WO2012141187A1 - Composant électronique, pâte conductrice pour électrode d'aluminium pour application dans ledit composant, et composition de verre pour électrode d'aluminium - Google Patents
Composant électronique, pâte conductrice pour électrode d'aluminium pour application dans ledit composant, et composition de verre pour électrode d'aluminium Download PDFInfo
- Publication number
- WO2012141187A1 WO2012141187A1 PCT/JP2012/059850 JP2012059850W WO2012141187A1 WO 2012141187 A1 WO2012141187 A1 WO 2012141187A1 JP 2012059850 W JP2012059850 W JP 2012059850W WO 2012141187 A1 WO2012141187 A1 WO 2012141187A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- weight
- glass
- aluminum
- electrode
- conductive paste
- Prior art date
Links
- 239000011521 glass Substances 0.000 title claims abstract description 286
- 229910052782 aluminium Inorganic materials 0.000 title claims abstract description 178
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 title claims abstract description 178
- 239000000203 mixture Substances 0.000 title claims abstract description 69
- 239000000758 substrate Substances 0.000 claims abstract description 80
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 claims abstract description 57
- 229910052710 silicon Inorganic materials 0.000 claims abstract description 57
- 239000010703 silicon Substances 0.000 claims abstract description 57
- 239000002245 particle Substances 0.000 claims description 78
- 239000004065 semiconductor Substances 0.000 claims description 31
- 229910000838 Al alloy Inorganic materials 0.000 claims description 29
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 claims description 26
- 239000002923 metal particle Substances 0.000 claims description 26
- 229910052796 boron Inorganic materials 0.000 claims description 24
- 229910015902 Bi 2 O 3 Inorganic materials 0.000 claims description 21
- 229910052698 phosphorus Inorganic materials 0.000 claims description 19
- 239000011574 phosphorus Substances 0.000 claims description 18
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 claims description 17
- 229910052787 antimony Inorganic materials 0.000 claims description 16
- 229910052720 vanadium Inorganic materials 0.000 claims description 16
- 239000011230 binding agent Substances 0.000 claims description 15
- 238000000034 method Methods 0.000 claims description 15
- 239000000075 oxide glass Substances 0.000 claims description 15
- 229910052797 bismuth Inorganic materials 0.000 claims description 14
- 229910052725 zinc Inorganic materials 0.000 claims description 13
- 239000011701 zinc Substances 0.000 claims description 13
- 239000011347 resin Substances 0.000 claims description 12
- 229920005989 resin Polymers 0.000 claims description 12
- 239000002904 solvent Substances 0.000 claims description 12
- JCXGWMGPZLAOME-UHFFFAOYSA-N bismuth atom Chemical compound [Bi] JCXGWMGPZLAOME-UHFFFAOYSA-N 0.000 claims description 10
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 claims description 9
- WATWJIUSRGPENY-UHFFFAOYSA-N antimony atom Chemical compound [Sb] WATWJIUSRGPENY-UHFFFAOYSA-N 0.000 claims description 9
- 229910052714 tellurium Inorganic materials 0.000 claims description 9
- PORWMNRCUJJQNO-UHFFFAOYSA-N tellurium atom Chemical compound [Te] PORWMNRCUJJQNO-UHFFFAOYSA-N 0.000 claims description 9
- 239000000843 powder Substances 0.000 claims description 7
- LEONUFNNVUYDNQ-UHFFFAOYSA-N vanadium atom Chemical compound [V] LEONUFNNVUYDNQ-UHFFFAOYSA-N 0.000 claims 3
- 238000002844 melting Methods 0.000 abstract description 33
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 abstract description 19
- 229910052760 oxygen Inorganic materials 0.000 abstract description 19
- 239000001301 oxygen Substances 0.000 abstract description 19
- 238000009792 diffusion process Methods 0.000 abstract description 15
- 230000009257 reactivity Effects 0.000 abstract description 3
- 238000006243 chemical reaction Methods 0.000 description 62
- 239000010410 layer Substances 0.000 description 56
- 230000008018 melting Effects 0.000 description 27
- 229910052709 silver Inorganic materials 0.000 description 22
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 21
- 239000004332 silver Substances 0.000 description 21
- 229910018557 Si O Inorganic materials 0.000 description 17
- LIVNPJMFVYWSIS-UHFFFAOYSA-N silicon monoxide Inorganic materials [Si-]#[O+] LIVNPJMFVYWSIS-UHFFFAOYSA-N 0.000 description 17
- 229910045601 alloy Inorganic materials 0.000 description 11
- 239000000956 alloy Substances 0.000 description 11
- GPPXJZIENCGNKB-UHFFFAOYSA-N vanadium Chemical compound [V]#[V] GPPXJZIENCGNKB-UHFFFAOYSA-N 0.000 description 11
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 10
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 9
- 238000010304 firing Methods 0.000 description 9
- 239000000463 material Substances 0.000 description 9
- 230000007613 environmental effect Effects 0.000 description 8
- 238000011156 evaluation Methods 0.000 description 8
- 239000003566 sealing material Substances 0.000 description 8
- 230000000052 comparative effect Effects 0.000 description 7
- 238000004455 differential thermal analysis Methods 0.000 description 7
- 238000010438 heat treatment Methods 0.000 description 7
- 238000004519 manufacturing process Methods 0.000 description 7
- 230000003647 oxidation Effects 0.000 description 7
- 238000007254 oxidation reaction Methods 0.000 description 7
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 6
- 239000012298 atmosphere Substances 0.000 description 6
- 229910052802 copper Inorganic materials 0.000 description 6
- 239000010949 copper Substances 0.000 description 6
- 238000007639 printing Methods 0.000 description 6
- 230000001105 regulatory effect Effects 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 238000002425 crystallisation Methods 0.000 description 5
- 230000008025 crystallization Effects 0.000 description 5
- 230000007423 decrease Effects 0.000 description 5
- 238000010586 diagram Methods 0.000 description 5
- 230000000694 effects Effects 0.000 description 5
- 238000005192 partition Methods 0.000 description 5
- 238000007650 screen-printing Methods 0.000 description 5
- 230000003667 anti-reflective effect Effects 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 239000000599 controlled substance Substances 0.000 description 4
- 239000007789 gas Substances 0.000 description 4
- 229910021421 monocrystalline silicon Inorganic materials 0.000 description 4
- 229910021420 polycrystalline silicon Inorganic materials 0.000 description 4
- 238000007789 sealing Methods 0.000 description 4
- 239000001856 Ethyl cellulose Substances 0.000 description 3
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- 229910052581 Si3N4 Inorganic materials 0.000 description 3
- 239000000919 ceramic Substances 0.000 description 3
- 229940125368 controlled substance Drugs 0.000 description 3
- 229920001249 ethyl cellulose Polymers 0.000 description 3
- 235000019325 ethyl cellulose Nutrition 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 229910000464 lead oxide Inorganic materials 0.000 description 3
- 230000005012 migration Effects 0.000 description 3
- 238000013508 migration Methods 0.000 description 3
- YEXPOXQUZXUXJW-UHFFFAOYSA-N oxolead Chemical compound [Pb]=O YEXPOXQUZXUXJW-UHFFFAOYSA-N 0.000 description 3
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Substances [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 3
- 230000000630 rising effect Effects 0.000 description 3
- HQVNEWCFYHHQES-UHFFFAOYSA-N silicon nitride Chemical compound N12[Si]34N5[Si]62N3[Si]51N64 HQVNEWCFYHHQES-UHFFFAOYSA-N 0.000 description 3
- 235000011121 sodium hydroxide Nutrition 0.000 description 3
- WUOACPNHFRMFPN-SECBINFHSA-N (S)-(-)-alpha-terpineol Chemical compound CC1=CC[C@@H](C(C)(C)O)CC1 WUOACPNHFRMFPN-SECBINFHSA-N 0.000 description 2
- VXQBJTKSVGFQOL-UHFFFAOYSA-N 2-(2-butoxyethoxy)ethyl acetate Chemical compound CCCCOCCOCCOC(C)=O VXQBJTKSVGFQOL-UHFFFAOYSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- 239000000020 Nitrocellulose Substances 0.000 description 2
- 229910007541 Zn O Inorganic materials 0.000 description 2
- 230000002159 abnormal effect Effects 0.000 description 2
- OVKDFILSBMEKLT-UHFFFAOYSA-N alpha-Terpineol Natural products CC(=C)C1(O)CCC(C)=CC1 OVKDFILSBMEKLT-UHFFFAOYSA-N 0.000 description 2
- 229940088601 alpha-terpineol Drugs 0.000 description 2
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 2
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 229910000416 bismuth oxide Inorganic materials 0.000 description 2
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 description 2
- 239000004327 boric acid Substances 0.000 description 2
- 238000004364 calculation method Methods 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 230000007547 defect Effects 0.000 description 2
- TYIXMATWDRGMPF-UHFFFAOYSA-N dibismuth;oxygen(2-) Chemical compound [O-2].[O-2].[O-2].[Bi+3].[Bi+3] TYIXMATWDRGMPF-UHFFFAOYSA-N 0.000 description 2
- YWEUIGNSBFLMFL-UHFFFAOYSA-N diphosphonate Chemical compound O=P(=O)OP(=O)=O YWEUIGNSBFLMFL-UHFFFAOYSA-N 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- 239000005355 lead glass Substances 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 229910052759 nickel Inorganic materials 0.000 description 2
- 229920001220 nitrocellulos Polymers 0.000 description 2
- DLYUQMMRRRQYAE-UHFFFAOYSA-N phosphorus pentoxide Inorganic materials O1P(O2)(=O)OP3(=O)OP1(=O)OP2(=O)O3 DLYUQMMRRRQYAE-UHFFFAOYSA-N 0.000 description 2
- 229910052697 platinum Inorganic materials 0.000 description 2
- 238000010248 power generation Methods 0.000 description 2
- 239000011241 protective layer Substances 0.000 description 2
- 239000002994 raw material Substances 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 238000005245 sintering Methods 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 229910052718 tin Inorganic materials 0.000 description 2
- 238000007740 vapor deposition Methods 0.000 description 2
- 230000000007 visual effect Effects 0.000 description 2
- 239000011800 void material Substances 0.000 description 2
- 238000007088 Archimedes method Methods 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 229920000298 Cellophane Polymers 0.000 description 1
- 229910017135 Fe—O Inorganic materials 0.000 description 1
- BLRPTPMANUNPDV-UHFFFAOYSA-N Silane Chemical compound [SiH4] BLRPTPMANUNPDV-UHFFFAOYSA-N 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 1
- 238000002441 X-ray diffraction Methods 0.000 description 1
- XHCLAFWTIXFWPH-UHFFFAOYSA-N [O-2].[O-2].[O-2].[O-2].[O-2].[V+5].[V+5] Chemical compound [O-2].[O-2].[O-2].[O-2].[O-2].[V+5].[V+5] XHCLAFWTIXFWPH-UHFFFAOYSA-N 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 238000000889 atomisation Methods 0.000 description 1
- 229910052788 barium Inorganic materials 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 229910052810 boron oxide Inorganic materials 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 238000003486 chemical etching Methods 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- JKWMSGQKBLHBQQ-UHFFFAOYSA-N diboron trioxide Chemical compound O=BOB=O JKWMSGQKBLHBQQ-UHFFFAOYSA-N 0.000 description 1
- 238000005530 etching Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 229910052732 germanium Inorganic materials 0.000 description 1
- GNPVGFCGXDBREM-UHFFFAOYSA-N germanium atom Chemical compound [Ge] GNPVGFCGXDBREM-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 239000000383 hazardous chemical Substances 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- XEEYBQQBJWHFJM-UHFFFAOYSA-N iron Substances [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 1
- WABPQHHGFIMREM-UHFFFAOYSA-N lead(0) Chemical compound [Pb] WABPQHHGFIMREM-UHFFFAOYSA-N 0.000 description 1
- 229910052987 metal hydride Inorganic materials 0.000 description 1
- -1 metal hydride compound Chemical class 0.000 description 1
- 229910052750 molybdenum Inorganic materials 0.000 description 1
- PXHVJJICTQNCMI-UHFFFAOYSA-N nickel Substances [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 1
- 229910052758 niobium Inorganic materials 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 239000012299 nitrogen atmosphere Substances 0.000 description 1
- 229910052756 noble gas Inorganic materials 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 238000000206 photolithography Methods 0.000 description 1
- 238000005268 plasma chemical vapour deposition Methods 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 239000000523 sample Substances 0.000 description 1
- 238000000550 scanning electron microscopy energy dispersive X-ray spectroscopy Methods 0.000 description 1
- 229910000077 silane Inorganic materials 0.000 description 1
- 229910052814 silicon oxide Inorganic materials 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 238000004544 sputter deposition Methods 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 238000004381 surface treatment Methods 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 229910052721 tungsten Inorganic materials 0.000 description 1
- 229910001935 vanadium oxide Inorganic materials 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L31/00—Semiconductor devices sensitive to infrared radiation, light, electromagnetic radiation of shorter wavelength or corpuscular radiation and specially adapted either for the conversion of the energy of such radiation into electrical energy or for the control of electrical energy by such radiation; Processes or apparatus specially adapted for the manufacture or treatment thereof or of parts thereof; Details thereof
- H01L31/02—Details
- H01L31/0224—Electrodes
- H01L31/022408—Electrodes for devices characterised by at least one potential jump barrier or surface barrier
- H01L31/022425—Electrodes for devices characterised by at least one potential jump barrier or surface barrier for solar cells
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C3/00—Glass compositions
- C03C3/12—Silica-free oxide glass compositions
- C03C3/16—Silica-free oxide glass compositions containing phosphorus
- C03C3/21—Silica-free oxide glass compositions containing phosphorus containing titanium, zirconium, vanadium, tungsten or molybdenum
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C8/00—Enamels; Glazes; Fusion seal compositions being frit compositions having non-frit additions
- C03C8/02—Frit compositions, i.e. in a powdered or comminuted form
- C03C8/08—Frit compositions, i.e. in a powdered or comminuted form containing phosphorus
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C8/00—Enamels; Glazes; Fusion seal compositions being frit compositions having non-frit additions
- C03C8/14—Glass frit mixtures having non-frit additions, e.g. opacifiers, colorants, mill-additions
- C03C8/18—Glass frit mixtures having non-frit additions, e.g. opacifiers, colorants, mill-additions containing free metals
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B1/00—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors
- H01B1/20—Conductive material dispersed in non-conductive organic material
- H01B1/22—Conductive material dispersed in non-conductive organic material the conductive material comprising metals or alloys
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01J—ELECTRIC DISCHARGE TUBES OR DISCHARGE LAMPS
- H01J2211/00—Plasma display panels with alternate current induction of the discharge, e.g. AC-PDPs
- H01J2211/10—AC-PDPs with at least one main electrode being out of contact with the plasma
- H01J2211/12—AC-PDPs with at least one main electrode being out of contact with the plasma with main electrodes provided on both sides of the discharge space
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01J—ELECTRIC DISCHARGE TUBES OR DISCHARGE LAMPS
- H01J2211/00—Plasma display panels with alternate current induction of the discharge, e.g. AC-PDPs
- H01J2211/20—Constructional details
- H01J2211/22—Electrodes
- H01J2211/225—Material of electrodes
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E10/00—Energy generation through renewable energy sources
- Y02E10/50—Photovoltaic [PV] energy
Definitions
- the present invention relates to a conductive paste for an aluminum electrode formed on a silicon substrate, a glass composition for an aluminum electrode contained therein, and an electronic component manufactured using the conductive paste for an aluminum electrode.
- a silver electrode or an aluminum electrode is formed.
- These electrodes are formed on a silicon substrate or the like by applying, drying, and firing a conductive paste containing a large number of metal particles of silver or aluminum.
- this conductive paste mainly comprises the metal particles, and is composed of glass particles, a binder resin, a solvent and the like.
- the glass particles soften and flow to form a dense electrode, and firmly adhere to a substrate or the like.
- Patent Document 1 proposes a lead-free low-melting glass which contains bismuth oxide and silicon oxide in a silver electrode or an aluminum electrode formed in a solar battery cell.
- Patent Document 2 proposes a low melting point glass containing bismuth oxide and boron oxide.
- a conductive paste mainly composed of metal particles such as aluminum particles and aluminum alloy particles can not be fired precisely due to the oxide film on the surface of the metal particles, and there is a problem in reducing resistance.
- a method of improving the sinterability of metal particles and reducing resistance by adding particles of vanadium or vanadium oxide to a conductive paste has been proposed (Patent Document 3).
- a method of improving resistance to oxidation and improving resistance by adding carbon, germanium, tin, a metal hydride compound, a metal phosphide compound and the like has also been proposed (Patent Document 4).
- Patent Documents 1 to 4 have both the performance and reliability of the electronic component. It was by no means fully considered in improving.
- JP, 2008-543080 A Japanese Patent Application Publication No. 2006-332032 Japanese Patent Application Laid-Open No. 7-73731 Unexamined-Japanese-Patent No. 5-298917
- an object of the present invention is to simultaneously achieve both high performance and high reliability of electronic parts such as solar cells using a silicon substrate having a pn junction.
- Another object of the present invention is to provide a conductive paste for an aluminum electrode and a glass composition for an aluminum electrode applied thereto which can achieve the above simultaneously.
- the means for solving the problems of the present invention are as follows.
- the glass phase comprises 60 to 80% by weight of V 2 O 5 , 10 to 25% by weight of P 2 O 5 and 5 to 15% by weight of B 2 O 3 in terms of the following oxide conversion. %, And the amount of P 2 O 5 is greater than the amount of B 2 O 3 .
- the glass phase comprises 70 to 80% by weight of V 2 O 5 , 10 to 20% by weight of P 2 O 5 , and B 2 O 3 in terms of the following oxide conversion.
- the glass phase has 40 to 80% by weight of V 2 O 5 , 10 to 25% by weight of P 2 O 5 and 5 to 15% by weight of B 2 O 3 in terms of oxide conversion %, TeO 2 0-25 wt%, Sb 2 O 3 0-20 wt%, Bi 2 O 3 0-20 wt%, ZnO 0-20 wt%, P 2 O 5 content
- An electronic component characterized by having a B 2 O 3 amount and a total amount of P 2 O 5 , B 2 O 3 and TeO 2 of 20 to 50% by weight of the glass phase.
- the glass phase comprises 60 to 80% by weight of V 2 O 5 , 10 to 20% by weight of P 2 O 5 , and B 2 O 3 in terms of the following oxide conversion. 5 to 10% by weight, 0 to 15% by weight of TeO 2 , 0 to 10% by weight of Sb 2 O 3 , 0 to 10% by weight of Bi 2 O 3 and 0 to 10% by weight of ZnO, P 2 Electronic parts characterized in that the total amount of O 5 , B 2 O 3 and TeO 2 is 20 to 40% by weight of the glass phase. (7) The electron according to any one of the above (1) to (6), wherein the glass phase is contained in a ratio of 0.2 to 2 parts by weight with respect to 100 parts by weight of the metal particles. parts.
- the metal particles are aluminum or an aluminum alloy
- the silicon substrate has a p-type semiconductor
- the electrode is formed on the p-type semiconductor.
- Electronic parts characterized by (9) The electronic component according to any one of the above (1) to (8), wherein the silicon substrate is a solar battery cell having a pn junction. (10) The electronic component according to any one of the above (1) to (9), wherein the content of lead in the glass composition for an aluminum electrode is 1000 ppm or less.
- a conductive paste for aluminum electrodes wherein metal particles of aluminum or aluminum alloy and glass particles are dispersed in a solvent in which a binder resin is dissolved, wherein the glass particles are an oxide containing vanadium, phosphorus and boron
- a conductive paste for aluminum electrodes characterized in that it is glass.
- the glass particles have a V 2 O 5 content of 60 to 80% by weight, a P 2 O 5 content of 10 to 25% by weight, and a B 2 O 3 content of 5 to 15%. %, And the amount of P 2 O 5 is greater than the amount of B 2 O 3 .
- the above-mentioned glass particles are 70 to 80 wt% of V 2 O 5 , 10 to 20 wt% of P 2 O 5, and 5 to 10 wt% of B 2 O 3 in terms of the following oxide conversion. %, And the amount of P 2 O 5 is greater than the amount of B 2 O 3 .
- the glass particles have a V 2 O 5 content of 40 to 80% by weight, a P 2 O 5 content of 10 to 25% by weight, and a B 2 O 3 content of 5 to 15%.
- the glass particles are contained in a ratio of 0.2 to 2 parts by weight with respect to 100 parts by weight of the metal particles.
- Conductive paste for electrodes (19) The conductive paste for aluminum electrodes according to any one of the above (11) to (18), wherein the content of lead in the glass composition for aluminum electrodes is 1000 ppm or less. (20) A glass composition contained in an aluminum electrode containing aluminum or aluminum alloy powder, wherein the glass composition contains vanadium, phosphorus and boron, and further one or more of tellurium, antimony, bismuth and zinc A glass composition for an aluminum electrode, which is an oxide glass containing, which has a softening point of 420 ° C. or less and flows at 500 ° C.
- the glass composition for an aluminum electrode has a V 2 O 5 content of 40 to 80% by weight, a P 2 O 5 content of 10 to 25% by weight, and a B 2 O 3 content of 5 ⁇ 15 wt% TeO 2 0-25 wt%, Sb 2 O 3 0-20 wt%, Bi 2 O 3 0-20 wt%, and ZnO 0-20 wt%, P 2 O 5 weight greater than the amount of B 2 O 3, and P 2 O 5, B 2 O 3 , and aluminum electrode glass composition, wherein the total amount of TeO 2 is from 20 to 50 wt%.
- the glass composition for an aluminum electrode has 60 to 80% by weight of V 2 O 5 , 10 to 20% by weight of P 2 O 5 , and B 2 O 3 in terms of the following oxide conversion. 5 to 10% by weight, 0 to 15% by weight of TeO 2 , 0 to 10% by weight of Sb 2 O 3 , 0 to 10% by weight of Bi 2 O 3 and 0 to 10% by weight of ZnO, P 2 A glass composition for an aluminum electrode, wherein the total amount of O 5 , B 2 O 3 and TeO 2 is 20 to 40% by weight. (23) The glass composition for an aluminum electrode according to any one of the above (20) to (22), wherein the content of lead in the glass composition for an aluminum electrode is 1,000 ppm or less.
- metal particles in addition to aluminum, silver, copper and their respective alloys, Fe, Ni, Pt, Au are used, and aluminum, copper and silver are preferable.
- a silicon substrate such as a solar cell
- Ba, Cr, Fe, Co, Ni, W which act as a killer for silicon, should not be contained.
- composition range of the above glass phase is 40 to 80% by weight of V 2 O 5 , 10 to 25% by weight of P 2 O 5 , 5 to 15% by weight of B 2 O 3 , TeO in terms of the following oxides.
- 2 is 0-25% by weight
- Sb 2 O 3 is 0-20% by weight
- Bi 2 O 3 is 0-20% by weight
- ZnO is 0-20% by weight
- the amount of P 2 O 5 is B 2 O greater than 3 weight, yet it is preferred that the total amount of P 2 O 5, B 2 O 3 and TeO 2 is from 20 to 50 wt%.
- a particularly effective composition range is 60-80 wt% V 2 O 5, 10-20 wt% P 2 O 5 , 5-10 wt% B 2 O 3 , 0-15 wt% TeO 2 , Sb 0 to 10% by weight of 2 O 3 , 0 to 10% by weight of Bi 2 O 3 , and 0 to 10% by weight of ZnO, and the total amount of P 2 O 5 , B 2 O 3 and TeO 2 is 20 to 20 It is 40% by weight.
- the ratio of 0.2 to 2 parts by weight of the glass phase is preferable with respect to 100 parts by weight of the metal particles contained in the electrode.
- the metal particles a large effect can be obtained particularly for aluminum or an aluminum alloy, and it is effective that the electrode is formed on the p-type semiconductor side of the silicon substrate.
- the solar cell using the silicon substrate which has pn junction is mentioned as a representative example.
- the present invention is a conductive paste for aluminum electrode in which a plurality of metal particles made of aluminum or aluminum alloy and a plurality of glass particles are dispersed in a solvent in which a binder resin is dissolved, and the glass particles are vanadium. It is characterized in that it is an oxide glass containing phosphorus and boron. Furthermore, it is desirable that the glass particles contain one or more of tellurium, antimony, bismuth and zinc.
- the preferable composition range of the glass particles is 40 to 80% by weight of V 2 O 5 , 10 to 25% by weight of P 2 O 5 , 5 to 15% by weight of B 2 O 3 , TeO 2 in terms of the following oxides.
- a particularly effective composition range is 60-80 wt% V 2 O 5, 10-20 wt% P 2 O 5 , 5-10 wt% B 2 O 3 , 0-15 wt% TeO 2 , Sb 0 to 10% by weight of 2 O 3 , 0 to 10% by weight of Bi 2 O 3 , and 0 to 10% by weight of ZnO, and the total amount of P 2 O 5 , B 2 O 3 and TeO 2 is 20 to 20 It is 40% by weight.
- the content of the glass particles contained in the conductive paste for an aluminum electrode is 0.2 to 15 parts by weight with respect to 100 parts by weight of the metal particles.
- a glass composition having a softening point of 420 ° C. or less and showing good fluidity at 500 ° C. is effective.
- the glass composition is substantially free of lead, and if the lead content is 1000 ppm or less, the impact on the environment can be reduced.
- the solid content consisting of metal particles and glass particles is preferably 70 to 75% by weight
- the binder component (resin and solvent) is preferably 30 to 25% by weight
- the binder component is 2 to 5% by weight of resin It is preferred that the solvent is 98 to 95% by weight.
- an oxide glass containing vanadium, phosphorus and boron to an electrode, it is possible to simultaneously achieve high performance and high reliability of an electronic component.
- the cell conversion efficiency and the lifetime can be simultaneously improved by forming an aluminum electrode containing the above-mentioned oxide glass on the p-type semiconductor side. it can.
- FIG. 3 is a schematic cross-sectional view taken along the line AA 'in FIG.
- FIG. 3 is an enlarged cross-sectional schematic view of the vicinity of the back surface along the line AA 'in FIG. 2;
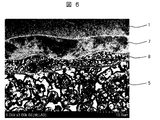
- It is a cross-sectional SEM observation image of the back surface vicinity of a representative photovoltaic cell.
- It is a graph which shows the relationship between the softening point of a glass composition, and the aluminum concentration in a silicon substrate.
- LTCC Low Temperature Co-fired Ceramics
- the present inventor has found that when an electrode containing an oxide glass containing vanadium, phosphorus and boron is fired on a silicon substrate to form the electrode, both the performance and the reliability of the electronic component to which the silicon substrate is applied are improved.
- the glass does not contain lead. It has been clarified that the cell conversion efficiency can be improved to the same level as conventional lead oxide glass.
- the aluminum electrode containing an oxide glass containing vanadium, phosphorus and boron promotes the diffusion of aluminum to the silicon substrate while suppressing the diffusion of oxygen. It is considered that the cell conversion efficiency is improved by forming a good p + layer (Back Surface Field: BSF layer) which is good on the p-type semiconductor surface.
- BSF layer Back Surface Field
- the moisture resistance and water resistance reliability of the aluminum electrode which could not be achieved with lead oxide glass, can be improved at the same time, which also contributes to prolonging the life.
- the oxide glass containing vanadium, phosphorus and boron exhibits good wettability and reactivity with aluminum particles, and prevents the aluminum electrode from being corroded by water to form aluminum hydroxide.
- this glass is applied to an aluminum electrode, defects due to the generation of foreign matter from the aluminum electrode and irregularities can be reduced, which can also contribute to the improvement of the productivity of electronic parts such as solar cells.
- the adhesion of the aluminum electrode to the silicon substrate was good.
- the above-mentioned oxide glass of the present invention is further improved in weatherability by containing at least one of tellurium, antimony, bismuth and zinc, and the produced glass is crushed or the crushed glass particles are stored. It is advantageous to That is, the handleability of the glass particles is improved.
- the preferable composition range of the above-mentioned oxide glass is 40 to 80% by weight of V 2 O 5 , 10 to 25% by weight of P 2 O 5 , 5 to 15% by weight of B 2 O 3 in terms of the following oxide conversion 2 is 0-25% by weight, Sb 2 O 3 is 0-20% by weight, Bi 2 O 3 is 0-20% by weight, and ZnO is 0-20% by weight, and the amount of P 2 O 5 is B 2 O greater than 3 weight, yet the total amount of P 2 O 5, B 2 O 3 and TeO 2 is from 20 to 50 wt%.
- V 2 O 5 When V 2 O 5 is less than 40 wt%, the firing temperature of the aluminum electrode becomes high, adhesion and water resistance of the aluminum electrode is reduced. In a solar cell, conversion efficiency is reduced. On the other hand, if V 2 O 5 exceeds 80% by weight, crystallization tends to occur, and good softening and fluidity can not be obtained at low temperatures. In addition, the weather resistance of the glass itself is significantly deteriorated, and the workability is reduced when the glass is crushed or the crushed glass particles are handled. When P 2 O 5 is less than 10% by weight, crystallization tends to occur easily and good softening and fluidity can not be obtained. On the other hand, when it exceeds 25% by weight, the conversion efficiency of the solar cell tends to decrease.
- B 2 O 3 is less than 5% by weight, the conversion efficiency of the solar cell can not be improved, while if it exceeds 15% by weight, the conversion efficiency of the solar cell is lowered. Moreover, even if TeO 2 exceeded 25 weight%, the conversion efficiency of the photovoltaic cell fell.
- Sb 2 O 3 , Bi 2 O 3 and ZnO each exceed 20% by weight, the softening point of the glass may become high or crystallization may occur, so that it is difficult to obtain good softening fluidity at low temperatures. Become. Furthermore, when the amount of B 2 O 3 is larger than the amount of P 2 O 5 , the crystallization tendency is increased, and the conversion efficiency of the solar battery cell is reduced.
- the most effective glass composition range is 60 to 80% by weight of V 2 O 5 in terms of the following oxide, P 10 to 20% by weight of 2 O 5, 5 to 10% by weight of B 2 O 3 , 0 to 15% by weight of TeO 2 , 0 to 10% by weight of Sb 2 O 3 , 0 to 10% by weight of Bi 2 O 3 % And ZnO were 0-10 wt%, and the total amount of P 2 O 5 , B 2 O 3 and TeO 2 was 20-40 wt%.
- the content of glass in the aluminum electrode formed in the solar battery cell is preferably 0.2 to 2 parts by weight, less than 0.2 parts by weight, or 2 parts by weight with respect to 100 parts by weight of aluminum particles. Cell conversion efficiency decreased. However, when it develops to the electrode of electronic components other than a photovoltaic cell, glass can be contained to 15 weight part. If it exceeds 15 parts by weight, the electrical resistance of the aluminum electrode will increase.
- the softening point of the glass is 420 ° C. or less and the softening flowability at 500 ° C. is good, the reliability of the adhesion of the aluminum electrode to the substrate and the moisture resistance are high, and when applied to a solar battery cell. Showed a high conversion efficiency.
- Table 1 shows the glass system examined in this example, its main component oxide, and its characteristics.
- G-01 is the glass of the example
- G-02 to 10 are the glasses of the comparative example.
- the “presence or absence of a hazardously controlled substance” in Table 1 was judged based on whether the hazardous substance regulated by the RoHS directive or the Joint Industry Guide Line was included.
- the "softening point” was measured by differential thermal analysis (DTA) using each glass powder.
- the analysis temperature rising condition of DTA was 5 ° C./min in air.
- FIG. 1 shows an example of a typical glass DTA curve.
- the start temperature of the first endothermic peak is the transition point T g
- the peak temperature is the sag point M g
- the second endothermic peak temperature is the softening point T s
- the respective characteristic points are defined by the viscosity.
- M g is 10 11 poise
- T s corresponds to 10 7.65 poise.
- the "softening flowability" was evaluated by producing a green compact having a diameter of 10 mm and a thickness of 5 mm using each glass powder, and heating on an alumina substrate.
- the heating conditions were such that the green compact mounted on the alumina substrate was placed in an electric furnace held at 400 ° C., 500 ° C., 600 ° C., 700 ° C., and 800 ° C. in the air for 1 minute, respectively, and taken out.
- good fluidity was obtained by visual observation, "o”, good fluidity was not obtained, but when softened, " ⁇ ”, and as a green compact, softening was also possible. When it did not, it evaluated as "x".
- the glass containing the harmful regulatory substance is only G-09.
- This Pb-B-Si-O-based glass has been widely applied to various electrodes of electronic parts such as solar cells and plasma display panels.
- the softening point was also relatively low at 390 ° C., and the flowability at 500 to 800 ° C. was good.
- G-10 is Pb-free glass which has been widely studied and is beginning to be put into practical use.
- this Bi-B-Si-O-based glass does not contain any harmful regulatory substance, its softening point and softening flowability have been raised in temperature as compared to G-09.
- the glass with a softening point higher than G-10 is G-06 in the Zn-B-V-O system and G-08 in the P-Zn-K-O system, and the softening flowability is also increased.
- Glass with a softening point between G-09 and G-10, G-02 of the V-P-Ba-O system, G-03 of the V-P-Fe-O system and Sn-P-Zn-O Although it was G-07 of the system, the softening flowability was almost equivalent to G-10.
- Glass with a softening point lower than G-09 is G-01 for V-P-B-O, G-04 for V-P-Te-O, and G-05 for V-Te-Zn-O. And the softening flowability was also lowered.
- the electroconductive paste for aluminum electrodes was produced using each glass shown in Table 1, and conversion efficiency and environmental conservation were evaluated by mounting in a photovoltaic cell. In addition, the appearance, adhesion and water resistance of the formed aluminum electrodes were also evaluated.
- the conductive paste for aluminum electrodes was prepared for each of the glasses G-01 to G-10 in Table 1.
- the glass was crushed into particles of 3 ⁇ m or less by a stamp mill and a jet mill.
- the aluminum particles those having an average particle diameter of 3 ⁇ m prepared by the atomizing method are used, and with respect to 100 parts by weight of the aluminum particles, 0.4 parts by weight of the glass particles of G-01 to 08, G-09 and -10 In the case of glass particles, 0.7 parts by weight was mixed respectively.
- the reason for changing the mixing amount of the glass particles is that the specific gravity of the glass of G-09 and -10 was about twice as large as that of the glass of G-01-8, and the glass content was almost the same in volume ratio .
- a solvent in which 2% by weight of a binder resin had been dissolved in advance was added and kneaded to prepare a conductive paste for an aluminum electrode.
- ethyl cellulose was used as the binder resin
- ⁇ -terpineol was used as the solvent.
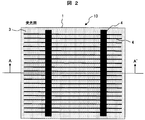
- FIG. 2 is a schematic plan view showing an example of a light receiving surface of a representative solar battery cell.
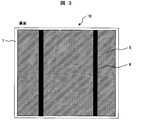
- 3 is a schematic plan view showing an example of the back surface,
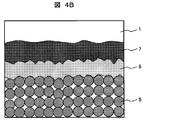
- FIG. 4A is a schematic sectional view taken along the line AA 'in FIG. 2, and
- FIG. 4B is an enlarged sectional schematic view in the vicinity of the back surface (shown by 4 in FIG. Part).
- a single crystal silicon substrate or a polycrystalline silicon substrate is usually used for the semiconductor substrate 1 of the solar battery cell 10 and contains boron or the like to be a p-type semiconductor. Irregularities are formed on the light receiving surface side by chemical etching or the like in order to suppress the reflection of sunlight.
- phosphorus or the like is doped on the light receiving surface to form an n-type semiconductor layer 2 having a thickness of about 1 ⁇ m. Then, a pn junction is formed at the boundary with the p-type bulk portion.
- an antireflective layer 3 such as silicon nitride is formed to a thickness of about 100 nm by vapor deposition or the like.
- a conductive paste for silver electrode containing silver particles and glass particles is used to form light receiving surface electrode 4 and output electrode 6, and aluminum containing aluminum particles and glass particles is used to form back surface electrode 5.
- Conductive pastes for electrodes are used. Each conductive paste is applied on the surface of the anti-reflection layer 3 formed on the light receiving surface of the semiconductor substrate 1 or the back surface of the semiconductor substrate 1 by a screen printing method or the like.
- the conductive paste After the conductive paste is dried, it is fired at around 800 ° C. in the atmosphere to form the respective electrodes.
- the glass composition contained in the light receiving surface electrode 4 reacts with the antireflective layer 3 to electrically connect the light receiving surface electrode 4 and the n-type semiconductor layer 2.
- the aluminum component in the back surface electrode 5 reacts with the semiconductor substrate 1 to form an alloy layer 8 of aluminum and silicon, and further aluminum diffuses into the semiconductor substrate 1 (Back Surface Field: BSF layer) ) 7 is formed.
- BSF layer 7 Back Surface Field: BSF layer 7
- the alloy layer 8 also has an effect of reflecting the light incident on the solar battery cell 10 on the back surface and confining the light in the semiconductor substrate 1 and is useful for improving the performance of the solar battery cell.
- the solar battery cell according to the electronic component of the present invention was produced.
- a p-type single crystal silicon substrate was used as the semiconductor substrate 1.
- the silicon substrate had a size of 125 mm square and a thickness of 200 ⁇ m.
- light reception of the semiconductor substrate 1 is performed using a strongly alkaline aqueous solution consisting of 1% caustic soda (sodium hydroxide: NaOH) and 10% isopropyl alcohol (CH 3 CH (OH) CH 3 ).
- the surface was etched to form asperities.
- a solution containing phosphorus pentoxide (P 2 O 5 ) is applied to the light receiving surface, and heat treatment is performed at 900 ° C. for 30 minutes to diffuse phosphorus (P) into the semiconductor substrate 1.
- Type semiconductor layer 2 was formed. After removing the phosphorus pentoxide, a silicon nitride film was uniformly formed on the n-type semiconductor layer 2 as an antireflective layer 3 with a thickness of about 100 nm.
- the silicon nitride film can be formed by a plasma CVD method using a mixed gas of silane (SiH 4 ) and ammonia (NH 3 ) as a raw material.
- a conductive paste for silver electrode containing silver particles and glass particles is applied in a grid shape by screen printing on the antireflection layer 3 and dried at 150 ° C. for 10 minutes I did.
- Silver particles having an average particle diameter of about 2 ⁇ m were used.
- glass particles a V-Ag-P-Te-O-based low melting glass having an average particle diameter of about 2 ⁇ m and containing no harmful lead was used.
- the same conductive paste for silver electrode as that described above was used for the output electrode 6 formed on the back surface of the semiconductor substrate 1, and it was similarly applied by screen printing and dried.
- a conductive paste for aluminum electrode containing aluminum particles and glass particles for the back electrode 5 was similarly applied and dried.
- the conductive paste for aluminum electrodes the conductive paste for aluminum electrodes produced using the example glass G-01 described above and the comparative glasses G-02 to 10 was used. Moreover, the conductive paste for aluminum electrodes which does not contain glass particles was also used for comparison.
- the solar cell 10 was manufactured by simultaneously firing and forming the light receiving surface electrode 4, the back surface electrode 5, and the output electrode 6 by rapidly heating to 800 ° C. in the atmosphere using a tunnel furnace and holding for 30 seconds. .
- the film thickness of the light-receiving surface electrode 4 and the output electrode 6 after firing was about 20 ⁇ m, and the film thickness of the back surface electrode was about 40 ⁇ m.
- the cell conversion efficiency was measured using the solar simulator with respect to the solar battery cell 10 which changed and produced the electrically conductive paste for aluminum electrodes for back surface electrodes 5 using a solar simulator.
- the produced photovoltaic cell 10 was also evaluated from the viewpoint of environmental protection (presence or absence of a harmful control substance). Furthermore, the appearance, adhesion and water resistance of the aluminum electrode formed for the back electrode 5 were also evaluated.
- the "adhesiveness" of the aluminum electrode was evaluated by the peel test.
- a commercially available cellophane tape is attached to an aluminum electrode, and when peeling off the aluminum electrode does not peel off, " ⁇ ", if slightly peeled off, “ ⁇ ”, if peeled off significantly It was set as “x” to.
- the "water resistance” is obtained by immersing the produced solar battery cell in warm water at 50 ° C. for 8 hours, " ⁇ ” when the aluminum electrode is hardly discolored in appearance, and " ⁇ when partially blackened”. When it was blackened on one side, it was evaluated as "x”.
- the above evaluation results are comprehensively examined and judged, “Good” for practically good solar cells, “Good” for insufficient solar cells, “good” for problematic solar cells It evaluated as x ".
- Example V-P-B-O-based glass G-01 and comparative example Pb-B-Si-O-based glass G-09 as a back surface electrode High conversion efficiency.
- comparative example Bi-B-Si-O-based glass G-10 relatively high conversion efficiency was shown. The conversion efficiency was lower when other glasses were used. Also, the conversion efficiency was lower when no glass was used than when using any glass.
- G-09 contains a large amount of harmful lead, so there are environmental problems even with solar cells, but there is no problem when using other glass.
- the solar cell with the highest conversion efficiency, ie, the highest performance electronic component was the case where the VPHO glass of Example G-01 was applied to the electrode.
- the appearance of the aluminum electrode formed in the solar battery cell was all good when using low melting point glass G-01 to 05 containing V 2 O 5 as the main component .
- the glass other than that was used and when it did not contain glass, many foreign materials and unevenness were recognized in the aluminum electrode surface part.
- the adhesion of the aluminum electrode was relatively low when the softening point was relatively low and glasses G-01 to 05, -07, -09 and -10 which softened and flowed at 500 ° C were used.
- the glass with good appearance, adhesion and water resistance of the aluminum electrode is G-01 to 05, and if it is a low melting point glass containing V 2 O 5 as the main component, it relates to the aluminum electrode It turned out that productivity and reliability can be improved or improved. It is considered that this is because low melting point glass particles containing aluminum particles and V 2 O 5 as main components have good wettability and reactivity when firing and forming an electrode. However, most of the low melting point glasses containing V 2 O 5 as a main component have reduced cell conversion efficiency. Only the oxide glass containing vanadium, phosphorus and boron of Example G-01 was the only glass whose cell conversion efficiency could be improved to the level of the Pb-B-Si-O-based glass G-09.
- the glass is substantially free of harmful controlled substances such as lead, and is sufficiently considered in the environment, and the conductive paste for electrodes to which the glass is applied, and the conductive paste Also in the electronic component having the formed electrode, the influence on the environmental load can be reduced.
- the lead content of electronic parts is regulated to 1000 ppm or less.
- the materials that make up the electronic component should not intentionally contain harmful lead.
- lead may be mixed as an impurity, and it is preferable to set the concentration to 1000 ppm or less as in the case of the electronic component also in each material constituting the electronic component.
- the V—P—B—O glass for an electrode according to the present invention has been developed on the premise of this in order to reduce the influence of environmental loads, and a conventional Pb—B—Si—O type glass or Bi—B— is used. It has been found that the performance and reliability of electronic parts can be improved compared to the case of using Si-O-based glass.
- Example 1 it was found that the conversion efficiency of the solar battery cell was different depending on the glass contained in the aluminum electrode. In order to investigate this cause, the state of the BSF layer 7 and the alloy layer 8 which are said to affect the cell conversion efficiency was observed and analyzed in detail by SEM-EDX.
- SEM-EDX The cross-sectional SEM photograph of the back surface vicinity of the photovoltaic cell produced using FIG. 5 using the electrically conductive paste for aluminum electrodes containing VPBO glass G-01 shown by Table 1 and Table 2 is shown. Since the BSF layer 7 is not usually observed, it was observed by etching using a hydrofluoric-nitric acid aqueous solution.
- the BSF layer 7 is formed by the diffusion of the aluminum component from the back electrode 5 to the semiconductor substrate 1 made of a silicon substrate.
- an alloy layer 8 is also formed between the back electrode 5 and the BSF layer 7 by the reaction of aluminum and silicon.
- glass G-02-10 other than G-01 was also made when using glass G-02-10 other than G-01.
- the compositions of the BSF layer 7 and the alloy layer 8 were analyzed by high sensitivity EDX.
- both aluminum and silicon were detected regardless of which glass was used.
- the composition of the analysis was very high, such as 90 atomic% or more of aluminum, while silicon was as low as 10 atomic% or less. The variation within this composition range is large even within the same solar battery cell, and a clear relationship between the conversion efficiency of the solar battery cell and the composition of the alloy layer 8 could not be found.
- the composition of the BSF layer 7 was similarly analyzed by high sensitivity EDX.
- a difference in the composition of the BSF layer 7 was observed depending on the glass.
- the relationship between the aluminum (Al) concentration in the BSF layer 7 and the softening point of the glass composition for aluminum electrodes is shown in FIG. It was found that the lower the softening point of the glass, the higher the aluminum concentration in the BSF layer 7. However, as shown in Tables 1 and 2, the higher the aluminum concentration, that is, the lower the softening point of the glass, the higher the conversion efficiency of the solar battery cell. Therefore, the oxygen concentration in the BSF layer 7 was also examined.
- FIG. 7 shows the relationship between the oxygen (O) concentration in the BSF layer 7 and the softening point of the glass composition for an aluminum electrode.
- the VPHO glass G-01 of the example With regard to the Pb-B-Si-O-based glass G-09 and the Bi-B-Si-O-based glass G-10 of the comparative example, this tendency is not met, and even if the softening point is low, the oxygen concentration in the BSF layer 7 is It was low. That is, it can be said that these glasses are difficult to oxidize the silicon substrate.
- the glass composition for aluminum electrodes which can make the aluminum concentration of BSF layer 7 high, and can make oxygen concentration low can improve the conversion efficiency of a photovoltaic cell.
- the softening point of the glass composition contained in the electrode may be lowered.
- the Pb-B-Si-O-based glass G-09 and the Bi-B-Si-O-based glass G-10 of the comparative example are very easy to be reduced, they are easily reduced in the aluminum electrode and oxygen is deprived. It is believed that the silicon substrate did not reach to oxidize the silicon substrate. As evidence of this, when X-ray diffraction of the back electrode 5 containing G-09 and -10, respectively, precipitation of lead metal and metal bismuth was observed. This is the result of the G-09 and -10 glasses being reduced in the aluminum electrode and deposited from these glasses.
- Low-melting glass G-02 to 05 containing V 2 O 5 as the main component is difficult to reduce compared to G-09 and -10, and such an effect can not be obtained, so it is thought that it is easy to oxidize the silicon substrate Be
- the V-P-B-O-based glass G-01 of the example is also a low melting point glass having V 2 O 5 as a main component, similarly to G-02 to 05 of the comparative example.
- the oxidation of the silicon substrate was suppressed. This is because G-01 and G-02 to 05 have different glass structures.
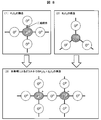
- the state of P 2 O 5 and B 2 O 3 in the glass structure is schematically shown in FIG.
- P 2 O 5 has one double bond oxygen (O) to one phosphorus (P) as shown in FIG. 8 (1) in the glass structure, and three crosslink oxygen (O) s make the glass It forms a mesh structure.
- B 2 O 3 forms a glass network structure from three bridging oxygen (O) to one boron (B) as shown in FIG. 8 (2) in the glass structure.
- This phenomenon was adopted in the glass structure of the V-P-B-O-based low melting point glass G-01 of the example. This suppressed the diffusion of oxygen to the silicon substrate, that is, the oxidation of the silicon substrate. Needless to say, this is a technology that can be effectively used other than the aluminum electrode if it is an electrode formed on a silicon substrate. In addition, the softening point is lowered by using V 2 O 5 as the main component, so that the amount of aluminum diffusion to the silicon substrate can be increased. Thus, the conversion efficiency of the solar cell was improved by the decrease of the amount of oxygen diffusion to the silicon substrate and the increase of the amount of aluminum diffusion.
- the solar battery cell has been described as an example, it is a technology that can be applied to all electronic components using a silicon substrate.
- the glass is a low melting point glass containing V 2 O 5 as a main component, it is possible to reduce the rate of defects such as the appearance of the aluminum electrode, and to improve the reliability such as adhesion and moisture resistance. This is due to the wettability and reaction with aluminum, and it goes without saying that the same effect can be obtained also with an aluminum alloy containing aluminum as a main component.
- the example applied to the back surface electrode of a photovoltaic cell was demonstrated, it can not be overemphasized that it is applicable also to the aluminum electrode and aluminum alloy electrode of other electronic components.
- Table 3 shows the composition and characteristics of the prepared VPOB low-melting glass. The glass production method of GA-01 to 30 shown in Table 3 will be described.
- the oxide shown by the composition of Table 3 was made into the glass raw material, and 200 g of each was mix
- the produced glass was ground by a stamp mill and a jet mill to an average particle size of 1 to 2 ⁇ m to obtain respective glass particles.
- the density of the produced glass was measured by the Archimedes method, and the softening point and the softening flowability were evaluated in the same manner as in Example 1.
- the density of the prepared V-P-B-O type low melting glass GA-01 to 30 is in the range of 2.60 to 3.60 g / cm 3 , and the conventional Pb-B-Si-O type low melting point The density was about half that of the glass G-09 and the Bi-B-Si-O low melting point glass G-10.
- the glass having a low density was GA-27 to 30, and the content of P 2 O 5 was equal to or less than that of B 2 O 3 .
- the content of P 2 O 5 was increased about twice as much as the content of B 2 O 3 to try to actively utilize the boric acid abnormal phenomenon described in Example 2.
- glasses having a high density were GA-15, -22 to 24 and -26 containing 30% by weight or more of TeO 2 .
- the softening point tends to be lower as the content of V 2 O 5 or TeO 2 is higher and the amount of P 2 O 5 is lower.
- the softening flowability was that all glasses had good flowability at 600 ° C. or higher. At 500 ° C., except for GA-27, -28 and -30, it had good fluidity. Also, at 400 ° C, GA-05 to 07, -09, -13 and -16 softened, and only GA-01 to 04, -14, -15, -22 and -23 showed good fluidity. . Similar to the softening point, the glass having a larger content of V 2 O 5 and TeO 2 and a smaller amount of P 2 O 5 tends to have a better softening flow at a low temperature.
- a conductive paste for an aluminum electrode was produced in the same manner as in Example 1 using the glass particles of GA-01 to 30. However, nitrocellulose was used as the binder resin, and butyl carbitol acetate was used as the solvent. Further, for comparison, in the same manner as in Example 1, the conductivity for aluminum electrodes containing Pb-B-Si-O low melting point glass G-09 and Bi-B-Si-O low melting point glass G-10 respectively A paste and a conductive paste for aluminum electrode without glass were also prepared.
- the solar cell shown in FIGS. 2 to 4 was produced and evaluated in the same manner as in Example 1 using the produced conductive paste for an aluminum electrode.
- the semiconductor substrate 1 a 150 mm square p-type polycrystalline silicon substrate having a thickness of 200 ⁇ m was used. Table 4 shows the evaluation results of the manufactured solar battery cell.
- a polycrystalline silicon substrate having a cell conversion efficiency lower than that of a single crystal silicon substrate is used as the semiconductor substrate 1 and therefore very high as a polycrystalline silicon substrate.
- Conversion efficiency 17.0% or more “ ⁇ ”, 16.5 to 17.0% “ ⁇ ”, 16.0 to 16.5% “ ⁇ ”, less than 16.0% “X” did.
- Other evaluations were performed in the same manner as in Example 1. However, in the “overall evaluation”, the solar cell that was particularly excellent in conversion efficiency in the “o” evaluation was evaluated as “o”.
- the glass composition for an aluminum electrode may contain one or more of Te, Sb, Bi, and Zn based on the VPBO system. .
- Te, Sb, Bi, and Zn the weather resistance of the glass is improved, and the handleability of the glass particles until the production of the conductive paste for an aluminum electrode is improved.
- the overall evaluation of the solar battery cell is 40 to 80 wt% of V 2 O 5 , 10 to 25 wt% of P 2 O 5 , and B 2 O 3 in terms of the following oxides. 5 to 15% by weight, 0 to 25% by weight of TeO 2 , 0 to 20% by weight of Sb 2 O 3 , 0 to 20% by weight of Bi 2 O 3 , and 0 to 20% by weight of ZnO, P 2
- the amount of O 5 was larger than the amount of B 2 O 3 , and the total amount of P 2 O 5 , B 2 O 3 and TeO 2 was 20 to 50% by weight.
- V-P-B-O type low melting glass having a density of 2.81 to 3.25 g / cm 3 , a softening point of 420 ° C. or less, and a good fluidity at 500 ° C. It is mentioned that it is effective.
- the overall evaluated glass composition range of “ ⁇ ” was 60 to 80 wt% of V 2 O 5 , 10 to 20 wt% of P 2 O 5 , B 2 O 3 in terms of the following oxides.
- the glass composition for aluminum electrodes in the above composition range can promote the diffusion of the aluminum component to the silicon substrate and can suppress the diffusion of oxygen, so it is effective not only for solar cells but also for all electronic components using silicon substrates. It goes without saying that it can be applied to Moreover, although it is effective especially to an aluminum electrode, it can utilize also other than an aluminum electrode.
- the effect of the content of the V—P—B—O low-melting glass in the aluminum electrode on the conversion efficiency of the solar battery cell was examined in detail.
- GA-05 in Example 3 shown in Table 3 and Table 4 was used as this glass.
- the glass content of GA-05 was examined in the range of 0 to 5 parts by weight with respect to 100 parts by weight of aluminum particles (0, 0.2, 0.4, 0.7, 1.0, 1.5, 2 .0, 2.5, 3.0, 3.5, 4.0 and 5.0 parts by weight).
- Example 3 In the same manner as in Example 3, the glass content was changed, and 12 types of conductive pastes for aluminum electrodes were produced.
- the solar cell shown in FIG. 2, FIG. 3, FIG. 4A and FIG. 4B was produced like Example 3 using the produced electrically conductive paste for aluminum electrodes for the back surface electrode, and cell conversion efficiency was measured.
- FIG. 9 shows the relationship between the conversion efficiency of the solar battery cell and the content of the V—P—B—O glass composition contained in the aluminum electrode. When glass was not contained in the aluminum electrode which is the back surface electrode, the conversion efficiency of the solar cell was extremely low, but when only 0.2 parts by weight of GA-05 glass was contained, the conversion efficiency was improved at once.
- a very high conversion efficiency of 17% or more was obtained in the range of 0.2 to 0.7 parts by weight of GA-05 glass. At a higher content, the conversion efficiency decreased slightly to 2 parts by weight, but the conversion efficiency was as good as 16.5% or more. When the amount exceeds 2 parts by weight, the conversion efficiency is significantly reduced, and at 2.5 parts by weight or more, it falls below 16.0%.
- the reason why the conversion efficiency decreases with the glass content in the aluminum electrode is considered to be the increase in the diffusion amount of oxygen to the silicon substrate, that is, the progress of the oxidation of the silicon substrate.
- the content of the V—P—B—O low-melting glass in the aluminum electrode applied as the back electrode of the solar battery cell was preferably in the range of 0.2 to 2 parts by weight.
- the range of 0.2 to 0.7 parts by weight was effective.
- this can be effectively applied not only to solar cells but also to electronic components in general using a silicon substrate.
- it is effective especially to an aluminum electrode, it can not be overemphasized that it can utilize also except an aluminum electrode.
- the glass content of GA-09 was examined in the range of 0 to 25 parts by weight with respect to 100 parts by weight of aluminum alloy particles (0, 0.2, 2.0, 5.0, 10.0, 15.0, 20.0 and 25.0 parts by weight).
- Example 3 Eight kinds of conductive pastes for aluminum alloy electrodes were prepared by changing the glass content. However, ethyl cellulose was used in place of nitrocellulose as the binder resin. The solvent is butyl carbitol acetate.
- the prepared conductive paste for an aluminum alloy electrode was applied to a single crystal silicon substrate by screen printing and dried at 150 ° C. for 10 minutes. Then, it was put into an electric furnace, heated to 500 ° C. at a temperature rising rate of 10 ° C./minute in the atmosphere, and held for 10 minutes, and then the furnace was cooled. The film thickness of the aluminum alloy electrode was about 20 ⁇ m. The specific resistance of the aluminum alloy electrode formed on the silicon substrate was measured by the four-point probe method.
- FIG. 10 shows the relationship between the specific resistance of the aluminum alloy electrode and the content of the glass composition contained in the electrode.
- the specific resistance is extremely high, but when only 0.2 parts by weight of GA-09 glass is contained, the specific resistance decreases rapidly. did.
- the specific resistance on the order of 10 -5 ⁇ cm was achieved in the range of 0.2 to 15 parts by weight of GA-05 glass. At a content of 20 parts by weight or more, the specific resistance of the aluminum alloy electrode became large again.
- the content of the V—P—B—O low melting point glass in the electrode is 0.2 It has been found that a range of ⁇ 15 parts by weight is preferred.
- a V—P—B—O low melting point glass was examined in the aluminum alloy electrode, but it goes without saying that it can be used for an aluminum electrode and other electrodes.
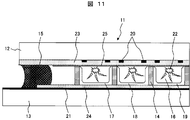
- FIG. 11 is a schematic cross-sectional view showing an example of a plasma display panel. This will be described below with reference to FIG.
- the front plate 12 and the back plate 13 are disposed to face each other with a gap of 100 to 150 ⁇ m, and the gap between the substrates (the front plate 12 and the back plate 13) is maintained by the partition wall.
- the peripheral portions of the front plate 12 and the back plate 13 are airtightly sealed with a sealing material 15, and the inside of the panel is filled with a rare gas.
- a display electrode 20 is formed on the front plate 12, a dielectric layer 23 is formed on the display electrode 20, and a protective layer 25 (eg, MgO) for protecting the display electrode 20 and the like from discharge on the dielectric layer 23.
- the partition 14 is made of a structure obtained by sintering a material containing at least a glass composition and a filler at 500 to 600 ° C., and is usually a stripe or box structure. Further, the address electrodes 21 of the back plate 13 are formed to be orthogonal to the display electrodes 20 of the front plate 12.
- the minute space (cell 16) partitioned by the partition 14 is filled with a fluorescent substance.
- the phosphor in the cell 16 is formed by filling the paste for the phosphor into the cell 16 and baking it at 450 to 500.degree.
- One pixel is constituted by three color cells of a cell filled with the red phosphor 17, a cell filled with the green phosphor 18, and a cell filled with the blue phosphor 19. Each pixel emits various colors in accordance with the signal applied to the display electrode 20 and the address electrode 21.
- the sealing material 15 is previously applied to the periphery of either the front plate 12 or the back plate 13 by a dispenser method, a printing method, or the like.
- the applied sealing material 15 may be pre-fired simultaneously with the firing of the phosphors 17-19.
- By temporarily baking the applied sealing material air bubbles in the glass sealing portion can be remarkably reduced, and a highly reliable (that is, highly airtight) glass sealing portion can be obtained.
- the sealing of the front plate 12 and the back plate 13 is performed by arranging the front plate 12 and the back plate 13 separately manufactured to face each other with accurate alignment and heating to 420 to 500.degree. At this time, the gas in the cell 16 is exhausted while heating, and a noble gas is filled instead, thereby completing the plasma display panel as an electronic component.
- the sealing material 15 may be in direct contact with the display electrode 20 or the address electrode 21 at the time of pre-sintering of the sealing material or at the time of glass sealing, the electrode wiring material and the sealing material do not react chemically. It is important that it is configured.
- a voltage is applied between the display electrode 20 and the address electrode 21 of the cell 16 to be lighted to perform address discharge in the cell 16 to plasma rare gas It excites to the state and accumulates the wall charge in the cell.
- display discharge occurs only in the cells in which the wall charges are accumulated, and ultraviolet light 22 is generated.
- image information is displayed by causing the fluorescent materials 17 to 19 to emit light using the ultraviolet light 22.
- the display electrode 20 and the address electrode 21 a silver thick film electrode wiring has been conventionally used in consideration of good electrical properties and oxidation resistance during manufacturing.
- the formation of the display electrodes 20 and the address electrodes 21 can also be performed by a sputtering method, but the printing method is advantageous for reducing the manufacturing cost.
- the dielectric layers 23 and 24 are usually formed by printing. Further, the display electrode 20, the address electrode 21 and the dielectric layers 23, 24 formed by the printing method are generally fired at a temperature range of 550 to 620 ° C. in an oxidizing atmosphere.
- the silver thick film electrode wiring has a problem that silver is apt to cause a migration phenomenon and also has a problem that the material cost is high.
- the specific resistance of the electrode wiring is low, the chemical reaction between the electrode wiring and the dielectric layer does not occur, and the vicinity of the formed electrode wiring It is necessary to satisfy the condition that the air pressure (air bubbles etc.) is generated and the electrical pressure resistance does not decrease.
- the aluminum alloy particles (Al-10 wt% Ag) used in Example 5 were prepared as metal particles to be contained in the conductive paste for aluminum electrode.
- a binder resin and a solvent were added and mixed to prepare a conductive paste for aluminum electrode.
- ethyl cellulose was used as a binder resin
- ⁇ -terpineol was used as a solvent.
- the plasma display panel according to the present invention was produced.
- the conductive paste for an aluminum electrode was applied to the entire surface of the front plate 12 and the back plate 13 by screen printing, and dried at 150 ° C. in the atmosphere. Excess portions of the coating film were removed by photolithography to pattern the electrode wiring, and then baked in the air at 580 ° C. for 10 minutes to form the display electrode 20 and the address electrode 21.
- dielectric layers 23 and 24 were respectively applied and baked at 560 ° C. for 30 minutes in the air.
- the front plate 12 and the back plate 13 produced in this manner were disposed opposite to each other, and the outer edge was sealed with glass to fabricate a plasma display panel having a structure as shown in FIG.
- the electrode wiring (the display electrode 20 and the address electrode 21) formed by using the conductive paste for aluminum electrode according to the present invention includes the interface between the display electrode 20 and the dielectric layer 23, the address electrode 21 and the dielectric layer 24. No generation of voids was observed at the interface of the above, and the plasma display panel could be manufactured in a good appearance.
- the conductive paste for an aluminum electrode of the present invention can be applied as an electrode wiring of a plasma display panel. Moreover, since it can be an alternative to expensive silver thick film electrode wiring, it can greatly contribute to cost reduction.
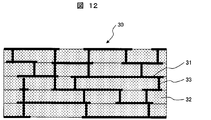
- FIG. 12 is a schematic cross-sectional view showing a structural example before firing of a multilayer wiring board (five layers) of LTCC (Low Temperature Co-fired Ceramics).
- the multilayer wiring board 30 is a wiring board on which wiring (conductive paste 31 for wiring) is three-dimensionally formed. This will be described below with reference to FIG.
- the production of a multilayer wiring board is usually performed in the following procedure.
- a green sheet 32 containing glass powder, ceramic powder and a binder is prepared, and the through holes 33 are opened at desired positions.
- the conductive paste 31 for wiring is applied to a desired wiring pattern by a printing method on the green sheet 32 in which the through holes 33 are opened, and the through holes 33 are also filled. If necessary, the wiring conductive paste 31 is applied to the back surface of the green sheet 32 by a printing method. When applying on the back surface of the green sheet 32, it is performed after drying the conductive paste 31 for wiring applied on the surface.
- a plurality of green sheets 32 on which a predetermined wiring pattern is formed are stacked and integrally fired to manufacture an LTCC multilayer wiring board.
- atmosphere is common.
- a conductive paste for wiring a conductive paste of silver is usually used in consideration of good electrical properties and oxidation resistance during production.
- the glass phase is easily softened / flows at the portion where the green sheet 32 and the conductive paste 31 are in contact during firing, the copper particles are oxidized, and the specific resistance of the electrode wiring is There was an increasing problem. Furthermore, a void may be generated at the interface due to a chemical reaction with the glass phase.
- a multilayer wiring board according to the present invention was produced.
- the conductive paste 31 for wiring the conductive paste for aluminum electrode examined in Example 6 is used to form a multilayer wiring stack as shown in FIG. Bake for 30 minutes.
- SYMBOLS 10 Solar cell, 1 ... p-type semiconductor substrate, 2 ... n-type semiconductor layer, 3 ... anti-reflective layer, 4 ... light-receiving surface electrode, 5 ... back surface electrode, 6 ... output electrode, 7 ... BSF layer, 8 ... alloy Layers 11 Plasma display panel 12 Front plate 13 Rear plate 14 Partition wall 15 Sealing material 16 Cell 17 Red phosphor 18 Green phosphor 19 Blue phosphor Reference Signs List 20 display electrode 21 address electrode 22 ultraviolet light 23 24 dielectric layer 25 protective layer 30 multilayer wiring board 31 conductive paste for wiring 32 green sheet 33 through hole .
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Geochemistry & Mineralogy (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Physics & Mathematics (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Sustainable Energy (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- Electromagnetism (AREA)
- General Physics & Mathematics (AREA)
- Computer Hardware Design (AREA)
- Sustainable Development (AREA)
- Power Engineering (AREA)
- Dispersion Chemistry (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Glass Compositions (AREA)
- Photovoltaic Devices (AREA)
- Conductive Materials (AREA)
- Gas-Filled Discharge Tubes (AREA)
Abstract
La présente invention concerne une pâte conductrice pour électrode d'aluminium qui permet d'obtenir de grandes performances et une fiabilité élevée dans une cellule solaire ou un autre composant électronique employant un substrat de silicium ; une composition de verre contenue à l'intérieur de ladite pâte ; et un composant électronique dont les performances et la fiabilité sont améliorées par celle-ci. Ladite pâte conductrice pour électrode d'aluminium contient une électrode d'aluminium et un verre à base de V-P-B-O à bas point de fusion, ce qui permet d'augmenter la quantité de diffusion d'aluminium dans le substrat de silicium tout en réduisant la quantité de diffusion d'oxygène, et de rendre ainsi le composant électronique plus fiable. De plus, comme le verre et l'aluminium ont une bonne mouillabilité et une bonne réactivité, la fiabilité de l'électrode peut être améliorée.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2011087120A JP5673316B2 (ja) | 2011-04-11 | 2011-04-11 | 電子部品、それに適用されるアルミニウム電極用導電性ペースト、及びアルミニウム電極用ガラス組成物 |
| JP2011-087120 | 2011-04-11 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012141187A1 true WO2012141187A1 (fr) | 2012-10-18 |
Family
ID=47009358
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2012/059850 WO2012141187A1 (fr) | 2011-04-11 | 2012-04-11 | Composant électronique, pâte conductrice pour électrode d'aluminium pour application dans ledit composant, et composition de verre pour électrode d'aluminium |
Country Status (3)
| Country | Link |
|---|---|
| JP (1) | JP5673316B2 (fr) |
| TW (1) | TWI478890B (fr) |
| WO (1) | WO2012141187A1 (fr) |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2750140A1 (fr) * | 2012-12-28 | 2014-07-02 | Heraeus Precious Metals GmbH & Co. KG | Pâte électroconductrice comprenant un vanadium contenant un composé et un phosphore contenant le matériau dans la préparation d'électrodes pour cellules solaires MWT |
| EP2750142A1 (fr) * | 2012-12-28 | 2014-07-02 | Heraeus Precious Metals GmbH & Co. KG | Pâte électroconductrice comprenant un système de réaction inorganique avec une température de transition vitreuse élevée dans la préparation d'électrodes pour cellules solaires MWT |
| EP2750139A1 (fr) * | 2012-12-28 | 2014-07-02 | Heraeus Precious Metals GmbH & Co. KG | Pâte électroconductrice comprenant un vanadium contenant un composé dans la préparation d'électrodes pour cellules solaires MWT |
| EP2750141A1 (fr) * | 2012-12-28 | 2014-07-02 | Heraeus Precious Metals GmbH & Co. KG | Pâte électroconductrice comprenant des particules d'oxyde inorganiques grossières dans la préparation d'électrodes pour cellules solaires MWT |
| CN109564945A (zh) * | 2016-08-23 | 2019-04-02 | 纳美仕有限公司 | 导电性糊剂和太阳能电池 |
| TWI687941B (zh) * | 2019-01-14 | 2020-03-11 | 磐采股份有限公司 | 導電膠及應用該導電膠之太陽能電池 |
| US10720260B2 (en) | 2014-11-13 | 2020-07-21 | Samsung Sdi Co., Ltd. | Paste for forming solar cell electrode and electrode prepared using the same |
| GB2589703A (en) * | 2019-10-17 | 2021-06-09 | Johnson Matthey Plc | Composition, paste and methods |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101587683B1 (ko) * | 2013-02-15 | 2016-01-21 | 제일모직주식회사 | 태양전지 전극 형성용 조성물 및 이로부터 제조된 전극 |
| JP6272332B2 (ja) * | 2013-08-30 | 2018-01-31 | 京セラ株式会社 | 太陽電池素子およびその製造方法 |
| CN104835606B (zh) * | 2015-04-03 | 2017-10-10 | 兴勤(常州)电子有限公司 | 电子元器件多层合金电极及其制备方法 |
| EP3355340B1 (fr) * | 2015-09-24 | 2020-09-09 | Toyo Aluminium Kabushiki Kaisha | Utilisation d'une composition de pâte et procédé de formation de couche de silicium-germanium |
| WO2017126378A1 (fr) * | 2016-01-18 | 2017-07-27 | 株式会社日立製作所 | Composition de verre sans plomb, matériau composite en verre, pâte de verre, structure d'étanchéité, composant électrique/électronique et composant revêtu |
| JP2017218335A (ja) * | 2016-06-03 | 2017-12-14 | 旭硝子株式会社 | ガラス、導電ペーストおよび太陽電池 |
| WO2022176519A1 (fr) * | 2021-02-16 | 2022-08-25 | 昭和電工マテリアルズ株式会社 | Composition pour formation d'électrode, élément de cellule solaire, et électrode stratifiée aluminium/argent |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009530845A (ja) * | 2006-03-20 | 2009-08-27 | フエロ コーポレーション | アルミニウム−ボロン太陽電池コンタクト |
| JP2010161331A (ja) * | 2008-12-12 | 2010-07-22 | Hitachi Ltd | 電極,電極ペースト及びそれを用いた電子部品 |
| JP2010533379A (ja) * | 2007-07-09 | 2010-10-21 | フエロ コーポレーション | アルミニウムと、ホウ素、チタン、ニッケル、錫、銀、ガリウム、亜鉛、インジウム、及び銅のうち少なくとも1種とを含有する太陽電池コンタクト |
| WO2010147160A1 (fr) * | 2009-06-17 | 2010-12-23 | 旭硝子株式会社 | Fritte de verre pour la formation d'une électrode et pâte électriquement conductrice pour la formation d'une électrode et photopile utilisant chacune celle-ci |
| JP2011035024A (ja) * | 2009-07-30 | 2011-02-17 | Toyo Aluminium Kk | ペースト組成物およびそれを用いた太陽電池素子 |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101265023B (zh) * | 2007-03-15 | 2010-05-26 | 北京印刷学院 | 钒银低熔玻璃和含有该玻璃的导电浆料 |
-
2011
- 2011-04-11 JP JP2011087120A patent/JP5673316B2/ja not_active Expired - Fee Related
-
2012
- 2012-04-11 TW TW101112860A patent/TWI478890B/zh active
- 2012-04-11 WO PCT/JP2012/059850 patent/WO2012141187A1/fr active Application Filing
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009530845A (ja) * | 2006-03-20 | 2009-08-27 | フエロ コーポレーション | アルミニウム−ボロン太陽電池コンタクト |
| JP2010533379A (ja) * | 2007-07-09 | 2010-10-21 | フエロ コーポレーション | アルミニウムと、ホウ素、チタン、ニッケル、錫、銀、ガリウム、亜鉛、インジウム、及び銅のうち少なくとも1種とを含有する太陽電池コンタクト |
| JP2010161331A (ja) * | 2008-12-12 | 2010-07-22 | Hitachi Ltd | 電極,電極ペースト及びそれを用いた電子部品 |
| WO2010147160A1 (fr) * | 2009-06-17 | 2010-12-23 | 旭硝子株式会社 | Fritte de verre pour la formation d'une électrode et pâte électriquement conductrice pour la formation d'une électrode et photopile utilisant chacune celle-ci |
| JP2011035024A (ja) * | 2009-07-30 | 2011-02-17 | Toyo Aluminium Kk | ペースト組成物およびそれを用いた太陽電池素子 |
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2014101999A1 (fr) * | 2012-12-28 | 2014-07-03 | Heraeus Precious Metals Gmbh & Co. Kg | Pâte électroconductrice contenant des particules d'oxyde inorganique grossières, utilisée dans la préparation d'électrodes pour cellules solaires mwt |
| CN105144304A (zh) * | 2012-12-28 | 2015-12-09 | 赫劳斯德国有限两和公司 | 制备mwt太阳能电池电极中的包含含钒化合物的导电浆料 |
| EP2750139A1 (fr) * | 2012-12-28 | 2014-07-02 | Heraeus Precious Metals GmbH & Co. KG | Pâte électroconductrice comprenant un vanadium contenant un composé dans la préparation d'électrodes pour cellules solaires MWT |
| EP2750141A1 (fr) * | 2012-12-28 | 2014-07-02 | Heraeus Precious Metals GmbH & Co. KG | Pâte électroconductrice comprenant des particules d'oxyde inorganiques grossières dans la préparation d'électrodes pour cellules solaires MWT |
| WO2014102000A1 (fr) * | 2012-12-28 | 2014-07-03 | Heraeus Precious Metals Gmbh & Co. Kg | Pâte électro-conductrice comprenant un système de réaction inorganique à température de transition vitreuse élevée utilisée dans la préparation d'électrodes dans des cellules photovoltaïques mwt |
| WO2014102004A1 (fr) * | 2012-12-28 | 2014-07-03 | Heraeus Precious Metals Gmbh & Co. Kg | Pâte électroconductrice comprenant un composé contenant du vanadium et un matériau contenant du phosphore utilisée dans la fabrication d'électrodes pour cellules solaires mwt |
| EP2750142A1 (fr) * | 2012-12-28 | 2014-07-02 | Heraeus Precious Metals GmbH & Co. KG | Pâte électroconductrice comprenant un système de réaction inorganique avec une température de transition vitreuse élevée dans la préparation d'électrodes pour cellules solaires MWT |
| WO2014102002A1 (fr) * | 2012-12-28 | 2014-07-03 | Heraeus Precious Metals Gmbh & Co. Kg | Pâte conductrice électrique comprenant un composé contenant du vanadium pour la préparation d'électrodes dans des cellules solaires mwt |
| EP2750140A1 (fr) * | 2012-12-28 | 2014-07-02 | Heraeus Precious Metals GmbH & Co. KG | Pâte électroconductrice comprenant un vanadium contenant un composé et un phosphore contenant le matériau dans la préparation d'électrodes pour cellules solaires MWT |
| US10002977B2 (en) | 2012-12-28 | 2018-06-19 | Heraeus Deutschland GmbH & Co. KG | Electro-conductive paste comprising coarse inorganic oxide particles in the preparation of electrodes in MWT solar cells |
| US10720260B2 (en) | 2014-11-13 | 2020-07-21 | Samsung Sdi Co., Ltd. | Paste for forming solar cell electrode and electrode prepared using the same |
| CN109564945A (zh) * | 2016-08-23 | 2019-04-02 | 纳美仕有限公司 | 导电性糊剂和太阳能电池 |
| TWI687941B (zh) * | 2019-01-14 | 2020-03-11 | 磐采股份有限公司 | 導電膠及應用該導電膠之太陽能電池 |
| GB2589703A (en) * | 2019-10-17 | 2021-06-09 | Johnson Matthey Plc | Composition, paste and methods |
| GB2589703B (en) * | 2019-10-17 | 2022-01-12 | Johnson Matthey Advanced Glass Tech B V | Composition for sealing inorganic substrates |
Also Published As
| Publication number | Publication date |
|---|---|
| JP5673316B2 (ja) | 2015-02-18 |
| JP2012218982A (ja) | 2012-11-12 |
| TWI478890B (zh) | 2015-04-01 |
| TW201249771A (en) | 2012-12-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2012141187A1 (fr) | Composant électronique, pâte conductrice pour électrode d'aluminium pour application dans ledit composant, et composition de verre pour électrode d'aluminium | |
| JP5699933B2 (ja) | ガラス組成物およびそれを用いた導電性ペースト組成物、電極配線部材と電子部品 | |
| JP5826178B2 (ja) | 電極用ガラス組成物、及びそれを用いた電極用ペースト、並びにそれを適用した電子部品 | |
| JP5497504B2 (ja) | 電子部品 | |
| JP5215458B2 (ja) | 導電性ペースト及びそれを用いた電極配線を具備する電子部品 | |
| KR101155536B1 (ko) | 저융점 유리조성물, 그것을 사용한 저융점 봉착재료 및 전자부품 | |
| WO2013069668A1 (fr) | Composant électronique, pâte conductrice pour des électrodes d'aluminium utilisées dans celui-ci et composition de verre pour des électrodes d'aluminium | |
| JP5416631B2 (ja) | アルミニウム電極配線用のガラス組成物と導電性ペースト、そのアルミニウム電極配線を具備する電子部品、及び、この電子部品の製造方法 | |
| CN109564945B (zh) | 导电性糊剂和太阳能电池 | |
| EP2461366A1 (fr) | Composition de pâte et élément de cellule solaire l'utilisant | |
| US8884277B2 (en) | Thick film conductive composition and use thereof | |
| JP5747096B2 (ja) | 導電性ペースト | |
| JP5546074B2 (ja) | 導電性ペースト及びそれを用いた電極配線を具備する電子部品 | |
| WO2011104859A1 (fr) | Composant électronique, pâte conductrice, et procédé de fabrication de composant électronique | |
| WO2024100947A1 (fr) | Cellule solaire | |
| WO2023190282A1 (fr) | Pâte électriquement conductrice, cellule solaire, et procédé destiné à fabriquer une cellule solaire | |
| WO2024101223A1 (fr) | Pâte électriquement conductrice, cellule solaire, et procédé destiné à fabriquer une cellule solaire |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12771387 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 12771387 Country of ref document: EP Kind code of ref document: A1 |