EP0441574B1 - Gleitschienenteil benutzend dispersionsverstärkte Eisen-Chrom-Basis-Legierungen - Google Patents
Gleitschienenteil benutzend dispersionsverstärkte Eisen-Chrom-Basis-Legierungen Download PDFInfo
- Publication number
- EP0441574B1 EP0441574B1 EP91300888A EP91300888A EP0441574B1 EP 0441574 B1 EP0441574 B1 EP 0441574B1 EP 91300888 A EP91300888 A EP 91300888A EP 91300888 A EP91300888 A EP 91300888A EP 0441574 B1 EP0441574 B1 EP 0441574B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- skid
- oxide
- alloy
- dispersion strengthened
- resistance
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C32/00—Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of oxides, carbides, borides, nitrides, silicides or other metal compounds, e.g. oxynitrides, sulfides, whether added as such or formed in situ
- C22C32/001—Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of oxides, carbides, borides, nitrides, silicides or other metal compounds, e.g. oxynitrides, sulfides, whether added as such or formed in situ with only oxides
- C22C32/0015—Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of oxides, carbides, borides, nitrides, silicides or other metal compounds, e.g. oxynitrides, sulfides, whether added as such or formed in situ with only oxides with only single oxides as main non-metallic constituents
- C22C32/0026—Matrix based on Ni, Co, Cr or alloys thereof
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F27—FURNACES; KILNS; OVENS; RETORTS
- F27D—DETAILS OR ACCESSORIES OF FURNACES, KILNS, OVENS OR RETORTS, IN SO FAR AS THEY ARE OF KINDS OCCURRING IN MORE THAN ONE KIND OF FURNACE
- F27D3/00—Charging; Discharging; Manipulation of charge
- F27D3/02—Skids or tracks for heavy objects
- F27D3/026—Skids or tracks for heavy objects transport or conveyor rolls for furnaces; roller rails
- F27D3/028—Roller rails or succession of small sized rollers
Definitions
- the present invention concerns skid rails having a component which is exposed to furnace atmospheres made of a heat-resistant alloy having good strength, and corrosion and oxidation properties at high temperature.
- Steel plates and steel wires are produced by rolling steel pieces called slabs or billets after uniformly heating them in a heating furnace such as a walking beam furnace or pusher furnace. If the temperature of the steel piece is lower at the position where the steel piece contacts the furnace bed than at the remaining positions, then uneven thickness of the rolled steel plate or even cracking may occur. In order to avoid these troubles, it is necessary to raise the temperature of the furnace bed at the position of contact with the heated piece to a temperature near the average heating temperature. Thus, at the highest temperatures of use the furnace bed metal attains a high temperature such as 1300°C or higher.
- super alloys of the oxide-dispersion strengthened type i.e., Ni-based super alloys in which fine particles of an oxide having a high melting point such as Y 2 O 3 are dispersed, are useful as components in gas-turbines and jet-engines (for example, Japanese Patent Publication No. 38665/1981).
- high temperature furnaces it has been proposed to use an oxide-dispersion strengthened type super alloy of the composition consisting of 12.5-20% Cr, up to 1% Al, up to 0.1% C and up to 0.5% (volume) Y 2 O 3 , the balance being Ni, as the material for mesh belts (Japanese Patent Publication No. 9610/1984).
- Ni-based super alloys are easily corroded owing to high temperature sulfidation attack by the sulfur in the heavy oil. Furthermore, Ni-based alloys are expensive, and therefore, it is desirable to construct the skid rails with a less expensive alloy. If an Fe-based alloy having equal performance in skid rail service to that of a Ni-based alloy were available, the above desire would be satisfied.
- Prior art alloys also include those described in JP-A-63157827 and US-A-4427447.
- the former discloses a heat-resistant Co-based alloy useful for the manufacture of furnace parts such as skid rails, the alloy containing ⁇ 0.1% C, ⁇ 0.8% Si ⁇ 0.8% Mn, 25-30% Cr, 20-30% Ni, 0.5-2% Mo, only 10-20% Fe and ⁇ 0.04% P and S. Ceramic particles are dispersed in the alloy.
- the latter document (US-A-4427447) relates to metal powder mixtures containing 0-30 wt % Cr, 0-3 wt % Ti, 0.3-10 wt % Al and 0.3-10 wt % of particles of a specified aluminayttria mixed oxide.
- the mixtures can be mechanically processed into high temperature alloys.
- Cr contents of no higher than 20 wt % are present.
- the general object of the present invention is to provide metal components for furnace construction, particularly, skid rails, of higher performance by using a heat-resistant oxide-dispersion strengthened iron-based alloy (or steel).
- the furnace component according to the present invention comprises a furnace atmosphere contacting surface made of an oxide-dispersion strengthened type heat resistant alloy consisting, apart from impurities, of 25-40 wt % Cr, up to 5 wt % Al, up to 5 wt % Ti, and the balance of Fe, and containing 0.1-2% of fine particles of a high melting point metal oxide dispersed in the ferrite matrix.
- an oxide-dispersion strengthened type heat resistant alloy consisting, apart from impurities, of 25-40 wt % Cr, up to 5 wt % Al, up to 5 wt % Ti, and the balance of Fe, and containing 0.1-2% of fine particles of a high melting point metal oxide dispersed in the ferrite matrix.
- a preferable range of Cr content is 25-35%. Percentages are by weight.
- the high melting point metal oxide may be one or more selected from Y 2 O 3 , ZrO 2 and Al 2 O 3. Y 2 O 3 gives the best results.
- Skid members or rails embodying the invention have been found to exhibit, when used in various furnaces such as heating furnaces for hot processing of steel, excellent properties against heat deformation, oxidation resistance, abrasion resistance, sulfidation resistance and thermal shock resistance, and therefore, can be used for long periods of time. This will decrease maintenance labor of the heating furnaces and facilitates continuous operation thereof. Decreased costs for energy and maintenance result in lower production costs in the hot processing of steel.
- the above mentioned oxide-dispersion strengthened type alloy so-called mechanical alloying technology developed by INCO (The International Nickel Co., Inc.) is useful.
- the technology comprises subjecting powders of metal components and fine crystals of a high melting point metal oxide in a ball mill, for example, a high kinetic energy type ball mill, so as to produce by repeated welding and fracturing a granular product comprising an intimate and uniform mixture of very fine particles of the components.
- the product prepared by mechanical alloying is then compacted and sintered by hot extrusion or hot isostatic pressing and, if necessary, machined to provide the component of the skid rail.
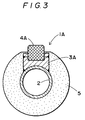
- a typical embodiment of the skid rail of the present invention is, as shown in Figure 1 to Figure 3, a skid rail 1A made by welding metal saddles 3A on a water-cooled skid pipe 2, attaching skid members 4A made of the oxidedispersion strengthened heat-resistant alloy to the saddles and covering all the members except for the skid members 4A with refractory insulator 5.
- the skid rails may be of other configurations.
- a skid structure may use cylindrical saddles to attach button shaped skid members.
- nickel-basedoxide-dispersion strengthened type super alloys are stable even at a high temperature
- the above mentioned known nickel-base alloys have alloy compositions suitable for uses such as turbine blades (Japanese Patent Publication No. 56-38665) or mesh belts (Japanese Patent Publication No. 59-9610) and contain suitable amounts of oxide particles.
- these known nickel-base alloys do not have sufficient corrosion-resistance against high temperature sulfidation attack occurring in furnaces having atmosphere resulting from combustion of heavy oil.
- skid member made of the above described iron-base oxide-dispersion strengthened alloy it is possible to achieve a high compresssion creep strength, as shown in the working example described later, in addition to the heat-resistance and oxidation-resistance. Thus, less expensive, but more durable skid members are provided.
- Criteria associated with the selected alloy compositions employed in the skid members of the present invention are as follows:
- the content of Cr is less than the lower limit, the desired heat-resistance is not obtained. On the other hand, if it exceeds the upper limit, an intermetallic compound called "sigma phase" is formed and the material becomes brittle. Preferable range of Cr content is 25-35%.
- Ti also contributes to the strength of the alloy and, therefore, is optionally added preferably in amounts up to 5%. Additions in amounts over 10% also causes formation of large inclusions.
- the most preferred metal oxide is, as noted above, Y 2 O 3 .
- the whole or a portion of the Y 2 O 3 may be replaced with ZrO 2 or Al 2 O 3 .
- ZrO 2 or Al 2 O 3 is possible.
- Contents of the high melting point metal oxide should be 0.1% or more. Otherwise, the effect of stabilizing the alloy at a high temperature will not be satisfactory. As the content increases, the effect slows down at about 1% and saturates at 2%, and therefore, a suitable content in this range should be chosen.
- Y 2 O 3 may convert to various yttria-alumina compounds (e.g., YAG) if alumina is copresent.
- FIG. 1 to Figure 3 illustrate a typical embodiment of the skid rail using an alloy embodying the invention:
- Oxide-dispersion strengthened type alloys INCOLOY MA956 and improved MA956 groups and having the composition as shown in Table 1 (weight %, the balance being Fe) were prepared by the above noted mechanical alloying process, and the alloys were hot extruded and machined to give testing materials.
- the above obtained materials and a conventional skid rail material "TH101" were subjected to compression creep test at a very high temperature for determining their durability as a material for the skid rail.
- the compression creep test is carried out by cramping a columnar test piece of 3mm in diameter and 6.5mm high between a fitting plate and a receiving plate, and applying compressing load at a high temperature. After a certain period of time, the height of the test piece is measured, and the deformation is calculated as the percentage of decrease in height.
Landscapes
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Mechanical Engineering (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- General Engineering & Computer Science (AREA)
- Heat Treatments In General, Especially Conveying And Cooling (AREA)
- Furnace Charging Or Discharging (AREA)
- Heat Treatment Of Articles (AREA)
- Sliding-Contact Bearings (AREA)
- Pistons, Piston Rings, And Cylinders (AREA)
Claims (5)
- Gleitschienenelement zur Verwendung in einem bei hohen Temperaturen betriebenen Ofen, umfassend zumindest eine der Hochtemperaturatmosphäre des Ofens ausgesetzte Oberfläche, dadurch gekennzeichnet, daß das Gleitschienenelement aus einer mit Oxiddispersion verstärkten, hitzebeständigen Legierung besteht, die abgesehen von Verunreinigungen aus 25-40 Gew.-% Cr, bis zu 5 Gew.-% Al, bis zu 5 Gew.-% Ti und ansonsten aus Fe besteht sowie 0,1-2% Feinteilchen eines hochschmelzenden Metalloxids enthält, die in der Ferritmatrix dispergiert sind.
- Gleitschienenelement nach Anspruch 1, worin das hochschmelzende Metalloxid in der hitzebeständigen Legierung Y2O3 umfaßt.
- Ofengleitschiene mit Gleitschienenelementen (4A) nach Anspruch 1 oder 2, die über Sättel (3A) entlang eines Gleitschienenrohrs (2) befestigt sind.
- Verwendung einer in Anspruch 1 oder 2 definierten, mit Oxiddispersion verstärkten, hitzebeständigen Legierung als Element, das in einem Hochtemperaturofen Hitze und Abrieb ausgesetzt ist.
- Verwendung nach Anspruch 4, worin das Metalloxid Y2O3, ZrO2, Al2O3 ist.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP26967/90 | 1990-02-06 | ||
| JP2026967A JPH03232920A (ja) | 1990-02-06 | 1990-02-06 | 鉄/クロム分散強化合金を使用したスキッドレール |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0441574A1 EP0441574A1 (de) | 1991-08-14 |
| EP0441574B1 true EP0441574B1 (de) | 1997-05-02 |
Family
ID=12207928
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP91300888A Expired - Lifetime EP0441574B1 (de) | 1990-02-06 | 1991-02-04 | Gleitschienenteil benutzend dispersionsverstärkte Eisen-Chrom-Basis-Legierungen |
Country Status (6)
| Country | Link |
|---|---|
| EP (1) | EP0441574B1 (de) |
| JP (1) | JPH03232920A (de) |
| KR (1) | KR100190551B1 (de) |
| AT (1) | ATE152485T1 (de) |
| CA (1) | CA2035634A1 (de) |
| DE (1) | DE69125868T2 (de) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB9206548D0 (en) * | 1992-03-26 | 1992-05-06 | British Ceramic Service Co | Improvements in or relating to kilns |
| DE4337189C2 (de) * | 1993-10-30 | 1995-11-09 | Pm Hochtemperatur Metall Gmbh | Chargiergestell für das Brennen von Gegenständen aus keramischen und glaskeramischen Werkstoffen |
| FR2779806B1 (fr) * | 1998-06-15 | 2000-07-21 | Air Liquide | Bruleur a injecteur perfectionne et procede de fabrication de cet injecteur |
| WO2012016649A1 (de) * | 2010-08-02 | 2012-02-09 | Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. | Verfahren zur herstellung von bauteilen, die endformnah aus einer dispersionsverstärkten eisen- oder nickelbasislegierung gebildet sind |
| KR101429641B1 (ko) * | 2012-12-27 | 2014-08-14 | 주식회사 포스코 | 가열로의 스키드레일 |
| GB201318660D0 (en) * | 2013-10-22 | 2013-12-04 | Materials Ct Leoben Forschung Gmbh | Ferritic alloys and methods for preparing the same |
| JP2018070897A (ja) * | 2015-03-02 | 2018-05-10 | 国立大学法人北海道大学 | 鉄−クロム−アルミニウム系酸化物分散強化型鋼およびその製造方法 |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1162882A (en) * | 1966-02-02 | 1969-08-27 | Gen Electric | Improvements in Chromium-Containing Alloys of Improved Resistance to Oxidation and Nitrification |
| US4427447A (en) * | 1982-03-31 | 1984-01-24 | Exxon Research And Engineering Co. | Alumina-yttria mixed oxides in dispersion strengthened high temperature alloy powders |
| CA1329320C (en) * | 1988-01-26 | 1994-05-10 | Kazuto Terai | Skid rail |
-
1990
- 1990-02-06 JP JP2026967A patent/JPH03232920A/ja active Pending
-
1991
- 1991-02-04 CA CA002035634A patent/CA2035634A1/en not_active Abandoned
- 1991-02-04 DE DE69125868T patent/DE69125868T2/de not_active Expired - Fee Related
- 1991-02-04 EP EP91300888A patent/EP0441574B1/de not_active Expired - Lifetime
- 1991-02-04 AT AT91300888T patent/ATE152485T1/de active
- 1991-02-06 KR KR1019910002050A patent/KR100190551B1/ko not_active Expired - Fee Related
Non-Patent Citations (1)
| Title |
|---|
| edition 1976, Newnes-Butterworths, Londen, GB, page 7:7, lines 19-38 * |
Also Published As
| Publication number | Publication date |
|---|---|
| DE69125868D1 (de) | 1997-06-05 |
| JPH03232920A (ja) | 1991-10-16 |
| KR100190551B1 (ko) | 1999-06-01 |
| DE69125868T2 (de) | 1997-10-16 |
| ATE152485T1 (de) | 1997-05-15 |
| CA2035634A1 (en) | 1991-08-07 |
| KR910015714A (ko) | 1991-09-30 |
| EP0441574A1 (de) | 1991-08-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0510495B1 (de) | Wärmebeständige gesinterte Oxiddispersionsgehärtete Legierung | |
| US4900248A (en) | Skid rail | |
| EP0441574B1 (de) | Gleitschienenteil benutzend dispersionsverstärkte Eisen-Chrom-Basis-Legierungen | |
| EP0429796A1 (de) | Hitzebeständige Werkstoffe | |
| EP0441573B1 (de) | Hitzebeständige Legierung | |
| US5226980A (en) | Skid rail alloy | |
| CA2215447C (en) | Heat-resistant alloy steel for hearth metal members of steel material heating furnaces | |
| KR960010603B1 (ko) | 스키드 레일 | |
| JP2932653B2 (ja) | 加熱炉用スキッドレールとその製造方法 | |
| JP2802768B2 (ja) | 複合焼結合金および加熱炉内鋼材支持部材 | |
| JPS6310114B2 (de) | ||
| JPH04301049A (ja) | 加熱炉内被加熱鋼材支持面部材用耐熱合金 | |
| JPH0693328A (ja) | 酸化物分散強化型合金を用いたハースロール | |
| JPH04301048A (ja) | 加熱炉内被加熱鋼材支持面部材用耐熱合金 | |
| JPH05463B2 (de) | ||
| JPH0394042A (ja) | 高温耐酸化・耐圧縮性材料 | |
| JPH04301047A (ja) | 加熱炉内被加熱鋼材支持面部材用耐熱合金 | |
| JPS6315341B2 (de) | ||
| JPH01222033A (ja) | 炉床部材用耐熱合金 | |
| JPS629180B2 (de) | ||
| JPH10226841A (ja) | 超耐熱クロム基合金および鋼材加熱炉の炉床金物 | |
| JPH06264173A (ja) | 加熱炉における被加熱体支持部材用複合材料 | |
| JPH03211220A (ja) | 耐熱支持部材 | |
| JPS60190544A (ja) | 炉床部材用耐熱合金 | |
| JPH062065A (ja) | 加熱炉内支持面部材用耐熱合金 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE FR GB IT LI SE |
|
| 17P | Request for examination filed |
Effective date: 19920211 |
|
| 17Q | First examination report despatched |
Effective date: 19940606 |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE FR GB IT LI SE |
|
| REF | Corresponds to: |
Ref document number: 152485 Country of ref document: AT Date of ref document: 19970515 Kind code of ref document: T |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REF | Corresponds to: |
Ref document number: 69125868 Country of ref document: DE Date of ref document: 19970605 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20010125 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20010216 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 20010219 Year of fee payment: 11 Ref country code: CH Payment date: 20010219 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20010220 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 20010226 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20010228 Year of fee payment: 11 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020204 Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020204 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020205 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020228 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020228 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020228 |
|
| BERE | Be: lapsed |
Owner name: INCO ALLOYS INTERNATIONAL INC. Effective date: 20020228 Owner name: DAIDO TOKUSHUKO K.K. Effective date: 20020228 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020903 |
|
| EUG | Se: european patent has lapsed |
Ref document number: 91300888.4 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20020204 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20021031 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED. Effective date: 20050204 |