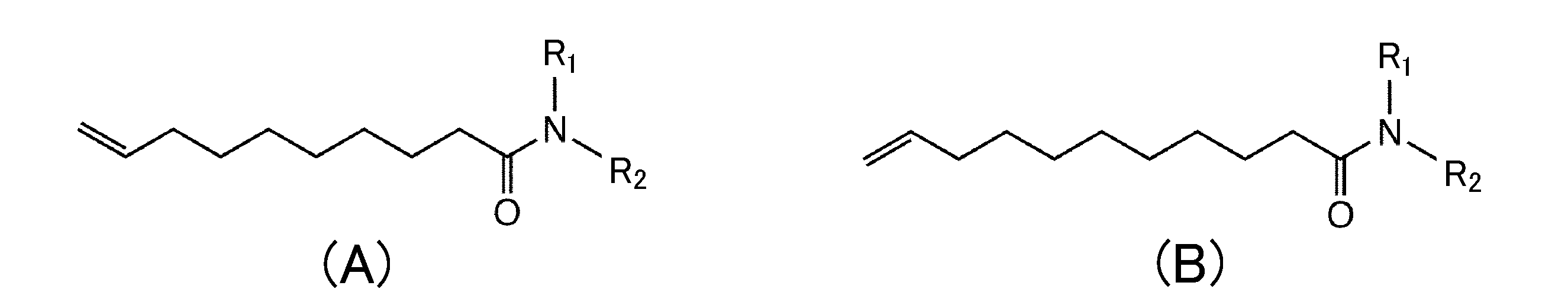WO2014148136A1 - Compound having anti-allergic activity and use of same - Google Patents
Compound having anti-allergic activity and use of same Download PDFInfo
- Publication number
- WO2014148136A1 WO2014148136A1 PCT/JP2014/052754 JP2014052754W WO2014148136A1 WO 2014148136 A1 WO2014148136 A1 WO 2014148136A1 JP 2014052754 W JP2014052754 W JP 2014052754W WO 2014148136 A1 WO2014148136 A1 WO 2014148136A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- tic
- unsubstituted
- substituted
- group
- antiallergic
- Prior art date
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C57/00—Unsaturated compounds having carboxyl groups bound to acyclic carbon atoms
- C07C57/02—Unsaturated compounds having carboxyl groups bound to acyclic carbon atoms with only carbon-to-carbon double bonds as unsaturation
- C07C57/03—Monocarboxylic acids
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS, OR NON-ALCOHOLIC BEVERAGES, NOT COVERED BY SUBCLASSES A21D OR A23B-A23J; THEIR PREPARATION OR TREATMENT, e.g. COOKING, MODIFICATION OF NUTRITIVE QUALITIES, PHYSICAL TREATMENT; PRESERVATION OF FOODS OR FOODSTUFFS, IN GENERAL
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/115—Fatty acids or derivatives thereof; Fats or oils
- A23L33/12—Fatty acids or derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C233/00—Carboxylic acid amides
- C07C233/01—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C233/02—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having nitrogen atoms of carboxamide groups bound to hydrogen atoms or to carbon atoms of unsubstituted hydrocarbon radicals
- C07C233/09—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having nitrogen atoms of carboxamide groups bound to hydrogen atoms or to carbon atoms of unsubstituted hydrocarbon radicals with carbon atoms of carboxamide groups bound to carbon atoms of an acyclic unsaturated carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C233/00—Carboxylic acid amides
- C07C233/01—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C233/34—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by amino groups
- C07C233/35—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by amino groups with the substituted hydrocarbon radical bound to the nitrogen atom of the carboxamide group by an acyclic carbon atom
- C07C233/38—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by amino groups with the substituted hydrocarbon radical bound to the nitrogen atom of the carboxamide group by an acyclic carbon atom having the carbon atom of the carboxamide group bound to a carbon atom of an acyclic unsaturated carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D295/00—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms
- C07D295/22—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with hetero atoms directly attached to ring nitrogen atoms
- C07D295/28—Nitrogen atoms
- C07D295/32—Nitrogen atoms acylated with carboxylic or carbonic acids, or their nitrogen or sulfur analogues
Definitions
- the present invention relates to a compound exhibiting antiallergic activity and its use.
- This application claims priority based on Japanese Patent Application No. 2013-056798 filed on Mar. 19, 2013, the entire contents of which are incorporated by reference.
- Non-patent Document 1 Treatment guidelines for allergic diseases have been recently created (Non-patent Document 1), and the usage rate of inhaled steroids and the like has increased accordingly, but such as allergic rhinitis, food allergy, atopic dermatitis, etc. In patients with diseases, patient satisfaction with treatment is still low. In Europe and the United States, the relationship between these allergic diseases and decreased QOL and productivity has been pointed out, and the development of new therapeutic agents is desired.
- IgE antibodies are known to play a central role not only in the activation of mast cells but also in the presentation of antigens and the activation of various inflammatory cells. Particularly, anti-IgE antibodies have recently been used. Its role has been regained attention since the previous treatment was applied to the treatment of patients with atopic refractory asthma. On the other hand, some of the components derived from natural products have an antiallergic action, but the medium chain fatty acids for which various actions have been reported so far are unclear. Accordingly, the present inventors have focused on medium-chain fatty acid derivatives and have conducted research aimed at creating compounds having antiallergic activity.
- Antiallergic compound represented by the following chemical formula (A) or (B): Where R1 is a hydrogen atom, an unsubstituted or substituted methyl group, an unsubstituted or substituted ethyl group, an unsubstituted or substituted pentyl group, or an unsubstituted or substituted phenyl group, and R2 is unsubstituted or substituted A substituted ethyl group, an unsubstituted or substituted aminoethyl group, an unsubstituted or substituted pentyl group, an unsubstituted or substituted phenyl group, or an unsubstituted or substituted piperidinyl group.
- TIC medium chain fatty acid derivative
- ACA mouse active skin anaphylaxis
- IPD suplatast tosylate
- OVA ovalbumin
- B shows the effect of various TIC compounds on serum OVA-specific IgE levels. Compared with some IPD. Comparison was made using the following formula.
- Ratio inhibition rate by TIC compound (%) / inhibition rate by IPD (%).
- DPH diphenhydramine
- PCA passive skin anaphylaxis
- PCA (-); group in which purified water was orally administered to non-sensitized mice, PCA (+); group in which purified water was orally administered to mouse-sensitized mice, Vehi; vehicle (0.2% EtOH / PBS diluted solution) )
- #, ## p ⁇ 0.05, 0.005 (by vs Vehi: Dunnett ’s multiple comparison test)
- ACA active skin anaphylaxis
- PCA passive skin anaphylaxis
- the first aspect of the present invention relates to an antiallergic active compound.
- the antiallergic activity compound of the present invention is represented by the following chemical formula (A) or (B).
- R1 is a hydrogen atom, an unsubstituted or substituted methyl group, an unsubstituted or substituted ethyl group, an unsubstituted or substituted pentyl group, or an unsubstituted or substituted phenyl group
- R2 is unsubstituted or substituted A substituted ethyl group, an unsubstituted or substituted aminoethyl group, an unsubstituted or substituted pentyl group, an unsubstituted or substituted phenyl group, or an unsubstituted or substituted piperidinyl group.
- the substitution position when R1 and / or R2 is substituted is not particularly limited. It may be substituted at two or more positions. Examples of the substituent include F, Cl, Br, I
- the compound of the present invention comprises (1) a derivative of a medium chain fatty acid having 10 or 11 carbon atoms, (2) a double bond at the end of the medium chain fatty acid, and (3) an amino group in the carboxylic acid moiety It has the feature of being bonded (it is an amide).
- TIC-11006 N-2- (diisopropylamino) ethyl) undecen-10-enamide
- TIC-11014 N-2- (diethylamino) ethyl) decen-9-enamide: N-2- (diethylamino) ethyl) dec-9-enamide
- TIC-11022 N- (2- (diethylamino) ethyl) -N-methylundecen-10-enamide: N- (2- (diethylamino) ethyl) -N-methylundec-10-enamide
- TIC-11030 N- (piperidin-1-yl) undecen-10-enamide :: N- (piperidin-1-yl) undec-10-enamide)
- TIC-11035 N- (piperidin-1-yl) undecen-10-enamide :: N- (piperidin-1-yl) undec-10-enamide)
- TIC-11006, TIC-11014, TIC-11022, TIC-11030, and TIC-11035 are compounds that have strong IgE production-suppressing activity and also have type-I allergic reaction-suppressing activity, and are highly useful. Among them, the action of TIC-11030 and TIC-11035 is strong. In addition, the detail of the synthesis
- Another aspect of the present invention provides an antiallergic composition containing the antiallergic activity compound of the present invention or a pharmacologically acceptable salt thereof as an active ingredient.
- the active ingredient of the composition of the present invention is one or more selected from the group consisting of TIC-11006, TIC-11014, TIC-11022, TIC-11030, TIC-11035, 11039 and TIC-11043.
- a compound or a salt thereof is used.
- these compounds have been identified as having both IgE production inhibitory activity and type I allergic reaction inhibitory activity, so their use can suppress IgE production and type I allergic reaction. The effect can be exerted by two actions of suppressing the above. In other words, both the source and symptoms of an allergic reaction can be suppressed.
- the compound having a strong IgE production inhibitory action and also having a type I allergic reaction inhibitory action that is, one or more selected from the group consisting of TIC-11006, TIC-11014, TIC-11022, TIC-11030, and TIC-11035
- a compound having a strong action that is, TIC-11030 and / or TIC-11035 or a pharmaceutically acceptable salt thereof is used as an active ingredient.
- the composition of the present invention is used for the purpose of treating or preventing allergy.
- the form of the composition of this invention is not specifically limited, Preferably it is a pharmaceutical, a quasi-drug, cosmetics, or a foodstuff. That is, the present invention provides, as a preferred embodiment, a pharmaceutical composition, a quasi-drug composition, a cosmetic composition, and a food composition containing the anti-allergic activity compound of the present invention or a pharmacologically acceptable salt thereof as an active ingredient. Offer things. Two or more kinds of antiallergic active compounds may be used in combination.
- Examples of “pharmacologically acceptable salts” in this specification include salts (inorganic acid salts) with hydrochloric acid, phosphoric acid, sulfuric acid, nitric acid, boric acid, etc., formic acid, acetic acid, lactic acid, fumaric acid, maleic acid And salts (organic acid salts) with tartaric acid, citric acid, succinic acid, malonic acid and the like.
- These salts can be prepared by conventional means.
- the above illustration should not be used because “pharmacologically acceptable salt” is limitedly interpreted. That is, “pharmacologically acceptable salt” is to be interpreted broadly and is a term that includes various salts.
- Preparation of the pharmaceutical composition and quasi-drug composition of the present invention can be performed according to a conventional method.
- other pharmaceutically acceptable ingredients for example, carriers, excipients, disintegrants, buffers, emulsifiers, suspending agents, soothing agents, stabilizers, preservatives, preservatives, physiological Saline solution and the like.
- excipient lactose, starch, sorbitol, D-mannitol, sucrose and the like can be used.
- disintegrant starch, carboxymethylcellulose, calcium carbonate and the like can be used. Phosphate, citrate, acetate, etc. can be used as the buffer.
- emulsifier gum arabic, sodium alginate, tragacanth and the like can be used.
- suspending agent glyceryl monostearate, aluminum monostearate, methyl cellulose, carboxymethyl cellulose, hydroxymethyl cellulose, sodium lauryl sulfate and the like can be used.
- soothing agent benzyl alcohol, chlorobutanol, sorbitol and the like can be used.
- stabilizer propylene glycol, ascorbic acid or the like can be used.
- preservatives phenol, benzalkonium chloride, benzyl alcohol, chlorobutanol, methylparaben, and the like can be used.
- preservatives benzalkonium chloride, paraoxybenzoic acid, chlorobutanol and the like can be used.
- the dosage form in the case of formulation is not particularly limited, for example, as nasal drops, eye drops, tablets, powders, fine granules, granules, capsules, syrups, injections, external preparations, suppositories, etc.
- the pharmaceutical composition or quasi-drug composition of the invention can be provided.
- the pharmaceutical composition of the present invention contains an active ingredient in an amount necessary for obtaining an expected therapeutic effect or preventive effect (that is, a therapeutically effective amount).
- the quasi-drug composition of the present invention contains an active ingredient in an amount necessary for obtaining the expected improvement effect, prevention effect and the like.
- the amount of the active ingredient contained in the pharmaceutical composition or quasi-drug composition of the present invention generally varies depending on the dosage form and form, but the amount of the active ingredient is, for example, about 0.1% by weight to about 95% so that a desired dosage can be achieved. Set within the weight% range.
- the pharmaceutical composition and quasi-drug composition of the present invention are oral or parenteral (intravenous, intraarterial, subcutaneous, intramuscular, or intraperitoneal injection, transdermal, nasal, transmucosal depending on the dosage form and form. , Application, etc.).
- the “subject” here is not particularly limited, and includes humans and non-human mammals (including pet animals, domestic animals, laboratory animals. Specifically, for example, mice, rats, guinea pigs, hamsters, monkeys, cows, pigs, goats. , Sheep, dogs, cats, chickens, quails, etc.).
- the application subject is a human.
- the dosage and usage of the pharmaceutical composition and quasi-drug composition of the present invention are set so as to obtain the expected effect.
- the symptom, age, sex, weight, etc. of the subject of application are generally considered.
- a person skilled in the art can set an appropriate dose in consideration of these matters.
- As the administration schedule for example, once to several times a day, once every two days, or once every three days can be adopted. In the preparation of the administration / use schedule, it is possible to consider the symptoms of the application target, the duration of effect of the active ingredient, and the like.
- the present application also provides a method for treating and preventing allergic diseases, which comprises administering a therapeutically effective amount of the pharmaceutical composition of the present invention to patients with allergic diseases.
- the cosmetic composition of the present invention comprises an antiallergic active compound or a pharmacologically acceptable salt thereof, and ingredients / bases usually used in cosmetics (for example, various oils and fats, mineral oil, petrolatum, squalane, lanolin, Beeswax, denatured alcohol, dextrin palmitate, glycerin, glycerin fatty acid ester, ethylene glycol, paraben, camphor, menthol, various vitamins, zinc oxide, titanium oxide, benzoic acid, edetic acid, chamomile oil, carrageenan, chitin powder, chitosan, fragrance , Coloring agents, etc.).
- ingredients / bases usually used in cosmetics (for example, various oils and fats, mineral oil, petrolatum, squalane, lanolin, Beeswax, denatured alcohol, dextrin palmitate, glycerin, glycerin fatty acid ester, ethylene glycol, paraben, camphor, menthol, various
- the cosmetic composition examples include emulsions for face or body, lotions, creams, lotions, essences, oils, packs, sheets, cleaning agents, and the like.
- the addition amount of the active ingredient in the cosmetic composition is not particularly limited.
- the active ingredient may be added so as to be 0.1 wt% to 60 wt%.
- one embodiment of the present invention is a food composition.
- the “food composition” in the present invention include general foods (cereals, vegetables, meat, various processed foods, confectionery (such as strawberries, gums), milk, soft drinks, alcoholic beverages, etc.), dietary supplements (supplements) , Nutritional drinks, etc.), food additives, foods for pets, nutritional supplements for pets.
- a dietary supplement or food additive it can be provided in the form of powder, granule powder, tablet, paste, liquid or the like.
- the food composition of the present invention preferably contains an active ingredient in an amount that can be expected to have a therapeutic or preventive effect.
- the amount added can be determined in consideration of the medical condition, health status, age, sex, weight, etc. of the person to whom it is used.
- test compound was intraperitoneally administered every day for 2 weeks from the day of the first immunization (500 ⁇ g / kg).
- target drug suplatast tosylate (IPD)
- IPD suplatast tosylate
- TIC-11006, TIC-11014, TIC-11022, TIC-11030, and TIC-11035 have a strong IgE production inhibitory action and can be expected to have a high therapeutic or preventive effect.
- TIC-11030 and TIC-11035 have the strong IgE production inhibitory action and are the most effective compounds.
- strong antiallergic activity was also observed in the known compounds TIC-11044 and TIC-11045.
- the novel compound of the present invention can suppress both IgE production and type I allergic reaction. Therefore, it can be used as a novel antiallergic agent that can suppress both the source and symptoms of an allergic reaction.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- General Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Pulmonology (AREA)
- Animal Behavior & Ethology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Mycology (AREA)
- Nutrition Science (AREA)
- Food Science & Technology (AREA)
- Polymers & Plastics (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
The purpose is to provide a novel compound for suppressing both IgE production and mast cell activation, and the use thereof. Provided is a novel compound that (1) is a derivative of a medium-chain fatty acid having 10 or 11 carbon atoms and (2) has a double bond at an end of the medium chain fatty acid, and in which (3) an amino group bonds to the carboxylic acid moiety.
Description
本発明は抗アレルギー活性を示す化合物及びその用途に関する。本出願は、2013年3月19日に出願された日本国特許出願第2013-056798号に基づく優先権を主張するものであり、当該特許出願の全内容は参照により援用される。
The present invention relates to a compound exhibiting antiallergic activity and its use. This application claims priority based on Japanese Patent Application No. 2013-056798 filed on Mar. 19, 2013, the entire contents of which are incorporated by reference.
アレルギー疾患の患者数は漸増しており、疫学調査では国民の約1/3は何らかのアレルギー疾患に罹患しているとの報告がある。これに対し、近年、アレルギー疾患の治療ガイドラインが作成され(非特許文献1)、それに伴い吸入ステロイド薬などの使用率は増加しているが、アレルギー性鼻炎・食物アレルギー・アトピー性皮膚炎などの疾患では治療に対する患者満足度がいまだ低く、欧米においても、これらアレルギー疾患とQOL・生産性の低下との関連性が指摘されており、新規治療薬の開発が望まれている。
The number of patients with allergic diseases is increasing gradually, and an epidemiological survey reports that about 1/3 of the population suffers from allergic diseases. On the other hand, treatment guidelines for allergic diseases have been recently created (Non-patent Document 1), and the usage rate of inhaled steroids and the like has increased accordingly, but such as allergic rhinitis, food allergy, atopic dermatitis, etc. In patients with diseases, patient satisfaction with treatment is still low. In Europe and the United States, the relationship between these allergic diseases and decreased QOL and productivity has been pointed out, and the development of new therapeutic agents is desired.
これまでに開発上市されてきた多くの抗アレルギー薬は肥満細胞脱顆粒抑制薬、ヒスタミンH1拮抗薬、ロイコトリエン拮抗薬、Th2サイトカン産生抑制薬などであるが、アトピー性疾患の発症および病態形成に重要なIgE産生と肥満細胞活性化の両者を抑制する化合物は上市されていない。このような化合物の創製が可能となれば、IgE産生を抑制することにより特定抗原に対するアレルギー反応の軽減、また、肥満細胞活性化を抑制することにより症状の抑制が期待できる。そこで本発明は、IgE産生と肥満細胞活性化の両者を抑制する新規化合物及びその用途を提供することを課題とする。
Many antiallergic drugs that have been put on the market are mast cell degranulation inhibitors, histamine H1 antagonists, leukotriene antagonists, Th2 cytocan production inhibitors, etc., but they are useful for the development of atopic diseases and pathogenesis. There are no commercially available compounds that suppress both important IgE production and mast cell activation. If such a compound can be created, it is possible to reduce the allergic reaction to a specific antigen by suppressing IgE production, and to suppress the symptoms by suppressing mast cell activation. Then, this invention makes it a subject to provide the novel compound which suppresses both IgE production and mast cell activation, and its use.
アレルギー反応においてIgE抗体は肥満細胞の活性化のみならず、抗原の提示、種々の炎症性細胞の活性化など、中心的な役割を有することが知られており、特に近年、抗IgE抗体を用いた治療がアトピー性の難治性喘息患者の治療に適用されて以来、その役割が再び注目されている。一方、天然物由来成分には抗アレルギー作用を有するものがあるが、これまでに多様な作用が報告されている中鎖脂肪酸については不明である。そこで、本発明者らは、中鎖脂肪酸誘導体に着目し、抗アレルギー活性を有する化合物の創出を目指して研究を進めた。詳細な検討の結果、IgE産生とI型アレルギー反応(肥満細胞に結合したIgE抗体に特異抗原が結合した際に生ずるアレルギー反応)の両者を抑制しうる新規化合物を見出した。
以下に示す発明は上記成果に基づく。
[1]以下の化学式(A)又は(B)で表される抗アレルギー活性化合物:
但し、式中のR1は水素原子、非置換又は置換のメチル基、非置換又は置換のエチル基、非置換又は置換のペンチル基、或いは非置換又は置換のフェニル基であり、R2は非置換又は置換のエチル基、非置換又は置換のアミノエチル基、非置換又は置換のペンチル基、非置換又は置換のフェニル基、或いは非置換又は置換のピペリジニル基である。
[2]以下の化学式2~8のいずれかの化学式で表される、[1]に記載の抗アレルギー活性化合物:
[3][1]又は[2]に記載の抗アレルギー活性化合物又はその薬理学的に許容可能な塩を有効成分として含有する、抗アレルギー組成物。
[4]以下の化学式9又は10で表される化合物又はその薬理学的に許容可能な塩を有効成分として含有する、抗アレルギー組成物。
[5]医薬、医薬部外品、化粧料又は食品である、[3]又は[4]に記載の組成物。
In allergic reactions, IgE antibodies are known to play a central role not only in the activation of mast cells but also in the presentation of antigens and the activation of various inflammatory cells. Particularly, anti-IgE antibodies have recently been used. Its role has been regained attention since the previous treatment was applied to the treatment of patients with atopic refractory asthma. On the other hand, some of the components derived from natural products have an antiallergic action, but the medium chain fatty acids for which various actions have been reported so far are unclear. Accordingly, the present inventors have focused on medium-chain fatty acid derivatives and have conducted research aimed at creating compounds having antiallergic activity. As a result of detailed studies, a novel compound that can suppress both IgE production and type I allergic reaction (allergic reaction that occurs when a specific antigen binds to an IgE antibody bound to mast cells) has been found.
The following invention is based on the above results.
[1] Antiallergic compound represented by the following chemical formula (A) or (B):
Where R1 is a hydrogen atom, an unsubstituted or substituted methyl group, an unsubstituted or substituted ethyl group, an unsubstituted or substituted pentyl group, or an unsubstituted or substituted phenyl group, and R2 is unsubstituted or substituted A substituted ethyl group, an unsubstituted or substituted aminoethyl group, an unsubstituted or substituted pentyl group, an unsubstituted or substituted phenyl group, or an unsubstituted or substituted piperidinyl group.
[2] The antiallergic active compound according to [1], represented by any one of the following chemical formulas 2 to 8:
[3] An antiallergic composition comprising the antiallergic activity compound according to [1] or [2] or a pharmacologically acceptable salt thereof as an active ingredient.
[4] An antiallergic composition comprising a compound represented by the followingchemical formula 9 or 10 or a pharmacologically acceptable salt thereof as an active ingredient.
[5] The composition according to [3] or [4], which is a medicine, quasi drug, cosmetic or food.
以下に示す発明は上記成果に基づく。
[1]以下の化学式(A)又は(B)で表される抗アレルギー活性化合物:
[2]以下の化学式2~8のいずれかの化学式で表される、[1]に記載の抗アレルギー活性化合物:
[4]以下の化学式9又は10で表される化合物又はその薬理学的に許容可能な塩を有効成分として含有する、抗アレルギー組成物。
The following invention is based on the above results.
[1] Antiallergic compound represented by the following chemical formula (A) or (B):
[2] The antiallergic active compound according to [1], represented by any one of the following chemical formulas 2 to 8:
[4] An antiallergic composition comprising a compound represented by the following
本発明の第1の局面は抗アレルギー活性化合物に関する。本発明の抗アレルギー活性化合物は以下の化学式(A)又は(B)で表される。
但し、式中のR1は水素原子、非置換又は置換のメチル基、非置換又は置換のエチル基、非置換又は置換のペンチル基、或いは非置換又は置換のフェニル基であり、R2は非置換又は置換のエチル基、非置換又は置換のアミノエチル基、非置換又は置換のペンチル基、非置換又は置換のフェニル基、或いは非置換又は置換のピペリジニル基である。R1及び/又はR2が置換されている場合の置換位置は特に限定されない。2以上の位置で置換されていてもよい。置換基としてF、Cl、Br、I、炭素数1~5のアルキル基を例示できる。
The first aspect of the present invention relates to an antiallergic active compound. The antiallergic activity compound of the present invention is represented by the following chemical formula (A) or (B).
Where R1 is a hydrogen atom, an unsubstituted or substituted methyl group, an unsubstituted or substituted ethyl group, an unsubstituted or substituted pentyl group, or an unsubstituted or substituted phenyl group, and R2 is unsubstituted or substituted A substituted ethyl group, an unsubstituted or substituted aminoethyl group, an unsubstituted or substituted pentyl group, an unsubstituted or substituted phenyl group, or an unsubstituted or substituted piperidinyl group. The substitution position when R1 and / or R2 is substituted is not particularly limited. It may be substituted at two or more positions. Examples of the substituent include F, Cl, Br, I, and an alkyl group having 1 to 5 carbon atoms.
本発明の化合物は、(1)炭素数が10又は11の中鎖脂肪酸の誘導体である、(2)中鎖脂肪酸の末端に二重結合を有する、及び(3)カルボン酸部分にアミノ基が結合している(アミド体である)、という特徴を備える。
The compound of the present invention comprises (1) a derivative of a medium chain fatty acid having 10 or 11 carbon atoms, (2) a double bond at the end of the medium chain fatty acid, and (3) an amino group in the carboxylic acid moiety It has the feature of being bonded (it is an amide).
本願が提供する化合物の具体例を以下に示す。尚、説明の便宜上、化合物名とその合成及び活性評価に際して付した特有の番号(後述の実施例を参照)によって各化合物を特定する。
TIC-11006(N-2-(ジイソプロピルアミノ)エチル)ウンデセン-10-エナミド: N-2-(diisopropylamino)ethyl)undec-10-enamide)
TIC-11014(N-2-(ジエチルアミノ)エチル)デセン-9-エナミド: N-2-(diethylamino)ethyl)dec-9-enamide)
TIC-11022(N-(2-(ジエチルアミノ)エチル)-N-メチルウンデセン-10-エナミド: N-(2-(diethylamino)ethyl)-N-methylundec-10-enamide)
TIC-11030(N-(ピペリジン-1-イル)ウンデセン-10-エナミド::N-(piperidin-1-yl)undec-10-enamide)
TIC-11035(N,N-ジエチルウンデセン-10-エナミド: N,N-diethylundec-10-enamide)
TIC-11039(N,N-ジペンチルウンデセン-10-エナミド: N,N-dipentylundec-10-enamide)
TIC-11043(N,N-ジフェニルウンデセン-10-エナミド: N,N-diphenylundec-10-enamide)
Specific examples of the compounds provided by the present application are shown below. For convenience of explanation, each compound is specified by a compound name and a unique number assigned in the synthesis and activity evaluation (see examples described later).
TIC-11006 (N-2- (diisopropylamino) ethyl) undecen-10-enamide)
TIC-11014 (N-2- (diethylamino) ethyl) decen-9-enamide: N-2- (diethylamino) ethyl) dec-9-enamide)
TIC-11022 (N- (2- (diethylamino) ethyl) -N-methylundecen-10-enamide: N- (2- (diethylamino) ethyl) -N-methylundec-10-enamide)
TIC-11030 (N- (piperidin-1-yl) undecen-10-enamide :: N- (piperidin-1-yl) undec-10-enamide)
TIC-11035 (N, N-diethylundecen-10-enamide)
TIC-11039 (N, N-dipentylundecen-10-enamide)
TIC-11043 (N, N-diphenylundecen-10-enamide)
後述の実施例に示す通り、上記の7化合物はIgE産生抑制作用とI型アレルギー反応抑制作用も併せ持つ化合物として同定された。この中でTIC-11006、TIC-11014、TIC-11022、TIC-11030及びTIC-11035の5化合物はIgE産生抑制作用が強く、I型アレルギー反応抑制作用も併せ持つ化合物であり有用性が高い。中でも、TIC-11030及びTIC-11035の作用は強力である。尚、以上の化合物の合成方法及び活性評価の結果の詳細は後述する。
As described in Examples below, the above seven compounds were identified as compounds having both an IgE production inhibitory action and a type I allergic reaction inhibitory action. Of these, TIC-11006, TIC-11014, TIC-11022, TIC-11030, and TIC-11035 are compounds that have strong IgE production-suppressing activity and also have type-I allergic reaction-suppressing activity, and are highly useful. Among them, the action of TIC-11030 and TIC-11035 is strong. In addition, the detail of the synthesis | combining method of the above compound and the result of activity evaluation is mentioned later.
本発明の他の局面は、本発明の抗アレルギー活性化合物又はその薬理学的に許容可能な塩を有効成分として含有する抗アレルギー組成物を提供する。一態様では、本発明の組成物の有効成分としてTIC-11006、TIC-11014、TIC-11022、TIC-11030、TIC-11035、11039及びTIC-11043からなる群より選択される一又は二以上の化合物又はその塩が用いられる。理論に拘泥する訳ではないが、これらの化合物は、IgE産生抑制作用とI型アレルギー反応抑制作用も併せ持つとして同定されたものであることから、その使用によってIgE産生の抑制と、I型アレルギー反応の抑制という二つの作用によって効果を発揮し得る。換言すれば、アレルギー反応の源流と症状の両者を抑制し得る。
Another aspect of the present invention provides an antiallergic composition containing the antiallergic activity compound of the present invention or a pharmacologically acceptable salt thereof as an active ingredient. In one embodiment, the active ingredient of the composition of the present invention is one or more selected from the group consisting of TIC-11006, TIC-11014, TIC-11022, TIC-11030, TIC-11035, 11039 and TIC-11043. A compound or a salt thereof is used. Without being bound by theory, these compounds have been identified as having both IgE production inhibitory activity and type I allergic reaction inhibitory activity, so their use can suppress IgE production and type I allergic reaction. The effect can be exerted by two actions of suppressing the above. In other words, both the source and symptoms of an allergic reaction can be suppressed.
好ましくは、IgE産生抑制作用が強く、I型アレルギー反応抑制作用も併せ持つ化合物、即ち、TIC-11006、TIC-11014、TIC-11022、TIC-11030及びTIC-11035からなる群より選択される一又は二以上の化合物、或いはその薬理学的に許容可能な塩を有効成分とする。更に好ましくは、強い作用を認めた化合物、即ち、TIC-11030及び/又はTIC-11035或いはその薬理学的に許容可能な塩を有効成分とする。
Preferably, the compound having a strong IgE production inhibitory action and also having a type I allergic reaction inhibitory action, that is, one or more selected from the group consisting of TIC-11006, TIC-11014, TIC-11022, TIC-11030, and TIC-11035 Two or more compounds or pharmacologically acceptable salts thereof are used as active ingredients. More preferably, a compound having a strong action, that is, TIC-11030 and / or TIC-11035 or a pharmaceutically acceptable salt thereof is used as an active ingredient.
本発明の組成物はアレルギーの治療又は予防目的で使用される。本発明の組成物の形態は特に限定されないが、好ましくは医薬、医薬部外品、化粧料又は食品である。即ち、本発明は好ましい態様として、本発明の抗アレルギー活性化合物又はその薬理学的に許容可能な塩を有効成分として含有する医薬組成物、医薬部外品組成物、化粧料組成物及び食品組成物を提供する。尚、2種類以上の抗アレルギー活性化合物を併用することにしてもよい。
The composition of the present invention is used for the purpose of treating or preventing allergy. Although the form of the composition of this invention is not specifically limited, Preferably it is a pharmaceutical, a quasi-drug, cosmetics, or a foodstuff. That is, the present invention provides, as a preferred embodiment, a pharmaceutical composition, a quasi-drug composition, a cosmetic composition, and a food composition containing the anti-allergic activity compound of the present invention or a pharmacologically acceptable salt thereof as an active ingredient. Offer things. Two or more kinds of antiallergic active compounds may be used in combination.
ここで、後述の実施例に示す通り、以下の化学式9に示す化合物(9-デセン酸)及び化学式10に示す化合物(10-ウンデセン酸)も高い抗アレルギー活性を示すことが明らかになった。そこで、上記抗アレルギー活性化合物に代えて又は加えて、これらの化合物のいずれか又は両者、或いはその薬理学的に許容可能な塩を有効成分として用いて各種組成物(医薬組成物、医薬部外品組成物、化粧料組成物及び食品組成物)を構成することにしてもよい。
TIC-11044(9-デセン酸:dec-9-enoic acid)
TIC-11045(10-ウンデセン酸:undec-10-enoic acid)
Here, as shown in Examples described later, it was revealed that the compound represented by the following chemical formula 9 (9-decenoic acid) and the compound represented by the chemical formula 10 (10-undecenoic acid) also exhibited high antiallergic activity. Therefore, in place of or in addition to the above-mentioned antiallergic active compound, various compositions (pharmaceutical compositions, quasi-drugs) using either or both of these compounds or pharmacologically acceptable salts thereof as active ingredients. Product composition, cosmetic composition and food composition).
TIC-11044 (9-decenoic acid)
TIC-11045 (10-undecenoic acid)
本明細書における「薬理学的に許容される塩」の例として塩酸、リン酸、硫酸、硝酸、ホウ酸等との塩(無機酸塩)や、ギ酸、酢酸、乳酸、フマル酸、マレイン酸、酒石酸、クエン酸、コハク酸、マロン酸等との塩(有機酸塩)を挙げることができる。これらの塩の調製は慣用手段によって行なうことができる。尚、以上の例示は、「薬理学的に許容される塩」が限定解釈されるために用いられるべきではない。即ち、「薬理学的に許容される塩」は、広義に解釈されるべきであり、各種の塩を含む用語である。
Examples of “pharmacologically acceptable salts” in this specification include salts (inorganic acid salts) with hydrochloric acid, phosphoric acid, sulfuric acid, nitric acid, boric acid, etc., formic acid, acetic acid, lactic acid, fumaric acid, maleic acid And salts (organic acid salts) with tartaric acid, citric acid, succinic acid, malonic acid and the like. These salts can be prepared by conventional means. In addition, the above illustration should not be used because “pharmacologically acceptable salt” is limitedly interpreted. That is, “pharmacologically acceptable salt” is to be interpreted broadly and is a term that includes various salts.
本発明の医薬組成物及び医薬部外品組成物の製剤化は常法に従って行うことができる。製剤化する場合には、製剤上許容される他の成分(例えば、担体、賦形剤、崩壊剤、緩衝剤、乳化剤、懸濁剤、無痛化剤、安定剤、保存剤、防腐剤、生理食塩水など)を含有させることができる。賦形剤としては乳糖、デンプン、ソルビトール、D-マンニトール、白糖等を用いることができる。崩壊剤としてはデンプン、カルボキシメチルセルロース、炭酸カルシウム等を用いることができる。緩衝剤としてはリン酸塩、クエン酸塩、酢酸塩等を用いることができる。乳化剤としてはアラビアゴム、アルギン酸ナトリウム、トラガント等を用いることができる。懸濁剤としてはモノステアリン酸グリセリン、モノステアリン酸アルミニウム、メチルセルロース、カルボキシメチルセルロース、ヒドロキシメチルセルロース、ラウリル硫酸ナトリウム等を用いることができる。無痛化剤としてはベンジルアルコール、クロロブタノール、ソルビトール等を用いることができる。安定剤としてはプロピレングリコール、アスコルビン酸等を用いることができる。保存剤としてはフェノール、塩化ベンザルコニウム、ベンジルアルコール、クロロブタノール、メチルパラベン等を用いることができる。防腐剤としては塩化ベンザルコニウム、パラオキシ安息香酸、クロロブタノール等と用いることができる。
Preparation of the pharmaceutical composition and quasi-drug composition of the present invention can be performed according to a conventional method. In the case of formulating, other pharmaceutically acceptable ingredients (for example, carriers, excipients, disintegrants, buffers, emulsifiers, suspending agents, soothing agents, stabilizers, preservatives, preservatives, physiological Saline solution and the like). As the excipient, lactose, starch, sorbitol, D-mannitol, sucrose and the like can be used. As the disintegrant, starch, carboxymethylcellulose, calcium carbonate and the like can be used. Phosphate, citrate, acetate, etc. can be used as the buffer. As the emulsifier, gum arabic, sodium alginate, tragacanth and the like can be used. As the suspending agent, glyceryl monostearate, aluminum monostearate, methyl cellulose, carboxymethyl cellulose, hydroxymethyl cellulose, sodium lauryl sulfate and the like can be used. As the soothing agent, benzyl alcohol, chlorobutanol, sorbitol and the like can be used. As the stabilizer, propylene glycol, ascorbic acid or the like can be used. As preservatives, phenol, benzalkonium chloride, benzyl alcohol, chlorobutanol, methylparaben, and the like can be used. As preservatives, benzalkonium chloride, paraoxybenzoic acid, chlorobutanol and the like can be used.
製剤化する場合の剤型も特に限定されず、例えば点鼻剤、点眼剤、錠剤、散剤、細粒剤、顆粒剤、カプセル剤、シロップ剤、注射剤、外用剤、及び座剤などとして本発明の医薬組成物又は医薬部外品組成物を提供できる。
The dosage form in the case of formulation is not particularly limited, for example, as nasal drops, eye drops, tablets, powders, fine granules, granules, capsules, syrups, injections, external preparations, suppositories, etc. The pharmaceutical composition or quasi-drug composition of the invention can be provided.
本発明の医薬組成物には、期待される治療効果や予防効果を得るために必要な量(即ち治療上有効量)の有効成分が含有される。同様に本発明の医薬部外品組成物には、期待される改善効果や予防効果等を得るために必要な量の有効成分が含有される。本発明の医薬組成物又は医薬部外品組成物に含まれる有効成分量は一般に剤型や形態によって異なるが、所望の投与量を達成できるように有効成分量を例えば約0.1重量%~約95重量%の範囲内で設定する。
The pharmaceutical composition of the present invention contains an active ingredient in an amount necessary for obtaining an expected therapeutic effect or preventive effect (that is, a therapeutically effective amount). Similarly, the quasi-drug composition of the present invention contains an active ingredient in an amount necessary for obtaining the expected improvement effect, prevention effect and the like. The amount of the active ingredient contained in the pharmaceutical composition or quasi-drug composition of the present invention generally varies depending on the dosage form and form, but the amount of the active ingredient is, for example, about 0.1% by weight to about 95% so that a desired dosage can be achieved. Set within the weight% range.
本発明の医薬組成物及び医薬部外品組成物はその剤型・形態に応じて経口又は非経口(静脈内、動脈内、皮下、筋肉、又は腹腔内注射、経皮、経鼻、経粘膜、塗布など)で対象に適用される。ここでの「対象」は特に限定されず、ヒト及びヒト以外の哺乳動物(ペット動物、家畜、実験動物を含む。具体的には例えばマウス、ラット、モルモット、ハムスター、サル、ウシ、ブタ、ヤギ、ヒツジ、イヌ、ネコ、ニワトリ、ウズラ等である)を含む。好ましい一態様では、適用対象はヒトである。
The pharmaceutical composition and quasi-drug composition of the present invention are oral or parenteral (intravenous, intraarterial, subcutaneous, intramuscular, or intraperitoneal injection, transdermal, nasal, transmucosal depending on the dosage form and form. , Application, etc.). The “subject” here is not particularly limited, and includes humans and non-human mammals (including pet animals, domestic animals, laboratory animals. Specifically, for example, mice, rats, guinea pigs, hamsters, monkeys, cows, pigs, goats. , Sheep, dogs, cats, chickens, quails, etc.). In a preferred embodiment, the application subject is a human.
本発明の医薬組成物及び医薬部外品組成物の投与量・使用量は、期待される効果が得られるように設定される。有効な投与量の設定においては一般に適用対象の症状、年齢、性別、体重などが考慮される。尚、当業者であればこれらの事項を考慮して適当な投与量を設定することが可能である。投与スケジュールとしては例えば一日一回~数回、二日に一回、或いは三日に一回などを採用できる。投与・使用スケジュールの作成においては、適用対象の症状や有効成分の効果持続時間などを考慮することができる。
The dosage and usage of the pharmaceutical composition and quasi-drug composition of the present invention are set so as to obtain the expected effect. In setting an effective dose, the symptom, age, sex, weight, etc. of the subject of application are generally considered. A person skilled in the art can set an appropriate dose in consideration of these matters. As the administration schedule, for example, once to several times a day, once every two days, or once every three days can be adopted. In the preparation of the administration / use schedule, it is possible to consider the symptoms of the application target, the duration of effect of the active ingredient, and the like.
以上の記述から明らかな通り、本出願は、アレルギー疾患の患者に対して本発明の医薬組成物を治療上有効量投与することを特徴とする、アレルギー疾患の治療・予防法も提供する。
As is apparent from the above description, the present application also provides a method for treating and preventing allergic diseases, which comprises administering a therapeutically effective amount of the pharmaceutical composition of the present invention to patients with allergic diseases.
上記の通り本発明の一態様は化粧料組成物である。本発明の化粧料組成物は抗アレルギー活性化合物又はその薬理学的に許容可能な塩と、化粧料に通常使用される成分・基材(例えば、各種油脂、ミネラルオイル、ワセリン、スクワラン、ラノリン、ミツロウ、変性アルコール、パルミチン酸デキストリン、グリセリン、グリセリン脂肪酸エステル、エチレングリコール、パラベン、カンフル、メントール、各種ビタミン、酸化亜鉛、酸化チタン、安息香酸、エデト酸、カミツレ油、カラギーナン、キチン末、キトサン、香料、着色料など)を配合することによって得ることができる。
As described above, one aspect of the present invention is a cosmetic composition. The cosmetic composition of the present invention comprises an antiallergic active compound or a pharmacologically acceptable salt thereof, and ingredients / bases usually used in cosmetics (for example, various oils and fats, mineral oil, petrolatum, squalane, lanolin, Beeswax, denatured alcohol, dextrin palmitate, glycerin, glycerin fatty acid ester, ethylene glycol, paraben, camphor, menthol, various vitamins, zinc oxide, titanium oxide, benzoic acid, edetic acid, chamomile oil, carrageenan, chitin powder, chitosan, fragrance , Coloring agents, etc.).
化粧料組成物の形態として、フェイス又はボディー用の乳液、化粧水、クリーム、ローション、エッセンス、オイル、パック、シート、洗浄料などを例示できる。化粧料組成物における有効成分の添加量は特に限定されない。例えば0.1重量%~60重量%となるように有効成分を添加するとよい。
Examples of the cosmetic composition include emulsions for face or body, lotions, creams, lotions, essences, oils, packs, sheets, cleaning agents, and the like. The addition amount of the active ingredient in the cosmetic composition is not particularly limited. For example, the active ingredient may be added so as to be 0.1 wt% to 60 wt%.
上記の通り本発明の一態様は食品組成物である。本発明での「食品組成物」の例として一般食品(穀類、野菜、食肉、各種加工食品、菓子類(飴、ガムなど)、牛乳、清涼飲料水、アルコール飲料等)、栄養補助食品(サプリメント、栄養ドリンク等)、食品添加物、愛玩動物用食品、愛玩動物用栄養補助食品を挙げることができる。栄養補助食品又は食品添加物の場合、粉末、顆粒末、タブレット、ペースト、液体等の形状で提供することができる。食品組成物の形態で提供することによって、本発明の有効成分を日常的に摂取したり、継続的に摂取したりすることが容易となる。
As described above, one embodiment of the present invention is a food composition. Examples of the “food composition” in the present invention include general foods (cereals, vegetables, meat, various processed foods, confectionery (such as strawberries, gums), milk, soft drinks, alcoholic beverages, etc.), dietary supplements (supplements) , Nutritional drinks, etc.), food additives, foods for pets, nutritional supplements for pets. In the case of a dietary supplement or food additive, it can be provided in the form of powder, granule powder, tablet, paste, liquid or the like. By providing in the form of a food composition, it becomes easy to ingest the active ingredient of the present invention on a daily basis or continuously.
本発明の食品組成物には治療的又は予防的効果が期待できる量の有効成分が含有されることが好ましい。添加量は、それが使用される対象となる者の病状、健康状態、年齢、性別、体重などを考慮して定めることができる。
The food composition of the present invention preferably contains an active ingredient in an amount that can be expected to have a therapeutic or preventive effect. The amount added can be determined in consideration of the medical condition, health status, age, sex, weight, etc. of the person to whom it is used.
1.中鎖脂肪酸誘導体の合成
(1)TIC-11014(N-2-(ジエチルアミノ)エチル)デセン-9-エナミド: N-2-(diethylamino)ethyl)dec-9-enamide)の合成
過剰の塩化チオニールと処理して得た9-デセン酸クロライド (C10H16OCl 分子量187.5) 1.0 g (5 mMol)を含むテトラヒドロフラン溶液(30 mL)にN,N-diethylethylenediamine (C6H16N2 分子量116) 1.2 g (10 mMol)を含むテトラヒロドフラン溶液(30 mL)を加え、水浴上で3時間加熱還流した。減圧下にテトラヒドロフランを除き、酢酸エチルエステルで抽出した。酢酸エチル層は濃縮後、シリカゲルカラムクロマトグラフィー(展開溶媒:クロロホルム-メタノール= 10 : 1)で精製し、淡褐色のN- (2-diethylamino)ethyl)dec-9-enamide (1.8 g)を油状物質として得た。
Pale brown oil; HR-ESIMS (positive ion mode): m/z 269.2509 [M+H]+ (Calcd for C16H32N2O, 269.2587); 1H-NMR (500 MHz CDCl3): δH 1.02 (6H, t, J = 7.2 Hz), 1.26 - 1.32 (6H, m), 1.37 (2H, quin, J = 7.0 Hz), 1.62 (2H, q , J = 7.7 Hz), 2.03 (2H, brqt, J = 8.0, 1.4 Hz), 2.17 (2H, t, J = 7.7 Hz), 2.51 - 2.56 (6H, m), 3.29 (2H, q, J = 5.4 Hz), 4.93 (1H, dq, J = 10.2, 1.2 Hz), 4.99 (1H, dq, J = 17.2, 1.7 Hz), 5.80 (1H, m), 6.15 (1H, br s). 1. Synthesis of medium-chain fatty acid derivatives (1) Synthesis of TIC-11014 (N-2- (diethylamino) ethyl) decen-9-enamide: N-2- (diethylamino) ethyl) dec-9-enamide) N, N-diethylethylenediamine (C 6 H 16 N 2 molecular weight 116) is added to a tetrahydrofuran solution (30 mL) containing 1.0 g (5 mMol) of 9-decenoic acid chloride (C 10 H 16 OCl molecular weight 187.5) obtained by treatment. 1.2 A tetrahydrofuran solution (30 mL) containing g (10 mMol) was added, and the mixture was heated to reflux for 3 hours on a water bath. Tetrahydrofuran was removed under reduced pressure and extracted with ethyl acetate. The ethyl acetate layer was concentrated and purified by silica gel column chromatography (developing solvent: chloroform-methanol = 10: 1) to give pale brown N- (2-diethylamino) ethyl) dec-9-enamide (1.8 g) as an oil Obtained as material.
Pale brown oil; HR-ESIMS (positive ion mode): m / z 269.2509 [M + H] + (Calcd for C 16 H 32 N 2 O, 269.2587); 1 H-NMR (500 MHz CDCl 3 ): δ H 1.02 (6H, t, J = 7.2 Hz), 1.26-1.32 (6H, m), 1.37 (2H, quin, J = 7.0 Hz), 1.62 (2H, q, J = 7.7 Hz), 2.03 (2H, brqt , J = 8.0, 1.4 Hz), 2.17 (2H, t, J = 7.7 Hz), 2.51-2.56 (6H, m), 3.29 (2H, q, J = 5.4 Hz), 4.93 (1H, dq, J = 10.2, 1.2 Hz), 4.99 (1H, dq, J = 17.2, 1.7 Hz), 5.80 (1H, m), 6.15 (1H, br s).
(1)TIC-11014(N-2-(ジエチルアミノ)エチル)デセン-9-エナミド: N-2-(diethylamino)ethyl)dec-9-enamide)の合成
過剰の塩化チオニールと処理して得た9-デセン酸クロライド (C10H16OCl 分子量187.5) 1.0 g (5 mMol)を含むテトラヒドロフラン溶液(30 mL)にN,N-diethylethylenediamine (C6H16N2 分子量116) 1.2 g (10 mMol)を含むテトラヒロドフラン溶液(30 mL)を加え、水浴上で3時間加熱還流した。減圧下にテトラヒドロフランを除き、酢酸エチルエステルで抽出した。酢酸エチル層は濃縮後、シリカゲルカラムクロマトグラフィー(展開溶媒:クロロホルム-メタノール= 10 : 1)で精製し、淡褐色のN- (2-diethylamino)ethyl)dec-9-enamide (1.8 g)を油状物質として得た。
Pale brown oil; HR-ESIMS (positive ion mode): m/z 269.2509 [M+H]+ (Calcd for C16H32N2O, 269.2587); 1H-NMR (500 MHz CDCl3): δH 1.02 (6H, t, J = 7.2 Hz), 1.26 - 1.32 (6H, m), 1.37 (2H, quin, J = 7.0 Hz), 1.62 (2H, q , J = 7.7 Hz), 2.03 (2H, brqt, J = 8.0, 1.4 Hz), 2.17 (2H, t, J = 7.7 Hz), 2.51 - 2.56 (6H, m), 3.29 (2H, q, J = 5.4 Hz), 4.93 (1H, dq, J = 10.2, 1.2 Hz), 4.99 (1H, dq, J = 17.2, 1.7 Hz), 5.80 (1H, m), 6.15 (1H, br s). 1. Synthesis of medium-chain fatty acid derivatives (1) Synthesis of TIC-11014 (N-2- (diethylamino) ethyl) decen-9-enamide: N-2- (diethylamino) ethyl) dec-9-enamide) N, N-diethylethylenediamine (C 6 H 16 N 2 molecular weight 116) is added to a tetrahydrofuran solution (30 mL) containing 1.0 g (5 mMol) of 9-decenoic acid chloride (C 10 H 16 OCl molecular weight 187.5) obtained by treatment. 1.2 A tetrahydrofuran solution (30 mL) containing g (10 mMol) was added, and the mixture was heated to reflux for 3 hours on a water bath. Tetrahydrofuran was removed under reduced pressure and extracted with ethyl acetate. The ethyl acetate layer was concentrated and purified by silica gel column chromatography (developing solvent: chloroform-methanol = 10: 1) to give pale brown N- (2-diethylamino) ethyl) dec-9-enamide (1.8 g) as an oil Obtained as material.
Pale brown oil; HR-ESIMS (positive ion mode): m / z 269.2509 [M + H] + (Calcd for C 16 H 32 N 2 O, 269.2587); 1 H-NMR (500 MHz CDCl 3 ): δ H 1.02 (6H, t, J = 7.2 Hz), 1.26-1.32 (6H, m), 1.37 (2H, quin, J = 7.0 Hz), 1.62 (2H, q, J = 7.7 Hz), 2.03 (2H, brqt , J = 8.0, 1.4 Hz), 2.17 (2H, t, J = 7.7 Hz), 2.51-2.56 (6H, m), 3.29 (2H, q, J = 5.4 Hz), 4.93 (1H, dq, J = 10.2, 1.2 Hz), 4.99 (1H, dq, J = 17.2, 1.7 Hz), 5.80 (1H, m), 6.15 (1H, br s).
(2)TIC-11022(N-(2-(ジエチルアミノ)エチル)-N-メチルウンデセン-10-エナミド: N-(2-(diethylamino)ethyl)-N-methylundec-10-enamide)の合成
過剰の塩化チオニールと処理して得た10-ウンデセン酸クロライド(C11H18OCl分子量 201.5) 1.2 g (6 mMol)を含むテトラヒドロフラン溶液(30 mL)にN,N-diethyl-N-methylene-1,2-diamine (C7H18N2 分子量130) 780 mg (6 mMol)を含むテトラヒロドフラン溶液(30 mL)およびpyridine (474 mg, 6 mMol)を加え、水浴上で4時間加熱還流した。減圧下にテトラヒドロフランを除き、反応液に少量の炭酸カリウム試液を加え、酢酸エチルエステルで抽出した。酢酸エチル層は濃縮後、シリカゲルカラムクロマトグラフィー(展開溶媒:クロロホルム-メタノール= 5 : 1)で精製し、淡褐色のN-(2-(diethylamino)ethyl)-N-methylundec-10-enamide (1.4 g)を油状物質として得た。
Pale brown oil; HR-ESIMS (positive ion mode): m/z 296.25352 [M+H]+ (calcd for C18H36N2O, 296.28276), 1H-NMR (500 MHz, CDCl3): δH 1.01 (6H, t, J = 6.8 Hz), 1.28-1.39 (10H, m), 1.63 (2H, m), 2.02 (2H, dd, J = 6.9, 14.3 Hz), 2.28 (1H, t, J = 7.4 Hz), 2.33 (1H, t, J = 7.4 Hz), 2.54 (6H, m), 3.02 (3H, s), 3.36 (1H, t, J = 7.4 Hz), 3.43 (1H, t, J = 6.8 Hz), 4.93 (1H, dd, J = 10.3, 1.7 Hz), 5.00 (1H, dd, J = 17.2, 1.7 Hz), 5.81 (1H, m). (2) Synthesis of TIC-11022 (N- (2- (diethylamino) ethyl) -N-methylundecen-10-enamide: N- (2- (diethylamino) ethyl) -N-methylundec-10-enamide) N, N-diethyl-N-methylene-1, in a tetrahydrofuran solution (30 mL) containing 1.2 g (6 mMol) of 10-undecenoic acid chloride (C 11 H 18 OCl molecular weight 201.5) obtained by treating with thionyl chloride Tetrahydrofuran solution (30 mL) and pyridine (474 mg, 6 mMol) containing 780 mg (6 mMol) of 2-diamine (C 7 H 18 N 2 molecular weight 130) were added, and the mixture was heated to reflux for 4 hours on a water bath. . Tetrahydrofuran was removed under reduced pressure, a small amount of potassium carbonate test solution was added to the reaction solution, and the mixture was extracted with ethyl acetate. The ethyl acetate layer was concentrated and purified by silica gel column chromatography (developing solvent: chloroform-methanol = 5: 1) to give pale brown N- (2- (diethylamino) ethyl) -N-methylundec-10-enamide (1.4 g) was obtained as an oil.
Pale brown oil; HR-ESIMS (positive ion mode): m / z 296.25352 [M + H] + (calcd for C 18 H 36 N 2 O, 296.28276), 1 H-NMR (500 MHz, CDCl 3 ): δ H 1.01 (6H, t, J = 6.8 Hz), 1.28-1.39 (10H, m), 1.63 (2H, m), 2.02 (2H, dd, J = 6.9, 14.3 Hz), 2.28 (1H, t, J = 7.4 Hz), 2.33 (1H, t, J = 7.4 Hz), 2.54 (6H, m), 3.02 (3H, s), 3.36 (1H, t, J = 7.4 Hz), 3.43 (1H, t, J = 6.8 Hz), 4.93 (1H, dd, J = 10.3, 1.7 Hz), 5.00 (1H, dd, J = 17.2, 1.7 Hz), 5.81 (1H, m).
過剰の塩化チオニールと処理して得た10-ウンデセン酸クロライド(C11H18OCl分子量 201.5) 1.2 g (6 mMol)を含むテトラヒドロフラン溶液(30 mL)にN,N-diethyl-N-methylene-1,2-diamine (C7H18N2 分子量130) 780 mg (6 mMol)を含むテトラヒロドフラン溶液(30 mL)およびpyridine (474 mg, 6 mMol)を加え、水浴上で4時間加熱還流した。減圧下にテトラヒドロフランを除き、反応液に少量の炭酸カリウム試液を加え、酢酸エチルエステルで抽出した。酢酸エチル層は濃縮後、シリカゲルカラムクロマトグラフィー(展開溶媒:クロロホルム-メタノール= 5 : 1)で精製し、淡褐色のN-(2-(diethylamino)ethyl)-N-methylundec-10-enamide (1.4 g)を油状物質として得た。
Pale brown oil; HR-ESIMS (positive ion mode): m/z 296.25352 [M+H]+ (calcd for C18H36N2O, 296.28276), 1H-NMR (500 MHz, CDCl3): δH 1.01 (6H, t, J = 6.8 Hz), 1.28-1.39 (10H, m), 1.63 (2H, m), 2.02 (2H, dd, J = 6.9, 14.3 Hz), 2.28 (1H, t, J = 7.4 Hz), 2.33 (1H, t, J = 7.4 Hz), 2.54 (6H, m), 3.02 (3H, s), 3.36 (1H, t, J = 7.4 Hz), 3.43 (1H, t, J = 6.8 Hz), 4.93 (1H, dd, J = 10.3, 1.7 Hz), 5.00 (1H, dd, J = 17.2, 1.7 Hz), 5.81 (1H, m). (2) Synthesis of TIC-11022 (N- (2- (diethylamino) ethyl) -N-methylundecen-10-enamide: N- (2- (diethylamino) ethyl) -N-methylundec-10-enamide) N, N-diethyl-N-methylene-1, in a tetrahydrofuran solution (30 mL) containing 1.2 g (6 mMol) of 10-undecenoic acid chloride (C 11 H 18 OCl molecular weight 201.5) obtained by treating with thionyl chloride Tetrahydrofuran solution (30 mL) and pyridine (474 mg, 6 mMol) containing 780 mg (6 mMol) of 2-diamine (C 7 H 18 N 2 molecular weight 130) were added, and the mixture was heated to reflux for 4 hours on a water bath. . Tetrahydrofuran was removed under reduced pressure, a small amount of potassium carbonate test solution was added to the reaction solution, and the mixture was extracted with ethyl acetate. The ethyl acetate layer was concentrated and purified by silica gel column chromatography (developing solvent: chloroform-methanol = 5: 1) to give pale brown N- (2- (diethylamino) ethyl) -N-methylundec-10-enamide (1.4 g) was obtained as an oil.
Pale brown oil; HR-ESIMS (positive ion mode): m / z 296.25352 [M + H] + (calcd for C 18 H 36 N 2 O, 296.28276), 1 H-NMR (500 MHz, CDCl 3 ): δ H 1.01 (6H, t, J = 6.8 Hz), 1.28-1.39 (10H, m), 1.63 (2H, m), 2.02 (2H, dd, J = 6.9, 14.3 Hz), 2.28 (1H, t, J = 7.4 Hz), 2.33 (1H, t, J = 7.4 Hz), 2.54 (6H, m), 3.02 (3H, s), 3.36 (1H, t, J = 7.4 Hz), 3.43 (1H, t, J = 6.8 Hz), 4.93 (1H, dd, J = 10.3, 1.7 Hz), 5.00 (1H, dd, J = 17.2, 1.7 Hz), 5.81 (1H, m).
(3)その他の化合物の合成
上記方法(1)、(2)に準じて各種中鎖脂肪酸誘導体を合成した。合成した化合物の一部の分析データを以下に示す。
TIC-11006(N-2-(ジイソプロピルアミノ)エチル)ウンデセン-10-エナミド: N-2-(diisopropylamino)ethyl)undec-10-enamide)
Pale brown oil; HR-ESIMS (positive ion mode): m/z 311.2993 [M+H]+ (Calcd for C19H38N2O, 311.3057) ;1H-NMR (500 MHz CDCl3): δH 1.02 (12H, d, J = 6.9 Hz), 1.26 - 1.32 (8H, m), 1.36 (2H, quin, J = 7.2 Hz), 1.61 (2H, q, J = 7.2 Hz), 2.03 (2H, q, J = 6.9 Hz), 2.17 (2H, t, J = 7.4 Hz), 2.58 (2H, t, J = 6.3 Hz), 3.02 (2H, sep, J = 6.9 Hz), 3.22 (2H, q, J = 5.8 Hz), 4.93 (1H, dq, J = 10.2, 2.2 Hz), 4.99 (1H, dq, J = 17.2, 2.0 Hz), 5.80 (1H, m), 6,20 (1H, br s). (3) Synthesis of other compounds Various medium chain fatty acid derivatives were synthesized according to the above methods (1) and (2). The analytical data of some of the synthesized compounds are shown below.
TIC-11006 (N-2- (diisopropylamino) ethyl) undecen-10-enamide)
Pale brown oil; HR-ESIMS (positive ion mode): m / z 311.2993 [M + H] + (Calcd for C 19 H 38 N 2 O, 311.3057); 1 H-NMR (500 MHz CDCl 3 ): δ H 1.02 (12H, d, J = 6.9 Hz), 1.26-1.32 (8H, m), 1.36 (2H, quin, J = 7.2 Hz), 1.61 (2H, q, J = 7.2 Hz), 2.03 (2H, q , J = 6.9 Hz), 2.17 (2H, t, J = 7.4 Hz), 2.58 (2H, t, J = 6.3 Hz), 3.02 (2H, sep, J = 6.9 Hz), 3.22 (2H, q, J = 5.8 Hz), 4.93 (1H, dq, J = 10.2, 2.2 Hz), 4.99 (1H, dq, J = 17.2, 2.0 Hz), 5.80 (1H, m), 6,20 (1H, br s).
上記方法(1)、(2)に準じて各種中鎖脂肪酸誘導体を合成した。合成した化合物の一部の分析データを以下に示す。
TIC-11006(N-2-(ジイソプロピルアミノ)エチル)ウンデセン-10-エナミド: N-2-(diisopropylamino)ethyl)undec-10-enamide)
Pale brown oil; HR-ESIMS (positive ion mode): m/z 311.2993 [M+H]+ (Calcd for C19H38N2O, 311.3057) ;1H-NMR (500 MHz CDCl3): δH 1.02 (12H, d, J = 6.9 Hz), 1.26 - 1.32 (8H, m), 1.36 (2H, quin, J = 7.2 Hz), 1.61 (2H, q, J = 7.2 Hz), 2.03 (2H, q, J = 6.9 Hz), 2.17 (2H, t, J = 7.4 Hz), 2.58 (2H, t, J = 6.3 Hz), 3.02 (2H, sep, J = 6.9 Hz), 3.22 (2H, q, J = 5.8 Hz), 4.93 (1H, dq, J = 10.2, 2.2 Hz), 4.99 (1H, dq, J = 17.2, 2.0 Hz), 5.80 (1H, m), 6,20 (1H, br s). (3) Synthesis of other compounds Various medium chain fatty acid derivatives were synthesized according to the above methods (1) and (2). The analytical data of some of the synthesized compounds are shown below.
TIC-11006 (N-2- (diisopropylamino) ethyl) undecen-10-enamide)
Pale brown oil; HR-ESIMS (positive ion mode): m / z 311.2993 [M + H] + (Calcd for C 19 H 38 N 2 O, 311.3057); 1 H-NMR (500 MHz CDCl 3 ): δ H 1.02 (12H, d, J = 6.9 Hz), 1.26-1.32 (8H, m), 1.36 (2H, quin, J = 7.2 Hz), 1.61 (2H, q, J = 7.2 Hz), 2.03 (2H, q , J = 6.9 Hz), 2.17 (2H, t, J = 7.4 Hz), 2.58 (2H, t, J = 6.3 Hz), 3.02 (2H, sep, J = 6.9 Hz), 3.22 (2H, q, J = 5.8 Hz), 4.93 (1H, dq, J = 10.2, 2.2 Hz), 4.99 (1H, dq, J = 17.2, 2.0 Hz), 5.80 (1H, m), 6,20 (1H, br s).
TIC-11030(N-(ピペリジン-1-イル)ウンデセン-10-エナミド::N-(piperidin-1-yl)undec-10-enamide)
Brown oil; HR-ESIMS (negative ion mode): m/z 269.24007 [M-H]- (calcd for C16H31N2O, 269.23771), 1H-NMR (500 MHz, CDCl3): δH 1.28-1.38 (10H, m), 1.63-1.72 (8H, m), 2.00-2.05 (4H, m), 2.44 (4H, t, J = 7.4 Hz), 4.93 (1H, dd, J = 10.3, 1.7 Hz), 5.00 (1H, dd, J = 17.2, 1.7 Hz), 5.80 (1H, m), 6.27 (3H, brs). TIC-11030 (N- (piperidin-1-yl) undecen-10-enamide :: N- (piperidin-1-yl) undec-10-enamide)
Brown oil; HR-ESIMS (negative ion mode): m / z 269.24007 [MH] - (calcd for C 16 H 31 N 2 O, 269.23771), 1 H-NMR (500 MHz, CDCl 3 ): δ H 1.28- 1.38 (10H, m), 1.63-1.72 (8H, m), 2.00-2.05 (4H, m), 2.44 (4H, t, J = 7.4 Hz), 4.93 (1H, dd, J = 10.3, 1.7 Hz) , 5.00 (1H, dd, J = 17.2, 1.7 Hz), 5.80 (1H, m), 6.27 (3H, brs).
Brown oil; HR-ESIMS (negative ion mode): m/z 269.24007 [M-H]- (calcd for C16H31N2O, 269.23771), 1H-NMR (500 MHz, CDCl3): δH 1.28-1.38 (10H, m), 1.63-1.72 (8H, m), 2.00-2.05 (4H, m), 2.44 (4H, t, J = 7.4 Hz), 4.93 (1H, dd, J = 10.3, 1.7 Hz), 5.00 (1H, dd, J = 17.2, 1.7 Hz), 5.80 (1H, m), 6.27 (3H, brs). TIC-11030 (N- (piperidin-1-yl) undecen-10-enamide :: N- (piperidin-1-yl) undec-10-enamide)
Brown oil; HR-ESIMS (negative ion mode): m / z 269.24007 [MH] - (calcd for C 16 H 31 N 2 O, 269.23771), 1 H-NMR (500 MHz, CDCl 3 ): δ H 1.28- 1.38 (10H, m), 1.63-1.72 (8H, m), 2.00-2.05 (4H, m), 2.44 (4H, t, J = 7.4 Hz), 4.93 (1H, dd, J = 10.3, 1.7 Hz) , 5.00 (1H, dd, J = 17.2, 1.7 Hz), 5.80 (1H, m), 6.27 (3H, brs).
TIC-11035(N,N-ジエチルウンデセン-10-エナミド: N,N-diethylundec-10-enamide)
Pale brown oil; HR-ESIMS (positive ion mode): m/z 262.30625 [M+Na]+ (calcd for C16H29N1Na1O1, 262.21468), 1H-NMR (500 MHz, CDCl3): δH 1.10 (3H, t, J = 6.8 Hz), 1.17 (3H, t, J = 7.4 Hz), 1.28-1.38 (10H, m), 1.64 (2H, m), 2.03 (2H, dd, J = 6.9, 14.3 Hz), 2.28 (2H, t, J = 7.4 Hz), 3.29 (2H, dd, J = 6.8, 11.6 Hz), 3.36 (2H, dd J = 6.8, 11.8 Hz), 4.93 (1H, dd, J = 10.3, 1.7 Hz), 5.00 (1H, dd, J = 17.2, 1.7 Hz), 5.81 (1H, m). TIC-11035 (N, N-diethylundecen-10-enamide)
Pale brown oil; HR-ESIMS (positive ion mode): m / z 262.30625 [M + Na] + (calcd for C 16 H 29 N 1 Na 1 O 1 , 262.21468), 1 H-NMR (500 MHz, CDCl 3 ): δ H 1.10 (3H, t, J = 6.8 Hz), 1.17 (3H, t, J = 7.4 Hz), 1.28-1.38 (10H, m), 1.64 (2H, m), 2.03 (2H, dd, J = 6.9, 14.3 Hz), 2.28 (2H, t, J = 7.4 Hz), 3.29 (2H, dd, J = 6.8, 11.6 Hz), 3.36 (2H, dd J = 6.8, 11.8 Hz), 4.93 (1H , dd, J = 10.3, 1.7 Hz), 5.00 (1H, dd, J = 17.2, 1.7 Hz), 5.81 (1H, m).
Pale brown oil; HR-ESIMS (positive ion mode): m/z 262.30625 [M+Na]+ (calcd for C16H29N1Na1O1, 262.21468), 1H-NMR (500 MHz, CDCl3): δH 1.10 (3H, t, J = 6.8 Hz), 1.17 (3H, t, J = 7.4 Hz), 1.28-1.38 (10H, m), 1.64 (2H, m), 2.03 (2H, dd, J = 6.9, 14.3 Hz), 2.28 (2H, t, J = 7.4 Hz), 3.29 (2H, dd, J = 6.8, 11.6 Hz), 3.36 (2H, dd J = 6.8, 11.8 Hz), 4.93 (1H, dd, J = 10.3, 1.7 Hz), 5.00 (1H, dd, J = 17.2, 1.7 Hz), 5.81 (1H, m). TIC-11035 (N, N-diethylundecen-10-enamide)
Pale brown oil; HR-ESIMS (positive ion mode): m / z 262.30625 [M + Na] + (calcd for C 16 H 29 N 1 Na 1 O 1 , 262.21468), 1 H-NMR (500 MHz, CDCl 3 ): δ H 1.10 (3H, t, J = 6.8 Hz), 1.17 (3H, t, J = 7.4 Hz), 1.28-1.38 (10H, m), 1.64 (2H, m), 2.03 (2H, dd, J = 6.9, 14.3 Hz), 2.28 (2H, t, J = 7.4 Hz), 3.29 (2H, dd, J = 6.8, 11.6 Hz), 3.36 (2H, dd J = 6.8, 11.8 Hz), 4.93 (1H , dd, J = 10.3, 1.7 Hz), 5.00 (1H, dd, J = 17.2, 1.7 Hz), 5.81 (1H, m).
TIC-11039(N,N-ジペンチルウンデセン-10-エナミド: N,N-dipentylundec-10-enamide)
Pale brown oil; HR-ESIMS (negative ion mode): m/z 323.25307 [M-H]- (calcd for C21H40NO, 323.30802), 1H-NMR (500 MHz, CDCl3): δH 0.89 (3H, t, J = 7.4 Hz), 0.91 (3H, t, J = 7.4 Hz), 1.20-1.43 (18H, m), 1.52 (4H, m), 1.63 (2H, m), 2.03 (2H, dd, J = 6.9, 14.3 Hz), 2.30 (2H, m), 3.20 (2H, m), 3.29 (2H, m), 4.93 (1H, dd, J = 10.3, 1.7 Hz), 5.00 (1H, dd, J = 17.2, 1.7 Hz), 5.80 (1H, m). TIC-11039 (N, N-dipentylundecen-10-enamide)
Pale brown oil; HR-ESIMS (negative ion mode): m / z 323.25307 [MH] - (calcd for C 21 H 40 NO, 323.30802), 1 H-NMR (500 MHz, CDCl 3 ): δ H 0.89 (3H , t, J = 7.4 Hz), 0.91 (3H, t, J = 7.4 Hz), 1.20-1.43 (18H, m), 1.52 (4H, m), 1.63 (2H, m), 2.03 (2H, dd, J = 6.9, 14.3 Hz), 2.30 (2H, m), 3.20 (2H, m), 3.29 (2H, m), 4.93 (1H, dd, J = 10.3, 1.7 Hz), 5.00 (1H, dd, J = 17.2, 1.7 Hz), 5.80 (1H, m).
Pale brown oil; HR-ESIMS (negative ion mode): m/z 323.25307 [M-H]- (calcd for C21H40NO, 323.30802), 1H-NMR (500 MHz, CDCl3): δH 0.89 (3H, t, J = 7.4 Hz), 0.91 (3H, t, J = 7.4 Hz), 1.20-1.43 (18H, m), 1.52 (4H, m), 1.63 (2H, m), 2.03 (2H, dd, J = 6.9, 14.3 Hz), 2.30 (2H, m), 3.20 (2H, m), 3.29 (2H, m), 4.93 (1H, dd, J = 10.3, 1.7 Hz), 5.00 (1H, dd, J = 17.2, 1.7 Hz), 5.80 (1H, m). TIC-11039 (N, N-dipentylundecen-10-enamide)
Pale brown oil; HR-ESIMS (negative ion mode): m / z 323.25307 [MH] - (calcd for C 21 H 40 NO, 323.30802), 1 H-NMR (500 MHz, CDCl 3 ): δ H 0.89 (3H , t, J = 7.4 Hz), 0.91 (3H, t, J = 7.4 Hz), 1.20-1.43 (18H, m), 1.52 (4H, m), 1.63 (2H, m), 2.03 (2H, dd, J = 6.9, 14.3 Hz), 2.30 (2H, m), 3.20 (2H, m), 3.29 (2H, m), 4.93 (1H, dd, J = 10.3, 1.7 Hz), 5.00 (1H, dd, J = 17.2, 1.7 Hz), 5.80 (1H, m).
TIC-11043(N,N-ジフェニルウンデセン-10-エナミド: N,N-diphenylundec-10-enamide)
Brown oil; HR-ESIMS (positive ion mode): m/z 336.2322 [M+H]+ (calcd for C23H29NO, 336.2288), 1H-NMR (500 MHz, CDCl3): δH 1.19-1.30 (8H, m), 1.33 (2H, m), 1.65 (2H, m), 2.02 (2H, dd, J = 6.9, 14.3 Hz), 2.25 (2H, t, J = 7.5 Hz), 4.92 (1H, dd, J = 10.3, 1.7 Hz), 4.98 (1H, dd, J = 17.2, 1.7 Hz), 5.80 (1H, m), 7.25 (6H, d, J = 8.02 Hz), 7.34 (4H, brs). TIC-11043 (N, N-diphenylundecen-10-enamide)
Brown oil; HR-ESIMS (positive ion mode): m / z 336.2322 [M + H] + (calcd for C 23 H 29 NO, 336.2288), 1 H-NMR (500 MHz, CDCl 3 ): δ H 1.19- 1.30 (8H, m), 1.33 (2H, m), 1.65 (2H, m), 2.02 (2H, dd, J = 6.9, 14.3 Hz), 2.25 (2H, t, J = 7.5 Hz), 4.92 (1H , dd, J = 10.3, 1.7 Hz), 4.98 (1H, dd, J = 17.2, 1.7 Hz), 5.80 (1H, m), 7.25 (6H, d, J = 8.02 Hz), 7.34 (4H, brs) .
Brown oil; HR-ESIMS (positive ion mode): m/z 336.2322 [M+H]+ (calcd for C23H29NO, 336.2288), 1H-NMR (500 MHz, CDCl3): δH 1.19-1.30 (8H, m), 1.33 (2H, m), 1.65 (2H, m), 2.02 (2H, dd, J = 6.9, 14.3 Hz), 2.25 (2H, t, J = 7.5 Hz), 4.92 (1H, dd, J = 10.3, 1.7 Hz), 4.98 (1H, dd, J = 17.2, 1.7 Hz), 5.80 (1H, m), 7.25 (6H, d, J = 8.02 Hz), 7.34 (4H, brs). TIC-11043 (N, N-diphenylundecen-10-enamide)
Brown oil; HR-ESIMS (positive ion mode): m / z 336.2322 [M + H] + (calcd for C 23 H 29 NO, 336.2288), 1 H-NMR (500 MHz, CDCl 3 ): δ H 1.19- 1.30 (8H, m), 1.33 (2H, m), 1.65 (2H, m), 2.02 (2H, dd, J = 6.9, 14.3 Hz), 2.25 (2H, t, J = 7.5 Hz), 4.92 (1H , dd, J = 10.3, 1.7 Hz), 4.98 (1H, dd, J = 17.2, 1.7 Hz), 5.80 (1H, m), 7.25 (6H, d, J = 8.02 Hz), 7.34 (4H, brs) .
2.抗アレルギー活性の検討
新たに合成した各種中鎖脂肪酸誘導体43種とTIC-11044(9-デセン酸:dec-9-enoic acid、東京化成工業株式会社より購入)及びTIC-11045(10-ウンデセン酸:undec-10-enoic acid、東京化成工業株式会社より購入)の抗アレルギー活性を評価した。 2. Examination of antiallergic activity 43 newly synthesized various medium chain fatty acid derivatives and TIC-11044 (9-decenoic acid: dec-9-enoic acid, purchased from Tokyo Chemical Industry Co., Ltd.) and TIC-11045 (10-undecenoic acid) : Undec-10-enoic acid, purchased from Tokyo Chemical Industry Co., Ltd.)
新たに合成した各種中鎖脂肪酸誘導体43種とTIC-11044(9-デセン酸:dec-9-enoic acid、東京化成工業株式会社より購入)及びTIC-11045(10-ウンデセン酸:undec-10-enoic acid、東京化成工業株式会社より購入)の抗アレルギー活性を評価した。 2. Examination of antiallergic activity 43 newly synthesized various medium chain fatty acid derivatives and TIC-11044 (9-decenoic acid: dec-9-enoic acid, purchased from Tokyo Chemical Industry Co., Ltd.) and TIC-11045 (10-undecenoic acid) : Undec-10-enoic acid, purchased from Tokyo Chemical Industry Co., Ltd.)
2-1.方法
(1)マウス能動皮膚アナフィラキシー(active cutaneous anaphylaxis;ACA)の誘発(図4)
雌性BALB/cマウスの腹腔内に卵白アルブミン(OVA) 1μgと水酸化アルミニウムゲル(alum) 1 mgを注射して免疫し、2週間後に両耳介にOVA 0.1μg (10μL)を注射して反応を誘発した。誘発30分後にマウスを頸椎脱臼死させて耳介を採取した。OVA注射による反応誘発と同時に0.5 % Evans blue生理食塩水溶液(0.25 mL)を尾静脈内へ投与し、反応によって耳介へ漏出したEvans blueを抽出定量した。また、反応誘発の前日に採血し、血清中IgEを定量した。被験化合物は、初回免疫日から2週間連日腹腔内投与した(500 μg/kg)。対象薬であるトシル酸スプラタスト(IPD)は初回免疫日から2週間連日経口投与した(100 mg/kg)。 2-1. Method (1) Induction of mouse active cutaneous anaphylaxis (ACA) (Fig. 4)
Female BALB / c mice were immunized with 1 μg of ovalbumin (OVA) and 1 mg of aluminum hydroxide gel (alum) intraperitoneally, and after 2 weeks, OVA 0.1 μg (10 μL) was injected into both ears. Induced. Thirty minutes after the induction, the mouse was killed by cervical dislocation and the auricle was collected. Simultaneously with the reaction induction by OVA injection, 0.5% Evans blue physiological saline solution (0.25 mL) was administered into the tail vein, and Evans blue leaked into the auricle by the reaction was extracted and quantified. In addition, blood was collected on the day before the reaction induction, and serum IgE was quantified. The test compound was intraperitoneally administered every day for 2 weeks from the day of the first immunization (500 μg / kg). The target drug, suplatast tosylate (IPD), was orally administered daily for 2 weeks (100 mg / kg) from the first immunization day.
(1)マウス能動皮膚アナフィラキシー(active cutaneous anaphylaxis;ACA)の誘発(図4)
雌性BALB/cマウスの腹腔内に卵白アルブミン(OVA) 1μgと水酸化アルミニウムゲル(alum) 1 mgを注射して免疫し、2週間後に両耳介にOVA 0.1μg (10μL)を注射して反応を誘発した。誘発30分後にマウスを頸椎脱臼死させて耳介を採取した。OVA注射による反応誘発と同時に0.5 % Evans blue生理食塩水溶液(0.25 mL)を尾静脈内へ投与し、反応によって耳介へ漏出したEvans blueを抽出定量した。また、反応誘発の前日に採血し、血清中IgEを定量した。被験化合物は、初回免疫日から2週間連日腹腔内投与した(500 μg/kg)。対象薬であるトシル酸スプラタスト(IPD)は初回免疫日から2週間連日経口投与した(100 mg/kg)。 2-1. Method (1) Induction of mouse active cutaneous anaphylaxis (ACA) (Fig. 4)
Female BALB / c mice were immunized with 1 μg of ovalbumin (OVA) and 1 mg of aluminum hydroxide gel (alum) intraperitoneally, and after 2 weeks, OVA 0.1 μg (10 μL) was injected into both ears. Induced. Thirty minutes after the induction, the mouse was killed by cervical dislocation and the auricle was collected. Simultaneously with the reaction induction by OVA injection, 0.5% Evans blue physiological saline solution (0.25 mL) was administered into the tail vein, and Evans blue leaked into the auricle by the reaction was extracted and quantified. In addition, blood was collected on the day before the reaction induction, and serum IgE was quantified. The test compound was intraperitoneally administered every day for 2 weeks from the day of the first immunization (500 μg / kg). The target drug, suplatast tosylate (IPD), was orally administered daily for 2 weeks (100 mg / kg) from the first immunization day.
(2)マウス受動皮膚アナフィラキシー(passive cutaneous anaphylaxis;PCA)の誘発(図5)
雌性BALB/cマウスの両耳介に10倍希釈したマウス抗ジニトロフェニル(DNP) IgE抗体 20μLを皮内注射して受動的に感作した。24時間後に0.1 mg/mLのDNP-BSAを含む0.5 % Evans blue生理食塩水溶液0.25 mLを尾静脈内へ注射して反応を惹起した。反応惹起直後から30分後にマウスを頸椎脱臼死させ、反応部位を採取してEvans blue漏出量を測定した。被験化合物は抗原静脈内投与1時間前に腹腔内投与した(500 μg/kg)。対象薬であるジフェンヒドラミン(DPH)は抗原静脈内投与1時間前に経口投与した(20 mg/kg)。 (2) Induction of mouse passive cutaneous anaphylaxis (PCA) (Fig. 5)
Passive sensitization was performed by intradermal injection of 20 μL of mouse anti-dinitrophenyl (DNP) IgE antibody diluted 10-fold into both ears of female BALB / c mice. 24 hours later, 0.25 mL of 0.5% Evans blue physiological saline solution containing 0.1 mg / mL DNP-BSA was injected into the tail vein to induce a reaction. Thirty minutes after the initiation of the reaction, the mouse was killed by cervical dislocation, the reaction site was collected, and the amount of Evans blue leakage was measured. The test compound was administered intraperitoneally 1 hour before intravenous administration of antigen (500 μg / kg). Diphenhydramine (DPH), a target drug, was orally administered (20 mg / kg) 1 hour before intravenous administration of antigen.
雌性BALB/cマウスの両耳介に10倍希釈したマウス抗ジニトロフェニル(DNP) IgE抗体 20μLを皮内注射して受動的に感作した。24時間後に0.1 mg/mLのDNP-BSAを含む0.5 % Evans blue生理食塩水溶液0.25 mLを尾静脈内へ注射して反応を惹起した。反応惹起直後から30分後にマウスを頸椎脱臼死させ、反応部位を採取してEvans blue漏出量を測定した。被験化合物は抗原静脈内投与1時間前に腹腔内投与した(500 μg/kg)。対象薬であるジフェンヒドラミン(DPH)は抗原静脈内投与1時間前に経口投与した(20 mg/kg)。 (2) Induction of mouse passive cutaneous anaphylaxis (PCA) (Fig. 5)
Passive sensitization was performed by intradermal injection of 20 μL of mouse anti-dinitrophenyl (DNP) IgE antibody diluted 10-fold into both ears of female BALB / c mice. 24 hours later, 0.25 mL of 0.5% Evans blue physiological saline solution containing 0.1 mg / mL DNP-BSA was injected into the tail vein to induce a reaction. Thirty minutes after the initiation of the reaction, the mouse was killed by cervical dislocation, the reaction site was collected, and the amount of Evans blue leakage was measured. The test compound was administered intraperitoneally 1 hour before intravenous administration of antigen (500 μg / kg). Diphenhydramine (DPH), a target drug, was orally administered (20 mg / kg) 1 hour before intravenous administration of antigen.
2-2.結果・考察
45化合物の能動皮膚アナフィラキシー反応(ACA)に及ぼす影響を比較・評価した(図1、2)。IgE産生抑制作用が強く、I型アレルギー反応抑制作用も併せ持つ化合物(図1において星印で示したTIC-11006、TIC-11014、TIC-11022、TIC-11030、TIC-11035)とIgE産生抑制作用は弱いものの、I型アレルギー反応抑制作用を併せ持つ化合物(図1において黒矢印で示したTIC-11039、TIC-11043)を有望な化合物(合計7種)として選出した。図2は、IgE産生抑制作用を併せ持つ5化合物の成績を示したものである。5種類の化合物のうち、特にTIC-11030及びTIC-11035の作用が強力であることがわかる。 2-2. Results and Discussion The effects of 45 compounds on the active skin anaphylactic reaction (ACA) were compared and evaluated (FIGS. 1 and 2). Compounds with strong IgE production inhibitory activity and type I allergic reaction inhibitory effect (TIC-11006, TIC-11014, TIC-11022, TIC-11030, TIC-11035 shown with an asterisk in Fig. 1) and IgE production inhibitory activity Were selected as promising compounds (7 types in total), which are weak, but also have an inhibitory effect on type I allergic reactions (TIC-11039 and TIC-11043 indicated by black arrows in FIG. 1). FIG. 2 shows the results of five compounds having an IgE production inhibitory effect. Of the five compounds, it can be seen that the action of TIC-11030 and TIC-11035 is particularly strong.
45化合物の能動皮膚アナフィラキシー反応(ACA)に及ぼす影響を比較・評価した(図1、2)。IgE産生抑制作用が強く、I型アレルギー反応抑制作用も併せ持つ化合物(図1において星印で示したTIC-11006、TIC-11014、TIC-11022、TIC-11030、TIC-11035)とIgE産生抑制作用は弱いものの、I型アレルギー反応抑制作用を併せ持つ化合物(図1において黒矢印で示したTIC-11039、TIC-11043)を有望な化合物(合計7種)として選出した。図2は、IgE産生抑制作用を併せ持つ5化合物の成績を示したものである。5種類の化合物のうち、特にTIC-11030及びTIC-11035の作用が強力であることがわかる。 2-2. Results and Discussion The effects of 45 compounds on the active skin anaphylactic reaction (ACA) were compared and evaluated (FIGS. 1 and 2). Compounds with strong IgE production inhibitory activity and type I allergic reaction inhibitory effect (TIC-11006, TIC-11014, TIC-11022, TIC-11030, TIC-11035 shown with an asterisk in Fig. 1) and IgE production inhibitory activity Were selected as promising compounds (7 types in total), which are weak, but also have an inhibitory effect on type I allergic reactions (TIC-11039 and TIC-11043 indicated by black arrows in FIG. 1). FIG. 2 shows the results of five compounds having an IgE production inhibitory effect. Of the five compounds, it can be seen that the action of TIC-11030 and TIC-11035 is particularly strong.
IgE産生抑制作用が強く、I型アレルギー反応抑制作用も併せ持つ5種類の化合物が受動皮膚アナフィラキシー反応(PCA)に及ぼす影響を検討した。図3に示す通り、これら5種の化合物は優れた抑制作用(0.5 mg/kgの腹腔内投与によりジフェンヒドラミン20 mg/kgの経口投与群と同等の抑制作用)を示すことが明らかとなった。
Investigated the effects of five compounds with strong IgE production inhibitory activity and type I allergic reaction inhibitory effect on passive skin anaphylactic reaction (PCA). As shown in FIG. 3, it was revealed that these five compounds exhibited excellent inhibitory action (inhibitory action equivalent to that of the oral administration group of diphenhydramine 20 mg / kg by intraperitoneal administration of 0.5 mg / kg).
以上の通り、IgE産生作用とI型アレルギー反応抑制作用を併せ持つ新規化合物7種類を同定することに成功した。これらの化合物は新規アレルギー疾患治療薬として有望である。中でも5種類の化合物、即ちTIC-11006、TIC-11014、TIC-11022、TIC-11030及びTIC-11035には強いIgE産生抑制作用が認められ、高い治療又は予防効果を期待できる。特に、TIC-11030及びTIC-11035のIgE産生抑制作用は強力であり、最も有効な化合物であると評価できる。一方、今回の検討によって、既知化合物であるTIC-11044及びTIC-11045にも強い抗アレルギー活性が認められた。これらの化合物もアレルギー疾患治療薬として有望といえる。
As described above, we have succeeded in identifying seven kinds of novel compounds having both an IgE producing action and a type I allergic reaction inhibiting action. These compounds are promising as novel therapeutic agents for allergic diseases. Among them, five kinds of compounds, that is, TIC-11006, TIC-11014, TIC-11022, TIC-11030, and TIC-11035 have a strong IgE production inhibitory action and can be expected to have a high therapeutic or preventive effect. In particular, it can be evaluated that TIC-11030 and TIC-11035 have the strong IgE production inhibitory action and are the most effective compounds. On the other hand, as a result of this study, strong antiallergic activity was also observed in the known compounds TIC-11044 and TIC-11045. These compounds are also promising as therapeutic agents for allergic diseases.
本発明の新規化合物はIgE産生とI型アレルギー反応の両者を抑制し得る。従って、アレルギー反応の源流と症状の両者を抑制しうる新規抗アレルギー薬として利用され得る。
The novel compound of the present invention can suppress both IgE production and type I allergic reaction. Therefore, it can be used as a novel antiallergic agent that can suppress both the source and symptoms of an allergic reaction.
この発明は、上記発明の実施の形態及び実施例の説明に何ら限定されるものではない。特許請求の範囲の記載を逸脱せず、当業者が容易に想到できる範囲で種々の変形態様もこの発明に含まれる。本明細書の中で明示した論文、公開特許公報、及び特許公報などの内容は、その全ての内容を援用によって引用することとする。
The present invention is not limited to the description of the embodiments and examples of the above invention. Various modifications may be included in the present invention as long as those skilled in the art can easily conceive without departing from the description of the scope of claims. The contents of papers, published patent gazettes, patent gazettes, and the like specified in this specification are incorporated by reference in their entirety.
Claims (5)
- 以下の化学式(A)又は(B)で表される抗アレルギー活性化合物:
- 請求項1又は2に記載の抗アレルギー活性化合物又はその薬理学的に許容可能な塩を有効成分として含有する、抗アレルギー組成物。 An antiallergic composition comprising the antiallergic active compound according to claim 1 or 2 or a pharmacologically acceptable salt thereof as an active ingredient.
- 医薬、医薬部外品、化粧料又は食品である、請求項3又は4に記載の組成物。 The composition according to claim 3 or 4, which is a medicine, quasi-drug, cosmetic or food.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013-056798 | 2013-03-19 | ||
| JP2013056798 | 2013-03-19 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014148136A1 true WO2014148136A1 (en) | 2014-09-25 |
Family
ID=51579831
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/052754 WO2014148136A1 (en) | 2013-03-19 | 2014-02-06 | Compound having anti-allergic activity and use of same |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2014148136A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112194594A (en) * | 2020-08-20 | 2021-01-08 | 广东省科学院动物研究所 | N, N-diethyl 10-undeceneamide, preparation method thereof and application thereof in mosquito repelling |
| CN112898176A (en) * | 2021-01-22 | 2021-06-04 | 广东省科学院动物研究所 | 10-undecylenoyl amino ethyl propionate, preparation method thereof and application thereof in mosquito repelling |
Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS51127002A (en) * | 1975-04-08 | 1976-11-05 | Bayer Ag | Carboxylic amide preparation method thereof and pharmaceutical composition and medicine containing same |
| JPS5218822A (en) * | 1976-07-15 | 1977-02-12 | Ajinomoto Co Inc | Germicides for agricultural and gardening use |
| JPS56123923A (en) * | 1980-06-21 | 1981-09-29 | Japan Synthetic Rubber Co Ltd | Conversion of unsaturated compound having functional group |
| JPH07196671A (en) * | 1993-12-28 | 1995-08-01 | Shin Etsu Chem Co Ltd | Amide group-modified fluorine-containing organopolysiloxane |
| JP2000143730A (en) * | 1998-06-19 | 2000-05-26 | Ciba Specialty Chem Holding Inc | Polymerization of unsaturated polyalkylpiperidine |
| JP2003509499A (en) * | 1999-09-21 | 2003-03-11 | アストラゼネカ アクチボラグ | Quinazoline derivatives and their use as pharmaceuticals |
| JP2005506947A (en) * | 1999-01-12 | 2005-03-10 | ケンブリッジ ユニバーシティ テクニカル サービシズ リミティド | Compositions and methods for inhibiting or enhancing inflammatory responses |
| JP2007530481A (en) * | 2004-03-24 | 2007-11-01 | ベーリンガー インゲルハイム インターナショナル ゲゼルシャフト ミット ベシュレンクテル ハフツング | Pharmaceutical composition for the treatment of skin diseases comprising a combination of epinastine and one or more additional minerals or one or more herbal medicines |
| JP2008013486A (en) * | 2006-07-05 | 2008-01-24 | Soda Aromatic Co Ltd | Arachidonate metabolism inhibitor |
| KR20100039532A (en) * | 2008-10-08 | 2010-04-16 | 주식회사 제닉 | Hydrogel composition for healing atopy and matrix patch for healing atopy using the hydrogel composition as a matrix |
| WO2012026886A1 (en) * | 2010-08-27 | 2012-03-01 | National University Of Singapore | Chalcone structure fluorescence dye for embryonic stem cell probe |
-
2014
- 2014-02-06 WO PCT/JP2014/052754 patent/WO2014148136A1/en active Application Filing
Patent Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS51127002A (en) * | 1975-04-08 | 1976-11-05 | Bayer Ag | Carboxylic amide preparation method thereof and pharmaceutical composition and medicine containing same |
| JPS5218822A (en) * | 1976-07-15 | 1977-02-12 | Ajinomoto Co Inc | Germicides for agricultural and gardening use |
| JPS56123923A (en) * | 1980-06-21 | 1981-09-29 | Japan Synthetic Rubber Co Ltd | Conversion of unsaturated compound having functional group |
| JPH07196671A (en) * | 1993-12-28 | 1995-08-01 | Shin Etsu Chem Co Ltd | Amide group-modified fluorine-containing organopolysiloxane |
| JP2000143730A (en) * | 1998-06-19 | 2000-05-26 | Ciba Specialty Chem Holding Inc | Polymerization of unsaturated polyalkylpiperidine |
| JP2005506947A (en) * | 1999-01-12 | 2005-03-10 | ケンブリッジ ユニバーシティ テクニカル サービシズ リミティド | Compositions and methods for inhibiting or enhancing inflammatory responses |
| JP2003509499A (en) * | 1999-09-21 | 2003-03-11 | アストラゼネカ アクチボラグ | Quinazoline derivatives and their use as pharmaceuticals |
| JP2007530481A (en) * | 2004-03-24 | 2007-11-01 | ベーリンガー インゲルハイム インターナショナル ゲゼルシャフト ミット ベシュレンクテル ハフツング | Pharmaceutical composition for the treatment of skin diseases comprising a combination of epinastine and one or more additional minerals or one or more herbal medicines |
| JP2008013486A (en) * | 2006-07-05 | 2008-01-24 | Soda Aromatic Co Ltd | Arachidonate metabolism inhibitor |
| KR20100039532A (en) * | 2008-10-08 | 2010-04-16 | 주식회사 제닉 | Hydrogel composition for healing atopy and matrix patch for healing atopy using the hydrogel composition as a matrix |
| WO2012026886A1 (en) * | 2010-08-27 | 2012-03-01 | National University Of Singapore | Chalcone structure fluorescence dye for embryonic stem cell probe |
Non-Patent Citations (4)
| Title |
|---|
| HAKALA, KIMMO ET AL.: "Synthesis of nitrogen- functionalized polyolefins with metallocene/ methylaluminoxane catalysts", POLYMER BULLETIN, vol. 46, no. 2-3, 2001, BERLIN, GERMANY, pages 123 - 130, XP001017502, DOI: doi:10.1007/s002890170065 * |
| MUKHERJEE, P. ET AL.: "Possible antifungal agents", JOURNAL OF THE INDIAN CHEMICAL SOCIETY, vol. 50, no. 4, 1973, pages 290 - 292 * |
| VIG, O. P. ET AL.: "Insect juvenile hormone analogs. Part VII. Syntheses of long chain aromatic ethers and nitrogen analogues", INDIAN JOURNAL OF CHEMISTRY, SECTION B: ORGANIC CHEMISTRY INCLUDING MEDICINAL CHEMISTRY, vol. 19B, no. 8, 1980, pages 688 - 691 * |
| VYAS, L. K. ET AL.: "Formulation and development of anti-blemish preparation using microsponge technology", JOURNAL OF CHEMICAL AND PHARMACEUTICAL RESEARCH, vol. 2, no. 5, 2010, pages 562 - 571 * |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112194594A (en) * | 2020-08-20 | 2021-01-08 | 广东省科学院动物研究所 | N, N-diethyl 10-undeceneamide, preparation method thereof and application thereof in mosquito repelling |
| WO2021159691A1 (en) * | 2020-08-20 | 2021-08-19 | 广东省科学院动物研究所 | N,n-diethyl-10-undecenamide, preparation method therefor, and use thereof in repelling mosquitos |
| CN112898176A (en) * | 2021-01-22 | 2021-06-04 | 广东省科学院动物研究所 | 10-undecylenoyl amino ethyl propionate, preparation method thereof and application thereof in mosquito repelling |
| WO2021258733A1 (en) * | 2021-01-22 | 2021-12-30 | 广东省科学院动物研究所 | 10-undecylenylamino methyl ethyl propionate, and preparation method therefor and use thereof in repelling mosquitos |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6636108B2 (en) | Astaxanthin anti-inflammatory synergistic combination | |
| US20110311478A1 (en) | Combinatorial Methods For Treating Cellular Proliferative Disorders And Immune Deficiencies Using Salicinium | |
| US11149014B2 (en) | Method for treating pain or associated condition or symptom | |
| KR101710474B1 (en) | Gallamide derivatives and use thereof | |
| JP2019182881A (en) | Composition for preventing or improving peripheral neuropathy | |
| JP6343000B2 (en) | Composition for preventing or treating atopic dermatitis containing eugenol as an active ingredient | |
| US20220273600A1 (en) | Lithium salts of n-substituted glycine compounds and uses thereof | |
| US20070244079A1 (en) | Preventive/remedy for allergic diseases | |
| JPWO2002055091A1 (en) | Antiallergic agent | |
| WO2017111069A1 (en) | Antipruritic | |
| WO2014148136A1 (en) | Compound having anti-allergic activity and use of same | |
| JP2021510728A (en) | Stroke preventive or therapeutic composition | |
| WO2020130146A1 (en) | Agent for improving muscle quality | |
| JP2007031302A (en) | Adiponectin production accelerator and metabolic syndrome preventive | |
| US20160008388A1 (en) | Compositions and methods for immunotherapy | |
| JP2007145741A (en) | Ige-capturing agent, and anti-allergic medicine composition, cosmetic composition, food composition, drink composition and feed composition | |
| CN104177318A (en) | L-ascorbic acid-6-(10-hydroxyl-2-caproleic acid) esters or derivatives thereof and application of esters or derivatives | |
| CN104262302A (en) | L-ascorbic acid-6-(E-2-caproleic acid)ester or derivatives thereof and application thereof | |
| JP7264540B2 (en) | Composition for prevention or treatment of asthma, rhinitis or conjunctivitis containing an N-acyl-amino acid as an active ingredient | |
| US11932593B1 (en) | 8-(3-bromobenzylideneamino)naphthalene-1,3-disulfonic acid as an antioxidant compound | |
| WO2021079987A1 (en) | Muscle quality improvement agent | |
| JP6981311B2 (en) | Composition for improving anxiety-like symptoms | |
| WO2019146652A1 (en) | Anti-i-type allergy agent, degranulation inhibitor for basophils and mast cells, anti-dementia agent, agent for improving/inhibiting short-term memory impairment | |
| JP2003313127A (en) | External preparation for treating allergic disease | |
| TW202333756A (en) | Placental composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14769822 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14769822 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: JP |