JP2005298330A - Synthetic quartz glass and its manufacturing method - Google Patents
Synthetic quartz glass and its manufacturing method Download PDFInfo
- Publication number
- JP2005298330A JP2005298330A JP2005131342A JP2005131342A JP2005298330A JP 2005298330 A JP2005298330 A JP 2005298330A JP 2005131342 A JP2005131342 A JP 2005131342A JP 2005131342 A JP2005131342 A JP 2005131342A JP 2005298330 A JP2005298330 A JP 2005298330A
- Authority
- JP
- Japan
- Prior art keywords
- quartz glass
- fluorine
- synthetic quartz
- concentration
- less
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03B—MANUFACTURE, SHAPING, OR SUPPLEMENTARY PROCESSES
- C03B19/00—Other methods of shaping glass
- C03B19/14—Other methods of shaping glass by gas- or vapour- phase reaction processes
- C03B19/1453—Thermal after-treatment of the shaped article, e.g. dehydrating, consolidating, sintering
- C03B19/1461—Thermal after-treatment of the shaped article, e.g. dehydrating, consolidating, sintering for doping the shaped article with flourine
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03B—MANUFACTURE, SHAPING, OR SUPPLEMENTARY PROCESSES
- C03B19/00—Other methods of shaping glass
- C03B19/14—Other methods of shaping glass by gas- or vapour- phase reaction processes
- C03B19/1453—Thermal after-treatment of the shaped article, e.g. dehydrating, consolidating, sintering
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03B—MANUFACTURE, SHAPING, OR SUPPLEMENTARY PROCESSES
- C03B2201/00—Type of glass produced
- C03B2201/06—Doped silica-based glasses
- C03B2201/07—Impurity concentration specified
- C03B2201/075—Hydroxyl ion (OH)
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03B—MANUFACTURE, SHAPING, OR SUPPLEMENTARY PROCESSES
- C03B2201/00—Type of glass produced
- C03B2201/06—Doped silica-based glasses
- C03B2201/08—Doped silica-based glasses doped with boron or fluorine or other refractive index decreasing dopant
- C03B2201/12—Doped silica-based glasses doped with boron or fluorine or other refractive index decreasing dopant doped with fluorine
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P40/00—Technologies relating to the processing of minerals
- Y02P40/50—Glass production, e.g. reusing waste heat during processing or shaping
- Y02P40/57—Improving the yield, e-g- reduction of reject rates
Abstract
Description
本発明は、波長400nm以下の紫外線を光源とする装置の光学部材に用いられる合成石英ガラスおよびその製造方法に関する。より詳細にはエキシマレーザ(XeCl:308nm、KrF:248nm、ArF:193nm)、F2レーザ(157nm)、低圧水銀ランプ(185nm)、Xe2 *エキシマランプ(172nm)、重水素ランプ(110〜400nm)などの光源から発せられる紫外域から真空紫外域までの光に用いられるレンズ(投影系、照明系)、プリズム、エタロン、フォトマスク、ペリクル(ペリクル材、ペリクルフレームまたはその両者)、窓材などの光学部材(製品化したものと半製品化したものとを含む)として用いられる合成石英ガラスおよびその製造方法に関する。 The present invention relates to a synthetic quartz glass used for an optical member of an apparatus using an ultraviolet ray having a wavelength of 400 nm or less as a light source, and a method for producing the same. More specifically, excimer laser (XeCl: 308 nm, KrF: 248 nm, ArF: 193 nm), F 2 laser (157 nm), low-pressure mercury lamp (185 nm), Xe 2 * excimer lamp (172 nm), deuterium lamp (110-400 nm) ) Etc. Lenses (projection system, illumination system), prism, etalon, photomask, pellicle (pellicle material, pellicle frame or both), window material, etc. The present invention relates to a synthetic quartz glass used as an optical member (including a commercialized product and a semifinished product) and a method for producing the same.
合成石英ガラスは、近赤外域から真空紫外域までの広範囲の波長域にわたって透明な材料であること、熱膨張係数がきわめて小さく寸法安定性に優れていること、また、金属不純物をほとんど含有しておらず高純度であることなどの特徴がある。そのため、従来のg線(436nm)、i線(365nm)を光源として用いた光学装置の光学部材には合成石英ガラスが主に用いられてきた。 Synthetic quartz glass is a transparent material over a wide wavelength range from the near infrared region to the vacuum ultraviolet region, has a very low thermal expansion coefficient and excellent dimensional stability, and contains almost no metal impurities. It is characterized by high purity. Therefore, synthetic quartz glass has been mainly used as an optical member of an optical device using conventional g-line (436 nm) and i-line (365 nm) as a light source.
近年、LSIの高集積化に伴い、ウエハ上に集積回路パターンを描画する光リソグラフィ技術において、より線幅の細い微細な描画技術が要求されており、これに対応するために露光光源の短波長化が進められている。例えばリソグラフィ用ステッパの光源には、従来のg線、i線に代わって、KrFエキシマレーザ、ArFエキシマレーザが用いられつつあり、さらにはF2レーザが用いられようとしている。 In recent years, along with the high integration of LSI, optical lithography technology that draws an integrated circuit pattern on a wafer requires a finer drawing technology with a narrower line width. Is being promoted. For example, KrF excimer lasers and ArF excimer lasers are being used in place of conventional g-line and i-line as light sources for lithography steppers, and F 2 lasers are being used.
また、低圧水銀ランプ、Xe2 *エキシマランプや重水素ランプは、1)光CVDなどの装置、2)シリコンウェハのアッシング装置やエッチング装置、または3)オゾン発生装置などに用いられており、また今後光リソグラフィ技術に適用すべく開発が進められている。低圧水銀ランプ、エキシマランプ、重水素ランプなどに用いられるガス封入管、または前述の短波長光源を用いた光学装置など、これらの短波長光を照射して用いる光学部材にも合成石英ガラスを用いる必要がある。 Low-pressure mercury lamps, Xe 2 * excimer lamps and deuterium lamps are used in 1) equipment for photo-CVD, 2) ashing and etching equipment for silicon wafers, or 3) ozone generators, etc. Development is underway to apply to photolithography technology. Synthetic quartz glass is also used for optical members that are irradiated with these short-wavelength light, such as gas-filled tubes used in low-pressure mercury lamps, excimer lamps, deuterium lamps, or optical devices using the short-wavelength light sources described above. There is a need.
これらの光学部材に用いられる合成石英ガラスは、紫外域から真空紫外域にわたる波長での光透過性が要求されるとともに、紫外線照射により透過率が低下しないこと(以下、単に「耐紫外線性」という)が要求される。また、ArFエキシマレーザ、F2レーザ、低圧水銀ランプ、Xe2 *エキシマランプ、重水素ランプ等等の光を照射して用いられる光学部材には、波長200nm以下の真空紫外域での光透過性(以下、単に「真空紫外線透過性」という)に優れることが要求される。また、波長200nm以下の光に使用する光学部材では、従来よりもさらに屈折率変動幅(Δn)が小さいこと(以下、「均質性」という)も要求される。 Synthetic quartz glass used in these optical members is required to have light transmittance at wavelengths ranging from the ultraviolet region to the vacuum ultraviolet region, and the transmittance is not lowered by ultraviolet irradiation (hereinafter simply referred to as “ultraviolet resistance”). ) Is required. Optical members used for irradiation with light such as ArF excimer laser, F 2 laser, low-pressure mercury lamp, Xe 2 * excimer lamp, deuterium lamp, etc. are light transmissive in the vacuum ultraviolet region with a wavelength of 200 nm or less. (Hereinafter simply referred to as “vacuum ultraviolet ray permeability”). In addition, an optical member used for light with a wavelength of 200 nm or less is required to have a smaller refractive index fluctuation range (Δn) than before (hereinafter referred to as “homogeneity”).
従来の合成石英ガラスでは、例えばKrFエキシマレーザやArFエキシマレーザなどの光源から発せられる高エネルギ光を照射すると、紫外域に新たな吸収帯を生じ、紫外線を光源とした光学系を構築する際の光学部材としては問題があった。すなわち、紫外線が長時間照射されると、いわゆるE’センタ(≡Si・)と呼ばれる略215nmの吸収帯とNBOHC(非架橋酸素ラジカル:≡Si−O・)と呼ばれる略260nmの吸収帯が生起する。 In conventional synthetic quartz glass, for example, when high energy light emitted from a light source such as a KrF excimer laser or an ArF excimer laser is irradiated, a new absorption band is generated in the ultraviolet region, and an optical system using ultraviolet light as a light source is constructed. There was a problem as an optical member. That is, when ultraviolet rays are irradiated for a long time, an absorption band of about 215 nm called E ′ center (≡Si ·) and an absorption band of about 260 nm called NBOHC (non-bridging oxygen radical: ≡Si—O ·) are generated. To do.
これらの吸収帯が生起する原因は大きく二つに分類でき、一つは合成石英ガラス中の構造欠陥、すなわち≡Si−Si≡(酸素欠乏型欠陥)や≡Si−Hなどの還元型欠陥、あるいは≡Si−O−O−Si≡などの酸化型欠陥によるもの、別の一つは合成石英ガラス中の不安定な構造、すなわち三員環構造や四員環構造によるものである。これらの欠陥が、次式(1)〜(4)に示すように、紫外線照射により切断され、常磁性欠陥(E’センタおよびNBOHC)が生成し、常磁性欠陥があると透過率の低下、耐紫外線性の低下、絶対屈折率の上昇、屈折率分布の変動や蛍光が生じると考えられている。 The causes of these absorption bands can be broadly classified into two types: one is a structural defect in synthetic quartz glass, that is, a reduced defect such as ≡Si—Si≡ (oxygen-deficient defect) or ≡Si—H, Alternatively, it is due to an oxidation type defect such as ≡Si—O—O—Si≡, and another is due to an unstable structure in the synthetic quartz glass, that is, a three-membered ring structure or a four-membered ring structure. As shown in the following formulas (1) to (4), these defects are cut by irradiation with ultraviolet rays to generate paramagnetic defects (E ′ center and NBOHC). If there are paramagnetic defects, the transmittance decreases. It is considered that the ultraviolet resistance decreases, the absolute refractive index increases, the refractive index distribution fluctuates and fluorescence occurs.
≡Si−Si≡ + hν → 2≡Si・ (1)
≡Si−H + hν → ≡Si・ + H・ (2)
≡Si−O−O−Si≡ + hν → 2≡Si−O・ (3)
≡Si−O−Si≡ + hν →≡Si・ + ≡Si−O・ (4)
≡Si-Si≡ + hν → 2≡Si · (1)
≡Si-H + hν → ≡Si · + H · (2)
≡Si-O-O-Si≡ + hν → 2≡Si-O. (3)
≡Si-O-Si≡ + hν → ≡Si · + ≡Si-O · (4)
これらの問題を解決するために種々の方法が検討されており、合成石英ガラス中に水素分子を何らかの形で含有させればよいことが知られている。例えば、特許文献1には、合成石英ガラス中に水素分子を5×1016分子/cm3以上含有し、かつOH基を100ppm以上含有させることにより、紫外線照射による透過率低下を抑制する方法が開示されている。 Various methods have been studied in order to solve these problems, and it is known that hydrogen molecules may be contained in some form in synthetic quartz glass. For example, Patent Document 1 discloses a method for suppressing a decrease in transmittance due to ultraviolet irradiation by containing 5 × 10 16 molecules / cm 3 or more of hydrogen molecules in synthetic quartz glass and 100 ppm or more of OH groups. It is disclosed.
しかし合成石英ガラス中のOH基は、紫外線照射により下記式(5)の反応が進んでNBOHCが生じ260nm吸収および650nm蛍光が生成するため問題であった。
≡Si−OH + hν → ≡Si−O・(NBOHC)+H・ (5)
However, the OH group in the synthetic quartz glass is a problem because the reaction of the following formula (5) proceeds due to ultraviolet irradiation, NBOHC is generated, and 260 nm absorption and 650 nm fluorescence are generated.
≡Si-OH + hν → ≡Si-O · (NBOHC) + H · (5)
たとえ水素分子を含有させても式(5)の反応を完全には防げず、特にOH基濃度が多い場合には650nm蛍光が強くなり、問題であった。またOH基濃度が多いと、150〜180nmにおける光の透過率が低下するため、特に低圧水銀ランプ、Xe2 *エキシマランプ、F2レーザなどを光源とする装置に使用される場合には問題であった。 Even if hydrogen molecules were contained, the reaction of formula (5) could not be completely prevented, and particularly when the OH group concentration was high, the 650 nm fluorescence became strong, which was a problem. In addition, when the OH group concentration is high, the light transmittance at 150 to 180 nm is lowered, which is a problem particularly when used in an apparatus using a low pressure mercury lamp, Xe 2 * excimer lamp, F 2 laser or the like as a light source. there were.
こうした問題を解決すべく、特許文献2には、OH基濃度が10ppm以下かつハロゲン濃度が400ppm以上であり水素分子を含有する合成石英ガラスが提案されている。この合成石英ガラスによれば、OH基濃度が少ないため耐紫外線性にも優れ、さらに150〜180nmにおいて高い透過率が得られる。 In order to solve such a problem, Patent Document 2 proposes a synthetic quartz glass having an OH group concentration of 10 ppm or less and a halogen concentration of 400 ppm or more and containing hydrogen molecules. According to this synthetic quartz glass, since the OH group concentration is low, it is excellent in ultraviolet resistance, and a high transmittance is obtained at 150 to 180 nm.
この特許文献2には、(1)ガラス形成原料を火炎加水分解して多孔質石英ガラス体を形成する工程と、(2)多孔質石英ガラス体をハロゲン含有雰囲気下にて800〜1250℃の温度で加熱し脱水処理する工程と、(3)脱水処理した多孔質石英ガラス体を透明ガラス化温度まで昇温し透明ガラス化する工程と、(4)透明ガラス化した合成石英ガラスを水素を含む雰囲気下で500〜1100℃の温度にて加熱処理し水素を含有させる工程とからなることを特徴とする製造方法が提案されている。 This Patent Document 2 includes (1) a step of flame hydrolysis of a glass forming raw material to form a porous quartz glass body, and (2) a porous quartz glass body at 800 to 1250 ° C. in a halogen-containing atmosphere. A step of dehydrating by heating at a temperature; (3) a step of raising the temperature of the dehydrated porous quartz glass body to a transparent vitrification temperature and converting it to transparent vitrification; and (4) hydrogenation of the synthetic quartz glass converted to transparent glass. The manufacturing method characterized by including the process of heat-processing at the temperature of 500-1100 degreeC in the atmosphere to contain and containing hydrogen is proposed.
さらに、高温で水素を含有した雰囲気下に合成石英ガラスを保持すると≡Si−Si≡および≡Si−Hの還元型欠陥が生成しやすくなるため、特許文献3には、特許文献2に開示の方法とほぼ同様に、透明ガラス化したフッ素含有石英ガラスを形成し、さらに500℃以下の温度で水素を含有した雰囲気下で水素を含有させる製造方法が提案されている。 Furthermore, when synthetic quartz glass is held in an atmosphere containing hydrogen at a high temperature, reduced defects of ≡Si—Si≡ and ≡Si—H are likely to be generated. Almost the same as the method, there has been proposed a production method in which a fluorine-containing quartz glass that has been made into a transparent glass is formed, and hydrogen is contained in an atmosphere containing hydrogen at a temperature of 500 ° C. or lower.
しかし本発明者らが、特許文献2や特許文献3に記載される方法について検討した結果、必ずしも充分な耐紫外線性が得られない場合があることが判明した。すなわち、多孔質石英ガラス体をフッ素化合物を含んだ雰囲気下で800〜1250℃の高温で処理すると、前記≡Si−Si≡欠陥が生成する。この≡Si−Si≡欠陥は、前述のように紫外線照射によりE’センタを生成するだけでなく、245nmおよび163nmに吸収を持つため問題であった。 However, as a result of studying the methods described in Patent Document 2 and Patent Document 3, the present inventors have found that sufficient UV resistance may not always be obtained. That is, when the porous quartz glass body is treated at a high temperature of 800 to 1250 ° C. in an atmosphere containing a fluorine compound, the ≡Si—Si≡ defect is generated. This ≡Si—Si≡ defect is a problem because it not only generates an E ′ center by ultraviolet irradiation as described above but also has absorption at 245 nm and 163 nm.
また≡Si−Si≡欠陥は、たとえ水素含有処理を行っても下記式(6)により≡Si−Hが生成し、この≡Si−Hも紫外線照射によりE’センタを生成するため問題であった。
≡Si−Si≡ + H2 → ≡Si−H + ≡Si−H (6)
The ≡Si—Si≡ defect is a problem because ≡Si—H is generated by the following formula (6) even if hydrogen-containing treatment is performed, and this ≡Si—H also generates an E ′ center by ultraviolet irradiation. It was.
≡Si-Si≡ + H 2 → ≡Si-H + ≡Si-H (6)
一方、真空紫外線透過性の向上を図るために、特許文献4には、OH基濃度が200ppm以下、塩素濃度が2ppm以下、かつ≡Si−Si≡濃度が1×1015個/cm3以下である合成石英ガラスが提案されている。特許文献5には、OH基濃度が10〜400ppmかつ還元型欠陥および酸化型欠陥の濃度がそれぞれ5×1016個/cm3以下である合成石英ガラスが提案されている。特許文献6には、OH基濃度が100〜2000ppm、かつ遷移金属、アルカリ金属やアルカリ土類金属をそれぞれ所定濃度以下含む合成石英ガラスが提案されている。
これら従来の合成石英ガラスは、いずれもOH基濃度を所定の範囲にすることにより真空紫外線透過性の向上を図るものであるが、必ずしも真空紫外域において高い透過率が得られなかった。
On the other hand, in order to improve the vacuum ultraviolet ray permeability, Patent Document 4 discloses that the OH group concentration is 200 ppm or less, the chlorine concentration is 2 ppm or less, and the ≡Si—Si≡ concentration is 1 × 10 15 atoms / cm 3 or less. A synthetic quartz glass has been proposed. Patent Document 5 proposes a synthetic quartz glass having an OH group concentration of 10 to 400 ppm and a concentration of reduced defects and oxidized defects of 5 × 10 16 pieces / cm 3 or less. Patent Document 6 proposes a synthetic quartz glass having an OH group concentration of 100 to 2000 ppm and containing a transition metal, an alkali metal, and an alkaline earth metal at a predetermined concentration or less.
All of these conventional synthetic quartz glasses are intended to improve the vacuum ultraviolet transmittance by setting the OH group concentration within a predetermined range, but a high transmittance is not necessarily obtained in the vacuum ultraviolet region.
また、合成石英ガラスの均質性を確保する方法として、特許文献7には、合成石英ガラス中にOH基および塩素を含有させ、OH基および塩素濃度の変動幅を調整する方法が提案されている。しかしながら、塩素は≡Si−Clの形で合成石英ガラス中に存在し、この≡Si−Clの結合は結合エネルギーが7〜8eVと弱く、紫外線照射によって下式に示すように容易に開裂し、やはりE’センタが生起する。
≡Si−Cl + hν → ≡Si・(E’センタ) + Cl・
したがって、上記特許文献に示される方法では均質性に優れた合成石英ガラスが得られるものの、耐紫外線性に問題があった。
Further, as a method for ensuring the homogeneity of the synthetic quartz glass, Patent Document 7 proposes a method of adjusting the fluctuation range of the OH group and chlorine concentration by including OH groups and chlorine in the synthetic quartz glass. . However, chlorine is present in synthetic quartz glass in the form of ≡Si—Cl, and this ≡Si—Cl bond has a weak bond energy of 7 to 8 eV and is easily cleaved as shown in the following formula by ultraviolet irradiation. After all, E 'center occurs.
≡Si-Cl + hν → ≡Si · (E 'center) + Cl ·
Therefore, although the method disclosed in the above-mentioned patent document can obtain a synthetic quartz glass excellent in homogeneity, there is a problem in ultraviolet resistance.
本発明は、E’センタおよび蛍光発光の発生が低減され、耐紫外線性に優れる合成石英ガラスを提供する。
本発明は、また、真空紫外線透過性に優れる合成石英ガラスを提供する。
本発明は、均質性に優れる合成石英ガラスを提供する。
本発明は、これらの合成石英ガラスを製造するために好適な方法を提供する。
The present invention provides a synthetic quartz glass with reduced generation of E ′ center and fluorescence and excellent in ultraviolet resistance.
The present invention also provides a synthetic quartz glass excellent in vacuum ultraviolet ray permeability.
The present invention provides a synthetic quartz glass excellent in homogeneity.
The present invention provides a suitable method for producing these synthetic quartz glasses.
本発明者らは、耐紫外線性および紫外線透過性に対して、合成石英ガラス中のハロゲン濃度が及ぼす影響、ならびに合成石英ガラス中の不安定な構造が及ぼす影響について、詳細な検討を行なった。その結果、合成石英ガラス中において、フッ素は≡Si−Fの形で存在し、この≡Si−F結合は結合エネルギーが20eV以上と非常に強く紫外線照射によっても開裂しないため、耐紫外線性については問題ないことを知見した。さらにフッ素は、その機構は定かではないが、石英ガラス中の歪んだ構造を低減し、耐紫外線性を改善することを知見した。 The present inventors have conducted detailed studies on the influence of the halogen concentration in the synthetic quartz glass and the influence of the unstable structure in the synthetic quartz glass on the ultraviolet resistance and ultraviolet transmittance. As a result, in synthetic quartz glass, fluorine exists in the form of ≡Si-F, and this ≡Si-F bond is very strong with a bond energy of 20 eV or more and is not cleaved by ultraviolet irradiation. I found that there was no problem. Furthermore, although the mechanism is not clear, it has been found that fluorine reduces the distorted structure in quartz glass and improves the UV resistance.
そこで、本発明は、フッ素を含有し、レーザラマンスペクトルにおける800cm-1の散乱ピーク強度I800に対する2250cm-1の散乱ピーク強度I2250の値(I2250/I800)が1×10-4以下であり、かつ245nmの光の吸収係数(以下、単に245nmの吸収係数という)が2×10-3cm-1以下である合成石英ガラスを提供する。 The present invention contain fluorine, the value of the scattering peak intensity I 2250 of 2250 cm -1 relative to the scattering peak intensity I 800 of the 800 cm -1 in the laser Raman spectrum (I 2250 / I 800) is 1 × 10 -4 or less A synthetic quartz glass having a light absorption coefficient of 245 nm (hereinafter simply referred to as an absorption coefficient of 245 nm) is 2 × 10 −3 cm −1 or less.
800cm-1の散乱ピークは≡Si−O−の結合(ケイ素と酸素との間の基本振動)を示すピークであり、2250cm-1の散乱ピークは還元型欠陥である≡Si−Hの結合を示すピークであって、I2250/I800の値は、≡Si−H欠陥の濃度(≡Si−H濃度)の指標となる。本発明においては、I2250/I800が1×10-4以下であることが重要である。1×10-4超では、E’センタを生起しやすい。 Scattering peak of 800 cm -1 is a peak indicating the bond of ≡Si-O-(fundamental vibration between silicon and oxygen), the scattering peak of 2250 cm -1 is the binding of ≡Si-H is a reduction type defects The value of I 2250 / I 800 is an index of ≡Si—H defect concentration (≡Si—H concentration). In the present invention, it is important that I 2250 / I 800 is 1 × 10 −4 or less. If it exceeds 1 × 10 −4 , the E ′ center is likely to occur.
245nmの吸収係数は、やはり還元型欠陥である≡Si−Si≡欠陥の濃度の指標となる。本発明においては、245nmの吸収係数が2×10-3cm-1以下であることが重要である。2×10-3cm-1超では、やはりE’センタを生起しやすい。また、2×10-3cm-1超では、150〜180nmにおける高透過性の達成が困難となる。また、163nmの光の吸収も低減されていることが好ましい。
本発明における2250cm-1の散乱ピークの規定、および245nmの吸収係数の規定は、還元型欠陥量を規定するものである。
The absorption coefficient of 245 nm is an index of the concentration of ≡Si—Si≡ defect, which is also a reduced defect. In the present invention, it is important that the absorption coefficient at 245 nm is 2 × 10 −3 cm −1 or less. If it exceeds 2 × 10 −3 cm −1 , the E ′ center is likely to occur. If it exceeds 2 × 10 −3 cm −1 , it will be difficult to achieve high transparency at 150 to 180 nm. Moreover, it is preferable that absorption of light of 163 nm is also reduced.
The definition of the scattering peak at 2250 cm −1 and the absorption coefficient at 245 nm in the present invention define the amount of reduced defects.
E’センタの濃度は、KrFエキシマレーザ光をショット照射した直後の214nmの光の透過率を紫外可視分光光度計により測定し、照射前後での吸収係数変化量Δk214[cm-1]を求めることで評価できる。Δk214は、1×10-1以下であることが好ましい。特に、1×10-2以下であることが好ましい。 The concentration of the E ′ center is obtained by measuring the transmittance of 214 nm light immediately after the shot irradiation with KrF excimer laser light with an ultraviolet-visible spectrophotometer, and obtaining the absorption coefficient change Δk 214 [cm −1 ] before and after the irradiation. Can be evaluated. Δk 214 is preferably 1 × 10 −1 or less. In particular, it is preferably 1 × 10 −2 or less.
蛍光発光の程度は、KrFエキシマレーザ光をショット照射した場合の650nm蛍光強度L650およびKrFエキシマレーザ散乱光強度S248をKrFレーザ光の入射軸の直角方向から測定し、KrFレーザ(248nm)散乱光強度に対する650nm蛍光強度の比L650/S248を求めることにより評価できる。L650/S248は、5×10-4以下であることが好ましく、1×10-4以下であることが特に好ましい。 The degree of fluorescence emission is measured by measuring 650 nm fluorescence intensity L 650 and KrF excimer laser scattered light intensity S 248 when shot with KrF excimer laser light from a direction perpendicular to the incident axis of KrF laser light, and KrF laser (248 nm) scattering. It can be evaluated by determining the ratio L 650 / S 248 of the fluorescence intensity at 650 nm to the light intensity. L 650 / S 248 is preferably 5 × 10 −4 or less, and particularly preferably 1 × 10 −4 or less.
また、本発明者らは、耐紫外線性に対する合成石英ガラス中のハロゲン濃度およびOH基濃度の影響についてさらに詳細な検討を行った結果、合成石英ガラス中において、フッ素と塩素はその作用が異なり、塩素は≡Si−Clの形で合成石英ガラス中に存在し、この≡Si−Cl結合は結合エネルギが7〜8eVと弱く、紫外線照射によって次式(7)
≡Si−Cl + hν → ≡Si・(E'センター)+ Cl・ (7)
に示すように容易に開裂し、前記E'センターを生じるため、耐紫外線性を低下させることを知見した。
In addition, as a result of further detailed examination of the influence of the halogen concentration and OH group concentration in the synthetic quartz glass on the ultraviolet resistance, the present inventors have different effects in fluorine and chlorine in the synthetic quartz glass, Chlorine is present in synthetic quartz glass in the form of ≡Si—Cl, and this ≡Si—Cl bond has a weak bond energy of 7-8 eV.
≡Si-Cl + hν → ≡Si · (E 'center) + Cl · (7)
It was found that it was easily cleaved to produce the E ′ center as shown in FIG.
なお塩素を含有しないガラス原料を用いて製造した、塩素を含有しない合成石英ガラスも提案されている(特開平7−291635号公報)。これは高エネルギ光線の照射による透過率の低下抑止のためにフッ素濃度を1000ppm以上とし、酸素欠乏型欠陥≡Si−Si≡による245nmでの吸収を抑制するためにOH基濃度を50ppm以上としたものであるが、その反面150〜180nmにおける透過率の低下問題に言及しておらず、低圧水銀ランプ、Xe2 *エキシマランプおよびF2レーザなどを光源とする装置に使用するに際し支障があった。 A synthetic quartz glass containing no chlorine and produced using a glass raw material not containing chlorine has also been proposed (Japanese Patent Laid-Open No. 7-291635). This is because the fluorine concentration is set to 1000 ppm or more in order to suppress the decrease in the transmittance due to the irradiation of the high energy beam, and the OH group concentration is set to 50 ppm or more in order to suppress the absorption at 245 nm due to the oxygen deficient defect ≡Si—Si≡. However, it does not mention the problem of a decrease in transmittance at 150 to 180 nm, and there is a problem in using it in a device using a low-pressure mercury lamp, Xe 2 * excimer lamp, F 2 laser or the like as a light source. .
そこで常磁性欠陥の生成自体を抑制して、本質的な耐紫外線性の向上を達成するためには、合成石英ガラス中のOH基、塩素、フッ素濃度の最適化を図る必要があると考え、この点に関する検討をさらに行った結果、合成石英ガラス中のフッ素濃度を増やし、塩素濃度を低減すれば、OH基濃度がやや少なくなっても耐紫外線性に優れた合成石英ガラスが得られることを見い出した。 Therefore, in order to suppress the formation of paramagnetic defects and achieve an essential improvement in UV resistance, it is necessary to optimize the OH group, chlorine and fluorine concentrations in the synthetic quartz glass. As a result of further studies on this point, it was found that if the fluorine concentration in the synthetic quartz glass is increased and the chlorine concentration is reduced, a synthetic quartz glass excellent in UV resistance can be obtained even if the OH group concentration is slightly reduced. I found it.
すなわち、本発明は、フッ素を含有して還元型欠陥を特定量以下とし、塩素濃度が100ppm以下である合成石英ガラスを提供する。特に、合成石英における不安定な構造、E’センター、蛍光発光の抑制に有効で、優れた耐紫外線性を示す合成石英ガラスとして、合成石英ガラス中のOH基濃度が50ppm未満、フッ素濃度が100ppm以上、塩素濃度が100ppm以下、水素分子濃度が5×1016分子/cm3以上であることを特徴とする合成石英ガラスが好ましい。 That is, the present invention provides a synthetic quartz glass containing fluorine, having reduced defects below a specific amount, and having a chlorine concentration of 100 ppm or less. In particular, the synthetic quartz glass is effective in suppressing the unstable structure, E ′ center, and fluorescence emission in synthetic quartz, and exhibits excellent UV resistance. The synthetic quartz glass has an OH group concentration of less than 50 ppm and a fluorine concentration of 100 ppm. As described above, a synthetic quartz glass having a chlorine concentration of 100 ppm or less and a hydrogen molecule concentration of 5 × 10 16 molecules / cm 3 or more is preferable.
また、合成石英ガラス中のハロゲン濃度および水素分子濃度が及ぼす影響、ならびに石英ガラス中の不安定な構造が及ぼす影響の相互の関係について検討した。その結果、フッ素ドープにより不安定な構造の存在量を一定の限度まで低減するとともに、水素分子の含有による常磁性欠陥の修復作用を併用すれば、短波長光源から発せられる光に対する合成石英ガラスの紫外線透過性および耐紫外線性を満足できるレベルまで向上できることを知見した。 In addition, the relationship between the effects of halogen concentration and hydrogen molecule concentration in synthetic quartz glass and the influence of unstable structure in quartz glass were investigated. As a result, the amount of unstable structure due to fluorine doping is reduced to a certain limit, and when combined with the repairing of paramagnetic defects due to the inclusion of hydrogen molecules, synthetic quartz glass with respect to light emitted from short-wavelength light sources can be used. It was found that the UV transmittance and UV resistance can be improved to a satisfactory level.
そこで、本発明の合成石英ガラスの中でも、石英ガラス中の不安定な構造に帰属されるレーザラマンスペクトルの495cm-1の散乱ピーク強度(I1)および606cm-1の散乱ピーク強度(I2)と、440cm-1の散乱ピーク強度(I0)との強度比I1/I0およびI2/I0が特定の範囲にある合成石英ガラスが、紫外線透過性および耐紫外線性の向上に有効であることを知見した。 Therefore, among the synthetic quartz glass of the present invention, the scattering peak intensity of the quartz glass unstable structure to the scattering peak intensity of the laser Raman spectrum of 495cm -1 attributed in (I 1) and 606 cm -1 and (I 2) Synthetic quartz glass in which the intensity ratios I 1 / I 0 and I 2 / I 0 with respect to the scattering peak intensity (I 0 ) of 440 cm −1 are in a specific range is effective in improving ultraviolet transmittance and ultraviolet resistance. I found out that there was.
そこで、本発明は、前記知見に基づき、フッ素を含有して、還元型欠陥を特定量以下として、かつレーザーラマンスペクトルにおける495cm-1の散乱ピーク強度(I1)および606cm-1の散乱ピーク強度(I2)が、440cm-1の散乱ピーク強度(I0)に対してそれぞれI1/I0≦0.585、I2/I0≦0.136であることを特徴とする合成石英ガラスをも提供する。特にフッ素を100ppm以上、水素分子を5×1016分子/cm3以上含有することが好ましい。 The present invention is based on the findings, it contains fluorine, scattering peak intensity of scattering peak intensity of 495cm -1 to oxygen deficient defects as a specific amount or less, and in the laser Raman spectrum (I 1) and 606 cm -1 Synthetic quartz glass characterized in that (I 2 ) is I 1 / I 0 ≦ 0.585 and I 2 / I 0 ≦ 0.136, respectively, with respect to a scattering peak intensity (I 0 ) of 440 cm −1. Also provide. In particular, it is preferable to contain 100 ppm or more of fluorine and 5 × 10 16 molecules / cm 3 or more of hydrogen molecules.
本発明の合成石英ガラスは、フッ素濃度が100ppm(重量ppmの意であり、以下も同様。ppbについても同様。)以上であることが好ましい。100ppm未満では、合成石英ガラス中の不安定な構造を低減する作用が充分でない場合がある。フッ素濃度が400ppm以上であることがより好ましく、400〜3000ppmの範囲が特に好ましい。フッ素の濃度が3000ppmを超えて含有する場合には、還元型欠陥が生成して耐紫外線性が低下するおそれがある。 The synthetic quartz glass of the present invention preferably has a fluorine concentration of 100 ppm (meaning ppm by weight, the same applies to the following, and the same applies to ppb) or higher. If it is less than 100 ppm, the action of reducing the unstable structure in the synthetic quartz glass may not be sufficient. The fluorine concentration is more preferably 400 ppm or more, and particularly preferably in the range of 400 to 3000 ppm. When the concentration of fluorine exceeds 3000 ppm, there is a possibility that reduced defects are generated and the ultraviolet resistance is lowered.
本発明の合成石英ガラスは、OH基濃度が100ppm以下であることが好ましい。100ppm超では、略170nm以下の波長領域での透過率が低下し、例えば、Xe2 *エキシマランプ、F2レーザ、重水素ランプを光源とする装置の光学部材として適さないおそれがある。OH基濃度が50ppm以下であれば、良好な耐紫外線性が得られ、真空紫外域において高い透過率が得られる点で、20ppm以下、さらには10ppm未満が好ましい。特に、OH基濃度は波長200nm以下の真空紫外域における光透過性に影響を及ぼし、波長175nm以下の真空紫外域の光に使用される合成石英ガラスでは、OH基濃度が10ppm未満であることが好ましい。さらに、波長160nm以下の真空紫外域の光に使用される合成石英ガラスでは、OH基濃度が5ppm以下であることが好ましい。 The synthetic quartz glass of the present invention preferably has an OH group concentration of 100 ppm or less. If it exceeds 100 ppm, the transmittance in a wavelength region of about 170 nm or less is lowered, and for example, there is a possibility that it is not suitable as an optical member of an apparatus using a Xe 2 * excimer lamp, F 2 laser, or deuterium lamp as a light source. When the OH group concentration is 50 ppm or less, good ultraviolet resistance is obtained, and in terms of obtaining high transmittance in the vacuum ultraviolet region, it is preferably 20 ppm or less, and more preferably less than 10 ppm. In particular, the OH group concentration affects the light transmittance in the vacuum ultraviolet region with a wavelength of 200 nm or less, and the synthetic quartz glass used for light in the vacuum ultraviolet region with a wavelength of 175 nm or less may have an OH group concentration of less than 10 ppm. preferable. Furthermore, in the synthetic quartz glass used for light in the vacuum ultraviolet region having a wavelength of 160 nm or less, the OH group concentration is preferably 5 ppm or less.
また、合成石英ガラス中の酸素欠乏型欠陥(≡Si−Si≡)は、真空紫外線透過性に大きな影響を及ぼし、この酸素欠乏型欠陥は、波長163nmを中心とする吸収帯を有する。波長163nmにおける内部透過率T163(%/cm)は、合成石英ガラス中のOH基濃度COH(ppm)により次式(i)のように推測される。
T163(%/cm)≧exp(−0.02COH 0.85)×100 (i)
In addition, oxygen-deficient defects (≡Si—Si≡) in the synthetic quartz glass have a great influence on the vacuum ultraviolet transmittance, and the oxygen-deficient defects have an absorption band centered at a wavelength of 163 nm. The internal transmittance T 163 (% / cm) at a wavelength of 163 nm is estimated as in the following formula (i) by the OH group concentration C OH (ppm) in the synthetic quartz glass.
T 163 (% / cm) ≧ exp (−0.02C OH 0.85 ) × 100 (i)
しかし、酸素欠乏型欠陥があると、163nmを中心とした吸収帯があるため、実際の波長163nmにおける透過率(T163)は、式(i)の右辺の値よりも小さくなり、さらに、その吸収帯の大きさにもよるが、波長200nm以下の透過率が低下する。したがって、酸素欠乏型欠陥を実質的に含有しないことが、優れた真空紫外線透過性を得るために重要であり、酸素欠乏型欠陥を実質的に含有しないこと、すなわち、波長163nmにおける内部透過率に関する式(i)を満足することが好ましい。 However, if there is an oxygen-deficient defect, since there is an absorption band centered at 163 nm, the transmittance (T 163 ) at the actual wavelength of 163 nm is smaller than the value on the right side of the equation (i), Although it depends on the size of the absorption band, the transmittance at a wavelength of 200 nm or less decreases. Accordingly, it is important to obtain substantially no oxygen-deficient defects, in order to obtain excellent vacuum ultraviolet transmittance, and substantially no oxygen-deficient defects, that is, internal transmittance at a wavelength of 163 nm. It is preferable that the formula (i) is satisfied.
また、本発明による合成石英ガラスは、真空紫外線透過性の観点から、157nmにおける内部透過率が70%/cmであることが好ましく、特に内部透過率が80%/cm以上であることが好ましい。 In addition, the synthetic quartz glass according to the present invention preferably has an internal transmittance at 157 nm of 70% / cm, particularly preferably 80% / cm or more, from the viewpoint of vacuum ultraviolet light transmittance.
本発明の合成石英ガラスにおいて、塩素濃度は、少なければ少ないほど好ましく、塩素濃度は100ppm以下であれば、良好な耐紫外線性が得られ、均質性の点からは25ppm以下が好ましく、特に良好な真空紫外線透過性が得られる点から10ppm以下であることが好ましい。さらに、波長175nm以下の真空紫外域における耐紫外線性の点では、塩素は極力少ない方が好ましく、具体的には100ppb以下、特に50ppb以下が好ましい。 In the synthetic quartz glass of the present invention, the smaller the chlorine concentration, the better. The chlorine concentration of 100 ppm or less provides good UV resistance, and from the point of homogeneity, 25 ppm or less is preferred, and particularly good. It is preferably 10 ppm or less from the viewpoint of obtaining vacuum ultraviolet transmittance. Furthermore, from the viewpoint of ultraviolet resistance in the vacuum ultraviolet region having a wavelength of 175 nm or less, chlorine is preferably as little as possible, specifically 100 ppb or less, particularly 50 ppb or less.
本発明の合成石英ガラスにおいて、水素分子濃度が5×1016分子/cm3以上にすると、紫外線照射により生成した常磁性欠陥を修復する作用を生じる。特に、水素分子濃度が1×1017分子/cm3以上、さらには1×1017〜5×1018分子/cm3、特に5×1017〜5×1018分子/cm3であることが好ましい。 In the synthetic quartz glass of the present invention, when the hydrogen molecule concentration is 5 × 10 16 molecules / cm 3 or more, an action of repairing paramagnetic defects generated by ultraviolet irradiation occurs. In particular, the hydrogen molecule concentration is 1 × 10 17 molecules / cm 3 or more, further 1 × 10 17 to 5 × 10 18 molecules / cm 3 , particularly 5 × 10 17 to 5 × 10 18 molecules / cm 3. preferable.
一方、石英ガラスネットワーク中の≡Si−O−Si≡結合におけるSi−O−Si結合角はある分布を有している。合成石英ガラス中の不安定な構造とは、歪んだ≡Si−O−Si≡結合のことをいう。合成石英ガラス中の不安定な構造は、正常な構造に比べて結合エネルギーが弱いため、不安定な構造が多いほど真空紫外線透過性が低下する。この不安定な構造は、合成石英ガラスの仮想温度に依存するとともに、合成石英ガラス中のフッ素濃度に影響を受ける。すなわち、合成石英ガラス中にフッ素をドープすると不安定な構造を低減することができ、また仮想温度が低いほど不安定な構造は低減される。具体的には合成石英ガラスの仮想温度が1100℃以下であれば、不安定な構造を低減することができ、優れた真空紫外線透過性が得られる。この場合、フッ素濃度は100ppm以上であることが好ましい。本発明において、仮想温度とは、A.Agarwalらの方法(J.Non−Cryst.,185,191,1995)を用いて求めた仮想温度をいう。 On the other hand, the Si—O—Si bond angle in the ≡Si—O—Si≡ bond in the quartz glass network has a certain distribution. An unstable structure in synthetic quartz glass means a distorted ≡Si—O—Si≡ bond. The unstable structure in the synthetic quartz glass has a weaker binding energy than the normal structure. Therefore, the more the unstable structure is, the lower the vacuum ultraviolet ray transmittance is. This unstable structure depends on the fictive temperature of the synthetic quartz glass and is affected by the fluorine concentration in the synthetic quartz glass. That is, if synthetic quartz glass is doped with fluorine, unstable structures can be reduced, and the lower the fictive temperature, the more unstable structures are reduced. Specifically, if the fictive temperature of synthetic quartz glass is 1100 ° C. or lower, unstable structures can be reduced, and excellent vacuum ultraviolet ray permeability can be obtained. In this case, the fluorine concentration is preferably 100 ppm or more. In the present invention, the fictive temperature means A.I. The fictive temperature calculated | required using the method (J.Non-Cryst., 185,191,1995) of Agarwal et al.
本発明の合成石英ガラス中のアルカリ金属、アルカリ土類金属、遷移金属等の金属不純物は、紫外域から真空紫外域における透過率を低下させるだけでなく、耐紫外線性を低下させる原因ともなるため、その濃度は極力少ない方が好ましい。具体的には金属不純物の合計量が100ppb以下、特に50ppb以下が好ましい。 Metal impurities such as alkali metals, alkaline earth metals, and transition metals in the synthetic quartz glass of the present invention not only decrease the transmittance from the ultraviolet region to the vacuum ultraviolet region, but also cause a decrease in ultraviolet resistance. The concentration is preferably as low as possible. Specifically, the total amount of metal impurities is preferably 100 ppb or less, particularly preferably 50 ppb or less.
また、合成石英ガラスにおいて、石英ガラス中のOH基およびフッ素は屈折率に影響を与えるため、OH基およびフッ素の濃度に分布が存在すると、均質性が悪化する。 In addition, in synthetic quartz glass, OH groups and fluorine in the quartz glass affect the refractive index. Therefore, if there is a distribution in the concentration of OH groups and fluorine, the homogeneity deteriorates.
そこで常磁性欠陥の生成自体を抑制して耐紫外線性を向上させ、かつ、均質性の向上を達成するためには、OH基、フッ素濃度の分布を最適化する必要があると考え、この点に関する検討を行った。その結果、光が透過する領域、すなわち光使用領域におけるフッ素濃度およびOH基濃度の分布を制御することにより、フッ素濃度およびOH基濃度の変動幅をともに、15ppm以下の範囲とすると、均質性を向上できるという知見を得た。
また、光が通過する領域において、OH基とフッ素が互いに濃度分布を打ち消しあうように分布する場合には、フッ素濃度およびOH基濃度の変動幅の上限を25ppm以下としても、均質性を向上できるという知見も得た。
Therefore, it is necessary to optimize the distribution of OH group and fluorine concentration in order to suppress the generation of paramagnetic defects and improve the UV resistance and to improve the homogeneity. Was examined. As a result, by controlling the distribution of the fluorine concentration and the OH group concentration in the region where light is transmitted, that is, the light use region, if the variation range of the fluorine concentration and the OH group concentration is both within 15 ppm, the homogeneity is improved. The knowledge that it can improve was obtained.
Further, in the region where light passes, when OH groups and fluorine are distributed so as to cancel out the concentration distribution, homogeneity can be improved even if the upper limit of the fluctuation range of the fluorine concentration and the OH group concentration is 25 ppm or less. I also got the knowledge.
よって、本発明は、紫外域から真空紫外域の光を照射して使用される光学用合成石英ガラスにおいて、OH基およびフッ素を含有する合成石英ガラスで形成され、光使用領域においてOH基濃度の変動幅が15ppm以下、フッ素濃度の変動幅が15ppm以下であり、かつ塩素濃度が25ppm以下である合成石英ガラスを提供する。
さらに、均質性および耐紫外線性に優れた合成石英ガラスとして、OH基およびフッ素を含有する合成石英ガラスで形成され、光使用領域において、OH基とフッ素が互いに濃度分布を打ち消しあうように分布し、かつOH基濃度の変動幅が25ppm以下、フッ素濃度の変動幅が25ppm以下、および塩素濃度が25ppm以下である合成石英ガラスを提供する。
Therefore, the present invention is an optical synthetic quartz glass used by irradiating light in the ultraviolet region to the vacuum ultraviolet region, and is formed of synthetic quartz glass containing OH groups and fluorine, and has an OH group concentration in the light use region. Provided is a synthetic quartz glass having a fluctuation range of 15 ppm or less, a fluorine concentration fluctuation range of 15 ppm or less, and a chlorine concentration of 25 ppm or less.
Furthermore, as a synthetic quartz glass excellent in homogeneity and UV resistance, it is formed of synthetic quartz glass containing OH groups and fluorine, and in the light usage region, OH groups and fluorine are distributed so as to cancel each other's concentration distribution. And a synthetic quartz glass having a fluctuation range of OH group concentration of 25 ppm or less, a fluctuation range of fluorine concentration of 25 ppm or less, and a chlorine concentration of 25 ppm or less.
本発明において、光使用領域におけるOH基濃度の変動幅およびフッ素濃度の変動幅が、ともに15ppm以下である合成石英ガラスは、優れた均質性を安定して発現できるため、好ましい。また、光使用領域において、OH基とフッ素が互いに濃度分布を打ち消しあうように分布する場合には、OH基濃度の変動幅が25ppm以下、およびフッ素濃度の変動幅が25ppm以下である合成石英ガラスであっても、優れた均質性を安定して発現できる。
このとき、入射光に直交する平面内における屈折率変動幅(Δn)が20×10-6以下であることが好ましく、特に10×10-6以下、さらには5×10-6以下であることが好ましく、最も好ましくは2×10-6以下である。
このΔnの観点からは、光使用領域におけるフッ素濃度の変動幅とOH基濃度の変動幅との合計が5ppm以下であることが、特に好ましい。
In the present invention, a synthetic quartz glass in which both the fluctuation range of the OH group concentration and the fluctuation range of the fluorine concentration in the light use region are 15 ppm or less is preferable because excellent homogeneity can be stably expressed. Further, when the OH groups and fluorine are distributed so as to cancel each other in the light use region, the synthetic quartz glass having a fluctuation range of the OH group concentration of 25 ppm or less and a fluctuation range of the fluorine concentration of 25 ppm or less. Even so, excellent homogeneity can be stably expressed.
At this time, the refractive index fluctuation range (Δn) in a plane orthogonal to the incident light is preferably 20 × 10 −6 or less, particularly 10 × 10 −6 or less, and further 5 × 10 −6 or less. And most preferably 2 × 10 −6 or less.
From the viewpoint of Δn, it is particularly preferable that the total of the fluctuation range of the fluorine concentration and the fluctuation range of the OH group concentration in the light use region is 5 ppm or less.
本発明によれば、紫外線透過性に優れるとともに、エキシマレーザなどの光源からの高エネルギ光や放射線などの照射によるE’センタの発生に基づく透過率の低下や蛍光発光が低減され、耐紫外線性に優れる合成石英ガラスが得られる。
また、本発明によれば、真空紫外線透過性に優れた合成石英ガラスを得ることができる。特に、波長200nm以下の真空紫外域でも透過率の高い合成石英ガラスを得ることができる。
さらに、本発明によれば、均質性および耐紫外線性に優れた合成石英ガラスを得ることができる。
したがって、本発明の合成石英ガラスは、紫外域から真空紫外域までの光に使用される光学系を構成する部材としてきわめて好適である。
また、本発明によれば,上記の耐紫外線性、真空紫外線透過性、または均質性に優れる合成石英ガラスを容易に製造することができる。
According to the present invention, the ultraviolet ray transmission is excellent, and the transmittance reduction and the fluorescence emission due to the generation of the E ′ center due to the irradiation of high energy light or radiation from a light source such as an excimer laser are reduced, and the ultraviolet light resistance is reduced. Synthetic quartz glass excellent in performance can be obtained.
Moreover, according to this invention, the synthetic quartz glass excellent in vacuum ultraviolet-ray permeability can be obtained. In particular, a synthetic quartz glass having a high transmittance can be obtained even in a vacuum ultraviolet region having a wavelength of 200 nm or less.
Furthermore, according to the present invention, a synthetic quartz glass excellent in homogeneity and ultraviolet resistance can be obtained.
Therefore, the synthetic quartz glass of the present invention is extremely suitable as a member constituting an optical system used for light from the ultraviolet region to the vacuum ultraviolet region.
Further, according to the present invention, it is possible to easily produce the synthetic quartz glass having excellent ultraviolet resistance, vacuum ultraviolet transmittance, or homogeneity.
本発明において、光使用領域とは、合成石英ガラスの使用時に、紫外域から真空紫外域までの光が透過または反射する領域をいう。さらに、本発明において、OH基とフッ素が互いに濃度分布を打ち消しあうように分布するとは、合成石英ガラスの光が通過する領域において、入射光に直交する任意の平面におけるフッ素濃度およびOH基濃度が互いに増減を相補する分布状態にあることをいう。すなわち、例えば、フッ素濃度が任意の平面の中心から外側に向けて増加する場合には、OH基濃度は平面の中心から外側に向けて減少するように分布している状態、またはその逆の分布状態をいう。具体的には、後記の例82〜94の合成石英ガラスについて、表14〜17に示すフッ素濃度およびOH基濃度の分布状態を表すグラフに図示されるように、入射光に直交する平面において、フッ素濃度が中心で最小値となる下に凸のグラフを示すのに対して、OH基濃度が中心で最大値となる上に凸のグラフを示し、両者の濃度が相補関係にある分布状態であること、またはその逆の相補関係にある分布状態をいう。 In the present invention, the light use region refers to a region through which light from the ultraviolet region to the vacuum ultraviolet region is transmitted or reflected when synthetic quartz glass is used. Furthermore, in the present invention, the distribution of OH groups and fluorine so that the concentration distributions cancel each other out means that the fluorine concentration and OH group concentration in an arbitrary plane orthogonal to the incident light in the region through which the light of synthetic quartz glass passes. It means that they are in a distribution state in which the increase and decrease are complementary. That is, for example, when the fluorine concentration increases from the center of any plane toward the outside, the OH group concentration is distributed so as to decrease from the center of the plane toward the outside, or vice versa. State. Specifically, for the synthetic quartz glasses of Examples 82 to 94 described later, as shown in the graphs showing the distribution states of the fluorine concentration and the OH group concentration shown in Tables 14 to 17, in a plane orthogonal to the incident light, In contrast to the distribution curve in which the concentration of fluorine is in a complementary relationship, the concentration of fluorine is shown as an upward convex graph in which the concentration of fluorine is maximum at the center. A distribution state in which there is a complementary relationship or the reverse.
本発明において、合成石英ガラスを製造する方法としては、直接法、スート法(VAD法、OVD法)、プラズマ法等を挙げることができる。製造時の温度が低く、塩素および金属などの不純物の混入を避けることができる観点で、スート法が特に好ましい。また、スート法によれば、フッ素をドープすることで、OH基がフッ素により置換される。スート法によれば、フッ素ドープ量と置換されるOH基量とはほぼ等しく、OH基を効率よく減少させることができるため、OH基濃度の少ない紫外線透過性に優れた合成石英ガラスを生産性よく得ることができる。 In the present invention, examples of the method for producing synthetic quartz glass include a direct method, a soot method (VAD method, OVD method), a plasma method, and the like. The soot method is particularly preferable from the viewpoint that the temperature during production is low and contamination of impurities such as chlorine and metal can be avoided. According to the soot method, the OH group is substituted with fluorine by doping with fluorine. According to the soot method, the amount of fluorine doped and the amount of substituted OH groups are almost the same, and OH groups can be reduced efficiently, so synthetic quartz glass with low UV concentration and low OH group concentration is productive. Can get well.
スート法によって、本発明の合成石英ガラスを製造する方法を具体的に説明する。
このスート法による合成石英ガラスの製造は、下記の(a)、(b)および(c)の工程を含む方法である。
(a)石英ガラス形成原料を火炎加水分解させて得られる石英ガラス微粒子を基材に堆積・成長させて多孔質石英ガラス体を形成させる工程と、
(b)多孔質石英ガラス体をフッ素含有雰囲気下にて保持し、フッ素を含有した多孔質石英ガラス体を得る工程と、
(c)フッ素を含有した多孔質石英ガラス体を透明ガラス化温度まで昇温して透明ガラス化し、フッ素を含有した透明石英ガラス体を得る工程
A method for producing the synthetic quartz glass of the present invention by the soot method will be specifically described.
The production of synthetic quartz glass by this soot method is a method including the following steps (a), (b) and (c).
(A) a step of depositing and growing quartz glass fine particles obtained by flame hydrolysis of a quartz glass forming raw material on a substrate to form a porous quartz glass body;
(B) holding the porous quartz glass body in a fluorine-containing atmosphere to obtain a porous quartz glass body containing fluorine;
(C) Step of obtaining a transparent quartz glass body containing fluorine by raising the temperature of the porous quartz glass body containing fluorine to a transparent vitrification temperature to form transparent glass
水素分子が含有される場合には、下記(a)、(b’)、(c’)、(d)の各工程をこの順で行うことで製造される。
(a)石英ガラス形成原料を火炎加水分解させて得られる石英ガラス微粒子を基材に堆積・成長させて多孔質石英ガラス体を形成させる工程。
(b’)多孔質石英ガラス体を600℃以下の温度にフッ素含有雰囲気下にて保持し、フッ素を含有した多孔質石英ガラス体を得る工程。
(c’)フッ素を含有した多孔質石英ガラス体を実質的にフッ素を含まない雰囲気下にて透明ガラス化温度まで昇温して透明ガラス化し、フッ素を含有した透明石英ガラス体を得る工程。
(d)フッ素を含有した透明石英ガラス体を600℃以下の温度に水素ガス含有雰囲気下にて保持し、フッ素を含有した透明石英ガラス体に水素を含有させて合成石英ガラスを得る工程。
When a hydrogen molecule is contained, it is produced by performing the following steps (a), (b ′), (c ′), and (d) in this order.
(A) A step of forming a porous quartz glass body by depositing and growing quartz glass fine particles obtained by subjecting a quartz glass forming raw material to flame hydrolysis on a substrate.
(B ′) A step of holding the porous quartz glass body at a temperature of 600 ° C. or less in a fluorine-containing atmosphere to obtain a porous quartz glass body containing fluorine.
(C ′) A step of raising the temperature of the porous quartz glass body containing fluorine to a transparent vitrification temperature in an atmosphere substantially free of fluorine to form transparent glass, thereby obtaining a transparent quartz glass body containing fluorine.
(D) A step of obtaining a synthetic quartz glass by holding a transparent quartz glass body containing fluorine at a temperature of 600 ° C. or less in an atmosphere containing hydrogen gas, and allowing the transparent quartz glass body containing fluorine to contain hydrogen.
多孔質石英ガラス体をフッ素化合物を含んだ雰囲気下で保持する際の温度が高いと、≡Si−Si≡欠陥が生成しやすい。すなわち、多孔質石英ガラス体をフッ素化合物を含んだ雰囲気下で高温で処理すると、フッ素化合物の活性が強く下記式(8)、(9)により≡Si−Si≡欠陥が生成する傾向にある。 If the temperature at which the porous quartz glass body is held in an atmosphere containing a fluorine compound is high, ≡Si—Si≡ defects are likely to be generated. That is, when a porous quartz glass body is treated at a high temperature in an atmosphere containing a fluorine compound, the fluorine compound has a strong activity and tends to generate ≡Si—Si≡ defects according to the following formulas (8) and (9).
≡Si−O−Si≡ → ≡Si−Si≡ (8)
フッ素化合物
≡Si−OH → ≡Si−F (9)
フッ素化合物
≡Si-O-Si≡ → ≡Si-Si≡ (8)
Fluorine compound ≡Si-OH → ≡Si-F (9)
Fluorine compound
したがって600℃以下の低温にてフッ素化合物を含んだ雰囲気にて多孔質石英ガラス体を処理すれば、フッ素化合物の活性を抑制でき、式(8)の反応が生じることなく上記式(9)の反応のみ起こるため、≡Si−Si≡欠陥は生成しない。 Therefore, if the porous quartz glass body is treated in an atmosphere containing a fluorine compound at a low temperature of 600 ° C. or lower, the activity of the fluorine compound can be suppressed, and the reaction of the above formula (9) can occur without causing the reaction of the formula (8). Since only the reaction occurs, no ≡Si—Si≡ defect is generated.
以下、各工程について説明する。工程(a)においては、石英ガラス形成原料を酸素ガスおよび水素ガスを多重管バーナーに供給し、火炎加水分解させて得られる石英ガラス微粒子を基材に堆積・成長させて多孔質石英ガラス体を形成させる。石英ガラス形成原料としては、ガス化可能な原料であれば特に限定されないが、SiCl4、SiHCl3、SiH2Cl2、SiCH3Cl3などの塩化物、SiF4、SiHF3、SiH2F2などのフッ化物、SiBr4、SiHBr3などの臭化物、SiI4などのヨウ化物、といったハロゲン化ケイ素化合物、またはRnSi(OR)4-n(ここにRは炭素数1〜4のアルキル基、nは0〜3の整数)で示されるアルコキシシランが挙げられる。また前記基材としては石英ガラス製の種棒(例えば特公昭63−24973記載の種棒)を使用できる。また棒状に限らず板状の基材を使用してもよい。また、酸素ガスと水素ガスとの比率は、水素過剰雰囲気であると還元型欠陥が生成するため、酸素過剰雰囲気が好ましく、具体的には酸素ガスに対する水素ガスの比率は1.6〜1.9が好ましい。 Hereinafter, each step will be described. In the step (a), a quartz glass forming raw material is supplied to a multi-tube burner by supplying oxygen gas and hydrogen gas to a multi-tube burner, and quartz glass fine particles obtained by flame hydrolysis are deposited and grown on a substrate to form a porous quartz glass body. Let it form. The quartz glass forming raw material is not particularly limited as long as it is a gasifiable raw material, but chlorides such as SiCl 4 , SiHCl 3 , SiH 2 Cl 2 , and SiCH 3 Cl 3 , SiF 4 , SiHF 3 , SiH 2 F 2 are used. fluorides such as, SiBr 4, bromides such as SiHBr 3, an iodide such as SiI 4, halogenated silicon compounds such or R n Si (oR) 4- n ( here R, is an alkyl group having 1 to 4 carbon atoms , N is an integer of 0 to 3). As the substrate, a quartz glass seed rod (for example, a seed rod described in Japanese Patent Publication No. 63-24974) can be used. Moreover, you may use not only rod shape but a plate-shaped base material. The oxygen gas / hydrogen gas ratio is preferably an oxygen-excess atmosphere because reduced defects are generated in an hydrogen-excess atmosphere. Specifically, the ratio of hydrogen gas to oxygen gas is 1.6 to 1. 9 is preferred.
次に、工程(b)において、前記多孔質石英ガラス体を600℃以下の温度にフッ素含有雰囲気下にて保持し、フッ素を含有した多孔質石英ガラス体を得る。このフッ素含有雰囲気としては、含フッ素ガス(例えばSiF4、SF6、CHF3、CF4、F2)を0.1〜100体積%、特に1〜20体積%含有する不活性ガス雰囲気が好ましい。これらの雰囲気下、600℃以下の温度にて圧力0.1〜10気圧で数十分〜数時間処理することが好ましい。特に、500〜100℃の高温下でフッ素ドープを行なう場合は酸素を5〜90体積%含有する雰囲気とし、還元型欠陥の生成を抑制することが好ましい。なお、本明細書において、「気圧」および後述する「Torr」は、ともにゲージ圧ではなく絶対圧の意である。 Next, in the step (b), the porous quartz glass body is held at a temperature of 600 ° C. or less in a fluorine-containing atmosphere to obtain a porous quartz glass body containing fluorine. As this fluorine-containing atmosphere, an inert gas atmosphere containing 0.1 to 100% by volume, particularly 1 to 20% by volume of a fluorine-containing gas (for example, SiF 4 , SF 6 , CHF 3 , CF 4 , F 2 ) is preferable. . In these atmospheres, the treatment is preferably performed at a temperature of 600 ° C. or lower and a pressure of 0.1 to 10 atm for several tens of minutes to several hours. In particular, when fluorine doping is performed at a high temperature of 500 to 100 ° C., it is preferable to set the atmosphere containing 5 to 90% by volume of oxygen to suppress the generation of reduced defects. In the present specification, “atmospheric pressure” and “Torr”, which will be described later, both mean absolute pressure, not gauge pressure.
さらに、工程(b)においては、多孔質石英ガラス体へ均一に短時間でフッ素をドープできることから、1200℃以下、好ましくは600℃以下の所定温度に減圧下(好ましくは100Torr以下、特に10Torr以下)で保持した状態とし、次いで、含フッ素ガスを常圧になるまで導入し、フッ素含有雰囲気とすることが好ましい。 Further, in the step (b), since the porous quartz glass body can be doped with fluorine uniformly in a short time, it is reduced to a predetermined temperature of 1200 ° C. or less, preferably 600 ° C. or less (preferably 100 Torr or less, particularly 10 Torr or less). ), And then it is preferable to introduce a fluorine-containing gas until a normal pressure is reached to obtain a fluorine-containing atmosphere.
次に、工程(c)において、前記フッ素を含有した多孔質石英ガラス体を実質的にフッ素を含まない雰囲気下にて透明ガラス化温度まで昇温して透明ガラス化し、フッ素を含有した透明石英ガラス体を得る。透明ガラス化温度は、1300℃以上であり、好ましくは1300〜1600℃であり、1350〜1500℃であることが特に好ましい。 Next, in the step (c), the porous quartz glass body containing fluorine is heated to a transparent vitrification temperature in an atmosphere substantially free of fluorine to form a transparent glass, and the transparent quartz containing fluorine is obtained. A glass body is obtained. Transparent vitrification temperature is 1300 degreeC or more, Preferably it is 1300-1600 degreeC, It is especially preferable that it is 1350-1500 degreeC.
実質的にフッ素を含まない雰囲気としては、工程(c)による処理開始時において、含フッ素ガス(例えばSiF4、SF6、CHF3、CF4、F2)が0.1体積%以下であれば特に限定されず、ヘリウムなどの不活性ガス100%の雰囲気、またはヘリウムなどの不活性ガスを主成分とする雰囲気であることが好ましい。圧力については、減圧または常圧であればよい。特に常圧の場合はヘリウムガスを使用できる。また、減圧の場合は100Torr以下、特に10Torr以下が好ましい。 The atmosphere that does not substantially contain fluorine is that the fluorine-containing gas (for example, SiF 4 , SF 6 , CHF 3 , CF 4 , F 2 ) is 0.1% by volume or less at the start of the treatment in step (c). There is no particular limitation, and an atmosphere containing a 100% inert gas such as helium or an atmosphere containing an inert gas such as helium as a main component is preferable. The pressure may be reduced pressure or normal pressure. In particular, helium gas can be used at normal pressure. In the case of reduced pressure, it is preferably 100 Torr or less, particularly 10 Torr or less.
また、工程(b)と(c)との間に、雰囲気を減圧し、フッ素を含有した多孔質石英ガラス体を減圧下に所定時間放置する工程(e)をさらに有することが好ましい。具体的には、フッ素を含有した多孔質石英ガラス体を、前記工程(b)のフッ素ドープを行う温度において、圧力100Torr以下、より好ましくは10Torr以下の不活性ガス雰囲気中で数十分〜数時間保持する工程を含むことが好ましい。工程(b)の後には、雰囲気からフッ素を取り除くことが必要である。常圧でもよいが長時間を要するため、工程(e)のように減圧にすれば短時間でフッ素を取り除ける。 Further, it is preferable to further include a step (e) between the steps (b) and (c), wherein the atmosphere is reduced in pressure and the porous quartz glass body containing fluorine is allowed to stand for a predetermined time under reduced pressure. Specifically, the porous quartz glass body containing fluorine is several tens to several in an inert gas atmosphere at a pressure of 100 Torr or less, more preferably 10 Torr or less, at the temperature at which fluorine doping in the step (b) is performed. It is preferable to include a step of holding time. After step (b), it is necessary to remove fluorine from the atmosphere. Although normal pressure may be used, it takes a long time, so fluorine can be removed in a short time by reducing the pressure as in step (e).
次いで、工程(d)においては、工程(c)で得られたフッ素を含有した透明石英ガラス体を水素ガスを含んだ雰囲気中にて、温度600℃以下で加熱処理して、合成石英ガラスを得る。圧力は、例えば1〜30気圧である。600℃以下で水素処理を行うことにより、≡Si−Hおよび≡Si−Si≡の還元型欠陥の生成を防止できる。水素ガスを含んだ雰囲気としては、水素ガスを0.1〜100体積%含有する不活性ガス雰囲気とすることが好ましい。さらに仮想温度を制御するためには以下の工程(f)を透明石英ガラス体に行なうことが好ましい。 Next, in the step (d), the transparent quartz glass body containing fluorine obtained in the step (c) is heat-treated at a temperature of 600 ° C. or lower in an atmosphere containing hydrogen gas to obtain a synthetic quartz glass. obtain. The pressure is, for example, 1 to 30 atmospheres. By performing the hydrogen treatment at 600 ° C. or lower, it is possible to prevent the generation of reduced defects of ≡Si—H and ≡Si—Si≡. The atmosphere containing hydrogen gas is preferably an inert gas atmosphere containing 0.1 to 100% by volume of hydrogen gas. Further, in order to control the fictive temperature, it is preferable to perform the following step (f) on the transparent quartz glass body.
工程(f):フッ素を含有した透明石英ガラス体を、800℃〜1100℃の温度にて5時間以上保持した後、10℃/hr以下の降温速度で750℃以下まで降温する熱処理を行ない、合成石英ガラスの仮想温度を制御する。750℃以下まで降温した後は放冷できる。この場合の雰囲気は、ヘリウム、アルゴン、窒素などの不活性ガス100%の雰囲気下、これらの不活性ガスを主成分とする雰囲気下、又は空気雰囲気下で、圧力は減圧又は常圧が好ましい。 Step (f): After holding the transparent quartz glass body containing fluorine at a temperature of 800 ° C. to 1100 ° C. for 5 hours or more, a heat treatment is performed to lower the temperature to 750 ° C. or less at a temperature lowering rate of 10 ° C./hr or less, Controls the fictive temperature of synthetic quartz glass. After cooling down to 750 ° C. or lower, it can be allowed to cool. The atmosphere in this case is preferably an atmosphere of 100% inert gas such as helium, argon or nitrogen, an atmosphere containing these inert gases as a main component, or an air atmosphere, and the pressure is preferably reduced or normal.
また、本発明の合成石英ガラスにおいてOH基を極力低減するためには、工程(a)の後に、多孔質石英ガラス体を1Torr以下の圧力で1000〜1300℃の温度にて所定時間保持して脱水を行った後、引き続き1Torr以下の圧力で透明ガラス化温度まで昇温して透明ガラス化することによってもよい。 In order to reduce OH groups as much as possible in the synthetic quartz glass of the present invention, after step (a), the porous quartz glass body is held at a temperature of 1000 to 1300 ° C. at a pressure of 1 Torr or less for a predetermined time. After dehydration, the temperature may be raised to a transparent vitrification temperature at a pressure of 1 Torr or less to form a transparent glass.
本発明の合成石英ガラスは、ステッパレンズその他の光学部品に用いられる。
この光学部品として必要な光学特性を与えるため、均質化、成形、アニールなどの各熱処理(以下、光学的熱処理という)を適宜行う必要があるが、光学的熱処理は工程(d)の前でもよく後でもよい。
The synthetic quartz glass of the present invention is used for stepper lenses and other optical components.
In order to provide the optical characteristics necessary for this optical component, it is necessary to appropriately perform each heat treatment (hereinafter referred to as optical heat treatment) such as homogenization, molding, and annealing, but the optical heat treatment may be performed before step (d). It may be later.
ただし光学的熱処理には800〜1500℃の高温を要するため、工程(d)で水素を含有させたとしても、その後の光学的熱処理により水素分子濃度が低下する可能性がある。したがって、工程(d)以後に光学的熱処理を行う場合は、水素ガスを0.1〜100体積%含み、圧力1〜30気圧の雰囲気下にて行うことが好ましい。
また、工程(d)以降に光学的熱処理を行う場合は、光学的熱処理のための炉を防爆構造とする必要がある。したがって、工程(d)の前に光学的熱処理を行う方が好ましい。
However, since the optical heat treatment requires a high temperature of 800 to 1500 ° C., even if hydrogen is contained in the step (d), the concentration of hydrogen molecules may be lowered by the subsequent optical heat treatment. Therefore, when the optical heat treatment is performed after the step (d), it is preferably performed in an atmosphere containing 0.1 to 100% by volume of hydrogen gas and a pressure of 1 to 30 atm.
Moreover, when performing optical heat processing after a process (d), it is necessary to make the furnace for optical heat processing into an explosion-proof structure. Therefore, it is preferable to perform an optical heat treatment before the step (d).
本発明においては、ホウ素をドープすることにより、より多くのフッ素をドープできる。ホウ素をドープする場合のホウ素源としては、BF3、BCl3、ホウ素のアルコキシドなどが挙げられる。
また、ホウ素とフッ素とをドープする方法としては、例えば、まず、ホウ素をドープし、次いで、フッ素とをドープする方法が挙げられる。
具体的には、例えば以下の1)または2)のような方法でホウ素とフッ素とをドープする。
In the present invention, more fluorine can be doped by doping boron. Examples of the boron source in the case of doping with boron include BF 3 , BCl 3 , and boron alkoxide.
Examples of the method of doping boron and fluorine include a method of first doping boron and then doping fluorine.
Specifically, for example, boron and fluorine are doped by the following method 1) or 2).
1)工程(a)で得られた多孔質石英ガラス体を圧力容器内にセットし、圧力容器内の圧力を1Torr程度にまで減圧し、次いで、ホウ素源を含有するガス(例えば、He等の不活性ガスで5体積%程度に希釈されたBCl3蒸気)を導入する。
常圧付近になったところで、前記のホウ素源を含有するガスの導入を停止し、所定時間放置することで多孔質石英ガラス体にホウ素をドープする。
次いで、工程(b)に従ってフッ素をドープする。
1) The porous quartz glass body obtained in step (a) is set in a pressure vessel, the pressure in the pressure vessel is reduced to about 1 Torr, and then a gas containing a boron source (for example, He or the like) BCl 3 vapor diluted to about 5% by volume with an inert gas) is introduced.
When the pressure is close to normal pressure, the introduction of the gas containing the boron source is stopped, and the porous quartz glass body is doped with boron by leaving it for a predetermined time.
Next, fluorine is doped according to step (b).
2)工程(a)で得られた多孔質石英ガラス体をホウ素のアルコキシドの蒸気で処理し、次いで、加湿雰囲気にして、ホウ素のアルコキシドの加水分解を行わせて多孔質石英ガラス体中にB2O3微粒子を析出させる。
次いで、工程(b)に従ってフッ素をドープする。
2) The porous quartz glass body obtained in the step (a) is treated with a boron alkoxide vapor, and then in a humidified atmosphere, the boron alkoxide is hydrolyzed to form B in the porous quartz glass body. 2 O 3 fine particles are deposited.
Next, fluorine is doped according to step (b).
以上の1)または2)の方法により、ホウ素をドープした多孔質石英ガラス体に、さらにフッ素をもドープでき、しかも、より多くのフッ素をドープできる。フッ素ドープ後は、工程(c)、(d)に従って、光学部材用合成石英ガラスを得ることができる。 By the above method 1) or 2), the porous quartz glass body doped with boron can be further doped with fluorine, and more fluorine can be doped. After fluorine doping, synthetic quartz glass for optical members can be obtained according to steps (c) and (d).
なお、この場合のフッ素ドープは、例えば以下のような手順で行う。
前記圧力容器内に不活性ガスを(例えばHeやN2等)を導入し圧力を常圧とする。再度、圧力容器内の圧力を1Torr程度にまで減圧し、次いで、不活性ガス(例えばHe等)で希釈したSiF4ガスを導入する。
常圧付近になったところで、前記の不活性ガスで希釈したSiF4ガスの導入を停止し、所定時間放置することでホウ素含有多孔質石英ガラス体にフッ素をドープする。
In this case, fluorine doping is performed by the following procedure, for example.
An inert gas (such as He or N 2 ) is introduced into the pressure vessel, and the pressure is set to normal pressure. Again, the pressure in the pressure vessel is reduced to about 1 Torr, and then SiF 4 gas diluted with an inert gas (such as He) is introduced.
When the pressure is close to normal pressure, the introduction of the SiF 4 gas diluted with the inert gas is stopped and left for a predetermined time to dope the boron-containing porous quartz glass body with fluorine.
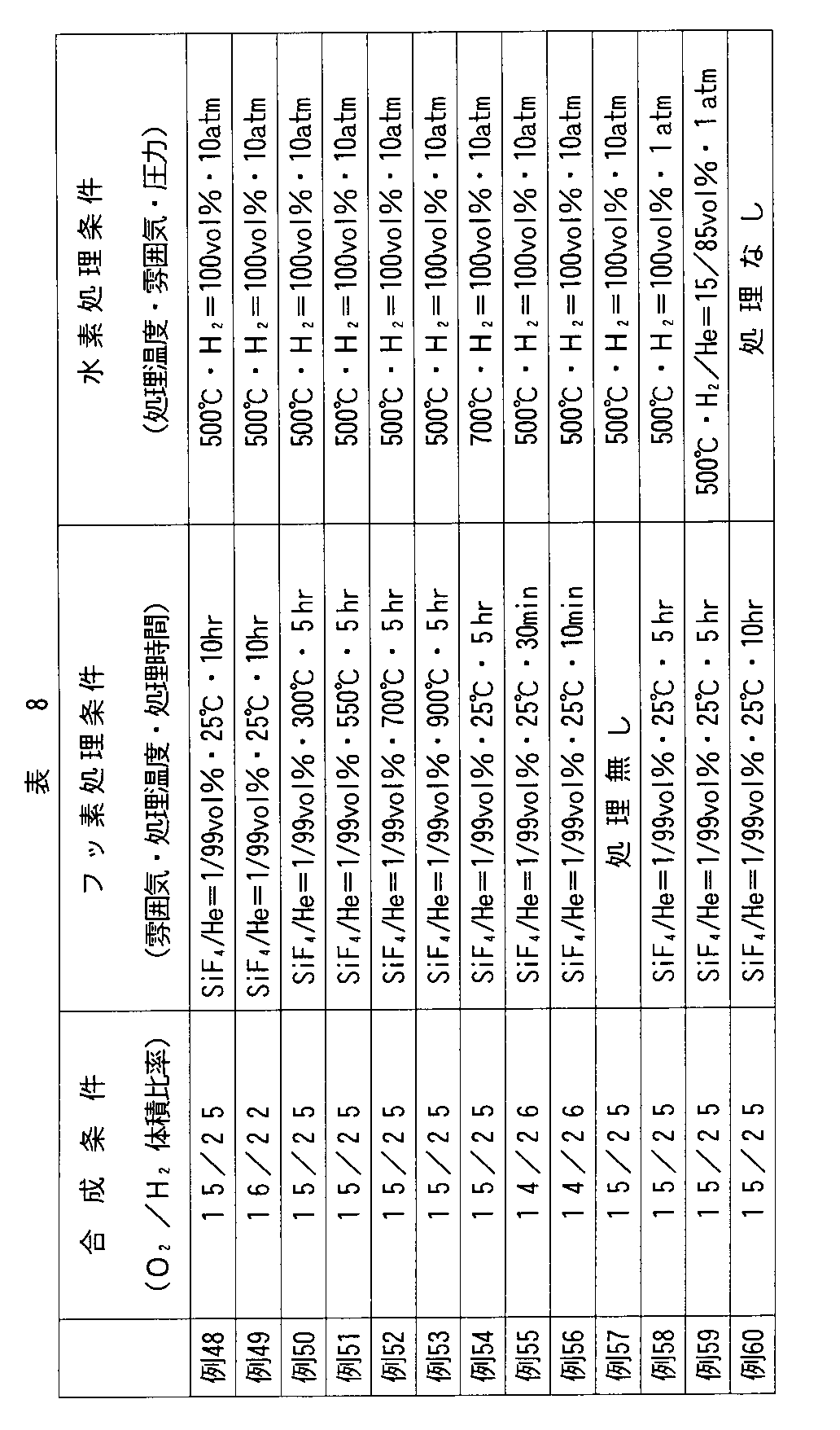
以下、実施例により本発明をさらに詳細に説明するが、本発明はこれらに限定されない。なお、以下の例で製造した合成石英ガラスの評価は、下記の方法にしたがって、行った。 EXAMPLES Hereinafter, although an Example demonstrates this invention further in detail, this invention is not limited to these. The synthetic quartz glass produced in the following examples was evaluated according to the following method.
(評価)
(評価1)フッ素濃度の測定
合成石英ガラスを無水炭酸ナトリウムにより加熱融解し、得られた融液に蒸留水および塩酸(体積比1:1)を加えて試料液を調製した。試料液の起電力を、フッ素イオン選択性電極および比較電極としてラジオメータトレーディング社製No945−220およびNo945−468をそれぞれ用いてラジオメータにより測定し、フッ素イオン標準溶液を用いてあらかじめ作製した検量線に基づいて、フッ素濃度を求めた(日本化学会誌,1972(2),350)。
(Evaluation)
(Evaluation 1) Measurement of fluorine concentration A synthetic quartz glass was heated and melted with anhydrous sodium carbonate, and distilled water and hydrochloric acid (volume ratio 1: 1) were added to the obtained melt to prepare a sample solution. An electromotive force of the sample solution was measured with a radiometer using No. 945-220 and No. 945-468 manufactured by Radiometer Trading Co. as a fluorine ion selective electrode and a reference electrode, respectively, and a calibration curve prepared in advance using a fluorine ion standard solution Based on the above, the fluorine concentration was obtained (Journal of the Chemical Society of Japan, 1972 (2), 350).
(評価2)水素分子濃度の測定
ラマン分光測定を行い、レーザラマンスペクトルの4135cm-1の散乱ピークにより検出した強度I4135と、800cm-1の散乱ピークの強度I800との強度比(=I4135/I800)より、水素分子濃度[分子/cm3]を求めた(V.S.Khotimchenko et.al.,Zhurnal Prikladnoi Spektroskopii,46(6),987〜997(1986))。なお本法による検出限界は1×1016分子/cm3である。
(Evaluation 2) Measurement of Hydrogen Molecule Concentration A Raman spectroscopic measurement was performed, and an intensity ratio between an intensity I 4135 detected by a scattering peak at 4135 cm −1 of a laser Raman spectrum and an intensity I 800 of a scattering peak at 800 cm −1 (= I 4135 / I 800 ), the hydrogen molecule concentration [molecules / cm 3 ] was determined (VS Khotimchenko et.al., Zhurnal Prikladno Specktroskii, 46 (6), 987-997 (1986)). The detection limit by this method is 1 × 10 16 molecules / cm 3 .
(評価3)OH基濃度の測定
赤外分光光度計による測定を行い、2.7μm波長での吸収ピークからOH基濃度を求めた(J.P.Wiiliams et.al.,Ceram. Bull.,55(5),524(1976))。
(Evaluation 3) Measurement of OH group concentration Measurement was carried out using an infrared spectrophotometer, and the OH group concentration was determined from the absorption peak at a wavelength of 2.7 μm (JP P. Williams et. Al., Ceram. Bull., 55 (5), 524 (1976)).
(評価4)
ラマン分光測定を行い、レーザラマンスペクトルの2250cm-1の散乱ピークにより検出した強度I2250を、800cm-1の散乱ピークの強度I800で割った値(I2250/I800)から≡Si−H欠陥の濃度(≡Si−H濃度)を評価した。ここで検出限界はI2250/I800=1×10-4である。I2250/I800の値が小さい方が良好な結果である。
(Evaluation 4)
Perform Raman spectroscopy, the intensity I 2250 detected by scattering peak of the laser Raman spectrum of 2250 cm -1, ≡Si-H defects from divided by the intensity I 800 of the scattering peak of 800cm -1 (I 2250 / I 800 ) Concentration (≡Si—H concentration) was evaluated. Here, the detection limit is I 2250 / I 800 = 1 × 10 −4 . A smaller value of I 2250 / I 800 is a better result.
(評価5)
紫外可視分光光度計を用いて、厚み10mmの試料と厚み35mmの試料の245nmの光の透過率を測定し、これらの透過率から245nmの吸収係数を算出し、≡Si−Si≡欠陥の生成の有無を評価した。245nmの吸収係数の値が小さい方が良好な結果である。
(Evaluation 5)
Using a UV-visible spectrophotometer, the transmittance of light at 245 nm is measured for a sample with a thickness of 10 mm and a sample with a thickness of 35 mm, and an absorption coefficient at 245 nm is calculated from these transmittances to generate ≡Si-Si≡ defects. The presence or absence of was evaluated. A smaller value of the absorption coefficient at 245 nm is a better result.
(評価6)還元型欠陥
真空紫外分光光度計(アクトンリサーチ社製VTMS−502)を用いて、厚さ10mmと4mmの試料について、波長163nmの透過率を測定し、その測定結果から波長163nmの吸収係数(k163)を求めた。該試料中に含まれるOH基濃度(COH、単位はppm)との関係が、k163≧0.02×(COH)0.85を満たすとき、還元型欠陥「有り」とし、満たさない場合は還元型欠陥「無し」とした。
(Evaluation 6) Reduction type defect Using a vacuum ultraviolet spectrophotometer (VTMS-502 manufactured by Acton Research Co., Ltd.), the transmittance at a wavelength of 163 nm was measured for samples having a thickness of 10 mm and a thickness of 4 mm. The absorption coefficient (k 163 ) was determined. When the relationship with the OH group concentration (C OH , the unit is ppm) contained in the sample satisfies k 163 ≧ 0.02 × (C OH ) 0.85 , the reduction type defect is “present”. Reduced defect “None”.
(評価7)
KrFエキシマレーザ(ラムダフィジーク社製LPX−120i)からの光をエネルギ密度100mJ/cm2/Pulse、周波数200Hzの条件にて試料に照射した。KrFエキシマレーザ光を5×106ショット照射した直後の214nmでの透過率を紫外可視分光光度計により測定し、KrFエキシマレーザ照射により生じる常磁性欠陥E’センタによる214nm吸収強度を、照射前後での吸収係数変化量Δk214[cm-1]により、評価した。Δk214の値が小さい方がE'センターが低減されていることを示し、良好な結果である。
(Evaluation 7)
The sample was irradiated with light from a KrF excimer laser (LPX-120i manufactured by Lambda Fijk) under the conditions of an energy density of 100 mJ / cm 2 / Pulse and a frequency of 200 Hz. The transmittance at 214 nm immediately after irradiation with 5 × 10 6 shots of KrF excimer laser light was measured with an ultraviolet-visible spectrophotometer, and the 214 nm absorption intensity by the paramagnetic defect E ′ center caused by KrF excimer laser irradiation was measured before and after irradiation. The absorption coefficient change amount Δk 214 [cm −1 ] was evaluated. A smaller value of Δk 214 indicates that the E ′ center is reduced, which is a good result.
(評価8)蛍光発光の評価
KrFエキシマレーザ(ラムダフィジーク社製LPX−120i)をエネルギ密度100mJ/cm2/Pulse、周波数200Hzの条件にて試料に照射した。KrFエキシマレーザを1×106ショット照射した場合の650nmの蛍光強度L650および248nmの散乱光強度S248をファイバ導光タイプの分光光度計を用いてそれぞれ測定し、248nmの散乱光強度S248に対する650nmの蛍光強度L650の比L650/S248を求めることにより、650nmの蛍光強度を評価した。L650/S248の値が小さい方が蛍光発光が抑制されていることを示し、良好な結果である。
(Evaluation 8) Evaluation of fluorescence emission The sample was irradiated with a KrF excimer laser (LPX-120i manufactured by Lambda Fijk) under the conditions of an energy density of 100 mJ / cm 2 / Pulse and a frequency of 200 Hz. The fluorescence intensity L 650 at 650 nm and the scattered light intensity S 248 at 248 nm when the KrF excimer laser was irradiated with 1 × 10 6 shots were respectively measured using a fiber light guide type spectrophotometer, and the scattered light intensity S 248 at 248 nm. The fluorescence intensity at 650 nm was evaluated by determining the ratio L 650 / S 248 of the fluorescence intensity L 650 at 650 nm relative to. A smaller value of L 650 / S 248 indicates that fluorescence emission is suppressed, which is a good result.
(評価9)172nmの内部透過率
真空紫外分光光度計(アクトンリサーチ社製VTMS−502)を用いて、厚さ10mmと4mmの試料について、波長175nm以下の真空紫外域の透過率の指標として172nmの内部透過率を測定した。
(評価10)157nmの内部透過率
真空紫外分光光度計(アクトンリサーチ社製VTMS−502)を用いて厚さ10mmと4mmの試料について、波長160nm以下の真空紫外域の透過率の指標として157nmの内部透過率を測定し、次式により同波長における内部透過率を求めた。
内部透過率(%/cm)=exp(ln(T1/T2)/(d1−d2))×100
ここで
T1:厚みd1[cm]の試料の透過率(%)
T2:厚みd2[cm]の試料の透過率(%)
透過率が高い方が良好な結果である。
(Evaluation 9) Internal transmittance of 172 nm Using a vacuum ultraviolet spectrophotometer (VTMS-502 manufactured by Acton Research), 172 nm as an index of the transmittance in the vacuum ultraviolet region with a wavelength of 175 nm or less for samples having a thickness of 10 mm and 4 mm The internal transmittance of was measured.
(Evaluation 10) Internal transmittance of 157 nm Using a vacuum ultraviolet spectrophotometer (VTMS-502 manufactured by Acton Research Co., Ltd.) for a sample of 10 mm thickness and 4 mm, 157 nm as an index of transmittance in the vacuum ultraviolet region of wavelength 160 nm or less The internal transmittance was measured, and the internal transmittance at the same wavelength was determined by the following equation.
Internal transmittance (% / cm) = exp (ln (T 1 / T 2 ) / (d 1 −d 2 )) × 100
Here, T 1 : transmittance (%) of a sample having a thickness d 1 [cm]
T 2 : Transmittance (%) of sample with thickness d 2 [cm]
A higher transmittance is a better result.
(評価11)
Xe2 *エキシマランプを10mW/cm2の条件で、厚さ10mmの試料に3時間照射した。照射前後での163nmにおける透過率を測定し、照射による163nmにおける透過率の変化(ΔT163)を算出した。ΔT163が小さいほど耐紫外線性に優れている。
(Evaluation 11)
A sample having a thickness of 10 mm was irradiated with a Xe 2 * excimer lamp under the condition of 10 mW / cm 2 for 3 hours. The transmittance at 163 nm before and after irradiation was measured, and the change in transmittance at 163 nm (ΔT 163 ) due to irradiation was calculated. As [Delta] T 163 is smaller has excellent ultraviolet resistance.
(評価12)仮想温度の測定
仮想温度の測定はA.Agarwalらの方法(J.Non−Cryst.,185,191,1995)を用いて求めた。鏡面研磨された石英ガラスを10%HF−2.5%H2SO4水溶液に浸漬し、表面に残留した研磨砥粒や傷などを除去する。その表面の反射スペクトルを赤外分光計(Nikolet社製Magna760)を用いて取得する。この際の赤外光入射角は6.5度に固定し、データ間隔は約0.5cm-1とし、64回スキャンさせた平均値を用いる。このようにして得られた赤外反射スペクトルにおいて、約1120cm-1に観察される最も大きなピークが石英ガラスのSi−O−Si結合による伸縮振動に起因する。このピーク位置をν(cm-1)とすると、仮想温度(Tf、単位:K)は下記の相関式により求められる。
ν=1114.51+(11603.51/Tf)。
(Evaluation 12) Measurement of virtual temperature It calculated | required using the method (J.Non-Cryst., 185,191,1995) of Agarwal et al. The mirror-polished quartz glass is immersed in a 10% HF-2.5% H 2 SO 4 aqueous solution to remove abrasive grains and scratches remaining on the surface. The reflection spectrum of the surface is acquired using an infrared spectrometer (Magna 760 manufactured by Nikolet). At this time, the incident angle of infrared light is fixed at 6.5 degrees, the data interval is about 0.5 cm −1, and an average value obtained by scanning 64 times is used. In the infrared reflection spectrum thus obtained, the largest peak observed at about 1120 cm −1 is due to stretching vibration due to the Si—O—Si bond of quartz glass. When this peak position is ν (cm −1 ), the fictive temperature (T f , unit: K) is obtained by the following correlation equation.
ν = 1114.51 + (11603.51 / T f ).
(評価13)塩素濃度の測定
Crのkα線を用いた蛍光X線分析を行い、塩素の特性X線強度を測定することにより、合成石英ガラス中の塩素濃度を求めた。なお本法による検出限界は2ppmである。
(Evaluation 13) Measurement of chlorine concentration Fluorescence X-ray analysis using the kα ray of Cr was performed, and the chlorine concentration in synthetic quartz glass was determined by measuring the characteristic X-ray intensity of chlorine. The detection limit by this method is 2 ppm.
(評価14)不安定構造の評価
ラマン分光測定(Jobin Ybon製 Ramonor T64000,励起光源:アルゴンイオンレーザ(波長514.5nm))を行い、レーザラマンスペクトルにおける495cm-1の散乱ピーク強度I1および605cm-1の散乱ピーク強度I2と、440cm-1の散乱ピーク強度I0との強度比I1/I0およびI2/I0を求めた。強度比I1/I0、強度比I2/I0の値が小さいほど良好である。
(Evaluation 14) Evaluation of unstable structure Raman spectroscopic measurement (Ramonor T64000 manufactured by Jobin Ybon, excitation light source: argon ion laser (wavelength 514.5 nm)) was performed, and a scattering peak intensity I 1 of 495 cm −1 and 605 cm − in the laser Raman spectrum. 1 of the scattering peak intensity I 2, was determined intensity ratio I 1 / I 0 and I 2 / I 0 of the scattering peak intensity I 0 of 440 cm -1. The smaller the intensity ratio I 1 / I 0 and the intensity ratio I 2 / I 0 , the better.
なお、各散乱ピーク強度I1、I2、I0の求め方は以下の通りである。
495cm-1の散乱ピークおよび605cm-1の散乱ピークに対してそれぞれ1本のローレンツ関数によりカーブフィッティングを行い、実スペクトルとの最小二乗誤差が最低となるように近似を行って各関数の係数を決定した。
440cm-1の散乱ピークに対しては3本のガウス関数の合成により、また495cm-1の散乱ピークと605cm-1の散乱ピークと440cm-1の散乱ピークとを除いた残余(ベースライン)に対しては2次関数により、それぞれカーブフィッティングを行い、実スペクトルとの最小二乗誤差が最低となるように近似を行って各関数の係数を決定した。
以上により求められた関数を用いて各散乱ピークの強度を求めた。
Incidentally, how to obtain the respective scattering peak intensity I 1, I 2, I 0 is as follows.
Perform curve fitting by a single Lorentzian respectively scattering peak of scattering peak and 605 cm -1 of 495cm -1, the coefficients of the functions performed approximated as least square error is minimum between the actual spectrum Decided.
The synthesis of the Gaussian function of the three for scattering peak of 440 cm -1, also the residual (baseline), excluding the scattering peak of scattering peak and 440 cm -1 of the scattering peak and 605 cm -1 of 495cm -1 On the other hand, curve fitting was performed using a quadratic function, and approximation was performed so that the least square error with the actual spectrum was minimized, and the coefficient of each function was determined.
The intensity | strength of each scattering peak was calculated | required using the function calculated | required by the above.
(評価15)
ICP質量分析法(セイコーインスツルメンツ社製SPQ9000)により、合成石英ガラス中のNa、Ca、Mg、Fe、Ni、Cu、Zn、Ti濃度を分析した。これら不純物の検出限界は、NiおよびCuについては0.1ppb、その他は0.3ppbである。
(Evaluation 15)
The concentrations of Na, Ca, Mg, Fe, Ni, Cu, Zn, and Ti in the synthetic quartz glass were analyzed by ICP mass spectrometry (SPQ9000 manufactured by Seiko Instruments Inc.). The detection limit of these impurities is 0.1 ppb for Ni and Cu, and 0.3 ppb for the others.
(評価16)
フィゾー干渉計にて、オイルオンプレート法で、合成石英ガラス試料の200mmφの面にヘリウムネオンレーザ光を垂直にあて、200mmφの面内での屈折率分布を測定した。
(Evaluation 16)
With a Fizeau interferometer, helium neon laser light was perpendicularly applied to a 200 mmφ surface of a synthetic quartz glass sample by an oil-on-plate method, and the refractive index distribution in the 200 mmφ surface was measured.
(例1)
公知の方法により、石英ガラス形成原料であるSiCl4を酸水素火炎中で加熱加水分解(火炎加水分解)させて得られる石英ガラス微粒子を基材に堆積・成長させて、直径35cm、長さ100cmの多孔質石英ガラス体を形成した(工程(a))。得られた多孔質石英ガラス体を雰囲気制御可能な電気炉に設置し、室温にて10Torrまで減圧して1時間保持した後、He/SiF4=99/1(体積比)の混合ガスを導入しながら、この雰囲気にて室温常圧下5時間保持しフッ素ドープを行った(工程(b))。その後SiF4の供給を遮断しHe100%雰囲気下で10時間保持した後、He100%雰囲気下1450℃まで昇温し、この温度で10時間保持し透明ガラス化し、フッ素を含有した透明石英ガラス体を得た(工程(c))。
(Example 1)
By a known method, quartz glass fine particles obtained by subjecting SiCl 4 as a raw material for forming quartz glass to heat hydrolysis (flame hydrolysis) in an oxyhydrogen flame are deposited and grown on a base material, and the diameter is 35 cm and the length is 100 cm. A porous quartz glass body was formed (step (a)). The obtained porous quartz glass body was placed in an electric furnace capable of controlling the atmosphere, and after reducing the pressure to 10 Torr at room temperature and holding it for 1 hour, a mixed gas of He / SiF 4 = 99/1 (volume ratio) was introduced. However, this atmosphere was maintained at room temperature and normal pressure for 5 hours to carry out fluorine doping (step (b)). Thereafter, the supply of SiF 4 was shut off and held in a He 100% atmosphere for 10 hours, and then heated to 1450 ° C. in a He 100% atmosphere, held at this temperature for 10 hours to form a transparent glass, and a transparent quartz glass body containing fluorine was obtained. Obtained (step (c)).
得られたフッ素を含有した透明石英ガラス体を、カーボン製発熱体を有する電気炉内で、軟化点以上の1750℃に加熱して自重変形を行わせ、250mm×250mm×120mmのブロック形状に成形した後、厚み30mmのブロックにスライスした。得られた250mm×250mm×30mmのブロックを水素100%、10気圧、500℃の雰囲気下で250時間保持し、水素ドープ処理を行い、合成石英ガラスを得た(工程(d))。 The obtained transparent quartz glass body containing fluorine is heated to 1750 ° C. above the softening point in an electric furnace having a carbon heating element, and is deformed by its own weight to form a block shape of 250 mm × 250 mm × 120 mm. Then, it was sliced into blocks having a thickness of 30 mm. The obtained 250 mm × 250 mm × 30 mm block was held in an atmosphere of 100% hydrogen, 10 atm, and 500 ° C. for 250 hours, and subjected to hydrogen doping treatment to obtain synthetic quartz glass (step (d)).
(例2)
例1における工程(b)において、多孔質石英ガラス体を電気炉に設置し、まず300℃に昇温し、10Torrまで減圧して1時間保持した後、He/SiF4=99/1(体積比)の混合ガスを導入し、この雰囲気にて300℃常圧下5時間保持しフッ素ドープを行った。これ以外は例1と全く同様の方法により合成石英ガラスを作製した。
(Example 2)
In step (b) in Example 1, the porous quartz glass body was placed in an electric furnace, first heated to 300 ° C., depressurized to 10 Torr and held for 1 hour, and then He / SiF 4 = 99/1 (volume). Ratio) gas mixture was introduced, and fluorine doping was performed in this atmosphere at 300 ° C. under normal pressure for 5 hours. A synthetic quartz glass was produced in the same manner as in Example 1 except for this.
(例3)
例1における工程(b)において、多孔質石英ガラス体を電気炉に設置し、まず500℃に昇温し、10Torrまで減圧して1時間保持し、He/SiF4=99/1(体積比)の混合ガスを導入し、この雰囲気にて500℃常圧下5時間保持しフッ素ドープを行った。これ以外は例1と全く同様の方法により合成石英ガラスを作製した。
(Example 3)
In step (b) in Example 1, the porous quartz glass body was placed in an electric furnace, first heated to 500 ° C., depressurized to 10 Torr and held for 1 hour, and He / SiF 4 = 99/1 (volume ratio). ) Was introduced and held in this atmosphere at 500 ° C. under normal pressure for 5 hours for fluorine doping. A synthetic quartz glass was produced in the same manner as in Example 1 except for this.
(例4)
例1における工程(b)において、多孔質石英ガラス体を電気炉に設置し、まず700℃に昇温し、10Torrまで減圧して1時間保持し、He/SiF4=99/1(体積比)の混合ガスを導入し、この雰囲気にて700℃常圧下5時間保持しフッ素ドープを行った。これ以外は例1と全く同様の方法により合成石英ガラスを作製した。
(Example 4)
In the step (b) in Example 1, the porous quartz glass body was placed in an electric furnace, first heated to 700 ° C., depressurized to 10 Torr and held for 1 hour, He / SiF 4 = 99/1 (volume ratio) ) Was introduced, and this atmosphere was maintained at 700 ° C. under normal pressure for 5 hours for fluorine doping. A synthetic quartz glass was produced in the same manner as in Example 1 except for this.
(例5)
例1における工程(b)において、多孔質石英ガラス体を電気炉に設置し、まず1200℃に昇温し、10Torrまで減圧して1時間保持し、He/SiF4=99/1(体積比)の混合ガスを導入し、この雰囲気にて1200℃常圧下5時間保持しフッ素ドープを行った。これ以外は例1と全く同様の方法により合成石英ガラスを作製した。
(Example 5)
In step (b) in Example 1, the porous quartz glass body was placed in an electric furnace, first heated to 1200 ° C., depressurized to 10 Torr and held for 1 hour, and He / SiF 4 = 99/1 (volume ratio). ) Was introduced, and this atmosphere was maintained at 1200 ° C. under normal pressure for 5 hours for fluorine doping. A synthetic quartz glass was produced in the same manner as in Example 1 except for this.
(例6)
例1における工程(b)において、多孔質石英ガラス体を電気炉に設置し、まず300℃に昇温し、10Torrまで減圧して1時間保持し、He/SiF4=99.9/0.1(体積比)の混合ガスを導入し、この雰囲気にて300℃、常圧で1時間保持しフッ素ドープを行った。これ以外は例1と全く同様の方法により合成石英ガラスを作製した。
(Example 6)
In step (b) in Example 1, the porous quartz glass body was placed in an electric furnace, first heated to 300 ° C., depressurized to 10 Torr and held for 1 hour, and He / SiF 4 = 99.9 / 0. A mixed gas of 1 (volume ratio) was introduced, and fluorine doping was performed in this atmosphere by maintaining at 300 ° C. and normal pressure for 1 hour. A synthetic quartz glass was produced in the same manner as in Example 1 except for this.
(例7)
例1における工程(b)において、多孔質石英ガラス体を電気炉に設置し、まず300℃に昇温し、10Torrまで減圧して1時間保持し、He/SiF4=99.9/0.1(体積比)の混合ガスを導入し、この雰囲気にて300℃、300Torrで1時間保持しフッ素ドープを行った。これ以外は例1と全く同様の方法により合成石英ガラスを作製した。
(Example 7)
In step (b) in Example 1, the porous quartz glass body was placed in an electric furnace, first heated to 300 ° C., depressurized to 10 Torr and held for 1 hour, and He / SiF 4 = 99.9 / 0. A mixed gas of 1 (volume ratio) was introduced, and fluorine doping was performed by maintaining at 300 ° C. and 300 Torr for 1 hour in this atmosphere. A synthetic quartz glass was produced in the same manner as in Example 1 except for this.
(例8)
例1における工程(b)において、多孔質石英ガラス体を電気炉に設置し、まず300℃に昇温し、10Torrまで減圧して1時間保持し、He/SiF4=99.9/0.1(体積比)の混合ガスを導入し、この雰囲気にて300℃、100Torrで1時間保持しフッ素ドープを行った。これ以外は例1と全く同様の方法により合成石英ガラスを作製した。
(Example 8)
In step (b) in Example 1, the porous quartz glass body was placed in an electric furnace, first heated to 300 ° C., depressurized to 10 Torr and held for 1 hour, and He / SiF 4 = 99.9 / 0. A mixed gas of 1 (volume ratio) was introduced, and fluorine doping was performed in this atmosphere at 300 ° C. and 100 Torr for 1 hour. A synthetic quartz glass was produced in the same manner as in Example 1 except for this.
(例9)
例1における工程(d)において、水素100%、1気圧、温度500℃の雰囲気下で250時間保持し、水素ドープ処理を行った。これ以外は例1と全く同様の方法により合成石英ガラスを作製した。
(Example 9)
In the step (d) in Example 1, hydrogen doping treatment was performed by maintaining for 250 hours in an atmosphere of 100% hydrogen, 1 atm, and 500 ° C. A synthetic quartz glass was produced in the same manner as in Example 1 except for this.
(例10)
例1における工程(d)において、水素/ヘリウム=10/90(体積比)の混合ガス1気圧、温度500℃の雰囲気下で250時間保持し、水素ドープ処理を行った。これ以外は例1と全く同様の方法により合成石英ガラスを作製した。
(Example 10)
In the step (d) in Example 1, hydrogen doping treatment was performed by holding for 250 hours in an atmosphere of a mixed gas of hydrogen / helium = 10/90 (volume ratio) at 1 atmosphere and a temperature of 500 ° C. A synthetic quartz glass was produced in the same manner as in Example 1 except for this.
(例11)
例1における工程(d)において、水素100%、10気圧、温度700℃の雰囲気下で250時間保持し、水素ドープ処理を行った。これ以外は例1と全く同様の方法により合成石英ガラスを作製した。
(Example 11)
In the step (d) in Example 1, hydrogen doping treatment was performed by maintaining for 250 hours in an atmosphere of 100% hydrogen, 10 atm, and 700 ° C. A synthetic quartz glass was produced in the same manner as in Example 1 except for this.
(例12)
例1における工程(d)において、水素100%、10気圧、温度900℃の雰囲気下で250時間保持し、水素ドープ処理を行った。これ以外は例1と全く同様の方法により合成石英ガラスを作製した。
(Example 12)
In the step (d) in Example 1, hydrogen doping treatment was performed by holding for 250 hours in an atmosphere of 100% hydrogen, 10 atm, and 900 ° C. A synthetic quartz glass was produced in the same manner as in Example 1 except for this.
(例13)
例1において工程(b)を実施せず、He100%雰囲気下で1450℃まで昇温し、この温度で10時間保持し透明ガラス化した。これ以外は例1と全く同様の方法により合成石英ガラスを作製した。
(Example 13)
In Example 1, the step (b) was not carried out, and the temperature was raised to 1450 ° C. in a He 100% atmosphere, and this temperature was maintained for 10 hours to form a transparent glass. A synthetic quartz glass was produced in the same manner as in Example 1 except for this.
(例14)
例1における工程(b)の後、SiF4の供給を遮断し、1Torrまで減圧して、この状態で1時間保持した(工程(e))。次いで、He100%を導入し常圧まで戻した後、再度1Torrまで減圧して実質的にフッ素を含まない雰囲気とした。該雰囲気下にて1450℃まで昇温し、1450℃で10時間保持し、透明ガラス化し、フッ素を含有した透明石英ガラス体を得た(工程(c))。これ以降は例1と同様に処理し、合成石英ガラスを作製した。
(Example 14)
After step (b) in Example 1, the supply of SiF 4 was cut off, the pressure was reduced to 1 Torr, and this state was maintained for 1 hour (step (e)). Subsequently, He 100% was introduced and the pressure was returned to normal pressure, and then the pressure was reduced again to 1 Torr to obtain an atmosphere substantially free of fluorine. The temperature was raised to 1450 ° C. under the atmosphere, and the glass was kept at 1450 ° C. for 10 hours to be transparent vitrified to obtain a transparent quartz glass body containing fluorine (step (c)). Thereafter, the same processing as in Example 1 was performed to produce a synthetic quartz glass.
(例15)
例1における工程(b)の後、SiF4の供給を遮断し、He100%雰囲気下で10時間保持し、さらにHe/SiF4=99.95/0.05(体積比)の混合ガス雰囲気にて10時間保持し、続いて1450℃まで昇温し、1450℃で10時間保持し、透明ガラス化し、フッ素を含有した透明石英ガラス体を得た(工程(c))。これ以降は例1と同様に処理し、合成石英ガラスを作製した。
(Example 15)
After the step (b) in Example 1, the supply of SiF 4 is cut off, maintained in a He 100% atmosphere for 10 hours, and further mixed gas atmosphere of He / SiF 4 = 99.95 / 0.05 (volume ratio). And then heated to 1450 ° C. and held at 1450 ° C. for 10 hours to form a transparent glass to obtain a transparent quartz glass body containing fluorine (step (c)). Thereafter, the same processing as in Example 1 was performed to produce a synthetic quartz glass.
(例16)
例1における工程(b)の後、SiF4の供給を遮断し、He100%雰囲気下で10時間保持し、さらにHe/SiF4=99.8/0.2(体積比)の混合ガス雰囲気にて10時間保持し、続いて1450℃まで昇温し、1450℃で10時間保持し、透明ガラス化し、フッ素を含有した透明石英ガラス体を得た(工程(c))。これ以降は例1と同様に処理し、合成石英ガラスを作製した。
(Example 16)
After the step (b) in Example 1, the supply of SiF 4 is cut off, maintained in a He 100% atmosphere for 10 hours, and further mixed gas atmosphere of He / SiF 4 = 99.8 / 0.2 (volume ratio). And then heated to 1450 ° C. and held at 1450 ° C. for 10 hours to form a transparent glass to obtain a transparent quartz glass body containing fluorine (step (c)). Thereafter, the same processing as in Example 1 was performed to produce a synthetic quartz glass.
これらの例1〜16で得られた合成石英ガラスを評価した。各評価の結果を表1に示す。NDは検出限界以下であることを示す。なお例1〜3、例6〜7、例9〜10および例14〜15は実施例、例4〜5、例8、例11〜13および例16は比較例に相当する。 The synthetic quartz glass obtained in Examples 1 to 16 was evaluated. The results of each evaluation are shown in Table 1. ND indicates that it is below the detection limit. Examples 1-3, Examples 6-7, Examples 9-10, and Examples 14-15 correspond to Examples, Examples 4-5, Example 8, Examples 11-13, and Example 16 correspond to comparative examples.
例17〜例34はOH基濃度、塩素濃度およびフッ素濃度の合成石英ガラスの特性への影響を調べた実験例である。
(例17〜31)
公知のスート法により、SiCl4またはSi(CH3O)4を1200〜1500℃の酸水素火炎中で加水分解させて形成されたSiO2微粒子を基材上に堆積させて直径500mm、長さ600mmの多孔質石英ガラス体を製造した。多孔質石英ガラス体を雰囲気制御可能な電気炉に設置し、圧力10Torr以下の減圧下で、表2に示す割合のSiF4を含んだヘリウムガスを導入し、この雰囲気下にて常圧・室温で表2に示す時間保持することにより、多孔質石英ガラス体中の脱水を行うと同時にフッ素をドープした。続いて圧力10Torr以下の減圧に保持した状態で1450℃まで昇温し、この温度にて10時間保持し透明石英ガラス体(直径200mm、長さ450mm)を製造した。さらに得られた透明石英ガラス体を直径200mm、厚さ10mmに切断し、表2に示す割合の水素含有雰囲気下、表2に示す圧力で500℃にて30時間保持した。上記の製法において、石英ガラス中のOH基濃度およびフッ素濃度の制御は、多孔質石英ガラスを製造する際の原料ガスに対する酸素および水素ガスの流量比、またはフッ素化合物を含んだ雰囲気下に多孔質石英ガラス体を保持する際のフッ素化合物の濃度および保持時間を調整することにより実施した。また石英ガラス中の水素分子濃度は、水素雰囲気下に保持する時の雰囲気中の水素濃度および全圧を調整することにより制御した。なお製造条件(ガラス形成原料、酸素および水素ガスの流量比、フッ素化合物濃度と圧力、水素濃度と圧力)の詳細を表2に示した。
Examples 17 to 34 are experimental examples in which the influence of the OH group concentration, the chlorine concentration, and the fluorine concentration on the characteristics of the synthetic quartz glass was examined.
(Examples 17 to 31)
SiO 2 fine particles formed by hydrolyzing SiCl 4 or Si (CH 3 O) 4 in a 1200 to 1500 ° C. oxyhydrogen flame by a known soot method are deposited on a substrate to have a diameter of 500 mm and a length of A 600 mm porous quartz glass body was produced. The porous quartz glass body is installed in an electric furnace capable of controlling the atmosphere, and helium gas containing SiF 4 in the ratio shown in Table 2 is introduced under a reduced pressure of 10 Torr or less. By holding for the time shown in Table 2, the porous quartz glass body was dehydrated and simultaneously doped with fluorine. Subsequently, the temperature was raised to 1450 ° C. while maintaining a reduced pressure of 10 Torr or less, and this temperature was maintained for 10 hours to produce a transparent quartz glass body (diameter 200 mm, length 450 mm). Further, the obtained transparent quartz glass body was cut into a diameter of 200 mm and a thickness of 10 mm, and held at 500 ° C. for 30 hours under the hydrogen-containing atmosphere at the ratio shown in Table 2 at the pressure shown in Table 2. In the above production method, the OH group concentration and the fluorine concentration in the quartz glass are controlled by the flow rate ratio of oxygen and hydrogen gas to the raw material gas when producing the porous quartz glass, or in an atmosphere containing a fluorine compound. It carried out by adjusting the density | concentration and holding time of the fluorine compound at the time of hold | maintaining a quartz glass body. Further, the concentration of hydrogen molecules in the quartz glass was controlled by adjusting the hydrogen concentration and the total pressure in the atmosphere when kept in a hydrogen atmosphere. Details of the production conditions (glass forming raw material, flow rate ratio of oxygen and hydrogen gas, fluorine compound concentration and pressure, hydrogen concentration and pressure) are shown in Table 2.
(例32〜34)
公知の直接法により、ガラス形成原料としてSiCl4を用い、SiF4を表3に示す割合の原料ガスに対する酸素および水素ガスの流量で、1800〜2000℃の酸水素火炎中で加水分解・酸化させ、基材上に直接透明石英ガラス体を製造した。この製法において、得られた石英ガラス中のフッ素濃度の制御は、SiCl4とSiF4との混合比を調整することにより行い、またOH基濃度および水素濃度は酸素と水素の流量比を調整することにより行った。なお、製造条件(SiF4、酸素および水素ガスの流量比)の詳細を表3に示した。
(Examples 32-34)
By using a known direct method, SiCl 4 is used as a glass forming raw material, and SiF 4 is hydrolyzed and oxidized in an oxyhydrogen flame at 1800 to 2000 ° C. at a flow rate of oxygen and hydrogen gas with respect to the raw material gas in the ratio shown in Table 3. A transparent quartz glass body was produced directly on the substrate. In this manufacturing method, the fluorine concentration in the obtained quartz glass is controlled by adjusting the mixing ratio of SiCl 4 and SiF 4, and the OH group concentration and the hydrogen concentration adjust the flow rate ratio of oxygen and hydrogen. Was done. Details of the production conditions (flow rate ratio of SiF 4 , oxygen and hydrogen gas) are shown in Table 3.
例17〜34により製造された合成石英ガラス中のOH基濃度、塩素濃度、フッ素濃度、水素分子濃度を表4に示す。なお各濃度は前記の方法により求め、NDは検出限界以下を示す。 Table 4 shows the OH group concentration, chlorine concentration, fluorine concentration, and hydrogen molecule concentration in the synthetic quartz glass produced in Examples 17 to 34. In addition, each density | concentration is calculated | required by the said method, ND shows below a detection limit.
次に、例17〜34により製造された合成石英ガラスについて、それぞれ散乱ピーク強度比(I1/I0、I2/I0)、Δk214、L650/S248、波長157nmにおける内部透過率、合成石英ガラス中の不純物濃度を測定して評価した。
評価結果を表5に示す。例17〜34の内、例20、21、22、32および34はOH基濃度が高いため、例29は塩素濃度が高いため、また例23および34はフッ素を含有しないため、他のものより特性が劣っている。
Next, with respect to the synthetic quartz glass produced according to Examples 17 to 34, the scattering peak intensity ratio (I 1 / I 0 , I 2 / I 0 ), Δk 214 , L 650 / S 248 , and internal transmittance at a wavelength of 157 nm, respectively. The impurity concentration in the synthetic quartz glass was measured and evaluated.
The evaluation results are shown in Table 5. Of Examples 17-34, Examples 20, 21, 22, 32 and 34 have higher OH group concentration, Example 29 has higher chlorine concentration, and Examples 23 and 34 do not contain fluorine. The characteristics are inferior.
例35〜47は、I1/I0、I2/I0の合成石英ガラスの特性への影響を調べた実験例である。
(例35〜例47)
公知のスート法により、SiCl4を酸水素火炎中で加水分解させ、形成されたSiO2微粒子を基材上に堆積させて500mmφ×長さ600mmの多孔質石英ガラス体を作製した(工程(a))。多孔質石英ガラス体を雰囲気制御可能な電気炉に設置し、10Torr以下の減圧状態から室温下で、フッ素化合物を含んだヘリウムガスを常圧になるまで導入した。この雰囲気下にて常圧・室温で数時間保持することにより、多孔質石英ガラス中の脱水を行うと同時にフッ素をドープした(工程(b))。続いて圧力10Torr以下の減圧に保持した状態で1450℃まで昇温し、この温度にて10時間保持し透明石英ガラス体(200mmφ×長さ450mm)を作製した。
Examples 35 to 47 are experimental examples in which the influence of I 1 / I 0 and I 2 / I 0 on the characteristics of the synthetic quartz glass was examined.
(Example 35 to Example 47)
By a known soot method, SiCl 4 was hydrolyzed in an oxyhydrogen flame, and the formed SiO 2 fine particles were deposited on a substrate to produce a porous quartz glass body of 500 mmφ × 600 mm in length (step (a )). The porous quartz glass body was installed in an electric furnace capable of controlling the atmosphere, and helium gas containing a fluorine compound was introduced at normal pressure from a reduced pressure state of 10 Torr or less at room temperature. By holding at atmospheric pressure and room temperature for several hours under this atmosphere, the porous quartz glass was dehydrated and simultaneously doped with fluorine (step (b)). Subsequently, the temperature was raised to 1450 ° C. while maintaining a reduced pressure of 10 Torr or less, and this temperature was maintained for 10 hours to produce a transparent quartz glass body (200 mmφ × 450 mm in length).
さらに、得られた透明石英ガラス体を200mmφ×厚さ10mmに切断し、水素含有雰囲気下、表6に示す条件で500℃にて30時間保持して石英ガラス中に水素ドープを行って、表7に示す例35〜47の合成石英ガラスを得た(工程(c))。
上記の製法において、石英ガラス中のOH基濃度およびフッ素濃度の制御は、工程(a)における原料ガスに対する酸素および水素ガスの流量比、工程(b)におけるフッ素化合物の濃度および処理時間を調整することにより実施した。また石英ガラス中の水素分子濃度は、工程(c)における水素処理時の雰囲気中の水素濃度および全圧を調整することにより制御した。なお、各例の工程(a)、工程(b)および工程(c)における処理条件の詳細を表6に示した。
Further, the obtained transparent quartz glass body was cut into 200 mmφ × 10 mm thickness, held in a hydrogen-containing atmosphere for 30 hours at 500 ° C. under the conditions shown in Table 6, and doped with hydrogen in the quartz glass. The synthetic quartz glass of Examples 35-47 shown in 7 was obtained (step (c)).
In the above manufacturing method, the OH group concentration and the fluorine concentration in the quartz glass are controlled by adjusting the flow ratio of oxygen and hydrogen gas to the raw material gas in the step (a), the concentration of the fluorine compound in the step (b), and the treatment time. Was carried out. The concentration of hydrogen molecules in the quartz glass was controlled by adjusting the hydrogen concentration and total pressure in the atmosphere during the hydrogen treatment in step (c). Table 6 shows the details of the processing conditions in step (a), step (b), and step (c) of each example.
次に、例35〜47の合成石英ガラスから調製された試料について、OH基濃度、フッ素濃度および水素分子濃度を、下記の方法にしたがって測定した。また、散乱ピーク強度比(I1/I0、I2/I0)、Δk214、L650/S248、波長157nmにおける内部透過率を測定して評価した。評価結果を表7に示す。例35〜47の内、例45〜47はI1/I0、I2/I0が高いため、他のものより特性が劣っている。 Next, for the samples prepared from the synthetic quartz glass of Examples 35 to 47, the OH group concentration, fluorine concentration, and hydrogen molecule concentration were measured according to the following methods. The scattering peak intensity ratios (I 1 / I 0 , I 2 / I 0 ), Δk 214 , L 650 / S 248 , and internal transmittance at a wavelength of 157 nm were measured and evaluated. Table 7 shows the evaluation results. Among Examples 35 to 47, Examples 45 to 47 have inferior characteristics than the others because I 1 / I 0 and I 2 / I 0 are high.
例48〜65は、OH基濃度、還元性欠陥の濃度の合成石英ガラスの特性への影響を調べた実験例である。
(例48〜60)
スート法により、SiCl4を酸水素火炎中で加水分解させて、形成されたSiO2微粒子を基材上に堆積させて400mmφ×長さ600mmの多孔質石英ガラス体を作製した。多孔質石英ガラス体を雰囲気制御可能な電気炉に設置し、室温で10Torr以下の減圧状態に保持した後、SiF4を含んだヘリウムガスを常圧になるまで導入した。この雰囲気下にて常圧・室温で数時間保持することにより、多孔質石英ガラス体中の脱水を行った。続いて、実質的にフッ素を含まない雰囲気下にて圧力10Torr以下の減圧に保持した状態で1450℃まで昇温し、この温度にて10時間保持し合成石英ガラス(200mmφ×長さ450mm)を作製した。
Examples 48 to 65 are experimental examples in which the influence of the OH group concentration and the concentration of reducing defects on the characteristics of the synthetic quartz glass was examined.
(Examples 48-60)
By a soot method, SiCl 4 was hydrolyzed in an oxyhydrogen flame, and the formed SiO 2 fine particles were deposited on a substrate to produce a porous quartz glass body of 400 mmφ × 600 mm in length. The porous quartz glass body was placed in an electric furnace capable of controlling the atmosphere, kept at a reduced pressure of 10 Torr or less at room temperature, and then helium gas containing SiF 4 was introduced until the pressure became normal. Under this atmosphere, the porous quartz glass body was dehydrated by maintaining at atmospheric pressure and room temperature for several hours. Subsequently, the temperature was raised to 1450 ° C. while maintaining a reduced pressure of 10 Torr or less in an atmosphere substantially free of fluorine, and this temperature was maintained for 10 hours to obtain a synthetic quartz glass (200 mmφ × length 450 mm). Produced.
さらに、得られた合成石英ガラスを、200mmφ×厚さ10mmに切断し、水素含有雰囲気下、表8に示す条件に30時間保持して合成石英ガラス中に水素ドープを行った。 Furthermore, the obtained synthetic quartz glass was cut into 200 mmφ × thickness 10 mm, and held for 30 hours in a hydrogen-containing atmosphere under the conditions shown in Table 8 to dope the synthetic quartz glass with hydrogen.
上記の製造工程において、多孔質石英ガラス体を製造する際の酸水素炎の酸素および水素ガスの体積比、ならびにフッ素化合物を含んだ雰囲気で多孔質ガラス体を保持する際のフッ素化合物の濃度、処理時間、および処理温度を調整することにより、得られる合成石英ガラス中のOH基濃度および還元型欠陥濃度を制御した。また、合成石英ガラス中の水素分子濃度は、水素ドープを行う際の処理温度、雰囲気中の水素濃度および全圧を調整することにより制御した。なお、各例の製造工程における処理条件の詳細を表8に示した。 In the production process described above, the volume ratio of oxygen and hydrogen gas of the oxyhydrogen flame when producing the porous quartz glass body, and the concentration of the fluorine compound when holding the porous glass body in an atmosphere containing the fluorine compound, By adjusting the treatment time and treatment temperature, the OH group concentration and the reduced defect concentration in the resulting synthetic quartz glass were controlled. In addition, the concentration of hydrogen molecules in the synthetic quartz glass was controlled by adjusting the treatment temperature during hydrogen doping, the hydrogen concentration in the atmosphere, and the total pressure. The details of the processing conditions in the manufacturing process of each example are shown in Table 8.
(例61〜65)
スート法により、SiCl4を酸水素火炎中で加水分解させて、形成されたSiO2微粒子を基材上に堆積させて400mmφ×長さ600mmの多孔質石英ガラス体を作製した。多孔質石英ガラス体を、雰囲気制御可能な電気炉に設置し、1Torr以下の減圧下で昇温し、1200℃にて所定時間保持し、続いて1450℃まで昇温し、この温度にて10時間保持し合成石英ガラス(200mmφ×長さ450mm)を作製した。
得られた合成石英ガラスを200mmφ×厚さ10mmに切断し、水素含有雰囲気下、表9に示す条件にて30時間保持して合成石英ガラス中に水素ドープを行った。
(Examples 61 to 65)
By a soot method, SiCl 4 was hydrolyzed in an oxyhydrogen flame, and the formed SiO 2 fine particles were deposited on a substrate to produce a porous quartz glass body of 400 mmφ × 600 mm in length. The porous quartz glass body is placed in an electric furnace capable of controlling the atmosphere, heated at a reduced pressure of 1 Torr or less, held at 1200 ° C. for a predetermined time, subsequently heated to 1450 ° C., and heated to 10 ° C. at this temperature. Synthetic quartz glass (200 mmφ × 450 mm in length) was produced by maintaining the time.
The obtained synthetic quartz glass was cut into 200 mmφ × 10 mm thickness, and kept in a hydrogen-containing atmosphere for 30 hours under the conditions shown in Table 9 to dope hydrogen into the synthetic quartz glass.
上記の製造工程において、1200℃での保持時間を調整することにより、合成石英ガラス中のOH基濃度および還元型欠陥の濃度を制御した。また、合成石英ガラス中の水素分子濃度は、水素ドープを行う際の処理温度、雰囲気中の水素濃度および全圧を調整することにより制御した。なお各例の製造工程における処理条件の詳細を表9に示した。 In the production process described above, by adjusting the holding time at 1200 ° C., the OH group concentration and the concentration of reduced defects in the synthetic quartz glass were controlled. In addition, the concentration of hydrogen molecules in the synthetic quartz glass was controlled by adjusting the treatment temperature during hydrogen doping, the hydrogen concentration in the atmosphere, and the total pressure. The details of the processing conditions in the manufacturing process of each example are shown in Table 9.
例48〜65で得られた合成石英ガラスのOH基濃度、水素分子濃度、163nm内部透過率および還元型欠陥の有無を、前記の方法にしたがって求めた。また、172nmの内部透過率、157nmの内部透過率、および耐紫外線性の指標としてΔT163を測定し、それぞれ波長175nm以下の真空紫外透過性、波長160nm以下の真空紫外線透過性、および耐紫外線性を評価した。各評価結果を表10および表11に示す。例48〜65の内、例52〜54は還元性欠陥があるため、例55〜58および例64はOH基濃度が比較的高いため、他のものより内部透過率が低い。 The OH group concentration, hydrogen molecule concentration, 163 nm internal transmittance, and presence or absence of reduced defects of the synthetic quartz glass obtained in Examples 48 to 65 were determined according to the above-described methods. Also, ΔT 163 was measured as an internal transmittance of 172 nm, an internal transmittance of 157 nm, and an ultraviolet resistance index, and vacuum ultraviolet transmittance of wavelength 175 nm or less, vacuum ultraviolet transmittance of wavelength 160 nm or less, and ultraviolet resistance, respectively. Evaluated. Each evaluation result is shown in Table 10 and Table 11. Of Examples 48 to 65, Examples 52 to 54 have reductive defects, so Examples 55 to 58 and Example 64 have a relatively high OH group concentration and therefore have lower internal transmittance than the others.
例66〜81は、フッ素濃度、OH基濃度、仮想温度および還元性欠陥の有無の合成石英ガラスの特性への影響を調べた実験例である。
(例66〜81)
ガラス形成材料としてSiCl4を用い、公知のスート法によりSiCl4を1200〜1500℃の酸水素火炎中で加水分解させて形成されたSiO2微粒子を基材上に堆積させて300mmφ×長さ800mmの多孔質石英ガラス体を作製した。酸水素火炎の条件は表12の工程(a)に示した。表12の工程(a)は、ガラス形成原料であるSiCl4に対する酸素と水素の体積比率を示している。
多孔質石英ガラス体を雰囲気制御可能な電気炉に設置し、表12の工程(b)に示した雰囲気、処理温度、処理時間にて多孔質石英ガラス体中の脱水(OH基低減)を行なうと同時にフッ素をドープした。なお、表12の工程(b)において、雰囲気は体積%で示している。続いて10Torr以下の減圧に保持した状態で1450℃まで昇温し、この温度にて10時間保持し透明石英ガラス体(105mmφ×長さ650mm)を作製した。
さらに得られた透明石英ガラス体をカーボン製発熱体を有する電気炉内で、窒素ガス100%、常圧下で、軟化点以上の1750℃に加熱して成長軸方向に自重変形を行なわせ円柱状のブロックに成形した。引き続き電気炉内に成形ブロックを設置したまま電気炉の温度を表12の工程(d)に記載した処理温度、処理時間で処理した後、表12の工程(d)に記載した降温プロファイルで室温まで降温させ仮想温度を制御した。
(Examples 66 to 81)
SiCl 4 is used as a glass forming material, and SiO 2 fine particles formed by hydrolyzing SiCl 4 in an oxyhydrogen flame at 1200 to 1500 ° C. by a known soot method are deposited on a substrate to be 300 mmφ × length 800 mm A porous quartz glass body was prepared. The conditions of the oxyhydrogen flame are shown in step (a) of Table 12. Step (a) in Table 12 shows the volume ratio of oxygen and hydrogen to SiCl 4 which is a glass forming raw material.
The porous quartz glass body is installed in an electric furnace capable of controlling the atmosphere, and dehydration (OH group reduction) is performed in the porous quartz glass body in the atmosphere, processing temperature, and processing time shown in step (b) of Table 12. At the same time, fluorine was doped. In step (b) of Table 12, the atmosphere is indicated by volume%. Subsequently, the temperature was raised to 1450 ° C. while maintaining a reduced pressure of 10 Torr or less, and this temperature was maintained for 10 hours to produce a transparent quartz glass body (105 mmφ × length 650 mm).
Further, the obtained transparent quartz glass body is heated to 1750 ° C. above the softening point in an electric furnace having a carbon heating element at 100% nitrogen gas and normal pressure, and is deformed by its own weight in the growth axis direction. Molded into blocks. Subsequently, the temperature of the electric furnace was treated at the treatment temperature and treatment time described in the step (d) of Table 12 with the forming block being installed in the electric furnace, and then the room temperature was measured according to the temperature drop profile described in the step (d) of Table 12. The virtual temperature was controlled by lowering the temperature.
上記製造工程において、多孔質石英ガラス体を製造する際の原料ガスに対する酸素および水素のガスの流量比、またはフッ素化合物を含んだ雰囲気下で多孔質体石英ガラス体を保持する際の雰囲気ガス組成、温度を調整することにより、得られる合成石英ガラス中のOH基濃度およびフッ素濃度を制御した。また仮想温度は、成形した円柱状ブロックを高温保持する際の温度および降温プロファイルを調整することにより、制御した。
各例で得られた合成石英ガラスのフッ素濃度、OH基濃度、仮想温度、還元型欠陥の有無を測定して表13に示した。
また、波長200nm以下の真空紫外域の透過率の指標として波長157nmの内部透過率を測定した。評価結果を表14に示す。
なお表12から14を通じて、例67〜例81の内、例66および例73はOH基濃度が高いため、例73はフッ素濃度が低いため、例74は仮想温度が高いため、また、例81は還元性欠陥があるため、他のものより特性が劣っている。
In the above manufacturing process, the flow rate ratio of oxygen and hydrogen gas to the raw material gas when manufacturing the porous quartz glass body, or the atmospheric gas composition when holding the porous quartz glass body in an atmosphere containing a fluorine compound The OH group concentration and fluorine concentration in the resulting synthetic quartz glass were controlled by adjusting the temperature. The fictive temperature was controlled by adjusting the temperature and temperature drop profile when the molded cylindrical block was kept at a high temperature.
The synthetic quartz glass obtained in each example was measured for fluorine concentration, OH group concentration, fictive temperature, and presence or absence of reducing defects, and are shown in Table 13.
Further, the internal transmittance at a wavelength of 157 nm was measured as an index of the transmittance in the vacuum ultraviolet region having a wavelength of 200 nm or less. The evaluation results are shown in Table 14.
In Tables 12 to 14, of Example 67 to Example 81, Example 66 and Example 73 have a high OH group concentration, Example 73 has a low fluorine concentration, and Example 74 has a high fictive temperature. Are inferior in properties to others due to reductive defects.
(例82〜例86)
公知の直接法により、SiCl4およびSiF4を1800〜2000℃の酸水素火炎中で加水分解および酸化させ、基材上に250mmφの透明石英ガラスを直接合成した。透明石英ガラスを200mmφの棒状体に延伸した後、横型帯域融解法(FZ法)により混練し均質化させた。次に、電気炉内にセットして1250℃にて一定時間保持し、800℃まで1℃/hrの冷却速度で徐冷を行い、その後放冷して合成石英ガラスを得た。
上記の製造工程において、SiCl4とSiF4との混合比を調整することによりフッ素濃度およびその分布を制御し、また、酸素と水素との流量比を調整することによりOH基濃度およびその分布と、水素分子濃度とを制御し、表15および16の例82〜86に示す合成石英ガラスを得た。
(Example 82 to Example 86)
By a known direct method, SiCl 4 and SiF 4 were hydrolyzed and oxidized in an oxyhydrogen flame at 1800 to 2000 ° C., and 250 mmφ transparent quartz glass was directly synthesized on the substrate. The transparent quartz glass was stretched into a 200 mmφ rod-shaped body, and then kneaded and homogenized by a horizontal zone melting method (FZ method). Next, it was set in an electric furnace, held at 1250 ° C. for a certain time, slowly cooled to 800 ° C. at a cooling rate of 1 ° C./hr, and then allowed to cool to obtain a synthetic quartz glass.
In the above manufacturing process, the fluorine concentration and its distribution are controlled by adjusting the mixing ratio of SiCl 4 and SiF 4, and the OH group concentration and its distribution are controlled by adjusting the flow rate ratio of oxygen and hydrogen. The synthetic quartz glass shown in Examples 82 to 86 in Tables 15 and 16 was obtained by controlling the hydrogen molecule concentration.
(例87〜例94)
公知のスート法により、SiCl4を1200〜1500℃の酸水素火炎中で加水分解させて形成されたSiO2微粒子を基材上に堆積させて300mmφ×長さ800mmの多孔質石英ガラス体を作製した。多孔質石英ガラス体を雰囲気制御可能な電気炉に設置し、圧力10Torr以下の減圧下で、フッ素化合物を1体積%含んだヘリウムガスを導入した。この雰囲気下にて常圧・室温で数時間保持することにより、多孔質石英ガラス中の脱水を行うと同時にフッ素をドープした。続いて圧力10Torr以下の減圧に保持した状態で1450℃まで昇温し、この温度にて10時間保持し透明石英ガラス体(105mmφ×長さ650mm)を作製した。
(Example 87 to Example 94)
A porous quartz glass body of 300 mmφ × 800 mm in length is produced by depositing SiO 2 fine particles formed by hydrolyzing SiCl 4 in an oxyhydrogen flame at 1200 to 1500 ° C. by a known soot method. did. The porous quartz glass body was placed in an electric furnace capable of controlling the atmosphere, and helium gas containing 1% by volume of a fluorine compound was introduced under a reduced pressure of 10 Torr or less. By holding at atmospheric pressure and room temperature for several hours under this atmosphere, the porous quartz glass was dehydrated and simultaneously doped with fluorine. Subsequently, the temperature was raised to 1450 ° C. while maintaining a reduced pressure of 10 Torr or less, and this temperature was maintained for 10 hours to produce a transparent quartz glass body (105 mmφ × length 650 mm).
さらに、得られた透明石英ガラス体を、カーボン製発熱体を有する電気炉内で、軟化点以上の1750℃に加熱して成長軸方向に自重変形を行わせ円柱状のブロックに成形した。引き続き、電気炉内に成形ブロックを設置したまま電気炉の温度を1250℃まで降温させ、以後1℃/hrの冷却速度で徐冷を行い、炉内温度が800℃になったところで給電を停止した。得られた石英ブロックを厚さ30mmに切断し、水素含有雰囲気下、500℃にて240時間保持して石英ガラス中に水素ドープを行って、表16〜19の例87〜94の合成石英ガラスを得た。 Furthermore, the obtained transparent quartz glass body was heated to 1750 ° C. above the softening point in an electric furnace having a carbon heating element, and was deformed by its own weight in the growth axis direction to form a cylindrical block. Subsequently, the temperature of the electric furnace is lowered to 1250 ° C. with the molding block installed in the electric furnace, and then gradually cooled at a cooling rate of 1 ° C./hr. When the temperature in the furnace reaches 800 ° C., power supply is stopped. did. The obtained quartz block was cut to a thickness of 30 mm, held in a hydrogen-containing atmosphere at 500 ° C. for 240 hours to dope hydrogen into the quartz glass, and the synthetic quartz glass of Examples 87 to 94 in Tables 16 to 19 Got.
上記の製造工程において、多孔質石英ガラス体を製造する際の原料ガスに対する酸素および水素ガスの流量比、またはフッ素化合物を含んだ雰囲気下で多孔質石英ガラス体を保持する際のフッ素化合物の濃度および保持時間を調整することにより、得られる合成石英ガラス中のOH基濃度およびフッ素濃度を制御した。また、合成石英ガラス中のOH基濃度およびフッ素濃度の変動幅の制御は、成形時のサイズを調整することにより実施した。さらに合成石英ガラス中の水素分子濃度は、水素含有雰囲気において熱処理する際の条件を調整することにより制御した。 In the above manufacturing process, the flow rate ratio of oxygen and hydrogen gas to the raw material gas when producing the porous quartz glass body, or the concentration of the fluorine compound when holding the porous quartz glass body in an atmosphere containing the fluorine compound And by adjusting the holding time, the OH group concentration and the fluorine concentration in the resulting synthetic quartz glass were controlled. In addition, the fluctuation range of the OH group concentration and the fluorine concentration in the synthetic quartz glass was controlled by adjusting the size at the time of molding. Furthermore, the hydrogen molecule concentration in the synthetic quartz glass was controlled by adjusting the conditions for heat treatment in a hydrogen-containing atmosphere.
例82〜94で得られた合成石英ガラスのフッ素濃度およびその変動幅、OH基濃度およびその変動幅、塩素濃度ならびに水素分子濃度を測定した。 The synthetic quartz glass obtained in Examples 82 to 94 was measured for fluorine concentration and its fluctuation range, OH group concentration and its fluctuation range, chlorine concentration, and hydrogen molecule concentration.
次に、例82〜94の合成石英ガラスから調製された試料について、屈折率分布、L650/S248、157nmにおける内部透過率を測定し、評価した。
各評価の結果を表15〜表21に示す。なお例82〜84および例87〜91および例94は、本発明の実施例に相当し、その他は比較例に相当する。
Next, the refractive index distribution, L 650 / S 248 , and internal transmittance at 157 nm were measured and evaluated for samples prepared from the synthetic quartz glasses of Examples 82 to 94.
The results of each evaluation are shown in Tables 15-21. Examples 82 to 84, Examples 87 to 91, and Example 94 correspond to examples of the present invention, and the others correspond to comparative examples.
Claims (8)
(a)石英ガラス形成原料を火炎加水分解させて得られる石英ガラス微粒子を基材に堆積・成長させて多孔質石英ガラス体を形成する工程と、
(b)多孔質石英ガラス体を600℃以下のフッ素含有雰囲気下にて保持し、フッ素を含有した多孔質石英ガラス体を得る工程と、
(c)フッ素を含有した多孔質石英ガラス体を透明ガラス化温度まで昇温して透明ガラス化し、フッ素を含有した透明石英ガラス体を得る工程と
を含むことを特徴とする合成石英ガラスの製造方法。 A method for producing the synthetic quartz glass according to any one of claims 1 to 5,
(A) a step of depositing and growing quartz glass fine particles obtained by hydrolyzing a quartz glass forming raw material on a substrate to form a porous quartz glass body;
(B) holding the porous quartz glass body in a fluorine-containing atmosphere at 600 ° C. or less to obtain a porous quartz glass body containing fluorine;
(C) The production of synthetic quartz glass, comprising a step of heating a porous quartz glass body containing fluorine to a transparent vitrification temperature to obtain a transparent quartz glass body containing fluorine. Method.
(a)石英ガラス形成原料を火炎加水分解させて得られる石英ガラス微粒子を基材に堆積・成長させて多孔質石英ガラス体を形成する工程と、
(b)多孔質石英ガラス体を酸素を5〜90体積%含有するフッ素含有雰囲気下にて保持し、フッ素を含有した多孔質石英ガラス体を得る工程と、
(c)フッ素を含有した多孔質石英ガラス体を透明ガラス化温度まで昇温して透明ガラス化し、フッ素を含有した透明石英ガラス体を得る工程と
を含むことを特徴とする合成石英ガラスの製造方法。 A method for producing the synthetic quartz glass according to any one of claims 1 to 5,
(A) a step of depositing and growing quartz glass fine particles obtained by hydrolyzing a quartz glass forming raw material on a substrate to form a porous quartz glass body;
(B) holding the porous quartz glass body in a fluorine-containing atmosphere containing 5 to 90% by volume of oxygen to obtain a porous quartz glass body containing fluorine;
(C) The production of synthetic quartz glass, comprising a step of heating a porous quartz glass body containing fluorine to a transparent vitrification temperature to obtain a transparent quartz glass body containing fluorine. Method.
(e)雰囲気を減圧し、フッ素を含有した多孔質石英ガラス体を減圧下に所定時間放置する工程 The method for producing synthetic quartz glass according to claim 6 or 7, wherein the following step (e) is further performed between step (b) and step (c).
(E) A step of reducing the atmosphere and leaving the porous quartz glass body containing fluorine under reduced pressure for a predetermined time.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2005131342A JP2005298330A (en) | 1998-10-28 | 2005-04-28 | Synthetic quartz glass and its manufacturing method |
Applications Claiming Priority (10)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP30747698 | 1998-10-28 | ||
| JP33863698 | 1998-11-30 | ||
| JP35011698 | 1998-12-09 | ||
| JP35333998 | 1998-12-11 | ||
| JP35335198 | 1998-12-11 | ||
| JP36767198 | 1998-12-24 | ||
| JP37001498 | 1998-12-25 | ||
| JP9361399 | 1999-03-31 | ||
| JP27503099 | 1999-09-28 | ||
| JP2005131342A JP2005298330A (en) | 1998-10-28 | 2005-04-28 | Synthetic quartz glass and its manufacturing method |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP30778399A Division JP3893816B2 (en) | 1998-10-28 | 1999-10-28 | Synthetic quartz glass and manufacturing method thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| JP2005298330A true JP2005298330A (en) | 2005-10-27 |
Family
ID=35330323
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2005131342A Pending JP2005298330A (en) | 1998-10-28 | 2005-04-28 | Synthetic quartz glass and its manufacturing method |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP2005298330A (en) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2008192351A (en) * | 2007-02-01 | 2008-08-21 | Ushio Inc | Discharge lamp |
| JP2011088815A (en) * | 2009-10-21 | 2011-05-06 | Corning Inc | Synthetic silica glass with uniform fictive temperature |
| JP2011225437A (en) * | 2010-04-01 | 2011-11-10 | Asahi Glass Co Ltd | Method for manufacturing synthetic quartz glass |
| JP2011225438A (en) * | 2010-04-01 | 2011-11-10 | Asahi Glass Co Ltd | Method for manufacturing synthetic quartz glass |
| WO2014073389A1 (en) * | 2012-11-08 | 2014-05-15 | Hoya株式会社 | Process for producing mask blank and process for producing transfer mask |
| JP2014209200A (en) * | 2013-03-22 | 2014-11-06 | Hoya株式会社 | Method of manufacturing mask blank and method of manufacturing transfer mask |
| WO2022176833A1 (en) * | 2021-02-17 | 2022-08-25 | エア・ウォーター株式会社 | Pellicle, and method for manufacturing pellicle |
-
2005
- 2005-04-28 JP JP2005131342A patent/JP2005298330A/en active Pending
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2008192351A (en) * | 2007-02-01 | 2008-08-21 | Ushio Inc | Discharge lamp |
| JP2011088815A (en) * | 2009-10-21 | 2011-05-06 | Corning Inc | Synthetic silica glass with uniform fictive temperature |
| JP2011225437A (en) * | 2010-04-01 | 2011-11-10 | Asahi Glass Co Ltd | Method for manufacturing synthetic quartz glass |
| JP2011225438A (en) * | 2010-04-01 | 2011-11-10 | Asahi Glass Co Ltd | Method for manufacturing synthetic quartz glass |
| JP2016011252A (en) * | 2010-04-01 | 2016-01-21 | 旭硝子株式会社 | Optical member |
| WO2014073389A1 (en) * | 2012-11-08 | 2014-05-15 | Hoya株式会社 | Process for producing mask blank and process for producing transfer mask |
| JPWO2014073389A1 (en) * | 2012-11-08 | 2016-09-08 | Hoya株式会社 | Mask blank manufacturing method and transfer mask manufacturing method |
| US9664997B2 (en) | 2012-11-08 | 2017-05-30 | Hoya Corporation | Method of manufacturing mask blank and method of manufacturing transfer mask |
| TWI610126B (en) * | 2012-11-08 | 2018-01-01 | Hoya Corp | Method for manufacturing photomask substrate and method for manufacturing transfer mask |
| JP2014209200A (en) * | 2013-03-22 | 2014-11-06 | Hoya株式会社 | Method of manufacturing mask blank and method of manufacturing transfer mask |
| WO2022176833A1 (en) * | 2021-02-17 | 2022-08-25 | エア・ウォーター株式会社 | Pellicle, and method for manufacturing pellicle |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP3893816B2 (en) | Synthetic quartz glass and manufacturing method thereof | |
| US6499317B1 (en) | Synthetic quartz glass and method for production thereof | |
| US7491475B2 (en) | Photomask substrate made of synthetic quartz glass and photomask | |
| US7514382B2 (en) | Synthetic quartz glass for optical member and its production method | |
| JP3069562B1 (en) | Silica glass optical material for excimer laser and excimer lamp and method for producing the same | |
| US6576578B1 (en) | Synthetic quartz glass and method for preparing the same | |
| EP2495220A1 (en) | Optical member for deep ultraviolet and process for producing same | |
| JP4066632B2 (en) | Synthetic quartz glass optical body and manufacturing method thereof | |
| JP2005298330A (en) | Synthetic quartz glass and its manufacturing method | |
| JP4946960B2 (en) | Synthetic quartz glass and manufacturing method thereof | |
| JP3472234B2 (en) | Silica glass optical material for excimer laser and excimer lamp | |
| JP3531870B2 (en) | Synthetic quartz glass | |
| EP1067097A1 (en) | Synthetic quartz glass and method for preparation thereof | |
| JP4085633B2 (en) | Synthetic quartz glass for optical components | |
| JP4240709B2 (en) | Synthetic quartz glass and manufacturing method thereof | |
| JPH11302025A (en) | Synthetic quartz glass optical member and its production | |
| JP4085490B2 (en) | Synthetic quartz glass for optical members and manufacturing method thereof | |
| JP4228493B2 (en) | Synthetic quartz glass | |
| JP2000007349A (en) | Synthetic silica glass optical member and its production | |
| JP2003201126A (en) | Synthetic quartz glass for optical member and method of manufacturing the same | |
| JP2001180962A (en) | Synthesized quartz glass and manufacturing method | |
| JP2003201125A (en) | Synthetic quartz glass and its manufacturing method | |
| JP2003183034A (en) | Synthetic quartz glass for optical member and its manufacturing method |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20080422 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20080617 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20090421 |