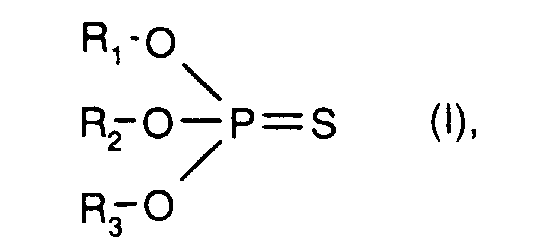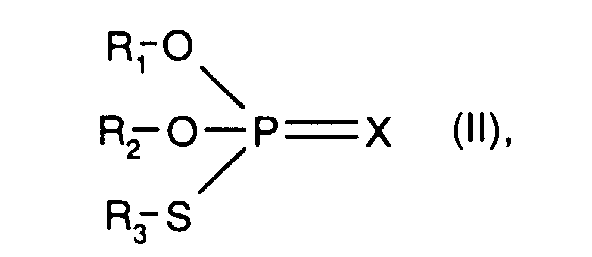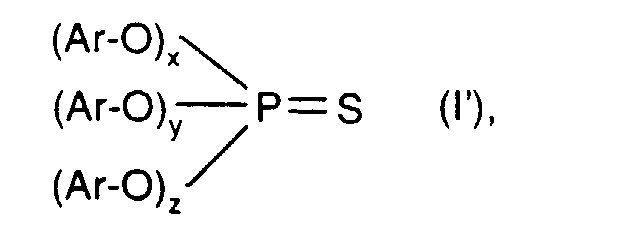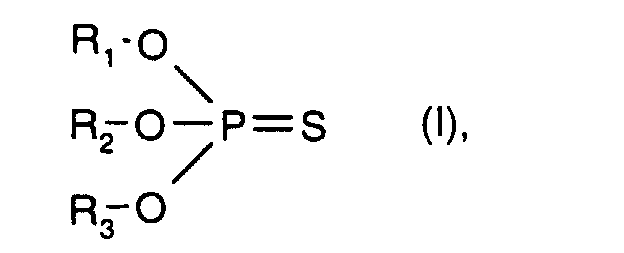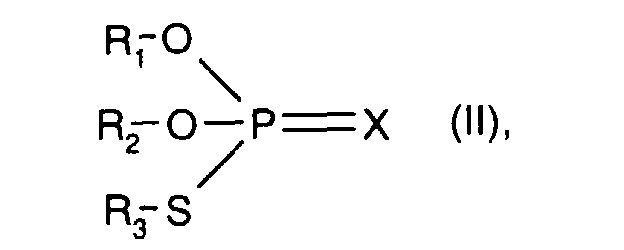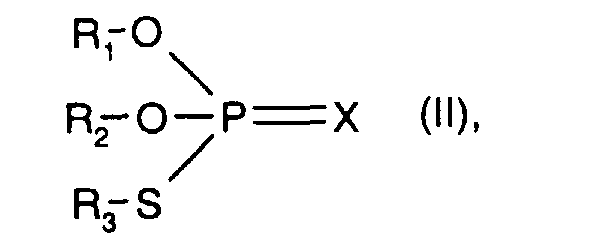EP0903399A1 - Lubricant compositions containing thiophosphoric and dithiophosphoric acid esters - Google Patents
Lubricant compositions containing thiophosphoric and dithiophosphoric acid esters Download PDFInfo
- Publication number
- EP0903399A1 EP0903399A1 EP98810894A EP98810894A EP0903399A1 EP 0903399 A1 EP0903399 A1 EP 0903399A1 EP 98810894 A EP98810894 A EP 98810894A EP 98810894 A EP98810894 A EP 98810894A EP 0903399 A1 EP0903399 A1 EP 0903399A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- formula
- ester
- thiophosphoric
- alkyl
- composition according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 0 C*(*(C)(C)C)N Chemical compound C*(*(C)(C)C)N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M141/00—Lubricating compositions characterised by the additive being a mixture of two or more compounds covered by more than one of the main groups C10M125/00 - C10M139/00, each of these compounds being essential
- C10M141/10—Lubricating compositions characterised by the additive being a mixture of two or more compounds covered by more than one of the main groups C10M125/00 - C10M139/00, each of these compounds being essential at least one of them being an organic phosphorus-containing compound
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M129/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing oxygen
- C10M129/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing oxygen having a carbon chain of less than 30 atoms
- C10M129/16—Ethers
- C10M129/18—Epoxides
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M129/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing oxygen
- C10M129/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing oxygen having a carbon chain of less than 30 atoms
- C10M129/68—Esters
- C10M129/76—Esters containing free hydroxy or carboxyl groups
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M133/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing nitrogen
- C10M133/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing nitrogen having a carbon chain of less than 30 atoms
- C10M133/04—Amines, e.g. polyalkylene polyamines; Quaternary amines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M133/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing nitrogen
- C10M133/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing nitrogen having a carbon chain of less than 30 atoms
- C10M133/04—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M133/06—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to acyclic or cycloaliphatic carbon atoms
- C10M133/08—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to acyclic or cycloaliphatic carbon atoms containing hydroxy groups
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M137/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing phosphorus
- C10M137/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing phosphorus having no phosphorus-to-carbon bond
- C10M137/04—Phosphate esters
- C10M137/08—Ammonium or amine salts
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M137/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing phosphorus
- C10M137/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing phosphorus having no phosphorus-to-carbon bond
- C10M137/04—Phosphate esters
- C10M137/10—Thio derivatives
- C10M137/105—Thio derivatives not containing metal
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/04—Ethers; Acetals; Ortho-esters; Ortho-carbonates
- C10M2207/042—Epoxides
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/28—Esters
- C10M2207/287—Partial esters
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/28—Esters
- C10M2207/287—Partial esters
- C10M2207/288—Partial esters containing free carboxyl groups
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/28—Esters
- C10M2207/287—Partial esters
- C10M2207/289—Partial esters containing free hydroxy groups
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2209/00—Organic macromolecular compounds containing oxygen as ingredients in lubricant compositions
- C10M2209/10—Macromolecular compoundss obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
- C10M2209/103—Polyethers, i.e. containing di- or higher polyoxyalkylene groups
- C10M2209/104—Polyethers, i.e. containing di- or higher polyoxyalkylene groups of alkylene oxides containing two carbon atoms only
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M2215/04—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to acyclic or cycloaliphatic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M2215/04—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to acyclic or cycloaliphatic carbon atoms
- C10M2215/042—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to acyclic or cycloaliphatic carbon atoms containing hydroxy groups; Alkoxylated derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2215/26—Amines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2219/00—Organic non-macromolecular compounds containing sulfur, selenium or tellurium as ingredients in lubricant compositions
- C10M2219/04—Organic non-macromolecular compounds containing sulfur, selenium or tellurium as ingredients in lubricant compositions containing sulfur-to-oxygen bonds, i.e. sulfones, sulfoxides
- C10M2219/044—Sulfonic acids, Derivatives thereof, e.g. neutral salts
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2223/00—Organic non-macromolecular compounds containing phosphorus as ingredients in lubricant compositions
- C10M2223/02—Organic non-macromolecular compounds containing phosphorus as ingredients in lubricant compositions having no phosphorus-to-carbon bonds
- C10M2223/04—Phosphate esters
- C10M2223/043—Ammonium or amine salts thereof
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2223/00—Organic non-macromolecular compounds containing phosphorus as ingredients in lubricant compositions
- C10M2223/02—Organic non-macromolecular compounds containing phosphorus as ingredients in lubricant compositions having no phosphorus-to-carbon bonds
- C10M2223/04—Phosphate esters
- C10M2223/047—Thioderivatives not containing metallic elements
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2290/00—Mixtures of base materials or thickeners or additives
- C10M2290/02—Mineral base oils; Mixtures of fractions
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2040/00—Specified use or application for which the lubricating composition is intended
- C10N2040/04—Oil-bath; Gear-boxes; Automatic transmissions; Traction drives
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2040/00—Specified use or application for which the lubricating composition is intended
- C10N2040/04—Oil-bath; Gear-boxes; Automatic transmissions; Traction drives
- C10N2040/042—Oil-bath; Gear-boxes; Automatic transmissions; Traction drives for automatic transmissions
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2040/00—Specified use or application for which the lubricating composition is intended
- C10N2040/04—Oil-bath; Gear-boxes; Automatic transmissions; Traction drives
- C10N2040/044—Oil-bath; Gear-boxes; Automatic transmissions; Traction drives for manual transmissions
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2040/00—Specified use or application for which the lubricating composition is intended
- C10N2040/04—Oil-bath; Gear-boxes; Automatic transmissions; Traction drives
- C10N2040/046—Oil-bath; Gear-boxes; Automatic transmissions; Traction drives for traction drives
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2040/00—Specified use or application for which the lubricating composition is intended
- C10N2040/08—Hydraulic fluids, e.g. brake-fluids
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2040/00—Specified use or application for which the lubricating composition is intended
- C10N2040/20—Metal working
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2050/00—Form in which the lubricant is applied to the material being lubricated
- C10N2050/10—Semi-solids; greasy
Definitions
- the invention relates to improved compositions with thiophosphoric acid esters and Dithiophosphoric acid esters or phosphoric acid thioesters and the use of these Lubricant compositions to improve the performance properties of Lubricants such as greases, metalworking, gear or hydraulic fluids.
- Additives are added to the lubricants mentioned, which perform demanding tasks, such as high load-bearing capacity, wear and corrosion protection as well as antioxidant effects, have to meet.
- Zinc dialkyldithiophosphates are suitable for this, but one strives to replace them with metal-free connections for reasons of environmental protection.
- Suitable hydraulic fluids the specifications of the leading hydraulic machine manufacturers, e.g. Meet Denison HFO (Denison Hydraulics) or Vickers M-2980-S (Vickers) and be compatible with water. Furthermore, they should be in accordance with the specifications of DIN 51524 and Denison HFO achieve an error load level (FLS) of at least 10 in the FZG test.
- FLS error load level
- dithiophosphoric acid esters of the type: known which are commercially available under the trade name Irgalube®63 (trademark of Ciba Specialty Chemicals).
- US Pat. No. 5,531,911 describes zinc-free hydraulic fluids which contain phosphorus and sulfur-containing additive components.
- One component is a triphenylthiophosphate (IRGALUBE TPPT) thiophosphoric acid ester. This is combined with IRGALUBE 63 dithiophosphoric acid esters and optional oil additive components, eg ammonium sulfonates.
- IRGALUBE TPPT triphenylthiophosphate
- Such formulations are disadvantageous because of their lack of compatibility with water.
- the contamination of a hydraulic oil with water is particularly important in mobile hydraulic systems often.
- hydrolytic degradation takes place with formation of corrosive decomposition products, which the metals used in the hydraulic systems, e.g. Steel and copper alloys, attack and cause damage to the hydraulic pumps. It can also agglomerations of these decomposition products, the filters of bypass filtration systems To block. Since the service life of hydraulic systems is characterized by fine filtering The filter mesh sizes of modern bypass filtration systems are significantly longer reduced from 30 ⁇ m to 6 ⁇ m today. Therefore, only such are Hydraulic oils to be used which, when contaminated with water, contain only the smallest quantities of Form hydrolytic decomposition products.
- the present invention has for its object compositions with improved Establish compatibility with water, which has a much lower tendency to Have formation of unwanted hydrolysis products.
- compositions with thiophosphoric acid esters combined with dithiophosphoric acid esters or phosphoric acid thioesters Addition of another additive component from the group of polyol partial esters, amines and Epoxies have a significantly lower tendency to form when contaminated with water have corrosive hydrolysis products and very good filtration properties.
- oil additives e.g. B. ammonium phosphate esters, can Increase load-bearing capacity and reach FZG error load levels ⁇ 10.
- a particularly preferred embodiment relates to compositions in which the phosphorus content the thiophosphoric acid component b), combined with the dithiophosphoric acid ester or phosphoric acid thioester component c), based on the composition with components a), b) and c), is less than 400 ppm.
- a very particularly preferred embodiment relates to compositions in which the Phosphorus content of the thiophosphoric acid component b), combined with the dithiophosphoric acid ester or phosphoric acid thioester component c) and the Ammonium phosphate ester component e), based on the total composition, less than 400 ppm.
- compositions are multifunctional wear protection additives - with additional ones antioxidant effect - for lubricants such as greases, metalworking, gear or hydraulic fluids are particularly suitable. They are largely free of metal and ash and meet the specified specifications.
- These mixtures have the addition of Additive component from the group of polyol partial esters, amines and epoxides (component d)) very good filtration properties when contaminated with water.
- FZG error load levels ⁇ 10 can be achieved with other oil additives (components e).
- Such mixtures meet the hydraulic machine specifications of leading manufacturers, especially Denison HFO.
- a base oil with a lubricating viscosity is used for the production of greases, metalworking, Gear and hydraulic fluids can be used.
- Suitable greases, metalworking, gear and hydraulic fluids are based, for example, on mineral or synthetic oils or mixtures thereof.
- the lubricants are familiar to the person skilled in the art and in the relevant specialist literature, for example in Chemistry and Technology of Lubricants; Mortier, RM and Orszulik, ST (Editors); 1992 Blackie and Son Ltd. for GB, VCH-Publishers NY for US, ISBN 0-216-92921-0, see pages 208 ff and 269 ff; in Kirk-Othmer Encyclopedia of Chemical Technology, Fourth Edition 1969, J. Wiley & Sons, New York, Vol. 13, page 533 ff. (Hydraulic Fluids); Performance Testing of Hydraulic Fluids; R.
- the lubricants are in particular oils and fats, for example based on mineral oil or vegetable and animal oils, fats, tallow and wax or their mixtures.
- Vegetable and animal oils, fats, tallow and wax are, for example, palm kernel oil, palm oil, Olive oil, rape oil, rapeseed oil, linseed oil, soybean oil, cotton oil, sunflower oil, coconut oil, Corn oil, castor oil, walnut oil and mixtures thereof, fish oils, and their chemically modified, e.g. epoxidized and sulfoxidized, or genetically engineered Molds, such as genetically engineered soybean oil.
- Examples of synthetic lubricants include aliphatic based lubricants or aromatic carboxy esters, polymeric esters, polyalkylene oxides, phosphoric acid esters, Poly- ⁇ -olefins or silicones, the diester of a dibasic acid with a monohydric alcohol, such as Dioctyl sebacate or dinonyl adipate, a trieste of Trimethylolpropane with a monohydric acid or with a mixture of such acids, such as e.g.
- mineral oils e.g. Poly-a-olefins
- the lubricants mentioned or mixtures thereof can also be mixed with an organic one. or inorganic. Thickener must be offset (basic grease). Metalworking fluids and hydraulic fluids can be made from the same substances as described above for the lubricants. Often these are also Emulsions of such substances in water or other liquids.
- R 1 , R 2 and R 3 with the meaning C 3 -C 20 hydrocarbon radical are preferably C 3 -C 20 alkyl, C 5 -C 12 cycloalkyl, C 5 -C 12 cycloalkyl-C 1 -C 4 - alkyl, phenyl, C 7 -C 20 alkylphenyl, C 7 -C 20 alkoxyphenyl, naphthyl and C 7 -C 9 phenylalkyl.
- C 3 -C 20 alkyl comprises branched or unbranched alkyl radicals.
- Examples include n-propyl, isopropyl, n-butyl, isobutyl, t-butyl, n-pentyl, isopentyl, n-hexyl, 2-ethylbutyl, 1-methylpentyl, 1,3-dimethylbutyl, n-heptyl, 3-heptyl , 1-methylhexyl, isoheptyl, n-octyl, 2-ethylhexyl, 1,1,3,3-tetramethylbutyl, 1-methylheptyl, n-nonyl, 1,1,3-trimethylhexyl, n-decyl, n-undecyl, n -Dodecyl, 1-methylundecyl, n-tridecyl, n-tetradecyl, n-pent
- a particularly preferred radical for R 1 , R 2 and R 3 is isopropyl.
- the meanings of R 1 , R 2 and R 3 can be the same or different.
- Thiophosphoric acid esters of the formula I are known, for example US Pat. No. 5,531,911.
- C 5 -C 12 cycloalkyl are, for example, cyclopentyl or hexyl.
- C 5 -C 12 cycloalkyl-C 1 -C 4 alkyl is, for example, cyclopentylmethyl, 2-cyclopentylethyl, cyclohexylmethyl or 2-cyclohexylethyl.
- C 7 -C 20 alkylphenyl is phenyl which is substituted, for example, by one to three of the C 1 -C 4 alkyl radicals described above or one or two C 1 -C 6 alkyl radicals or a C 1 -C 12 alkyl radical.
- C 7 -C 20 alkoxyphenyl is phenyl which is substituted, for example, by one to three C 1 -C 4 alkoxy radicals, in particular methoxy or ethoxy, or one or two C 1 -C 6 alkoxy radicals or a C 1 -C 12 alkoxy radical is, which are analogous to the alkyl radicals mentioned above.
- C 7 -C 9 phenylalkyl is, for example, benzyl, 1-phenyl-1-ethyl or 2-phenyl-1-ethyl.
- the preparation of these thiophosphoric esters is described in EP-A-368 803 .
- Preferred thiophosphoric acid esters of the formula I ' are triarylthiophosphate mixtures of the IRGALUBE 211 type with the ingredients such as n-decylphenyl-nnonylphenylphenylthiophosphate, o-tert.-butylphenyl-o-isopropylphenylphenylthiophosphate or n-hexylphenylphenylthiophosphate.
- component b) from a triphenylthiophosphate (IRGALUBE TPPT) thiophosphoric acid ester IRGALUBE TPPT
- X is preferably sulfur.
- R 1 , R 2 and R 3 with the meaning of unsubstituted C 3 -C 20 hydrocarbon radical have the meanings given above under component b) - thiophosphoric acid esters -, in particular C 3 -C 20 alkyl.
- R 1 and R 2 are unsubstituted C 3 -C 10 hydrocarbon radicals and R 3 represents a substituted C 3 -C 10 hydrocarbon radical.
- a substituted C 3 -C 10 hydrocarbon radical R 3 is preferably by Carboxy or esterified carboxy substituted C 2 -C 4 alkyl, for example of the sub-formula: wherein R x and R y are hydrogen or C 1 -C 4 alkyl, or the corresponding carboxylate salt.
- A is 2-carboxyeth-1-yl or 2-C 1 -C 4 -alkoxycarbonyleth-1-yl, for example methoxycarbonyleth-1-yl or ethoxycarbonylethl -yl, or carboxylate salts thereof.
- the component b) used is a dithiophosphoric acid ester of the IRGALUBE 63 type, which has the structural formula given further, optionally in a mixture with another dithiophosphoric acid ester of the formula II, in which R 1 and R 2 is isopropyl, isobutyl or 2 -Ethylhexyl mean, and R 3 has the meaning of partial formula A, wherein R x and R y are hydrogen, and 2-carboxy-1-ethyl.
- Dithiophosphoric acid esters and phosphoric acid thioesters are known. Their manufacture is described, for example, in US Pat. Nos. 4,333,841; 4,544,492 and 3,784,588 and in British Patent 1,569,730 .
- the phosphorus content of the components is b) and c), based on the composition with components a), b) and c), less than 400 ppm.
- the phosphorus content is of components b) and c) based on the composition with the components a), b) and c) 150 - 390 ppm, especially 160 - 370 ppm.
- the weight ratio of components b) and c) with one another can range from approximately 10:90 to 95: 5 Weight percent vary.
- Suitable oil additives are polyol partial esters, e.g. B. from the group the mono- and diglycerides, monoacetylated or diacetylated monoglycerides, polyglycerol fatty acid esters, Sorbitan fatty acid esters and partial fatty acid esters of polyoxyethylene sorbitan. These oil additives are added in a concentration of approx. 0.01 - 2.0%.
- Suitable mono- and diglycerides are from glycerin by esterification of one or two Hydroxy groups with one or two acid residues of saturated or unsaturated carboxylic acids and an even number of 8-20 C atoms.
- the acid residue of a saturated carboxylic acid with an even number of 8-20 C atoms, which esterified the polyglycerol base, is preferably straight-chain with 12, 14, 16 and 18 carbon atoms, e.g. n-dodecanoyl, n-tetradecanoyl, n-hexadecanoyl or n-octadecanoyl.
- the acid residue of an unsaturated carboxylic acid with an even number of 8-20 C atoms, which esterifies the glycerol base is preferably straight-chain with 12, 14, 16 and 18 C atoms and has 1 double bond, e.g. 9-cis-dodecenoyl, 9-cis-tetradecenoyl, 9-cis-hexadecenoyl or 9-cis-octadecenoyl.
- Particularly suitable mono- and diglycerides are Loxiol® G 10 and G 16 (Henkel), Nutrisoft® 100 (Grünau), Kessco GMO (Akzo) or Edenor®GMO, GDO (Henkel) commercially available.
- a suitable monoacetylated or diacetylated monoglyceride is a monoglyceride that in addition to the acyl residue of a fatty acid, preferably one or two acetyl residues having.
- the acyl radical is preferably derived from one of the unsaturated fatty acids mentioned with more than ten and an even number of carbon atoms.
- a monoglyceride is preferred, which consists of a mixture of monoacetylated or diacetylated monoglycerides using the usual separation methods, e.g. fractional distillation, available is.
- acetylated monoglycerides which are commercially available under the trademark MYVACET (Eastman) are available.
- Acetylated MYVACET series monoglycerides find as lubricants, plasticizers, non-ionic emulsifiers and solubilizers technical use. They are particularly preferred commercially under the name MYVACET 5-07, 7-00, 7-07, 9-08, 9-40 and 9-45 K products available.
- a suitable polyglycerol fatty acid ester consists of an essentially pure or a mixture of different polyglycerol fatty acid esters, in which the polyglycerol base preferably contains up to and including 10 glycerol units with 1-10 acid residues the stated saturated or unsaturated carboxylic acids and an even number of 8-20 C atoms are esterified.
- Suitable polyglycerol fatty acid esters with a uniformly defined structure are, for example diglycerol monocaprate, diglycerol monolaurate, diglycerol diisostearate, diglycerol monoisostearate, diglycerol tetrastearate (polyglycerol 2-tetrastearate), triglycerol monooleate (polyglyceryl 3-monooleate), triglycerol monolaurate, triglycerol monostearate (polyglycerol 3-stearate), triglycerol monoisosterate, hexaglycerol dioleate (polyglycerol 6-dioleates), hexaglycerol distearate (polyglycerol 6-distearate), decaglycerol dioleate (polyglycerol 10-dioleates), decaglycerol tetraoleates (polyglycerol 10-tetra
- CTFA nomenclature is given in brackets. These products are commercially under the word trademark Caprol® (trademark of Karlshamns USA Inc., Columbus Ohio). Exact product names: CAPROL 2G4S, 3GO, 3GS, 6G2O, 6G2S, 10G2O, 10G4O, 10G10O, 10G10S. Other products are under the names DGLC-MC, DGLC-ML, DGLC-DISOS, DGLC-MISOS, TGLC-ML and TGLC-MISOS available from Solvay Alkali GmbH, D-3002 Hanover.
- a suitable sorbitan fatty acid ester preferably consists of an essentially pure one or a mixture of different sorbitan fatty acid esters, wherein the sorbitan base with 1-3 acid residues of one of the above-mentioned saturated or unsaturated, straight-chain Carboxylic acids and an even number of 8-20 carbon atoms is esterified.
- Suitable sorbitan fatty acid esters are especially sorbitan monolaurate, monopalmitate, monostearate, tristearate, monooleate, sesquioleate and trioleate.
- Span® trademark of Atlas, Wilmington USA
- exact product names: ARLACEL 20, 40, 60, 80, 83, 85 and C Crill / (trademark of Croda Chemicals Ltd., Cowick Hall, Snaith Goole GB)
- Crill / trademark of Croda Chemicals Ltd., Cowick Hall, Snaith Goole GB
- Dehymuls® trademark of Henkel, Düsseldorf DE
- exact product names: DEHYMULS SML, SMO, SMS, SSO, Famodan® trademark of Grindsted Products, Grindsted Denmark
- the partial fatty acid ester of polyoxyethylene sorbitan mentioned preferably consists of a substantially pure or a mixture of different esters of sorbitan, wherein the structure of the fatty acid groups and the length of the polyoxyethylene chains vary.
- the Sorbitan is preferably etherified by three polyoxyethylene chains and by a fatty acid group esterified.
- the sorbitan can only be produced by one or two polyoxyethylene chains etherified and correspondingly esterified by two or three fatty acid groups.
- the sorbitan base body is hydrophilic by at least two and at most four Groups substituted, the polyoxyethylene chains under the term hydrophilic group and fatty acid groups can be summarized.
- the polyoxyethylene chain is straight-chain and preferably has 4-10, in particular 4-8, Ethylene oxide units.
- the ester groups on the sorbitan base are of a saturated one or unsaturated, straight-chain carboxylic acid with an even number of 8-20 C atoms derived.
- the ester group derived from this carboxylic acid is preferably straight chain with 12, 14, 16 and 18 carbon atoms, e.g. n-dodecanoyl, n-tetradecanoyl, n-hexadecanoyl or n-octadecanoyl.
- An even numbered unsaturated carboxylic acid Ester group derived from 8-20 C atoms is preferably straight-chain with 12, 14, 16 and 18 carbon atoms, e.g. Oleoyl.
- Suitable partial fatty acid esters of polyoxyethylene sorbitan are under the word sign Tween® commercially available from ICI and the chemical names polyoxyethylene (20 or 4) sorbitan monolaurate (TWEEN 20 and 21), polyoxyethylene (20) sorbitan monopalmitate or monostearate (TWEEN 40 and 60), polyoxyethylene (4 or 20) sorbitan monostearate or tristearate (TWEEN 61 and 65), polyoxyethylene (20 or 5) sorbitan monooleate (TWEEN 80 or 81) or polyoxyethylene (20) sorbitan trioleate (TWEEN 85) are known.
- Suitable amines are, for example, primary or secondary amines with the C 1 -C 20 -alkyl radicals described above, in particular C 2 -C 20 -alkyl, which can be substituted by hydroxyl (alkanolamines) or interrupted by oxygen (etheramines), or polyoxyalkylene diamines or polyoxyalkylene polyamines, furthermore primary or secondary amines with C 5 -C 6 cycloalkyl radicals, for example cyclopentyl or cyclohexyl radicals.
- Suitable alkanolamines are e.g. Ethanolamine, isopropanolamine, 2-amino-2-methyl-1-propanol, 2- (2-aminoethoxy) ethanol, 3-amino-1-propanol, 2-amino-1-butanol, 2-amino-2-methyl-1,3-propanediol or 2-amino-2-ethyl-1,3-propanediol.
- Suitable alkanolamines are e.g. under the word signs Ethomeen and Propomeen (Armak Chemical Div. Of Akzona, Inc., Chicago USA) commercially available, e.g. the products ETHOMEEN C / 15, C / 20, C / 25, O / 12, S / 15, S / 20, T / 12, T / 15 or T / 25 or the corresponding Products from the PROPOMEEN range.
- Ethomeen and Propomeen Armak Chemical Div. Of Akzona, Inc., Chicago USA
- Suitable ether amines are primary ether amines, which come under the word Surfam (Mars Chemical Co., Atlanta USA) are commercially available, e.g. the products SURFAM P14B (decyloxypropylamine) or P16A or P17B (tridecyloxypropylamine).
- Suitable polyoxyalkylene diamines are e.g. B. Ethoxylomeen® alkoxylated diamines (Armak), e.g. the products T / 13 and T / 20.
- Suitable polyoxyalkylene polyamines are e.g. commercially available under the word mark JEFFAMINE (Jefferson Chemical Co.), e.g. the products D-230, D-400, D-1000, D-2000 or T-403.
- Suitable epoxides are, for example, C 4 -C 20 epoxyalkanes, for example epoxybutane, or esters of C 10 -C 20 fatty acids, namely esters of epoxidized fatty acids with monohydric alcohols or polyhydric alcohols, for example glycerides.
- Epoxidized esters of fatty acids with monohydric alcohols for example straight-chain and branched C 1 -C 10 alkyl-C 1 -C 10 alkoxy, aryl or C 5 -C 8 cycloalkyl esters of C 10 -C 20 fatty acids, for example, are preferred Cyclopentyl or cyclohexyl, n-butyl, n-hexyl, benzyl, methoxyethyl, n-octyl, phenyl or tert-butylphenyl epoxy stearate or oleate, and epoxidized soybean oil or linseed oil or epoxidized natural oils and fats, the are known for a high content of unsaturated fatty acids.
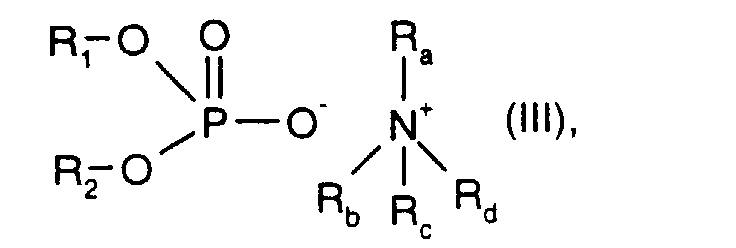
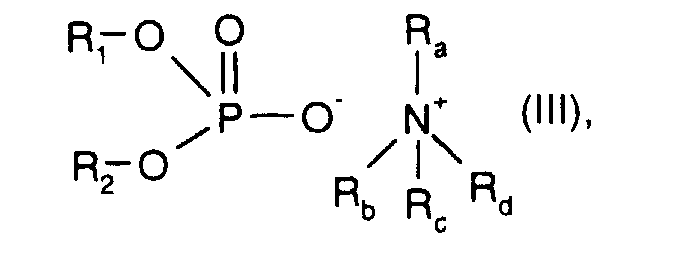
- R 1 and R 2 are C 1 -C 20 hydrocarbon radicals and R a , R b , R c and R d independently of one another are hydrogen or C 1 -C 20 hydrocarbon radicals.
- R 1 and R 2 and R a , R b , R c and R d with the meaning C 1 -C 20 hydrocarbon radical are preferably C 1 -C 7 alkyl, for example methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, t- Butyl, n-pentyl, isopentyl, n-hexyl, 2-ethylbutyl, 1-methylpentyl, 1,3-dimethylbutyl or n-heptyl.
- the ammonium phosphate ester is used in a low concentration of approximately 200 to 500 ppm added. This additive gives the hydraulic oil a particularly good load-bearing capacity (FZG error load levels ⁇ 10).
- the total phosphorus content is components b), c) and e), based on the total composition, less than 400 ppm.
- the lubricant compositions mentioned, e.g. Greases, gear, metalworking and hydraulic fluids can additionally contain other additives that are added to improve their basic properties even further. These include: antioxidants, Metal deactivators, rust inhibitors, viscosity index improvers, pour point depressants, Dispersants, detergents, high-pressure additives and antiwear additives. Such additives are added in the amounts customary for them in the range of each about 0.01 to 10.0% by weight. The following are examples of other additives:
- Alkylated monophenols e.g. 2,6-di-tert-butyl-4-methylphenol, 2-butyl-4,6-dimethylphenol, 2,6-di-tert-butyl-4-ethylphenol, 2,6-di-tert- butyl-4-n-butylphenol, 2,6-di-tert-butyl-4-isobutylphenol, 2,6-di-cyclopentyl-4-methylphenol, 2- (a-methylcyclohexyl) -4,6-dimethylphenol, 2, 6-di-octadecyl-4-methylphenol, 2,4,6-tri-cyclohexylphenol, 2,6-di-tert-butyl-4-methoxymethylphenol, linear or branched nonylphenols such as 2,6-di-nonyl, for example -4-methylphenol, 2,4-dimethyl-6- (1'-methyl-undec-1'-yl) phenol, 2,
- Alkylthiomethylphenols for example 2,4-dioctylthiomethyl-6-tert-butylphenol, 2,4-dioctylthiomethyl-6-methylphenol, 2,4-dioctylthiomethyl-6-ethylphenol, 2,6-didodecylthiomethyl-4 -nonylphenol.
- Hydroquinones and alkali hydroquinones for example 2,6-di-tert-butyl-4-methoxyphenol, 2,5-di-tert-butyl-hydroquinone, 2,5-di-tert-amyl-hydroquinone, 2,6-diphenyl- 4-octadecyloxyphenol, 2,6-di-tert-butyl-hydroquinone, 2,5-di-tert-butyl-4-hydroxyanisole, 3,5-di-tert-butyl-4-hydroxyanisole, 3,5-di- tert-butyl-4-hydroxyphenyl stearate, bis (3,5-di-tert-butyl-4-hydroxyphenyl) adipate.
- Tocopherols e.g. ⁇ -, ⁇ -, ⁇ - or ⁇ - and mixtures thereof (vitamin E).
- Hydroxylated thiodiphenyl ethers for example 2,2'-thio-bis (6-tert-butyl-4-methylphenol), 2,2'-thio-bis (4-octylphenol), 4,4'-thio-bis (6-tert -butyl-3-methylphenol), 4,4'-thio-bis (6-tert-butyl-2-methylphenol), 4,4'-thio-bis (3,6-di-sec.-amylphenol), 4,4'-bis (2,6-dimethyl-4-hydroxyphenyl) disulfide.
- Alkylidene bisphenols for example 2,2'-methylene-bis (6-tert-butyl-4-methylphenol), 2,2'-methylene-bis (6-tert-butyl-4-ethylphenol), 2,2'- Methylene bis [4-methyl-6- (a-methylcyclohexyl) phenol], 2,2'-methylene bis (4-methyl-6-cyclohexylphenol), 2,2'-methylene bis (6-nonyl- 4-methyphenol), 2,2'-methylene-bis (4,6-di-tert-butylphenol), 2,2'-ethylidene-bis (4,6-di-tert-butylphenol), 2,2'- Ethylidene-bis (6-tert-butyl-4-isobutylphenol), 2,2'-methylene-bis [6- (a-methylbenzyl) -4-nonylphenol], 2,2'-methylene-bis [6- (a , a-dimethylbenzyl)
- O-, N- and S-benzyl compounds for example 3,5,3 ', 5'-tetra-tert-butyl-4,4'-dihydroxydibenzyl ether, octadecyl-4-hydroxy-3,5-dimethylbenzyl-mercaptoacetate, tridecyl- 4-hydroxy-3,5-di-tert-butylbenzyl mercaptoacetate, tris (3,5-di-tert-butyl-4-hydroxybenzyl) amine, bis (4-tert-butyl-3-hydroxy-2,6) -dimethylbenzyl) dithioterephthalate, bis (3,5-di-tert-butyl-4-hydroxybenzyl) sulfide, isooctyl-3,5-di-tert-butyl-4-hydroxybenzyl-mercaptoacetate.
- hydroxybenzylated malonates e.g. dioctadecyl-2,2-bis (3,5-di-tert-butyl-2-hydroxybenzyl) malonate, di-octadecyl-2- (3-tert-butyl-4-hydroxy-5-methylbenzyl) -malonate, di-dodecyl-mercaptoethyl-2,2-bis (3,5-di-tert-butyl-4-hydroxybenzyl) -malonate, di- [4- (1,1,3,3-tetramethyl-butyl) -phenyl] -2,2-bis (3,5-di-tert-butyl-4-hydroxybenzyl) malonate.
- Hydroxybenzyl aromatics for example 1,3,5-tris (3,5-di-tert-butyl-4-hydroxybenzyl) -2,4,6-trimethylbenzene, 1,4-bis (3,5-di-tert- butyl-4-hydroxybenzyl) -2,3,5,6-tetramethylbenzene, 2,4,6-tris (3,5-di-tert-butyl-4-hydroxybenzyl) phenol.
- Triazine compounds e.g. 2,4-bis-octylmercapto-6- (3,5-di-tert-butyl-4-hydroxyanilino) -1,3,5-triazine, 2-octylmercapto-4,6-bis (3,5 -di-tert-butyl-4-hydroxyanilino) -1,3,5-triazine, 2-octylmercapto-4,6-bis (3,5-di-tert-butyl-4-hydroxyphenoxy) -1,3,5 -triazine, 2,4,6-tris (3,5-di-tert-butyl-4-hydroxyphenoxy) -1,2,3-triazine, 1,3,5-tris (3,5-di-tert- butyl-4-hydroxybenzyl) isocyanurate, 1,3,5-tris (4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl) isocyanurate, 2,
- esters of beta (3,5-di-tert-butyl-4-hydroxyphenyl) propionic acid with mono- or polyhydric alcohols such as with methanol, ethanol, n-octanol, i-octanol, octadecanol, 1,6-hexanediol , 1,9-nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethylene glycol, triethylene glycol, pentaerythritol, tris (hydroxyethyl) isocyanurate, N, N'-bis (hydroxyethyl) oxalic acid diamide, 3-thiaundecanol, 3 -Thiapentadecanol, trimethylhexanediol, trimethylolpropane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabi
- esters of beta (5-tert-butyl-4-hydroxy-3-methylphenyl) propionic acid with mono- or polyhydric alcohols such as with methanol, ethanol, n-octanol, i-octanol, octadecanol, 1,6-hexanediol , 1,9-nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethylene glycol, triethylene glycol, pentaerythritol, tris (hydroxyethyl) isocyanurate, N, N'-bis (hydroxyethyl) oxalic acid diamide, 3-thiaundecanol, 3- Thiapentadecanol, trimethylhexanediol, trimethylolpropane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo [2.
- esters of beta- (3,5-dicyclohexyl-4-hydroxyphenyl) propionic acid with mono- or polyhydric alcohols such as with methanol, ethanol, octanol, octadecanol, 1,6-hexanediol, 1,9-nonanediol, ethylene glycol, 1 , 2-propanediol, neopentylglycol, thiodiethylene glycol, diethylene glycol, triethylene glycol, pentaerythritol, tris (hydroxyethyl) isocyanurate, N, N'-bis (hydroxyethyl) oxalic acid diamide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol, trimethylolpropane, trimethyl 1-phospha-2,6,7-trioxabicyclo [2.2.2] octane.
- esters of 3,5-di-tert-butyl-4-hydroxyphenylacetic acid with mono- or polyhydric alcohols such as with methanol, ethanol, octanol, octadecanol, 1,6-hexanediol, 1,9-nonanediol, ethylene glycol, 1,2 Propanediol, neopentyl glycol, thiodiethylene glycol, diethylene glycol, triethylene glycol, pentaerythritol, tris (hydroxyethyl) isocyanurate, N, N'-bis (hydroxyethyl) oxalic acid diamide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol, trimethylolpropanediol, trimethylolpropanediol, trimethylolpropanediol phospha-2,6,7-trio
- Amine antioxidants such as N, N'-di-isopropyl-p-phenylenediamine, N, N'-di-sec-butyl-p-phenylenediamine, N, N'-bis (1,4-dimethyl-pentyl) -p -phenylenediamine, N, N'-bis (1-ethyl-3-methylpentyl) -p-phenylenediamine, N, N'-bis (1-methyl-heptyl) -p-phenylenediamine, N, N'-dicyclohexyl- p-phenylenediamine, N, N'-diphenyl-p-phenylenediamine, N, N'-di- (naphthyl-2) -p-phenylenediamine, N-isopropyl-N'-phenyl-p-phenylenediamine, N- (1, 3-dimethyl-butyl) -N'
- metal deactivators e.g. for copper:
- viscosity index improvers polyacrylates, polymethacrylates, vinyl pyrrolidone / methacrylate copolymers, polyvinyl pyrrolidones, polybutenes, olefin copolymers, styrene / acrylate copolymers, polyethers.
- pour point depressants poly (meth) acrylates, ethylene-vinyl acetate copolymer, alkylpolystyrenes, fumarate copolymers, alkylated naphthalene derivatives.
- dispersants / surfactants polybutenylsuccinic acid amides or imides, polybutenylphosphonic acid derivatives, basic magnesium, calcium and barium sulfonates and phenolates.
- Sulfur and halogen containing compounds such as chlorinated paraffins, sulfurized olefins or vegetable oils (soybean oil, rapeseed oil), alkyl or aryl di- or trisulfides, benzotriazoles or their derivatives such as bis (2-ethylhexyl) aminomethyl tolutriazole, dithiocarbamates such as methylene bis-dibutyldithiocarbamate, Derivatives of 2-mercaptobenzothiazole such as 1- [N, N-bis (2-ethylhexyl) aminomethyl] -2-mercapto-1H-1,3-benzothiazole, Derivatives of 2,5-dimercapto-1,3,4-thiadiazole such as 2,5-bis (tert.nonyldithio) -1,3,4-thiadiazole.
- benzotriazoles or their derivatives such as bis (2-ethylhexyl) aminomethyl to
- friction reducers eg oil from lard, oleic acid, tallow, rapeseed oil, sulfurized fats, amines. Further examples are mentioned in EP 565487.
- Emulsifiers petroleum sulfonates, amines such as polyoxyethylated fatty amines, nonionic surface-active substances; Buffer: alkanolamines; Biocides: triazines, thiazolinones, tris-nitromethane, morpholine, sodium pyridene ethiol ; Processing speed improvers: calcium and barium sulfonates;
- the components mentioned can be added to the lubricants in a manner known per se become. It is also possible to use a concentrate or a so-called additive package produce that according to the consumption at the use concentrations for the corresponding Lubricant can be diluted.
- the components are put together in the concentrate so that it is at room temperature is liquid without the addition of base oil a) or a solvent.
- the invention also relates to a method for improving the performance properties of lubricants, characterized by adding components b), c) and d), preferably in a concentration related to the phosphorus content of these components on the total composition, is less than 400 ppm.
- the invention also relates to the use of compounds of the components b) c) and d), preferably in the concentration mentioned, as additives in motor oils, Turbine oils, gear oils, hydraulic fluids, metal working fluids or greases.
- the various blends were made using an ISO VG 46 mineral oil (kinematic viscosity at 40 ° C: 42 - 50 CSt) and a basic additive mixture, which typically used for hydraulic fluids.
- This basic additive mixture is free of metal salts and is used with 0.29 - 0.47% (wt.%).
- she is a combination of an aromatic amine antioxidant (e.g. Irganox® L 57), a hindered Phenol antioxidant (e.g. Irganox® L 135) and smaller proportions of other common ones Additives such as pour point depressants (e.g. Plexol® 154), anti-foaming agents (e.g. Mobilad® C402), demulsifying additives (e.g.
- Synperonic® PEL81 corrosion inhibitors (e.g. Irgacor® NPA) and metal deactivators (e.g. Irgamet® 39).
- corrosion inhibitors e.g. Irgacor® NPA
- metal deactivators e.g. Irgamet® 39.
- the additives and mixtures used and the results of the tests performed are shown in the table. Wording 9, 10, 11, 12, 13, 14 correspond to the claimed composition of the present Invention.
- the other formulations are for comparison, especially with the state of the art (state of T. I and II).
Abstract
Description
Die Erfindung betrifft verbesserte Zusammensetzungen mit Thiophosphorsäureestern und Dithiophosphorsäureestern oder Phosphorsäurethioestern sowie die Verwendung dieser Schmierstoffzusammensetzungen zur Verbesserung der Gebrauchseigenschaften von Schmierstoffen, wie Fetten, Metallbearbeitungs-, Getriebe- oder Hydraulikflüssigkeiten.The invention relates to improved compositions with thiophosphoric acid esters and Dithiophosphoric acid esters or phosphoric acid thioesters and the use of these Lubricant compositions to improve the performance properties of Lubricants such as greases, metalworking, gear or hydraulic fluids.
Den genannten Schmierstoffen werden Additive zugesetzt, welche anspruchsvolle Aufgaben, wie hohes Lasttragevermögen, Verschleiss- und Korrosionsschutz sowie Antioxidationswirkung, erfüllen müssen. Hierfür eignen sich Zinkdialkyldithiophosphate, die man aber aus Gründen des Umweltschutzes durch metallfreie Verbindungen zu ersetzen anstrebt. Insbesondere in der Landwirtschaft oder generell bei mobilen Hydraulikanlagen, bei denen durch Undichtigkeiten die Gefahr einer Boden- oder Gewässerkontamination mit Zinkverbindungen besteht, wird die Verwendung von metallfreien Hydraulikflüssigkeiten gefordert. Es besteht daher ein Bedürfnis an metallfreien und aschefreien Additiven. Geeignete Hydraulikflüssigkeiten müssen auch die Spezifikationen der führenden Hydraulikmaschinenherstellter, z.B. Denison HFO (Denison Hydraulics) oder Vickers M-2980-S (Vickers) erfüllen und mit Wasser kompatibel sein. Weiterhin sollten sie gemäss den Spezifikationen der DIN 51524 und Denison HFO eine Fehlerlaststufe (FLS) im FZG-Test von mindestens 10 erreichen.Additives are added to the lubricants mentioned, which perform demanding tasks, such as high load-bearing capacity, wear and corrosion protection as well as antioxidant effects, have to meet. Zinc dialkyldithiophosphates are suitable for this, but one strives to replace them with metal-free connections for reasons of environmental protection. Especially in agriculture or in general in mobile hydraulic systems where due to leaks the risk of soil or water contamination with zinc compounds the use of metal-free hydraulic fluids is required. It there is therefore a need for metal-free and ash-free additives. Suitable hydraulic fluids the specifications of the leading hydraulic machine manufacturers, e.g. Meet Denison HFO (Denison Hydraulics) or Vickers M-2980-S (Vickers) and be compatible with water. Furthermore, they should be in accordance with the specifications of DIN 51524 and Denison HFO achieve an error load level (FLS) of at least 10 in the FZG test.
Als Öladditive für Flüssigkeiten sind Dithiophosphosphorsäureester des Typs: bekannt, welche unter dem Handelsnamen Irgalube®63 (Warenzeichen der Ciba Spezialitätenchemie) kommerziell erhältlich sind.As oil additives for liquids are dithiophosphoric acid esters of the type: known, which are commercially available under the trade name Irgalube®63 (trademark of Ciba Specialty Chemicals).
In der U.S. Patentschrift Nr. 5 531 911 sind zinkfreie Hydraulikflüssigkeiten beschrieben, die phosphor- und schwefelhaltige Additivkomponenten enthalten. Eine Komponente ist ein Thiophosphorsäureester vom Typ Triphenylthiophosphat (IRGALUBE TPPT). Diese wird mit Dithiophosphorsäureestern vom Typ IRGALUBE 63 und fakultativen Öladditivkomponenten, z.B. Ammoniumsulfonaten, kombiniert. US Pat. No. 5,531,911 describes zinc-free hydraulic fluids which contain phosphorus and sulfur-containing additive components. One component is a triphenylthiophosphate (IRGALUBE TPPT) thiophosphoric acid ester. This is combined with IRGALUBE 63 dithiophosphoric acid esters and optional oil additive components, eg ammonium sulfonates.
Solche Formulierungen sind wegen ihrer mangelnden Kompatibilität mit Wasser nachteilig. Die Kontamination eines Hydrauliköls mit Wasser ist insbesondere bei mobilen Hydraulikanlagen häufig. Wegen der Verwendung von phosphor- und schwefelhaltigen Additiven erfolgt ein hydrolytischer Abbau unter Bildung von korrodierenden Zersetzungsprodukten, welche die in den Hydraulikanlagen verwendeten Metalle, z.B. Stahl und Kupferlegierungen, angreifen und Schäden an den Hydraulikpumpen verursachen können. Es können ausserdem auch Agglomerationen dieser Zersetzungsprodukte die Filter von Bypass-Filtrationsanlagen blockieren. Da die Lebensdauer von Hydraulikanlagen sich durch Feinstfilterung bedeutend verlängern lässt, sind die Filtermaschengrössen moderner Bypass-Filtrationsanlagen von früher 30 µm auf derzeit 6 µm herabgesetzt worden. Daher sind nur solche Hydrauliköle zu verwenden, die bei Kontamination mit Wasser nur geringste Mengen von hydrolytischen Zersetzungsprodukten bilden.Such formulations are disadvantageous because of their lack of compatibility with water. The contamination of a hydraulic oil with water is particularly important in mobile hydraulic systems often. Because of the use of phosphorus and sulfur-containing additives hydrolytic degradation takes place with formation of corrosive decomposition products, which the metals used in the hydraulic systems, e.g. Steel and copper alloys, attack and cause damage to the hydraulic pumps. It can also agglomerations of these decomposition products, the filters of bypass filtration systems To block. Since the service life of hydraulic systems is characterized by fine filtering The filter mesh sizes of modern bypass filtration systems are significantly longer reduced from 30 µm to 6 µm today. Therefore, only such are Hydraulic oils to be used which, when contaminated with water, contain only the smallest quantities of Form hydrolytic decomposition products.
Der vorliegenden Erfindung liegt die Aufgabe zugrunde, Zusammensetzungen mit verbesserter Kompatibilität mit Wasser herzustellen, welche eine wesentlich geringere Neigung zur Bildung von unerwünschten Hydrolyseprodukten aufweisen.The present invention has for its object compositions with improved Establish compatibility with water, which has a much lower tendency to Have formation of unwanted hydrolysis products.
Es wurde überraschenderweise gefunden, dass Zusammensetzungen mit Thiophosphorsäureestern kombiniert mit Dithiophosphorsäureestern oder Phosphorsäurethioestern durch Zusatz einer weiteren Additivkomponente aus der Gruppe der Polyolpartialester, Amine und Epoxide bei Kontamination mit Wasser eine wesentlich geringere Neigung zur Bildung von korrodierenden Hydrolyseprodukten und sehr gute Filtrationseigenschaften aufweisen. Durch Zusatz von weiteren Öladditiven, z. B. Ammoniumphosphatestern, lässt sich das Lasttragevermögen steigern und FZG Fehlerlaststufen ≥ 10 erreichen.It has surprisingly been found that compositions with thiophosphoric acid esters combined with dithiophosphoric acid esters or phosphoric acid thioesters Addition of another additive component from the group of polyol partial esters, amines and Epoxies have a significantly lower tendency to form when contaminated with water have corrosive hydrolysis products and very good filtration properties. By adding other oil additives, e.g. B. ammonium phosphate esters, can Increase load-bearing capacity and reach FZG error load levels ≥ 10.
Gegenstand der Erfindung sind Zusammensetzungen enthaltend:
Eine bevorzugte Ausführungsform betrifft Zusammensetzungen enthaltend:
Eine besonders bevorzugte Ausführungsform betrifft Zusammensetzungen, worin der Phosphorgehalt der Thiophosphorsäureester-Komponente b), kombiniert mit der Dithiophosphorsäureester- oder Phosphorsäurethioester-Komponente c), bezogen auf die Zusammensetzung mit den Komponenten a), b) und c), weniger als 400 ppm beträgt.A particularly preferred embodiment relates to compositions in which the phosphorus content the thiophosphoric acid component b), combined with the dithiophosphoric acid ester or phosphoric acid thioester component c), based on the composition with components a), b) and c), is less than 400 ppm.
Eine ganz besonders bevorzugte Ausführungsform betrifft Zusammensetzungen, worin der Phosphorgehalt der Thiophosphorsäureester-Komponente b), kombiniert mit der Dithiophosphorsäureester- oder Phosphorsäurethioester-Komponente c) und der Ammoniumphosphatesterkomponente e), bezogen auf die Gesamtzusammensetzung, weniger als 400 ppm beträgt.A very particularly preferred embodiment relates to compositions in which the Phosphorus content of the thiophosphoric acid component b), combined with the dithiophosphoric acid ester or phosphoric acid thioester component c) and the Ammonium phosphate ester component e), based on the total composition, less than 400 ppm.
Die Zusammensetzungen sind als multifunktionale Verschleissschutz-Additive - mit zusätzlicher antioxydativer Wirkung - für Schmierstoffe, wie Fette, Metallbearbeitungs-, Getriebe- oder Hydraulikflüssigkeiten besonders geeignet. Sie sind weitgehend metall- und aschefrei und erfüllen die genannten Spezifikationen. Überraschenderweise ergeben Mischungen der Komponenten b) und c) im Grundöl a) bei Phosphorkonzentrationen kleiner als 400 ppm Phosphor sehr gute Antiverschleisseigenschaften, d.h. sehr gute VKA (Test im Vierkugelapparat) und SRV (Schwing-Reib-Verschleiss)-Werte. Diese Mischungen haben bei Zusatz der Additivkomponente aus der Gruppe der Polyolpartialester, Amine und Epoxide (Komponente d)) bei Kontamination mit Wasser sehr gute Filtrationseigenschaften. Durch Zusatz von weiteren Öladditiven (Komponenten e) lassen sich FZG Fehlerlaststufen ≥ 10 erreichen. Solche Mischungen erfüllen die Hydraulikmaschinenspezifikationen führender Hersteller, insbesondere Denison HFO.The compositions are multifunctional wear protection additives - with additional ones antioxidant effect - for lubricants such as greases, metalworking, gear or hydraulic fluids are particularly suitable. They are largely free of metal and ash and meet the specified specifications. Surprisingly, mixtures of Components b) and c) in base oil a) at phosphorus concentrations less than 400 ppm Phosphorus very good antiwear properties, i.e. very good VKA (test in a four-ball device) and SRV (vibration-friction-wear) values. These mixtures have the addition of Additive component from the group of polyol partial esters, amines and epoxides (component d)) very good filtration properties when contaminated with water. By adding FZG error load levels ≥ 10 can be achieved with other oil additives (components e). Such mixtures meet the hydraulic machine specifications of leading manufacturers, especially Denison HFO.
Die im Rahmen der Beschreibung der vorliegenden Erfindung verwendeten Begriffe und Definitionen haben vorzugsweise die folgenden Bedeutungen:The terms and definitions used in the description of the present invention preferably have the following meanings:
Ein Grundöl mit schmierender Viskosität ist für die Herstellung von Fetten, Metallbearbeitungs-, Getriebe- und Hydraulikflüssigkeiten verwendbar.A base oil with a lubricating viscosity is used for the production of greases, metalworking, Gear and hydraulic fluids can be used.
Geeignete Fette, Metallbearbeitungs-, Getriebe- und Hydraulikflüssigkeiten basieren beispielsweise auf mineralischen oder synthetischen Ölen oder Mischungen davon. Die Schmierstoffe sind dem Fachmann geläufig und in der einschlägigen Fachliteratur, wie beispielsweise in Chemistry and Technology of Lubricants; Mortier, R.M. and Orszulik, S.T (Editors); 1992 Blackie and Son Ltd. for GB, VCH-Publishers N. Y. for U.S., ISBN 0-216-92921-0, siehe Seiten 208 ff und 269 ff; in Kirk-Othmer Encyclopedia of Chemical Technology, Fourth Edition 1969, J. Wiley & Sons, New York, Vol. 13, Seite 533 ff. (Hydraulic Fluids); Performance Testing of Hydraulic Fluids; R. Tourret and E.P. Wright, Hyden & Son Ltd. GB, on behalf of The Institute of Petroleum London, ISBN 0 85501 317 6; Ullmann's Encyclopedia of Ind. Chem., Fifth Completely Revised Edition, Verlag Chemie, DE-Weinheim, VCH-Publishers for U.S., Vol. A 15, Seite 423 ff(lubricants), Vol. A 13, Seite 165 ff (hydraulic fluids) beschrieben.Suitable greases, metalworking, gear and hydraulic fluids are based, for example, on mineral or synthetic oils or mixtures thereof. The lubricants are familiar to the person skilled in the art and in the relevant specialist literature, for example in Chemistry and Technology of Lubricants; Mortier, RM and Orszulik, ST (Editors); 1992 Blackie and Son Ltd. for GB, VCH-Publishers NY for US, ISBN 0-216-92921-0, see pages 208 ff and 269 ff; in Kirk-Othmer Encyclopedia of Chemical Technology, Fourth Edition 1969, J. Wiley & Sons, New York, Vol. 13, page 533 ff. (Hydraulic Fluids); Performance Testing of Hydraulic Fluids; R. Tourret and EP Wright, Hyden & Son Ltd. GB, on behalf of The Institute of Petroleum London, ISBN 0 85501 317 6; Ullmann's Encyclopedia of Ind. Chem., Fifth Completely Revised Edition, Verlag Chemie, DE-Weinheim, VCH-Publishers for US, Vol. A 15, page 423 ff (lubricants), Vol. A 13, page 165 ff (hydraulic fluids) described.
Die Schmierstoffe sind insbesondere Öle und Fette, beispielsweise basierend auf Mineralöl oder pflanzlichen und tierischen Ölen, Fetten, Talg und Wachs oder deren Gemische. Pflanzliche und tierische Öle, Fette, Talg und Wachs sind beispielsweise Palmkernöl, Palmöl, Olivenöl, Rüböl, Rapsöl, Leinöl, Sojabohnenöl, Baumwollöl, Sonnenblumenöl, Kokosnussöl, Maisöl, Rizinusöl, Baumnussöl und Mischungen davon, Fischöle, sowie deren chemisch modifizierte, z.B. epoxidierte und sulfoxidierte, oder gentechnisch hergestellte Formen, beispielsweise gentechnisch hergestelltes Sojabohnenöl.The lubricants are in particular oils and fats, for example based on mineral oil or vegetable and animal oils, fats, tallow and wax or their mixtures. Vegetable and animal oils, fats, tallow and wax are, for example, palm kernel oil, palm oil, Olive oil, rape oil, rapeseed oil, linseed oil, soybean oil, cotton oil, sunflower oil, coconut oil, Corn oil, castor oil, walnut oil and mixtures thereof, fish oils, and their chemically modified, e.g. epoxidized and sulfoxidized, or genetically engineered Molds, such as genetically engineered soybean oil.
Beispiele von synthetischen Schmierstoffen umfassen Schmierstoffe auf der Basis von aliphatischen oder aromatischen Carboxylestern, polymeren Ester, Polyalkylenoxide, Phosphorsäureester, Poly-α-olefine oder Silicone, des Diesters einer zweiwertigen Säure mit einem einwertigen Alkohol, wie z.B. Dioctylsebacat oder Dinonyladipat, eines Triesters von Trimethylolpropan mit einer einwertigen Säure oder mit einem Gemisch solcher Säuren, wie z.B. Trimethylolpropantripelargonat, Trimethylolpropan-tricaprylat oder Gemische davon, eines Tetraesters von Pentaerythrit mit einer einwertigen Säure oder mit einem Gemisch solcher Säuren, wie z.B. Pentaerythrit-tetracaprylat, oder eines komplexen Esters von einwertigen und zweiwertigen Säuren mit mehrwertigen Alkoholen, z.B. ein komplexer Ester von Trimethylolpropan mit Capryl- und Sebacinsäure oder von einem Gemisch davon. Besonders geeignet sind neben Mineralölen z.B. Poly-a-Olefine, Schmierstoffe auf Esterbasis, Phosphate, Glycole, Polyglycole und Polyalkylenglycole, sowie deren Mischungen mit Wasser.Examples of synthetic lubricants include aliphatic based lubricants or aromatic carboxy esters, polymeric esters, polyalkylene oxides, phosphoric acid esters, Poly-α-olefins or silicones, the diester of a dibasic acid with a monohydric alcohol, such as Dioctyl sebacate or dinonyl adipate, a trieste of Trimethylolpropane with a monohydric acid or with a mixture of such acids, such as e.g. Trimethylolpropane tripelargonate, trimethylolpropane tricaprylate or mixtures thereof, a tetraester of pentaerythritol with a monovalent acid or with a mixture such acids, e.g. Pentaerythritol tetracaprylate, or a complex ester of monovalent and dihydric acids with polyhydric alcohols, e.g. a complex ester trimethylolpropane with caprylic and sebacic acid or a mixture thereof. Especially In addition to mineral oils, e.g. Poly-a-olefins, lubricants based on esters, Phosphates, glycols, polyglycols and polyalkylene glycols, as well as their mixtures with Water.
Die genannten Schmierstoffe oder Mischungen davon können auch mit einem organischen. oder anorganischen. Verdicker versetzt sein (Grundfett). Metallbearbeitungsflüssigkeiten und Hydraulikflüssigkeiten können auf Basis der gleichen Substanzen hergestellt werden wie vorstehend für die Schmiermittel beschrieben. Häufig handelt es sich dabei auch um Emulsionen solcher Substanzen in Wasser oder anderen Flüssigkeiten.The lubricants mentioned or mixtures thereof can also be mixed with an organic one. or inorganic. Thickener must be offset (basic grease). Metalworking fluids and hydraulic fluids can be made from the same substances as described above for the lubricants. Often these are also Emulsions of such substances in water or other liquids.
R1, R2 und R3 mit der Bedeutung C3-C20-Kohlenwasserstoffrest sind vorzugsweise C3-C20-Alkyl, C5-C12-Cycloalkyl, C5-C12-Cycloalkyl-C1-C4-alkyl, Phenyl, C7-C20-Alkylphenyl, C7-C20-Alkoxyphenyl, Naphthyl und C7-C9-Phenylalkyl.R 1 , R 2 and R 3 with the meaning C 3 -C 20 hydrocarbon radical are preferably C 3 -C 20 alkyl, C 5 -C 12 cycloalkyl, C 5 -C 12 cycloalkyl-C 1 -C 4 - alkyl, phenyl, C 7 -C 20 alkylphenyl, C 7 -C 20 alkoxyphenyl, naphthyl and C 7 -C 9 phenylalkyl.
C3-C20-Alkyl umfasst verzweigte oder unverzweigte Alkylreste. Beispiele hierfür sind n-Propyl, Isopropyl, n-Butyl, Isobutyl, t-Butyl, n-Pentyl, Isopentyl, n-Hexyl, 2-Ethylbutyl, 1-Methylpentyl, 1,3-Dimethylbutyl, n-Heptyl, 3-Heptyl, 1-Methylhexyl, Isoheptyl, n-Octyl, 2-Ethylhexyl, 1,1,3,3-Tetramethylbutyl, 1-Methylheptyl, n-Nonyl, 1,1,3-Trimethylhexyl, n-Decyl, n-Undecyl, n-Dodecyl, 1-Methylundecyl, n-Tridecyl, n-Tetradecyl, n-Pentadecyl, n-Hexadecyl, n-Heptadecyl oder n-Octadecyl. Ein besonders bevorzugter Rest für R1, R2 und R3 ist Isopropyl. Die Bedeutungen von R1, R2 und R3 können gleich oder verschieden sein. Thiophosphorsäureester der Formel I sind bekannt, z.B. U.S. Patentschrift 5,531,911. C 3 -C 20 alkyl comprises branched or unbranched alkyl radicals. Examples include n-propyl, isopropyl, n-butyl, isobutyl, t-butyl, n-pentyl, isopentyl, n-hexyl, 2-ethylbutyl, 1-methylpentyl, 1,3-dimethylbutyl, n-heptyl, 3-heptyl , 1-methylhexyl, isoheptyl, n-octyl, 2-ethylhexyl, 1,1,3,3-tetramethylbutyl, 1-methylheptyl, n-nonyl, 1,1,3-trimethylhexyl, n-decyl, n-undecyl, n -Dodecyl, 1-methylundecyl, n-tridecyl, n-tetradecyl, n-pentadecyl, n-hexadecyl, n-heptadecyl or n-octadecyl. A particularly preferred radical for R 1 , R 2 and R 3 is isopropyl. The meanings of R 1 , R 2 and R 3 can be the same or different. Thiophosphoric acid esters of the formula I are known, for example US Pat. No. 5,531,911.
C5-C12-Cycloalkyl sind z.B. Cyclopentyl oder -hexyl. C5-C12-Cycloalkyl-C1-C4-alkyl ist z.B. Cyclopentylmethyl, 2-Cyclopentylethyl, Cyclohexylmethyl oder 2-Cyclohexylethyl.C 5 -C 12 cycloalkyl are, for example, cyclopentyl or hexyl. C 5 -C 12 cycloalkyl-C 1 -C 4 alkyl is, for example, cyclopentylmethyl, 2-cyclopentylethyl, cyclohexylmethyl or 2-cyclohexylethyl.
C7-C20-Alkylphenyl ist Phenyl, das z.B. durch ein bis drei der weiter vorn beschriebenen C1-C4-Alkylreste oder ein bis zwei C1-C6-Alkylreste oder einen C1-C12-Alkylreste substituiert ist.C 7 -C 20 alkylphenyl is phenyl which is substituted, for example, by one to three of the C 1 -C 4 alkyl radicals described above or one or two C 1 -C 6 alkyl radicals or a C 1 -C 12 alkyl radical.
C7-C20-Alkoxyphenyl ist Phenyl, das z.B. durch ein bis drei C1-C4-Alkoxyreste, insbesondere Methoxy oder Ethoxy, oder ein bis zwei C1-C6-Alkoxyreste oder einen C1-C12-Alkoxyreste substituiert ist, die den weiter vorn genannten Alkylresten analog sind.C 7 -C 20 alkoxyphenyl is phenyl which is substituted, for example, by one to three C 1 -C 4 alkoxy radicals, in particular methoxy or ethoxy, or one or two C 1 -C 6 alkoxy radicals or a C 1 -C 12 alkoxy radical is, which are analogous to the alkyl radicals mentioned above.
C7-C9-Phenylalkyl ist z.B. Benzyl, 1-Phenyl-1-ethyl oder 2-Phenyl-1-ethyl. C 7 -C 9 phenylalkyl is, for example, benzyl, 1-phenyl-1-ethyl or 2-phenyl-1-ethyl.
In einer bevorzugten Ausführungsform der Erfindung besteht die Komponente b) aus einer Mischung von Thiophosphorsäureestern der Formel: worin x 0 bis 2,7, y 3 - (x + z), z 0 bis 3 - (x + y) und x + y + z = 3 ist, und Ar Phenyl, C7-C18-Alkylphenyl, C7-C18-Alkoxyphenyl, Naphthyl und C7-C9-Phenylalkyl mit den weiter vorn angegebenen Bedeutungen darstellen. Die Herstellung dieser Thiophosphorsäureester ist in EP-A-368 803 beschrieben. Als Thiophosphorsäureester der Formel I' sind Triarylthiophosphatgemische vom Typ IRGALUBE 211 mit den Inhaltsstoffen wie n-Decylphenyl-nnonylphenyl-phenylthiophosphat, o-tert.-Butylphenyl-o-isopropylphenyl- phenylthiophosphat oder n-Hexylphenyl-phenylthiophosphat-Gemische bevorzugt.In a preferred embodiment of the invention, component b) consists of a mixture of thiophosphoric esters of the formula: wherein x is 0 to 2.7, y 3 - (x + z), z 0 to 3 - (x + y) and x + y + z = 3, and Ar is phenyl, C 7 -C 18 -alkylphenyl, C Represent 7 -C 18 alkoxyphenyl, naphthyl and C 7 -C 9 phenylalkyl with the meanings given above. The preparation of these thiophosphoric esters is described in EP-A-368 803 . Preferred thiophosphoric acid esters of the formula I 'are triarylthiophosphate mixtures of the IRGALUBE 211 type with the ingredients such as n-decylphenyl-nnonylphenylphenylthiophosphate, o-tert.-butylphenyl-o-isopropylphenylphenylthiophosphate or n-hexylphenylphenylphenylthiophosphate.
In einer weiteren bevorzugten Ausführungsform der Erfindung besteht die Komponente b) aus einem Thiophosphorsäureester vom Typ Triphenylthiophosphat (IRGALUBE TPPT).In a further preferred embodiment of the invention, component b) from a triphenylthiophosphate (IRGALUBE TPPT) thiophosphoric acid ester.
In einer Verbindung (II) ist X bevorzugt Schwefel. R1, R2 und R3 mit der Bedeutung unsubstituierter C3-C20-Kohlenwasserstoffrest haben die weiter vorn unter der Komponente b) - Thiophosphorsäureester -, angegebenen Bedeutungen, insbesondere C3-C20-Alkyl.In a compound (II), X is preferably sulfur. R 1 , R 2 and R 3 with the meaning of unsubstituted C 3 -C 20 hydrocarbon radical have the meanings given above under component b) - thiophosphoric acid esters -, in particular C 3 -C 20 alkyl.
In einer bevorzugten Verbindung (II) sind R1 und R2 unsubstituierte C3-C10-Kohlenwasserstoffreste und R3 stellt einen substituierten C3-C10-Kohlenwasserstoffrest dar. Ein substituierter C3-C10-Kohlenwasserstoffrest R3 ist vorzugsweise durch Carboxy oder verestertes Carboxy substituiertes C2-C4-Alkyl, z.B. der Teilformel: worin Rx und Ry Wasserstoff oder C1-C4-Alkyl bedeuten, oder das entsprechende Carboxylatsalz. Bevorzugte Bedeutungen von A sind 2-Carboxyeth-1-yl oder 2-C1-C4-Alkoxycarbonyleth-1-yl, z.B. Methoxycarbonyleth-1-yl oder Ethoxycarbonylethl -yl, oder Carboxylatsalze davon.In a preferred compound (II) R 1 and R 2 are unsubstituted C 3 -C 10 hydrocarbon radicals and R 3 represents a substituted C 3 -C 10 hydrocarbon radical. A substituted C 3 -C 10 hydrocarbon radical R 3 is preferably by Carboxy or esterified carboxy substituted C 2 -C 4 alkyl, for example of the sub-formula: wherein R x and R y are hydrogen or C 1 -C 4 alkyl, or the corresponding carboxylate salt. Preferred meanings of A are 2-carboxyeth-1-yl or 2-C 1 -C 4 -alkoxycarbonyleth-1-yl, for example methoxycarbonyleth-1-yl or ethoxycarbonylethl -yl, or carboxylate salts thereof.
In einer besonders bevorzugten Ausführungsform der Erfindung verwendet man als Komponente b) einen Dithiophosphorsäureester vom Typ IRGALUBE 63, welcher die weiter vom angegebene Strukturformel hat, gegebenenfalls im Gemisch mit einem weiteren Dithiophosphorsäureester der Formel II, worin R1 und R2 Isopropyl, Isobutyl oder 2-Ethylhexyl bedeuten, und R3 die Bedeutung der Teilformel A hat, worin Rx und Ry Wasserstoff bedeuten, und 2-Carboxy-1-ethyl bedeutet.In a particularly preferred embodiment of the invention, the component b) used is a dithiophosphoric acid ester of the IRGALUBE 63 type, which has the structural formula given further, optionally in a mixture with another dithiophosphoric acid ester of the formula II, in which R 1 and R 2 is isopropyl, isobutyl or 2 -Ethylhexyl mean, and R 3 has the meaning of partial formula A, wherein R x and R y are hydrogen, and 2-carboxy-1-ethyl.
Dithiophosphorsäureester und Phosphorsäurethioester sind bekannt. Ihre Herstellung ist z.B. in den U.S. Patentschriften 4,333,841; 4,544,492 und 3,784,588 sowie in der Britischen Patentschrift 1 569 730 beschrieben.Dithiophosphoric acid esters and phosphoric acid thioesters are known. Their manufacture is described, for example, in US Pat. Nos. 4,333,841; 4,544,492 and 3,784,588 and in British Patent 1,569,730 .
In einer bevorzugten Ausführungsform der Erfindung beträgt der Phosphorgehalt der Komponenten b) und c), bezogen auf die Zusammensetzung mit den Komponenten a), b) und c), weniger als 400 ppm. In einer besonders bevorzugten Ausführungsform beträgt der Phosphorgehalt der Komponenten b) und c) bezogen auf die Zusammensetzung mit den Komponenten a), b) und c) 150 - 390 ppm, insbesondere 160 - 370 ppm. Das Gewichtsverhältnis der Komponenten b) und c) untereinander kann in Bereichen von ca. 10 : 90 bis 95 : 5 Gewichtsprozent variieren.In a preferred embodiment of the invention, the phosphorus content of the components is b) and c), based on the composition with components a), b) and c), less than 400 ppm. In a particularly preferred embodiment, the phosphorus content is of components b) and c) based on the composition with the components a), b) and c) 150 - 390 ppm, especially 160 - 370 ppm. The weight ratio of components b) and c) with one another can range from approximately 10:90 to 95: 5 Weight percent vary.
Durch Zusatz einer weiteren Additivkomponente aus der Gruppe der Polyolpartialester, Amine und Epoxide erhalten die Zusammensetzungen bei Kontamination eine bessere Kompatibilität mit Wasser. Geeignete Öladditive sind Polyolpartialester, z. B. aus der Gruppe der Mono- und Diglyceride, monoacetylierten oder diacetylierten Monoglyceride, Polyglycerinfettsäureester, Sorbitanfettsäureester und Partialfettsäureester des Polyoxyethylensorbitans. Diese Öladditive setzt man in einer Konzentration von ca. 0,01 - 2,0 % hinzu.By adding another additive component from the group of the polyol partial esters, Amines and epoxies get better compositions when contaminated Compatibility with water. Suitable oil additives are polyol partial esters, e.g. B. from the group the mono- and diglycerides, monoacetylated or diacetylated monoglycerides, polyglycerol fatty acid esters, Sorbitan fatty acid esters and partial fatty acid esters of polyoxyethylene sorbitan. These oil additives are added in a concentration of approx. 0.01 - 2.0%.
Geeignete Mono- und Diglyceride sind vom Glycerin durch Veresterung von ein oder zwei Hydroxygruppen mit ein oder zwei Säureresten von gesättigten oder ungesättigten Carbonsäuren und gerader Anzahl von 8-20 C-Atomen abgeleitet.Suitable mono- and diglycerides are from glycerin by esterification of one or two Hydroxy groups with one or two acid residues of saturated or unsaturated carboxylic acids and an even number of 8-20 C atoms.
Der Säurerest einer gesättigten Carbonsäure mit gerader Anzahl von 8-20 C-Atomen, welcher den Polyglyceringrundkörper verestert, ist vorzugsweise geradkettig mit 12, 14, 16 und 18 C-Atomen, z.B. n-Dodecanoyl, n-Tetradecanoyl, n-Hexadecanoyl oder n-Octadecanoyl.The acid residue of a saturated carboxylic acid with an even number of 8-20 C atoms, which esterified the polyglycerol base, is preferably straight-chain with 12, 14, 16 and 18 carbon atoms, e.g. n-dodecanoyl, n-tetradecanoyl, n-hexadecanoyl or n-octadecanoyl.
Der Säurerest einer ungesättigten Carbonsäure mit gerader Anzahl von 8-20 C-Atomen, welcher den Glyceringrundkörper verestert, ist vorzugsweise geradkettig mit 12, 14, 16 und 18 C-Atomen und weist 1 Doppelbindung auf, z.B. 9-cis-Dodecenoyl, 9-cis-Tetradecenoyl, 9-cis-Hexadecenoyl oder 9-cis-Octadecenoyl.The acid residue of an unsaturated carboxylic acid with an even number of 8-20 C atoms, which esterifies the glycerol base is preferably straight-chain with 12, 14, 16 and 18 C atoms and has 1 double bond, e.g. 9-cis-dodecenoyl, 9-cis-tetradecenoyl, 9-cis-hexadecenoyl or 9-cis-octadecenoyl.
Für die genannten Säurereste sind ausserdem folgende Bezeichnungen gebräuchlich: 9-cis-Dodecenoyl (Lauroleoyl), 9-cis-Tetradecenoyl (Myristoleoyl), 9-cis-Hexadecenoyl (Palmitoleoyl), 6-cis-Octadecenoyl (Petroseloyl), 6-trans-Octadecenoyl (Petroselaidoyl), 9-cis-Octadecenoyl (Oleoyl), 9-trans-Octadecenoyl (Elaidoyl), 11-cis-Octadecenoyl (Vaccenoyl), 9-cis-lcosenoyl (Gadoleoyl), n-Dodecanoyl (Lauroyl), n-Tetradecanoyl (Myristoyl), n-Hexadecanoyl (Palmitoyl), n-Octadecanoyl (Stearoyl), n-lcosanoyl (Arachidoyl).The following terms are also used for the acid residues mentioned: 9-cis-dodecenoyl (Lauroleoyl), 9-cis-tetradecenoyl (Myristoleoyl), 9-cis-hexadecenoyl (Palmitoleoyl), 6-cis-octadecenoyl (petroseloyl), 6-trans-octadecenoyl (petroselaidoyl), 9-cis-octadecenoyl (Oleoyl), 9-trans octadecenoyl (elaidoyl), 11-cis octadecenoyl (Vaccenoyl), 9-cis-lcosenoyl (Gadoleoyl), n-dodecanoyl (Lauroyl), n-tetradecanoyl (Myristoyl), n-hexadecanoyl (palmitoyl), n-octadecanoyl (stearoyl), n-lcosanoyl (arachidoyl).
Besonders geeignete Mono- und Diglyceride sind unter den Bezeichnungen Loxiol® G 10 und G 16 (Henkel), Nutrisoft® 100 (Grünau), Kessco GMO (Akzo) oder Edenor®GMO, GDO (Henkel) kommerziell erhältlich.Particularly suitable mono- and diglycerides are Loxiol® G 10 and G 16 (Henkel), Nutrisoft® 100 (Grünau), Kessco GMO (Akzo) or Edenor®GMO, GDO (Henkel) commercially available.
Ein geeignetes monoacetyliertes oder diacetyliertes Monoglycerid ist ein Monoglycerid, das zusätzlich zum Acylrest einer Fettsäure noch vorzugsweise einen oder auch zwei Acetylreste aufweist. Der Acylrest leitet sich vorzugsweise von einer der genannten ungesättigten Fettsäuren mit mehr als zehn und einer geraden Anzahl an C-Atomen ab. Bevorzugt ist ein Monoglycerid, welches aus einem Gemisch von monoacetylierten oder diacetylierten Monoglyceriden unter Anwendung der üblichen Trennmethoden, z.B. fraktionierte Destillation, erhältlich ist.A suitable monoacetylated or diacetylated monoglyceride is a monoglyceride that in addition to the acyl residue of a fatty acid, preferably one or two acetyl residues having. The acyl radical is preferably derived from one of the unsaturated fatty acids mentioned with more than ten and an even number of carbon atoms. A monoglyceride is preferred, which consists of a mixture of monoacetylated or diacetylated monoglycerides using the usual separation methods, e.g. fractional distillation, available is.
Besonders bevorzugt sind acetylierte Monoglyceride, welche kommerziell unter dem Warenzeichen MYVACET (Eastman) erhältlich sind. Acetylierte Monoglyceride der MYVACET-Reihe finden als Schmiermittel, Weichmacher, nicht-ionische Emulgatoren und Lösungsvermittler technische Verwendung. Besonders bevorzugt sind die kommerziell unter der Bezeichnung MYVACET 5-07, 7-00, 7-07, 9-08, 9-40 und 9-45 K erhältlichen Produkte.Particularly preferred are acetylated monoglycerides, which are commercially available under the trademark MYVACET (Eastman) are available. Acetylated MYVACET series monoglycerides find as lubricants, plasticizers, non-ionic emulsifiers and solubilizers technical use. They are particularly preferred commercially under the name MYVACET 5-07, 7-00, 7-07, 9-08, 9-40 and 9-45 K products available.
Ein geeigneter Polyglycerinfettsäureester besteht aus einem im wesentlichen reinen oder einem Gemisch verschiedener Polyglycerinfettsäureester, worin der Polyglyceringrundkörper vorzugsweise bis einschliesslich 10 Glycerineinheiten enthält, welche mit 1-10 Säureresten der genannten gesättigten oder ungesättigten Carbonsäuren und gerader Anzahl von 8-20 C-Atomen verestert sind.A suitable polyglycerol fatty acid ester consists of an essentially pure or a mixture of different polyglycerol fatty acid esters, in which the polyglycerol base preferably contains up to and including 10 glycerol units with 1-10 acid residues the stated saturated or unsaturated carboxylic acids and an even number of 8-20 C atoms are esterified.
Geeignete Polyglycerinfettsäureester mit einheitlich definierter Struktur sind beispielsweise (in engl.Bezeichnung) diglycerol monocaprate, diglycerol monolaurate, diglycerol diisostearate, diglycerol monoisostearate, diglycerol tetrastearate (polyglycerol 2-tetrastearate), triglycerol monooleate (polyglyceryl 3-monooleate), triglycerol monolaurate, triglycerol monostearate (polyglycerol 3-stearate), triglycerol monoisosterate, hexaglycerol dioleate (polyglycerol 6-dioleate), hexaglycerol distearate (polyglycerol 6-distearate), decaglycerol dioleate (polyglycerol 10-dioleate), decaglycerol tetraoleate (polyglycerol 10-tetraoleate), decaglycerol decaoleate (polyglycerol 10-decaoleate), decaglycerol decastearate (polyglycerol 10-decastearate). In Klammern ist die CTFA-Nomenklatur angegeben. Diese Produkte sind kommerziell unter den Wortzeichen Caprol® (Warenzeichen der Fa. Karlshamns USA Inc., Columbus Ohio) erhältlich. Exakte Produktbezeichnungen: CAPROL 2G4S, 3GO, 3GS, 6G2O, 6G2S, 10G2O, 10G4O, 10G10O, 10G10S. Weitere Produkte sind unter den Bezeichungen DGLC-MC, DGLC-ML, DGLC-DISOS, DGLC-MISOS, TGLC-ML und TGLC-MISOS bei der Fa. Solvay Alkali GmbH, D-3002 Hannover erhältlich.Suitable polyglycerol fatty acid esters with a uniformly defined structure are, for example diglycerol monocaprate, diglycerol monolaurate, diglycerol diisostearate, diglycerol monoisostearate, diglycerol tetrastearate (polyglycerol 2-tetrastearate), triglycerol monooleate (polyglyceryl 3-monooleate), triglycerol monolaurate, triglycerol monostearate (polyglycerol 3-stearate), triglycerol monoisosterate, hexaglycerol dioleate (polyglycerol 6-dioleates), hexaglycerol distearate (polyglycerol 6-distearate), decaglycerol dioleate (polyglycerol 10-dioleates), decaglycerol tetraoleates (polyglycerol 10-tetraoleates), decaglycerol decaoleate (polyglycerol 10-decaoleate), decaglycerol decastearate (polyglycerol 10-decastearate). The CTFA nomenclature is given in brackets. These products are commercially under the word trademark Caprol® (trademark of Karlshamns USA Inc., Columbus Ohio). Exact product names: CAPROL 2G4S, 3GO, 3GS, 6G2O, 6G2S, 10G2O, 10G4O, 10G10O, 10G10S. Other products are under the names DGLC-MC, DGLC-ML, DGLC-DISOS, DGLC-MISOS, TGLC-ML and TGLC-MISOS available from Solvay Alkali GmbH, D-3002 Hanover.
Gemische verschiedener Polyglycerinfettsäureester sind unter Bezeichnungen wie decaglycerol mono-, di-oleate, polyglycerol ester of mixed fatty acids, Polyglycerolester der Fettsäuren, polyglycerol caprate, cocoate, laurate, lanolinate, isostearate oder rizinolate definiert und kommerziell unter den Wortzeichen Triodan® und Homodan® (Warenzeichen der Fa. Grindsted Products, Grindsted Dänemark), exakte Produktbezeichnungen: TRIODAN 20, 55, R90 und HOMODAN MO, Radiamuls® (Warenzeichen der Fa. Petrofina (FINA), Bruxelles Belgien), exakte Produktbezeichnung RADIAMULS Poly 2253, der Bezeichnung CAPROL PGE860 oder ET, oder den Wortzeichen Plurol® (Warenzeichen Gattefossé Etablissements, Saint-Priest, Frankreich), exakte Produktbezeichnung PLUROL Stearique WL1009 oder PLUROL Oleique WL1173, erhältlich. Weitere Produkte sind unter den Bezeichungen PGLC-C1010S, PGLC-C0810, PGLC-C1010/S, PGLC-LT2010, PGLC-LAN0510/S, PGLC-CT2010/90, PGLC-ISOSTUE, PGLC-RUE, PGLC-ISOS0410 bei der Fa. Solvay Alkali GmbH, D-3002 Hannover erhältlich.Mixtures of various polyglycerol fatty acid esters are available under names such as decaglycerol mono-, di-oleates, polyglycerol esters of mixed fatty acids, polyglycerol esters of fatty acids, polyglycerol caprate, cocoate, laurate, lanolinate, isostearate or rizinolate defined and commercially under the word marks Triodan® and Homodan® (trademark of Grindsted Products, Grindsted Denmark), exact product names: TRIODAN 20, 55, R90 and HOMODAN MO, Radiamuls® (trademark of Petrofina (FINA), Bruxelles Belgium), exact product name RADIAMULS Poly 2253, the name CAPROL PGE860 or ET, or the word mark Plurol® (trademark Gattefossé Etablissements, Saint-Priest, France), exact product name PLUROL Stearique WL1009 or PLUROL Oleique WL1173 available. Other products are under the names PGLC-C1010S, PGLC-C0810, PGLC-C1010 / S, PGLC-LT2010, PGLC-LAN0510 / S, PGLC-CT2010 / 90, PGLC-ISOSTUE, PGLC-RUE, PGLC-ISOS0410 from Solvay Alkali GmbH, D-3002 Hannover available.
Ein geeigneter Sorbitanfettsäureester besteht vorzugsweise aus einem im wesentlichen reinen oder einem Gemisch verschiedener Sorbitanfettsäureester, worin der Sorbitangrundkörper mit 1-3 Säureresten einer der genannten gesättigten oder ungesättigten, geradkettigen Carbonsäuren und gerader Anzahl von 8-20 C-Atomen verestert ist.A suitable sorbitan fatty acid ester preferably consists of an essentially pure one or a mixture of different sorbitan fatty acid esters, wherein the sorbitan base with 1-3 acid residues of one of the above-mentioned saturated or unsaturated, straight-chain Carboxylic acids and an even number of 8-20 carbon atoms is esterified.
Geeignete Sorbitanfettsäureester sind insbesondere Sorbitan-Monolaurat, -Monopalmitat,
-Monostearat, -Tristearat, -Monooleat, -Sesquioleat und -Trioleat. Diese Produkte sind
kommerziell unter den Wortzeichen Span® (Warenzeichen der Fa.Atlas, Wilmington USA),
exakte Produktbezeichnungen: SPAN 20, 40, 60, 65, 80 und 85, Arlacel® (Warenzeichen der
Fa.Atlas), exakte Produktbezeichnungen: ARLACEL 20, 40, 60, 80, 83, 85 und C, Crill/
(Warenzeichen der Fa. Croda Chemicals Ltd., Cowick Hall, Snaith Goole GB), exakte Produktbezeichnungen:
CRILL 1, 3 und 4, Dehymuls® (Warenzeichen der Fa. Henkel, Düsseldorf
DE), exakte Produktbezeichnungen: DEHYMULS SML, SMO, SMS, SSO, Famodan®
(Warenzeichen der Fa. Grindsted Products, Grindsted Dänemark), exakte Produktbezeichnungen:
FAMODAN MS und TS, Capmul® (Warenzeichen der Fa.Karlshamns USA Inc., Columbus
Ohio), exakte Produktbezeichnungen: CAPMULS und O, Radiasurf® (Warenzeichen
der Fa. Petrofina (FINA), Bruxelles Belgien), exakte Produktbezeichnungen:
RADIASURF7125, 7135, 7145 und 7155, erhältlich.Suitable sorbitan fatty acid esters are especially sorbitan monolaurate, monopalmitate, monostearate, tristearate, monooleate, sesquioleate and trioleate. These products are commercially available under the word marks Span® (trademark of Atlas, Wilmington USA), exact product names: SPAN 20, 40, 60, 65, 80 and 85, Arlacel® (trademark of Atlas), exact product names: ARLACEL 20, 40, 60, 80, 83, 85 and C, Crill / (trademark of Croda Chemicals Ltd., Cowick Hall, Snaith Goole GB), exact product names: CRILL 1, 3 and 4, Dehymuls® (trademark of Henkel, Düsseldorf DE), exact product names: DEHYMULS SML, SMO, SMS, SSO, Famodan® (trademark of Grindsted Products, Grindsted Denmark), exact product names: FAMODAN MS and TS, Capmul® (trademark of Karlshamns USA Inc., Columbus Ohio), exact product names: CAPMULS and O, Radiasurf® (trademark of Petrofina (FINA), Bruxelles Belgium), exact product names:
RADIASURF7125, 7135, 7145 and 7155 available.
Der genannte Partialfettsäureester des Polyoxyethylensorbitans besteht vorzugsweise aus einem im wesentlichen reinen oder dem Gemisch verschiedener Ester des Sorbitans, worin die Struktur der Fettsäuregruppen und die Länge der Polyoxyethylenketten variieren. Das Sorbitan ist vorzugsweise durch drei Polyoxyethylenketten veräthert und durch eine Fettsäuregruppe verestert. Das Sorbitan kann aber auch nur durch ein oder zwei Polyoxyethylenketten veräthert und entsprechend durch zwei oder drei Fettsäuregruppen verestert sein. Insgesamt ist der Sorbitangrundkörper durch mindestens zwei und maximal vier hydrophile Gruppen substitutiert, wobei unter dem Begriff hydrophile Gruppe die Polyoxyethylenketten und Fettsäuregruppen zusammengefasst werden.The partial fatty acid ester of polyoxyethylene sorbitan mentioned preferably consists of a substantially pure or a mixture of different esters of sorbitan, wherein the structure of the fatty acid groups and the length of the polyoxyethylene chains vary. The Sorbitan is preferably etherified by three polyoxyethylene chains and by a fatty acid group esterified. However, the sorbitan can only be produced by one or two polyoxyethylene chains etherified and correspondingly esterified by two or three fatty acid groups. Overall, the sorbitan base body is hydrophilic by at least two and at most four Groups substituted, the polyoxyethylene chains under the term hydrophilic group and fatty acid groups can be summarized.
Die Polyoxyethylenkette ist geradkettig und weist vorzugsweise 4-10, insbesondere 4-8, Ethylenoxideinheiten auf. Die Estergruppen am Sorbitangrundkörper sind von einer gesättigten oder ungesättigten, geradkettigen Carbonsäure mit gerader Anzahl von 8-20 C-Atomen abgeleitet. Die von dieser Carbonsäure abgeleitete Estergruppe ist vorzugsweise geradkettig mit 12, 14, 16 und 18 C-Atomen, z.B. n-Dodecanoyl, n-Tetradecanoyl, n-Hexadecanoyl oder n-Octadecanoyl. Die von einer ungesättigten Carbonsäure mit gerader Anzahl von 8-20 C-Atomen abgeleitete Estergruppe ist vorzugsweise geradkettig mit 12, 14, 16 und 18 C-Atomen, z.B. Oleoyl.The polyoxyethylene chain is straight-chain and preferably has 4-10, in particular 4-8, Ethylene oxide units. The ester groups on the sorbitan base are of a saturated one or unsaturated, straight-chain carboxylic acid with an even number of 8-20 C atoms derived. The ester group derived from this carboxylic acid is preferably straight chain with 12, 14, 16 and 18 carbon atoms, e.g. n-dodecanoyl, n-tetradecanoyl, n-hexadecanoyl or n-octadecanoyl. An even numbered unsaturated carboxylic acid Ester group derived from 8-20 C atoms is preferably straight-chain with 12, 14, 16 and 18 carbon atoms, e.g. Oleoyl.
Geeignete Partialfettsäureester des Polyoxyethylensorbitans sind unter dem Wortzeichen Tween® der Fa.lCI kommerziell erhältlich und den chemischen Bezeichnungen Polyoxyethylen-(20 oder 4)-sorbitanmonolaurat (TWEEN 20 und 21), Polyoxyethylen-(20)-sorbitanmonopalmitat oder -monostearat (TWEEN 40 und 60), Polyoxyethylen-(4 oder 20)-sorbitanmonostearat oder -tristearat (TWEEN 61 und 65), Polyoxyethylen-(20 oder 5)-sorbitanmonooleat (TWEEN 80 oder 81) oder Polyoxyethylen-(20)-sorbitantrioleat (TWEEN 85) bekannt.Suitable partial fatty acid esters of polyoxyethylene sorbitan are under the word sign Tween® commercially available from ICI and the chemical names polyoxyethylene (20 or 4) sorbitan monolaurate (TWEEN 20 and 21), polyoxyethylene (20) sorbitan monopalmitate or monostearate (TWEEN 40 and 60), polyoxyethylene (4 or 20) sorbitan monostearate or tristearate (TWEEN 61 and 65), polyoxyethylene (20 or 5) sorbitan monooleate (TWEEN 80 or 81) or polyoxyethylene (20) sorbitan trioleate (TWEEN 85) are known.
Geeignete Amine sind z.B. primäre oder sekundäre Amine mit den weiter vorn beschriebenen C1-C20-Alkylresten, insbesondere C2-C20-Alkyl, die z.B. durch Hydroxy substituiert (Alkanolamine) oder durch Sauerstoff (Etheramine) unterbrochen sein können, oder Polyoxyalkylendiamine oder Polyoxyalkylenpolyamine, ferner primäre oder sekundäre Amine mit C5-C6-Cycloalkylresten, z.B. Cyclopentyl- oder Cyclohexylresten.Suitable amines are, for example, primary or secondary amines with the C 1 -C 20 -alkyl radicals described above, in particular C 2 -C 20 -alkyl, which can be substituted by hydroxyl (alkanolamines) or interrupted by oxygen (etheramines), or polyoxyalkylene diamines or polyoxyalkylene polyamines, furthermore primary or secondary amines with C 5 -C 6 cycloalkyl radicals, for example cyclopentyl or cyclohexyl radicals.
Geeignete Alkanolamine sind z.B. Ethanolamin, Isopropanolamin, 2-Amino-2-methyl-1-propanol, 2-(2-Aminoethoxy)-ethanol, 3-Amino-1-propanol, 2-Amino-1-butanol, 2-Amino-2-methyl-1,3-propandiol oder 2-Amino-2-ethyl-1,3-propandiol.Suitable alkanolamines are e.g. Ethanolamine, isopropanolamine, 2-amino-2-methyl-1-propanol, 2- (2-aminoethoxy) ethanol, 3-amino-1-propanol, 2-amino-1-butanol, 2-amino-2-methyl-1,3-propanediol or 2-amino-2-ethyl-1,3-propanediol.
Geeignete Alkanolamine sind z.B. unter den Wortzeichen Ethomeen und Propomeen (Armak Chemical Div. of Akzona, Inc., Chicago USA) kommerziell erhältlich, z.B. die Produkte ETHOMEEN C/15, C/20, C/25, O/12, S/15, S/20, T/12, T/15 oder T/25 oder die entsprechenden Produkte der PROPOMEEN-Reihe. Suitable alkanolamines are e.g. under the word signs Ethomeen and Propomeen (Armak Chemical Div. Of Akzona, Inc., Chicago USA) commercially available, e.g. the products ETHOMEEN C / 15, C / 20, C / 25, O / 12, S / 15, S / 20, T / 12, T / 15 or T / 25 or the corresponding Products from the PROPOMEEN range.
Geeignete Etheramine sind primäre Etheramine, welche unter den Wortzeichen Surfam (Mars Chemical Co., Atlanta USA) kommerziell erhältlich sind, z.B. die Produkte SURFAM P14B (Decyloxypropylamin) oder P16A oder P17B (Tridecyloxypropylamin).Suitable ether amines are primary ether amines, which come under the word Surfam (Mars Chemical Co., Atlanta USA) are commercially available, e.g. the products SURFAM P14B (decyloxypropylamine) or P16A or P17B (tridecyloxypropylamine).
Geeignete Polyoxyalkylendiamine sind z. B. alkoxylierte Diamine vom Typ Ethoduomeen® (Armak), z.B. die Produkte T/13 und T/20. Geeignete Polyoxyalkylenpolyamine sind z.B. unter dem Wortzeichen JEFFAMINE (Jefferson Chemical Co.) kommerziell erhältlich, z.B. die Produkte D-230, D-400, D-1000, D-2000 oder T-403.Suitable polyoxyalkylene diamines are e.g. B. Ethoxylomeen® alkoxylated diamines (Armak), e.g. the products T / 13 and T / 20. Suitable polyoxyalkylene polyamines are e.g. commercially available under the word mark JEFFAMINE (Jefferson Chemical Co.), e.g. the products D-230, D-400, D-1000, D-2000 or T-403.
Geeignete Epoxide sind z.B. C4-C20-Epoxyalkane, z.B. Epoxybutan, oder Ester von C10-C20-Fettsäuren und zwar Ester epoxydierter Fettsäuren mit einwertigen Alkoholen oder mehrwertigen Alkoholen, z.B. Glyceride. Bevorzugt sind epoxydierte Ester von Fettsäuren mit einwertigen Alkoholen, z.B. geradkettige und verzweigte C1-C10-Alkyl- C1-C10-Alkoxy, Aryl- oder C5-C8-Cycloalkylester von C10-C20-Fettsäuren, z.B. Cyclopentyl- oder Cyclohexyl-, n-Butyl-, n-Hexyl-, Benzyl-, Methoxyethyl-, n-Octyl-, Phenyl- oder tert.-Butylphenylepoxystearat oder -oleat sowie epoxydiertes Sojaöl oder Leinöl oder epoxydierte natürliche Öle und Fette, die für einen hohen Gehalt an ungesättigten Fettsäuren bekannt sind.Suitable epoxides are, for example, C 4 -C 20 epoxyalkanes, for example epoxybutane, or esters of C 10 -C 20 fatty acids, namely esters of epoxidized fatty acids with monohydric alcohols or polyhydric alcohols, for example glycerides. Epoxidized esters of fatty acids with monohydric alcohols, for example straight-chain and branched C 1 -C 10 alkyl-C 1 -C 10 alkoxy, aryl or C 5 -C 8 cycloalkyl esters of C 10 -C 20 fatty acids, for example, are preferred Cyclopentyl or cyclohexyl, n-butyl, n-hexyl, benzyl, methoxyethyl, n-octyl, phenyl or tert-butylphenyl epoxy stearate or oleate, and epoxidized soybean oil or linseed oil or epoxidized natural oils and fats, the are known for a high content of unsaturated fatty acids.
In einem Ammoniumphosphatester der Formel III stellen R1 und R2 C1-C20-Kohlenwasserstoffreste und Ra, Rb, Rc und Rd unabhängig voneinander Wasserstoff oder C1-C20-Kohlenwasserstoffreste dar. R1 und R2 sowie Ra, Rb, Rc und Rd mit der Bedeutung C1-C20-Kohlenwasserstoffrest sind vorzugsweise C1-C7-Alkyl, z.B. Methyl, Ethyl, n-Propyl, Isopropyl, n-Butyl, Isobutyl, t-Butyl, n-Pentyl, Isopentyl, n-Hexyl, 2-Ethylbutyl, 1-Methylpentyl, 1,3-Dimethylbutyl oder n-Heptyl.In an ammonium phosphate ester of the formula III, R 1 and R 2 are C 1 -C 20 hydrocarbon radicals and R a , R b , R c and R d independently of one another are hydrogen or C 1 -C 20 hydrocarbon radicals. R 1 and R 2 and R a , R b , R c and R d with the meaning C 1 -C 20 hydrocarbon radical are preferably C 1 -C 7 alkyl, for example methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, t- Butyl, n-pentyl, isopentyl, n-hexyl, 2-ethylbutyl, 1-methylpentyl, 1,3-dimethylbutyl or n-heptyl.
Der Ammoniumphosphatester wird in einer niedrigen Konzentration von ca. 200 bis 500 ppm zugesetzt. Durch diesen Zusatz erhält das Hydrauliköl ein besonders gutes Lasttragevermögen (FZG Fehlerlaststufen ≥ 10). In den bevorzugten Ausführungsform beträgt der Gesamtphosphorgehalt der Komponenten b), c) und e), bezogen auf die Gesamtzusammensetzung, weniger als 400 ppm.The ammonium phosphate ester is used in a low concentration of approximately 200 to 500 ppm added. This additive gives the hydraulic oil a particularly good load-bearing capacity (FZG error load levels ≥ 10). In the preferred embodiment, the total phosphorus content is components b), c) and e), based on the total composition, less than 400 ppm.
Die genannten Schmierstoffzusammensetzungen, z.B. Fette, Getriebe-, Metallbearbeitungs- und Hydraulikflüssigkeiten, können zusätzlich weitere Additive enthalten, die zugegeben werden, um ihre Grundeigenschaften noch weiter zu verbessern. Dazu gehören: Antioxidantien, Metalldesaktivatoren, Rostinhibitoren, Viskositätsindex-Verbesserer, Stockpunkterniedriger, Dispergiermittel, Detergentien, Hochdruck-Zusätze und Antiverschleissadditive. Solche Additive gibt man in den jeweils dafür üblichen Mengen im Bereich von je etwa 0,01 bis 10,0 Gew.% zu. Es folgen Beispiele für weitere Zusatzstoffe:The lubricant compositions mentioned, e.g. Greases, gear, metalworking and hydraulic fluids can additionally contain other additives that are added to improve their basic properties even further. These include: antioxidants, Metal deactivators, rust inhibitors, viscosity index improvers, pour point depressants, Dispersants, detergents, high-pressure additives and antiwear additives. Such additives are added in the amounts customary for them in the range of each about 0.01 to 10.0% by weight. The following are examples of other additives:
1.1. Alkylierte Monophenole, z.B. 2,6-Di-tert-butyl-4-methylphenol, 2-Butyl-4,6-dimethylphenol, 2,6-Di-tert-butyl-4-ethylphenol, 2,6-Di-tert-butyl-4-n-butylphenol, 2,6-Di-tert-butyl-4-isobutylphenol, 2,6-Di-cyclopentyl-4-methylphenol, 2-(a-Methylcyclohexyl)-4,6-dimethylphenol, 2,6-Di-octadecyl-4-methylphenol, 2,4,6-Tri-cyclohexylphenol, 2,6-Di-tert-butyl-4-methoxymethylphenol, lineare oder in der Seitenkette verzweigte Nonylphenole wie z.B. 2,6-Di-nonyl-4-methylphenol, 2,4-Dimethyl-6-(1'-methyl-undec-1'-yl)-phenol, 2,4-Dimethyl-6-(1'-methyl-heptadec-1'-yl)-phenol, 2,4-Dimethyl-6-(1'-methyl-tridec-1'-yl)-phenol und Mischungen davon. 1.1. Alkylated monophenols, e.g. 2,6-di-tert-butyl-4-methylphenol, 2-butyl-4,6-dimethylphenol, 2,6-di-tert-butyl-4-ethylphenol, 2,6-di-tert- butyl-4-n-butylphenol, 2,6-di-tert-butyl-4-isobutylphenol, 2,6-di-cyclopentyl-4-methylphenol, 2- (a-methylcyclohexyl) -4,6-dimethylphenol, 2, 6-di-octadecyl-4-methylphenol, 2,4,6-tri-cyclohexylphenol, 2,6-di-tert-butyl-4-methoxymethylphenol, linear or branched nonylphenols such as 2,6-di-nonyl, for example -4-methylphenol, 2,4-dimethyl-6- (1'-methyl-undec-1'-yl) phenol, 2,4-dimethyl-6- (1'-methyl-heptadec-1'-yl) -phenol, 2,4-dimethyl-6- (1'-methyl-tridec-1'-yl) phenol and mixtures thereof.
1.2. Alkylthiomethylphenole, z.B. 2,4-Di-octylthiomethyl-6-tert-butylphenol, 2,4-Di-octylthiomethyl-6-methylphenol, 2,4-Di-octylthiomethyl-6-ethylphenol, 2,6-Di-dodecylthiomethyl-4-nonylphenol. 1.2. Alkylthiomethylphenols, for example 2,4-dioctylthiomethyl-6-tert-butylphenol, 2,4-dioctylthiomethyl-6-methylphenol, 2,4-dioctylthiomethyl-6-ethylphenol, 2,6-didodecylthiomethyl-4 -nonylphenol.
1.3. Hydrochinone und alkvlierte Hydrochinone, z.B. 2,6-Di-tert-butyl-4-methoxyphenol, 2,5-Di-tert-butyl-hydrochinon, 2,5-Di-tert-amyl-hydrochinon, 2,6-Diphenyl-4-octadecyloxyphenol, 2,6-Di-tert-butyl-hydrochinon, 2,5-Di-tert-butyl-4-hydroxyanisol, 3,5-Di-tert-butyl-4-hydroxyanisol, 3,5-Di-tert-butyl-4-hydroxyphenyl-stearat, Bis(3,5-di-tert-butyl-4-hydroxyphenyl)adipat. 1.3. Hydroquinones and alkali hydroquinones, for example 2,6-di-tert-butyl-4-methoxyphenol, 2,5-di-tert-butyl-hydroquinone, 2,5-di-tert-amyl-hydroquinone, 2,6-diphenyl- 4-octadecyloxyphenol, 2,6-di-tert-butyl-hydroquinone, 2,5-di-tert-butyl-4-hydroxyanisole, 3,5-di-tert-butyl-4-hydroxyanisole, 3,5-di- tert-butyl-4-hydroxyphenyl stearate, bis (3,5-di-tert-butyl-4-hydroxyphenyl) adipate.
1.4. Tocopherole, z.B. α-, β-, γ- oder δ- und Mischungen davon (Vitamin E). 1.4. Tocopherols, e.g. α-, β-, γ- or δ- and mixtures thereof (vitamin E).
1.5. Hydroxylierte Thiodiphenylether, z.B. 2,2'-Thio-bis(6-tert-butyl-4-methylphenol), 2,2'-Thio-bis(4-octylphenol), 4,4'-Thio-bis(6-tert-butyl-3-methylphenol), 4,4'-Thio-bis-(6-tert-butyl-2-methylphenol), 4,4'-Thio-bis(3,6-di-sec.-amylphenol), 4,4'-Bis(2,6-dimethyl-4-hydroxyphenyl)-disulfid. 1.5. Hydroxylated thiodiphenyl ethers, for example 2,2'-thio-bis (6-tert-butyl-4-methylphenol), 2,2'-thio-bis (4-octylphenol), 4,4'-thio-bis (6-tert -butyl-3-methylphenol), 4,4'-thio-bis (6-tert-butyl-2-methylphenol), 4,4'-thio-bis (3,6-di-sec.-amylphenol), 4,4'-bis (2,6-dimethyl-4-hydroxyphenyl) disulfide.
1.6. Alkyliden-Bisphenole, z.B. 2,2'-Methylen-bis(6-tert-butyl-4-methylphenol), 2,2'-Methylen-bis(6-tert-butyl-4-ethylphenol), 2,2'-Methylen-bis[4-methyl-6-(a-methylcyclohexyl)-phenol], 2,2'-Methylen-bis(4-methyl-6-cyclohexylphenol), 2,2'-Methylen-bis(6-nonyl-4-methyphenol), 2,2'-Methylen-bis(4,6-di-tert-butylphenol), 2,2'-Ethyliden-bis(4,6-di-tert-butylphenol), 2,2'-Ethyliden-bis(6-tert-butyl-4-isobutylphenol), 2,2'-Methylen-bis[6-(a-methylbenzyl)-4-nonylphenol], 2,2'-Methylen-bis[6-(a,a-dimethylbenzyl)-4-nonylphenol], 4,4'-Methylen-bis(2,6-di-tert-butylphenol), 4,4'-Methylen-bis(6-tert-butyl-2-methylphenol), 1,1-Bis(5-tert-butyl-4-hydroxy-2-methylphenyl)-butan, 2,6-Bis(3-tert-butyl-5-methyl-2-hydroxybenzyl)-4-methylphenol, 1,1 ,3-Tris(5-tert-butyl-4-hydroxy-2-methylphenyl)-butan, 1,1-Bis(5-tert-butyl-4-hydroxy-2-methyl-phenyl)-3-n-dodecylmercaptobutan, Ethylenglycol-bis[3,3-bis(3'-tert-butyl-4'-hydroxyphenyl)-butyrat], Bis(3-tert-butyl-4-hydroxy-5-methyl-phenyl)-dicyclopentadien, Bis[2-(3'-tert-butyl-2'-hydroxy-5'-methyl-benzyl)-6-tert-butyl-4-methyl-phenyl]-terephthalat, 1,1-Bis(3,5-dimethyl-2-hydroxyphenyl)-butan, 2,2-Bis(3,5-di-tert-butyl-4-hydroxyphenyl)-propan, 2,2-Bis(5-tert-butyl-4-hydroxy-2-methylphenyl)-4-n-dodecylmercapto-butan, 1,1,5,5-Tetra-(5-tert-butyl-4-hydroxy-2-methylphenyl)-pentan. 1.6. Alkylidene bisphenols, for example 2,2'-methylene-bis (6-tert-butyl-4-methylphenol), 2,2'-methylene-bis (6-tert-butyl-4-ethylphenol), 2,2'- Methylene bis [4-methyl-6- (a-methylcyclohexyl) phenol], 2,2'-methylene bis (4-methyl-6-cyclohexylphenol), 2,2'-methylene bis (6-nonyl- 4-methyphenol), 2,2'-methylene-bis (4,6-di-tert-butylphenol), 2,2'-ethylidene-bis (4,6-di-tert-butylphenol), 2,2'- Ethylidene-bis (6-tert-butyl-4-isobutylphenol), 2,2'-methylene-bis [6- (a-methylbenzyl) -4-nonylphenol], 2,2'-methylene-bis [6- (a , a-dimethylbenzyl) -4-nonylphenol], 4,4'-methylene-bis (2,6-di-tert-butylphenol), 4,4'-methylene-bis (6-tert-butyl-2-methylphenol) , 1,1-bis (5-tert-butyl-4-hydroxy-2-methylphenyl) butane, 2,6-bis (3-tert-butyl-5-methyl-2-hydroxybenzyl) -4-methylphenol, 1 , 1,3-tris (5-tert-butyl-4-hydroxy-2-methylphenyl) butane, 1,1-bis (5-tert-butyl-4-hydroxy-2-methylphenyl) -3-n -dodecyl mercaptobutane, ethylene glycol bis [3,3-bis (3'-tert-butyl-4'-hydroxyphenyl) butyrate], bis (3-tert-butyl-4-hydroxy-5-methylphenyl) dicyclopentadiene, To [2- (3'- tert-butyl-2'-hydroxy-5'-methyl-benzyl) -6-tert-butyl-4-methyl-phenyl] terephthalate, 1,1-bis (3,5-dimethyl-2-hydroxyphenyl) butane , 2,2-bis (3,5-di-tert-butyl-4-hydroxyphenyl) propane, 2,2-bis (5-tert-butyl-4-hydroxy-2-methylphenyl) -4-n-dodecylmercapto -butane, 1,1,5,5-tetra- (5-tert-butyl-4-hydroxy-2-methylphenyl) pentane.
1.7. O-, N- und S-Benzylverbindungen, z.B. 3,5,3',5'-Tetra-tert-butyl-4,4'-dihydroxydibenzylether, Octadecyl-4-hydroxy-3,5-dimethylbenzyl-mercaptoacetat, Tridecyl-4-hydroxy-3,5-di-tert-butylbenzyl-mercaptoacetat, Tris(3,5-di-tert-butyl-4-hydroxybenzyl)-amin, Bis(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)-dithioterephthalat, Bis(3,5-di-tert-butyl-4-hydroxybenzyl)-sulfid, Isooctyl-3,5-di-tert-butyl-4-hydroxybenzyl-mercaptoacetat. 1.7. O-, N- and S-benzyl compounds, for example 3,5,3 ', 5'-tetra-tert-butyl-4,4'-dihydroxydibenzyl ether, octadecyl-4-hydroxy-3,5-dimethylbenzyl-mercaptoacetate, tridecyl- 4-hydroxy-3,5-di-tert-butylbenzyl mercaptoacetate, tris (3,5-di-tert-butyl-4-hydroxybenzyl) amine, bis (4-tert-butyl-3-hydroxy-2,6) -dimethylbenzyl) dithioterephthalate, bis (3,5-di-tert-butyl-4-hydroxybenzyl) sulfide, isooctyl-3,5-di-tert-butyl-4-hydroxybenzyl-mercaptoacetate.
1.8. Hydroxybenzylierte Malonate, z.B. Dioctadecyl-2,2-bis(3,5-di-tert-butyl-2-hydroxybenzyl)-malonat, Di-octadecyl-2-(3-tert-butyl-4-hydroxy-5-methylbenzyl)-malonat, Di-dodecyl-mercaptoethyl-2,2-bis(3,5-di-tert-butyl-4-hydroxybenzyl)-malonat, Di-[4-(1,1,3,3-tetramethyl-butyl)-phenyl]-2,2-bis(3,5-di-tert-butyl-4-hydroxybenzyl)-malonat. 1.8. Hydroxybenzylated malonates, e.g. dioctadecyl-2,2-bis (3,5-di-tert-butyl-2-hydroxybenzyl) malonate, di-octadecyl-2- (3-tert-butyl-4-hydroxy-5-methylbenzyl) -malonate, di-dodecyl-mercaptoethyl-2,2-bis (3,5-di-tert-butyl-4-hydroxybenzyl) -malonate, di- [4- (1,1,3,3-tetramethyl-butyl) -phenyl] -2,2-bis (3,5-di-tert-butyl-4-hydroxybenzyl) malonate.
1.9. Hydroxybenzyl-Aromaten, z.B. 1,3,5-Tris(3,5-di-tert-butyl-4-hydroxybenzyl)-2,4,6-trimethylbenzol, 1,4-Bis(3,5-di-tert-butyl-4-hydroxybenzyl)-2,3,5,6-tetramethylbenzol, 2,4,6-Tris(3,5-di-tert-butyl-4-hydroxybenzyl)-phenol. 1.9. Hydroxybenzyl aromatics, for example 1,3,5-tris (3,5-di-tert-butyl-4-hydroxybenzyl) -2,4,6-trimethylbenzene, 1,4-bis (3,5-di-tert- butyl-4-hydroxybenzyl) -2,3,5,6-tetramethylbenzene, 2,4,6-tris (3,5-di-tert-butyl-4-hydroxybenzyl) phenol.
1.10. Triazinverbindungen, z.B. 2,4-Bis-octylmercapto-6-(3,5-di-tert-butyl-4-hydroxyanilino)-1,3,5-triazin, 2-Octylmercapto-4,6-bis(3,5-di-tert-butyl-4-hydroxyanilino)-1,3,5-triazin, 2-Octylmercapto-4,6-bis(3,5-di-tert-butyl-4-hydroxyphenoxy)-1,3,5-triazin, 2,4,6-Tris(3,5-di-tert-butyl-4-hydroxyphenoxy)-1,2,3-triazin, 1,3,5-Tris(3,5-di-tert-butyl-4-hydroxybenzyl)-isocyanurat, 1,3,5-Tris(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)-isocyanurat, 2,4,6-Tris(3,5-di-tert-butyl-4-hydroxyphenylethyl)-1,3,5-triazin, 1,3,5-Tris(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)-hexahydro-1,3,5-triazin, 1,3,5-Tris(3,5-dicyclohexyl-4-hydroxybenzyl)-isocyanurat. 1.10. Triazine compounds, e.g. 2,4-bis-octylmercapto-6- (3,5-di-tert-butyl-4-hydroxyanilino) -1,3,5-triazine, 2-octylmercapto-4,6-bis (3,5 -di-tert-butyl-4-hydroxyanilino) -1,3,5-triazine, 2-octylmercapto-4,6-bis (3,5-di-tert-butyl-4-hydroxyphenoxy) -1,3,5 -triazine, 2,4,6-tris (3,5-di-tert-butyl-4-hydroxyphenoxy) -1,2,3-triazine, 1,3,5-tris (3,5-di-tert- butyl-4-hydroxybenzyl) isocyanurate, 1,3,5-tris (4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl) isocyanurate, 2,4,6-tris (3,5-di- tert-butyl-4-hydroxyphenylethyl) -1,3,5-triazine, 1,3,5-tris (3,5-di-tert-butyl-4-hydroxyphenylpropionyl) hexahydro-1,3,5-triazine, 1,3,5-tris (3,5-dicyclohexyl-4-hydroxybenzyl) isocyanurate.
1.11. Acylaminophenole, z.B. 4-Hydroxy-laurinsäureanilid, 4-Hydroxystearinsäureanilid, N-(3,5-di-tert-butyl-4-hydroxyphenyl)-carbaminsäureoctylester. 1.11. Acylaminophenols, for example 4-hydroxylauric anilide, 4-hydroxystearic anilide, N- (3,5-di-tert-butyl-4-hydroxyphenyl) carbamic acid octyl ester.
1.12. Ester der beta-(3,5-Di-tert-butyl-4-hydroxyphenyl)-propionsäure mit ein- oder mehrwertigen Alkoholen, wie z.B. mit Methanol, Ethanol, n-Octanol, i-Octanol, Octadecanol, 1,6-Hexandiol, 1,9-Nonandiol, Ethylenglycol, 1,2-Propandiol, Neopentylglycol, Thiodiethylenglycol, Diethylenglycol, Triethylenglycol, Pentaerythrit, Tris(hydroxyethyl)-isocyanurat, N,N'-Bis-(hydroxyethyl)-oxalsäurediamid, 3-Thiaundecanol, 3-Thiapentadecanol, Trimethylhexandiol, Trimethylolpropan, 4-Hydroxymethyl-1-phospha-2,6,7-trioxabicyclo-[2.2.2]-octan. 1.12. Esters of beta (3,5-di-tert-butyl-4-hydroxyphenyl) propionic acid with mono- or polyhydric alcohols, such as with methanol, ethanol, n-octanol, i-octanol, octadecanol, 1,6-hexanediol , 1,9-nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethylene glycol, triethylene glycol, pentaerythritol, tris (hydroxyethyl) isocyanurate, N, N'-bis (hydroxyethyl) oxalic acid diamide, 3-thiaundecanol, 3 -Thiapentadecanol, trimethylhexanediol, trimethylolpropane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo- [2.2.2] octane.
1.13. Ester der beta-(5-tert-Butyl-4-hydroxy-3-methylphenyl)-propionsäure mit ein- oder mehrwertigen Alkoholen, wie z.B. mit Methanol, Ethanol, n-Octanol, i-Octanol, Octadecanol, 1,6-Hexandiol, 1,9-Nonandiol, Ethylenglycol, 1,2-Propandiol, Neopentylglycol, Thiodiethylenglycol, Diethylenglycol, Triethylenglycol, Pentaerythrit, Tris(hydroxyethyl)-isocyanurat, N,N'-Bis(hydroxyethyl)-oxalsäurediamid, 3-Thiaundecanol, 3-Thiapentadecanol, Trimethylhexandiol, Trimethylolpropan, 4-Hydroxymethyl-1-phospha-2,6,7-trioxabicyclo-[2.2.2]-octan. 1.13. Esters of beta (5-tert-butyl-4-hydroxy-3-methylphenyl) propionic acid with mono- or polyhydric alcohols, such as with methanol, ethanol, n-octanol, i-octanol, octadecanol, 1,6-hexanediol , 1,9-nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethylene glycol, triethylene glycol, pentaerythritol, tris (hydroxyethyl) isocyanurate, N, N'-bis (hydroxyethyl) oxalic acid diamide, 3-thiaundecanol, 3- Thiapentadecanol, trimethylhexanediol, trimethylolpropane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo [2.2.2] octane.
1.14. Ester der beta-(3,5-Dicyclohexyl-4-hydroxyphenyl)-propionsäure mit ein- oder mehrwertigen Alkoholen, wie z.B. mit Methanol, Ethanol, Octanol, Octadecanol, 1,6-Hexandiol, 1,9-Nonandiol, Ethylenglycol, 1,2-Propandiol, Neopentylglycol, Thiodiethylenglycol, Diethylenglycol, Triethylenglycol, Pentaerythrit, Tris(hydroxyethyl)-isocyanurat, N,N'-Bis(hydroxyethyl)-oxalsäurediamid, 3-Thiaundecanol, 3-Thiapentadecanol, Trimethylhexandiol, Trimethylolpropan, 4-Hydroxymethyl-1-phospha-2,6,7-trioxabicyclo-[2.2.2]-octan. 1.14. Esters of beta- (3,5-dicyclohexyl-4-hydroxyphenyl) propionic acid with mono- or polyhydric alcohols, such as with methanol, ethanol, octanol, octadecanol, 1,6-hexanediol, 1,9-nonanediol, ethylene glycol, 1 , 2-propanediol, neopentylglycol, thiodiethylene glycol, diethylene glycol, triethylene glycol, pentaerythritol, tris (hydroxyethyl) isocyanurate, N, N'-bis (hydroxyethyl) oxalic acid diamide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol, trimethylolpropane, trimethyl 1-phospha-2,6,7-trioxabicyclo [2.2.2] octane.
1.15. Ester der 3,5-Di-tert-butyl-4-hydroxyphenylessigsäure mit ein- oder mehrwertigen Alkoholen, wie z.B. mit Methanol, Ethanol, Octanol, Octadecanol, 1,6-Hexandiol, 1,9-Nonandiol, Ethylenglycol, 1,2-Propandiol, Neopentylglycol, Thiodiethylenglycol, Diethylenglycol, Triethylenglycol, Pentaerythrit, Tris(hydroxyethyl)-isocyanurat, N,N'-Bis(hydroxyethyl)-oxalsäurediamid, 3-Thiaundecanol, 3-Thiapentadecanol, Trimethylhexandiol, Trimethylolpropan, 4-Hydroxymethyl-1 -phospha-2,6,7-trioxabicyclo-[2.2.2]-octan. 1.15. Esters of 3,5-di-tert-butyl-4-hydroxyphenylacetic acid with mono- or polyhydric alcohols, such as with methanol, ethanol, octanol, octadecanol, 1,6-hexanediol, 1,9-nonanediol, ethylene glycol, 1,2 Propanediol, neopentyl glycol, thiodiethylene glycol, diethylene glycol, triethylene glycol, pentaerythritol, tris (hydroxyethyl) isocyanurate, N, N'-bis (hydroxyethyl) oxalic acid diamide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol, trimethylolpropanediol, trimethylolpropanediol, trimethylolpropanediol phospha-2,6,7-trioxabicyclo- [2.2.2] octane.
1.16. Amide der beta-(3,5-Di-tert-butyl-4-hydroxyphenyl)-propionsäure, wie z.B. N,N'-Bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)-hexamethylendiamin, N,N'-Bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)-trimethylendiamin, N,N'-Bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)-hydrazin. 1.16. Amides of beta (3,5-di-tert-butyl-4-hydroxyphenyl) propionic acid, such as, for example, N, N'-bis (3,5-di-tert-butyl-4-hydroxyphenylpropionyl) hexamethylene diamine, N, N'-bis (3,5-di-tert-butyl-4-hydroxyphenylpropionyl) trimethylene diamine, N, N'-bis (3,5-di-tert-butyl-4-hydroxyphenylpropionyl) hydrazine.
1.17. Ascorbinsäure (Vitamin C).1.17. Ascorbic acid (vitamin C).
1.18. Aminische Antioxidantien, wie z.B. N,N'-Di-isopropyl-p-phenylendiamin, N,N'-Di-sec-butyl-p-phenylendiamin, N,N'-Bis(1,4-dimethyl-pentyl)-p-phenylendiamin, N,N'-Bis(1-ethyl-3-methyl-pentyl)-p-phenylendiamin, N,N'-Bis(1-methyl-heptyl)-p-phenylendiamin, N,N'-Dicyclohexyl-p-phenylendiamin, N,N'-Diphenyl-p-phenylendiamin, N,N'-Di-(naphthyl-2)-p-phenylendiamin, N-lsopropyl-N'-phenyl-p-phenylendiamin, N-(1,3-Dimethyl-butyl)-N'-phenyl-p-phenylendiamin, N-(1-Methyl-heptyl)-N'-phenyl-p-phenylendiamin, N-Cyclohexyl-N'-phenyl-p-phenylendiamin, 4-(p-Toluol-sulfonamido)-diphenylamin, N,N'-Dimethyl-N,N'-di-sec-butyl-p-phenylendiamin, Diphenylamin, N-Allyldiphenylamin, 4-lsopropoxy-diphenylamin, N-Phenyl-1-naphthylamin, N-(4-tert-Octylphenyl)-1-naphthylamin, N-Phenyl-2-naphthylamin, octyliertes Diphenylamin, z.B. p,p'-Di-tert-octyldiphenylamin, 4-n-Butylaminophenol, 4-Butyrylaminophenol, 4-Nonanoylamino-phenol, 4-Dodecanoylamino-phenol, 4-Octadecanoylaminophenol, Di-(4-methoxyphenyl)-amin, 2,6-Di-tert-butyl-4-dimethylamino-methyl-phenol, 2,4'-Diamino-diphenylmethan, 4,4'-Diamino-diphenylmethan, N,N,N',N'-Tetramethyl-4,4'-diamino-diphenylmethan, 1,2-Di-[(2-methyl-phenyl)-amino]-ethan, 1,2-Di-(phenylamino)-propan, (o-Tolyl)-biguanid, Di-[4-(1',3'-dimethyl-butyl)-phenyl]amin, tert-octyliertes N-Phenyl-1-naphthylamin, Gemisch aus mono- und dialkylierten tert-Butyl/tert-Octyldiphenylaminen, Gemisch aus mono- und dialkylierten Nonyldiphenylaminen, Gemisch aus mono- und dialkylierten Dodecyldiphenylaminen, Gemisch aus mono- und dialkylierten Isopropyl/ Isohexyl-diphenylaminen, Gemische aus mono- und dialkylierten tert-Butyldiphenylaminen, 2,3-Dihydro-3,3-dimethyl-4H-1,4-benzothiazin, Phenothiazin, Gemisch aus mono- und dialkylierten tert-Butyl/tert-Octyl-phenothiazinen, Gemisch aus mono- und dialkylierten tert-Octyl-phenothiazinen, N-Allylphenothiazin, N,N,N',N'-Tetraphenyl-1,4-diaminobut-2-en, N,N-Bis-(2,2,6,6-tetramethyl-piperidin-4-yl-hexamethylendiamin, Bis-(2,2,6,6-tetramethylpiperidin-4-yl)-sebacat, 2,2,6,6-Tetramethypiperidin-4-on, 2,2,6,6-Tetramethylpiperidin-4-ol. 1.18. Amine antioxidants such as N, N'-di-isopropyl-p-phenylenediamine, N, N'-di-sec-butyl-p-phenylenediamine, N, N'-bis (1,4-dimethyl-pentyl) -p -phenylenediamine, N, N'-bis (1-ethyl-3-methylpentyl) -p-phenylenediamine, N, N'-bis (1-methyl-heptyl) -p-phenylenediamine, N, N'-dicyclohexyl- p-phenylenediamine, N, N'-diphenyl-p-phenylenediamine, N, N'-di- (naphthyl-2) -p-phenylenediamine, N-isopropyl-N'-phenyl-p-phenylenediamine, N- (1, 3-dimethyl-butyl) -N'-phenyl-p-phenylenediamine, N- (1-methyl-heptyl) -N'-phenyl-p-phenylenediamine, N-cyclohexyl-N'-phenyl-p-phenylenediamine, 4- (p-toluenesulfonamido) diphenylamine, N, N'-dimethyl-N, N'-di-sec-butyl-p-phenylenediamine, diphenylamine, N-allyldiphenylamine, 4-isopropoxy-diphenylamine, N-phenyl-1- naphthylamine, N- (4-tert-octylphenyl) -1-naphthylamine, N-phenyl-2-naphthylamine, octylated diphenylamine, e.g. p, p'-di-tert-octyldiphenylamine, 4-n-butylaminophenol, 4-butyrylaminophenol, 4 -Nonanoylamino-phenol, 4-dodecanoylamino-phenol, 4-octadecanoylaminophenol, di- (4-methoxyphenyl) amine, 2,6-di-tert-but yl-4-dimethylamino-methyl-phenol, 2,4'-diamino-diphenylmethane, 4,4'-diamino-diphenylmethane, N, N, N ', N'-tetramethyl-4,4'-diamino-diphenylmethane, 1 , 2-Di - [(2-methylphenyl) amino] ethane, 1,2-di- (phenylamino) propane, (o-tolyl) biguanide, di- [4- (1 ', 3' -dimethyl-butyl) -phenyl] amine, tert-octylated N-phenyl-1-naphthylamine, mixture of mono- and dialkylated tert-butyl / tert-octyldiphenylamines, mixture of mono- and dialkylated nonyldiphenylamines, mixture of mono- and dialkylated dodecyldiphenylamines , Mixture of mono- and dialkylated isopropyl / isohexyl-diphenylamines, mixtures of mono- and dialkylated tert-butyldiphenylamines, 2,3-dihydro-3,3-dimethyl-4H-1,4-benzothiazine, phenothiazine, mixture of mono- and dialkylated tert-butyl / tert-octyl-phenothiazines, mixture of mono- and dialkylated tert-octyl-phenothiazines, N-allylphenothiazine, N, N, N ', N'-tetraphenyl-1,4-diaminobut-2-ene, N , N-bis- (2,2,6,6-tetramethyl-piperidin-4-yl-hexamethylenediamine, bis- (2,2,6,6-tetramethyl-piperidine-4 -yl) sebacate, 2,2,6,6-tetramethypiperidin-4-one, 2,2,6,6-tetramethylpiperidin-4-ol.
Aliphatische oder aromatische Phosphite, Ester der Thiodipropionsäure oder Thiodiessigsäure, oder Salze der Dithiocarbamid- oder Dithiophosphorsäure, 2,2,12,12-Tetramethyl-5,9-dihydroxy-3,7,11-trithiatridecan und 2,2,15,15-Tetramethyl-5,12-dihydroxy-3,7,10,14-tetrathiahexadecan.Aliphatic or aromatic phosphites, esters of thiodipropionic acid or thiodiacetic acid, or salts of dithiocarbamic or dithiophosphoric acid, 2,2,12,12-tetramethyl-5,9-dihydroxy-3,7,11-trithiatridecane and 2,2,15,15-tetramethyl-5,12-dihydroxy-3,7,10,14-tetrathiahexadecane.
Beispiele für Viskositätsindex-Verbesserer: Polyacrylate, Polymethacrylate, Vinylpyrrolidon/Methacrylat-Copolymere, Polyvinylpyrrolidone, Polybutene, Olefin-Copolymere, Styrol/Acrylat-Copolymere, Polyether. Examples of viscosity index improvers: polyacrylates, polymethacrylates, vinyl pyrrolidone / methacrylate copolymers, polyvinyl pyrrolidones, polybutenes, olefin copolymers, styrene / acrylate copolymers, polyethers.
Beispiele für Stockpunkterniedriger: Poly(meth)acrylate, Ethylen-Vinylacetat-Copolymer, Alkylpolystyrole, Fumaratcopolymere, alkylierte Naphthalinderivate. Examples of pour point depressants: poly (meth) acrylates, ethylene-vinyl acetate copolymer, alkylpolystyrenes, fumarate copolymers, alkylated naphthalene derivatives.
Beispiele für Dispergiermittel/Tenside: Polybutenylbernsteinsäureamide oder -imide, Polybutenylphosphonsäurederivate, basische Magnesium-, Calcium-, und Bariumsulfonate und - phenolate. Examples of dispersants / surfactants: polybutenylsuccinic acid amides or imides, polybutenylphosphonic acid derivatives, basic magnesium, calcium and barium sulfonates and phenolates.
Schwefel- und halogenhaltige Verbindungen wie z.B. chlorierte Paraffine, sulfurierte Olefine oder pflanzliche Öle (Soja-, Rapsöl), Alkyl- oder Aryl-di- oder -trisulfide, Benzotriazole oder deren Derivate wie bis(2-Ethylhexyl)aminomethyl tolutriazole, Dithiocarbamate wie Methylen-bis-dibutyldithiocarbamat, Derivate des 2-Mercaptobenzothiazols wie 1-[N,N-bis(2-ethylhexyl)aminomethyl]-2-mercapto-1H-1,3-benzothiazol, Derivate des 2,5-dimercapto-1,3,4-thiadiazols wie 2,5-bis(tert.nonyldithio)-1,3,4-thiadiazol.Sulfur and halogen containing compounds such as chlorinated paraffins, sulfurized olefins or vegetable oils (soybean oil, rapeseed oil), alkyl or aryl di- or trisulfides, benzotriazoles or their derivatives such as bis (2-ethylhexyl) aminomethyl tolutriazole, dithiocarbamates such as methylene bis-dibutyldithiocarbamate, Derivatives of 2-mercaptobenzothiazole such as 1- [N, N-bis (2-ethylhexyl) aminomethyl] -2-mercapto-1H-1,3-benzothiazole, Derivatives of 2,5-dimercapto-1,3,4-thiadiazole such as 2,5-bis (tert.nonyldithio) -1,3,4-thiadiazole.
Beispiele für Reibwertverminderer, z.B. Öl aus Schmalz, Ölsäure, Talg, Rapsöl, geschwefelte Fette, Amine. Weitere Beispiele sind in EP 565487 genannt. Examples of friction reducers , eg oil from lard, oleic acid, tallow, rapeseed oil, sulfurized fats, amines. Further examples are mentioned in EP 565487.
Beispiele für besondere Additive zur Anwendung in Wasser/Öl-Metallbearbeitungs- und Hydraulikflüssigkeiten sind: Examples of special additives for use in water / oil metal working and hydraulic fluids are:
Emulgatoren: Petroleumsulfonate, Amine wie polyoxyethylierte Fettamine, nichtionische oberflächenaktive Substanzen; Puffer: Alkanolamine; Biocide: Triazine, Thiazolinone, Tris-Nitromethan, Morpholin, Natriumpyridenethiol; Verarbeitungsgeschwindigkeitsverbesserer: Calcium- und Bariumsulfonate; Emulsifiers : petroleum sulfonates, amines such as polyoxyethylated fatty amines, nonionic surface-active substances; Buffer: alkanolamines; Biocides: triazines, thiazolinones, tris-nitromethane, morpholine, sodium pyridene ethiol ; Processing speed improvers: calcium and barium sulfonates;
Die genannten Komponenten können den Schmierstoffen auf an sich bekannte Weise beigemischt werden. Es ist auch möglich, ein Konzentrat oder ein sogenanntes Additivpaket herzustellen, das nach Massgabe des Verbrauchs auf Einsatzkonzentrationen für den entsprechenden Schmierstoff verdünnt werden kann.The components mentioned can be added to the lubricants in a manner known per se become. It is also possible to use a concentrate or a so-called additive package produce that according to the consumption at the use concentrations for the corresponding Lubricant can be diluted.
Eine bevorzugte Ausführungsform der Erfindung betrifft eine Zusammensetzung enthaltend
Eine weitere besonders bevorzugte Ausführungsform der Erfindung betrifft eine Zusammensetzung
enthaltend
Ebenfalls Gegenstand der Erfindung ist ein Konzentrat verwendbar für die Herstellung der
Zusammensetzung enthaltend
Das Konzentrat kann folgende zusätzliche Bestandteile enthalten:
Die Komponenten werden so im Konzentrat zusammengesetzt, dass dieses bei Raumtemperatur ohne Zusatz des Grundöls a) oder eines Lösungsmittels flüssig ist.The components are put together in the concentrate so that it is at room temperature is liquid without the addition of base oil a) or a solvent.
Die Erfindung betrifft ebenfalls ein Verfahren zur Verbesserung der Gebrauchseigenschaften von Schmierstoffen, gekennzeichnet durch Zugabe der Komponenten b), c) und d), vorzugsweise in einer Konzentration, dass der Phosphorgehalt dieser Komponenten, bezogen auf die Gesamtzusammensetzung, weniger als 400 ppm beträgt.The invention also relates to a method for improving the performance properties of lubricants, characterized by adding components b), c) and d), preferably in a concentration related to the phosphorus content of these components on the total composition, is less than 400 ppm.
Ebenfalls Gegenstand der Erfindung ist auch die Verwendung von Verbindungen der Komponenten b) c) und d), vorzugsweise in der genannten Konzentration, als Additive in Motorenölen, Turbinenölen, Getriebeölen, Hydraulikflüssigkeiten, Metallbearbeitungsflüssigkeiten oder Schmierfetten.The invention also relates to the use of compounds of the components b) c) and d), preferably in the concentration mentioned, as additives in motor oils, Turbine oils, gear oils, hydraulic fluids, metal working fluids or greases.
Die folgenden Beispiele illustrieren die Erfindung:The following examples illustrate the invention:
Die verschiedenen Mischungen wurden unter Verwendung eines ISO VG 46 Mineralöls (kinematische Viskosität bei 40°C: 42 - 50 CSt) und einer Basis-Additiv-Mischung, welche typischerweise für Hydraulikflüssigkeiten zum Einsatz kommt, hergestellt. Diese Basis-Additiv-Mischung ist frei von Metallsalzen und wird mit 0,29 - 0,47% (Gew.%) eingesetzt. Sie ist eine Kombination von einem aromatischen Amin-Antioxidant (z.B. Irganox® L 57), einem gehinderten Phenol-Antioxidant (z.B. Irganox® L 135) und kleineren Anteilen von anderen üblichen Additiven wie Stockpunkterniedriger (z.B: Plexol® 154), Antischaummittel (z.B. Mobilad® C402), Demulgieradditive (z.B. Synperonic® PEL81), Korrosionsinhibitoren (z.B. Irgacor® NPA) und Metalldesaktivatoren (z.B. Irgamet® 39). Die verwendeten Additive und Mischungen sowie die Ergebnisse der durchgeführten Tests sind in der Tabelle aufgeführt. Formulierungen 9, 10 , 11, 12, 13, 14 entsprechen der beanspruchten Zusammensetzung der vorliegenden Erfindung. Die anderen Formulierungen dienen dem Vergleich, insbesondere mit dem Stand der Technik (Stand d. T. I und II).The various blends were made using an ISO VG 46 mineral oil (kinematic viscosity at 40 ° C: 42 - 50 CSt) and a basic additive mixture, which typically used for hydraulic fluids. This basic additive mixture is free of metal salts and is used with 0.29 - 0.47% (wt.%). she is a combination of an aromatic amine antioxidant (e.g. Irganox® L 57), a hindered Phenol antioxidant (e.g. Irganox® L 135) and smaller proportions of other common ones Additives such as pour point depressants (e.g. Plexol® 154), anti-foaming agents (e.g. Mobilad® C402), demulsifying additives (e.g. Synperonic® PEL81), corrosion inhibitors (e.g. Irgacor® NPA) and metal deactivators (e.g. Irgamet® 39). The additives and mixtures used and the results of the tests performed are shown in the table. Wording 9, 10, 11, 12, 13, 14 correspond to the claimed composition of the present Invention. The other formulations are for comparison, especially with the state of the art (state of T. I and II).
Folgende Tests wurden durchgeführt: Prüfung von: Antiverschleisseigenschaften: VKA und
SRV, Kompatibilität mit Wasser (spezielle Lagerung des Öls mit Wasser, danach Filtration
und Rosttest), Lasttragevermögen (extreme pressure): FZG.
< 0,45 mm
<0.45 mm
Claims (16)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CH2202/97 | 1997-09-18 | ||
| CH220297 | 1997-09-18 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0903399A1 true EP0903399A1 (en) | 1999-03-24 |
| EP0903399B1 EP0903399B1 (en) | 2007-02-14 |
Family
ID=4228284
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP98810894A Expired - Lifetime EP0903399B1 (en) | 1997-09-18 | 1998-09-09 | Lubricant compositions containing thiophosphoric and dithiophosphoric acid esters |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US6531429B2 (en) |
| EP (1) | EP0903399B1 (en) |
| JP (1) | JP4114180B2 (en) |
| KR (1) | KR100538777B1 (en) |
| DE (1) | DE59813902D1 (en) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002102945A1 (en) * | 2001-06-14 | 2002-12-27 | Ciba Specialty Chemicals Holding Inc. | Improved antiwear performance of engine oils with $g(b)-dithiophosphorylated propionic acids |
| EP1529830A1 (en) * | 2003-11-04 | 2005-05-11 | Chevron Oronite S.A. | Ashless additive formulations suitable for hydraulic oil applications |
| WO2006100188A1 (en) * | 2005-03-21 | 2006-09-28 | Ciba Specialty Chemicals Holding Inc. | Antiwear lubricant compositions for use in combustion engines |
| EP2147967A1 (en) * | 2008-07-14 | 2010-01-27 | Afton Chemical Corporation | Thermally stable zinc-free antiwear agent |
| WO2011035865A1 (en) * | 2009-09-23 | 2011-03-31 | Cognis Ip Management Gmbh | Lubricant compositions |
| CN112080333A (en) * | 2020-08-27 | 2020-12-15 | 统一石油化工有限公司 | Long-life manual transmission oil composition |
Families Citing this family (40)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2353290A (en) * | 1999-08-17 | 2001-02-21 | Exxon Research Engineering Co | Stabilized lubricating formulation and method |
| JP2001303086A (en) * | 2000-04-18 | 2001-10-31 | Chevron Oronite Ltd | Lubricating oil composition and additive composition |
| MXPA04001356A (en) * | 2001-08-14 | 2004-10-27 | United Soybean Board | Soy-based methyl ester high performance metal working fluids. |
| WO2003020855A1 (en) * | 2001-09-05 | 2003-03-13 | United Soybean Board | Soybean oil based metalworking fluids |
| US20040241309A1 (en) * | 2003-05-30 | 2004-12-02 | Renewable Lubricants. | Food-grade-lubricant |
| US20060211585A1 (en) * | 2003-09-12 | 2006-09-21 | Renewable Lubricants, Inc. | Vegetable oil lubricant comprising Fischer Tropsch synthetic oils |
| JP4625456B2 (en) * | 2003-09-12 | 2011-02-02 | リニューアブル リューブリカンツ インコーポレーテッド | Vegetable oil lubricant containing all hydrotreated synthetic oil |
| US7759294B2 (en) * | 2003-10-24 | 2010-07-20 | Afton Chemical Corporation | Lubricant compositions |
| CA2496230C (en) * | 2004-02-06 | 2015-11-24 | Henkel Kommanditgesellschaft Auf Aktien | Antimicrobial metal working fluids |
| JP2005307202A (en) * | 2004-03-25 | 2005-11-04 | Nippon Oil Corp | Lubricating oil composition |
| JP2005290181A (en) * | 2004-03-31 | 2005-10-20 | Nippon Oil Corp | Gear oil composition |
| JP2005307203A (en) * | 2004-03-25 | 2005-11-04 | Nippon Oil Corp | Lubricating oil composition |
| WO2005093020A1 (en) * | 2004-03-25 | 2005-10-06 | Nippon Oil Corporation | Lubricating oil composition for industrial machinery and equipment |
| JP4641381B2 (en) * | 2004-03-31 | 2011-03-02 | Jx日鉱日石エネルギー株式会社 | Lubricating oil composition for paper machine |
| US20060090393A1 (en) * | 2004-10-29 | 2006-05-04 | Rowland Robert G | Epoxidized ester additives for reducing lead corrosion in lubricants and fuels |
| CN101107347B (en) * | 2005-01-24 | 2011-08-17 | 日本精工株式会社 | Grease composition for hub unit bearing, and hub unit bearing for vehicles |
| US7579306B2 (en) * | 2005-03-02 | 2009-08-25 | Chemtura Corporation | Method for improving the oxidative stability of industrial fluids |
| US7648948B2 (en) | 2005-04-08 | 2010-01-19 | Exxonmobil Chemical Patents Inc. | Additive system for lubricants |
| CN101218331B (en) * | 2005-04-26 | 2013-04-24 | 可再生润滑油有限公司 | High temperature biobased lubricant compositions comprising boron nitride |
| US7754664B2 (en) * | 2006-09-19 | 2010-07-13 | Ut-Battelle, Llc | Lubricants or lubricant additives composed of ionic liquids containing ammonium cations |
| EP2067846B1 (en) * | 2006-09-28 | 2012-03-21 | Idemitsu Kosan Co., Ltd. | Lubricating oil composition |
| EP2121880B1 (en) * | 2006-11-30 | 2013-01-30 | R.T. Vanderbilt Company, Inc. | Vegetable oil lubricating composition |
| AU2010206868B2 (en) * | 2009-01-20 | 2016-11-03 | The Lubrizol Corporation | Hydraulic composition with improved wear properties |
| US20100210487A1 (en) * | 2009-02-16 | 2010-08-19 | Chemtura Coproration | Fatty sorbitan ester based friction modifiers |
| US9296969B2 (en) * | 2009-02-16 | 2016-03-29 | Chemtura Corporation | Fatty sorbitan ester based friction modifiers |
| JP5965231B2 (en) * | 2012-07-12 | 2016-08-03 | 出光興産株式会社 | Lubricating oil composition for shock absorbers |
| KR20150086303A (en) | 2012-11-16 | 2015-07-27 | 바스프 에스이 | Lubricant compositions comprising epoxide compounds to improve fluoropolymer seal compatibility |
| US20140171348A1 (en) * | 2012-12-14 | 2014-06-19 | Exxonmobil Research And Engineering Company | Ionic liquids as lubricating oil base stocks, cobase stocks and multifunctional functional fluids |
| GB201317278D0 (en) * | 2013-09-30 | 2013-11-13 | Croda Int Plc | Gear oil composition |
| JP6050213B2 (en) * | 2013-11-01 | 2016-12-21 | Jxエネルギー株式会社 | Lubricating oil composition |
| WO2016072296A1 (en) * | 2014-11-04 | 2016-05-12 | Jx日鉱日石エネルギー株式会社 | Refrigerator oil |
| US9732301B2 (en) * | 2014-11-05 | 2017-08-15 | Infineum International Limited | Power transmitting fluids with improved materials compatibility |
| JP6669343B2 (en) * | 2015-02-27 | 2020-03-18 | 出光興産株式会社 | Biodegradable lubricating oil composition |
| BR112018001430A2 (en) * | 2015-07-24 | 2018-09-11 | Evonik Oil Additives Gmbh | lubricating oil composition, method for lubricating and reducing friction in an engine |
| KR102401413B1 (en) * | 2015-12-23 | 2022-05-24 | 헨켈 아게 운트 코. 카게아아 | metalworking fluid |
| CN108884407A (en) * | 2016-03-31 | 2018-11-23 | 出光兴产株式会社 | Lubricating oil composition and precision speed reducer using same |
| EP3438232B1 (en) | 2016-03-31 | 2022-05-04 | Idemitsu Kosan Co., Ltd. | Lubricating oil composition, and precision reduction gear using same |
| US9995110B2 (en) * | 2016-06-29 | 2018-06-12 | Peter Kris Cleven | Methods and systems for stimulating and restimulating a well |
| CA3066365A1 (en) * | 2017-06-20 | 2018-12-27 | The Lubrizol Corporation | Lubricating composition |
| CN114657012B (en) * | 2022-04-20 | 2023-01-03 | 卡松科技股份有限公司 | High-cleanness high-pressure ashless anti-wear hydraulic oil composition and preparation method thereof |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1043488A (en) * | 1963-12-27 | 1966-09-21 | Shell Int Research | Lubricating oil compositions containing organo phosphorus complexes, the preparation thereof and the complexes thus prepared |
| GB1506196A (en) * | 1974-02-11 | 1978-04-05 | Stauffer Chemical Co | Fluids in particular hydraulic fluids containing ammonium salts of phosphorus acids |
| US4431552A (en) * | 1982-11-26 | 1984-02-14 | Chevron Research Company | Lubricant composition containing an alkali-metal borate and a mixture of phosphates, monothiophosphates and dithiophosphates in a critical ratio |
| GB2178446A (en) * | 1985-07-31 | 1987-02-11 | Chevron Res | Lubricant additive composition containing a neutralized mixture of phosphates |
| US5531911A (en) * | 1994-02-11 | 1996-07-02 | The Lubrizol Corporation | Metal free hydraulic fluid with amine salt |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2250049A (en) | 1938-12-19 | 1941-07-22 | Dow Chemical Co | Mixed triaryl thiophosphates |
| US3254027A (en) | 1962-04-05 | 1966-05-31 | Sinclair Research Inc | Lubricating oil compositions |
| GB1287331A (en) | 1970-01-29 | 1972-08-31 | Ciba Geigy Uk Ltd | Phosphorodithioic acid esters |
| US4333841A (en) | 1978-10-19 | 1982-06-08 | Ciba-Geigy Corporation | Dithiophosphate lubricant additives |
| US4544492A (en) | 1983-05-09 | 1985-10-01 | Ciba-Geigy Corporation | Lubricant compositions |
| ES2055156T3 (en) | 1988-11-08 | 1994-08-16 | Ciba Geigy Ag | LUBRICATING COMPOSITIONS. |
| TW279839B (en) | 1992-06-02 | 1996-07-01 | Ciba Geigy Ag | |
| JPH0680981A (en) * | 1992-08-31 | 1994-03-22 | Tonen Corp | Lubricating oil composition for internal combustion engine |
| WO1995007964A1 (en) * | 1993-09-13 | 1995-03-23 | Exxon Research And Engineering Company | Lubricant composition containing antiwear additive combination |
| JPH08183986A (en) | 1994-12-27 | 1996-07-16 | Tonen Corp | Fluid composition for fluid coupling |
-
1998
- 1998-09-09 DE DE59813902T patent/DE59813902D1/en not_active Expired - Lifetime
- 1998-09-09 EP EP98810894A patent/EP0903399B1/en not_active Expired - Lifetime
- 1998-09-18 KR KR1019980038646A patent/KR100538777B1/en not_active IP Right Cessation
- 1998-09-18 JP JP28339698A patent/JP4114180B2/en not_active Expired - Fee Related
-
2001
- 2001-08-17 US US09/932,762 patent/US6531429B2/en not_active Expired - Lifetime
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1043488A (en) * | 1963-12-27 | 1966-09-21 | Shell Int Research | Lubricating oil compositions containing organo phosphorus complexes, the preparation thereof and the complexes thus prepared |
| GB1506196A (en) * | 1974-02-11 | 1978-04-05 | Stauffer Chemical Co | Fluids in particular hydraulic fluids containing ammonium salts of phosphorus acids |
| US4431552A (en) * | 1982-11-26 | 1984-02-14 | Chevron Research Company | Lubricant composition containing an alkali-metal borate and a mixture of phosphates, monothiophosphates and dithiophosphates in a critical ratio |
| GB2178446A (en) * | 1985-07-31 | 1987-02-11 | Chevron Res | Lubricant additive composition containing a neutralized mixture of phosphates |
| US5531911A (en) * | 1994-02-11 | 1996-07-02 | The Lubrizol Corporation | Metal free hydraulic fluid with amine salt |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002102945A1 (en) * | 2001-06-14 | 2002-12-27 | Ciba Specialty Chemicals Holding Inc. | Improved antiwear performance of engine oils with $g(b)-dithiophosphorylated propionic acids |
| EP1529830A1 (en) * | 2003-11-04 | 2005-05-11 | Chevron Oronite S.A. | Ashless additive formulations suitable for hydraulic oil applications |
| WO2006100188A1 (en) * | 2005-03-21 | 2006-09-28 | Ciba Specialty Chemicals Holding Inc. | Antiwear lubricant compositions for use in combustion engines |
| CN101146898B (en) * | 2005-03-21 | 2012-01-11 | 西巴特殊化学品控股有限公司 | Antiwear lubricant compositions for use in combustion engines |
| EP2147967A1 (en) * | 2008-07-14 | 2010-01-27 | Afton Chemical Corporation | Thermally stable zinc-free antiwear agent |
| WO2011035865A1 (en) * | 2009-09-23 | 2011-03-31 | Cognis Ip Management Gmbh | Lubricant compositions |
| EP2305782A1 (en) * | 2009-09-23 | 2011-04-06 | Cognis IP Management GmbH | Lubricant compositions |
| US8969267B2 (en) | 2009-09-23 | 2015-03-03 | Cognis Ip Management Gmbh | Lubricant compositions |
| CN112080333A (en) * | 2020-08-27 | 2020-12-15 | 统一石油化工有限公司 | Long-life manual transmission oil composition |
| CN112080333B (en) * | 2020-08-27 | 2022-09-13 | 统一石油化工有限公司 | Long-life manual transmission oil composition |
Also Published As
| Publication number | Publication date |
|---|---|
| JP4114180B2 (en) | 2008-07-09 |
| US20020016266A1 (en) | 2002-02-07 |
| KR100538777B1 (en) | 2006-04-28 |
| DE59813902D1 (en) | 2007-03-29 |
| KR19990029924A (en) | 1999-04-26 |
| US6531429B2 (en) | 2003-03-11 |
| EP0903399B1 (en) | 2007-02-14 |
| JPH11217577A (en) | 1999-08-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0903399B1 (en) | Lubricant compositions containing thiophosphoric and dithiophosphoric acid esters | |
| EP0819754B1 (en) | Beta-dithiophosphorilated propionic acid in lubricants | |
| DE60029049T2 (en) | Stabilized hydrorefined and hydrodewaxed lubricant compositions | |
| DE4317943B4 (en) | Dithiophosphoric acid derivatives as lubricant additives | |
| DE10145952B4 (en) | Lubricant containing 5-tert.-butyl-4-hydroxy-3-methylphenyl substituted fatty acid esters and their use | |
| EP1861485B1 (en) | Antiwear lubricant compositions for use in combustion engines | |
| EP1713891B1 (en) | Lubricant compositions comprising an antioxidant blend | |
| EP2142624A2 (en) | Lubricant blend composition | |
| US8329625B2 (en) | Aqueous functional fluids with antioxidants | |
| US20090221458A1 (en) | Succinic acid semi-amides as anti-corrosive agents | |
| MX2009001124A (en) | Lubricant composition. | |
| US7026438B2 (en) | Liquid phenolic sulphur-containing antioxidants | |
| DE19834951A1 (en) | Heterocyclic thioethers as additives for lubricants | |
| EP0849282A2 (en) | Multifunctional polymeric lubricant additives | |
| DE4317980B4 (en) | Bis-Dithiophosphorsäurederivate as lubricant additives | |
| EP0894793A1 (en) | Phosphorus-free multifunctional lubricant additives | |
| EP0595771B1 (en) | Phosphorfree lubricant-additives | |
| DE102015204009B4 (en) | Use of a lubricant composition for lubricating a dual-clutch transmission | |
| DE4317911A1 (en) | Trisamidodithionodiphosphate | |
| US20110212864A1 (en) | Benzotriazole compositions |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): DE FR GB IT SE |
|
| AX | Request for extension of the european patent |
Free format text: AL;LT;LV;MK;RO;SI |
|
| AKX | Designation fees paid |
Free format text: DE FR GB IT SE |
|
| 17P | Request for examination filed |
Effective date: 19990820 |
|
| 17Q | First examination report despatched |
Effective date: 20010308 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: C10M 141/10 20060101AFI20060731BHEP |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): DE FR GB IT SE |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 20070214 |
|
| REF | Corresponds to: |
Ref document number: 59813902 Country of ref document: DE Date of ref document: 20070329 Kind code of ref document: P |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: TRGR |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20071115 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 20120927 Year of fee payment: 15 |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: EUG |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130910 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20150930 Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20150928 Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20150925 Year of fee payment: 18 Ref country code: DE Payment date: 20151130 Year of fee payment: 18 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 59813902 Country of ref document: DE |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20160909 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20170531 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170401 Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160930 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160909 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160909 |