EP0489726B1 - Verfahren und einrichtung zum dampfkracken von kohlenwasserstoffen in der wirbelschichtphase - Google Patents
Verfahren und einrichtung zum dampfkracken von kohlenwasserstoffen in der wirbelschichtphase Download PDFInfo
- Publication number
- EP0489726B1 EP0489726B1 EP89910120A EP89910120A EP0489726B1 EP 0489726 B1 EP0489726 B1 EP 0489726B1 EP 89910120 A EP89910120 A EP 89910120A EP 89910120 A EP89910120 A EP 89910120A EP 0489726 B1 EP0489726 B1 EP 0489726B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- temperature
- reactor
- particles
- process according
- effluents
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 229930195733 hydrocarbon Natural products 0.000 title claims abstract description 75
- 150000002430 hydrocarbons Chemical class 0.000 title claims abstract description 75
- 238000000034 method Methods 0.000 title claims abstract description 37
- 238000005336 cracking Methods 0.000 title abstract description 10
- 239000002245 particle Substances 0.000 claims abstract description 76
- 239000004215 Carbon black (E152) Substances 0.000 claims abstract description 31
- 238000004821 distillation Methods 0.000 claims abstract description 28
- 238000000926 separation method Methods 0.000 claims abstract description 25
- 238000009835 boiling Methods 0.000 claims abstract description 21
- 150000001875 compounds Chemical class 0.000 claims abstract description 14
- 229910052751 metal Inorganic materials 0.000 claims abstract description 5
- 239000002184 metal Substances 0.000 claims abstract description 5
- 150000002739 metals Chemical class 0.000 claims abstract description 5
- 238000006243 chemical reaction Methods 0.000 claims description 61
- 238000004230 steam cracking Methods 0.000 claims description 45
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N Propane Chemical compound CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 claims description 30
- 239000000571 coke Substances 0.000 claims description 27
- 239000007789 gas Substances 0.000 claims description 25
- OTMSDBZUPAUEDD-UHFFFAOYSA-N Ethane Chemical compound CC OTMSDBZUPAUEDD-UHFFFAOYSA-N 0.000 claims description 21
- 238000002347 injection Methods 0.000 claims description 20
- 239000007924 injection Substances 0.000 claims description 20
- 239000003921 oil Substances 0.000 claims description 16
- 239000001294 propane Substances 0.000 claims description 15
- 239000012071 phase Substances 0.000 claims description 14
- 230000003197 catalytic effect Effects 0.000 claims description 13
- 235000013844 butane Nutrition 0.000 claims description 12
- 239000000203 mixture Substances 0.000 claims description 12
- IJDNQMDRQITEOD-UHFFFAOYSA-N n-butane Chemical class CCCC IJDNQMDRQITEOD-UHFFFAOYSA-N 0.000 claims description 12
- 238000011144 upstream manufacturing Methods 0.000 claims description 12
- 230000001174 ascending effect Effects 0.000 claims description 10
- 238000002485 combustion reaction Methods 0.000 claims description 10
- 239000007788 liquid Substances 0.000 claims description 10
- 239000003208 petroleum Substances 0.000 claims description 10
- 238000004064 recycling Methods 0.000 claims description 10
- 230000003247 decreasing effect Effects 0.000 claims description 7
- 239000001273 butane Substances 0.000 claims description 6
- OFBQJSOFQDEBGM-UHFFFAOYSA-N n-pentane Natural products CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 claims description 6
- 230000000694 effects Effects 0.000 claims description 5
- 239000000446 fuel Substances 0.000 claims description 5
- 239000003209 petroleum derivative Substances 0.000 claims description 5
- 239000002002 slurry Substances 0.000 claims description 4
- 238000005292 vacuum distillation Methods 0.000 claims description 4
- 239000007791 liquid phase Substances 0.000 claims description 3
- 239000011295 pitch Substances 0.000 claims description 3
- 239000010913 used oil Substances 0.000 claims description 2
- 238000004508 fractional distillation Methods 0.000 claims 5
- 238000011109 contamination Methods 0.000 claims 2
- 238000000889 atomisation Methods 0.000 claims 1
- 238000009833 condensation Methods 0.000 claims 1
- 230000005494 condensation Effects 0.000 claims 1
- 230000001172 regenerating effect Effects 0.000 claims 1
- 239000011369 resultant mixture Substances 0.000 claims 1
- 238000005194 fractionation Methods 0.000 abstract description 27
- 238000012546 transfer Methods 0.000 description 32
- 150000001336 alkenes Chemical class 0.000 description 16
- 238000004519 manufacturing process Methods 0.000 description 13
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 13
- 230000008929 regeneration Effects 0.000 description 12
- 238000011069 regeneration method Methods 0.000 description 12
- 230000000171 quenching effect Effects 0.000 description 11
- 238000010791 quenching Methods 0.000 description 10
- 239000003054 catalyst Substances 0.000 description 9
- 239000000686 essence Substances 0.000 description 9
- 239000003502 gasoline Substances 0.000 description 8
- KAKZBPTYRLMSJV-UHFFFAOYSA-N Butadiene Chemical compound C=CC=C KAKZBPTYRLMSJV-UHFFFAOYSA-N 0.000 description 6
- 230000015572 biosynthetic process Effects 0.000 description 6
- 239000003915 liquefied petroleum gas Substances 0.000 description 6
- 239000000047 product Substances 0.000 description 6
- 238000005235 decoking Methods 0.000 description 5
- 239000007787 solid Substances 0.000 description 5
- 238000004227 thermal cracking Methods 0.000 description 5
- 238000004523 catalytic cracking Methods 0.000 description 4
- 239000000945 filler Substances 0.000 description 4
- 238000005507 spraying Methods 0.000 description 4
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- 240000008042 Zea mays Species 0.000 description 3
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 3
- -1 benzene Chemical class 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 238000007796 conventional method Methods 0.000 description 3
- 230000007423 decrease Effects 0.000 description 3
- 238000004090 dissolution Methods 0.000 description 3
- 239000012530 fluid Substances 0.000 description 3
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 3
- 238000006384 oligomerization reaction Methods 0.000 description 3
- 239000001301 oxygen Substances 0.000 description 3
- 229910052760 oxygen Inorganic materials 0.000 description 3
- 230000001737 promoting effect Effects 0.000 description 3
- 238000009834 vaporization Methods 0.000 description 3
- 230000008016 vaporization Effects 0.000 description 3
- 239000005995 Aluminium silicate Substances 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 2
- 239000005977 Ethylene Substances 0.000 description 2
- CPLXHLVBOLITMK-UHFFFAOYSA-N Magnesium oxide Chemical compound [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 2
- QQONPFPTGQHPMA-UHFFFAOYSA-N Propene Chemical compound CC=C QQONPFPTGQHPMA-UHFFFAOYSA-N 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 235000012211 aluminium silicate Nutrition 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 239000000356 contaminant Substances 0.000 description 2
- 239000010779 crude oil Substances 0.000 description 2
- 239000007857 degradation product Substances 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- 230000008030 elimination Effects 0.000 description 2
- 238000003379 elimination reaction Methods 0.000 description 2
- 229940082150 encore Drugs 0.000 description 2
- 238000005243 fluidization Methods 0.000 description 2
- 230000004907 flux Effects 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 2
- 239000004005 microsphere Substances 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- JTJMJGYZQZDUJJ-UHFFFAOYSA-N phencyclidine Chemical class C1CCCCN1C1(C=2C=CC=CC=2)CCCCC1 JTJMJGYZQZDUJJ-UHFFFAOYSA-N 0.000 description 2
- 238000006116 polymerization reaction Methods 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- 230000035484 reaction time Effects 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 229920006395 saturated elastomer Polymers 0.000 description 2
- 229930195734 saturated hydrocarbon Natural products 0.000 description 2
- 239000004575 stone Substances 0.000 description 2
- 238000010408 sweeping Methods 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 125000000383 tetramethylene group Chemical group [H]C([H])([*:1])C([H])([H])C([H])([H])C([H])([H])[*:2] 0.000 description 2
- 230000009466 transformation Effects 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 101100536354 Drosophila melanogaster tant gene Proteins 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- BPQQTUXANYXVAA-UHFFFAOYSA-N Orthosilicate Chemical compound [O-][Si]([O-])([O-])[O-] BPQQTUXANYXVAA-UHFFFAOYSA-N 0.000 description 1
- 241001080024 Telles Species 0.000 description 1
- 239000008186 active pharmaceutical agent Substances 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- 229910000323 aluminium silicate Inorganic materials 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 239000010426 asphalt Substances 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 238000004517 catalytic hydrocracking Methods 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 238000004939 coking Methods 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 239000002283 diesel fuel Substances 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 235000021183 entrée Nutrition 0.000 description 1
- 239000000284 extract Substances 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 239000003517 fume Substances 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 150000002431 hydrogen Chemical class 0.000 description 1
- 238000009434 installation Methods 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 239000000395 magnesium oxide Substances 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 229910044991 metal oxide Inorganic materials 0.000 description 1
- 150000004706 metal oxides Chemical class 0.000 description 1
- 238000005272 metallurgy Methods 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 125000004805 propylene group Chemical group [H]C([H])([H])C([H])([*:1])C([H])([H])[*:2] 0.000 description 1
- 238000010926 purge Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 238000007363 ring formation reaction Methods 0.000 description 1
- 239000000523 sample Substances 0.000 description 1
- 239000009671 shengli Substances 0.000 description 1
- 238000007086 side reaction Methods 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 230000009897 systematic effect Effects 0.000 description 1
- 239000011269 tar Substances 0.000 description 1
- 238000005829 trimerization reaction Methods 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
- 150000003738 xylenes Chemical class 0.000 description 1
- 239000010457 zeolite Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G11/00—Catalytic cracking, in the absence of hydrogen, of hydrocarbon oils
- C10G11/14—Catalytic cracking, in the absence of hydrogen, of hydrocarbon oils with preheated moving solid catalysts
- C10G11/18—Catalytic cracking, in the absence of hydrogen, of hydrocarbon oils with preheated moving solid catalysts according to the "fluidised-bed" technique
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G9/00—Thermal non-catalytic cracking, in the absence of hydrogen, of hydrocarbon oils
- C10G9/002—Cooling of cracked gases
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G9/00—Thermal non-catalytic cracking, in the absence of hydrogen, of hydrocarbon oils
- C10G9/28—Thermal non-catalytic cracking, in the absence of hydrogen, of hydrocarbon oils with preheated moving solid material
- C10G9/32—Thermal non-catalytic cracking, in the absence of hydrogen, of hydrocarbon oils with preheated moving solid material according to the "fluidised-bed" technique
Definitions
- the present invention relates to a steam cracking method and device for converting petroleum hydrocarbon fractions, in the fluidized phase of heat-carrying particles and at high temperature, for the production of olefins and, in particular, olefins comprising 2 to 4 carbon atoms, butadiene and monoaromatic compounds, such as benzene, or which can be branched, such as toluene, xylenes, etc.
- hydrocarbon cracking processes are commonly used in the petroleum and oil services industries; they consist of dividing, by increasing the temperature, hydrocarbon molecules into smaller molecules.
- thermal cracking and catalytic cracking, which involve either the only influence of temperature or the active sites of a catalyst.
- thermocracking reaction is very endothermic. There are therefore problems of temperature regulation and maintenance and, consequently, of selectivity, which are very difficult to solve.
- thermocrack petroleum hydrocarbon fractions which include light paraffins such as butanes, propane and especially ethane, as well as petroleum fractions such as gasolines, naphthas and diesel fuels
- reaction temperature it is necessary to maintain the reaction temperature at a very high level, generally of the order of 750 to 850 ° C, for a very short time, but strictly controlled.
- olefin molecules formed during the conversion could polymerize to the detriment of the overall selectivity of the reaction.
- the present invention aims to remedy these drawbacks by proposing a process for the conversion by steam cracking at high temperature of cuts of petroleum hydrocarbons into olefins such as ethylene, propylene and butenes, butadiene and monoaromatic compounds, by introduction said cuts in a dilute fluidized phase of heat transfer particles and water vapor at high temperature, under reaction conditions of fluidization, temperature and duration strictly determined.
- olefins such as ethylene, propylene and butenes, butadiene and monoaromatic compounds
- the invention also aims to allow satisfactory conversion, by cracking the cuts introduced into the reactor, with a high selectivity for light olefins, butadiene and monoaromatic compounds.
- the invention also aims to allow effective control of the polymerization reactions of the reaction products.
- the invention finally aims to produce coke only reduced quantity, but sufficient to satisfy the thermal balance of the unit.
- the subject of the invention is a process of conversion by steam cracking, at high temperature and in the presence of a dilute fluidized phase of essentially heat-transferable particles, on the one hand, of at least one cut of light hydrocarbons little contaminated with metals, the boiling point of which is less than approximately 400 ° C and, on the other hand, a heavier hydrocarbon charge, consisting essentially of compounds whose boiling point is greater than approximately 400 ° C, this process comprising a step of bringing said cut, then said load, in a staged manner and with decreasing severity, with heat transfer particles, catalytic or not, in a continuous reactor of tubular type with ascending or descending flow , a separation and stripping step making it possible to separate, on the one hand, at least 90% of said particles, which are then preferably regenerated by combustion of the coke deposited on before recycling them at a higher temperature to the feed to said continuous reactor and, on the other hand, the effluent hydrocarbons, which are recovered during a fractionation stage by distillation
- the cut or cuts of lightly contaminated light hydrocarbons distilling at less than 400 ° C. may be advantageously chosen from the group consisting of light paraffins such as ethane, propane and butanes, and heavier hydrocarbons such as essences, naphthas and gas oils, or even certain cuts with higher boiling point, but strongly paraffinic or naphthenic, such as paraffins or slack oil or oil recycles.
- These hydrocarbon fractions can come either from different refinery units, such as atmospheric distillation, visbreaking, hydrocracking, oil manufacturing or olefin oligomerization units, or effluents from the conversion itself.
- These various cuts can also be injected alone or in combination with steam and possibly other fluidizing gases such as hydrogen and light gases.
- the steam cracking will be carried out in the continuous reactor in several zones of decreasing severity, by successive injections in the presence of steam and / or gaseous fluids of several distinct cuts, the first cut must necessarily have a boiling temperature lower than that of the next.
- This temperature profile is in fact particularly advantageous for optimizing the selectivity of the reactions involved. It is possible, for example, to successively inject a first cut containing mainly ethane, then optionally propane and butane, then in the liquid phase , a cut containing light gasolines, then possibly naphthas or gas oils and finally the heavier hydrocarbon charge, having a boiling point higher than about 400 ° C.
- the latter can advantageously be chosen from the group consisting of atmospheric or vacuum distillation residues, deasphalting pitches, catalytic slurries, or synthetic hydrocarbons.
- These charges can therefore be very heavy charges containing hydrocarbons whose boiling point can go up to 750 ° C. and more, and whose density can vary between 0 and 25 ° API.
- the quantity injected of these heavy hydrocarbon charges may advantageously, depending on the desired temperature profile and the needs of the thermal balance, represent 0.25 to 4 times the quantity of light cut injected in upstream.
- the continuous reactor will therefore, in fact, comprise several distinct reaction zones, operating successively under conditions of decreasing severity (decrease in temperature, in duration of contact with the heat transfer mass, the possibly catalytic activity of the heat transfer mass and the ratio between the flow rate of this mass and that of the hydrocarbons) and adapted to the nature of the charges to be treated and of the products sought.
- heavier and heavier cuts for example ethane or LPG (liquefied petroleum gas)
- petrol or diesel liquefied petroleum gas
- the heavier load of the distillation residue type
- the present invention aims to remedy the problems associated with the formation of coke and degradation products in the conduits leading the reaction effluents from the steam cracking units to the fractionation zone of these effluents.
- the temperature of these In order to be able to fractionate the effluent hydrocarbons from steam cracking units of the conventional type by distillation, the temperature of these must be lowered very sharply and above all very quickly, so as to obtain a fractionation tower inlet temperature lower than the "dew point" of effluents (ie at a temperature at which the heaviest fractions condense).
- the heaviest compounds produced by the steam cracking reaction tend to deposit on the wall of the pipes, which results in regular and costly stopping of the steam cracking units for decoking these pipes. .
- the present invention also makes it possible to remedy the drawbacks mentioned above, insofar as the injection of a major part of the heaviest charge necessary for the thermal equilibrium of the steam cracking reaction is carried out after step separation of at least 90% of the particles and of the hydrocarbons and before that of fractionation by distillation.
- the entrained heat transfer particles can be fully recycled and it is then possible, without excessive loss of heat transfer particles, to promote the rapid separation between the heat transfer particles and the gaseous effluents, even if this must be done at the expense of the efficiency of separation.
- an almost instantaneous heat transfer is provided between the heavier hydrocarbon charge and the effluents of the steam cracking reaction by spraying in a manner known per se (see, for this purpose , European Patent No. 312,428) said filler in the liquid state.
- the injector (s) will be adapted to allow spraying of the charge in drops of diameter less than 200 microns and, preferably, 100 microns.
- Said injectors may advantageously be equipped with mixing chambers making it possible to introduce certain quantities of water or steam, or other petroleum fractions, with the charge.
- the quality of the quenching will be optimum, insofar as the temperature of the hydrocarbons entering the fractionation zone and resulting from the dissolution of the steam cracking effluents in the feed. the heaviest to steam will be below the "dew point" of these hydrocarbons.
- the heat transfer thus carried out makes it possible to bring the hydrocarbons to a temperature which will preferably be between 300 and 450 ° C. in less than 0.3 seconds and, preferably, in less than 0.1 seconds.
- the charge of hydrocarbons entering the fractionation zone will have a temperature less than 100 ° C and, preferably, less than 50 ° C than the temperature corresponding to the "bubble point" of said charge (c (i.e. at a temperature where it is in the liquid state, but at which the first bubbles of gaseous hydrocarbons are formed).
- the production of olefins and of monoaromatic compounds can be notably increased by judicious reuse of the saturated hydrocarbons produced during the reaction: it will suffice, for this purpose, to separate from each C2, C3, C4 and other cuts produced, these hydrocarbons saturated with olefins and to recycle the hydrocarbons in the corresponding injection zone of the upstream part of the reactor previously described.
- An additional advantage arising from the present invention resides in the fact that the hydrogen necessarily produced by steam cracking in the upstream part of the reactor is capable of reacting under the reaction conditions of the downstream part of the reactor, and, therefore, of improving the selectivity of the effluents from the conversion unit into better-valued and possibly more stable products.
- the deposition of coke resulting from thermal or catalytic cracking must, for economic reasons, be minimized, but nevertheless be sufficient to ensure the thermal balance in the upstream and downstream parts of the tubular reactor (failing this, the thermal balance can be ensured by introducing an additional fuel into the regenerator); also, at least 50% and preferably 80% by weight of the heavy feed should preferably have a boiling temperature above about 400 ° C. This value of approximately 400 ° C. being mainly linked to the cutting point of the distillation residues, it may of course vary between 300 and 550 ° C. without departing from the scope of the present invention.
- fillers include vacuum gas oils and heavier hydrocarbon oils such as crude oils, whether or not topped, as well as residues from atmospheric distillation or vacuum distillation, pitches, bitumen emulsions, aromatic extracts , catalytic slurries, or synthetic or used oils.
- These fillers may, if necessary, have received a preliminary treatment such as, for example, a hydrotreatment. They may, in particular, contain fractions whose boiling point can go up to 750 ° C. and more, which may contain high percentages of asphaltenic products, and have a carbon content. Conradson high (10% and above).
- lighter cuts which may include cuts of hydrocarbons which have already undergone a cracking operation and which are recycled, such as LCOs ("Light Cycle Oils")
- LCOs Light Cycle Oils
- the interval d boiling is generally between 160-220 ° C (start of cut) and 320-380 ° C (end of cut)
- heavy recycling oils or HCOs Heavy Cycle Oils
- the boiling range is generally between 300-380 ° C (start of cut) and 460-500 ° C (end of cut)
- catalytic residues slurries
- the charges can advantageously be preheated in a temperature range generally between 100 and 400 ° C., preferably close to the bubble point, so as to promote instant and homogeneous vaporization when brought into contact with the hot solid grains.
- inert heat transfer particles of a type known per se such as kaolin or silicate microspheres; it is also possible to use all the classes of catalysts having catalytic cracking capacities.
- a particularly advantageous class is constituted by catalysts having porous structures in which molecules can be brought into contact with active sites located in the pores; in this class, there are in particular silicates or aluminosilicates.
- catalysts comprising stable zeolites are commercially available with supports containing a variety of metal oxides and combinations of said oxides, in particular silica, alumina, magnesia and mixtures of these substances, as well as mixtures of said oxides with clays.
- the catalytic composition can naturally contain one or more agents promoting one or more the other step of the process; the catalyst may therefore contain, in particular, agents promoting the combustion of coke, during regeneration, or contain agents capable of promoting the cyclization of olefins into aromatics and vice versa, if the production of aromatics becomes a priority objective .
- the invention therefore also relates to a steam cracking device, by conversion by direct contact, in the fluidized phase of heat-carrying particles and at high temperature, of petroleum hydrocarbon charges comprising, on the one hand, at least one light section which is little contaminated with metals, the boiling temperature of which is less than approximately 400 ° C.
- this device comprising a continuous reactor for bringing petroleum fractions at high temperature into contact with heat-carrying particles, catalytic or not, the continuous reactor being of tubular type with essentially ascending or descending flow, means in particular ballistic capable of effecting the separation of at least 90 % of said particles and cracked hydrocarbons, means for stripping separate particles, control means generation under combustion conditions of the coke deposited on these particles by air or water vapor, and means for recycling the regenerated particles to the supply of said reactor, as well as means for fractionating the gaseous effluents by distillation, said device being characterized in that it comprises, on the one hand, between said separation means and said fractionation means, means for injecting a fraction of said heavier hydrocarbon charge into the effluents and, on the other hand , means for recycling and injecting at least part of the distillation residue in the liquid phase, but at a temperature close to
- This device may include means for successively injecting into the reactor, from upstream to downstream, light gases comprising ethane, then optionally, propane and / or butanes, in an amount such as the temperature of the mixture with the particles.
- heat transfer is greater than 800 ° C and preferably at 825 ° C, then cuts of hydrocarbons such as light gasolines and / or possibly naphthas and gas oils, in an amount such as the temperature of the resulting mixture, immediately in downstream of the injection point, ie greater than 750 ° C and, preferably, 800 ° C, finally, in the downstream part of the reactor, in the form of fine liquid droplets with an average diameter preferably less than 100 microns, the or heavier hydrocarbon charges.
- the injectors of the fractionation residues recycled in the downstream part of the continuous reactor will be adapted to allow spraying of the charge in drops with a diameter of less than 200 microns and, preferably, less than 100 microns. They will preferably be of the venturi type with a wide neck (see European patent n ° 312 428) to limit as much as possible the problems of attrition linked to the presence of recycled heat transfer particles.
- certain types of devices for separating effluents from steam cracking intended to limit the duration of the transfer of effluents to the fractionation zone by distillation may be more advantageously be used in accordance with the present invention.
- the reactor will operate in ascending mode ("riser")
- the heat-transfer particles will reach the reaction zone at high speeds (between 20 and 200 m / s and, preferably, between 40 and 100 m / s), and a simple device for separation by centrifugation can therefore possibly be used.
- the generally expensive use of cyclonic systems can thus be avoided.
- the heat-transfer particles will be collected in an enclosure arranged at the base of the dropper, where they will be stripped after being separated from the steam cracking effluents by simple ballistic effect.
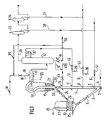
- the ascending fluidized phase steam cracking device shown diagrammatically in FIG. 1 comprises a reaction column 1, known as a load riser, or “riser”. This is supplied at its base, via line 2, with regenerated heat-transfer particles, in a quantity determined for example by the opening or closing of a valve 3.
- the regenerated particles are fluidized by injection at the base of the riser, using a diffuser 5, steam arriving via line 4, or any other suitable gas flow.
- a first line 6 here supplies a diffuser 7, making it possible to inject a saturated light gas such as ethane into the upstream part of the reactor.
- a cut which here is a propane cut, but which could just as well be a butane cut or a mixture of the two, can then be injected identically by line 8 using the diffuser 9.
- a cut of gasoline or diesel can finally be vaporized here using an injector 11 supplied by line 10.
- a charge of distillation residue coming from line 14 from the fractionation zone 12 is introduced, possibly mixed with a little of fresh charge supplied by the line 40, using an injector 13, in the downstream part of the reactor, under temperature conditions close to the bubble point of said residue, in order to facilitate instant and homogeneous vaporization.
- Column 1 opens at its top into an enclosure 15, which is for example concentric with it and in which, on the one hand, thanks to the ballistic separator 16, the separation between the reaction effluents and the heat-carrying particles takes place and, on the other hand, the stripping of coked particles.
- the effluent hydrocarbons are evacuated from the centrifugal system 16 by the evacuation line 17, in which the cold charge arriving by the line 18 is sprayed using the injectors 19, while at least 90% of the heat-carrying particles descend to the base of enclosure 15, where a line 20 supplies stripping gas, generally water vapor, to diffusers 21, regularly arranged at the base of enclosure 15.
- the quenching effect of the effluents from the steam cracking reaction, caused in 17 by the direct contact between the droplets of the fresh charge and said effluents, is here reinforced by the injection by line 50 of a residue recycle of the distillation carried out in the fractionation zone 12.
- the distillation residue can be cooled by passing through a heat exchanger 51 and the calories thus recovered can be used to form water vapor for the entire installation, without that it is necessary to use additional quenching, as is the case with conventional methods.
- the presence of a small amount of grains or fines of the heat transfer solid in the reactor effluents not only allows effective sweeping of the walls, but also constitutes a means of adsorption of the gum precursors and of coke deposits. To this end, it is possible to modify the content of particles circulating in line 17 by providing an injector of fresh particles arriving via line 53.
- the heat transfer particles stripped at the base of the enclosure 15 are discharged to a regenerator 22, via a conduit 23, on which a regulating valve 24 is provided here.
- the regenerator 22 shown in this figure only has '' a single combustion zone for the coke deposited on the heat transfer particles in the presence of oxygen or water vapor. This regeneration is carried out in such a way that a large part of the heat released by the combustion of the coke is transferred to the particles to enable them to reach the high temperatures necessary for the reaction in zone 1.
- the coke deposited on the particles is thus eliminated, using air injected at the base of the regenerator by a line 25, which feeds the diffuser 26.
- additional fuel can be injected.
- the regeneration gas is separated from the heat-transfer particles entrained in the cyclone 27, from which the regeneration gas is evacuated by a line 28, while the regenerated and hot heat-transfer particles are extracted from the regulator, from where they are recycled by the conduit 2 to the elevator supply or riser 1.
- Part of this fractionation residue is therefore injected at 13 into the steam cracking reactor, in accordance with the present invention; depending on the case and after recovery of the heat by passing through the exchanger 51, another part can either be recycled for quenching, by the line 50, in mixture with the charge to be steam cracked, or withdrawn from the device by the purge line 52.
- ethane, propane, gasoline cut, from this fractionation device can be recycled in the reaction section by lines 30, 31, 36, then 6, 8 and 10, while the olefins in C2, C3 produced by steam cracking are isolated by lines 33 and 34 respectively.
- Regulatory systems, 41 to 44 can also make it possible, in a manner known per se, to modulate the quantities injected into the reaction zone, in order to maintain the desired temperature profiles thanks to temperature probes placed for this purpose in said zones. .
- FIGS. 2 and 3 are variants of that of FIG. 1, in which the reaction zone is operating this time in descending mode.
- the reactor will therefore be called “dropper”, by reference to the English term.
- the parts of this device identical to those of FIG. 1 are designated therein by the same reference numerals, assigned the index 'for Figure 2 and the index' 'for Figure 3.
- regenerator a different type of regenerator is used, capable of better withstanding the high temperatures required by steam cracking.
- the regeneration fumes leave the unit in 28 'after passing through a cyclone 27' external to the regeneration chamber 22 '.
- the regeneration chamber 22' is located in the upper part of the unit, and the particles to be regenerated come from the stripping zone by line 23 'must be transported by an ascending column 55'. This transport is carried out by fluidization with a gas diffused in 26 'by line 25'.
- a primary combustion of the coke deposited on the catalyst particles can take place under conditions known per se with a fluidizing gas containing air or oxygen.
- the particles of the catalyst and the fluidizing gas are then separated ballistically by means of the device 56 'and the particles of the catalyst are regenerated in a manner known per se in the chamber 22', where the particles are burned against the flow of oxygen. brought by line 45 'to diffuser 46'.
- the regenerated particles of the catalyst can be introduced without thermal losses into the upstream part of the reactor 1 ′ in an amount determined by the flow rate of the diffuser 57 ′; the homogeneity of the grain distribution is ensured by a device of a type known per se and not shown here.
- the particles dive directly into the dense fluidized stripping zone 15 ′, while the hydrocarbon vapors as well as the stripping vapor coming from the diffuser 21 ′ supplied by the line 20 ′ and the stripped hydrocarbons are evacuated almost instantaneously by line 17 ', where they are immediately quenched by dissolution in the heavy load which enters the unit by line 18'.
- the regenerated and hot particles coming from the line 2 '' are first transported inside an ascending column 58 '' by injection of a fluid such as water vapor arriving via line 4 ''.
- a fluid such as water vapor arriving via line 4 ''.
- the particles are discharged homogeneously into the dropper 1 '', where, for example, are successively injected, in 7 '' and 8 '', from the ethane and gasoline.
- the effluents are quenched by the heavy load in 19 '', and the distillation residue from the fractionation zone 12 '' is injected in 13 '' at a temperature close to its bubble point.
- the particles are stripped and leave zone 15 '' by line 23 '', at the bottom of which an injection of fluid, such as water vapor or air, allows them to be transfer via line 55 '' to the regeneration chamber 22 ''.
- a ballistic separation device 56 '' allows them to be poured into the combustion zone in a fluidized bed.
- This example shows the advantages of a device in accordance with the present invention, of the type represented in FIG. 3.
- the tests were carried out using ethane, a gasoline cut (straight-run cut) and two charges A and B, which are respectively an atmospheric distillation residue and a vacuum distillation residue of a crude oil of the SHENGLI type.
- the charges have the following characteristics: ESSENCE LOAD A LOAD B - density (at 15 ° C) 0.675 0.955 0.985 -% by volume distilled at 50 ° C 20 - - at 70 ° C 70 - - at 100 ° C 99 - - -% by weight distilled at 450 ° C - 20 - at 550 ° C - 45 10 at 650 ° C - 70 55 - P / N / A (% by weight) 77/17/6 - - - H2 (% by weight) 15.4 12.1 11.7 - S (% by weight) - 1.0 1.3 - Total nitrogen (% by weight) - 0.6 0.8 - Carbon (% by weight) - 8.1 14.2 - Ni + V (ppm) - 40 70
- contact mass particles composed of microspheres, mainly kaolin, with a specific surface of approximately 10 m2 / g and an average diameter of approximately 70 microns are used.
- the charge injectors in the quenching zone and in the reactor are of the type described in European patent application No. 312,428.
Landscapes
- Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Physics & Mathematics (AREA)
- Thermal Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Production Of Liquid Hydrocarbon Mixture For Refining Petroleum (AREA)
Claims (19)
- Verfahren zur Umwandlung mindestens einer durch Metalle wenig verunreinigten leichten Kohlenwasserstofffraktion mit einer Siedetemperatur unter etwa 400oC einerseits und einer im wesentlichen aus Verbindungen mit einer Siedetemperatur über etwa 400oC bestehenden schwereren Kohlenwasserstoffcharge andererseits durch Dampfkracken bei hoher Temperatur und in Gegenwart einer verdünnten Wirbelschicht im wesentlichen wärmeführender Teilchen, wobei ein Schritt vorgesehen ist, um die Fraktion und dann die Charge stufenweise und unter Bedingungen abnehmender Härte mit den katalytischen oder nicht katalytischen wärmeführenden Teilchen in einem kontinuierlichen Reaktor vom rohrförmigen Typ mit steigender oder fallender Strömung in Berührung zu bringen, und ein Trenn- und Strippschritt, welcher es ermöglicht, einerseits wenigstens 90 % der Teilchen, die anschließend vorzugsweise durch Reaktion des auf ihnen abgelagerten Kokses vor ihrer Rückführung mit höherer Temperatur zur Speisung des kontinuierlichen Reaktors regeneriert werden, und andererseits die abgehenden Kohlenwasserstoffe, die während eines Schrittes zum Fraktionieren durch Destillation gewonnen werden, zu trennen, dadurch gekennzeichnet, daß mindestens ein Teil der schwereren Kohlenwasserstoffcharge zwischen dem Schritt zur Trennung der Teilchen und der abgehenden Kohlenwasserstoffe und dem Fraktionierschritt eingespritzt wird und daß wenigstens ein Teil des Rückstandes des Schrittes zum Fraktionieren durch Destillation in den stromabwärts gelegenen Teil des Reaktors rückgeführt wird.
- Verfahren nach Anspruch 1, dadurch gekennzeichnet, daß die schwerere Kohlenwasserstoffcharge in flüssigem Zustand durch Zerstäubung in Tropfen mit einem Durchmesser kleiner als 200 Mikron, vorzugsweise kleiner als 100 Mikron, eingespritzt wird.
- Verfahren nach Anspruch 1 oder 2, dadurch gekennzeichnet, daß die abgehenden Produkte der Dampfkrackreaktion durch Einspritzen der schwereren Charge auf eine Temperatur unter dem Taupunkt gebracht werden.
- Verfahren nach Anspruch 1, 2 oder 3, dadurch gekennzeichnet, daß die in die Fraktionierzone eintretenden Kohlenwasserstoffe zwischen 0,01 und 10 Gewichtsprozent, vorzugsweise zwischen 0,05 und 5 Gewichtsprozent, wärmeführende Teilchen enthalten.
- Verfahren nach Anspruch 1, 2, 3 oder 4, dadurch gekennzeichnet, daß die abgehenden Produkte der Dampfkrackreaktion vor dem Schritt zu ihrer Fraktionierung durch Destillation auf eine Temperatur vorzugsweise zwischen 300 und 450oC gebracht werden.
- Verfahren nach einem der Ansprüche 1 bis 5, dadurch gekennzeichnet, daß die von der Dampfkrackzone abgehenden Produkte in flüssigen Zustand auf eine Temperatur zwischen 300 und 450oC in weniger als 0,3 Sekunde, vorzugsweise in weniger als 0,1 Sekunde, gebracht werden.
- Verfahren nach einem der Ansprüche 1 bis 6, dadurch gekennzeichnet, daß die schwerere Kohlenwasserstoffcharge aus der Gruppe ausgewählt wird, welche aus den Rückständen der Destillation bei Atmosphärendruck bzw. bei Unterdruck, den katalytischen Schlämmen, den Entasphaltierungspechen, den synthetischen Ölen und den Altölen besteht.
- Verfahren nach einem der Ansprüche 1 bis 7, dadurch gekennzeichnet, daß der in den Reaktor rückgeführte Teil des Destillationsrückstandes sich auf einer Temperatur befindet, welche um weniger als 100oC, vorzugsweise um weniger als 50oC, über der Blasenpunkttemperatur des Teils liegt.
- Verfahren nach einem der Ansprüche 1 bis 8, dadurch gekennzeichnet, daß die flüssige schwere Charge wenigstens teilweise aus wenigstens einem Teil des Destillationsrückstandes des Fraktionierschrittes besteht.
- Verfahren nach Anspruch 9, dadurch gekennzeichnet, daß der Destillationsrückstand des Fraktionierschrittes durch Wärmeaustausch beim Verlassen der Fraktionierkolonne abgekühlt wird.
- Verfahren nach einem der Ansprüche 1 bis 10, dadurch gekennzeichnet, daß die leichte Kohlenwasserstofffraktion bzw. die leichten Kohlenwasserstofffraktionen aus der Gruppe ausgewählt wird bzw. werden, welche aus den leichten Paraffinen, wei Äthan, Propan, den Butanen, und den Benzinen, den Naphthas und den Gasölen besteht.
- Verfahren nach Anspruch 11, wobei mehrere leichte Kohlenwasserstofffraktionen in den stromaufwärts gelegenen Teil des Reaktors eingespritzt werden, dadurch gekennzeichnet, daß das Einspritzen stufenweise im Sinne abnehmender Härte der Fraktionen erfolgt.
- Verfahren nach Anspruch 12, dadurch gekennzeichnet, daß in den Reaktor von stromaufwärts nach stromabwärts nacheinander leichte Gase, welche Äthan umfassen, und dann gegebenenfalls Propan und Butane, in einer solchen Menge, daß die Temperatur der Mischung mit den wärmeführenden Teilchen über 800oC, vorzugsweise über 825oC, bleibt, dann Kohlenwasserstofffraktionen, wie leichte Benzine und/oder gegebenenfalls Naphthas oder Gasöle, in einer solchen Menge, daß die Temperatur der Mischung unmittelbar stromabwärts über 750oC, vorzugsweise über 800oC, liegt, und schließlich ein Teil des Destillationsrückstandes eingespritzt werden, um durch letzteres die Reaktionstemperatur auf eine Temperatur zwischen 650 und 750oC zu bringen.
- Verfahren nach einem der Ansprüche 1 bis 13, dadurch gekennzeichnet, daß wenigstens ein Teil des durch Dampfkracken erzeugten Äthans und/oder Propans und/oder Butans zum Reaktor rückgeführt wird.
- Verfahren nach einem der Ansprüche 1 bis 14, dadurch gekennzeichnet, daß der Arbeitsdruck der Reaktionszone zwischen 0,3 und 5 kg/cm² liegt.
- Einrichtung zur Umwandlung von Erdölkohlenwasserstoffchargen, welche einerseits mindestens eine durch Metalle wenig verunreinigte leichte Fraktion mit einer Siedetemperatur unter etwa 400oC und andererseits eine im wesentlichen aus Verbindungen mit einer Siedetemperatur über 400oC bestehende Charge schwererer Kohlenwasserstoffe umfassen, durch Dampfkracken infolge direkter Berührung mit einer Wirbelschicht wärmeführender Teilchen bei hoher Temperatur, wobei ein kontinuierlicher Reaktor (1) vom rohrförmigen Typ mit im wesentlichen steigender oder fallender Strömung vorgesehen ist, um Erdölfraktionen mit katalytischen oder nicht katalytischen wärmeführenden Teilchen bei hoher Temperatur in Berührung zu bringen, ferner Mittel (16), insbesondere solche ballistischer Art, welche zur Abtrennung von Wenigstens 90 % der Teilchen aus den gekrackten Kohlenwasserstoffen in der Lage sind, Mittel (20, 21) zum Strippen der abgetrennten Teilchen, Mittel (22) zum Regenerieren unter Bedingungen, bei denen der auf den Teilchen abgelagerte Koks mit Luft oder Wasserdampf verbrennt, Mittel (2) zum Rückführen der regenerierten Teilchen zur Speisung des Reaktors (1) und Mittel (12) zum Fraktionieren der abgehenden gasförmigen Produkte durch Destillation, dadurch gekennzeichnet, daß einerseits zwischen den Abtrennmitteln (16) und den Fraktioniermitteln (12) Mittel (18, 19) zum Einspritzen eines Teils der schwereren Kohlenwasserstoffcharge in die abgehenden Produkte und andererseits Mittel (14, 13) zum Rückführen und Einspritzen wenigstens eines Teils des Destillationsrückstandes in flüssiger Phase, jedoch mit einer Temperatur in der Nähe seines Blasenpunktes, in den stromabwärts gelegenen Abschnitt des Reaktors (1) vorgesehen sind.
- Einrichtung nach Anspruch 16, dadurch gekennzeichnet, daß die Mittel (18, 19; 14, 13) zum Einspritzen der schwersten Kohlenwasserstoffcharge zwischen den ballistischen Abtrennmitteln (16) und den Fraktioniermitteln (12) einerseits und in den stromabwärts gelegenen Abschnitt des Reaktors (1) andererseits auf der Grundlage des von den Mitteln (12) zum Fraktionieren der von der Reaktionszone abgehenden Produkte durch Destillation stammenden Rückstandes gespeist werden.
- Einrichtung nach Anspruch 16 oder 17, gekennzeichnet durch Mittel (7, 9, 11, 13), um in den Reaktor von stromaufwärts nach stromabwärts nacheinander leichte Gase, welche Äthan umfassen, und dann gegebenenfalls Propan und/oder Butane, in solcher Menge, daß die Temperatur der Mischung mit den wärmeführenden Teilchen über 800oC, vorzugsweise über 825oC, liegt, dann Kohlenwasserstofffraktionen, wie leichte Benzine und/oder gegebenenfalls Naphthas und Gasöle, in solcher Menge, daß die Temperatur der resultierenden Mischung unmittelbar stromabwärts von der Einspritzstelle über 750oC, vorzugsweise über 800oC, liegt, und schließlich die schwerere Kohlenwasserstoffcharge oder die schwereren Kohlenwasserstoffchargen in Form kleiner flüssiger Tröpfchen mit einem mittleren Durchmesser vorzugsweise kleiner als 100 Mikron in den stromabwärts gelegenen Teil des Reaktors einzuspritzen.
- Einrichtung nach Anspruch 16, 17 oder 18, gekennzeichnet durch Mittel (53) zum Einspritzen von frischen körnigen wärmeführenden Teilchen in die vom Dampfkracken abgehenden Produkte.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT89910120T ATE103628T1 (de) | 1989-09-01 | 1989-09-01 | Verfahren und einrichtung zum dampfkracken von kohlenwasserstoffen in der wirbelschichtphase. |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/FR1989/000437 WO1991003527A1 (fr) | 1989-09-01 | 1989-09-01 | Procede et dispositif de vapocraquage d'hydrocarbures en phase fluidisee |
| AU42252/89A AU641367B2 (en) | 1989-09-01 | 1989-09-01 | Method and device for vapor-cracking of hydrocarbons in fluidized phase |
| CA000610736A CA1337477C (fr) | 1989-09-01 | 1989-09-08 | Procede et dispositif de vapocraquage d'hydrocarbures en phase fluidisee |
| US07/836,330 US5538625A (en) | 1989-09-01 | 1992-04-10 | Process and apparatus for the steam cracking of hydrocarbons in the fluidized phase |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0489726A1 EP0489726A1 (de) | 1992-06-17 |
| EP0489726B1 true EP0489726B1 (de) | 1994-03-30 |
Family
ID=27154122
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP89910120A Expired - Lifetime EP0489726B1 (de) | 1989-09-01 | 1989-09-01 | Verfahren und einrichtung zum dampfkracken von kohlenwasserstoffen in der wirbelschichtphase |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US5538625A (de) |
| EP (1) | EP0489726B1 (de) |
| CA (1) | CA1337477C (de) |
| DE (1) | DE68914291T2 (de) |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5904837A (en) * | 1996-10-07 | 1999-05-18 | Nippon Oil Co., Ltd. | Process for fluid catalytic cracking of oils |
| US6045690A (en) * | 1996-11-15 | 2000-04-04 | Nippon Oil Co., Ltd. | Process for fluid catalytic cracking of heavy fraction oils |
| EP0909804B1 (de) * | 1997-10-15 | 2010-09-08 | China Petro-Chemical Corporation | Verfahren zur Herstellung von Ethylen und Propylen durch katalytische Pyrolyse von schweren Kohlenwasserstoffen |
| US6013852A (en) * | 1997-11-21 | 2000-01-11 | Shell Oil Company | Producing light olefins from a contaminated liquid hydrocarbon stream by means of thermal cracking |
| US8105482B1 (en) * | 1999-04-07 | 2012-01-31 | Ivanhoe Energy, Inc. | Rapid thermal processing of heavy hydrocarbon feedstocks |
| US20040238422A1 (en) * | 2003-04-25 | 2004-12-02 | Launer Brian R. | Filter apparatus and associated method |
| US8940955B2 (en) * | 2008-12-19 | 2015-01-27 | Uop Llc | Fluid catalytic cracking system and process |
| US8246914B2 (en) * | 2008-12-22 | 2012-08-21 | Uop Llc | Fluid catalytic cracking system |
| US8726663B2 (en) * | 2010-01-05 | 2014-05-20 | General Electric Company | Combined cycle system employing phase change material |
| KR101389011B1 (ko) * | 2012-03-28 | 2014-04-24 | 주식회사 유니텍스 | 소스 컨테이너 및 기상 증착용 반응로 |
| US9522376B2 (en) * | 2012-06-08 | 2016-12-20 | Uop Llc | Process for fluid catalytic cracking and a riser related thereto |
| US9707532B1 (en) | 2013-03-04 | 2017-07-18 | Ivanhoe Htl Petroleum Ltd. | HTL reactor geometry |
| WO2019164609A1 (en) * | 2018-02-21 | 2019-08-29 | Exxonmobil Chemical Patents Inc. | Fluid bed steam cracking using direct heating |
| US12018220B2 (en) | 2019-05-24 | 2024-06-25 | Eastman Chemical Company | Thermal pyoil to a gas fed cracker furnace |
| US11365357B2 (en) | 2019-05-24 | 2022-06-21 | Eastman Chemical Company | Cracking C8+ fraction of pyoil |
| EP4424800A1 (de) | 2023-03-02 | 2024-09-04 | Indian Oil Corporation Limited | Mit kokshandhabungssystem integriertes vorheizprozessmodul zum dampfkracken von kohlenwasserstoffeinsätzen |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2906695A (en) * | 1956-08-07 | 1959-09-29 | Exxon Research Engineering Co | High temperature short time hydrocarbon conversion process |
| US3579601A (en) * | 1968-06-10 | 1971-05-18 | Exxon Research Engineering Co | Pyrolysis of hydrocarbons |
| US4061562A (en) * | 1976-07-12 | 1977-12-06 | Gulf Research & Development Company | Thermal cracking of hydrodesulfurized residual petroleum oils |
| US4213848A (en) * | 1978-07-27 | 1980-07-22 | Exxon Research & Engineering Co. | Fluid coking and gasification process |
| JPS585225B2 (ja) * | 1978-12-21 | 1983-01-29 | 工業技術院長 | コ−クス粒子の加熱方法 |
| US5045176A (en) * | 1981-05-13 | 1991-09-03 | Ashland Oil, Inc. | Carbometallic oil conversion with ballistic separation |
| US4405445A (en) * | 1981-08-24 | 1983-09-20 | Ashland Oil, Inc. | Homogenization of water and reduced crude for catalytic cracking |
| US4716958A (en) * | 1981-09-01 | 1988-01-05 | Ashland Oil, Inc. | Method and apparatus for cooling fluid solid particles used in a regeneration system |
| US4552645A (en) * | 1984-03-09 | 1985-11-12 | Stone & Webster Engineering Corporation | Process for cracking heavy hydrocarbon to produce olefins and liquid hydrocarbon fuels |
| FR2584732B1 (fr) * | 1985-07-10 | 1988-08-19 | Raffinage Cie Francaise | Procede et dispositif pour le craquage catalytique de charges d'hydrocarbures, avec controle de la temperature de reaction |
| US4836909A (en) * | 1985-11-25 | 1989-06-06 | Research Association For Residual Oil Processing | Process of thermally cracking heavy petroleum oil |
| US5089496A (en) * | 1986-10-31 | 1992-02-18 | Schering Corporation | Benzo[5,6]cycloheptapyridine compounds, compositions and method of treating allergies |
| FR2647804A1 (fr) * | 1989-06-05 | 1990-12-07 | Procedes Petroliers Petrochim | Procede et installation de vapocraquage d'hydrocarbures |
-
1989
- 1989-09-01 DE DE68914291T patent/DE68914291T2/de not_active Expired - Fee Related
- 1989-09-01 EP EP89910120A patent/EP0489726B1/de not_active Expired - Lifetime
- 1989-09-08 CA CA000610736A patent/CA1337477C/fr not_active Expired - Fee Related
-
1992
- 1992-04-10 US US07/836,330 patent/US5538625A/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| DE68914291D1 (de) | 1994-05-05 |
| CA1337477C (fr) | 1995-10-31 |
| EP0489726A1 (de) | 1992-06-17 |
| US5538625A (en) | 1996-07-23 |
| DE68914291T2 (de) | 1994-09-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0323297B1 (de) | Wirbelschichtverfahren zur Kohlenwasserstoffumwandlung | |
| EP1800742B1 (de) | Reaktor mit zwei fluidisierbaren Reaktionsstufen und einem integrierten Gas/Feststofftrennsystem | |
| EP0208609B2 (de) | Verfahren und Einrichtung für das katalytische Kracken von Kohlenwasserstoffen mit Kontrolle der Reaktionstemperatur | |
| EP0489726B1 (de) | Verfahren und einrichtung zum dampfkracken von kohlenwasserstoffen in der wirbelschichtphase | |
| EP1413622B1 (de) | Verfahren zur flüssigen katalytischen Spaltung in zwei integrierten, mit unterschiedlicher Strenge, bei einer Abkühlungszone gefolgten Spaltenzonen | |
| EP1656989B1 (de) | Einrichtung und Verfahren zur katalytischen Spaltung von zwei unterschiedlichen Kohlenwasserstoffeinsätzen | |
| EP1170355B1 (de) | Verfahren und Einrichtung zum Cracken von Kohlenwasserstoffen in zwei aufeinanderfolgenden Reaktionstufen | |
| EP2658950B1 (de) | Katalytisches krackverfahren zur behandlung einer fraktion mit niedrigem gehalt an conradson-kohlenstoff | |
| EP0191695B1 (de) | Verfahren und Einrichtung für die Injektion von Katalysator in ein katalytisches Wirbelschichtkrackverfahren, insbesondere von schweren Einsätzen | |
| FR2659346A1 (fr) | Procede de craquage avec oligomerisation ou trimerisation des olefines presentes dans les effluents. | |
| FR2654435A1 (fr) | Procede et dispositif de mise en contact d'une charge d'hydrocarbures avec des particules solides chaudes, dans un reacteur tubulaire a lit fluidise ascendant. | |
| JPH06322377A (ja) | 高および低コンカーボン成分を含むパラフィンリッチ供給原料を接触的にクラッキングする方法および装置 | |
| EP0485259B1 (de) | Verfahren und Einrichtung für Homogenisierung in ein röhrenformige Kohlenwasserstoff-Krackreaktor mit Wirbelbett von feste Teilchen, von das Gemisch von diese Teilchen und die zu behandeln Kohlenwasserstoffen | |
| JPH0645787B2 (ja) | 炭化水素の接触分解方法 | |
| CA2236839C (fr) | Procede et dispositif de craquage catalytique en lit fluidise d'une charge d'hydrocarbures, mettant en oeuvre une zone de mise en contact amelioree | |
| EP0291408B1 (de) | Dampfspaltungsverfahren in einer Wirbelschicht-Reaktionszone | |
| EP0911379A1 (de) | Verfahren und Einrichtung zum selektiven Verdampfen von Kohlenwasserstoffeinsätzen in katalytischen Kracken | |
| WO1991003527A1 (fr) | Procede et dispositif de vapocraquage d'hydrocarbures en phase fluidisee | |
| FR2682119A1 (fr) | Perfectionnements aux dispositifs de craquage catalytique a l'etat fluide de charges d'hydrocarbures. | |
| FR2628436A1 (fr) | Procede et dispositif de vapocraquage d'hydrocarbures en phase fluidisee de particules caloporteuses | |
| FR2658833A1 (fr) | Procede de craquage a l'etat fluide d'une charge d'hydrocarbures. | |
| EP0265347A1 (de) | Verfahren und Vorrichtung für die katalytische Wirbelschichtspaltung von Kohlenwasserstoffeinsätzen | |
| FR2659976A1 (fr) | Craquage catalytique avec refroidissement brusque. | |
| FR2521157A1 (fr) | Preparation d'une charge de craquage catalytique fluide par vaporisation selective | |
| FR3090683A1 (fr) | Conversion d’un brut pétrolier en lit fluidisé compartimenté |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19920130 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE FR GB IT LI NL SE |
|
| 17Q | First examination report despatched |
Effective date: 19930902 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| ITF | It: translation for a ep patent filed | ||
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE FR GB IT LI NL SE |
|
| REF | Corresponds to: |
Ref document number: 103628 Country of ref document: AT Date of ref document: 19940415 Kind code of ref document: T |
|
| REF | Corresponds to: |
Ref document number: 68914291 Country of ref document: DE Date of ref document: 19940505 |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 19940706 |
|
| EAL | Se: european patent in force in sweden |
Ref document number: 89910120.8 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 19980826 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 19980831 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19980907 Year of fee payment: 10 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990901 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: THE PATENT HAS BEEN ANNULLED BY A DECISION OF A NATIONAL AUTHORITY Effective date: 19990929 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990930 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990930 |
|
| EUG | Se: european patent has lapsed |
Ref document number: 89910120.8 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20050822 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20050912 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20050914 Year of fee payment: 17 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: FC Ref country code: FR Ref legal event code: RN |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20051013 Year of fee payment: 17 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20060930 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20060930 Year of fee payment: 18 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20070401 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20070403 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20060901 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 20070401 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20060901 |
|
| BERE | Be: lapsed |
Owner name: S.A. *TOTAL RAFFINAGE DISTRIBUTION Effective date: 20060930 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20080929 Year of fee payment: 20 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20070901 |