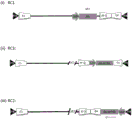
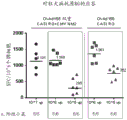
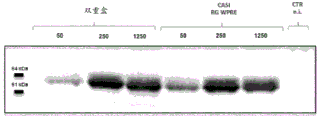
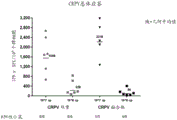
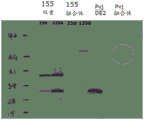
CN111479926A - 具有两个表达盒的猿猴腺病毒载体 - Google Patents
具有两个表达盒的猿猴腺病毒载体 Download PDFInfo
- Publication number
- CN111479926A CN111479926A CN201880081337.6A CN201880081337A CN111479926A CN 111479926 A CN111479926 A CN 111479926A CN 201880081337 A CN201880081337 A CN 201880081337A CN 111479926 A CN111479926 A CN 111479926A
- Authority
- CN
- China
- Prior art keywords
- vector
- adenovirus vector
- expression cassette
- chad155
- simian
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N7/00—Viruses; Bacteriophages; Compositions thereof; Preparation or purification thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
- A61K39/235—Adenoviridae
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/525—Virus
- A61K2039/5256—Virus expressing foreign proteins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/53—DNA (RNA) vaccination
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2710/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA dsDNA viruses
- C12N2710/00011—Details
- C12N2710/10011—Adenoviridae
- C12N2710/10311—Mastadenovirus, e.g. human or simian adenoviruses
- C12N2710/10334—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2710/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA dsDNA viruses
- C12N2710/00011—Details
- C12N2710/10011—Adenoviridae
- C12N2710/10311—Mastadenovirus, e.g. human or simian adenoviruses
- C12N2710/10341—Use of virus, viral particle or viral elements as a vector
- C12N2710/10342—Use of virus, viral particle or viral elements as a vector virus or viral particle as vehicle, e.g. encapsulating small organic molecule
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2710/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA dsDNA viruses
- C12N2710/00011—Details
- C12N2710/10011—Adenoviridae
- C12N2710/10311—Mastadenovirus, e.g. human or simian adenoviruses
- C12N2710/10341—Use of virus, viral particle or viral elements as a vector
- C12N2710/10343—Use of virus, viral particle or viral elements as a vector viral genome or elements thereof as genetic vector
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2710/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA dsDNA viruses
- C12N2710/00011—Details
- C12N2710/16011—Herpesviridae
- C12N2710/16111—Cytomegalovirus, e.g. human herpesvirus 5
- C12N2710/16121—Viruses as such, e.g. new isolates, mutants or their genomic sequences
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2760/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses negative-sense
- C12N2760/00011—Details
- C12N2760/18011—Paramyxoviridae
- C12N2760/18511—Pneumovirus, e.g. human respiratory syncytial virus
- C12N2760/18534—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2760/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses negative-sense
- C12N2760/00011—Details
- C12N2760/20011—Rhabdoviridae
- C12N2760/20111—Lyssavirus, e.g. rabies virus
- C12N2760/20134—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2830/00—Vector systems having a special element relevant for transcription
- C12N2830/48—Vector systems having a special element relevant for transcription regulating transport or export of RNA, e.g. RRE, PRE, WPRE, CTE
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Genetics & Genomics (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Organic Chemistry (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Biotechnology (AREA)
- General Health & Medical Sciences (AREA)
- General Engineering & Computer Science (AREA)
- Microbiology (AREA)
- Biomedical Technology (AREA)
- Virology (AREA)
- Medicinal Chemistry (AREA)
- Immunology (AREA)
- Biochemistry (AREA)
- Mycology (AREA)
- Animal Behavior & Ethology (AREA)
- Biophysics (AREA)
- Plant Pathology (AREA)
- Molecular Biology (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Physics & Mathematics (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Breeding Of Plants And Reproduction By Means Of Culturing (AREA)
- Information Retrieval, Db Structures And Fs Structures Therefor (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762572944P | 2017-10-16 | 2017-10-16 | |
| US62/572944 | 2017-10-16 | ||
| PCT/EP2018/078210 WO2019076880A1 (en) | 2017-10-16 | 2018-10-16 | SIMIENS ADENOVIRAL VECTORS COMPRISING TWO EXPRESSION CASSETTES |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN111479926A true CN111479926A (zh) | 2020-07-31 |
Family
ID=63896161
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201880081337.6A Pending CN111479926A (zh) | 2017-10-16 | 2018-10-16 | 具有两个表达盒的猿猴腺病毒载体 |
Country Status (9)
| Country | Link |
|---|---|
| US (2) | US20200299651A1 (cg-RX-API-DMAC7.html) |
| EP (1) | EP3697918B1 (cg-RX-API-DMAC7.html) |
| JP (1) | JP2021500880A (cg-RX-API-DMAC7.html) |
| CN (1) | CN111479926A (cg-RX-API-DMAC7.html) |
| AR (1) | AR114989A1 (cg-RX-API-DMAC7.html) |
| BR (1) | BR112020007413A2 (cg-RX-API-DMAC7.html) |
| CA (1) | CA3084346A1 (cg-RX-API-DMAC7.html) |
| MX (1) | MX2020003746A (cg-RX-API-DMAC7.html) |
| WO (1) | WO2019076880A1 (cg-RX-API-DMAC7.html) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7454645B2 (ja) | 2019-07-16 | 2024-03-22 | ギリアード サイエンシーズ, インコーポレイテッド | Hivワクチン並びにその作製方法及び使用方法 |
| IL315295A (en) | 2019-09-30 | 2024-10-01 | Gilead Sciences Inc | Hbv vaccines and methods treating hbv |
| TW202144570A (zh) * | 2020-01-28 | 2021-12-01 | 美商科達金尼克斯有限公司 | 去最佳化(DEOPTIMIZED)SARS-CoV-2及其方法及用途 |
| CA3259040A1 (en) | 2022-07-12 | 2024-01-18 | Gilead Sciences, Inc. | HIV Immunogenic Polypeptides and Vaccines and Their Uses |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040136963A1 (en) * | 2001-06-22 | 2004-07-15 | The Trustees Of The University Of Pennsylvania | Simian adenovirus vectors and methods of use |
| CN1965086A (zh) * | 2004-04-28 | 2007-05-16 | 宾夕法尼亚州立大学托管会 | 多价病毒载体和产生它的系统 |
| WO2017017049A1 (en) * | 2015-07-27 | 2017-02-02 | Glaxosmithkline Biologicals S.A. | Novel adenovirus |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008095027A2 (en) | 2007-01-30 | 2008-08-07 | Cedars-Sinai Medical Center | Adenoviral vector comprising herpes simplex virus type 1 thymidine kinase and a transgene for increasing the expression of the transgene |
| EP2181121A4 (en) | 2007-03-21 | 2012-07-11 | Id Biomedical Corp Quebec | CHIMÄRE ANTIGENE |
| CA2738654C (en) | 2008-09-27 | 2019-02-26 | Witricity Corporation | Wireless energy transfer systems |
| EP3385387B1 (en) | 2009-02-02 | 2021-08-25 | GlaxoSmithKline Biologicals SA | Simian adenovirus nucleic acid- and amino acid-sequences, vectors containing same, and uses thereof |
| WO2012021730A2 (en) * | 2010-08-11 | 2012-02-16 | Genvec, Inc. | Respiratory syncytial virus (rsv) vaccine |
| WO2012089231A1 (en) | 2010-12-30 | 2012-07-05 | Okairòs Ag | Paramyxovirus vaccines |
| CN103492574B (zh) | 2011-02-22 | 2015-12-09 | 加州理工学院 | 使用腺相关病毒(aav)载体递送蛋白 |
| EP2764011B1 (en) * | 2011-10-05 | 2021-04-07 | GenVec, Inc. | Adenoviral vector-based respiratory syncytial virus (rsv) vaccine |
| MX384992B (es) * | 2014-06-13 | 2025-03-14 | Glaxosmithkline Biologicals Sa | Combinaciones inmunógenas. |
| BE1024420B1 (fr) | 2015-06-12 | 2018-02-19 | Glaxosmithkline Biologicals Sa | Polynucleotides et polypeptides d'adenovirus |
| BE1024774B1 (fr) | 2016-09-29 | 2018-07-02 | Glaxosmithkline Biologicals Sa | Compositions et procedes de traitement |
-
2018
- 2018-10-16 CN CN201880081337.6A patent/CN111479926A/zh active Pending
- 2018-10-16 EP EP18789094.2A patent/EP3697918B1/en active Active
- 2018-10-16 WO PCT/EP2018/078210 patent/WO2019076880A1/en not_active Ceased
- 2018-10-16 BR BR112020007413-6A patent/BR112020007413A2/pt not_active IP Right Cessation
- 2018-10-16 US US16/756,377 patent/US20200299651A1/en not_active Abandoned
- 2018-10-16 JP JP2020521360A patent/JP2021500880A/ja not_active Ceased
- 2018-10-16 CA CA3084346A patent/CA3084346A1/en not_active Abandoned
- 2018-10-16 MX MX2020003746A patent/MX2020003746A/es unknown
- 2018-10-16 AR ARP180103001A patent/AR114989A1/es not_active Application Discontinuation
-
2023
- 2023-08-21 US US18/453,078 patent/US12180512B2/en active Active
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040136963A1 (en) * | 2001-06-22 | 2004-07-15 | The Trustees Of The University Of Pennsylvania | Simian adenovirus vectors and methods of use |
| CN1965086A (zh) * | 2004-04-28 | 2007-05-16 | 宾夕法尼亚州立大学托管会 | 多价病毒载体和产生它的系统 |
| WO2017017049A1 (en) * | 2015-07-27 | 2017-02-02 | Glaxosmithkline Biologicals S.A. | Novel adenovirus |
Non-Patent Citations (2)
| Title |
|---|
| D. L. LI等: "Modified recombinant adenoviruses increase porcine circovirus 2 capsid protein expression and induce enhanced immune responses in mice" * |
| JULIANA C. SMALL等: "Construction and Characterization of E1- and E3-Deleted Adenovirus Vectors Expressing Two Antigens from Two Separate Expression Cassettes" * |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2021500880A (ja) | 2021-01-14 |
| WO2019076880A1 (en) | 2019-04-25 |
| AR114989A1 (es) | 2020-11-18 |
| EP3697918A1 (en) | 2020-08-26 |
| MX2020003746A (es) | 2020-11-06 |
| US20240093161A1 (en) | 2024-03-21 |
| US12180512B2 (en) | 2024-12-31 |
| EP3697918B1 (en) | 2025-11-26 |
| US20200299651A1 (en) | 2020-09-24 |
| CA3084346A1 (en) | 2019-04-25 |
| BR112020007413A2 (pt) | 2020-12-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20230158134A1 (en) | Chimpanzee adenovirus constructs with lyssavirus antigens | |
| JP6230527B2 (ja) | サルアデノウイルス及び雑種アデノウイルスベクター | |
| US12180512B2 (en) | Simian adenoviral vectors with two expression cassettes | |
| US11268108B2 (en) | Replication competent adenoviral vectors | |
| US11859199B2 (en) | Adenoviral vectors with two expression cassettes encoding RSV antigenic proteins or fragments thereof | |
| US20220364115A1 (en) | Enhanced promoter | |
| EA039001B1 (ru) | Аденовирусные полинуклеотиды и полипептиды |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| WD01 | Invention patent application deemed withdrawn after publication | ||
| WD01 | Invention patent application deemed withdrawn after publication |
Application publication date: 20200731 |