WO2019221114A1 - Agent de réparation du tissu cutané contenant de la bétanine ou un composé analogue à cette dernière - Google Patents
Agent de réparation du tissu cutané contenant de la bétanine ou un composé analogue à cette dernière Download PDFInfo
- Publication number
- WO2019221114A1 WO2019221114A1 PCT/JP2019/019104 JP2019019104W WO2019221114A1 WO 2019221114 A1 WO2019221114 A1 WO 2019221114A1 JP 2019019104 W JP2019019104 W JP 2019019104W WO 2019221114 A1 WO2019221114 A1 WO 2019221114A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- haloalkyl
- formula
- hydrogen
- optionally substituted
- Prior art date
Links
- 0 *CC([C@@](*)C(*)=C1*)O[C@]1Oc1c(*)cc2N(CC=C3C=C(*)NC(*)C3)[C@](*)Cc2c1 Chemical compound *CC([C@@](*)C(*)=C1*)O[C@]1Oc1c(*)cc2N(CC=C3C=C(*)NC(*)C3)[C@](*)Cc2c1 0.000 description 3
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/21—Amaranthaceae (Amaranth family), e.g. pigweed, rockwort or globe amaranth
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/04—Antipruritics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
Definitions
- the present invention relates to a skin tissue repair agent containing betanin or a similar compound. More specifically, the present invention relates to a skin tissue repair agent comprising a compound represented by formula (I) or a salt thereof, and a medicament for treating a skin disease comprising a compound represented by formula (I) or a salt thereof.
- the present invention relates to a composition and a cosmetic comprising the skin tissue repair agent.
- the skin has a three-layer structure of epidermis, dermis and subcutaneous tissue.
- the epidermis is composed of four layers, a basal layer, a spiny layer, a granule layer, and a stratum corneum, and prevents water in the body from being released outside the body in the stratum corneum in which corneocytes are stacked in a brick shape, and Protects against irritation from outside the body.
- the skin tissue has a large number of immune cells as well as the skin barrier, the cells responsible for the skin barrier and immunity suppress and prevent entry of foreign substances. When these defense systems become abnormal, irritation such as invasion of foreign matter from the outside of the body causes inflammation and dryness of the skin, resulting in skin diseases such as atopic dermatitis.
- Atopic dermatitis a type of skin disease, manifests various symptoms such as eczema, itching and cracking due to dryness.
- Red beet that contains a large amount of betanin pigment is edible, and its extract is widely known as a natural red colorant, and it is also known to have functions such as antioxidant action, liver function enhancement, and fat burning. Yes.
- betanin which is a water-soluble natural pigment that is easily oxidized and easily dissociates from the imine binding site, and there has been little research on the action of betanin itself.
- Patent Literature 1 ice cream, powdered beverages, etc.
- Patent Document 2 For other use examples other than the use as a red colorant for food addition that can be used even in a mixture with a large amount of stabilizer (such as dextran) There is no report in particular (Patent Document 2).
- the object of the present invention is to provide a composition useful for the treatment of skin diseases, comprising betanin or a similar compound thereof.
- the present inventors When purifying the water-soluble red pigment betanin from red beet, the present inventors accidentally deposited the betanine pigment on the affected area of the rough hand, and after several days, the affected area where the betanin pigment was deposited dramatically Found to heal. Therefore, as a result of investigating the effect of the betanin pigment in the eczema and finger joint cracks caused by rough skin of subjects who suffer from skin diseases on a daily basis and examining the effect of the betanin pigment, the betanin pigment was immediately washed with soapy water. It was found that the inflammatory skin colored with the betanin pigment did not fall off, and the old keratin exfoliated to heal eczema and cracks, thereby completing the present invention.
- R 2 is hydrogen, C 1-6 alkyl or C 1-6 haloalkyl
- R 3 is hydrogen, C 1-6 alkyl, C 1-6 haloalkyl or Wherein R 3a is each independently hydrogen, C 1-6 alkyl or C 1-6 haloalkyl.
- each R 4 is independently hydrogen, C 1-6 alkyl or C 1-6 haloalkyl]
- a skin tissue repair agent comprising a compound represented by the formula: [2]
- Formula (I) is converted to formula (Ia): [Wherein R 1 , R 2 , R 3 and R 4 have the same meanings as [1]] The skin tissue repair agent according to [1].
- R 1 is (1) C 2-6 alkenyl (wherein the group is a substitutable position selected from the group consisting of a halogen atom, C 1-6 alkyl and C 1-6 haloalkyl, or two or more same or different Optionally substituted with a group), (2) COOH, (3) C 3-6 cycloalkyl (wherein the group is a substitutable one selected from the group consisting of a halogen atom, C 1-6 alkyl, C 1-6 haloalkyl and C 2-6 alkenyl, or Which may be substituted with two or more groups identical or different), (4) -C 2-10 alkenylene -C 3-6 cycloalkyl (said C 2-10 alkenylene and C 3-6 cycloalkyl, in substitutable positions, halogen atom, C 1-6 alkyl and C 1- 1 or a group selected from the group consisting of 6 haloalkyls (which may be substituted with two or more groups identical or different), or (5)
- R 1 is —CH (OR 1a ) —CH (OR 1a ) —CH (OR 1a ) —CH (OR 1a ) —CH (OR 1a ) —CH 2 OR 1a and the carbon atom to which R 1 is bonded; Together The skin tissue repair agent according to [1] or [2].
- R 1 is COOH, 1-pentene, Or —CH (OH) —CH (OH) —CH (OH) —CH (OH) —CH (OH) —CH 2 OH, and R 2 , R 3 and R 4 are hydrogen, [1] to [3] The skin tissue repair agent according to any one of 1.
- Formula (I) is converted to formula (Ib): [Wherein, R 1b , R 2 , R 3 , R 4 have the same meanings as [1]] The skin tissue repair agent according to [1]. [6] The skin tissue repair agent according to [5], wherein R 1b , R 2 , R 3 and R 4 are hydrogen, C 1-6 alkyl or C 1-6 haloalkyl. [7] Formula (Ib) is The skin tissue repair agent according to [5] or [6].
- R 2 is hydrogen, C 1-6 alkyl or C 1-6 haloalkyl
- R 3 is hydrogen, C 1-6 alkyl, C 1-6 haloalkyl or Wherein R 3a is each independently hydrogen, C 1-6 alkyl or C 1-6 haloalkyl.
- each R 4 is independently hydrogen, C 1-6 alkyl or C 1-6 haloalkyl]
- a pharmaceutical composition for treating a skin disease comprising a compound represented by the formula: [9] Formula (I) is converted to formula (Ia): [Wherein R 1 , R 2 , R 3 and R 4 have the same meanings as [8]]
- R 1 is —CH (OR 1a ) —CH (OR 1a ) —CH (OR 1a ) —CH (OR 1a ) —CH (OR 1a ) —CH 2 OR 1a and the carbon atom to which R 1 is bonded; Together The pharmaceutical composition according to [8] or [9]. [11] R 1 is COOH, 1-pentene, Or —CH (OH) —CH (OH) —CH (OH) —CH (OH) —CH 2 OH, and R 2 , R 3 and R 4 are hydrogen, [8] to [10] A pharmaceutical composition according to any one of the above.
- Formula (I) is converted to formula (Ib): [Wherein R 1b , R 2 , R 3 and R 4 have the same meanings as [8]] The pharmaceutical composition according to [8], wherein [13] The pharmaceutical composition according to [12], wherein R 1b , R 2 , R 3 and R 4 are hydrogen, C 1-6 alkyl or C 1-6 haloalkyl. [14] Formula (Ib) is The pharmaceutical composition according to [12] or [13]. [15] The pharmaceutical composition according to any one of [8] to [14], wherein the skin disease is an inflammatory skin disease or a dry skin disease.
- the pharmaceutical composition according to [15], wherein the inflammatory skin disease is atopic dermatitis, contact dermatitis, seborrheic dermatitis, psoriasis or acne vulgaris.
- the pharmaceutical composition according to [15], wherein the dry skin disease is xeroderma or progressive palmokeratoderma.
- a cosmetic comprising the skin tissue repair agent according to any one of [1] to [7].
- a therapeutically effective amount of a compound represented by the formula (I) according to any one of [1] to [7] or a salt thereof is administered to a patient in need of treatment, How to treat skin diseases.
- [20] Use of the compound represented by the formula (I) or a salt thereof according to any one of [1] to [7] in the manufacture of a medicament for treating a skin disease.
- a method for repairing skin tissue comprising administering the skin tissue repair agent according to any one of [1] to [7] to a skin tissue of a patient in need of treatment.
- a method for enhancing the ability to regenerate skin tissue comprising administering the skin tissue repair agent according to any one of [1] to [7] to the skin tissue of a patient in need of treatment.
- a method for promoting proliferation of skin cells comprising administering the skin tissue repair agent according to any one of [1] to [7] to skin tissue of a patient in need of treatment.
- a method for improving skin quality wherein the cosmetic according to [18] is directly applied to a patient in need of treatment.
- a skin tissue repair agent comprising a red beet extract.
- a pharmaceutical composition for treating skin diseases comprising a red beet extract.
- the compound represented by the formula (I) or a salt thereof used in the skin tissue repair agent, pharmaceutical composition and cosmetics of the present invention was not cured by a steroid drug by repairing or regenerating the skin tissue of the affected area. It can be expected to exert an excellent therapeutic effect on chronic skin diseases such as atopic dermatitis or symptoms thereof. Moreover, since the cosmetics of the present invention improve symptoms such as dry skin and rough skin where betanin appears in patients with skin diseases, it can also be expected to exhibit an effect of improving skin quality. Furthermore, since high-purity betanin is a natural pigment derived from food, even if it is directly applied to the skin or wound site, it does not become an allergen and can be used safely.
- photographed the state of the affected part on the 10th day and 3 weeks after a betanin application to the crack of the base of the right hand little finger is shown.
- photographed the state of the thumb of both legs immediately after betaine application to the chicken eye of the left thumb and one week after is shown.
- coated betanin, or the palm of the commercial medicated toothpaste is shown.
- the photomicrograph of the intact human epidermis model of the 0th day, the 1st day, and the 2nd day after betanin administration is shown. By administering betanin, migration of basal cells begins on the first day after administration, and on the second day, redifferentiation into dendritic melanocytes is observed.
- slice of the mechanical wound opening vicinity of the 9th day after 0.4 mM and 4 mM betanin administration is shown.
- the upper figure is an epidermis model administered with 4 mM betanin, and a cell line redifferentiated into basal cells is observed after melanocyte formation (black arrow).
- the lower right line (-) indicates that the length is 300 ⁇ m.
- the upper figure shows the repair process of the wound site after administration of 4 mM betanin
- the upper figure shows the repair process of the wound site administered with 0.4 mM cyclodopa 5-O- ⁇ glucoside
- the lower figure shows the repair process of the wound site administered with 4 mM cyclodopa 5-O- ⁇ glucoside.
- red beet is a water-soluble red pigment having the following structure and a main pigment component of red beet.
- Red beet means a group of varieties called red beet or table beet among the Amaranthaceae.
- the red beet is not limited, but varieties such as Moneta, Crosby's Egyptian, Cylindra, Pablo F1, Cardal, Chioggia, Moulin Rouge, Detroit2, Detroit darkred, and Boltardy can be used.
- Cylindra or Detroit darkred which has a high betanin content and does not contain much impurities.
- the purity of betanin is 90% or more, preferably 95% or more, and more preferably 98% or more.
- R 2 is hydrogen, C 1-6 alkyl or C 1-6 haloalkyl
- R 3 is hydrogen, C 1-6 alkyl, C 1-6 haloalkyl or Wherein R 3a is each independently hydrogen, C 1-6 alkyl or C 1-6 haloalkyl.
- each R 4 is independently hydrogen, C 1-6 alkyl or C 1-6 haloalkyl] Or a salt thereof.
- Formula (I) is preferably of formula (Ia): Or formula (Ib): It is.
- R 1 is preferably (1) C 2-6 alkenyl (wherein the group is a substitutable position selected from the group consisting of a halogen atom, C 1-6 alkyl and C 1-6 haloalkyl, or two or more same or different Optionally substituted with a group), (2) COOH, (3) C 3-6 cycloalkyl (wherein the group is a substitutable one selected from the group consisting of a halogen atom, C 1-6 alkyl, C 1-6 haloalkyl and C 2-6 alkenyl, or Which may be substituted with two or more groups identical or different), (4) -C 2-10 alkenylene -C 3-6 cycloalkyl (said C 2-10 alkenylene and C 3-6 cycloalkyl, in substitutable positions, halogen atom, C 1-6 alkyl and C 1- 1 or a group selected from the group consisting of 6 haloalkyls (which may be substituted with two or more groups identical or different), or (5)
- R 1 is —CH (OR 1a ) —CH (OR 1a ) —CH (OR 1a ) —CH (OR 1a ) —CH (OR 1a ) —CH 2 OR 1a , together with the carbon atom to which R 1 is attached, Can be formed. More preferably, R 1 is COOH, 1-pentene, Or —CH (OH) —CH (OH) —CH (OH) —CH (OH) —CH 2 OH.
- R 1b , R 2 , R 3 and R 4 are preferably hydrogen, C 1-6 alkyl or C 1-6 haloalkyl, more preferably hydrogen.
- halogen atom means a fluorine atom, a chlorine atom, a bromine atom or an iodine atom.
- C 1-6 alkyl means a linear or branched saturated hydrocarbon group having 1 to 6 carbon atoms. Preferred is “C 1-4 alkyl”. Specific examples of “C 1-6 alkyl” include, for example, methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl, neopentyl, 1-ethylpropyl, hexyl, isohexyl, Examples include 1,1-dimethylbutyl, 2,2-dimethylbutyl, 3,3-dimethylbutyl, 2-ethylbutyl and the like.
- C 1-6 haloalkyl means C 1-6 alkyl as defined above, wherein one or more hydrogen atoms are replaced by halogen atoms.
- the number of hydrogen atoms replaced can range from one up to the total number of hydrogen atoms that may otherwise be present in the parent alkyl group. In the case of having a plurality of halogen atoms, they may be substituted with the same or different halogen atoms.
- Examples of “C 1-6 haloalkyl” include, but are not limited to, chloromethyl, trifluoromethyl, 2,2,2-trifluoroethyl, and the like.
- C 2-6 alkenyl means a linear or branched unsaturated hydrocarbon group having 2 to 6 carbon atoms and containing 1 to 3 carbon-carbon double bonds. Means.
- Examples of “C 2-6 alkenyl” include, but are not limited to, ethenyl, propenyl, butenyl, pentenyl, hexenyl, butadienyl, heptadienyl, and the like.
- C 2-10 alkenylene means a linear or branched divalent unsaturated hydrocarbon group having 2 to 6 carbon atoms and containing 1 to 5 carbon-carbon bonds. Means.
- Examples of “C 2-10 alkenylene” include, but are not limited to, propenylene, butenylene, pentenylene, hexenylene, butazinylene, hexatrienylene, nonatetraenylene, decapentaenylene, and the like.
- C 3-6 cycloalkyl means a 3- to 6-membered monocyclic or polycyclic saturated or partially unsaturated hydrocarbon group. Preferred is “C 5-6 cycloalkyl”, more preferred is cyclohexenyl. Specific examples of “C 3-6 cycloalkyl” include, for example, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclopentenyl, cyclohexenyl and the like.
- substituent in “optionally C 2-10 alkenylene” include a halogen atom, C 1-6 alkyl, C 1-6 haloalkyl, C 2-6 alkenyl, etc., preferably C 1-6 alkyl And C 2-6 alkenyl, more preferably methyl and propenyl.
- betanin is extracted from red beet root with a 0.3% citric acid solution, added with 70% saturated ammonium sulfate solution, centrifuged or filtered to remove protein, and a clear supernatant (a dye pretreatment solution).
- the dye pretreatment solution preferably pH 4.5 to 5.0
- the dye pretreatment solution is adsorbed onto the carrier using a reverse phase silica gel ODS column or a separate bead, and the dye adsorption carrier in the column is quenched.
- ethanol at a final concentration of 20% or 40% (v / v) is added to the acidic aqueous citric acid solution. Elution and concentration under reduced pressure to obtain a coprecipitate of citric acid and dye, or newly resuspend the concentrate in 95% ethanol and separate well with ultrasound. After is, the precipitate was repeated several times washed with filtered or 95% ethanol collected by centrifugation and vacuum dried to obtain a dry powder.
- An analog of betanin can be produced from purified betanin by a known method. Moreover, you may use a commercially available betanin solution with comparatively high purity for betanin preparation.
- the compound represented by the formula (I) may be in a salt form, and is not particularly limited as long as it is a pharmaceutically acceptable salt.
- the salt include salts of alkali metals (eg, potassium, sodium, lithium, etc.), salts of alkaline earth metals (eg, calcium, magnesium, etc.), ammonium salts (eg, tetramethylammonium salt, tetrabutylammonium salt) Etc.), organic amines (for example, triethylamine, methylamine, dimethylamine, cyclopentylamine, benzylamine, phenethylamine, piperidine, monoethanolamine, diethanolamine, tris (hydroxymethyl) methylamine, lysine, arginine, N-methyl-D- Salt of glucamine and the like).
- alkali metals eg, potassium, sodium, lithium, etc.
- alkaline earth metals eg, calcium, magnesium, etc.
- ammonium salts e
- the compound represented by the formula (I) may exist as an isomer such as an optically active substance and a stereoisomer. Accordingly, the compound represented by the formula (I) includes racemates, optically active isomers, stereoisomers, mixtures thereof and isolated ones.
- betanin in the present invention, the purity of betanin or a similar compound is assayed by mass spectrometry (MALDI-TOF-MS and ESI-MS), 1 H-NMR, and UV spectrum, but is not particularly limited to these methods. Furthermore, betanin can be recovered from 98 to 99.5% of its completely decomposed product by quantification of glucose released by ⁇ -glucosidase treatment, and further, no inorganic salt remains even if ashed by flame incineration. The purified powder betanin is confirmed to be a highly purified product.
- the “red beet extract” is a low-molecular substance obtained by extraction by a commonly used extraction method using red beet root as a raw material.
- the extraction method include, but are not limited to, a method of adding an acidic substance or an alkaline substance as appropriate with water or hydrous ethanol, heating the mixture appropriately, treating the red beet root, and concentrating the extract. It is not something.
- skin disease means inflammatory skin disease or dry skin disease and symptoms thereof.
- inflammatory skin diseases include atopic dermatitis, contact dermatitis, seborrheic dermatitis, psoriasis, acne vulgaris, and examples of dry skin diseases include psoriasis. Progressive palmar keratoderma and the like.
- atopic dermatitis is preferable.
- Symptoms of skin diseases include, for example, dry skin, rough skin, cracks, chicken eyes, eczema, rash, and pigmentation of healing scars caused by inflammatory skin diseases, so-called rough skin called sasare or akagile, or metabolic inhibitors As a side effect at the time of taking, there is a finger joint crack.
- the “skin tissue repair agent” is a function capable of repairing (healing) a skin tissue (affected part) in which an abnormality or defect has occurred and improving the state of the affected part and the surrounding skin tissue to a healthier one.
- Means a drug with Also included are drugs that exert therapeutic effects by inhibiting the destruction of skin tissue, enhancing the ability to regenerate skin tissue, and promoting the proliferation of skin cells.
- treatment means healing and amelioration of skin diseases, particularly skin wound sites in mammals, particularly humans. It also includes alleviation, alleviation and suppression of progression of the disease and its symptoms.
- patient means humans and other animals such as dogs, cats, horses and the like.
- the patient is preferably a mammal, more preferably a human.
- the “therapeutically effective amount” means an amount that provides a therapeutic effect on a disease and its symptoms or an amount that causes a delay in the progression of a disease and its symptoms, as compared to an untreated subject.
- the “therapeutically effective amount” also includes an amount effective to promote repair (regeneration) of skin tissue. The amount will vary depending on the type of compound, the disease and its severity, and the age, weight, etc., of the mammal to be treated.
- the skin tissue repair agent, pharmaceutical composition and cosmetics of the present invention may be other pharmaceutically active ingredients such as steroid drugs, as long as they do not interfere with the effect on the skin disease caused by the compound represented by formula (I) or a salt thereof.
- You may combine drugs, such as an antiallergic drug, an immunosuppressant, and an anti-inflammatory drug.
- the other pharmaceutically active ingredient may be blended in an amount of, for example, 0.01 to 10% by weight with respect to the total weight of the skin tissue repair agent, pharmaceutical composition or cosmetic. Two or more types may be used in combination.
- the skin tissue repair agent and pharmaceutical composition of the present invention can be administered in an oral or parenteral (for example, topical) dosage form, and is preferably administered in a form in which an active ingredient can be directly administered to the local surface of the skin.
- the dosage form is not particularly limited as long as it can be used as a pharmaceutical product. Examples thereof include injections, ointments (eg, oily and water-soluble ointments), creams, and gels. , Lotions, poultices, tapes, sprays and the like. ⁇
- the dosage form of the present invention is preferably an ointment, cream, gel or lotion. These can be produced by known methods.
- the cosmetic of the present invention is not particularly limited as long as it can be used as a cosmetic, and examples thereof include ointments, creams, gels, lotions, and emulsions.
- Cosmetics of the present invention include, for example, lotions; makeup base lotions, makeup base creams, whitening makeup cosmetics such as liquid or creamy or ointment-type foundations; sunscreen lotions, sunscreen creams, etc. Can be used as a stop cosmetic; hand cream; leg cream; and body lotion.
- the skin tissue repair agent, pharmaceutical composition and cosmetics of the present invention are usually additives used for pharmaceuticals and cosmetics, such as bases, absorption enhancers, humectants, thickeners, emulsifiers, antioxidants, stable Agents, bactericides, preservatives and the like may be appropriately blended by a method known per se.
- bases examples include white petrolatum, stearyl alcohol, stearic acid, liquid paraffin, squalene, medium chain fatty acid triglycerides and the like.
- absorption promoters examples include urea, diethyl sebacate, isopropyl alcohol, l-menthol, salicylic acid, medium chain fatty acid esters and the like.
- humectants include polyhydric alcohols such as glycerin, sorbitol, propylene glycol, 1,3-butanediol, and sodium hyaluronate.
- thickeners examples include gum arabic, guar gum, carrageenan, carboxymethylcellulose, and hydroxypropylmethylcellulose.
- emulsifiers examples include glycerin fatty acid ester, polyoxyethylene castor oil, polyoxyethylene sorbitan fatty acid ester, sodium lauryl sulfate and the like.
- antioxidant examples include L-ascorbic acid, dl- ⁇ -tocopherol, dibutylhydroxytoluene (BHT), butylhydroxyanisole (BHA) and the like.
- stabilizers examples include polysorbate, polyethylene glycol, and light anhydrous silicic acid.
- preservatives examples include paraoxybenzoates, benzalkonium chloride, sorbic acid, phenol and the like.
- the compound represented by the formula (I) or a salt thereof contained as an active ingredient in the pharmaceutical composition, skin tissue repair agent and cosmetics of the present invention may be any concentration that provides an effect for treating skin diseases.
- concentration of the compound represented by formula (I) or a salt thereof is not limited, but is 0.1 to 100 mM, preferably 0.2 to 50 mM, more preferably 0.3 to 20 mM, and even more. Preferably, it is 0.4 to 15 mM.
- the daily dose varies depending on various factors such as the type of compound, administration method, patient symptom and age, but is usually 0.1 mg to 100 mg, preferably 0.5 mg to 50 mg in an adult (body weight 60 kg), More preferably, the amount may be in the range of 1 mg to 10 mg. The amount may be applied directly to the affected area once a day, or once every other day.
- Example 1 Preparation of aqueous solution of betanin A red beet tuberous root (variety: Cylindra) root was washed with water and crushed in an equal amount of 0.3% citric acid solution (w / v) to obtain a crude pigment extract. To the crude dye extract, ammonium sulfate was added so as to be 70% saturated, and centrifuged (5000 ⁇ g, 5 minutes, 4 ° C.) to perform protein removal treatment to obtain a clear supernatant (dye pretreatment solution).
- the dye pretreatment liquid was sucked through Sepabead 70SP or a reverse phase silica gel ODS column (SV2 ⁇ 24 hours, 4 ° C.) to adsorb the dye component to the reverse phase resin.
- the reverse phase silica gel adsorbed with the dye component was thoroughly washed with 0.3% aqueous citric acid solution and further with 0.3% aqueous citric acid solution containing 1% ethanol (SV2 ⁇ 24 hours, 4 ° C.), and then 0.3% Elution and concentration by adding 10-20% ethanol or 40% fermented ethanol to an aqueous citric acid solution and dispersing the concentrate as a suspension in 95% ethanol, or with citric acid produced by storage at 4 ° C
- the black coprecipitate was washed with 95% ethanol to obtain betanin as an insoluble matter.
- the obtained betanin was dissolved in sterilized water so as to have a concentration of 10 mM to 15 mM to prepare an aqueous solution of betan
- Example 2 Determination of purity of betanin
- the purity of betanin obtained in Example 1 was measured using glucose CII-Test Wako. When measured using 200.0 mg (0.363 mmol) of betanin powder (theoretical value of free glucose: 65.39 mg), glucose levels of 64.18 mg (98.1%: 505 nm) and 65.09 mg (99.5%: 320 nm) were obtained. It was confirmed that 98-99.5% of glucose was released. That is, it was shown that the purity of betanin is 98 to 99.5%. In this test, the molecular weight of betanin was confirmed by mass spectrometry (MALDI-TOF-MS and ESI-MS), and the absence of impurities was confirmed by 1 H-NMR measurement.
- MALDI-TOF-MS mass spectrometry
- Test Example 1 Evaluation of skin tissue repair effect of betanin (1) As below, the betanin aqueous solution of Example 1 was apply
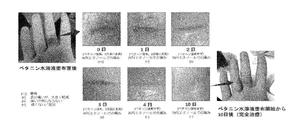
- FIG. 1 shows photographs taken of the affected area on the first day, the second day, the third day, the fourth day, and the tenth day immediately after the application of betanin.
- the wound was completely closed on the 4th day, and the crack was completely healed on the 10th day.
- Test Example 2 Evaluation of betanin skin tissue repair effect (2) As below, the betanin aqueous solution of Example 1 was apply
- One to two drops of the aqueous betanin solution of Example 1 was applied directly to the cracked part of the left footpad once a day, and the condition of the heels of both feet was compared for 14 days without treating the cracked right footpad. Thereafter, from the 14th day after application of the betanin aqueous solution to the left footpad, 1-2 drops of the aqueous solution of Example 1 were applied directly to the cracked portion of the right footpad once a day for 14 days, and the state was observed. During the betanin application period, both feet were not polished during bathing, and no wrinkle was treated with the urea-containing cream after bathing.
- 1-2 drops of the aqueous betanin solution of Example 1 were applied once a day for 7 days to the cracked portion of the base of the right little finger, and the state was observed for 3 weeks after the application.
- the betanin aqueous solution of Example 1 was applied to the chicken eye of the left big toe only once or twice a day for 7 days, and the effect of betanin was evaluated by a left-right coating method.
- photographed the state of the heel of both feet 2 weeks after the application of betanin to the left footpad is shown in FIG.
- the number of footpads on both heels was counted. The results are shown in Table 2.
- FIG. 3 is a photograph showing the state of the heels of both feet immediately after application of betanin to the right footpad (A), 1 day after application (B), 11 days after application (C), and 14 days after application (D). Shown in In order to confirm the state of the heel, the number of vertical cracks in the circle in Fig. 3 (A) was counted, and the cracks in the side and diagonal directions of both feet on the first day after applying betanin to the starboard Counted the number. The counted number of cracks is shown in Table 3.
- the test subject realized that the application of the betanin solution made the port skin softened in one week.
- the skin on the starboard was softened from the 3rd day after application, the keratin was reduced from about the 10th day, the appearance was improved, the number of toe crests was restored on the 14th day, and cracked The number was greatly reduced and the symptoms improved.
- Fig. 4 shows photographs of the condition of the affected area on the 10th day and 3 weeks after the application of betanin to the crack of the base of the right hand little finger.
- the subject was not worried about pain after the 4th day after application, but the periphery became swollen and hard (right figure). Later, a lump-like swelling began to heal and healed completely in 3 weeks.
- Fig. 5 shows photographs of the thumbs of both legs immediately after applying betanin to the chicken eye of the left big toe and one week later.
- the test subject realized that the skin of the left big toe became soft from the 4th day after application, and the symptoms improved after one week.
- Test Example 3 Evaluation of skin tissue repair effect of betanin (3)
- the betanin aqueous solution of Example 1 was apply
- the cleaning effect of soap and medicinal toothpaste betanin was confirmed.
- [Method] Betanin was applied to the right hand. Thereafter, the palm was washed with soap only, and the cracked finger part was washed only with a commercially available toothpaste foam, and the effect of removing the deposit of betanin was confirmed.
- FIG. 6 shows a photograph of the palm coated with betanin and soap or the palm washed with a commercially available toothpaste foam.
- Test Example 4 Effect of high-purity betanin administration in an intact human epidermis model
- a human epidermis model preparation kit / lab site Epikit (Japan Tissue Engineering Co., Ltd. (J-TEC)) was purchased to prepare a human epidermis model.
- the prepared human epidermis model was administered with 4 mM of the betanin solution of Example 1 to confirm the effect of high-purity betanin administration.
- FIG. 7 shows micrographs of intact human epidermis models on day 0, day 1 and day 2 after betanin administration. On the first day after the administration of betanin, migration of basal cells started, and on the second day after the administration, redifferentiation into dendritic melanocytes was observed.
- Test Example 5 Evaluation of skin tissue repair effect of betanin in an injured human epidermis model
- the skin tissue repair effect of betanin was examined using the prepared human skin model. Specifically, a wound is made on the prepared epidermis model with a cutter, and 0.4 ⁇ m or 4 mM of the aqueous betanin solution of Example 1 is dropped into the wound site with a micropipette and cultured for 14 days. The skin repair effect of was evaluated. Water was used as a control.
- FIG. 8 shows a micrograph of a vertical cross section of the epidermis model section in the vicinity of the mechanically injured opening on day 9 after administration of 0.4 mM and 4 mM betanin.
- betanin brings about the outstanding skin tissue repair effect. Moreover, it was suggested that the skin tissue repair effect is enhanced by increasing the concentration of betanin to about 4 mM.
- Test Example 6 Evaluation of skin tissue repair effect of cyclodopa 5-O- ⁇ glucoside (cycloDOPA 5-O-Glc) in an injured human epidermis model Using the prepared human epidermis model, cyclodopa 5-O- ⁇ glucoside (cycloDOPA The skin tissue repair effect of 5-O-Glc) was examined. Specifically, a wound was made on the prepared epidermis model with a cutter, and 0.4 ⁇ m or 4 mM cyclodopa 5-O- ⁇ glucoside was dropped directly into the wound site with a micropipette and cultured for 14 days. The skin repair effect of cyclodopa 5-O- ⁇ glucoside was evaluated.
- the pharmaceutical composition containing the compound represented by the formula (I) or the salt thereof and the skin tissue repair agent of the present invention are extremely effective particularly for the treatment of atopic skin salt, which is said to be intractable, and betanin is a food. Since it is a natural pigment derived from it, it can be used for patients who have not been effective with steroid drugs or who have not been able to use steroid drugs due to side effects. Therefore, the present invention can be expected to provide a new therapeutic agent for skin diseases. In addition, since the texture of the skin surface is finely adjusted and the touch becomes soft, the skin is dried and the barrier function of the skin is prevented from being lowered. Therefore, it can be expected to be provided as a skin care cosmetic that improves the skin quality.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Natural Medicines & Medicinal Plants (AREA)
- Dermatology (AREA)
- Epidemiology (AREA)
- Pulmonology (AREA)
- Medical Informatics (AREA)
- Immunology (AREA)
- Botany (AREA)
- Alternative & Traditional Medicine (AREA)
- Mycology (AREA)
- Microbiology (AREA)
- Molecular Biology (AREA)
- Biotechnology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Cosmetics (AREA)
- Medicines Containing Plant Substances (AREA)
Abstract
La présente invention concerne une composition (en particulier, un agent de réparation du tissu cutané) qui est utilisable pour le traitement de maladies de la peau et qui contient un composé représenté par la formule (I) : [dans la formule, R1, R1', R2, R3 et R4 sont tels que définis dans la description de la présente demande] ou un sel correspondant.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2020519653A JPWO2019221114A1 (ja) | 2018-05-14 | 2019-05-14 | ベタニンまたはその類似化合物を含む皮膚組織修復剤 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2018-093061 | 2018-05-14 | ||
| JP2018093061 | 2018-05-14 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019221114A1 true WO2019221114A1 (fr) | 2019-11-21 |
Family
ID=68540044
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2019/019104 WO2019221114A1 (fr) | 2018-05-14 | 2019-05-14 | Agent de réparation du tissu cutané contenant de la bétanine ou un composé analogue à cette dernière |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JPWO2019221114A1 (fr) |
| WO (1) | WO2019221114A1 (fr) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP4146012A4 (fr) * | 2020-05-04 | 2023-11-15 | Sensient Colors LLC | Procédé d'augmentation de la teneur en bétalaïne dans une plante cultivée |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0717848A (ja) * | 1993-06-30 | 1995-01-20 | Sansho Seiyaku Co Ltd | 皮膚外用剤 |
| WO1998026792A1 (fr) * | 1996-12-19 | 1998-06-25 | State Of Israel - Ministry Of Agriculture | Compositions contenant des antioxydants et procede de preparation desdits antioxydants |
| JP2011529906A (ja) * | 2008-07-30 | 2011-12-15 | ブイ・デイ・エフ・フユーチヤーシユーテイカルズ・インコーポレイテツド | ベタレイン組成物およびそれらの使用 |
| JP2015221790A (ja) * | 2014-05-01 | 2015-12-10 | 日本メナード化粧品株式会社 | 特定の波長域を有する光を照射して栽培したレッドビートの抽出物を含有する皮膚外用剤や内用剤。 |
-
2019
- 2019-05-14 WO PCT/JP2019/019104 patent/WO2019221114A1/fr active Application Filing
- 2019-05-14 JP JP2020519653A patent/JPWO2019221114A1/ja active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0717848A (ja) * | 1993-06-30 | 1995-01-20 | Sansho Seiyaku Co Ltd | 皮膚外用剤 |
| WO1998026792A1 (fr) * | 1996-12-19 | 1998-06-25 | State Of Israel - Ministry Of Agriculture | Compositions contenant des antioxydants et procede de preparation desdits antioxydants |
| JP2011529906A (ja) * | 2008-07-30 | 2011-12-15 | ブイ・デイ・エフ・フユーチヤーシユーテイカルズ・インコーポレイテツド | ベタレイン組成物およびそれらの使用 |
| JP2015221790A (ja) * | 2014-05-01 | 2015-12-10 | 日本メナード化粧品株式会社 | 特定の波長域を有する光を照射して栽培したレッドビートの抽出物を含有する皮膚外用剤や内用剤。 |
Non-Patent Citations (5)
| Title |
|---|
| KANNER J. ET AL.: "BETALAINS-A NEW CLASS OF DIETARY CATIONIZED ANTIOXIDANTS", JOURNAL OF AGRICULTURAL AND CHEMISTRY, vol. 9, no. 11, 4 November 2001 (2001-11-04), pages 5178 - 5185, XP002549925, DOI: 10.1021/jf010456f * |
| KAWANO K. ET AL.: "Oral intake of beet extract provides protection against skin barrier impairment in hairless mice", PHYTOTHER RES., vol. 27, no. 5, 2013, pages 775 - 783, XP018507448, DOI: 10.1002/ptr.4792 * |
| KITAMURA SEIICHI ET AL: "Development of skin moisturizer using 'Barafu' the brand vegetable produced by Saga University", FRAGRANCE JOURNAL, vol. 42, no. 2, 2014, pages 25 - 29 * |
| RENATA M. M. ET AL.: "Anti-inflammatory activity of betalain-rich dye of Beta vulgaris: effect on edema, leukocyte recruitment, superoxide anion and cytokine production", ARCHIVES OF PHARMACAL RESEARCH, vol. 38, no. 4, 2015, pages 494 - 504, XP035445182, DOI: 10.1007/s12272-014-0473-7 * |
| YONG-HAN H. ET AL.: "Djulis (Chenopodium formosanum Koidz. ) Water Extract and Its Bioactive Components Ameliorate Dermal Damage in UVB-Irradiated Skin Models", BIOMED RESEARCH INTERNATIONAL, vol. 2016, 2016, pages 1 - 8, XP055656129 * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP4146012A4 (fr) * | 2020-05-04 | 2023-11-15 | Sensient Colors LLC | Procédé d'augmentation de la teneur en bétalaïne dans une plante cultivée |
Also Published As
| Publication number | Publication date |
|---|---|
| JPWO2019221114A1 (ja) | 2021-06-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11058735B2 (en) | Formulations containing pomegranate seed oil, rosa canina fruit oil and inula viscosa oleoresin or extract | |
| KR101514970B1 (ko) | 피토스핑고신-1-포스페이트 또는 그 유도체를 포함하는 아토피 또는 피부상처 치료 또는 예방용 조성물 | |
| AU1657201A (en) | Therapeutic compositions and methods of use thereof | |
| US20110009486A1 (en) | Method for treating pruritus by administering fullerenes | |
| US11090205B2 (en) | Methods and compositions for treatment of skin conditions | |
| JP5685315B2 (ja) | ニコチン酸アデニンディヌクレオチドリン酸又はその誘導体を含む薬学又は化粧料組成物 | |
| US20060205699A1 (en) | Topical treatment for psoriasis | |
| JP5622775B2 (ja) | 皮膚病の治療用組成物、並びにそれを用いた製剤及びその調製方法 | |
| MXPA06004081A (es) | Lipolisis local medicamentosa dirigida. | |
| EP0531495A1 (fr) | Compositions therapeutiques et cosmetiques pour le traitement de la peau. | |
| WO2017111069A1 (fr) | Agent antiprurigineux | |
| WO2019221114A1 (fr) | Agent de réparation du tissu cutané contenant de la bétanine ou un composé analogue à cette dernière | |
| CN115551516A (zh) | 烟酰胺单核苷酸或其某些衍生物用于预防和/或治疗由身体活动引起的肌肉、韧带或肌腱疼痛的用途,以及相应的组合物 | |
| JPH04500824A (ja) | 光老化の作用を回復させるための皮膚の治療方法 | |
| CA2754008A1 (fr) | Lipoatrophie cosmetique medicinale | |
| WO2002058640A1 (fr) | Systeme therapeutique topique pour les soins de la peau | |
| JPH10175868A (ja) | 津液改善組成物 | |
| CA3223022A1 (fr) | Utilisation d'un extrait de coque de fruit de mangoustan dans la preparation d'un medicament pour le traitement du psoriasis | |
| DE102017215154A1 (de) | Zusammensetzung zur topischen Behandlung von nicht-Mikroorganismus-verursachten entzündlichen Haut- und Schleimhauterkrankungen | |
| CA2809793C (fr) | Traitement d'infections fongiques | |
| DE102013018565A1 (de) | Körperpflegemittel und dessen Verwendung | |
| MICHELSON et al. | Treatment of cutaneous tuberculosis with large doses of vitamin D2 | |
| RU2506944C1 (ru) | Способ повышения трансдермальной проницаемости лечебных или косметических препаратов для наружного применения, способ введения в кожу газообразного ксенона | |
| JP2002241308A (ja) | 皮膚そう痒症治療薬 | |
| EP1747782A1 (fr) | Produits pour l'application topique comprenant de lysophospholipids et d'acides gras |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19803837 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| ENP | Entry into the national phase |
Ref document number: 2020519653 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 19803837 Country of ref document: EP Kind code of ref document: A1 |