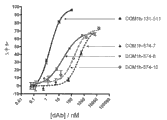
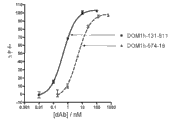
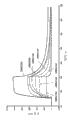
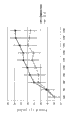
CN102405236A - 改进的抗-tnfr1多肽,抗体可变结构域和拮抗剂 - Google Patents
改进的抗-tnfr1多肽,抗体可变结构域和拮抗剂 Download PDFInfo
- Publication number
- CN102405236A CN102405236A CN2010800173301A CN201080017330A CN102405236A CN 102405236 A CN102405236 A CN 102405236A CN 2010800173301 A CN2010800173301 A CN 2010800173301A CN 201080017330 A CN201080017330 A CN 201080017330A CN 102405236 A CN102405236 A CN 102405236A
- Authority
- CN
- China
- Prior art keywords
- dom1h
- seq
- tnfr1
- single variable
- variable domains
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2878—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30, CD40, CD95
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/02—Nasal agents, e.g. decongestants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/16—Antivirals for RNA viruses for influenza or rhinoviruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/31—Immunoglobulins specific features characterized by aspects of specificity or valency multispecific
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/33—Crossreactivity, e.g. for species or epitope, or lack of said crossreactivity
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/34—Identification of a linear epitope shorter than 20 amino acid residues or of a conformational epitope defined by amino acid residues
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/40—Immunoglobulins specific features characterized by post-translational modification
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/569—Single domain, e.g. dAb, sdAb, VHH, VNAR or nanobody®
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/94—Stability, e.g. half-life, pH, temperature or enzyme-resistance
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/31—Fusion polypeptide fusions, other than Fc, for prolonged plasma life, e.g. albumin
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Immunology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Pulmonology (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Virology (AREA)
- Rheumatology (AREA)
- Biomedical Technology (AREA)
- Otolaryngology (AREA)
- Neurosurgery (AREA)
- Pain & Pain Management (AREA)
- Heart & Thoracic Surgery (AREA)
- Oncology (AREA)
- Transplantation (AREA)
- Communicable Diseases (AREA)
- Cardiology (AREA)
- Neurology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Physical Education & Sports Medicine (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15374609P | 2009-02-19 | 2009-02-19 | |
| US61/153746 | 2009-02-19 | ||
| US24119809P | 2009-09-10 | 2009-09-10 | |
| US61/241198 | 2009-09-10 | ||
| PCT/EP2010/052005 WO2010094720A2 (en) | 2009-02-19 | 2010-02-17 | Improved anti-tnfr1 polypeptides, antibody variable domains & antagonists |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN102405236A true CN102405236A (zh) | 2012-04-04 |
Family
ID=42136168
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN2010800173301A Pending CN102405236A (zh) | 2009-02-19 | 2010-02-17 | 改进的抗-tnfr1多肽,抗体可变结构域和拮抗剂 |
Country Status (14)
| Country | Link |
|---|---|
| US (1) | US20110301335A1 (cg-RX-API-DMAC7.html) |
| EP (1) | EP2398827A2 (cg-RX-API-DMAC7.html) |
| JP (2) | JP2012517818A (cg-RX-API-DMAC7.html) |
| KR (1) | KR20110119806A (cg-RX-API-DMAC7.html) |
| CN (1) | CN102405236A (cg-RX-API-DMAC7.html) |
| AU (1) | AU2010215479B2 (cg-RX-API-DMAC7.html) |
| BR (1) | BRPI1008014A2 (cg-RX-API-DMAC7.html) |
| CA (1) | CA2750477A1 (cg-RX-API-DMAC7.html) |
| EA (1) | EA022925B1 (cg-RX-API-DMAC7.html) |
| IL (1) | IL214648A0 (cg-RX-API-DMAC7.html) |
| MX (1) | MX2011008799A (cg-RX-API-DMAC7.html) |
| SG (1) | SG173173A1 (cg-RX-API-DMAC7.html) |
| WO (1) | WO2010094720A2 (cg-RX-API-DMAC7.html) |
| ZA (1) | ZA201105627B (cg-RX-API-DMAC7.html) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107043413A (zh) * | 2016-02-05 | 2017-08-15 | 华中农业大学 | 一种肺结核相关的尿液生物标志蛋白及其应用 |
| CN108473563A (zh) * | 2015-11-12 | 2018-08-31 | 埃博灵克斯股份有限公司 | 改进的tnf结合剂 |
| CN113711038A (zh) * | 2019-01-31 | 2021-11-26 | 积水医疗株式会社 | 用于生物样品中游离aim的免疫分析方法及用于检测对象中nash的方法 |
| WO2025021184A1 (zh) * | 2023-07-27 | 2025-01-30 | 上海复宏汉霖生物医药有限公司 | 编码尿酸氧化酶的多核苷酸及其相关组合物和方法 |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9028822B2 (en) * | 2002-06-28 | 2015-05-12 | Domantis Limited | Antagonists against TNFR1 and methods of use therefor |
| EA028178B1 (ru) | 2009-02-19 | 2017-10-31 | Глэксо Груп Лимитед | Улучшенные связывающие сывороточный альбумин варианты |
| WO2011008814A2 (en) * | 2009-07-14 | 2011-01-20 | Immune Tolerance Institute, Inc., A California Not-For-Profit Corporation | Multiplexed measurement of exogenous and endogenous dna |
| US20120107330A1 (en) * | 2009-07-16 | 2012-05-03 | Adriaan Allart Stoop | Antagonists, uses & methods for partially inhibiting tnfr1 |
| AU2010311640B2 (en) * | 2009-10-27 | 2015-01-29 | Glaxo Group Limited | Stable anti-TNFR1 polypeptides, antibody variable domains & antagonists |
| WO2011144751A1 (en) * | 2010-05-20 | 2011-11-24 | Glaxo Group Limited | Improved anti-serum albumin binding variants |
| ES2618586T3 (es) * | 2010-09-16 | 2017-06-21 | Baliopharm Ag | Anticuerpo anti-huTNFR1 |
| EP2721066A1 (en) * | 2011-06-17 | 2014-04-23 | Glaxo Group Limited | Tumour necrosis factor receptor 1 antagonists |
| WO2015104322A1 (en) | 2014-01-09 | 2015-07-16 | Glaxosmithkline Intellectual Property Development Limited | Treatment of inflammatory diseases with non-competitive tnfr1 antagonists |
| US9107906B1 (en) | 2014-10-28 | 2015-08-18 | Adma Biologics, Inc. | Compositions and methods for the treatment of immunodeficiency |
| WO2016094309A1 (en) * | 2014-12-10 | 2016-06-16 | Myosotis | Inhibition of tnf signaling in cancer immunotherapy |
| US10259865B2 (en) | 2017-03-15 | 2019-04-16 | Adma Biologics, Inc. | Anti-pneumococcal hyperimmune globulin for the treatment and prevention of pneumococcal infection |
| EP3927828A4 (en) | 2019-02-21 | 2023-02-01 | Enosi Life Sciences Corp. | ANTIBODIES AND ENONOMERS |
| CN116847887A (zh) | 2020-08-27 | 2023-10-03 | 伊诺西治疗公司 | 治疗自身免疫性疾病和癌症的方法和组合物 |
| WO2022117569A1 (en) | 2020-12-02 | 2022-06-09 | Oncurious Nv | A ccr8 antagonist antibody in combination with a lymphotoxin beta receptor agonist antibody in therapy against cancer |
| WO2025049818A1 (en) | 2023-08-29 | 2025-03-06 | Enosi Therapeutics Corporation | Tnfr1 antagonists lacking agonist activity and uses thereof |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007063308A2 (en) * | 2005-12-01 | 2007-06-07 | Domantis Limited | Noncompetitive domain antibody formats that bind interleukin 1 receptor type 1 |
| WO2007066106A1 (en) * | 2005-12-06 | 2007-06-14 | Domantis Limited | Ligands that have binding specificity for egfr and/or vegf and methods of use therefor |
| CN101084014A (zh) * | 2004-10-08 | 2007-12-05 | 杜门蒂斯有限公司 | 抗肿瘤坏死因子受体1的单域抗体及其使用方法 |
| WO2008096158A2 (en) * | 2007-02-08 | 2008-08-14 | Domantis Limited | Antibody single variable domains against serum albumin |
| WO2008149148A2 (en) * | 2007-06-06 | 2008-12-11 | Domantis Limited | Polypeptides, antibody variable domains and antagonists |
Family Cites Families (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3883899T3 (de) | 1987-03-18 | 1999-04-22 | Sb2, Inc., Danville, Calif. | Geänderte antikörper. |
| GB8725529D0 (en) | 1987-10-30 | 1987-12-02 | Delta Biotechnology Ltd | Polypeptides |
| JP3095168B2 (ja) | 1988-02-05 | 2000-10-03 | エル. モリソン,シェリー | ドメイン‐変性不変部を有する抗体 |
| DE68927933T2 (de) | 1988-09-02 | 1997-08-14 | Dyax Corp | Herstellung und auswahl von rekombinantproteinen mit verschiedenen bindestellen |
| ATE92107T1 (de) | 1989-04-29 | 1993-08-15 | Delta Biotechnology Ltd | N-terminale fragmente von menschliches serumalbumin enthaltenden fusionsproteinen. |
| US5977307A (en) | 1989-09-07 | 1999-11-02 | Alkermes, Inc. | Transferrin receptor specific ligand-neuropharmaceutical agent fusion proteins |
| US6172197B1 (en) | 1991-07-10 | 2001-01-09 | Medical Research Council | Methods for producing members of specific binding pairs |
| WO1994026087A2 (en) | 1993-05-14 | 1994-11-24 | Connor Kim C O | Recombinant protein production and insect cell culture and process |
| CA2163345A1 (en) | 1993-06-16 | 1994-12-22 | Susan Adrienne Morgan | Antibodies |
| EP1019496B1 (en) | 1997-07-07 | 2004-09-29 | Medical Research Council | In vitro sorting method |
| IL127127A0 (en) | 1998-11-18 | 1999-09-22 | Peptor Ltd | Small functional units of antibody heavy chain variable regions |
| EP1263788A2 (en) | 2000-02-11 | 2002-12-11 | THE GOVERNMENT OF THE UNITED STATES OF AMERICA, as represented by THE SECRETARY, DEPARTMENT OF HEALTH AND HUMAN SERVICES | Identification of a domain in the tumor necrosis factor receptor family that mediates pre-ligand receptor assembly and function |
| DK1399484T3 (da) | 2001-06-28 | 2010-11-08 | Domantis Ltd | Dobbelt-specifik ligand og anvendelse af denne |
| US20080108560A1 (en) | 2002-03-05 | 2008-05-08 | Eli Lilly And Company | Heterologous G-Csf Fusion Proteins |
| US20060002935A1 (en) * | 2002-06-28 | 2006-01-05 | Domantis Limited | Tumor Necrosis Factor Receptor 1 antagonists and methods of use therefor |
| EP2135879A3 (en) | 2002-06-28 | 2010-06-23 | Domantis Limited | Ligand |
| US7696320B2 (en) | 2004-08-24 | 2010-04-13 | Domantis Limited | Ligands that have binding specificity for VEGF and/or EGFR and methods of use therefor |
| CA2505326A1 (en) | 2002-11-08 | 2004-05-21 | Ablynx N.V. | Camelidae antibodies against immunoglobulin e and use thereof for the treatment of allergic disorders |
| EP1578801A2 (en) | 2002-12-27 | 2005-09-28 | Domantis Limited | Dual specific single domain antibodies specific for a ligand and for the receptor of the ligand |
| GB0230203D0 (en) | 2002-12-27 | 2003-02-05 | Domantis Ltd | Fc fusion |
| US20090005257A1 (en) | 2003-05-14 | 2009-01-01 | Jespers Laurent S | Process for Recovering Polypeptides that Unfold Reversibly from a Polypeptide Repertoire |
| DE602004017726D1 (de) | 2003-06-30 | 2008-12-24 | Domantis Ltd | Pegylierte Single-domain-antikörper (dAb) |
| AU2005211725B2 (en) | 2004-02-09 | 2010-07-15 | Human Genome Sciences, Inc. | Albumin fusion proteins |
| DK1737962T3 (da) | 2004-03-24 | 2010-12-13 | Domantis Ltd | GAS1-universel leder |
| GB0418651D0 (en) | 2004-08-20 | 2004-09-22 | Medical Res Council | Method |
| GB0521621D0 (en) | 2005-10-24 | 2005-11-30 | Domantis Ltd | Tumor necrosis factor receptor 1 antagonists for treating respiratory diseases |
| GB0724331D0 (en) | 2007-12-13 | 2008-01-23 | Domantis Ltd | Compositions for pulmonary delivery |
| US7868885B2 (en) | 2007-06-22 | 2011-01-11 | Microsoft Corporation | Direct manipulation of subdivision surfaces using a graphics processing unit |
| US20120107330A1 (en) * | 2009-07-16 | 2012-05-03 | Adriaan Allart Stoop | Antagonists, uses & methods for partially inhibiting tnfr1 |
| BR112012001681A2 (pt) * | 2009-07-16 | 2019-09-24 | Graxo Group Ltd | domínios variáveis de ligação de anti-albumina sérica aperfeiçoados |
| AU2010311640B2 (en) * | 2009-10-27 | 2015-01-29 | Glaxo Group Limited | Stable anti-TNFR1 polypeptides, antibody variable domains & antagonists |
-
2010
- 2010-02-17 CA CA2750477A patent/CA2750477A1/en not_active Abandoned
- 2010-02-17 MX MX2011008799A patent/MX2011008799A/es not_active Application Discontinuation
- 2010-02-17 KR KR1020117021824A patent/KR20110119806A/ko not_active Ceased
- 2010-02-17 WO PCT/EP2010/052005 patent/WO2010094720A2/en not_active Ceased
- 2010-02-17 EP EP10704151A patent/EP2398827A2/en not_active Withdrawn
- 2010-02-17 US US13/202,349 patent/US20110301335A1/en not_active Abandoned
- 2010-02-17 SG SG2011054459A patent/SG173173A1/en unknown
- 2010-02-17 JP JP2011550552A patent/JP2012517818A/ja active Pending
- 2010-02-17 BR BRPI1008014A patent/BRPI1008014A2/pt not_active IP Right Cessation
- 2010-02-17 AU AU2010215479A patent/AU2010215479B2/en not_active Ceased
- 2010-02-17 EA EA201190116A patent/EA022925B1/ru not_active IP Right Cessation
- 2010-02-17 CN CN2010800173301A patent/CN102405236A/zh active Pending
-
2011
- 2011-07-29 ZA ZA2011/05627A patent/ZA201105627B/en unknown
- 2011-08-15 IL IL214648A patent/IL214648A0/en unknown
-
2015
- 2015-07-31 JP JP2015151376A patent/JP2016007218A/ja active Pending
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101084014A (zh) * | 2004-10-08 | 2007-12-05 | 杜门蒂斯有限公司 | 抗肿瘤坏死因子受体1的单域抗体及其使用方法 |
| WO2007063308A2 (en) * | 2005-12-01 | 2007-06-07 | Domantis Limited | Noncompetitive domain antibody formats that bind interleukin 1 receptor type 1 |
| WO2007066106A1 (en) * | 2005-12-06 | 2007-06-14 | Domantis Limited | Ligands that have binding specificity for egfr and/or vegf and methods of use therefor |
| WO2008096158A2 (en) * | 2007-02-08 | 2008-08-14 | Domantis Limited | Antibody single variable domains against serum albumin |
| WO2008149148A2 (en) * | 2007-06-06 | 2008-12-11 | Domantis Limited | Polypeptides, antibody variable domains and antagonists |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN108473563A (zh) * | 2015-11-12 | 2018-08-31 | 埃博灵克斯股份有限公司 | 改进的tnf结合剂 |
| CN108473563B (zh) * | 2015-11-12 | 2022-06-14 | 埃博灵克斯股份有限公司 | 改进的tnf结合剂 |
| CN107043413A (zh) * | 2016-02-05 | 2017-08-15 | 华中农业大学 | 一种肺结核相关的尿液生物标志蛋白及其应用 |
| CN113711038A (zh) * | 2019-01-31 | 2021-11-26 | 积水医疗株式会社 | 用于生物样品中游离aim的免疫分析方法及用于检测对象中nash的方法 |
| WO2025021184A1 (zh) * | 2023-07-27 | 2025-01-30 | 上海复宏汉霖生物医药有限公司 | 编码尿酸氧化酶的多核苷酸及其相关组合物和方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2398827A2 (en) | 2011-12-28 |
| AU2010215479B2 (en) | 2015-01-22 |
| WO2010094720A3 (en) | 2011-01-13 |
| EA022925B1 (ru) | 2016-03-31 |
| CA2750477A1 (en) | 2010-08-26 |
| WO2010094720A2 (en) | 2010-08-26 |
| BRPI1008014A2 (pt) | 2016-10-04 |
| SG173173A1 (en) | 2011-08-29 |
| AU2010215479A1 (en) | 2011-08-25 |
| US20110301335A1 (en) | 2011-12-08 |
| JP2012517818A (ja) | 2012-08-09 |
| EA201190116A1 (ru) | 2012-06-29 |
| IL214648A0 (en) | 2011-09-27 |
| JP2016007218A (ja) | 2016-01-18 |
| MX2011008799A (es) | 2011-09-27 |
| KR20110119806A (ko) | 2011-11-02 |
| ZA201105627B (en) | 2013-01-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2010215479B2 (en) | Improved anti-TNFR1 polypeptides, antibody variable domains & antagonists | |
| US20120107330A1 (en) | Antagonists, uses & methods for partially inhibiting tnfr1 | |
| JP5444215B2 (ja) | ポリペプチド、抗体可変ドメインおよびアンタゴニスト | |
| CN102686239B (zh) | 稳定的抗-tnfr1多肽、抗体可变结构域和拮抗剂 | |
| JP2010528646A (ja) | ポリペプチド、抗体可変ドメイン、およびアンタゴニスト | |
| JP2010528647A (ja) | ポリペプチド、抗体可変ドメインおよびアンタゴニスト | |
| US9028817B2 (en) | Stable anti-TNFR1 polypeptides, antibody variable domains and antagonists | |
| KR20070084069A (ko) | Tnfr1에 대한 단일 도메인 항체 및 이의 사용 방법 | |
| US20110236380A1 (en) | Ligands that bind il-13 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C02 | Deemed withdrawal of patent application after publication (patent law 2001) | ||
| WD01 | Invention patent application deemed withdrawn after publication |
Application publication date: 20120404 |