WO2015022560A1 - Stable pharmaceutical composition containing bisoprolol and ramipril - Google Patents
Stable pharmaceutical composition containing bisoprolol and ramipril Download PDFInfo
- Publication number
- WO2015022560A1 WO2015022560A1 PCT/HU2014/000072 HU2014000072W WO2015022560A1 WO 2015022560 A1 WO2015022560 A1 WO 2015022560A1 HU 2014000072 W HU2014000072 W HU 2014000072W WO 2015022560 A1 WO2015022560 A1 WO 2015022560A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- weight
- ramipril
- bisoprolol
- coated
- compact
- Prior art date
Links
- HDACQVRGBOVJII-JBDAPHQKSA-N ramipril Chemical compound C([C@@H](C(=O)OCC)N[C@@H](C)C(=O)N1[C@@H](C[C@@H]2CCC[C@@H]21)C(O)=O)CC1=CC=CC=C1 HDACQVRGBOVJII-JBDAPHQKSA-N 0.000 title claims abstract description 214
- 229960003401 ramipril Drugs 0.000 title claims abstract description 213
- 229960002781 bisoprolol Drugs 0.000 title claims abstract description 185
- VHYCDWMUTMEGQY-UHFFFAOYSA-N bisoprolol Chemical compound CC(C)NCC(O)COC1=CC=C(COCCOC(C)C)C=C1 VHYCDWMUTMEGQY-UHFFFAOYSA-N 0.000 title claims abstract description 138
- 239000008194 pharmaceutical composition Substances 0.000 title claims description 67
- 239000000203 mixture Substances 0.000 claims abstract description 174
- 239000004480 active ingredient Substances 0.000 claims abstract description 136
- 238000002360 preparation method Methods 0.000 claims abstract description 31
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 claims description 92
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 claims description 92
- 239000008187 granular material Substances 0.000 claims description 83
- 239000003826 tablet Substances 0.000 claims description 73
- RZPZLFIUFMNCLY-WLHGVMLRSA-N (e)-but-2-enedioic acid;1-(propan-2-ylamino)-3-[4-(2-propan-2-yloxyethoxymethyl)phenoxy]propan-2-ol Chemical compound OC(=O)\C=C\C(O)=O.CC(C)NCC(O)COC1=CC=C(COCCOC(C)C)C=C1 RZPZLFIUFMNCLY-WLHGVMLRSA-N 0.000 claims description 56
- 239000002775 capsule Substances 0.000 claims description 56
- 229920000168 Microcrystalline cellulose Polymers 0.000 claims description 50
- 229940016286 microcrystalline cellulose Drugs 0.000 claims description 50
- 235000019813 microcrystalline cellulose Nutrition 0.000 claims description 50
- 239000008108 microcrystalline cellulose Substances 0.000 claims description 50
- 239000002245 particle Substances 0.000 claims description 47
- 150000003839 salts Chemical class 0.000 claims description 40
- 238000000034 method Methods 0.000 claims description 37
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 claims description 37
- 229920000036 polyvinylpyrrolidone Polymers 0.000 claims description 35
- 206010020772 Hypertension Diseases 0.000 claims description 32
- 239000011248 coating agent Substances 0.000 claims description 29
- 239000001267 polyvinylpyrrolidone Substances 0.000 claims description 29
- 238000011282 treatment Methods 0.000 claims description 27
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims description 26
- 239000000243 solution Substances 0.000 claims description 26
- 239000007931 coated granule Substances 0.000 claims description 25
- 239000012752 auxiliary agent Substances 0.000 claims description 24
- 238000000576 coating method Methods 0.000 claims description 24
- 229920000620 organic polymer Polymers 0.000 claims description 24
- 239000007864 aqueous solution Substances 0.000 claims description 21
- 239000008185 minitablet Substances 0.000 claims description 21
- 230000008569 process Effects 0.000 claims description 21
- 239000000945 filler Substances 0.000 claims description 20
- 239000007884 disintegrant Substances 0.000 claims description 15
- 238000005469 granulation Methods 0.000 claims description 15
- 239000008188 pellet Substances 0.000 claims description 15
- 230000003179 granulation Effects 0.000 claims description 14
- 238000003825 pressing Methods 0.000 claims description 14
- 239000008119 colloidal silica Substances 0.000 claims description 13
- 239000000314 lubricant Substances 0.000 claims description 13
- 229910002012 Aerosil® Inorganic materials 0.000 claims description 11
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 claims description 10
- 102100028255 Renin Human genes 0.000 claims description 9
- 108090000783 Renin Proteins 0.000 claims description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 9
- OKMWKBLSFKFYGZ-UHFFFAOYSA-N 1-behenoylglycerol Chemical compound CCCCCCCCCCCCCCCCCCCCCC(=O)OCC(O)CO OKMWKBLSFKFYGZ-UHFFFAOYSA-N 0.000 claims description 8
- 229940049654 glyceryl behenate Drugs 0.000 claims description 8
- 239000001828 Gelatine Substances 0.000 claims description 7
- 229920000159 gelatin Polymers 0.000 claims description 7
- 235000019322 gelatine Nutrition 0.000 claims description 7
- 229920000642 polymer Polymers 0.000 claims description 7
- 238000011049 filling Methods 0.000 claims description 6
- 239000007787 solid Substances 0.000 claims description 6
- 235000019359 magnesium stearate Nutrition 0.000 claims description 5
- 239000010410 layer Substances 0.000 description 74
- 239000000047 product Substances 0.000 description 39
- 235000002639 sodium chloride Nutrition 0.000 description 30
- 238000012360 testing method Methods 0.000 description 28
- 238000004090 dissolution Methods 0.000 description 23
- 230000000694 effects Effects 0.000 description 17
- 238000003860 storage Methods 0.000 description 14
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 13
- 238000000354 decomposition reaction Methods 0.000 description 13
- 235000019888 Vivapur Nutrition 0.000 description 12
- 230000007423 decrease Effects 0.000 description 12
- 239000012535 impurity Substances 0.000 description 12
- DMBUODUULYCPAK-UHFFFAOYSA-N 1,3-bis(docosanoyloxy)propan-2-yl docosanoate Chemical compound CCCCCCCCCCCCCCCCCCCCCC(=O)OCC(OC(=O)CCCCCCCCCCCCCCCCCCCCC)COC(=O)CCCCCCCCCCCCCCCCCCCCC DMBUODUULYCPAK-UHFFFAOYSA-N 0.000 description 10
- 230000008859 change Effects 0.000 description 9
- 239000012530 fluid Substances 0.000 description 9
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 8
- 201000010099 disease Diseases 0.000 description 7
- 238000009472 formulation Methods 0.000 description 7
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 7
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- 229960005400 bisoprolol fumarate Drugs 0.000 description 6
- 238000005243 fluidization Methods 0.000 description 6
- 239000001530 fumaric acid Substances 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 6
- 238000004704 ultra performance liquid chromatography Methods 0.000 description 6
- 239000005541 ACE inhibitor Substances 0.000 description 5
- 229940044094 angiotensin-converting-enzyme inhibitor Drugs 0.000 description 5
- 239000003643 water by type Substances 0.000 description 5
- 206010019280 Heart failures Diseases 0.000 description 4
- 229920002472 Starch Polymers 0.000 description 4
- 230000000181 anti-adherent effect Effects 0.000 description 4
- 239000002876 beta blocker Substances 0.000 description 4
- 229940097320 beta blocking agent Drugs 0.000 description 4
- 239000011230 binding agent Substances 0.000 description 4
- 230000036772 blood pressure Effects 0.000 description 4
- 238000011109 contamination Methods 0.000 description 4
- 239000003814 drug Substances 0.000 description 4
- 229960003943 hypromellose Drugs 0.000 description 4
- 230000003993 interaction Effects 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- VLTRZXGMWDSKGL-UHFFFAOYSA-N perchloric acid Chemical compound OCl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-N 0.000 description 4
- 239000000843 powder Substances 0.000 description 4
- KOVMAAYRBJCASY-JBDAPHQKSA-N ramipril diketopiperazine Chemical compound C([C@@H](C(=O)OCC)N1C([C@@H]2C[C@@H]3CCC[C@@H]3N2C(=O)[C@@H]1C)=O)CC1=CC=CC=C1 KOVMAAYRBJCASY-JBDAPHQKSA-N 0.000 description 4
- AAEQXEDPVFIFDK-UHFFFAOYSA-N 3-(4-fluorobenzoyl)-2-(2-methylpropanoyl)-n,3-diphenyloxirane-2-carboxamide Chemical compound C=1C=CC=CC=1NC(=O)C1(C(=O)C(C)C)OC1(C=1C=CC=CC=1)C(=O)C1=CC=C(F)C=C1 AAEQXEDPVFIFDK-UHFFFAOYSA-N 0.000 description 3
- 229940127291 Calcium channel antagonist Drugs 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 239000000480 calcium channel blocker Substances 0.000 description 3
- 239000001506 calcium phosphate Substances 0.000 description 3
- 238000004587 chromatography analysis Methods 0.000 description 3
- -1 compact Substances 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 239000002934 diuretic Substances 0.000 description 3
- 230000001882 diuretic effect Effects 0.000 description 3
- 239000003480 eluent Substances 0.000 description 3
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 3
- BXRNXXXXHLBUKK-UHFFFAOYSA-N piperazine-2,5-dione Chemical compound O=C1CNC(=O)CN1 BXRNXXXXHLBUKK-UHFFFAOYSA-N 0.000 description 3
- KEDYTOTWMPBSLG-HILJTLORSA-N ramiprilat Chemical compound C([C@H](N[C@@H](C)C(=O)N1[C@@H](C[C@@H]2CCC[C@@H]21)C(O)=O)C(O)=O)CC1=CC=CC=C1 KEDYTOTWMPBSLG-HILJTLORSA-N 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 239000002356 single layer Substances 0.000 description 3
- 230000000087 stabilizing effect Effects 0.000 description 3
- 239000008107 starch Substances 0.000 description 3
- 235000019698 starch Nutrition 0.000 description 3
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 3
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 2
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 239000002333 angiotensin II receptor antagonist Substances 0.000 description 2
- 229940126317 angiotensin II receptor antagonist Drugs 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 230000000747 cardiac effect Effects 0.000 description 2
- 235000010980 cellulose Nutrition 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 230000001684 chronic effect Effects 0.000 description 2
- 238000011284 combination treatment Methods 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 238000007922 dissolution test Methods 0.000 description 2
- 238000001647 drug administration Methods 0.000 description 2
- 238000007908 dry granulation Methods 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- MVPICKVDHDWCJQ-UHFFFAOYSA-N ethyl 3-pyrrolidin-1-ylpropanoate Chemical compound CCOC(=O)CCN1CCCC1 MVPICKVDHDWCJQ-UHFFFAOYSA-N 0.000 description 2
- 230000003203 everyday effect Effects 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 2
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 2
- 208000017169 kidney disease Diseases 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 239000008101 lactose Substances 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 239000000395 magnesium oxide Substances 0.000 description 2
- CPLXHLVBOLITMK-UHFFFAOYSA-N magnesium oxide Inorganic materials [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 2
- AXZKOIWUVFPNLO-UHFFFAOYSA-N magnesium;oxygen(2-) Chemical compound [O-2].[Mg+2] AXZKOIWUVFPNLO-UHFFFAOYSA-N 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 229920000609 methyl cellulose Polymers 0.000 description 2
- 239000001923 methylcellulose Substances 0.000 description 2
- 235000010981 methylcellulose Nutrition 0.000 description 2
- 210000004165 myocardium Anatomy 0.000 description 2
- 230000036284 oxygen consumption Effects 0.000 description 2
- 239000008177 pharmaceutical agent Substances 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 229940045902 sodium stearyl fumarate Drugs 0.000 description 2
- 230000006641 stabilisation Effects 0.000 description 2
- 238000011105 stabilization Methods 0.000 description 2
- 239000000454 talc Substances 0.000 description 2
- 229910052623 talc Inorganic materials 0.000 description 2
- 229910000391 tricalcium phosphate Inorganic materials 0.000 description 2
- 229940078499 tricalcium phosphate Drugs 0.000 description 2
- 235000019731 tricalcium phosphate Nutrition 0.000 description 2
- 108060003345 Adrenergic Receptor Proteins 0.000 description 1
- 102000017910 Adrenergic receptor Human genes 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 206010002383 Angina Pectoris Diseases 0.000 description 1
- 239000004072 C09CA03 - Valsartan Substances 0.000 description 1
- 0 CCOC(O)[S@](CCC1CCCCC1)N(C(C(C[C@@]1CCC2)*[C@]12N)O)[C@@](C)CN=O Chemical compound CCOC(O)[S@](CCC1CCCCC1)N(C(C(C[C@@]1CCC2)*[C@]12N)O)[C@@](C)CN=O 0.000 description 1
- 208000024172 Cardiovascular disease Diseases 0.000 description 1
- 229920000623 Cellulose acetate phthalate Polymers 0.000 description 1
- JZUFKLXOESDKRF-UHFFFAOYSA-N Chlorothiazide Chemical compound C1=C(Cl)C(S(=O)(=O)N)=CC2=C1NCNS2(=O)=O JZUFKLXOESDKRF-UHFFFAOYSA-N 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- 239000004150 EU approved colour Substances 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 239000001856 Ethyl cellulose Substances 0.000 description 1
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 1
- 206010019233 Headaches Diseases 0.000 description 1
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 description 1
- 239000004354 Hydroxyethyl cellulose Substances 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 206010061216 Infarction Diseases 0.000 description 1
- 235000019759 Maize starch Nutrition 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- CERQOIWHTDAKMF-UHFFFAOYSA-M Methacrylate Chemical compound CC(=C)C([O-])=O CERQOIWHTDAKMF-UHFFFAOYSA-M 0.000 description 1
- 229920000881 Modified starch Polymers 0.000 description 1
- GIYXAJPCNFJEHY-UHFFFAOYSA-N N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]-1-propanamine hydrochloride (1:1) Chemical compound Cl.C=1C=CC=CC=1C(CCNC)OC1=CC=C(C(F)(F)F)C=C1 GIYXAJPCNFJEHY-UHFFFAOYSA-N 0.000 description 1
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 1
- MHQJUHSHQGQVTM-HNENSFHCSA-N Octadecyl fumarate Chemical compound CCCCCCCCCCCCCCCCCCOC(=O)\C=C/C(O)=O MHQJUHSHQGQVTM-HNENSFHCSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 229940077927 altace Drugs 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- HTIQEAQVCYTUBX-UHFFFAOYSA-N amlodipine Chemical compound CCOC(=O)C1=C(COCCN)NC(C)=C(C(=O)OC)C1C1=CC=CC=C1Cl HTIQEAQVCYTUBX-UHFFFAOYSA-N 0.000 description 1
- 229960000528 amlodipine Drugs 0.000 description 1
- 238000000540 analysis of variance Methods 0.000 description 1
- JYPVVOOBQVVUQV-CGHBYZBKSA-N angiotensinamide Chemical compound C([C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(N)=O)C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1N=CNC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=C(O)C=C1 JYPVVOOBQVVUQV-CGHBYZBKSA-N 0.000 description 1
- 230000003276 anti-hypertensive effect Effects 0.000 description 1
- 208000037849 arterial hypertension Diseases 0.000 description 1
- 210000001367 artery Anatomy 0.000 description 1
- 230000003416 augmentation Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-M benzenesulfonate Chemical compound [O-]S(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-M 0.000 description 1
- 102000012740 beta Adrenergic Receptors Human genes 0.000 description 1
- 108010079452 beta Adrenergic Receptors Proteins 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 235000010216 calcium carbonate Nutrition 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- PASHVRUKOFIRIK-UHFFFAOYSA-L calcium sulfate dihydrate Chemical compound O.O.[Ca+2].[O-]S([O-])(=O)=O PASHVRUKOFIRIK-UHFFFAOYSA-L 0.000 description 1
- 239000001175 calcium sulphate Substances 0.000 description 1
- 235000011132 calcium sulphate Nutrition 0.000 description 1
- NPAKNKYSJIDKMW-UHFFFAOYSA-N carvedilol Chemical compound COC1=CC=CC=C1OCCNCC(O)COC1=CC=CC2=NC3=CC=C[CH]C3=C12 NPAKNKYSJIDKMW-UHFFFAOYSA-N 0.000 description 1
- 229960004195 carvedilol Drugs 0.000 description 1
- 229940081734 cellulose acetate phthalate Drugs 0.000 description 1
- 239000011247 coating layer Substances 0.000 description 1
- 238000004040 coloring Methods 0.000 description 1
- 229940000425 combination drug Drugs 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 210000004351 coronary vessel Anatomy 0.000 description 1
- 238000007872 degassing Methods 0.000 description 1
- 230000000368 destabilizing effect Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 235000013681 dietary sucrose Nutrition 0.000 description 1
- 238000002845 discoloration Methods 0.000 description 1
- 208000002173 dizziness Diseases 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 230000002526 effect on cardiovascular system Effects 0.000 description 1
- 125000004185 ester group Chemical group 0.000 description 1
- 229920001249 ethyl cellulose Polymers 0.000 description 1
- 235000019325 ethyl cellulose Nutrition 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 229960000389 fluoxetine hydrochloride Drugs 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 125000003976 glyceryl group Chemical group [H]C([*])([H])C(O[H])([H])C(O[H])([H])[H] 0.000 description 1
- 231100000869 headache Toxicity 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 238000000589 high-performance liquid chromatography-mass spectrometry Methods 0.000 description 1
- 238000000265 homogenisation Methods 0.000 description 1
- 229960002003 hydrochlorothiazide Drugs 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 description 1
- 229920003063 hydroxymethyl cellulose Polymers 0.000 description 1
- 229940031574 hydroxymethyl cellulose Drugs 0.000 description 1
- 229920003125 hypromellose 2910 Polymers 0.000 description 1
- 229940031672 hypromellose 2910 Drugs 0.000 description 1
- 230000007574 infarction Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 description 1
- 239000001095 magnesium carbonate Substances 0.000 description 1
- 229910000021 magnesium carbonate Inorganic materials 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 229960003194 meglumine Drugs 0.000 description 1
- 230000000936 membranestabilizing effect Effects 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- 238000010606 normalization Methods 0.000 description 1
- KVWDHTXUZHCGIO-UHFFFAOYSA-N olanzapine Chemical compound C1CN(C)CCN1C1=NC2=CC=CC=C2NC2=C1C=C(C)S2 KVWDHTXUZHCGIO-UHFFFAOYSA-N 0.000 description 1
- 229940006093 opthalmologic coloring agent diagnostic Drugs 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 238000006864 oxidative decomposition reaction Methods 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- IPVQLZZIHOAWMC-QXKUPLGCSA-N perindopril Chemical compound C1CCC[C@H]2C[C@@H](C(O)=O)N(C(=O)[C@H](C)N[C@@H](CCC)C(=O)OCC)[C@H]21 IPVQLZZIHOAWMC-QXKUPLGCSA-N 0.000 description 1
- 229960002582 perindopril Drugs 0.000 description 1
- 230000036581 peripheral resistance Effects 0.000 description 1
- 239000008019 pharmaceutical lubricant Substances 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- XNGIFLGASWRNHJ-UHFFFAOYSA-L phthalate(2-) Chemical compound [O-]C(=O)C1=CC=CC=C1C([O-])=O XNGIFLGASWRNHJ-UHFFFAOYSA-L 0.000 description 1
- 230000036470 plasma concentration Effects 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 239000004810 polytetrafluoroethylene Substances 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- XAEFZNCEHLXOMS-UHFFFAOYSA-M potassium benzoate Chemical compound [K+].[O-]C(=O)C1=CC=CC=C1 XAEFZNCEHLXOMS-UHFFFAOYSA-M 0.000 description 1
- 239000003087 receptor blocking agent Substances 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000000250 revascularization Effects 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 238000009097 single-agent therapy Methods 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000007909 solid dosage form Substances 0.000 description 1
- 239000008247 solid mixture Substances 0.000 description 1
- 238000013112 stability test Methods 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 229940032147 starch Drugs 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 229940071138 stearyl fumarate Drugs 0.000 description 1
- 229960004793 sucrose Drugs 0.000 description 1
- 150000005846 sugar alcohols Chemical class 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 230000002110 toxicologic effect Effects 0.000 description 1
- 231100000027 toxicology Toxicity 0.000 description 1
- SJSNUMAYCRRIOM-QFIPXVFZSA-N valsartan Chemical compound C1=CC(CN(C(=O)CCCC)[C@@H](C(C)C)C(O)=O)=CC=C1C1=CC=CC=C1C1=NN=N[N]1 SJSNUMAYCRRIOM-QFIPXVFZSA-N 0.000 description 1
- 229960004699 valsartan Drugs 0.000 description 1
- 238000005550 wet granulation Methods 0.000 description 1
- 229940100445 wheat starch Drugs 0.000 description 1
- XOOUIPVCVHRTMJ-UHFFFAOYSA-L zinc stearate Chemical class [Zn+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O XOOUIPVCVHRTMJ-UHFFFAOYSA-L 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2072—Pills, tablets, discs, rods characterised by shape, structure or size; Tablets with holes, special break lines or identification marks; Partially coated tablets; Disintegrating flat shaped forms
- A61K9/2086—Layered tablets, e.g. bilayer tablets; Tablets of the type inert core-active coat
- A61K9/209—Layered tablets, e.g. bilayer tablets; Tablets of the type inert core-active coat containing drug in at least two layers or in the core and in at least one outer layer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/403—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil condensed with carbocyclic rings, e.g. carbazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2095—Tabletting processes; Dosage units made by direct compression of powders or specially processed granules, by eliminating solvents, by melt-extrusion, by injection molding, by 3D printing
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4808—Preparations in capsules, e.g. of gelatin, of chocolate characterised by the form of the capsule or the structure of the filling; Capsules containing small tablets; Capsules with outer layer for immediate drug release
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5005—Wall or coating material
- A61K9/5021—Organic macromolecular compounds
- A61K9/5036—Polysaccharides, e.g. gums, alginate; Cyclodextrin
- A61K9/5042—Cellulose; Cellulose derivatives, e.g. phthalate or acetate succinate esters of hydroxypropyl methylcellulose
- A61K9/5047—Cellulose ethers containing no ester groups, e.g. hydroxypropyl methylcellulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5084—Mixtures of one or more drugs in different galenical forms, at least one of which being granules, microcapsules or (coated) microparticles according to A61K9/16 or A61K9/50, e.g. for obtaining a specific release pattern or for combining different drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/06—Antiarrhythmics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
Definitions
- the invention relates to a stable pharmaceutical composition suitable for the treatment of cardiac insuffiency, namely comprising ramipril of the Formula (I)
- compositions were developed for the treatment of hypertension which decrease blood pressure by various types of mechanism.
- high blood pressure is often associated with other diseases, in many cases monotherapy is not sufficient, as disclosed in the Directive issued in Hungary by the Hungarian Hypertension Company.
- the use of combined pharmaceutical compositions as first treatment may be justified.
- combinations such as a diuretic (e.g. hydrochlorothiazide) and a beta blocker (e.g. carvediol), or a diuretic and an ACE- inhibitor (e.g.
- perindopril or a diuretic and an angiotensin-II-receptor antagonist (e.g. valsartan), or a calcium antagonist (e.g.amlodipine) and a beta blocker, or a calcium antagonist and an ACE-inhibitor, or a calcium antagonist and an angiotensin-II- receptor- antagonist, or an alpha- 1-adrenerg receptor blocker and a beta blocker can be used.
- an angiotensin-II-receptor antagonist e.g. valsartan
- calcium antagonist e.g.amlodipine
- beta blocker e.g.amlodipine
- a combined therapy may become necessary, namely if the efficiency of the first medicine is unsatisfactory or the increase of the dosage of the first pharmaceutical agent causes the augmentation of the number or strength of the side effects.
- Ramipril and bisoprolol are generally used in the treatment of hypertension and cardiac insufficiency. Said two active ingredients are also applied together. Only in Hungary about 40 000 hypertension patients receive such combination treatment which comprises the simultaneous use of bisoprolol and ramipril. In Hungary 16 % of the patients treated with ramipril receive bisoprolol as well, while 12 % of the patents treated with bisoprolol receive 12 % of ramipril too.
- the components of the combination treatment differ significantly from each other both in their chemical structure and mechanism of effect. Both active ingredients are sensitive and for this reason the preparation of stable pharmaceutical compositions is not simple, even if the composition comprises only ramipril or bisoprolol.
- Ramipril of the Formula (I) /chemical name: (2S,3aS,6aS)-l-[(S)-2-[[(S)-l- ethoxycarbonyl)-3-phenyl-propyl]-amino]-propanoyl/-octahydrocyclopenta[b]- pyrrole-2-carboxylic acid] is a known pharmaceutical active ingredient which is used in therapy for the treatment of hypertension, cardiac insufficiency and nephropathy, the promotion of revascularization, the decrease of the risk of cardiovascular diseases and events, particularly of stroke and myocardic infarction, and the reduction of cardiovascular mortality.
- Ramipril exhibits its effect first of all via an active metabolit -namely ramiprilate - which is an effective inhibitor of the angiotensine converting enzyme (ACE).
- ACE angiotensine converting enzyme
- Ramipril is a pharmaceutical active ingredient sensitive to moisture and mechanical effects (e.g. pressing) and decomposes easily. Said decomposition is due partly to the hydrolysis of the ester group which gives rise to the formation of a contamination corresponding to the active metabolite, namely ramiprilate /European Pharmacopoeia: Impurity E). The decomposition of ramipril can also be due to the formation of the diketopiperazine contamination of the Formula (III)
- a polymeric coating is applied and the product is then subjected to further pharmaceutical technological processing (e.g. to tableting).
- further pharmaceutical technological processing e.g. to tableting.
- 3-25 % of hydroxypropyl cellulose, hydroxpropyl methyl cellulose phthalate, hydroxyethyl cellulose, ethyl cellulose, cellulose acetate phthalate, polyvinyl pyrrolidone or a methacrylate type agent or a different coating agent is applied on the surface of the particles of the ramipril active ingredient as a layer formed from a pharmaceutically suitable coasting system.
- WO 2006050533 in course of the preparation of the ramipril particles coated with a stabilizing layer aggregation which takes place before the coating step had been eliminated and therefore each individual particle received a separate coating.
- coating agent preferably hydroxymethyl cellulose is used.
- the coating layer is applied by means of a fluidization method.
- Ramipril can also be stabilized by formulating the active ingredient in the presence of a stabilizing auxiliary agent and omitting the auxiliary agents generally used in pharmaceutical technology which are incompatible with ramipril.
- auxiliary agents of acidic character which have a large specific surface are incompatible with ramipril and can not be used therefore in the formulating procedure
- Such an auxiliary agent is e.g. colloidal silica (Aerosil) frequently used in formulating methods.
- EP 1 734 931 relates to ramipril containing compositions which comprise calcium sulphate dihydrate as auxiliary agent.
- concentration of the ramipril diketopiperazine decomposition product amounts to 0.478-1.06 % which is significantly lower than that of the pharmaceutical composition put on the market.
- ramipril is stabilized simultaneously with sodium hydrogen carbonate and calcium sulphate whereby particularly the decrease of the concentration of the ramiprilate impurity was observed.
- a similar process is described in US 20030215526.
- ramipril and other ACE-inhibitors are stabilized with meglumine and optionally lower substituted hydroxypropyl cellulose.
- the product stored at 60°C for 15 days contains 0.75-1.59 % of the decomposition product.
- the disadvantage of this process is that when stored the composition at higher temperature already for a short period of time decomposition of the product becomes significant.
- WO 2005002548 relates to stabilized pharmaceutical compositions which contain a significantly homogenized mixture of an ACE inhibitor and an effective amount of a pharmaceutical lubricant and if desired further auxiliary agents.
- lubricant conventional auxiliary agents can be used.
- the composition according to said international patent application contains decomposition product in an amount similar to that of the marketed reference composition.
- WO 2007120930 is concerned with stable pharmaceutical compositions comprising ramipril and a stabilizing auxiliary agent.
- the stability of a pharmaceutical composition filled in hard gelatine capsules and containing ramipril (1.25 mg), gelatinized starch (23.35 mg), lactose (25.0 mg) and stearyl fumarate (0.4 mg) was tested and it has been found that after a storage of 3 months at 40°C under a relative moisture content of 75 % the concentration of the diketopiperazine impurity amounted to 3.5 %. Under similar storing conditions in the ALTACE composition of the originator the concentration of the same impurity was 4.9 %.
- WO 2008021875 tablets comprising more than one active ingredients are disclosed in which the different active ingredients are formulated in different layers which are physically separated and are in contact with each other only at a small surface.
- WO 2008095263 relates to pharmaceutical compositions comprising more than one active ingredients in which the active ingredients are formulated in physically different pharmaceutical forms (e.g. powder, granule, pellet, particle, minitablet, tablet) in order to minimize the interaction between the individual active ingredients.
- the active ingredients are formulated in physically different pharmaceutical forms (e.g. powder, granule, pellet, particle, minitablet, tablet) in order to minimize the interaction between the individual active ingredients.
- the active ingredients are formulated in physically different pharmaceutical forms (e.g. powder, granule, pellet, particle, minitablet, tablet) in order to minimize the interaction between the individual active ingredients.
- tablets were prepared in which the fluoxetine hydrochloride was formulated in form of a powder mixture and the olanzapin active ingredient in form of a minitablet.
- compositions comprising amlodipin and an ACE-inhibitor described in WO2008065485 are prepared by admixing the active ingredients and adjusting the pH of the composition to a value higher than 6.0. This is achieved by preparing from a mixture of amlodipin besylate, microcrystalline cellulose, magnesium carbonate and a colouring agent pellets by wet granulation and filling said pellets in capsules.
- the marketed TRITACE product described in prior art comprises in addition to ramipril also sodium stearyl fumarate, hypromellose, microcrystalline cellulose, starch which swells in the cold and in case of compositions of a certain strength also colouring agents.
- this composition can be stored only at a temperature not exceeding 25°C. It can be deemed as probable that at a higher temperature and/or moisture content the product rapidly decomposes.
- the health authorities raise strict requirements relating to the active ingredient content, stability and purity of pharmaceutical compositions.
- ramipril containing pharmaceutical compositions which can be stored for a longer period of time in such a manner that the active ingredient content does not decrease below the allowed limit value and the amount of the decomposition products does not exceed the corresponding limit value.
- Bisoprolol (chemical name l-[4-[[2-(l-methyl-ethoxy)-ethoxy]-methyl]-phenoxy]-3- [(l-methyl-ethyl)-amino]-2-propanol) is an adrenoceptor blocker of high beta-1- selectivity.
- Bisoprolol is cardioselective and exhibits neither a sympatomimetic activity (ISA) nor a clinically significant membrane stabilizing effect.
- the decrease of the renin activity of the plasma also contributes to the antihypertensive effect of the beta-blockers. It decreases the heart rate and the pulse volume, consequently also the minute volume and the oxygen consumption. In course of chronic treatment the peripheral resistance increases at the beginning and decreases later.
- Bisoprolol inhibits the response given to the sympato-adrenerg activation through blockade of the beta- receptors of the heart. This results in the decrease of the heart rate and contractility and thereby the reduction of the oxygen consumption of the heart muscle which is a desired effect in the treatment of Angina pectoris derived from the disease of the coronary artery.
- the marketed pharmaceutical compositions contain bisoprolol hemifumarate of the Formula (IV) as bisoprolol salt due to the relative stability and favourable processing properties thereof.
- the use of other salts is encountered with several problems such as the insufficient stability, and in some cases the different absorption of the products which makes their interchangeability with the products on the market impossible.
- the hemifumarate salt of bisoprolol has the drawback that the other acid function of fumaric acid can destabilize the other active ingredient of the composition.
- the double bond of fumaric acid can react with amines, as described in WO 110038091 which relates to tablets containing amlodipin and bisoprolol.
- the object of the invention is to develop a combined pharmaceutical composition which contains ramipril and bisoprolol or a salt or complex thereof in form of a dosage unit, and whereby a) the active ingredients remain stable under the conditions of manufacture and storage and thereby the amount of the impurities being present in the composition or formed during the manufacturing process does not exceed the impurity limit authorized for the mono compositions having the given active ingredient content; b) the bioavailability of the combined pharmaceutical composition is similar to or identical with that measured in case of simultaneous administration of two mono compositions which have an active ingredient content corresponding to that of the combined composition; and c) the dissolution profile of each active ingredient does not change during storage.
- bioequivalent compositions corresponding to the above objects of the invention make possible to continue the treatment of patients treated by simultaneous administration of two mono compositions with the use of the combined pharmaceutical composition according to the present invention without any risks.
- This is particularly important in case of patients suffering from high blood pressure because when such patients are treated with more than one active ingredients the blood pressure can be adjusted generally only within a longer period of time and in case of the use of a new combination there is a risk that the desired effect can not be achieved.
- the selection and adjustment of the new medicine can last several months and during this time the side effects caused by high blood pressure (dizziness, head ache) significantly deteriorate the quality of life of the patient and the development and/or progress of the accompanying diseases are not prevented.
- the combined pharmaceutical composition is bioequivalent, the lengthy procedure of adjustment and the risks can be avoided.
- a further object of the present invention is that the dissolution profiles should not change during the storage. Namely, if the dissolution profile of one or both active ingredient(s) change, the bioavailability of the product may differ from the prescribed value and therefore the combined pharmaceutical composition will not only become unsuitable for the replacement of the corresponding mono compositions but can not be even used as a medicine.
- a combined composition comprising ramipril and bisoprolol can be suitable for use as a once-a-day pharmaceutical composition for the treatment of hypertension, preferably high renin level hypertension.
- the two active ingredients are incompatible.
- the percental impurity content of the active ingredient is disclosed. It can be seen that the amount of impurity D of the Formula III of ramipril is increased to a small extent during storage of pure ramipril but in the presence of bisoprolol hemifumarate the amount of said impurity augments to more than 32 % and this is unacceptable. It has been found in a surprising manner that it is still possible to develop a pharmaceutical composition suitable for the treatment of patients suffering from high blood pressure, particularly of high renin level high blood pressure by separating the active ingredients from each other physically in the composition.
- the invention relates to a solid combined pharmaceutical composition which comprises ramipril and bisoprolol or a pharmaceutically acceptable salt or complex thereof.
- the pharmaceutical composition according to the present invention contains both active ingredients in form of a single dosage unit which can be e.g. tablet or capsule.
- the dosage units in table form can be mono- or two-layer tablets.
- the invention relates to a solid pharmaceutical composition in which the particles of the active ingredients ramipril and bisoprolol or a pharmaceutically acceptable salt or complex thereof are separated from each other.
- the particles of the active ingredients ramipril and bisopolol or a pharmaceutically acceptable salt or complex thereof can be in form of separate phases whereby the particles of the active ingredients are in contact with each other only on the edge surface of the phases.
- two- layer tablets in which one layer contains the ramipril active ingredient and the other the bisoprolol.
- the active ingredients are in contact with each other on the edge surface of the layers, the amount of the contacting active ingredients on the surface is so small that the decomposition related to the total active ingredient content can be neglected.
- composition according to the present invention comprises preferably as active ingredient ramipril or a salt, e.g. sodium or potassium salt thereof and biosoprolol or a salt thereof, e.g. bisoprolol hemifumarate.
- ramipril and bisoprolol hemifumarate are advantageous embodiments of the present invention.
- the pharmaceutical composition according to the present invention may also contain auxiliary agents generally used in pharmaceutical industry.
- auxiliary agents e.g. fillers, glidants (antiadhesives), binders, disintegrants, lubricants etc. can be used.
- the pharmaceutical compositions according to the present invention may contain fillers, in an amount of 20-90 % by weight.
- the amount of the filler is preferably 40- 80 % by weight, particularly 60-70 % by weight.
- Any filler generally used in pharmaceutical industry can be used which does not affect the stability of the active ingredients.
- filler e.g. organic polymers, such as microcrystalline cellulose; organic mono- or polysaccharides or sugar alcohols, such as lactose, mannitol or saccharose , or inorganic salts, e.g. tricalcium phosphate, calcium phosphate, calcium carbonate or sodium chloride can be used.
- the composition contains as filler microcrystalline cellulose.
- the pharmaceutical composition according to the present invention may also contain disintegrants in order to promote dissolution of the active ingredients.
- Disintegrants are substances which swell under the effect of moisture and burst the tablet or capsules open whereby the active ingredients are set free.
- the disintegrants are generally organic polymers.
- the disintegrant content of the composition is generally 1-30 % weight, preferably 10-25 % by weight %, particularly 10-20 % by weight %. Any disintegrant generally used in pharmaceutical industry can be applied which does not enter into interaction with the active ingredients.
- organic polymers such as cross-linked polyvinyl pyrrolidone (Poliplasdon, particularly Polyplasdon XL- 10), microcrystalline cellulose or starch (e.g.
- the external phase can preferably contain microcrystalline cellulose of larger particle size (e.g. Vivapur 200) and the internal phase can preferably contain microcrystalline cellulose of smaller particle size (e.g. Vivapur 101) e.g. for compacting bisoprolol.
- the composition may also contain binders, if necessary.
- Any binder generally used in pharmaceutical industry can be applied which does not enter into interaction with the active ingredients.
- organic polymers such as hydroxypropyl methyl cellulose (hypromellose, referred to further on as HPMC) can be used, preferably a product which has a viscosity lower than 1000 mPas in a 2 % aqueous solution at 20°C. It is still more preferable to use HMPC having a viscosity of 3000- 6000.
- HPMC types are Pharmacoat 603 or 606.
- PVP polyvinyl pyrrolidone - referred to furtheron as PVP.
- the amount of the binder in the pharmaceutical composition is preferably 1-10 % by weight, preferably 1-5 % by weight, particularly 1-3 % by weight. Formulation of the pharmaceutical composition can be rendered easier by using a lubricant and a glidant.
- the lubricant content of the pharmaceutical composition according to the present invention is 0.1-10 % by weight, preferably 0.1-5 % by weight, particularly 0.2-2 weight by weight %.
- Any organic or inorganic lubricant generally used in pharmaceutical industry can be applied such as stearates (e.g. magnesium stearate, sodium stearyl fumarate), glyceryl behenate, (such as compritol 888) or inorganic substances (e.g. talc).
- Any glidant generally used in pharmaceutical industry can be applied e.g. talc, colloidal silica or tricalcium phosphate etc.
- the glidant content of the pharmaceutical composition according to the present invention is 0.1-10 % by weight, preferably 0.1-5 % by weight, particularly 0.1-2 weight by weight %.
- the essence of the invention is that the active ingredients are separated from each other in the composition. If the active ingredients are contained in the same layer, then at least one of the active ingredients is (are) to be protected by a coating.
- Said coating may contain any coating agent generally used in pharmaceutical industry - e.g. an organic polymer - which does not enter into interaction with the active ingredients. It is preferred to use polymers which swell or are dissolved in water and thereby ensure the rapid dissolution of the active ingredients from the tablet. As polymer e.g.
- HPMC preferably low viscosity HMPC having a viscosity under 10000 mPas in 2% aqueous solution at 20°C, particularly HPMC having a viscosity of 3000-6000 mPas can be used.
- HPMC types are Pharmacoat 603 or 606.
- polyvinyl pyrrolidone can also be applied.
- products of lower polymerisation degree e.g. K-15 or K-30 can be used.
- the pharmaceutical composition contains the coating agent in an amount of 1-10 % by weight, preferably 1-5 % by weight, particularly 1-3 % by weight.
- phase which contain the active ingredients are named as internal phase and the added further ingredients are named as components of the external phase.
- the internal phase of the composition according to the present invention can be a premix (powder mixture), uncoated or coated granule, compact, pellet or minitablet.
- the internal phase contains 10-50 % by weight, preferably 10-30 % by weight, particularly 15-25 % by weight of the active ingredients, related to the weight of the internal phase.
- the amount of the filler is 40-90 % by weight.
- the internal phase can contain further auxiliary agents
- the internal phase contain 1-15 % by weight, preferably 1-10 % by weight, particularly 2-6 weight by weight % of a coating, related to the weight of the coated internal phase.
- At least one of the two active ingredients or pharmaceutically acceptable salts or complexes thereof is (are) incorporated into dry granules or compacts.
- the particles or granules or compacts of at least one of the active ingredients is (are) coated.
- the particles of ramipril or salts thereof or granules, impacts or minitablets containing the same and particles of biosoprolol or a salt thereof and granules, minitablets or compacts containing the same are coated.
- compositions of the present invention are mono- or two-layer tablets or capsules, regarding their pharmaceutical form.
- mono-layer tablets are those which contain both active ingredients in the same layer of the tablet.
- Tablets consisting of two or more layers which contain in addition to ramipril and bisoprolol optionally further active ingredients also belong to this group provided that ramipril and bisoprolol are incorporated into the same layer of the tablet.
- the invention also relates to mono-or two-layer tablets in which bisoprolol and ramipril are incorporated into the same layer whereby the particles of the active ingredient are separated from each other in the layer.
- the active ingredients are separated from each other as follows: at least one of the active ingredients of the mixture to be pressed are used in form of coated particles, coated granules or coated pellets. It is preferred to use both active ingredients in coated form.
- the mono-layer tablets contain the ramipril and bisoprolol active ingredients in form of coated granules or compacts.
- bisoprolol is compacted with microcrystalline cellulose and coated with HPMC, while ramipril is granulated or compacted with polyplasdon and coated with HPMC.
- the invention also relates to multilayer tablets which contain bisoprolol and ramipril or pharmaceutically acceptable salts thereof in separate layers.
- multi-layer tablets relates to tablets which - independently from the number of the layers - contain the two active ingredients in two separate contacting layers, or in layers separated from each other by one or more layers. In this case it is not necessary that the active ingredients should be coated.
- ramipril is granulated or compacted with polyplasdon, and coated with HPMC.
- Capsules according to the present invention contain coated particles, granules, pellets or coated or uncoated minitablets of one of the active ingredients, and coated or uncoated particles, or coated or uncoated granules, pellets or coated or uncoated minitablets of the other active ingredient and optionally further auxiliary agents (e.g. fillers, glidants and antiadhesives).
- auxiliary agents e.g. fillers, glidants and antiadhesives.
- capsules contain the active ingredients in form of coated granules, and also contain fillers, antiadhesives and lubricants.
- Such compacts contain 5-30 % by weight, preferably 10-25 % by weight, particularly 20-25 % by weight of bisoprolol hemifumarate, 60-93 % by weight, preferably 65-80 % by weight, particularly 75-80 % by weight of microcrystalline cellulose and 2-10 % by weight, preferably 3-7 % by weight, particularly 4-5 % by weight of an organic polymer, e.g. polyvinyl pyrrolidone or HPMC, most preferably HPMC - related to the weight of the coated compact. It has been surprisingly found that although the compact is not isodimensional, a layer is formed on said compact which significantly decreases or hinders the decomposition reactions caused by fumaric acid.
- the present invention also relates to the coated bisoprolol compacts described above.
- the compact according to the present invention can be incorporated into combined pharmaceutical compositions whereby the strong decomposing effect of fumaric acid exerted on the other active ingredient can be avoided.
- a process for the preparation of pharmaceutical compositions which contain bisoprolol and ramipril or a pharmaceutically acceptable salt or complex thereof.
- HPMC or polyvinyl pyrrolidone HPMC or polyvinyl pyrrolidone
- auxiliary agents preferably fillers, if necessary with disintegrants, lubricants, glidants and antiadhesives
- pressing the mixture thus obtained to tablets or filling in capsules It is preferred to fill the mixture in hard gelatine capsules.
- At first ramipril is granulated and coated, the coated or uncoated bisoprolol particles and the auxiliary agents are admixed with the coated granules and the mixture thus obtained is homogenized and filled in capsules or pressed to tablets.
- bisoprolol is converted into coated granules or compacts and is used in this pharmaceutical form.
- Ramipril is preferably granulated by subjecting a mixture of ramipril and a disintegrant, preferably cross-linked PVP (e.g. polyplasdone XL 10) to pre-granulation with a solution of HPMC in a vortex granulating machine and thereafter coating the granules thus obtained in a fluidization granulator with HPMC.
- a disintegrant preferably cross-linked PVP (e.g. polyplasdone XL 10) to pre-granulation with a solution of HPMC in a vortex granulating machine and thereafter coating the granules thus obtained in a fluidization granulator with HPMC.
- a disintegrant preferably cross-linked PVP (e.g. polyplasdone XL 10) to pre-granulation with a solution of HPMC in a vortex granulating machine and thereafter coating the granules thus obtained in a fluidization granulator with HPMC.
- One may proceed by
- a disintegrant preferably cross-linked PVP (e.g. polyplasdone XL 10) to dry granulation (compacting), if necessary regranulating the compact thus obtained and coating the granules with a 5-10 % by weight, preferably 5-8 % by weight aqueous solution of HPMC.
- Bisoprolol can be used not only as particles but also in form of granules or compacts. Thus one may proceed by homogenizing and compacting bisoprolol with the filler and subjecting the compact to re-granulation e.g. on a sieve. It is preferred to regranulate the compacts in more than one step. Thus e.g. regranulation may be carried out at first on a 1.2 mm sieve and thereafter on a e.g. 0.5 mm sieve. The compacted granules may be directly used. One may also proceed by applying the solution of bisoprolol on a carrier in a fluidization granulating or vortex granulating apparatus.
- Bisoprolol can also be coated by applying on the granules an organic polymer (preferably HPMC or polyvinyl pyrrolidone layer) in a fluidization granulating apparatus. It is preferred to use a 5-10 % by weight, preferably 5-8 % by weight aqueous HPMC solution in the coating step.
- an organic polymer preferably HPMC or polyvinyl pyrrolidone layer
- the bisoprolol hemifumarate containing coated compact according to the present invention can be prepared by homogenizing 5-30 % by weight, preferably 10-25 % by weight, particularly 20-25 % by weight of bisoprolol hemifumarate, and 60-93 % by weight, preferably 65-80 % by weight, particularly 75-80 % by weight of microcrystalline cellulose - related to the weight of the coated compact - whereupon the mixture is compacted. If necessary the compact thus obtained is regranulated first on a 1.2 mm sieve and thereafter on a 0.5 mm sieve.
- the optionally regranulated bisoprolol hemifumarate containing compacts are coated with an organic polymer, preferably HPMC or polyvinyl pyrrolidone so that a 5-10 % by weight, preferably 5-8 % by weight aqueous solution of 2-10 % by weight, preferably 3-7 % by weight, particularly 4-5 % by weight of an organic polymer, preferably HPMC or polyvinyl pyrrolidone - related to the weight of the coated compact - is used.
- an organic polymer preferably HPMC or polyvinyl pyrrolidone
- the coated compact thus obtained is not isodimensional, it prevents the fumaric acid component of bisoprolol hemifumarate from exerting the decomposing effect on other active ingredients, e.g. on ramipril.
- the present invention also relates to tablets which consist of layers and contain the two active ingredients in separate layers.
- Such tablet can also be prepared by homogenizing the active ingredients with the auxiliary agents in separate layers and thereafter pressing to tablets consisting of layers on a suitable apparatus (e.g. on a sandwich tableting equipment). Since ramipril is sensitive to pressure it is advisable to press first the bisoprolol containing layer, thereafter applying the components of the ramipril containing layer and finally applying the product thus obtained on the bisoprolol containing layer.
- the layer containing one of the active ingredients preferably on the layer comprising bisoprolol or a salt thereof, particularly the hemifumarate salt
- a separating layer consisting of the auxiliary agents
- the above tablets consisting of layers can be preferably prepared by using in each layer granules or coated granules of the active ingredients in place of the particles of the active ingredients.
- the coated or uncoated bisoprolol granules are homogenized with the components of the external phase, the mixture is pressed and finally the tablets consisting of layers are obtained by applying the homogenized mixture of the uncoated or coated ramipril granules and the external phase.
- the preparation of the corresponding coated or uncoated granules is described above.
- capsules which comprise at least one of the active ingredients in form of a minitablet or as minitablets.
- the other active ingredient may be in form of coated or uncoated particles, granules, pellets or minitablets.
- Minitablets may be prepared by homogenizing one of the active ingredients (preferably ramipril) or granules or coated or uncoated pellets prepared therefrom as described above with the components of the external phase and pressing the mixture to minitablets.
- the size of the minitablets should be such that they can be placed into the capsule.
- one or more minitablet(s) obtained is (are) placed in the capsule (preferably in hard gelatine capsule) whereupon the mixture comprising the other active ingredient, which contains the coated or uncoated particles of the active ingredient or the coated or uncoated granules or pellets containing the active ingredients and if necessary the components of the external phase, are introduced in the capsule which is finally closed.
- One may also proceed by preparing minitablets from both active ingredients as described above and placing said minitablets in soft gelatine capsules.
- the invention relates to a solid combined pharmaceutical composition which contains ramipril and bisoprolol or a pharmaceutically acceptable salt or complex thereof.
- composition according to the present invention contains preferably 1.25-10 mg of ramipril and preferably 1.25-10 mg of bisoprolol.
- the ratio of bisoprolol and ramipril - related to a combination of bisoprolol hemifumarate and ramipril base, in mg/mg - is as follows: 1.25/5; 2.5/2.5; 2.5/5; 2.5/10; 5/1.25; 5/2.5; 5/5; 5/10; 10/2.5; 10/5; 10/10; 10/5 mg/mg.
- the combined product containing bisoprolol hemifumarate and ramipril has generally most preferably the following composition: 5/5 - 2.5/5 and 10/5.
- compositions fall under the scope of the present invention in which the particles of the ramipril and bisoprolol active ingredients or pharmaceutically acceptable salts thereof are in separate phases and the particles of the active ingredient are in contact with each other only at the limit surface of the layers.
- the pharmaceutical composition according to the present invention may be preferably in form of tablets or capsules.
- the pharmaceutical composition according to the present invention may also be in form of multi-layer tablets in which bisoprolol and ramipril or pharmaceutically acceptable salts thereof are formulated in separate layers.
- the tablet according to the present invention can also be a mono- or multi-layer tablet in which the bisoprolol and ramipril active ingredients are in the very same layer of the tablet and the particles of the active ingredients are separated from each other in the layer.
- at least one of both active ingredients or pharmaceutically acceptable salts thereof is (are) in form of a granule, preferably a dry granule or a compact.
- the particles of at least one active ingredient or granules or compacts containing the same is (are) coated.
- particles of ramipril or a salt thereof or granules, compacts or minitablets containing the same and particles of bisoprolol or a salt thereof or granules, minitablets or compacts containing the same are coated.
- the composition contains as active ingredient ramipril and bisoprolol hemifumarate.
- a ramipril containing coated granule which contains cross-linked polyvinyl- pyrrolidone and optionally HPMC and other auxiliary agents, whereby said granules are coated with an organic polymer, preferably HPMC;
- a bisoprolol hemifumarate containing coated compact which contains bisoprolol hemifumarate, microcrystalline cellulose and optionally further auxiliary agents generally used in pharmaceutical industry, whereby said granules are coated with an organic polymer, preferably HPMC, and c) an external phase which contains a filler, preferably microcrystalline cellulose, a glidant preferably colloidal silica and a lubricant, preferably magnesium stearate or glyceryl behenate.
- the ramipril containing coated granules, the bisoprolol hemifumarate containing coated compacts and the components of the external phase are filled in a capsule.
- the present invention also relates to a coated compact comprising bisoprolol hemifumarate active ingredient which contain a filler, preferably microcrystalline cellulose, as coating agent an organic polymer such as polyvinyl pyrrolidone, HPMC or a mixture thereof, preferably HPMC.
- a filler preferably microcrystalline cellulose
- an organic polymer such as polyvinyl pyrrolidone, HPMC or a mixture thereof, preferably HPMC.
- a bisoprolol hemifumarate containing coated compact which contains 5-30 % by weight, preferably 10-25 % by weight, particularly 20-25 % by weight of bisoprolol hemifumarate, 60-93 % by weight, preferably 65-80 % by weight, particularly 75-80 % by weight of a filler, preferably microcrystalline cellulose and 2-10 % by weight, preferably 3-7 % by weight., particularly 4-5 % by weight of an organic polymer, e.g. polyvinyl pyrrolidone or HPMC, particularly HPMC - related to the weight of the coated compact.
- an organic polymer e.g. polyvinyl pyrrolidone or HPMC, particularly HPMC - related to the weight of the coated compact.
- compositions of varying strength which contain the active ingredients separately in granulated or compacted form. It is very advantageous to use ramipril containing granules or compacts of the following composition: 72
- Ramipril containing granules or compacts and bisoprolol containing compacts can be used for the preparation of combined pharmaceutical compositions of varying active ingredient content which comprise in addition to the active ingredient containing compacts or granules further auxiliary agents.
- the advantageous composition of such capsules of varying strength is shown in the following Table
- the solid pharmaceutical composition according to the present invention contains the active ingredients substantially separated from each other and on the other characterized by the fact that their dissolution profile is similar to the dissolution profiles observed on simultaneously administration of the mono compositions i.e. the pharmaceutical composition according to the present invention can directly replace the simultaneous administration of the mono compositions.
- the dissolution of the active ingredients from the compositions according to the present invention was tested under standard conditions according to European Pharmacopoeia (2.9.3. dissolution test for solid dosage forms) at 37°C in 900 ml of a 0.1 N HCl solution by using a 50 r.p.m. paddle stirrer.
- compositions comprising bisoprolol and ramipril in a ratio of 10/5 mg and 10/10 mg meet the requirements .
- Bisoprolol and ramipril were formulated in form of coated compacts as described above
- the plasma ramipril concentration obtained from the combined composition is substantially bioequivalent to the ramipril containing reference composition. Since we have carried out a so-called "pilot" test, the case number was significantly lower than justified by the variability of the ramipril C max values among the patients. Due to the small case number the 90 % confidence interval of the C max test/reference ratio was broad. However the lower limit of the confidence interval (79.4 %) was just below the desired value of 80 %. At the same time the higher value (117.8 %) is within the acceptable limit value, but falls into the upper range of the interval. It is evident that the broad confidence interval is due to the small case number.
- the logarithmically transformed test/reference ratio (96.7 %) is within the expected value and is near to 100 %. If the case number is increased the confidence interval becomes narrower and therefore also the lower value falls in the acceptable interval. However the deviation is not so high and when the parallel treatment with two mono compositions is replaced by the administration of the combined composition according to the present invention no unexpected effect or side effect was observed.
- the 90 % confidence interval of the test/reference ratio falls in the prescribed interval of 80-125 % and on the basis of the test/reference ratio (99.96 %) the AUC values can be considered to be almost identical and differ from the 100 % value only to a small extent.
- the above data prove that the two mono compositions of the corresponding active ingredient content can be replaced by the combined composition according to the present invention substantially without any risk.
- a solid composition in which ramipril and bisoprolol are separated from each other, and which contain 10 mg of ramipril and 10 mg of bisoprolol, preferably bisoprolol fumarate, whereby the C max value of the composition related to bisoprolol is 35-55 ng/ml, preferably 39-48 ng/ml, particularly 43.8 ng/ml, the C max value related to ramipril is 13-21 ng/ml, preferably 14,82-18.15 mg/ml, particularly 16.5 ng/ml and/or the AUCT value related to bisoprolol is 503-790 ng.h/ml, preferably 566-692 ng.h/ml, particularly 629 ng.h/ml and the AUC T value related to ramipril is 9.3-14.7 ng.h/ml, preferably 10.5-12.9 ng.h/ml, particularly 11.7
- a solid pharmaceutical composition in which ramipril and bisoprolol are separated from each other and the composition contains 10 mg of ramipril and 10 mg of bisoprolol, preferably bisoprolol fumarate, whereby the C raax value of the composition related to bisoprolol is 43.8 ng/ml and the C max value related to ramipril is 16.5 ng/ml and/or the AUCT value related to bisoprolol is 629 ng.h/ml and the AUCT value related to ramipril is 11.7 ng.h/ml or is bioequivalent to the above values.
- compositions containing 10 mg of bisoprolol and 10 mg of ramipril are considered as bioequivalent which show the following C max and AUC T values: C max related to bisoprolol 43.8 ng/ml, C max related to ramipril 16.5 ng/ml, AUC T related to bisoprolol 629 ng.h/ml and the AUC T value related to ramipril 11.7 ng.h/ml, fall in the range of 80-125 %, preferably 90-110 %.
- the dissolution of the products according to the present invention does not considerably change during storage. On storing the combined composition according to the present invention at 40°C under a moisture content of 75 % the dissolution profile dose not significantly change. Table 12/A Test of the dissolution of capsules containing 10/5 mg of bisoprolol/ramipil .
- Bisoprolol is formulated as coated compacts and ramipril is in form of coated granules, prepared as described above (130RB0314K)
- the concentration of ramipril impurity D is below 1 %.
- the invention relates to a process for the preparation of a combined pharmaceutical composition which contains as active ingredient ramipril and bisoprolol or a salt or complex thereof which comprises
- HPMC HPMC or polyvinyl pyrrolidone or a compact, granule or pellet containing the same and with further auxiliary agents, preferably fillers, and if necessary disintegrants, lubricants and glidants and pressing the mixture thus obtained to tablets or filling in capsules.
- auxiliary agents preferably fillers, and if necessary disintegrants, lubricants and glidants and pressing the mixture thus obtained to tablets or filling in capsules.
- the composition contains both active ingredients in the form coated granules.
- ramipril and bisoprolol hemifumarate can be used as active ingredient.
- the combined pharmaceutical composition according to the present invention can be prepared by
- a disintegrant preferably cross-linked PVP (e.g.
- polyplasdone XL 10 re-granulating the compacts and coating with a 5-10 % by weight, preferably 5-8 % by weight aqueous solution of 1-15 % by weight, preferably 1-10 % by weight, particularly 2-6 % by weight - related to the weight of the coated granules - off HPMC; b) preparing bisoprolol hemifumarate containing a coated compact by homogenizing - related to the weight of the coated compact - 5-30 % by weight, preferably 10-25 % by weight, particularly 20-25 % by weight of bisoprolol hemifumarate a and 60-93 % by weight, preferably 65-80 % by weight, particularly 75-80 % by weight of microcrystalline cellulose, compacting the mixture, re-granulating the compact thus obtained optionally at first on a 1.2 mm sieve and then on an e.g.
- a process for the preparation of a bisoprolol hemifumarate containing coated compact which comprises homogenizing - related to the weight of the coated compact - 5-30 % by weight, preferably 10-25 % by weight, particularly 20-25 % by weight of bisoprolol hemifumarate and 60-93 % by weight, preferably 65-80 % by weight, particularly 75- 80 % by weight of microcrystallines cellulose, compacting the homogenized mixture, if necessary re- granulating the compact thus obtained and coating the bisoprolol hemifumarate containing compact thus obtained with an organic polymer, preferably HPMC or polyvinyl pyrrolidone by using - related to the weight of the coated compact - 2-10 % by weight, preferably 3-7 % by weight, particularly 4-5 % by weight of an organic polymer, such as polyvinyl pyrrolidone or HPMC, most preferably HPMC in form of a 5-10
- Re- granulation can be also carried out -if necessary - by re-granulating the compact at first on a e.g. 1.2 mm sieve and then on an e.g. 0.5 mm sieve. Thereafter the re- granulated compact is coated as described above.
- ramipril containing granules or compacts and bisoprolol containing compacts are prepared as follows: a) Preparation of ramipril granules
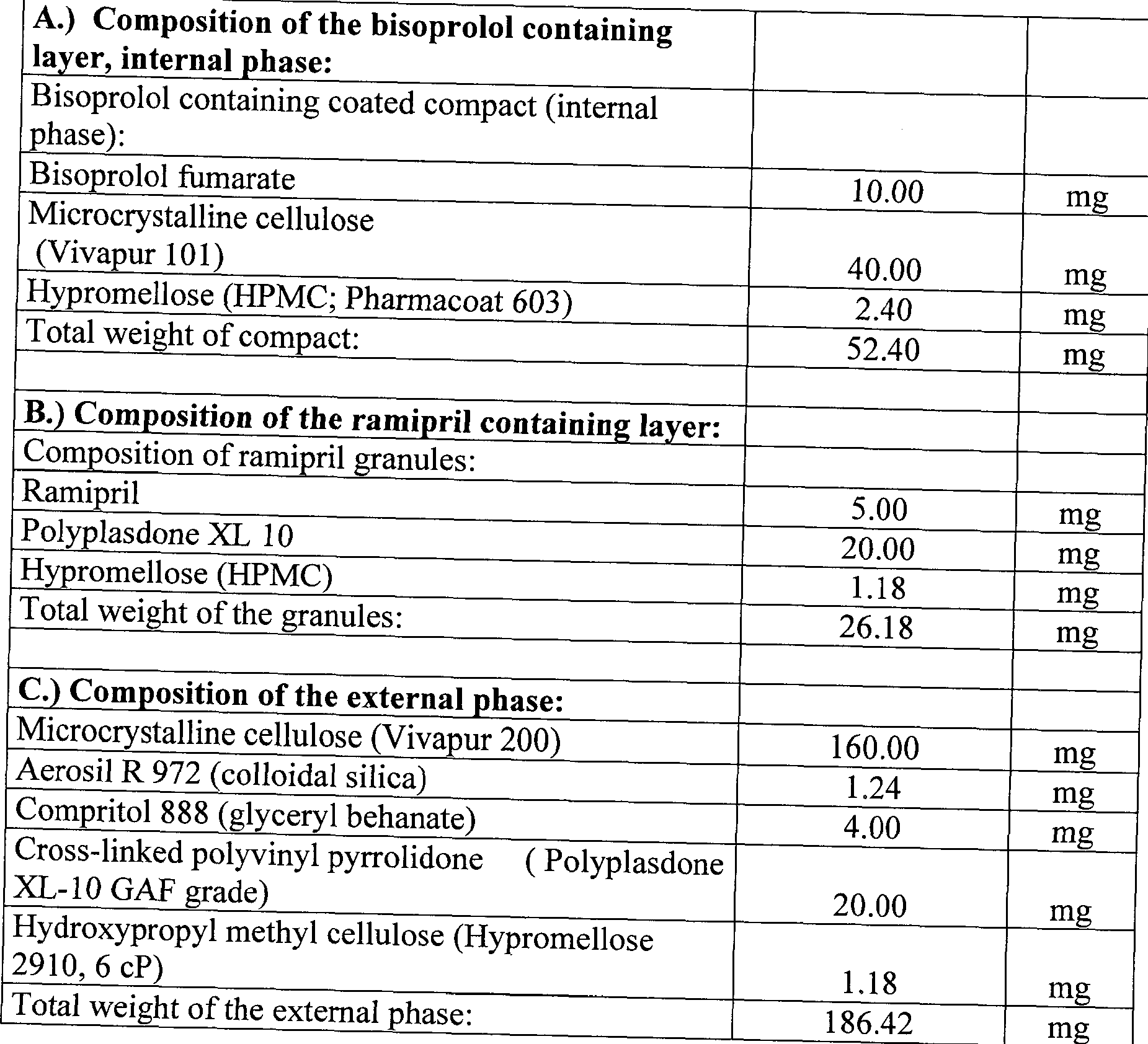
- a bisoprolol-ramipril 10/5 mg capsule can be prepared by the following process from the ramipril containing granules or compacts and the bisoprolol containing coated compacts obtained as described above.
- the first step - related to the weight of a capsule - 52.4 mg of a coated bisoprolol compact 26,18 mg of the ramipril granules or compact prepared according to the above paragraphs a) and b) and 83.2 mg of Vivapur 200 (microcrystalline cellulose) are passed through a 0.8 mm sieve whereupon 112 mg of Vivapur 200 (microcrystalline cellulose), 20 mg of polyplasdone XL- 10 (GAF granule) and 1.18 mg of hydroxypropyl methyl cellulose (Pharmacoat 606) are added and the mixture obtained is homogenized.
- Vivapur 200 microcrystalline cellulose
- Compritol 888 microcrystalline cellulose
- colloidal silica colloidal silica
- a pharmaceutical composition suitable for the treatment of hypertension preferably high renin level hypertension can be prepared by using bisoprolol and ramipril or a salt or complex thereof.
- a medical treatment of hypertension, particularly high renin level hypertension which comprises administering to the patient in need of such treatment a combined pharmaceutical composition comprising bisoprolol and ramipril or a salt or complex thereof.
- ramipril and bisoprolol in the following doses (calculated for bisoprolol hemifumarate/ramipril base, in mg/mg). 1.25/5; 2.5/2.5; 2.5/5; 2.5/10; 5/1.25; 5/2.5; 5/5; 5/10; 10/2.5; 10/5; 10/10 mg.
- composition according to the present invention can be summarized as follows:
- the patients in need of the treatment can receive both active ingredients in a single composition which improves the patient compliance.
- the risk that the patient incidentally forgets to swallow one of the active ingredients is eliminated.
- the combined composition according to the present invention is bioequivalent to the simultaneous administration of the corresponding mono compositions and can therefore directly replace the mono compositions.
- the process according to the present invention makes the formulation of the two incompatible pharmaceutical agents in a single composition possible.
- Our invention also relates to a bisoprolol hemifumarate containing coated compact which hinders the decomposition of the other or further active ingredients in the combined composition, because the coating prevents fumaric acid from exerting the effect thereof.
- the present invention enables the use of the bisoprolol fumarate salt of the composition; the advantageous dissolution and pharmacological effects of the fumarate salt are well-known. There is no need to carry out expensive biological tests of new bisoprolol salts for the development of combined compositions.
- compositions according to the present invention preserve not only their chemical stability but also the dissolution properties.
- Blood samples were collected prior to drug administration and at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 12, 24, 36, and 48 hours after dosing for assessment of bisoprolol, and at 0.17, 0.33, 0.5, 0.67, 0.83, 1, 1.25, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 8 and 12 hours after dosing for assessment of ramipril plasma concentration in each study period.
- Testl formulation bisoprolol/ramipril 10/10 mg FDC-1
- ramipril the pharmacokinetic parameters were normalized to the ramipril contents of the formulations.
- the Test to Reference ratio of geometric LSmeans and corresponding 90% confidence interval for AUC T was within the range of 80.00 to 125.00% for ramipril indicating equivalence of this parameter.
- the Test to Reference ratio of geometric LSmeans for C max was within the range of 80.00 to 125.00% ⁇ and only the lower end of the 90% confidence interval is ranged out marginally the 80 - 125% limit due to the relatively low sample size of this pilot study indicating that in appropriately powered pivotal study the bioequivalence could be proved for C max of ramipril, too.
- Rotation 50 r.p.m, +15 minutes of rotation (250 r.p.m.).
- Test liquid 900 ml 0.1 M HC1.
- Test temperature 37°C ⁇ 0.5°C.
- Duration of the test 5 10, 15, 30, 45, 60 minutes.
- Apparatus Waters Acquity UPLC or Waters Acquity UPLC H-class system, or any equivalent equipment.
- Two-layer tablet containing ramipril and bisoprolol Two-layer tablet containing ramipril and bisoprolol:
- the active ingredient and the other components are used in amounts corresponding to 10 000 tablets.
- Bisoprolol and microcrystalline cellulose used in the internal phase are homogenized and the homogenized mixture is compacted.
- the compacts thus obtained are re- granulated on sieves of 1.2 mm and 0.5 mm.
- the granules thus obtained are coated with a 8 % by weight aqueous solution of HPMC in a fluidization granulation apparatus.
- the coated compact thus obtained is homogenized with a portion of microcrystalline cellulose used in the external phase, whereupon the residual microcrystalline cellulose, Aerosil R972 and Compritol (glyceryl behenate) are added to the mixture thus obtained to yield the final homogenized mixture.
- the active ingredient and the other components are used in amounts corresponding to 10,000 tablets.
- Ramipril and Poliplasdon XL 10 cross-linked polyvinyl pyrrolidone
- the mixture is pre-granulated with a 5 % by weight HPMC solution of 45 % of the HPMC used in the internal phase in a vortex granulating apparatus, whereupon the granules are passed through a 0.5 mm sieve.
- the re-granulated product thus obtained is granulated with a 5 % by weight aqueous solution of the residual microcrystalline cellulose in a fluidized bed granulator.
- the granules thus obtained are homogenized first with a portion of the microcrystalline cellulose and thereafter with a mixture of the residual microcrystalline cellulose and Aerosil R-972.
- Compritol 888 is added to yield the final homogenized product.
- C) The homogenized products prepared according to steps A) and B) are poured into two feeding containers whereupon sandwich-tablets are prepared on a tabletting machine by pressing first the bisoprolol containing layer and thereafter pressing the ramipril containing layer on the bisoprolol layer. The tablets thus obtained are packed.
- Ramipril and poliplasdon XL 10 cross-linked polyvinyl pyrrolidone are homogenized and the mixture obtained is compacted.
- the compacts thus obtained are re-granulated first on a 1.2 mm sieve and then on a 0.5 mm sieve.
- the granules thus obtained are coated with a 8 % by weight aqueous solution of HPMC in a fluidization granulating apparatus.
- the coated granules are homogenized with a portion of the microcrystalline cellulose used in the external phase whereupon the residual microcrystalline cellulose and the Aerosil R972 are added to the mixture thus obtained.
- the above components are homogenized whereupon Compritol 888 (glyceryl behenate) is added.
- Compritol 888 glyceryl behenate
- Example 3/A Three-layer tablet containing ramipril and bisoprolol Example 3/A One proceeds as described in Example 1 except that that not only the bisoprolol and ramipril containing layers but also the components of the active ingredient free external phase are homogenized.
- the tablets contain 93.2 mg of microcrystalline cellulose, 1 mg of Aerosil R 972 (colloidal silica) and 1 mg of Compritol 888.
- Tabletting is carried out by introducing the bisoprolol containing homogenized layer, the ramipril containing homogenized layer and the active ingredient free homogenized layer into three feeding containers of a sandwich-tabletting machine and pressing the tablets in the following order: at first the bisoprolol containing layer, then the active ingredient free separating layer and finally the ramipril containing layer.
- Example 3/B
- Example 2 One proceeds as described in Example 2 except that not only the bisoprolol and ramipril containing layers but also the components of the external phase are separately homogenized.
- the tablets contain 93.2 mg of microcrystalline cellulose, 1 mg of Aerosil R 972 and 1 mg of Compritol 888.
- Tabletting is carried out by introducing the bisoprolol containing homogenized layer, the ramipril containing homogenized layer and the active ingredient free homogenized layer into three feeding containers of a sandwich-tabletting machine and pressing the tablets in the following order: at first the bisoprolol containing layer, then the active ingredient free separating layer and finally the ramipril containing layer.
- Hypromellose (HPMC; Pharmacoat 603) 2.40 mg
- composition of ramipril granules Composition of ramipril granules:
- Hypromellose (HPMC) 2.36 mg
- Aerosil R 972 (colloidal silica) 1.00 mg
- the active ingredient and the other components are used in amounts corresponding to 10 000 tablets.
- Bisoprolol and the microcrystalline cellulose used in the internal phase are homogenized and the homogenized product is compacted.
- the compact thus obtained is re-granulated first on a 1.2 mm sieve and thereafter on a 0.5 mm sieve.
- the granules thus obtained are coated with a 8 % by weight aqueous solution of HPMC.
- B) Preparation of ramipril containing granules When preparing the ramipril containing layer the active ingredient and the other components are used in amounts corresponding to 10 000 tablets.
- Ramipril and Polyplasdon XL 10 cross-linked polyvinyl pyrrolidone
- the mixture obtained is pre-granulated in a vortex granulating machine with a 8 % by weight HPMC solution prepared from 45 % of the HPMC used in the internal phase and the granules thus obtained are re-granulated on a 0.5 mm sieve.
- the re-granulated product is granulated with an 8 % aqueous solution of the residual HPMC in a fluid bed granulating apparatus.
- step C is carried out as follows:
- ramipril granules are replaced by a ramipril compact prepared by the following process: 19 % by weight of ramipril and 76.4 % by weight of Polyplasdone XL- 100 (GAF quality) - related to the weight of the compact - are passed through a 0.8 mm sieve, homogenized and compacted. The compacts thus obtained are passed through a 0.8 mm sieve and homogenized.
- a 5.8 % by weight HPMC solution is prepared by using 4.5 % by weight of HPMC -related to the weight of the compact. The compacts are coated with this solution, preferably by using a fluid granulation method. The granules thus obtained are granulated on a 0.5 mm sieve and homogenized.
- Example 6 Capsules (6/A) or tablets (6/B) comprising bisoprolol and ramipril (strength 10/5 mg/mg) W
- the mixture is homogenized and to the homogenized mixture thus obtained 8 mg of Vivapur 200 (microcrystalline cellulose), 4 mg of Compritol 888 (microcrystalline cellulose) and 1.24 mg of colloidal silica (Aerosil R972) are added.
- the homogenized mixture thus obtained is filled in capsules. 6/B
- Capsule (7/A) or tablet (7/B) containing bisoprolol and ramipril (strength 10/5 mg) One proceeds as described in Example 6 (Examples 6/A and 6/B) except that ramipril granules are replaced by a ramipril compact prepared according to the following process: 19 % by weight of ramipril and 76.4 % by weight of a Polyplasdone XL- 10 (GAF grade) are passed through a 0.8 mm sieve. The mixture is homogenized and compacted. The compact thus obtained is passed through a 0.8 mm sieve and homogenized.
- HPMC solution is prepared by using 4.5 % by weight of HPMC (Pharmacoat 603) - related to the weight of the compact.
- the compacts are coated with this solution, preferably with the aid of a fluid granulating procedure.
- the granules thus obtained are re-granulated on a 0.5 mm sieve and homogenized.
- composition of the dosage unit is composition of the dosage unit
- Example 6/A One proceeds as described in Example 6/A except that the homogenized mixture is not filled in capsules but pressed to tablets.
Landscapes
- Health & Medical Sciences (AREA)
- Public Health (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Heart & Thoracic Surgery (AREA)
- Cardiology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Abstract
The present invention relates to a combination composition containing ramipril and bisoprolol and preparation thereof. The active ingredients are separated from each other in the composition.
Description
STABLE PHARMACEUTICAL COMPOSITION CONTAINING
BISOPROLOL AND RAMIPRIL
Technical field of the invention
The invention relates to a stable pharmaceutical composition suitable for the treatment of cardiac insuffiency, namely comprising ramipril of the Formula (I)
Hypertension - also called as high blood pressure or sometimes arterial hypertension - is a very widespread chronic pathological condition, whereby the blood pressure is increased in the arteries. According to certain estimations annually 7 million people die because of this disease and the life of a further 1.5 billion people is made difficult by the unduly high blood pressure and the accompanying complications. High blood pressure is one of the mortality causes worldwide. High blood pressure is a significant risk in the development of blood vessel disease, cardiac insufficiency and can also invoke renal diseases. Cardiac insufficiency is a state in which the decrease of the contracting strength of the cardiac muscle and the reduction of the performance results in a limited everyday activity of the cardiac patient.
Several pharmaceutical compositions were developed for the treatment of hypertension which decrease blood pressure by various types of mechanism. However, since high blood pressure is often associated with other diseases, in many cases monotherapy is not sufficient, as disclosed in the Directive issued in Hungary by the Hungarian Hypertension Company. According to said Directive if an associated disease or condition is observed and the normalization of blood pressure probably requires more than medicines, the use of combined pharmaceutical compositions as first treatment may be justified. For this purpose combinations such as a diuretic (e.g. hydrochlorothiazide) and a beta blocker (e.g. carvediol), or a diuretic and an ACE- inhibitor (e.g. perindopril), or a diuretic and an angiotensin-II-receptor antagonist (e.g. valsartan), or a calcium antagonist (e.g.amlodipine) and a beta blocker, or a calcium antagonist and an ACE-inhibitor, or a calcium antagonist and an angiotensin-II- receptor- antagonist, or an alpha- 1-adrenerg receptor blocker and a beta blocker can be used.
Additionally in case of hypertension free from complications and associated diseases a combined therapy may become necessary, namely if the efficiency of the first medicine is unsatisfactory or the increase of the dosage of the first pharmaceutical
agent causes the augmentation of the number or strength of the side effects. In order to improve compliance once-a-day compositions which exert their effect for 24 hours are recommended. Ramipril and bisoprolol are generally used in the treatment of hypertension and cardiac insufficiency. Said two active ingredients are also applied together. Only in Hungary about 40 000 hypertension patients receive such combination treatment which comprises the simultaneous use of bisoprolol and ramipril. In Hungary 16 % of the patients treated with ramipril receive bisoprolol as well, while 12 % of the patents treated with bisoprolol receive 12 % of ramipril too.
The components of the combination treatment differ significantly from each other both in their chemical structure and mechanism of effect. Both active ingredients are sensitive and for this reason the preparation of stable pharmaceutical compositions is not simple, even if the composition comprises only ramipril or bisoprolol.
Ramipril of the Formula (I) /chemical name: (2S,3aS,6aS)-l-[(S)-2-[[(S)-l- ethoxycarbonyl)-3-phenyl-propyl]-amino]-propanoyl/-octahydrocyclopenta[b]- pyrrole-2-carboxylic acid] is a known pharmaceutical active ingredient which is used in therapy for the treatment of hypertension, cardiac insufficiency and nephropathy, the promotion of revascularization, the decrease of the risk of cardiovascular diseases and events, particularly of stroke and myocardic infarction, and the reduction of cardiovascular mortality. Ramipril exhibits its effect first of all via an active metabolit -namely ramiprilate - which is an effective inhibitor of the angiotensine converting enzyme (ACE).
Compounds analogous to ramipril were first disclosed in EP 50800 and ramipril was first published in EP 79022. Ramipril is a pharmaceutical active ingredient sensitive to moisture and mechanical effects (e.g. pressing) and decomposes easily. Said decomposition is due partly to the
hydrolysis of the ester group which gives rise to the formation of a contamination corresponding to the active metabolite, namely ramiprilate /European Pharmacopoeia: Impurity E). The decomposition of ramipril can also be due to the formation of the diketopiperazine contamination of the Formula (III)
(European Pharmacopoeia: Impurity D). According to US patent 5 442 008 when ramipril tablets having an active ingredient content of 2.5 mg and prepared by dry granulation are stored at 40°C for 6 months the concentration of the diketopiperazine impurity amounts to 22.8 %, while in case of ramipril capsules containing 2.5 mg of the active ingredient and filled with the powder mixture the concentration of said contamination is 6.4 %. WO 2005041940 teaches that if the above storage is carried out in the presence of air the oxidative decomposition of ramipril can cause an undesirable discoloration.
According to US patent 5 442 008 on the ramipril active ingredient particles a polymeric coating is applied and the product is then subjected to further pharmaceutical technological processing (e.g. to tableting). During the coating procedure 3-25 % of hydroxypropyl cellulose, hydroxpropyl methyl cellulose phthalate, hydroxyethyl cellulose, ethyl cellulose, cellulose acetate phthalate, polyvinyl pyrrolidone or a methacrylate type agent or a different coating agent is applied on the surface of the particles of the ramipril active ingredient as a layer formed from a pharmaceutically suitable coasting system.
According to WO 2006050533 in course of the preparation of the ramipril particles coated with a stabilizing layer aggregation which takes place before the coating step
had been eliminated and therefore each individual particle received a separate coating. As coating agent preferably hydroxymethyl cellulose is used. The coating layer is applied by means of a fluidization method. Ramipril can also be stabilized by formulating the active ingredient in the presence of a stabilizing auxiliary agent and omitting the auxiliary agents generally used in pharmaceutical technology which are incompatible with ramipril.
According to GB 2 394 660 auxiliary agents of acidic character which have a large specific surface are incompatible with ramipril and can not be used therefore in the formulating procedure, Such an auxiliary agent is e.g. colloidal silica (Aerosil) frequently used in formulating methods.
EP 1 734 931 relates to ramipril containing compositions which comprise calcium sulphate dihydrate as auxiliary agent. On storing said pharmaceutical compositions at 40°C for 6 months under a moisture content of 75 %, the concentration of the ramipril diketopiperazine decomposition product amounts to 0.478-1.06 % which is significantly lower than that of the pharmaceutical composition put on the market. According to WO 2007056907 ramipril is stabilized simultaneously with sodium hydrogen carbonate and calcium sulphate whereby particularly the decrease of the concentration of the ramiprilate impurity was observed. A similar process is described in US 20030215526. According to WO 20055041940 ramipril and other ACE-inhibitors are stabilized with meglumine and optionally lower substituted hydroxypropyl cellulose. The product stored at 60°C for 15 days contains 0.75-1.59 % of the decomposition product. The disadvantage of this process is that when stored the composition at higher temperature already for a short period of time decomposition of the product becomes significant.
WO 2005002548 relates to stabilized pharmaceutical compositions which contain a significantly homogenized mixture of an ACE inhibitor and an effective amount of a pharmaceutical lubricant and if desired further auxiliary agents. As lubricant conventional auxiliary agents can be used. However after a storage of already 48 hours the composition according to said international patent application contains decomposition product in an amount similar to that of the marketed reference composition.
According to US 20070232680 and WO 2008000040 magnesium oxide was used to stabilize ramipril. WO 2008132756 described the use of magnesium oxide and lactic acid for the stabilization of ramipril. In these patent publications however the results of the stability tests of the composition were not disclosed.
WO 2007120930 is concerned with stable pharmaceutical compositions comprising ramipril and a stabilizing auxiliary agent. The stability of a pharmaceutical composition filled in hard gelatine capsules and containing ramipril (1.25 mg), gelatinized starch (23.35 mg), lactose (25.0 mg) and stearyl fumarate (0.4 mg) was tested and it has been found that after a storage of 3 months at 40°C under a relative moisture content of 75 % the concentration of the diketopiperazine impurity amounted to 3.5 %. Under similar storing conditions in the ALTACE composition of the originator the concentration of the same impurity was 4.9 %.
In case of combined pharmaceutical compositions comprising more than one active ingredients the incompatibility of the different active ingredients is to be taken into consideration. In WO 2008021875 tablets comprising more than one active ingredients are disclosed in which the different active ingredients are formulated in different layers which are physically separated and are in contact with each other only at a small surface. WO 2008095263 relates to pharmaceutical compositions comprising more than one active ingredients in which the active ingredients are formulated in physically different
pharmaceutical forms (e.g. powder, granule, pellet, particle, minitablet, tablet) in order to minimize the interaction between the individual active ingredients. Thus e.g. tablets were prepared in which the fluoxetine hydrochloride was formulated in form of a powder mixture and the olanzapin active ingredient in form of a minitablet.
The pharmaceutical compositions comprising amlodipin and an ACE-inhibitor described in WO2008065485 are prepared by admixing the active ingredients and adjusting the pH of the composition to a value higher than 6.0. This is achieved by preparing from a mixture of amlodipin besylate, microcrystalline cellulose, magnesium carbonate and a colouring agent pellets by wet granulation and filling said pellets in capsules.
The marketed TRITACE product described in prior art comprises in addition to ramipril also sodium stearyl fumarate, hypromellose, microcrystalline cellulose, starch which swells in the cold and in case of compositions of a certain strength also colouring agents. According to the prescription leaflet this composition can be stored only at a temperature not exceeding 25°C. It can be deemed as probable that at a higher temperature and/or moisture content the product rapidly decomposes. The health authorities raise strict requirements relating to the active ingredient content, stability and purity of pharmaceutical compositions. In order to meet these requirements ramipril containing pharmaceutical compositions are needed which can be stored for a longer period of time in such a manner that the active ingredient content does not decrease below the allowed limit value and the amount of the decomposition products does not exceed the corresponding limit value.
Although in prior art a large number of procedures directed to the stabilization of ramipril are set forth, in most publications the stability data, storability and impurity profile of the pharmaceutical composition were not disclosed.
Bisoprolol (chemical name l-[4-[[2-(l-methyl-ethoxy)-ethoxy]-methyl]-phenoxy]-3- [(l-methyl-ethyl)-amino]-2-propanol) is an adrenoceptor blocker of high beta-1- selectivity. Bisoprolol is cardioselective and exhibits neither a sympatomimetic activity (ISA) nor a clinically significant membrane stabilizing effect. The decrease of the renin activity of the plasma also contributes to the antihypertensive effect of the beta-blockers. It decreases the heart rate and the pulse volume, consequently also the minute volume and the oxygen consumption. In course of chronic treatment the peripheral resistance increases at the beginning and decreases later. Bisoprolol inhibits the response given to the sympato-adrenerg activation through blockade of the beta- receptors of the heart. This results in the decrease of the heart rate and contractility and thereby the reduction of the oxygen consumption of the heart muscle which is a desired effect in the treatment of Angina pectoris derived from the disease of the coronary artery. The marketed pharmaceutical compositions contain bisoprolol hemifumarate of the Formula (IV) as bisoprolol salt due to the relative stability and favourable processing properties thereof. The use of other salts is encountered with several problems such as the insufficient stability, and in some cases the different absorption of the products which makes their interchangeability with the products on the market impossible. However also the hemifumarate salt of bisoprolol has the drawback that the other acid function of fumaric acid can destabilize the other active ingredient of the composition. Also the double bond of fumaric acid can react with amines, as described in WO 110038091 which relates to tablets containing amlodipin and bisoprolol. The large number of the patients treated with the combination of the two active ingredient would justify the marketing of combined pharmaceutical compositions in order to improve the compliance. However the preparation of such compositions meets serious difficulties and therefore no suitable method has been elaborated so far for the manufacture of a combined composition.
Brief summary of the invention
There is a need for new combined pharmaceutical compositions which are suitable for the treatment of patients suffering from hypertension (high blood pressure disease), particularly high hypertension of high renin level. Our further aim is to use such compositions for this purposes which are suitable for once-a-day use. Thus the object of the invention is to develop a combined pharmaceutical composition which contains ramipril and bisoprolol or a salt or complex thereof in form of a dosage unit, and whereby a) the active ingredients remain stable under the conditions of manufacture and storage and thereby the amount of the impurities being present in the composition or formed during the manufacturing process does not exceed the impurity limit authorized for the mono compositions having the given active ingredient content; b) the bioavailability of the combined pharmaceutical composition is similar to or identical with that measured in case of simultaneous administration of two mono compositions which have an active ingredient content corresponding to that of the combined composition; and c) the dissolution profile of each active ingredient does not change during storage.
Preferably bioequivalent compositions corresponding to the above objects of the invention make possible to continue the treatment of patients treated by simultaneous administration of two mono compositions with the use of the combined pharmaceutical composition according to the present invention without any risks. This is particularly important in case of patients suffering from high blood pressure because when such patients are treated with more than one active ingredients the blood pressure can be adjusted generally only within a longer period of time and in case of the use of a new combination there is a risk that the
desired effect can not be achieved. In such cases the selection and adjustment of the new medicine can last several months and during this time the side effects caused by high blood pressure (dizziness, head ache) significantly deteriorate the quality of life of the patient and the development and/or progress of the accompanying diseases are not prevented. However if the combined pharmaceutical composition is bioequivalent, the lengthy procedure of adjustment and the risks can be avoided.
A further object of the present invention is that the dissolution profiles should not change during the storage. Namely, if the dissolution profile of one or both active ingredient(s) change, the bioavailability of the product may differ from the prescribed value and therefore the combined pharmaceutical composition will not only become unsuitable for the replacement of the corresponding mono compositions but can not be even used as a medicine.
It has been found that a combined composition comprising ramipril and bisoprolol can be suitable for use as a once-a-day pharmaceutical composition for the treatment of hypertension, preferably high renin level hypertension. However the two active ingredients are incompatible.
Our preliminary tests have shown that when contacting ramipril and bisoproilol the ramipril component suffer significant decomposition.
Table 1
*During storage of the samples the moisture content was not regulated and the corresponding values were not registered.
In the above Table the percental impurity content of the active ingredient is disclosed. It can be seen that the amount of impurity D of the Formula III of ramipril is increased to a small extent during storage of pure ramipril but in the presence of bisoprolol hemifumarate the amount of said impurity augments to more than 32 % and this is unacceptable. It has been found in a surprising manner that it is still possible to develop a pharmaceutical composition suitable for the treatment of patients suffering from high blood pressure, particularly of high renin level high blood pressure by separating the active ingredients from each other physically in the composition. Our experiments have shown (Table 2) that if ramipril granules coated with HPMC are admixed with particles of bisoprolol hemifumarate the decomposition of the product
significantly decreases as compared to the decomposition of the uncoated particles. This is shown in Table 1.
Table 2:
*Bisoprolol hemifumarate was used per se as particles of the active ingredient. During storage the moisture content was not regulated and the value thereof was not registered. It has been surprisingly found that when separating the two active ingredients physically from each other a stable combined pharmaceutical composition can be prepared which comprises ramipril or a salt thereof, preferably ramipril and bisoprolol hemifumarate. However it has been observed that despite of the separation the amount of impurity D of ramipril is still formed in a rather high amount. We have surprisingly found that when coated bisoprolol compact according to the present invention is used, the amount of the impurity ramipril D is significantly decreased in the invention capsules (examples 4/II and 5/II).
Table 3
Although on the basis of toxicological tests the authorized upper limit of ramipril impurity D in the ramipril mono compositions is relatively high (maximum 5 %), this impurity decreases the active ingredient content as well. It is therefore advisable to elaborate a composition in which the amount of said contamination is below 2.5 % at the expiry date of the use of the pharmaceutical composition, but it is still more preferable if the amount of the ramipril impurity D is below 1 %. Brief description of the drawings
Figure 1. Change of the dissolution rate of bisoprolol during storage of the product according to Example 6/A (the data are shown in Table 12/A).
Figure 2. Change of the dissolution of ramipril during storage of the product according to Example 6/A (the data are shown in Table 12/A).
Figure 3. Change of the dissolution of bisoprolol during storage of the product according to Example 7/A (the data are shown in Table 12/B).
Figure 4. Change of dissolution of ramipril during storage of the product according to Example 7/A (the data are shown in Table 12/B).
Detailed description of the invention
The invention relates to a solid combined pharmaceutical composition which comprises ramipril and bisoprolol or a pharmaceutically acceptable salt or complex thereof. The pharmaceutical composition according to the present invention contains
both active ingredients in form of a single dosage unit which can be e.g. tablet or capsule. The dosage units in table form can be mono- or two-layer tablets.
More particularly the invention relates to a solid pharmaceutical composition in which the particles of the active ingredients ramipril and bisoprolol or a pharmaceutically acceptable salt or complex thereof are separated from each other.
In the composition according to the present invention the particles of the active ingredients ramipril and bisopolol or a pharmaceutically acceptable salt or complex thereof can be in form of separate phases whereby the particles of the active ingredients are in contact with each other only on the edge surface of the phases.
According to an embodiment of the invention there are provided two- layer tablets in which one layer contains the ramipril active ingredient and the other the bisoprolol.
Although in this embodiment of the invention the active ingredients are in contact with each other on the edge surface of the layers, the amount of the contacting active ingredients on the surface is so small that the decomposition related to the total active ingredient content can be neglected.
The composition according to the present invention comprises preferably as active ingredient ramipril or a salt, e.g. sodium or potassium salt thereof and biosoprolol or a salt thereof, e.g. bisoprolol hemifumarate. In an advantageous embodiment of the present invention comprises ramipril and bisoprolol hemifumarate.
The pharmaceutical composition according to the present invention may also contain auxiliary agents generally used in pharmaceutical industry. As auxiliary agents e.g. fillers, glidants (antiadhesives), binders, disintegrants, lubricants etc. can be used.
The pharmaceutical compositions according to the present invention may contain fillers, in an amount of 20-90 % by weight. The amount of the filler is preferably 40- 80 % by weight, particularly 60-70 % by weight.
Any filler generally used in pharmaceutical industry can be used which does not affect the stability of the active ingredients. Thus as filler e.g. organic polymers, such as microcrystalline cellulose; organic mono- or polysaccharides or sugar alcohols, such as lactose, mannitol or saccharose , or inorganic salts, e.g. tricalcium phosphate, calcium phosphate, calcium carbonate or sodium chloride can be used. According to the most preferable embodiment of the invention the composition contains as filler microcrystalline cellulose.
The pharmaceutical composition according to the present invention may also contain disintegrants in order to promote dissolution of the active ingredients. Disintegrants are substances which swell under the effect of moisture and burst the tablet or capsules open whereby the active ingredients are set free. The disintegrants are generally organic polymers. The disintegrant content of the composition is generally 1-30 % weight, preferably 10-25 % by weight %, particularly 10-20 % by weight %. Any disintegrant generally used in pharmaceutical industry can be applied which does not enter into interaction with the active ingredients. For this purpose e.g. organic polymers, such as cross-linked polyvinyl pyrrolidone (Poliplasdon, particularly Polyplasdon XL- 10), microcrystalline cellulose or starch (e.g. maize starch, wheat starch or pre-gelatinized starch) can be used. The external phase can preferably contain microcrystalline cellulose of larger particle size (e.g. Vivapur 200) and the internal phase can preferably contain microcrystalline cellulose of smaller particle size (e.g. Vivapur 101) e.g. for compacting bisoprolol.
The composition may also contain binders, if necessary. Any binder generally used in pharmaceutical industry can be applied which does not enter into interaction with the active ingredients. For this purpose e.g. organic polymers, such as hydroxypropyl methyl cellulose (hypromellose, referred to further on as HPMC) can be used, preferably a product which has a viscosity lower than 1000 mPas in a 2 % aqueous solution at 20°C. It is still more preferable to use HMPC having a viscosity of 3000- 6000. Such preferred HPMC types are Pharmacoat 603 or 606. One can also use for this purpose polyvinyl pyrrolidone - referred to furtheron as PVP. It is advantageous
to use PVP products of lower polymerisation degree, e.g. K-15 or K-30. The amount of the binder in the pharmaceutical composition is preferably 1-10 % by weight, preferably 1-5 % by weight, particularly 1-3 % by weight. Formulation of the pharmaceutical composition can be rendered easier by using a lubricant and a glidant.
The lubricant content of the pharmaceutical composition according to the present invention is 0.1-10 % by weight, preferably 0.1-5 % by weight, particularly 0.2-2 weight by weight %. Any organic or inorganic lubricant generally used in pharmaceutical industry can be applied such as stearates (e.g. magnesium stearate, sodium stearyl fumarate), glyceryl behenate, (such as compritol 888) or inorganic substances (e.g. talc). Any glidant generally used in pharmaceutical industry can be applied e.g. talc, colloidal silica or tricalcium phosphate etc. The glidant content of the pharmaceutical composition according to the present invention is 0.1-10 % by weight, preferably 0.1-5 % by weight, particularly 0.1-2 weight by weight %. The essence of the invention is that the active ingredients are separated from each other in the composition. If the active ingredients are contained in the same layer, then at least one of the active ingredients is (are) to be protected by a coating. Said coating may contain any coating agent generally used in pharmaceutical industry - e.g. an organic polymer - which does not enter into interaction with the active ingredients. It is preferred to use polymers which swell or are dissolved in water and thereby ensure the rapid dissolution of the active ingredients from the tablet. As polymer e.g. HPMC, preferably low viscosity HMPC having a viscosity under 10000 mPas in 2% aqueous solution at 20°C, particularly HPMC having a viscosity of 3000-6000 mPas can be used. Such HPMC types are Pharmacoat 603 or 606. For this purpose polyvinyl pyrrolidone can also be applied. According to a preferred embodiment of the invention products of lower polymerisation degree, e.g. K-15 or K-30 can be used. The
pharmaceutical composition contains the coating agent in an amount of 1-10 % by weight, preferably 1-5 % by weight, particularly 1-3 % by weight.
According to the present patent specification the phases which contain the active ingredients are named as internal phase and the added further ingredients are named as components of the external phase.
The internal phase of the composition according to the present invention can be a premix (powder mixture), uncoated or coated granule, compact, pellet or minitablet. The internal phase contains 10-50 % by weight, preferably 10-30 % by weight, particularly 15-25 % by weight of the active ingredients, related to the weight of the internal phase. The amount of the filler is 40-90 % by weight. The internal phase can contain further auxiliary agents The internal phase contain 1-15 % by weight, preferably 1-10 % by weight, particularly 2-6 weight by weight % of a coating, related to the weight of the coated internal phase.
According to a preferred embodiment of the present invention at least one of the two active ingredients or pharmaceutically acceptable salts or complexes thereof is (are) incorporated into dry granules or compacts.
According to a preferred embodiment of the invention the particles or granules or compacts of at least one of the active ingredients is (are) coated.
According to the most preferred embodiment of the invention the particles of ramipril or salts thereof or granules, impacts or minitablets containing the same and particles of biosoprolol or a salt thereof and granules, minitablets or compacts containing the same are coated.
The pharmaceutical compositions of the present invention are mono- or two-layer tablets or capsules, regarding their pharmaceutical form. According to the present patent specification mono-layer tablets are those which contain both active ingredients
in the same layer of the tablet. Tablets consisting of two or more layers which contain in addition to ramipril and bisoprolol optionally further active ingredients also belong to this group provided that ramipril and bisoprolol are incorporated into the same layer of the tablet.
Thus the invention also relates to mono-or two-layer tablets in which bisoprolol and ramipril are incorporated into the same layer whereby the particles of the active ingredient are separated from each other in the layer. In case of mono-layer tablets the active ingredients are separated from each other as follows: at least one of the active ingredients of the mixture to be pressed are used in form of coated particles, coated granules or coated pellets. It is preferred to use both active ingredients in coated form. According to the most preferred embodiment of the invention the mono-layer tablets contain the ramipril and bisoprolol active ingredients in form of coated granules or compacts. According to the most preferred embodiment of the invention bisoprolol is compacted with microcrystalline cellulose and coated with HPMC, while ramipril is granulated or compacted with polyplasdon and coated with HPMC. The invention also relates to multilayer tablets which contain bisoprolol and ramipril or pharmaceutically acceptable salts thereof in separate layers.
According to the present patent specification the term "multi-layer tablets" relates to tablets which - independently from the number of the layers - contain the two active ingredients in two separate contacting layers, or in layers separated from each other by one or more layers. In this case it is not necessary that the active ingredients should be coated. At the same time according to a preferred embodiment of the invention also in this case ramipril is granulated or compacted with polyplasdon, and coated with HPMC.
Capsules according to the present invention contain coated particles, granules, pellets or coated or uncoated minitablets of one of the active ingredients, and coated or uncoated particles, or coated or uncoated granules, pellets or coated or uncoated minitablets of the other active ingredient and optionally further auxiliary agents (e.g. fillers, glidants and antiadhesives).
According to the most preferable embodiment of the invention capsules contain the active ingredients in form of coated granules, and also contain fillers, antiadhesives and lubricants.
On elaborating the present invention it has been found that the very strong destabilizing effect of bisoprolol fumarate can be significantly decreased by preparing coated compacts of bisoprolol fumarate. Such compacts contain 5-30 % by weight, preferably 10-25 % by weight, particularly 20-25 % by weight of bisoprolol hemifumarate, 60-93 % by weight, preferably 65-80 % by weight, particularly 75-80 % by weight of microcrystalline cellulose and 2-10 % by weight, preferably 3-7 % by weight, particularly 4-5 % by weight of an organic polymer, e.g. polyvinyl pyrrolidone or HPMC, most preferably HPMC - related to the weight of the coated compact. It has been surprisingly found that although the compact is not isodimensional, a layer is formed on said compact which significantly decreases or hinders the decomposition reactions caused by fumaric acid.
Accordingly the present invention also relates to the coated bisoprolol compacts described above.
The compact according to the present invention can be incorporated into combined pharmaceutical compositions whereby the strong decomposing effect of fumaric acid exerted on the other active ingredient can be avoided.
According to a further aspect of the present invention there is provided a process for the preparation of pharmaceutical compositions which contain bisoprolol and ramipril or a pharmaceutically acceptable salt or complex thereof. According to an embodiment of the present invention one may proceed by coating the particles of ramipril or bisoprolol with a water soluble or in water swellable polymer (e.g. HPMC or polyvinyl pyrrolidone), homogenizing the coated particles thus obtained with the uncoated particles of the other active ingredient and with further auxiliary agents (preferably fillers, if necessary with disintegrants, lubricants, glidants and antiadhesives) and pressing the mixture thus obtained to tablets or filling in capsules, It is preferred to fill the mixture in hard gelatine capsules.
It has been surprisingly found that the granulation and compacting of the active ingredients, and the coating of the granules or compacts can be carried out in such a manner that the absorption of the compositions obtained is bioequivalent with that obtained on simultaneous administration of the corresponding mono-compositions.
According to a more advantageous embodiment of the present invention at first ramipril is granulated and coated, the coated or uncoated bisoprolol particles and the auxiliary agents are admixed with the coated granules and the mixture thus obtained is homogenized and filled in capsules or pressed to tablets.
According to a still more advantageous embodiment of the present invention also bisoprolol is converted into coated granules or compacts and is used in this pharmaceutical form.
Ramipril is preferably granulated by subjecting a mixture of ramipril and a disintegrant, preferably cross-linked PVP (e.g. polyplasdone XL 10) to pre-granulation with a solution of HPMC in a vortex granulating machine and thereafter coating the granules thus obtained in a fluidization granulator with HPMC.
One may proceed by carrying out pre-granulation with a 5-10 % by weight, preferably 5-8 % by weight aqueous solution of 40-60 % of the HPMC and thereafter coating the granules with a 5-10 % by weight, preferably 5-8 % by weight aqueous solution of the residual HPMC.
One may also proceed by subjecting a mixture of ramipril and a disintegrant, preferably cross-linked PVP (e.g. polyplasdone XL 10) to dry granulation (compacting), if necessary regranulating the compact thus obtained and coating the granules with a 5-10 % by weight, preferably 5-8 % by weight aqueous solution of HPMC.
Bisoprolol can be used not only as particles but also in form of granules or compacts. Thus one may proceed by homogenizing and compacting bisoprolol with the filler and subjecting the compact to re-granulation e.g. on a sieve. It is preferred to regranulate the compacts in more than one step. Thus e.g. regranulation may be carried out at first on a 1.2 mm sieve and thereafter on a e.g. 0.5 mm sieve. The compacted granules may be directly used. One may also proceed by applying the solution of bisoprolol on a carrier in a fluidization granulating or vortex granulating apparatus. Bisoprolol can also be coated by applying on the granules an organic polymer (preferably HPMC or polyvinyl pyrrolidone layer) in a fluidization granulating apparatus. It is preferred to use a 5-10 % by weight, preferably 5-8 % by weight aqueous HPMC solution in the coating step. The bisoprolol hemifumarate containing coated compact according to the present invention can be prepared by homogenizing 5-30 % by weight, preferably 10-25 % by weight, particularly 20-25 % by weight of bisoprolol hemifumarate, and 60-93 % by weight, preferably 65-80 % by weight, particularly 75-80 % by weight of microcrystalline cellulose - related to the weight of the coated compact - whereupon the mixture is compacted. If necessary the compact thus obtained is regranulated first on a 1.2 mm sieve and thereafter on a 0.5 mm sieve. The optionally regranulated
bisoprolol hemifumarate containing compacts are coated with an organic polymer, preferably HPMC or polyvinyl pyrrolidone so that a 5-10 % by weight, preferably 5-8 % by weight aqueous solution of 2-10 % by weight, preferably 3-7 % by weight, particularly 4-5 % by weight of an organic polymer, preferably HPMC or polyvinyl pyrrolidone - related to the weight of the coated compact - is used.
Although the coated compact thus obtained is not isodimensional, it prevents the fumaric acid component of bisoprolol hemifumarate from exerting the decomposing effect on other active ingredients, e.g. on ramipril.
The present invention also relates to tablets which consist of layers and contain the two active ingredients in separate layers. Such tablet can also be prepared by homogenizing the active ingredients with the auxiliary agents in separate layers and thereafter pressing to tablets consisting of layers on a suitable apparatus (e.g. on a sandwich tableting equipment). Since ramipril is sensitive to pressure it is advisable to press first the bisoprolol containing layer, thereafter applying the components of the ramipril containing layer and finally applying the product thus obtained on the bisoprolol containing layer. One may also proceed by pressing in the first step on the layer containing one of the active ingredients (preferably on the layer comprising bisoprolol or a salt thereof, particularly the hemifumarate salt) a separating layer consisting of the auxiliary agents, thereafter applying the components of the layer containing the other active ingredient (preferably ramipril) and finally preparing tablets by pressing.
The above tablets consisting of layers can be preferably prepared by using in each layer granules or coated granules of the active ingredients in place of the particles of the active ingredients. According to this aspect of the invention the coated or uncoated bisoprolol granules are homogenized with the components of the external phase, the mixture is pressed and finally the tablets consisting of layers are obtained by applying the homogenized mixture of the uncoated or coated ramipril granules and the external
phase. The preparation of the corresponding coated or uncoated granules is described above.
According to a further aspect of the present invention there are provided capsules which comprise at least one of the active ingredients in form of a minitablet or as minitablets. The other active ingredient may be in form of coated or uncoated particles, granules, pellets or minitablets.
Minitablets may be prepared by homogenizing one of the active ingredients (preferably ramipril) or granules or coated or uncoated pellets prepared therefrom as described above with the components of the external phase and pressing the mixture to minitablets. The size of the minitablets should be such that they can be placed into the capsule. Thus according to the process one or more minitablet(s) obtained is (are) placed in the capsule (preferably in hard gelatine capsule) whereupon the mixture comprising the other active ingredient, which contains the coated or uncoated particles of the active ingredient or the coated or uncoated granules or pellets containing the active ingredients and if necessary the components of the external phase, are introduced in the capsule which is finally closed. One may also proceed by preparing minitablets from both active ingredients as described above and placing said minitablets in soft gelatine capsules.
The processes suitable for the preparation of the compositions according to the present invention and the apparatus used in said procedures are generally known and used in pharmaceutical industry. The optimization of the technological steps and the determination of the suitable parameters belong to the general knowledge of the person skilled in the art. The procedures and equipments used for the preparation of tablets, capsules, compacts, granules, pellets and minitablets and the corresponding technological details are described in a large number of manuals, such as Encyclopedia of Pharmaceutical Technology (editor: James Swarbrick; Infoma HealthCare USA Inc, 2007).
The most important embodiments of the invention can be summarized as follows:
The invention relates to a solid combined pharmaceutical composition which contains ramipril and bisoprolol or a pharmaceutically acceptable salt or complex thereof.
The composition according to the present invention contains preferably 1.25-10 mg of ramipril and preferably 1.25-10 mg of bisoprolol.
According to a more preferred embodiment of the present invention in the dosage units of the combined compositions the ratio of bisoprolol and ramipril - related to a combination of bisoprolol hemifumarate and ramipril base, in mg/mg - is as follows: 1.25/5; 2.5/2.5; 2.5/5; 2.5/10; 5/1.25; 5/2.5; 5/5; 5/10; 10/2.5; 10/5; 10/10; 10/5 mg/mg.
The combined product containing bisoprolol hemifumarate and ramipril has generally most preferably the following composition: 5/5 - 2.5/5 and 10/5.
According to a very preferable embodiment of the invention the products of various strength have the following quantitative composition:
Table 3:
More particularly the particles of ramipril and bisoprolol or pharmaceutically acceptable salts there are separated from each other in the pharmaceutical composition.
However also such pharmaceutical compositions fall under the scope of the present invention in which the particles of the ramipril and bisoprolol active ingredients or pharmaceutically acceptable salts thereof are in separate phases and the particles of the active ingredient are in contact with each other only at the limit surface of the layers.
The pharmaceutical composition according to the present invention may be preferably in form of tablets or capsules.
The pharmaceutical composition according to the present invention may also be in form of multi-layer tablets in which bisoprolol and ramipril or pharmaceutically acceptable salts thereof are formulated in separate layers. The tablet according to the present invention can also be a mono- or multi-layer tablet in which the bisoprolol and ramipril active ingredients are in the very same layer of the tablet and the particles of the active ingredients are separated from each other in the layer. According to a preferred embodiment of the invention at least one of both active ingredients or pharmaceutically acceptable salts thereof is (are) in form of a granule, preferably a dry granule or a compact. According to a preferred embodiment the particles of at least one active ingredient or granules or compacts containing the same is (are) coated. According to a still more advantageous embodiment of the invention particles of ramipril or a salt thereof or granules, compacts or minitablets containing the same and particles of bisoprolol or a salt thereof or granules, minitablets or compacts containing the same are coated.
According to a preferred embodiment of the invention the composition contains as active ingredient ramipril and bisoprolol hemifumarate.
According to the most preferred embodiment of the invention there are provided combined pharmaceutical compositions comprising
a) a ramipril containing coated granule which contains cross-linked polyvinyl- pyrrolidone and optionally HPMC and other auxiliary agents, whereby said granules are coated with an organic polymer, preferably HPMC;
b) a bisoprolol hemifumarate containing coated compact which contains bisoprolol hemifumarate, microcrystalline cellulose and optionally further auxiliary agents generally used in pharmaceutical industry, whereby said granules are coated with an organic polymer, preferably HPMC, and
c) an external phase which contains a filler, preferably microcrystalline cellulose, a glidant preferably colloidal silica and a lubricant, preferably magnesium stearate or glyceryl behenate. According to a still more preferred embodiment of the present invention the ramipril containing coated granules, the bisoprolol hemifumarate containing coated compacts and the components of the external phase are filled in a capsule.
The present invention also relates to a coated compact comprising bisoprolol hemifumarate active ingredient which contain a filler, preferably microcrystalline cellulose, as coating agent an organic polymer such as polyvinyl pyrrolidone, HPMC or a mixture thereof, preferably HPMC.
According to the present invention there is provided a bisoprolol hemifumarate containing coated compact which contains 5-30 % by weight, preferably 10-25 % by weight, particularly 20-25 % by weight of bisoprolol hemifumarate, 60-93 % by weight, preferably 65-80 % by weight, particularly 75-80 % by weight of a filler, preferably microcrystalline cellulose and 2-10 % by weight, preferably 3-7 % by weight., particularly 4-5 % by weight of an organic polymer, e.g. polyvinyl pyrrolidone or HPMC, particularly HPMC - related to the weight of the coated compact.
According to a highly preferred embodiment of the present invention pharmaceutical compositions of varying strength are provided which contain the active ingredients separately in granulated or compacted form. It is very advantageous to use ramipril containing granules or compacts of the following composition:
72
Table 4
It is highly advantageous to use a bisoprolol containing compact of the following composition:
Table 5
Ramipril containing granules or compacts and bisoprolol containing compacts can be used for the preparation of combined pharmaceutical compositions of varying active ingredient content which comprise in addition to the active ingredient containing compacts or granules further auxiliary agents. The advantageous composition of such capsules of varying strength is shown in the following Table
Table 6
On the one hand the solid pharmaceutical composition according to the present invention contains the active ingredients substantially separated from each other and on the other characterized by the fact that their dissolution profile is similar to the dissolution profiles observed on simultaneously administration of the mono compositions i.e. the pharmaceutical composition according to the present invention can directly replace the simultaneous administration of the mono compositions. The dissolution of the active ingredients from the compositions according to the present invention was tested under standard conditions according to European Pharmacopoeia (2.9.3. dissolution test for solid dosage forms) at 37°C in 900 ml of a 0.1 N HCl solution by using a 50 r.p.m. paddle stirrer. According to this test in the first
15 minutes on an average 90-100 %, preferably 95-100 % of the bisoprolol and 90-100 %, preferably 95-100 % of the ramipril were dissolved. The term "dissolution on an average" means that the average value of the dissolution of 12 capsules or tablets was taken into consideration.
On testing capsules it has been found that although according to a very preferred embodiment of the invention both active ingredients were coated, the rate of dissolution was within the desired limits. The following Tables show that compositions comprising bisoprolol and ramipril in a ratio of 10/5 mg and 10/10 mg meet the requirements .
Bisoprolol and ramipril were formulated in form of coated compacts as described above
0.1 N HCl, 900 ml, Average of dissolution [%]
paddle, 50 rpm
T [min] 0 5 10 15 30 45 60
BISOPROLOL Average: 0 80.8 99.5 101.0 100.9 100.7 100.4 (135RB0314K) 10/5 standard 0 7.32 1.71 1.44 1.48 1.46 1.54 mg deviation
starting
RAMIPRIL Average: 0 82.2 97.3 98.5 98.5 98.9 99.0
(135RB0314K) 10/5 standard 0 5.72 1.02 1.41 1.55 1.54 1.47 mg deviation
starting
Table 9
Pilot bioequivalence tests carried out with compositions containing 10 mg/10 mg of bisoprolol and ramipril have shown that the combined pharmaceutical compositions according to the present invention can directly replace the mono compositions having the corresponding active ingredient content previously used for the treatment of the patients. Thus e.g. the treatment of a patient who previously received simultaneously every day a composition containing 10 mg of ramipril and a composition containing 10 mg of bisoprolol hemifumarate can be continued by administering a combined composition according to the present invention containing 10 mg/10 mg of both active ingredients without any risks. Thus the administration of two mono compositions can be eliminated.
The results of the bioequivalence tests are summarized in the following Tables: Table 10
Statistic comparison of the bioavailability of bisoprolol between the Test 1 (T) and Reference (R) formulations
Table 11
Statistic comparison of the bioavailability of ramipril between the Test 1 (T) and the Reference (R) formulations.
GEOMETRIC T/R 90% CONFIDENCE
PARAMETE LSMEANS Ratio LIMITS (%)
R
TEST REFERENCE (%) LOWER UPPER c 16.0 16.5 96.72 79.40 1 17.80
AUCx 11.8 1 1.7 99.96 90.32 110.62
* UNITS OF MEASUREMENT: in case of Cmax /ng/ml/ and AUCT ng.h/ml
The plasma ramipril concentration obtained from the combined composition is substantially bioequivalent to the ramipril containing reference composition. Since we have carried out a so-called "pilot" test, the case number was significantly lower than justified by the variability of the ramipril Cmax values among the patients. Due to the small case number the 90 % confidence interval of the Cmax test/reference ratio was broad. However the lower limit of the confidence interval (79.4 %) was just below the desired value of 80 %. At the same time the higher value (117.8 %) is within the acceptable limit value, but falls into the upper range of the interval. It is evident that the broad confidence interval is due to the small case number. The logarithmically transformed test/reference ratio (96.7 %) is within the expected value and is near to 100 %. If the case number is increased the confidence interval becomes narrower and therefore also the lower value falls in the acceptable interval. However the deviation is not so high and when the parallel treatment with two mono compositions is replaced by the administration of the combined composition according to the present invention no unexpected effect or side effect was observed. In case of ramipril AUC the 90 % confidence interval of the test/reference ratio falls in the prescribed interval of 80-125 % and on the basis of the test/reference ratio (99.96 %) the AUC values can be considered to be almost identical and differ from the 100 % value only to a small extent. The above data prove that the two mono compositions of the corresponding active ingredient content can be replaced by the combined composition according to the present invention substantially without any risk.
According to a highly preferred embodiment of the present invention there is provided a solid composition in which ramipril and bisoprolol are separated from each other,
and which contain 10 mg of ramipril and 10 mg of bisoprolol, preferably bisoprolol fumarate, whereby the Cmax value of the composition related to bisoprolol is 35-55 ng/ml, preferably 39-48 ng/ml, particularly 43.8 ng/ml, the Cmax value related to ramipril is 13-21 ng/ml, preferably 14,82-18.15 mg/ml, particularly 16.5 ng/ml and/or the AUCT value related to bisoprolol is 503-790 ng.h/ml, preferably 566-692 ng.h/ml, particularly 629 ng.h/ml and the AUCT value related to ramipril is 9.3-14.7 ng.h/ml, preferably 10.5-12.9 ng.h/ml, particularly 11.7 ng.h ml.
According to a highly preferred embodiment of the present invention there is provided a solid pharmaceutical composition in which ramipril and bisoprolol are separated from each other and the composition contains 10 mg of ramipril and 10 mg of bisoprolol, preferably bisoprolol fumarate, whereby the Craax value of the composition related to bisoprolol is 43.8 ng/ml and the Cmax value related to ramipril is 16.5 ng/ml and/or the AUCT value related to bisoprolol is 629 ng.h/ml and the AUCT value related to ramipril is 11.7 ng.h/ml or is bioequivalent to the above values.
According to the present invention those combined compositions containing 10 mg of bisoprolol and 10 mg of ramipril are considered as bioequivalent which show the following Cmax and AUCT values: Cmax related to bisoprolol 43.8 ng/ml, Cmax related to ramipril 16.5 ng/ml, AUCT related to bisoprolol 629 ng.h/ml and the AUCT value related to ramipril 11.7 ng.h/ml, fall in the range of 80-125 %, preferably 90-110 %.
The dissolution of the products according to the present invention does not considerably change during storage. On storing the combined composition according to the present invention at 40°C under a moisture content of 75 % the dissolution profile dose not significantly change.
Table 12/A Test of the dissolution of capsules containing 10/5 mg of bisoprolol/ramipil .
Bisoprolol is formulated as coated compacts and ramipril is in form of coated granules, prepared as described above (130RB0314K)
Product prepared according to Example 6/A
0.1 N HCl, 900 ml, Average of dissolution [%]
paddle, 50 rpm
T [min] 0 5 10 15 30 45
BISOPROLOL Average: 0 89.4 100.5 101.4 101.6 101.4 (130RB0314K) 10/5 standard 0 6.50 1.02 1.05 1.00 1.08 mg deviation:
starting
2 weeks / 40°C / 75% Average: 0 86.2 101.7 102.3 102.3 102.0 moisture content standard 0 9.11 1.19 1.06 0.99 1.01 deviation:
1 month / 40°C / 75% Average: 0 88.6 99.5 100.3 100.6 100.3 moisture content standard 0 5.14 0.88 1.19 1.44 1.54 deviation:
RAMIPRIL Average: 0 88.6 98.2 99.2 99.9 100.3 130RB0314K 10/5 mg standard 0 6.86 1.97 2.00 1.81 1.86 starting deviation:
2 weeks / 40°C / 75% Average: 0 87.1 100.9 101.5 101.9 102.1 moisture content standard 0 8.47 1.88 1.86 1.86 1.78 deviation:
1 month / 40°C / 75% Average: 0 88.1 97.6 98.4 98.8 99.0 moisture content standard 0 4.76 1.49 1.64 1.73 1.77 deviation:
deviation:
In the composition according to the present invention the concentration of ramipril impurity D is below 1 %.
Table 13
The invention relates to a process for the preparation of a combined pharmaceutical composition which contains as active ingredient ramipril and bisoprolol or a salt or complex thereof which comprises
coating the particles of one of the active ingredients, namely ramipril or bisoprolol or a compact, granule or pellet contaming the same with a polymer soluble or swellable in water - such as HPMC or polyvinyl pyrrolidone - and
admixing the product thus obtained with particles of the other active ingredient which can be uncoated or coated with a polymer soluble or swellable in water, such as
HPMC or polyvinyl pyrrolidone or a compact, granule or pellet containing the same and with further auxiliary agents, preferably fillers, and if necessary disintegrants, lubricants and glidants and pressing the mixture thus obtained to tablets or filling in capsules.
For this purpose preferably hard gelatine capsules can be used.
According to a preferred embodiment of the invention the composition contains both active ingredients in the form coated granules.
According to a further preferred embodiment of the invention ramipril and bisoprolol hemifumarate can be used as active ingredient.
According to the most preferred form of realization of the present invention the combined pharmaceutical composition according to the present invention can be prepared by
a) preparing at first a ramipril containing coated granule by i. related to the weight of the coated granule, pre-granulating 10-50 % by weight, preferably 10-30 % by weight, particularly 15-25 % by weight of ramipril and 60-93 % by weight, preferably 65-80 % by weight, particularly 75-80 % by weight of a disintegrant, preferably cross-linked PVP (such as polyplasdone XL 110) in a vortex granulator so that pre-granulation is carried out with a 5-10 % by weight, preferably 5-8 % by weight of an aqueous solution of 30-50 % by weight, preferably 40-50 % by weight of the HPMC used in an amount of 1-15 % by weight, preferably 1-10 % by weight, particularly 2-6 % by weight - related to the weight of the coated granules - coating the granules thus obtained with a 5-10 % by weight, preferably 5-8 % by weight aqueous solution of the residual amount of HPMC; or ii. related to the weight of the coated granules, compacting in the dry a mixture of 10-50 % by weight, preferably 10-30 % by weight, particularly 15-25 % by weight of ramipril and 60-93 % by weight, preferably 65-80 % by weight, particularly 75-80 % by weight of a disintegrant, preferably cross-linked PVP (e.g. polyplasdone XL 10), re-granulating the compacts and coating with a 5-10 % by weight, preferably 5-8 % by weight aqueous solution of 1-15 % by weight, preferably 1-10 % by weight, particularly 2-6 % by weight - related to the weight of the coated granules - off HPMC;
b) preparing bisoprolol hemifumarate containing a coated compact by homogenizing - related to the weight of the coated compact - 5-30 % by weight, preferably 10-25 % by weight, particularly 20-25 % by weight of bisoprolol hemifumarate a and 60-93 % by weight, preferably 65-80 % by weight, particularly 75-80 % by weight of microcrystalline cellulose, compacting the mixture, re-granulating the compact thus obtained optionally at first on a 1.2 mm sieve and then on an e.g. 0.5 mm sieve, coating the compact thus obtained or optionally the re-granulated bisoprolol hemifumarate containing compact with an organic polymer, preferably HPMC or polyvinyl pyrrolidone by using - related to the weight of the compact to be coated - 2- 10 % by weight, preferably 3-7 % by weight, particularly 4-5 % by weight of an organic polymer, such polyvinyl pyrrolidone or HPMC, preferably HPMC in form of a 5-10 % by weight, preferably 5-8 % by weight aqueous solution; and thereafter c) homogenizing the ramipril containing granules prepared according to step a) and the bisoprolol hemifumarate containing compact prepared according to step b) with the components of the external phase, preferably fillers, such as microcrystalline cellulose, glidants, preferably Aerosol (colloidal silica) and lubricants, preferably magnesium stearate or glyceryl behenate and pressing the homogenized product to tablets or filling in capsules.
According to the present invention there is also provided a process for the preparation of a bisoprolol hemifumarate containing coated compact which comprises homogenizing - related to the weight of the coated compact - 5-30 % by weight, preferably 10-25 % by weight, particularly 20-25 % by weight of bisoprolol hemifumarate and 60-93 % by weight, preferably 65-80 % by weight, particularly 75- 80 % by weight of microcrystallines cellulose, compacting the homogenized mixture, if necessary re- granulating the compact thus obtained and coating the bisoprolol hemifumarate containing compact thus obtained with an organic polymer, preferably HPMC or polyvinyl pyrrolidone by using - related to the weight of the coated compact - 2-10 % by weight, preferably 3-7 % by weight, particularly 4-5 % by weight of an
organic polymer, such as polyvinyl pyrrolidone or HPMC, most preferably HPMC in form of a 5-10 % by weight, particularly 5-6 % by weight aqueous solution.
Re- granulation can be also carried out -if necessary - by re-granulating the compact at first on a e.g. 1.2 mm sieve and then on an e.g. 0.5 mm sieve. Thereafter the re- granulated compact is coated as described above.
According to the most preferable embodiment of the composition according to the present invention ramipril containing granules or compacts and bisoprolol containing compacts are prepared as follows: a) Preparation of ramipril granules
Related to the weight of the final granules 19 % by weight of ramipril and 76.4 % by weight of Polyplasdone XL- 10 (GAP quality) are passed through a 0.8 mm sieve, whereupon a 5.8 % by weight solution of HPMC is prepared by using 4.5 % by weight of HPMC (Pharmacoat 603), whereupon 45 % of the sieved mixture of ramipril and Polyplasdone XL- 10 is granulated on a vortex granulator. The granules thus obtained are re-granulated on a 0.5 mm sieve and coated with the residual HPMC solution by fluid granulation. The granules thus obtained are re-granulated on a 0.5 mm sieve and homogenized. b) Preparation of coated ramipril compacts Related to the weight of the final compact 19 % by weight of ramipril and 76.4 % by weight of Polyplasdone XL- 10 (GAF grade) are passed through a 0.8 mm sieve, the mixture is homogenized and thereafter compacted. The compacts thus obtained are passed through a 0.8 mm sieve, homogenized and a 5.8 % by weight HPMC solution is prepared by using 4.5 % by weight of HPMC (Pharmacoat 603) - related to the weight of the compact. The compacts are coated with this solution, preferably by fluid
granulation. The granules thus obtained are re-granulated on a 0.5 mm sieve and homogenized. c) Preparation of a bisoprolol containing compact
Related to the weight of the final compact 19 % by weight of bisoprolol and 76.4 % by weight of microcrystalline cellulose (PH 101) are passed through a 0.8 mm sieve, homogenized and compacted. The compacts thus obtained are re-granulated at first on a 1.2 mm sieve, thereafter on a 0.8 mm sieve. A 5.8 % by weight HPMC solution is prepared from 4.6 % by weight of HPMC (Pharmacoat 603) - related to the weight of the compact - and the compacts are coated with this solution, preferably by fluid granulation. The granules thus obtained are re-granulated on a 0.5 mm sieve and homogenized. A bisoprolol-ramipril 10/5 mg capsule can be prepared by the following process from the ramipril containing granules or compacts and the bisoprolol containing coated compacts obtained as described above.
In the first step - related to the weight of a capsule - 52.4 mg of a coated bisoprolol compact, 26,18 mg of the ramipril granules or compact prepared according to the above paragraphs a) and b) and 83.2 mg of Vivapur 200 (microcrystalline cellulose) are passed through a 0.8 mm sieve whereupon 112 mg of Vivapur 200 (microcrystalline cellulose), 20 mg of polyplasdone XL- 10 (GAF granule) and 1.18 mg of hydroxypropyl methyl cellulose (Pharmacoat 606) are added and the mixture obtained is homogenized.
To the homogenized mixture thus obtained 8 mg of Vivapur 200 (microcrystalline cellulose), 4 mg of Compritol 888 (microcrystalline cellulose) and 1.24 mg of colloidal silica (Aerosil R972) are added. The homogenized mixture thus obtained is filled in capsules or pressed to tablets. Compositions containing 5/5, 10/10 and 5/10 mg/mg of
the active ingredients can be prepared in a similar way by using the components and ratio disclosed in Table 6.
According to another aspect of the present invention there is provided the use of bisoprolol and ramipril or a salt or complex thereof for the preparation of a combined pharmaceutical composition suitable for the treatment of hypertension, preferably high renin level hypertension.
It has been found that a pharmaceutical composition suitable for the treatment of hypertension, preferably high renin level hypertension can be prepared by using bisoprolol and ramipril or a salt or complex thereof. Based on the above recognition there is also provided a medical treatment of hypertension, particularly high renin level hypertension which comprises administering to the patient in need of such treatment a combined pharmaceutical composition comprising bisoprolol and ramipril or a salt or complex thereof.
It is most preferable to use ramipril and bisoprolol in the following doses (calculated for bisoprolol hemifumarate/ramipril base, in mg/mg). 1.25/5; 2.5/2.5; 2.5/5; 2.5/10; 5/1.25; 5/2.5; 5/5; 5/10; 10/2.5; 10/5; 10/10 mg.
In most cases the doses of 5/5-2.5/5, 10/5 and 10/10 proved to be advantageous.
It has also been found that in course of the treatment the once-a-day administration of the combined pharmaceutical composition can also be used.
The advantages of the composition according to the present invention can be summarized as follows:
The patients in need of the treatment can receive both active ingredients in a single composition which improves the patient compliance. The risk that the patient incidentally forgets to swallow one of the active ingredients is eliminated. - The combined composition according to the present invention is bioequivalent to the simultaneous administration of the corresponding mono compositions and can therefore directly replace the mono compositions.
The process according to the present invention makes the formulation of the two incompatible pharmaceutical agents in a single composition possible.
Our invention also relates to a bisoprolol hemifumarate containing coated compact which hinders the decomposition of the other or further active ingredients in the combined composition, because the coating prevents fumaric acid from exerting the effect thereof. At the same time the present invention enables the use of the bisoprolol fumarate salt of the composition; the advantageous dissolution and pharmacological effects of the fumarate salt are well-known. There is no need to carry out expensive biological tests of new bisoprolol salts for the development of combined compositions.
The combined compositions according to the present invention preserve not only their chemical stability but also the dissolution properties.
Further details and preferred embodiments of the present invention are to be found in the Examples without limiting the scope of protection to the Examples.
Pilot pharmacokinetic tests
Pilot pharmacokinetic study to evaluate the bioavailability of bisoprolol and ramipril from two different type of fixed dose combination of bisoprolol and ramipril compared to co-administered marketed bisoprolol and ramipril mono-component tablets has been
conducted. In a single dose, 3 -period, 3 -treatment, 3 -sequence crossover study 24 healthy subjects were randomly assigned to a treatment sequence and received bisoprolol/ramipril 10/10 mg FDC-1 (Test 1), bisoprolol/ramipril 10/10 mg FDC-2 (Test 2) and 10 mg bisoprolol + 10 mg ramipril mono-component tablets (Reference).
Subjects fasted overnight for at least 10 hours prior to drug administration. Blood samples were collected prior to drug administration and at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 12, 24, 36, and 48 hours after dosing for assessment of bisoprolol, and at 0.17, 0.33, 0.5, 0.67, 0.83, 1, 1.25, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 8 and 12 hours after dosing for assessment of ramipril plasma concentration in each study period.
The samples were analysed for ramipril and bisoprolol by validated HPLC-MS/MS method and the following pharmacokinetic parameters were calculated: Cmax, AUCj, AUC∞, AUCT/∞, Kei and T½ei. Statistical analysis was based on a parametric ANOVA model and two-sided 90% confidence interval of the ratio of geometric means for the ln-transformed Cmax and AUCT.
As between the two Test products Testl formulation (bisoprolol/ramipril 10/10 mg FDC-1) was produced according to the manufacturing procedure described in the present patent application, only the result of this formulation versus reference treatment is shown. (In case of ramipril the pharmacokinetic parameters were normalized to the ramipril contents of the formulations).
The results provided in Table 10. indicate that the Test to Reference ratio of geometric LSmeans and corresponding 90% confidence interval for the Cmax, AUCT were all within the range of 80.00 to 125.00% for bisoprolol indicating bioequivalence.
According to the results provided in Table 11. the Test to Reference ratio of geometric LSmeans and corresponding 90% confidence interval for AUCT was within the range of 80.00 to 125.00% for ramipril indicating equivalence of this parameter. The Test to Reference ratio of geometric LSmeans for Cmax was within the range of 80.00 to 125.00%· and only the lower end of the 90% confidence interval is ranged out
marginally the 80 - 125% limit due to the relatively low sample size of this pilot study indicating that in appropriately powered pivotal study the bioequivalence could be proved for Cmax of ramipril, too. Dissolution tests
1. Conditions of the test
Apparatus Ph. Eur. System, rotating paddle.
Rotation: 50 r.p.m, +15 minutes of rotation (250 r.p.m.).
Test liquid: 900 ml 0.1 M HC1.
Test temperature: 37°C ± 0.5°C.
Determination: UPLC method.
Duration of the test: 5, 10, 15, 30, 45, 60 minutes.
Number of tested capsules: 6 or 12.
2. Process.
900 ml of the dissolving medium are poured in a glass and the temperature of the solution is kept at 37°C ± 0.5°C. 6 x 1 or 12 x 1 capsules each are placed in glasses and thereafter the apparatus and the watch are simultaneously started. In the 5th, 10th, 15th, 30th 45th and 60th minutes samples are taken at the middle of the distance between the top of the rotating paddle and the surface of the liquid at a distance of at least 1 cm from the wall of the vessel through a HPDE filter having a pore diameter of 10 micrometers. The samples are pressed through a 0.20 micrometer Millex LG 0.20 micrometer /PTFE) filter. The first part of the filtrate is discarded and the solution is subjected to chromatography.
3. Conditions of chromatography.
Apparatus: Waters Acquity UPLC or Waters Acquity UPLC H-class system, or any equivalent equipment.
PDA detector
Quaternary gradient pump
Automatic sample feeder
Means for degassing
Column thermostat
Empower chromatography software or an equivalent product
Column: ACQUITY UPLC BEH C 18 1.7 micrometer, 50x21 mm (Waters) Pre-column: ACQUITY UPLC BEH C 18 Van GuardT Pre-Column 5x2.1 mm, 1,7 micrometer (Waters)
Column temperature: 45°C
Column pressure: about 685 bar
"A" eluent: acetonitrile: water :perchloric acid = 100:900:1 V/V/V %
"B" eluent: acetonitrile:water:methanol:perchloric acid = 400:200:400:1 V/V/V/V % After homogenization the eluents are filtered through a 0.22 um filter-paper.
Streaming speed: 0.7 ml/minute Method of separation: gradient
Duration of chromatography: 6.00 minutes
Material: stainless steel
Injected volume: 10.0 pi
Method of injecting: Waters Acquity UPLC:Full Loop
Detection
Sampling rate 20 points/sec
Filtration Time Constant: Normal (0, 1000 sec) Exposure time: Auto
215 nm, 4.8 nm resolution
Ίη = 226 nm, 4.8 nm resolution
Temperature of sample area: 20°C
Evaluation, External standard calibration.
Example 1
Two-layer tablet containing ramipril and bisoprolol:
Brief description of the process
A) Preparation of the layer containing bisoprolol active ingredient:
The active ingredient and the other components are used in amounts corresponding to 10 000 tablets.
Bisoprolol and microcrystalline cellulose used in the internal phase are homogenized and the homogenized mixture is compacted. The compacts thus obtained are re- granulated on sieves of 1.2 mm and 0.5 mm. The granules thus obtained are coated with a 8 % by weight aqueous solution of HPMC in a fluidization granulation apparatus. The coated compact thus obtained is homogenized with a portion of microcrystalline cellulose used in the external phase, whereupon the residual microcrystalline cellulose, Aerosil R972 and Compritol (glyceryl behenate) are added to the mixture thus obtained to yield the final homogenized mixture. B) Preparation of ramipril granules:
The active ingredient and the other components are used in amounts corresponding to 10,000 tablets. Ramipril and Poliplasdon XL 10 (cross-linked polyvinyl pyrrolidone) are homogenized, the mixture is pre-granulated with a 5 % by weight HPMC solution of 45 % of the HPMC used in the internal phase in a vortex granulating apparatus, whereupon the granules are passed through a 0.5 mm sieve. The re-granulated product thus obtained is granulated with a 5 % by weight aqueous solution of the residual microcrystalline cellulose in a fluidized bed granulator. The granules thus obtained are homogenized first with a portion of the microcrystalline cellulose and thereafter with a mixture of the residual microcrystalline cellulose and Aerosil R-972. To the homogenized product thus obtained Compritol 888 is added to yield the final homogenized product.
C) The homogenized products prepared according to steps A) and B) are poured into two feeding containers whereupon sandwich-tablets are prepared on a tabletting machine by pressing first the bisoprolol containing layer and thereafter pressing the ramipril containing layer on the bisoprolol layer. The tablets thus obtained are packed.
Example 2
Two-layer tablets containing ramipril and bisoprolol One proceeds as described in Example 1 except that the internal phase of the ramipril containing layer is prepared as follows:
Ramipril and poliplasdon XL 10 (cross-linked polyvinyl pyrrolidone) are homogenized and the mixture obtained is compacted. The compacts thus obtained are re-granulated first on a 1.2 mm sieve and then on a 0.5 mm sieve. The granules thus obtained are coated with a 8 % by weight aqueous solution of HPMC in a fluidization granulating apparatus. The coated granules are homogenized with a portion of the microcrystalline cellulose used in the external phase whereupon the residual microcrystalline cellulose and the Aerosil R972 are added to the mixture thus obtained. The above components are homogenized whereupon Compritol 888 (glyceryl behenate) is added. Thus the final homogenized product is obtained.
Example 3
Three-layer tablet containing ramipril and bisoprolol Example 3/A One proceeds as described in Example 1 except that that not only the bisoprolol and ramipril containing layers but also the components of the active ingredient free
external phase are homogenized. The tablets contain 93.2 mg of microcrystalline cellulose, 1 mg of Aerosil R 972 (colloidal silica) and 1 mg of Compritol 888.
Tabletting is carried out by introducing the bisoprolol containing homogenized layer, the ramipril containing homogenized layer and the active ingredient free homogenized layer into three feeding containers of a sandwich-tabletting machine and pressing the tablets in the following order: at first the bisoprolol containing layer, then the active ingredient free separating layer and finally the ramipril containing layer. Example 3/B
One proceeds as described in Example 2 except that not only the bisoprolol and ramipril containing layers but also the components of the external phase are separately homogenized. The tablets contain 93.2 mg of microcrystalline cellulose, 1 mg of Aerosil R 972 and 1 mg of Compritol 888.
Tabletting is carried out by introducing the bisoprolol containing homogenized layer, the ramipril containing homogenized layer and the active ingredient free homogenized layer into three feeding containers of a sandwich-tabletting machine and pressing the tablets in the following order: at first the bisoprolol containing layer, then the active ingredient free separating layer and finally the ramipril containing layer.
Example 4 Preparation of ramipril and bisoprolol containing one-layer tablets (4.1.) and capsules (4.II)
4.1. Preparation of tablets
A.) Composition of the bisoprolol containing layer,
(internal phase)
Composition of coated bisoprolol hemifumarate
containing compact (internal layer):
Bisoprolol fumarate 10.00 mg
Microcrystalline cellulose (Vivapur 101) 40.00 mg
Hypromellose (HPMC; Pharmacoat 603) 2.40 mg
Total weight of compact: 52.40 mg
B.) Composition of the ramipril containing layer:
Composition of ramipril granules:
Ramipril 10.00 mg
Polyplasdone XL 10 40.00 mg
Hypromellose (HPMC) 2.36 mg
Total weight of granules total: 52.36 mg
C.) Composition of the external phase:
Microcrystalline cellulose (Vivapur 200) 93.20 mg
Aerosil R 972 (colloidal silica) 1.00 mg
Compritol 888 (glyceryl behanate) 1.04 mg
Total weight of the external phase : 95.24 mg
Brief description of the process A) Preparation of the bisoprolol containing layer
When preparing the bisoprolol containing layer the active ingredient and the other components are used in amounts corresponding to 10 000 tablets. Bisoprolol and the microcrystalline cellulose used in the internal phase are homogenized and the homogenized product is compacted. The compact thus obtained is re-granulated first on a 1.2 mm sieve and thereafter on a 0.5 mm sieve. The granules thus obtained are coated with a 8 % by weight aqueous solution of HPMC. B) Preparation of ramipril containing granules
When preparing the ramipril containing layer the active ingredient and the other components are used in amounts corresponding to 10 000 tablets.
Ramipril and Polyplasdon XL 10 (cross-linked polyvinyl pyrrolidone) are homogenized, the mixture obtained is pre-granulated in a vortex granulating machine with a 8 % by weight HPMC solution prepared from 45 % of the HPMC used in the internal phase and the granules thus obtained are re-granulated on a 0.5 mm sieve. The re-granulated product is granulated with an 8 % aqueous solution of the residual HPMC in a fluid bed granulating apparatus.
C) The bisoprolol fumarate containing coated compact and the ramipril containing granules are homogenized, the microcrystalline cellulose is added and the mixture is again homogenized. To the homogenized mixture the colloidal silica (Aerosil 972) and Compritol 888 are added and the mixture is homogenized. The final homogenized product thus obtained is tabletted and the tablets are packed.
4.Π. Preparation of capsules
One proceeds as described in 4.1. directed to the preparation of tablets except that step C) is carried out as follows:
C) The bisoprolol containing coated compact and the ramipril containing granules are homogenized, microcrystalline cellulose is added and the mixture is again homogenized. To the homogenized mixture microcrystalline cellulose is added, the mixture is again homogenized, whereupon to the homogenized product colloidal silica (Aerosil 972) and Compritol are added and the mixture obtained is homogenized. The final homogenized product thus obtained is filled in hard gelatine capsules and packed.
Example 5
One-layer tablet (5/1) and capsule (5/B) containing ramipril and bisoprolol
Tablet 5/1
One proceeds as described in Example 4.1. except that ramipril granules are replaced by a ramipril compact prepared by the following process:
19 % by weight of ramipril and 76.4 % by weight of Polyplasdone XL- 10 (GAF grade) - related to the weight of the final compact - are passed through a 0.8 mm sieve, homogenized and compacted. The compacts thus obtained are passed through a 0.8 mm sieve and homogenized. A 5.8 % by weight HPMC solution prepared from 4.5 % by weight of HPMC (Pharmacoat 603) - related to the weight of the compact - is prepared. The compacts are coated with this solution, preferably by fluid granulation. The granules thus obtained are re-granulated on a 0.5 mm sieve and homogenized. Capsule 5/II (batch number 01019 0713)
One proceeds as described in Example 4.II except that ramipril granules are replaced by a ramipril compact prepared by the following process: 19 % by weight of ramipril and 76.4 % by weight of Polyplasdone XL- 100 (GAF quality) - related to the weight of the compact - are passed through a 0.8 mm sieve, homogenized and compacted. The compacts thus obtained are passed through a 0.8 mm sieve and homogenized. A 5.8 % by weight HPMC solution is prepared by using 4.5 % by weight of HPMC -related to the weight of the compact. The compacts are coated with this solution, preferably by using a fluid granulation method. The granules thus obtained are granulated on a 0.5 mm sieve and homogenized.
Example 6 Capsules (6/A) or tablets (6/B) comprising bisoprolol and ramipril (strength 10/5 mg/mg)
W
Composition
Preparation of ramipril granules
19 % by weight of ramipril and 76.4 % by weight of Polyplasdone XL-10 (GAF grade) - related to the final weight of the granules - are passed through a 0.8 mm sieve. A 5.8 % by weight HPMC solution is prepared by using 4.5 % by weight of HPMC (Pharmacoat 603) - related to the weight of the final granules). The sieved mixture of ramipril and Polyplasdone XL-10 is granulated with the above HPMC solution in a
vortex granulator. The granules thus obtained are re-granulated on a 0.5 mm sieve and coated with the residual HPMC solution by fluid granulation. The granules thus obtained are re-granulated on a 0.5 mm sieve and homogenized. Preparation of a coated bisoprolol containing compact
19 % by weight of bisoprolol and 76.4 % by weight of microcrystalline cellulose (PH 101) - related to the weight of the final compact - are passed through a 0.8 mm sieve, homogenized and compacted. The compacts thus obtained are re-granulated first on a 1.2 mm and thereafter on a 0.8 mm sieve. A 5.8 % by weight HPMC solution is prepared by using 4.6 % by weight of HPMC (Pharmacoat 603) - related to the weight of the compact - and this solution is used to coat the compacts, preferably by fluid granulation. The granules thus obtained are re-granulated on a 0.5 mm sieve and homogenized.
6/A
Preparation of capsules Related to one capsule 52.4 mg of coated bisoprolol containing compact, 26.18 mg of ramipril containing granules and 83.2 mg of Vivapur 200 (microcrystalline cellulose) are passed through a 0.8 mm sieve. To the homogenized mixture thus obtained 112 mg of Vivapur 200 (microcrystalline cellulose), 20 mg of Polyplasdone (GAF grade) and 1.18 mg of hydroxypropyl methylcellulose (Hypromellose 2910, 6 cP) are added. The mixture is homogenized and to the homogenized mixture thus obtained 8 mg of Vivapur 200 (microcrystalline cellulose), 4 mg of Compritol 888 (microcrystalline cellulose) and 1.24 mg of colloidal silica (Aerosil R972) are added. The homogenized mixture thus obtained is filled in capsules. 6/B
Preparation of tablets
One proceeds as described in Example 6/A except that the homogenized mixture is not filled in capsules but is pressed to tablets.
Example 7
Capsule (7/A) or tablet (7/B) containing bisoprolol and ramipril (strength 10/5 mg) One proceeds as described in Example 6 (Examples 6/A and 6/B) except that ramipril granules are replaced by a ramipril compact prepared according to the following process: 19 % by weight of ramipril and 76.4 % by weight of a Polyplasdone XL- 10 (GAF grade) are passed through a 0.8 mm sieve. The mixture is homogenized and compacted. The compact thus obtained is passed through a 0.8 mm sieve and homogenized. A 5.8 % by weight HPMC solution is prepared by using 4.5 % by weight of HPMC (Pharmacoat 603) - related to the weight of the compact. The compacts are coated with this solution, preferably with the aid of a fluid granulating procedure. The granules thus obtained are re-granulated on a 0.5 mm sieve and homogenized.
Example 8
Capsule (8/A) or tablet (8/B) containing bisoprolol and ramipril (strength 5/10 mg) strength
Composition of the dosage unit
Preparation of capsules:
Related to the weight of one capsule 26.20 mg of bisoprolol containing compact prepared according to Example 6, 52.36 g of ramipril compact prepared according to Example 7 and 80.00 g of Vivapur 200 (macrocrystalline cellulose) are passed through a 0.8 mm sieve. To the homogenized mixture thus obtained 80.00 mg of Vivapur 200 (microcrystalline cellulose and 1.20mg of hydroxypropil methylcellulose (Pharmacoat 603) are added and the mixture thus obtained is homogenized. To the homogenized mixture 20 mg of microcrystalline cellulose (PH 101), 4 mg of Compritol 888 (glyceryl behenate) and 1.24 mg of colloidal silica (Aerosil R972) are added. The homogenized mixture thus obtained is filled in capsules.
8/B
One proceeds as described in Example 6/A except that the homogenized mixture is not filled in capsules but pressed to tablets.
Example 9
One-layer tablets (9/A) and capsules (9/B) containing ramipril and bisoprolol
One proceeds as described in Examples 5/1 and 5/II except that the ratio of the bisoprolol compact, the ramipril compact and the auxiliary agents is as shown in the following Table:
Claims
1. Stable solid pharmaceutical composition comprising ramipril and bisoprolol or a pharmaceutically acceptable salt or complex thereof.
2. Composition according to claim 1 wherein the particles of the ramipril and bisoprolol active ingredients or pharmaceutically acceptable salts thereof are separated from each other in the composition.
3. Composition according to claim 1 wherein the particles of the ramipril and bisoprolol active ingredients or pharmaceutically acceptable salts thereof are in separate phases and the particles of the active ingredients are in contact with each other only at the edge surface of the phases.
4. Composition according to claim 2 or 3 wherein the composition is in tablet or capsule form.
5. Composition according to claim 3 wherein the composition is a multi-layer tablet in which the bisoprolol and ramipril or pharmaceutically acceptable salts thereof are in separate layers.
6. Composition according to claim 3 wherein the composition is a one-layer or multi-layer tablet in which bisoprolol and ramipril are in the same layer of the tablet and the particles of the active ingredients are separated from each other within said layer.
7. Composition according to any of claims 1-6 wherein at least one of the two active ingredients or pharmaceutically acceptable salts thereof is (are) in form of granules, preferably as dry granule or compact.
8. Composition according to claim 7 wherein the particles of at least one of the active ingredients or granules or compacts containing the same is (are) coated.
9. Composition according to claim 7 wherein the particles of ramipril or a salt thereof or granules, compacts or minitablets containing the same and particles of bisoprolol or a salt thereof or granules, minitablets or compacts containing the same are coated.
10. Composition according to claims 1-9 comprising ramipril and bisoprolol hemifumarate as active ingredient.
1 1. Pharmaceutical composition according to claim 1 or 2 comprising a) a ramipril containing coated granule which contains ramipril and cross-linked polyvinyl-pyrrolidone and optionally HPMC and other auxiliary agents, whereby said granules are coated with an organic polymer, preferably HPMC; b) a bisoprolol hemifumarate containing coated compact which contains bisoprolol hemifumarate, microcrystalline cellulose and optionally further auxiliary agents generally used in pharmaceutical industry, whereby said granules are coated with an organic polymer, preferably HPMC, and c) an external phase which contains a filler, preferably microcrystalline cellulose, a glidant preferably colloidal silica and a lubricant, preferably magnesium stearate or glyceryl behenate.
12. Composition according to claim 11 wherein the ramipril containing coated granule, the bisoprolol hemifumarate containing coated compact and the components of the external phase are in a capsule.
13. Composition according to a any of claims 1-12 wherein ramipril and bisoprolol are separated from each other in the composition; the composition contains the active ingredients in an amount corresponding to 10 mg of ramipril and 10 mg of bisopropol base; the Cmax value of the composition related to bisoprolol is 35-55 ng/ml, preferably 39-48 ng/ml, particularly 43.8 ng/ml and the Cmax value related to ramipril is 13-21 ng/ml, preferably 14.82-18.15 ng/ml, particularly 16.5 ng/ml and/or the AUCT value related to bisoprolol is 503-790 ng.h/ml, preferably 566-692 ng.h/ml, particularly 629 ng.h/ml and the AUCx value related to ramipril is 9.3-14.7 ng.h/ml, preferably 10.5- 12.9 ng.h ml, particularly 11.7 ng.h/ml.
14. Composition according to claim 13 which comprises a salt of bisoprolol, preferably bisoprolol hemifumarate .
15. Bisoprolol hemifumarate containing coated compact which contains - related to the weight of the coated compact - 5-30 % by weight, preferably 10-25 % by weight, particularly 20-25 % by weight of bisoprolol hemifumarate, 60-93 % by weight, preferably 65-80 % by weight, particularly 75-80 % by weight of a filler, preferably microcrystalline cellulose and 2-10 % by weight, preferably 3-7 % by weight., particularly 4-5 % by weight of an organic polymer, such as polyvinyl pyrrolidone or HPMC, particularly HPMC or a mixture thereof.
16. Process for the preparation of a combined pharmaceutical composition which contains as active ingredient ramipril and bisoprolol or a salt or complex thereof which comprises
- coating the particles of one of the active ingredients, namely ramipril or bisoprolol or a compact, granule or pellet containing the same with a polymer soluble or swellable in water, such as HPMC or polyvinyl pyrrolidone and admixing the coated particles thus obtained with particles of the other active ingredient which can be uncoated or coated with a polymer soluble or swellable in water, such as HPMC or polyvinyl pyrrolidone or a compact, granule or pellet
containing the same and with further auxiliary agents, preferably fillers, if necessary disintegrants, lubricants and glidants and pressing the mixture obtained into tablets or filling into capsules, preferably in hard gelatine capsules.
17. Process according to claim 16 wherein both active ingredients are in form of coated granules.
18. Process according to claim 16 or 17 which comprises using as active ingredient ramipril and bisoprolol hemifumarate.
19. Process according to any of claims 16-18 which comprises
a) preparing a ramipril containing coated granule by i. related to the weight of the coated granule, pre-granulating a mixture of 10- 50 % by weight, preferably 10-30 % by weight, particularly 15-25 % by weight of ramipril and 60-93 % by weight, preferably 65-80 % by weight, particularly 75-80 % by weight of a disintegrant, preferably cross-linked PVP (such as polyplasdone XL 10) with a solution of HPMC in a vortex granulator so that pre- granulation is carried out with a 5-10 % by weight, preferably 5-8 % by weight of an aqueous solution of 30-50 % by weight, preferably 40-50 % by weight of the HPMC used in an amount of 1-15 % by weight, preferably 1-10 % by weight, particularly 2-6 % by weight - related to the weight of the coated granules - coating the granules thus obtained with a 5-10 % by weight, preferably 5-8 % by weight aqueous solution of the residual amount of HPMC; or ii. related to the weight of the coated granules, compacting in the dry a mixture of 10-50 % by weight, preferably 10-30 % by weight, particularly 15-25 % by weight of ramipril and 60-93 % by weight, preferably 65-80 % by weight, particularly 75-80 % by weight of a disintegrant, preferably cross- linked PVP (such as polyplasdone XL 10), re-granulating the compact and
coating with a 5-10 % by weight, preferably 5-8 % by weight aqueous solution of 1-15 % by weight, preferably 1-10 % by weight, particularly 2-6 % by weight - related to the weight of the coated granules - of HPMC; b) preparing bisoprolol hemifumarate containing coated compact by homogenizing - related to the weight of the coated compact - 5-30 % by weight, preferably 10-25 % by weight, particularly 20-25 % by weight of bisoprolol hemifumarate and 60-93 % by weight, preferably 65-80 % by weight, particularly 75-80 % by weight of microcrystalline cellulose, compacting the mixture, re-granulating the compact thus obtained at first on a 1.2 mm sieve and then on an e.g. 0.5 mm sieve, coating the re- granulated bisoprolol hemifumarate containing compact thus obtained with an organic polymer, preferably HPMC or polyvinyl pyrrolidone by using - related to the weight of the coated compact - 2-10 % by weight, preferably 3-7 % by weight, particularly 4- 5 % by weight of an organic polymer, such polyvinyl pyrrolidone or HPMC, preferably HPMC in form of a 5-10 % by weight, preferably 5-8 % by weight aqueous solution; and thereafter c) homogenizing the ramipril containing granules prepared according to step a) and the bisoprolol hemifumarate containing compact prepared according to step b) with the components of the external phase, preferably fillers, such as microcrystalline cellulose, glidants, preferably Aerosil (colloidal silica) and lubricants, preferably magnesium stearate or glyceryl behenate and pressing the homogenized product to tablets or filling in capsules.
20. Process for the preparation of a bisoprolol hemifumarate containing coated compact which comprises homogenizing - related to the weight of the coated compact - 5-30 % by weight, preferably 10-25 % by weight, particularly 20-25 % by weight of bisoprolol hemifumarate and 60-93 % by weight, 65-80 % by weight, particularly 75- 80 % by weight of microcrystalline cellulose,, compacting the mixture, if necessary re- granulating the compact thus obtained, coating the compact thus obtained or the re- granulated bisoprolol hemifumarate containing compact with an organic polymer, preferably HPMC or polyvinyl pyrrolidone by using a 5-10 % by weight, preferably 5-
8 % by weight aqueous solution with - related to the weight of the coated compact - 2-10 % by weight, preferably 3-7 % by weight, particularly 4-5 % by weight of an organic polymer, such as polyvinyl pyrrolidone or HPMC, preferably HPMC.
21. Use of bisoprolol and ramipril or salts or complexes thereof for the preparation of a combined pharmaceutical composition suitable for the treatment of hypertension, preferably high renin hypertension level hypertension.
22. Medical treatment of hypertension, particularly high renin level hypertension which comprises administering to the patient in need of such treatment a combined pharmaceutical composition which contains an effective dose of busoprolol and ramipril or a salt or complex thereof, preferably bisoprolol hemifumarate.
23. Method of treatment according to claim 22 which comprises administering the pharmaceutical composition once-a-day.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| HU1600414A HU231052B1 (en) | 2013-08-16 | 2014-08-15 | Stable pharmaceutical composition containing bisoprolol and ramipril |
| EA201690395A EA033291B1 (en) | 2013-08-16 | 2014-08-15 | Dosage unit comprising ramipril and bisoprolol hemifumarate, process for the preparation thereof and method of treating hypertension |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| HU1300496A HUP1300496A2 (en) | 2013-08-16 | 2013-08-16 | Stable pharmaceutical composition |
| HUP1300496 | 2013-08-16 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2015022560A1 true WO2015022560A1 (en) | 2015-02-19 |
| WO2015022560A8 WO2015022560A8 (en) | 2015-05-14 |
Family
ID=89991232
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/HU2014/000072 WO2015022560A1 (en) | 2013-08-16 | 2014-08-15 | Stable pharmaceutical composition containing bisoprolol and ramipril |
| PCT/HU2014/000071 WO2015022559A1 (en) | 2013-08-16 | 2014-08-15 | Pharmaceutical composition containing rosuvastatin and ramipril |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/HU2014/000071 WO2015022559A1 (en) | 2013-08-16 | 2014-08-15 | Pharmaceutical composition containing rosuvastatin and ramipril |
Country Status (3)
| Country | Link |
|---|---|
| EA (1) | EA033291B1 (en) |
| HU (2) | HUP1300496A2 (en) |
| WO (2) | WO2015022560A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2021162562A2 (en) | 2020-02-10 | 2021-08-19 | Adamed Pharma S.A. | Stable ramipril composition and fixed dose composition comprising thereof |
| CN115068434A (en) * | 2022-08-03 | 2022-09-20 | 昆山龙灯瑞迪制药有限公司 | Preparation method of ramipril tablets |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3501502A1 (en) * | 2017-12-20 | 2019-06-26 | Midas Pharma GmbH | Fixed dosed pharmaceutical compositions comprising amlodipine, ramipril and atorvastatin |
Citations (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0050800A1 (en) | 1980-10-23 | 1982-05-05 | Schering Corporation | Carboxyalkyl dipeptides, processes for their production and pharmaceutical compositions containing them |
| EP0079022A2 (en) | 1981-11-05 | 1983-05-18 | Hoechst Aktiengesellschaft | Derivatives of cis, endo-2-azabicyclo-(3.3.0)-octane-3-carboxylic acid, process for their preparation, medicines containing them and their use |
| US5442008A (en) | 1987-11-24 | 1995-08-15 | Hoechst Aktiengesellschaft | Stabilized polymer film coated compounds and stabilized formulations in compressed from using same |
| US20030215526A1 (en) | 2002-03-08 | 2003-11-20 | Scott Stofik | Stable formulations of angiotensin converting enzyme (ACE) inhibitors |
| GB2394660A (en) | 2003-12-17 | 2004-05-05 | Niche Generics Ltd | Stabilisation of pharmaceutical compositions comprising ACE inhibitor by absence of acidic excipients having large specific surface area, eg silicon dioxide |
| WO2005002548A1 (en) | 2003-06-26 | 2005-01-13 | Teva Pharmaceutical Industries Ltd. | Stable pharmaceutical compositions of 2-aza-bicyclo[3.3.0]-octane-3-carboxylic acid derivatives |
| WO2005041940A1 (en) | 2003-10-30 | 2005-05-12 | Lupin Ltd. | Stable formulations of ace inhibitors and methods for preparation thereof |
| WO2006050533A2 (en) | 2004-11-05 | 2006-05-11 | King Pharmaceuticals Research & Development, Inc. | Stabilized individually coated ramipril particles, compositions and methods |
| EP1734931A2 (en) | 2004-03-24 | 2006-12-27 | Actavis Group | Formulations of ramipril |
| WO2007056442A1 (en) * | 2005-11-07 | 2007-05-18 | King Pharmaceuticals Research & Development, Inc. | Compositions of stabilized ramipril in combination with another active agent |
| WO2007056907A1 (en) | 2005-11-21 | 2007-05-24 | Dalian D.N Bio-Engineering Co., Ltd | TRUNCATED GLUCAGON-LIKE PEPTIDE-1 (sGLP-1) AND ITS PREPARING METHOD AND USE |
| US20070232680A1 (en) | 2006-04-04 | 2007-10-04 | Vijayabhaskar Bolugoddu | Preparation of ramipril and stable pharmaceutical compositions |
| WO2007120930A2 (en) | 2006-04-19 | 2007-10-25 | Teva Pharmaceutical Industries Ltd. | Stable pharmaceutical compositions of 2-aza-bicyclo[3.3.0]-octane-3-carboxylic acid derivatives |
| WO2008000040A1 (en) | 2006-06-30 | 2008-01-03 | Alphapharm Pty Ltd | A stabilised composition comprising ace inhibitors |
| WO2008021875A2 (en) | 2006-08-08 | 2008-02-21 | Accu-Break Technologies, Inc. | Pharmaceutical tablets containing a plurality of active segments |
| WO2008065485A2 (en) | 2006-10-19 | 2008-06-05 | Torrent Pharmaceuticals Limited | Stable pharmaceutical compositions of a calcium channel blocker and an ace inhibitor |
| WO2008095263A1 (en) | 2007-02-09 | 2008-08-14 | Alphapharm Pty Ltd | A dosage form containing two or more active pharmaceutical ingredients in different physical forms |
| WO2008132756A1 (en) | 2007-05-01 | 2008-11-06 | Lupin Limited | Stable pharmaceutical compositions of ramipril |
| WO2011003809A1 (en) | 2009-07-10 | 2011-01-13 | Csl Behring Gmbh | Role of pld1 in thrombus formation and integrin alpha iib beta 3 activation |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003092729A1 (en) * | 2002-05-03 | 2003-11-13 | Hexal Ag | Stable pharmaceutical formulation for a combination of a statin and an ace inhibitor |
| HU230877B1 (en) | 2008-09-30 | 2018-11-29 | EGIS Gyógyszergyár NyR | Stable pharmaceutical combination |
| CN101612403A (en) | 2009-08-13 | 2009-12-30 | 王丽燕 | The pharmaceutical composition that contains calcium antagonist, ACE inhibitor and statins |
| EP2814465B1 (en) | 2012-02-17 | 2019-07-03 | Egis Gyógyszergyár Zrt. | Pharmaceutical formulation having improved stability |
-
2013
- 2013-08-16 HU HU1300496A patent/HUP1300496A2/en not_active Application Discontinuation
-
2014
- 2014-08-15 HU HU1600414A patent/HU231052B1/en unknown
- 2014-08-15 EA EA201690395A patent/EA033291B1/en not_active IP Right Cessation
- 2014-08-15 WO PCT/HU2014/000072 patent/WO2015022560A1/en active Application Filing
- 2014-08-15 WO PCT/HU2014/000071 patent/WO2015022559A1/en active Application Filing
Patent Citations (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0050800A1 (en) | 1980-10-23 | 1982-05-05 | Schering Corporation | Carboxyalkyl dipeptides, processes for their production and pharmaceutical compositions containing them |
| EP0079022A2 (en) | 1981-11-05 | 1983-05-18 | Hoechst Aktiengesellschaft | Derivatives of cis, endo-2-azabicyclo-(3.3.0)-octane-3-carboxylic acid, process for their preparation, medicines containing them and their use |
| US5442008A (en) | 1987-11-24 | 1995-08-15 | Hoechst Aktiengesellschaft | Stabilized polymer film coated compounds and stabilized formulations in compressed from using same |
| US20030215526A1 (en) | 2002-03-08 | 2003-11-20 | Scott Stofik | Stable formulations of angiotensin converting enzyme (ACE) inhibitors |
| WO2005002548A1 (en) | 2003-06-26 | 2005-01-13 | Teva Pharmaceutical Industries Ltd. | Stable pharmaceutical compositions of 2-aza-bicyclo[3.3.0]-octane-3-carboxylic acid derivatives |
| WO2005041940A1 (en) | 2003-10-30 | 2005-05-12 | Lupin Ltd. | Stable formulations of ace inhibitors and methods for preparation thereof |
| GB2394660A (en) | 2003-12-17 | 2004-05-05 | Niche Generics Ltd | Stabilisation of pharmaceutical compositions comprising ACE inhibitor by absence of acidic excipients having large specific surface area, eg silicon dioxide |
| EP1734931A2 (en) | 2004-03-24 | 2006-12-27 | Actavis Group | Formulations of ramipril |
| WO2006050533A2 (en) | 2004-11-05 | 2006-05-11 | King Pharmaceuticals Research & Development, Inc. | Stabilized individually coated ramipril particles, compositions and methods |
| WO2007056442A1 (en) * | 2005-11-07 | 2007-05-18 | King Pharmaceuticals Research & Development, Inc. | Compositions of stabilized ramipril in combination with another active agent |
| WO2007056907A1 (en) | 2005-11-21 | 2007-05-24 | Dalian D.N Bio-Engineering Co., Ltd | TRUNCATED GLUCAGON-LIKE PEPTIDE-1 (sGLP-1) AND ITS PREPARING METHOD AND USE |
| US20070232680A1 (en) | 2006-04-04 | 2007-10-04 | Vijayabhaskar Bolugoddu | Preparation of ramipril and stable pharmaceutical compositions |
| WO2007120930A2 (en) | 2006-04-19 | 2007-10-25 | Teva Pharmaceutical Industries Ltd. | Stable pharmaceutical compositions of 2-aza-bicyclo[3.3.0]-octane-3-carboxylic acid derivatives |
| WO2008000040A1 (en) | 2006-06-30 | 2008-01-03 | Alphapharm Pty Ltd | A stabilised composition comprising ace inhibitors |
| WO2008021875A2 (en) | 2006-08-08 | 2008-02-21 | Accu-Break Technologies, Inc. | Pharmaceutical tablets containing a plurality of active segments |
| WO2008065485A2 (en) | 2006-10-19 | 2008-06-05 | Torrent Pharmaceuticals Limited | Stable pharmaceutical compositions of a calcium channel blocker and an ace inhibitor |
| WO2008095263A1 (en) | 2007-02-09 | 2008-08-14 | Alphapharm Pty Ltd | A dosage form containing two or more active pharmaceutical ingredients in different physical forms |
| WO2008132756A1 (en) | 2007-05-01 | 2008-11-06 | Lupin Limited | Stable pharmaceutical compositions of ramipril |
| WO2011003809A1 (en) | 2009-07-10 | 2011-01-13 | Csl Behring Gmbh | Role of pld1 in thrombus formation and integrin alpha iib beta 3 activation |
Non-Patent Citations (4)
| Title |
|---|
| "Encyclopedia of Pharmaceutical Technology", 2007, INFOMA HEALTHCARE USA INC |
| FETZNER A ET AL: "Degradation of raw or film-incorporated beta-cyclodextrin by enzymes and colonic bacteria", EUROPEAN JOURNAL OF PHARMACEUTICS AND BIOPHARMACEUTICS, ELSEVIER SCIENCE PUBLISHERS B.V., AMSTERDAM, NL, vol. 58, no. 1, 1 July 2004 (2004-07-01), pages 91 - 97, XP004519832, ISSN: 0939-6411, DOI: 10.1016/J.EJPB.2004.02.001 * |
| FINSTERER JOSEF ET AL: "Successful heart failure therapy in mitochondrial disorder with noncompaction cardiomyopathy", THE INTERNATIONAL JOURNAL OF CARDIAC IMAGING, KLUWER ACADEMIC PUBLISHERS, DO, vol. 22, no. 3-4, 25 February 2006 (2006-02-25), pages 393 - 398, XP019392601, ISSN: 1573-0743, DOI: 10.1007/S10554-005-9073-4 * |
| THERES H P ET AL: "Combined treatment with ramipril and metoprolol prevents changes in the creatine kinase isoenzyme system and improves hemodynamic function in rat hearts after myocardial infarction", CARDIOVASCULAR DRUGS AND THERAPY, KLUWER ACADEMIC PUBLISHERS, BOSTON, US, vol. 14, no. 6, 1 January 2000 (2000-01-01), pages 597 - 606, XP002983205, ISSN: 0920-3206, DOI: 10.1023/A:1007846311040 * |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2021162562A2 (en) | 2020-02-10 | 2021-08-19 | Adamed Pharma S.A. | Stable ramipril composition and fixed dose composition comprising thereof |
| WO2021162562A3 (en) * | 2020-02-10 | 2021-12-02 | Adamed Pharma S.A. | Stable ramipril composition and fixed dose composition comprising thereof |
| CN115068434A (en) * | 2022-08-03 | 2022-09-20 | 昆山龙灯瑞迪制药有限公司 | Preparation method of ramipril tablets |
| CN115068434B (en) * | 2022-08-03 | 2023-05-09 | 昆山龙灯瑞迪制药有限公司 | Preparation method of ramipril tablet |
Also Published As
| Publication number | Publication date |
|---|---|
| HUP1600414A2 (en) | 2016-09-28 |
| EA033291B1 (en) | 2019-09-30 |
| WO2015022559A1 (en) | 2015-02-19 |
| HUP1300496A2 (en) | 2015-03-02 |
| EA201690395A1 (en) | 2016-07-29 |
| HU231052B1 (en) | 2020-02-28 |
| WO2015022560A8 (en) | 2015-05-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2445092C2 (en) | Composition containing dementia drug | |
| EP2884967B1 (en) | Pharmaceutical compositions of memantine | |
| MX2007001850A (en) | Extended release pellet formulation containing pramipexole or a pharmaceutically acceptable salt thereof, method for manufacturing the same and use thereof. | |
| EA020870B1 (en) | Solid preparation comprising alogliptin and metformin hydrochloride | |
| MXPA05006513A (en) | Solid drug for oral use. | |
| EP3744320A1 (en) | Pharmaceutical tablet composition comprising edoxaban | |
| JP2011507973A (en) | Pharmaceutical composition of amlodipine and valsartan | |
| WO2013100630A1 (en) | Fixed dose combination formulation comprising losartan, amlodipine and hydrochlorothiazide | |
| WO2015022560A1 (en) | Stable pharmaceutical composition containing bisoprolol and ramipril | |
| EP2538924B1 (en) | Solid pharmaceutical formulations of ramipril and amlodipine besylate, and their preparation | |
| CA2869281A1 (en) | Prasugrel-containing immediate release stable oral pharmaceutical compositions | |
| RU2613192C1 (en) | Tablets of clozapine with sustained release | |
| WO2023111880A1 (en) | An extended release pharmaceutical composition of clozapine | |
| US20230040902A1 (en) | Pharmaceutical Formulation Comprising Cibenzoline or Salt Thereof | |
| WO2015063670A1 (en) | Solid oral modified-release composition comprising oxcarbazepine or a pharmaceutically acceptable salt thereof | |
| PL227900B1 (en) | Pharmaceutical composition comprising an ACE inhibitor and a calcium channel blocker, a method for its preparation and the dosage unit comprising the composition | |
| EP3932398B1 (en) | Pharmaceutical composition of single dosage form for treating or preventing hypertension and hyperlipidemia | |
| EP4205730A1 (en) | Pharmaceutical composition of single dosage form for treating or preventing hypertension and hyperlipidemia | |
| KR20220026520A (en) | A single dosage form of a pharmaceutical composition for the treatment or prevention of hypertension and hypercholesterolemia | |
| CA3226799A1 (en) | Multiparticulate pharmaceutical composition | |
| US20080085311A1 (en) | Antihistamine-decongestant combinations | |
| WO2024047208A1 (en) | Anticoagulant therapy with an improved dosage regimen | |
| WO2023012817A1 (en) | A pharmaceutical composition comprising combination of dapagliflozin and sitagliptin | |
| KR20220007446A (en) | Composite formulation comprising sitagliptin and dapagliflozin and a process for the preparation thereof | |
| KR20150055986A (en) | Extended Release Formulation for Oral Administration of Bosentan |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14777376 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 201690395 Country of ref document: EA |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14777376 Country of ref document: EP Kind code of ref document: A1 |