EP3143115B2 - Agent de lavage et de nettoyage avec des performances de blanchiment amélioré - Google Patents
Agent de lavage et de nettoyage avec des performances de blanchiment amélioré Download PDFInfo
- Publication number
- EP3143115B2 EP3143115B2 EP15720976.8A EP15720976A EP3143115B2 EP 3143115 B2 EP3143115 B2 EP 3143115B2 EP 15720976 A EP15720976 A EP 15720976A EP 3143115 B2 EP3143115 B2 EP 3143115B2
- Authority
- EP
- European Patent Office
- Prior art keywords
- amino acid
- alkyl
- seq
- acid sequence
- phenyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/38—Products with no well-defined composition, e.g. natural products
- C11D3/386—Preparations containing enzymes, e.g. protease or amylase
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/39—Organic or inorganic per-compounds
- C11D3/3902—Organic or inorganic per-compounds combined with specific additives
- C11D3/3905—Bleach activators or bleach catalysts
- C11D3/3907—Organic compounds
- C11D3/3917—Nitrogen-containing compounds
- C11D3/392—Heterocyclic compounds, e.g. cyclic imides or lactames
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/39—Organic or inorganic per-compounds
- C11D3/3902—Organic or inorganic per-compounds combined with specific additives
- C11D3/3905—Bleach activators or bleach catalysts
- C11D3/3907—Organic compounds
- C11D3/3917—Nitrogen-containing compounds
- C11D3/3927—Quarternary ammonium compounds
Definitions
- the present invention relates to the use of a specific protease for enhancing the bleaching performance of certain acylhydrazones when washing textiles or cleaning hard surfaces, and to washing and cleaning agents which contain such a protease and such acylhydrazone.
- the acylhydrazones may exist in E or Z configuration; when R 2 is hydrogen, the compound of general formula (I) may exist in one of its tautomeric forms or as a mixture thereof.
- R 2 is preferably hydrogen.
- R 1 and/or R 3 is preferably a methyl, phenyl or naphthyl group substituted by an electron-withdrawing group.
- R 4 is preferably hydrogen.
- the electron-withdrawing group is preferably an ammonium group which optionally carries alkyl or hydroxyalkyl groups or, including the N atom carrying an alkyl group, as optionally carrying further heterocycloalkyl groups.
- the anion A - is preferably carboxylate such as lactate, citrate, tartrate or succinate, perchlorate, tetrafluoroborate, hexafluorophosphate, alkylsulfonate, alkyl sulfate, hydrogen sulfate, sulfate, dihydrogen phosphate, hydrogen phosphate, phosphate, isocyanate, thiocyanate, nitrate, fluoride, chloride, bromide, hydrogen carbonate or carbonate, whereby in the case of polyvalent anions the charge balance can be achieved by the presence of additional cations such as sodium or ammonium ions.
- carboxylate such as lactate, citrate, tartrate or succinate, perchlorate, tetrafluoroborate, hexafluorophosphate, alkylsulfonate, alkyl sulfate, hydrogen sulfate, sulfate, dihydrogen phosphate, hydrogen phosphate, phosphate
- proteases are enzymes which catalyze the hydrolysis of amide bonds in protein substrates.
- the protease used according to the invention preferably has the amino acid V at position 193 in the numbering according to SEQ ID NO:1 and the amino acid L at position 211, or it has the amino acid R at position 9 in the numbering according to SEQ ID NO:2, the amino acid T at position 15, the amino acid A at position 66, the amino acid D at position 212 and/or the amino acid R at position 239, whereby several, for example 2, 3, 4 or 5, such proteases can also be used.
- those of the subtilisin type can also be used.
- subtilisins BPN' and Carlsberg examples of these are the subtilisins BPN' and Carlsberg, the protease PB92, the subtilisins 147 and 309, the alkaline protease from Bacillus lentus, subtilisin DY and the enzymes thermitase, proteinase K and the proteases TW3 and TW7, which are classified as subtilases but no longer as subtilisins in the narrower sense.
- Subtilisin Carlsberg is available in a further developed form under the trade name Alcalase ® from Novozymes A/S, Bagsvaerd, Denmark.
- the subtilisins 147 and 309 are sold under the trade names Esperase ® and Savinase ® by Novozymes.
- protease variants known under the name BLAP ® are derived from the protease from Bacillus lentus DSM 5483.
- Other useful proteases are, for example, those sold under the trade names Durazym ® , Relase ® , Everlase ® , Nafizym ® , Natalase ® and Kannase ® by Novozymes, those sold under the trade names Purafect ® , Purafect ® OxP, Purafect ® Prime, Excellase ® and Properase ® by Genencor, those sold under the trade name Biotouch ® ROC 250 L by AB-Enzymes, Darmstadt, those sold under the trade name Protosol ® by Advanced Biochemicals Ltd., Thane, India, those sold under the trade name Wuxi ® by Wuxi Snyder Bioproducts Ltd., China, those sold under the trade names Proleather ® and Protease P ® by Amano Pharmaceuticals Ltd., Nagoya, Japan, those sold
- proteases from Bacillus gibsonii and Bacillus pumilus which are disclosed in the international patent applications WO2008/086916 and WO2007/131656
- the effect of increasing the bleaching performance according to the invention is not observed when using the protease according to SEQ ID NO: 4.
- sequence comparison is based on the BLAST algorithm, which is established in the state of the art and is commonly used (see, for example, Altschul, SF, Gish, W., Miller, W., Myers, EW & Lipman, DJ (1990) "Basic local alignment search tool.” J Mol Biol 215:403-410 , and Altschul, Stephan F., Thomas L. Madden, Alejandro A. Schaffer, Jinghui Zhang, Hheng Zhang, Webb Miller, and David J.
- T-Coffee see for example Notredame et al. (2000): T-Coffee: A novel method for multiple sequence alignments. J. Mol. Biol. 302, 205-217 ) or programs based on these programs or algorithms.
- all sequence comparisons were created using the computer program Vector NTI ® Suite 10.3 (Invitrogen Corporation, 1600 Faraday Avenue, Carlsbad, California, USA) with the specified standard parameters, whose AlignX module for sequence comparisons is based on ClustalW.
- Such a comparison also allows a statement to be made about the similarity of the sequences being compared to one another. This is usually expressed as percent identity, i.e. the proportion of identical nucleotides or amino acid residues at the same positions or positions that correspond to one another in an alignment.
- the broader term homology includes conserved amino acid exchanges in amino acid sequences, i.e. amino acids with similar chemical activity, since these usually perform similar chemical activities within the protein. Therefore, the similarity of the sequences being compared can also be expressed as percent homology or percent similarity. Identity and/or homology statements can be made for entire polypeptides or genes or just for individual regions. Homologous or identical regions of different nucleic acid or amino acid sequences are therefore defined by similarities in the sequences.
- Such regions often have identical functions. They can be small and contain only a few nucleotides or amino acids. Such small regions often perform essential functions for the overall activity of the protein. It can therefore be useful to relate sequence similarities only to individual, possibly small regions. Unless otherwise stated, however, information on identity or homology in the present application refers to the total length of the respective nucleic acid or amino acid sequence indicated.
- the proportion of the protease by weight, based on active protein, in the total weight of washing or cleaning agents according to the invention is preferably 0.005 to 1.0% by weight, in particular 0.01 to 0.5% by weight and particularly preferably 0.02 to 0.2% by weight.
- the protein concentration can be determined using known methods, for example the BCA method (bicinchoninic acid; 2,2'-biquinolyl-4,4'-dicarboxylic acid) or the biuret method ( AG Gornall, CS Bardawill and MM David, J. Biol. Chem., 177 (1948), pp. 751-766 ).
- the active protein concentration was determined by titrating the active centers using a suitable irreversible inhibitor (for proteases, for example, phenylmethylsulfonyl fluoride (PMSF)) and determining the residual activity (cf. M. Bender et al., J. Am. Chem. Soc. 88, 24 (1966), pp. 5890-5913 ).
- a suitable irreversible inhibitor for proteases, for example, phenylmethylsulfonyl fluoride (PMSF)
- the cleaning effects of the protease are retained in the presence of the acylhydrazone present at the same time.
- the cleaning effects of the acylhydrazone are enhanced in the presence of the protease present at the same time if the protease is selected from the above-mentioned enzymes.
- the combined use of such a protease and acylhydrazone also results in a larger number of improved soilings being removed than the sum of improved soilings being removed when using each of the two active ingredients individually, particularly at low washing temperatures of, for example, 40 °C and below, and even at room temperature.
- the performance of compounds of general formula (I) may optionally be enhanced by the presence of Manganese, titanium, cobalt, nickel or copper ions, preferably Mn(II)-(III)-(IV)-(V), Cu(I)-(II)-(III), Fe(I)-(II)-(III)-(IV), Co(I)-(II)-(III), Ni(I)-(II)-(III), Ti(II)-(III)-(IV) and particularly preferably those selected from Mn(II)-(III)-(IV)-(V), Cu(I)-(II)-(III), Fe(I)-(II)-(III)-(IV) and Co(I)-(II)-(III); if desired, the acylhydrazone can also be used in the form of complex compounds of the said metal central atoms with ligands of the general formula (I) and in particular of the general formula (II).
- a bleach-enhancing complex which has a ligand with a framework according to formula (I) can have the corresponding ligand once or multiple times, in particular twice. It can be mono- or optionally di- or polynuclear. It can also have further neutral, anion or cation ligands, such as, for example, H 2 O, NH 3 , CH 3 OH, acetylacetone, terpyridine, organic anions, such as, for example, citrate, oxalate, tartrate, formate, a C 2-18 carboxylate, a C 1-18 alkyl sulfate, in particular methosulfate, or a corresponding alkanesulfonate, inorganic anions, such as, for example, halide, in particular chloride, perchlorate, tetrafluoroborate, hexafluorophosphate, nitrate, hydrogen sulfate, hydroxide or hydroperoxide. It can also have bridging ligands
- the concentration of the compound according to formula (I) in aqueous liquor is 0.5 ⁇ mol/l to 500 ⁇ mol/l, in particular 5 ⁇ mol/l to 100 ⁇ mol/l.
- the above-mentioned complex-forming metal ions are preferably not added intentionally, but they can be present from possible sources of such metal ions, which include in particular tap water, the washing machine itself, adhesions to textiles and soiling on the textiles. Metal ions introduced unintentionally with other detergent ingredients may also be considered.
- Preferred peroxygen concentrations (calculated as H 2 O 2 ) in the liquor are in the range from 0.001 g/l to 10 g/l, in particular from 0.1 g/l to 1 g/l and particularly preferably from 0.2 g/l to 0.5 g/l.
- the use according to the invention is preferably carried out at temperatures in the range from 10 °C to 95 °C, in particular 20 °C to 40 °C and particularly preferably at temperatures below 30 °C.
- the water hardness of the water used to prepare the aqueous liquor is preferably in the range from 0°dH to 21°dH, in particular 0°dH to 3°dH.
- the water hardness is preferably in the range from 0°dH to 16°dH, in particular 0°dH to 3°dH, which can be achieved, for example, by using conventional builder materials or water softeners.
- the use according to the invention is preferably carried out at pH values in the range from pH 5 to pH 12, in particular from pH 7 to pH 11.
- the use according to the invention is preferably carried out in such a way that a peroxygen compound and a detergent which contains an acylhydrazone of the general formula (I) and the above-mentioned protease are allowed to act on a contaminated textile during a machine or hand-washing process.
- the use according to the invention can be implemented particularly easily by using a detergent which contains a peroxygen compound, the above-mentioned protease and a compound of the formula (I) or, if desired, a bleach catalyst which can be obtained from this by complex formation with a transition metal ion mentioned, when washing textiles which require cleaning.
- the peroxygen compound and/or the compound of the formula (I) and/or a complex which can be obtained from this and/or the protease can also be added separately to a washing liquor which contains a detergent without the respective ingredient mentioned.
- a washing liquor which contains a detergent without the respective ingredient mentioned.
- a further subject matter of the invention is therefore a washing or cleaning agent containing a peroxygen-containing bleach, a compound of the formula (I) and a protease selected from a) proteases comprising an amino acid sequence which is at least 98.5% and preferably at least 99% identical to the amino acid sequence given in SEQ ID NO:1 over its entire length and has the amino acid V at position 193 and/or the amino acid L at position 211 in the numbering according to SEQ ID NO:1, b) proteases comprising an amino acid sequence which is at least 70% and increasingly preferably at least 75%, 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 90.5%, 91%, 91.5%, 92%, 92.5%, 93%, 93.5%, 94%, 94.5%, 95%, 95.5%, 96%, 96.5%, 97%, 97.5%, 98%, 98.5% and 99% identical and in the number
- lipases or cutinases particularly because of their triglyceride-cleaving activities, but also to produce peracids in situ from suitable precursors.
- lipases or cutinases include, for example, the lipases originally obtained from Humicola lanuginosa (Thermomyces lanuginosus) or further developed lipases, particularly those with the amino acid exchange D96L. Cutinases that were originally isolated from Fusarium solani pisi and Humicola insolens can also be used. Lipases or cutinases whose starting enzymes were originally isolated from Pseudomonas mendocina and Fusarium solanii can also be used.
- oxidoreductases for example oxidases, oxygenases, catalases, peroxidases such as halo-, chloro-, bromo-, lignin-, glucose- or manganese-peroxidases, dioxygenases or laccases (phenol oxidases, polyphenol oxidases) can be used to increase the bleaching effect. It is advantageous to also add organic, particularly aromatic, compounds that interact with the enzymes in order to increase the activity of the oxidoreductases in question (enhancers) or to ensure the flow of electrons when the redox potentials between the oxidizing enzymes and the soiling differ greatly (mediators).
- the cleaning agents according to the invention particularly preferably contain at least one amylase as a further enzyme.
- An amylase is an enzyme as described in the introduction. Synonymous terms can be used for amylases, for example 1,4-alpha-D-glucan glucanohydrolase or glycogenase.
- Amylases preferred according to the invention are ⁇ -amylases. The decisive factor as to whether an enzyme is an ⁇ -amylase within the meaning of the invention is its ability to hydrolyze ⁇ (1-4) glycoside bonds in the amylose of starch.
- amylases are the ⁇ -amylases from Bacillus licheniformis, from Bacillus amyloliquefaciens or from Bacillus stearothermophilus and in particular their further developments which have been improved for use in washing or cleaning agents.
- the enzyme from Bacillus licheniformis is available from Novozymes under the name Termamyl ® and from Danisco/Genencor under the name Purastar ® ST.
- this ⁇ -amylase is available from Novozymes under the trade names Duramyl ® and Termamy ® ultra, from Danisco/Genencor under the name Purastar ® OxAm and from Daiwa Seiko Inc., Tokyo, Japan, as Keistase ® .
- the ⁇ -amylase from Bacillus amyloliquefaciens is sold by the company Novozymes under the name BAN ® , and derived variants of the ⁇ -amylase from Bacillus stearothermophilus under the names BSG ® and Novamyl ® , also by the company Novozymes.
- Other products that are particularly suitable for this purpose are the ⁇ -amylase from Bacillus sp.
- a 7-7 (DSM 12368) and the cyclodextrin glucanotransferase (CGTase) from Bacillus agaradherens (DSM 9948). Fusion products of all of the molecules mentioned can also be used.
- CGTase cyclodextrin glucanotransferase
- DSM 9948 Bacillus agaradherens
- Fusion products of all of the molecules mentioned can also be used.
- the further developments of the ⁇ -amylase from Aspergillus niger and A. oryzae available under the trade name Fungamyl ® from the company Novozymes are suitable.
- Other commercial products that can be used advantageously are, for example, Amylase-LT ® and Stainzyme ® or Stainzyme ultra ® or Stainzyme plus ® , the latter also from the company Novozymes.
- Variants of these enzymes that can be obtained by point mutations can also be used according to the invention.
- Particularly preferred amylases are disclosed in the international publications WO 00/60060 , WO 03/002711 , WO 03/054177 and WO07/079938 .
- preferred cleaning agents are characterized in that at least one enzyme from the group of amylases, cellulases, hemicellulases, mannanases, tannases, xylanases, xanthanases, xyloglucanases, ⁇ -glucosidases, pectinases, carrageenases, perhydrolases, oxidases, oxidoreductases or a lipase, as well as mixtures thereof, preferably from the group of amylases, is used as the additional enzyme.
- the weight proportion of the additional enzymes based on active protein in the total weight of the washing and cleaning agents is preferably 0.0005 to 1.0% by weight, in particular 0.001 to 0.5% by weight and particularly preferably 0.002 to 0.2% by weight.
- Detergents or cleaning agents preferably contain 0.001% by weight to 5% by weight, in particular 0.05% by weight to 0.15% by weight and particularly preferably 0.03% by weight to 0.09% by weight of the compound according to formula (I).
- the agent additionally contains a manganese, titanium, cobalt, nickel or copper salt and/or a manganese, titanium, cobalt, nickel or copper complex without a ligand which corresponds to a compound according to formula (I).
- the molar ratio of the transition metal mentioned or the sum of the transition metals mentioned to the compound according to formula (I) is then preferably in the range from 0.001:1 to 2:1, in particular 0.01:1 to 1:1.
- they contain 0.05% by weight to 1% by weight, in particular 0.1% by weight to 0.5% by weight of bleach-enhancing complex which has a ligand according to formula (I).
- the preferred transition metal is Mn.
- the peroxygen compounds contained in the agents are in particular organic peracids or peracidic salts of organic acids, such as phthalimidopercaproic acid, perbenzoic acid or salts of diperoxododecanedioic acid, other peroxo acids or peroxo acid salts, such as alkali persulfates or peroxodisulfates or caroates, or diacyl peroxides or tetraacyl diperoxides, hydrogen peroxide and substances which release hydrogen peroxide under the washing conditions, such as alkali perborates, alkali percarbonates, alkali persilicates and urea perhydrate.
- organic peracids or peracidic salts of organic acids such as phthalimidopercaproic acid, perbenzoic acid or salts of diperoxododecanedioic acid, other peroxo acids or peroxo acid salts, such as alkali persulfates or peroxodisulf
- Hydrogen peroxide can also be produced with the aid of an enzymatic system, i.e. an oxidase and its substrate.
- an enzymatic system i.e. an oxidase and its substrate.
- solid peroxygen compounds are to be used, these can be used in the form of powders or granules, which can also be coated in a manner known in principle.
- peroxygen compounds are present in the agents in amounts of up to 50 wt.%, in particular from 2 wt.% to 45 wt.% and particularly preferably from 5 wt.% to 20 wt.%.
- a conventional bleach activator is used together with the acyl hydrazone of the general formula (I), the general formula (II) and in particular the formula (III), in particular in the presence of a peroxygen compound which releases H 2 O 2 .
- bleach activators are preferably contained in amounts of up to 10% by weight, in particular from 1.5% by weight to 5% by weight.
- the compound which forms peroxocarboxylic acid under perhydrolysis conditions and the acyl hydrazone are used in molar ratios in the range from 4:1 to 100:1, in particular from 25:1 to 50:1.
- Compounds which can be used as peroxocarboxylic acid-yielding compounds under perhydrolysis conditions are in particular compounds which, under perhydrolysis conditions, yield optionally substituted perbenzoic acid and/or aliphatic peroxocarboxylic acids having 1 to 12 carbon atoms, in particular 2 to 4 carbon atoms, alone or in mixtures.
- Suitable bleach activators are those which carry O- and/or N-acyl groups, in particular of the stated number of carbon atoms, and/or optionally substituted benzoyl groups.
- acylated alkylenediamines in particular tetraacetylethylenediamine (TAED), acylated glycolurils, in particular tetraacetylglycoluril (TAGU), acylated triazine derivatives, in particular 1,5-diacetyl-2,4-dioxohexahydro-1,3,5-triazine (DADHT), N-acylimides, in particular N-nonanoylsuccinimide (NOSI), acylated phenolsulfonates or carboxylates or the sulfonic or carboxylic acids thereof, in particular nonanoyl or isononanoyl or lauroyloxybenzenesulfonate (NOBS or iso-NOBS or LOBS) or decanoyloxybenzoate (DOBA), their formal carbonic acid ester derivatives such as 4-(2-decanoyloxyethoxycarbonyloxy)benz
- bleach-activating compounds such as nitriles from which perimidic acids are formed under perhydrolysis conditions
- nitriles from which perimidic acids are formed under perhydrolysis conditions include in particular aminoacetonitrile derivatives with a quaternized nitrogen atom according to the formula in which R 11 is -H, -CH 3 , a C 2-24 alkyl or alkenyl radical, a substituted C 1-24 alkyl or C 2-24 alkenyl radical with at least one substituent from the group -Cl, -Br, -OH, -NH 2 , -CN and -N (+) -CH 2 -CN, an alkyl or alkenylaryl radical with a C 1-24 alkyl group, or a substituted alkyl or alkenylaryl radical with at least one, preferably two, optionally substituted C 1-24 alkyl group(s) and optionally further substituents on the aromatic ring, R
- the bleach activators may be coated or granulated in a known manner with coating substances, whereby granulated tetraacetylethylenediamine with average grain sizes of 0.01 mm to 0.8 mm, granulated 1,5-diacetyl-2,4-dioxohexahydro-1,3,5-triazine, and/or trialkylammonium acetonitrile in particle form is particularly preferred.
- transition metal complexes can also be used. These are preferably selected from among the cobalt, iron, copper, titanium, vanadium, manganese and ruthenium complexes.
- Both inorganic and organic compounds can be used as ligands in such transition metal complexes, which include, in addition to carboxylates, in particular compounds with primary, secondary and/or tertiary amine and/or alcohol functions, such as pyridine, pyridazine, pyrimidine, pyrazine, imidazole, pyrazole, triazole, 2,2'-bispyridylamine, tris-(2-pyridylmethyl)amine, 1,4,7-triazacyclononane and its substituted derivatives such as 1,4,7-trimethyl-1,4,7-triazacyclononane, 1,5,9-triazacyclododecane and its substituted derivatives such as 1,5,9-trimethyl-1,5,9-tritri
- the inorganic neutral ligands include in particular ammonia and water. If not all coordination sites of the transition metal central atom are occupied by neutral ligands, the complex contains further, preferably anionic and among these in particular mono- or bidentate ligands. These include in particular the halides such as fluoride, chloride, bromide and iodide, and the (NO 2 ) - group, i.e. a nitro ligand or a nitrito ligand.
- the (NO 2 ) - group can also be bound to a transition metal in a chelate-forming manner, or it can bridge two transition metal atoms asymmetrically or ⁇ 1 -O.
- the transition metal complexes can also carry other, usually simpler ligands, in particular mono- or polyvalent anion ligands.
- examples of possible anions are nitrate, acetate, trifluoroacetate, formate, carbonate, citrate, oxalate, perchlorate and complex anions such as hexafluorophosphate.
- the anion ligands are intended to ensure charge balance between the transition metal central atom and the ligand system.
- the presence of oxo ligands, peroxo ligands and imino ligands is also possible. In particular, such ligands can also have a bridging effect, so that polynuclear complexes are formed.
- both metal atoms in the complex do not have to be the same.
- the use of binuclear complexes in which the two central transition metal atoms have different oxidation numbers is also possible. If anion ligands are missing or the presence of anion ligands does not lead to charge equalization in the complex, anionic counterions are present in the transition metal complex compounds to be used according to the invention, which neutralize the cationic transition metal complex.
- anionic counterions include in particular nitrate, hydroxide, hexafluorophosphate, sulfate, chlorate, perchlorate, the halides such as chloride or the anions of carboxylic acids such as formate, acetate, oxalate, benzoate or citrate.
- transition metal complex compounds are Mn(IV) 2 ( ⁇ -O) 3 (1,4,7-trimethyl-1,4,7-triazacyclononane)-di-hexafluorophosphate, [N,N'-bis[(2-hydroxy-5-vinylphenyl)-methylene]-1,2-diaminocyclohexane]-manganese-(III) chloride, [N,N'-bis[(2-hydroxy-5-nitrophenyl)-methylene]-1,2-diaminocyclohexane]-manganese-(III) acetate, [N,N'-bis[(2-hydroxyphenyl)-methylene]-1,2-phenylenediamine]-manganese-(III) acetate, [N,N'-bis[(2-hydroxyphenyl)-methylene]-1,2-diaminocyclohexane]-manganese-(III) chloride, [N ) chloride,
- Detergents containing peroxygen compounds which can be present in particular as powdered solids, in post-compacted particle form, as homogeneous solutions or suspensions, can in principle contain all known ingredients that are customary in such agents, in addition to the combination of protease and compound according to formula (I) to be used according to the invention and optionally the bleach activators and catalysts mentioned.
- the agents can in particular contain builder substances, surface-active surfactants, water-miscible organic solvents, other enzymes, sequestering agents, electrolytes, pH regulators, polymers with special effects, such as soil release polymers, color transfer inhibitors, graying inhibitors, crease-reducing polymeric active ingredients and shape-retaining polymeric active ingredients, and other auxiliary substances, such as optical brighteners, foam regulators, dyes and fragrances.
- an agent can additionally contain common antimicrobial agents, such as alcohols, aldehydes, acids, carboxylic acid esters, acid amides, phenols and phenol derivatives, diphenyls, diphenylalkanes, urea derivatives, O-acetates and O-formals bound to organic frameworks, benzamidines, isothiazolines, phthalimide derivatives, pyridine derivatives, amines, quaternary ammonium compounds, guanidines, amphoteric compounds, quinolines, benzimidazoles, IPBC, dithiocarbamates, metals and metal compounds, such as silver and silver salts, halogens, such as chlorine, iodine and their Compounds, other oxidizing agents and inorganic nitrogen compounds.
- antimicrobial additives are preferably contained in amounts of up to 10% by weight, in particular from 0.01% by weight to 5% by weight, in each case based on the
- the agents can contain one or more surfactants, whereby anionic surfactants, non-ionic surfactants and mixtures thereof are particularly suitable, but cationic and/or amphoteric surfactants can also be included.
- Suitable non-ionic surfactants are in particular alkyl glycosides and ethoxylation and/or propoxylation products of alkyl glycosides or linear or branched alcohols, each with 12 to 18 C atoms in the alkyl part and 3 to 20, preferably 4 to 10 alkyl ether groups.
- Suitable anionic surfactants are in particular soaps and those that contain sulfate or sulfonate groups with preferably alkali ions as cations.
- Soaps that can be used are preferably the alkali salts of saturated or unsaturated fatty acids with 12 to 18 carbon atoms. Such fatty acids can also be used in a form that is not completely neutralized.
- Useful sulfate-type surfactants include the salts of the sulfuric acid semiesters of fatty alcohols with 12 to 18 carbon atoms and the sulfation products of the nonionic surfactants mentioned with a low degree of ethoxylation.
- the sulfonate-type surfactants that can be used include linear alkylbenzenesulfonates with 9 to 14 C atoms in the alkyl part, alkanesulfonates with 12 to 18 C atoms, as well as olefinsulfonates with 12 to 18 C atoms, which are formed during the reaction of corresponding monoolefins with sulfur trioxide, as well as alpha-sulfofatty acid esters, which are formed during the sulfonation of fatty acid methyl or ethyl esters.
- Such surfactants are contained in detergents in amounts of preferably 5% by weight to 50% by weight, in particular 8% by weight to 30% by weight,
- a detergent preferably contains at least one water-soluble and/or water-insoluble, organic and/or inorganic builder.
- the water-soluble organic builder substances include polycarboxylic acids, in particular citric acid and sugar acids, monomeric and polymeric aminopolycarboxylic acids, in particular glycinediacetic acid, methylglycinediacetic acid, nitrilotriacetic acid, iminodisuccinates such as ethylenediamine-N,N'-disuccinic acid and hydroxyiminodisuccinates, ethylenediaminetetraacetic acid and polyaspartic acid, polyphosphonic acids, in particular aminotris(methylenephosphonic acid), ethylenediaminetetrakis(methylenephosphonic acid), lysinetetra(methylenephosphonic acid) and 1-hydroxyethane-1,1-diphosphonic acid, polymeric hydroxy compounds such as dextrin and polymeric (poly)carboxylic acids, in particular polycar
- the relative average molecular mass (here and hereinafter: weight average) of the homopolymers of unsaturated carboxylic acids is generally between 5,000 g/mol and 200,000 g/mol, that of the copolymers between 2,000 g/mol and 200,000 g/mol, preferably 50,000 g/mol to 120,000 g/mol, in each case based on the free acid.
- a particularly preferred acrylic acid-maleic acid copolymer has a relative average molecular mass of 50,000 to 100,000.
- Suitable, although less preferred, compounds of this class are copolymers of acrylic acid or methacrylic acid with vinyl ethers, such as vinyl methyl ethers, vinyl esters, ethylene, propylene and styrene, in which the proportion of acid is at least 50% by weight.
- vinyl ethers such as vinyl methyl ethers, vinyl esters, ethylene, propylene and styrene
- Terpolymers which contain two unsaturated acids and/or their salts as monomers and vinyl alcohol and/or a vinyl alcohol derivative or a carbohydrate as the third monomer can also be used as water-soluble organic builder substances.
- the first acidic monomer or its salt is derived from a monoethylenically unsaturated C 3 -C 8 carboxylic acid and preferably from a C 3 -C 4 monocarboxylic acid, in particular from (meth)acrylic acid.
- the second acidic monomer or its salt can be a derivative of a C 4 -C 8 dicarboxylic acid, with maleic acid being particularly preferred.
- the third monomeric unit is in this case formed from vinyl alcohol and/or preferably an esterified vinyl alcohol.
- Vinyl alcohol derivatives which are an ester of short-chain carboxylic acids, for example of C 1 -C 4 carboxylic acids, with vinyl alcohol are particularly preferred.
- Preferred polymers contain 60% by weight to 95% by weight, in particular 70% by weight to 90% by weight of (meth)acrylic acid or (meth)acrylate, particularly preferably acrylic acid or acrylate, and maleic acid or maleate, as well as 5% by weight to 40% by weight, preferably 10% by weight to 30% by weight, of vinyl alcohol and/or vinyl acetate.
- the second acidic monomer or its salt can also be a derivative of an allylsulfonic acid which is substituted in the 2-position with an alkyl radical, preferably with a C 1 -C 4 alkyl radical, or an aromatic radical which is preferably derived from benzene or benzene derivatives.
- Preferred terpolymers contain 40% by weight to 60% by weight, in particular 45 to 55% by weight of (meth)acrylic acid or (meth)acrylate, particularly preferably acrylic acid or acrylate, 10% by weight to 30% by weight, preferably 15% by weight to 25% by weight Methallylsulfonic acid or methallylsulfonate and as a third monomer 15 wt.% to 40 wt.%, preferably 20 wt.% to 40 wt.% of a carbohydrate.
- This carbohydrate can be, for example, a mono-, di-, oligo- or polysaccharide, with mono-, di- or oligosaccharides being preferred. Sucrose is particularly preferred.
- the use of the third monomer presumably builds predetermined breaking points into the polymer, which are responsible for the good biodegradability of the polymer.
- These terpolymers generally have a relative average molecular mass of between 1,000 g/mol and 200,000 g/mol, preferably between 200 g/mol and 50,000 g/mol.
- Other preferred copolymers are those which have acrolein and acrylic acid/acrylic acid salts or vinyl acetate as monomers.
- the organic builder substances can be used, particularly for the production of liquid agents, in the form of aqueous solutions, preferably in the form of 30 to 50 percent by weight aqueous solutions. All of the acids mentioned are generally used in the form of their water-soluble salts, particularly their alkali salts.
- Such organic builder substances can, if desired, be present in amounts of up to 40% by weight, in particular up to 25% by weight and preferably from 1% to 8% by weight. Amounts close to the upper limit mentioned are preferably used in paste-like or liquid, in particular water-containing, agents.
- Polyphosphates preferably sodium triphosphate, are particularly suitable as water-soluble inorganic builder materials.
- Crystalline or amorphous, water-dispersible alkali aluminosilicates are particularly used as water-insoluble inorganic builder materials, in amounts of not more than 25% by weight, preferably from 3% by weight to 20% by weight and in particular in amounts of from 5% by weight to 15% by weight.
- crystalline sodium aluminosilicates in detergent quality in particular zeolite A, zeolite P and zeolite MAP and optionally zeolite X, are preferred. Amounts close to the upper limit mentioned are preferably used in solid, particulate agents.
- Suitable aluminosilicates in particular have no particles with a grain size of more than 30 ⁇ m and preferably consist of at least 80% by weight of particles with a size of less than 10 ⁇ m.
- Their calcium binding capacity is generally in the range from 100 to 200 mg CaO per gram.
- water-soluble inorganic builder materials can be included.
- polyphosphates such as sodium triphosphate
- these include in particular the water-soluble crystalline and/or amorphous alkali silicate builders.
- Such water-soluble inorganic builder materials are preferably included in the agents in amounts of 1 wt.% to 20 wt.%, in particular 5 wt.% to 15 wt.%
- the alkali silicates that can be used as builder materials preferably have a molar ratio of alkali oxide to SiO 2 of less than 0.95, in particular of 1:1.1 to 1:12, and can be amorphous or crystalline.
- Preferred alkali silicates are the sodium silicates, in particular the amorphous sodium silicates, with a molar ratio Na 2 O:SiO 2 of 1:2 to 1:2.8.
- Crystalline silicates which can be present alone or in a mixture with amorphous silicates are preferably crystalline layered silicates of the general formula Na 2 Si x O 2x+1 ⁇ y H 2 O, in which x, the so-called modulus, is a number from 1.9 to 4 and y is a number from 0 to 20 and preferred values for x are 2, 3 or 4.
- Preferred crystalline layered silicates are those in which x in the general formula mentioned assumes the values 2 or 3.
- both ⁇ - and ⁇ -sodium disilicates are preferred.
- Virtually anhydrous crystalline alkali silicates of the above general formula, in which x is a number from 1.9 to 2.1, produced from amorphous alkali silicates, can also be used in the agents.
- a crystalline sodium layer silicate with a modulus of 2 to 3 is used, as can be produced from sand and soda.
- Sodium silicates with a modulus in the range of 1.9 to 3.5 are used in a further embodiment.
- a granular compound of alkali silicate and alkali carbonate is used, as is commercially available, for example, under the name Nabion ® 15.
- Enzymes that may be contained in the detergents are particularly those from the class of proteases, cutinases, amylases, pullulanases, xylanases, hemicellulases, cellulases, peroxidases and oxidases or mixtures thereof, with the use of protease, amylase and/or cellulase being particularly preferred.
- the proportion is preferably 0.2% by weight to 1.5% by weight, in particular 0.5% by weight to 1% by weight.
- the enzymes can be adsorbed in the usual way on carriers and/or embedded in coating substances or incorporated as concentrated, preferably water-free liquid formulations.
- Suitable graying inhibitors or soil-release agents are cellulose ethers such as carboxymethylcellulose, methylcellulose, hydroxyalkylcelluloses and cellulose mixed ethers such as methylhydroxyethylcellulose, methylhydroxypropylcellulose and methylcarboxymethylcellulose. Sodium carboxymethylcellulose and mixtures thereof with methylcellulose are preferably used.
- the soil-release agents commonly used include copolyesters containing dicarboxylic acid units, alkylene glycol units and polyalkylene glycol units.
- the proportion of graying inhibitors and/or soil-release agents in the agents is generally not more than 2% by weight and is preferably 0.5% to 1.5% by weight.
- Detergents can contain, for example, derivatives of diaminostilbenedisulfonic acid or its alkali metal salts as optical brighteners, particularly for textiles made of cellulose fibers (for example cotton).
- Suitable examples include salts of 4,4'-bis(2-anilino-4-morpholino-1,3,5-triazin-6-yl-amino)-stilbene-2,2'-disulfonic acid or similarly structured compounds which carry a diethanolamino group, a methylamino group or a 2-methoxyethylamino group instead of the morpholino group.
- Brighteners of the substituted 4,4'-distyryl-diphenyl type may also be present, for example 4,4'-bis-(4-chloro-3-sulfostyryl)-diphenyl. Mixtures of brighteners may also be used.
- Brighteners of the 1,3-diaryl-2-pyrazoline type for example 1-(p-sulfoamoylphenyl)-3-(p-chlorophenyl)-2-pyrazoline, and similarly structured compounds are particularly suitable for polyamide fibers.
- the content of optical brighteners or brightener mixtures in the agent is generally not more than 1% by weight and preferably in the range from 0.05% by weight to 0.5% by weight.
- the usual foam regulators that can be used in detergents include, for example, polysiloxane-silica mixtures, whereby the finely divided silica contained therein is preferably silanized or otherwise hydrophobized.
- the polysiloxanes can consist of linear compounds as well as of cross-linked polysiloxane resins and mixtures thereof.
- Other defoamers are paraffin hydrocarbons, in particular microparaffins and paraffin waxes, whose melting point is above 40 °C, saturated fatty acids or soaps with in particular 20 to 22 C atoms, for example sodium behenate, and alkali salts of phosphoric acid mono- and/or dialkyl esters, in which the alkyl chains each have 12 to 22 C atoms.
- sodium monoalkyl phosphate and/or dialkyl phosphate with C 16 to C 18 alkyl groups is preferably used.
- the proportion of foam regulators can preferably be 0.2% by weight to 2% by weight.
- the agents can contain water as a solvent.
- the organic solvents that can be used in the agents include alcohols with 1 to 4 carbon atoms, especially methanol, ethanol, isopropanol and tert-butanol, diols with 2 to 4 carbon atoms, especially ethylene glycol and propylene glycol, and mixtures thereof and the ethers that can be derived from the above-mentioned classes of compounds.
- Such water-miscible solvents are present in the agents in amounts of preferably not more than 20% by weight, especially from 1% to 15% by weight.
- the agents can contain system- and environmentally-compatible acids, in particular citric acid, acetic acid, tartaric acid, malic acid, lactic acid, glycolic acid, succinic acid, glutaric acid and/or adipic acid, but also mineral acids, in particular sulfuric acid or alkali hydrogen sulfates, or bases, in particular ammonium or alkali hydroxides.
- Such pH regulators are preferably contained in amounts of no more than 10% by weight, in particular from 0.5% by weight to 6% by weight.
- Agents in the form of aqueous solutions or solutions containing other conventional solvents are particularly advantageously prepared by simply mixing the ingredients, which can be added in bulk or as a solution into an automatic mixer.
- the agents are preferably in the form of powdered, granular or tablet-shaped preparations, which can be produced in a manner known per se, for example by mixing, granulating, roller compacting and/or spray drying the thermally resilient components and admixing the more sensitive components, which include in particular enzymes, bleaching agents and bleach-activating agents.
- a process comprising an extrusion step is preferred.
- the preferred procedure is to mix all the components together in a mixer and then compress the mixture using conventional tablet presses, for example eccentric presses or rotary presses, with pressures in the range from 200 ⁇ 10 5 Pa to 1 500 ⁇ 10 5 Pa.
- a tablet produced in this way preferably has a weight of 15 g to 40 g, in particular 20 g to 30 g, and a diameter of 35 mm to 40 mm.
- a protease-free and acylhydrazone-containing powder detergent V1 the protease-containing and acylhydrazone-free detergents V2, V3, V4 and V5, which are otherwise identical to V1
- the protease- and acylhydrazone-containing detergents E1 and E2 with the compositions given in Table 1 below (ingredients in % by weight) were tested at 20 °C in washing tests at a dosage of 67.5 g per 17 l of water in a Miele ® W1935 washing machine.
- agents V6 and V7 were used, which contained other proteases and otherwise corresponded in their composition to agents E1 and E2.
- the proteases were used in the agents with the same activity.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Detergent Compositions (AREA)
Claims (10)
- Utilisation d'une protéase sélectionnée parmia) les protéases comprenant une séquence d'acides aminés qui est identique à raison d'au moins 98,5 % à la séquence d'acides aminés donnée dans SEQ ID NO:1 sur toute la longueur de celle-ci et qui a l'acide aminé V à la position 193 et/ou l'acide aminé L à la position 211 dans le compte selon SEQ ID NO:1,b) les protéases comprenant une séquence d'acides aminés qui est identique à raison d'au moins 70 % à la séquence d'acides aminés donnée dans SEQ ID NO:2 sur toute sa longueur et qui, dans le compte selon SEQ ID NO:2, a l'acide aminé R à la position 9 et/ou l'acide aminé T à la position 15 et/ou l'acide aminé A à la position 66 et/ou l'acide aminé D à la position 212 et/ou l'acide aminé R à la position 239, etc) leurs mélanges,dans laquelle R1 représente un groupe CF3 ou un groupe alkyle en C1-28, un groupe alcényle en C2-28, un groupe alcynyle en C2-22, un groupe cycloalkyle en C3-12, un groupe cycloalcényle en C3-12, un groupe phényle, un groupe naphtyle, un groupe aralkyle en C7-9, un groupe hétéroalkyle en C3-20 ou un groupe cyclohétéroalkyle en C3-12,R2 et R3 représentent, indépendamment l'un de l'autre, l'hydrogène ou un groupe alkyle en C1-28, un groupe alcényle en C2-28, un groupe alcynyle en C2-22, un groupe cycloalkyle en C3-12, un groupe cycloalcényle en C3-12, un groupe aralkyle en C7-9, un groupe hétéroalkyle en C3-28, un groupe cyclohétéroalkyle en C3-12, un groupe hétéroaralkyle en C5-16, un groupe phényle, un groupe naphtyle ou un groupe hétéroaryle éventuellement substitué, ou bien R2 et R3 représentent, avec l'atome de carbone qui les relie, un noyau à 5, 6, 7, 8 ou 9 chaînons éventuellement substitué, qui peut éventuellement contenir des hétéroatomes, etR4 représente l'hydrogène ou un groupe alkyle en C1-28, un groupe alcényle en C2-28, un groupe alcynyle en C2-22, un groupe cycloalkyle en C3-12, un groupe cycloalcényle en C3-12, un groupe aralkyle en C7-9, un groupe hétéroalkyle en C3-20, un groupe cyclohétéroalkyle en C3-12, un groupe hétéroaralkyle en C5-16 ou un groupe phényle ou un groupe naphtyle ou un groupe hétéroaryle éventuellement substitué, pour le nettoyage de textiles ou de surfaces dures en présence de détergents ou de produits de nettoyage contenant de l'oxygène.
- Utilisation selon la revendication 1, caractérisée en ce que la concentration du composé selon la formule (I) dans la liqueur aqueuse est de 0,5 µmol/l à 500 µmol/l, en particulier de 5 µmol/l à 100 µmol/l.
- Utilisation selon la revendication 1 ou 2, caractérisée en ce que la concentration en peroxygène (calculée comme H2O2) dans la liqueur est dans la plage de 0,001 g/l à 10 g/l, en particulier de 0,1 g/l à 1 g/l.
- Agent de lavage ou de nettoyage contenant un agent de blanchiment contenant du peroxygène, une protéase choisie parmia) les protéases comprenant une séquence d'acides aminés qui est identique à raison d'au moins 98,5 % à la séquence d'acides aminés donnée dans SEQ ID NO:1 sur toute la longueur de celle-ci et qui a l'acide aminé V à la position 193 et/ou l'acide aminé L à la position 211 dans le compte selon SEQ ID NO:1,b) les protéases comprenant une séquence d'acides aminés qui est identique à raison d'au moins 70 % à la séquence d'acides aminés donnée dans SEQ ID NO:2 sur toute sa longueur et qui, dans le compte selon SEQ ID NO:2, a l'acide aminé R à la position 9 et/ou l'acide aminé T à la position 15 et/ou l'acide aminé A à la position 66 et/ou l'acide aminé D à la position 212 et/ou l'acide aminé R à la position 239, etc) leurs mélanges,et un composé de formule (I)dans laquelle R1 représente un groupe CF3 ou un groupe alkyle en C1-28, un groupe alcényle en C2-28, un groupe alcynyle en C2-22, un groupe cycloalkyle en C3-12, un groupe cycloalcényle en C3-12, un groupe phényle, un groupe naphtyle, un groupe aralkyle en C7-9, un groupe hétéroalkyle en C3-20 ou un groupe cyclohétéroalkyle en C3-12,R2 et R3 représentent, indépendamment l'un de l'autre, l'hydrogène ou un groupe alkyle en C1-28, un groupe alcényle en C2-28, un groupe alcynyle en C2-22, un groupe cycloalkyle en C3-12, un groupe cycloalcényle en C3-12, un groupe aralkyle en C7-9, un groupe hétéroalkyle en C3-28, un groupe cyclohétéroalkyle en C3-12, un groupe hétéroaralkyle en C5-16, un groupe phényle, un groupe naphtyle ou un groupe hétéroaryle éventuellement substitué, ou R2 et R3 représentent, avec l'atome de carbone qui les relie, un noyau à 5, 6, 7, 8 ou 9 chaînons éventuellement substitué, qui peut éventuellement contenir des hétéroatomes, etR4 représente un atome d'hydrogène ou un groupe alkyle en C1-28, un groupe alcényle en C2-28, un groupe alcynyle en C2-22, un groupe cycloalkyle en C3-12, un groupe cycloalcényle en C3-12, un groupe aralkyle en C7-9, un groupe hétéroalkyle en C3-20, un groupe cyclohétéroalkyle en C3-12, un groupe hétéroaralkyle en C5-16 ou un groupe phényle ou un groupe naphtyle ou un groupe hétéroaryle éventuellement substitué.
- Agent selon la revendication 4, caractérisé en ce qu'il contient 0,001 à 5 % en poids, en particulier 0,05 à 0,15 % en poids du composé de formule (I).
- Agent selon la revendication 4 ou 5, caractérisé en ce qu'il contient une protéase dans des quantités de 0,005 % à 1,0 % en poids, en particulier de 0,01 % à 0,5 % en poids, dans chaque cas par rapport à la protéine active.
- Agent selon l'une quelconque des revendications 4 à 6, caractérisé en ce qu'il contient des composés peroxygénés dans des quantités allant jusqu'à 50 % en poids, en particulier de 2 % à 45 % en poids.
- Agent selon l'une quelconque des revendications 4 à 7, caractérisé en ce qu'il contient des activateurs de blanchiment dans des quantités allant jusqu'à 10 % en poids, en particulier de 1,5 % à 5 % en poids.
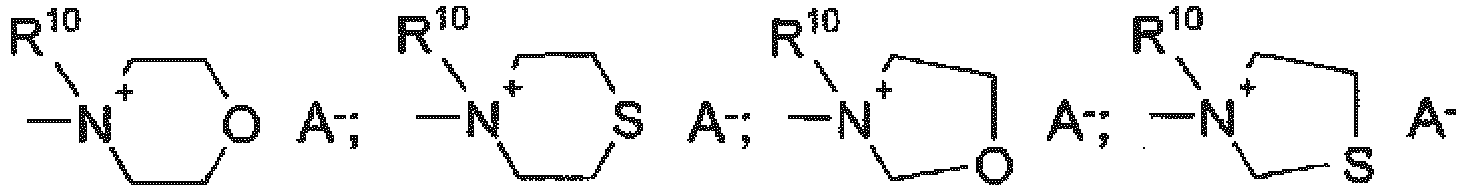
- Utilisation selon l'une quelconque des revendications 1 à 3 ou agent selon l'une quelconque des revendications 4 à 8, caractérisé(e) en ce que le composé de formule générale (I) correspond à la formule générale (II),dans lequel R10 est un hydrogène ou un groupe alkyle en C1-28, un groupe alcényle en C2-28, un groupe alcynyle en C2-22, un groupe cycloalkyle en C3-12, un groupe cycloalcényle en C3-12, un groupe aralkyle en C7-9, un groupe hétéroalkyle en C3-20, un groupe cyclohétéroalkyle en C3-12, un groupe hétéroaralkyle en C5-16 et A- est l'anion d'un acide organique ou inorganique, R2 et R4 sont tels que définis pour la formule (I) etR5, R6, R7 et R8 représentent, indépendamment les uns des autres, R1, un atome d'hydrogène, un atome d'halogène, un groupe hydroxy, un groupe amino, un groupe N-mono ou di-alkyle en C1-4 éventuellement substitué ou un groupe hydroxyalkyl-amino en C2-4, un groupe N-phényl ou un groupe N-naphtyl-amino, un groupe alkyle en C1-28, un groupe alcoxy en C1-28, un groupe phénoxy, un groupe alcényle en C2-28, un groupe alcynyle en C2-22, un groupe cycloalkyle en C3-12, un groupe cycloalcényle en C3-12, un groupe aralkyle en C7-9, un groupe hétéroalkyle en C3-20, un groupe cyclohétéroalkyle en C3-12, un groupe hétéroaralkyle en C5-16, un groupe phényle ou un groupe naphtyle, où les substituants sont choisis parmi un groupe alkyle C1-4, un groupe alcoxy C1-4, un groupe hydroxy, un groupe sulfo, un groupe sulfato, un groupe halogéno, un groupe cyano, un groupe nitro, un groupe carboxy, un groupe phényle, un groupe phénoxy, un groupe naphtoxy, un groupe amino, un groupe N-mono ou di-alkyle en C1-4 ou un groupe hydroxyalkylamino en C2-4, un groupe N-phényle ou N-naphtylamino, ou R5 et R6 ou R6 et R7 ou R7 et R8 sont reliés entre eux pour former 1, 2 ou 3 noyaux carbocycliques ou un noyau O-, un noyau NR10 ou un noyau S-hétérocyclique, éventuellement aromatiques et/ou éventuellement substitués par un alkyle en C1-6.
- Utilisation ou agent selon l'une quelconque des revendications précédentes, caractérisé(e) en ce que la protéase est choisie parmia) les protéases comprenant une séquence d'acides aminés identique à la séquence présentée dans SEQ ID NO:1 sur toute sa longueur, de préférence à raison d'au moins et 99 %,b) les protéases comprenant une séquence d'acides aminés identique à la séquence présentée dans SEQ ID NO:2 sur toute sa longueur, de préférence à raison d'au moins 75 %, 80 %, 81 %, 82 %, 83 %, 84 %, 85 %, 86 %, 87 %, 88 %, 89 %, 90 %, 90,5 %, 91 %, 91,5 %, 92 %, 92,5 %, 93 %, 93,5 %, 94 %, 94,5 %, 95 %, 95,5 %, 96 %, 96,5 %, 97 %, 97,5 %, 98 %, 98,5 % et 99 %, etc) leurs mélanges.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PL15720976.8T PL3143115T5 (pl) | 2014-05-15 | 2015-05-05 | Środek piorący i czyszczący o zwiększonej mocy wybielającej |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE102014209241.8A DE102014209241A1 (de) | 2014-05-15 | 2014-05-15 | Wasch- und Reinigungsmittel mit erhöhter Bleichleistung |
| PCT/EP2015/059766 WO2015173055A1 (fr) | 2014-05-15 | 2015-05-05 | Produits de lavage et de nettoyage à blanchiment amélioré |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP3143115A1 EP3143115A1 (fr) | 2017-03-22 |
| EP3143115B1 EP3143115B1 (fr) | 2020-02-19 |
| EP3143115B2 true EP3143115B2 (fr) | 2024-08-14 |
Family
ID=53055045
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP15720976.8A Active EP3143115B2 (fr) | 2014-05-15 | 2015-05-05 | Agent de lavage et de nettoyage avec des performances de blanchiment amélioré |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP3143115B2 (fr) |
| DE (1) | DE102014209241A1 (fr) |
| PL (1) | PL3143115T5 (fr) |
| WO (1) | WO2015173055A1 (fr) |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102014220663A1 (de) * | 2014-10-13 | 2016-04-14 | Henkel Ag & Co. Kgaa | Farbschützende Waschmittel |
| DE102015210416A1 (de) * | 2015-06-08 | 2016-12-08 | Henkel Ag & Co. Kgaa | Acylhydrazone als Enzymstabilisatoren |
| DE102015225874A1 (de) * | 2015-12-18 | 2017-06-22 | Henkel Ag & Co. Kgaa | Flüssige Wasch- oder Reinigungsmittel enthaltend Acylhydrazon |
| DE102015225881A1 (de) * | 2015-12-18 | 2017-06-22 | Henkel Ag & Co. Kgaa | Flüssige Wasch- oder Reinigungsmittel enthaltend Acylhydrazon und Reduktionsmittel |
| EP3249032A1 (fr) * | 2016-05-26 | 2017-11-29 | The Procter and Gamble Company | Composition détergente comprenant un catalyseur de blanchiment et une protéase |
Family Cites Families (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1991002792A1 (fr) | 1989-08-25 | 1991-03-07 | Henkel Research Corporation | Enzyme proteolytique alcaline et procede de production |
| KR100787392B1 (ko) | 1999-03-31 | 2007-12-21 | 노보자임스 에이/에스 | 알칼리 α-아밀라제 활성을 가지는 폴리펩티드 및 그것을코드하는 핵산 |
| DE10131441A1 (de) | 2001-06-29 | 2003-01-30 | Henkel Kgaa | Eine neue Gruppe von alpha-Amylasen sowie ein Verfahren zur Identifizierung und Gewinnung neuer alpha-Amylasen |
| DE10163748A1 (de) | 2001-12-21 | 2003-07-17 | Henkel Kgaa | Neue Glykosylhydrolasen |
| DE102006038448A1 (de) | 2005-12-28 | 2008-02-21 | Henkel Kgaa | Enzym-haltiges Reinigungsmittel |
| DE102006022224A1 (de) | 2006-05-11 | 2007-11-15 | Henkel Kgaa | Subtilisin aus Bacillus pumilus und Wasch- und Reinigungsmittel enthaltend dieses neue Subtilisin |
| DE102007003143A1 (de) | 2007-01-16 | 2008-07-17 | Henkel Kgaa | Neue Alkalische Protease aus Bacillus gibsonii und Wasch- und Reinigungsmittel enthaltend diese neue Alkalische Protease |
| US8492324B2 (en) | 2008-04-09 | 2013-07-23 | Basf Se | Use of metal hydrazide complex compounds as oxidation catalysts |
| RU2651525C2 (ru) | 2009-09-25 | 2018-04-19 | Новозимс А/С | Варианты субтилаз |
| EP2395071A1 (fr) | 2010-06-10 | 2011-12-14 | The Procter & Gamble Company | Composition détergente solide comprenant une lipase d'origine bactérienne |
| CN103328455B (zh) | 2010-12-13 | 2016-02-24 | 巴斯夫欧洲公司 | 漂白催化剂 |
| DE102011076417A1 (de) * | 2011-05-24 | 2012-11-29 | Henkel Ag & Co. Kgaa | Aktivatorsysteme für Persauerstoffverbindungen |
| DE102011118037A1 (de) * | 2011-06-16 | 2012-12-20 | Henkel Ag & Co. Kgaa | Geschirrspülmittel mit Bleichkatalysator und Protease |
| DE102012200333A1 (de) | 2012-01-11 | 2013-07-11 | Henkel Ag & Co. Kgaa | Acylhydrazone als bleichverstärkende Wirkstoffe |
-
2014
- 2014-05-15 DE DE102014209241.8A patent/DE102014209241A1/de active Pending
-
2015
- 2015-05-05 WO PCT/EP2015/059766 patent/WO2015173055A1/fr not_active Ceased
- 2015-05-05 EP EP15720976.8A patent/EP3143115B2/fr active Active
- 2015-05-05 PL PL15720976.8T patent/PL3143115T5/pl unknown
Also Published As
| Publication number | Publication date |
|---|---|
| EP3143115A1 (fr) | 2017-03-22 |
| PL3143115T3 (pl) | 2020-08-10 |
| DE102014209241A1 (de) | 2015-11-19 |
| WO2015173055A1 (fr) | 2015-11-19 |
| EP3143115B1 (fr) | 2020-02-19 |
| PL3143115T5 (pl) | 2024-11-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2912156B1 (fr) | Utilisation d'un acyl hydrazone dans les compositions détergentes | |
| EP2714877B1 (fr) | Systèmes activateurs pour composés peroxygénés | |
| EP3143115B2 (fr) | Agent de lavage et de nettoyage avec des performances de blanchiment amélioré | |
| DE102014221889B4 (de) | Waschmittel mit Mannosylerythritollipid, Verstärkung der Reinigungsleistung von Waschmitteln durch Mannosylerythritollipid, und Waschverfahren unter Einsatz von Mannosylerythritollipid | |
| EP3030642A1 (fr) | Détergent présentant une puissance de lavage primaire augmentée | |
| EP3406698A1 (fr) | Composition de blanchiment pour lavage ou nettoyage comprenant un précurseur d'oxaziridine | |
| EP3152289A1 (fr) | Détergents contenant au moins une laccase en tant qu'inhibiteur de transfert de couleur | |
| DE102014218507A1 (de) | Spinnenseidenproteine als Enzymstabilisatoren | |
| EP3303572B1 (fr) | Détergent contenant au moins une laccase, dont la performance de lavage est améliorée | |
| EP4061918B1 (fr) | Activateur de blanchiment ayant un groupe cationique et produit détergent ou nettoyant le contenant | |
| DE102012219405A1 (de) | Katalytische Verstärkung der Bleichwirkung von Persauerstoffverbindungen | |
| EP3095847B1 (fr) | Produit de lavage et de nettoyage a hygiene amelioree | |
| DE102019217852A1 (de) | Bleichaktivator mit kationischer Gruppe und diesen enthaltendes Wasch- oder Reinigungsmittel IV | |
| DE102015210368A1 (de) | Waschmittel mit verbesserter Waschleistung, enthaltend mindestens eine Laccase | |
| EP3409757B1 (fr) | Composition detergente blanchissante | |
| EP2727987B1 (fr) | Polymères pour équipement antimicrobien | |
| DE102015225882A1 (de) | Partikuläres Mittel zur Verstärkung der Bleichwirkung | |
| EP3075835A2 (fr) | Lessive particulaire comprenant un catalyseur de blanchiment | |
| EP3409756A1 (fr) | Composition detergente blanchissante | |
| DE102019217850A1 (de) | Bleichaktivator mit kationischer Gruppe und diesen enthaltendes Wasch- oder Reinigungsmittel II | |
| DE102015210369A1 (de) | Waschmittel mit verbesserter Waschleistung, enthaltend mindestens eine Laccase | |
| DE102015210367A1 (de) | Waschmittel mit verbesserter Waschleistung, enthaltend mindestens eine Laccase | |
| DE102015210370A1 (de) | Waschmittel mit verbesserter Waschleistung, enthaltend mindestens eine Laccase |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE INTERNATIONAL PUBLICATION HAS BEEN MADE |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20161109 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| DAV | Request for validation of the european patent (deleted) | ||
| DAX | Request for extension of the european patent (deleted) | ||
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20190108 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20191008 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 502015011773 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 1234972 Country of ref document: AT Kind code of ref document: T Effective date: 20200315 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: GERMAN |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20200219 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200519 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200519 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200619 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200520 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200712 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R026 Ref document number: 502015011773 Country of ref document: DE |
|
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| PLAX | Notice of opposition and request to file observation + time limit sent |
Free format text: ORIGINAL CODE: EPIDOSNOBS2 |
|
| 26 | Opposition filed |
Opponent name: NOVOZYMES A/S Effective date: 20201117 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200531 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200531 Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20200531 |
|
| PLBB | Reply of patent proprietor to notice(s) of opposition received |
Free format text: ORIGINAL CODE: EPIDOSNOBS3 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20200519 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200505 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200505 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200519 Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200531 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200531 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MM01 Ref document number: 1234972 Country of ref document: AT Kind code of ref document: T Effective date: 20200505 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200505 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20200219 |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230530 |
|
| PUAH | Patent maintained in amended form |
Free format text: ORIGINAL CODE: 0009272 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: PATENT MAINTAINED AS AMENDED |
|
| 27A | Patent maintained in amended form |
Effective date: 20240814 |
|
| AK | Designated contracting states |
Kind code of ref document: B2 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R102 Ref document number: 502015011773 Country of ref document: DE |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: PL Payment date: 20250425 Year of fee payment: 11 Ref country code: DE Payment date: 20250521 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20250527 Year of fee payment: 11 |