EP2645994B1 - Formulierungen enthaltend methylthioninium chlorid - Google Patents
Formulierungen enthaltend methylthioninium chlorid Download PDFInfo
- Publication number
- EP2645994B1 EP2645994B1 EP11802119.5A EP11802119A EP2645994B1 EP 2645994 B1 EP2645994 B1 EP 2645994B1 EP 11802119 A EP11802119 A EP 11802119A EP 2645994 B1 EP2645994 B1 EP 2645994B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- mtc
- composition
- diluent
- tablets
- tablet
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 229960000907 methylthioninium chloride Drugs 0.000 title claims description 146
- 239000000203 mixture Substances 0.000 title claims description 79
- 238000009472 formulation Methods 0.000 title description 13
- RBTBFTRPCNLSDE-UHFFFAOYSA-N 3,7-bis(dimethylamino)phenothiazin-5-ium Chemical compound C1=CC(N(C)C)=CC2=[S+]C3=CC(N(C)C)=CC=C3N=C21 RBTBFTRPCNLSDE-UHFFFAOYSA-N 0.000 title 1
- 238000000034 method Methods 0.000 claims description 78
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 claims description 70
- 239000003085 diluting agent Substances 0.000 claims description 44
- 238000007907 direct compression Methods 0.000 claims description 36
- 230000008569 process Effects 0.000 claims description 36
- 229920000168 Microcrystalline cellulose Polymers 0.000 claims description 35
- 235000019359 magnesium stearate Nutrition 0.000 claims description 35
- 235000019813 microcrystalline cellulose Nutrition 0.000 claims description 35
- 239000008108 microcrystalline cellulose Substances 0.000 claims description 34
- 229940016286 microcrystalline cellulose Drugs 0.000 claims description 34
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 33
- 239000000314 lubricant Substances 0.000 claims description 25
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 claims description 23
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 claims description 23
- 239000008101 lactose Substances 0.000 claims description 23
- 239000007909 solid dosage form Substances 0.000 claims description 22
- 239000007888 film coating Substances 0.000 claims description 18
- 238000009501 film coating Methods 0.000 claims description 18
- 238000007908 dry granulation Methods 0.000 claims description 17
- 239000007787 solid Substances 0.000 claims description 17
- 238000011282 treatment Methods 0.000 claims description 17
- 235000010947 crosslinked sodium carboxy methyl cellulose Nutrition 0.000 claims description 16
- 239000007884 disintegrant Substances 0.000 claims description 16
- 229920000036 polyvinylpyrrolidone Polymers 0.000 claims description 16
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 claims description 16
- 229920002785 Croscarmellose sodium Polymers 0.000 claims description 14
- 239000011248 coating agent Substances 0.000 claims description 14
- 238000000576 coating method Methods 0.000 claims description 14
- 201000010099 disease Diseases 0.000 claims description 14
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 14
- 238000004519 manufacturing process Methods 0.000 claims description 14
- 239000000843 powder Substances 0.000 claims description 14
- 239000008194 pharmaceutical composition Substances 0.000 claims description 13
- 239000001267 polyvinylpyrrolidone Substances 0.000 claims description 13
- 238000007906 compression Methods 0.000 claims description 12
- 230000006835 compression Effects 0.000 claims description 12
- 229920003109 sodium starch glycolate Polymers 0.000 claims description 12
- 239000008109 sodium starch glycolate Substances 0.000 claims description 12
- 229940079832 sodium starch glycolate Drugs 0.000 claims description 12
- 239000008187 granular material Substances 0.000 claims description 11
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 claims description 10
- 229930195725 Mannitol Natural products 0.000 claims description 10
- 239000004480 active ingredient Substances 0.000 claims description 10
- 239000011230 binding agent Substances 0.000 claims description 10
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 claims description 10
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 claims description 10
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 claims description 10
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 claims description 10
- 239000000594 mannitol Substances 0.000 claims description 10
- 235000010355 mannitol Nutrition 0.000 claims description 10
- 208000034799 Tauopathies Diseases 0.000 claims description 9
- 238000011321 prophylaxis Methods 0.000 claims description 9
- 229920001223 polyethylene glycol Polymers 0.000 claims description 7
- 239000012530 fluid Substances 0.000 claims description 6
- 238000009481 moist granulation Methods 0.000 claims description 6
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 claims description 5
- 239000002202 Polyethylene glycol Substances 0.000 claims description 5
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 claims description 5
- 235000000346 sugar Nutrition 0.000 claims description 5
- 229920000881 Modified starch Polymers 0.000 claims description 4
- 239000004372 Polyvinyl alcohol Substances 0.000 claims description 4
- 229920002451 polyvinyl alcohol Polymers 0.000 claims description 4
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 claims description 3
- 239000005913 Maltodextrin Substances 0.000 claims description 3
- 229920002774 Maltodextrin Polymers 0.000 claims description 3
- 235000021355 Stearic acid Nutrition 0.000 claims description 3
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 claims description 3
- 229930006000 Sucrose Natural products 0.000 claims description 3
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 claims description 3
- 239000008116 calcium stearate Substances 0.000 claims description 3
- 235000013539 calcium stearate Nutrition 0.000 claims description 3
- 239000008121 dextrose Substances 0.000 claims description 3
- 239000001863 hydroxypropyl cellulose Substances 0.000 claims description 3
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 claims description 3
- 239000011872 intimate mixture Substances 0.000 claims description 3
- 229940035034 maltodextrin Drugs 0.000 claims description 3
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 claims description 3
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 claims description 3
- 239000008117 stearic acid Substances 0.000 claims description 3
- 239000005720 sucrose Substances 0.000 claims description 3
- 150000008163 sugars Chemical class 0.000 claims description 3
- 229920002554 vinyl polymer Polymers 0.000 claims description 3
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 claims description 2
- 229920002134 Carboxymethyl cellulose Polymers 0.000 claims description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 claims description 2
- 239000001856 Ethyl cellulose Substances 0.000 claims description 2
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 claims description 2
- 239000004354 Hydroxyethyl cellulose Substances 0.000 claims description 2
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 claims description 2
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 claims description 2
- 235000010443 alginic acid Nutrition 0.000 claims description 2
- 229920000615 alginic acid Polymers 0.000 claims description 2
- 150000004781 alginic acids Chemical class 0.000 claims description 2
- SNAAJJQQZSMGQD-UHFFFAOYSA-N aluminum magnesium Chemical compound [Mg].[Al] SNAAJJQQZSMGQD-UHFFFAOYSA-N 0.000 claims description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 claims description 2
- 235000010948 carboxy methyl cellulose Nutrition 0.000 claims description 2
- 239000008112 carboxymethyl-cellulose Substances 0.000 claims description 2
- 235000010980 cellulose Nutrition 0.000 claims description 2
- 229920002678 cellulose Polymers 0.000 claims description 2
- 239000001913 cellulose Substances 0.000 claims description 2
- 239000001767 crosslinked sodium carboxy methyl cellulose Substances 0.000 claims description 2
- 235000019325 ethyl cellulose Nutrition 0.000 claims description 2
- 229920001249 ethyl cellulose Polymers 0.000 claims description 2
- 239000008103 glucose Substances 0.000 claims description 2
- 238000010438 heat treatment Methods 0.000 claims description 2
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 claims description 2
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 claims description 2
- 229920000193 polymethacrylate Polymers 0.000 claims description 2
- 150000003839 salts Chemical class 0.000 claims description 2
- 235000010413 sodium alginate Nutrition 0.000 claims description 2
- 239000000661 sodium alginate Substances 0.000 claims description 2
- 229940005550 sodium alginate Drugs 0.000 claims description 2
- 235000002639 sodium chloride Nutrition 0.000 claims description 2
- 239000000600 sorbitol Substances 0.000 claims description 2
- 239000000811 xylitol Substances 0.000 claims description 2
- 229960002675 xylitol Drugs 0.000 claims description 2
- 235000010447 xylitol Nutrition 0.000 claims description 2
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 claims description 2
- CXKWCBBOMKCUKX-UHFFFAOYSA-M methylene blue Chemical compound [Cl-].C1=CC(N(C)C)=CC2=[S+]C3=CC(N(C)C)=CC=C3N=C21 CXKWCBBOMKCUKX-UHFFFAOYSA-M 0.000 claims 15
- XQAXGZLFSSPBMK-UHFFFAOYSA-M [7-(dimethylamino)phenothiazin-3-ylidene]-dimethylazanium;chloride;trihydrate Chemical compound O.O.O.[Cl-].C1=CC(=[N+](C)C)C=C2SC3=CC(N(C)C)=CC=C3N=C21 XQAXGZLFSSPBMK-UHFFFAOYSA-M 0.000 description 124
- 239000000463 material Substances 0.000 description 25
- 239000003814 drug Substances 0.000 description 23
- 229940079593 drug Drugs 0.000 description 21
- 229960001375 lactose Drugs 0.000 description 21
- 239000004615 ingredient Substances 0.000 description 19
- 238000004090 dissolution Methods 0.000 description 16
- 201000011240 Frontotemporal dementia Diseases 0.000 description 15
- 238000003860 storage Methods 0.000 description 15
- 208000024827 Alzheimer disease Diseases 0.000 description 14
- 230000006870 function Effects 0.000 description 14
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 13
- -1 81.6 mg Chemical compound 0.000 description 12
- 229920003084 Avicel® PH-102 Polymers 0.000 description 12
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 12
- 239000002775 capsule Substances 0.000 description 12
- 229960001681 croscarmellose sodium Drugs 0.000 description 12
- 102000013498 tau Proteins Human genes 0.000 description 11
- 108010026424 tau Proteins Proteins 0.000 description 11
- 239000012729 immediate-release (IR) formulation Substances 0.000 description 9
- 238000002360 preparation method Methods 0.000 description 9
- 239000007921 spray Substances 0.000 description 9
- 150000004684 trihydrates Chemical class 0.000 description 9
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 8
- 239000002552 dosage form Substances 0.000 description 8
- 238000005259 measurement Methods 0.000 description 8
- 210000002682 neurofibrillary tangle Anatomy 0.000 description 8
- 230000002776 aggregation Effects 0.000 description 7
- 238000004220 aggregation Methods 0.000 description 7
- 238000009491 slugging Methods 0.000 description 7
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 6
- 239000012458 free base Substances 0.000 description 6
- 239000006186 oral dosage form Substances 0.000 description 6
- 238000000634 powder X-ray diffraction Methods 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 238000010521 absorption reaction Methods 0.000 description 5
- 239000013078 crystal Substances 0.000 description 5
- 229940126534 drug product Drugs 0.000 description 5
- 238000001035 drying Methods 0.000 description 5
- 150000004686 pentahydrates Chemical class 0.000 description 5
- 230000035699 permeability Effects 0.000 description 5
- 239000000047 product Substances 0.000 description 5
- 230000004845 protein aggregation Effects 0.000 description 5
- 238000009490 roller compaction Methods 0.000 description 5
- 239000007916 tablet composition Substances 0.000 description 5
- 230000004584 weight gain Effects 0.000 description 5
- 235000019786 weight gain Nutrition 0.000 description 5
- 238000005550 wet granulation Methods 0.000 description 5
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 4
- 208000011990 Corticobasal Degeneration Diseases 0.000 description 4
- 206010012289 Dementia Diseases 0.000 description 4
- 201000010374 Down Syndrome Diseases 0.000 description 4
- 108010010803 Gelatin Proteins 0.000 description 4
- 241000124008 Mammalia Species 0.000 description 4
- 208000036757 Postencephalitic parkinsonism Diseases 0.000 description 4
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 4
- 206010044688 Trisomy 21 Diseases 0.000 description 4
- 206010002026 amyotrophic lateral sclerosis Diseases 0.000 description 4
- 210000004556 brain Anatomy 0.000 description 4
- 208000010877 cognitive disease Diseases 0.000 description 4
- 208000017004 dementia pugilistica Diseases 0.000 description 4
- 238000009506 drug dissolution testing Methods 0.000 description 4
- 239000000975 dye Substances 0.000 description 4
- 229920000159 gelatin Polymers 0.000 description 4
- 239000008273 gelatin Substances 0.000 description 4
- 235000019322 gelatine Nutrition 0.000 description 4
- 235000011852 gelatine desserts Nutrition 0.000 description 4
- 238000005469 granulation Methods 0.000 description 4
- 230000003179 granulation Effects 0.000 description 4
- 208000027061 mild cognitive impairment Diseases 0.000 description 4
- 208000015122 neurodegenerative disease Diseases 0.000 description 4
- 239000002245 particle Substances 0.000 description 4
- 239000000825 pharmaceutical preparation Substances 0.000 description 4
- 208000000170 postencephalitic Parkinson disease Diseases 0.000 description 4
- 201000002212 progressive supranuclear palsy Diseases 0.000 description 4
- 102000004169 proteins and genes Human genes 0.000 description 4
- 108090000623 proteins and genes Proteins 0.000 description 4
- 238000002411 thermogravimetry Methods 0.000 description 4
- WSVLPVUVIUVCRA-KPKNDVKVSA-N Alpha-lactose monohydrate Chemical compound O.O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O WSVLPVUVIUVCRA-KPKNDVKVSA-N 0.000 description 3
- 206010067889 Dementia with Lewy bodies Diseases 0.000 description 3
- 241000237858 Gastropoda Species 0.000 description 3
- 201000002832 Lewy body dementia Diseases 0.000 description 3
- 229920003072 Plasdone™ povidone Polymers 0.000 description 3
- 238000009825 accumulation Methods 0.000 description 3
- 239000008186 active pharmaceutical agent Substances 0.000 description 3
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 3
- 229910052782 aluminium Inorganic materials 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 239000007900 aqueous suspension Substances 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 238000007922 dissolution test Methods 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 229940088679 drug related substance Drugs 0.000 description 3
- 230000007613 environmental effect Effects 0.000 description 3
- 210000001035 gastrointestinal tract Anatomy 0.000 description 3
- 229960001031 glucose Drugs 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 229960001021 lactose monohydrate Drugs 0.000 description 3
- 230000000670 limiting effect Effects 0.000 description 3
- 230000007774 longterm Effects 0.000 description 3
- 229960001855 mannitol Drugs 0.000 description 3
- 229940069328 povidone Drugs 0.000 description 3
- 230000001105 regulatory effect Effects 0.000 description 3
- 208000011580 syndromic disease Diseases 0.000 description 3
- 239000000454 talc Substances 0.000 description 3
- 229910052623 talc Inorganic materials 0.000 description 3
- 229940033134 talc Drugs 0.000 description 3
- 235000012222 talc Nutrition 0.000 description 3
- 239000004408 titanium dioxide Substances 0.000 description 3
- 230000009466 transformation Effects 0.000 description 3
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N Aniline Chemical compound NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- 208000005870 Lafora disease Diseases 0.000 description 2
- 208000014161 Lafora myoclonic epilepsy Diseases 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 208000010577 Niemann-Pick disease type C Diseases 0.000 description 2
- 208000000609 Pick Disease of the Brain Diseases 0.000 description 2
- DLRVVLDZNNYCBX-UHFFFAOYSA-N Polydextrose Polymers OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(O)O1 DLRVVLDZNNYCBX-UHFFFAOYSA-N 0.000 description 2
- 208000010291 Primary Progressive Nonfluent Aphasia Diseases 0.000 description 2
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 2
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 2
- 239000004141 Sodium laurylsulphate Substances 0.000 description 2
- 208000037065 Subacute sclerosing leukoencephalitis Diseases 0.000 description 2
- 206010042297 Subacute sclerosing panencephalitis Diseases 0.000 description 2
- 101710150875 TAR DNA-binding protein 43 Proteins 0.000 description 2
- 102100040347 TAR DNA-binding protein 43 Human genes 0.000 description 2
- 208000036278 TDP-43 proteinopathy Diseases 0.000 description 2
- 208000007930 Type C Niemann-Pick Disease Diseases 0.000 description 2
- 230000002159 abnormal effect Effects 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 230000002421 anti-septic effect Effects 0.000 description 2
- 229960000074 biopharmaceutical Drugs 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- 210000003710 cerebral cortex Anatomy 0.000 description 2
- 239000003086 colorant Substances 0.000 description 2
- 238000004040 coloring Methods 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 239000000470 constituent Substances 0.000 description 2
- 229960000913 crospovidone Drugs 0.000 description 2
- 238000000354 decomposition reaction Methods 0.000 description 2
- 230000007850 degeneration Effects 0.000 description 2
- 150000004683 dihydrates Chemical class 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- MVPICKVDHDWCJQ-UHFFFAOYSA-N ethyl 3-pyrrolidin-1-ylpropanoate Chemical compound CCOC(=O)CCN1CCCC1 MVPICKVDHDWCJQ-UHFFFAOYSA-N 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 235000013305 food Nutrition 0.000 description 2
- 230000002518 glial effect Effects 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 150000004677 hydrates Chemical class 0.000 description 2
- 230000036571 hydration Effects 0.000 description 2
- 238000006703 hydration reaction Methods 0.000 description 2
- 238000010348 incorporation Methods 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 229940057948 magnesium stearate Drugs 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 208000005340 mucopolysaccharidosis III Diseases 0.000 description 2
- 208000036709 mucopolysaccharidosis type 3B Diseases 0.000 description 2
- 230000004770 neurodegeneration Effects 0.000 description 2
- 239000002547 new drug Substances 0.000 description 2
- 230000007170 pathology Effects 0.000 description 2
- ZQBAKBUEJOMQEX-UHFFFAOYSA-N phenyl salicylate Chemical compound OC1=CC=CC=C1C(=O)OC1=CC=CC=C1 ZQBAKBUEJOMQEX-UHFFFAOYSA-N 0.000 description 2
- 230000036470 plasma concentration Effects 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 235000013809 polyvinylpolypyrrolidone Nutrition 0.000 description 2
- 229920000523 polyvinylpolypyrrolidone Polymers 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 230000002035 prolonged effect Effects 0.000 description 2
- 230000005855 radiation Effects 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 229910052710 silicon Inorganic materials 0.000 description 2
- 239000010703 silicon Substances 0.000 description 2
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 2
- 229940045902 sodium stearyl fumarate Drugs 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 238000001228 spectrum Methods 0.000 description 2
- 238000005507 spraying Methods 0.000 description 2
- 229960004793 sucrose Drugs 0.000 description 2
- 230000009747 swallowing Effects 0.000 description 2
- 239000007885 tablet disintegrant Substances 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 238000000003 thermogravimetry coupled to Fourier transform infrared spectroscopy Methods 0.000 description 2
- URAYPUMNDPQOKB-UHFFFAOYSA-N triacetin Chemical compound CC(=O)OCC(OC(C)=O)COC(C)=O URAYPUMNDPQOKB-UHFFFAOYSA-N 0.000 description 2
- OTHYPAMNTUGKDK-UHFFFAOYSA-N (3-acetylphenyl) acetate Chemical compound CC(=O)OC1=CC=CC(C(C)=O)=C1 OTHYPAMNTUGKDK-UHFFFAOYSA-N 0.000 description 1
- KPYHKEZPEDJERZ-UHFFFAOYSA-N 10h-phenothiazine-1,2-diamine Chemical class C1=CC=C2NC3=C(N)C(N)=CC=C3SC2=C1 KPYHKEZPEDJERZ-UHFFFAOYSA-N 0.000 description 1
- 229910002012 Aerosil® Inorganic materials 0.000 description 1
- 229910002016 Aerosil® 200 Inorganic materials 0.000 description 1
- 208000037259 Amyloid Plaque Diseases 0.000 description 1
- GUBGYTABKSRVRQ-DCSYEGIMSA-N Beta-Lactose Chemical compound OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-DCSYEGIMSA-N 0.000 description 1
- VONKRKBGTZDZNV-UHFFFAOYSA-N CC=C1CCCC1 Chemical compound CC=C1CCCC1 VONKRKBGTZDZNV-UHFFFAOYSA-N 0.000 description 1
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 description 1
- 235000019739 Dicalciumphosphate Nutrition 0.000 description 1
- 239000004150 EU approved colour Substances 0.000 description 1
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 208000000913 Kidney Calculi Diseases 0.000 description 1
- 125000000174 L-prolyl group Chemical group [H]N1C([H])([H])C([H])([H])C([H])([H])[C@@]1([H])C(*)=O 0.000 description 1
- 101710192391 Laforin Proteins 0.000 description 1
- 102100038889 Laforin, isoform 9 Human genes 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 206010068871 Myotonic dystrophy Diseases 0.000 description 1
- 206010029148 Nephrolithiasis Diseases 0.000 description 1
- IOVCWXUNBOPUCH-UHFFFAOYSA-M Nitrite anion Chemical compound [O-]N=O IOVCWXUNBOPUCH-UHFFFAOYSA-M 0.000 description 1
- 241000276398 Opsanus tau Species 0.000 description 1
- 208000027089 Parkinsonian disease Diseases 0.000 description 1
- 206010034010 Parkinsonism Diseases 0.000 description 1
- 229920001100 Polydextrose Polymers 0.000 description 1
- 229920003081 Povidone K 30 Polymers 0.000 description 1
- 208000025820 Sanfilippo syndrome type B Diseases 0.000 description 1
- 208000018642 Semantic dementia Diseases 0.000 description 1
- 206010039966 Senile dementia Diseases 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 235000019892 Stellar Nutrition 0.000 description 1
- 229940122777 Tau aggregation inhibitor Drugs 0.000 description 1
- 208000036142 Viral infection Diseases 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- VREFGVBLTWBCJP-UHFFFAOYSA-N alprazolam Chemical compound C12=CC(Cl)=CC=C2N2C(C)=NN=C2CN=C1C1=CC=CC=C1 VREFGVBLTWBCJP-UHFFFAOYSA-N 0.000 description 1
- 239000004411 aluminium Substances 0.000 description 1
- 229960004977 anhydrous lactose Drugs 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 239000000729 antidote Substances 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 230000003542 behavioural effect Effects 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 239000007963 capsule composition Substances 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 210000000349 chromosome Anatomy 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- 239000008199 coating composition Substances 0.000 description 1
- 230000007278 cognition impairment Effects 0.000 description 1
- 230000006999 cognitive decline Effects 0.000 description 1
- 239000008119 colloidal silica Substances 0.000 description 1
- 229940075614 colloidal silicon dioxide Drugs 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 210000001947 dentate gyrus Anatomy 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- NEFBYIFKOOEVPA-UHFFFAOYSA-K dicalcium phosphate Chemical compound [Ca+2].[Ca+2].[O-]P([O-])([O-])=O NEFBYIFKOOEVPA-UHFFFAOYSA-K 0.000 description 1
- 229940038472 dicalcium phosphate Drugs 0.000 description 1
- 229910000390 dicalcium phosphate Inorganic materials 0.000 description 1
- 238000000113 differential scanning calorimetry Methods 0.000 description 1
- 239000012738 dissolution medium Substances 0.000 description 1
- QXYCYSAGHGKNHM-UHFFFAOYSA-N dodecyl octadecanoate;magnesium Chemical compound [Mg].CCCCCCCCCCCCCCCCCC(=O)OCCCCCCCCCCCC QXYCYSAGHGKNHM-UHFFFAOYSA-N 0.000 description 1
- 238000001647 drug administration Methods 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 229940083280 fd&c blue #1 aluminum lake Drugs 0.000 description 1
- 239000003337 fertilizer Substances 0.000 description 1
- 230000003619 fibrillary effect Effects 0.000 description 1
- 239000012467 final product Substances 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 239000011888 foil Substances 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 235000013773 glyceryl triacetate Nutrition 0.000 description 1
- 239000001087 glyceryl triacetate Substances 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 235000010299 hexamethylene tetramine Nutrition 0.000 description 1
- VKYKSIONXSXAKP-UHFFFAOYSA-N hexamethylenetetramine Chemical compound C1N(C2)CN3CN1CN2C3 VKYKSIONXSXAKP-UHFFFAOYSA-N 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 239000008172 hydrogenated vegetable oil Substances 0.000 description 1
- 229960001550 hyoscyamine sulfate Drugs 0.000 description 1
- 230000006951 hyperphosphorylation Effects 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 239000005414 inactive ingredient Substances 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 210000004558 lewy body Anatomy 0.000 description 1
- 239000007791 liquid phase Substances 0.000 description 1
- 201000004792 malaria Diseases 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 230000003340 mental effect Effects 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 229960004011 methenamine Drugs 0.000 description 1
- 238000003801 milling Methods 0.000 description 1
- 208000012227 mucopolysaccharidosis type IIIB Diseases 0.000 description 1
- 210000000478 neocortex Anatomy 0.000 description 1
- 210000004248 oligodendroglia Anatomy 0.000 description 1
- 229940006093 opthalmologic coloring agent diagnostic Drugs 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 239000013588 oral product Substances 0.000 description 1
- 229940023486 oral product Drugs 0.000 description 1
- 239000007800 oxidant agent Substances 0.000 description 1
- 230000008506 pathogenesis Effects 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 150000002990 phenothiazines Chemical class 0.000 description 1
- 229960000969 phenyl salicylate Drugs 0.000 description 1
- 229940063331 phosphasal Drugs 0.000 description 1
- 230000026731 phosphorylation Effects 0.000 description 1
- 238000006366 phosphorylation reaction Methods 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 231100000572 poisoning Toxicity 0.000 description 1
- 230000000607 poisoning effect Effects 0.000 description 1
- 239000001259 polydextrose Substances 0.000 description 1
- 235000013856 polydextrose Nutrition 0.000 description 1
- 229940035035 polydextrose Drugs 0.000 description 1
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- 229940068968 polysorbate 80 Drugs 0.000 description 1
- 229920002689 polyvinyl acetate Polymers 0.000 description 1
- 239000011118 polyvinyl acetate Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 210000002763 pyramidal cell Anatomy 0.000 description 1
- HNJBEVLQSNELDL-UHFFFAOYSA-N pyrrolidin-2-one Chemical compound O=C1CCCN1 HNJBEVLQSNELDL-UHFFFAOYSA-N 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 239000002824 redox indicator Substances 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 238000013341 scale-up Methods 0.000 description 1
- 238000005204 segregation Methods 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- AJPJDKMHJJGVTQ-UHFFFAOYSA-M sodium dihydrogen phosphate Chemical compound [Na+].OP(O)([O-])=O AJPJDKMHJJGVTQ-UHFFFAOYSA-M 0.000 description 1
- 238000007614 solvation Methods 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 229940032147 starch Drugs 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 150000004685 tetrahydrates Chemical class 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 238000012876 topography Methods 0.000 description 1
- 229960002622 triacetin Drugs 0.000 description 1
- 210000002438 upper gastrointestinal tract Anatomy 0.000 description 1
- 229920006163 vinyl copolymer Polymers 0.000 description 1
- 230000009385 viral infection Effects 0.000 description 1
- 230000004580 weight loss Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/54—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one sulfur as the ring hetero atoms, e.g. sulthiame
- A61K31/5415—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one sulfur as the ring hetero atoms, e.g. sulthiame ortho- or peri-condensed with carbocyclic ring systems, e.g. phenothiazine, chlorpromazine, piroxicam
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1629—Organic macromolecular compounds
- A61K9/1652—Polysaccharides, e.g. alginate, cellulose derivatives; Cyclodextrin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/205—Polysaccharides, e.g. alginate, gums; Cyclodextrin
- A61K9/2054—Cellulose; Cellulose derivatives, e.g. hydroxypropyl methylcellulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2095—Tabletting processes; Dosage units made by direct compression of powders or specially processed granules, by eliminating solvents, by melt-extrusion, by injection molding, by 3D printing
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4841—Filling excipients; Inactive ingredients
- A61K9/4866—Organic macromolecular compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/02—Muscle relaxants, e.g. for tetanus or cramps
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D279/00—Heterocyclic compounds containing six-membered rings having one nitrogen atom and one sulfur atom as the only ring hetero atoms
- C07D279/10—1,4-Thiazines; Hydrogenated 1,4-thiazines
- C07D279/14—1,4-Thiazines; Hydrogenated 1,4-thiazines condensed with carbocyclic rings or ring systems
- C07D279/18—[b, e]-condensed with two six-membered rings
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Definitions
- the present invention relates to solid dosage forms of methylthioninium chloride and to methods of preparing such solid dosage forms.
- the invention relates to tablet formulations in which the polymorphic form of the active ingredient is stable.
- Methylthioninium chloride [3,7-bisdimethylaminophenazothionium chloride, C 16 H 18 ClN 3 S, 319.85 g/mol], commonly known as methylene blue, was prepared for the first time in 1876 ( The Merck Index, 13th edition, Merck & Co., Inc., 2001, entry 6085 ).
- MTC Methylthioninium chloride
- WO 2006/032879 also discloses a number of applications of methylene blue, which include use as a medical dye, as a redox indicator, as an antiseptic, for the treatment and prevention of kidney stones, and for the treatment of melanoma, malaria, and viral infections.
- MTC has also been used as an oxidizing agent and as an antidote in the case of carbon monoxide, nitrite and aniline poisoning.
- PhosphasalTM for oral administration (see www.drugs.com/pro/phosphasal.html; PhosphasalTM is a trademark of Stellar Pharmacal).
- PhosphasalTM is a trademark of Stellar Pharmacal.
- Each tablet is reported to contain Methenamine, 81.6 mg, Sodium Phosphate Monobasic, 40.8 mg, Phenyl Salicylate, 36.2 mg, Methylene Blue, 10.8 mg, and Hyoscyamine Sulfate, 0.12 mg as well as inactive ingredients: Crospovidone, Dicalcium Phosphate, FD&C Blue #1 Aluminum Lake, Magnesium Stearate, Microcrystalline Cellulose, Polyethylene Glycol, Polyvinyl Alcohol, Stearic Acid, Talc, and Titanium Dioxide. The drug is apparently not approved for any specific indication, but the methylene blue ingredient is reported to possess weak antiseptic properties.
- MTC has also been proposed for treatment of mild to moderate dementia of the Alzheimer's type (DAT), also known as Alzheimer's disease (AD), a severe irreversible neurodegenerative disease resulting in complete loss of mental faculties.
- DAT Alzheimer's type
- AD Alzheimer's disease
- WO96/30766 describes the use of tau aggregation inhibitors - including MTC for the treatment of various diseases of protein aggregation including AD.
- Other disclosures of phenothiazines in the area of neurodegenerative disorders include WO 02/075318 , WO 2005/030676 , WO 02/055720 , WO2007110627 , WO2009/060191 , WO2009/044127 .
- MTC in common with many solid substances useful as active pharmaceutical ingredients, exhibits polymorphism, i.e. it exists in more than one physical form, known as polymorphs, which are typically different crystalline and hydrate forms of the drug substance. Hydrates are crystalline solids containing differing amounts of water incorporated into the crystal structure.
- MTC Fluka catalogue states in very general terms that MTC may contain up to 22% water (Fluka Catalogue 1997/1998, Fluka Chemie AG, 1997]. MTC is generally considered to exist as a trihydrate, but this was disputed as long as 80 years ago, and non-specific adsorption of water by MTC was proposed instead ( H. Wales, O.A. Nelson, J. Am. Chem. Soc. 45 (1923) 1657 ). A pentahydrate including single crystal X-ray data was described later by several authors ( J.O. Warwicker, J. Chem. Soc. (1955) 2531 and H.E. Marr III, J.M. Stewart, M.F. Chiu, Acta Cryst. B29 (1973) 847 ).
- This pentahydrate consists of ⁇ -stacked columns of MTC molecules arranged in planes perpendicular to the ⁇ -axis of the crystal.
- the water molecules and chloride ions are located between these layers, whereas the chloride ions are concentrated in planes almost perpendicular to the water planes and parallel to the axis of the columns.
- the chloride ions are coordinated with three hydrogen bonds from 3/2 water molecules. Presumably, the same structure was earlier attributed to a tetrahydrate ( W.H. Taylor, Z. Krist. 91 (1935) 450 ).
- Form A has been identified as a pentahydrate and "Form B” as a dihydrate.
- Form A is considered stable at high relative humidity (RH) down to approximately 35% RH.
- RH relative humidity
- Form A is even observed down to RH less than 20%.
- Forms C, D and E Several other polymorphs of MTC, referred to as Forms C, D and E, have also been identified. It has been found that the dihydrates B and D are metastable forms over the whole range of water activity and are only kinetically favoured under certain preparation conditions. Form B seems to be the product of incomplete drying of Form A. Form D was obtained in precipitation experiments. No anhydrate form has been identified, but X-ray diffractograms include peaks that cannot be attributed to any of the five known forms. It is therefore likely that the polymorphism of MTC is even more complex than has hitherto been established.
- polymorphic forms of a drug molecule may have different chemical and/or physical properties.
- polymorphs can differ substantially in melting point, chemical reactivity, particle size, shape, flow characteristics, caking, degree of hydration or solvation, optical and electrical properties vapour pressure, and density.
- certain polymorphs of a drug molecule can be more stable than others under given environmental conditions.
- MTC has a number of properties which render its formulation into a dosage form quite difficult.
- the distinct blue colour presents processing and cleaning challenges.
- the existence of numerous polymorphic forms is problematic.
- the physical stability of the polymorphic Form A is problematic, as during heating and/or storage it can be converted into the polymorphic Forms B and D.
- the interconversion of the individual crystalline polymorphic forms of MTC in a medicament, during manufacture and/or storage is undesirable as it is a general regulatory requirement that the identity of the medicament must be guaranteed throughout its entire shelf life.
- the limited stability domain of all MTC polymorphic forms requires an innovative approach to formulation to produce a consistent dosage form.
- the product quality should be consistent and reproducible at the time of manufacture and on storage at temperatures and relative humidity levels typically encountered in most countries of the world.
- One may additionally seek other desirable properties in a formulation such as fast dissolution so that the tablet quickly dissolves and the medicine is available for absorption, and also properties such as good compressibility and robustness, and ease of manufacture. Accordingly, good storage stability and fast dissolution are important and desirable attributes for immediate release tablet formulations and capsules.
- Processes used for tablet formulation and film coating often require the use of heat accompanied by low humidity during the drying process.
- a material such as MTC, with complex polymorphism, such processes can lead to changes in the physical form of the active ingredient, and hence potentially to instability in the performance of the product.
- the most commonly used method for the preparation of solid dosage forms is wet granulation. This involves adding a granulating fluid to a powder.
- the granulating fluid may be water or some other solvent that is sufficiently volatile that it can subsequently be removed by drying.
- the granulating fluid may also include a binder. Once the solvent has been removed, the resulting mass is milled.
- wet granulation is often preferred over direct compression because wet granulation is more likely to overcome any problems associated with the physical characteristics of various ingredients in the formulation.
- Wet granulation provides material which has the required flow and cohesive properties necessary to obtain an acceptable solid dosage form.
- the content uniformity of the solid dosage form is generally improved with wet granulation because all of the granules usually contain the same amount of drug. Segregation of the drug from excipients is also avoided.
- the present invention provides novel formulations (tablets and capsules), particularly immediate release formulations, of MTC having one or more desirable properties such as those described above e.g. a consistent and reproducible composition at the time of manufacture, stability on storage at temperatures and relative humidity levels typically encountered in most countries of the world, fast dissolution, robustness, and ease of manufacture.
- tablet dosage forms and processes for the production thereof provided by direct compression (e.g. simple direct compression), dry granulation, or moist granulation of excipients, followed by drying and addition of the active ingredient extra-granularly.
- direct compression e.g. simple direct compression
- dry granulation e.g. dry granulation
- moist granulation e.g. moist granulation of excipients
- drying and addition of the active ingredient extra-granularly e.g. simple direct compression
- such processes can avoid the application of heat or excessive moisture to the MTC, but nevertheless produce a formulation having one or more of the desirable properties above.
- MTC notably MTC of polymorphic Form A but also other Forms or mixtures of Forms, such as a mixture of Forms B and C
- MTC has also been found to be stable in a directly compressed solid dosage form such as a tablet, during manufacture and storage, and is not converted into another polymorphic form (in particular Form A is not converted to the Forms B, C or D).
- MTC when processed by conventional granulation processes, in which, for instance, MTC of Form A can be converted to a substantial extent into Forms B, C and/or D.
- a pharmaceutical composition in solid dosage form comprising, as active ingredient, MTC, said composition further comprising at least one diluent suitable for direct compression which is microcrystalline cellulose or a sugar, wherein the MTC exists in a substantially pure and stable polymorphic form, which is polymorph Form A wherein the amount of MTC in the composition is more than 10% w/w, and wherein the composition is obtained by a process comprising the dry compression of an intimate powder mixture of the MTC in a substantially pure polymorphic form with the at least one diluent suitable for direct compression, and optionally one or more other excipients.
- a process for the manufacture of a pharmaceutical composition according to the first aspect comprises the direct compression of an intimate powder mixture of MTC in a substantially pure polymorphic form, which is polymorph Form A, with at least one diluent suitable for direct compression, and optionally one or more other excipients.
- a free-flowing, cohesive powder comprising MTC in a substantially pure polymorphic form, at least one diluent suitable for direct compression, and optionally one or more other excipients, said powder being capable of being directly compressed into a pharmaceutical composition according to the first aspect.
- the invention provides pharmaceutical compositions comprising MTC in solid dosage form, the composition further comprising at least one diluent suitable for other dry compression methods e.g. based on dry granulation, or moist granulation of excipients, followed by drying and addition of the active ingredient extra-granularly.
- diluent suitable for other dry compression methods e.g. based on dry granulation, or moist granulation of excipients, followed by drying and addition of the active ingredient extra-granularly.
- 'Dry compression' refers to compression techniques which do not involve the use of excessive heat or moisture in respect of the active ingredient (here: MTC).
- the free-flowing, cohesive powder comprising a compound of the invention (here: MTC) and at least one diluent suitable for dry granulation and optionally one or more other excipients (particularly a lubricant) can be used in methods such as "slugging" or those using a "roller compactor". Such processes form further embodiments of the invention as defined in the claims.
- the dry granulation is carried out by blending MTC. optionally with a lubricant, with wet-massed excipients, which are dried before the blending step.
- Solid dosage forms according to the invention exhibit long term chemical and crystallographic stability of the active ingredient, MTC.
- the pharmaceutical compositions according to the invention have very good dissolution rates even after long term storage.
- a "substantially pure" polymorphic form of MTC is one in which the predominant polymorphic form of MTC accounts for at least 80% w/w of the material, or at least 90% w/w, or at least 95% w/w. In other words, the material contains less than 20% w/w, or less than 10% w/w or less than 5% w/w of polymorphic forms other than the predominant form.
- a "substantially pure and stable polymorphic form" of MTC is one in which the predominant polymorphic form remains substantially pure in the composition even after prolonged storage under controlled conditions of temperature and humidity.
- the MTC in the solid dosage form will substantially maintain its polymorphic identity when stored under chosen environmental conditions.
- the predominant polymorphic form of the MTC in the composition will exhibit less than 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10 % conversion to polymorphic forms other than the predominant form when stored under controlled conditions for a defined period of time.
- Preferred controlled conditions are storage in a blister pack (preferably one having aluminum cavity and aluminum foil) which is thus substantially moisture-impervious.
- the controlled temperature is 25°C and the controlled storage humidity is 65% RH.
- the defined period of time is 9 months.
- preferred embodiments of the invention may demonstrate stability even at higher temperatures or different humidities (e.g. 30 or 40°C / 75% RH) and longer periods e.g. 10.5 months, 12 months, 24 months, or 36 months.
- the conversion between polymorphic forms may conveniently be assessed by X-ray powder diffractograms.
- the blister pack is opened and the tablet cores are slightly crushed in order to obtain a suitable powder that can be prepared on an XRPD sample holder.
- the different polymorphs show characteristic signals in the 2q range below 12°, which will be essentially free of signals from the excipients.
- An evaluation of the XRPD signals in this 2q range permits an unambiguous assignment of the solid state polymorphic forms of MTC in the sample.
- the absence of any signals from lower hydrates may be verified (here the absence of a signal would typically be defined by a signal intensity which does not exceed three times the noise).
- Initial assessment may be followed by quantification of each polymorph (if present) by use of appropriate reference measurements of mixtures of different polymorphs.
- Also described herein is a system comprising a diluent and direct compression (or dry granulation) for enhancing the stability of a substantially pure MTC polymorph in a pharmaceutical composition.
- the MTC is used in polymorph Form A.
- use of the present invention minimises conversion of Form A to the Forms B, C or D.
- the amount of MTC in the uncoated composition is more than about 10% w/w, but can be more than 20%, or more than 30% w/w.
- the amount of MTC is generally less than about 70% w/w, and usually less than 60% or less than 50% w/w.
- the amount of MTC in the uncoated composition is thus from about 10% w/w (or 20% or 30%) to about 70% w/w (or 60% or 50%).
- MTC is not inherently compressible and thus requires addition of suitable diluents to aid compression.
- compositions of the invention therefore commonly comprise at least 15% w/w, more commonly at least 20%, at least 30%, at least 40% or at least 50% w/w of diluent(s).
- Diluents that may be used include one or more of microcrystalline cellulose and sugars such as lactose, sucrose, dextrose and maltodextrin.
- Preferred diluents are microcrystalline cellulose, lactose and mannitol. Spray-dried forms of lactose and mannitol are particularly suitable forms of those compounds.
- MTC of a particular polymorphic form here: Form A
- direct compression diluents such as one or more of microcrystalline cellulose, spray dried lactose, anhydrous lactose and mannitol
- the resulting solid dosage forms are stable in the sense that the polymorphic form of the MTC is preserved, and the MTC remains chemically stable, even after extended storage.
- the invention thus provides a method of preparing low-, medium- or high-dose MTC tablets that are stable and have acceptable dissolution profiles, acceptable degrees of hardness and resistance to chipping, as well as a short disintegration time.
- the pharmaceutical composition will generally also include a lubricant.
- lubricants include magnesium stearate, calcium stearate, sodium stearyl fumarate, stearic acid, glycerylbehaptate, polyethylene glycol, ethylene oxide polymers (for example, those available under the registered trademark Carbowax from Union Carbide, Inc., Danbury, CT), sodium lauryl sulphate, magnesium lauryl stearate, mixtures of magnesium stearate with sodium lauryl sulphate, and hydrogenated vegetable oil.
- Preferred lubricants include calcium stearate, magnesium stearate and sodium stearyl fumarate. Most preferred as the lubricant is magnesium stearate.
- Lubricants generally comprise from about 0.5 to about 5.0% of the total (uncoated) tablet weight. The amount of lubricant employed is generally from about 1.0 to about 2.0%, preferably 0.5 to 2.0% w/w.
- excipients may also be present in the pharmaceutical compositions of the invention.
- additional excipients include disintegrants, binders, flavouring agents, colours and glidants.
- Some excipients can serve multiple functions, for example as both binder and tablet disintegrant.
- a tablet disintegrant may be present in an amount necessary to achieve rapid dissolution.
- Disintegrants are excipients which oppose the physical forces of particle bonding in a tablet or capsule when the dosage form is placed in an aqueous environment.
- examples of disintegrants include crosslinked polyvinylpyrrolidone, sodium starch glycolate, crosslinked sodium carboxymethyl cellulose (sodium croscarmellose), and pregelatinized starch.
- the amount of disintegrant can be from 0 to about 25% w/w, more commonly from about 1% to about 15% w/w, and usually less than 10% or less than 5% w/w, of the composition.
- Binders are excipients which contribute to particle adhesion in a solid formulation.
- binders include cellulose derivatives (carboxymethylcellulose, hydroxypropyl methylcellulose, hydroxypropyl cellulose, hydroxyethylcellulose, ethylcellulose, microcrystalline cellulose) and sugars such as lactose, sucrose, dextrose, glucose, maltodextrin, and mannitol, xylitol, polymethacrylates, polyvinylpyrrolidone, sorbitol, pregelatinized starch, alginic acids, and salts thereof such as sodium alginate, magnesium aluminum silicate, polyethylene glycol, and the like.
- the amount of binder can vary widely, e.g. from 0% to 95% w/w of the composition.
- excipients may serve multiple functions.
- the tabletting diluent may also serve as a binder.
- Glidants are substances added to a powder to improve its flowability.
- examples of glidants include magnesium stearate, colloidal silicon dioxide (such as the grades sold as Aerosil), starch and talc.
- Glidants may be present in the pharmaceutical composition at a level of from 0 to about 5% w/w. Again, however, it should be noted that excipients may serve multiple functions.
- the lubricant for example magnesium stearate, may also function as a glidant.
- colours examples include titanium dioxide and/or dyes suitable for food such as those known as FD&C dyes and natural colouring agents.
- a colouring agent is unlikely to be used in the powder mixture that is compressed in accordance with the second aspect of the invention, but may form part of a coating applied to the composition, as described below, in which case the colouring agent may be present in the film coat in an amount up to about 2.0% w/w.
- composition is a tablet
- this is desirably coated with a conventional film coating which imparts toughness, ease of swallowing, and an elegant appearance to the final product.
- Many polymeric film-coating materials are known in the art.
- a preferred film-coating material is hydroxypropylmethylcellulose (HPMC) or polyvinyl alcohol-part hydrolysed (PVA).
- HPMC and PVA may be obtained commercially, for example from Colorcon, in coating formulations containing excipients which serve as coating aids, under the registered trademark Opadry.
- Opadry formulations may contain talc, polydextrose, triacetin, polyethyleneglycol, polysorbate 80, titanium dioxide, and one or more dyes or lakes.
- film-forming polymers may also be used, including hydroxypropylcellulose, vinyl copolymers such as polyvinyl pyrollidone and polyvinyl acetate, and acrylate-methacrylate copolymers.
- Use of a film coating is beneficial for ease of handling and because a blue coloured uncoated core may stain the inside of the mouth during swallowing. Coating also improves light stability of the dosage form.
- Coating of the tablets may conveniently be carried out using a conventional coating pan.
- the coating pan is pre-heated using heated inlet air until the exhaust temperature reaches 35-55°C, more preferably 40-50°C. This may typically require application of heated inlet air at an inlet temperature of 45-75°C, preferably 50-65°C, for 10-15 minutes.
- the MTC tablets are then added to the coating pan and the aqueous film coat applied.
- the spray rate is controlled such that the bed temperature is maintained at 38-48°C, more preferably 42-44°C, until the desired weight gain (coating weight) has been achieved.
- Described herein is a method of treatment or prophylaxis of a disease condition in a patient, comprising administering to said patient a therapeutically-effective amount of a solid dosage form composition as described herein.
- Described herein is a method of treatment or prophylaxis of a tauopathy condition in a patient, comprising administering to said patient a therapeutically-effective amount of a solid dosage form composition as described herein.
- prophylaxis in the context of the present specification should not be understood to circumscribe complete success i.e. complete protection or complete prevention. Rather prophylaxis in the present context refers to a measure which is administered in advance of detection of a symptomatic condition with the aim preserving health by helping to delay, mitigate or avoid that particular condition.
- tauopathies As well as Alzheimer's disease (AD), the pathogenesis of neurodegenerative disorders such as Pick's disease and Progressive Supranuclear Palsy (PSP) appears to correlate with an accumulation of pathological truncated tau aggregates in the dentate gyrus and stellate pyramidal cells of the neocortex, respectively.
- Other dementias include fronto-temporal dementia (FTD); FTD with parkinsonism linked to chromosome 17 (FTDP-17); behavioural variant FTD (bvFTD); progressive nonfluent aphasia (PNFA) Josephs, KA, Petersen, RC, Knopman, DS, et al.
- FTD fronto-temporal dementia
- FTDP-17 FTD with parkinsonism linked to chromosome 17
- bvFTD behavioural variant FTD
- PNFA progressive nonfluent aphasia
- Senile dementia of Lewy body type and Alzheimer type are biochemically distinct in terms of paired helical filaments and hyperphosphorylated tau protein. Dementia 5, 215-228 ).
- Tau-positive NFTs are also found in Postencephalitic parkinsonism (PEP) ( Hof P.R., Charpiot, A., Delacourte A., Buee, L., Purohit, D., Perl D.P. and Bouras, C. (1992) Distribution of neurofibrillary tangles and senile plaques in the cerebral cortex in postencephalitic parkinsonism. Neurosci. Lett. 139, 10-14 ).
- Glial tau tangles are observed in Subacute sclerosing panencephalitis (SSPE) ( Ikeda K., Akiyama H., Kondo H., Arai T., Arai N. and Yagishita S. (1995) Numerous glial fibrillary tangles in oligodendroglia in cases of subacute sclerosing panencephalitis with neurofibrillary tangles. Neurosci. Lett., 194, 133-135 ).
- SSPE Subacute sclerosing panencephalitis
- tauopathies include Niemann-Pick disease type C (NPC) ( Love, S., Bridges, L.R. & Case, C.P. (1995), Brain, 118, 119-129 ); Sanfilippo syndrome type B (or mucopolysaccharidosis III B, MPS III B) ( Ohmi, K., Kudo, L.C., Ryazantsev, S., et al. (2009) PNAS, 106, 8332-8337 ; myotonic dystrophies (DM), DM1 ( Sergeant, N., Sablonniere, B., Schraen-Maschke, S., et al.
- NPC Niemann-Pick disease type C
- MPS III B mucopolysaccharidosis III B
- DM1 myotonic dystrophies
- DM1 Sergeant, N., Sablonniere, B., Schraen-Maschke, S., et al.
- tau pathology may also contribute more generally to cognitive deficits and decline, including in mild cognitive impairment (MCI) (see e.g. Braak, H., Del Tredici, K, Braak, E. (2003) Spectrum of pathology. In Mild cognitive impairment: Aging to Alzheimer's disease edited by Petersen, R.C.; pp. 149-189 ).
- MCI mild cognitive impairment
- tauopathies or “diseases of tau protein aggregation”.
- the tauopathy is selected from the list consisting of the indications above, i.e., AD, Pick's disease, PSP, FTD, FTDP-17, DDPAC, PPND, Guam-ALS syndrome, PNLD, and CBD and AgD, DS, SSPE, DP, PEP, DLB and MCI.
- the tauopathy is Alzheimer's disease (AD).
- compositions of the invention may be used in the treatment or prophylaxis of TDP-43 proteinopathies e.g. frontotemporal lobe dementia associated with TDP-43 (FTLD-TDP43), amyotrophic lateral sclerosis (ALS) and overlapping syndromes, and semantic dementia and FTDP-17 ( Chen-Plotkin, AS, Lee, VMY, Trojanowski, JQ (2010) TAR DNA-binding protein 43 in neurodegenerative disease. Nature Reviews Neurology 6:211-220 ).
- TDP-43 proteinopathies e.g. frontotemporal lobe dementia associated with TDP-43 (FTLD-TDP43), amyotrophic lateral sclerosis (ALS) and overlapping syndromes, and semantic dementia and FTDP-17 ( Chen-Plotkin, AS, Lee, VMY, Trojanowski, JQ (2010) TAR DNA-binding protein 43 in neurodegenerative disease. Nature Reviews Neurology 6:211-220 ).
- one aspect of the present invention pertains to a solid dosage form composition, as described in the claims herein, for use in a method of treatment or prophylaxis (e.g. of a tauopathy condition or TDP-43 proteinopathy) of the human or animal body by therapy.
- a method of treatment or prophylaxis e.g. of a tauopathy condition or TDP-43 proteinopathy
- Also described herein is a method of treatment or prophylaxis of a disease of protein aggregation as described herein, which method comprises administering to a subject a solid dosage form composition such as to inhibit the aggregation of the protein (e.g. tau protein) associated with said disease state.
- a solid dosage form composition such as to inhibit the aggregation of the protein (e.g. tau protein) associated with said disease state.
- a solid dosage form composition of the invention for use in a method of treatment or prophylaxis of a disease of protein aggregation as described above, which method comprises administering to a subject the solid dosage form composition such as to inhibit the aggregation of the protein associated with said disease state.
- a method of inhibiting production of protein aggregates e.g. in the form of paired helical filaments (PHFs), optionally in neurofibrillary tangles (NFTs)
- PHFs paired helical filaments
- NFTs neurofibrillary tangles
- a drug product for the treatment of a disease state associated with protein aggregation in a mammal suffering therefrom comprising a container labeled or accompanied by a label indicating that the drug product is for the treatment of said disease, the container containing one or more dosage units each comprising a solid dosage form composition of the invention.
- a first embodiment of the manufacturing process of the second aspect of this invention is simple direct compression.
- the tablet ingredients i.e. MTC, diluent(s) and other optional excipients
- MTC solid, particulate form
- diluent(s) and other optional excipients are blended together in solid, particulate form to create an intimate mixture, e.g. in a tumbling blender, and then compressed using a tablet machine.
- the composition is prepared by a dry granulation process.
- Dry granulation refers to the process of granulating without the use of granulating fluids.
- at least one of its constituents, either the active ingredient or a diluent must have cohesive properties. Dry granulation may be performed by a process known as "slugging". In “slugging”, the material to be granulated is first made into a large compressed mass or "slug”, typically using a tablet press with large flat-faced tooling (an example of a linear press is illustrated in US-4,880,373 ).
- a fairly dense slug may be formed by allowing sufficient time for the air to escape from the material to be compacted. Compressed slugs are then milled through a desired mesh screen manually or automatically as, for example, by way of a comminuting mill. Formation of granules by "slugging” is also known as precompression. When tablets are made from the granulated slugged material, the process is referred to as the "double compression method".
- Dry granulation may also be performed using a "roller compactor".
- a roller compactor material particles are consolidated and densified by passing the material between two high-pressure rollers.
- the densified material from a roller compactor is then reduced to a uniform granule size by milling.
- the uniform granules may then be mixed with other substances, such as a lubricant, to tablet the material (as, for example, by way of a rotary tableting machine).
- roller compaction is used in other industries, such as the food industry, animal feed industry and fertilizer industry.
- Dry granulation is nowadays generally understood to mean roller compaction or slugging, and is well known to those skilled in the art (see, for instance, Pharmaceutical Dosage Forms: Tablets (Lieberman, Lachman, and Schwartz (Eds); Marcel Dekker, Inc, 2nd Edition, 1989 ) and Remington's Pharmaceutical Sciences (A. R. Gennaro (Ed); Mack Publishing Co, Easton, PA, 18th edition, 1990 )).
- MTC tablets are prepared by moist granulation of the excipients and incorporation of the MTC extra-granularly.
- a process involves wet massing diluents such as lactose and/or microcrystalline cellulose with water, optionally with the addition of a binder such as polyvinyl pyrrolidone.
- the wet mass is passed through a mesh and dried to form granules.
- the MTC and any remaining excipients, such as a lubricant, are then blended with the dry granules and compressed to form tablets.
- capsules comprising MTC which has been formulated with diluents and other optionally other excipients as described herein.
- Such capsules may be prepared from materials well known in the art e.g. Gelatin, Gelatin ⁇ PEG, HPMC and so on - see " Pharmaceutical Capsules; 2nd Revised Edition” Podczeck & Jones (Eds), Pharmaceutical Press 2004 .
- X-ray powder diffractograms were obtained using a Bruker D8 Advance diffractometer with LynxEye detector. Measurements were performed in the Bragg-Brentano reflection geometry with Cu K ⁇ radiation ( ⁇ 1.54180 ⁇ at 40 kV/40mA. Data points were collected with 0.02 step size in 20 and 37s accumulation time per step. The samples were prepared on silicon single crystal sample holders with 1.0mm depth and 12mm diameter. All samples were rotated at 30 rpm during the measurement.
- Figures 1A-1E show respectively the X-ray diffractograms of Forms A-E of MTC.
- TG and TG-FTIR of hydrate form A consistently indicated a weight loss of 21.7%, which corresponds with good approximation to 5 molecules of water per molecule of MTC.
- the separation between water loss and decomposition was much more distinct in TG than in TG-FTIR, which can be explained by the different measurement setup (open pan versus pan with micro hole).
- a DSC measurement was performed with a sample of MTC pentahydrate in a closed gold pan with a scanning rate of 100 K/min (see Figure 2 ).
- AH 116 J/g
- a much smaller, broadened peak was observed between approximately 150°C and 180°C which can be attributed to the dissolution of the remaining solid in the liquid phase. Above 190°C there is evidence of decomposition (exothermic signals).
- thermodynamic stability of MTC hydrates is defined for a given set of environmental conditions such as temperature (T) and relative humidity (RH).
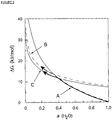
- Figure 3 shows the free energy of Forms A, B and C as a function of RH at a fixed T (25°C).
- MTC Form B is not thermodynamically stable, though it is kinetically stable in a certain range of T and RH.
- Form A is thermodynamically stable at high RH, and at relatively low T.
- Form A may also exist as a metastable state in a broader range of T and RH.
- Form B can transform into Form C (and probably even Form D). There is a risk that on prolonged storage and during processing such as film coating, transformation of Form B into Form C or D could also occur.
- FIG 4 shows the stability domain of MTC Form A.
- the shaded area represents all combinations of water activity (RH) and temperature at which Form A is thermodynamically stable.
- the black dots represent experimental data points for the equilibrium T and RH values for the transformation between form A and form C.
- the Figure shows that Form A is relatively unstable below 50% RH at 40°C and also below 40% RH at 25°C.
- Tablets having the following compositions were prepared by a direct compression method: Tablet strength (mg MTC/tablet) 50mg 75mg 100mg 125mg Ingredient mg/tablet MTC (free base equivalent) 73.61 110.41 147.22 184.02 Microcrystalline cellulose (Avicel PH 102) 230.00 230.00 230.00 287.50 Spray-dried lactose 127.39 90.59 53.78 67.23 Crosslinked polyvinylpyrrolidone 15.00 15.00 15.00 18.75 magnesium stearate 4.00 4.00 4.00 5.00 TOTAL TABLET WEIGHT/mg 450 450 450 562.5
- the MTC (Form A), microcrystalline cellulose, spray-dried lactose, cross-linked polyvinylpyrrolidone and magnesium stearate were blended in a tumbling blender, and then compressed using a tablet machine.
- Similar formulations may be prepared by a dry granulation method.
- the mix of microcrystalline cellulose, spray-dried lactose, cross-linked polyvinylpyrrolidone and magnesium stearate can be dry granulated using a roller compactor and then milled, e.g. with an oscillating granulator.
- half of the magnesium stearate is used prior to roller compaction and half of the magnesium stearate may be added to the granulation and blended prior to compression on a conventional tablet machine.
- the tablet cores may then be film coated with an aqueous suspension of Opadry blue (registered trademark of Colorcon for a series of film coating systems).
- Tablets having the following compositions were prepared by a direct compression method: Tablet strength (mg MTC/tablet) 50mg 75mg 100mg 125mg Ingredient mg/tablet MTC (free base equivalent) 73.61 110.41 147.22 184.02 Spray-dried mannitol 357.39 320.59 283.78 354.73 Crosslinked polyvinylpyrrolidone 15.00 15.00 15.00 18.75 Magnesium stearate 4.00 4.00 4.00 5.00 TOTAL TABLET WEIGHT/mg 450 450 450 562.5
- the MTC (Form A), microcrystalline cellulose, spray-dried mannitol, crosslinked polyvinylpyrrolidone and magnesium stearate were blended in a tumbling blender then compressed using a tablet machine.
- Similar formulations may be prepared by a dry granulation method.
- the mix of microcrystalline cellulose, spray-dried lactose, cross-linked polyvinylpyrrolidone and magnesium stearate can also be dry granulated using a roller compactor and then milled with an oscillating granulator using a 20 mesh screen.
- half of magnesium stearate is used prior to roller compaction and half of the magnesium stearate may be added to the granules and blended prior to compression on a conventional tablet machine.
- the tablet cores may then be film coated with an aqueous suspension of Opadry blue (registered trademark of Colorcon for a series of film coating systems).
- Tablets having the following compositions were prepared by a direct compression method: Tablet strength (mg MTC/tablet) 50mg 50mg 50mg 75mg 75mg 75mg Food mg/tablet MTC (free base equivalent) 73.6 73.6 73.6 110.4 1 110.4 1 110.4 1 Microcrystalline cellulose (Avicel PH 102) 115 172.5 Spray-dried lactose 26.9 141.9 40.35 212.8 5 Spray-dried mannitol 141.9 212.8 5 Crosslinked polyvinylpyrrolidone 7.5 7.5 7.5 11.25 11.25 11.25 Magnesium Stearate 2 2 2 3.00 3.00 3.00 TOTAL TABLET WEIGHT/mg 225 225 225 337.5 337.5 337.5 337.5 337.5 337.5
- the MTC (Form A) and excipients were blended in a tumbling blender, and then compressed using a tablet machine.
- Similar formulations may be prepared by a dry granulation method.
- the mix of excipients may alternatively be dry granulated using a roller compactor and then milled with an oscillating granulator using a 20 mesh screen.
- half of magnesium stearate may be used prior to roller compaction and half of the magnesium stearate is added to the granues and blended prior to compression on a conventional tablet machine.
- the tablet cores may then be film coated with an aqueous suspension of Opadry blue (registered trademark of Colorcon for a series of film coating systems).
- Tablets having the following compositions were prepared by a direct compression method: Tablet strength (mg MTC/tablet) 25 50 75 100 Ingredient mg/tablet MTC (free base equivalent) 36.80 73.60 110.43 147.24 Microcrystalline cellulose (Avicel PH 102) 230 230 230 230.00 Spray-dried Lactose 149.20 112.40 75.57 38.76 Croscarmellose sodium 30.00 30.00 30.00 30.00 Magnesium stearate 4.00 4.00 4.00 4.00 TOTAL TABLET WEIGHT/mg 450 450 450 450 450 450
- Tablet strength (mg) Hardness range (kg) Thickness range (mm) Mean weight of 20 tablets (mg) Table weight range of 20 tablets (mg) Disintegration time (min) 25 13.4-16.4 4.8-4.9 451 447-455 2 50 14.31-18.60 4.8 451.77 446-455 1.5 75 19.5-22.0 4.7-4.9 451.5 445-458 1.5 100 19.2-23.8 4.8-5.1 451 446-457 1.5
- MTC 30 mg tablets prepared by direct compression with microcrystalline cellulose (155mg/tablet), spray dried lactose (50mg) and sodium starch glycolate; 250mg round:
- the blended material was compressed to a tablet weight of 250mg at three different pressures.
- Properties of the resulting tablets were: Pressure 1 Pressure 2 Pressure 3 Weight, mean 249mg 250mg 251mg Hardness, mean 10.8kg 14.5kg 15.3kg Thickness, mean 4.5mm 4.3mm 4.3mm Disintegration time, mean 50 seconds 2.40 minutes 1 minute Friability 2% 0.39% 1.3%
- MTC 30mg tablets prepared by direct compression with microcrystalline cellulose and croscarmellose sodium; tablet weight 250mg
- the blended material was compressed to a tablet weight of 250mg.
- Properties of the resulting tablets were: Parameter Result Description Blue round tablet Thickness range mm 4.3 - 4.4 Hardness range 5.0 - 10.5kg Weight range for 20 tablets 212 - 270mg Mean weight of 20 tablets 247.51mg Friability 0.26% Disintegration time 1 to 5 minutes
- MTC 30 mg tablets prepared by direct compression with microcrystalline cellulose, spray dried lactose and croscarmellose sodium; tablet weight 250mg:
- the blended material was compressed to a tablet weight of 250mg.
- Properties of the resulting tablets were: Parameter Results Description Blue round tablets Thickness, mean 4.8mm Hardness, mean 7.5kg Hardness range for 20 tablets 6.0 to 8.8kg Weight of 20 tablets, mean 248.4mg Friability 0.31% Disintegration time, mean About 1minute
- the blended material was compressed to a tablet weight of 210mg.
- Properties of the resulting tablets were: Parameter Result Weight 202.5mg Hardness. mean 7.9kg Thickness, mean 4.2mm Disintegration time, mean 36 seconds Friability 0.13%
- the disintegrant used can be replaced by crospovidone without affecting compressibility, disintegration and dissolution properties.
- the blended material was compressed to a weight of 420mg (Oblong tablets).
- Properties of the resulting tablets were: Parameter Result Weight, mean 420.1mg Hardness mean 12.9Kg Thickness mean 5.2mm Disintegration time mean 50 seconds Friability 0.1%
- the resulting tablets had the following properties: Parameter Result Description Blue round tablets Thickness range 4.5 - 4.6mm Hardness range 7.1 - 10.1kg Weight of 20 tablets, mean 252.03mg Friability 0.22% Disintegration time 2.0 - 2.5 minutes
- the resulting tablets had the following properties: Parameters Results Description Blue round tablets Thickness 4.5 - 4.6mm Hardness 12.0 - 16.4kg Tablet weight range (20 tablets) 245-254mg Friability loss Less than 0.1%
- Ingredient Tablet strength (mg MTC/tablet) 30mg 60mg MTC (expressed as trihydrate) 30mg 60mg Microcrystalline cellulose, Avicel PH 102 92.5mg 77.5mg Lactose monohydrate 60mg 45mg Povidone (Plasdone K30) 2.5mg 2.5mg Croscarmellose sodium 22.5 22.5mg Magnesium stearate 2.5mg 2.5mg
- bioavailability The rate and extent to which the active ingredient is absorbed from a pharmaceutical dosage form and becomes available at the site of action is defined as bioavailability ( Chen, M. L. et al. Bioavailability and bioequivalence: an FDA regulatory overview, Pharm. Res. 2001, 18, 1645-1648 ).
- bioavailability is assessed based on drug concentrations in the general circulation.
- the systemic exposure is determined by measuring the blood or plasma concentrations of the active drug at numerous time points following the drug administration and calculation of the area under the concentration-time curve (AUC). Blood/plasma drug concentration time profiles are affected by the dynamics of dissolution, solubility, absorption, metabolism, distribution, and elimination.
- Absorption from a solid oral dosage form after administration can depend on the dissolution of the solid oral dosage form, which results from a series of simultaneous and successive processes, and the permeability across the gut wall of the gastrointestinal tract.
- in vitro dissolution may be relevant to the prediction of in vivo plasma concentrations and therefore bioavailability (Guidance for Industry, Dissolution Testing of Immediate Release Solid Oral Dosage Forms, U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), August 1997).
- in vitro dissolution tests for immediate release solid oral dosage forms are used to assess the quality of a drug product.
- An immediate release product allows the ingredient or active moiety to dissolve in the gastrointestinal tract, without causing any delay or prolongation of the dissolution or absorption of the drug.
- Requirements for dissolution testing of immediate release products are set out in the Guidance for Industry (CDER 1997) "Dissolution testing for immediate release solid oral dosage forms ", ( CDER 1997) “Immediate release solid oral dosage forms - Scale up and Postapproval Changes", ICH Guidance Q6A , Specifications: Test Procedures and Acceptance Criteria For New Drug Substances And New Drug Products.
- compositions according to the invention can be dissolution tested in a USP-2 apparatus in 900ml of 0.1N HCl, with paddles rotating at 50-75 rpm.
- Compositions according to the invention exhibit at least the acceptance criteria cited for Stage 1 (S1) testing in the USP 32 (The United States Pharmacopeia, edited by the United States Pharmacopeial Convention, Inc., 12601 Twinbrook Parkway, Rockville, MD 20852; Published by Rand McNally, Inc., 32nd Edition, 2008 ):
- the coating pan was warmed without the tablets for 10-15 minutes with an inlet air temperature between 45°C and 75° C, preferably 50 - 65° C, until the exhaust temperature reaches 35-55°C, preferably 40-50°C.
- the MTC tablets were sprayed with the aqueous film coat at a spray rate such that the bed temperature is maintained at 38-48°C, preferably 42-44°C. Once the required weight gain was achieved, spraying was stopped and the temperature inlet control was switched off. The tablets were allowed to cool on a jog cycle until bed temperature reached room temperature.
- a similar method may be used for non-aqueous film coating.
- the coating pan was warmed with the tablets for 10 minutes with an inlet air temperature around 50°C, preferably around 40°C, until the bed temperature reached 30-45°C, preferably 35-40°C.
- the MTC tablets were sprayed with the aqueous film coat at a starting spray rate such that the bed temperature was maintained at 28-44°C, preferably 30-35°C.
- the inlet temperature was increased to around 50°C, preferably 45°C, the spray rate was used to keep the bed temperature at 28-44°C, preferably 35-40°C.
- the inlet temperature was increased to around 60°C, preferably 50°C, and the spray rate increased to keep the bed temperature at 28-44°C, preferably 35-40°C.
- spraying was stopped and the temperature inlet control was switched off. The tablets were allowed to cool on a jog cycle until the bed temperature reached room temperature.
- a similar method may be used for non-aqueous film coating.
- An example of a tablet formulation that may be film-coated by one of the above methods is: Ingredient mg/tablet MTC 147.24 MCC 230.00 Spray-dried lactose 38.76 Crosscarmelose sodium 30.00 Magnesium stearate 4.00 Tablet weight 450.00
- MTC tablets were manufactured at various tablet strengths, stored under controlled conditions of temperature and relative humidity, and the polymorphic forms analysed by XRPD: MTC Form A tablets; stored in aluminium blisters Tablet strength Storage conditions Storage time / months Polymorphic form 50mg 25°C / 65% RH 10.5 A 25mg 25°C / 65% RH 9 A 25mg 40°C / 75% RH 6 A
- MTC capsules for example in Gelatin or HPMC, may be prepared using compositions of MTC with diluents, disintegrants and lubricants which may be qualitatively similar to those discussed above in respect of the tablet formulations.
- An example capsule composition containing 25, 50, 75, 100 mg of MTC as free base equivalent in Gelatin or HPMC capsule shells is as follows: Capsule strength in (mg of MTC) 25 50 75 100 Ingredient Function mg/capsule MTC (free base equivalent) Drug 36.80 73.61 110.41 147.22 Spray-dried mannitol Diluent 238.70 201.89 165.09 128.28 Cross-linked polyvinylpyrrolidone Disintegrant 3.00 3.00 3.00 3.00 Magnesium stearate Lubricant 1.50 1.50 1.50 1.50 Total 280.00 280.00 280.00 280.00 280.00 280.00 280.00 280.00 280.00
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Epidemiology (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Neurology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Neurosurgery (AREA)
- Biomedical Technology (AREA)
- Psychiatry (AREA)
- Hospice & Palliative Care (AREA)
- Pain & Pain Management (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Physical Education & Sports Medicine (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Claims (18)
- Pharmazeutische Zusammensetzung in fester Dosierungsform, die eine Tablette ist, die als Wirkstoff Methylthioniumchlorid (MTC) umfasst, wobei die Zusammensetzung weiters zumindest einen für Direktpressung geeigneten Verdünner umfasst, der mikrokristalline Cellulose oder ein Zucker ist,
wobei das MTC in einer im Wesentlichen reinen und stabilen polymorphen Form vorliegt, die die polymorphe Form A ist,
wobei die Menge an MTC in der Zusammensetzung mehr als 10 Gew.-% beträgt und
wobei die Zusammensetzung durch ein Verfahren erhalten wird, das die Trockenpressung eines innigen Pulvergemischs des MTC in einer im Wesentlichen reinen polymorphen Form mit dem zumindest einen für Direktkompression geeigneten Verdünner und gegebenenfalls einem oder mehreren weiteren Exzipienten umfasst. - Zusammensetzung nach Anspruch 1, wobei das Verfahren ein Direktpressverfahren ist, in dem MTC, ein oder mehrere Verdünner und weitere optionale Exzipienten in Form fester Teilchen vermischt werden, um ein inniges Gemisch zu bilden, und anschließend unter Verwendung einer Tablettiermaschine verpresst werden.
- Zusammensetzung nach Anspruch 1, wobei das Verfahren ein Trockengranulationsverfahren ist, in dem MTC, ein oder mehrere Verdünner und weitere optionale Exzipienten zu einer gepressten Masse geformt, gemahlen und anschließend unter Verwendung einer Tablettiermaschine verpresst werden.
- Zusammensetzung nach Anspruch 1, wobei das Verfahren ein Nassgranulationsverfahren ist, in dem der/die Verdünner unter Verwendung eines Granulationsmediums nass geknetet wird/werden und die nasse Masse anschließend getrocknet wird, um ein Granulat zu bilden, das daraufhin mit MTC vermischt und zu Tabletten verpresst wird.
- Zusammensetzung nach einem der vorangegangenen Ansprüche, wobei:(i) die Menge an MTC in der Zusammensetzung mehr als 20 Gew.-% oder mehr als 30 Gew.-% und gegebenenfalls weniger als 75 Gew.-% oder weniger als 60 Gew.-% oder weniger als 50 Gew.-% beträgt, beispielsweise zwischen 10 Gew.-% (oder 20 Gew.-% oder 30 Gew.-%) und 70 Gew.-% (oder 60 Gew.-% oder 50 Gew.-%); und/oder(ii) die Zusammensetzung zumindest 15 Gew.-%, zumindest 20 Gew.-%, zumindest 30 Gew.-%, zumindest 40 Gew.-% oder zumindest 50 Gew.-% an Verdünner(n) umfasst.
- Zusammensetzung nach einem der vorangegangenen Ansprüche, wobei der/ die Verdünner aus der aus mikrokristalliner Cellulose, Lactose und Mannit bestehenden Gruppe ausgewählt ist/sind.
- Zusammensetzung nach einem der vorangegangenen Ansprüche, die weiters ein Schmiermittel, gegebenenfalls 0,5 bis 2,0 Gew.-% Schmiermittel, umfasst,
wobei das Schmiermittel gegebenenfalls aus der aus Magnesiumstearat, Calciumstearat und Stearinsäure bestehenden Gruppe ausgewählt ist. - Zusammensetzung nach einem der vorangegangenen Ansprüche, die weiters Folgendes umfasst:(i) einen oder mehrere Zerfallsbeschleuniger, der/die aus der aus vernetztem Polyvinylpyrrolidon, Natriumstärkeglykolat, vernetzter Natriumcarboxymethylcellulose (Natriumcroscarmellose) und vorgelatinierter Stärke bestehenden Gruppe ausgewählt ist/sind; und/oder(ii) ein Bindemittel, das aus der aus Cellulosederivaten (Carboxymethylcellulose, Hydroxypropylmethylcellulose, Hydroxypropylcellulose, Hydroxyethylcellulose, Ethylcellulose, mikrokristalliner Cellulose) und Zuckern wie z.B. Lactose, Saccharose, Dextrose, Glucose, Maltodextrin und Mannit, Xylit, Polymethacrylaten, Polyvinylpyrrolidon, Sorbit, vorgelatinierter Stärke, Alginsäuren und Salzen davon wie z.B. Natriumalginat, Magnesiumaluminiumsilikat, Polyethylenglykol bestehenden Gruppe ausgewählt ist.
- Zusammensetzung nach einem der vorangegangenen Ansprüche, wobei eine Filmbeschichtung aufgetragen wurde, wobei die Filmbeschichtung gegebenenfalls Hydroxypropylmethylcellulose (HPMC) oder partiell hydrolysierten Polyvinylalkohol (PVA) umfasst.
- Verfahren zur Herstellung einer pharmazeutischen Zusammensetzung nach Anspruch 1, wobei das Verfahren die Trockenpressung eines innigen Pulvergemischs von Methylthioniumchlorid (MTC) in einer im Wesentlichen reinen polymorphen Form, die die polymorphe Form A ist, mit zumindest einem für Direktpressung geeigneten Verdünner und gegebenenfalls einem oder mehreren weiteren Exzipienten umfasst.
- Verfahren nach Anspruch 10, wobei das Verfahren die Direktpressung eines innigen Pulvergemischs von MTC in einer im Wesentlichen reinen polymorphen Form mit zumindest einem für Direktpressung geeigneten Verdünner und gegebenenfalls einem oder mehreren weiteren Exzipienten umfasst.
- Verfahren nach Anspruch 10, das ein Direktpressverfahren ist, in dem MTC, ein oder mehrere Verdünner und weitere optionale Exzipienten in Form fester Teilchen vermischt werden, um ein inniges Gemisch zu erzeugen, und anschließend unter Verwendung einer Tablettiermaschine verpresst werden.
- Verfahren nach Anspruch 10, das ein Trockengranulationsverfahren ist, in dem MTC, ein oder mehrere Verdünner und weitere optionale Exzipienten zu einer gepressten Masse geformt, gemahlen und anschließend unter Verwendung einer Tablettiermaschine verpresst werden.
- Verfahren nach Anspruch 10, das ein Feuchtgranulationsverfahren ist, in dem der/die Verdünner unter Verwendung eines Granulationsmediums nass geknetet wird/werden und die nasse Masse anschließend getrocknet wird, um ein Granulat zu bilden, das daraufhin mit MTC vermischt und zu Tabletten verpresst wird.
- Verfahren nach einem der Ansprüche 10 bis 14, das weiters den Schritt des Auftragens einer Filmbeschichtung auf die Tabletten umfasst,
wobei der Schritt des Auftragens einer Filmbeschichtung in einer Beschichtungstrommel durchgeführt wird und das Vorwärmen der Beschichtungstrommel vor dem Zuführen der zu beschichtenden Tabletten umfasst,
wobei gegebenenfalls die Temperatur der Tabletten während der Beschichtung bei einer Temperatur von 38-48 °C, noch bevorzugter 42-44 °C gehalten wird. - Zusammensetzung in fester Dosierungsform nach einem der Ansprüche 1 bis 9 zur Verwendung in einem Verfahren zur Behandlung oder Prophylaxe eines Krankheitszustands in einem Patienten, wobei das Verfahren das Verabreichen einer therapeutisch wirksamen Menge der Zusammensetzung an den Patienten umfasst.
- Zusammensetzung zur Verwendung nach Anspruch 16, wobei die Krankheit eine Tauopathie ist.
- Im Wesentlichen feuchtigkeitsundurchlässige Blisterpackung, die eine Zusammensetzung nach einem der Ansprüche 1 bis 9 umfasst.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US41816410P | 2010-11-30 | 2010-11-30 | |
| PCT/GB2011/001662 WO2012072977A2 (en) | 2010-11-30 | 2011-11-30 | Compound formulations |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP2645994A2 EP2645994A2 (de) | 2013-10-09 |
| EP2645994B1 true EP2645994B1 (de) | 2017-11-01 |
Family
ID=45418703
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP11802119.5A Active EP2645994B1 (de) | 2010-11-30 | 2011-11-30 | Formulierungen enthaltend methylthioninium chlorid |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US9192611B2 (de) |
| EP (1) | EP2645994B1 (de) |
| JP (1) | JP6093707B2 (de) |
| CN (1) | CN103379901B (de) |
| AU (1) | AU2011334679B2 (de) |
| CA (1) | CA2818068C (de) |
| ES (1) | ES2655492T3 (de) |
| MY (1) | MY163074A (de) |
| SG (2) | SG190406A1 (de) |
| WO (1) | WO2012072977A2 (de) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2021116611A1 (fr) * | 2019-12-12 | 2021-06-17 | Universite de Bordeaux | Formulation pour le bleu de methylene et procede |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014153444A (ja) * | 2013-02-06 | 2014-08-25 | Konica Minolta Inc | 位相差フィルム、偏光板及び液晶表示装置 |
| MX2019000946A (es) | 2016-07-25 | 2019-05-13 | Wista Lab Ltd | Administracion y dosificacion de diaminofenotiazinas. |
| WO2023232764A1 (en) | 2022-05-31 | 2023-12-07 | Wista Laboratories Ltd. | Treatment of neurodegenerative disorders utilising methylthioninium (mt)-containing compounds |
| WO2024063700A1 (en) | 2022-09-21 | 2024-03-28 | Yin Sze Loh | Oral formulation of diaminophenothiazines and methods of making and using the same in the treatment and/or prevention of diseases |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4880373A (en) | 1988-04-28 | 1989-11-14 | The Upjohn Company | Tablet press |
| JPH0840942A (ja) * | 1994-07-29 | 1996-02-13 | Kanegafuchi Chem Ind Co Ltd | 薬物放出制御製剤 |
| GB9506197D0 (en) | 1995-03-27 | 1995-05-17 | Hoffmann La Roche | Inhibition of tau-tau association. |
| GB0101049D0 (en) | 2001-01-15 | 2001-02-28 | Univ Aberdeen | Materials and methods relating to protein aggregation in neurodegenerative disease |
| GB0106953D0 (en) | 2001-03-20 | 2001-05-09 | Univ Aberdeen | Neufofibrillary labels |
| GB0322756D0 (en) | 2003-09-29 | 2003-10-29 | Univ Aberdeen | Methods of chemical synthesis |
| EP2322517B1 (de) | 2004-09-23 | 2019-04-24 | WisTa Laboratories Ltd. | Verfahren zur chemischen synthese und aufreinigung von diaminophenothiaziniumverbindungen einschliesslich methylthioniniumchlorid (mtc) |
| US7790881B2 (en) * | 2004-09-23 | 2010-09-07 | Wista Laboratories Ltd. | Methods of chemical synthesis and purification of diaminophenothiazinium compounds including methylthioninium chloride (MTC) |
| DE602005011326D1 (de) * | 2004-10-19 | 2009-01-08 | Krka Tovarna Zdravil D D | Feste pharmazeutische zusammensetzung mit donepezilhydrochlorid |
| CN101304731B (zh) * | 2005-08-24 | 2012-06-20 | 惠氏公司 | 醋酸苯卓昔芬制剂及其生产方法 |
| DK1808165T3 (da) * | 2006-01-05 | 2009-08-03 | Teva Pharma | Törre formuleringer af aripiprazol |
| PT2013191E (pt) | 2006-03-29 | 2010-10-29 | Wista Lab Ltd | Sais de 3,7-diamino-10h-fenotiazina e a sua utilização |
| US20080317678A1 (en) * | 2007-06-22 | 2008-12-25 | Szymczak Christopher E | Laser Marked Dosage Forms |
| ES2546819T3 (es) | 2007-10-03 | 2015-09-28 | Wista Laboratories Ltd. | Uso terapéutico de las diaminofenotiazinas |
| EP2227766A2 (de) | 2007-11-05 | 2010-09-15 | Wista Laboratories Ltd. | Systeme für klinische versuche |
| MY161463A (en) * | 2009-09-24 | 2017-04-14 | Wista Lab Ltd | Process for the preparation of substantially pure methylthionium chloride pentahydrate form a |
-
2011
- 2011-11-30 US US13/989,953 patent/US9192611B2/en active Active
- 2011-11-30 CN CN201180057700.9A patent/CN103379901B/zh active Active
- 2011-11-30 WO PCT/GB2011/001662 patent/WO2012072977A2/en active Application Filing
- 2011-11-30 SG SG2013040357A patent/SG190406A1/en unknown
- 2011-11-30 JP JP2013541414A patent/JP6093707B2/ja active Active
- 2011-11-30 EP EP11802119.5A patent/EP2645994B1/de active Active
- 2011-11-30 SG SG10201602111VA patent/SG10201602111VA/en unknown
- 2011-11-30 AU AU2011334679A patent/AU2011334679B2/en active Active
- 2011-11-30 ES ES11802119.5T patent/ES2655492T3/es active Active
- 2011-11-30 MY MYPI2013001934A patent/MY163074A/en unknown
- 2011-11-30 CA CA2818068A patent/CA2818068C/en active Active
Non-Patent Citations (1)
| Title |
|---|
| None * |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2021116611A1 (fr) * | 2019-12-12 | 2021-06-17 | Universite de Bordeaux | Formulation pour le bleu de methylene et procede |
| FR3104438A1 (fr) * | 2019-12-12 | 2021-06-18 | Universite de Bordeaux | Formulation pour le bleu de methylene et procede |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2645994A2 (de) | 2013-10-09 |
| CA2818068C (en) | 2019-06-11 |
| ES2655492T3 (es) | 2018-02-20 |
| JP6093707B2 (ja) | 2017-03-08 |
| US9192611B2 (en) | 2015-11-24 |
| AU2011334679A1 (en) | 2013-07-18 |
| MY163074A (en) | 2017-08-15 |
| US20130243858A1 (en) | 2013-09-19 |
| AU2011334679B2 (en) | 2016-12-15 |
| CN103379901B (zh) | 2018-04-03 |
| JP2013544272A (ja) | 2013-12-12 |
| CA2818068A1 (en) | 2012-06-07 |
| WO2012072977A2 (en) | 2012-06-07 |
| CN103379901A (zh) | 2013-10-30 |
| WO2012072977A8 (en) | 2012-09-20 |
| SG10201602111VA (en) | 2016-04-28 |
| SG190406A1 (en) | 2013-06-28 |
| WO2012072977A3 (en) | 2012-08-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| TWI778983B (zh) | 包含2-羥基-6-((2-(1-異丙基-1h-吡唑-5-基)吡啶-3-基)甲氧基)-苯甲醛之片劑 | |
| EP3024442B1 (de) | Zusammensetzungen mit amorphem dapagliflozin | |
| EP2974720B1 (de) | Präparat mit verzögerter mosaprid-freisetzung zur erreichung pharmakologischer klinischer wirkungen zur täglichen verabreichung | |
| EP2645994B1 (de) | Formulierungen enthaltend methylthioninium chlorid | |
| MX2009002336A (es) | Composiciones de imatinib. | |
| JP2008536922A (ja) | オランザピンの医薬用経口崩壊錠 | |
| JP6215940B2 (ja) | 8−[(3r)−3−アミノ−1−ピペリジニル]−7−(2−ブチン−1−イル)−3,7−ジヒドロ−3−メチル−1−[(4−メチル−2−キナゾリニル)メチル]−1h−プリン−2,6−ジオンまたはその医薬的に許容され得る塩を含む安定な医薬組成物 | |
| AU2019232937B2 (en) | Ceritinib formulation | |
| CN106822112B (zh) | 一种替米沙坦氨氯地平双层片的制备方法 | |
| CN106511291A (zh) | 一种盐酸阿考替胺缓释片剂及其制备方法 | |
| Buwade et al. | Advantages of immediate release tablets over the other tablet forms | |
| WO2020111089A1 (ja) | 医薬組成物 | |
| US9949964B2 (en) | Tesofensine compositions | |
| JP2012180280A (ja) | 貯蔵安定性が改善された固形製剤 | |
| BRPI0621739A2 (pt) | formulação estável que consiste em drogas sensìveis à umectação e seu procedimento de fabricação | |
| EP4183390A1 (de) | Pharmazeutische schmelztablettendosierungsform von edoxaban | |
| JP2009536643A (ja) | 2−n−{5−[[4−[2−(メチル−2−ピリジニルアミノ)エトキシ]フェニル]メチル]−2,4−チアゾリジンジオン}−ブタン二酸、製造方法、および、ロシグリタゾンマレアートとの組成物 | |
| Bhaskar et al. | A review on formulation approaches in immediate release tablet | |
| CN107648237B (zh) | 氨基嘧啶类化合物的药物组合物及其制备方法 | |
| CN118356426A (zh) | 一种包含并环化合物的药物组合物及其制备方法和应用 | |
| WO2019170795A1 (en) | A pharmaceutical solid oral dosage form of solifenacin | |
| JP2021104939A (ja) | ラメルテオンを含む錠剤および医薬組成物 | |
| BR112019020269A2 (pt) | formulação farmacêutica |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20130620 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: WISTA LABORATORIES LTD. |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: KHAN, KARRAR, AHMAD |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: DE Ref document number: 1184386 Country of ref document: HK |
|
| DAX | Request for extension of the european patent (deleted) | ||
| 17Q | First examination report despatched |
Effective date: 20140623 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: WISTA LABORATORIES LTD. |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: A61K 9/48 20060101ALI20170419BHEP Ipc: A61K 9/20 20060101ALI20170419BHEP Ipc: C07D 279/18 20060101ALI20170419BHEP Ipc: A61K 9/14 20060101AFI20170419BHEP Ipc: A61K 9/16 20060101ALI20170419BHEP Ipc: A61P 25/28 20060101ALI20170419BHEP Ipc: A61K 31/5415 20060101ALI20170419BHEP |
|
| INTG | Intention to grant announced |
Effective date: 20170515 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP Ref country code: AT Ref legal event code: REF Ref document number: 941305 Country of ref document: AT Kind code of ref document: T Effective date: 20171115 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602011042984 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 7 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: FP |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2655492 Country of ref document: ES Kind code of ref document: T3 Effective date: 20180220 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 941305 Country of ref document: AT Kind code of ref document: T Effective date: 20171101 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180201 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180201 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180301 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180202 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602011042984 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20171130 Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: GR Ref document number: 1184386 Country of ref document: HK |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 8 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20171130 |
|
| 26N | No opposition filed |
Effective date: 20180802 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20111130 Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171101 |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230427 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20231023 Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20231120 Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20231201 Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20231121 Year of fee payment: 13 Ref country code: IE Payment date: 20231023 Year of fee payment: 13 Ref country code: FR Payment date: 20231027 Year of fee payment: 13 Ref country code: DE Payment date: 20231031 Year of fee payment: 13 Ref country code: CH Payment date: 20231201 Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20231031 Year of fee payment: 13 |