CN102126994A - 一种二苯酮腙衍生物、其制备方法和用途 - Google Patents
一种二苯酮腙衍生物、其制备方法和用途 Download PDFInfo
- Publication number
- CN102126994A CN102126994A CN2010100400400A CN201010040040A CN102126994A CN 102126994 A CN102126994 A CN 102126994A CN 2010100400400 A CN2010100400400 A CN 2010100400400A CN 201010040040 A CN201010040040 A CN 201010040040A CN 102126994 A CN102126994 A CN 102126994A
- Authority
- CN
- China
- Prior art keywords
- direct key
- alkyl
- benzophenone
- hydrazone derivatives
- benzophenone hydrazone
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Landscapes
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
Description
发明领域
本发明涉及一种二苯酮腙类化合物、其制备方法和用途。
背景技术
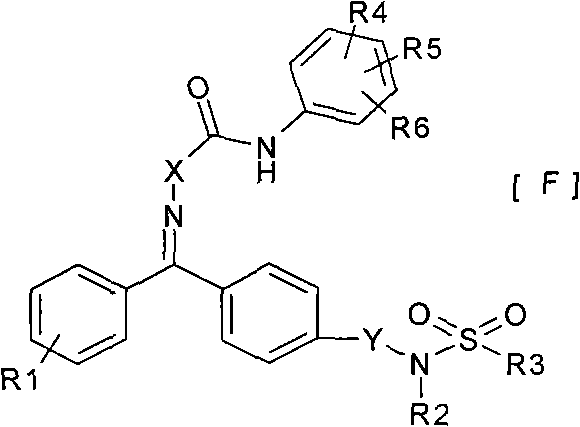
对二苯酮腙类化合物的研究始于上世纪80年代,但高活性化合物专利报导却集中在近十年时间,近几年甚至有超高活性化合物(10ppm以下)被发现。这些化合物都具有下述结构通式:
依据取代基R1、R2、R3、R4和取代基数目m、n的不同而形成不同的化合物,美国专利US5340837(结构式II)、US6077866(结构式III)和日本专利JP8041013A(结构式IV)、JP10182625A(结构式V)、JP10109971A(结构式VI)等均有报导。这些不同的化合物对线虫、螨虫和粘虫均具良好的杀虫活性,特别是对鳞翅目害虫普遍有效,而对其它有机生物体和人畜则大多表现为中低毒。
2001年,浙江化工科技集团有限公司(国家南方农药创制中心浙江基地)发现了一类新的苯基甲磺酸酯类化合物,专利号:ZL 01119493.6,发明名称:杀虫剂二苯甲酮腙衍生物。经过几年的研究和评价认为这是一类具有良好杀虫活性的、低毒环境相容性好的、合成工艺简单的、开发前景看好的苯基甲磺酸酯类杀虫剂。代表化合物化学结构式如下:
对此类化合物的报道有:巴斯夫公司2007年商品化的MEtaflumiZon(US5543573),诺华公司1993年化合物(EP662472),日本住友公司1994年化合物(JP7149708),日本拜耳公司1996年化合物(JP10182625),日本农药申请的专利(US 5358965),日本Otsuka公司于1998年申请的专利(JP10109971)和Agro-KanBsho公司于2000年申请的专利(WO0023422),类似专利还有EP462456、US6306798、JP07053501、WO2001001781、WO9206076和WO2002032226等。
发明内容
本发明的目的在于提供一种具备生物活性的二苯酮腙衍生物、其制备方法和用途。
为实现上述目的,本发明提供如下技术方案:
一种二苯酮腙衍生物,具有式[F]所示结构式:
其中:
R1为卤素、C1-C4烷基、C1-C4烷氧基、C1-C4卤代烷基或C1-C4卤代烷氧基;
R2为氢、C1-C4烷基或环丙基;
R3为C1-C4烷基或C1-C4卤代烷基;
R4、R5、R6独立地选自氢、卤素、硝基、腈基、羟基、C1-C4烷基、C1-C4卤代烷基、C1-C4烷氧基、(取代)苯氧基、C1-C4卤代烷氧基、甲酸及其碱金属盐、甲酸C1~6酯基、甲酰胺或N-C1-4烷基或苯基取代甲酰胺;
X为胺基或氧;
Y为亚甲基或直接键。
上述R1可以位于苯环上任一未被取代的位置,优选位于苯环的3位或4位;R1优选为氯、氟或三氟甲基。
上述R2优选为甲基。
上述R3优选为甲基或三氟甲基。
上述X优选为胺基。
上述Y优选为直接键。
上述R4、R5、R6可以位于苯环上任一未被取代的位置;R4、R5、R6独立地优选为氢、卤素、硝基、甲基、三氟甲基、甲氧基、三氟甲氧基或甲酸酯。
本发明所述二苯酮腙衍生物,包括N-X为顺式异构体和/或反式异构体,两种异构体可以以任何比例混合。
本发明所述二苯酮腙衍生物可以用如下的反应步骤合成:
中间体A的合成可以采取以下方法:
(1)R1取代的苯,对硝基苯甲酰氯在氯代烷烃溶剂中分批加入三氯化铝,反应得中间体A;
(2)R1取代的溴苯在醚类溶剂中和镁反应得格氏试剂,再和对硝基苯甲酰氯反应得中间体A。
中间体A用氯化亚锡,多硫化钠或钯碳-氢气还原硝基得到中间体B。
中间体B和R3取代磺酰氯或者磺酸酐在缚酸剂存在下反应得到中间体C,缚酸剂可以选用三乙胺或碳酸钾。
中间体C和卤代烷或者硫酸二甲(乙)酯在缚酸剂存在下反应得到中间体D,卤代烷可以选用碘甲烷,缚酸剂可以选用三乙胺或碳酸钾。
由中间体D合成目标产物F可以有两条合成工艺路线,一条为:
4(或3或2)-R1-4’-R3SO2N(R2)Y-二苯甲酮(即中间体D)与1-10当量的水合肼或盐酸羟胺,在醇类溶剂中,在0℃到溶剂回流温度下,反应得到4(或3或2)-R1-4’-R3SO2N(R2)Y-二苯甲酮的水合肼脱水缩合物中间体(即中间体E);
当使用盐酸羟胺时,需要加入缚酸剂;
4(或3或2)-R1-4’-R3SO2N(R2)Y-二苯甲酮的水合肼脱水缩合物中间体(即中间体E)与结构式[I]所表示的R4R5R6取代的苯基异氰酸酯在醚类溶剂中,在0℃到溶剂回流温度下,反应得到结构式[F]所述的二苯酮腙衍生物。
上述合成工艺路线中,R1、R2、R3、R4、R5、R6、X和Y基团与目标产物结构式[F]所述的二苯酮腙衍生物上的基团相对应,其中:
R1为卤素、C1-C4烷基、C1-C4烷氧基、C1-C4卤代烷基或C1-C4卤代烷氧基;
R2为氢、C1-C4烷基或环丙基;
R3为C1-C4烷基或C1-C4卤代烷基;
R4、R5、R6独立地选自氢、卤素、硝基、腈基、羟基、C1-C4烷基、C1-C4卤代烷基、C1-C4烷氧基、(取代)苯氧基、C1-C4卤代烷氧基、甲酸及其碱金属盐、甲酸C1~6酯基、甲酰胺或N-C1-4烷基或苯基取代甲酰胺;
X为胺基或氧;
Y为亚甲基或直接键。
上述R1可以位于苯环上任一未被取代的位置,优选位于苯环的3位或4位;R1优选为氯、氟或三氟甲基。
上述R2优选为甲基。
上述R3优选为甲基或三氟甲基。
上述X优选为胺基。
上述Y优选为直接键。
上述R4、R5、R6可以位于苯环上任一未被取代的位置;R4、R5、R6独立地优选为氢、卤素、硝基、甲基、三氟甲基、甲氧基、三氟甲氧基或甲酸酯。
上述缚酸剂优选为醋酸钠、三乙胺或碳酸钾。
上述醚类优选为乙醚、四氢呋喃或二氧六环。
另一条合成工艺路线为:
4(或3或2)-R1-4’-R3SO2N(R2)Y-二苯甲酮(即中间体D)与1-5当量的肼基甲酸甲酯,在苯(苯、甲苯、二甲苯、乙苯)类或醇类溶剂中,在0℃到溶剂回流温度下,反应得到4(或3或2)-R1-4’-R3SO2N(R2)Y-二苯甲酮的肼基甲酸甲酯脱水缩合物中间体(即中间体G);
4(或3或2)-R1-4’-R3SO2N(R2)Y-二苯甲酮的肼基甲酸甲酯脱水缩合物中间体(即中间体G)与R4R5R6取代的苯胺在苯类或醚类溶剂中,在0℃到溶剂回流温度下,反应得到结构式[F]所述的二苯酮腙衍生物。
上述合成工艺路线中,R1、R2、R3、R4、R5、R6、X和Y基团与目标产物结构式[F]所述的二苯酮腙衍生物上的基团相对应,其中:
R1为卤素、C1-C4烷基、C1-C4烷氧基、C1-C4卤代烷基或C1-C4卤代烷氧基;
R2为氢、C1-C4烷基或环丙基;
R3为C1-C4烷基或C1-C4卤代烷基;
R4、R5、R6独立地选自氢、卤素、硝基、腈基、羟基、C1-C4烷基、C1-C4卤代烷基、C1-C4烷氧基、(取代)苯氧基、C1-C4卤代烷氧基、甲酸及其碱金属盐、甲酸C1~6酯基、甲酰胺或N-C1-4烷基或苯基取代甲酰胺;
X为胺基或氧;
Y为亚甲基或直接键。
上述R1可以位于苯环上任一未被取代的位置,优选位于苯环的3位或4位;R1优选为氯、氟或三氟甲基。
上述R2优选为甲基。
上述R3优选为甲基或三氟甲基。
上述X优选为胺基。
上述Y优选为直接键。
上述R4、R5、R6可以位于苯环上任一未被取代的位置;R4、R5、R6独立地优选为氢、卤素、硝基、甲基、三氟甲基、甲氧基、三氟甲氧基或甲酸酯。
上述苯类优选为苯、甲苯、二甲苯或乙苯。
上述醚类优选为乙醚、四氢呋喃或二氧六环。
本发明将结构式(I)所涉及的典型化合物列于表1,AB分别代表顺反异构体中的其中一种。
表1:结构式(I)所涉及的典型化合物
| 编号 | R1 | R2 | R3 | X | Y | R4R5R6-Ph | H1NMR(核磁氢谱) |
| F1 | 4-Cl | CH3 | CH3 | NH | 直接键 | 苯胺 | (B)2.96(s,3H),3.43(s,3H),7.23-9.12(m,15H). |
| F2 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3,4-二氯苯胺 | (B)2.97(s,3H),3.43(s,3H),7.25-9.38(m,13H). |
| F3 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2,4-二甲基苯胺 | (A)2.31(s,3H),2.36(s,3H),2.86(s,3H),3.35(s,3H),7.05-8.14(m,13H).(B)2.31(s,3H),2.35(s,3H),2.97(s,3H),3.43(s,3H),7.05-8.14(m,13H).A∶B=1∶1 |
| F4 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2-甲基-3-氯苯胺 | (A)2.45(s,3H),2.87(s,3H),3.35(s,3H),7.16-8.31(m,13H).(B)2.45(s,3H),2.97(s,3H),3.43(s,3H),7.15-8.30(m,13H).A∶B=1∶1 |
| F5 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2-甲氧基苯胺 | (A)2.87(s,3H),3.36(s,3H),3.99(s,3H),6.93-9.02(m,14H)(B)2.97(s,3H),3.43(s,3H),3.98(s,3H),6.93-9.04(m,14H)A∶B=1∶1 |
| F6 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-三氟甲氧基苯胺 | (A)2.87(s,3H),3.36(s,3H),7.168.29(m,14H)(B)2.97(s,3H),3.43(s,3H),7.20-8.28(m,14H)A∶B=1∶1 |
| F7 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2,4-二氟苯胺 | (B)2.95(s,3H),3.43(s,3H),7.03-9.41(m,13H). |
| F8 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3,5-二甲基苯胺 | (B)2.23(s,6H),2.95(s,3H),3.42(s,3H),7.16-9.00(m,13H). |
| F9 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2-三氟甲氧基苯胺 | (B)2.96(s,3H),3.43(s,3H),7.33-9.72(m,14H) |
| F10 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3-三氟甲氧基苯胺 | (A)2.88(s,3H),3.36(s,3H),6.93-8.34(m,14H)(B)2.97(s,3H),3.43(s,3H),6.96-8.32(m,14H)A∶B=1∶1 |
| F11 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-二氟甲氧基苯胺 | (B)2.97(s,3H),3.43(s,3H),6.36-9.18(m,15H) |
| F12 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3,4-二氟苯胺 | |
| F13 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3-三氟甲基-4-氯苯胺 | (B)2.96(s,3H),3.43(s,3H),7.36-9.46(m,13H) |
| F14 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3-二氟甲氧基苯胺 | (A)2.89(s,3H),3.36(s,3H),6.30-8.33(m,15H) |
| (B)2.97(s,3H),3.43(s,3H),6.37-8.30(m,15H)A∶B=1∶1 |
| F15 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3-三氟甲基苯胺 | (A)2.87(s,3H),3.36(s,3H),7.24-8.38(m,14H)(B)2.98(s,3H),3.44(s,3H),7.32-8.37(m,14H)A∶B=1∶1 |
| F16 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3-甲基苯胺 | (B)2.28(s,3H),2.96(s,3H),3.43(s,3H),7.34-9.02(m,14H). |
| F17 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2-甲基苯胺 | (B)2.27(s,3H),2.96(s,3H),3.43(s,3H),7.34-9.08(m,14H). |
| F18 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2,5-二甲基苯胺 | (A)2.35(s,3H),2.36(s,3H),2.87(s,3H),3.35(s,3H),6.87-8.23(m,13H). |
| F19 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2,5-二甲基苯胺 | (B)2.20(s,3H),2.35(s,3H),2.98(s,3H),3.44(s,3H),6.88-8.23(m,13H). |
| F20 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-七氟异丙基苯胺 | (A)2.88(s,3H),3.36(s,3H),7.42-8.41(m,14H). |
| F21 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-七氟异丙基苯胺 | (B)2.98(s,3H),3.44(s,3H),7.32-8.41(m,14H). |
| F22 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3,4-二甲基苯胺 | (A)2.25(s,3H),2.29(s,3H),2.87(s,3H),3.36(s,3H),7.12-8.14(m,13H). |
| F23 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3,4-二甲基苯胺 | (B)2.25(s,3H),2.29(s,3H),2.97(s,3H),3.43(s,3H),7.11-8.14(m,13H). |
| F24 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-苯氧基苯胺 | (A)2.87(s,3H),3.36(s,3H),7.00-8.22(m,19H). |
| F25 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-苯氧基苯胺 | (A)2.87(s,3H),3.36(s,3H),7.00-8.22(m,19H).(B)2.98(s,3H),3.44(s,3H),7.02-8.22(m,19H).A∶B=3∶5 |
| F26 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3-氯苯胺 | (A)2.87(s,3H),3.36(s,3H),7.09-8.28(m,14H). |
| F27 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3-氯苯胺 | (B)2.97(s,3H),3.43(s,3H),7.10-8.28(m,14H). |
| F28 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2,3-二甲基苯胺 | (A)2.30(s,3H),2.35(s,3H),2.87(s,3H),3.35(s,3H),7.02-8.21(m,13H). |
| F29 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2,3-二甲基苯胺 | (B)2.30(s,3H),2.35(s,3H),2.98(s,3H),3.44(s,3H),7.03-8.21(m,13H). |
| F30 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2,3-二氯苯胺 | (A)2.88(s,3H),3.36(s,3H),7.21-9.23(m,13H).(B)2.91(s,3H),3.40(s,3H),7.21-9.23(m,13H).A∶B=1∶1.3 |
| F31 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2-氯苯胺 | (A)2.87(s,3H),3.36(s,3H),7.01-9.12(m,14H). |
| F32 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2-氯苯胺 | (B)2.98(s,3H),3.44(s,3H),7.01-9.12(m,14H). |
| F33 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3.5-二氯苯胺 | (A)2.88(s,3H),3.36(s,3H),7.09-8.31(m,13H). |
| F34 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3.5-二氯苯胺 | (B)2.97(s,3H),3.43(s,3H),7.06-8.30(m,13H). |
| F35 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2,6-二甲基苯胺 | (A)2.27(s,6H),2.86(s,3H),3.34(s,3H),7.09-8.45(m,13H). |
| F36 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2,6-二甲基苯胺 | (B)2.34(s,6H),2.96(s,3H),3.42(s,3H),7.13-7.81(m,12H). |
| F37 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-乙氧基苯胺 | (A)1.40-1.43(t,3H),2.87(s,3H),3.35(s,3H),4.0-4.05(q,2H),6.88-8.09(m,14H) |
| F38 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-乙氧基苯胺 | (B)1.40-1.44(t,3H),2.97(s,3H),3.43(s,3H),4.0-4.06(q,2H),6.89-8.09(m,14H) |
| F39 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2,4-二甲基苯胺 | (A)2.31(s,3H),2.36(s,3H),2.86(s,3H),3.35(s,3H),7.05-8.14(m,13H). |
| F40 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2,4-二甲基苯胺 | (B)2.31(s,3H),2.35(s,3H),2.97(s,3H),3.43(s,3H),7.05-8.14(m,13H). |
| F41 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2-甲基-3-氯苯胺 | (A)2.45(s,3H),2.87(s,3H),3.35(s,3H),7.16-8.31(m,13H). |
| F42 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2-甲基-3-氯苯胺 | (B)2.45(s,3H),2.97(s,3H),3.43(s,3H),7.15-8.30(m,13H). |
| F43 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3-三氟甲氧基苯胺 | (A)2.88(s,3H),3.36(s,3H),6.93-8.34(m,14H) |
| F44 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3-三氟甲氧基苯胺 | (B)2.97(s,3H),3.43(s,3H),6.96-8.32(m,14H) |
| F45 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4三氟甲氧基苯胺 | (A)2.87(s,3H),3.36(s,3H),7.16-8.29(m,14H) |
| F46 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-三氟甲氧基苯胺 | (B)2.97(s,3H),3.43(s,3H),7.20-8.28(m,14H) |
| F47 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3-二氟甲氧基苯胺 | (A)2.89(s,3H),3.36(s,3H),6.30-8.33(m,15H) |
| F48 | 4-Cl | CH3 | CH3 | NH | 直接键 | 厂二氟甲氧基苯胺 | (B)2.97(s,3H),3.43(s,3H),6.378.30(m,15H) |
| F49 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3-三氟甲基苯胺 | (A)2.87(s,3H),3.36(s,3H),7.24-8.38(m,14H) |
| F50 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3-三氟甲基苯胺 | (B)2.98(s,3H),3.44(s,3H),7.32-8.37(m,14H) |
| F51 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-三氟甲基苯胺 | (A)2.88(s,3H),3.36(s,3H),7.39-8.43(m,14H) |
| F52 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4三氟甲基苯胺 | (B)2.97(s,3H),3.44(s,3H),7.32-8.42(m,14H) |
| F53 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2-甲氧基苯胺 | (A)2.87(s,3H),3.36(s,3H),3.99(s,3H),6.93-9.02(m,14H) |
| F54 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2-甲氧基苯胺 | (B)2.97(s,3H),3.43(s,3H),3.98(s,3H),6.93-9.04(m,14H) |
| F55 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2-氟苯胺 | (A)2.87(s,3H),3.36(s,3H),7.00-8.62(m,14H) |
| F56 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2-氟苯胺 | (B)2.97(s,3H),3.43(s,3H),7.04-8.62(m,14H) |
| F57 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2,5-二氯苯胺 | (B)2.98(s,3H),3.44(s,3H),7.33-9.15(m,13H) |
| F58 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-硝基苯胺 | (A)2.88(s,3H),3.36(s,3H),7.32-8.62(m,14H) |
| F59 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-硝基苯胺 | (B)2.98(s,3H),3.44(s,3H),7.32-8.62(m,14H) |
| F60 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3-硝基苯胺 | (A)2.88(s,3H),3.37(s,3H),7.41-8.50(m,14H) |
| F61 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3-硝基苯胺 | (B)2.98(s,3H),3.44(s,3H),7.32-8.38(m,14H) |
| F62 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2-甲基-4-硝基苯胺 | (A)2.51(s,3H),2.88(s,3H),3.36(s,3H),7.33-8.73(m,13H) |
| F63 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2-甲基-4-硝基苯胺 | (B)2.51(s,3H),2.99(s,3H),3.44(s,3H),7.35-8.74(m,13H) |
| F64 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-氯苯胺 | (A)2.87(s,3H),3.36(s,3H),7.31-8.25(m,14H) |
| F65 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-氯苯胺 | (B)2.98(s,3H),3.43(s,3H),7.31-8.25(m,14H) |
| F66 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-叔丁基苯胺 | (A)1.32(s,9H),2.86(s,3H),3.35(s,3H),7.31-8.17(m,14H) |
| F67 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-叔厂基基苯胺 | (B)1.32(s,9H),2.96(s,3H),3.42(s,3H),7.31-8.16(m,14H) |
| F68 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3-氟苯胺 | (A)2.87(s,3H),3.35(s,3H),6.78-8.31(m,14H) |
| F69 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3-氟苯胺 | (B)2.97(s,3H),3.43(s,3H),6.80-8.30(m,14H) |
| F70 | 4-Cl | CH3 | CH3 | 0 | 直接键 | 2-甲基苯胺 | (A)2.35(s,3H),2.89(s,3H),3.38(s,3H),7.09-8.37(m,13H).(B)2.35(s,3H),2.94(s,3H),3.41(s,3H),7.09-8.37(m,13H).A∶B=1∶1 |
| F71 | 4-Cl | CH3 | CH3 | O | 直接键 | 4-叔丁基苯胺 | (A)1.32(s,9H),2.89(s,3H),3.37(s,3H),7.35-8.11(m,13H).(B)1.32(s,9H),2.94(s,3H),3.40(s,3H),7.35-8.11(m,13H).A∶B=1∶1 |
| F72 | 4-Cl | CH3 | CH3 | O | 直接键 | 2,6-二乙基苯胺 | (A)1.23-1.27(t,3H),2.67-2.73(q,2H),2.94(s,3H),3.40(s,3H),7.167.85(m,12H). |
| F73 | 4-Cl | CH3 | CH3 | O | 直接键 | 2,6-二乙基苯胺 | (B)1.23-1.27(t,3H),2.67-2.72(q,2H),2.88(s,3H),3.36(s,3H),7.15-7.76(m,12H). |
| F74 | 4-Cl | CH3 | CH3 | O | 直接键 | 3-三氟甲基苯胺 | 2.95(s,3H),3.41(s,3H),7.38-8.32(m,13H) |
| F75 | 4Cl | CH3 | CH3 | O | 直接键 | 3-三氟甲基-4-氯苯胺 | (A)2.89(s,3H),3.38(s,3H),7.26-8.30(m,12H).(B)2.95(s,3H),3.40(s,3H),7.26-8.30(m,12H).A∶B=1∶1 |
| F76 | 4-Cl | CH3 | CH3 | O | 直接键 | 2-氟苯胺 | (A)2.89(s,3H),3.38(s,3H),7.06-8.57(m,13H).(B)2.95(s,3H),3.41(s,3H),7.06-8.57(m,12H).A∶B=4∶1 |
| F77 | 4-Cl | CH3 | CH3 | O | 直接键 | 3-甲基苯胺 | (B)2.37(s,3H),2.95(s,3H),3.41(s,3H),6.96-8.13(m,13H). |
| F78 | 4-Cl | Bt | CH3 | NH | 直接键 | 2-甲基苯胺 | (A)1.13-1.17(t,3H),2.25(s,3H),2.89 |
| (s,3H),3.72-3.78(q,2H),7.02-8.28(m,13H).(B)1.18-1.22(t,3H),2.40(s,3H),2.99(s,3H),3.78-3.83(q,2H),7.02-8.28(m,13H).A∶B=3∶1 | |||||||
| F79 | 4-Cl | Bt | CH3 | NH | 直接键 | 4-叔丁基苯胺 | (B)1.25-1.28(t,3H),1.30(s,9H),,2.99(s,3H)3.81-3.86(q,2H),7.25-8.12(m,13H). |
| F80 | 4-Cl | Bt | CH3 | NH | 直接键 | 2,6-二乙基苯胺 | (A)1.20-1.23(t,6H),1.23-1.27(t,3H),2.94(s,3H),2.60-2.64(q,4H),3.82-3.86(q,2H),7,11-8.43(m,14H). |
| F81 | 4-Cl | Bt | CH3 | NH | 直接键 | 3-三氟甲基苯胺 | (A)1.16-1.19(t,3H),2.91(s,3H),3.75-3.81(q,2H),7.25-8.38(m,14H).(B)1.25-1.29(t,3H),3.00(s,3H),3.81-3.86(q,2H),7.25-8.38(m,14H).A∶B=2∶1 |
| F82 | 4-Cl | Bt | CH3 | NH | 直接键 | 3-甲基苯胺 | (A)1.15-1.19(t,3H),2.34(s,3H),2.90(s,3H),3.75-3.80(q,2H),6.94-8.21(m,14H).(B)1.18-1.22(t,3H),2.37(s,3H),2.94(s,3H),3.80-3.85(q,2H),6.94-8.21(m,14H).A∶B=4∶1 |
| F83 | 4-Cl | H | CH3 | NH | 直接键 | 2-甲基苯胺 | (A)2.40(s,3H),3.16(s,3H),6.94-8.27(m,14H). |
| F84 | 4Cl | H | CH3 | NH | 直接键 | 2,6-二乙基苯胺 | (A)1.231.27(t,3H),3.04(s,3H),2.68-2.74(q,2H),7.14-7.93(m,14H).(B)1.24-1.27(t,3H),3.14(s,3H),2.69-2.74(q,2H),7.14-7.93(m,14H).A∶B=1∶1 |
| F85 | 4-Cl | H | CH3 | NH | 直接键 | 3-三氟甲基苯胺 | (A)3.18(s,3H),6.79-8.38(m,15H). |
| F86 | 4-Cl | H | CH3 | NH | 直接键 | 3-三氟甲基-4-氯苯胺 | (A)3.17(s,3H),7.15-8.39(m,14H). |
| F87 | 4-Cl | H | CH3 | NH | 直接键 | 2-氟苯胺 | (A)3.18(s,3H),7.01-8.63(m,15H). |
| F88 | 4-Cl | H | CH3 | NH | 直接键 | 2-甲基苯胺 | (A)2.38(s,3H),3.18(s,3H),6.95-8.22(m,14H). |
| F89 | 4-Cl | CH3 | CH3 | O | 直接键 | 2.4-二氟苯胺 | (A)2.89(s,3H),3.38(s,3H),6.89-8.46(m,12H).(B)2.94(s,3H),3.41(s,3H),6.89-8.46(m,12H).A∶B=1∶2 |
| F90 | 4-Cl | CH3 | CH3 | O | 直接键 | 3.4-二氯苯胺 | (A)2.89(s,3H),3.37(s,3H),7.26-8.25(m,12H).(B)2.94(s,3H),3.40(s,3H),7.26-8.25(m,12H).A∶B=1∶1 |
| F91 | 4-Cl | CH3 | CH3 | O | 直接键 | 2.3-二氯苯胺 | (A)2.90(s,3H),3.38(s,3H),7.24-9.28(m,12H).(B)2.95(s,3H),3.41(s,3H),7.24-9.28(m,12H).A∶B=1∶2 |
| F92 | 4-Cl | CH3 | CH3 | O | 直接键 | 2-甲氧基苯胺 | (A)2.89(s,3H),3.38(s,3H),3.93(s,3H),6.91-8.95(m,13H).(B)2.94(s,3H),3.41(s,3H),6.91-8.95(m,13H).A∶B=1∶1 |
| F93 | 4-Cl | CH3 | CH3 | O | 直接键 | 2,5-二甲基苯胺 | (B)2.29(s,3H),2.35(s,3H),2.94(s,3H),3.41(s,3H),6.91-8.32(m,12H). |
| F94 | 4-Cl | CH3 | CH3 | O | 直接键 | 2-三氟甲氧基苯胺 | (A)2.89(s,3H),3.38(s,3H),7.12-9.10(m,13H). |
| F95 | 4-Cl | CH3 | CH3 | O | 直接键 | 2,3,4-三氟苯胺 | (A)2.90(s,3H),3.38(s,3H),6.97-8.40(m,11H). |
| F96 | 4-Cl | CH3 | CH3 | O | 直接键 | 4-三氟甲基苯胺 | (A)2.89(s,3H),3.38(s,3H),7.27-8.20(m,13H). |
| F97 | 4-Cl | CH3 | CH3 | O | 直接键 | 3-三氟甲基苯胺 | (B)2.95(s,3H),3.41(s,3H),7.27-8.31(m,13H). |
| F98 | 4-Cl | CH3 | CH3 | O | 直接键 | 3,5-二氟苯胺 | (B)2.95(s,3H),3.41(s,3H),6.57-8.26(m,12H). |
| F99 | 4-Cl | CH3 | CH3 | O | 直接键 | 3,5-二甲基苯胺 | (B)2.32(s,6H),2.94(s,3H),3.41(s,3H),6.79-8.10(m,12H). |
| F100 | 4-Cl | CH3 | CH3 | O | 直接键 | 3,4-二氟苯胺 | (A)2.89(s,3H),3.38(s,3H),7.12-8.20(m,12H).(B)2.95(s,3H),3.41(s,3H),7.12-8.20(m,12H).A∶B=1∶1 |
| F101 | 4-Cl | CH3 | CH3 | O | 直接键 | 3-甲氧基苯胺 | (A)2.90(s,3H),3.38(s,3H),3.83(s,3H),6.698.13(m,13H).(B)2.95(s,3H),3.41(s,3H),3.83(s,3H),6.69-8.13(m,13H).A∶B=1∶1 |
| F102 | 4-Cl | CH3 | CH3 | O | 直接键 | 苯胺 | (A)2.89(s,3H),3.38(s,3H),7.13-8.17(m,14H).(B)2.94(s,3H),3.41(s,3H),7.13-8.17(m,14H).A∶B=1∶1 |
| F103 | 4-Cl | CH3 | CH3 | O | 直接键 | 4-甲基苯胺 | (A)2.89(s,3H),3.37(s,3H),2.34(s,3H),7.15-8.13(m,13H).(B)2.94(s,3H),3.40(s,3H),2.34(s,3H),7.15-8.13(m,13H).A∶B=1∶1 |
| F104 | 4-Cl | CH3 | CH3 | O | 直接键 | 4-三氟甲氧基苯胺 | (B)2.95(s,3H),3.41(s,3H),7.22-8.22(m,13H). |
| F105 | 4-Cl | CH3 | CH3 | O | 直接键 | 3-三氟甲氧基苯胺 | (B)2.95(s,3H),3.41(s,3H),7.00-8.23(m,13H). |
| F106 | 4-Cl | CH3 | CH3 | O | 直接键 | 2,4-二甲基苯胺 | (A)2.89(s,3H),3.37(s,3H),2.30(s,3H),2.31(s,3H),7.00-8.12(m,12H). |
| F107 | 4-Cl | CH3 | CH3 | O | 直接键 | 2-甲基-3-氯苯胺 | (A)2.88(s,3H),3.36(s,3H),2.42(s,3H),7.20-8.40(m,12H). |
| F108 | 4-Cl | CH3 | CH3 | O | 直接键 | 3-二氟甲氧基苯胺 | (B)2.95(s,3H),3.41(s,3H),6.36-8.21(m,14H). |
| F109 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-甲基苯胺 | (A)2.34(s,3H),2.87(s,3H),3.34(s,3H),7.16-8.17(m,14H). |
| F110 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3,5-二氟苯 | (A)2.87(s,3H),3.34(s,3H),7.01-8.35 |
| 胺 | (m,13H). | ||||||
| F111 | 4-Cl | CH3 | CH3 | NH | 直接键 | 2,3,4-三氟苯胺 | (A)2.88(s,3H),3.36(s,3H),6.95-8.51(m,12H). |
| F112 | 4-Cl | CH3 | CH3 | NH | 直接键 | 3-甲氧基苯胺 | (A)2.84(s,3H),3.36(s,3H),3.84(s,3H),6.66-8.25(m,13H).(B)2.98(s,3H),3.44(s,3H),3.84(s,3H),6.66-8.25(m,13H).A∶B=1∶1 |
| F113 | 4-Cl | Bt | CH3 | NH | 直接键 | 3-三氟甲基-4-氯苯胺 | (A)1.18-1.22(t,3H),3.77-3.82(q,2H),2.91(s,3H),7.27-8.37(m,14H).(B)1.26-1.30(t,3H),3.81-3.86(q,2H),3.00(s,3H),7.27-8.37(m,14H).A∶B=2∶1 |
| F114 | 4-Cl | Bt | CH3 | NH | 直接键 | 2-氟苯胺 | (A)1.14-1.18(t,3H),3.73-3.78(q,2H),2.90(s,3H),7.06-8.63(m,14H).(B)1.26-1.30(t,3H),3.83-3.86(q,2H),3.00(s,3H),7.06-8.63(m,14H).A∶B=4∶1 |
| F115 | 4-Cl | Bt | CH3 | NH | 直接键 | 2,4-二氟苯胺 | (A)1.15-1.19(t,3H),3.75-3.80(q,2H),2.90(s,3H),6.88-8.48(m,13H).(B)1.26-1.30(t,3H),3.82-3.88(q,2H),3.00(s,3H),7.06-8.63(m,13H).A∶B=1∶2 |
| F116 | 4Cl | Bt | CH3 | NH | 直接键 | 4-氯苯胺 | (A)1.16-1.20(t,3H),3.76-3.81(q,2H),2.92(s,3H),7.25-8.28(m,14H).(B)1.26-1.30(t,3H),3.81-3.86(q,2H),3.00(s,3H),7.25-8.28(m,14H).A∶B=2∶1 |
| F117 | 4Cl | Bt | CH3 | NH | 直接键 | 2,3-二氯苯胺 | (A)1.15-1.18(t,3H),3.75-3.81(q,2H),2.90(s,3H),7.16-8.11(m,13H).(B)1.22-1.28(t,3H),3.773.85(q,2H),3.00(s,3H),7.16-8.11(m,13H).A∶B=2∶1 |
| F118 | 4-Cl | Bt | CH3 | NH | 直接键 | 2-甲氧基苯胺 | (A)1.17-1.20(t,3H),3.77-3.82(q,2H),2.91(s,3H),6.94-9.06(m,14H).(B)1.26-1.30(t,3H),3.82-3.87(q,2H),3.00(s,3H),6.94-9.06(m,14H).A∶B=1∶2 |
| F119 | 4-Cl | Bt | CH3 | NH | 直接键 | 2,5-二甲基苯胺 | (A)1.16-1.19(t,3H),3.75-3.80(q,2H),2.33(s,6H),2.91(s,3H),6.88-8.23(m,13H).(B)1.27-1.30(t,3H),3.82-3.87(q,2H),3.00(s,3H),2.36(s,3H),6.88-8.23(m,13H).A∶B=2∶1 |
| F120 | 4-Cl | Bt | CH3 | NH | 直接键 | 2-三氟甲氧基苯胺 | (A)1.14-1.18(t,3H),3.74-3.78(q,2H),2.90(s,3H),7.07-8.95(m,14H).(B)1.22-1.27(t,3H),3.82-3.86(q,2H),3.00(s,3H),7.07-8.95(m,14H).A∶B=2∶1 |
| F121 | 4-Cl | Bt | CH3 | NH | 直接键 | 2,3,4-三氟 | (A)1.14-1.18(t,3H),3.76-3.80 |
| 苯胺 | (q,2H),2.90(s,3H),6.93-8.51(m,12H).(B)1.24-1.29(t,3H),3.82-3.86(q,2H),3.00(s,3H),7.07-8.95(m,12H).A∶B=1∶1 |
| F122 | 4-Cl | Bt | CH3 | NH | 直接键 | 4-三氟甲基苯胺 | (A)1.16-1.20(t,3H),3.76-3.80(q,2H),2.91(s,3H),7.25-8.43(m,14H).(B)1.26-1.30(t,3H),3.81-3.86(q,2H),3.00(s,3H),7.25-8.43(m,14H).A∶B=3∶1 |
| F123 | 4-Cl | Bt | CH3 | NH | 直接键 | 3-三氟甲基苯胺 | (A)1.17-1.20(t,3H),3.76-3.81(q,2H),2.92(s,3H),7.26-8.39(m,14H).(B)1.26-1.30(t,3H),3.84-3.87(q,2H),3.01(s,3H),7.26-8.39(m,14H).A∶B=3∶1 |
| F124 | 4-Cl | CH3 | CF3 | NH | 直接键 | 3-三氟甲氧基苯胺 | (A)3.51(s,3H),6.98-8.33(m,14H).(B)3.59(s,3H),6.98-8.33(m,14H).A∶B=1∶1 |
| F125 | 4-Cl | CH3 | CF3 | NH | 直接键 | 2-甲基-3-氯苯胺 | (A)2.47(s,3H),3.50(s,3H),7.16-8.30(m,13H).(B)2.47(s,3H),3.59(s,3H),7.16-8.30(m,13H).A∶B=1∶1 |
| F126 | 4Cl | CH3 | CF3 | NH | 直接键 | 2,3-二氯苯胺 | (B)3.59(s,3H),7.219.23(m,13H). |
| F127 | 4-Cl | CH3 | CF3 | NH | 直接键 | 3-二氟甲氧基苯胺 | (A)3.51(s,3H),6.38-8.31(m,15H).(B)3.59(s,3H),6.38-8.31(m,15H).A∶B=1∶1 |
| F128 | 4F | CH3 | CH3 | NH | 直接键 | 苯胺 | (A)2.88(s,3H),3.36(s,3H),7.128.27(m,15H). |
| F129 | 4-F | CH3 | CH3 | NH | 直接键 | 苯胺 | (B)2.98(s,3H),3.44(s,3H),7.07-8.27(m,15H). |
| F130 | 4-F | CH3 | CH3 | NH | 直接键 | 2-甲基-3-氯苯胺 | (A)2.47(s,3H),2.88(s,3H),3.36(s,3H),7.18-8.33(m,13H). |
| F131 | 4-F | CH3 | CH3 | NH | 直接键 | 2-甲基-3-氯苯胺 | (B)2.47(s,3H),2.98(s,3H),3.44(s,3H),7.06-8.33(m,13H). |
| F132 | 4-F | CH3 | CH3 | NH | 直接键 | 2,5-二甲基苯胺 | (A)2.35(s,3H),2.36(s,3H),2.86(s,3H),3.35(s,3H),6.87-8.24(m,13H). |
| F133 | 4-F | CH3 | CH3 | NH | 直接键 | 2,5-二甲基苯胺 | (B)2.33(s,3H),3.35(s,3H),2.97(s,3H),3.43(s,3H),6.87-8.23(m,13H). |
| F134 | 4-F | CH3 | CH3 | NH | 直接键 | 2,3-二氯苯胺 | (B)3.44(s,3H),2.98(s,3H),7.07-8.32(m,13H). |
| F135 | 4-F | CH3 | CH3 | NH | 直接键 | 2,6-二乙基苯胺 | (A)1.24-1.28(t,3H),2.69-2.75(q,2H),2.86(s,3H),3.35(s,3H),7.17-7.92(m,13H). |
| F136 | 4-F | CH3 | CH3 | NH | 直接键 | 2,6-二乙基苯胺 | (B)1.24-1.28(t,3H),2.69-2.75(q,2H),2.96(s,3H),3.43(s,3H),7.04-7.88(m,13H). |
| F137 | 4-F | CH3 | CH3 | NH | 直接键 | 2,4-二甲基苯胺 | (A)2.31(s,3H),2.36(s,3H),2.86(s,3H),3.35(s,3H),7.05-8.15(m,13H). |
| F138 | 4-F | CH3 | CH3 | NH | 直接键 | 2,4-二甲基苯胺 | (B)2.31(s,3H),2.36(s,3H),2.97(s,3H),3.43(s,3H),7.05-8.15(m,13H). |
| F139 | 4-F | CH3 | CH3 | NH | 直接键 | 3-三氟甲氧基苯胺 | (A)2.87(s,3H),3.36(s,3H),6.96-8.34(m,14H). |
| F140 | 4-F | CH3 | CH3 | NH | 直接键 | 3-三氟甲氧基苯胺 | (B)2.97(s,3H),3.43(s,3H),6.96-8.34(m,14H). |
| F141 | 4-F | CH3 | CH3 | NH | 直接键 | 3-二氟甲氧基苯胺 | (A)2.87(s,3H),3.36(s,3H),6.37-8.32(m,15H).(B)2.97(s,3H),3.43(s,3H),6.37-8.32(m,15H)A∶B=1∶1 |
| F142 | 4-F | CH3 | CH3 | NH | 直接键 | 3,5-二氟苯胺 | (A)2.88(s,3H),3.36(s,3H),7.16-8.36(m,13H).(B)2.98(s,3H),3.44(s,3H),7.16-8.36(m,13H)A∶B=2∶1 |
| F143 | 4-F | CH3 | CH3 | NH | 直接键 | 3-甲氧基苯胺 | (A)2.87(s,3H),3.36(s,3H),3.84(s,3H),6.66-8.26(m,14H).(B)2.97(s,3H),3.43(s,3H),3.84(s,3H),6.66-8.26(m,14H)A∶B=1∶1 |
| F144 | 4-F | CH3 | CH3 | NH | 直接键 | 4甲基苯胺 | (A)2.34(s,3H),2.88(s,3H),3.36(s,3H),7.16-8.20(m,14H). |
| F145 | 4-F | CH3 | CH3 | NH | 直接键 | 4-甲基苯胺 | (B)2.34(s,3H),2.97(s,3H),3.43(s,3H),7.09-8.17(m,14H). |
| F146 | 4F | CH3 | CH3 | NH | 直接键 | 3,5-二甲基苯胺 | (A)2.33(s,6H),2.87(s,3H),3.36(s,3H),6.76-8.17(m,13H). |
| F147 | 4F | CH3 | CH3 | NH | 直接键 | 3,5-二甲基苯胺 | (B)2.34(s,3H),2.97(s,3H),3.43(s,3H),6.76-8.17(m,13H). |
| F148 | 4-F | CH3 | CH3 | NH | 直接键 | 3,4二氟苯胺 | (A)2.87(s,3H),3.36(s,3H),7.11-8.24(m,13H). |
| F149 | 4F | CH3 | CH3 | NH | 直接键 | 3,4-二氟苯胺 | (B)2.97(s,3H),3.43(s,3H),7.10-8.24(m,13H). |
| F150 | 4-Cl | CH3 | CF3 | NH | 直接键 | 3,5-二氟苯胺 | (A)3.50(s,3H),6.56-8.32(m,13H). |
| F151 | 4-Cl | CH3 | CF3 | NH | 直接键 | 3-甲氧基苯胺 | (A)3.50(s,3H),3.84(s,3H),6.67-8.22(m,14H). |
| F152 | 4-Cl | CH3 | CF3 | NH | 直接键 | 3,5-二甲基苯胺 | (A)3.50(s,3H),2.34(s,6H),6.77-8.14(m,13H). |
| F153 | 4-Cl | CH3 | CF3 | NH | 直接键 | 3,4-二氟苯胺 | (A)3.50(s,3H),7.14-8.22(m,13H).(B)3.58(s,3H),7.14-8.22(m,13H).A∶B=1∶3 |
| F154 | 4F | CH3 | CH3 | NH | 直接键 | 3-三氟甲基苯胺 | (A)2.88(s,3H),3.36(s,3H),7.26-8.39(m,14H). |
| F155 | 4-F | CH3 | CH3 | NH | 直接键 | 3-三氟甲基苯胺 | (B)2.98(s,3H),3.43(s,3H),6.98-8.39(m,14H). |
| F156 | 4-F | CH3 | CH3 | NH | 直接键 | 3-氟苯胺 | (A)2.87(s,3H),3.35(s,3H),6.78-8.32(m,14H). |
| F157 | 4-F | CH3 | CH3 | NH | 直接键 | 3氟苯胺 | (B)2.97(s,3H),3.43(s,3H),6.78-8.32(m,14H). |
| F158 | 4-F | CH3 | CH3 | NH | 直接键 | 2,4-二氟苯胺 | (B)2.96(s,3H),3.42(s,3H),6.86-8.82(m,13H). |
| F159 | 4-F | CH3 | CH3 | NH | 直接键 | 2,3,4-三氟苯胺 | (B)2.96(s,3H),3.42(s,3H),6.93-8.50(m,12H). |
| F160 | 4-F | CH3 | CH3 | NH | CH2 | 4-三氟甲基苯胺 | |
| F161 | 4-Cl | CH3 | CH3 | NH | CH2 | 4-三氟甲氧基苯胺 | |
| F162 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-羟基苯胺 | |
| F163 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-氰基苯胺 | |
| F164 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-溴苯胺 | |
| F165 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-碘苯胺 | |
| F166 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-甲酸基苯胺 | |
| F167 | 4Cl | CH3 | CH3 | NH | 直接键 | 4-甲酸甲酯基苯胺 |
| F168 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-甲酰胺基苯胺 | |
| F169 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-(N,N-二甲基)甲酰胺基苯胺 | |
| F170 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-(N-苯基)甲酰胺基苯胺 | |
| F171 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-三氟甲基苯胺 | (A)2.89(s,3H),3.36(s,3H),7.22-8.44(m,14H). |
| F172 | 4-Cl | CH3 | CH3 | NH | 直接键 | 4-三氟甲基苯胺 | (B)2.98(s,3H),3.44(s,3H),7.26-8.43(m,14H). |
| F173 | 4-Cl | 环丙基 | CH3 | NH | 直接键 | 4-三氟甲基苯胺 |
本发明所述的二苯酮腙衍生物,可以用于农用化学杀虫剂。
本发明所述的二苯酮腙衍生物可以作为农药化学杀虫剂的活性组份,配制成各种液剂、乳油、悬浮剂、水悬剂、微乳剂、(水)乳剂、粉剂、可湿性粉剂、可溶性粉剂、(水分散性)颗粒剂或胶囊剂等,用于水稻、大豆、小麦、棉花、玉米、蔬菜和油菜等农作物的害虫防治。
当配置成农药化学杀虫剂时,活性组分的重量百分含量即本发明所述的二苯酮腙衍生物的重量百分含量优选为5~90%,其余为可接受的载体,载体至少包括两种,其中至少-种是表面活性剂。
载体可以是固体或液体。合适的固体载体包括天然的或合成的粘土和硅酸盐,例如天然硅石和硅藻土;硅酸镁例如滑石;硅酸铝镁例如高岭石、高岭土、蒙脱土和云母;白碳黑、碳酸钙、轻质碳酸钙;硫酸钙;石灰石;硫酸钠;胺盐如硫酸铵、六甲撑二胺。液体载体包括水和有机溶剂,当用水做溶剂或稀释剂时,有机溶剂也能用做辅助剂或防冻添加剂。合适的有机溶剂包括芳烃例如苯、二甲苯、甲苯等;氯代烃,例如氯代苯、氯乙烯、三氯甲烷、二氯甲烷等;脂肪烃,例如石油馏分、环己烷、轻质矿物油;醇类,例如异丙醇、丁醇、乙二醇、丙三醇和环己醇等;以及它们的醚和酯;还有酮类,例如丙酮、环己酮以及二甲基甲酰胺和N-甲基-吡咯烷酮。
表面活性剂可以是乳化剂、分散剂或湿润剂;可以是离子型的或非离子型的。非离子型乳化剂例如聚氧乙烯脂肪酸脂、聚氧乙烯脂肪醇醚、聚氧乙烯脂肪氨,以及市售的乳化剂:农乳2201B、农乳0203B、农乳100#、农乳500#、农乳600#、农乳600-2#、农乳1601、农乳2201、农乳NP-10、农乳NP-15、农乳507#、农乳OX-635、农乳OX-622、农乳OX-653、农乳OX-667、宁乳36#。分散剂包括木质素磺酸钠、拉开粉、木质素磺酸钙、甲基萘磺酸甲醛缩合物等。湿润剂为:月桂醇硫酸钠、十二烷基苯磺酸钠、烷基萘磺酸钠等。
上述农药化学杀虫剂可由通用的方法制备。例如,将活性物质与液体溶剂和/或固体载体混合,同时加入表面活性剂如乳化剂、分散剂、稳定剂、湿润剂,还可以加入其它助剂如:粘合剂、消泡剂、氧化剂等。
本发明所述的二苯酮腙衍生物还可以与除草剂、杀菌剂、杀线虫剂、植物生长调节剂、肥料,以及其它杀虫剂或其他农用化学品混配使用。
本发明所述的二苯酮腙衍生物及由其配置的农药化学杀虫剂,具有如下优点:
(1)具有较好的杀好的选择性,对部分作物如小麦、大豆、棉花、水稻等安全性好;
(3)具有合理的毒性、生态毒性和环境相容性,属低毒环境友好型农药。
具体实施方式
下文就部分实例给出详细的反应条件、纯化方法、物理常数和结构确认所需的分析数据,需要指出的是本发明并不仅仅局限在下述实施例的范围内。
下述实施例以本发明的化合物作为活性物质组份,加工配制几种除草剂剂型的实施例中,所有的“%”均指重量百分比,“gai/ha”均指每克活性物/公顷。
实施例1 中间体A的合成
在烧瓶加入25克氯苯、41.2克对硝基苯甲酰氯和200ml二氯乙烷,机械搅拌升温到50摄氏度,2小时内分批加入34克无水三氯化铝,再回流1小时,冷却后倒入冰水中,分层,萃取,水层用100毫升二氯乙烷分2次萃取,合并有机层,30毫升饱和食盐水洗涤一次,硫酸钠干燥后过滤,减压脱溶后甲醇结晶得到中间体4-氯-4‘-硝基二苯甲酮,产率85%。
用同样的方法,使用不同的原料——氟苯可以合成中间体4-氟-4‘-硝基二苯甲酮。产率80%。
在烧瓶中加入2.45克镁粉和100毫升无水乙醚,滴入22.5克间三氟甲基溴苯,控制滴入速度保持体系微沸,滴完后回流1小时得到间三氟甲基溴苯的格氏试剂,加入18.55克对硝基苯甲酰氯,搅拌8小时,加入200毫升1N稀盐酸,分层,萃取,水层用100毫升乙醚分2次萃取,合并有机层,30毫升饱和食盐水洗涤一次,硫酸钠干燥后过滤,减压脱溶后甲醇结晶得到中间体3-三氟甲基-4‘-硝基二苯甲酮,产率45%。
实施例2 中间体B的制备
烧瓶中加入350毫升水,145克Na2S,9H2O,和17.3可硫粉,加热搅拌至硫粉完全溶解得Na2S2水溶液备用。
烧瓶中加入75克中间体4-氯-4‘-硝基二苯甲酮和100毫升Na2S2水溶液,搅拌下升温到回流,将其余的Na2S2水溶液在2小时内滴入,继续搅拌3小时,溶液颜色由红变黄,冷却,有大量固体出现,过滤水洗,晾干得中间体4-氯-4‘-胺基二苯甲酮,产率90%。
用同样的方法,使用不同的原料——4-氟-4‘-硝基二苯甲酮和3-三氟甲基-4‘-硝基二苯甲酮可以合成中间体4-氟-4‘-氨基二苯甲酮和3-三氟甲基-4‘-胺基二苯甲酮。
实施例3 中间体C的制备
烧瓶中加入9.7克中间体4-氯-4‘-胺基二苯甲酮50毫升吡啶,冰浴下滴入4.8克甲磺酰氯,半小时滴完后继续搅拌1小时,倒入冰的2N稀盐酸中,过滤水洗,晾干得中间体4-氯-4‘-甲磺酰胺基二苯甲酮,产率92%。
用同样的方法,使用不同的原料——4-氟-4‘-氨基二苯甲酮和3-三氟甲基-4‘-胺基二苯甲酮可以合成中间体4-氟-4‘-甲磺酰胺基二苯甲酮和3-三氟甲基-4‘-甲磺酰胺基二苯甲酮。
烧瓶中加入12.65克中间体4-氯-4‘-氨基二苯甲酮、50毫升三氯甲烷和5.52克三乙胺,冰浴下滴入15.41三氟甲磺酸酐,自然升至室温,加入10%过量的氢氧化钠水溶液搅拌,分层萃取,碱水层分离,20毫升氯仿洗涤,盐酸酸化,析出固体,过滤水洗,晾干得中间体3-三氟甲基-4‘-甲磺酰胺基二苯甲酮,产率50%。
实施例4 中间体D的制备
烧瓶中加入10克4-氟-4‘-甲磺酰胺基二苯甲酮、4.73克硫酸二甲酯、11克无水碳酸钾和100毫升丙酮,回流8小时后冷却,过滤,脱溶后甲醇结晶得中间体4-氟-4‘-(N-甲基)甲磺酰胺基二苯甲酮,产率70%。
用同样的方法,使用不同的原料——4-氯-4‘-甲磺酰胺基二苯甲酮、3-三氟甲基-4‘-甲磺酰胺基二苯甲酮、硫酸二乙酯,可以合成各种不同的中间体(如结构式(D)所示),(R1、R2、R3代表结构式(D)中的其它取代基)。
实施例5 中间体E的制备
烧瓶中加入4克4-氯-4‘-(N-甲基)三氟甲磺酰胺基二苯甲酮、6.5克85%水合肼和50毫升乙醇,室温搅拌1小时,回流6小时,冷却,冰冻后过滤,得中间体E(R1=氯、R2=甲基、R3=三氟甲基、Y=NH)。
烧瓶中加入2.39克盐酸羟胺、2.79克醋酸钠和60毫升乙醇,分批加入7.35克4-氯-4‘-(N-甲基)甲磺酰胺基二苯甲酮,室温搅拌1小时,回流4小时,冷却过滤,水洗得中间体E(R1=氯、R2=甲基、R3=甲基、Y=0)。
用同样的方法,使用不同的原料和盐酸羟胺,可以合成各种不同的中间体(如结构式(E)所示),(R1、R2、R3、Y代表结构式(E)中的其它取代基)。
实施例6 中间体G的制备
烧瓶中加入中间体D,等当量肼基甲酸甲酯和溶剂乙醇,回流8小时,冷却,过滤的中间体G,(R1、R2、R3、代表结构式(G)中的其它取代基)。
实施例7 目标化合物F的合成
烧瓶中加入中间体E、等当量的R4R5R6取代的苯基异氰酸酯和无水四氢呋喃,室温搅拌12小时,减压蒸去溶剂,硅胶层析或者甲醇结晶即得目标化合物。
烧瓶中加入中间体G、等当量的R4R5R6取代的苯胺和甲苯,回流搅拌12小时,减压蒸去溶剂,硅胶层析或者甲醇结晶即得目标化合物。
以下实施例8至实施例10给出以本发明的化合物作为活性物质组份,加工配制几种除草剂剂型的实际例子,需要指出的是本发明并不仅仅局限在下述实例的范围内。在这些配方例子中,所有的“%”均指重量百分比,“gai/ha”均指每克活性物/公顷。
实施例8 可湿性粉剂配方
将15%的表1所述化合物F1、5%的木质素磺酸盐(Mq)、1%的月桂醇聚氧乙烯醚(JFC)、40%的硅藻土和44%的轻质碳酸钙均匀地混合,粉碎,即得可湿性粉剂。
实施例9 乳油配方
将10%的表1所述化合物F1、5%的农乳500号(钙盐)、5%的农乳602号、5%的N-甲基-2-吡咯烷酮和75%的二甲苯加热搅拌均匀,即得乳油。
实施例10 粒剂配方
将5%的表1所述化合物F1、1%的聚乙烯醇(PVA)、4%的萘磺酸钠甲醛缩合物(NMO)和90%粘土均匀地混合,粉碎,然后向此100份混合物加入20份水,捏合,用挤压成粒机,制成14-32目的颗粒,干燥,即得颗粒剂。
实施例11 生物活性测定:
以下实施给出下面给出使用本发明的化合物进行生物活性测定的实例,需要指出的是本发明并不仅仅局限在下述实例的范围内。
杀虫活性评价试验根据下列方法进行:
称取一定质量的制剂,加蒸馏水稀释配制成测定所需浓度的药液。筛选浓度为500、100、20mg/L,药剂处理药液量10mL。试验靶标为东方粘虫(Mythima sBparata)、稻褐飞虱(Nilaparvata lugBns)、朱砂叶螨(TBtranychus cinnabarnus)、苜蓿蚜(Aphis mBdicagini)、小菜蛾(PlutBlla xylostBlla)、斜纹夜蛾(ProdBnia litura)等。
(1)水稻褐飞虱筛选——喷雾法
将水稻苗用白石英沙固定于培养皿内,接用CO2麻醉3龄中期若虫,置于POTTBR喷雾塔下喷雾。喷雾后用透明塑料杯罩住,标记后放于观察室内。72h后检查结果。
(2)东方粘虫筛选——浸苗饲喂法
将玉米叶在药液中充分浸润后自然阴干,放入培养皿中,接3龄中期幼虫,加盖标记后置于观察室内。72h后检查结果。
(3)小菜蛾筛选——浸渍法
将甘蓝片剪下,打孔成圆片,然后浸于药液中20s,放于Φ9cm塑料培养皿内(5片/皿),接小菜蛾2龄幼虫15头/皿,放一张滤纸,加盖。置于26℃室内培养,72h后检查结果。试验重复4次。以尖头镊子轻触虫体,无反应视为死虫
(4)朱砂叶螨筛选——浸渍法
将蚕豆叶片打成叶碟,背面朝上放在小块棉花上,置于塑料培养皿内,加少量水,接朱砂叶螨成螨。待成螨于叶片上稳定后,将叶片在药液中充分浸润5s后迅速用吸水纸吸去叶片表面水滴,重新置于棉花上,风干。72h后检查结果。
(5)苜蓿蚜筛选——浸渍法
将蚕豆叶片剪去两端,背面朝上放在小块棉花上,置于塑料培养皿内,加少量水,接苜蓿蚜成蚜以产若蚜。24h后去除成蚜,继续培养2d后将叶片在药液中充分浸润5s后,重新置于棉花上,自然凉干。24h后检查结果。
(6)斜纹夜蛾筛选——浸渍法
将甘蓝片剪下,用打孔器打取甘蓝圆叶,于药液中浸20s,晾干。放入带有圆孔(Φ2cm)的六孔塑料盒内,每孔3片。接入斜纹夜蛾3龄中期幼虫1头/孔,放入观察室内。72h后检查结果。试验重复4次。以尖头镊子轻触虫体,无反应视为死虫。
试验统计:统计各个处理的死虫数和活虫数,计算死亡率。
CK对照死亡率<20%,试验结果可信,试验结果进行校正,CK对照死亡率<5%时可不校正。
生测试验结果表明:本发明化合物具有良好的杀虫活性,特别是对鳞翅目害虫,如东方粘虫、小菜蛾、斜纹夜蛾等具有广谱的杀虫活性,“mg/L”均指每毫克活性物/升。
500mg/L浓度下,F1-F30,F33,F34,F37-F88,F110,F111,F115,F116,F120,F122,F123对东方粘虫的死亡率大于80%。
100mg/L浓度下,F19,F171,F172,F21,F26-F30,F33,F34,F36,F38-F47,对斜纹夜蛾的死亡率大于80%。
100mg/L浓度下,F55-F69,F74,F81,F85,F86,F88,F111,F115,F116,F122,F123对小菜蛾的死亡率大于80%。
Claims (11)
2.按照权利要求1所述的一种二苯酮腙衍生物,其特征在于所述R1为氯、氟或三氟甲基,R1位于苯环的3位或4位。
3.按照权利要求1所述的一种二苯酮腙衍生物,其特征在于所述R2为甲基,所述R3为甲基或三氟甲基。
4.按照权利要求1所述的一种二苯酮腙衍生物,其特征在于所述X为胺基,所述Y为直接键。
5.按照权利要求1所述的一种二苯酮腙衍生物,其特征在于所述R4、R5、R6独立地选自氢、卤素、硝基、甲基、三氟甲基、甲氧基、三氟甲氧基或甲酸酯。
6.按照权利要求1所述的一种二苯酮腙衍生物,其特征在于所述N-X为顺式异构体和/或反式异构体。
7.一种制备按照权利要求1所述的二苯酮腙衍生物的方法,其特征在于包括以下步骤:
4(或3或2)-R1-4’-R3SO2N(R2)Y-二苯甲酮与1-10当量的水合肼或盐酸羟胺,在醇类溶剂中,在0℃到溶剂回流温度下,反应得到4(或3或2)-R1-4’-R3SO2N(R2)Y-二苯甲酮的水合肼脱水缩合物中间体;
当使用盐酸羟胺时,需要加入缚酸剂;
4(或3或2)-R1-4’-R3SO2N(R2)Y-二苯甲酮的水合肼脱水缩合物中间体与结构式[I]所表示的R4R5R6取代的苯基异氰酸酯在醚类溶剂中,在0℃到溶剂回流温度下,反应得到按照权利要求1所述的二苯酮腙衍生物;
其中:
R1为卤素、C1-C4烷基、C1-C4烷氧基、C1-C4卤代烷基或C1-C4卤代烷氧基;
R2为氢、C1-C4烷基或环丙基;
R3为C1-C4烷基或C1-C4卤代烷基;
R4、R5、R6独立地选自氢、卤素、硝基、腈基、羟基、C1-C4烷基、C1-C4卤代烷基、C1-C4烷氧基、(取代)苯氧基、C1-C4卤代烷氧基、甲酸及其碱金属盐、甲酸C1~6酯基、甲酰胺或N-C1-4烷基或苯基取代甲酰胺;
X为胺基或氧;
Y为亚甲基或直接键。
8.按照权利要求7所述的制备按照权利要求1所述的二苯酮腙衍生物的方法,其特征在于所述缚酸剂为醋酸钠、三乙胺或碳酸钾,所述醚类为乙醚、四氢呋喃或二氧六环。
9.一种制备按照权利要求1所述的二苯酮腙衍生物的方法,其特征在于包括以下步骤:
4(或3或2)-R14’-R3SO2N(R2)Y-二苯甲酮与1-5当量的肼基甲酸甲酯,在苯(苯、甲苯、二甲苯、乙苯)类或醇类溶剂中,在0℃到溶剂回流温度下,反应得到4(或3或2)-R1-4’-R3SO2N(R2)Y-二苯甲酮的肼基甲酸甲酯脱水缩合物中间体;
4(或3或2)-R1-4’-R3SO2N(R2)Y-二苯甲酮的肼基甲酸甲酯脱水缩合物中间体与R4R5R6取代的苯胺在苯类或醚类溶剂中,在0℃到溶剂回流温度下,反应得到按照权利要求1所述的二苯酮腙衍生物;
其中:
R1为卤素、C1-C4烷基、C1-C4烷氧基、C1-C4卤代烷基或C1-C4卤代烷氧基;
R2为氢、C1-C4烷基或环丙基;
R3为C1-C4烷基或C1-C4卤代烷基;
R4、R5、R6独立地选自氢、卤素、硝基、腈基、羟基、C1-C4烷基、C1-C4卤代烷基、C1-C4烷氧基、(取代)苯氧基、C1-C4卤代烷氧基、甲酸及其碱金属盐、甲酸C1~6酯基、甲酰胺或N-C1-4烷基或苯基取代甲酰胺;
X为胺基或氧;
Y为亚甲基或直接键。
10.按照权利要求9所述的制备按照权利要求1所述的二苯酮腙衍生物的方法,其特征在于所述苯类为苯、甲苯、二甲苯或乙苯,所述醚类为乙醚、四氢呋喃或二氧六环。
11.一种按照权利要求1所述的二苯酮腙衍生物的的用途,其特征在于用于农用化学杀虫剂。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201010040040.0A CN102126994B (zh) | 2010-01-19 | 2010-01-19 | 一种二苯酮腙衍生物、其制备方法和用途 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201010040040.0A CN102126994B (zh) | 2010-01-19 | 2010-01-19 | 一种二苯酮腙衍生物、其制备方法和用途 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN102126994A true CN102126994A (zh) | 2011-07-20 |
| CN102126994B CN102126994B (zh) | 2014-07-09 |
Family
ID=44265312
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201010040040.0A Active CN102126994B (zh) | 2010-01-19 | 2010-01-19 | 一种二苯酮腙衍生物、其制备方法和用途 |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN102126994B (zh) |
Cited By (85)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2014170300A1 (en) | 2013-04-19 | 2014-10-23 | Basf Se | N-substituted acyl-imino-pyridine compounds and derivatives for combating animal pests |
| WO2015055757A1 (en) | 2013-10-18 | 2015-04-23 | Basf Se | Use of pesticidal active carboxamide derivative in soil and seed application and treatment methods |
| CN104725288A (zh) * | 2013-12-20 | 2015-06-24 | 兰州大学 | 一种二苯酮腙磺酰脲类化合物及其制备方法和用途 |
| WO2016071499A1 (en) | 2014-11-06 | 2016-05-12 | Basf Se | 3-pyridyl heterobicyclic compound for controlling invertebrate pests |
| WO2016128261A2 (en) | 2015-02-11 | 2016-08-18 | Basf Se | Pesticidal mixture comprising a pyrazole compound, an insecticide and a fungicide |
| WO2016162371A1 (en) | 2015-04-07 | 2016-10-13 | Basf Agrochemical Products B.V. | Use of an insecticidal carboxamide compound against pests on cultivated plants |
| WO2016198611A1 (en) | 2015-06-11 | 2016-12-15 | Basf Se | N-(thio)acylimino heterocyclic compounds |
| WO2016198613A1 (en) | 2015-06-11 | 2016-12-15 | Basf Se | N-(thio)acylimino compounds |
| WO2017016883A1 (en) | 2015-07-24 | 2017-02-02 | Basf Se | Process for preparation of cyclopentene compounds |
| WO2017093163A1 (en) | 2015-11-30 | 2017-06-08 | Basf Se | Mixtures of cis-jasmone and bacillus amyloliquefaciens |
| WO2017140614A1 (en) | 2016-02-19 | 2017-08-24 | Basf Se | Method for controlling pests of soybean, corn, and cotton plants |
| WO2017153217A1 (en) | 2016-03-09 | 2017-09-14 | Basf Se | Spirocyclic derivatives |
| WO2017153218A1 (en) | 2016-03-11 | 2017-09-14 | Basf Se | Method for controlling pests of plants |
| WO2017167832A1 (en) | 2016-04-01 | 2017-10-05 | Basf Se | Bicyclic compounds |
| WO2017198588A1 (en) | 2016-05-18 | 2017-11-23 | Basf Se | Capsules comprising benzylpropargylethers for use as nitrification inhibitors |
| WO2018108671A1 (en) | 2016-12-16 | 2018-06-21 | Basf Se | Pesticidal compounds |
| WO2018162312A1 (en) | 2017-03-10 | 2018-09-13 | Basf Se | Spirocyclic derivatives |
| WO2018166855A1 (en) | 2017-03-16 | 2018-09-20 | Basf Se | Heterobicyclic substituted dihydroisoxazoles |
| WO2018177781A1 (en) | 2017-03-28 | 2018-10-04 | Basf Se | Pesticidal compounds |
| WO2018177970A1 (en) | 2017-03-31 | 2018-10-04 | Basf Se | Process for preparing chiral 2,3-dihydrothiazolo[3,2-a]pyrimidin-4-ium compounds |
| WO2018192793A1 (en) | 2017-04-20 | 2018-10-25 | Basf Se | Substituted rhodanine derivatives |
| WO2018197466A1 (en) | 2017-04-26 | 2018-11-01 | Basf Se | Substituted succinimide derivatives as pesticides |
| WO2018206479A1 (en) | 2017-05-10 | 2018-11-15 | Basf Se | Bicyclic pesticidal compounds |
| US10149477B2 (en) | 2014-10-06 | 2018-12-11 | Basf Se | Substituted pyrimidinium compounds for combating animal pests |
| WO2018224455A1 (en) | 2017-06-07 | 2018-12-13 | Basf Se | Substituted cyclopropyl derivatives |
| WO2018229202A1 (en) | 2017-06-16 | 2018-12-20 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| WO2018234488A1 (en) | 2017-06-23 | 2018-12-27 | Basf Se | SUBSTITUTED CYCLOPROPYL DERIVATIVES |
| WO2018234202A1 (en) | 2017-06-19 | 2018-12-27 | Basf Se | SUBSTITUTED PYRIMIDINIUM COMPOUNDS AND DERIVATIVES FOR CONTROLLING HARMFUL ANIMALS |
| WO2019043183A1 (en) | 2017-08-31 | 2019-03-07 | Basf Se | METHOD FOR CONTROLLING PESTS OF RICE IN RICE |
| EP3453706A1 (en) | 2017-09-08 | 2019-03-13 | Basf Se | Pesticidal imidazole compounds |
| WO2019072906A1 (en) | 2017-10-13 | 2019-04-18 | Basf Se | IMIDAZOLIDINE PYRIMIDINIUM COMPOUNDS FOR CONTROL OF HARMFUL ANIMALS |
| WO2019121143A1 (en) | 2017-12-20 | 2019-06-27 | Basf Se | Substituted cyclopropyl derivatives |
| WO2019121159A1 (en) | 2017-12-21 | 2019-06-27 | Basf Se | Pesticidal compounds |
| WO2019134840A1 (en) | 2018-01-05 | 2019-07-11 | Basf Se | Control of pests of soybean plants with mesoionic compounds |
| WO2019137995A1 (en) | 2018-01-11 | 2019-07-18 | Basf Se | Novel pyridazine compounds for controlling invertebrate pests |
| WO2019145140A1 (en) | 2018-01-09 | 2019-08-01 | Basf Se | Silylethynyl hetaryl compounds as nitrification inhibitors |
| WO2019166560A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of n-functionalized alkoxy pyrazole compounds as nitrification inhibitors |
| WO2019166558A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of pyrazole propargyl ethers as nitrification inhibitors |
| WO2019166561A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of alkoxypyrazoles as nitrification inhibitors |
| WO2019175712A1 (en) | 2018-03-14 | 2019-09-19 | Basf Corporation | New uses for catechol molecules as inhibitors to glutathione s-transferase metabolic pathways |
| WO2019175713A1 (en) | 2018-03-14 | 2019-09-19 | Basf Corporation | New catechol molecules and their use as inhibitors to p450 related metabolic pathways |
| WO2019185413A1 (en) | 2018-03-27 | 2019-10-03 | Basf Se | Pesticidal substituted cyclopropyl derivatives |
| WO2019211106A1 (en) | 2018-04-30 | 2019-11-07 | Basf Se | Control of pests of soybean plants with mesoionic compounds |
| WO2019219529A1 (en) | 2018-05-15 | 2019-11-21 | Basf Se | Mixtures comprising benzpyrimoxan and oxazosulfyl and uses and methods of applying them |
| WO2019224092A1 (en) | 2018-05-22 | 2019-11-28 | Basf Se | Pesticidally active c15-derivatives of ginkgolides |
| WO2020002472A1 (en) | 2018-06-28 | 2020-01-02 | Basf Se | Use of alkynylthiophenes as nitrification inhibitors |
| WO2020020777A1 (en) | 2018-07-23 | 2020-01-30 | Basf Se | Use of substituted 2-thiazolines as nitrification inhibitors |
| WO2020020765A1 (en) | 2018-07-23 | 2020-01-30 | Basf Se | Use of a substituted thiazolidine compound as nitrification inhibitor |
| US10556844B2 (en) | 2015-02-06 | 2020-02-11 | Basf Se | Pyrazole compounds as nitrification inhibitors |
| EP3613736A1 (en) | 2018-08-22 | 2020-02-26 | Basf Se | Substituted glutarimide derivatives |
| EP3628156A1 (en) | 2018-09-28 | 2020-04-01 | Basf Se | Method for controlling pests of sugarcane, citrus, rapeseed, and potato plants |
| EP3628157A1 (en) | 2018-09-28 | 2020-04-01 | Basf Se | Method of controlling insecticide resistant insects and virus transmission to plants |
| EP3628158A1 (en) | 2018-09-28 | 2020-04-01 | Basf Se | Pesticidal mixture comprising a mesoionic compound and a biopesticide |
| WO2020064492A1 (en) | 2018-09-28 | 2020-04-02 | Basf Se | Method of controlling pests by seed treatment application of a mesoionic compound or mixture thereof |
| EP3643705A1 (en) | 2018-10-24 | 2020-04-29 | Basf Se | Pesticidal compounds |
| WO2020109039A1 (en) | 2018-11-28 | 2020-06-04 | Basf Se | Pesticidal compounds |
| WO2020126591A1 (en) | 2018-12-18 | 2020-06-25 | Basf Se | Substituted pyrimidinium compounds for combating animal pests |
| EP3696177A1 (en) | 2019-02-12 | 2020-08-19 | Basf Se | Heterocyclic compounds for the control of invertebrate pests |
| WO2020239517A1 (en) | 2019-05-29 | 2020-12-03 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| EP3766879A1 (en) | 2019-07-19 | 2021-01-20 | Basf Se | Pesticidal pyrazole derivatives |
| EP3769623A1 (en) | 2019-07-22 | 2021-01-27 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| WO2021130143A1 (en) | 2019-12-23 | 2021-07-01 | Basf Se | Enzyme enhanced root uptake of agrochemical active compound |
| US11053175B2 (en) | 2015-05-12 | 2021-07-06 | Basf Se | Thioether compounds as nitrification inhibitors |
| WO2021170463A1 (en) | 2020-02-28 | 2021-09-02 | BASF Agro B.V. | Methods and uses of a mixture comprising alpha-cypermethrin and dinotefuran for controlling invertebrate pests in turf |
| US11142514B2 (en) | 2015-10-02 | 2021-10-12 | Basf Se | Imino compounds with a 2-chloropyrimidin-5-yl substituent as pest-control agents |
| WO2021219513A1 (en) | 2020-04-28 | 2021-11-04 | Basf Se | Pesticidal compounds |
| EP3909950A1 (en) | 2020-05-13 | 2021-11-17 | Basf Se | Heterocyclic compounds for the control of invertebrate pests |
| WO2022167488A1 (en) | 2021-02-02 | 2022-08-11 | Basf Se | Synergistic action of dcd and alkoxypyrazoles as nitrification inhibitors |
| EP4043444A1 (en) | 2021-02-11 | 2022-08-17 | Basf Se | Substituted isoxazoline derivatives |
| WO2022243523A1 (en) | 2021-05-21 | 2022-11-24 | Basf Se | Use of an n-functionalized alkoxy pyrazole compound as nitrification inhibitor |
| WO2022243521A1 (en) | 2021-05-21 | 2022-11-24 | Basf Se | Use of ethynylpyridine compounds as nitrification inhibitors |
| WO2022268810A1 (en) | 2021-06-21 | 2022-12-29 | Basf Se | Metal-organic frameworks with pyrazole-based building blocks |
| EP4119547A1 (en) | 2021-07-12 | 2023-01-18 | Basf Se | Triazole compounds for the control of invertebrate pests |
| EP4140986A1 (en) | 2021-08-23 | 2023-03-01 | Basf Se | Pyrazine compounds for the control of invertebrate pests |
| EP4140995A1 (en) | 2021-08-27 | 2023-03-01 | Basf Se | Pyrazine compounds for the control of invertebrate pests |
| EP4151631A1 (en) | 2021-09-20 | 2023-03-22 | Basf Se | Heterocyclic compounds for the control of invertebrate pests |
| EP4194453A1 (en) | 2021-12-08 | 2023-06-14 | Basf Se | Pyrazine compounds for the control of invertebrate pests |
| EP4198023A1 (en) | 2021-12-16 | 2023-06-21 | Basf Se | Pesticidally active thiosemicarbazone compounds |
| EP4198033A1 (en) | 2021-12-14 | 2023-06-21 | Basf Se | Heterocyclic compounds for the control of invertebrate pests |
| EP4238971A1 (en) | 2022-03-02 | 2023-09-06 | Basf Se | Substituted isoxazoline derivatives |
| WO2023203066A1 (en) | 2022-04-21 | 2023-10-26 | Basf Se | Synergistic action as nitrification inhibitors of dcd oligomers with alkoxypyrazole and its oligomers |
| WO2023208447A1 (en) | 2022-04-25 | 2023-11-02 | Basf Se | An emulsifiable concentrate having a (substituted) benzaldehyde-based solvent system |
| WO2024028243A1 (en) | 2022-08-02 | 2024-02-08 | Basf Se | Pyrazolo pesticidal compounds |
| EP4342885A1 (en) | 2022-09-20 | 2024-03-27 | Basf Se | N-(3-(aminomethyl)-phenyl)-5-(4-phenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-amine derivatives and similar compounds as pesticides |
| EP4389210A1 (en) | 2022-12-21 | 2024-06-26 | Basf Se | Heteroaryl compounds for the control of invertebrate pests |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TW268881B (zh) * | 1992-07-07 | 1996-01-21 | Ciba Geigy Ag | |
| ATE187165T1 (de) * | 1995-05-12 | 1999-12-15 | Bayer Agrochem Kk | Benzophenonhydrazon-derivate als insektizide |
-
2010
- 2010-01-19 CN CN201010040040.0A patent/CN102126994B/zh active Active
Cited By (93)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2014170300A1 (en) | 2013-04-19 | 2014-10-23 | Basf Se | N-substituted acyl-imino-pyridine compounds and derivatives for combating animal pests |
| EP3456201A1 (en) | 2013-10-18 | 2019-03-20 | BASF Agrochemical Products B.V. | Use of pesticidal active carboxamide derivative in soil and seed application and treatment meth-ods |
| WO2015055757A1 (en) | 2013-10-18 | 2015-04-23 | Basf Se | Use of pesticidal active carboxamide derivative in soil and seed application and treatment methods |
| CN104725288A (zh) * | 2013-12-20 | 2015-06-24 | 兰州大学 | 一种二苯酮腙磺酰脲类化合物及其制备方法和用途 |
| US10149477B2 (en) | 2014-10-06 | 2018-12-11 | Basf Se | Substituted pyrimidinium compounds for combating animal pests |
| WO2016071499A1 (en) | 2014-11-06 | 2016-05-12 | Basf Se | 3-pyridyl heterobicyclic compound for controlling invertebrate pests |
| US10556844B2 (en) | 2015-02-06 | 2020-02-11 | Basf Se | Pyrazole compounds as nitrification inhibitors |
| WO2016128261A2 (en) | 2015-02-11 | 2016-08-18 | Basf Se | Pesticidal mixture comprising a pyrazole compound, an insecticide and a fungicide |
| US10701937B2 (en) | 2015-02-11 | 2020-07-07 | Basf Se | Pesticidal mixture comprising a pyrazole compound, an insecticide and a fungicide |
| WO2016162371A1 (en) | 2015-04-07 | 2016-10-13 | Basf Agrochemical Products B.V. | Use of an insecticidal carboxamide compound against pests on cultivated plants |
| US11053175B2 (en) | 2015-05-12 | 2021-07-06 | Basf Se | Thioether compounds as nitrification inhibitors |
| WO2016198613A1 (en) | 2015-06-11 | 2016-12-15 | Basf Se | N-(thio)acylimino compounds |
| WO2016198611A1 (en) | 2015-06-11 | 2016-12-15 | Basf Se | N-(thio)acylimino heterocyclic compounds |
| WO2017016883A1 (en) | 2015-07-24 | 2017-02-02 | Basf Se | Process for preparation of cyclopentene compounds |
| US11142514B2 (en) | 2015-10-02 | 2021-10-12 | Basf Se | Imino compounds with a 2-chloropyrimidin-5-yl substituent as pest-control agents |
| WO2017093163A1 (en) | 2015-11-30 | 2017-06-08 | Basf Se | Mixtures of cis-jasmone and bacillus amyloliquefaciens |
| WO2017140614A1 (en) | 2016-02-19 | 2017-08-24 | Basf Se | Method for controlling pests of soybean, corn, and cotton plants |
| WO2017153217A1 (en) | 2016-03-09 | 2017-09-14 | Basf Se | Spirocyclic derivatives |
| WO2017153218A1 (en) | 2016-03-11 | 2017-09-14 | Basf Se | Method for controlling pests of plants |
| WO2017167832A1 (en) | 2016-04-01 | 2017-10-05 | Basf Se | Bicyclic compounds |
| WO2017198588A1 (en) | 2016-05-18 | 2017-11-23 | Basf Se | Capsules comprising benzylpropargylethers for use as nitrification inhibitors |
| WO2018108671A1 (en) | 2016-12-16 | 2018-06-21 | Basf Se | Pesticidal compounds |
| WO2018162312A1 (en) | 2017-03-10 | 2018-09-13 | Basf Se | Spirocyclic derivatives |
| WO2018166855A1 (en) | 2017-03-16 | 2018-09-20 | Basf Se | Heterobicyclic substituted dihydroisoxazoles |
| WO2018177781A1 (en) | 2017-03-28 | 2018-10-04 | Basf Se | Pesticidal compounds |
| WO2018177970A1 (en) | 2017-03-31 | 2018-10-04 | Basf Se | Process for preparing chiral 2,3-dihydrothiazolo[3,2-a]pyrimidin-4-ium compounds |
| EP3978504A1 (en) | 2017-03-31 | 2022-04-06 | Basf Se | Chiral 2,3-dihydrothiazolo[3,2-a]pyrimidine derivatives for combating animal pests |
| WO2018192793A1 (en) | 2017-04-20 | 2018-10-25 | Basf Se | Substituted rhodanine derivatives |
| WO2018197466A1 (en) | 2017-04-26 | 2018-11-01 | Basf Se | Substituted succinimide derivatives as pesticides |
| WO2018206479A1 (en) | 2017-05-10 | 2018-11-15 | Basf Se | Bicyclic pesticidal compounds |
| WO2018224455A1 (en) | 2017-06-07 | 2018-12-13 | Basf Se | Substituted cyclopropyl derivatives |
| WO2018229202A1 (en) | 2017-06-16 | 2018-12-20 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| WO2018234202A1 (en) | 2017-06-19 | 2018-12-27 | Basf Se | SUBSTITUTED PYRIMIDINIUM COMPOUNDS AND DERIVATIVES FOR CONTROLLING HARMFUL ANIMALS |
| WO2018234488A1 (en) | 2017-06-23 | 2018-12-27 | Basf Se | SUBSTITUTED CYCLOPROPYL DERIVATIVES |
| WO2019042932A1 (en) | 2017-08-31 | 2019-03-07 | Basf Se | METHOD FOR CONTROLLING RICE PARASITES IN RICE |
| WO2019043183A1 (en) | 2017-08-31 | 2019-03-07 | Basf Se | METHOD FOR CONTROLLING PESTS OF RICE IN RICE |
| EP3453706A1 (en) | 2017-09-08 | 2019-03-13 | Basf Se | Pesticidal imidazole compounds |
| WO2019072906A1 (en) | 2017-10-13 | 2019-04-18 | Basf Se | IMIDAZOLIDINE PYRIMIDINIUM COMPOUNDS FOR CONTROL OF HARMFUL ANIMALS |
| WO2019121143A1 (en) | 2017-12-20 | 2019-06-27 | Basf Se | Substituted cyclopropyl derivatives |
| WO2019121159A1 (en) | 2017-12-21 | 2019-06-27 | Basf Se | Pesticidal compounds |
| WO2019134840A1 (en) | 2018-01-05 | 2019-07-11 | Basf Se | Control of pests of soybean plants with mesoionic compounds |
| WO2019145140A1 (en) | 2018-01-09 | 2019-08-01 | Basf Se | Silylethynyl hetaryl compounds as nitrification inhibitors |
| WO2019137995A1 (en) | 2018-01-11 | 2019-07-18 | Basf Se | Novel pyridazine compounds for controlling invertebrate pests |
| WO2019166561A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of alkoxypyrazoles as nitrification inhibitors |
| WO2019166558A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of pyrazole propargyl ethers as nitrification inhibitors |
| WO2019166560A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of n-functionalized alkoxy pyrazole compounds as nitrification inhibitors |
| WO2019175713A1 (en) | 2018-03-14 | 2019-09-19 | Basf Corporation | New catechol molecules and their use as inhibitors to p450 related metabolic pathways |
| WO2019175712A1 (en) | 2018-03-14 | 2019-09-19 | Basf Corporation | New uses for catechol molecules as inhibitors to glutathione s-transferase metabolic pathways |
| WO2019185413A1 (en) | 2018-03-27 | 2019-10-03 | Basf Se | Pesticidal substituted cyclopropyl derivatives |
| WO2019211106A1 (en) | 2018-04-30 | 2019-11-07 | Basf Se | Control of pests of soybean plants with mesoionic compounds |
| WO2019219529A1 (en) | 2018-05-15 | 2019-11-21 | Basf Se | Mixtures comprising benzpyrimoxan and oxazosulfyl and uses and methods of applying them |
| WO2019224092A1 (en) | 2018-05-22 | 2019-11-28 | Basf Se | Pesticidally active c15-derivatives of ginkgolides |
| WO2020002472A1 (en) | 2018-06-28 | 2020-01-02 | Basf Se | Use of alkynylthiophenes as nitrification inhibitors |
| WO2020020777A1 (en) | 2018-07-23 | 2020-01-30 | Basf Se | Use of substituted 2-thiazolines as nitrification inhibitors |
| WO2020020765A1 (en) | 2018-07-23 | 2020-01-30 | Basf Se | Use of a substituted thiazolidine compound as nitrification inhibitor |
| EP3613736A1 (en) | 2018-08-22 | 2020-02-26 | Basf Se | Substituted glutarimide derivatives |
| WO2020064480A1 (en) | 2018-09-28 | 2020-04-02 | Basf Se | Pesticidal mixture comprising a mesoionic compound and a biopesticide |
| EP3628158A1 (en) | 2018-09-28 | 2020-04-01 | Basf Se | Pesticidal mixture comprising a mesoionic compound and a biopesticide |
| WO2020064492A1 (en) | 2018-09-28 | 2020-04-02 | Basf Se | Method of controlling pests by seed treatment application of a mesoionic compound or mixture thereof |
| WO2020064408A1 (en) | 2018-09-28 | 2020-04-02 | Basf Se | Method of controlling insecticide resistant insects and virus transmission to plants |
| EP3628156A1 (en) | 2018-09-28 | 2020-04-01 | Basf Se | Method for controlling pests of sugarcane, citrus, rapeseed, and potato plants |
| EP3628157A1 (en) | 2018-09-28 | 2020-04-01 | Basf Se | Method of controlling insecticide resistant insects and virus transmission to plants |
| EP3643705A1 (en) | 2018-10-24 | 2020-04-29 | Basf Se | Pesticidal compounds |
| WO2020083733A1 (en) | 2018-10-24 | 2020-04-30 | Basf Se | Pesticidal compounds |
| WO2020109039A1 (en) | 2018-11-28 | 2020-06-04 | Basf Se | Pesticidal compounds |
| WO2020126591A1 (en) | 2018-12-18 | 2020-06-25 | Basf Se | Substituted pyrimidinium compounds for combating animal pests |
| EP3696177A1 (en) | 2019-02-12 | 2020-08-19 | Basf Se | Heterocyclic compounds for the control of invertebrate pests |
| WO2020239517A1 (en) | 2019-05-29 | 2020-12-03 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| WO2021013561A1 (en) | 2019-07-19 | 2021-01-28 | Basf Se | Pesticidal pyrazole and triazole derivatives |
| EP3766879A1 (en) | 2019-07-19 | 2021-01-20 | Basf Se | Pesticidal pyrazole derivatives |
| EP3769623A1 (en) | 2019-07-22 | 2021-01-27 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| WO2021130143A1 (en) | 2019-12-23 | 2021-07-01 | Basf Se | Enzyme enhanced root uptake of agrochemical active compound |
| WO2021170463A1 (en) | 2020-02-28 | 2021-09-02 | BASF Agro B.V. | Methods and uses of a mixture comprising alpha-cypermethrin and dinotefuran for controlling invertebrate pests in turf |
| WO2021219513A1 (en) | 2020-04-28 | 2021-11-04 | Basf Se | Pesticidal compounds |
| EP3909950A1 (en) | 2020-05-13 | 2021-11-17 | Basf Se | Heterocyclic compounds for the control of invertebrate pests |
| WO2022167488A1 (en) | 2021-02-02 | 2022-08-11 | Basf Se | Synergistic action of dcd and alkoxypyrazoles as nitrification inhibitors |
| EP4043444A1 (en) | 2021-02-11 | 2022-08-17 | Basf Se | Substituted isoxazoline derivatives |
| WO2022243523A1 (en) | 2021-05-21 | 2022-11-24 | Basf Se | Use of an n-functionalized alkoxy pyrazole compound as nitrification inhibitor |
| WO2022243521A1 (en) | 2021-05-21 | 2022-11-24 | Basf Se | Use of ethynylpyridine compounds as nitrification inhibitors |
| WO2022268810A1 (en) | 2021-06-21 | 2022-12-29 | Basf Se | Metal-organic frameworks with pyrazole-based building blocks |
| EP4119547A1 (en) | 2021-07-12 | 2023-01-18 | Basf Se | Triazole compounds for the control of invertebrate pests |
| EP4140986A1 (en) | 2021-08-23 | 2023-03-01 | Basf Se | Pyrazine compounds for the control of invertebrate pests |
| EP4140995A1 (en) | 2021-08-27 | 2023-03-01 | Basf Se | Pyrazine compounds for the control of invertebrate pests |
| EP4151631A1 (en) | 2021-09-20 | 2023-03-22 | Basf Se | Heterocyclic compounds for the control of invertebrate pests |
| EP4194453A1 (en) | 2021-12-08 | 2023-06-14 | Basf Se | Pyrazine compounds for the control of invertebrate pests |
| EP4198033A1 (en) | 2021-12-14 | 2023-06-21 | Basf Se | Heterocyclic compounds for the control of invertebrate pests |
| EP4198023A1 (en) | 2021-12-16 | 2023-06-21 | Basf Se | Pesticidally active thiosemicarbazone compounds |
| EP4238971A1 (en) | 2022-03-02 | 2023-09-06 | Basf Se | Substituted isoxazoline derivatives |
| WO2023203066A1 (en) | 2022-04-21 | 2023-10-26 | Basf Se | Synergistic action as nitrification inhibitors of dcd oligomers with alkoxypyrazole and its oligomers |
| WO2023208447A1 (en) | 2022-04-25 | 2023-11-02 | Basf Se | An emulsifiable concentrate having a (substituted) benzaldehyde-based solvent system |
| WO2024028243A1 (en) | 2022-08-02 | 2024-02-08 | Basf Se | Pyrazolo pesticidal compounds |
| EP4342885A1 (en) | 2022-09-20 | 2024-03-27 | Basf Se | N-(3-(aminomethyl)-phenyl)-5-(4-phenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-amine derivatives and similar compounds as pesticides |
| EP4389210A1 (en) | 2022-12-21 | 2024-06-26 | Basf Se | Heteroaryl compounds for the control of invertebrate pests |
Also Published As
| Publication number | Publication date |
|---|---|
| CN102126994B (zh) | 2014-07-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN102126994B (zh) | 一种二苯酮腙衍生物、其制备方法和用途 | |
| CN102718700B (zh) | 3,5,6-三氯-2-吡啶氧乙酸衍生物制备及应用研究 | |
| CN101967139A (zh) | 一种含一氟甲氧基吡唑的邻甲酰氨基苯甲酰胺类化合物、其合成方法及应用 | |
| BRPI0809815A2 (pt) | "composição artropodicida sólida e propágulo" | |
| CN102267935A (zh) | 一类吲哚满二酮衍生物及其制备方法和用途 | |
| CN103059006A (zh) | 具有抗菌活性的白杨素-1,2,3-三氮唑类化合物及其制备方法 | |
| CN111087345B (zh) | 偶氮苯类杂环酰胺衍生物及其制备方法和应用 | |
| BRPI1006544B1 (pt) | Fertilizante granular contendo agente agroquímico herbicida e método para erradicar ervas daninhas | |
| CN108084108A (zh) | 一种5-氯苯并噁唑衍生物及其制备方法、除草组合物和应用 | |
| CN101723943B (zh) | 1,3,4-噻二唑基氟尿嘧啶类化合物及其制备方法和应用 | |
| CN106632122B (zh) | 苯并噁唑苯氧羧酸酰胺类化合物及其制备方法 | |
| CN108419804A (zh) | 一种杀菌组合物及其应用 | |
| CN103450179B (zh) | N-(1,3,4-噻二唑基)噻唑甲酰胺类化合物及其用途 | |
| CN104803929B (zh) | 一种取代肟醚类化合物及其用途 | |
| US2779669A (en) | Acyl pseudourea herbicidal composition and a method for killing weeds therewith | |
| JP5563964B2 (ja) | 水面浮遊性農薬製剤 | |
| CN103874691B (zh) | 具有除草活性的基于苯基异噁唑啉的化合物及其用途 | |
| JPS59176243A (ja) | 新規ベンゾイル尿素化合物およびその製造方法並びにこの化合物を含有する殺虫−殺ダニ剤 | |
| WO2019148851A1 (zh) | 五元环取代的哒嗪醇类化合物及其衍生物、制备方法、除草组合物和应用 | |
| WO2019047978A1 (zh) | 含氟氯吡啶肟酯结构的化合物及其制备方法和应用及一种除草剂 | |
| CN105037395B (zh) | 5H‑噻唑并[3,2‑a]嘧啶‑5‑酮类衍生物及其制备方法与作为抗菌药物的应用 | |
| CN104725276B (zh) | 一种含七氟异丙基的羰基肟醚类化合物、其制备方法及应用 | |
| CN101381347B (zh) | 一种嘧啶类化合物、制备方法及其用途 | |
| CN103275029B (zh) | 一种水油兼溶的芳氧苯氧丙酸酯类衍生物制备及应用研究 | |
| CN109232534B (zh) | 含杂环二芳胺基吡唑甲酰胺类化合物及其制备方法与应用 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| TR01 | Transfer of patent right |
Effective date of registration: 20200426 Address after: 310023 No. 926, Xixi Road, Hangzhou, Zhejiang, Xihu District Co-patentee after: SINOCHEM LANTIAN Co.,Ltd. Patentee after: ZHEJIANG RESEARCH INSTITUTE OF CHEMICAL INDUSTRY Ltd. Co-patentee after: SINOCHEM Corp. Address before: 310012 No. 487, Stadium Road, Hangzhou, Zhejiang Patentee before: SINOCHEM LANTIAN Co.,Ltd. |
|
| TR01 | Transfer of patent right |