WO2024005144A1 - 体表面潰瘍治癒促進装置 - Google Patents
体表面潰瘍治癒促進装置 Download PDFInfo
- Publication number
- WO2024005144A1 WO2024005144A1 PCT/JP2023/024188 JP2023024188W WO2024005144A1 WO 2024005144 A1 WO2024005144 A1 WO 2024005144A1 JP 2023024188 W JP2023024188 W JP 2023024188W WO 2024005144 A1 WO2024005144 A1 WO 2024005144A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- body surface
- ulcer
- vibration
- glucose concentration
- healing promoting
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/48—Other medical applications
- A61B5/4836—Diagnosis combined with treatment in closed-loop systems or methods
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/02—Detecting, measuring or recording for evaluating the cardiovascular system, e.g. pulse, heart rate, blood pressure or blood flow
- A61B5/026—Measuring blood flow
- A61B5/0261—Measuring blood flow using optical means, e.g. infrared light
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/145—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue
- A61B5/14532—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue for measuring glucose, e.g. by tissue impedance measurement
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/44—Detecting, measuring or recording for evaluating the integumentary system, e.g. skin, hair or nails
- A61B5/441—Skin evaluation, e.g. for skin disorder diagnosis
- A61B5/445—Evaluating skin irritation or skin trauma, e.g. rash, eczema, wound, bed sore
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/44—Detecting, measuring or recording for evaluating the integumentary system, e.g. skin, hair or nails
- A61B5/441—Skin evaluation, e.g. for skin disorder diagnosis
- A61B5/447—Skin evaluation, e.g. for skin disorder diagnosis specially adapted for aiding the prevention of ulcer or pressure sore development, i.e. before the ulcer or sore has developed
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/68—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient
- A61B5/6801—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient specially adapted to be attached to or worn on the body surface
- A61B5/6813—Specially adapted to be attached to a specific body part
- A61B5/6829—Foot or ankle
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/74—Details of notification to user or communication with user or patient; User input means
- A61B5/7455—Details of notification to user or communication with user or patient; User input means characterised by tactile indication, e.g. vibration or electrical stimulation
Definitions
- the present invention relates to treatment technology, and relates to a body surface ulcer healing promotion device.
- a method of treating foot ulcers includes local administration of platelet-derived growth factors.
- one of the objects of the present invention is to provide a device that promotes healing of ulcers on the body surface.
- a body surface ulcer healing promoting device includes a glucose concentration sensor that detects the glucose concentration in the exudate of a body surface ulcer, a vibrator that vibrates the ulcer, and a body surface ulcer based on the glucose concentration in the exudate. , and a control device that controls vibration of the vibrator.
- the ulcer may be an ulcer caused by diabetes.
- the ulcer may be a foot ulcer caused by diabetes.
- the above body surface ulcer healing promotion device may include a plurality of vibrators.
- control device may control the vibration of the vibrator so that the production of arginine in cells is promoted.
- control device may control the vibration of the vibrator so that the glucose concentration in the exudate is equal to or higher than the concentration that increases blood flow.
- control device may vibrate the vibrator until the glucose concentration in the exudate falls below a concentration at which the amount of arginine produced in the cells is equal to or higher than a threshold value.
- the above body surface ulcer healing promoting device may further include a blood flow sensor that detects blood flow near the ulcer, and the control device may control the vibration of the vibrator based on the blood flow.
- control device may control the vibration of the vibrator so that blood flow increases.
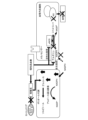
- FIG. 1 is a schematic diagram showing a body surface ulcer healing promoting device according to an embodiment.
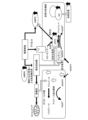
- FIG. 2 is a schematic diagram showing the relationship between hyperglycemia and metabolism according to an embodiment.
- FIG. 2 is a schematic diagram showing the relationship between hyperglycemia and metabolism according to an embodiment.
- 2 is a graph showing the amount of 2-deoxyglucose taken up in Examples.
- 2 is a graph showing the amount of 2-deoxyglucose taken up in Examples.
- 2 is a graph showing the amount of 2-deoxyglucose taken up in Examples.
- 2 is a graph showing the amount of 2-deoxyglucose taken up in Examples.
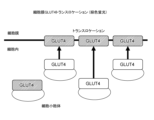
- FIG. 2 is a schematic diagram showing GLUT4 translocation according to an example.
- FIG. 2 is a schematic diagram and a photograph showing GLUT4 translocation according to an example. It is a photograph showing the presence or absence of GLUT4 translocation according to an example. It is a graph of the ratio of the intensity of GLUT4-derived fluorescence in the cell membrane to the intensity of GLUT4-derived fluorescence in the cytoplasm according to Examples. It is a photograph of a rat according to an example. 1 is a photograph of a rat wound according to an example. 1 is a photograph of a rat wound according to an example. It is a graph of the wound area of a rat according to an example. It is a photograph of the tissue of a rat wound according to an example.
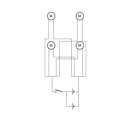
- FIG. 2 is a circuit diagram of an excitation device according to an example.
- 1 is a photograph of a rat wound according to an example. It is a graph of the wound area of a rat according to an example. It is a graph of blood volume of a rat according to an example. It is a photograph of the tissue of a rat wound according to an example. 1 is a graph of gene expression levels in rats according to Examples. 1 is a graph of gene expression levels in rats according to Examples. 1 is a graph of gene expression levels in rats according to Examples.
- 1 is a photograph of rat wound tissue stained using an anti-PTX3 antibody according to an example. It is a graph of the value obtained by dividing the number of CD68-positive cells by the number of CD163-positive cells (M1/M2 macrophage ratio) according to Examples.
- the body surface ulcer healing promoting device includes a glucose concentration sensor 11 that detects the glucose concentration in the exudate of an ulcer 2 on the body surface 1, and a vibrator 21A that vibrates the ulcer 2. , 21B, and 21C, and a control device 31 that controls the vibrations of the vibrators 21A, 21B, and 21C based on the glucose concentration in the exudate.
- the vibrators 21A, 21B, and 21C can be used as the vibrators 21A, 21B, and 21C.
- the number of vibrators 21A, 21B, and 21C included in the body surface ulcer healing promoting device according to the embodiment is not particularly limited, and may be one or more than one.
- the vibrators 21A, 21B, 21C are placed so as to surround the patient's ulcer 2.
- the vibrators 21A, 21B, and 21C generate, for example, low-frequency vibrations.
- the low frequency is, for example, 20 Hz or more and 100 Hz or less, 30 Hz or more and 90 Hz or less, 40 Hz or more and 80 Hz or less, 40 Hz or more and 70 Hz or less, or 40 Hz or more and 60 Hz or less, but is not particularly limited.
- the intensity of the vibration is, for example, 100 mVpp or more and 3000 mVpp or less, 500 mVpp or more and 2500 mVpp or less, 500 mVpp or more and 2000 mVpp or less, or 800 mVpp or more and 2000 mVpp or less, but is not particularly limited.
- the vibrators 21A, 21B, and 21C may be placed on the body surface 1 via the dressing material 41.
- materials for dressing material 41 include silicone, polyurethane, hydrophilic membrane, hydrophilic fiber, hydrocolloid, hydrogel, cellulose acetate, and polyvinyl alcohol.
- the glucose concentration sensor 11 is placed, for example, in contact with the patient's ulcer 2.
- the principle by which the glucose concentration sensor 11 detects the glucose concentration is not particularly limited.
- the glucose concentration sensor 11 includes a membrane to which glucose oxidase is immobilized, a platinum electrode, and a silver electrode.
- glucose is decomposed into gluconic acid and hydrogen peroxide by glucose oxidase
- hydrogen peroxide is oxidized with a platinum electrode and reduced with a silver electrode.
- the current produced by this at the electrode is proportional to the glucose concentration.
- the glucose concentration sensor 11 detects the glucose concentration in the exudate of the ulcer 2 and transmits the glucose concentration in the exudate of the ulcer 2 to the control device 31 .
- the control device 31 controls the vibrations of the vibrators 21A, 21B, and 21C so that the vibrations of the vibrators 21A, 21B, and 21C satisfy conditions for promoting the production of arginine in cells.
- conditions that promote arginine production in cells include vibration intensity and frequency.
- the control device 31 may store the relationship between the glucose concentration in the exudate and the amount of intracellular arginine produced, which has been obtained in advance. Further, the control device 31 may store a pre-obtained threshold value for the amount of intracellular arginine produced necessary for properly synthesizing nitric oxide. Generally, there is a negative proportional relationship between the glucose concentration in the exudate and the amount of intracellular arginine produced. Therefore, when the glucose concentration in the exudate is high, the amount of intracellular arginine produced is small, and when the glucose concentration in the exudate is low, the amount of intracellular arginine produced is large.
- the control device 31 controls the vibrations of the vibrators 21A, 21B, and 21C so that the glucose concentration in the exudate detected by the glucose concentration sensor 11 is equal to or lower than the concentration at which the amount of arginine produced in the cells is equal to or higher than a threshold value. do.
- the control device 31 continues to vibrate the vibrators 21A, 21B, and 21C until the glucose concentration in the exudate detected by the glucose concentration sensor 11 falls below a concentration at which the amount of arginine produced in cells exceeds a threshold value. good.
- the control device 31 may store the relationship between the glucose concentration in the exudate and the blood flow, which is obtained in advance. Further, the control device 31 may store an appropriate blood flow threshold obtained in advance. Generally, the glucose concentration in exudate and blood flow are in a negative proportional relationship. Therefore, if the glucose concentration in the exudate is high, there will be less blood flow, and if the glucose concentration in the exudate is low, there will be more blood flow.
- the control device 31 controls the vibrations of the vibrators 21A, 21B, and 21C so that the glucose concentration in the exudate detected by the glucose concentration sensor 11 is equal to or lower than the concentration at which the blood flow is equal to or higher than the threshold value.
- the control device 31 may vibrate the vibrators 21A, 21B, and 21C until the glucose concentration in the exudate detected by the glucose concentration sensor 11 falls below a concentration at which the blood flow is equal to or higher than a threshold value.
- the body surface ulcer healing promotion device may further include a blood flow sensor 12 that detects blood flow near the ulcer.
- the principle by which the blood flow sensor 12 detects blood flow is not particularly limited.
- the blood flow sensor 12 includes a light source and a light receiving element. Laser light emitted from a light source toward the human body is scattered by red blood cells in blood vessels. The blood flow sensor 12 receives scattered light with a light receiving element, and detects blood flow from the frequency spectrum of the scattered light. The blood flow sensor 12 transmits the detected blood flow to the control device 31.
- the control device 31 may control the vibrations of the vibrators 21A, 21B, and 21C based on blood flow. For example, the control device 31 may control the vibrations of the vibrators 21A, 21B, and 21C so as to satisfy conditions for increasing blood flow. Examples of conditions that increase blood flow include vibration intensity and frequency.
- the ulcer may be an ulcer caused by diabetes.
- the ulcer may be a foot ulcer.
- glucose taken into cells from blood vessels is used for the production of ATP in the glycolytic system and the citric acid cycle.
- glucose is taken up into cells due to hyperglycemia, glucose is taken into the polyol metabolic pathway.
- glucose is converted to sorbitol by aldose reductase.
- NADPH reduced nicotinamide adenine dinucleotide phosphate
- NADPH is also used in vascular endothelial cells to synthesize nitric oxide (NO) using arginine, but when NADPH is consumed in the polyol metabolic pathway, nitric oxide synthesis decreases.
- high glucose concentrations inhibit arginine production. When arginine production is inhibited, nitric oxide synthesis decreases. Decreased nitric oxide results in decreased blood flow and ischemia.
- the body surface ulcer healing promoting device according to the embodiment applies vibration to the ulcer 2, as shown in FIG. It is thought that insulin resistance in adipocytes is improved by activating protein kinase (protein kinase). Furthermore, it is thought that by improving the insulin resistance of adipocytes, the glucose concentration near the adipocytes is optimized, nitric oxide is appropriately synthesized in vascular endothelial cells, and blood flow is promoted. Note that diabetic foot ulcers tend to form in areas with poor muscle mass, such as metatarsals and heels. In addition, diabetic patients also progress to muscle atrophy due to peripheral neuropathy, resulting in poor muscle function. Therefore, the body surface ulcer healing promoting device according to the embodiment is considered to activate AMPK in fat cells near the ulcer 2.
- protein kinase protein kinase
- mechanosensors in vascular endothelial cells respond to mechanical stress caused by the body surface ulcer healing promoting device according to the embodiment, and nitric oxide synthase (NOS) in vascular endothelial cells ) is thought to promote the synthesis of Furthermore, activation of AMPK in adipocytes is thought to lead to activation of nitric oxide synthase in vascular endothelial cells. It is thought that by promoting and activating the synthesis of nitric oxide synthase, nitric oxide is appropriately synthesized in vascular endothelial cells and blood flow is promoted.
- NOS nitric oxide synthase
- the body surface ulcer healing promoting device according to the embodiment can be miniaturized, it can be fixed to the patient's body surface at all times while the patient leads an independent daily life. Also, a patient's insulin resistance is not constant and may fluctuate.
- the body surface ulcer healing promoting device according to the embodiment detects the glucose concentration in the exudate, which varies depending on insulin resistance, and controls the vibrations of the vibrators 21A, 21B, and 21C, so it does not impose unnecessary load on the patient. It is possible to suppress this.
- the stages of ulcer healing are divided into the inflammatory phase and the proliferative phase.
- the ulcer is infiltrated with neutrophils and M1 inflammatory macrophages, resulting in edema.
- fibroblasts migrate from the periphery of the ulcer to rebuild the extracellular matrix, angiogenesis occurs, and granulation tissue is formed.
- prolonged inflammation and inability to transition to the proliferative phase are the causes of intractable foot ulcers.
- the body surface ulcer healing promoting device vibrates locally near the ulcer to promote wound contraction at an early stage, thereby causing the ulcer to quickly transition to the growth phase without prolonging inflammation.
- Example 1 3T3-L1 adipocytes that had been differentiated into adipocytes for 8 days were prepared.
- the adipocytes were divided into a group with vibrations and a group without vibrations and cultured.
- the vibration groups received 50 Hz vibration at an intensity of 600 mVpp, 800 mVpp, 1000 mVpp, 1500 mVpp, or 2000 mVpp for 40 minutes every day.
- Example 2 The protein GLUT4, which is a sugar transporter, is responsible for the intracellular uptake of blood sugar promoted by insulin and exercise. As shown in FIGS. 8 and 9, GLUT4 is normally stored in the cytoplasmic GLUT4 storage compartment. When cells are stimulated by insulin or exercise, GLUT4 is transported from the storage compartment to the cell membrane. This phenomenon, called GLUT4 translocation, is known to be inhibited by insulin resistance due to type 2 diabetes. GLUT4 translocation can be observed by immunofluorescent staining of GLUT4.
- GLUT4 was present in the cytoplasm in cells to which vibration was not applied, but in cells to which vibration was applied, GLUT4 was localized to the cell membrane.
- GLUT4 was localized at the cell membrane both in cells not given vibration and in cells given vibration.
- wortmannin GLUT4 was present in the cytoplasm in cells to which vibration was not applied, but in cells to which vibration was applied, GLUT4 was localized to the cell membrane.
- GLUT4 was present in the cytoplasm in cells to which vibration was not applied, and GLUT4 was present in the cytoplasm in cells to which vibration was applied.
- FIG. 11 shows a graph of the ratio of the fluorescence intensity in the cell membrane to the fluorescence intensity in the cytoplasm.
- Compound C In the cells to which Compound C was applied, no significant difference was observed between the cells to which vibration was not applied and the cells to which vibration was applied.
- wortmannin In the cells given wortmannin, a significant difference was observed between the cells not given vibration and the cells given vibration. This result also suggests that the effect of vibration on promoting 2-deoxyglucose uptake is due to activation of AMPK by vibration.
- Example 3 Seven-week-old male SD rats were prepared. 55 mg/kg of streptozotocin was intraperitoneally administered to rats, and rats with blood glucose levels of 300 mg/dL or higher 7 and 14 days later were selected as diabetic model rats.
- a full-thickness wound with a diameter of 2 cm was formed on the flank of a diabetic model rat under anesthesia, a dressing material (Foamlite, ConvaTec) was applied to the wound, and the trunk was fixed with gauze. . Dressings were changed daily.
- a vibrator was placed on the gauze above the wound every day for 14 days, and low-frequency vibrations were locally applied to the wound for 40 minutes while the diabetic model rat was put to sleep with isoflurane inhalation anesthesia.
- the period of vibration was 50 Hz, and the intensity of vibration was 0 mVpp, 300 mVpp, 600 mVpp, or 1000 mVpp.
- Example 4 Non-diabetic rats and diabetic model rats were anesthetized and a wound was formed in the same manner as in Example 3, and the total tissue blood volume at the time of anesthesia, the tissue total blood volume at the time of wound formation under anesthesia, and the amount from anesthesia. Total tissue blood volume after awakening was measured with a laser tissue blood oxygen monitor (OMEGAMONITOR, BOM-L1 TR SF, OMEGAWAVE, INC.). As a result, as shown in FIG. 18, in both non-diabetic rats and diabetic model rats, the total tissue blood volume was higher when awake than during anesthesia. This result suggests that changes in total tissue blood volume can be measured more accurately during wakefulness than during anesthesia.
- OMEGAMONITOR laser tissue blood oxygen monitor
- Example 5 A vibrating device having a circuit equivalent to the circuit diagram shown in FIG. 19 was manufactured using a C1034 small vibration motor (Shinsou Denki Co., Ltd.). In the same manner as in Example 3, a full-thickness wound with a diameter of 2 cm was formed on the flank of an anesthetized diabetic model rat. A vibration device was attached to the. Rats were subjected to vibrations of 50 Hz and 600 to 1000 mVpp from a vibrator for 40 minutes a day for 7 days. The rats were not anesthetized and were awake when the vibrations were applied. In the control group, a vibration device was attached to the rat, but the vibration device was not activated.
- FIG. 22 shows a graph of the blood volume of the rat, where the blood volume of the control rat on day 0 after the wound was formed is set to 1.
- the blood volume of rats exposed to vibration increased day by day. Therefore, it was shown that vibration not only temporarily dilated blood vessels, but also increased steady-state blood volume in rats.
- Example 6 In the rats subjected to vibration in Example 3, gene expression was examined on the fourth day. As shown in FIG. 24, the gene marker NOS3 of the NO synthase eNOS related to vasodilation and the angiogenesis marker Vegfa It was rising at a vibration of 1000 mVpp. Furthermore, the decrease in TNF-a suggests that inflammation is suppressed and vascular dynamics are improved.
- Example 7 A diabetic model rat was prepared in the same manner as in Example 3, a full-thickness wound with a diameter of 2 cm was formed on the flank of the diabetic model rat under anesthesia, and a dressing material (Foamlite, ConvaTec) was applied to the wound. The trunk was fixed with gauze. Dressings were changed daily. Thereafter, a vibrator was placed on the gauze above the wound every day for 14 days, and low-frequency vibrations were locally applied to the wound for 40 minutes while the diabetic model rat was put to sleep with isoflurane inhalation anesthesia. The period of vibration was 50 Hz, and the intensity of vibration was 0 mVpp or 1000 mVpp.
- a dressing material Foamlite, ConvaTec
- tissue from the wound was collected, and the collected tissue was immersed in a 10% formalin solution overnight at room temperature to fix the tissue.
- the tissues were then dehydrated using G-Nox (Genostaff), an alternative to ethanol and xylene, and then the tissues were embedded in paraffin to create paraffin blocks of the tissues. Furthermore, the paraffin-embedded tissue was sliced to a thickness of 3 ⁇ m to prepare tissue sections.
- tissue section was immersed in G-Nox for 5 minutes x 3 times to remove paraffin from the tissue section.
- tissue section was immersed in ethanol for 5 minutes x 3 times to remove G-Nox from the tissue section. Thereafter, the tissue sections were washed twice for 5 minutes with purified water. Additionally, tissue sections were incubated in 3% hydrogen peroxide diluted in methanol for 30 minutes to inactivate endogenous peroxidase in the tissue sections. Further, the tissue section was placed in 0.01 mol/L citrate buffer (pH 6.0) and autoclaved at 121° C. for 15 minutes to activate the antigen in the tissue section.
- citrate buffer pH 6.0
- tissue sections were washed with phosphate buffered saline (PBS), and anti-Pentraxin 3 (PTX3) antibody (rabbit-polyclonal, 13797-1- AP, Novus Biological) and tissue sections were reacted overnight. Thereafter, the anti-PTX3 antibody not bound to the antigen was removed from the tissue section, and the tissue section was washed 3 times for 5 minutes with PBS.
- PBS phosphate buffered saline
- PTX3 antibody rabbit-polyclonal, 13797-1- AP, Novus Biological
- tissue sections and horseradish peroxidase (HRP)-labeled anti-rabbit immunoglobulin antibody (Jackson ImmunoResearch) diluted 1000 times were incubated at room temperature for 1 hour.
- the tissue sections were mixed with 0.2 mg/mL 3,3'-diaminobenzidine [DAB] in 0.05 mol/L Tris-HCl buffer (pH 7.4) supplemented with 2% hydrogen peroxide.
- HRP was visualized, and the reaction was stopped with purified water. Thereafter, counterstaining with hematoxylin was performed. After dehydrating the tissue section with ethanol and clearing the tissue section with G-Nox, the tissue section was mounted with a mounting medium.
- the stained tissue sections were observed under a microscope (BZ-X800, Keyence). As a result, as shown in FIG. 27, it was confirmed that the inflammatory marker PTX3 was significantly reduced in the rats to which vibration was applied, compared to the rats to which no vibration was applied.
- Example 8 Tissue sections were prepared in the same manner as in Example 7. However, in this example, endogenous peroxidase in the tissue section was not inactivated. The tissue sections were washed with phosphate buffered saline (PBS), and anti-CD68 antibody (CD68/SR-D1 antibody (ED1), mouse monoclonal, diluted 100 times with bovine serum albumin/phosphate buffered saline) was added. The tissue sections were reacted with NB600-985-0.025 (Novus Biological) and anti-CD163 antibody (CD163, EPR19518, rabbit monoclonal, ab182422, Abcam) overnight.
- CD68 is a marker for M1-type macrophages that promotes inflammatory responses.
- CD163 is a marker for M2-type macrophages that suppresses inflammatory responses.
- tissue sections were washed 3 times for 5 minutes with PBS. Furthermore, tissue sections and 1000-fold diluted green fluorescent dye-labeled anti-rabbit IgG antibody (Alexa Fluor 488, registered trademark, donkey, #711-545-152, Jackson ImmunoResearch) and red fluorescent dye-labeled anti-mouse IgG antibody (Alexa Fluor 594) were used. , Donkey, #715-585-151, Jackson ImmunoResearch) was incubated for 1 hour at room temperature. Next, the tissue section was washed with PBS, the nucleus was stained with a blue fluorescent dye using DAPI, and the tissue section was mounted.
- Green fluorescent dye-labeled anti-rabbit IgG antibody Alexa Fluor 488, registered trademark, donkey, #711-545-152, Jackson ImmunoResearch
- red fluorescent dye-labeled anti-mouse IgG antibody Alexa Fluor 594
- Donkey, #715-585-151, Jackson ImmunoResearch was incubated for
- the stained tissue sections were observed under a microscope (BZ-X800, Keyence).
- Cells that emit green fluorescence are CD163-positive cells.
- Cells that emit red fluorescence are CD68 positive cells.
- the number of CD68-positive cells and the number of CD163-positive cells were measured. Furthermore, the value obtained by dividing the number of CD68-positive cells by the number of CD163-positive cells (M1/M2 macrophage ratio) was calculated. The smaller M1/M2 indicates that the inflammation is improved. As a result, as shown in FIG. 28, M1/M2 was significantly decreased in rats to which vibrations were applied, compared to rats to which no vibrations were applied.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Physics & Mathematics (AREA)
- Surgery (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Animal Behavior & Ethology (AREA)
- Pathology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Dermatology (AREA)
- Hematology (AREA)
- Cardiology (AREA)
- Physiology (AREA)
- Emergency Medicine (AREA)
- Optics & Photonics (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Measuring And Recording Apparatus For Diagnosis (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP23831583.2A EP4548898A1 (en) | 2022-07-01 | 2023-06-29 | Body surface ulcer healing promoting device |
| CN202380051277.4A CN119403529A (zh) | 2022-07-01 | 2023-06-29 | 体表面溃疡治愈促进装置 |
| JP2024530965A JPWO2024005144A1 (enExample) | 2022-07-01 | 2023-06-29 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2022107292 | 2022-07-01 | ||
| JP2022-107292 | 2022-07-01 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2024005144A1 true WO2024005144A1 (ja) | 2024-01-04 |
Family
ID=89382433
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2023/024188 Ceased WO2024005144A1 (ja) | 2022-07-01 | 2023-06-29 | 体表面潰瘍治癒促進装置 |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP4548898A1 (enExample) |
| JP (1) | JPWO2024005144A1 (enExample) |
| CN (1) | CN119403529A (enExample) |
| WO (1) | WO2024005144A1 (enExample) |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008059810A1 (en) * | 2006-11-14 | 2008-05-22 | Kagoshima University | Drug injecting device |
| JP2012517324A (ja) * | 2009-02-12 | 2012-08-02 | パーフュジア メディカル インコーポレーテッド | 患者の循環系の循環を操作するための装置及び方法 |
| JP5252585B2 (ja) | 2006-06-09 | 2013-07-31 | ウェルスタット セラピューティクス コーポレイション | 代謝障害の治療のための化合物 |
| JP5558573B2 (ja) | 2009-09-22 | 2014-07-23 | ヴイライフ サイエンシズ テクノロジーズ プライベート リミテッド | 糖尿病性足潰瘍用の局所用製剤 |
| JP5715326B2 (ja) | 2005-12-29 | 2015-05-07 | セントロ デ インジエニエリア ジエネテイカ イ バイオテクノロジア | 糖尿病による足切断を予防するための、上皮成長因子(egf)を含有する局所組成物の使用 |
| WO2022137756A1 (ja) * | 2020-12-23 | 2022-06-30 | 株式会社ウイルステージ | マッサージ機、および、それを用いた血糖値管理システム |
-
2023
- 2023-06-29 EP EP23831583.2A patent/EP4548898A1/en active Pending
- 2023-06-29 CN CN202380051277.4A patent/CN119403529A/zh active Pending
- 2023-06-29 WO PCT/JP2023/024188 patent/WO2024005144A1/ja not_active Ceased
- 2023-06-29 JP JP2024530965A patent/JPWO2024005144A1/ja active Pending
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5715326B2 (ja) | 2005-12-29 | 2015-05-07 | セントロ デ インジエニエリア ジエネテイカ イ バイオテクノロジア | 糖尿病による足切断を予防するための、上皮成長因子(egf)を含有する局所組成物の使用 |
| JP5252585B2 (ja) | 2006-06-09 | 2013-07-31 | ウェルスタット セラピューティクス コーポレイション | 代謝障害の治療のための化合物 |
| WO2008059810A1 (en) * | 2006-11-14 | 2008-05-22 | Kagoshima University | Drug injecting device |
| JP2012517324A (ja) * | 2009-02-12 | 2012-08-02 | パーフュジア メディカル インコーポレーテッド | 患者の循環系の循環を操作するための装置及び方法 |
| JP5558573B2 (ja) | 2009-09-22 | 2014-07-23 | ヴイライフ サイエンシズ テクノロジーズ プライベート リミテッド | 糖尿病性足潰瘍用の局所用製剤 |
| WO2022137756A1 (ja) * | 2020-12-23 | 2022-06-30 | 株式会社ウイルステージ | マッサージ機、および、それを用いた血糖値管理システム |
Also Published As
| Publication number | Publication date |
|---|---|
| CN119403529A (zh) | 2025-02-07 |
| EP4548898A1 (en) | 2025-05-07 |
| JPWO2024005144A1 (enExample) | 2024-01-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Xiang et al. | In situ regulation of macrophage polarization to enhance osseointegration under diabetic conditions using injectable silk/sitagliptin gel scaffolds | |
| Tan et al. | Improvement of diabetic wound healing by topical application of Vicenin-2 hydrocolloid film on Sprague Dawley rats | |
| Davies et al. | Pathophysiological mechanisms of vascular calcification in end-stage renal disease | |
| Tandara et al. | Oxygen in wound healing—more than a nutrient | |
| Neidert et al. | Enhanced fibrin remodeling in vitro with TGF-β1, insulin and plasmin for improved tissue-equivalents | |
| Blair et al. | Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. | |
| Wu et al. | Stem cell-derived exosomes: a new method for reversing skin aging | |
| Syeda et al. | Prostaglandin transporter modulates wound healing in diabetes by regulating prostaglandin-induced angiogenesis | |
| La Padula et al. | Striae distensae: in vitro study and assessment of combined treatment with sodium ascorbate and platelet-rich plasma on fibroblasts | |
| Sun et al. | Effects of metformin on the osteogenesis of alveolar BMSCs from diabetic patients and implant osseointegration in rats | |
| JP2011523355A (ja) | 生体模倣細胞足場 | |
| Lu et al. | Erythropoietin-activated mesenchymal stem cells promote healing ulcers by improving microenvironment | |
| Cosentino et al. | Effects of blood pressure and glucose on endothelial function | |
| Luo et al. | Polycaprolactone nanofibrous mesh reduces foreign body reaction and induces adipose flap expansion in tissue engineering chamber | |
| Liu et al. | Role of SIRT3 in Angiotensin II-induced human umbilical vein endothelial cells dysfunction | |
| Natorska | Diabetes mellitus as a risk factor for aortic stenosis: from new mechanisms to clinical implications | |
| Yoshizawa et al. | Ex vivo produced human conjunctiva and oral mucosa equivalents grown in a serum-free culture system | |
| WO2024005144A1 (ja) | 体表面潰瘍治癒促進装置 | |
| Joiner et al. | Bone marrow stromal cells from aged male rats have delayed mineralization and reduced response to mechanical stimulation through nitric oxide and ERK1/2 signaling during osteogenic differentiation | |
| Gao et al. | Aldo-keto reductase family 1 member B induces aortic valve calcification by activating hippo signaling in valvular interstitial cells | |
| Danielsen et al. | Platelet-rich fibrin versus albumin in surgical wound repair: a randomized trial with paired design | |
| Petrenko et al. | Topical application of autologous plasma-derived plasminogen accelerates healing of chronic foot ulcers in type 2 diabetes patients | |
| do Nascimento et al. | Both obesity-prone and obesity-resistant rats present delayed cutaneous wound healing | |
| Gracioso Martins et al. | Bioreactors for vocal fold tissue engineering | |
| Wang et al. | Enhancement of Bone Repair in Diabetic Rats with Metformin‐Modified Silicified Collagen Scaffolds |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 23831583 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2024530965 Country of ref document: JP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 202380051277.4 Country of ref document: CN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2023831583 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWP | Wipo information: published in national office |
Ref document number: 202380051277.4 Country of ref document: CN |
|
| ENP | Entry into the national phase |
Ref document number: 2023831583 Country of ref document: EP Effective date: 20250203 |
|
| WWP | Wipo information: published in national office |
Ref document number: 2023831583 Country of ref document: EP |