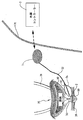
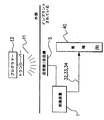
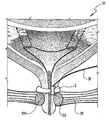
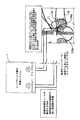
JP2008531138A - 失禁を治療するための改良された方法および装置 - Google Patents
失禁を治療するための改良された方法および装置 Download PDFInfo
- Publication number
- JP2008531138A JP2008531138A JP2007557279A JP2007557279A JP2008531138A JP 2008531138 A JP2008531138 A JP 2008531138A JP 2007557279 A JP2007557279 A JP 2007557279A JP 2007557279 A JP2007557279 A JP 2007557279A JP 2008531138 A JP2008531138 A JP 2008531138A
- Authority
- JP
- Japan
- Prior art keywords
- electrode
- signal
- sphincter
- stimulating
- nerves
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 238000000034 method Methods 0.000 title claims abstract description 92
- 206010021639 Incontinence Diseases 0.000 title description 12
- 210000005070 sphincter Anatomy 0.000 claims abstract description 162
- 210000005036 nerve Anatomy 0.000 claims abstract description 120
- 230000000638 stimulation Effects 0.000 claims abstract description 114
- 210000001519 tissue Anatomy 0.000 claims abstract description 74
- 210000002460 smooth muscle Anatomy 0.000 claims abstract description 36
- 208000000921 Urge Urinary Incontinence Diseases 0.000 claims abstract description 30
- 210000003708 urethra Anatomy 0.000 claims abstract description 18
- 210000004197 pelvis Anatomy 0.000 claims abstract description 3
- 230000004936 stimulating effect Effects 0.000 claims description 86
- 208000024891 symptom Diseases 0.000 claims description 35
- 206010046543 Urinary incontinence Diseases 0.000 claims description 24
- 210000003205 muscle Anatomy 0.000 claims description 21
- 238000011282 treatment Methods 0.000 claims description 19
- 210000003484 anatomy Anatomy 0.000 claims description 16
- 201000010099 disease Diseases 0.000 claims description 15
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 15
- 206010046494 urge incontinence Diseases 0.000 claims description 14
- 230000001154 acute effect Effects 0.000 claims description 11
- 210000003903 pelvic floor Anatomy 0.000 claims description 10
- 208000034347 Faecal incontinence Diseases 0.000 claims description 9
- 210000004126 nerve fiber Anatomy 0.000 claims description 8
- 230000004007 neuromodulation Effects 0.000 claims description 8
- 239000000835 fiber Substances 0.000 claims description 6
- 210000002429 large intestine Anatomy 0.000 claims description 5
- 208000002193 Pain Diseases 0.000 claims description 4
- 210000002255 anal canal Anatomy 0.000 claims description 4
- 230000008058 pain sensation Effects 0.000 claims description 3
- 239000012528 membrane Substances 0.000 claims 2
- 230000003227 neuromodulating effect Effects 0.000 abstract description 6
- 230000004044 response Effects 0.000 abstract description 5
- 210000003932 urinary bladder Anatomy 0.000 description 18
- 239000004020 conductor Substances 0.000 description 15
- 230000007383 nerve stimulation Effects 0.000 description 12
- 238000010586 diagram Methods 0.000 description 10
- 230000001537 neural effect Effects 0.000 description 9
- 206010066218 Stress Urinary Incontinence Diseases 0.000 description 7
- 230000000694 effects Effects 0.000 description 7
- 239000007943 implant Substances 0.000 description 7
- 230000027939 micturition Effects 0.000 description 6
- 230000005540 biological transmission Effects 0.000 description 5
- 230000006870 function Effects 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 229920001296 polysiloxane Polymers 0.000 description 5
- 230000001953 sensory effect Effects 0.000 description 5
- 230000008447 perception Effects 0.000 description 4
- 230000002485 urinary effect Effects 0.000 description 4
- 241001465754 Metazoa Species 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- WABPQHHGFIMREM-UHFFFAOYSA-N lead(0) Chemical compound [Pb] WABPQHHGFIMREM-UHFFFAOYSA-N 0.000 description 3
- 230000011514 reflex Effects 0.000 description 3
- 238000001356 surgical procedure Methods 0.000 description 3
- 241000282412 Homo Species 0.000 description 2
- 210000003489 abdominal muscle Anatomy 0.000 description 2
- 239000000560 biocompatible material Substances 0.000 description 2
- 230000002051 biphasic effect Effects 0.000 description 2
- 210000001072 colon Anatomy 0.000 description 2
- 230000008602 contraction Effects 0.000 description 2
- 210000005071 external anal sphincter Anatomy 0.000 description 2
- 239000011810 insulating material Substances 0.000 description 2
- 210000005072 internal anal sphincter Anatomy 0.000 description 2
- 230000036453 micturition reflex Effects 0.000 description 2
- 210000000653 nervous system Anatomy 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 210000000278 spinal cord Anatomy 0.000 description 2
- 210000002700 urine Anatomy 0.000 description 2
- 206010053236 Mixed incontinence Diseases 0.000 description 1
- 208000028389 Nerve injury Diseases 0.000 description 1
- 206010035148 Plague Diseases 0.000 description 1
- 229910000566 Platinum-iridium alloy Inorganic materials 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 241000607479 Yersinia pestis Species 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 210000000436 anus Anatomy 0.000 description 1
- 210000003169 central nervous system Anatomy 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 238000002224 dissection Methods 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 230000005684 electric field Effects 0.000 description 1
- 230000005611 electricity Effects 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 210000003195 fascia Anatomy 0.000 description 1
- 230000002550 fecal effect Effects 0.000 description 1
- 230000007274 generation of a signal involved in cell-cell signaling Effects 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 239000012212 insulator Substances 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- 210000004705 lumbosacral region Anatomy 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000008764 nerve damage Effects 0.000 description 1
- 210000001640 nerve ending Anatomy 0.000 description 1
- 235000001968 nicotinic acid Nutrition 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 1
- HWLDNSXPUQTBOD-UHFFFAOYSA-N platinum-iridium alloy Chemical class [Ir].[Pt] HWLDNSXPUQTBOD-UHFFFAOYSA-N 0.000 description 1
- 239000004810 polytetrafluoroethylene Substances 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000009158 reflex pathway Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 230000033764 rhythmic process Effects 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 230000009155 sensory pathway Effects 0.000 description 1
- 230000009131 signaling function Effects 0.000 description 1
- 239000004447 silicone coating Substances 0.000 description 1
- 229920002379 silicone rubber Polymers 0.000 description 1
- 239000004945 silicone rubber Substances 0.000 description 1
- 208000022170 stress incontinence Diseases 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 210000000626 ureter Anatomy 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/02—Details
- A61N1/04—Electrodes
- A61N1/05—Electrodes for implantation or insertion into the body, e.g. heart electrode
- A61N1/0507—Electrodes for the digestive system
- A61N1/0514—Electrodes for the urinary tract
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/36007—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation of urogenital or gastrointestinal organs, e.g. for incontinence control
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/3605—Implantable neurostimulators for stimulating central or peripheral nerve system
- A61N1/36128—Control systems
- A61N1/36146—Control systems specified by the stimulation parameters
- A61N1/3615—Intensity
- A61N1/36153—Voltage
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/3605—Implantable neurostimulators for stimulating central or peripheral nerve system
- A61N1/36128—Control systems
- A61N1/36146—Control systems specified by the stimulation parameters
- A61N1/3615—Intensity
- A61N1/36157—Current
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/3605—Implantable neurostimulators for stimulating central or peripheral nerve system
- A61N1/36128—Control systems
- A61N1/36146—Control systems specified by the stimulation parameters
- A61N1/36167—Timing, e.g. stimulation onset
- A61N1/36171—Frequency
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/372—Arrangements in connection with the implantation of stimulators
- A61N1/37211—Means for communicating with stimulators
- A61N1/37235—Aspects of the external programmer
Landscapes
- Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Radiology & Medical Imaging (AREA)
- Veterinary Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Gastroenterology & Hepatology (AREA)
- Urology & Nephrology (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Electrotherapy Devices (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2005900957A AU2005900957A0 (en) | 2005-03-02 | Improved method and device for managing urinary incontinence | |
| PCT/AU2006/000258 WO2006092007A1 (en) | 2005-03-02 | 2006-03-02 | Improved method and apparatus for treating incontinence |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2008531138A true JP2008531138A (ja) | 2008-08-14 |
| JP2008531138A5 JP2008531138A5 (cg-RX-API-DMAC7.html) | 2009-04-23 |
Family
ID=36940775
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2007557279A Abandoned JP2008531138A (ja) | 2005-03-02 | 2006-03-02 | 失禁を治療するための改良された方法および装置 |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US20090054950A1 (cg-RX-API-DMAC7.html) |
| EP (1) | EP1853344A4 (cg-RX-API-DMAC7.html) |
| JP (1) | JP2008531138A (cg-RX-API-DMAC7.html) |
| WO (1) | WO2006092007A1 (cg-RX-API-DMAC7.html) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2021531852A (ja) * | 2018-07-11 | 2021-11-25 | ディグニファイ セラピューティクス エルエルシー | 排泄機能障害の治療方法 |
Families Citing this family (93)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7695427B2 (en) | 2002-04-26 | 2010-04-13 | Torax Medical, Inc. | Methods and apparatus for treating body tissue sphincters and the like |
| US7346382B2 (en) | 2004-07-07 | 2008-03-18 | The Cleveland Clinic Foundation | Brain stimulation models, systems, devices, and methods |
| US9610459B2 (en) * | 2009-07-24 | 2017-04-04 | Emkinetics, Inc. | Cooling systems and methods for conductive coils |
| US9339641B2 (en) | 2006-01-17 | 2016-05-17 | Emkinetics, Inc. | Method and apparatus for transdermal stimulation over the palmar and plantar surfaces |
| US20100168501A1 (en) * | 2006-10-02 | 2010-07-01 | Daniel Rogers Burnett | Method and apparatus for magnetic induction therapy |
| US9005102B2 (en) | 2006-10-02 | 2015-04-14 | Emkinetics, Inc. | Method and apparatus for electrical stimulation therapy |
| US11224742B2 (en) | 2006-10-02 | 2022-01-18 | Emkinetics, Inc. | Methods and devices for performing electrical stimulation to treat various conditions |
| US10786669B2 (en) | 2006-10-02 | 2020-09-29 | Emkinetics, Inc. | Method and apparatus for transdermal stimulation over the palmar and plantar surfaces |
| US8805508B2 (en) | 2007-05-30 | 2014-08-12 | Medtronic, Inc. | Collecting activity data for evaluation of patient incontinence |
| US8204597B2 (en) | 2007-05-30 | 2012-06-19 | Medtronic, Inc. | Evaluating patient incontinence |
| US20100298906A1 (en) * | 2007-09-20 | 2010-11-25 | Continence Control Systems International Pty Ltd | System, method and apparatus for control of enterostomies |
| US8352026B2 (en) * | 2007-10-03 | 2013-01-08 | Ethicon, Inc. | Implantable pulse generators and methods for selective nerve stimulation |
| US9220889B2 (en) | 2008-02-11 | 2015-12-29 | Intelect Medical, Inc. | Directional electrode devices with locating features |
| US8019440B2 (en) | 2008-02-12 | 2011-09-13 | Intelect Medical, Inc. | Directional lead assembly |
| US9272153B2 (en) | 2008-05-15 | 2016-03-01 | Boston Scientific Neuromodulation Corporation | VOA generation system and method using a fiber specific analysis |
| US9310985B2 (en) | 2008-05-15 | 2016-04-12 | Boston Scientific Neuromodulation Corporation | System and method for determining target stimulation volumes |
| US10603489B2 (en) | 2008-10-09 | 2020-03-31 | Virender K. Sharma | Methods and apparatuses for stimulating blood vessels in order to control, treat, and/or prevent a hemorrhage |
| WO2010042686A1 (en) | 2008-10-09 | 2010-04-15 | Sharma Virender K | Method and apparatus for stimulating the vascular system |
| US8923970B2 (en) | 2008-12-09 | 2014-12-30 | Nephera Ltd. | Stimulation of the urinary system |
| US8725249B2 (en) | 2008-12-09 | 2014-05-13 | Nephera Ltd. | Stimulation of the urinary system |
| JP5734295B2 (ja) | 2009-08-27 | 2015-06-17 | ザ クリーブランド クリニック ファウンデーション | 組織活性の部位を推定するためのシステムおよび方法 |
| JP2013508119A (ja) | 2009-10-26 | 2013-03-07 | エムキネティクス, インコーポレイテッド | 神経、筋肉および身体組織の電磁刺激のための方法および装置 |
| WO2011068997A1 (en) | 2009-12-02 | 2011-06-09 | The Cleveland Clinic Foundation | Reversing cognitive-motor impairments in patients having a neuro-degenerative disease using a computational modeling approach to deep brain stimulation programming |
| WO2011156287A2 (en) | 2010-06-07 | 2011-12-15 | Medtronic, Inc. | Selective termination of stimulation to deliver post-stimulation therapeutic effect |
| US9089699B2 (en) * | 2010-06-07 | 2015-07-28 | Medtronic, Inc. | Adaptive stimulation for treating urgency or incontinence |
| WO2011159688A2 (en) | 2010-06-14 | 2011-12-22 | Boston Scientific Neuromodulation Corporation | Programming interface for spinal cord neuromodulation |
| US9592389B2 (en) | 2011-05-27 | 2017-03-14 | Boston Scientific Neuromodulation Corporation | Visualization of relevant stimulation leadwire electrodes relative to selected stimulation information |
| US8751008B2 (en) | 2011-08-09 | 2014-06-10 | Boston Scientific Neuromodulation Corporation | Remote control data management with correlation of patient condition to stimulation settings and/or with clinical mode providing a mismatch between settings and interface data |
| US8712534B2 (en) | 2011-10-28 | 2014-04-29 | Medtronic, Inc. | Combined high and low frequency stimulation therapy |
| US8818515B2 (en) * | 2012-01-13 | 2014-08-26 | Research Foundation Of The City University Of New York | Voltage limited neurostimulation |
| US10576278B2 (en) | 2012-02-21 | 2020-03-03 | Virender K. Sharma | System and method for electrical stimulation of anorectal structures to treat urinary dysfunction |
| US8706234B2 (en) * | 2012-02-21 | 2014-04-22 | Virender K. Sharma | System and method for electrical stimulation of anorectal structures to treat anal dysfunction |
| US9782583B2 (en) | 2012-02-21 | 2017-10-10 | Virender K. Sharma | System and method for electrical stimulation of anorectal structures to treat urinary dysfunction |
| US9604067B2 (en) | 2012-08-04 | 2017-03-28 | Boston Scientific Neuromodulation Corporation | Techniques and methods for storing and transferring registration, atlas, and lead information between medical devices |
| WO2014036079A2 (en) | 2012-08-28 | 2014-03-06 | Boston Scientific Neuromodulation Corporation | Parameter visualization, selection, and annotation interface |
| US9792412B2 (en) | 2012-11-01 | 2017-10-17 | Boston Scientific Neuromodulation Corporation | Systems and methods for VOA model generation and use |
| CN105142714B (zh) | 2013-01-21 | 2018-02-16 | 卡拉健康公司 | 用于控制震颤的设备和方法 |
| US12453853B2 (en) | 2013-01-21 | 2025-10-28 | Cala Health, Inc. | Multi-modal stimulation for treating tremor |
| CA2906558A1 (en) * | 2013-03-15 | 2014-09-25 | Amit Rajguru | Method and apparatus for transdermal stimulation over the palmar and plantar surfaces |
| US11229789B2 (en) | 2013-05-30 | 2022-01-25 | Neurostim Oab, Inc. | Neuro activator with controller |
| PL3003473T3 (pl) | 2013-05-30 | 2019-05-31 | Neurostim Solutions LLC | Miejscowa stymulacja neurologiczna |
| EP3827874A1 (en) | 2013-11-14 | 2021-06-02 | Boston Scientific Neuromodulation Corporation | Systems and visualization tools for stimulation and sensing of neural systems with system-level interaction models |
| BR112016025203B1 (pt) | 2014-06-02 | 2022-09-06 | Cala Health, Inc | Sistema transcutâneo para tratar um paciente que sofre de tremor |
| US9959388B2 (en) | 2014-07-24 | 2018-05-01 | Boston Scientific Neuromodulation Corporation | Systems, devices, and methods for providing electrical stimulation therapy feedback |
| US10265528B2 (en) | 2014-07-30 | 2019-04-23 | Boston Scientific Neuromodulation Corporation | Systems and methods for electrical stimulation-related patient population volume analysis and use |
| US10272247B2 (en) | 2014-07-30 | 2019-04-30 | Boston Scientific Neuromodulation Corporation | Systems and methods for stimulation-related volume analysis, creation, and sharing with integrated surgical planning and stimulation programming |
| WO2021134085A1 (en) | 2019-12-28 | 2021-07-01 | Jeff Bennett | Apparatus and method of treating an approaching physiological event and increasing an intensity of the event |
| US9974959B2 (en) | 2014-10-07 | 2018-05-22 | Boston Scientific Neuromodulation Corporation | Systems, devices, and methods for electrical stimulation using feedback to adjust stimulation parameters |
| US11077301B2 (en) | 2015-02-21 | 2021-08-03 | NeurostimOAB, Inc. | Topical nerve stimulator and sensor for bladder control |
| CN111494793A (zh) * | 2015-03-20 | 2020-08-07 | 维兰德.K.沙马 | 用于电刺激肛肠结构以治疗排尿功能障碍的系统和方法 |
| US10780283B2 (en) | 2015-05-26 | 2020-09-22 | Boston Scientific Neuromodulation Corporation | Systems and methods for analyzing electrical stimulation and selecting or manipulating volumes of activation |
| AU2016268259B2 (en) | 2015-05-26 | 2019-01-31 | Boston Scientific Neuromodulation Corporation | Systems and methods for analyzing electrical stimulation and selecting or manipulating volumes of activation |
| JP2018516702A (ja) | 2015-06-10 | 2018-06-28 | カラ ヘルス, インコーポレイテッドCala Health, Inc. | 取り外し可能な治療装置および監視装置で振戦を治療するための末梢神経刺激用のシステムおよび方法 |
| US20160375248A1 (en) | 2015-06-29 | 2016-12-29 | Boston Scientific Neuromodulation Corporation | Systems and methods for selecting stimulation parameters based on stimulation target region, effects, or side effects |
| EP3280491B1 (en) | 2015-06-29 | 2023-03-01 | Boston Scientific Neuromodulation Corporation | Systems for selecting stimulation parameters by targeting and steering |
| WO2017053847A1 (en) | 2015-09-23 | 2017-03-30 | Cala Health, Inc. | Systems and methods for peripheral nerve stimulation in the finger or hand to treat hand tremors |
| WO2017062378A1 (en) | 2015-10-09 | 2017-04-13 | Boston Scientific Neuromodulation Corporation | System and methods for clinical effects mapping for directional stimulations leads |
| US11318310B1 (en) | 2015-10-26 | 2022-05-03 | Nevro Corp. | Neuromodulation for altering autonomic functions, and associated systems and methods |
| IL286747B2 (en) | 2016-01-21 | 2024-05-01 | Cala Health Inc | A wearable device for the treatment of symptoms related to the urinary system |
| US11045649B2 (en) | 2016-02-19 | 2021-06-29 | Medtronic, Inc. | Incontinence therapy |
| US10589093B2 (en) | 2016-03-02 | 2020-03-17 | Incube Labs, Llc | Urethral catheters and methods for facilitated introduction into the urinary tract |
| US10716942B2 (en) | 2016-04-25 | 2020-07-21 | Boston Scientific Neuromodulation Corporation | System and methods for directional steering of electrical stimulation |
| CN105769380B (zh) * | 2016-05-30 | 2017-09-22 | 周建 | 骨盆腔内膀胱底外支架和植入方法 |
| EP3458152B1 (en) | 2016-06-24 | 2021-04-21 | Boston Scientific Neuromodulation Corporation | Systems and methods for visual analytics of clinical effects |
| CN117959601A (zh) | 2016-08-25 | 2024-05-03 | 卡拉健康公司 | 通过周围神经刺激治疗心脏机能障碍的系统和方法 |
| US10350404B2 (en) | 2016-09-02 | 2019-07-16 | Boston Scientific Neuromodulation Corporation | Systems and methods for visualizing and directing stimulation of neural elements |
| US10780282B2 (en) | 2016-09-20 | 2020-09-22 | Boston Scientific Neuromodulation Corporation | Systems and methods for steering electrical stimulation of patient tissue and determining stimulation parameters |
| WO2018071865A1 (en) | 2016-10-14 | 2018-04-19 | Boston Scientific Neuromodulation Corporation | Systems and methods for closed-loop determination of stimulation parameter settings for an electrical simulation system |
| CN106512217A (zh) * | 2016-12-21 | 2017-03-22 | 北京品驰医疗设备有限公司 | 一种多个植入式医疗设备协同工作的系统 |
| CN106512218A (zh) * | 2016-12-31 | 2017-03-22 | 北京品驰医疗设备有限公司 | 一种温控充电的植入式医疗设备协同工作的系统 |
| CN106730340A (zh) * | 2016-12-31 | 2017-05-31 | 北京品驰医疗设备有限公司 | 一种可充电的植入式医疗设备协同工作的系统 |
| JP6834005B2 (ja) | 2017-01-03 | 2021-02-24 | ボストン サイエンティフィック ニューロモデュレイション コーポレイション | Mri適合刺激パラメータを選択するためのシステム及び方法 |
| US10589104B2 (en) | 2017-01-10 | 2020-03-17 | Boston Scientific Neuromodulation Corporation | Systems and methods for creating stimulation programs based on user-defined areas or volumes |
| CN106580517B (zh) * | 2017-01-23 | 2018-05-25 | 夏生俊 | 一种人工膀胱装置 |
| US10625082B2 (en) | 2017-03-15 | 2020-04-21 | Boston Scientific Neuromodulation Corporation | Visualization of deep brain stimulation efficacy |
| CA3058786A1 (en) | 2017-04-03 | 2018-10-11 | Cala Health, Inc. | Systems, methods and devices for peripheral neuromodulation for treating diseases related to overactive bladder |
| WO2018187090A1 (en) | 2017-04-03 | 2018-10-11 | Boston Scientific Neuromodulation Corporation | Systems and methods for estimating a volume of activation using a compressed database of threshold values |
| JP6932835B2 (ja) | 2017-07-14 | 2021-09-08 | ボストン サイエンティフィック ニューロモデュレイション コーポレイション | 電気刺激の臨床効果を推定するシステム及び方法 |
| WO2019036180A1 (en) | 2017-08-15 | 2019-02-21 | Boston Scientific Neuromodulation Corporation | SYSTEMS AND METHODS FOR CONTROLLING ELECTRICAL STIMULATION USING MULTIPLE STIMULATION FIELDS |
| CN111601636A (zh) | 2017-11-07 | 2020-08-28 | Oab神经电疗科技公司 | 具有自适应电路的非侵入性神经激活器 |
| EP3784332B1 (en) | 2018-04-27 | 2023-04-26 | Boston Scientific Neuromodulation Corporation | Systems for visualizing and programming electrical stimulation |
| JP7295141B2 (ja) | 2018-04-27 | 2023-06-20 | ボストン サイエンティフィック ニューロモデュレイション コーポレイション | マルチモード電気刺激システム及び製造する及び使用する方法 |
| US11590352B2 (en) | 2019-01-29 | 2023-02-28 | Nevro Corp. | Ramped therapeutic signals for modulating inhibitory interneurons, and associated systems and methods |
| JP2022538419A (ja) | 2019-06-26 | 2022-09-02 | ニューロスティム テクノロジーズ エルエルシー | 適応回路を備えた非侵襲性神経活性化装置 |
| US11311729B2 (en) | 2019-07-30 | 2022-04-26 | Medtronic, Inc. | Titration for sub-threshold electrical stimulation therapy |
| US11383086B2 (en) | 2019-07-30 | 2022-07-12 | Medtronic, Inc. | Posture-based paresthesia threshold or perception threshold determination |
| US11638821B2 (en) | 2019-07-30 | 2023-05-02 | Medtronic, Inc. | Incontinence therapy |
| US12251560B1 (en) | 2019-08-13 | 2025-03-18 | Cala Health, Inc. | Connection quality determination for wearable neurostimulation systems |
| WO2021126921A1 (en) | 2019-12-16 | 2021-06-24 | Neurostim Solutions, Llc | Non-invasive nerve activator with boosted charge delivery |
| JP2024513688A (ja) | 2021-03-12 | 2024-03-27 | アンバー セラピューティクス リミテッド | 失禁制御のためのデバイス、システム、および方法 |
| EP4291294A1 (en) | 2021-04-27 | 2023-12-20 | Boston Scientific Neuromodulation Corporation | Systems and methods for automated programming of electrical stimulation |
| WO2022265977A1 (en) | 2021-06-15 | 2022-12-22 | Boston Scientific Neuromodulation Corporation | Methods and systems for estimating neural activation by stimulation using a stimulation system |
| US12471831B2 (en) | 2021-12-10 | 2025-11-18 | Boston Scientific Neuromodulation Corporation | Systems and methods for generating and using response maps for electrical stimulation |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4607639A (en) * | 1984-05-18 | 1986-08-26 | Regents Of The University Of California | Method and system for controlling bladder evacuation |
| US4881526A (en) * | 1988-05-27 | 1989-11-21 | Empi, Inc. | Intravaginal electrode and stimulation system for controlling female urinary incontinence |
| US5447526A (en) * | 1992-12-24 | 1995-09-05 | Karsdon; Jeffrey | Transcutaneous electric muscle/nerve controller/feedback unit |
| EP1319422A3 (en) * | 1996-02-15 | 2004-01-28 | Nihon Kohden Corporation | An apparatus for treating urinary incontinence |
| US7225019B2 (en) * | 1996-04-30 | 2007-05-29 | Medtronic, Inc. | Method and system for nerve stimulation and cardiac sensing prior to and during a medical procedure |
| US6735474B1 (en) * | 1998-07-06 | 2004-05-11 | Advanced Bionics Corporation | Implantable stimulator system and method for treatment of incontinence and pain |
| CA2336190A1 (en) * | 1998-07-06 | 2000-01-13 | Advanced Bionics Corporation | Implantable stimulator system and method for treatment of urinary incontinence |
| AUPQ202699A0 (en) * | 1999-08-04 | 1999-08-26 | University Of Melbourne, The | Prosthetic device for incontinence |
| US6393323B1 (en) * | 2000-01-31 | 2002-05-21 | Mcgill University | Electronic stimulator implant for modulating and synchronizing bladder and sphincter function |
| US6907293B2 (en) * | 2001-03-30 | 2005-06-14 | Case Western Reserve University | Systems and methods for selectively stimulating components in, on, or near the pudendal nerve or its branches to achieve selective physiologic responses |
| WO2004047914A1 (en) * | 2001-11-29 | 2004-06-10 | Biocontrol Medical Ltd. | Pelvic disorder treatment device |
| US20050010260A1 (en) * | 2002-09-06 | 2005-01-13 | Medtronic, Inc. | Method, system and device for treating disorders of the pelvic floor by electrical stimulation of and drug delivery to the pudendal and sacral nerves |
| US6990376B2 (en) * | 2002-12-06 | 2006-01-24 | The Regents Of The University Of California | Methods and systems for selective control of bladder function |
-
2006
- 2006-03-02 JP JP2007557279A patent/JP2008531138A/ja not_active Abandoned
- 2006-03-02 US US11/885,503 patent/US20090054950A1/en not_active Abandoned
- 2006-03-02 EP EP06704933A patent/EP1853344A4/en not_active Withdrawn
- 2006-03-02 WO PCT/AU2006/000258 patent/WO2006092007A1/en not_active Ceased
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2021531852A (ja) * | 2018-07-11 | 2021-11-25 | ディグニファイ セラピューティクス エルエルシー | 排泄機能障害の治療方法 |
| JP7485379B2 (ja) | 2018-07-11 | 2024-05-16 | ディグニファイ セラピューティクス エルエルシー | 排泄物貯留を制御するための刺激デバイス |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1853344A4 (en) | 2008-05-28 |
| WO2006092007A1 (en) | 2006-09-08 |
| US20090054950A1 (en) | 2009-02-26 |
| EP1853344A1 (en) | 2007-11-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2008531138A (ja) | 失禁を治療するための改良された方法および装置 | |
| US11471670B2 (en) | Electrical stimulator for treatment of back pain and methods of use | |
| US11052254B2 (en) | Methods and systems of electrode polarity switching in electrical stimulation therapy | |
| US6735474B1 (en) | Implantable stimulator system and method for treatment of incontinence and pain | |
| US20080275524A1 (en) | Implantable Electrode Arrangement | |
| JP2006508768A (ja) | 膀胱機能の選択的な調整のための方法とシステム | |
| US20080071321A1 (en) | Systems and methods of neuromodulation stimulation for the restoration of sexual function | |
| WO2009023543A2 (en) | Systems and methods of neuromodulation stimulation for the restoration of sexual function | |
| JP2009534133A (ja) | 勃起不全に対処するための方法および装置 | |
| US11684774B2 (en) | Electrical stimulator for treatment of back pain and methods of use | |
| US20230067424A1 (en) | Electrical stimulator for the treatment of back pain and methods of use | |
| US20100010563A1 (en) | Method and Apparatus for Treating Fecal Incontinence | |
| JP2010502406A (ja) | 脱出症に関連する状態を治療するための方法および装置 | |
| AU2005301103A1 (en) | An implantable electrode arrangement | |
| AU2006220228A1 (en) | Improved method and apparatus for treating incontinence | |
| US12496448B2 (en) | Method and system for electrical nerve stimulation | |
| US20100298906A1 (en) | System, method and apparatus for control of enterostomies | |
| AU2006301939A1 (en) | A method and apparatus for treating fecal incontinence |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090302 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20090302 |
|
| A762 | Written abandonment of application |
Free format text: JAPANESE INTERMEDIATE CODE: A762 Effective date: 20110307 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20110308 |