WO2013079174A1 - Anti-pd-l1 antibodies and uses thereof - Google Patents
Anti-pd-l1 antibodies and uses thereof Download PDFInfo
- Publication number
- WO2013079174A1 WO2013079174A1 PCT/EP2012/004822 EP2012004822W WO2013079174A1 WO 2013079174 A1 WO2013079174 A1 WO 2013079174A1 EP 2012004822 W EP2012004822 W EP 2012004822W WO 2013079174 A1 WO2013079174 A1 WO 2013079174A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- antibody
- seq
- hvr
- sequence
- human
- Prior art date
Links
- 230000027455 binding Effects 0.000 claims abstract description 116
- 239000000427 antigen Substances 0.000 claims abstract description 112
- 108091007433 antigens Proteins 0.000 claims abstract description 109
- 102000036639 antigens Human genes 0.000 claims abstract description 109
- 210000001744 T-lymphocyte Anatomy 0.000 claims abstract description 104
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 79
- 239000000203 mixture Substances 0.000 claims abstract description 58
- 239000012634 fragment Substances 0.000 claims abstract description 51
- 238000011282 treatment Methods 0.000 claims abstract description 39
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 36
- 150000007523 nucleic acids Chemical class 0.000 claims abstract description 29
- 108020004707 nucleic acids Proteins 0.000 claims abstract description 28
- 102000039446 nucleic acids Human genes 0.000 claims abstract description 28
- 201000011510 cancer Diseases 0.000 claims abstract description 19
- 230000036039 immunity Effects 0.000 claims abstract description 18
- 210000004027 cell Anatomy 0.000 claims description 121
- 241000282414 Homo sapiens Species 0.000 claims description 99
- 102000008096 B7-H1 Antigen Human genes 0.000 claims description 96
- 108010074708 B7-H1 Antigen Proteins 0.000 claims description 96
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 63
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 56
- 238000000034 method Methods 0.000 claims description 48
- 229920001184 polypeptide Polymers 0.000 claims description 45
- 102100040678 Programmed cell death protein 1 Human genes 0.000 claims description 41
- 102000008394 Immunoglobulin Fragments Human genes 0.000 claims description 40
- 108010021625 Immunoglobulin Fragments Proteins 0.000 claims description 40
- 101710089372 Programmed cell death protein 1 Proteins 0.000 claims description 40
- 229910052757 nitrogen Inorganic materials 0.000 claims description 33
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical group ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 claims description 29
- 229960004397 cyclophosphamide Drugs 0.000 claims description 29
- 230000010056 antibody-dependent cellular cytotoxicity Effects 0.000 claims description 26
- 229910052731 fluorine Inorganic materials 0.000 claims description 26
- 208000035475 disorder Diseases 0.000 claims description 23
- 230000003993 interaction Effects 0.000 claims description 19
- 229960005486 vaccine Drugs 0.000 claims description 19
- 108091007491 NSP3 Papain-like protease domains Proteins 0.000 claims description 17
- 150000001413 amino acids Chemical class 0.000 claims description 17
- 101001117317 Homo sapiens Programmed cell death 1 ligand 1 Proteins 0.000 claims description 16
- 229960005277 gemcitabine Drugs 0.000 claims description 16
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 claims description 16
- 229910052727 yttrium Inorganic materials 0.000 claims description 16
- 102000048776 human CD274 Human genes 0.000 claims description 14
- 241000699666 Mus <mouse, genus> Species 0.000 claims description 13
- 229940127089 cytotoxic agent Drugs 0.000 claims description 13
- 241000282567 Macaca fascicularis Species 0.000 claims description 12
- 241001529936 Murinae Species 0.000 claims description 12
- 239000002246 antineoplastic agent Substances 0.000 claims description 12
- 239000003814 drug Substances 0.000 claims description 12
- 101100454807 Caenorhabditis elegans lgg-1 gene Proteins 0.000 claims description 11
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 claims description 11
- 229960002949 fluorouracil Drugs 0.000 claims description 11
- 210000004602 germ cell Anatomy 0.000 claims description 10
- 229960001756 oxaliplatin Drugs 0.000 claims description 10
- DWAFYCQODLXJNR-BNTLRKBRSA-L oxaliplatin Chemical compound O1C(=O)C(=O)O[Pt]11N[C@@H]2CCCC[C@H]2N1 DWAFYCQODLXJNR-BNTLRKBRSA-L 0.000 claims description 10
- 229910052739 hydrogen Inorganic materials 0.000 claims description 9
- 229940124597 therapeutic agent Drugs 0.000 claims description 9
- 230000000903 blocking effect Effects 0.000 claims description 8
- 210000004185 liver Anatomy 0.000 claims description 7
- 238000001959 radiotherapy Methods 0.000 claims description 7
- 229910052720 vanadium Inorganic materials 0.000 claims description 7
- 101100454808 Caenorhabditis elegans lgg-2 gene Proteins 0.000 claims description 6
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims description 6
- 229910052805 deuterium Inorganic materials 0.000 claims description 6
- 230000002496 gastric effect Effects 0.000 claims description 6
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims description 6
- 201000001441 melanoma Diseases 0.000 claims description 6
- 201000002528 pancreatic cancer Diseases 0.000 claims description 6
- 208000008443 pancreatic carcinoma Diseases 0.000 claims description 6
- 101100217502 Caenorhabditis elegans lgg-3 gene Proteins 0.000 claims description 5
- 201000009030 Carcinoma Diseases 0.000 claims description 5
- 230000002708 enhancing effect Effects 0.000 claims description 5
- 210000004072 lung Anatomy 0.000 claims description 5
- 210000001685 thyroid gland Anatomy 0.000 claims description 5
- 229910052721 tungsten Inorganic materials 0.000 claims description 5
- 206010018338 Glioma Diseases 0.000 claims description 4
- 208000005718 Stomach Neoplasms Diseases 0.000 claims description 4
- 208000000728 Thymus Neoplasms Diseases 0.000 claims description 4
- 208000024770 Thyroid neoplasm Diseases 0.000 claims description 4
- 210000000481 breast Anatomy 0.000 claims description 4
- 210000001072 colon Anatomy 0.000 claims description 4
- 206010017758 gastric cancer Diseases 0.000 claims description 4
- 201000010536 head and neck cancer Diseases 0.000 claims description 4
- 208000014829 head and neck neoplasm Diseases 0.000 claims description 4
- 210000003734 kidney Anatomy 0.000 claims description 4
- 230000002611 ovarian Effects 0.000 claims description 4
- 210000002784 stomach Anatomy 0.000 claims description 4
- 201000011549 stomach cancer Diseases 0.000 claims description 4
- 230000002992 thymic effect Effects 0.000 claims description 4
- 238000011269 treatment regimen Methods 0.000 claims description 4
- 210000003932 urinary bladder Anatomy 0.000 claims description 4
- 238000002512 chemotherapy Methods 0.000 claims description 3
- 239000003937 drug carrier Substances 0.000 claims description 3
- 238000009169 immunotherapy Methods 0.000 claims description 3
- 230000008569 process Effects 0.000 claims description 3
- 229940021747 therapeutic vaccine Drugs 0.000 claims description 3
- 241000699802 Cricetulus griseus Species 0.000 claims description 2
- 241000588724 Escherichia coli Species 0.000 claims description 2
- 230000033115 angiogenesis Effects 0.000 claims description 2
- 238000012258 culturing Methods 0.000 claims description 2
- 238000001794 hormone therapy Methods 0.000 claims description 2
- 210000001672 ovary Anatomy 0.000 claims description 2
- 238000002638 palliative care Methods 0.000 claims description 2
- 238000001356 surgical procedure Methods 0.000 claims description 2
- 238000002626 targeted therapy Methods 0.000 claims description 2
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims 1
- 230000001225 therapeutic effect Effects 0.000 abstract description 7
- 230000021633 leukocyte mediated immunity Effects 0.000 abstract description 3
- 108090000623 proteins and genes Proteins 0.000 description 41
- 235000018102 proteins Nutrition 0.000 description 38
- 102000004169 proteins and genes Human genes 0.000 description 38
- 238000009472 formulation Methods 0.000 description 33
- 230000001965 increasing effect Effects 0.000 description 32
- 241000699670 Mus sp. Species 0.000 description 27
- 230000000694 effects Effects 0.000 description 26
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 description 23
- 102100027222 T-lymphocyte activation antigen CD80 Human genes 0.000 description 23
- 238000003556 assay Methods 0.000 description 21
- 108060003951 Immunoglobulin Proteins 0.000 description 20
- 102000018358 immunoglobulin Human genes 0.000 description 20
- 210000003719 b-lymphocyte Anatomy 0.000 description 19
- 239000012636 effector Substances 0.000 description 19
- 230000006870 function Effects 0.000 description 19
- 102000005962 receptors Human genes 0.000 description 18
- 108020003175 receptors Proteins 0.000 description 18
- 108010087819 Fc receptors Proteins 0.000 description 17
- 102000009109 Fc receptors Human genes 0.000 description 17
- 235000001014 amino acid Nutrition 0.000 description 17
- 102200076656 rs34991226 Human genes 0.000 description 17
- 102220316120 rs763702846 Human genes 0.000 description 17
- 230000011664 signaling Effects 0.000 description 16
- 229940024606 amino acid Drugs 0.000 description 15
- 230000005764 inhibitory process Effects 0.000 description 15
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 14
- -1 IFNa Proteins 0.000 description 14
- 210000004698 lymphocyte Anatomy 0.000 description 14
- 230000004044 response Effects 0.000 description 14
- 102100024213 Programmed cell death 1 ligand 2 Human genes 0.000 description 13
- 230000004913 activation Effects 0.000 description 13
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 13
- 101100407308 Mus musculus Pdcd1lg2 gene Proteins 0.000 description 12
- 108700030875 Programmed Cell Death 1 Ligand 2 Proteins 0.000 description 12
- 125000003275 alpha amino acid group Chemical group 0.000 description 12
- 230000000259 anti-tumor effect Effects 0.000 description 12
- 201000010099 disease Diseases 0.000 description 12
- 238000004519 manufacturing process Methods 0.000 description 12
- 238000010494 dissociation reaction Methods 0.000 description 11
- 230000005593 dissociations Effects 0.000 description 11
- 241001465754 Metazoa Species 0.000 description 10
- 230000000875 corresponding effect Effects 0.000 description 10
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 10
- 238000002347 injection Methods 0.000 description 10
- 239000007924 injection Substances 0.000 description 10
- 210000002540 macrophage Anatomy 0.000 description 10
- 239000002953 phosphate buffered saline Substances 0.000 description 10
- 238000002560 therapeutic procedure Methods 0.000 description 10
- 210000004881 tumor cell Anatomy 0.000 description 10
- 206010009944 Colon cancer Diseases 0.000 description 9
- 108091008874 T cell receptors Proteins 0.000 description 9
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 9
- 125000000539 amino acid group Chemical group 0.000 description 9
- 239000000872 buffer Substances 0.000 description 9
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 9
- 230000000284 resting effect Effects 0.000 description 9
- 235000000346 sugar Nutrition 0.000 description 9
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 8
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 8
- 108010074328 Interferon-gamma Proteins 0.000 description 8
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 8
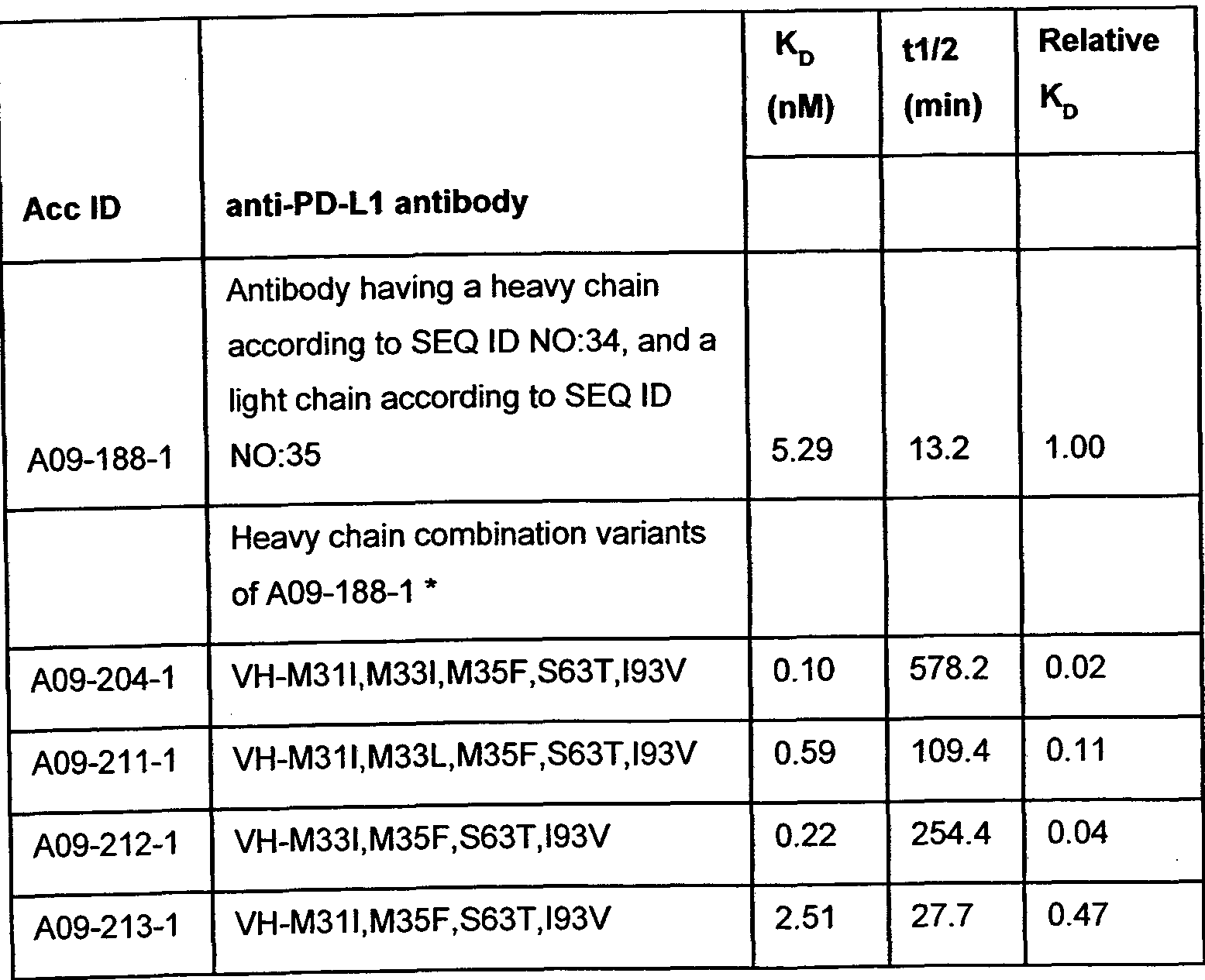
- 102220579302 Transthyretin_M33I_mutation Human genes 0.000 description 8
- 210000000612 antigen-presenting cell Anatomy 0.000 description 8
- 230000003247 decreasing effect Effects 0.000 description 8
- 231100000673 dose–response relationship Toxicity 0.000 description 8
- 230000002401 inhibitory effect Effects 0.000 description 8
- 239000003446 ligand Substances 0.000 description 8
- 244000052769 pathogen Species 0.000 description 8
- 230000001717 pathogenic effect Effects 0.000 description 8
- 150000003839 salts Chemical class 0.000 description 8
- 150000008163 sugars Chemical class 0.000 description 8
- YXTKHLHCVFUPPT-YYFJYKOTSA-N (2s)-2-[[4-[(2-amino-5-formyl-4-oxo-1,6,7,8-tetrahydropteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid;(1r,2r)-1,2-dimethanidylcyclohexane;5-fluoro-1h-pyrimidine-2,4-dione;oxalic acid;platinum(2+) Chemical compound [Pt+2].OC(=O)C(O)=O.[CH2-][C@@H]1CCCC[C@H]1[CH2-].FC1=CNC(=O)NC1=O.C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 YXTKHLHCVFUPPT-YYFJYKOTSA-N 0.000 description 7
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 7
- 239000002253 acid Substances 0.000 description 7
- 230000001363 autoimmune Effects 0.000 description 7
- 210000001185 bone marrow Anatomy 0.000 description 7
- 239000000306 component Substances 0.000 description 7
- 210000004443 dendritic cell Anatomy 0.000 description 7
- 238000011161 development Methods 0.000 description 7
- 230000002163 immunogen Effects 0.000 description 7
- 238000013394 immunophenotyping Methods 0.000 description 7
- 238000000746 purification Methods 0.000 description 7
- 238000003860 storage Methods 0.000 description 7
- 239000000126 substance Substances 0.000 description 7
- 150000005846 sugar alcohols Chemical class 0.000 description 7
- 238000012360 testing method Methods 0.000 description 7
- 108010021064 CTLA-4 Antigen Proteins 0.000 description 6
- 102000008203 CTLA-4 Antigen Human genes 0.000 description 6
- 229940045513 CTLA4 antagonist Drugs 0.000 description 6
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 6
- 101001133056 Homo sapiens Mucin-1 Proteins 0.000 description 6
- 102100037850 Interferon gamma Human genes 0.000 description 6
- 102100034256 Mucin-1 Human genes 0.000 description 6
- 102220485514 Rhodopsin_M33L_mutation Human genes 0.000 description 6
- 230000005867 T cell response Effects 0.000 description 6
- 210000004369 blood Anatomy 0.000 description 6
- 239000008280 blood Substances 0.000 description 6
- 150000001875 compounds Chemical class 0.000 description 6
- 230000016396 cytokine production Effects 0.000 description 6
- 230000001472 cytotoxic effect Effects 0.000 description 6
- 238000005516 engineering process Methods 0.000 description 6
- 230000001900 immune effect Effects 0.000 description 6
- 231100000252 nontoxic Toxicity 0.000 description 6
- 230000003000 nontoxic effect Effects 0.000 description 6
- 239000000047 product Substances 0.000 description 6
- 230000002829 reductive effect Effects 0.000 description 6
- 238000009097 single-agent therapy Methods 0.000 description 6
- 230000003393 splenic effect Effects 0.000 description 6
- 230000004936 stimulating effect Effects 0.000 description 6
- 230000000638 stimulation Effects 0.000 description 6
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 6
- 102100033400 4F2 cell-surface antigen heavy chain Human genes 0.000 description 5
- 102000004127 Cytokines Human genes 0.000 description 5
- 108090000695 Cytokines Proteins 0.000 description 5
- 108020004414 DNA Proteins 0.000 description 5
- 101000800023 Homo sapiens 4F2 cell-surface antigen heavy chain Proteins 0.000 description 5
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 5
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 5
- 241000283973 Oryctolagus cuniculus Species 0.000 description 5
- 241000700159 Rattus Species 0.000 description 5
- 108010003723 Single-Domain Antibodies Proteins 0.000 description 5
- 238000004458 analytical method Methods 0.000 description 5
- 239000012148 binding buffer Substances 0.000 description 5
- 238000006073 displacement reaction Methods 0.000 description 5
- 210000002443 helper t lymphocyte Anatomy 0.000 description 5
- 210000004408 hybridoma Anatomy 0.000 description 5
- 210000000987 immune system Anatomy 0.000 description 5
- 229940072221 immunoglobulins Drugs 0.000 description 5
- 208000015181 infectious disease Diseases 0.000 description 5
- 239000003112 inhibitor Substances 0.000 description 5
- 238000003780 insertion Methods 0.000 description 5
- 230000037431 insertion Effects 0.000 description 5
- 230000004073 interleukin-2 production Effects 0.000 description 5
- 230000015654 memory Effects 0.000 description 5
- 210000000056 organ Anatomy 0.000 description 5
- 239000003755 preservative agent Substances 0.000 description 5
- 238000002203 pretreatment Methods 0.000 description 5
- 230000035755 proliferation Effects 0.000 description 5
- 206010039073 rheumatoid arthritis Diseases 0.000 description 5
- 238000013207 serial dilution Methods 0.000 description 5
- 210000000952 spleen Anatomy 0.000 description 5
- 238000006467 substitution reaction Methods 0.000 description 5
- 230000004083 survival effect Effects 0.000 description 5
- 230000004614 tumor growth Effects 0.000 description 5
- 230000003442 weekly effect Effects 0.000 description 5
- FPQQSJJWHUJYPU-UHFFFAOYSA-N 3-(dimethylamino)propyliminomethylidene-ethylazanium;chloride Chemical compound Cl.CCN=C=NCCCN(C)C FPQQSJJWHUJYPU-UHFFFAOYSA-N 0.000 description 4
- 241000282472 Canis lupus familiaris Species 0.000 description 4
- 238000002965 ELISA Methods 0.000 description 4
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 4
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 4
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 4
- 239000004472 Lysine Substances 0.000 description 4
- 108700018351 Major Histocompatibility Complex Proteins 0.000 description 4
- 241000124008 Mammalia Species 0.000 description 4
- 108010021466 Mutant Proteins Proteins 0.000 description 4
- 102000008300 Mutant Proteins Human genes 0.000 description 4
- 108091028043 Nucleic acid sequence Proteins 0.000 description 4
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 4
- 230000006044 T cell activation Effects 0.000 description 4
- 230000006052 T cell proliferation Effects 0.000 description 4
- 150000007513 acids Chemical class 0.000 description 4
- 230000003213 activating effect Effects 0.000 description 4
- 239000002671 adjuvant Substances 0.000 description 4
- 230000009824 affinity maturation Effects 0.000 description 4
- 239000005557 antagonist Substances 0.000 description 4
- 239000012131 assay buffer Substances 0.000 description 4
- 239000011324 bead Substances 0.000 description 4
- 230000004071 biological effect Effects 0.000 description 4
- 210000004899 c-terminal region Anatomy 0.000 description 4
- RYYVLZVUVIJVGH-UHFFFAOYSA-N caffeine Chemical compound CN1C(=O)N(C)C(=O)C2=C1N=CN2C RYYVLZVUVIJVGH-UHFFFAOYSA-N 0.000 description 4
- 238000002648 combination therapy Methods 0.000 description 4
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 4
- 230000001086 cytosolic effect Effects 0.000 description 4
- 231100000433 cytotoxic Toxicity 0.000 description 4
- 239000003085 diluting agent Substances 0.000 description 4
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 4
- 230000006872 improvement Effects 0.000 description 4
- 238000001727 in vivo Methods 0.000 description 4
- 238000011081 inoculation Methods 0.000 description 4
- 125000005647 linker group Chemical group 0.000 description 4
- 235000018977 lysine Nutrition 0.000 description 4
- 230000010534 mechanism of action Effects 0.000 description 4
- 210000001616 monocyte Anatomy 0.000 description 4
- 230000035772 mutation Effects 0.000 description 4
- 230000003204 osmotic effect Effects 0.000 description 4
- 230000037361 pathway Effects 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- RXWNCPJZOCPEPQ-NVWDDTSBSA-N puromycin Chemical compound C1=CC(OC)=CC=C1C[C@H](N)C(=O)N[C@H]1[C@@H](O)[C@H](N2C3=NC=NC(=C3N=C2)N(C)C)O[C@@H]1CO RXWNCPJZOCPEPQ-NVWDDTSBSA-N 0.000 description 4
- 230000002285 radioactive effect Effects 0.000 description 4
- 230000004043 responsiveness Effects 0.000 description 4
- 102220309994 rs1554198179 Human genes 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- 238000007920 subcutaneous administration Methods 0.000 description 4
- 208000024891 symptom Diseases 0.000 description 4
- 210000001541 thymus gland Anatomy 0.000 description 4
- WYWHKKSPHMUBEB-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 4
- 230000003612 virological effect Effects 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- 208000023275 Autoimmune disease Diseases 0.000 description 3
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 3
- 108091026890 Coding region Proteins 0.000 description 3
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 3
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 3
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 3
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- 101100346929 Homo sapiens MUC1 gene Proteins 0.000 description 3
- 102100026120 IgG receptor FcRn large subunit p51 Human genes 0.000 description 3
- 101710177940 IgG receptor FcRn large subunit p51 Proteins 0.000 description 3
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 description 3
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 description 3
- 108010002350 Interleukin-2 Proteins 0.000 description 3
- 102000000588 Interleukin-2 Human genes 0.000 description 3
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 3
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 3
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 3
- 229930195725 Mannitol Natural products 0.000 description 3
- 101001117316 Mus musculus Programmed cell death 1 ligand 1 Proteins 0.000 description 3
- 239000002202 Polyethylene glycol Substances 0.000 description 3
- 229920001213 Polysorbate 20 Polymers 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 239000004365 Protease Substances 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 3
- 230000001154 acute effect Effects 0.000 description 3
- 235000004279 alanine Nutrition 0.000 description 3
- 230000001580 bacterial effect Effects 0.000 description 3
- HXCHCVDVKSCDHU-LULTVBGHSA-N calicheamicin Chemical compound C1[C@H](OC)[C@@H](NCC)CO[C@H]1O[C@H]1[C@H](O[C@@H]2C\3=C(NC(=O)OC)C(=O)C[C@](C/3=C/CSSSC)(O)C#C\C=C/C#C2)O[C@H](C)[C@@H](NO[C@@H]2O[C@H](C)[C@@H](SC(=O)C=3C(=C(OC)C(O[C@H]4[C@@H]([C@H](OC)[C@@H](O)[C@H](C)O4)O)=C(I)C=3C)OC)[C@@H](O)C2)[C@@H]1O HXCHCVDVKSCDHU-LULTVBGHSA-N 0.000 description 3
- 229930195731 calicheamicin Natural products 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- 230000004663 cell proliferation Effects 0.000 description 3
- 229960005395 cetuximab Drugs 0.000 description 3
- 239000008358 core component Substances 0.000 description 3
- 230000004940 costimulation Effects 0.000 description 3
- 235000018417 cysteine Nutrition 0.000 description 3
- 230000001419 dependent effect Effects 0.000 description 3
- 230000001687 destabilization Effects 0.000 description 3
- 229960004679 doxorubicin Drugs 0.000 description 3
- 239000003623 enhancer Substances 0.000 description 3
- 238000003114 enzyme-linked immunosorbent spot assay Methods 0.000 description 3
- 210000003527 eukaryotic cell Anatomy 0.000 description 3
- 235000011187 glycerol Nutrition 0.000 description 3
- 229940093915 gynecological organic acid Drugs 0.000 description 3
- 230000036541 health Effects 0.000 description 3
- 102000057860 human MUC1 Human genes 0.000 description 3
- 229910052588 hydroxylapatite Inorganic materials 0.000 description 3
- 230000028993 immune response Effects 0.000 description 3
- 230000005847 immunogenicity Effects 0.000 description 3
- 230000006698 induction Effects 0.000 description 3
- 238000001990 intravenous administration Methods 0.000 description 3
- FZWBNHMXJMCXLU-BLAUPYHCSA-N isomaltotriose Chemical class O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O)O1 FZWBNHMXJMCXLU-BLAUPYHCSA-N 0.000 description 3
- 208000032839 leukemia Diseases 0.000 description 3
- 210000000265 leukocyte Anatomy 0.000 description 3
- 239000012516 mab select resin Substances 0.000 description 3
- 239000000594 mannitol Substances 0.000 description 3
- 235000010355 mannitol Nutrition 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 3
- 229960000485 methotrexate Drugs 0.000 description 3
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 3
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 150000007524 organic acids Chemical class 0.000 description 3
- 235000005985 organic acids Nutrition 0.000 description 3
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 description 3
- 229920001223 polyethylene glycol Polymers 0.000 description 3
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 3
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 3
- 230000002335 preservative effect Effects 0.000 description 3
- 238000000159 protein binding assay Methods 0.000 description 3
- 238000003127 radioimmunoassay Methods 0.000 description 3
- 230000001105 regulatory effect Effects 0.000 description 3
- 230000003248 secreting effect Effects 0.000 description 3
- 230000028327 secretion Effects 0.000 description 3
- 238000001542 size-exclusion chromatography Methods 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 241000894007 species Species 0.000 description 3
- 239000006228 supernatant Substances 0.000 description 3
- 230000020382 suppression by virus of host antigen processing and presentation of peptide antigen via MHC class I Effects 0.000 description 3
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 3
- 239000003053 toxin Substances 0.000 description 3
- 231100000765 toxin Toxicity 0.000 description 3
- 108700012359 toxins Proteins 0.000 description 3
- 210000003171 tumor-infiltrating lymphocyte Anatomy 0.000 description 3
- 208000035408 type 1 diabetes mellitus 1 Diseases 0.000 description 3
- 238000002255 vaccination Methods 0.000 description 3
- JVJGCCBAOOWGEO-RUTPOYCXSA-N (2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-4-amino-2-[[(2s,3s)-2-[[(2s,3s)-2-[[(2s)-2-azaniumyl-3-hydroxypropanoyl]amino]-3-methylpentanoyl]amino]-3-methylpentanoyl]amino]-4-oxobutanoyl]amino]-3-phenylpropanoyl]amino]-4-carboxylatobutanoyl]amino]-6-azaniumy Chemical compound OC[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O)CC1=CC=CC=C1 JVJGCCBAOOWGEO-RUTPOYCXSA-N 0.000 description 2
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 2
- PVXPPJIGRGXGCY-TZLCEDOOSA-N 6-O-alpha-D-glucopyranosyl-D-fructofuranose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)C(O)(CO)O1 PVXPPJIGRGXGCY-TZLCEDOOSA-N 0.000 description 2
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 2
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 2
- 208000002267 Anti-neutrophil cytoplasmic antibody-associated vasculitis Diseases 0.000 description 2
- 239000004475 Arginine Substances 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 2
- 208000023328 Basedow disease Diseases 0.000 description 2
- KWIUHFFTVRNATP-UHFFFAOYSA-N Betaine Natural products C[N+](C)(C)CC([O-])=O KWIUHFFTVRNATP-UHFFFAOYSA-N 0.000 description 2
- 238000011740 C57BL/6 mouse Methods 0.000 description 2
- 241000282693 Cercopithecidae Species 0.000 description 2
- 206010009900 Colitis ulcerative Diseases 0.000 description 2
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 2
- RGSFGYAAUTVSQA-UHFFFAOYSA-N Cyclopentane Chemical compound C1CCCC1 RGSFGYAAUTVSQA-UHFFFAOYSA-N 0.000 description 2
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- 108010092160 Dactinomycin Proteins 0.000 description 2
- XBPCUCUWBYBCDP-UHFFFAOYSA-N Dicyclohexylamine Chemical compound C1CCCCC1NC1CCCCC1 XBPCUCUWBYBCDP-UHFFFAOYSA-N 0.000 description 2
- 206010061818 Disease progression Diseases 0.000 description 2
- 102000001301 EGF receptor Human genes 0.000 description 2
- 108060006698 EGF receptor Proteins 0.000 description 2
- 238000011510 Elispot assay Methods 0.000 description 2
- 108700024394 Exon Proteins 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- 206010018364 Glomerulonephritis Diseases 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- 102000003886 Glycoproteins Human genes 0.000 description 2
- 108090000288 Glycoproteins Proteins 0.000 description 2
- 208000015023 Graves' disease Diseases 0.000 description 2
- XLYOFNOQVPJJNP-ZSJDYOACSA-N Heavy water Chemical compound [2H]O[2H] XLYOFNOQVPJJNP-ZSJDYOACSA-N 0.000 description 2
- 108010027412 Histocompatibility Antigens Class II Proteins 0.000 description 2
- 102000018713 Histocompatibility Antigens Class II Human genes 0.000 description 2
- 101000840258 Homo sapiens Immunoglobulin J chain Proteins 0.000 description 2
- 101001002657 Homo sapiens Interleukin-2 Proteins 0.000 description 2
- 101000611936 Homo sapiens Programmed cell death protein 1 Proteins 0.000 description 2
- 241000725303 Human immunodeficiency virus Species 0.000 description 2
- 206010020751 Hypersensitivity Diseases 0.000 description 2
- 108010073807 IgG Receptors Proteins 0.000 description 2
- 102000009490 IgG Receptors Human genes 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- 102000009786 Immunoglobulin Constant Regions Human genes 0.000 description 2
- 108010009817 Immunoglobulin Constant Regions Proteins 0.000 description 2
- 102100029571 Immunoglobulin J chain Human genes 0.000 description 2
- 102000008070 Interferon-gamma Human genes 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- LPHGQDQBBGAPDZ-UHFFFAOYSA-N Isocaffeine Natural products CN1C(=O)N(C)C(=O)C2=C1N(C)C=N2 LPHGQDQBBGAPDZ-UHFFFAOYSA-N 0.000 description 2
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 2
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 2
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 2
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 2
- 239000005517 L01XE01 - Imatinib Substances 0.000 description 2
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 2
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 2
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 2
- NBGXQZRRLOGAJF-UHFFFAOYSA-N Maltulose Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)(CO)OCC1O NBGXQZRRLOGAJF-UHFFFAOYSA-N 0.000 description 2
- 102000018697 Membrane Proteins Human genes 0.000 description 2
- 108010052285 Membrane Proteins Proteins 0.000 description 2
- BAVYZALUXZFZLV-UHFFFAOYSA-N Methylamine Chemical compound NC BAVYZALUXZFZLV-UHFFFAOYSA-N 0.000 description 2
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 2
- 101000686985 Mouse mammary tumor virus (strain C3H) Protein PR73 Proteins 0.000 description 2
- 241000699660 Mus musculus Species 0.000 description 2
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 2
- KWIUHFFTVRNATP-UHFFFAOYSA-O N,N,N-trimethylglycinium Chemical compound C[N+](C)(C)CC(O)=O KWIUHFFTVRNATP-UHFFFAOYSA-O 0.000 description 2
- NQTADLQHYWFPDB-UHFFFAOYSA-N N-Hydroxysuccinimide Chemical compound ON1C(=O)CCC1=O NQTADLQHYWFPDB-UHFFFAOYSA-N 0.000 description 2
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 2
- 229930012538 Paclitaxel Natural products 0.000 description 2
- 108091005804 Peptidases Proteins 0.000 description 2
- 208000031845 Pernicious anaemia Diseases 0.000 description 2
- 208000037581 Persistent Infection Diseases 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Natural products OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 102100032543 Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN Human genes 0.000 description 2
- 101710132081 Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN Proteins 0.000 description 2
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 2
- 102100024216 Programmed cell death 1 ligand 1 Human genes 0.000 description 2
- 206010060862 Prostate cancer Diseases 0.000 description 2
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 2
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 2
- IIDJRNMFWXDHID-UHFFFAOYSA-N Risedronic acid Chemical compound OP(=O)(O)C(P(O)(O)=O)(O)CC1=CC=CN=C1 IIDJRNMFWXDHID-UHFFFAOYSA-N 0.000 description 2
- 208000021386 Sjogren Syndrome Diseases 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 2
- 230000024932 T cell mediated immunity Effects 0.000 description 2
- 210000000662 T-lymphocyte subset Anatomy 0.000 description 2
- CYQFCXCEBYINGO-UHFFFAOYSA-N THC Natural products C1=C(C)CCC2C(C)(C)OC3=CC(CCCCC)=CC(O)=C3C21 CYQFCXCEBYINGO-UHFFFAOYSA-N 0.000 description 2
- FOCVUCIESVLUNU-UHFFFAOYSA-N Thiotepa Chemical compound C1CN1P(N1CC1)(=S)N1CC1 FOCVUCIESVLUNU-UHFFFAOYSA-N 0.000 description 2
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 2
- 102100040247 Tumor necrosis factor Human genes 0.000 description 2
- 201000006704 Ulcerative Colitis Diseases 0.000 description 2
- 208000024780 Urticaria Diseases 0.000 description 2
- 206010047115 Vasculitis Diseases 0.000 description 2
- 241000700605 Viruses Species 0.000 description 2
- 238000009825 accumulation Methods 0.000 description 2
- RJURFGZVJUQBHK-UHFFFAOYSA-N actinomycin D Natural products CC1OC(=O)C(C(C)C)N(C)C(=O)CN(C)C(=O)C2CCCN2C(=O)C(C(C)C)NC(=O)C1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)NC4C(=O)NC(C(N5CCCC5C(=O)N(C)CC(=O)N(C)C(C(C)C)C(=O)OC4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-UHFFFAOYSA-N 0.000 description 2
- 239000000556 agonist Substances 0.000 description 2
- 125000000217 alkyl group Chemical group 0.000 description 2
- 208000026935 allergic disease Diseases 0.000 description 2
- 230000007815 allergy Effects 0.000 description 2
- 230000004075 alteration Effects 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 229940088710 antibiotic agent Drugs 0.000 description 2
- 230000000890 antigenic effect Effects 0.000 description 2
- 230000007503 antigenic stimulation Effects 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 2
- 235000009697 arginine Nutrition 0.000 description 2
- 235000009582 asparagine Nutrition 0.000 description 2
- 229960001230 asparagine Drugs 0.000 description 2
- 208000006673 asthma Diseases 0.000 description 2
- 230000002238 attenuated effect Effects 0.000 description 2
- 230000005784 autoimmunity Effects 0.000 description 2
- 239000008228 bacteriostatic water for injection Substances 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid group Chemical group C(C1=CC=CC=C1)(=O)O WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- QZPQTZZNNJUOLS-UHFFFAOYSA-N beta-lapachone Chemical compound C12=CC=CC=C2C(=O)C(=O)C2=C1OC(C)(C)CC2 QZPQTZZNNJUOLS-UHFFFAOYSA-N 0.000 description 2
- GUBGYTABKSRVRQ-QUYVBRFLSA-N beta-maltose Chemical compound OC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O GUBGYTABKSRVRQ-QUYVBRFLSA-N 0.000 description 2
- 229960003237 betaine Drugs 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 239000008366 buffered solution Substances 0.000 description 2
- VJEONQKOZGKCAK-UHFFFAOYSA-N caffeine Natural products CN1C(=O)N(C)C(=O)C2=C1C=CN2C VJEONQKOZGKCAK-UHFFFAOYSA-N 0.000 description 2
- 229960001948 caffeine Drugs 0.000 description 2
- 238000009566 cancer vaccine Methods 0.000 description 2
- 229940022399 cancer vaccine Drugs 0.000 description 2
- 150000001720 carbohydrates Chemical class 0.000 description 2
- 235000014633 carbohydrates Nutrition 0.000 description 2
- YCIMNLLNPGFGHC-UHFFFAOYSA-N catechol Chemical compound OC1=CC=CC=C1O YCIMNLLNPGFGHC-UHFFFAOYSA-N 0.000 description 2
- 238000000423 cell based assay Methods 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 238000012512 characterization method Methods 0.000 description 2
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 2
- 229960001231 choline Drugs 0.000 description 2
- 238000011210 chromatographic step Methods 0.000 description 2
- 230000001684 chronic effect Effects 0.000 description 2
- 238000011284 combination treatment Methods 0.000 description 2
- 239000000356 contaminant Substances 0.000 description 2
- 230000009260 cross reactivity Effects 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- 230000009089 cytolysis Effects 0.000 description 2
- OPTASPLRGRRNAP-UHFFFAOYSA-N cytosine Chemical compound NC=1C=CNC(=O)N=1 OPTASPLRGRRNAP-UHFFFAOYSA-N 0.000 description 2
- 231100000599 cytotoxic agent Toxicity 0.000 description 2
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- CYQFCXCEBYINGO-IAGOWNOFSA-N delta1-THC Chemical compound C1=C(C)CC[C@H]2C(C)(C)OC3=CC(CCCCC)=CC(O)=C3[C@@H]21 CYQFCXCEBYINGO-IAGOWNOFSA-N 0.000 description 2
- 238000004925 denaturation Methods 0.000 description 2
- 230000036425 denaturation Effects 0.000 description 2
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 2
- 230000029087 digestion Effects 0.000 description 2
- 150000002016 disaccharides Chemical class 0.000 description 2
- 230000005750 disease progression Effects 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 238000012377 drug delivery Methods 0.000 description 2
- 230000004064 dysfunction Effects 0.000 description 2
- 238000010828 elution Methods 0.000 description 2
- 230000005284 excitation Effects 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- OVBPIULPVIDEAO-LBPRGKRZSA-N folic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-LBPRGKRZSA-N 0.000 description 2
- 238000011404 fractionated radiotherapy Methods 0.000 description 2
- 238000004108 freeze drying Methods 0.000 description 2
- CHPZKNULDCNCBW-UHFFFAOYSA-N gallium nitrate Chemical compound [Ga+3].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O CHPZKNULDCNCBW-UHFFFAOYSA-N 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 229930182470 glycoside Natural products 0.000 description 2
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 2
- 210000003630 histaminocyte Anatomy 0.000 description 2
- 229960001101 ifosfamide Drugs 0.000 description 2
- HOMGKSMUEGBAAB-UHFFFAOYSA-N ifosfamide Chemical compound ClCCNP1(=O)OCCCN1CCCl HOMGKSMUEGBAAB-UHFFFAOYSA-N 0.000 description 2
- KTUFNOKKBVMGRW-UHFFFAOYSA-N imatinib Chemical compound C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 KTUFNOKKBVMGRW-UHFFFAOYSA-N 0.000 description 2
- 229960002411 imatinib Drugs 0.000 description 2
- 210000002865 immune cell Anatomy 0.000 description 2
- 230000036737 immune function Effects 0.000 description 2
- 230000001506 immunosuppresive effect Effects 0.000 description 2
- 230000001976 improved effect Effects 0.000 description 2
- 239000012535 impurity Substances 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 150000007529 inorganic bases Chemical class 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- GURKHSYORGJETM-WAQYZQTGSA-N irinotecan hydrochloride (anhydrous) Chemical compound Cl.C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 GURKHSYORGJETM-WAQYZQTGSA-N 0.000 description 2
- 238000006317 isomerization reaction Methods 0.000 description 2
- JJWLVOIRVHMVIS-UHFFFAOYSA-N isopropylamine Chemical compound CC(C)N JJWLVOIRVHMVIS-UHFFFAOYSA-N 0.000 description 2
- 230000002147 killing effect Effects 0.000 description 2
- 239000008101 lactose Substances 0.000 description 2
- JCQLYHFGKNRPGE-FCVZTGTOSA-N lactulose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 JCQLYHFGKNRPGE-FCVZTGTOSA-N 0.000 description 2
- 229960000511 lactulose Drugs 0.000 description 2
- PFCRQPBOOFTZGQ-UHFFFAOYSA-N lactulose keto form Natural products OCC(=O)C(O)C(C(O)CO)OC1OC(CO)C(O)C(O)C1O PFCRQPBOOFTZGQ-UHFFFAOYSA-N 0.000 description 2
- BCFGMOOMADDAQU-UHFFFAOYSA-N lapatinib Chemical compound O1C(CNCCS(=O)(=O)C)=CC=C1C1=CC=C(N=CN=C2NC=3C=C(Cl)C(OCC=4C=C(F)C=CC=4)=CC=3)C2=C1 BCFGMOOMADDAQU-UHFFFAOYSA-N 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 239000012669 liquid formulation Substances 0.000 description 2
- 206010025135 lupus erythematosus Diseases 0.000 description 2
- 238000002794 lymphocyte assay Methods 0.000 description 2
- 239000011777 magnesium Substances 0.000 description 2
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 2
- JCQLYHFGKNRPGE-HFZVAGMNSA-N maltulose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 JCQLYHFGKNRPGE-HFZVAGMNSA-N 0.000 description 2
- 238000013507 mapping Methods 0.000 description 2
- 238000004949 mass spectrometry Methods 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- 229960001428 mercaptopurine Drugs 0.000 description 2
- 210000002901 mesenchymal stem cell Anatomy 0.000 description 2
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 description 2
- 150000007522 mineralic acids Chemical class 0.000 description 2
- 229960001156 mitoxantrone Drugs 0.000 description 2
- 201000006417 multiple sclerosis Diseases 0.000 description 2
- 210000000822 natural killer cell Anatomy 0.000 description 2
- QZGIWPZCWHMVQL-UIYAJPBUSA-N neocarzinostatin chromophore Chemical compound O1[C@H](C)[C@H](O)[C@H](O)[C@@H](NC)[C@H]1O[C@@H]1C/2=C/C#C[C@H]3O[C@@]3([C@@H]3OC(=O)OC3)C#CC\2=C[C@H]1OC(=O)C1=C(O)C=CC2=C(C)C=C(OC)C=C12 QZGIWPZCWHMVQL-UIYAJPBUSA-N 0.000 description 2
- 108010068617 neonatal Fc receptor Proteins 0.000 description 2
- 210000000440 neutrophil Anatomy 0.000 description 2
- 231100000062 no-observed-adverse-effect level Toxicity 0.000 description 2
- 210000004967 non-hematopoietic stem cell Anatomy 0.000 description 2
- 238000005457 optimization Methods 0.000 description 2
- 210000004789 organ system Anatomy 0.000 description 2
- 229960001592 paclitaxel Drugs 0.000 description 2
- 210000000496 pancreas Anatomy 0.000 description 2
- AQIXEPGDORPWBJ-UHFFFAOYSA-N pentan-3-ol Chemical compound CCC(O)CC AQIXEPGDORPWBJ-UHFFFAOYSA-N 0.000 description 2
- 238000002823 phage display Methods 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 229960001539 poliomyelitis vaccine Drugs 0.000 description 2
- 238000005498 polishing Methods 0.000 description 2
- 230000001737 promoting effect Effects 0.000 description 2
- 230000000069 prophylactic effect Effects 0.000 description 2
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 2
- 235000019419 proteases Nutrition 0.000 description 2
- 230000005180 public health Effects 0.000 description 2
- 150000003212 purines Chemical class 0.000 description 2
- 229950010131 puromycin Drugs 0.000 description 2
- 238000010791 quenching Methods 0.000 description 2
- 230000000171 quenching effect Effects 0.000 description 2
- 230000009257 reactivity Effects 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 210000003289 regulatory T cell Anatomy 0.000 description 2
- GHMLBKRAJCXXBS-UHFFFAOYSA-N resorcinol Chemical compound OC1=CC=CC(O)=C1 GHMLBKRAJCXXBS-UHFFFAOYSA-N 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 238000003998 size exclusion chromatography high performance liquid chromatography Methods 0.000 description 2
- 150000003384 small molecules Chemical class 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 239000001632 sodium acetate Substances 0.000 description 2
- 235000017281 sodium acetate Nutrition 0.000 description 2
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 235000010356 sorbitol Nutrition 0.000 description 2
- 210000004988 splenocyte Anatomy 0.000 description 2
- PVYJZLYGTZKPJE-UHFFFAOYSA-N streptonigrin Chemical compound C=1C=C2C(=O)C(OC)=C(N)C(=O)C2=NC=1C(C=1N)=NC(C(O)=O)=C(C)C=1C1=CC=C(OC)C(OC)=C1O PVYJZLYGTZKPJE-UHFFFAOYSA-N 0.000 description 2
- 231100000617 superantigen Toxicity 0.000 description 2
- 230000002195 synergetic effect Effects 0.000 description 2
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 2
- YAPQBXQYLJRXSA-UHFFFAOYSA-N theobromine Chemical compound CN1C(=O)NC(=O)C2=C1N=CN2C YAPQBXQYLJRXSA-UHFFFAOYSA-N 0.000 description 2
- 229960001196 thiotepa Drugs 0.000 description 2
- 206010043778 thyroiditis Diseases 0.000 description 2
- 229960003087 tioguanine Drugs 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 231100000331 toxic Toxicity 0.000 description 2
- 230000002588 toxic effect Effects 0.000 description 2
- 230000009261 transgenic effect Effects 0.000 description 2
- 238000011830 transgenic mouse model Methods 0.000 description 2
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 2
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 2
- 230000002792 vascular Effects 0.000 description 2
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 2
- 229960004528 vincristine Drugs 0.000 description 2
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 2
- XRASPMIURGNCCH-UHFFFAOYSA-N zoledronic acid Chemical compound OP(=O)(O)C(P(O)(O)=O)(O)CN1C=CN=C1 XRASPMIURGNCCH-UHFFFAOYSA-N 0.000 description 2
- 229960004276 zoledronic acid Drugs 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- NNJPGOLRFBJNIW-HNNXBMFYSA-N (-)-demecolcine Chemical compound C1=C(OC)C(=O)C=C2[C@@H](NC)CCC3=CC(OC)=C(OC)C(OC)=C3C2=C1 NNJPGOLRFBJNIW-HNNXBMFYSA-N 0.000 description 1
- WDQLRUYAYXDIFW-RWKIJVEZSA-N (2r,3r,4s,5r,6r)-4-[(2s,3r,4s,5r,6r)-3,5-dihydroxy-4-[(2r,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-6-[[(2r,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]oxan-2-yl]oxy-6-(hydroxymethyl)oxane-2,3,5-triol Chemical compound O[C@@H]1[C@@H](CO)O[C@@H](O)[C@H](O)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O2)O)[C@H](O)[C@@H](CO[C@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O2)O)O1 WDQLRUYAYXDIFW-RWKIJVEZSA-N 0.000 description 1
- FLWWDYNPWOSLEO-HQVZTVAUSA-N (2s)-2-[[4-[1-(2-amino-4-oxo-1h-pteridin-6-yl)ethyl-methylamino]benzoyl]amino]pentanedioic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1C(C)N(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FLWWDYNPWOSLEO-HQVZTVAUSA-N 0.000 description 1
- CGMTUJFWROPELF-YPAAEMCBSA-N (3E,5S)-5-[(2S)-butan-2-yl]-3-(1-hydroxyethylidene)pyrrolidine-2,4-dione Chemical compound CC[C@H](C)[C@@H]1NC(=O)\C(=C(/C)O)C1=O CGMTUJFWROPELF-YPAAEMCBSA-N 0.000 description 1
- TVIRNGFXQVMMGB-OFWIHYRESA-N (3s,6r,10r,13e,16s)-16-[(2r,3r,4s)-4-chloro-3-hydroxy-4-phenylbutan-2-yl]-10-[(3-chloro-4-methoxyphenyl)methyl]-6-methyl-3-(2-methylpropyl)-1,4-dioxa-8,11-diazacyclohexadec-13-ene-2,5,9,12-tetrone Chemical compound C1=C(Cl)C(OC)=CC=C1C[C@@H]1C(=O)NC[C@@H](C)C(=O)O[C@@H](CC(C)C)C(=O)O[C@H]([C@H](C)[C@@H](O)[C@@H](Cl)C=2C=CC=CC=2)C/C=C/C(=O)N1 TVIRNGFXQVMMGB-OFWIHYRESA-N 0.000 description 1
- XRBSKUSTLXISAB-XVVDYKMHSA-N (5r,6r,7r,8r)-8-hydroxy-7-(hydroxymethyl)-5-(3,4,5-trimethoxyphenyl)-5,6,7,8-tetrahydrobenzo[f][1,3]benzodioxole-6-carboxylic acid Chemical compound COC1=C(OC)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@H](O)[C@@H](CO)[C@@H]2C(O)=O)=C1 XRBSKUSTLXISAB-XVVDYKMHSA-N 0.000 description 1
- XRBSKUSTLXISAB-UHFFFAOYSA-N (7R,7'R,8R,8'R)-form-Podophyllic acid Natural products COC1=C(OC)C(OC)=CC(C2C3=CC=4OCOC=4C=C3C(O)C(CO)C2C(O)=O)=C1 XRBSKUSTLXISAB-UHFFFAOYSA-N 0.000 description 1
- AESVUZLWRXEGEX-DKCAWCKPSA-N (7S,9R)-7-[(2S,4R,5R,6R)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione iron(3+) Chemical compound [Fe+3].COc1cccc2C(=O)c3c(O)c4C[C@@](O)(C[C@H](O[C@@H]5C[C@@H](N)[C@@H](O)[C@@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO AESVUZLWRXEGEX-DKCAWCKPSA-N 0.000 description 1
- JXVAMODRWBNUSF-KZQKBALLSA-N (7s,9r,10r)-7-[(2r,4s,5s,6s)-5-[[(2s,4as,5as,7s,9s,9ar,10ar)-2,9-dimethyl-3-oxo-4,4a,5a,6,7,9,9a,10a-octahydrodipyrano[4,2-a:4',3'-e][1,4]dioxin-7-yl]oxy]-4-(dimethylamino)-6-methyloxan-2-yl]oxy-10-[(2s,4s,5s,6s)-4-(dimethylamino)-5-hydroxy-6-methyloxan-2 Chemical compound O([C@@H]1C2=C(O)C=3C(=O)C4=CC=CC(O)=C4C(=O)C=3C(O)=C2[C@@H](O[C@@H]2O[C@@H](C)[C@@H](O[C@@H]3O[C@@H](C)[C@H]4O[C@@H]5O[C@@H](C)C(=O)C[C@@H]5O[C@H]4C3)[C@H](C2)N(C)C)C[C@]1(O)CC)[C@H]1C[C@H](N(C)C)[C@H](O)[C@H](C)O1 JXVAMODRWBNUSF-KZQKBALLSA-N 0.000 description 1
- INAUWOVKEZHHDM-PEDBPRJASA-N (7s,9s)-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-7-[(2r,4s,5s,6s)-5-hydroxy-6-methyl-4-morpholin-4-yloxan-2-yl]oxy-4-methoxy-8,10-dihydro-7h-tetracene-5,12-dione;hydrochloride Chemical compound Cl.N1([C@H]2C[C@@H](O[C@@H](C)[C@H]2O)O[C@H]2C[C@@](O)(CC=3C(O)=C4C(=O)C=5C=CC=C(C=5C(=O)C4=C(O)C=32)OC)C(=O)CO)CCOCC1 INAUWOVKEZHHDM-PEDBPRJASA-N 0.000 description 1
- RCFNNLSZHVHCEK-IMHLAKCZSA-N (7s,9s)-7-(4-amino-6-methyloxan-2-yl)oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7h-tetracene-5,12-dione;hydrochloride Chemical compound [Cl-].O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)C1CC([NH3+])CC(C)O1 RCFNNLSZHVHCEK-IMHLAKCZSA-N 0.000 description 1
- NOPNWHSMQOXAEI-PUCKCBAPSA-N (7s,9s)-7-[(2r,4s,5s,6s)-4-(2,3-dihydropyrrol-1-yl)-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7h-tetracene-5,12-dione Chemical compound N1([C@H]2C[C@@H](O[C@@H](C)[C@H]2O)O[C@H]2C[C@@](O)(CC=3C(O)=C4C(=O)C=5C=CC=C(C=5C(=O)C4=C(O)C=32)OC)C(=O)CO)CCC=C1 NOPNWHSMQOXAEI-PUCKCBAPSA-N 0.000 description 1
- FPVKHBSQESCIEP-UHFFFAOYSA-N (8S)-3-(2-deoxy-beta-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol Natural products C1C(O)C(CO)OC1N1C(NC=NCC2O)=C2N=C1 FPVKHBSQESCIEP-UHFFFAOYSA-N 0.000 description 1
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 description 1
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 description 1
- AGNGYMCLFWQVGX-AGFFZDDWSA-N (e)-1-[(2s)-2-amino-2-carboxyethoxy]-2-diazonioethenolate Chemical compound OC(=O)[C@@H](N)CO\C([O-])=C\[N+]#N AGNGYMCLFWQVGX-AGFFZDDWSA-N 0.000 description 1
- FONKWHRXTPJODV-DNQXCXABSA-N 1,3-bis[2-[(8s)-8-(chloromethyl)-4-hydroxy-1-methyl-7,8-dihydro-3h-pyrrolo[3,2-e]indole-6-carbonyl]-1h-indol-5-yl]urea Chemical compound C1([C@H](CCl)CN2C(=O)C=3NC4=CC=C(C=C4C=3)NC(=O)NC=3C=C4C=C(NC4=CC=3)C(=O)N3C4=CC(O)=C5NC=C(C5=C4[C@H](CCl)C3)C)=C2C=C(O)C2=C1C(C)=CN2 FONKWHRXTPJODV-DNQXCXABSA-N 0.000 description 1
- WNXJIVFYUVYPPR-UHFFFAOYSA-N 1,3-dioxolane Chemical compound C1COCO1 WNXJIVFYUVYPPR-UHFFFAOYSA-N 0.000 description 1
- AMMPLVWPWSYRDR-UHFFFAOYSA-N 1-methylbicyclo[2.2.2]oct-2-ene-4-carboxylic acid Chemical compound C1CC2(C(O)=O)CCC1(C)C=C2 AMMPLVWPWSYRDR-UHFFFAOYSA-N 0.000 description 1
- DGHHQBMTXTWTJV-BQAIUKQQSA-N 119413-54-6 Chemical compound Cl.C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 DGHHQBMTXTWTJV-BQAIUKQQSA-N 0.000 description 1
- AYRXSINWFIIFAE-UHFFFAOYSA-N 2,3,4,5-tetrahydroxy-6-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyhexanal Chemical compound OCC1OC(OCC(O)C(O)C(O)C(O)C=O)C(O)C(O)C1O AYRXSINWFIIFAE-UHFFFAOYSA-N 0.000 description 1
- BTOTXLJHDSNXMW-POYBYMJQSA-N 2,3-dideoxyuridine Chemical compound O1[C@H](CO)CC[C@@H]1N1C(=O)NC(=O)C=C1 BTOTXLJHDSNXMW-POYBYMJQSA-N 0.000 description 1
- BOMZMNZEXMAQQW-UHFFFAOYSA-N 2,5,11-trimethyl-6h-pyrido[4,3-b]carbazol-2-ium-9-ol;acetate Chemical compound CC([O-])=O.C[N+]1=CC=C2C(C)=C(NC=3C4=CC(O)=CC=3)C4=C(C)C2=C1 BOMZMNZEXMAQQW-UHFFFAOYSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- 125000005273 2-acetoxybenzoic acid group Chemical group 0.000 description 1
- QCXJFISCRQIYID-IAEPZHFASA-N 2-amino-1-n-[(3s,6s,7r,10s,16s)-3-[(2s)-butan-2-yl]-7,11,14-trimethyl-2,5,9,12,15-pentaoxo-10-propan-2-yl-8-oxa-1,4,11,14-tetrazabicyclo[14.3.0]nonadecan-6-yl]-4,6-dimethyl-3-oxo-9-n-[(3s,6s,7r,10s,16s)-7,11,14-trimethyl-2,5,9,12,15-pentaoxo-3,10-di(propa Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N=C2C(C(=O)N[C@@H]3C(=O)N[C@H](C(N4CCC[C@H]4C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]3C)=O)[C@@H](C)CC)=C(N)C(=O)C(C)=C2O2)C2=C(C)C=C1 QCXJFISCRQIYID-IAEPZHFASA-N 0.000 description 1
- MSWZFWKMSRAUBD-IVMDWMLBSA-N 2-amino-2-deoxy-D-glucopyranose Chemical compound N[C@H]1C(O)O[C@H](CO)[C@@H](O)[C@@H]1O MSWZFWKMSRAUBD-IVMDWMLBSA-N 0.000 description 1
- VNBAOSVONFJBKP-UHFFFAOYSA-N 2-chloro-n,n-bis(2-chloroethyl)propan-1-amine;hydrochloride Chemical compound Cl.CC(Cl)CN(CCCl)CCCl VNBAOSVONFJBKP-UHFFFAOYSA-N 0.000 description 1
- BFSVOASYOCHEOV-UHFFFAOYSA-N 2-diethylaminoethanol Chemical compound CCN(CC)CCO BFSVOASYOCHEOV-UHFFFAOYSA-N 0.000 description 1
- 229940013085 2-diethylaminoethanol Drugs 0.000 description 1
- YIMDLWDNDGKDTJ-QLKYHASDSA-N 3'-deamino-3'-(3-cyanomorpholin-4-yl)doxorubicin Chemical compound N1([C@H]2C[C@@H](O[C@@H](C)[C@H]2O)O[C@H]2C[C@@](O)(CC=3C(O)=C4C(=O)C=5C=CC=C(C=5C(=O)C4=C(O)C=32)OC)C(=O)CO)CCOCC1C#N YIMDLWDNDGKDTJ-QLKYHASDSA-N 0.000 description 1
- NDMPLJNOPCLANR-UHFFFAOYSA-N 3,4-dihydroxy-15-(4-hydroxy-18-methoxycarbonyl-5,18-seco-ibogamin-18-yl)-16-methoxy-1-methyl-6,7-didehydro-aspidospermidine-3-carboxylic acid methyl ester Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 NDMPLJNOPCLANR-UHFFFAOYSA-N 0.000 description 1
- XMTQQYYKAHVGBJ-UHFFFAOYSA-N 3-(3,4-DICHLOROPHENYL)-1,1-DIMETHYLUREA Chemical compound CN(C)C(=O)NC1=CC=C(Cl)C(Cl)=C1 XMTQQYYKAHVGBJ-UHFFFAOYSA-N 0.000 description 1
- PWMYMKOUNYTVQN-UHFFFAOYSA-N 3-(8,8-diethyl-2-aza-8-germaspiro[4.5]decan-2-yl)-n,n-dimethylpropan-1-amine Chemical compound C1C[Ge](CC)(CC)CCC11CN(CCCN(C)C)CC1 PWMYMKOUNYTVQN-UHFFFAOYSA-N 0.000 description 1
- QGJZLNKBHJESQX-UHFFFAOYSA-N 3-Epi-Betulin-Saeure Natural products C1CC(O)C(C)(C)C2CCC3(C)C4(C)CCC5(C(O)=O)CCC(C(=C)C)C5C4CCC3C21C QGJZLNKBHJESQX-UHFFFAOYSA-N 0.000 description 1
- CLOUCVRNYSHRCF-UHFFFAOYSA-N 3beta-Hydroxy-20(29)-Lupen-3,27-oic acid Natural products C1CC(O)C(C)(C)C2CCC3(C)C4(C(O)=O)CCC5(C)CCC(C(=C)C)C5C4CCC3C21C CLOUCVRNYSHRCF-UHFFFAOYSA-N 0.000 description 1
- AOJJSUZBOXZQNB-VTZDEGQISA-N 4'-epidoxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-VTZDEGQISA-N 0.000 description 1
- QFVHZQCOUORWEI-UHFFFAOYSA-N 4-[(4-anilino-5-sulfonaphthalen-1-yl)diazenyl]-5-hydroxynaphthalene-2,7-disulfonic acid Chemical compound C=12C(O)=CC(S(O)(=O)=O)=CC2=CC(S(O)(=O)=O)=CC=1N=NC(C1=CC=CC(=C11)S(O)(=O)=O)=CC=C1NC1=CC=CC=C1 QFVHZQCOUORWEI-UHFFFAOYSA-N 0.000 description 1
- TVZGACDUOSZQKY-LBPRGKRZSA-N 4-aminofolic acid Chemical compound C1=NC2=NC(N)=NC(N)=C2N=C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 TVZGACDUOSZQKY-LBPRGKRZSA-N 0.000 description 1
- IDPUKCWIGUEADI-UHFFFAOYSA-N 5-[bis(2-chloroethyl)amino]uracil Chemical compound ClCCN(CCCl)C1=CNC(=O)NC1=O IDPUKCWIGUEADI-UHFFFAOYSA-N 0.000 description 1
- NMUSYJAQQFHJEW-KVTDHHQDSA-N 5-azacytidine Chemical compound O=C1N=C(N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 NMUSYJAQQFHJEW-KVTDHHQDSA-N 0.000 description 1
- WYXSYVWAUAUWLD-SHUUEZRQSA-N 6-azauridine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=N1 WYXSYVWAUAUWLD-SHUUEZRQSA-N 0.000 description 1
- 229960005538 6-diazo-5-oxo-L-norleucine Drugs 0.000 description 1
- YCWQAMGASJSUIP-YFKPBYRVSA-N 6-diazo-5-oxo-L-norleucine Chemical compound OC(=O)[C@@H](N)CCC(=O)C=[N+]=[N-] YCWQAMGASJSUIP-YFKPBYRVSA-N 0.000 description 1
- VVIAGPKUTFNRDU-UHFFFAOYSA-N 6S-folinic acid Natural products C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 VVIAGPKUTFNRDU-UHFFFAOYSA-N 0.000 description 1
- ZGXJTSGNIOSYLO-UHFFFAOYSA-N 88755TAZ87 Chemical compound NCC(=O)CCC(O)=O ZGXJTSGNIOSYLO-UHFFFAOYSA-N 0.000 description 1
- FUXVKZWTXQUGMW-FQEVSTJZSA-N 9-Aminocamptothecin Chemical compound C1=CC(N)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 FUXVKZWTXQUGMW-FQEVSTJZSA-N 0.000 description 1
- HDZZVAMISRMYHH-UHFFFAOYSA-N 9beta-Ribofuranosyl-7-deazaadenin Natural products C1=CC=2C(N)=NC=NC=2N1C1OC(CO)C(O)C1O HDZZVAMISRMYHH-UHFFFAOYSA-N 0.000 description 1
- 208000026872 Addison Disease Diseases 0.000 description 1
- 208000010507 Adenocarcinoma of Lung Diseases 0.000 description 1
- 206010052747 Adenocarcinoma pancreas Diseases 0.000 description 1
- 102100034540 Adenomatous polyposis coli protein Human genes 0.000 description 1
- 230000007730 Akt signaling Effects 0.000 description 1
- OGSPWJRAVKPPFI-UHFFFAOYSA-N Alendronic Acid Chemical compound NCCCC(O)(P(O)(O)=O)P(O)(O)=O OGSPWJRAVKPPFI-UHFFFAOYSA-N 0.000 description 1
- CEIZFXOZIQNICU-UHFFFAOYSA-N Alternaria alternata Crofton-weed toxin Natural products CCC(C)C1NC(=O)C(C(C)=O)=C1O CEIZFXOZIQNICU-UHFFFAOYSA-N 0.000 description 1
- 208000024827 Alzheimer disease Diseases 0.000 description 1
- 208000003343 Antiphospholipid Syndrome Diseases 0.000 description 1
- 108020000948 Antisense Oligonucleotides Proteins 0.000 description 1
- 206010003571 Astrocytoma Diseases 0.000 description 1
- 201000001320 Atherosclerosis Diseases 0.000 description 1
- 206010003827 Autoimmune hepatitis Diseases 0.000 description 1
- NOWKCMXCCJGMRR-UHFFFAOYSA-N Aziridine Chemical class C1CN1 NOWKCMXCCJGMRR-UHFFFAOYSA-N 0.000 description 1
- 102000019260 B-Cell Antigen Receptors Human genes 0.000 description 1
- 108010012919 B-Cell Antigen Receptors Proteins 0.000 description 1
- 230000003844 B-cell-activation Effects 0.000 description 1
- 102000005738 B7 Antigens Human genes 0.000 description 1
- 108010045634 B7 Antigens Proteins 0.000 description 1
- 231100000699 Bacterial toxin Toxicity 0.000 description 1
- 208000009137 Behcet syndrome Diseases 0.000 description 1
- VGGGPCQERPFHOB-MCIONIFRSA-N Bestatin Chemical compound CC(C)C[C@H](C(O)=O)NC(=O)[C@@H](O)[C@H](N)CC1=CC=CC=C1 VGGGPCQERPFHOB-MCIONIFRSA-N 0.000 description 1
- DIZWSDNSTNAYHK-XGWVBXMLSA-N Betulinic acid Natural products CC(=C)[C@@H]1C[C@H]([C@H]2CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(=O)O DIZWSDNSTNAYHK-XGWVBXMLSA-N 0.000 description 1
- 208000008439 Biliary Liver Cirrhosis Diseases 0.000 description 1
- 208000033222 Biliary cirrhosis primary Diseases 0.000 description 1
- 229940122361 Bisphosphonate Drugs 0.000 description 1
- 108010006654 Bleomycin Proteins 0.000 description 1
- 208000019838 Blood disease Diseases 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 108700031361 Brachyury Proteins 0.000 description 1
- 102100026008 Breakpoint cluster region protein Human genes 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- MBABCNBNDNGODA-LTGLSHGVSA-N Bullatacin Natural products O=C1C(C[C@H](O)CCCCCCCCCC[C@@H](O)[C@@H]2O[C@@H]([C@@H]3O[C@H]([C@@H](O)CCCCCCCCCC)CC3)CC2)=C[C@H](C)O1 MBABCNBNDNGODA-LTGLSHGVSA-N 0.000 description 1
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 1
- 108010029697 CD40 Ligand Proteins 0.000 description 1
- 102100032937 CD40 ligand Human genes 0.000 description 1
- 210000001266 CD8-positive T-lymphocyte Anatomy 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- KLWPJMFMVPTNCC-UHFFFAOYSA-N Camptothecin Natural products CCC1(O)C(=O)OCC2=C1C=C3C4Nc5ccccc5C=C4CN3C2=O KLWPJMFMVPTNCC-UHFFFAOYSA-N 0.000 description 1
- GAGWJHPBXLXJQN-UHFFFAOYSA-N Capecitabine Natural products C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1C1C(O)C(O)C(C)O1 GAGWJHPBXLXJQN-UHFFFAOYSA-N 0.000 description 1
- GAGWJHPBXLXJQN-UORFTKCHSA-N Capecitabine Chemical compound C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1[C@H]1[C@H](O)[C@H](O)[C@@H](C)O1 GAGWJHPBXLXJQN-UORFTKCHSA-N 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- SHHKQEUPHAENFK-UHFFFAOYSA-N Carboquone Chemical compound O=C1C(C)=C(N2CC2)C(=O)C(C(COC(N)=O)OC)=C1N1CC1 SHHKQEUPHAENFK-UHFFFAOYSA-N 0.000 description 1
- AOCCBINRVIKJHY-UHFFFAOYSA-N Carmofur Chemical compound CCCCCCNC(=O)N1C=C(F)C(=O)NC1=O AOCCBINRVIKJHY-UHFFFAOYSA-N 0.000 description 1
- DLGOEMSEDOSKAD-UHFFFAOYSA-N Carmustine Chemical compound ClCCNC(=O)N(N=O)CCCl DLGOEMSEDOSKAD-UHFFFAOYSA-N 0.000 description 1
- 102100025064 Cellular tumor antigen p53 Human genes 0.000 description 1
- JWBOIMRXGHLCPP-UHFFFAOYSA-N Chloditan Chemical compound C=1C=CC=C(Cl)C=1C(C(Cl)Cl)C1=CC=C(Cl)C=C1 JWBOIMRXGHLCPP-UHFFFAOYSA-N 0.000 description 1
- XCDXSSFOJZZGQC-UHFFFAOYSA-N Chlornaphazine Chemical compound C1=CC=CC2=CC(N(CCCl)CCCl)=CC=C21 XCDXSSFOJZZGQC-UHFFFAOYSA-N 0.000 description 1
- MKQWTWSXVILIKJ-LXGUWJNJSA-N Chlorozotocin Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](C=O)NC(=O)N(N=O)CCCl MKQWTWSXVILIKJ-LXGUWJNJSA-N 0.000 description 1
- 206010008609 Cholangitis sclerosing Diseases 0.000 description 1
- 208000006344 Churg-Strauss Syndrome Diseases 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 108020004705 Codon Proteins 0.000 description 1
- 208000015943 Coeliac disease Diseases 0.000 description 1
- 108010060123 Conjugate Vaccines Proteins 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- 229930188224 Cryptophycin Natural products 0.000 description 1
- HEBKCHPVOIAQTA-QWWZWVQMSA-N D-arabinitol Chemical compound OC[C@@H](O)C(O)[C@H](O)CO HEBKCHPVOIAQTA-QWWZWVQMSA-N 0.000 description 1
- WQZGKKKJIJFFOK-QTVWNMPRSA-N D-mannopyranose Chemical compound OC[C@H]1OC(O)[C@@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-QTVWNMPRSA-N 0.000 description 1
- QWIZNVHXZXRPDR-UHFFFAOYSA-N D-melezitose Natural products O1C(CO)C(O)C(O)C(O)C1OC1C(O)C(CO)OC1(CO)OC1OC(CO)C(O)C(O)C1O QWIZNVHXZXRPDR-UHFFFAOYSA-N 0.000 description 1
- 108010041986 DNA Vaccines Proteins 0.000 description 1
- 229940021995 DNA vaccine Drugs 0.000 description 1
- WEAHRLBPCANXCN-UHFFFAOYSA-N Daunomycin Natural products CCC1(O)CC(OC2CC(N)C(O)C(C)O2)c3cc4C(=O)c5c(OC)cccc5C(=O)c4c(O)c3C1 WEAHRLBPCANXCN-UHFFFAOYSA-N 0.000 description 1
- 206010011878 Deafness Diseases 0.000 description 1
- 206010011968 Decreased immune responsiveness Diseases 0.000 description 1
- XXGMIHXASFDFSM-UHFFFAOYSA-N Delta9-tetrahydrocannabinol Natural products CCCCCc1cc2OC(C)(C)C3CCC(=CC3c2c(O)c1O)C XXGMIHXASFDFSM-UHFFFAOYSA-N 0.000 description 1
- NNJPGOLRFBJNIW-UHFFFAOYSA-N Demecolcine Natural products C1=C(OC)C(=O)C=C2C(NC)CCC3=CC(OC)=C(OC)C(OC)=C3C2=C1 NNJPGOLRFBJNIW-UHFFFAOYSA-N 0.000 description 1
- 108010002156 Depsipeptides Proteins 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 239000004375 Dextrin Substances 0.000 description 1
- 229920001353 Dextrin Polymers 0.000 description 1
- AUGQEEXBDZWUJY-ZLJUKNTDSA-N Diacetoxyscirpenol Chemical compound C([C@]12[C@]3(C)[C@H](OC(C)=O)[C@@H](O)[C@H]1O[C@@H]1C=C(C)CC[C@@]13COC(=O)C)O2 AUGQEEXBDZWUJY-ZLJUKNTDSA-N 0.000 description 1
- AUGQEEXBDZWUJY-UHFFFAOYSA-N Diacetoxyscirpenol Natural products CC(=O)OCC12CCC(C)=CC1OC1C(O)C(OC(C)=O)C2(C)C11CO1 AUGQEEXBDZWUJY-UHFFFAOYSA-N 0.000 description 1
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 1
- CYQFCXCEBYINGO-DLBZAZTESA-N Dronabinol Natural products C1=C(C)CC[C@H]2C(C)(C)OC3=CC(CCCCC)=CC(O)=C3[C@H]21 CYQFCXCEBYINGO-DLBZAZTESA-N 0.000 description 1
- 229930193152 Dynemicin Natural products 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 101150029707 ERBB2 gene Proteins 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 208000017701 Endocrine disease Diseases 0.000 description 1
- AFMYMMXSQGUCBK-UHFFFAOYSA-N Endynamicin A Natural products C1#CC=CC#CC2NC(C=3C(=O)C4=C(O)C=CC(O)=C4C(=O)C=3C(O)=C3)=C3C34OC32C(C)C(C(O)=O)=C(OC)C41 AFMYMMXSQGUCBK-UHFFFAOYSA-N 0.000 description 1
- SAMRUMKYXPVKPA-VFKOLLTISA-N Enocitabine Chemical compound O=C1N=C(NC(=O)CCCCCCCCCCCCCCCCCCCCC)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 SAMRUMKYXPVKPA-VFKOLLTISA-N 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 208000018428 Eosinophilic granulomatosis with polyangiitis Diseases 0.000 description 1
- 206010014982 Epidermal and dermal conditions Diseases 0.000 description 1
- HTIJFSOGRVMCQR-UHFFFAOYSA-N Epirubicin Natural products COc1cccc2C(=O)c3c(O)c4CC(O)(CC(OC5CC(N)C(=O)C(C)O5)c4c(O)c3C(=O)c12)C(=O)CO HTIJFSOGRVMCQR-UHFFFAOYSA-N 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 239000004386 Erythritol Substances 0.000 description 1
- UNXHWFMMPAWVPI-UHFFFAOYSA-N Erythritol Natural products OCC(O)C(O)CO UNXHWFMMPAWVPI-UHFFFAOYSA-N 0.000 description 1
- 229930189413 Esperamicin Natural products 0.000 description 1
- JOYRKODLDBILNP-UHFFFAOYSA-N Ethyl urethane Chemical compound CCOC(N)=O JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 206010015548 Euthanasia Diseases 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- MPJKWIXIYCLVCU-UHFFFAOYSA-N Folinic acid Natural products NC1=NC2=C(N(C=O)C(CNc3ccc(cc3)C(=O)NC(CCC(=O)O)CC(=O)O)CN2)C(=O)N1 MPJKWIXIYCLVCU-UHFFFAOYSA-N 0.000 description 1
- VWUXBMIQPBEWFH-WCCTWKNTSA-N Fulvestrant Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)CC2=C1 VWUXBMIQPBEWFH-WCCTWKNTSA-N 0.000 description 1
- 208000018522 Gastrointestinal disease Diseases 0.000 description 1
- 241000699694 Gerbillinae Species 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- 208000024869 Goodpasture syndrome Diseases 0.000 description 1
- 206010072579 Granulomatosis with polyangiitis Diseases 0.000 description 1
- 208000016621 Hearing disease Diseases 0.000 description 1
- 108010004889 Heat-Shock Proteins Proteins 0.000 description 1
- 102000002812 Heat-Shock Proteins Human genes 0.000 description 1
- 208000035186 Hemolytic Autoimmune Anemia Diseases 0.000 description 1
- 208000032843 Hemorrhage Diseases 0.000 description 1
- 101000924577 Homo sapiens Adenomatous polyposis coli protein Proteins 0.000 description 1
- 101100005713 Homo sapiens CD4 gene Proteins 0.000 description 1
- 101000935043 Homo sapiens Integrin beta-1 Proteins 0.000 description 1
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 description 1
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 1
- 101000878605 Homo sapiens Low affinity immunoglobulin epsilon Fc receptor Proteins 0.000 description 1
- 101000997832 Homo sapiens Tyrosine-protein kinase JAK2 Proteins 0.000 description 1
- 241000701806 Human papillomavirus Species 0.000 description 1
- VSNHCAURESNICA-UHFFFAOYSA-N Hydroxyurea Chemical compound NC(=O)NO VSNHCAURESNICA-UHFFFAOYSA-N 0.000 description 1
- 101150007193 IFNB1 gene Proteins 0.000 description 1
- MPBVHIBUJCELCL-UHFFFAOYSA-N Ibandronate Chemical compound CCCCCN(C)CCC(O)(P(O)(O)=O)P(O)(O)=O MPBVHIBUJCELCL-UHFFFAOYSA-N 0.000 description 1
- XDXDZDZNSLXDNA-TZNDIEGXSA-N Idarubicin Chemical compound C1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(C)=O)C1 XDXDZDZNSLXDNA-TZNDIEGXSA-N 0.000 description 1
- XDXDZDZNSLXDNA-UHFFFAOYSA-N Idarubicin Natural products C1C(N)C(O)C(C)OC1OC1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2CC(O)(C(C)=O)C1 XDXDZDZNSLXDNA-UHFFFAOYSA-N 0.000 description 1
- 102000018071 Immunoglobulin Fc Fragments Human genes 0.000 description 1
- 108010091135 Immunoglobulin Fc Fragments Proteins 0.000 description 1
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 1
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 1
- 206010022095 Injection Site reaction Diseases 0.000 description 1
- 208000027601 Inner ear disease Diseases 0.000 description 1
- 108010041012 Integrin alpha4 Proteins 0.000 description 1
- 102100025304 Integrin beta-1 Human genes 0.000 description 1
- 102100027268 Interferon-stimulated gene 20 kDa protein Human genes 0.000 description 1
- 108010050904 Interferons Proteins 0.000 description 1
- 102000014150 Interferons Human genes 0.000 description 1
- 108010065805 Interleukin-12 Proteins 0.000 description 1
- 108090000978 Interleukin-4 Proteins 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- 241000274177 Juniperus sabina Species 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- 239000005551 L01XE03 - Erlotinib Substances 0.000 description 1
- 239000002136 L01XE07 - Lapatinib Substances 0.000 description 1
- 208000017119 Labyrinth disease Diseases 0.000 description 1
- 229920001491 Lentinan Polymers 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- MEPSBMMZQBMKHM-UHFFFAOYSA-N Lomatiol Natural products CC(=C/CC1=C(O)C(=O)c2ccccc2C1=O)CO MEPSBMMZQBMKHM-UHFFFAOYSA-N 0.000 description 1
- GQYIWUVLTXOXAJ-UHFFFAOYSA-N Lomustine Chemical compound ClCCN(N=O)C(=O)NC1CCCCC1 GQYIWUVLTXOXAJ-UHFFFAOYSA-N 0.000 description 1
- 102100038007 Low affinity immunoglobulin epsilon Fc receptor Human genes 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 208000005777 Lupus Nephritis Diseases 0.000 description 1
- 102000008072 Lymphokines Human genes 0.000 description 1
- 108010074338 Lymphokines Proteins 0.000 description 1
- 108091054437 MHC class I family Proteins 0.000 description 1
- 102000043129 MHC class I family Human genes 0.000 description 1
- 102000043131 MHC class II family Human genes 0.000 description 1
- 108091054438 MHC class II family Proteins 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- VJRAUFKOOPNFIQ-UHFFFAOYSA-N Marcellomycin Natural products C12=C(O)C=3C(=O)C4=C(O)C=CC(O)=C4C(=O)C=3C=C2C(C(=O)OC)C(CC)(O)CC1OC(OC1C)CC(N(C)C)C1OC(OC1C)CC(O)C1OC1CC(O)C(O)C(C)O1 VJRAUFKOOPNFIQ-UHFFFAOYSA-N 0.000 description 1
- 229930126263 Maytansine Natural products 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- VFKZTMPDYBFSTM-KVTDHHQDSA-N Mitobronitol Chemical compound BrC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CBr VFKZTMPDYBFSTM-KVTDHHQDSA-N 0.000 description 1
- 102000004232 Mitogen-Activated Protein Kinase Kinases Human genes 0.000 description 1
- 108090000744 Mitogen-Activated Protein Kinase Kinases Proteins 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- 101100519207 Mus musculus Pdcd1 gene Proteins 0.000 description 1
- 241000282339 Mustela Species 0.000 description 1
- 102000010168 Myeloid Differentiation Factor 88 Human genes 0.000 description 1
- 108010077432 Myeloid Differentiation Factor 88 Proteins 0.000 description 1
- OVBPIULPVIDEAO-UHFFFAOYSA-N N-Pteroyl-L-glutaminsaeure Natural products C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-UHFFFAOYSA-N 0.000 description 1
- HTLZVHNRZJPSMI-UHFFFAOYSA-N N-ethylpiperidine Chemical compound CCN1CCCCC1 HTLZVHNRZJPSMI-UHFFFAOYSA-N 0.000 description 1
- 208000012902 Nervous system disease Diseases 0.000 description 1
- 208000025966 Neurological disease Diseases 0.000 description 1
- SYNHCENRCUAUNM-UHFFFAOYSA-N Nitrogen mustard N-oxide hydrochloride Chemical compound Cl.ClCC[N+]([O-])(C)CCCl SYNHCENRCUAUNM-UHFFFAOYSA-N 0.000 description 1
- KGTDRFCXGRULNK-UHFFFAOYSA-N Nogalamycin Natural products COC1C(OC)(C)C(OC)C(C)OC1OC1C2=C(O)C(C(=O)C3=C(O)C=C4C5(C)OC(C(C(C5O)N(C)C)O)OC4=C3C3=O)=C3C=C2C(C(=O)OC)C(C)(O)C1 KGTDRFCXGRULNK-UHFFFAOYSA-N 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 229930187135 Olivomycin Natural products 0.000 description 1
- 208000005225 Opsoclonus-Myoclonus Syndrome Diseases 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 102220502507 PX domain-containing protein kinase-like protein_Y56A_mutation Human genes 0.000 description 1
- 241000609499 Palicourea Species 0.000 description 1
- VREZDOWOLGNDPW-ALTGWBOUSA-N Pancratistatin Chemical compound C1=C2[C@H]3[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O)[C@@H]3NC(=O)C2=C(O)C2=C1OCO2 VREZDOWOLGNDPW-ALTGWBOUSA-N 0.000 description 1
- VREZDOWOLGNDPW-MYVCAWNPSA-N Pancratistatin Natural products O=C1N[C@H]2[C@H](O)[C@H](O)[C@H](O)[C@H](O)[C@@H]2c2c1c(O)c1OCOc1c2 VREZDOWOLGNDPW-MYVCAWNPSA-N 0.000 description 1
- 108090000526 Papain Proteins 0.000 description 1
- 208000018737 Parkinson disease Diseases 0.000 description 1
- 206010034277 Pemphigoid Diseases 0.000 description 1
- 201000011152 Pemphigus Diseases 0.000 description 1
- 108010057150 Peplomycin Proteins 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- 206010057249 Phagocytosis Diseases 0.000 description 1
- 102000004160 Phosphoric Monoester Hydrolases Human genes 0.000 description 1
- 108090000608 Phosphoric Monoester Hydrolases Proteins 0.000 description 1
- KMSKQZKKOZQFFG-HSUXVGOQSA-N Pirarubicin Chemical compound O([C@H]1[C@@H](N)C[C@@H](O[C@H]1C)O[C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1CCCCO1 KMSKQZKKOZQFFG-HSUXVGOQSA-N 0.000 description 1
- 241000276498 Pollachius virens Species 0.000 description 1
- 206010036105 Polyneuropathy Diseases 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- HFVNWDWLWUCIHC-GUPDPFMOSA-N Prednimustine Chemical compound O=C([C@@]1(O)CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)[C@@H](O)C[C@@]21C)COC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 HFVNWDWLWUCIHC-GUPDPFMOSA-N 0.000 description 1
- 208000012654 Primary biliary cholangitis Diseases 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 101710094000 Programmed cell death 1 ligand 1 Proteins 0.000 description 1
- 102100024924 Protein kinase C alpha type Human genes 0.000 description 1
- 101710109947 Protein kinase C alpha type Proteins 0.000 description 1
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 1
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 1
- 102000016971 Proto-Oncogene Proteins c-kit Human genes 0.000 description 1
- 108010014608 Proto-Oncogene Proteins c-kit Proteins 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- 201000001263 Psoriatic Arthritis Diseases 0.000 description 1
- 208000036824 Psoriatic arthropathy Diseases 0.000 description 1
- 208000003782 Raynaud disease Diseases 0.000 description 1
- 208000012322 Raynaud phenomenon Diseases 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 208000025747 Rheumatic disease Diseases 0.000 description 1
- OWPCHSCAPHNHAV-UHFFFAOYSA-N Rhizoxin Natural products C1C(O)C2(C)OC2C=CC(C)C(OC(=O)C2)CC2CC2OC2C(=O)OC1C(C)C(OC)C(C)=CC=CC(C)=CC1=COC(C)=N1 OWPCHSCAPHNHAV-UHFFFAOYSA-N 0.000 description 1
- 102220477434 Ribosome biogenesis protein BOP1_D52E_mutation Human genes 0.000 description 1
- NSFWWJIQIKBZMJ-YKNYLIOZSA-N Roridin A Chemical compound C([C@]12[C@]3(C)[C@H]4C[C@H]1O[C@@H]1C=C(C)CC[C@@]13COC(=O)[C@@H](O)[C@H](C)CCO[C@H](\C=C\C=C/C(=O)O4)[C@H](O)C)O2 NSFWWJIQIKBZMJ-YKNYLIOZSA-N 0.000 description 1
- CIEYTVIYYGTCCI-UHFFFAOYSA-N SJ000286565 Natural products C1=CC=C2C(=O)C(CC=C(C)C)=C(O)C(=O)C2=C1 CIEYTVIYYGTCCI-UHFFFAOYSA-N 0.000 description 1
- 206010039710 Scleroderma Diseases 0.000 description 1
- 108010071390 Serum Albumin Proteins 0.000 description 1
- 102000007562 Serum Albumin Human genes 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 229920000519 Sizofiran Polymers 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- UQZIYBXSHAGNOE-USOSMYMVSA-N Stachyose Natural products O(C[C@H]1[C@@H](O)[C@H](O)[C@H](O)[C@@H](O[C@@]2(CO)[C@H](O)[C@@H](O)[C@@H](CO)O2)O1)[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@H](CO[C@@H]2[C@@H](O)[C@@H](O)[C@@H](O)[C@H](CO)O2)O1 UQZIYBXSHAGNOE-USOSMYMVSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- BXFOFFBJRFZBQZ-QYWOHJEZSA-N T-2 toxin Chemical compound C([C@@]12[C@]3(C)[C@H](OC(C)=O)[C@@H](O)[C@H]1O[C@H]1[C@]3(COC(C)=O)C[C@@H](C(=C1)C)OC(=O)CC(C)C)O2 BXFOFFBJRFZBQZ-QYWOHJEZSA-N 0.000 description 1
- 108700011582 TER 286 Proteins 0.000 description 1
- 108700012920 TNF Proteins 0.000 description 1
- 102000003714 TNF receptor-associated factor 6 Human genes 0.000 description 1
- 108090000009 TNF receptor-associated factor 6 Proteins 0.000 description 1
- CGMTUJFWROPELF-UHFFFAOYSA-N Tenuazonic acid Natural products CCC(C)C1NC(=O)C(=C(C)/O)C1=O CGMTUJFWROPELF-UHFFFAOYSA-N 0.000 description 1
- 206010043376 Tetanus Diseases 0.000 description 1
- 206010043561 Thrombocytopenic purpura Diseases 0.000 description 1
- 201000007023 Thrombotic Thrombocytopenic Purpura Diseases 0.000 description 1
- DKJJVAGXPKPDRL-UHFFFAOYSA-N Tiludronic acid Chemical compound OP(O)(=O)C(P(O)(O)=O)SC1=CC=C(Cl)C=C1 DKJJVAGXPKPDRL-UHFFFAOYSA-N 0.000 description 1
- 101710183280 Topoisomerase Proteins 0.000 description 1
- 102000007238 Transferrin Receptors Human genes 0.000 description 1
- 108010033576 Transferrin Receptors Proteins 0.000 description 1
- 208000003441 Transfusion reaction Diseases 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- UMILHIMHKXVDGH-UHFFFAOYSA-N Triethylene glycol diglycidyl ether Chemical compound C1OC1COCCOCCOCCOCC1CO1 UMILHIMHKXVDGH-UHFFFAOYSA-N 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 102100033444 Tyrosine-protein kinase JAK2 Human genes 0.000 description 1
- 206010046851 Uveitis Diseases 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- 208000036142 Viral infection Diseases 0.000 description 1
- 208000033559 Waldenström macroglobulinemia Diseases 0.000 description 1
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- IFJUINDAXYAPTO-UUBSBJJBSA-N [(8r,9s,13s,14s,17s)-17-[2-[4-[4-[bis(2-chloroethyl)amino]phenyl]butanoyloxy]acetyl]oxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-3-yl] benzoate Chemical compound C([C@@H]1[C@@H](C2=CC=3)CC[C@]4([C@H]1CC[C@@H]4OC(=O)COC(=O)CCCC=1C=CC(=CC=1)N(CCCl)CCCl)C)CC2=CC=3OC(=O)C1=CC=CC=C1 IFJUINDAXYAPTO-UUBSBJJBSA-N 0.000 description 1
- XZSRRNFBEIOBDA-CFNBKWCHSA-N [2-[(2s,4s)-4-[(2r,4s,5s,6s)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-3,4-dihydro-1h-tetracen-2-yl]-2-oxoethyl] 2,2-diethoxyacetate Chemical compound O([C@H]1C[C@](CC2=C(O)C=3C(=O)C4=CC=CC(OC)=C4C(=O)C=3C(O)=C21)(O)C(=O)COC(=O)C(OCC)OCC)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 XZSRRNFBEIOBDA-CFNBKWCHSA-N 0.000 description 1
- ZOZKYEHVNDEUCO-XUTVFYLZSA-N aceglatone Chemical compound O1C(=O)[C@H](OC(C)=O)[C@@H]2OC(=O)[C@@H](OC(=O)C)[C@@H]21 ZOZKYEHVNDEUCO-XUTVFYLZSA-N 0.000 description 1
- 229950002684 aceglatone Drugs 0.000 description 1
- 229930183665 actinomycin Natural products 0.000 description 1
- RJURFGZVJUQBHK-IIXSONLDSA-N actinomycin D Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-IIXSONLDSA-N 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 229950004955 adozelesin Drugs 0.000 description 1
- BYRVKDUQDLJUBX-JJCDCTGGSA-N adozelesin Chemical compound C1=CC=C2OC(C(=O)NC=3C=C4C=C(NC4=CC=3)C(=O)N3C[C@H]4C[C@]44C5=C(C(C=C43)=O)NC=C5C)=CC2=C1 BYRVKDUQDLJUBX-JJCDCTGGSA-N 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 229940062527 alendronate Drugs 0.000 description 1
- 229940045714 alkyl sulfonate alkylating agent Drugs 0.000 description 1
- 150000008052 alkyl sulfonates Chemical class 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 1
- 239000013566 allergen Substances 0.000 description 1
- 201000009961 allergic asthma Diseases 0.000 description 1
- 230000003281 allosteric effect Effects 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- LHAOFBCHXGZGOR-NAVBLJQLSA-N alpha-D-Manp-(1->3)-alpha-D-Manp-(1->2)-alpha-D-Manp Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@H](O)[C@H]1O[C@@H]1[C@@H](O)[C@@H](O[C@@H]2[C@H]([C@@H](O)[C@H](O)[C@@H](CO)O2)O)[C@H](O)[C@@H](CO)O1 LHAOFBCHXGZGOR-NAVBLJQLSA-N 0.000 description 1
- 238000003016 alphascreen Methods 0.000 description 1
- 229960000473 altretamine Drugs 0.000 description 1
- AZDRQVAHHNSJOQ-UHFFFAOYSA-N alumane Chemical class [AlH3] AZDRQVAHHNSJOQ-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 230000009435 amidation Effects 0.000 description 1
- 238000007112 amidation reaction Methods 0.000 description 1
- 150000001408 amides Chemical group 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 229960003437 aminoglutethimide Drugs 0.000 description 1
- ROBVIMPUHSLWNV-UHFFFAOYSA-N aminoglutethimide Chemical compound C=1C=C(N)C=CC=1C1(CC)CCC(=O)NC1=O ROBVIMPUHSLWNV-UHFFFAOYSA-N 0.000 description 1
- 229960002749 aminolevulinic acid Drugs 0.000 description 1
- 229960003896 aminopterin Drugs 0.000 description 1
- 229960001220 amsacrine Drugs 0.000 description 1
- XCPGHVQEEXUHNC-UHFFFAOYSA-N amsacrine Chemical compound COC1=CC(NS(C)(=O)=O)=CC=C1NC1=C(C=CC=C2)C2=NC2=CC=CC=C12 XCPGHVQEEXUHNC-UHFFFAOYSA-N 0.000 description 1
- 238000012436 analytical size exclusion chromatography Methods 0.000 description 1
- BBDAGFIXKZCXAH-CCXZUQQUSA-N ancitabine Chemical compound N=C1C=CN2[C@@H]3O[C@H](CO)[C@@H](O)[C@@H]3OC2=N1 BBDAGFIXKZCXAH-CCXZUQQUSA-N 0.000 description 1
- 229950000242 ancitabine Drugs 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 230000008485 antagonism Effects 0.000 description 1
- 229940046836 anti-estrogen Drugs 0.000 description 1
- 230000001833 anti-estrogenic effect Effects 0.000 description 1
- 230000000340 anti-metabolite Effects 0.000 description 1
- 230000001147 anti-toxic effect Effects 0.000 description 1
- 230000030741 antigen processing and presentation Effects 0.000 description 1
- 229940100197 antimetabolite Drugs 0.000 description 1
- 239000002256 antimetabolite Substances 0.000 description 1
- 229940045687 antimetabolites folic acid analogs Drugs 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 239000000074 antisense oligonucleotide Substances 0.000 description 1
- 238000012230 antisense oligonucleotides Methods 0.000 description 1
- 230000005975 antitumor immune response Effects 0.000 description 1
- 230000001640 apoptogenic effect Effects 0.000 description 1
- 150000008209 arabinosides Chemical class 0.000 description 1
- 229960003121 arginine Drugs 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 201000005000 autoimmune gastritis Diseases 0.000 description 1
- 201000000448 autoimmune hemolytic anemia Diseases 0.000 description 1
- 208000010928 autoimmune thyroid disease Diseases 0.000 description 1
- 229960002756 azacitidine Drugs 0.000 description 1
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 1
- 229950011321 azaserine Drugs 0.000 description 1
- 150000001541 aziridines Chemical class 0.000 description 1
- 239000000688 bacterial toxin Substances 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 229960000686 benzalkonium chloride Drugs 0.000 description 1
- UREZNYTWGJKWBI-UHFFFAOYSA-M benzethonium chloride Chemical compound [Cl-].C1=CC(C(C)(C)CC(C)(C)C)=CC=C1OCCOCC[N+](C)(C)CC1=CC=CC=C1 UREZNYTWGJKWBI-UHFFFAOYSA-M 0.000 description 1
- 229960001950 benzethonium chloride Drugs 0.000 description 1
- 235000019445 benzyl alcohol Nutrition 0.000 description 1
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 1
- MSWZFWKMSRAUBD-UHFFFAOYSA-N beta-D-galactosamine Natural products NC1C(O)OC(CO)C(O)C1O MSWZFWKMSRAUBD-UHFFFAOYSA-N 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- QGJZLNKBHJESQX-FZFNOLFKSA-N betulinic acid Chemical compound C1C[C@H](O)C(C)(C)[C@@H]2CC[C@@]3(C)[C@]4(C)CC[C@@]5(C(O)=O)CC[C@@H](C(=C)C)[C@@H]5[C@H]4CC[C@@H]3[C@]21C QGJZLNKBHJESQX-FZFNOLFKSA-N 0.000 description 1
- 229960000397 bevacizumab Drugs 0.000 description 1
- 230000002457 bidirectional effect Effects 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 230000008827 biological function Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000002051 biphasic effect Effects 0.000 description 1
- HUTDDBSSHVOYJR-UHFFFAOYSA-H bis[(2-oxo-1,3,2$l^{5},4$l^{2}-dioxaphosphaplumbetan-2-yl)oxy]lead Chemical compound [Pb+2].[Pb+2].[Pb+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O HUTDDBSSHVOYJR-UHFFFAOYSA-H 0.000 description 1
- 229950008548 bisantrene Drugs 0.000 description 1
- 150000004663 bisphosphonates Chemical class 0.000 description 1
- 229950006844 bizelesin Drugs 0.000 description 1
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical class N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- 229960005520 bryostatin Drugs 0.000 description 1
- MJQUEDHRCUIRLF-TVIXENOKSA-N bryostatin 1 Chemical compound C([C@@H]1CC(/[C@@H]([C@@](C(C)(C)/C=C/2)(O)O1)OC(=O)/C=C/C=C/CCC)=C\C(=O)OC)[C@H]([C@@H](C)O)OC(=O)C[C@H](O)C[C@@H](O1)C[C@H](OC(C)=O)C(C)(C)[C@]1(O)C[C@@H]1C\C(=C\C(=O)OC)C[C@H]\2O1 MJQUEDHRCUIRLF-TVIXENOKSA-N 0.000 description 1
- MUIWQCKLQMOUAT-AKUNNTHJSA-N bryostatin 20 Natural products COC(=O)C=C1C[C@@]2(C)C[C@]3(O)O[C@](C)(C[C@@H](O)CC(=O)O[C@](C)(C[C@@]4(C)O[C@](O)(CC5=CC(=O)O[C@]45C)C(C)(C)C=C[C@@](C)(C1)O2)[C@@H](C)O)C[C@H](OC(=O)C(C)(C)C)C3(C)C MUIWQCKLQMOUAT-AKUNNTHJSA-N 0.000 description 1
- MBABCNBNDNGODA-LUVUIASKSA-N bullatacin Chemical compound O1[C@@H]([C@@H](O)CCCCCCCCCC)CC[C@@H]1[C@@H]1O[C@@H]([C@H](O)CCCCCCCCCC[C@@H](O)CC=2C(O[C@@H](C)C=2)=O)CC1 MBABCNBNDNGODA-LUVUIASKSA-N 0.000 description 1
- 208000000594 bullous pemphigoid Diseases 0.000 description 1
- 229960002092 busulfan Drugs 0.000 description 1
- LRHPLDYGYMQRHN-UHFFFAOYSA-N butyl alcohol Substances CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 108700002839 cactinomycin Proteins 0.000 description 1
- 229950009908 cactinomycin Drugs 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 229940127093 camptothecin Drugs 0.000 description 1
- VSJKWCGYPAHWDS-FQEVSTJZSA-N camptothecin Chemical compound C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-FQEVSTJZSA-N 0.000 description 1
- 229960004117 capecitabine Drugs 0.000 description 1
- 229960004562 carboplatin Drugs 0.000 description 1
- 229960002115 carboquone Drugs 0.000 description 1
- JLQUFIHWVLZVTJ-UHFFFAOYSA-N carbosulfan Chemical compound CCCCN(CCCC)SN(C)C(=O)OC1=CC=CC2=C1OC(C)(C)C2 JLQUFIHWVLZVTJ-UHFFFAOYSA-N 0.000 description 1
- 230000002612 cardiopulmonary effect Effects 0.000 description 1
- XREUEWVEMYWFFA-CSKJXFQVSA-N carminomycin Chemical compound C1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=C(O)C=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(C)=O)C1 XREUEWVEMYWFFA-CSKJXFQVSA-N 0.000 description 1
- 229930188550 carminomycin Natural products 0.000 description 1
- XREUEWVEMYWFFA-UHFFFAOYSA-N carminomycin I Natural products C1C(N)C(O)C(C)OC1OC1C2=C(O)C(C(=O)C3=C(O)C=CC=C3C3=O)=C3C(O)=C2CC(O)(C(C)=O)C1 XREUEWVEMYWFFA-UHFFFAOYSA-N 0.000 description 1
- 229960003261 carmofur Drugs 0.000 description 1
- 229960005243 carmustine Drugs 0.000 description 1
- 229950001725 carubicin Drugs 0.000 description 1
- BBZDXMBRAFTCAA-AREMUKBSSA-N carzelesin Chemical compound C1=2NC=C(C)C=2C([C@H](CCl)CN2C(=O)C=3NC4=CC=C(C=C4C=3)NC(=O)C3=CC4=CC=C(C=C4O3)N(CC)CC)=C2C=C1OC(=O)NC1=CC=CC=C1 BBZDXMBRAFTCAA-AREMUKBSSA-N 0.000 description 1
- 229950007509 carzelesin Drugs 0.000 description 1
- 108010047060 carzinophilin Proteins 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000032823 cell division Effects 0.000 description 1
- 230000003915 cell function Effects 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000007969 cellular immunity Effects 0.000 description 1
- 230000007541 cellular toxicity Effects 0.000 description 1
- 210000003169 central nervous system Anatomy 0.000 description 1
- TVFDJXOCXUVLDH-RNFDNDRNSA-N cesium-137 Chemical compound [137Cs] TVFDJXOCXUVLDH-RNFDNDRNSA-N 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 238000012412 chemical coupling Methods 0.000 description 1
- 238000010382 chemical cross-linking Methods 0.000 description 1
- 238000009104 chemotherapy regimen Methods 0.000 description 1
- 229960004630 chlorambucil Drugs 0.000 description 1
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 1
- 150000001805 chlorine compounds Chemical class 0.000 description 1
- 229950008249 chlornaphazine Drugs 0.000 description 1
- 229960001480 chlorozotocin Drugs 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 208000025302 chronic primary adrenal insufficiency Diseases 0.000 description 1
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 1
- 229960004316 cisplatin Drugs 0.000 description 1
- 238000005352 clarification Methods 0.000 description 1
- ACSIXWWBWUQEHA-UHFFFAOYSA-N clodronic acid Chemical compound OP(O)(=O)C(Cl)(Cl)P(O)(O)=O ACSIXWWBWUQEHA-UHFFFAOYSA-N 0.000 description 1
- 229960002286 clodronic acid Drugs 0.000 description 1
- 201000010989 colorectal carcinoma Diseases 0.000 description 1
- 238000009096 combination chemotherapy Methods 0.000 description 1
- 238000012875 competitive assay Methods 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 239000003636 conditioned culture medium Substances 0.000 description 1
- 229940031670 conjugate vaccine Drugs 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 210000004087 cornea Anatomy 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 201000003740 cowpox Diseases 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 201000003278 cryoglobulinemia Diseases 0.000 description 1
- 108010089438 cryptophycin 1 Proteins 0.000 description 1
- PSNOPSMXOBPNNV-VVCTWANISA-N cryptophycin 1 Chemical compound C1=C(Cl)C(OC)=CC=C1C[C@@H]1C(=O)NC[C@@H](C)C(=O)O[C@@H](CC(C)C)C(=O)O[C@H]([C@H](C)[C@@H]2[C@H](O2)C=2C=CC=CC=2)C/C=C/C(=O)N1 PSNOPSMXOBPNNV-VVCTWANISA-N 0.000 description 1
- 108010090203 cryptophycin 8 Proteins 0.000 description 1
- PSNOPSMXOBPNNV-UHFFFAOYSA-N cryptophycin-327 Natural products C1=C(Cl)C(OC)=CC=C1CC1C(=O)NCC(C)C(=O)OC(CC(C)C)C(=O)OC(C(C)C2C(O2)C=2C=CC=CC=2)CC=CC(=O)N1 PSNOPSMXOBPNNV-UHFFFAOYSA-N 0.000 description 1
- 208000004921 cutaneous lupus erythematosus Diseases 0.000 description 1
- WZHCOOQXZCIUNC-UHFFFAOYSA-N cyclandelate Chemical compound C1C(C)(C)CC(C)CC1OC(=O)C(O)C1=CC=CC=C1 WZHCOOQXZCIUNC-UHFFFAOYSA-N 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- HPXRVTGHNJAIIH-UHFFFAOYSA-N cyclohexanol Chemical compound OC1CCCCC1 HPXRVTGHNJAIIH-UHFFFAOYSA-N 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 229960000684 cytarabine Drugs 0.000 description 1
- 230000001461 cytolytic effect Effects 0.000 description 1
- 229940104302 cytosine Drugs 0.000 description 1
- 239000002254 cytotoxic agent Substances 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 239000002619 cytotoxin Substances 0.000 description 1
- 229960003901 dacarbazine Drugs 0.000 description 1
- 229960000640 dactinomycin Drugs 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 229960000975 daunorubicin Drugs 0.000 description 1
- 230000007123 defense Effects 0.000 description 1
- 230000005786 degenerative changes Effects 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 229960005052 demecolcine Drugs 0.000 description 1
- 230000000779 depleting effect Effects 0.000 description 1
- 201000001981 dermatomyositis Diseases 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 229950003913 detorubicin Drugs 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 235000019425 dextrin Nutrition 0.000 description 1
- NIJJYAXOARWZEE-UHFFFAOYSA-N di-n-propyl-acetic acid Natural products CCCC(C(O)=O)CCC NIJJYAXOARWZEE-UHFFFAOYSA-N 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- WVYXNIXAMZOZFK-UHFFFAOYSA-N diaziquone Chemical compound O=C1C(NC(=O)OCC)=C(N2CC2)C(=O)C(NC(=O)OCC)=C1N1CC1 WVYXNIXAMZOZFK-UHFFFAOYSA-N 0.000 description 1
- 229950002389 diaziquone Drugs 0.000 description 1
- PZXJOHSZQAEJFE-UHFFFAOYSA-N dihydrobetulinic acid Natural products C1CC(O)C(C)(C)C2CCC3(C)C4(C)CCC5(C(O)=O)CCC(C(C)C)C5C4CCC3C21C PZXJOHSZQAEJFE-UHFFFAOYSA-N 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 239000000539 dimer Substances 0.000 description 1
- 230000003292 diminished effect Effects 0.000 description 1
- 206010013023 diphtheria Diseases 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- 150000002019 disulfides Chemical class 0.000 description 1
- VSJKWCGYPAHWDS-UHFFFAOYSA-N dl-camptothecin Natural products C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)C5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-UHFFFAOYSA-N 0.000 description 1
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 1
- 125000003438 dodecyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- AMRJKAQTDDKMCE-UHFFFAOYSA-N dolastatin Chemical compound CC(C)C(N(C)C)C(=O)NC(C(C)C)C(=O)N(C)C(C(C)C)C(OC)CC(=O)N1CCCC1C(OC)C(C)C(=O)NC(C=1SC=CN=1)CC1=CC=CC=C1 AMRJKAQTDDKMCE-UHFFFAOYSA-N 0.000 description 1
- 229930188854 dolastatin Natural products 0.000 description 1
- ZWAOHEXOSAUJHY-ZIYNGMLESA-N doxifluridine Chemical compound O[C@@H]1[C@H](O)[C@@H](C)O[C@H]1N1C(=O)NC(=O)C(F)=C1 ZWAOHEXOSAUJHY-ZIYNGMLESA-N 0.000 description 1
- 229950005454 doxifluridine Drugs 0.000 description 1
- 229960004242 dronabinol Drugs 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 229960005501 duocarmycin Drugs 0.000 description 1
- VQNATVDKACXKTF-XELLLNAOSA-N duocarmycin Chemical compound COC1=C(OC)C(OC)=C2NC(C(=O)N3C4=CC(=O)C5=C([C@@]64C[C@@H]6C3)C=C(N5)C(=O)OC)=CC2=C1 VQNATVDKACXKTF-XELLLNAOSA-N 0.000 description 1
- 229930184221 duocarmycin Natural products 0.000 description 1
- AFMYMMXSQGUCBK-AKMKHHNQSA-N dynemicin a Chemical compound C1#C\C=C/C#C[C@@H]2NC(C=3C(=O)C4=C(O)C=CC(O)=C4C(=O)C=3C(O)=C3)=C3[C@@]34O[C@]32[C@@H](C)C(C(O)=O)=C(OC)[C@H]41 AFMYMMXSQGUCBK-AKMKHHNQSA-N 0.000 description 1
- 210000005069 ears Anatomy 0.000 description 1
- FSIRXIHZBIXHKT-MHTVFEQDSA-N edatrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CC(CC)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FSIRXIHZBIXHKT-MHTVFEQDSA-N 0.000 description 1
- 229950006700 edatrexate Drugs 0.000 description 1
- 210000003162 effector t lymphocyte Anatomy 0.000 description 1
- VLCYCQAOQCDTCN-UHFFFAOYSA-N eflornithine Chemical compound NCCCC(N)(C(F)F)C(O)=O VLCYCQAOQCDTCN-UHFFFAOYSA-N 0.000 description 1
- 229960002759 eflornithine Drugs 0.000 description 1
- 229940121647 egfr inhibitor Drugs 0.000 description 1
- XOPYFXBZMVTEJF-UHFFFAOYSA-N eleutherobin Natural products C1=CC2(OC)OC1(C)C(OC(=O)C=CC=1N=CN(C)C=1)CC(C(=CCC1C(C)C)C)C1C=C2COC1OCC(O)C(O)C1OC(C)=O XOPYFXBZMVTEJF-UHFFFAOYSA-N 0.000 description 1
- XOPYFXBZMVTEJF-PDACKIITSA-N eleutherobin Chemical compound C(/[C@H]1[C@H](C(=CC[C@@H]1C(C)C)C)C[C@@H]([C@@]1(C)O[C@@]2(C=C1)OC)OC(=O)\C=C\C=1N=CN(C)C=1)=C2\CO[C@@H]1OC[C@@H](O)[C@@H](O)[C@@H]1OC(C)=O XOPYFXBZMVTEJF-PDACKIITSA-N 0.000 description 1
- 229950000549 elliptinium acetate Drugs 0.000 description 1
- 210000000750 endocrine system Anatomy 0.000 description 1
- 239000002158 endotoxin Substances 0.000 description 1
- JOZGNYDSEBIJDH-UHFFFAOYSA-N eniluracil Chemical compound O=C1NC=C(C#C)C(=O)N1 JOZGNYDSEBIJDH-UHFFFAOYSA-N 0.000 description 1
- 229950010213 eniluracil Drugs 0.000 description 1
- 229950011487 enocitabine Drugs 0.000 description 1
- 231100000655 enterotoxin Toxicity 0.000 description 1
- 229940088598 enzyme Drugs 0.000 description 1
- 102000052116 epidermal growth factor receptor activity proteins Human genes 0.000 description 1
- 108700015053 epidermal growth factor receptor activity proteins Proteins 0.000 description 1
- YJGVMLPVUAXIQN-UHFFFAOYSA-N epipodophyllotoxin Natural products COC1=C(OC)C(OC)=CC(C2C3=CC=4OCOC=4C=C3C(O)C3C2C(OC3)=O)=C1 YJGVMLPVUAXIQN-UHFFFAOYSA-N 0.000 description 1
- 229960001904 epirubicin Drugs 0.000 description 1
- 208000037828 epithelial carcinoma Diseases 0.000 description 1
- 210000002919 epithelial cell Anatomy 0.000 description 1
- 210000000981 epithelium Anatomy 0.000 description 1
- 229930013356 epothilone Natural products 0.000 description 1
- 150000003883 epothilone derivatives Chemical class 0.000 description 1
- AAKJLRGGTJKAMG-UHFFFAOYSA-N erlotinib Chemical compound C=12C=C(OCCOC)C(OCCOC)=CC2=NC=NC=1NC1=CC=CC(C#C)=C1 AAKJLRGGTJKAMG-UHFFFAOYSA-N 0.000 description 1
- 229960001433 erlotinib Drugs 0.000 description 1
- UNXHWFMMPAWVPI-ZXZARUISSA-N erythritol Chemical compound OC[C@H](O)[C@H](O)CO UNXHWFMMPAWVPI-ZXZARUISSA-N 0.000 description 1
- 235000019414 erythritol Nutrition 0.000 description 1
- 229940009714 erythritol Drugs 0.000 description 1
- ITSGNOIFAJAQHJ-BMFNZSJVSA-N esorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)C[C@H](C)O1 ITSGNOIFAJAQHJ-BMFNZSJVSA-N 0.000 description 1
- 229950002017 esorubicin Drugs 0.000 description 1
- LJQQFQHBKUKHIS-WJHRIEJJSA-N esperamicin Chemical compound O1CC(NC(C)C)C(OC)CC1OC1C(O)C(NOC2OC(C)C(SC)C(O)C2)C(C)OC1OC1C(\C2=C/CSSSC)=C(NC(=O)OC)C(=O)C(OC3OC(C)C(O)C(OC(=O)C=4C(=CC(OC)=C(OC)C=4)NC(=O)C(=C)OC)C3)C2(O)C#C\C=C/C#C1 LJQQFQHBKUKHIS-WJHRIEJJSA-N 0.000 description 1
- 229960001842 estramustine Drugs 0.000 description 1
- FRPJXPJMRWBBIH-RBRWEJTLSA-N estramustine Chemical compound ClCCN(CCCl)C(=O)OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 FRPJXPJMRWBBIH-RBRWEJTLSA-N 0.000 description 1
- 239000000328 estrogen antagonist Substances 0.000 description 1
- QCYAXXZCQKMTMO-QFIPXVFZSA-N ethyl (2s)-2-[(2-bromo-3-oxospiro[3.5]non-1-en-1-yl)amino]-3-[4-(2,7-naphthyridin-1-ylamino)phenyl]propanoate Chemical compound N([C@@H](CC=1C=CC(NC=2C3=CN=CC=C3C=CN=2)=CC=1)C(=O)OCC)C1=C(Br)C(=O)C11CCCCC1 QCYAXXZCQKMTMO-QFIPXVFZSA-N 0.000 description 1
- QSRLNKCNOLVZIR-KRWDZBQOSA-N ethyl (2s)-2-[[2-[4-[bis(2-chloroethyl)amino]phenyl]acetyl]amino]-4-methylsulfanylbutanoate Chemical compound CCOC(=O)[C@H](CCSC)NC(=O)CC1=CC=C(N(CCCl)CCCl)C=C1 QSRLNKCNOLVZIR-KRWDZBQOSA-N 0.000 description 1
- 229940012017 ethylenediamine Drugs 0.000 description 1
- 229960005237 etoglucid Drugs 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000001605 fetal effect Effects 0.000 description 1
- 210000003754 fetus Anatomy 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- ODKNJVUHOIMIIZ-RRKCRQDMSA-N floxuridine Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(F)=C1 ODKNJVUHOIMIIZ-RRKCRQDMSA-N 0.000 description 1
- 229960000961 floxuridine Drugs 0.000 description 1
- 229960000390 fludarabine Drugs 0.000 description 1
- GIUYCYHIANZCFB-FJFJXFQQSA-N fludarabine phosphate Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@@H]1O GIUYCYHIANZCFB-FJFJXFQQSA-N 0.000 description 1
- 235000019152 folic acid Nutrition 0.000 description 1
- 239000011724 folic acid Substances 0.000 description 1
- 229960000304 folic acid Drugs 0.000 description 1
- 150000002224 folic acids Chemical class 0.000 description 1
- VVIAGPKUTFNRDU-ABLWVSNPSA-N folinic acid Chemical compound C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 VVIAGPKUTFNRDU-ABLWVSNPSA-N 0.000 description 1
- 235000008191 folinic acid Nutrition 0.000 description 1
- 239000011672 folinic acid Substances 0.000 description 1
- 239000012537 formulation buffer Substances 0.000 description 1
- 229960004783 fotemustine Drugs 0.000 description 1
- YAKWPXVTIGTRJH-UHFFFAOYSA-N fotemustine Chemical compound CCOP(=O)(OCC)C(C)NC(=O)N(CCCl)N=O YAKWPXVTIGTRJH-UHFFFAOYSA-N 0.000 description 1
- 238000007710 freezing Methods 0.000 description 1
- 229960002258 fulvestrant Drugs 0.000 description 1
- 230000002538 fungal effect Effects 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 229940044658 gallium nitrate Drugs 0.000 description 1
- 229940044627 gamma-interferon Drugs 0.000 description 1
- 229940083124 ganglion-blocking antiadrenergic secondary and tertiary amines Drugs 0.000 description 1
- QTQAWLPCGQOSGP-GBTDJJJQSA-N geldanamycin Chemical class N1C(=O)\C(C)=C/C=C\[C@@H](OC)[C@H](OC(N)=O)\C(C)=C/[C@@H](C)[C@@H](O)[C@H](OC)C[C@@H](C)CC2=C(OC)C(=O)C=C1C2=O QTQAWLPCGQOSGP-GBTDJJJQSA-N 0.000 description 1
- 238000001415 gene therapy Methods 0.000 description 1
- 208000005017 glioblastoma Diseases 0.000 description 1
- 229960002442 glucosamine Drugs 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 235000004554 glutamine Nutrition 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 150000002338 glycosides Chemical class 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 238000003306 harvesting Methods 0.000 description 1
- 230000010370 hearing loss Effects 0.000 description 1
- 231100000888 hearing loss Toxicity 0.000 description 1
- 208000016354 hearing loss disease Diseases 0.000 description 1
- 208000014951 hematologic disease Diseases 0.000 description 1
- 210000000777 hematopoietic system Anatomy 0.000 description 1
- 208000002672 hepatitis B Diseases 0.000 description 1
- DMEGYFMYUHOHGS-UHFFFAOYSA-N heptamethylene Natural products C1CCCCCC1 DMEGYFMYUHOHGS-UHFFFAOYSA-N 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- UUVWYPNAQBNQJQ-UHFFFAOYSA-N hexamethylmelamine Chemical compound CN(C)C1=NC(N(C)C)=NC(N(C)C)=N1 UUVWYPNAQBNQJQ-UHFFFAOYSA-N 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 229960002885 histidine Drugs 0.000 description 1
- 230000036732 histological change Effects 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 102000048362 human PDCD1 Human genes 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- XGIHQYAWBCFNPY-AZOCGYLKSA-N hydrabamine Chemical compound C([C@@H]12)CC3=CC(C(C)C)=CC=C3[C@@]2(C)CCC[C@@]1(C)CNCCNC[C@@]1(C)[C@@H]2CCC3=CC(C(C)C)=CC=C3[C@@]2(C)CCC1 XGIHQYAWBCFNPY-AZOCGYLKSA-N 0.000 description 1
- 229920001477 hydrophilic polymer Polymers 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 125000001165 hydrophobic group Chemical group 0.000 description 1
- 229960001330 hydroxycarbamide Drugs 0.000 description 1
- UWYVPFMHMJIBHE-OWOJBTEDSA-N hydroxymaleic acid group Chemical group O/C(/C(=O)O)=C/C(=O)O UWYVPFMHMJIBHE-OWOJBTEDSA-N 0.000 description 1
- KNOSIOWNDGUGFJ-UHFFFAOYSA-N hydroxysesamone Natural products C1=CC(O)=C2C(=O)C(CC=C(C)C)=C(O)C(=O)C2=C1O KNOSIOWNDGUGFJ-UHFFFAOYSA-N 0.000 description 1
- 229940015872 ibandronate Drugs 0.000 description 1
- 229960000908 idarubicin Drugs 0.000 description 1
- 230000002519 immonomodulatory effect Effects 0.000 description 1
- 230000008073 immune recognition Effects 0.000 description 1
- 208000026278 immune system disease Diseases 0.000 description 1
- 229940127130 immunocytokine Drugs 0.000 description 1
- 230000016784 immunoglobulin production Effects 0.000 description 1
- 238000002991 immunohistochemical analysis Methods 0.000 description 1
- 230000006054 immunological memory Effects 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- DBIGHPPNXATHOF-UHFFFAOYSA-N improsulfan Chemical compound CS(=O)(=O)OCCCNCCCOS(C)(=O)=O DBIGHPPNXATHOF-UHFFFAOYSA-N 0.000 description 1
- 229950008097 improsulfan Drugs 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 230000004941 influx Effects 0.000 description 1
- 230000015788 innate immune response Effects 0.000 description 1
- 238000007689 inspection Methods 0.000 description 1
- 229940125798 integrin inhibitor Drugs 0.000 description 1
- 229940079322 interferon Drugs 0.000 description 1
- 229960003130 interferon gamma Drugs 0.000 description 1
- 230000031261 interleukin-10 production Effects 0.000 description 1
- 230000002601 intratumoral effect Effects 0.000 description 1
- 239000003456 ion exchange resin Substances 0.000 description 1
- 229920003303 ion-exchange polymer Polymers 0.000 description 1
- 229960004768 irinotecan Drugs 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 210000004153 islets of langerhan Anatomy 0.000 description 1
- 210000002510 keratinocyte Anatomy 0.000 description 1
- 208000017169 kidney disease Diseases 0.000 description 1
- 239000000832 lactitol Substances 0.000 description 1
- 235000010448 lactitol Nutrition 0.000 description 1
- VQHSOMBJVWLPSR-JVCRWLNRSA-N lactitol Chemical compound OC[C@H](O)[C@@H](O)[C@@H]([C@H](O)CO)O[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O VQHSOMBJVWLPSR-JVCRWLNRSA-N 0.000 description 1
- 229960003451 lactitol Drugs 0.000 description 1
- SIUGQQMOYSVTAT-UHFFFAOYSA-N lapachol Natural products CC(=CCC1C(O)C(=O)c2ccccc2C1=O)C SIUGQQMOYSVTAT-UHFFFAOYSA-N 0.000 description 1
- CWPGNVFCJOPXFB-UHFFFAOYSA-N lapachol Chemical compound C1=CC=C2C(=O)C(=O)C(CC=C(C)C)=C(O)C2=C1 CWPGNVFCJOPXFB-UHFFFAOYSA-N 0.000 description 1
- 229960004891 lapatinib Drugs 0.000 description 1
- 229960001320 lapatinib ditosylate Drugs 0.000 description 1
- 229940115286 lentinan Drugs 0.000 description 1
- 229960001691 leucovorin Drugs 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 208000019423 liver disease Diseases 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 229960002247 lomustine Drugs 0.000 description 1
- YROQEQPFUCPDCP-UHFFFAOYSA-N losoxantrone Chemical compound OCCNCCN1N=C2C3=CC=CC(O)=C3C(=O)C3=C2C1=CC=C3NCCNCCO YROQEQPFUCPDCP-UHFFFAOYSA-N 0.000 description 1
- 229950008745 losoxantrone Drugs 0.000 description 1
- 238000004020 luminiscence type Methods 0.000 description 1
- 201000005249 lung adenocarcinoma Diseases 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 210000001165 lymph node Anatomy 0.000 description 1
- 210000005210 lymphoid organ Anatomy 0.000 description 1
- 230000002535 lyotropic effect Effects 0.000 description 1
- 229960003646 lysine Drugs 0.000 description 1
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 235000019341 magnesium sulphate Nutrition 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 239000000845 maltitol Substances 0.000 description 1
- 235000010449 maltitol Nutrition 0.000 description 1
- VQHSOMBJVWLPSR-WUJBLJFYSA-N maltitol Chemical compound OC[C@H](O)[C@@H](O)[C@@H]([C@H](O)CO)O[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O VQHSOMBJVWLPSR-WUJBLJFYSA-N 0.000 description 1
- 229940035436 maltitol Drugs 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 1
- MQXVYODZCMMZEM-ZYUZMQFOSA-N mannomustine Chemical compound ClCCNC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CNCCCl MQXVYODZCMMZEM-ZYUZMQFOSA-N 0.000 description 1
- 229950008612 mannomustine Drugs 0.000 description 1
- FYGDTMLNYKFZSV-UHFFFAOYSA-N mannotriose Natural products OC1C(O)C(O)C(CO)OC1OC1C(CO)OC(OC2C(OC(O)C(O)C2O)CO)C(O)C1O FYGDTMLNYKFZSV-UHFFFAOYSA-N 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 230000008774 maternal effect Effects 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- WKPWGQKGSOKKOO-RSFHAFMBSA-N maytansine Chemical compound CO[C@@H]([C@@]1(O)C[C@](OC(=O)N1)([C@H]([C@@H]1O[C@@]1(C)[C@@H](OC(=O)[C@H](C)N(C)C(C)=O)CC(=O)N1C)C)[H])\C=C\C=C(C)\CC2=CC(OC)=C(Cl)C1=C2 WKPWGQKGSOKKOO-RSFHAFMBSA-N 0.000 description 1
- 229960004961 mechlorethamine Drugs 0.000 description 1
- HAWPXGHAZFHHAD-UHFFFAOYSA-N mechlorethamine Chemical compound ClCCN(C)CCCl HAWPXGHAZFHHAD-UHFFFAOYSA-N 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- QWIZNVHXZXRPDR-WSCXOGSTSA-N melezitose Chemical compound O([C@@]1(O[C@@H]([C@H]([C@@H]1O[C@@H]1[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O1)O)O)CO)CO)[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O QWIZNVHXZXRPDR-WSCXOGSTSA-N 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 210000003071 memory t lymphocyte Anatomy 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 230000001394 metastastic effect Effects 0.000 description 1
- 206010061289 metastatic neoplasm Diseases 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- VJRAUFKOOPNFIQ-TVEKBUMESA-N methyl (1r,2r,4s)-4-[(2r,4s,5s,6s)-5-[(2s,4s,5s,6s)-5-[(2s,4s,5s,6s)-4,5-dihydroxy-6-methyloxan-2-yl]oxy-4-hydroxy-6-methyloxan-2-yl]oxy-4-(dimethylamino)-6-methyloxan-2-yl]oxy-2-ethyl-2,5,7,10-tetrahydroxy-6,11-dioxo-3,4-dihydro-1h-tetracene-1-carboxylat Chemical compound O([C@H]1[C@@H](O)C[C@@H](O[C@H]1C)O[C@H]1[C@H](C[C@@H](O[C@H]1C)O[C@H]1C[C@]([C@@H](C2=CC=3C(=O)C4=C(O)C=CC(O)=C4C(=O)C=3C(O)=C21)C(=O)OC)(O)CC)N(C)C)[C@H]1C[C@H](O)[C@H](O)[C@H](C)O1 VJRAUFKOOPNFIQ-TVEKBUMESA-N 0.000 description 1
- QRMNENFZDDYDEF-GOSISDBHSA-N methyl (8s)-8-(bromomethyl)-2-methyl-4-(4-methylpiperazine-1-carbonyl)oxy-6-(5,6,7-trimethoxy-1h-indole-2-carbonyl)-7,8-dihydro-3h-pyrrolo[3,2-e]indole-1-carboxylate Chemical compound C1([C@H](CBr)CN(C1=C1)C(=O)C=2NC3=C(OC)C(OC)=C(OC)C=C3C=2)=C2C(C(=O)OC)=C(C)NC2=C1OC(=O)N1CCN(C)CC1 QRMNENFZDDYDEF-GOSISDBHSA-N 0.000 description 1
- 239000004292 methyl p-hydroxybenzoate Substances 0.000 description 1
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 1
- 229960002216 methylparaben Drugs 0.000 description 1
- HPNSFSBZBAHARI-UHFFFAOYSA-N micophenolic acid Natural products OC1=C(CC=C(C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-UHFFFAOYSA-N 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 230000004089 microcirculation Effects 0.000 description 1
- 206010063344 microscopic polyangiitis Diseases 0.000 description 1
- 229960005485 mitobronitol Drugs 0.000 description 1
- 229960003539 mitoguazone Drugs 0.000 description 1
- MXWHMTNPTTVWDM-NXOFHUPFSA-N mitoguazone Chemical compound NC(N)=N\N=C(/C)\C=N\N=C(N)N MXWHMTNPTTVWDM-NXOFHUPFSA-N 0.000 description 1
- VFKZTMPDYBFSTM-GUCUJZIJSA-N mitolactol Chemical compound BrC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)CBr VFKZTMPDYBFSTM-GUCUJZIJSA-N 0.000 description 1
- 229950010913 mitolactol Drugs 0.000 description 1
- 229960004857 mitomycin Drugs 0.000 description 1
- 229960000350 mitotane Drugs 0.000 description 1
- 230000000394 mitotic effect Effects 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 238000000302 molecular modelling Methods 0.000 description 1
- 210000005087 mononuclear cell Anatomy 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- LPUQAYUQRXPFSQ-DFWYDOINSA-M monosodium L-glutamate Chemical compound [Na+].[O-]C(=O)[C@@H](N)CCC(O)=O LPUQAYUQRXPFSQ-DFWYDOINSA-M 0.000 description 1
- 235000013923 monosodium glutamate Nutrition 0.000 description 1
- 239000004223 monosodium glutamate Substances 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 125000001446 muramyl group Chemical group N[C@@H](C=O)[C@@H](O[C@@H](C(=O)*)C)[C@H](O)[C@H](O)CO 0.000 description 1
- 238000002703 mutagenesis Methods 0.000 description 1
- 231100000350 mutagenesis Toxicity 0.000 description 1
- 206010028417 myasthenia gravis Diseases 0.000 description 1
- 229960000951 mycophenolic acid Drugs 0.000 description 1
- HPNSFSBZBAHARI-RUDMXATFSA-N mycophenolic acid Chemical compound OC1=C(C\C=C(/C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-RUDMXATFSA-N 0.000 description 1
- 210000000066 myeloid cell Anatomy 0.000 description 1
- AZBFJBJXUQUQLF-UHFFFAOYSA-N n-(1,5-dimethylpyrrolidin-3-yl)pyrrolidine-1-carboxamide Chemical compound C1N(C)C(C)CC1NC(=O)N1CCCC1 AZBFJBJXUQUQLF-UHFFFAOYSA-N 0.000 description 1
- NJSMWLQOCQIOPE-OCHFTUDZSA-N n-[(e)-[10-[(e)-(4,5-dihydro-1h-imidazol-2-ylhydrazinylidene)methyl]anthracen-9-yl]methylideneamino]-4,5-dihydro-1h-imidazol-2-amine Chemical compound N1CCN=C1N\N=C\C(C1=CC=CC=C11)=C(C=CC=C2)C2=C1\C=N\NC1=NCCN1 NJSMWLQOCQIOPE-OCHFTUDZSA-N 0.000 description 1
- YOHYSYJDKVYCJI-UHFFFAOYSA-N n-[3-[[6-[3-(trifluoromethyl)anilino]pyrimidin-4-yl]amino]phenyl]cyclopropanecarboxamide Chemical compound FC(F)(F)C1=CC=CC(NC=2N=CN=C(NC=3C=C(NC(=O)C4CC4)C=CC=3)C=2)=C1 YOHYSYJDKVYCJI-UHFFFAOYSA-N 0.000 description 1
- XGXNTJHZPBRBHJ-UHFFFAOYSA-N n-phenylpyrimidin-2-amine Chemical class N=1C=CC=NC=1NC1=CC=CC=C1 XGXNTJHZPBRBHJ-UHFFFAOYSA-N 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 210000000581 natural killer T-cell Anatomy 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- MQYXUWHLBZFQQO-UHFFFAOYSA-N nepehinol Natural products C1CC(O)C(C)(C)C2CCC3(C)C4(C)CCC5(C)CCC(C(=C)C)C5C4CCC3C21C MQYXUWHLBZFQQO-UHFFFAOYSA-N 0.000 description 1
- 230000002232 neuromuscular Effects 0.000 description 1
- 208000008795 neuromyelitis optica Diseases 0.000 description 1
- 230000003472 neutralizing effect Effects 0.000 description 1
- 229960001420 nimustine Drugs 0.000 description 1
- VFEDRRNHLBGPNN-UHFFFAOYSA-N nimustine Chemical compound CC1=NC=C(CNC(=O)N(CCCl)N=O)C(N)=N1 VFEDRRNHLBGPNN-UHFFFAOYSA-N 0.000 description 1
- KGTDRFCXGRULNK-JYOBTZKQSA-N nogalamycin Chemical compound CO[C@@H]1[C@@](OC)(C)[C@@H](OC)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=C(O)C=C4[C@@]5(C)O[C@H]([C@H]([C@@H]([C@H]5O)N(C)C)O)OC4=C3C3=O)=C3C=C2[C@@H](C(=O)OC)[C@@](C)(O)C1 KGTDRFCXGRULNK-JYOBTZKQSA-N 0.000 description 1
- 229950009266 nogalamycin Drugs 0.000 description 1
- 239000002736 nonionic surfactant Substances 0.000 description 1
- 230000009871 nonspecific binding Effects 0.000 description 1
- 239000002777 nucleoside Substances 0.000 description 1
- CZDBNBLGZNWKMC-MWQNXGTOSA-N olivomycin Chemical class O([C@@H]1C[C@@H](O[C@H](C)[C@@H]1O)OC=1C=C2C=C3C[C@H]([C@@H](C(=O)C3=C(O)C2=C(O)C=1)O[C@H]1O[C@@H](C)[C@H](O)[C@@H](OC2O[C@@H](C)[C@H](O)[C@@H](O)C2)C1)[C@H](OC)C(=O)[C@@H](O)[C@@H](C)O)[C@H]1C[C@H](O)[C@H](OC)[C@H](C)O1 CZDBNBLGZNWKMC-MWQNXGTOSA-N 0.000 description 1
- 238000011275 oncology therapy Methods 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 229940043515 other immunoglobulins in atc Drugs 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- LXCFILQKKLGQFO-UHFFFAOYSA-N p-hydroxybenzoic acid methyl ester Natural products COC(=O)C1=CC=C(O)C=C1 LXCFILQKKLGQFO-UHFFFAOYSA-N 0.000 description 1
- 239000006174 pH buffer Substances 0.000 description 1
- 210000002741 palatine tonsil Anatomy 0.000 description 1
- WRUUGTRCQOWXEG-UHFFFAOYSA-N pamidronate Chemical compound NCCC(O)(P(O)(O)=O)P(O)(O)=O WRUUGTRCQOWXEG-UHFFFAOYSA-N 0.000 description 1
- 229940046231 pamidronate Drugs 0.000 description 1
- VREZDOWOLGNDPW-UHFFFAOYSA-N pancratistatine Natural products C1=C2C3C(O)C(O)C(O)C(O)C3NC(=O)C2=C(O)C2=C1OCO2 VREZDOWOLGNDPW-UHFFFAOYSA-N 0.000 description 1
- 201000002094 pancreatic adenocarcinoma Diseases 0.000 description 1
- 229940055729 papain Drugs 0.000 description 1
- 235000019834 papain Nutrition 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 229960005079 pemetrexed Drugs 0.000 description 1
- WBXPDJSOTKVWSJ-ZDUSSCGKSA-N pemetrexed Chemical compound C=1NC=2NC(N)=NC(=O)C=2C=1CCC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 WBXPDJSOTKVWSJ-ZDUSSCGKSA-N 0.000 description 1
- 201000001976 pemphigus vulgaris Diseases 0.000 description 1
- 229960002340 pentostatin Drugs 0.000 description 1
- FPVKHBSQESCIEP-JQCXWYLXSA-N pentostatin Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC[C@H]2O)=C2N=C1 FPVKHBSQESCIEP-JQCXWYLXSA-N 0.000 description 1
- QIMGFXOHTOXMQP-GFAGFCTOSA-N peplomycin Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCCN[C@@H](C)C=1C=CC=CC=1)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1NC=NC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C QIMGFXOHTOXMQP-GFAGFCTOSA-N 0.000 description 1
- 229950003180 peplomycin Drugs 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 230000008782 phagocytosis Effects 0.000 description 1
- 239000008194 pharmaceutical composition Substances 0.000 description 1
- WLJVXDMOQOGPHL-UHFFFAOYSA-N phenylacetic acid Chemical compound OC(=O)CC1=CC=CC=C1 WLJVXDMOQOGPHL-UHFFFAOYSA-N 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- 235000008729 phenylalanine Nutrition 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 description 1
- 229960000952 pipobroman Drugs 0.000 description 1
- NJBFOOCLYDNZJN-UHFFFAOYSA-N pipobroman Chemical compound BrCCC(=O)N1CCN(C(=O)CCBr)CC1 NJBFOOCLYDNZJN-UHFFFAOYSA-N 0.000 description 1
- NUKCGLDCWQXYOQ-UHFFFAOYSA-N piposulfan Chemical compound CS(=O)(=O)OCCC(=O)N1CCN(C(=O)CCOS(C)(=O)=O)CC1 NUKCGLDCWQXYOQ-UHFFFAOYSA-N 0.000 description 1
- 229950001100 piposulfan Drugs 0.000 description 1
- 229960001221 pirarubicin Drugs 0.000 description 1
- 230000003169 placental effect Effects 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 150000003057 platinum Chemical class 0.000 description 1
- 229960001237 podophyllotoxin Drugs 0.000 description 1
- YJGVMLPVUAXIQN-XVVDYKMHSA-N podophyllotoxin Chemical compound COC1=C(OC)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@H](O)[C@@H]3[C@@H]2C(OC3)=O)=C1 YJGVMLPVUAXIQN-XVVDYKMHSA-N 0.000 description 1
- YVCVYCSAAZQOJI-UHFFFAOYSA-N podophyllotoxin Natural products COC1=C(O)C(OC)=CC(C2C3=CC=4OCOC=4C=C3C(O)C3C2C(OC3)=O)=C1 YVCVYCSAAZQOJI-UHFFFAOYSA-N 0.000 description 1
- 231100000614 poison Toxicity 0.000 description 1
- 239000002574 poison Substances 0.000 description 1
- 229920001983 poloxamer Polymers 0.000 description 1
- 230000008488 polyadenylation Effects 0.000 description 1
- 229920000768 polyamine Polymers 0.000 description 1
- 208000005987 polymyositis Diseases 0.000 description 1
- 230000007824 polyneuropathy Effects 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 230000004481 post-translational protein modification Effects 0.000 description 1
- 230000001124 posttranscriptional effect Effects 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 229960004694 prednimustine Drugs 0.000 description 1
- 229960005205 prednisolone Drugs 0.000 description 1
- OIGNJSKKLXVSLS-VWUMJDOOSA-N prednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OIGNJSKKLXVSLS-VWUMJDOOSA-N 0.000 description 1
- 230000002028 premature Effects 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 150000003141 primary amines Chemical class 0.000 description 1
- 201000000742 primary sclerosing cholangitis Diseases 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 229960004919 procaine Drugs 0.000 description 1
- CPTBDICYNRMXFX-UHFFFAOYSA-N procarbazine Chemical compound CNNCC1=CC=C(C(=O)NC(C)C)C=C1 CPTBDICYNRMXFX-UHFFFAOYSA-N 0.000 description 1
- 229960000624 procarbazine Drugs 0.000 description 1
- 238000004393 prognosis Methods 0.000 description 1
- 230000000770 proinflammatory effect Effects 0.000 description 1
- 210000001236 prokaryotic cell Anatomy 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 239000004405 propyl p-hydroxybenzoate Substances 0.000 description 1
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 1
- 229960003415 propylparaben Drugs 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- WOLQREOUPKZMEX-UHFFFAOYSA-N pteroyltriglutamic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)NC(CCC(=O)NC(CCC(=O)NC(CCC(O)=O)C(O)=O)C(O)=O)C(O)=O)C=C1 WOLQREOUPKZMEX-UHFFFAOYSA-N 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 238000002708 random mutagenesis Methods 0.000 description 1
- BMKDZUISNHGIBY-UHFFFAOYSA-N razoxane Chemical compound C1C(=O)NC(=O)CN1C(C)CN1CC(=O)NC(=O)C1 BMKDZUISNHGIBY-UHFFFAOYSA-N 0.000 description 1
- 229960000460 razoxane Drugs 0.000 description 1
- 210000005227 renal system Anatomy 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 229930002330 retinoic acid Natural products 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- OWPCHSCAPHNHAV-LMONGJCWSA-N rhizoxin Chemical compound C/C([C@H](OC)[C@@H](C)[C@@H]1C[C@H](O)[C@]2(C)O[C@@H]2/C=C/[C@@H](C)[C@]2([H])OC(=O)C[C@@](C2)(C[C@@H]2O[C@H]2C(=O)O1)[H])=C\C=C\C(\C)=C\C1=COC(C)=N1 OWPCHSCAPHNHAV-LMONGJCWSA-N 0.000 description 1
- 229940089617 risedronate Drugs 0.000 description 1
- 229950004892 rodorubicin Drugs 0.000 description 1
- MBABCNBNDNGODA-WPZDJQSSSA-N rolliniastatin 1 Natural products O1[C@@H]([C@@H](O)CCCCCCCCCC)CC[C@H]1[C@H]1O[C@@H]([C@H](O)CCCCCCCCCC[C@@H](O)CC=2C(O[C@@H](C)C=2)=O)CC1 MBABCNBNDNGODA-WPZDJQSSSA-N 0.000 description 1
- IMUQLZLGWJSVMV-UOBFQKKOSA-N roridin A Natural products CC(O)C1OCCC(C)C(O)C(=O)OCC2CC(=CC3OC4CC(OC(=O)C=C/C=C/1)C(C)(C23)C45CO5)C IMUQLZLGWJSVMV-UOBFQKKOSA-N 0.000 description 1
- 102220053806 rs121918461 Human genes 0.000 description 1
- VHXNKPBCCMUMSW-FQEVSTJZSA-N rubitecan Chemical compound C1=CC([N+]([O-])=O)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VHXNKPBCCMUMSW-FQEVSTJZSA-N 0.000 description 1
- 229930182947 sarcodictyin Natural products 0.000 description 1
- 239000013017 sartobind Substances 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 208000010157 sclerosing cholangitis Diseases 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 238000005204 segregation Methods 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 229950001403 sizofiran Drugs 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 230000009870 specific binding Effects 0.000 description 1
- 238000004611 spectroscopical analysis Methods 0.000 description 1
- 238000009987 spinning Methods 0.000 description 1
- 229950006315 spirogermanium Drugs 0.000 description 1
- ICXJVZHDZFXYQC-UHFFFAOYSA-N spongistatin 1 Natural products OC1C(O2)(O)CC(O)C(C)C2CCCC=CC(O2)CC(O)CC2(O2)CC(OC)CC2CC(=O)C(C)C(OC(C)=O)C(C)C(=C)CC(O2)CC(C)(O)CC2(O2)CC(OC(C)=O)CC2CC(=O)OC2C(O)C(CC(=C)CC(O)C=CC(Cl)=C)OC1C2C ICXJVZHDZFXYQC-UHFFFAOYSA-N 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- UQZIYBXSHAGNOE-XNSRJBNMSA-N stachyose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO[C@@H]2[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO[C@@H]3[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O3)O)O2)O)O1 UQZIYBXSHAGNOE-XNSRJBNMSA-N 0.000 description 1
- SFVFIFLLYFPGHH-UHFFFAOYSA-M stearalkonium chloride Chemical compound [Cl-].CCCCCCCCCCCCCCCCCC[N+](C)(C)CC1=CC=CC=C1 SFVFIFLLYFPGHH-UHFFFAOYSA-M 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 229960001052 streptozocin Drugs 0.000 description 1
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 1
- 229940031626 subunit vaccine Drugs 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 210000001179 synovial fluid Anatomy 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- AYUNIORJHRXIBJ-TXHRRWQRSA-N tanespimycin Chemical compound N1C(=O)\C(C)=C\C=C/[C@H](OC)[C@@H](OC(N)=O)\C(C)=C\[C@H](C)[C@@H](O)[C@@H](OC)C[C@H](C)CC2=C(NCC=C)C(=O)C=C1C2=O AYUNIORJHRXIBJ-TXHRRWQRSA-N 0.000 description 1
- 229950007866 tanespimycin Drugs 0.000 description 1
- 229960001674 tegafur Drugs 0.000 description 1
- WFWLQNSHRPWKFK-ZCFIWIBFSA-N tegafur Chemical compound O=C1NC(=O)C(F)=CN1[C@@H]1OCCC1 WFWLQNSHRPWKFK-ZCFIWIBFSA-N 0.000 description 1
- NRUKOCRGYNPUPR-QBPJDGROSA-N teniposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@@H](OC[C@H]4O3)C=3SC=CC=3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 NRUKOCRGYNPUPR-QBPJDGROSA-N 0.000 description 1
- 229960001278 teniposide Drugs 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 229960000814 tetanus toxoid Drugs 0.000 description 1
- 229960004559 theobromine Drugs 0.000 description 1
- 125000003396 thiol group Chemical group [H]S* 0.000 description 1
- YFTWHEBLORWGNI-UHFFFAOYSA-N tiamiprine Chemical compound CN1C=NC([N+]([O-])=O)=C1SC1=NC(N)=NC2=C1NC=N2 YFTWHEBLORWGNI-UHFFFAOYSA-N 0.000 description 1
- 229950011457 tiamiprine Drugs 0.000 description 1
- 229940019375 tiludronate Drugs 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 1
- 229960000303 topotecan Drugs 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 231100000041 toxicology testing Toxicity 0.000 description 1
- 229940031572 toxoid vaccine Drugs 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- IUCJMVBFZDHPDX-UHFFFAOYSA-N tretamine Chemical compound C1CN1C1=NC(N2CC2)=NC(N2CC2)=N1 IUCJMVBFZDHPDX-UHFFFAOYSA-N 0.000 description 1
- 229950001353 tretamine Drugs 0.000 description 1
- 229960001727 tretinoin Drugs 0.000 description 1
- PXSOHRWMIRDKMP-UHFFFAOYSA-N triaziquone Chemical compound O=C1C(N2CC2)=C(N2CC2)C(=O)C=C1N1CC1 PXSOHRWMIRDKMP-UHFFFAOYSA-N 0.000 description 1
- 229960004560 triaziquone Drugs 0.000 description 1
- 229930013292 trichothecene Natural products 0.000 description 1
- 150000003327 trichothecene derivatives Chemical class 0.000 description 1
- 229960001670 trilostane Drugs 0.000 description 1
- KVJXBPDAXMEYOA-CXANFOAXSA-N trilostane Chemical compound OC1=C(C#N)C[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CC[C@@]32O[C@@H]31 KVJXBPDAXMEYOA-CXANFOAXSA-N 0.000 description 1
- NOYPYLRCIDNJJB-UHFFFAOYSA-N trimetrexate Chemical compound COC1=C(OC)C(OC)=CC(NCC=2C(=C3C(N)=NC(N)=NC3=CC=2)C)=C1 NOYPYLRCIDNJJB-UHFFFAOYSA-N 0.000 description 1
- 229960001099 trimetrexate Drugs 0.000 description 1
- YFTHZRPMJXBUME-UHFFFAOYSA-N tripropylamine Chemical compound CCCN(CCC)CCC YFTHZRPMJXBUME-UHFFFAOYSA-N 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 229960000875 trofosfamide Drugs 0.000 description 1
- UMKFEPPTGMDVMI-UHFFFAOYSA-N trofosfamide Chemical compound ClCCN(CCCl)P1(=O)OCCCN1CCCl UMKFEPPTGMDVMI-UHFFFAOYSA-N 0.000 description 1
- 229950010147 troxacitabine Drugs 0.000 description 1
- RXRGZNYSEHTMHC-BQBZGAKWSA-N troxacitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1O[C@@H](CO)OC1 RXRGZNYSEHTMHC-BQBZGAKWSA-N 0.000 description 1
- HDZZVAMISRMYHH-LITAXDCLSA-N tubercidin Chemical compound C1=CC=2C(N)=NC=NC=2N1[C@@H]1O[C@@H](CO)[C@H](O)[C@H]1O HDZZVAMISRMYHH-LITAXDCLSA-N 0.000 description 1
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 description 1
- 239000005483 tyrosine kinase inhibitor Substances 0.000 description 1
- 150000004917 tyrosine kinase inhibitor derivatives Chemical class 0.000 description 1
- 229950009811 ubenimex Drugs 0.000 description 1
- 238000013060 ultrafiltration and diafiltration Methods 0.000 description 1
- 241001529453 unidentified herpesvirus Species 0.000 description 1
- 229960001055 uracil mustard Drugs 0.000 description 1
- MSRILKIQRXUYCT-UHFFFAOYSA-M valproate semisodium Chemical compound [Na+].CCCC(C(O)=O)CCC.CCCC(C([O-])=O)CCC MSRILKIQRXUYCT-UHFFFAOYSA-M 0.000 description 1
- 229960000604 valproic acid Drugs 0.000 description 1
- 239000002525 vasculotropin inhibitor Substances 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- UGGWPQSBPIFKDZ-KOTLKJBCSA-N vindesine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(N)=O)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1N=C1[C]2C=CC=C1 UGGWPQSBPIFKDZ-KOTLKJBCSA-N 0.000 description 1
- 229960004355 vindesine Drugs 0.000 description 1
- GBABOYUKABKIAF-GHYRFKGUSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-GHYRFKGUSA-N 0.000 description 1
- 229960002066 vinorelbine Drugs 0.000 description 1
- 230000009385 viral infection Effects 0.000 description 1
- 239000000811 xylitol Substances 0.000 description 1
- 235000010447 xylitol Nutrition 0.000 description 1
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 description 1
- 229960002675 xylitol Drugs 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229950009268 zinostatin Drugs 0.000 description 1
- FBTUMDXHSRTGRV-ALTNURHMSA-N zorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(\C)=N\NC(=O)C=1C=CC=CC=1)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 FBTUMDXHSRTGRV-ALTNURHMSA-N 0.000 description 1
- 229960000641 zorubicin Drugs 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/28—Compounds containing heavy metals
- A61K31/282—Platinum compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/513—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim having oxo groups directly attached to the heterocyclic ring, e.g. cytosine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/519—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/555—Heterocyclic compounds containing heavy metals, e.g. hemin, hematin, melarsoprol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/66—Phosphorus compounds
- A61K31/675—Phosphorus compounds having nitrogen as a ring hetero atom, e.g. pyridoxal phosphate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7068—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines having oxo groups directly attached to the pyrimidine ring, e.g. cytidine, cytidylic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/18—Growth factors; Growth regulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/19—Cytokines; Lymphokines; Interferons
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001102—Receptors, cell surface antigens or cell surface determinants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/3955—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against proteinaceous materials, e.g. enzymes, hormones, lymphokines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/39558—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against tumor tissues, cells, antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39591—Stabilisation, fragmentation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
- A61K49/06—Nuclear magnetic resonance [NMR] contrast preparations; Magnetic resonance imaging [MRI] contrast preparations
- A61K49/08—Nuclear magnetic resonance [NMR] contrast preparations; Magnetic resonance imaging [MRI] contrast preparations characterised by the carrier
- A61K49/10—Organic compounds
- A61K49/14—Peptides, e.g. proteins
- A61K49/16—Antibodies; Immunoglobulins; Fragments thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/02—Stomatological preparations, e.g. drugs for caries, aphtae, periodontitis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/18—Drugs for disorders of the alimentary tract or the digestive system for pancreatic disorders, e.g. pancreatic enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/10—Drugs for disorders of the urinary system of the bladder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/475—Growth factors; Growth regulators
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/22—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against growth factors ; against growth regulators
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2827—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against B7 molecules, e.g. CD80, CD86
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/34—Identification of a linear epitope shorter than 20 amino acid residues or of a conformational epitope defined by amino acid residues
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/55—Fab or Fab'
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
- C07K2317/732—Antibody-dependent cellular cytotoxicity [ADCC]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
Definitions
- the present application relates to anti-PD-L1 antibodies or antigen binding fragments thereof, nucleic acid encoding the same, therapeutic compositions thereof, and their use to enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, such as tumor immunity, for the treatment of and cancer.
- lymphocyte development and activation The two major types of lymphocytes in humans are T (thymus-derived) and B (bone marrow derived. These cells are derived from hematopoietic stem cells in the bone marrow and fetal liver that have committed to the lymphoid development pathway. The progeny of these stem cells follow divergent pathways to mature into either B or T lymphocytes. Human B-lymphocyte development takes place entirely within the bone marrow. T cells, on the other hand, develop from immature precursors that leave the marrow and travel through the bloodstream to the thymus, where they proliferate and differentiate into mature T lymphocytes.
- Mature lymphocytes that emerge from the thymus or bone marrow are in a
- quiescent, or "resting” state i.e., they are mitotically inactive.
- these "naive” or “virgin” lymphocytes travel into various secondary or peripheral lymphoid organs, such as the spleen, lymph nodes or tonsils.
- Most virgin lymphocytes have an inherently short life span and die without a few days after leaving the marrow or thymus. However, if such a cell receives signals that indicate the presence of an antigen, they may activate and undergo successive rounds of cell division. Some of the resulting progeny cells then revert to the resting state to become memory lymphocytes - B and T cells that are essentially primed for the next encounter with the stimulating allergen.
- Lymphocyte activation refers to an ordered series of events through which a resting lymphocyte passes as it is stimulated to divide and produce progeny, some of which become effector cells.
- a full response includes both the induction of cell proliferation (mitogenesis) and the expression of immunologic functions.
- Lymphocytes become activated when specific ligands bind to receptors on their surfaces. The ligands are different for T cells and B cells, but the resulting intracellular physiological
- These more common antigens activate B cells when they are co-stimulated with nearby activated helper T- lymphocytes. Such stimulation may occur from lymphokines secreted by the T-cell, but is transmitted most efficiently by direct contact of the B cell with T-cell surface proteins that interact with certain B-cell surface receptors to generate a secondary signal.
- T lymphocytes do not express immunoglobulins, but, instead detect the presence of foreign substances by way of surface proteins called T-cell receptors (TCR). These receptors recognize antigens by either direct contact or through influencing the activity of other immune cells. Together with macrophages, T cells are the primary cell type involved in the cell-mediated immunity.
- T-cells can detect foreign substances only in specific contexts.
- T-lymphocytes will recognize a foreign protein only if it first cleaved into small peptides, which are then displayed on the surface of a second host cell, called an antigen- presenting cell (APC).
- APC antigen-presenting cell
- Many types of host cells can present antigens under some conditions but certain types are more specifically adapted for this purpose and are particularly important in controlling T-cell activity, including macrophages and other B-cells.
- Antigen presentation depends in part on specific proteins, called major histocompatibility complex (MHC) proteins, on the surface of the presenting cells.
- MHC major histocompatibility complex
- T c cells cytotoxic T lymphocytes
- T H helper T cells
- T c cells are important in viral defense, and can kill viruses directly by recognizing certain cell surface expressed viral peptides.
- TH cells promote proliferation, maturation and immunologic function of other cell types, e.g. , lymphokine secretion to control activities of B cells,
- cytotoxic T cells Both virgin and memory T-lymphocytes ordinarily remain in the resting state, and in this state they do not exhibit significant helper or cytotoxic activity. When activated, these cells undergo several rounds of mitotic division to produce daughter cells. Some of these daughter cells return to the resting state as memory cells, but others become effector cells that actively express helper or cytotoxic activity. These daughter cells resemble their parents: CD4+ cells can only product CD4+ progeny, while CD8+ cells yield only CD8+ progeny. Effector T- cells express cell surface markers that are not expressed on resting T-cells, such as CD25, CD28, CD29, CD40L, transferrin receptors and class II MHC proteins.
- T-lymphocyte responses to most antigens also require two types of simultaneous stimuli.
- the first is the antigen, which if appropriately displayed by MHC proteins on an antigen-presenting cell, can be recognized and bound by T-cell receptors. While this antigen-MHC complex does send a signal to the cell interior, it is usually insufficient to result in T-cell activation.
- Full activation such as occurs with helper T-cells, requires costimulation with other specific ligands called costimulators that are expressed on the surface of the antigen-presenting cell.
- Activation of a cytotoxic T cell generally requires IL -2, a cytokine secreted by activated helper T cells.
- PD-1 programmed death-1 receptor
- B7-H1, CD274 PD-L1
- PD-L2 B7-DC, CD273
- the negative regulatory role of PD-1 was revealed by PD-1 knock outs (Pdcdl ' ' ' ), which are prone to autoimmunity.
- PD-1 is related to CD28 and CTLA-4, but lacks the membrane proximal cysteine that allows homodimerization.
- the cytoplasmic domain of PD-1 contains an immunoreceptor tyorine-based inhibition motif (ITIM, V/lxYxxL/V).
- ITIM immunoreceptor tyorine-based inhibition motif
- PD-1 only binds to PD-L1 and PD-L2.
- PD-1 can be expressed on T cells, B cells, natural killer T cells, activated monocytes and dendritic cells (DCs). PD-1 is expressed by activated, but not by unstimulated human CD4 + and CD8 + T cells, B cells and myeloid cells. This stands in contrast to the more restricted expression of CD28 and CTLA-4. Nishimura et al, Int. Immunol. 8: 773-80 (1996); Boettler et al, J. Virol. 80: 3532-40 (2006).
- PD-1 PD-1 that have been cloned from activated human T cells, including transcripts lacking (i) exon 2, (ii) exon 3, (iii) exons 2 and 3 or (iv) exons 2 through 4. Nielsen et al, Cell. Immunol. 235: 109-16 (2005). With the exception of PD-IAex3, all variants are expressed at similar levels as full length PD-1 in resting peripheral blood mononuclear cells (PBMCs). Expression of all variants is significantly induced upon activation of human T cells with anti-CD3 and anti-CD28.
- PBMCs peripheral blood mononuclear cells
- the PD-1Aex3 variants lacks a transmembrane domain, and resembles soluble CTLA-4, which plays an important role in autoimmunity. Ueda et al, Nature 423: 506-11 (2003). This variant is enriched in the synovial fluid and sera of patients with rheumatoid arthritis. Wan et al, J. Immunol. 177: 8844-50 (2006).
- the two PD-1 ligands differ in their expression patterns.
- PD-L1 is constitutively expressed on mouse T and B cells, CDs,
- PD-L1 is expressed on a wide range of nonhematopoietic cells (e.g., cornea, lung, vascular epithelium, liver nonpar enchymal cells, mesenchymal stem cells, pancreatic islets, placental
- JAK2 has also been implicated in PD-L1 induction.
- Loss or inhibition of phosphatase and tensin homolog (PTEN), a cellular phosphatase that modified phosphatidylinosital 3-kinase (PI3K) and Akt signaling increased post- transcriptional PD-L1 expression in cancers.
- PTEN phosphatase and tensin homolog
- PI3K a cellular phosphatase that modified phosphatidylinosital 3-kinase
- Akt signaling increased post- transcriptional PD-L1 expression in cancers.
- PD-L2 expression is more restricted than PD-L1.
- PD-L2 is inducibly expressed on DCs, macrophag
- PD-L2 is also expressed on about half to two-thirds of resting peritoneal Bl cells, but not on conventional B2 B cells. Zhong et al, Eur. J. Immunol. 37: 2405-10 (2007). PD-L2+ Bl cells bind phosphatidylcholine and may be important for innate immune responses against bacterial antigens. Induction of PD-L2 by IFN- ⁇ is partially dependent upon NF -KB. Liang et al, Eur. J. Immunol. 33_: 2706-16 (2003). PD-L2 can also be induced on monocytes and macrophages by GM-CF, IL-4 and and IFN- ⁇ . Yamazaki et al., J. Immunol. 169: 5538-45 (2002); Loke et al, PNAS 100:5336 ⁇ 1 (2003).
- PD-1 signaling typically has a greater effect on cytokine production than on cellular proliferation, with significant effects on IFN- ⁇ , TNF-a and IL-2 production.
- PD-1 mediated inhibitory signaling also depends on the strength of the TCR signaling, with greater inhibition delivered at low levels of TCR stimulation. This reduction can be overcome by costimulation through CD28 [Freeman et al, J. Exp. Med. 192: 1027-34 (2000)] or the presence of IL-2 [Carter et al, Eur. J. Immunol. 32: 634-43 (2002)]- Evidence is mounting that signaling through PD-L1 and PD-L2 may be bidirectional.
- signaling may also be delivered back to the cells expressing PD-L1 and PD-L2.
- a naturally human anti-PD-L2 antibody isolated from a patient with Waldenstrom's macroglobulinemia was not found to upregulate MHC II or B7 costimulatory molecules, such cells did produce greater amount of proinflammatory cytokines, particularly TNF-a and IL-6, and stimulated T cell proliferation.
- Treatment of mice with this antibody also (1) enhanced resistance to transplated bl6 melanoma and rapidly induced tumor- specific CTL.
- Radhakrishnan et al J. Immunol. 170: 1830-38 (2003); Radhakrishnan et al, Cancer Res. 64: 4965-72 (2004); Heckman et al, Eur. J. Immunol. 37: 1827-35 (2007); (2) blocked development of airway inflammatory disease in a mouse model of allergic asthma. Radhakrishnan et al, J. Immunol. 173: 1360-65 (2004);
- B7.1 has already been identified as a binding partner for PD-L1. Butte et al, Immunity 27: 111-22 (2007). Chemical crosslinking studies suggest that PD-L1 and B7.1 can interact through their IgV-like domains. B7.1 :PD-L1 interactions can induce an inhibitory signal into T cells.
- T cells lacking CD28 and CTLA-4 show decreased proliferation and cytokine production when stimulated by anti-CD3 plus B7.1 coated beads.
- T cells lacking all the receptors for B7.1 i.e., CD28, CTLA-4 and PD-L1
- T-cell proliferation and cytokine production were no longer inhibited by anti-CD3 plus B7.1 coated beads. This indicates that B7.1 acts specifically through PD-L1 on the T- cell in the absence of CD28 and CTLA-4.
- T cells lacking PD-1 showed decreased proliferation and cytokine production when stimulated in the presence of anti-CD3 plus PD-L1 coated beads, demonstrating the inhibitory effect of PD-L1 ligation on B7.1 on T cells.
- T cells lacking all known receptors for PD-L1 i.e., no PD-1 and B7.1
- T cell proliferation was no longer impaired by anti-CD3 plus PD- L1 coated beads.
- PD-L1 can exert an inhibitory effect on T cells either through B7.1 or PD-1.
- T cell PD-L1 may trap or segregate away APC B7.1 from interaction with CD28.
- the antagonism of signaling through PD-L1 including blocking PD-L1 from interacting with either PD-1 , B7.1 or both, thereby preventing PD-L1 from sending a negative co- stimulatory signal to T-cells and other antigen presenting cells is likely to enhance immunity in response to infection (e.g., acute and chronic) and tumor immunity.
- the anti-PD-L1 antibodies of the present invention may be combined with antagonists of other components of PD-1 : PD-L1 signaling, for example, antagonist anti-PD-1 and anti-PD-L2 antibodies.
- the inhibition of PD-L1 signaling has been proposed as a means to enhance T cell immunity for the treatment of cancer (e.g., tumor immunity) and infection, including both acute and chronic (e.g., persistent) infection.
- cancer e.g., tumor immunity
- infection including both acute and chronic (e.g., persistent) infection.
- Inhibitors blocking the PD-L1 : PD-1 interaction are known from, i.a.,
- anti-PD-L1 antibodies including nucleic acids encoding and compositions containing such antibodies, and for their use to enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, such as tumor immunity.
- ADCC antibody dependent cell-mediated cytotoxicity
- the anti-PD-L1 antibodies according to the present invention which have antibody dependent cell-mediated cytotoxicity (ADCC) activity, directly act on PD-L1 bearing tumor cells by inducing their lysis without showing any significant toxicity.
- ADCC antibody dependent cell-mediated cytotoxicity
- the antibodies do not only block the interaction between human PD-L1 and human PD-1 , but also the interactions between the respective mouse and cynomolgus monkey proteins.
- the invention provides for an isolated heavy chain variable region polypeptide comprising an HVR-H1 , HVR-H2 and HVR-H3 sequence, wherein:
- HVR-H1 sequence is X YX 2 MX 3 (SEQ ID NO:1);
- the HVR-H2 sequence is SIYPSGGX 4 TFYADX 5 VKG (SEQ ID NO:2);
- the HVR-H3 sequence is IKLGTVTTVX 6 Y (SEQ ID NO:3); further wherein: X ⁇ is K, R, T, Q, G, A, W, M, I or S; X 2 is V, R, K, L, or I; X 3 is H ( T, N, Q, A, V, Y, W, F or M; X4 is F or I; X 5 is S or T; X 6 is E or D.
- Xi is M, I or S;
- X 2 is R, K, L, M or I;
- X 3 is F or M;
- X4 is F or l;
- X 5 is S or T;
- X 6 is E or D.
- Xi is M, I or S; X 2 is L, M or I; X 3 is F or M; X4 is I; X 5 is S or T; Xe is D.
- Xi is S; X 2 is I; X3 is M; X4 is I; X5 is T; Xe is D.
- polypeptide further comprises variable region heavy chain framework sequences juxtaposed between the HVRs according to the formula: (HC- FR1)-(HVR-H1)-(HC-FR2)-(HVR-H2)-(HC-FR3)-(HVR-H3)- (HC-FR4).
- the framework sequences are derived from human consensus framework sequences or human germline framework sequences.
- HC-FR1 is EVQLLESGGGLVQPGGSLRLSCAASGFTFS (SEQ ID NO:4);
- HC-FR2 is WVRQAPGKG LEWVS (SEQ ID NO:5);
- HC-FR3 is RFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR (SEQ ID NO:6);
- HC-FR4 is WGQGTLVTVSS (SEQ ID NO:7).
- the heavy chain polypeptide is further combined with a variable region light chain comprising an an HVR-L1 , HVR-L2 and HVR-L3, wherein: (a) the HVR-L1 sequence is (SEQ ID NO.8);
- HVR-L2 sequence is XioVXnX 12 RPS (SEQ ID NO:9);
- X 7 is N or S
- Xe is T, R or S
- X 9 is A or G
- X10 is E or D
- Xn is I, N or
- the light chain further comprises variable region light chain framework sequences juxtaposed between the HVRs according to the formula: (LC- FR1 MHVR-L1 )-(LC-FR2)-(HVR-L2)-(LC-FR3)-(HVR-L3)-(LC-FR4).
- the light chain framework sequences are derived from human consensus framework sequences or human germline framework sequences.
- the light chain framework sequences are lambda light chain sequences.
- at least one of the framework sequence is the following:
- LC-FR1 is QSALTQPASVSGSPGQSITISC (SEQ ID NO:11);
- LC-FR2 is WYQQHPGKAPKLMIY (SEQ ID NO:12);
- LC-FR3 is GVSNRFSGSKSGNTASLTISGLQAEDEADYYC (SEQ ID NO:13);
- LC-FR4 is FGTGTKVTVL (SEQ ID NO: 14).
- the invention provides an isolated anti-PD-L1 antibody or antigen binding fragment comprising a heavy chain and a light chain variable region sequence, wherein:
- the heavy chain comprises an HVR-H1 , HVR-H2 and HVR-H3, wherein further: (i) the HVR-H1 sequence is X1YX2MX3 (SEQ ID NO:1); (ii) the HVR-H2 sequence is
- the light chain comprises an HVR-L1 , HVR-L2 and HVR-L3, wherein further: (iv) the HVR-L1 sequence is (SEQ ID NO:8); (v) the HVR-L2 sequence is X 10 VX 11 X 12 RPS (SEQ ID NO:9); (vi) the HVR-L3 sequence is
- Xi is K, R, T, Q, G, A, W, M, I or S
- X 2 is V, R, K, L, M or I
- X 3 is H, T, N, Q, A, V, Y, W, F or M
- X4 is F or I
- X 5 is S or T
- Xe is E or D
- X 7 is N or S
- X 8 is T, R or S
- X 9 is A or G
- X 0 is E or D
- Xn is I, N or S
- X 1 2 is D, H or N
- X 1 3 is F or Y
- X 14 is N or S
- X 15 is R, T or S
- X 6 is G or S
- Xi 7 is I or T.
- Xi is M, I or S; X 2 is R, K, L, M or I; X 3 is F or M; X4 is F or I; X 5 is S or T; Xe is E or D; X 7 is N or S; X 8 is T, R or S; X 9 is A or G; X i0 is E or D; Xn is N or S; X 12 is N; X13 is F or Y; X 14 is S; X15 is S; X 6 is G or S; X 17 is T.
- Xi is M, I or S; X 2 is L, M or I; X 3 is F or M; X4 is I; X 5 is S or T; X 6 is D; X 7 is N or S; X 8 is T, R or S; X 9 is A or G; X 10 is E or D; Xn is N or S; X12 is N; X 3 is F or Y; X14 is S; X15 is S; Xi 6 is G or S; X 17 is T.
- the heavy chain variable region comprises one or more
- framework sequences juxtaposed between the HVRs as: (HC-FR1)-(HVR-H1)-(HC- FR2)-(HVR-H2)-(HC-FR3)-(HVR- H3)-(HC-FR4), and the light chain variable regions comprises one or more framework sequences juxtaposed between the HVRs as: (LC-FR1 MHVR-L1 )-(LC-FR2)-(HVR-l_2)-(LC-FR3)-(HVR- L3)-(LC-FR4).
- framework sequences are derived from human
- one or more of the heavy chain framework sequences is the following:
- HC-FR1 is EVQLLESGGGLVQPGGSLRLSCAASGFTFS (SEQ ID NO:4);
- HC-FR2 is WVRQAPGKGLEWVS (SEQ ID NO:5);
- HC-FR3 is RFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR (SEQ ID NO:6);
- HC-FR4 is WGQGTLVTVSS (SEQ ID NO:7).
- the light chain framework sequences are lambda light chain sequences.
- one or more of the light chain framework sequences is the following:
- LC-FR1 is QSALTQPASVSGSPGQSITISC (SEQ ID NO:11);
- LC-FR2 is WYQQHPGKAPKLMIY (SEQ ID NO:12);
- LC-FR3 is GVSNRFSGSKSGNTASLTISGLQAEDEADYYC; (SEQ ID NO: 13);
- LC-FR4 is FGTGTKVTVL (SEQ ID NO: 14).
- the heavy chain variable region polypeptide, antibody or antibody fragment further comprises at least a C H 1 domain. In a more specific aspect, the heavy chain variable region polypeptide, antibody or antibody fragment further comprises a C H 1, a C H 2 and a C H 3 domain.
- variable region light chain, antibody or antibody fragment further comprises a C L domain.
- the antibody further comprises a C H 1, a C H 2, a C H 3 and a C L domain. In a still further specific aspect, the antibody further comprises a human or murine constant region.
- the human constant region is selected from the group consisting of lgG1, lgG2, lgG2, lgG3, lgG4.
- the human or murine constant region is lgG1.
- the invention provides for an anti-PD-L1 antibody comprising a heavy chain and a light chain variable region sequence, wherein:
- the heavy chain comprises an HVR-H1, HVR-H2 and an HVR-H3, having at least 80% overall sequence identity to SYIMM (SEQ ID NO:15), SIYPSGGITFYADTVKG
- the light chain comprises an HVR-L1 , HVR-L2 and an HVR-L3, having at least 80% overall sequence identity to TGTSSDVGGYNYVS (SEQ ID NO:18), DVSNRPS (SEQ ID NO:19) and SSYTSSSTRV (SEQ ID NO:20), respectively.
- sequence identity is 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99% or 100%.
- the invention provides for an anti-PD-L1 antibody comprising a heavy chain and a light chain variable region sequence, wherein:
- the heavy chain comprises an HVR-H1 , HVR-H2 and an HVR-H3, having at least 80% overall sequence identity to MYMMM (SEQ ID NO:21), SIYPSGGITFYADSVKG (SEQ ID NO:22) and IKLGTVTTVDY (SEQ ID NO: 17), respectively, and
- the light chain comprises an HVR-L1, HVR-L2 and an HVR-L3, having at least 80% overall sequence identity to TGTSSDVGAYNYVS (SEQ ID NO:23), DVSNRPS (SEQ ID NO: 19) and SSYTSSSTRV (SEQ ID NO.20), respectively.
- sequence identity is 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99% or 100%.
- HVR-H1 SEQ ID NO:15
- HVR-H2 SEQ ID NO:16
- HVR-H3 SEQ ID NO:17
- HVR-L1 SEQ ID NO: 18
- HVR-L2 SEQ ID NO: 19
- HVR-L3 SEQ ID NO:20
- the heavy chain variable region comprises one or more framework sequences juxtaposed between the HVRs as: (HC-FR1)- (HVR-H1)-(HC-FR2)-(HVR- H2)-(HC-FR3)-(HVR-H3)-(HC-FR4), and the light chain variable regions comprises one or more framework sequences juxtaposed between the HVRs as: (LC- FR1)- (HVR-L1 )-(LC-FR2)-(HVR-l_2)-(LC-FR3)-(HVR-L3)-(LC-FR4).
- the framework sequences are derived from human germline sequences.
- one or more of the heavy chain framework sequences is the following:
- HC-FR1 is EVQLLESGGGLVQPGGSLRLSCAASGFTFS (SEQ ID NO:4);
- HC-FR2 is WVRQAPGKG LEWVS (SEQ ID NO:5);
- HC-FR3 is RFTISRDNSKNTLYLQMNSLRAEDTAVYYCAR (SEQ ID NO:6); HC-FR4 is WGQGTLVTVSS (SEQ ID NO:7).
- the light chain framework sequences are derived from a lambda light chain sequence.
- one or more of the light chain framework sequences is the following:
- LC-FR1 is QSALTQPASVSGSPGQSITISC (SEQ ID NO:11);
- LC-FR2 is WYQQHPGKAPKLMIY (SEQ ID NO: 12);
- LC-FR3 is GVSNRFSGSKSGNTASLTISGLQAEDEADYYC (SEQ ID NO:13);
- LC-FR4 is FGTGTKVTVL (SEQ ID NO:14).
- the antibody further comprises a human or murine constant region.
- the human constant region is selected from the group consisting of lgG1, lgG2, lgG2, lgG3, lgG4.
- the invention provides for an isolated anti-PD-L1 antibody comprising a heavy chain and a light chain variable region sequence, wherein:
- the heavy chain sequence has at least 85% sequence identity to the heavy chain sequence:
- the light chain sequence has at least 85% sequence identity to the light chain sequence:
- sequence identity is 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99% or 100%.
- the invention provides for an isolated anti-PD-L1 antibody comprising a heavy chain and a light chain variable region sequence, wherein:
- the heavy chain sequence has at least 85% sequence identity to the heavy chain sequence:
- the light chain sequence has at least 85% sequence identity to the light chain sequence:
- sequence identity is 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99% or 100%.
- the antibody binds to human, mouse or cynomolgus monkey PD-L1.
- the antibody is capable of blocking the interaction between human, mouse or cynomolgus monkey PD-L1 and the respective human, mouse or cynomolgus monkey PD-1 receptors.
- the antibody binds to human PD-L1 with a KD of 5x10 "9 M or less, preferably with a KD of 2x10 "9 M or less, and even more preferred with a KD of 1x10 "9 M or less.
- the invention concerns an isolated anti-PD-L1 antibody or antigen binding fragment thereof which binds to a functional epitope comprising residues Y56 and D61 of human PD-L1 (SEQ ID NO:28).
- the functional epitope further comprises E58, E60, Q66, R113 and M115 of human PD-L1 (SEQ ID NO:28).
- the antibody binds to a conformational epitope, comprising residues 54-66 and 1 2-122 of human PD-L1 (SEQ ID NO:28).
- the invention is related to an anti-PD-L1 antibody, or antigen binding fragment thereof, which cross-competes for binding to PD-L1 with an antibody according to the invention as described herein.
- the invention provides for compositions comprising any of the above described anti-PD-L1 antibodies in combination with at least one pharmaceutically acceptable carrier.
- the invention provides for an isolated nucleic acid encoding a polypeptide, or light chain or a heavy chain variable region sequence of an anti-PD-L1 antibody, or antigen binding fragment thereof, as described herein. In a still further embodiment, the invention provides for an isolated nucleic acid encoding a light chain or a heavy chain variable region sequence of an anti-PD-L1 antibody, wherein:
- the heavy chain comprises an HVR-H1 , HVR-H2 and an HVR-H3 sequence having at least 80% sequence identity to SYIMM (SEQ ID NO: 15),
- SIYPSGGITFYADTVKG SEQ ID NO: 16
- IKLGTVTTVDY SEQ ID NO: 17
- the light chain comprises an HVR-L1 , HVR-L2 and an HVR-L3 sequence having at least 80% sequence identity to TGTSSDVGGYNYVS (SEQ ID NO: 18), DVSNRPS (SEQ ID NO:19) and SSYTSSSTRV (SEQ ID NO:20), respectively.
- sequence identity is 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99% or 100%.
- nucleic acid is SEQ ID NO:30 for the heavy chain, and SEQ ID NO:31 for the light chain.
- the nucleic acid further comprises a vector suitable for expression of the nucleic acid encoding any of the previously described anti-PD-L1 antibodies.
- the vector further comprises a host cell suitable for expression of the nucleic acid.
- the host cell is a eukaryotic cell or a prokaryotic cell.
- the eukaryotic cell is a mammalian cell, such as Chinese Hamster Ovary (CHO).
- CHO Chinese Hamster Ovary
- the invention provides for a process of making an anti- PD- L1 antibody or antigen binding fragment thereof, comprising culturing a host cell containing nucleic acid encoding any of the previously described anti-PD-L1 antibodies or antigen-binding fragment in a form suitable for expression, under conditions suitable to produce such antibody or fragment, and recovering the antibody or fragment.
- the invention provides a kit of parts comprising a container enclosing a therapeutically effective amount of a composition disclosed herein and a package insert indicating use for the treatment of a T-cell dysfunctional disorder.
- the invention provides for a kit of parts comprising any of the above described anti-PD-L1 compositions in combination with at least one further therapeutic agent or vaccine, such as a chemotherapeutic agent.
- the at least one chemotherapeutic agent is gemcitabine
- the vaccine is Stimuvax.
- the invention provides for a method of enhancing T-cell function comprising administering an effective amount of any of the above described anti-PD-L1 antibodies or compositions.
- the anti-PD-L1 antibody or composition renders dysfunctional T-cells non-dysfunctional.
- the antibody or composition treats of prevents a symptom of persistent infection, such as viral infection, e.g. by human immunodeficiency virus (HIV), herpes virus, Eppstein-Barr virus or human papilloma virus.
- HIV human immunodeficiency virus
- the invention provides for a method of treating a T-cell dysfunctional disorder comprising administering a therapeutically effective amount of any of the above described anti-PD-L1 antibodies or compositions.
- the T-cell dysfunctional disorder is tumor immunity.
- the method further comprises treatment with a vaccine.
- the PD-L1 antibody or composition is combined with a treatment regimen further comprising a traditional therapy selected from the group consisting of: surgery, radiation therapy, chemotherapy, targeted therapy,
- the tumor immunity results from a cancer selected from the group consisting of: breast, lung, colon, ovarian, melanoma, bladder, kidney, liver, salivary, stomach, gliomas, thyroid, thymic, epithelial, head and neck cancers, gastric, and pancreatic cancer.
- Another aspect of the invention relates to the use of antibody dependent cell- mediated cytotoxicity (ADCC) of an anti-PD-L1 antibody disclosed herein or composition in the treatment of cancer.
- ADCC antibody dependent cell- mediated cytotoxicity
- the invention pertains to method of treating cancer comprising
- an anti-PD-L1 antibody which induces antibody dependent cell-mediated cytotoxicity (ADCC).
- ADCC antibody dependent cell-mediated cytotoxicity
- the constant region of the anti-PD-L1 antibody is lgG1.
- the cancer is selected from the group consisting of: breast, lung, colon, ovarian, melanoma, bladder, kidney, liver, salivary, stomach, gliomas, thyroid, thymic, epithelial, head and neck cancers, gastric and pancreatic cancer.
- the invention relates likewise to the use of an anti-PD-L1 antibody or composition as described above and below for the manufacture of a medicament for ehancing T-cell function, treating a T-cell dysfunctional disorder or treating cancer;
- the invention is directed to engineered antibodies, or engineered antibody fragments, which are fused directly or via a linker molecule to therapeutic agents, such as cytokines (e.g. IL-2, IL-12, TNFa, IFNa, IFNb), or growth factors; which engineered antibodies or engineered antibody fragments may also be used in tumor therapy and immune system related diseases.
- cytokines e.g. IL-2, IL-12, TNFa, IFNa, IFNb
- growth factors e.g. IL-2, IL-12, TNFa, IFNa, IFNb
- Antibody fusion proteins, especially immunocytokines are well known in the art.
- the fusion partner can be bound to the N-terminus of the antibody or antibody fragment or, preferably, to its C-terminus.
- Disfunction in the context of immune dysfunction, refers to a state of immune reduced responsiveness to antigenic stimulation.
- the term includes the common elements of both exhaustion and/or anergy in which antigen recognition may occur, but the ensuing immune response is ineffective to control infection or tumor growth.
- Enhancing T-cell function means to induce, cause or stimulate a T-cell to have a sustained or amplified biological function, or renew or reactivate exhausted or inactive T-cells.
- enhancing T-cell function include: increased secretion of ⁇ -interferon from CD8 + T- cells, increased proliferation, increased antigen responsiveness (e.g., viral or pathogen clearance) relative to such levels before the intervention.
- the level of enhancement is as least 50%, alternatively 60%, 70%, 80%, 90%, 100%, 120%, 150%, 200%. The manner of measuring this enhancement is known to one of ordinary skill in the art.
- T cell dysfunctional disorder is a disorder or condition of T-cells characterized by decreased responsiveness to antigenic stimulation.
- a T- cell dysfunctional disorder is a disorder that is specifically associated with
- T -cell dysfunctional disorder is one in which T-cells are anergic or have decreased ability to secrete cytokines, proliferate, or execute cytolytic activity.
- the decreased responsiveness results in ineffective control of a pathogen or tumor expressing an immunogen.
- T cell dysfunctional disorders characterized by T-cell dysfunction include unresolved acute infection, chronic infection and tumor immunity.
- 'Tumor immunity refers to the process in which tumors evade immune recognition and clearance.
- tumor immunity is "treated” when such evasion is attenuated, and the tumors are recognized and attacked by the immune system.
- tumor recognition include tumor binding, tumor shrinkage and tumor clearance.
- vaccine includes any nonpathogenic immunogen that, when inoculated into a host, induces protective immunity against a specific pathogen.
- Vaccines can take many forms. Vaccines can be whole organisms that share important antigens with the pathogem, but are not pathogenic themselves (e.g., cowpox). Vaccines can also be prepared from killed (e.g., Salk polio vaccine) or attenuated (lost ability to produce disease - e.g., Sabin polio vaccine). Vaccines can also be prepared from purified macromolecules isolated from the pathogenic organism. For example, toxoid vaccines (e.g.
- Subunit vaccines e.g., Hepatitis B
- Hapten conjugate vaccines attaches certain carbohydrate or polypeptide epitopes isolated from the pathogen of interest to immunogenic carriers, such as tetanus toxoid. These strategies essentially use the epitopes as haptens to induce antibody production, which then recognize the same epitope in the native pathogen.
- DNA vaccines exploit the ability of host cells to take up and express DNA encoding pathogenic proteins that is injected intramuscularly. Host responses to immunogens can be enhanced if administered as a mixture with adjuvants.
- Immune adjuvants function in one or more of the following ways: (1) prolonging retention of the immunogen, (2) increased effective size of the immunogen (and hence promoting phagocytosis and presentation to macrophages), (3) stimulating the influx of macrophage or other immune cells to the injection site, or (4) promoting local cytokine production and other immunologic activities.
- Example adjuvants include: complete Freund's adjuvant (CFA), aluminum salts, and mycobacterial derived proteins such as muramyl di- or tri-peptides.
- antibody includes monoclonal antibodies (including full length antibodies which have an immunoglobulin Fc region), antibody compositions with polyepitopic specificity, multispecific antibodies ⁇ e.g., bispecific antibodies, diabodies, and single- chain molecules, as well as antibody fragments (e.g., Fab, F(ab')2, and Fv).
- immunoglobulin Ig
- the basic 4- chain antibody unit is a heterotetrameric glycoprotein composed of two identical light (L) chains and two identical heavy (H) chains.
- IgM antibody consists of 5 of the basic heterotetramer units along with an additional polypeptide called a J chain, and contains 10 antigen binding sites, while IgA antibodies comprise from 2-5 of the basic 4-chain units which can polymerize to form polyvalent assemblages in combination with the J chain.
- the 4-chain unit is generally about 150,000 daltons.
- Each L chain is linked to an H chain by one covalent disulfide bond, while the two H chains are linked to each other by one or more disulfide bonds depending on the H chain isotype.
- Each H and L chain also has regularly spaced intrachain disulfide bridges.
- Each H chain has at the N-terminus, a variable domain (V H ) followed by three constant domains (C H ) for each of the a and ⁇ chains and four C H domains for ⁇ and ⁇ isotypes.
- Each L chain has at the N-terminus, a variable domain (V L ) followed by a constant domain at its other end.
- the V L is aligned with the V H and the C L is aligned with the first constant domain of the heavy chain (C H 1).
- Particular amino acid residues are believed to form an interface between the light chain and heavy chain variable domains.
- the pairing of a V H and V L together forms a single antigen-binding site.
- L chain from any vertebrate species can be assigned to one of two clearly distinct types, called kappa and lambda, based on the amino acid sequences of their constant domains.
- immunoglobulins can be assigned to different classes or isotypes.
- immunoglobulins There are five classes of immunoglobulins: IgA, IgD, IgE, IgG and IgM, having heavy chains designated ⁇ , ⁇ , ⁇ , ⁇ and ⁇ , respectively.
- the Y and a classes are further divided into subclasses on the basis of relatively minor differences in the CH sequence and function, e.g., humans express the following subclasses: lgG1, lgG2A, lgG2B, lgG3, lgG4, lgA1 and lgK1.
- An “isolated” antibody is one that has been identified, separated and/or recovered from a component of its production environment (E.g., natural or recombinant).
- the isolated polypeptide is free of association with all other components from its production environment.
- Contaminant components of its production environment such as that resulting from recombinant transfected cells, are materials that would typically interfere with research, diagnostic or therapeutic uses for the antibody, and may include enzymes, hormones, and other proteinaceous or non- proteinaceous solutes.
- the polypeptide will be purified: (1) to greater than 95% by weight of antibody as determined by, for example, the Lowry method, and in some embodiments, to greater than 99% by weight; (1) to a degree sufficient to obtain at least 15 residues of N-terminal or internal amino acid sequence by use of a spinning cup sequenator, or (3) to homogeneity by SDS-PAGE under non-reducing or reducing conditions using Coomassie blue or, preferably, silver stain.
- Isolated antibody includes the antibody in situ within recombinant cells since at least one component of the antibody's natural environment will not be present.
- variable region or “variable domain” of an antibody refers to the amino-terminal domains of the heavy or light chain of the antibody.
- the variable domains of the heavy chain and light chain may be referred to as "VH” and “VL”, respectively. These domains are generally the most variable parts of the antibody (relative to other antibodies of the same class) and contain the antigen binding sites.
- variable refers to the fact that certain segments of the variable domains differ extensively in sequence among antibodies.
- the V domain mediates antigen binding and defines the specificity of a particular antibody for its particular antigen.
- variability is not evenly distributed across the entire span of the variable domains. Instead, it is concentrated in three segments called hypervariable regions (HVRs) both in the light-chain and the heavy chain variable domains.
- HVRs hypervariable regions
- the more highly conserved portions of variable domains are called the framework regions (FR).
- the variable domains of native heavy and light chains each comprise four FR regions, largely adopting a beta-sheet configuration, connected by three HVRs, which form loops connecting, and in some cases forming part of, the beta-sheet structure.
- the HVRs in each chain are held together in close proximity by the FR regions and, with the HVRs from the other chain, contribute to the formation of the antigen binding site of antibodies (see Kabat et al, Sequences of Immunological Interest, Fifth Edition, National Institute of Health, Bethesda, MD (1991)).
- the constant domains are not involved directly in the binding of antibody to an antigen, but exhibit various effector functions, such as participation of the antibody in antibody-dependent cellular toxicity.
- monoclonal antibody refers to an antibody obtained from a population of substantially homogeneous antibodies, i.e., the individual antibodies comprising the population are identical except for possible naturally occurring mutations and/or post-translation modifications (e.g., isomerizations, amidations) that may be present in minor amounts. Monoclonal antibodies are highly specific, being directed against a single antigenic site. In contrast to polyclonal antibody
- each monoclonal antibody is directed against a single determinant on the antigen.
- the monoclonal antibodies are advantageous in that they are synthesized by the hybridoma culture, uncontaminated by other immunoglobulins.
- the modifier "monoclonal” indicates the character of the antibody as being obtained from a substantially homogeneous population of antibodies, and is not to be construed as requiring production of the antibody by any particular method.
- the monoclonal antibodies to be used in accordance with the present invention may be made by a variety of techniques, including, for example, the hybridoma method (e.g., Kohler and Milstein., Nature, 256:495-97 (1975); Hongo et al, Hybridoma, 14 (3): 253-260 (1995), Harlow et al, Antibodies: A Laboratory Manual, (Cold Spring Harbor Laboratory Press, 2 nd ed. 1988); Hammerling et al, in: Monoclonal Antibodies and T-Cell Hybridomas 563- 681 (Elsevier, N. Y., 1981)), recombinant DNA methods (see, e.g., U.S. Patent No.
- phage-display technologies see, e.g., Clackson et al, Nature, 352: 624- 628 (1991); Marks et al, J. Mol Biol. 222: 581-597 (1992); Sidhu et al, J. Mol Biol. 338(2): 299-310 (2004); Lee et al, J. Mol Biol. 340(5): 1073-1093 (2004); Fellouse, Proc. Natl. Acad. ScL USA 101(34): 12467-12472 (2004); and Lee et al, J. Immunol. Methods 284(1-2): 119-132 (2004), and technologies for producing human or humanlike antibodies in animals that have parts or all of the human immunoglobulin loci or genes encoding human immunoglobulin sequences (see, e.g., WO
- antibody fragment comprises a portion of an intact antibody, preferably the antigen binding and/or the variable region of the intact antibody.
- antibody fragments include Fab, Fab * , F(ab') 2 and Fv fragments; diabodies; linear antibodies (see U.S. Patent 5,641,870, Example 2; Zapata et al, Protein Eng. 8HO): 1057-1062 [1995]); single-chain antibody molecules and multispecific antibodies formed from antibody fragments. Papain digestion of antibodies produced two identical antigen-binding fragments, called “Fab” fragments, and a residual "Fc” fragment, a designation reflecting the ability to crystallize readily.
- the Fab fragment consists of an entire L chain along with the variable region domain of the H chain (V H ), and the first constant domain of one heavy chain (CH1).
- V H variable region domain
- CH1 first constant domain of one heavy chain
- Each Fab fragment is monovalent with respect to antigen binding, i.e., it has a single antigen-binding site.
- Pepsin treatment of an antibody yields a single large F(ab')2 fragment which roughly corresponds to two disulfide linked Fab fragments having different antigen-binding activity and is still capable of cross-linking antigen.
- Fab' fragments differ from Fab fragments by having a few additional residues at the carboxy terminus of the CH1 domain including one or more cysteines from the antibody hinge region.
- Fab '-SH is the designation herein for Fab' in which the cysteine residue(s) of the constant domains bear a free thiol group.
- F(ab')2 antibody fragments originally were produced as pairs of Fab' fragments which have hinge cysteines between them. Other chemical couplings of antibody fragments are also known.
- the Fc fragment comprises the carboxy-terminal portions of both H chains held together by disulfides.
- the effector functions of antibodies are determined by sequences in the Fc region, the region which is also recognized by Fc receptors (FcR) found on certain types of cells.
- Fv is the minimum antibody fragment which contains a complete antigen- recognition and -binding site. This fragment consists of a dimer of one heavy- and one light-chain variable region domain in tight, non-covalent association. From the folding of these two domains emanate six hypervariable loops (3 bops each from the H and L chain) that contribute the amino acid residues for antigen binding and confer antigen binding specificity to the antibody. However, even a single variable domain (or half of an Fv comprising only three HVRs specific for an antigen) has the ability to recognize and bind antigen, although at a lower affinity than the entire binding site.
- Single-chain Fv also abbreviated as “sFv” or “scFv” are antibody fragments that comprise the VH and V
- the sFv polypeptide further comprises a polypeptide linker between the VH and V u domains which enables the sFv to form the desired structure for antigen binding.
- sFv Single-chain Fv
- “Functional fragments” of the antibodies of the invention comprise a portion of an intact antibody, generally including the antigen binding or variable region of the intact antibody or the Fc region of an antibody which retains or has modified FcR binding capability.
- antibody fragments include linear antibody, single-chain antibody molecules and multispecific antibodies formed from antibody fragments.
- diabodies refers to small antibody fragments prepared by constructing sFv fragments (see preceding paragraph) with short linkers (about 5-10) residues) between the VH and VL domains such that inter-chain but not intra-chain pairing of the V domains is achieved, thereby resulting in a bivalent fragment, i.e., a fragment having two antigen-binding sites.
- Bispecific diabodies are heterodimers of two "crossover" sFv fragments in which the VH and VL domains of the two antibodies are present on different polypeptide chains.
- Diabodies are described in greater detail in, for example, EP 404,097; WO 93/11161 ; Hollinger et al, Proc. Natl. Acad. ScL USA 90: 6444-6448 (1993).
- the term "nanobodies” refers to single-domain antibodies which are antibody fragments consisting of a single monomeric variable antibody domain. Like a whole antibody, they are able to bind selectively to a specific antigen. With a molecular weight of only 12-15 kDa, single-domain antibodies are much smaller than common antibodies (150-160 kDa). The first single-domain antibodies were engineered from heavy-chain antibodies found in camelids. Gibbs, W. Wayt (August 2005).
- the monoclonal antibodies herein specifically include “chimeric” antibodies
- immunoglobulins in which a portion of the heavy and/or light chain is identical with or homologous to corresponding sequences in antibodies derived from a particular species or belonging to a particular antibody class or subclass, while the remainder of the chain(s) is(are) identical with or homologous to corresponding sequences in antibodies derived from another species or belonging to another antibody class or subclass, as well as fragments of such antibodies, so long as they exhibit the desired biological activity (U.S. Patent No. 4,816,567; Morrison et al, Proc. Natl. Acad. ScL USA, 81 :6851-6855 (1984)).
- humanized antibody is used a subset of “chimeric antibodies.”
- “Humanized” forms of non-human (e.g., murine) antibodies are chimeric antibodies that contain minimal sequence derived from non-human immunoglobulin.
- a humanized antibody is a human immunoglobulin (recipient antibody) in which residues from an HVR (hereinafter defined) of the recipient are replaced by residues from an HVR of a non-human species (donor antibody) such as mouse, rat, rabbit or non-human primate having the desired specificity, affinity, and/or capacity.
- donor antibody such as mouse, rat, rabbit or non-human primate having the desired specificity, affinity, and/or capacity.
- framework (“FR") residues of the human immunoglobulin are replaced by corresponding non-human residues.
- humanized antibodies may comprise residues that are not found in the recipient antibody or in the donor antibody. These modifications may be made to further refine antibody performance, such as binding affinity.
- a humanized antibody will comprise substantially all of at least one, and typically two, variable domains, in which all or substantially all of the hypervariable loops correspond to those of a non-human immunoglobulin sequence, and all or substantially all of the FR regions are those of a human immunoglobulin sequence, although the FR regions may include one or more individual FR residue substitutions that improve antibody performance, such as binding affinity, isomerization, immunogenicity, etc.
- the number of these amino acid substitutions in the FR are typically no more than 6 in the H chain, and in the L chain, no more than 3.
- the humanized antibody optionally will also comprise at least a portion of an immunoglobulin constant region (Fc), typically that of a human immunoglobulin.
- Fc immunoglobulin constant region
- human antibody is an antibody that possesses an amino-acid sequence corresponding to that of an antibody produced by a human and/or has been made using any of the techniques for making human antibodies as disclosed herein. This definition of a human antibody specifically excludes a humanized antibody
- Human antibodies can be produced using various techniques known in the art, including phage-display libraries.
- Human antibodies can be prepared by administering the antigen to a transgenic animal that has been modified to produce such antibodies in response to antigenic challenge, but whose endogenous loci have been disabled, e.g., immunized xenomice (see, e.g., U.S. Pat. Nos. 6,075,181 and 6,150,584 regarding XENOMOUSETM
- hypervariable region when used herein refers to the regions of an antibody variable domain which are hypervariable in sequence and/or form structurally defined loops.
- antibodies comprise six HVRs; three in the VH (H1, H2, H3), and three in the VL (L1, 12, L3).
- H3 and L3 display the most diversity of the six HVRs, and H3 in particular is believed to play a unique role in conferring fine specificity to antibodies. See, e.g., Xu et al, Immunity 13:37-45 (2000); Johnson and Wu, in Methods in Molecular Biology 248:1-25 (Lo, ed., Human Press, Totowa, NJ, 2003).
- camelid antibodies consisting of a heavy chain only are functional and stable in the absence of light chain. See, e.g., Hamers-Casterman et al., Nature 363:446-448 (1993); Sheriff et al, Nature Struct. Biol. 3:733-736 (1996).
- CDRs Kabat Complementarity Determining Regions
- HVRs may comprise "extended HVRs" as follows: 24-36 or 24-34 (L1), 46-56 or 50- 56 (L2) and 89-97 or 89-96 (L3) in the VL and 26-35 (H1), 50-65 or 49-65 (H2) and 93-102, 94-102, or 95-102 (H3) in the VH.
- the variable domain residues are numbered according to Kabat et al., supra, for each of these definitions.
- variable-domain residue -numbering as in Kabat or “amino-acid- position numbering as in Kabat,” and variations thereof, refers to the numbering system used for heavy-chain variable domains or light-chain variable domains of the compilation of antibodies in Kabat et al., supra. Using this numbering system, the actual linear amino acid sequence may contain fewer or additional amino acids corresponding to a shortening of, or insertion into, a FR or HVR of the variable domain.
- a heavy-chain variable domain may include a single amino acid insert (residue 52a according to Kabat) after residue 52 of H2 and inserted residues (e.g. residues 82a, 82b, and 82c, etc.
- a "human consensus framework” or “acceptor human framework” is a framework that represents the most commonly occurring amino acid residues in a selection of human immunoglobulin VL or VH framework sequences. Generally, the selection of human immunoglobulin VL or VH sequences is from a subgroup of variable domain sequences.
- the subgroup of sequences is a subgroup as in Kabat et al., Sequences of Proteins of Immunological Interest, 5 th Ed. Public Health Service, National Institutes of Health, Bethesda, D (1991). Examples include for the VL, the subgroup may be subgroup kappa I, kappa II, kappa III or kappa IV as in Kabat et al, supra. Additionally, for the VH, the subgroup may be subgroup I, subgroup II, or subgroup III as in Kabat et al., supra.
- a human consensus framework can be derived from the above in which particular residues, such as when a human framework residue is selected based on its homology to the donor framework by aligning the donor framework sequence with a collection of various human framework sequences.
- An acceptor human framework "derived from" a human immunoglobulin framework or a human consensus framework may comprise the same amino acid sequence thereof, or it may contain preexisting amino acid sequence changes. In some embodiments, the number of pre-existing amino acid changes are 10 or less, 9 or less, 8 or less, 7 or less, 6 or less, 5 or less, 4 or less, 3 or less, or 2 or less.
- amino-acid modification at a specified position, e.g. of the Fc region, refers to the substitution or deletion of the specified residue, or the insertion of at least one amino acid residue adjacent the specified residue. Insertion "adjacent" to a specified residue means insertion within one to two residues thereof. The insertion may be N- terminal or C-terminal to the specified residue.
- the preferred amino acid modification herein is a substitution.
- an “affinity-matured” antibody is one with one or more alterations in one or more HVRs thereof that result in an improvement in the affinity of the antibody for antigen, compared to a parent antibody that does not possess those alteration(s).
- an affinity-matured antibody has nanomolar or even picomolar affinities for the target antigen.
- Affinity-matured antibodies are produced by procedures known in the art. For example, Marks et al, Bio/Technology 10:779-783 (1992) describes affinity maturation by VH- and VL-domain shuffling. Random mutagenesis of HVR and/or framework residues is described by, for example: Barbas et al. Proc Nat. Acad. ScL USA 91 :3809-3813 (1994); Schier et al. Gene 169:147-155 (1995);
- the term “specifically binds to” or is "specific for” refers to measurable and reproducible interactions such as binding between a target and an antibody, which is determinative of the presence of the target in the presence of a
- an antibody that specifically binds to a target is an antibody that binds this target with greater affinity, avidity, more readily, and/or with greater duration than it binds to other targets.
- the extent of binding of an antibody to an unrelated target is less than about 10% of the binding of the antibody to the target as measured, e.g., by a radioimmunoassay (RIA).
- RIA radioimmunoassay
- an antibody specifically binds to an epitope on a protein that is conserved among the protein from different species.
- specific binding can include, but does not require exclusive binding.
- ADCC antibody-dependent cell-mediated cytotoxicity
- FcRs Fc receptors
- cytotoxic cells e.g., natural killer (NK) cells, neutrophils and macrophages
- NK cells natural killer cells
- monocytes express FcyRI, FcyRII and FcyRIII.
- Fc expression on hematopoietic cells is summarized in Table 3 on page 464 of Ravetch and Kinet, Annu. Rev. Immunol. 9: 457-92 (1991).
- an in vitro ADCC assay such as that described in U.S. Patent No. 5,500,362 or 5,821,337 may be performed.
- Useful effector cells for such assays include peripheral blood
- PBMC mononuclear cells
- NK natural killer cells
- ADCC activity of the molecule of interest may be assessed in vivo, e.g., in an animal model such as that disclosed in Clynes et al, PNAS USA 95:652-656 (1998).
- the numbering of the residues in an immunoglobulin heavy chain is that of the EU index as in Kabat et al, supra.
- the "EU index as in Kabat” refers to the residue numbering of the human tgG1 EU antibody. In many cancers the tumor cells express high levels of PD-L1 on their surface.
- anti-PD-L1 antibodies with ADCC potential can trigger ADCC which may lead to the death of these tumor cells.
- Fc region herein is used to define a C-terminal region of an
- immunoglobulin heavy chain including native-sequence Fc regions and variant Fc regions.
- the boundaries of the Fc region of an immunoglobulin heavy chain might vary, the human IgG heavy- chain Fc region is usually defined to stretch from an amino acid residue at position Cys226, or from Pro230, to the carboxyl-terminus thereof.
- the C-terminal lysine (residue 447 according to the EU numbering system) of the Fc region may be removed, for example, during production or purification of the antibody, or by recombinantly engineering the nucleic acid encoding a heavy chain of the antibody.
- a composition of intact antibodies may comprise antibody populations with all K447 residues removed, antibody populations with no K447 residues removed, and antibody populations having a mixture of antibodies with and without the K447 residue.
- Suitable native-sequence Fc regions for use in the antibodies of the invention include human lgG1, lgG2 (lgG2A, JgG2B), lgG3 and lgG4.
- "Fc receptor” or "FcR” describes a receptor that binds to the Fc region of an antibody.
- the preferred FcR is a native sequence human FcR.
- a preferred FcR is one which binds an IgG antibody (a gamma receptor) and includes receptors of the FcyRI, FcyRII, and FcyRIII subclasses, including allelic variants and
- FcyRII receptors include FcyRIIA (an “activating receptor”) and FcyRI IB (an “inhibiting receptor”), which have similar amino acid sequences that differ primarily in the cytoplasmic domains thereof.
- Activating receptor FcyRIIA contains an immunoreceptor tyrosine -based activation motif (ITAM) in its cytoplasmic domain.
- Inhibiting receptor FcyRIIB contains an immunoreceptor tyrosine -based activation motif
- ITIM immunoreceptor tyrosine -based inhibition motif
- Fc receptor or “FcR” also includes the neonatal receptor, FcRn, which is responsible for the transfer of maternal IgGs to the fetus.
- FcRn the neonatal receptor
- Methods of measuring binding to FcRn are known (see, e.g., Ghetie and Ward, Immunol. Today 18: (12): 592-8 (1997); Ghetie et al, Nature Biotechnology 15 (7): 637-40 (1997); Hinton et al, J. Biol. Chem.
- Binding to FcRn in vivo and serum half-life of human FcRn high- affinity binding polypeptides can be assayed, e.g., in transgenic mice or transfected human cell lines expressing human FcRn, or in primates to which the polypeptides having a variant Fc region are administered.
- WO 2004/42072 (Presta) describes antibody variants which improved or diminished binding to FcRs. See also, e.g., Shields et al, J. Biol. Chem. 9(2):
- “Effector cells” are leukocytes which express one or more FcRs and perform effector functions.
- the effector cells express at least FcyRIII and perform ADCC effector function.
- human leukocytes which mediate ADCC include peripheral blood mononuclear cells (PBMC), natural killer (NK) cells, monocytes, cytotoxic T cells and neutrophils.
- PBMC peripheral blood mononuclear cells
- NK natural killer cells
- monocytes cytotoxic T cells
- neutrophils neutrophils.
- the effector cells may be isolated from a native source, e.g., blood. Effector cells generally are lymphocytes associated with the effector phase, and function to produce cytokines (helper T cells), killing cells in infected with pathogens (cytotoxic T cells) or secreting antibodies (differentiated B cells).
- Binding affinity generally refers to the strength of the sum total of non-covalent interactions between a single binding site of a molecule (e.g., of an antibody) and its binding partner (e.g., an antigen). Unless indicated otherwise, as used herein, "binding affinity”, “bind to”, “binds to” or “binding to” refers to intrinsic binding affinity that reflects a 1 : 1 interaction between members of a binding pair (e.g. , antibody Fab fragment and antigen).
- the affinity of a molecule X for its partner Y can generally be represented by the dissociation constant (K D ). Affinity can be measured by common methods known in the art, including those described herein.
- binding affinity generally bind antigen slowly and tend to dissociate readily, whereas high- affinity antibodies generally bind antigen faster and tend to remain bound longer.
- a variety of methods of measuring binding affinity are known in the art, any of which can be used for purposes of the present invention. Specific illustrative and exemplary embodiments for measuring binding affinity, i.e. binding strength are described in the following.
- the "KD" or "KD value” according to this invention is in one embodiment measured by a radiolabeled antigen binding assay (RIA) performed with the Fab version of the antibody and antigen molecule as described by the following assay that measures solution binding affinity of Fabs for antigen by equilibrating Fab with a minimal concentration of ( 125 l)-labeled antigen in the presence of a titration series of unlabeled antigen, then capturing bound antigen with an anti- Fab antibody-coated plate (Chen, et al, (1999) J. ol Biol 293:865-881).
- RIA radiolabeled antigen binding assay
- microtiter plates (Dynex) are coated overnight with 5 ug/ml of a capturing anti- Fab antibody (Cappel Labs) in 50 mM sodium carbonate (pH 9.6), and subsequently blocked with 2% (w v) bovine serum albumin in PBS for two to five hours at room temperature (approximately 23°C).
- a non-adsorbant plate (Nunc #269620) 100 pM or 26 pM [ 125 l]-antigen are mixed with serial dilutions of a Fab of interest
- the K D is measured by using surface-plasmon resonance assays using a BIACORE ® -2000 or a
- CM5 chips immobilized antigen CM5 chips at -10 response units (RU).
- carboxymethylated dextran biosensor chips CM5, BIAcore Inc.
- EDC N- ethyl-N'- (3-dimethylaminopropyl)-carbodiimide hydrochloride
- NHS N- hydroxysuccinimide
- Antigen is diluted with 10 mM sodium acetate, pH 4.8, to 5 pg/ml (-0.2 ⁇ ) before injection at a flow rate of 5 ptJminute to achieve approximately 10 response units (RU) of coupled protein.
- 1 M ethanolamine is injected to block unreacted groups.
- K D equilibrium dissociation constant
- a spectrometer such as a stop-flow- equipped spectrophotometer (Aviv Instruments) or a 8000-series SLM-AMINCOTM spectrophotometer (ThermoSpectronic) with a stirred cuvette.
- a spectrometer such as a stop-flow- equipped spectrophotometer (Aviv Instruments) or a 8000-series SLM-AMINCOTM spectrophotometer (ThermoSpectronic) with a stirred cuvette.
- CM5 carboxymethylated dextran biosensor ships
- ECD N-ethyl-N'-(3- dimethylamino propyl )-carbodiimide hydrochloride
- NHS N-hydroxysuccinimide
- Antigen is diluted with 10 mM sodium acetate, ph 4.8, into 5 mg/ml ( ⁇ 0.2 mM) before injection at a flow rate of 5 ml/min. to achieve approximately 10 response units (RU) of coupled protein.
- IM ethanolamine is added to block unreacted groups.
- two-fold serial dilutions of Fab (0.78 nM to 500 nM) are injected in PBS with 0.05% Tween 20 (PBST) at 25°C at a flow rate of approximately 25ul/min.
- association rates (k on ) and dissociation rates (k 0 ff) are calculated using a simple one-to-one Langmuir binding model (BIAcore Evaluation Software version 3.2) by simultaneous fitting the association and dissociation sensorgram.
- the equilibrium dissociation constant (KD) was calculated as the ratio k 0f i/k on . See, e.g., Chen, Y., et al, (1999) J. ol Biol 293:865-881.
- the term "functional epitope” as used herein refers to amino acid residues of an antigen that contribute energetically to the binding of an antibody, i.e. forming an "energetic epitope”. Mutation of any one of the energetically contributing residues of the antigen to alanine will disrupt the binding of the antibody such that the relative KD ratio (KD mutant PD-L1 / KD wild type PD-L1) of the antibody will be greater than 4 (see Example 3.x(b)).
- conformation epitope refers to amino acid residues of the PD-L1 antigen that come together on the surface when the polypeptide chain folds to form the native protein, and show a significantly reduced rate of HD exchange due to Fab binding, as described in the experimental section.
- the conformation epitope contains, but is not limited to, the functional epitope.
- the difference between the two values is, for example, greater than about 10%, greater than about 20%, greater than about 30%, greater than about 40%, and/or greater than about 50% as a function of the value for the reference/comparator molecule.
- substantially similar denotes a sufficiently high degree of similarity between two numeric values (for example, one associated with an antibody of the invention and the other associated with a reference/comparator antibody), such that one of skill in the art would consider the difference between the two values to be of little or no biological and/or statistical significance within the context of the biological characteristic measured by said values ⁇ e.g., KD values).
- the difference between said two values is, for example, less than about 50%, less than about 40%, less than about 30%, less than about 20%, and/or less than about 10% as a function of the reference/comparator value.
- Percent (%) amino acid sequence identity and “homology” with respect to a peptide, polypeptide or antibody sequence are defined as the percentage of amino acid residues in a candidate sequence that are identical with the amino acid residues in the specific peptide or polypeptide sequence, after aligning the sequences and introducing gaps, if necessary, to achieve the maximum percent sequence identity, and not considering any conservative substitutions as part of the sequence identity. Alignment for purposes of determining percent amino acid sequence identity can be achieved in various ways that are within the skill in the art, for instance, using publicly available computer software such as BLAST, BLAST-2 or ALIGN software. Those skilled in the art can determine appropriate parameters for measuring alignment, including any algorithms needed to achieve maximal alignment over the full length of the sequences being compared.
- blocking antibody or an “antagonist” antibody is one that inhibits or reduces a biological activity of the antigen it binds to.
- blocking antibodies or antagonist antibodies substantially or completely inhibit the biological activity of the antigen.
- the anti-PD-L1 antibodies of the invention block the
- agonist or activating antibody is one that enhances or initiates signaling by the antigen to which it binds.
- agonist antibodies cause or activate signaling without the presence of the natural ligand.
- cross-compete means the ability of an antibody or fragment thereof to interfere with the binding directly or indirectly through allosteric modulation of the anti-PD-L1 antibodies of the invention to the target human PD-L1.
- the extent to which an an antibody or fragment thereof is able to interfere with the binding of another to the target, and therefore whether it can be said to cross-block or cross-compete according to the invention, can be determined using competition binding assays.
- One particularly suitable quantitative cross-competition assay uses a FACS- or an AlphaScreen-based approach to measure competition between the labelled (e.g.
- a suitable assay for determining whether a binding molecule cross-competes or is capable of cross-competing with an antibody or fragment thereof.
- a cross-competing antibody or fragment thereof is for example one which will bind to the target in the cross-competition assay such that, during the assay and in the presence of a second antibody or fragment thereof, the recorded displacement of the immunoglobulin single variable domain or polypeptide according to the invention is up to 100% (e.g. in FACS based
- cross-competing antibodies or fragments thereof have a recorded displacement that is between 10% and 100%, more preferred between 50% to 100%.
- an "isolated" nucleic acid molecule encoding the antibodies herein is a nucleic acid molecule that is identified and separated from at least one contaminant nucleic acid molecule with which it is ordinarily associated in the environment in which it was produced. Preferably, the isolated nucleic acid is free of association with all components associated with the production environment.
- the isolated nucleic acid molecules encoding the polypeptides and antibodies herein is in a form other than in the form or setting in which it is found in nature. Isolated nucleic acid molecules therefore are distinguished from nucleic acid encoding the polypeptides and antibodies herein existing naturally in cells.
- control sequences refers to DNA sequences necessary for the expression of an operably linked coding sequence in a particular host organism.
- control sequences that are suitable for prokaryotes include a promoter, optionally an operator sequence, and a ribosome binding site.
- Eukaryotic cells are known to utilize promoters, polyadenylation signals, and enhancers.
- Nucleic acid is "operably linked" when it is placed into a functional relationship with another nucleic acid sequence.
- DNA for a presequence or secretory leader is operably linked to DNA for a polypeptide if it is expressed as a preprotein that participates in the secretion of the polypeptide; a promoter or enhancer is operably linked to a coding sequence if it affects the transcription of the sequence; or a ribosome binding site is operably linked to a coding sequence if it is positioned so as to facilitate translation.
- "operably linked" means that the DNA sequences being linked are contiguous, and, in the case of a secretory leader, contiguous and in reading phase. However, enhancers do not have to be contiguous. Linking is accomplished by ligation at convenient restriction sites.
- a “stable" formulation is one in which the protein therein essentially retains its physical and chemical stability and integrity upon storage.
- Various analytical techniques for measuring protein stability are available in the art and are reviewed in Peptide and Protein Drug Delivery, 247-301 , Vincent Lee Ed., Marcel Dekker, Inc., New York, New York, Pubs. (1991) and Jones, A. Adv. Drug Delivery Rev. 10: 29-90 (1993). Stability can be measured at a selected temperature for a selected time period. For rapid screening, the formulation may be kept at 40°C for 2 weeks to 1 month, at which time stability is measured.
- the formulation should be stable at 30°C or 40°C for at least 1 month and/or stable at 2-8°C for at least 2 years.
- the formulation should be stable for at least 2 years at 30°C and/or stable at 40°C for at least 6 months.
- the extent of aggregation during storage can be used as an indicator of protein stability.
- a "stable" formulation may be one wherein less than about 10% and preferably less than about 5% of the protein are present as an aggregate in the formulation. In other embodiments, any increase in aggregate formation during storage of the formulation can be determined.
- a "reconstituted" formulation is one which has been prepared by dissolving a lyophilized protein or antibody formulation in a diluent such that the protein is dispersed throughout.
- the reconstituted formulation is suitable for administration (e.g. subscutaneous administration) to a patient to be treated with the protein of interest and, in certain embodiments of the invention, may be one which is suitable for parenteral or intravenous administration.
- An "isotonic" formulation is one which has essentially the same osmotic pressure as human blood. Isotonic formulations will generally have an osmotic pressure from about 250 to 350 mOsm.
- the term “hypotonic” describes a formulation with an osmotic pressure below that of human blood.
- the term “hypertonic” is used to describe a formulation with an osmotic pressure above that of human blood. Isotonicity can be measured using a vapor pressure or ice-freezing type osmometer, for example.
- the formulations of the present invention are hypertonic as a result of the addition of salt and/or buffer.
- Carriers as used herein include pharmaceutically acceptable carriers, excipients, or stabilizers that are nontoxic to the cell or mammal being exposed thereto at the dosages and concentrations employed. Often the physiologically acceptable carrier is an aqueous pH buffered solution.
- physiologically acceptable carriers include buffers such as phosphate, citrate, and other organic acids; antioxidants including ascorbic acid; low molecular weight (less than about 10 residues) polypeptide; proteins, such as serum albumin, gelatin, or immunoglobulins; hydrophilic polymers such as
- polyvinylpyrrolidone amino acids such as glycine, glutamine, asparagine, arginine or lysine; monosaccharides, disaccharides, and other carbohydrates including glucose, mannose, or dextrins; chelating agents such as EDTA; sugar alcohols such as mannitol or sorbitol; salt- forming counterions such as sodium; and/or nonionic surfactants such as TWEENTM, polyethylene glycol (PEG), and PLURONICSTM.
- amino acids such as glycine, glutamine, asparagine, arginine or lysine
- monosaccharides, disaccharides, and other carbohydrates including glucose, mannose, or dextrins chelating agents such as EDTA
- sugar alcohols such as mannitol or sorbitol
- salt- forming counterions such as sodium
- nonionic surfactants such as TWEENTM, polyethylene glycol (PEG), and PLURONICSTM
- a “pharmaceutically acceptable acid” includes inorganic and organic acids which are non toxic at the concentration and manner in which they are formulated.
- suitable inorganic acids include hydrochloric, perchloric, hydrobromic, hydroiodic, nitric, sulfuric, sulfonic, sulfuric, sulfanilic, phosphoric, carbonic, etc.
- Suitable organic acids include straight and branched-chain alkyl, aromatic, cyclic, cycloaliphatic, arylaliphatic, heterocyclic, saturated, unsaturated, mono, di- and tricarboxylic, including for example, formic, acetic, 2-hydroxyacetic, trifluoroacetic, phenylacetic, trimethylacetic, t-butyl acetic, anthranilic, propanoic, 2- hydroxypropanoic, 2-oxopropanoic, propandioic, cyclopentanepropionic,
- camphorsulphonic 4-methylbicyclo[2.2.2]-oct-2-ene-l-carboxylic, glucoheptonic, 4,4'- methylenebis-3-(hydroxy-2-ene-l-carboxylic acid), hydroxynapthoic.
- “Pharmaceutically-acceptable bases” include inorganic and organic bases which are non-toxic at the concentration and manner in which they are formulated.
- suitable bases include those formed from inorganic base forming metals such as lithium, sodium, potassium, magnesium, calcium, ammonium, iron, zinc, copper, manganese, aluminum, N- methylglucamine, morpholine, piperidine and organic nontoxic bases including, primary, secondary and tertiary amines, substituted amines, cyclic amines and basic ion exchange resins, [e.g., N(R') 4 + (where R' is independently H or C1-4 alkyl, e.g., ammonium, Tris)], for example, isopropylamine, trimethylamine, diethylamine, triethylamine, tripropylamine, ethanolamine, 2- diethylaminoethanol, trimethamine, dicyclohexylamine, lysine, arginine, histidine, caffeine, pro
- organic non-toxic bases are isopropylamine, diethylamine, ethanolamine, trimethamine, dicyclohexylamine, choline, and caffeine.
- Additional pharmaceutically acceptable acids and bases useable with the present invention include those which are derived from the amino acids, for example, histidine, glycine, phenylalanine, aspartic acid, glutamic acid, lysine and asparagine.
- "Pharmaceutically acceptable" buffers and salts include those derived from both acid and base addition salts of the above indicated acids and bases. Specific buffers and/ or salts include histidine, succinate and acetate.
- a "pharmaceutically acceptable sugar” is a molecule which, when combined with a protein of interest, significantly prevents or reduces chemical and/or physical instability of the protein upon storage.
- “pharmaceutically acceptable sugars” may also be known as a "lyoprotectant”.
- “Exemplary sugars and their corresponding sugar alcohols include: an amino acid such as monosodium glutamate or histidine; a methylamine such as betaine; a lyotropic salt such as magnesium sulfate; a polyol such as trihydric or higher molecular weight sugar alcohols, e.g. glycerin, dextran, erythritol, glycerol, arabitol, xylitol, sorbitol, and mannitol; propylene glycol;
- polyethylene glycol polyethylene glycol; PLURONICS ® ; and combinations thereof.
- Additional exemplary lyoprotectants include glycerin and gelatin, and the sugars mellibiose, melezitose, raffmose, mannotriose and stachyose.
- reducing sugars include glucose, maltose, lactose, maltulose, iso-maltulose and lactulose.
- non-reducing sugars include non-reducing glycosides of polyhydroxy compounds selected from sugar alcohols and other straight chain polyalcohols.
- Preferred sugar alcohols are monoglycosides, especially those compounds obtained by reduction of disaccharides such as lactose, maltose, lactulose and maltulose.
- the glycosidic side group can be either glucosidic or galactosidic. Additional examples of sugar alcohols are glucitol, maltitol, lactitol and iso-maltulose.
- the preferred pharmaceutically-acceptable sugars are the non-reducing sugars trehalose or sucrose.
- Pharmaceutically acceptable sugars are added to the formulation in a "protecting amount" (e.g. pre-lyophilization) which means that the protein essentially retains its physical and chemical stability and integrity during storage (e.g., after reconstitution and storage).
- the "diluent" of interest herein is one which is pharmaceutically acceptable (safe and non-toxic for administration to a human) and is useful for the preparation of a liquid formulation, such as a formulation reconstituted after lyophilization.
- exemplary diluents include sterile water, bacteriostatic water for injection (BWFI), a pH buffered solution (e.g. phosphate-buffered saline), sterile saline solution, Ringer's solution or dextrose solution.
- BWFI bacteriostatic water for injection
- a pH buffered solution e.g. phosphate-buffered saline
- sterile saline solution e.g. phosphate-buffered saline
- Ringer's solution or dextrose solution e.g. phosphate-buffered saline
- diluents can include aqueous solutions of salts and/or buffers.
- a "preservative” is a compound which can be added to the formulations herein to reduce bacterial activity.
- the addition of a preservative may, for example, facilitate the production of a multi-use (multiple-dose) formulation.
- potential preservatives include octadecyldimethylbenzyl ammonium chloride, hexamethonium chloride, benzalkonium chloride (a mixture of alkylbenzyldimethylammonium chlorides in which the alkyl groups are long-chain compounds), and benzethonium chloride.
- preservatives include aromatic alcohols such as phenol, butyl and benzyl alcohol, alkyl parabens such as methyl or propyl paraben, catechol, resorcinol, cyclohexanol, 3-pentanol, and w-cresol.
- aromatic alcohols such as phenol, butyl and benzyl alcohol
- alkyl parabens such as methyl or propyl paraben
- catechol resorcinol
- cyclohexanol 3-pentanol
- w-cresol w-cresol
- the most preferred preservative herein is benzyl alcohol.
- 'Treatment' refers to clinical intervention designed to alter the natural course of the individual or cell being treated, and can be performed either for prophylaxis or during the course of clinical pathology.
- Desirable effects of treatment include preventing occurrence or recurrence of disease, preventing metastasis, decreasing the rate of disease progression, ameliorating or palliating the disease state, and remission or improved prognosis.
- antibodies of the invention are used to delay development of a disease or disorder.
- a subject is successfully "treated", for example, using the apoptotic anti-PD-L1 antibodies of the invention if one or more symptoms associated with a T-cell dysfunctional disorder is mitigated.
- an “effective amount' refers to at least an amount effective, at dosages and for periods of time necessary, to achieve the desired or indicated effect, including a therapeutic or prophylactic result.
- an effective amount of the anti-PD-L1 antibodies of the present invention is at least the minimum concentration that results in inhibition of signaling from PD-L1, either through PD-1 on T-cells or B7.1 on other APCs or both.
- a “therapeutically effective amount' is at least the minimum concentration required to effect a measurable improvement or prevention of a particular disorder.
- therapeutically effective amount herein may vary according to factors such as the disease state, age, sex, and weight of the patient, and the ability of the antibody to elicit a desired response in the individual.
- a therapeutically effective amount is also one in which any toxic or detrimental effects of the antibody are outweighed by the therapeutically beneficial effects.
- a therapeutically effective amount of the anti-PD-L1 antibodies of the present invention is at least the minimum
- prophylactically effective amount refers to an amount effective, at the dosages and for periods of time necessary, to achieve the desired prophylactic result.
- a prophylactically effective amount of the anti-PD-L1 antibodies of the present invention is at least the minimum concentration that prevents or attenuates the development of at least one symptom of a T cell dysfunctional disorder.
- “Mammal” for purposes of treatment refers to any animal classified as a mammal, including humans, domestic and farm animals, and zoo, sports, or pet animals, such as dogs, horses, rabbits, cattle, pigs, hamsters, gerbils, mice, ferrets, rats, cats, etc.
- the mammal is human.
- 'pharmaceutical formulation refers to a preparation that is in such form as to permit the biological activity of the active ingredient to be effective, and that contains no additional components that are unacceptably toxic to a subject to which the formulation would be administered. Such formulations are sterile.
- a "sterile" formulation is aseptic or free from all living microorganisms and their spores.
- autoimmune disorder is a disease or disorder arising from and directed against an individual's own tissues or organs or a co-segregation or manifestation thereof or resulting condition therefrom.
- Autoimmune diseases can be an organ-specific disease (i.e., the immune response is specifically directed against an organ system such as the endocrine system, the hematopoietic system, the skin, the cardiopulmonary system, the gastrointestinal and liver systems, the renal system, the thyroid, the ears, the neuromuscular system, the central nervous system, etc.) or a systemic disease that can affect multiple organ systems (for example, systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), polymyositis, etc.).
- SLE systemic lupus erythematosus
- RA rheumatoid arthritis
- Preferred such diseases include autoimmune rheumatologic disorders (such as, for example, RA, Sjogren's syndrome, scleroderma, lupus such as SLE and lupus nephritis,
- polymyositis-dermatomyositis, cryoglobulinemia, anti-phospholipid antibody syndrome, and psoriatic arthritis autoimmune gastrointestinal and liver disorders (such as, for example, inflammatory bowel diseases ⁇ e.g., ulcerative colitis and Crohn's disease), autoimmune gastritis and pernicious anemia, autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, and celiac disease), vasculitis (such as, for example, ANCA-negative vasculitis and ANCA-associated vasculitis, including Churg-Strauss vasculitis, Wegener's granulomatosis, and microscopic polyangiitis), autoimmune neurological disorders (such as, for example, multiple sclerosis, opsoclonus myoclonus syndrome, myasthenia gravis,
- neuromyelitis optica Parkinson's disease, Alzheimer's disease, and autoimmune polyneuropathies
- renal disorders such as, for example, glomerulonephritis
- autoimmune dermatologic disorders such as, for example, psoriasis, urticaria, hives, pemphigus vulgaris, bullous pemphigoid, and cutaneous lupus erythematosus
- hematologic disorders such as, for example, thrombocytopenic purpura, thrombotic thrombocytopenic purpura, post-transfusion purpura, and autoimmune hemolytic anemia
- Atherosclerosis uveitis
- autoimmune hearing diseases such as, for example, inner ear disease and hearing loss
- Behcet's disease Raynaud's syndrome
- organ transplant organ transplant
- autoimmune endocrine disorders such as, for example, diabetic- related autoimmune diseases such as insulin-dependent diabetes mellitus (IDDM), Addison's disease, and autoimmune thyroid disease (e.g., Graves' disease and thyroiditis)
- IDDM insulin-dependent diabetes mellitus
- Addison's disease e.g., Graves' disease and thyroiditis
- More preferred such diseases include, for example, RA, ulcerative colitis, ANCA-associated vasculitis, lupus, multiple sclerosis, Sjogren's syndrome, Graves' disease, IDDM, pernicious anemia, thyroiditis, and glomerulonephritis.
- cytotoxic agent refers to a substance that inhibits or prevents the function of cells and/or causes destruction of cells.
- the term includes radioactive isotopes (e.g. At 211 , 1 131 , 1 125 , Y 90 , Re 186 , Re 188 , Sm 153 , Bi 212 , P 32 and radioactive isotopes of Lu), and toxins such as small-molecule toxins or enzymatically active toxins of bacterial, fungal, plant or animal origin, or fragments thereof.
- a "chemotherapeutic agent” is a chemical compound useful in the treatment of cancer.
- chemotherapeutic agents include alkylating agents such as thiotepa and cyclophosphamide; alkyl sulfonates such as busulfan, improsulfan, and piposulfan; aziridines such as benzodopa, carboquone, meturedopa, and uredopa; ethylenimines and methylamelamines including altretamine, triethylenemelamine, trietylenephosphoramide, triethiylenethiophosphoramide and trimethylolomelamine; acetogenins (especially bullatacin and butlatacinone); delta-9-tetrahydrocannabinol (dronabinol); beta-lapachone; lapachol; colchicines; betulinic acid; a camptothecin (including the synthetic analogue topotecan (CPT-11 (irinotecan),
- pemetrexed including its adozelesin, carzelesin and bizelesin synthetic analogues); podophyllotoxin; podophyllinic acid; teniposide; cryptophycins (particularly cryptophycin 1 and cryptophycin 8); dolastatin; duocarmycin (including the synthetic analogues, KW-2189 and CB1-TM1); eleutherobin; pancratistatin; TLK- 286; CDP323, an oral alpha-4 integrin inhibitor; a sarcodictyin; spongistatin; nitrogen mustards such as chlorambucil, chlornaphazine, cholophosphamide, estramustine, ifosfamide, mechlorethamine, mechlorethamine oxide hydrochloride, melphalan, novembichin, phenestenne, prednimustine, trofosfamide, uracil mustard
- antibiotics such as the enediyne antibiotics (e. g., calicheamicin, especially calicheamicin gammall and calicheamicin omegall (see, e.g., Nicolaou et ah, Angew. Chem Intl. Ed.
- dynemicin including dynemicin A; an esperamicin; as well as neocarzinostatin chromophore and related chromoprotein enediyne antibiotic chromophores), aclacinomysins, actinomycin, authramycin, azaserine, bleomycins, cactinomycin, carabicin, carminomycin, carzinophilin, chromomycinis, dactinomycin, daunorubicin, detorubicin, 6-diazo-5- oxo-L-norleucine, doxorubicin (including morpholino-doxorubicin, cyanomorpholino- doxorubicin, 2-pyrrolino- doxorubicin, doxorubicin HCI liposome injection and deoxydoxorubicin), epirubicin, esorubicin
- amsacrine bestrabucil
- bisantrene edatraxate
- defofamine demecolcine
- diaziquone diaziquone; elfornithine; elliptinium acetate; etoglucid; gallium nitrate; hydroxyurea; lentinan; lonidainine; maytansinoids such as maytansine and ansamitocins;
- mitoguazone mitoxantrone; mopidanmol; nitraerine; pentostatin; phenamet;
- trichothecenes especially T-2 toxin, verracurin A, roridin A and anguidine
- urethan vindesine; dacarbazine; mannomustine; mitobronitol; mitolactol; pipobroman;
- gacytosine arabinoside ("Ara-C”); thiotepa; taxoids, e.g., paclitaxel, albumin- engineered nanoparticle formulation of paclitaxel, and doxetaxel; chloranbucil; 6- thioguanine; mercaptopurine; methotrexate; platinum analogs such as cisplatin and carboplatin; vinblastine; platinum; etoposide (VP-16); ifosfamide; mitoxantrone;
- retinoids such as retinoic acid
- pharmaceutically acceptable salts, acids or derivatives of any of the above as well as combinations of two or more of the above such as CHOP, an abbreviation for a combined therapy of cyclophosphamide, doxorubicin, vincristine, and prednisolone, and FOLFOX, an abbreviation for a treatment regimen with oxaliplatin combined with 5-FU and leucovovin.
- anti-PD-L1 antibodies of the invention are bisphosphonates such as clodronate, NE-58095, zoledronic acid/zoledronate, alendronate, pamidronate, tiludronate, or risedronate; as well as troxacitabine (a 1 ,3-dioxolane nucleoside cytosine analog); anti-sense oligonucleotides, particularly those that inhibit expression of genes in signaling pathways implicated in abherant cell proliferation, such as, for example, PKC-alpha, Raf, H-Ras, and epidermal growth factor receptor (EGF-R); vaccines such as
- Stimuvax vaccine for example, Allovectin vaccine, Leuvectin vaccine, and Vaxid vaccine; topoisomerase 1 inhibitor; an anti-estrogen such as fulvestrant; a Kit inhibitor such as imatinib or EXEL-0862 (a tyrosine kinase inhibitor); EGFR inhibitor such as erlotinib or cetuximab; an anti- VEGF inhibitor such as bevacizumab; arinotecan; rmRH; lapatinib and lapatinib ditosylate (an ErbB-2 and EGFR dual tyrosine kinase small-molecule inhibitor also known as GW572016); 17AAG (geldanamycin derivative that is a heat shock protein (Hsp) 90 poison), and pharmaceutically acceptable salts, acids or derivatives of any of the above.
- Hsp heat shock protein
- Stimuvax is a BLP25 liposome cancer vaccine designed to induce an immune response to cancer cells that express MUC1 , a protein antigen widely expressed on common cancers. MUC1 is over expressed on many cancers such as lung cancer, breast cancer, prostate cancer and colorectal cancer. Stimuvax is thought to work by stimulating the body's immune system to identify and destroy cancer cells expressing MUC1.
- Figure 1 shows that A09-246-2 efficiently blocks 125 I-PD-L1 binding to immobilized PD-1-Fc .
- Inactive mutant Mutant VL-A31G,D52E,R99Y of A09-188-1.
- Figure 2 shows sequence of the extracellular domain (fused to a 6 amino acid His tag, SEQ ID NO:29) of PD-L1. Peptides that could be identified by MS are indicated by grey bars. Those that showed protection from HD exchange in the presence of Fab are represented by black bars. Peptides that could not be analyzed are highlighted by underlining and italicizing in the sequence.
- Figure 3 shows the epitope of A09-246-2 on PD-L1. The backbone of PD-L1 is shown in a ribbon representation. Amino acids which, when mutated to alanine, destabilize the A09-246-2 - PD-L1 binding by more than 0.7 kcal/mol are shown as sticks.
- Figure 4 shows that A09-246-2 efficiently enhances T cell activities represented by IL-2 production as shown by SEA human PBMC assay.
- FIGS. 5-16 show that A09-246-2 increases ADCC in different tumor lines
- Antibodies were selected from phage Fab display libraries. The selection included two different arms one utilizing biotinylated human PD-L1 on the different selection rounds and other alternating human and mouse PD-L1 as target on different rounds. 3840 clones were screened by ELISA to identify 170 individual PD-L1 binders. Based on the inhibition of PD-1 ligand binding, 48 hits were selected and were expressed in medium scale for further characterization.
- Hit optimization candidates were selected based on the potency to block binding of PD-1 to PD-L1 and the ability of binding to both human and mouse versions of PD-L1. Binding to PD-L1 was originally determined by ELISA and later quantified by Biacore and binding to PD-L1 expressing cells by FACS. Four candidates fitted the predefined profile, including A09-188-1 which contained a lambda light chain.
- A09-188-1 was chosen for affinity maturation and sequence optimization.
- the goals of the affinity maturation were increased affinity to the human target, cross-reactivity to the murine target, and improvement of manufacturabiiity.
- Heavy chain mutations in the HVR's were introduced by codon based randomization. This heavy chain diversity was combined with light chain diversity introduced by light chain shuffling to generate the affinity maturation library. Further heavy and light chain FR and HVR residues were mutated to increase stability of the antibody and introduce amino acids found in the germline, such as the heavy chain FR mutation 193V.
- HVR-H1 sequence is X1YX2MX3 (SEQ ID NO:1);
- HVR-H2 sequence is SIYPSGGX4TFYADX5VKG (SEQ ID NO:2);
- HVR-H3 sequence is IKLGTVTTVX 6 Y (SEQ ID NO:3);
- X ! is K, R, T, Q, G, A, W, M, I or S
- X 2 is V, R, K, L, M or I
- X 3 is H, T, N, Q, A, V, Y, W, F or M
- X4 is F or I
- X5 is S or T
- Xe is E or D
- HVR-L1 sequence is TGTXyXeDVGXgYNYVS (SEQ ID NO:8);
- HVR-L2 sequence is X 10 VXnXi 2 RPS (SEQ ID NO:9);
- HVR-L3 sequence is SSX 13 TX 14 Xi5Xi6Xi7RV (SEQ ID NO: 10);
- X 7 is N or S
- X 8 is T, R or S
- X 9 is A or G
- X 10 is E or D
- Xn is I, N or S
- Xi 2 is D, H or N
- X13 is F or Y
- X i4 is N or S
- X15 is R, T or S
- X i6 is G or S
- X17 is I or T.
- Cell cultures were conducted in batch mode in a 250L Single-use-Bioreactor (SUB) (Table 2-2). Cells were grown in ProCH05 growth media supplemented with 4 m L- Glutamine + 25 pg/mL puromycin at 37 °C. The cultures were fed with 15% Efficient Feed B and 1.0 mM valproic acid 3 days after inoculation.
- Millistak+ Pod D0HC (Millipore MD0HC10FS1) and 0.11 m2 Millistak+ Pod A1HC (Millipore MA1HC01FS1) filters, followed by terminal filtration with a Sartopore 2 filter (Sartorius 5445307H8-SS).
- Pod D0HC (Millipore MD0HC10FS1)
- 0.11 m2 Millistak+ Pod A1HC (Millipore MA1HC01FS1) filters
- terminal filtration with a Sartopore 2 filter (Sartorius 5445307H8-SS).
- the purification process consisted of two chromatography steps; (a) MabSelect Protein A to capture the antibody from the clarified harvest, and (b) Hydroxyapatite Type II polish step to remove remaining aggregated product, host cell proteins and DNA, and product related impurities.
- An intermediate Q-filtration step was inserted between the 2 chromatography steps to further reduce DNA.
- SDS-PAGE and size exclusion chromatography SE-HPLC were used to analyze in-process samples during purification. Protein content of the Mabselect in-process samples was performed using the Protein A HPLC method while UVA is spectroscopy was used for all other process steps.
- Post Mabselect eluates were subjected to 30 min of low pH viral inactivation (pH 3.7) and subsequently neutralized to pH 7.0 prior to the next purification step.
- the final polishing step was the hydroxyapatite Type II chromatography.
- the conductivity of the Sartobind Q filtrate was adjusted to ⁇ 3 mS/cm with water, and pH reduced to 6.5 with acetic acid before sample loading.
- Bound anti-PD-L1 product was eluted with a NaCI step gradient. Aggregated product-related impurities was eluted with the Strip Buffer.
- Purified anti-PDL1 from the hydroxyapatite polishing step were concentrated and then diafiltered into their respective buffers according to the Table below.
- the bulk products were then sterile-filtered through 0.2 filter units and further diluted with formulation buffer to their final concentrations.
- Formulated bulk substance were further tested for endotoxin and checked by SE-HPLC.
- the formulation contains antioxidative excipients and was shown to be sufficiently stable at the following stress conditions:
- Binding affinity and selectivity was determined by Biacore assays.
- the affinity of the lead antibody candidate for human and non human orthologues is summarized in the table below.
- the binding affinity of anti PD-L1 antibody A09-246-2 according to this invention for human, mouse and cynomolgus monkey proteins was statistically similar but highly reduced for dog, rat and rabbit proteins that displayed a very fast dissociation profile.
- ka d/M sl kd d/sl * KD(M> KD(nM) : .+/- STD
- VL-A31G VH-
- VL-A31G VH-
- VL-A31G VH-
- VL-A31G VH-
- VL-A31G VH-
- VL-A31G VH-
- VL-A31G VH-
- VL-A31G VH-
- VL-A31G VH-
- Selectivity was determined by evaluating the binding to members of the B7 family including hu-PD- L1-huFc, hu-PDL-2-huFc, hu-B7.1-huFc, hu-B7.2-huFc, huB7-H2- huFc and huB7-H3-huFc by Biacore.
- Binding Buffer 50 mM Hepes, pH 7.5, 130 mM NaCI, 5.1 mM KCI, 1.3 mM MgS0 4 Assay buffer: binding buffer + 0.5% BSA
- A09-246-2 The ability of A09-246-2 to block soluble B7.1 binding to PD-L1 on cell surface was measured by FACS. Results indicated A09-246-2 efficiently blocks the interaction of B7.1 and PD-L1 with an IC50 of 0.2 ⁇ 0.004nM (0.03 ⁇ 0.0006 ⁇ g/ml).
- the extracellular domain of PD-L1 antigen (SEQ ID NO:29) was incubated in heavy water (D 2 0) solution to allow amide protons on the protein backbone to exchange with deuterons from the solvent, in either the presence or absence of excess anti- PD-L1 Fab or a non-specific Fab.
- the samples were digested with protease and analysed by liquid chromatography-mass spectrometry (LC-MS) to determine the level of deuteration in each peptide.
- the Fab corresponding to A09-246-2 was used instead of the full IgG in order to simplify the mass spectrometry analysis by decreasing the number of peptides generated by protease digestion.
- HD exchange identified two peptides
- the antigens were injected at concentrations of 100 nM, 50 nM, 25 nM, 12.5 nM and 6.25 nM (except for Y56 and D61 mutants, which were injected at 1 uM, 500 nM, 250 nM, 125 nM and 62.5 nM). Between each cycle, the chip was regenerated with low pH buffer and fresh A09-246-2 was captured prior to injecting the next concentration of antigen. The rate constants were determined by iterative fitting of the data to a 1 :1 binding model by an algorithm that minimizes Chi- squared.
- K D The equilibrium dissociation constant
- Mutants of Y56 and D61 displayed minimal destabilization of the antigen indicated by a small decrease in the T V2 of fluorescence monitored unfolding (table above). This confirms that Y56 and D61 are true binding hotspots for A09-246-2. The structural integrity of most of the other mutant proteins was also confirmed by this method (table above). The observation that most mutant proteins behaved similarly to wild type on analytical size exclusion chromatography (last column in the above table) provides further support for native structure of mutant antigen proteins. 3.6 Binding to Tumor Cells and Primary Cells
- A09-246-2 demonstrated reactivity to human PD-L1 on all seven tested human tumor lines (A431 , epithelial carcinoma cell line; A549, lung adenocarcinoma epithelial cells; BxPC3, pancreatic cancer cells; HCT116, colorectal carcinoma; M24, melanoma cell lines; PC3mm2, prostate cancer cell line; U-87 MG, glioblastoma- astrocytoma) of which PD-L1 was up-regulated by interferon treatment to enable detection.
- human tumor lines A431 , epithelial carcinoma cell line; A549, lung adenocarcinoma epithelial cells; BxPC3, pancreatic cancer cells; HCT116, colorectal carcinoma; M24, melanoma cell lines; PC3mm2, prostate cancer cell line; U-87 MG, glioblastoma- astrocytoma
- A09-246-2 efficiently binds to human PD-L1 expressed on the HEK cell surface with an EC50 of 0.3 ⁇ 0.02nM (0.04 ⁇ 0.003 Mg/ml); to cynomolgus monkey expressed on the HEK cell surface with an EC50 of 0.94 ⁇ 0.015nM (0.14 ⁇ 0.002 ⁇ g/ml); to mouse PD-L1 expressed on the HEK293 cell surface with an EC50 of 0.34 ⁇ 0.08 nM (0.05 ⁇ 0.012 ⁇ g/ml) and mouse PD-L1 expressed on the EL4 cell surface with an EC50 of 0.91 ⁇ 0.21 nM (0.13 ⁇ 0.03 ⁇ g/ml).
- the assays qualitatively described the dose dependent binding characteristics of anti-PD-L1.
- Antigen-specific CD8 T cells were generated by stimulating splenocytes from OT-1 transgenic mice with Ova peptide SIINFEKL and cyropreserved. mPD-L1 over- expressing EL4 cells were used as antigen presenting cells. Serial dilutions of tested compounds were incubated with thawed OT-1 T cells and SIINFEKL-loaded APC for 48 hours. IFN- ⁇ in the supernatant was measured using mlFN ⁇ y ELISA. Anti-PD-L1 (A09-246-2) efficiently enhanced T cell activities represented by IFN- ⁇ production with an EC50 of 0.28 ⁇ 0.1 nM (0.04 ⁇ 0.015 ⁇ g/ml) b) SEA Assay
- ADCC Antibody dependent cell-mediated Cytotoxicity
- ADCC was measured utilizing two different human tumor lines A431 and A549 as target cells and human PBMC as effector cells. In some cases, tests were performed using target cells following stimulation with Interferon-gamma to increase the expression of PD-L1.
- the anti-EGFR antibody, cetuximab was used as an ADCC positive control. Given the fact that the Fcyllla receptor 158V allotype displays a higher affinity for human lgG1 and increases ADCC, the observed results were correlated with the donor's allotype.
- ADCC activity of A09-246-2 was comparable to that mediated with the anti-EGFR antibody cetuximab, inducing approximately 50% of maximum lysis in both cell lines.
- INF- ⁇ treatment did not alter the response of A431 cells for all the different allotypes tested (VA , V/F and F/F).
- a significant difference (almost twice) between stimulated and not stimulated cells was observed when A549 cells were employed for PBMC from V V and V/F donors. No ADCC was observed when PBMC from F/F donors were analyzed with A549 cells.
- the selected antibody has demonstrated significant activity as a monotherapy and in various combination therapy settings.
- the antitumor activity of the anti-PD-L1 antibody demonstrated a dose-dependent trend when given as a monotherapy against MC38 tumors.
- the anti-PD-L1 antibody In combination with fractionated radiotherapy against MC38 tumors, the anti-PD-L1 antibody showed strong synergistic activity, with curative potential. Combination with a single low-dose of cyclophosphamide resulted in enhanced anti-tumor effects in the MC-38 model that were associated with an increased frequency of tumor-antigen specific CD8 + T cells. Anti-PD-L1 therapy significantly extended survival time when combined with Gemcitabine in the PANC02 orthotopic tumor model of pancreatic cancer. When anti-PD-L1 was combined with cyclophosphamide pre-treatment followed by vaccination with Stimuvax, a significant increase in tumor growth inhibition was achieved in both the MC38/MUC1 and PANC02/MUC1 tumor models.
- mice were inoculated subcutaneously in the right flank with 1x10 6 MC38 colon carcinoma cells.
- N 14
- Groups were administered A09-246-2 intravenously at dose levels of 100, 200, 400, or 800 pg on days 0, 3, and 6.
- a control group was treated with 200 pg of an inactive isotype antibody. Tumors were measured twice weekly for the study duration. All treatment groups demonstrated significant efficacy (P ⁇ 0.050) when compared to the isotype control group.
- the 800 pg dose group did not show enhanced efficacy over the 400 pg group, a significant trend toward a dose-dependent effect was observed.
- the primary endpoint of this study was survival based on the onset of clinical signs, indicative of metastatic dissemination, which warranted euthanasia.
- At the end of the study (day 76), 20% of mice (1/5) were still alive in the isotype antibody treated group, and 80% (4/5) survivors remained in the A09-246-2 treated group.
- GEM was dosed at 150 mg/kg in all studies and A09-246-2 was dosed at 400 pg per mouse.
- a 28 day cycle of GEM was modeled (administration on days 5, 19, 26), with a 14 day holiday period during which A09-246-2 was given on days 8, 11, 14.
- a 21 day cycle of GEM was modeled (administration on days 5, 12, 26, 33), with a 14 day holiday period during which A09-246-2 was given on days 13, 16, 19.
- Monotherapy with GEM or anti-PD-L1 failed to extend survival time in this model.
- the combination of GEM and A09-246-2 significantly extended mean survival time (P ⁇ 0.02).
- Immunophenotyping revealed several effects in groups receiving A09-246-2, both as a monotherapy and in combination with GEM, that were consistent with the proposed MOA of anti-PD-L1 including increased percentages of CD8 + T EM in spleens, an increased ratio of splenic CD8 + T E M to T REG cells, and increased percentages of splenic PD-1 + CD8 + T cells. Furthermore, immunophenotyping of tumor infiltrating lymphocytes (TIL) showed significantly increased percentages of CD8 + TIL in the combination group.
- TIL tumor infiltrating lymphocytes
- Low-dose CPA is known to enhance anti-tumor immune responses through the inhibition of immunosuppressive regulatory T cells.
- the potential for low-dose CPA pre-treatment was investigated to enhance the efficacy of the anti-PD-L1 Ab (A09- 246-2) in the MC38 subcutaneous tumor model. Mice were inoculated
- a control treatment group received 100 pg of an inactive isotype antibody in combination with CPA pretreatment.
- the combination treatment group demonstrated a statistically significant enhancement (p ⁇ 0.050) of anti-tumor activity when compared against the isotype and monotherapy control groups.
- ELISPOT assay the effects of treatment on the magnitude of CD8 + T cell responses directed against the well-characterized p15E tumor antigen were measured.
- Both CPA and A09-246-2 showed substantially increased levels of p15E- reactive CD8 + T cells ( ⁇ 100 spots in both groups) when compared to the isotype control (-25 spots), with the combination group showing a further enhancement (-250 spots).
- the anti-tumor efficacy of the CPA plus A09-246-2 combination was associated with increased frequencies of tumor-antigen reactive CTL.
- Stimuvax is a vaccine against the human MUC1 antigen, which is commonly overexpressed by solid tumors.
- Mice transgenic for the human MUC1 protein (MUCl .tg mice) are immunologically tolerant of the antigen, and, when inoculated with murine tumors that also express human MUC1, provide a relevant model of the clinical vaccination setting.
- cyclophosphamide (CPA) pre-treatment is used in combination with Stimuvax as a strategy for transiently depleting immunosuppressive T reg cells that can inhibit the vaccine response.
- mice were inoculated subcutaneously in the right rear flank with 1x10 6 MC38/ UC1 colon carcinoma cells.
- Five days after tumor cell inoculation, mice were randomized into treatment groups (N 10) on day -3.
- Vaccination was initiated on day 0 and was repeated weekly.
- Anti-PD-L1 Ab (A09-246-2) was dosed by i.p. injection on days 0, 3, and 6. Tumors were measured twice weekly.
- the combination of CPA/Stimuvax and A09-246-2 demonstrated significantly enhanced (p ⁇ 0.050) tumor growth inhibition when compared against treatment with CPA/Stimuvax.
- Radiotherapy has been demonstrated to enhance the immunogenicity of tumor cells, through increased expression of MHC class I and diversification of the intracellular peptide pool.
- MC38 colon carcinoma cells (1x10 5 ) were inoculated intramuscularly into the right quadriceps of C57BL/6 female mice.
- the tumor-bearing legs were isolated and treated with 360 cGy of gamma irradiation from a cesium-137 source on days 0, 1, 2, 3, and 4 (total dose of 1800 cGy).
- Anti-PD-L1 Ab (A09-246-2) was dosed i.v. at 400 pg on days 3, 6, and 9.
- the A09-246-2 and radiotherapy combination resulted in a high rate of tumor regressions, ultimately leading to 6/10 complete responses (CR).
- Mice with CR were re-challenged by inoculation of MC38 tumor cells, and 3/6 mice remained tumor-free seventy-four days after the re-challenge, indicating that effective immune memory was generated by the combination therapy.
- a control group treated with an isotype control antibody in combination with radiation showed significant tumor growth inhibition, but did not induce regressions.
- CD8 + T cells completely abrogated the synergy of the combination, confirming that the mechanism involves the stimulation of anti-tumor CD8 + T cell responses. This observation was further supported by increased frequencies of CD8 + T cells reactive to the p15E tumor antigen. Immunophenotyping by FACS revealed increased percentages of CD8 + T cell proliferation in spleens, and increased splenic percentages of CD8 + T E M and CD8+ T CM -
- FOLFOX is a combination chemotherapy regimen, consisting of folinic acid, 5- fluorouracil (5-FU), and oxaliplatin (OX), used in the treatment of stage III colorectal cancer.
- 5- fluorouracil 5-FU
- OX oxaliplatin
- FOLFOX 5-fluorouracil and oxaliplatin
- mice were inoculated in the right subcutaneous flank with 1x10 6 MC38 colon carcinoma cells.
- 5-FU 60 mg/kg i.v.
- OX 5 mg/kg i.p.
- 5-FU 60 mg/kg i.v.
- OX 5 mg/kg i.p.
- Anti-PD-L1 Ab (A09-246-2) (400 pg i.v.) was given on days 3, 6, and 9.
- the combination treatment was observed to have significantly greater efficacy (p ⁇ 0.050) when compared to A09-246-2 given alone, or 5-FU and OX given in combination with an isotype antibody.
- a repeat of the anti-PD-L1 Ab and FOLFOX combination study was performed and, again, the combination demonstrated significantly greater (p ⁇ 0.050) anti-tumor activity than either of the monotherapy regimens.
- FACS-based immunophenotyping conducted in these studies revealed increases in several immunological markers consistent with a CD8 + T cell driven MOA, including increased splenic levels of p15E tumor antigen specific CD8 + T cells, an increase in the splenic ratio of TEM to regulatory T cells (T re g), and increased splenic percentages of CD8 + PD-1 + T cells. Furthermore, the percentage of tumor infiltrating natural killer (NK) cells and CD8 + T cells was observed to increase significantly in the combination group.
- NK tumor infiltrating natural killer
- the TK evaluation indicates that all animals were exposed to the test material throughout the study.
- the exposure levels increased roughly proportionally to dose increasing at both 1 st and 4 th dose, without any relevant accumulation or gender- dependency at any dose.
- Anti drug antibody were detected in 2/4 and 1/4 monkeys at 20 and 140 mg/kg levels respectively. There was no premature animal death in the study. No treatment related changes were noted in the 20 and 60 mg/kg dosing groups for all parameters evaluated in the study.
- treatment related findings include slight decrease of lymphocytes in haematology testing, slight decrease in lymphocyte count together with a decrease in NK cell count on study day 30. There were no significant histological changes in major organs/tissues except moderate perivascular hemorrhage and inflammation/vessel necrosis observed at local injection site at the 140 mg/kg. There was no clear trend or change observed in multicytokine analysis at this dose level. Based on the results from this study the No Observable Adverse Effect Level (NOAEL) was identified as 140 mg/kg.
- NOAEL No Observable Adverse Effect Level
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Epidemiology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Mycology (AREA)
- Microbiology (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Gastroenterology & Hepatology (AREA)
- Oncology (AREA)
- Endocrinology (AREA)
- Zoology (AREA)
- Biomedical Technology (AREA)
- Cell Biology (AREA)
- Urology & Nephrology (AREA)
- Toxicology (AREA)
- Diabetes (AREA)
- Neurosurgery (AREA)
- Pulmonology (AREA)
- Reproductive Health (AREA)
- Dermatology (AREA)
- Neurology (AREA)
- Radiology & Medical Imaging (AREA)
- Biotechnology (AREA)
Abstract
Description
Claims
Priority Applications (33)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201280058422.3A CN103987405B (en) | 2011-11-28 | 2012-11-21 | Anti- PD L1 antibody and application thereof |
| EA201400625A EA036814B9 (en) | 2011-11-28 | 2012-11-21 | Anti-pd-l1 antibody (embodiments), composition comprising this antibody and use thereof |
| US14/360,775 US9624298B2 (en) | 2011-11-28 | 2012-11-21 | Anti-PD-L1 antibodies and uses thereof |
| BR112014012819-7A BR112014012819B1 (en) | 2011-11-28 | 2012-11-21 | ANTI-PD-L1 ANTIBODY OR ANTIGEN BINDING FRAGMENT AND COMPOSITION |
| EP12790432.4A EP2785375B1 (en) | 2011-11-28 | 2012-11-21 | Anti-pd-l1 antibodies and uses thereof |
| MX2014006316A MX349096B (en) | 2011-11-28 | 2012-11-21 | Anti-pd-l1 antibodies and uses thereof. |
| KR1020177021068A KR101981873B1 (en) | 2011-11-28 | 2012-11-21 | Anti-pd-l1 antibodies and uses thereof |
| SG11201402603WA SG11201402603WA (en) | 2011-11-28 | 2012-11-21 | Anti-pd-l1 antibodies and uses thereof |
| AU2012344260A AU2012344260B2 (en) | 2011-11-28 | 2012-11-21 | Anti-PD-L1 antibodies and uses thereof |
| KR1020147017974A KR101764096B1 (en) | 2011-11-28 | 2012-11-21 | Anti-pd-l1 antibodies and uses thereof |
| SI201231849T SI2785375T1 (en) | 2011-11-28 | 2012-11-21 | Anti-pd-l1 antibodies and uses thereof |
| LTEP12790432.4T LT2785375T (en) | 2011-11-28 | 2012-11-21 | Anti-pd-l1 antibodies and uses thereof |
| DK12790432.4T DK2785375T3 (en) | 2011-11-28 | 2012-11-21 | ANTI-PD-L1 ANTIBODIES AND USES THEREOF |
| CA2856895A CA2856895C (en) | 2011-11-28 | 2012-11-21 | Anti-pd-l1 antibodies and uses thereof |
| JP2014542728A JP6138813B2 (en) | 2011-11-28 | 2012-11-21 | Anti-PD-L1 antibody and use thereof |
| EP20186754.6A EP3763741A1 (en) | 2011-11-28 | 2012-11-21 | Anti-pd-l1 antibodies and uses thereof |
| RS20201263A RS61033B1 (en) | 2011-11-28 | 2012-11-21 | Anti-pd-l1 antibodies and uses thereof |
| ES12790432T ES2808152T3 (en) | 2011-11-28 | 2012-11-21 | Anti-PD-L1 antibodies and their uses |
| IL232778A IL232778B (en) | 2011-11-28 | 2014-05-25 | Anti-pd-l1 antibodies and uses thereof |
| ZA2014/04790A ZA201404790B (en) | 2011-11-28 | 2014-06-27 | Anti-pd-l1 antibodies and uses thereof |
| HK15101432.7A HK1200736A1 (en) | 2011-11-28 | 2015-02-10 | Anti-pd-l1 antibodies and uses thereof pd-l1 |
| US15/454,959 US10487147B2 (en) | 2011-11-28 | 2017-03-09 | Anti-PD-L1 antibodies and uses thereof |
| US15/454,939 US10759856B2 (en) | 2011-11-28 | 2017-03-09 | Anti-PD-L1 antibodies and uses thereof |
| AU2017268603A AU2017268603B2 (en) | 2011-11-28 | 2017-11-30 | Anti-PD-L1 antibodies and uses thereof |
| US16/984,365 US11884724B2 (en) | 2011-11-28 | 2020-08-04 | Anti-PD-L1 antibodies and uses thereof |
| IL276573A IL276573A (en) | 2011-11-28 | 2020-08-09 | Anti-pd-l1 antibodies and uses thereof |
| HRP20201595TT HRP20201595T1 (en) | 2011-11-28 | 2020-10-07 | Anti-pd-l1 antibodies and uses thereof |
| CY20201100946T CY1123435T1 (en) | 2011-11-28 | 2020-10-08 | ANTI-PD-L1 ANTIBODIES AND USES THEREOF |
| NL301081C NL301081I2 (en) | 2011-11-28 | 2020-12-18 | Avelumab |
| CY2020045C CY2020045I2 (en) | 2011-11-28 | 2020-12-23 | ANTI-PD-L1 ANTIBODIES AND USES THEREOF |
| FR20C1068C FR20C1068I2 (en) | 2011-11-28 | 2020-12-29 | ANTI-PD-L1 ANTIBODIES AND THEIR USE |
| LTPA2021001C LTC2785375I2 (en) | 2011-11-28 | 2021-01-05 | ANTI-PD-L1 ANTIBODIES AND THEIR USE |
| NO2021003C NO2021003I1 (en) | 2011-11-28 | 2021-01-19 | avelumab |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161563903P | 2011-11-28 | 2011-11-28 | |
| US61/563,903 | 2011-11-28 |
Related Child Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/360,775 A-371-Of-International US9624298B2 (en) | 2011-11-28 | 2012-11-21 | Anti-PD-L1 antibodies and uses thereof |
| US15/454,959 Division US10487147B2 (en) | 2011-11-28 | 2017-03-09 | Anti-PD-L1 antibodies and uses thereof |
| US15/454,939 Division US10759856B2 (en) | 2011-11-28 | 2017-03-09 | Anti-PD-L1 antibodies and uses thereof |
| US15/454,939 Continuation US10759856B2 (en) | 2011-11-28 | 2017-03-09 | Anti-PD-L1 antibodies and uses thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013079174A1 true WO2013079174A1 (en) | 2013-06-06 |
Family
ID=47221305
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2012/004822 WO2013079174A1 (en) | 2011-11-28 | 2012-11-21 | Anti-pd-l1 antibodies and uses thereof |
Country Status (28)
| Country | Link |
|---|---|
| US (4) | US9624298B2 (en) |
| EP (2) | EP3763741A1 (en) |
| JP (2) | JP6138813B2 (en) |
| KR (2) | KR101981873B1 (en) |
| CN (1) | CN103987405B (en) |
| AR (1) | AR089010A1 (en) |
| AU (2) | AU2012344260B2 (en) |
| BR (1) | BR112014012819B1 (en) |
| CA (1) | CA2856895C (en) |
| CY (2) | CY1123435T1 (en) |
| DK (1) | DK2785375T3 (en) |
| EA (1) | EA036814B9 (en) |
| ES (1) | ES2808152T3 (en) |
| FR (1) | FR20C1068I2 (en) |
| HK (1) | HK1200736A1 (en) |
| HR (1) | HRP20201595T1 (en) |
| HU (2) | HUE051954T2 (en) |
| IL (2) | IL232778B (en) |
| LT (2) | LT2785375T (en) |
| MX (1) | MX349096B (en) |
| NL (1) | NL301081I2 (en) |
| NO (1) | NO2021003I1 (en) |
| PT (1) | PT2785375T (en) |
| RS (1) | RS61033B1 (en) |
| SG (1) | SG11201402603WA (en) |
| SI (1) | SI2785375T1 (en) |
| WO (1) | WO2013079174A1 (en) |
| ZA (1) | ZA201404790B (en) |
Cited By (666)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2014195852A1 (en) | 2013-06-03 | 2014-12-11 | Glaxosmithkline Intellectual Property (No.2) Limited | Combinations of an anti-pd-l1 antibody and a mek inhibitor and/or a braf inhibitor |
| WO2015036511A1 (en) * | 2013-09-12 | 2015-03-19 | F. Hoffmann-La Roche Ag | Combination therapy of antibodies against human csf-1r and antibodies against human pd-l1 |
| WO2015036499A1 (en) * | 2013-09-11 | 2015-03-19 | Medimmune Limited | Anti-b7-h1 antibodies for treating tumors |
| WO2015048520A1 (en) * | 2013-09-27 | 2015-04-02 | Genentech, Inc. | Anti-pdl1 antibody formulations |
| WO2015061668A1 (en) * | 2013-10-25 | 2015-04-30 | Dana-Farber Cancer Institute, Inc. | Anti-pd-l1 monoclonal antibodies and fragments thereof |
| WO2015066413A1 (en) | 2013-11-01 | 2015-05-07 | Novartis Ag | Oxazolidinone hydroxamic acid compounds for the treatment of bacterial infections |
| WO2015100282A1 (en) | 2013-12-24 | 2015-07-02 | Bristol-Myers Squibb Company | Tricyclic compounds as anticancer agents |
| WO2015107495A1 (en) | 2014-01-17 | 2015-07-23 | Novartis Ag | N-azaspirocycloalkane substituted n-heteroaryl compounds and compositions for inhibiting the activity of shp2 |
| WO2015118175A2 (en) | 2014-02-10 | 2015-08-13 | Merck Patent Gmbh | TARGETED TGFβ INHIBITION |
| WO2015138920A1 (en) | 2014-03-14 | 2015-09-17 | Novartis Ag | Antibody molecules to lag-3 and uses thereof |
| WO2015109124A3 (en) * | 2014-01-15 | 2015-09-24 | Kadmon Corporation, Llc | Immunomodulatory agents |
| WO2015148379A1 (en) | 2014-03-24 | 2015-10-01 | Novartis Ag | Monobactam organic compounds for the treatment of bacterial infections |
| US9192667B2 (en) | 2013-04-22 | 2015-11-24 | Hoffmann-La Roche Inc. | Method of treating cancer by administering CSF-1R antibodies and a TLR9 agonist |
| WO2015187835A2 (en) | 2014-06-06 | 2015-12-10 | Bristol-Myers Squibb Company | Antibodies against glucocorticoid-induced tumor necrosis factor receptor (gitr) and uses thereof |
| WO2015193352A1 (en) | 2014-06-17 | 2015-12-23 | Medimmune Limited | Methods of cancer treatment with antagonists against pd-1 and pd-l1 in combination with radiation therapy |
| WO2016004876A1 (en) | 2014-07-09 | 2016-01-14 | Shanghai Birdie Biotech, Inc. | Anti-pd-l1 combinations for treating tumors |
| WO2016020836A1 (en) | 2014-08-06 | 2016-02-11 | Novartis Ag | Quinolone derivatives as antibacterials |
| WO2016040880A1 (en) | 2014-09-13 | 2016-03-17 | Novartis Ag | Combination therapies of alk inhibitors |
| WO2016050721A1 (en) * | 2014-09-30 | 2016-04-07 | Intervet International B.V. | Pd-l1 antibodies binding canine pd-l1 |
| WO2016054555A2 (en) | 2014-10-03 | 2016-04-07 | Novartis Ag | Combination therapies |
| WO2016057846A1 (en) | 2014-10-08 | 2016-04-14 | Novartis Ag | Compositions and methods of use for augmented immune response and cancer therapy |
| WO2016061142A1 (en) | 2014-10-14 | 2016-04-21 | Novartis Ag | Antibody molecules to pd-l1 and uses thereof |
| CN105695406A (en) * | 2016-04-27 | 2016-06-22 | 天津普瑞赛尔生物科技有限公司 | Method for preparing DC-CIK immune cells with high-efficiency tumor killing property and prepared DC-CIK immune cells |
| WO2016100882A1 (en) | 2014-12-19 | 2016-06-23 | Novartis Ag | Combination therapies |
| WO2016097995A1 (en) | 2014-12-16 | 2016-06-23 | Novartis Ag | Isoxazole hydroxamic acid compounds as lpxc inhibitors |
| WO2016106266A1 (en) | 2014-12-22 | 2016-06-30 | Bristol-Myers Squibb Company | TGFβ RECEPTOR ANTAGONISTS |
| WO2016123454A1 (en) | 2015-01-29 | 2016-08-04 | Board Of Trustees Of Miching State University | Cryptic polypeptides and uses thereof |
| WO2016127052A1 (en) | 2015-02-05 | 2016-08-11 | Bristol-Myers Squibb Company | Cxcl11 and smica as predictive biomarkers for efficacy of anti-ctla4 immunotherapy |
| WO2016081518A3 (en) * | 2014-11-17 | 2016-08-18 | Adicet Bio, Inc. | Engineered gamma delta t-cells |
| WO2016137985A1 (en) | 2015-02-26 | 2016-09-01 | Merck Patent Gmbh | Pd-1 / pd-l1 inhibitors for the treatment of cancer |
| WO2016140884A1 (en) | 2015-03-02 | 2016-09-09 | Rigel Pharmaceuticals, Inc. | TGF-β INHIBITORS |
| WO2016145102A1 (en) | 2015-03-10 | 2016-09-15 | Aduro Biotech, Inc. | Compositions and methods for activating "stimulator of interferon gene" -dependent signalling |
| WO2016145085A2 (en) | 2015-03-09 | 2016-09-15 | Celldex Therapeutics, Inc. | Cd27 agonists |
| WO2016161269A1 (en) | 2015-04-03 | 2016-10-06 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase for the treatment of cancer |
| WO2016162505A1 (en) | 2015-04-08 | 2016-10-13 | F-Star Biotechnology Limited | Her2 binding agent therapies |
| WO2016183118A1 (en) | 2015-05-12 | 2016-11-17 | Bristol-Myers Squibb Company | Tricyclic compounds as anticancer agents |
| WO2016183114A1 (en) | 2015-05-11 | 2016-11-17 | Bristol-Myers Squibb Company | Tricyclic compounds as anticancer agents |
| WO2016181348A1 (en) | 2015-05-14 | 2016-11-17 | Pfizer Inc. | Combinations comprising a pyrrolidine-2,5-dione ido1 inhibitor and an anti-body |
| WO2016183115A1 (en) | 2015-05-12 | 2016-11-17 | Bristol-Myers Squibb Company | 5h-pyrido[3,2-b]indole compounds as anticancer agents |
| WO2016196218A1 (en) | 2015-05-31 | 2016-12-08 | Curegenix Corporation | Combination compositions for immunotherapy |
| WO2016196228A1 (en) | 2015-05-29 | 2016-12-08 | Bristol-Myers Squibb Company | Antibodies against ox40 and uses thereof |
| WO2016201425A1 (en) | 2015-06-12 | 2016-12-15 | Bristol-Myers Squibb Company | Treatment of cancer by combined blockade of the pd-1 and cxcr4 signaling pathways |
| WO2016205277A1 (en) * | 2015-06-16 | 2016-12-22 | Merck Patent Gmbh | Pd-l1 antagonist combination treatments |
| WO2017004016A1 (en) | 2015-06-29 | 2017-01-05 | The Rockefeller University | Antibodies to cd40 with enhanced agonist activity |
| WO2017000983A1 (en) | 2015-06-29 | 2017-01-05 | Ose Immunotherapeutics | Method for inducing early t memory response with short peptides anti-tumor vaccine |
| WO2017019894A1 (en) | 2015-07-29 | 2017-02-02 | Novartis Ag | Combination therapies comprising antibody molecules to lag-3 |
| WO2017019757A1 (en) | 2015-07-28 | 2017-02-02 | Bristol-Myers Squibb Company | Tgf beta receptor antagonists |
| WO2017019897A1 (en) | 2015-07-29 | 2017-02-02 | Novartis Ag | Combination therapies comprising antibody molecules to tim-3 |
| WO2017035118A1 (en) | 2015-08-25 | 2017-03-02 | Bristol-Myers Squibb Company | Tgf beta receptor antagonists |
| US9598422B2 (en) | 2014-11-05 | 2017-03-21 | Flexus Biosciences, Inc. | Immunoregulatory agents |
| US9605070B2 (en) | 2014-01-31 | 2017-03-28 | Novartis Ag | Antibody molecules to TIM-3 and uses thereof |
| WO2017058780A1 (en) | 2015-09-30 | 2017-04-06 | Merck Patent Gmbh | Combination of a pd-1 axis binding antagonist and an alk inhibitor for treating alk-negative cancer |
| WO2017055484A1 (en) | 2015-09-29 | 2017-04-06 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for determining the metabolic status of lymphomas |
| KR20170039706A (en) * | 2014-08-05 | 2017-04-11 | 씨비 테라퓨틱스, 인코포레이티드 | Anti-pd-l1 antibodies |
| US20170100425A1 (en) * | 2014-07-10 | 2017-04-13 | Biothera, Inc. | Beta-glucan in combination with anti-cancer agents affecting the tumor microenvironment |
| WO2017069291A1 (en) | 2015-10-23 | 2017-04-27 | Canbas Co., Ltd. | Peptides and peptidomimetics in combination with t cell activating and/or checkpoint inhibiting agents for cancer treatment |
| US9643972B2 (en) | 2014-11-05 | 2017-05-09 | Flexus Biosciences, Inc. | Immunoregulatory agents |
| WO2017087678A2 (en) | 2015-11-19 | 2017-05-26 | Bristol-Myers Squibb Company | Antibodies against glucocorticoid-induced tumor necrosis factor receptor (gitr) and uses thereof |
| WO2017093933A1 (en) | 2015-12-03 | 2017-06-08 | Glaxosmithkline Intellectual Property Development Limited | Cyclic purine dinucleotides as modulators of sting |
| WO2017098421A1 (en) | 2015-12-08 | 2017-06-15 | Glaxosmithkline Intellectual Property Development Limited | Benzothiadiazine compounds |
| WO2017097407A1 (en) * | 2015-12-07 | 2017-06-15 | Merck Patent Gmbh | Aqueous pharmaceutical formulation comprising anti-pd-1 antibody avelumab |
| US9683048B2 (en) | 2014-01-24 | 2017-06-20 | Novartis Ag | Antibody molecules to PD-1 and uses thereof |
| WO2017106453A1 (en) | 2015-12-17 | 2017-06-22 | University Of Maryland, Baltimore County | A recombinant bi-specific polypeptide for coordinately activating tumor-reactive t-cells and neutralizing immune suppression |
| WO2017106656A1 (en) | 2015-12-17 | 2017-06-22 | Novartis Ag | Antibody molecules to pd-1 and uses thereof |
| WO2017103283A1 (en) | 2015-12-17 | 2017-06-22 | Photocure Asa | Neoadjuvant therapy for bladder cancer |
| WO2017106291A1 (en) | 2015-12-15 | 2017-06-22 | Bristol-Myers Squibb Company | Cxcr4 receptor antagonists |
| WO2017103895A1 (en) | 2015-12-18 | 2017-06-22 | Novartis Ag | Antibodies targeting cd32b and methods of use thereof |
| WO2017112741A1 (en) | 2015-12-22 | 2017-06-29 | Novartis Ag | Mesothelin chimeric antigen receptor (car) and antibody against pd-l1 inhibitor for combined use in anticancer therapy |
| WO2017118634A1 (en) | 2016-01-04 | 2017-07-13 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Use of pd-1 and tim-3 as a measure for cd8+ cells in predicting and treating renal cell carcinoma |
| JP2017522905A (en) * | 2014-07-03 | 2017-08-17 | ベイジーン リミテッド | Anti-PD-L1 antibodies and their use for therapy and diagnosis |
| WO2017140821A1 (en) | 2016-02-19 | 2017-08-24 | Novartis Ag | Tetracyclic pyridone compounds as antivirals |
| WO2017144681A1 (en) | 2016-02-25 | 2017-08-31 | Cell Medica Switzerland Ag | Binding members to pd-l1 |
| WO2017153952A1 (en) | 2016-03-10 | 2017-09-14 | Glaxosmithkline Intellectual Property Development Limited | 5-sulfamoyl-2-hydroxybenzamide derivatives |
| WO2017160754A1 (en) | 2016-03-15 | 2017-09-21 | Mersana Therapeutics,Inc. | Napi2b-targeted antibody-drug conjugates and methods of use thereof |
| WO2017159699A1 (en) | 2016-03-15 | 2017-09-21 | Chugai Seiyaku Kabushiki Kaisha | Methods of treating cancers using pd-1 axis binding antagonists and anti-gpc3 antibodies |
| WO2017163186A1 (en) | 2016-03-24 | 2017-09-28 | Novartis Ag | Alkynyl nucleoside analogs as inhibitors of human rhinovirus |
| WO2017178572A1 (en) | 2016-04-13 | 2017-10-19 | Vivia Biotech, S.L | Ex vivo bite-activated t cells |
| EP3094736A4 (en) * | 2014-01-14 | 2017-10-25 | Dana-Farber Cancer Institute, Inc. | Compositions and methods for identification, assessment, prevention, and treatment of melanoma using pd-l1 isoforms |
| WO2017184619A2 (en) | 2016-04-18 | 2017-10-26 | Celldex Therapeutics, Inc. | Agonistic antibodies that bind human cd40 and uses thereof |
| WO2017191545A1 (en) | 2016-05-05 | 2017-11-09 | Glaxosmithkline Intellectual Property (No.2) Limited | Enhancer of zeste homolog 2 inhibitors |
| WO2017196867A1 (en) * | 2016-05-09 | 2017-11-16 | Igm Biosciences, Inc. | Anti-pd-l1 antibodies |
| WO2017200969A1 (en) | 2016-05-20 | 2017-11-23 | Eli Lilly And Company | Combination therapy with notch and pd-1 or pd-l1 inhibitors |
| WO2017202744A1 (en) | 2016-05-26 | 2017-11-30 | Merck Patent Gmbh | Pd-1 / pd-l1 inhibitors for cancer treatment |
| WO2017212425A1 (en) | 2016-06-08 | 2017-12-14 | Glaxosmithkline Intellectual Property Development Limited | Chemical compounds as atf4 pathway inhibitors |
| WO2017216685A1 (en) | 2016-06-16 | 2017-12-21 | Novartis Ag | Pentacyclic pyridone compounds as antivirals |
| WO2017216705A1 (en) | 2016-06-14 | 2017-12-21 | Novartis Ag | Crystalline form of (r)-4-(5-(cyclopropylethynyl)isoxazol-3-yl)-n-hydroxy-2-methyl-2-(methylsulfonyl)butanamide as an antibacterial agent |
| WO2017216686A1 (en) | 2016-06-16 | 2017-12-21 | Novartis Ag | 8,9-fused 2-oxo-6,7-dihydropyrido-isoquinoline compounds as antivirals |
| WO2017218533A1 (en) | 2016-06-13 | 2017-12-21 | Torque Therapeutics, Inc. | Methods and compositions for promoting immune cell function |
| WO2017220989A1 (en) | 2016-06-20 | 2017-12-28 | Kymab Limited | Anti-pd-l1 and il-2 cytokines |
| WO2017223422A1 (en) | 2016-06-24 | 2017-12-28 | Infinity Pharmaceuticals, Inc. | Combination therapies |
| KR20180004277A (en) * | 2016-03-04 | 2018-01-10 | 쓰촨 케룬-바이오테크 바이오파마수티컬 컴퍼니 리미티드 | PDL-1 antibody, pharmaceutical composition thereof and uses thereof |
| WO2018013818A2 (en) | 2016-07-14 | 2018-01-18 | Bristol-Myers Squibb Company | Antibodies against tim3 and uses thereof |
| WO2018015879A1 (en) | 2016-07-20 | 2018-01-25 | Glaxosmithkline Intellectual Property Development Limited | Isoquinoline derivatives as perk inhibitors |
| WO2018017633A1 (en) | 2016-07-21 | 2018-01-25 | Bristol-Myers Squibb Company | TGF Beta RECEPTOR ANTAGONISTS |
| US9885721B2 (en) | 2014-05-29 | 2018-02-06 | Spring Bioscience Corporation | PD-L1 antibodies and uses thereof |
| WO2018029367A1 (en) | 2016-08-12 | 2018-02-15 | Merck Patent Gmbh | Combination therapy for cancer |
| WO2018029474A2 (en) | 2016-08-09 | 2018-02-15 | Kymab Limited | Anti-icos antibodies |
| US9895441B2 (en) | 2012-05-31 | 2018-02-20 | Genentech, Inc. | Methods of treating cancer using PD-L1 axis binding antagonists and VEGF antagonists |
| US9913461B2 (en) | 2014-12-09 | 2018-03-13 | Regeneron Pharmaceuticals, Inc. | Genetically modified mouse whose genome comprises a humanized CD274 gene |
| WO2018047109A1 (en) | 2016-09-09 | 2018-03-15 | Novartis Ag | Polycyclic pyridone compounds as antivirals |
| US9920123B2 (en) | 2008-12-09 | 2018-03-20 | Genentech, Inc. | Anti-PD-L1 antibodies, compositions and articles of manufacture |
| WO2018057955A1 (en) | 2016-09-23 | 2018-03-29 | Elstar Therapeutics, Inc. | Multispecific antibody molecules comprising lambda and kappa light chains |
| WO2018060926A1 (en) | 2016-09-28 | 2018-04-05 | Novartis Ag | Beta-lactamase inhibitors |
| US9938345B2 (en) | 2014-01-23 | 2018-04-10 | Regeneron Pharmaceuticals, Inc. | Human antibodies to PD-L1 |
| WO2018065938A1 (en) | 2016-10-06 | 2018-04-12 | Pfizer Inc. | Dosing regimen of avelumab for the treatment of cancer |
| WO2018068336A1 (en) * | 2016-10-15 | 2018-04-19 | Innovent Biologics (Suzhou) Co., Ltd | Pd-1 antibodies |
| WO2018073753A1 (en) | 2016-10-18 | 2018-04-26 | Novartis Ag | Fused tetracyclic pyridone compounds as antivirals |
| US9957323B2 (en) | 2016-06-20 | 2018-05-01 | Kymab Limited | Anti-ICOS antibodies |
| WO2018081621A1 (en) | 2016-10-28 | 2018-05-03 | Bristol-Myers Squibb Company | Methods of treating urothelial carcinoma using an anti-pd-1 antibody |
| WO2018085555A1 (en) | 2016-11-03 | 2018-05-11 | Bristol-Myers Squibb Company | Activatable anti-ctla-4 antibodies and uses thereof |
| WO2018098269A2 (en) | 2016-11-23 | 2018-05-31 | Mersana Therapeutics, Inc. | Peptide-containing linkers for antibody-drug conjugates |
| WO2018098352A2 (en) | 2016-11-22 | 2018-05-31 | Jun Oishi | Targeting kras induced immune checkpoint expression |
| US9987500B2 (en) | 2014-01-23 | 2018-06-05 | Regeneron Pharmaceuticals, Inc. | Human antibodies to PD-1 |
| WO2018100535A1 (en) | 2016-12-01 | 2018-06-07 | Glaxosmithkline Intellectual Property Development Limited | Combination therapy |
| WO2018100534A1 (en) | 2016-12-01 | 2018-06-07 | Glaxosmithkline Intellectual Property Development Limited | Combination therapy |
| WO2018110515A1 (en) | 2016-12-12 | 2018-06-21 | 第一三共株式会社 | Combination of antibody-drug conjugate and immune checkpoint inhibitor |
| WO2018132279A1 (en) | 2017-01-05 | 2018-07-19 | Bristol-Myers Squibb Company | Tgf beta receptor antagonists |
| WO2018136700A1 (en) | 2017-01-20 | 2018-07-26 | Arcus Biosciences, Inc. | Azolopyrimidine for the treatment of cancer-related disorders |
| EP3277320A4 (en) * | 2015-03-30 | 2018-08-01 | Stcube, Inc. | Antibodies specific to glycosylated pd-l1 and methods of use thereof |
| WO2018138110A1 (en) | 2017-01-25 | 2018-08-02 | Ose Immunotherapeutics | Method for manufacturing a stable emulsion for peptide delivery |
| WO2018138591A1 (en) | 2017-01-24 | 2018-08-02 | Pfizer Inc. | Calicheamicin derivatives and antibody drug conjugates thereof |
| WO2018146612A1 (en) | 2017-02-10 | 2018-08-16 | Novartis Ag | 1-(4-amino-5-bromo-6-(1 h-pyrazol-1-yl)pyrimidin-2-yl)-1 h-pyrazol-4-ol and use thereof in the treatment of cancer |
| WO2018151820A1 (en) | 2017-02-16 | 2018-08-23 | Elstar Therapeutics, Inc. | Multifunctional molecules comprising a trimeric ligand and uses thereof |
| WO2018152396A1 (en) | 2017-02-17 | 2018-08-23 | Innate Tumor Immunity, Inc. | Substituted imidazo-quinolines as nlrp3 modulators |
| WO2018160538A1 (en) | 2017-02-28 | 2018-09-07 | Mersana Therapeutics, Inc. | Combination therapies of her2-targeted antibody-drug conjugates |
| EP3372615A1 (en) | 2017-03-06 | 2018-09-12 | Merck Patent GmbH | Composition comprising avelumab |
| WO2018162446A1 (en) | 2017-03-06 | 2018-09-13 | Merck Patent Gmbh | Aqueous anti-pd-l1 antibody formulation |
| WO2018162749A1 (en) | 2017-03-09 | 2018-09-13 | Genmab A/S | Antibodies against pd-l1 |
| WO2018172508A1 (en) | 2017-03-24 | 2018-09-27 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating melanoma |
| WO2018183928A1 (en) | 2017-03-31 | 2018-10-04 | Bristol-Myers Squibb Company | Methods of treating tumor |
| WO2018178040A1 (en) | 2017-03-30 | 2018-10-04 | Merck Patent Gmbh | Combination of an anti-pd-l1 antibody and a dna-pk inhibitor for the treatment of cancer |
| WO2018187613A2 (en) | 2017-04-07 | 2018-10-11 | Bristol-Myers Squibb Company | Anti-icos agonist antibodies and uses thereof |
| WO2018195397A2 (en) | 2017-04-21 | 2018-10-25 | Kyn Therapeutics | Indole ahr inhibitors and uses thereof |
| WO2018194496A2 (en) | 2017-04-17 | 2018-10-25 | Закрытое Акционерное Общество "Биокад" | Monoclonal antibody to pd-l1 |
| WO2018195283A1 (en) | 2017-04-19 | 2018-10-25 | Elstar Therapeutics, Inc. | Multispecific molecules and uses thereof |
| WO2018198076A1 (en) | 2017-04-28 | 2018-11-01 | Aduro Biotech, Inc. | Bis 2'-5'-rr-(3'f-a)(3'f-a) cyclic dinucleotide compound and uses thereof |
| WO2018200430A1 (en) | 2017-04-26 | 2018-11-01 | Bristol-Myers Squibb Company | Methods of antibody production that minimize disulfide bond reduction |
| WO2018198091A1 (en) | 2017-04-28 | 2018-11-01 | Novartis Ag | Antibody conjugates comprising toll-like receptor agonist and combination therapies |
| WO2018201047A1 (en) | 2017-04-28 | 2018-11-01 | Elstar Therapeutics, Inc. | Multispecific molecules comprising a non-immunoglobulin heterodimerization domain and uses thereof |
| WO2018198079A1 (en) | 2017-04-27 | 2018-11-01 | Novartis Ag | Fused indazole pyridone compounds as antivirals |
| WO2018203302A1 (en) | 2017-05-05 | 2018-11-08 | Novartis Ag | Tricyclic 2-quinolinones as antibacterials |
| CN108779180A (en) * | 2016-03-23 | 2018-11-09 | 迈博斯生物医药(苏州)有限公司 | Novel anti-PD-L1 antibody |
| WO2018209049A1 (en) | 2017-05-12 | 2018-11-15 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2018213377A1 (en) | 2017-05-17 | 2018-11-22 | Arcus Biosciences, Inc. | Quinazoline-pyrazole derivatives for the treatment of cancer-related disorders |
| WO2018213297A1 (en) | 2017-05-16 | 2018-11-22 | Bristol-Myers Squibb Company | Treatment of cancer with anti-gitr agonist antibodies |
| WO2018220446A1 (en) | 2017-06-01 | 2018-12-06 | Compugen Ltd. | Triple combination antibody therapies |
| WO2018223002A1 (en) | 2017-06-01 | 2018-12-06 | Xencor, Inc. | Bispecific antibodies that bind cd 123 cd3 |
| WO2018222901A1 (en) | 2017-05-31 | 2018-12-06 | Elstar Therapeutics, Inc. | Multispecific molecules that bind to myeloproliferative leukemia (mpl) protein and uses thereof |
| WO2018220546A1 (en) | 2017-05-31 | 2018-12-06 | Novartis Ag | Crystalline forms of 5-bromo-2,6-di(1 h-pyrazol-1-yl)pyrimidin-4-amine and new salts |
| WO2018222718A1 (en) | 2017-05-30 | 2018-12-06 | Bristol-Myers Squibb Company | Treatment of lag-3 positive tumors |
| WO2018223004A1 (en) | 2017-06-01 | 2018-12-06 | Xencor, Inc. | Bispecific antibodies that bind cd20 and cd3 |
| WO2018222711A2 (en) | 2017-05-30 | 2018-12-06 | Bristol-Myers Squibb Company | Compositions comprising a combination of an anti-lag-3 antibody, a pd-1 pathway inhibitor, and an immunotherapeutic agent |
| WO2018222722A2 (en) | 2017-05-30 | 2018-12-06 | Bristol-Myers Squibb Company | Compositions comprising an anti-lag-3 antibody or an anti-lag-3 antibody and an anti-pd-1 or anti-pd-l1 antibody |
| WO2018223040A1 (en) | 2017-06-01 | 2018-12-06 | Bristol-Myers Squibb Company | Methods of treating a tumor using an anti-pd-1 antibody |
| WO2018225033A1 (en) | 2017-06-09 | 2018-12-13 | Glaxosmithkline Intellectual Property Development Limited | Combination therapy |
| WO2018225093A1 (en) | 2017-06-07 | 2018-12-13 | Glaxosmithkline Intellectual Property Development Limited | Chemical compounds as atf4 pathway inhibitors |
| WO2018229715A1 (en) | 2017-06-16 | 2018-12-20 | Novartis Ag | Compositions comprising anti-cd32b antibodies and methods of use thereof |
| WO2018237173A1 (en) | 2017-06-22 | 2018-12-27 | Novartis Ag | Antibody molecules to cd73 and uses thereof |
| WO2018235056A1 (en) | 2017-06-22 | 2018-12-27 | Novartis Ag | Il-1beta binding antibodies for use in treating cancer |
| WO2018234879A1 (en) | 2017-06-22 | 2018-12-27 | Novartis Ag | Il-1beta binding antibodies for use in treating cancer |
| WO2018237157A1 (en) | 2017-06-22 | 2018-12-27 | Novartis Ag | Antibody molecules to cd73 and uses thereof |
| WO2019006283A1 (en) | 2017-06-30 | 2019-01-03 | Bristol-Myers Squibb Company | Amorphous and crystalline forms of ido inhibitors |
| WO2019006007A1 (en) | 2017-06-27 | 2019-01-03 | Novartis Ag | Dosage regimens for anti-tim-3 antibodies and uses thereof |
| WO2019008507A1 (en) | 2017-07-03 | 2019-01-10 | Glaxosmithkline Intellectual Property Development Limited | 2-(4-chlorophenoxy)-n-((1 -(2-(4-chlorophenoxy)ethynazetidin-3-yl)methyl)acetamide derivatives and related compounds as atf4 inhibitors for treating cancer and other diseases |
| WO2019008506A1 (en) | 2017-07-03 | 2019-01-10 | Glaxosmithkline Intellectual Property Development Limited | N-(3-(2-(4-chlorophenoxy)acetamido)bicyclo[1.1.1]pentan-1-yl)-2-cyclobutane-1-carboxamide derivatives and related compounds as atf4 inhibitors for treating cancer and other diseases |
| WO2019014402A1 (en) | 2017-07-14 | 2019-01-17 | Innate Tumor Immunity, Inc. | Nlrp3 modulators |
| EP3431088A1 (en) * | 2017-07-20 | 2019-01-23 | Paris Sciences et Lettres - Quartier Latin | Mntbap for use in inducing a transient immune tolerance |
| WO2019018730A1 (en) | 2017-07-20 | 2019-01-24 | Novartis Ag | Dosage regimens of anti-lag-3 antibodies and uses thereof |
| WO2019021208A1 (en) | 2017-07-27 | 2019-01-31 | Glaxosmithkline Intellectual Property Development Limited | Indazole derivatives useful as perk inhibitors |
| WO2019025545A1 (en) | 2017-08-04 | 2019-02-07 | Genmab A/S | Binding agents binding to pd-l1 and cd137 and use thereof |
| WO2019035938A1 (en) | 2017-08-16 | 2019-02-21 | Elstar Therapeutics, Inc. | Multispecific molecules that bind to bcma and uses thereof |
| US10214586B2 (en) | 2015-08-24 | 2019-02-26 | Eli Lilly And Company | PD-L1 antibodies |
| WO2019040780A1 (en) | 2017-08-25 | 2019-02-28 | Five Prime Therapeutics Inc. | B7-h4 antibodies and methods of use thereof |
| WO2019049061A1 (en) | 2017-09-07 | 2019-03-14 | Glaxosmithkline Intellectual Property Development Limited | 5-(1 h-benzo[d]imidazo-2-yl)-pyridin-2-amine and 5-(3h-imidazo[4,5-b]pyridin-6-yl)-pyridin-2-amine derivatives as c-myc and p300/cbp histone acetyltransferase inhibitors for treating cancer |
| WO2019053617A1 (en) | 2017-09-12 | 2019-03-21 | Glaxosmithkline Intellectual Property Development Limited | Chemical compounds |
| EP2944323B1 (en) | 2013-01-11 | 2019-03-27 | Dingfu Biotarget Co., Ltd | Agents for treating tumours, use and method thereof |
| WO2019059411A1 (en) | 2017-09-20 | 2019-03-28 | Chugai Seiyaku Kabushiki Kaisha | Dosage regimen for combination therapy using pd-1 axis binding antagonists and gpc3 targeting agent |
| EP3470426A1 (en) | 2017-10-10 | 2019-04-17 | Numab Therapeutics AG | Multispecific antibody |
| EP3470429A1 (en) * | 2017-10-10 | 2019-04-17 | Numab Innovation AG | Antibodies targeting pdl1 and methods of use thereof |
| WO2019072870A1 (en) | 2017-10-10 | 2019-04-18 | Numab Innovation Ag | Antibodies targeting cd137 and methods of use thereof |
| WO2019072869A1 (en) | 2017-10-10 | 2019-04-18 | Numab Innovation Ag | Antibodies targeting pdl1 and methods of use thereof |
| WO2019075032A1 (en) | 2017-10-13 | 2019-04-18 | Merck Patent Gmbh | Combination of a parp inhibitor and a pd-1 axis binding antagonist |
| WO2019072868A1 (en) | 2017-10-10 | 2019-04-18 | Numab Therapeutics AG | Multispecific antibody |
| WO2019074824A1 (en) | 2017-10-09 | 2019-04-18 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2019075090A1 (en) | 2017-10-10 | 2019-04-18 | Tilos Therapeutics, Inc. | Anti-lap antibodies and uses thereof |
| WO2019075468A1 (en) | 2017-10-15 | 2019-04-18 | Bristol-Myers Squibb Company | Methods of treating tumor |
| WO2019074822A1 (en) | 2017-10-09 | 2019-04-18 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2019077062A1 (en) | 2017-10-18 | 2019-04-25 | Vivia Biotech, S.L. | Bite-activated car-t cells |
| WO2019079520A2 (en) | 2017-10-18 | 2019-04-25 | Alpine Immune Sciences, Inc. | Variant icos ligand immunomodulatory proteins and related compositions and methods |
| WO2019077132A1 (en) | 2017-10-19 | 2019-04-25 | Debiopharm International S.A. | Combination product for the treatment of cancer |
| WO2019081983A1 (en) | 2017-10-25 | 2019-05-02 | Novartis Ag | Antibodies targeting cd32b and methods of use thereof |
| WO2019089753A2 (en) | 2017-10-31 | 2019-05-09 | Compass Therapeutics Llc | Cd137 antibodies and pd-1 antagonists and uses thereof |
| WO2019089921A1 (en) | 2017-11-01 | 2019-05-09 | Bristol-Myers Squibb Company | Immunostimulatory agonistic antibodies for use in treating cancer |
| WO2019090198A1 (en) | 2017-11-06 | 2019-05-09 | Bristol-Myers Squibb Company | Isofuranone compounds useful as hpk1 inhibitors |
| WO2019090330A1 (en) | 2017-11-06 | 2019-05-09 | Bristol-Myers Squibb Company | Methods of treating a tumor |
| WO2019097369A1 (en) | 2017-11-14 | 2019-05-23 | Pfizer Inc. | Ezh2 inhibitor combination therapies |
| WO2019097479A1 (en) | 2017-11-17 | 2019-05-23 | Novartis Ag | Novel dihydroisoxazole compounds and their use for the treatment of hepatitis b |
| WO2019104289A1 (en) | 2017-11-27 | 2019-05-31 | Mersana Therapeutics, Inc. | Pyrrolobenzodiazepine antibody conjugates |
| WO2019101347A1 (en) | 2017-11-27 | 2019-05-31 | Ose Immunotherapeutics | Improved treatment of cancer |
| WO2019108900A1 (en) | 2017-11-30 | 2019-06-06 | Novartis Ag | Bcma-targeting chimeric antigen receptor, and uses thereof |
| WO2019113464A1 (en) | 2017-12-08 | 2019-06-13 | Elstar Therapeutics, Inc. | Multispecific molecules and uses thereof |
| US10323004B2 (en) | 2016-05-04 | 2019-06-18 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2019123285A1 (en) | 2017-12-20 | 2019-06-27 | Novartis Ag | Fused tricyclic pyrazolo-dihydropyrazinyl-pyridone compounds as antivirals |
| WO2019123207A1 (en) | 2017-12-18 | 2019-06-27 | Pfizer Inc. | Methods and combination therapy to treat cancer |
| WO2019126691A1 (en) | 2017-12-21 | 2019-06-27 | Mersana Therapeutics, Inc. | Pyrrolobenzodiazepine antibody conjugates |
| WO2019122884A1 (en) | 2017-12-19 | 2019-06-27 | Kymab Limited | Antibodies to icos |
| CN109963589A (en) * | 2016-10-30 | 2019-07-02 | 上海复宏汉霖生物技术股份有限公司 | Anti- PD-L1 antibody and variant |
| WO2019129211A1 (en) * | 2017-12-28 | 2019-07-04 | Nanjing Legend Biotech Co., Ltd. | Antibodies and variants thereof against pd-l1 |
| WO2019133747A1 (en) | 2017-12-27 | 2019-07-04 | Bristol-Myers Squibb Company | Anti-cd40 antibodies and uses thereof |
| WO2019136432A1 (en) | 2018-01-08 | 2019-07-11 | Novartis Ag | Immune-enhancing rnas for combination with chimeric antigen receptor therapy |
| WO2019134946A1 (en) | 2018-01-04 | 2019-07-11 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating melanoma resistant |
| WO2019136112A1 (en) | 2018-01-05 | 2019-07-11 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2019140229A1 (en) | 2018-01-12 | 2019-07-18 | Bristol-Myers Squibb Company | Antibodies against tim3 and uses thereof |
| WO2019140150A1 (en) | 2018-01-12 | 2019-07-18 | Bristol-Myers Squibb Company | Combination therapy with anti-il-8 antibodies and anti-pd-1 antibodies for treating cancer |
| WO2019143607A1 (en) | 2018-01-16 | 2019-07-25 | Bristol-Myers Squibb Company | Methods of treating cancer with antibodies against tim3 |
| WO2019144098A1 (en) | 2018-01-22 | 2019-07-25 | Bristol-Myers Squibb Company | Compositions and methods of treating cancer |
| WO2019144126A1 (en) | 2018-01-22 | 2019-07-25 | Pascal Biosciences Inc. | Cannabinoids and derivatives for promoting immunogenicity of tumor and infected cells |
| WO2019148132A1 (en) | 2018-01-29 | 2019-08-01 | Merck Patent Gmbh | Gcn2 inhibitors and uses thereof |
| WO2019152743A1 (en) | 2018-01-31 | 2019-08-08 | Celgene Corporation | Combination therapy using adoptive cell therapy and checkpoint inhibitor |
| WO2019152660A1 (en) | 2018-01-31 | 2019-08-08 | Novartis Ag | Combination therapy using a chimeric antigen receptor |
| WO2019160956A1 (en) | 2018-02-13 | 2019-08-22 | Novartis Ag | Chimeric antigen receptor therapy in combination with il-15r and il15 |
| WO2019162325A1 (en) | 2018-02-21 | 2019-08-29 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Use of sk1 as biomarker for predicting response to immunecheckpoint inhibitors |
| WO2019165315A1 (en) | 2018-02-23 | 2019-08-29 | Syntrix Biosystems Inc. | Method for treating cancer using chemokine antagonists alone or in combination |
| WO2019166951A1 (en) | 2018-02-28 | 2019-09-06 | Novartis Ag | Indole-2-carbonyl compounds and their use for the treatment of hepatitis b |
| WO2019178364A2 (en) | 2018-03-14 | 2019-09-19 | Elstar Therapeutics, Inc. | Multifunctional molecules and uses thereof |
| WO2019178269A2 (en) | 2018-03-14 | 2019-09-19 | Surface Oncology, Inc. | Antibodies that bind cd39 and uses thereof |
| WO2019175243A1 (en) | 2018-03-14 | 2019-09-19 | Merck Patent Gmbh | Compounds and uses thereof to treat tumors in a subject |
| WO2019178362A1 (en) | 2018-03-14 | 2019-09-19 | Elstar Therapeutics, Inc. | Multifunctional molecules that bind to calreticulin and uses thereof |
| WO2019183040A1 (en) | 2018-03-21 | 2019-09-26 | Five Prime Therapeutics, Inc. | ANTIBODIES BINDING TO VISTA AT ACIDIC pH |
| US10428146B2 (en) | 2014-07-22 | 2019-10-01 | Cb Therapeutics, Inc. | Anti PD-1 antibodies |
| WO2019190327A2 (en) | 2018-03-30 | 2019-10-03 | Merus N.V. | Multivalent antibody |
| WO2019191676A1 (en) | 2018-03-30 | 2019-10-03 | Bristol-Myers Squibb Company | Methods of treating tumor |
| WO2019193540A1 (en) | 2018-04-06 | 2019-10-10 | Glaxosmithkline Intellectual Property Development Limited | Heteroaryl derivatives of formula (i) as atf4 inhibitors |
| WO2019193541A1 (en) | 2018-04-06 | 2019-10-10 | Glaxosmithkline Intellectual Property Development Limited | Bicyclic aromatic ring derivatives of formula (i) as atf4 inhibitors |
| US10441654B2 (en) | 2014-01-24 | 2019-10-15 | Children's Hospital Of Eastern Ontario Research Institute Inc. | SMC combination therapy for the treatment of cancer |
| WO2019200229A1 (en) | 2018-04-13 | 2019-10-17 | Novartis Ag | Dosage regimens for anti-pd-l1 antibodies and uses thereof |
| WO2019200256A1 (en) | 2018-04-12 | 2019-10-17 | Bristol-Myers Squibb Company | Anticancer combination therapy with cd73 antagonist antibody and pd-1/pd-l1 axis antagonist antibody |
| EP3083686B1 (en) | 2013-12-17 | 2019-10-23 | F.Hoffmann-La Roche Ag | Methods of treating cancers using pd-1 axis binding antagonists and taxanes |
| WO2019204257A1 (en) | 2018-04-16 | 2019-10-24 | Arrys Therapeutics, Inc. | Ep4 inhibitors and use thereof |
| WO2019204743A1 (en) | 2018-04-19 | 2019-10-24 | Checkmate Pharmaceuticals, Inc. | Synthetic rig-i-like receptor agonists |
| WO2019204592A1 (en) | 2018-04-18 | 2019-10-24 | Xencor, Inc. | Il-15/il-15ra heterodimeric fc fusion proteins and uses thereof |
| WO2019204665A1 (en) | 2018-04-18 | 2019-10-24 | Xencor, Inc. | Pd-1 targeted heterodimeric fusion proteins containing il-15/il-15ra fc-fusion proteins and pd-1 antigen binding domains and uses thereof |
| US10457725B2 (en) | 2016-05-13 | 2019-10-29 | Regeneron Pharmaceuticals, Inc. | Methods of treating skin cancer by administering a PD-1 inhibitor |
| WO2019209896A1 (en) | 2018-04-25 | 2019-10-31 | Innate Tumor Immunity, Inc. | Nlrp3 modulators |
| WO2019211489A1 (en) | 2018-05-04 | 2019-11-07 | Merck Patent Gmbh | COMBINED INHIBITION OF PD-1/PD-L1, TGFβ AND DNA-PK FOR THE TREATMENT OF CANCER |
| WO2019213340A1 (en) | 2018-05-03 | 2019-11-07 | Bristol-Myers Squibb Company | Uracil derivatives as mer-axl inhibitors |
| WO2019229701A2 (en) | 2018-06-01 | 2019-12-05 | Novartis Ag | Binding molecules against bcma and uses thereof |
| WO2019229658A1 (en) | 2018-05-30 | 2019-12-05 | Novartis Ag | Entpd2 antibodies, combination therapies, and methods of using the antibodies and combination therapies |
| WO2019232244A2 (en) | 2018-05-31 | 2019-12-05 | Novartis Ag | Antibody molecules to cd73 and uses thereof |
| WO2019232528A1 (en) | 2018-06-01 | 2019-12-05 | Xencor, Inc. | Dosing of a bispecific antibody that bind cd123 and cd3 |
| WO2019246528A1 (en) * | 2018-06-22 | 2019-12-26 | Vyriad | Methods of treating cancer using combination therapy |
| WO2019243832A1 (en) | 2018-06-22 | 2019-12-26 | Bicycletx Limited | Bicyclic peptide ligands specific for nectin-4 |
| WO2020006018A1 (en) | 2018-06-27 | 2020-01-02 | Bristol-Myers Squibb Company | Substituted naphthyridinone compounds useful as t cell activators |
| WO2020006016A1 (en) | 2018-06-27 | 2020-01-02 | Bristol-Myers Squibb Company | Naphthyridinone compounds useful as t cell activators |
| WO2020010177A1 (en) | 2018-07-06 | 2020-01-09 | Kymera Therapeutics, Inc. | Tricyclic crbn ligands and uses thereof |
| WO2020010250A2 (en) | 2018-07-03 | 2020-01-09 | Elstar Therapeutics, Inc. | Anti-tcr antibody molecules and uses thereof |
| WO2020012334A1 (en) | 2018-07-10 | 2020-01-16 | Novartis Ag | 3-(5-hydroxy-1-oxoisoindolin-2-yl)piperidine-2,6-dione derivatives and their use in the treatment of ikaros family zinc finger 2 (ikzf2)-dependent diseases |
| WO2020012337A1 (en) | 2018-07-10 | 2020-01-16 | Novartis Ag | 3-(5-amino-1-oxoisoindolin-2-yl)piperidine-2,6-dione derivatives and their use in the treatment of i karos family zinc finger 2 (ikzf2)-dependent diseases |
| WO2020014132A2 (en) | 2018-07-09 | 2020-01-16 | Five Prime Therapeutics, Inc. | Antibodies binding to ilt4 |
| WO2020014327A2 (en) | 2018-07-11 | 2020-01-16 | Five Prime Therapeutics, Inc. | Antibodies binding to vista at acidic ph |
| WO2020012339A1 (en) | 2018-07-09 | 2020-01-16 | Glaxosmithkline Intellectual Property Development Limited | Chemical compounds |
| WO2020018680A1 (en) | 2018-07-18 | 2020-01-23 | Arcus Biosciences, Inc. | Solid forms of an azolopyrimidine compound |
| US10544099B2 (en) | 2016-05-04 | 2020-01-28 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2020023355A1 (en) | 2018-07-23 | 2020-01-30 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2020023707A1 (en) | 2018-07-26 | 2020-01-30 | Bristol-Myers Squibb Company | Lag-3 combination therapy for the treatment of cancer |
| WO2020023356A1 (en) | 2018-07-23 | 2020-01-30 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2020027224A1 (en) | 2018-07-31 | 2020-02-06 | 国立大学法人東京大学 | Super versatile method for imparting new binding specificity to antibody |
| WO2020031107A1 (en) | 2018-08-08 | 2020-02-13 | Glaxosmithkline Intellectual Property Development Limited | Chemical compounds |
| WO2020037092A1 (en) | 2018-08-16 | 2020-02-20 | Innate Tumor Immunity, Inc. | Imidazo[4,5-c]quinoline derived nlrp3-modulators |
| WO2020037091A1 (en) | 2018-08-16 | 2020-02-20 | Innate Tumor Immunity, Inc. | Imidazo[4,5-c]quinoline derived nlrp3-modulators |
| WO2020037094A1 (en) | 2018-08-16 | 2020-02-20 | Innate Tumor Immunity, Inc. | Substitued 4-amino-1h-imidazo[4,5-c]quinoline compounds and improved methods for their preparation |
| US10570204B2 (en) | 2013-09-26 | 2020-02-25 | The Medical College Of Wisconsin, Inc. | Methods for treating hematologic cancers |
| WO2020039321A2 (en) | 2018-08-20 | 2020-02-27 | Pfizer Inc. | Anti-gdf15 antibodies, compositions and methods of use |
| WO2020043683A1 (en) | 2018-08-27 | 2020-03-05 | Pieris Pharmaceuticals Gmbh | Combination therapies comprising cd137/her2 bispecific agents and pd-1 axis inhibitors and uses thereof |
| WO2020051424A1 (en) | 2018-09-07 | 2020-03-12 | Pic Therapeutics | Eif4e inhibitors and uses thereof |
| WO2020053654A1 (en) | 2018-09-12 | 2020-03-19 | Novartis Ag | Antiviral pyridopyrazinedione compounds |
| US10604574B2 (en) | 2016-06-30 | 2020-03-31 | Oncorus, Inc. | Oncolytic viral delivery of therapeutic polypeptides |
| WO2020069372A1 (en) | 2018-09-27 | 2020-04-02 | Elstar Therapeutics, Inc. | Csf1r/ccr2 multispecific antibodies |
| WO2020064971A1 (en) | 2018-09-26 | 2020-04-02 | Merck Patent Gmbh | Combination of a pd-1 antagonist, an atr inhibitor and a platinating agent for the treatment of cancer |
| WO2020072821A2 (en) | 2018-10-03 | 2020-04-09 | Xencor, Inc. | Il-12 heterodimeric fc-fusion proteins |
| US10620211B2 (en) | 2015-02-03 | 2020-04-14 | Ventana Medical Systems, Inc. | Histochemical assay for evaluating expression of programmed death ligand 1 (PD-L1) |
| WO2020076969A2 (en) | 2018-10-10 | 2020-04-16 | Tilos Therapeutics, Inc. | Anti-lap antibody variants and uses thereof |
| WO2020077276A2 (en) | 2018-10-12 | 2020-04-16 | Xencor, Inc. | Pd-1 targeted il-15/il-15ralpha fc fusion proteins and uses in combination therapies thereof |
| US10626155B2 (en) | 2011-06-24 | 2020-04-21 | Cytune Pharma | IL-15 and IL-15R\alpha sushi domain based immunocytokines |
| WO2020081928A1 (en) | 2018-10-19 | 2020-04-23 | Bristol-Myers Squibb Company | Combination therapy for melanoma |
| WO2020079581A1 (en) | 2018-10-16 | 2020-04-23 | Novartis Ag | Tumor mutation burden alone or in combination with immune markers as biomarkers for predicting response to targeted therapy |
| US10633342B2 (en) | 2016-05-04 | 2020-04-28 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| EP3643727A1 (en) | 2014-08-15 | 2020-04-29 | Merck Patent GmbH | Sirp-alpha immunoglobulin fusion proteins |
| WO2020086476A1 (en) | 2018-10-22 | 2020-04-30 | Glaxosmithkline Intellectual Property Development Limited | Dosing |
| WO2020086724A1 (en) | 2018-10-23 | 2020-04-30 | Bristol-Myers Squibb Company | Methods of treating tumor |
| WO2020092385A1 (en) | 2018-10-29 | 2020-05-07 | Mersana Therapeutics, Inc. | Cysteine engineered antibody-drug conjugates with peptide-containing linkers |
| WO2020093024A2 (en) | 2018-11-01 | 2020-05-07 | Merck Patent Gmbh | Methods of administering anti-tim-3 antibodies |
| WO2020093023A1 (en) | 2018-11-01 | 2020-05-07 | Merck Patent Gmbh | Anti-tim-3 antibodies |
| WO2020089811A1 (en) | 2018-10-31 | 2020-05-07 | Novartis Ag | Dc-sign antibody drug conjugates |
| WO2020095183A1 (en) | 2018-11-05 | 2020-05-14 | Pfizer Inc. | Combination for treating cancer |
| WO2020094744A1 (en) | 2018-11-06 | 2020-05-14 | Genmab A/S | Antibody formulation |
| WO2020095184A1 (en) | 2018-11-05 | 2020-05-14 | Pfizer Inc. | Combinations for treating cancer |
| WO2020102501A1 (en) | 2018-11-16 | 2020-05-22 | Bristol-Myers Squibb Company | Anti-nkg2a antibodies and uses thereof |
| US10660909B2 (en) | 2016-11-17 | 2020-05-26 | Syntrix Biosystems Inc. | Method for treating cancer using chemokine antagonists |
| WO2020104479A1 (en) | 2018-11-20 | 2020-05-28 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating cancers and resistant cancers with anti transferrin receptor 1 antibodies |
| WO2020104496A1 (en) | 2018-11-20 | 2020-05-28 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Bispecific antibody targeting transferrin receptor 1 and soluble antigen |
| WO2020110056A1 (en) | 2018-11-30 | 2020-06-04 | Glaxosmithkline Intellectual Property Development Limited | Compounds useful in hiv therapy |
| WO2020109328A1 (en) | 2018-11-26 | 2020-06-04 | Debiopharm International S.A. | Combination treatment of hiv infections |
| WO2020115261A1 (en) | 2018-12-07 | 2020-06-11 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating melanoma |
| WO2020117849A1 (en) | 2018-12-04 | 2020-06-11 | Bristol-Myers Squibb Company | Methods of analysis using in-sample calibration curve by multiple isotopologue reaction monitoring |
| WO2020117988A1 (en) | 2018-12-04 | 2020-06-11 | Tolero Pharmaceuticals, Inc. | Cdk9 inhibitors and polymorphs thereof for use as agents for treatment of cancer |
| WO2020120592A1 (en) | 2018-12-12 | 2020-06-18 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for predicting and treating melanoma |
| EP3670659A1 (en) | 2018-12-20 | 2020-06-24 | Abivax | Biomarkers, and uses in treatment of viral infections, inflammations, or cancer |
| WO2020128637A1 (en) | 2018-12-21 | 2020-06-25 | Novartis Ag | Use of il-1 binding antibodies in the treatment of a msi-h cancer |
| WO2020127885A1 (en) | 2018-12-21 | 2020-06-25 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Compositions for treating cancers and resistant cancers |
| WO2020128893A1 (en) | 2018-12-21 | 2020-06-25 | Pfizer Inc. | Combination treatments of cancer comprising a tlr agonist |
| WO2020128620A1 (en) | 2018-12-21 | 2020-06-25 | Novartis Ag | Use of il-1beta binding antibodies |
| WO2020128636A1 (en) | 2018-12-21 | 2020-06-25 | Novartis Ag | Use of il-1 beta antibodies in the treatment or prevention of myelodysplastic syndrome |
| WO2020128972A1 (en) | 2018-12-20 | 2020-06-25 | Novartis Ag | Dosing regimen and pharmaceutical combination comprising 3-(1-oxoisoindolin-2-yl)piperidine-2,6-dione derivatives |
| WO2020127411A1 (en) | 2018-12-19 | 2020-06-25 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating cancers by immuno-modulation using antibodies against cathespin-d |
| WO2020128613A1 (en) | 2018-12-21 | 2020-06-25 | Novartis Ag | Use of il-1beta binding antibodies |
| WO2020132646A1 (en) | 2018-12-20 | 2020-06-25 | Xencor, Inc. | Targeted heterodimeric fc fusion proteins containing il-15/il-15ra and nkg2d antigen binding domains |
| WO2020127965A1 (en) | 2018-12-21 | 2020-06-25 | Onxeo | New conjugated nucleic acid molecules and their uses |
| US10696648B2 (en) | 2016-05-04 | 2020-06-30 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| US10696650B2 (en) | 2017-08-17 | 2020-06-30 | Ikena Oncology, Inc. | AHR inhibitors and uses thereof |
| WO2020136235A1 (en) | 2018-12-28 | 2020-07-02 | Transgene Sa | M2-defective poxvirus |
| WO2020150113A1 (en) | 2019-01-14 | 2020-07-23 | Innate Tumor Immunity, Inc. | Substituted quinazolines as nlrp3 modulators, for use in the treatment of cancer |
| WO2020150114A1 (en) | 2019-01-14 | 2020-07-23 | Innate Tumor Immunity, Inc. | Heterocyclic nlrp3 modulators, for use in the treatment of cancer |
| WO2020150116A1 (en) | 2019-01-14 | 2020-07-23 | Innate Tumor Immunity, Inc. | Nlrp3 modulators |
| WO2020150115A1 (en) | 2019-01-14 | 2020-07-23 | Innate Tumor Immunity, Inc. | Nlrp3 modulators |
| US10723799B2 (en) | 2016-06-13 | 2020-07-28 | I-Mab Biopharma Us Limited | Anti-PD-L1 antibodies and uses thereof |
| EP3478723A4 (en) * | 2016-06-29 | 2020-07-29 | Checkpoint Therapeutics, Inc. | Pd-l1-specific antibodies and methods of using the same |
| WO2020157131A1 (en) | 2019-01-30 | 2020-08-06 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for identifying whether a subject suffering from a cancer will achieve a response with an immune-checkpoint inhibitor |
| WO2020161083A1 (en) | 2019-02-04 | 2020-08-13 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for modulating blood-brain barrier |
| EP3559045A4 (en) * | 2016-12-23 | 2020-08-19 | REMD Biotherapeutics, Inc. | Immunotherapy using antibodies that bind programmed death ligand-1 (pd-l1) |
| WO2020165834A1 (en) | 2019-02-15 | 2020-08-20 | Novartis Ag | Substituted 3-(1-oxoisoindolin-2-yl)piperidine-2,6-dione derivatives and uses thereof |
| WO2020165833A1 (en) | 2019-02-15 | 2020-08-20 | Novartis Ag | 3-(1-oxo-5-(piperidin-4-yl)isoindolin-2-yl)piperidine-2,6-dione derivatives and uses thereof |
| WO2020165370A1 (en) | 2019-02-13 | 2020-08-20 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for selecting a cancer treatment in a subject suffering from cancer |
| WO2020167990A1 (en) | 2019-02-12 | 2020-08-20 | Tolero Pharmaceuticals, Inc. | Formulations comprising heterocyclic protein kinase inhibitors |
| WO2020171757A1 (en) * | 2019-02-18 | 2020-08-27 | Medivir Aktiebolag | Methods for treating liver cancers using an orally administered dioxolane nucleotide in combination with an anti-pd1 or anti-pdl1 monoclonal antibody |
| WO2020187998A1 (en) | 2019-03-19 | 2020-09-24 | Fundació Privada Institut D'investigació Oncològica De Vall Hebron | Combination therapy with omomyc and an antibody binding pd-1 or ctla-4 for the treatment of cancer |
| WO2020198672A1 (en) | 2019-03-28 | 2020-10-01 | Bristol-Myers Squibb Company | Methods of treating tumor |
| WO2020198077A1 (en) | 2019-03-22 | 2020-10-01 | Sumitomo Dainippon Pharma Oncology, Inc. | Compositions comprising pkm2 modulators and methods of treatment using the same |
| WO2020198676A1 (en) | 2019-03-28 | 2020-10-01 | Bristol-Myers Squibb Company | Methods of treating tumor |
| US10793563B2 (en) | 2018-01-29 | 2020-10-06 | Merck Patent Gmbh | GCN2 inhibitors and uses thereof |
| WO2020201753A1 (en) | 2019-04-02 | 2020-10-08 | Bicycletx Limited | Bicycle toxin conjugates and uses thereof |
| WO2020216379A1 (en) | 2019-04-26 | 2020-10-29 | I-Mab | Human pd-l1 antibodies |
| WO2020221796A1 (en) | 2019-04-30 | 2020-11-05 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating melanoma |
| EP3736287A1 (en) | 2015-05-11 | 2020-11-11 | The Johns Hopkins University | Autoimmune antibodies for use in inhibiting cancer cell growth |
| WO2020243568A1 (en) | 2019-05-30 | 2020-12-03 | Bristol-Myers Squibb Company | Methods of identifying a subject suitable for an immuno-oncology (i-o) therapy |
| WO2020243570A1 (en) | 2019-05-30 | 2020-12-03 | Bristol-Myers Squibb Company | Cell localization signature and combination therapy |
| WO2020243423A1 (en) | 2019-05-31 | 2020-12-03 | Ikena Oncology, Inc. | Tead inhibitors and uses thereof |
| WO2020239558A1 (en) | 2019-05-24 | 2020-12-03 | Pfizer Inc. | Combination therapies using cdk inhibitors |
| WO2020243563A1 (en) | 2019-05-30 | 2020-12-03 | Bristol-Myers Squibb Company | Multi-tumor gene signatures for suitability to immuno-oncology therapy |
| US10857181B2 (en) | 2015-04-21 | 2020-12-08 | Enlivex Therapeutics Ltd | Therapeutic pooled blood apoptotic cell preparations and uses thereof |
| US10858432B2 (en) | 2015-12-02 | 2020-12-08 | Stcube, Inc. | Antibodies specific to glycosylated PD-1 and methods of use thereof |
| US10864203B2 (en) | 2016-07-05 | 2020-12-15 | Beigene, Ltd. | Combination of a PD-1 antagonist and a RAF inhibitor for treating cancer |
| WO2020249071A1 (en) * | 2019-06-12 | 2020-12-17 | Nanjing GenScript Biotech Co., Ltd. | Anti-pd-l1/anti-lag-3 multiple antigen binding proteins and methods of use thereof |
| US10874743B2 (en) | 2017-12-26 | 2020-12-29 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US10875864B2 (en) | 2011-07-21 | 2020-12-29 | Sumitomo Dainippon Pharma Oncology, Inc. | Substituted imidazo[1,2-B]pyridazines as protein kinase inhibitors |
| WO2021003417A1 (en) | 2019-07-03 | 2021-01-07 | Sumitomo Dainippon Pharma Oncology, Inc. | Tyrosine kinase non-receptor 1 (tnk1) inhibitors and uses thereof |
| EP3763742A1 (en) | 2014-09-01 | 2021-01-13 | Birdie Biopharmaceuticals Inc. | Anti-pd-l1 conjugates for treating tumors |
| RU2742312C1 (en) * | 2015-02-26 | 2021-02-04 | Мерк Патент Гмбх | Pd-1/pd-l1 inhibitors for treating cancer |
| WO2021041588A1 (en) | 2019-08-28 | 2021-03-04 | Bristol-Myers Squibb Company | Substituted pyridopyrimidinonyl compounds useful as t cell activators |
| WO2021048292A1 (en) | 2019-09-11 | 2021-03-18 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating melanoma |
| WO2021053559A1 (en) | 2019-09-18 | 2021-03-25 | Novartis Ag | Entpd2 antibodies, combination therapies, and methods of using the antibodies and combination therapies |
| WO2021055994A1 (en) | 2019-09-22 | 2021-03-25 | Bristol-Myers Squibb Company | Quantitative spatial profiling for lag-3 antagonist therapy |
| WO2021053560A1 (en) | 2019-09-18 | 2021-03-25 | Novartis Ag | Combination therapy with entpd2 and cd73 antibodies |
| WO2021053207A1 (en) | 2019-09-20 | 2021-03-25 | Transgene | Combination of a poxvirus encoding hpv polypeptides and il-2 with an anti-pd-l1 antibody |
| WO2021055698A1 (en) | 2019-09-19 | 2021-03-25 | Bristol-Myers Squibb Company | Antibodies binding to vista at acidic ph |
| US10959986B2 (en) | 2018-08-29 | 2021-03-30 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2021058711A2 (en) | 2019-09-27 | 2021-04-01 | Glaxosmithkline Intellectual Property Development Limited | Antigen binding proteins |
| WO2021062244A1 (en) | 2019-09-25 | 2021-04-01 | Surface Oncology, Inc. | Anti-il-27 antibodies and uses thereof |
| WO2021062018A1 (en) | 2019-09-25 | 2021-04-01 | Bristol-Myers Squibb Company | Composite biomarker for cancer therapy |
| WO2021067863A2 (en) | 2019-10-03 | 2021-04-08 | Xencor, Inc. | Targeted il-12 heterodimeric fc-fusion proteins |
| WO2021064180A1 (en) | 2019-10-03 | 2021-04-08 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for modulating macrophages polarization |
| WO2021072298A1 (en) | 2019-10-11 | 2021-04-15 | Genentech, Inc. | Pd-1 targeted il-15/il-15ralpha fc fusion proteins with improved properties |
| WO2021074391A1 (en) | 2019-10-17 | 2021-04-22 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for diagnosing nasal intestinal type adenocarcinomas |
| US10987322B2 (en) | 2014-06-06 | 2021-04-27 | Flexus Biosciences, Inc. | Immunoregulatory agents |
| WO2021079958A1 (en) | 2019-10-25 | 2021-04-29 | 第一三共株式会社 | Combination of anti-garp antibody and immunoregulator |
| WO2021079195A1 (en) | 2019-10-21 | 2021-04-29 | Novartis Ag | Tim-3 inhibitors and uses thereof |
| WO2021081353A1 (en) | 2019-10-23 | 2021-04-29 | Checkmate Pharmaceuticals, Inc. | Synthetic rig-i-like receptor agonists |
| WO2021079188A1 (en) | 2019-10-21 | 2021-04-29 | Novartis Ag | Combination therapies with venetoclax and tim-3 inhibitors |
| WO2021083959A1 (en) | 2019-10-29 | 2021-05-06 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating uveal melanoma |
| US11000548B2 (en) | 2015-02-18 | 2021-05-11 | Enlivex Therapeutics Ltd | Combination immune therapy and cytokine control therapy for cancer treatment |
| WO2021092221A1 (en) | 2019-11-06 | 2021-05-14 | Bristol-Myers Squibb Company | Methods of identifying a subject with a tumor suitable for a checkpoint inhibitor therapy |
| WO2021092220A1 (en) | 2019-11-06 | 2021-05-14 | Bristol-Myers Squibb Company | Methods of identifying a subject with a tumor suitable for a checkpoint inhibitor therapy |
| WO2021091885A2 (en) | 2019-11-04 | 2021-05-14 | Alector Llc | Siglec-9 ecd fusion molecules and methods of use thereof |
| WO2021092380A1 (en) | 2019-11-08 | 2021-05-14 | Bristol-Myers Squibb Company | Lag-3 antagonist therapy for melanoma |
| WO2021097256A1 (en) | 2019-11-14 | 2021-05-20 | Cohbar, Inc. | Cxcr4 antagonist peptides |
| WO2021101919A1 (en) | 2019-11-19 | 2021-05-27 | Bristol-Myers Squibb Company | Compounds useful as inhibitors of helios protein |
| WO2021102343A1 (en) | 2019-11-22 | 2021-05-27 | Sumitomo Dainippon Pharma Oncology, Inc. | Solid dose pharmaceutical composition |
| US11021481B2 (en) | 2019-09-13 | 2021-06-01 | Nimbus Saturn, Inc. | Substituted isoindolin-1-ones and 2,3-dihydro-1h-pyrrolo[3,4-c]pyridin-1-ones as HPK1 antagonists |
| WO2021108288A1 (en) | 2019-11-26 | 2021-06-03 | Bristol-Myers Squibb Company | Salts/cocrystals of (r)-n-(4-chlorophenyl)-2-((1s,4s)-4-(6-fluoroquinolin-4-yl)cyclohexyl)propanamide |
| WO2021108528A1 (en) | 2019-11-26 | 2021-06-03 | Ikena Oncology, Inc. | Polymorphic carbazole derivatives and uses thereof |
| WO2021106978A1 (en) | 2019-11-27 | 2021-06-03 | サイトリミック株式会社 | Pharmaceutical composition |
| US11034771B2 (en) | 2018-07-25 | 2021-06-15 | I-Mab Biopharma Us Limited | Anti-CD73 anti-PD-L1 bispecific antibodies |
| WO2021123243A1 (en) | 2019-12-19 | 2021-06-24 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and vaccine compositions to treat cancers |
| WO2021123996A1 (en) | 2019-12-20 | 2021-06-24 | Novartis Ag | Uses of anti-tgf-beta antibodies and checkpoint inhibitors for the treatment of proliferative diseases |
| WO2021127554A1 (en) | 2019-12-19 | 2021-06-24 | Bristol-Myers Squibb Company | Combinations of dgk inhibitors and checkpoint antagonists |
| WO2021133748A1 (en) | 2019-12-23 | 2021-07-01 | Bristol-Myers Squibb Company | Substituted quinolinonyl piperazine compounds useful as t cell activators |
| WO2021133751A1 (en) | 2019-12-23 | 2021-07-01 | Bristol-Myers Squibb Company | Substituted quinazolinyl compounds useful as t cell activators |
| WO2021133749A1 (en) | 2019-12-23 | 2021-07-01 | Bristol-Myers Squibb Company | Substituted piperazine derivatives useful as t cell activators |
| WO2021133750A1 (en) | 2019-12-23 | 2021-07-01 | Bristol-Myers Squibb Company | Substituted bicyclic piperidine derivatives useful as t cell activators |
| WO2021133752A1 (en) | 2019-12-23 | 2021-07-01 | Bristol-Myers Squibb Company | Substituted heteroaryl compounds useful as t cell activators |
| EP3587453A4 (en) * | 2017-02-21 | 2021-07-07 | Shanghai Junshi Bioscience Co., Ltd. | Anti-pd-l1 antibody and application thereof |
| WO2021138407A2 (en) | 2020-01-03 | 2021-07-08 | Marengo Therapeutics, Inc. | Multifunctional molecules that bind to cd33 and uses thereof |
| WO2021142203A1 (en) | 2020-01-10 | 2021-07-15 | Innate Tumor Immunity, Inc. | Nlrp3 modulators |
| WO2021141907A1 (en) | 2020-01-06 | 2021-07-15 | Hifibio (Hong Kong) Limited | Anti-tnfr2 antibody and uses thereof |
| WO2021139682A1 (en) | 2020-01-07 | 2021-07-15 | Hifibio (Hk) Limited | Anti-galectin-9 antibody and uses thereof |
| US11066478B2 (en) | 2015-12-17 | 2021-07-20 | Photocure Asa | Intravesical therapy for bladder cancer |
| US11066383B2 (en) | 2016-05-04 | 2021-07-20 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2021146370A1 (en) | 2020-01-15 | 2021-07-22 | Blueprint Medicines Corporation | Map4k1 inhibitors |
| WO2021144426A1 (en) | 2020-01-17 | 2021-07-22 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating melanoma |
| WO2021144657A1 (en) | 2020-01-17 | 2021-07-22 | Novartis Ag | Combination comprising a tim-3 inhibitor and a hypomethylating agent for use in treating myelodysplastic syndrome or chronic myelomonocytic leukemia |
| WO2021152005A1 (en) | 2020-01-28 | 2021-08-05 | Universite De Strasbourg | Antisense oligonucleotide targeting linc00518 for treating melanoma |
| WO2021154073A1 (en) | 2020-01-29 | 2021-08-05 | Merus N.V. | Means and method for modulating immune cell engaging effects. |
| WO2021152548A1 (en) | 2020-01-30 | 2021-08-05 | Benitah Salvador Aznar | Combination therapy for treatment of cancer and cancer metastasis |
| WO2021156360A1 (en) | 2020-02-05 | 2021-08-12 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for discontinuing a treatment with a tyrosine kinase inhibitor (tki) |
| WO2021158938A1 (en) | 2020-02-06 | 2021-08-12 | Bristol-Myers Squibb Company | Il-10 and uses thereof |
| US11090367B2 (en) | 2016-11-09 | 2021-08-17 | The Brigham And Women's Hospital, Inc. | Restoration of tumor suppression using mRNA-based delivery system |
| US11098077B2 (en) | 2016-07-05 | 2021-08-24 | Chinook Therapeutics, Inc. | Locked nucleic acid cyclic dinucleotide compounds and uses thereof |
| WO2021167964A1 (en) | 2020-02-18 | 2021-08-26 | Alector Llc | Pilra antibodies and methods of use thereof |
| US11104712B2 (en) | 2017-03-15 | 2021-08-31 | Cue Biopharma, Inc. | Methods for modulating an immune response |
| WO2021170777A1 (en) | 2020-02-28 | 2021-09-02 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for diagnosing, prognosing and managing treatment of breast cancer |
| WO2021171264A1 (en) | 2020-02-28 | 2021-09-02 | Novartis Ag | Dosing of a bispecific antibody that binds cd123 and cd3 |
| WO2021174045A1 (en) | 2020-02-28 | 2021-09-02 | Bristol-Myers Squibb Company | Radiolabeled fibronectin based scaffolds and antibodies and theranostic uses thereof |
| WO2021178807A1 (en) | 2020-03-06 | 2021-09-10 | Celgene Quanticel Research, Inc. | Combination of an lsd-1 inhibitor and nivolumab for use in treating sclc or sqnsclc |
| WO2021178488A1 (en) | 2020-03-03 | 2021-09-10 | PIC Therapeutics, Inc. | Eif4e inhibitors and uses thereof |
| WO2021176424A1 (en) | 2020-03-06 | 2021-09-10 | Ona Therapeutics, S.L. | Anti-cd36 antibodies and their use to treat cancer |
| US11117889B1 (en) | 2018-11-30 | 2021-09-14 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11117945B2 (en) | 2016-12-22 | 2021-09-14 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides and methods of use thereof |
| EP3878446A1 (en) | 2020-03-09 | 2021-09-15 | Universite De Geneve | Hsd11b1 inhibitors for use in immunotherapy and uses thereof |
| WO2021183428A1 (en) | 2020-03-09 | 2021-09-16 | Bristol-Myers Squibb Company | Antibodies to cd40 with enhanced agonist activity |
| WO2021188769A1 (en) | 2020-03-19 | 2021-09-23 | Arcus Biosciences, Inc. | Tetralin and tetrahydroquinoline compounds as inhibitors of hif-2alpha |
| WO2021194914A1 (en) | 2020-03-23 | 2021-09-30 | Bristol-Myers Squibb Company | Substituted oxoisoindoline compounds for the treatment of cancer |
| US11142800B2 (en) | 2010-10-07 | 2021-10-12 | The General Hospital Corporation | Biomarkers of cancer |
| WO2021207449A1 (en) | 2020-04-09 | 2021-10-14 | Merck Sharp & Dohme Corp. | Affinity matured anti-lap antibodies and uses thereof |
| WO2021209358A1 (en) | 2020-04-14 | 2021-10-21 | Glaxosmithkline Intellectual Property Development Limited | Combination treatment for cancer based upon an icos antibody and a pd-l1 antibody tgf-beta-receptor fusion protein |
| US11154616B2 (en) | 2015-06-17 | 2021-10-26 | Genentech, Inc. | Methods of treating locally advanced or metastatic breast cancers using PD-1 axis binding antagonists and taxanes |
| US11168144B2 (en) | 2017-06-01 | 2021-11-09 | Cytomx Therapeutics, Inc. | Activatable anti-PDL1 antibodies, and methods of use thereof |
| WO2021224215A1 (en) | 2020-05-05 | 2021-11-11 | F. Hoffmann-La Roche Ag | Predicting response to pd-1 axis inhibitors |
| US11174316B2 (en) | 2015-03-13 | 2021-11-16 | Cytomx Therapeutics, Inc. | Anti-PDL1 antibodies, activatable anti-PDL1 antibodies, and methods of use thereof |
| WO2021231732A1 (en) | 2020-05-15 | 2021-11-18 | Bristol-Myers Squibb Company | Antibodies to garp |
| US11186637B2 (en) | 2013-09-13 | 2021-11-30 | Beigene Switzerland Gmbh | Anti-PD1 antibodies and their use as therapeutics and diagnostics |
| EP3694545A4 (en) * | 2017-10-11 | 2021-12-01 | Board Of Regents, The University Of Texas System | Human pd-l1 antibodies and methods of use therefor |
| WO2021247591A1 (en) | 2020-06-02 | 2021-12-09 | Arcus Biosciences, Inc. | Antibodies to tigit |
| WO2021249969A1 (en) | 2020-06-10 | 2021-12-16 | Merck Patent Gmbh | Combination product for the treatment of cancer diseases |
| WO2021258010A1 (en) | 2020-06-19 | 2021-12-23 | Gossamer Bio Services, Inc. | Oxime compounds useful as t cell activators |
| WO2021255223A1 (en) | 2020-06-19 | 2021-12-23 | Onxeo | New conjugated nucleic acid molecules and their uses |
| WO2021260528A1 (en) | 2020-06-23 | 2021-12-30 | Novartis Ag | Dosing regimen comprising 3-(1-oxoisoindolin-2-yl)piperidine-2,6-dione derivatives |
| US11214619B2 (en) | 2018-07-20 | 2022-01-04 | Surface Oncology, Inc. | Anti-CD112R compositions and methods |
| WO2022003554A1 (en) | 2020-07-01 | 2022-01-06 | Pfizer Inc. | Biomarkers for pd-1 axis binding antagonist therapy |
| US11226339B2 (en) * | 2012-12-11 | 2022-01-18 | Albert Einstein College Of Medicine | Methods for high throughput receptor:ligand identification |
| WO2022023379A1 (en) | 2020-07-28 | 2022-02-03 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for preventing and treating a cancer |
| US11242393B2 (en) | 2018-03-23 | 2022-02-08 | Bristol-Myers Squibb Company | Antibodies against MICA and/or MICB and uses thereof |
| US11242319B2 (en) | 2014-11-05 | 2022-02-08 | Flexus Biosciences, Inc. | Immunoregulatory agents |
| WO2022029573A1 (en) | 2020-08-03 | 2022-02-10 | Novartis Ag | Heteroaryl substituted 3-(1-oxoisoindolin-2-yl)piperidine-2,6-dione derivatives and uses thereof |
| WO2022033419A2 (en) | 2020-08-10 | 2022-02-17 | Shanghai Xbh Biotechnology Co., Ltd. | Compositions and methods for treating autoimmune diseases and cancers by targeting igsf8 |
| US11253525B2 (en) | 2018-08-29 | 2022-02-22 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2022038158A1 (en) | 2020-08-17 | 2022-02-24 | Bicycletx Limited | Bicycle conjugates specific for nectin-4 and uses thereof |
| WO2022047046A1 (en) | 2020-08-26 | 2022-03-03 | Marengo Therapeutics, Inc. | Methods of detecting trbc1 or trbc2 |
| WO2022047412A1 (en) | 2020-08-31 | 2022-03-03 | Bristol-Myers Squibb Company | Cell localization signature and immunotherapy |
| WO2022047189A1 (en) | 2020-08-28 | 2022-03-03 | Bristol-Myers Squibb Company | Lag-3 antagonist therapy for hepatocellular carcinoma |
| WO2022053703A1 (en) | 2020-09-14 | 2022-03-17 | Boehringer Ingelheim International Gmbh | Heterologous prime boost vaccine |
| US11279694B2 (en) | 2016-11-18 | 2022-03-22 | Sumitomo Dainippon Pharma Oncology, Inc. | Alvocidib prodrugs and their use as protein kinase inhibitors |
| US11279766B2 (en) | 2016-04-14 | 2022-03-22 | Ose Immunotherapeutics | Anti-SIRPa antibodies and their therapeutic applications |
| US11291718B2 (en) | 2016-10-11 | 2022-04-05 | Cytlimic Inc. | Method for treating cancer by administering a toll-like receptor agonist and LAG-3 IgG fusion protein |
| US11299708B2 (en) | 2016-05-12 | 2022-04-12 | Adicet Bio, Inc. | Methods for selective expansion of γδ T-cell populations and compositions thereof |
| US11299544B2 (en) | 2013-03-15 | 2022-04-12 | Genentech, Inc. | Biomarkers and methods of treating PD-1 and PD-L1 related conditions |
| WO2022076596A1 (en) | 2020-10-06 | 2022-04-14 | Codiak Biosciences, Inc. | Extracellular vesicle-aso constructs targeting stat6 |
| WO2022076318A1 (en) | 2020-10-05 | 2022-04-14 | Bristol-Myers Squibb Company | Methods for concentrating proteins |
| US11304976B2 (en) | 2015-02-18 | 2022-04-19 | Enlivex Therapeutics Ltd | Combination immune therapy and cytokine control therapy for cancer treatment |
| EP3768719A4 (en) * | 2018-03-19 | 2022-04-27 | Abeome Corporation | High affinity neutralizing monoclonal antibodies to programmed death ligand 1 (pd-l1) and uses thereof |
| WO2022087402A1 (en) | 2020-10-23 | 2022-04-28 | Bristol-Myers Squibb Company | Lag-3 antagonist therapy for lung cancer |
| WO2022084531A1 (en) | 2020-10-23 | 2022-04-28 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating glioma |
| US11318163B2 (en) | 2015-02-18 | 2022-05-03 | Enlivex Therapeutics Ltd | Combination immune therapy and cytokine control therapy for cancer treatment |
| EP3992208A1 (en) * | 2020-11-03 | 2022-05-04 | Randox Laboratories Ltd | Anti-pd-l1 vhh antibodies |
| WO2022094567A1 (en) | 2020-10-28 | 2022-05-05 | Ikena Oncology, Inc. | Combination of an ahr inhibitor with a pdx inhibitor or doxorubicine |
| US11326170B2 (en) | 2017-04-18 | 2022-05-10 | Changchun Huapu Biotechnology Co. Ltd. | Immunomodulatory polynucleotides and uses thereof |
| WO2022097060A1 (en) | 2020-11-06 | 2022-05-12 | Novartis Ag | Cd19 binding molecules and uses thereof |
| US11332524B2 (en) | 2018-03-22 | 2022-05-17 | Surface Oncology, Inc. | Anti-IL-27 antibodies and uses thereof |
| WO2022101481A1 (en) | 2020-11-16 | 2022-05-19 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for predicting and treating uveal melanoma |
| WO2022101484A1 (en) | 2020-11-16 | 2022-05-19 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for predicting and treating uveal melanoma |
| US11351164B2 (en) | 2016-08-26 | 2022-06-07 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2022120353A1 (en) | 2020-12-02 | 2022-06-09 | Ikena Oncology, Inc. | Tead inhibitors and uses thereof |
| WO2022120179A1 (en) | 2020-12-03 | 2022-06-09 | Bristol-Myers Squibb Company | Multi-tumor gene signatures and uses thereof |
| WO2022120354A1 (en) | 2020-12-02 | 2022-06-09 | Ikena Oncology, Inc. | Tead inhibitors and uses thereof |
| US11358948B2 (en) | 2017-09-22 | 2022-06-14 | Kymera Therapeutics, Inc. | CRBN ligands and uses thereof |
| EP3929215A4 (en) * | 2019-06-10 | 2022-06-22 | Shandong Boan Biotechnology Co., Ltd. | Bifunctional fusion protein against pdl1 and tgf? and use thereof |
| WO2022133083A1 (en) | 2020-12-16 | 2022-06-23 | Gossamer Bio Services, Inc. | Compounds useful as t cell activators |
| WO2022146947A1 (en) | 2020-12-28 | 2022-07-07 | Bristol-Myers Squibb Company | Antibody compositions and methods of use thereof |
| WO2022146948A1 (en) | 2020-12-28 | 2022-07-07 | Bristol-Myers Squibb Company | Subcutaneous administration of pd1/pd-l1 antibodies |
| US11384131B2 (en) | 2014-04-24 | 2022-07-12 | The Board Of Trustees Of The Leland Stanford Junior University | Superagonists, partial agonists and antagonists of interleukin-2 |
| WO2022148979A1 (en) | 2021-01-11 | 2022-07-14 | Bicycletx Limited | Methods for treating cancer |
| WO2022148736A1 (en) | 2021-01-05 | 2022-07-14 | Transgene | Vectorization of muc1 t cell engager |
| EP4029508A1 (en) | 2014-10-10 | 2022-07-20 | Idera Pharmaceuticals, Inc. | Treatment of cancer using tlr9 agonists and checkpoint inhibitors |
| WO2022162569A1 (en) | 2021-01-29 | 2022-08-04 | Novartis Ag | Dosage regimes for anti-cd73 and anti-entpd2 antibodies and uses thereof |
| EP3841126A4 (en) * | 2018-08-20 | 2022-08-10 | 1Globe Biomedical Co., Ltd. | Novel cancer immunotherapy antibody compositions |
| WO2022167457A1 (en) | 2021-02-02 | 2022-08-11 | Liminal Biosciences Limited | Gpr84 antagonists and uses thereof |
| WO2022167445A1 (en) | 2021-02-02 | 2022-08-11 | Liminal Biosciences Limited | Gpr84 antagonists and uses thereof |
| WO2022171745A1 (en) | 2021-02-12 | 2022-08-18 | F. Hoffmann-La Roche Ag | Bicyclic tetrahydroazepine derivatives for the treatment of cancer |
| WO2022171121A1 (en) | 2021-02-10 | 2022-08-18 | 同润生物医药(上海)有限公司 | Method and combination for treating tumors |
| WO2022184937A1 (en) | 2021-03-05 | 2022-09-09 | Leadartis, S.L. | Trimeric polypeptides and uses thereof in the treatment of cancer |
| WO2022185160A1 (en) | 2021-03-02 | 2022-09-09 | Glaxosmithkline Intellectual Property Development Limited | Substituted pyridines as dnmt1 inhibitors |
| US11440960B2 (en) | 2017-06-20 | 2022-09-13 | Kymab Limited | TIGIT antibodies, encoding nucleic acids and methods of using said antibodies in vivo |
| WO2022192145A1 (en) | 2021-03-08 | 2022-09-15 | Blueprint Medicines Corporation | Map4k1 inhibitors |
| WO2022194908A1 (en) | 2021-03-17 | 2022-09-22 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating melanoma |
| WO2022195551A1 (en) | 2021-03-18 | 2022-09-22 | Novartis Ag | Biomarkers for cancer and methods of use thereof |
| WO2022197641A1 (en) | 2021-03-15 | 2022-09-22 | Rapt Therapeutics, Inc. | 1h-pyrazolo[3,4-d]pyrimidin-6-yl-amine derivatives as hematopoietic progenitor kinase 1 (hpk1) modulators and/or inhibitors for the treatment of cancer and other diseases |
| US11459394B2 (en) | 2017-02-24 | 2022-10-04 | Macrogenics, Inc. | Bispecific binding molecules that are capable of binding CD137 and tumor antigens, and uses thereof |
| WO2022212876A1 (en) | 2021-04-02 | 2022-10-06 | The Regents Of The University Of California | Antibodies against cleaved cdcp1 and uses thereof |
| WO2022212400A1 (en) | 2021-03-29 | 2022-10-06 | Juno Therapeutics, Inc. | Methods for dosing and treatment with a combination of a checkpoint inhibitor therapy and a car t cell therapy |
| WO2022208353A1 (en) | 2021-03-31 | 2022-10-06 | Glaxosmithkline Intellectual Property Development Limited | Antigen binding proteins and combinations thereof |
| WO2022214653A1 (en) | 2021-04-09 | 2022-10-13 | Ose Immunotherapeutics | New scaffold for bifunctional molecules with improved properties |
| WO2022216573A1 (en) | 2021-04-05 | 2022-10-13 | Bristol-Myers Squibb Company | Pyridinyl substituted oxoisoindoline compounds for the treatment of cancer |
| WO2022214652A1 (en) | 2021-04-09 | 2022-10-13 | Ose Immunotherapeutics | Scaffold for bifunctioanl molecules comprising pd-1 or cd28 and sirp binding domains |
| WO2022216644A1 (en) | 2021-04-06 | 2022-10-13 | Bristol-Myers Squibb Company | Pyridinyl substituted oxoisoindoline compounds |
| WO2022216993A2 (en) | 2021-04-08 | 2022-10-13 | Marengo Therapeutics, Inc. | Multifuntional molecules binding to tcr and uses thereof |
| WO2022215011A1 (en) | 2021-04-07 | 2022-10-13 | Novartis Ag | USES OF ANTI-TGFβ ANTIBODIES AND OTHER THERAPEUTIC AGENTS FOR THE TREATMENT OF PROLIFERATIVE DISEASES |
| WO2022221227A1 (en) | 2021-04-13 | 2022-10-20 | Nuvalent, Inc. | Amino-substituted heterocycles for treating cancers with egfr mutations |
| WO2022221866A1 (en) | 2021-04-16 | 2022-10-20 | Ikena Oncology, Inc. | Mek inhibitors and uses thereof |
| WO2022219080A1 (en) | 2021-04-14 | 2022-10-20 | INSERM (Institut National de la Santé et de la Recherche Médicale) | New method to improve nk cells cytotoxicity |
| WO2022223791A1 (en) | 2021-04-23 | 2022-10-27 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating cell senescence accumulation related disease |
| US11485743B2 (en) | 2018-01-12 | 2022-11-01 | Kymera Therapeutics, Inc. | Protein degraders and uses thereof |
| US11485750B1 (en) | 2019-04-05 | 2022-11-01 | Kymera Therapeutics, Inc. | STAT degraders and uses thereof |
| US11491204B2 (en) | 2015-04-07 | 2022-11-08 | Cytlimic Inc. | Composition comprising poly I:C and LAG-3-IGG fusion protein |
| WO2022236134A1 (en) | 2021-05-07 | 2022-11-10 | Surface Oncology, Inc. | Anti-il-27 antibodies and uses thereof |
| US11498968B2 (en) | 2016-12-22 | 2022-11-15 | Hoffmann-La Roche Inc. | Treatment of tumors with an anti-CSF-1R antibody in combination with an anti-PD-L1 antibody after failure of anti-PD-L1/PD1 treatment |
| US11497767B2 (en) | 2015-02-18 | 2022-11-15 | Enlivex Therapeutics R&D Ltd | Combination immune therapy and cytokine control therapy for cancer treatment |
| US11497756B2 (en) | 2017-09-12 | 2022-11-15 | Sumitomo Pharma Oncology, Inc. | Treatment regimen for cancers that are insensitive to BCL-2 inhibitors using the MCL-1 inhibitor alvocidib |
| US11505591B2 (en) | 2016-05-18 | 2022-11-22 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides and methods of use thereof |
| WO2022246179A1 (en) | 2021-05-21 | 2022-11-24 | Arcus Biosciences, Inc. | Axl inhibitor compounds |
| WO2022243846A1 (en) | 2021-05-18 | 2022-11-24 | Novartis Ag | Combination therapies |
| WO2022243378A1 (en) | 2021-05-18 | 2022-11-24 | Kymab Limited | Uses of anti-icos antibodies |
| WO2022246177A1 (en) | 2021-05-21 | 2022-11-24 | Arcus Biosciences, Inc. | Axl compounds |
| US11512289B2 (en) | 2015-02-18 | 2022-11-29 | Enlivex Therapeutics Rdo Ltd | Combination immune therapy and cytokine control therapy for cancer treatment |
| US11512080B2 (en) | 2018-01-12 | 2022-11-29 | Kymera Therapeutics, Inc. | CRBN ligands and uses thereof |
| WO2022254227A1 (en) | 2021-06-04 | 2022-12-08 | Kymab Limited | Treatment of pd-l1 negative or low expressing cancer with anti-icos antibodies |
| US11530269B2 (en) | 2014-07-11 | 2022-12-20 | Ventana Medical Systems, Inc. | Anti-PD-L1 antibodies and diagnostic uses thereof |
| US11542312B2 (en) | 2017-06-19 | 2023-01-03 | Medicenna Therapeutics, Inc. | IL-2 superagonists in combination with anti-PD-1 antibodies |
| US11542335B2 (en) | 2016-08-25 | 2023-01-03 | Hoffmann-La Roche Inc. | Method of treating cancer in a patient by administering an antibody which binds colony stimulating factor-1 receptor (CSF-1R) |
| US11555038B2 (en) | 2017-01-25 | 2023-01-17 | Beigene, Ltd. | Crystalline forms of (S)-7-(1-(but-2-ynoyl)piperidin-4-yl)-2-(4-phenoxyphenyl)-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidine-3-carboxamide, preparation, and uses thereof |
| WO2023288254A1 (en) | 2021-07-14 | 2023-01-19 | Blueprint Medicines Corporation | Heterocyclic compounds as map4k1 inhibitors |
| WO2023288264A1 (en) | 2021-07-15 | 2023-01-19 | Blueprint Medicines Corporation | Map4k1 inhibitors |
| US11560425B2 (en) | 2017-06-27 | 2023-01-24 | Neuracle Science Co., Ltd. | Use of anti-FAM19A5 antibodies for treating cancers |
| WO2023007472A1 (en) | 2021-07-30 | 2023-02-02 | ONA Therapeutics S.L. | Anti-cd36 antibodies and their use to treat cancer |
| US11572412B2 (en) | 2021-06-04 | 2023-02-07 | Boehringer Ingelheim International Gmbh | Anti-SIRP-alpha antibodies |
| EP3999534A4 (en) * | 2019-07-19 | 2023-02-08 | Beijing Sinotau Bio-Pharmaceuticals Technology Co., Ltd. | A novel anti-pd-l1 antibody and use thereof |
| US11584793B2 (en) | 2015-06-24 | 2023-02-21 | Hoffmann-La Roche Inc. | Anti-transferrin receptor antibodies with tailored affinity |
| US11591332B2 (en) | 2019-12-17 | 2023-02-28 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| WO2023028235A1 (en) | 2021-08-25 | 2023-03-02 | PIC Therapeutics, Inc. | Eif4e inhibitors and uses thereof |
| WO2023028238A1 (en) | 2021-08-25 | 2023-03-02 | PIC Therapeutics, Inc. | Eif4e inhibitors and uses thereof |
| US11596652B2 (en) | 2015-02-18 | 2023-03-07 | Enlivex Therapeutics R&D Ltd | Early apoptotic cells for use in treating sepsis |
| US11597768B2 (en) | 2017-06-26 | 2023-03-07 | Beigene, Ltd. | Immunotherapy for hepatocellular carcinoma |
| EP3929213A4 (en) * | 2019-02-21 | 2023-03-08 | Eucure (Beijing) Biopharma Co., Ltd | Anti-pd-l1 antibody and use thereof |
| US11603407B2 (en) | 2017-04-06 | 2023-03-14 | Regeneron Pharmaceuticals, Inc. | Stable antibody formulation |
| US11603411B2 (en) | 2015-10-02 | 2023-03-14 | Hoffmann-La Roche Inc. | Bispecific anti-human CD20/human transferrin receptor antibodies and methods of use |
| WO2023039089A1 (en) | 2021-09-08 | 2023-03-16 | Twentyeight-Seven, Inc. | Papd5 and/or papd7 inhibiting 4-oxo-1,4-dihydroquinoline-3-carboxylic acid derivatives |
| US11607453B2 (en) | 2017-05-12 | 2023-03-21 | Harpoon Therapeutics, Inc. | Mesothelin binding proteins |
| US11623932B2 (en) | 2017-09-22 | 2023-04-11 | Kymera Therapeutics, Inc. | Protein degraders and uses thereof |
| US11623958B2 (en) | 2016-05-20 | 2023-04-11 | Harpoon Therapeutics, Inc. | Single chain variable fragment CD3 binding proteins |
| US11629189B2 (en) | 2017-12-19 | 2023-04-18 | Kymab Limited | Bispecific antibody for ICOS and PD-L1 |
| WO2023077090A1 (en) | 2021-10-29 | 2023-05-04 | Bristol-Myers Squibb Company | Lag-3 antagonist therapy for hematological cancer |
| WO2023079430A1 (en) | 2021-11-02 | 2023-05-11 | Pfizer Inc. | Methods of treating mitochondrial myopathies using anti-gdf15 antibodies |
| WO2023078900A1 (en) | 2021-11-03 | 2023-05-11 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating triple negative breast cancer (tnbc) |
| US11655303B2 (en) | 2019-09-16 | 2023-05-23 | Surface Oncology, Inc. | Anti-CD39 antibody compositions and methods |
| US11660352B2 (en) | 2016-03-29 | 2023-05-30 | Stcube, Inc. | Dual function antibodies specific to glycosylated PD-L1 and methods of use thereof |
| US11667613B2 (en) | 2019-09-26 | 2023-06-06 | Novartis Ag | Antiviral pyrazolopyridinone compounds |
| US11679109B2 (en) | 2019-12-23 | 2023-06-20 | Kymera Therapeutics, Inc. | SMARCA degraders and uses thereof |
| US11680090B2 (en) | 2013-09-24 | 2023-06-20 | Medicenna Therapeutics, Inc. | Interleukin-2 fusion proteins and uses thereof |
| WO2023111203A1 (en) | 2021-12-16 | 2023-06-22 | Onxeo | New conjugated nucleic acid molecules and their uses |
| WO2023114984A1 (en) | 2021-12-17 | 2023-06-22 | Ikena Oncology, Inc. | Tead inhibitors and uses thereof |
| US11685750B2 (en) | 2020-06-03 | 2023-06-27 | Kymera Therapeutics, Inc. | Crystalline forms of IRAK degraders |
| WO2023122772A1 (en) | 2021-12-22 | 2023-06-29 | Gossamer Bio Services, Inc. | Oxime derivatives useful as t cell activators |
| WO2023122778A1 (en) | 2021-12-22 | 2023-06-29 | Gossamer Bio Services, Inc. | Pyridazinone derivatives useful as t cell activators |
| WO2023122777A1 (en) | 2021-12-22 | 2023-06-29 | Gossamer Bio Services, Inc. | Oxime derivatives useful as t cell activators |
| WO2023118165A1 (en) | 2021-12-21 | 2023-06-29 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating melanoma |
| US11701357B2 (en) | 2016-08-19 | 2023-07-18 | Beigene Switzerland Gmbh | Treatment of B cell cancers using a combination comprising Btk inhibitors |
| US11702461B2 (en) | 2018-01-09 | 2023-07-18 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides comprising reduced-affinity immunomodulatory polypeptides |
| US11707457B2 (en) | 2019-12-17 | 2023-07-25 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11713356B2 (en) | 2017-10-13 | 2023-08-01 | Ose Immunotherapeutics | Modified bifunctional anti-human signal regulatory protein alpha (SIRPa) antibody and method of use thereof for treating cancer |
| WO2023147371A1 (en) | 2022-01-26 | 2023-08-03 | Bristol-Myers Squibb Company | Combination therapy for hepatocellular carcinoma |
| WO2023150186A1 (en) | 2022-02-01 | 2023-08-10 | Arvinas Operations, Inc. | Dgk targeting compounds and uses thereof |
| WO2023154799A1 (en) | 2022-02-14 | 2023-08-17 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Combination immunotherapy for treating cancer |
| WO2023154905A1 (en) | 2022-02-14 | 2023-08-17 | Gilead Sciences, Inc. | Antiviral pyrazolopyridinone compounds |
| US11730761B2 (en) | 2016-02-18 | 2023-08-22 | Enlivex Therapeutics Rdo Ltd | Combination immune therapy and cytokine control therapy for cancer treatment |
| WO2023164638A1 (en) | 2022-02-25 | 2023-08-31 | Bristol-Myers Squibb Company | Combination therapy for colorectal carcinoma |
| US11746152B2 (en) | 2016-07-20 | 2023-09-05 | Stcube, Inc. | Methods of cancer treatment and therapy using a combination of antibodies that bind glycosylated PD-L1 |
| US11746103B2 (en) | 2020-12-10 | 2023-09-05 | Sumitomo Pharma Oncology, Inc. | ALK-5 inhibitors and uses thereof |
| WO2023168404A1 (en) | 2022-03-04 | 2023-09-07 | Bristol-Myers Squibb Company | Methods of treating a tumor |
| WO2023166418A2 (en) | 2022-03-03 | 2023-09-07 | Pfizer Inc. | Multispecific antibodies and uses thereof |
| US11753479B2 (en) | 2014-03-04 | 2023-09-12 | Kymab Limited | Nucleic acids encoding anti-OX40L antibodies |
| WO2023173053A1 (en) | 2022-03-10 | 2023-09-14 | Ikena Oncology, Inc. | Mek inhibitors and uses thereof |
| WO2023173057A1 (en) | 2022-03-10 | 2023-09-14 | Ikena Oncology, Inc. | Mek inhibitors and uses thereof |
| WO2023170606A1 (en) | 2022-03-08 | 2023-09-14 | Alentis Therapeutics Ag | Use of anti-claudin-1 antibodies to increase t cell availability |
| WO2023178192A1 (en) | 2022-03-15 | 2023-09-21 | Compugen Ltd. | Il-18bp antagonist antibodies and their use in monotherapy and combination therapy in the treatment of cancer |
| WO2023174210A1 (en) | 2022-03-14 | 2023-09-21 | Laekna Limited | Combination treatment for cancer |
| WO2023178329A1 (en) | 2022-03-18 | 2023-09-21 | Bristol-Myers Squibb Company | Methods of isolating polypeptides |
| EP4249066A2 (en) | 2014-12-23 | 2023-09-27 | Bristol-Myers Squibb Company | Antibodies to tigit |
| US11773103B2 (en) | 2021-02-15 | 2023-10-03 | Kymera Therapeutics, Inc. | IRAK4 degraders and uses thereof |
| WO2023192478A1 (en) | 2022-04-01 | 2023-10-05 | Bristol-Myers Squibb Company | Combination therapy with anti-il-8 antibodies and anti-pd-1 antibodies for treating cancer |
| US11779604B2 (en) | 2016-11-03 | 2023-10-10 | Kymab Limited | Antibodies, combinations comprising antibodies, biomarkers, uses and methods |
| WO2023196964A1 (en) | 2022-04-08 | 2023-10-12 | Bristol-Myers Squibb Company | Machine learning identification, classification, and quantification of tertiary lymphoid structures |
| WO2023196987A1 (en) | 2022-04-07 | 2023-10-12 | Bristol-Myers Squibb Company | Methods of treating tumor |
| US11786529B2 (en) | 2017-11-29 | 2023-10-17 | Beigene Switzerland Gmbh | Treatment of indolent or aggressive B-cell lymphomas using a combination comprising BTK inhibitors |
| US11787868B2 (en) | 2015-10-02 | 2023-10-17 | Hoffmann-La Roche Inc. | Bispecific anti-human A-beta/human transferrin receptor antibodies and methods of use |
| US11793802B2 (en) | 2019-03-20 | 2023-10-24 | Sumitomo Pharma Oncology, Inc. | Treatment of acute myeloid leukemia (AML) with venetoclax failure |
| WO2023211889A1 (en) | 2022-04-25 | 2023-11-02 | Ikena Oncology, Inc. | Polymorphic compounds and uses thereof |
| US11807692B2 (en) | 2018-09-25 | 2023-11-07 | Harpoon Therapeutics, Inc. | DLL3 binding proteins and methods of use |
| WO2023213763A1 (en) * | 2022-05-02 | 2023-11-09 | Transgene | Poxvirus encoding a binding agent comprising an anti- pd-l1 sdab |
| WO2023213764A1 (en) * | 2022-05-02 | 2023-11-09 | Transgene | Fusion polypeptide comprising an anti-pd-l1 sdab and a member of the tnfsf |
| WO2023214325A1 (en) | 2022-05-05 | 2023-11-09 | Novartis Ag | Pyrazolopyrimidine derivatives and uses thereof as tet2 inhibitors |
| US11815435B2 (en) | 2017-02-24 | 2023-11-14 | Hibercell, Inc. | Beta glucan immunopharmacodynamics |
| WO2023222854A1 (en) | 2022-05-18 | 2023-11-23 | Kymab Limited | Uses of anti-icos antibodies |
| WO2023228095A1 (en) | 2022-05-24 | 2023-11-30 | Daiichi Sankyo Company, Limited | Dosage regimen of an anti-cdh6 antibody-drug conjugate |
| WO2023230205A1 (en) | 2022-05-25 | 2023-11-30 | Ikena Oncology, Inc. | Mek inhibitors and uses thereof |
| WO2023235847A1 (en) | 2022-06-02 | 2023-12-07 | Bristol-Myers Squibb Company | Antibody compositions and methods of use thereof |
| US11851471B2 (en) | 2017-01-09 | 2023-12-26 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides and methods of use thereof |
| US11858996B2 (en) | 2016-08-09 | 2024-01-02 | Kymab Limited | Anti-ICOS antibodies |
| US11857535B2 (en) | 2020-07-30 | 2024-01-02 | Kymera Therapeutics, Inc. | Methods of treating mutant lymphomas |
| US11865081B2 (en) | 2017-12-29 | 2024-01-09 | Virogin Biotech Canada Ltd. | Oncolytic viral delivery of therapeutic polypeptides |
| US11878062B2 (en) | 2020-05-12 | 2024-01-23 | Cue Biopharma, Inc. | Multimeric T-cell modulatory polypeptides and methods of use thereof |
| US11884723B2 (en) | 2018-03-13 | 2024-01-30 | Ose Immunotherapeutics | Use of anti-human SIRPa v1 antibodies and method for producing anti-SIRPa v1 antibodies |
| WO2024023740A1 (en) | 2022-07-27 | 2024-02-01 | Astrazeneca Ab | Combinations of recombinant virus expressing interleukin-12 with pd-1/pd-l1 inhibitors |
| WO2024023750A1 (en) | 2022-07-28 | 2024-02-01 | Astrazeneca Uk Limited | Combination of antibody-drug conjugate and bispecific checkpoint inhibitor |
| EP4317972A2 (en) | 2018-02-06 | 2024-02-07 | The General Hospital Corporation | Repeat rna as biomarkers of tumor immune response |
| WO2024028364A1 (en) | 2022-08-02 | 2024-02-08 | Liminal Biosciences Limited | Aryl-triazolyl and related gpr84 antagonists and uses thereof |
| WO2024028363A1 (en) | 2022-08-02 | 2024-02-08 | Liminal Biosciences Limited | Heteroaryl carboxamide and related gpr84 antagonists and uses thereof |
| WO2024028365A1 (en) | 2022-08-02 | 2024-02-08 | Liminal Biosciences Limited | Substituted pyridone gpr84 antagonists and uses thereof |
| WO2024036100A1 (en) | 2022-08-08 | 2024-02-15 | Bristol-Myers Squibb Company | Substituted tetrazolyl compounds useful as t cell activators |
| WO2024033399A1 (en) | 2022-08-10 | 2024-02-15 | Institut National de la Santé et de la Recherche Médicale | Sigmar1 ligand for the treatment of pancreatic cancer |
| WO2024033458A1 (en) | 2022-08-11 | 2024-02-15 | F. Hoffmann-La Roche Ag | Bicyclic tetrahydroazepine derivatives |
| WO2024036101A1 (en) | 2022-08-09 | 2024-02-15 | Bristol-Myers Squibb Company | Tertiary amine substituted bicyclic compounds useful as t cell activators |
| WO2024033388A1 (en) | 2022-08-11 | 2024-02-15 | F. Hoffmann-La Roche Ag | Bicyclic tetrahydrothiazepine derivatives |
| WO2024033457A1 (en) | 2022-08-11 | 2024-02-15 | F. Hoffmann-La Roche Ag | Bicyclic tetrahydrothiazepine derivatives |
| WO2024033400A1 (en) | 2022-08-10 | 2024-02-15 | Institut National de la Santé et de la Recherche Médicale | Sk2 inhibitor for the treatment of pancreatic cancer |
| US11926625B2 (en) | 2021-03-05 | 2024-03-12 | Nimbus Saturn, Inc. | HPK1 antagonists and uses thereof |
| WO2024054992A1 (en) | 2022-09-09 | 2024-03-14 | Bristol-Myers Squibb Company | Methods of separating chelator |
| US11933786B2 (en) | 2015-03-30 | 2024-03-19 | Stcube, Inc. | Antibodies specific to glycosylated PD-L1 and methods of use thereof |
| US11932624B2 (en) | 2020-03-19 | 2024-03-19 | Kymera Therapeutics, Inc. | MDM2 degraders and uses thereof |
| WO2024056716A1 (en) | 2022-09-14 | 2024-03-21 | Institut National de la Santé et de la Recherche Médicale | Methods and pharmaceutical compositions for the treatment of dilated cardiomyopathy |
| US11939383B2 (en) | 2018-03-02 | 2024-03-26 | Five Prime Therapeutics, Inc. | B7-H4 antibodies and methods and use thereof |
| WO2024069009A1 (en) | 2022-09-30 | 2024-04-04 | Alentis Therapeutics Ag | Treatment of drug-resistant hepatocellular carcinoma |
| US11964015B2 (en) | 2016-04-01 | 2024-04-23 | Deutsches Krebsforschungszentrum | Cancer therapy with an oncolytic virus combined with a checkpoint inhibitor |
| WO2024084034A1 (en) | 2022-10-21 | 2024-04-25 | Institut National de la Santé et de la Recherche Médicale | Methods and pharmaceutical compositions for the treatment of osteoarthritis |
| US11976128B2 (en) | 2018-03-23 | 2024-05-07 | Board Of Regents, The University Of Texas System | Human PD-L2 antibodies and methods of use therefor |
| US11976125B2 (en) | 2017-10-13 | 2024-05-07 | Harpoon Therapeutics, Inc. | B cell maturation antigen binding proteins |
| WO2024094688A1 (en) | 2022-11-01 | 2024-05-10 | Heidelberg Pharma Research Gmbh | Anti-gucy2c antibody and uses thereof |
| US11981705B2 (en) | 2020-01-10 | 2024-05-14 | The Brigham And Women's Hospital, Inc. | Methods and compositions for delivery of immunotherapy agents across the blood-brain barrier to treat brain cancer |
| WO2024102635A1 (en) | 2022-11-07 | 2024-05-16 | Alector Llc | Uses of siglec-9 ecd fusion molecules in cancer treatment |
| WO2024112894A1 (en) | 2022-11-22 | 2024-05-30 | PIC Therapeutics, Inc. | Eif4e inhibitors and uses thereof |
| EP4378957A2 (en) | 2015-07-29 | 2024-06-05 | Novartis AG | Combination therapies comprising antibody molecules to pd-1 |
| US12006347B2 (en) | 2010-12-22 | 2024-06-11 | The Board Of Trustees Of The Leland Stanford Junior University | Superagonists and antagonists of interleukin-2 |
| WO2024137776A1 (en) | 2022-12-21 | 2024-06-27 | Bristol-Myers Squibb Company | Combination therapy for lung cancer |
| WO2024137865A1 (en) | 2022-12-22 | 2024-06-27 | Gossamer Bio Services, Inc. | Compounds useful as t cell activators |
| US12029782B2 (en) | 2020-09-09 | 2024-07-09 | Cue Biopharma, Inc. | MHC class II T-cell modulatory multimeric polypeptides for treating type 1 diabetes mellitus (T1D) and methods of use thereof |
| US12049520B2 (en) | 2017-08-04 | 2024-07-30 | Bicycletx Limited | Bicyclic peptide ligands specific for CD137 |
| EP4165086A4 (en) * | 2020-09-16 | 2024-07-31 | Suzhou Neologics Bioscience Co Ltd | Pd-l1 antibodies, fusion proteins, and uses thereof |
| US12054557B2 (en) | 2015-12-22 | 2024-08-06 | Regeneron Pharmaceuticals, Inc. | Combination of anti-PD-1 antibodies and bispecific anti-CD20/anti-CD3 antibodies to treat cancer |
| WO2024160721A1 (en) | 2023-01-30 | 2024-08-08 | Kymab Limited | Antibodies |
| WO2024163477A1 (en) | 2023-01-31 | 2024-08-08 | University Of Rochester | Immune checkpoint blockade therapy for treating staphylococcus aureus infections |
| US12059474B2 (en) | 2016-03-29 | 2024-08-13 | Stcube & Co., Inc. | Methods for selecting antibodies that specifically bind glycosylated immune checkpoint proteins |
| US12071442B2 (en) | 2021-03-29 | 2024-08-27 | Nimbus Saturn, Inc. | Substituted pyrrolo[3,4-c]pyridines as HPK1 antagonists |
| US12084518B2 (en) | 2015-05-21 | 2024-09-10 | Harpoon Therapeutics, Inc. | Trispecific binding proteins and methods of use |
| WO2024184417A1 (en) | 2023-03-07 | 2024-09-12 | Photocure Asa | Therapy for bladder cancer |
| US12091411B2 (en) | 2022-01-31 | 2024-09-17 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US12097261B2 (en) | 2021-05-07 | 2024-09-24 | Kymera Therapeutics, Inc. | CDK2 degraders and uses thereof |
| WO2024196952A1 (en) | 2023-03-20 | 2024-09-26 | Bristol-Myers Squibb Company | Tumor subtype assessment for cancer therapy |
| WO2024200571A1 (en) | 2023-03-28 | 2024-10-03 | Institut National de la Santé et de la Recherche Médicale | Method for discriminating mono-immunotherapy from combined immunotherapy in cancers |
| US12123003B2 (en) | 2020-12-23 | 2024-10-22 | Checkmate Pharmaceuticals, Inc. | Synthetic RIG-I-like receptor agonists |
Families Citing this family (209)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8741591B2 (en) | 2009-10-09 | 2014-06-03 | The Research Foundation For The State University Of New York | pH-insensitive glucose indicator protein |
| SI2785375T1 (en) * | 2011-11-28 | 2020-11-30 | Merck Patent Gmbh | Anti-pd-l1 antibodies and uses thereof |
| SG11201504410PA (en) | 2012-12-07 | 2015-07-30 | Chemocentryx Inc | Diazole lactams |
| KR20230144099A (en) | 2014-05-15 | 2023-10-13 | 브리스톨-마이어스 스큅 컴퍼니 | Treatment of lung cancer using a combination of an anti-pd-1 antibody and another anti-cancer agent |
| US11186640B2 (en) * | 2014-07-31 | 2021-11-30 | The University Of Western Australia | Method for the identification of immunotherapy-drug combinations using a network approach |
| TW201618775A (en) | 2014-08-11 | 2016-06-01 | 艾森塔製藥公司 | Therapeutic combinations of a BTK inhibitor, a PI3K inhibitor, a JAK-2 inhibitor, a PD-1 inhibitor and/or a PD-L1 inhibitor |
| KR101619862B1 (en) * | 2014-09-30 | 2016-05-12 | 주식회사 녹십자 | Kit and Method of Measuring Titers of Protein Comprising Human Fc Using Indirect Enzyme-linked Immunosorbent Assay |
| HRP20220531T1 (en) | 2014-12-05 | 2022-06-10 | Xyphos Biosciences Inc. | Insertable variable fragments of antibodies and modified a1-a2 domains of nkg2d ligands |
| CN105777906B (en) * | 2014-12-19 | 2019-04-23 | 苏州丁孚靶点生物技术有限公司 | Anti- PD-L1 human antibody and its application |
| GB201500319D0 (en) * | 2015-01-09 | 2015-02-25 | Agency Science Tech & Res | Anti-PD-L1 antibodies |
| WO2016128912A1 (en) | 2015-02-12 | 2016-08-18 | Acerta Pharma B.V. | Therapeutic combinations of a btk inhibitor, a pi3k inhibitor, a jak-2 inhibitor, a pd-1 inhibitor, and/or a pd-l1 inhibitor |
| EP3067062A1 (en) * | 2015-03-13 | 2016-09-14 | Ipsen Pharma S.A.S. | Combination of tasquinimod or a pharmaceutically acceptable salt thereof and a pd1 and/or pdl1 inhibitor, for use as a medicament |
| WO2016150899A2 (en) | 2015-03-23 | 2016-09-29 | Bayer Pharma Aktiengesellschaft | Anti-ceacam6 antibodies and uses thereof |
| AU2016249395B2 (en) | 2015-04-17 | 2022-04-07 | Bristol-Myers Squibb Company | Compositions comprising a combination of an anti-PD-1 antibody and another antibody |
| CN108135168B (en) | 2015-05-21 | 2021-07-20 | 凯莫森特里克斯股份有限公司 | CCR2 modulators |
| US20180155429A1 (en) | 2015-05-28 | 2018-06-07 | Bristol-Myers Squibb Company | Treatment of pd-l1 positive lung cancer using an anti-pd-1 antibody |
| US11078278B2 (en) | 2015-05-29 | 2021-08-03 | Bristol-Myers Squibb Company | Treatment of renal cell carcinoma |
| MA53355A (en) | 2015-05-29 | 2022-03-16 | Agenus Inc | ANTI-CTLA-4 ANTIBODIES AND METHODS OF USE THEREOF |
| JP2018516947A (en) * | 2015-06-09 | 2018-06-28 | レクサン ファーマシューティカルズ インコーポレイテッド | Process of use and preparation of fluorocyclopentenylcytosine |
| CN106243225B (en) * | 2015-06-11 | 2021-01-19 | 智翔(上海)医药科技有限公司 | Novel anti-PD-L1 antibodies |
| WO2016197367A1 (en) | 2015-06-11 | 2016-12-15 | Wuxi Biologics (Shanghai) Co. Ltd. | Novel anti-pd-l1 antibodies |
| EP3858859A1 (en) | 2015-07-14 | 2021-08-04 | Bristol-Myers Squibb Company | Method of treating cancer using immune checkpoint inhibitor; antibody that binds to programmed death-1 receptor (pd-1) or programmed death ligand 1 (pd-l1) |
| CN108136025B (en) | 2015-07-16 | 2022-09-06 | 比奥克斯塞尔医疗股份有限公司 | A novel method of treating cancer using immunomodulation |
| CN106397592A (en) * | 2015-07-31 | 2017-02-15 | 苏州康宁杰瑞生物科技有限公司 | Single-domain antibody directed at programmed death ligand (PD-L1) and derived protein thereof |
| WO2017020291A1 (en) | 2015-08-06 | 2017-02-09 | Wuxi Biologics (Shanghai) Co. Ltd. | Novel anti-pd-l1 antibodies |
| KR20180049015A (en) | 2015-09-04 | 2018-05-10 | 렉산 파마슈티컬스, 인코포레이티드 | Method for using quinoxalinyl-piperazinamide |
| MA44909A (en) | 2015-09-15 | 2018-07-25 | Acerta Pharma Bv | THERAPEUTIC ASSOCIATION OF A CD19 INHIBITOR AND A BTK INHIBITOR |
| CN106540255A (en) * | 2015-09-18 | 2017-03-29 | 聊城市奥润生物医药科技有限公司 | Ring dinucleotide cGAMP combines application of the Avastin in antitumor |
| CN106565836B (en) * | 2015-10-10 | 2020-08-18 | 中国科学院广州生物医药与健康研究院 | High affinity soluble PDL-1 molecules |
| CN108135934B (en) | 2015-10-19 | 2024-09-10 | 永恒生物科技股份有限公司 | Methods of treating solid or lymphoid tumors by combination therapy |
| LT3370733T (en) | 2015-11-02 | 2021-10-25 | Board Of Regents, The University Of Texas System | Methods of cd40 activation and immune checkpoint blockade |
| BR112018009064A8 (en) * | 2015-11-17 | 2019-02-26 | Jiangsu Hengrui Medicine Co | pd-11 antibody, antigen binding fragment thereof and medical application thereof |
| TWI795347B (en) | 2015-11-18 | 2023-03-11 | 美商必治妥施貴寶公司 | Treatment of lung cancer using a combination of an anti-pd-1 antibody and an anti-ctla-4 antibody |
| RS63135B1 (en) | 2015-12-23 | 2022-05-31 | Modernatx Inc | Methods of using ox40 ligand encoding polynucleotides |
| CN105461808B (en) * | 2015-12-24 | 2019-03-19 | 长春金赛药业股份有限公司 | Monoclonal antibody and its application |
| CN106939047B (en) * | 2016-01-04 | 2021-08-31 | 江苏怀瑜药业有限公司 | PD-L1 antibody and preparation method thereof |
| EP3402503B1 (en) | 2016-01-13 | 2020-10-21 | Acerta Pharma B.V. | Therapeutic combinations of an antifolate and a btk inhibitor |
| WO2017136562A2 (en) * | 2016-02-02 | 2017-08-10 | Kadmon Corporation, Llc | Bispecific binding proteins for pd-l1 and kdr |
| WO2017156349A1 (en) | 2016-03-10 | 2017-09-14 | Cold Genesys, Inc. | Methods of treating solid or lymphatic tumors by combination therapy |
| CN107216389B (en) | 2016-03-18 | 2022-03-29 | 和迈生物科技有限公司 | anti-PD-L1 nano antibody and coding sequence and application thereof |
| WO2017176925A1 (en) | 2016-04-05 | 2017-10-12 | Bristol-Myers Squibb Company | Cytokine profiling analysis for predicting prognosis of a patient in need of an anti-cancer treatment |
| EP3439653B1 (en) | 2016-04-07 | 2021-01-20 | ChemoCentryx, Inc. | Reducing tumor burden by administering ccr1 antagonists in combination with pd-1 inhibitors or pd-l1 inhibitors |
| WO2017181073A1 (en) * | 2016-04-14 | 2017-10-19 | Creatv Microtech, Inc. | Methods of using pd-l1 expression in treatment decisions for cancer therapy |
| PT3458083T (en) | 2016-05-18 | 2023-03-06 | Modernatx Inc | Polynucleotides encoding interleukin-12 (il12) and uses thereof |
| KR102482867B1 (en) | 2016-05-18 | 2023-01-02 | 모더나티엑스, 인크. | Combinations of mRNAs encoding immune modulatory polypeptides and uses thereof |
| CN109476751B (en) | 2016-05-27 | 2024-04-19 | 艾吉纳斯公司 | Anti-TIM-3 antibodies and methods of use thereof |
| WO2017210453A1 (en) | 2016-06-02 | 2017-12-07 | Bristol-Myers Squibb Company | Pd-1 blockade with nivolumab in refractory hodgkin's lymphoma |
| HUE063911T2 (en) | 2016-06-02 | 2024-02-28 | Bristol Myers Squibb Co | Use of an anti-pd-1 antibody in combination with an anti-cd30 antibody in lymphoma treatment |
| CN109476753A (en) | 2016-06-03 | 2019-03-15 | 百时美施贵宝公司 | For treating the anti-PD-1 antibody of the method for tumour |
| KR102515509B1 (en) | 2016-06-03 | 2023-03-28 | 브리스톨-마이어스 스큅 컴퍼니 | Use of Anti-PD-1 Antibodies in the Treatment of Patients with Colorectal Cancer |
| WO2017210631A1 (en) | 2016-06-03 | 2017-12-07 | Bristol-Myers Squibb Company | Anti-pd-1 antibody for use in a method of treatment of recurrent small cell lung cancer |
| CN109414500B (en) * | 2016-06-13 | 2022-02-25 | 奥美药业有限公司 | PD-L1 specific monoclonal antibody for treatment and diagnosis |
| WO2017222556A2 (en) | 2016-06-24 | 2017-12-28 | Avidbiotics Corp. | Insertable variable fragments of antibodies and modified a1-a2 domains of nkg2d ligands |
| GB201612520D0 (en) | 2016-07-19 | 2016-08-31 | F-Star Beta Ltd | Binding molecules |
| US11274153B2 (en) | 2016-08-04 | 2022-03-15 | Innovent Biologics (Suzhou) Co., Ltd. | Anti-PD-L1 nanobody and use thereof |
| EP3496707A4 (en) * | 2016-08-09 | 2020-03-25 | Angimmune, LLC | Treatment of cancer using a combination of immunomodulation and check point inhibitors |
| CN106248955A (en) * | 2016-09-03 | 2016-12-21 | 长春工业大学 | A kind of test kit detecting humanization PD L1 antibody |
| AU2017322501A1 (en) | 2016-09-09 | 2019-03-28 | Laboratoire Francais Du Fractionnement Et Des Biotechnologies | Combination of an anti-CD20 antibody, PI3 kinase-delta inhibitor, and anti-PD-1 or anti-PD-L1 antibody for treating hematological cancers |
| WO2018048975A1 (en) | 2016-09-09 | 2018-03-15 | Bristol-Myers Squibb Company | Use of an anti-pd-1 antibody in combination with an anti-mesothelin antibody in cancer treatment |
| AU2017343621B2 (en) | 2016-10-11 | 2021-12-02 | Agenus Inc. | Anti-LAG-3 antibodies and methods of use thereof |
| TWI788307B (en) | 2016-10-31 | 2023-01-01 | 美商艾歐凡斯生物治療公司 | Engineered artificial antigen presenting cells for tumor infiltrating lymphocyte expansion |
| AU2017356350B2 (en) * | 2016-11-09 | 2024-02-29 | Engene, Inc. | Intestinal expression of programmed death ligand 1 |
| CN106496327B (en) * | 2016-11-18 | 2019-01-15 | 昆山百尔泰生物科技有限公司 | Human antibody or antibody fragment and purposes, nucleotide sequence and carrier for PD-L1 extracellular fragment |
| JOP20190100A1 (en) | 2016-11-19 | 2019-05-01 | Potenza Therapeutics Inc | Anti-gitr antigen-binding proteins and methods of use thereof |
| CA3045508A1 (en) | 2016-12-03 | 2018-06-07 | Juno Therapeutics, Inc. | Methods for modulation of car-t cells |
| MA50948A (en) | 2016-12-07 | 2020-10-14 | Agenus Inc | ANTIBODIES AND METHODS OF USING THE SAME |
| PT3551660T (en) | 2016-12-07 | 2023-11-30 | Ludwig Inst For Cancer Res Ltd | Anti-ctla-4 antibodies and methods of use thereof |
| CA3046963A1 (en) | 2016-12-14 | 2018-06-21 | Janssen Biotech, Inc. | Cd8a-binding fibronectin type iii domains |
| WO2018111978A1 (en) | 2016-12-14 | 2018-06-21 | Janssen Biotech, Inc. | Cd137 binding fibronectin type iii domains |
| US10597438B2 (en) | 2016-12-14 | 2020-03-24 | Janssen Biotech, Inc. | PD-L1 binding fibronectin type III domains |
| WO2018112364A1 (en) | 2016-12-16 | 2018-06-21 | Evelo Biosciences, Inc. | Combination therapies for treating melanoma |
| WO2018112360A1 (en) | 2016-12-16 | 2018-06-21 | Evelo Biosciences, Inc. | Combination therapies for treating cancer |
| CN110139922B (en) | 2016-12-22 | 2021-05-25 | 香港大学 | Compositions and methods for clarifying tissue |
| EP3565560B1 (en) | 2017-01-09 | 2024-05-29 | OnkosXcel Therapeutics, LLC | Predictive and diagnostic methods for prostate cancer |
| US11034667B2 (en) | 2017-01-09 | 2021-06-15 | Shuttle Pharmaceuticals, Inc. | Selective histone deacetylase inhibitors for the treatment of human disease |
| US11584733B2 (en) | 2017-01-09 | 2023-02-21 | Shuttle Pharmaceuticals, Inc. | Selective histone deacetylase inhibitors for the treatment of human disease |
| MX2019008346A (en) | 2017-01-13 | 2019-09-09 | Agenus Inc | T cell receptors that bind to ny-eso-1 and methods of use thereof. |
| EP3573657A4 (en) * | 2017-01-29 | 2021-04-14 | Zequn Tang | Methods of immune modulation against foreign and/or auto antigens |
| RS64870B1 (en) * | 2017-03-16 | 2023-12-29 | Alpine Immune Sciences Inc | Pd-l1 variant immunomodulatory proteins and uses thereof |
| CN111315776A (en) * | 2017-03-29 | 2020-06-19 | 葛莱高托普有限公司 | PD-L1 and TA-MUC1 antibodies |
| MX2019012223A (en) | 2017-04-13 | 2019-12-09 | Agenus Inc | Anti-cd137 antibodies and methods of use thereof. |
| TWI805582B (en) | 2017-05-01 | 2023-06-21 | 美商艾吉納斯公司 | Anti-tigit antibodies and methods of use thereof |
| CN110050000B (en) | 2017-05-12 | 2022-07-26 | 苏州盛迪亚生物医药有限公司 | Fusion protein containing TGF-beta receptor and medical application thereof |
| CN110913911A (en) | 2017-05-18 | 2020-03-24 | 特沙诺有限公司 | Combination therapy for the treatment of cancer |
| JP7285220B2 (en) | 2017-05-18 | 2023-06-01 | モデルナティエックス インコーポレイテッド | Lipid nanoparticles comprising linked interleukin-12 (IL12) polypeptide-encoding polynucleotides |
| WO2019005634A2 (en) * | 2017-06-25 | 2019-01-03 | Systimmune, Inc. | Anti-pd-l1 antibodies and methods of making and using thereof |
| US11899017B2 (en) | 2017-07-28 | 2024-02-13 | Bristol-Myers Squibb Company | Predictive peripheral blood biomarker for checkpoint inhibitors |
| CN111356477B (en) | 2017-08-01 | 2024-08-30 | Ab工作室有限公司 | Bispecific antibodies and uses thereof |
| CN111372934B (en) | 2017-08-18 | 2024-04-26 | 科瑞华生物技术有限公司 | Polymorphic forms of TG02 |
| MX2020001980A (en) | 2017-08-28 | 2020-03-24 | Bristol Myers Squibb Co | Tim-3 antagonists for the treatment and diagnosis of cancers. |
| CA3073055A1 (en) | 2017-09-04 | 2019-03-07 | Agenus Inc. | T cell receptors that bind to mixed lineage leukemia (mll)-specific phosphopeptides and methods of use thereof |
| IL273188B2 (en) | 2017-09-25 | 2023-03-01 | Chemocentryx Inc | Combination therapy using a chemokine receptor 2 (ccr2) antagonist and a pd-1/pd-l1 inhibitor |
| MX2020004185A (en) * | 2017-09-27 | 2021-01-08 | Univ Southern California | Novel platforms for co-stimulation, novel car designs and other enhancements for adoptive cellular therapy. |
| EP3697442A4 (en) | 2017-09-30 | 2021-07-07 | Tesaro, Inc. | Combination therapies for treating cancer |
| CA3076859A1 (en) | 2017-10-06 | 2019-04-11 | Tesaro, Inc. | Combination therapies and uses thereof |
| US11685782B2 (en) | 2017-10-23 | 2023-06-27 | Children's Medical Center Corporation | Methods of treating cancer using LSD1 inhibitors in combination with immunotherapy |
| US20190269664A1 (en) | 2018-01-08 | 2019-09-05 | Chemocentryx, Inc. | Methods of treating solid tumors with ccr2 antagonists |
| EP3737367B1 (en) | 2018-01-08 | 2024-10-02 | ChemoCentryx, Inc. | Ccr2 antagonists for the treatment of cutaneous t-cell lymphoma |
| CN112004537A (en) | 2018-01-09 | 2020-11-27 | 穿梭药业公司 | Selective histone deacetylase inhibitors for the treatment of human diseases |
| JP2021510078A (en) | 2018-01-10 | 2021-04-15 | 江▲蘇▼恒瑞医▲薬▼股▲フン▼有限公司Jiangsu Hengrui Medicine Co., Ltd. | PD-L1 antibody, its antigen-binding fragment, and its pharmaceutical use |
| WO2019157124A1 (en) | 2018-02-08 | 2019-08-15 | Bristol-Myers Squibb Company | Combination of a tetanus toxoid, anti-ox40 antibody and/or anti-pd-1 antibody to treat tumors |
| CA3093742A1 (en) | 2018-03-12 | 2019-09-19 | Inserm (Institut National De La Sante Et De La Recherche Medicale) | Use of caloric restriction mimetics for potentiating chemo-immunotherapy for the treatment of cancers |
| US12110337B2 (en) | 2018-04-04 | 2024-10-08 | Bristol-Myers Squibb Company | Anti-CD27 antibodies and uses thereof |
| BR112020021271A2 (en) | 2018-04-17 | 2021-01-26 | Celldex Therapeutics, Inc. | bispecific constructs and anti-cd27 and anti-pd-l1 antibodies |
| JP2021522239A (en) | 2018-04-26 | 2021-08-30 | アジェナス インコーポレイテッド | Heat shock protein-binding peptide composition and how to use it |
| WO2019232533A1 (en) | 2018-06-01 | 2019-12-05 | Massachusetts Institute Of Technology | Combination treatments of hsp90 inhibitors for enhancing tumor immunogenicity and methods of use thereof |
| CN113195525B (en) * | 2018-06-29 | 2024-10-01 | 国民大学校产学协力团 | PD-L1 variants with increased binding affinity for PD-1 |
| US20210147549A1 (en) * | 2018-07-11 | 2021-05-20 | Momenta Pharmaceuticals, Inc. | COMPOSITIONS AND METHODS RELATED TO ENGINEERED Fc-ANTIGEN BINDING DOMAIN CONSTRUCTS TARGETED TO PD-L1 |
| GB201811408D0 (en) | 2018-07-12 | 2018-08-29 | F Star Beta Ltd | CD137 Binding Molecules |
| WO2020014583A1 (en) | 2018-07-13 | 2020-01-16 | Bristol-Myers Squibb Company | Ox-40 agonist, pd-1 pathway inhibitor and ctla-4 inhibitor combination for use in a mehtod of treating a cancer or a solid tumor |
| TW202031273A (en) | 2018-08-31 | 2020-09-01 | 美商艾歐凡斯生物治療公司 | Treatment of nsclc patients refractory for anti-pd-1 antibody |
| WO2020048942A1 (en) | 2018-09-04 | 2020-03-12 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and pharmaceutical compositions for enhancing cytotoxic t lymphocyte-dependent immune responses |
| US11331269B2 (en) | 2018-09-06 | 2022-05-17 | Massachusetts Institute Of Technology | Methods and compositions targeting lung microbiota and its responding immune pathways for lung cancer treatment |
| WO2020068398A2 (en) * | 2018-09-07 | 2020-04-02 | The General Hospital Corporation | Compositions and methods for immune checkpoint inhibition |
| CN109053891B (en) * | 2018-09-17 | 2021-12-21 | 苏州泓迅生物科技股份有限公司 | anti-PD-L1 antibody, and preparation method and application thereof |
| WO2020058372A1 (en) | 2018-09-19 | 2020-03-26 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and pharmaceutical composition for the treatment of cancers resistant to immune checkpoint therapy |
| WO2020061429A1 (en) | 2018-09-20 | 2020-03-26 | Iovance Biotherapeutics, Inc. | Expansion of tils from cryopreserved tumor samples |
| US20220040183A1 (en) | 2018-10-01 | 2022-02-10 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Use of inhibitors of stress granule formation for targeting the regulation of immune responses |
| JP2022512642A (en) | 2018-10-09 | 2022-02-07 | ブリストル-マイヤーズ スクイブ カンパニー | Anti-MerTK antibody to treat cancer |
| WO2020081493A1 (en) | 2018-10-16 | 2020-04-23 | Molecular Templates, Inc. | Pd-l1 binding proteins |
| US20220001026A1 (en) | 2018-11-08 | 2022-01-06 | Modernatx, Inc. | Use of mrna encoding ox40l to treat cancer in human patients |
| MX2021005018A (en) | 2018-11-09 | 2021-06-15 | Jiangsu Hengrui Medicine Co | Tgf-î² receptor fusion protein pharmaceutical composition and use thereof. |
| CA3119563A1 (en) | 2018-11-14 | 2020-05-22 | Bayer Aktiengesellschaft | Pharmaceutical combination of anti-ceacam6 and either anti-pd-1 or anti-pd-l1 antibodies for the treatment of cancer |
| JP7513604B2 (en) | 2018-11-14 | 2024-07-09 | リジェネロン・ファーマシューティカルズ・インコーポレイテッド | Intralesional administration of PD-1 inhibitors to treat skin cancer |
| US20220008515A1 (en) | 2018-11-16 | 2022-01-13 | Neoimmunetech, Inc. | Method of treating a tumor with a combination of il-7 protein and an immune checkpoint inhibitor |
| MX2021005808A (en) | 2018-11-20 | 2021-07-02 | Univ Cornell | Macrocyclic complexes of radionuclides and their use in radiotherapy of cancer. |
| ES2971964T3 (en) | 2018-11-28 | 2024-06-10 | Inst Nat Sante Rech Med | Methods and kit for testing the lytic potential of immune effector cells |
| WO2020115262A1 (en) | 2018-12-07 | 2020-06-11 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Use of cd26 and cd39 as new phenotypic markers for assessing maturation of foxp3+ t cells and uses thereof for diagnostic purposes |
| WO2020127059A1 (en) | 2018-12-17 | 2020-06-25 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Use of sulconazole as a furin inhibitor |
| SG11202107606VA (en) | 2019-01-15 | 2021-08-30 | Inst Nat Sante Rech Med | Mutated interleukin-34 (il-34) polypeptides and uses thereof in therapy |
| CN113365659B (en) * | 2019-01-31 | 2023-08-18 | 正大天晴药业集团股份有限公司 | Use of anti-PD-L1 antibodies for the treatment of head and neck cancer |
| WO2020169472A2 (en) | 2019-02-18 | 2020-08-27 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods of inducing phenotypic changes in macrophages |
| PT3927370T (en) | 2019-02-19 | 2024-06-03 | Turnstone Biologics Corp | Methods for producing autologous t cells useful to treat cancers and compositions thereof |
| CN113490529A (en) | 2019-02-28 | 2021-10-08 | 瑞泽恩制药公司 | Administration of PD-1 inhibitors for the treatment of skin cancer |
| KR20210136071A (en) | 2019-03-06 | 2021-11-16 | 리제너론 파아마슈티컬스, 인크. | IL-4/IL-13 Pathway Inhibitors for Enhanced Efficacy in Treating Cancer |
| MX2021011753A (en) | 2019-03-26 | 2022-01-31 | Univ Michigan Regents | Small molecule degraders of stat3. |
| EP3946437A1 (en) | 2019-03-29 | 2022-02-09 | Myst Therapeutics, LLC | Ex vivo methods for producing a t cell therapeutic and related compositions and methods |
| WO2020205467A1 (en) | 2019-03-29 | 2020-10-08 | The Regents Of The University Of Michigan | Stat3 protein degraders |
| JP2022527972A (en) | 2019-04-02 | 2022-06-07 | アンスティチュ ナショナル ドゥ ラ サンテ エ ドゥ ラ ルシェルシュ メディカル | How to predict and prevent cancer in patients with premalignant lesions |
| WO2020208060A1 (en) | 2019-04-09 | 2020-10-15 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Use of sk2 inhibitors in combination with immune checkpoint blockade therapy for the treatment of cancer |
| SG11202109441UA (en) | 2019-04-12 | 2021-09-29 | Vascular Biogenics Ltd | Methods of anti-tumor therapy |
| US20220220480A1 (en) | 2019-04-17 | 2022-07-14 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treatment of nlrp3 inflammasome mediated il-1beta dependent disorders |
| JP2022531444A (en) * | 2019-05-06 | 2022-07-06 | ブラウン ユニバーシティ | Bispecific antibodies to CHI3L1 and PD1 with increased T cell-mediated cytotoxic effects on tumor cells |
| CN114269715A (en) | 2019-06-12 | 2022-04-01 | 范德比尔特大学 | Dibenzylamines as amino acid transport inhibitors |
| CA3141405A1 (en) | 2019-06-12 | 2020-12-17 | H. Charles Manning | Amino acid transport inhibitors and the uses thereof |
| CA3138560A1 (en) | 2019-07-16 | 2021-01-21 | Shaomeng Wang | Imidazopyrimidines as eed inhibitors and the use thereof |
| WO2021024020A1 (en) | 2019-08-06 | 2021-02-11 | Astellas Pharma Inc. | Combination therapy involving antibodies against claudin 18.2 and immune checkpoint inhibitors for treatment of cancer |
| CN114641337A (en) | 2019-08-27 | 2022-06-17 | 密歇根大学董事会 | CEREBLON E3 ligase inhibitors |
| CA3152027A1 (en) | 2019-08-30 | 2021-03-04 | Agenus Inc. | Anti-cd96 antibodies and methods of use thereof |
| JP2022548078A (en) | 2019-09-18 | 2022-11-16 | モレキュラー テンプレーツ,インク. | PD-L1 binding molecules containing Shiga toxin A subunit scaffolds |
| WO2021055816A1 (en) | 2019-09-18 | 2021-03-25 | Molecular Templates, Inc. | Pd-l1 binding molecules comprising shiga toxin a subunit scaffolds |
| JP2022548775A (en) | 2019-09-19 | 2022-11-21 | ザ リージェンツ オブ ザ ユニヴァシティ オブ ミシガン | Spirocyclic Androgen Receptor Proteolytic Agent |
| EP3800201A1 (en) | 2019-10-01 | 2021-04-07 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Cd28h stimulation enhances nk cell killing activities |
| EP4037710A1 (en) | 2019-10-04 | 2022-08-10 | Institut National de la Santé et de la Recherche Médicale (INSERM) | Methods and pharmaceutical composition for the treatment of ovarian cancer, breast cancer or pancreatic cancer |
| WO2021076546A1 (en) | 2019-10-14 | 2021-04-22 | Aro Biotherapeutics Company | Cd71 binding fibronectin type iii domains |
| US11781138B2 (en) | 2019-10-14 | 2023-10-10 | Aro Biotherapeutics Company | FN3 domain-siRNA conjugates and uses thereof |
| WO2021108727A1 (en) | 2019-11-27 | 2021-06-03 | Myst Therapeutics, Inc. | Method of producing tumor-reactive t cell composition using modulatory agents |
| CA3172902A1 (en) | 2020-02-27 | 2021-09-02 | Myst Therapeutics, Llc | Methods for ex vivo enrichment and expansion of tumor reactive t cells and related compositions thereof |
| WO2021194942A1 (en) | 2020-03-23 | 2021-09-30 | Bristol-Myers Squibb Company | Anti-ccr8 antibodies for treating cancer |
| US20230159573A1 (en) | 2020-03-26 | 2023-05-25 | The Regents Of The University Of Michigan | Small molecule stat protein degraders |
| CA3176356A1 (en) | 2020-04-22 | 2021-10-28 | Anne BROOKS | Systems and methods for coordinating manufacturing of cells for patient-specific immunotherapy |
| JP2023528017A (en) | 2020-05-26 | 2023-07-03 | アンセルム(アンスティチュート・ナシオナル・ドゥ・ラ・サンテ・エ・ドゥ・ラ・ルシェルシュ・メディカル) | Severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) polypeptide and its use for vaccine purposes |
| MX2022014734A (en) | 2020-05-26 | 2023-03-15 | Regeneron Pharma | Methods of treating cervical cancer by administering the pd-1 inhibitor antibody cemiplimab. |
| EP4157319A1 (en) | 2020-05-28 | 2023-04-05 | Modernatx, Inc. | Use of mrnas encoding ox40l, il-23 and il-36gamma for treating cancer |
| MX2023000197A (en) | 2020-07-07 | 2023-02-22 | BioNTech SE | Therapeutic rna for hpv-positive cancer. |
| WO2022011204A1 (en) | 2020-07-10 | 2022-01-13 | The Regents Of The University Of Michigan | Small molecule androgen receptor protein degraders |
| WO2022011205A1 (en) | 2020-07-10 | 2022-01-13 | The Regents Of The University Of Michigan | Androgen receptor protein degraders |
| CA3168743A1 (en) | 2020-08-26 | 2022-03-03 | Matthew G. Fury | Methods of treating cancer by administering a pd-1 inhibitor |
| CN111808196B (en) * | 2020-08-31 | 2020-12-29 | 北京百奥赛图基因生物技术有限公司 | anti-PD-1 antibodies and uses thereof |
| CN116194142A (en) | 2020-09-03 | 2023-05-30 | 瑞泽恩制药公司 | Methods of treating cancer pain by administering PD-1 inhibitors |
| CN111920948B (en) * | 2020-09-25 | 2021-02-02 | 安可瑞(山西)生物细胞有限公司 | Pharmaceutical composition comprising immune cells for treating cancer |
| JP2023554587A (en) | 2020-11-12 | 2023-12-28 | アンセルム(アンスティチュート・ナシオナル・ドゥ・ラ・サンテ・エ・ドゥ・ラ・ルシェルシュ・メディカル) | Antibodies conjugated or fused to the receptor binding domain of the SARS-COV-2 spike protein and their use for vaccine purposes |
| WO2022101463A1 (en) | 2020-11-16 | 2022-05-19 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Use of the last c-terminal residues m31/41 of zikv m ectodomain for triggering apoptotic cell death |
| WO2022135666A1 (en) | 2020-12-21 | 2022-06-30 | BioNTech SE | Treatment schedule for cytokine proteins |
| TW202245808A (en) | 2020-12-21 | 2022-12-01 | 德商拜恩迪克公司 | Therapeutic rna for treating cancer |
| WO2022135667A1 (en) | 2020-12-21 | 2022-06-30 | BioNTech SE | Therapeutic rna for treating cancer |
| CN116963773A (en) | 2021-01-21 | 2023-10-27 | 浙江养生堂天然药物研究所有限公司 | Compositions and methods for treating tumors |
| US20240190874A1 (en) | 2021-03-03 | 2024-06-13 | The Regents Of The University Of Michigan | Small molecule degraders of androgen receptor |
| US20240166647A1 (en) | 2021-03-03 | 2024-05-23 | The Regents Of The University Of Michigan | Cereblon Ligands |
| EP4313123A1 (en) | 2021-03-23 | 2024-02-07 | Regeneron Pharmaceuticals, Inc. | Methods of treating cancer in immunosuppressed or immunocompromised patients by administering a pd-1 inhibitor |
| TW202304506A (en) | 2021-03-25 | 2023-02-01 | 日商安斯泰來製藥公司 | Combination therapy involving antibodies against claudin 18.2 for treatment of cancer |
| BR112023021325A2 (en) | 2021-04-14 | 2023-12-19 | Aro Biotherapeutics Company | CD71-BINDING TYPE III FIBRONECTIN DOMAINS |
| CN117396222A (en) | 2021-05-21 | 2024-01-12 | 天津立博美华基因科技有限责任公司 | Pharmaceutical combination and use thereof |
| CN117412767A (en) | 2021-05-25 | 2024-01-16 | 雪绒花免疫公司 | C-X-C motif chemokine receptor 6 (CXCR 6) binding molecules and methods of use thereof |
| WO2022247972A2 (en) | 2021-05-26 | 2022-12-01 | Centro De Inmunologia Molecular | Use of therapeutic compositions for the treatment of patients with tumours of epithelial origin |
| WO2023280790A1 (en) | 2021-07-05 | 2023-01-12 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Gene signatures for predicting survival time in patients suffering from renal cell carcinoma |
| KR20240046323A (en) | 2021-07-13 | 2024-04-08 | 비온테크 에스이 | Multispecific binding agent for CD40 and CD137 in combination therapy for cancer |
| US20230139492A1 (en) | 2021-07-19 | 2023-05-04 | Regeneron Pharmaceuticals, Inc. | Combination of checkpoint inhibitors and an oncolytic virus for treating cancer |
| KR20240051162A (en) | 2021-09-02 | 2024-04-19 | 도이체스크레브스포르슝스젠트룸스티프퉁데스외펜트리헨레크츠 | Anti-CECAM6 antibody with reduced side effects |
| WO2023051926A1 (en) | 2021-09-30 | 2023-04-06 | BioNTech SE | Treatment involving non-immunogenic rna for antigen vaccination and pd-1 axis binding antagonists |
| TW202333802A (en) | 2021-10-11 | 2023-09-01 | 德商拜恩迪克公司 | Therapeutic rna for lung cancer |
| CA3234821A1 (en) | 2021-10-28 | 2023-05-04 | Suman Kumar VODNALA | Methods for culturing immune cells |
| WO2023083439A1 (en) | 2021-11-09 | 2023-05-19 | BioNTech SE | Tlr7 agonist and combinations for cancer treatment |
| CN118765283A (en) | 2021-11-17 | 2024-10-11 | 国家健康科学研究所 | Universal sand Bei Bingdu vaccine |
| WO2023130081A1 (en) | 2021-12-30 | 2023-07-06 | Neoimmunetech, Inc. | Method of treating a tumor with a combination of il-7 protein and vegf antagonist |
| WO2023159102A1 (en) | 2022-02-17 | 2023-08-24 | Regeneron Pharmaceuticals, Inc. | Combinations of checkpoint inhibitors and oncolytic virus for treating cancer |
| KR20230130911A (en) | 2022-03-04 | 2023-09-12 | 주식회사 온코인사이트 | Pharmaceutical composition for combination therapy for cancer prevention or treatment |
| WO2023196988A1 (en) | 2022-04-07 | 2023-10-12 | Modernatx, Inc. | Methods of use of mrnas encoding il-12 |
| WO2023201369A1 (en) | 2022-04-15 | 2023-10-19 | Iovance Biotherapeutics, Inc. | Til expansion processes using specific cytokine combinations and/or akti treatment |
| WO2024052356A1 (en) | 2022-09-06 | 2024-03-14 | Institut National de la Santé et de la Recherche Médicale | Inhibitors of the ceramide metabolic pathway for overcoming immunotherapy resistance in cancer |
| US20240190982A1 (en) | 2022-11-03 | 2024-06-13 | Incyte Corporation | Combination therapies comprising an anti-gitr antibody for treating cancers |
| WO2024102722A1 (en) | 2022-11-07 | 2024-05-16 | Neoimmunetech, Inc. | Methods of treating a tumor with an unmethylated mgmt promoter |
| WO2024112571A2 (en) | 2022-11-21 | 2024-05-30 | Iovance Biotherapeutics, Inc. | Two-dimensional processes for the expansion of tumor infiltrating lymphocytes and therapies therefrom |
| WO2024115725A1 (en) | 2022-12-01 | 2024-06-06 | BioNTech SE | Multispecific antibody against cd40 and cd137 in combination therapy with anti-pd1 ab and chemotherapy |
| WO2024126457A1 (en) | 2022-12-14 | 2024-06-20 | Astellas Pharma Europe Bv | Combination therapy involving bispecific binding agents binding to cldn18.2 and cd3 and immune checkpoint inhibitors |
| CN116077645A (en) * | 2022-12-28 | 2023-05-09 | 广州誉衡生物科技有限公司 | anti-PD-1 antibodies and their use in the preparation of a medicament for treating non-small cell lung cancer patients |
| WO2024151885A1 (en) | 2023-01-13 | 2024-07-18 | Iovance Biotherapeutics, Inc. | Use of til as maintenance therapy for nsclc patients who achieved pr/cr after prior therapy |
| WO2024192051A1 (en) | 2023-03-13 | 2024-09-19 | Turnstone Biologics Corp. | Composition of selected tumor infiltrating lymphocytes and related methods of producing and using the same |
| CN118077649B (en) * | 2024-02-29 | 2024-09-10 | 复旦大学附属中山医院 | Method for constructing atherosclerosis mouse model by immune checkpoint inhibitor |
Citations (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| EP0404097A2 (en) | 1989-06-22 | 1990-12-27 | BEHRINGWERKE Aktiengesellschaft | Bispecific and oligospecific, mono- and oligovalent receptors, production and applications thereof |
| WO1991010741A1 (en) | 1990-01-12 | 1991-07-25 | Cell Genesys, Inc. | Generation of xenogeneic antibodies |
| WO1993011161A1 (en) | 1991-11-25 | 1993-06-10 | Enzon, Inc. | Multivalent antigen-binding proteins |
| US5500362A (en) | 1987-01-08 | 1996-03-19 | Xoma Corporation | Chimeric antibody with specificity to human B cell surface antigen |
| US5545806A (en) | 1990-08-29 | 1996-08-13 | Genpharm International, Inc. | Ransgenic non-human animals for producing heterologous antibodies |
| US5545807A (en) | 1988-10-12 | 1996-08-13 | The Babraham Institute | Production of antibodies from transgenic animals |
| US5569825A (en) | 1990-08-29 | 1996-10-29 | Genpharm International | Transgenic non-human animals capable of producing heterologous antibodies of various isotypes |
| WO1996033735A1 (en) | 1995-04-27 | 1996-10-31 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| WO1996034096A1 (en) | 1995-04-28 | 1996-10-31 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| US5625126A (en) | 1990-08-29 | 1997-04-29 | Genpharm International, Inc. | Transgenic non-human animals for producing heterologous antibodies |
| US5633425A (en) | 1990-08-29 | 1997-05-27 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| US5641870A (en) | 1995-04-20 | 1997-06-24 | Genentech, Inc. | Low pH hydrophobic interaction chromatography for antibody purification |
| US5661016A (en) | 1990-08-29 | 1997-08-26 | Genpharm International Inc. | Transgenic non-human animals capable of producing heterologous antibodies of various isotypes |
| WO1998024893A2 (en) | 1996-12-03 | 1998-06-11 | Abgenix, Inc. | TRANSGENIC MAMMALS HAVING HUMAN IG LOCI INCLUDING PLURAL VH AND Vλ REGIONS AND ANTIBODIES PRODUCED THEREFROM |
| US5821337A (en) | 1991-06-14 | 1998-10-13 | Genentech, Inc. | Immunoglobulin variants |
| US6075181A (en) | 1990-01-12 | 2000-06-13 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| US6150584A (en) | 1990-01-12 | 2000-11-21 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| WO2001014557A1 (en) | 1999-08-23 | 2001-03-01 | Dana-Farber Cancer Institute, Inc. | Pd-1, a receptor for b7-4, and uses therefor |
| WO2002086083A2 (en) | 2001-04-20 | 2002-10-31 | Mayo Foundation For Medical Education And Research | Methods of enhancing cell responsiveness |
| WO2004042072A2 (en) | 2002-11-01 | 2004-05-21 | The Regents Of The University Of Colorado, A Body Corporate | Quantitative analysis of protein isoforms using matrix-assisted laser desorption/ionization time of flight mass spectrometry |
| WO2004092219A2 (en) | 2003-04-10 | 2004-10-28 | Protein Design Labs, Inc | Alteration of fcrn binding affinities or serum half-lives of antibodies by mutagenesis |
| EP1537878A1 (en) * | 2002-07-03 | 2005-06-08 | Ono Pharmaceutical Co., Ltd. | Immunopotentiating compositions |
| US6982321B2 (en) | 1986-03-27 | 2006-01-03 | Medical Research Council | Altered antibodies |
| US7087409B2 (en) | 1997-12-05 | 2006-08-08 | The Scripps Research Institute | Humanization of murine antibody |
| WO2007005874A2 (en) | 2005-07-01 | 2007-01-11 | Medarex, Inc. | Human monoclonal antibodies to programmed death ligand 1 (pd-l1) |
| WO2008083174A2 (en) * | 2006-12-27 | 2008-07-10 | Emory University | Compositions and methods for the treatment of infections and tumors |
| WO2010036959A2 (en) | 2008-09-26 | 2010-04-01 | Dana-Farber Cancer Institute | Human anti-pd-1, pd-l1, and pd-l2 antibodies and uses therefor |
| WO2010077634A1 (en) | 2008-12-09 | 2010-07-08 | Genentech, Inc. | Anti-pd-l1 antibodies and their use to enhance t-cell function |
| WO2011066389A1 (en) | 2009-11-24 | 2011-06-03 | Medimmmune, Limited | Targeted binding agents against b7-h1 |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2575791A1 (en) * | 2004-08-03 | 2006-02-16 | Dyax Corp. | Hk1-binding proteins |
| KR101050829B1 (en) * | 2008-10-02 | 2011-07-20 | 서울대학교산학협력단 | Anticancer agents comprising an anti-PD-1 antibody or an anti-PD-L1 antibody |
| US9115388B2 (en) * | 2011-11-01 | 2015-08-25 | H. Lee Moffitt Cancer Center And Research Institute, Inc. | Gene signature for the prediction of NF-kappaB activity |
| SI2785375T1 (en) | 2011-11-28 | 2020-11-30 | Merck Patent Gmbh | Anti-pd-l1 antibodies and uses thereof |
| AU2016222928B2 (en) * | 2015-02-26 | 2021-05-13 | Merck Patent Gmbh | PD-1 / PD-L1 inhibitors for the treatment of cancer |
| MY193229A (en) * | 2015-06-16 | 2022-09-26 | Merck Patent Gmbh | Pd-l1 antagonist combination treatments |
| WO2017058780A1 (en) * | 2015-09-30 | 2017-04-06 | Merck Patent Gmbh | Combination of a pd-1 axis binding antagonist and an alk inhibitor for treating alk-negative cancer |
| RS61029B1 (en) * | 2015-12-07 | 2020-12-31 | Merck Patent Gmbh | Aqueous pharmaceutical formulation comprising anti-pd-1 antibody avelumab |
| EP3464356A1 (en) * | 2016-05-26 | 2019-04-10 | Merck Patent GmbH | Pd-1 / pd-l1 inhibitors for cancer treatment |
-
2012
- 2012-11-21 SI SI201231849T patent/SI2785375T1/en unknown
- 2012-11-21 KR KR1020177021068A patent/KR101981873B1/en active IP Right Grant
- 2012-11-21 LT LTEP12790432.4T patent/LT2785375T/en unknown
- 2012-11-21 DK DK12790432.4T patent/DK2785375T3/en active
- 2012-11-21 SG SG11201402603WA patent/SG11201402603WA/en unknown
- 2012-11-21 EP EP20186754.6A patent/EP3763741A1/en not_active Withdrawn
- 2012-11-21 MX MX2014006316A patent/MX349096B/en active IP Right Grant
- 2012-11-21 CA CA2856895A patent/CA2856895C/en active Active
- 2012-11-21 KR KR1020147017974A patent/KR101764096B1/en active IP Right Grant
- 2012-11-21 PT PT127904324T patent/PT2785375T/en unknown
- 2012-11-21 EP EP12790432.4A patent/EP2785375B1/en active Active
- 2012-11-21 HU HUE12790432A patent/HUE051954T2/en unknown
- 2012-11-21 EA EA201400625A patent/EA036814B9/en active Protection Beyond IP Right Term
- 2012-11-21 AU AU2012344260A patent/AU2012344260B2/en active Active
- 2012-11-21 US US14/360,775 patent/US9624298B2/en active Active
- 2012-11-21 JP JP2014542728A patent/JP6138813B2/en active Active
- 2012-11-21 BR BR112014012819-7A patent/BR112014012819B1/en active IP Right Grant
- 2012-11-21 ES ES12790432T patent/ES2808152T3/en active Active
- 2012-11-21 RS RS20201263A patent/RS61033B1/en unknown
- 2012-11-21 CN CN201280058422.3A patent/CN103987405B/en active Active
- 2012-11-21 WO PCT/EP2012/004822 patent/WO2013079174A1/en active Application Filing
- 2012-11-28 AR ARP120104464A patent/AR089010A1/en active IP Right Grant
-
2014
- 2014-05-25 IL IL232778A patent/IL232778B/en active IP Right Grant
- 2014-06-27 ZA ZA2014/04790A patent/ZA201404790B/en unknown
-
2015
- 2015-02-10 HK HK15101432.7A patent/HK1200736A1/en unknown
-
2017
- 2017-03-09 US US15/454,959 patent/US10487147B2/en active Active
- 2017-03-09 US US15/454,939 patent/US10759856B2/en active Active
- 2017-03-27 JP JP2017061474A patent/JP2017158553A/en not_active Withdrawn
- 2017-11-30 AU AU2017268603A patent/AU2017268603B2/en active Active
-
2020
- 2020-08-04 US US16/984,365 patent/US11884724B2/en active Active
- 2020-08-09 IL IL276573A patent/IL276573A/en unknown
- 2020-10-07 HR HRP20201595TT patent/HRP20201595T1/en unknown
- 2020-10-08 CY CY20201100946T patent/CY1123435T1/en unknown
- 2020-12-18 NL NL301081C patent/NL301081I2/en unknown
- 2020-12-21 HU HUS2000056C patent/HUS2000056I1/en unknown
- 2020-12-23 CY CY2020045C patent/CY2020045I2/en unknown
- 2020-12-29 FR FR20C1068C patent/FR20C1068I2/en active Active
-
2021
- 2021-01-05 LT LTPA2021001C patent/LTC2785375I2/en unknown
- 2021-01-19 NO NO2021003C patent/NO2021003I1/en unknown
Patent Citations (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US6982321B2 (en) | 1986-03-27 | 2006-01-03 | Medical Research Council | Altered antibodies |
| US5500362A (en) | 1987-01-08 | 1996-03-19 | Xoma Corporation | Chimeric antibody with specificity to human B cell surface antigen |
| US5545807A (en) | 1988-10-12 | 1996-08-13 | The Babraham Institute | Production of antibodies from transgenic animals |
| EP0404097A2 (en) | 1989-06-22 | 1990-12-27 | BEHRINGWERKE Aktiengesellschaft | Bispecific and oligospecific, mono- and oligovalent receptors, production and applications thereof |
| US6075181A (en) | 1990-01-12 | 2000-06-13 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| US6150584A (en) | 1990-01-12 | 2000-11-21 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| WO1991010741A1 (en) | 1990-01-12 | 1991-07-25 | Cell Genesys, Inc. | Generation of xenogeneic antibodies |
| US5545806A (en) | 1990-08-29 | 1996-08-13 | Genpharm International, Inc. | Ransgenic non-human animals for producing heterologous antibodies |
| US5569825A (en) | 1990-08-29 | 1996-10-29 | Genpharm International | Transgenic non-human animals capable of producing heterologous antibodies of various isotypes |
| US5625126A (en) | 1990-08-29 | 1997-04-29 | Genpharm International, Inc. | Transgenic non-human animals for producing heterologous antibodies |
| US5633425A (en) | 1990-08-29 | 1997-05-27 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| US5661016A (en) | 1990-08-29 | 1997-08-26 | Genpharm International Inc. | Transgenic non-human animals capable of producing heterologous antibodies of various isotypes |
| US5821337A (en) | 1991-06-14 | 1998-10-13 | Genentech, Inc. | Immunoglobulin variants |
| WO1993011161A1 (en) | 1991-11-25 | 1993-06-10 | Enzon, Inc. | Multivalent antigen-binding proteins |
| US5641870A (en) | 1995-04-20 | 1997-06-24 | Genentech, Inc. | Low pH hydrophobic interaction chromatography for antibody purification |
| WO1996033735A1 (en) | 1995-04-27 | 1996-10-31 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| WO1996034096A1 (en) | 1995-04-28 | 1996-10-31 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| WO1998024893A2 (en) | 1996-12-03 | 1998-06-11 | Abgenix, Inc. | TRANSGENIC MAMMALS HAVING HUMAN IG LOCI INCLUDING PLURAL VH AND Vλ REGIONS AND ANTIBODIES PRODUCED THEREFROM |
| US7087409B2 (en) | 1997-12-05 | 2006-08-08 | The Scripps Research Institute | Humanization of murine antibody |
| WO2001014557A1 (en) | 1999-08-23 | 2001-03-01 | Dana-Farber Cancer Institute, Inc. | Pd-1, a receptor for b7-4, and uses therefor |
| WO2002086083A2 (en) | 2001-04-20 | 2002-10-31 | Mayo Foundation For Medical Education And Research | Methods of enhancing cell responsiveness |
| EP1537878A1 (en) * | 2002-07-03 | 2005-06-08 | Ono Pharmaceutical Co., Ltd. | Immunopotentiating compositions |
| WO2004042072A2 (en) | 2002-11-01 | 2004-05-21 | The Regents Of The University Of Colorado, A Body Corporate | Quantitative analysis of protein isoforms using matrix-assisted laser desorption/ionization time of flight mass spectrometry |
| WO2004092219A2 (en) | 2003-04-10 | 2004-10-28 | Protein Design Labs, Inc | Alteration of fcrn binding affinities or serum half-lives of antibodies by mutagenesis |
| WO2007005874A2 (en) | 2005-07-01 | 2007-01-11 | Medarex, Inc. | Human monoclonal antibodies to programmed death ligand 1 (pd-l1) |
| WO2008083174A2 (en) * | 2006-12-27 | 2008-07-10 | Emory University | Compositions and methods for the treatment of infections and tumors |
| WO2010036959A2 (en) | 2008-09-26 | 2010-04-01 | Dana-Farber Cancer Institute | Human anti-pd-1, pd-l1, and pd-l2 antibodies and uses therefor |
| WO2010077634A1 (en) | 2008-12-09 | 2010-07-08 | Genentech, Inc. | Anti-pd-l1 antibodies and their use to enhance t-cell function |
| WO2011066389A1 (en) | 2009-11-24 | 2011-06-03 | Medimmmune, Limited | Targeted binding agents against b7-h1 |
Non-Patent Citations (114)
| Title |
|---|
| "Basic and Clinical Immunology", 1994, APPLETON & LANGE, pages: 71 |
| "Peptide and Protein Drug Delivery", 1991, MARCEL DEKKER, INC., pages: 247 - 301 |
| BARBAS ET AL., PROC NAT. ACAD. SCL USA, vol. 91, 1994, pages 3809 - 3813 |
| BLANK CHRISTIAN ET AL: "Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro", INTERNATIONAL JOURNAL OF CANCER, JOHN WILEY & SONS, INC, NEW YORK, NY; US, vol. 119, no. 2, 1 July 2006 (2006-07-01), pages 317 - 327, XP002557386, ISSN: 0020-7136, DOI: 10.1002/IJC.21775 * |
| BOERNER, J. IMMUNOL, vol. 147, no. 1, 1991, pages 86 - 95 |
| BOETTLER ET AL., J. VIROL., vol. 80, 2006, pages 3532 - 40 |
| BRUGGEMANN ET AL., YEAR IN IMMUNOL., vol. 7, 1993, pages 33 |
| BULLOCK, A. N. ET AL.: "Thermodynamic stability of wild-type and mutant p53 core domain", PNAS, vol. 94, 1997, pages 14338 - 14342 |
| BUTTE ET AL., IMMUNITY, vol. 27, 2007, pages 111 - 22 |
| CAPEL ET AL., IMMUNOMETHODS, vol. 4, 1994, pages 25 - 34 |
| CARTER ET AL., EUR. J. IMMUNOL., vol. 32, 2002, pages 634 - 43 |
| CHEN ET AL., J. MOL BIOL, vol. 293, 1999, pages 865 - 881 |
| CHEN ET AL., J. MOL. BIOL., vol. 293, 1999, pages 865 - 881 |
| CHEN, Y. ET AL., J. MOL BIOL, vol. 293, 1999, pages 865 - 881 |
| CHOTHIA; LESK, J. MOL. BIOL., vol. 196, 1987, pages 901 - 917 |
| CLACKSON ET AL., NATURE, vol. 352, 1991, pages 624 - 628 |
| CLYNES, PNAS USA, vol. 95, 1998, pages 652 - 656 |
| COLE: "Monoclonal Antibodies and Cancer Therapy", 1985, ALAN R. LISS, pages: 77 |
| DE HAAS, J. LAB. CLIN. MED., vol. 126, 1995, pages 330 - 41 |
| DONG HAIDONG ET AL: "Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion", NATURE MEDICINE, NATURE PUBLISHING GROUP, NEW YORK, NY, US, vol. 8, no. 8, 24 June 2002 (2002-06-24), pages 793 - 800, XP002397368, ISSN: 1078-8956 * |
| DONG, NATURE MED., vol. 5, 1999, pages 1365 - 1369 |
| EPPIHIMER ET AL., MICROCIRCULATION, vol. 9, 2002, pages 133 - 45 |
| FELLOUSE, PROC. NATL. ACAD. SCL USA, vol. 101, no. 34, 2004, pages 12467 - 12472 |
| FISHWILD ET AL., NATURE BIOTECHNOL, vol. 14, 1996, pages 845 - 851 |
| FREEMAN ET AL., J. EXP. MED., vol. 192, 2000, pages 1 - 9 |
| FREEMAN ET AL., J. EXP. MED., vol. 192, 2000, pages 1027 - 34 |
| GHETIE ET AL., NATURE BIOTECHNOLOGY, vol. 15, no. 7, 1997, pages 637 - 40 |
| GHETIE; WARD, IMMUNOL. TODAY, vol. 18, no. 12, 1997, pages 592 - 8 |
| GIBBS, W. WAYT: "Nanobodies", SCIENTIFIC AMERICAN MAGAZINE, August 2005 (2005-08-01) |
| GUYER ET AL., J. IMMUNOL., vol. 117, 1976, pages 587 |
| HAMERS-CASTERMAN ET AL., NATURE, vol. 363, 1993, pages 446 - 448 |
| HAMMERLING: "Monoclonal Antibodies and T-Cell Hybridomas", 1981, ELSEVIER, pages: 563 - 681 |
| HARLOW ET AL.: "Antibodies: A Laboratory Manual", 1988, COLD SPRING HARBOR LABORATORY PRESS |
| HARRIS, BIOCHEM. SOC. TRANSACTIONS, vol. 23, 1995, pages 1035 - 1038 |
| HAWKINS ET AL., J. MOL. BIOL., vol. 226, 1992, pages 889 - 896 |
| HECKMAN ET AL., EUR. J. IMMUNOL., vol. 37, 2007, pages 1827 - 35 |
| HINTON, J. BIOL. CHEM., vol. TJI, no. 8, 2004, pages 6213 - 6 |
| HOLLINGER ET AL., PROC. NATL. ACAD. SCL USA, vol. 90, 1993, pages 6444 - 6448 |
| HOLT L J ET AL: "Domain antibodies: proteins for therapy", TRENDS IN BIOTECHNOLOGY, ELSEVIER PUBLICATIONS, CAMBRIDGE, GB, vol. 21, no. 11, 1 November 2003 (2003-11-01), pages 484 - 490, XP004467495, ISSN: 0167-7799, DOI: 10.1016/J.TIBTECH.2003.08.007 * |
| HONGO, HYBRIDOMA, vol. 14, no. 3, 1995, pages 253 - 260 |
| HOOGENBOOM; WINTER, J. MOL. BIOL, vol. 227, 1991, pages 381 |
| HURLE; GROSS, CURR. OP. BIOTECH., vol. 5, 1994, pages 428 - 433 |
| IWAI YOSHIKO ET AL: "Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade", PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES, NATIONAL ACADEMY OF SCIENCES, US, vol. 99, no. 19, 17 September 2002 (2002-09-17), pages 12293 - 12297, XP002398159, ISSN: 0027-8424, DOI: 10.1073/PNAS.192461099 * |
| JACKSON ET AL., J. IMMUNOL., vol. 154, no. 7, 1995, pages 3310 - 9 |
| JAKOBOVITS ET AL., NATURE, vol. 362, 1993, pages 255 - 258 |
| JAKOBOVITS ET AL., PROC. NATL. ACAD. SCL USA, vol. 90, 1993, pages 2551 |
| JOHNSON; WU: "Methods in Molecular Biology", vol. 248, 2003, HUMAN PRESS, pages: 1 - 25 |
| JONES ET AL., NATURE, vol. 321, 1986, pages 522 - 525 |
| JONES, A., ADV. DRUG DELIVERY REV., vol. 10, 1993, pages 29 - 90 |
| KABAT ET AL.: "Sequences of Proteins of Immunological Interest", 1991 |
| KABAT ET AL.: "Sequences of Proteins of Immunological Interest", 1991, NATIONAL INSTITUTES OF HEALTH |
| KABAT: "Sequences of Immunological Interest", 1991, NATIONAL INSTITUTE OF HEALTH |
| KEIR, ANNU. REV. IMMUNOL., vol. 26, 2008, pages 677 - 704 |
| KIM ET AL., J. IMMUNOL., vol. 24, 1994, pages 249 |
| KOHLER; MILSTEIN, NATURE, vol. 256, 1975, pages 495 - 97 |
| KUIPERS ET AL., EUR. J. IMMUNOL., vol. 36, 2006, pages 2472 - 82 |
| L. ZHANG ET AL: "PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model", BLOOD, vol. 114, no. 8, 20 August 2009 (2009-08-20), pages 1545 - 1552, XP055053821, ISSN: 0006-4971, DOI: 10.1182/blood-2009-03-206672 * |
| LATCHMAN ET AL., NATURE IMMUNOL, vol. 2, 2001, pages 261 - 268 |
| LATCHMAN ET AL., PROC. NATL. ACAD. SCI. USA, vol. 101, 2004, pages 10691 - 96 |
| LEE ET AL., FEBS LETT, vol. 580, 2006, pages 755 - 62 |
| LEE ET AL., J. IMMUNOL. METHODS, vol. 284, no. 1-2, 2004, pages 119 - 132 |
| LEE ET AL., J. MOL BIOL., vol. 340, no. 5, 2004, pages 1073 - 1093 |
| LI, PROC. NATL. ACAD. SCI. USA, vol. 103, 2006, pages 3557 - 3562 |
| LIANG, EUR. J. IMMUNOL., vol. 33, 2003, pages 2706 - 16 |
| LIN, D.Y.-W. ET AL., PNAS, vol. 105, 2008, pages 3011 - 6 |
| LIU ET AL., BLOOD, vol. HO, 2007, pages 296 - 304 |
| LIU, BLOOD, vol. HO, 2007, pages 296 - 304 |
| LOKE ET AL., PNAS, vol. 100, 2003, pages 5336 - 41 |
| LONBERG ET AL., NATURE, vol. 368, 1994, pages 856 - 859 |
| LONBERG; HUSZAR, INTERN. REV. IMMUNOL., vol. 13, 1995, pages 65 - 93 |
| M. DAERON, ANNU. REV. IMMUNOL., vol. 15, 1997, pages 203 - 234 |
| M. V. GOLDBERG ET AL: "Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells", BLOOD, vol. 110, no. 1, 1 July 2007 (2007-07-01), pages 186 - 192, XP055053809, ISSN: 0006-4971, DOI: 10.1182/blood-2006-12-062422 * |
| MARKS ET AL., BIOFTECHNOLOGY, vol. 10, 1992, pages 779 - 783 |
| MARKS ET AL., BIORRECHNOLOGY, vol. 10, 1992, pages 779 - 783 |
| MARKS, J. MOL BIOL., vol. 222, 1992, pages 581 - 597 |
| MARKS, J. MOL. BIOL, vol. 222, 1991, pages 581 |
| MORRISON ET AL., PROC. NATL. ACAD. SCL USA, vol. 81, 1984, pages 6851 - 6855 |
| MORRISON, NATURE, vol. 368, 1994, pages 812 - 813 |
| NEUBERGER, NATURE BIOTECHNOL., vol. 14, 1996, pages 826 |
| NGUYEN ET AL., J. EXP. MED., vol. 196, 2002, pages 1393 - 98 |
| NICOLAOU, ANGEW. CHEM INTL. ED. ENGL., vol. 33, 1994, pages 183 - 186 |
| NIELSEN ET AL., CELL. LMMUNOL., vol. 235, 2005, pages 109 - 16 |
| NISHIMURA ET AL., IMMUNITY JJ., 1999, pages 141 - 51 |
| NISHIMURA ET AL., INT. IMMUNOL., vol. 8, 1996, pages 773 - 80 |
| NISHIMURA ET AL., SCIENCE, vol. 291, 2001, pages 319 - 22 |
| NOMI TAKEO ET AL: "Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer", CLINICAL CANCER RESEARCH, THE AMERICAN ASSOCIATION FOR CANCER RESEARCH, US, vol. 13, no. 7, 1 April 2007 (2007-04-01), pages 2151 - 2157, XP002533527, ISSN: 1078-0432, DOI: 10.1158/1078-0432.CCR-06-2746 * |
| OKUDAIRA: "Blockade of B7-H1 or B7-DC induces an anti-tumor effect in a mouse pancreatic cancer model", INTERNATIONAL JOURNAL OF ONCOLOGY, vol. 35, no. 4, 1 September 2009 (2009-09-01), XP055052567, ISSN: 1019-6439, DOI: 10.3892/ijo_00000387 * |
| PARSA, NAT. MED., vol. 13, 2007, pages 84 - 88 |
| PLUCKTHUN: "The Pharmacology of Monoclonal Antibodies", vol. 113, 1994, SPRINGER- VERLAG, pages: 269 - 315 |
| PRESTA ET AL., CANCER RES., vol. 57, 1997, pages 4593 - 4599 |
| PRESTA, CURR. OP. STRUCT. BIOL., vol. 2, 1992, pages 593 - 596 |
| RADHAKRISHNAN ET AL., CANCER RES., vol. 64, 2004, pages 4965 - 72 |
| RADHAKRISHNAN ET AL., J. ALLERGY CLIN. IMMUNOL., 2005, pages 668 - 74 |
| RADHAKRISHNAN ET AL., J. IMMUNOL., vol. 170, 2003, pages 1830 - 38 |
| RADHAKRISHNAN, J. LMMUNOL., vol. 173, 2004, pages 1360 - 65 |
| RAVETCH; KINET, ANNU. REV. IMMUNOL., vol. 9, 1991, pages 457 - 92 |
| RIECHMANN ET AL., NATURE, vol. 332, 1988, pages 323 - 329 |
| SCHIER ET AL., GENE, vol. 169, 1995, pages 147 - 155 |
| SCHREINER ET AL., J. NEUROIMMUNOL, vol. 155, 2004, pages 172 - 82 |
| SHERIFF ET AL., NATURE STRUCT. BIOL., vol. 3, 1996, pages 733 - 736 |
| SHIELDS ET AL., J. BIOL. CHEM., vol. 9, no. 2, 2001, pages 6591 - 6604 |
| SIDHU ET AL., J. MOL BIOL., vol. 338, no. 2, 2004, pages 299 - 310 |
| SOPHIE R. SIERRO ET AL: "Combination of lentivector immunization and low-dose chemotherapy or PD-1/PD-L1 blocking primes self-reactive T cells and induces anti-tumor immunity", EUROPEAN JOURNAL OF IMMUNOLOGY, vol. 41, no. 8, 1 August 2011 (2011-08-01), pages 2217 - 2228, XP055053701, ISSN: 0014-2980, DOI: 10.1002/eji.201041235 * |
| TSENG, J. EXP. MED., vol. 193, 2001, pages 839 - 846 |
| UEDA ET AL., NATURE, vol. 423, 2003, pages 506 - 11 |
| VAN DIJK; VAN DE WINKEL, CURR. OPIN. PHARMACOL, vol. 5, 2001, pages 368 - 74 |
| VASWANI; HAMILTON, ANN. ALLERGY, ASTHMA & IMMUNOL., vol. 1, 1998, pages 105 - 115 |
| WAN ET AL., J. IMMUNOL., vol. 177, 2006, pages 8844 - 50 |
| WELLS. J.A., PNAS, vol. 93, 1996, pages 1 - 6 |
| XU ET AL., IMMUNITY, vol. 13, 2000, pages 37 - 45 |
| YAMAZAKI ET AL., J. IMMUNOL., vol. 169, 2002, pages 5538 - 45 |
| YDXON ET AL., J. IMMUNOL., vol. 155, 1995, pages 1994 - 2004 |
| ZAPATA ET AL., PROTEIN ENG., 1995, pages 1057 - 1062 |
| ZHONG ET AL., EUR. J. IMMUNOL., vol. 37, 2007, pages 2405 - 10 |
Cited By (970)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9920123B2 (en) | 2008-12-09 | 2018-03-20 | Genentech, Inc. | Anti-PD-L1 antibodies, compositions and articles of manufacture |
| US11142800B2 (en) | 2010-10-07 | 2021-10-12 | The General Hospital Corporation | Biomarkers of cancer |
| US12006347B2 (en) | 2010-12-22 | 2024-06-11 | The Board Of Trustees Of The Leland Stanford Junior University | Superagonists and antagonists of interleukin-2 |
| US10626155B2 (en) | 2011-06-24 | 2020-04-21 | Cytune Pharma | IL-15 and IL-15R\alpha sushi domain based immunocytokines |
| US10899816B2 (en) | 2011-06-24 | 2021-01-26 | Inserm (Institut National De La Santé Et De La Recherche Medicale) | IL-15 and IL-15Rα sushi domain based immunocytokines |
| US11753454B2 (en) | 2011-06-24 | 2023-09-12 | Cytune Pharma | IL-15 and IL-15R\alpha sushi domain based immunocytokines |
| US10875864B2 (en) | 2011-07-21 | 2020-12-29 | Sumitomo Dainippon Pharma Oncology, Inc. | Substituted imidazo[1,2-B]pyridazines as protein kinase inhibitors |
| US9895441B2 (en) | 2012-05-31 | 2018-02-20 | Genentech, Inc. | Methods of treating cancer using PD-L1 axis binding antagonists and VEGF antagonists |
| US11226339B2 (en) * | 2012-12-11 | 2022-01-18 | Albert Einstein College Of Medicine | Methods for high throughput receptor:ligand identification |
| EP2944323B1 (en) | 2013-01-11 | 2019-03-27 | Dingfu Biotarget Co., Ltd | Agents for treating tumours, use and method thereof |
| US11299544B2 (en) | 2013-03-15 | 2022-04-12 | Genentech, Inc. | Biomarkers and methods of treating PD-1 and PD-L1 related conditions |
| US9192667B2 (en) | 2013-04-22 | 2015-11-24 | Hoffmann-La Roche Inc. | Method of treating cancer by administering CSF-1R antibodies and a TLR9 agonist |
| WO2014195852A1 (en) | 2013-06-03 | 2014-12-11 | Glaxosmithkline Intellectual Property (No.2) Limited | Combinations of an anti-pd-l1 antibody and a mek inhibitor and/or a braf inhibitor |
| WO2015036499A1 (en) * | 2013-09-11 | 2015-03-19 | Medimmune Limited | Anti-b7-h1 antibodies for treating tumors |
| IL274462A (en) * | 2013-09-11 | 2020-06-30 | Medimmune Ltd | Anti-b7-h1 antibodies for treating tumors |
| US10336823B2 (en) | 2013-09-11 | 2019-07-02 | Medimmune Limited | Anti-B7-H1 antibodies for treating tumors |
| US10829557B2 (en) | 2013-09-11 | 2020-11-10 | Medimmune Limited | Anti-B7-H1 antibodies for treating tumors |
| KR20160044030A (en) * | 2013-09-11 | 2016-04-22 | 메디뮨 리미티드 | Anti-b7-h1 antibodies for treating tumors |
| CN105873606A (en) * | 2013-09-11 | 2016-08-17 | 米迪缪尼有限公司 | Anti-b7-h1 antibodies for treating tumors |
| RU2701327C2 (en) * | 2013-09-11 | 2019-09-25 | Медиммьюн Лимитед | Antibodies to b7-h1 for treating tumours |
| KR102329584B1 (en) | 2013-09-11 | 2021-11-22 | 메디뮨 리미티드 | Anti-b7-h1 antibodies for treating tumors |
| CN105873606B (en) * | 2013-09-11 | 2020-12-29 | 米迪缪尼有限公司 | anti-B7-H1 antibodies for the treatment of tumors |
| EP3656398A1 (en) * | 2013-09-11 | 2020-05-27 | MedImmune Limited | Anti-b7-h1 antibodies for treating tumors |
| US11827706B2 (en) | 2013-09-11 | 2023-11-28 | Medimmune Limited | Anti-B7-H1 antibodies for treating tumors |
| WO2015036511A1 (en) * | 2013-09-12 | 2015-03-19 | F. Hoffmann-La Roche Ag | Combination therapy of antibodies against human csf-1r and antibodies against human pd-l1 |
| US11512133B2 (en) | 2013-09-12 | 2022-11-29 | Hoffmann-La Roche Inc. | Methods for treating colon cancer or inhibiting cell proliferation by administering a combination of antibodies against human CSF-1R and antibodies against human PD-L1 |
| JP2016531150A (en) * | 2013-09-12 | 2016-10-06 | エフ・ホフマン−ラ・ロシュ・アクチェンゲゼルシャフト | Combination therapy of antibody against human CSF-1R and antibody against human PD-L1 |
| US11673951B2 (en) | 2013-09-13 | 2023-06-13 | Beigene Switzerland Gmbh | Anti-PD1 antibodies and their use as therapeutics and diagnostics |
| US11186637B2 (en) | 2013-09-13 | 2021-11-30 | Beigene Switzerland Gmbh | Anti-PD1 antibodies and their use as therapeutics and diagnostics |
| US11680090B2 (en) | 2013-09-24 | 2023-06-20 | Medicenna Therapeutics, Inc. | Interleukin-2 fusion proteins and uses thereof |
| US11708412B2 (en) | 2013-09-26 | 2023-07-25 | Novartis Ag | Methods for treating hematologic cancers |
| US10570204B2 (en) | 2013-09-26 | 2020-02-25 | The Medical College Of Wisconsin, Inc. | Methods for treating hematologic cancers |
| WO2015048520A1 (en) * | 2013-09-27 | 2015-04-02 | Genentech, Inc. | Anti-pdl1 antibody formulations |
| EA033817B1 (en) * | 2013-09-27 | 2019-11-28 | Genentech Inc | Anti-pdl1 antibody formulations |
| US10875922B2 (en) | 2013-09-27 | 2020-12-29 | Genentech, Inc. | Anti-PDL1 antibody formulations |
| AU2014339900B2 (en) * | 2013-10-25 | 2019-10-24 | Dana-Farber Cancer Institute, Inc. | Anti-PD-L1 monoclonal antibodies and fragments thereof |
| WO2015061668A1 (en) * | 2013-10-25 | 2015-04-30 | Dana-Farber Cancer Institute, Inc. | Anti-pd-l1 monoclonal antibodies and fragments thereof |
| US10202454B2 (en) | 2013-10-25 | 2019-02-12 | Dana-Farber Cancer Institute, Inc. | Anti-PD-L1 monoclonal antibodies and fragments thereof |
| WO2015066413A1 (en) | 2013-11-01 | 2015-05-07 | Novartis Ag | Oxazolidinone hydroxamic acid compounds for the treatment of bacterial infections |
| EP3083686B1 (en) | 2013-12-17 | 2019-10-23 | F.Hoffmann-La Roche Ag | Methods of treating cancers using pd-1 axis binding antagonists and taxanes |
| EP3466949A1 (en) | 2013-12-24 | 2019-04-10 | Bristol-Myers Squibb Company | Tricyclic compound as anticancer agents |
| WO2015100282A1 (en) | 2013-12-24 | 2015-07-02 | Bristol-Myers Squibb Company | Tricyclic compounds as anticancer agents |
| AU2015206603B2 (en) * | 2014-01-14 | 2019-07-11 | Dana-Farber Cancer Institute, Inc. | Compositions and methods for identification, assessment, prevention, and treatment of melanoma using PD-L1 isoforms |
| US11584788B2 (en) | 2014-01-14 | 2023-02-21 | Dana-Farber Cancer Institute, Inc. | Compositions and methods for identification, assessment, prevention, and treatment of melanoma using PD-L1 isoforms |
| AU2015206603B9 (en) * | 2014-01-14 | 2019-07-18 | Dana-Farber Cancer Institute, Inc. | Compositions and methods for identification, assessment, prevention, and treatment of melanoma using PD-L1 isoforms |
| EP3094736A4 (en) * | 2014-01-14 | 2017-10-25 | Dana-Farber Cancer Institute, Inc. | Compositions and methods for identification, assessment, prevention, and treatment of melanoma using pd-l1 isoforms |
| EP4079321A1 (en) * | 2014-01-15 | 2022-10-26 | Kadmon Corporation, LLC | Immunomodulatory agents |
| CN105263521A (en) * | 2014-01-15 | 2016-01-20 | 卡德门企业有限公司 | Immunomodulatory agents |
| CN113637692A (en) * | 2014-01-15 | 2021-11-12 | 卡德门企业有限公司 | Immunomodulator |
| CN113603788A (en) * | 2014-01-15 | 2021-11-05 | 卡德门企业有限公司 | Immunomodulator |
| JP2017509319A (en) * | 2014-01-15 | 2017-04-06 | カドモン コーポレイション,リミティド ライアビリティ カンパニー | Immunomodulator |
| CN105263521B (en) * | 2014-01-15 | 2021-06-29 | 卡德门企业有限公司 | Immunomodulator |
| WO2015109124A3 (en) * | 2014-01-15 | 2015-09-24 | Kadmon Corporation, Llc | Immunomodulatory agents |
| EP3094351A4 (en) * | 2014-01-15 | 2017-06-28 | Kadmon Corporation, LLC | Immunomodulatory agents |
| US10407502B2 (en) | 2014-01-15 | 2019-09-10 | Kadmon Corporation, Llc | Immunomodulatory agents |
| WO2015107495A1 (en) | 2014-01-17 | 2015-07-23 | Novartis Ag | N-azaspirocycloalkane substituted n-heteroaryl compounds and compositions for inhibiting the activity of shp2 |
| US10737113B2 (en) | 2014-01-23 | 2020-08-11 | Regeneron Pharmaceuticals, Inc. | Human antibodies to PD-1 |
| US9938345B2 (en) | 2014-01-23 | 2018-04-10 | Regeneron Pharmaceuticals, Inc. | Human antibodies to PD-L1 |
| US9987500B2 (en) | 2014-01-23 | 2018-06-05 | Regeneron Pharmaceuticals, Inc. | Human antibodies to PD-1 |
| US11117970B2 (en) | 2014-01-23 | 2021-09-14 | Regeneron Pharmaceuticals, Inc. | Human antibodies to PD-L1 |
| US10441654B2 (en) | 2014-01-24 | 2019-10-15 | Children's Hospital Of Eastern Ontario Research Institute Inc. | SMC combination therapy for the treatment of cancer |
| US11827704B2 (en) | 2014-01-24 | 2023-11-28 | Novartis Ag | Antibody molecules to PD-1 and uses thereof |
| US9815898B2 (en) | 2014-01-24 | 2017-11-14 | Novartis Ag | Antibody molecules to PD-1 and uses thereof |
| US9683048B2 (en) | 2014-01-24 | 2017-06-20 | Novartis Ag | Antibody molecules to PD-1 and uses thereof |
| US10752687B2 (en) | 2014-01-24 | 2020-08-25 | Novartis Ag | Antibody molecules to PD-1 and uses thereof |
| US9605070B2 (en) | 2014-01-31 | 2017-03-28 | Novartis Ag | Antibody molecules to TIM-3 and uses thereof |
| US10981990B2 (en) | 2014-01-31 | 2021-04-20 | Novartis Ag | Antibody molecules to TIM-3 and uses thereof |
| US11155620B2 (en) | 2014-01-31 | 2021-10-26 | Novartis Ag | Method of detecting TIM-3 using antibody molecules to TIM-3 |
| US10472419B2 (en) | 2014-01-31 | 2019-11-12 | Novartis Ag | Antibody molecules to TIM-3 and uses thereof |
| US9884913B2 (en) | 2014-01-31 | 2018-02-06 | Novartis Ag | Antibody molecules to TIM-3 and uses thereof |
| KR102363008B1 (en) | 2014-02-10 | 2022-02-16 | 메르크 파텐트 게엠베하 | TARGETED TGFβ INHIBITION |
| WO2015118175A3 (en) * | 2014-02-10 | 2015-10-08 | Merck Patent Gmbh | TARGETED TGFβ INHIBITION |
| US9676863B2 (en) | 2014-02-10 | 2017-06-13 | Merck Patent Gmbh | Targeted TGFβ inhibitors |
| CN106103488A (en) * | 2014-02-10 | 2016-11-09 | 默克专利有限公司 | Targeting TGF β suppresses |
| CN113549159A (en) * | 2014-02-10 | 2021-10-26 | 默克专利有限公司 | Targeted TGF-beta inhibition |
| KR20160119197A (en) * | 2014-02-10 | 2016-10-12 | 메르크 파텐트 게엠베하 | Tgf targeted tgf inhibition |
| EP3889172A1 (en) | 2014-02-10 | 2021-10-06 | Merck Patent GmbH | Targeted tgf beta inhibition |
| WO2015118175A2 (en) | 2014-02-10 | 2015-08-13 | Merck Patent Gmbh | TARGETED TGFβ INHIBITION |
| JP2020174674A (en) * | 2014-02-10 | 2020-10-29 | メルク パテント ゲーエムベーハー | TARGETED TGFβ INHIBITION |
| JP2017506217A (en) * | 2014-02-10 | 2017-03-02 | メルク パテント ゲーエムベーハー | Target TGFβ inhibition |
| US11370819B2 (en) | 2014-02-10 | 2022-06-28 | Merck Patent Gmbh | Targeted TGFβ inhibition |
| US11773175B2 (en) | 2014-03-04 | 2023-10-03 | Kymab Limited | Antibodies, uses and methods |
| US11753479B2 (en) | 2014-03-04 | 2023-09-12 | Kymab Limited | Nucleic acids encoding anti-OX40L antibodies |
| WO2015138920A1 (en) | 2014-03-14 | 2015-09-17 | Novartis Ag | Antibody molecules to lag-3 and uses thereof |
| EP3660050A1 (en) | 2014-03-14 | 2020-06-03 | Novartis AG | Antibody molecules to lag-3 and uses thereof |
| WO2015148379A1 (en) | 2014-03-24 | 2015-10-01 | Novartis Ag | Monobactam organic compounds for the treatment of bacterial infections |
| EP3511328A1 (en) | 2014-03-24 | 2019-07-17 | Novartis AG | Monobactam organic compounds for the treatment of bacterial infections |
| US11384131B2 (en) | 2014-04-24 | 2022-07-12 | The Board Of Trustees Of The Leland Stanford Junior University | Superagonists, partial agonists and antagonists of interleukin-2 |
| US9885721B2 (en) | 2014-05-29 | 2018-02-06 | Spring Bioscience Corporation | PD-L1 antibodies and uses thereof |
| US10775383B2 (en) | 2014-05-29 | 2020-09-15 | Ventana Medical Systems, Inc. | PD-L1 antibodies and uses thereof |
| EP3998079A1 (en) | 2014-06-06 | 2022-05-18 | Bristol-Myers Squibb Company | Antibodies against glucocorticoid-induced tumor necrosis factor receptor (gitr) and uses thereof |
| US10987322B2 (en) | 2014-06-06 | 2021-04-27 | Flexus Biosciences, Inc. | Immunoregulatory agents |
| WO2015187835A2 (en) | 2014-06-06 | 2015-12-10 | Bristol-Myers Squibb Company | Antibodies against glucocorticoid-induced tumor necrosis factor receptor (gitr) and uses thereof |
| EP3610924A1 (en) | 2014-06-06 | 2020-02-19 | Bristol-Myers Squibb Company | Antibodies against glucocorticoid-induced tumor necrosis factor receptor (gitr) and uses thereof |
| WO2015193352A1 (en) | 2014-06-17 | 2015-12-23 | Medimmune Limited | Methods of cancer treatment with antagonists against pd-1 and pd-l1 in combination with radiation therapy |
| EP3157629B1 (en) * | 2014-06-17 | 2022-10-05 | MedImmune Limited | Methods of cancer treatment with antagonists against pd-1 and pd-l1 in combination with radiation therapy |
| EP4144412A1 (en) * | 2014-06-17 | 2023-03-08 | MedImmune Limited | Methods of cancer treatment with antagonists against pd-1 and pd-l1 in combination with radiation therapy |
| CN107073107A (en) * | 2014-06-17 | 2017-08-18 | 免疫医疗有限公司 | Use the cancer treatment method of the combination of antagonist and radiotherapy for PD 1 and PD L1 |
| EP3157629A1 (en) * | 2014-06-17 | 2017-04-26 | Medimmune Limited | Methods of cancer treatment with antagonists against pd-1 and pd-l1 in combination with radiation therapy |
| US11512132B2 (en) | 2014-07-03 | 2022-11-29 | Beigene, Ltd. | Anti-PD-L1 antibodies and their use as therapeutics and diagnostics |
| JP2017522905A (en) * | 2014-07-03 | 2017-08-17 | ベイジーン リミテッド | Anti-PD-L1 antibodies and their use for therapy and diagnosis |
| JP2019172677A (en) * | 2014-07-03 | 2019-10-10 | ベイジーン リミテッド | Anti-pd-l1 antibodies and their use for treatment and diagnosis |
| EP4001311A1 (en) | 2014-07-09 | 2022-05-25 | Birdie Biopharmaceuticals Inc. | Anti-pd-l1 combinations for treating tumors |
| WO2016004876A1 (en) | 2014-07-09 | 2016-01-14 | Shanghai Birdie Biotech, Inc. | Anti-pd-l1 combinations for treating tumors |
| US20170100425A1 (en) * | 2014-07-10 | 2017-04-13 | Biothera, Inc. | Beta-glucan in combination with anti-cancer agents affecting the tumor microenvironment |
| US10111901B2 (en) * | 2014-07-10 | 2018-10-30 | Biothera, Inc. | Beta-glucan in combination with anti-cancer agents affecting the tumor microenvironment |
| US11530269B2 (en) | 2014-07-11 | 2022-12-20 | Ventana Medical Systems, Inc. | Anti-PD-L1 antibodies and diagnostic uses thereof |
| US11560429B2 (en) | 2014-07-22 | 2023-01-24 | Apollomics Inc. | Anti PD-1 antibodies |
| US10981994B2 (en) | 2014-07-22 | 2021-04-20 | Apollomics Inc. | Anti PD-1 antibodies |
| US10428146B2 (en) | 2014-07-22 | 2019-10-01 | Cb Therapeutics, Inc. | Anti PD-1 antibodies |
| US11111300B2 (en) | 2014-08-05 | 2021-09-07 | Apollomics Inc. | Anti PD-L1 antibodies |
| JP7144477B2 (en) | 2014-08-05 | 2022-09-29 | アポロミクス インコーポレイテッド | Anti-PD-L1 antibody |
| US10435470B2 (en) | 2014-08-05 | 2019-10-08 | Cb Therapeutics, Inc. | Anti-PD-L1 antibodies |
| US11827707B2 (en) | 2014-08-05 | 2023-11-28 | Apollomics Inc. | Anti PD-L1 antibodies |
| EP3177649A4 (en) * | 2014-08-05 | 2018-04-25 | CB Therapeutics, Inc. | Anti-pd-l1 antibodies |
| KR20170039706A (en) * | 2014-08-05 | 2017-04-11 | 씨비 테라퓨틱스, 인코포레이티드 | Anti-pd-l1 antibodies |
| JP2020141672A (en) * | 2014-08-05 | 2020-09-10 | シービー セラピューティックス インコーポレイテッド | Anti-pd-l1 antibodies |
| JP2017523786A (en) * | 2014-08-05 | 2017-08-24 | シービー セラピューティックス インコーポレイテッド | Anti-PD-L1 antibody |
| KR102476226B1 (en) | 2014-08-05 | 2022-12-12 | 아폴로믹스 인코포레이티드 | Anti-pd-l1 antibodies |
| WO2016020836A1 (en) | 2014-08-06 | 2016-02-11 | Novartis Ag | Quinolone derivatives as antibacterials |
| EP3643727A1 (en) | 2014-08-15 | 2020-04-29 | Merck Patent GmbH | Sirp-alpha immunoglobulin fusion proteins |
| US11021694B2 (en) | 2014-08-15 | 2021-06-01 | Merck Patent Gmbh | SIRP-α immunoglobulin fusion proteins |
| EP4148069A1 (en) | 2014-09-01 | 2023-03-15 | Birdie Biopharmaceuticals Inc. | Anti-pd-l1 conjugates for treating tumors |
| EP3763742A1 (en) | 2014-09-01 | 2021-01-13 | Birdie Biopharmaceuticals Inc. | Anti-pd-l1 conjugates for treating tumors |
| EP3659621A1 (en) | 2014-09-13 | 2020-06-03 | Novartis AG | Combination therapies for cancer |
| WO2016040880A1 (en) | 2014-09-13 | 2016-03-17 | Novartis Ag | Combination therapies of alk inhibitors |
| WO2016040892A1 (en) | 2014-09-13 | 2016-03-17 | Novartis Ag | Combination therapies |
| EP3925622A1 (en) | 2014-09-13 | 2021-12-22 | Novartis AG | Combination therapies |
| US11344620B2 (en) | 2014-09-13 | 2022-05-31 | Novartis Ag | Combination therapies |
| AU2015326996B2 (en) * | 2014-09-30 | 2021-05-20 | Intervet International B.V. | PD-L1 antibodies binding canine PD-L1 |
| US10550194B2 (en) | 2014-09-30 | 2020-02-04 | Intervet Inc. | PD-L1 antibodies binding canine PD-L1 |
| WO2016050721A1 (en) * | 2014-09-30 | 2016-04-07 | Intervet International B.V. | Pd-l1 antibodies binding canine pd-l1 |
| US11447561B2 (en) | 2014-09-30 | 2022-09-20 | Intervet, Inc. | PD-L1 antibodies binding canine PD-L1 |
| CN112851816A (en) * | 2014-09-30 | 2021-05-28 | 英特维特国际股份有限公司 | PD-L1 antibodies that bind canine PD-L1 |
| CN112851816B (en) * | 2014-09-30 | 2024-03-08 | 英特维特国际股份有限公司 | PD-L1 antibodies that bind canine PD-L1 |
| AU2021218094B2 (en) * | 2014-09-30 | 2023-04-20 | Intervet International B.V. | PD-L1 antibodies binding canine PD-L1 |
| EP3662903A2 (en) | 2014-10-03 | 2020-06-10 | Novartis AG | Combination therapies |
| WO2016054555A2 (en) | 2014-10-03 | 2016-04-07 | Novartis Ag | Combination therapies |
| WO2016057846A1 (en) | 2014-10-08 | 2016-04-14 | Novartis Ag | Compositions and methods of use for augmented immune response and cancer therapy |
| WO2016057841A1 (en) | 2014-10-08 | 2016-04-14 | Novartis Ag | Compositions and methods of use for augmented immune response and cancer therapy |
| EP4029508A1 (en) | 2014-10-10 | 2022-07-20 | Idera Pharmaceuticals, Inc. | Treatment of cancer using tlr9 agonists and checkpoint inhibitors |
| US10851165B2 (en) | 2014-10-14 | 2020-12-01 | Novartis Ag | Antibody molecules to PD-L1 and methods of treating cancer |
| US9988452B2 (en) | 2014-10-14 | 2018-06-05 | Novartis Ag | Antibody molecules to PD-L1 and uses thereof |
| WO2016061142A1 (en) | 2014-10-14 | 2016-04-21 | Novartis Ag | Antibody molecules to pd-l1 and uses thereof |
| EP4245376A2 (en) | 2014-10-14 | 2023-09-20 | Novartis AG | Antibody molecules to pd-l1 and uses thereof |
| US9643972B2 (en) | 2014-11-05 | 2017-05-09 | Flexus Biosciences, Inc. | Immunoregulatory agents |
| US9598422B2 (en) | 2014-11-05 | 2017-03-21 | Flexus Biosciences, Inc. | Immunoregulatory agents |
| US10106546B2 (en) | 2014-11-05 | 2018-10-23 | Flexus Biosciences, Inc. | Immunoregulatory agents |
| US11242319B2 (en) | 2014-11-05 | 2022-02-08 | Flexus Biosciences, Inc. | Immunoregulatory agents |
| US11932601B2 (en) | 2014-11-05 | 2024-03-19 | Flexus Biosciences, Inc. | Immunoregulatory agents |
| US10533014B2 (en) | 2014-11-05 | 2020-01-14 | Flexus Biosciences, Inc. | Immunoregulatory agents |
| US10206893B2 (en) | 2014-11-05 | 2019-02-19 | Flexus Biosciences, Inc. | Immunoregulatory agents |
| EP3854394A1 (en) | 2014-11-05 | 2021-07-28 | Flexus Biosciences, Inc. | Immunoregulatory agents |
| US11135245B2 (en) | 2014-11-17 | 2021-10-05 | Adicet Bio, Inc. | Engineered γδ T-cells |
| WO2016081518A3 (en) * | 2014-11-17 | 2016-08-18 | Adicet Bio, Inc. | Engineered gamma delta t-cells |
| US10881086B2 (en) | 2014-12-09 | 2021-01-05 | Regeneron Pharmaceuticals, Inc. | Genetically modified mouse whose genome comprises a humanized CD274 gene |
| US12089575B2 (en) | 2014-12-09 | 2024-09-17 | Regeneron Pharmaceuticals, Inc. | Genetically modified mouse that expresses humanized PD1 and PD-L1 proteins |
| US9913461B2 (en) | 2014-12-09 | 2018-03-13 | Regeneron Pharmaceuticals, Inc. | Genetically modified mouse whose genome comprises a humanized CD274 gene |
| WO2016097995A1 (en) | 2014-12-16 | 2016-06-23 | Novartis Ag | Isoxazole hydroxamic acid compounds as lpxc inhibitors |
| WO2016100882A1 (en) | 2014-12-19 | 2016-06-23 | Novartis Ag | Combination therapies |
| WO2016106266A1 (en) | 2014-12-22 | 2016-06-30 | Bristol-Myers Squibb Company | TGFβ RECEPTOR ANTAGONISTS |
| EP4249066A2 (en) | 2014-12-23 | 2023-09-27 | Bristol-Myers Squibb Company | Antibodies to tigit |
| WO2016123454A1 (en) | 2015-01-29 | 2016-08-04 | Board Of Trustees Of Miching State University | Cryptic polypeptides and uses thereof |
| US10620211B2 (en) | 2015-02-03 | 2020-04-14 | Ventana Medical Systems, Inc. | Histochemical assay for evaluating expression of programmed death ligand 1 (PD-L1) |
| WO2016127052A1 (en) | 2015-02-05 | 2016-08-11 | Bristol-Myers Squibb Company | Cxcl11 and smica as predictive biomarkers for efficacy of anti-ctla4 immunotherapy |
| US11000548B2 (en) | 2015-02-18 | 2021-05-11 | Enlivex Therapeutics Ltd | Combination immune therapy and cytokine control therapy for cancer treatment |
| US11304976B2 (en) | 2015-02-18 | 2022-04-19 | Enlivex Therapeutics Ltd | Combination immune therapy and cytokine control therapy for cancer treatment |
| US11717539B2 (en) | 2015-02-18 | 2023-08-08 | Enlivex Therapeutics RDO Ltd. | Combination immune therapy and cytokine control therapy for cancer treatment |
| US11318163B2 (en) | 2015-02-18 | 2022-05-03 | Enlivex Therapeutics Ltd | Combination immune therapy and cytokine control therapy for cancer treatment |
| US11596652B2 (en) | 2015-02-18 | 2023-03-07 | Enlivex Therapeutics R&D Ltd | Early apoptotic cells for use in treating sepsis |
| US11497767B2 (en) | 2015-02-18 | 2022-11-15 | Enlivex Therapeutics R&D Ltd | Combination immune therapy and cytokine control therapy for cancer treatment |
| US11512289B2 (en) | 2015-02-18 | 2022-11-29 | Enlivex Therapeutics Rdo Ltd | Combination immune therapy and cytokine control therapy for cancer treatment |
| RU2714233C2 (en) * | 2015-02-26 | 2020-02-13 | Мерк Патент Гмбх | Pd-1/pd-l1 inhibitors for treating cancer |
| WO2016137985A1 (en) | 2015-02-26 | 2016-09-01 | Merck Patent Gmbh | Pd-1 / pd-l1 inhibitors for the treatment of cancer |
| EP4279087A2 (en) | 2015-02-26 | 2023-11-22 | Merck Patent GmbH | Pd-1 / pd-l1 inhibitors for the treatment of cancer |
| US10800846B2 (en) | 2015-02-26 | 2020-10-13 | Merck Patent Gmbh | PD-1/PD-L1 inhibitors for the treatment of cancer |
| RU2742312C1 (en) * | 2015-02-26 | 2021-02-04 | Мерк Патент Гмбх | Pd-1/pd-l1 inhibitors for treating cancer |
| WO2016140884A1 (en) | 2015-03-02 | 2016-09-09 | Rigel Pharmaceuticals, Inc. | TGF-β INHIBITORS |
| WO2016145085A2 (en) | 2015-03-09 | 2016-09-15 | Celldex Therapeutics, Inc. | Cd27 agonists |
| US11040053B2 (en) | 2015-03-10 | 2021-06-22 | Chinook Therapeutics, Inc. | Compositions and methods for activating “stimulator of interferon gene”13 dependent signalling |
| US10449211B2 (en) | 2015-03-10 | 2019-10-22 | Aduro Biotech, Inc. | Compositions and methods for activating “stimulator of interferon gene”—dependent signalling |
| WO2016145102A1 (en) | 2015-03-10 | 2016-09-15 | Aduro Biotech, Inc. | Compositions and methods for activating "stimulator of interferon gene" -dependent signalling |
| US11174316B2 (en) | 2015-03-13 | 2021-11-16 | Cytomx Therapeutics, Inc. | Anti-PDL1 antibodies, activatable anti-PDL1 antibodies, and methods of use thereof |
| EP3277320A4 (en) * | 2015-03-30 | 2018-08-01 | Stcube, Inc. | Antibodies specific to glycosylated pd-l1 and methods of use thereof |
| US11933786B2 (en) | 2015-03-30 | 2024-03-19 | Stcube, Inc. | Antibodies specific to glycosylated PD-L1 and methods of use thereof |
| US10836827B2 (en) | 2015-03-30 | 2020-11-17 | Stcube, Inc. | Antibodies specific to glycosylated PD-L1 and methods of use thereof |
| US10399933B2 (en) | 2015-04-03 | 2019-09-03 | Bristol-Myers Squibb Company | Inhibitors of indoleamine-2,3-dioxygenase for the treatment of cancer |
| WO2016161269A1 (en) | 2015-04-03 | 2016-10-06 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase for the treatment of cancer |
| WO2016161279A1 (en) | 2015-04-03 | 2016-10-06 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase for the treatment of cancer |
| US10399932B2 (en) | 2015-04-03 | 2019-09-03 | Bristol-Myers Squibb Company | Inhibitors of indoleamine-2,3-dioxygenase for the treatment of cancer |
| US9790169B2 (en) | 2015-04-03 | 2017-10-17 | Bristol-Myers Squibb Company | IDO inhibitors |
| US10167254B2 (en) | 2015-04-03 | 2019-01-01 | Bristol-Myers Squibb Company | IDO inhibitors |
| US11491204B2 (en) | 2015-04-07 | 2022-11-08 | Cytlimic Inc. | Composition comprising poly I:C and LAG-3-IGG fusion protein |
| WO2016162505A1 (en) | 2015-04-08 | 2016-10-13 | F-Star Biotechnology Limited | Her2 binding agent therapies |
| US10857181B2 (en) | 2015-04-21 | 2020-12-08 | Enlivex Therapeutics Ltd | Therapeutic pooled blood apoptotic cell preparations and uses thereof |
| US11883429B2 (en) | 2015-04-21 | 2024-01-30 | Enlivex Therapeutics Rdo Ltd | Therapeutic pooled blood apoptotic cell preparations and uses thereof |
| EP3736287A1 (en) | 2015-05-11 | 2020-11-11 | The Johns Hopkins University | Autoimmune antibodies for use in inhibiting cancer cell growth |
| US11938183B2 (en) | 2015-05-11 | 2024-03-26 | The Johns Hopkins University | Autoimmune antibodies for use in inhibiting cancer cell growth |
| WO2016183114A1 (en) | 2015-05-11 | 2016-11-17 | Bristol-Myers Squibb Company | Tricyclic compounds as anticancer agents |
| WO2016183115A1 (en) | 2015-05-12 | 2016-11-17 | Bristol-Myers Squibb Company | 5h-pyrido[3,2-b]indole compounds as anticancer agents |
| WO2016183118A1 (en) | 2015-05-12 | 2016-11-17 | Bristol-Myers Squibb Company | Tricyclic compounds as anticancer agents |
| WO2016181348A1 (en) | 2015-05-14 | 2016-11-17 | Pfizer Inc. | Combinations comprising a pyrrolidine-2,5-dione ido1 inhibitor and an anti-body |
| US10945994B2 (en) | 2015-05-14 | 2021-03-16 | Pfizer Inc. | Combinations comprising a pyrrolidine-2,5-dione IDO1 inhibitor and an anti-body |
| US12084518B2 (en) | 2015-05-21 | 2024-09-10 | Harpoon Therapeutics, Inc. | Trispecific binding proteins and methods of use |
| WO2016196228A1 (en) | 2015-05-29 | 2016-12-08 | Bristol-Myers Squibb Company | Antibodies against ox40 and uses thereof |
| WO2016196218A1 (en) | 2015-05-31 | 2016-12-08 | Curegenix Corporation | Combination compositions for immunotherapy |
| WO2016201425A1 (en) | 2015-06-12 | 2016-12-15 | Bristol-Myers Squibb Company | Treatment of cancer by combined blockade of the pd-1 and cxcr4 signaling pathways |
| TWI772261B (en) * | 2015-06-16 | 2022-08-01 | 德商馬克專利公司 | Pd-l1 antagonist combination treatments |
| IL256245B (en) * | 2015-06-16 | 2022-09-01 | Merck Patent Gmbh | Pd-l1 antagonist combination treatments |
| WO2016205277A1 (en) * | 2015-06-16 | 2016-12-22 | Merck Patent Gmbh | Pd-l1 antagonist combination treatments |
| AU2016280003B2 (en) * | 2015-06-16 | 2021-09-16 | Merck Patent Gmbh | PD-L1 antagonist combination treatments |
| US11154616B2 (en) | 2015-06-17 | 2021-10-26 | Genentech, Inc. | Methods of treating locally advanced or metastatic breast cancers using PD-1 axis binding antagonists and taxanes |
| US11584793B2 (en) | 2015-06-24 | 2023-02-21 | Hoffmann-La Roche Inc. | Anti-transferrin receptor antibodies with tailored affinity |
| WO2017000983A1 (en) | 2015-06-29 | 2017-01-05 | Ose Immunotherapeutics | Method for inducing early t memory response with short peptides anti-tumor vaccine |
| WO2017004016A1 (en) | 2015-06-29 | 2017-01-05 | The Rockefeller University | Antibodies to cd40 with enhanced agonist activity |
| WO2017019757A1 (en) | 2015-07-28 | 2017-02-02 | Bristol-Myers Squibb Company | Tgf beta receptor antagonists |
| WO2017019894A1 (en) | 2015-07-29 | 2017-02-02 | Novartis Ag | Combination therapies comprising antibody molecules to lag-3 |
| WO2017019897A1 (en) | 2015-07-29 | 2017-02-02 | Novartis Ag | Combination therapies comprising antibody molecules to tim-3 |
| EP4378957A2 (en) | 2015-07-29 | 2024-06-05 | Novartis AG | Combination therapies comprising antibody molecules to pd-1 |
| EP3878465A1 (en) | 2015-07-29 | 2021-09-15 | Novartis AG | Combination therapies comprising antibody molecules to tim-3 |
| EP3964528A1 (en) | 2015-07-29 | 2022-03-09 | Novartis AG | Combination therapies comprising antibody molecules to lag-3 |
| US10214586B2 (en) | 2015-08-24 | 2019-02-26 | Eli Lilly And Company | PD-L1 antibodies |
| WO2017035118A1 (en) | 2015-08-25 | 2017-03-02 | Bristol-Myers Squibb Company | Tgf beta receptor antagonists |
| WO2017055484A1 (en) | 2015-09-29 | 2017-04-06 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for determining the metabolic status of lymphomas |
| WO2017058780A1 (en) | 2015-09-30 | 2017-04-06 | Merck Patent Gmbh | Combination of a pd-1 axis binding antagonist and an alk inhibitor for treating alk-negative cancer |
| US11603411B2 (en) | 2015-10-02 | 2023-03-14 | Hoffmann-La Roche Inc. | Bispecific anti-human CD20/human transferrin receptor antibodies and methods of use |
| US11787868B2 (en) | 2015-10-02 | 2023-10-17 | Hoffmann-La Roche Inc. | Bispecific anti-human A-beta/human transferrin receptor antibodies and methods of use |
| US12030952B2 (en) | 2015-10-02 | 2024-07-09 | Hoffmann-La Roche Inc. | Bispecific anti-human CD20/human transferrin receptor antibodies and methods of use |
| WO2017069291A1 (en) | 2015-10-23 | 2017-04-27 | Canbas Co., Ltd. | Peptides and peptidomimetics in combination with t cell activating and/or checkpoint inhibiting agents for cancer treatment |
| WO2017087678A2 (en) | 2015-11-19 | 2017-05-26 | Bristol-Myers Squibb Company | Antibodies against glucocorticoid-induced tumor necrosis factor receptor (gitr) and uses thereof |
| US10858432B2 (en) | 2015-12-02 | 2020-12-08 | Stcube, Inc. | Antibodies specific to glycosylated PD-1 and methods of use thereof |
| US11981736B2 (en) | 2015-12-02 | 2024-05-14 | St Cube Inc. | Antibodies specific to glycosylated PD-1 and methods of use thereof |
| EP3366691A1 (en) | 2015-12-03 | 2018-08-29 | GlaxoSmithKline Intellectual Property Development Limited | Cyclic purine dinucleotides as modulators of sting |
| WO2017093933A1 (en) | 2015-12-03 | 2017-06-08 | Glaxosmithkline Intellectual Property Development Limited | Cyclic purine dinucleotides as modulators of sting |
| EP3747466A1 (en) | 2015-12-07 | 2020-12-09 | Merck Patent GmbH | Aqueous pharmaceutical formulation |
| KR20180085801A (en) * | 2015-12-07 | 2018-07-27 | 메르크 파텐트 게엠베하 | An aqueous pharmaceutical formulation comprising an anti-PD-1 antibody aveludine |
| AU2016368099B2 (en) * | 2015-12-07 | 2023-03-09 | Merck Patent Gmbh | Aqueous Pharmaceutical Formulation Comprising anti-PD-L1 Antibody Avelumab |
| WO2017097407A1 (en) * | 2015-12-07 | 2017-06-15 | Merck Patent Gmbh | Aqueous pharmaceutical formulation comprising anti-pd-1 antibody avelumab |
| AU2016368099C1 (en) * | 2015-12-07 | 2023-10-12 | Merck Patent Gmbh | Aqueous Pharmaceutical Formulation Comprising anti-PD-L1 Antibody Avelumab |
| KR102697026B1 (en) * | 2015-12-07 | 2024-08-20 | 메르크 파텐트 게엠베하 | Aqueous pharmaceutical formulation comprising anti-pd-l1 antibody avelumab |
| US11058769B2 (en) | 2015-12-07 | 2021-07-13 | Merck Patent Gmbh | Aqueous pharmaceutical formulation comprising anti-PD-L1 antibody Avelumab |
| TWI630917B (en) * | 2015-12-07 | 2018-08-01 | 馬克專利公司 | Aqueous pharmaceutical formulation |
| WO2017098421A1 (en) | 2015-12-08 | 2017-06-15 | Glaxosmithkline Intellectual Property Development Limited | Benzothiadiazine compounds |
| WO2017106291A1 (en) | 2015-12-15 | 2017-06-22 | Bristol-Myers Squibb Company | Cxcr4 receptor antagonists |
| US10556010B2 (en) | 2015-12-17 | 2020-02-11 | Photocure Asa | Neoadjuvant therapy for bladder cancer |
| US11066478B2 (en) | 2015-12-17 | 2021-07-20 | Photocure Asa | Intravesical therapy for bladder cancer |
| EP4424322A2 (en) | 2015-12-17 | 2024-09-04 | Novartis AG | Antibody molecules to pd-1 and uses thereof |
| WO2017106453A1 (en) | 2015-12-17 | 2017-06-22 | University Of Maryland, Baltimore County | A recombinant bi-specific polypeptide for coordinately activating tumor-reactive t-cells and neutralizing immune suppression |
| US11311620B2 (en) | 2015-12-17 | 2022-04-26 | Photocure Asa | Neoadjuvant therapy for bladder cancer |
| WO2017103283A1 (en) | 2015-12-17 | 2017-06-22 | Photocure Asa | Neoadjuvant therapy for bladder cancer |
| WO2017106656A1 (en) | 2015-12-17 | 2017-06-22 | Novartis Ag | Antibody molecules to pd-1 and uses thereof |
| WO2017103895A1 (en) | 2015-12-18 | 2017-06-22 | Novartis Ag | Antibodies targeting cd32b and methods of use thereof |
| WO2017112741A1 (en) | 2015-12-22 | 2017-06-29 | Novartis Ag | Mesothelin chimeric antigen receptor (car) and antibody against pd-l1 inhibitor for combined use in anticancer therapy |
| US12054557B2 (en) | 2015-12-22 | 2024-08-06 | Regeneron Pharmaceuticals, Inc. | Combination of anti-PD-1 antibodies and bispecific anti-CD20/anti-CD3 antibodies to treat cancer |
| WO2017118634A1 (en) | 2016-01-04 | 2017-07-13 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Use of pd-1 and tim-3 as a measure for cd8+ cells in predicting and treating renal cell carcinoma |
| US11730761B2 (en) | 2016-02-18 | 2023-08-22 | Enlivex Therapeutics Rdo Ltd | Combination immune therapy and cytokine control therapy for cancer treatment |
| WO2017140821A1 (en) | 2016-02-19 | 2017-08-24 | Novartis Ag | Tetracyclic pyridone compounds as antivirals |
| US11434294B2 (en) | 2016-02-25 | 2022-09-06 | Cell Medica, Inc. | Binding members to PD-L1 |
| EP4086285A1 (en) | 2016-02-25 | 2022-11-09 | Cell Medica Switzerland AG | Binding members to pd-l1 |
| WO2017144681A1 (en) | 2016-02-25 | 2017-08-31 | Cell Medica Switzerland Ag | Binding members to pd-l1 |
| US10465014B2 (en) | 2016-03-04 | 2019-11-05 | Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. | PDL-1 antibody, pharmaceutical composition thereof, and uses thereof |
| KR102068600B1 (en) | 2016-03-04 | 2020-01-21 | 쓰촨 케룬-바이오테크 바이오파마수티컬 컴퍼니 리미티드 | PDL-1 antibody, pharmaceutical composition thereof and use thereof |
| EP3309177A4 (en) * | 2016-03-04 | 2018-10-10 | Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. | Pdl-1 antibody, pharmaceutical composition thereof, and uses thereof |
| US11136413B2 (en) | 2016-03-04 | 2021-10-05 | Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. | PDL-1 antibody, pharmaceutical composition thereof, and uses thereof |
| KR20180004277A (en) * | 2016-03-04 | 2018-01-10 | 쓰촨 케룬-바이오테크 바이오파마수티컬 컴퍼니 리미티드 | PDL-1 antibody, pharmaceutical composition thereof and uses thereof |
| WO2017153952A1 (en) | 2016-03-10 | 2017-09-14 | Glaxosmithkline Intellectual Property Development Limited | 5-sulfamoyl-2-hydroxybenzamide derivatives |
| WO2017159699A1 (en) | 2016-03-15 | 2017-09-21 | Chugai Seiyaku Kabushiki Kaisha | Methods of treating cancers using pd-1 axis binding antagonists and anti-gpc3 antibodies |
| EP4112641A1 (en) | 2016-03-15 | 2023-01-04 | Chugai Seiyaku Kabushiki Kaisha | Methods of treating cancers using pd-1 axis binding antagonists and anti-gpc3 antibodies |
| EP4302782A2 (en) | 2016-03-15 | 2024-01-10 | Mersana Therapeutics, Inc. | Napi2b-targeted antibody-drug conjugates and methods of use thereof |
| WO2017160754A1 (en) | 2016-03-15 | 2017-09-21 | Mersana Therapeutics,Inc. | Napi2b-targeted antibody-drug conjugates and methods of use thereof |
| CN108779180A (en) * | 2016-03-23 | 2018-11-09 | 迈博斯生物医药(苏州)有限公司 | Novel anti-PD-L1 antibody |
| US11753473B2 (en) | 2016-03-23 | 2023-09-12 | Suzhou Transcenta Therapeutics Co., Ltd. | Anti-PD-L1 antibodies |
| CN111363041B (en) * | 2016-03-23 | 2022-02-22 | 苏州创胜医药集团有限公司 | Novel anti-PD-L1 antibodies |
| CN108779180B (en) * | 2016-03-23 | 2020-10-16 | 迈博斯生物医药(苏州)有限公司 | Novel anti-PD-L1 antibodies |
| CN111363041A (en) * | 2016-03-23 | 2020-07-03 | 迈博斯生物医药(苏州)有限公司 | Novel anti-PD-L1 antibodies |
| WO2017163186A1 (en) | 2016-03-24 | 2017-09-28 | Novartis Ag | Alkynyl nucleoside analogs as inhibitors of human rhinovirus |
| EP4292658A2 (en) | 2016-03-24 | 2023-12-20 | Novartis AG | Alkynyl nucleoside analogs as inhibitors of human rhinovirus |
| US12059474B2 (en) | 2016-03-29 | 2024-08-13 | Stcube & Co., Inc. | Methods for selecting antibodies that specifically bind glycosylated immune checkpoint proteins |
| US11660352B2 (en) | 2016-03-29 | 2023-05-30 | Stcube, Inc. | Dual function antibodies specific to glycosylated PD-L1 and methods of use thereof |
| US11964015B2 (en) | 2016-04-01 | 2024-04-23 | Deutsches Krebsforschungszentrum | Cancer therapy with an oncolytic virus combined with a checkpoint inhibitor |
| WO2017178572A1 (en) | 2016-04-13 | 2017-10-19 | Vivia Biotech, S.L | Ex vivo bite-activated t cells |
| US11279766B2 (en) | 2016-04-14 | 2022-03-22 | Ose Immunotherapeutics | Anti-SIRPa antibodies and their therapeutic applications |
| WO2017184619A2 (en) | 2016-04-18 | 2017-10-26 | Celldex Therapeutics, Inc. | Agonistic antibodies that bind human cd40 and uses thereof |
| CN105695406A (en) * | 2016-04-27 | 2016-06-22 | 天津普瑞赛尔生物科技有限公司 | Method for preparing DC-CIK immune cells with high-efficiency tumor killing property and prepared DC-CIK immune cells |
| US10323004B2 (en) | 2016-05-04 | 2019-06-18 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| US10696648B2 (en) | 2016-05-04 | 2020-06-30 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| US11066383B2 (en) | 2016-05-04 | 2021-07-20 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| US10633342B2 (en) | 2016-05-04 | 2020-04-28 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| US10544099B2 (en) | 2016-05-04 | 2020-01-28 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2017191545A1 (en) | 2016-05-05 | 2017-11-09 | Glaxosmithkline Intellectual Property (No.2) Limited | Enhancer of zeste homolog 2 inhibitors |
| WO2017196867A1 (en) * | 2016-05-09 | 2017-11-16 | Igm Biosciences, Inc. | Anti-pd-l1 antibodies |
| AU2017264683B2 (en) * | 2016-05-09 | 2024-02-29 | Igm Biosciences, Inc. | Anti-PD-L1 antibodies |
| IL262295B2 (en) * | 2016-05-09 | 2023-09-01 | Igm Biosciences Inc | Anti-pd-l1 antibodies |
| IL262295B1 (en) * | 2016-05-09 | 2023-05-01 | Igm Biosciences Inc | Anti-pd-l1 antibodies |
| US10954302B2 (en) | 2016-05-09 | 2021-03-23 | Igm Biosciences, Inc. | Anti-PD-L1 antibodies |
| US11299708B2 (en) | 2016-05-12 | 2022-04-12 | Adicet Bio, Inc. | Methods for selective expansion of γδ T-cell populations and compositions thereof |
| US11505600B2 (en) | 2016-05-13 | 2022-11-22 | Regeneron Pharmaceuticals, Inc. | Methods of treating skin cancer by administering a PD-1 inhibitor |
| US10457725B2 (en) | 2016-05-13 | 2019-10-29 | Regeneron Pharmaceuticals, Inc. | Methods of treating skin cancer by administering a PD-1 inhibitor |
| US11505591B2 (en) | 2016-05-18 | 2022-11-22 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides and methods of use thereof |
| WO2017200969A1 (en) | 2016-05-20 | 2017-11-23 | Eli Lilly And Company | Combination therapy with notch and pd-1 or pd-l1 inhibitors |
| US11826317B2 (en) | 2016-05-20 | 2023-11-28 | Eli Lilly And Company | Combination therapy with notch and PD-1 or PD-L1 inhibitors |
| US11623958B2 (en) | 2016-05-20 | 2023-04-11 | Harpoon Therapeutics, Inc. | Single chain variable fragment CD3 binding proteins |
| US10688104B2 (en) | 2016-05-20 | 2020-06-23 | Eli Lilly And Company | Combination therapy with Notch and PD-1 or PD-L1 inhibitors |
| CN109195989A (en) * | 2016-05-26 | 2019-01-11 | 默克专利股份有限公司 | PD-1/PD-L1 inhibitors for cancer treatment |
| WO2017202744A1 (en) | 2016-05-26 | 2017-11-30 | Merck Patent Gmbh | Pd-1 / pd-l1 inhibitors for cancer treatment |
| WO2017212425A1 (en) | 2016-06-08 | 2017-12-14 | Glaxosmithkline Intellectual Property Development Limited | Chemical compounds as atf4 pathway inhibitors |
| US10723799B2 (en) | 2016-06-13 | 2020-07-28 | I-Mab Biopharma Us Limited | Anti-PD-L1 antibodies and uses thereof |
| WO2017218533A1 (en) | 2016-06-13 | 2017-12-21 | Torque Therapeutics, Inc. | Methods and compositions for promoting immune cell function |
| WO2017216705A1 (en) | 2016-06-14 | 2017-12-21 | Novartis Ag | Crystalline form of (r)-4-(5-(cyclopropylethynyl)isoxazol-3-yl)-n-hydroxy-2-methyl-2-(methylsulfonyl)butanamide as an antibacterial agent |
| US10071973B2 (en) | 2016-06-14 | 2018-09-11 | Novartis Ag | Crystalline isoxazole hydroxamic acid compounds |
| WO2017216685A1 (en) | 2016-06-16 | 2017-12-21 | Novartis Ag | Pentacyclic pyridone compounds as antivirals |
| WO2017216686A1 (en) | 2016-06-16 | 2017-12-21 | Novartis Ag | 8,9-fused 2-oxo-6,7-dihydropyrido-isoquinoline compounds as antivirals |
| WO2017220990A1 (en) | 2016-06-20 | 2017-12-28 | Kymab Limited | Anti-pd-l1 antibodies |
| KR102531889B1 (en) | 2016-06-20 | 2023-05-17 | 키맵 리미티드 | Anti-PD-L1 and IL-2 cytokines |
| US11965026B2 (en) | 2016-06-20 | 2024-04-23 | Kymab Limited | Anti-PD-L1 and IL-2 cytokines |
| KR20190032355A (en) * | 2016-06-20 | 2019-03-27 | 키맵 리미티드 | Anti-PD-L1 and IL-2 cytokines |
| WO2017220988A1 (en) | 2016-06-20 | 2017-12-28 | Kymab Limited | Multispecific antibodies for immuno-oncology |
| US10604576B2 (en) | 2016-06-20 | 2020-03-31 | Kymab Limited | Antibodies and immunocytokines |
| US9957323B2 (en) | 2016-06-20 | 2018-05-01 | Kymab Limited | Anti-ICOS antibodies |
| WO2017220989A1 (en) | 2016-06-20 | 2017-12-28 | Kymab Limited | Anti-pd-l1 and il-2 cytokines |
| WO2017223422A1 (en) | 2016-06-24 | 2017-12-28 | Infinity Pharmaceuticals, Inc. | Combination therapies |
| EP3478723A4 (en) * | 2016-06-29 | 2020-07-29 | Checkpoint Therapeutics, Inc. | Pd-l1-specific antibodies and methods of using the same |
| US11834505B2 (en) | 2016-06-29 | 2023-12-05 | Checkpoint Therapeutics, Inc. | PD-L1-specific antibodies and methods of using the same |
| RU2749109C2 (en) * | 2016-06-29 | 2021-06-04 | Чекпойнт Терапьютикс, Инк. | Specific antibodies to pd-l1 and methods for their application |
| US10604574B2 (en) | 2016-06-30 | 2020-03-31 | Oncorus, Inc. | Oncolytic viral delivery of therapeutic polypeptides |
| US11078280B2 (en) | 2016-06-30 | 2021-08-03 | Oncorus, Inc. | Oncolytic viral delivery of therapeutic polypeptides |
| US11534431B2 (en) | 2016-07-05 | 2022-12-27 | Beigene Switzerland Gmbh | Combination of a PD-1 antagonist and a RAF inhibitor for treating cancer |
| US10864203B2 (en) | 2016-07-05 | 2020-12-15 | Beigene, Ltd. | Combination of a PD-1 antagonist and a RAF inhibitor for treating cancer |
| US11098077B2 (en) | 2016-07-05 | 2021-08-24 | Chinook Therapeutics, Inc. | Locked nucleic acid cyclic dinucleotide compounds and uses thereof |
| WO2018013818A2 (en) | 2016-07-14 | 2018-01-18 | Bristol-Myers Squibb Company | Antibodies against tim3 and uses thereof |
| US10077306B2 (en) | 2016-07-14 | 2018-09-18 | Bristol-Myers Squibb Company | Antibodies against TIM3 and uses thereof |
| US11591392B2 (en) | 2016-07-14 | 2023-02-28 | Bristol-Myers Squibb Company | Antibodies against TIM3 and uses thereof |
| US10533052B2 (en) | 2016-07-14 | 2020-01-14 | Bristol-Myers Squibb Company | Antibodies against TIM3 and uses thereof |
| WO2018015879A1 (en) | 2016-07-20 | 2018-01-25 | Glaxosmithkline Intellectual Property Development Limited | Isoquinoline derivatives as perk inhibitors |
| US11746152B2 (en) | 2016-07-20 | 2023-09-05 | Stcube, Inc. | Methods of cancer treatment and therapy using a combination of antibodies that bind glycosylated PD-L1 |
| WO2018017633A1 (en) | 2016-07-21 | 2018-01-25 | Bristol-Myers Squibb Company | TGF Beta RECEPTOR ANTAGONISTS |
| US11858996B2 (en) | 2016-08-09 | 2024-01-02 | Kymab Limited | Anti-ICOS antibodies |
| WO2018029474A2 (en) | 2016-08-09 | 2018-02-15 | Kymab Limited | Anti-icos antibodies |
| IL264730B2 (en) * | 2016-08-12 | 2024-09-01 | Merck Patent Gmbh | PHARMACEUTICAL COMPOSITION COMPRISING TGFbetaRII AND ANOTHER ANTI-CANCER AGENT |
| IL264730B1 (en) * | 2016-08-12 | 2024-05-01 | Merck Patent Gmbh | PHARMACEUTICAL COMPOSITION COMPRISING TGFbetaRII AND ANOTHER ANTI-CANCER AGENT |
| WO2018029367A1 (en) | 2016-08-12 | 2018-02-15 | Merck Patent Gmbh | Combination therapy for cancer |
| US11701357B2 (en) | 2016-08-19 | 2023-07-18 | Beigene Switzerland Gmbh | Treatment of B cell cancers using a combination comprising Btk inhibitors |
| US11542335B2 (en) | 2016-08-25 | 2023-01-03 | Hoffmann-La Roche Inc. | Method of treating cancer in a patient by administering an antibody which binds colony stimulating factor-1 receptor (CSF-1R) |
| US11351164B2 (en) | 2016-08-26 | 2022-06-07 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2018047109A1 (en) | 2016-09-09 | 2018-03-15 | Novartis Ag | Polycyclic pyridone compounds as antivirals |
| WO2018057955A1 (en) | 2016-09-23 | 2018-03-29 | Elstar Therapeutics, Inc. | Multispecific antibody molecules comprising lambda and kappa light chains |
| US11673971B2 (en) | 2016-09-23 | 2023-06-13 | Marengo Therapeutics, Inc. | Multispecific antibody molecules comprising lambda and kappa light chains |
| WO2018060926A1 (en) | 2016-09-28 | 2018-04-05 | Novartis Ag | Beta-lactamase inhibitors |
| EP3698796A1 (en) | 2016-09-28 | 2020-08-26 | Novartis AG | Pharmaceutical combination of a tricyclic beta-lactamase inhibitor with specific beta-lactam antibiotics |
| US11274154B2 (en) | 2016-10-06 | 2022-03-15 | Pfizer Inc. | Dosing regimen of avelumab for the treatment of cancer |
| WO2018065938A1 (en) | 2016-10-06 | 2018-04-12 | Pfizer Inc. | Dosing regimen of avelumab for the treatment of cancer |
| US11759518B2 (en) | 2016-10-11 | 2023-09-19 | Nec Corporation | Medicine for treating cancer by administering a toll-like receptor agonist and LAG-3 IgG fusion protein |
| US11291718B2 (en) | 2016-10-11 | 2022-04-05 | Cytlimic Inc. | Method for treating cancer by administering a toll-like receptor agonist and LAG-3 IgG fusion protein |
| US10597454B2 (en) | 2016-10-15 | 2020-03-24 | Innovent Biologics (Suzhou) Co., Ltd | PD-1 antibodies |
| CN107955072A (en) * | 2016-10-15 | 2018-04-24 | 信达生物制药(苏州)有限公司 | Pd-1 antibody |
| WO2018068336A1 (en) * | 2016-10-15 | 2018-04-19 | Innovent Biologics (Suzhou) Co., Ltd | Pd-1 antibodies |
| CN107955072B (en) * | 2016-10-15 | 2022-10-18 | 信达生物制药(苏州)有限公司 | PD-1 antibodies |
| WO2018073753A1 (en) | 2016-10-18 | 2018-04-26 | Novartis Ag | Fused tetracyclic pyridone compounds as antivirals |
| WO2018081621A1 (en) | 2016-10-28 | 2018-05-03 | Bristol-Myers Squibb Company | Methods of treating urothelial carcinoma using an anti-pd-1 antibody |
| TWI812600B (en) * | 2016-10-30 | 2023-08-21 | 中國商上海復宏漢霖生物技術股份有限公司 | Anti-pd-l1 antibodies and variants |
| US11618786B2 (en) | 2016-10-30 | 2023-04-04 | Shanghai Henlius Biotech Inc. | Anti-PD-L1 antibodies and variants |
| AU2017348475B2 (en) * | 2016-10-30 | 2024-02-08 | Shanghai Henlius Biotech, Inc. | Anti-PD-L1 antibodies and variants |
| CN109963589A (en) * | 2016-10-30 | 2019-07-02 | 上海复宏汉霖生物技术股份有限公司 | Anti- PD-L1 antibody and variant |
| US11274155B2 (en) | 2016-10-30 | 2022-03-15 | Shanghai Henlius Biotech Inc. | Anti-PD-L1 antibodies and variants |
| CN109963589B (en) * | 2016-10-30 | 2023-05-05 | 上海复宏汉霖生物技术股份有限公司 | anti-PD-L1 antibodies and variants |
| EP3532100A4 (en) * | 2016-10-30 | 2020-11-25 | Shanghai Henlius Biotech, Inc. | Anti-pd-l1 antibodies and variants |
| US11117968B2 (en) | 2016-11-03 | 2021-09-14 | Bristol-Myers Squibb Company | Activatable anti-CTLA-4 antibodies and uses thereof |
| US11779604B2 (en) | 2016-11-03 | 2023-10-10 | Kymab Limited | Antibodies, combinations comprising antibodies, biomarkers, uses and methods |
| WO2018085555A1 (en) | 2016-11-03 | 2018-05-11 | Bristol-Myers Squibb Company | Activatable anti-ctla-4 antibodies and uses thereof |
| US11471515B2 (en) | 2016-11-09 | 2022-10-18 | The Brigham And Women's Hospital, Inc. | Restoration of tumor suppression using MRNA-based delivery system |
| US11090367B2 (en) | 2016-11-09 | 2021-08-17 | The Brigham And Women's Hospital, Inc. | Restoration of tumor suppression using mRNA-based delivery system |
| US10660909B2 (en) | 2016-11-17 | 2020-05-26 | Syntrix Biosystems Inc. | Method for treating cancer using chemokine antagonists |
| US11279694B2 (en) | 2016-11-18 | 2022-03-22 | Sumitomo Dainippon Pharma Oncology, Inc. | Alvocidib prodrugs and their use as protein kinase inhibitors |
| WO2018098352A2 (en) | 2016-11-22 | 2018-05-31 | Jun Oishi | Targeting kras induced immune checkpoint expression |
| WO2018098269A2 (en) | 2016-11-23 | 2018-05-31 | Mersana Therapeutics, Inc. | Peptide-containing linkers for antibody-drug conjugates |
| WO2018100534A1 (en) | 2016-12-01 | 2018-06-07 | Glaxosmithkline Intellectual Property Development Limited | Combination therapy |
| WO2018100535A1 (en) | 2016-12-01 | 2018-06-07 | Glaxosmithkline Intellectual Property Development Limited | Combination therapy |
| KR20190095280A (en) | 2016-12-12 | 2019-08-14 | 다이이찌 산쿄 가부시키가이샤 | Combination of Antibody-Drug Conjugates and Immune Checkpoint Inhibitors |
| US11273155B2 (en) | 2016-12-12 | 2022-03-15 | Daiichi Sankyo Company, Limited | Combination of antibody-drug conjugate and immune checkpoint inhibitor |
| WO2018110515A1 (en) | 2016-12-12 | 2018-06-21 | 第一三共株式会社 | Combination of antibody-drug conjugate and immune checkpoint inhibitor |
| US11739133B2 (en) | 2016-12-22 | 2023-08-29 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides and methods of use thereof |
| US11117945B2 (en) | 2016-12-22 | 2021-09-14 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides and methods of use thereof |
| US11905320B2 (en) | 2016-12-22 | 2024-02-20 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides and methods of use thereof |
| US11505588B2 (en) | 2016-12-22 | 2022-11-22 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides and methods of use thereof |
| US11851467B2 (en) | 2016-12-22 | 2023-12-26 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides and methods of use thereof |
| US11401314B2 (en) | 2016-12-22 | 2022-08-02 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides and methods of use thereof |
| US11370821B2 (en) | 2016-12-22 | 2022-06-28 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides and methods of use thereof |
| US11987610B2 (en) | 2016-12-22 | 2024-05-21 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides and methods of use thereof |
| US11377478B2 (en) | 2016-12-22 | 2022-07-05 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides and methods of use thereof |
| US11498968B2 (en) | 2016-12-22 | 2022-11-15 | Hoffmann-La Roche Inc. | Treatment of tumors with an anti-CSF-1R antibody in combination with an anti-PD-L1 antibody after failure of anti-PD-L1/PD1 treatment |
| US11708400B2 (en) | 2016-12-22 | 2023-07-25 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides and methods of use thereof |
| US11530248B2 (en) | 2016-12-22 | 2022-12-20 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides and methods of use thereof |
| RU2766582C2 (en) * | 2016-12-23 | 2022-03-15 | Ремд Биотерапьютикс, Инк. | Immunotherapy using antibodies binding programmed cell death protein ligand 1 (pd-l1) |
| EP3559045A4 (en) * | 2016-12-23 | 2020-08-19 | REMD Biotherapeutics, Inc. | Immunotherapy using antibodies that bind programmed death ligand-1 (pd-l1) |
| WO2018132279A1 (en) | 2017-01-05 | 2018-07-19 | Bristol-Myers Squibb Company | Tgf beta receptor antagonists |
| US11851471B2 (en) | 2017-01-09 | 2023-12-26 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides and methods of use thereof |
| EP4310082A2 (en) | 2017-01-20 | 2024-01-24 | Arcus Biosciences, Inc. | Azolopyrimidine for the treatment of cancer-related disorders |
| WO2018136700A1 (en) | 2017-01-20 | 2018-07-26 | Arcus Biosciences, Inc. | Azolopyrimidine for the treatment of cancer-related disorders |
| WO2018138591A1 (en) | 2017-01-24 | 2018-08-02 | Pfizer Inc. | Calicheamicin derivatives and antibody drug conjugates thereof |
| US11993625B2 (en) | 2017-01-24 | 2024-05-28 | Pfizer, Inc. | Calicheamicin derivatives and antibody drug conjugates thereof |
| US11555038B2 (en) | 2017-01-25 | 2023-01-17 | Beigene, Ltd. | Crystalline forms of (S)-7-(1-(but-2-ynoyl)piperidin-4-yl)-2-(4-phenoxyphenyl)-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidine-3-carboxamide, preparation, and uses thereof |
| EP4029494A1 (en) | 2017-01-25 | 2022-07-20 | OSE Immunotherapeutics | Method for manufacturing a stable emulsion for peptide delivery |
| WO2018138110A1 (en) | 2017-01-25 | 2018-08-02 | Ose Immunotherapeutics | Method for manufacturing a stable emulsion for peptide delivery |
| WO2018146612A1 (en) | 2017-02-10 | 2018-08-16 | Novartis Ag | 1-(4-amino-5-bromo-6-(1 h-pyrazol-1-yl)pyrimidin-2-yl)-1 h-pyrazol-4-ol and use thereof in the treatment of cancer |
| WO2018151820A1 (en) | 2017-02-16 | 2018-08-23 | Elstar Therapeutics, Inc. | Multifunctional molecules comprising a trimeric ligand and uses thereof |
| WO2018152396A1 (en) | 2017-02-17 | 2018-08-23 | Innate Tumor Immunity, Inc. | Substituted imidazo-quinolines as nlrp3 modulators |
| EP3753938A1 (en) | 2017-02-17 | 2020-12-23 | Innate Tumor Immunity, Inc. | Substituted imidazo-quinolines as nlrp3 modulators |
| EP3587453A4 (en) * | 2017-02-21 | 2021-07-07 | Shanghai Junshi Bioscience Co., Ltd. | Anti-pd-l1 antibody and application thereof |
| US11267890B2 (en) | 2017-02-21 | 2022-03-08 | Shanghai Junshi Biosciences Inc. | Anti-PD-L1 antibody and application thereof |
| EP4389226A2 (en) | 2017-02-24 | 2024-06-26 | MacroGenics, Inc. | Bispecific binding molecules that are capable of binding cd137 and tumor antigens, and uses thereof |
| US11815435B2 (en) | 2017-02-24 | 2023-11-14 | Hibercell, Inc. | Beta glucan immunopharmacodynamics |
| US11459394B2 (en) | 2017-02-24 | 2022-10-04 | Macrogenics, Inc. | Bispecific binding molecules that are capable of binding CD137 and tumor antigens, and uses thereof |
| US11942149B2 (en) | 2017-02-24 | 2024-03-26 | Macrogenics, Inc. | Bispecific binding molecules that are capable of binding CD137 and tumor antigens, and uses thereof |
| WO2018160538A1 (en) | 2017-02-28 | 2018-09-07 | Mersana Therapeutics, Inc. | Combination therapies of her2-targeted antibody-drug conjugates |
| WO2018162446A1 (en) | 2017-03-06 | 2018-09-13 | Merck Patent Gmbh | Aqueous anti-pd-l1 antibody formulation |
| IL268943B2 (en) * | 2017-03-06 | 2023-06-01 | Merck Patent Gmbh | Aqueous anti-pd-l1 antibody formulation |
| WO2018162430A1 (en) | 2017-03-06 | 2018-09-13 | Merck Patent Gmbh | Composition comprising avelumab |
| EP3372615A1 (en) | 2017-03-06 | 2018-09-12 | Merck Patent GmbH | Composition comprising avelumab |
| WO2018162749A1 (en) | 2017-03-09 | 2018-09-13 | Genmab A/S | Antibodies against pd-l1 |
| US11767355B2 (en) | 2017-03-15 | 2023-09-26 | Cue Biopharma, Inc. | Methods for modulating an immune response |
| US11993641B2 (en) | 2017-03-15 | 2024-05-28 | Cue Biopharma, Inc. | Methods for modulating an immune response |
| US11958893B2 (en) | 2017-03-15 | 2024-04-16 | Cue Biopharma, Inc. | Methods for modulating an immune response |
| US11104712B2 (en) | 2017-03-15 | 2021-08-31 | Cue Biopharma, Inc. | Methods for modulating an immune response |
| US11479595B2 (en) | 2017-03-15 | 2022-10-25 | Cue Biopharma, Inc. | Methods for modulating an immune response |
| WO2018172508A1 (en) | 2017-03-24 | 2018-09-27 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating melanoma |
| WO2018178040A1 (en) | 2017-03-30 | 2018-10-04 | Merck Patent Gmbh | Combination of an anti-pd-l1 antibody and a dna-pk inhibitor for the treatment of cancer |
| WO2018183928A1 (en) | 2017-03-31 | 2018-10-04 | Bristol-Myers Squibb Company | Methods of treating tumor |
| US11603407B2 (en) | 2017-04-06 | 2023-03-14 | Regeneron Pharmaceuticals, Inc. | Stable antibody formulation |
| WO2018187613A2 (en) | 2017-04-07 | 2018-10-11 | Bristol-Myers Squibb Company | Anti-icos agonist antibodies and uses thereof |
| KR102679554B1 (en) | 2017-04-17 | 2024-07-01 | 조인트 스탁 컴퍼니 “바이오케드” | Monoclonal antibodies against PD-L1 |
| WO2018194496A2 (en) | 2017-04-17 | 2018-10-25 | Закрытое Акционерное Общество "Биокад" | Monoclonal antibody to pd-l1 |
| EP3613770A4 (en) * | 2017-04-17 | 2021-01-27 | Joint Stock Company "Biocad" | Monoclonal antibody to pd-l1 |
| US11236167B2 (en) | 2017-04-17 | 2022-02-01 | Joint Stock Company “Biocad” | Monoclonal antibody to PD-L1 |
| KR20190140979A (en) * | 2017-04-17 | 2019-12-20 | 조인트 스탁 컴퍼니 "바이오케드" | Monoclonal antibody against PD-L1 |
| US11326170B2 (en) | 2017-04-18 | 2022-05-10 | Changchun Huapu Biotechnology Co. Ltd. | Immunomodulatory polynucleotides and uses thereof |
| WO2018195283A1 (en) | 2017-04-19 | 2018-10-25 | Elstar Therapeutics, Inc. | Multispecific molecules and uses thereof |
| US10570138B2 (en) | 2017-04-21 | 2020-02-25 | Kyn Therapeutics | Indole AHR inhibitors and uses thereof |
| US12077542B2 (en) | 2017-04-21 | 2024-09-03 | Ikena Oncology, Inc. | Indole AHR inhibitors and uses thereof |
| US11358969B2 (en) | 2017-04-21 | 2022-06-14 | Ikena Oncology, Inc. | Indole AHR inhibitors and uses thereof |
| US10689388B1 (en) | 2017-04-21 | 2020-06-23 | Ikena Oncology, Inc. | Indole AHR inhibitors and uses thereof |
| WO2018195397A2 (en) | 2017-04-21 | 2018-10-25 | Kyn Therapeutics | Indole ahr inhibitors and uses thereof |
| WO2018200430A1 (en) | 2017-04-26 | 2018-11-01 | Bristol-Myers Squibb Company | Methods of antibody production that minimize disulfide bond reduction |
| WO2018198079A1 (en) | 2017-04-27 | 2018-11-01 | Novartis Ag | Fused indazole pyridone compounds as antivirals |
| US10301312B2 (en) | 2017-04-27 | 2019-05-28 | Novartis Ag | Fused indazole pyridone compounds as antivirals |
| EP3998269A1 (en) | 2017-04-27 | 2022-05-18 | Novartis AG | Fused indazole pyridone compounds as antivirals |
| US10975078B2 (en) | 2017-04-27 | 2021-04-13 | Novartis Ag | Fused indazole pyridone compounds as antivirals |
| WO2018198076A1 (en) | 2017-04-28 | 2018-11-01 | Aduro Biotech, Inc. | Bis 2'-5'-rr-(3'f-a)(3'f-a) cyclic dinucleotide compound and uses thereof |
| EP4328241A2 (en) | 2017-04-28 | 2024-02-28 | Marengo Therapeutics, Inc. | Multispecific molecules comprising a non-immunoglobulin heterodimerization domain and uses thereof |
| WO2018198091A1 (en) | 2017-04-28 | 2018-11-01 | Novartis Ag | Antibody conjugates comprising toll-like receptor agonist and combination therapies |
| WO2018201047A1 (en) | 2017-04-28 | 2018-11-01 | Elstar Therapeutics, Inc. | Multispecific molecules comprising a non-immunoglobulin heterodimerization domain and uses thereof |
| US10975114B2 (en) | 2017-04-28 | 2021-04-13 | Chinook Therapeutics, Inc. | Bis 2′-5′-RR-(3′F-A)(3′F-A) cyclic dinucleotide compound and uses thereof |
| WO2018203302A1 (en) | 2017-05-05 | 2018-11-08 | Novartis Ag | Tricyclic 2-quinolinones as antibacterials |
| WO2018209049A1 (en) | 2017-05-12 | 2018-11-15 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| US11607453B2 (en) | 2017-05-12 | 2023-03-21 | Harpoon Therapeutics, Inc. | Mesothelin binding proteins |
| US11066392B2 (en) | 2017-05-12 | 2021-07-20 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2018213297A1 (en) | 2017-05-16 | 2018-11-22 | Bristol-Myers Squibb Company | Treatment of cancer with anti-gitr agonist antibodies |
| WO2018213377A1 (en) | 2017-05-17 | 2018-11-22 | Arcus Biosciences, Inc. | Quinazoline-pyrazole derivatives for the treatment of cancer-related disorders |
| WO2018222718A1 (en) | 2017-05-30 | 2018-12-06 | Bristol-Myers Squibb Company | Treatment of lag-3 positive tumors |
| US11723975B2 (en) | 2017-05-30 | 2023-08-15 | Bristol-Myers Squibb Company | Compositions comprising an anti-LAG-3 antibody or an anti-LAG-3 antibody and an anti-PD-1 or anti-PD-L1 antibody |
| US12049503B2 (en) | 2017-05-30 | 2024-07-30 | Bristol-Myers Squibb Company | Treatment of LAG-3 positive tumors |
| WO2018222711A2 (en) | 2017-05-30 | 2018-12-06 | Bristol-Myers Squibb Company | Compositions comprising a combination of an anti-lag-3 antibody, a pd-1 pathway inhibitor, and an immunotherapeutic agent |
| WO2018222722A2 (en) | 2017-05-30 | 2018-12-06 | Bristol-Myers Squibb Company | Compositions comprising an anti-lag-3 antibody or an anti-lag-3 antibody and an anti-pd-1 or anti-pd-l1 antibody |
| EP4245375A2 (en) | 2017-05-30 | 2023-09-20 | Bristol-Myers Squibb Company | Compositions comprising a combination of an anti-lag-3 antibody, a pd-1 pathway inhibitor, and an immunotherapeutic agent |
| US11807686B2 (en) | 2017-05-30 | 2023-11-07 | Bristol-Myers Squibb Company | Treatment of LAG-3 positive tumors |
| EP4306542A2 (en) | 2017-05-30 | 2024-01-17 | Bristol-Myers Squibb Company | Treatment of lag-3 positive tumors |
| WO2018220546A1 (en) | 2017-05-31 | 2018-12-06 | Novartis Ag | Crystalline forms of 5-bromo-2,6-di(1 h-pyrazol-1-yl)pyrimidin-4-amine and new salts |
| WO2018222901A1 (en) | 2017-05-31 | 2018-12-06 | Elstar Therapeutics, Inc. | Multispecific molecules that bind to myeloproliferative leukemia (mpl) protein and uses thereof |
| US11168144B2 (en) | 2017-06-01 | 2021-11-09 | Cytomx Therapeutics, Inc. | Activatable anti-PDL1 antibodies, and methods of use thereof |
| WO2018220446A1 (en) | 2017-06-01 | 2018-12-06 | Compugen Ltd. | Triple combination antibody therapies |
| WO2018223002A1 (en) | 2017-06-01 | 2018-12-06 | Xencor, Inc. | Bispecific antibodies that bind cd 123 cd3 |
| WO2018223004A1 (en) | 2017-06-01 | 2018-12-06 | Xencor, Inc. | Bispecific antibodies that bind cd20 and cd3 |
| WO2018223040A1 (en) | 2017-06-01 | 2018-12-06 | Bristol-Myers Squibb Company | Methods of treating a tumor using an anti-pd-1 antibody |
| US11566073B2 (en) | 2017-06-01 | 2023-01-31 | Bristol-Myers Squibb Company | Methods of treating a tumor using an anti-PD-1 antibody |
| WO2018225093A1 (en) | 2017-06-07 | 2018-12-13 | Glaxosmithkline Intellectual Property Development Limited | Chemical compounds as atf4 pathway inhibitors |
| WO2018225033A1 (en) | 2017-06-09 | 2018-12-13 | Glaxosmithkline Intellectual Property Development Limited | Combination therapy |
| WO2018229715A1 (en) | 2017-06-16 | 2018-12-20 | Novartis Ag | Compositions comprising anti-cd32b antibodies and methods of use thereof |
| US11542312B2 (en) | 2017-06-19 | 2023-01-03 | Medicenna Therapeutics, Inc. | IL-2 superagonists in combination with anti-PD-1 antibodies |
| US11440960B2 (en) | 2017-06-20 | 2022-09-13 | Kymab Limited | TIGIT antibodies, encoding nucleic acids and methods of using said antibodies in vivo |
| WO2018237173A1 (en) | 2017-06-22 | 2018-12-27 | Novartis Ag | Antibody molecules to cd73 and uses thereof |
| WO2018235056A1 (en) | 2017-06-22 | 2018-12-27 | Novartis Ag | Il-1beta binding antibodies for use in treating cancer |
| WO2018234879A1 (en) | 2017-06-22 | 2018-12-27 | Novartis Ag | Il-1beta binding antibodies for use in treating cancer |
| WO2018237157A1 (en) | 2017-06-22 | 2018-12-27 | Novartis Ag | Antibody molecules to cd73 and uses thereof |
| US11597768B2 (en) | 2017-06-26 | 2023-03-07 | Beigene, Ltd. | Immunotherapy for hepatocellular carcinoma |
| US11560425B2 (en) | 2017-06-27 | 2023-01-24 | Neuracle Science Co., Ltd. | Use of anti-FAM19A5 antibodies for treating cancers |
| WO2019006007A1 (en) | 2017-06-27 | 2019-01-03 | Novartis Ag | Dosage regimens for anti-tim-3 antibodies and uses thereof |
| US11236049B2 (en) | 2017-06-30 | 2022-02-01 | Bristol-Myers Squibb Company | Amorphous and crystalline forms of IDO inhibitors |
| WO2019006283A1 (en) | 2017-06-30 | 2019-01-03 | Bristol-Myers Squibb Company | Amorphous and crystalline forms of ido inhibitors |
| WO2019008506A1 (en) | 2017-07-03 | 2019-01-10 | Glaxosmithkline Intellectual Property Development Limited | N-(3-(2-(4-chlorophenoxy)acetamido)bicyclo[1.1.1]pentan-1-yl)-2-cyclobutane-1-carboxamide derivatives and related compounds as atf4 inhibitors for treating cancer and other diseases |
| WO2019008507A1 (en) | 2017-07-03 | 2019-01-10 | Glaxosmithkline Intellectual Property Development Limited | 2-(4-chlorophenoxy)-n-((1 -(2-(4-chlorophenoxy)ethynazetidin-3-yl)methyl)acetamide derivatives and related compounds as atf4 inhibitors for treating cancer and other diseases |
| WO2019014402A1 (en) | 2017-07-14 | 2019-01-17 | Innate Tumor Immunity, Inc. | Nlrp3 modulators |
| EP3431088A1 (en) * | 2017-07-20 | 2019-01-23 | Paris Sciences et Lettres - Quartier Latin | Mntbap for use in inducing a transient immune tolerance |
| WO2019018730A1 (en) | 2017-07-20 | 2019-01-24 | Novartis Ag | Dosage regimens of anti-lag-3 antibodies and uses thereof |
| WO2019021208A1 (en) | 2017-07-27 | 2019-01-31 | Glaxosmithkline Intellectual Property Development Limited | Indazole derivatives useful as perk inhibitors |
| WO2019025545A1 (en) | 2017-08-04 | 2019-02-07 | Genmab A/S | Binding agents binding to pd-l1 and cd137 and use thereof |
| US12049520B2 (en) | 2017-08-04 | 2024-07-30 | Bicycletx Limited | Bicyclic peptide ligands specific for CD137 |
| WO2019035938A1 (en) | 2017-08-16 | 2019-02-21 | Elstar Therapeutics, Inc. | Multispecific molecules that bind to bcma and uses thereof |
| US11555026B2 (en) | 2017-08-17 | 2023-01-17 | Ikena Oncology, Inc. | AHR inhibitors and uses thereof |
| US10696650B2 (en) | 2017-08-17 | 2020-06-30 | Ikena Oncology, Inc. | AHR inhibitors and uses thereof |
| WO2019040780A1 (en) | 2017-08-25 | 2019-02-28 | Five Prime Therapeutics Inc. | B7-h4 antibodies and methods of use thereof |
| US11306144B2 (en) | 2017-08-25 | 2022-04-19 | Five Prime Therapeutics, Inc. | B7-H4 antibodies and methods of use thereof |
| US11814431B2 (en) | 2017-08-25 | 2023-11-14 | Five Prime Therapeutics, Inc. | B7-H4 antibodies and methods of use thereof |
| WO2019049061A1 (en) | 2017-09-07 | 2019-03-14 | Glaxosmithkline Intellectual Property Development Limited | 5-(1 h-benzo[d]imidazo-2-yl)-pyridin-2-amine and 5-(3h-imidazo[4,5-b]pyridin-6-yl)-pyridin-2-amine derivatives as c-myc and p300/cbp histone acetyltransferase inhibitors for treating cancer |
| WO2019053617A1 (en) | 2017-09-12 | 2019-03-21 | Glaxosmithkline Intellectual Property Development Limited | Chemical compounds |
| US11497756B2 (en) | 2017-09-12 | 2022-11-15 | Sumitomo Pharma Oncology, Inc. | Treatment regimen for cancers that are insensitive to BCL-2 inhibitors using the MCL-1 inhibitor alvocidib |
| WO2019059411A1 (en) | 2017-09-20 | 2019-03-28 | Chugai Seiyaku Kabushiki Kaisha | Dosage regimen for combination therapy using pd-1 axis binding antagonists and gpc3 targeting agent |
| US11358948B2 (en) | 2017-09-22 | 2022-06-14 | Kymera Therapeutics, Inc. | CRBN ligands and uses thereof |
| US11623932B2 (en) | 2017-09-22 | 2023-04-11 | Kymera Therapeutics, Inc. | Protein degraders and uses thereof |
| WO2019074822A1 (en) | 2017-10-09 | 2019-04-18 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2019074824A1 (en) | 2017-10-09 | 2019-04-18 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| US11203592B2 (en) | 2017-10-09 | 2021-12-21 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2019072868A1 (en) | 2017-10-10 | 2019-04-18 | Numab Therapeutics AG | Multispecific antibody |
| WO2019075090A1 (en) | 2017-10-10 | 2019-04-18 | Tilos Therapeutics, Inc. | Anti-lap antibodies and uses thereof |
| EP3470429A1 (en) * | 2017-10-10 | 2019-04-17 | Numab Innovation AG | Antibodies targeting pdl1 and methods of use thereof |
| US12077588B2 (en) | 2017-10-10 | 2024-09-03 | Numab Therapeutics AG | Antibodies targeting PDL1 and methods of use thereof |
| US11230601B2 (en) | 2017-10-10 | 2022-01-25 | Tilos Therapeutics, Inc. | Methods of using anti-lap antibodies |
| CN111225926A (en) * | 2017-10-10 | 2020-06-02 | 努玛治疗有限公司 | Antibodies targeting PDL1 and methods of use thereof |
| WO2019072869A1 (en) | 2017-10-10 | 2019-04-18 | Numab Innovation Ag | Antibodies targeting pdl1 and methods of use thereof |
| EP3470426A1 (en) | 2017-10-10 | 2019-04-17 | Numab Therapeutics AG | Multispecific antibody |
| WO2019072870A1 (en) | 2017-10-10 | 2019-04-18 | Numab Innovation Ag | Antibodies targeting cd137 and methods of use thereof |
| US11525002B2 (en) | 2017-10-11 | 2022-12-13 | Board Of Regents, The University Of Texas System | Human PD-L1 antibodies and methods of use therefor |
| EP3694545A4 (en) * | 2017-10-11 | 2021-12-01 | Board Of Regents, The University Of Texas System | Human pd-l1 antibodies and methods of use therefor |
| EP4116327A1 (en) * | 2017-10-11 | 2023-01-11 | Board Of Regents, The University Of Texas System | Human pd-l1 antibodies and methods of use therefor |
| WO2019075032A1 (en) | 2017-10-13 | 2019-04-18 | Merck Patent Gmbh | Combination of a parp inhibitor and a pd-1 axis binding antagonist |
| US11976125B2 (en) | 2017-10-13 | 2024-05-07 | Harpoon Therapeutics, Inc. | B cell maturation antigen binding proteins |
| US11713356B2 (en) | 2017-10-13 | 2023-08-01 | Ose Immunotherapeutics | Modified bifunctional anti-human signal regulatory protein alpha (SIRPa) antibody and method of use thereof for treating cancer |
| WO2019075468A1 (en) | 2017-10-15 | 2019-04-18 | Bristol-Myers Squibb Company | Methods of treating tumor |
| US11919957B2 (en) | 2017-10-15 | 2024-03-05 | Bristol-Myers Squibb Company | Methods of treating tumor |
| WO2019077062A1 (en) | 2017-10-18 | 2019-04-25 | Vivia Biotech, S.L. | Bite-activated car-t cells |
| WO2019079520A2 (en) | 2017-10-18 | 2019-04-25 | Alpine Immune Sciences, Inc. | Variant icos ligand immunomodulatory proteins and related compositions and methods |
| US11613566B2 (en) | 2017-10-18 | 2023-03-28 | Alpine Immune Sciences, Inc. | Variant ICOS ligand immunomodulatory proteins and related compositions and methods |
| WO2019077132A1 (en) | 2017-10-19 | 2019-04-25 | Debiopharm International S.A. | Combination product for the treatment of cancer |
| WO2019081983A1 (en) | 2017-10-25 | 2019-05-02 | Novartis Ag | Antibodies targeting cd32b and methods of use thereof |
| WO2019089753A2 (en) | 2017-10-31 | 2019-05-09 | Compass Therapeutics Llc | Cd137 antibodies and pd-1 antagonists and uses thereof |
| WO2019089921A1 (en) | 2017-11-01 | 2019-05-09 | Bristol-Myers Squibb Company | Immunostimulatory agonistic antibodies for use in treating cancer |
| WO2019090330A1 (en) | 2017-11-06 | 2019-05-09 | Bristol-Myers Squibb Company | Methods of treating a tumor |
| WO2019090198A1 (en) | 2017-11-06 | 2019-05-09 | Bristol-Myers Squibb Company | Isofuranone compounds useful as hpk1 inhibitors |
| WO2019097369A1 (en) | 2017-11-14 | 2019-05-23 | Pfizer Inc. | Ezh2 inhibitor combination therapies |
| WO2019097479A1 (en) | 2017-11-17 | 2019-05-23 | Novartis Ag | Novel dihydroisoxazole compounds and their use for the treatment of hepatitis b |
| WO2019101347A1 (en) | 2017-11-27 | 2019-05-31 | Ose Immunotherapeutics | Improved treatment of cancer |
| US11638760B2 (en) | 2017-11-27 | 2023-05-02 | Mersana Therapeutics, Inc. | Pyrrolobenzodiazepine antibody conjugates |
| WO2019104289A1 (en) | 2017-11-27 | 2019-05-31 | Mersana Therapeutics, Inc. | Pyrrolobenzodiazepine antibody conjugates |
| US11786529B2 (en) | 2017-11-29 | 2023-10-17 | Beigene Switzerland Gmbh | Treatment of indolent or aggressive B-cell lymphomas using a combination comprising BTK inhibitors |
| WO2019108900A1 (en) | 2017-11-30 | 2019-06-06 | Novartis Ag | Bcma-targeting chimeric antigen receptor, and uses thereof |
| WO2019113464A1 (en) | 2017-12-08 | 2019-06-13 | Elstar Therapeutics, Inc. | Multispecific molecules and uses thereof |
| WO2019123207A1 (en) | 2017-12-18 | 2019-06-27 | Pfizer Inc. | Methods and combination therapy to treat cancer |
| US11629189B2 (en) | 2017-12-19 | 2023-04-18 | Kymab Limited | Bispecific antibody for ICOS and PD-L1 |
| WO2019122884A1 (en) | 2017-12-19 | 2019-06-27 | Kymab Limited | Antibodies to icos |
| WO2019123285A1 (en) | 2017-12-20 | 2019-06-27 | Novartis Ag | Fused tricyclic pyrazolo-dihydropyrazinyl-pyridone compounds as antivirals |
| US11234977B2 (en) | 2017-12-20 | 2022-02-01 | Novartis Ag | Fused tricyclic pyrazolo-dihydropyrazinyl-pyridone compounds as antivirals |
| WO2019126691A1 (en) | 2017-12-21 | 2019-06-27 | Mersana Therapeutics, Inc. | Pyrrolobenzodiazepine antibody conjugates |
| US10874743B2 (en) | 2017-12-26 | 2020-12-29 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11318205B1 (en) | 2017-12-26 | 2022-05-03 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11723980B2 (en) | 2017-12-26 | 2023-08-15 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| WO2019133747A1 (en) | 2017-12-27 | 2019-07-04 | Bristol-Myers Squibb Company | Anti-cd40 antibodies and uses thereof |
| US11306149B2 (en) | 2017-12-27 | 2022-04-19 | Bristol-Myers Squibb Company | Anti-CD40 antibodies and uses thereof |
| US11952427B2 (en) | 2017-12-27 | 2024-04-09 | Bristol-Myers Squibb Company | Anti-CD40 antibodies and uses thereof |
| WO2019129211A1 (en) * | 2017-12-28 | 2019-07-04 | Nanjing Legend Biotech Co., Ltd. | Antibodies and variants thereof against pd-l1 |
| US11865081B2 (en) | 2017-12-29 | 2024-01-09 | Virogin Biotech Canada Ltd. | Oncolytic viral delivery of therapeutic polypeptides |
| WO2019134946A1 (en) | 2018-01-04 | 2019-07-11 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating melanoma resistant |
| US11447449B2 (en) | 2018-01-05 | 2022-09-20 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2019136112A1 (en) | 2018-01-05 | 2019-07-11 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2019136432A1 (en) | 2018-01-08 | 2019-07-11 | Novartis Ag | Immune-enhancing rnas for combination with chimeric antigen receptor therapy |
| US11702461B2 (en) | 2018-01-09 | 2023-07-18 | Cue Biopharma, Inc. | T-cell modulatory multimeric polypeptides comprising reduced-affinity immunomodulatory polypeptides |
| WO2019140229A1 (en) | 2018-01-12 | 2019-07-18 | Bristol-Myers Squibb Company | Antibodies against tim3 and uses thereof |
| WO2019140150A1 (en) | 2018-01-12 | 2019-07-18 | Bristol-Myers Squibb Company | Combination therapy with anti-il-8 antibodies and anti-pd-1 antibodies for treating cancer |
| US11485743B2 (en) | 2018-01-12 | 2022-11-01 | Kymera Therapeutics, Inc. | Protein degraders and uses thereof |
| US11512080B2 (en) | 2018-01-12 | 2022-11-29 | Kymera Therapeutics, Inc. | CRBN ligands and uses thereof |
| US11932635B2 (en) | 2018-01-12 | 2024-03-19 | Kymera Therapeutics, Inc. | CRBN ligands and uses thereof |
| US12006329B2 (en) | 2018-01-12 | 2024-06-11 | Kymera Therapeutics, Inc. | Protein degraders and uses thereof |
| WO2019143607A1 (en) | 2018-01-16 | 2019-07-25 | Bristol-Myers Squibb Company | Methods of treating cancer with antibodies against tim3 |
| WO2019144126A1 (en) | 2018-01-22 | 2019-07-25 | Pascal Biosciences Inc. | Cannabinoids and derivatives for promoting immunogenicity of tumor and infected cells |
| WO2019144098A1 (en) | 2018-01-22 | 2019-07-25 | Bristol-Myers Squibb Company | Compositions and methods of treating cancer |
| WO2019148132A1 (en) | 2018-01-29 | 2019-08-01 | Merck Patent Gmbh | Gcn2 inhibitors and uses thereof |
| US10793563B2 (en) | 2018-01-29 | 2020-10-06 | Merck Patent Gmbh | GCN2 inhibitors and uses thereof |
| US10988477B2 (en) | 2018-01-29 | 2021-04-27 | Merck Patent Gmbh | GCN2 inhibitors and uses thereof |
| US12084438B2 (en) | 2018-01-29 | 2024-09-10 | Merck Patent Gmbh | GCN2 inhibitors and uses thereof |
| WO2019152660A1 (en) | 2018-01-31 | 2019-08-08 | Novartis Ag | Combination therapy using a chimeric antigen receptor |
| WO2019152743A1 (en) | 2018-01-31 | 2019-08-08 | Celgene Corporation | Combination therapy using adoptive cell therapy and checkpoint inhibitor |
| EP4317972A2 (en) | 2018-02-06 | 2024-02-07 | The General Hospital Corporation | Repeat rna as biomarkers of tumor immune response |
| WO2019160956A1 (en) | 2018-02-13 | 2019-08-22 | Novartis Ag | Chimeric antigen receptor therapy in combination with il-15r and il15 |
| WO2019162325A1 (en) | 2018-02-21 | 2019-08-29 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Use of sk1 as biomarker for predicting response to immunecheckpoint inhibitors |
| WO2019165315A1 (en) | 2018-02-23 | 2019-08-29 | Syntrix Biosystems Inc. | Method for treating cancer using chemokine antagonists alone or in combination |
| WO2019166951A1 (en) | 2018-02-28 | 2019-09-06 | Novartis Ag | Indole-2-carbonyl compounds and their use for the treatment of hepatitis b |
| US11939383B2 (en) | 2018-03-02 | 2024-03-26 | Five Prime Therapeutics, Inc. | B7-H4 antibodies and methods and use thereof |
| US11884723B2 (en) | 2018-03-13 | 2024-01-30 | Ose Immunotherapeutics | Use of anti-human SIRPa v1 antibodies and method for producing anti-SIRPa v1 antibodies |
| US10738128B2 (en) | 2018-03-14 | 2020-08-11 | Surface Oncology, Inc. | Antibodies that bind CD39 and uses thereof |
| WO2019178364A2 (en) | 2018-03-14 | 2019-09-19 | Elstar Therapeutics, Inc. | Multifunctional molecules and uses thereof |
| EP4043496A1 (en) | 2018-03-14 | 2022-08-17 | Surface Oncology, Inc. | Antibodies that bind cd39 and uses thereof |
| WO2019178362A1 (en) | 2018-03-14 | 2019-09-19 | Elstar Therapeutics, Inc. | Multifunctional molecules that bind to calreticulin and uses thereof |
| WO2019175243A1 (en) | 2018-03-14 | 2019-09-19 | Merck Patent Gmbh | Compounds and uses thereof to treat tumors in a subject |
| US10793637B2 (en) | 2018-03-14 | 2020-10-06 | Surface Oncology, Inc. | Antibodies that bind CD39 and uses thereof |
| WO2019178269A2 (en) | 2018-03-14 | 2019-09-19 | Surface Oncology, Inc. | Antibodies that bind cd39 and uses thereof |
| EP3768719A4 (en) * | 2018-03-19 | 2022-04-27 | Abeome Corporation | High affinity neutralizing monoclonal antibodies to programmed death ligand 1 (pd-l1) and uses thereof |
| WO2019183040A1 (en) | 2018-03-21 | 2019-09-26 | Five Prime Therapeutics, Inc. | ANTIBODIES BINDING TO VISTA AT ACIDIC pH |
| US11332524B2 (en) | 2018-03-22 | 2022-05-17 | Surface Oncology, Inc. | Anti-IL-27 antibodies and uses thereof |
| US11976128B2 (en) | 2018-03-23 | 2024-05-07 | Board Of Regents, The University Of Texas System | Human PD-L2 antibodies and methods of use therefor |
| US11242393B2 (en) | 2018-03-23 | 2022-02-08 | Bristol-Myers Squibb Company | Antibodies against MICA and/or MICB and uses thereof |
| US11952424B2 (en) | 2018-03-30 | 2024-04-09 | Merus N.V. | Multivalent antibody |
| WO2019191676A1 (en) | 2018-03-30 | 2019-10-03 | Bristol-Myers Squibb Company | Methods of treating tumor |
| WO2019190327A2 (en) | 2018-03-30 | 2019-10-03 | Merus N.V. | Multivalent antibody |
| WO2019193541A1 (en) | 2018-04-06 | 2019-10-10 | Glaxosmithkline Intellectual Property Development Limited | Bicyclic aromatic ring derivatives of formula (i) as atf4 inhibitors |
| WO2019193540A1 (en) | 2018-04-06 | 2019-10-10 | Glaxosmithkline Intellectual Property Development Limited | Heteroaryl derivatives of formula (i) as atf4 inhibitors |
| WO2019200256A1 (en) | 2018-04-12 | 2019-10-17 | Bristol-Myers Squibb Company | Anticancer combination therapy with cd73 antagonist antibody and pd-1/pd-l1 axis antagonist antibody |
| WO2019200229A1 (en) | 2018-04-13 | 2019-10-17 | Novartis Ag | Dosage regimens for anti-pd-l1 antibodies and uses thereof |
| WO2019204257A1 (en) | 2018-04-16 | 2019-10-24 | Arrys Therapeutics, Inc. | Ep4 inhibitors and use thereof |
| WO2019204592A1 (en) | 2018-04-18 | 2019-10-24 | Xencor, Inc. | Il-15/il-15ra heterodimeric fc fusion proteins and uses thereof |
| WO2019204665A1 (en) | 2018-04-18 | 2019-10-24 | Xencor, Inc. | Pd-1 targeted heterodimeric fusion proteins containing il-15/il-15ra fc-fusion proteins and pd-1 antigen binding domains and uses thereof |
| WO2019204743A1 (en) | 2018-04-19 | 2019-10-24 | Checkmate Pharmaceuticals, Inc. | Synthetic rig-i-like receptor agonists |
| WO2019209896A1 (en) | 2018-04-25 | 2019-10-31 | Innate Tumor Immunity, Inc. | Nlrp3 modulators |
| EP4353235A2 (en) | 2018-04-25 | 2024-04-17 | Innate Tumor Immunity, Inc. | Nlrp3 modulators |
| WO2019213340A1 (en) | 2018-05-03 | 2019-11-07 | Bristol-Myers Squibb Company | Uracil derivatives as mer-axl inhibitors |
| WO2019211489A1 (en) | 2018-05-04 | 2019-11-07 | Merck Patent Gmbh | COMBINED INHIBITION OF PD-1/PD-L1, TGFβ AND DNA-PK FOR THE TREATMENT OF CANCER |
| WO2019229658A1 (en) | 2018-05-30 | 2019-12-05 | Novartis Ag | Entpd2 antibodies, combination therapies, and methods of using the antibodies and combination therapies |
| WO2019232244A2 (en) | 2018-05-31 | 2019-12-05 | Novartis Ag | Antibody molecules to cd73 and uses thereof |
| WO2019229701A2 (en) | 2018-06-01 | 2019-12-05 | Novartis Ag | Binding molecules against bcma and uses thereof |
| WO2019232528A1 (en) | 2018-06-01 | 2019-12-05 | Xencor, Inc. | Dosing of a bispecific antibody that bind cd123 and cd3 |
| WO2019246528A1 (en) * | 2018-06-22 | 2019-12-26 | Vyriad | Methods of treating cancer using combination therapy |
| US11453702B2 (en) | 2018-06-22 | 2022-09-27 | Bicycletx Limited | Bicyclic peptide ligands specific for Nectin-4 |
| US11180531B2 (en) | 2018-06-22 | 2021-11-23 | Bicycletx Limited | Bicyclic peptide ligands specific for Nectin-4 |
| WO2019243832A1 (en) | 2018-06-22 | 2019-12-26 | Bicycletx Limited | Bicyclic peptide ligands specific for nectin-4 |
| WO2019243833A1 (en) | 2018-06-22 | 2019-12-26 | Bicycletx Limited | Bicyclic peptide ligands specific for nectin-4 |
| US11912792B2 (en) | 2018-06-22 | 2024-02-27 | Bicycletx Limited | Bicyclic peptide ligands specific for nectin-4 |
| WO2020006018A1 (en) | 2018-06-27 | 2020-01-02 | Bristol-Myers Squibb Company | Substituted naphthyridinone compounds useful as t cell activators |
| WO2020006016A1 (en) | 2018-06-27 | 2020-01-02 | Bristol-Myers Squibb Company | Naphthyridinone compounds useful as t cell activators |
| WO2020010250A2 (en) | 2018-07-03 | 2020-01-09 | Elstar Therapeutics, Inc. | Anti-tcr antibody molecules and uses thereof |
| US11845797B2 (en) | 2018-07-03 | 2023-12-19 | Marengo Therapeutics, Inc. | Anti-TCR antibody molecules and uses thereof |
| DE202019005887U1 (en) | 2018-07-03 | 2023-06-14 | Marengo Therapeutics, Inc. | Anti-TCR antibody molecules and uses thereof |
| US11965025B2 (en) | 2018-07-03 | 2024-04-23 | Marengo Therapeutics, Inc. | Method of treating solid cancers with bispecific interleukin-anti-TCRß molecules |
| US11292792B2 (en) | 2018-07-06 | 2022-04-05 | Kymera Therapeutics, Inc. | Tricyclic CRBN ligands and uses thereof |
| WO2020010177A1 (en) | 2018-07-06 | 2020-01-09 | Kymera Therapeutics, Inc. | Tricyclic crbn ligands and uses thereof |
| US11897882B2 (en) | 2018-07-06 | 2024-02-13 | Kymera Therapeutics, Inc. | Tricyclic crbn ligands and uses thereof |
| WO2020012339A1 (en) | 2018-07-09 | 2020-01-16 | Glaxosmithkline Intellectual Property Development Limited | Chemical compounds |
| US11401328B2 (en) | 2018-07-09 | 2022-08-02 | Five Prime Therapeutics, Inc. | Antibodies binding to ILT4 |
| WO2020014132A2 (en) | 2018-07-09 | 2020-01-16 | Five Prime Therapeutics, Inc. | Antibodies binding to ilt4 |
| EP4306111A2 (en) | 2018-07-10 | 2024-01-17 | Novartis AG | 3-(5-hydroxy-1-oxoisoindolin-2-yl)piperidine-2,6-dione derivatives and uses thereof |
| WO2020012334A1 (en) | 2018-07-10 | 2020-01-16 | Novartis Ag | 3-(5-hydroxy-1-oxoisoindolin-2-yl)piperidine-2,6-dione derivatives and their use in the treatment of ikaros family zinc finger 2 (ikzf2)-dependent diseases |
| WO2020012337A1 (en) | 2018-07-10 | 2020-01-16 | Novartis Ag | 3-(5-amino-1-oxoisoindolin-2-yl)piperidine-2,6-dione derivatives and their use in the treatment of i karos family zinc finger 2 (ikzf2)-dependent diseases |
| WO2020014327A2 (en) | 2018-07-11 | 2020-01-16 | Five Prime Therapeutics, Inc. | Antibodies binding to vista at acidic ph |
| WO2020018680A1 (en) | 2018-07-18 | 2020-01-23 | Arcus Biosciences, Inc. | Solid forms of an azolopyrimidine compound |
| US11279758B2 (en) | 2018-07-20 | 2022-03-22 | Surface Oncology, Inc. | Anti-CD112R compositions and methods |
| US11214619B2 (en) | 2018-07-20 | 2022-01-04 | Surface Oncology, Inc. | Anti-CD112R compositions and methods |
| US12059420B2 (en) | 2018-07-23 | 2024-08-13 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2020023356A1 (en) | 2018-07-23 | 2020-01-30 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2020023355A1 (en) | 2018-07-23 | 2020-01-30 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| US11034771B2 (en) | 2018-07-25 | 2021-06-15 | I-Mab Biopharma Us Limited | Anti-CD73 anti-PD-L1 bispecific antibodies |
| WO2020023707A1 (en) | 2018-07-26 | 2020-01-30 | Bristol-Myers Squibb Company | Lag-3 combination therapy for the treatment of cancer |
| WO2020027224A1 (en) | 2018-07-31 | 2020-02-06 | 国立大学法人東京大学 | Super versatile method for imparting new binding specificity to antibody |
| WO2020031107A1 (en) | 2018-08-08 | 2020-02-13 | Glaxosmithkline Intellectual Property Development Limited | Chemical compounds |
| WO2020037092A1 (en) | 2018-08-16 | 2020-02-20 | Innate Tumor Immunity, Inc. | Imidazo[4,5-c]quinoline derived nlrp3-modulators |
| WO2020037091A1 (en) | 2018-08-16 | 2020-02-20 | Innate Tumor Immunity, Inc. | Imidazo[4,5-c]quinoline derived nlrp3-modulators |
| WO2020037094A1 (en) | 2018-08-16 | 2020-02-20 | Innate Tumor Immunity, Inc. | Substitued 4-amino-1h-imidazo[4,5-c]quinoline compounds and improved methods for their preparation |
| EP3841126A4 (en) * | 2018-08-20 | 2022-08-10 | 1Globe Biomedical Co., Ltd. | Novel cancer immunotherapy antibody compositions |
| US11566066B2 (en) | 2018-08-20 | 2023-01-31 | Pfizer Inc. | Anti-GDF15 antibodies, compositions and methods of use |
| WO2020039321A2 (en) | 2018-08-20 | 2020-02-27 | Pfizer Inc. | Anti-gdf15 antibodies, compositions and methods of use |
| WO2020043683A1 (en) | 2018-08-27 | 2020-03-05 | Pieris Pharmaceuticals Gmbh | Combination therapies comprising cd137/her2 bispecific agents and pd-1 axis inhibitors and uses thereof |
| US11253525B2 (en) | 2018-08-29 | 2022-02-22 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| US10959986B2 (en) | 2018-08-29 | 2021-03-30 | Bristol-Myers Squibb Company | Inhibitors of indoleamine 2,3-dioxygenase and methods of their use |
| WO2020051424A1 (en) | 2018-09-07 | 2020-03-12 | Pic Therapeutics | Eif4e inhibitors and uses thereof |
| US11072610B2 (en) | 2018-09-12 | 2021-07-27 | Novartis Ag | Antiviral pyridopyrazinedione compounds |
| WO2020053654A1 (en) | 2018-09-12 | 2020-03-19 | Novartis Ag | Antiviral pyridopyrazinedione compounds |
| US11807692B2 (en) | 2018-09-25 | 2023-11-07 | Harpoon Therapeutics, Inc. | DLL3 binding proteins and methods of use |
| WO2020064971A1 (en) | 2018-09-26 | 2020-04-02 | Merck Patent Gmbh | Combination of a pd-1 antagonist, an atr inhibitor and a platinating agent for the treatment of cancer |
| WO2020069372A1 (en) | 2018-09-27 | 2020-04-02 | Elstar Therapeutics, Inc. | Csf1r/ccr2 multispecific antibodies |
| WO2020072821A2 (en) | 2018-10-03 | 2020-04-09 | Xencor, Inc. | Il-12 heterodimeric fc-fusion proteins |
| US11130802B2 (en) | 2018-10-10 | 2021-09-28 | Tilos Therapeutics, Inc. | Anti-lap antibody variants |
| WO2020076969A2 (en) | 2018-10-10 | 2020-04-16 | Tilos Therapeutics, Inc. | Anti-lap antibody variants and uses thereof |
| WO2020077276A2 (en) | 2018-10-12 | 2020-04-16 | Xencor, Inc. | Pd-1 targeted il-15/il-15ralpha fc fusion proteins and uses in combination therapies thereof |
| WO2020079581A1 (en) | 2018-10-16 | 2020-04-23 | Novartis Ag | Tumor mutation burden alone or in combination with immune markers as biomarkers for predicting response to targeted therapy |
| WO2020081928A1 (en) | 2018-10-19 | 2020-04-23 | Bristol-Myers Squibb Company | Combination therapy for melanoma |
| EP4445958A2 (en) | 2018-10-19 | 2024-10-16 | Bristol-Myers Squibb Company | Combination therapy for melanoma |
| WO2020086476A1 (en) | 2018-10-22 | 2020-04-30 | Glaxosmithkline Intellectual Property Development Limited | Dosing |
| WO2020086479A1 (en) | 2018-10-22 | 2020-04-30 | Glaxosmithkline Intellectual Property Development Limited | Dosing |
| WO2020086724A1 (en) | 2018-10-23 | 2020-04-30 | Bristol-Myers Squibb Company | Methods of treating tumor |
| WO2020092385A1 (en) | 2018-10-29 | 2020-05-07 | Mersana Therapeutics, Inc. | Cysteine engineered antibody-drug conjugates with peptide-containing linkers |
| WO2020089811A1 (en) | 2018-10-31 | 2020-05-07 | Novartis Ag | Dc-sign antibody drug conjugates |
| WO2020093024A2 (en) | 2018-11-01 | 2020-05-07 | Merck Patent Gmbh | Methods of administering anti-tim-3 antibodies |
| WO2020093023A1 (en) | 2018-11-01 | 2020-05-07 | Merck Patent Gmbh | Anti-tim-3 antibodies |
| WO2020095184A1 (en) | 2018-11-05 | 2020-05-14 | Pfizer Inc. | Combinations for treating cancer |
| WO2020095183A1 (en) | 2018-11-05 | 2020-05-14 | Pfizer Inc. | Combination for treating cancer |
| WO2020094744A1 (en) | 2018-11-06 | 2020-05-14 | Genmab A/S | Antibody formulation |
| WO2020102501A1 (en) | 2018-11-16 | 2020-05-22 | Bristol-Myers Squibb Company | Anti-nkg2a antibodies and uses thereof |
| WO2020104496A1 (en) | 2018-11-20 | 2020-05-28 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Bispecific antibody targeting transferrin receptor 1 and soluble antigen |
| WO2020104479A1 (en) | 2018-11-20 | 2020-05-28 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating cancers and resistant cancers with anti transferrin receptor 1 antibodies |
| WO2020109328A1 (en) | 2018-11-26 | 2020-06-04 | Debiopharm International S.A. | Combination treatment of hiv infections |
| WO2020110056A1 (en) | 2018-11-30 | 2020-06-04 | Glaxosmithkline Intellectual Property Development Limited | Compounds useful in hiv therapy |
| US11117889B1 (en) | 2018-11-30 | 2021-09-14 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11807636B2 (en) | 2018-11-30 | 2023-11-07 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11352350B2 (en) | 2018-11-30 | 2022-06-07 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| WO2020117849A1 (en) | 2018-12-04 | 2020-06-11 | Bristol-Myers Squibb Company | Methods of analysis using in-sample calibration curve by multiple isotopologue reaction monitoring |
| US11530231B2 (en) | 2018-12-04 | 2022-12-20 | Sumitomo Pharma Oncology, Inc. | CDK9 inhibitors and polymorphs thereof for use as agents for treatment of cancer |
| WO2020117988A1 (en) | 2018-12-04 | 2020-06-11 | Tolero Pharmaceuticals, Inc. | Cdk9 inhibitors and polymorphs thereof for use as agents for treatment of cancer |
| US11034710B2 (en) | 2018-12-04 | 2021-06-15 | Sumitomo Dainippon Pharma Oncology, Inc. | CDK9 inhibitors and polymorphs thereof for use as agents for treatment of cancer |
| US12077554B2 (en) | 2018-12-04 | 2024-09-03 | Sumitomo Pharma Oncology, Inc. | CDK9 inhibitors and polymorphs thereof for use as agents for treatment of cancer |
| WO2020115261A1 (en) | 2018-12-07 | 2020-06-11 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating melanoma |
| WO2020120592A1 (en) | 2018-12-12 | 2020-06-18 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for predicting and treating melanoma |
| WO2020127411A1 (en) | 2018-12-19 | 2020-06-25 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating cancers by immuno-modulation using antibodies against cathespin-d |
| WO2020132646A1 (en) | 2018-12-20 | 2020-06-25 | Xencor, Inc. | Targeted heterodimeric fc fusion proteins containing il-15/il-15ra and nkg2d antigen binding domains |
| WO2020128972A1 (en) | 2018-12-20 | 2020-06-25 | Novartis Ag | Dosing regimen and pharmaceutical combination comprising 3-(1-oxoisoindolin-2-yl)piperidine-2,6-dione derivatives |
| EP3670659A1 (en) | 2018-12-20 | 2020-06-24 | Abivax | Biomarkers, and uses in treatment of viral infections, inflammations, or cancer |
| WO2020127853A1 (en) | 2018-12-20 | 2020-06-25 | Abivax | Biomarkers, and uses in treatment of viral infections, inflammations, or cancer |
| WO2020128620A1 (en) | 2018-12-21 | 2020-06-25 | Novartis Ag | Use of il-1beta binding antibodies |
| WO2020128613A1 (en) | 2018-12-21 | 2020-06-25 | Novartis Ag | Use of il-1beta binding antibodies |
| WO2020128637A1 (en) | 2018-12-21 | 2020-06-25 | Novartis Ag | Use of il-1 binding antibodies in the treatment of a msi-h cancer |
| WO2020128893A1 (en) | 2018-12-21 | 2020-06-25 | Pfizer Inc. | Combination treatments of cancer comprising a tlr agonist |
| WO2020127965A1 (en) | 2018-12-21 | 2020-06-25 | Onxeo | New conjugated nucleic acid molecules and their uses |
| WO2020127885A1 (en) | 2018-12-21 | 2020-06-25 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Compositions for treating cancers and resistant cancers |
| WO2020128636A1 (en) | 2018-12-21 | 2020-06-25 | Novartis Ag | Use of il-1 beta antibodies in the treatment or prevention of myelodysplastic syndrome |
| KR20210110838A (en) | 2018-12-28 | 2021-09-09 | 트랜스진 에스.에이. | M2 defective poxvirus |
| WO2020136235A1 (en) | 2018-12-28 | 2020-07-02 | Transgene Sa | M2-defective poxvirus |
| WO2020150115A1 (en) | 2019-01-14 | 2020-07-23 | Innate Tumor Immunity, Inc. | Nlrp3 modulators |
| WO2020150116A1 (en) | 2019-01-14 | 2020-07-23 | Innate Tumor Immunity, Inc. | Nlrp3 modulators |
| WO2020150114A1 (en) | 2019-01-14 | 2020-07-23 | Innate Tumor Immunity, Inc. | Heterocyclic nlrp3 modulators, for use in the treatment of cancer |
| WO2020150113A1 (en) | 2019-01-14 | 2020-07-23 | Innate Tumor Immunity, Inc. | Substituted quinazolines as nlrp3 modulators, for use in the treatment of cancer |
| WO2020157131A1 (en) | 2019-01-30 | 2020-08-06 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for identifying whether a subject suffering from a cancer will achieve a response with an immune-checkpoint inhibitor |
| WO2020161083A1 (en) | 2019-02-04 | 2020-08-13 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for modulating blood-brain barrier |
| US11471456B2 (en) | 2019-02-12 | 2022-10-18 | Sumitomo Pharma Oncology, Inc. | Formulations comprising heterocyclic protein kinase inhibitors |
| WO2020167990A1 (en) | 2019-02-12 | 2020-08-20 | Tolero Pharmaceuticals, Inc. | Formulations comprising heterocyclic protein kinase inhibitors |
| WO2020165370A1 (en) | 2019-02-13 | 2020-08-20 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for selecting a cancer treatment in a subject suffering from cancer |
| WO2020165834A1 (en) | 2019-02-15 | 2020-08-20 | Novartis Ag | Substituted 3-(1-oxoisoindolin-2-yl)piperidine-2,6-dione derivatives and uses thereof |
| WO2020165833A1 (en) | 2019-02-15 | 2020-08-20 | Novartis Ag | 3-(1-oxo-5-(piperidin-4-yl)isoindolin-2-yl)piperidine-2,6-dione derivatives and uses thereof |
| WO2020171757A1 (en) * | 2019-02-18 | 2020-08-27 | Medivir Aktiebolag | Methods for treating liver cancers using an orally administered dioxolane nucleotide in combination with an anti-pd1 or anti-pdl1 monoclonal antibody |
| EP3929213A4 (en) * | 2019-02-21 | 2023-03-08 | Eucure (Beijing) Biopharma Co., Ltd | Anti-pd-l1 antibody and use thereof |
| WO2020187998A1 (en) | 2019-03-19 | 2020-09-24 | Fundació Privada Institut D'investigació Oncològica De Vall Hebron | Combination therapy with omomyc and an antibody binding pd-1 or ctla-4 for the treatment of cancer |
| US11793802B2 (en) | 2019-03-20 | 2023-10-24 | Sumitomo Pharma Oncology, Inc. | Treatment of acute myeloid leukemia (AML) with venetoclax failure |
| WO2020198077A1 (en) | 2019-03-22 | 2020-10-01 | Sumitomo Dainippon Pharma Oncology, Inc. | Compositions comprising pkm2 modulators and methods of treatment using the same |
| US11712433B2 (en) | 2019-03-22 | 2023-08-01 | Sumitomo Pharma Oncology, Inc. | Compositions comprising PKM2 modulators and methods of treatment using the same |
| WO2020198672A1 (en) | 2019-03-28 | 2020-10-01 | Bristol-Myers Squibb Company | Methods of treating tumor |
| WO2020198676A1 (en) | 2019-03-28 | 2020-10-01 | Bristol-Myers Squibb Company | Methods of treating tumor |
| WO2020201753A1 (en) | 2019-04-02 | 2020-10-08 | Bicycletx Limited | Bicycle toxin conjugates and uses thereof |
| US11746120B2 (en) | 2019-04-05 | 2023-09-05 | Kymera Therapeutics, Inc. | Stat degraders and uses thereof |
| US12077555B2 (en) | 2019-04-05 | 2024-09-03 | Kymera Therapeutics, Inc. | STAT degraders and uses thereof |
| US11485750B1 (en) | 2019-04-05 | 2022-11-01 | Kymera Therapeutics, Inc. | STAT degraders and uses thereof |
| EP3833691A4 (en) * | 2019-04-26 | 2022-05-18 | I-Mab Biopharma US Limited | Human pd-l1 antibodies |
| WO2020216379A1 (en) | 2019-04-26 | 2020-10-29 | I-Mab | Human pd-l1 antibodies |
| WO2020221796A1 (en) | 2019-04-30 | 2020-11-05 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating melanoma |
| WO2020239558A1 (en) | 2019-05-24 | 2020-12-03 | Pfizer Inc. | Combination therapies using cdk inhibitors |
| WO2020243563A1 (en) | 2019-05-30 | 2020-12-03 | Bristol-Myers Squibb Company | Multi-tumor gene signatures for suitability to immuno-oncology therapy |
| WO2020243568A1 (en) | 2019-05-30 | 2020-12-03 | Bristol-Myers Squibb Company | Methods of identifying a subject suitable for an immuno-oncology (i-o) therapy |
| WO2020243570A1 (en) | 2019-05-30 | 2020-12-03 | Bristol-Myers Squibb Company | Cell localization signature and combination therapy |
| WO2020243423A1 (en) | 2019-05-31 | 2020-12-03 | Ikena Oncology, Inc. | Tead inhibitors and uses thereof |
| EP3929215A4 (en) * | 2019-06-10 | 2022-06-22 | Shandong Boan Biotechnology Co., Ltd. | Bifunctional fusion protein against pdl1 and tgf? and use thereof |
| WO2020249071A1 (en) * | 2019-06-12 | 2020-12-17 | Nanjing GenScript Biotech Co., Ltd. | Anti-pd-l1/anti-lag-3 multiple antigen binding proteins and methods of use thereof |
| CN114008080B (en) * | 2019-06-12 | 2023-06-09 | 南京金斯瑞生物科技有限公司 | anti-PD-L1/anti-LAG-3 multi-antigen binding proteins and methods of use thereof |
| CN114008080A (en) * | 2019-06-12 | 2022-02-01 | 南京金斯瑞生物科技有限公司 | anti-PD-L1/anti-LAG-3 multiple antigen binding proteins and methods of use thereof |
| US11529350B2 (en) | 2019-07-03 | 2022-12-20 | Sumitomo Pharma Oncology, Inc. | Tyrosine kinase non-receptor 1 (TNK1) inhibitors and uses thereof |
| WO2021003417A1 (en) | 2019-07-03 | 2021-01-07 | Sumitomo Dainippon Pharma Oncology, Inc. | Tyrosine kinase non-receptor 1 (tnk1) inhibitors and uses thereof |
| EP3999534A4 (en) * | 2019-07-19 | 2023-02-08 | Beijing Sinotau Bio-Pharmaceuticals Technology Co., Ltd. | A novel anti-pd-l1 antibody and use thereof |
| WO2021041588A1 (en) | 2019-08-28 | 2021-03-04 | Bristol-Myers Squibb Company | Substituted pyridopyrimidinonyl compounds useful as t cell activators |
| WO2021048292A1 (en) | 2019-09-11 | 2021-03-18 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating melanoma |
| US11034694B2 (en) | 2019-09-13 | 2021-06-15 | Nimbus Saturn, Inc. | Substituted isoindolin-1-ones and 2,3-dihydro-1H-pyrrolo[3,4-c]pyridin-1-ones as HPK1 antagonists |
| US11078201B2 (en) | 2019-09-13 | 2021-08-03 | Nimbus Saturn, Inc. | Substituted isoindolin-1-ones and 2,3-dihydro-1H-pyrrol[3,4-c]pyridin-1-ones as HPK1 antagonists |
| US11548890B1 (en) | 2019-09-13 | 2023-01-10 | Nimbus Saturn, Inc. | HPK1 antagonists and uses thereof |
| US11021481B2 (en) | 2019-09-13 | 2021-06-01 | Nimbus Saturn, Inc. | Substituted isoindolin-1-ones and 2,3-dihydro-1h-pyrrolo[3,4-c]pyridin-1-ones as HPK1 antagonists |
| US11028085B2 (en) | 2019-09-13 | 2021-06-08 | Nimbus Saturn, Inc. | Substituted isoindolin-1-ones and 2,3-dihydro-1h-pyrrolo[3,4-c]pyridin-1-ones as hpk1 antagonists |
| US11655303B2 (en) | 2019-09-16 | 2023-05-23 | Surface Oncology, Inc. | Anti-CD39 antibody compositions and methods |
| WO2021053559A1 (en) | 2019-09-18 | 2021-03-25 | Novartis Ag | Entpd2 antibodies, combination therapies, and methods of using the antibodies and combination therapies |
| WO2021053560A1 (en) | 2019-09-18 | 2021-03-25 | Novartis Ag | Combination therapy with entpd2 and cd73 antibodies |
| WO2021055698A1 (en) | 2019-09-19 | 2021-03-25 | Bristol-Myers Squibb Company | Antibodies binding to vista at acidic ph |
| WO2021053207A1 (en) | 2019-09-20 | 2021-03-25 | Transgene | Combination of a poxvirus encoding hpv polypeptides and il-2 with an anti-pd-l1 antibody |
| WO2021055994A1 (en) | 2019-09-22 | 2021-03-25 | Bristol-Myers Squibb Company | Quantitative spatial profiling for lag-3 antagonist therapy |
| WO2021062018A1 (en) | 2019-09-25 | 2021-04-01 | Bristol-Myers Squibb Company | Composite biomarker for cancer therapy |
| WO2021062244A1 (en) | 2019-09-25 | 2021-04-01 | Surface Oncology, Inc. | Anti-il-27 antibodies and uses thereof |
| US11667613B2 (en) | 2019-09-26 | 2023-06-06 | Novartis Ag | Antiviral pyrazolopyridinone compounds |
| WO2021058711A2 (en) | 2019-09-27 | 2021-04-01 | Glaxosmithkline Intellectual Property Development Limited | Antigen binding proteins |
| WO2021067863A2 (en) | 2019-10-03 | 2021-04-08 | Xencor, Inc. | Targeted il-12 heterodimeric fc-fusion proteins |
| WO2021064180A1 (en) | 2019-10-03 | 2021-04-08 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for modulating macrophages polarization |
| WO2021072298A1 (en) | 2019-10-11 | 2021-04-15 | Genentech, Inc. | Pd-1 targeted il-15/il-15ralpha fc fusion proteins with improved properties |
| WO2021074391A1 (en) | 2019-10-17 | 2021-04-22 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for diagnosing nasal intestinal type adenocarcinomas |
| WO2021079195A1 (en) | 2019-10-21 | 2021-04-29 | Novartis Ag | Tim-3 inhibitors and uses thereof |
| WO2021079188A1 (en) | 2019-10-21 | 2021-04-29 | Novartis Ag | Combination therapies with venetoclax and tim-3 inhibitors |
| WO2021081353A1 (en) | 2019-10-23 | 2021-04-29 | Checkmate Pharmaceuticals, Inc. | Synthetic rig-i-like receptor agonists |
| WO2021079958A1 (en) | 2019-10-25 | 2021-04-29 | 第一三共株式会社 | Combination of anti-garp antibody and immunoregulator |
| KR20220088425A (en) | 2019-10-25 | 2022-06-27 | 다이이찌 산쿄 가부시키가이샤 | Combination of anti-GARP antibodies and immunomodulators |
| WO2021083959A1 (en) | 2019-10-29 | 2021-05-06 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating uveal melanoma |
| WO2021091885A2 (en) | 2019-11-04 | 2021-05-14 | Alector Llc | Siglec-9 ecd fusion molecules and methods of use thereof |
| WO2021092221A1 (en) | 2019-11-06 | 2021-05-14 | Bristol-Myers Squibb Company | Methods of identifying a subject with a tumor suitable for a checkpoint inhibitor therapy |
| WO2021092220A1 (en) | 2019-11-06 | 2021-05-14 | Bristol-Myers Squibb Company | Methods of identifying a subject with a tumor suitable for a checkpoint inhibitor therapy |
| WO2021092380A1 (en) | 2019-11-08 | 2021-05-14 | Bristol-Myers Squibb Company | Lag-3 antagonist therapy for melanoma |
| WO2021097256A1 (en) | 2019-11-14 | 2021-05-20 | Cohbar, Inc. | Cxcr4 antagonist peptides |
| WO2021101919A1 (en) | 2019-11-19 | 2021-05-27 | Bristol-Myers Squibb Company | Compounds useful as inhibitors of helios protein |
| WO2021102343A1 (en) | 2019-11-22 | 2021-05-27 | Sumitomo Dainippon Pharma Oncology, Inc. | Solid dose pharmaceutical composition |
| US11591339B2 (en) | 2019-11-26 | 2023-02-28 | Ikena Oncology, Inc. | Solid forms of (R)-N-(2-(5-fluoropyridin-3-yl)-8-isopropylpyrazolo[ 1,5-a][1,3,5]triazin-4-yl)-2,3,4,9-tetrahydro-1H-carbazol-3-amine maleate as aryl hydrocarbon receptor (AHR) inhibitors |
| WO2021108288A1 (en) | 2019-11-26 | 2021-06-03 | Bristol-Myers Squibb Company | Salts/cocrystals of (r)-n-(4-chlorophenyl)-2-((1s,4s)-4-(6-fluoroquinolin-4-yl)cyclohexyl)propanamide |
| WO2021108528A1 (en) | 2019-11-26 | 2021-06-03 | Ikena Oncology, Inc. | Polymorphic carbazole derivatives and uses thereof |
| WO2021106978A1 (en) | 2019-11-27 | 2021-06-03 | サイトリミック株式会社 | Pharmaceutical composition |
| US11707457B2 (en) | 2019-12-17 | 2023-07-25 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11591332B2 (en) | 2019-12-17 | 2023-02-28 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| US11779578B2 (en) | 2019-12-17 | 2023-10-10 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| WO2021127554A1 (en) | 2019-12-19 | 2021-06-24 | Bristol-Myers Squibb Company | Combinations of dgk inhibitors and checkpoint antagonists |
| WO2021123243A1 (en) | 2019-12-19 | 2021-06-24 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and vaccine compositions to treat cancers |
| WO2021123902A1 (en) | 2019-12-20 | 2021-06-24 | Novartis Ag | Combination of anti tim-3 antibody mbg453 and anti tgf-beta antibody nis793, with or without decitabine or the anti pd-1 antibody spartalizumab, for treating myelofibrosis and myelodysplastic syndrome |
| WO2021123996A1 (en) | 2019-12-20 | 2021-06-24 | Novartis Ag | Uses of anti-tgf-beta antibodies and checkpoint inhibitors for the treatment of proliferative diseases |
| US11679109B2 (en) | 2019-12-23 | 2023-06-20 | Kymera Therapeutics, Inc. | SMARCA degraders and uses thereof |
| WO2021133752A1 (en) | 2019-12-23 | 2021-07-01 | Bristol-Myers Squibb Company | Substituted heteroaryl compounds useful as t cell activators |
| WO2021133750A1 (en) | 2019-12-23 | 2021-07-01 | Bristol-Myers Squibb Company | Substituted bicyclic piperidine derivatives useful as t cell activators |
| WO2021133751A1 (en) | 2019-12-23 | 2021-07-01 | Bristol-Myers Squibb Company | Substituted quinazolinyl compounds useful as t cell activators |
| WO2021133749A1 (en) | 2019-12-23 | 2021-07-01 | Bristol-Myers Squibb Company | Substituted piperazine derivatives useful as t cell activators |
| WO2021133748A1 (en) | 2019-12-23 | 2021-07-01 | Bristol-Myers Squibb Company | Substituted quinolinonyl piperazine compounds useful as t cell activators |
| WO2021138407A2 (en) | 2020-01-03 | 2021-07-08 | Marengo Therapeutics, Inc. | Multifunctional molecules that bind to cd33 and uses thereof |
| WO2021141907A1 (en) | 2020-01-06 | 2021-07-15 | Hifibio (Hong Kong) Limited | Anti-tnfr2 antibody and uses thereof |
| WO2021139682A1 (en) | 2020-01-07 | 2021-07-15 | Hifibio (Hk) Limited | Anti-galectin-9 antibody and uses thereof |
| WO2021142203A1 (en) | 2020-01-10 | 2021-07-15 | Innate Tumor Immunity, Inc. | Nlrp3 modulators |
| US11981705B2 (en) | 2020-01-10 | 2024-05-14 | The Brigham And Women's Hospital, Inc. | Methods and compositions for delivery of immunotherapy agents across the blood-brain barrier to treat brain cancer |
| WO2021146370A1 (en) | 2020-01-15 | 2021-07-22 | Blueprint Medicines Corporation | Map4k1 inhibitors |
| WO2021144426A1 (en) | 2020-01-17 | 2021-07-22 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating melanoma |
| WO2021144657A1 (en) | 2020-01-17 | 2021-07-22 | Novartis Ag | Combination comprising a tim-3 inhibitor and a hypomethylating agent for use in treating myelodysplastic syndrome or chronic myelomonocytic leukemia |
| WO2021152005A1 (en) | 2020-01-28 | 2021-08-05 | Universite De Strasbourg | Antisense oligonucleotide targeting linc00518 for treating melanoma |
| WO2021154073A1 (en) | 2020-01-29 | 2021-08-05 | Merus N.V. | Means and method for modulating immune cell engaging effects. |
| WO2021152548A1 (en) | 2020-01-30 | 2021-08-05 | Benitah Salvador Aznar | Combination therapy for treatment of cancer and cancer metastasis |
| WO2021156360A1 (en) | 2020-02-05 | 2021-08-12 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for discontinuing a treatment with a tyrosine kinase inhibitor (tki) |
| WO2021158938A1 (en) | 2020-02-06 | 2021-08-12 | Bristol-Myers Squibb Company | Il-10 and uses thereof |
| WO2021167964A1 (en) | 2020-02-18 | 2021-08-26 | Alector Llc | Pilra antibodies and methods of use thereof |
| WO2021174045A1 (en) | 2020-02-28 | 2021-09-02 | Bristol-Myers Squibb Company | Radiolabeled fibronectin based scaffolds and antibodies and theranostic uses thereof |
| WO2021170777A1 (en) | 2020-02-28 | 2021-09-02 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for diagnosing, prognosing and managing treatment of breast cancer |
| WO2021171264A1 (en) | 2020-02-28 | 2021-09-02 | Novartis Ag | Dosing of a bispecific antibody that binds cd123 and cd3 |
| WO2021178488A1 (en) | 2020-03-03 | 2021-09-10 | PIC Therapeutics, Inc. | Eif4e inhibitors and uses thereof |
| WO2021178807A1 (en) | 2020-03-06 | 2021-09-10 | Celgene Quanticel Research, Inc. | Combination of an lsd-1 inhibitor and nivolumab for use in treating sclc or sqnsclc |
| WO2021176424A1 (en) | 2020-03-06 | 2021-09-10 | Ona Therapeutics, S.L. | Anti-cd36 antibodies and their use to treat cancer |
| WO2021180643A1 (en) | 2020-03-09 | 2021-09-16 | Universite De Geneve | Hsd11b1 inhibitors for use in immunotherapy and uses thereof |
| WO2021183428A1 (en) | 2020-03-09 | 2021-09-16 | Bristol-Myers Squibb Company | Antibodies to cd40 with enhanced agonist activity |
| EP3878446A1 (en) | 2020-03-09 | 2021-09-15 | Universite De Geneve | Hsd11b1 inhibitors for use in immunotherapy and uses thereof |
| WO2021188769A1 (en) | 2020-03-19 | 2021-09-23 | Arcus Biosciences, Inc. | Tetralin and tetrahydroquinoline compounds as inhibitors of hif-2alpha |
| US11932624B2 (en) | 2020-03-19 | 2024-03-19 | Kymera Therapeutics, Inc. | MDM2 degraders and uses thereof |
| WO2021194914A1 (en) | 2020-03-23 | 2021-09-30 | Bristol-Myers Squibb Company | Substituted oxoisoindoline compounds for the treatment of cancer |
| WO2021207449A1 (en) | 2020-04-09 | 2021-10-14 | Merck Sharp & Dohme Corp. | Affinity matured anti-lap antibodies and uses thereof |
| WO2021209358A1 (en) | 2020-04-14 | 2021-10-21 | Glaxosmithkline Intellectual Property Development Limited | Combination treatment for cancer based upon an icos antibody and a pd-l1 antibody tgf-beta-receptor fusion protein |
| WO2021224215A1 (en) | 2020-05-05 | 2021-11-11 | F. Hoffmann-La Roche Ag | Predicting response to pd-1 axis inhibitors |
| US11878062B2 (en) | 2020-05-12 | 2024-01-23 | Cue Biopharma, Inc. | Multimeric T-cell modulatory polypeptides and methods of use thereof |
| WO2021231732A1 (en) | 2020-05-15 | 2021-11-18 | Bristol-Myers Squibb Company | Antibodies to garp |
| WO2021247591A1 (en) | 2020-06-02 | 2021-12-09 | Arcus Biosciences, Inc. | Antibodies to tigit |
| US11685750B2 (en) | 2020-06-03 | 2023-06-27 | Kymera Therapeutics, Inc. | Crystalline forms of IRAK degraders |
| WO2021249969A1 (en) | 2020-06-10 | 2021-12-16 | Merck Patent Gmbh | Combination product for the treatment of cancer diseases |
| WO2021258010A1 (en) | 2020-06-19 | 2021-12-23 | Gossamer Bio Services, Inc. | Oxime compounds useful as t cell activators |
| WO2021255223A1 (en) | 2020-06-19 | 2021-12-23 | Onxeo | New conjugated nucleic acid molecules and their uses |
| WO2021260528A1 (en) | 2020-06-23 | 2021-12-30 | Novartis Ag | Dosing regimen comprising 3-(1-oxoisoindolin-2-yl)piperidine-2,6-dione derivatives |
| WO2022003554A1 (en) | 2020-07-01 | 2022-01-06 | Pfizer Inc. | Biomarkers for pd-1 axis binding antagonist therapy |
| WO2022023379A1 (en) | 2020-07-28 | 2022-02-03 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for preventing and treating a cancer |
| US11857535B2 (en) | 2020-07-30 | 2024-01-02 | Kymera Therapeutics, Inc. | Methods of treating mutant lymphomas |
| WO2022029573A1 (en) | 2020-08-03 | 2022-02-10 | Novartis Ag | Heteroaryl substituted 3-(1-oxoisoindolin-2-yl)piperidine-2,6-dione derivatives and uses thereof |
| WO2022033419A2 (en) | 2020-08-10 | 2022-02-17 | Shanghai Xbh Biotechnology Co., Ltd. | Compositions and methods for treating autoimmune diseases and cancers by targeting igsf8 |
| WO2022038158A1 (en) | 2020-08-17 | 2022-02-24 | Bicycletx Limited | Bicycle conjugates specific for nectin-4 and uses thereof |
| WO2022047046A1 (en) | 2020-08-26 | 2022-03-03 | Marengo Therapeutics, Inc. | Methods of detecting trbc1 or trbc2 |
| WO2022047189A1 (en) | 2020-08-28 | 2022-03-03 | Bristol-Myers Squibb Company | Lag-3 antagonist therapy for hepatocellular carcinoma |
| WO2022047412A1 (en) | 2020-08-31 | 2022-03-03 | Bristol-Myers Squibb Company | Cell localization signature and immunotherapy |
| US12029782B2 (en) | 2020-09-09 | 2024-07-09 | Cue Biopharma, Inc. | MHC class II T-cell modulatory multimeric polypeptides for treating type 1 diabetes mellitus (T1D) and methods of use thereof |
| WO2022053703A1 (en) | 2020-09-14 | 2022-03-17 | Boehringer Ingelheim International Gmbh | Heterologous prime boost vaccine |
| EP4165086A4 (en) * | 2020-09-16 | 2024-07-31 | Suzhou Neologics Bioscience Co Ltd | Pd-l1 antibodies, fusion proteins, and uses thereof |
| WO2022076318A1 (en) | 2020-10-05 | 2022-04-14 | Bristol-Myers Squibb Company | Methods for concentrating proteins |
| WO2022076596A1 (en) | 2020-10-06 | 2022-04-14 | Codiak Biosciences, Inc. | Extracellular vesicle-aso constructs targeting stat6 |
| WO2022087402A1 (en) | 2020-10-23 | 2022-04-28 | Bristol-Myers Squibb Company | Lag-3 antagonist therapy for lung cancer |
| WO2022084531A1 (en) | 2020-10-23 | 2022-04-28 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating glioma |
| WO2022094567A1 (en) | 2020-10-28 | 2022-05-05 | Ikena Oncology, Inc. | Combination of an ahr inhibitor with a pdx inhibitor or doxorubicine |
| WO2022096470A1 (en) * | 2020-11-03 | 2022-05-12 | Randox Laboratories Ltd | Immune checkpoint inhibitor |
| EP3992208A1 (en) * | 2020-11-03 | 2022-05-04 | Randox Laboratories Ltd | Anti-pd-l1 vhh antibodies |
| WO2022097060A1 (en) | 2020-11-06 | 2022-05-12 | Novartis Ag | Cd19 binding molecules and uses thereof |
| WO2022101481A1 (en) | 2020-11-16 | 2022-05-19 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for predicting and treating uveal melanoma |
| WO2022101484A1 (en) | 2020-11-16 | 2022-05-19 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for predicting and treating uveal melanoma |
| WO2022120354A1 (en) | 2020-12-02 | 2022-06-09 | Ikena Oncology, Inc. | Tead inhibitors and uses thereof |
| WO2022120353A1 (en) | 2020-12-02 | 2022-06-09 | Ikena Oncology, Inc. | Tead inhibitors and uses thereof |
| WO2022120179A1 (en) | 2020-12-03 | 2022-06-09 | Bristol-Myers Squibb Company | Multi-tumor gene signatures and uses thereof |
| US11746103B2 (en) | 2020-12-10 | 2023-09-05 | Sumitomo Pharma Oncology, Inc. | ALK-5 inhibitors and uses thereof |
| WO2022133083A1 (en) | 2020-12-16 | 2022-06-23 | Gossamer Bio Services, Inc. | Compounds useful as t cell activators |
| US12123003B2 (en) | 2020-12-23 | 2024-10-22 | Checkmate Pharmaceuticals, Inc. | Synthetic RIG-I-like receptor agonists |
| WO2022146947A1 (en) | 2020-12-28 | 2022-07-07 | Bristol-Myers Squibb Company | Antibody compositions and methods of use thereof |
| WO2022146948A1 (en) | 2020-12-28 | 2022-07-07 | Bristol-Myers Squibb Company | Subcutaneous administration of pd1/pd-l1 antibodies |
| WO2022148736A1 (en) | 2021-01-05 | 2022-07-14 | Transgene | Vectorization of muc1 t cell engager |
| WO2022148979A1 (en) | 2021-01-11 | 2022-07-14 | Bicycletx Limited | Methods for treating cancer |
| WO2022162569A1 (en) | 2021-01-29 | 2022-08-04 | Novartis Ag | Dosage regimes for anti-cd73 and anti-entpd2 antibodies and uses thereof |
| WO2022167457A1 (en) | 2021-02-02 | 2022-08-11 | Liminal Biosciences Limited | Gpr84 antagonists and uses thereof |
| WO2022167445A1 (en) | 2021-02-02 | 2022-08-11 | Liminal Biosciences Limited | Gpr84 antagonists and uses thereof |
| WO2022171121A1 (en) | 2021-02-10 | 2022-08-18 | 同润生物医药(上海)有限公司 | Method and combination for treating tumors |
| WO2022171745A1 (en) | 2021-02-12 | 2022-08-18 | F. Hoffmann-La Roche Ag | Bicyclic tetrahydroazepine derivatives for the treatment of cancer |
| US11773103B2 (en) | 2021-02-15 | 2023-10-03 | Kymera Therapeutics, Inc. | IRAK4 degraders and uses thereof |
| WO2022185160A1 (en) | 2021-03-02 | 2022-09-09 | Glaxosmithkline Intellectual Property Development Limited | Substituted pyridines as dnmt1 inhibitors |
| WO2022184937A1 (en) | 2021-03-05 | 2022-09-09 | Leadartis, S.L. | Trimeric polypeptides and uses thereof in the treatment of cancer |
| US11926625B2 (en) | 2021-03-05 | 2024-03-12 | Nimbus Saturn, Inc. | HPK1 antagonists and uses thereof |
| WO2022192145A1 (en) | 2021-03-08 | 2022-09-15 | Blueprint Medicines Corporation | Map4k1 inhibitors |
| WO2022197641A1 (en) | 2021-03-15 | 2022-09-22 | Rapt Therapeutics, Inc. | 1h-pyrazolo[3,4-d]pyrimidin-6-yl-amine derivatives as hematopoietic progenitor kinase 1 (hpk1) modulators and/or inhibitors for the treatment of cancer and other diseases |
| WO2022194908A1 (en) | 2021-03-17 | 2022-09-22 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating melanoma |
| WO2022195551A1 (en) | 2021-03-18 | 2022-09-22 | Novartis Ag | Biomarkers for cancer and methods of use thereof |
| WO2022212400A1 (en) | 2021-03-29 | 2022-10-06 | Juno Therapeutics, Inc. | Methods for dosing and treatment with a combination of a checkpoint inhibitor therapy and a car t cell therapy |
| US12071442B2 (en) | 2021-03-29 | 2024-08-27 | Nimbus Saturn, Inc. | Substituted pyrrolo[3,4-c]pyridines as HPK1 antagonists |
| WO2022208353A1 (en) | 2021-03-31 | 2022-10-06 | Glaxosmithkline Intellectual Property Development Limited | Antigen binding proteins and combinations thereof |
| WO2022212876A1 (en) | 2021-04-02 | 2022-10-06 | The Regents Of The University Of California | Antibodies against cleaved cdcp1 and uses thereof |
| WO2022216573A1 (en) | 2021-04-05 | 2022-10-13 | Bristol-Myers Squibb Company | Pyridinyl substituted oxoisoindoline compounds for the treatment of cancer |
| WO2022216644A1 (en) | 2021-04-06 | 2022-10-13 | Bristol-Myers Squibb Company | Pyridinyl substituted oxoisoindoline compounds |
| WO2022215011A1 (en) | 2021-04-07 | 2022-10-13 | Novartis Ag | USES OF ANTI-TGFβ ANTIBODIES AND OTHER THERAPEUTIC AGENTS FOR THE TREATMENT OF PROLIFERATIVE DISEASES |
| WO2022216993A2 (en) | 2021-04-08 | 2022-10-13 | Marengo Therapeutics, Inc. | Multifuntional molecules binding to tcr and uses thereof |
| WO2022214652A1 (en) | 2021-04-09 | 2022-10-13 | Ose Immunotherapeutics | Scaffold for bifunctioanl molecules comprising pd-1 or cd28 and sirp binding domains |
| WO2022214653A1 (en) | 2021-04-09 | 2022-10-13 | Ose Immunotherapeutics | New scaffold for bifunctional molecules with improved properties |
| US12037346B2 (en) | 2021-04-13 | 2024-07-16 | Nuvalent, Inc. | Amino-substituted heteroaryls for treating cancers with EGFR mutations |
| WO2022221227A1 (en) | 2021-04-13 | 2022-10-20 | Nuvalent, Inc. | Amino-substituted heterocycles for treating cancers with egfr mutations |
| WO2022219080A1 (en) | 2021-04-14 | 2022-10-20 | INSERM (Institut National de la Santé et de la Recherche Médicale) | New method to improve nk cells cytotoxicity |
| WO2022221866A1 (en) | 2021-04-16 | 2022-10-20 | Ikena Oncology, Inc. | Mek inhibitors and uses thereof |
| WO2022223791A1 (en) | 2021-04-23 | 2022-10-27 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating cell senescence accumulation related disease |
| US12097261B2 (en) | 2021-05-07 | 2024-09-24 | Kymera Therapeutics, Inc. | CDK2 degraders and uses thereof |
| WO2022236134A1 (en) | 2021-05-07 | 2022-11-10 | Surface Oncology, Inc. | Anti-il-27 antibodies and uses thereof |
| WO2022243846A1 (en) | 2021-05-18 | 2022-11-24 | Novartis Ag | Combination therapies |
| WO2022243378A1 (en) | 2021-05-18 | 2022-11-24 | Kymab Limited | Uses of anti-icos antibodies |
| WO2022246177A1 (en) | 2021-05-21 | 2022-11-24 | Arcus Biosciences, Inc. | Axl compounds |
| WO2022246179A1 (en) | 2021-05-21 | 2022-11-24 | Arcus Biosciences, Inc. | Axl inhibitor compounds |
| WO2022254227A1 (en) | 2021-06-04 | 2022-12-08 | Kymab Limited | Treatment of pd-l1 negative or low expressing cancer with anti-icos antibodies |
| US11572412B2 (en) | 2021-06-04 | 2023-02-07 | Boehringer Ingelheim International Gmbh | Anti-SIRP-alpha antibodies |
| WO2023288254A1 (en) | 2021-07-14 | 2023-01-19 | Blueprint Medicines Corporation | Heterocyclic compounds as map4k1 inhibitors |
| WO2023288264A1 (en) | 2021-07-15 | 2023-01-19 | Blueprint Medicines Corporation | Map4k1 inhibitors |
| WO2023007472A1 (en) | 2021-07-30 | 2023-02-02 | ONA Therapeutics S.L. | Anti-cd36 antibodies and their use to treat cancer |
| WO2023028238A1 (en) | 2021-08-25 | 2023-03-02 | PIC Therapeutics, Inc. | Eif4e inhibitors and uses thereof |
| WO2023028235A1 (en) | 2021-08-25 | 2023-03-02 | PIC Therapeutics, Inc. | Eif4e inhibitors and uses thereof |
| WO2023039089A1 (en) | 2021-09-08 | 2023-03-16 | Twentyeight-Seven, Inc. | Papd5 and/or papd7 inhibiting 4-oxo-1,4-dihydroquinoline-3-carboxylic acid derivatives |
| WO2023077090A1 (en) | 2021-10-29 | 2023-05-04 | Bristol-Myers Squibb Company | Lag-3 antagonist therapy for hematological cancer |
| WO2023079430A1 (en) | 2021-11-02 | 2023-05-11 | Pfizer Inc. | Methods of treating mitochondrial myopathies using anti-gdf15 antibodies |
| WO2023078900A1 (en) | 2021-11-03 | 2023-05-11 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating triple negative breast cancer (tnbc) |
| WO2023111203A1 (en) | 2021-12-16 | 2023-06-22 | Onxeo | New conjugated nucleic acid molecules and their uses |
| WO2023114984A1 (en) | 2021-12-17 | 2023-06-22 | Ikena Oncology, Inc. | Tead inhibitors and uses thereof |
| WO2023118165A1 (en) | 2021-12-21 | 2023-06-29 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and compositions for treating melanoma |
| WO2023122777A1 (en) | 2021-12-22 | 2023-06-29 | Gossamer Bio Services, Inc. | Oxime derivatives useful as t cell activators |
| WO2023122778A1 (en) | 2021-12-22 | 2023-06-29 | Gossamer Bio Services, Inc. | Pyridazinone derivatives useful as t cell activators |
| WO2023122772A1 (en) | 2021-12-22 | 2023-06-29 | Gossamer Bio Services, Inc. | Oxime derivatives useful as t cell activators |
| WO2023147371A1 (en) | 2022-01-26 | 2023-08-03 | Bristol-Myers Squibb Company | Combination therapy for hepatocellular carcinoma |
| US12091411B2 (en) | 2022-01-31 | 2024-09-17 | Kymera Therapeutics, Inc. | IRAK degraders and uses thereof |
| WO2023150186A1 (en) | 2022-02-01 | 2023-08-10 | Arvinas Operations, Inc. | Dgk targeting compounds and uses thereof |
| WO2023154799A1 (en) | 2022-02-14 | 2023-08-17 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Combination immunotherapy for treating cancer |
| WO2023154905A1 (en) | 2022-02-14 | 2023-08-17 | Gilead Sciences, Inc. | Antiviral pyrazolopyridinone compounds |
| WO2023164638A1 (en) | 2022-02-25 | 2023-08-31 | Bristol-Myers Squibb Company | Combination therapy for colorectal carcinoma |
| WO2023166418A2 (en) | 2022-03-03 | 2023-09-07 | Pfizer Inc. | Multispecific antibodies and uses thereof |
| WO2023166420A1 (en) | 2022-03-03 | 2023-09-07 | Pfizer Inc. | Multispecific antibodies and uses thereof |
| WO2023168404A1 (en) | 2022-03-04 | 2023-09-07 | Bristol-Myers Squibb Company | Methods of treating a tumor |
| WO2023170606A1 (en) | 2022-03-08 | 2023-09-14 | Alentis Therapeutics Ag | Use of anti-claudin-1 antibodies to increase t cell availability |
| WO2023173057A1 (en) | 2022-03-10 | 2023-09-14 | Ikena Oncology, Inc. | Mek inhibitors and uses thereof |
| WO2023173053A1 (en) | 2022-03-10 | 2023-09-14 | Ikena Oncology, Inc. | Mek inhibitors and uses thereof |
| WO2023174210A1 (en) | 2022-03-14 | 2023-09-21 | Laekna Limited | Combination treatment for cancer |
| WO2023178192A1 (en) | 2022-03-15 | 2023-09-21 | Compugen Ltd. | Il-18bp antagonist antibodies and their use in monotherapy and combination therapy in the treatment of cancer |
| WO2023178329A1 (en) | 2022-03-18 | 2023-09-21 | Bristol-Myers Squibb Company | Methods of isolating polypeptides |
| WO2023192478A1 (en) | 2022-04-01 | 2023-10-05 | Bristol-Myers Squibb Company | Combination therapy with anti-il-8 antibodies and anti-pd-1 antibodies for treating cancer |
| WO2023196987A1 (en) | 2022-04-07 | 2023-10-12 | Bristol-Myers Squibb Company | Methods of treating tumor |
| WO2023196964A1 (en) | 2022-04-08 | 2023-10-12 | Bristol-Myers Squibb Company | Machine learning identification, classification, and quantification of tertiary lymphoid structures |
| WO2023211889A1 (en) | 2022-04-25 | 2023-11-02 | Ikena Oncology, Inc. | Polymorphic compounds and uses thereof |
| WO2023213764A1 (en) * | 2022-05-02 | 2023-11-09 | Transgene | Fusion polypeptide comprising an anti-pd-l1 sdab and a member of the tnfsf |
| WO2023213763A1 (en) * | 2022-05-02 | 2023-11-09 | Transgene | Poxvirus encoding a binding agent comprising an anti- pd-l1 sdab |
| WO2023214325A1 (en) | 2022-05-05 | 2023-11-09 | Novartis Ag | Pyrazolopyrimidine derivatives and uses thereof as tet2 inhibitors |
| WO2023222854A1 (en) | 2022-05-18 | 2023-11-23 | Kymab Limited | Uses of anti-icos antibodies |
| WO2023228095A1 (en) | 2022-05-24 | 2023-11-30 | Daiichi Sankyo Company, Limited | Dosage regimen of an anti-cdh6 antibody-drug conjugate |
| WO2023230205A1 (en) | 2022-05-25 | 2023-11-30 | Ikena Oncology, Inc. | Mek inhibitors and uses thereof |
| WO2023235847A1 (en) | 2022-06-02 | 2023-12-07 | Bristol-Myers Squibb Company | Antibody compositions and methods of use thereof |
| WO2024023740A1 (en) | 2022-07-27 | 2024-02-01 | Astrazeneca Ab | Combinations of recombinant virus expressing interleukin-12 with pd-1/pd-l1 inhibitors |
| WO2024023750A1 (en) | 2022-07-28 | 2024-02-01 | Astrazeneca Uk Limited | Combination of antibody-drug conjugate and bispecific checkpoint inhibitor |
| WO2024028365A1 (en) | 2022-08-02 | 2024-02-08 | Liminal Biosciences Limited | Substituted pyridone gpr84 antagonists and uses thereof |
| WO2024028363A1 (en) | 2022-08-02 | 2024-02-08 | Liminal Biosciences Limited | Heteroaryl carboxamide and related gpr84 antagonists and uses thereof |
| WO2024028364A1 (en) | 2022-08-02 | 2024-02-08 | Liminal Biosciences Limited | Aryl-triazolyl and related gpr84 antagonists and uses thereof |
| WO2024036100A1 (en) | 2022-08-08 | 2024-02-15 | Bristol-Myers Squibb Company | Substituted tetrazolyl compounds useful as t cell activators |
| WO2024036101A1 (en) | 2022-08-09 | 2024-02-15 | Bristol-Myers Squibb Company | Tertiary amine substituted bicyclic compounds useful as t cell activators |
| WO2024033400A1 (en) | 2022-08-10 | 2024-02-15 | Institut National de la Santé et de la Recherche Médicale | Sk2 inhibitor for the treatment of pancreatic cancer |
| WO2024033399A1 (en) | 2022-08-10 | 2024-02-15 | Institut National de la Santé et de la Recherche Médicale | Sigmar1 ligand for the treatment of pancreatic cancer |
| WO2024033458A1 (en) | 2022-08-11 | 2024-02-15 | F. Hoffmann-La Roche Ag | Bicyclic tetrahydroazepine derivatives |
| WO2024033388A1 (en) | 2022-08-11 | 2024-02-15 | F. Hoffmann-La Roche Ag | Bicyclic tetrahydrothiazepine derivatives |
| WO2024033457A1 (en) | 2022-08-11 | 2024-02-15 | F. Hoffmann-La Roche Ag | Bicyclic tetrahydrothiazepine derivatives |
| WO2024054992A1 (en) | 2022-09-09 | 2024-03-14 | Bristol-Myers Squibb Company | Methods of separating chelator |
| WO2024056716A1 (en) | 2022-09-14 | 2024-03-21 | Institut National de la Santé et de la Recherche Médicale | Methods and pharmaceutical compositions for the treatment of dilated cardiomyopathy |
| WO2024069009A1 (en) | 2022-09-30 | 2024-04-04 | Alentis Therapeutics Ag | Treatment of drug-resistant hepatocellular carcinoma |
| WO2024084034A1 (en) | 2022-10-21 | 2024-04-25 | Institut National de la Santé et de la Recherche Médicale | Methods and pharmaceutical compositions for the treatment of osteoarthritis |
| WO2024094688A1 (en) | 2022-11-01 | 2024-05-10 | Heidelberg Pharma Research Gmbh | Anti-gucy2c antibody and uses thereof |
| WO2024102635A1 (en) | 2022-11-07 | 2024-05-16 | Alector Llc | Uses of siglec-9 ecd fusion molecules in cancer treatment |
| WO2024112894A1 (en) | 2022-11-22 | 2024-05-30 | PIC Therapeutics, Inc. | Eif4e inhibitors and uses thereof |
| WO2024137776A1 (en) | 2022-12-21 | 2024-06-27 | Bristol-Myers Squibb Company | Combination therapy for lung cancer |
| WO2024137865A1 (en) | 2022-12-22 | 2024-06-27 | Gossamer Bio Services, Inc. | Compounds useful as t cell activators |
| WO2024160721A1 (en) | 2023-01-30 | 2024-08-08 | Kymab Limited | Antibodies |
| WO2024163477A1 (en) | 2023-01-31 | 2024-08-08 | University Of Rochester | Immune checkpoint blockade therapy for treating staphylococcus aureus infections |
| WO2024184417A1 (en) | 2023-03-07 | 2024-09-12 | Photocure Asa | Therapy for bladder cancer |
| WO2024196952A1 (en) | 2023-03-20 | 2024-09-26 | Bristol-Myers Squibb Company | Tumor subtype assessment for cancer therapy |
| WO2024200571A1 (en) | 2023-03-28 | 2024-10-03 | Institut National de la Santé et de la Recherche Médicale | Method for discriminating mono-immunotherapy from combined immunotherapy in cancers |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11884724B2 (en) | Anti-PD-L1 antibodies and uses thereof | |
| US20240166741A1 (en) | Methods of treating cancer using pd-1 axis binding antagonists and tigit inhibitors | |
| US20180171025A1 (en) | Methods of treating cancer using pd-1 axis binding antagonists and an anti-cd20 antibody | |
| CA3000386A1 (en) | Combination of a pd-1 axis binding antagonist and an alk inhibitor for treating alk-negative cancer | |
| US12054548B2 (en) | Anti-PD-L1 antibodies and use thereof | |
| NZ755387B2 (en) | Methods of treating cancer using pd-1 axis binding antagonists and tigit inhibitors |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12790432 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012790432 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 232778 Country of ref document: IL |
|
| ENP | Entry into the national phase |
Ref document number: 2856895 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/A/2014/006316 Country of ref document: MX |
|
| ENP | Entry into the national phase |
Ref document number: 2014542728 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14360775 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20147017974 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 201400625 Country of ref document: EA |
|
| ENP | Entry into the national phase |
Ref document number: 2012344260 Country of ref document: AU Date of ref document: 20121121 Kind code of ref document: A |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112014012819 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 112014012819 Country of ref document: BR Kind code of ref document: A2 Effective date: 20140528 |