EP0000009B1 - Verfahren zur Oxydation von Chinin zu Chininone und Chinidinone. - Google Patents
Verfahren zur Oxydation von Chinin zu Chininone und Chinidinone. Download PDFInfo
- Publication number
- EP0000009B1 EP0000009B1 EP78400019A EP78400019A EP0000009B1 EP 0000009 B1 EP0000009 B1 EP 0000009B1 EP 78400019 A EP78400019 A EP 78400019A EP 78400019 A EP78400019 A EP 78400019A EP 0000009 B1 EP0000009 B1 EP 0000009B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- quininone
- quinidinone
- quinine
- solution
- oxidation
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- LOUPRKONTZGTKE-WZBLMQSHSA-N Quinine Chemical compound C([C@H]([C@H](C1)C=C)C2)C[N@@]1[C@@H]2[C@H](O)C1=CC=NC2=CC=C(OC)C=C21 LOUPRKONTZGTKE-WZBLMQSHSA-N 0.000 title claims description 22
- LOUPRKONTZGTKE-UHFFFAOYSA-N cinchonine Natural products C1C(C(C2)C=C)CCN2C1C(O)C1=CC=NC2=CC=C(OC)C=C21 LOUPRKONTZGTKE-UHFFFAOYSA-N 0.000 title claims description 21
- SRFCUPVBYYAMIL-UHFFFAOYSA-N Quinidinon Natural products C1C(C(C2)C=C)CCN2C1C(=O)C1=CC=NC2=CC=C(OC)C=C21 SRFCUPVBYYAMIL-UHFFFAOYSA-N 0.000 title claims description 15
- SRFCUPVBYYAMIL-CKFHNAJUSA-N [(2r,4s,5r)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methanone Chemical compound C([C@H]([C@H](C1)C=C)C2)CN1[C@H]2C(=O)C1=CC=NC2=CC=C(OC)C=C21 SRFCUPVBYYAMIL-CKFHNAJUSA-N 0.000 title claims description 15
- 235000001258 Cinchona calisaya Nutrition 0.000 title claims description 11
- 229960000948 quinine Drugs 0.000 title claims description 11
- 238000007254 oxidation reaction Methods 0.000 title claims description 10
- 230000003647 oxidation Effects 0.000 title claims description 8
- 238000000034 method Methods 0.000 title claims description 5
- 238000006243 chemical reaction Methods 0.000 claims description 11
- RWCCWEUUXYIKHB-UHFFFAOYSA-N benzophenone Chemical compound C=1C=CC=CC=1C(=O)C1=CC=CC=C1 RWCCWEUUXYIKHB-UHFFFAOYSA-N 0.000 claims description 10
- 239000012965 benzophenone Substances 0.000 claims description 8
- 239000002904 solvent Substances 0.000 claims description 6
- 230000008569 process Effects 0.000 claims description 4
- 150000008366 benzophenones Chemical group 0.000 claims description 3
- 150000001338 aliphatic hydrocarbons Chemical class 0.000 claims description 2
- 150000004945 aromatic hydrocarbons Chemical class 0.000 claims description 2
- 239000003153 chemical reaction reagent Substances 0.000 claims description 2
- 125000000753 cycloalkyl group Chemical group 0.000 claims description 2
- 229910052751 metal Inorganic materials 0.000 claims description 2
- 239000002184 metal Substances 0.000 claims description 2
- 238000002360 preparation method Methods 0.000 claims description 2
- 238000005520 cutting process Methods 0.000 claims 1
- 150000008376 fluorenones Chemical class 0.000 claims 1
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 21
- 239000000243 solution Substances 0.000 description 20
- LOUPRKONTZGTKE-LHHVKLHASA-N quinidine Chemical compound C([C@H]([C@H](C1)C=C)C2)C[N@@]1[C@H]2[C@@H](O)C1=CC=NC2=CC=C(OC)C=C21 LOUPRKONTZGTKE-LHHVKLHASA-N 0.000 description 16
- 241000157855 Cinchona Species 0.000 description 12
- 239000002585 base Substances 0.000 description 10
- 150000002576 ketones Chemical class 0.000 description 10
- 229960001404 quinidine Drugs 0.000 description 8
- YLQWCDOCJODRMT-UHFFFAOYSA-N fluoren-9-one Chemical compound C1=CC=C2C(=O)C3=CC=CC=C3C2=C1 YLQWCDOCJODRMT-UHFFFAOYSA-N 0.000 description 7
- 239000000047 product Substances 0.000 description 7
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 6
- 238000010992 reflux Methods 0.000 description 6
- 229910052708 sodium Inorganic materials 0.000 description 6
- 239000011734 sodium Substances 0.000 description 6
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 5
- 239000000203 mixture Substances 0.000 description 5
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 4
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 4
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 4
- 229910052783 alkali metal Inorganic materials 0.000 description 4
- 150000001340 alkali metals Chemical group 0.000 description 4
- 239000006227 byproduct Substances 0.000 description 4
- 239000008096 xylene Substances 0.000 description 4
- 235000021513 Cinchona Nutrition 0.000 description 3
- 238000003786 synthesis reaction Methods 0.000 description 3
- 238000011282 treatment Methods 0.000 description 3
- 0 *C(c1ccccc1)c1ccccc1 Chemical compound *C(c1ccccc1)c1ccccc1 0.000 description 2
- KWOLFJPFCHCOCG-UHFFFAOYSA-N Acetophenone Chemical compound CC(=O)C1=CC=CC=C1 KWOLFJPFCHCOCG-UHFFFAOYSA-N 0.000 description 2
- 244000186140 Asperula odorata Species 0.000 description 2
- 235000008526 Galium odoratum Nutrition 0.000 description 2
- -1 aliphatic ketones Chemical class 0.000 description 2
- 229930013930 alkaloid Natural products 0.000 description 2
- 229910021529 ammonia Inorganic materials 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 2
- 239000012535 impurity Substances 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 238000004064 recycling Methods 0.000 description 2
- 238000004809 thin layer chromatography Methods 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- DNIAPMSPPWPWGF-GSVOUGTGSA-N (R)-(-)-Propylene glycol Chemical compound C[C@@H](O)CO DNIAPMSPPWPWGF-GSVOUGTGSA-N 0.000 description 1
- LOUPRKONTZGTKE-FEBSWUBLSA-N (S)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxy-4-quinolinyl)methanol Chemical compound C1=C(OC)C=C2C([C@H](O)[C@H]3N4CC[C@]([C@H](C4)C=C)(C3)[H])=CC=NC2=C1 LOUPRKONTZGTKE-FEBSWUBLSA-N 0.000 description 1
- LOUPRKONTZGTKE-AFHBHXEDSA-N (r)-[(2r,4s,5r)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methanol Chemical compound C([C@H]([C@H](C1)C=C)C2)CN1[C@H]2[C@H](O)C1=CC=NC2=CC=C(OC)C=C21 LOUPRKONTZGTKE-AFHBHXEDSA-N 0.000 description 1
- 229910000497 Amalgam Inorganic materials 0.000 description 1
- JGLMVXWAHNTPRF-CMDGGOBGSA-N CCN1N=C(C)C=C1C(=O)NC1=NC2=CC(=CC(OC)=C2N1C\C=C\CN1C(NC(=O)C2=CC(C)=NN2CC)=NC2=CC(=CC(OCCCN3CCOCC3)=C12)C(N)=O)C(N)=O Chemical compound CCN1N=C(C)C=C1C(=O)NC1=NC2=CC(=CC(OC)=C2N1C\C=C\CN1C(NC(=O)C2=CC(C)=NN2CC)=NC2=CC(=CC(OCCCN3CCOCC3)=C12)C(N)=O)C(N)=O JGLMVXWAHNTPRF-CMDGGOBGSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- AEFOLTVWSRMXMW-LWINWCPBSA-N Cinchonidinone Chemical compound C1=CC=C2C(C(=O)[C@@H]3CC4CCN3C[C@@H]4C=C)=CC=NC2=C1 AEFOLTVWSRMXMW-LWINWCPBSA-N 0.000 description 1
- AEFOLTVWSRMXMW-UHFFFAOYSA-N Cinchonidinone Natural products C1=CC=C2C(C(=O)C3CC4CCN3CC4C=C)=CC=NC2=C1 AEFOLTVWSRMXMW-UHFFFAOYSA-N 0.000 description 1
- AEFOLTVWSRMXMW-IXRXBBNISA-N Cinchoninone Chemical compound C1=CC=C2C(C(=O)[C@H]3CC4CCN3C[C@@H]4C=C)=CC=NC2=C1 AEFOLTVWSRMXMW-IXRXBBNISA-N 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 239000003849 aromatic solvent Substances 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 238000002983 circular dichroism Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- DKRSEIPLAZTSFD-UHFFFAOYSA-N d-quinotoxine Natural products C12=CC(OC)=CC=C2N=CC=C1C(=O)CCC1CCNCC1C=C DKRSEIPLAZTSFD-UHFFFAOYSA-N 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 239000003480 eluent Substances 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000004508 fractional distillation Methods 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 239000000543 intermediate Substances 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- MJGFBOZCAJSGQW-UHFFFAOYSA-N mercury sodium Chemical compound [Na].[Hg] MJGFBOZCAJSGQW-UHFFFAOYSA-N 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 239000011259 mixed solution Substances 0.000 description 1
- 238000010899 nucleation Methods 0.000 description 1
- 239000007800 oxidant agent Substances 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 244000045947 parasite Species 0.000 description 1
- 230000003071 parasitic effect Effects 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 150000003138 primary alcohols Chemical class 0.000 description 1
- 150000008584 quinuclidines Chemical class 0.000 description 1
- 150000005838 radical anions Chemical class 0.000 description 1
- 239000012429 reaction media Substances 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 239000012047 saturated solution Substances 0.000 description 1
- 238000007086 side reaction Methods 0.000 description 1
- 229910001023 sodium amalgam Inorganic materials 0.000 description 1
- 238000009331 sowing Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 238000003419 tautomerization reaction Methods 0.000 description 1
- DKRSEIPLAZTSFD-LSDHHAIUSA-N viquidil Chemical compound C12=CC(OC)=CC=C2N=CC=C1C(=O)CC[C@@H]1CCNC[C@@H]1C=C DKRSEIPLAZTSFD-LSDHHAIUSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D453/00—Heterocyclic compounds containing quinuclidine or iso-quinuclidine ring systems, e.g. quinine alkaloids
- C07D453/02—Heterocyclic compounds containing quinuclidine or iso-quinuclidine ring systems, e.g. quinine alkaloids containing not further condensed quinuclidine ring systems
- C07D453/04—Heterocyclic compounds containing quinuclidine or iso-quinuclidine ring systems, e.g. quinine alkaloids containing not further condensed quinuclidine ring systems having a quinolyl-4, a substituted quinolyl-4 or a alkylenedioxy-quinolyl-4 radical linked through only one carbon atom, attached in position 2, e.g. quinine
Definitions
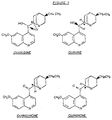
- the present invention relates to an oxidation process of the Oppenauer type with a new reagent and its application to the manufacture of quininone and quinidinone, intermediates for the synthesis of quinidine.
- the quininone and quinidinone differentiated by the asymmetric carbon isomerism at C s of the quinuclidine nucleus are generally two forms in equilibrium, which explains the phenomena of mutarotation in solution hitherto observed.
- the quininone formed is found in the presence of variable amounts of impurities, mainly: quinine, quinidine, epiquinine, epiquinidine, quinicine (quinotoxin ), which has the effect of reducing the reaction yield, the by-products formed having to be separated and purified before being recycled (it is noted in particular that the presence - even in small proportions - of epibases considerably reduces the crystallization yields of quinine and quinidine from their saturated solutions.
- impurities mainly: quinine, quinidine, epiquinine, epiquinidine, quinicine (quinotoxin )
- the reaction being carried out in enolic form it is necessary to introduce a base of PK sufficient to enolate all of the ketone forms contributing to the reaction.
- a base of PK sufficient to enolate all of the ketone forms contributing to the reaction.
- An amount of 2 to 7 equivalent moles has proved necessary in the various experiments described in the literature.
- the bases used, because of their PKI are the alcoholates, and mainly the sodium, potassium and aluminum alcoholates, methyl, ethyl, isopropyl and tert-butyl alcohols.
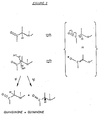
- the object of the invention is mainly to use a base having the ideal characteristics PK and compatibility which is an "in situ" derivative of the ketone used as proton acceptor.
- Ketyls are prepared by simple addition of the alkali metal to a solution of ketone in an appropriate aromatic solvent.
- a “Ketyl” obtained by interaction of an alkali metal with a diphenylketone, more particularly with benzophenone or fluorenone, will therefore be used as the quinine oxidant.
- the reaction of quinine and "Ketyl” is carried out in a solvent medium; said solvent is selected from aromatic hydrocarbons, C 6 -C 9 saturated cyclic hydrocarbons, C 6 -C 9 or aliphatic hydrocarbons corresponding to naphtha or gas oil cuts.
- the xylene solution is treated with water (20 ml), then extracted with 100 ml of sulfuric acid diluted to 20%.
- the cold sulfuric solution is neutralized by adding ammonia.
- the crystals that form are composed of quinidinone; as quininone and quinidinone are in equilibrium in the oil obtained, the precipitation of quinidinone has the consequence of shifting said equilibrium towards the production of quinidinone.
- the cold sulfuric solution is neutralized by adding ammonia. Decanting oil. It is separated and crystallized by seeding and appropriate treatment.
- the mixed solutions are kept at reflux for 5 hours.
- a “Ketyl” fluorenone solution is produced from 9 g of fluorenone in anhydrous toluene.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Quinoline Compounds (AREA)
Claims (3)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR7718398 | 1977-06-15 | ||
| FR7718398A FR2394545A1 (fr) | 1977-06-15 | 1977-06-15 | Procede de fabrication de quininone et de quinidinone, intermediaires de synthese de la quinidine |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0000009A1 EP0000009A1 (de) | 1978-12-20 |
| EP0000009B1 true EP0000009B1 (de) | 1980-07-23 |
Family
ID=9192140
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP78400019A Expired EP0000009B1 (de) | 1977-06-15 | 1978-06-14 | Verfahren zur Oxydation von Chinin zu Chininone und Chinidinone. |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP0000009B1 (de) |
| DE (1) | DE2860032D1 (de) |
| FR (1) | FR2394545A1 (de) |
| IE (1) | IE46935B1 (de) |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE1165604B (de) * | 1957-12-18 | 1964-03-19 | Chininfabrik Braunschweig Buch | Verfahren zur sterischen Umlagerung von Chinaalkaloiden |
| FR2332278A1 (fr) * | 1975-11-19 | 1977-06-17 | Nativelle Sa Ets | Procede de transformation stereochimique des alcaloides du quinquina |
| FR2332279A1 (fr) * | 1975-11-19 | 1977-06-17 | Nativelle Sa Ets | Procede d'oxydation des alcaloides du quinquina |
-
1977
- 1977-06-15 FR FR7718398A patent/FR2394545A1/fr not_active Withdrawn
-
1978
- 1978-06-14 DE DE7878400019T patent/DE2860032D1/de not_active Expired
- 1978-06-14 EP EP78400019A patent/EP0000009B1/de not_active Expired
- 1978-06-15 IE IE120478A patent/IE46935B1/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| DE2860032D1 (en) | 1980-11-13 |
| EP0000009A1 (de) | 1978-12-20 |
| FR2394545A1 (fr) | 1979-01-12 |
| IE781204L (en) | 1978-12-15 |
| IE46935B1 (en) | 1983-11-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CH616161A5 (de) | ||
| EP0000009B1 (de) | Verfahren zur Oxydation von Chinin zu Chininone und Chinidinone. | |
| EP0001029B1 (de) | Verfahren zur Herstellung von Steroid-Derivaten die in Stellung 3 eine Oximgruppe enthalten | |
| CA1231939A (fr) | PROCEDE DE PREPARATION DE DERIVES DE LA SERIE DE LA 17.alpha.-HYDROXY 19-NOR-PROGESTERONE | |
| EP0013995B1 (de) | Verfahren zur Herstellung acetylenischer macrocyclischer Ketone | |
| CH642373A5 (fr) | Compose pyrannique, procede pour sa preparation et son utilisation a titre d'intermediaire de synthese. | |
| FR2510991A1 (fr) | Procede pour la preparation de methacrylamides et d'acrylamides n-substitues | |
| EP0184572B1 (de) | Verfahren zur Herstellung von alpha-Hydroxyalkancarbonsäuren | |
| FR2759079A1 (fr) | Procede de preparation d'anthraquinones substituees et application a la preparation de rheines | |
| EP0000302B1 (de) | Verfahren zur Herstellung von Chinidin | |
| EP0362309B1 (de) | (ethylenedioxo-3,3 zyclohexyl)-4 aketophenon und derivate davon, verfahren zu deren herstellung und verwendung dieser verbindungen | |
| CH648483A5 (fr) | Composition pharmaceutique contre l'arthrite rhumatoide. | |
| CH623818A5 (en) | Process for producing derivatives of thiochroman | |
| EP0082782B1 (de) | Verfahren zur Herstellung von ethylenischen Acetalen | |
| BE884977A (fr) | Nouveau n-phenyl-benzamides utiles dans le traitement de l'inflammation et de la douleur des mammiferes | |
| FR2494696A1 (fr) | Nouveau procede de preparation de steroides 3-amines et leurs sels | |
| CH662571A5 (fr) | Procede de preparation de derives de la prednisone et produits intermediaires de ce procede. | |
| CH330478A (fr) | Procédé de préparation de nouveaux thiodérivés de colchicéines | |
| BE561749A (de) | ||
| CH421098A (fr) | Procédé de préparation de stéroïdes fluorés | |
| EP0184573A1 (de) | Verfahren zur Herstellung von alpha-Hydroxyalkancarbonsäuren | |
| BE525128A (de) | ||
| BE837643A (fr) | Composes pour la reduction ou la prevention des dommages causes au foie par l'alcool | |
| BE460230A (de) | ||
| BE535243A (de) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): BE CH DE FR GB LU NL SE |
|
| 17P | Request for examination filed | ||
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): BE CH DE FR GB LU NL SE |
|
| REF | Corresponds to: |
Ref document number: 2860032 Country of ref document: DE Date of ref document: 19801113 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Effective date: 19810614 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Effective date: 19810615 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19810630 Ref country code: CH Effective date: 19810630 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Effective date: 19820101 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 19820101 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19820501 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19830331 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Effective date: 19881117 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GR Payment date: 19910619 Year of fee payment: 5 |
|
| EUG | Se: european patent has lapsed |
Ref document number: 78400019.2 Effective date: 19820119 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |