WO2019087993A1 - 癌性悪液質抑制作用を有する発酵乳および多糖類 - Google Patents
癌性悪液質抑制作用を有する発酵乳および多糖類 Download PDFInfo
- Publication number
- WO2019087993A1 WO2019087993A1 PCT/JP2018/040016 JP2018040016W WO2019087993A1 WO 2019087993 A1 WO2019087993 A1 WO 2019087993A1 JP 2018040016 W JP2018040016 W JP 2018040016W WO 2019087993 A1 WO2019087993 A1 WO 2019087993A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- cachexia
- fermented milk
- cancer
- bulgaricus
- lactobacillus
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23C—DAIRY PRODUCTS, e.g. MILK, BUTTER OR CHEESE; MILK OR CHEESE SUBSTITUTES; MAKING OR TREATMENT THEREOF
- A23C9/00—Milk preparations; Milk powder or milk powder preparations
- A23C9/12—Fermented milk preparations; Treatment using microorganisms or enzymes
- A23C9/123—Fermented milk preparations; Treatment using microorganisms or enzymes using only microorganisms of the genus lactobacteriaceae; Yoghurt
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23C—DAIRY PRODUCTS, e.g. MILK, BUTTER OR CHEESE; MILK OR CHEESE SUBSTITUTES; MAKING OR TREATMENT THEREOF
- A23C9/00—Milk preparations; Milk powder or milk powder preparations
- A23C9/12—Fermented milk preparations; Treatment using microorganisms or enzymes
- A23C9/123—Fermented milk preparations; Treatment using microorganisms or enzymes using only microorganisms of the genus lactobacteriaceae; Yoghurt
- A23C9/1234—Fermented milk preparations; Treatment using microorganisms or enzymes using only microorganisms of the genus lactobacteriaceae; Yoghurt characterised by using a Lactobacillus sp. other than Lactobacillus Bulgaricus, including Bificlobacterium sp.
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/125—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives containing carbohydrate syrups; containing sugars; containing sugar alcohols; containing starch hydrolysates
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/135—Bacteria or derivatives thereof, e.g. probiotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/20—Milk; Whey; Colostrum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/74—Bacteria
- A61K35/741—Probiotics
- A61K35/744—Lactic acid bacteria, e.g. enterococci, pediococci, lactococci, streptococci or leuconostocs
- A61K35/747—Lactobacilli, e.g. L. acidophilus or L. brevis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08B—POLYSACCHARIDES; DERIVATIVES THEREOF
- C08B37/00—Preparation of polysaccharides not provided for in groups C08B1/00 - C08B35/00; Derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N1/00—Microorganisms, e.g. protozoa; Compositions thereof; Processes of propagating, maintaining or preserving microorganisms or compositions thereof; Processes of preparing or isolating a composition containing a microorganism; Culture media therefor
- C12N1/20—Bacteria; Culture media therefor
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2002/00—Food compositions, function of food ingredients or processes for food or foodstuffs
Definitions
- the present invention relates to fermented milk (yoghurt) having a cancerous cachexia inhibitory action.
- the present invention also relates to polysaccharides having cancer cachexia inhibitory activity.
- cancerous cachexia is characterized by the decrease in skeletal muscle found in patients with advanced cancer. Patients with cancer cachexia suffer from weight loss, loss of appetite, and are debilitated, sometimes leading to immediate death.
- An object of the present invention is to provide fermented milk and polysaccharides capable of suppressing cancerous cachexia.
- a fermented milk having a cancerous cachexia inhibitory action comprises Lactobacillus delbrueckii subsp. Bulgaricus as an active ingredient.
- Lactobacillus delbrooki subspecies bulgaricus is Lactobacillus delbrooki subspecies bulgaricus OLL 1073 R-1 (Accession No .: FERM BP-10741).
- the above-mentioned "fermented milk having a cancerous cachexia suppressing action” is “fermented milk having a survival improving action for cancerous cachexia patients", and “fermented milk having a weight loss suppressing action for cancerous cachexia patients” "Fermented milk with serum concentration lowering effect of inflammatory cytokines", “Fermented milk with serum concentration lowering effect of inflammation related chemokine”, “Hydraulicity with excessive elevation of serum concentration of IL-1 ⁇ accompanied by cancer” Also described as “fermented milk”, “fermented milk having an inhibitory effect on excessive serum concentration elevation of TNF- ⁇ associated with cancer”, “fermented milk having an inhibitory effect on excessive serum concentration elevation of CXCL1 associated with cancer” it can.
- the polysaccharide having a cancer cachexia-suppressing action according to another aspect of the present invention is produced from Lactobacillus delbrueckii subspecies bulgaricus as described above.
- polysaccharide having a cancer cachexia suppressing action is a “polysaccharide having a cancer cachexia patient survival improving action”
- a polysaccharide having a weight loss suppression action of a cancer cachexia patient is a “polysaccharide having a cancer cachexia patient survival improving action”
- Polysaccharides that lower the serum concentration of inflammatory cytokines “polysaccharides that lower the serum concentration of inflammation-related chemokines”, and “multiples that suppress excessive serum concentrations of IL-1 ⁇ associated with cancer It can also be expressed as a saccharide, "a polysaccharide having an inhibitory effect on excessive serum concentration elevation of TNF- ⁇ associated with cancer", or "a polysaccharide having an inhibitory effect on excessive serum concentration elevation of CXCL1 associated with cancer” .
- the cancerous cachexia-suppressing composition comprises, as an active ingredient, the above-mentioned Lactobacillus delbruchii subspecies bulgaricus or polysaccharide. That is, the above-mentioned Lactobacillus delbruchii subspecies bulgaricus or polysaccharide is used as a cancerous cachexia inhibitory action composition or as one component thereof.
- composition refers to preparations such as pharmaceuticals, supplements and food additives, food and drink (excluding animals and plants themselves) and food and drink compositions (including processed food and drink). It includes things that can be ingested (including humans).
- the above-mentioned invention is a method of using "Lactobacillus del Brucky subspecies vulgaricus or polysaccharide as a cancerous cachexia suppressing agent", for use as a cancerous cachexia suppressing agent
- Bacterial cachexia is orally administered to patients with cancerous cachexia by orally administering Lactobacillus delbulcian subspecies bulgaricus or polysaccharides, Lactobacillus delbricchi subspecies bulgaricus or polysaccharides to patients with cancerous cachexia It can also be expressed as “a method of suppressing”. In addition, it can also be expressed as "the use of Lactobacillus delbrueckii subspecies bulgaricus or polysaccharide for the production of a composition for suppressing cancerous cachexia" from another viewpoint.
- the composition for suppressing cancerous cachexia can be a composition for improving the survival rate of cancerous cachexia patients, the composition for suppressing weight loss for cancerous cachexia patients, and serum concentrations of inflammatory cytokines for patients with cancerous cachexia. It can also be expressed as a hypotensive composition, a serum concentration acting composition of inflammation-related chemokine in cancer cachexia patients.
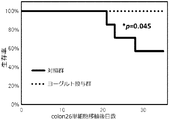
- FIG. 7 is a graph showing the time course of the survival rates of mice belonging to the control group and mice belonging to the yogurt administration group in Example 1.
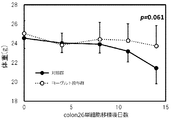
- FIG. 6 is a graph showing the time-dependent changes in body weight of mice belonging to the control group and mice belonging to the yogurt administration group in Example 2.
- the fermented milk having a cancer cachexia suppressing effect according to the embodiment of the present invention contains Lactobacillus delbrueckii subspecies bulgaricus as an active ingredient.
- Bulgaricus examples include, for example, Lactobacillus delbrucco subsp.
- Bulgaricus OLL 1073R-1 (Accession No .: FERM BP-10741).
- Lactobacillus del brucca subspecies bulgaricus OLL 1073R-1 is an independent administrative corporation National Institute of Advanced Industrial Science and Technology Patent Organism Depositary (Tsukuba City East Ibaraki 1) -1-1 This is a lactic acid bacterium that has been internationally deposited under the Budapest Treaty under the accession number of FERM BP-10741 at Central Center No. 6).
- the exopolysaccharide having a cancer cachexia suppressing action is a raw material containing milk (hereinafter sometimes referred to as "milk-containing raw material") as Lactobacillus delbruchii subspecies ⁇ ⁇ It is produced in the process of fermenting B. vulgaricus. That is, the fermented milk having the above-mentioned cancerous cachexia inhibitory action contains this exopolysaccharide.
- exopolysaccharides can be contained in a culture product, a metabolite, etc.
- Lactobacillus delbrueckii subspecies bulgaricus is cultured in a milk-containing medium.
- Milk is obtained from mammals.
- the type of mammal is not particularly limited.
- primates such as humans, monkeys, gorillas, baboons, chimpanzees, etc.
- domestic animals such as horses, cattle, buffalo, sheep, goats, pigs, camels, deer, etc. Examples include dogs, cats and other companion animals.
- the milk is preferably raw milk, but its processed product pasteurized milk, skimmed milk, whole fat milk powder, partially skimmed milk, skimmed milk powder, whole fat concentrated milk, skimmed concentrated milk, cream, butter, butter milk Whey, whey protein concentrate (WPC), whey protein isolate (WPI), etc.
- WPC whey protein concentrate
- WPI whey protein isolate
- the fermented milk or exopolysaccharide having a cancer cachexia suppressing action exerts its function by being consumed by an animal having a cancer cachexia symptom, in particular, a human.
- the "human” mentioned here may be a human of a wide age group from infants to elderly people regardless of age and sex.
- the fermented milk or exopolysaccharide having the cancer cachexia suppressing function exerts its function effectively by being taken in particular by cancer patients who are losing weight or cancer patients who are expected to lose weight in the future. Do.
- ingestion is not limited as long as it enters the human body, and can be achieved by any of the known intake methods such as oral ingestion, tube feeding and enteral ingestion. At this time, typically, oral intake via the gastrointestinal tract and enteral intake may be mentioned, but oral intake is preferable, and oral intake by eating and drinking is more preferable.
- the concentration of extracellular polysaccharides in fermented milk having a cancer cachexia suppressing effect according to the embodiment of the present invention is preferably 1 ⁇ g / mL or more, more preferably 5 ⁇ g / mL or more, It is more preferably 10 ⁇ g / mL or more, further preferably 20 ⁇ g / mL or more, and particularly preferably 30 ⁇ g / mL or more.
- an upper limit is 0.5 mg / mL, for example.
- the mass per unit package of the fermented milk according to the embodiment of the present invention is not particularly limited, but in the range of 50 g or more and 500 g or less from the viewpoint that the effect can be sufficiently obtained and easily consumed once. It is preferably in the range of 60 g to 200 g, more preferably in the range of 70 g to 150 g, and most preferably in the range of 80 g to 120 g.
- the above-mentioned unit packaging may be not only unit packaging per bag, a box, and a container, but may be unit packaging per unit contained in them, and may be unit packaging per day. A plurality of days, for example, an amount obtained by packaging a suitable quantity for intake for one week may be packaged, or a plurality of individual packages may be included.
- the mass per unit package of the exopolysaccharide according to the embodiment of the present invention is not particularly limited, but it is 50 g or more and 500 g or less from the viewpoint that an effect can be sufficiently obtained and it is easy to ingest once and cut off. Is preferably in the range of 60 g to 200 g, more preferably in the range of 70 g to 150 g, and most preferably in the range of 80 g to 120 g. .
- the above-mentioned unit packaging may be not only unit packaging per bag, a box, and a container, but may be unit packaging per unit contained in them, and may be unit packaging per day. A plurality of days, for example, an amount obtained by packaging a suitable quantity for intake for one week may be packaged, or a plurality of individual packages may be included.
- the fermented milk or exopolysaccharide having a cancer cachexia suppressing action according to the embodiment of the present invention may be continuously consumed for one week or more, preferably two weeks or more, more preferably four weeks or more. desirable.
- an ingestion period is not specifically limited, It can be continued permanently.
- the fermented milk or exopolysaccharide having a cancer cachexia inhibitory action may be continuously taken only for a part of the period, or may be taken intermittently for an arbitrary period.
- composition Containing the Fermented Milk and Extracellular Polysaccharide ⁇ Composition Containing the Fermented Milk and Extracellular Polysaccharide>
- a fermented milk or exopolysaccharide having a cancerous cachexia suppressing action according to an embodiment of the present invention is included in the composition (hereinafter, such a composition is referred to as “cancerous cachexia suppressing action composition
- the fermented milk and the content of extracellular polysaccharides are not particularly limited, but when the cancerous cachexia inhibitory action composition is a liquid, 1 ⁇ g of extracellular polysaccharides is used.
- fermented milk and extracellular polysaccharides are contained so as to be contained at / mL or more, and fermented milk and extracellular polysaccharides are contained so that extracellular polysaccharides are contained at 5 ⁇ g / mL or more. It is more preferable that the fermented milk and the exopolysaccharides are contained so that the exopolysaccharides are contained at 10 ⁇ g / mL or more, and the exopolysaccharides are contained at 20 ⁇ g / mL or more.
- fermented milk or extracellular polysaccharides are included, Particularly preferably fermented milk and extracellular polysaccharides are included, as are contained more than 30 [mu] g / mL.
- an upper limit is 0.5 mg / mL, for example.
- the mass per unit package of the cancerous cachexia suppressing action composition according to the embodiment of the present invention is not particularly limited, but it is 50 g from the viewpoint that the effect can be sufficiently obtained and it is easy to take it once. It is preferably in the range of not less than 500 g, more preferably in the range of not less than 60 g and not more than 200 g, still more preferably in the range of not less than 70 g and not more than 150 g, and in the range of not less than 80 g and not more than 120 g. Is most preferred.
- the above-mentioned unit packaging may be not only unit packaging per bag, a box, and a container, but may be unit packaging per unit contained in them, and may be unit packaging per day. A plurality of days, for example, an amount obtained by packaging a suitable quantity for intake for one week may be packaged, or a plurality of individual packages may be included.
- the cancerous cachexia suppressing action composition according to the embodiment of the present invention be continuously taken for 1 week or more, preferably 2 weeks or more, more preferably 4 weeks or more.
- an ingestion period is not specifically limited, It can be continued permanently.
- the cancerous cachexia suppressive action composition may be taken continuously only for a part of the time, or may be taken intermittently for any time.
- the fermented milk or exopolysaccharide having a cancerous cachexia suppressing action according to the embodiment of the present invention or the cancerous cachexia suppressing action composition can be used as a medicine or a food and drink.
- the pharmaceuticals or food and drink are useful in that they have a cancer cachexia suppressing effect.
- the fermented milk or exopolysaccharide having cancerous cachexia suppressing action according to the embodiment of the present invention or the cancerous cachexia suppressing action composition is used as a medicine or a food and drink, a single lactobacilli.
- Extracellular polysaccharides obtained from Drubricchi subsp. Vulgaricus may also be used, or extrafungal polyhedra from two or more types of Lactobacillus delbulcchi subsp. You may use combining saccharides.
- the fermented milk or exopolysaccharide having cancerous cachexia suppressing action according to the embodiment of the present invention or the cancerous cachexia suppressing action composition is used as a medicine or a food and drink
- those conditions are particularly limited. State such as paste, spray-dried, lyophilizate, vacuum-dried, drum-dried, liquid dispersed in medium, diluted diluted with diluent, crushed material obtained by crushing dried material in a mill etc. It can be
- the fermented milk or extracellular polysaccharide or cancerous cachexia suppressing composition according to the embodiment of the present invention has a cancerous cachexia suppressing action, a food for health functional food and a food for patients (cancerous cachexia) It can also be used as a food with suppressed action).
- the Health Function Food System was established not only for ordinary foods but also for foods in the form of tablets, capsules, etc., in consideration of internal and external trends and consistency with the conventional food system for specific health use. . And in the same system, three types of food for specific health (individual permission type), nutrition functional food (standard standard type), and functional indication food are defined.
- the fermented milk or exopolysaccharide having cancerous cachexia suppressing action according to the embodiment of the present invention or the cancerous cachexia suppressing action composition is used as a food for special use such as a food for specified health use or a nutritive function food, By administering to animals such as humans, cancerous cachexia can be suppressed.
- the present invention relates to a fermented milk or exopolysaccharide having cancerous cachexia inhibitory activity or a cancerous cachexia inhibitory composition according to an embodiment of the present invention, its use, efficacy, function, type of active ingredient, functional component It is preferable to display a description of the type of food, intake method, and the like. "Indication” in this context should be appropriate for medicines, quasi-drugs, health food, food for specified health use, food for functional indication, general food, health supplements, health food and supplements. . Also, the term “indication” as used herein includes all indications for informing the consumer of the above description.
- This display may be any display that recalls or infers the above-mentioned display contents, and may include all display regardless of the purpose of display, the contents of display, the object / medium to be displayed, and the like. For example, displaying the above description on a product packaging / container, displaying or distributing the above description on an advertisement / price list or transaction document relating to the product, or electromagnetically (for example, the Internet) Provided by the method).
- the product formed by packaging the fermented milk or exopolysaccharide having cancerous cachexia suppressing action according to the embodiment of the present invention or the cancerous cachexia suppressing working composition is, for example, a food or drink
- a display of “cancerous cachexia suppression” or a display of “cancerous cachectic symptom progression suppression” be attached.
- the wording used in order to perform the above display is not limited to the above-mentioned example, You may be a wording synonymous with such a meaning. Such terms include, for example, for consumers, such as “suppressing cancerous cachexia”, “helping to suppress the onset of cancerous cachexia” or “helping to suppress the progression of cancerous cachexia”, etc. Various wordings are acceptable.
- the type of the food or drink is not particularly limited.
- Food and drink include, for example, milk, processed milk, soft drinks, cheese, other dairy products, bread, biscuits, crackers, pizza crust, prepared milk powder, liquid food, food for the sick, infant milk, infant food, pregnant women, nursing Milk powder for women, nutritional foods, etc.
- the extracellular polysaccharide or cancerous cachexia suppressing action composition having a cancer cachexia suppressing action according to the embodiment of the present invention is used as it is, or other food and drink
- the preparation method in a usual food composition, such as mixing with food ingredients.
- the shape of the food or drink is not particularly limited, as long as it is the shape of a commonly used food or drink.
- it may be in any form such as solid (including powder and granular), paste, liquid, suspension and the like, and is not limited thereto.
- milk drinks, soft drinks, jelly drinks, tablets and powdered foods are more preferable.
- the fermented milk or exopolysaccharide having cancerous cachexia suppressing action according to the embodiment of the present invention or the cancerous cachexia suppressing action composition water, protein, carbohydrate, lipid, vitamins, minerals If it is an ingredient contained in usual foodstuffs, such as organic acid, an organic base, fruit juice, a flavor, a functional ingredient, a food additive, it can be added without problem.
- a protein source for example, protein or protein-containing raw material usually used for food production, such as soy protein, milk protein, chicken egg protein, animal and vegetable proteins such as meat protein, and hydrolysates thereof are used can do.
- sugar sources include processed starch (in addition to texturin, soluble starch, British starch, oxidized starch, starch ester, starch ether, etc.), dietary fiber and the like.
- lipid sources include animal fats and oils such as lard, fish oil and the like, fractionated oils thereof, hydrogenated oils, transesterified oils and the like; palm oil, safflower oil, corn oil, rapeseed oil, coconut oil, fractionated oils thereof And vegetable fats and oils such as hydrogenated oils and transesterified oils.
- vitamins for example, vitamin A, carotenes, vitamin B group, vitamin C, vitamin D group, vitamin E, vitamin K group, vitamin P, vitamin Q, niacin, nicotinic acid, pantothenic acid, biotin, inositol, choline And folic acid and the like
- minerals for example, calcium, potassium, magnesium, sodium, copper, iron, manganese, zinc, selenium and the like
- examples of the organic acid include malic acid, citric acid, lactic acid and tartaric acid.
- functional components include oligosaccharides, glucosamine, collagen, ceramide, royal jelly, polyphenol and the like.
- Examples of food additives include emulsifiers, stabilizers, thickeners, gelling agents, sweeteners, acidulants, preservatives, antioxidants, pH adjusters, colorants, flavors and the like.
- Various milk-derived components such as butter, milk mineral, cream, whey, non-protein nitrogen, sialic acid, phospholipids, lactose and the like can be suitably used for producing the food and drink according to the embodiment of the present invention
- any component having a cancerous cachexia suppression effect is included in the fermented milk or extracellular polysaccharide or cancerous cachexia suppressing composition according to the embodiment of the present invention. It may be added.
- the raw material may be any of natural products, processed natural products, synthetic products and / or foods rich in these.
- the inventors of the present invention can improve the survival rate of cancer patients or the weight of cancer patients by using the above-mentioned fermented milk or extracellular polysaccharides or cancerous cachexia suppressing compositions having the cancer cachexia suppressing action. It has been confirmed to suppress the decrease and to lower the serum levels of inflammatory cytokines and inflammation-related chemokines in cancer patients. Therefore, the fermented milk or exopolysaccharide having cancerous cachexia suppressing action or the cancerous cachexia suppressing action composition according to the embodiment of the present invention is a fermented milk having a weight loss suppressing action of a cancer patient.
- Extracellular polysaccharides or composition for suppressing weight loss of cancer patients Fermented milk or extracellular polysaccharides having a therapeutic activity for reducing serum levels of inflammatory cytokines in cancer patients, or compositions for reducing serum concentrations of inflammatory cytokines in cancer patients Also, it can be referred to as a fermented milk or exopolysaccharide having an action of reducing inflammation-related chemokine serum concentration in cancer patients or a composition for reducing inflammation-related chemokine serum concentration in cancer patients.
- the product formed by packaging the fermented milk or extracellular polysaccharide having cancerous cachexia suppressing action according to the embodiment of the present invention or the cancerous cachexia suppressing composition is, for example, a food or drink
- a display similar to these displays may be attached.
- the inventors of the present invention as a specific action and effect of the above-mentioned fermented milk or exopolysaccharide having the effect of reducing serum levels of inflammatory cytokines in cancer patients or the composition for reducing serum levels of inflammatory cytokines in cancer patients. It has been confirmed to lower serum concentrations of IL-1 ⁇ and TNF- ⁇ .
- the fermented milk or exopolysaccharide having cancerous cachexia suppressing action according to the embodiment of the present invention or the cancerous cachexia suppressing action composition is a fermented milk having IL-1 ⁇ serum concentration lowering action or Extracellular polysaccharides or IL-1 ⁇ serum concentration lowering action composition for cancer patients, fermented milk or exopolysaccharide having TNF- ⁇ serum concentration lowering action activity, or TNF- ⁇ serum concentration lowering action compositions for cancer patients I can say that.
- the inventors of the present invention as a specific action and effect of the above-mentioned fermented milk or exopolysaccharide having the effect of reducing serum concentration of chemokines in cancer patients or inflammation-related chemokine serum concentration acting composition of cancer patients , Has been confirmed to lower the serum concentration of CXCL1.
- the fermented milk or extracellular polysaccharide having cancerous cachexia suppressing action according to the embodiment of the present invention or the cancerous cachexia suppressing action composition is a fermented milk or bacterial cell having the CXCL1 serum concentration lowering action. It can also be referred to as exopolysaccharide or CXCL1 serum concentration-lowering composition for cancer patients.
- the extracellular polysaccharide or Lactobacillus delbrueckii subspecies Bulgaricus bacillus is used as a cancer cachexia suppressant drug, a weight loss suppressant drug for cancer patients, inflammatory cytokine serum of cancer patients
- the drug may be used as a drug for lowering concentration, a drug for lowering serum concentration of IL-1 ⁇ in cancer patients, a drug for lowering TNF- ⁇ serum concentration in cancer patients, or a drug for lowering CXCL1 serum concentration in cancer patients.
- Bulgaricus can be used as crushed or uncrushed processed products.
- the exopolysaccharide used in this case may be an exopolysaccharide obtained from Lactobacillus delbrueckii subspecies bulgaricus alone, or two or more types of lactobacilli. -It may be a combination of exopolysaccharides obtained from Delbrukchi subspecies vulgaricus.
- the Lactobacillus delbruchii subspecies bulgaricus used in this case may be a single Lactobacillus delbulchysubsp. Bulgaricus bacteria, or two or more types of Lactobacillus delphircius may be used. It may be a combination of bulky subspecies bulgaricus.
- the content of exopolysaccharide in the above-mentioned medicine can be arbitrarily determined according to the purpose and use.
- One example of the content is to occupy 1 ⁇ g / mL, but the embodiment of the present invention is not limited thereto.
- the dose of the pharmaceutical preparation containing the above-mentioned extracellular polysaccharide as an active ingredient can be appropriately set in consideration of various factors such as the administration route, the age, body weight, and symptoms of the subject of administration including humans.
- a suitable dose 0.01 mg to 1000 mg / kg / day can be mentioned as an active ingredient, but the embodiment of the present invention is not limited thereto.
- the exopolysaccharide which is the present active ingredient may be used in a larger amount than the above range.
- the dosage form of the above-mentioned medicine is preferably a dosage form which can be orally administered in order to allow extracellular polysaccharides or Lactobacillus delbrucchi subsp. Bulgaricus to reach the intestine.
- Examples of preferable dosage forms of the pharmaceutical product according to the embodiment of the present invention include, for example, tablets, pills, coated tablets, capsules, granules, powders, solutions, suspensions, emulsions, syrups, troches and the like. be able to.
- compositions can be added to the main component extracellular polysaccharides or Lactobacillus delbrueckii subspecies bulgaricus in accordance with the conventional method as excipients, binders, disintegrants, lubricants, stabilizers, coloring It can be formulated by mixing adjuvants which can be generally used in the pharmaceutical formulation art such as pharmaceutical agents, flavoring agents, solubilizers, suspensions, surfactants, coatings and the like.
- extracellular polysaccharides or Lactobacillus delbrueckii subspecies bulgaricus when it is orally administered, extracellular polysaccharides or Lactobacillus delbrucky subspecies
- the B. vulgaricus can be taken as it is, but it can be, for example, a tablet, a granule, a powder, a capsule or a powder according to a general preparation method of pharmaceuticals.
- the content of the exopolysaccharide or Lactobacillus delbrueckii subspecies bulgaricus in the above-mentioned pharmaceutical preparation is a dosage form, dosage, patient's age, sex, type and progress of cancer, degree of weight loss, It can be set appropriately according to other conditions and the like.
- the present pharmaceutical comprising the exopolysaccharide or Lactobacillus delbrueckii subsp.
- a drug may be used in combination.
- Preparation Example 2 Raw milk, skimmed milk powder, cream, sugar, a mixture containing Stevia, as a lactic acid bacteria starter, Lactobacillus delbrueckii subsp. Bulgaricus (Lactobacillus delbrueckii subsp. Bulgaricus) OLL 1073 R-1 (Accession No .: FERM BP-10741) And Streptococcus thermophilus bacteria were added, and the skimmed milk powder medium was fermented under a temperature environment of 43 ° C. for 3.5 hours to prepare a target yogurt.
- FERM BP-10741 FERM BP-10741
- Streptococcus thermophilus bacteria were added, and the skimmed milk powder medium was fermented under a temperature environment of 43 ° C. for 3.5 hours to prepare a target yogurt.
- Example 1 First, a total of 15 animals of BALB / c mice, male and 7 weeks old (sold by Japan Charles River) were divided into a control group (7 animals) and a yogurt administration group (8 animals). Next, a colon 26 single cell suspension (in PBS), which is a mouse colon cancer-derived cell line, is prepared, and the colon 26 single cell suspension is prepared at 6.0 ⁇ 10 5 cells / 200 ⁇ L of all mice (ie, 15 mice). Each mouse was transplanted with a 26G injection needle (manufactured by Terumo Corp.) subcutaneously on the left side of the abdomen.
- PBS a colon 26 single cell suspension
- mice in the control group were orally administered 400 ⁇ L of 10% non-fat dry milk medium per mouse by gavage
- mice in the yogurt administration group were orally administered 400 ⁇ L of the yoghurt prepared in Preparation Example 1 by oral gavage.
- oral gavage administration to mice in the yogurt administration group was performed once daily from the day of colon 26 cell transplantation into the mice to the day before dissection of the mice.
- body weight measurement of each mouse was started at a pace of 3 times a week, and when cachexia progressed seriously and the body weight fell below 20 g, humane endpoint As euthanized that mouse.
- the mice were followed up to 35 days after colon 26 cell transplantation, and all surviving mice were euthanized at 35 days.
- Example 2 First, a total of 12 animals of BALB / c mice, male and 5 weeks old (sold by Japan Charles River) were divided into a control group (6 animals) and a yogurt administration group (6 animals). Next, 400 ⁇ L of distilled water was orally administered orally to a control group, and 400 ⁇ L of yogurt prepared in Preparation Example 2 was orally administered orally to a yogurt administration group. Oral gavage to mice in each group was performed once daily until the day before dissection. Then, 13 days after the start of oral gavage administration, a colon 26 single cell suspension (in PBS), which is a mouse colon cancer-derived cell line, is prepared, and the colon 26 single cell suspension is subjected to 8.0 ⁇ 10 5 cells / cell.
- PBS colon 26 single cell suspension
- mice Each mouse was implanted with 200 ⁇ L of all mice (ie, 12 mice) right axillary subcutaneously using a 26G injection needle (manufactured by Terumo Corporation).
- free water administration (5 mg / mL) of metformin (manufactured by Wako Pure Chemical Industries, Ltd.), which is a metabolism improving agent, was performed on all mice from the day of colon26 cell transplantation to the day of mouse dissection.
- metformin is known to improve cancer cachexia (improvement by nutrition / metabolic action) through the improvement of sugar and protein metabolism, Lactobacillus delbruchie subspecies Bulgari.
- serum cytokine concentrations in the blood of mice belonging to both groups were compared, representative inflammatory cytokines IL-1 ⁇ and TNF ⁇ , and inflammation related chemokines having a function of mobilizing neutrophils to the site of inflammation
- concentration of CXCL1 was significantly lower in mice belonging to the yogurt administration group than to mice belonging to the control group (see Table 1).
- the fermented milk and polysaccharide having a cancerous cachexia inhibitory action according to the present invention can suppress cancerous cachexia and is expected to contribute to the survival of a cancer patient.
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Mycology (AREA)
- Microbiology (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Food Science & Technology (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Organic Chemistry (AREA)
- Molecular Biology (AREA)
- Nutrition Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Epidemiology (AREA)
- Biotechnology (AREA)
- Zoology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biomedical Technology (AREA)
- Wood Science & Technology (AREA)
- Virology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biochemistry (AREA)
- Genetics & Genomics (AREA)
- Cell Biology (AREA)
- Materials Engineering (AREA)
- General Engineering & Computer Science (AREA)
- Tropical Medicine & Parasitology (AREA)
- Developmental Biology & Embryology (AREA)
- Immunology (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Dairy Products (AREA)
- Coloring Foods And Improving Nutritive Qualities (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Polysaccharides And Polysaccharide Derivatives (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US16/650,184 US11638431B2 (en) | 2017-10-31 | 2018-10-29 | Fermented milk and polysaccharide with cancerous cachexia inhibitory effect |
| CN201880064288.5A CN111201026B (zh) | 2017-10-31 | 2018-10-29 | 具有癌性恶病质抑制作用的发酵乳和多糖类 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2017-210401 | 2017-10-31 | ||
| JP2017210401A JP7065589B2 (ja) | 2017-10-31 | 2017-10-31 | IL-1β血清濃度低下用発酵乳、CXCL1血清濃度低下用発酵乳、癌に伴うIL-1βの過度な血清濃度上昇の抑制用発酵乳、または、癌に伴うCXCL1の過度な血清濃度上昇の抑制用発酵乳 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019087993A1 true WO2019087993A1 (ja) | 2019-05-09 |
Family
ID=66331935
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2018/040016 Ceased WO2019087993A1 (ja) | 2017-10-31 | 2018-10-29 | 癌性悪液質抑制作用を有する発酵乳および多糖類 |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US11638431B2 (enExample) |
| JP (1) | JP7065589B2 (enExample) |
| CN (1) | CN111201026B (enExample) |
| WO (1) | WO2019087993A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11638431B2 (en) | 2017-10-31 | 2023-05-02 | Meiji Co., Ltd. | Fermented milk and polysaccharide with cancerous cachexia inhibitory effect |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113861303B (zh) * | 2021-10-21 | 2023-04-21 | 华南理工大学 | 一种从德氏乳杆菌和嗜热链球菌发酵酸奶中分离出的胞外多糖及其应用 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20130236600A1 (en) * | 2012-03-07 | 2013-09-12 | Daflorn Ltd., Bulgaria | Probiotics for dietary dairy product |
| JP2014027925A (ja) * | 2012-06-25 | 2014-02-13 | Meiji Co Ltd | 菌体外多糖産生乳酸菌用培地、菌体外多糖産生菌の製造方法、菌体外多糖、菌体外多糖の製造方法、及びヨーグルトの製造方法 |
| WO2017135364A1 (ja) * | 2016-02-03 | 2017-08-10 | 株式会社明治 | 発酵乳の製造方法及び発酵乳 |
| WO2018194149A1 (ja) * | 2017-04-21 | 2018-10-25 | 株式会社明治 | サイトカイン産生制御剤 |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2211578C (en) * | 1995-02-13 | 2010-09-21 | Chugai Seiyaku Kabushiki Kaisha | Muscle protein proteolysis inhibiting agent containing il-6 receptor antibody |
| JP2009537582A (ja) | 2006-05-24 | 2009-10-29 | ディーエスエム アイピー アセッツ ビー.ブイ. | 有機化合物の新しい用途 |
| WO2007140613A1 (en) | 2006-06-06 | 2007-12-13 | Mcgill University | Fermented milk product and use thereof |
| US7901925B2 (en) * | 2006-06-23 | 2011-03-08 | Bojrab Gregory G | Lactobacillus delbrueckii ssp. bulgaricus strain and compositions |
| EP2203551B1 (en) | 2007-10-20 | 2013-08-21 | Université de Liège | Bifidobacterial species |
| AU2009240643B2 (en) | 2008-04-23 | 2014-03-06 | Rigel Pharmaceuticals, Inc. | Carboxamide compounds for the treatment of metabolic disorders |
| CA2761573A1 (en) | 2009-05-11 | 2010-11-18 | Nestec S.A. | Bifidobacterium longum ncc2705 (cncm i-2618) and immune disorders |
| CA2818467A1 (en) | 2009-11-18 | 2011-05-26 | Murray Goulburn Co-Operative Co. Limited | Recombinant microorganisms |
| US9585920B2 (en) * | 2011-02-04 | 2017-03-07 | Katherine Rose Kovarik | Method and system for treating cancer cachexia |
| JP6016326B2 (ja) * | 2009-12-09 | 2016-10-26 | 株式会社明治 | 乳酸菌のスクリーニング方法 |
| CA2921820C (en) * | 2013-08-26 | 2021-10-12 | Meiji Co., Ltd. | Antibody titer-increasing agent using lactic acid bacterium |
| CN104905246A (zh) * | 2015-04-24 | 2015-09-16 | 劲膳美生物科技股份有限公司 | 恶性肿瘤特定全营养配方食品 |
| JP7065589B2 (ja) | 2017-10-31 | 2022-05-12 | 株式会社明治 | IL-1β血清濃度低下用発酵乳、CXCL1血清濃度低下用発酵乳、癌に伴うIL-1βの過度な血清濃度上昇の抑制用発酵乳、または、癌に伴うCXCL1の過度な血清濃度上昇の抑制用発酵乳 |
-
2017
- 2017-10-31 JP JP2017210401A patent/JP7065589B2/ja active Active
-
2018
- 2018-10-29 WO PCT/JP2018/040016 patent/WO2019087993A1/ja not_active Ceased
- 2018-10-29 US US16/650,184 patent/US11638431B2/en active Active
- 2018-10-29 CN CN201880064288.5A patent/CN111201026B/zh active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20130236600A1 (en) * | 2012-03-07 | 2013-09-12 | Daflorn Ltd., Bulgaria | Probiotics for dietary dairy product |
| JP2014027925A (ja) * | 2012-06-25 | 2014-02-13 | Meiji Co Ltd | 菌体外多糖産生乳酸菌用培地、菌体外多糖産生菌の製造方法、菌体外多糖、菌体外多糖の製造方法、及びヨーグルトの製造方法 |
| WO2017135364A1 (ja) * | 2016-02-03 | 2017-08-10 | 株式会社明治 | 発酵乳の製造方法及び発酵乳 |
| WO2018194149A1 (ja) * | 2017-04-21 | 2018-10-25 | 株式会社明治 | サイトカイン産生制御剤 |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11638431B2 (en) | 2017-10-31 | 2023-05-02 | Meiji Co., Ltd. | Fermented milk and polysaccharide with cancerous cachexia inhibitory effect |
Also Published As
| Publication number | Publication date |
|---|---|
| CN111201026A (zh) | 2020-05-26 |
| JP7065589B2 (ja) | 2022-05-12 |
| US11638431B2 (en) | 2023-05-02 |
| US20200296979A1 (en) | 2020-09-24 |
| CN111201026B (zh) | 2023-09-19 |
| JP2019081733A (ja) | 2019-05-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| MXPA05006266A (es) | Composiciones prebioticas. | |
| AU2017249660B2 (en) | Bifidobacteria for increasing lean body mass | |
| US20240115630A1 (en) | Bifidobacteria for reducing food, energy and/or fat intake | |
| JP2023175938A (ja) | 糖代謝改善用組成物 | |
| JP2024111238A (ja) | インターロイキン-23産生促進用組成物およびReg3ファミリータンパク質の発現誘導用組成物 | |
| CN108367034A (zh) | 面向婴儿的感染防御剂 | |
| JP6916042B2 (ja) | 抗原特異的インターフェロンγ産生促進用組成物 | |
| JP5971893B2 (ja) | ミネラル吸収改善剤 | |
| US11638431B2 (en) | Fermented milk and polysaccharide with cancerous cachexia inhibitory effect | |
| JP2019081733A5 (enExample) | ||
| JP2021107400A (ja) | 肥満抑制剤 | |
| JP2013119546A (ja) | 不安及び/又は多動性の改善剤 | |
| JP7181954B2 (ja) | 高いAhR活性化能を有する乳酸菌 | |
| CN120225074A (zh) | 肌肉肌醇和预防加速生长 | |
| WO2011025019A1 (ja) | チオレドキシン誘導活性を有する乳酸菌ならびにチオレドキシンを介する生体傷害の予防および/または改善用の飲食品および医薬品 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 18872224 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 18872224 Country of ref document: EP Kind code of ref document: A1 |