EP0034515B1 - Application de mélanges autodestructibles à base de résines et de charges oxydantes à la réalisation de liaisons temporaires d'éléments de construction - Google Patents
Application de mélanges autodestructibles à base de résines et de charges oxydantes à la réalisation de liaisons temporaires d'éléments de construction Download PDFInfo
- Publication number
- EP0034515B1 EP0034515B1 EP81400132A EP81400132A EP0034515B1 EP 0034515 B1 EP0034515 B1 EP 0034515B1 EP 81400132 A EP81400132 A EP 81400132A EP 81400132 A EP81400132 A EP 81400132A EP 0034515 B1 EP0034515 B1 EP 0034515B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- resin
- application according
- inorganic filler
- combustion
- resins
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- 229920005989 resin Polymers 0.000 title claims abstract description 60
- 239000011347 resin Substances 0.000 title claims abstract description 60
- 239000000203 mixture Substances 0.000 title claims abstract description 21
- 238000002485 combustion reaction Methods 0.000 claims abstract description 23
- 230000001590 oxidative effect Effects 0.000 claims abstract description 14
- 238000010276 construction Methods 0.000 claims abstract description 11
- 239000011256 inorganic filler Substances 0.000 claims abstract 8
- 229910003475 inorganic filler Inorganic materials 0.000 claims abstract 8
- 238000004519 manufacturing process Methods 0.000 claims abstract 2
- AXZAYXJCENRGIM-UHFFFAOYSA-J dipotassium;tetrabromoplatinum(2-) Chemical compound [K+].[K+].[Br-].[Br-].[Br-].[Br-].[Pt+2] AXZAYXJCENRGIM-UHFFFAOYSA-J 0.000 claims description 6
- FGIUAXJPYTZDNR-UHFFFAOYSA-N potassium nitrate Chemical compound [K+].[O-][N+]([O-])=O FGIUAXJPYTZDNR-UHFFFAOYSA-N 0.000 claims description 6
- 229910001487 potassium perchlorate Inorganic materials 0.000 claims description 6
- 150000002823 nitrates Chemical class 0.000 claims description 5
- 229910052784 alkaline earth metal Inorganic materials 0.000 claims description 4
- 150000001768 cations Chemical class 0.000 claims description 4
- VLTRZXGMWDSKGL-UHFFFAOYSA-N perchloric acid Chemical class OCl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-N 0.000 claims description 4
- 229920001225 polyester resin Polymers 0.000 claims description 4
- 239000004645 polyester resin Substances 0.000 claims description 4
- 239000003513 alkali Substances 0.000 claims description 3
- 235000010333 potassium nitrate Nutrition 0.000 claims description 3
- 239000004323 potassium nitrate Substances 0.000 claims description 3
- GDDNTTHUKVNJRA-UHFFFAOYSA-N 3-bromo-3,3-difluoroprop-1-ene Chemical compound FC(F)(Br)C=C GDDNTTHUKVNJRA-UHFFFAOYSA-N 0.000 claims description 2
- 150000001342 alkaline earth metals Chemical class 0.000 claims description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-O ammonium group Chemical group [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 claims description 2
- 229920005749 polyurethane resin Polymers 0.000 claims description 2
- 206010061218 Inflammation Diseases 0.000 claims 1
- 239000000853 adhesive Substances 0.000 claims 1
- 230000001070 adhesive effect Effects 0.000 claims 1
- 238000001033 granulometry Methods 0.000 claims 1
- 230000004054 inflammatory process Effects 0.000 claims 1
- 238000007789 sealing Methods 0.000 claims 1
- 229910052751 metal Inorganic materials 0.000 description 12
- 239000012764 mineral filler Substances 0.000 description 12
- 239000002184 metal Substances 0.000 description 11
- 239000003054 catalyst Substances 0.000 description 6
- 230000037452 priming Effects 0.000 description 6
- 239000007788 liquid Substances 0.000 description 5
- 230000006378 damage Effects 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 150000001412 amines Chemical class 0.000 description 2
- 238000004873 anchoring Methods 0.000 description 2
- 239000004568 cement Substances 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 239000000446 fuel Substances 0.000 description 2
- 229920000728 polyester Polymers 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 241000422252 Cales Species 0.000 description 1
- 229920000114 Corrugated plastic Polymers 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 206010035148 Plague Diseases 0.000 description 1
- 229910000831 Steel Inorganic materials 0.000 description 1
- 241000607479 Yersinia pestis Species 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 238000009412 basement excavation Methods 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 239000000567 combustion gas Substances 0.000 description 1
- 238000007596 consolidation process Methods 0.000 description 1
- 238000005474 detonation Methods 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 230000008034 disappearance Effects 0.000 description 1
- 238000005553 drilling Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 239000003822 epoxy resin Substances 0.000 description 1
- 239000002360 explosive Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 239000011440 grout Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 150000002978 peroxides Chemical class 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 229920000647 polyepoxide Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- VKJKEPKFPUWCAS-UHFFFAOYSA-M potassium chlorate Chemical compound [K+].[O-]Cl(=O)=O VKJKEPKFPUWCAS-UHFFFAOYSA-M 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 230000002787 reinforcement Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 239000011435 rock Substances 0.000 description 1
- 238000010008 shearing Methods 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 239000011343 solid material Substances 0.000 description 1
- 239000010959 steel Substances 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- 239000003832 thermite Substances 0.000 description 1
- 229920005992 thermoplastic resin Polymers 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 230000003313 weakening effect Effects 0.000 description 1
- 238000003466 welding Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C06—EXPLOSIVES; MATCHES
- C06B—EXPLOSIVES OR THERMIC COMPOSITIONS; MANUFACTURE THEREOF; USE OF SINGLE SUBSTANCES AS EXPLOSIVES
- C06B45/00—Compositions or products which are defined by structure or arrangement of component of product
- C06B45/04—Compositions or products which are defined by structure or arrangement of component of product comprising solid particles dispersed in solid solution or matrix not used for explosives where the matrix consists essentially of nitrated carbohydrates or a low molecular organic explosive
- C06B45/06—Compositions or products which are defined by structure or arrangement of component of product comprising solid particles dispersed in solid solution or matrix not used for explosives where the matrix consists essentially of nitrated carbohydrates or a low molecular organic explosive the solid solution or matrix containing an organic component
- C06B45/10—Compositions or products which are defined by structure or arrangement of component of product comprising solid particles dispersed in solid solution or matrix not used for explosives where the matrix consists essentially of nitrated carbohydrates or a low molecular organic explosive the solid solution or matrix containing an organic component the organic component containing a resin
-
- E—FIXED CONSTRUCTIONS
- E01—CONSTRUCTION OF ROADS, RAILWAYS, OR BRIDGES
- E01D—CONSTRUCTION OF BRIDGES, ELEVATED ROADWAYS OR VIADUCTS; ASSEMBLY OF BRIDGES
- E01D21/00—Methods or apparatus specially adapted for erecting or assembling bridges
-
- E—FIXED CONSTRUCTIONS
- E02—HYDRAULIC ENGINEERING; FOUNDATIONS; SOIL SHIFTING
- E02D—FOUNDATIONS; EXCAVATIONS; EMBANKMENTS; UNDERGROUND OR UNDERWATER STRUCTURES
- E02D5/00—Bulkheads, piles, or other structural elements specially adapted to foundation engineering
- E02D5/74—Means for anchoring structural elements or bulkheads
- E02D5/76—Anchorings for bulkheads or sections thereof in as much as specially adapted therefor
- E02D5/765—Anchorings for bulkheads or sections thereof in as much as specially adapted therefor removable
Definitions
- the present invention relates to the application to the realization of temporary connections of building elements, by means of cold-hardenable resin and mineral filler.
- the invention relates more particularly to the fields of building and public works.
- a major problem in the field of construction is that of temporary connections, necessary to ensure the holding of a structure for a given time during which work is carried out on said structure or around it and which become useless once said work is finished.
- tie rods which are used to hold a wall of a building or building while work is being carried out on the building or building, or while excavations are being carried out. proximity, and which become useless after the end of the work.
- wedges intended to hold props where to temporarily compensate, during the construction phase, for the effects of pressure.
- the anchors are made up of cables or metal bars fixed at one of their ends to the structure which must be maintained and retained in the ground by the other of their ends.
- the buried end is generally coated with a bulb of solid material such as concrete or a curable resin.
- a bulb of solid material such as concrete or a curable resin.
- a first solution proposed in FR-A-2 274 740, consists in not coating the buried end of the tie rods in a bulb, but in welding this end to a buried metal plate and in placing around the welds an aluminum-thermal composition whose ignition causes the tie rods to shear and allows their withdrawal.
- This solution is however not very satisfactory insofar as the welds are always weak points and do not make it possible to give a high mechanical strength to the tie rods; moreover, after shearing, the metal anchoring plate remains in the ground.
- a second solution proposed in US-A-3,936,924, consists in coating the tie rods with a thermoplastic resin which is melted and possibly burned using an auxiliary heat source, at the end of the work, to release the tie rods and remove them.
- This resin is made combustible by adding a mineral filler such as black powder or thermite.
- This solution has several drawbacks: it requires a significant additional source of heat and is therefore relatively expensive, and if it does allow the ground rods to be removed, it leaves agglomerates of molten resin, which does not entirely answer the problem posed and furthermore makes any subsequent reuse of drilling impossible for the laying of a new tie rod, finally it does not ensure regular and non-explosive combustion.
- the present invention aims to remedy these drawbacks and, for this purpose, it provides, for the application to the realization of temporary connections of building elements by means of cold-hardenable resin and mineral filler, that the connection is made. by a hardened mixture, self-destructing by combustion and not detonating, of the resin and of an oxidizing mineral filler, the quantity by weight of resin being between 20 and 40% of the total mixture, an ignition system triggering the combustion.
- an ignition device By placing an ignition device in contact with said temporary connection, constituted for example by an electric igniter and a pyrotechnic ignition composition, it is possible to cause, at the appropriate time, the complete combustion of the combustible connection which will be completely destroyed by very simple way.
- the temporary connections according to the invention are produced from mixtures consisting of a cold-hardening resin made combustible by the addition of an oxidizing mineral filler, the quantity by weight of resin being between 20 and 40% of the total mixture, said mixture being hardened and preferably having an ignition temperature equal to or greater than 500 ° C.
- a temporary bond according to the invention is therefore essentially constituted by a cold-hardening resin and made combustible by the addition of an oxidizing mineral filler.
- the resin must be cold hardenable, usually in the presence of a catalyst, so that it can be easily poured and hardened on a site.
- the resin must have two essential qualities: it must, once hardened, have good mechanical strength and must not be corrosive with respect to the elements which it makes integral, in particular when the latter are metallic elements.
- the preferred resins according to the invention are polyurethane, polyester or polyepoxy resins.
- These resins are two-component resins which harden in the presence of a catalyst which is generally a tertiary amine or a metal salt of fatty acid for polyurethane resins, a peroxide associated with a metal salt or an amine for resins polyesters, an amine for polyepoxy resins.
- a catalyst which is generally a tertiary amine or a metal salt of fatty acid for polyurethane resins, a peroxide associated with a metal salt or an amine for resins polyesters, an amine for polyepoxy resins.
- Said resin is mixed in the liquid state with an oxidizing mineral filler which increases its mechanical strength after hardening and which makes the mixture combustible.
- an oxidizing mineral filler which increases its mechanical strength after hardening and which makes the mixture combustible.
- the hardened mixture of charged resin has an ignition temperature equal to or higher than 500 ° C so as not to risk accidental ignition.
- the charged resin must be perfectly combustible but must not, for safety reasons, present risks of detonation during combustion. Oxidizing charges such as potassium chlorate are therefore not recommended.
- oxidizing mineral fillers chosen from the group consisting of perchlorates and nitrates of an alkaline nature meet the criteria set out above.
- perchlorates or nitrates of an alkaline character means perchlorates or nitrates in which the cation is a true alkali or alkaline earth metal, or in which the cation is the ammonium group.
- the preferred alkali perchlorates and nitrates are potassium perchlorate, ammonium perchlorate and potassium nitrate.
- the oxidizing mineral filler must advantageously have a particle size between 40 and 200 microns.
- the quantity by weight of resin must be between 20 and 40% of the weight of the mixture and preferably between 23 and 30%. The Applicant has observed that mixtures consisting of 25% resin and 75% oxidizing mineral filler give very good results.
- the oxidizing mineral filler is mixed, in a manner known per se, in a kneader, either with one of the liquid components of the resin, in which case the second liquid component generally containing the catalyst is then added, or directly with the liquid resin not containing the catalyst, which is only added immediately before use.
- the loaded liquid resin containing the curing catalyst is then applied to the elements which should be made integral.
- the hardening of the charged resin is then very rapid.
- an ignition device which generally consists of a pyrotechnic priming paste and an electric igniter.
- the electric igniter which can be controlled remotely, ignites the priming paste, which itself ignites and burns the charged resin. After combustion of the resin, the elements made temporarily united, regain their individuality.
- the temporary connections according to the invention find multiple applications in the field of construction.
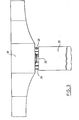
- a tie rod 11 made up of several metallic cables sealed inside a corrugated plastic sheath 13 by means of a charged resin 14 according to the invention, and constituting the steel-sheath seal, crosses a retaining wall 12 to anchor in the ground by means of a bulb constituted by a cement grout 19 injected in place.
- the free part of the tie rod is protected, over its entire buried length, by a second smooth sheath 18.
- a priming paste 15 composed, for example, of 75% by weight of potassium nitrate, 15% of magnesium and 10% of a resin serving as a binder is placed on the external surface of the resin.
- An electric igniter 16 connected to a control station 17 and embedded in the priming paste, allows the latter to be ignited; this thus ignites the resin 14 which, on burning, will release the complete tie rod 11 is lying. It is then possible to remove the tie rod 11 1 over its entire length either permanently or to replace it in the event of damage.
- the resin 14 can also be used to seal the tie rod 11 directly in a material without passing through a cement slurry or a sheath 13 when said material is sufficiently homogeneous as in the case of rock for example.
- FIG. 3 Another interesting application of the invention, represented in FIG. 3, relates to the cantilever execution of a bridge deck by beams constructed symmetrically on either side of the piers. It is necessary in this case, if one wants to avoid shoring, to securely secure the central segment 21 of each beam on its pile 22, around the final bearings 23 of the deck on the piles, to stabilize the plagues as long as these are not interconnected. This fixing is advantageously carried out by interposition, between the segment 21 and the stack 22, of fuel wedges 24 produced from a charged resin according to the invention. When the construction of the bridge is finished, it is easy to get rid of the wedges that have become useless by causing them to burn.
- Another interesting application of the invention is in the field of underwater work.
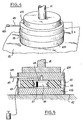
- a simple example is the temporary setting of a forestay which will have to be removed at the end of the realization of a metal or concrete structure, as shown in Figures 4 and 5.
- the forces transmitted to the forestay 41, during the construction of the structure, pass from the forestay to the support 42 by means of a wedge produced according to the invention.
- the fuse block may have a circular or rectangular shape or any other shape suitable for the use. It consists of two support plates 431 and 432 between which is placed a ring of combustible resin 44 charged according to the invention and bonded to the two plates so as to create a combustion chamber 45. An ignition composition 46 is applied on the walls of this chamber and an igniter 47 allows the fuel crown to be ignited. A nozzle 48 allows the evacuation of combustion gases. The nozzle 48 can advantageously be equipped at the outlet with a non-return valve 481 preventing the penetration of water into the hold or be extended by a vent pipe. The flexible seals 49 seal the assembly.
- FIG. 6 represents another possible application of the invention.
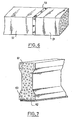
- Two prefabricated elements 51 and 52 are temporarily made integral by bonding with an intermediate layer 53 of combustible resin loaded according to the invention. After combustion of the intermediate layer the two elements regain their individuality.
- FIG. 7 represents another possibility of applying the invention.
- a concrete beam 61, having a weakening, is temporarily reinforced by a metal plate 62 bonded by means of a layer of combustible mixture 63 according to the invention.
- This example concerns anchor rods. Two types of test were carried out on tie rods coated with charged resin according to the invention.
- a 12 mm diameter metal cable is trapped in a plastic sheath filled with resin loaded with 75% by weight of potassium perchlorate.
- a second test piece is produced using an unfilled resin.
- a polyester resin was used on the one hand and a polyepoxy resin on the other hand.
- the cable is pulled using a cylinder in 50 bar steps with a stop time of one minute between each level. It was considered that the adhesion break is reached when the pressure drop is recorded. The following results were obtained.
- the tests were carried out on 12 strands sealed on one meter by resin loaded inside a metal sheath of 65 mm in diameter.
- the ignition was carried out using a wick and a priming paste.
- the purpose of this example is to verify the possibility of burning a charged resin according to the invention in a confined atmosphere.
- the blocks were placed in a metal combustion chamber having roughly the outside dimensions of the block and closed by a metal nozzle.
- the ignition was carried out using a wick and a priming paste.
Landscapes
- Engineering & Computer Science (AREA)
- Structural Engineering (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Civil Engineering (AREA)
- Mining & Mineral Resources (AREA)
- Architecture (AREA)
- Crystallography & Structural Chemistry (AREA)
- Molecular Biology (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Paleontology (AREA)
- Dispersion Chemistry (AREA)
- General Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Adhesives Or Adhesive Processes (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Building Environments (AREA)
- Conveying And Assembling Of Building Elements In Situ (AREA)
- Working Measures On Existing Buildindgs (AREA)
- Furnace Housings, Linings, Walls, And Ceilings (AREA)
- Glass Compositions (AREA)
- Organic Insulating Materials (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT81400132T ATE5783T1 (de) | 1980-02-08 | 1981-01-29 | Verwendung selbstvernichtender harzmischungen und oxydierender ladungen zum verwirklichen vorlaeufiger verbindungen von bauelementen. |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR8002749 | 1980-02-08 | ||
| FR8002749A FR2475598A1 (fr) | 1980-02-08 | 1980-02-08 | Application de melanges autodestructibles a base de resines et de charges oxydantes a la realisation de liaisons temporaires d'elements de construction |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0034515A1 EP0034515A1 (fr) | 1981-08-26 |
| EP0034515B1 true EP0034515B1 (fr) | 1984-01-04 |
Family
ID=9238343
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP81400132A Expired EP0034515B1 (fr) | 1980-02-08 | 1981-01-29 | Application de mélanges autodestructibles à base de résines et de charges oxydantes à la réalisation de liaisons temporaires d'éléments de construction |
Country Status (5)
| Country | Link |
|---|---|
| EP (1) | EP0034515B1 (enExample) |
| JP (1) | JPS607106B2 (enExample) |
| AT (1) | ATE5783T1 (enExample) |
| DE (1) | DE3161811D1 (enExample) |
| FR (1) | FR2475598A1 (enExample) |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20090000736A1 (en) * | 2006-01-19 | 2009-01-01 | Asahi Kasei Chemicals Corporation | Oxidant-Containing Adhesive Enabling Disassembly |
| US20100190013A1 (en) * | 2007-07-19 | 2010-07-29 | Asahi Kasei Chemicals Corporation | Detachable adhesive containing reaction product of oxidizing agent and amine compound |
| CN102828470B (zh) * | 2012-09-05 | 2014-06-11 | 中建七局第二建筑有限公司 | 一种斜拉桥主梁拉索套管的定位方法 |
| CN108952211A (zh) * | 2018-07-03 | 2018-12-07 | 中国人民解放军陆军工程大学 | 一种筒状钢柱结构建筑物的爆破拆除装置 |
| CN111335165B (zh) * | 2020-03-04 | 2021-11-16 | 中铁第四勘察设计院集团有限公司 | 一种封锚的施工方法及其辅助封锚构造 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3615960A (en) * | 1968-02-26 | 1971-10-26 | Fujikura Ltd | Bonding using epoxy resin composition and nonactivated blowing agent |
| US4156700A (en) * | 1975-08-11 | 1979-05-29 | Her Majesty The Queen In Right Of Canada, As Represented By The Minister Of National Defence | Solid propellants containing polyether or polyester binders |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3936924A (en) * | 1973-09-21 | 1976-02-10 | Yoshio Ichise | Releaseable steel cable anchor and method for withdrawing the same |
-
1980
- 1980-02-08 FR FR8002749A patent/FR2475598A1/fr active Granted
-
1981
- 1981-01-29 DE DE8181400132T patent/DE3161811D1/de not_active Expired
- 1981-01-29 AT AT81400132T patent/ATE5783T1/de not_active IP Right Cessation
- 1981-01-29 EP EP81400132A patent/EP0034515B1/fr not_active Expired
- 1981-02-06 JP JP56016798A patent/JPS607106B2/ja not_active Expired
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3615960A (en) * | 1968-02-26 | 1971-10-26 | Fujikura Ltd | Bonding using epoxy resin composition and nonactivated blowing agent |
| US4156700A (en) * | 1975-08-11 | 1979-05-29 | Her Majesty The Queen In Right Of Canada, As Represented By The Minister Of National Defence | Solid propellants containing polyether or polyester binders |
Also Published As
| Publication number | Publication date |
|---|---|
| ATE5783T1 (de) | 1984-01-15 |
| FR2475598A1 (fr) | 1981-08-14 |
| DE3161811D1 (en) | 1984-02-09 |
| JPS607106B2 (ja) | 1985-02-22 |
| EP0034515A1 (fr) | 1981-08-26 |
| JPS56163175A (en) | 1981-12-15 |
| FR2475598B1 (enExample) | 1983-02-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CH633058A5 (fr) | Dispositif pour la realisation de tirants ancres dans le sol et utilisation dudit dispositif. | |
| EP0034515B1 (fr) | Application de mélanges autodestructibles à base de résines et de charges oxydantes à la réalisation de liaisons temporaires d'éléments de construction | |
| US4140428A (en) | Tie rod support for mine | |
| EP0389375B1 (fr) | Système d'enfoncement d'ancrage dans un sol | |
| US3936924A (en) | Releaseable steel cable anchor and method for withdrawing the same | |
| US4884377A (en) | Removable tension member | |
| EP0263803B1 (fr) | Procédé et dispositif d'ancrage sous contrainte | |
| US6380508B1 (en) | Apparatus and method for severing a tendon used in post-tension construction | |
| EP0251887B1 (fr) | Boulon de soutènement extensible, méthode de soutènement, utilisation du boulon | |
| FR2642158A1 (fr) | Procede de preparation d'un ensemble retardateur pour detonateur et ensemble retardateur | |
| EP1101076B1 (fr) | Procede de mise en oeuvre d'une substance pyrotechnique et initiateur pyrotechnique obtenu avec un tel procede | |
| EP1243702B1 (fr) | Dispositif d'étayage d'une paroi de soutènement de fouille et utilisation d'un tel dispositif | |
| AT393406B (de) | Vorrichtung zum trennen des zugglieds eines vorgespannten verpressankers durch bilden einer sollbruchstelle | |
| CA1073721A (fr) | Element pour la construction de joints de retrait ou de dilatation et element composite obtenu avec cet element | |
| FR2461190A1 (fr) | Bouchon pour obturation de tubes par explosion | |
| FR2524030A1 (fr) | Tete d'ancrage pour tirant composite precomprime a raccourcissement progressif | |
| CN117822746B (zh) | 一种不同型号混凝土连接装置及其连接工艺 | |
| EP0049646A1 (fr) | Procédé de scellement de boulons d'ancrage dans des terrains, au moyen d'un bloc de colle thermofusible chauffé in situ, et compositions correspondantes | |
| JPS5819814B2 (ja) | ドドメアンカ−コウホウ | |
| JP2832720B2 (ja) | 降雨時等の発破作業における飛石防止方法 | |
| EP3508654A1 (fr) | Pieu composite | |
| EP1194465A1 (fr) | Composition de protection des torons de cables pour ouvrages d'art | |
| AT526621A2 (de) | Ankeranordnung | |
| BE1006194A5 (fr) | Dispositif et procede de forage pour la formation de pieux ou analogues. | |
| DE10108597A1 (de) | Aluminothermisches Schweißverfahren und elektrischer Anzünder |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT LU |
|
| 17P | Request for examination filed |
Effective date: 19811029 |
|
| ITF | It: translation for a ep patent filed | ||
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT LI LU |
|
| REF | Corresponds to: |
Ref document number: 5783 Country of ref document: AT Date of ref document: 19840115 Kind code of ref document: T |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19840131 |
|
| REF | Corresponds to: |
Ref document number: 3161811 Country of ref document: DE Date of ref document: 19840209 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19900126 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19900130 Year of fee payment: 10 Ref country code: LU Payment date: 19900130 Year of fee payment: 10 |
|
| ITTA | It: last paid annual fee | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 19900131 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19900202 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19900222 Year of fee payment: 10 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Effective date: 19910129 Ref country code: AT Effective date: 19910129 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Effective date: 19910131 Ref country code: LI Effective date: 19910131 Ref country code: CH Effective date: 19910131 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Effective date: 19910930 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19911001 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 19930121 Year of fee payment: 13 |