteria Species 0.000 description 4
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 4
- 210000003484 anatomy Anatomy 0.000 description 4
- 229960002949 fluorouracil Drugs 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- 238000003780 insertion Methods 0.000 description 4
- 230000037431 insertion Effects 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 102000008186 Collagen Human genes 0.000 description 3
- 108010035532 Collagen Proteins 0.000 description 3
- 206010030348 Open-Angle Glaucoma Diseases 0.000 description 3
- 239000004743 Polypropylene Substances 0.000 description 3
- 229920001436 collagen Polymers 0.000 description 3
- 239000008358 core component Substances 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 210000001232 limbus corneae Anatomy 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- -1 polypropylene Polymers 0.000 description 3
- 229920001155 polypropylene Polymers 0.000 description 3
- 201000006366 primary open angle glaucoma Diseases 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 210000003462 vein Anatomy 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- LEBVLXFERQHONN-UHFFFAOYSA-N 1-butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide Chemical compound CCCCN1CCCCC1C(=O)NC1=C(C)C=CC=C1C LEBVLXFERQHONN-UHFFFAOYSA-N 0.000 description 2
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 2
- 201000002862 Angle-Closure Glaucoma Diseases 0.000 description 2
- 208000001953 Hypotension Diseases 0.000 description 2
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 description 2
- 235000004522 Pentaglottis sempervirens Nutrition 0.000 description 2
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 2
- 229910000831 Steel Inorganic materials 0.000 description 2
- 230000003444 anaesthetic effect Effects 0.000 description 2
- 229940093906 antibiotic and corticosteroids Drugs 0.000 description 2
- 239000003855 balanced salt solution Substances 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 229960003150 bupivacaine Drugs 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 230000001886 ciliary effect Effects 0.000 description 2
- 239000000306 component Substances 0.000 description 2
- 239000000645 desinfectant Substances 0.000 description 2
- 238000001125 extrusion Methods 0.000 description 2
- 238000002695 general anesthesia Methods 0.000 description 2
- 230000023597 hemostasis Effects 0.000 description 2
- 230000036543 hypotension Effects 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 229910052740 iodine Inorganic materials 0.000 description 2
- 239000011630 iodine Substances 0.000 description 2
- 229960004194 lidocaine Drugs 0.000 description 2
- 238000002690 local anesthesia Methods 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 210000003205 muscle Anatomy 0.000 description 2
- 210000005036 nerve Anatomy 0.000 description 2
- 208000020911 optic nerve disease Diseases 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 230000001681 protective effect Effects 0.000 description 2
- 210000001747 pupil Anatomy 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 239000010959 steel Substances 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 229940124597 therapeutic agent Drugs 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- 230000004393 visual impairment Effects 0.000 description 2
- KIUKXJAPPMFGSW-DNGZLQJQSA-N (2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-Acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-DNGZLQJQSA-N 0.000 description 1
- WCDDVEOXEIYWFB-VXORFPGASA-N (2s,3s,4r,5r,6r)-3-[(2s,3r,5s,6r)-3-acetamido-5-hydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4,5,6-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@@H]1C[C@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](C(O)=O)O[C@@H](O)[C@H](O)[C@H]1O WCDDVEOXEIYWFB-VXORFPGASA-N 0.000 description 1
- SQDAZGGFXASXDW-UHFFFAOYSA-N 5-bromo-2-(trifluoromethoxy)pyridine Chemical compound FC(F)(F)OC1=CC=C(Br)C=N1 SQDAZGGFXASXDW-UHFFFAOYSA-N 0.000 description 1
- 208000021959 Abnormal metabolism Diseases 0.000 description 1
- 208000032467 Aplastic anaemia Diseases 0.000 description 1
- 206010007559 Cardiac failure congestive Diseases 0.000 description 1
- 229920001287 Chondroitin sulfate Polymers 0.000 description 1
- 208000003164 Diplopia Diseases 0.000 description 1
- 208000000059 Dyspnea Diseases 0.000 description 1
- 206010013975 Dyspnoeas Diseases 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 206010016654 Fibrosis Diseases 0.000 description 1
- 206010051283 Fluid imbalance Diseases 0.000 description 1
- 206010019280 Heart failures Diseases 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- 208000000913 Kidney Calculi Diseases 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- 206010029148 Nephrolithiasis Diseases 0.000 description 1
- 240000004050 Pentaglottis sempervirens Species 0.000 description 1
- 201000001880 Sexual dysfunction Diseases 0.000 description 1
- 229920006362 Teflon® Polymers 0.000 description 1
- 241000905137 Veronica schmidtiana Species 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- OIPILFWXSMYKGL-UHFFFAOYSA-N acetylcholine Chemical compound CC(=O)OCC[N+](C)(C)C OIPILFWXSMYKGL-UHFFFAOYSA-N 0.000 description 1
- 229960004373 acetylcholine Drugs 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000003510 anti-fibrotic effect Effects 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 239000002260 anti-inflammatory agent Substances 0.000 description 1
- 230000000259 anti-tumor effect Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 230000004509 aqueous humor production Effects 0.000 description 1
- 210000003050 axon Anatomy 0.000 description 1
- 230000002457 bidirectional effect Effects 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- AIXAANGOTKPUOY-UHFFFAOYSA-N carbachol Chemical compound [Cl-].C[N+](C)(C)CCOC(N)=O AIXAANGOTKPUOY-UHFFFAOYSA-N 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000019522 cellular metabolic process Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 230000005465 channeling Effects 0.000 description 1
- 229940059329 chondroitin sulfate Drugs 0.000 description 1
- 210000004081 cilia Anatomy 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 210000000744 eyelid Anatomy 0.000 description 1
- 230000004438 eyesight Effects 0.000 description 1
- 230000004761 fibrosis Effects 0.000 description 1
- 230000003176 fibrotic effect Effects 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 229920002674 hyaluronan Polymers 0.000 description 1
- 229940014041 hyaluronate Drugs 0.000 description 1
- 229960003160 hyaluronic acid Drugs 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 230000002262 irrigation Effects 0.000 description 1
- 238000003973 irrigation Methods 0.000 description 1
- 230000000622 irritating effect Effects 0.000 description 1
- 238000002430 laser surgery Methods 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 230000007257 malfunction Effects 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- 230000006371 metabolic abnormality Effects 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 230000003547 miosis Effects 0.000 description 1
- 230000000394 mitotic effect Effects 0.000 description 1
- 238000000465 moulding Methods 0.000 description 1
- 210000001087 myotubule Anatomy 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- 210000003516 pericardium Anatomy 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 230000005043 peripheral vision Effects 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 230000005180 public health Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000002271 resection Methods 0.000 description 1
- 231100000241 scar Toxicity 0.000 description 1
- 231100000872 sexual dysfunction Toxicity 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 238000004513 sizing Methods 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 238000011477 surgical intervention Methods 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- 210000001745 uvea Anatomy 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- 239000011345 viscous material Substances 0.000 description 1
- 230000004304 visual acuity Effects 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F9/00—Methods or devices for treatment of the eyes; Devices for putting-in contact lenses; Devices to correct squinting; Apparatus to guide the blind; Protective devices for the eyes, carried on the body or in the hand
- A61F9/007—Methods or devices for eye surgery
- A61F9/00781—Apparatus for modifying intraocular pressure, e.g. for glaucoma treatment
Landscapes
- Health & Medical Sciences (AREA)
- Ophthalmology & Optometry (AREA)
- Heart & Thoracic Surgery (AREA)
- Surgery (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Prostheses (AREA)
- External Artificial Organs (AREA)
Abstract
Description
表1
表1:Eyepass(登録商標)Glaucoma Implantを使用した第2段階及び第3段階の臨床研究が不首尾に終わった後に代替手術を受けた患者のフォローアップデータ及び眼圧(mmHg)データ
Claims (20)
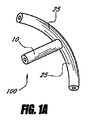
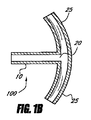
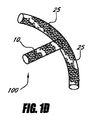
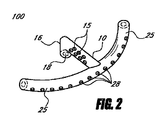
- 眼圧を下げるための装置であって、
間に延びるルーメンを伴って近位部分と遠位部分とを有するインプラントを備え、ルーメンは、移植されるとき眼の前房から眼内の脈絡膜上腔内へ房水を排出するのに十分な長さを有し、前記インプラントは前記近位部分に配置されるアンカー部分を有する装置。 - 前記アンカー部分は移植されるとき眼組織に隣接して係合するように構成される、請求項1に記載の装置。
- 前記アンカー部分は細胞の増殖を促進する表面を備える、請求項1に記載の装置。
- 前記アンカー部分は遠位部分から離隔されている、請求項1に記載の装置。
- 前記アンカー部分は移植されるとき前房の外側に位置づけられる、請求項1に記載の装置。
- 前記アンカー部分は前記近位部分の近位端から離隔されている、請求項1に記載の装置。
- 前記ルーメンの長さは約4mm〜約6mmである、請求項1に記載の装置。
- 前記アンカー部分は前記インプラントの外面に形成される少なくとも1つの溝を備える、請求項1に記載の装置。
- 前記ルーメンを介する少なくとも一方向への流れを抑制するように配置される弁をさらに備える、請求項1に記載の装置。
- 眼圧を下げるための方法であって:
前房からシュレム管へ房水を排出するための第1の流出ルートを確立する工程であって、小柱網を介して移植可能な部材を挿入する工程を含む工程;及び
前房から脈絡膜上腔へ房水を排出するための第2の流出ルートを確立する工程であって、移植可能な部材の遠位端が房水を前記脈絡膜上腔へ排出するように当該脈絡膜上腔に近接する組織に移植可能な部材を挿入する工程;
を含む方法。 - 前記流出ルートを形成する前記移植可能な部材は互いに接続されている、請求項10に記載の方法。
- 前記第1の流出ルートと前記第2の流出ルートとは部分的に重なる、請求項10に記載の方法。
- 前記第1の流出ルートは前記第2の流出ルートより前に確立される、請求項10に記載の方法。
- 前記第1及び第2の流出ルートのうちの少なくとも一方を介する流れを抑制することをさらに含む、請求項10に記載の方法。
- 眼圧を下げるための方法であって:
眼の前房から眼の脈絡膜上腔へ房水を排出するために、インプラントを、当該インプラントの近位部分の近位端が眼の前房内に存在するように、位置合わせする工程;及び
前記インプラントの近位部分を当該インプラントの近位部分に隣接する組織内に固定する工程;
を含む方法。 - 前記固定する工程は、前記インプラントに、細胞の増殖を促進する表面を設ける工程を含む、請求項15に記載の方法。
- 前記固定する工程は、眼の前房の外側の位置に固定する工程を含む、請求項15に記載の方法。
- 前記固定する工程は、前記インプラントの遠位端から離隔された位置に固定する工程を含む、請求項15に記載の方法。
- 房水を約4mm〜約6mmの長さを有するルーメンを介して導く工程をさらに含む、請求項15に記載の方法。
- 前記インプラントを介する少なくとも一方向への流れを抑制する工程をさらに含む、請求項15に記載の方法。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US79642406P | 2006-05-01 | 2006-05-01 | |
| PCT/US2007/010525 WO2007130393A2 (en) | 2006-05-01 | 2007-05-01 | Dual drainage pathway shunt device and method for treating glaucoma |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2009535162A true JP2009535162A (ja) | 2009-10-01 |
| JP2009535162A5 JP2009535162A5 (ja) | 2010-06-17 |
Family
ID=38582122
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009509656A Pending JP2009535162A (ja) | 2006-05-01 | 2007-05-01 | 緑内障治療用二重排出経路シャントデバイス及び方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (2) | US20070293807A1 (ja) |
| EP (1) | EP2012724A2 (ja) |
| JP (1) | JP2009535162A (ja) |
| AU (1) | AU2007248710A1 (ja) |
| CA (1) | CA2650726A1 (ja) |
| WO (1) | WO2007130393A2 (ja) |
Families Citing this family (83)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8313454B2 (en) | 1997-11-20 | 2012-11-20 | Optonol Ltd. | Fluid drainage device, delivery device, and associated methods of use and manufacture |
| AU767526B2 (en) | 1999-04-26 | 2003-11-13 | Gmp Vision Solutions, Inc. | Trabeculotomy device and method for treating glaucoma |
| US7867186B2 (en) | 2002-04-08 | 2011-01-11 | Glaukos Corporation | Devices and methods for treatment of ocular disorders |
| US6638239B1 (en) | 2000-04-14 | 2003-10-28 | Glaukos Corporation | Apparatus and method for treating glaucoma |
| US7488303B1 (en) | 2002-09-21 | 2009-02-10 | Glaukos Corporation | Ocular implant with anchor and multiple openings |
| EP1977724A1 (en) | 2001-04-07 | 2008-10-08 | Glaukos Corporation | System for treating ocular disorders |
| US7431710B2 (en) | 2002-04-08 | 2008-10-07 | Glaukos Corporation | Ocular implants with anchors and methods thereof |
| US7331984B2 (en) | 2001-08-28 | 2008-02-19 | Glaukos Corporation | Glaucoma stent for treating glaucoma and methods of use |
| US9301875B2 (en) | 2002-04-08 | 2016-04-05 | Glaukos Corporation | Ocular disorder treatment implants with multiple opening |
| US20040225250A1 (en) | 2003-05-05 | 2004-11-11 | Michael Yablonski | Internal shunt and method for treating glaucoma |
| US7291125B2 (en) | 2003-11-14 | 2007-11-06 | Transcend Medical, Inc. | Ocular pressure regulation |
| US9084662B2 (en) | 2006-01-17 | 2015-07-21 | Transcend Medical, Inc. | Drug delivery treatment device |
| PT2526910E (pt) | 2006-01-17 | 2015-11-18 | Transcend Medical Inc | Dispositivo para tratamento de glaucoma |
| US8506515B2 (en) * | 2006-11-10 | 2013-08-13 | Glaukos Corporation | Uveoscleral shunt and methods for implanting same |
| WO2009012406A1 (en) | 2007-07-17 | 2009-01-22 | Transcend Medical, Inc. | Ocular implant with hydrogel expansion capabilities reference to priority document |
| US20170360609A9 (en) | 2007-09-24 | 2017-12-21 | Ivantis, Inc. | Methods and devices for increasing aqueous humor outflow |
| US7740604B2 (en) | 2007-09-24 | 2010-06-22 | Ivantis, Inc. | Ocular implants for placement in schlemm's canal |
| US8734377B2 (en) | 2007-09-24 | 2014-05-27 | Ivantis, Inc. | Ocular implants with asymmetric flexibility |
| US20090082862A1 (en) | 2007-09-24 | 2009-03-26 | Schieber Andrew T | Ocular Implant Architectures |
| US8512404B2 (en) | 2007-11-20 | 2013-08-20 | Ivantis, Inc. | Ocular implant delivery system and method |
| US8808222B2 (en) | 2007-11-20 | 2014-08-19 | Ivantis, Inc. | Methods and apparatus for delivering ocular implants into the eye |
| EP3970664A1 (en) * | 2008-01-28 | 2022-03-23 | Implantica Patent Ltd. | A fluid movement device |
| US8109896B2 (en) | 2008-02-11 | 2012-02-07 | Optonol Ltd. | Devices and methods for opening fluid passageways |
| JP2011513002A (ja) | 2008-03-05 | 2011-04-28 | イバンティス インコーポレイテッド | 緑内障を治療する方法及び器具 |
| EP3298995A1 (en) | 2008-06-25 | 2018-03-28 | Novartis AG | Ocular implant with shape change capabilities |
| EP3960135A1 (en) | 2008-12-05 | 2022-03-02 | Ivantis, Inc. | Apparatus for delivering ocular implants into the eye |
| WO2010088258A2 (en) | 2009-01-28 | 2010-08-05 | Transcend Medical, Inc. | Ocular implant with stiffness qualities, methods of implantation and system |
| US10206813B2 (en) | 2009-05-18 | 2019-02-19 | Dose Medical Corporation | Implants with controlled drug delivery features and methods of using same |
| US8764696B2 (en) * | 2009-06-16 | 2014-07-01 | Mobius Therapeutics, Inc. | Medical drainage devices with carbon-based structures for inhibiting growth of fibroblasts |
| CN102481404B (zh) | 2009-07-09 | 2014-03-05 | 伊万提斯公司 | 眼部植入物 |
| US9693899B2 (en) | 2009-07-09 | 2017-07-04 | Ivantis, Inc. | Single operator device for delivering an ocular implant |
| US20110118835A1 (en) * | 2009-08-13 | 2011-05-19 | Matthew Silvestrini | Branched ocular implant |
| JP2013508096A (ja) | 2009-10-23 | 2013-03-07 | イバンティス インコーポレイテッド | 眼内移植システムおよび眼内移植方法 |
| US8529492B2 (en) | 2009-12-23 | 2013-09-10 | Trascend Medical, Inc. | Drug delivery devices and methods |
| US9510973B2 (en) | 2010-06-23 | 2016-12-06 | Ivantis, Inc. | Ocular implants deployed in schlemm's canal of the eye |
| US8915877B2 (en) | 2010-10-12 | 2014-12-23 | Emmett T. Cunningham, JR. | Glaucoma drainage device and uses thereof |
| US9370444B2 (en) * | 2010-10-12 | 2016-06-21 | Emmett T. Cunningham, JR. | Subconjunctival conformer device and uses thereof |
| US8657776B2 (en) | 2011-06-14 | 2014-02-25 | Ivantis, Inc. | Ocular implants for delivery into the eye |
| US10307292B2 (en) | 2011-07-18 | 2019-06-04 | Mor Research Applications Ltd | Device for adjusting the intraocular pressure |
| WO2013069018A2 (en) * | 2011-11-11 | 2013-05-16 | Opr Group Ltd. | Ocular implant with intraocular fluid pressure regulation |
| US8765210B2 (en) | 2011-12-08 | 2014-07-01 | Aquesys, Inc. | Systems and methods for making gelatin shunts |
| US8663150B2 (en) | 2011-12-19 | 2014-03-04 | Ivantis, Inc. | Delivering ocular implants into the eye |
| EP2830553B1 (en) | 2012-03-26 | 2017-12-27 | Glaukos Corporation | Apparatus for delivering multiple ocular implants |
| US9358156B2 (en) | 2012-04-18 | 2016-06-07 | Invantis, Inc. | Ocular implants for delivery into an anterior chamber of the eye |
| US10085633B2 (en) | 2012-04-19 | 2018-10-02 | Novartis Ag | Direct visualization system for glaucoma treatment |
| US9241832B2 (en) | 2012-04-24 | 2016-01-26 | Transcend Medical, Inc. | Delivery system for ocular implant |
| US9480598B2 (en) | 2012-09-17 | 2016-11-01 | Novartis Ag | Expanding ocular implant devices and methods |
| WO2014078288A1 (en) | 2012-11-14 | 2014-05-22 | Transcend Medical, Inc. | Flow promoting ocular implant |
| WO2014085450A1 (en) | 2012-11-28 | 2014-06-05 | Ivantis, Inc. | Apparatus for delivering ocular implants into an anterior chamber of the eye |
| US9125723B2 (en) | 2013-02-19 | 2015-09-08 | Aquesys, Inc. | Adjustable glaucoma implant |
| US10159600B2 (en) | 2013-02-19 | 2018-12-25 | Aquesys, Inc. | Adjustable intraocular flow regulation |
| US10517759B2 (en) | 2013-03-15 | 2019-12-31 | Glaukos Corporation | Glaucoma stent and methods thereof for glaucoma treatment |
| US9592151B2 (en) | 2013-03-15 | 2017-03-14 | Glaukos Corporation | Systems and methods for delivering an ocular implant to the suprachoroidal space within an eye |
| US9987163B2 (en) | 2013-04-16 | 2018-06-05 | Novartis Ag | Device for dispensing intraocular substances |
| US9585790B2 (en) | 2013-11-14 | 2017-03-07 | Aquesys, Inc. | Intraocular shunt inserter |
| RU2562553C1 (ru) * | 2014-04-30 | 2015-09-10 | Общество с ограниченной ответственностью "Вертикаль-М" (ООО "Вертикаль-М") | Композитный пористый дренаж для хирургического лечения глаукомы |
| EP3148491B1 (en) | 2014-05-29 | 2020-07-01 | Glaukos Corporation | Implants with controlled drug delivery features and manufacturing method for said implants |
| WO2016011056A1 (en) | 2014-07-14 | 2016-01-21 | Ivantis, Inc. | Ocular implant delivery system and method |
| EP3270765B1 (en) | 2015-03-20 | 2020-05-06 | Glaukos Corporation | Gonioscopic devices |
| ES2962607T3 (es) | 2015-08-14 | 2024-03-20 | Alcon Inc | Implante ocular con sensor de presión |
| US11925578B2 (en) | 2015-09-02 | 2024-03-12 | Glaukos Corporation | Drug delivery implants with bi-directional delivery capacity |
| WO2017053885A1 (en) | 2015-09-25 | 2017-03-30 | Glaukos Corporation | Punctal implants with controlled drug delivery features and methods of using same |
| WO2017106517A1 (en) | 2015-12-15 | 2017-06-22 | Ivantis, Inc. | Ocular implant and delivery system |
| EP3442479A1 (en) | 2016-04-20 | 2019-02-20 | Harold Alexander Heitzmann | Bioresorbable ocular drug delivery device |
| CN105997341B (zh) * | 2016-04-21 | 2019-03-08 | 温州医科大学附属眼视光医院 | 一种青光眼内引流替代仿生支架的制备及其使用方法 |
| US10674906B2 (en) | 2017-02-24 | 2020-06-09 | Glaukos Corporation | Gonioscopes |
| US11833077B2 (en) * | 2017-06-16 | 2023-12-05 | Massachusetts Institute Of Technology | Modular glaucoma implant |
| WO2019036025A2 (en) * | 2017-08-17 | 2019-02-21 | Aspip Inc. | METHOD, DEVICE AND SYSTEM FOR TREATING HIGH INTRAOCULAR PRESSURE |
| US11116625B2 (en) | 2017-09-28 | 2021-09-14 | Glaukos Corporation | Apparatus and method for controlling placement of intraocular implants |
| AU2018346229B2 (en) | 2017-10-06 | 2024-07-18 | Glaukos Corporation | Systems and methods for delivering multiple ocular implants |
| USD846738S1 (en) | 2017-10-27 | 2019-04-23 | Glaukos Corporation | Implant delivery apparatus |
| CN107981969B (zh) * | 2017-12-29 | 2023-07-14 | 苏州朗目医疗科技有限公司 | 一种青光眼内引流替代仿生支架 |
| EP4371535A3 (en) * | 2018-02-22 | 2024-08-14 | Alcon Inc. | Ocular implant |
| EP3930647A4 (en) * | 2019-02-27 | 2022-11-30 | Innfocus, Inc. | GLAUCOMA DEVICE DEPLOYMENT DEVICE |
| US11517477B2 (en) | 2019-10-10 | 2022-12-06 | Shifamed Holdings, Llc | Adjustable flow glaucoma shunts and associated systems and methods |
| CA3165037A1 (en) | 2020-01-23 | 2021-07-29 | Robert Chang | Adjustable flow glaucoma shunts and associated systems and methods |
| EP4103117A4 (en) | 2020-02-14 | 2024-03-20 | Shifamed Holdings, LLC | MARGINING SYSTEMS WITH ROTATION-BASED FLOW CONTROL DEVICES AND ASSOCIATED SYSTEMS AND METHODS |
| US11737920B2 (en) | 2020-02-18 | 2023-08-29 | Shifamed Holdings, Llc | Adjustable flow glaucoma shunts having non-linearly arranged flow control elements, and associated systems and methods |
| CN115715214A (zh) * | 2020-03-04 | 2023-02-24 | 悉尼西部地方卫生区 | 眼部植入物和其制造方法 |
| US11766355B2 (en) | 2020-03-19 | 2023-09-26 | Shifamed Holdings, Llc | Intraocular shunts with low-profile actuation elements and associated systems and methods |
| WO2021212007A2 (en) | 2020-04-16 | 2021-10-21 | Shifamed Holdings, Llc | Adjustable glaucoma treatment devices and associated systems and methods |
| WO2022150684A1 (en) | 2021-01-11 | 2022-07-14 | Ivantis, Inc. | Systems and methods for viscoelastic delivery |
| WO2022159723A1 (en) | 2021-01-22 | 2022-07-28 | Shifamed Holdings, Llc | Adjustable shunting systems with plate assemblies, and associated systems and methods |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003180730A (ja) * | 2001-11-08 | 2003-07-02 | Glaukos Corp | 緑内障治療用の薬物放出小柱インプラント |
| WO2004110391A2 (en) * | 2003-06-16 | 2004-12-23 | Solx, Inc. | Shunt for the treatment of glaucoma |
| WO2005055873A2 (en) * | 2003-12-05 | 2005-06-23 | Innfocus, Llc | Improved glaucoma implant device |
| JP2005525835A (ja) * | 2001-08-16 | 2005-09-02 | ジーエムピー ヴィジョン ソルーションズ インコーポレイテッド | 緑内障を治療するための改良型短絡装置および改良式短絡方法 |
| WO2005107664A2 (en) * | 2004-04-29 | 2005-11-17 | Iscience Interventional Corporation | Apparatus and method for surgical enhancement of aqueous humor drainage |
Family Cites Families (105)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3788327A (en) * | 1971-03-30 | 1974-01-29 | H Donowitz | Surgical implant device |
| US4037604A (en) * | 1976-01-05 | 1977-07-26 | Newkirk John B | Artifical biological drainage device |
| US4168697A (en) * | 1977-01-17 | 1979-09-25 | Cantekin Erdem I | Middle ear ventilating tube and method |
| US4113088A (en) * | 1977-06-06 | 1978-09-12 | Binkhorst Richard D | Sterile package |
| US4175563A (en) * | 1977-10-05 | 1979-11-27 | Arenberg Irving K | Biological drainage shunt |
| US4402681A (en) * | 1980-08-23 | 1983-09-06 | Haas Joseph S | Artificial implant valve for the regulation of intraocular pressure |
| NO147900C (no) * | 1981-03-12 | 1983-07-06 | Finn Skjaerpe | Mikrokirurgisk instrument. |
| US4428746A (en) * | 1981-07-29 | 1984-01-31 | Antonio Mendez | Glaucoma treatment device |
| US4554918A (en) * | 1982-07-28 | 1985-11-26 | White Thomas C | Ocular pressure relief device |
| JPS5985153A (ja) * | 1982-11-08 | 1984-05-17 | Hitachi Ltd | 冗長化制御装置 |
| US4521210A (en) * | 1982-12-27 | 1985-06-04 | Wong Vernon G | Eye implant for relieving glaucoma, and device and method for use therewith |
| US4634418A (en) * | 1984-04-06 | 1987-01-06 | Binder Perry S | Hydrogel seton |
| US4604087A (en) * | 1985-02-26 | 1986-08-05 | Joseph Neil H | Aqueous humor drainage device |
| US4820626A (en) * | 1985-06-06 | 1989-04-11 | Thomas Jefferson University | Method of treating a synthetic or naturally occuring surface with microvascular endothelial cells, and the treated surface itself |
| US4718907A (en) * | 1985-06-20 | 1988-01-12 | Atrium Medical Corporation | Vascular prosthesis having fluorinated coating with varying F/C ratio |
| US4733665C2 (en) * | 1985-11-07 | 2002-01-29 | Expandable Grafts Partnership | Expandable intraluminal graft and method and apparatus for implanting an expandable intraluminal graft |
| NZ215409A (en) * | 1986-03-07 | 1989-02-24 | Anthony Christopher Be Molteno | Implant for drainage of aqueous humour in glaucoma |
| CH670760A5 (ja) * | 1986-06-02 | 1989-07-14 | Sulzer Ag | |
| US4722724A (en) * | 1986-06-23 | 1988-02-02 | Stanley Schocket | Anterior chamber tube shunt to an encircling band, and related surgical procedure |
| US4846793A (en) * | 1987-03-18 | 1989-07-11 | Endocon, Inc. | Injector for implanting multiple pellet medicaments |
| US4846172A (en) * | 1987-05-26 | 1989-07-11 | Berlin Michael S | Laser-delivery eye-treatment method |
| US4900300A (en) * | 1987-07-06 | 1990-02-13 | Lee David A | Surgical instrument |
| US4997652A (en) * | 1987-12-22 | 1991-03-05 | Visionex | Biodegradable ocular implants |
| US4853224A (en) * | 1987-12-22 | 1989-08-01 | Visionex | Biodegradable ocular implants |
| US4936825A (en) * | 1988-04-11 | 1990-06-26 | Ungerleider Bruce A | Method for reducing intraocular pressure caused by glaucoma |
| US5005577A (en) * | 1988-08-23 | 1991-04-09 | Frenkel Ronald E P | Intraocular lens pressure monitoring device |
| US5785674A (en) * | 1988-10-07 | 1998-07-28 | Mateen; Ahmed Abdul | Device and method for treating glaucoma |
| FR2651668B1 (fr) * | 1989-09-12 | 1991-12-27 | Leon Claude | Ensemble microscope-endoscope utile notamment en chirurgie. |
| US4946436A (en) * | 1989-11-17 | 1990-08-07 | Smith Stewart G | Pressure-relieving device and process for implanting |
| USRE35390E (en) * | 1989-11-17 | 1996-12-03 | Smith; Stewart G. | Pressure relieving device and process for implanting |
| US5092837A (en) * | 1989-12-20 | 1992-03-03 | Robert Ritch | Method for the treatment of glaucoma |
| US5180362A (en) * | 1990-04-03 | 1993-01-19 | Worst J G F | Gonio seton |
| US5129895A (en) * | 1990-05-16 | 1992-07-14 | Sunrise Technologies, Inc. | Laser sclerostomy procedure |
| US5127901A (en) * | 1990-05-18 | 1992-07-07 | Odrich Ronald B | Implant with subconjunctival arch |
| US5397300A (en) * | 1990-05-31 | 1995-03-14 | Iovision, Inc. | Glaucoma implant |
| US5178604A (en) * | 1990-05-31 | 1993-01-12 | Iovision, Inc. | Glaucoma implant |
| US5300020A (en) * | 1991-05-31 | 1994-04-05 | Medflex Corporation | Surgically implantable device for glaucoma relief |
| US5360399A (en) * | 1992-01-10 | 1994-11-01 | Robert Stegmann | Method and apparatus for maintaining the normal intraocular pressure |
| US5207685A (en) * | 1992-02-11 | 1993-05-04 | Cinberg James Z | Tympanic ventilation tube and related technique |
| US6197056B1 (en) * | 1992-07-15 | 2001-03-06 | Ras Holding Corp. | Segmented scleral band for treatment of presbyopia and other eye disorders |
| US5290295A (en) * | 1992-07-15 | 1994-03-01 | Querals & Fine, Inc. | Insertion tool for an intraluminal graft procedure |
| US5318513A (en) * | 1992-09-24 | 1994-06-07 | Leib Martin L | Canalicular balloon fixation stent |
| US5639278A (en) * | 1993-10-21 | 1997-06-17 | Corvita Corporation | Expandable supportive bifurcated endoluminal grafts |
| US5443505A (en) * | 1993-11-15 | 1995-08-22 | Oculex Pharmaceuticals, Inc. | Biocompatible ocular implants |
| US5743868A (en) * | 1994-02-14 | 1998-04-28 | Brown; Reay H. | Corneal pressure-regulating implant device |
| US5516522A (en) * | 1994-03-14 | 1996-05-14 | Board Of Supervisors Of Louisiana State University | Biodegradable porous device for long-term drug delivery with constant rate release and method of making the same |
| US5716394A (en) * | 1994-04-29 | 1998-02-10 | W. L. Gore & Associates, Inc. | Blood contact surfaces using extracellular matrix synthesized in vitro |
| IL109499A (en) * | 1994-05-02 | 1998-01-04 | Univ Ramot | Implant device for draining excess intraocular fluid |
| FR2721499B1 (fr) * | 1994-06-22 | 1997-01-03 | Opsia | Implant de trabéculectomie. |
| US5520631A (en) * | 1994-07-22 | 1996-05-28 | Wound Healing Of Oklahoma | Method and apparatus for lowering the intraocular pressure of an eye |
| US5704907A (en) * | 1994-07-22 | 1998-01-06 | Wound Healing Of Oklahoma | Method and apparatus for lowering the intraocular pressure of an eye |
| US6063396A (en) * | 1994-10-26 | 2000-05-16 | Houston Biotechnology Incorporated | Methods and compositions for the modulation of cell proliferation and wound healing |
| US6063116A (en) * | 1994-10-26 | 2000-05-16 | Medarex, Inc. | Modulation of cell proliferation and wound healing |
| JP3642812B2 (ja) * | 1994-11-17 | 2005-04-27 | 株式会社町田製作所 | 医療用観察装置 |
| US5601094A (en) * | 1994-11-22 | 1997-02-11 | Reiss; George R. | Ophthalmic shunt |
| US6228873B1 (en) * | 1994-12-09 | 2001-05-08 | The Regents Of The University Of California | Method for enhancing outflow of aqueous humor in treatment of glaucoma |
| US5725493A (en) * | 1994-12-12 | 1998-03-10 | Avery; Robert Logan | Intravitreal medicine delivery |
| US5433701A (en) * | 1994-12-21 | 1995-07-18 | Rubinstein; Mark H. | Apparatus for reducing ocular pressure |
| US6059772A (en) * | 1995-03-10 | 2000-05-09 | Candela Corporation | Apparatus and method for treating glaucoma using a gonioscopic laser trabecular ablation procedure |
| BE1009278A3 (fr) * | 1995-04-12 | 1997-01-07 | Corvita Europ | Tuteur auto-expansible pour dispositif medical a introduire dans une cavite d'un corps, et dispositif medical muni d'un tel tuteur. |
| US5626558A (en) * | 1995-05-05 | 1997-05-06 | Suson; John | Adjustable flow rate glaucoma shunt and method of using same |
| IL113723A (en) * | 1995-05-14 | 2002-11-10 | Optonol Ltd | Intraocular implant |
| US5723005A (en) * | 1995-06-07 | 1998-03-03 | Herrick Family Limited Partnership | Punctum plug having a collapsible flared section and method |
| EP0830109B1 (en) * | 1995-06-08 | 2003-10-15 | Ave Galway Limited | Bifurcated endovascular stent |
| US6045557A (en) * | 1995-11-10 | 2000-04-04 | Baxter International Inc. | Delivery catheter and method for positioning an intraluminal graft |
| US5651783A (en) * | 1995-12-20 | 1997-07-29 | Reynard; Michael | Fiber optic sleeve for surgical instruments |
| US5830179A (en) * | 1996-04-09 | 1998-11-03 | Endocare, Inc. | Urological stent therapy system and method |
| US5865831A (en) * | 1996-04-17 | 1999-02-02 | Premier Laser Systems, Inc. | Laser surgical procedures for treatment of glaucoma |
| US6530896B1 (en) * | 1996-05-13 | 2003-03-11 | James B. Elliott | Apparatus and method for introducing an implant |
| US5886822A (en) * | 1996-10-08 | 1999-03-23 | The Microoptical Corporation | Image combining system for eyeglasses and face masks |
| AUPO394496A0 (en) * | 1996-11-29 | 1997-01-02 | Lions Eye Institute | Biological microfistula tube and implantation method and apparatus |
| US6261256B1 (en) * | 1996-12-20 | 2001-07-17 | Abdul Mateen Ahmed | Pocket medical valve & method |
| US5713844A (en) * | 1997-01-10 | 1998-02-03 | Peyman; Gholam A. | Device and method for regulating intraocular pressure |
| US6071286A (en) * | 1997-02-19 | 2000-06-06 | Mawad; Michel E. | Combination angioplasty balloon/stent deployment device |
| US5893837A (en) * | 1997-02-28 | 1999-04-13 | Staar Surgical Company, Inc. | Glaucoma drain implanting device and method |
| US6059812A (en) * | 1997-03-21 | 2000-05-09 | Schneider (Usa) Inc. | Self-expanding medical device for centering radioactive treatment sources in body vessels |
| JP3827429B2 (ja) * | 1997-04-03 | 2006-09-27 | オリンパス株式会社 | 手術用顕微鏡 |
| US5882327A (en) * | 1997-04-17 | 1999-03-16 | Jacob; Jean T. | Long-term glaucoma drainage implant |
| US6050970A (en) * | 1997-05-08 | 2000-04-18 | Pharmacia & Upjohn Company | Method and apparatus for inserting a glaucoma implant in an anterior and posterior segment of the eye |
| US5752928A (en) * | 1997-07-14 | 1998-05-19 | Rdo Medical, Inc. | Glaucoma pressure regulator |
| US6203513B1 (en) * | 1997-11-20 | 2001-03-20 | Optonol Ltd. | Flow regulating implant, method of manufacture, and delivery device |
| US6050999A (en) * | 1997-12-18 | 2000-04-18 | Keravision, Inc. | Corneal implant introducer and method of use |
| US6168575B1 (en) * | 1998-01-29 | 2001-01-02 | David Pyam Soltanpour | Method and apparatus for controlling intraocular pressure |
| EP1071414A1 (en) * | 1998-04-24 | 2001-01-31 | Mitokor | Compounds and methods for treating mitochondria-associated diseases |
| US6077299A (en) * | 1998-06-22 | 2000-06-20 | Eyetronic, Llc | Non-invasively adjustable valve implant for the drainage of aqueous humor in glaucoma |
| US6241721B1 (en) * | 1998-10-09 | 2001-06-05 | Colette Cozean | Laser surgical procedures for treatment of glaucoma |
| US6254612B1 (en) * | 1998-10-22 | 2001-07-03 | Cordis Neurovascular, Inc. | Hydraulic stent deployment system |
| US6348042B1 (en) * | 1999-02-02 | 2002-02-19 | W. Lee Warren, Jr. | Bioactive shunt |
| US6193656B1 (en) * | 1999-02-08 | 2001-02-27 | Robert E. Jeffries | Intraocular pressure monitoring/measuring apparatus and method |
| US6231597B1 (en) * | 1999-02-16 | 2001-05-15 | Mark E. Deem | Apparatus and methods for selectively stenting a portion of a vessel wall |
| US6217895B1 (en) * | 1999-03-22 | 2001-04-17 | Control Delivery Systems | Method for treating and/or preventing retinal diseases with sustained release corticosteroids |
| AU767526B2 (en) * | 1999-04-26 | 2003-11-13 | Gmp Vision Solutions, Inc. | Trabeculotomy device and method for treating glaucoma |
| US6342058B1 (en) * | 1999-05-14 | 2002-01-29 | Valdemar Portney | Iris fixated intraocular lens and instrument for attaching same to an iris |
| US6187016B1 (en) * | 1999-09-14 | 2001-02-13 | Daniel G. Hedges | Stent retrieval device |
| US6416777B1 (en) * | 1999-10-21 | 2002-07-09 | Alcon Universal Ltd. | Ophthalmic drug delivery device |
| MXPA02002338A (es) * | 1999-10-21 | 2002-07-30 | Alcon Universal Ltd | Dispositivo para la entrega de drogas. |
| US6579235B1 (en) * | 1999-11-01 | 2003-06-17 | The Johns Hopkins University | Method for monitoring intraocular pressure using a passive intraocular pressure sensor and patient worn monitoring recorder |
| DE29920949U1 (de) * | 1999-11-29 | 2000-04-27 | Bugge, Mogens, Göteborg | Saugrohr für chirurgische Zwecke |
| US6375642B1 (en) * | 2000-02-15 | 2002-04-23 | Grieshaber & Co. Ag Schaffhausen | Method of and device for improving a drainage of aqueous humor within the eye |
| US6533768B1 (en) * | 2000-04-14 | 2003-03-18 | The Regents Of The University Of California | Device for glaucoma treatment and methods thereof |
| US6638239B1 (en) * | 2000-04-14 | 2003-10-28 | Glaukos Corporation | Apparatus and method for treating glaucoma |
| US6699211B2 (en) * | 2000-08-22 | 2004-03-02 | James A. Savage | Method and apparatus for treatment of glaucoma |
| US6595945B2 (en) * | 2001-01-09 | 2003-07-22 | J. David Brown | Glaucoma treatment device and method |
| EP1977724A1 (en) * | 2001-04-07 | 2008-10-08 | Glaukos Corporation | System for treating ocular disorders |
| USD490152S1 (en) * | 2003-02-28 | 2004-05-18 | Glaukos Corporation | Surgical handpiece |
-
2007
- 2007-04-30 US US11/742,484 patent/US20070293807A1/en not_active Abandoned
- 2007-05-01 CA CA002650726A patent/CA2650726A1/en not_active Abandoned
- 2007-05-01 EP EP07794452A patent/EP2012724A2/en not_active Withdrawn
- 2007-05-01 WO PCT/US2007/010525 patent/WO2007130393A2/en active Application Filing
- 2007-05-01 JP JP2009509656A patent/JP2009535162A/ja active Pending
- 2007-05-01 AU AU2007248710A patent/AU2007248710A1/en not_active Abandoned
-
2010
- 2010-05-21 US US12/785,306 patent/US20100234791A1/en not_active Abandoned
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005525835A (ja) * | 2001-08-16 | 2005-09-02 | ジーエムピー ヴィジョン ソルーションズ インコーポレイテッド | 緑内障を治療するための改良型短絡装置および改良式短絡方法 |
| JP2003180730A (ja) * | 2001-11-08 | 2003-07-02 | Glaukos Corp | 緑内障治療用の薬物放出小柱インプラント |
| WO2004110391A2 (en) * | 2003-06-16 | 2004-12-23 | Solx, Inc. | Shunt for the treatment of glaucoma |
| WO2005055873A2 (en) * | 2003-12-05 | 2005-06-23 | Innfocus, Llc | Improved glaucoma implant device |
| WO2005107664A2 (en) * | 2004-04-29 | 2005-11-17 | Iscience Interventional Corporation | Apparatus and method for surgical enhancement of aqueous humor drainage |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2650726A1 (en) | 2007-11-15 |
| US20070293807A1 (en) | 2007-12-20 |
| AU2007248710A1 (en) | 2007-11-15 |
| EP2012724A2 (en) | 2009-01-14 |
| WO2007130393A3 (en) | 2008-01-17 |
| WO2007130393A2 (en) | 2007-11-15 |
| US20100234791A1 (en) | 2010-09-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7220238B2 (en) | Shunt device and method for treating glaucoma | |
| JP2009535162A (ja) | 緑内障治療用二重排出経路シャントデバイス及び方法 | |
| US10492950B2 (en) | Shunt device and method for treating ocular disorders | |
| CA2457137A1 (en) | Improved shunt device and method for treating glaucoma | |
| AU2002323194A1 (en) | Improved shunt device and method for treating glaucoma |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100427 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20100427 |
|
| RD02 | Notification of acceptance of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7422 Effective date: 20100810 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20100908 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20120412 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120417 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120717 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120724 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120815 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120822 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120914 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120924 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20121017 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20130604 |