EP3337496B1 - B7-h1 fusion polypeptides for use in treating and preventing organ failure - Google Patents
B7-h1 fusion polypeptides for use in treating and preventing organ failure Download PDFInfo
- Publication number
- EP3337496B1 EP3337496B1 EP16757610.7A EP16757610A EP3337496B1 EP 3337496 B1 EP3337496 B1 EP 3337496B1 EP 16757610 A EP16757610 A EP 16757610A EP 3337496 B1 EP3337496 B1 EP 3337496B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- polypeptide
- fusion polypeptide
- cells
- sepsis
- expression
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 108090000765 processed proteins & peptides Proteins 0.000 title claims description 131
- 102000004196 processed proteins & peptides Human genes 0.000 title claims description 121
- 229920001184 polypeptide Polymers 0.000 title claims description 119
- 230000004927 fusion Effects 0.000 title claims description 80
- 206010053159 Organ failure Diseases 0.000 title claims description 27
- 230000014509 gene expression Effects 0.000 claims description 82
- 206010040047 Sepsis Diseases 0.000 claims description 57
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 claims description 46
- 241000282414 Homo sapiens Species 0.000 claims description 45
- 150000001413 amino acids Chemical class 0.000 claims description 35
- 102000040430 polynucleotide Human genes 0.000 claims description 34
- 108091033319 polynucleotide Proteins 0.000 claims description 34
- 239000002157 polynucleotide Substances 0.000 claims description 34
- 239000003814 drug Substances 0.000 claims description 33
- 101001117317 Homo sapiens Programmed cell death 1 ligand 1 Proteins 0.000 claims description 22
- 150000007523 nucleic acids Chemical group 0.000 claims description 21
- 102000048776 human CD274 Human genes 0.000 claims description 20
- 229940079593 drug Drugs 0.000 claims description 19
- 108060003951 Immunoglobulin Proteins 0.000 claims description 14
- 102000018358 immunoglobulin Human genes 0.000 claims description 14
- 230000001419 dependent effect Effects 0.000 claims description 13
- 239000012634 fragment Substances 0.000 claims description 8
- 241000124008 Mammalia Species 0.000 claims description 7
- 108091028043 Nucleic acid sequence Proteins 0.000 claims description 7
- 108010084313 CD58 Antigens Proteins 0.000 claims description 6
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 claims description 6
- 102100030984 Lymphocyte function-associated antigen 3 Human genes 0.000 claims description 6
- 208000034486 Multi-organ failure Diseases 0.000 claims description 6
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 claims description 6
- 239000003112 inhibitor Substances 0.000 claims description 6
- 208000029744 multiple organ dysfunction syndrome Diseases 0.000 claims description 6
- 230000000770 proinflammatory effect Effects 0.000 claims description 5
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 4
- 101710187898 60S ribosomal protein L28 Proteins 0.000 claims description 4
- 102220539261 Programmed cell death 1 ligand 2_A98F_mutation Human genes 0.000 claims description 4
- 102220539294 Programmed cell death 1 ligand 2_D49S_mutation Human genes 0.000 claims description 4
- 102220539291 Programmed cell death 1 ligand 2_E58S_mutation Human genes 0.000 claims description 4
- 102220539286 Programmed cell death 1 ligand 2_E72S_mutation Human genes 0.000 claims description 4
- 102220539288 Programmed cell death 1 ligand 2_K75S_mutation Human genes 0.000 claims description 4
- 102220539262 Programmed cell death 1 ligand 2_K89S_mutation Human genes 0.000 claims description 4
- 102220539506 Programmed cell death 1 ligand 2_L27A_mutation Human genes 0.000 claims description 4
- 102220539264 Programmed cell death 1 ligand 2_Q100S_mutation Human genes 0.000 claims description 4
- 102220539292 Programmed cell death 1 ligand 2_S34Y_mutation Human genes 0.000 claims description 4
- 102220539289 Programmed cell death 1 ligand 2_Y56S_mutation Human genes 0.000 claims description 4
- 102220470351 Charged multivesicular body protein 5_K62S_mutation Human genes 0.000 claims description 3
- 102000004127 Cytokines Human genes 0.000 claims description 3
- 108090000695 Cytokines Proteins 0.000 claims description 3
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 claims description 3
- 102100025237 T-cell surface antigen CD2 Human genes 0.000 claims description 3
- 102100027222 T-lymphocyte activation antigen CD80 Human genes 0.000 claims description 3
- 239000003242 anti bacterial agent Substances 0.000 claims description 3
- 230000002785 anti-thrombosis Effects 0.000 claims description 3
- 229940088710 antibiotic agent Drugs 0.000 claims description 3
- 239000003146 anticoagulant agent Substances 0.000 claims description 3
- 229940127219 anticoagulant drug Drugs 0.000 claims description 3
- 229960004676 antithrombotic agent Drugs 0.000 claims description 3
- 150000003431 steroids Chemical class 0.000 claims description 3
- 125000003275 alpha amino acid group Chemical group 0.000 claims 5
- 210000004027 cell Anatomy 0.000 description 104
- 102100024216 Programmed cell death 1 ligand 1 Human genes 0.000 description 96
- 101710094000 Programmed cell death 1 ligand 1 Proteins 0.000 description 92
- 108020004414 DNA Proteins 0.000 description 47
- 241000699670 Mus sp. Species 0.000 description 44
- 238000000034 method Methods 0.000 description 26
- 210000004185 liver Anatomy 0.000 description 22
- 239000013598 vector Substances 0.000 description 22
- 230000002441 reversible effect Effects 0.000 description 19
- 108090000623 proteins and genes Proteins 0.000 description 18
- 241000699660 Mus musculus Species 0.000 description 17
- 238000003753 real-time PCR Methods 0.000 description 17
- 102000004070 NADPH Oxidase 4 Human genes 0.000 description 16
- 108010082699 NADPH Oxidase 4 Proteins 0.000 description 16
- 239000002158 endotoxin Substances 0.000 description 16
- 210000003494 hepatocyte Anatomy 0.000 description 15
- 102000004169 proteins and genes Human genes 0.000 description 15
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 13
- 206010067125 Liver injury Diseases 0.000 description 13
- 231100000234 hepatic damage Toxicity 0.000 description 13
- 230000008818 liver damage Effects 0.000 description 13
- 229940024606 amino acid Drugs 0.000 description 12
- 241001529936 Murinae Species 0.000 description 11
- 102100040678 Programmed cell death protein 1 Human genes 0.000 description 11
- 241000700605 Viruses Species 0.000 description 11
- 238000013459 approach Methods 0.000 description 11
- 239000012636 effector Substances 0.000 description 11
- 210000001266 CD8-positive T-lymphocyte Anatomy 0.000 description 10
- AIYUHDOJVYHVIT-UHFFFAOYSA-M caesium chloride Chemical compound [Cl-].[Cs+] AIYUHDOJVYHVIT-UHFFFAOYSA-M 0.000 description 10
- 230000003013 cytotoxicity Effects 0.000 description 10
- 231100000135 cytotoxicity Toxicity 0.000 description 10
- 238000002474 experimental method Methods 0.000 description 10
- 239000002953 phosphate buffered saline Substances 0.000 description 10
- 239000003642 reactive oxygen metabolite Substances 0.000 description 10
- 208000024891 symptom Diseases 0.000 description 10
- 101710089372 Programmed cell death protein 1 Proteins 0.000 description 9
- 230000004913 activation Effects 0.000 description 9
- 201000010099 disease Diseases 0.000 description 9
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 9
- 108010048367 enhanced green fluorescent protein Proteins 0.000 description 9
- 108020004999 messenger RNA Proteins 0.000 description 9
- 102100024213 Programmed cell death 1 ligand 2 Human genes 0.000 description 8
- 210000004369 blood Anatomy 0.000 description 8
- 239000008280 blood Substances 0.000 description 8
- 238000009472 formulation Methods 0.000 description 8
- 238000011813 knockout mouse model Methods 0.000 description 8
- 239000000203 mixture Substances 0.000 description 8
- 238000011002 quantification Methods 0.000 description 8
- 210000002966 serum Anatomy 0.000 description 8
- 230000001225 therapeutic effect Effects 0.000 description 8
- 241001465754 Metazoa Species 0.000 description 7
- 101100407308 Mus musculus Pdcd1lg2 gene Proteins 0.000 description 7
- 108700030875 Programmed Cell Death 1 Ligand 2 Proteins 0.000 description 7
- 239000006285 cell suspension Substances 0.000 description 7
- 238000012217 deletion Methods 0.000 description 7
- 239000001963 growth medium Substances 0.000 description 7
- 239000000463 material Substances 0.000 description 7
- 102000039446 nucleic acids Human genes 0.000 description 7
- 108020004707 nucleic acids Proteins 0.000 description 7
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- PWKSKIMOESPYIA-BYPYZUCNSA-N L-N-acetyl-Cysteine Chemical compound CC(=O)N[C@@H](CS)C(O)=O PWKSKIMOESPYIA-BYPYZUCNSA-N 0.000 description 6
- 108010082739 NADPH Oxidase 2 Proteins 0.000 description 6
- 102000004180 NADPH Oxidase 2 Human genes 0.000 description 6
- 229960004308 acetylcysteine Drugs 0.000 description 6
- 230000001363 autoimmune Effects 0.000 description 6
- 238000002784 cytotoxicity assay Methods 0.000 description 6
- 231100000263 cytotoxicity test Toxicity 0.000 description 6
- 230000037430 deletion Effects 0.000 description 6
- 230000003828 downregulation Effects 0.000 description 6
- 230000000694 effects Effects 0.000 description 6
- 230000001404 mediated effect Effects 0.000 description 6
- 210000000056 organ Anatomy 0.000 description 6
- 239000002245 particle Substances 0.000 description 6
- 230000001105 regulatory effect Effects 0.000 description 6
- 230000004083 survival effect Effects 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- 241000700721 Hepatitis B virus Species 0.000 description 5
- 210000001744 T-lymphocyte Anatomy 0.000 description 5
- 238000007792 addition Methods 0.000 description 5
- 238000009396 hybridization Methods 0.000 description 5
- 238000000338 in vitro Methods 0.000 description 5
- 208000015181 infectious disease Diseases 0.000 description 5
- 230000010354 integration Effects 0.000 description 5
- 230000002265 prevention Effects 0.000 description 5
- 230000003449 preventive effect Effects 0.000 description 5
- 210000000952 spleen Anatomy 0.000 description 5
- 230000000638 stimulation Effects 0.000 description 5
- 230000008685 targeting Effects 0.000 description 5
- 238000013518 transcription Methods 0.000 description 5
- 230000035897 transcription Effects 0.000 description 5
- 230000026683 transduction Effects 0.000 description 5
- 238000010361 transduction Methods 0.000 description 5
- 238000001262 western blot Methods 0.000 description 5
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 4
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 4
- 108020004635 Complementary DNA Proteins 0.000 description 4
- 230000002159 abnormal effect Effects 0.000 description 4
- 230000001580 bacterial effect Effects 0.000 description 4
- 238000010804 cDNA synthesis Methods 0.000 description 4
- 239000000969 carrier Substances 0.000 description 4
- 239000002299 complementary DNA Substances 0.000 description 4
- 230000002950 deficient Effects 0.000 description 4
- 239000003085 diluting agent Substances 0.000 description 4
- 239000013604 expression vector Substances 0.000 description 4
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 4
- 230000006870 function Effects 0.000 description 4
- 102000054766 genetic haplotypes Human genes 0.000 description 4
- 210000002865 immune cell Anatomy 0.000 description 4
- 238000001727 in vivo Methods 0.000 description 4
- 230000001965 increasing effect Effects 0.000 description 4
- 230000005764 inhibitory process Effects 0.000 description 4
- 230000000977 initiatory effect Effects 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 239000006166 lysate Substances 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 230000007246 mechanism Effects 0.000 description 4
- 239000002609 medium Substances 0.000 description 4
- 239000008194 pharmaceutical composition Substances 0.000 description 4
- 230000004044 response Effects 0.000 description 4
- 238000002560 therapeutic procedure Methods 0.000 description 4
- 108010074708 B7-H1 Antigen Proteins 0.000 description 3
- 241000894006 Bacteria Species 0.000 description 3
- 101100044298 Drosophila melanogaster fand gene Proteins 0.000 description 3
- 101150064015 FAS gene Proteins 0.000 description 3
- 102000001398 Granzyme Human genes 0.000 description 3
- 108060005986 Granzyme Proteins 0.000 description 3
- 101000831567 Homo sapiens Toll-like receptor 2 Proteins 0.000 description 3
- 108010002350 Interleukin-2 Proteins 0.000 description 3
- 241000699666 Mus <mouse, genus> Species 0.000 description 3
- 108010058846 Ovalbumin Proteins 0.000 description 3
- KHGNFPUMBJSZSM-UHFFFAOYSA-N Perforine Natural products COC1=C2CCC(O)C(CCC(C)(C)O)(OC)C2=NC2=C1C=CO2 KHGNFPUMBJSZSM-UHFFFAOYSA-N 0.000 description 3
- 101100335198 Pneumocystis carinii fol1 gene Proteins 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 3
- 206010051379 Systemic Inflammatory Response Syndrome Diseases 0.000 description 3
- 102000002689 Toll-like receptor Human genes 0.000 description 3
- 108020000411 Toll-like receptor Proteins 0.000 description 3
- 102100024333 Toll-like receptor 2 Human genes 0.000 description 3
- 239000000427 antigen Substances 0.000 description 3
- 108091007433 antigens Proteins 0.000 description 3
- 102000036639 antigens Human genes 0.000 description 3
- 230000004071 biological effect Effects 0.000 description 3
- 210000002421 cell wall Anatomy 0.000 description 3
- 238000010367 cloning Methods 0.000 description 3
- 230000034994 death Effects 0.000 description 3
- 239000003937 drug carrier Substances 0.000 description 3
- 239000003623 enhancer Substances 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- 210000002216 heart Anatomy 0.000 description 3
- 229940072221 immunoglobulins Drugs 0.000 description 3
- 230000001939 inductive effect Effects 0.000 description 3
- 230000002147 killing effect Effects 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 238000010172 mouse model Methods 0.000 description 3
- 239000002773 nucleotide Substances 0.000 description 3
- 125000003729 nucleotide group Chemical group 0.000 description 3
- 229940092253 ovalbumin Drugs 0.000 description 3
- 239000008188 pellet Substances 0.000 description 3
- 229930192851 perforin Natural products 0.000 description 3
- 239000002244 precipitate Substances 0.000 description 3
- 102000005962 receptors Human genes 0.000 description 3
- 108020003175 receptors Proteins 0.000 description 3
- 238000001356 surgical procedure Methods 0.000 description 3
- 208000011580 syndromic disease Diseases 0.000 description 3
- 241000701161 unidentified adenovirus Species 0.000 description 3
- 230000003612 virological effect Effects 0.000 description 3
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- 108020004463 18S ribosomal RNA Proteins 0.000 description 2
- IZHVBANLECCAGF-UHFFFAOYSA-N 2-hydroxy-3-(octadecanoyloxy)propyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)COC(=O)CCCCCCCCCCCCCCCCC IZHVBANLECCAGF-UHFFFAOYSA-N 0.000 description 2
- 102000053602 DNA Human genes 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- 206010061218 Inflammation Diseases 0.000 description 2
- 102220470475 L-seryl-tRNA(Sec) kinase_C57L_mutation Human genes 0.000 description 2
- 108010016731 PPAR gamma Proteins 0.000 description 2
- 206010033799 Paralysis Diseases 0.000 description 2
- 239000006180 TBST buffer Substances 0.000 description 2
- 108010060804 Toll-Like Receptor 4 Proteins 0.000 description 2
- 102100039360 Toll-like receptor 4 Human genes 0.000 description 2
- 102000004142 Trypsin Human genes 0.000 description 2
- 108090000631 Trypsin Proteins 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 210000000612 antigen-presenting cell Anatomy 0.000 description 2
- 230000033228 biological regulation Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 229960002685 biotin Drugs 0.000 description 2
- 235000020958 biotin Nutrition 0.000 description 2
- 239000011616 biotin Substances 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- RMRJXGBAOAMLHD-IHFGGWKQSA-N buprenorphine Chemical compound C([C@]12[C@H]3OC=4C(O)=CC=C(C2=4)C[C@@H]2[C@]11CC[C@]3([C@H](C1)[C@](C)(O)C(C)(C)C)OC)CN2CC1CC1 RMRJXGBAOAMLHD-IHFGGWKQSA-N 0.000 description 2
- 238000004113 cell culture Methods 0.000 description 2
- 239000013592 cell lysate Substances 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 230000004064 dysfunction Effects 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 229940088598 enzyme Drugs 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 230000002068 genetic effect Effects 0.000 description 2
- 230000002440 hepatic effect Effects 0.000 description 2
- 230000028993 immune response Effects 0.000 description 2
- 230000001976 improved effect Effects 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 239000003446 ligand Substances 0.000 description 2
- 238000011068 loading method Methods 0.000 description 2
- 230000005923 long-lasting effect Effects 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 239000001788 mono and diglycerides of fatty acids Substances 0.000 description 2
- 210000001616 monocyte Anatomy 0.000 description 2
- 230000008816 organ damage Effects 0.000 description 2
- GSSMIHQEWAQUPM-AOLPDKKJSA-N ovalbumin peptide Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)[C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C1=CN=CN1 GSSMIHQEWAQUPM-AOLPDKKJSA-N 0.000 description 2
- 230000002018 overexpression Effects 0.000 description 2
- 239000013600 plasmid vector Substances 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 239000003531 protein hydrolysate Substances 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 230000010076 replication Effects 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 230000029058 respiratory gaseous exchange Effects 0.000 description 2
- 230000001177 retroviral effect Effects 0.000 description 2
- FGDZQCVHDSGLHJ-UHFFFAOYSA-M rubidium chloride Chemical compound [Cl-].[Rb+] FGDZQCVHDSGLHJ-UHFFFAOYSA-M 0.000 description 2
- 238000002864 sequence alignment Methods 0.000 description 2
- 238000012163 sequencing technique Methods 0.000 description 2
- 230000035939 shock Effects 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 238000010186 staining Methods 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 239000006228 supernatant Substances 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 230000036962 time dependent Effects 0.000 description 2
- 230000005030 transcription termination Effects 0.000 description 2
- 239000012588 trypsin Substances 0.000 description 2
- NJZHEQOUHLZCOX-ZENOOKHLSA-N (3aR,4R,9bS)-golgicide A Chemical compound C1([C@@H]2NC3=C(F)C=C(C=C3[C@H]3C=CC[C@H]32)F)=CC=CN=C1 NJZHEQOUHLZCOX-ZENOOKHLSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- 108020005345 3' Untranslated Regions Proteins 0.000 description 1
- 208000010444 Acidosis Diseases 0.000 description 1
- 241000251468 Actinopterygii Species 0.000 description 1
- 102000007469 Actins Human genes 0.000 description 1
- 108010085238 Actins Proteins 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- HJCMDXDYPOUFDY-WHFBIAKZSA-N Ala-Gln Chemical compound C[C@H](N)C(=O)N[C@H](C(O)=O)CCC(N)=O HJCMDXDYPOUFDY-WHFBIAKZSA-N 0.000 description 1
- 102100036475 Alanine aminotransferase 1 Human genes 0.000 description 1
- 108010082126 Alanine transaminase Proteins 0.000 description 1
- 108700028369 Alleles Proteins 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 241001156002 Anthonomus pomorum Species 0.000 description 1
- 108010003415 Aspartate Aminotransferases Proteins 0.000 description 1
- 102000004625 Aspartate Aminotransferases Human genes 0.000 description 1
- 241000271566 Aves Species 0.000 description 1
- 108090001008 Avidin Proteins 0.000 description 1
- 208000035143 Bacterial infection Diseases 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 241000701822 Bovine papillomavirus Species 0.000 description 1
- 108091033409 CRISPR Proteins 0.000 description 1
- 238000010354 CRISPR gene editing Methods 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- UNPLRYRWJLTVAE-UHFFFAOYSA-N Cloperastine hydrochloride Chemical compound Cl.C1=CC(Cl)=CC=C1C(C=1C=CC=CC=1)OCCN1CCCCC1 UNPLRYRWJLTVAE-UHFFFAOYSA-N 0.000 description 1
- 208000003322 Coinfection Diseases 0.000 description 1
- 206010010305 Confusional state Diseases 0.000 description 1
- 108010051219 Cre recombinase Proteins 0.000 description 1
- 241000938605 Crocodylia Species 0.000 description 1
- 241000702421 Dependoparvovirus Species 0.000 description 1
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 108700039887 Essential Genes Proteins 0.000 description 1
- 108010087819 Fc receptors Proteins 0.000 description 1
- 102000009109 Fc receptors Human genes 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 241000192125 Firmicutes Species 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 108700028146 Genetic Enhancer Elements Proteins 0.000 description 1
- 101100175482 Glycine max CG-3 gene Proteins 0.000 description 1
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 206010018910 Haemolysis Diseases 0.000 description 1
- 239000012981 Hank's balanced salt solution Substances 0.000 description 1
- 241000238631 Hexapoda Species 0.000 description 1
- 241001272567 Hominoidea Species 0.000 description 1
- 101000917826 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor II-a Proteins 0.000 description 1
- 101000917824 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor II-b Proteins 0.000 description 1
- 101000917858 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-A Proteins 0.000 description 1
- 101000917839 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-B Proteins 0.000 description 1
- 101000582320 Homo sapiens Neurogenic differentiation factor 6 Proteins 0.000 description 1
- 101000611023 Homo sapiens Tumor necrosis factor receptor superfamily member 6 Proteins 0.000 description 1
- 108091006905 Human Serum Albumin Proteins 0.000 description 1
- 102000008100 Human Serum Albumin Human genes 0.000 description 1
- 208000001953 Hypotension Diseases 0.000 description 1
- 102220475419 Iduronate 2-sulfatase_S117Y_mutation Human genes 0.000 description 1
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 1
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 1
- 206010062016 Immunosuppression Diseases 0.000 description 1
- YQEZLKZALYSWHR-UHFFFAOYSA-N Ketamine Chemical compound C=1C=CC=C(Cl)C=1C1(NC)CCCCC1=O YQEZLKZALYSWHR-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- 102100029204 Low affinity immunoglobulin gamma Fc region receptor II-a Human genes 0.000 description 1
- 102100029185 Low affinity immunoglobulin gamma Fc region receptor III-B Human genes 0.000 description 1
- 206010027417 Metabolic acidosis Diseases 0.000 description 1
- 101000713102 Mus musculus C-C motif chemokine 1 Proteins 0.000 description 1
- 102000004722 NADPH Oxidases Human genes 0.000 description 1
- 108010002998 NADPH Oxidases Proteins 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 102100030589 Neurogenic differentiation factor 6 Human genes 0.000 description 1
- 239000000020 Nitrocellulose Substances 0.000 description 1
- 206010030113 Oedema Diseases 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 108090000526 Papain Proteins 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 102000012132 Peroxisome proliferator-activated receptor gamma Human genes 0.000 description 1
- 102100038825 Peroxisome proliferator-activated receptor gamma Human genes 0.000 description 1
- 101710182846 Polyhedrin Proteins 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 102220539284 Programmed cell death 1 ligand 2_A69F_mutation Human genes 0.000 description 1
- 102220539290 Programmed cell death 1 ligand 2_E62S_mutation Human genes 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 229940124158 Protease/peptidase inhibitor Drugs 0.000 description 1
- 206010037660 Pyrexia Diseases 0.000 description 1
- 239000012083 RIPA buffer Substances 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 241000714474 Rous sarcoma virus Species 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 108020004682 Single-Stranded DNA Proteins 0.000 description 1
- 102100035254 Sodium- and chloride-dependent GABA transporter 3 Human genes 0.000 description 1
- 101710104417 Sodium- and chloride-dependent GABA transporter 3 Proteins 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 208000001871 Tachycardia Diseases 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 1
- 102100031988 Tumor necrosis factor ligand superfamily member 6 Human genes 0.000 description 1
- 108050002568 Tumor necrosis factor ligand superfamily member 6 Proteins 0.000 description 1
- 102100040403 Tumor necrosis factor receptor superfamily member 6 Human genes 0.000 description 1
- 241000700618 Vaccinia virus Species 0.000 description 1
- 238000005411 Van der Waals force Methods 0.000 description 1
- 230000001133 acceleration Effects 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 235000010419 agar Nutrition 0.000 description 1
- 230000004520 agglutination Effects 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 230000003042 antagnostic effect Effects 0.000 description 1
- 230000007416 antiviral immune response Effects 0.000 description 1
- 210000004436 artificial bacterial chromosome Anatomy 0.000 description 1
- 210000004507 artificial chromosome Anatomy 0.000 description 1
- 210000001106 artificial yeast chromosome Anatomy 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 238000013475 authorization Methods 0.000 description 1
- 208000022362 bacterial infectious disease Diseases 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 230000008827 biological function Effects 0.000 description 1
- 230000036760 body temperature Effects 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- UDSAIICHUKSCKT-UHFFFAOYSA-N bromophenol blue Chemical compound C1=C(Br)C(O)=C(Br)C=C1C1(C=2C=C(Br)C(O)=C(Br)C=2)C2=CC=CC=C2S(=O)(=O)O1 UDSAIICHUKSCKT-UHFFFAOYSA-N 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 229960001736 buprenorphine Drugs 0.000 description 1
- 238000010805 cDNA synthesis kit Methods 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 230000001364 causal effect Effects 0.000 description 1
- 210000004534 cecum Anatomy 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 238000003501 co-culture Methods 0.000 description 1
- 230000015271 coagulation Effects 0.000 description 1
- 238000005345 coagulation Methods 0.000 description 1
- 230000001332 colony forming effect Effects 0.000 description 1
- 230000024203 complement activation Effects 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000004590 computer program Methods 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 238000007405 data analysis Methods 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000002939 deleterious effect Effects 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 239000003797 essential amino acid Substances 0.000 description 1
- 235000020776 essential amino acid Nutrition 0.000 description 1
- 238000009313 farming Methods 0.000 description 1
- 239000012894 fetal calf serum Substances 0.000 description 1
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 1
- 238000000799 fluorescence microscopy Methods 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 210000005095 gastrointestinal system Anatomy 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 102000018146 globin Human genes 0.000 description 1
- 108060003196 globin Proteins 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 229940074045 glyceryl distearate Drugs 0.000 description 1
- 229940075507 glyceryl monostearate Drugs 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 230000008588 hemolysis Effects 0.000 description 1
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 1
- 210000003630 histaminocyte Anatomy 0.000 description 1
- -1 hnRNA Proteins 0.000 description 1
- 230000013632 homeostatic process Effects 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 210000003767 ileocecal valve Anatomy 0.000 description 1
- 230000002163 immunogen Effects 0.000 description 1
- 230000001506 immunosuppresive effect Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 230000028709 inflammatory response Effects 0.000 description 1
- 238000003331 infrared imaging Methods 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 229960003299 ketamine Drugs 0.000 description 1
- VCMGMSHEPQENPE-UHFFFAOYSA-N ketamine hydrochloride Chemical compound [Cl-].C=1C=CC=C(Cl)C=1C1([NH2+]C)CCCCC1=O VCMGMSHEPQENPE-UHFFFAOYSA-N 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 238000002350 laparotomy Methods 0.000 description 1
- 231100000518 lethal Toxicity 0.000 description 1
- 230000001665 lethal effect Effects 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 210000005229 liver cell Anatomy 0.000 description 1
- 210000005228 liver tissue Anatomy 0.000 description 1
- 208000012866 low blood pressure Diseases 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 210000000653 nervous system Anatomy 0.000 description 1
- 230000003472 neutralizing effect Effects 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 238000007899 nucleic acid hybridization Methods 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 239000011022 opal Substances 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 238000007427 paired t-test Methods 0.000 description 1
- 229940055729 papain Drugs 0.000 description 1
- 235000019834 papain Nutrition 0.000 description 1
- 244000045947 parasite Species 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 231100000915 pathological change Toxicity 0.000 description 1
- 230000036285 pathological change Effects 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 230000001991 pathophysiological effect Effects 0.000 description 1
- 239000001814 pectin Substances 0.000 description 1
- 235000010987 pectin Nutrition 0.000 description 1
- 229920001277 pectin Polymers 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 230000036513 peripheral conductance Effects 0.000 description 1
- 230000035479 physiological effects, processes and functions Effects 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 239000013612 plasmid Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 229920002401 polyacrylamide Polymers 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 238000002331 protein detection Methods 0.000 description 1
- 229940124272 protein stabilizer Drugs 0.000 description 1
- 230000006337 proteolytic cleavage Effects 0.000 description 1
- 230000006950 reactive oxygen species formation Effects 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 230000010282 redox signaling Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000036387 respiratory rate Effects 0.000 description 1
- 238000011808 rodent model Methods 0.000 description 1
- 229940069575 rompun Drugs 0.000 description 1
- 102200117930 rs33932908 Human genes 0.000 description 1
- 238000010079 rubber tapping Methods 0.000 description 1
- 229940102127 rubidium chloride Drugs 0.000 description 1
- 239000012723 sample buffer Substances 0.000 description 1
- RGYQPQARIQKJKH-UHFFFAOYSA-N setanaxib Chemical compound CN(C)C1=CC=CC(C2=C3C(=O)N(C=4C(=CC=CC=4)Cl)NC3=CC(=O)N2C)=C1 RGYQPQARIQKJKH-UHFFFAOYSA-N 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 125000006850 spacer group Chemical group 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 230000006794 tachycardia Effects 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 231100001274 therapeutic index Toxicity 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000002103 transcriptional effect Effects 0.000 description 1
- 238000001890 transfection Methods 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 238000011830 transgenic mouse model Methods 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- 102000035160 transmembrane proteins Human genes 0.000 description 1
- 108091005703 transmembrane proteins Proteins 0.000 description 1
- 238000012384 transportation and delivery Methods 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 241000701447 unidentified baculovirus Species 0.000 description 1
- 241001529453 unidentified herpesvirus Species 0.000 description 1
- 241001430294 unidentified retrovirus Species 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 239000013603 viral vector Substances 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- BPICBUSOMSTKRF-UHFFFAOYSA-N xylazine Chemical compound CC1=CC=CC(C)=C1NC1=NCCCS1 BPICBUSOMSTKRF-UHFFFAOYSA-N 0.000 description 1
- 229960001600 xylazine Drugs 0.000 description 1
- QYEFBJRXKKSABU-UHFFFAOYSA-N xylazine hydrochloride Chemical compound Cl.CC1=CC=CC(C)=C1NC1=NCCCS1 QYEFBJRXKKSABU-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/70532—B7 molecules, e.g. CD80, CD86
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/177—Receptors; Cell surface antigens; Cell surface determinants
- A61K38/1774—Immunoglobulin superfamily (e.g. CD2, CD4, CD8, ICAM molecules, B7 molecules, Fc-receptors, MHC-molecules)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/62—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being a protein, peptide or polyamino acid
- A61K47/64—Drug-peptide, drug-protein or drug-polyamino acid conjugates, i.e. the modifying agent being a peptide, protein or polyamino acid which is covalently bonded or complexed to a therapeutically active agent
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
- A61K47/6811—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates the drug being a protein or peptide, e.g. transferrin or bleomycin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6849—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a receptor, a cell surface antigen or a cell surface determinant
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/30—Non-immunoglobulin-derived peptide or protein having an immunoglobulin constant or Fc region, or a fragment thereof, attached thereto
Definitions
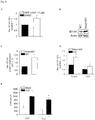
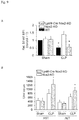
- the present invention pertains to a fusion polypeptide for use in treating and/or preventing organ failure in a subject suffering from sepsis, said fusion polypeptide comprises at least (i) a first portion being an Fc portion of an immunoglobulin and (ii) a second portion comprising the extracellular portion of the human B7-H1 polypeptide or a variant thereof.
- a polynucleotide encoding said fusion polypeptide for use in treating and/or preventing organ failure in a subject suffering from sepsis.
- Sepsis is a life-threatening illness that can occur when the whole body reacts to an infection.
- sepsis remains the third leading cause of mortality in intensive care units (Balk, 2004; Dellinger et al., 2008; Kaukonen et al., 2014; Vincent et al., 2014).
- Recent therapy approaches mainly focus on the treatment of the hyper-inflammatory response to confine the release of pro-inflammatory mediators, block their function, or remove them from the circulation.
- TNF ⁇ Marquez-Velasco et al., 2006
- neutralizing antibodies this approach was translated into the human situation, but failed to improve sepsis survival (Clark et al., 1998; Reinbart et al., 2001).
- the hyper-inflammation is limited and most patients survive this phase.
- the present invention relates to a fusion polypeptide for use in treating and/or preventing organ failure in a subject suffering from sepsis, said fusion polypeptide comprises at least (i) a first portion being a Fc portion of an immunoglobulin and (ii) a second portion comprising the extracellular portion of the human B7-H1 polypeptide or a variant thereof.
- polypeptide refers to a molecule consisting of several, typically, at least 20, at least 30, at least 40, at least 50 or at least 60 amino acids that are covalently linked to each other by peptide bonds. Molecules consisting less than 20 amino acids covalently linked by peptide bonds are usually considered to be peptides.
- a “fusion polypeptide” in accordance with the present invention refers to a polypeptide that is composed of at least two polypeptides or peptides.
- a fusion polypeptide as referred to herein may comprise two, three, four, five or even more polypeptide or peptide portions.
- the fusion polypeptide shall comprise at least a first and a second portion as specified herein.
- the polypeptide or peptide portions comprised in the fusion polypeptide may be linked to each other either permanently, typically by peptide bonds, or reversibly, e.g., via disulfide bonds or affinity binding based on ionic bonds, hydrogen bonds and/or van der Waals forces.
- Permanent binding between the portions of the fusion polypeptide according to the present invention can be achieved typically by recombinant manufacture.
- a polynucleotide encoding the said portions is synthesized either chemically or by recombinant DNA techniques and expressed in a suitable expression system.
- the expressed fusion polypeptide can be purified from the expression system.
- affinity binding systems comprising a ligand and a receptor portion are known in the art such that the skilled artisan can be used in order to construe fusion polypeptides comprising the different peptide or polypeptide portions reversibly bound to each other without further ado.
- Typical examples of such affinity binding systems are those based on antibodies/antigens, streptavidin/biotin, avidin/biotin, and others well known in the art.
- the fusion polypeptide applied in accordance with the present invention shall comprise a first portion being an Fc portion of an immunoglobulin and a second portion comprising the extracellular portion of the human B7-H1 polypeptide or a variant thereof.
- the extracellular portion of the human B7-H1 polypeptide or a variant thereof is located N-terminally in the fusion polypeptide while the Fc portion of the immunoglobulin is located C-terminally.
- Fc portion of an immunoglobulin refers to antibody fragments which comprise the C H 2 and C H 3 domains of an antibody and which can be obtained by proteolytic cleavage of an antibody using, e.g., papain.
- Various immunoglobulins are known in the art from a variety of different species. These immunoglobulins encompass IgA, IgD, IgE, IgG, IgM, IgW or IgY. Preferred in accordance with the present invention among the immunoglobulins are, however, those which appear in mammals and, in particular, in humans, i.e. IgA, IgD, IgE, IgG, IgM.
- the Fc portion of an antibody determines the class effect. Since only the constant domains of the heavy chains form the Fc portion of an antibody, the classes of the heavy chains determine the class effects. Possible classes of heavy chains in antibodies encompass alpha, gamma, delta, epsilon, and mu. These heavy chain classes define the isotype. Different isotypes of antibodies have different class effects due to their respective Fc portions. Such Fc mediated class effects include those affecting effector cells or effector molecules, e.g., opsonisation, agglutination, haemolysis, complement activation, and mast cell degranulation.
- Amino acid sequences for C H 2 and C H 3 domains forming the Fc portions are well known in the art for different antibody isotypes and can be provided by the skilled artisan without further ado.
- the Fc portion as referred to in accordance with the present invention may be, preferably, posttranslationally modified and, more preferably, glycosylated.
- said immunoglobulin in accordance with the present invention is IgG and, more preferably, human IgG.
- Amino acid sequences encoding human IgG are well known in the art as well as the nucleic acid sequences encoding them.
- amino acids correspond to the Fc portions in the said amino acid sequences.
- extracellular portion of the human B7-H1 polypeptide refers to the extracellular part of the human B7 homolog1 protein also known as Programmed death-ligand 1 (PD-L1) or Cluster of differentiation 274 (CD274).
- the B7-H1 protein is a 40 kDa transmembrane protein known to bind to the PD-1 receptor found on activated T cells.
- the B7-H1/PD-1 complex has been suggested to be involved in the control of the proliferation of CD8+ T cells.
- Amino acid sequences for the human B7-H1 protein are well known in the art.
- an amino acid sequence for human B7-H1 to be used in accordance with the present invention is publicly available under UniprotKB No: Q9NZQ7, is shown in SEQ ID NO: 4 or is encoded by the nucleic acid sequence shown in SEQ ID NO: 3 or under GenBank Accession number AF177937.
- the extracellular portion of the said human B7-H1 encompasses the amino acids 19 to 239 of the sequence publicly available under UniprotKB No: Q9NZQ7, is shown in SEQ ID NO: 2, is encoded by the nucleic acid sequence shown in SEQ ID NO: 1 or is encoded by nucleotides 55 to 717 of the sequence under GenBank Accession number AF177937.
- an amino acid sequence for murine B7-H1 to be used in accordance with the present invention is publicly available under UniprotKB No: Q9EP73, is shown in SEQ ID NO: 8 or is encoded by the nucleic acid sequence shown in SEQ ID NO: 7 or under GenBank Accession number NM_021893.
- the extracellular portion of the said murine B7-H1 encompasses the amino acids 19 to 239 of the sequence publicly available under UniprotKB No: Q9EP73, is shown in SEQ ID NO: 6, is encoded by the nucleic acid sequence shown in SEQ ID NO: 5 or is encoded by nucleotides 55 to 717 of the sequence under GenBank Accession number NM_021893.
- extracellular portions of the human B7-H1 polypeptide according to the present invention are, preferably, variants of any of the aforementioned specific extracellular domains. Such a variant, typically, differs from the specific amino acid sequences referred to before by at least one amino acid substitution, deletion and/or addition. Nevertheless, the variant of the extracellular portion of the human B7-H1 polypeptide shall still be capable of exerting the biological effects mediated by the extracellular portion of the human B7-H1 polypeptide and, in particular, remaining capable of binding to PD-1.
- Said variant shall, preferably, have an amino acid sequence which is at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least 98% or at least 99% identical to the amino acid sequence shown in SEQ ID NO: 2 or 6 or to an amino acid sequence encoded by the nucleic acid sequences shown in SEQ ID NO: 1 or 5.
- Sequence identity between amino acid sequences or nucleic acid sequences as used herein can be assessed by determining the number of identical nucleotides or amino acids between two nucleic acid sequences or amino acid sequences wherein the sequences are aligned so that the highest order match is obtained.
- a comparison window preferably, is the length of the entire sequence of the shorter sequence to be aligned or at least half of said sequence.
- a series of programs based on a variety of algorithms is available to the skilled worker for comparing different sequences. In this context, the algorithms of Needleman and Wunsch or Smith and Waterman give particularly reliable results.
- the program PileUp Higgins 1989, CABIOS 5, 151
- the programs Gap and BestFit Needleman 1970, J Mol Biol 48: 443 ; Smith 1981, Adv Appl Math 2: 482
- the sequence identity values recited above in percent (%) are to be determined, in another aspect of the invention, using the program GAP over the entire sequence region with the following settings: Gap Weight: 50, Length Weight: 3, Average Match: 10.000 and Average Mismatch: 0.000, which, unless otherwise specified, shall always be used as standard settings for sequence alignments.
- variants of the aforementioned specific extracellular portions of the human B7-H1 polypeptide are those which are encoded by polynucleotides having a nucleic acid sequence which is capable of hybridizing to the nucleic acid sequences encoding the aforementioned specific extracellular portions of the human or murine B7-H1 polypeptide under stringent hybridization conditions.
- Stringent hybridization conditions as used herein are those which allow for identifying polynucleotides encoding polypeptides which have essentially the same biological function as B7-H1.
- stringent hybridization conditions in accordance with the present invention are: hybridization in 6x sodium chloride/sodium citrate (SSC) at 45°C, followed by one or more wash steps in 0.2x SSC, 0.1% SDS at a temperature between 50°C to 65°C.
- SSC sodium chloride/sodium citrate
- SDS 0.1% SDS
- polynucleotide encoding the aforementioned variants can also be obtained by PCR-based techniques such as mixed oligonucleotide primer- based amplification of DNA, i.e. using degenerated primers against conserved domains of the extracellular portions of the human or murine B7-H1 polypeptide.
- conserved domains may be identified by a sequence comparison of the amino acid or nucleic acid sequences of the extracellular portion of human or murine B7-H1..
- the aforementioned variants of the extracellular portion of the human B7-H1 polypeptide comprise at least one of the following amino acid exchanges L27A, S34Y, D49S, Y56S, E58S, K62S, H69F, E72S, K75S, K89S, A98F, Q100S, R113Y, and S117Y.
- amino acid exchanges in the extracellular portion of the human B7-H1 polypeptide correspond to the following amino acid exchanges in the extracellular portion of the murine B7-H1 polypeptide: L27A, S34Y, D49S, Y56S, E58S, E62S, A69F, E72S, K75S, K89S, A98F, Q100S, C113Y, and S117Y.
- amino acid exchanges have been previously reported to enhance binding and/or activity of B7-H1 protein to PD-1; see Wang 2003, J. Exp. Med. 197 No. 9: 1083-1091 .
- the fusion polypeptide to be applied in accordance with the present invention has an amino acid sequence as shown in SEQ ID NO: 9 or is a variant thereof which has at least one at least one amino acid substitution, deletion and/or addition.
- the said variant fusion protein shall still be capable of exerting the biological effects mediated by the extracellular portion of the human B7-H1 polypeptide and, in particular, remaining capable of binding to PD-1.
- Said variant shall, preferably, have an amino acid sequence which is at least at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least 98% or at least 99% identical to the amino acid sequence shown in SEQ ID NO: 9.
- the fusion polypeptide according to the present invention may also comprise further portions such as linker or spacer sequences between the individual portions. Moreover, also encompassed are portions contributing further functions. Such portions include, e.g., portions which allow for targeting of certain cells in an organism, portions which increase stability of the fusion polypeptide as well as portions which facilitate manufacture and/or purification of the fusion polypeptide such as tags.
- the said fusion polypeptide comprises a third portion for targeting and, in particular, a third portion being a polypeptide capable of binding specifically to cytotoxic T-cells.
- said polypeptide capable of binding specifically to cytotoxic T-cells is selected from the group consisting of: a polypeptide comprising a portion of the MHC-I complex which is capable of binding to CD8, a portion of the CD80 which is capable of binding to CD28, a polypeptide being an antibody or fragment thereof capable of specifically binding to CD8, a polypeptide being an antibody or fragment thereof capable of specifically binding to CD28, and CD2-binding portion of lymphocyte function associated antigen-3 (LFA-3). How such portions can be derived from the respective proteins is well known to the person skilled in the art.
- treating refers to ameliorating or curing a disease or at least one symptom associated therewith. Thus, if there is amelioration or cure of the disease or at least a symptom associated therewith, the treatment shall be deemed to be effective. It will be understood that treating might not be effective in all subjects. However, according to the present invention it is envisaged that treatment will be effective in at least a statistically significant portion of subjects to be treated. It is well known to the skilled artisan how to determine a statistically significant portion of subjects that can be effectively treated.
- Whether a portion is statistically significant can be determined without further ado by the person skilled in the art using various well known statistic evaluation tools, e.g., determination of confidence intervals, p-value determination, Student's t-test, Mann-Whitney test etc.. Details are found in Dowdy and Wearden, Statistics for Research, John Wiley & Sons, New York 1983 .
- Preferred confidence intervals are at least 90%, at least 95%, at least 97%, at least 98% or at least 99%.
- the p-values are, preferably, 0.1, 0.05, 0.01, 0.005, or 0.0001.
- the probability envisaged by the present invention allows that the finding of effective treatment will be correct for at least 60%, at least 70%, at least 80%, or at least 90% of the subjects of a given cohort or population.
- preventing refers to avoiding the onset of the disease or at least one symptom associated therewith or to prevent the worsening of the disease or the said at least one symptom.
- the prevention as referred to herein can be typically achieved either for the period during which a drug is administered. If the administration of the drug is stopped, however, the prevention may not persist for an unlimited time but may remain present for a certain preventive time window after application of the drug.
- a preventive time window in accordance with the present invention may be at least 1 day, at least 2 days, at least 3 days, at least 4 days, at least 5 days, at least 6 days, or at least 7 days.
- the preventive time window may also depend on the dosage of a drug as well as the mode of administration or the kind of formulation. For example, if a high dosage is applied, usually longer preventive time windows can be achieved. The same holds true if slow release formulations of a drug are administered or the drug is administered via routes that do not lead to immediate metabolization of a drug in the subject. In such cases, the predictive time window may be increased up to several weeks, months or even years. It will be understood that prevention might not be effective in all subjects. However, according to the present invention it is envisaged that prevention will be effective in at least a statistically significant portion of subjects. It is well known to the skilled artisan how to determine a statistically significant portion of subjects that can be effectively prevented. Whether a portion is statistically significant can be determined without further ado by the person skilled in the art using various well known statistic evaluation tools as discussed above.
- the fusion polypeptide according to the present invention shall be used for treating and/or preventing organ failure, it shall, preferably, be formulated as a medicament.
- a medicament in the sense of the present invention refers, preferably, to a pharmaceutical composition containing the biologically active fusion polypeptide according to the invention as pharmaceutical active compound and one or more other components such as one or more pharmaceutically acceptable carrier(s).
- the fusion polypeptide can be present in liquid or lyophilized form.
- the fusion polypeptide can be present together with glycerol and/or protein stabilizers (e.g., human serum albumin).
- the medicament is, typically, administered systemically and, preferably, intravenously or intramuscularly. However, depending on the nature of the formulation and the desired therapeutic application, the medicament may be administered by other routes as well.
- the fusion polypeptide is the active ingredient or drug of the medicament, and is preferably administered in conventional dosage forms prepared by combining the drug with standard pharmaceutical carriers according to conventional procedures. These procedures may involve mixing, granulating, and compression, or dissolving the ingredients as appropriate to the desired preparation. It will be appreciated that the form and character of the pharmaceutical acceptable carrier or diluent is dictated by the amount of active ingredient with which it is to be combined, the route of administration, and other well-known variables.
- the carrier(s) must be acceptable in the sense of being compatible with the other ingredients of the formulation and being not deleterious to the recipient thereof.
- the pharmaceutical carrier employed may include a solid, a gel, or a liquid.
- Exemplary of solid carriers are lactose, terra alba, sucrose, talc, gelatin, agar, pectin, acacia, magnesium stearate, stearic acid and the like.
- Exemplary of liquid carriers are phosphate buffered saline solution, syrup, oil, water, emulsions, various types of wetting agents, and the like.
- the carrier or diluent may include time delay material well known to the art, such as glyceryl mono-stearate or glyceryl distearate alone or with a wax.
- time delay material well known to the art, such as glyceryl mono-stearate or glyceryl distearate alone or with a wax.
- suitable carriers comprise those mentioned above and others well known in the art, see, e.g., Remington's Pharmaceutical Sciences, Mack Publishing Company, Easton, Pennsylvania.
- the diluent(s) is/are selected so as not to affect the biological activity of the combination.
- examples of such diluents are distilled water, physiological saline, Ringer's solutions, dextrose solution, and Hank's solution.
- the pharmaceutical composition or formulation may also include other carriers, adjuvants, or non-toxic, non-therapeutic, non-immunogenic stabilizers and the like.
- a therapeutically effective dose refers to an amount of the fusion polypeptide according to the invention to be used in medicament which prevents, ameliorates or cures the symptoms accompanying a disease or condition referred to in this specification.
- Therapeutic efficacy and toxicity of a drug can be determined by standard pharmaceutical procedures in cell cultures or experimental animals, e.g., ED50 (the dose therapeutically effective in 50% of the population) and LD50 (the dose lethal to 50% of the population).
- the dose ratio between therapeutic and toxic effects is the therapeutic index, and it can be expressed as the ratio, LD50/ED50.
- the dosage regimen will be determined by the attending physician and by clinical factors.
- dosages for any one patient depends upon many factors, including the patient's size, age, the particular formulation of the medicament to be administered, sex, time and route of administration, general health, and other drugs being administered concurrently. Progress can be monitored by periodic assessment. Dosage recommendations shall be indicated in the prescribers or users instructions in order to anticipate dose adjustments depending on the considered recipient.
- the medicament referred to herein is, preferably, administered at least once, e.g. as a bolus.
- the said medicament may be administered more than one time and, preferably, at least twice, e.g. permanently or periodically after defined time windows.
- the medicament according to the present invention may in a further aspect of the invention comprise drugs in addition to the fusion polypeptide which are added during its formulation.
- the fusion polypeptide according to the invention is to be applied together with at least one further drug and, thus, may be formulated together with these other drugs as a medicament.
- said at least one further drug is selected from the group consisting of: antibiotics, vasopressors, steroids, anticoagulants, antithrombotics, proinflammatory cytokines and DAMP inhibitors.
- organ failure refers to any dysfunction of the organ which affects the physiologically expected function of an organ to such an extent that normal homeostasis can neither be maintained nor endogenously compensated. Organ failure may be acute or chronic. Symptoms associated with organ failure depend on the affected organ usually become apparent by a pathological physiology in the subject which can be determined, e.g., by clinical or biochemical parameters. Symptoms of organ failure are also well known in the art and are described in medicinal text books.
- organ failure as referred to herein is multi organ failure. Multi organ failure is characterized by the failure of two or more organs at the same time or sequentially within a short period of time.
- SIRS systemic inflammatory response syndrome
- Typical organs which fail during SIRS or sepsis are lung, kidney, heart and/or the entire circulation system, the gastrointestinal system as well as the nervous system.
- the multi organ failure referred to herein is caused by autoreactive cytotoxic cells and, more preferably, is CD8 cytotoxic T-cell dependent multi-organ failure.
- subject refers to any kind of animal encompassing, e.g., mammals, birds, fish or reptiles.
- the said animal is a mammal such as a mammals used as pets including dogs, cats, horses, or rodents, laboratory animals, e.g., rats, mice or apes, or farming animals such as pigs, cows, goats, or sheep.
- the mammal is a primate and, most preferably, a human.
- the subject according to the present invention shall suffer from sepsis, i.e. it shall exhibit at least one or more pathological changes such as clinically apparent symptoms or changes of physiological or molecular parameters which are typically associated with sepsis.
- sepsis refers to an inflammatory response affecting the entire organism. Typical symptoms associated with sepsis are well known in the art and described in standard textbooks of medicine. They include a significantly altered body temperature (low temperature or fever), rapid breathing, tachycardia, low blood pressure due to decreased peripheral vascular resistance, mental confusion and edema formation. Biochemical parameters such as coagulation dysfunction or metabolic acidosis are also typical signs of sepsis. Sepsis is caused by severe infection by bacteria, viruses, parasites or fungi. Moreover, there are cofounding factors which inferior influence the onset or outcome of sepsis such as diabetes or cancer.
- sepsis is referred to in accordance with the present invention is characterized by the presence of two or more of the following symptoms in response to an infection: abnormal temperature (preferably, below 36°C or above 38°C), abnormal heart rate (preferably, above 90 beats/min), abnormal respiratory rate (preferably, above 20 breathings/min) or blood gas composition (preferably, CO 2 less than 4.3 kPa) and abnormal white blood cell number (preferably, less than 4x10 9 /L or more than 12x10 9 /L or histological presence of band neutrophils).
- abnormal temperature preferably, below 36°C or above 38°C
- abnormal heart rate preferably, above 90 beats/min
- abnormal respiratory rate preferably, above 20 breathings/min
- blood gas composition preferably, CO 2 less than 4.3 kPa
- abnormal white blood cell number preferably, less than 4x10 9 /L or more than 12x10 9 /L or histological presence of band neutrophils.
- a fusion polypeptide comprising an Fc portion of an immunoglobulin and the extracellular portion of the human B7-H1 polypeptide can be used for efficiently treating and/or preventing organ failure in a subject suffering from sepsis.
- the aforementioned fusion polypeptide or variants thereof are capable of binding to cytotoxic T cells, thereby inhibiting sepsis-induced T-cell cytotoxicity and preventing organ failure.
- downregulation of the B7-H1 protein allows autoimmune CTL activation during sepsis. Maintaining B7-H1 expression or applying the fusion polypeptide of the invention significantly improves liver damage.
- the fusion polypeptide according to the invention shall, preferably, upon administration inhibit sepsis-induced cytotoxic T-cells in the subject. Moreover, it shall, preferably, upon administration induce a long-lasting tolerance in cytotoxic T-cells in the subject against sepsis-caused activation. Consequently, it can be surprisingly applied in a therapeutic as well as preventive manner.
- said organ failure is CD8 cytotoxic T-cell dependent multi-organ failure.
- said immunoglobulin is human IgG.
- said extracellular portion of the human B7-H1 polypeptide or variant thereof is selected from the group consisting of:
- said fusion polypeptide comprises (iii) a third portion being a polypeptide capable of binding specifically to cytotoxic T-cells.
- said polypeptide capable of binding specifically to cytotoxic T-cells is selected from the group consisting of: a polypeptide comprising a portion of the MHC-I complex which is capable of binding to CD8, a portion of the CD80 which is capable of binding to CD28, a polypeptide being an antibody or fragment thereof capable of specifically binding to CD8, a polypeptide being an antibody or fragment thereof capable of specifically binding to CD28, and CD2-binding portion of lymphocyte function associated antigen-3 (LFA-3).
- LFA-3 lymphocyte function associated antigen-3
- said at least the first portion and at least the second portion are permanently or reversible linked to each other.
- said subject is a mammal, preferably a human.
- said fusion polypeptide is to be applied once as a bolus or is to be applied at least twice.
- fusion polypeptide for use according to the invention said fusion polypeptide is to be applied together with at least one further drug.
- said at least one further drug is selected from the group consisting of: antibiotics, vasopressors, steroids, anticoagulants, antithrombotics, proinflammatory cytokines and DAMP inhibitors.
- said fusion polypeptide upon administration inhibits sepsis-induced cytotoxic T-cells in the subject.
- said fusion polypeptide upon administration induces a long-lasting tolerance in cytotoxic T-cells in the subject against sepsis-caused activation.
- the present invention furthermore, relates to a polynucleotide for use in treating and/or preventing organ failure in a subject suffering from sepsis, said polynucleotide encoding a fusion polypeptide defined in accordance with the present invention.
- polynucleotide refers to single- or double-stranded DNA molecules as well as to RNA molecules. Encompassed by the said term is genomic DNA, cDNA, hnRNA, mRNA as well as all naturally occurring or artificially modified derivatives of such molecular species.
- the polynucleotide may be, preferably, a linear or circular molecule.
- a polynucleotide according to the present invention may comprise additional sequences required for proper transcription and/or translation such as 5'- or 3'-UTR sequences or sequences required for splicing or RNA stability. Preferred polynucleotides encoding the fusion polypeptide according to the present invention are also described above in more detail.
- said polynucleotide is comprised in an expression construct allowing for expression of the said polynucleotide in the said subject.
- expression construct refers to a heterologous polynucleotide comprising the aforementioned polynucleotide encoding the fusion polypeptide as well as nucleic acids being heterologous thereto which are required for expression of the polynucleotide encoding the fusion polypeptide.
- heterologous nucleic acids may be promoter sequences, enhancer sequence and/or transcription termination sequences such as terminators.
- the expression construct may also comprise further nucleic acids required for introducing the expression construct into a host. For example, if expression in host cells is desired, the expression construct may comprise further nucleic acids required for transformation or transfection and for propagation of the introduced expression construct in the host cells.
- the expression construct referred to herein may be a vector.
- a vector as meant herein preferably, encompasses phage, plasmid, viral or retroviral vectors as well as artificial chromosomes, such as bacterial or yeast artificial chromosomes.
- the vector encompassing the polynucleotide encoding the fusion polypeptide preferably, further comprises selectable markers for propagation and/or selection in a host.
- the vector may be incorporated into a host cell by various techniques well known in the art.
- a plasmid vector can be introduced in a precipitate such as a calcium phosphate precipitate or rubidium chloride precipitate, or in a complex with a charged lipid or in carbon-based clusters, such as fullerens.
- a plasmid vector may be introduced by heat shock or electroporation techniques.
- the vector may be packaged in vitro using an appropriate packaging cell line prior to application to host cells.
- Retroviral vectors may be replication competent or replication defective. In the latter case, viral propagation generally will occur only in complementing host/cells.
- the polynucleotide is usually operatively linked to expression control sequences allowing expression in prokaryotic or eukaryotic host cells or isolated fractions thereof in the said vector.
- Expression of the polynucleotide comprises transcription of the polynucleotide into a translatable mRNA.
- Regulatory elements ensuring expression in host cells are well known in the art.
- they comprise regulatory sequences ensuring initiation of transcription and/or poly-A signals ensuring termination of transcription and stabilization of the transcript.
- Additional regulatory elements may include transcriptional as well as translational enhancers.
- Possible regulatory elements permitting expression in prokaryotic host cells comprise, e.g., the lac-, trp- or tac- promoter in E.
- inducible expression control sequences may be used in an vector encompassed by the present invention. Such inducible vectors may comprise tet or lac operator sequences or sequences inducible by heat shock or other environmental factors. Suitable expression control sequences are well known in the art.
- Beside elements which are responsible for the initiation of transcription such regulatory elements may also comprise transcription termination signals, such as the SV40-poly-A site or the tk-poly-A site, downstream of the polynucleotide.
- suitable expression vectors are known in the art such as Okayama-Berg cDNA expression vector pcDV1 (Pharmacia), pBluescript (Stratagene), pCDM8, pRc/CMV, pcDNA1, pcDNA3 (Invitrogen) or pSPORT1 (Invitrogen) or baculovirus-derived vectors.
- said vector is an expression vector and a gene transfer or targeting vector.
- Expression vectors derived from viruses such as retroviruses, vaccinia virus, adeno-associated virus, herpes viruses, or bovine papilloma virus, may be used for delivery of the expression constructs according to the invention into targeted cell population, e.g. also in gene therapeutic approaches.
- Methods which are well known to those skilled in the art can be used to construct recombinant viral vectors; see, for example, the techniques described in Sambrook, Molecular Cloning A Laboratory Manual, Cold Spring Harbor Laboratory (1989) N.Y . and Ausubel, Current Protocols in Molecular Biology, Green Publishing Associates and Wiley Interscience, N.Y. (1994 ).
- the expression construct may also comprise nucleic acids that allow for either heterologous or homologous integration of the said expression construct.
- the expression construct referred to herein may also be a targeting constructs which allow for random or site-directed integration of the targeting construct into genomic DNA.
- target constructs preferably, comprise DNA of sufficient length for either homologous or heterologous recombination flanking the expression cassette with the polynucleotide encoding the fusion polypeptide.
- the expression construct may also be introduced using integration systems like Cre/LoxP or CRISPR/CAS. In such cases, the expression construct may comprise further nucleic acids allowing for the use of such integration systems. Suitable modifications/additions depend on the envisaged integration system and are well known for those skilled in the art.
- the present invention furthermore provides for the use of the above defined fusion polypeptide or polynucleotide for the manufacture of a medicament for treating and/or preventing organ failure in a subject.
- the fusion polypeptide or polynucleotide as well as the subjects to be treated or the diseases referred to have the preferred characteristics defined above.
- the present invention also provides for a method of treating and/or preventing organ failure in a subject.
- a method of treating organ failure in a subject suffering from sepsis comprising (a) administering to said subject a therapeutically effective amount of a fusion polypeptide comprises at least (i) a first portion being an Fc portion of an immunoglobulin and (ii) a second portion comprising the extracellular portion of the human B7-H1 polypeptide or a variant thereof or (b) administering a therapeutically effective amount of a polynucleotide encoding said fusion polypeptide.
- Typical aspects of the invention with respect to the kind of organ failure, the subjects to be treated, sepsis, and the fusion polypeptide or the polynucleotide are described above and apply mutatis mutandis for the present method of treating and/or preventing organ failure in a subject.
- said method may encompass identification of a subject to be treated by determining the presence of sepsis prior to administering the fusion polypeptide or polynucleotide encoding it.
- said method comprises monitoring the subjects for signs of organ failure after administration of the fusion polypeptide and, if necessary, administering the fusion polypeptide or polynucleotide encoding it again or at a difference dosage.
- Example 1 Cytotoxic T cells (CTLs) accumulate in the liver of septic mice
- Example 2 Expression of B7-H1 is downregulated in a polymicrobial sepsis model
- B7-H1 also named CD274 or PD-L1, or B7-DC designated CD273 or PD-L2 as well
- B7-inhibitory proteins are typically expressed on antigen presenting cells (APC), which are most likely hepatocytes in the case of the polymicrobial sepsis model (Ueki et al., 2011).
- APC antigen presenting cells
- B7-H1 was downregulated on total protein level ( Figure 2A ) and on the cell surface ( Figure 2B ).
- Example 3 Cell wall components of gram-positive and gram-negative bacteria downregulate B7-H1 expression in Hepal-6 cells
- Example 4 LPS-treated Hepa1-6 cells are more susceptible to CTL-dependent cytotoxicity
- cells were pulsed for 2 h with the ovalbumin peptide 257-264 (OVA257-264) or the hepatitis B-virus (HBV)-derived peptide ILSPFLPLL derived from the HBV surface antigen (HBsAg) as control.
- OVA257-264 ovalbumin peptide 257-264
- HBV hepatitis B-virus
- ILSPFLPLL hepatitis B-virus
- HBV surface antigen HBV surface antigen
- Hepa1-6 cells Following LPS-stimulation of Hepa 1-6 cells, cytotoxicity was enhanced to approximately 70% dead target cells when Hepa1-6 cells were pulsed with the OVA257-264 peptide. In contrast, HBV peptide pulsed Hepa 1-6 cells were killed significantly less by CTLs.
- FIG. 5A shows that a hepatocyte transduction efficiency of around 70% could be achieved. Mice were kept for four days untreated to recover from the adenoviral transduction, which consequently induces an anti-viral immune response of the mice. After that time, CLP was initiated for 24 h. Then, mice were sacrificed and serum was isolated from mice blood to determine disease severity by analyzing the liver damage markers ALT and AST. As shown in Figure 5B , overexpression of B7-H1 improved liver damage, i.e. significantly reduced ALT/AST levels.
- Maintaining B7-H1 expression as a therapeutic approach can be achieved by exogenously adding B7-H1.
- B7-H1 Fc chimera In an in vitro cytotoxicity assay with OVA257-264 pulsed Hepa1-6 as target cells and OT-I mice derived CTLs as effector cells, simultaneous addition of recombinant B7-H1 Fc chimera inhibited CTL-mediated cytotoxicity ( Fig. 6A ). While 1 ⁇ g/ml recombinant B7-H1 Fc chimera did not alter killing of target cells, 5 ⁇ g/ml enhanced target cell survival up to approximately 50%. Increasing recombinant B7-H1 Fc chimera concentration up to 20 ⁇ g/ml does not enhance target cell survival.
- recombinant B7-H1 Fc chimera was applied intravenously (i.v.) into the tail vein, directly after the CLP operation. Twenty-four hours afterwards, blood of mice was collected and serum was prepared. The release of liver damage markers ALT/AST was determined. As shown in Figure 6B , the application of recombinant B7-H1 Fc chimera significantly reduced ALT/AST release, which is indicative of an improvement in septic outcome in vivo.
- LPS and LTA act via different Toll-like receptors (TLRs) on target cells i.e. TLR2 for LTA and TLR4 for LPS. Binding to these receptors has been shown to trigger various signaling cascades, i.e. NADPH oxidase-mediated redox signaling.
- NADPH oxidase NOX4 has been shown to constitutively generate reactive oxygen species (ROS) such as 02- or H2O2 (Dikalov et al., 2008). Recent data support the assumption that 02- is not only generated to kill pathogens but acts as second messenger as well (Brune et al., 2013).
- ROS reactive oxygen species
- ROS formation was determined in Hepa 1-6 cells treated with LPS alone or in combination with N-acetylcysteine (NAC). Treatment with the ROS inhibitor NAC restored expression of B7-H1 (see Figure 7 vs. Fig. 3B ).
- Nox4 The NADPH oxidase 4 (Nox4) has been shown to be expressed in Hepa1-6 cells (Boudreau et al., 2009) and to provoke a constitutive production of H2O2, which - upon stimulation - may be enhanced.
- Nox4 plays a role in the regulation of B7-H1 expression
- Hepa1-6 cells were incubated with the specific Nox4 inhibitor GKT137S31 [10 ⁇ M] for 24 h without any further treatment (Jiang et al., 2012).
- Nox4 inhibition increased B7-H1 surface expression up to roughly 50% in Hepa1-6 cells.
- livers from global NOX4-knockout mice were isolated and a total lysate Western analysis was performed.
- FIG. 8B shows that B7-H1 is upregulated in livers derived from Nox4-deficient mice compared to wild type controls.
- a densitometric quantification provided in Fig. 8C demonstrated a roughly two-fold higher expression of B7-H1 in NOX4-knockout mice derived livers.
- An analysis of B7-H1 surface expression in hepatocytes showed a similar rise in B7-H1 expression ( Fig. 8D , left columns). Using these mice with the CLP model, a downregulation of B7-H1 expression could be observed in both, the wild type as well as the knockout mice ( Fig. 8D , right columns).
- Example 9 Global NOX2 deletion prevents B7-H1 downregulation during polymicrobial sepsis
- the experimental sepsis model was next evaluated using global NOX2-deficient (NOX2-KO) as well as mice with a NOX2-knockout specific for the myeloid lineage (LysM-Cre Nox2-KO).
- NOX2-KO global NOX2-deficient mice
- LysM-Cre Nox2-KO mice with a NOX2-knockout specific for the myeloid lineage
- Fig. 9A expression of B7-H1 in hepatocytes was similar 24 h following sham operation in all three genotypes.
- 24 h after CLP expression of B7-H1 was downregulated in wild type (black column) and mice with a Nox-2 deletion in the myeloid lineage (grey column), whereas in mice with a global NOX2-knockout (white column) B7-H1 expression remained high.
- liver damage markers into the serum revealed a significant increase in ALT and AST in mice with a myeloid lineage NOX2-deletion ( Fig. 9B , grey columns), but remained weak in global NOX2-knockout mice ( Fig. 9B , white columns).
- mice with a specific NOX2 knockout for the myeloid lineage were generated by crossing C57Bl/6 mice bearing conditional loxP-flanked alleles of NOX2 (NOX2fl/fl), kindly provided by Prof. Shah (King's College London BHF Centre of Excellence, London, UK) with C57Bl/6N-(Tg) LysM-Cre transgenic mice, where the Cre recombinase has been knocked in behind the LysM promoter (Akiyama et al., 2002; Cui et al., 2002; Hennet et al., 1995; Hume, 2011; Schmidt et al., 2011).
- mice Global NOX2- and NOX4 knockout mice as well as wild type mice were used on a C57Bl/6 background as well. Mice were kept in a temperature-controlled room with 12 h light and 12 h dark diurnal cycle. They were housed in filter-topped cages and were fed standard laboratory chow and water ad libidum. Genotypes were determined by PCR of tail DNA and deletion of NOX2 and NOX4 was confirmed by mRNA analysis (data not shown). All animal experiments followed the guidelines of the Hessian animal care and use committee (authorization no. F144/15).
- CLP cecal ligation and puncture model
- mice were sacrificed, spleens were dissected and a single cell suspension was prepared.
- CD8+ T cells were enriched to >95% by positive selection from spleens using the Dynabeads FlowComp Mouse CD8 Kit (Life Sciences, Heidelberg, Germany) following the distributors instructions. Purification was verified by FACS analysis using an anti-CD8 ⁇ -FITC-labeled antibody (EuroBioScience, Friesoythe, Germany).
- liver damage markers alanine- and aspartate aminotransferase.
- the amounts of the two enzymes were analyzed using a Reflotron Plus hematology analyzer (Roche Diagnostics, Mannheim, Germany) with the corresponding test strips.
- the organ was flushed with PBS before.
- the liver was dissected and a single cell suspension prepared. Following CellTrackerTM Orange (Life Technologies GmbH, Frankfurt, Germany) staining, these cells were directly used for the co-culture cytotoxicity assay with enriched CTL.
- Hepa1-6 cells were cultured in RPMI1640 (PAA Laboratories) supplemented with 100 U/ml penicillin (PAA Laboratories), 100 ⁇ g/ml streptomycin (PAA Laboratories), and 10% heat inactivated fetal calf serum (PAA Laboratories).
- B7-H1 To overexpress murine B7-H1 in vitro, we amplified B7-H1 from murine mRNA of Hepa1-6 cells by PCR using the following primer pair (NM_021893): forward 5'-CGC CCG GGG GGG ATC ATG AGG ATA TTT GCT GGC ATT ATA TTC ACA-3'; reverse 5' -TCA AGC TTG CAT GCC TTA CTT GTA CAG CTC GTC CA-3'.
- the primers were used to clone mB7-H1 into the lentiviral vector pSEW (Demaison et al., 2002) in front of the EGFP encoding sequence, already present in the pSEW vector.
- Coding sequences of B7-H1 are shown in italics. Following linearization of pSEW with BamHI, the amplified mB7-H1 fragment was inserted with the InFusion system (Takara Bio Europe, Saint-Germain-en-Laye, France). Correct sequence was verified by sequencing. For in vivo transduction of B7-H1 into the liver of mice, B7-H1 EGFP in the pSEW vector was subcloned into the pShuttle-CMV vector of the adEasy adenoviral vector system (Luo et al., 2007).
- the following primer pair was used containing flanking sequence appropriate InFusion cloning into the BglII/EcoRV site of pShuttle-CMV: forward 5'-GAT CCG CTA GAG ATC GCC ACC ATG AGG ATA TTT GCT GGC ATT ATA TTC ACA GC-3', reverse 5'-TCC GGT GGA TCG GAT TTA CTT GTA CAG CTC GTC CAT GCC-3'. Coding sequences of B7-H1 (forward primer) and EGFP (reverse primer) are displayed in italics. Correct sequence was verified by sequencing. As a negative control the pAdTrack vector, only encoding EGFP, was used.
- Ad-293 cells were seeded in a 75 cm 2 flask in DMEM (high glucose, Glutamax) + 10% FCS + pen/strep 1:100 + HEPES 1:100 + non-essential amino acids 1:100. After 4 days, cells were detached with 1 ml trypsin. 9 ml culture medium were added and cells were centrifuged at 500g for 5 min. Following pellet resuspension in 10 ml of culture medium, 3.5 ml were seeded in 2 x 175 cm 2 flasks. After three days, cells were detached from both 175 cm 2 flasks with 1 x 2 ml trypsin.
- Flasks were incubated for 4 h in an incubator. Then, 15 ml prewarmed culture medium was added to each flask. After 3 days, most infected cells were detached as clusters. Non-infected cells were still spread-out and attached to the plastic. The medium was yellowish. Cells were detached by tapping the flasks against the hand. Medium was transferred into 10 x 50 ml tubes and spun at 500g for 5min at 4°C. Supernatant was discarded. Tubes were lightly shaken. 3 x 1 ml culture medium was transferred to 3 tubes. Using a 1 ml filter tip, first three pellets were resuspended in 1 ml culture medium.
- Resuspendend cells were transferred into a cryo-vial, which was snap-frozen in liquid N2 and transferred to a -80°C freezer.
- cell lysates were prepared by putting cells of 10 vials through 4 rapid thaw/freeze cycles. Lysates were transferred into a 15 ml tube and spun at 1500 g for 10 min at 4°C.
- 6 CsCl gradients were prepared: To each ultracentrifuge tube 4 ml of 40% CsCl in PBS was added. Then, this was overlayed carefully with 4.5 ml 15% CsCl in PBS.
- the cleared cell lysate was transferred to a 50 ml tube, filled up to 18 ml with culture medium and mixed by pipeting. Three ml cleared lysate were transferred onto each CsCl gradient. Ultracentrifuge tubes were transferred into buckets of the rotor. The weight of corresponding bucket pairs was adjusted. CsCl gradients were centrifuged at 25,400 rpm for about 17 h at 4°C. Acceleration and deceleration were both set as 1. After centrifugation, tubes were carefully removed. Virus was collected by inserting a 23G needle connected to a 2 ml syringe below the lower virus band.
- Viruses from 3 ultracentrifuge tubes were collected and loaded into a 3 ml slide-a-lyzer cassette (10 kDa) (Thermo Scientifc, Darmstadt, Germany). The viral particles were dialyzed against 2 1 cold PBS for 4 h. Then PBS was changed and dialyzing prolonged for 12 h. Afterwards, virus was collected and transferred to a 15 ml tube on ice. Aliquots were transferred to cryovials, snap-frozen and stored at -80°C. To determine the colony forming unit capacity, a plaque assay was applied. Evaluation was performed by fluorescence-microscopy due to the EGFP-tag of transduced genes. For mouse transduction, 5x1010 infectious particle were administered in 100 ⁇ l PBS.
- CD8+ T cells derived from the spleen of C57Bl/6N OT-I mice (haplotype H-2b) as effector cells were co-incubated with Hepa1-6 cells, originating from the C57L strain (haplotype H-2b) as target cells for 24 h.
- Hepa1-6 cells Prior to this, Hepa1-6 cells were pulsed for 2 h with the ovalbumin (OVA) peptide 257-264 (AnaSpec, Fremont, U.S.A.), or the hepatitis B virus (HBV) peptide ILSPFLPLL derived from the HBsAg as control (IBA, Goettingen, Germany) or remained untreated as control.
- OVA ovalbumin
- HBV hepatitis B virus
- Hepa1-6 cells were stained with CellTrackerTM Orange (Life Technologies GmbH, Frankfurt, Germany) before CD8+ T cell addition. After 24 h, surviving target cells were determined by FACS analyzes (FACS Fortessa, BD, Heidelberg, Germany).
- RNA from 5 ⁇ 10 5 CD8+ T cells, Hepa1-6 cells, or primary liver cells was isolated by using peqGOLD RNAPure Kit (Peqlab, Er Weg, Germany) as instructed by the manufacturer's protocol. Two ⁇ g RNA was reverse transcribed into complementary DNA (cDNA) with the iScriptTM cDNA Synthesis kit (Bio-Rad, Kunststoff, Germany). Quantitative PCR (qPCR) was performed with the iQTM SYBR® Green Supermix (Bio-Rad) according to the distributor's instructions. qPCR measurement and data analysis were performed with the CFX real-time PCR system from Bio-Rad.
- B7-H1 (NM_21893) forward: 5'-TGC AGC AGT AAA CGC CTG CG-3', reverse: 5'CGC TGC CAA AGG ACC AGC TT-3'; IL-2 (NM_008366) forward: 5'TGA GCA TCC TGG GGA GTT TC-3', reverse: 5'-GTG ACC TCA AGT CCT GCA GG-3'; Fas-L (NM_010177) forward: 5'-ACC AAC CAA AGC CTT AAA-3', reverse: 5'-ATA CTT CAC TCC AGA GAT-3'; granzyme B (NM_013542) forward: 5'-CTC CAC GTG CTT TCA CCA AA-3', reverse: 5'-GGA AAA TAG TAC AGA GAG GCA-3'; perforin (NM_011073) forward: 5'-TGC TAC ACT GCC ACT CGG
- OT 1 CD8+ T cells were identified by FACS analysis utilizing anti-mouse V alpha 2 TCR FITC (eBioscience, San Diego, CA, USA) as well as anti-mouse V ⁇ 5.1, 5.2 TCR-PE (BD Bioscience Heidelberg, Germany) antibodies. Fc receptor binding on OT 1 CD8+ T cells was blocked by CD16/CD32 anti mouse antibody for 15 min on ice followed by 20 min incubations of anti-CD8 ⁇ -APC for T cells on ice. Surface expression of B7-H1 and B7-DC on the surface of primary hepatocytes was determined by FACS analysis, using anti-B7-H-PE or anti-B7-DC-PE. Immune cells were excluded by CD45-FITC staining, consequently analyzing CD45- cells only.
- B7-H1, B7-DC, PD-1, Fas and EGFP expression were analyzed by Western analysis. Briefly, equivalent numbers of primary hepatocytes or Hepa1-6 cells were washed twice with PBS, lysed in RIPA buffer containing 1x complete Protease Inhibitor Cocktail Tablets (Roche, Basel, Switzerland) and sonicated for 10 impulses, followed by centrifugation for 10 min at 16.000 g (4°C). Supernatants were denaturated with SDS-PAGE sample buffer (250 mM Tris pH 6.8, 40% glycerol, 10% 2-ME, 8% SDS, 0.02% bromphenol blue) for 10 minutes at 95°C. Comparable protein concentrations were maintained by Lowry (Bio-Rad).
- Proteins were separated on 10% SDS-polyacrylamide gels and transferred onto a nitrocellulose membrane by semi-dry blotting. Membranes were blocked with 5% BSA/TTBS followed by incubation with anti-B7-H1- (R&D systems), anti-B7-DC- (Santa Cruz), anti-PD-1- (Santa Cruz), a-Fas-antibody (Santa Cruz) in 5% BSA/TBS at 4°C overnight. Loading was normalized to ⁇ -actin (anti-actin, Sigma-Aldrich). For protein detection, membrane was incubated with IRDye secondary antibodies (LI-COR, Bad Homburg, Germany) in 5% BSA/TTBS. Proteins were visualized and densitometrically analyzed with the Odyssey infrared imaging system.
- IRDye secondary antibodies LI-COR, Bad Homburg, Germany
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Immunology (AREA)
- Epidemiology (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Cell Biology (AREA)
- Gastroenterology & Hepatology (AREA)
- Zoology (AREA)
- Molecular Biology (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Toxicology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicinal Preparation (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP15181800 | 2015-08-20 | ||
| PCT/EP2016/069680 WO2017029389A1 (en) | 2015-08-20 | 2016-08-19 | B7-h1 fusion polypeptides for treating and preventing organ failure |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP3337496A1 EP3337496A1 (en) | 2018-06-27 |
| EP3337496B1 true EP3337496B1 (en) | 2020-11-25 |
Family
ID=54014503
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP16757610.7A Active EP3337496B1 (en) | 2015-08-20 | 2016-08-19 | B7-h1 fusion polypeptides for use in treating and preventing organ failure |
Country Status (7)
| Country | Link |
|---|---|
| US (2) | US10632174B2 (zh) |
| EP (1) | EP3337496B1 (zh) |
| JP (1) | JP6859320B2 (zh) |
| CN (2) | CN107921093B (zh) |
| CA (1) | CA2995987C (zh) |
| ES (1) | ES2851474T3 (zh) |
| WO (1) | WO2017029389A1 (zh) |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016168771A2 (en) | 2015-04-17 | 2016-10-20 | Alpine Immune Sciences, Inc. | Immunomodulatory proteins with tunable affinities |
| US11078282B2 (en) | 2016-04-15 | 2021-08-03 | Alpine Immune Sciences, Inc. | CD80 variant immunomodulatory proteins and uses thereof |
| US11834490B2 (en) | 2016-07-28 | 2023-12-05 | Alpine Immune Sciences, Inc. | CD112 variant immunomodulatory proteins and uses thereof |
| US11471488B2 (en) | 2016-07-28 | 2022-10-18 | Alpine Immune Sciences, Inc. | CD155 variant immunomodulatory proteins and uses thereof |
| EP3596116B1 (en) * | 2017-03-16 | 2023-09-06 | Alpine Immune Sciences, Inc. | Pd-l1 variant immunomodulatory proteins and uses thereof |
| CA3054068A1 (en) | 2017-03-16 | 2018-09-20 | Alpine Immune Sciences, Inc. | Cd80 variant immunomodulatory proteins and uses thereof |
| JP2020511144A (ja) | 2017-03-16 | 2020-04-16 | アルパイン イミューン サイエンシズ インコーポレイテッド | Pd−l2バリアント免疫調節タンパク質及びその使用 |
| US10650210B1 (en) | 2019-03-18 | 2020-05-12 | Haier Us Appliance Solutions, Inc. | Method for authenticating a filter cartridge for a refrigerator appliance |
| EP4143221A1 (en) | 2020-04-30 | 2023-03-08 | Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. | Pd-l1 variants with improved affinity towards pd-1 |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2950814A4 (en) * | 2013-01-31 | 2016-06-08 | Univ Jefferson | PD-L1 AND PD-L2 BASED FUSION PROTEINS AND USES THEREOF |
| EP3052131B1 (en) | 2013-10-01 | 2018-12-05 | Mayo Foundation for Medical Education and Research | Methods for treating cancer in patients with elevated levels of bim |
-
2016
- 2016-08-19 US US15/753,541 patent/US10632174B2/en active Active
- 2016-08-19 JP JP2018509494A patent/JP6859320B2/ja active Active
- 2016-08-19 CA CA2995987A patent/CA2995987C/en active Active
- 2016-08-19 WO PCT/EP2016/069680 patent/WO2017029389A1/en active Application Filing
- 2016-08-19 EP EP16757610.7A patent/EP3337496B1/en active Active
- 2016-08-19 ES ES16757610T patent/ES2851474T3/es active Active
- 2016-08-19 CN CN201680044831.6A patent/CN107921093B/zh active Active
- 2016-08-19 CN CN202210458693.3A patent/CN114767833A/zh active Pending
-
2020
- 2020-03-18 US US16/822,240 patent/US11679142B2/en active Active
Non-Patent Citations (1)
| Title |
|---|
| None * |
Also Published As
| Publication number | Publication date |
|---|---|
| CN107921093A (zh) | 2018-04-17 |
| US10632174B2 (en) | 2020-04-28 |
| CA2995987C (en) | 2023-10-24 |
| JP6859320B2 (ja) | 2021-04-14 |
| CN107921093B (zh) | 2022-04-01 |
| JP2018523483A (ja) | 2018-08-23 |
| CN114767833A (zh) | 2022-07-22 |
| ES2851474T3 (es) | 2021-09-07 |
| US20180243372A1 (en) | 2018-08-30 |
| US20200215159A1 (en) | 2020-07-09 |
| WO2017029389A1 (en) | 2017-02-23 |
| CA2995987A1 (en) | 2017-02-23 |
| EP3337496A1 (en) | 2018-06-27 |
| US11679142B2 (en) | 2023-06-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11679142B2 (en) | B7-H1 fusion polypeptides for treating and preventing organ failure | |
| RU2763798C1 (ru) | Искусственные антигенпрезентирующие клетки и способы их применения | |
| Lehuen et al. | Immune cell crosstalk in type 1 diabetes | |
| US9937205B2 (en) | Inhibition of diacylglycerol kinase to augment adoptive T cell transfer | |
| US20130183308A1 (en) | Therapeutic nuclease compositions and methods | |
| Elhag et al. | Reconstructed adeno-associated virus with the extracellular domain of murine PD-1 induces antitumor immunity | |
| Guo et al. | Evasion of natural killer cells by influenza virus | |
| AU2015362748A1 (en) | Methods for controlled elimination of therapeutic cells | |
| WO2016073704A1 (en) | Immunotherapeutics for cancer and autoimmune diseases | |
| US11547741B2 (en) | Methods of use of soluble CD24 for treating immune related adverse events in cancer therapies | |
| Von Knethen et al. | Tolerizing CTL by sustained hepatic PD-L1 expression provides a new therapy approach in mouse sepsis | |
| Denner | Xenotransplantation-progress and problems: a review | |
| Yan et al. | Parenchymal expression of CD40 exacerbates adenovirus‐induced hepatitis in mice | |
| US20110135672A1 (en) | Hla-g compositions and methods of use thereof | |
| Kimura et al. | Contribution of alloantigens to hepatic ischemia/reperfusion injury: roles of natural killer cells and innate immune recognition of nonself | |
| JP2024508920A (ja) | 多武装の粘液腫ウイルス | |
| Adachi et al. | Exogenous expression of Fas-ligand or CrmA prolongs the survival in rat liver transplantation | |
| Borges et al. | Interactions of LIRs, a family of immunoreceptors expressed in myeloid and lymphoid cells, with viral and cellular MHC class I antigens | |
| Chen et al. | In vivo analysis of adenovirus-specific cytotoxic T lymphocyte response in mice deficient in CD28, fas ligand, and perforin | |
| JP7334249B2 (ja) | 敗血症の治療に使用するための初期アポトーシス細胞 | |
| ES2972793T3 (es) | Células diseñadas para inducir tolerancia | |
| KR101263103B1 (ko) | CD1d 발현을 감소조절하는 US2 단백질 유전자를 포함하는 자연 살해 T 세포활성 억제용 조성물 및 이를 이용한 자연 살해 T 세포 활성 억제 방법 | |
| KR20230038496A (ko) | 면역 억제의 치료 | |
| KR20110011595A (ko) | 면역 반응 증진을 위한 타파신 증대 | |
| van Altena | Tumor surveillance and immune evasion: the interplay between the immune system and tumor cells |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: UNKNOWN |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE INTERNATIONAL PUBLICATION HAS BEEN MADE |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20180313 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: VON KNETHEN, ANDREAS Inventor name: SHA, LISA Inventor name: PARNHAM, MICHAEL |
|
| DAV | Request for validation of the european patent (deleted) | ||
| DAX | Request for extension of the european patent (deleted) | ||
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20191001 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20200605 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602016048595 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 1337506 Country of ref document: AT Kind code of ref document: T Effective date: 20201215 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: NV Representative=s name: SCHMAUDER AND PARTNER AG PATENT- UND MARKENANW, CH |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: FP |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210225 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210325 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210226 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210225 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210325 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG9D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602016048595 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2851474 Country of ref document: ES Kind code of ref document: T3 Effective date: 20210907 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 |
|
| 26N | No opposition filed |
Effective date: 20210826 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20210831 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210325 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210819 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210819 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210831 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20160819 |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230524 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20230831 Year of fee payment: 8 Ref country code: ES Payment date: 20230918 Year of fee payment: 8 Ref country code: CH Payment date: 20230902 Year of fee payment: 8 Ref country code: AT Payment date: 20230818 Year of fee payment: 8 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20240821 Year of fee payment: 9 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20201125 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20240819 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20240822 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20240823 Year of fee payment: 9 |