CN105891090B - Blood cell analyzer, body fluid analysis method, and control system therefor - Google Patents
Blood cell analyzer, body fluid analysis method, and control system therefor Download PDFInfo
- Publication number
- CN105891090B CN105891090B CN201610212407.XA CN201610212407A CN105891090B CN 105891090 B CN105891090 B CN 105891090B CN 201610212407 A CN201610212407 A CN 201610212407A CN 105891090 B CN105891090 B CN 105891090B
- Authority
- CN
- China
- Prior art keywords
- body fluid
- measurement
- blood cell
- measurement mode
- sample
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 210000001124 body fluid Anatomy 0.000 title claims abstract description 116
- 239000010839 body fluid Substances 0.000 title claims abstract description 116
- 210000000601 blood cell Anatomy 0.000 title claims abstract description 97
- 238000004458 analytical method Methods 0.000 title description 46
- 238000005259 measurement Methods 0.000 claims abstract description 276
- 210000000265 leukocyte Anatomy 0.000 claims abstract description 119
- 210000004027 cell Anatomy 0.000 claims abstract description 72
- 239000002245 particle Substances 0.000 claims abstract description 52
- 210000004369 blood Anatomy 0.000 claims abstract description 49
- 239000008280 blood Substances 0.000 claims abstract description 49
- 238000001514 detection method Methods 0.000 claims abstract description 43
- 210000003743 erythrocyte Anatomy 0.000 claims abstract description 35
- 238000000034 method Methods 0.000 claims abstract description 31
- 230000002159 abnormal effect Effects 0.000 claims abstract description 20
- 230000008569 process Effects 0.000 claims abstract description 13
- 210000001175 cerebrospinal fluid Anatomy 0.000 claims description 16
- 239000012530 fluid Substances 0.000 claims description 16
- 210000002433 mononuclear leukocyte Anatomy 0.000 claims description 16
- 210000003617 erythrocyte membrane Anatomy 0.000 claims description 9
- 206010003445 Ascites Diseases 0.000 claims description 8
- 210000000224 granular leucocyte Anatomy 0.000 claims description 8
- 210000002540 macrophage Anatomy 0.000 claims description 6
- 210000004881 tumor cell Anatomy 0.000 claims description 6
- 238000003556 assay Methods 0.000 claims description 5
- 210000005033 mesothelial cell Anatomy 0.000 claims description 5
- 239000000126 substance Substances 0.000 claims description 3
- 230000000747 cardiac effect Effects 0.000 claims description 2
- 206010048612 Hydrothorax Diseases 0.000 claims 1
- 239000000523 sample Substances 0.000 description 150
- 238000012545 processing Methods 0.000 description 38
- 239000003153 chemical reaction reagent Substances 0.000 description 30
- 230000003287 optical effect Effects 0.000 description 21
- 238000010586 diagram Methods 0.000 description 19
- 102000001554 Hemoglobins Human genes 0.000 description 18
- 108010054147 Hemoglobins Proteins 0.000 description 18
- 238000005070 sampling Methods 0.000 description 13
- 210000003651 basophil Anatomy 0.000 description 12
- 210000001616 monocyte Anatomy 0.000 description 12
- 210000001995 reticulocyte Anatomy 0.000 description 12
- 238000006243 chemical reaction Methods 0.000 description 11
- 239000003085 diluting agent Substances 0.000 description 9
- 210000000440 neutrophil Anatomy 0.000 description 8
- 210000004910 pleural fluid Anatomy 0.000 description 8
- 210000003979 eosinophil Anatomy 0.000 description 7
- 238000011084 recovery Methods 0.000 description 7
- 210000001772 blood platelet Anatomy 0.000 description 6
- 238000007405 data analysis Methods 0.000 description 6
- 210000004698 lymphocyte Anatomy 0.000 description 6
- 239000003795 chemical substances by application Substances 0.000 description 5
- 239000012470 diluted sample Substances 0.000 description 5
- 238000009826 distribution Methods 0.000 description 5
- 239000007788 liquid Substances 0.000 description 5
- 239000012192 staining solution Substances 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- 206010028980 Neoplasm Diseases 0.000 description 4
- 238000004140 cleaning Methods 0.000 description 4
- 201000010099 disease Diseases 0.000 description 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 4
- 230000002949 hemolytic effect Effects 0.000 description 4
- 238000010186 staining Methods 0.000 description 4
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- 208000002151 Pleural effusion Diseases 0.000 description 3
- 238000002835 absorbance Methods 0.000 description 3
- 238000004820 blood count Methods 0.000 description 3
- 238000012790 confirmation Methods 0.000 description 3
- 230000006870 function Effects 0.000 description 3
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 241000894006 Bacteria Species 0.000 description 2
- 208000005228 Pericardial Effusion Diseases 0.000 description 2
- 230000003187 abdominal effect Effects 0.000 description 2
- 239000012496 blank sample Substances 0.000 description 2
- 238000000502 dialysis Methods 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- 238000000684 flow cytometry Methods 0.000 description 2
- 239000003219 hemolytic agent Substances 0.000 description 2
- 230000001678 irradiating effect Effects 0.000 description 2
- 239000004973 liquid crystal related substance Substances 0.000 description 2
- 210000002751 lymph Anatomy 0.000 description 2
- 210000005088 multinucleated cell Anatomy 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 210000004912 pericardial fluid Anatomy 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 210000001179 synovial fluid Anatomy 0.000 description 2
- 230000032258 transport Effects 0.000 description 2
- 208000035473 Communicable disease Diseases 0.000 description 1
- 229920000858 Cyclodextrin Polymers 0.000 description 1
- 108700035531 EC 3.4.24.6 Proteins 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 206010018910 Haemolysis Diseases 0.000 description 1
- 208000032843 Hemorrhage Diseases 0.000 description 1
- 201000009906 Meningitis Diseases 0.000 description 1
- 206010027259 Meningitis tuberculous Diseases 0.000 description 1
- 206010027260 Meningitis viral Diseases 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 1
- 208000032851 Subarachnoid Hemorrhage Diseases 0.000 description 1
- 208000022971 Tuberculous meningitis Diseases 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 239000008186 active pharmaceutical agent Substances 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 210000003567 ascitic fluid Anatomy 0.000 description 1
- 208000034158 bleeding Diseases 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 238000009534 blood test Methods 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000004163 cytometry Methods 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 230000002327 eosinophilic effect Effects 0.000 description 1
- 125000002485 formyl group Chemical class [H]C(*)=O 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 230000008588 hemolysis Effects 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 210000001503 joint Anatomy 0.000 description 1
- 210000000281 joint capsule Anatomy 0.000 description 1
- 230000031700 light absorption Effects 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 208000001223 meningeal tuberculosis Diseases 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 230000003448 neutrophilic effect Effects 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 239000012788 optical film Substances 0.000 description 1
- 244000045947 parasite Species 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- HFHDHCJBZVLPGP-UHFFFAOYSA-N schardinger α-dextrin Chemical compound O1C(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(O)C2O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC2C(O)C(O)C1OC2CO HFHDHCJBZVLPGP-UHFFFAOYSA-N 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 210000002330 subarachnoid space Anatomy 0.000 description 1
- 210000002435 tendon Anatomy 0.000 description 1
- 238000002834 transmittance Methods 0.000 description 1
- 238000010200 validation analysis Methods 0.000 description 1
- 201000010044 viral meningitis Diseases 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N15/00—Investigating characteristics of particles; Investigating permeability, pore-volume, or surface-area of porous materials
- G01N15/10—Investigating individual particles
- G01N15/12—Coulter-counters
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N15/00—Investigating characteristics of particles; Investigating permeability, pore-volume, or surface-area of porous materials
- G01N15/10—Investigating individual particles
- G01N15/14—Electro-optical investigation, e.g. flow cytometers
- G01N15/1456—Electro-optical investigation, e.g. flow cytometers without spatial resolution of the texture or inner structure of the particle, e.g. processing of pulse signals
- G01N15/1459—Electro-optical investigation, e.g. flow cytometers without spatial resolution of the texture or inner structure of the particle, e.g. processing of pulse signals the analysis being performed on a sample stream
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N35/00—Automatic analysis not limited to methods or materials provided for in any single one of groups G01N1/00 - G01N33/00; Handling materials therefor
- G01N35/00584—Control arrangements for automatic analysers
-
- G01N2015/012—
-
- G01N2015/014—
-
- G01N2015/016—
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N15/00—Investigating characteristics of particles; Investigating permeability, pore-volume, or surface-area of porous materials
- G01N15/10—Investigating individual particles
- G01N15/14—Electro-optical investigation, e.g. flow cytometers
- G01N2015/1486—Counting the particles
Abstract
The present invention provides a blood cell analyzer for measuring a specimen, including: a detection section capable of detecting the side scattered light and the fluorescence obtained from the particles flowing through the sheath flow cell; a controller that processes a detection result output from the detection section; wherein the controller is capable of receiving settings for a blood measurement mode and a body fluid measurement mode; when the body fluid measurement mode is set, the controller classifies and counts particles, from which abnormal particles other than red blood cell ghosts and blood cells are removed, as white blood cells on the basis of the intensity of the side scattered light and the intensity of the fluorescence.
Description
The present application is a divisional application of a chinese invention patent application filed by the same applicant under the name of "blood cell analyzer, body fluid analyzing method and control system" with the application number of 200810005239.2, the application date of 31/2008/1.
The technical field is as follows:
the present invention relates to a blood cell analyzer, a body fluid analyzing method, and a control system thereof, which can measure not only blood but also body fluids other than blood, such as cerebrospinal fluid (medullary fluid), pleural fluid (pleural fluid), and ascites, as a sample.
Background art:
in the field of clinical examination, a blood cell analyzer is often used to analyze blood collected from a body as a test sample and to use the analysis result as one of references for diagnosis and monitoring.
Meanwhile, in the field of clinical examination, it is desired to measure body fluids other than blood such as cerebrospinal fluid, easily and conveniently. Generally, body fluids contain almost no cells, but when a disease or an organ concerned has a tumor or an injury, bleeding (blood cells) and abnormal cells, bacteria and other cells are found.
U.S. patent application publication No. 2003/0215890 discloses a technique for measuring cells in a body fluid with a blood cell analyzer, and U.S. patent application publication No. 2003/0215890 discloses that a reagent component containing an aldehyde, a surfactant and cyclodextrin is mixed with cerebrospinal fluid (CSF) to prepare a measurement sample, and the measurement sample prepared is analyzed with an ADVIA120 cell analyzer, and the cells in the cerebrospinal fluid are classified and counted based on a cytogram shown in FIG. 11A ~ and FIG. 11G of this publication.
However, according to the technique disclosed in U.S. patent application publication No. 2003/0215890, only cerebrospinal fluid (CSF) is actually analyzed as a body fluid, and no analysis is performed with respect to body fluids such as ascites and pleural fluid. In general, the cerebrospinal fluid does not contain particulate components other than blood cells in many cases, and there are cases where mesothelial cells, macrophages, tumor cells, and the like are contained in body fluids other than cerebrospinal fluid, such as ascites and pleural effusion, depending on the disease of a patient. Thus, when a body fluid containing a particulate component other than blood cells is analyzed by the technique disclosed in U.S. patent application publication No. 2003/0215890, there is a possibility that the particulate component other than blood cells appears in a certain cell region of a cytogram, for example, and an accurate analysis result cannot be obtained.
The invention content is as follows:
the scope of the invention is to be determined solely by the appended claims, and not by the statements within this summary to any degree.
The blood cell analyzer for measuring blood cells according to the first aspect of the present invention includes: a measurement mode setting unit for setting a body fluid measurement mode; measurement start instruction means for receiving an instruction to start measurement; an optical information acquisition unit that irradiates a measurement sample with light and acquires optical information from cells contained in the measurement sample; and an analyzing unit that classifies cells contained in the measurement sample into at least leukocytes and nucleated cells other than leukocytes based on the optical information acquired from the measurement sample prepared from the body fluid specimen and the reagent for measuring leukocytes, and counts the leukocytes and the nucleated cells other than leukocytes, when the instruction to start measurement is received by the measurement start instructing unit after the setting of the body fluid measurement mode.
The analysis unit divides the leukocytes into polymorphonuclear leukocytes and mononuclear leukocytes, and counts the polymorphonuclear leukocytes and the mononuclear leukocytes, respectively.
The analysis unit calculates the proportion of polymorphonuclear leukocytes or mononuclear leukocytes in the leukocytes.
The analyzing means obtains the number of all nucleated cells from the number of leukocytes and the number of nucleated cells other than leukocytes, and further obtains the ratio of the nucleated cells other than leukocytes to the all nucleated cells.
The analysis unit further separates a erythrocyte ghost from the cells contained in the measurement sample.
When the body fluid measurement mode is not set, the measurement start instruction means receives an instruction to start measurement, and the analysis means classifies leukocytes contained in a measurement sample prepared from a blood sample and a reagent for measuring leukocytes into several subclasses based on the optical information obtained from the measurement sample, and counts the subclasses.
The analysis device further comprises an output unit for outputting a display screen for displaying the analysis result of the analysis unit.
The optical information is selected from the group consisting of scattered light information emitted by the cell, fluorescence information emitted by the cell, light absorption information absorbed by the cell, and combinations thereof.
The body fluid specimen is selected from the group consisting of cerebrospinal fluid, pleural fluid, ascites, pericardial fluid, joint fluid, dialysate for peritoneal dialysis, and intra-abdominal wash.
The nucleated cells are selected from the group consisting of macrophages, mesothelial cells, tumor cells, erythrocyte ghosts, and combinations thereof.
The blood cell analyzer according to the second aspect of the present invention comprises: a measurement mode setting unit for setting a body fluid measurement mode; measurement start instruction means for receiving an instruction to start measurement; a specimen pipetting unit for pipetting a specimen; a measurement sample preparation unit for preparing a measurement sample from the sample aspirated by the sample aspirating unit and the reagent for measuring leukocytes; an optical information acquisition unit that irradiates a measurement sample with light and acquires optical information from cells contained in the measurement sample; and an analyzing unit that classifies cells contained in the measurement sample based on the acquired optical information and counts the classified cells. After the body fluid measurement mode is set by the measurement mode setting means, when the measurement start instruction means receives an instruction to start measurement, the measurement sample preparation means prepares the measurement sample from the body fluid sample aspirated by the sample aspirating means and the reagent for measuring leukocytes, and the analysis means classifies cells contained in the measurement sample into leukocytes and cells other than leukocytes based on the optical information acquired from the measurement sample and counts the leukocytes and nucleated cells other than the leukocytes.
When the body fluid measurement mode is not set, the measurement start instruction unit receives an instruction to start measurement, the measurement sample preparation unit prepares a measurement sample from the blood sample aspirated by the sample aspirating unit and the reagent for measuring leukocytes, and the analysis unit classifies leukocytes contained in the measurement sample into a plurality of subclasses based on the optical information acquired from the measurement sample and counts the subclasses.
The body fluid analyzing method according to the third aspect of the present invention comprises: (a) the method comprises the following steps: setting a measurement mode to set a body fluid measurement mode; (b) the method comprises the following steps: receiving an instruction to start measurement after the body fluid measurement mode is set; (c) the method comprises the following steps: receiving the instruction to start measurement, irradiating a measurement sample prepared from a body fluid sample and a reagent for measuring leukocytes with light, and acquiring optical information from cells contained in the measurement sample; (d) the method comprises the following steps: the cells contained in the measurement sample are classified into at least leukocytes and nucleated cells other than leukocytes on the basis of the acquired optical information, and the leukocytes and the nucleated cells other than leukocytes are counted.
The body fluid analysis method further comprises a step e of: after receiving the instruction to start measurement, the body fluid sample is aspirated, and the measurement sample is prepared from the aspirated body fluid sample and the reagent for leukocyte measurement.
The (d) step includes the steps of classifying the leukocytes into polymorphonuclear leukocytes and mononuclear leukocytes, and counting the polymorphonuclear leukocytes and the mononuclear leukocytes separately.
The step (d) includes a step of determining a ratio of mononuclear leukocytes in the leukocytes or a ratio of mononuclear leukocytes in the leukocytes.
A fourth aspect of the present invention provides a control system for a body fluid analyzer, comprising: a measurement mode setting control system sets a measurement mode to set a body fluid measurement mode; a measurement instruction reception control system receives an instruction to start measurement after a body fluid measurement mode is set; an optical information acquisition control system for receiving the instruction to start measurement, irradiating a measurement sample prepared from a body fluid sample and a reagent for measuring leukocytes with light, and acquiring optical information from cells contained in the measurement sample; and a cell classification and counting control system for classifying cells contained in the measurement sample into at least leukocytes and nucleated cells other than leukocytes based on the acquired optical information, and counting the leukocytes and the nucleated cells other than leukocytes.
The cell sorting and counting control system may sort the leukocytes into and count the polymorphonuclear leukocytes and the mononuclear leukocytes, respectively.
The cell sorting and counting control system may calculate the ratio of mononuclear leukocytes in leukocytes or the ratio of mononuclear leukocytes in leukocytes.
The cell sorting and counting control system may further separate a erythrocyte ghost from the cells contained in the assay sample.
Description of the drawings:
FIG. 1 is an external view of a blood cell analyzer according to an embodiment of the present invention.
Fig. 2 is a block diagram of an analyzer measurement device.
Fig. 3 is a block diagram of a fluidic device.
FIG. 4 is a diagram of the optical system of the leukocyte detector.
FIG. 5 is a display of the RBC/PLT detector.
FIG. 6 is a diagram of an HGB detector.
Fig. 7 is a flowchart of the sample measurement process.
Fig. 8 is a diagram of a display screen for setting a measurement mode.
Fig. 9 is a flowchart of the pre-sequence processing.
Fig. 10 is a schematic diagram of a scattergram for measuring a measurement sample for DIFF prepared from a body fluid.
FIG. 11 is a graph showing a comparison between the measurement results of the blood cell analyzer according to the embodiment and the measurement results of the control method.
Fig. 12 is a schematic diagram of a scattergram for measuring a measurement sample for DIFF prepared from blood.
Fig. 13 is a display screen of the measurement result in the blood measurement mode.
Fig. 14 is a display screen showing the measurement results in the body fluid measurement mode.
Fig. 15 is a display screen showing the measurement results in the body fluid measurement mode.
Fig. 16 is a display screen showing the measurement results in the body fluid measurement mode.
Fig. 17 is a confirmation screen for starting blank detection displayed in the body fluid measurement mode.
The specific implementation mode is as follows:
the following describes embodiments of the present invention with reference to the drawings.
Fig. 1 shows a blood cell analyzer 1. The blood cell analyzer 1 is a multi-item fully automatic blood cell analyzer for blood test, and is capable of measuring a blood sample in a sample container (blood collection tube), acquiring characteristic information indicating characteristics of blood cells contained in the sample, and analyzing and processing the characteristic information. The blood cell analyzer 1 can also analyze body fluid. In the blood cell analyzer of the present embodiment, the body fluid to be analyzed refers to a body cavity fluid present in a body cavity other than blood. In particular cerebrospinal fluid (medullary fluid, CSF: effusion of ventricles of brain and subarachnoid space), pleural fluid (pleural fluid, PE: pleural effusion), ascites (peritoneal effusion), pericardial fluid (cardiac cavity effusion), synovial fluid (synovial fluid: fluid in joints, synovial capsule and tendon sheath), etc. Dialysate for peritoneal dialysis (CAPD), intra-abdominal cleaning fluid, and the like can also be analyzed as one of the body fluids. These fluids usually contain few cells, but when the diseased or associated organ has a tumor or is damaged, they may contain blood cells, abnormal cells, bacteria and other cells. Such as cerebrospinal fluid, from which the following clinical inferences can be made. For example, subarachnoid hemorrhage may be if red blood cells are increased, meningitis may be suspected if neutrophils are increased, infectious diseases (parasites and fungi) may be suspected if eosinophils are increased, tuberculous and viral meningitis may be suspected if monocytes are increased, and metastasis of the tumor to the medulla membrane may be suspected if other cells are increased. In the case of ascites and pleural fluid, if nucleated cells such as mesothelial cells, macrophages and tumor cells are contained in addition to blood cells, they can be used as an index for diseases such as cancer, and these indices can be obtained by analyzing nucleated cells other than blood cells.
The blood cell analyzer 1 includes a measurement device 2 that can measure blood and a body fluid as a sample, and a data processing device 3 that processes measurement results output from the measurement device 2 and obtains analysis results. The data processing apparatus 3 includes a controller 301, a display 302, and an input device 303. In fig. 1, the measurement device 2 and the data processing device 3 are present as one device, respectively, or may be integrated into one device.
Fig. 2 is a block diagram of the measurement device 2 of the blood cell analyzer 1. As shown in fig. 2, the measurement device 2 includes a blood cell detection section 4, an analog signal processor 5 that processes an output signal (analog signal) of the detection section 4, a microcomputer 6, a display/operation section 7, and a device mechanical section 8 that measures blood and body fluid. The apparatus mechanical portion 8 includes the following fluid devices 81.
Fig. 3 is a block diagram of a configuration of a fluid device 81, as shown in fig. 3, the fluid device 81 includes a sample pipette 18, a plurality of reagent containers 11, a sampling valve 12, and a reaction chamber 13 ~ 17. the sample pipette 18 pipettes a sample from the sample container and sends the sample to the sampling valve 12. the sampling valve 12 divides the introduced sample into a plurality of equal divisions of a predetermined amount, the division number differs depending on a measurement mode (each measurement mode), and the CBC mode sample for measuring the red blood cell count, the white blood cell count, the platelet count, and the hemoglobin concentration is divided into three equal divisions.
A reagent (diluent) is introduced into the sampling valve 12 from the reagent container 11, and the divided sample and the like are transferred to the reaction chamber 13 ~ 17 and the HGB detector 43 described later, together with the reagent, the reaction chamber 13 supplies a predetermined amount of the sample (aliquot) extracted by the sampling valve 12, a predetermined amount of the diluent, and a predetermined amount of the staining solution by a non-illustrated quantitative pump, and these sample and the reagent are mixed to prepare a measurement sample for four-class white blood cell (DIFF).
As the diluent, a reagent "leucolysin STROMMATOLYSER-4 DL" available from Sysmex corporation can be suitably used. The reagent contains surfactant, and can dissolve erythrocyte. As the staining solution, a reagent "leukocyte four-classification solution STROMMATOLYSER-4 DS" available from Sysmex corporation can be used as appropriate. This staining solution contains ethylene glycol, lower alcohol and polymethine, and after hemolysis of the above dilution, blood cell components were stained, and finally a 50-fold diluted sample was prepared.
When the body fluid measurement mode is selected, the measurement sample for classifying leukocytes is prepared under the conditions of the same sample amount, the same reagent amount, and the same reagent amount as the measurement sample for classifying leukocytes. However, as described later, the leukocyte classification in the body fluid measurement mode is not four types but two types.
The reaction chamber 14 is supplied with a predetermined amount of the sample collected by the sampling valve 12, a predetermined amount of the diluted hemolyzing agent, and a predetermined amount of the staining solution by a non-illustrated metering pump, and mixes these sample and reagents to prepare a measurement sample for measuring Nucleated Red Blood Cell (NRBC).
The reaction chamber 15 is supplied with a predetermined amount of the sample collected by the sampling valve 12, a predetermined amount of the diluent hemolyzing agent, and a predetermined amount of the staining solution by a non-illustrated metering pump, and mixes these sample and reagents to prepare a measurement sample for measuring Reticulocytes (RET).
The reaction chamber 16 is supplied with a predetermined amount of the sample and a predetermined amount of the diluent hemolyzing agent collected by the sampling valve 12 by a non-illustrated quantitative pump, and mixes these sample and the reagent to prepare a measurement sample for measuring white blood cells and basophils (WBC/BASO).
The reaction chamber 17 is supplied with a predetermined amount of the sample and a predetermined amount of the diluent collected by the sampling valve 12 by a non-illustrated quantitative pump, and mixes these samples with a reagent to prepare a measurement sample for measuring red blood cells and platelets (RET/PLT).
The predetermined amount of sample and the predetermined amount of diluent hemolyzing agent collected by the sampling valve 12 are also supplied to an HGB detector 43 described later.
The detecting section 4 has a white blood cell detector 41 for detecting white blood cells. This leukocyte detector 41 is also used for the detection of nucleated red blood cells and reticulocytes. The detecting section 4 includes, in addition to the white blood cell detector 41, an RBC/PLT detector 42 for measuring the number of red blood cells and the number of platelets, and an HGB detector 43 for measuring the amount of hemoglobin in blood.
The white blood cell detector 41 is mainly composed of an optical detector, and specifically, a detector using flow cytometry. Herein, cytometry is the measurement of physical and chemical properties of cells and other biological particles, and flow cytometry is the measurement of: and (c) passing the particles through a stream of particles to perform the assay. Fig. 4 shows the optical system of the white blood cell detector 41. In this figure, a light beam emitted from a laser light emitting diode 401 is irradiated to a blood cell flowing through a sheath flow cell 403 by a collimator mirror 402. The leukocyte detector 41 detects the intensity of forward scattered light, the intensity of side scattered light, and the intensity of side fluorescence emitted from blood cells in the sheath flow cell 403 under laser irradiation, as characteristic parameters of the blood cells.
Here, the scattering of light is a phenomenon in which particles such as blood cells become obstacles in the traveling direction of light, and the traveling direction of light is changed. Detection of this scattered light allows information to be obtained about the particle characteristics of the particle size and composition. Forward scattered light refers to scattered light emitted by a particle in substantially the same direction of travel as the light being irradiated. Characteristic information about the size of the particles (blood cells) can be obtained from the forward scattered light. The side scattered light is the scattered light emitted by the particles in a direction approximately perpendicular to the light irradiated. Characteristic information about the interior of the particle can be obtained from the laterally scattered light. When laser light is irradiated onto blood cell particles, the intensity of the side scattered light depends on the complexity of the inside of the cell (shape, size, density of nuclei and number of particles). Therefore, it is possible to classify (identify) blood cells and to determine the number of blood cells by using this characteristic of the side scattered light intensity. In addition, although the present embodiment has been described as using the forward scattered light and the side scattered light as the scattered light, the present invention is not limited to this, and the angle of the scattered light with respect to the optical axis of the light irradiated from the light source through the sheath flow cell is not limited as long as the scattered light signal reflecting the particle characteristics required for the analysis can be obtained.
When a fluorescent substance such as stained blood cells is irradiated with light, light having a wavelength longer than that of the irradiated light is emitted. The fluorescence intensity is the better and stronger the staining is, and the measurement of the fluorescence intensity can obtain characteristic information about the staining degree of blood cells. Therefore, the measurement such as the classification of leukocytes can be performed based on the difference in (lateral) fluorescence intensity.
As shown in fig. 4, forward scattered light emitted from blood cells (white blood cells and nucleated red blood cells) flowing through the sheath flow cell 403 is received by a light emitting diode (forward scattered light collector) 406 through a condenser 404 and a pinhole 405. The side scattered light is received by a photomultiplier (side scattered light collector) 411 through a condenser 407, a dichroic mirror 408, an optical film 409 and a pinhole 410. The lateral fluorescence is received by a photomultiplier (lateral fluorescence collector) 412 through a condenser 407 and a dichroic mirror 408. The received optical signals output from the respective light collectors 406, 411, and 412 are subjected to analog signal processing such as amplification and waveform processing by analog signal processors 5 including amplifiers 51, 52, and 53, respectively, and then sent to a microcomputer 6.
Next, the structure of the RBC/PLT detector 42 will be explained. Fig. 5 is a schematic diagram of the structure of the RBC/PLT detector 42. The RBC/PLT detector 42 can measure red blood cell count and platelet count using sheath flow DC detection. The RBC/PLT detector 42 has a sheath flow cell 42a as shown in fig. 5. The sheath flow cell 42a is provided with an upward opening sample port 42b, and a sample can be added from the reaction chamber 17 to the sample port 42 b. The sheath flow cell 42a further has a tapered sample chamber 42c which is tapered upward, and the sample addition port 42b is disposed at the inner center of the sample chamber 42 c. The upper end of the sample chamber 42c is provided with a hole 42d, and the hole 42d is exactly opposite to the center of the sample addition port 42 b. The measurement sample supplied from the sample feeder is transported upward from the distal end of the sample addition port 42b, and at the same time, the sheath liquid is supplied to the sample chamber 42c, and the sheath liquid flows upward to the well 42 d. Here, the measurement sample flows under the enclosure of the sheath fluid, the tapered sample chamber 42c narrows the flow of the measurement sample, and the blood cells in the measurement sample pass through the holes 42d one by one. The hole 42d is provided with an electrode between which a direct current is supplied. When the measurement sample flows through the hole 42d, the change in the direct current resistance of the hole 42d is detected, and the electric signal is output to the controller 25. Since the direct current resistance increases when the blood cell passes through the hole 42d, the electric signal reflects information of the blood cell passing through the hole 42d, and the red blood cell and the platelet are counted by signal processing the electric signal.
A recovery pipe 42e extending vertically is provided above the hole 42 d. The recovery tube 42e is disposed in a sample chamber 42f connected to the sample chamber 42c through a hole 42 d. The lower end of the recovery pipe 42e is separated from the inner wall of the sample chamber 42 f. The sample chamber 42f is supplied with a sheath liquid, which then flows down along the outside area of the recovery tube 42e of the sample chamber 42 f. The sheath liquid flowing from the outside of the recovery tube 42e reaches the lower end of the sample chamber 42f, passes between the lower end of the recovery tube 42e and the inner wall of the sample chamber 42f, and flows into the recovery tube 42 e. Therefore, backflow of blood cells through the hole 42d can be prevented, and erroneous detection of blood cells can be prevented.
The structure of the HGB detector 43 will be explained below. The HGB detector 43 can measure the amount of Hemoglobin (HGB) by the SLS hemoglobin method. Fig. 6 is an oblique view of the structure of the HGB detector 43. The HGB detector 43 includes a cuvette 43a containing a diluted sample, a light emitting diode 43b emitting light to the cuvette 43a, and a light collecting element 43c receiving light transmitted through the cuvette 43 a. The blood quantified by the sampling valve 12 is diluted with a diluent and a hemolytic agent at a predetermined dilution ratio to prepare a diluted sample. The hemolytic agent has a property of converting hemoglobin in blood into SLS-hemoglobin. The diluted sample is supplied to the well 43a and stored in the well 43 a. In this state, the light emitting diode 43b emits light, and the transmitted light is received by the light collecting element 43c disposed so that the cuvette 43a and the light emitting diode 43b face each other. The light of the wavelength emitted from the light emitting diode 43b is easily absorbed by SLS-hemoglobin, and the sample cell 43a is made of a plastic material having high light transmittance, so that the light collecting element 43c receives the transmitted light of the light emitting diode 43b after the light is absorbed only by the diluted sample. The light collecting element 43c outputs an electric signal corresponding to the amount of collected light (absorbance) to the microcomputer 6, and the microcomputer 6 compares the absorbance with the absorbance of only the diluent measured in advance to calculate the hemoglobin value.
The microcomputer 6 has an a/D converter 61 that converts the analog signal supplied from the analog signal processor 5 into a digital signal. The output value of the a/D converter 61 is sent to the arithmetic unit 62 of the microcomputer 6, and is calculated by the arithmetic unit 62 to perform a predetermined process on the collected light signal. The calculator 62 creates distribution data (a two-dimensional scattergram (unclassified) and a one-dimensional histogram) from the output value of the detection section 4.
The microcomputer 6 includes a controller 63 including a control processor and a control processor operation memory, and a data analysis unit 64 including an analysis processor and an analysis processor operation memory. The controller 63 controls the instrument mechanism 8, which is composed of a sample feeder (not shown) for automatically feeding a blood collection tube, a fluid system for preparing and measuring a sample, and other parts. The data analysis unit 64 is used to perform analysis processing such as screening on each distribution data. The analysis result is transmitted to the external data processing apparatus 3 through the interface 65, and is processed, such as displaying a data screen and storing data.
The microcomputer 6 has an interface 66 connected to the display/operation section 7 and an interface 67 connected to the device mechanism section 8. The calculator 62, the controller 63, and the interfaces 66 and 67 are connected by a bus 68, and the controller 63 and the data analysis unit 64 are connected by a bus 69. The display/operation section 7 includes a start switch for an operator to give an instruction to start measurement, a display device state, various setting values and analysis results, and a touch panel type liquid crystal display for receiving an input from the operator.
The operation of the blood cell analyzer 1 of the present embodiment will be described below. Fig. 7 is a flowchart showing the operation of the sample analyzer according to the present embodiment. The user (operator) turns on the power supply of the blood cell analyzer 1 (step S1), and starts the blood cell analyzer 1. When the blood cell analyzer 1 is started, a self-test is performed (step S2). In the self-test, not only the operation of each operating mechanism of the microcomputer 6 and the blood cell analyzer 1 is tested, but also blank detection is performed to measure a blank sample containing no sample. Then, the microcomputer 6 performs initial setting of the measurement mode (step S3). This initial setting is CBC + DIFF mode. Specifically, in the processing of step S3, parameters (operating conditions) for measuring blood, such as a reaction chamber to be used and the setting of measurement time, are set. In this manner, the sample analyzer of the present embodiment uses the blood measurement mode as the initial operation mode. Accordingly, the blood cell analyzer 1 is in a standby state where it is acceptable to start measurement. The microcomputer 6 displays a screen for notifying the standby state on the liquid crystal display (step S4).
In this standby state, the operator can switch the measurement mode by operating the display/operation section 7. Fig. 8 is a schematic diagram of an input screen for setting a measurement mode. The screen includes display screens of a specimen number 120, a specimen loading mode type 121, a partial examination (measurement mode) type 122, and a specimen type 123. The specimen putting mode is provided with three modes: a manual mode in which the operator manually inserts the sample container into the sample suction nozzle 18 to suction the sample; a micro blood predilution mode in which an operator mixes a sample and a reagent in advance to prepare a measurement sample and suctions the measurement sample by a sample suction nozzle 18; the closed mode of the specimen is provided by a transport device that automatically transports specimen containers. The specimen types include Normal blood specimen (Normal), HPC (hematopoietic progenitor cell) specimen (HPC), and Body Fluid (Body Fluid). The operator can specify the specimen loading mode, the measurement mode, and the specimen type, respectively. If the operator designates the blood measurement mode, the operator designates the type of specimen as Normal (Normal), and designates the arbitrary specimen set mode and measurement mode. If the Body Fluid measurement mode is designated, the operator designates the "manual mode" in the put-in mode, designates one of "CBC + DIFF", "CBC + DIFF + RET", "CBC + DIFF + NRBC", and "CBC + DIFF + NRBC + RET" in the itemized tests, and designates the Body Fluid (Body Fluid) in the specimen type. In step S4, the operator thus designates a desired measurement mode. If the operator performs blood measurement without changing the initially set measurement mode (N is selected in step S5), the operator presses the start switch to issue a measurement start instruction. The microcomputer 6 receives the measurement start instruction (step S6), and aspirates the blood sample from the sample aspirating nozzle (step S7).
After the blood sample is aspirated, the sample is introduced into the sampling valve 12 as described above, and a sample necessary for measurement is prepared according to the type of item detection in the measurement mode (step S14). Then, a measurement operation for measuring the sample is performed (step S16). For example, when the type of the itemized detection is set to "7", various measurement samples for HGB, WBC/BASO, DIFF, RET, NRBC, RBC/PLT are prepared. Then, the measurement samples for WBC/BASO, DIFF, RET, and NRBC are measured by the leukocyte detector 41, the measurement samples for RBC/PLT are measured by the RBC/PLT detector 42, and the measurement samples for HGB are measured by the HGB detector 43. In this case, since only one white blood cell detector 41 is provided, the measurement samples NRBC, WBC/BASO, DIFF, and RET are introduced into the white blood cell detector 41 in order of NRBC, WBC/BASO, DIFF, and RET, and are measured one by one. In this measurement operation, the calculator 62 draws a particle distribution map (scattergram, histogram). Here, a description will be given of a process of drawing a scattergram from optical information obtained by the DIFF measurement. The calculator 62 draws a two-dimensional scattergram (particle distribution map) using the side scatter signal and the side fluorescence signal in the collected light signal output from the white blood cell detector 41 in the DIFF measurement as characteristic parameters. The scattergram (hereinafter referred to as DIFF scattergram) is drawn with the side scattered light intensity as X-axis and the side fluorescence intensity as Y-axis, and generally includes "erythrocyte ghosts", "lymph groups", "mononuclear groups", "neutrophilic + basophilic groups", and "eosinophilic groups". These particle groups are identified by the data analysis unit 64 by processing the DIFF scattergram.
Then, an analysis process is performed based on the particle distribution map obtained by the measurement (step S18). In this analysis process, the DIFF scattergram rendered by the calculator 62 when the data analysis unit 64 of the microcomputer 6 measures the DIFF measurement sample with respect to the white blood cell detector 41 is classified into: four leukocyte populations (lymphocyte population, monocyte population, neutrophil + basophil population and eosinophil population) and erythrocyte ghost populations are shown in FIG. 12. In the analysis processing according to the present embodiment, the degree of attribution of each particle to each group can be obtained from the distance between each particle and the position of the center of gravity of each group divided on the scattergram. The particles are classified into groups according to the degree of attribution. Such a method of classifying particles is described in detail in Japanese patent laid-open publication No. 5-149863. On the scattergram obtained by the WBC/BASO measurement, the cells were classified into a basophil group, a leukocyte group other than basophils, and a erythrocyte ghost group. Then, based on the results of the DIFF scattergram analysis processing for classifying and counting four white blood cells (see FIG. 12) and the results of the WBC/BASO scattergram analysis processing for classifying and counting two white blood cells, five white blood cells contained in the blood sample were classified. Specifically, the data analysis unit 64 subtracts the "blood cell number of basophil" obtained by the WBC/BASO scattergram analysis processing from the "blood cell number of neutrophil + basophil" obtained by the DIFF scattergram analysis processing, and thereby obtains the blood cell number of neutrophil and the blood cell number of basophil, respectively. Accordingly, leukocytes were classified into five categories (lymphocytes, monocytes, neutrophils, basophils, and eosinophils), and the number of blood cells of each item was obtained. In addition, in the RBC/PLT measurement, the trough of a one-dimensional histogram drawn based on the characteristic information measured by the detector 42 is detected, and red blood cells and platelets are classified. The analysis result thus obtained is output to the display 302 of the data processing device 3 (step S20).
On the other hand, if the microcomputer 6 receives an input designating the measurement mode as the body fluid measurement mode in step S5 as described above, it sets parameters (operating conditions) for performing body fluid measurement, such as a reaction chamber to be used, a measurement time, and the like (step S8). In the present embodiment, the measurement time is three times as long as that in blood measurement as described later.
When the measurement mode is switched from the other measurement mode (here, the blood measurement mode) to the body fluid measurement mode (step S9), the measurement device 2 starts the pre-sequence processing (step S10). This pre-sequence processing provides for the measurement of body fluids. Since the body fluid measurement mode is a sample having a low blood cell component concentration, a pre-sequence process is performed to ensure that the background does not affect the body fluid measurement result when the body fluid measurement mode is switched from the blood measurement mode (shown as "1: normal" in fig. 8).
Pre-sequence processing includes blank detection. The blank detection judgment criterion in the previous sequence processing is more strict than the judgment criterion of blank detection (for example, performed after power-on and after automatic washing) performed in the blood cell measurement mode, and is set to a value of a fraction or less. In addition, when the setting is switched from the body fluid measurement mode to the blood measurement mode, since the background influence (influence of the residue) does not generally affect the blood measurement result, the pre-sequence processing is not performed. When a body fluid sample is repeatedly measured in the body fluid measurement mode, the pre-sequence processing is not performed because the body fluid sample is not generally affected by the background. However, since some body fluid samples contain a large amount of particles, if the analysis result of the body fluid sample exceeds a certain value, "the measurement result is too high on the interface, and the next measurement of the sample may be affected, and blank detection may be performed. Please press "OK". "and the like, notifying the operator of the possibility of affecting the result of the following sample analysis. The operator presses the "confirm" button to perform blank detection. In this case, the interface is provided with a "stop" button, and the operator may move to the standby interface without performing blank detection by pressing the "stop" button. If the blank detection is not performed, it is preferable to mark a symbol having low reliability on the measurement result. This allows the blank detection to be added only when necessary, thereby preventing waste of time and reagents.
Fig. 9 is a flowchart of the pre-sequence processing steps performed when the measurement mode is switched from the blood measurement mode to the body fluid measurement mode. The blood cell analyzer 1 performs blank detection by measuring a blank sample in the measuring device 2 (step S31), and the microcomputer 6 compares the measurement result with a predetermined allowable value and determines whether or not the measurement result is lower than the allowable value (step S32). When the measurement result is lower than the allowable value, the microcomputer 6 ends the pre-sequence operation and resumes the processing. If the measured value is greater than the allowable value, the microcomputer 6 judges whether or not the blank detection is performed a predetermined number of times (for example, three times) (step S33), and if the number of blank detections does not reach the predetermined number of times, the process returns to step S31, and the blank detection is performed again within the predetermined number of times. If the blank detection measurement result has not been lower than the allowable value for the predetermined number of times, the blank measurement result and a screen including a "confirm" button, a "blank detection" button, and an "automatic wash" button are displayed on the display and operation section 7 (step S34). If the operator presses the "ok" button (step S35), the microcomputer 6 ends the previous sequence operation and resumes the processing. If the "blank detection" button is pressed (step S36), the microcomputer 6 returns the process to step S31 to perform blank detection again. If the "automatic cleaning" button is pressed (step S37), the microcomputer 6 performs automatic cleaning with the dedicated cleaning liquid (step S38), returns the processing to step S31, and performs blank detection again.
After the pre-sequence processing is completed, the blood cell analyzer 1 returns to the standby state (step S11). When the operator starts the measurement of the body fluid, the specimen aspirating nozzle 18 of the measuring apparatus 2 is inserted into the body fluid specimen in the specimen container, and the start switch is pressed, as in the case of the manual measurement of the blood specimen. Upon receiving the measurement start instruction (step S12), the microcomputer 6 starts aspirating the body fluid specimen (step S13).
After the body fluid specimen is aspirated, the body fluid specimen is introduced into the sampling valve 91 as in the case of the blood specimen, the RBC/PLT measurement sample is prepared from the reaction chamber 13 (step S15), then the DIFF measurement sample is measured by the leukocyte detector 41, and the RBC/PLT measurement sample is measured by the RBC/PLT detector 42 (step S17). in the state of the body fluid measurement mode, only the DIFF measurement sample is measured by the leukocyte detector 41, and therefore, even if the measurement time is longer than the measurement time in the blood measurement mode, the measurement can be completed in a shorter time than the time in the blood measurement mode.
On the other hand, the RBC/PLT measurement sample is introduced into the resistance detector 41 in any measurement mode, and is measured under a constant flow rate condition. Then, analysis processing is performed based on the measured characteristic information (step S19), and the analysis result is output to the display 302 of the data processing device 3 (step S21). In the analysis processing in the blood measurement mode, information (quantity and ratio) of five leukocyte subsets (neutral cells: NEUT, lymphocytes: LYMPH, monocytes: MONO, eosinophils: EO, basophils: BASO) was calculated by analyzing DIFF scattergrams and the like, but in the analysis processing in the body fluid measurement mode, the number of blood cells was small or damaged, and thus, the blood cells were classified into two subsets (monocytes: MN, multinucleated cells: PMN) in a partially integrated manner. Lymphocytes and monocytes belong to the monocyte family, and neutrophils, eosinophils, and basophils belong to the multinucleated cell family. This sort algorithm is the same as the algorithm described in the analysis processing in the blood measurement mode, and therefore, the description thereof is omitted.
Next, the analysis result obtained in step S19 is compared with an allowable value (predetermined threshold) (step S22). This allowable value is the same as the allowable value used for the blank detection in the previous sequence processing of step S10. When the analysis result is larger than the allowable value (yes in step S22), the confirmation interface 151 shown in fig. 17 to start blank detection is displayed in step S23. This confirmation interface 151 displays: it was shown that "the measurement result was too high, and the following measurement of the specimen was likely to be affected. A blank detection will be performed. Please press "OK". "information display of information 152, confirm button 153, and cancel button 154. Next, it is determined whether the user inputs the ok button 153 or the cancel button 154 (step S24), and if the user inputs the ok button (selects "ok" in step S24), blank detection is performed (step S25). When the analysis result acquired in step S19 is smaller than the allowable value (no is selected in step S22) or when a cancel button is input (cancel is selected in step S24), the blank detection is not performed, and the process returns to step S5.
Abnormal particles (macrophages, mesodermal cells, tumor cells, and the like) other than blood cells may be present in the body fluid sample. The presence of these abnormal particles in cerebrospinal fluid is rare, but more common in other body fluids, pleural and abdominal fluids. Therefore, regardless of the kind of the body fluid, the influence of these abnormal particles is excluded to accurately classify and count the blood cells in the body fluid. Therefore, the present invention makes it possible to more accurately measure leukocytes in a target body fluid sample based on the novel recognition that abnormal particles appear on the upper side of the DIFF scattergram of the present blood cell analyzer. This is not considered in the aforementioned conventional art.
Fig. 10 is a schematic diagram of a scattergram obtained by measuring and analyzing a DIFF measurement sample prepared from a body fluid and a reagent for measuring leukocytes in the body fluid measurement mode by the blood cell analyzer 1 according to the present embodiment. The vertical axis of the scattergram indicates the side fluorescence intensity (the higher the fluorescence intensity), and the horizontal axis indicates the side scattered light intensity (the higher the scattered light intensity, the farther to the right). In the scatter diagram, hemolyzed erythrocyte ghosts Gc are distributed in a region LF with weak fluorescence intensity, abnormal particles such as mesothelial cells are distributed in a region HF with strong fluorescence intensity, and mononuclear leukocytes Mc and multinuclear leukocytes Pc are distributed in a middle region MF. Therefore, in the analysis of the scattergram, the particle components distributed in the region MF other than the regions LF and HF are analyzed as leukocytes, classified into the above two types, and counted. Furthermore, the mononuclear leukocyte Mc includes lymphocytes and monocytes, and the polymorphonuclear leukocyte Pc includes neutrophils, eosinophils, and basophils.
When leukocytes in a body fluid are analyzed in this manner, there are cases where the number of blood cells contained in the body fluid is small or damaged, and therefore, the leukocytes are classified into mononuclear leukocytes and counted as clinically significant information.
In addition, abnormal particles (macrophages, mesodermal cells, tumor cells, and the like) other than blood cells may be present in the body fluid. The presence of these abnormal particles in cerebrospinal fluid is rare, but more common in other body fluids, pleural and abdominal fluids. In the scatter diagram of fig. 10, nucleated cells other than such leukocytes are distributed in the region HF. In the present embodiment, since the nucleated cells other than leukocytes can be distinguished from leukocytes, the accurate number of leukocytes can be obtained even when the body fluid contains such nucleated cells other than leukocytes. By counting the cells present in the region HF, the degree of abnormal cell presence can be provided. In the present embodiment, each cell is divided into the regions LF, MF, and HF according to a threshold value for distinguishing each region, and this threshold value may be manually changed.
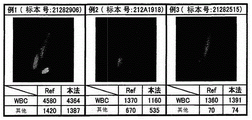
Fig. 11 is a diagram for comparing the analysis result obtained by the blood cell analyzer 1 according to the present embodiment with the counting result obtained by the control method, in order to show the appropriateness of the scattergram analysis method. The test sample is pleural fluid, the "present method" in the figure indicates the number of White Blood Cells (WBC) and the number of other abnormal particles (other) calculated by the blood cell analyzer 1 of the present embodiment, and the "Ref" indicates the results calculated by the control method (Fuchs-rosenttal plate) and site spin method). Examples 1, 2 and 3 are results of analyzing pleural effusion with a large number of abnormal particles, and it can be seen that the analysis results obtained by the blood cell analyzer 1 of the present embodiment have a correlation with the control method.
Fig. 13 is a screen 100 displayed on the display 302 of the data processing apparatus 3 as the analysis result of the measurement sample for DIFF prepared from blood. The upper part of the screen 100 has a specimen number display area for displaying the specimen number 101, and an attribute display area for displaying the attributes of the patient is provided adjacent to the specimen number display area. The attribute display area specifically displays the specimen number, patient ID, patient name, date of birth, sex, ward, attending physician, date of measurement, time of measurement, remarks, and the like. The lower part of the attribute display area is provided with a measurement result display area for displaying the measurement result. The measurement result display area is constituted by a plurality of pages, and these pages can display a screen by selecting a plurality of tabs 102. Tags are provisioned for main pages, chart screens and other items. Fig. 12 is a display screen at the time of selection of a chart tab. The left half of the measurement result display area is provided with a measurement value display area 103 for displaying the measurement value of the measurement result and a graph display area 104 for displaying a graph, and the right half is provided with a profile display area 105 for displaying a profile of the measurement result. The measured value display area displays the items, data and units of WBC, RBC, …, neit #, … BASO #, neit%, …, BASO%, etc., and the chart display area 104 displays the labeled results of specimen abnormalities and disease suspicions regarding WBC, PLT, RBC, or RET, which can be useful information in clinical examinations.
The profile display area 105 displays six profiles. The scatter diagram at the upper left is a scatter diagram for DIFF. Respective dot plots for WBC/BASO in the upper right, for naive cells (IMI) in the middle left, and for RET in the middle right. The left lower part is a histogram for RBC, and the right lower part is a histogram for PLT.
Fig. 14 is a screen 110 displayed on the display 302 of the data processing device 3 as the measurement result of the measurement sample for DIFF prepared from the body fluid. The upper part of the screen 110 has a specimen number display area 111 for displaying a specimen number, and a patient attribute display area is provided adjacent to the specimen number display area. On the left side of the specimen number display area 111, "F" indicating that measurement is performed in the body fluid measurement mode is displayed. It is clear from this that the analysis result is a body fluid measurement result. The measurement result display area is constituted by pages selectable by the tab 112. In this example, a label for "body fluid measurement (body fluid)" is selected.
In the measurement value display area 113, body fluids different from the measurement results in the blood measurement mode are displayed in association with measurement item names WBC — BF (WBC number), RBC — BF (RBC number), MN # (monocyte number (lymphocyte + single cell)), PMN # (monocyte number (neutrophil + basophil + eosinophil)), MN% (monocyte ratio in leukocyte), PMN% (monocyte ratio in leukocyte), and measurement values and units, respectively. The body fluid measurement is also provided with a graph display area 114 in the same manner as the blood measurement. The histogram display area displays two histograms 115, and the upper scatter diagram is a scatter diagram for DIFF. The lower part is a histogram for RBC.
Fig. 15 shows an example in which the "search BF (BF)) tag is selected from the tags 112 on the screen 110 of fig. 14. This screen displays the same items as those on the screen 110, in addition to the search parameter display area 116. As shown in fig. 10, the search parameter display area 116 displays the number of particles "HF-BF #" existing in the region HF, the ratio "HF-BF%" of the number of particles in the region HF to the number of particles existing in the region including the region HF and the region MF, and the number of particles "TC-BF #" existing in the region including the region HF and the region MF. Further, "HF-BF%" is the ratio of HF-BF to TC-BF.
Fig. 16 is a stored specimen list display screen 140 displayed on the display 302 of the data processing apparatus 3. 130 is a patient attribute display area. A measurement result display area for selectively displaying the measurement result through the label is arranged above the base plate. The leftmost column 131 of the assay result display area is used to display that the validation work of the assay result has not been done or has been done. V represents verified. The right column 132 is used to show the measurement mode. "F" represents the measurement result of the body fluid measurement mode. In the body fluid measurement mode, if the specimen is a high-value specimen requiring blank detection, but blank detection is not performed, the F-inversion flag may be set to indicate this.
The configuration and function of the blood cell analyzer of the present invention have been described above by taking the blood cell analyzer as an example, but the function may be realized by a control system incorporated in a conventional blood cell analyzer to allow the conventional blood cell analyzer to exhibit the function of the present invention.
In the configuration of the present embodiment, the sample amount, the type of reagent, and the reagent amount in preparing the measurement sample are the same for each of the leukocyte classification in the blood measurement mode and the leukocyte classification in the body fluid measurement mode. The sample amount and reagent amount of the measurement sample for classifying leukocytes in the prepared body fluid measurement mode may be larger than those of the measurement sample for classifying leukocytes in the prepared blood measurement mode. Since the time for the measurement of the leukocyte classification in the body fluid measurement mode is longer than that in the blood measurement mode and the amount of the measurement sample required for the measurement is large, it is possible to prepare an appropriate amount of the measurement sample for each of the leukocyte classification in the blood measurement mode and the leukocyte classification in the body fluid measurement mode.
In the present embodiment, the structure for classifying white blood cells in the body fluid measurement mode using scattered light and fluorescence is explained, but the structure is not limited thereto, and white blood cells may be classified in the body fluid measurement mode using scattered light and absorbed light, for example. In the measurement of the absorbed light, a staining agent for staining leukocytes is mixed with other reagents into a specimen to prepare a measurement sample, the measurement sample is supplied to a flow cell to form a sample flow in the flow cell, the sample flow is irradiated with light, and the light emitted from the sample flow is received by a light-collecting element such as a photodiode. When the white blood cells pass through the flow cell, the light is absorbed by the white blood cells, and the degree of absorption can be captured as the light collection amount of the light collection element. For such measurement of the absorbed light, U.S. Pat. No. 5122453 and U.S. Pat. No. 5138181 disclose the measurement. Instead of scattered light, the resistance value may be measured, and leukocytes may be classified by the resistance value and the absorbed light.
Claims (15)
1. A blood cell analyzer for performing an assay on a sample, comprising:
a detection section capable of detecting the side scattered light and the fluorescence obtained from the particles flowing through the sheath flow cell;
a controller that processes a detection result output from the detection section;
a display;
wherein the content of the first and second substances,
the controller is capable of receiving settings of a blood measurement mode and a body fluid measurement mode for measuring a body fluid other than blood;
when the body fluid measurement mode is set, the controller displays a scattergram on the display, the scattergram having the intensity of the side scattered light and the intensity of the fluorescence as axes, and classifies and counts particles, from which red blood cell ghosts and abnormal particles other than blood cells are removed, as white blood cells;
wherein the scattergram includes a first region with a small fluorescence intensity, a second region with a large fluorescence intensity, and a third region with a fluorescence intensity between the first region and the second region, the third region being a region of the scattergram excluding the first region and the second region;
the erythrocyte ghosts are distributed in the first area;
abnormal particles other than the blood cells are distributed in the second region;
analyzing the particles in the third region as the white blood cells.
2. The blood cell analyzer according to claim 1, wherein:
in the body fluid measurement mode, the controller classifies and counts mononuclear leukocytes among the leukocytes according to the intensity of the side scattered light and the intensity of the fluorescence.
3. The blood cell analyzer according to claim 1, wherein:
in the body fluid measurement mode, the controller classifies and counts polymorphonuclear leukocytes among the leukocytes according to the intensity of the side scattered light and the intensity of the fluorescence.
4. The blood cell analyzer according to claim 1, wherein:
if the blood measurement mode is set, the controller classifies leukocytes in the measurement sample into at least 4 subgroups based on the intensity of the side scattered light and the intensity of the fluorescence and counts the leukocytes.
5. The blood cell analyzer according to claim 1, wherein:
in the body fluid measurement mode, the controller counts the number of particles after the removal of the red blood cell angiogram.
6. The blood cell analyzer according to claim 1, wherein:
in the body fluid measurement mode, the controller counts the number of abnormal particles other than the blood cells.
7. The blood cell analyzer according to claim 2, wherein:
in the body fluid measurement mode, the controller calculates a ratio of the mononuclear leukocytes in the leukocytes.
8. The blood cell analyzer according to claim 3, wherein:
in the body fluid measurement mode, the controller calculates a ratio of the mononuclear leukocytes in the leukocytes.
9. The blood cell analyzer according to claim 5, wherein:
in the body fluid measurement mode, the controller calculates a ratio of the number of abnormal particles other than the blood cells to the number of particles from which the erythrocyte ghost is removed.
10. The blood cell analyzer according to claim 1, further comprising a resistive detection portion, wherein:
in the body fluid measurement mode, the controller classifies and counts red blood cells in the measurement sample based on the characteristic information obtained by the measurement by the resistance detection section.
11. The blood cell analyzer according to claim 1, wherein:
in the body fluid measurement mode, the controller removes particles having the fluorescence intensity greater than a threshold value as abnormal particles other than the blood cells, classifies the abnormal particles, and counts leukocytes.
12. The blood cell analyzer according to claim 1, wherein:
the controller displays a measurement result screen on the display;
when the measurement is performed in the body fluid measurement mode, the controller displays a content indicating that the measurement is performed in the body fluid measurement mode on the measurement result screen together with a sample number.
13. The blood cell analyzer according to claim 1, wherein:
the controller displays a list display screen of the stored specimens on the display;
when the measurement is performed in the body fluid measurement mode, the controller displays a content indicating that the measurement is performed in the body fluid measurement mode on the list display screen together with the sample number.
14. The blood cell analyzer according to claim 1, wherein:
the abnormal particles other than blood cells include at least one of macrophages, mesothelial cells and tumor cells.
15. The blood cell analyzer according to claim 1, wherein:
the body fluid is cerebrospinal fluid, hydrothorax, ascites, cardiac sac fluid or joint fluid.
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2007022524 | 2007-02-01 | ||
| JP2007-022524 | 2007-02-01 | ||
| JP2007-119012 | 2007-04-27 | ||
| JP2007119012A JP4926812B2 (en) | 2007-02-01 | 2007-04-27 | Blood cell analyzer and body fluid analysis method |
| CN200810005239.2A CN101236195B (en) | 2007-02-01 | 2008-01-31 | Cellanalyzer, method for analyzing body fluid and control system thereof |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN200810005239.2A Division CN101236195B (en) | 2007-02-01 | 2008-01-31 | Cellanalyzer, method for analyzing body fluid and control system thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN105891090A CN105891090A (en) | 2016-08-24 |
| CN105891090B true CN105891090B (en) | 2020-01-17 |
Family
ID=39785791
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201610208992.6A Active CN105807037B (en) | 2007-02-01 | 2008-01-31 | Cellanalyzer, method for analyzing body fluid and its control system |
| CN201610212407.XA Active CN105891090B (en) | 2007-02-01 | 2008-01-31 | Blood cell analyzer, body fluid analysis method, and control system therefor |
| CN201610209662.9A Active CN105807038B (en) | 2007-02-01 | 2008-01-31 | Cellanalyzer, method for analyzing body fluid and its control system |
| CN200810005239.2A Active CN101236195B (en) | 2007-02-01 | 2008-01-31 | Cellanalyzer, method for analyzing body fluid and control system thereof |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201610208992.6A Active CN105807037B (en) | 2007-02-01 | 2008-01-31 | Cellanalyzer, method for analyzing body fluid and its control system |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201610209662.9A Active CN105807038B (en) | 2007-02-01 | 2008-01-31 | Cellanalyzer, method for analyzing body fluid and its control system |
| CN200810005239.2A Active CN101236195B (en) | 2007-02-01 | 2008-01-31 | Cellanalyzer, method for analyzing body fluid and control system thereof |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP4926812B2 (en) |
| CN (4) | CN105807037B (en) |
Families Citing this family (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5337806B2 (en) * | 2008-09-03 | 2013-11-06 | 株式会社日立ハイテクノロジーズ | Automatic analyzer |
| DE102011076238A1 (en) | 2011-05-20 | 2012-11-22 | Siemens Ag | Arrangement and method for optical analysis and specific isolation of biological samples |
| CN102331397A (en) * | 2011-07-08 | 2012-01-25 | 无锡荣兴科技有限公司 | Photoelectric sensor for statistic analysis of blood cells |
| JP6085419B2 (en) * | 2012-03-30 | 2017-02-22 | シスメックス株式会社 | Sample analyzer |
| ES2560311T3 (en) * | 2013-02-28 | 2016-02-18 | Sysmex Corporation | Urine sample analyzer, sample analysis method, and sample analysis control program |
| CN103436439B (en) * | 2013-09-06 | 2014-12-17 | 朱耀辉 | Calibration device for cell counter based on image identification method |
| JP6092132B2 (en) * | 2014-01-29 | 2017-03-08 | シスメックス株式会社 | Blood cell analyzer |
| JP6170865B2 (en) * | 2014-03-31 | 2017-07-26 | シスメックス株式会社 | Blood analyzer |
| CN104535298B (en) * | 2014-12-29 | 2017-07-11 | 中国科学院长春光学精密机械与物理研究所 | Side scattered light analogue means applied to imaging flow cytometer |
| US9566605B2 (en) | 2015-01-20 | 2017-02-14 | R.J. Reynolds Tobacco Products | Humidity control insert for cigarette packs |
| JP6680492B2 (en) | 2015-09-11 | 2020-04-15 | シスメックス株式会社 | Cell analyzer and cell analysis method |
| CN110249223B (en) | 2017-02-17 | 2020-10-20 | 深圳迈瑞生物医疗电子股份有限公司 | Blood cell analysis method and blood cell analyzer |
| EP4246148A3 (en) * | 2017-03-07 | 2023-12-06 | Hitachi High-Tech Corporation | Automatic analysis device |
| JP7002245B2 (en) * | 2017-08-10 | 2022-01-20 | シスメックス株式会社 | Blood analyzers, blood analysis methods and programs |
| CN111033254A (en) * | 2017-08-31 | 2020-04-17 | 深圳迈瑞生物医疗电子股份有限公司 | Sample detection device, sample analyzer and sample detection method |
| JP6434114B1 (en) * | 2017-11-30 | 2018-12-05 | シスメックス株式会社 | Measuring method and measuring device |
| CN109991430A (en) * | 2017-12-30 | 2019-07-09 | 深圳迈瑞生物医疗电子股份有限公司 | Sample analyser and method of sample analysis |
| US11538566B2 (en) * | 2018-05-23 | 2022-12-27 | Beckman Coulter, Inc. | Sample analysis with test determination based on identified condition |
| EP3789757A4 (en) * | 2018-04-28 | 2021-07-07 | Shenzhen Mindray Bio-Medical Electronics Co., Ltd. | Blood analyzer and analysis method |
| CN109612911B (en) * | 2018-12-26 | 2023-07-21 | 深圳天依生命健康科技有限公司 | Full-automatic sperm cell detector |
| WO2020133257A1 (en) * | 2018-12-28 | 2020-07-02 | 深圳迈瑞生物医疗电子股份有限公司 | Method for processing detection value of object under measurement, hemocyte analyzer, and storage medium |
| CN114450589A (en) * | 2019-10-23 | 2022-05-06 | 深圳迈瑞生物医疗电子股份有限公司 | Method for analyzing red blood cells in blood sample and blood analysis system |
| CN114829944A (en) * | 2019-12-31 | 2022-07-29 | 深圳迈瑞生物医疗电子股份有限公司 | Sample analysis system and method |
| CN113959912A (en) * | 2020-07-20 | 2022-01-21 | 深圳迈瑞生物医疗电子股份有限公司 | Leukocyte detection method and reagent for resisting platelet aggregation interference and application thereof |
| CN115885166A (en) * | 2020-09-24 | 2023-03-31 | 深圳迈瑞生物医疗电子股份有限公司 | Sample analyzer, sample analyzing method, and computer-readable storage medium |
| CN112304816A (en) * | 2020-10-28 | 2021-02-02 | 宁夏医科大学总医院 | Cerebrospinal fluid cell characteristic collecting method and device |
| CN113640195B (en) * | 2021-02-03 | 2022-10-11 | 深圳市帝迈生物技术有限公司 | Kit and POCT blood cell analyzer |
| EP4174488A4 (en) * | 2021-08-31 | 2023-10-11 | Shenzhen Mindray Animal Medical Technology Co., Ltd. | Specimen analysis apparatus and specimen analysis method |
| CN113759129A (en) * | 2021-09-26 | 2021-12-07 | 北京倍肯恒业科技发展股份有限公司 | Rapid detection card for leucocyte-bound C-reactive protein and preparation method thereof |
| CN114791502B (en) * | 2022-06-13 | 2022-10-28 | 深圳市帝迈生物技术有限公司 | Sample detection method and sample analyzer |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5812419A (en) * | 1994-08-01 | 1998-09-22 | Abbott Laboratories | Fully automated analysis method with optical system for blood cell analyzer |
| JP2002207035A (en) * | 2001-01-10 | 2002-07-26 | Sysmex Corp | Method for counting tumorigenic cell |
| US6979550B1 (en) * | 2002-09-05 | 2005-12-27 | Rivas Ariel L | Method for diagnosis of, and determination of susceptibility to bovine mastitis |
| US7580120B2 (en) * | 2005-04-07 | 2009-08-25 | Sysmex Corporation | Blood analyzer, sample analyzer, and flow cytometer |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3070968B2 (en) * | 1991-05-14 | 2000-07-31 | シスメックス株式会社 | Urine cell analysis reagents and methods |
| JP3875754B2 (en) * | 1995-11-17 | 2007-01-31 | シスメックス株式会社 | Standard solution for flow cytometer |
| US6524858B1 (en) * | 1999-03-31 | 2003-02-25 | Bayer Corporation | Single channel, single dilution detection method for the identification and quantification of blood cells and platelets in a whole blood sample using an automated hematology analyzer |
| US7630063B2 (en) * | 2000-08-02 | 2009-12-08 | Honeywell International Inc. | Miniaturized cytometer for detecting multiple species in a sample |
| AU2001288425A1 (en) * | 2000-08-25 | 2002-03-04 | Cytometrics, Inc. | System, method and computer program product for measuring blood properties form a spectral image |
| EP1189059B1 (en) * | 2000-09-18 | 2009-06-24 | Sysmex Corporation | Blood cell detector, blood analyzer and blood analyzing method using the detector |
| AU2002360305A1 (en) * | 2001-10-26 | 2003-05-06 | Immunivest Corporation | Multiparameter analysis of comprehensive nucleic acids and morphological features on the same sample |
| US6653137B2 (en) * | 2001-12-03 | 2003-11-25 | Streck Laboratories Inc. | Hematology reference control |
| JP2003287491A (en) * | 2002-01-28 | 2003-10-10 | Sysmex Corp | Apparatus and method for analyzing particle |
| EP1348943A3 (en) * | 2002-03-25 | 2003-12-17 | Sysmex Corporation | Sheath liquid for particle analyzer |
| CA2428740A1 (en) * | 2002-05-20 | 2003-11-20 | Bayer Corporation | Automated method and reagent therefor for assaying body fluid samples such as cerebrospinal fluid (csf) |
| JP4873969B2 (en) * | 2005-03-17 | 2012-02-08 | シスメックス株式会社 | Sample analyzer and sample analysis method |
| CN1834659A (en) * | 2005-03-17 | 2006-09-20 | 希森美康株式会社 | Sample analyzer and sample analyzing method |
| EP1744145B1 (en) * | 2005-07-12 | 2015-09-09 | Sysmex Corporation | Standard material for particle analyzer |
| JP4994920B2 (en) * | 2007-02-01 | 2012-08-08 | シスメックス株式会社 | Sample analyzer |
-
2007
- 2007-04-27 JP JP2007119012A patent/JP4926812B2/en active Active
-
2008
- 2008-01-31 CN CN201610208992.6A patent/CN105807037B/en active Active
- 2008-01-31 CN CN201610212407.XA patent/CN105891090B/en active Active
- 2008-01-31 CN CN201610209662.9A patent/CN105807038B/en active Active
- 2008-01-31 CN CN200810005239.2A patent/CN101236195B/en active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5812419A (en) * | 1994-08-01 | 1998-09-22 | Abbott Laboratories | Fully automated analysis method with optical system for blood cell analyzer |
| JP2002207035A (en) * | 2001-01-10 | 2002-07-26 | Sysmex Corp | Method for counting tumorigenic cell |
| US6979550B1 (en) * | 2002-09-05 | 2005-12-27 | Rivas Ariel L | Method for diagnosis of, and determination of susceptibility to bovine mastitis |
| US7580120B2 (en) * | 2005-04-07 | 2009-08-25 | Sysmex Corporation | Blood analyzer, sample analyzer, and flow cytometer |
Non-Patent Citations (1)
| Title |
|---|
| 《Automated counting of cells in cerebrospinal fluid using the CellDyn-4000 haematology analyser》;Hoffmann et al.;《Clinical Chemistry and Laboratory Medicine》;20021231;第40卷(第11期);第1168页右栏倒数第2段,第1169页左栏第4段 * |
Also Published As
| Publication number | Publication date |
|---|---|
| CN105807038B (en) | 2019-06-18 |
| CN105807038A (en) | 2016-07-27 |
| CN105807037B (en) | 2019-05-28 |
| CN105807037A (en) | 2016-07-27 |
| JP2008209386A (en) | 2008-09-11 |
| CN105866012A (en) | 2016-08-17 |
| CN105891090A (en) | 2016-08-24 |
| CN101236195A (en) | 2008-08-06 |
| CN101236195B (en) | 2016-05-04 |
| JP4926812B2 (en) | 2012-05-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN105891090B (en) | Blood cell analyzer, body fluid analysis method, and control system therefor | |
| US11921106B2 (en) | Sample analyzer and computer program product | |
| JP5581283B2 (en) | Sample analyzer | |
| EP3260842B1 (en) | Hematological analyzer and method for analyzing a sample |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |