EP3982960B1 - Treatments of hereditary angioedema - Google Patents
Treatments of hereditary angioedema Download PDFInfo
- Publication number
- EP3982960B1 EP3982960B1 EP20734283.3A EP20734283A EP3982960B1 EP 3982960 B1 EP3982960 B1 EP 3982960B1 EP 20734283 A EP20734283 A EP 20734283A EP 3982960 B1 EP3982960 B1 EP 3982960B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- compound
- formula
- solvate
- pharmaceutically acceptable
- acceptable salt
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/444—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a six-membered ring with nitrogen as a ring heteroatom, e.g. amrinone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/08—Drugs for disorders of the alimentary tract or the digestive system for nausea, cinetosis or vertigo; Antiemetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/12—Antidiarrhoeals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/10—Antioedematous agents; Diuretics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
Definitions
- the second dosage amount can be administered between about 4 and about 12 hours of the first (more specifically, between about 4 and about 8 hours, or at about 6 hours, following the first dosage amount), and the third dosage amount can be administered between about 4 and about 12 hours of the second (more specifically, between about 4 and about 8 hours, or at about 6 hours, following the second dosage amount). Even more specifically, the second dosage amount can be administered about 2 hours following the first dosage amount and the third dosage amount can be administered about 4 hours following the first dosage amount. In some embodiments, the second and third dosage amounts can both be administered within about 8 hours of the first. More specifically, the second dosage amount can be administered between about 3 and 5 hours of the first dosage amount and the third dosage amount can be administered between about 7 and about 8 hours following the first dosage amount.
- the on-demand treatment can comprise administering four dosage amounts of the compound of Formula A within a 24 hour period starting from the time of taking the first dosage amount.
- the dosage amount can be evenly spaced apart such that there is an approximately equal time period between each dosage amount e.g. taking the subsequent dosage amount at 8 hours, 16 hours and 24 hours following the initial dosage amount.
- the second dosage amount can be administered between about 4 and about 12 hours of the first (more specifically, between about 4 and about 8 hours, or at about 6 hours, following the first dosage amount), and the third dosage amount can be administered between about 4 and about 12 hours of the second (more specifically, between about 4 and about 8 hours, or at about 6 hours, following the second dosage amount). Even more specifically, the second dosage amount can be administered about 2 hours following the first dosage amount and the third dosage amount can be administered about 4 hours following the first dosage amount. In some embodiments, the second and third dosage amounts can both be administered within about 8 hours of the first. More specifically, the second dosage amount can be administered between about 3 and 5 hours of the first dosage amount and the third dosage amount can be administered between about 7 and about 8 hours following the first dosage amount.
- molecular ions were obtained using LCMS which was carried out using an Agilent Poroshell 120 EC-C18 (2.7 ⁇ m, 3.0 ⁇ 50mm) column with 0.1% v/v Formic acid in water [eluent A]; MeCN [eluent B]; Flow rate 0.8mL/min and 1.5 minutes equilibration time between samples, gradient shown below. Mass detection was afforded with API 2000 mass spectrometer (electrospray).
- Plasma free fraction was determined using "Rapid Equilibrium Dialysis” system (Thermo Scientific), test compounds were prepared at 5 ⁇ M in neat human plasma and dialysed against phosphate buffer for 5 hrs at 37°C. Quantification of the compound partitioned in two chambers of the dialysis device was performed via LCMS/ MS. Fraction of compound unbound to plasma proteins presented as % of total.
- the compound of Formula A appears to be a highly potent inhibitor of PKa with 17-fold and 20-fold potency vs. exogenously added C1-INH in diluted plasma ( Figure 2A ) and undiluted plasma ( Figure 2B ), respectively.
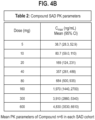
- Table 1 showing the biochemical profile of the therapies tested in this example.
- IC 50 purified enzyme nM IC 50 plasma enzyme nM K on ( ⁇ M -1 sec -1 ) Plasma Free Fraction % HK protection % C1-INH 50 1700 0.04
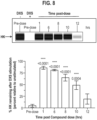
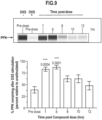
- Figures 13A and 13B show the time course of dextran sulfate-activated cleavage of HK in HAE whole undiluted plasma determined using western blotting, and a representative blot.
- the study is a randomized, double-blind, placebo-controlled, phase 2, cross-over clinical trial evaluating the efficacy and safety of the compound of formula A ("the compound”), an oral plasma kallikrein inhibitor, in the on-demand treatment of angioedema attacks in adult subjects with hereditary angioedema type I or II (EudraCT number: 2018-004489-32).
- the compound an oral plasma kallikrein inhibitor
- the PK parameters of the compound will be determined from the individual concentration versus time data using Phoenix WinNonlin. In case of a deviation from the theoretical time, the actual time of blood sample will be used in the calculation of the derived PK parameters. Individual concentrations and derived PK parameters of the compound in plasma will be listed and summarized for each treatment. Individual and geometric mean concentration-time data will be plotted on linear and semi-logarithmic scales.
Landscapes
- Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Dermatology (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Pulmonology (AREA)
- Hospice & Palliative Care (AREA)
- Otolaryngology (AREA)
- Pain & Pain Management (AREA)
- Rheumatology (AREA)
- Diabetes (AREA)
- Hematology (AREA)
- Neurosurgery (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicinal Preparation (AREA)
- Plural Heterocyclic Compounds (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Nutrition Science (AREA)
- Physiology (AREA)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| HRP20230696TT HRP20230696T1 (hr) | 2019-06-14 | 2020-06-15 | Liječenje hereditarnog angioedema |
| EP23181261.1A EP4282474A3 (en) | 2019-06-14 | 2020-06-15 | Treatments of hereditary angioedema |
| RS20230620A RS64412B1 (sr) | 2019-06-14 | 2020-06-15 | Tretmani naslednog angioedema |
| SI202030247T SI3982960T1 (sl) | 2019-06-14 | 2020-06-15 | Zdravljenja hereditarnega angioedema |
| SM20230261T SMT202300261T1 (it) | 2019-06-14 | 2020-06-15 | Trattamenti dell’angioedema ereditario |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201962861725P | 2019-06-14 | 2019-06-14 | |
| GBGB1910116.1A GB201910116D0 (en) | 2019-07-15 | 2019-07-15 | Treatments of hereditary angioedema |
| PCT/GB2020/051439 WO2020249977A1 (en) | 2019-06-14 | 2020-06-15 | Treatments of hereditary angioedema |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP23181261.1A Division EP4282474A3 (en) | 2019-06-14 | 2020-06-15 | Treatments of hereditary angioedema |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP3982960A1 EP3982960A1 (en) | 2022-04-20 |
| EP3982960B1 true EP3982960B1 (en) | 2023-06-28 |
Family
ID=67700145
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP20734283.3A Active EP3982960B1 (en) | 2019-06-14 | 2020-06-15 | Treatments of hereditary angioedema |
| EP23181261.1A Pending EP4282474A3 (en) | 2019-06-14 | 2020-06-15 | Treatments of hereditary angioedema |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP23181261.1A Pending EP4282474A3 (en) | 2019-06-14 | 2020-06-15 | Treatments of hereditary angioedema |
Country Status (34)
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB201609607D0 (en) | 2016-06-01 | 2016-07-13 | Kalvista Pharmaceuticals Ltd | Polymorphs of N-(3-Fluoro-4-methoxypyridin-2-yl)methyl)-3-(methoxymethyl)-1-({4-((2-oxopy ridin-1-yl)methyl)phenyl}methyl)pyrazole-4-carboxamide and salts |
| GB201719881D0 (en) | 2017-11-29 | 2018-01-10 | Kalvista Pharmaceuticals Ltd | Solid forms of plasma kallikrein inhibitor and salts thereof |
| GB201910116D0 (en) * | 2019-07-15 | 2019-08-28 | Kalvista Pharmaceuticals Ltd | Treatments of hereditary angioedema |
| EP4010333A1 (en) | 2019-08-09 | 2022-06-15 | Kalvista Pharmaceuticals Limited | Plasma kallikrein inhibitors |
| WO2022084693A1 (en) * | 2020-10-23 | 2022-04-28 | Kalvista Pharmaceuticals Limited | Treatments of angioedema |
| WO2022172006A1 (en) | 2021-02-09 | 2022-08-18 | Kalvista Pharmaceuticals Limited | Treatments of hereditary angioedema |
| SI4288036T1 (sl) | 2022-04-27 | 2024-10-30 | Kalvista Pharmaceuticals Limited | Formulacije zaviralca plazemskega kalikreina |
| CN116003386B (zh) * | 2022-11-20 | 2024-03-26 | 药康众拓(北京)医药科技有限公司 | 一种氘代n-苄基吡啶酮吡唑甲酰胺类化合物、药物组合物和用途 |
| WO2025172693A1 (en) | 2024-02-13 | 2025-08-21 | Kalvista Pharmaceuticals Limited | Oral sebetralstat for the treatment of an attack of hereditary angioedema |
| WO2025172692A1 (en) | 2024-02-13 | 2025-08-21 | Kalvista Pharmaceuticals Limited | Oral sebetralstat for the treatment of an attack of hereditary angioedema |
Family Cites Families (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5187157A (en) | 1987-06-05 | 1993-02-16 | Du Pont Merck Pharmaceutical Company | Peptide boronic acid inhibitors of trypsin-like proteases |
| GB9019558D0 (en) | 1990-09-07 | 1990-10-24 | Szelke Michael | Enzyme inhibitors |
| SE9301911D0 (sv) | 1993-06-03 | 1993-06-03 | Ab Astra | New peptide derivatives |
| US5589467A (en) | 1993-09-17 | 1996-12-31 | Novo Nordisk A/S | 2,5',N6-trisubstituted adenosine derivatives |
| GB0205527D0 (en) | 2002-03-08 | 2002-04-24 | Ferring Bv | Inhibitors |
| US7429604B2 (en) | 2004-06-15 | 2008-09-30 | Bristol Myers Squibb Company | Six-membered heterocycles useful as serine protease inhibitors |
| CA2658523C (en) | 2006-07-31 | 2012-06-12 | Activesite Pharmaceuticals, Inc. | Inhibitors of plasma kallikrein |
| DE102006050672A1 (de) | 2006-10-24 | 2008-04-30 | Curacyte Discovery Gmbh | Hemmstoffe des Plasmins und des Plasmakallikreins |
| JP2013121919A (ja) | 2010-03-25 | 2013-06-20 | Astellas Pharma Inc | 血漿カリクレイン阻害剤 |
| WO2012004678A2 (en) | 2010-07-07 | 2012-01-12 | The Medicines Company (Leipzig) Gmbh | Serine protease inhibitors |
| WO2012009009A2 (en) | 2010-07-14 | 2012-01-19 | Addex Pharma S.A. | Novel 2-amino-4-pyrazolyl-thiazole derivatives and their use as allosteric modulators of metabotropic glutamate receptors |
| US9290485B2 (en) | 2010-08-04 | 2016-03-22 | Novartis Ag | N-((6-amino-pyridin-3-yl)methyl)-heteroaryl-carboxamides |
| GB2494851A (en) | 2011-07-07 | 2013-03-27 | Kalvista Pharmaceuticals Ltd | Plasma kallikrein inhibitors |
| GB201300304D0 (en) | 2013-01-08 | 2013-02-20 | Kalvista Pharmaceuticals Ltd | Benzylamine derivatives |
| PT3305778T (pt) | 2013-05-23 | 2022-05-02 | Kalvista Pharmaceuticals Ltd | Inibidores de calicreína plasmática |
| EP2815749A1 (en) * | 2013-06-20 | 2014-12-24 | IP Gesellschaft für Management mbH | Solid form of 4-amino-2-(2,6-dioxopiperidine-3-yl)isoindoline-1,3-dione having specified X-ray diffraction pattern |
| EP2886107A1 (en) * | 2013-12-17 | 2015-06-24 | ObsEva S.A. | Oral formulations of pyrrolydine derivatives |
| GB201421083D0 (en) | 2014-11-27 | 2015-01-14 | Kalvista Pharmaceuticals Ltd | Enzyme inhibitors |
| GB201721515D0 (en) * | 2017-12-21 | 2018-02-07 | Kalvista Pharmaceuticals Ltd | Dosage forms comprising a plasma kallikrein inhibtor |
| GB201910116D0 (en) | 2019-07-15 | 2019-08-28 | Kalvista Pharmaceuticals Ltd | Treatments of hereditary angioedema |
-
2019
- 2019-07-15 GB GBGB1910116.1A patent/GB201910116D0/en not_active Ceased
-
2020
- 2020-06-15 AU AU2020293614A patent/AU2020293614B2/en active Active
- 2020-06-15 HU HUE20734283A patent/HUE063163T2/hu unknown
- 2020-06-15 UA UAA202106869A patent/UA129869C2/uk unknown
- 2020-06-15 EA EA202193019A patent/EA202193019A1/ru unknown
- 2020-06-15 MX MX2021014557A patent/MX2021014557A/es unknown
- 2020-06-15 TW TW109120108A patent/TW202112370A/zh unknown
- 2020-06-15 HR HRP20230696TT patent/HRP20230696T1/hr unknown
- 2020-06-15 SG SG11202113304YA patent/SG11202113304YA/en unknown
- 2020-06-15 EP EP20734283.3A patent/EP3982960B1/en active Active
- 2020-06-15 US US17/617,439 patent/US20220218680A1/en active Pending
- 2020-06-15 PH PH1/2021/552966A patent/PH12021552966A1/en unknown
- 2020-06-15 FI FIEP20734283.3T patent/FI3982960T3/fi active
- 2020-06-15 MD MDE20220440T patent/MD3982960T2/ro unknown
- 2020-06-15 WO PCT/GB2020/051439 patent/WO2020249977A1/en not_active Ceased
- 2020-06-15 BR BR112021024664A patent/BR112021024664A2/pt not_active Application Discontinuation
- 2020-06-15 CA CA3142218A patent/CA3142218A1/en active Pending
- 2020-06-15 MY MYPI2021007320A patent/MY205687A/en unknown
- 2020-06-15 PL PL20734283.3T patent/PL3982960T3/pl unknown
- 2020-06-15 KR KR1020217043374A patent/KR102748728B1/ko active Active
- 2020-06-15 PT PT207342833T patent/PT3982960T/pt unknown
- 2020-06-15 LT LTEPPCT/GB2020/051439T patent/LT3982960T/lt unknown
- 2020-06-15 MA MA56187A patent/MA56187B1/fr unknown
- 2020-06-15 EP EP23181261.1A patent/EP4282474A3/en active Pending
- 2020-06-15 RS RS20230620A patent/RS64412B1/sr unknown
- 2020-06-15 ES ES20734283T patent/ES2956471T3/es active Active
- 2020-06-15 JP JP2021571931A patent/JP7356518B2/ja active Active
- 2020-06-15 SI SI202030247T patent/SI3982960T1/sl unknown
- 2020-06-15 SM SM20230261T patent/SMT202300261T1/it unknown
- 2020-06-15 CN CN202080043365.6A patent/CN114126612A/zh active Pending
- 2020-06-15 DK DK20734283.3T patent/DK3982960T3/da active
- 2020-06-16 AR ARP200101681A patent/AR119158A1/es unknown
-
2021
- 2021-12-02 IL IL288615A patent/IL288615A/en unknown
- 2021-12-06 CL CL2021003244A patent/CL2021003244A1/es unknown
- 2021-12-20 ZA ZA2021/10685A patent/ZA202110685B/en unknown
-
2023
- 2023-03-06 CL CL2023000639A patent/CL2023000639A1/es unknown
- 2023-08-14 JP JP2023131860A patent/JP7688078B2/ja active Active
-
2025
- 2025-05-21 JP JP2025084646A patent/JP2025124716A/ja active Pending
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3982960B1 (en) | Treatments of hereditary angioedema | |
| JP7640474B2 (ja) | 血管性浮腫の治療 | |
| US20240122909A1 (en) | Treatments of hereditary angioedema | |
| US20230381162A1 (en) | Treatments of angioedema | |
| WO2022079446A1 (en) | Treatments of angioedema | |
| US20250281397A1 (en) | Formulations of a plasma kallikrein inhibitor | |
| HK40101443A (en) | Treatments of hereditary angioedema | |
| HK40070596B (en) | Treatments of hereditary angioedema | |
| HK40070596A (en) | Treatments of hereditary angioedema | |
| EA048676B1 (ru) | Лечение наследственного ангионевротического отека | |
| WO2024180100A1 (en) | New solid form of a plasma kallikrein inhibitor | |
| JP2022069377A (ja) | 血管性浮腫の処置 | |
| WO2025172692A1 (en) | Oral sebetralstat for the treatment of an attack of hereditary angioedema | |
| WO2025172693A1 (en) | Oral sebetralstat for the treatment of an attack of hereditary angioedema | |
| WO2023002219A1 (en) | Treatments of hereditary angioedema |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| REG | Reference to a national code |
Ref country code: HR Ref legal event code: TUEP Ref document number: P20230696T Country of ref document: HR |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: UNKNOWN |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE INTERNATIONAL PUBLICATION HAS BEEN MADE |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20220112 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: DE Ref document number: 40070596 Country of ref document: HK |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R079 Free format text: PREVIOUS MAIN CLASS: A61K0031443900 Ipc: A61K0031444000 Ref country code: DE Ref legal event code: R079 Ref document number: 602020013054 Country of ref document: DE Free format text: PREVIOUS MAIN CLASS: A61K0031443900 Ipc: A61K0031444000 |
|
| 17Q | First examination report despatched |
Effective date: 20230309 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: A61P 7/00 20060101ALI20230405BHEP Ipc: A61K 31/444 20060101AFI20230405BHEP |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| INTG | Intention to grant announced |
Effective date: 20230508 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: TN Ref legal event code: VAGR Ref document number: TN/P/2023/000160 Country of ref document: TN |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 1582126 Country of ref document: AT Kind code of ref document: T Effective date: 20230715 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602020013054 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: T3 Effective date: 20230818 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: FP |
|
| REG | Reference to a national code |
Ref country code: NO Ref legal event code: T2 Effective date: 20230628 |
|
| REG | Reference to a national code |
Ref country code: FI Ref legal event code: FGE |
|
| REG | Reference to a national code |
Ref country code: RO Ref legal event code: EPE |
|
| REG | Reference to a national code |
Ref country code: PT Ref legal event code: SC4A Ref document number: 3982960 Country of ref document: PT Date of ref document: 20230926 Kind code of ref document: T Free format text: AVAILABILITY OF NATIONAL TRANSLATION Effective date: 20230919 Ref country code: SE Ref legal event code: TRGR |
|
| REG | Reference to a national code |
Ref country code: MA Ref legal event code: VAGR Ref document number: 56187 Country of ref document: MA Kind code of ref document: B1 |
|
| REG | Reference to a national code |
Ref country code: HR Ref legal event code: T1PR Ref document number: P20230696 Country of ref document: HR |
|
| REG | Reference to a national code |
Ref country code: EE Ref legal event code: FG4A Ref document number: E023532 Country of ref document: EE Effective date: 20230921 |
|
| REG | Reference to a national code |
Ref country code: GR Ref legal event code: EP Ref document number: 20230401590 Country of ref document: GR Effective date: 20231113 |
|
| REG | Reference to a national code |
Ref country code: SK Ref legal event code: T3 Ref document number: E 42400 Country of ref document: SK |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2956471 Country of ref document: ES Kind code of ref document: T3 Effective date: 20231221 |
|
| REG | Reference to a national code |
Ref country code: HU Ref legal event code: AG4A Ref document number: E063163 Country of ref document: HU |
|
| REG | Reference to a national code |
Ref country code: MD Ref legal event code: VAGR Ref document number: 3982960 Country of ref document: MD Date of ref document: 20231231 Kind code of ref document: T2 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: UEP Ref document number: 1582126 Country of ref document: AT Kind code of ref document: T Effective date: 20230628 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602020013054 Country of ref document: DE |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20240402 |
|
| REG | Reference to a national code |
Ref country code: HR Ref legal event code: ODRP Ref document number: P20230696 Country of ref document: HR Payment date: 20240429 Year of fee payment: 5 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CY Payment date: 20240509 Year of fee payment: 5 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 732E Free format text: REGISTERED BETWEEN 20241114 AND 20241120 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 20250310 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20250409 Year of fee payment: 6 |
|
| REG | Reference to a national code |
Ref country code: HR Ref legal event code: ODRP Ref document number: P20230696 Country of ref document: HR Payment date: 20250428 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SM Payment date: 20250529 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: MC Payment date: 20250602 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FI Payment date: 20250620 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: PL Payment date: 20250407 Year of fee payment: 6 Ref country code: DE Payment date: 20250402 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20250401 Year of fee payment: 6 Ref country code: DK Payment date: 20250618 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: LT Payment date: 20250424 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IS Payment date: 20250602 Year of fee payment: 6 Ref country code: RS Payment date: 20250516 Year of fee payment: 6 Ref country code: NO Payment date: 20250610 Year of fee payment: 6 |
|
| VSFP | Annual fee paid to validation state [announced via postgrant information from national office to epo] |
Ref country code: MD Payment date: 20250422 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: LU Payment date: 20250611 Year of fee payment: 6 Ref country code: AL Payment date: 20250616 Year of fee payment: 6 Ref country code: IT Payment date: 20250522 Year of fee payment: 6 Ref country code: BE Payment date: 20250410 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: HR Payment date: 20250428 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: PT Payment date: 20250606 Year of fee payment: 6 Ref country code: LV Payment date: 20250423 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: HU Payment date: 20250513 Year of fee payment: 6 Ref country code: FR Payment date: 20250409 Year of fee payment: 6 Ref country code: EE Payment date: 20250514 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: MT Payment date: 20250618 Year of fee payment: 6 Ref country code: GR Payment date: 20250516 Year of fee payment: 6 Ref country code: BG Payment date: 20250415 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: RO Payment date: 20250508 Year of fee payment: 6 Ref country code: AT Payment date: 20250528 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: TR Payment date: 20250612 Year of fee payment: 6 Ref country code: SK Payment date: 20250513 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CZ Payment date: 20250530 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IE Payment date: 20250402 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SI Payment date: 20250424 Year of fee payment: 6 |
|
| VSFP | Annual fee paid to validation state [announced via postgrant information from national office to epo] |
Ref country code: MD Payment date: 20240429 Year of fee payment: 5 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20250707 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 20250701 Year of fee payment: 6 |
|
| VSFP | Annual fee paid to validation state [announced via postgrant information from national office to epo] |
Ref country code: MA Payment date: 20240522 Year of fee payment: 5 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: MK Payment date: 20250428 Year of fee payment: 6 |
|
| VSFP | Annual fee paid to validation state [announced via postgrant information from national office to epo] |
Ref country code: MA Payment date: 20250612 Year of fee payment: 6 |