EP2769321B1 - Verfahren zur verbesserung der diagnose einer entzündlichen darmerkrankung - Google Patents
Verfahren zur verbesserung der diagnose einer entzündlichen darmerkrankung Download PDFInfo
- Publication number
- EP2769321B1 EP2769321B1 EP12841790.4A EP12841790A EP2769321B1 EP 2769321 B1 EP2769321 B1 EP 2769321B1 EP 12841790 A EP12841790 A EP 12841790A EP 2769321 B1 EP2769321 B1 EP 2769321B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- sample
- ibd
- antibody
- markers
- disease
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
- G01N33/54306—Solid-phase reaction mechanisms
-
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16B—BIOINFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR GENETIC OR PROTEIN-RELATED DATA PROCESSING IN COMPUTATIONAL MOLECULAR BIOLOGY
- G16B20/00—ICT specially adapted for functional genomics or proteomics, e.g. genotype-phenotype associations
- G16B20/20—Allele or variant detection, e.g. single nucleotide polymorphism [SNP] detection
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6883—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6854—Immunoglobulins
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6893—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids related to diseases not provided for elsewhere
-
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16B—BIOINFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR GENETIC OR PROTEIN-RELATED DATA PROCESSING IN COMPUTATIONAL MOLECULAR BIOLOGY
- G16B20/00—ICT specially adapted for functional genomics or proteomics, e.g. genotype-phenotype associations
- G16B20/40—Population genetics; Linkage disequilibrium
-
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16B—BIOINFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR GENETIC OR PROTEIN-RELATED DATA PROCESSING IN COMPUTATIONAL MOLECULAR BIOLOGY
- G16B40/00—ICT specially adapted for biostatistics; ICT specially adapted for bioinformatics-related machine learning or data mining, e.g. knowledge discovery or pattern finding
-
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16H—HEALTHCARE INFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR THE HANDLING OR PROCESSING OF MEDICAL OR HEALTHCARE DATA
- G16H50/00—ICT specially adapted for medical diagnosis, medical simulation or medical data mining; ICT specially adapted for detecting, monitoring or modelling epidemics or pandemics
- G16H50/30—ICT specially adapted for medical diagnosis, medical simulation or medical data mining; ICT specially adapted for detecting, monitoring or modelling epidemics or pandemics for calculating health indices; for individual health risk assessment
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/112—Disease subtyping, staging or classification
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/156—Polymorphic or mutational markers
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/158—Expression markers
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/46—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans from vertebrates
- G01N2333/47—Assays involving proteins of known structure or function as defined in the subgroups
- G01N2333/4701—Details
- G01N2333/4737—C-reactive protein
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/475—Assays involving growth factors
- G01N2333/49—Platelet-derived growth factor [PDGF]
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/705—Assays involving receptors, cell surface antigens or cell surface determinants
- G01N2333/70503—Immunoglobulin superfamily, e.g. VCAMs, PECAM, LFA-3
- G01N2333/70525—ICAM molecules, e.g. CD50, CD54, CD102
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/705—Assays involving receptors, cell surface antigens or cell surface determinants
- G01N2333/70503—Immunoglobulin superfamily, e.g. VCAMs, PECAM, LFA-3
- G01N2333/70542—CD106
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/775—Apolipopeptides
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/06—Gastro-intestinal diseases
- G01N2800/065—Bowel diseases, e.g. Crohn, ulcerative colitis, IBS
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/60—Complex ways of combining multiple protein biomarkers for diagnosis
-
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16B—BIOINFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR GENETIC OR PROTEIN-RELATED DATA PROCESSING IN COMPUTATIONAL MOLECULAR BIOLOGY
- G16B20/00—ICT specially adapted for functional genomics or proteomics, e.g. genotype-phenotype associations
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A90/00—Technologies having an indirect contribution to adaptation to climate change
- Y02A90/10—Information and communication technologies [ICT] supporting adaptation to climate change, e.g. for weather forecasting or climate simulation
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Physics & Mathematics (AREA)
- Molecular Biology (AREA)
- Immunology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- General Health & Medical Sciences (AREA)
- Analytical Chemistry (AREA)
- Biotechnology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biomedical Technology (AREA)
- Genetics & Genomics (AREA)
- Medical Informatics (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Pathology (AREA)
- Biophysics (AREA)
- Organic Chemistry (AREA)
- Microbiology (AREA)
- Biochemistry (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Evolutionary Biology (AREA)
- Bioinformatics & Computational Biology (AREA)
- Public Health (AREA)
- Theoretical Computer Science (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Medicinal Chemistry (AREA)
- Cell Biology (AREA)
- Food Science & Technology (AREA)
- General Physics & Mathematics (AREA)
- Data Mining & Analysis (AREA)
- Databases & Information Systems (AREA)
- Epidemiology (AREA)
- General Engineering & Computer Science (AREA)
- Ecology (AREA)
- Primary Health Care (AREA)
- Physiology (AREA)
Claims (14)
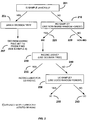
- Verfahren zum Diagnostizieren entzündlicher Darmerkrankung (IBD) und/oder eines klinischen Untertyps davon in einem Individuum, wobei das Verfahren Folgendes umfasst:(a) Analysieren einer von dem Individuum erhaltenen Probe, um Anwesenheit oder Niveau oder Genotyp von mindestens jedem der folgenden Marker zu bestimmen, um ein Markerprofil zu erhalten: (i) Anwesenheit oder Niveau von jedem der serologischen Marker ASCA-A, ASCA-G, ANCA, pANCA, Anti-OmpC-Antikörper, Anti-CBirl-Antikörper, Anti-FlaX-Antikörper, und Anti-A4-Fla2-Antikörper; (ii) Anwesenheit oder Niveau von jedem der Entzündungsmarker VEGF, ICAM, VCAM, SAA, und CRP; und (iii) Genotyp von jedem der genetischen Marker ATG16L1, ECMI, NKX2-3, und STAT3;(b) Anwenden einer ersten statistischen Stichprobenanalyse auf das Markerprofil, um eine erste Modellpunktzahl zu berechnen und die erste Modellpunktzahl mit einem ersten Grenzwert zu vergleichen, um eine Entscheidung zu erhalten, ob die Probe eine IBD-Probe oder eine Nicht-IBD-Probe ist;(c) wenn die Probe eine IBD-Probe ist, Anwenden eines Entscheidungsbaumes auf die IBD-Probe, um zu bestimmen, ob die IBD-Probe eine oder keine nicht eindeutige Probe ist,
wobei die IBD-Probe eine nicht eindeutige Probe ist, wenn die IBD-Probe ein ANCA-Niveau größer als eine Quartilpunktzahl von 3 (> Q3) aufweist, pANCA2-positiv ist, und ein Niveau von Anti-CBirl-Antikörpern oder Anti-A4-Fla2-Antikörpern oder Anti-FlaX-Antikörpern > Q3 aufweist, oder
wobei die IBD-Probe eine nicht eindeutige Probe ist, wenn die IBD-Probe pANCA2-positiv ist und zwei von drei Markern, ausgewählt aus Anti-CBirl-Antikörpern, Anti-A4-Fla2-Antikörpern, und Anti-FlaX-Antikörpern > Q3, exprimiert, und
wobei die Anwesenheit oder das Niveau von pANCA zur der Bestimmung des Wertes von pANCA2 verwendet wird; und(d) wenn die IBD-Probe keine nicht eindeutige Probe ist, Anwenden einer zweiten statistischen Stichprobenanalyse auf die IBD-Probe, um eine zweite Modellpunktzahl zu berechnen und die zweite Modellpunktzahl mit einem zweiten Grenzwert zu vergleichen, um einen klinischen Untertyp von IBDi zu bestimmen,wobei die zweite statistische Stichprobenanalyse (i) auf der Anwesenheit oder dem Niveau von ASCA-A, ASCA-G, ANCA, pANCA, Anti-OmpC-Antikörpern, Anti-CBirl-Antikörpern, Anti-FlaX-Antikörpern, Anti-A4-Fla2-Antikörpern und VEGF in der Probe und (ii) des Genotyps von ECMI und STAT3 in der Probe basiert. - Verfahren nach Anspruch 1, wobei die Anwesenheit oder das Niveau jedes der serologischen Marker oder Entzündungsmarker unabhängig voneinander durch ein Hybridisierungsassay, verstärkungsbasiertes Assay, Immunoassay, oder immunohistochemisches Assay bestimmt werden.
- Verfahren nach Anspruch 1, wobei der Genotyp eines jeden der genetischen Marker unabhängig voneinander durch Genotypisieren für die Anwesenheit oder Abwesenheit eines Einzel-Nucleotid-Polymorphismus (SNP) in jedem der genetischen Marker bestimmt wird.
- Verfahren nach Anspruch 3, wobei der SNP rs2241880 für ATG16L1, rs3737240 für ECMI, rs10883365 für NKX2-3, und/oder rs744166 für STAT3 ist.
- Verfahren nach Anspruch 1, wobei der klinische Untertyp von IBD Morbus Crohn (CD) oder Colitis ulcerosa (UC) ist.
- Verfahren nach Anspruch 1, wobei die Probe ausgewählt ist aus der Gruppe bestehend aus Serum, Plasma, Vollblut, und Stuhl.
- Verfahren nach Anspruch 1, wobei- wenn pANCA-Werte 0 oder +1 sind, der pANCA2-Wert negativ ist; oder- wenn pANCA-Werte +2, +3 oder +4 sind, der pANCA2-Wert positiv ist.
- Verfahren nach Anspruch 7, wobei in der pANCA2-negativen Probe, weiterhin umfassend:(a) Bestimmen, ob die pANCA2-negative Probe Morbus Crohn direkt durch Messen eines Serummarkerpanels vorhersagt, wobei das Serummarkerpanel ein Teil der Gruppe bestehend aus ASCA-IgA, ASCA-IgG, und OmpC-IgA ist und Vergleichen eines jeden der Serummarker des Serummarkerpanels mit einem Grenzwert, um zu bestimmen, ob die Probe mit Morbus Crohn konsistent ist; oder(b) Bestimmen, ob die pANCA2-negative Probe mit Morbus Crohn konsistent ist durch Messen eines CD-Zählpanels, wobei das CD-Zählpanel ein Teil der Gruppe bestehend aus ASCA-IgA, ASCA-IgG, OmpC-IgA, CBirl-IgG, A4-Fla2-IgG und FlaX-IgG zum Bilden eines CD-Zählwerts ist, wobei, wenn der CD-Zählwert größer als oder gleich 2 ist, die Probe mit Morbus Crohn konsistent ist; oder(c) Bestimmen, ob die pANCA2-negative Probe, die einen pANCA-Wert von null aufweist, mit IBD konsistent oder inkonsistent ist, durch Messen von ANCA ELISA und Vergleichen des ANCA-ELISA-Werts mit einem Referenzwert unter Berücksichtigung, dass der CD-Zählwert bestimmt, ob die Probe mit IBD konsistent oder inkonsistent ist; oder(d) Bestimmen, ob die pANCA2-negative Probe, die einen pANCA-Wert von null aufweist, mit IBD konsistent oder für IBD nicht eindeutig ist, durch Messen von ANCA ELISA und Vergleichen des ANCA-ELISA-Werts unter Berücksichtigung, dass der CD-Zählwert bestimmt, ob die Probe mit IBD konsistent oder inkonsistent oder mit IBD konsistent und nicht eindeutig für Morbus Crohn oder Colitis ulcerosa ist; oder(e) Bestimmen, ob die pANCA2-negative Probe, die einen pANCA-Wert von eins aufweist, mit IBD konsistent oder inkonsistent ist durch Messen von ANCA ELISA und Vergleichen des ANCA-ELISA-Werts mit einem Grenzwert, um zu bestimmen, ob die Probe mit IBD konsistent oder inkonsistent ist.
- Verfahren nach Anspruch 8, wobei in Abschnitt (a) vorstehend, wenn der Wert für- ASCA-IgA größer oder gleich 69 EU/mL ist; oder- ASCA-IgG größer oder gleich 40 EU/mL ist; oder- OmpC-IgA größer oder gleich 60 EU/mL ist;die Probe als konsistent mit Morbus Crohn bezeichnet wird.
- Verfahren nach Anspruch 8, wobei in Abschnitt (b) vorstehend, weiterhin umfassend das Zuweisen des CD-Zählwerts, durch Bestimmen, obi. ASCA-IgA größer oder gleich 8,5 EU/mL ist, dann Addieren von +1 zu dem CD-Zählwert;ii. ASCA-IgG größer oder gleich 17,8 EU/mL ist, dann Addieren von +1 zu dem CD-Zählwert;iii. OmpC-IgA größer oder gleich 10,9 EU/mL ist, dann Addieren von +1 zu dem CD-Zählwert;iv. 2 oder mehr Flagellin-Marker über ihren jeweiligen Referenzbereichen liegen, Addieren von +1 zu dem CD-Zählwert;v. jeder Flagellin größer oder gleich 100 EU/mL ist, dann Addieren von +1 zu dem CD-Zählwert;und wenn der Gesamt-CD-Zählwert größer oder gleich 2 ist, dann Bezeichnen der Probe als konsistent mit Morbus Crohn.
- Verfahren nach Anspruch 10, wobei der Referenzwert für- CBirl-IgG größer oder gleich 78,4 EU/mL ist; oder- A4-Fla2-IgG größer oder gleich 44,8 EU/mL ist; oder- FlaX-IgG größer oder gleich 33,4 EU/mL ist.
- Verfahren nach Anspruch 8, wobei in Abschnitt (c) vorstehend, wenn pANCA null ist (nicht nachgewiesen), weiterhin umfassend:i. Bezeichnen der Probe als mit IBD inkonsistent, wenn ANCA ELISA geringer als 20 EU/mL ist; oderii. Bezeichnen der Probe als konsistent mit Colitis ulcerosa, wenn ANCA ELISA größer als 27,4 EU/mL ist; oderiii. Bezeichnen der Probe als mit IBD inkonsistent, wenn ANCA ELISA größer oder gleich 20 und geringer oder gleich 27,4 EU/mL ist und der CD-Zählwert null ist; oderiv. Bezeichnen der Probe als mit IBD konsistent, jedoch als nicht eindeutig für Morbus Crohn oder Colitis ulcerosa, wenn ANCA ELISA größer oder gleich 20 und geringer oder gleich 27,4 EU/mL ist und der CD-Zählwert eins ist.
- Verfahren nach Anspruch 8, wobei, wenn pANCA eins ist, weiterhin umfassend:i. Bezeichnen der Probe als mit IBD inkonsistent, wenn ANCA ELISA geringer als 13,7 EU/mL ist; oderii. Bezeichnen der Probe als vereinbar mit Colitis ulcerosa, wenn ANCA ELISA größer als der gleich 13,7 EU/mL ist; oder
- Verfahren zum Diagnostizieren entzündlicher Darmerkrankung (IBD) und/oder eines klinischen Untertyps davon in einem Individuum, wobei das Verfahren Folgendes umfasst:(a) Analysieren einer von dem Individuum erhaltenen Probe, um Anwesenheit oder Niveau oder Genotyp von mindestens jedem der folgenden Marker zu bestimmen, um ein Markerprofil zu erhalten: (i) Die Anwesenheit oder das Niveau von jedem der serologischen Marker ASCA-A, ASCA-G, ANCA, pANCA, Anti-OmpC-Antikörper, Anti-CBirl-Antikörper, Anti-FlaX-Antikörper, und Anti-A4-Fla2-Antikörper; (ii) Anwesenheit oder Niveau von jedem der Entzündungsmarker VEGF, ICAM, VCAM, SAA, und CRP; und (iii) des Genotyps von jedem der genetischen Marker ATG16L1, ECMI, NKX2-3, und STAT3;(b) Anwenden einer ersten statistischen Stichprobenanalyse auf das Markerprofil, um zu bestimmen, ob diese Probe eine IBD-Probe oder eine Nicht-IBD-Probe ist mit einer Empfindlichkeit von mindestens 70 %;(c) wenn die Probe eine IBD-Probe ist, Anwenden eines Entscheidungsbaumes auf die IBD-Probe, um zu bestimmen, ob die IBD-Probe eine oder keine nicht eindeutige Probe ist,
wobei die IBD-Probe eine nicht eindeutige Probe ist, wenn die IBD-Probe ein ANCA-Niveau größer als eine Quartilpunktzahl von 3 (> Q3) aufweist, pANCA2-positiv ist, und ein Niveau von Anti-CBirl-Antikörper oder Anti-A4-Fla2-Antikörper oder Anti-FlaX-Antikörper > Q3 aufweist, oder
wobei die IBD-Probe eine nicht eindeutige Probe ist, wenn die IBD-Probe pANCA2-positiv ist und zwei von drei Markern, ausgewählt aus Anti-CBirl-Antikörper, Anti-A4-Fla2-Antikörper, und Anti-FlaX-Antikörper > Q3, exprimiert, und
wobei die Anwesenheit oder das Niveau von pANCA zu der Bestimmung des Wertes von pANCA2 verwendet wird; und(d) wenn die IBD-Probe keine nicht eindeutige Probe ist, dann Anwenden einer zweiten statistischen Stichprobenanalyse auf die IBD-Probe zum Bestimmen eines klinischen Untertyps von IBD mit einer Empfindlichkeit von mindestens 85 % für Morbus Crohn (CD) und einer Empfindlichkeit von mindestens 95 % für Colitis ulcerosa (UC),wobei die zweite statistische Stichprobenanalyse (i) auf der Anwesenheit oder dem Niveau von ASCA-A, ASCA-G, ANCA, pANCA, Anti-OmpC-Antikörper, Anti-CBirl-Antikörper, Anti-FlaX-Antikörper, Anti-A4-Fla2-Antikörper und VEGF in der Probe basiert und (ii) des Genotyps von ECMI und STAT3 in der Probe.
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161550293P | 2011-10-21 | 2011-10-21 | |
| US201161553853P | 2011-10-31 | 2011-10-31 | |
| US201161567096P | 2011-12-05 | 2011-12-05 | |
| US201161570271P | 2011-12-13 | 2011-12-13 | |
| PCT/US2012/061202 WO2013059732A1 (en) | 2011-10-21 | 2012-10-19 | Methods for improving inflammatory bowel disease diagnosis |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP2769321A1 EP2769321A1 (de) | 2014-08-27 |
| EP2769321A4 EP2769321A4 (de) | 2015-05-13 |
| EP2769321B1 true EP2769321B1 (de) | 2016-06-01 |
Family
ID=48141435
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP12841790.4A Active EP2769321B1 (de) | 2011-10-21 | 2012-10-19 | Verfahren zur verbesserung der diagnose einer entzündlichen darmerkrankung |
Country Status (10)
| Country | Link |
|---|---|
| US (2) | US8715943B2 (de) |
| EP (1) | EP2769321B1 (de) |
| JP (1) | JP2015502740A (de) |
| AU (1) | AU2012325798B2 (de) |
| CA (1) | CA2852954A1 (de) |
| HK (1) | HK1200933A1 (de) |
| IL (1) | IL232167A0 (de) |
| MX (1) | MX352274B (de) |
| SG (1) | SG11201401536QA (de) |
| WO (1) | WO2013059732A1 (de) |
Families Citing this family (44)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010062960A2 (en) | 2008-11-26 | 2010-06-03 | Cedars-Sinai Medical Center | METHODS OF DETERMINING RESPONSIVENESS TO ANTI-TNFα THERAPY IN INFLAMMATORY BOWEL DISEASE |
| NZ595471A (en) | 2009-04-14 | 2014-01-31 | Nestec Sa | Inflammatory bowel disease prognostics |
| WO2015067913A1 (en) | 2013-11-07 | 2015-05-14 | Diagnodus Limited | Biomarkers |
| SG194710A1 (en) | 2011-05-10 | 2013-12-30 | Nestec Sa | Methods of disease activity profiling for personalized therapy management |
| CN105246501B (zh) | 2013-03-27 | 2021-01-12 | 雪松-西奈医学中心 | 通过抑制tl1a的功能和相关信号传导途径来减轻并逆转纤维化和炎症 |
| WO2014182689A1 (en) * | 2013-05-06 | 2014-11-13 | Yacyshyn Bruce R | Method of using biomarkers in predicting inflammatory bowel disease |
| BR112015029320A8 (pt) * | 2013-05-24 | 2023-01-03 | Nestec Sa | Ensaios específicos de vias para predição de diagnóstico de síndrome do intestino irritável |
| EP3022295A4 (de) | 2013-07-19 | 2017-03-01 | Cedars-Sinai Medical Center | Signatur des tl1a (tnfsf15)-signalweges |
| US11568982B1 (en) | 2014-02-17 | 2023-01-31 | Health at Scale Corporation | System to improve the logistics of clinical care by selectively matching patients to providers |
| EP3167392A4 (de) * | 2014-07-11 | 2018-08-01 | Matatu Inc. | Verwendung von darmmikrobiom als prädiktor des wachstums oder der gesundheit von tieren |
| CN104131102B (zh) * | 2014-08-07 | 2016-06-15 | 马飞 | 一种判断nsclc患者对吉非替尼治疗反应性的试剂盒 |
| US11809501B2 (en) * | 2014-08-28 | 2023-11-07 | Ebay Inc. | Systems, apparatuses, and methods for providing a ranking based recommendation |
| WO2016088068A1 (en) * | 2014-12-02 | 2016-06-09 | Nestec S.A. | Methods for establishing a vedolizumab dosing regimen to treat patients with irritable bowel disease |
| CN107548498A (zh) | 2015-01-20 | 2018-01-05 | 南托米克斯有限责任公司 | 用于反应预测高级别膀胱癌中的化疗的系统和方法 |
| CA2978708A1 (en) * | 2015-03-03 | 2016-09-09 | Nantomics, Llc | Ensemble-based research recommendation systems and methods |
| US11037070B2 (en) * | 2015-04-29 | 2021-06-15 | Siemens Healthcare Gmbh | Diagnostic test planning using machine learning techniques |
| US11946927B2 (en) | 2016-03-14 | 2024-04-02 | Musidora Biotechnology Llc | Process and system for identifying individuals having a high risk of inflammatory bowel disease and a method of treatment |
| US10295527B2 (en) | 2016-03-14 | 2019-05-21 | Bruce Yacyshyn | Process and system for predicting responders and non-responders to mesalamine treatment of ulcerative colitis |
| EP3430172A4 (de) | 2016-03-17 | 2019-08-21 | Cedars-Sinai Medical Center | Verfahren zur diagnose einer entzündlichen darmerkrankung durch rnaset2 |
| KR20230003433A (ko) * | 2016-05-20 | 2023-01-05 | 세다르스-신나이 메디칼 센터 | 유전자에 기반한 염증성 장 질환의 진단 |
| JP7111322B2 (ja) * | 2016-12-02 | 2022-08-02 | 国立大学法人 東京大学 | 線維化関連分子に対する抗体およびその医療応用 |
| EP3554362A4 (de) * | 2016-12-14 | 2020-08-12 | Warren, Tracy | System und verfahren zur entwicklung und verwendung einer mikrobiombasierten aktionskomponente für die patientengesundheit |
| US11978543B2 (en) | 2016-12-14 | 2024-05-07 | Astarte Medical Partners Inc. | System and methods for developing and using a microbiome-based action component |
| US10409734B1 (en) * | 2017-03-27 | 2019-09-10 | Symantec Corporation | Systems and methods for controlling auxiliary device access to computing devices based on device functionality descriptors |
| CN108784666B (zh) * | 2017-05-04 | 2023-01-13 | 深圳市生码医疗科技有限公司 | 连续监测心血管的精准医疗系统及数据处理方法 |
| JP2020523560A (ja) * | 2017-05-31 | 2020-08-06 | プロメテウス バイオサイエンシーズ インコーポレイテッド | クローン病患者の粘膜治癒を評価する方法 |
| US11244454B2 (en) * | 2018-04-03 | 2022-02-08 | Boston Scientific Scimed, Inc. | Systems and methods for diagnosing and/or monitoring disease |
| EP3818544A1 (de) * | 2018-07-05 | 2021-05-12 | Boston Scientific Scimed, Inc. | Vorrichtungen, systeme und verfahren zur bestimmung von entzündungen und/oder fibrose |
| WO2020041287A1 (en) * | 2018-08-21 | 2020-02-27 | Yacyshyn Bruce R | Process and system for identifying individuals having a high risk of inflammatory bowel disease and a method of treatment |
| CA3121167A1 (en) * | 2018-11-29 | 2020-06-04 | Cedars-Sinai Medical Center | Methods of stratifying and treating a sub-population of inflammatory bowel disease patients |
| WO2020117795A1 (en) * | 2018-12-04 | 2020-06-11 | Prometheus Biosciences, Inc. | Assessment and monitoring of mucosal healing in children and adults with crohn's disease |
| EP3935581A4 (de) | 2019-03-04 | 2022-11-30 | Iocurrents, Inc. | Datenkompression und -kommunikation mit maschinenlernung |
| WO2020219549A1 (en) * | 2019-04-23 | 2020-10-29 | Cedars-Sinai Medical Center | Methods and systems for assessing inflammatory disease with deep learning |
| JP2022532381A (ja) * | 2019-05-16 | 2022-07-14 | プロサイセデクス インコーポレイティド | Vcam-1及びカルプロテクチンのための分析検出方法 |
| CN110373457B (zh) * | 2019-06-20 | 2023-03-14 | 镇江市第一人民医院 | 一种用于溃疡性结肠炎诊断的mRNA标志物及其应用 |
| CN111417124A (zh) * | 2019-06-28 | 2020-07-14 | 西南交通大学 | 在认知无线网络环境下频谱感知的方法 |
| US11610679B1 (en) * | 2020-04-20 | 2023-03-21 | Health at Scale Corporation | Prediction and prevention of medical events using machine-learning algorithms |
| WO2021247548A1 (en) * | 2020-06-03 | 2021-12-09 | Cedars-Sinai Medical Center | Treatments for a sub-population of inflammatory bowel disease patients |
| WO2022061346A1 (en) * | 2020-09-16 | 2022-03-24 | Northwestern University | Classification of functional lumen imaging probe data |
| WO2022076372A1 (en) * | 2020-10-05 | 2022-04-14 | Cedars-Sinai Medical Center | Methods and systems for stratifying inflammatory bowel disease patients |
| US20240044916A1 (en) * | 2020-12-11 | 2024-02-08 | Icahn School Of Medicine At Mount Sinai | Methods of monitoring inflammatory bowel diseases |
| WO2022177963A1 (en) * | 2021-02-17 | 2022-08-25 | Prometheus Biosciences, Inc. | Anti-cd30l antibodies and uses thereof |
| WO2023047257A1 (en) * | 2021-09-22 | 2023-03-30 | Janssen Research & Development, Llc | Automated estimation of ulcerative colitis severity from endoscopy videos using ordinal multi-instance learning |
| WO2023147139A1 (en) * | 2022-01-28 | 2023-08-03 | University Of Southern California | Identifying non-disease patients using a disease related assay and analysis in the liquid biopsy |
Family Cites Families (60)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4277250A (en) | 1979-12-10 | 1981-07-07 | Baylor College Of Medicine | Methods for detecting and quantifying occult blood |
| JPH0684970B2 (ja) | 1987-03-31 | 1994-10-26 | 株式会社京都医科学研究所 | 糞便中の潜血検出方法 |
| US5081040A (en) | 1987-06-29 | 1992-01-14 | Helena Laboratories Corporation | Composition and kit for testing for occult blood in human and animal excretions, fluids, or tissue matrixes |
| US6867195B1 (en) | 1989-03-21 | 2005-03-15 | Vical Incorporated | Lipid-mediated polynucleotide administration to reduce likelihood of subject's becoming infected |
| JPH0736013B2 (ja) | 1991-04-27 | 1995-04-19 | 一男 田畑 | 潜血検出用試薬 |
| US5885527A (en) | 1992-05-21 | 1999-03-23 | Biosite Diagnostics, Inc. | Diagnostic devices and apparatus for the controlled movement of reagents without membrances |
| DK0615129T3 (da) | 1993-03-10 | 2000-08-07 | Cedars Sinai Medical Center | Fremgangsmåder til selektiv påvisning af perinukleært anti-neutrophilt cytoplasmatisk antistof fra ulcerativ colitis eller |
| US5830675A (en) | 1993-03-10 | 1998-11-03 | Cedars-Sinai Medical Center | Methods for selectively detecting perinuclear anti-neutrophil cytoplasmic antibody of ulcerative colitis, primary sclerosing cholangitis, or type 1 autoimmune hepatitis |
| US5972901A (en) | 1994-03-23 | 1999-10-26 | Case Western Reserve University | Serpin enzyme complex receptor--mediated gene transfer |
| US6077835A (en) | 1994-03-23 | 2000-06-20 | Case Western Reserve University | Delivery of compacted nucleic acid to cells |
| EP0752005B1 (de) | 1994-03-23 | 2008-10-08 | Ohio University | Kompaktnukleinsäure und ihre verabreichung in zellen |
| US5844107A (en) | 1994-03-23 | 1998-12-01 | Case Western Reserve University | Compacted nucleic acids and their delivery to cells |
| US5916748A (en) | 1996-04-12 | 1999-06-29 | Cedars-Sinai Medical Center | Method of diagnosing a clinical subtype of crohn's disease with features of ulcerative colitis |
| US5932429A (en) | 1996-04-12 | 1999-08-03 | Cedars-Sinai Medical Center | Methods of diagnosing clinical subtypes of crohn's disease |
| US6074835A (en) | 1996-04-12 | 2000-06-13 | Regents Of The Univ. Of California | Diagnosis, prevention and treatment of ulcerative colitis, and clinical subtypes thereof, using histone H1 |
| US6033864A (en) | 1996-04-12 | 2000-03-07 | The Regents Of The University Of California | Diagnosis, prevention and treatment of ulcerative colitis, and clinical subtypes thereof, using microbial UC pANCA antigens |
| US5750479A (en) | 1997-02-14 | 1998-05-12 | Bandon Corp. | Enclosed fluid system conditioner and process therefor |
| AU4145597A (en) | 1997-02-20 | 1998-09-09 | Cedars-Sinai Medical Center | Ulcerative colitis panca secretory vesicle antigen and methods of using sa me |
| US6218129B1 (en) | 1998-05-15 | 2001-04-17 | Prometheus Laboratories, Inc. | Inflammatory bowel disease first step assay system |
| US6406862B1 (en) | 1998-10-06 | 2002-06-18 | The United States Of America As Represented By The Secretary Of The Army | Dip-stick assay for C-reactive protein |
| US6309643B1 (en) | 1999-04-30 | 2001-10-30 | The Regents Of The University Of California | IBD-associated microbial antigens and methods of using same |
| AU5161800A (en) | 1999-05-24 | 2000-12-12 | Board Of Regents, The University Of Texas System | Methods and compositions for non-viral gene therapy for treatment of hyperproliferative diseases |
| FR2802536B1 (fr) | 1999-11-23 | 2003-06-13 | Chru Lille | Oligomannosides de synthese, leur preparation et leur utilisation a la detection d'anticorps et a la prevention d'infections |
| FR2806739B1 (fr) | 2000-03-27 | 2005-02-18 | Fond Jean Dausset Ceph | Genes impliques dans les maladies inflammatoires de l'intestin et leur utilisation |
| US6838250B2 (en) | 2000-03-31 | 2005-01-04 | Ortho-Clinical Diagnostics, Inc. | Immunoassay for C-reactive protein |
| US7138237B1 (en) | 2000-05-19 | 2006-11-21 | Cedars-Sinai Medical Center | Diagnosis, prevention and treatment of Crohn's disease using the OmpC antigen |
| US20020042388A1 (en) | 2001-05-01 | 2002-04-11 | Cooper Mark J. | Lyophilizable and enhanced compacted nucleic acids |
| US6821739B2 (en) | 2000-10-13 | 2004-11-23 | The Regents Of The University Of California | Methods of diagnosing and treating Crohn's disease using Pseudomonas antigens |
| WO2002032396A2 (en) | 2000-10-16 | 2002-04-25 | Massachusetts Institute Of Technology | Lipid-protein-sugar particles for delivery of nucleic acids |
| AU2735002A (en) | 2000-10-30 | 2002-05-15 | Univ Michigan | Nod2 nucleic acids and proteins |
| AU4341502A (en) | 2000-10-30 | 2002-06-11 | Univ Michigan | Nod2 nucleic acids and proteins |
| US7060442B2 (en) | 2000-10-30 | 2006-06-13 | Regents Of The University Of Michigan | Modulators on Nod2 signaling |
| US20050164929A1 (en) | 2000-11-06 | 2005-07-28 | Lupine Logic, Inc. | Methods of preventing and treating inflammatory bowel disease |
| US7192724B2 (en) | 2000-11-14 | 2007-03-20 | Techlab, Inc. | Method for differentiating irritable bowel syndrome from inflammatory bowel disease (IBD) and for monitoring persons with IBD using total endogenous lactoferrin as a marker |
| JP2004535388A (ja) | 2001-04-30 | 2004-11-25 | ターゲティッド ジェネティクス コーポレイション | 脂質含有薬物送達複合体およびそれらの生成方法 |
| AU2002365279B2 (en) | 2001-12-17 | 2009-08-13 | Corixa Corporation | Compositions and methods for the therapy and diagnosis of inflammatory bowel disease |
| US20030204507A1 (en) | 2002-04-25 | 2003-10-30 | Li Jonathan Qiang | Classification of rare events with high reliability |
| US20040053263A1 (en) | 2002-08-30 | 2004-03-18 | Abreu Maria T. | Mutations in NOD2 are associated with fibrostenosing disease in patients with Crohn's disease |
| CA2502976C (en) | 2002-10-25 | 2012-05-22 | Techlab, Inc. | Inflammatory bowel disease and irritable bowel syndrome ibd-first chek diagnostic panel |
| AU2003298703A1 (en) | 2002-11-22 | 2004-06-18 | Emory University | Diagnostic tests and methods for diagnosing inflammatory bowel disease |
| US7662569B2 (en) | 2003-04-11 | 2010-02-16 | Cedars-Sinai Medical Center | Methods of assessing Crohn's disease patient phenotype by I2 serologic response |
| EP1617214A4 (de) | 2003-04-23 | 2008-01-23 | Eisai R&D Man Co Ltd | Verfahren zur erzeugung eines krankheitsprognosemodells, verfahren zur vorhersage einer krankheitsprognose unter verwendung des modells, vorrichtung zur vorhersage einer krankheitsprognose unter verwendung des modells, deren programm und aufzeichnungsmedium |
| US20040242972A1 (en) | 2003-05-28 | 2004-12-02 | General Electric Company | Method, system and computer product for prognosis of a medical disorder |
| US20050060295A1 (en) | 2003-09-12 | 2005-03-17 | Sensory Networks, Inc. | Statistical classification of high-speed network data through content inspection |
| WO2005041896A2 (en) | 2003-11-03 | 2005-05-12 | Duke University | Methods of identifying individuals at risk of perioperative bleeding, renal dysfunction or stroke |
| CA2554836A1 (en) | 2004-02-05 | 2005-08-25 | Medtronic, Inc. | Methods and apparatus for identifying patients at risk for life threatening arrhythmias |
| US7759079B2 (en) | 2004-05-13 | 2010-07-20 | Prometheus Laboratories Inc. | Methods of diagnosing inflammatory bowel disease |
| US20060154276A1 (en) * | 2004-05-13 | 2006-07-13 | Prometheus Laboratories Inc. | Methods of diagnosing inflammatory bowel disease |
| WO2006012472A1 (en) | 2004-07-21 | 2006-02-02 | Qualyst, Inc. | Apparatus, kits and methods for evaluating binding interactions, for detecting and quantifying binding molecules, and for sample preparation |
| CA3204588A1 (en) | 2004-12-08 | 2006-06-15 | Cedars-Sinai Medical Center | Methods for diagnosis and treatment of crohn's disease |
| US7873479B2 (en) | 2005-12-01 | 2011-01-18 | Prometheus Laboratories Inc. | Methods of diagnosing inflammatory bowel disease |
| US20080085524A1 (en) * | 2006-08-15 | 2008-04-10 | Prometheus Laboratories Inc. | Methods for diagnosing irritable bowel syndrome |
| US20100015156A1 (en) | 2007-03-06 | 2010-01-21 | Cedars-Sinai Medical Center | Diagnosis of inflammatory bowel disease in children |
| US8153443B2 (en) | 2007-05-10 | 2012-04-10 | Cedars-Sinai Medical Center | Characterization of the CBir1 antigenic response for diagnosis and treatment of Crohn's disease |
| KR20100138995A (ko) | 2008-03-27 | 2010-12-31 | 제이엑스 닛코닛세키에너지주식회사 | 연료 전지 시스템과 그 부하 추종 운전 방법 |
| WO2010056682A2 (en) * | 2008-11-11 | 2010-05-20 | Prometheus Laboratories Inc. | Methods for prediction of inflammatory bowel disease (ibd) using serologic markers |
| NZ595471A (en) * | 2009-04-14 | 2014-01-31 | Nestec Sa | Inflammatory bowel disease prognostics |
| AU2010266028B2 (en) * | 2009-06-25 | 2015-04-30 | Société des Produits Nestlé S.A. | Methods for diagnosing irritable bowel syndrome |
| CA2781654A1 (en) * | 2009-11-25 | 2011-06-03 | Hua Gong | Novel genomic biomarkers for irritable bowel syndrome diagnosis |
| CA2801575A1 (en) | 2010-06-04 | 2011-12-08 | Prometheus Laboratories Inc. | Methods for improving inflammatory bowel disease diagnosis |
-
2012
- 2012-10-19 AU AU2012325798A patent/AU2012325798B2/en not_active Ceased
- 2012-10-19 WO PCT/US2012/061202 patent/WO2013059732A1/en active Application Filing
- 2012-10-19 JP JP2014537335A patent/JP2015502740A/ja active Pending
- 2012-10-19 MX MX2014004712A patent/MX352274B/es active IP Right Grant
- 2012-10-19 SG SG11201401536QA patent/SG11201401536QA/en unknown
- 2012-10-19 CA CA2852954A patent/CA2852954A1/en not_active Abandoned
- 2012-10-19 EP EP12841790.4A patent/EP2769321B1/de active Active
-
2013
- 2013-03-15 US US13/840,779 patent/US8715943B2/en active Active
-
2014
- 2014-03-28 US US14/229,715 patent/US20150072879A1/en not_active Abandoned
- 2014-04-22 IL IL232167A patent/IL232167A0/en unknown
-
2015
- 2015-02-04 HK HK15101185.6A patent/HK1200933A1/zh not_active IP Right Cessation
Also Published As
| Publication number | Publication date |
|---|---|
| HK1200933A1 (zh) | 2015-08-14 |
| IL232167A0 (en) | 2014-06-30 |
| CA2852954A1 (en) | 2013-04-25 |
| AU2012325798A1 (en) | 2013-05-16 |
| JP2015502740A (ja) | 2015-01-29 |
| US8715943B2 (en) | 2014-05-06 |
| US20150072879A1 (en) | 2015-03-12 |
| EP2769321A4 (de) | 2015-05-13 |
| SG11201401536QA (en) | 2014-05-29 |
| AU2012325798B2 (en) | 2015-11-26 |
| WO2013059732A1 (en) | 2013-04-25 |
| MX2014004712A (es) | 2014-11-14 |
| US20130225439A1 (en) | 2013-08-29 |
| EP2769321A1 (de) | 2014-08-27 |
| MX352274B (es) | 2017-11-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2769321B1 (de) | Verfahren zur verbesserung der diagnose einer entzündlichen darmerkrankung | |
| US9732385B2 (en) | Method for determining the risk of crohn's disease-related complications | |
| US20120171672A1 (en) | Inflammatory bowel disease prognostics | |
| JP5634023B2 (ja) | 試料が炎症性腸疾患と関連しているか分類する方法 | |
| US7873479B2 (en) | Methods of diagnosing inflammatory bowel disease | |
| US20130203053A1 (en) | Methods for improving inflammatory bowel disease diagnosis | |
| US20130005596A1 (en) | Novel genomic biomarkers for irritable bowel syndrome diagnosis | |
| JP5749652B2 (ja) | 血清学的マーカーを用いた炎症性腸疾患(ibd)の予測方法 | |
| EP2710383B1 (de) | Leistungsfähigkeit eines Biomarkerpanels für Reizdarmsyndrom | |
| WO2011060098A1 (en) | Methods for predicting post-surgery risk associated with ileal pouch-anal anastomosis |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20140417 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| RA4 | Supplementary search report drawn up and despatched (corrected) |
Effective date: 20150414 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: G06F 19/00 20110101ALI20150408BHEP Ipc: G01N 33/53 20060101ALI20150408BHEP Ipc: C12Q 1/68 20060101AFI20150408BHEP Ipc: G01N 33/48 20060101ALI20150408BHEP |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: DE Ref document number: 1200933 Country of ref document: HK |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R079 Ref document number: 602012019275 Country of ref document: DE Free format text: PREVIOUS MAIN CLASS: G06F0019000000 Ipc: C12Q0001680000 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: G01N 33/543 20060101ALI20151223BHEP Ipc: C12Q 1/68 20060101AFI20151223BHEP Ipc: G06F 19/18 20110101ALI20151223BHEP Ipc: G06F 19/24 20110101ALI20151223BHEP |
|
| INTG | Intention to grant announced |
Effective date: 20160128 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP Ref country code: AT Ref legal event code: REF Ref document number: 803925 Country of ref document: AT Kind code of ref document: T Effective date: 20160615 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R082 Ref document number: 602012019275 Country of ref document: DE Representative=s name: MITSCHERLICH, PATENT- UND RECHTSANWAELTE PARTM, DE |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602012019275 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 5 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20160601 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160901 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 803925 Country of ref document: AT Kind code of ref document: T Effective date: 20160601 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160902 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161001 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160601 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161003 Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602012019275 Country of ref document: DE |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20170302 |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: GR Ref document number: 1200933 Country of ref document: HK |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20161019 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 6 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20161019 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20121019 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 Ref country code: MT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20161031 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 7 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20160601 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PFUS Owner name: SOCIETE DES PRODUITS NESTLE S.A., CH Free format text: FORMER OWNER: NESTEC S.A., CH |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 732E Free format text: REGISTERED BETWEEN 20190801 AND 20190807 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20191029 Year of fee payment: 8 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R082 Ref document number: 602012019275 Country of ref document: DE Representative=s name: MITSCHERLICH, PATENT- UND RECHTSANWAELTE PARTM, DE Ref country code: DE Ref legal event code: R081 Ref document number: 602012019275 Country of ref document: DE Owner name: SOCIETE DES PRODUITS NESTLE S.A., CH Free format text: FORMER OWNER: NESTEC S.A., VEVEY, CH Ref country code: DE Ref legal event code: R081 Ref document number: 602012019275 Country of ref document: DE Owner name: PROMETHEUS BIOSCIENCES, INC., SAN DIEGO, US Free format text: FORMER OWNER: NESTEC S.A., VEVEY, CH |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20191025 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 20191015 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20191018 Year of fee payment: 8 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R082 Ref document number: 602012019275 Country of ref document: DE Representative=s name: MITSCHERLICH, PATENT- UND RECHTSANWAELTE PARTM, DE Ref country code: DE Ref legal event code: R081 Ref document number: 602012019275 Country of ref document: DE Owner name: PROMETHEUS BIOSCIENCES, INC., SAN DIEGO, US Free format text: FORMER OWNER: SOCIETE DES PRODUITS NESTLE S.A., VEVEY, CH |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 732E Free format text: REGISTERED BETWEEN 20200618 AND 20200624 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 602012019275 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20201019 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210501 Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20201031 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20201031 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20201031 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20201019 |