WO2013117759A1 - Procédé d'anodisation de pièces en alliage d'aluminium - Google Patents
Procédé d'anodisation de pièces en alliage d'aluminium Download PDFInfo
- Publication number
- WO2013117759A1 WO2013117759A1 PCT/EP2013/052686 EP2013052686W WO2013117759A1 WO 2013117759 A1 WO2013117759 A1 WO 2013117759A1 EP 2013052686 W EP2013052686 W EP 2013052686W WO 2013117759 A1 WO2013117759 A1 WO 2013117759A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- voltage
- anodizing
- bath
- temperature
- aluminum alloy
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25D—PROCESSES FOR THE ELECTROLYTIC OR ELECTROPHORETIC PRODUCTION OF COATINGS; ELECTROFORMING; APPARATUS THEREFOR
- C25D11/00—Electrolytic coating by surface reaction, i.e. forming conversion layers
- C25D11/02—Anodisation
- C25D11/024—Anodisation under pulsed or modulated current or potential
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25D—PROCESSES FOR THE ELECTROLYTIC OR ELECTROPHORETIC PRODUCTION OF COATINGS; ELECTROFORMING; APPARATUS THEREFOR
- C25D11/00—Electrolytic coating by surface reaction, i.e. forming conversion layers
- C25D11/02—Anodisation
- C25D11/04—Anodisation of aluminium or alloys based thereon
- C25D11/06—Anodisation of aluminium or alloys based thereon characterised by the electrolytes used
- C25D11/08—Anodisation of aluminium or alloys based thereon characterised by the electrolytes used containing inorganic acids
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25D—PROCESSES FOR THE ELECTROLYTIC OR ELECTROPHORETIC PRODUCTION OF COATINGS; ELECTROFORMING; APPARATUS THEREFOR
- C25D11/00—Electrolytic coating by surface reaction, i.e. forming conversion layers
- C25D11/02—Anodisation
- C25D11/04—Anodisation of aluminium or alloys based thereon
- C25D11/16—Pretreatment, e.g. desmutting
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25D—PROCESSES FOR THE ELECTROLYTIC OR ELECTROPHORETIC PRODUCTION OF COATINGS; ELECTROFORMING; APPARATUS THEREFOR
- C25D11/00—Electrolytic coating by surface reaction, i.e. forming conversion layers
- C25D11/02—Anodisation
- C25D11/04—Anodisation of aluminium or alloys based thereon
- C25D11/18—After-treatment, e.g. pore-sealing
- C25D11/24—Chemical after-treatment
- C25D11/246—Chemical after-treatment for sealing layers
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25D—PROCESSES FOR THE ELECTROLYTIC OR ELECTROPHORETIC PRODUCTION OF COATINGS; ELECTROFORMING; APPARATUS THEREFOR
- C25D11/00—Electrolytic coating by surface reaction, i.e. forming conversion layers
- C25D11/38—Chromatising
Definitions
- the present invention is in the field of surface treatment of aluminum or aluminum alloy parts, aimed at improving their corrosion resistance properties. More particularly, it relates to a method of anodizing an aluminum part or one of its alloys, as well as a more general method of surface treatment of such a part implementing said method of anodization followed by a clogging step.
- anodization also called anodic oxidation
- anodic layer consists in forming on the surface of the part a porous aluminum oxide / hydroxide layer, called anodic layer, by applying a current to the piece immersed in an electrolytic bath containing a strong acid electrolyte, the piece constituting the anode of the electrolytic device.
- the anodic layer thus formed on the surface of the workpiece, after having been subjected to a clogging after-treatment, protects the workpiece against corrosion.
- This anodic layer is also a support for the attachment of conventional paint systems.
- the electrolytic baths currently used for the anodizing of aluminum alloy parts which provide the most advantageous performance in terms of, in particular, protection against corrosion of the part, mechanical hanging of paint coatings on the surface of the workpiece, and fatigue abatement, are formed with hexavalent chromium.
- chemicals containing hexavalent chromium are harmful to health and the environment.
- the present invention aims to remedy the disadvantages of anodizing processes of aluminum alloy parts of the prior art, in particular to those described above, by proposing such a process that does not implement any harmful substance, particularly to hexavalent chromium base, while having at least equivalent performance to the processes of the prior art using hexavalent chromium, in particular in terms of resistance to corrosion of the treated part, fatigue fatigue of the part and adhesion of conventional paint systems on its surface.
- a method of anodizing a piece of aluminum or aluminum alloy wherein the part is immersed in an aqueous bath comprising essentially sulfuric acid at a concentration of between 150 and 250 g / L and maintained at a constant temperature of between 5 and 25 ° C.
- essentially comprising sulfuric acid is meant that the bath contains no other active electrolytic substance, especially strong acid, in an amount sufficient to intervene in the anodization.
- the bath contains in particular no phosphoric, boric, chromic or tartaric acid, or only in the form of traces.
- This process according to the invention is characterized by the application to the piece immersed in the bath of a DC voltage according to a voltage profile comprising a rise in voltage, from a starting value of 0 V, at a speed of between 1 and 32 V / min, then maintaining the voltage at a value a so-called plateau voltage of between 12 and 20 V for a period of time sufficient to obtain an anodic layer of aluminum oxides / hydroxides at the surface of the part, of a thickness of between 3 and 7 ⁇ , preferably of between 3 and 7 ⁇ . and 5 ⁇ , and / or a layer weight of between 20 and 150 mg / dm 2 .
- Such an anodic layer exhibits properties of adhesion to paint and corrosion resistance after clogging equivalent to those of the anode layers obtained by prior art chromic anodizing processes, while not using any substance. based on hexavalent chromium.
- the method according to the invention has an additional advantage, which is to overcome the problems of resizing and fatigue reduction generated by standard sulfuric anodization processes of the prior art.
- the voltage profile applied to the part comprises a rise in voltage at a speed of between 1 and 32 V / min until reaching the so-called plateau voltage value between 12 and 20 V, then maintaining the voltage at said plateau voltage value for a period of time sufficient to obtain an anodic layer of aluminum oxides / hydroxides of thickness between 3 and 7 on the surface of the part.
- ⁇ preferably between 3 and 5 ⁇ - ⁇ , and / or weight of layer between 20 and 150 mg / dm 2 .
- the voltage profile applied to the part comprises a plurality of voltage rise phases, at least one of which is carried out at a speed of between 1 and 32 V / min, and which can be separated. two by two by a bearing during which the voltage is temporarily maintained at a fixed value, before the implementation of the final phase of maintaining the voltage at the plateau voltage value between 12 and 20 V.
- the voltage is maintained at the plateau value for a period of between 5 and 30 minutes, depending on the aluminum alloy and the thickness of the desired anodic layer. .
- the rate of rise in voltage is between 1 and 6 V / min, preferably equal to 3 V / min.
- the plateau voltage value is between 14 and 16 V. It is within the competence of those skilled in the art to determine the optimum voltage value within this range, depending in particular on the characteristics of the alloy. constituting the room.
- the concentration of sulfuric acid in the bath is preferably from 180 to 220 g / l, for example equal to 200 g / l.
- the bath temperature is between 15 and 25 ° C, preferably between 18 and 20 ° C, and for example equal to 19 ° C. All these preferential parameters ensure the best performance of the bath from the point of view of the properties of the anodic layer formed on the surface of the part.
- the part may be subjected to a surface preparation step by degreasing and / or pickling prior to immersion in the bath, so as to remove grease, dirt and oxides present on its surface.
- This preliminary surface preparation step can comprise one or more of the following operations:
- - Solvent degreasing to dissolve fats present on the surface of the room.
- This operation may be carried out by dipping, spraying, or any other technique known in itself. It may for example be carried out by soaking in methoklone or acetone, at a temperature below 42 ° C, for a period of between 5 seconds and 3 minutes; - alkaline degreasing, to dissolve fats present on the surface of the room. This operation may be carried out by dipping, spraying, or any other technique known in itself.
- This operation may be carried out by dipping, spraying, or any other technique known in itself. It may for example be carried out by soaking in a sodium hydroxide solution at 30 to 70 g / l, at a temperature between 20 and 60 ° C, for a period of between 10 seconds and 2 minutes.
- a sodium hydroxide solution at 30 to 70 g / l, at a temperature between 20 and 60 ° C, for a period of between 10 seconds and 2 minutes.
- the part is covered with a powdery layer formed of oxidation products of intermetallic compounds, which should be removed by an acid pickling step; acid pickling, for dissolving the oxides naturally formed on the surface of the part, and / or the oxidation layer formed on the surface of the part during the alkaline pickling step.
- This operation may be carried out by dipping, spraying, or any other technique known in itself. It may for example be carried out by dipping in a 15 to 25% v / v solution of SMUT-GO NC (Henkel) at a temperature of between 10 and 50 ° C for a period of between 1 and 10 minutes; or by soaking in a solution of ARDROX 295GD (Chemetall) at 15 to 30% v / v, at a temperature between 10 and 30 ° C, for a period of between 1 and 10 minutes. Interleaved rinses, especially with water, are preferably made between the successive steps above, and before the treatment of the piece by anodizing.
- Another aspect of the invention is a more general method of surface treatment of an aluminum or aluminum alloy part, according to which the part is subjected to an anodizing process corresponding to one or more of the characteristics above, then to a clogging step of the anodic layer then formed on the workpiece.
- the sealing step of the porous anodic layer may be of any type known to those skilled in the art. It can for example be a hydrothermal clogging, hot clogging with hexavalent chromium salts or nickel salts, etc. Clogging processes not involving any substance harmful to the environment and / or health are particularly preferred in the context of the invention.
- this sealing step comprises immersing the part in an aqueous bath containing a trivalent chromium salt and an oxidizing compound, with a temperature of between 20 ° and 80 ° C., preferably between 20 and 60 ° C, more particularly between 35 and 45 ° C, and / or the immersion of the piece in water at a temperature between 98 and 100 ° C, and pH for example between 4 , 5 and 8.
- conventional trivalent chromium is understood to mean chromium in the +3 oxidation state.
- Hexavalent chromium means chromium in the +6 oxidation state.
- the oxidizing compound may be of any type known in itself for post-anodizing clogging baths of aluminum or its alloys. Compounds having no adverse effect on the environment are particularly preferred in the context of the invention.
- Nonlimiting examples of such oxidizing compounds are fluoride-based substances, such as ammonium fluoride or potassium fluoro-zirconate K 2 ZrF 6 , of permanganate, such as potassium permanganate, hydrogen peroxide H 2 O 2. etc.
- the concentration of oxidizing compound in the bath may especially be between 0.1 and 50 g / l.
- the trivalent chromium salt and the oxidizing compound present in the bath may consist of two different compounds, or of one and the same compound capable of ensuring on its own the two functions of corrosion inhibition and oxidation, for example by trivalent chromium fluoride CrF 3 .
- the trivalent chromium salt can be brought in any conventional form in itself for post-anodization sealing treatments of aluminum, especially in the form of fluoride, chloride, nitrate, acetate, acetate hydroxide, sulfate, potassium sulfate, etc. of trivalent chromium, for example CrF 3 , xH 2 O, CrCl 3 , xH 2 O, Cr (NO 3 ) 3, xH 2 O, (CH 3 CO 2 ) 2 Cr, xH 2 O, (CH 3 CO 2 ) 7 Cr 3 (OH) 2 , xH 2 O, Cr 2 (SO 4 ) 3 , xH 2 O, CrK (SO 4 ) 2 , xH 2 O, etc.
- trivalent chromium for example CrF 3 , xH 2 O, CrCl 3 , xH 2 O, Cr (NO 3 ) 3, xH 2 O, (CH 3 CO 2 ) 2 Cr, xH 2 O, (CH
- the trivalent chromium salt present in the bath is a fluoride. This is, for example, chromium trifluoride CrF 3 .
- the immersion step in the aqueous bath meets one or more of the following operating parameters:
- the temperature of the bath is between 20 and 80 ° C, preferably between 20 and 60 ° C, more preferably between 35 and 60 ° C, and preferably between 35 and 45 ° C, for example equal to 40 ° C;
- the bath pH is between 3 and 4.5, preferably between 3 and 4, for example equal to 3.5;
- the immersion time in the bath is between 5 and 40 min, preferably between 10 and 30 minutes, for example equal to 15 or
- the concentration of trivalent chromium salt in the bath is preferably between 0.5 and 50 g / l.
- the immersion of the piece in water at a temperature between 98 and 100 ° C can be carried out with an immersion time of between 10 and 60 minutes, in accordance with the operating parameters of the so-called traditional hydrothermal sealing processes.
- the sealing step comprises immersing the part successively in the aqueous bath containing a trivalent chromium salt and an oxidizing compound, and in water at a temperature of between 98 and 100 ° C.
- steps can be carried out in any order, and in particular be separated by one or more interleaved water rinses.
- the clogging step may include immersing the workpiece in the aqueous bath containing a trivalent chromium salt and an oxidizing compound, and then, after rinsing (s), in water at a temperature of 98 ° C. at 100 ° C.
- the clogging step may include immersing the piece in water at a temperature of 98 to 100 ° C, and then, after rinsing (s), in the aqueous bath containing a trivalent chromium salt and an oxidizing compound.
- FIGS. 1A to 1E show micrographs of anodic layers formed on the surface of aluminum parts by FIG. 1A, chromic anodizing (OAC), FIG. 1B, standard sulfuric anodization (OASstandard), FIG. 1C, sulfo-tartaric anodization (OAST), FIG. 1D, sulfoboric anodization (OASB) and FIG. 1E, anodizing according to an implementation mode of the invention.
- OAC chromic anodizing
- FIG. 1B standard sulfuric anodization
- FIG. 1C sulfo-tartaric anodization
- FIG. 1D sulfoboric anodization
- FIG. 1E anodizing according to an implementation mode of the invention.
- Parts of 2024 T3 aluminum alloy laminated 120x80x2 mm dimensions are treated by anodization according to the methods below.
- Steps for surface preparation of the part are firstly carried out successively:
- Parts are then subjected to an anodizing process according to an embodiment of the invention, as follows.
- a bath is prepared by diluting a solution of sulfuric acid in water to obtain a sulfuric acid concentration of 200 g / L, excluding any other compound. This bath is heated and maintained at a temperature of 19 ° C.
- the part is immersed in the bath, and it is applied a DC voltage according to the following voltage profile: voltage rise, from an initial value of 0 V, at a speed of 3 V / min, to a value called 16 V tray. The tension is maintained at the plateau value for 16 minutes.
- an anodic oxide / aluminum hydroxide layer with a thickness of approximately 4 to 5 ⁇ is formed.
- FIG. 1 E corresponding to the anode layer obtained by a method according to an embodiment of the invention, shows a homogeneous morphology in the thickness of the layer, with the absence of micro-precipitates from the substrate within the layer. From the micrographic observations, the pore diameters were measured for each of the anode layers and the results are shown in Table 2 below.
- the various anodized parts are subjected to a fatigue test in order to evaluate the fatigue reduction related to the formation of the anodic layer on their surface.
- the parameters of the fatigue test are as follows:
- Parts anodized by the method according to an embodiment of the invention, as indicated above, are subjected to tests adhesion of conventional paint systems.
- Two paint systems are tested: a water-based epoxy system (P60 + F70) and a solvent-based polyurethane system (PAC33 + PU66).
- the tests are carried out according to the ISO 2409 standard, for dry adhesion, after drying of the paint system, and for wet adhesion: after drying of the paint system, the samples are immersed in demineralized water for 14 hours. days and then dried before undergoing the adhesion test according to the standard.
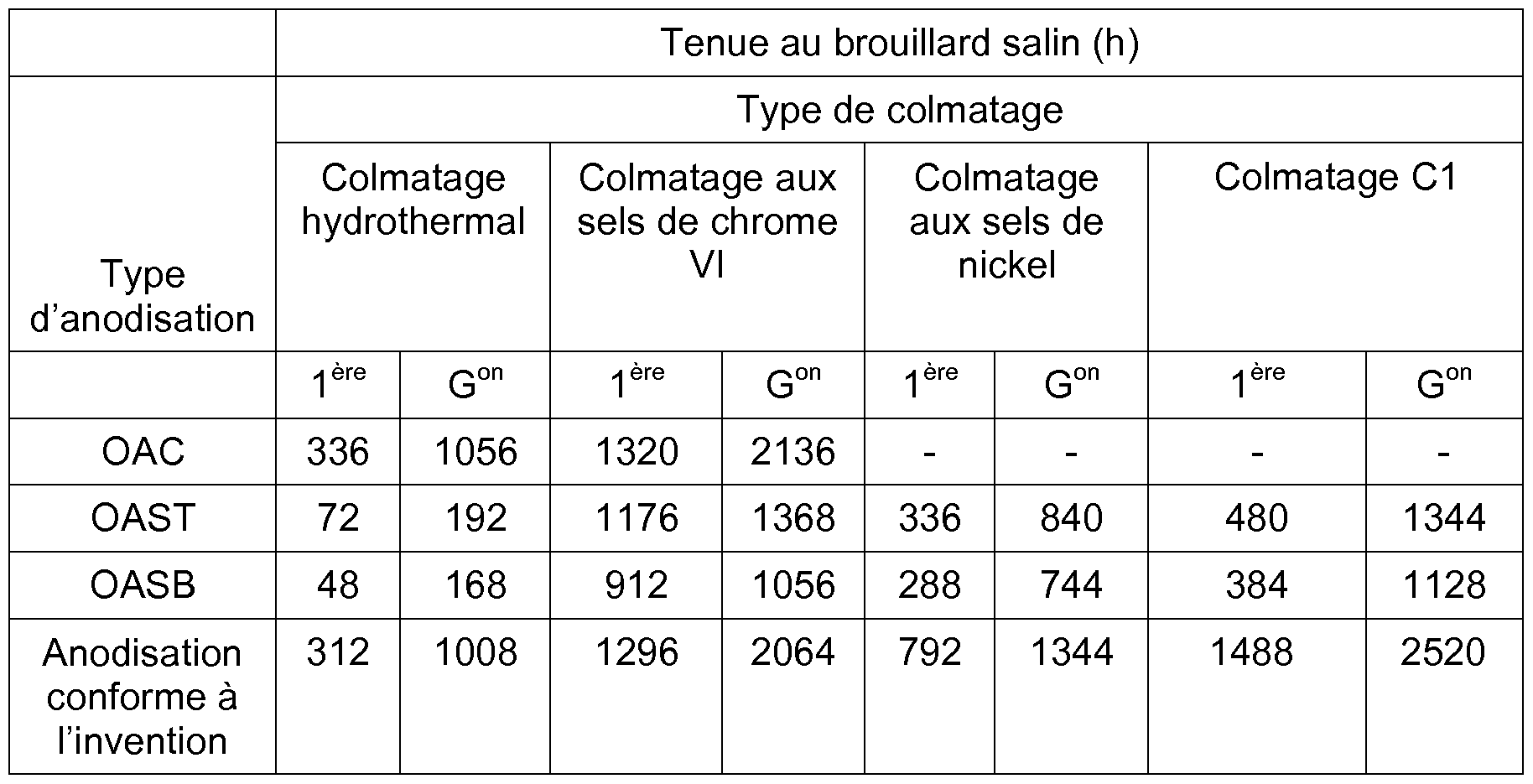
- anodized parts are also subjected to the following conventional sealing methods: hydrothermal clogging, hot clogging with hexavalent chromium salts, hot clogging with nickel salts, according to the operating conditions indicated in Table 6 below. Clogging Clogging at hydrothermal clogging chromium salts nickel salts
- the parts thus treated are subjected to a salt spray test according to the ISO 9227 standard.
- Table 7 Salt spray resistance of 2024 T3 aluminum alloy rolled parts treated by anodizing and clogging, the anodization being carried out by a method according to an embodiment of the invention or by methods of anodizing of the prior art
- Table 8 Salt spray resistance of 2024 T3 aluminum alloy rolled parts treated by anodization and clogging, the anodization being carried out by a method according to an embodiment of the invention or by methods of anodizing of the prior art
- the method of anodizing according to an embodiment of the invention has anticorrosion performance equivalent to chromic anodization (OAC) in combination with hydrothermal sealing or hot clogging with hexavalent chromium salts, and much better than dilute sulfo-tartaric (OAST) or sulfoboric (OASB) anodizations.
- OAC chromic anodization
- ASB sulfoboric
- Aluminum alloy parts similar to those of Example 1, having previously been subjected to surface preparation steps as indicated in Example 1 above, are subjected to an anodizing process according to the invention.
- anodizing process according to the invention.
- the tension is maintained at the plateau value for 16 minutes.
- the anode layer is then sealed by immersing the part in a water bath at a temperature between 98 and 100 ° C for 40 min.
- an anodic layer of aluminum oxide / hydroxide with a thickness of approximately 3.5 to 4.5 ⁇ .
- Aluminum alloy parts similar to those of Example 1, having previously been subjected to surface preparation steps as indicated in Example 1 above, are subjected to an anodizing process according to the invention by immersion in a bath at 19 ° C. containing sulfuric acid at a concentration of 200 g / l, excluding any other compound. It is then applied to each piece a DC voltage according to the following voltage profile: voltage rise, from an initial value of 0 V, to a so-called plateau value of 16 V. The voltage is then maintained at the value of plateau for 16 minutes. Different speeds of rise in voltage are tested: 1 V / min, 20 V / min, 32 V / min.
- the anode layer is then sealed by immersing the part in a water bath at a temperature between 98 and 100 ° C for 40 min.
- anodic oxide / aluminum hydroxide layer with a thickness of approximately 4 to 4.5 ⁇ is formed.
- Aluminum alloy parts similar to those of Example 1, having previously been subjected to surface preparation steps as indicated in Example 1 above, are subjected to an anodizing process according to the invention.
- anodizing process according to the invention.
- the anode layer is then sealed by the clogging method C1 described in Example 1 above.
- anodic oxide / aluminum hydroxide layer approximately 4 to 5 ⁇ thick is formed.
- Aluminum alloy parts similar to those of Example 1, having previously been subjected to surface preparation steps as indicated in Example 1 above, are subjected to an anodizing process according to the invention. by immersion in a bath containing sulfuric acid at a concentration of 200 g / l, to the exclusion of any other compound. Several bath temperatures are tested, more particularly 6 ° C, 12 ° C and 25 ° C.
- the anode layer is then sealed by the clogging method C1 described in Example 1 above.
- anodizing aluminum alloy parts which avoids the use of substances based on hexavalent chromium, while exhibiting performance, in particular in terms of corrosion resistance of the treated part, fatigue reduction and adhesion of paint coatings on the surface of the part, which are at least equivalent to those of chromic anodizing processes, and superior to those sulfuric anodizing processes proposed by the prior art .
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Treatment Of Metals (AREA)
- Other Surface Treatments For Metallic Materials (AREA)
- Application Of Or Painting With Fluid Materials (AREA)
Abstract
Description
Claims
Priority Applications (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| BR112014019652A BR112014019652B8 (pt) | 2012-02-10 | 2013-02-11 | processo de anodização de uma peça de alumínio e processo de tratamento superficial |
| CA2864107A CA2864107C (fr) | 2012-02-10 | 2013-02-11 | Procede d'anodisation de pieces en alliage d'aluminium |
| US14/377,503 US9879355B2 (en) | 2012-02-10 | 2013-02-11 | Method for anodizing parts made of an aluminum alloy |
| ES13703427T ES2711541T3 (es) | 2012-02-10 | 2013-02-11 | Procedimiento de anodización de piezas de aleación de aluminio |
| EP13703427.8A EP2812467B1 (fr) | 2012-02-10 | 2013-02-11 | Procédé d'anodisation de pièces en alliage d'aluminium |
| MX2014009607A MX368584B (es) | 2012-02-10 | 2013-02-11 | Procedimiento de anodización de piezas de aleación de aluminio. |
| TNP2014000339A TN2014000339A1 (fr) | 2012-02-10 | 2014-08-06 | Procédé d'anodisation de pièces en alliage d'aluminium |
| MA37272A MA35901B1 (fr) | 2012-02-10 | 2014-08-07 | Procédé d'anodisation de pièces en alliage d'aluminium |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR1251273 | 2012-02-10 | ||
| FR1251273A FR2986807B1 (fr) | 2012-02-10 | 2012-02-10 | Procede d'anodisation de pieces en alliage d'aluminium |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013117759A1 true WO2013117759A1 (fr) | 2013-08-15 |
Family
ID=47681927
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2013/052686 WO2013117759A1 (fr) | 2012-02-10 | 2013-02-11 | Procédé d'anodisation de pièces en alliage d'aluminium |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US9879355B2 (fr) |
| EP (1) | EP2812467B1 (fr) |
| BR (1) | BR112014019652B8 (fr) |
| CA (1) | CA2864107C (fr) |
| ES (1) | ES2711541T3 (fr) |
| FR (1) | FR2986807B1 (fr) |
| MA (1) | MA35901B1 (fr) |
| MX (1) | MX368584B (fr) |
| TN (1) | TN2014000339A1 (fr) |
| TR (1) | TR201902209T4 (fr) |
| WO (1) | WO2013117759A1 (fr) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103409784A (zh) * | 2013-08-22 | 2013-11-27 | 上海科秉电子科技有限公司 | 一种集成电路制造设备的零部件的阳极膜的制造方法 |
| EP3070190A1 (fr) * | 2015-03-17 | 2016-09-21 | Goodrich Corporation | Anodisation en alliage d'aluminium |
| WO2021152241A1 (fr) | 2020-01-31 | 2021-08-05 | Safran Aircraft Engines | Procede de colmatage des alliages d'aluminium |
| WO2021152240A1 (fr) | 2020-01-31 | 2021-08-05 | Safran Aerosystems | Procede de traitement de surface de pieces a base d'aluminium |
| WO2023094766A1 (fr) | 2021-11-29 | 2023-06-01 | Safran Aerotechnics | Procede de marquage laser contraste de surface de pieces en aluminium ou en alliage d'aluminium anodisees |
| FR3140382A1 (fr) | 2022-10-04 | 2024-04-05 | Safran Landing Systems | Procede de colmatage post-anodisation de l’aluminium et des alliages d’aluminium sans utiliser de chrome |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR3082528B1 (fr) | 2018-06-14 | 2021-02-12 | Liebherr Aerospace Toulouse Sas | Composition aqueuse et procede de traitement de surface d'une piece en alliage d'aluminium mettant en œuvre une telle composition |
| FR3111869A1 (fr) * | 2020-06-29 | 2021-12-31 | Airbus Operations | Rail hybride pour plancher d’aéronef |
| US11945569B2 (en) * | 2021-01-29 | 2024-04-02 | Airbus Operations (S.A.S.) | Hybrid anchor rail for aircraft floor |
| DE102021003140A1 (de) | 2021-06-18 | 2021-08-12 | Daimler Ag | Aluminiumgehäuse |
| US20230235472A1 (en) * | 2022-01-27 | 2023-07-27 | Divergent Technologies, Inc. | Electrocoating (e-coating) on a part by part basis |
| CN114737233B (zh) * | 2022-02-27 | 2024-04-02 | 陕西良鼎瑞金属新材料有限公司 | 一种铝材产品 |
| IT202200018684A1 (it) * | 2022-09-13 | 2024-03-13 | O M P M Officina Meridionale Di Prec Meccanica | Trattamento di ossidazione anodica e conversione chimica di alluminio o leghe di alluminio senza l’utilizzo di cromati |
| DE102022126251A1 (de) | 2022-10-11 | 2024-04-11 | Liebherr-Aerospace Lindenberg Gmbh | Verfahren zur Oberflächenbehandlung |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR1452852A (fr) * | 1965-08-04 | 1966-04-15 | Minnesota De France | Procédé de fabrication d'étiquettes auto-adhésives |
| EP0232211A1 (fr) * | 1986-02-05 | 1987-08-12 | Cegedur Pechiney Rhenalu | Procédé de traitement de surfaces en aluminium destinées à être revêtues d'un film fluocarbone |
| DE4213535C1 (en) * | 1992-04-24 | 1993-09-23 | Deutsche Aerospace Airbus Gmbh, 21129 Hamburg, De | Anodising aluminium@ and magnesium@ surfaces - by constantly increasing current to predetermined max. value and holding at this value so that ratio of charge in 1st stage to 2nd stage is approximately 0.5 |
| US20040050709A1 (en) * | 2002-09-17 | 2004-03-18 | The Boeing Company | Accelerated sulfuric acid and boric sulfuric acid anodize process |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES452499A1 (es) * | 1976-10-05 | 1978-04-01 | Brugarolas Sa | Procedimiento para colmatar peliculas obtenidas por oxida- cion anodica sobre el aluminio y sus aleaciones. |
| US5304257A (en) * | 1993-09-27 | 1994-04-19 | The United States Of America As Represented By The Secretary Of The Navy | Trivalent chromium conversion coatings for aluminum |
| EP1087038A1 (fr) * | 1999-09-23 | 2001-03-28 | Clariant International Ltd. | Procédé pour colorer des couches d'oxyde d'aluminium |
| US6511532B2 (en) * | 2000-10-31 | 2003-01-28 | The United States Of America As Represented By The Secretary Of The Navy | Post-treatment for anodized aluminum |
| FR2838754B1 (fr) * | 2002-04-22 | 2005-03-18 | Messier Bugatti | Procede d'anodisation d'une piece en alliage d'aluminium |
| DE10361888B3 (de) * | 2003-12-23 | 2005-09-22 | Airbus Deutschland Gmbh | Anodisierverfahren für Aluminiumwerkstoffe |
| US20060213589A1 (en) * | 2005-03-23 | 2006-09-28 | Fuji Photo Film Co., Ltd. | Method of manufacturing a support for a lithographic printing plate |
-
2012
- 2012-02-10 FR FR1251273A patent/FR2986807B1/fr active Active
-
2013
- 2013-02-11 ES ES13703427T patent/ES2711541T3/es active Active
- 2013-02-11 EP EP13703427.8A patent/EP2812467B1/fr active Active
- 2013-02-11 US US14/377,503 patent/US9879355B2/en active Active
- 2013-02-11 TR TR2019/02209T patent/TR201902209T4/tr unknown
- 2013-02-11 WO PCT/EP2013/052686 patent/WO2013117759A1/fr active Application Filing
- 2013-02-11 BR BR112014019652A patent/BR112014019652B8/pt active IP Right Grant
- 2013-02-11 MX MX2014009607A patent/MX368584B/es active IP Right Grant
- 2013-02-11 CA CA2864107A patent/CA2864107C/fr active Active
-
2014
- 2014-08-06 TN TNP2014000339A patent/TN2014000339A1/fr unknown
- 2014-08-07 MA MA37272A patent/MA35901B1/fr unknown
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR1452852A (fr) * | 1965-08-04 | 1966-04-15 | Minnesota De France | Procédé de fabrication d'étiquettes auto-adhésives |
| EP0232211A1 (fr) * | 1986-02-05 | 1987-08-12 | Cegedur Pechiney Rhenalu | Procédé de traitement de surfaces en aluminium destinées à être revêtues d'un film fluocarbone |
| DE4213535C1 (en) * | 1992-04-24 | 1993-09-23 | Deutsche Aerospace Airbus Gmbh, 21129 Hamburg, De | Anodising aluminium@ and magnesium@ surfaces - by constantly increasing current to predetermined max. value and holding at this value so that ratio of charge in 1st stage to 2nd stage is approximately 0.5 |
| US20040050709A1 (en) * | 2002-09-17 | 2004-03-18 | The Boeing Company | Accelerated sulfuric acid and boric sulfuric acid anodize process |
Non-Patent Citations (8)
| Title |
|---|
| ANONYMOUS: "AESF/EPA Conference for Environmental & Process Excellence - February 3-6, Adams Mark Resort, Daytona Beach, Fla.", MÉTAL FINISHING, vol. 101, no. 1, January 2003 (2003-01-01), pages 28 - 31,33-40, XP055425382, Retrieved from the Internet <URL:http://www.sciencedirect.com/science/article/pii/S0026057603800077> |
| AUGROS, M. ET AL.: "Innovative Cr-free anodizing & sealing processes for corrosion protection of aerospace aluminum alloys", SURFAIR, 2006, pages 1 - 8, XP055323527 |
| BECK E.: "Performance Validation of Thin-Film Sulfuric Acid Anodization(TFSAA) on Aluminum Alloys", PROCEEDINGS 2003 AESF/EPA CONFÉRENCE FOR ENVIRONMENTAL & PROCESS EXCELLENCE, 3 February 2003 (2003-02-03), pages 339 - 356, XP055323530 |
| BECK, ERIN: "Performance Validation of Thin-Film Sulfuric Acid Anodization (TFSAA) on Aluminum Alloys", AESF/EPA CONFERENCE FOR ENVIRONMENTAL & PROCESS EXCELLENCE,2003, 3 February 2003 (2003-02-03), pages 339 - 356, XP055323530, Retrieved from the Internet <URL:http://www.nmfrc.org/pdf/awk03/aw03o01.pdf> |
| MAJID SHAHZAD: "Influence de la rugosité et des traitements d’anodisationsur la tenue en fatigue des alliages d’aluminium aéronautiques 2214 et 7050", THÈSE EN VUE DE L'OBTENTION DU DOCTORAT DE L’UNIVERSITÉ DE TOULOUSE, 18 March 2011 (2011-03-18), pages 3 - 71, XP055425361, Retrieved from the Internet <URL:https://depozit.isae.fr/theses/2011/2011_Shahzad_Majid.pdf> |
| P.G.SHEASBY, R. PINNER: "The Surface Treatment and Finishing of Aluminium and its Alloys, Sixth Edition, Volume 2", vol. 2, 2001, FINISHING PUBLICATIONS LTD.,, ISBN: 978-0-904477-22-1, article "Ch 12- Properties and Tests of Anodic Oxide Coatings", pages: 1102 - 1111, XP055425372 |
| SAE AEROSPACE: "Anodic Treatment of Aluminum Alloys Sulfuric Acid Process, Undyed Coating", AMS2471G, 18 December 2008 (2008-12-18), XP055323543 |
| See also references of EP2812467A1 |
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103409784A (zh) * | 2013-08-22 | 2013-11-27 | 上海科秉电子科技有限公司 | 一种集成电路制造设备的零部件的阳极膜的制造方法 |
| CN103409784B (zh) * | 2013-08-22 | 2016-01-20 | 上海科秉电子科技有限公司 | 一种集成电路制造设备的零部件的阳极膜的制造方法 |
| EP3070190A1 (fr) * | 2015-03-17 | 2016-09-21 | Goodrich Corporation | Anodisation en alliage d'aluminium |
| US9790613B2 (en) | 2015-03-17 | 2017-10-17 | Goodrich Corporation | Aluminum alloy anodization |
| WO2021152241A1 (fr) | 2020-01-31 | 2021-08-05 | Safran Aircraft Engines | Procede de colmatage des alliages d'aluminium |
| WO2021152240A1 (fr) | 2020-01-31 | 2021-08-05 | Safran Aerosystems | Procede de traitement de surface de pieces a base d'aluminium |
| FR3106837A1 (fr) | 2020-01-31 | 2021-08-06 | Safran Aerosystems | Procede de traitement de surface de pieces a base d’aluminium |
| FR3106838A1 (fr) | 2020-01-31 | 2021-08-06 | Safran Aircraft Engines | Procede de colmatage des alliages d’aluminium |
| WO2023094766A1 (fr) | 2021-11-29 | 2023-06-01 | Safran Aerotechnics | Procede de marquage laser contraste de surface de pieces en aluminium ou en alliage d'aluminium anodisees |
| FR3129617A1 (fr) | 2021-11-29 | 2023-06-02 | Safran Aerotechnics | Procede de marquage laser contraste de surface de pieces en aluminium ou en alliage d’aluminium anodisees |
| FR3140382A1 (fr) | 2022-10-04 | 2024-04-05 | Safran Landing Systems | Procede de colmatage post-anodisation de l’aluminium et des alliages d’aluminium sans utiliser de chrome |
| WO2024074774A1 (fr) | 2022-10-04 | 2024-04-11 | Safran Landing Systems | Procede de colmatage post-anodisation de l'aluminium et des alliages d'aluminium sans utiliser de chrome |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2812467B1 (fr) | 2019-01-02 |
| US9879355B2 (en) | 2018-01-30 |
| ES2711541T3 (es) | 2019-05-06 |
| TR201902209T4 (tr) | 2019-03-21 |
| BR112014019652A8 (pt) | 2017-07-11 |
| CA2864107C (fr) | 2020-12-29 |
| MX2014009607A (es) | 2015-05-20 |
| FR2986807A1 (fr) | 2013-08-16 |
| EP2812467A1 (fr) | 2014-12-17 |
| US20160047057A1 (en) | 2016-02-18 |
| CA2864107A1 (fr) | 2013-08-15 |
| TN2014000339A1 (fr) | 2015-12-21 |
| MA35901B1 (fr) | 2014-12-01 |
| FR2986807B1 (fr) | 2015-05-15 |
| BR112014019652B1 (pt) | 2021-03-30 |
| BR112014019652B8 (pt) | 2021-05-18 |
| MX368584B (es) | 2019-10-08 |
| BR112014019652A2 (fr) | 2017-06-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2812467B1 (fr) | Procédé d'anodisation de pièces en alliage d'aluminium | |
| CA2864109C (fr) | Procede de traitement de surface de pieces en alliage d'aluminium ou de magnesium | |
| EP3810833B1 (fr) | Procédé de traitement de surface d'une pièce en aluminium ou alliage d'aluminium ou en magnésium ou alliage de magnésium | |
| EP4097277A1 (fr) | Procede de traitement de surface de pieces a base d'aluminium | |
| EP4097278A1 (fr) | Procédé de colmatage des alliages d'aluminium | |
| EP1357206A2 (fr) | Procédé d'anodisation d'une pièce en alliage d'aluminium | |
| EP2802682B1 (fr) | Utilisation d'une solution contenant des ions sulfates pour réduire le noircissement ou le ternissement d'une tôle lors de son stockage et tôle traitée par une telle solution | |
| US8470452B2 (en) | Wear resistant ceramic coated aluminum alloy article | |
| FR2822852A1 (fr) | Procede de traitement par carboxylatation de surfaces metalliques | |
| WO2020079358A1 (fr) | Procédé de traitement de surface de pièces en aluminium | |
| CN113423873A (zh) | 用于生产耐腐蚀铝硅合金铸件的方法、该耐腐蚀铝硅合金铸件及其用途 | |
| WO2024074774A1 (fr) | Procede de colmatage post-anodisation de l'aluminium et des alliages d'aluminium sans utiliser de chrome | |
| EP2682502B1 (fr) | Procédé de traitement avec anodisation d'alliages d'aluminium contenant du cuivre | |
| FR3073529A1 (fr) | Procede de traitement de surface d’une piece revetue d’un revetement de cadmium et composition pour la mise en œuvre d’un tel procede | |
| WO2024003504A1 (fr) | Piece en alliage d'aluminium et procede de fabrication associe | |
| WO2024134085A1 (fr) | Pièces comprenant de l'aluminium ou un de ses alliages revêtues d'une couche d'anodisation autolubrifiante, et procédé d'anodisation correspondant | |
| EP4440845A1 (fr) | Procede de marquage laser contraste de surface de pieces en aluminium ou en alliage d'aluminium anodisees | |
| EP3864195A1 (fr) | Composition pour le chromage d'un substrat et procédé de chromage mettant en ?uvre une telle composition | |
| JP2010180474A (ja) | 化成処理品の製造方法 | |
| EP3580374A1 (fr) | Traitement de substrats en alliage ayant des couches oxydées |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13703427 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2013703427 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2864107 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/A/2014/009607 Country of ref document: MX |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112014019652 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 112014019652 Country of ref document: BR Kind code of ref document: A2 Effective date: 20140808 |