WO2006102289A2 - Skin lightening compositions - Google Patents
Skin lightening compositions Download PDFInfo
- Publication number
- WO2006102289A2 WO2006102289A2 PCT/US2006/010149 US2006010149W WO2006102289A2 WO 2006102289 A2 WO2006102289 A2 WO 2006102289A2 US 2006010149 W US2006010149 W US 2006010149W WO 2006102289 A2 WO2006102289 A2 WO 2006102289A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- composition
- skin
- ascorbic acid
- extract
- glucoside
- Prior art date
Links
- 0 *C(NC(Cc1c(*)c(*)c(*)c(*)c1*)C(O)=O)=O Chemical compound *C(NC(Cc1c(*)c(*)c(*)c(*)c1*)C(O)=O)=O 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/60—Moraceae (Mulberry family), e.g. breadfruit or fig
- A61K36/605—Morus (mulberry)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/365—Lactones
- A61K31/375—Ascorbic acid, i.e. vitamin C; Salts thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/445—Non condensed piperidines, e.g. piperocaine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/25—Araliaceae (Ginseng family), e.g. ivy, aralia, schefflera or tetrapanax
- A61K36/258—Panax (ginseng)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/42—Cucurbitaceae (Cucumber family)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/48—Fabaceae or Leguminosae (Pea or Legume family); Caesalpiniaceae; Mimosaceae; Papilionaceae
- A61K36/484—Glycyrrhiza (licorice)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/53—Lamiaceae or Labiatae (Mint family), e.g. thyme, rosemary or lavender
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/73—Rosaceae (Rose family), e.g. strawberry, chokeberry, blackberry, pear or firethorn
- A61K36/739—Sanguisorba (burnet)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/75—Rutaceae (Rue family)
- A61K36/752—Citrus, e.g. lime, orange or lemon
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/82—Theaceae (Tea family), e.g. camellia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/88—Liliopsida (monocotyledons)
- A61K36/906—Zingiberaceae (Ginger family)
- A61K36/9062—Alpinia, e.g. red ginger or galangal
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/40—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing nitrogen
- A61K8/42—Amides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/40—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing nitrogen
- A61K8/44—Aminocarboxylic acids or derivatives thereof, e.g. aminocarboxylic acids containing sulfur; Salts; Esters or N-acylated derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/49—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds
- A61K8/494—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with more than one nitrogen as the only hetero atom
- A61K8/4946—Imidazoles or their condensed derivatives, e.g. benzimidazoles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/67—Vitamins
- A61K8/673—Vitamin B group
- A61K8/675—Vitamin B3 or vitamin B3 active, e.g. nicotinamide, nicotinic acid, nicotinyl aldehyde
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/67—Vitamins
- A61K8/676—Ascorbic acid, i.e. vitamin C
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/96—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution
- A61K8/97—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution from algae, fungi, lichens or plants; from derivatives thereof
- A61K8/9783—Angiosperms [Magnoliophyta]
- A61K8/9789—Magnoliopsida [dicotyledons]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/96—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution
- A61K8/97—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution from algae, fungi, lichens or plants; from derivatives thereof
- A61K8/9783—Angiosperms [Magnoliophyta]
- A61K8/9794—Liliopsida [monocotyledons]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/02—Preparations for care of the skin for chemically bleaching or whitening the skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2236/00—Isolation or extraction methods of medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicine
- A61K2236/30—Extraction of the material
- A61K2236/33—Extraction of the material involving extraction with hydrophilic solvents, e.g. lower alcohols, esters or ketones
- A61K2236/333—Extraction of the material involving extraction with hydrophilic solvents, e.g. lower alcohols, esters or ketones using mixed solvents, e.g. 70% EtOH
Definitions
- compositions of the present invention can include, for example, a combination of ingredients that can be used to whiten skin, even out skin color, or treat hyperpigmentation.
- the color in human skin is caused by the pigment melanin.
- Melanin is produced in special dendritic cells, melanocytes, which are found below or between the basal cells of the epidermis of the skin (U.S. Pat. No. 5,411,741). Melanin is synthesized by a reaction cascade triggered by the enzyme tyrosinase (U.S. Pat. No. 5,262,153).
- Typical pigmentation is characterized by an even, uniform coloration of the skin.
- Many individuals have excess melanin pigmentation or a hyperpigmentation patch which can cause pigmentary variation or abnormal pigmentation of the skin. This may lead to unwanted freckles or dark spots such as senile lentigo, liver spots, melasma, brown or age spots, vitiligo, sunburn pigmentation, post-inflammatory hyperpigmentation due to abrasion, burns, wounds or dermatitis, phototoxic reaction and other similar small, fixed pigmented lesions. It is often desirable to lighten these areas or even out the appearance of irregularly pigmented areas of skin. Individuals may also wish to increase fairness or reduce the overall level of pigmentation in the skin. In either case, the hyperpigmentation is usually viewed as cosmetically undesirable and individuals often wish to lighten the skin.
- the use of one skin lightening ingredient may not be effective for individuals with significant hyperpigmentation, freckles, or age spots, for example. Additionally, previous attempts to combine various skin lightening ingredients have been ineffective, and in some instance, have produced negative results (Talwar 1993).
- a composition of the present invention can include at least two of the following: (a) a vitamin C derivative; (b) niacinamide; (c) a composition comprising cucumber extract and lemon extract; (d) a compound comprising the formula:
- R 1 , R 2 , R 3 , R 4 , and R 5 are independently an H, an alkyl group, a hydroxyalkyl group, or a carboxyalkyl group, or (e) a compound comprising the fo ⁇ nula:
- R 1 , R 2 , R 3 , R 4 , and R 5 are independently an H, an alkyl group, a hydroxyallcyl group, or a carboxyalkyl group, and where x is an integer from 1 to 30.
- R 1 , R 2 , R 3 , R 4 , and R 5 are independently an H, an alkyl group, a hydroxyallcyl group, or a carboxyalkyl group, and where x is an integer from 1 to 30.
- a composition of the present invention can include, in non-limiting embodiments, any one of or a combination of any of the vitamin C derivatives, niacinamide, the composition comprising cucumber extract and lemon extract, or the above chemical compounds.
- a composition of the present invention may include all of these ingredients. Additionally, it is also contemplated that these ingredients may be applied to the skin in separate compositions at the same or different time periods or intervals.
- compositions of the present invention can be formulated into a cosmetic blend.
- the composition can, in certain embodiments, be chemically compatible.
- the composition can also include a pH of 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, or more. In certain aspects, the pH of the composition is between about 5 and 8.
- the compositions can also be included into a cosmetic vehicle.
- Non limiting examples of cosmetic vehicles include emulsions, creams, lotions, solutions, anhydrous bases, gels, and ointments, and other vehicles that are discussed throughout this document and that are known to those of ordinary skill in the art.
- the cosmetic vehicle is an oil- in-water emulsion, a water-in-oil emulsion, an aqueous solution, or hydro-alcoholic solution.
- the anhydrous base in non-limiting aspects can be a lipstick or powder.
- the compositions can be included into a cosmetic product.
- Non- limiting examples of cosmetic products include skin-whitening products, anti-aging products, moisturizing products, foundations, masks, lotions, skin softeners, cleansers, creams (e.g., day or night creams), or sunscreens, or other products that are disclosed throughout this document or are known to those of ordinary skill in the art which are incorporated into this section by reference.
- the composition of the present invention can be adapted or formulated for application at least once, twice, three, four, five, six, seven, eight, nine, ten, eleven, twelve, thirteen, fourteen, fifteen, sixteen, seventeen, or more times a day during use. It is contemplated that the composition of the present invention can be used in a regimen-like format.
- the regimen can include applying various products to a person's skin in the morning or evening or both.
- the various products can be applied at various intervals during any time of the day or evening. The intervals can vary depending on the desired effects of a given person or a product.
- a regimen can include applying a cleanser, a softener, a lotion, a sunscreen, and/or a foundation in the morning; it is contemplated that all, some, or one of these products include a composition of the present invention.
- Another non-limiting example of a regimen can include applying a cleanser, a mask, a softener, a lotion, and/or a night cream in the evening. It is also contemplated that all, some, or one of these products include a composition of the present invention.
- a regimen can include combining morning and evening regimens. It is contemplated that any type of regimen format can be used with the present invention.
- the regimens can also be varied to the specific needs or desires of a person using the product.
- the length of time of the regimens can vary.
- the regimen can by 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, days or more.
- the length of time of the regimen can be 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25, 30, 35, 40, 45, 50, 60, 70, 80, 90, 100, 200, weeks or longer.
- compositions of the present invention can be formulated as a leave-on composition, a rinsing composition, or as a cleansing composition.
- the compositions can also be formulated to include a sun protection factor (SPF) of 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25, 30, 35, 40, 45, 50, or more.
- SPF sun protection factor
- the compositions can also, for example, be formulated into a cosmetic product.
- Non-limiting examples of cosmetic products include skin softeners, day or night lotions, day or night creams, foundations, cleansers, masks, or sunscreens. It is contemplated, however, that the compositions of the present invention can be incorporated into any type of cosmetic product discussed in this document or known to those of ordinary skill in the art.
- the vitamin C derivative can be selected from the group consisting of ascorbyl glucoside, ascorbyl phosphate, and tetrahexydecyl ascorbate.
- ascorbyl phosphate include ascorbyl phosphate of an alkali metal, an alkaline earth metal, or a transition metal such as sodium ascorbyl phosphate, aminopropyl ascorbyl phosphate, or magnesium ascorbyl phosphate.
- Non-limiting examples of ascorbyl glucoside include ascorbic acid 1 -glucoside (e.g., 1-0- ⁇ -D- glucopyranosyl-L-ascorbic acid or l-O- ⁇ -D-glucopyranosyl-L-ascorbic acid), ascorbic acid 2-glucoside (e.g., 2-O- ⁇ -D-glucopyranosyl-L-ascorbic acid or 2-0/3-D-glucopyraiiosyl-L- ascorbic acid), ascorbic acid 3-glucoside (e.g., S-O-oD-glucopyranosyl-L-ascorbic acid or S-O- ⁇ -D-glucopyranosyl-L-ascorbic acid), ascorbic acid 5-glucoside (e.g., 5-Oa-D- glucopyranosyl-L-ascorbic acid or 5-O-/3-D-glucopyranosyl-L-a
- the composition can include, for example, a second, third, fourth, fifth or more vitamin C derivatives; for example, the first vitamin C derivative can be ascorbyl glucoside and the second vitamin C derivative can be ascorbyl phosphate.
- the above vitamin C derivatives are exemplary only, and that all types of vitamin C derivatives that are discussed throughout this document and known to those of ordinary skill in the art are contemplated as being useful with the present invention.
- the composition comprising cucumber extract and lemon extract can further include sodium citrate.
- Non-limiting examples of such a composition include a formulation called UNINONTAN U34TM.
- compositions of the present invention include a compound comprising the following formula:
- R 1 , R 2 , R 3 , R 4 , and R 5 are independently an H, an alkyl group, a hydroxyalkyl group, or a carboxyalkyl group.
- the alkyl group, hydroxyalkyl group, or carboxyalkyl group comprises 1 to 30 carbon atoms.
- R 1 can be CH 3
- R 2 , R 3 , R 4 , and R 5 can be H.
- R 1 , R 2 , R 3 , R 4 , and R 5 can be a variety of different chemical groups. Additionally, it is contemplated that derivatives or chemical modifications or both can be made to these groups.
- the compound includes the following formula:
- the composition can include from about 0.01% to about 5.0% of the vitamin C derivative, from about 0.01% to about 5.0% of niacinamide, from about 0.01% to about 5.0% of the extract formulation comprising cucumber extract and lemon extract, from about 0.01% to about 5.0% of the compound comprising the formula:
- Ri, R 2 , R 3 , R 4 , and R 5 are independently an H, an alkyl group, a hydroxyalkyl group, or a carboxyalkyl group, and/or from about 0.01% to about 5.0% of the compound comprising the formula:
- Ri, R 2 , R 3 , R 4 , and R 5 are independently an H, an alkyl group, a hydroxyalkyl group, or a carboxyalkyl group and where x is an integer from 1 to 30.
- the second vitamin C derivative can be present in an amount of about 0.01% to about 5.0%.
- the composition of the present invention may include a licorice extract, hi non-limiting embodiments, the licorice extract can be an oil-soluble licorice extract. The licorice can be present in an amount from about 0.01% to about 5.0% of the composition, hi other embodiments, the compositions of the present invention can include a botanical blend.
- the botanical blend can include any one of the following: lemon extract, cucumber extract, green tea extract, ginseng extract, mulberry extract, evening primrose seed extract, thyme extract, galangal extract, burnet extract, or licorice extract.
- Other ingredients discussed throughout this document and known to those of skill in the art can also be included in the botanical blend, hi certain aspects, the botanical blend can be present in the composition in an amount of from about 0.01% to about 5.0% of the composition.
- the amount of the ingredients and compounds in the composition of the present invention can be similar or different. Additionally, the amounts can vary below, in between, or above the ranges noted above.
- concentration ranges can also vary to achieve a specific desired result ⁇ e.g., a person may want to lighten their skin slightly or may want to achieve stronger results).
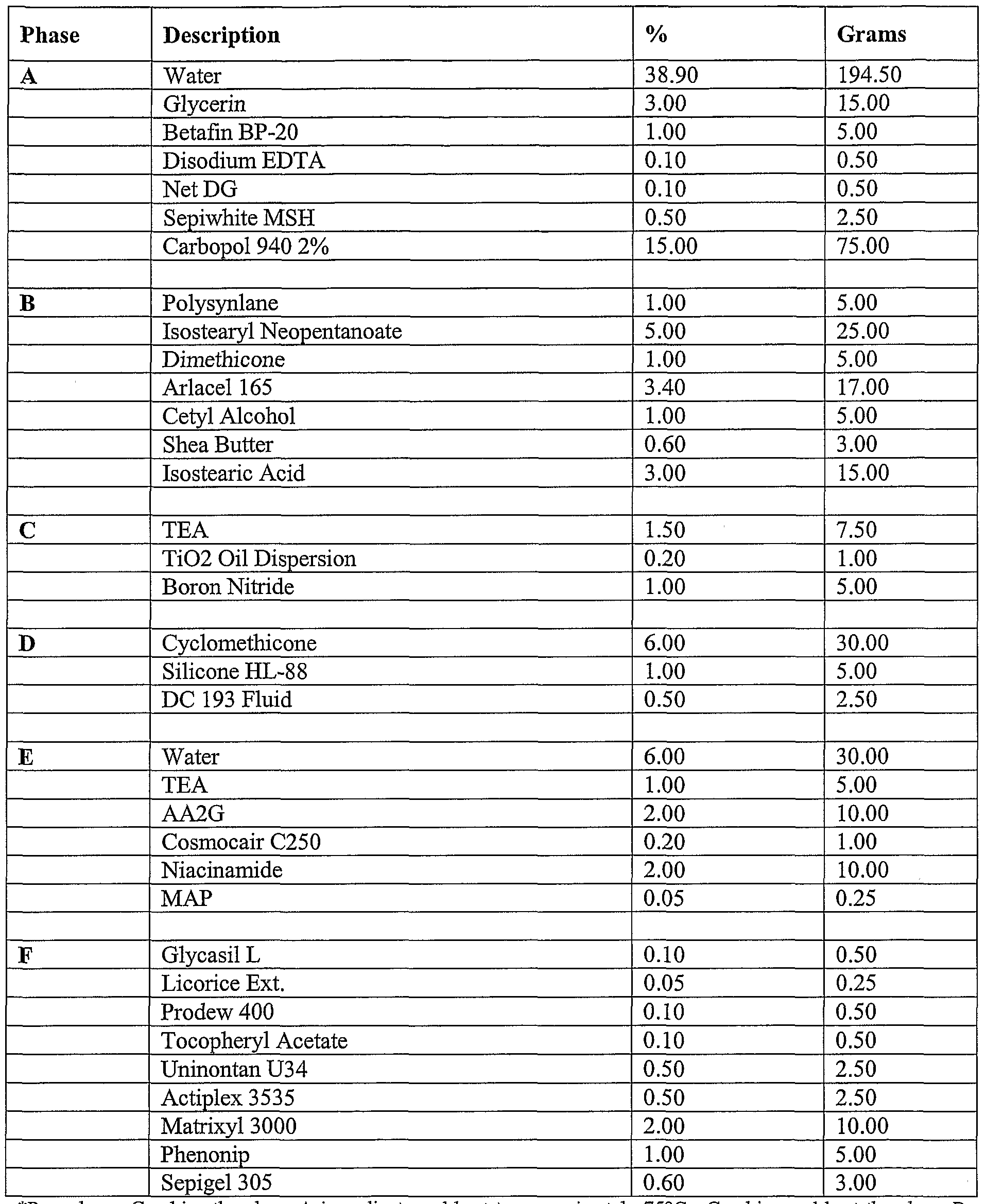
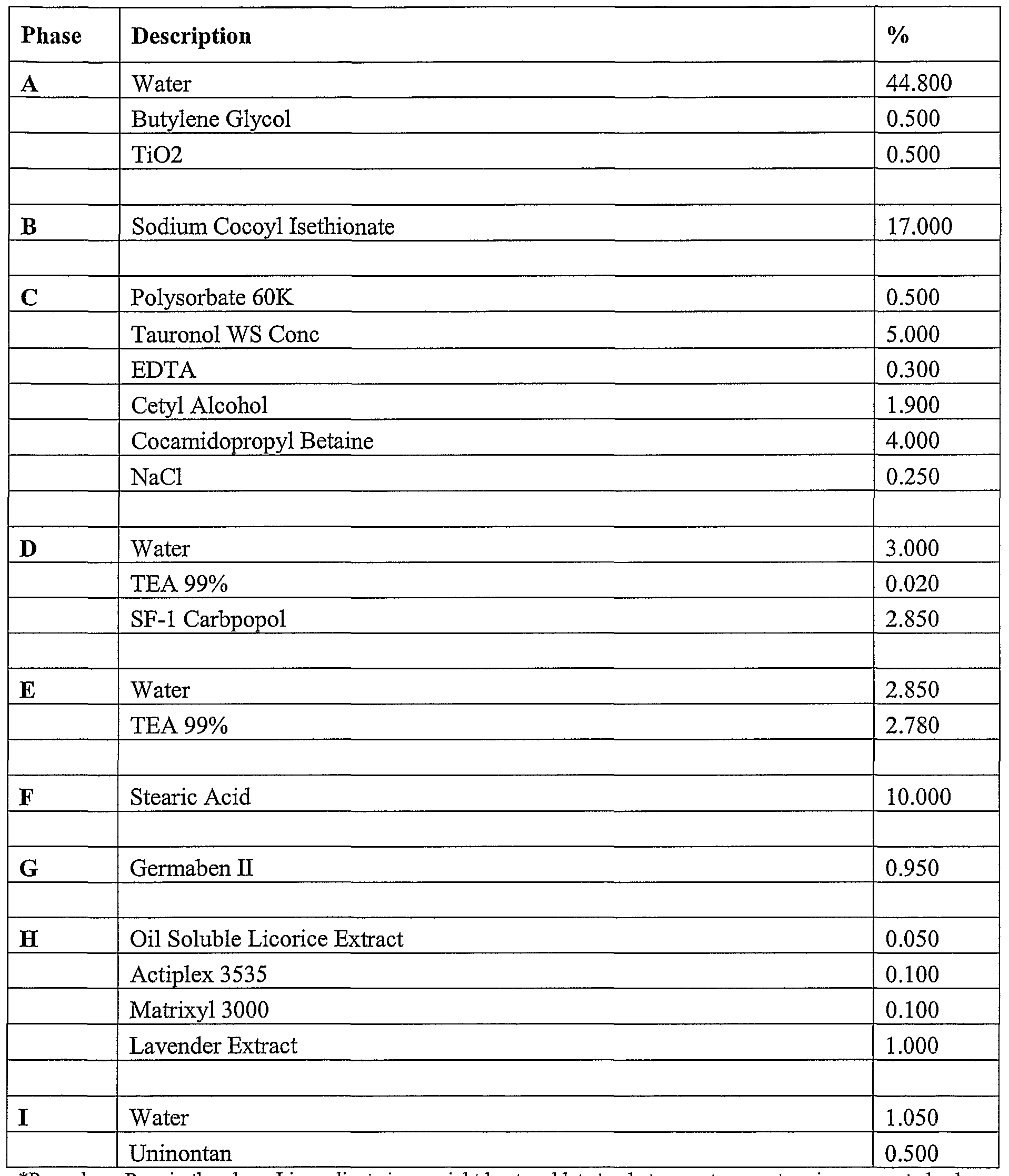
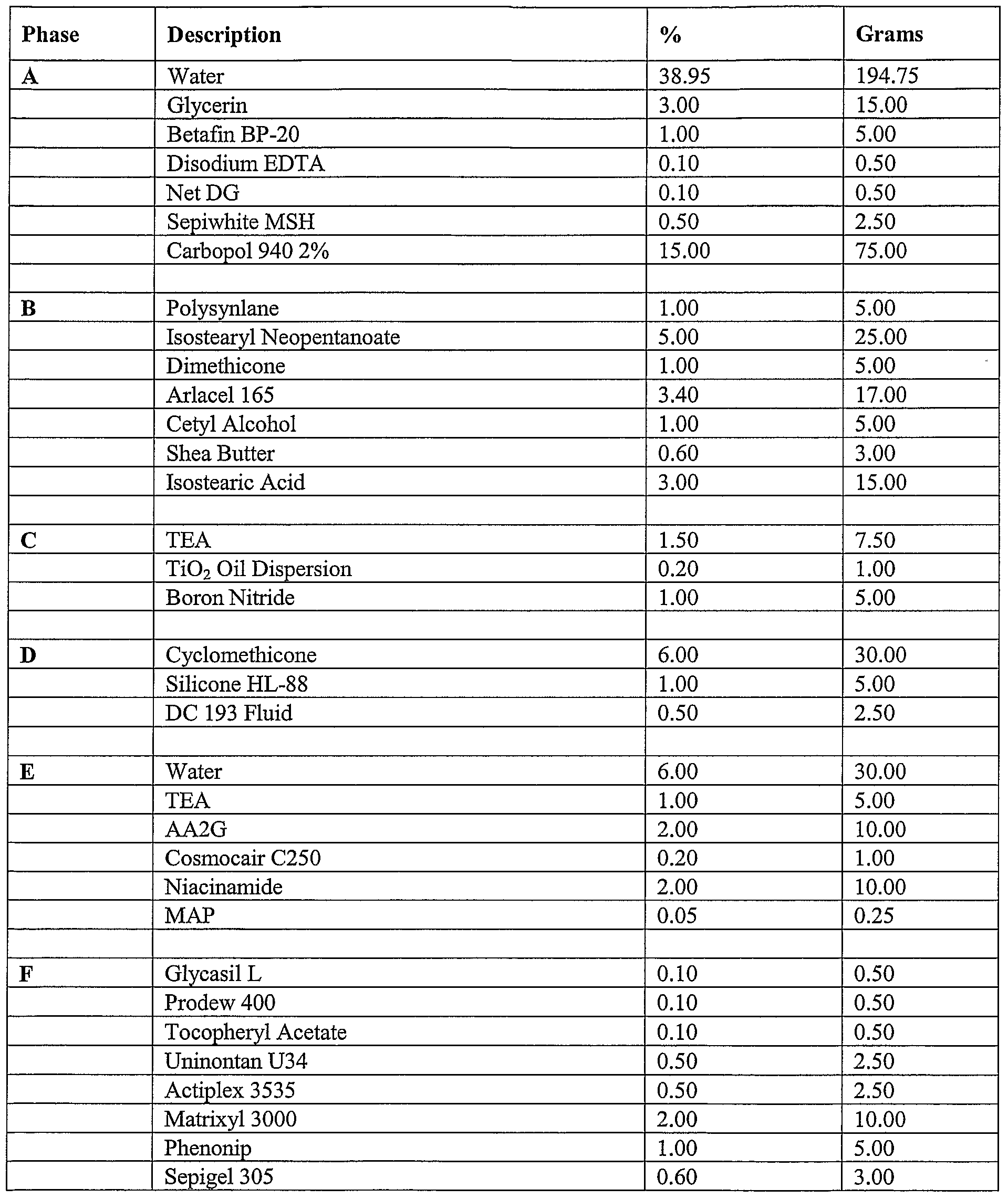
- compositions of the present invention can include a formulation selected from the group consisting of the formulation described in Table 1, Table, 2, Table 3, Table 4, Table 5, Table, 6, Table 7, Table 8, Table 9, Table 10, Table 16, and Table 17, below.
- the composition can include at least two of the following: (a) a vitamin C derivative; (b) niacinamide; (c) a composition comprising cucumber extract and lemon extract; (d) a compound comprising the formula:
- R 1 , R 2 , R 3 , R 4 , and R 5 are independently an H, an alkyl group, a hydroxyalkyl group, or a carboxyalkyl group, or (e) a compound comprising the formula:
- R 1 , R 2 , R 3 , R 4 , and R 5 are independently an H, an alkyl group, a hydroxyalkyl group, or a carboxyalkyl group, and where x is an integer from 1 to 30.
- the ingredients in the composition can be formulated into separate compositions, and the separate compositions can be applied to the skin at the same or different times.
- the composition in certain aspects, can inhibit, prevent, or reduce melanogenesis in a skin cell, tyrosinase or tyrosinase synthesis in a skin cell, or melanin transport to keratinocytes in a skin cell.
- the composition can act as an alpha melanin stimulatory hormone antagonist.
- the method can be further defined as a method of evening out the pigmentation of the skin.
- the method can include, in other non-limiting embodiments, applying the composition one, two, three, four, five six, seven, eight, nine, ten, eleven twelve, thirteen, fourteen, fifteen, sixteen, seventeen, or more times a day during use.
- the composition can be used in a regimen-like format.
- the regimen can include applying various products that have the composition of the present invention to a person's skin in the morning or evening or both. The intervals can vary depending on the desired effects of a given person or product.
- the method can include, for example, applying a cleanser product, a softener product, a lotion product, a sunscreen product, and/or a foundation product in the morning.
- the method can also include, in another aspect, applying a cleanser product, a mask product, a softener product, a lotion product, and/or a night cream product in the evening.
- the skin in certain non-limiting aspects, can be any skin that is on the human body. Non- limiting examples include facial, head, neck, back, chest, stomach, shoulder, arm, hand, finger, buttock, leg, foot, or toe skin.
- the method can further be defined, in non-limiting aspects, as reducing the appearance of an age spot, a skin discoloration, or a freckle on the skin.
- the compositions can include a formulation selected from the group consisting of the formulation described in Table 1, Table, 2, Table 3, Table 4, Table 5, Table, 6, Table 7, Table 8, Table 9, Table 10, Table 16, and Table 17, below.
- the composition includes the formulation described in Table 16.
- the method can also include applying at least a second, third, fourth, fifth, sixth, seventh, eight, ninth, tenth, eleventh, twelfth, thirteenth, fourteenth, fifteenth, sixteenth, seventeenth, or more skin-lightening composition to the skin.
- the additional skin lightening composition can include a skin-lightening formulation known to those of ordinary skill in the art and/or those described in this specification.
- the additional composition can be selected from the group consisting of the formulation described in Table 1, Table, 2, Table 3, Table 4, Table 5, Table, 6, Table 7, Table 8, Table 9, Table 10, Table 16, and Table 17, below.
- the additional composition is the formulation described in Table 17.
- compositions of the invention can be used to achieve methods of the invention.
- the terms “inhibiting,” “reducing,” or “prevention,” or any variation of these terms, when used in the claims and/or the specification includes any measurable decrease or complete inhibition to achieve a desired result.
- “about” can be within 10%, preferably within 5%, more preferably within 1%, and most preferably within 0.5%.
- the words “comprising” (and any form of comprising, such as “comprise” and “comprises”), “having” (and any form of having, such as “have” and “has”), "including” (and any form of including, such as “includes” and “include”) or “containing” (and any form of containing, such as “contains” and “contain”) are inclusive or open-ended and do not exclude additional, unrecited elements or method steps.
- the present invention is an effective alternative to the skin-whitening compounds and formulas that are currently used to lighten the skin, treat hyperpigmentation, or other skin tone disorders.
- compositions and methods of the present invention can be used, for example, for improving the skin's visual appearance, whitening or lightening the skin's color or tone, treating hyperpigmentation and other related disorders, and evening out a person's skin tone.
- the compositions of the present invention can include a combination of ingredients that can be used to lighten skin.
- Non-limiting examples of such ingredients and compounds include ascorbyl glucoside, niacinamide, undecylenoyl phenylalanine, creatinine, botanical extracts, and other extract formulations.
- Ascorbyl glucoside is a derivative of ascorbic acid (vitamin C) that includes an attached glucose sugar.
- vitamin C ascorbic acid
- the chemical structure of ascorbic acid (Cas. No. 50-81-7) is:
- glucose is typically attached at an OH group of ascorbic acid.
- ascorbyl glucoside typically, ascorbic acid-2 glucoside:
- ascorbyl glucoside examples include ascorbic acid 1 -glucoside (including l-O- ⁇ -D-glucopyranosyl-L-ascorbic acid and l-O-/3-D-glucopyranosyl-L- ascorbic acid), ascorbic acid 2-glucoside (including 2-O- ⁇ -D-glucopyranosyl-L-ascorbic acid and 2-O-/3-D-glucopyranosyl-L-ascorbic acid), ascorbic acid 3-glucoside (including 3- O- ⁇ -D-glucopyranosyl-L-ascorbic acid or 3-O-/3-D-glucopyranosyl-L-ascorbic acid), ascorbic acid 5-glucoside (including 5-O- ⁇ -D-glucopyranosyl-L-ascorbic acid or 5-O-/3-D- glucopyranosyl-L-ascorbic acid), and ascorbic acid 6-glucoside (
- Ascorbyl glucoside is commercially available (e.g., Hayashibara Biochemical Laboratories, Inc.). The preparation of ascorbyl glucoside is also known in the art (e.g. U.S. Pat. Nos. 5,084,563; 5,252,722; 5,272,136; 5,388,420; 5,432,161; 5,843,907; and 5,508,391).
- Niacinamide (Cas. No. 98-92-0), also known as nicotinamide or pyridine-3- carboxylic acid amide, is a water-soluble amide of nicotinic acid. It is one of the two forms of vitamin B3 and was first isolated from rice bran in 1911 ⁇ Niacinamide, Alternative
- Niacinamide is known to have skin-lightening properties (e.g. U.S. Pat. No. 4,096,240; Hakozaki et al, 2002).
- niacinamide includes a pyridine ring that has an amide group at position 3.
- the molecule formula for niacinamide is C 6 H 6 N 2 O, and its molecular weight is 122.12.
- the chemical structure of niacinamide is:
- Niacinamide is commercially available (e.g., Indian Chemical Industries, Inc.). The preparation of niacinamide is also known in the art.
- the compositions of the present invention can include an extract formulation comprising cucumber extract and lemon extract.
- the combination of these extracts has skin-lightening properties.
- the cucumber and lemon extract combination can be formulated into UNINONTAN U34TM.
- UNTNONTAN-U34TM is an effective lightening agent, and it can be combined with the compounds and ingredients that are described in the claims and other sections of this document.
- vitamin C derivatives such as MAP and ascorbyl glucoside, can be combined with UNINONTAN U34TM without affecting the vitamin C derivatives' stability. A synergistic effect is therefore observed, increasing the total skin lightening effects of the compositions of the present invention.
- compositions also provide the treatments for hyperpigmentation not responsive to traditional treatments.
- the present invention may be practiced by combining the raw materials comprising the UNTNONTAN- U34TM (extract formulation of cucumber extract and lemon extract) product in the amounts specified, or by creating reasonable variations in the ingredients.
- the specific ingredients in UNINONTAN U34TM include cucumber extract
- compositions of the present invention can also include a structure comprising the following formula:
- R 1 , R 2 , R 3 , R 4 , and R 5 can be independently an H, an alkyl group, a hydroxyalkyl group, or a carboxyalkyl group.
- This structure has been shown to have skin-lightening properties (e.g., U.S. Pat. Pub. 2003/0180237; U.S. Pat. No. 6,235,910; WO 03011241; WO 03011242; and WO 04064801).
- a person of ordinary skill in the art would know how to prepare such a structure by chemical synthesis (e.g., U.S. Pat. Pub. 2003/0180237 and U.S. Pat. No. 6,235,910).
- Creatinine or 2-imino-N- methylhydantoin is a cyclic condensation product which can be obtained by intramolecular elimination of water from creatine. Creatinine has the following structure:
- COSMOCAIR C250 can be formulated into COSMOCAIR C250TM.
- the international company, Degussa sells COSMOCAIR C250 under its Personal Care Specialties business unit.
- COSMOCAIR C250 is characterized as a natural amino acid derivative that belongs to the class of guanidino-compounds that can be used in skin brightening products.
- compositions of the present invention can also include a structure comprising the following formula:
- R 1 , R 2 , R 3 , R 4 , and R 5 are independently an H, an alkyl group, a hydroxyalkyl group, or a carboxyalkyl group, and x is an integer from 1 to 30.
- This structure has been shown to have skin-lightening properties (e.g., WO 03/061768). A person of ordinary skill in the art would know how to prepare such a structure by chemical synthesis. In preferred embodiments, the structure is formulated into undecylenoyl phenylalanine:
- SEPIWHITETM MSH SEPIWHITETM MSH
- SEPIWHITETM is a formulation that is sold by the French company, Societe D 'Exploitation De Produits Pour Les Industries Chemiques (SEPPIC) for use as a skin lightening active ingredient.
- SEPIWHITETM is characterized as an alpha-MSH (melanotropin) antagonist; it reduces the synthesis of melanin pigments effectively while maintaining skin integrity.
- the compounds, extracts, and active ingredients that are described in the claims and specification can be obtained by any means known to a person of ordinary skill in the art.
- the compounds, extracts, and active ingredients can be isolated by obtaining the source of such compounds and extracts.
- the compounds, extracts, and active ingredients are commercially available.
- the compounds, extracts, and active ingredients can be purified by any number of techniques known to a person of ordinary skill in the art. Non-limiting examples of purification techniques include Polyacrylamide Gel Electrophoresis, High Performance Liquid Chromatography (HPLC), Gel chromatography or Molecular Sieve Chromatography, and Affinity Chromatography.
- the compounds, extracts, and active ingredients can be obtained by chemical synthesis or by recombinant means by using conventional techniques.
- various automatic polypeptide synthesizers and chemical reactions are known and can be used in accordance with known protocols. See, for example, Stewart and Young, (1984); Tarn et ah, (1983); Merrifield, (1986); and Barany and Merrifield (1979), Houghten (1985).
- Derivatives may be prepared and the properties of such derivatives may be assayed for their desired properties by any method known to those of skill in the art.
- “derivative” refers to a chemically modified compound, inhibitor, or stimulator that still retains the desired effects of the prior to the chemical modification.
- Such derivatives may have the addition, removal, or substitution of one or more chemical moieties on the parent molecule.
- Non limiting examples of the types modifications that can be made to the compounds and structures disclosed throughout this document include the addition or removal of lower alkanes such as methyl, ethyl, propyl, or substituted lower alkanes such as hydroxymethyl or aminomethyl groups; carboxyl groups and carbonyl groups; hydroxyls; nitro, amino, amide, and azo groups; sulfate, sulfonate, sulfono, sulfhydryl, sulfonyl, sulfoxido, phosphate, phosphono, phosphoryl groups, and halide substituents.
- lower alkanes such as methyl, ethyl, propyl, or substituted lower alkanes
- carboxyl groups and carbonyl groups hydroxyls; nitro, amino, amide, and azo groups
- Additional modifications can include an addition or a deletion of one or more atoms of the atomic framework, for example, substitution of an ethyl by a propyl; substitution of a phenyl by a larger or smaller aromatic group.
- hetero atoms such as N, S, or O can be substituted into the structure instead of a carbon atom.
- compositions of the present invention can include any number of combinations of compounds and/or extracts, or derivatives therein. It is also contemplated that that the concentrations of the compounds and extracts can vary.
- the compositions may include in their final form, for example, at least about 0.0001%, 0.0002%, 0.0003%, 0.0004%, 0.0005%, 0.0006%, 0.0007%, 0.0008%, 0.0009%, 0.0010%, 0.0011%, 0.0012%, 0.0013%, 0.0014%, 0.0015%, 0.0016%, 0.0017%, 0.0018%, 0.0019%, 0.0020%, 0.0021%, 0.0022%, 0.0023%, 0.0024%, 0.0025%, 0.0026%, 0.0027%, 0.0028%, 0.0029%, 0.0030%, 0.0031%, 0.0032%, 0.0033%, 0.0034%, 0.0035%, 0.0036%, 0.003
- the percentage can be calculated by weight or volume of the total composition.
- concentrations can vary depending on the addition, substitution, and/or subtraction of the compounds, extracts, and substitutes to these compounds and extracts.
- compositions of the present invention may also include various antioxidants to retard oxidation of one or more components. Additionally, the prevention of the action of microorganisms can be brought about by preservatives such as various antibacterial and antifungal agents, including but not limited to parabens (e.g., methylparabens, propylparabens), chlorobutanol, phenol, sorbic acid, thimerosal or combinations thereof.
- preservatives such as various antibacterial and antifungal agents, including but not limited to parabens (e.g., methylparabens, propylparabens), chlorobutanol, phenol, sorbic acid, thimerosal or combinations thereof.
- compositions are effective in all types of cosmetic vehicles.
- suitable cosmetic vehicles include emulsions, creams, lotions, solutions (both aqueous and hydro-alcoholic), anhydrous bases (such as lipsticks and powders), gels, and ointments or by other method or any combination of the forgoing as would be known to one of ordinary skill in the art (Remington's, 1990). Variations and other appropriate vehicles will be apparent to the skilled artisan and are appropriate for use in the present invention.
- the cosmetic vehicle is selected from oil-in-water emulsions, hydro-alcoholic solutions, or encapsulated beads in anhydrous systems.
- oil-in-water emulsions such emulsions and their compositions and methods of making are well known in the art. It is important, however, that the concentrations and combinations of the compounds and extracts be selected in such a way that the combinations are chemically compatible and do not form complexes which precipitate from the finished product.
- composition of the present invention can also be used in many cosmetic products including, but not limited to, moisturizing creams, skin benefit creams and lotions, softeners, day lotions, gels, ointments, foundations, night creams, lipsticks, cleansers, toners, masks, or other known cosmetic products or applications. Additionally, the cosmetic products can be formulated as leave-on or rinse-off products. The compositions of the present invention is most preferably used in skin lightening products for the face and other body parts.
- compositions of the present invention can include other beneficial agents and compounds such as, for example, acute or chronic moisturizing agents (including, e.g., humectants, occlusive agents, and agents that affect the natural moisturization mechanisms of the skin), anti-oxidants, sunscreens having UVA and/or UVB protection, emollients, anti-irritants, vitamins, trace metals, anti-microbial agents, botanical extracts, fragrances, and/or dyes and color ingredients.
- moisturizing agents including, e.g., humectants, occlusive agents, and agents that affect the natural moisturization mechanisms of the skin
- sunscreens having UVA and/or UVB protection sunscreens having UVA and/or UVB protection
- emollients anti-irritants
- vitamins trace metals
- anti-microbial agents botanical extracts, fragrances, and/or dyes and color ingredients.
- Non-limiting examples of moisturizing agents that can be used with the compositions of the present invention include amino acids, chondroitin sulfate, diglycerin, erythritol, fructose, glucose, glycerin, glycerol polymers, glycol, 1,2,6-hexanetriol, honey, hyaluronic acid, hydrogenated honey, hydrogenated starch hydrolysate, inositol, lactitol, maltitol, maltose, mannitol, natural moisturizing factor, PEG- 15 butanediol, polyglyceryl sorbitol, salts of pyrollidone carboxylic acid, potassium PCA, propylene glycol, sodium glucuronate, sodium PCA, sorbitol, sucrose, trehalose, urea, and xylitol.
- acetylated lanolin examples include acetylated lanolin, acetylated lanolin alcohol, acrylates/C 10-30 alkyl acrylate crosspolymer, acrylates copolymer, alanine, algae extract, aloe barbadensis, aloe-barbadensis extract, aloe barbadensis gel, althea officinalis extract, aluminum starch octenylsuccinate, aluminum stearate, apricot (prunus armeniaca) kernel oil, argmine, arginine aspartate, arnica montana extract, ascorbic acid, ascorbyl palmitate, aspartic acid, avocado (persea gratissima) oil, barium sulfate, barrier sphingolipids, butyl alcohol, beeswax, behenyl alcohol, beta-sitosterol, BHT, birch (betula alba) bark extract, borage (borago officinal
- Non-limiting examples of antioxidants that can be used with the compositions of the present invention include acetyl cysteine, ascorbic acid, ascorbic acid polypeptide, ascorbyl dipalmitate, ascorbyl methylsilanol pectinate, ascorbyl palmitate, ascorbyl stearate, BHA, BHT, t-butyl hydroquinone, cysteine, cysteine HCI, diamylhydroquinone, di-t-butylhydroquinone, dicetyl thiodipropionate, dioleyl tocopheryl methylsilanol, disodium ascorbyl sulfate, distearyl thiodipropionate, ditridecyl thiodipropionate, dodecyl gallate, erythorbic acid, esters of ascorbic acid, ethyl ferulate, ferulic acid, gallic acid esters, hydroquinone, iso
- Non-limiting examples of compounds that have ultraviolet light absorbing properties that can be used with the compounds of the present invention include benzophenone, benzophenone-1, benzophenone-2, benzophenone-3, benzophenone-4 benzophenone-5, benzophenone-6, benzophenone-7, benzophenone-8, benzophenone-9, benzophenone- 10, benzophenone-11, benzophenone- 12, benzyl salicylate, butyl PABA, cinnamate esters, cinoxate, DEA-methoxycinnamate, diisopropyl methyl cinnamate, ethyl dihydroxypropyl PABA, ethyl diisopropylcinnamate, ethyl methoxycinnamate, ethyl
- PABA ethyl urocanate, glyceryl octanoate dimethoxycinnamate, glyceryl PABA, glycol salicylate, homosalate, isoamyl p-methoxycinnamate, PABA, PABA esters, Parsol 1789, and isopropylbenzyl salicylate.
- compositions of the present invention can include a structuring agent.
- Structuring agent in certain aspects, assist in providing rheological characteristics to the composition to contribute to the composition's stability.
- structuring agents can also function as an emulsifier or surfactant.
- Non-limiting examples of structuring agents include stearic acid, palmitic acid, stearyl alcohol, cetyl alcohol, behenyl alcohol, stearic acid, palmitic acid, the polyethylene glycol ether of stearyl alcohol having an average of about 1 to about 21 ethylene oxide units, the polyethylene glycol ether of cetyl alcohol having an average of about 1 to about 5 ethylene oxide units, and mixtures thereof.
- the compositions do not include an emulsifier. In other aspects, however, the compositions can include one or more emulsifiers. Emulsifiers can reduce the in interfacial tension between phases and improve the formulation and stability of an emulsion.
- the emulsifiers can be nonionic, cationic, anionic, and zwitterionic emulsifiers (See McCutcheon's (1986); U.S. Pat. Nos. 5,011,681; 4,421,769; 3,755,560).
- Non-limiting examples include esters of glycerin, esters of propylene glycol, fatty acid esters of polyethylene glycol, fatty acid esters of polypropylene glycol, esters of sorbitol, esters of sorbitan anhydrides, carboxylic acid copolymers, esters and ethers of glucose, ethoxylated ethers, ethoxylated alcohols, alkyl phosphates, polyoxyethylene fatty ether phosphates, fatty acid amides, acyl lactylates, soaps, TEA stearate, DEA oleth-3 phosphate, polyethylene glycol 20 sorbitan monolaurate (polysorbate 20), polyethylene glycol 5 soya sterol, steareth-2, steareth-20, steareth-21, ceteareth-20, PPG-2 methyl glucose ether distearate, ceteth-10, polysorbate 80, cetyl phosphate, potassium cetyl phosphat
- silicone containing compounds include any member of a family of polymeric products whose molecular backbone is made up of alternating silicon and oxygen atoms with side groups attached to the silicon atoms. By varying the -Si-O- chain lengths, side groups, and crosslinking, silicones can be synthesized into a wide variety of materials. They can vary in consistency from liquid to gel to solids.
- the silicone containing compounds that can be used in the context of the present invention include those described in this specification or those known to a person of ordinary skill in the art.
- Non-limiting examples include silicone oils (e.g., volatile and nonvolatile oils), gels, and solids, hi preferred aspects, the silicon containing compounds includes a silicone oils such as a polyorganosiloxane.
- silicone oils e.g., volatile and nonvolatile oils
- the silicon containing compounds includes a silicone oils such as a polyorganosiloxane.
- Non-limiting examples of polyorganosiloxanes include dimethicone, cyclomethicone, polysilicone-11, phenyl trimethicone, trimethylsilylamodimethicone, stearoxytrimethylsilane, or mixtures of these and other organosiloxane materials in any given ratio in order to achieve the desired consistency and application characteristics depending upon the intended application (e.g., to a particular area such as the skin, hair, or eyes).
- a "volatile silicone oil” includes a silicone oil have a low heat of vaporization, i.e. normally less than about 50 cal per gram of silicone oil.
- volatile silicone oils include: cyclomethicones such as Dow Corning 344 Fluid, Dow Corning 345 Fluid, Dow Corning 244 Fluid, and Dow Corning 245 Fluid, Volatile Silicon 7207 (Union Carbide Corp., Danbury, Conn.); low viscosity dimethicones, i.e. dimethicones having a viscosity of about 50 cst or less (e.g., dimethicones such as Dow Corning 200-0.5 cst Fluid).
- the Dow Corning Fluids are available from Dow Corning Corporation, Midland, Michigan.
- Cyclomethicone and dimethicone are described in the Third Edition of the CTFA Cosmetic Ingredient Dictionary (incorporated by reference) as cyclic dimethyl polysiloxane compounds and a mixture of fully methylated linear siloxane polymers end-blocked with trimethylsiloxy units, respectively.
- Other non-limiting volatile silicone oils that can be used in the context of the present invention include those available from General Electric Co., Silicone Products Div., Waterford, N. Y. and SWS Silicones Div. of Stauffer Chemical Co., Adrian, Michigan.
- Essential oils include oils derived from herbs, flowers, trees, and other plants. Such oils are typically present as tiny droplets between the plant's cells, and can be extracted by several method known to those of skill in the art (e.g., steam distilled, enfleurage (i.e., extraction by using fat), maceration, solvent extraction, or mechanical pressing). When these types of oils are exposed to air they tend to evaporate (i.e., a volatile oil). As a result, many essential oils are colorless, but with age they can oxidize and become darker. Essential oils are insoluble in water and are soluble in alcohol, ether, fixed oils (vegetal), and other organic solvents. Typical physical characteristics found in essential oils include boiling points that vary from about 160° to 240° C and densities ranging from about 0.759 to about 1.096.
- Essential oils typically are named by the plant from which the oil is found.
- rose oil or peppermint oil are derived from rose or peppermint plants, respectively.
- Non-limiting examples of essential oils that can be used in the context of the present invention include sesame oil, macadamia nut oil, tea tree oil, evening primrose oil, Spanish sage oil, Spanish rosemary oil, coriander oil, thyme oil, pimento berries oil, rose oil, anise oil, balsam oil, bergamot oil, rosewood oil, cedar oil, chamomile oil, sage oil, clary sage oil, clove oil, cypress oil, eucalyptus oil, fennel oil, sea fennel oil, frankincense oil, geranium oil, ginger oil, grapefruit oil, jasmine oil, juniper oil, lavender oil, lemon oil, lemongrass oil, lime oil, mandarin oil, marjoram oil, myrrh oil, neroli oil, orange oil, patch
- Thickening agents include substances which that can increase the viscosity of a composition.
- Preferred thickeners includes those that can increase the viscosity of a composition without substantially modifying the efficacy of the active ingredient within the composition.
- Thickeners can also increase the stability of the compositions of the present invention, hi certain aspects of the present invention, preferred thickeners include hydrogenated polyisobutene or trihydroxystearin, or a mixture of both.
- Non-limiting examples of additional thickening agents that can be used in the context of the present invention include carboxylic acid polymers, crosslinked polyacrylate polymers, polyacrylamide polymers, polysaccharides, and gums.
- carboxylic acid polymers include crosslinked compounds containing one or more monomers derived from acrylic acid, substituted acrylic acids, and salts and esters of these acrylic acids and the substituted acrylic acids, wherein the crosslinking agent contains two or more carbon- carbon double bonds and is derived from a polyhydric alcohol (see U.S. Pat. Nos. 5,087,445; 4,509,949; 2,798,053; CTFA International Cosmetic Ingredient Dictionary, Fourth edition, 1991, pp. 12 and 80).
- carboxylic acid polymers examples include carbomers, which are homopolymers of acrylic acid crosslinked with allyl ethers of sucrose or pentaerytritol (e.g., CarbopolTM 900 series from B. F. Goodrich).
- Non-limiting examples of crosslinked polyacrylate polymers include cationic and nonionic polymers. Examples are described in U.S. Pat. Nos. 5,100,660 ; 4,849,484; 4,835,206; 4,628,078; 4,599,379).
- Non-limiting examples of polyacrylamide polymers include polyacrylamide, isoparaffm and laureth-7, multi-block copolymers of acrylamides and substituted acrylamides with acrylic acids and substituted acrylic acids.
- Non-limiting examples of polysaccharides include cellulose, carboxymethyl hydroxyethylcellulose, cellulose acetate propionate carboxylate, hydroxyethylcellulose, hydroxyethyl ethylcellulose, hydroxypropylcellulose, hydroxypropyl methylcellulose, methyl hydroxyethylcellulose, microcrystalline cellulose, sodium cellulose sulfate, and mixtures thereof.
- alkyl substituted cellulose where the hydroxy groups of the cellulose polymer is hydroxyalkylated (preferably hydroxy ethylated or hydroxypropylated) to form a hydroxyalkylated cellulose which is then further modified with a C ⁇ o " ⁇ 30 straight chain or branched chain alkyl group through an ether linkage.
- these polymers are ethers of C10-C30 straight or branched chain alcohols with hydroxyalkylcelluloses.
- Other useful polysaccharides include scleroglucans comprising a linear chain of (1-3) linked glucose units with a (1-6) linked glucose every three unit.
- Non-limiting examples of gums that can be used with the present invention include acacia, agar, algin, alginic acid, ammonium alginate, amylopectin, calcium alginate, calcium carrageenan, carnitine, carrageenan, dextrin, gelatin, gellan gum, guar gum, guar hydroxypropyltrimonium chloride, hectorite, hyaluroinic acid, hydrated silica, hydroxypropyl chitosan, hydroxypropyl guar, karaya gum, kelp, locust bean gum, natto gum, potassium alginate, potassium carrageenan, propylene glycol alginate, sclerotium gum, sodium carboyxmethyl dextran, sodium carrageenan, tragacanth gum, xanthan gum, and mixtures thereof. 9. Additional Compounds and Agents
- Non-limiting examples of additional compounds and agents that can be used with the compositions of the present invention include additional skin lightening agents (e.g. kojic acid, hydroquinone, retinoids and their derivatives) and other known methods of lightening skin, emollients (e.g. esters and fatty acids), vitamins (e.g. D, E, A, K, and C), trace metals (e.g. zinc, calcium and selenium), anti-irritants (e.g. steroids and non-steroidal antiinflammatories), botanical extracts (e.g. aloe vera, chamomile, cucumber extract, ginkgo biloba, ginseng, and rosemary), dyes and color ingredients (e.g. D&C blue no.
- additional skin lightening agents e.g. kojic acid, hydroquinone, retinoids and their derivatives
- emollients e.g. esters and fatty acids
- vitamins e.g. D, E, A,
- D&C green no. 5 D&C orange no. 4
- D&C red no. 17, D&C red no. 33 D&C violet no. 2
- D&C yellow no. 10 D&C yellow no. 11 and DEA-cetyl phosphate
- preservatives e.g. BHA
- emollients i.e. organic esters, fatty acids, lanolin and its derivatives, plant and animal oils and fats, and di- and triglycerides
- antimicrobial agents e.g., triclosan and ethanol
- fragrances natural and artificial.
- Kits are also contemplated in certain aspects of the present invention.
- any of the compositions, compounds, agents, or ingredients described in this specification may be included in a kit.
- a kit can include a skin whitening composition, a corresponding cosmetic product, or other products and articles of manufacture.
- Containers of the kits can include a bottle, dispenser, package, compartment, or other types of containers, into which a component may be placed.
- the containers can dispense a pre-determined amount of the component (e.g. compositions of the present invention).
- the composition can be dispensed in a spray, an aerosol, or in a liquid form or semi-solid form.
- the containers can have spray, pump, or squeeze mechanisms.
- the container can include indicia on its surface.
- the indicia for example, can be a word, a phrase, an abbreviation, a picture, or a symbol.
- the word or phrase can be "Mary Kay,” "cosmetic," "sunscreen,” etc.
- kits of the present invention also can include a container housing the components in close confinement for commercial sale.
- Such containers may include injection or blow-molded plastic containers into which the desired bottles, dispensers, or packages are retained.
- a kit can also include instructions for employing the kit components as well the use of any other compositions, compounds, agents, ingredients, or objects not included in the kit. Instructions may include variations that can be implemented. For example, the instructions can include an explanation of how to apply, use, and maintain the products or compositions.
- Tables 1-3 provide non-limiting examples of the various types of skin whitening formulations of the present invention that can be comprised in various cosmetic products. As noted throughout this document, it is contemplated that these concentrations ranges can vary. A person of ordinary skill in the art, for example, would recognize that concentration ranges can vary by the addition, removal, or substitution of any one of the listed ingredients. These concentration ranges can also vary depending on the desired effects of any given skin whitening formulation. Further, it should be recognized that the concentration ranges of ingredients can go below or above the concentration ranges noted throughout this document in situations where a synergistic effect is observed between two or more ingredients.
- Botanical blend includes lemon extract, cucumber extract, green tea extract, ginseng extract, mulberry extract, evening primrose seed extract, thyme extract, galangal extract, burnet extract, and licorice extract.
- Table 3 Skin-lightening formulation included in a leave-on foundation, a rinse-off cleanser, and a rinse-off mask
- Botanical blend includes lemon extract, cucumber extract, green tea extract, ginseng extract, mulberry extract, evening primrose seed extract, thyme extract, galangal extract, burnet extract, and licorice extract.
- derivatives of these ingredients can be used as substitutes. Additionally, other ingredients with similar physiological activities are contemplated as being useful as substitutes or as additional ingredients that can be used with the compositions of the present invention.
- This example includes non-limiting procedures of how to make skin softeners, day time lotions, night time lotions, foundations, cleansers, and masks that are described throughout this document.
- a person of ordinary skill in the art would recognize that these procedures and the ingredients that are used can be varied, removed, added to, or substituted to conform with a specific product or to obtain a desired effect.
- phase A to a beaker in the order presented in the table (i.e. from top to bottom). Mix until all of the ingredients are dissolved. Premix phase B in the same manner as phase A. Add phase B to phase A and mix for approximately 20 minutes. These procedures can be performed at room temperature.
- Phase A--Heat water and add Hydroxyethylcellulose Mix with high speed mixing until batch is clear or transparent and thickened.
- Add the phase E mixture with the batch when the phase E mixture and the batch are approximately 7O-75C 0 .
- phase F Add the ingredients in phase F to the batch at approximately 6O-65C 0 .
- the batch should begin to thicken. Continue mixing the batch until it is uniform and then switch to sweep mixing. At approximately 40-45C° add the ingredients in phase G in order with continuous mixing.
- phase J ingredients in order and allow each ingredient to dissolve prior to adding the next ingredient. Add the phase J mixture into the batch. Mix the batch for approximately 10 minutes. Add the phase K ingredients and mix until the batch is uniform, and then mix for approximately 5-10 more minutes.
- phase I ingredients Premix the phase I ingredients in a weight boat and let stand at room temperature in a separate beaker. Mix the phase A ingredients and heat to approximately 85°C. Add the phase B ingredients with the phase A ingredients to form a batch and allow the batch to melt. Maintain the batch at approximately 80-85 0 C. Add the phase C ingredients one at a time into the batch by mixing. Premix the phase D ingredients one at a time and in order ⁇ i.e. top to bottom). Allow the batch to cool to approximately 65-7O 0 C. Add to the phase D ingredients to the batch. Premix the phase E ingredients and add to the batch by mixing. Add the phase E ingredients to the batch by mixing. Allow the batch to cool to approximately 60-65°C. Add the phase F ingredients and change to sweep mixing.
- phase A Add the ingredients in phase A to a beaker and mix for approximately 20 minutes to create a batch.
- Add the phase E ingredients into the batch. Premix the phase F ingredients and then add to the batch. Continue cooling the batch to room temperature (approximately 20-25 0 C).
- the whitening essence lotion was applied to the forearm twice a day.
- the scale ranged from the assessed parameter being much less improved, somewhat less improved, no change, somewhat greater improved, and much greater improved.
- the values represent the percent of panelists who perceived improvement at the given point in time.
- the study parameters included applying various combinations of these compositions to the panelist's skin in a regime-like format in the morning and evening.
- the panelists used the following compositions in the morning and in the following order: (i) the cleanser (Table 9); (ii) the softener (Table 4); (iii) the essence lotion (Table 7); (iv) the day lotion (Table 5); and (v) the foundation (Table 6).
- the foundation included three different shades or colors ⁇ e.g.., foundation ivory 105, foundation ivory soft, and foundation antique ivory) of which the subjects selected one.
- the evening regimen included applying the following compositions in order: (i) the cleanser (Table 9); (ii) the mask (table 10); (iii) the softener formulation (Table 4); (iv) the essence lotion (Table 7); and (v) the night cream (Table 8).
- the data in Table 12 were obtained by using objective methods that included instrumental measurements and/or expert grading systems. The results were obtained at 2, 4, 6, and 8 weeks during the regimen use by the subjects.
- Skin moisture/hydration was measured using impedance measurements with the Nova Dermal Phase Meter. The impedance meter measures changes in skin moisture content. The outer layer of the skin has distinct electrical properties. When skin is dry it conducts electricity very poorly. As it becomes more hydrated increasing conductivity results. Consequently, changes in skin impedance (related to conductivity) can be used to assess changes in skin hydration. In the present study, the unit was calibrated according to instrument instructions for each testing day. A notation of temperature and relative humidity was made.
- Subjects were evaluated as follows : prior to measurement they will equilibrate in a room with defined humidity (30-50%) and temperature (68-72C). Three separate impedance reading were made on each side of the face, recorded and averaged. The T5 setting was used on the impedance meter that averages the impedance values of every five seconds application to the face. Changes were reported with statistical variance and significance.
- Skin clarity and the reduction in freckles and age spots was evaluated using a Minolta Chromometer. Changes in skin color were assessed to determine irritation potential due to product treatment using the a* values of the Minolta Chroma Meter. The a* value measures changes in skin color in the red region. This is used to determine whether the product is inducing irritation. The measurements were made on each side of the face and averaged, as left and right facial values. Skin clarity can also be measured using the Minolta Meter. The measurement is a combination of the a*, b, and L values of the Minolta Meter and is related to skin brightness, and correlates well with skin smoothness and hydration. Skin reading is taken as above. Skin clarity is defined as L/C where C is chroma and is defined as (a + b ) .
- Skin dryness, surface fine lines, skin smoothness, and skin tone were evaluated with clinical grading techniques.
- clinical grading of skin dryness was determined by a five point standard Kligman Scale: (0) skin is soft and moist; (1) skin appears normal with no visible dryness; (2) skin feels slightly dry to the touch with no visible flaking; (3) skin feels dry, tough, and has a whitish appearance with some scaling; and (4) skin feels very dry, rough, and has a whitish appearance with scaling. Evaluations were made independently by two clinicians and averaged.
- Clinical grading of skin tone was performed via a ten point analog numerical scale:
- Scores are obtained for the eye area and mouth area (left and right sides) and added together as the total wrinkle score.
- Skin firmness was measured using a Hargens ballistometer, a device that evaluates the elasticity and firmness of the skin by dropping a small body onto the skin and recording its first two rebound peaks.
- the ballistometry is a small lightweight probe with a relatively blunt tip (4 square mm-contact area) was used. The probe penetrates slightly into the skin and results in measurements that are dependent upon the properties of the outer layers of the skin, including the stratum corneum and outer epidermis and some of the dermal layers.
- Skin softness/suppleness was evaluated using the Gas Bearing Electrodynamometer, an instrument that measures the stress/strain properties of the skin.
- the viscoelastic properties of skin correlate with skin moisturization. Measurements were obtained on the predetermined site on the cheek area by attaching the probe to the skin surface with double-stick tape. A force of approximately 3.5 gm is applied parallel to the skin surface and the skin displacement is accurately measured. Skin suppleness is then calculated and is expressed as DSR (Dynamic Spring Rate in gm/mm).
- the surface contour of the skin was measured by using the profilometer/Stylus method. This includes either shining a light or dragging a stylus across the replica surface. The vertical displacement of the stylus is fed into a computer via a distance transducer, and after scanning a fixed length of replica a cross-sectional analysis of skin profile is generated as a two-dimensional curve. This scan can be repeated any number of times along a fix axis to generate a simulated 3-D picture of the skin. Ten random sections of the replicas using the stylus technique were obtained and combined to generate average values.
- the values of interest include Ra which is the arithmetic mean of all roughness (height) values computed by integrating the profile height relative to the mean profile height.
- Rt which is the maximum vertical distance between the highest peak and lowest trough
- Rz which is the mean peak amplitude minus the mean peak height. Values are given as a calibrated value in mm. Equipment is standardized prior to each use by scanning metal standards of know values.
- the absolute value of the location of the profile relative to the mean profile height (x-axis).
- the efficacy of the compositions of the present invention can be evaluated by using a skin analog, such as, for example, MELANODERMTM.
- a skin analog such as, for example, MELANODERMTM.
- Melanocytes one of the cells in the skin analog, stain positively when exposed to L-dihydroxyphenyl alanine (L-DOPA), a precursor of melanin.
- L-DOPA L-dihydroxyphenyl alanine
- the skin analog, MELANODERMTM can be treated with a variety of bases containing the compositions and whitening agents of the present invention or with the base alone as a control. Alternatively, an untreated sample of the skin analog can be used as a control.
- Table 15 provides a summary of the subjective data that was obtained in the U.S. and Thailand Studies (Tables 13 and 14, respectively).
- Tables 16-17 provide non-limiting examples of TimeWise Essence and TimeWise Night Cream Formulations. As discussed above, these concentrations ranges can vary.
- Panelist Accountability 44 out of 49 subjects completed the study. Three subjects were dropped from the study for non-compliance and the other subject withdrew for personal reasons. There was no adverse event reported for this study.
- Panelist Accountability 46 out of 49 subjects completed the study. Three subjects withdrew from the study for personal reasons. There was no adverse event reported for this study.
- compositions and/or methods disclosed and claimed in this specification can be made and executed without undue experimentation in light of the present disclosure. While the compositions and methods of this invention have been described in terms of preferred embodiments, it will be apparent to those of skill in the art that variations may be applied to the compositions and/or methods and in the steps or in the sequence of steps of the method described herein without departing from the concept, spirit and scope of the invention. More specifically, it will be apparent that certain agents which are both chemically and physiologically related may be substituted for the agents described herein while the same or similar results would be achieved. All such similar substitutes and modifications apparent to those skilled in the art are deemed to be within the spirit, scope and concept of the invention as defined by the appended claims.
Landscapes
- Health & Medical Sciences (AREA)
- Natural Medicines & Medicinal Plants (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Mycology (AREA)
- Microbiology (AREA)
- Botany (AREA)
- Alternative & Traditional Medicine (AREA)
- Medical Informatics (AREA)
- Birds (AREA)
- Dermatology (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Cosmetics (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Abstract
Description
Claims
Priority Applications (9)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN2006800144571A CN101166506B (en) | 2005-03-23 | 2006-03-21 | Skin lightening compositions |
| BRPI0609575A BRPI0609575B1 (en) | 2005-03-23 | 2006-03-21 | skin lightening compositions |
| AU2006227205A AU2006227205A1 (en) | 2005-03-23 | 2006-03-21 | Skin lightening compositions |
| CA2601571A CA2601571C (en) | 2005-03-23 | 2006-03-21 | Skin lightening compositions |
| MX2007011784A MX2007011784A (en) | 2005-03-23 | 2006-03-21 | Skin lightening compositions. |
| AT06739081T ATE527985T1 (en) | 2005-03-23 | 2006-03-21 | COMPOSITIONS FOR SKIN LIGHTENING |
| EA200702056A EA015357B1 (en) | 2005-03-23 | 2006-03-21 | Skin lightening compositions |
| EP06739081A EP1871334B1 (en) | 2005-03-23 | 2006-03-21 | Skin lightening compositions |
| HK08107151.2A HK1112182A1 (en) | 2005-03-23 | 2008-06-27 | Skin lightening compositions |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US66433305P | 2005-03-23 | 2005-03-23 | |
| US60/664,333 | 2005-03-23 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2006102289A2 true WO2006102289A2 (en) | 2006-09-28 |
| WO2006102289A3 WO2006102289A3 (en) | 2006-11-30 |
Family
ID=37024509
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2006/010149 WO2006102289A2 (en) | 2005-03-23 | 2006-03-21 | Skin lightening compositions |
Country Status (13)
| Country | Link |
|---|---|
| US (1) | US20060216254A1 (en) |
| EP (1) | EP1871334B1 (en) |
| KR (1) | KR20080025036A (en) |
| CN (1) | CN101166506B (en) |
| AT (1) | ATE527985T1 (en) |
| AU (1) | AU2006227205A1 (en) |
| BR (1) | BRPI0609575B1 (en) |
| CA (1) | CA2601571C (en) |
| EA (1) | EA015357B1 (en) |
| HK (2) | HK1096241A2 (en) |
| MX (1) | MX2007011784A (en) |
| TW (1) | TW200716197A (en) |
| WO (1) | WO2006102289A2 (en) |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2911280A1 (en) * | 2007-01-16 | 2008-07-18 | Mayoly Spindler Soc Par Action | Combination for prevention and treatment of hyperpigmentation of the skin, contains an MC1-R receptor antagonist, a tyrosinase inhibitor derived from vitamin C and a melanocyte-transport inhibitor |
| WO2009033326A1 (en) * | 2007-09-14 | 2009-03-19 | Nanjing Zhongshi Chemical Co., Ltd. | Ascorbic acid derivates, their preparation methods, intermediates and uses in cosmetics |
| WO2011157640A3 (en) * | 2010-06-14 | 2012-09-07 | Unilever Plc | A high humectant high internal phase emulsion |
| GR1007699B (en) * | 2011-02-11 | 2012-09-19 | Ιφιγενεια Δημητριου Σιγαλα-Χαραλαμπιδου | Method for the preparation of an anti-blotch cream |
| US8821839B2 (en) | 2010-10-22 | 2014-09-02 | Conopco, Inc. | Compositions and methods for imparting a sunless tan with a vicinal diamine |
| US8961942B2 (en) | 2011-12-13 | 2015-02-24 | Conopco, Inc. | Sunless tanning compositions with adjuvants comprising sulfur comprising moieties |
| US9254276B2 (en) | 2011-01-25 | 2016-02-09 | The Procter & Gamble Company | Liposome and personal care composition comprising thereof |
| WO2016053289A1 (en) * | 2014-09-30 | 2016-04-07 | Avon Products, Inc. | Topical compositions and methods for skin lightening |
| WO2019120830A1 (en) * | 2017-12-21 | 2019-06-27 | L'oreal | Ascorbic 3-xyloside derivatives for cosmetic use thereof |
| RU2727807C2 (en) * | 2015-07-23 | 2020-07-24 | Джонсон энд Джонсон Консьюмер Инк. | Local delivery of skin care compositions having low ph |
| JP2021004215A (en) * | 2019-06-27 | 2021-01-14 | 小林製薬株式会社 | MC1R expression inhibitor |
Families Citing this family (46)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8128917B2 (en) | 2005-06-16 | 2012-03-06 | L'oréal | Aqueous polyamine-containing carrier systems for water-insoluble materials |
| US20060292100A1 (en) * | 2005-06-16 | 2006-12-28 | L'oreal | Aqueous phospholipid-containing carrier systems for water-insoluble materials |
| US8128916B2 (en) * | 2005-06-16 | 2012-03-06 | L'oréal | Aqueous fatty quaternary amine-containing carrier systems for water-insoluble materials |
| US8128915B2 (en) * | 2005-06-16 | 2012-03-06 | L'oréal | Aqueous fatty monoamine-containing carrier systems for water -insoluble materials |
| US20080096782A1 (en) * | 2006-10-19 | 2008-04-24 | L'oreal | Aqueous systems containing phospholipid, surfactant and phosphate ester for water-insoluble materials |
| US20080097070A1 (en) * | 2006-10-19 | 2008-04-24 | L'oreal | Aqueous polyamine-containing systems for water-insoluble materials |
| KR101319367B1 (en) | 2007-01-03 | 2013-10-16 | 주식회사 엘지생활건강 | Patch composition for skin-whitening |
| US20090035398A1 (en) * | 2007-03-29 | 2009-02-05 | Raymond Williams | Topical formulations |
| KR100865562B1 (en) * | 2008-03-27 | 2008-10-28 | (주)비에스인터내셔날 | Cmposition make use of beauty |
| FR2930145B1 (en) * | 2008-04-16 | 2012-08-03 | Fabre Pierre Dermo Cosmetique | USE OF MYRT EXTRACT AS DEPIGMENTING. |
| CN101401775B (en) * | 2008-11-18 | 2010-08-25 | 董萍 | Novel passive target skin whitening efficacy nanoemulsion for night and daylight and method of preparing the same |
| CA2769512C (en) | 2009-07-29 | 2019-07-09 | Duke University | Compositions comprising fp receptor antagonists and their use for inhibiting hair growth |
| WO2010000877A2 (en) * | 2009-10-14 | 2010-01-07 | Symrise Gmbh & Co. Kg | Formulation with irritation reducing action comprising bisabolol and [6]-paradol |
| US8722026B2 (en) * | 2010-01-06 | 2014-05-13 | Elc Management, Llc | Skin lightening compositions |
| WO2011087523A1 (en) * | 2010-01-17 | 2011-07-21 | The Procter & Gamble Company | Biomarker-based methods for identifying and formulating compositions that improve skin quality and reduce the visible signs of aging in skin |
| JP2011184308A (en) * | 2010-03-04 | 2011-09-22 | Shiseido Co Ltd | External preparation for skin |
| JP5676187B2 (en) * | 2010-09-15 | 2015-02-25 | 富士フイルム株式会社 | Oil-in-water cosmetic |
| MY175521A (en) * | 2011-01-07 | 2020-07-01 | Allergan Inc | Melanin modification compositions and methods of use |
| CN103561754A (en) | 2011-03-28 | 2014-02-05 | 玫琳凯有限公司 | Topical skin care formulations comprising plant extracts |
| CN102366397B (en) * | 2011-11-29 | 2013-01-30 | 宝健(中国)日用品有限公司 | Skin-whitening chloasma-reducing skin-brightening composition, skin care product therewith, and preparation method thereof |
| KR102245069B1 (en) | 2011-12-19 | 2021-04-26 | 마리 케이 인코포레이티드 | Combination of plant extrats to improve skin tone |
| WO2014103475A1 (en) * | 2012-12-27 | 2014-07-03 | 株式会社林原 | Skin-exterior anti-ageing composition and production method therefor |
| WO2014163896A1 (en) * | 2013-03-12 | 2014-10-09 | Avon Products, Inc | A topical lightening composition and methods of use thereof |
| US9511144B2 (en) | 2013-03-14 | 2016-12-06 | The Proctor & Gamble Company | Cosmetic compositions and methods providing enhanced penetration of skin care actives |
| JP6345176B2 (en) * | 2013-06-28 | 2018-06-20 | パナソニック インテレクチュアル プロパティ コーポレーション オブ アメリカPanasonic Intellectual Property Corporation of America | Skin sensory evaluation apparatus and skin evaluation method |
| EP2875806A1 (en) * | 2013-11-20 | 2015-05-27 | Infinitec Activos, S.L. | Targeted capsules for the delivery of whitening agents in the skin |
| KR102323049B1 (en) * | 2014-03-10 | 2021-11-05 | 마리 케이 인코포레이티드 | Skin lightening compositions |
| WO2015157692A1 (en) * | 2014-04-10 | 2015-10-15 | Cgtn C.V. | Skin care composition comprising plant extracts |
| MX2016015256A (en) * | 2014-06-02 | 2017-02-22 | Avon Prod Inc | Topical lightening composition and methods of use thereof. |
| DE102014211185A1 (en) * | 2014-06-11 | 2015-12-17 | Henkel Ag & Co. Kgaa | Cosmetic compositions for skin lightening |
| KR102569280B1 (en) * | 2014-12-04 | 2023-08-21 | 마리 케이 인코포레이티드 | Topical skin care composition comprising trifluoroacetyl tripeptide-2 |
| KR20170008367A (en) * | 2015-07-13 | 2017-01-24 | 씨제이제일제당 (주) | A protein having activity of glucose transfer and use thereof |
| US9833393B2 (en) * | 2015-09-24 | 2017-12-05 | Elc Management Llc | Method and compositions for treating skin |
| CN108697616B (en) * | 2015-12-15 | 2022-03-11 | 欧莱雅 | Iontophoretic method, iontophoretic composition, kit and iontophoretic device for delivering vitamin C |
| SG11201805765QA (en) | 2016-01-19 | 2018-08-30 | Achromaz Pte Ltd | A cosmetic composition and the use thereof for regulating skin quality |
| CN105902423A (en) * | 2016-04-15 | 2016-08-31 | 欧标(广州)化妆品有限公司 | Whitening mask composition for uniformizing skin color and brightening skin |
| US10363207B2 (en) * | 2016-04-29 | 2019-07-30 | Purvisha Patel | All-in-one skin-brightening formulations |
| US12097279B2 (en) | 2017-09-18 | 2024-09-24 | Mary Kay Inc. | Cosmetic compositions and methods |
| JP6900496B2 (en) * | 2017-09-28 | 2021-07-07 | 株式会社マンダム | Semi-solid cleaning agent |
| CN112218616A (en) * | 2018-03-23 | 2021-01-12 | 玫琳凯有限公司 | Topical compositions and methods |
| CN108324590A (en) * | 2018-05-17 | 2018-07-27 | 云南白药清逸堂实业有限公司 | A kind of whitening conditioning liquid and preparation method thereof |
| CN108619028A (en) * | 2018-06-30 | 2018-10-09 | 太原紫兰科技有限责任公司 | A kind of multiple-effect moisturizing daily renewal cream and preparation method thereof |
| CN109620782B (en) * | 2018-11-02 | 2021-12-21 | 广州市爱百伊生物技术有限公司 | Plant composition with whitening and spot-fading effects |
| CN109394561B (en) * | 2018-12-14 | 2021-11-02 | 完美(中国)有限公司 | Composition for removing yellow and whitening skin, application and preparation method thereof |
| CN114159545B (en) * | 2021-12-29 | 2022-11-25 | 天中康元药业集团有限公司 | Whitening anti-wrinkle composition containing enzyme and application of composition in medicines |
| WO2023141804A1 (en) * | 2022-01-26 | 2023-08-03 | L'oreal | Composition for brightening or whitening keratin materials |
Family Cites Families (41)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1533119A (en) * | 1975-04-10 | 1978-11-22 | Unilever Ltd | Skin lightening compositions |
| DE69019779T2 (en) * | 1989-05-19 | 1995-12-14 | Hayashibara Biochem Lab | Alpha-glycosyl-L-ascorbic acid and its production and uses. |
| JP2593714B2 (en) * | 1989-09-20 | 1997-03-26 | 株式会社 林原生物化学研究所 | Whitening agent |
| JP2832848B2 (en) * | 1989-10-21 | 1998-12-09 | 株式会社林原生物化学研究所 | Crystal 2-O-α-D-glucopyranosyl-L-ascorbic acid, its production method and use |
| US5272136A (en) * | 1991-10-12 | 1993-12-21 | Kabushiki Kaisha Hayashibara Seibutsu Kagaku Kenkyujo | 5-0-α-D-Glucopyranosyl-L-ascorbic acid, and its preparation and uses |
| JP3072535B2 (en) * | 1991-10-21 | 2000-07-31 | 株式会社林原生物化学研究所 | 5-O-α-D-glucopyranosyl-L-ascorbic acid, its production method and use |
| JP3134236B2 (en) * | 1992-01-30 | 2001-02-13 | 株式会社林原生物化学研究所 | Method for producing high α-glycosyl-L-ascorbic acid content and separation system for the production |
| US5411741A (en) * | 1993-07-29 | 1995-05-02 | Zaias; Nardo | Method and composition for skin depigmentation |
| US5759524A (en) * | 1996-02-09 | 1998-06-02 | The Procter & Gamble Company | Photoprotective compositions |
| CA2226996C (en) * | 1997-01-24 | 2006-08-15 | Kose Corporation | Whitening cosmetic composition comprising polyhydric alcohol |
| US5747006A (en) * | 1997-05-14 | 1998-05-05 | Amway Corporation | Skin whitener composition containing acerola cherry fermentate |
| US6153177A (en) * | 1997-12-15 | 2000-11-28 | Chesebrough-Ponds's Usa, Co., Division Of Conopco, Inc. | Skin lightening composition |
| TW581680B (en) * | 1998-03-31 | 2004-04-01 | Mary Kay Cosmetics Inc | A whitening cosmetic composition and a pharmaceutical composition for treating hyperpigmentation |
| DE19940211A1 (en) * | 1998-09-22 | 2000-03-23 | Degussa | Single stage, high yield preparation of imidazolidine-2,4-diones useful as aminoacid intermediates, from aldehyde, (thio)urea compound and carbon monoxide over metal compound catalyst |
| US6224888B1 (en) * | 1999-02-12 | 2001-05-01 | The Procter & Gamble Company | Cosmetic compositions |
| US6444647B1 (en) * | 1999-04-19 | 2002-09-03 | The Procter & Gamble Company | Skin care compositions containing combination of skin care actives |
| US6492326B1 (en) * | 1999-04-19 | 2002-12-10 | The Procter & Gamble Company | Skin care compositions containing combination of skin care actives |
| US6284802B1 (en) * | 1999-04-19 | 2001-09-04 | The Procter & Gamble Company | Methods for regulating the condition of mammalian keratinous tissue |
| US6551604B1 (en) * | 1999-06-28 | 2003-04-22 | The Procter & Gamble Company | Skin care compositions |
| US6413552B1 (en) * | 2000-11-27 | 2002-07-02 | David M. Stoll | Topically applied creatine containing composition |
| WO2002062312A1 (en) * | 2001-02-08 | 2002-08-15 | The Procter & Gamble Company | Mask composition |
| US6436378B1 (en) * | 2001-02-28 | 2002-08-20 | Colgate-Palmolive Company | Composition |
| DE10136077A1 (en) * | 2001-07-25 | 2003-02-13 | Beiersdorf Ag | Cosmetic or dermatological preparations containing creatinine and creatine, useful e.g. for combating skin aging symptoms or treating inflammatory conditions such as eczema or psoriasis |
| GB0119831D0 (en) * | 2001-08-14 | 2001-10-10 | Unilever Plc | Dual compartment packaged cosmetic composition |
| TWI220386B (en) * | 2002-01-21 | 2004-08-21 | Matsushita Electric Works Ltd | Ultrasonic transdermal permeation device |
| FR2835252B1 (en) * | 2002-01-25 | 2005-08-05 | Seppic Sa | USE OF A PROTEIN KINASE A INACTIFYING COMPOUND IN A COMPOSITION CONTAINING A COSMETICALLY ACCEPTABLE MEDIUM FOR LAMINATING THE SKIN |
| US20030147830A1 (en) * | 2002-01-30 | 2003-08-07 | The Procter & Gamble Company | Topical skin and/or hair compositions containing protein |
| ATE300280T1 (en) * | 2002-02-15 | 2005-08-15 | Goldschmidt Gmbh | USE OF CREATININE AND/OR CREATININE DERIVATIVES TO BRIGHTEN THE SKIN AND REDUCE PIGMENT DISORDERS |
| KR20030080869A (en) * | 2002-04-11 | 2003-10-17 | 주식회사 코리아나화장품 | Cosmetic Composition for Skin-Whitening Comprising Ascorbic Acid 2-Glucoside as Active Ingredient |
| US7226583B2 (en) * | 2002-08-12 | 2007-06-05 | Hair Systems, Inc. | Composition containing leukocyte extract for the whitening or lightening of skin |
| JP2003113070A (en) * | 2002-08-19 | 2003-04-18 | Mochida Pharmaceut Co Ltd | Stabilizer for oil-soluble licorice extract and method of stabilizing the same |
| US20040081672A1 (en) * | 2002-10-25 | 2004-04-29 | Gupta Shyam K. | Niacinamide, niacin, and niacin esters based delivery systems for treating topical disorders of skin and skin aging |
| DE10301632A1 (en) * | 2003-01-17 | 2004-07-29 | Beiersdorf Ag | Cosmetic and dermatological composition, useful e.g. for restructuring and rejuvenating the skin, contains soya bean germ extract, creatinine and creatine |
| JP2004262853A (en) * | 2003-03-03 | 2004-09-24 | Kanebo Ltd | Cosmetic |
| US20040175347A1 (en) * | 2003-03-04 | 2004-09-09 | The Procter & Gamble Company | Regulation of mammalian keratinous tissue using hexamidine compositions |
| US7285570B2 (en) * | 2003-04-17 | 2007-10-23 | The Procter & Gamble Company | Compositions and methods for regulating mammalian keratinous tissue |
| FR2855050B1 (en) * | 2003-05-22 | 2008-07-04 | Oreal | PROCESS FOR THE COSMETIC TREATMENT OF REDNESS |
| WO2005004830A1 (en) * | 2003-06-13 | 2005-01-20 | The Procter & Gamble Company | Skin care composition comprising skin lightening agent |
| JP4921351B2 (en) * | 2004-03-26 | 2012-04-25 | ディーエスエム アイピー アセッツ ビー.ブイ. | Composition comprising an HDAC inhibitor in combination with a retinoid |
| US20050271608A1 (en) * | 2004-06-05 | 2005-12-08 | Gupta Shyam K | Skin whitening compositions based on hydroxyaryl alkyl ketones and their isosteric derivatives |
| WO2006020164A1 (en) * | 2004-07-23 | 2006-02-23 | The Procter & Gamble Company | Skin care composition containing a flavonoid and vitamin b3 |
-
2006
- 2006-03-21 AU AU2006227205A patent/AU2006227205A1/en not_active Abandoned
- 2006-03-21 AT AT06739081T patent/ATE527985T1/en not_active IP Right Cessation
- 2006-03-21 BR BRPI0609575A patent/BRPI0609575B1/en not_active IP Right Cessation
- 2006-03-21 WO PCT/US2006/010149 patent/WO2006102289A2/en active Application Filing
- 2006-03-21 MX MX2007011784A patent/MX2007011784A/en active IP Right Grant
- 2006-03-21 CA CA2601571A patent/CA2601571C/en active Active
- 2006-03-21 EP EP06739081A patent/EP1871334B1/en active Active
- 2006-03-21 EA EA200702056A patent/EA015357B1/en not_active IP Right Cessation
- 2006-03-21 KR KR1020077024399A patent/KR20080025036A/en not_active Application Discontinuation
- 2006-03-21 US US11/385,550 patent/US20060216254A1/en not_active Abandoned
- 2006-03-21 CN CN2006800144571A patent/CN101166506B/en active Active
- 2006-03-23 TW TW095110058A patent/TW200716197A/en unknown
- 2006-03-23 HK HK06103676A patent/HK1096241A2/en not_active IP Right Cessation
-
2008
- 2008-06-27 HK HK08107151.2A patent/HK1112182A1/en not_active IP Right Cessation
Non-Patent Citations (1)
| Title |
|---|
| See references of EP1871334A4 * |
Cited By (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2911280A1 (en) * | 2007-01-16 | 2008-07-18 | Mayoly Spindler Soc Par Action | Combination for prevention and treatment of hyperpigmentation of the skin, contains an MC1-R receptor antagonist, a tyrosinase inhibitor derived from vitamin C and a melanocyte-transport inhibitor |
| WO2008107533A2 (en) | 2007-01-16 | 2008-09-12 | Laboratoires Mayoly Spindler | Association of compounds inhibiting melanogenesis and use thereof in cosmetics and dermatology |
| WO2008107533A3 (en) * | 2007-01-16 | 2009-04-09 | Mayoly Spindler Lab | Association of compounds inhibiting melanogenesis and use thereof in cosmetics and dermatology |
| JP2010515768A (en) * | 2007-01-16 | 2010-05-13 | ラボラトワール マヨリ スパンドレ | Combinations of compounds that inhibit melanogenesis and their use in cosmetics and skin products |
| EA016034B1 (en) * | 2007-01-16 | 2012-01-30 | Лаборатуар Мейоли Спэндлер | Association of compounds inhibiting melanogenesis and use thereof in cosmetics and dermatology |
| WO2009033326A1 (en) * | 2007-09-14 | 2009-03-19 | Nanjing Zhongshi Chemical Co., Ltd. | Ascorbic acid derivates, their preparation methods, intermediates and uses in cosmetics |
| WO2011157640A3 (en) * | 2010-06-14 | 2012-09-07 | Unilever Plc | A high humectant high internal phase emulsion |
| US8821839B2 (en) | 2010-10-22 | 2014-09-02 | Conopco, Inc. | Compositions and methods for imparting a sunless tan with a vicinal diamine |
| US9254276B2 (en) | 2011-01-25 | 2016-02-09 | The Procter & Gamble Company | Liposome and personal care composition comprising thereof |
| GR1007699B (en) * | 2011-02-11 | 2012-09-19 | Ιφιγενεια Δημητριου Σιγαλα-Χαραλαμπιδου | Method for the preparation of an anti-blotch cream |
| US8961942B2 (en) | 2011-12-13 | 2015-02-24 | Conopco, Inc. | Sunless tanning compositions with adjuvants comprising sulfur comprising moieties |
| WO2016053289A1 (en) * | 2014-09-30 | 2016-04-07 | Avon Products, Inc. | Topical compositions and methods for skin lightening |
| US9592186B2 (en) | 2014-09-30 | 2017-03-14 | Avon Products, Inc. | Topical compositions and methods for skin lightening |
| RU2727807C2 (en) * | 2015-07-23 | 2020-07-24 | Джонсон энд Джонсон Консьюмер Инк. | Local delivery of skin care compositions having low ph |
| WO2019120830A1 (en) * | 2017-12-21 | 2019-06-27 | L'oreal | Ascorbic 3-xyloside derivatives for cosmetic use thereof |
| FR3075797A1 (en) * | 2017-12-21 | 2019-06-28 | L'oreal | ASCROBIC 3-XYLOSIDE DERIVATIVES FOR THEIR COSMETIC USE |
| JP2021004215A (en) * | 2019-06-27 | 2021-01-14 | 小林製薬株式会社 | MC1R expression inhibitor |
Also Published As
| Publication number | Publication date |
|---|---|
| ATE527985T1 (en) | 2011-10-15 |
| CA2601571C (en) | 2012-07-31 |
| BRPI0609575A2 (en) | 2010-04-20 |
| BRPI0609575B1 (en) | 2016-07-12 |
| CN101166506B (en) | 2012-01-25 |
| EP1871334A2 (en) | 2008-01-02 |
| AU2006227205A1 (en) | 2006-09-28 |
| EP1871334A4 (en) | 2009-11-11 |
| EP1871334B1 (en) | 2011-10-12 |
| CA2601571A1 (en) | 2006-09-28 |
| HK1096241A2 (en) | 2007-05-25 |
| TW200716197A (en) | 2007-05-01 |
| HK1112182A1 (en) | 2008-08-29 |
| KR20080025036A (en) | 2008-03-19 |
| EA200702056A1 (en) | 2008-04-28 |
| MX2007011784A (en) | 2008-03-14 |
| WO2006102289A3 (en) | 2006-11-30 |
| EA015357B1 (en) | 2011-06-30 |
| CN101166506A (en) | 2008-04-23 |
| US20060216254A1 (en) | 2006-09-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1871334B1 (en) | Skin lightening compositions | |
| US11865202B2 (en) | Combination of plant extracts to improve skin tone | |
| US9592194B2 (en) | Lip stick | |
| US8318222B2 (en) | Topical skin care formulation | |
| US8372382B2 (en) | Skin moisturizer and age fighting formula | |
| EP2788010B1 (en) | Skin care formulation | |
| CA2823534C (en) | Topical composition comprising a psidium guajava extract and a kunzea ericoides extract for providing sebum control and treating acne | |
| US9402794B2 (en) | Topical skin care formulation | |
| US9295622B2 (en) | Substantive sunscreen formulation | |
| US9005588B2 (en) | Substantive sunscreen formulation | |
| US20070031357A1 (en) | High PH compositions |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 200680014457.1 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2006227205 Country of ref document: AU |
|
| ENP | Entry into the national phase |
Ref document number: 2601571 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/a/2007/011784 Country of ref document: MX |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2006739081 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2006227205 Country of ref document: AU Date of ref document: 20060321 Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: RU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020077024399 Country of ref document: KR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 200702056 Country of ref document: EA |
|
| ENP | Entry into the national phase |
Ref document number: PI0609575 Country of ref document: BR Kind code of ref document: A2 |