WO2014181642A1 - 安定した超弾性を示すCu-Al-Mn系棒材及び板材、その製造方法、それを用いた制震部材、並びに制震部材を用いた制震構造体 - Google Patents
安定した超弾性を示すCu-Al-Mn系棒材及び板材、その製造方法、それを用いた制震部材、並びに制震部材を用いた制震構造体 Download PDFInfo
- Publication number
- WO2014181642A1 WO2014181642A1 PCT/JP2014/060586 JP2014060586W WO2014181642A1 WO 2014181642 A1 WO2014181642 A1 WO 2014181642A1 JP 2014060586 W JP2014060586 W JP 2014060586W WO 2014181642 A1 WO2014181642 A1 WO 2014181642A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- temperature
- bar
- plate material
- heating
- heat treatment
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22F—CHANGING THE PHYSICAL STRUCTURE OF NON-FERROUS METALS AND NON-FERROUS ALLOYS
- C22F1/00—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working
- C22F1/006—Resulting in heat recoverable alloys with a memory effect
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22D—CASTING OF METALS; CASTING OF OTHER SUBSTANCES BY THE SAME PROCESSES OR DEVICES
- B22D21/00—Casting non-ferrous metals or metallic compounds so far as their metallurgical properties are of importance for the casting procedure; Selection of compositions therefor
- B22D21/02—Casting exceedingly oxidisable non-ferrous metals, e.g. in inert atmosphere
- B22D21/025—Casting heavy metals with high melting point, i.e. 1000 - 1600 degrees C, e.g. Co 1490 degrees C, Ni 1450 degrees C, Mn 1240 degrees C, Cu 1083 degrees C
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C9/00—Alloys based on copper
- C22C9/01—Alloys based on copper with aluminium as the next major constituent
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C9/00—Alloys based on copper
- C22C9/05—Alloys based on copper with manganese as the next major constituent
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22F—CHANGING THE PHYSICAL STRUCTURE OF NON-FERROUS METALS AND NON-FERROUS ALLOYS
- C22F1/00—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22F—CHANGING THE PHYSICAL STRUCTURE OF NON-FERROUS METALS AND NON-FERROUS ALLOYS
- C22F1/00—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working
- C22F1/08—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working of copper or alloys based thereon
Definitions
- Shape memory alloys and superelastic alloys exhibit remarkable shape memory effects and superelastic properties associated with the reverse transformation of thermoelastic martensitic transformation, and have excellent functions near the living environment temperature. In practical use.
- Typical materials for shape memory alloys and superelastic alloys include TiNi alloys and copper (Cu) alloys. Copper-based shape memory alloys / superelastic alloys (hereinafter collectively referred to simply as copper-based alloys) are inferior to TiNi alloys in terms of repeatability, corrosion resistance, and the like. On the other hand, since the cost is low, there is a movement to expand the application range of the copper-based alloy.
- the composition of the Cu—Al—Mn alloy contains 3 to 10 mass% Al, 5 to 20 mass% Mn, and optionally contains 1 mass% or less Ni, and further required 1 type selected from the group consisting of Co, Fe, Ti, V, Cr, Si, Nb, Mo, W, Sn, Mg, P, Be, Sb, Cd, As, Zr, Zn, and Ag.
- the bar according to item (1) or (2) comprising a total of 0.001 to 10% by mass of two or more types, the balance being Cu and inevitable impurities.
- the composition of the Cu—Al—Mn alloy contains 3 to 10% by mass of Al, 5 to 20% by mass of Mn, and optionally contains 1% by mass or less of Ni.
- a method for producing a bar material comprising 0.001 to 10% by mass in total of two or more kinds, the balance being Cu and inevitable impurities, Melting and casting the alloy material giving the above composition [step 1], Perform hot working [Step 2] and perform memory heat treatment [Step 3] [Step 1] to [Step 3] are performed in this order.
- the memory heat treatment in [Step 3] is performed by heating from room temperature to a temperature range that becomes a ⁇ phase [Step 3-1], and maintaining the heating temperature for 1 to 120 minutes, followed by cooling [ Step 3-2] and heating [Step 3-3] are repeated at least once each, and at the low temperature of the cooling [Step 3-2] and heating [Step 3-3], the temperature becomes ⁇ + ⁇ phase.
- the temperature becomes ⁇ phase, and heat treatment is performed so that the cooling rate and the heating rate during the cooling [Step 3-2] and heating [Step 3-3] are 0.1 to 100 ° C./min, respectively. Further, after the final heating, a heat treatment is performed to rapidly cool from the temperature that becomes the ⁇ phase [step 3-4].
- the composition of the Cu—Al—Mn alloy contains 3 to 10 mass% Al, 5 to 20 mass% Mn, and optionally contains 1 mass% or less Ni, and further required 1 type selected from the group consisting of Co, Fe, Ti, V, Cr, Si, Nb, Mo, W, Sn, Mg, P, Be, Sb, Cd, As, Zr, Zn, and Ag.
- the memory heat treatment in [Step 3] is performed by heating from room temperature to a temperature range that becomes a ⁇ phase [Step 3-1], and maintaining the heating temperature for 1 to 120 minutes, followed by cooling [ Step 3-2] and heating [Step 3-3] are repeated at least once each, and at the low temperature of the cooling [Step 3-2] and heating [Step 3-3], the temperature becomes ⁇ + ⁇ phase.
- the temperature becomes ⁇ phase, and heat treatment is performed so that the cooling rate and the heating rate during the cooling [Step 3-2] and heating [Step 3-3] are 0.1 to 100 ° C./min, respectively. Further, after the final heating, a heat treatment is performed to rapidly cool from the temperature that becomes the ⁇ phase [step 3-4].
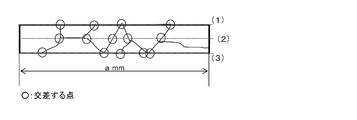
- FIG. 1 is a schematic diagram for explaining a method for evaluating a crystal grain size.
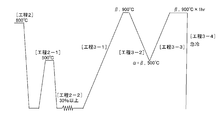
- FIG. 2-1 shows an example of a process chart of processing and heat treatment. In this example, neither intermediate annealing [step 2-1] nor cold working [step 2-2] is performed.
- FIG. 2-2 shows another example of a process chart of processing and heat treatment. In this example, after the hot working [Step 2], the intermediate annealing [Step 2-1] and the cold working [Step 2-2] are repeated at least once in this order, and then the memory heat treatment [Step 3].
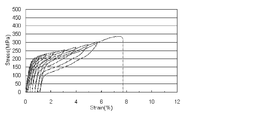
- FIG. 3a is a stress-strain curve (SS curve) showing the residual strain as the superelastic characteristic in the bar material of the present invention example (Invention Example 1) obtained by the example described later.
- FIG. SS curve stress-strain curve
- a small number of crystal grains having a small crystal grain size may exist, but most of them are crystal grains having a large crystal grain size. . That is, in the case of a bar, in the longitudinal section of the bar, the region where the grain size of each crystal grain is equal to or larger than the radius of the bar is 90% or more of the longitudinal section at an arbitrary position of the bar.
- the average grain size of crystal grains having a diameter greater than or equal to the radius of the rod is 80% or more of the diameter of the rod. It is preferable that the average crystal grain size is not less than the diameter of the rod.
- the structural characteristics are defined.
- the plate material is not a circular cross section and has low symmetry. Therefore, the reference for the crystal grain size is based on the plate thickness, not the plate width. The reason is that when the crystal grains penetrate the plate thickness or width, the driving force for the growth of the interface by the crystal grains is reduced thereafter, and not only the plate thickness but also the plate thickness is penetrated. This is due to the fact that it is difficult.
- the superelastic characteristics can be stabilized by controlling the particle size distribution and the average crystal grain size in the wire (bar) material or plate material in this way.
- the bar material and the plate material are defined as separate inventions, but from the viewpoint of a stretched material of Cu—Al—Mn superelastic alloy.
- the crystal grain size is defined with respect to the rod diameter or the crystal grain size is defined with respect to the plate thickness, both invention specific matters are common. Both can be said to have common technical features.
- the manufacturing method of the bar material and the manufacturing method of the plate material the same thing can be said as the invention of the product. Therefore, it is understood that both have common technical characteristics.
- the predetermined grain size distribution and crystal grains having a predetermined size or more have an average crystal grain size having a predetermined size or more.
- the crystal grain size of crystal grains of a predetermined size or more is defined, and crystal grains of less than a predetermined size are remarkably smaller than crystal grains of a predetermined size or more and have less influence on superelastic properties. This is because the influence of less than crystal grains is considered negligible.
- the Cu—Al—Mn rod and plate of the present invention are substantially ⁇ single phase.
- “substantially ⁇ single phase” means that the existence ratio of, for example, ⁇ phase other than ⁇ phase is usually 10% or less, preferably 5% or less.
- a Cu-8.1 mass% Al-11.1 mass% Mn alloy has a ⁇ (BCC) single phase at 900 ° C., but has two phases of ⁇ (FCC) phase and ⁇ phase at 700 ° C. or less.
- the grain size is sufficiently increased to the predetermined large size by controlling both the temperature lowering rate in the memory heat treatment and the temperature rising rate after once cooling to a predetermined slow range in the entire manufacturing process and its particle size.
- the distribution it is possible to obtain a Cu—Al—Mn alloy material that stably exhibits good superelastic characteristics.
- at least one repetition of the intermediate annealing at 400 to 600 ° C. for 1 to 120 minutes and the cold working in which the working rate of cold rolling or cold drawing is in the range of 30% or more is performed at least once. It may be performed after the processing and before the storage heat treatment. Alternatively, after the hot working, only the intermediate annealing at 400 to 600 ° C.
- the memory heat treatment may be performed without performing the cold working after the intermediate annealing.
- the temperature is raised by heating to a temperature range equal to or higher than the transformation temperature from the ⁇ + ⁇ phase to the ⁇ phase, which becomes the ⁇ phase, and is maintained at the heating temperature for 1 to 120 minutes.
- the heating to the temperature range above the transformation temperature from the first ⁇ + ⁇ phase to the ⁇ phase at the time of the memory heat treatment is usually cooled to room temperature and then from room temperature, but not cooled to room temperature after hot working
- heating can be performed immediately after hot working, or heating can be performed in the cooling process after hot working.
- both the temperature lowering rate (temperature decreasing rate in the cooling of [Step 3-2]) and the temperature increasing rate (temperature increasing rate in the heating of [Step 3-3]) in the memory heat treatment are slowed (in this document) This is also referred to as gradual temperature decrease or temperature increase).
- the rate of temperature decrease at the time of slow temperature decrease and the temperature increase rate at the time of gradually temperature increase are usually 0.1 to 100 ° C./min, preferably 0.1 to 10 ° C./min, more preferably 0.1 to 10 ° C./min. It is 3 ° C./min, particularly preferably 0.2 to 1 ° C./min.
- the heat treatment temperature rise to ⁇ single phase (abbreviated as “ ⁇ ” in the chart.
- Step 4 it is preferable to perform this arbitrary aging heat treatment [Step 4]. If the aging temperature is too low, the ⁇ phase is unstable, and if left at room temperature, the martensitic transformation temperature may change. On the other hand, if the aging temperature is too high, precipitation of ⁇ phase occurs, and the shape memory characteristics and superelasticity tend to be remarkably lowered.
- Co, Fe, and Sn are effective elements for strengthening the base structure. Co coarsens crystal grains due to the formation of CoAl, but if excessive, it lowers the toughness of the alloy.
- a preferable content of Co is 0.001 to 2% by mass.
- a preferable content of Fe is 0.001 to 3 mass%.
- a preferable content of Sn is 0.001 to 1% by mass.
- Ti combines with inhibitory elements N and O to form oxynitrides.
- a preferable content of Ti is 0.001 to 2% by mass.
- V, Nb, Mo, and Zr have the effect of increasing the hardness and improve the wear resistance. Moreover, since these elements hardly dissolve in the matrix, they are precipitated as a ⁇ phase (bcc crystal) to improve the strength.
- the preferred contents of V, Nb, Mo, and Zr are each 0.001 to 1 mass%.
- Cr is an effective element for maintaining wear resistance and corrosion resistance.
- a preferable content of Cr is 0.001 to 2% by mass.
- Si has the effect of improving the corrosion resistance.
- a preferable content of Si is 0.001 to 2% by mass. Since W hardly dissolves in the base, there is an effect of precipitation strengthening.
- a preferable content of W is 0.001 to 1% by mass.
- Zn has the effect of increasing the shape memory processing temperature.
- a preferable content of Zn is 0.001 to 5% by mass.
- Ag has the effect of improving cold workability.
- a preferable content of Ag is 0.001 to 2% by mass.
- the superelastic Cu—Al—Mn alloy material constituting the rod and plate of the present invention preferably has a Ni content of 1% by mass or less, more preferably 0.15% by mass or less. It is particularly preferable that no Ni is contained. This is because if the Ni content is too large, the hardenability described above is lowered.
- the superelastic Cu—Al—Mn alloy bar and plate of the present invention have the following physical properties.
- the superelastic property the residual strain after 6% deformation is usually 1.0% or less, preferably 0.5% or less, more preferably 0.2% or less.
- the elongation (breaking elongation) is usually 6% or more, preferably 8% or more, more preferably 10% or more.
- the residual strain and elongation as the superelastic characteristics are not uneven in performance even when several specimens are cut out from the same material and measured.
- the residual strain and elongation are measured by cutting, for example, three specimens from the same material, one or more specimens have a residual strain of 1.0%. Or the elongation is less than 6%.
- the size of the Cu-Al-Mn alloy bar and plate of the present invention there is no particular limitation on the size of the Cu-Al-Mn alloy bar and plate of the present invention.
- the diameter is usually 8 mm or more, for example, 8 to 50 mm.
- the diameter may be 8 mm to 16 mm.
- the thickness is usually 1 mm or more, and may be, for example, 1 mm to 15 mm.
- the bar of the present invention may be in the shape of a hollow tube having a tube wall.
- the vibration damping member of the present invention is composed of the bar or plate material.
- Examples of the damping member are not particularly limited, and examples thereof include a brace, a fastener, and an anchor bolt.
- the damping structure of the present invention is constructed from the damping member. Examples of the vibration control structure are not particularly limited, and any structure may be used as long as the structure is configured using the braces, fasteners, anchor bolts, and the like.
- Example 1 A sample (test material) of a bar (wire) was produced under the following conditions.
- the molten copper alloy was cooled to obtain an ingot having a diameter of 80 mm and a length of 300 mm.
- This ingot was hot forged at 800 ° C. to obtain a round bar with a diameter of 20 mm. If necessary, this round bar is further subjected to (1) hot forging or (2) cold wire drawing to obtain the bar having the diameter shown in Tables 2-1 and 2-2 as follows. Obtained. That is, according to the processing and heat treatment processes shown in FIG. 2-1 and FIG.
- the processing and heat treatment were performed under various conditions shown in Tables 2-1 and 2-2. Specifically, after the hot working [step 1], the memory heat treatment [step 3] is performed without performing the intermediate annealing [step 2-1] or the cold wire drawing [step 2-2] (Example 1 of the present invention). Invention Example 23, Invention Example 28, each comparative example) (process of FIG. 2-1), or intermediate annealing at 500 ° C. for 1 hour after hot working [Step 1] [Step 2-1] And subsequent cold wire drawing [Step 2-2] were repeated once or a plurality of times (Invention Example 24 to Invention Example 27) (process of FIG. 2-2). In any case, after that, the temperature is raised to 900 ° C.
- FIGS. 2-1 and 2-2 are charts showing examples of processes, respectively.
- the processing rate of cold working, and the number of repetitions of cold working and intermediate annealing are shown in Tables 2-1 to 2-2. The changes were made as shown in.
- the processing rate in each cold working (in this example, the processing rate by cold drawing) is the first from left to right in the column of “Cold working rate (%)”.
- the number of repetitions of the intermediate annealing and the cold working is indicated as “the number of cold working cycles (times)”. That is, before each cold wire drawing [Step 2-2], intermediate annealing [Step 2-1] is performed at 500 ° C.
- Each round bar obtained through the processes of the processing and the heat treatment in this way was quenched by the final water cooling to obtain a ⁇ (BCC) single-phase sample.
- Each sample was subjected to an aging heat treatment at 200 ° C. for 15 minutes.
- the bar materials of Comparative Examples 3 to 8 were obtained in the same manner as in Examples 1 to 23 of the present invention except for Comparative Examples 4 and 5 in which production was interrupted due to forging cracks.
- the bar materials of Comparative Examples 1 and 2 were subjected to a rapid temperature decrease at a temperature decreasing rate of 150 ° C./min in the temperature decreasing step [Step 3-2] ( ⁇ ⁇ ⁇ + ⁇ ) in the memory heat treatment in Examples 1 to 23 of the present invention.
- Comparative Examples 1 and 2 are test examples simulating Japanese Patent Application Laid-Open No. 2001-20026 (Patent Document 2) and International Publication WO2011 / 152009A1 (Patent Document 3).
- Patent Document 2 Japanese Patent Laid-Open No. 2001-20026 (Patent Document 2) and International Publication WO2011 / 152009A1 (Patent Document 3)
- no consideration has been given to the rate of temperature rise or the rate of temperature drop during memory heat treatment. For this reason, there is no description as to the specific temperature increase rate or temperature decrease rate. Therefore, the tests were conducted at a high speed (rapid temperature rise or rapid cooling) that is out of the gradual temperature rise or slow cooling specified in the present invention as the temperature rise rate or temperature drop rate that has been conventionally used.
- the bar material As in the case of the bar material, it was subjected to processing and heat treatment under various conditions shown in Table 2-3 to Table 2-4 according to the processing and heat treatment processes shown in FIGS. 2-1 and 2-2, respectively. . Specifically, after the hot working [Step 1], the memory heat treatment [Step 3] is performed without performing the intermediate annealing [Step 2-1] or the cold wire drawing [Step 2-2] (Invention Example 29). Invention Example 51, Invention Example 56, Comparative Examples) (process of FIG. 2-1), or hot annealing [Step 1] followed by intermediate annealing at 500 ° C.
- Invention Examples 29 to 40 are test examples of alloy compositions that consist of only essential additive elements and whose contents (composition ratio) are variously changed.
- Invention Examples 41 to 43 and 44 to 51 are test examples of various alloy compositions in which an optional additive element (a trace additive element) is added to an essential additive element.
- Inventive Examples 29 to 34 and 52 to 56 are test examples obtained by variously changing the manufacturing conditions with respect to Inventive Examples 35 to 51. As is clear from the results shown in each table, as shown in Examples 29 to 56 of the present invention, the predetermined manufacturing specified in the present invention is performed regardless of whether intermediate annealing after hot working or subsequent cold working is performed.
- a material satisfying the particle size distribution and the average crystal particle size can be obtained, exhibiting desired excellent superelastic characteristics, and excellent elongation and quenching sensitivity.
- the temperature increase rate in [Step 3-3] or the temperature decrease rate in [Step 3-2] at the time of the memory heat treatment is too fast.
- the grain size distribution of the large-diameter crystal grains cannot be satisfied, and the average crystal grain diameter cannot be satisfied. None of them exhibit the desired superelastic characteristics, and the improvement in elongation is small.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Physics & Mathematics (AREA)
- Thermal Sciences (AREA)
- Crystallography & Structural Chemistry (AREA)
- Conductive Materials (AREA)
- Heat Treatment Of Steel (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP14794166.0A EP2995694A4 (en) | 2013-05-10 | 2014-04-14 | Cu-Al-Mn-BASED BAR MATERIAL AND PLATE MATERIAL DEMONSTRATING STABLE SUPERELASTICITY, METHOD FOR MANUFACTURING SAID BAR MATERIAL AND PLATE MATERIAL, SEISMIC CONTROL MEMBER IN WHICH SAID BAR MATERIAL AND PLATE MATERIAL ARE USED, AND SEISMIC CONTROL STRUCTURE IN WHICH SEISMIC CONTROL MEMBER IS USED |
| CN201480024828.9A CN105164289A (zh) | 2013-05-10 | 2014-04-14 | 显示稳定的超弹性的Cu-Al-Mn系棒材和板材、其制造方法、使用该棒材和板材的减震部件、以及使用减震部件的减震结构体 |
| US14/937,512 US20160060740A1 (en) | 2013-05-10 | 2015-11-10 | Cu-AI-Mn-BASED ALLOY ROD AND SHEET EXHIBITING STABLE SUPERELASTICITY, METHOD OF PRODUCING THE SAME, VIBRATION DAMPING MATERIAL USING THE SAME, AND VIBRATION DAMPING STRUCTURE CONSTRUCTED BY USING VIBRATION DAMPING MATERIAL |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013-099996 | 2013-05-10 | ||
| JP2013099996A JP5912094B2 (ja) | 2013-05-10 | 2013-05-10 | 安定した超弾性を示すCu−Al−Mn系棒材及び板材の製造方法 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/937,512 Continuation US20160060740A1 (en) | 2013-05-10 | 2015-11-10 | Cu-AI-Mn-BASED ALLOY ROD AND SHEET EXHIBITING STABLE SUPERELASTICITY, METHOD OF PRODUCING THE SAME, VIBRATION DAMPING MATERIAL USING THE SAME, AND VIBRATION DAMPING STRUCTURE CONSTRUCTED BY USING VIBRATION DAMPING MATERIAL |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014181642A1 true WO2014181642A1 (ja) | 2014-11-13 |
Family
ID=51867124
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/060586 Ceased WO2014181642A1 (ja) | 2013-05-10 | 2014-04-14 | 安定した超弾性を示すCu-Al-Mn系棒材及び板材、その製造方法、それを用いた制震部材、並びに制震部材を用いた制震構造体 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US20160060740A1 (enExample) |
| EP (1) | EP2995694A4 (enExample) |
| JP (1) | JP5912094B2 (enExample) |
| CN (1) | CN105164289A (enExample) |
| WO (1) | WO2014181642A1 (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN111394611A (zh) * | 2020-04-08 | 2020-07-10 | 公牛集团股份有限公司 | 一种耐磨高弹性铜合金插套材料及其制备方法 |
| CN113862508A (zh) * | 2021-09-29 | 2021-12-31 | 哈尔滨工程大学 | 一种CuAlMnCoNi形状记忆合金及其制备方法 |
| CN114807648A (zh) * | 2022-05-27 | 2022-07-29 | 天津理工大学 | 一种高温形状记忆合金及其制备方法 |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5567093B2 (ja) * | 2012-09-16 | 2014-08-06 | 国立大学法人東北大学 | 安定した超弾性を示すCu−Al−Mn系合金材とその製造方法 |
| CN106460098B (zh) | 2014-03-14 | 2019-01-08 | 古河电气工业株式会社 | Cu-Al-Mn系合金材料及其制造方法、以及使用了该合金材料的棒材或板材 |
| CN105562448B (zh) * | 2016-01-11 | 2019-05-10 | 中国兵器工业第五九研究所 | 药型罩细晶材料的低温制备方法 |
| JP6490608B2 (ja) * | 2016-02-10 | 2019-03-27 | 国立大学法人東北大学 | Cu−Al−Mn系合金材の製造方法 |
| CN105690040B (zh) * | 2016-04-06 | 2018-04-03 | 台州市椒江永固船舶螺旋桨厂 | 螺旋桨浇注、打磨工艺及其铜合金配方 |
| CN105734337A (zh) * | 2016-05-05 | 2016-07-06 | 太仓小小精密模具有限公司 | 一种耐磨型铜合金模具材料 |
| CN106834796A (zh) * | 2017-01-25 | 2017-06-13 | 广东广信科技有限公司 | 一种用于配电柜的高强度铜合金材料及其制备方法 |
| CN107123811B (zh) * | 2017-04-11 | 2020-01-10 | 华南理工大学 | 双尺度多孔铜铝锰形状记忆合金复合材料及其制备方法与应用 |
| CN108998694A (zh) * | 2018-07-06 | 2018-12-14 | 武汉理工大学 | 一种超弹性合金局部增强混凝土抗震柱的制备方法 |
| CN108972862A (zh) * | 2018-07-28 | 2018-12-11 | 武汉理工大学 | 一种超弹性合金局部增强抗震自修复混凝土梁的制备方法 |
| JP7103588B2 (ja) * | 2019-01-31 | 2022-07-20 | 株式会社古河テクノマテリアル | ねじ部を有するCu-Al-Mn系形状記憶合金成形体及びその製造方法 |
| CN111139373B (zh) * | 2020-02-10 | 2021-11-05 | 江西理工大学 | 高强亚稳态弹性铜合金及其制备方法 |
| CN113234957B (zh) * | 2021-04-27 | 2022-04-01 | 中机智能装备创新研究院(宁波)有限公司 | 一种铜合金焊丝、制备方法及应用 |
| CN113373342B (zh) * | 2021-05-28 | 2022-07-22 | 上海理工大学 | 一种高超弹性CuAlMn形状记忆合金线材的制备方法 |
| CN113846244B (zh) * | 2021-09-20 | 2022-06-21 | 哈尔滨工程大学 | 一种CuAlMn形状记忆合金及制备方法 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001020026A (ja) | 1999-07-08 | 2001-01-23 | Kiyohito Ishida | 形状記憶特性及び超弾性を有する銅系合金、それからなる部材ならびにそれらの製造方法 |
| JP2005298952A (ja) | 2004-04-15 | 2005-10-27 | Chuo Spring Co Ltd | 制振材料およびその製造方法 |
| WO2011152009A1 (ja) | 2010-05-31 | 2011-12-08 | 社団法人 日本銅センター | 銅系合金及びそれを用いた構造材 |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4479510B2 (ja) * | 2005-01-17 | 2010-06-09 | 日立電線株式会社 | 銅合金導体及びそれを用いたトロリー線・ケーブル並びに銅合金導体の製造方法 |
| JP5569330B2 (ja) * | 2010-10-20 | 2014-08-13 | 日立金属株式会社 | 音楽・映像用ケーブル |
-
2013
- 2013-05-10 JP JP2013099996A patent/JP5912094B2/ja active Active
-
2014
- 2014-04-14 EP EP14794166.0A patent/EP2995694A4/en not_active Withdrawn
- 2014-04-14 WO PCT/JP2014/060586 patent/WO2014181642A1/ja not_active Ceased
- 2014-04-14 CN CN201480024828.9A patent/CN105164289A/zh active Pending
-
2015
- 2015-11-10 US US14/937,512 patent/US20160060740A1/en not_active Abandoned
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001020026A (ja) | 1999-07-08 | 2001-01-23 | Kiyohito Ishida | 形状記憶特性及び超弾性を有する銅系合金、それからなる部材ならびにそれらの製造方法 |
| JP2005298952A (ja) | 2004-04-15 | 2005-10-27 | Chuo Spring Co Ltd | 制振材料およびその製造方法 |
| WO2011152009A1 (ja) | 2010-05-31 | 2011-12-08 | 社団法人 日本銅センター | 銅系合金及びそれを用いた構造材 |
Non-Patent Citations (6)
| Title |
|---|
| K EIICHI ARAKI ET AL.: "Mechanical properties of Cu-Al-Mn superelastic alloys as a damping material for building structures", COPPER AND COPPER ALLOY, vol. 47, 1 August 2008 (2008-08-01), pages 73 - 77, XP008182583 * |
| See also references of EP2995694A4 * |
| TOSHIHIRO OMORI ET AL.: "Abnormal Grain Growth Induced by Cyclic Heat Treatment", SCIENCE, vol. 341, 27 September 2013 (2013-09-27), pages 1500 - 1502, XP055295450 * |
| TOSHIHIRO OMORI ET AL.: "Damping Characteristics of Ductile Cu-Al-Mn Shape Memory Alloys", COPPER AND COPPER ALLOY, vol. 42, 1 August 2003 (2003-08-01), pages 198 - 201, XP008182479 * |
| Y. SUTOU ET AL.: "Grain size dependence of pseudoelasticity in polycrystalline Cu-Al-Mn- based shape memory sheets", ACTA MATERIALIA, vol. 61, no. 10, pages 3842 - 3850, XP055086107 * |
| YUJI SUTO ET AL.: "Development of Cu-Al-Mn- based Shape Memory Alloys with Enhanced Ductility", MATERIA JAPAN, vol. 42, no. 11, 20 November 2003 (2003-11-20), pages 813 - 821, XP055294456 * |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN111394611A (zh) * | 2020-04-08 | 2020-07-10 | 公牛集团股份有限公司 | 一种耐磨高弹性铜合金插套材料及其制备方法 |
| CN113862508A (zh) * | 2021-09-29 | 2021-12-31 | 哈尔滨工程大学 | 一种CuAlMnCoNi形状记忆合金及其制备方法 |
| CN113862508B (zh) * | 2021-09-29 | 2022-09-02 | 哈尔滨工程大学 | 一种CuAlMnCoNi形状记忆合金及其制备方法 |
| CN114807648A (zh) * | 2022-05-27 | 2022-07-29 | 天津理工大学 | 一种高温形状记忆合金及其制备方法 |
| CN114807648B (zh) * | 2022-05-27 | 2023-08-18 | 天津理工大学 | 一种高温形状记忆合金及其制备方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2995694A1 (en) | 2016-03-16 |
| CN105164289A (zh) | 2015-12-16 |
| JP2014218717A (ja) | 2014-11-20 |
| JP5912094B2 (ja) | 2016-04-27 |
| EP2995694A4 (en) | 2017-05-17 |
| US20160060740A1 (en) | 2016-03-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5912094B2 (ja) | 安定した超弾性を示すCu−Al−Mn系棒材及び板材の製造方法 | |
| JP5567093B2 (ja) | 安定した超弾性を示すCu−Al−Mn系合金材とその製造方法 | |
| KR102237789B1 (ko) | 내응력부식성이 우수한 Cu-Al-Mn계 합금재료로 이루어지는 전신재와 그 용도 | |
| US11118255B2 (en) | Cu-Al-Mn-based alloy material, method of producing the same, and rod material or sheet material using the same | |
| JP5215855B2 (ja) | Fe基合金及びその製造方法 | |
| CN112639144B (zh) | 铜系合金材料及其制造方法以及由铜系合金材料构成的构件或部件 | |
| JP6490608B2 (ja) | Cu−Al−Mn系合金材の製造方法 | |
| JPWO2018047787A1 (ja) | Fe基形状記憶合金材及びその製造方法 | |
| JP6258644B2 (ja) | 破断伸びに優れたCu−Al−Mn系合金材及びそれを用いてなる制震部材 | |
| CN112840051B (zh) | Cu-Al-Mn系形状记忆合金的成型体及其制造方法 | |
| JP2016153532A (ja) | 安定した超弾性を示すCu−Al−Mn系棒材及び板材、それを用いた制震部材、並びに制震部材を用いた制震構造体 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201480024828.9 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14794166 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2014794166 Country of ref document: EP |