EP0633964B1 - Feuille antistatique - Google Patents
Feuille antistatique Download PDFInfo
- Publication number
- EP0633964B1 EP0633964B1 EP93907902A EP93907902A EP0633964B1 EP 0633964 B1 EP0633964 B1 EP 0633964B1 EP 93907902 A EP93907902 A EP 93907902A EP 93907902 A EP93907902 A EP 93907902A EP 0633964 B1 EP0633964 B1 EP 0633964B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- sheet
- conductive
- layer
- pigments
- sheet according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 239000000049 pigment Substances 0.000 claims abstract description 53
- XOLBLPGZBRYERU-UHFFFAOYSA-N tin dioxide Chemical compound O=[Sn]=O XOLBLPGZBRYERU-UHFFFAOYSA-N 0.000 claims abstract description 12
- 229910001887 tin oxide Inorganic materials 0.000 claims abstract description 12
- 239000011230 binding agent Substances 0.000 claims abstract description 10
- 229910052618 mica group Inorganic materials 0.000 claims abstract description 9
- 239000002245 particle Substances 0.000 claims abstract description 5
- 239000005995 Aluminium silicate Substances 0.000 claims abstract description 3
- 235000012211 aluminium silicate Nutrition 0.000 claims abstract description 3
- 235000012216 bentonite Nutrition 0.000 claims abstract description 3
- 239000011521 glass Substances 0.000 claims abstract description 3
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 claims abstract description 3
- 239000000454 talc Substances 0.000 claims abstract description 3
- 235000012222 talc Nutrition 0.000 claims abstract description 3
- 229910052623 talc Inorganic materials 0.000 claims abstract description 3
- 229910044991 metal oxide Inorganic materials 0.000 claims description 10
- 150000004706 metal oxides Chemical class 0.000 claims description 10
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims description 6
- 239000010445 mica Substances 0.000 claims description 6
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 claims description 5
- 239000000377 silicon dioxide Substances 0.000 claims description 3
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 claims description 3
- 229910052901 montmorillonite Inorganic materials 0.000 claims 1
- 229910052787 antimony Inorganic materials 0.000 abstract description 9
- WATWJIUSRGPENY-UHFFFAOYSA-N antimony atom Chemical compound [Sb] WATWJIUSRGPENY-UHFFFAOYSA-N 0.000 abstract description 9
- 239000000758 substrate Substances 0.000 abstract description 3
- 239000000123 paper Substances 0.000 description 18
- 239000000835 fiber Substances 0.000 description 8
- 239000000853 adhesive Substances 0.000 description 7
- 230000001070 adhesive effect Effects 0.000 description 7
- 239000006061 abrasive grain Substances 0.000 description 6
- 239000006229 carbon black Substances 0.000 description 5
- 229910052751 metal Inorganic materials 0.000 description 5
- 239000002184 metal Substances 0.000 description 5
- 229920005989 resin Polymers 0.000 description 5
- 239000011347 resin Substances 0.000 description 5
- 150000003839 salts Chemical class 0.000 description 5
- 239000012736 aqueous medium Substances 0.000 description 4
- 239000003795 chemical substances by application Substances 0.000 description 4
- 239000004020 conductor Substances 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 229920002451 polyvinyl alcohol Polymers 0.000 description 4
- 235000019422 polyvinyl alcohol Nutrition 0.000 description 4
- 239000004372 Polyvinyl alcohol Substances 0.000 description 3
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 3
- 229910052500 inorganic mineral Inorganic materials 0.000 description 3
- 239000011707 mineral Substances 0.000 description 3
- 239000000203 mixture Substances 0.000 description 3
- -1 polyethylene Polymers 0.000 description 3
- 230000001105 regulatory effect Effects 0.000 description 3
- 229920000049 Carbon (fiber) Polymers 0.000 description 2
- 229920000877 Melamine resin Polymers 0.000 description 2
- 229920002472 Starch Polymers 0.000 description 2
- 238000005299 abrasion Methods 0.000 description 2
- 239000003082 abrasive agent Substances 0.000 description 2
- 239000004917 carbon fiber Substances 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 239000000428 dust Substances 0.000 description 2
- 239000002655 kraft paper Substances 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 229910001092 metal group alloy Inorganic materials 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 150000003242 quaternary ammonium salts Chemical class 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 229920001187 thermosetting polymer Polymers 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 239000003232 water-soluble binding agent Substances 0.000 description 2
- KXGFMDJXCMQABM-UHFFFAOYSA-N 2-methoxy-6-methylphenol Chemical compound [CH]OC1=CC=CC([CH])=C1O KXGFMDJXCMQABM-UHFFFAOYSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 229920003043 Cellulose fiber Polymers 0.000 description 1
- 206010014357 Electric shock Diseases 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 229920000914 Metallic fiber Polymers 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 239000002518 antifoaming agent Substances 0.000 description 1
- 239000002216 antistatic agent Substances 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 239000007767 bonding agent Substances 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 239000011111 cardboard Substances 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 229920000547 conjugated polymer Polymers 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 239000002019 doping agent Substances 0.000 description 1
- 230000005611 electricity Effects 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- IVJISJACKSSFGE-UHFFFAOYSA-N formaldehyde;1,3,5-triazine-2,4,6-triamine Chemical compound O=C.NC1=NC(N)=NC(N)=N1 IVJISJACKSSFGE-UHFFFAOYSA-N 0.000 description 1
- 239000003365 glass fiber Substances 0.000 description 1
- 239000010439 graphite Substances 0.000 description 1
- 229910002804 graphite Inorganic materials 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 238000005470 impregnation Methods 0.000 description 1
- 229910052738 indium Inorganic materials 0.000 description 1
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 description 1
- 238000003475 lamination Methods 0.000 description 1
- 239000004816 latex Substances 0.000 description 1
- 229920000126 latex Polymers 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 239000002557 mineral fiber Substances 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 239000011087 paperboard Substances 0.000 description 1
- 239000005011 phenolic resin Substances 0.000 description 1
- 229920001568 phenolic resin Polymers 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920000767 polyaniline Polymers 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920000128 polypyrrole Polymers 0.000 description 1
- 229920000123 polythiophene Polymers 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 125000001453 quaternary ammonium group Chemical group 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 230000003068 static effect Effects 0.000 description 1
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B1/00—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors
- H01B1/20—Conductive material dispersed in non-conductive organic material
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H19/00—Coated paper; Coating material
- D21H19/36—Coatings with pigments
- D21H19/38—Coatings with pigments characterised by the pigments
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H21/00—Non-fibrous material added to the pulp, characterised by its function, form or properties; Paper-impregnating or coating material, characterised by its function, form or properties
- D21H21/14—Non-fibrous material added to the pulp, characterised by its function, form or properties; Paper-impregnating or coating material, characterised by its function, form or properties characterised by function or properties in or on the paper
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/24—Structurally defined web or sheet [e.g., overall dimension, etc.]
- Y10T428/24802—Discontinuous or differential coating, impregnation or bond [e.g., artwork, printing, retouched photograph, etc.]
- Y10T428/24851—Intermediate layer is discontinuous or differential
- Y10T428/24868—Translucent outer layer
- Y10T428/24884—Translucent layer comprises natural oil, wax, resin, gum, glue, gelatin
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/24—Structurally defined web or sheet [e.g., overall dimension, etc.]
- Y10T428/24802—Discontinuous or differential coating, impregnation or bond [e.g., artwork, printing, retouched photograph, etc.]
- Y10T428/24893—Discontinuous or differential coating, impregnation or bond [e.g., artwork, printing, retouched photograph, etc.] including particulate material
- Y10T428/24901—Discontinuous or differential coating, impregnation or bond [e.g., artwork, printing, retouched photograph, etc.] including particulate material including coloring matter
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/31504—Composite [nonstructural laminate]
- Y10T428/31971—Of carbohydrate
- Y10T428/31993—Of paper
Definitions
- the invention relates to a sheet of paper having properties. antistatic.
- Such a sheet can be used in various fields. It can be used to make items for which it it is necessary to dissipate the electrostatic charges produced when in use or for items which have as primary or secondary function of dissipating charges forming electrostatic and can be dangerous in a given environment.
- Flexible abrasives consist of a support sheet on which abrasive grains are bonded with an adhesive for which it is necessary to dissipate the electrostatic charges created during their use.
- the abrasive is not treated to dissipate the charges, the dust formed during the abrasion of an object is deposited and fouls the abrasive grains; it then reduces the abrasion yield.
- workers can suffer electric shocks which make them react with uncontrolled gestures putting them in danger during their work.
- abrasives In the field of abrasives we treat with a conductive product the support or the layer of adhesive or the surface of the grains abrasive.
- conductive products salts have been used quaternary ammonium, carbon black, powders or fibers metals, metal alloys, metal salts, conductive doped polymers or rendered mineral pigments conductors by coating them with an electrically conductive layer metal oxide.
- the abrasive is made conductive by incorporating carbon black into the adhesive used to bond abrasive grains.
- the abrasive is made conductive by to a layer of a conductive compound which can be a metal, a metallic alloy, metallic pigment, salt or complex metallic, this layer being placed between two layers insulating.
- the conductive product can be put on the back of the support, on the face of the support (below the adhesive), mixed with the adhesive or on the grains.
- an abrasive is made conductive by having a conductive layer on top of the abrasive grains, the conductive product being in particular graphite.
- the abrasive is made conductive by treating the surface of the layer containing the abrasive grains with a solution containing a quaternary ammonium salt.
- the abrasive is made conductive by treating the surface of the layer containing the abrasive grains by a doped conjugated polymer such as for example polythiophene, polyaniline, polypyrrole.
- the paper sheets constituting them are treated with a conductive product.
- the so-called high pressure laminates are produced from a core consisting of a stack of sheets, generally kraft paper, impregnated with a thermosetting resin, in particular a phenolic resin. Once the sheets of kraft paper impregnated with resin, they are dried, cut, then stacked on top of each other; the number of stacked sheets depends on the applications, it generally varies between three and nine.
- a decorative sheet which can be plain, with printed patterns or even presented an iridescent or metallic appearance and being impregnated with a thermosetting resin which does not blacken with heat (for example a resin melamine-formaldehyde).
- a protective covering sheet called “overlay”, also impregnated with a resin, devoid of pattern and transparent in the final laminate, is placed above the decorative sheet.
- the pile of the various types of impregnated sheets is placed in a press provided with a sheet conferring the surface appearance; the assembly is laminated under pressure and hot; an extremely hard unitary structure is obtained which has a decorative effect.
- low pressure laminates are produced in a similar manner to that of high pressure laminates, but the lamination of the decorative sheet directly on a particle board or any other basic support.
- the finished sheet which also belongs to the category of decorative papers.
- This sheet of paper that is pre or post impregnated (usually of a mixture of latex and melamine-formaldehyde resin) is intended to be glued on a particle board or any other support.
- a laminate is made conductive by the fact that part of the leaves constituting the core is conductive by incorporating a material into each sheet electrically conductive such as carbon black, metal or metallic salts or conductive fibers.
- fibers conductive in a paper are carbon fibers, metallic fibers or fibers covered with a metal.
- an anti-static agent for plastics, a conductive pigment obtained by deposition tin or indium on a non-conductive mineral base pigment followed by heating in an oxygen atmosphere to form their oxide.
- the metal oxide is therefore not doped.
- pigments As basic pigments which can be used, many pigments are cited without specifying any advantage linked to a particular pigment or to a geometric form of a family of pigments.
- US-A-5071676 describes a conductive pigment usable for imparting antistatic properties to paper and cardboard.
- This pigment consists of a non-substrate conductor covered with an electroconductive oxide layer antimony-doped tin, itself covered with a layer giving the pigment an isoelectric point between 5 and 9 in order to facilitate its dispersion.
- the substrate can be any, it is not critical to the invention.
- Application EP-A-415478 describes a colored and conductive pigment usable in laminated papers.
- This pigment consists of a base pigment of rutile titanium dioxide and mixed phase coated with a layer of tin oxide doped with antimony.
- Rutile has a spherical geometric shape, so this pigment is spherical.
- a disadvantage of certain products such as doped polymers is their high price.
- a disadvantage of certain products such as quaternary ammonium salts is that it gives the articles a conductivity too low to have a good flow of electrostatic charges.
- Another disadvantage of conductive salts is that the level of conductivity of the products containing them varies with relative humidity.
- a disadvantage of certain conductive products such as aluminum is the sensitivity to water; in the presence of water a dangerous release of hydrogen takes place. They cannot therefore be easily used in an aqueous medium.
- Drawbacks linked to the use of conductive fibers are, on the one hand, the mottled aesthetic appearance given to paper, in particular with carbon fibers, and, on the other hand, the reduction in the physical characteristics of the paper sheet.
- Certain inorganic conductive pigments made conductive by a layer of metal oxide can give a conductivity which is too low to ensure good dissipation of electrostatic charges, especially once used in a paper application.
- the object of the invention is to provide a sheet of paper which has a sufficient level of electrical conductivity giving it antistatic properties.
- Those skilled in the art know from experience that, for an article to effectively dissipate electrostatic charges, it is preferable that its surface resistivity is not greater than about 10 7 ohms, measurement made according to standard ASTM 257-66.
- a second aim is to provide a sheet having antistatic properties which can be produced entirely in an aqueous medium.
- a third object is to provide a sheet having antistatic properties which do not vary with the relative humidity.
- a fourth aim is to provide a sheet having antistatic properties which has a neutral aesthetic appearance, that is to say that the product which will make the sheet conductive must not or only slightly modify the appearance of the sheet.
- Another object is to provide a sheet having antistatic properties whose mechanical characteristics are good.
- Another object is to provide a sheet having antistatic properties which has a low cost price.
- the Applicant has found that the objects of the invention are achieved by making a sheet with pigments conductors having a basic structure of the lamellar type and provided with an electroconductive layer of doped metal oxide.
- the mineral pigments covered with doped metal oxides are known for their electroconductive properties, however the Applicant has found that, for pigments having an electroconductive layer based on the same oxide and for the same dopant, therefore having a priori comparable intrinsic conductivity levels, the sheets of paper containing these pigments have very different final conductivities depending on the basic structure (geometric shape) of the support pigment of the layer.
- the doped metal oxide chosen being tin oxide doped with antimony, this doped oxide is deposited on support pigments of different geometric shapes.
- the conductive pigments were coated in an aqueous medium under the same conditions using the same binder (polyvinyl alcohol PVA) and in the same 1: 1 ratio, on the surface of a sheet of paper.
- the surface resistivity of the sheets was measured according to standard ASTM 257-66 for a relative humidity of 50% (the conductivity of the sheets can be obtained by taking the inverse of the resistivity). From this study it seems that the basic structure of the pigment has an influence on the final conductivity of the sheet.
- the level of resistivity desirable for good dissipation of electrostatic charges (less than 10 7 ohms) is only achieved with pigments having a basic structure of the lamellar type (therefore of flat geometric shape); the resistivity is indeed of the order of 10 5 ohms.
- the invention therefore provides a sheet of paper having antistatic properties characterized by the fact that it contains conductive pigments having a basic structure of lamellar type and provided with at least one electrically conductive layer doped metal oxide.
- the doped metal oxide is a tin oxide spiked with antimony.
- the pigments having a lamellar type structure can be chosen for example from micas, talc, kaolin, bentonites, montmorillionites or glass particles.
- the conductive pigment is a mica coated with a layer of tin oxide doped with antimony.
- the mica pigments covered with a layer of tin oxide doped with antimony have good light transparency, they do not modify the aesthetic appearance of the paper which contains them.
- the pigment conductor is a mica coated with a layer of titanium oxide, optionally with a layer of silica, and coated with a layer of tin oxide doped with antimony.
- These pigments have some iridescence but they have little effect on appearance aesthetics of the paper containing them. It may be interesting to use them in areas where the decorative effect of iridescence is sought after, for example in the field of laminates.
- the conductive pigments can be incorporated en masse during the manufacture of the sheet on the paper machine or be deposited on the surface of the sheet by impregnation in a size press or by any coating means or even by printing.
- the conductive pigments are provided in an aqueous medium.
- the sheet is characterized in that it carries, on at least one face, a layer containing at least the said conductive pigments and at least one binder.
- the binder is a binder usually used in stationery such as water-soluble binders, latexes. It may be advantageous to use a water-soluble binder, such as, for example, polyvinyl alcohols or starches, to obtain an easily repulpable sheet.
- the layer may optionally contain other adjuvants commonly used in stationery such as viscosity regulating agents, for example carboxymethylcellulose, defoamers, etc.
- the quantity of conductive pigments deposited on the surface of the sheet is between 1 and 10 g / m 2 , by dry weight.
- the treated sheet is based on cellulose fibers, it can contain other organic fibers (polyethylene fibers, polypropylene, polyester ...) or mineral fibers such as glass fibers. It can also contain other additives used in stationery as fillers, bonding agents, binders, wet strength agents, retention, anti-foaming agents, regulating agents viscosity, pH regulating agents etc ....
- the invention also provides a flexible abrasive product having antistatic properties which is characterized by the fact that it is supported by said sheet with antistatic properties.
- the abrasive can obviously be in the form of a sheet, but also in other forms such as, for example, continuous tape, disc, etc.
- the sheet used contains the conductive pigments on the surface.
- the conductive pigments can be on the back of the abrasive or on the face carrying the grains, below the adhesive. Since it is known to use a conductive product in the adhesive or on the grains, one can envisage such a use of conductive pigments.
- the invention also relates to a decorative sheet obtained from said sheet with antistatic properties.
- the invention also relates to a laminate having antistatic properties which is characterized in that it comprises at least one sheet like the said sheet with antistatic properties.
- Said sheet can be used as a component of the core of the laminate or as a decorative sheet or possibly as an overlay. Said sheet can also be a finished sheet.
- the pigment-binder ratio is 5: 1.
- Samples are produced with different layer weights of conductive pigments (expressed in dry weight in the table below). The surface resistivity of each sample is measured according to standard ASTM 257-66 and this at relative humidity levels (denoted RH) of 50 and 20%. The results in the table below show that the resistivity of the sheet does not vary with the relative humidity.
- Samples are produced as in Example 1, but a conductive pigment is used, a mica covered with a layer of titanium oxide then with a layer of silica and finally coated with a layer of tin oxide doped with antimony, the tin / antimony ratio being 85:15.
- This pigment is marketed by MERCK. According to the results in the table below, it can be seen that the relative humidity has no influence on the level of conductivity of the sheets obtained according to the invention. The leaves have a slight iridescence which has little effect on their aesthetic appearance.
Landscapes
- Physics & Mathematics (AREA)
- Chemical & Material Sciences (AREA)
- Dispersion Chemistry (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Laminated Bodies (AREA)
- Paper (AREA)
- Polarising Elements (AREA)
- Elimination Of Static Electricity (AREA)
Description
En effet, si l'abrasif n'est pas traité pour dissiper les charges, la poussière formée lors de l'abrasion d'un objet se dépose et encrasse les grains abrasifs; elle diminue alors le rendement en abrasion. Par ailleurs les ouvriers peuvent subir des chocs électriques qui les font réagir avec des gestes incontrôlés les mettant en danger lors de leur travail.
Les produits obtenus sont notamment utilisés dans les salles d'opération, les salles blanches ou les salles d'ordinateurs. Dans ces salles il faut éviter que la poussière soit retenue notamment du fait de l'attraction exercée par les charges électrostatiques et il faut éviter aussi des décharges électrostatiques soudaines, il est donc nécessaire que les surfaces se trouvant dans la salle soient traitées pour dissiper régulièrement l'électricité statique.
Rappelons tout d'abord comment sont fabriqués en général les stratifiés en distinguant les deux types de stratifiés existant actuellement, les stratifiés dits haute pression et les stratifiés dits basse pression.
On produit les stratifiés dits haute pression à partir d'une âme constituée d'un empilement de feuilles, généralement du papier kraft, imprégnées d'une résine thermodurcissable, en particulier d'une résine phénolique.
Une fois les feuilles de papier kraft imprégnées de résine, on les sèche, on les découpe, puis on les empile les unes sur les autres; le nombre de feuilles empilées dépend des applications, il varie en général entre trois et neuf.
Ensuite on place sur la pile de feuilles constituant l'âme, une feuille décorative pouvant être unie, à motifs imprimés ou encore présentée un aspect iridescent ou métallique et étant imprégnée d'une résine thermodurcissable ne noircissant pas à la chaleur (par exemple une résine mélamine-formaldéhyde). Parfois on place au-dessus de la feuille décorative, une feuille protectrice de recouvrement, appelée "overlay", également imprégnée d'une résine, dépourvue de motif et transparente dans le stratifié final.
La pile des divers types de feuilles imprégnées est placée dans une presse munie d'une tôle conférant l'aspect de surface; on stratifie l'ensemble sous pression et à chaud; on obtient une structure unitaire extrêmement dure et ayant un effet décoratif.
Ce pigment est constitué d'un pigment de base de dioxyde de titane rutile et à phase mixte revêtu d'une couche d'oxyde d'étain dopé à l'antimoine. Le rutile a une forme géométrique sphérique, ce pigment est donc sphérique.
Un inconvénient de certains produits comme les polymères dopés est leur prix élevé.
Un inconvénient de certains produits comme les sels d'ammonium quarternaires est qu'il confère aux articles une conductivité trop faible pour avoir un bon écoulement des charges électrostatiques.
Un autre inconvénient des sels conducteurs est que le niveau de conductivité des produits les contenant varie avec l'humidité relative.
Un inconvénient de certains produits conducteurs comme par exemple l'aluminium est la sensibilité à l'eau; en présence d'eau il se produit un dégagement dangeureux d'hydrogène. On ne peut donc pas les utiliser aisément en milieu aqueux.
Des inconvénients liés à l'emploi de fibres conductrices sont d'une part l'aspect esthétique chiné donné au papier notamment avec des fibres de carbone et d'autre part la diminution des caractéristiques physiques de la feuille de papier.
Certains pigments conducteurs minéraux rendus conducteurs par une couche d'oxyde métallique peuvent donner une conductivité trop faible pour assurer une bonne dissipation des charges électrostatiques notamment une fois mis en oeuvre dans une application papetière.
L'homme du métier sait par expérience que, pour qu'un article dissipe efficacement les charges électrostatiques, il est préférable que sa résistivité de surface ne soit pas supérieure à environ 107 ohms, mesure faite selon la norme ASTM 257-66.
Un troisième but est de fournir une feuille ayant des propriétés antistatiques qui ne varient pas avec l'humidité relative.
Un quatrième but est de fournir une feuille ayant des propriétés antistatiques qui a un aspect esthéthique neutre c'est-à-dire que le produit qui va rendre la feuille conductrice ne doit pas ou peu modifier l'aspect de la feuille.
Un autre but est de fournir une feuille ayant des propriétés antistatiques dont les caractéristiques mécaniques sont bonnes. Un autre but est de fournir une feuille ayant des propriétés antistatiques qui a un prix de revient peu élevé.
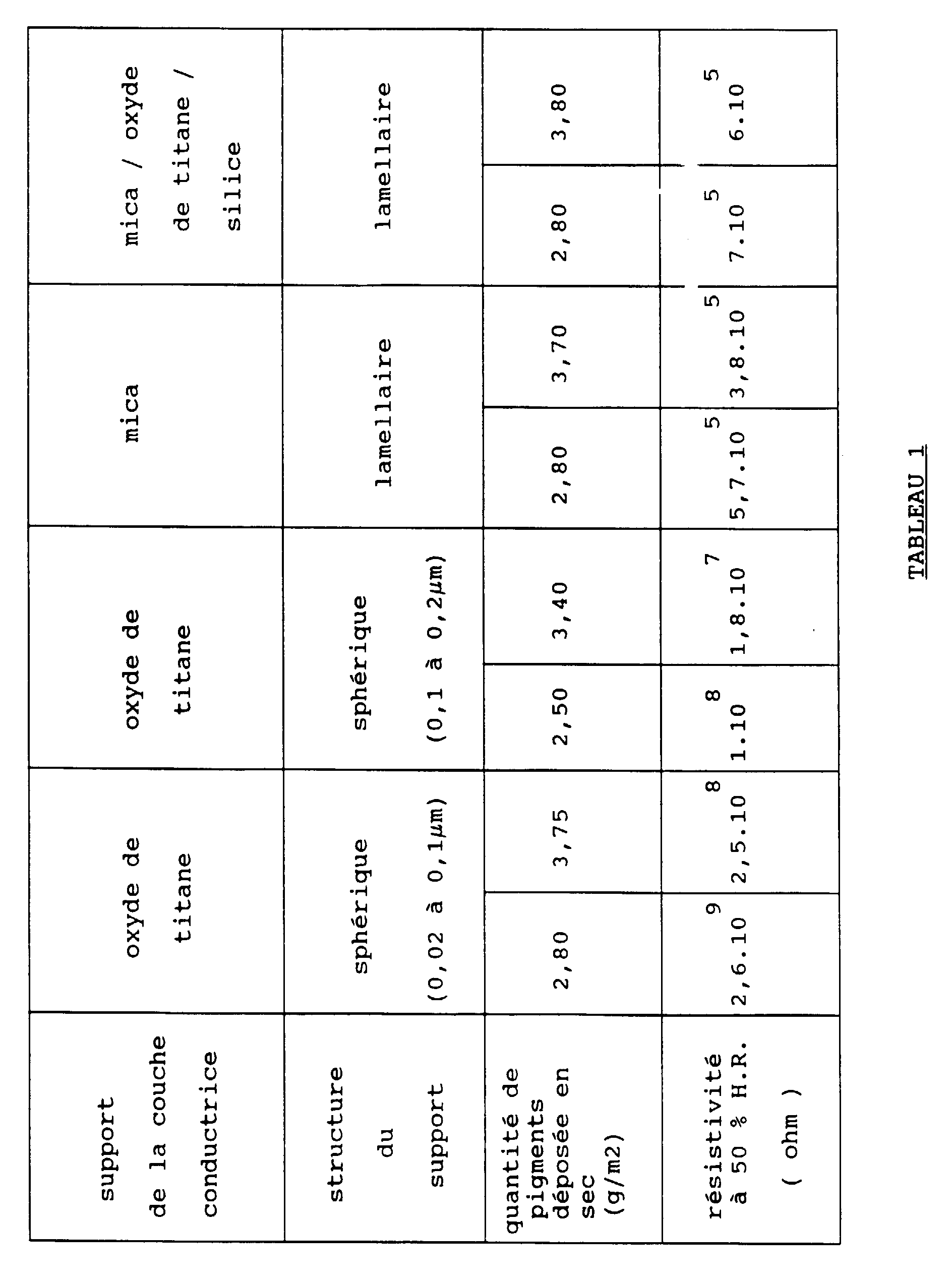
De tels résultats sont montrés dans le tableau 1 annexé, l'oxyde métallique dopé choisi étant l'oxyde d'étain dopé avec de l'antimoine, cet oxyde dopé est déposé sur des pigments supports de formes géométriques différentes.
Les pigments conducteurs ont été couchés en milieu aqueux dans les mêmes conditions à l'aide d'un même liant (l'alcool polyvinylique PVA) et dans le même ratio 1:1, sur la surface d'une feuille de papier.
La résistivité de surface des feuilles a été mesurée selon la norme ASTM 257-66 pour une humidité relative à 50% (la conductivité des feuilles pouvant être obtenue en prenant l'inverse de la résistivité).
De cette étude il semble que la structure de base du pigment ait une influence sur la conductivité finale de la feuille. Le niveau de résistivité souhaitable pour avoir une bonne dissipation des charges électrostatiques (inférieur à 107 ohms) n'est atteint qu'avec les pigments ayant une structure de base de type lamellaire (donc de forme géométrique plate); la résistivité est en effet de l'ordre de 105 ohms.
Dans un cas particulier de l'invention le pigment conducteur est un mica revêtu d'une couche d'oxyde d'étain dopé avec de l'antimoine.
Les pigments de mica recouverts d'une couche d'oxyde d'étain dopé avec de l'antimoine ont une bonne transparence à la lumière, ils ne modifient pas l'aspect esthétique du papier qui les comporte.
De préférence la feuille se caractérise par le fait qu'elle porte, sur au moins une face, une couche contenant au moins les dits pigments conducteurs et au moins un liant.
Le liant est un liant habituellement utilisé en papeterie comme les liants hydrosolubles, les latex.
Il peut être avantageux d'utiliser un liant hydrosoluble, comme par exemple les alcools polyvinyliques ou les amidons, pour obtenir une feuille facilement repulpable.
La couche peut contenir éventuellement d'autres adjuvants utilisés habituellement en papeterie comme les agents régulateurs de viscosité, par exemple la carboxyméthylcellulose, des antimousses, etc... .
De préférence la quantité de pigments conducteurs déposée à la surface de la feuille est comprise entre 1 et 10 g/m2, en poids sec.
De préférence la feuille utilisée comporte les pigments conducteurs en surface. Les pigments conducteurs peuvent être au dos de l'abrasif ou sur la face portant les grains, en dessous de l'adhésif.
Etant donné qu'il est connu d'utiliser un produit conducteur dans l'adhésif ou sur les grains, on peut envisager une telle utilisation des pigments conducteurs.
L'invention concerne également un stratifié ayant des propriétés antistatiques qui se caractérise par le fait qu'il comporte au moins une feuille comme la dite feuille à propriétés antistatiques. La dite feuille peut être utilisée comme composante de l'âme du stratifié ou comme feuille décorative ou éventuellement comme overlay.
La dite feuille peut être aussi une feuille finie.
Sur une autre feuille on couche une composition semblable mais le liant est un PVA.
On réalise des échantillons ayant des poids de couche différents en pigments conducteurs (exprimés en poids sec dans le tableau ci-dessous).
On mesure la résistivité de surface de chaque échantillon selon la norme ASTM 257-66 et ce à des taux d'humidité relative (notée H.R.) de 50 et de 20% . Les résultats dans le tableau ci-dessous montrent que la résistivité de la feuille ne varie pas avec l'humidité relative.
| LIANT | AMIDON | PVA | |||
| POIDS DE COUCHE en pigments (g/m2) | 6,0 | 4,6 | 2,2 | 5,7 | 2,75 |
| Résistivité de surface à 50% H.R. ( ohm ) | 1,2.105 | 5,1.105 | 6,3.105 | 2.105 | 3,6.105 |
| Résistivité de surface à 20% H.R. ( ohm ) | 1,6.105 | 3,8.105 | 6,7.105 | 2.105 | 3,7.105 |
Selon les résultats du tableau ci-dessous on constate que l'humidité relative n'a pas d'influence sur le niveau de conductivité des feuilles obtenues selon l'invention. Les feuilles présentent une légère iridescence qui affecte peu leur aspect esthétique.
| LIANT | AMIDON | PVA | |||
| POIDS DE COUCHE en pigments (g/m2) | 6,0 | 4,1 | 1,9 | 7,4 | 5,0 |
| Résistivité de surface à 50% H.R. ( ohm ) | 0,9.105 | 2,6.105 | 12.105 | 6.104 | 1,3.105 |
| Résistivité de surface à 20% H.R. ( ohm ) | 0,8.105 | 2,6.105 | 15.105 | 9.104 | 2,0.105 |
Claims (10)
- Feuille de papier, ayant des propriétés antistatiques, comportant des pigments conducteurs dotés d'une couche électroconductrice d'oxyde métallique dopé caractérisée par le fait que les dits pigments conducteurs ont une structure de base de type lamellaire.
- Feuille selon la revendication 1, caractérisée par le fait que les pigments ayant une structure lamellaire sont choisis parmi les micas, le talc, le kaolin, les bentonites, les montmorillionites ou les particules de verre.
- Feuille selon les revendications 1 ou 2, caractérisée par le fait que l'oxyde métallique dopé de la couche électroconductrice est un oxyde d'étain dopé avec de l'antimoine.
- Feuille selon les revendications 2 à 3, caractérisée par le fait que le pigment conducteur est un mica revêtu d'une couche d'oxyde d'étain dopé avec de l'antimoine.
- Feuille selon les revendications 2 à 4, caractérisée par le fait que le pigment conducteur est un mica recouvert d'une couche d'oxyde de titane, éventuellement d'une couche de silice, et revêtu d'une couche d'oxyde d'étain dopé avec de l'antimoine.
- Feuille selon les revendications 1 à 5, caractérisée par le fait qu'elle porte sur au moins une face une couche contenant au moins les dits pigments conducteurs et au moins un liant.
- Feuille selon la revendication 6, caractérisée par le fait que la quantité des dits pigments conducteurs déposée sur la feuille est comprise entre 1 et 10 g/m2, en poids sec.
- Abrasif flexible ayant des propriétés antistatiques caractérisé par le fait qu'il a pour support la feuille selon les revendications 1 à 7.
- Feuille décorative caractérisée par le fait qu'elle est obtenue à partir d'une feuille selon les revendications 1 à 7.
- Stratifié ayant des propriétés antistatiques caractérisé par le fait qu'il comporte au moins une feuille selon les revendications 1 à 7 ou 9.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR9204230A FR2689531B1 (fr) | 1992-04-07 | 1992-04-07 | Feuille antistatique. |
| FR9204230 | 1992-04-07 | ||
| PCT/FR1993/000310 WO1993020280A1 (fr) | 1992-04-07 | 1993-03-29 | Feuille antistatique |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0633964A1 EP0633964A1 (fr) | 1995-01-18 |
| EP0633964B1 true EP0633964B1 (fr) | 1998-01-14 |
Family
ID=9428579
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP93907902A Expired - Lifetime EP0633964B1 (fr) | 1992-04-07 | 1993-03-29 | Feuille antistatique |
Country Status (13)
| Country | Link |
|---|---|
| US (1) | US5677039A (fr) |
| EP (1) | EP0633964B1 (fr) |
| AT (1) | ATE162250T1 (fr) |
| AU (1) | AU661902B2 (fr) |
| BR (1) | BR9306198A (fr) |
| DE (1) | DE69316346T2 (fr) |
| DK (1) | DK0633964T3 (fr) |
| ES (1) | ES2111742T3 (fr) |
| FI (1) | FI114651B (fr) |
| FR (1) | FR2689531B1 (fr) |
| MX (1) | MX9302060A (fr) |
| NO (1) | NO302248B1 (fr) |
| WO (1) | WO1993020280A1 (fr) |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE19511012A1 (de) * | 1994-04-06 | 1995-10-12 | Merck Patent Gmbh | Oberflächenmodifiziertes, leitfähiges Pigment |
| ES2187140T3 (es) * | 1998-02-02 | 2003-05-16 | Int Paper Co | Laminado disipativo estatico independiente de la humedad. |
| US6114079A (en) * | 1998-04-01 | 2000-09-05 | Eastman Kodak Company | Electrically-conductive layer for imaging element containing composite metal-containing particles |
| DE60228500D1 (de) * | 2002-06-27 | 2008-10-02 | Upm Kymmene Oyj | Bedrucktes substrat und druckverfahren |
| AU2003299203A1 (en) * | 2002-10-03 | 2004-04-23 | Metss Corporation | Electrostatic charge dissipating hard laminate surfaces |
| DE202004002832U1 (de) * | 2004-02-20 | 2005-06-30 | Kronospan Technical Company Ltd., Engomi | Kohlenstoffhaltiges Papier nebst Paneel |
| US20090176074A1 (en) * | 2006-05-05 | 2009-07-09 | Meadwestvaco Corporation | Conductive/absorbtive sheet materials with enhanced properties |
| DE102008009716B4 (de) * | 2008-02-19 | 2013-08-29 | Nanogate Ag | Verfahren zur Herstellung einer Elektretbeschichtung und die Verwendung der damit hergestellten Beschichtung |
| PL2284019T3 (pl) * | 2009-06-22 | 2017-03-31 | Polska Wytwórnia Papierów Wartościowych S.A. | Papier zabezpieczony do grawerowania laserowego, dokument zabezpieczony i sposób wytwarzania dokumentów zabezpieczonych |
| JP5389568B2 (ja) * | 2009-08-19 | 2014-01-15 | 富士フイルム株式会社 | 透明導電性フィルム |
| ES2602708T3 (es) | 2011-06-21 | 2017-02-22 | Flooring Technologies Ltd. | Tablero de material derivado de la madera y procedimiento de producción |
| WO2013018111A1 (fr) | 2011-08-02 | 2013-02-07 | C.I.M. CALCI IDRATE MARCELLINA SpA | Peinture à l'eau autonettoyante, anti-smog et anti-moisissures à base de matières pulvérulentes ayant des propriétés photocatalytiques |
| ES2827452T3 (es) | 2014-10-10 | 2021-05-21 | Formica Corp | Materiales decorativos de revestimiento multicapa con materiales conductores incorporados, superficies sólidas elaboradas con los mismos, procedimientos para fabricar tales materiales de revestimiento y usos de los mismos |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5834861A (ja) * | 1981-08-27 | 1983-03-01 | Ricoh Co Ltd | 導電性顔料の製造法 |
| JPS6050813A (ja) * | 1983-08-31 | 1985-03-20 | 触媒化成工業株式会社 | プラスチック又は塗料配合用透光性導電性粉末素材 |
| US5071676A (en) * | 1989-08-03 | 1991-12-10 | E. I. Du Pont De Nemours And Company | Electroconductive particles and method for adjusting the isoelectric point thereof |
| DE3929057A1 (de) * | 1989-09-01 | 1991-03-07 | Metallgesellschaft Ag | Elektrisch leitfaehiges rutilmischphasen-pigment, verfahren zu seiner herstellung sowie dessen verwendung |
-
1992
- 1992-04-07 FR FR9204230A patent/FR2689531B1/fr not_active Expired - Fee Related
-
1993
- 1993-03-29 AT AT93907902T patent/ATE162250T1/de not_active IP Right Cessation
- 1993-03-29 US US08/307,792 patent/US5677039A/en not_active Expired - Fee Related
- 1993-03-29 WO PCT/FR1993/000310 patent/WO1993020280A1/fr not_active Ceased
- 1993-03-29 AU AU38929/93A patent/AU661902B2/en not_active Ceased
- 1993-03-29 DE DE69316346T patent/DE69316346T2/de not_active Expired - Lifetime
- 1993-03-29 EP EP93907902A patent/EP0633964B1/fr not_active Expired - Lifetime
- 1993-03-29 DK DK93907902T patent/DK0633964T3/da active
- 1993-03-29 BR BR9306198A patent/BR9306198A/pt not_active IP Right Cessation
- 1993-03-29 ES ES93907902T patent/ES2111742T3/es not_active Expired - Lifetime
- 1993-04-07 MX MX9302060A patent/MX9302060A/es not_active IP Right Cessation
-
1994
- 1994-10-05 FI FI944645A patent/FI114651B/fi active IP Right Grant
- 1994-10-06 NO NO943756A patent/NO302248B1/no unknown
Also Published As
| Publication number | Publication date |
|---|---|
| WO1993020280A1 (fr) | 1993-10-14 |
| DE69316346D1 (de) | 1998-02-19 |
| AU661902B2 (en) | 1995-08-10 |
| BR9306198A (pt) | 1998-06-23 |
| NO943756L (no) | 1994-12-01 |
| ES2111742T3 (es) | 1998-03-16 |
| EP0633964A1 (fr) | 1995-01-18 |
| DK0633964T3 (da) | 1998-09-14 |
| FI944645L (fi) | 1994-10-05 |
| FR2689531A1 (fr) | 1993-10-08 |
| AU3892993A (en) | 1993-11-08 |
| MX9302060A (es) | 1994-07-29 |
| DE69316346T2 (de) | 1998-05-14 |
| NO302248B1 (no) | 1998-02-09 |
| US5677039A (en) | 1997-10-14 |
| FR2689531B1 (fr) | 1994-12-23 |
| NO943756D0 (no) | 1994-10-06 |
| FI114651B (fi) | 2004-11-30 |
| ATE162250T1 (de) | 1998-01-15 |
| FI944645A0 (fi) | 1994-10-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0633964B1 (fr) | Feuille antistatique | |
| US4403224A (en) | Smudge-free electrosensitive recording medium and method of inhibiting smudge formation on said medium | |
| US4606790A (en) | Conductive paper and method | |
| FR2713249A1 (fr) | Feuille papetière pour stratifiés résistant à l'abrasion. | |
| US5384190A (en) | Conductive substrate comprising carbon black and inorganic powders | |
| EP0134811B1 (fr) | Lamine conducteur a haute pression | |
| AU2004225737A1 (en) | Multilayer product and method for the production thereof | |
| CN102634253B (zh) | 镭射铝浆的制备方法 | |
| US4455350A (en) | Conductive laminate sheet material and method of preparation | |
| US4472474A (en) | Electrically conductive laminate | |
| EP0513155B1 (fr) | Feuilles decoratives utilisables notamment pour la fabrication de panneaux stratifies et comprenant des particules metalliques ou des paillettes metallisees | |
| US4645717A (en) | Solution for use in impregnating paper for high-pressure antistatic laminates | |
| US4589954A (en) | Fibrous sheet material for conductive high-pressure laminate | |
| JP6783368B1 (ja) | 金属加工用シート | |
| JP2005533195A (ja) | 装飾積層板用用紙及びその製造方法 | |
| CN105862517A (zh) | 一种电磁屏蔽及抗静电的石头纸墙纸的制备方法 | |
| CN108026701B (zh) | 用于制造多孔式涂层原纸或预浸料的纤维载体材料及其制造方法 | |
| JP2565955B2 (ja) | オフ輪コート原紙の製造方法 | |
| JPS6384935A (ja) | 金属調光沢表面の化粧板とその製造方法 | |
| US5804308A (en) | Heat lag media | |
| JPH04240300A (ja) | 化粧紙および化粧板 | |
| JPS60167997A (ja) | 装飾ラミネ−ト形成に有用な導電紙 | |
| JPH0418542B2 (fr) | ||
| CN205167780U (zh) | 一种牛皮纸板 | |
| TWM366269U (en) | Soft printed circuit board structure |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19941021 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE DK ES FR GB GR IE IT LI LU NL PT SE |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: ARJO WIGGINS S.A. |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| 17Q | First examination report despatched |
Effective date: 19970414 |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE DK ES FR GB GR IE IT LI LU NL PT SE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 19980114 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 19980114 |
|
| REF | Corresponds to: |
Ref document number: 162250 Country of ref document: AT Date of ref document: 19980115 Kind code of ref document: T |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| ITF | It: translation for a ep patent filed | ||
| REF | Corresponds to: |
Ref document number: 69316346 Country of ref document: DE Date of ref document: 19980219 |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 19980129 |
|
| RAP2 | Party data changed (patent owner data changed or rights of a patent transferred) |
Owner name: ARJO WIGGINS S.A. |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2111742 Country of ref document: ES Kind code of ref document: T3 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980329 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980331 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980331 |
|
| NLT2 | Nl: modifications (of names), taken from the european patent patent bulletin |
Owner name: ARJO WIGGINS S.A. |
|
| REG | Reference to a national code |
Ref country code: PT Ref legal event code: SC4A Free format text: AVAILABILITY OF NATIONAL TRANSLATION Effective date: 19980304 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: 78345 |
|
| NLV1 | Nl: lapsed or annulled due to failure to fulfill the requirements of art. 29p and 29m of the patents act | ||
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: T3 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: CJ Ref country code: FR Ref legal event code: CD |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20080319 Year of fee payment: 16 Ref country code: DK Payment date: 20080222 Year of fee payment: 16 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: PT Payment date: 20080225 Year of fee payment: 16 Ref country code: IT Payment date: 20080326 Year of fee payment: 16 Ref country code: IE Payment date: 20080225 Year of fee payment: 16 Ref country code: GB Payment date: 20080307 Year of fee payment: 16 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 20080220 Year of fee payment: 16 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20080411 Year of fee payment: 16 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20080327 Year of fee payment: 16 |
|
| BERE | Be: lapsed |
Owner name: S.A. *ARJO WIGGINS Effective date: 20090331 |
|
| REG | Reference to a national code |
Ref country code: PT Ref legal event code: MM4A Free format text: LAPSE DUE TO NON-PAYMENT OF FEES Effective date: 20090929 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090929 Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090329 |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: EBP |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20090329 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20091130 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090330 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090329 Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20091123 Ref country code: DK Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090331 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20090330 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090330 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090329 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20120323 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 20120322 Year of fee payment: 20 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R071 Ref document number: 69316346 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R071 Ref document number: 69316346 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: EUG |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20130403 |