EP1430163B1 - Edelmetallrückgewinnung - Google Patents
Edelmetallrückgewinnung Download PDFInfo
- Publication number
- EP1430163B1 EP1430163B1 EP02800002A EP02800002A EP1430163B1 EP 1430163 B1 EP1430163 B1 EP 1430163B1 EP 02800002 A EP02800002 A EP 02800002A EP 02800002 A EP02800002 A EP 02800002A EP 1430163 B1 EP1430163 B1 EP 1430163B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- fluid
- palladium
- rinsing
- membrane filter
- work pieces
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C18/00—Chemical coating by decomposition of either liquid compounds or solutions of the coating forming compounds, without leaving reaction products of surface material in the coating; Contact plating
- C23C18/16—Chemical coating by decomposition of either liquid compounds or solutions of the coating forming compounds, without leaving reaction products of surface material in the coating; Contact plating by reduction or substitution, e.g. electroless plating
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C18/00—Chemical coating by decomposition of either liquid compounds or solutions of the coating forming compounds, without leaving reaction products of surface material in the coating; Contact plating
- C23C18/16—Chemical coating by decomposition of either liquid compounds or solutions of the coating forming compounds, without leaving reaction products of surface material in the coating; Contact plating by reduction or substitution, e.g. electroless plating
- C23C18/1601—Process or apparatus
- C23C18/1617—Purification and regeneration of coating baths
Definitions
- the invention relates to a method and a device for the plating of work pieces with a fluid containing palladium.
- the invention is especially applicable in processes for producing electrical circuit carriers.
- Ionic palladium may more specifically be present in the form of a salt, such as palladium chloride for example, which is generally dissolved in a hydrochloric acidic solution.
- Ionogenic palladium is present as a complex, an aminopyridine complex for example.
- Colloidal palladium may contain diverse protective colloids, e.g., a protective colloid formed from tin(II) chloride or consisting of an organic polymer.
- the palladium nuclei which are thereupon adsorbed on the surfaces of the work pieces serve for example as activators to initiate an electroless metal deposition that causes an electrically conductive layer to form on the surfaces so that the surfaces are then ready to be metal-plated with any metal.
- This method is utilized for producing printed circuit boards and other circuit carriers as well as metal-plated parts in the sanitary, automotive and furniture industry for example and more specifically for chromium-plating plastic parts.
- the palladium containing solution may also be used for forming an electrically conductive layer.
- further metal is electrolytically deposited after palladium treatment without a metal layer being previously formed with an electroless metal coating method.
- part of the palladium containing solution still adheres to the work pieces when the previously immersed work pieces are emersed from the solution.
- the adhering solution is usually rinsed with water.
- a solution is used that usually contains 50 - 400 mg/l of palladium.
- about 5 - 10 mg of palladium are typically adsorbed. This quantity is necessary to activate the plastic surface.
- about 0.2 1 activating solution per square meter is carried over from the bath and is still left on the surfaces. Therefore, approximately 10 - 50 mg of palladium get lost to the bath because the adhering solution is entrained out of the processing bath, then rinsed and transferred to waste water treatment.
- the palladium entrained from the processing solution amounts to about 50 mg/m 2 .
- the adsorption of the palladium particles may be increased from a relatively low value to about 50 mg/m 2 of the surface of the work pieces.
- about 60 - 70% of the palladium utilized in the solution gets lost by being entrained. 40 - 30% only can actually be used for metal-plating the surfaces of the work pieces.
- U.S. Patent No. 4,078,918 e.g., describes a recovery process for reclaiming e.g., palladium from various materials that contain dissolved or non-dissolved palladium.
- the materials are at first treated with an oxidizing agent to destroy possible organic components and are then treated with ammonium hydroxide in order to form amine complexes.
- the thus obtained palladium complexes are next reduced with ascorbic acid so that palladium deposits from the processing solution as a metal and may be filtered.
- U.S. Patent No. 4,435,258 discloses another method for recovering palladium from spent baths that are utilized for activating electrically non-conductive surfaces for the subsequent electroless metal-plating process.
- the activating solutions are reprocessed by causing the colloidal palladium to oxidize into the solution by adding an oxidizing agent such as hydrogen peroxide for example, by subsequently heating the solution to destroy the residual hydrogen peroxide and by thereafter electrolytically depositing palladium from these solutions onto a cathode.
- an oxidizing agent such as hydrogen peroxide for example
- DE 100 24 239 C1 describes a method of electroplating work pieces with a palladium colloid solution by contacting the work pieces with the colloid solution according to which palladium is recovered after the colloid solution was used, by separating palladium colloid particles from the colloid solution by means of a membrane filter.
- Materials made from ceramics for example may be used for filtration.
- the pore exclusion size of the membranes amounts to 200 to 10,000 Dalton. It is stated therein that the palladium particles pass through the membrane filter when the pore exclusion size used is in excess of 10,000 Dalton.
- the basic problem the present invention faces is to avoid the drawbacks of the known methods and to find a method for plating work pieces with a fluid containing palladium that may be carried out at low cost. Small quantities of additional chemicals only should be necessary to carry out the method. Furthermore, the method should involve little expense of energy and time and should more specifically require low maintenance.
- the method in accordance with the invention serves to plate work pieces with a fluid, said fluid containing palladium, the method comprising contacting the work pieces with the fluid.
- said fluid is mixed with chemical substances selected from the group comprising pH adjusting agents, reducing agents, sulfur compounds, selenium compounds and tellurium compounds such that the palladium is altered in such a manner that it is substantially entirely retained during filtration and is thereafter filtered through at least one ceramic membrane filter to separate the palladium from the fluid, the ceramic membrane filter having an exclusion pore size in excess of 10,000 Dalton. Due to the filtering the palladium is separated from the fluid.
- any treatment with fluids is meant that is directed to alter the surface of the work pieces, the fluid having to contain palladium.
- methods of coating work pieces with polymer coatings more specifically enamelling methods.
- the work pieces to be plated include metallic work pieces, non-metallic work pieces and work pieces consisting of both metallic and non-metallic materials.
- the work pieces may have all conceivable forms and be intended for all conceivable utilizations.
- Preferred pieces are semi-finished products for producing circuit carriers, more specifically for producing printed circuit boards and hybrid circuit carriers such as multichip modules for example.
- the fluid may be a solution. This is more specifically the case when the palladium is present in ionic or ionogenic form.
- ionic form of the palladium salts of palladium dissolved in water or in another solvent that promotes the dissociation of said salts is more specifically meant.
- ionogenic form of the palladium palladium complexes are meant, more specifically.complexes of the palladium ions with organic complex ligands.
- the complexes may be uncharged or be present in the form of ions.
- the fluid may be present in the form of a colloid, more specifically of a colloid of the elemental palladium.
- the palladium containing fluid may be both a processing fluid for treating the work pieces or a rinsing fluid.
- processing fluid a fluid is meant that serves to alter the surface properties of the work pieces, e.g., a coating fluid, including an activating fluid, a cleansing fluid, an etching fluid or the like.
- a rinsing fluid only serves, after treatment of a work piece with the processing fluid, to rinse off the processing fluid still adhering on the surface of the work piece.
- the palladium is filtered in the at least one ceramic membrane filter.
- the fluid is at first utilized for plating and is only filtered afterwards in the membrane filter for the purpose of recovering the palladium that it contains.
- the fluid can for example be contacted with a work piece by spraying, jetting, flooding or blasting, the fluid dripping off the work piece collected and the collected fluid be conducted to the membrane filter immediately thereafter.
- the collected fluid may also be first retained in a reservoir from where it is delivered back to the work piece, though.
- the fluid may be either conducted to the membrane filter after having been collected for a predetermined period of time (intermittent method), or part of the fluid may be tapped continuously from the reservoir and be transferred to the membrane filter (continuous method).
- new processing fluid is permanently introduced into the reservoir in a quantity per unit of time that equals the quantity of the fluid permeating the membrane filter per unit of time.
- the work pieces may also be contacted with processing fluid contained in a treatment container by immersing them thereinto.
- the processing fluid is, after use, either conducted to the membrane filter after having been collected for a predetermined period of time (intermittent method), or part of the fluid in the reservoir may be tapped continuously from the treatment container and be transferred to the membrane filter (continuous method).
- the method of the invention permits to achieve in a simple manner and with little expense of chemicals, energy and time as well as with little maintenance, a far-reaching separation of palladium from the exhausted processing solutions under continuous operation. It more specifically permits to regenerate the exhausted processing solutions after the fraction containing the palladium has been separated so that the entire palladium may be recirculated into the process.
- the present method has the advantage that the fractions are completely separated whereas with the precipitation method described in Chemical Abstracts a non-negligible part of the palladium is oxidized to form the bivalent, soluble stage thereof so that it cannot be completely separated from the solution by filtration. Accordingly, this part of the palladium cannot be recovered and will be lost.
- the method in accordance with the invention also has substantial advantages over the method described in U.S. Patent No. 4,435,258 , namely that palladium may be removed almost entirely from the solutions, whereas, by the method according to U.S. Patent No. 4,435,258 , only an extremely low current efficiency may be achieved, especially when the palladium concentration is low, which occurs after a long period of electrolysis. Therefore, it is either very complicated or not possible at all to completely remove palladium with this prior art method.
- Colloidal activators on the basis of palladium comprise palladium particles that are surrounded by a protective coating (protective colloid).
- Tests using high resolution transmission electron microscopy (HTEM) and atomic force microscopy (AFM) showed that the palladium particles have a diameter of at least 2.5 nm.
- the mean particle diameter amounts to approximately 4 nm, which corresponds to the gaussian distribution of particles.
- HTEM transmission electron microscopy
- AFM atomic force microscopy
- a wide particle size distribution that showed particles with a maximum size of about 18 nm as well as smaller particles (from 2 to 18 nm) was determined.
- colloid solutions are acidic, often highly hydrochloric acidic, and contain chloride ions as well as possibly tin in the oxidation stages (II) and (IV) or organic, polymeric stabilizers like gelatine or polyvinyl pyrrolidone and reducing agents. Except for the polymers, which are utilized in small quantities, all the other substances contained therein are ionic. It is presumed that these ionic constituents are much smaller than the palladium particles.
- palladium particles may be removed very selectively and completely from these colloid solutions by means of appropriate membrane filters comprising different porosities, although, in the case of the tin containing colloidal solutions, tin, which is simultaneously present, is contained in a high concentration (usually more than 70 times the palladium concentration) and although the tin compounds are known to form colloidal solutions that are difficult to filter.
- ceramic membrane filters may be utilized that have an exclusion pore size of from about 15,000 Dalton to about 25,000 Dalton, more specifically an exclusion pore size of from about 17,500 Dalton to about 22,500 Dalton and most preferably of approximately 20,000 Dalton.
- a preferably utilized ceramic membrane filter is made of a ceramic material containing aluminum oxide, more specifically ⁇ -Al 2 O 3 , titanium dioxide and possibly zirconium dioxide. In principle, other filter materials may also be utilized. As a rule, the filter material is deposited onto a highly porous supporting body that provides the filter with the required mechanical stability. This supporting body may consist of ⁇ -Al 2 O 3 or of SiC (silicon carbide) for example.
- the filter may be configured in the form of a disc or as a tube.
- a flow is directed onto the disc, approximately normal to the surface thereof, said flow being deviated in radial direction.
- a pressure difference is built up between the two surfaces of the disc so that permeate may pervade the disc.
- the filter has the shape of a tube, the fluid is conveyed through the tube in axial direction, a pressure difference being built up between the inner space and the outer space of the tube.
- permeate can pervade the wall of the tube e.g., from the interior volume of the tube to the space external of the tube.
- This second method is called dynamic filtration.
- the palladium is retained within the inner space of the tube, whereas the fluid, which has been largely freed from palladium permeates through the wall of the tube from the inner volume of the tube to the space external of the tube.
- Some fluids may be filtered directly without any further pretreatment. In this case, very good results are obtained with the ceramic membrane filters.
- the fluids to be reprocessed are chemically pretreated first. After having been used for plating and prior to being filtered through the membrane filter, the fluid is mixed for this purpose with chemical substances that are suited to alter the palladium in such a manner that it is almost completely retained during filtration. It is presumed that, by adding these chemical substances, the particle size of the palladium is altered in such a manner that the particles that contain palladium cannot pass through the pores of the membrane filter. For this purpose it should be sufficient to adjust the average particle size to a value in excess of about 10 nm when the particle size fits the Gaussian distribution. In this case, a membrane filter with an exclusion pore size in excess of 10,000 Dalton would already retain almost the entire quantity of palladium in the concentrate. Larger particles may be set accordingly by adding these chemical substances when membrane filters with a greater exclusion pore size are being used.
- the fluid may be mixed with chemical substances selected from the group comprised of reducing agents, sulfur compounds, selenium compounds and tellurium compounds.
- the chemical substances for pretreatment are most preferably selected from the group comprised of boron hydrides, amine boranes, hypophosphites, inorganic sulfides and organic thio compounds, more specifically the alkali and ammonium salts of dimethyl dithiocarbamate, of sulfides, of boron hydrides such as tetrahydroboranate for example, and of hypophosphites.
- the organic thio compounds considered are more specifically organic compounds in which sulfur is bonded to one or to two atoms of carbon to form a single or a double bond therewith i.e., thioles, sulfides, disulfides and polysulfides, thioamides and thioaldehydes for example.
- pH adjusting agents are used as chemical substances.
- the fluid is mixed with the pH adjusting agents in such a manner that solution pH ranges from 3 to 12.
- the work pieces which are preferably made of an electrically non-conductive material, are rinsed in a suitable device with a rinsing fluid by immersing them into it, by flooding or preferably by spraying said rinsing fluid onto said work pieces in order to keep the volume of rinsing solution as small as possible.
- the rinsing fluid is next conducted through a ceramic membrane filter by means of a pressure pump, said filter retaining the palladium particles and allowing the rinsing water to permeate. Said permeate may then be transferred to waste water treatment.
- the processing fluid and/or the rinsing fluid may be mixed with the chemical substances such as for example the reducing agents, sulfur compounds, selenium compounds, tellurium compounds or the pH adjusting agents.
- the rinsing fluid or a rinsing fluid containing preferably up to 5 percent by volume of processing fluid, is conducted through the membrane filter (preferably under pressure).
- the work pieces are contacted with fresh rinsing solution, a predetermined quantity of fresh rinsing solution per unit of time being permanently available.
- the quantity of the permeate fluid formed per unit of time may be more specifically adjusted to approximately equal the quantity of the rinsing fluid that is contacted with the work pieces per unit of time.
- Retained palladium which is present as a concentrate in the form of a homogeneous dispersion of metal or of a metal compound, e.g., in the form of a PdS dispersion, may be recycled.
- the retained palladium may e.g., be dissolved, converted to palladium chloride and be utilized to synthesize a new palladium containing processing fluid or for any other application.
- the palladium containing concentrate solution may also be concentrated to near dryness in a filter press.
- the concentrate fluid coming from the membrane filter is directed into a container in which palladium containing slurry that has formed during concentration deposits, the slurry suspension being directed to the filter press.
- the palladium containing filter cake obtained by means of the filter press may be utilized as a base substance for producing pure palladium and palladium compounds.
- the device for plating work pieces with a fluid containing palladium is typically provided with means for contacting the work pieces with the fluid as well as with holding means for the work pieces.
- the means for contacting the fluid with the work pieces are e.g., nozzles by means of which processing or rinsing fluid is sprayed, jetted, flooded or discharged onto the surfaces of the work pieces. This arrangement is used for example when the fluid is to reach the surface at a high flow velocity or when the quantity of the fluid needed is to be minimized.
- the contacting means are treatment containers in which the processing fluid is disposed and into which the work pieces are immersed.
- the holding means for the work pieces may also be embodied in very diverse forms: the work pieces may for example be retained in a conventional way by means of cramps, clamps, tongs or screw fastenings. Furthermore, the work pieces may also simply be held, transported and conducted in a horizontal position on rolls, wheels or cylinders or they may be clamped therein between.
- the device also comprises a facility for separating the palladium from the fluid.
- This facility comprises at least one ceramic membrane having an exclusion pore size in excess of 10,000 Dalton.
- the facility comprises at least one pump for delivering the fluid to the at least one membrane and fluid conduits for conducting the fluid from the means for contacting the work pieces with the fluid to the at least one ceramic membrane.
- a pump any pump which is not motor operated or simply delivery of the fluid by gravity is also meant.
- the facility for separating the palladium from the fluid is furthermore provided with a mixing facility.
- a mixing facility fluid coming from the means for contacting the work pieces with the fluid can be mixed with chemical substances.
- any conventional mixing facility known in the chemical reaction technique such as e.g., stirring facilities and mixing zones in flow reactors, may be utilized.
- the facility for separating the palladium from the fluid may also be provided with a multiphase separating unit in which slurry may deposit which is produced during separation from the fluid and which comes from the facility for separating the palladium from the fluid.
- a multiphase separating unit of this type is formed by a sedimentation tank for example in which virtually no fluid convection is taking place. Said slurry suspension may then be directed into a filter press in order to largely purify and dry the slurry, which mainly contains palladium.
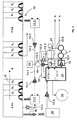
- Fig. 1 illustrates a ceramic membrane filter in the form of a tube 1 .
- the tube is made of a highly porous ceramic material that serves as support 3 and that is, in the present case, of aluminum oxide.
- the support 3 is provided, on its inner side, with another ceramic layer of an oxide that serves as a membrane filter layer 2 .
- Said membrane filter layer 2 in turn consists of two layers (not specifically shown), i.e., a first microfiltration layer made of ⁇ -Al 2 O 3 and of a second ultrafiltration layer made of ZrO 2 and TiO 2 , TiO 2 having the finest pore size so that filtration is also possible with an exclusion pore size of e.g., 20,000 Dalton.
- the membrane filter layer 2 has an exclusion pore size of about 20,000 Dalton. Accordingly, the mean pore size amounts to approximately 20 nm.
- the tube has an inside diameter of about 6 mm.
- the tube is about 1000 mm in length.
- the flow passes therethrough under pressure in the direction of flow referred to be reference numeral 4 .
- the pressure difference between the entrance and the exit of the tube ranges from 1.5 to 3 bar.
- the ceramic tube In order to collect the permeate passing through the internal wall of the tube, the ceramic tube is positioned concentrically within another tube.
- Fig. 2 comprises two of the filter tubes 1 represented in Fig. 1 in the lower part of the figure, the filter tubes 1 being part of ceramic tubes with several bores of the type shown in Fig. 1.
- 19 axial bores are for example drilled in a ceramic tube consisting of a highly porous ceramic material, said axial bores being paralleled.
- FIG. 2 In the upper part of Fig. 2 , the processing stations of a processing plant for printed circuit boards is partially shown. The printed circuit boards are successively conveyed through the different processing stations in the processing direction R .
- a typical example of such a method is described, inter alia, in WO 93/17153 A1 .
- the printed circuit boards (which are not shown herein) are immersed, in activating station A-Pd , into an activating bath containing palladium in colloidal form.
- the fluid is contained in an immersion bath tank.
- the printed circuit boards are conveyed through three successive rinsing stations S 1 , S 2 and S 3 .
- the activating fluid that adheres to the surfaces of the printed circuit boards is successively rinsed off.
- the different rinsing stations S 1 , S 2 and S 3 are provided with spray nozzles to serve this purpose.
- the rinsing stations S 1 , S 2 and S 3 are configured as open top containers that are provided with nozzles arranged on the walls of the long sides thereof.
- rinsing fluid is sprayed onto the surfaces of the printed circuit boards as the printed circuit boards are lowered into and/or are raised out of the stations S 1 , S 2 and S 3 .
- the rinsing fluid is collected at the bottom of a container in the respective one of the rinsing stations S 1 , S 2 and S 3 .
- Fresh rinsing fluid is dispensed to the rinsing station S 3 at an average flow rate of 200 l/h, from there it is conducted in a direction counter to the processing direction R of the printed circuit boards to the rinsing station S 2 arranged upstream thereof from where it is brought into the rinsing station S 1 , the flow rate remaining the same.
- Each rinsing station S 1 , S 2 and S 3 is also allocated a collecting tank (not shown) in which the respective rinsing fluid is collected.
- the collected rinsing fluid is drained at a flow rate of 200 l/h from the collecting tank of rinsing station S 1 toward further processing.
- processing fluids are for example solutions of sulphinic acids.
- the printed circuit boards are immersed for treatment into these solutions which are contained in the treatment containers.
- adhering posttreatment solution is rinsed off again in the further rinsing stations S 4 , S 5 and S 6 .
- the rinsing fluid is sprayed from nozzles arranged in the stations S 4 , S 5 and S 6 onto the surfaces of the printed circuit boards.
- the collected rinsing fluid is directed to collecting tanks (not shown) from where it is conducted successively back, in a direction counter to the processing direction of the printed circuit boards R , to the rinsing stations S 5 and S 4 which are arranged upstream thereof.
- the rinsing fluid is drained from rinsing station S 4 toward subsequent waste water treatment.
- the printed circuit boards are next immersed into an etch solution contained in a container in etch station C-Pd . There, palladium adsorbed to the copper surfaces is removed from activation by etching slightly the copper surfaces. In this case as well, the printed circuit boards are immersed into the etch solution.
- adhering processing fluid is again rinsed off the surfaces of the printed circuit boards.
- the printed circuit boards are conveyed to the rinsing stations S 7 , S 8 and S 9 .
- Etch solution adhering to the surfaces of the printed circuit boards is removed by means of rinsing fluid that is sprayed from nozzles onto the surfaces.
- fresh rinsing fluid is conducted into rinsing station S 9 at a flow rate of 200 l/h and the rinsing fluid gathering in this rinsing station is collected in collecting tanks (not shown).
- the collected rinsing fluid is conducted in a direction counter to the processing direction of the printed circuit boards R , from rinsing station S 9 to rinsing station S 8 and from there into rinsing station S 7 .
- the palladium enriched rinsing fluid is conducted to a regenerating arrangement at a flow rate of 200 l/h.
- the afore mentioned way of treating the printed circuit boards is but one possible alternative.
- the printed circuit boards may also be processed in a so called horizontal plant.
- the boards are hereby conducted through the various stations in a horizontal direction of transport and in a horizontal or vertical orientation.
- the fluids may be delivered to the surfaces by nozzles.
- the rinsing fluid originating from rinsing station S 4 contains virtually no precious metal and can be dispensed to the conventional waste water processing system.
- the rinsing fluid originating from the rinsing stations S 1 and S 7 contains palladium and is regenerated in the inventive way:
- the various rinsing waters are collected in buffer tanks 11.1 and 11.2 , respectively.
- Rinsing fluid drained from the buffer tanks 11.1 and 11.2 at a flow rate of 200 l/h is next conducted into the conduits 13.1 and 13.2 , respectively, by means of pumps 12.1 and 12.2 , respectively, and is delivered to a common conduit 13.3 .
- the combined rinsing fluids are - if necessary - mixed with a pH adjusting agent, in the present case with NaOH.
- NaOH solution is added from a reservoir 14 to the combined rinsing fluids.
- An electric control circuit serves to control the dosage of the NaOH solution.
- Said control circuit comprises a pH probe 15 , a pH measuring electrode for example, for controlling a dosing pump (not shown) for the NaOH solution.
- a dosing pump (not shown) for the NaOH solution.
- the pH of the rinsing fluid is near 7, the pH needs not be adjusted to the precise value of 7.
- the rinsing fluid is then directed by means of another pump 12.3 through a conduit 13.4 into a collecting tank 16 .
- a lower fill level sensor 17.1 and an upper fill level sensor 17.2 are provided in collecting tank 16 . If the fluid level is higher than the upper fill level sensor 17.2 , fluid is directed through conduit 13.5 from container 16 to pump 18 . If, by contrast, the fill level of the collecting tank 16 is below the lower fill level sensor 17.1 , the rinsing fluid is not pumped out of the collecting tank 16 .
- the fluid is conducted, under a pressure ranging from 1.5 to 3 bar, through two membrane filter tubes 1 connected in series.
- the permeate fluid pervading the walls of the tube is drained toward further waste water treatment A .
- the concentrate fluid remaining in the filter tube is recirculated via the closed circular conduit 13.6 so that the fluid is permanently and increasingly concentrated with regard to palladium.
- part of the concentrated rinsing fluid is permanently circulated back to the collecting tank 16 from where it is directed to the membrane filters by way of pump 18 so that palladium gradually enriches in this fluid.
- Fluid coming directly from the activating station A-Pd can also be discharged directly for regeneration and be directed toward ultrafiltration.
- said fluid may be either transferred by hand into a collecting tank 20 using the path referred to by reference numeral M or small quantities thereof may be conducted to the buffer tank 11.1 by means of a pump 12.4 . Fluid that has been removed and transferred by hand to collecting tank 20 may then be dispensed to collecting tank 16 by way of another pump 12.5 for example.
- the slurry suspension contained within the container 16 in the multiphase separating unit is directed to a filter press 21 for further separation of palladium.
- the filter press 21 is shown in dashed lines in Fig. 2 . It contains filter material having a pore size of about 50 ⁇ m. The pressure in the press amounts to about 4 bar. Excess fluid can either be circulated back to the collecting tank 16 by way of the additional conduit 22 or be dispensed to waste water treatment A .
- a colloidal, acidic activating fluid that contained 400 mg/l of colloidal palladium, a protective colloid in the form of a polymer and a reducing agent in the form of sodium hypophosphite.
- the mean particle diameter of the palladium colloid particles was about 4 nm.
- the printed circuit boards were treated with an posttreatment solution containing an organic sulphinic acid, were then rinsed again and finally treated in an etch solution containing 300 g/l of sodium persulfate.
- the amounts of palladium thereby removed from the copper surfaces were dispensed to the etch solution and, via the etch solution adhering to the surfaces of the printed circuit boards, to the subsequent rinsing fluid.
- the rinsing fluids obtained in the rinsing stations S 1 to S 3 and S 7 to S 9 (see Fig. 2 ) under the afore mentioned conditions were dispensed to the regeneration arrangement described at a flow rate of 200 l/h respectively.
- the fluids were separated at a filter membrane made of ceramic ( ⁇ -Al 2 O 3 as a support material with two ultrafiltration layers of ZrO 2 and TiO 2 applied thereon, TiO 2 being provided with the finest pore size and effecting a filtration with a pore exclusion size of approximately 20,000 Dalton; the TiO 2 layer was applied by a Sol Gel method).
- the concentration of palladium in the rinsing fluids as well as the pH of these fluids are indicated in Table 1 (tests No. 1 and 2).
- the pH of the fluids originating from rinsing stations S 1 to S 3 and S 7 to S 9 were not adjusted with pH adjusting agents.
- the concentrate fluid was conducted through the ceramic membrane filter at a flow rate of 2,800 l/h.
- the permeate flow rate obtained was of 40 to 45 l/h.
- a mixture of rinsing fluids from the colloidal activating fluid and from the etch solution was prepared at a volume ratio of 1 : 1 (test No. 3).
- the same ceramic membrane filter was used as in Example 1.
- the initial palladium concentration in the combined rinsing fluids and the pH of the mixture are indicated in Table 1.
- a NaOH solution was added to the rinsing fluid.
- the permeate solution obtained after having carried out ultrafiltration had a palladium concentration of ⁇ 0.5 mg/l.
- the palladium concentration in the concentrate was > 1 g/l (see Table 1).
- Example 4 In another test No. 4, the same ceramic membrane filter as in Example 1 was used. Colloidal activating fluid was added at a volume ratio of 1 : 100 to the mixture of rinsing fluids obtained according to Example 2. The palladium concentration in this fluid was equal to 15.0 mg/l. The pH of this fluid was adjusted to 7 by means of NaOH solution. The palladium concentrations in the permeate and in the concentrate after ultrafiltration was performed are indicated in Table 1.
- Example 5 the same ceramic membrane filter as in Example 1 was used.
- the solution of an ionogenic activator was used in place of a colloidal activating solution.
- the activator contained an organic palladium complex (Neoganth ® Activator, Atotech Deutschland GmbH, Germany), the palladium concentration in this solution was 250 mg/l.
- the printed circuit boards activated with this solution were again treated in a rinsing cascade of three rinsing stations S 1 , S 2 and S 3 , the direction of flow of the rinsing water corresponding to that shown in Fig. 2 .
- the palladium concentration in the rinsing water originating from rinsing station S 1 was about 1.5 mg/l.
- an aqueous solution of 467 g/l of sodium dimethyl dithiocarbamate was added to the rinsing water.
- the palladium concentrations obtained in the permeate and in the concentrate as a result of ultrafiltration of this solution are indicated in Table 1 (Test No. 5).
- Example 6 the same ceramic membrane filter as in Example 1 was used.
- the rinsing water obtained according to Example 4 was mixed at a volume ratio of 100 : 1 with the solution of the activating bath.
- An aqueous solution of 10 g/I of sodium sulfide was added to the mixture.
- the initial palladium concentration was 8.0 mg/l.
- the palladium concentrations in the filtrate and in the concentrate after ultrafiltration are indicated in Table 1.
- Example 7 In another test No. 7, the same ceramic membrane filter as in Example 1 was used. A mixture according to Example 5 was added at a volume ratio of 2 : 1 to the mixture of rinsing fluids obtained according to Example 2.
- the palladium concentration in this fluid was 4.2 mg/l.
- the pH was adjusted to 7 by means of NaOH solution.
- an aqueous solution of 467 g/I of sodium dimethyl dithiocarbamate was added to the fluid.
- the palladium concentrations in the permeate and in the concentrate after ultrafiltration had been performed are shown in Table 1.
Landscapes
- Chemical & Material Sciences (AREA)
- Metallurgy (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Separation Using Semi-Permeable Membranes (AREA)
- Chemically Coating (AREA)
- Manufacture And Refinement Of Metals (AREA)
- Porous Artificial Stone Or Porous Ceramic Products (AREA)
- Manufacturing Of Printed Wiring (AREA)
- Filtering Materials (AREA)
Claims (12)
- Verfahren zum Beschichten von Werkstücken mit einer Flüssigkeit, wobei die Flüssigkeit Palladium enthält und wobei das Verfahren umfasst: In-Kontakt-Bringen der Werkstücke mit der Flüssigkeit, danach Mischen der Flüssigkeit mit chemischen Substanzen, die ausgewählt sind aus der Gruppe, umfassend pH-Einstellmittel, Reduktionsmittel, Schwefelverbindungen, Selenverbindungen und Tellurverbindungen, und danach Filtrieren der Flüssigkeit durch mindestens einen Keramikmembran-Filter, um das Palladium von der Flüssigkeit zu trennen, wobei der Keramikmembran-Filter eine Ausschlussporengröße von über 10 000 Dalton hat und wobei die chemischen Substanzen so ausgewählt sind, dass Palladiumteilchen gebildet werden, deren Teilchengröße so groß ist, dass die Teilchen durch die Poren des mindestens einen Keramikmembran-Filters nicht passieren können.
- Verfahren nach Anspruch 1, wobei der mindestens eine Keramikmembran-Filter eine Ausschlussporengröße im Bereich von ungefähr 15 000 Dalton bis ungefähr 25 000 Dalton hat.
- Verfahren nach Anspruch 2, wobei der mindestens eine Keramikmembran-Filter eine Ausschlussporengröße von ungefähr 20 000 Dalton hat.
- Verfahren nach einem der vorstehenden Ansprüche, wobei der mindestens eine Keramikmembran-Filter aus einem Aluminiumoxid/Titandioxid/Zirkondioxid-Keramikmaterial hergestellt ist.
- Verfahren nach einem der vorstehenden Ansprüche, wobei die Werkstücke zur Herstellung von elektrischen Schaltungsträgern geeignet sind.
- Verfahren nach einem der vorstehenden Ansprüche, wobei Palladium in ionischer und/oder ionogener Form vorliegt und wobei die Flüssigkeit mit den chemischen Substanzen, die ausgewählt sind aus der Gruppe, umfassend Reduktionsmittel, Schwefelverbindungen, Selenverbindungen und Tellurverbindungen, gemischt wird.
- Verfahren nach Anspruch 6, wobei die chemischen Substanzen ausgewählt sind aus der Gruppe, umfassend Borhydride, Aminborane, Hypophosphite, anorganische Sulfide und organische Thioverbindungen.
- Verfahren nach Anspruch 7, wobei Palladium in kolloidaler Form vorliegt und wobei die chemischen Substanzen pH-Einstellmittel sind, die mit der Flüssigkeit in einer Art und Weise gemischt werden, dass der pH-Wert der Flüssigkeit im Bereich von 3 bis 12 liegt.
- Verfahren nach einem der Ansprüche 6-8, umfassend die folgenden Verfahrensschritte:a. die Werkstücke werden mit einer Palladium enthaltenden Behandlungsflüssigkeit in Kontakt gebracht,b. dann wird die an den Oberflächen der Werkstücke anhaftende Behandlungsflüssigkeit mit einer Spülflüssigkeit entfernt, undc. die Behandlungsflüssigkeit und/oder die Spülflüssigkeit werden durch den mindestens einen Keramikmembran-Filter zu deren Filtration geleitet, wobei die Flüssigkeit, die den mindestens einen Keramikmembran-Filter passiert hat, eine Permeatflüssigkeit ist und die Flüssigkeit, die den mindestens einen Keramikmembran-Filter nicht passiert hat, eine Konzentratflüssigkeit ist.
- Verfahren nach Anspruch 9, wobei die Behandlungsflüssigkeit und/oder Spülflüssigkeit mit den chemischen Substanzen gemischt wird, bevor sie durch den mindestens einen Keramikmembran-Filter geleitet wird.
- Verfahren nach einem der Ansprüche 9 und 10, wobei eine Spülflüssigkeit, die höchstens 5 Vol.-% der Behandlungsflüssigkeit enthält, durch den mindestens einen Keramikmembran-Filter geleitet wird.
- Verfahren nach Anspruch 11, wobei die Werkstücke pro Zeiteinheit mit einer vorbestimmten Menge von frischer Spülflüssigkeit in Kontakt gebracht werden und wobei die Menge der pro Zeiteinheit gebildeten Permeatflüssigkeit ungefähr zur Menge der Spülflüssigkeit gleich ist, die pro Zeiteinheit mit den Werkstücken in Kontakt gebracht wird.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE10148632 | 2001-09-26 | ||
| DE10148632A DE10148632C1 (de) | 2001-09-26 | 2001-09-26 | Verfahren und Vorrichtung zum galvanotechnischen Behandeln von Werkstücken mit einer Edelmetalle enthaltenden Flüssigkeit |
| PCT/EP2002/009125 WO2003029518A2 (en) | 2001-09-26 | 2002-08-15 | Precious metal recovery |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP1430163A2 EP1430163A2 (de) | 2004-06-23 |
| EP1430163B1 true EP1430163B1 (de) | 2007-12-05 |
Family
ID=7701145
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP02800002A Expired - Lifetime EP1430163B1 (de) | 2001-09-26 | 2002-08-15 | Edelmetallrückgewinnung |
Country Status (15)
| Country | Link |
|---|---|
| US (1) | US20040237717A1 (de) |
| EP (1) | EP1430163B1 (de) |
| JP (1) | JP2005504178A (de) |
| KR (1) | KR20040043168A (de) |
| CN (1) | CN1633521A (de) |
| AT (1) | ATE380264T1 (de) |
| AU (1) | AU2002333428A1 (de) |
| BR (1) | BR0212823A (de) |
| CA (1) | CA2460015A1 (de) |
| DE (2) | DE10148632C1 (de) |
| ES (1) | ES2297045T3 (de) |
| MX (1) | MXPA04002772A (de) |
| MY (1) | MY147747A (de) |
| TW (1) | TWI293999B (de) |
| WO (1) | WO2003029518A2 (de) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1884278A1 (de) * | 2006-07-24 | 2008-02-06 | ATOTECH Deutschland GmbH | Spülvorrichtung und -verfahren für das Spülen von Flüssigkeiten von Werkstücken |
| US20100108601A1 (en) * | 2007-03-30 | 2010-05-06 | Masahiro Saito | Method for Treating Ballast Water with a Membrane |
| CN103945654A (zh) * | 2013-01-18 | 2014-07-23 | 北大方正集团有限公司 | 钝化线路板非金属化孔中残留钯离子的方法及此方法制备的线路板 |
| JP6234070B2 (ja) * | 2013-06-03 | 2017-11-22 | 木田精工株式会社 | 表面処理排液の再生方法及び再生装置 |
| KR20170008309A (ko) * | 2014-07-10 | 2017-01-23 | 오꾸노 케미칼 인더스트리즈 컴파니,리미티드 | 수지 도금 방법 |
| JP6340302B2 (ja) * | 2014-10-24 | 2018-06-06 | 田中貴金属工業株式会社 | 廃液の処理方法、廃液の処理装置および廃液の再利用方法 |
| EP3133175A1 (de) * | 2015-08-19 | 2017-02-22 | Enthone, Inc. | System und verfahren zur wiedergewinnung von katalytischem edelmetall aus wässriger galvanischer verarbeitungslösung |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4078918A (en) * | 1976-11-26 | 1978-03-14 | Perman Craig A | Method for precious metal recovery |
| US4435258A (en) * | 1982-09-28 | 1984-03-06 | Western Electric Co., Inc. | Method and apparatus for the recovery of palladium from spent electroless catalytic baths |
| US4895739A (en) * | 1988-02-08 | 1990-01-23 | Shipley Company Inc. | Pretreatment for electroplating process |
| US5205937A (en) * | 1992-04-30 | 1993-04-27 | U.S. Filter Membralox | Recovery and reuse of water-based cleaners |
| DE10024239C1 (de) * | 2000-05-15 | 2001-09-20 | Atotech Deutschland Gmbh | Verfahren zum galvanotechnischen Behandeln von Werkstücken mit einer Palladiumkolloidlösung |
| EP1224972A1 (de) * | 2001-01-18 | 2002-07-24 | Shipley Co. L.L.C. | Verfahren zur Rückgewinnung von Katalysatormetallen aus kolloidaler Lösung |
-
2001
- 2001-09-26 DE DE10148632A patent/DE10148632C1/de not_active Expired - Fee Related
-
2002
- 2002-08-15 MX MXPA04002772A patent/MXPA04002772A/es unknown
- 2002-08-15 KR KR10-2004-7001290A patent/KR20040043168A/ko not_active Application Discontinuation
- 2002-08-15 JP JP2003532726A patent/JP2005504178A/ja not_active Withdrawn
- 2002-08-15 AT AT02800002T patent/ATE380264T1/de not_active IP Right Cessation
- 2002-08-15 CN CNA028188861A patent/CN1633521A/zh active Pending
- 2002-08-15 ES ES02800002T patent/ES2297045T3/es not_active Expired - Lifetime
- 2002-08-15 DE DE60223934T patent/DE60223934T2/de not_active Expired - Lifetime
- 2002-08-15 AU AU2002333428A patent/AU2002333428A1/en not_active Abandoned
- 2002-08-15 WO PCT/EP2002/009125 patent/WO2003029518A2/en active IP Right Grant
- 2002-08-15 BR BR0212823-3A patent/BR0212823A/pt not_active Application Discontinuation
- 2002-08-15 EP EP02800002A patent/EP1430163B1/de not_active Expired - Lifetime
- 2002-08-15 US US10/490,004 patent/US20040237717A1/en not_active Abandoned
- 2002-08-15 CA CA002460015A patent/CA2460015A1/en not_active Abandoned
- 2002-08-22 MY MYPI20023105A patent/MY147747A/en unknown
- 2002-08-23 TW TW091119189A patent/TWI293999B/zh not_active IP Right Cessation
Also Published As
| Publication number | Publication date |
|---|---|
| MXPA04002772A (es) | 2004-06-29 |
| AU2002333428A1 (en) | 2003-04-14 |
| KR20040043168A (ko) | 2004-05-22 |
| ATE380264T1 (de) | 2007-12-15 |
| TWI293999B (en) | 2008-03-01 |
| WO2003029518A2 (en) | 2003-04-10 |
| CN1633521A (zh) | 2005-06-29 |
| BR0212823A (pt) | 2004-10-13 |
| CA2460015A1 (en) | 2003-04-10 |
| DE10148632C1 (de) | 2003-06-05 |
| DE60223934T2 (de) | 2008-12-04 |
| MY147747A (en) | 2013-01-15 |
| ES2297045T3 (es) | 2008-05-01 |
| DE60223934D1 (de) | 2008-01-17 |
| US20040237717A1 (en) | 2004-12-02 |
| EP1430163A2 (de) | 2004-06-23 |
| JP2005504178A (ja) | 2005-02-10 |
| WO2003029518A3 (en) | 2004-03-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2001088228A1 (fr) | Appareil d'electrolyse pour feuille de cuivre electrolytique et feuille de cuivre electrolytique produite au moyen dudit appareil d'electrolyse | |
| JPH10317154A (ja) | 錫メッキ用溶液の再生方法およびその装置 | |
| EP1430163B1 (de) | Edelmetallrückgewinnung | |
| KR100839730B1 (ko) | 프로세스 용액 정제용 방법 및 장치 | |
| EP1427869B1 (de) | Regenerationsverfahren für eine plattierungslösung | |
| JP2006328536A (ja) | 電解質のイオン濃度を設定する方法及びその装置 | |
| US20030155250A1 (en) | Method for the treatment of work pieces with a palladium colloid solution | |
| EP1884278A1 (de) | Spülvorrichtung und -verfahren für das Spülen von Flüssigkeiten von Werkstücken | |
| JP6518834B2 (ja) | 水性ガルバニック処理溶液から触媒貴金属を回収するためのシステム及び方法 | |
| MXPA04012038A (es) | Dispositivo y metodo para la regeneracion de un bano de metalizacion sin electrodos. | |
| EP3426600A1 (de) | Verfahren zur rückgewinnung von phosphorsäure aus einer verbrauchten phosphorsäure-/alkalimetallsalzpermanganatätzlösung | |
| JP7359133B2 (ja) | 金めっき膜の形成方法 | |
| KR102102992B1 (ko) | 세라믹 파우더 유동화를 이용한 도금방법 | |
| JPH06145995A (ja) | メッキ液の処理方法 | |
| Pishin | CAUSES OF REDUCED EFFICIENCY OF TRILONATE CHEMICAL COPPER-PLATING SOLUTIONS | |
| CN102815828A (zh) | 表面处理湿制程清洗液中金及氰化物的循环利用系统 | |
| JP2014118612A (ja) | 無電解ニッケルりんめっき液の再生方法及び再生装置 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20040330 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR IE IT LI LU MC NL PT SE SK TR |
|
| AX | Request for extension of the european patent |
Extension state: AL LT LV MK RO SI |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: KOSTOUROS, DEMITRY Inventor name: HAHNDORF, INA |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: DE Ref document number: 1062581 Country of ref document: HK |
|
| 17Q | First examination report despatched |
Effective date: 20051121 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR IE IT LI LU MC NL PT SE SK TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REF | Corresponds to: |
Ref document number: 60223934 Country of ref document: DE Date of ref document: 20080117 Kind code of ref document: P |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: WD Ref document number: 1062581 Country of ref document: HK |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071205 Ref country code: CH Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071205 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20080305 Ref country code: LI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071205 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2297045 Country of ref document: ES Kind code of ref document: T3 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071205 |
|
| NLV1 | Nl: lapsed or annulled due to failure to fulfill the requirements of art. 29p and 29m of the patents act | ||
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071205 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071205 |
|
| ET | Fr: translation filed | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071205 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071205 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20080505 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071205 |
|
| 26N | No opposition filed |
Effective date: 20080908 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20080306 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20080831 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071205 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20080305 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071205 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20080815 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20090827 Year of fee payment: 8 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20080815 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20071205 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20100825 Year of fee payment: 9 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20080831 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20100815 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20100815 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20110901 Year of fee payment: 10 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R082 Ref document number: 60223934 Country of ref document: DE Representative=s name: , |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20130430 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120831 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20131029 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20110816 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20160822 Year of fee payment: 15 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 60223934 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180301 |