WO2021005693A1 - 炭化水素燃料から硫黄化合物を除去する吸着剤、吸着剤の製造方法、吸着剤の製造装置、硫黄化合物の除去方法および除去装置 - Google Patents
炭化水素燃料から硫黄化合物を除去する吸着剤、吸着剤の製造方法、吸着剤の製造装置、硫黄化合物の除去方法および除去装置 Download PDFInfo
- Publication number
- WO2021005693A1 WO2021005693A1 PCT/JP2019/027033 JP2019027033W WO2021005693A1 WO 2021005693 A1 WO2021005693 A1 WO 2021005693A1 JP 2019027033 W JP2019027033 W JP 2019027033W WO 2021005693 A1 WO2021005693 A1 WO 2021005693A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- adsorbent
- sulfur
- porous material
- silica
- compound
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/02—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material
- B01J20/10—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising silica or silicate
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/02—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material
- B01J20/10—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising silica or silicate
- B01J20/16—Alumino-silicates
- B01J20/18—Synthetic zeolitic molecular sieves
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/28—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/30—Processes for preparing, regenerating, or reactivating
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/30—Processes for preparing, regenerating, or reactivating
- B01J20/34—Regenerating or reactivating
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J31/00—Catalysts comprising hydrides, coordination complexes or organic compounds
- B01J31/02—Catalysts comprising hydrides, coordination complexes or organic compounds containing organic compounds or metal hydrides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C7/00—Purification; Separation; Use of additives
- C07C7/12—Purification; Separation; Use of additives by adsorption, i.e. purification or separation of hydrocarbons with the aid of solids, e.g. with ion-exchangers
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G25/00—Refining of hydrocarbon oils in the absence of hydrogen, with solid sorbents
- C10G25/02—Refining of hydrocarbon oils in the absence of hydrogen, with solid sorbents with ion-exchange material
- C10G25/03—Refining of hydrocarbon oils in the absence of hydrogen, with solid sorbents with ion-exchange material with crystalline alumino-silicates, e.g. molecular sieves
- C10G25/05—Removal of non-hydrocarbon compounds, e.g. sulfur compounds
Definitions
- the present invention relates to a novel technique for extremely removing sulfur by a synthesized functional porous silica base used as an adsorbent in petroleum and petroleum chemical fuels.
- Catalytic adsorbents remove sulfur from hydrocarbon fuels by superficial absorption processes or oxidation and absorption.
- a wide range of adsorbent bases are available worldwide.
- Patent Documents 1 and 2 describe alumina bases, copper oxide, zinc oxide and manganese.
- Patent Document 5 discloses that zeolites X and Y containing nickel (Ni) or molybdenum (Mo) can be used to remove sulfur compounds from a hydrocarbon stream. In a typical adsorption process, the desorption cycle is used to adsorb contaminants from the feedstock and then separate them from the adsorbent (Patent Documents 6-8).
- Zeolite powder as a strong hygroscopic agent for 3A, 4A and 5A and Zeolite 13X suitable for molecular adsorption are one of the zeolites consumed very much in the laboratory.
- porous materials are divided into three categories (types) based on their pore size ( ⁇ ). When the pore size ⁇ 20 ⁇ , it is a micropore type, when 20 ⁇ > pore diameter> 500 ⁇ , it is a mesopore, and when the pore diameter> 500 ⁇ , it is a macropore.
- Patent Document 9 high silica zeolite is used to remove sulfur from the naphtha stream.
- the amount of sulfur is reduced by using a porous zeolite-based catalyst with a different percentage of silica than the passage of naphtha flow.
- the present invention further develops such a conventional catalytic adsorbent to produce an adsorbent capable of removing sulfur compounds in a hydrocarbon fuel at room temperature and ambient pressure (atmospheric pressure), and an adsorbent capable of easily producing an adsorbent. It is an object of the present invention to provide a method, a manufacturing apparatus, a removing method and a removing apparatus for removing a sulfur compound in a hydrocarbon fuel at room temperature and ambient pressure (atmospheric pressure) using this adsorbent.
- a new super adsorbent synthesized by using a porous nanosilica adsorbent having different pore size distributions by the coprecipitation method was formed.
- Surface modifiers used to form porous and functional groups are used to adsorb sulfur from petroleum compounds.
- These new super adsorbents can adsorb categories of sulfone petroleum compounds, including naphtha, gasoline, gasoline and the like. These adsorbents are synthesized in one step and do not require sophisticated equipment.
- the adsorption properties of the porous silica base and the presence of fragile sulfur functional groups on the surface of the adsorbent are simultaneously used to remove sulfur compounds from petroleum compounds at ambient temperature.
- the adsorbent of the present invention is produced by a coprecipitation method and a surface porous organic modifier with various zeolites and silicate compounds having high porosity and ability to remove sulfur compounds.
- the surface of the porous material containing silica is functionalized to form a catalyst, which produces naphtha, diesel fuel, gasoline, jet fuel oil and coal liquor (condensate) and sulfur.
- a liquid hydrocarbon fuel containing similar petroleum products is used as the liquid fuel to be treated.
- the adsorbent has the ability to break the mercaptan and thiol bonds in the liquid hydrocarbon fuel by the catalyst, convert them into oxides, and adsorb the sulfur compounds remaining from the surface.
- the presence of thiol and mercaptan functional groups in the fuel produces a silica-based compound adsorbent suitable for adsorbing them on the surface, depending on the fuel concentration and density.
- the release of the fuel sulfur compound in the form of an oxide and the catalytic adsorption of the residual compound on a porous silica-based nanoadsorbent base significantly reduces the unwanted odor caused by the sulfur compound.
- adsorbents can adsorb sulfur atoms from compounds found in hydrocarbon fuels such as thiophene and thiols at ambient temperature and standard pressure (atmospheric pressure). They can be easily added and separated from hydrocarbon fuels in a simple way.
- the level of sulfur reduction in hydrocarbon fuels depends on the concentration of sulfur and the amount of adsorbent (more adsorbent if there is more silica in the structure), but the chemistry and fluidity of the fuel. The nature is different.
- a multi-feeder tank (first feeder 14, second feeder 15) having a liquid injection capacity into a main tank (reaction vessel 11) is used.
- the main tank is equipped with a stirrer (operating speed: 100 to 700 rpm) 16 capable of making its blades spiral or disk-shaped.
- the surface modifier is added to the system (water supply pipe 13) after mixing with water, with reference to FIG. 1, and the synthesis process is carried out according to the filling level in the synthesis reservoir (main tank). After washing and drying the sample in the second tank, a second surface modifier (hydrogen peroxide, CTAB, M2P (C 6 H 16 O 12 P 12) to functionalize the surface of the porous silica ), MP (C 24 N 4 O 15 H 54 ), sulfuric acid, urea, thiourea, mercaptopropionic acid, 3-ethanolamine, etc.) followed by impregnation. In the next step, the sample is transferred to the dryer. The resulting vapor is collected by submersion or redistillation to prevent air pollution. It is recommended to use preheated adsorbents in hydrocarbon fuels, as drying of the applied adsorbent is one of the most important issues.
- the preheating temperature is in the range of 60 ° C to 250 ° C, which depends on the ambient humidity at this stage.
- a mixer to shorten the adsorption process period.
- This mixer can be used as a continuous cycle or rotary mixer.
- the process of adsorbing sulfur from the hydrocarbon fuel is followed by the separation of the adsorbent from the fuel. Two separation methods are implemented for this purpose.
- the adsorbent settles to the bottom of the tank 31 due to its own weight over time, and the fuel is placed on it.
- the pump 32 can easily separate the fuel.
- the adsorbent is separated from the fuel by filtration and transferred to a recycling or burial stage. Then, after the adsorption step is complete, after the adsorption step, the adsorbent returns to the working cycle again by the reduction step with acetone or citric acid, nitric acid, formic acid, hydrochloric acid, sulfuric acid and hydrogen peroxide or even amine.
- Compounds such as triethanolamine have a 1: 2 and 1: 1 ratio. After re-reduction, the initial efficiency of the adsorbent will not be achieved.
- the sulfur-containing fuel is pumped onto a porous filter (called an adsorption screen) manufactured from the adsorbent and passed through a pressure pump.
- adsorption screen is fixed and the fuel moves between these ceramic functionalized filters to separate sulfur. After a period of time, these filters are replaced and reused in the previous process.
- a porous nanosilica catalyst adsorbent was produced by the coprecipitation method.
- surface modifiers such as hydrogen peroxide, CTAB, M2P, MP, sulfuric acid, urea, thiourea, mercaptopropionic acid and 3-ethanolamine
- the modified surface is functionalized and catalyzed.
- the sulfur compound was functionalized to adsorb and remove the sulfuric acid compound from the hydrocarbon fuel.
- the adsorption efficiency of the catalyst can be increased.
- adsorbent An inexpensive material and a commercially available silica precursor were used to manufacture this product (adsorbent).
- the functional groups on the silica base of the porous nanosilica were formed by using acidic and oxidizing and amino solutions to improve the adsorptive capacity.
- the obtained adsorbent can adsorb sulfur compounds from hydrocarbon fuels.
- the highest level of sulfur adsorption from sulfur-containing hydrocarbon fuels was about 79-100 mg per liter of adsorbent for the intended catalyst (when the adsorbent was filled in a 1 liter container). This catalyst was able to function at ambient temperature and atmospheric pressure.
- the adsorbent is effectively mixed with the hydrocarbon fuel by using an industrial mixer and mixer.
- the catalyst adsorbent
- the catalyst was easily separated from the hydrocarbon fuel by filtering and depositing at the bottom of the tank (see Figure 2).
- adsorbents according to the invention provide zeolite nanoparticles with an acidic surface that oxidizes sulfur compounds.
- the properties of this acidic surface were made by surface functionalizing the nanoparticles with specific hydrogen peroxide and various acid ratios.
- the acidity of the surface depends on the preparation method, dehydration temperature, structure and Si / Al ratio.
- Sodium cations are often replaced with ammonium cations to form Bronsted sites (acid spots) by the ion transfer method.
- ammonium gas is extracted from the zeolite, and protons remain and are converted into Bronsted-acid sites. These Bronsted acid points are unstable and are converted to temporary Bronsted acid points when dehydrated at temperatures above 550 ° C.
- the adsorbent according to the invention covers all mesoporous and aluminate-based adsorbents such as zeolite X-type, Y-type, and (Me) xSiOy compounds.
- the catalyst surface of these adsorbents can adsorb active metals such as iron, silver, molybdenum, nickel and copper.
- the adsorbent may be referred to as a catalyst, and both may be described together.
- the surface modifier may be referred to as a surfactant, and both may be described together.
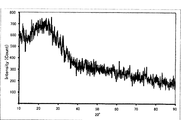
- FIG. 6 is a synthetic adsorbent-based X-ray diffraction pattern diagram in an amorphous phase. Scanning electron microscope (SEM) photograph of the adsorbent. Scanning electron microscope (SEM) photograph in the form of zeolite nanoparticles impregnated with silver ions.
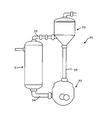
- FIG. 1 is a block diagram having a schematic configuration showing an embodiment of an adsorbent manufacturing apparatus.
- the manufacturing apparatus 10 supplies the water in the water storage tank 12, for example, deionized water, to the reaction vessel 11 via the water supply pipe 13.
- the first feeder 14 contains a porous material containing silica, for example, porous nanosilica
- the second feeder 15 contains a surface modifier.
- supply pipes 14a and 15b are connected to the water supply pipe 13, respectively.
- a predetermined amount of silica is supplied from the first feeder 14 to the reaction vessel 11 to which the ionized water is supplied from the supply pipe 14a via the water supply pipe 13 and mixed, and the water and silica filled in the reaction vessel 11 are mixed. Is stirred by the stirrer 16.
- the second feeder 15 supplies the surface modifier to the reaction vessel 11 via the water supply pipe 13 via the supply pipe 15b, mixes and stirs.
- the adsorbent is synthesized in the stirred and mixed solution in the reaction vessel 11, and the surface is functionalized.
- the synthesized adsorbent is moved to the dryer 19 by the liquid feed pump 17 via the liquid supply pipe 18.
- a one-way valve 20 that allows liquid to be fed to the dryer 19 but prevents backflow is arranged between the liquid feed pump 17 and the dryer 19.
- the dryer 19 allows the solution to be circulated to the dryer 19 by the bypass pipe 21.
- the dried adsorbent is transferred to the storage tank 22.
- a feeder (not shown) for accommodating the acid may be provided, and a supply pipe (not shown) may be connected to, for example, a water supply pipe 13 to add the acid to the reaction vessel 11.
- FIG. 2 is a block diagram showing an embodiment of a removal device that mixes a hydrocarbon fuel adsorbent and a hydrocarbon fuel to remove sulfur compounds.
- the mixing device 30, which is a removing device, has a fuel tank 31 for accommodating hydrocarbon fuel, a mixing pump 32, and an adsorbent feeder 33 for accommodating an adsorbent.
- the mixing pump 32 is arranged at the lowest stage position
- the adsorbent feeder 33 is arranged at the uppermost stage position
- the fuel tank 31 is arranged at the middle stage position.
- the lower part of the fuel tank 31 and the mixing pump 32 are connected by the first pipe 34
- the mixing pump 32 and the adsorbent feeder 33 are connected by the second pipe 35
- the fuel tank 31 and the adsorbent feeder 33 are connected by the third pipe 36. Connect with to form a circulation path.
- the mixing pump 32 When the mixing pump 32 is started and the hydrocarbon fuel and the adsorbent are circulated in the circulation path, the adsorbent and the hydrocarbon fuel are mixed, and the hydrocarbon is formed by a catalyst formed on the surface of the porous silica constituting the adsorbent. Hydrocarbon compounds in the fuel are converted to oxides and adsorb the remaining hydrocarbon compounds.
- TEOS tetraethyl orthosilicate: tetraethyl orthosilicate
- sodium silicate Sodiumsilicate
- mineral silica mineral silica (mineralsilica)
- An amorphous silica precursor is used as an initial material for forming the porous silica.
- a fluoride compound such as sodium fluoride or lithium fluoride can be used to form pores on the silica base.
- the advantages of using this method are the low cost of synthesis and the use of silica soil resources, but it should be considered that the resulting silica containing unexpected metal cation impurities was synthesized in a complex process. With this method, it was not possible to control the type and porosity using a combination of sodium hydroxide solution and Flora, and the adsorption efficiency was very low.
- tetraethyl orthosilicate (TEOS) and sodium silicate can be applied to eliminate the above conventional drawbacks.
- TEOS tetraethyl orthosilicate
- the tetraethyl orthosilicate (TEOS) solution has maximum production efficiency. It is possible to produce 1 kg of high-purity silica from 1 kg of TEOS on a nanoscale. The formation of pores in silica made from TEOS is much easier and operably operational according to this combination terminology.
- the method of the present invention it is carried out by using sodium silicate in mass production in order to achieve simultaneous control of material quality and porosity for synthesizing a porous adsorbent base.
- the ratio of SiOx to NaO varies at a rate of 15-40%.
- Using a precursor with the highest proportion of SiOx is more effective for the synthesis of silica.
- the synthesis process was carried out by adding to a non-acidic metal tank (stainless steel L316) or a plastic tank in a non-stoichiometric ratio of a silica source and a different acid.
- a non-acidic metal tank stainless steel L316
- a plastic tank in a non-stoichiometric ratio of a silica source and a different acid.
- different values of deionized water and silica sources were added to the tank in a ratio of 1: 1, 1: 2 to 1: 8 and in a velocity range of 300 to 1200 rpm. Stirred and mixed. After 15 minutes at 500 rpm at the optimum speed for mixing, the primary modifier was added to the above achieved solution.
- cetyltrimethylammonium bromide, hexadecyltrimethylammonium bromide (CTAB), D-mannitol-1,6-diphosphate (M2P), MP, urea, thiourea, sodium dodecyl sulfate (SDS) and sodium hexametaphosphate (SHMP) ) Is the most common of the most important modifiers.
- CAB hexadecyltrimethylammonium bromide
- M2P D-mannitol-1,6-diphosphate
- MP urea
- thiourea sodium dodecyl sulfate
- SHMP sodium hexametaphosphate
- Mercaptopropionic acid and 3-ethanolamine can also be applied as supplements.
- the approximate number of pores formed on the silica was determined by performing several tests with modifiers of various values in the range of 2.0-10% by weight. In some cases, both urea modifiers can be used in combination with others to control particle size, porosity and further reduce the amount of water consumed. The amount of urea used in this case can vary between 0.2-5% by weight of the raw material base, with the best percentage being about 2-3% by weight. Pentanols and propanols with different weight ratios ranging from 1 to 10 percent were used to improve and control the properties and size of the nanoparticles.
- FIG. 3 is a silica synthetic adsorbent-based Fourier spectrum diagram with absorption bands of Si-O-Si (1099 cm -1 ), Si-OH (908 cm -1 ) asymmetric vibrations, and Si-O-Si (800 cm). It arises from the symmetrical vibration of -1 ). Adsorbed water (H-OH, expansion and contraction vibration), residual intermolecular water (H-OH bending vibration), and moisture (H-OH bending vibration) in the sample are 3500 to 3400 and 1600 (H-OH bending vibration). It belongs to the vibration of cm -1 ).
- FIG. 4 is a synthetic adsorbent-based X-ray diffraction pattern diagram in an amorphous phase
- FIG. 5 is a scanning electron microscope (SEM) photograph of a nanosilica-based catalyst.
- the condensed phase of silica was dissolved and precipitated, and the pH of the solution was adjusted to acidic with various weight ratios of hydrochloric acid or acetic acid.
- the addition of acid and acidity are directly related to the amount of silica particles produced.
- the highest efficiency and particle size were due to chlorine (HCl) and small amounts of nitric acid, and for silica precursors due to a 1/2: 1 ratio. The best results occurred in a ratio of 1-1.
- the presence of chlorine at this stage helped to adsorb sulfur compounds.
- the obtained adsorbent was washed 3 to 5 times and dried at a temperature of about 80 to 120 ° C. This has the ability to adsorb sulfur from hydrocarbon fuels. Alkaline media can also be used to form silica precipitates, but this is not highly efficient.
- the type of surface modifier was investigated based on the type of silicate-based synthesis.
- the best precipitation conditions are hydrochloric acid and 2-4% by weight nitric acid.
- the pH of the solution needs to be adjusted to about 2-5.
- This synthetic method was performed for all surface modifiers.
- Figure 1 shows the manufacturing method and manufacturing equipment for this catalyst.
- a porous material containing silica such as porous nanosilica a synthetic porous material containing a surface organic metal surfactant (organic metal surfactant or amphoteric tenside), a surface modifier is added. No process is required and the catalyst is preformed on the surface.
- This synthetic porous material includes organically modified silica containing any functionalizing material of any of methyl, amino and sulfone and carboxyl groups.
- Example 1 Based on the above production method, steady-state silica having 2 to 15 wt% of surface modifier, hexadecyltrimethylammonium bromide (CTAB), was synthesized on a raw material basis.
- the adsorbent obtained from the above production method was subjected to a sulfur (S) adsorption test at an ambient temperature using 100 cc of fuel containing 100 mg of sulfur. The test results are shown in Table 1.
- Example 1 a sulfur adsorption test was conducted using adsorbents having different amounts of CTAB (2%, 15%).
- the adsorbent samples of sample numbers 1 to 4 had adsorbent amounts (wt%) of 2.0, 4.0, 8.0, and 10.0.
- Example 2 Based on the above production method, steady-state silica pregelatin having 2 to 15 wt% of surface modifier M2P on a raw material basis was synthesized.
- the adsorbent obtained from the above production method was subjected to a sulfur (S) adsorption test at an ambient temperature using 100 cc of fuel containing 100 mg of sulfur. The test results are shown in Table 2.
- S sulfur
- a sulfur adsorption test was conducted using adsorbents having different amounts of M2P (2%, 15%).
- the adsorbent samples of sample numbers 21 to 24 had adsorbent amounts (wt%) of 2.0, 4.0, 8.0, and 10.0.
- Example 3 Based on the above production method, steady-state silica having 2 to 15 wt% urea, which is a surface modifier, was synthesized on a raw material basis.
- the adsorbent obtained from the above production method was subjected to a sulfur (S) adsorption test at an ambient temperature using 100 cc of fuel containing 100 mg of sulfur.
- S sulfur
- Table 3 The test results are shown in Table 3.
- a sulfur adsorption test was conducted using adsorbents having different amounts of urea (2%, 15%).
- a sulfur adsorption test was performed on each of the adsorbent samples of sample numbers 31 to 34.
- the adsorbent samples of sample numbers 31 to 34 had adsorbent amounts (wt%) of 2.0, 4.0, 8.0, and 10.0.
- Example 4 Based on the above production method, steady-state silica having 2 to 15 wt% of surface modifier thiourea on a raw material basis was synthesized.
- the adsorbent obtained from the above production method was subjected to a sulfur (S) adsorption test at an ambient temperature using 100 cc of fuel containing 100 mg of sulfur.
- S sulfur
- Table 4 The test results are shown in Table 4.
- a sulfur adsorption test was carried out using adsorbents having different amounts of thiourea (2%, 15%).
- the adsorbent samples of sample numbers 41 to 44 had adsorbent amounts (wt%) of 2.0, 4.0, 8.0, and 10.0.
- Example 5 Based on the above production method, steady-state silica pregelatin having 2 to 15 wt% sodium dodecyl sulfate (SDS), which is a surface modifier, was synthesized on a raw material basis.
- SDS sodium dodecyl sulfate
- the adsorbent obtained from the above production method was subjected to a sulfur (S) adsorption test at an ambient temperature using 100 cc of fuel containing 100 mg of sulfur. The test results are shown in Table 5.
- S sulfur
- a sulfur adsorption test was performed using adsorbents having different amounts of SDS (2%, 15%). For the samples of sample numbers 51 to 54, the amount (wt%) of the adsorbent was 2.0, 4.0, 8.0, 10.0.
- Example 6 Based on the above production method, steady-state silica having 2 to 15 wt% of pentanol, which is a surface modifier, was synthesized on a raw material basis.
- the adsorbent obtained from the above production method was subjected to a sulfur (S) adsorption test at an ambient temperature using 100 cc of fuel containing 100 mg of sulfur.
- S sulfur
- Table 6 The test results are shown in Table 6.
- a sulfur adsorption test was conducted using adsorbents having different amounts of pentanol (2%, 15%). For the samples of sample numbers 61 to 64, the amount (wt%) of the adsorbent was 2.0, 4.0, 8.0, 10.0.
- Zeolite nanocrystals are often synthesized using a homogeneous transparent solution.
- the synthetic method results in the formation of a colloidal zeolite suspension with a uniform distribution and particle size of less than 100 nm.
- Subcolloids or separate zeolite particles are present in clear solution and prior to zeolite suspension formation.
- Appropriate hypersaturation conditions and spatial stability of the initial core are one of the factors that cause zeolite nanocrystals to form and avoid particle accumulation and grinding.
- the synthesis of zeolite nanoparticles is first carried out by dissolving a stoichiometrically liquid sodium silicate with a higher value of TEOS in deionized water. This is used as the first solution.
- a proportional amount of NaOH was dissolved in deionized water.
- Different amounts of sodium aluminate (NaAlO 2 ) were added to this second solution.
- the two solutions, the first solution and the second solution, were mixed and stirred using a magnetic stirrer for 30 minutes.
- the obtained solution was transferred to an autoclave having a volume of 50 ml.
- the resulting solution was stored in an oven at 120 ° C. for about 1 hour and used for synthesis in the next step.
- Zeolite Y having a Si / Al ratio of 1.5 is an opaque gel with the following composition: 17 (Na 2 O): 1 (Al 2 O 3 ): 12.80 (SiO 2 ): 975 (H 2 O). Synthesized from. The gel was prepared by mixing with stirring NaOH, Al a (OH) 3 and H 2 O basic clear solution in colloidal nanosilica particles and polypropylene bottles.
- FIG. 6 shows a micrograph (SEM) of synthetic zeolite, showing the morphology of zeolite nanoparticles impregnated with silver ions. Zeolite particles are spherical and have a size of less than 100 nanometers.
- Addition of small amounts of material can affect nucleation and crystallization.
- the addition of seeds to the reaction mixture improves the crystallization process.
- the pores can be organized by adding colloidal particles such as polystyrene that act as a template and nucleating agent.
- the particle size of polystyrene varies from 4 microns to 150 microns.
- catalytically adsorbed sulfur compounds are measured for 100 ml fuel containing 100 ppm sulfur compound (S), such as thiols and mercaptans. did.
- Example 7 By the above-mentioned production method (B), zeolite Y and zeolite X, which are adsorbents, were synthesized using 2 wt% of surface modifiers SDS, pentanol, M2P, urea, and CTAB on a raw material basis. The test results are shown in Table 7. This test was performed at ambient temperature and pressure for 20 minutes. The amounts of the adsorbents (catalysts) for Zeolite X and Zeolite Y were kept constant.
- the best surfactant (surface modifier) for sulfur adsorption is CTAB, and there is no difference between the two types of zeolite, zeolite Y and zeolite X, which are adsorbents.
- the obtained solution is refluxed at 80 to 100 ° C. for 2 to 5 hours to hydrolyze TEOS, and the liberated silica becomes a substantially metal cation.
- Hydrochloric acid was used to initiate the condensation of sodium silicate.
- pore formation was achieved by applying various amounts of pentanol, isopropanol, CTAB and sodium dodecyl sulfate.
- the sample was dried over a temperature range of 100-250 ° C.
- This method can also be carried out using other silicate sources such as sodium silicate.
- Several experiments were planned and repeated under equivalent conditions to determine the adsorption percentage and to detect the best sample.
- Example 8 In the experiment, 100 ml of fuel containing 100 ppm sulfur compound containing thiol and mercaptan was used. The experiment was carried out at ambient temperature and pressure for 20 minutes. The amount of adsorbent (catalyst amount) was constant in all tests. The test results are shown in Table 8. According to the experiments carried out, the average adsorption amount in the presence of urea, thiouric acid and M2P modifier was in the range of 30 to 32 mg per liter.
- FIG. 2 shows a method of using an adsorbent (catalyst) composed of these silicate compounds.
- a silicate) adsorbent is used as a sample, and as a surface modifier for each adsorbent, a sulfur compound (S) containing 2 wt% of a surface modifier (urea, pentanol, SDS, M2P, CTAB) on a raw material basis. The adsorption rate was measured. The test results are shown in Table 8.
- the porous material containing silica is composed of a porous silicate compound, a zeolite compound shown in Example 14 described later, a forsterite compound, zinc silicate mineral (Zn 2 SiO 4 ), and a small amount of zinc ore. Willemite compounds can be used.
- the adsorbent using CTAB as the surface modifier (surfactant) had a high adsorption rate for all the samples.
- a new method for surface functionalization of silicate-based nanoadsorbents uses an oxidizing solution of the resulting porous nanosilica-based adsorbent (catalyst), such as hydrogen peroxide, sodium hydroxide and acidic solutions, such as sulfuric acid, nitric acid, and even acetic acid and amino compounds. It was used to increase the sulfur adsorbent level of porous silica after drying for the first time to improve and enhance adsorptivity.
- catalyst an oxidizing solution of the resulting porous nanosilica-based adsorbent (catalyst), such as hydrogen peroxide, sodium hydroxide and acidic solutions, such as sulfuric acid, nitric acid, and even acetic acid and amino compounds. It was used to increase the sulfur adsorbent level of porous silica after drying for the first time to improve and enhance adsorptivity.
- the surface of the adsorbent can be functionalized with an organic acid and an oxidizing agent such as hydrogen peroxide or hydrazine hydrate to form a -OH or Si-O-Si group.
- an oxidizing agent such as hydrogen peroxide or hydrazine hydrate to form a -OH or Si-O-Si group.
- the methods described above help break and adsorb thiol-mercaptan bonds in hydrocarbon fuels. That is, it transforms the surface of the catalyst into a small reactor to break and adsorb thiol bonds.
- Sulfuric acid and hydrogen peroxide also oxidize sulfur compounds such as mercaptans and thiols.
- Oxidation process occurs on the surface due to the reaction between the functional group and active oxygen.
- the SOx gas generated from the hydrocarbon fuel is discharged as bubbles, and the odor of sulfur in the hydrocarbon fuel is significantly reduced.
- the remaining sulfur is also adsorbed by the silicon-based porous nanoadsorbent (catalyst). If it is also possible to use certain substances to produce trivalent oxygen, the efficiency of adsorption and destruction of sulfur compounds can be increased in hydrocarbon fuels.
- the drying temperature is 120 ° C to 150 ° C, and the ratio of oxidizing acids varies between 1 to 2 and 1 to 3 or 1 to 3.4.
- the best efficiency in this method is for 1: 2 (1/2) (1/3), (2/1), (1/1), (3/1) combinations in acid and hydrogen peroxide compounds.
- the resulting silica can be marketed in the industry as one of the important marketable adsorbents in the acid cleaning step.
- Example 9 The test results of Example 9 are shown in Table 9.
- a constant catalyst sample collected in 60 minutes was collected in 60 minutes at a set temperature of 25 ° C.
- the concentration of unknown sulfur in the fuel is 100 mg / l.
- the adsorbent samples numbers 70-79 of these proportions are different, the sulfur compounds in the fuel
- An experiment was conducted to measure the adsorption ratio of (S) at 100 ppm. The ratio of formic acid and hydrogen peroxide indicates wt% of the solution of formic acid and hydrogen peroxide.
- Example 10 In Example 10, a constant catalyst sample collected in 60 minutes was collected in 60 minutes at a set temperature of 25 ° C. The concentration of unknown sulfur in the fuel is 100 mg / l. The test results are shown in Table 10. Sulfuric acid with a concentration of 60% is used as the organic acid, and hydrogen peroxide (H 2 O 2 ) with a concentration of 96% is used, and the adsorbents of sample numbers 80 to 89 having different ratios thereof are sulfur compounds in the fuel. An experiment was conducted to measure the adsorption ratio of (S) at 100 ppm. The ratio of sulfuric acid to hydrogen peroxide indicates wt% of the solution of formic acid and hydrogen peroxide.
- Example 11 In Example 11, a constant catalyst sample collected in 60 minutes was collected in 60 minutes at a set temperature of 25 ° C. The concentration of unknown sulfur in the fuel is 100 mg / l. The test results are shown in Table 11. Nitric acid with a purity of 99% is used as the organic acid, and hydrogen peroxide (H 2 O 2 ) with a purity of 96% is used, and the sulfur compounds in the fuel for the adsorbents of sample numbers 90 to 99 having different ratios thereof. An experiment was conducted to measure the adsorption ratio of (S) at 100 ppm. The ratio of nitric acid and hydrogen peroxide indicates wt% of the solution of nitric acid and hydrogen peroxide.
- Example 12 In Example 12, a constant catalyst sample collected in 60 minutes was collected in 60 minutes at a set temperature of 25 ° C. The concentration of unknown sulfur in the fuel is 100 mg / l. The test results are shown in Table 12. Using 99% pure triethanolamine as organic acids, also using the purity of 96% hydrogen peroxide (H 2 O 2), the adsorbent sample numbers 100-109 that these proportions are different, the fuel of the An experiment was conducted to measure the adsorption ratio of the sulfur compound (S) at 100 ppm. The ratio of triethanolamine and hydrogen peroxide indicates wt% of the solution of triethanolamine and hydrogen peroxide.
- Example 13 In Example 13, a constant catalyst sample collected in 60 minutes was collected in 60 minutes at a set temperature of 25 ° C. The concentration of unknown sulfur in the fuel is 100 mg / l. The test results are shown in Table 13. Hydrochloric acid with a purity of 37% is used as the organic acid, and hydrogen peroxide (H 2 O 2 ) with a purity of 96% is used. A test was conducted to measure the adsorption rate of (S) at 100 ppm. The ratio of hydrochloric acid and hydrogen peroxide indicates wt% of the solution of hydrochloric acid and hydrogen peroxide.
- Example 14 Test Example 14 was performed to determine the optimal ratio SiO 4 silicate-based. Zeolite nanocatalyst and silicate-based efficiency level measurements were achieved by using optimal ratios obtained under equal and similar conditions. The test results of Example 14 are shown in Table 14. The test took constant catalyst samples at 60 minutes and 25 ° C. The concentration of unknown sulfur in the fuel is 100 mg / l.
- Table 14 shows a combination of zeolite XY (ZeoliteXY) (complex), a combination of barium (Ba) and orthosilicate (SrSiO 4 ) (complex), and magnesium (composite) as adsorbent samples of the Keisan salt base (SiO 4 ). It is a combination of Mg), zinc (Zn) and zircon (Zr).
- hydrochloric acid and H 2 O 2 are sample 115
- sulfuric acid and H 2 O 2 are sample 83
- nitric acid and H 2 O 2 are sample 99, and triethanolamine.
- H 2 O 2 are used as sample 105, and the one having the best adsorption rate in each experimental example is used.
- Table 14 shows that the best acid combination is sulfuric acid / H 2 O 2 with the highest adsorption in the Mg / Zn / ZrSiO 4 complex.
- the fuel can be pumped through the synthetic catalyst in a fixed state.
- This method does not require catalyst separation, but the catalyst efficiency is reduced and the sulfur adsorption process can be carried out in a period of 15-45 times.
- Nanocatalyst-based waste silica recycling After absorbing sulfur from the fuel, the color of the catalyst changes from brown to black, depending on the amount of sulfur absorbed and the dye used in the fuel. After the porous silica-based nanocatalysts are completely saturated, they can be recycled and reused.
- the waste nanocatalyst is transferred to the steel tank.
- the catalyst can be reduced by heat treatment where the required temperature is about 180-250 ° C., and the annealing time after heating depends on the color of the catalyst and the amount of residual sulfur.
- the tank material must be 316 low carbon steel grade.
- the catalyst is usually slowly cooled until the color of the catalyst turns white. This method releases a variety of dangerous gases, including SOx, which must be neutralized within the substrate of water. This can result in the production of by-products such as sulfuric acid at concentrations of 30-50%.
- an acetone solution such as ethyl acetate, acetone, acetylacetone or a solution capable of dissolving sulfur can be used.
- xylene and toluene is effective in current methods, but the acetone compound only removes sulfur from the catalyst surface instead of dissolving it.
- recycled catalysts show low initial yields.
- the catalyst can be functionalized and reused.
- 1 kg of these nanocatalysts 1 kg of acetone or toluene is required.
- the catalyst can be used after functionalization and drying. According to the experiments carried out, the yield of this product is high over up to 10 working cycles and then decreases.
- these porous nanocatalysts can be used in a variety of industries, including agriculture, glass production, or the production of ceramic pieces and building materials.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Analytical Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Inorganic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Crystallography & Structural Chemistry (AREA)
- Materials Engineering (AREA)
- Water Supply & Treatment (AREA)
- Solid-Sorbent Or Filter-Aiding Compositions (AREA)
- Production Of Liquid Hydrocarbon Mixture For Refining Petroleum (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/JP2019/027033 WO2021005693A1 (ja) | 2019-07-08 | 2019-07-08 | 炭化水素燃料から硫黄化合物を除去する吸着剤、吸着剤の製造方法、吸着剤の製造装置、硫黄化合物の除去方法および除去装置 |
| JP2021530381A JPWO2021005693A1 (enExample) | 2019-07-08 | 2019-07-08 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/JP2019/027033 WO2021005693A1 (ja) | 2019-07-08 | 2019-07-08 | 炭化水素燃料から硫黄化合物を除去する吸着剤、吸着剤の製造方法、吸着剤の製造装置、硫黄化合物の除去方法および除去装置 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2021005693A1 true WO2021005693A1 (ja) | 2021-01-14 |
Family
ID=74114460
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2019/027033 Ceased WO2021005693A1 (ja) | 2019-07-08 | 2019-07-08 | 炭化水素燃料から硫黄化合物を除去する吸着剤、吸着剤の製造方法、吸着剤の製造装置、硫黄化合物の除去方法および除去装置 |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JPWO2021005693A1 (enExample) |
| WO (1) | WO2021005693A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113340765A (zh) * | 2021-06-25 | 2021-09-03 | 西藏大学 | 一种分子筛材料吸附性能检测装置及方法 |
| KR102739300B1 (ko) * | 2023-11-14 | 2024-12-05 | 주식회사 블루랩스 | 굴패각을 이용한 흡착제 조성물의 제조방법 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH11147039A (ja) * | 1997-11-18 | 1999-06-02 | Tonen Corp | 水素化処理用触媒および該水素化処理用触媒を使用する水素化脱硫方法 |
| JP2008501522A (ja) * | 2004-06-08 | 2008-01-24 | エクソンモービル リサーチ アンド エンジニアリング カンパニー | メソ細孔性触媒を用いるfcc方法 |
| JP2011518101A (ja) * | 2008-03-31 | 2011-06-23 | イエフペ エネルジ ヌヴェル | 特定のサイズの球状粒子から作られたメソ構造化アルミノケイ酸塩材料 |
| JP2013521212A (ja) * | 2010-03-02 | 2013-06-10 | キング アブドゥーラ ユニバーシティ オブ サイエンス アンド テクノロジー | 高表面積の繊維状シリカナノ粒子 |
-
2019
- 2019-07-08 WO PCT/JP2019/027033 patent/WO2021005693A1/ja not_active Ceased
- 2019-07-08 JP JP2021530381A patent/JPWO2021005693A1/ja active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH11147039A (ja) * | 1997-11-18 | 1999-06-02 | Tonen Corp | 水素化処理用触媒および該水素化処理用触媒を使用する水素化脱硫方法 |
| JP2008501522A (ja) * | 2004-06-08 | 2008-01-24 | エクソンモービル リサーチ アンド エンジニアリング カンパニー | メソ細孔性触媒を用いるfcc方法 |
| JP2011518101A (ja) * | 2008-03-31 | 2011-06-23 | イエフペ エネルジ ヌヴェル | 特定のサイズの球状粒子から作られたメソ構造化アルミノケイ酸塩材料 |
| JP2013521212A (ja) * | 2010-03-02 | 2013-06-10 | キング アブドゥーラ ユニバーシティ オブ サイエンス アンド テクノロジー | 高表面積の繊維状シリカナノ粒子 |
Non-Patent Citations (1)
| Title |
|---|
| WANG, DANHONG ET AL.: "Oxidative desulfurization using ordered mesoporous silicas as catalysts", JOURNAL OF MOLECULAR CATALYSIS A: CHEMICAL, vol. 393, 12 June 2014 (2014-06-12), pages 47 - 55, XP029041289, DOI: 10.1016/j.molcata.2014.05.026 * |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113340765A (zh) * | 2021-06-25 | 2021-09-03 | 西藏大学 | 一种分子筛材料吸附性能检测装置及方法 |
| KR102739300B1 (ko) * | 2023-11-14 | 2024-12-05 | 주식회사 블루랩스 | 굴패각을 이용한 흡착제 조성물의 제조방법 |
Also Published As
| Publication number | Publication date |
|---|---|
| JPWO2021005693A1 (enExample) | 2021-01-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Chen et al. | Amine modified nano-sized hierarchical hollow system for highly effective and stable oxidative-adsorptive desulfurization | |
| Guan et al. | Preparation of mesoporous Al-MCM-41 from natural palygorskite and its adsorption performance for hazardous aniline dye-basic fuchsin | |
| Shen et al. | A nanocellulose template strategy for the controllable synthesis of tungsten-containing mesoporous silica for ultra-deep oxidative desulfurization | |
| RU2306177C2 (ru) | Способ получения микропористых материалов с покрытием из оксида редкоземельного металла | |
| CN112408402B (zh) | La活化的功能化树枝状介孔二氧化硅纳米球的制备方法及其应用 | |
| Chen et al. | Ionic liquid-supported 3DOM silica for efficient heterogeneous oxidative desulfurization | |
| Aslam et al. | Unusual nickel dispersion in confined spaces of mesoporous silica by one-pot strategy for deep desulfurization of sulfur compounds and FCC gasoline | |
| WO2009049280A2 (en) | Methods of making aluminosilicate coated alumina | |
| Yuan et al. | Morphology-controlled synthesis and sulfur modification of 3D hierarchical layered double hydroxides for gaseous elemental mercury removal | |
| Li et al. | H2O2-assisted hydrothermal synthesis of TiO2-SiO2 and its enhanced photocatalytic-adsorptive desulfurization performance for model fuel | |
| Guo et al. | Synthesis of chitosan-functionalized MCM-41-A and its performance in Pb (II) removal from synthetic water | |
| CN103464141B (zh) | 一种含高分散性钨的介孔材料及其制备方法和应用 | |
| CN111115651B (zh) | 纳米分子筛、合成方法及其用途 | |
| CN111203190A (zh) | 一种高不饱和配位体系三价铈除磷吸附剂的制备方法 | |
| WO2021005693A1 (ja) | 炭化水素燃料から硫黄化合物を除去する吸着剤、吸着剤の製造方法、吸着剤の製造装置、硫黄化合物の除去方法および除去装置 | |
| JP6879680B2 (ja) | 高シリカチャバザイト型ゼオライトの製造方法および高シリカチャバザイト型ゼオライト | |
| Su et al. | Facile synthesis of geopolymer-based hierarchical porous materials for efficient adsorption-photocatalysis of dye wastewater | |
| Baha et al. | Synergistic photocatalysis of bayerite/zeolite loaded TiO2 nanocomposites for highly efficient degradation of organic pollutants in aqueous environments | |
| WO2022010888A1 (en) | Method of producing a cracking catalyst | |
| Kamel et al. | Composite beads of molybdenum oxide supported on textured silicon as an oxidative desulfurization nanocatalyst | |
| Liu et al. | Monodispersed dendritic mesoporous silica/carbon nanospheres with enhanced active site accessibility for selective adsorptive desulfurization | |
| Shaafi et al. | Sulfated zirconium oxide-decorated magnetite KCC-1 as a durable and recyclable adsorbent for the efficient removal of asphaltene from crude oil | |
| CN107344112A (zh) | 一种生产优质催化重整原料的加氢裂化催化剂及其制备方法和应用 | |
| JP2000176277A (ja) | メソ多孔体からなる炭化水素ガス吸着剤 | |
| CN101172242A (zh) | 一种催化裂化催化剂及其制备方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19936913 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2021530381 Country of ref document: JP Kind code of ref document: A |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 19936913 Country of ref document: EP Kind code of ref document: A1 |