EP1314366A1 - Method for making a fusible interlining with dots of thermally fusible polymer and thermally fusible polymer used thereby - Google Patents
Method for making a fusible interlining with dots of thermally fusible polymer and thermally fusible polymer used thereby Download PDFInfo
- Publication number
- EP1314366A1 EP1314366A1 EP02370050A EP02370050A EP1314366A1 EP 1314366 A1 EP1314366 A1 EP 1314366A1 EP 02370050 A EP02370050 A EP 02370050A EP 02370050 A EP02370050 A EP 02370050A EP 1314366 A1 EP1314366 A1 EP 1314366A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- polymer
- functional
- functional groups
- interlining
- free radicals
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Classifications
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M15/00—Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with macromolecular compounds; Such treatment combined with mechanical treatment
- D06M15/19—Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with macromolecular compounds; Such treatment combined with mechanical treatment with synthetic macromolecular compounds
- D06M15/21—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- D06M15/263—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds of unsaturated carboxylic acids; Salts or esters thereof
-
- A—HUMAN NECESSITIES
- A41—WEARING APPAREL
- A41D—OUTERWEAR; PROTECTIVE GARMENTS; ACCESSORIES
- A41D27/00—Details of garments or of their making
- A41D27/02—Linings
- A41D27/06—Stiffening-pieces
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M10/00—Physical treatment of fibres, threads, yarns, fabrics, or fibrous goods made from such materials, e.g. ultrasonic, corona discharge, irradiation, electric currents, or magnetic fields; Physical treatment combined with treatment with chemical compounds or elements
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M10/00—Physical treatment of fibres, threads, yarns, fabrics, or fibrous goods made from such materials, e.g. ultrasonic, corona discharge, irradiation, electric currents, or magnetic fields; Physical treatment combined with treatment with chemical compounds or elements
- D06M10/04—Physical treatment combined with treatment with chemical compounds or elements
- D06M10/08—Organic compounds
- D06M10/10—Macromolecular compounds
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M14/00—Graft polymerisation of monomers containing carbon-to-carbon unsaturated bonds on to fibres, threads, yarns, fabrics, or fibrous goods made from such materials
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M14/00—Graft polymerisation of monomers containing carbon-to-carbon unsaturated bonds on to fibres, threads, yarns, fabrics, or fibrous goods made from such materials
- D06M14/18—Graft polymerisation of monomers containing carbon-to-carbon unsaturated bonds on to fibres, threads, yarns, fabrics, or fibrous goods made from such materials using wave energy or particle radiation
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06M—TREATMENT, NOT PROVIDED FOR ELSEWHERE IN CLASS D06, OF FIBRES, THREADS, YARNS, FABRICS, FEATHERS OR FIBROUS GOODS MADE FROM SUCH MATERIALS
- D06M17/00—Producing multi-layer textile fabrics
- D06M17/04—Producing multi-layer textile fabrics by applying synthetic resins as adhesives
Definitions
- the present invention relates to the field of interlinings fusible which are supports, textile or non-woven, on a on which are applied points of hot melt polymer, likely to adhere later on the piece of clothing to strengthen under the effect of the application of some hot pressure. It relates more particularly to a method of manufacturing such interlining using electronic bombardment in view to modify locally the melting temperature and / or the viscosity of the hot melt polymer; it also relates to a polymer hot melt specially designed for the implementation of said process.
- this piercing has the effect of increasing locally the rigidity of the interlining and therefore the piece of clothing, which may be contrary to the desired effect. It can also cause collages on dubbing fabrics such as lining and part of draperies in reverse, which causes a degradation of the quality of the garment.
- thermofusible interlining whose points of thermofusible polymer have two superimposed layers, namely a first layer in contact with the face of the interlining support and a second layer arranged precisely above the first. Good sure the constituents of the two layers are determined so that when application with hot pressure of the piece of clothing, only the thermofusible polymer of the second layer reacts to the action of temperature. The diffusion of the hot-melt polymer can in this only to the piece of clothing, being prevented from interlayer support, the first layer acting as kind of barrier.
- the means, of a chemical nature, capable of modifying the chemical structure of the hot melt polymer comprise at least a reactive material and at least one reactive means capable of starting, ensure, promote the reaction between the reactive material and the polymer melt.
- the contacting between the reactive material and the polymer hot melt is either by mixing these two elements that are deposited in the form of dots, in an intimate mixture, on the interlayer support, either by application of the reactive material on the interlining support before depositing the polymer dots (then free of reactive material).
- the reactive means are cited the heat inputs, ultraviolet radiation and bombardment electronic.
- the applicant also proposed in the document EP.0.855.146A1 a method according to which one deposits on the face location a interlining support of hot melt polymer dots of average thickness E and containing a radical activator and submits one of the faces of the support to an electronic bombardment by adjusting the penetration depth of the electrons in the points of the hot-melt polymer to obtain a modification of physicochemical properties of the hot-melt polymer, chosen among the melting temperature and the viscosity, on a thickness e by compared to the average thickness E.
- the radical activator has the function of creating radicals free to initiate the polymerization reaction on itself thermofusible polymer. It is not strictly speaking a reactive material in the sense of the document FR.2.606.603.
- thermofusible polymer and the radical activator the mixing of the thermofusible polymer and the radical activator, this mixing with successive operations of fusion, extrusion and grinding so as to obtain a powder which is used as it is for coating or that is diluted for the subsequent preparation of the aqueous dispersion in the form of a paste for depositing the points of polymer on the interlining support.
- the presence of the radical agent induces a certain number of difficulties.
- the technique of depositing points of polymer uses an aqueous dispersion in the form of a paste, it is important to obtain a good stability of the dough in time that the components used in the formulation of the dough are soluble in water.
- products suitable as agents radicals are for the most part insoluble in water at least in the proportions in which they intervene in the preparation of the aqueous dispersion, which can cause a relative instability dough in time.
- the products suitable as radical agents are usually in liquid form, with boiling temperatures that can possibly be incompatible with the temperatures used in the conditions procedures implemented when depositing points on the support interlining. So in this case there may be partial evaporation of the radical agent, which leads to a loss or even a disappearance of responsiveness to electronic bombardment.
- the products as radical agents are usually monomers of low molecular weight, their behavior in mixing with the Hot melt polymer is comparable to that of a plasticizer. This behavior can cause a change in the melt viscosity of the hot-melt polymer, can pose quality problems, coating and can also change the strength properties intrinsic mechanics of the polymer and thereby influence the collage performance.
- the aim of the applicant is to propose a method of manufacturing a fusible interlining an electronic bombardment to modify the structure chemistry of the hot-melt polymer that overcomes the disadvantages supra.
- the polymer points thermofusible are based on at least one functional polymer having functional groups capable of reacting with free radicals generated under the action of electronic bombing and / or themselves generators of free radicals under the action of electronic bombing; in addition, the depth of electron penetration into the polymer points to obtain, thanks to said functional groups, a self-crosslinking of said functional polymer on a limited thickness e with respect to the average thickness E of the polymer points.
- thermofusible polymer itself that includes both the adhesion function and the bombardment reactivity function electronic.
- thermofusible polymer for fusible interlinings, especially designed for the implementation of the aforementioned method.
- This polymer thermofusible is characterized in that it comprises groups functional groups able to react with free radicals under the action of a electronic bombardment and / or themselves generators of free radicals under the action of electronic bombardment.
- these functional groupings have functions with ethylenic unsaturation, for example acrylate, methacrylate, allyl, acrylamide, vinyl ether, styrenic, maleic or fumaric.
- said groupings functional entities include labile entities, that is entities whose binding energies are lower than the usual connections carbon - carbon or carbon - hydrogen.
- labile entities there may be mentioned a carbon-chlorine bond C-CI or a bond thiol S-H.
- Functional Hot Melt Polymers According to the Invention are obtained according to the two possible ways.
- first way we add directly, in the synthesis reaction medium of the polymer, monomers carrying the functional group or groups able to react with free radicals under the action of a electronic bombardment and / or themselves generators of free radicals under the action of electronic bombardment.
- second way we start from the already formed hot melt polymer and it is later transformed by grafting onto its polymer structure functional groups desired by grafting techniques known.
- the location of the functional group along the polymer chain significantly influences the reactivity of the polymer functional under the action of electronic bombardment as well as the structure of the crosslinked network obtained.
- the functional group can be located at the end of the chain, included along the chain or located on ramifications or grafts along the polymer chain main.
- the functional thermofusible polymer according to the invention must necessarily have the properties of adhesion or bonding necessary for the intended use which is fusible interlining. Of the more it must be functionalized either during its synthesis or by subsequent transformation, as indicated previously. It is therefore especially polyethylene (PE), copolyamide (coPA), polyester (Pes), polyurethane (PU) or copolyamide block ether (PBAX).
- PE polyethylene
- coPA copolyamide
- Pes polyester
- PU polyurethane
- PBAX copolyamide block ether
- the functional groups are located at the end of the chain; regarding of a skeleton of the polyethylene type, the functional groups are located on branches along the main chain; in the case of a skeleton of the polyester type, the groups functional are included along the main chain; in the case of a skeleton of the polyurethane type, the functional groups are grafted along the main chain.
- thermofusible polymer of the invention is selected so as to meet the constraints of use in fusible interlinings, constraints which are variables according to the techniques used.
- this polymer must be delivered in the form of a grinding-resistant powder for particle sizes from 10 to 200 ⁇ m or to be available in granules if the technique used is of the hot-melt type.
- the functional groups that comprise the hot melt polymer must be stable at the coating temperature, knowing that the technique used, this temperature can range from 150 to 225 ° C. This thermal stability is essential to prevent functional groups give rise to an uncontrolled start of self-crosslinking. This thermal stability can be improved by incorporating into the functional hot melt polymer an antioxidant.
- the melting temperature of the hot melt polymer functional of the invention should generally be between 70 and 150 ° C, knowing that the melting temperature of the same self-crosslinked polymer under the action of electronic bombing him is higher.
- the hot melt functional polymer of the invention is according to the applications, machine wash resistant, resistant to dry cleaning with chlorinated solvent and resistant to steam.
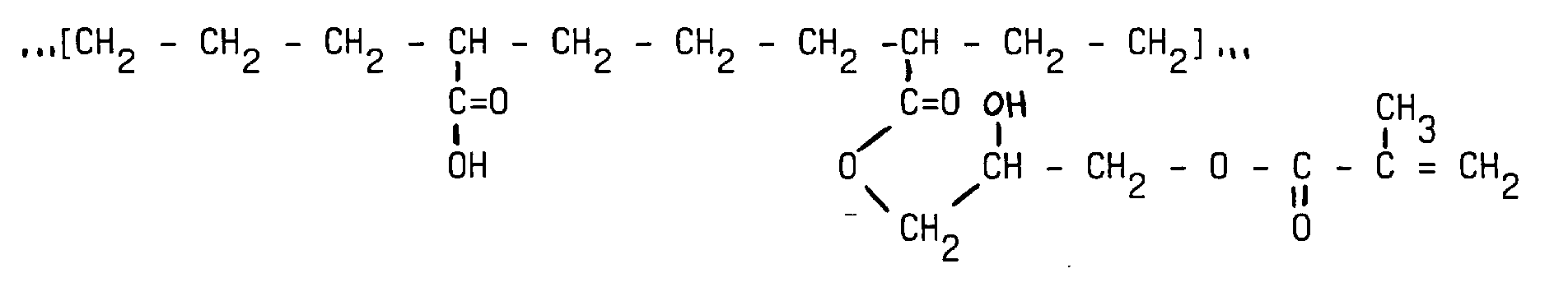
- the functional polymer has a polyethylene backbone and comprises functional groups of methacrylate type.
- This functional polymer is based on an initial polymer obtained from ethylene monomers and a small percentage, of the order of 3% by weight, of acrylic acid.
- This initial polymer of the polyethylene type contains acid functions attached to the carbon chain.
- This initial polymer is subjected to an esterification reaction with an epoxide type compound of formula: proposed by the firm Aldricch under the name GMA, in stachiometric proportion.
- the power and the dose used make it possible to limit the action of the electrons on a limited thickness e of the average thickness of the deposited points.
- the self-crosslinking of the functional polymer occurs only on this thickness e of the point, at the base of said point, that is to say that which is in contact with the interlining support.
- the self-crosslinked polymer has a higher melting temperature than the non-self-crosslinking functional polymer, so that when applying the interlining to the article to reinforce the self-crosslinked base of the polymer point less than than the rest of the item, which avoids piercing.
- a second and a third example of polymers functional polyethylene backbone can be cited.
- the functional groups are of the styrenic type.
- the initial polymer is obtained from ethylene monomer and of the order of 10% by weight of hydroxyethyl methacrylate.
- This may be the EHEMA polymer proposed by the company Neste Chemical under the reference NRT 354. It reacts with an isopropenyl compound of formula proposed by the firm American Cyanamid under the name TMI, to give the functional polymer of general form:
- the functional groups are of the acrylate type.
- the initial polymer is obtained from ethylene monomer and of the order of 16% by weight of vinyl alcohol. It may be the EVOH polymer proposed by Bayer under the reference Levasint S-31. It reacts with an acrylic acid compound to give the functional polymer of the general formula:
- the operating conditions of the different reactions implemented are determined in order to obtain a polymer functional that contains an adequate proportion of groupings functional, to obtain the desired result, namely to obtain, under the action of electrons, a localized increase in melting temperature due to the self-crosslinking of said polymer functional and which moreover meets the conditions imposed by application to the fusible interlining of the support on which the Functional polymer dots are deposited.
Landscapes
- Engineering & Computer Science (AREA)
- Textile Engineering (AREA)
- Health & Medical Sciences (AREA)
- Toxicology (AREA)
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Adhesives Or Adhesive Processes (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Treatments For Attaching Organic Compounds To Fibrous Goods (AREA)
- Laminated Bodies (AREA)
- Details Of Garments (AREA)
- Adhesive Tapes (AREA)
- Macromonomer-Based Addition Polymer (AREA)
- Manufacturing Of Multi-Layer Textile Fabrics (AREA)
Abstract
Description
La présente invention concerne le domaine des entoilages thermocollants qui sont des supports, textiles ou non-tissés , sur une face desquels sont appliqués des points de polymère thermofusible , susceptibles d'adhérer ultérieurement sur la pièce d'habillement à renforcer sous l'effet de l'application d'une certaine pression à chaud. Elle concerne plus particulièrement un procédé de fabrication d'un tel entoilage mettant en oeuvre un bombardement électronique en vue de modifier localement la température de fusion et/ou la viscosité du polymère thermofusible; elle concerne également un polymère thermofusible spécialement conçu pour la mise en oeuvre dudit procédé.The present invention relates to the field of interlinings fusible which are supports, textile or non-woven, on a on which are applied points of hot melt polymer, likely to adhere later on the piece of clothing to strengthen under the effect of the application of some hot pressure. It relates more particularly to a method of manufacturing such interlining using electronic bombardment in view to modify locally the melting temperature and / or the viscosity of the hot melt polymer; it also relates to a polymer hot melt specially designed for the implementation of said process.
Parmi tous les problèmes rencontrés dans le domaine de l'entoilage thermocollant , l'un des plus délicats à résoudre consiste dans le risque de transpercement du support d'entoilage lors de l'application par pression à chaud de l'entoilage thermocollant contre la pièce d'habillement à renforcer. En effet la température qui est choisie pour effectuer cette application à chaud doit permettre de réaliser la fusion du point de polymère de manière à ce que le polymère ainsi fondu puisse se répartir et adhérer sur les fibres ou filaments en surface de la pièce d'habillement. Il arrive cependant fréquemment que cette répartition ne se fasse pas uniquement en surface mais que le polymère fondu flue à travers les fibres ou filaments et apparaisse sur la face opposée du support d'entoilage. Ceci n'a pas d'incidence sur le plan esthétique , sauf si l'entoilage est destiné à être apparent et à former la face arrière du vêtement. En tout état de cause ce transpercement a pour effet d'augmenter localement la rigidité de l'entoilage et donc de la pièce d'habillement, ce qui peut être contraire à l'effet souhaité. Il peut également provoquer des collages sur les tissus de doublage tels que doublure et partie de draperies en revers, ce qui provoque une dégradation de la qualité du vêtement.Of all the problems encountered in the field of fusible interlining, one of the most delicate to solve is in the risk of penetration of the interlining support during the application by hot pressure of fusible interlining against the piece of clothing to reinforce. Indeed the temperature that is chosen to perform this hot application should allow to carry out the melting of the polymer point so that the polymer thus melted can be distributed and adhere to the fibers or filaments on the surface of the piece of clothing. It happens however frequently that this distribution is not only surface but that the molten polymer is flowing through the fibers or filaments and appears on the opposite side of the interlining support. This does not affect aesthetically, unless the stabilizer is intended to be apparent and to form the back side of the garment. In any event this piercing has the effect of increasing locally the rigidity of the interlining and therefore the piece of clothing, which may be contrary to the desired effect. It can also cause collages on dubbing fabrics such as lining and part of draperies in reverse, which causes a degradation of the quality of the garment.
Pour résoudre cette difficulté, on a déjà proposé de réaliser un entoilage thermocollant dont les points de polymère thermofusible comportent deux couches superposées, à savoir une première couche en contact avec la face endroit du support d'entoilage et une deuxième couche disposée précisément au-dessus de la première. Bien sûr les constituants des deux couches sont déterminés en sorte que lors de l'application avec pression à chaud de la pièce d'habillement, seul le polymère thermofusible de la seconde couche réagisse à l'action de la température. La diffusion du polymère thermofusible peut dans ce cas ne se faire que vers la pièce d'habillement, étant empêchée vers le support d'entoilage, la première couche faisant office en quelque sorte de barrière.To solve this problem, it has already been proposed to realize a fusible interlining whose points of thermofusible polymer have two superimposed layers, namely a first layer in contact with the face of the interlining support and a second layer arranged precisely above the first. Good sure the constituents of the two layers are determined so that when application with hot pressure of the piece of clothing, only the thermofusible polymer of the second layer reacts to the action of temperature. The diffusion of the hot-melt polymer can in this only to the piece of clothing, being prevented from interlayer support, the first layer acting as kind of barrier.
En pratique cette technique à deux couches superposées présente des inconvénients, notamment difficulté de réalisation de la superposition des deux couches et risque de délamination des deux couches.In practice this technique with two superposed layers has drawbacks, in particular the difficulty of carrying out the layering of both layers and risk of delamination of both layers.
Pour pallier ces inconvénients , le demandeur a déjà proposé, dans le document FR.2.606.603 de mettre en oeuvre des moyens de nature chimique, agissant sur le polymère thermofusible en vue de modifier sa structure chimique, au moins partiellement, au moins à l'interface avec le support d'entoilage , de manière à empêcher le polymère thermofusible de coller à travers le support d'entoilage sous l'effet de la chaleur et/ou de la pression et/ou de la vapeur. Les moyens , de nature chimique , propres à modifier la structure chimique du polymère thermofusible comportent au moins une matière réactive et au moins un moyen réactif apte à amorcer, assurer, favoriser la réaction entre la matière réactive et le polymère thermofusible.To overcome these disadvantages, the applicant has already proposed in document FR.2.606.603 to implement means of a chemical nature, acting on the hot-melt polymer to change its chemical structure, at least partially, to less at the interface with the interlining support, so as to prevent the hot-melt polymer from sticking through the support of interlining under the effect of heat and / or pressure and / or steam. The means, of a chemical nature, capable of modifying the chemical structure of the hot melt polymer comprise at least a reactive material and at least one reactive means capable of starting, ensure, promote the reaction between the reactive material and the polymer melt.
La mise en contact entre la matière réactive et le polymère thermofusible se fait soit par mélange de ces deux éléments qui sont alors déposés sous forme de points , en mélange intime, sur le support d'entoilage, soit par application de la matière réactive sur le support d'entoilage avant le dépôt des points de polymère (alors exempts de matière réactive). Parmi les moyens réactifs, sont cités les apports de chaleur, les radiations ultraviolettes et le bombardement électronique.The contacting between the reactive material and the polymer hot melt is either by mixing these two elements that are deposited in the form of dots, in an intimate mixture, on the interlayer support, either by application of the reactive material on the interlining support before depositing the polymer dots (then free of reactive material). Among the reactive means, are cited the heat inputs, ultraviolet radiation and bombardment electronic.
Le demandeur a également proposé dans le document EP.0.855.146A1 un procédé selon lequel on dépose sur la face endroit d'un support d'entoilage des points de polymères thermofusibles d'épaisseur moyenne E et contenant un activateur radicalaire et on soumet l'une des faces du support à un bombardement électronique en réglant la profondeur de pénétration des électrons dans les points du polymère thermofusible pour obtenir une modification des propriétés physico-chimiques du polymère thermofusible, choisies parmi la température de fusion et la viscosité, sur une épaisseur e par rapport à l'épaisseur moyenne E.The applicant also proposed in the document EP.0.855.146A1 a method according to which one deposits on the face location a interlining support of hot melt polymer dots of average thickness E and containing a radical activator and submits one of the faces of the support to an electronic bombardment by adjusting the penetration depth of the electrons in the points of the hot-melt polymer to obtain a modification of physicochemical properties of the hot-melt polymer, chosen among the melting temperature and the viscosity, on a thickness e by compared to the average thickness E.
L'activateur radicalaire a pour fonction de créer des radicaux libres permettant d'initier la réaction de polymérisation sur lui-même du polymère thermofusible. Il n'est donc pas à proprement parler une matière réactive au sens où le prévoyait le document FR.2.606.603.The radical activator has the function of creating radicals free to initiate the polymerization reaction on itself thermofusible polymer. It is not strictly speaking a reactive material in the sense of the document FR.2.606.603.
Les techniques enseignées par les deux documents précités présentent divers inconvénients. Selon le document FR.2.606.603, lorsque la matière réactive est appliquée sur le support d'entoilage avant le dépôt des points de polymère , la réaction qui intervient après la mise en oeuvre de l'apport de chaleur, de l'irradiation UV ou du bombardement électronique se fait à l'interface entre la matière réactive et le polymère thermofusible. Cette réaction n'intervient donc que sur une épaisseur très réduite . Dans tous les autres cas la matière réactive selon le document FR.2.606.603 ou l'agent radicalaire selon le document EP.0.855.146A1 est mélangée au polymère thermofusible avant le dépôt des points sur le support d'entoilage. Ce mélange est habituellement réalisé lors de la mise en dispersion du polymère sous forme d'une pâte , la matière réactive ou l'agent radicalaire étant alors incorporée comme n'importe quel autre produit de la formulation. Pour obtenir un mélange encore plus intime, selon le document EP.0.855.146A1, on réalise préalablement le mélange du polymère thermofusible et de l'activateur radicalaire, on soumet ce mélange à des opérations successives de fusion, extrusion et broyage de manière à obtenir une poudre qu'on utilise telle quelle pour I 'enduction ou que l'on dilue pour la préparation ultérieure de la dispersion aqueuse sous forme de pâte servant au dépôt des points de polymère sur le support d'entoilage. Cependant quel que soit le caractère intime du mélange, il y a toujours dans chaque point appliqué sur ledit support d'entoilage d'une part un polymère thermofusible qui apporte la fonction de collage qui est nécessaire pour l'adhérence du support d'entoilage sur la pièce d'habillement à renforcer et d' autre part une matière réactive ou un agent radicalaire qui apporte une fonction de réactivité sous l'action des moyens réactifs tels qu'un apport de chaleur , une irradiation UV ou un bombardement électronique , ce dernier en particulier s'agissant d'un agent radicalaire.The techniques taught by the two aforementioned documents have various disadvantages. According to the document FR.2.606.603, when the reactive material is applied to the interlining support before the deposition of the polymer points, the reaction that takes place after the implementation of heat input, UV irradiation or electron bombing is done at the interface between matter reactive and the hot melt polymer. This reaction does not intervene only on a very small thickness. In all other cases the material reactive according to the document FR.2.606.603 or the radical agent according to EP.0.855.146A1 is mixed with the hot melt polymer before depositing points on the interlining support. This mixture is usually made during the dispersion of the polymer under form of a paste, the reactive material or the radical agent being then incorporated like any other product of the formulation. To get an even more intimate mix, according to the document EP.0.855.146A1, the mixing of the thermofusible polymer and the radical activator, this mixing with successive operations of fusion, extrusion and grinding so as to obtain a powder which is used as it is for coating or that is diluted for the subsequent preparation of the aqueous dispersion in the form of a paste for depositing the points of polymer on the interlining support. However, whatever the Intimate nature of the mix, there is always in every point applied on said interlining support on the one hand a polymer hot melt which brings the bonding function that is needed for the adhesion of the interlining support on the piece of clothing to reinforce and on the other hand a reactive material or a radical agent which brings a function of reactivity under the action of the means reagents such as heat input, UV irradiation or electronic bombing, the latter in particular as regards a radical agent.
Dans le cas particulier d'un procédé de fabrication d'un entoilage thermocollant mettant en oeuvre un bombardement électronique pour modifier la structure chimique du polymère thermofusible , la présence de l'agent radicalaire induit un certain nombre de difficultés. Lorsque la technique de dépôt des points de polymère met en oeuvre une dispersion aqueuse sous forme de pâte, il importe pour obtenir une bonne stabilité de la pâte dans le temps que les composants entrant dans la formulation de la pâte soient solubles dans l'eau. Or les produits convenant comme agents radicalaires sont pour la plupart insolubles dans l'eau au moins dans les proportions dans lesquelles ils interviennent dans la préparation de la dispersion aqueuse , ce qui peut occasionner une relative instabilité de la pâte dans le temps. De plus les produits convenant comme agents radicalaires se présentent généralement sous forme liquide , avec des températures d'ébullition qui peuvent éventuellement être incompatibles avec les températures utilisées dans les conditions opératoires mises en oeuvre lors du dépôt des points sur le support d'entoilage. Ainsi dans ce cas il peut y avoir évaporation partielle de l'agent radicalaire , ce qui entraíne une perte voire même une disparition de la réactivité au bombardement électronique. Enfin il a également été remarqué que , du fait que les produits convenant comme agents radicalaires sont généralement des monomères de faible poids moléculaire , leur comportement dans le mélange avec le polymère thermofusible est comparable à celui d'un plastifiant. Ce comportement peut entraíner un changement de la viscosité fondue du polymère thermofusible , peut poser des problèmes de qualité , d'enduction et peut également changer les propriétés de résistance mécanique intrinsèques du polymère et de ce fait influer sur les performances de collage.In the particular case of a method of manufacturing a fusible interlining using a bombardment electronics to modify the chemical structure of the polymer thermofusible, the presence of the radical agent induces a certain number of difficulties. When the technique of depositing points of polymer uses an aqueous dispersion in the form of a paste, it is important to obtain a good stability of the dough in time that the components used in the formulation of the dough are soluble in water. Or products suitable as agents radicals are for the most part insoluble in water at least in the proportions in which they intervene in the preparation of the aqueous dispersion, which can cause a relative instability dough in time. In addition the products suitable as radical agents are usually in liquid form, with boiling temperatures that can possibly be incompatible with the temperatures used in the conditions procedures implemented when depositing points on the support interlining. So in this case there may be partial evaporation of the radical agent, which leads to a loss or even a disappearance of responsiveness to electronic bombardment. Finally he It has also been noticed that since the products as radical agents are usually monomers of low molecular weight, their behavior in mixing with the Hot melt polymer is comparable to that of a plasticizer. This behavior can cause a change in the melt viscosity of the hot-melt polymer, can pose quality problems, coating and can also change the strength properties intrinsic mechanics of the polymer and thereby influence the collage performance.
Le but que s'est fixé le demandeur est de proposer un procédé de fabrication d'un entoilage thermocollant mettant en oeuvre un bombardement électronique pour modifier la structure chimique du polymère thermofusible qui pallie les inconvénients précités. The aim of the applicant is to propose a method of manufacturing a fusible interlining an electronic bombardment to modify the structure chemistry of the hot-melt polymer that overcomes the disadvantages supra.
Ce but est parfaitement atteint par le procédé de l'invention, selon lequel de manière connue on dépose sur la face endroit d'un support d'entoilage , choisi parmi les supports textiles et les non-tissés, des points de polymère thermofusible et on soumet la face envers du support d'entoilage à un bombardement électronique. De manière caractéristique, selon l'invention, les points de polymère thermofusible sont à base d'au moins un polymère fonctionnel comportant des groupements fonctionnels aptes à réagir avec des radicaux libres générés sous l'action du bombardement électronique et/ou eux-mêmes générateurs de radicaux libres sous l'action du bombardement électronique; de plus on règle la profondeur de pénétration des électrons dans les points de polymère pour obtenir, grâce auxdits groupements fonctionnels une auto-réticulation dudit polymère fonctionnel sur une épaisseur limitée e par rapport à l'épaisseur moyenne E des points de polymère.This goal is perfectly achieved by the method of the invention, according to which in known manner is deposited on the face place of a interlining support, selected from textile supports and nonwovens, hot melt polymer dots and subject the face of the interlining support to an electronic bombardment. Typically, according to the invention, the polymer points thermofusible are based on at least one functional polymer having functional groups capable of reacting with free radicals generated under the action of electronic bombing and / or themselves generators of free radicals under the action of electronic bombing; in addition, the depth of electron penetration into the polymer points to obtain, thanks to said functional groups, a self-crosslinking of said functional polymer on a limited thickness e with respect to the average thickness E of the polymer points.
Ainsi tous les inconvénients précités liés au mélange du polymère thermofusible et de l'agent radicalaire sont éliminés puisque c'est le polymère thermofusible lui-même qui comporte à la fois la fonction d'adhérence et la fonction de réactivité au bombardement électronique.Thus all the aforementioned drawbacks relating to the mixing of the thermofusible polymer and the radical agent are eliminated since it is the thermofusible polymer itself that includes both the adhesion function and the bombardment reactivity function electronic.
C'est un autre objet de l'invention que de proposer un polymère thermofusible pour entoilage thermocollant, spécialement conçu pour la mise en oeuvre du procédé précité. Ce polymère thermofusible se caractérise en ce qu'il comporte des groupements fonctionnels aptes à réagir avec des radicaux libres sous l'action d'un bombardement électronique et/ou eux-mêmes générateurs de radicaux libres sous l'action du bombardement électronique.It is another object of the invention to provide a thermofusible polymer for fusible interlinings, especially designed for the implementation of the aforementioned method. This polymer thermofusible is characterized in that it comprises groups functional groups able to react with free radicals under the action of a electronic bombardment and / or themselves generators of free radicals under the action of electronic bombardment.
Selon une première version, ces groupements fonctionnels comportent des fonctions à insaturation éthylénique , par exemple du type acrylate, méthacrylate , allylique , acrylamide, vinyléther, styrénique, maléique ou fumarique.According to a first version, these functional groupings have functions with ethylenic unsaturation, for example acrylate, methacrylate, allyl, acrylamide, vinyl ether, styrenic, maleic or fumaric.
Selon une seconde version, lesdits groupements fonctionnels comportent des entités labiles c'est-à-dire des entités dont les énergies de liaison sont plus faibles que les liaisons usuelles carbone - carbone ou carbone - hydrogène. Comme exemple d'entité labile, on peut citer une liaison carbone - chlore C-CI ou une liaison thiol S-H.According to a second version, said groupings functional entities include labile entities, that is entities whose binding energies are lower than the usual connections carbon - carbon or carbon - hydrogen. As an example of an entity labile, there may be mentioned a carbon-chlorine bond C-CI or a bond thiol S-H.
Les polymères thermofusibles fonctionnels selon l'invention sont obtenus selon les deux voies possibles. Selon la première voie, on ajoute directement, dans le milieu réactionnel de synthèse du polymère, des monomères portant le ou les groupements fonctionnels aptes à réagir avec des radicaux libres sous l'action d'un bombardement électronique et/ou eux-mêmes générateurs de radicaux libres sous l'action du bombardement électronique. Selon la seconde voie, on part du polymère thermofusible déjà constitué et on le transforme ultérieurement en greffant sur sa structure polymère les groupements fonctionnels désirés par des techniques de greffage connues.Functional Hot Melt Polymers According to the Invention are obtained according to the two possible ways. According to the first way, we add directly, in the synthesis reaction medium of the polymer, monomers carrying the functional group or groups able to react with free radicals under the action of a electronic bombardment and / or themselves generators of free radicals under the action of electronic bombardment. According to second way, we start from the already formed hot melt polymer and it is later transformed by grafting onto its polymer structure functional groups desired by grafting techniques known.
L'emplacement du groupement fonctionnel le long de la chaíne polymère influence considérablement la réactivité du polymère fonctionnel sous l'action du bombardement électronique ainsi que la structure du réseau réticulé obtenu. Le groupement fonctionnel peut être situé en bout de chaíne , inclus le long de la chaíne ou encore situé sur des ramifications ou greffons le long de la chaíne polymère principale.The location of the functional group along the polymer chain significantly influences the reactivity of the polymer functional under the action of electronic bombardment as well as the structure of the crosslinked network obtained. The functional group can be located at the end of the chain, included along the chain or located on ramifications or grafts along the polymer chain main.
Le polymère thermofusible fonctionnel selon l'invention doit nécessairement présenter les propriétés d'adhérence ou collage nécessaires à l'utilisation visée qui est l'entoilage thermocollant. De plus il doit pouvoir être fonctionnalisé soit lors de sa synthèse soit par transformation ultérieure, comme indiqué précédemment. Il est donc notamment du type polyéthylène (PE), copolyamide (coPA), polyester (Pes), polyuréthanne (PU) ou copolyamide bloc éther (PBAX). A titre d'exemples non limitatifs s'agissant d'un squelette du type polyamide , les groupes fonctionnels sont situés en bout de chaíne ; s'agissant d'un squelette du type polyéthylène , les groupements fonctionnels sont situés sur des ramifications le long de la chaíne principale; s'agissant d'un squelette du type polyester , les groupements fonctionnels sont inclus le long de la chaíne principale ; s'agissant d'un squelette du type polyuréthanne , les groupements fonctionnels sont greffés le long de la chaíne principale.The functional thermofusible polymer according to the invention must necessarily have the properties of adhesion or bonding necessary for the intended use which is fusible interlining. Of the more it must be functionalized either during its synthesis or by subsequent transformation, as indicated previously. It is therefore especially polyethylene (PE), copolyamide (coPA), polyester (Pes), polyurethane (PU) or copolyamide block ether (PBAX). As non-limiting examples with regard to a skeleton of the polyamide type the functional groups are located at the end of the chain; regarding of a skeleton of the polyethylene type, the functional groups are located on branches along the main chain; in the case of a skeleton of the polyester type, the groups functional are included along the main chain; in the case of a skeleton of the polyurethane type, the functional groups are grafted along the main chain.
Bien sûr le polymère thermofusible fonctionnel de l'invention est sélectionné en sorte de répondre aux contraintes d'utilisation dans l'entoilage thermocollant , contraintes qui sont variables en fonction des techniques mises en oeuvre.Of course the functional thermofusible polymer of the invention is selected so as to meet the constraints of use in fusible interlinings, constraints which are variables according to the techniques used.
En particulier , s'agissant de sa présentation, ce polymère doit pouvoir être livré sous forme d'une poudre résistante au broyage pour des granulométries de 10 à 200 µm ou encore être livrables en granulés si la technique utilisée est du type hot-melt.In particular, as regards its presentation, this polymer must be delivered in the form of a grinding-resistant powder for particle sizes from 10 to 200 μm or to be available in granules if the technique used is of the hot-melt type.
Lorsque le dépôt des points de polymère se fait à partir d'une dispersion aqueuse sous forme de pâte, le polymère doit bien sûr être compatible pour une telle mise en dispersion aqueuse.When the deposit of polymer points is made from of an aqueous dispersion in the form of a paste, the polymer must be sure to be compatible for such an aqueous dispersion.
Lorsque le dépôt se fait sous forme d'une enduction, les groupements fonctionnels que comporte le polymère thermofusible doivent être stables à la température d'enduction, sachant que selon la technique utilisée , cette température peut aller de 150 à 225°C. Cette stabilité thermique est indispensable pour éviter que les groupements fonctionnels donnent lieu à un démarrage incontrôlé de l'auto-réticulation. Cette stabilité thermique peut être améliorée en incorporant au polymère thermofusible fonctionnel un anti-oxydant. When the deposit is in the form of a coating, the functional groups that comprise the hot melt polymer must be stable at the coating temperature, knowing that the technique used, this temperature can range from 150 to 225 ° C. This thermal stability is essential to prevent functional groups give rise to an uncontrolled start of self-crosslinking. This thermal stability can be improved by incorporating into the functional hot melt polymer an antioxidant.
La température de fusion du polymère thermofusible fonctionnel de l'invention, non soumis au bombardement électronique, doit être généralement comprise entre 70 et 150°C, sachant que la température de fusion du même polymère auto-réticulé sous l'action du bombardement électronique lui est supérieure.The melting temperature of the hot melt polymer functional of the invention, not subjected to bombardment electronic system, should generally be between 70 and 150 ° C, knowing that the melting temperature of the same self-crosslinked polymer under the action of electronic bombing him is higher.
Le polymère fonctionnel thermofusible de l'invention est, selon les applications , résistant au lavage machine, résistant au nettoyage à sec avec solvant chloré et résistant à la vapeur.The hot melt functional polymer of the invention is according to the applications, machine wash resistant, resistant to dry cleaning with chlorinated solvent and resistant to steam.
Selon un exemple de réalisation, le polymère fonctionnel a un squelette de type polyéthylène et comporte des groupements fonctionnels de type méthacrylate. Pour obtenir ce polymère fonctionnel on part d'un polymère initial obtenu à partir de monomères d'éthylène et d'un faible pourcentage, de l'ordre de 3% en poids, d'acide acrylique. Ce polymère initial du type polyéthylène comporte des fonctions acide attachées à la chaíne carbonée. Il s'agit notamment du polymère EAA, proposé par la firme DOW CHEMICAL sous la dénomination Primacor 3150. On fait subir à ce polymère initial une réaction d'estérification avec un composé de type époxyde de formule : proposé par la firme Aldricch sous l'appellation GMA, en proportion stachiométrique. On obtient le polymère fonctionnel de formule : dont les groupements fonctionnels méthacrylates comportent des liaisons insaturées éthyléniques aptes à réaliser une auto-réticulation du polymère sur lui-même , grâce aux radicaux libres générés par l'action des élections lors du bombardement électronique. Il s'agit notamment d'un bombardement électronique réalisé avec une puissance d'au moins 70kV, avec une dose de l'ordre de 10 à 100 kGray sur la face envers du support d'entoilage dont la face endroit comporte des points formés avec le polymère fonctionnel. La puissance et la dose retenue permettent de limiter l'action des électrons sur une épaisseur e limitée de l'épaisseur moyenne des points déposés. Ainsi l'auto-réticulation du polymère fonctionnel ne se produit que sur cette épaisseur e du point, au niveau de la base dudit point, c'est-à-dire celle qui est en contact avec le support d'entoilage . Le polymère auto-réticulé a une température de fusion plus élevée que celle du polymère fonctionnel non-autoréticulé, de sorte que lors de l'application de l'entoilage sur l'article à renforcer la base auto-réticulée du point de polymère flue moins que le reste du point, ce qui évite le transpercement.According to an exemplary embodiment, the functional polymer has a polyethylene backbone and comprises functional groups of methacrylate type. To obtain this functional polymer is based on an initial polymer obtained from ethylene monomers and a small percentage, of the order of 3% by weight, of acrylic acid. This initial polymer of the polyethylene type contains acid functions attached to the carbon chain. These include the EAA polymer, proposed by DOW CHEMICAL company under the name Primacor 3150. This initial polymer is subjected to an esterification reaction with an epoxide type compound of formula: proposed by the firm Aldricch under the name GMA, in stachiometric proportion. The functional polymer of formula whose methacrylate functional groups comprise ethylenic unsaturated bonds able to perform a self-crosslinking of the polymer on itself, thanks to the free radicals generated by the action of the elections during the electronic bombardment. This involves an electronic bombardment performed with a power of at least 70kV, with a dose of the order of 10 to 100 kGray on the reverse side of the interlining support whose face face has points formed with the functional polymer. The power and the dose used make it possible to limit the action of the electrons on a limited thickness e of the average thickness of the deposited points. Thus the self-crosslinking of the functional polymer occurs only on this thickness e of the point, at the base of said point, that is to say that which is in contact with the interlining support. The self-crosslinked polymer has a higher melting temperature than the non-self-crosslinking functional polymer, so that when applying the interlining to the article to reinforce the self-crosslinked base of the polymer point less than than the rest of the item, which avoids piercing.
Un second et un troisième exemples de polymères fonctionnels à squelette de type polyéthylène peuvent être cités.A second and a third example of polymers functional polyethylene backbone can be cited.
Dans le second exemple, les groupements fonctionnels sont de type styrénique. Le polymère initial est obtenu à partir de monomère d'éthylène et de l'ordre de 10% en poids d'hydroxyéthyl méthacrylate. Il peut s 'agir du polymère EHEMA proposé par la firme Neste Chemical sous la référence NRT 354. Il réagit avec un composé m.isopropenyl, de formule proposé par la firme American Cyanamid sous la dénomination TMI, pour donner le polymère fonctionnel de forme générale : In the second example, the functional groups are of the styrenic type. The initial polymer is obtained from ethylene monomer and of the order of 10% by weight of hydroxyethyl methacrylate. This may be the EHEMA polymer proposed by the company Neste Chemical under the reference NRT 354. It reacts with an isopropenyl compound of formula proposed by the firm American Cyanamid under the name TMI, to give the functional polymer of general form:
Dans le troisième exemple, les groupements fonctionnels sont du type acrylate. Le polymère initial est obtenu à partir de monomère d'éthylène et de l'ordre de 16% en poids de vinyl alcool. Il peut s'agir du polymère EVOH proposé par la firme Bayer sous la référence Levasint S-31. Il réagit avec un composé acide acrylique pour donner le polymère fonctionnel de formule générale : In the third example, the functional groups are of the acrylate type. The initial polymer is obtained from ethylene monomer and of the order of 16% by weight of vinyl alcohol. It may be the EVOH polymer proposed by Bayer under the reference Levasint S-31. It reacts with an acrylic acid compound to give the functional polymer of the general formula:
Dans tous les cas, les conditions opératoires des différentes réactions mises en oeuvre sont déterminées en sorte d'obtenir un polymère fonctionnel qui contient une proportion adéquate de groupements fonctionnels, pour obtenir le résultat recherché , à savoir d'obtenir, sous l'action des électrons , une augmentation localisée de la température de fusion due à l'auto-réticulation dudit polymère fonctionnel et qui de plus répond aux conditions imposées par l'application à l'entoilage thermocollant du support sur lequel les points de polymère fonctionnel sont déposés.In all cases, the operating conditions of the different reactions implemented are determined in order to obtain a polymer functional that contains an adequate proportion of groupings functional, to obtain the desired result, namely to obtain, under the action of electrons, a localized increase in melting temperature due to the self-crosslinking of said polymer functional and which moreover meets the conditions imposed by application to the fusible interlining of the support on which the Functional polymer dots are deposited.
Claims (5)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| SI200230421T SI1314366T1 (en) | 2001-11-26 | 2002-11-19 | Method for making a fusible interlining with dots of thermally fusible polymer and thermally fusible polymer used thereby |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR0115272 | 2001-11-26 | ||
| FR0115272A FR2832595B1 (en) | 2001-11-26 | 2001-11-26 | METHOD FOR MANUFACTURING A THERMOCOLLATING WEAR WITH POINTS OF THERMOFUSIBLE POLYMER AND THERMOFUSIBLE POLYMER SPECIALLY DESIGNED FOR CARRYING OUT SAID METHOD |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP1314366A1 true EP1314366A1 (en) | 2003-05-28 |
| EP1314366B1 EP1314366B1 (en) | 2006-08-09 |
Family
ID=8869793
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP02370050A Expired - Lifetime EP1314366B1 (en) | 2001-11-26 | 2002-11-19 | Method for making a fusible interlining with dots of thermally fusible polymer and thermally fusible polymer used thereby |
Country Status (23)
| Country | Link |
|---|---|
| US (1) | US6991832B2 (en) |
| EP (1) | EP1314366B1 (en) |
| JP (1) | JP2003193319A (en) |
| KR (1) | KR100948454B1 (en) |
| CN (1) | CN1318533C (en) |
| AR (1) | AR037419A1 (en) |
| AT (1) | ATE335415T1 (en) |
| AU (1) | AU2002304014B2 (en) |
| BR (1) | BR0204772B1 (en) |
| CA (1) | CA2412473C (en) |
| DE (1) | DE60213740T2 (en) |
| ES (1) | ES2269635T3 (en) |
| FR (1) | FR2832595B1 (en) |
| HU (1) | HUP0204045A2 (en) |
| MX (1) | MXPA02011642A (en) |
| MY (1) | MY131227A (en) |
| NO (1) | NO325648B1 (en) |
| PL (1) | PL212674B1 (en) |
| PT (1) | PT1314366E (en) |
| RU (1) | RU2317311C2 (en) |
| SI (1) | SI1314366T1 (en) |
| UA (1) | UA79579C2 (en) |
| ZA (1) | ZA200209564B (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2870433A1 (en) * | 2004-05-24 | 2005-11-25 | Lainiere De Picardie Bc Soc Pa | METHOD OF MANUFACTURING A THERMOCOLLATING WEAR AND THERMOCOLLANT WEARING OBTAINED |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2832595B1 (en) * | 2001-11-26 | 2004-03-19 | Lainiere De Picardie Bc | METHOD FOR MANUFACTURING A THERMOCOLLATING WEAR WITH POINTS OF THERMOFUSIBLE POLYMER AND THERMOFUSIBLE POLYMER SPECIALLY DESIGNED FOR CARRYING OUT SAID METHOD |
| JP2005146137A (en) * | 2003-11-17 | 2005-06-09 | Jsr Corp | Thermoplastic elastomer composition molded article and its manufacturing method |
| DE102005006335A1 (en) * | 2005-02-10 | 2006-08-24 | Bozzetto Gmbh | Crosslinkable melt adhesive mixture and method for coating and / or lamination of substrates |
| US20060258875A1 (en) * | 2005-05-10 | 2006-11-16 | Clementine Reyes | Methods for manufacturing supported nanocatalysts and methods for using supported nanocatalysts |
| US8097229B2 (en) * | 2006-01-17 | 2012-01-17 | Headwaters Technology Innovation, Llc | Methods for manufacturing functionalized inorganic oxides and polymers incorporating same |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4908229A (en) * | 1986-03-11 | 1990-03-13 | Union Oil Of California | Method for producing an article containing a radiation cross-linked polymer and the article produced thereby |
| JPH0711585A (en) * | 1993-04-30 | 1995-01-13 | Kotec Kk | Fusible interlining and its production |
| EP0775773A1 (en) * | 1994-08-09 | 1997-05-28 | Asahi Kasei Kogyo Kabushiki Kaisha | Adhesive padding cloth, method of manufacturing the same and bonding agent for adhesive cloths |
| EP0855146A1 (en) * | 1997-01-20 | 1998-07-29 | Lainiere De Picardie | Method for making a fusible interlining and interlining obtained thereby |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4234662A (en) * | 1979-04-26 | 1980-11-18 | National Starch And Chemical Corporation | Pressure sensitive hot melt adhesive curable by exposure to electron beam radiation |
| US4748044A (en) * | 1980-12-24 | 1988-05-31 | Rma Carl Freudenberg | Method for the simultaneous, continuous binding and coating of a nonwoven fabric |
| JPS6257986A (en) * | 1985-09-05 | 1987-03-13 | 工業技術院長 | Hot melt adhesive for ionizing radiation curable fiber |
| FR2606603B1 (en) * | 1986-11-14 | 1991-03-22 | Picardie Lainiere | THERMAL ADHESIVE PRODUCT AND MANUFACTURING METHOD |
| US5543214A (en) * | 1988-01-08 | 1996-08-06 | Laniere De Picarde | Thermo-adhesive cross-linkable textile product |
| JP2842626B2 (en) * | 1989-07-29 | 1999-01-06 | ダイニック株式会社 | Adhesive interlining and manufacturing method |
| JPH06257986A (en) * | 1993-03-04 | 1994-09-16 | Ishikawajima Harima Heavy Ind Co Ltd | Double heat transfer tube and manufacture thereof |
| JPH0959876A (en) * | 1995-08-11 | 1997-03-04 | Hayakawa Rubber Co Ltd | Adhesive interlining cloth and its production |
| DE19826093A1 (en) * | 1998-06-12 | 1999-12-23 | Beiersdorf Ag | Partially self-adhesive object with permanently deformed self-adhesive |
| US6232365B1 (en) * | 1998-07-17 | 2001-05-15 | 3M Innovative Properties Company | Low temperature electron beam polymerization |
| FR2781648B1 (en) * | 1998-07-31 | 2001-01-05 | Dhj Internat | THERMAL-ADHESIVE COVER AND MANUFACTURING METHOD, USE OF THE COVER, CLOTHES OR PARTS OF CLOTHING COMPRISING THIS COVER |
| DE10008844A1 (en) * | 2000-02-25 | 2001-09-06 | Beiersdorf Ag | Process for the crosslinking of polyacrylates by electron beams |
| US6780484B2 (en) * | 2001-02-02 | 2004-08-24 | 3M Innovative Properties Company | Adhesive article and method of preparing |
| US6858695B2 (en) * | 2001-04-27 | 2005-02-22 | National Starch And Chemical Investment Holding Corporation | Curable hot melt adhesive for casemaking |
| FR2832595B1 (en) * | 2001-11-26 | 2004-03-19 | Lainiere De Picardie Bc | METHOD FOR MANUFACTURING A THERMOCOLLATING WEAR WITH POINTS OF THERMOFUSIBLE POLYMER AND THERMOFUSIBLE POLYMER SPECIALLY DESIGNED FOR CARRYING OUT SAID METHOD |
-
2001
- 2001-11-26 FR FR0115272A patent/FR2832595B1/en not_active Expired - Fee Related
-
2002
- 2002-11-19 DE DE60213740T patent/DE60213740T2/en not_active Expired - Lifetime
- 2002-11-19 ES ES02370050T patent/ES2269635T3/en not_active Expired - Lifetime
- 2002-11-19 PT PT02370050T patent/PT1314366E/en unknown
- 2002-11-19 EP EP02370050A patent/EP1314366B1/en not_active Expired - Lifetime
- 2002-11-19 SI SI200230421T patent/SI1314366T1/en unknown
- 2002-11-19 AT AT02370050T patent/ATE335415T1/en not_active IP Right Cessation
- 2002-11-22 BR BRPI0204772-1A patent/BR0204772B1/en active IP Right Grant
- 2002-11-22 US US10/302,486 patent/US6991832B2/en not_active Expired - Lifetime
- 2002-11-25 UA UA2002119371A patent/UA79579C2/en unknown
- 2002-11-25 PL PL357334A patent/PL212674B1/en unknown
- 2002-11-25 CA CA2412473A patent/CA2412473C/en not_active Expired - Lifetime
- 2002-11-25 AU AU2002304014A patent/AU2002304014B2/en not_active Expired
- 2002-11-25 RU RU2002131673/04A patent/RU2317311C2/en active
- 2002-11-25 NO NO20025661A patent/NO325648B1/en unknown
- 2002-11-25 AR ARP020104517A patent/AR037419A1/en active IP Right Grant
- 2002-11-25 MY MYPI20024383A patent/MY131227A/en unknown
- 2002-11-25 MX MXPA02011642A patent/MXPA02011642A/en active IP Right Grant
- 2002-11-25 ZA ZA200209564A patent/ZA200209564B/en unknown
- 2002-11-25 CN CNB021542651A patent/CN1318533C/en not_active Expired - Lifetime
- 2002-11-25 HU HU0204045A patent/HUP0204045A2/en unknown
- 2002-11-26 JP JP2002341588A patent/JP2003193319A/en active Pending
- 2002-11-26 KR KR1020020073698A patent/KR100948454B1/en active IP Right Grant
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4908229A (en) * | 1986-03-11 | 1990-03-13 | Union Oil Of California | Method for producing an article containing a radiation cross-linked polymer and the article produced thereby |
| JPH0711585A (en) * | 1993-04-30 | 1995-01-13 | Kotec Kk | Fusible interlining and its production |
| EP0775773A1 (en) * | 1994-08-09 | 1997-05-28 | Asahi Kasei Kogyo Kabushiki Kaisha | Adhesive padding cloth, method of manufacturing the same and bonding agent for adhesive cloths |
| EP0855146A1 (en) * | 1997-01-20 | 1998-07-29 | Lainiere De Picardie | Method for making a fusible interlining and interlining obtained thereby |
Non-Patent Citations (1)
| Title |
|---|
| PATENT ABSTRACTS OF JAPAN vol. 1995, no. 04 31 May 1995 (1995-05-31) * |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2870433A1 (en) * | 2004-05-24 | 2005-11-25 | Lainiere De Picardie Bc Soc Pa | METHOD OF MANUFACTURING A THERMOCOLLATING WEAR AND THERMOCOLLANT WEARING OBTAINED |
| EP1600552A2 (en) * | 2004-05-24 | 2005-11-30 | Lainiere De Picardie Bc | Method for making a fusible interlining and fusible interlining obtained thereby |
| EP1600552A3 (en) * | 2004-05-24 | 2008-04-02 | Lainiere De Picardie Bc | Method for making a fusible interlining and fusible interlining obtained thereby |
Also Published As
| Publication number | Publication date |
|---|---|
| NO325648B1 (en) | 2008-06-30 |
| FR2832595B1 (en) | 2004-03-19 |
| CA2412473A1 (en) | 2003-05-26 |
| HU0204045D0 (en) | 2003-02-28 |
| CN1432616A (en) | 2003-07-30 |
| SI1314366T1 (en) | 2007-02-28 |
| PL212674B1 (en) | 2012-11-30 |
| RU2317311C2 (en) | 2008-02-20 |
| ATE335415T1 (en) | 2006-09-15 |
| EP1314366B1 (en) | 2006-08-09 |
| DE60213740D1 (en) | 2006-09-21 |
| ZA200209564B (en) | 2003-06-03 |
| CN1318533C (en) | 2007-05-30 |
| KR100948454B1 (en) | 2010-03-17 |
| PL357334A1 (en) | 2003-06-02 |
| DE60213740T2 (en) | 2007-03-29 |
| BR0204772B1 (en) | 2012-04-03 |
| US6991832B2 (en) | 2006-01-31 |
| PT1314366E (en) | 2006-12-29 |
| HUP0204045A2 (en) | 2003-06-28 |
| BR0204772A (en) | 2003-09-16 |
| AR037419A1 (en) | 2004-11-10 |
| ES2269635T3 (en) | 2007-04-01 |
| UA79579C2 (en) | 2007-07-10 |
| MXPA02011642A (en) | 2004-09-03 |
| US20030099781A1 (en) | 2003-05-29 |
| MY131227A (en) | 2007-07-31 |
| CA2412473C (en) | 2010-10-12 |
| KR20040029930A (en) | 2004-04-08 |
| AU2002304014B2 (en) | 2007-05-17 |
| FR2832595A1 (en) | 2003-05-30 |
| NO20025661L (en) | 2003-05-27 |
| JP2003193319A (en) | 2003-07-09 |
| NO20025661D0 (en) | 2002-11-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CA2641435C (en) | Structure made from a thermoplastic composition of a polyolefin functionalised by polyether grafts and use thereof | |
| EP1314366B1 (en) | Method for making a fusible interlining with dots of thermally fusible polymer and thermally fusible polymer used thereby | |
| EP2234944B1 (en) | Method for making a refractory carbide layer on a part made of c/c composite material | |
| WO2002012376A1 (en) | Breathable film | |
| CH638472A5 (en) | GLASS FIBER THREAD IMPREGNATED WITH A STABLE PRIMER, NOT CROSSLINKING. | |
| JPH10510487A (en) | Heat sealable multilayer film containing polyvinyl alcohol layer | |
| EP2108070B1 (en) | Method for functionalising a textile substrate by cross-linking bonding under a ionising radiation | |
| EP1600552B1 (en) | Method for making a fusible interlining and fusible interlining obtained thereby | |
| EP0797932B1 (en) | Method to make a fusible interlining | |
| EP0565394B1 (en) | Two-phase heat-sealable interlining and method for its manufacture | |
| FR2949679A1 (en) | METHOD FOR MAKING NAILS AND ARTICLE FOR IMPLEMENTING THE METHOD | |
| FR2606603A1 (en) | THERMOCOLLANT PRODUCT AND METHOD OF MANUFACTURE | |
| EP0966001A1 (en) | Manufacture of a mica tape and obtained product | |
| Funasaka et al. | Adhesive ability and solvent solubility of propylene-butene copolymers modified with maleic anhydride | |
| EP0288357A1 (en) | Method of making a textile stiffener, and fabric treated therewith | |
| EP0855146B1 (en) | Method for making a fusible interlining and interlining obtained thereby | |
| CH621830A5 (en) | ||
| WO1994014915A1 (en) | Polyolefin-acrylic bonded composites | |
| CA2324933A1 (en) | Composite absorbent materials for aqueous liquid | |
| WO2001004418A1 (en) | Cellulosic support treatment | |
| JP2000283369A (en) | Polyolefin joint | |
| FR2617494A1 (en) | Composition for adhesively bonding a textile coating onto plastic articles | |
| FR2949678A1 (en) | Make up of nails comprises applying adhesive article comprising pre-thermoformed support having thermoplastic materials and heating the article applied to the nail to soften the materials | |
| KR20140019671A (en) | Transfer paper and transfering method using the same | |
| FR3063500A1 (en) | PROCESS FOR MANUFACTURING COATED PART |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR IE IT LI LU MC NL PT SE SK TR |
|
| AX | Request for extension of the european patent |
Extension state: AL LT LV MK RO SI |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: MESNIL, BENOIT Inventor name: LEFEBVRE, JEAN |
|
| 17P | Request for examination filed |
Effective date: 20031007 |
|
| AKX | Designation fees paid |
Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR IE IT LI LU MC NL PT SE SK TR |
|
| AXX | Extension fees paid |
Extension state: SI Payment date: 20031007 Extension state: LT Payment date: 20031007 Extension state: RO Payment date: 20031007 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR IE IT LI LU MC NL PT SE SK TR |
|
| AX | Request for extension of the european patent |
Extension state: LT RO SI |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20060809 Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED. Effective date: 20060809 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20060809 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: FRENCH |
|
| REF | Corresponds to: |
Ref document number: 60213740 Country of ref document: DE Date of ref document: 20060921 Kind code of ref document: P |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 20061003 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20061109 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: NV Representative=s name: BOVARD AG PATENTANWAELTE |
|
| REG | Reference to a national code |
Ref country code: GR Ref legal event code: EP Ref document number: 20060403764 Country of ref document: GR |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: TRGR |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20061130 |
|
| REG | Reference to a national code |
Ref country code: PT Ref legal event code: SC4A Free format text: AVAILABILITY OF NATIONAL TRANSLATION Effective date: 20061024 |
|
| LTIE | Lt: invalidation of european patent or patent extension |
Effective date: 20060809 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2269635 Country of ref document: ES Kind code of ref document: T3 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20070510 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20060809 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20061119 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20060809 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IE Payment date: 20071023 Year of fee payment: 6 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20081119 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 20091020 Year of fee payment: 8 Ref country code: CH Payment date: 20091117 Year of fee payment: 8 Ref country code: SE Payment date: 20091021 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20091020 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GR Payment date: 20091021 Year of fee payment: 8 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PFA Owner name: LAINIERE DE PICARDIE BC Free format text: LAINIERE DE PICARDIE BC#BUIRE COURCELLES#80202 PERONNE (FR) -TRANSFER TO- LAINIERE DE PICARDIE BC#BUIRE COURCELLES#80202 PERONNE (FR) |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: V1 Effective date: 20110601 |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: EUG |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20101130 Ref country code: GR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20110602 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20101130 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20101119 Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20110601 |
|
| REG | Reference to a national code |
Ref country code: SI Ref legal event code: KO00 Effective date: 20110725 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20101120 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 14 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 15 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 16 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20211122 Year of fee payment: 20 Ref country code: ES Payment date: 20211203 Year of fee payment: 20 Ref country code: GB Payment date: 20211122 Year of fee payment: 20 Ref country code: TR Payment date: 20211020 Year of fee payment: 20 Ref country code: PT Payment date: 20211015 Year of fee payment: 20 Ref country code: CZ Payment date: 20211012 Year of fee payment: 20 Ref country code: BG Payment date: 20211012 Year of fee payment: 20 Ref country code: DE Payment date: 20211110 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20211110 Year of fee payment: 20 Ref country code: BE Payment date: 20211130 Year of fee payment: 20 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R071 Ref document number: 60213740 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MK Effective date: 20221119 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 Expiry date: 20221118 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20221130 Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20221118 Ref country code: CZ Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20221119 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20230503 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20221120 |