EP0034515B1 - Use of autodestructive mixtures of resins and oxydant charges in the temporary bonding of constructional elements - Google Patents
Use of autodestructive mixtures of resins and oxydant charges in the temporary bonding of constructional elements Download PDFInfo
- Publication number
- EP0034515B1 EP0034515B1 EP81400132A EP81400132A EP0034515B1 EP 0034515 B1 EP0034515 B1 EP 0034515B1 EP 81400132 A EP81400132 A EP 81400132A EP 81400132 A EP81400132 A EP 81400132A EP 0034515 B1 EP0034515 B1 EP 0034515B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- resin
- application according
- inorganic filler
- combustion
- resins
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C06—EXPLOSIVES; MATCHES
- C06B—EXPLOSIVES OR THERMIC COMPOSITIONS; MANUFACTURE THEREOF; USE OF SINGLE SUBSTANCES AS EXPLOSIVES
- C06B45/00—Compositions or products which are defined by structure or arrangement of component of product
- C06B45/04—Compositions or products which are defined by structure or arrangement of component of product comprising solid particles dispersed in solid solution or matrix not used for explosives where the matrix consists essentially of nitrated carbohydrates or a low molecular organic explosive
- C06B45/06—Compositions or products which are defined by structure or arrangement of component of product comprising solid particles dispersed in solid solution or matrix not used for explosives where the matrix consists essentially of nitrated carbohydrates or a low molecular organic explosive the solid solution or matrix containing an organic component
- C06B45/10—Compositions or products which are defined by structure or arrangement of component of product comprising solid particles dispersed in solid solution or matrix not used for explosives where the matrix consists essentially of nitrated carbohydrates or a low molecular organic explosive the solid solution or matrix containing an organic component the organic component containing a resin
-
- E—FIXED CONSTRUCTIONS
- E01—CONSTRUCTION OF ROADS, RAILWAYS, OR BRIDGES
- E01D—CONSTRUCTION OF BRIDGES, ELEVATED ROADWAYS OR VIADUCTS; ASSEMBLY OF BRIDGES
- E01D21/00—Methods or apparatus specially adapted for erecting or assembling bridges
-
- E—FIXED CONSTRUCTIONS
- E02—HYDRAULIC ENGINEERING; FOUNDATIONS; SOIL SHIFTING
- E02D—FOUNDATIONS; EXCAVATIONS; EMBANKMENTS; UNDERGROUND OR UNDERWATER STRUCTURES
- E02D5/00—Bulkheads, piles, or other structural elements specially adapted to foundation engineering
- E02D5/74—Means for anchoring structural elements or bulkheads
- E02D5/76—Anchorings for bulkheads or sections thereof in as much as specially adapted therefor
- E02D5/765—Anchorings for bulkheads or sections thereof in as much as specially adapted therefor removable
Definitions
- the present invention relates to the application to the realization of temporary connections of building elements, by means of cold-hardenable resin and mineral filler.
- the invention relates more particularly to the fields of building and public works.
- a major problem in the field of construction is that of temporary connections, necessary to ensure the holding of a structure for a given time during which work is carried out on said structure or around it and which become useless once said work is finished.
- tie rods which are used to hold a wall of a building or building while work is being carried out on the building or building, or while excavations are being carried out. proximity, and which become useless after the end of the work.
- wedges intended to hold props where to temporarily compensate, during the construction phase, for the effects of pressure.
- the anchors are made up of cables or metal bars fixed at one of their ends to the structure which must be maintained and retained in the ground by the other of their ends.
- the buried end is generally coated with a bulb of solid material such as concrete or a curable resin.
- a bulb of solid material such as concrete or a curable resin.
- a first solution proposed in FR-A-2 274 740, consists in not coating the buried end of the tie rods in a bulb, but in welding this end to a buried metal plate and in placing around the welds an aluminum-thermal composition whose ignition causes the tie rods to shear and allows their withdrawal.
- This solution is however not very satisfactory insofar as the welds are always weak points and do not make it possible to give a high mechanical strength to the tie rods; moreover, after shearing, the metal anchoring plate remains in the ground.
- a second solution proposed in US-A-3,936,924, consists in coating the tie rods with a thermoplastic resin which is melted and possibly burned using an auxiliary heat source, at the end of the work, to release the tie rods and remove them.
- This resin is made combustible by adding a mineral filler such as black powder or thermite.
- This solution has several drawbacks: it requires a significant additional source of heat and is therefore relatively expensive, and if it does allow the ground rods to be removed, it leaves agglomerates of molten resin, which does not entirely answer the problem posed and furthermore makes any subsequent reuse of drilling impossible for the laying of a new tie rod, finally it does not ensure regular and non-explosive combustion.
- the present invention aims to remedy these drawbacks and, for this purpose, it provides, for the application to the realization of temporary connections of building elements by means of cold-hardenable resin and mineral filler, that the connection is made. by a hardened mixture, self-destructing by combustion and not detonating, of the resin and of an oxidizing mineral filler, the quantity by weight of resin being between 20 and 40% of the total mixture, an ignition system triggering the combustion.
- an ignition device By placing an ignition device in contact with said temporary connection, constituted for example by an electric igniter and a pyrotechnic ignition composition, it is possible to cause, at the appropriate time, the complete combustion of the combustible connection which will be completely destroyed by very simple way.
- the temporary connections according to the invention are produced from mixtures consisting of a cold-hardening resin made combustible by the addition of an oxidizing mineral filler, the quantity by weight of resin being between 20 and 40% of the total mixture, said mixture being hardened and preferably having an ignition temperature equal to or greater than 500 ° C.
- a temporary bond according to the invention is therefore essentially constituted by a cold-hardening resin and made combustible by the addition of an oxidizing mineral filler.
- the resin must be cold hardenable, usually in the presence of a catalyst, so that it can be easily poured and hardened on a site.
- the resin must have two essential qualities: it must, once hardened, have good mechanical strength and must not be corrosive with respect to the elements which it makes integral, in particular when the latter are metallic elements.
- the preferred resins according to the invention are polyurethane, polyester or polyepoxy resins.
- These resins are two-component resins which harden in the presence of a catalyst which is generally a tertiary amine or a metal salt of fatty acid for polyurethane resins, a peroxide associated with a metal salt or an amine for resins polyesters, an amine for polyepoxy resins.
- a catalyst which is generally a tertiary amine or a metal salt of fatty acid for polyurethane resins, a peroxide associated with a metal salt or an amine for resins polyesters, an amine for polyepoxy resins.
- Said resin is mixed in the liquid state with an oxidizing mineral filler which increases its mechanical strength after hardening and which makes the mixture combustible.
- an oxidizing mineral filler which increases its mechanical strength after hardening and which makes the mixture combustible.
- the hardened mixture of charged resin has an ignition temperature equal to or higher than 500 ° C so as not to risk accidental ignition.
- the charged resin must be perfectly combustible but must not, for safety reasons, present risks of detonation during combustion. Oxidizing charges such as potassium chlorate are therefore not recommended.
- oxidizing mineral fillers chosen from the group consisting of perchlorates and nitrates of an alkaline nature meet the criteria set out above.
- perchlorates or nitrates of an alkaline character means perchlorates or nitrates in which the cation is a true alkali or alkaline earth metal, or in which the cation is the ammonium group.
- the preferred alkali perchlorates and nitrates are potassium perchlorate, ammonium perchlorate and potassium nitrate.
- the oxidizing mineral filler must advantageously have a particle size between 40 and 200 microns.
- the quantity by weight of resin must be between 20 and 40% of the weight of the mixture and preferably between 23 and 30%. The Applicant has observed that mixtures consisting of 25% resin and 75% oxidizing mineral filler give very good results.
- the oxidizing mineral filler is mixed, in a manner known per se, in a kneader, either with one of the liquid components of the resin, in which case the second liquid component generally containing the catalyst is then added, or directly with the liquid resin not containing the catalyst, which is only added immediately before use.
- the loaded liquid resin containing the curing catalyst is then applied to the elements which should be made integral.
- the hardening of the charged resin is then very rapid.
- an ignition device which generally consists of a pyrotechnic priming paste and an electric igniter.
- the electric igniter which can be controlled remotely, ignites the priming paste, which itself ignites and burns the charged resin. After combustion of the resin, the elements made temporarily united, regain their individuality.
- the temporary connections according to the invention find multiple applications in the field of construction.
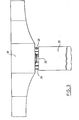
- a tie rod 11 made up of several metallic cables sealed inside a corrugated plastic sheath 13 by means of a charged resin 14 according to the invention, and constituting the steel-sheath seal, crosses a retaining wall 12 to anchor in the ground by means of a bulb constituted by a cement grout 19 injected in place.
- the free part of the tie rod is protected, over its entire buried length, by a second smooth sheath 18.
- a priming paste 15 composed, for example, of 75% by weight of potassium nitrate, 15% of magnesium and 10% of a resin serving as a binder is placed on the external surface of the resin.
- An electric igniter 16 connected to a control station 17 and embedded in the priming paste, allows the latter to be ignited; this thus ignites the resin 14 which, on burning, will release the complete tie rod 11 is lying. It is then possible to remove the tie rod 11 1 over its entire length either permanently or to replace it in the event of damage.
- the resin 14 can also be used to seal the tie rod 11 directly in a material without passing through a cement slurry or a sheath 13 when said material is sufficiently homogeneous as in the case of rock for example.
- FIG. 3 Another interesting application of the invention, represented in FIG. 3, relates to the cantilever execution of a bridge deck by beams constructed symmetrically on either side of the piers. It is necessary in this case, if one wants to avoid shoring, to securely secure the central segment 21 of each beam on its pile 22, around the final bearings 23 of the deck on the piles, to stabilize the plagues as long as these are not interconnected. This fixing is advantageously carried out by interposition, between the segment 21 and the stack 22, of fuel wedges 24 produced from a charged resin according to the invention. When the construction of the bridge is finished, it is easy to get rid of the wedges that have become useless by causing them to burn.
- Another interesting application of the invention is in the field of underwater work.
- a simple example is the temporary setting of a forestay which will have to be removed at the end of the realization of a metal or concrete structure, as shown in Figures 4 and 5.
- the forces transmitted to the forestay 41, during the construction of the structure, pass from the forestay to the support 42 by means of a wedge produced according to the invention.
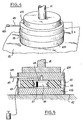
- the fuse block may have a circular or rectangular shape or any other shape suitable for the use. It consists of two support plates 431 and 432 between which is placed a ring of combustible resin 44 charged according to the invention and bonded to the two plates so as to create a combustion chamber 45. An ignition composition 46 is applied on the walls of this chamber and an igniter 47 allows the fuel crown to be ignited. A nozzle 48 allows the evacuation of combustion gases. The nozzle 48 can advantageously be equipped at the outlet with a non-return valve 481 preventing the penetration of water into the hold or be extended by a vent pipe. The flexible seals 49 seal the assembly.
- FIG. 6 represents another possible application of the invention.
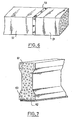
- Two prefabricated elements 51 and 52 are temporarily made integral by bonding with an intermediate layer 53 of combustible resin loaded according to the invention. After combustion of the intermediate layer the two elements regain their individuality.
- FIG. 7 represents another possibility of applying the invention.
- a concrete beam 61, having a weakening, is temporarily reinforced by a metal plate 62 bonded by means of a layer of combustible mixture 63 according to the invention.
- This example concerns anchor rods. Two types of test were carried out on tie rods coated with charged resin according to the invention.
- a 12 mm diameter metal cable is trapped in a plastic sheath filled with resin loaded with 75% by weight of potassium perchlorate.
- a second test piece is produced using an unfilled resin.
- a polyester resin was used on the one hand and a polyepoxy resin on the other hand.
- the cable is pulled using a cylinder in 50 bar steps with a stop time of one minute between each level. It was considered that the adhesion break is reached when the pressure drop is recorded. The following results were obtained.
- the tests were carried out on 12 strands sealed on one meter by resin loaded inside a metal sheath of 65 mm in diameter.
- the ignition was carried out using a wick and a priming paste.
- the purpose of this example is to verify the possibility of burning a charged resin according to the invention in a confined atmosphere.
- the blocks were placed in a metal combustion chamber having roughly the outside dimensions of the block and closed by a metal nozzle.
- the ignition was carried out using a wick and a priming paste.
Landscapes
- Engineering & Computer Science (AREA)
- Structural Engineering (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Civil Engineering (AREA)
- Organic Chemistry (AREA)
- General Engineering & Computer Science (AREA)
- Dispersion Chemistry (AREA)
- Molecular Biology (AREA)
- Crystallography & Structural Chemistry (AREA)
- Architecture (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Mining & Mineral Resources (AREA)
- Paleontology (AREA)
- Health & Medical Sciences (AREA)
- Adhesives Or Adhesive Processes (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Conveying And Assembling Of Building Elements In Situ (AREA)
- Working Measures On Existing Buildindgs (AREA)
- Building Environments (AREA)
- Glass Compositions (AREA)
- Organic Insulating Materials (AREA)
- Furnace Housings, Linings, Walls, And Ceilings (AREA)
Abstract
Description
La présente invention concerne l'application à la réalisation de liaisons temporaires d'éléments de construction, au moyen de résine durcissable à froid et de charge minérale. L'invention concerne plus spécialement les domaines du bâtiment et des travaux publics.The present invention relates to the application to the realization of temporary connections of building elements, by means of cold-hardenable resin and mineral filler. The invention relates more particularly to the fields of building and public works.
Un problème majeur dans le domaine de la construction est celui des liaisons temporaires, nécessaires pour assurer la tenue d'un ouvrage pendant un temps donné au cours duquel des travaux sont effectués sur ledit ouvrage ou alentour et qui deviennent inutiles une fois que lesdits travaux sont terminés. On peut ainsi citer l'exemple des tirants d'ancrage qui servent à maintenir un mur d'un immeuble ou d'un édifice pendant que des travaux sont effectués sur l'immeuble ou l'édifice, ou pendant que des fouilles sont effectuées à proximité, et qui deviennent inutiles après la fin des travaux. On peut encore citer à titre d'exemple le cas, dans la construction en encorbellement d'un tablier de pont, des celes placées provisoirement entre les piles ou les voussoirs centraux de chaque fléau ou celui, dans le domaine des travaux sous l'eau, des cales destinées à tenir des étais où compenser temporairement, en phase de construction, les effets de la pression.A major problem in the field of construction is that of temporary connections, necessary to ensure the holding of a structure for a given time during which work is carried out on said structure or around it and which become useless once said work is finished. We can thus cite the example of tie rods which are used to hold a wall of a building or building while work is being carried out on the building or building, or while excavations are being carried out. proximity, and which become useless after the end of the work. We can also cite as an example the case, in the cantilever construction of a bridge deck, those temporarily placed between the piers or the central segments of each beam or that, in the field of underwater works , wedges intended to hold props where to temporarily compensate, during the construction phase, for the effects of pressure.
Lorsque la présence desdites liaisons temporaires n'est plus nécessaire, se pose alors le problème de leur destruction qui est en règle générale complexe et onéreux de fait que ces liaisons sont souvent constituées à partir de matériaux résistants et difficilement destructibles, comme le béton ou l'acier. Dans certains cas on peut laisser subsister tout ou partie de ces liaisons, mais il est des ces où, pour des causes de fonctionnement ou pour des raisons réglementaires, il est impératif de procéder à la destruction de la liaison.When the presence of said temporary connections is no longer necessary, there then arises the problem of their destruction which is generally complex and expensive because these connections are often made from resistant and hardly destructible materials, such as concrete or l 'steel. In some cases, all or part of these links can be left to exist, but there are some where, for operational reasons or for regulatory reasons, it is imperative to destroy the link.
Il en est ainsi par exemple les tirants d'ancrage mentionnés plus haut. Les tirants d'ancrage sont constitués par des câbles ou barres métalliques fixés à une de leurs extrémités à l'ouvrage qui doit être maintenu et retenus en terre par l'autre de leurs extrémités. De manière à assurer une bonne retenue, l'extrémité enterrée est en général enrobée par un bulbe de matériau solide tel que du béton ou une résine durcissable. A la fin des travaux, il était d'usage de cisailler la partie aérienne des tirants et de laisser en terre la partie scellée. Il n'est cependant pas toujours possible ou permis de laisser en terre de tels éléments et un nombre croissant de législations nationales imposant aux utilisateurs de tirants de procéder au retrait complet de ceux-ci.This is the case, for example, with the anchors mentioned above. The anchors are made up of cables or metal bars fixed at one of their ends to the structure which must be maintained and retained in the ground by the other of their ends. In order to ensure good retention, the buried end is generally coated with a bulb of solid material such as concrete or a curable resin. At the end of the work, it was customary to shear the aerial part of the tie rods and leave the sealed part in the ground. However, it is not always possible or permissible to leave such elements and an increasing number of national legislations obliging users of tie rods to proceed to their complete withdrawal.
On a alors cherché des solutions qui permettent un tel retrait. Une première solution, proposée dans FR-A-2 274 740, consiste à ne pas enrober l'extrémité enterrée des tirants dans un bulbe, mais à souder cette extrémité à une plaque métallique enterrée et à placer autour des soudures une composition alumino- thermique dont la mise à feu provoque le cisaillement des tirants et permet leur retrait. Cette solution n'est cependant pas très satisfaisante dans la mesure où les soudures sont toujours des points faibles et ne permettent pas de donner une grande résistante mécanique aux tirants; de plus, après cisaillement, la plaque métallique d'ancrage demeure dans le terrain. Une deuxième solution, proposée dans US-A-3 936 924, consiste à enrober les tirants avec une résine thermoplastique que l'on fait fondre et éventuellement brûler à l'aide d'une source annexe de chaleur, à la fin des travaux, pour dégager les tirants et les retirer. Cette résine est rendue combustible par adjonction d'une charge minérale telle que de la poudre noire ou de la thermite. Cette solution présente plusieurs inconvénients: elle nécessite une source annexe importante de chaleur et est donc relativement onéreuse, et si elle permet bien de retirer les tirants de terre, elle laisse subsister des agglomérats de résine fondue, ce que ne répond pas entièrement au problème posé et rend impossible de surcroît toute réutil- lisation ultérieure de forage pour la pose d'un nouveau tirant, enfin elle ne permet pas d'assurer une combustion régulière et non détonante.We then sought solutions that allow such a withdrawal. A first solution, proposed in FR-A-2 274 740, consists in not coating the buried end of the tie rods in a bulb, but in welding this end to a buried metal plate and in placing around the welds an aluminum-thermal composition whose ignition causes the tie rods to shear and allows their withdrawal. This solution is however not very satisfactory insofar as the welds are always weak points and do not make it possible to give a high mechanical strength to the tie rods; moreover, after shearing, the metal anchoring plate remains in the ground. A second solution, proposed in US-A-3,936,924, consists in coating the tie rods with a thermoplastic resin which is melted and possibly burned using an auxiliary heat source, at the end of the work, to release the tie rods and remove them. This resin is made combustible by adding a mineral filler such as black powder or thermite. This solution has several drawbacks: it requires a significant additional source of heat and is therefore relatively expensive, and if it does allow the ground rods to be removed, it leaves agglomerates of molten resin, which does not entirely answer the problem posed and furthermore makes any subsequent reuse of drilling impossible for the laying of a new tie rod, finally it does not ensure regular and non-explosive combustion.
La présente invention vise à remédier à ces inconvénients et, à cet effet, elle prévoit, pour l'application à la réalisation de liaisons temporaires d'éléments de construction au moyen de résine durcissable à froid et de charge minérale, que fa liaison est réalisée par un mélange durci, autodestructible par combustion et non détonant, de la résine et d'une charge minérale oxydante, la quantité pondérale de résine étant comprise entre 20 et 40% du mélange total, un système d'allumage déclenchant la combustion.The present invention aims to remedy these drawbacks and, for this purpose, it provides, for the application to the realization of temporary connections of building elements by means of cold-hardenable resin and mineral filler, that the connection is made. by a hardened mixture, self-destructing by combustion and not detonating, of the resin and of an oxidizing mineral filler, the quantity by weight of resin being between 20 and 40% of the total mixture, an ignition system triggering the combustion.
Grâce à l'utilisation d'une charge minérale oxydante, on peut utiliser des mélanges de liaison assurant une combustion régulière et non détonante.Thanks to the use of an oxidizing mineral filler, it is possible to use bonding mixtures ensuring regular and non-detonating combustion.
En disposant au contact de ladite liaison temporaire un dispositif d'allumage, constitué par exemple par un allumeur électrique et une composition d'amorçage pyrotechnique, on peut provoquer, au moment voulu, la combustion complète de la liaison combustible qui se trouvera totalement détruite de façon très simple.By placing an ignition device in contact with said temporary connection, constituted for example by an electric igniter and a pyrotechnic ignition composition, it is possible to cause, at the appropriate time, the complete combustion of the combustible connection which will be completely destroyed by very simple way.
L'invention sera mieux comprise à l'aide de la description et des exemples qui suivant et qui font référence aux figures énumérées ci-après.
- La figure 1 représente un tirant d'ancrage dont la partie scellée est enrobée par une résine chargée selon l'invention.
- La figure 2 représente une variante d'exécution du dispositif représenté à la figure 1.
- La figure 3 représente des cales utilisées lors de la construction en encorbellement d'un tablier de pont par fléaux construits symétriquement de part et d'autre des piles.
- Les figures 4 et 5 représentent une cale pouvant être utilisée sous l'eau.
- La figure 6 représente des éléments préfabriqués dissociables, rendus provisoirement solidaires par une résine chargée selon l'invention.
- La figure 7 représente une pièce de renfort collée provisoirement à une poutre par une résine chargée selon l'invention.
- FIG. 1 represents an anchoring tie, the sealed part of which is coated with a charged resin according to the invention.
- FIG. 2 represents an alternative embodiment of the device shown in FIG. 1.
- Figure 3 shows wedges used during of the cantilever construction of a bridge deck by symmetrically constructed beams on either side of the piers.
- Figures 4 and 5 show a wedge that can be used underwater.
- FIG. 6 represents dissociable prefabricated elements, temporarily made integral by a charged resin according to the invention.
- FIG. 7 represents a reinforcement piece temporarily bonded to a beam with a charged resin according to the invention.
Comme il a déjà été dit plus haut, les liaisons temporaires selon l'invention sont réalisées à partir de mélanges constitués par une résine durcissable à froid rendue combustible par adjonction d'une charge minérale oxydante, la quantité pondérale de résine étant comprise entre 20 et 40% du mélange total, ledit mélange étant durci et présentant préférentiellement une température d'inflammation égale ou supérieure à 500°C.As already mentioned above, the temporary connections according to the invention are produced from mixtures consisting of a cold-hardening resin made combustible by the addition of an oxidizing mineral filler, the quantity by weight of resin being between 20 and 40% of the total mixture, said mixture being hardened and preferably having an ignition temperature equal to or greater than 500 ° C.
Une liaison temporaire selon l'invention est donc essentiellement constituée par une résine durcissable à froid et rendue combustible par adjonction d'une charge minérale oxydante. La résine doit être durcissable à froid, en général en présence d'un catalyseur, de manière à pouvoir être facilement coulée et durcie sur un chantier. La résine doit présenter deux qualités essentielles: elle doit, une fois durcie, présenter une bonne tenue mécanique et ne doit pas être corrosive vis à vis des élements qu'elle rend solidaires, notamment lorsque ces derniers sont des éléments métalliques. One peut dire qu'en règle générale les résines fortement acides ou basiques, éventuellement en raison de catalyseur employé, sont à proscrire. Les résines préférées selon l'invention sont les résines polyuréthannes, polyesters ou polyépoxy. Ces résines sont des résines bi-composants qui durcissent en présence d'un catalyseur qui est en général une amine tertiaire ou un sel métallique d'acide gras pour les résines polyuréthannes, un peroxyde associé à un sel métallique ou à une amine pour les résines polyesters, une amine pour les résines polyépoxy.A temporary bond according to the invention is therefore essentially constituted by a cold-hardening resin and made combustible by the addition of an oxidizing mineral filler. The resin must be cold hardenable, usually in the presence of a catalyst, so that it can be easily poured and hardened on a site. The resin must have two essential qualities: it must, once hardened, have good mechanical strength and must not be corrosive with respect to the elements which it makes integral, in particular when the latter are metallic elements. One can say that as a general rule strongly acidic or basic resins, possibly due to the catalyst used, are to be avoided. The preferred resins according to the invention are polyurethane, polyester or polyepoxy resins. These resins are two-component resins which harden in the presence of a catalyst which is generally a tertiary amine or a metal salt of fatty acid for polyurethane resins, a peroxide associated with a metal salt or an amine for resins polyesters, an amine for polyepoxy resins.
Ladite résine est mélangée à l'état liquide avec une charge minérale oxydante qui augmente sa tenue mécanique après durcissement et qui rend le mélange combustible. Pour des raisons de sécurité, il est préférable que le mélange durci de résine chargée présente une température d'inflammation égale ou supérieure à 500°C de manière à ne pas risquer une inflammation accidentelle. Par ailleurs, la résine chargée doit être parfaitement combustible mais ne doit pas, pour des raisons de sécurité, présenter des risques de détonation en cours de combustion. Des charges oxydantes comme le chlorate de potassium sont, pour cette raison, déconseillées. La demanderesse a constaté que des charges minérales oxydantes choisies dans le groupe constitué par les perchlorates et nitrates à caractère alcalin répondent bien aux critères énoncés ci-dessus. On entend par perchlorates ou nitrates à caractère alcalin les perchlorates ou les nitrates dont le cation est un métal alcalin vrai ou alcalino-terreux, ou encore dont le cation est le groupe ammonium.Said resin is mixed in the liquid state with an oxidizing mineral filler which increases its mechanical strength after hardening and which makes the mixture combustible. For safety reasons, it is preferable that the hardened mixture of charged resin has an ignition temperature equal to or higher than 500 ° C so as not to risk accidental ignition. Furthermore, the charged resin must be perfectly combustible but must not, for safety reasons, present risks of detonation during combustion. Oxidizing charges such as potassium chlorate are therefore not recommended. The Applicant has found that oxidizing mineral fillers chosen from the group consisting of perchlorates and nitrates of an alkaline nature meet the criteria set out above. The term “perchlorates or nitrates of an alkaline character” means perchlorates or nitrates in which the cation is a true alkali or alkaline earth metal, or in which the cation is the ammonium group.
Les perchlorates et nitrates à caractère alcalin préférés sont le perchlorate de potassium, le perchlorate d'ammonium et le nitrate de potassium. La charge minérale oxydante doit avantageusement avoir une granulométrie comprise entre 40 et 200 microns. La quantité pondérale de résine doit être comprise entre 20 et 40% du poids du mélange et préférentiellement entre 23 et 30%. La demanderesse a observé que des mélanges constitués de 25% de résine et de 75% de charge minérale oxydante donnent de très bons résultats.The preferred alkali perchlorates and nitrates are potassium perchlorate, ammonium perchlorate and potassium nitrate. The oxidizing mineral filler must advantageously have a particle size between 40 and 200 microns. The quantity by weight of resin must be between 20 and 40% of the weight of the mixture and preferably between 23 and 30%. The Applicant has observed that mixtures consisting of 25% resin and 75% oxidizing mineral filler give very good results.
La charge minérale oxydante est mélangée, d'une manière connue en soi, dans un malaxeur, soit à l'un des composants liquides de la résine, auquel cas on ajoute ensuite le deuxième composant liquide contenant en général le catalyseur, soit directement à la résine liquide ne contenant pas le catalyseur, qui n'est ajouté qu'immédiatement avant l'emploi. La résine liquide chargée et contenant le catalyseur de durcissement est alors appliquée aux éléments qu'il convient de render solidaires. Le durcissement de la résine chargée est alors très rapide. One dispose ainsi d'une liaison solide que l'on peut détruire facilement par combustion. Pour ce faire on dispose, ou contact de la liaison constituée par la résine chargée, un dispositif d'allumage qui se compose en général d'une pâte d'amorçage pyrotechnique et d'un alumeur électrique. L'allumeur électrique, qui peut être commandé à distance, provoque la mise à feu de la pâte d'amorçage qui provoque elle-même l'inflammation et la combustion de la résine chargée. Après combustion de la résine, les éléments rendus temporairement solidaires, retrouvent leur individualité.The oxidizing mineral filler is mixed, in a manner known per se, in a kneader, either with one of the liquid components of the resin, in which case the second liquid component generally containing the catalyst is then added, or directly with the liquid resin not containing the catalyst, which is only added immediately before use. The loaded liquid resin containing the curing catalyst is then applied to the elements which should be made integral. The hardening of the charged resin is then very rapid. One thus has a solid bond which can be easily destroyed by combustion. To do this, there is, or contact of the connection formed by the charged resin, an ignition device which generally consists of a pyrotechnic priming paste and an electric igniter. The electric igniter, which can be controlled remotely, ignites the priming paste, which itself ignites and burns the charged resin. After combustion of the resin, the elements made temporarily united, regain their individuality.
Les liaisons temporaires selon l'invention trouvent des applications multiples dans le domaine de la construction.The temporary connections according to the invention find multiple applications in the field of construction.
Ainsi, dans le cas des tirants d'ancrage, en se reportant à la figure 1, un tirant 11 constitué de plusieurs câbles métalliques scellés à l'intérieur d'une gaine plastique annelée 13 au moyen d'une résine chargée 14 selon l'invention, et constituant le scellement aciergaine, traverse .un mur de soutènement 12 pour s'ancrer dans le terrain au moyen d'un bulbe constitué par un coulis de ciment 19 injecté en place. La partie libre du tirant est protégée, sur toute sa longueur enterrée, par une deuxième gaine lisse 18. Une pâte, d'amorçage 15 composée, par exemple, de 75% en poids de nitrate de potassium, de 15% de magnésium et de 10% d'une résine servant de liant, est placée sur la surface externe de la résine. Un allumeur électrique 16, relié à un poste de commande 17 et noyé dans la pâte d'amorçage, permet la mise à feu de cette dernière; celle-ci allume ainsi la résine 14 qui, en brûlant, va libérer le tirant 11 complètement. Il est alors possible de retirer le tirant 11 1 sur toute sa longueur soit définitivement, soit pour le remplacer en cas d'avarie.Thus, in the case of anchor tie rods, with reference to FIG. 1, a
C'est en effet des avantages de l'invention, par rapport aux solutions antérieures de la technique, que de réaliser une destruction totale du scellement permettant le remplacement aisé d'un tirant en cas de besoin.It is indeed advantages of the invention, compared to the prior art solutions, to achieve total destruction of the seal allowing the easy replacement of a tie rod if necessary.
Comme représenté à la figure 2, la résine 14 peut également être utilisée pour sceller le tirant 11 directement dans un matériau sans passer par l'intermédiaire d'un coulis de ciment, ni d'une gaine 13 quand ledit matériau est suffisamment homogène comme dans le cas de rocher par exemple.As shown in Figure 2, the
Une autre application intéressante de l'invention, représentée à la figure 3, concerne l'exécution en encorbellement d'un tablier de pont par fléaux construits symétriquement de part et d'autre des piles. Il est nécessaire dans ce cas, si l'on veut éviter d'étayer, d'assujettir solidement le voussoir central 21 de chaque fléau sur sa pile 22, autour des appareils d'appui définitifs 23 du tablier sur les piles, pour stabiliser les fléaux tant que ceux-ci ne sont pas reliés entre eux. Cette fixation est avantageusement réalisée par interposition, entre le voussoir 21 et la pile 22, de cales 24 combustibles réalisées à partir d'une résine chargée selon l'invention. Lorsque la construction du pont est terminée, il est facile de se débarrasser des cales devenues inutiles en provoquant leur combustion.Another interesting application of the invention, represented in FIG. 3, relates to the cantilever execution of a bridge deck by beams constructed symmetrically on either side of the piers. It is necessary in this case, if one wants to avoid shoring, to securely secure the
Une autre application intéressante de l'invention se situe dans le domaine des travaux sous l'eau. Un exemple simple est le calage provisoire d'un étai de construction qui devra être supprimé en fin de réalisation d'une structure métallique ou en béton, comme représenté aux figures 4 et 5. Les efforts transmis à l'étai 41, lors de la construction de l'ouvrage, passent de l'étai au support 42 par l'intermédiaire d'une cale réalisée selon l'invention.Another interesting application of the invention is in the field of underwater work. A simple example is the temporary setting of a forestay which will have to be removed at the end of the realization of a metal or concrete structure, as shown in Figures 4 and 5. The forces transmitted to the
La cale fusible peut avoir une forme circulaire ou rectangulaire ou toute autre forme adaptée à l'utilisation. Elle est constituée par deux plaques d'appui 431 et 432 entre lesquelles est placée une couronne de résine combustible 44 chargée selon l'invention et collée aux deux plaques de manière à créer une chambre de combustion 45. Une composition d'allumage 46 est appliquée sur les parois de cette chambre et un allumeur 47 permet la mise à feu de la couronne combustible. Une tuyère 48 permet l'évacuation des gaz de combustion. La tuyère 48 peut avantageusement être équipée à la sortie d'un clapet anti-retour 481 évitant la pénétration de l'eau dans la cale ou être prolongée par un tuyau de mise à l'air libre. Les joints souples 49 rendent l'ensemble étanche.The fuse block may have a circular or rectangular shape or any other shape suitable for the use. It consists of two
Lorsque l'on veut retirer la cale, il suffit de provoquer, à l'aide de l'allumeur électrique 47, l'inflammation et la combustion de la couronne combustible 44. La disparition de cette couronne libèrera les contraintes entre les parois et la force appliquée et il deviendra aisé de retirer le vérin 41.When it is desired to remove the wedge, it suffices to cause, by means of the
La figure 6 représente une autre application possible de l'invention. Deux éléments préfabriqués 51 et 52 sont temporairement rendus solidaires par collage avec une couche inter- médaire 53 de résine combustible chargée selon l'invention. Après combustion de la couche intermédiaire les deux éléments retrouvent leur individualité.FIG. 6 represents another possible application of the invention. Two
La figure 7 représente une autre possibilité d'application de l'invention.FIG. 7 represents another possibility of applying the invention.
Une poutre en béton 61, présentant un affaiblissement, est renforcée de façon temporaire par une plaque métallique 62 collée au moyen d'une couche de mélange combustible 63 selon l'invention.A
Lorsque la consolidation temporaire n'est plus nécessaire, parce que la surcharge de la poudtre a été supprimée par exemple, il suffit de provoquer la combustion de la couche 63 pour retirer la plaque 62.When temporary consolidation is no longer necessary, for example because the overload of the powder has been removed, it suffices to cause the burning of the
On a indiqué ci-dessus un certain nombre de possibilités d'applications industrielles de l'invention; l'homme de métier peut, en fonction des problèmes auxquels il est confronté, en trouver d'autres sans pour autant sortir du cadre de la présente invention, l'étendue de la protection étant déterminée par la teneur des revendications.A number of possible industrial applications of the invention have been indicated above; those skilled in the art can, depending on the problems with which they are confronted, find others without departing from the scope of the present invention, the scope of protection being determined by the content of the claims.
On illustre dans les exemples qui suivent certaines réalisations particulières de l'invention.The following examples illustrate certain specific embodiments of the invention.
Cet exemple concerne des tirants d'ancrage. On a effectué sur des tirants enrobés de résine chargée selon l'invention deux types d'essai.This example concerns anchor rods. Two types of test were carried out on tie rods coated with charged resin according to the invention.
Un cable métallique de diamètre 12 mm est emprisonné dans une gaine plastique remplie de résine chargée à 75% en poids de perchlorate de potassium.A 12 mm diameter metal cable is trapped in a plastic sheath filled with resin loaded with 75% by weight of potassium perchlorate.
Un deuxième éprouvette est réalisée en utilisant une résine non chargée. On a employé d'une part une résine polyester et d'autre part une résine polyépoxy.A second test piece is produced using an unfilled resin. A polyester resin was used on the one hand and a polyepoxy resin on the other hand.
On tire sur le câble à l'aide d'un vérin par paliers de 50 bars avec un temps d'arrêt de une minutre entre chaque palier. On a considéré que la rupture d'adhérence est atteinte lorsque la chute de pression est enregistrée. Les résultants suivants ont été obtenus.
Les essais ont été effectués sur 12 torons scellés sur un mètre par de la résine chargée à l'intérieur d'une gaine métallique de 65 mm de diamètre. L'allumage a été réalisé à l'aide d'une mèche étoupille et d'une pâte d'amorçage.The tests were carried out on 12 strands sealed on one meter by resin loaded inside a metal sheath of 65 mm in diameter. The ignition was carried out using a wick and a priming paste.
On a chronométré les temps de combustion de la résine chargée. Avec une résine époxy chargée à 75% en poids de perchlorate de potassium on a observé 22 minutes de combustion régulière; avec une résine polyester chargée à 73% en poids de perchlorate de potassium on a observé 33 minutes de combustion régulière. Dans les deux cas la combustion a été complète et s'est faite pendant toute la durée à un régime uniforme.The combustion times of the charged resin were timed. With an epoxy resin loaded with 75% by weight of potassium perchlorate, 22 minutes of regular combustion were observed; with a polyester resin loaded with 73% by weight of potassium perchlorate, 33 minutes of regular combustion were observed. In both cases the combustion was complete and was carried out throughout the duration at a uniform speed.
Cet exemple a pour objet de vérifier la possibilité de faire brûler en atmosphère confinée une résine chargée selon l'invention. Pour ce faire, on a utilisé des blocs cylindriques pleins de diamètre 34 mm et de hauteur 28 mm constitués par une résine polyester chargée à 75% en poids de perchlorate de potassium. Les blocs ont été placés dans une chamber de combustion métallique possédant à peu prés les cotes extérieures du bloc et fermée par une tuyère métallique. L'allumage a été réalisé à l'aide d'une mèche étoupille et d'une pâte d'amorçage.The purpose of this example is to verify the possibility of burning a charged resin according to the invention in a confined atmosphere. To do this, we used solid cylindrical blocks with a diameter of 34 mm and a height of 28 mm consisting of a polyester resin loaded with 75% by weight of potassium perchlorate. The blocks were placed in a metal combustion chamber having roughly the outside dimensions of the block and closed by a metal nozzle. The ignition was carried out using a wick and a priming paste.
On a observé les durées de combustion suivantes en fonction du diamètre de la tuyère:
Dans tous les cas le combustion du bloc a été complète.In all cases the combustion of the block was complete.
Claims (10)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT81400132T ATE5783T1 (en) | 1980-02-08 | 1981-01-29 | USE OF SELF-DESTRUCTIVE RESIN MIXTURES AND OXIDIZING CHARGES TO REALIZE TEMPORARY JOINTS OF CONSTRUCTION ELEMENTS. |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR8002749A FR2475598A1 (en) | 1980-02-08 | 1980-02-08 | APPLICATION OF AUTODESTRUCTIBLE MIXTURES BASED ON RESINS AND OXIDIZING LOADS TO THE PRODUCTION OF TEMPORARY LINKS OF CONSTRUCTION ELEMENTS |
| FR8002749 | 1980-02-08 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0034515A1 EP0034515A1 (en) | 1981-08-26 |
| EP0034515B1 true EP0034515B1 (en) | 1984-01-04 |
Family
ID=9238343
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP81400132A Expired EP0034515B1 (en) | 1980-02-08 | 1981-01-29 | Use of autodestructive mixtures of resins and oxydant charges in the temporary bonding of constructional elements |
Country Status (5)
| Country | Link |
|---|---|
| EP (1) | EP0034515B1 (en) |
| JP (1) | JPS607106B2 (en) |
| AT (1) | ATE5783T1 (en) |
| DE (1) | DE3161811D1 (en) |
| FR (1) | FR2475598A1 (en) |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5099767B2 (en) * | 2006-01-19 | 2012-12-19 | 旭化成ケミカルズ株式会社 | Decomposable oxidant-containing adhesive |
| CN101743288B (en) * | 2007-07-19 | 2012-07-18 | 旭化成化学株式会社 | Detachable adhesive containing reaction product of oxidizing agent and amine compound |
| CN102828470B (en) * | 2012-09-05 | 2014-06-11 | 中建七局第二建筑有限公司 | Method for locating main girder cable sleeve of cable-stayed bridge |
| CN108952211A (en) * | 2018-07-03 | 2018-12-07 | 中国人民解放军陆军工程大学 | A kind of demolition blasting device of tubular steel column structure building |
| CN111335165B (en) * | 2020-03-04 | 2021-11-16 | 中铁第四勘察设计院集团有限公司 | Construction method for sealing anchor and auxiliary anchor sealing structure thereof |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3615960A (en) * | 1968-02-26 | 1971-10-26 | Fujikura Ltd | Bonding using epoxy resin composition and nonactivated blowing agent |
| US4156700A (en) * | 1975-08-11 | 1979-05-29 | Her Majesty The Queen In Right Of Canada, As Represented By The Minister Of National Defence | Solid propellants containing polyether or polyester binders |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3936924A (en) * | 1973-09-21 | 1976-02-10 | Yoshio Ichise | Releaseable steel cable anchor and method for withdrawing the same |
-
1980
- 1980-02-08 FR FR8002749A patent/FR2475598A1/en active Granted
-
1981
- 1981-01-29 EP EP81400132A patent/EP0034515B1/en not_active Expired
- 1981-01-29 DE DE8181400132T patent/DE3161811D1/en not_active Expired
- 1981-01-29 AT AT81400132T patent/ATE5783T1/en not_active IP Right Cessation
- 1981-02-06 JP JP56016798A patent/JPS607106B2/en not_active Expired
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3615960A (en) * | 1968-02-26 | 1971-10-26 | Fujikura Ltd | Bonding using epoxy resin composition and nonactivated blowing agent |
| US4156700A (en) * | 1975-08-11 | 1979-05-29 | Her Majesty The Queen In Right Of Canada, As Represented By The Minister Of National Defence | Solid propellants containing polyether or polyester binders |
Also Published As
| Publication number | Publication date |
|---|---|
| FR2475598B1 (en) | 1983-02-18 |
| DE3161811D1 (en) | 1984-02-09 |
| FR2475598A1 (en) | 1981-08-14 |
| JPS56163175A (en) | 1981-12-15 |
| EP0034515A1 (en) | 1981-08-26 |
| ATE5783T1 (en) | 1984-01-15 |
| JPS607106B2 (en) | 1985-02-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CH633058A5 (en) | DEVICE FOR MAKING DRAWERS ANCHORED IN THE GROUND AND USE OF SAID DEVICE. | |
| EP0034515B1 (en) | Use of autodestructive mixtures of resins and oxydant charges in the temporary bonding of constructional elements | |
| EP0389375B1 (en) | System for driving anchors into the ground | |
| US3936924A (en) | Releaseable steel cable anchor and method for withdrawing the same | |
| US4884377A (en) | Removable tension member | |
| EP0320393A1 (en) | Method of storing waste underground in a cavern formed by salt dissolution | |
| EP0263803B1 (en) | Process and device for anchoring under tension | |
| US6380508B1 (en) | Apparatus and method for severing a tendon used in post-tension construction | |
| WO1988004343A1 (en) | Device for the assembly or mechanical reinforcement and the anticorrosion treatment of immersed structure elements and related treatment and assembly method | |
| FR2642158A1 (en) | Process for preparing a delay assembly for a detonator and delay assembly | |
| EP1243702B1 (en) | Buttressing apparatus for an excavation shoring and use of the apparatus | |
| AT393406B (en) | DEVICE FOR DISCONNECTING THE TENSION MEMBER OF A PRELOADED VESSEL ANCHOR BY FORMING A TARGET BREAKING POINT | |
| FR2545151A1 (en) | ROCK BOLTING METHOD AND MATERIAL | |
| FR2461190A1 (en) | CAP FOR TUBE FILLING BY EXPLOSION | |
| JP2655820B2 (en) | Earth anchor method | |
| FR2524030A1 (en) | Anchor head for progressively contracted composite tie rod - has truncated anchoring pin wedged into anchoring ring orifice | |
| CH711029B1 (en) | Assembly for anchoring device at least partially removable. | |
| CH682150A5 (en) | ||
| EP0049646A1 (en) | Process for fixing anchor bolts in rocks by means of a thermoplastic glue-block heated in situ, and relevant compositions | |
| JPS5819814B2 (en) | Dodome Anchor Kouhou | |
| JP2832720B2 (en) | Stepping prevention method for blasting work during rainfall etc. | |
| FR2503787A2 (en) | Fixing anchor bolts etc. into rock - using hot melt adhesive melted by rotation of the bolt during insertion into the borehole | |
| WO2008009861A2 (en) | Sheath section for a structural cable and associated processes | |
| JPH05214899A (en) | Adhesive cartridge for fixed dowel and manufacture of adhesive cartridge | |
| CA2365581A1 (en) | Compositions for protecting cable strands for highway structures |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT LU |
|
| 17P | Request for examination filed |
Effective date: 19811029 |
|
| ITF | It: translation for a ep patent filed |
Owner name: STUDIO TORTA SOCIETA' SEMPLICE |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT LI LU |
|
| REF | Corresponds to: |
Ref document number: 5783 Country of ref document: AT Date of ref document: 19840115 Kind code of ref document: T |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19840131 |
|
| REF | Corresponds to: |
Ref document number: 3161811 Country of ref document: DE Date of ref document: 19840209 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19900126 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19900130 Year of fee payment: 10 Ref country code: LU Payment date: 19900130 Year of fee payment: 10 |
|
| ITTA | It: last paid annual fee | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 19900131 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19900202 Year of fee payment: 10 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19900222 Year of fee payment: 10 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Effective date: 19910129 Ref country code: AT Effective date: 19910129 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Effective date: 19910131 Ref country code: LI Effective date: 19910131 Ref country code: CH Effective date: 19910131 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Effective date: 19910930 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19911001 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 19930121 Year of fee payment: 13 |