EP0245201A1 - Anode for electrolyses - Google Patents
Anode for electrolyses Download PDFInfo
- Publication number
- EP0245201A1 EP0245201A1 EP87810254A EP87810254A EP0245201A1 EP 0245201 A1 EP0245201 A1 EP 0245201A1 EP 87810254 A EP87810254 A EP 87810254A EP 87810254 A EP87810254 A EP 87810254A EP 0245201 A1 EP0245201 A1 EP 0245201A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- substrate
- titanium
- anode
- framework
- electrochemically active
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B11/00—Electrodes; Manufacture thereof not otherwise provided for
- C25B11/02—Electrodes; Manufacture thereof not otherwise provided for characterised by shape or form
- C25B11/03—Electrodes; Manufacture thereof not otherwise provided for characterised by shape or form perforated or foraminous
- C25B11/031—Porous electrodes
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B11/00—Electrodes; Manufacture thereof not otherwise provided for
- C25B11/04—Electrodes; Manufacture thereof not otherwise provided for characterised by the material
- C25B11/051—Electrodes formed of electrocatalysts on a substrate or carrier
- C25B11/073—Electrodes formed of electrocatalysts on a substrate or carrier characterised by the electrocatalyst material
- C25B11/091—Electrodes formed of electrocatalysts on a substrate or carrier characterised by the electrocatalyst material consisting of at least one catalytic element and at least one catalytic compound; consisting of two or more catalytic elements or catalytic compounds
Definitions
- the invention relates to an anode for aqueous electrolysis, consisting of a framework which is resistant to the electrolyte and the electrolysis products, a porous substrate containing titanium which is firmly connected to the framework and electrochemically active substances which are distributed in the pores of the substrate.
- metal anodes In chlor-alkali electrolysis and other electrolyses with aqueous electrolytes, metal anodes have been used for some time, which essentially contain a framework or a base made of a passivable metal, on which one or more electrochemically active substances are firmly anchored. Usually, because of its availability and the comparatively low price, titanium scaffolds are used which are resistant to the electrolyte and the electrolysis products.
- Preferred electrochemically active substances are oxides of platinum group metals, alone or in mixtures with other metal oxides, spinels, perovskites and other mixed oxides. Coatings that do not contain platinum metal oxides have also become known for special electrolyses.
- the service life of the coated anodes is essentially determined by the resistance of the electrochemically active coating, which also depends on the resistance in contact with mercury, depending on the type of substance and the electrolysis conditions, the adhesion to the metal structure and in chlor-alkali electrolysis in mercury cells.
- Numerous proposals have been published for extending the anode service life, which secure the active substance against damage caused by short circuit, improve their anchoring on the titanium framework and ultimately provide the largest possible amount of the electrochemically active substance. These proposals have in common porous support layers or substrates that are firmly attached to the framework and absorb the electrochemically active substance.
- the porous substrate is a better primer than the more or less smooth surface of the framework, it protects the active substance in the event of short circuits, and its absorption capacity can be adapted to the needs of electrolysis in a wide range via the porosity and thickness of the substrate.
- the substrate consists of various titanium oxides, which are applied to the anode frame in an amount of 100 to 6000 g / m 2 by flame or plasma spraying.
- Oxides of the composition TiO 2-x should behave particularly advantageously, with 0.1>x> 0.
- the porous substrate is impregnated with salts of the solution containing platinum metals, which are thermally decomposed after evaporation of the solvent. It is also known to apply the electrochemically active substance together with oxides, nitrides, phosphides, borides or carbides of a metal from the group of passivatable metals, preferably with titanium oxide, to the surface of the anode structure in a single operation (EP-OS 0 058 832 ).
- Another anode has a substrate which, in addition to titanium oxides, contains oxides of other non-noble metals, such as niobium oxide or nickel oxide (DE-OS 32 08 835). Compounds of at least one element of the platinum group are added to the substrate applied by flame spraying. Finally, a substrate is known which consists of a sintered layer of titanium oxides of the composition TiO x , with 0.25 ⁇ x ⁇ 1.50 (DE-OS 24 12 828). The porous substrate known from DE-OS 20 35 212, sintered onto the support frame, is made of metallic titanium.
- the invention relates to an anode for aqueous electrolysis, consisting of a framework which is resistant to the electrolyte and the electrolysis products, a porous substrate containing titanium connected to the framework and electrochemically active substances which are distributed in the pores of the substrate, characterized in that porous titanium-containing substrate with a metal from the group chromium, nickel is doped.
- the invention is based on the surprising finding that, under the conditions of aqueous electrolysis, titanium doped with chromium and / or nickel also transports the current in the anodic direction, even if it does not contain any electrochemically active substances.
- the passivation is greatly reduced compared to substrates made of titanium or other passivable metals or valve metals. Anodic metal dissolutions are practically not observed.
- the character of the layer according to the invention is comparable to that of a noble metal.
- the proportion of the doping elements added to the titanium can e.g. 0.5 to 40% by weight and is preferably 2 to 20% by weight, especially 2 to 10% by weight.
- the effect of the doping is small below approximately 2%, and above 20%, partial doping of the doping metals can occur under the conditions of oxygen-developing anodes.
- chromium and / or nickel in the form of fine powders can be mixed with powdered titanium and the mixture can be applied to the framework, for example by flame spraying. Under these conditions, mixed crystals of titanium and the doping metal are only formed to a limited extent.
- the powder mixture mixed with a temporary binder is sprayed or brushed onto the framework and a porous sintered layer firmly connected to the framework is formed by heating in an inert atmosphere.
- Mixed crystals can form to a large extent during sintering, but are thermodynamically unstable at room temperature and therefore disintegrate on cooling.
- the functionality of the doped substrates is practically independent of the different manufacturing processes.
- the thickness of the substrate is preferably 0.2 to 1 mm.
- the porosity can be, for example, 20 to 60% by volume, in particular 30 to 50% by volume. With an average porosity of approx. 40% by volume, the substrate has a holding capacity for the electrochemically active substances, which is appropriate for the known aqueous electrolysis.
- the substrate can be impregnated with solutions or suspensions containing these substances.
- the type of electrochemically active substances used is determined in a known manner by the electrolysis conditions. Suitable metals include platinum metals, oxides of platinum metals, spinels, perovskites, ⁇ -manganese dioxide alone or in mixtures.
- Anodes according to the invention are particularly suitable for chloralkali electrolysis and for electrolysis in which oxygen is generated anodically.
- the anodes have a long service life and their reactivation is particularly simple, since obviously no oxides which do not conduct electricity are formed during the electrolysis.
- the anode is reactivated by introducing electrochemically active substances into the porous substrate.
- Example 1 Titanium sheets are degreased, sandblasted and coated with a fine-grained mixture of titanium and chrome powder.
- the mixture contains 9% by weight of chromium and 91% by weight of titanium (maximum grain size 0.1 mm) and is made into an injectable paste with an aqueous tylose solution.
- a 0.5 mm thick layer is applied to the sheets with a gravity cup gun; the sheets are dried at room temperature and, by heating to 1200 ° C. in argon, a porous substrate layer is adhered to the sheets, the porosity of which is approximately 25% by volume.
- titanium sheets without substrates and titanium sheets with undoped substrate layers made of porous sintered titanium were coated with the same amounts of the electrochemically active substances and the service life of the anodes was measured in 20% sulfuric acid at room temperature under the same conditions.
- Example 2 An approximately 0.4 mm thick substrate layer of doped titanium is applied to titanium sheets by flame spraying a mixture containing 9% by weight of nickel and 91% by weight of titanium powder. The grain size of the powder is less than 0.05 mm. As described in Example 1, the substrate layers are impregnated with solutions a, b and c and tested in comparison with anodes which contain the same amount of electrochemically active substances but no substrate or no doped substrate.
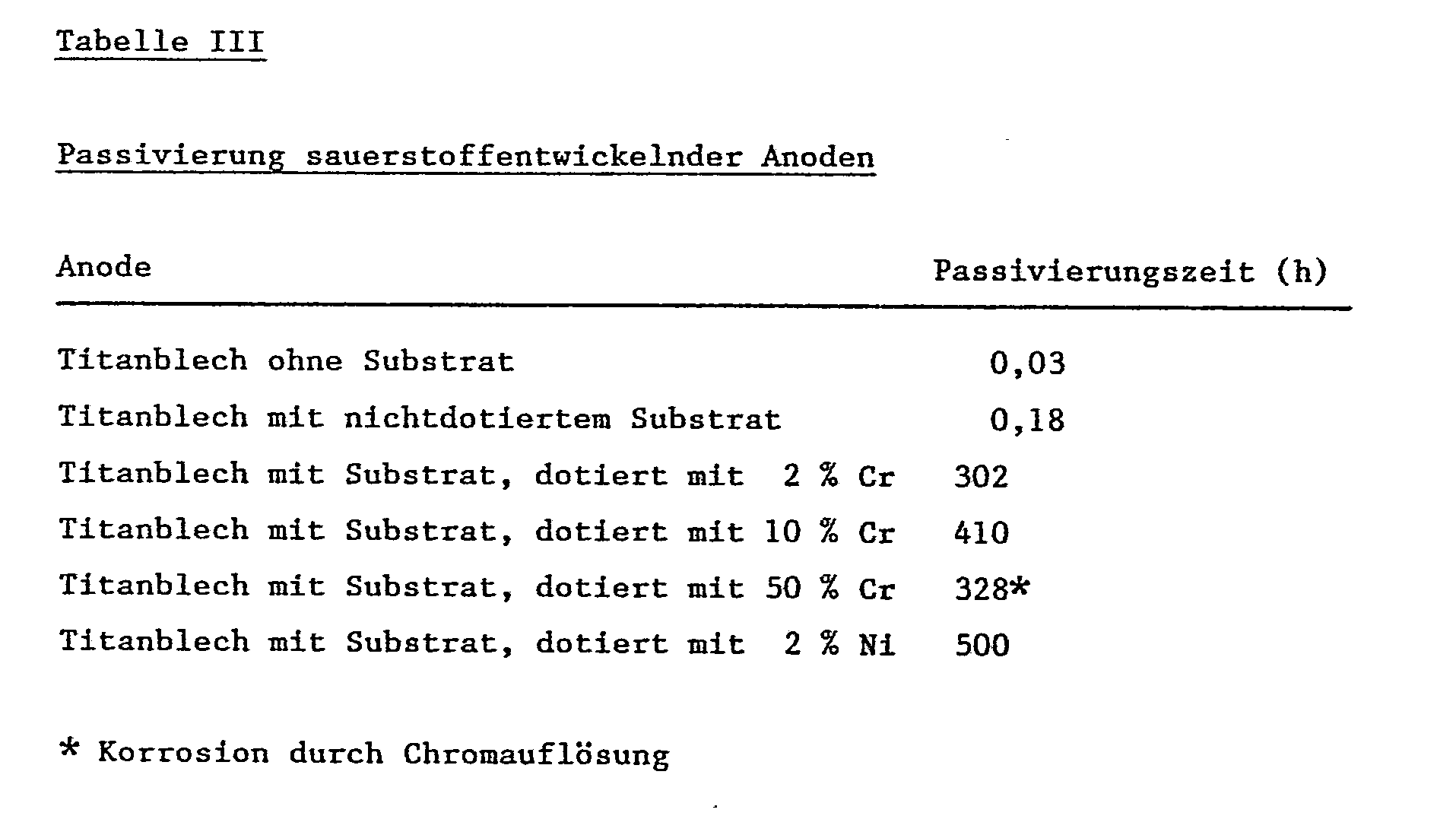
- Example 3 The passivation rate of different anodes, which have no coatings with electrochemically active substances, is measured in 20% sulfuric acid at room temperature and a current density of 0.2 kA / m2. Passivation is indicated by an increase in cell voltage to 10 V.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Electrodes For Compound Or Non-Metal Manufacture (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Secondary Cells (AREA)
Abstract
Anode für wässerige Elektrolysen, bestehend aus einem Gerüst oder Träger und einem mit dem Gerüst verbundenem porösen Substrat, in dem elektrochemisch aktive Substanzen dispergiert sind. Das Substrat besteht aus Titan, das mit Chrom oder Nickel dotiert ist.Anode for aqueous electrolysis, consisting of a framework or carrier and a porous substrate connected to the framework, in which electrochemically active substances are dispersed. The substrate is made of titanium, which is doped with chrome or nickel.
Description
Die Erfindung betrifft eine Anode für wässerige Elektrolysen, bestehend aus einem gegen den Elektrolyten und die Elektrolyseprodukte beständigen Gerüst, einem mit dem Gerüst fest verbundenen, Titan enthaltenden porösen Substrat und elektrochemisch aktiven Substanzen, die in den Poren des Substrates verteilt sind.The invention relates to an anode for aqueous electrolysis, consisting of a framework which is resistant to the electrolyte and the electrolysis products, a porous substrate containing titanium which is firmly connected to the framework and electrochemically active substances which are distributed in the pores of the substrate.
Bei der Chloralkali-Elektrolyse und anderen Elektrolysen mit wässerigen Elektrolyten werden seit geraumer Zeit Metallanoden eingesetzt, die im wesentlichen ein Gerüst oder eine Basis aus einem passivierbaren Metall enthalten, auf dem eine oder mehrere elektrochemisch aktive Substanzen fest verankert sind. Ueblicherweise verwendet man wegen seiner Verfügbarkeit und des vergleichsweise niedrigen Preises Gerüste aus Titan, die gegen den Elektrolyten und die Elektrolyseprodukte beständig sind. Bevorzugte elektrochemisch aktive Substanzen sind Oxide von Metallen der Platingruppe, allein oder in Gemischen mit anderen Metalloxiden, Spinellen, Perowskiten und anderen Mischoxiden. Für spezielle Elektrolysen sind auch Beschichtungen bekanntgeworden, die keine Platinmetalloxide enthalten. Die Lebensdauer der beschichteten Anoden wird im wesentlichen durch die Beständigkeit der elektrochemisch aktiven Beschichtung bestimmt, die von der Art der Substanz und den Elektrolysebedingungen, der Haftung auf dem Metallgerüst und bei der Chloralkali-Elektrolyse in Quecksilberzellen auch von der Beständigkeit im Kontakt mit Quecksilber abhängt. Zur Verlängerung der Anoden-Lebensdauer sind zahlreiche Vorschläge bekanntgeworden, die die aktive Substanz gegen Schädigungen durch Kurzschluss sichern, ihre Verankerung auf dem Titangerüst verbessern und schliesslich eine möglichst grosse Menge der elektrochemisch aktiven Substanz bereitstellen sollen. Diesen Vorschlägen gemeinsam sind poröse Trägerschichten oder Substrate, die fest mit dem Gerüst verbunden sind und die elektrochemisch aktive Substanz aufnehmen. Das poröse Substrat ist ein besserer Haftgrund als die mehr oder weniger glatte Oberfläche des Gerüsts, sie schützt die aktive Substanz bei Kurzschlüssen und ihr Aufnahmevermögen kann über Porosität und Dicke des Substrats in weiten Bereichen den Bedürfnissen der Elektrolyse angepasst werden.In chlor-alkali electrolysis and other electrolyses with aqueous electrolytes, metal anodes have been used for some time, which essentially contain a framework or a base made of a passivable metal, on which one or more electrochemically active substances are firmly anchored. Usually, because of its availability and the comparatively low price, titanium scaffolds are used which are resistant to the electrolyte and the electrolysis products. Preferred electrochemically active substances are oxides of platinum group metals, alone or in mixtures with other metal oxides, spinels, perovskites and other mixed oxides. Coatings that do not contain platinum metal oxides have also become known for special electrolyses. The service life of the coated anodes is essentially determined by the resistance of the electrochemically active coating, which also depends on the resistance in contact with mercury, depending on the type of substance and the electrolysis conditions, the adhesion to the metal structure and in chlor-alkali electrolysis in mercury cells. Numerous proposals have been published for extending the anode service life, which secure the active substance against damage caused by short circuit, improve their anchoring on the titanium framework and ultimately provide the largest possible amount of the electrochemically active substance. These proposals have in common porous support layers or substrates that are firmly attached to the framework and absorb the electrochemically active substance. The porous substrate is a better primer than the more or less smooth surface of the framework, it protects the active substance in the event of short circuits, and its absorption capacity can be adapted to the needs of electrolysis in a wide range via the porosity and thickness of the substrate.
Das Substrat besteht nach der DE-PS 2 300 422 aus verschiedenen Titanoxiden, die durch Flamm- oder Plasmaspritzen in einer Menge von 100 bis 6000 g/m² auf das Anodengestell aufgetragen werden. Besonders vorteilhaft sollen sich Oxide der Zusammensetzung TiO2-x verhalten, mit 0,1 > x > 0. Das poröse Substrat wird mit einer Salze der Platinmetalle enthaltenden Lösung imprägniert, die nach Verdampfen des Lösemittels thermisch zersetzt werden. Es ist auch bekannt, die elektrochemisch aktive Substanz gemeinsam mit Oxiden, Nitriden, Phosphiden, Boriden oder Carbiden eines Metalls aus der Gruppe der passivierbaren Metalle, bevorzugt mit Titanoxid, in einem einzigen Arbeitsgang auf die Oberfläche des Anodengerüsts aufzutragen (EP-OS 0 058 832). Eine andere Anode hat ein Substrat, das ausser Titanoxiden Oxide anderer Nichtedelmetalle, wie Nioboxid oder Nickeloxid, enthält (DE-OS 32 08 835). Dem durch Flammspritzen aufgebrachten Substrat sind Verbindungen wenigstens eines Elements der Platingruppe zugesetzt. Schliesslich ist ein Substrat bekannt, das aus einer gesinterten Schicht aus Titanoxiden der Zusammensetzung TiOx besteht, mit 0,25 < x < 1,50 (DE-OS 24 12 828). Das durch die DE-OS 20 35 212 bekanntgewordene, auf das Trägergerüst aufgesinterte poröse Substrat besteht aus metallischem Titan.According to DE-PS 2 300 422, the substrate consists of various titanium oxides, which are applied to the anode frame in an amount of 100 to 6000 g / m 2 by flame or plasma spraying. Oxides of the composition TiO 2-x should behave particularly advantageously, with 0.1>x> 0. The porous substrate is impregnated with salts of the solution containing platinum metals, which are thermally decomposed after evaporation of the solvent. It is also known to apply the electrochemically active substance together with oxides, nitrides, phosphides, borides or carbides of a metal from the group of passivatable metals, preferably with titanium oxide, to the surface of the anode structure in a single operation (EP-OS 0 058 832 ). Another anode has a substrate which, in addition to titanium oxides, contains oxides of other non-noble metals, such as niobium oxide or nickel oxide (DE-OS 32 08 835). Compounds of at least one element of the platinum group are added to the substrate applied by flame spraying. Finally, a substrate is known which consists of a sintered layer of titanium oxides of the composition TiO x , with 0.25 <x <1.50 (DE-OS 24 12 828). The porous substrate known from DE-OS 20 35 212, sintered onto the support frame, is made of metallic titanium.
Alle Substratschichten bilden bei der Elektrolyse elektrisch nichtleitende Oxide an der Grenzfläche zwischen dem im allgemeinen aus metallischem Titan bestehenden Gerüst und der Basis des Substrats, die eine mit der Betriebszeit zunehmende Passivierung der Anode und gegebenenfalls sogar das Ablösen der Substratschichten bewirken. Die Passivierungsschicht ist schliesslich auch Ursache dafür, dass vor einer Reaktivierung der passivierten Anode, das gesamte Substrat entfernt werden muss, wobei Edelmetalle verloren gehen. Zur Verhinderung der Passivierung ist vorgeschlagen worden, zwischen dem metallischen Gerüst und dem die elektrochemisch aktiven Substanzen enthaltenden Substrat eine besondere Zwischenschicht anzuordnen, die aus Mischoxiden mit den Valenzzahlen 4 und 3 und in den Oxiden dispergiertem Platin besteht (DE-OS 29 36 033). Diese Anoden haben eine vergleichsweise lange Lebensdauer, nachteilig ist aber ihre technisch aufwendige Herstellung.During the electrolysis, all substrate layers form electrically non-conductive oxides at the interface between the framework, which is generally made of metallic titanium, and the base of the substrate, which passivation of the anode and increases with the operating time possibly even cause the substrate layers to detach. Finally, the passivation layer is also the reason why, before the passivated anode is reactivated, the entire substrate has to be removed, precious metals being lost. To prevent passivation, it has been proposed to arrange a special intermediate layer between the metallic framework and the substrate containing the electrochemically active substances, which consists of mixed oxides with valence numbers 4 and 3 and platinum dispersed in the oxides (DE-OS 29 36 033). These anodes have a comparatively long service life, but their technically complex manufacture is disadvantageous.
Es besteht ein Bedürfnis, ein einfach herstellbares Substrat zur Aufnahme elektrochemisch aktiver Substanzen zu schaffen, das ein guter Haftgrund für die Substanzen ist, sie gegen Kurzschlüsse sichert, bei Verwendung als sauerstoffbildender Anode die Ausbildung einer Passivierungsschicht wesentlich verzögert und mit geringem Aufwand reaktiviert werden kann.There is a need to create a simple-to-produce substrate for receiving electrochemically active substances, which is a good base for the substances to be adhered to, protects them against short circuits, and when used as an oxygen-forming anode, the formation of a passivation layer can be significantly delayed and reactivated with little effort.
Gegenstand der Erfindung ist eine Anode für wässerige Elektrolysen, bestehend aus einem gegen den Elektrolyten und die Elektrolyseprodukte beständigen Gerüst, einem mit dem Gerüst verbundenen Titan enthaltenden porösen Substrat und elektrochemisch aktiven Substanzen, die in den Poren des Substrats verteilt sind, dadurch gekennzeichnet, dass das poröse titanhaltige Substrat mit einem Metall aus der Gruppe Chrom, Nickel dotiert ist.The invention relates to an anode for aqueous electrolysis, consisting of a framework which is resistant to the electrolyte and the electrolysis products, a porous substrate containing titanium connected to the framework and electrochemically active substances which are distributed in the pores of the substrate, characterized in that porous titanium-containing substrate with a metal from the group chromium, nickel is doped.
Die Erfindung geht auf die überraschende Erkenntnis zurück, das mit Chrom und/oder Nickel dotiertes Titan unter den Bedingungen wässeriger Elektrolysen den Strom auch in anodischer Richtung transportiert, selbst wenn es keine elektrochemisch aktiven Substanzen enthält. Die Passivierung ist gegenüber Substraten aus Titan oder anderen passivierbaren Metallen oder Ventilmetallen stark vermindert. Anodische Metallauflösungen werden praktisch nicht beobachtet. Der Charakter der erfindungsgemässen Schicht ist mit dem eines Edelmetalls vergleichbar.The invention is based on the surprising finding that, under the conditions of aqueous electrolysis, titanium doped with chromium and / or nickel also transports the current in the anodic direction, even if it does not contain any electrochemically active substances. The passivation is greatly reduced compared to substrates made of titanium or other passivable metals or valve metals. Anodic metal dissolutions are practically not observed. The character of the layer according to the invention is comparable to that of a noble metal.
Der Anteil der dem Titan zugesetzten Dotierungselemente kann z.B. 0,5 bis 40 Gew.% sein und beträgt vorzugsweise 2 bis 20 Gew.%, besonders 2 bis 10 Gew.%. Unterhalb etwa 2 % ist die Wirkung der Dotierung klein, oberhalb 20 % kann es unter den Bedingungen Sauerstoff-entwickelnder Anoden zur partiellen Lösung der Dotierungsmetalle kommen. Zur Herstellung des dotierten Substrats können zum Beispiel Chrom und/oder Nickel in Form feiner Pulver mit pulverförmigem Titan gemischt und das Gemisch beispielsweise durch Flammspritzen auf das Gerüst aufgetragen werden. Unter diesen Bedingungen bilden sich nur begrenzt Mischkristalle aus Titan und dem Dotierungsmetall. Bei einem anderen Verfahren wird das mit einem temporären Binder versetzte Pulvergemisch auf das Gerüst gespritzt oder aufgepinselt und durch Erhitzen in inerter Atmosphäre eine poröse, mit dem Gerüst fest verbundene Sinterschicht gebildet. Beim Sintern können sich in grösserem Umfang Mischkristalle bilden, die aber bei Raumtemperatur thermodynamisch instabil sind und beim Abkühlen daher zerfallen. Die Funktionalität der dotierten Substrate ist von den verschiedenen Herstellungsverfahren praktisch unabhängig.The proportion of the doping elements added to the titanium can e.g. 0.5 to 40% by weight and is preferably 2 to 20% by weight, especially 2 to 10% by weight. The effect of the doping is small below approximately 2%, and above 20%, partial doping of the doping metals can occur under the conditions of oxygen-developing anodes. To produce the doped substrate, for example, chromium and / or nickel in the form of fine powders can be mixed with powdered titanium and the mixture can be applied to the framework, for example by flame spraying. Under these conditions, mixed crystals of titanium and the doping metal are only formed to a limited extent. In another method, the powder mixture mixed with a temporary binder is sprayed or brushed onto the framework and a porous sintered layer firmly connected to the framework is formed by heating in an inert atmosphere. Mixed crystals can form to a large extent during sintering, but are thermodynamically unstable at room temperature and therefore disintegrate on cooling. The functionality of the doped substrates is practically independent of the different manufacturing processes.
Die Dicke des Substrats beträgt vorzugsweise 0,2 bis 1 mm. Die Porosität kann z.B. 20 bis 60 Vol.-%, besonders 30 bis 50 Vol.-% betragen. Bei einer durchschnittlichen Porosität von ca. 40 Vol.-% hat das Substrat eine Aufnahmekapazität für die elektrochemisch aktiven substanzen, die den bekannten wässerigen Elektrolysen angemessen ist. Zum Einbringen der aktiven Substanzen kann das Substrat mit Lösungen oder Suspensionen imprägniert werden, die diese Substanzen enthalten. Die Art der verwendeten elektrochemisch aktiven Substanzen wird in bekannter Weise durch die Elektrolysebedingungen bestimmt. Geeignet sind u.a. Platinmetalle, Oxide von Platinmetallen, Spinelle, Perowskite, β-Mangandioxid allein oder in Gemischen.The thickness of the substrate is preferably 0.2 to 1 mm. The porosity can be, for example, 20 to 60% by volume, in particular 30 to 50% by volume. With an average porosity of approx. 40% by volume, the substrate has a holding capacity for the electrochemically active substances, which is appropriate for the known aqueous electrolysis. To introduce the active substances, the substrate can be impregnated with solutions or suspensions containing these substances. The type of electrochemically active substances used is determined in a known manner by the electrolysis conditions. Suitable metals include platinum metals, oxides of platinum metals, spinels, perovskites, β-manganese dioxide alone or in mixtures.
Erfindungsgemässe Anoden eignen sich besonders für die Chloralkali-Elektrolyse und für Elektrolysen, bei denen anodisch Sauerstoff erzeugt wird. Die Anoden haben eine lange Lebensdauer und ihre Reaktivierung ist besonders einfach, da bei der Elektrolyse offensichtlich keine, den elektrischen Strom nicht leitende Oxide gebildet werden. Nach einer Reinigung, z.B. durch Dampfstrahlen, wird die Anode durch das Einbringen elektrochemisch aktiver Substanzen in das poröse Substrat reaktiviert.Anodes according to the invention are particularly suitable for chloralkali electrolysis and for electrolysis in which oxygen is generated anodically. The anodes have a long service life and their reactivation is particularly simple, since obviously no oxides which do not conduct electricity are formed during the electrolysis. After cleaning, e.g. by steam jets, the anode is reactivated by introducing electrochemically active substances into the porous substrate.
Die Erfindung wird nachfolgend beispielhaft erläutert:The invention is explained below by way of example:
Beispiel 1: Titanbleche werden entfettet, sandgestrahlt und mit einem feinkörnigen Gemisch aus Titan- und Chrompulver beschichtet. Das Gemisch enthält 9 Gew.% Chrom und 91 Gew.% Titan (maximale Korngrösse 0,1 mm) und ist mit einer wässerigen Tyloselösung zu einer spritzfähigen Paste angeteigt. Mit einer Fliessbecherpistole wird eine 0,5 mm dicke Schicht auf die Bleche aufgetragen; die Bleche werden bei Raumtemperatur getrocknet und durch Erhitzen auf 1200°C in Argon eine fest auf den Blechen haftende poröse Substratschicht erzeugt, deren Porosität etwa 25 Vol.-% beträgt. Example 1: Titanium sheets are degreased, sandblasted and coated with a fine-grained mixture of titanium and chrome powder. The mixture contains 9% by weight of chromium and 91% by weight of titanium (maximum grain size 0.1 mm) and is made into an injectable paste with an aqueous tylose solution. A 0.5 mm thick layer is applied to the sheets with a gravity cup gun; the sheets are dried at room temperature and, by heating to 1200 ° C. in argon, a porous substrate layer is adhered to the sheets, the porosity of which is approximately 25% by volume.
Die Bleche werden in 50 x 100 mm grosse Abschnitte zerlegt und die Substratschichten wie folgt mit elektrochemisch aktiven Substanzen imprägniert:
- a) Eine 40%ige wässerige Lösung von Mangan (II)-Nitrat wird auf das poröse Substrat aufgetragen und die Anode nach Trocknung zur Zersetzung des Salzes auf 300°C erhitzt (Verweilzeit 10 min.). Nach fünfmaliger Wiederholung enthält die Anode etwa 300 g/m² β-MnO₂.
- b) Das Substrat wird mit einer Lösung enthaltend 48,17 mg H₂IrCl₆, 37,27 mg TaCl₅, und 278,2 mg Ethanol imprägniert und zur Zersetzung der Salze auf 550°C erhitzt (Verweilzeit 10 min.). Nach viermaliger Wiederholung der Verfahrensschritte enthält das Substrat 23 g/m² IrO₂ und 2 g/m² TaO₂.
- c) Das Substrat wird mit einer Lösung imprägniert, die 1,93 g RuCl₃, 7,23 g Butyltitanat, 1,43 g HCl und 7,31 g Butanol enthält. Die Anoden werden getrocknet, auf 520°C erhitzt und die Verfahrensschritte dreimal wiederholt. Die Anode enthält dann verteilt in dem Substrat 11,8 g/m² RuO₂ und 21,3 g/m² TiO₂,
- a) A 40% aqueous solution of manganese (II) nitrate is applied to the porous substrate and the anode is heated to 300 ° C. after drying to decompose the salt (residence time 10 min.). After five repetitions, the anode contains about 300 g / m² of β-MnO₂.
- b) The substrate is impregnated with a solution containing 48.17 mg H₂IrCl₆, 37.27 mg TaCl₅, and 278.2 mg ethanol and heated to 550 ° C to decompose the salts (residence time 10 min.). After repeating the process steps four times, the substrate contains 23 g / m² of IrO₂ and 2 g / m² of TaO₂.
- c) The substrate is impregnated with a solution containing 1.93 g RuCl₃, 7.23 g butyl titanate, 1.43 g HCl and 7.31 g butanol. The anodes are dried, heated to 520 ° C and the process steps repeated three times. The anode then contains 11.8 g / m² RuO₂ and 21.3 g / m² TiO₂ distributed in the substrate,
Zum Vergleich wurden Titanbleche ohne Substrate und Titanbleche mit nichtdotierten Substratschichten aus porösem Sintertitan mit den gleichen Mengen der elektrochemisch aktiven Substanzen beschichtet und unter den gleichen Bedingungen die Lebensdauer der Anoden in 20%iger Schwefelsäure bei Raumtemperatur gemessen.
Beispiel 2: auf Titanbleche wird durch Flammspritzen eines 9 Gew.% Nickel- und 91 Gew.% Titanpulver enthaltenden Gemischs eine etwa 0,4 mm dicke Substratschicht aus dotiertem Titan aufgetragen. Die Korngrösse der Pulver ist kleiner als 0,05 mm. Wie in Beispiel 1 beschrieben werden die Substratschichten mit den Lösungen a, b und c imprägniert und vergleichend mit Anoden getestet, die die gleiche Menge elektrochemisch aktiver Substanzen aber kein Substrat bzw. kein dotiertes Substrat enthalten.
Beispiel 3: Die Passivierungsgeschwindigkeit verschiedener Anoden, die keine Beschichtungen mit elektrochemisch aktiven Substanzen aufweisen, wird in 20%iger Schwefelsäure bei Raumtemperatur und einer Stromdichte von 0,2 kA/m² gemessen. Indikator der Passivierung ist ein Anstieg der Zellenspannung auf 10 V.
Claims (4)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE3613997 | 1986-04-25 | ||
| DE19863613997 DE3613997A1 (en) | 1986-04-25 | 1986-04-25 | ANODE FOR ELECTROLYTIC PROCESSES |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0245201A1 true EP0245201A1 (en) | 1987-11-11 |
| EP0245201B1 EP0245201B1 (en) | 1991-05-22 |
Family
ID=6299502
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP87810254A Expired - Lifetime EP0245201B1 (en) | 1986-04-25 | 1987-04-22 | Anode for electrolyses |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US4849085A (en) |
| EP (1) | EP0245201B1 (en) |
| JP (1) | JPS62270790A (en) |
| DE (2) | DE3613997A1 (en) |
| NO (1) | NO166496C (en) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5314601A (en) * | 1989-06-30 | 1994-05-24 | Eltech Systems Corporation | Electrodes of improved service life |
| US5324407A (en) * | 1989-06-30 | 1994-06-28 | Eltech Systems Corporation | Substrate of improved plasma sprayed surface morphology and its use as an electrode in an electrolytic cell |
| US5879817A (en) * | 1994-02-15 | 1999-03-09 | Eltech Systems Corporation | Reinforced concrete structure |
| US5964993A (en) * | 1996-12-19 | 1999-10-12 | Implanted Biosystems Inc. | Glucose sensor |
| US5914026A (en) * | 1997-01-06 | 1999-06-22 | Implanted Biosystems Inc. | Implantable sensor employing an auxiliary electrode |
| US20030010649A1 (en) * | 2001-07-16 | 2003-01-16 | Waite Michael D. | Inert anode for electrochemical process |
| EP1850412A1 (en) * | 2006-04-26 | 2007-10-31 | Technical University of Denmark | A multi-layer coating |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE1812522A1 (en) * | 1968-12-04 | 1970-06-18 | Basf Ag | Anode for alkali chloride electrolysis |
| GB1233590A (en) * | 1967-08-16 | 1971-05-26 | ||
| FR2098464A1 (en) * | 1970-07-16 | 1972-03-10 | Conradty Fa C | |
| FR2215268A1 (en) * | 1973-01-26 | 1974-08-23 | Electronor Corp | |
| FR2216021A1 (en) * | 1973-02-02 | 1974-08-30 | Sigri Elektrographit Gmbh | Porous electrodes for electrolytic cells - with a non-passivatable metal coating |
| US4138510A (en) * | 1973-09-27 | 1979-02-06 | Firma C. Conradty | Metal anode for electrochemical processing and method of making same |
| US4140615A (en) * | 1977-03-28 | 1979-02-20 | Olin Corporation | Cell and process for electrolyzing aqueous solutions using a porous anode separator |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3926773A (en) * | 1970-07-16 | 1975-12-16 | Conradty Fa C | Metal anode for electrochemical processes and method of making same |
| DE2300422C3 (en) * | 1973-01-05 | 1981-10-15 | Hoechst Ag, 6000 Frankfurt | Method of making an electrode |
| US4013525A (en) * | 1973-09-24 | 1977-03-22 | Imperial Chemical Industries Limited | Electrolytic cells |
| DE2405010C3 (en) * | 1974-02-02 | 1982-08-05 | Sigri Elektrographit Gmbh, 8901 Meitingen | Sintered electrode for electrochemical processes and methods of manufacturing the electrode |
| DD137365A5 (en) * | 1976-03-31 | 1979-08-29 | Diamond Shamrock Techn | ELECTRODE |
| JPS5544514A (en) * | 1978-09-22 | 1980-03-28 | Permelec Electrode Ltd | Electrode for electrolysis and production thereof |
| DE3106587A1 (en) * | 1981-02-21 | 1982-09-02 | Heraeus-Elektroden Gmbh, 6450 Hanau | "ELECTRODE" |
| JPS6017834B2 (en) * | 1981-03-11 | 1985-05-07 | 昭和電工株式会社 | Electrochemical device with insoluble electrodes |
-
1986
- 1986-04-25 DE DE19863613997 patent/DE3613997A1/en not_active Withdrawn
-
1987
- 1987-04-22 EP EP87810254A patent/EP0245201B1/en not_active Expired - Lifetime
- 1987-04-22 US US07/041,888 patent/US4849085A/en not_active Expired - Fee Related
- 1987-04-22 DE DE8787810254T patent/DE3770193D1/en not_active Expired - Lifetime
- 1987-04-24 NO NO871717A patent/NO166496C/en unknown
- 1987-04-24 JP JP62100196A patent/JPS62270790A/en active Pending
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1233590A (en) * | 1967-08-16 | 1971-05-26 | ||
| DE1812522A1 (en) * | 1968-12-04 | 1970-06-18 | Basf Ag | Anode for alkali chloride electrolysis |
| FR2098464A1 (en) * | 1970-07-16 | 1972-03-10 | Conradty Fa C | |
| FR2215268A1 (en) * | 1973-01-26 | 1974-08-23 | Electronor Corp | |
| FR2216021A1 (en) * | 1973-02-02 | 1974-08-30 | Sigri Elektrographit Gmbh | Porous electrodes for electrolytic cells - with a non-passivatable metal coating |
| US4138510A (en) * | 1973-09-27 | 1979-02-06 | Firma C. Conradty | Metal anode for electrochemical processing and method of making same |
| US4140615A (en) * | 1977-03-28 | 1979-02-20 | Olin Corporation | Cell and process for electrolyzing aqueous solutions using a porous anode separator |
Also Published As
| Publication number | Publication date |
|---|---|
| DE3613997A1 (en) | 1987-10-29 |
| US4849085A (en) | 1989-07-18 |
| NO871717L (en) | 1987-10-26 |
| EP0245201B1 (en) | 1991-05-22 |
| JPS62270790A (en) | 1987-11-25 |
| DE3770193D1 (en) | 1991-06-27 |
| NO166496C (en) | 1991-07-31 |
| NO166496B (en) | 1991-04-22 |
| NO871717D0 (en) | 1987-04-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0169301B1 (en) | Composite electrode and method of production and applications thereof | |
| DE2113795C3 (en) | Electrode for electrolytic processes as an oxygen anode | |
| DE2331949C3 (en) | Method of making an electrode | |
| DE2532553A1 (en) | ANODE FOR ELECTROLYTIC PROCEDURES | |
| DE2657979A1 (en) | ELECTRODE FOR ELECTROCHEMICAL PROCESSES AND PROCESS FOR THEIR PRODUCTION | |
| DE1952484B2 (en) | COATING ELECTRODE FOR USE IN ELECTROCHEMICAL PROCESSES | |
| CA1058552A (en) | Electrodes | |
| JPH0694597B2 (en) | Electrode used in electrochemical process and manufacturing method thereof | |
| DE2619670A1 (en) | ANODE FOR ELECTROLYTIC PROCESSES | |
| EP0715002A1 (en) | Stable coating solutions for preparing electrocatalytic mixed oxide coatings on metal substrates or metal-coated conductive substrates, and dimensionally stable anodes produced from such solutions | |
| DE2752875A1 (en) | ELECTRODE FOR ELECTROCHEMICAL PROCESSES AND METHOD FOR THE PRODUCTION THEREOF | |
| DD253648A1 (en) | METHOD FOR PRODUCING A CATHODE WITH LOW HYDROGEN SUPPLY VOLTAGE | |
| DE2113676C2 (en) | Electrode for electrochemical processes | |
| EP0245201B1 (en) | Anode for electrolyses | |
| DE3322169C2 (en) | ||
| DD153397A5 (en) | ELECTRODE WITH AN ELECTROCATALYTIC COVER | |
| EP0033363B1 (en) | Process for coating a porous electrode | |
| DE2852136C2 (en) | ||
| EP0042984B1 (en) | Electrode free from noble metals and process for its manufacture | |
| DE2527386A1 (en) | CATHODE SURFACES WITH LOW HYDROGEN OVERVOLTAGE | |
| EP0205631A1 (en) | Process for coating a porous electrode | |
| DE3612790C2 (en) | ||
| DE2714605A1 (en) | Lead di:oxide electrode having sub:oxide-coated titanium support - used in fuel and galvanic cells, for electrochemical reactions and for anticorrosion purposes | |
| DE2750029A1 (en) | ELECTRODES FOR ELECTROLYSIS PURPOSES | |
| JP2836890B2 (en) | Electrode for organic matter electrolysis and method for producing the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19870424 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): BE CH DE ES FR GB IT LI NL SE |
|
| 17Q | First examination report despatched |
Effective date: 19890522 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): BE CH DE ES FR GB IT LI NL SE |
|
| ITF | It: translation for a ep patent filed |
Owner name: BARZANO' E ZANARDO MILANO S.P.A. |
|
| REF | Corresponds to: |
Ref document number: 3770193 Country of ref document: DE Date of ref document: 19910627 |
|
| ET | Fr: translation filed | ||
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 19910902 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19920202 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19920217 Year of fee payment: 6 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 19920327 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 19920428 Year of fee payment: 6 Ref country code: BE Payment date: 19920428 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19920430 Year of fee payment: 6 |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19920721 Year of fee payment: 6 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Effective date: 19930422 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Effective date: 19930423 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Effective date: 19930430 Ref country code: CH Effective date: 19930430 Ref country code: BE Effective date: 19930430 |
|
| BERE | Be: lapsed |
Owner name: CIBA-GEIGY A.G. Effective date: 19930430 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Effective date: 19931101 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee | ||
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 19930422 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Effective date: 19931229 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19940101 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| EUG | Se: european patent has lapsed |
Ref document number: 87810254.0 Effective date: 19931110 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED. Effective date: 20050422 |