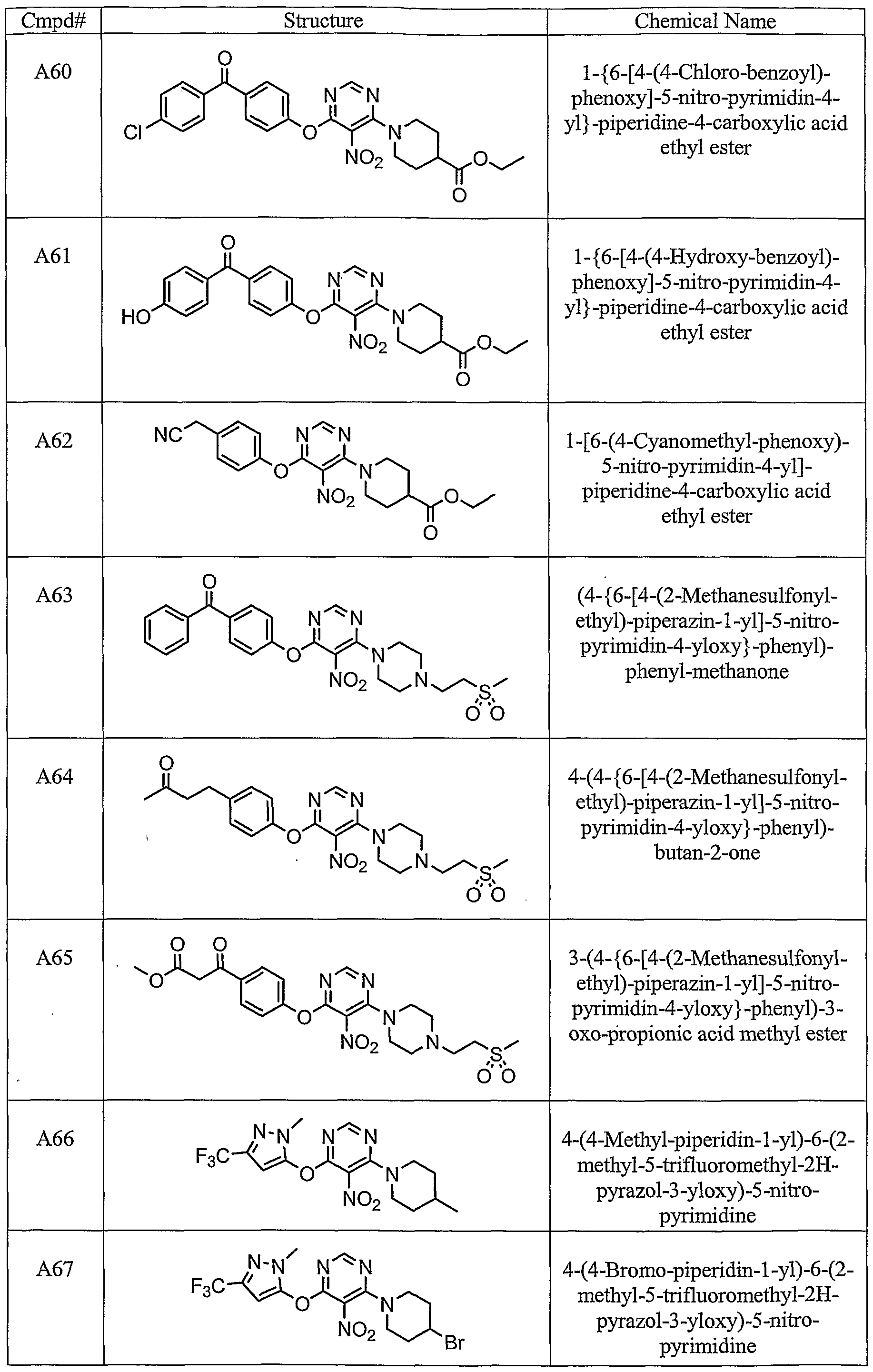
WO2004065380A1 - 1,2,3-trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prpphylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia - Google Patents
1,2,3-trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prpphylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia Download PDFInfo
- Publication number
- WO2004065380A1 WO2004065380A1 PCT/US2004/001267 US2004001267W WO2004065380A1 WO 2004065380 A1 WO2004065380 A1 WO 2004065380A1 US 2004001267 W US2004001267 W US 2004001267W WO 2004065380 A1 WO2004065380 A1 WO 2004065380A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- pyrimidin
- nitro
- carboxylic acid
- piperidine
- ethyl ester
- Prior art date
Links
- 0 C[C@@](C*)CC[C@@](C1)C1[C@]1C=CCC1 Chemical compound C[C@@](C*)CC[C@@](C1)C1[C@]1C=CCC1 0.000 description 5
- UFCJOPTZRITGIL-UHFFFAOYSA-N CC(C(N1C)=S)N(C)C1=O Chemical compound CC(C(N1C)=S)N(C)C1=O UFCJOPTZRITGIL-UHFFFAOYSA-N 0.000 description 1
- OHADEECROMTLRQ-UHFFFAOYSA-N CCN(C)C(C(C)NC)=S Chemical compound CCN(C)C(C(C)NC)=S OHADEECROMTLRQ-UHFFFAOYSA-N 0.000 description 1
- RHYBFKMFHLPQPH-UHFFFAOYSA-N CN(CC(N1)=O)C1=O Chemical compound CN(CC(N1)=O)C1=O RHYBFKMFHLPQPH-UHFFFAOYSA-N 0.000 description 1
- JTPZTKBRUCILQD-UHFFFAOYSA-N CN(CCN1)C1=O Chemical compound CN(CCN1)C1=O JTPZTKBRUCILQD-UHFFFAOYSA-N 0.000 description 1
- VWIIJDNADIEEDB-UHFFFAOYSA-N CN(CCO1)C1=O Chemical compound CN(CCO1)C1=O VWIIJDNADIEEDB-UHFFFAOYSA-N 0.000 description 1
- ITKKIAIATBKBEO-UHFFFAOYSA-N CN(CCS1)C1=O Chemical compound CN(CCS1)C1=O ITKKIAIATBKBEO-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/62—Oxygen or sulfur atoms
- C07D213/63—One oxygen atom
- C07D213/68—One oxygen atom attached in position 4
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/04—Inotropic agents, i.e. stimulants of cardiac contraction; Drugs for heart failure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/78—Carbon atoms having three bonds to hetero atoms, with at the most one bond to halogen, e.g. ester or nitrile radicals
- C07D213/79—Acids; Esters
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/02—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings
- C07D239/24—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members
- C07D239/28—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, directly attached to ring carbon atoms
- C07D239/32—One oxygen, sulfur or nitrogen atom
- C07D239/34—One oxygen atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/02—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings
- C07D239/24—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members
- C07D239/28—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, directly attached to ring carbon atoms
- C07D239/46—Two or more oxygen, sulphur or nitrogen atoms
- C07D239/50—Three nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D295/00—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms
- C07D295/04—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms
- C07D295/08—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by singly bound oxygen or sulfur atoms
- C07D295/096—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by singly bound oxygen or sulfur atoms with the ring nitrogen atoms and the oxygen or sulfur atoms separated by carbocyclic rings or by carbon chains interrupted by carbocyclic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/04—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/14—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D411/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen and sulfur atoms as the only ring hetero atoms
- C07D411/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen and sulfur atoms as the only ring hetero atoms containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing three or more hetero rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D419/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen, oxygen, and sulfur atoms as the only ring hetero atoms
- C07D419/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen, oxygen, and sulfur atoms as the only ring hetero atoms containing three or more hetero rings
Definitions
- the present invention relates to certain 1,2,3-trisubstituted aryl and heteroaryl derivatives that are modulators of glucose metabolism. Accordingly, compounds of the present invention are useful in the prophylaxis or treatment of metabolic disorders and complications thereof, such as, diabetes and obesity.
- Diabetes mellitus is a serious disease afflicting over 100 million people worldwide. In the United States, there are more than 12 million diabetics, with 600,000 new cases diagnosed each year.
- Diabetes mellitus is a diagnostic term for a group of disorders characterized by abnormal glucose homeostasis resulting in elevated blood sugar.
- IDDM insulin-dependent diabetes mellitus
- NIDDM non-insulin-dependent diabetes mellitus
- the etiology of the different types of diabetes is not the same; however, everyone with diabetes has two things in common: overproduction of glucose by the liver and little or no ability to move glucose out of the blood into the cells where it becomes the body's primary fuel.
- Diabetes is a syndrome with interrelated metabolic, vascular, and neuropathic components.
- the metabolic syndrome generally characterized by hyperglycemia, comprises alterations in carbohydrate, fat and protein metabolism caused by absent or markedly reduced insulin secretion and/or ineffective insulin action.
- the vascular syndrome consists of abnormalities in the blood vessels leading to cardiovascular, retinal and renal complications. Abnormalities in the peripheral and autonomic nervous systems are also part of the diabetic syndrome.
- IDDM People with IDDM, which accounts for about 5% to 10% of those who have diabetes, don't produce insulin and therefore must inject insulin to keep their blood glucose levels normal. IDDM is characterized by low or undetectable levels' of endogenous insulin production caused by destruction of the insulin-producing ⁇ cells of the pancreas, the characteristic that most readily distinguishes IDDM from NIDDM. IDDM, once termed juvenile-onset diabetes, strikes young and older adults alike.
- NIDDM Type II diabetes Approximately 90 to 95% of people with diabetes have Type II (or NIDDM). NIDDM subjects produce insulin, but the cells in their bodies are insulin resistant: the cells don't respond properly to the hormone, so glucose accumulates in their blood. NIDDM is characterized by a relative disparity between endogenous insulin production and insulin requirements, leading to elevated blood glucose levels. In contrast to IDDM, there is always some endogenous insulin production in NIDDM; many NTDDM patients have normal or even elevated blood insulin levels, while other NIDDM patients have inadequate insulin production (Rotwein, R. et al. N. Engl. J. Med. 308, 65-71 (1983)). Most people diagnosed with ⁇ TDDM are age 30 or older, and half of all new cases are age 55 and older. Compared with whites and Asians, ⁇ TJDDM is more common among Native Americans, African-Americans, Latinos, and Hispanics. In addition, the onset can be insidious or even clinically inapparent, making diagnosis difficult
- NIDDM neurodegenerative disease
- Obesity and diabetes are among the most common human health problems in industrialized societies. In industrialized countries a third of the population is at least 20% overweight. In the United States, the percentage of obese people has increased from 25% at the end of the 1970s, to 33% at the beginning the 1990s. Obesity is one of the most important risk factors for NIDDM. Definitions of obesity differ, but in general, a subject weighing at least 20% more than the recommended weight for his/her height and build is considered obese. The risk of developing NIDDM is tripled in subjects 30% overweight, and three-quarters with NIDDM are overweight.
- Obesity which is the result of an imbalance between caloric intake and energy expenditure, is highly correlated with insulin resistance and diabetes in experimental animals and human.
- the molecular mechanisms that are involved in obesity-diabetes syndromes are not clear.
- increase insulin secretion balances insulin resistance and protects patients from hyperglycemia (Le Stunf ⁇ et al. Diabetes 43, 696-702 (1989)).
- ⁇ cell function deteriorates and non-insulin-dependent diabetes develops in about 20% of the obese population (Pederson, P. Diab. Metab. Rev. 5, 505- 509 (1989)) and (Brancati, F. L., et al., Arch. Intern. Med.
- BMI body mass index
- m 2 body weight index
- Overweight is defined as a BMI in the range 25-30 kg/m 2
- obesity is a BMI greater than 30 kg/m 2 (see TABLE below).
- Orlistat a lipase inhibitor
- Sibutramine a mixed 5-HT/noradrenaline reuptake inhibitor
- fenfluramine Pieris-associated fenfluramine
- ReduxTM dexfenfluramine
- Kidney disease also called nephropathy
- Diabetes occurs when the kidney's "filter mechanism” is damaged and protein leaks into urine in excessive amounts and eventually the kidney fails. Diabetes is also a leading cause of damage to the retina at the back of the eye and increases risk of cataracts and glaucoma.
- diabetes is associated with nerve damage, especially in the legs and feet, which interferes with the ability to sense pain and contributes to serious infections. Taken together, diabetes complications are one of the nation's leading causes of death.
- the present invention is drawn to compounds, which bind to and modulate the activity of a GPCR referred to herein as RUP3, and uses thereof.
- RUP3, as used herein includes the human sequences found in GeneBank accession number XM_066873, naturally-occurring allelic variants, mammalian orthologs, and recombinant mutants thereof.
- a preferred human RUP3 for use in screening and testing of the compounds of the invention is provided in the nucleotide sequence of Seq. ID .No: 1 and the corresponding amino acid sequence in Seq. ID.No:2.
- One aspect of the present invention encompasses 1,2,3-trisubstituted aryl and heteroaryl derivatives as shown in Formula (la):
- a and B are independently C ⁇ - 3 alkylene optionally substituted with 1 to 4 methyl groups
- D is O, S, S(O), S(0) 2 , CR 2 R 3 or N-R 2 ;
- V is selected from the group consisting of C1. 3 alkylene, ethynylene and C1- 2 heteroalkylene wherein each are optionally substituted with 1 to 4 substituents selected from the group consisting of C1- 3 alkyl, C ⁇ ialkoxy, carboxy, cyano, - 3 haloalkyl and halogen; or V is absent;
- W is NR,, O, S, S(O) or S(0) 2 ; or W is absent;
- X is N or CR 5 ;
- Y is or CRe
- Z is selected from the group consisting of C 1 - 5 acyl, C1- 5 acyloxy, C ⁇ alkoxy, C ⁇ - 8 alkyl, C 1 - 4 alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ alkylthioureyl, C alkylureyl, amino, C ⁇ - 2 alkylamino, C 2 - 4 dialkylamino, carbo-Ci- ⁇ -alkoxy, carboxamide, carboxy, cyano, -s diacylamino, C 2 - 6 dialkylcarboxamide, C ⁇ dialkylthiocarboxamide, C 2 _ 6 dialkylsulfonamide, C ⁇ dialkylsulfonylamino, formyl, C ⁇ haloalkoxy, C ⁇ haloalkyl,
- Z is a group of Formula (A):
- R 7 is H, Ci-s alkyl or C3-6 cycloalkyl
- R 8 is H, nitro or nitrile
- a ⁇ is aryl or heteroaryl wherein each are optionally substituted with R9-R1 3 ;
- Ri is selected from the group consisting of H, C1- 5 acyloxy, C 2 . 6 alkenyl, C ⁇ alkoxy, -g alkyl, C ⁇ alkylcarboxamide, C 2 .
- R 2 is selected from the group consisting of H, C,- 5 acyl, C1- 5 acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, amino, carbo-C -alkoxy, carboxamide, carboxy, cyano, C 3 - 6 -cycloalkyl, C 2 .
- R 2 is -Ar 2 -Ar 3 wherein Ar 2 and Ar 3 are independently aryl or heteroaryl each optionally substituted with 1 to 5 substituents selected from the group consisting of H, C ⁇ acyl, C 1 - 5 acyloxy, C ⁇ alkoxy, C ⁇ - 8 alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, amino, carbo-C -alkoxy, carboxamide, carboxy, cyano, C 3 . 6 -cycloa.kyl, C 2 . 6 dialkylcarboxamide, C ⁇ haloalkoxy, C ⁇ haloalkyl, halogen, hydroxyl and nitro; or
- R 2 is a group of Formula (B):
- R ⁇ is C ⁇ . 8 alkyl or C 3 . 6 cycloalkyl; and R 15 is F, Cl, Br or CN; or R 2 is a group of Formula (C):
- Ar 4 is phenyl or heteroaryl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ alkylthioureyl, C ⁇ alkylureyl, amino, carbo-C ⁇ -alkoxy, carboxamide, carboxy, cyano, C 3 .
- R 3 is H, C ⁇ alkyl, C ⁇ alkoxy, halogen or hydroxyl
- R t is H or C ⁇ alkyl
- R 5 and Re are independently H, C alkyl or halogen
- R 9 is selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C 2 . 6 alkenyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylamino, C ⁇ alkylcarboxamide, C 2 .g alkynyl, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ alkylureyl, amino, arylsulfonyl, carbo-C ⁇ -alkoxy, carboxamide, carboxy, cyano, C 3 . 6 cycloalkyl, C 2 . 6 dialkylamino, C 2 .
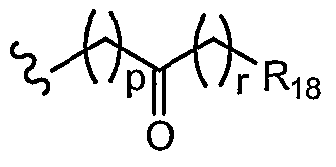
- R 9 is a group of Formula (D): ⁇ p ⁇ R. 18
- R 18 is H, C ⁇ acyl, C 2 . 6 alkenyl, C ⁇ alkyl, C ⁇ alkylcarboxamide, C 2 - 6 alkynyl, C ⁇ alkylsulfonamide, carbo-C ⁇ -alkoxy, carboxamide, carboxy, cyano, C 3 . s cycloalkyl, C 2 .
- Some embodiments of the present invention include pharmaceutical compositions comprising at least one compound of the present invention and a pharmaceutically acceptable carrier.
- Some embodiments of the present invention include methods for prophylaxis or treatment of a metabolic disorder in an individual comprising administering to the individual a therapeutically effective amount of a compound of the present invention or a pharmaceutical composition thereof.
- Some embodiments of the present invention include methods of controlling or decreasing weight gain of an individual comprising administering to the individual a therapeutically effective amount of a compound of the present invention or pharmaceutical composition thereof.
- Some embodiments of the present invention include methods of modulating a RUP3 receptor comprising contacting the receptor with a compound of the present invention.
- Some embodiments of the present invention include methods of modulating a RUP3 receptor in an individual comprising contacting the receptor with a compound of the present invention.
- the compound is an agonist.
- the compound is an inverse agonist.
- Some embodiments of the present invention include methods of modulating a RUP3 receptor in an individual comprising contacting the receptor with a compound of the present invention wherein the modulation of the RUP3 receptor is prophylaxis or treatment of a metabolic disorder.
- Some embodiments of the present invention include methods of modulating a RUP3 receptor in an individual comprising contacting the receptor with a compound of the present invention wherein the modulation of the RUP3 receptor controls or reduces weight gain of the individual.
- Some embodiments of the present invention include the use of compounds of the present invention for production of a medicament for use in prophylaxis or treatment of a metabolic disorder.
- Some embodiments of the present invention include the use of compounds of the present invention for production of a medicament for use in controlling or decreasing weight gain in an individual.
- One aspect of the present invention pertains to compounds of the present invention, as described herein, for use in methods of treatment of the human or animal body by therapy.
- One aspect of the present invention pertains to compounds of the present invention, as described herein, for use in methods of prophylaxis or treatment of a metabolic disorder of the human or animal body by therapy.
- the metabolic disorder is type I, type II diabetes, inadequate glucose tolerance, insulin resistance, hyperglycemia, hyperlipidemia, hypertriglyceridemia, hypercholesterolemia, dyslipidemia, syndrome X or metabolic syndrome.
- One aspect of the present invention pertains to methods of producing a pharmaceutical compositions comprising admixing at least one compound of the present invention and a pharmaceutically acceptable carrier.
- Figure 1A shows RT-PCR analysis of RUP3 expression in human tissues. A total of twenty-two (22) human tissues were analyzed.
- Figure IB shows the cDNA Dot-Blot analysis of RUP 3 expression in human tissues.
- Figure 1C shows analysis of RUP3 by RT-PCR with isolated huyman pancreatic islets of Langerhans.
- Figure ID shows analysis of RUP3 expression with cDNAs of rat origin by RT-PCR.
- Figure 2 A shows a polyclonal anti-RUP3 antibody prepared in Rabbits.
- Figure 2B shows the expression of RUP3 in insulin-producing ⁇ cells of pancreatic islets.
- Figure 3 shows functional activities of RUP3 In vitro.
- Figure 4A shows a RUP3 RNA blot.
- Figure 4B shows RUP3 agonist, Compound B84, stimulates cAMP production in HIT cells, at a level comparable to that seen with the adenyl cyclase activator forskolin.
- Figure 4C shows RUP3 agonist, Compound B84, stimulates insulin secretion in HIT cells exposed to 15 mM glucose, at a level comparable to that seen with the adenylcyclase activator forskolin.
- Figure 4D shows two RUP3 compounds, Compounds A48 and A51 (at 10 ⁇ M concentration), and the enhanced glucose-dependent insulin release compared to control.
- Figure 5A shows the In vivo effects of a RUP3 agonist (Compound B70) on glucose homeostasis in mice and specifically the effect by a RUP3 agonist in a dose-dependent manner on the lowering of blood glucose after glucose challenge.
- a RUP3 agonist Compound B70
- Figure 5B shows the acute response of db mice to a RUP 3 agonist and Ex-4.
- Figure 5B shows Compound B70 and Ex-4 significantly reduces glucose levels compared to vehicle control.
- Figure 6 shows a representative scheme for the syntheses of compounds of the present invention.
- AGONISTS shall mean moieties that activate the intracellular response when they bind to the receptor, or enhance GTP binding to membranes.
- AMTNO ACID ABBREVIATIONS used herein are set out in Table 1 :
- C ⁇ acyl denotes an alkyl radical attached to a carbonyl wherein the definition of alkyl has the same definition as described herein; some examples include formyl, acetyl, propionyl, butanoyl, iso-butanoyl. pentanoyl, hexanoyl, heptanoyl, and the like.
- C ⁇ acyloxy denotes an acyl radical attached to an oxygen atom wherein acyl has the same definition has described herein; some examples include acetyloxy, propionyloxy, butanoyloxy, zw-butanoyloxy and the like.
- C 2 -6 alkenyl denotes a radical containing 2 to 6 carbons wherein at least one carbon-carbon double bond is present, some embodiments are 2 to 4 carbons, some embodiments are 2 to 3 carbons, and some embodiments have 2 carbons. Both E and Z isomers are embraced by the term “alkenyl.” Furthermore, the term “alkenyl” includes di- and tri-alkenyls. Accordingly, if more than one double bond is present then the bonds may be all E or Z or a mixtures of E and Z.
- alkenyl examples include vinyl, allyl, 2-butenyl, 3-butenyl, 2-pentenyl, 3- pentenyl, 4-pentenyl, 2-hexenyl, 3-hexenyl, 4-hexenyl, 5-hexanyl, 2,4-hexadienyl and the like.
- C ⁇ alkoxy denotes a radical alkyl, as defined herein, attached directly to an oxygen atom.
- Example include methoxy, ethoxy, n- propoxy, z ' s ⁇ -propoxy, n-butoxy, t-butoxy, iso-butoxy and the like.
- C ⁇ alkyl denotes a straight or branched carbon radical containing 1 to 8 carbons, some embodiments are 1 to 6 carbons, some embodiments are 1 to 3 carbons, and some embodiments are 1 or 2 carbons.
- Examples of an alkyl include methyl, ethyl, n-propyl, iso-propyl, n-butyl, sec-butyl, t-butyl, amyl, t-amyl, n-pentyl and the like.
- C ⁇ alkylcarboxamido denotes a single alkyl group attached to an amide, wherein alkyl has the same definition as found herein.
- the C ⁇ alkylcarboxamido may be represented by the following:
- Examples include N-methylcarboxamide, N-ethylcarboxamide, N-(iso- pro ⁇ yl)carboxamide and the like.
- C 1 -C 3 alkylene refers to a divalent straight carbon group, such as, -CH 2 -, -CH 2 CH 2 -, -CH 2 CH 2 CH 2 -.
- C ⁇ alkylsulfinyl denotes an alkyl radical attached to a sulfoxide radical of the formula: -S(O)- wherein the alkyl radical has the same definition as described herein. Examples include methylsulfinyl, ethylsulfinyl and the like.
- C ⁇ alkylsulfonamide refers to the groups
- C ⁇ alkylsulfonyl denotes an alkyl radical attached to a sulfone radical of the formula: -S(O) 2 - wherein the alkyl radical has the same definition as described herein. Examples include methylsulfonyl, ethylsulfonyl and the like.
- C ⁇ alkylthio denotes an alkyl radical attached to a sulfide of the formula: -S- wherein the alkyl radical has the same definition as described herein. Examples include methylsulfanyl (i.e., CH 3 S-), ethylsulfanyl, isopropylsulfanyl and the like.
- C ⁇ alkylthiocarboxamide denotes a thioamide of the following formulae:
- C ⁇ alkylthioureyl denotes the group of the formula: - ⁇ C(S) ⁇ - wherein one are both of the nitrogens are substituted with the same or different alkyl group and alkyl has the same definition as described herein.
- alkylthioureyl examples include, CH 3 NHC(0)NH-, NH 2 C(0)NCH 3 -, (CH 3 ) 2 N(0)NH-, (CH 3 ) 2 N(0)NH-, (CH 3 ) 2 N(0)NCH 3 -, CH 3 CH 2 NHC(0)NH-, CH 3 CH 2 NHC(0)NCH 3 -, and the like.
- C ⁇ alkylureyl denotes the group of the formula: -NC(0)N- wherein one are both of the nitrogens are substituted with the same or different alkyl group wherein alkyl has the same definition as described herein.
- alkylureyl examples include, CH 3 NHC(0)NH-, NH 2 C(0)NCH 3 -, (CH 3 ) 2 N(0)NH-, (CH 3 ) 2 N(0)NH-, (CH 3 ) 2 N(0)NCH 3 -, CH 3 CH 2 NHC(0)NH-, CH 3 CH 2 NHC(0)NCH 3 -, and the like.
- C 2 _ 6 alkynyl denotes a radical containing 2 to 6 carbons and at least one carbon-carbon triple bond, some embodiments are 2 to 4 carbons, some embodiments are 2 to 3 carbons, and some embodiments have 2 carbons.
- alkynyl examples include ethynyl, ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl, 3- butynyl, 1-pentynyl, 2-pentynyl, 3-pentynyl, 4-pentynyl, 1-hexynyl, 2-hexynyl, 3- hexynyl, 4-hexynyl, 5-hexynyl and the like.
- alkynyl includes di- and tri- ynes.
- amino denotes the group -NH 2 .
- C ⁇ alkylamino denotes one alkyl radical attached to an amino radical wherein the alkyl radical has the same meaning as described herein. Some examples include methylamino, ethylamino, propylamino and the like.
- aryl denotes an aromatic ring radical containing 6 to 10 ring carbons. Examples include phenyl and naphthyl.
- arylalkyl defines a C 1 -C 4 alkylene, such as -CH 2 -, -CH 2 CH 2 - and the like, which is further substituted with an aryl group.
- Examples of an “arylalkyl” include benzyl, phenethylene and the like.
- ary ⁇ carboxamido denotes a single aryl group attached to the amine of an amide, wherein aryl has the same definition as found herein.
- the example is N-phenylcarboxamide.
- arylureyl denotes the group - ⁇ C(O) ⁇ - where one of the nitrogens are substituted with an aryl.
- benzyl denotes the group -CH 2 C-6H 5 .
- carbo-C ⁇ -alkoxy refers to an alkyl ester of a carboxylic acid, wherein the alkyl group is C ⁇ . Examples include carbomethoxy, carboethoxy, carboisopropoxy and the like.
- carboxylate refers to the group -CONH 2 .
- carboxy or “carboxyl” denotes the group -CO 2 H; also referred to as a carboxylic acid.
- cyano denotes the group -CN.
- C 3 . 7 cycloalkenyl denotes a non-aromatic ring radical containing 3 to 6 ring carbons and at least one double bond; some embodiments contain 3 to 5 carbons; some embodiments contain 3 to 4 carbons. Examples include cyclopropenyl, cyclobutenyl, cyclopentenyl, cyclopentenyl, cyclohexenyl, and the like.
- C 3 . 7 cycloalkyl denotes a saturated ring radical containing 3 to 6 carbons; some embodiments contain 3 to 5 carbons; some embodiments contain 3 to 4 carbons. Examples include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, and the like.
- C 3 - 6 cycloalkyl-C ⁇ -alkylene denotes a saturated ring radical containing 3 to 6 carbons bonded to a C ⁇ -alkylene as described herein; some embodiments contain 3 to 5 carbons; some embodiments contain 3 to 4 carbons. Examples include cyclopropyl-CH 2 -, cyclopropyl-CH 2 CH 2 -, cyclobutyl-CH 2 -, cyclopentyl-CH 2 -, cyclohexyl-CH 2 -, and the like.
- G t -s diacylamino denotes an amino group bonded with two acyl groups defined herein wherein the acyl groups may be the same or different, such as:
- Represented dialkylamino groups include diacetylamino, dipropionylamino, acetylpropionylamino and the like.
- C 2 - 6 dialkylamino denotes an amino substituted with two of the same or different alkyl radicals wherein alkyl radical has the same definition as described herein. Some embodiments are C ⁇ dialkylamino groups. Some examples include dimethylamino, methylethylamino, diethylamino and the like.
- C ⁇ dialkylcarboxamido or "C ⁇ dialkylcarboxamide”denotes two alkyl radicals, that are the same or different, attached to an amide group, wherein alkyl has the same definition as described herein.
- a C ⁇ dialkylcarboxamido may be represented by the following groups:
- dialkylcarboxamide examples include N,N-dimethylcarboxamide, N-methyl-N- ethylcarboxamide and the like.
- C 2 . 6 dialkylsulfonamide refers to one of the following groups shown below:
- C ⁇ dialkylthiocarboxamido or "C ⁇ dialkylthiocarboxamide” denotes two alkyl radicals, that are the same or different, attached to a thioamide group, wherein alkyl has the same definition as described herein.
- a C ⁇ dialkylthiocarboxamido may be represented by the following groups: S S if N N C ⁇ alkyl
- dialkylthiocarboxamide examples include NN-dimethylthiocarboxamide, N- methyl-N-ethylthiocarboxamide and the like.
- C ⁇ dialkylsulfonylamino refers to an amino group bonded with two C ⁇ alkylsulfonyl groups as defined herein.
- ethynylene refers to the carbon-carbon triple bond group as represented below:
- C ⁇ haloalkoxy denotes a haloalkyl, as defined herein, that is directly attached to an oxygen to form a difluoromethoxy, trifluoromethoxy, 2,2,2- trifluoroethoxy, pentafluoroethoxy and the like.
- C ⁇ haloalkyl denotes an alkyl group, defined herein, wherein the alkyl is substituted with one halogen up to fully substituted represented by the formula C n F 2n+I ; when more than one halogen is present they may be the same or different and selected from F, Cl, Br or I. Some embodiments are 1 to 3 carbons. Examples include fluoromethyl, difluoromethyl, trifluoromethyl, chlorodifluoromethyl, 2,2,2- trifluoroethyl, pentafluoroethyl and the like.
- C ⁇ haloalkylcarboxamide denotes an alkylcarboxamide group, defined herein, wherein the alkyl is substituted with one halogen up to fully substituted represented by the formula C n F 2n+ ⁇ and "n" is 1, 2, 3 or 4.
- n is 1, 2, 3 or 4.
- halogen When more than one halogen is present they may be the same or different and selected from F, Cl, Br or I. Examples include 2-fluoroacetyl, 2,2-difluoroacetyl, 2,2,2-trifluoroacetyl, 2-chloro-2,2- difluoroacetyl, 3,3,3-trifluoropropionyl, 2,2,3,3,3-pentafluoropropionyl and the like.
- C ⁇ haloalkylsulfinyl denotes a haloalkyl radical attached to a sulfoxide of the formula: -S(O)- wherein the alkyl radical has the same definition as described herein. Examples include trifluoromethylsulfinyl, 2,2,2-trifluoroethylsulfinyl, 2,2-difluoroethylsulfinyl and the like.
- C ⁇ haloalkylsulfonyl denotes a haloalkyl attached to a sulfone of the formula: -S(O) 2 - wherein haloalkyl has the same definition as described herein. Examples include trifluoromethylsulfonyl, 2,2,2-trifluoroethylsulfonyl, 2,2- difiuoroethylsulfonyl and the like.
- C ⁇ haloalkylthio denotes an alkylthio radical substituted with one or more halogens. Examples include trifluoromethylthio, 1,1-difluoroethylthio, 2,2,2-trifluoroethylthio and the like.
- halogen or halo denotes to a fluoro, chloro, bromo or iodo group.
- C ⁇ heteroalkylene refers to a C ⁇ alkylene bonded to a heteroatom selected from O, S, S(O), S(0) 2 and NH.
- heteroaryl denotes an aromatic ring system that may be a single ring, two fused rings or three fused rings containing carbons and at least one ring heteroatom selected from O, S and N.
- heteroaryl groups include, but not limited to, pyridyl, benzofuranyl, pyrazinyl, pyridazinyl, pyrimidinyl, triazinyl, quinoline, benzoxazole, benzothiazole, lH-benzimidazole, isoquinoline, quinazoline, quinoxaline, l ⁇ -imidazolyl, [l,2,4]triazolyl, [l,2,3]triazolyl, [l,2,4jtriazolyl, pyrrolyl, pyrazolyl, l ⁇ -pyrazolyl, imidazolyl, oxazolyl, oxazolyl, [l,3,4]oxadiazolyl, [l,3,4]thi
- heterocyclic denotes a non-aromatic carbon ring (i.e., cycloalkyl or cycloalkenyl as defined herein) wherein one, two or three ring carbons are replaced a heteroatom, such as, O, S, N, wherein the N can be optionally substituted with ⁇ , C ⁇ acyl or C ⁇ alkyl, and ring carbon atoms optionally substituted with oxo or a thiooxo thus forming a carbonyl or thiocarbonyl group.
- the heterocyclic group is a 3-, 4-, 5-, 6- or 7-membered containing ring.
- heterocyclic group examples include but not limited to aziridin-1-yl, aziridin-2-yl, azetidin-1-yl, azetidin-2-yl, azetidin-3-yl, piperidin-1-yl, piperidin-4-yl, morpholin-4-yl, piperzin-1-yl, piperzin-4- yl, pyrrolidin-1-yl, pyrrolidin-3-yl, [l,3]-dioxolan-2-yl and the like. Additional examples of heterocyclic groups are shown in Tables 2B, 2C, 2D, 2E, 2F and 2G, infra.
- heterocycliccarboxamido denotes a heterocyclic group with a ring nitrogen where the ring nitrogen is bonded directly to the carbonyl forming an amide. Examples include:
- heterocyclicsulfonyl denotes a heterocyclic group with a ring nitrogen where the ring nitrogen is bonded directly to an S0 2 group forming an sulfonamide. Examples include:
- hydroxyl refers to the group -OH.
- hydroxylamino refers to the group -NHOH.
- nitro refers to the group -N0 2 .
- Q- 7 oxo-cycloalkyl refers to a C 4 - 7 cycloalkyl, as defined herein, wherein one of the ring carbons is replaced with a carbonyl.
- G ⁇ ? oxo- cycloalkyl include but are not limited to: 2-oxo-cyclobutyl, 3-oxo-cyclobutyl, 3-oxo- cyclopentyl, 4-oxo-cyclohexyl, and the like and represented the following structures respectively:
- perfluoroalkyl denotes the group of the formula -C n F 2n+ ⁇ ; stated differently, a perfluoroalkyl is an alkyl as defined herein herein wherein the alkyl is fully substituted with fluorine atoms and is therefore considered a subset of haloalkyl.
- perfluoroalkyls include CF 3 , CF 2 CF 3 , CF 2 CF 2 CF 3 , CF(CF 3 ) 2 , CF 2 CF 2 CF 2 CF 3 , CF 2 CF(CF 3 ) 2 , CF(CF 3 )CF 2 CF 3 and the like.
- phenoxy refers to the group C ⁇ HsO-.
- phenyl refers to the group CgHs-.
- sulfonic acid refers to the group -S0 3 H.
- tetrazolyl refers to the five membered heteroaryl of the following formulae:
- the tetrazolyl group is further substituted at either the 1 or 5 position resepectively.
- thiol denotes the group -SH.
- CODON shall mean a grouping of three nucleotides (or equivalents to nucleotides) which generally comprise a nucleoside (adenosine (A), guanosine (G), cytidine (C), uridine (U) and thymidine (T)) coupled to a phosphate group and which, when translated, encodes an amino acid.
- A adenosine
- G guanosine
- C cytidine
- U uridine
- T thymidine
- COMPOSITION shall mean a material comprising at least two compounds or two components; for example, and not limitation, a Pharmaceutical Composition is a Composition.
- COMPOUND EFFICACY shall mean a. measurement of the ability of a compound to inhibit or stimulate receptor functionality, as opposed to receptor binding affinity.
- CONSTITUTIVELY ACTIVATED RECEPTOR shall mean a receptor subject to constitutive receptor activation.
- CONSTITUTIVE RECEPTOR ACTIVATION shall mean stabilization of a receptor in the active state by means other than binding of the receptor with its endogenous ligand or a chemical equivalent thereof.
- CONTACT or CONTACTING shall mean bringing at least two moieties together, whether in an in vitro system or an in vivo system.
- ENDOGENOUS shall mean a material that a mammal naturally produces.
- ENDOGENOUS in reference to, for example and not limitation, the term "receptor" shall mean that which is naturally produced by a mammal (for example, and not limitation, a human) or a virus.
- the term NON-ENDOGENOUS in this context shall mean that which is not naturally produced by a mammal (for example, and not limitation, a human) or a virus.
- a receptor which is not constitutively active in its endogenous form, but when manipulated becomes constitutively active is most preferably referred to herein as a "non-endogenous, constitutively activated receptor.”
- Both terms can be utilized to describe both "in vivo" and “in vitro" systems.
- the endogenous or non-endogenous receptor may be in reference to an in vitro screening system.
- screening of a candidate compound by means of an in vivo system is viable.
- INDIVIDUAL refers to any animal, including mammals, preferably mice, rats, other rodents, rabbits, dogs, cats, swine, cattle, sheep, horses, or primates, and most preferably humans.
- INHIBIT or INHIBITING in relationship to the term "response” shall mean that a response is decreased or prevented in the presence of a compound as opposed to in the absence of the compound.
- INVERSE AGONISTS shall mean moieties that bind the endogenous form of the receptor or to the constitutively activated form of the receptor, and which inhibit the baseline intracellular response initiated by the active form of the receptor below the normal base level of activity which is observed in the absence of agonists or partial agonists, or decrease GTP binding to membranes.
- the baseline intracellular response is inhibited in the presence of the inverse agonist by at least 30%, more preferably by at least 50%, and most preferably by at least 75%), as compared with the baseline response in the absence of the inverse agonist.
- LIGAND shall mean an endogenous, naturally occurring molecule specific for an endogenous, naturally occurring receptor.
- MODULATE or MODULATING shall mean to refer to an increase or decrease in the amount, quality, response or effect of a particular activity, function or molecule.
- PHARMACEUTICAL COMPOSITION shall mean a composition comprising at least one active ingredient, whereby the composition is amenable to investigation for a specified, efficacious outcome in a mammal (for example, and not limitation, a human).
- a mammal for example, and not limitation, a human.
- One aspect of the present invention pertains to certain 1,2,3-trisubstituted aryl and heteroaryl derivatives as shown in Formula (la):
- One aspect of the present invention encompasses N-oxides of 1,2,3-trisubstituted aryl and heteroaryl derivatives of Formula (la).
- One aspect of the present invention encompasses 1,2,3-trisubstituted aryl and heteroaryl derivatives as shown in Formula (la) wherein W is NR 4 and compounds may be represented by Formula (lb) as shown below:
- P ⁇ is H. In some embodiments, is CH 3 or CH 2 CH 3 .
- One aspect of the present invention encompasses 1,2,3-trisubstituted aryl and heteroaryl derivatives as shown in Formula (la) wherein W is O, (i.e., an oxygen atom) and compounds may be represented by Formula (Ic) as shown below:
- One aspect of the present invention encompasses 1,2,3-trisubstituted aryl and heteroaryl derivatives as shown in Formula (la) wherein W is S, S(O) or S(0) 2 and compounds may be represented by Formulae (Id), (le) and (If) respectively as shown below:
- One aspect of the present invention encompasses 1,2,3-trisubstituted aryl and heteroaryl derivatives as shown in Formula (la) wherein W is absent and compounds may be represented by Formula (Ig) as shown below: dg) wherein each variable in Formula (Ig) has the same meaning as described herein.
- compounds of the present invention are of Formula (Ig) wherein V is absent and accordingly these compounds may be represented by Formula (Ih) as shown below:
- One aspect of the present invention encompasses 1,2,3-trisubstituted aryl and heteroaryl derivatives as shown in Formula (la) wherein W is absent and V is ethynylene.
- Compounds may be represented by Formula (Ii) as shown below:
- V is C ⁇ alkylene optionally substituted with 1 to 4 substituents selected from the group consisting of C ⁇ alkyl, C ⁇ alkoxy and halogen.
- V is a methylene group (i.e., -CH 2 -).
- V is an ethylene group (i.e., -CH 2 CH 2 -).
- V is a methylene and W is an oxygen atom.
- V is methylene and W is a N j group.
- V is methylene and W is a NH group.
- V is ethylene and W is an oxygen atom.
- V is ethylene and W is a R group.
- V is ethylene and W is a NH group.
- V is C ⁇ heteroalkyle ⁇ e optionally substituted with 1 to 4 substituents selected from the group consisting of C ⁇ alkyl, C ⁇ alkoxy and halogen.
- V is -OCH 2 CH 2 -.
- V is -OCH 2 CH 2 - and W is an oxygen atom and may be represented by the formula: -OCH 2 CH 2 0-.
- N is -OCH 2 CH 2 - and W is a ⁇ H group and may be represented by the formula: - OCH 2 CH 2 ⁇ H-.
- V is absent and may be represented by Formula (Ij) as shown below:
- a and B are both methylene wherein A and B are optionally substituted with 1 to 2 methyl groups and therefore form a four-membered nitrogen containing ring.
- compounds of the invention may be represented by Formula (Ik) as shown below:
- A is ethylene and B is methylene wherein A is optionally substituted with 1 to 4 methyl groups and B is optionally substituted with 1 to 2 methyl groups.
- compounds of the invention may be represented by Formula (Im) as shown below:
- D is -CHR 2 -.
- R 2 is C ⁇ alkylsulfonyl.
- A is propylene and B is methylene wherein A is optionally substituted with 1 to 4 methyl groups and B is optionally substituted with 1 to 2 methyl groups.
- compounds of the invention may be represented by Formula (In) as shown below:
- a and B are both ethylene wherein A and B are optionally substituted with 1 to 4 methyl groups.
- compounds of the invention may be represented by Formula (Io) as shown below:
- A is propylene and B is ethylene wherein A and B are optionally substituted with 1 to 4 methyl groups.
- compounds of the invention may be represented by Formula (Ip) as shown below:
- a and B are both propylene wherein A and B are optionally substituted with 1 to 4 methyl groups.
- compounds of the invention may be represented by Formula (Iq) as shown below:
- D is O, S, S(O) or S(0) 2 . In some embodiments, D is S, S(O) or S(0) 2 ; and A and B are independently optionally substituted with 1 or 2 methyl groups. In some embodiments, A and B are ethylene groups. In some embodiments, A and B are ethylene groups substituted with 2 methyl groups and D is an oxygen atom (i.e., forming a 2,6-dimethyl-morpholin-4-yl group).
- D is CR 2 R3.
- R 2 is selected from the group consisting of H, C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ allcylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, amino, carbo-C ⁇ -alkoxy, carboxamide, carboxyl, C 3 . ⁇ cycloalkyl, C ⁇ haloalkoxy, C ⁇ haloalkyl, halogen and hydroxyl.
- R 2 is selected from the group consisting of C(0)CH 3 , C(0)CH 2 CH 3 , C(0)CH 2 CH 2 CH 3 , C(0)CH(CH 3 ) 2 , C(0)CH 2 CH 2 CH 2 CH 3; OC(0)CH 3 , 0C(0)CH 2 CH 3j OC(0)CH 2 CH 2 CH 3 , OCH 3 , OCH 2 CH 3 , OCH 2 CH 2 CH 3 , OCH(CH 3 ) 2 , OCH 2 (CH 2 ) 2 CH 3 , CH 3 , CH 2 CH 3 , CH 2 CH 2 CH 3 , CH(CH 3 ) 2 , CH(CH 3 )(CH 2 CH 3 ), CH 2 (CH 2 ) 2 CH 3 , CH 2 (CH 2 ) 3 CH 3 , C(0)NHCH 3 , C(0)NHCH 2 CH 3 , C(0)NHCH 2 CH 2 CH 3 , C(0)NHCH(CH 3 ) 2 , C(0)NHCH 2 (CH 2 ) 2 CH 3 , C0 2 CH 3
- R 2 is selected from the group consisting of C(0)CH 3 , C(0)CH 2 CH 3 , C(0)CH 2 CH 2 CH 3 , C(0)CH(CH 3 ) 2 , C(0)CH 2 CH 2 CH 2 CH 3 , OC(0)CH 3 , OC(0)CH 2 CH 3 , OC(0)CH 2 CH 2 CH 3 , OCH 3 , OCH 2 CH 3 , OCH 2 CH 2 CH 3 , OCH(CH 3 ) 2 , OCH 2 (CH 2 ) 2 CH 3 , CH 3 , CH 2 CH 3 , CH(CH 3 ) 2 , CH(CH 3 )(CH 2 CH 3 ), CH 2 (CH 2 ) 2 CH 3 , CH 2 (CH 2 ) 3 CH 3 , C(0)NH 2 , C0 2 CH 3 , C0 2 CH 2 CH 3 , C0 2 CH 2 CH 2 CH 3 , C0 2 CH(CH 3 ) 2 , C0 2 CH 2 (CH 2 ) 2 CH 3 , C(0)NH 2 , C0 2 CH
- R 2 is selected from the group consisting of SCH 3 , SCH 2 CH 3 , SCH 2 CH 2 CH 3 , SCH(CH 3 ) 2 , SCH 2 (CH 2 ) 2 CH 3 , S(0)CH 3 , S(0)CH 2 CH 3 , S(0)CH 2 CH 2 CH 3 , S(0)CH(CH 3 ) 2 , S(0)CH 2 (CH 2 ) 2 CH 3 , S(0) 2 CH 3 , S(0) 2 CH 2 CH 3 , S(0) 2 CH 2 CH 2 CH 3 , S(0) 2 CH(CH 3 ) 2 , S(0) 2 CH 2 (CH 2 ) 2 CH 3 , cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, OCF3, OCHF 2 , CF 3 , CHF 2 and F.
- R 2 is selected from the group consisting of S(0) 2 CH 3 , S(0) 2 CH 2 CH 3 , S(0) 2 CH 2 CH 2 CH 3 , S(0) 2 CH(CH 3 ) 2 , S(0) 2 CH 2 (CH 2 ) 2 CH 3 , cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, hydroxyl, and F.
- R 2 is C ⁇ alkyl, or heteroaryl each optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylsulfonyl, carbo-C ⁇ -alkoxy, carboxamide, carboxy, C 3 - 6 -cycloalkyl, Q ⁇ -cycloalkyl- C ⁇ -alkylene, C 3 . 6 -cycloalkyl-C ⁇ - 3 -heteroalkylene, and hydroxyl.
- R 2 is selected from the group consisting of CH 2 OCH 3 , CH 2 CH 2 OCH 3 , CH 2 OCH 2 CH 3 , CH 2 OCH 2 CH 2 CH 3 , CH 2 CH 2 OCH 2 CH 3 , CH 2 CH 2 OCH 2 CH 2 CH 3) CH 2 OCH(CH 3 ) 2 , CH 2 OCH 2 CH(CH 3 ) 2 , CH 2 C0 2 H, CH 2 CH 2 C0 2 H, CH 2 OH, CH 2 CH 2 OH and CH 2 CH 2 CH 2 0H.
- R 2 is C ⁇ - 8 alkyl, heteroaryl or phenyl each optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylamino, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C alkylthioureyl, C ⁇ alkylureyl, amino, carbo-C ⁇ -alkoxy, carboxamide, carboxy, cyano, C 3 _ 6 -cycloalkyl-C ⁇ -heteroalkylene, C 2 - 8 dialkylamino, C 2 .
- R 2 is selected from the group consisting of CH 2 OCH 3 , CH 2 CH 2 OCH 3 , CH 2 OCH 2 CH 3 , CH 2 OCH 2 CH 2 CH 3 , CH 2 CH 2 OCH 2 CH 3 , CH 2 CH 2 OCH 2 CH 2 CH 3 , CH 2 OCH(CH 3 ) 2 , CH 2 OCH 2 CH(CH 3 ) 2 , CH 2 C0 2 H, CH 2 CH 2 C0 2 H, CH 2 OH, CH 2 CH 2 OH and CH 2 CH 2 CH 2 OH.
- R 2 is selected from the group consisting of CH 2 SCH , CH 2 SCH 2 CH 3 , CH 2 SCH 2 CH 2 CH 3 , CH 2 SCH(CH 3 ) 2 , CH 2 SCH 2 (CH 2 ) 2 CH 3 , CH 2 CH 2 SCH 3 , CH 2 CH 2 SCH 2 CH 3 , CH 2 CH 2 SCH(CH 3 ) 2 , CH 2 CH 2 SCH 2 (CH 2 ) 2 CH 3 , CH 2 S(0)CH 3 , CH 2 S(0)CH 2 CH 3 , CH 2 S(0)CH 2 CH 2 CH 3 , CH 2 S(0)CH(CH 3 ) 2 , CH 2 S(0)CH 2 (CH 2 ) 2 CH 3 , CH 2 CH 2 S(0)CH 3 , CH 2 CH 2 S(0)CH 2 CH 3 , CH 2 CH 2 S(0)CH 3 , CH 2 CH 2 S(0)CH 2 CH 3 , CH 2 CH 2 S(0)CH 3 , CH 2 CH 2 S(0)CH 2 CH 3 , CH 2 CH 2 S(0)CH 3 , CH 2 CH 2 S
- R 2 is selected from the group consisting of CH 2 0CH 2 ⁇ cyclopropyl, CH 2 OCH 2 -cyclobutyl, CH 2 OCH 2 -cyclopentyl, CH 2 OCH 2 -cyclohexyl, CH 2 OCH 2 CH 2 -cyclopropyl, CH 2 OCH 2 CH 2 -cyclobutyl, CH 2 OCH 2 CH 2 -cyclopentyl, CH 2 OCH 2 CH 2 -cyclohexyl, CH 2 CH 2 OCH 2 -cyclopropyl, CH 2 CH 2 ⁇ CH 2 -cyclobutyl, CH 2 CH 2 ⁇ CH 2 -cyclopentyl, CH 2 CH 2 OCH 2 -cyclohexyl, CH 2 CH 2 OCH 2 CH 2 -cyclopropyl, CH 2 CH 2 OCH 2 CH 2 -cyclobutyl, CH 2 CH 2 OCH 2 CH 2 -cyclopropyl, CH 2 CH 2 OCH 2 CH 2 -cyclobut
- R 2 is selected from the group consisting of l,2,4-oxadiazol-3- yl, l,2,4-oxadiazol-5-yl, l,3,4-oxadiazol-2-yl, l,2,4-triazol-5-yl and 1,2,4-triazol-l-yl, 3- methyl-l,2,4-oxadiazol-5-yl, 3-methyl-l,2,4-oxadiazol-5-yl, 3-ethyl-l,2,4-oxadiazol-5-yl, 5- ethyl-l,2,4-oxadiazol-3-yl, 5-methyl-l,3,4-oxadiazol-2-yl, 5-ethyl-l,3,4-oxadiazol-2-yl, 3- methyl-l,2,4-triazol-5-yl, 3-ethyl-l,2,4-triazol-5-yl, 3-ethyl-l,2,4-triazol-5-yl, 3-methyl-
- R 2 is selected from the group consisting of l,2,4-oxadiazol-3- yl, l,2,4-oxadiazol-5-yl, l,3,4-oxadiazol-2-yl, 3-methyl-l,2,4-oxadiazol-5-yl, 3 -ethyl- 1,2,4- oxadiazol-5-yl, 3-isopropyl-l,2,4-oxadiazol-5-yl, 3-propyl-l,2,4-oxadiazol-5-yl, 3-t-butyl- l,2,4-oxadiazol-5-yl, and 3-cyclopropyl-l,2,4-oxadiazol-5-yl.
- R 2 is selected from the group consisting of 3-methyl-l,2,4- oxadiazol-5-yl, 3-ethyl-l,2,4-oxadiazol-5-yl, 3-propyl-l,2,4-oxadiazol-5-yl, 3-isopropyl- l,2,4-oxadiazol-5-yl, 3-butyl-l,2,4-oxadiazol-5-yl, and 3-(t-butyl)-l,2,4-oxadiazol-5-yl.
- R 2 is a heteroaryl comprising 5 -atoms in the aromatic ring and are represented by the following formulae:
- a imidazolyl ring can be bonded at one of the ring nitrogens (i.e., imidazol-1-yl group) or at one of the ring carbons (i.e., imidazol-2-yl, imidazol-4-yl or imiadazol-5-yl group).
- R 2 is a 5-membered heteroaryl optionally substituted with 1 to 4 substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylamino, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ alkylthioureyl, C ⁇ alkylureyl, amino, carbo-C ⁇ -alkoxy, carboxamide, carboxy, cyano, C 3 _ 6 -cycloalkyl-C ⁇ - heteroalkylene, C 2 .
- R 2 is a 5 -membered heteroaryl optionally substituted with 1 to 4 substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylamino, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ alkylthioureyl, C ⁇ alkylureyl, amino, carbo-C ⁇ - alkoxy, carboxamide, carboxy, cyano, C 3 - 6 -cycloalkyl-C ⁇ -heteroalkylene, C 2 .
- R 2 is a 5-membered heteroaryl optionally substituted with 1 or 2 substituents selected from the group consisting of C ⁇ alkyl, C ⁇ haloalkyl and halogen; and R 3 is hydrogen.
- R 2 is a 5-membered heteroaryl optionally substituted with lor 2 C ⁇ alkyl substituents; and R 3 is hydrogen.
- R 2 is a 5-membered heteroaryl optionally substituted with CH 3 , CH 2 CH 3 . CH(CH 3 ) 2 , CH 2 CH 2 CH 3 , C(CH 3 ) 3 ; and R 3 is hydrogen.
- R 2 is a fused heteroaryl group containing to aromatic rings wherein at least one is a heteroaryl ring, such as, benzofuranyl, benzimidazole, benzoxazole, benzothiazole, indole, benzothiophenyl.
- R 2 is a benzofuran-2-yl group.
- R 2 is a heterocyclic represented, for example, by the formulae in TABLE 2B.
- any one of the heterocyclic groups shown in TABLES 2B to 2E may be bonded at any available ring carbon or ring nitrogen as allowed by the respective formula.
- a 2,5-dioxo-imidazolidinyl group may be bonded at the ring carbon or at either of the two ring nitrogens to give the following formulae respectively:
- R 2 is a heterocyclic represented, for example, by the formulae in TABLE 2C.
- R 2 is a heterocyclic represented, for example, by the formulae in TABLE 2D.
- R 2 is a heterocyclic represented, for example, by the formulae in TABLE 2E.
- R 2 is a heterocyclic represented, for example, by the formulae in TABLE 2F wherein the C ⁇ alkyl group on the respective ring nitrogen atoms may be the same or different.
- R 2 is a heterocyclic represented, for example, by the formulae in TABLE 2G wherein the C ⁇ alkyl group on the respective ring nitrogen atoms may be the same or different.
- D is CR 2 R 3 and R 2 is -Ar 2 -Ar 3 wherein Ar 2 and Ar 3 are independently aryl or heteroaryl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, amino, carbo- C ⁇ -alkoxy, carboxamide, carboxy, cyano, C 3 .
- substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, amino, carbo- C
- Ar 2 is a heteroaryl comprising 5-atoms in the aromatic ring and are represented by the following formulae:
- a imidazolyl ring can be bonded at one of the ring nitrogens (i.e., imidazol-1-yl group) or at one of the ring carbons (i.e., imidazol-2-yl, imidazol-4-yl or imiadazol-5-yl group) and Ar 3 is bonded to any remaining available ring atom.
- Ar 2 is a heteroaryl and Ar 3 is phenyl.
- the heteroaryl and phenyl are optionally substituted with 1 to 5 substituents selected from the .. group consisting of H, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ haloalkoxy, C ⁇ haloalkyl, halogen, hydroxyl and nitro.
- D is CR 2 R 3 and R 2 is Formula (B):
- R ⁇ 4 is C ⁇ ' alkyl or C 3 - 6 cycloalkyl; and R1 5 is F, Cl, Br or CN. Li some embodiments, R M is C ⁇ alkyl and R 15 is F, Cl or CN.
- D is CR 2 R 3 and R 2 is Formula (C):
- Ar 4 is selected from the group consisting of pyridinyl, pyridazinyl, pyrimidinyl and pyrazinyl. Li some embodiments, Ar is 2-pyridyl.
- compounds of the present invention are represented by Formula (Is) as shown below:
- D is CR 2 R 3
- Ar 4 is phenyl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ alkylthioureyl, C ⁇ alkylureyl, amino, carbo-C ⁇ -alkoxy, carboxamide, carboxy, cyano, C 3 - 6 -cycloalkyl-C 1 - 3 -heteroalkylene, C 2 _ 6 dialkylcarboxamide, C ⁇ dialkylthiocarboxamide, C 2 .
- Ar 4 is phenyl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, carboxamide, C ⁇ haloalkoxy, C ⁇ haloalkyl, C ⁇ haloalkylsulfinyl, C ⁇ haloalkylsulfonyl, C ⁇ haloalkyl, halogen and hydroxyl.
- Ar is phenyl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ haloalkoxy, C ⁇ haloalkyl, C ⁇ haloalkyl, halogen and hydroxyl.
- Ar 4 is heteroaryl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ alkylthioureyl, C ⁇ alkylureyl, amino, carbo-C ⁇ -alkoxy, carboxamide, carboxy, cyano, C ⁇ -cycloalkyl-C ⁇ -heteroalkylene, C 2 -6 dialkylcarboxamide, C ⁇ dialkylthiocarboxamide, C 2 - 6 dialkylsulfonamide, C ⁇ alkylthioureyl, C ⁇ haloalkoxy, - 4 haloalkyl, C ⁇ haloalkylsul
- Ar is heteroaryl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, carboxamide, C ⁇ haloalkoxy, C ⁇ haloalkyl, C ⁇ haloalkylsulfinyl, C ⁇ haloalkylsulfonyl, C ⁇ haloalkyl, halogen and hydroxyl.
- Ar 4 is heteroaryl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ haloalkoxy, C ⁇ haloalkyl, C ⁇ haloalkyl, halogen and hydroxyl.
- Ar 4 is a 5-membered heteroaryl, for example, as shown in TABLE 2A supra.
- Ar is a 6-membered heteroaryl, for example, the 6-membered heteroaryls as shown in TABLE 4:
- Ar is selected from the group consisting of pyridinyl, pyridazinyl, pyrimidinyl and pyrazinyl.
- Ar is 2-pyridyl.
- D is CR 2 R 3
- R 2 is Formula (C)
- G is CR ⁇ 6 R ⁇ 7 and R 16 and R !7 are independently H or C ⁇ alkyl.
- D is CR 2 R 3
- R 2 is Formula (C)
- G is S, S(0) or S(0) .
- Ar 4 is phenyl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ alkylthioureyl, C ⁇ alkylureyl, amino, carbo-C ⁇ -alkoxy, carboxamide, carboxy, cyano, C 3 - 6 -cycloalkyl-C ⁇ -heteroalkylene, C 2 - 6 dialkylcarboxamide, C ⁇ dialkylthiocarboxamide, C 2 .
- substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇
- Ar 4 is phenyl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, carboxamide, C ⁇ haloalkoxy, C ⁇ haloalkyl, C ⁇ haloalkylsulfinyl, C ⁇ haloalkylsulfonyl, C ⁇ haloalkyl, halogen and hydroxyl.
- Ar 4 is phenyl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ haloalkoxy, C ⁇ haloalkyl, C ⁇ haloalkyl, halogen and hydroxyl.
- Ar 4 is heteroaryl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ alkylthioureyl, C ⁇ alkylureyl, amino, carbo-C ⁇ -alkoxy, carboxamide, carboxy, cyano, C ⁇ -cycloalkyl-C ⁇ -heteroalkylene, C 2 .
- substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulf
- dialkylcarboxamide C ⁇ dialkylthiocarboxamide, C 2 - 6 dialkylsulfonamide, C ⁇ alkylthioureyl, C ⁇ haloalkoxy, Ci- 4 haloalkyl, C ⁇ haloalkylsulfinyl, C ⁇ haloalkylsulfonyl, C ⁇ haloalkyl, C ⁇ haloalkylthio, halogen, heteroaryl, hydroxyl, hydroxylamino and nitro.
- Ar 4 is heteroaryl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, carboxamide, C ⁇ haloalkoxy, C ⁇ haloalkyl, C ⁇ haloalkylsulfinyl, C ⁇ haloalkylsulfonyl, C ⁇ haloalkyl, halogen and hydroxyl.
- substituents selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, carboxamide, C ⁇ halo
- Ar 4 is heteroaryl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ haloalkoxy, C ⁇ haloalkyl, C ⁇ haloalkyl, halogen and hydroxyl.
- Ar 4 is a 5-membered heteroaryl, for example, as shown in TABLE 2A, supra.
- Ar 4 is a 6-membered heteroaryl, for example, as shown in TABLE 4, supra.
- Ar 4 is selected from the group consisting of pyridinyl, pyridazinyl, pyrimidinyl and pyrazinyl. Li some embodiments, Ar is 2-pyridyl.
- R 3 is H.
- D is N-R 2 .
- R 2 is selected from the group consisting of H, C ⁇ acyl, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylsulfonyl, carbo- C ⁇ -alkoxy, carboxamide, C 3 . 6 -cycloalkyl and C ⁇ haloalkyl.
- R 2 is selected from the group consisting of C(0)CH 3 , C(0)CH 2 CH 3 , C(0)CH 2 CH 2 CH 3 , C(0)CH(CH 3 ) 2 , C(0)CH 2 CH 2 CH 2 CH 3 , CH 3 , CH 2 CH 3) CH 2 CH 2 CH 3 , CH(CH 3 ) 2 , CH(CH 3 )(CH 2 CH 3 ), CH 2 (CH 2 ) 2 CH 3; CH 2 (CH 2 ) 3 CH 3 , C(0)NHCH 3 , C(0)NHCH 2 CH 3 , C(0)NHCH 2 CH 2 CH 3 , C(0)NHCH(CH 3 ) 2 , C(0)NHCH 2 (CH 2 ) 2 CH 3 , C0 2 CH 3 , C0 2 CH 2 CH 3 , C0 2 CH 2 CH 2 CH 3 , C0 2 CH(CH 3 ) 2 and C0 2 CH 2 (CH 2 ) 2 CH 3 .
- R 2 is selected from the group consisting of S(0) 2 CH 3 , S(0) 2 CH 2 CH 3 , S(0) 2 CH 2 CH 2 CH 3 , S(0) 2 CH(CH 3 ) 2 , S(0) 2 CH 2 (CH 2 ) 2 CH 3 , cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, CH 2 CF 3 , CF 3 and CHF 2 .
- D is N-R 2 and R 2 is H, or carbo-C ⁇ -alkoxy.
- R 2 is selected from the group consisting of C0 2 CH 3 , C0 2 CH 2 CH 3 , C0 2 CH 2 CH 2 CH 3 , C0 2 CH(CH 3 ) 2 and C0 2 CH 2 (CH 2 ) 2 CH 3 .
- R 2 is C ⁇ alkyl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ alkylsulfonyl, carbo-C ⁇ -alkoxy, and carboxy.
- R 2 is CH 2 C0 2 Et,.or , CH 2 CH 2 C0 2 H.
- R 2 is selected from the group consisting of CH 2 CH 2 S(0) 2 CH 3 , CH 2 CH 2 S(0) 2 CH 2 CH 3 , CH 2 CH 2 S(0) 2 CH 2 CH 2 CH 3 , CH 2 CH 2 S(0) 2 CH(CH 3 ) 2 and CH 2 CH 2 S(0) 2 CH 2 (CH 2 ) 2 CH 3 .
- D is N-R 2 wherein R 2 is C ⁇ alkyl, heteroaryl or phenyl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylamino, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ alkylthioureyl, C ⁇ alkylureyl, amino, carbo-C ⁇ -alkoxy, carboxamide, carboxy, cyano, C ⁇ -cycloalkyl-C ⁇ -heteroalkylene, C 2 .
- R 2 is C ⁇ alkyl, heteroaryl or phenyl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy
- dialkylamino C 2 . 6 dialkylcarboxamide, C ⁇ dialkylthiocarboxamide, C 2 - 6 dialkylsulfonamide, C ⁇ allcylthioureyl, C ⁇ haloalkoxy, C ⁇ haloalkyl, C ⁇ haloalkylsulfinyl, C ⁇ haloalkylsulfonyl, C ⁇ haloalkyl, C ⁇ haloalkylthio, halogen, heterocyclic, hydroxyl, hydroxylamino and nitro.
- R 2 is selected from the group consisting of CH 2 CH 2 OCH 3 , CH 2 CH 2 OCH 2 CH 3 , CH 2 CH 2 OCH 2 CH 2 CH 3 , CH 2 C0 2 H, CH 2 CH 2 C0 2 H, CH 2 CH 2 OH and CH 2 CH 2 CH 2 OH.
- R 2 is selected from the group consisting of CH 2 CH 2 SCH 3 , CH 2 CH 2 SCH 2 CH 3 , CH 2 CH 2 SCH 2 CH 2 CH 3 , CH 2 CH 2 SCH(CH 3 ) 2 , CH 2 CH 2 SCH 2 (CH 2 ) 2 CH 3 , CH 2 CH 2 S(0)CH 3 , CH 2 CH 2 S(0)CH 2 CH3, CH 2 CH 2 S(0)CH 2 CH 2 CH 3J CH 2 CH 2 S(0)CH(CH 3 ) 2 , CH 2 CH 2 S(0)CH 2 (CH 2 ) 2 CH 3 , CH 2 CH 2 S(0) 2 CH 3 , CH 2 CH 2 S(0) 2 CH 3 , CH 2 CH 2 S(0) 2 CH 2 CH 3 , CH 2 CH 2 S(0) 2 CH 2 CH 3 , CH 2 CH 2 S(0) 2 CH 2 CH 3 , CH 2 CH 2 S(0) 2 CH(CH 3 ) 2 and CH 2 CH 2 S(0) 2 CH 2 (CH 2 ) 2 CH 3 .
- R 2 is selected from the group consisting of l,2,4-oxadiazol-3-yl, l,2,4-oxadiazol-5-yl, 1,3,4- oxadiazol-2-yl, l,2,4-triazol-5-yl and 1,2,4-triazol-l-yl, 3-methyl-l,2,4-oxadiazol-5-yl, 3- methyl-l,2,4-oxadiazol-5-yl, 3-ethyl-l,2,4-oxadiazol-5-yl, 3-ethyl-l,2,4-oxadiazol-5-yl, 5- methyl-l,3,4-oxadiazol-2-yl, 5-ethyl-l,3,4-oxadiazol-2-yl, 3 -methyl- l,2,4-xriazol-5-yl, 3- ethyl-l,2,4-triazol-5-yl, 3 -methyl- 1,2,4-triazol-l-
- D is N-R 2 and R 2 is -Ar 2 -Ar 3 wherein Ar 2 and Ar 3 are independently aryl or heteroaryl optionally substituted with 1 to 5 substituents selected from the group consisting of H, C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, amino, carbo-C ⁇ -alkoxy, carboxamide, carboxy, cyano, C 3 . 6 -cycloalkyl, C 2 . 6 dialkylcarboxamide, C ⁇ haloalkoxy, C ⁇ haloalkyl, halogen, hydroxyl and nitro.
- Formula (It) as shown below:
- Ar 2 is a heteroaryl and Ar 3 is phenyl.
- the heteroaryl and phenyl are optionally substituted with 1 to 5 substituents selected from the group consisting of H, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ haloalkoxy, C ⁇ haloalkyl, halogen, hydroxyl and nitro.
- D is N-R 2 wherein R 2 is Formula (C):
- D is N-R 2
- Ar 4 is phenyl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ alkylthioureyl, C ⁇ alkylureyl, amino, carbo ⁇ C ⁇ -alkoxy, carboxamide, carboxy, cyano, C ⁇ -cycloalkyl-C ⁇ -heteroalkylene, C 2 .
- Ar 4 is phenyl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, carboxamide, C ⁇ haloalkoxy, C ⁇ haloalkyl, C ⁇ haloalkylsulfinyl, C ⁇ haloalkylsulfonyl, C ⁇ haloalkyl, halogen and hydroxyl.
- Ar is phenyl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ haloalkoxy, C ⁇ haloalkyl, C ⁇ haloalkyl, halogen and hydroxyl.
- Ar is heteroaryl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ aikylthio, C ⁇ alkylthioureyl, C ⁇ alkylureyl, amino, carbo-C ⁇ -alkoxy, carboxamide, carboxy, cyano, C 3 .6-cycloalkyl-C ⁇ -heteroalkylene, C 2 .
- substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfonamide, C ⁇ alky
- Ar 4 is heteroaryl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, carboxamide, C ⁇ haloalkoxy, C ⁇ haloalkyl, C ⁇ haloalkylsulfinyl, C ⁇ haloalkylsulfonyl, C ⁇ haloalkyl, halogen and hydroxyl.
- substituents selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, carboxamide, C ⁇ halo
- Ar 4 is heteroaryl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ haloalkoxy, C ⁇ haloalkyl, C ⁇ haloalkyl, halogen and hydroxyl.
- Ar is a 5-membered heteroaryl, for example, as shown in TABLE 2A, supra.
- Ar 4 is a 6-membered heteroaryl, for example, as shown in TABLE 4, supra.
- Ar is selected from the group consisting of pyridinyl, pyridazinyl, pyrimidinyl and pyrazinyl.
- Ar is 2-pyridyl.
- D is N-R 2 wherein R 2 is Formula (C) and G is CR 16 R ⁇ -
- Ar 4 is phenyl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ alkylthioureyl, C ⁇ alkylureyl, amino, carbo-C ⁇ -alkoxy, carboxamide, carboxy, cyano, C 3 .
- Ar 4 is phenyl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, carboxamide, C ⁇ haloalkoxy, C ⁇ haloalkyl, C ⁇ haloalkylsulfinyl, C ⁇ haloalkylsulfonyl, C ⁇ haloalkyl, halogen and hydroxyl.
- Ar 4 is phenyl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ haloalkoxy, C ⁇ haloalkyl, C ⁇ haloalkyl, halogen and hydroxyl.
- Ar 4 is heteroaryl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ allcylthioureyl, C ⁇ alkylureyl, amino, carbo-C ⁇ -alkoxy, carboxamide, carboxy, cyano, C ⁇ -cycloalkyl-C ⁇ -heteroalkylene, C 2 .
- substituents selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsul
- Ar 4 is heteroaryl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, carboxamide, C ⁇ haloalkoxy, C ⁇ haloalkyl, C ⁇ haloalkylsulfinyl, C ⁇ haloalkylsulfonyl, C ⁇ haloalkyl, halogen and hydroxyl.
- substituents selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, carboxamide, C ⁇ halo
- Ar 4 is heteroaryl optionally substituted with 1 to 5 substituents selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ haloalkoxy, C ⁇ haloalkyl, C ⁇ haloalkyl, halogen and hydroxyl.
- Ar 4 is a 5-membered heteroaryl, for example, as shown in TABLE 2A, supra.
- Ar 4 is a 6-membered heteroaryl, for example, as shown in TABLE 4, supra.
- Ar 4 is selected from the group consisting of pyridinyl, pyridazinyl, pyrimidinyl and pyrazinyl.
- Ar is 2-pyridyl.
- D is N-R 2 wherein R 2 is Formula (C), G is CR ⁇ 6 R ⁇ 7 and R ⁇ 6 and R i7 are independently H or C ⁇ alkyl.
- Z is selected from the group consisting of C ⁇ acyl, C ⁇ alkyl, C ⁇ alkylcarboxamide, amino, cyano, Q-s diacylamino, C ⁇ dialkylsulfonamide, formyl, halogen, heterocyclic, and nitro wherein C ⁇ alkyl and C ⁇ acyl are each optionally substituted with 1, or 2 groups selected from the group consisting of C 2 . 4 dialkylmino, hydroxy, and halogen.
- Z is selected from the group consisting of nitro, amino, formyl, NHC(0)CF 3 , Br, NHC(0)CH 3 , N(C(0)CH 3 ) 2 , N(S(0) 2 CH 3 ) 2 , CH 3 , [l,3]dioxolan-2- yl, CH 2 OH, CH 2 N(CH 3 ) 2 , and C(0)CH 3 .
- Z is selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, C ⁇ alkylthioureyl, C ⁇ alkylureyl, carboxamide, carboxy, cyano, aryl, C ⁇ haloalkyl, C ⁇ haloalkylcarboxamide, heteroaryl, hydroxyl, hydroxylamino, nitro and tetrazolyl, wherein C ⁇ alkyl is optionally substituted with 1, 2, 3 or 4 groups selected from the group consisting of C ⁇ acyl, C ⁇ acyloxy, C ⁇ alkoxy, C ⁇ alkylcarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsul
- Z is selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C ⁇ alkylthiocarboxamide, C ⁇ alkylthioureyl, C ⁇ alkylureyl, carboxamide, carboxy, cyano, aryl, C ⁇ haloalkyl, C ⁇ haloalkylcarboxamide, heteroaryl, hydroxyl, hydroxylamino, nitro and tetrazolyl.
- Z is a heterocyclic group.
- Z is a 5- membered heterocyclic group containing two oxygen atoms.
- Z is an alkyl group optionally substituted C 2 . 4 -dialkylamino or hydroxy.
- Z is selected from the group consisting of formyl, NHC(0)CH 3 , NHC(0)CH 2 CH 3 , NHC(0)CH(CH 3 ) 2 , CH 3 , CH 2 CH 3 , CH(CH 3 ) 2 , CH 2 CH 2 CH 2 CH 3 , NHC(0)CF 3 , carboxy, CF 3 , CF 2 CF 3 , nitro and lH-tetrazol-5-yl.
- Z is selected from the group consisting of carboxy, CF 3 , nitro and lH-tetrazol-5-yl.
- Z is [l,3]-dioxolan-2-yl.
- Z is a formyl group.
- Z is a carboxy group.
- Z is a -C ⁇ 2 O ⁇ group.
- Z is a -CH 2 N(CH 3 ) 2 group.
- Z is Formula (A):
- R 7 is H, C ⁇ - 8 alkyl or C 3 - 6 cycloalkyl; and R 8 is H, nitro or nitrile. Li some embodiments, R 7 is H or C ⁇ alkyl.
- Ri is selected from the group consisting of H, C ⁇ alkoxy, C ⁇ alkyl, C 2 - 6 alkynyl, amino, C 3 . 6 cycloalkyl and C ⁇ haloalkyl. Li some embodiments, Ri is H or amino.
- Ri is selected from the group consisting of H, C ⁇ alkyl, and amino.
- Ari is aryl optionally substituted with R 9 -R ]3 .
- Ari is phenyl.
- ⁇ is heteroaryl.
- Ari is heteroaryl optionally substituted with R 9 -R ⁇ 3 .
- Ari is a heteroaryl selected from TABLE 2A.
- Ari is a heteroaryl selected from TABLE 4 or an N-oxide thereof.
- compounds of the invention are of Formula (Iv):
- Ari is heteroaryl and R 9 is selected from the group consisting of H, C ⁇ acyl, C alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C 2 . 6 alkynyl, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, carboxamide and sulfonamide.
- R 9 is selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C 2 - 6 alkynyl, C ⁇ alkylsulfonamide, C 2 . 6 dialkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, amino, arylsulfonyl, C 2 . 6 dialkylamino, C ⁇ dialkylsulfonamide, and carboxamide.
- R 9 is selected from the group consisting of C(0)CH 3 , C(0)CH 2 CH 3 , C(0)CH 2 CH 2 CH 3 , C(0)CH(CH 3 ) 2 , C(0)CH 2 CH 2 CH 2 CH 3 , OCH 3 , OCH 2 CH 3 , OCH 2 CH 2 CH 3 , OCH(CH 3 ) 2 , OCH 2 CH 2 CH 2 CH 3 , CH 3 , CH 2 CH 3 , CH 2 CH 2 CH 3 , CH(CH 3 ) 2 , CH(CH 3 )(CH 2 CH 3 ), CH 2 (CH 2 ) 2 CH 3 , CH 2 (CH 2 ) 3 CH 3 , CH 2 (CH 2 ) 4 CH 3 , CH 2 (CH 2 ) 5 CH 3 , C(0)NHCH 3 , C(0)NHCH 2 CH 3 , C(0)NHCH 2 CH 2 CH 3 , C(0)NHCH(CH 3 ) 2 , C ⁇ CH, S(0) 2 NHCH 3 , S(0) 2 NHCH 2 CH 3 ,
- R 9 is selected from the group consisting of cyano, C 3 . 6 cycloalkyl, halogen, C ⁇ haloalkoxy, C ⁇ haloalkyl, C ⁇ haloalkylsulfonyl, and C ⁇ haloalkylthio.
- R 9 is selected from the group consisting of cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, Cl, F, Br, OCF 3 , OCHF 2 , OCH 2 CF 3 , CF 3 , CHF 2 , CH 2 CF 3 , SCF 3 , SCHF 2 and SCH 2 CF 3 .
- R 9 is selected from the group consisting of CN, C0 2 Me, C0 2 Et, S(0) 2 CH 3 , S(0) 2 CF 3 , N(CH 3 ) 2 , N(Et) 2 , C(0)NHCH 3 , C(0)NHEt, C(0)N(CH 3 ) 2 , OH, OCH 3 , and OEt.
- R 9 is selected from the group consisting of heterocyclic, heterocyclicsulfonyl, heteroaryl, hydroxy, C 4 - 7 oxo-cycloalkyl, phenoxy and phenyl.
- R 9 is selected from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C 2 . 6 alkynyl, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, carboxamide, halogen "and sulfonamide.
- R 9 is selected from the group consisting of C(0)CH 3 , C(0)CH 2 CH 3 , C(0)CH 2 CH 2 CH 3 , C(0)CH(CH 3 ) 2 , C(0)CH 2 CH 2 CH 2 CH 3 , OCH 3 , OCH 2 CH 3 , OCH 2 CH 2 CH 3 , OCH(CH 3 ) 2 , OCH 2 CH 2 CH 2 CH 3 , CH 3 , CH 2 CH 3 , CH 2 CH 2 CH 3 , CH(CH 3 ) 2 , CH(CH 3 )(CH 2 CH 3 ), CH 2 (CH 2 ) 2 CH 3 , CH 2 (CH 2 ) 3 CH 3 , CH 2 (CH 2 ) 4 CH 3 , CH 2 (CH 2 ) 5 CH 3; C(0)NHCH 3 , C(0)NHCH 2 CH 3 , C(0)NHCH 2 CH 2 CH 3 , C(0)NHCH(CH 3 ) 2 , C(0)NHCH 2 (CH 2 ) 2 CH 3 , CCH, S(0) 2 NHCH 3
- R 9 is selected from the group consisting of amino, arylsulfonyl, carboxy, cyano, C 3 . 6 cycloalkyl, halogen, C ⁇ haloalkoxy, C ⁇ haloalkyl and C ⁇ haloalkylthio.
- R 9 is selected from the group consisting of phenylsulfonyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, Cl, F, Br, OCF 3 , OCHF 2 , OCH 2 CF 3 , CF 3 , CHF 2 , CH 2 CF 3 , SCF 3 , SCHF 2 and SCH 2 CF 3 .
- R 9 is selected from the group consisting of heterocyclic, heteroaryl, C 4 - 7 oxo-cycloalkyl, phenoxy and phenyl.
- R 9 is selected from the group consisting of morpholin-4-yl, thiomorpholin-4-yl, l-oxo-l ⁇ 4 -thiomorpholin-4- yl, l,l-Dioxo-l ⁇ 6 -thiomorpholin-4-yl, piperazin-1-yl, 4-methyl-piperazin-l-yl, 4-ethyl- piperazin-1-yl, 4-propyl-piperazin-l-yl, piperidin-1-yl, pyrrolidin-1-yl, 2,5-dioxo- imidazolidin-4-yl, 2,4-dioxo-thiazolidin-5-yl, 4-oxo-2-thioxo-thiazolidin-5-yl, 3-methyl-2,
- R 9 is selected from the group consisting of lH-imidazol-4-yl, [l,2,4]triazol-l-yl, [l,2,3]triazol-l-yl, [l,2,4]triazol-4-yl, pyrrol- 1-yl, pyrazol-1-yl, 1H- pyrazol-3-yl, imidazol-1-yl, oxazol-5-yl, oxazol-2-yl, [l,3,4]oxadiazol-2-yl, [l,3,4]thiadiazol- 2-yl, [l,2,4]oxadiazol-3-yl, [l,2,4]thiadiazol-3-yl, tetrazol-1-yl, pyrimidin-5-yl, pyrimidin-2- yl, pyrimidin-4-yl, pyridazin-3-yl, pyridazin-4-yl, pyrazin-2-yl, pyr
- R 9 is C ⁇ - 8 alkyl or C ⁇ alkoxy optionally substituted with 1 to 5 substituents selected independently from the group consisting of C ⁇ alkoxy, C ⁇ alkylcarboxamide, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, carbo-C ⁇ -alkoxy, carboxamide, carboxy, cyano, heterocyclic, hydroxyl and phenyl.
- R 9 is C ⁇ alkylsulfonyl optionally substituted with 1 to 5 substituents selected independently from the group consisting of C ⁇ alkoxy, carboxamide, heteroaryl, heterocyclic and phenyl.
- R 9 is C ⁇ alkylsulfonyl substituted with the heteroaryl group.
- the heteroaryl is selected from the group consisting of lH-imidazol-4- yl, [l,2,4jtriazol-l-yl, [l,2,3]triazol-l-yl, [l,2,4]triazol-4-yl, pyrrol-1-yl, pyrazol-1-yl, 1H- pyrazol-3-yl, imidazol-1-yl, oxazol-5-yl, oxazol-2-yl, [l,3,4]oxadiazol-2-yl, * [l,3,4]thiadiazol- 2-yl, [l,2,4]oxadiazol-3-yl, [l,2,4]thiadiazol-3-yl, tetrazol-1-yl, pyrimidin-5-yl, pyrimidin-2- yl, pyrimidin-4-
- R 9 is arylsulfonyl, heteroaryl, phenoxy or phenyl optionally substituted with 1 to 5 substituents selected independently from the group consisting of C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, C ⁇ alkylthio, carboxamide, carboxy, cyano, halogen, C ⁇ haloalkoxy, C ⁇ haloalkyl, C ⁇ haloalkylthio and hydroxyl.
- R 9 is arylsulfonyl, heteroaryl, phenoxy or phenyl each optionally substituted with 1 to 5 substituents selected independently from the group consisting of C ⁇ alkoxy, C ⁇ alkyl, cyano, halogen, C ⁇ haloalkoxy, C ⁇ haloalkyl and hydroxyl.
- R 9 is a heterocyclic group as described herein.
- R 9 is a heterocyclic group represented by the formulae shown in Table 2B, supra. Li some embodiments, R 9 is a heterocyclic group represented by the formulae shown in Table 2C, supra. Li some embodiments, R 9 is a heterocyclic group represented by the formulae shown in Table 2D, supra. i some embodiments, R 9 is a heterocyclic group represented by the formulae shown in Table 2E, supra. Li some embodiments, R 9 is a heterocyclic group represented by the formulae shown in Table 2F, supra. Li some embodiments, R 9 is a heterocyclic group represented by the formulae shown in Table 2G, supra.
- R 9 is of Formula (D):
- R 18 is H, C ⁇ acyl, C 2 - 6 alkenyl, C ⁇ alkyl, C ⁇ alkylcarboxamide, C 2 - 6 alkynyl, C ⁇ alkylsulfonamide, carbo-C ⁇ -alkoxy, carboxamide, carboxy, cyano, C 3 . 6 cycloalkyl, C 2 . 6 dialkylcarboxamide, halogen, heteroaryl or phenyl, and wherein the heteroaryl or phenyl may be optionally substituted with 1 to 5 substituents selected independently from the group consisting of C ⁇ alkoxy, amino, C ⁇ alkylamino, C 2 . 6 alkynyl, C 2 . 8 dialkylamino, halogen, C ⁇ haloalkoxy, C ⁇ haloalkyl and hydroxyl.
- R 9 is of Formula (D) wherein "p" and “r” are independently 0, or 1; and R ⁇ 8 is H, carbo-C ⁇ -alkoxy, heteroaryl or phenyl, and wherein the heteroaryl and phenyl are each optionally substituted with 1 to 5 substituents selected independently from the group consisting of C ⁇ alkoxy, amino, C ⁇ alkylamino, C 2 - 6 alkynyl, C 2 . 8 dialkylamino, halogen, C ⁇ haloalkoxy, C ⁇ haloalkyl and hydroxyl.
- R 18 is phenyl optionally substituted with 1 to 5 substituents selected independently from the group consisting of C ⁇ alkoxy, amino, C ⁇ alkylamino, C 2 - ⁇ alkynyl, C 2 - 8 dialkylamino, halogen, C ⁇ haloalkoxy, C ⁇ haloalkyl and hydroxyl.
- R 18 is carbo-C ⁇ -alkoxy or carboxy.
- R 18 is heteroaryl or phenyl optionally substituted with 1 to 5 substituents selected independently from the group consisting of C ⁇ alkoxy, amino, C ⁇ alkylamino, C 2 . 6 alkynyl, C 2 . 8 dialkylamino, halogen, C ⁇ haloalkoxy, C ⁇ haloalkyl and hydroxyl.
- the heteroaryl is selected from the group consisting of 1H- imidazol-4-yl, [l,2,4]triazol-l-yl, [l,2,3]triazol-l-yl, [l,2,4]triazol-4-yl, pyrrol-1-yl, pyrazol- 1-yl, lH-pyrazol-3-yl, imidazol-1-yl, oxazol-5-yl, oxazol-2-yl, [l,3,4]oxadiazol-2-yl, [l,3,4]thiadiazol-2-yl, [l,2,4]oxadiazol-3-yl, [l,2,4]thiadiazol-3-yl, tetrazol-1-yl, pyrimidin-5- yl, pyrimidin-2-yl, pyrimidin-4-yl, pyridazin-3-yl, pyridazin-4-yl, pyrazin-2-
- R 18 is H, C ⁇ acyl or C ⁇ alkyl.
- R 10 -R 13 are independently C ⁇ acyl, C ⁇ alkoxy, C ⁇ alkyl, Ci- 4 alkylcarboxamide, C ⁇ alkylureyl, carbo-C ⁇ -alkoxy, carboxamide, carboxy, cyano, C 3 . 6 cycloalkyl, halogen, C ⁇ haloalkoxy and C ⁇ haloalkyl.
- one or two R 10 -R1 3 groups are independently halogen. In some embodiments, one R10-R 1 3 group is a halogen.
- Ari is phenyl and R 9 is substituted at the para position on the phenyl.
- Ari is phenyl optionally substituted with R 9 , Rio and R u .
- the compounds of the invention are of Formula (Iw):
- R 9 is cyano, carbo-C ⁇ -alkoxy, carboxy, C 2 . 6 dialkylamino, C ⁇ alkylcarboxamide, C ⁇ dialkylsulfonamide, C ⁇ alkylsulfonyl, hydroxyl, C ⁇ alkoxy, 5-membered heteroaryl, 6- membered heteroaryl, or heterocyclic, wherein the 6-membered heteoaryl is optionally an N- oxide; and Rio and R ⁇ are independently H or halogen.
- R 9 is cyano, carbomethoxy, carboethoxy, carboisopropoxy, carboxy, dimethylamino, diethylamino, methylethylamino, C(0)NHCH 3 , C(0)NHCH 2 CH 3 , C(0)NH(CH 3 ) 2 , S(0) 2 CH 3 , S(0) 2 CH 2 CH 3 , hydroxyl, OCH 3 , [l,2,4]triazol-4-yl, thiazol-2-yl, 3H-imidazol-4-yl, 1H- imidazol-2-yl, lH-imidazol-4-yl, pyridin-2-yl, pyridin-3-yl, pyridin-4-yl, l-oxy-pyridin-2-yl, l-oxy-pyridin-3-yl, l-oxy-pyridin-4-yl, or 2-oxo-oxazolidin-4-yl.
- Ari is phenyl and two adjacent R ⁇ 0 -R ⁇ groups form a 5, 6 or 7 membered cycloalkyl, cycloalkenyl or heterocyclic group with the phenyl group wherein the 5, 6 or 7 membered group is optionally substituted with halogen.
- Ari is phenyl and two adjacent R1 0 -R 11 groups form a 5, 6 or 7 membered cycloalkyl group with the phenyl group and is of the formulae shown below:
- a is 1, 2 or 3 to give a 5, 6 or 7 membered cycloalkyl fused together with the phenyl group where two of the ring carbons are shared between the cycloalkyl and phenyl group.
- the cycloalkyl is optionally substituted with halogen.
- the halogen is fluorine.
- Ari is phenyl and two adjacent R1 0 -R 11 groups form a 5, 6 or 7 membered cycloalkenyl group with the phenyl group and is of the formulae shown in TABLE 5 and has at least one carbon-carbon ring double bond present that is not part of the phenyl group (i.e., cycloalkenyl), for example, lH-Lidenyl and dihydro-naphthyl.
- the cycloalkenyl is optionally substituted with halogen.
- the halogen is fluorine.
- Ari is phenyl and two adjacent R 10 -R ⁇ 1 groups form a 5, 6 or 7 heterocyclic group with the phenyl group and is of the formulae in TABLE 5 wherein one or more ring cycloalkyl carbons are replaced by a O, S, S(O), S(0) 2 , N ⁇ or N-C ⁇ -alkyl group.
- the heterocyclic group is optionally substituted with halogen.
- the halogen is fluorine.
- Ari is phenyl and two adjacent R1 0 -R. 1 groups form a 5 membered heterocyclic group with the phenyl group.
- the 5 membered heterocyclic group with the phenyl group together form a 2,3-dihydro-benzofuran-5-yl or benzo[l,3]dioxol-5-yl group.
- the two adjacent groups form a 6 membered heterocyclic group with the phenyl group.
- the 6 membered heterocyclic group with the phenyl group together form a 2,3-dihydro-benzo[l,4]dioxin-6-yl or 2,3-dihydro-benzo[l,4]dioxin-2-yl group.
- the two adjacent groups form a 7 membered heterocyclic group with the phenyl group.
- the 7 membered heterocyclic group with the phenyl group together form a 3,4-dihydro-2H- benzo[b][l,4]dioxepin-7-yl group.
- Ari is heteroaryl and two adjacent R10-R.1 groups form a 5, 6 or 7 membered cycloalkyl, cycloalkenyl or heterocyclic group with the heteroaryl group wherein the 5, 6 or 7 membered group is optionally substituted with halogen.
- Ari is a heteroaryl selected from TABLE 2A.
- Arj is a heteroaryl selected from TABLE 4.
- the two adjacent groups form a 5 membered heterocyclic group with the heteroaryl group.
- the two adjacent groups form a 6 membered heterocyclic group with the heteroaryl group.
- the two adjacent groups form a 7 membered heterocyclic group with the heteroaryl group.
- R 5 is H, C ⁇ acyl, C ⁇ acyloxy, C 2 - 6 alkenyl, C ⁇ alkoxy, C ⁇ alkyl, C ⁇ alkylcarboxamide, C 2 . 6 alkynyl, C ⁇ alkylsulfonamide, C ⁇ alkylsulfinyl, C ⁇ alkylsulfonyl, Ci- 4 alkylthio, C ⁇ alkylureyl, amino, arylsulfonyl, carbo-C ⁇ -alkoxy, carboxamide, carboxy, cyano, C 3 . 6 cycloalkyl, C 2 .
- R 5 and e are independently H or F.
- X is N and Y is CH.
- X is N and Y is CF.
- X is CH and Y is N.
- Li some embodiments, X and Y are N.
- X and Y are CH.
- X is CH and Y is CF.
- Some embodiments of the present invention include compounds illustrated in TABLES A, B, C, D and E; these TABLES are shown below. TABLE A
- A128 4-[4-(2-Methoxy-ethyl)- piperidin-1 -yl]-6-(2-methyl-5- trifluoromethyl-2H- ⁇ yrazol-3 - yloxy)-5-nitro-pyrimidine
- Some embodiments of the present invention include a pharmaceutical composition comprising at least one compound according to any of the compound embodiments disclosed herein and a pharmaceutically acceptable carrier.
- compounds of Formula (la) encompass all pharmaceutically acceptable solvates, particularly hydrates, thereof.
- the present invention also encompasses diastereomers as well as optical isomers, e.g. mixtures of enantiomers including racemic mixtures, as well as individual enantiomers and diastereomers, which arise as a consequence of structural asymmetry in certain compounds of Formula (la). Separation of the individual isomers or selective synthesis of the individual isomers is accomplished by application of various methods which are well known to practitioners in the art.
- compounds of the invention are useful in the prophylaxis or treatment of additional diseases. Without limitation, these include the following.
- Type II diabetes The most significant pathologies in Type II diabetes are impaired insulin signaling at its target tissues ("insulin resistance") and failure of the insulin-producing cells of the pancreas to secrete an appropriate degree of insulin in response to a hyperglycemic signal.
- Current therapies to treat the latter include inhibitors of the ⁇ -cell ATP-sensitive potassium channel to trigger the release of endogenous insulin stores, or administration of exogenous insulin. Neither of these achieves accurate normalization of blood glucose levels and both carry the risk of inducing hypoglycemia. For these reasons, there has been intense interest in the development of pharmaceuticals that function in a glucose-dependent action, i.e. potentiators of glucose signaling.
- Physiological signaling systems which function in this manner are well-characterized and include the gut peptides GLP1, GIP and PACAP. These hormones act via their cognate G-protein coupled receptor to stimulate the production of cAMP in pancreatic ⁇ -cells. The increased cAMP does not appear to result in stimulation of insulin release during the fasting or preprandial state.
- a series of biochemical targets of cAMP signaling including the ATP-sensitive potassium channel, voltage-sensitive potassium channels and the exocytotic machinery, are modified in such a way that the insulin secretory response to a postprandial glucose stimulus is markedly enhanced.
- agonists of novel, similarly functioning, ⁇ -cell GPCRs, including RUP3 would also stimulate the release of endogenous insulin and consequently promote normoglycemia in Type II diabetes.
- cAMP for example as a result of GLP1 stimulation, promotes ⁇ -cell proliferation, inhibits ⁇ -cell death and thus improves islet mass.
- This positive effect on ⁇ -cell mass is expected to be beneficial in both Type II diabetes, where insufficient insulin is produced, and Type I diabetes, where ⁇ -cells are destroyed by an inappropriate autoimmune response.
- metabolic diseases exert a negative influence on other physiological systems. Thus, there is often the codevelopment of multiple disease states (e.g.
- Some embodiments of the present invention include a method for prophylaxis or treatment of a metabolic disorder or complications thereof in an individual comprising administering to the individual a therapeutically effective amount of a compound of the present invention or a pharmaceutical composition thereof.
- the metabolic disorder or complications thereof is type I, type II diabetes, inadequate glucose tolerance, insulin resistance, hyperglycemia, hyperlipidemia, hypertriglyceridemia, hypercholesterolemia, dyslipidemia syndrome X, or metabolic syndrome.
- the metabolic disorder is type II diabetes.
- the metabolic disorder is hyperglycemia.
- the metabolic disorder is hyperlipidemia.
- the metabolic disorder is hypertriglyceridemia.
- ' the metabolic disorder is type I diabetes.
- the metabolic disorder is dyslipidemia.
- the metabolic disorder is syndrome X.
- the individual is a mammal. In some embodiments, the mammal is a human.
- Some embodiments of the present invention include a method of controlling or decreasing weight gain of an individual comprising administering to the individual a therapeutically effective amount of a compound of the present invention or pharmaceutical composition thereof.
- the individual is a mammal.
- the mammal is a human.
- the human has a body mass index of about 18.5 to about 45. In some embodiments, the human has a body mass index of about 25 to about 45. In some embodiments, the human has a body mass index of about 30 to about 45. In some embodiments, the human has a body mass index of about 35 to about 45.
- One aspect of the present invention pertains to a compound of Formula (la), as described herein, for use in a method of treatment of the human or animal body by therapy.
- Some embodiments of the present invention include a method of modulating a RUP3 receptor comprising contacting the receptor with a compound of the present invention.
- Some embodiments of the present invention include a method of modulating a RUP3 receptor in an individual comprising contacting the receptor with a compound of the present , invention.
- the compound is an agonist.
- the compound is an inverse agonist.
- Some embodiments of the present invention include a method of modulating a RUP3 receptor in an individual comprising contacting the receptor with a compound of the present invention wherein the modulation of the RUP3 receptor is prophylaxis or treatment of a metabolic disorder and complications thereof.
- the metabolic disorder is type I, type ⁇ diabetes, inadequate glucose tolerance, insulin resistance, hyperglycemia, hyperlipidemia, hypertriglyceridemia, hypercholesterolemia, dyslipidemia syndrome X, or metabolic syndrome.
- the metabolic disorder is type II diabetes. In some embodiments, the metabolic disorder is hyperglycemia. In some embodiments, the metabolic disorder is hyperlipidemia. In some embodiments, the metabolic disorder is hypertriglyceridemia. In some embodiments, the metabolic disorder is type I diabetes. In some embodiments, the metabolic disorder is dyslipidemia. ⁇ n some embodiments, the metabolic disorder is syndrome X. In some embodiments, the individual is a mammal. In some embodiments, the mammal is a human.
- Some embodiments of the present invention include a method of modulating a RUP3 receptor in an individual comprising contacting the receptor with a compound of the present invention wherein the modulation of the RUP3 receptor controls or reduces weight gain o the , individual.
- the individual is a mammal.
- the mammal is a human.
- the human has a body mass index of about 18.5 to about 45. In some embodiments, the human has a body mass index of about 25 to about 45. In some embodiments, the human has a body mass index of about 30 to about 45. In some embodiments, the human has a body mass index of about 35 to about 45.
- Some embodiments of the present invention include the use of a compound of the present invention for production of a medicament for use in prophylaxis or treatment of a metabolic disorder.
- the metabolic disorder is type ⁇ diabetes, inadequate glucose tolerance, insulin resistance, hyperglycemia, hyperlipidemia, hypertriglyceridemia, hypercholesterolemia, dyslipidemia syndrome X, or metabolic syndrome.
- Some embodiments of the present invention include the use of a compound of the present invention for production of a medicament for use in controlling or decreasing weight gain in an individual.
- the individual is a mammal.
- the mammal is a human.
- the human has a body mass index of about 18.5 to about 45. In some embodiments, the human has a body mass index of about 25 to about 45. In some embodiments, the human has a body mass index of about 30 to about 45. In some embodiments, the human has a body mass index of about 35 to about 45.
- Compounds of the present invention are identified as an agonist or an inverse agonist using methods known to those skilled in art, such as an assay as described in Example 1. Accordingly, representative examples of compounds of the present invention that are agonists include the following:
- Representative examples of compounds of the present invention that are inverse agonists include the following:
- Some embodiments of the present invention include a method of producing a pharmaceutical composition comprising admixing at least one compound according to any of the compound embodiments disclosed herein and a pharmaceutically acceptable carrier.
- a compound of the present invention can be formulated into pharmaceutical compositions using techniques well known to those in the art. Suitable pharmaceutically- acceptable carriers, outside those mentioned herein, are available to those in the art; for example, see Remington's Pharmaceutical Sciences, 16 th Edition, 1980, Mack Publishing Co., (Oslo et al., eds.) and the most current version.
- a compound of the invention may in an alternative use be administered as a raw or pure chemical, it is preferable however to present the compound or active ingredient as a pharmaceutical formulation or composition further comprising a pharmaceutically acceptable carrier.
- the invention thus further provides pharmaceutical formulations comprising a compound of the invention or a pharmaceutically acceptable salt or derivative thereof together with one or more pharmaceutically acceptable carriers therefor and/or prophylactic ingredients.
- the carrier(s) must be acceptable" in the sense of being compatible with the other ingredients of the formulation and not overly deleterious to the recipient thereof.
- compositions include those suitable for oral, rectal, nasal, topical (including buccal and sub-lingual), vaginal or parenteral (including intramuscular, subcutaneous and intravenous) administration or in a form suitable for administration by inhalation or insufflation.
- the compounds of the invention may thus be placed into the form of pharmaceutical formulations and unit dosages thereof, and in such form may be employed as solids, such as tablets or filled capsules, or liquids such as solutions, suspensions, emulsions, elixirs, gels or capsules filled with the same, all for oral use, in the form of suppositories for rectal administration; or in the form of sterile injectable solutions for parenteral (including subcutaneous) use.
- Such pharmaceutical compositions and unit dosage forms thereof may comprise conventional ingredients in conventional proportions, with or without additional active compounds or principles, and such ⁇ 4
- unit dosage forms may contain any suitable effective amount of the active ingredient commensurate with the intended daily dosage range to be employed.
- the pharmaceutical composition may be in the form of, for example, a tablet, capsule, suspension or liquid.
- the pharmaceutical composition is preferably made in the form of a dosage unit containing a particular amount of the active ingredient.
- dosage units are capsules, tablets, powders, granules or a suspension, with conventional additives such as lactose, mannitol, corn starch or potato starch; with binders such as crystalline cellulose, cellulose derivatives, acacia, corn starch or gelatins; with disintegrators such as corn starch, potato starch or sodium carboxymethyl- cellulose; and with lubricants such as talc or magnesium stearate.
- the active ingredient may also be administered by injection as a composition wherein, for example, saline, dextrose or water may be used as a suitable pharmaceutically acceptable carrier.
- the dose when using the compounds of Formula (la) can vary within wide limits, and as is customary and is known to the physician, it is to be tailored to the individual conditions in each individual case. It depends, for example, on the nature and severity of the illness to be treated, on the condition of the patient, on the compound employed or on whether an acute or chronic disease state is treated or prophylaxis is conducted or on whether further active compounds are administered in addition to the compounds of the Formula (la).
- Representative doses of the present invention include, about 0.01 mg to about 1000 mg, about 0.01 to about 750 mg, about 0.01 to about 500 mg, 0.01 to about 250 mg, 0.01 mg to about 200 mg, about 0.01 mg to 150 mg, about 0.01 mg to about 100 mg, and about 0.01 mg to about 75 mg.
- Multiple doses may be administered during the day, especially when relatively large amounts are deemed to be needed, for example 2, 3 or 4, doses. If appropriate, depending on individual behavior and as appropriate from the patients physician or care-giver it may be necessary to deviate upward or downward from the daily dose.
- the amount of active ingredient, or an active salt or derivative thereof, required for use in treatment will vary not only with the particular salt selected but also with the route of administration, the nature of the condition being treated and the age and condition of the patient and will ultimately be at the discretion of the attendant physician or clinician.
- animal models include, but are not limited to, the rodents diabetes models as described in Example 6, infra (other animal models have been reported by Reed and Scribner in Diabetes, Obesity and Metabolism, 1, 1999, 75-86).
- these extrapolations may merely be based on the weight of the animal model in comparison to another, such as a mammal, preferably a human, however, more often, these extrapolations are not simply based on weights, but rather incorporate a variety of factors. Representative factors include the type, age, weight, sex, diet and medical condition of the patient, the severity of the disease, the route of administration, pharmacological considerations such as the activity, efficacy, pharmacokinetic and toxicology profiles of the particular compound employed, whether a drug delivery system is utilized, on whether an acute or chronic disease state is being treated or prophylaxis is conducted or on whether further active compounds are administered in addition to the compounds of the Formula (la) and as part of a drug combination.
- factors include the type, age, weight, sex, diet and medical condition of the patient, the severity of the disease, the route of administration, pharmacological considerations such as the activity, efficacy, pharmacokinetic and toxicology profiles of the particular compound employed, whether a drug delivery system is utilized, on
- the dosage regimen for treating a disease condition with the compounds and/or compositions of this invention is selected in accordance with a variety factors as cited above.
- the actual dosage regimen employed may vary widely and therefore may deviate from a preferred dosage regimen and one skilled in the art will recognize that dosage and dosage regimen outside these typical ranges can be tested and, where appropriate, may be used in the methods of this invention.
- the desired dose may conveniently be presented in a single dose or as divided doses administered at appropriate intervals, for example, as two, three, four or more sub-doses per day.
- the sub-dose itself may be further divided, e.g., into a number of discrete loosely spaced administrations.
- the daily dose can be divided, especially when relatively large amounts are administered as deemed appropriate, into several, for example 2, 3 or 4, part administrations. If appropriate, depending on individual behavior, it may be necessary to deviate upward or downward from the daily dose indicated.
- the compounds of the present invention can be administrated in a wide variety of oral and parenteral dosage forms. It will be obvious to those skilled in the art that the following dosage forms may comprise, as the active component, either a compound of the invention or a pharmaceutically acceptable salt of a compound of the invention.
- a suitable pharmaceutically acceptable carrier can be either solid, liquid or a mixture of both.
- Solid form preparations include powders, tablets, pills, capsules, cachets, suppositories, and dispersible granules.
- a solid carrier can be one or more substances which may also act as diluents, flavouring agents, solubilizers, lubricants, suspending agents, binders, preservatives, tablet disintegrating agents, or an encapsulating material.
- the carrier is a finely divided solid which is in a mixture with the finely divided active component.
- the active component is mixed with the carrier having the necessary binding capacity in suitable proportions and compacted to the desire shape and size.
- the powders and tablets may contain varying percentage amounts of the active compound.
- a representative amount in a powder or tablet may contain from 0.5 to about 90 percent of the active compound; however, an artisan would know when amounts outside of réelle-_ -, ⁇ . ft
- Suitable carriers for powders and tablets are magnesium carbonate, magnesium stearate, talc, sugar, lactose, pectin, dextrin, starch, gelatin, tragacanth, methylcellulose, sodium carboxymethylcellulose, a low melting wax, cocoa butter, and the like.
- the term "preparation" is intended to include the formulation of the active compound with encapsulating material as carrier providing a capsule in which the active component, with or without carriers, is surrounded by a carrier, which is thus in association with it.
- cachets and lozenges are included. Tablets, powders, capsules, pills, cachets, and lozenges can be used as solid forms suitable for oral administration.
- a low melting wax such as an admixture of fatty acid glycerides or cocoa butter
- the active component is dispersed homogeneously therein, as by stirring.
- the molten homogenous mixture is then poured into convenient sized molds, allowed to cool, and thereby to solidify.
- Formulations suitable for vaginal administration may be presented as pessaries, tampons, creams, gels, pastes, foams or sprays containing in addition to the active ingredient such carriers as are known in the art to be appropriate.
- Liquid form preparations include solutions, suspensions, and emulsions, for example, water or water-propylene glycol solutions.
- parenteral injection liquid preparations can be formulated as solutions in aqueous polyethylene glycol solution.
- injectable preparations for example, sterile injectable aqueous or oleaginous suspensions may be formulated according to the known art using suitable dispersing or wetting agents and suspending agents.
- the sterile injectable preparation may also be a sterile injectable solution or suspension in a nontoxic parenterally acceptable diluent or solvent, for example, as a solution in 1,3-butanediol.
- Suitable vehicles and solvents that may be employed are water, Ringer's solution, and isotonic sodium chloride solution.
- sterile, fixed oils are conventionally employed as a solvent or suspending medium.
- any bland fixed oil may be employed including synthetic mono- or diglycerides.
- fatty acids such as oleic acid find use in the preparation of injectables.
- the compounds according to the present invention may thus be formulated for parenteral administration (e.g. by injection, for example bolus injection or continuous infusion) and may be presented in unit dose form in ampoules, pre-filled syringes, small volume infusion or in multi-dose containers with an added preservative.
- the pharmaceutical compositions may take such forms as suspensions, solutions, or emulsions in oily or aqueous vehicles, and may contain formulatory agents such as suspending, stabilizing and/or dispersing agents.
- the active ingredient may be in powder form, obtained by aseptic isolation of sterile solid or by lyophilization from solution, for constitution with a suitable vehicle, e.g. sterile, pyrogen-free water, before use.
- Aqueous solutions suitable for oral use can be prepared by dissolving the active component in water and adding suitable colorants, flavours, stabilizing and thickening agents, as desired.
- Aqueous suspensions suitable for oral use can be made by dispersing the finely divided active component in water with viscous material, such as natural or synthetic gums, resins, methylcellulose, sodium carboxymethylcellulose, or other well known suspending agents.
- viscous material such as natural or synthetic gums, resins, methylcellulose, sodium carboxymethylcellulose, or other well known suspending agents.
- solid form preparations which are intended to be converted, shortly before use, to liquid form preparations for oral administration.
- liquid forms include solutions, suspensions, and emulsions.
- These preparations may contain, in addition to the active component, colorants, flavors, stabilizers, buffers, artificial and natural sweeteners, dispersants, thickeners, solubilizing agents, and the like.
- the compounds according to the invention may be formulated as ointments, creams or lotions, or as a transdermal patch.
- Ointments and creams may, for example, be formulated with an aqueous or oily base with the addition of suitable thickening and or gelling agents.
- Lotions may be formulated . with an aqueous or oily base and will in general also contain one or more emulsifying agents, stabilizing agents, dispersing agents, suspending agents, thickening agents, or coloring agents.
- Formulations suitable for topical administration in the mouth include lozenges comprising active agent in a flavored base, usually sucrose and acacia or tragacanth; pastilles comprising the active ingredient in an inert base such as gelatin and glycerin or sucrose and acacia; and mouthwashes comprising the active ingredient in a suitable liquid carrier.
- Solutions or suspensions are applied directly to the nasal cavity by conventional means, for example with a dropper, pipette or spray.
- the formulations may be provided in single or multi-dose form. In the latter case of a dropper or pipette, this may be achieved by the patient administering an appropriate, predetermined volume of the solution or suspension. In the case of a spray, this may be achieved for example by means of a metering atomizing spray pump.
- Administration to the respiratory tract may also be achieved by means of an aerosol formulation in which the active ingredient is provided in a pressurized pack with a suitable propellant.
- the compounds of the Formula (la) or pharmaceutical compositions comprising them are administered as aerosols, for example as nasal aerosols or by inhalation, this can be carried out, for example, using a spray, a nebulizer, a pump nebulizer, an inhalation apparatus, a metered inhaler or a dry powder inhaler.
- Pharmaceutical forms for administration of the compounds of the Formula (la) as an aerosol can be prepared by processes well-known to the person skilled in the art.
- solutions or dispersions of the compounds of the Formula (la) in water, water/alcohol mixtures or suitable saline solutions can be employed using customary additives, for example benzyl alcohol or other suitable preservatives, absorption enhancers for increasing the bioavailability, solubilizers, dispersants and others, and, if appropriate, customary propellants, for example include carbon dioxide, CFC's, such as, dichlorodifluoromethane, trichlorofluoromethane, or dichlorotetrafluoroethane; and the like.
- the aerosol may conveniently also contain a surfactant such as lecithin.
- the dose of drug may be controlled by provision of a metered valve.
- the compound In formulations intended for administration to the respiratory tract, including intranasal formulations, the compound will generally have a small particle size for example of the order of 10 microns or less. Such a particle size may be obtained by means known in the art, for example by micronization. When desired, formulations adapted to give sustained release of the active ingredient may be employed.
- the active ingredients may be provided in the form of a dry powder, for example, a powder mix of the compound in a suitable powder base such as lactose, starch, starch derivatives such as hydroxypropylmethyl cellulose and polyvinylpyrrolidone (PNP).
- a powder base such as lactose, starch, starch derivatives such as hydroxypropylmethyl cellulose and polyvinylpyrrolidone (PNP).
- PNP polyvinylpyrrolidone
- the powder carrier will form a gel in the nasal cavity.
- the powder composition may be presented in unit dose form for example in capsules or cartridges of, e.g., gelatin, or blister packs from which the powder may be administered by means of an inhaler.
- the pharmaceutical preparations are preferably in unit dosage forms.
- the preparation is subdivided into unit doses containing appropriate quantities of the active component.
- the unit dosage form can be a packaged preparation, the package containing discrete quantities of preparation, such as packeted tablets, capsules, and powders in vials or ampoules.
- the unit dosage form can be a capsule, tablet, cachet, or lozenge itself, or it can be the appropriate number ofany of these in packaged form.
- Tablets or capsules for oral administration and liquids for intravenous administration are preferred compositions.
- prodrug refers to compounds that are rapidly transformed in vivo to yield the parent compound of the above formulae, for example, by hydrolysis in blood.
- a thorough discussion is provided in T. Higuchi and N. Stella, "Pro-drugs as Novel Delivery Systems,” Vol. 14 of the A.C.S. Symposium Series, and in Bioreversible Carriers in Drug Design, ed. Edward B. Roche, American Pharmaceutical Association and Pergamon Press, 1987, both of which are hereby incorporated by reference.
- the compounds of the invention can be administered as the sole active pharmaceutical agent as described herein above, they can also be used in combination with one or more agents belonging to the class of drugs known as ⁇ -glucosidase inhibitors, aldose reductase inhibitors, biguanides, HMG-CoA reductase inhibitors, squalene synthesis inhibitors, fibrate compounds, LDL catabolism enhancers and angiotensin converting enzyme (ACE) inhibitors.
- ⁇ -Glucosidase inhibitors belong to the class of drugs which competitively inhibit digestive enzymes such as ⁇ -amylase, maltase, ⁇ -dextrinase, sucrase, etc. in the pancreas and or small infesting.
- ⁇ -glucosidase inhibitors include acarbose, N-(l,3-dihydroxy-2- propyl)valiolamine (generic name; voglibose), miglitol, and ⁇ -glucosidase inhibitors known in the art.
- the class of aldose reductase inhibitors are drugs which inhibit the first-stage rate- limiting enzyme in the polyol pathway that prevent or arrest diabetic complications.
- the utilization of glucose in the polyol pathway is increased and the excess csorbitol accumulated intracellularly as a consequence acts as a tissue toxin and hence evokes the onset of complications such as diabetic neuropathy, retinopathy, and nephropathy.
- aldose reductase inhibitors examples include tolurestat; epalrestat; 3,4- dihydro-2,8-diisopropyl-3-thioxo-2H-l ,4-benzoxazine-4-acetic acid; 2,7-difluorospiro(9H- fluorene-9,4'-imidazolidine)-2',5'-dione (generic name: imirestat); 3-[(4-bromo-2- flurophenyl)methy]-7-chloro-3,4-dihydro-2,4-dioxo-l(2H)-qumazoline acetic acid (generic name: zenarestat); 6-fluoro-2,3-dihydro-2',5'-dioxo-spiro[4H-l-benzopyran-4,4'- imidazolidine]-2-carboxamide (SNK-860); zopolrestat; sorbinil; and
- the biguanides are a class of drugs that stimulate anaerobic glycolysis, increase the sensitivity to insulin in the peripheral tissues, inhibit glucose absorption from the intestine, suppress of hepatic gluconeogenesis, and inhibit fatty acid oxidation.
- Examples of biguanides include phenformin, metformin, buformin, and biguanides l ⁇ iown in the art.
- Statin compounds belong to a class of drugs that lower blood cholesterol levels by inhibiting hydroxymethylglutalyl CoA ( ⁇ MG-CoA) reductase.
- ⁇ MG-CoA reductase is the rate-limiting enzyme in cholesterol biosynthesis.
- a statin that inhibits this reductase lowers serum LDL concentrations by upregulating the activity of LDL receptors and responsible for clearing LDL from the blood.
- the statin compounds include rosuvastatin, pravastatin and its sodium salt, simvastatin, lovastatin, atorvastatin, fluvastatin, cerivastatin, and ⁇ MG-CoA reductase inhibitors known in the art.
- Squalene synthesis inhibitors belong to a class of drugs that lower blood cholesterol levels by inhibiting synthesis of squalene.
- examples of the squalene synthesis inhibitors include (S)- ⁇ -[Bis[2,2-dimethyl-l -oxopropoxy)methoxy] phosphinyl]-3- phenoxybenzenebutanesulfonic acid, mono potassium salt (BMS- 188494) and squalene synthesis inhibitors known in the art.
- Fibrate compounds belong to a class of drugs that lower blood cholesterol levels by inhibiting synthesis and secretion of triglycerides in the liver and activating a lipoprotein lipase. Fibrates have been known to activate peroxisome proliferators-activated receptors and induce lipoprotein lipase expression.
- fibrate compounds include bezafibrate, beclobrate, binifibrate, ciplof ⁇ brate, clinofibrate, clofibrate, clofibric acid, etofibrate, fenofibrate, gemfibrozil, nicof ⁇ brate, pirifibrate, ronifibrate, simfibrate, theof ⁇ brate, and fibrates known in the art.
- LDL (low-density lipoprotein) catabolism enhancers belong to a class of drugs that lower blood cholesterol levels by increasing the number of LDL (low-density lipoprotein) receptors, examples include LDL catabolism enhancers known in the art.
- Angiotensin converting enzyme (ACE) inhibitors belong to the class of drugs that partially lower blood glucose levels as well as lowering blood pressure by inhibiting angiotensin converting enzymes.
- the angiotensin converting enzyme inhibitors include captopril, enalapril, alacepril, delapril; ramipril, lisinopril, imidapril, benazepril, ceronapril, cilazapril, enalaprilat, fosinopril, moveltopril, perindopril, quinapril, spirapril, temocapril, trandolapril, and angiotensin converting enzyme inhibitors known in the art.
- Insulin secretion enhancers belong to the class of drugs having the property to promote secretion of insulin from pancreatic ⁇ cells.
- Examples of the insulin secretion enhancers include sulfonylureas (SU).
- the sulfonylureas (SU) are drugs which promote secretion of insulin from pancreatic ⁇ cells by transmitting signals of insulin secretion via SU receptors in the cell membranes.
- sulfonylureas examples include tolbutamide; chlorpropamide; tolazamide; acetohexamide; 4-chloro-N-[(l-pyrolidinylamino) carbonyl]- benzenesulfonamide (generic name: glycopyramide) or its ammonium salt; glibenclamide (glyburide); gliclazide; l-butyl-3-metanilylurea; carbutamide; glibonuride; glipizide; gliquidone; glisoxepid; glybuthiazole; glibuzole; glyhexamide; glymidine; glypinamide; phenbutamide; tolcyclamide, glimepiride, and other insulin secretion enhancers known in the art.
- insulin secretion enhancers include N-[[4-(l-methylethyl)cyclohexyl)carbonyl]-D- phenylalanine (Nateglinide); calcium (2S)-2-benzyl-3-(cis-hexahydro-2- isoindolinylcarbonyl)propionate dihydrate (Mitiglinide, KAD-1229); and other insulin secretion enhancers known in the art.
- Thiazolidinediones belong to the class of drugs more commoningly known as TZDs.
- Examples of thiazolidinediones include rosiglitazone, pioglitazone, and thiazolidinediones known in the art.
- Some embodiments of the invention include, a pharmaceutical composition comprising a compound of Formula (la) or a pharmaceutically acceptable salt thereof in combination with at least one member selected from the group consisting of an ⁇ -glucosidase inhibitor, an aldose reductase inhibitor, a biguanide, a HMG-CoA reductase inhibitor, a squalene synthesis inhibitor, a fibrate compound, a LDL catabolism enhancer and an angiotensin converting enzyme inhibitor.
- a pharmaceutical composition comprising a compound of Formula (la) or a pharmaceutically acceptable salt thereof in combination with at least one member selected from the group consisting of an ⁇ -glucosidase inhibitor, an aldose reductase
- the pharmaceutical composition is a compound of Formula (la) or a pharmaceutically acceptable salt thereof in combination with a HMG-CoA reductase inhibitor.
- the HMG- CoA reductase inhibitor is selected from the group consisting of prevastatin, simvastatin, lovastatin, atorvastatin, fluvastatin and lipitor.
- the combination can be used by mixing the respective active components either all together or independently with a physiologically acceptable carrier, excipient, binder, diluent, etc., as described herein above, and administering the mixture or mixtures either orally or non-orally as a pharmaceutical composition.
- a compound or a mixture of compounds of Formula (la) are administered as a combination therapy or prophylaxis with another active compound the therapeutic agents can be formulated as a separate pharmaceutical compositions given at the same time or at different times, or the therapeutic agents can be given as a single composition.
- Another object of the present invention relates to radiolabelled compounds of Formula (la) that would be useful not only in radio-imaging but also in assays, both in vitro and in vivo, for localizing and quantitating RUP3 in tissue samples, including human, and for identifying RUP3 ligands by inhibition binding of a radiolabelled compound. It is a further object of this invention to develop novel RUP3 assays of which comprise such radiolabelled compounds.
- Suitable radionuclides that may be incorporated in compounds of the present invention include but are not limited to 3 H (also written as T), n C, 14 C, 18 F, 125 1, 82 Br, 123 1, 124 I, 125 1, 131 1, 75 Br, 76 Br, 15 0, 13 N, 35 S and 77 Br.
- the radionuclide that is incorporated in the instant radiolabelled compounds will depend on the specific application of that radiolabelled compound. Thus, for in vitro RUP3 labeling and competition assays, compounds that incorporate 3 H, 14 C, 125 1 , 131 1, 35 S or 82 Br will generally be most useful.
- n C, 18 F, ,25 1, 123 1, 124 1, 131 1, 75 Br, 76 Br or 77 Br will generally be most useful.
- a “radio-labelled " or “labelled compound” is a compound of Formula (la) that has incorporated at least one radionuclide;
- the radionuclide is selected from the group consisting of 3 H, 14 C, 125 1 , 35 S and 82 Br; In some embodiments, the radionuclide 3 H or 14 C.
- all of the atoms represented in the compounds of the invention can be either the most commonly occurring isotope of such atoms or the more scarce radio-isotope or nonradio-active isotope.
- Synthetic methods for incorporating radio-isotopes into organic compounds including those applicable to those compounds of the invention are well known in the art and include incorporating activity levels of tritium into target molecules include: A. Catalytic Reduction with Tritium Gas - This procedure normally yields high specific activity products and requires halogenated or unsaturated precursors. B. Reduction with Sodium Borohydride [ 3 H] - This procedure is rather inexpensive and requires precursors containing reducible functional groups such as aldehydes, ketones, lactones, esters, and the like. C. Reduction with Lithium Aluminum Hydride [ 3 H ] - This procedure offers products at almost theoretical specific activities.
- D. Tritium Gas Exposure Labeling This procedure involves exposing precursors containing exchangeable protons to tritium gas in the presence of a suitable catalyst.
- Synthetic methods for incorporating activity levels of 125 I into target molecules include: A. Sandmeyer and like reactions - This procedure transforms an aryl or heteroaryl amine into a diazonium salt, such as a tetrafluoroborate salt, and subsequently to 125 I labelled compound using Na 125 I. A represented procedure was reported by Zhu, D.-G. and co-workers in J. Org. Chem. 2002, 67, 943-948. B. Ortho 125 Iodination of phenols - This procedure . allows for the incorporation of 125 I at the ortho position of a phenol as reported by Collier, T. L. and co-workers in J. Labelled Compd Radiopharm.
- This method is generally a two step process.
- the first step is the conversion of the aryl or heteroaryl bromide to the corresponding tri-alkyltin intermediate using for example, a Pd catalyzed reaction [i.e. Pd(Ph 3 P) 4 ] or through an aryl or heteroaryl lithium, in the presence of a xri-alkyltinhalide or hexaalkylditin [e.g., (CH 3 ) 3 SnSn(CH 3 ) 3 ].
- a xri-alkyltinhalide or hexaalkylditin e.g., (CH 3 ) 3 SnSn(CH 3 ) 3 ].
- a radiolabelled RUP3 compound as described herein can be used in a screening assay to identify/evaluate compounds.
- a newly synthesized or identified compound i.e., test compound
- the ability of a test compound to compete with the "radio-labelled compound of Formula (la)" for the binding to the RUP3 receptor directly correlates to its binding affinity.
- the labelled compounds of the present invention bind to the RUP3 receptor.
- the labelled compound has an IC5 0 less than about 500 ⁇ M, in another embodiment the labelled compound has an IC50 less than about 100 ⁇ M, in yet another embodiment the labelled compound has an IC50 less than about 10 ⁇ M, in yet another embodiment the labelled compound has an IC5 0 less than about 1 ⁇ M, and in still yet another embodiment the labelled inhibitor has an IC 50 less than about 0.1 ⁇ M.
- Adenlyl cyclase Activation Flashplate Assay kit from Perkin Elmer — 96 wells (SMP004B) and 125 I tracer (NEX130) which comes with the kit. Keep in refrigerator, in a box, and do not expose the Flashplates to light.
- Regeneration buffer can be aliquotted into 40-45 ml portions (in 50 ml sterile tubes) and kept frozen for up to 2 months. Simply put the tube in a beaker with room temperature water to thaw out the regeneration buffer on the day of the assay.
- Flashplate can therefore be set up to handle 3 compounds, (i.e., columns 2, 3, and 4 are for compound #1, columns 5, 6, and 7 are for compound #2, and columns 8, 9, and 10 are for compound #3.)
- An acceptable signal-to-noise ratio for RUP3 can vary from 4 to 6.
- the raw cpms are approximately 1800 to 2500 for RUP3 and 3500-4500 for CMN.
- the cpm (or ultimately pmoles of cAMP/well) cannot be outside the standard curve, and should not approach well A of the standard curve (50 pmole/well) and well H (no cAMP).
- the pmoles of cAMP produced by RUP3 receptor are around 11 to 13 pmole/well (for 15 ug/well protein), and for CMN are between 2 to 3 pmole/well (for 15 ug protein /well).
- the slope should be linear and the error bars for duplicates should be very small.
- the receptor and CMN controls cannot be off scale of the standard curve, as described above. If the receptor controls are off the high end of the standard curve,i.e. 50 pmole/well or higher, one must repeat the experiment using less protein. However, such a case has not been observed with transiently transfected RUP3 membranes (10 ug D ⁇ A/ 15 cm plate, using 60 ul Lipofectamine, and preparing membranes after 24 hour of transfection.)
- the IC 50 or EC 50 curve should be at 100% (+ or - 20%) of control RUP3 membranes at the top, and should go down to 0 (or up to 20%) at the bottom.
- the standard error of the triplicate determinations should be + or - 10%.
- H1T-T15 (ATCC CRL#1777) is an immortalized hamster insulin-producing cell line. These cells express RUP3 and therefore can be used to assess the ability of RUP3 ligands to stimulate or inhibit cAMP accumulation via its endogenously expressed receptor.
- cells are grown to 80% confluence and then distributed into a 96-well Flashplate (50,000 cells/ well) for detection of cAMP via a "cAMP Flashplate Assay" (NEN, Cat # SMP004). Briefly, cells are placed into anti-cAMP antibody-coated wells that contain either vehicle, the test ligand(s) at a concentration of interest, or 1 uM forskolin.
- the latter is a direct activator of adenylyl cyclase and serves as a positive control for stimulation of cAMP in HIT-T15 cells. All conditions are tested in triplicate. After a 1 hour incubation to allow for stimulation of cAMP, a Detection Mix containing 125 I-cAMP is added to each well and the plate is allowed to incubate for another 1 hour. The wells are then aspirated to remove unbound 125 I-cAMP. Bound 125 I-cAMP is detected using a Wallac Microbeta Counter. The amount of cAMP in each sample is determined by comparison to a standard curve, obtained by placing known concentrations of cAMP in some wells on the plate.
- RUP3 ligands can also be tested for their ability to stimulate glucose-dependent insulin secretion (GSIS) in HIT-T15 cells.
- GSIS glucose-dependent insulin secretion
- 30,000 cells/well in a 12-well plate are incubated in culture media containing 3 mM glucose and no serum for 2 hours. The media is then changed; wells receive media containing either 3 mM or 15 mM glucose, and in both cases the media contains either vehicle (DMSO) or RUP3 ligand at a concentration of interest. Some wells receive media containing 1 uM forskolin as a positive control.
- RUP3 is an endogenously expressed GPCR in the till-_ -, ⁇ . ft
- RUP3 ligands can also be tested for their ability to stimulate GSIS in rat islet cultures. This assay is performed as follows:
- IEQ islet equivalents
- RT-PCR was applied to determine the tissue distribution of RUP3.
- Ohgonucleotides used for PCR had the following sequences:
- ZC47 5'-CATTGCCGGGCTGTGGTTAGTGTC-3' (forward primer), (SEQ ID NO:3);
- ZC48 5'-GGCATAGATGAGTGGGTTGAGCAG-3' (reverse primer), (SEQ ID NO:4); and the human multiple tissue cDNA panels (MTC, Clontech) were used as templates (1 ng cDNA per PCR amplification). Twenty-two (22) human tissues were analyzed. PCR was performed using Platinum PCR SuperMix (Life Technologies, Inc.; manufacture instructions were followed) in a 50 ⁇ l reaction by the following sequences: step 1, 95°C for 4 min; step 2, 95°C for 1 min; step 3, 60°C for 30 sec; step 4, 72°C for 1 min; and step 5, 72°C for 7 min. Steps 2 through 4 were repeated 35 times.
- the resulting PCR reactions (15 ⁇ l) were loaded on a 1.5% agarose gel to analyze the RT-PCR products, and a specific 466 base-pair DNA fragment representing RUP3 was specifically amplified from cDNA of pancreas origin. Low expression was also evident in subregions of brain.
- results from RT-PCR analysis were further confirmed in cDNA dot-blot analysis.
- a dot-blot membrane containing cDNA from 50 human tissues (Clontech) was hybridized with a 32 P-radiolabelled DNA probe having sequences derived from human RUP3. Hybridyzation signals were seen in pancreas and fetal liver, suggesting these tissues express RTJP3. No significant expression was detected in other tissues analyzed.
- RUP3 expression was further analyzed with cDNAs of rat origin by RT-PCR technique.
- Tissue cDNAs used for this assay were obtained from Clontech except those for hypothalamus and islets, which were prepared in house. Concentrations of each cDNA sample were normalized via a control RT-PCR analysis of the house-keeping gene GAPDH before assaying for RUP3 expression.
- Ohgonucleotides used for PCR had the following sequences: rat RUP3 ("rRUP3") forward: 5'-CATGGGCCCTGCACCTTCTTTG-3' (SEQ ID NO:5); rRUP3 reverse: 5'-GCTCCGGATGGCTGATGATAGTGA-3' (SEQ ID NO:6).
- PCR was performed using Platinum PCR SuperMix (Life Technologies, Inc.; manufacture instructions were followed) in a 50 ⁇ l reaction by the following sequences: step 1, 95°C for 4 min; step 2, 95°C for 1 min; step 3, 60°C for 30 sec; step 4, 72°C for 1 min; and step 5, 72°C for 7 min. Steps 2 through 4 were repeated 35 times.
- PCR reactions (15 ⁇ l) were loaded on a 1.5% agarose gel to analyze the RT-PCR products, and a specific 547 base-pair DNA fragment representing rat RUP3 was specifically amplified from cDNA of pancreas origin, revealing a similar expression profile with human. Of particular note, robust expression was seen in isolated islets and hypothalamus.
- RUP3 protein expression is restricted to ⁇ cell lineage of pancreatic islets ( Figure 2).
- a polyclonal anti-RUP3 antibody was prepared in rabbits ( Figure 2A). Rabbits were immunized with an antigenic peptide with sequence derived from rat
- RUP3 (“rRUP3").
- the peptide sequence was RGPERTRESAYHINTISHPELDG and shared 100% identity with mouse RUP3 in the corresponding region.
- a cysteine residue was incorporated at the ⁇ -terminal end of this antigenic peptide to facilitate KLH crosslinking before injecting into rabbits.
- the resulting antisera (“anti-rRUP3”) and the corresponding preimmune sera (“pre-rRUP3”) were tested for immune reactivity to mouse RUP3 in immunobloting assays (lanes 1 thought 4).
- the GST-RUP3 fusion protein was readily recognized by the anti-rRUP3 antisera (lane 4), but not by the preimmurie sera (lane 2).
- the immunoreactive signal could be efficiently eliminated when the immunobloting assay was performed in the presence of excess antigenic peptide (lane 6).
- Rat pancreas was perfused with 4% paraformaldehyde (PFA) in PBS and embedded in OCT embedding medium.
- PFA paraformaldehyde
- Ten micron sections were prepared, fixed on glass slides, and immunostained with either pre-rRUP3 (Figure 2B, panel a) or with anti-rRUP3 antisera ( Figure 2B, panels c and e) followed by secondary staining with donkey anti-rabbit IgG conjugated to the fluorochrome Cy-3.
- Each section was also co-immunostained with a dire-_ fame, ftyl /ft «
- RUP3 stimulates the production of cAMP by cotransfection of 293 cells with: (1) a CRE-Luciferase reporter, wherein the ability to stimulate the production of firefly luciferase depends on increased cAMP in cells, and (2) an expression plasmid encoding the human form of RUP3 (Figure 3A).
- a CRE-Luciferase reporter wherein the ability to stimulate the production of firefly luciferase depends on increased cAMP in cells
- an expression plasmid encoding the human form of RUP3 Figure 3A. Note that cells co-transfected with an expression plasmid containing no RUP3 sequences (“CMN" in Figure 3A) produce very little luciferase activity, whereas cells transfected with an expression plasmid encoding RUP3 (“RUP3" in Figure 3A) have at least a 10-fold increase in luciferase activity.
- RUP3 stimulates the production of cAMP when introduced into 293 cells. This property of RUP3 is conserved across species, because hamster RUP3 stimulates luciferase activity when introduced into 293 cells in a manner analogous to that described for human RUP3 ( Figure 3B).
- Tu6 cells that express high levels of RUP3. Tu6 cells produce insulin, but do not express appreciable levels of RUP3 and do not normally exhibit an increase in insulin release when increased glucose is present in the culture media. As shown in Figure 3C, Tu6 cells transduced with a control virus that contains no receptor are still able to produce insulin, but do not show an increase in insulin secretion when the concentration of glucose in the culture media is shifted from 1 mM to 16 mM. By contrast, Tu6 cells transduced with RUP3-containing retrovirus display significant glucose-dependent insulin secretion (Figure 3C).
- RUP3 agonists stimulate endogenously expressed RUP3 in insulin-producing cells
- two in vitro models can be used.
- RUP3 agonists are used to stimulate HIT-T15 cells, which express RUP3 at significant levels, as indicated in the Northern blot shown in Figure 4A.
- these cells are known to exhibit enhanced glucose-dependent insulin release when intracellular cAMP concentrations are elevated.
- the RUP3 agonist Compound B84 stimulates cAMP production in HIT cells, at a level comparable to that seen with the adenyl cyclase activator forskolin. This indicates that Compound B84 is a very robust stimulator of cAMP in HIT- T15 cells.
- Compound B84 also stimulates insulin secretion in HIT cells exposed to 15 mM glucose, once again at a level comparable to that seen with the adenyl cyclase activator forskolin. This indicates that Compound B 84 is a very robust potentiator of insulin secretion in HIT-T15 cells.
- Isolated rat islets are the other in vitro model used to demonstrate the efficacy of RUP3 agonists.
- agents that induce cAMP are not expected to stimulate insulin secretion when glucose concentrations are low (e.g. 60 mg/dl). However, when glucose concentrations are increased (e.g. to 300 mg/dl), these agents are expected to enhance insulin secretion to levels above those seen with glucose alone.
- both RUP3 agonists Compounds 48 and 51 at 10 uM concentration
- the level of enhancement was comparable to that seen with 25 nM GLP-1, a gut hormone known to act on islets in this manner.
- Compound B70 was delivered orally via a gavage needle (p.o. volume at 100 ⁇ l).
- Control Ex-4 was delivered intraperitoneally. Thirty minutes after administration of test compound and control ex-4, mice were administered orally with dextrose at 5 g/kg dose. Levels of blood glucose were determined at the indicated time points using Glucometer Elite XL (Bayer).
- Figure 5A shows the mean glucose concentration averaged from eleven animals in each treatment group.
- 293 cells were plated in 96-well tissue culture plates at a concentration of 20,000 cells per well. The following day, the cells are transfected with a mixture of pCRE-Luc (Stratagene, Cat. # 219076), the indicated expression plasmid, and pEGFP-Nl (Clontech, Cat. # 6085-1) at a ratio of 5:1:0.25 using Lipofectamine Reagent (Invitrogen, Cat. #18324-020) according to the manufacturer's directions.
- pEGFP-Nl encodes a "green fluorescent protein" and was used as a control to determine that most cells were successfully transfected.
- the cells were lysed in situ with 100 ul/ well reconstituted Luclite buffer (Luclite- Reporter Gene Assay Kit, Packard, Cat. # 6016911), according to the manufacturer's directions. After a 10 minute incubation in the dark, luminescence was measured using a TRJLUX 1450 Microbeta Counter (Wallac).
- Retrovirus bearing an expression cassette for RUP3 was generated. Briefly, RUP3 coding sequence was cloned into the retroviral vector pLNCX2 (Clontech, Cat # 6102-1). The amphotropic packaging cell line PT-67 (Clontech, K1060-D) was then transfected with either the parental vector pLNCX2 or pLNCX2/RTJP3 using Lipofectamine and stable lines were established using guidelines provided by the PT-67 vendor. Retrovirus-containing supernatant was obtained by collecting media from the resultant stables according to the manufacturer's directions.
- Tu6 cells in a 10 cm dish, were then infected with retroviras by incubating in a solution of 1 ml viral supernatant/ 9 ml culture media containing 40 ug/ml polybrene for 24 hours. The medium was then changed to culture media containing 300 ug/ml G418. G418-resistant clones were ultimately created by virtue of the neomycin-resistance gene cassette present in the pLNCX2 vector, thus indicating the successful integration of retroviras into the Tu6 genome. The expression of RUP3 in the Tu6/RUP3 G418-resistant colonies was confirmed by Northern blot.
- EIA Rat Insulin Enzyme-Immunoassay
- the denatured probe, 10 ml ExpressHyb solution (Clontech, Cat # 8015-2) and the RNA blot were incubated in a hybridization oven, washed and exposed to film using standard molecular biology practices.
- another means for evaluating a test compound is by determining binding affinities to the RUP3 receptor.
- This type of assay generally requires a radiolabelled ligand to the RUP3 receptor. Absent the use of known ligands for the RUP3 receptor and radiolabels thereof, compounds of Formula (la) can be labelled with a radioisotope and used in an assay for evaluating the affinity of a test compound to the RUP3 receptor.
- a radiolabelled RUP3 compound as described herein can be used in a screening assay to identify/evaluate compounds.
- a newly synthesized or identified compound i.e., test compound
- the ability to compete with the "radio-labelled compound of Formula (la)" or Radiolabelled RUP3 Ligand for the binding to the RUP3 receptor directly correlates to its binding affinity of the test compound to the RUP3 receptor.
- 293 cells human kidney, ATCC
- transiently transfected with 10 ug human RUP3 receptor and 60 ul Lipofectamine per 15-cm dish
- 10 ml/dish of Hepes-EDTA buffer 20mM Hepes + 10 mM EDTA, pH 7.4
- the cells were then centrifuged in a Beckman Coulter centrifuge for 20 minutes, 17,000 rpm (JA-25.50 rotor).
- the pellet was resuspended in 20 mM Hepes + 1 mM EDTA, pH 7.4 and homogenized with a 50- ml Dounce homogenizer and again centrifuged. After removing the supernatant, the pellets were stored at -80°C, until used in binding assay.
- membranes were thawed on ice for 20 minutes and then 10 mL of incubation buffer (20 mM Hepes, 1 mM MgCl 2 ,100 mM NaCl, pH 7.4) added. The membranes were then vortexed to resuspend the crude membrane pellet and homogenized with a Brinkmann PT-3100 Polytron homogenizer for 15 seconds at setting 6. The concentration of membrane protein was determined using the BRL Bradford protein assay.
- a total volume of 50ul of appropriately diluted membranes (diluted in assay buffer containing 50mM Tris HCl (pH 7.4), lOmM MgCl 2 , and ImM EDTA; 5-50ug protein) is added to 96-well polyproylene microtiter plates followed by addition of lOOul of assay buffer and 50ul of Radiolabelled RUP3 Ligand.
- 50 ul of assay buffer is added instead of lOOul and an additional 50ul of lOuM cold RUP3 is added before 50ul of Radiolabelled RUP3 Ligand is added. Plates are then incubated at room temperature for 60-120 minutes.
- the binding reaction is terminated by filtering assay plates through a Microplate Devices GF/C Unifilter filtration plate with a Brandell 96-well plate harvester followed by washing with cold 50 mM Tris HCl, pH 7.4 containing 0.9% NaCl. Then, the bottom of the filtration plate are sealed, 50ul of Optiphase Supermix is added to each well, the top of the plates are sealed, and plates are counted in a Trilux MicroBeta scintillation counter. For compound competition studies, instead of adding lOOul of assay buffer, lOOul of appropriately diluted test compound is added to appropriate wells followed by addition of 50ul of Radiolabelled RTJP3 Ligand.
- test compounds are initially assayed at 1 and 0.1 ⁇ M and then at a range of concentrations chosen such that the middle dose would cause about 50% inhibition of a Radio-RUP3 Ligand binding (i.e., IC 50 ).
- IC 50 Specific binding in the absence of test compound (Bo) is the difference of total binding (B ⁇ ) minus non-specific binding (NSB) and similarly specific binding (in the presence of test compound) (B) is the difference of displacement binding (Bp) minus non-specific binding (NSB).
- IC 50 is determined from an inhibition response curve, logit-log plot of % B/B 0 vs concentration of test compound.
- Ki ⁇ C 5 o / (i + [Lj ⁇ D )
- [L] is the concentration of a Radio-RUP3 Ligand used in the assay and K D is the dissociation constant of a Radio-RUP3 Ligand determined independently under the same binding conditions.
- N-oxides can be prepared at normal or elevated pressure, in the presence of an oxidizing agent, such as, hydrogen peroxide, peracetic acid, perbenzoic, m- chloroperbenzoic acid (mCPBA), ozone, oxygen and the like, in the presence or absence of solvent, such as chloroform, dichlormethane, acetic acid, trifluoroacetic acid, and the like or mixtures thereof.
- an oxidizing agent such as, hydrogen peroxide, peracetic acid, perbenzoic, m- chloroperbenzoic acid (mCPBA), ozone, oxygen and the like
- solvent such as chloroform, dichlormethane, acetic acid, trifluoroacetic acid, and the like or mixtures thereof.
- TLC Thin-layer chromatography
- PK6F silica gel 60 A 1 mm plates (Whatman)
- column chromatography was carried out on a silica gel column using Kieselgel 60, 0.063-0.200 mm (Merck). Evaporation was done in vacuo on a Buchi rotary evaporator. Celite 545 ® was used during palladium filtrations. '
- LCMS specs 1) PC: HPLC-pumps: LC-IOAD VP, Shimadzu Inc.; HPLC system controller: SCL-10A VP, Shimadzu Inc; UN-Detector: SPD-10A VP, Shimadzu Pnc; Autosampler: CTC HTS, PAL, Leap Scientific; Mass spectrometer: API 150EX with Turbo Ion Spray source, AB/MDS Sciex; Software: Analyst 1.2. 2) Mac: HPLC-pumps: LC-8A VP, Shimadzu Inc; HPLC system controller: SCL-10A VP, Shimadzu Inc.
- UN-Detector SPD-10A VP, Shimadzu Inc; Autosampler: 215 Liquid Handler, Gilson Inc; Mass, spectrometer: API 150EX with Turbo Ion Spray source, AB/MDS Sciex Software: Masschrom 1.5.2.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Diabetes (AREA)
- Hematology (AREA)
- Obesity (AREA)
- Cardiology (AREA)
- Urology & Nephrology (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Endocrinology (AREA)
- Emergency Medicine (AREA)
- Hospice & Palliative Care (AREA)
- Child & Adolescent Psychology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Hydrogenated Pyridines (AREA)
- Peptides Or Proteins (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
Abstract
Description
Claims
Priority Applications (22)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP04702248A EP1599468B1 (en) | 2003-01-14 | 2004-01-14 | 1,2,3-trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
| SI200430529T SI1599468T1 (en) | 2003-01-14 | 2004-01-14 | 1,2,3-trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
| BR0406761-4A BRPI0406761A (en) | 2003-01-14 | 2004-01-14 | 1,2,3-Trisubstituted aryl and heteroaryl derivatives as metabolism modulators and the prophylaxis and treatment of related disorders such as diabetes and hyperglycemia |
| DK04702248T DK1599468T3 (en) | 2003-01-14 | 2004-01-14 | 1,2,3-Trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prevention and treatment of disorders associated therewith such as diabetes and hyperglycemia |
| EA200501132A EA011009B1 (en) | 2003-01-14 | 2004-01-14 | 1,2,3-substituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
| DE602004009295T DE602004009295T2 (en) | 2003-01-14 | 2004-01-14 | 1,2,3-TRISUBSTITUTED ARYL AND HETEROARYL DERIVATIVES AS MODULATORS OF METABOLISM FOR THE PREVENTION AND TREATMENT OF METABOLISM-CONDITIONAL DISEASES SUCH AS DIABETES OR HYPERGLYKEMIA |
| CA002512899A CA2512899A1 (en) | 2003-01-14 | 2004-01-14 | 1,2,3-trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
| NZ540612A NZ540612A (en) | 2003-01-14 | 2004-01-14 | 1,2,3-Trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
| US10/541,657 US8293751B2 (en) | 2003-01-14 | 2004-01-14 | 1,2,3-trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
| MXPA05007485A MXPA05007485A (en) | 2003-01-14 | 2004-01-14 | 1,2,3-trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prpphylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia. |
| YUP-2005/0532A RS20050532A (en) | 2003-01-14 | 2004-01-14 | 1,2,3-trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prpphylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
| CN2004800022039A CN1835943B (en) | 2003-01-14 | 2004-01-14 | 1,2,3-trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and uses thereof |
| JP2006501019A JP2006516572A (en) | 2003-01-14 | 2004-01-14 | Prevention and treatment of 1,2,3-trisubstituted aryl derivatives and 1,2,3-trisubstituted heteroaryl derivatives as metabolic modulators and disorders associated with these derivatives such as diabetes and hyperglycemia |
| AU2004205642A AU2004205642C1 (en) | 2003-01-14 | 2004-01-14 | 1,2,3-trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
| IL169081A IL169081A (en) | 2003-01-14 | 2005-06-08 | 4,5,6-trisubstituted pyrimidine compounds, pharmaceutical compositions containing them and use thereof in the preparation of medicaments for modulating metabolism |
| HR20050696A HRP20050696B1 (en) | 2003-01-14 | 2005-08-03 | 1,2,3-trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prpphylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
| NO20053803A NO20053803L (en) | 2003-01-14 | 2005-08-11 | 1,2,3-trisubstituted aryl and heteroaryl derivatives such as metabolism modulators and prophylaxis and treatment of related disorders such as diabetes and hyperglycemia |
| IS7979A IS2713B (en) | 2003-01-14 | 2005-08-12 | 1,2,3-triple aryl and heteroaryl derivatives as metabolic regulators and prevention and treatment of related diseases such as diabetes and hyperglycaemia |
| HK05111332A HK1076815A1 (en) | 2003-01-14 | 2005-12-09 | 1,2,3-trisubstitued aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such asdiabetes and hyperglycemia |
| US12/945,712 US20110082134A1 (en) | 2003-01-14 | 2010-11-12 | 1,2,3-Trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
| AU2010257356A AU2010257356A1 (en) | 2003-01-14 | 2010-12-21 | 1,2,3-trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
| US13/618,592 US8933083B2 (en) | 2003-01-14 | 2012-09-14 | 1,2,3-trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
Applications Claiming Priority (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US44039403P | 2003-01-14 | 2003-01-14 | |
| US60/440,394 | 2003-01-14 | ||
| US44982903P | 2003-02-24 | 2003-02-24 | |
| US60/449,829 | 2003-02-24 | ||
| US45339003P | 2003-03-06 | 2003-03-06 | |
| US60/453,390 | 2003-03-06 | ||
| US47087503P | 2003-05-14 | 2003-05-14 | |
| US60/470,875 | 2003-05-14 |
Related Child Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/541,657 A-371-Of-International US8293751B2 (en) | 2003-01-14 | 2004-01-14 | 1,2,3-trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
| US12/945,712 Continuation US20110082134A1 (en) | 2003-01-14 | 2010-11-12 | 1,2,3-Trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
| US13/618,592 Continuation US8933083B2 (en) | 2003-01-14 | 2012-09-14 | 1,2,3-trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2004065380A1 true WO2004065380A1 (en) | 2004-08-05 |
| WO2004065380A8 WO2004065380A8 (en) | 2004-10-21 |
Family
ID=32777226
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2004/001267 WO2004065380A1 (en) | 2003-01-14 | 2004-01-14 | 1,2,3-trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prpphylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
Country Status (30)
| Country | Link |
|---|---|
| US (3) | US8293751B2 (en) |
| EP (1) | EP1599468B1 (en) |
| JP (2) | JP2006516572A (en) |
| KR (2) | KR20110010824A (en) |
| CN (1) | CN102558155A (en) |
| AT (1) | ATE374766T1 (en) |
| AU (2) | AU2004205642C1 (en) |
| BR (1) | BRPI0406761A (en) |
| CA (1) | CA2512899A1 (en) |
| CO (1) | CO5640119A2 (en) |
| CY (1) | CY1107085T1 (en) |
| DE (1) | DE602004009295T2 (en) |
| DK (1) | DK1599468T3 (en) |
| EA (1) | EA011009B1 (en) |
| EC (1) | ECSP055916A (en) |
| ES (1) | ES2295816T3 (en) |
| GE (1) | GEP20084540B (en) |
| HK (1) | HK1076815A1 (en) |
| HR (1) | HRP20050696B1 (en) |
| IL (1) | IL169081A (en) |
| IS (2) | IS2713B (en) |
| MA (1) | MA27707A1 (en) |
| MX (1) | MXPA05007485A (en) |
| NO (1) | NO20053803L (en) |
| NZ (1) | NZ540612A (en) |
| PL (1) | PL377847A1 (en) |
| PT (1) | PT1599468E (en) |
| RS (1) | RS20050532A (en) |
| SG (1) | SG182004A1 (en) |
| WO (1) | WO2004065380A1 (en) |
Cited By (124)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005007647A1 (en) * | 2003-07-11 | 2005-01-27 | Arena Pharmaceuticals, Inc. | Trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto |
| WO2006067531A1 (en) * | 2004-12-24 | 2006-06-29 | Prosidion Ltd | G-protein coupled receptor (gpr116) agonists and use thereof for treating obesity and diabetes |
| WO2006076243A1 (en) * | 2005-01-10 | 2006-07-20 | Arena Pharmaceuticals, Inc. | Processes for preparing 4-(phenoxy-5-methyl-pyrimidin-4-yloxy)piperidine-1-carboxylic acid derivatives and related compounds |
| WO2006076231A2 (en) * | 2005-01-10 | 2006-07-20 | Arena Pharmaceuticals, Inc. | Combination therapy for the treatment of diabetes and conditions related thereto and for the treatment of conditions ameliorated by increasing a blood glp-1 level |
| JP2006522143A (en) * | 2003-04-04 | 2006-09-28 | アイアールエム・リミテッド・ライアビリティ・カンパニー | Novel compounds and compositions as protein kinase inhibitors |
| WO2007027225A2 (en) | 2005-04-27 | 2007-03-08 | Arena Pharmaceuticals, Inc. | Combination therapy for the treatment of obesity and diabetes and conditions related thereto and for the treatment of conditions ameliorated by increasing a blood glp-1 level |
| WO2007120702A2 (en) | 2006-04-11 | 2007-10-25 | Arena Pharmaceuticals, Inc. | Use of gpr119 receptor agonists for increasing bone mass and for treating osteoporosis, and combination therapy relating thereto |
| WO2007120689A2 (en) | 2006-04-11 | 2007-10-25 | Arena Pharmaceuticals, Inc. | Methods of using gpr119 receptor to identify compounds useful for increasing bone mass in an individual |
| WO2008017381A1 (en) | 2006-08-08 | 2008-02-14 | Sanofi-Aventis | Arylaminoaryl-alkyl-substituted imidazolidine-2,4-diones, processes for preparing them, medicaments comprising these compounds, and their use |
| US7335672B2 (en) | 2004-09-13 | 2008-02-26 | Amgen Inc. | Vanilloid receptor ligands and their use in treatments |
| WO2008070692A2 (en) | 2006-12-06 | 2008-06-12 | Smithkline Beecham Corporation | Bicyclic compounds and use as antidiabetics |
| JP2008526724A (en) * | 2004-12-31 | 2008-07-24 | プロシディオン・リミテッド | Derivatives of pyridine, pyrimidine, and pyrazine as GPCR agonists |
| WO2009021740A2 (en) | 2007-08-15 | 2009-02-19 | Sanofis-Aventis | Substituted tetrahydronaphthalenes, process for the preparation thereof and the use thereof as medicaments |
| WO2009055331A2 (en) | 2007-10-22 | 2009-04-30 | Schering Corporation | Bicyclic heterocycle derivatives and their use as modulators of the activity of gpr119 |
| EP2108960A1 (en) | 2008-04-07 | 2009-10-14 | Arena Pharmaceuticals, Inc. | Methods of using A G protein-coupled receptor to identify peptide YY (PYY) secretagogues and compounds useful in the treatment of conditons modulated by PYY |
| WO2009143049A1 (en) | 2008-05-19 | 2009-11-26 | Schering Corporation | Bicyclic heterocycle derivatives and use thereof as gpr119 modulators |
| WO2010003624A2 (en) | 2008-07-09 | 2010-01-14 | Sanofi-Aventis | Heterocyclic compounds, processes for their preparation, medicaments comprising these compounds, and the use thereof |
| WO2010009195A1 (en) | 2008-07-16 | 2010-01-21 | Schering Corporation | Bicyclic heterocycle derivatives and use thereof as gpr119 modulators |
| WO2010013849A1 (en) | 2008-08-01 | 2010-02-04 | 日本ケミファ株式会社 | Gpr119 agonist |
| WO2010029089A2 (en) * | 2008-09-10 | 2010-03-18 | Boehringer Ingelheim International Gmbh | Combination therapy for the treatment of diabetes and related conditions |
| WO2010068601A1 (en) | 2008-12-08 | 2010-06-17 | Sanofi-Aventis | A crystalline heteroaromatic fluoroglycoside hydrate, processes for making, methods of use and pharmaceutical compositions thereof |
| WO2010075273A1 (en) | 2008-12-23 | 2010-07-01 | Schering Corporation | Bicyclic heterocycle derivatives and methods of use thereof |
| WO2010075269A1 (en) | 2008-12-23 | 2010-07-01 | Schering Corporation | Pyrimidine derivatives as gpcr modttlators for use in the treatment of obesity and diabetes |
| WO2010075271A1 (en) | 2008-12-23 | 2010-07-01 | Schering Corporation | Bicyclic heterocycle derivatives and methods of use thereof |
| US7767677B2 (en) | 2004-09-20 | 2010-08-03 | Xenon Pharmaceuticals Inc. | Heterocyclic derivatives and their use as stearoyl-CoA desaturase inhibitors |
| US7777036B2 (en) | 2004-09-20 | 2010-08-17 | Xenon Pharmaceuticals Inc. | Heterocyclic derivatives and their use as therapeutic agents |
| WO2010114957A1 (en) | 2009-04-03 | 2010-10-07 | Schering Corporation | Bicyclic piperidine and piperazine derivatives as gpcr modulators for the treatment of obesity, diabetes and other metabolic disorders |
| WO2010114958A1 (en) | 2009-04-03 | 2010-10-07 | Schering Corporation | Bridged bicyclic heterocycle derivatives and methods of use thereof |
| US7829712B2 (en) | 2004-09-20 | 2010-11-09 | Xenon Pharmaceuticals Inc. | Pyridazine derivatives for inhibiting human stearoyl-CoA-desaturase |
| WO2010146605A1 (en) * | 2009-06-18 | 2010-12-23 | Cadila Healthcare Limited | Novel gpr 119 agonists |
| WO2011005929A1 (en) | 2009-07-09 | 2011-01-13 | Arena Pharmaceuticals, Inc. | Piperidine derivative and its use for the treatment of diabets and obesity |
| WO2011023754A1 (en) | 2009-08-26 | 2011-03-03 | Sanofi-Aventis | Novel crystalline heteroaromatic fluoroglycoside hydrates, pharmaceuticals comprising these compounds and their use |
| WO2011025006A1 (en) | 2009-08-31 | 2011-03-03 | 日本ケミファ株式会社 | Gpr119 agonist |
| US7910583B2 (en) | 2007-05-04 | 2011-03-22 | Bristol-Myers Squibb Company | [6,6] and [6,7]-bicyclic GPR119 G protein-coupled receptor agonists |
| US7919496B2 (en) | 2004-09-20 | 2011-04-05 | Xenon Pharmaceuticals Inc. | Heterocyclic derivatives for the treatment of diseases mediated by stearoyl-CoA desaturase enzymes |
| US7928230B2 (en) | 2007-07-17 | 2011-04-19 | Bristol-Myers Squibb Company | Method for modulating GPR119 G protein-coupled receptor and selected compounds |
| EP2316457A1 (en) | 2004-09-20 | 2011-05-04 | Xenon Pharmaceuticals Inc. | Pyridine derivatives for inhibiting human stearoyl-coa-desaturase |
| WO2011053688A1 (en) | 2009-10-29 | 2011-05-05 | Schering Corporation | Bridged bicyclic piperidine derivatives and methods of use thereof |
| WO2011062885A1 (en) | 2009-11-23 | 2011-05-26 | Schering Corporation | Fused bicyclic pyrimidine derivatives and methods of use thereof |
| WO2011062889A1 (en) | 2009-11-23 | 2011-05-26 | Schering Corporation | Pyrimidine ether derivatives and methods of use thereof |
| US7951805B2 (en) | 2004-09-20 | 2011-05-31 | Xenon Pharmaceuticals Inc. | Heterocyclic derivatives and their use as mediators of stearoyl-CoA desaturase |
| WO2011066137A1 (en) | 2009-11-24 | 2011-06-03 | Schering Corporation | Substituted biaryl derivatives and methods of use thereof |
| WO2011107494A1 (en) | 2010-03-03 | 2011-09-09 | Sanofi | Novel aromatic glycoside derivatives, medicaments containing said compounds, and the use thereof |
| WO2011113947A1 (en) | 2010-03-18 | 2011-09-22 | Boehringer Ingelheim International Gmbh | Combination of a gpr119 agonist and the dpp-iv inhibitor linagliptin for use in the treatment of diabetes and related conditions |
| US8026360B2 (en) | 2004-09-20 | 2011-09-27 | Xenon Pharmaceuticals Inc. | Substituted pyridazines as stearoyl-CoA desaturase inhibitors |
| WO2011127106A1 (en) | 2010-04-08 | 2011-10-13 | Bristol-Myers Squibb Company | Pyrimidinylpiperidinyloxypyridinone analogues as gpr119 modulators |
| WO2011127051A1 (en) | 2010-04-06 | 2011-10-13 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| WO2011140161A1 (en) | 2010-05-06 | 2011-11-10 | Bristol-Myers Squibb Company | Benzofuranyl analogues as gpr119 modulators |
| WO2011140160A1 (en) | 2010-05-06 | 2011-11-10 | Bristol-Myers Squibb Company | Bicyclic heteroaryl compounds as gpr119 modulators |
| US8071603B2 (en) | 2004-09-20 | 2011-12-06 | Xenon Pharmaceuticals Inc. | Heterocyclic derivatives and their use as stearoyl-CoA desaturase inhibitors |
| WO2011157827A1 (en) | 2010-06-18 | 2011-12-22 | Sanofi | Azolopyridin-3-one derivatives as inhibitors of lipases and phospholipases |
| WO2011161030A1 (en) | 2010-06-21 | 2011-12-29 | Sanofi | Heterocyclic substituted methoxyphenyl derivatives having an oxo group, method for producing same, and use thereof as gpr40 receptor modulators |
| US8093257B2 (en) | 2007-05-04 | 2012-01-10 | Bristol-Myers Squibb Company | [6,5]-bicyclic GPR119 G protein-coupled receptor agonists |
| WO2012004269A1 (en) | 2010-07-05 | 2012-01-12 | Sanofi | (2-aryloxy-acetylamino)-phenyl-propionic acid derivatives, method for producing same and use thereof as pharmaceuticals |
| WO2012004270A1 (en) | 2010-07-05 | 2012-01-12 | Sanofi | Spirocyclically substituted 1,3-propane dioxide derivatives, methods for the production thereof and use of the same as medicament |
| WO2012010413A1 (en) | 2010-07-05 | 2012-01-26 | Sanofi | Aryloxy-alkylene substituted hydroxyphenyl hexynoic acids, methods for the production thereof and use of the same as medicament |
| WO2012025811A1 (en) | 2010-08-23 | 2012-03-01 | Lupin Limited | Indolylpyrimidines as modulators of gpr119 |
| WO2012040279A1 (en) | 2010-09-22 | 2012-03-29 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| US8153635B2 (en) | 2007-09-20 | 2012-04-10 | Irm Llc | Compounds and compositions as modulators of GPR119 activity |
| WO2012069917A1 (en) | 2010-11-26 | 2012-05-31 | Lupin Limited | Bicyclic gpr119 modulators |
| US20120157443A1 (en) * | 2009-09-04 | 2012-06-21 | Sunesis Pharmaceuticals, Inc. | Bruton's tyrosine kinase inhibitors |
| WO2012120054A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Di- and tri-substituted oxathiazine derivates, method for the production thereof, use thereof as medicine and drug containing said derivatives and use thereof |
| WO2012120053A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Branched oxathiazine derivatives, method for the production thereof, use thereof as medicine and drug containing said derivatives and use thereof |
| WO2012120052A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Oxathiazine derivatives substituted with carbocycles or heterocycles, method for producing same, drugs containing said compounds, and use thereof |
| WO2012120056A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Tetrasubstituted oxathiazine derivatives, method for producing them, their use as medicine and drug containing said derivatives and the use thereof |
| WO2012120055A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Di- and tri-substituted oxathiazine derivates, method for the production thereof, use thereof as medicine and drug containing said derivatives and use thereof |
| WO2012135570A1 (en) | 2011-04-01 | 2012-10-04 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| WO2012145604A1 (en) | 2011-04-22 | 2012-10-26 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| WO2012145603A1 (en) | 2011-04-22 | 2012-10-26 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| WO2012145361A1 (en) | 2011-04-19 | 2012-10-26 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| CN101123964B (en) * | 2004-12-24 | 2012-11-28 | 普罗西迪恩有限公司 | G-protein coupled receptor(GPR116) agonists and use thereof for treating obesity and diabetes |
| WO2012170702A1 (en) | 2011-06-08 | 2012-12-13 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| WO2012170867A1 (en) | 2011-06-09 | 2012-12-13 | Rhizen Pharmaceuticals Sa | Novel compounds as modulators of gpr-119 |
| JP2012533546A (en) * | 2009-07-15 | 2012-12-27 | イーライ リリー アンド カンパニー | GPR119 agonist |
| US8372837B2 (en) | 2008-07-16 | 2013-02-12 | Bristol-Myers Squibb Company | Pyridone and pyridazone analogues as GPR119 modulators |
| EP2567959A1 (en) | 2011-09-12 | 2013-03-13 | Sanofi | 6-(4-Hydroxy-phenyl)-3-styryl-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| US8399485B2 (en) | 2006-12-14 | 2013-03-19 | Merck Sharp & Dohme Corp. | Acyl bipiperidinyl compounds useful as GPR 119 agonists |
| WO2013037390A1 (en) | 2011-09-12 | 2013-03-21 | Sanofi | 6-(4-hydroxy-phenyl)-3-styryl-1h-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| WO2013045413A1 (en) | 2011-09-27 | 2013-04-04 | Sanofi | 6-(4-hydroxy-phenyl)-3-alkyl-1h-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| WO2013055910A1 (en) | 2011-10-12 | 2013-04-18 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| US8481731B2 (en) | 2009-06-24 | 2013-07-09 | Boehringer Ingelheim International Gmbh | Compounds, pharmaceutical composition and methods relating thereto |
| US8541457B2 (en) | 2005-06-03 | 2013-09-24 | Xenon Pharmaceuticals Inc. | Aminothiazole derivatives as human stearoyl-CoA desaturase inhibitors |
| US8551957B2 (en) | 2007-08-16 | 2013-10-08 | Boehringer Ingelheim International Gmbh | Pharmaceutical composition comprising a glucopyranosyl-substituted benzene derivate |
| WO2013151982A1 (en) | 2012-04-03 | 2013-10-10 | Arena Pharmaceuticals, Inc. | Methods and compounds useful in treating pruritus, and methods for identifying such compounds |
| US8637530B2 (en) | 2005-07-30 | 2014-01-28 | Boehringer Ingelheim International Gmbh | 8-(3-amino-piperidin-1-yl)-xanthines, their preparation, and their use as pharmaceuticals |
| US8664232B2 (en) | 2002-08-21 | 2014-03-04 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | 8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions |
| US8697868B2 (en) | 2004-02-18 | 2014-04-15 | Boehringer Ingelheim International Gmbh | 8-[3-amino-piperidin-1-yl]-xanthines, their preparation and their use as pharmaceutical compositions |
| WO2014074668A1 (en) | 2012-11-08 | 2014-05-15 | Arena Pharmaceuticals, Inc. | Modulators of gpr119 and the treatment of disorders related thereto |
| US8815876B2 (en) | 2008-07-16 | 2014-08-26 | Merck Sharp & Dohme Corp. | Bicyclic heterocycle derivatives and methods of use thereof |
| US8883800B2 (en) | 2011-07-15 | 2014-11-11 | Boehringer Ingelheim International Gmbh | Substituted quinazolines, the preparation thereof and the use thereof in pharmaceutical compositions |
| US8883805B2 (en) | 2004-11-05 | 2014-11-11 | Boehringer Ingelheim International Gmbh | Process for the preparation of chiral 8-(3-aminopiperidin-1-yl)-xanthines |
| US9034883B2 (en) | 2010-11-15 | 2015-05-19 | Boehringer Ingelheim International Gmbh | Vasoprotective and cardioprotective antidiabetic therapy |
| US9067891B2 (en) | 2007-03-07 | 2015-06-30 | Janssen Pharmaceuticals, Inc. | 1,4-disubstituted 3-cyano-pyridone derivatives and their use as positive allosteric modulators of mGluR2-receptors |
| US9174965B2 (en) | 2012-05-16 | 2015-11-03 | Bristol-Myers Squibb Company | Pyrimidinylpiperidinyloxypyridone analogues as GPR119 modulators |
| US9173859B2 (en) | 2006-05-04 | 2015-11-03 | Boehringer Ingelheim International Gmbh | Uses of DPP IV inhibitors |
| KR20150126564A (en) | 2014-05-02 | 2015-11-12 | 현대약품 주식회사 | Novel cyclohexene derivatives, preparation method thereof and pharmaceutical composition for prevention or treatment of the metabolic diseases containing the same as an active ingredient |
| US9186392B2 (en) | 2010-05-05 | 2015-11-17 | Boehringer Ingelheim International Gmbh | Combination therapy |
| US9212183B2 (en) | 2008-12-23 | 2015-12-15 | Boehringer Ingelheim International Gmbh | Salt forms of 1-[(4-methyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-(R)-amino-piperidin-1-yl)-xanthine |
| US9266888B2 (en) | 2006-05-04 | 2016-02-23 | Boehringer Ingelheim International Gmbh | Polymorphs |
| US9415016B2 (en) | 2008-04-03 | 2016-08-16 | Boehringer Ingelheim International Gmbh | DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation |
| US9457029B2 (en) | 2009-11-27 | 2016-10-04 | Boehringer Ingelheim International Gmbh | Treatment of genotyped diabetic patients with DPP-IV inhibitors such as linagliptin |
| US9486526B2 (en) | 2008-08-06 | 2016-11-08 | Boehringer Ingelheim International Gmbh | Treatment for diabetes in patients inappropriate for metformin therapy |
| US9526728B2 (en) | 2014-02-28 | 2016-12-27 | Boehringer Ingelheim International Gmbh | Medical use of a DPP-4 inhibitor |
| US9526730B2 (en) | 2012-05-14 | 2016-12-27 | Boehringer Ingelheim International Gmbh | Use of a DPP-4 inhibitor in podocytes related disorders and/or nephrotic syndrome |
| US9555001B2 (en) | 2012-03-07 | 2017-01-31 | Boehringer Ingelheim International Gmbh | Pharmaceutical composition and uses thereof |
| US9708315B2 (en) | 2013-09-06 | 2017-07-18 | Janssen Pharmaceutica Nv | 1,2,4-triazolo[4,3-a]pyridine compounds and their use as positive allosteric modulators of MGLUR2 receptors |
| US9713618B2 (en) | 2012-05-24 | 2017-07-25 | Boehringer Ingelheim International Gmbh | Method for modifying food intake and regulating food preference with a DPP-4 inhibitor |
| US9737533B2 (en) | 2009-05-12 | 2017-08-22 | Janssen Pharmaceuticals. Inc. | 1,2,4-triazolo [4,3-A] pyridine derivatives and their use for the treatment of prevention of neurological and psychiatric disorders |
| US20180185291A1 (en) | 2011-03-07 | 2018-07-05 | Boehringer Ingelheim International Gmbh | Pharmaceutical compositions |
| US10106542B2 (en) | 2013-06-04 | 2018-10-23 | Janssen Pharmaceutica Nv | Substituted 6,7-dihydropyrazolo[1,5-a]pyrazines as negative allosteric modulators of mGluR2 receptors |
| US10155000B2 (en) | 2016-06-10 | 2018-12-18 | Boehringer Ingelheim International Gmbh | Medical use of pharmaceutical combination or composition |
| WO2018236896A1 (en) | 2017-06-19 | 2018-12-27 | Arena Pharmaceuticals, Inc. | Compounds and methods for treatment of nafld and nash |
| US10406172B2 (en) | 2009-02-13 | 2019-09-10 | Boehringer Ingelheim International Gmbh | Pharmaceutical composition, methods for treating and uses thereof |
| US10537573B2 (en) | 2014-01-21 | 2020-01-21 | Janssen Pharmaceutica Nv | Combinations comprising positive allosteric modulators or orthosteric agonists of metabotropic glutamatergic receptor subtype 2 and their use |
| US10618887B2 (en) | 2012-06-08 | 2020-04-14 | Sunesis Pharmaceuticals, Inc. | Pyrimidinyl tyrosine kinase inhibitors |
| US10723699B2 (en) | 2015-11-04 | 2020-07-28 | Hyundai Pharm Co., Ltd. | Cyclohexene derivative, preparation method thereof, and pharmaceutical composition for preventing or treating metabolic disease comprising the same as active ingredient |
| US11007175B2 (en) | 2015-01-06 | 2021-05-18 | Arena Pharmaceuticals, Inc. | Methods of treating conditions related to the S1P1 receptor |
| US11033552B2 (en) | 2006-05-04 | 2021-06-15 | Boehringer Ingelheim International Gmbh | DPP IV inhibitor formulations |
| US11071729B2 (en) | 2007-09-14 | 2021-07-27 | Addex Pharmaceuticals S.A. | 1′,3′-disubstituted-4-phenyl-3,4,5,6-tetrahydro-2H,1′H-[1,4′]bipyridinyl-2′-ones |
| US11174243B2 (en) | 2016-07-21 | 2021-11-16 | Sunesis Pharmaceuticals, Inc. | Succinate forms and compositions of Bruton's tyrosine kinase inhibitors |
| US11369606B2 (en) | 2014-01-21 | 2022-06-28 | Janssen Pharmaceutica Nv | Combinations comprising positive allosteric modulators or orthosteric agonists of metabotropic glutamatergic receptor subtype 2 and their use |
| US11534424B2 (en) | 2017-02-16 | 2022-12-27 | Arena Pharmaceuticals, Inc. | Compounds and methods for treatment of primary biliary cholangitis |
| US11884626B2 (en) | 2015-06-22 | 2024-01-30 | Arena Pharmaceuticals, Inc. | Crystalline L-arginine salt of (R)-2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclo-penta [b]indol-3-yl)acetic acid(Compound1) for use in S1P1 receptor-associated disorders |
| US11911388B2 (en) | 2008-10-16 | 2024-02-27 | Boehringer Ingelheim International Gmbh | Treatment for diabetes in patients with insufficient glycemic control despite therapy with an oral or non-oral antidiabetic drug |
Families Citing this family (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| NZ540612A (en) | 2003-01-14 | 2008-02-29 | Arena Pharm Inc | 1,2,3-Trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
| PL378295A1 (en) * | 2003-02-24 | 2006-03-20 | Arena Pharmaceuticals, Inc. | Substituted aryl and heteroaryl derivatives as modulators of glucose metabolism and the prophylaxis and treatment of disorders thereof |
| AU2004257267B2 (en) | 2003-07-14 | 2009-12-03 | Arena Pharmaceuticals,Inc | Fused-aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto |
| WO2005121121A2 (en) * | 2004-06-04 | 2005-12-22 | Arena Pharmaceuticals, Inc. | Substituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto |
| MY148521A (en) | 2005-01-10 | 2013-04-30 | Arena Pharm Inc | Substituted pyridinyl and pyrimidinyl derivatives as modulators of metabolism and the treatment of disorders related thereto |
| AU2006244203B2 (en) | 2005-05-09 | 2012-05-03 | Achillion Pharmaceuticals, Inc. | Thiazole compounds and methods of use |
| WO2007035355A2 (en) * | 2005-09-16 | 2007-03-29 | Arena Pharmaceuticals, Inc. | Modulators of metabolism and the treatment of disorders related thereto |
| AR059898A1 (en) | 2006-03-15 | 2008-05-07 | Janssen Pharmaceutica Nv | DERIVATIVES OF 3-CIANO-PIRIDONA 1,4-DISUSTITUTED AND ITS USE AS ALLOSTERIC MODULATORS OF MGLUR2 RECEIVERS |
| TW200811140A (en) * | 2006-07-06 | 2008-03-01 | Arena Pharm Inc | Modulators of metabolism and the treatment of disorders related thereto |
| TW200811147A (en) * | 2006-07-06 | 2008-03-01 | Arena Pharm Inc | Modulators of metabolism and the treatment of disorders related thereto |
| TW200845978A (en) | 2007-03-07 | 2008-12-01 | Janssen Pharmaceutica Nv | 3-cyano-4-(4-tetrahydropyran-phenyl)-pyridin-2-one derivatives |
| EP2164846A2 (en) | 2007-05-22 | 2010-03-24 | Achillion Pharmaceuticals, Inc. | Heteroaryl substituted thiazoles and their use as antiviral agents. |
| CN101801930B (en) | 2007-09-14 | 2013-01-30 | 奥梅-杨森制药有限公司 | 1,3-disubstituted-4-phenyl-1 h-pyridin-2-ones |
| AU2008297876B2 (en) | 2007-09-14 | 2011-07-07 | Addex Pharma S.A. | 1,3-disubstituted 4-(aryl-x-phenyl)-1h-pyridin-2-ones |
| ES2637794T3 (en) | 2007-11-14 | 2017-10-17 | Janssen Pharmaceuticals, Inc. | Imidazo [1,2-A] pyridine derivatives and their use as positive allosteric modulators of MGLUR2 receptors |
| US8106209B2 (en) | 2008-06-06 | 2012-01-31 | Achillion Pharmaceuticals, Inc. | Substituted aminothiazole prodrugs of compounds with anti-HCV activity |
| AU2009289784B2 (en) | 2008-09-02 | 2012-03-22 | Addex Pharma S.A. | 3-azabicyclo[3.1.0]hexyl derivatives as modulators of metabotropic glutamate receptors |
| WO2010043396A1 (en) | 2008-10-16 | 2010-04-22 | Ortho-Mcneil-Janssen Pharmaceuticals, Inc. | Indole and benzomorpholine derivatives as modulators of metabotropic glutamate receptors |
| WO2010060589A1 (en) | 2008-11-28 | 2010-06-03 | Ortho-Mcneil-Janssen Pharmaceuticals, Inc. | Indole and benzoxazine derivatives as modulators of metabotropic glutamate receptors |
| MY153913A (en) | 2009-05-12 | 2015-04-15 | Janssen Pharmaceuticals Inc | 7-aryl-1,2,4-triazolo[4,3-a]pyridine derivatives and their use as positive allosteric modulators of mglur2 receptors |
| US8946205B2 (en) | 2009-05-12 | 2015-02-03 | Janssen Pharmaceuticals, Inc. | 1,2,4-triazolo[4,3-a]pyridine derivatives and their use as positive allosteric modulators of mGluR2 receptors |
| AU2010313397B2 (en) * | 2009-10-30 | 2015-07-02 | Janssen Pharmaceutica Nv | Phenoxy-substituted pyrimidines as opioid receptor modulators |
| US20110269759A1 (en) * | 2009-10-30 | 2011-11-03 | Coats Steven J | Pyrimidine compounds as delta opioid receptor modulators |
| WO2011094008A1 (en) | 2010-01-27 | 2011-08-04 | Arena Pharmaceuticals, Inc. | Processes for the preparation of (r)-2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic acid and salts thereof |
| PL2649069T3 (en) | 2010-11-08 | 2016-01-29 | Janssen Pharmaceuticals Inc | 1,2,4-TRIAZOLO[4,3-a]PYRIDINE DERIVATIVES AND THEIR USE AS POSITIVE ALLOSTERIC MODULATORS OF MGLUR2 RECEPTORS |
| AU2011328203B2 (en) | 2010-11-08 | 2015-03-19 | Janssen Pharmaceuticals, Inc. | 1,2,4-triazolo[4,3-a]pyridine derivatives and their use as positive allosteric modulators of mGluR2 receptors |
| CN103261195B (en) | 2010-11-08 | 2015-09-02 | 杨森制药公司 | The purposes of 1,2,4-triazolo [4,3-a] pyridine derivate and the positive allosteric modulators as MGLUR2 acceptor thereof |
| JP2014094886A (en) * | 2011-02-28 | 2014-05-22 | Nippon Chemiphar Co Ltd | Gpr119 agonist |
| WO2013052395A1 (en) * | 2011-10-06 | 2013-04-11 | Merck Sharp & Dohme Corp. | 1,3-substituted azetidine pde10 inhibitors |
| WO2014013014A1 (en) | 2012-07-18 | 2014-01-23 | Fundació Privada Centre De Regulació Genòmica (Crg) | Jak inhibitors for activation of epidermal stem cell populations |
| US9353078B2 (en) * | 2013-10-01 | 2016-05-31 | New York University | Amino, amido and heterocyclic compounds as modulators of rage activity and uses thereof |
| WO2018041989A1 (en) | 2016-09-02 | 2018-03-08 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for diagnosing and treating refractory celiac disease type 2 |
| WO2020201362A2 (en) | 2019-04-02 | 2020-10-08 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods of predicting and preventing cancer in patients having premalignant lesions |
| US20220202820A1 (en) | 2019-04-16 | 2022-06-30 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Use of jak inhibitors for the treatment of painful conditions involving nav1.7 channels |
| CN115340526B (en) * | 2021-05-12 | 2024-02-02 | 中国科学院上海药物研究所 | Phthalimide compound, pharmaceutical composition, preparation method and application thereof |
| WO2023222565A1 (en) | 2022-05-16 | 2023-11-23 | Institut National de la Santé et de la Recherche Médicale | Methods for assessing the exhaustion of hematopoietic stems cells induced by chronic inflammation |
Citations (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2341925A1 (en) * | 1973-08-20 | 1975-03-06 | Thomae Gmbh Dr K | 2,4, (opt.5-), 6-substd. pyriminidines as antithrombic agents - e.g. 6-methyl-5-nitro-2-piperazino-4-thiomorpolino-pyrimidine |
| DE2831850A1 (en) * | 1978-07-20 | 1980-02-07 | Basf Ag | Diuretic n-carboxy:phenyl:sulphonyl pyrrole derivs. - prepd. from substd. 3-sulphonamido:benzoic acid |
| EP0556889A1 (en) * | 1992-02-18 | 1993-08-25 | Duphar International Research B.V | Method of preparing aryl(homo)piperazines |
| RU938559C (en) * | 1980-12-12 | 1993-11-30 | Всесоюзный научно-исследовательский химико-фармацевтический институт им.Серго Орджоникидзе | S-derivatives of 5-amyno-6-mercaptopyrimidine possessing antitumoral and cytostatic activity |
| EP0667343A1 (en) * | 1994-01-18 | 1995-08-16 | Sandoz Ltd. | Alpha-pyrimidinyl acrylic acid derivatives with fungicidal activity |
| WO1996033994A1 (en) * | 1995-04-28 | 1996-10-31 | Nippon Soda Co., Ltd. | Amino-substituted derivatives, process for the preparation thereof, and herbicide |
| WO1996036613A1 (en) * | 1995-05-19 | 1996-11-21 | Nippon Soda Co., Ltd. | Substituted benzoic acid derivatives, process for the production thereof, and herbicides |
| WO1997026252A1 (en) * | 1996-01-19 | 1997-07-24 | Fmc Corporation | Insecticidal n-heterocyclylalkyl- or n-[(polycyclyl)-alkyl]-n'-substituted piperazines |
| EP0801059A1 (en) * | 1994-11-29 | 1997-10-15 | Dainippon Pharmaceutical Co., Ltd. | Indole derivative |
| US5849759A (en) * | 1995-12-08 | 1998-12-15 | Berlex Laboratories, Inc. | Naphthyl-substituted benzimidazole derivatives as anti-coagulants |
| EP0940387A1 (en) * | 1995-10-26 | 1999-09-08 | Tokyo Tanabe Company Limited | Phenylethanolamine compounds useful as beta3 agonist, process for producing the same, and intermediates in the production of the same |
| US6187777B1 (en) * | 1998-02-06 | 2001-02-13 | Amgen Inc. | Compounds and methods which modulate feeding behavior and related diseases |
| WO2001027107A2 (en) * | 1999-10-12 | 2001-04-19 | Bristol-Myers Squibb Company | Heterocyclic sodium/proton exchange inhibitors and method |
| WO2001049677A1 (en) * | 1999-12-30 | 2001-07-12 | H. Lundbeck A/S | Phenylpiperazinyl derivatives |
| WO2001062233A2 (en) * | 2000-02-25 | 2001-08-30 | F. Hoffmann La Roche Ag | Adenosine receptor modulators |
| WO2002002549A1 (en) * | 2000-07-05 | 2002-01-10 | Taisho Pharmaceutical Co., Ltd. | Tetrahydropyridino or piperidino heterocyclic derivatives |
| WO2002006274A1 (en) * | 2000-07-17 | 2002-01-24 | Wyeth | N-(4-sulfonylaryl)cyclylamine-2-hydroxyethylamines as beta-3 adrenergic receptor agonists |
| US6414002B1 (en) * | 1999-09-22 | 2002-07-02 | Bristol-Myers Squibb Company | Substituted acid derivatives useful as antidiabetic and antiobesity agents and method |
| WO2002098864A1 (en) * | 2001-06-01 | 2002-12-12 | F. Hoffmann-La Roche Ag | Pyrimidine, triazine and pyrazine derivatives as glutamate receptors |
| WO2003002544A1 (en) * | 2001-06-26 | 2003-01-09 | Bristol-Myers Squibb Company | N-heterocyclic inhibitors of tnf-alpha expression |
| WO2003077656A1 (en) * | 2002-03-15 | 2003-09-25 | Ciba Specialty Chemicals Holding Inc. | 4-aminopyrimidines and their use for the antimicrobial treatment of surfaces |
| WO2004000843A1 (en) * | 2002-06-24 | 2003-12-31 | Astrazeneca Ab | NOVEL PURINE- OR PYRROLOL[2,3-d]PYRIMIDINE-2-CARBONITILES FOR TREATING DISEASES ASSOCIATED WITH CYSTEINE PROTEASE ACTIVITY |
| WO2004000819A1 (en) * | 2002-06-24 | 2003-12-31 | Astrazeneca Ab | New use of pyrimidine - or triazine- 2-carbonitiles for treating diseases associated with cysteine protease activity and novel pyrimidine-2-carbonitile derivatives |
| WO2004029204A2 (en) * | 2002-09-27 | 2004-04-08 | Merck & Co., Inc. | Substituted pyrimidines |
Family Cites Families (320)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CH389134A (en) | 1960-03-15 | 1965-03-15 | Ciba Geigy | Process for the production of new anthraquinone vat dyes |
| CH478818A (en) * | 1965-10-22 | 1969-09-30 | Ciba Geigy | Process for the preparation of new N, N'-di- (pyrimidyl- (4) -aminoalkyl) -diazacycloalkanes |
| CH480410A (en) | 1967-01-09 | 1969-10-31 | Geigy Ag J R | Process for the preparation of water-soluble azopyrimidine dyes |
| CA961052A (en) * | 1967-01-12 | 1975-01-14 | Max Schellenbaum | N-2-ethylhexyl-n'-aryl ureas and preparation containing them |
| FR1593586A (en) | 1967-10-17 | 1970-06-01 | ||
| US3608087A (en) * | 1968-06-21 | 1971-09-21 | Merck & Co Inc | Feed compositions |
| US3887329A (en) * | 1969-05-05 | 1975-06-03 | Ciba Geigy Ag | Hexamethyl phosphotriamide-dye compositions |
| BE756953A (en) | 1969-10-02 | 1971-04-01 | Merck & Co Inc | POTENTIALIZATION OF ANTIBIOTICS |
| US3686238A (en) * | 1970-01-19 | 1972-08-22 | Syntex Corp | Glycerol esterified with 2-naphthyl-acetic acids and fatty acids |
| US3852434A (en) * | 1970-09-11 | 1974-12-03 | Merck & Co Inc | Potentiation of ({31 ) cis-1,2-epoxypropyl)phosphonic acid and analogues thereof |
| US3966744A (en) * | 1971-01-11 | 1976-06-29 | Syva Company | Spin labeled compounds |
| US3690834A (en) * | 1971-01-11 | 1972-09-12 | Syva Co | Ligand determination with spin labeled compounds by receptor displacement |
| DE2106585A1 (en) * | 1971-02-11 | 1972-08-24 | Farbenfabriken Bayer Ag, 5090 Leverkusen | Aminothiodiazoles and thiodiazole azo dyes |
| CH560197A5 (en) | 1971-05-17 | 1975-03-27 | Ciba Geigy Ag | 2-alkylthio-4,6-bis (subst amino)-5-nitropyrimidines - - herbicides |
| CH558137A (en) | 1971-05-17 | 1975-01-31 | Ciba Geigy Ag | MEANS OF INFLUENCING PLANT GROWTH. |
| US3966764A (en) * | 1972-07-10 | 1976-06-29 | Syva Company | Ligand determination of spin labeled compounds by receptor displacement-amphetamine analogs |
| US3849420A (en) * | 1972-10-20 | 1974-11-19 | Dow Chemical Co | Bis-(alkylthio-and alkylsulfonyl)-pentachloroquinolines |
| CH574206A5 (en) | 1972-11-16 | 1976-04-15 | Ciba Geigy Ag | |
| DE2340569C2 (en) * | 1973-08-10 | 1982-12-02 | Bayer Ag, 5090 Leverkusen | Azo dyes |
| AT340933B (en) * | 1973-08-20 | 1978-01-10 | Thomae Gmbh Dr K | PROCESS FOR THE PRODUCTION OF NEW PYRIMIDE DERIVATIVES AND THEIR ACID ADDITIONAL SALTS |
| JPS5517382Y2 (en) | 1973-11-14 | 1980-04-22 | ||
| US4101541A (en) * | 1973-12-21 | 1978-07-18 | Ciba-Geigy Corporation | 3-Cyano-1,2,4-thiadiazolyl-5-czo dyestuffs |
| CH584739A5 (en) | 1973-12-21 | 1977-02-15 | Ciba Geigy Ag | |
| FR2258841B1 (en) | 1974-01-29 | 1977-11-04 | Ugine Kuhlmann | |
| AT327605B (en) | 1974-05-06 | 1976-02-10 | Ciba Geigy Ag | AGENTS FOR INHIBITING PLANT GROWTH |
| AU492126B2 (en) | 1974-05-14 | 1975-11-20 | Ciba-Geigy Ag | Nitropyrimidine derivatives |
| FR2306697A1 (en) | 1975-04-10 | 1976-11-05 | Sogeras | NEW PYRIMIDINES FOR USE AS ANTIDIABETIC AND HYPOCHOLESTEROLEMANT MEDICINAL PRODUCTS |
| US4139705A (en) | 1977-05-20 | 1979-02-13 | The Dow Chemical Company | Pyrazolopyrimidines |
| US4189579A (en) | 1977-05-20 | 1980-02-19 | The Dow Chemical Company | Aminoalkylthiopurines |
| DE2731264A1 (en) | 1977-07-11 | 1979-02-01 | Boehringer Mannheim Gmbh | NEW 1-ACYL-2-CYANAZIRIDINE, METHOD FOR THE PRODUCTION THEREOF AND PHARMACEUTICAL PREPARATIONS CONTAINING THESE COMPOUNDS |
| JPS6038696B2 (en) * | 1977-12-09 | 1985-09-02 | コニカ株式会社 | Silver halide color photographic material |
| US4242507A (en) * | 1978-02-23 | 1980-12-30 | Fujisawa Pharmaceutical Co., Ltd. | Sulfonic acid esters |
| JPS556283A (en) | 1978-06-29 | 1980-01-17 | Sanyo Electric Co Ltd | Electronic wrist watch |
| DE2831580C2 (en) | 1978-07-18 | 1980-09-18 | Boehringer Mannheim Gmbh, 6800 Mannheim | Method and reagent for the determination of glycerin |
| DE2906603A1 (en) * | 1979-02-21 | 1980-09-04 | Boehringer Mannheim Gmbh | N-SUBSTITUTED AZIRIDINE-2-CARBONIC ACID DERIVATIVES, METHOD FOR THE PRODUCTION THEREOF AND MEDICINAL PRODUCTS CONTAINING THESE SUBSTANCES |
| US4343804A (en) | 1979-03-26 | 1982-08-10 | A. H. Robins Company, Inc. | 4-Amino-3-quinolinecarboxylic acids and esters-antisecretory anti-ulcer compounds |
| DE3070304D1 (en) | 1980-04-28 | 1985-04-25 | Teijin Ltd | Thiazolo(3,2-a)pyrimidine derivatives, process for preparing same, and drug containing same |
| EP0053678A1 (en) | 1980-12-05 | 1982-06-16 | BASF Aktiengesellschaft | 5-Amino-1-phenyl-4-cyano pyrazoles, herbicides containing them, process for their preparation and their use as herbicides |
| DOP1981004033A (en) | 1980-12-23 | 1990-12-29 | Ciba Geigy Ag | PROCEDURE TO PROTECT CROP PLANTS FROM PHYTOTOXIC ACTION OF HERBICIDES. |
| DE3334455A1 (en) | 1983-03-04 | 1984-09-06 | Bayer Ag, 5090 Leverkusen | GUANIDIN DERIVATIVES |
| US4612376A (en) | 1983-03-25 | 1986-09-16 | Fujisawa Pharmaceutical Co., Ltd. | Substituted-3,4-dihydro-4-(2,4,6-trimethoxyphenylimino)-2(1H)-pyrimidones useful as cardiotonic, antihypertensive, cerebrovascular vasodilator and anti-platelet agent |
| ZA848275B (en) | 1983-12-28 | 1985-08-28 | Degussa | New piridine-2-ethers or pyridine-2-thioethers having a nitrogen-containing cycloaliphatic ring |
| DE3406329A1 (en) | 1984-02-22 | 1985-08-22 | Merck Patent Gmbh, 6100 Darmstadt | PYRIDONE |
| JPS6157587A (en) | 1984-08-29 | 1986-03-24 | Shionogi & Co Ltd | Condensed heterocyclic derivative and antiulcerative |
| PH22302A (en) | 1985-02-11 | 1988-07-22 | Fujisawa Pharmaceutical Co | Piperidine compounds |
| AU601145B2 (en) | 1985-03-01 | 1990-09-06 | Duphar International Research B.V. | Benzoyl urea derivatives having anti-tumor activity |
| DE3601196A1 (en) | 1986-01-17 | 1987-07-23 | Merck Patent Gmbh | 1,4-DIHYDROPYRIDINE |
| JPH0533359Y2 (en) | 1986-08-04 | 1993-08-25 | ||
| CA1340284C (en) | 1987-03-19 | 1998-12-22 | Zeneca Inc. | Herbicidal substituted cyclic diones |
| DE68926687T2 (en) | 1988-01-11 | 1997-03-06 | Fuji Photo Film Co Ltd | Process for the generation of extremely high-contrast negative images |
| NZ238863A (en) | 1990-07-19 | 1993-03-26 | Janssen Pharmaceutica Nv | Substituted thiazolyl and pyridinyl derivatives |
| WO1992012976A1 (en) | 1991-01-16 | 1992-08-06 | Yoshitomi Pharmaceutical Industries, Ltd. | Use of pyridine compound as selective drug and novel pyridine compound |
| CA2070978A1 (en) | 1991-06-11 | 1992-12-12 | William J. Greenlee | Cyclic renin inhibitors |
| GB9114760D0 (en) | 1991-07-09 | 1991-08-28 | Pfizer Ltd | Therapeutic agents |
| JPH0533359A (en) | 1991-08-02 | 1993-02-09 | Kubota Corp | Small-sized back hoe of full swing type |
| DE4208254A1 (en) | 1992-03-14 | 1993-09-16 | Hoechst Ag | SUBSTITUTED PYRIMIDINE, METHOD FOR THE PRODUCTION THEREOF AND THEIR USE AS A PEST CONTROL AND FUNGICIDE |
| TW237456B (en) | 1992-04-09 | 1995-01-01 | Ciba Geigy | |
| FR2692575B1 (en) | 1992-06-23 | 1995-06-30 | Sanofi Elf | NOVEL PYRAZOLE DERIVATIVES, PROCESS FOR THEIR PREPARATION AND PHARMACEUTICAL COMPOSITIONS CONTAINING THEM. |
| NZ314207A (en) | 1992-09-28 | 2000-12-22 | Vertex Pharma | 1-(2-Oxoacetyl)-piperidine-2-carboxylic acid derivatives as multi drug resistant cancer cell sensitizers |
| DE4241632A1 (en) | 1992-12-10 | 1994-06-16 | Thomae Gmbh Dr K | New (aza)aromatic or alicyclic carboxylic acid cpds. - used e.g. for treating thrombosis, inflammation, osteoporosis, cardiac infarct or tumour metastasis |
| TW370529B (en) | 1992-12-17 | 1999-09-21 | Pfizer | Pyrazolopyrimidines |
| JPH0753546B2 (en) | 1992-12-28 | 1995-06-07 | 山本 優 | Manufacturing method of coreless paper roll, device used for it and winding shaft |
| JPH0753546A (en) | 1993-08-09 | 1995-02-28 | Kuraray Co Ltd | Diaryl-substituted heterocyclic compound and its medical use |
| CZ101496A3 (en) | 1993-10-12 | 1996-11-13 | Du Pont Merck Pharma | N-alkyl-n-aryl-pyrimidinamines and derivatives thereof |
| DE69430581D1 (en) * | 1993-10-14 | 2002-06-13 | Abbott Lab | CHINOLIZINONE TYPE COMPOUNDS |
| TW574214B (en) | 1994-06-08 | 2004-02-01 | Pfizer | Corticotropin releasing factor antagonists |
| HU221855B1 (en) | 1994-09-09 | 2003-02-28 | Nippon Shinyaku Co., Ltd. | Substituted piridin-, pyrimidin- and triazine derivatives and pharmaceutical compositions containing such compounds |
| WO1996028427A1 (en) | 1995-03-10 | 1996-09-19 | Berlex Laboratories, Inc. | Benzamidine derivatives their preparation and their use as anti-coagulants |
| US5691364A (en) * | 1995-03-10 | 1997-11-25 | Berlex Laboratories, Inc. | Benzamidine derivatives and their use as anti-coagulants |
| IL117659A (en) | 1995-04-13 | 2000-12-06 | Dainippon Pharmaceutical Co | Substituted 2-phenyl pyrimidino amino acetamide derivative process for preparing the same and a pharmaceutical composition containing same |
| GB9508538D0 (en) | 1995-04-27 | 1995-06-14 | Zeneca Ltd | Quinazoline derivatives |
| JPH10506126A (en) | 1995-05-12 | 1998-06-16 | ニューロゲン コーポレイション | Novel deazapurine derivatives; a new class of CRF1 specific ligands |
| US6403599B1 (en) | 1995-11-08 | 2002-06-11 | Pfizer Inc | Corticotropin releasing factor antagonists |
| US6956047B1 (en) | 1995-06-06 | 2005-10-18 | Pfizer Inc. | Corticotropin releasing factor antagonists |
| TW438587B (en) | 1995-06-20 | 2001-06-07 | Takeda Chemical Industries Ltd | A pharmaceutical composition for prophylaxis and treatment of diabetes |
| KR0169813B1 (en) | 1995-07-12 | 1999-01-15 | 김종인 | 4-amino-3-acylnaphthyridine derivatives |
| US6248571B1 (en) | 1995-08-31 | 2001-06-19 | Lonza Ag | Method of producing dihydroxypyrimidine derivatives |
| JP4022271B2 (en) | 1995-10-31 | 2007-12-12 | 富士フイルム株式会社 | Pyrazolylazophenol dye |
| DE19602095A1 (en) | 1996-01-22 | 1997-07-24 | Bayer Ag | Halopyrimidines |
| AU708055B2 (en) | 1996-02-02 | 1999-07-29 | Merck & Co., Inc. | Heterocyclic derivatives as antidiabetic and antiobesity agents |
| PL191271B1 (en) | 1996-02-07 | 2006-04-28 | Neurocrine Biosciences Inc | Pyrazole pyrimidines as antagonists of crf receptor |
| US5948786A (en) * | 1996-04-12 | 1999-09-07 | Sumitomo Pharmaceuticals Company, Limited | Piperidinylpyrimidine derivatives |
| DE122010000020I1 (en) | 1996-04-25 | 2010-07-08 | Prosidion Ltd | Method for lowering the blood glucose level in mammals |
| DE19624659A1 (en) | 1996-06-20 | 1998-01-08 | Klinge Co Chem Pharm Fab | New pyridylalkene and pyridylalkanoic acid amides |
| AU3176297A (en) | 1996-06-25 | 1998-01-14 | Novartis Ag | Substituted 7-amino-pyrrolo{3,2-d}pyrimidines and the use thereof |
| US6060478A (en) | 1996-07-24 | 2000-05-09 | Dupont Pharmaceuticals | Azolo triazines and pyrimidines |
| AR008789A1 (en) | 1996-07-31 | 2000-02-23 | Bayer Corp | PIRIDINES AND SUBSTITUTED BIPHENYLS |
| TW477787B (en) | 1996-08-27 | 2002-03-01 | Pfizer | Pyrido six-membered nitrogen-containing cyclic ring derivatives having corticotropin releasing factor antagonist activity and pharmaceutical composition containing same |
| EP0923582B1 (en) | 1996-08-28 | 2006-09-20 | Pfizer Inc. | Substituted 6,5-hetero-bicyclic derivatives |
| JP3565864B2 (en) | 1996-09-12 | 2004-09-15 | シエーリング アクチエンゲゼルシヤフト | Benzamidine derivatives substituted by cyclic amino acids or cyclic hydroxy acid derivatives and their use as anti-pseudo-solids |
| US6008234A (en) | 1996-09-12 | 1999-12-28 | Berlex Laboratories, Inc. | Benzamidine derivatives substituted by cyclic amino acid and cyclic hydroxy acid derivatives and their use as anti-coagulants |
| GB9718972D0 (en) | 1996-09-25 | 1997-11-12 | Zeneca Ltd | Chemical compounds |
| TW492957B (en) | 1996-11-07 | 2002-07-01 | Novartis Ag | N-substituted 2-cyanopyrrolidnes |
| DZ2376A1 (en) | 1996-12-19 | 2002-12-28 | Smithkline Beecham Plc | New sulfonamide derivatives process for their preparation and pharmaceutical compositions containing them. |
| CA2229123A1 (en) | 1997-02-11 | 1998-08-11 | Mitchell Irvin Steinberg | Pharmaceutical agents |
| WO1998035967A2 (en) | 1997-02-18 | 1998-08-20 | Neurocrine Biosciences, Inc. | Biazacyclic crf antagonists |
| ATE250035T1 (en) | 1997-04-22 | 2003-10-15 | Janssen Pharmaceutica Nv | QUINOLINE AND QUINAZOLINE DERIVATIVES AS CRF ANTAGONISTS |
| JP2002501493A (en) | 1997-04-22 | 2002-01-15 | ジヤンセン・フアーマシユーチカ・ナームローゼ・フエンノートシヤツプ | Thiophenopyridines as CRF antagonists |
| JPH11193277A (en) | 1997-05-14 | 1999-07-21 | Nippon Soda Co Ltd | Pyrimidine derivative, its production and harmful organism controller |
| DE19737723A1 (en) | 1997-08-14 | 1999-02-18 | Bayer Ag | New methoximino:methyl-di:hydro-1,2,4-oxadiazine derivatives |
| BR9811939A (en) | 1997-08-14 | 2000-09-05 | Bayer Ag | Methoxyiminomethyloxadiazines as pesticides |
| NL1010018C2 (en) | 1997-09-09 | 1999-03-10 | Duphar Int Res | Quinoline and quinazoline derivatives with corticotropin releasing factor (CRF) antagonistic effect. |
| EP1068206A1 (en) | 1998-04-02 | 2001-01-17 | Neurogen Corporation | Aminoalkyl substituted pyrrolo 2,3-b]pyridine and pyrrolo 2,3-d]pyrimidine derivatives: modulators of crf1 receptors |
| JP2000038350A (en) | 1998-05-18 | 2000-02-08 | Yoshitomi Pharmaceut Ind Ltd | Diabetes therapeutic drug |
| AU5777299A (en) | 1998-08-21 | 2000-03-14 | Du Pont Pharmaceuticals Company | Isoxazolo(4,5-d)pyrimidines as CRF antagonists |
| KR100658489B1 (en) | 1998-11-10 | 2006-12-18 | 얀센 파마슈티카 엔.브이. | HIV replication inhibiting pyrimidines |
| US6262088B1 (en) | 1998-11-19 | 2001-07-17 | Berlex Laboratories, Inc. | Polyhydroxylated monocyclic N-heterocyclic derivatives as anti-coagulants |
| CA2645717A1 (en) | 1998-11-20 | 2000-06-02 | Arena Pharmaceuticals, Inc. | Human orphan g protein-coupled receptors |
| CO5150173A1 (en) | 1998-12-10 | 2002-04-29 | Novartis Ag | COMPOUNDS N- (REPLACED GLYCLE) -2-DIPEPTIDYL-IV PEPTIDASE INHIBITING CYANOPIRROLIDINS (DPP-IV) WHICH ARE EFFECTIVE IN THE TREATMENT OF CONDITIONS MEDIATED BY DPP-IV INHIBITION |
| AU3123500A (en) | 1998-12-17 | 2000-07-03 | American Home Products Corporation | Arylpiperidine and aryl-1,2,5,6-tetrahydropyridine urea derivatives having 5ht1areceptor activity |
| US6239126B1 (en) | 1998-12-17 | 2001-05-29 | American Home Products Corporation | Arylpiperidine and aryl-1,2,5,6-tetra-hydropyridine urea derivatives |
| US6867200B1 (en) | 1998-12-18 | 2005-03-15 | Axys Pharmaceuticals, Inc. | (Hetero)aryl-bicyclic heteroaryl derivatives, their preparation and their use as protease inhibitors |
| CZ27399A3 (en) | 1999-01-26 | 2000-08-16 | Ústav Experimentální Botaniky Av Čr | Substituted nitrogen heterocyclic derivatives process of their preparation, the derivatives employed as medicaments, pharmaceutical composition and a compound pharmaceutical preparation in which these derivatives are comprised as well as use of these derivatives for preparing medicaments |
| US6267985B1 (en) | 1999-06-30 | 2001-07-31 | Lipocine Inc. | Clear oil-containing pharmaceutical compositions |
| PT1163237E (en) | 1999-03-17 | 2004-08-31 | Astrazeneca Ab | AMIDA DERIVATIVES |
| EP1040831A3 (en) | 1999-04-02 | 2003-05-02 | Pfizer Products Inc. | Use of corticotropin releasing factor (CRF) antagonists to prevent sudden death |
| EP1074549B1 (en) | 1999-08-06 | 2003-11-19 | F. Hoffmann-La Roche Ag | Tetrahydro-benzo(d)azepines and their use as antagonists at metabotropic glutamate receptors |
| US6921763B2 (en) | 1999-09-17 | 2005-07-26 | Abbott Laboratories | Pyrazolopyrimidines as therapeutic agents |
| DK1212327T3 (en) | 1999-09-17 | 2003-12-15 | Abbott Gmbh & Co Kg | Pyrazolopyrimidines as therapeutic agents |
| JP2001089452A (en) | 1999-09-22 | 2001-04-03 | Sankyo Co Ltd | Pyrimidine derivative |
| PT1225874E (en) | 1999-09-24 | 2006-06-30 | Janssen Pharmaceutica Nv | ANTIVIRAL COMPOSITIONS. |
| IL148905A0 (en) | 1999-09-30 | 2002-09-12 | Neurogen Corp Pfizer Inc | Certain alkylene diamine-substituted pyrazolo{1,5,-a}-1,5-pyrimidines and pyrazolo{1,5,-a}-1,3,5-triazines |
| DE60026155T2 (en) | 1999-09-30 | 2006-08-10 | Neurogen Corp., Branford | SOME ALKYLENDIAMINE-SUBSTITUTED HETEROCYCLES |
| OA12049A (en) | 1999-09-30 | 2006-05-02 | Neurogen Corp | Amino substituted pyrazoloÄ1,5,-aÜ-1,5-pyrimidinesand pyrazoloÄ1,5,-aÜ-1,3,5-triazines. |
| DE19947154A1 (en) | 1999-10-01 | 2001-10-04 | Bayer Ag | Substituted 2-thio-3,5-dicyano-4-aryl-6-aminopyridines and their use |
| CO5271670A1 (en) | 1999-10-29 | 2003-04-30 | Pfizer Prod Inc | ANTIGONISTS OF THE CORTICITROPINE RELEASE FACTOR AND RELATED COMPOSITIONS |
| GR990100388A (en) | 1999-11-09 | 2001-07-31 | PHARMACEUTICAL COMBINATION COMPRISING SEPARATE DOSAGE FORMS IN A COMPLIANCE PACKAGE OF AN INHIBITOR OF HMG CoA REDUCTASE AND A FIBRIC ACID DERIVATIVE, PROVIDING FOR PATIENT'S COMPLIANCE AND ANTIPATED TO RETAIN EFFICACY, IMPROVE SAFETY AND REDUCE.... | |
| JP2003518128A (en) | 1999-12-22 | 2003-06-03 | メルク フロスト カナダ アンド カンパニー | Aromatic phosphonates as inhibitors of protein tyrosine phosphatase 1B (PTP-1B) |
| DE19962936A1 (en) | 1999-12-24 | 2001-06-28 | Bayer Ag | New beta-aminoacid amide derivatives, are integrin antagonists useful for treating inflammatory, autoimmune or immunological disorders e.g. asthma, diabetes or rheumatoid arthritis |
| OA12157A (en) | 2000-01-18 | 2006-05-08 | Pfizer Prod Inc | Corticotropin releasing factor antagonists. |
| IL150841A0 (en) | 2000-02-09 | 2003-02-12 | Hokuriku Pharmaceutical | 1h-imidazopyridine derivatives |
| WO2001060870A1 (en) | 2000-02-15 | 2001-08-23 | Foster Miller, Inc. | No voc radiation curable resin compositions |
| AU784722B2 (en) | 2000-02-18 | 2006-06-01 | Merck & Co., Inc. | Aryloxyacetic acids for diabetes and lipid disorders |
| US6569879B2 (en) | 2000-02-18 | 2003-05-27 | Merck & Co., Inc. | Aryloxyacetic acids for diabetes and lipid disorders |
| EP1326604A2 (en) | 2000-04-12 | 2003-07-16 | Novartis AG | Combination of at least two compounds selected from an at1-receptorantagonist or an ace inhibitor or a hmg-co-a reductase inhibitor groups |
| WO2001085699A2 (en) | 2000-05-08 | 2001-11-15 | Janssen Pharmaceutica N.V. | Prodrugs of hiv replication inhibiting pyrimidines |
| DE10024319A1 (en) | 2000-05-17 | 2001-11-22 | Merck Patent Gmbh | New bis-acylguanidine compounds are subtype-1 cellular sodium ion-proton antiporter inhibitors useful e.g. for treating arrhythmia, angina pectoris, infarction and insulin-independent diabetes mellitus |
| WO2001087892A1 (en) | 2000-05-18 | 2001-11-22 | Neurocrine Biosciences, Inc. | Crf receptor antagonists |
| ATE348162T1 (en) | 2000-05-18 | 2007-01-15 | Bayer Healthcare Ag | REGULATION OF A HUMAN DOPAMINE-LIKE G-PROTEIN COUPLED RECEPTOR. |
| YU89002A (en) | 2000-05-25 | 2006-01-16 | F. Hoffmann-La Roche Ag. | Substituted 1-aminoalkyl-lactams and their use as muscarinic receptors antagonists |
| US6620821B2 (en) | 2000-06-15 | 2003-09-16 | Bristol-Myers Squibb Company | HMG-CoA reductase inhibitors and method |
| MXPA02012795A (en) * | 2000-06-28 | 2004-07-30 | Teva Pharma | Carvedilol. |
| WO2002002539A1 (en) | 2000-06-29 | 2002-01-10 | Abbott Laboratories | Aryl phenylheterocyclyl sulfide derivatives and their use as cell adhesion-inhibiting anti-inflammatory and immune-suppressive agents |
| CN1441783A (en) | 2000-07-18 | 2003-09-10 | 山之内制药株式会社 | Medicine comprising dicyanopyridine derivative |
| ATE362468T1 (en) | 2000-07-25 | 2007-06-15 | Merck & Co Inc | N-SUBSTITUTED INDOLES WITH APPLICATION IN THE TREATMENT OF DIABETES |
| WO2002019975A1 (en) | 2000-09-05 | 2002-03-14 | Taisho Pharmaceutical Co., Ltd. | Hair growth stimulants |
| AU2001262945B2 (en) | 2000-09-20 | 2006-02-02 | Skyepharma Canada Inc. | Spray drying process and compositions of fenofibrate |
| US7276249B2 (en) | 2002-05-24 | 2007-10-02 | Elan Pharma International, Ltd. | Nanoparticulate fibrate formulations |
| US20030224058A1 (en) | 2002-05-24 | 2003-12-04 | Elan Pharma International, Ltd. | Nanoparticulate fibrate formulations |
| PE20020507A1 (en) | 2000-10-17 | 2002-06-25 | Schering Corp | NON-IMIDAZOLE COMPOUNDS AS ANTAGONISTS OF THE HISTAMINE H3 RECEPTOR |
| AU2002223626A1 (en) | 2000-10-20 | 2002-04-29 | Novartis Ag | Combinations of a thyromimetic compound and a statin |
| DK1333833T3 (en) | 2000-10-23 | 2011-12-12 | Glaxosmithkline Llc | New trisubstituted 8H-pyridol [2,3-d] pyrimidin-7-one compound for the treatment of CSBP / RK / p38 kinase-mediated diseases |
| EP1347755A2 (en) | 2000-10-31 | 2003-10-01 | Merck & Co., Inc. | Benzopyrancarboxylic acid derivatives for the treatment of diabetes and lipid disorders |
| JP2004529075A (en) | 2000-11-03 | 2004-09-24 | ニューロクライン バイオサイエンシーズ, インコーポレイテッド | CRF receptor antagonists and methods related thereto |
| US20020058026A1 (en) | 2000-11-13 | 2002-05-16 | Milton Hammerly | HMG CoA reductase inhibitor medications combined wih CoEnzyme Q-10 |
| EP1340749A4 (en) | 2000-11-17 | 2007-09-05 | Takeda Pharmaceutical | Isoxazole derivatives |
| UA77165C2 (en) | 2000-11-17 | 2006-11-15 | Lilly Co Eli | (n)-((s)-2-hydroxy-3-methyl-butyryl)-1-(l-alaninyl)-(s)-1-amino-3-methyl-4,5,6,7-tetrahydro-2h-3-benzazepin-2-one dihydrate, processes for manufacturing and pharmaceutical composition |
| CA2448729A1 (en) | 2000-11-20 | 2002-05-23 | Biovitrum Ab | Piperazinyl and piperidyl substituted heterocyclic compounds |
| US20030180813A1 (en) | 2000-12-01 | 2003-09-25 | Takahide Ohishi | Method of screening remedy for diabetes |
| US20020137755A1 (en) * | 2000-12-04 | 2002-09-26 | Bilodeau Mark T. | Tyrosine kinase inhibitors |
| US6545017B1 (en) | 2000-12-08 | 2003-04-08 | 3M Innovative Properties Company | Urea substituted imidazopyridines |
| US6525064B1 (en) | 2000-12-08 | 2003-02-25 | 3M Innovative Properties Company | Sulfonamido substituted imidazopyridines |
| US6545016B1 (en) | 2000-12-08 | 2003-04-08 | 3M Innovative Properties Company | Amide substituted imidazopyridines |
| US20020107262A1 (en) | 2000-12-08 | 2002-08-08 | 3M Innovative Properties Company | Substituted imidazopyridines |
| AU2002231139B2 (en) | 2000-12-21 | 2007-03-22 | Bristol-Myers Squibb Company | Thiazolyl inhibitors of tec family tyrosine kinases |
| CZ305202B6 (en) | 2001-01-26 | 2015-06-10 | Merck Sharp & Dohme Corp. | Pharmaceutical composition |
| EP1357908A4 (en) | 2001-01-30 | 2009-07-15 | Merck & Co Inc | Acyl sulfamides for treatment of obesity, diabetes and lipid disorders |
| WO2002098878A1 (en) | 2001-02-08 | 2002-12-12 | Memory Pharmaceuticals Corporation | Trifluoromethylpurines as phosphodiesterase 4 inhibitors |
| AU2002251978B2 (en) | 2001-02-09 | 2007-07-19 | Merck & Co., Inc. | 2-aryloxy-2-arylalkanoic acids for diabetes and lipid disorders |
| DE10110754A1 (en) | 2001-03-07 | 2002-09-19 | Bayer Ag | Substituted 2-thio-3,5-dicyano-4-aryl-6-aminopyridines and their use |
| WO2002072101A1 (en) | 2001-03-13 | 2002-09-19 | Bristol-Myers Squibb Pharma Company | A corticotropin releasing factor receptor ligand, its enantiomer and pharmaceutically acceptable salts |
| WO2002081454A1 (en) | 2001-04-09 | 2002-10-17 | Dr. Reddy's Laboratories Ltd. | Derivatives of aryl acids, their use in medicine, process for their preparation and pharmaceutical compositions containing them |
| JP4323810B2 (en) | 2001-04-20 | 2009-09-02 | ワイス | Heterocyclyloxy-, -thioxy- and -aminobenzazole derivatives as 5-hydroxytryptamine-6 ligands |
| HUP0402352A2 (en) | 2001-06-19 | 2005-02-28 | Bristol-Myers Squibb Co. | Pyrimidine derivatives for use as phosphodiesterase (pde)7 inhibitors and pharmaceutical compositions containing them |
| US6825198B2 (en) | 2001-06-21 | 2004-11-30 | Pfizer Inc | 5-HT receptor ligands and uses thereof |
| UA74912C2 (en) | 2001-07-06 | 2006-02-15 | Merck & Co Inc | Beta-aminotetrahydroimidazo-(1,2-a)-pyrazines and tetratriazolo-(4,3-a)-pyrazines as inhibitors of dipeptylpeptidase for the treatment or prevention of diabetes |
| GB0117899D0 (en) | 2001-07-23 | 2001-09-12 | Astrazeneca Ab | Chemical compounds |
| WO2003026661A1 (en) | 2001-09-14 | 2003-04-03 | Yamanouchi Pharmaceutical Co., Ltd. | Insulin secretion accelerator and novel pyrimidine derivative |
| EP1438048A1 (en) | 2001-10-18 | 2004-07-21 | Boehringer Ingelheim Pharmaceuticals Inc. | 1,4-disubstituted benzo-fused urea compounds as cytokine inhibitors |
| GB0229931D0 (en) | 2002-12-21 | 2003-01-29 | Astrazeneca Ab | Therapeutic agents |
| SE0104334D0 (en) | 2001-12-19 | 2001-12-19 | Astrazeneca Ab | Therapeutic agents |
| JP2005518408A (en) | 2001-12-29 | 2005-06-23 | ノボ ノルディスク アクティーゼルスカブ | Combination use of GLP-1 compounds and other drugs to treat dyslipidemia |
| US20050043315A1 (en) | 2002-01-02 | 2005-02-24 | Hideo Tsutsumi | Aminopyrimidine compounds, processes for their preparation and pharmaceutical compositions containing them |
| US20070225351A1 (en) | 2002-01-11 | 2007-09-27 | Lippa Arnold S | Methods and compositions for controlling body weight and appetite |
| GB0201850D0 (en) | 2002-01-26 | 2002-03-13 | Astrazeneca Ab | Therapeutic treatment |
| WO2003076418A1 (en) | 2002-03-07 | 2003-09-18 | X-Ceptor Therapeutics, Inc. | Quinazolinone modulators of nuclear receptors |
| AR039090A1 (en) | 2002-03-22 | 2005-02-09 | Novartis Ag | COMBINATION OF ORGANIC COMPOUNDS |
| WO2003088962A1 (en) | 2002-04-16 | 2003-10-30 | Merck & Co., Inc. | Combination therapy using a ppar alpha/gamma agonist |
| EP1499598A1 (en) | 2002-04-18 | 2005-01-26 | Ucb, S.A. | Chemical compounds with dual activity, processes for their preparation and pharmaceutical compositions |
| DE10219435A1 (en) | 2002-05-02 | 2003-11-13 | Bayer Cropscience Ag | Substituted pyrazolo-pyrimidin-4-ones |
| AU2003225305A1 (en) | 2002-05-08 | 2003-11-11 | Bristol-Myers Squibb Company | Pyridine-based thyroid receptor ligands |
| US7057046B2 (en) | 2002-05-20 | 2006-06-06 | Bristol-Myers Squibb Company | Lactam glycogen phosphorylase inhibitors and method of use |
| CA2488617A1 (en) | 2002-06-10 | 2003-12-18 | Eugene R. Cooper | Nanoparticulate sterol formulations and sterol combinations |
| JP4831965B2 (en) | 2002-06-10 | 2011-12-07 | エラン ファーマ インターナショナル,リミティド | Nanoparticle formulations comprising HMG-CoA reductase inhibitor derivatives ("statins"), novel combinations thereof, and the manufacture of these pharmaceutical compositions |
| AR040241A1 (en) | 2002-06-10 | 2005-03-23 | Merck & Co Inc | INHIBITORS OF 11-BETA-HYDROXIESTEROID DEHYDROGRENASE 1 FOR THE TREATMENT OF DIABETES OBESITY AND DISLIPIDEMIA |
| WO2003103632A1 (en) | 2002-06-10 | 2003-12-18 | Elan Pharma International, Ltd. | Nanoparticulate polycosanol formulations and novel polycosanol combinations |
| ATE494002T1 (en) | 2002-06-14 | 2011-01-15 | Amylin Pharmaceuticals Inc | PREVENTION AND/OR TREATMENT OF ULCEROSAL COLITIS WITH PYY OR PYYÄ3-36Ü |
| DE10226943A1 (en) | 2002-06-17 | 2004-01-08 | Bayer Ag | Phenylaminopyrimidines and their use |
| GB0214254D0 (en) | 2002-06-20 | 2002-07-31 | Glaxo Group Ltd | Chemical compounds |
| EP1531832B1 (en) | 2002-06-28 | 2009-04-15 | Glykos Finland Oy | Therapeutic compositions for use in prophylaxis or treatment of diarrheas |
| US7071210B2 (en) | 2002-07-02 | 2006-07-04 | Pfizer Inc. | CETP inhibitors in combination with antihypertensive agents and uses thereof |
| US20040053842A1 (en) | 2002-07-02 | 2004-03-18 | Pfizer Inc. | Methods of treatment with CETP inhibitors and antihypertensive agents |
| AU2003254053A1 (en) | 2002-07-23 | 2004-02-09 | Smithkline Beecham Corporation | Pyrazolopyrimidines as protein kinase inhibitors |
| EP1534389A2 (en) | 2002-07-23 | 2005-06-01 | SmithKline Beecham Corporation | Pyrazolopyrimidines as kinase inhibitors |
| JP2005536517A (en) | 2002-07-23 | 2005-12-02 | スミスクライン ビーチャム コーポレーション | Pyrazolopyrimidines as kinase inhibitors |
| EP1527345A2 (en) | 2002-07-29 | 2005-05-04 | Axxima Pharmaceuticals AG | Method for isolating atp binding proteins by means of immobilized protein inhibitors |
| EP1539136B1 (en) | 2002-07-30 | 2008-07-09 | Merck & Co., Inc. | Ppar alpha selective compounds for the treatment of dyslipidemia and other lipid disorders |
| DE60332738D1 (en) | 2002-07-30 | 2010-07-08 | Merck Sharp & Dohme | PPAR ALPHA SELECTIVE COMPOUNDS FOR THE TREATMENT OF DYSLIPIDEMIA AND OTHER LIPID DISORDERS |
| BR0313255A (en) | 2002-08-08 | 2005-07-12 | Amgen Inc | Compound, pharmaceutical composition, use of a compound and methods of making a medicament and preparing a compound |
| DE10237722A1 (en) | 2002-08-17 | 2004-08-19 | Aventis Pharma Deutschland Gmbh | Indole or benzimidazole derivatives for the modulation of IKappaB kinase |
| WO2004017896A2 (en) | 2002-08-21 | 2004-03-04 | Merck & Co., Inc. | Combination therapy using a dual ppar alpha/gamma agonist and an angiotensin ii type i receptor antagonist |
| CN100457730C (en) | 2002-08-29 | 2009-02-04 | 默克公司 | Indoles having anti-diabetic activity |
| US7393960B2 (en) | 2002-08-29 | 2008-07-01 | Merck & Co., Inc. | Indoles having anti-diabetic activity |
| CA2489405A1 (en) | 2002-09-11 | 2004-03-25 | Yamanouchi Pharmaceutical Co., Ltd. | Screening method of agents for increasing insulin content |
| US7067658B2 (en) | 2002-09-30 | 2006-06-27 | Bristol-Myers Squibb Company | Pyridino and pyrimidino pyrazinones |
| WO2004033431A2 (en) | 2002-10-04 | 2004-04-22 | Arena Pharmaceuticals, Inc. | Hydroxypyrazoles for use against metabolic-related disorders |
| WO2004033710A2 (en) | 2002-10-09 | 2004-04-22 | Genaissance Pharmaceuticals, Inc. | Itgb3 gene haplotypes and atorvastatin dose effects on hdl cholesterol |
| EP1551842A1 (en) | 2002-10-15 | 2005-07-13 | Smithkline Beecham Corporation | Pyradazine compounds as gsk-3 inhibitors |
| US7129239B2 (en) * | 2002-10-28 | 2006-10-31 | Pfizer Inc. | Purine compounds and uses thereof |
| JP2006507302A (en) | 2002-10-30 | 2006-03-02 | メルク エンド カムパニー インコーポレーテッド | Kinase inhibitor |
| US20040110241A1 (en) | 2002-12-06 | 2004-06-10 | Segal Mark S. | Materials and methods for monitoring vascular endothelial function |
| AU2003299791A1 (en) | 2002-12-20 | 2004-07-22 | Bayer Pharmaceuticals Corporation | Substituted 3,5-dihydro-4h-imidazol-4-ones for the treatment of obesity |
| JO2397B1 (en) | 2002-12-20 | 2007-06-17 | ميرك شارب اند دوم كوربوريشن | Triazole Derivatives As Inhibitors Of 11-Beta -Hydroxysteriod Dehydrogenase-1 |
| DE60336264D1 (en) | 2002-12-20 | 2011-04-14 | Bayer Pharmaceuticals Corp | INDIC ACID DERIVATIVES AND THEIR USE AS PHARMACEUTICAL AGENTS AND INTERMEDIATE PRODUCTS AND METHODS FOR THEIR MANUFACTURE |
| GB0230021D0 (en) | 2002-12-23 | 2003-01-29 | Syngenta Ltd | Fungicides |
| GB0230020D0 (en) | 2002-12-23 | 2003-01-29 | Syngenta Ltd | Fungicides |
| NZ540612A (en) | 2003-01-14 | 2008-02-29 | Arena Pharm Inc | 1,2,3-Trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
| EP1583531B1 (en) | 2003-01-16 | 2007-04-18 | SB Pharmco Puerto Rico Inc | Heteroaryl-substituted pyrrolo[2, 3- b]pyridine derivatives as crf receptor antagonists |
| JP2007504285A (en) | 2003-01-17 | 2007-03-01 | メルク エンド カムパニー インコーポレーテッド | N-cyclohexylaminocarbonylbenzenesulfonamide derivative |
| TW200418829A (en) | 2003-02-14 | 2004-10-01 | Avanir Pharmaceutics | Inhibitors of macrophage migration inhibitory factor and methods for identifying the same |
| PL378295A1 (en) | 2003-02-24 | 2006-03-20 | Arena Pharmaceuticals, Inc. | Substituted aryl and heteroaryl derivatives as modulators of glucose metabolism and the prophylaxis and treatment of disorders thereof |
| JP2004269469A (en) | 2003-03-12 | 2004-09-30 | Yamanouchi Pharmaceut Co Ltd | Pyrimidine derivative or salt thereof |
| JP2004269468A (en) | 2003-03-12 | 2004-09-30 | Yamanouchi Pharmaceut Co Ltd | Pyrimidine derivative or salt thereof |
| MXPA05010456A (en) | 2003-03-28 | 2006-03-21 | Pfizer Prod Inc | 1,2,4-substituerte 1,2,3,4-tetrahydro-and 1,2 dihydro-quinoline and 1,2,3,4-tetrahydro-quinoxaline derivatives as cetp inhibitors for the treatment of atherosclerosis and obesity. |
| TWI393560B (en) | 2003-05-02 | 2013-04-21 | Japan Tobacco Inc | Combination comprising s-[2-([[1-(2-ethylbutyl)cyclohexyl]carbonyl]amino)phenyl]2-methylpropanethioate and an hmg coa reductase inhibitor |
| DK1475094T3 (en) | 2003-05-06 | 2010-11-22 | Univ Palackeho | Pyrazolo [4,3-d] pyrimidines, processes for their preparation and their use |
| SE0301368D0 (en) | 2003-05-09 | 2003-05-09 | Astrazeneca Ab | Chemical compounds |
| US7083933B1 (en) | 2003-05-09 | 2006-08-01 | Prosidion Limited | Methods for identification of modulators of OSGPR116 activity |
| US6987118B2 (en) | 2003-05-21 | 2006-01-17 | Pfizer Inc. | Tetrahydroisoquinoline derivatives as PPAR-alpha activators |
| WO2004110368A2 (en) | 2003-06-06 | 2004-12-23 | Merck & Co., Inc. | Combination therapy for the treatment of hypertension |
| AU2003902882A0 (en) | 2003-06-10 | 2003-06-26 | Fujisawa Pharmaceutical Co., Ltd. | Piperidyl derivatives |
| AR045047A1 (en) | 2003-07-11 | 2005-10-12 | Arena Pharm Inc | ARILO AND HETEROARILO DERIVATIVES TRISUSTITUIDOS AS MODULATORS OF METABOLISM AND PROFILAXIS AND TREATMENT OF DISORDERS RELATED TO THEMSELVES |
| CN100509798C (en) | 2003-07-11 | 2009-07-08 | 艾尼纳制药公司 | Trisubstituted aryl and heteroaryl derivatives as modulatorsof metabolism and the prophylaxis and treatment of disorders related thereto |
| AU2004257267B2 (en) | 2003-07-14 | 2009-12-03 | Arena Pharmaceuticals,Inc | Fused-aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto |
| EP2287156B1 (en) | 2003-08-15 | 2013-05-29 | Novartis AG | 2,4-Di(phenylamino)-pyrimidines useful in the treatment of neoplastic diseases, inflammatory and immune system disorders |
| US20050043327A1 (en) | 2003-08-21 | 2005-02-24 | Pfizer Inc | Pharmaceutical composition for the prevention and treatment of addiction in a mammal |
| CN100457108C (en) | 2003-09-02 | 2009-02-04 | 默克公司 | Novel crystalline forms of a phosphoric acid salt of a dipeptidyl peptidase-iv inhibitor |
| WO2005023762A1 (en) | 2003-09-04 | 2005-03-17 | Abbott Laboratories | Pyrrolidine-2-carbonitrile derivatives and their use as inhibitors of dipeptidyl peptidase-iv (dpp-iv) |
| PE20050374A1 (en) | 2003-09-05 | 2005-05-30 | Vertex Pharma | SERINE PROTEASE INHIBITORS, IN PARTICULAR HCV PROTEASE NS3-NS4A |
| US20050065144A1 (en) | 2003-09-08 | 2005-03-24 | Syrrx, Inc. | Dipeptidyl peptidase inhibitors |
| US7790734B2 (en) | 2003-09-08 | 2010-09-07 | Takeda Pharmaceutical Company Limited | Dipeptidyl peptidase inhibitors |
| WO2005025554A2 (en) | 2003-09-09 | 2005-03-24 | Japan Tobacco Inc. | Dipeptidyl peptidase iv inhibitor |
| AU2004275719B2 (en) | 2003-09-23 | 2010-08-19 | Merck Sharp & Dohme Corp. | Quinoline potassium channel inhibitors |
| WO2005030127A2 (en) | 2003-09-23 | 2005-04-07 | Merck & Co., Inc. | Novel crystalline form of a phosphoric acid salt of a dipeptidyl peptidase-iv inhibitor |
| WO2005033099A2 (en) | 2003-10-03 | 2005-04-14 | Glenmark Pharmaceuticals Ltd. | Novel dipeptidyl peptidase iv inhibitors; processes for their preparation and compositions thereof |
| US20060089335A1 (en) | 2003-10-14 | 2006-04-27 | Guosong Liu | Compositions and methods for enhancing cognitive function and synaptic plasticity |
| GB0324236D0 (en) | 2003-10-16 | 2003-11-19 | Astrazeneca Ab | Chemical compounds |
| PE20050444A1 (en) | 2003-10-31 | 2005-08-09 | Takeda Pharmaceutical | PYRIDINE COMPOUNDS AS PEPTIDASE INHIBITORS |
| AU2004289304A1 (en) | 2003-11-10 | 2005-05-26 | Synta Pharmaceuticals, Corp. | Pyridine compounds |
| KR20070054762A (en) | 2003-11-12 | 2007-05-29 | 페노믹스 코포레이션 | Heterocyclic boronic acid compounds |
| MY141220A (en) | 2003-11-17 | 2010-03-31 | Astrazeneca Ab | Pyrazole derivatives as inhibitors of receptor tyrosine kinases |
| WO2005058315A1 (en) | 2003-12-12 | 2005-06-30 | Ribapharm, Inc. | Novel heterocyclic compounds as ikk2 inhibitors with anti-hbv activity |
| WO2005058849A1 (en) | 2003-12-15 | 2005-06-30 | Glenmark Pharmaceuticals Ltd. | New dipeptidyl peptidase in inhibitors; process for their preparation and compositions containing them |
| DE10360835A1 (en) | 2003-12-23 | 2005-07-21 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | New bicyclic imidazole derivatives are dipeptidylpeptidase-IV inhibitors useful to treat e.g. arthritis, obesity, allograft transplantation and calcitonin-induced osteoporosis |
| KR101154830B1 (en) | 2003-12-24 | 2012-06-18 | 프로시디온 리미티드 | Heterocyclic derivatives as GPCR receptor agonists |
| EP1708571A4 (en) | 2004-01-16 | 2009-07-08 | Merck & Co Inc | Novel crystalline salts of a dipeptidyl peptidase-iv inhibitor |
| WO2005075426A1 (en) | 2004-02-03 | 2005-08-18 | Glenmark Pharmaceuticals Ltd. | Novel dipeptidyl peptidase iv inhibitors; processes for their preparation and compositions thereof |
| CA2556239A1 (en) | 2004-02-11 | 2005-08-25 | Amgen Inc. | Vanilloid receptor ligands and their use in treatments |
| TW200530236A (en) | 2004-02-23 | 2005-09-16 | Chugai Pharmaceutical Co Ltd | Heteroaryl phenylurea |
| JP4609080B2 (en) | 2004-03-10 | 2011-01-12 | 富士ゼロックス株式会社 | Fixing device and image forming apparatus using the same |
| GB0405933D0 (en) | 2004-03-16 | 2004-04-21 | Glaxo Group Ltd | Compounds |
| MXPA06011799A (en) | 2004-04-12 | 2006-12-15 | Sankyo Co | Thienopyridine derivatives. |
| US8076338B2 (en) | 2004-04-23 | 2011-12-13 | Exelixis, Inc. | Kinase modulators and methods of use |
| WO2005121121A2 (en) | 2004-06-04 | 2005-12-22 | Arena Pharmaceuticals, Inc. | Substituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto |
| US20080167321A1 (en) | 2004-09-20 | 2008-07-10 | Xenon Pharmaceuticals Inc. | Pyridine Derivatives For Inhibiting Human Stearoyl-Coa-Desaturase |
| ATE481969T1 (en) | 2004-10-01 | 2010-10-15 | Merck Sharp & Dohme | AMINOPIPERIDINES AS DIPEPTIDYLPEPTIDASE IV INHIBITORS FOR THE TREATMENT OR PREVENTION OF DIABETES |
| JP4862654B2 (en) | 2004-10-08 | 2012-01-25 | アステラス製薬株式会社 | Aromatic ring fused pyrimidine derivatives |
| TW200621257A (en) | 2004-10-20 | 2006-07-01 | Astellas Pharma Inc | Pyrimidine derivative fused with nonaromatic ring |
| AR051596A1 (en) | 2004-10-26 | 2007-01-24 | Irm Llc | CONDENSED HETEROCICLIC COMPOUNDS NITROGENATED AS INHIBITORS OF THE ACTIVITY OF THE CANABINOID RECEIVER 1; PHARMACEUTICAL COMPOSITIONS THAT CONTAIN THEM AND THEIR EMPLOYMENT IN THE PREPARATION OF MEDICINES FOR THE TREATMENT OF FOOD DISORDERS |
| ES2498972T3 (en) | 2004-11-03 | 2014-09-26 | Arena Pharmaceuticals, Inc. | GPR41 and its modulators for the treatment of insulin-related disorders |
| GB0425035D0 (en) | 2004-11-12 | 2004-12-15 | Novartis Ag | Organic compounds |
| BRPI0516407A (en) | 2004-12-24 | 2008-09-02 | Prosidion Ltd | G-protein coupled receptor agonists (gpr116) and their use for the treatment of obesity and diabetes |
| JP5065908B2 (en) | 2004-12-24 | 2012-11-07 | プロシディオン・リミテッド | G protein-coupled receptor agonist |
| GB0428514D0 (en) | 2004-12-31 | 2005-02-09 | Prosidion Ltd | Compounds |
| DOP2006000010A (en) | 2005-01-10 | 2006-07-31 | Arena Pharm Inc | PROCEDURE TO PREPARE AROMATIC ETERES |
| MY148521A (en) | 2005-01-10 | 2013-04-30 | Arena Pharm Inc | Substituted pyridinyl and pyrimidinyl derivatives as modulators of metabolism and the treatment of disorders related thereto |
| DOP2006000008A (en) | 2005-01-10 | 2006-08-31 | Arena Pharm Inc | COMBINED THERAPY FOR THE TREATMENT OF DIABETES AND RELATED AFFECTIONS AND FOR THE TREATMENT OF AFFECTIONS THAT IMPROVE THROUGH AN INCREASE IN THE BLOOD CONCENTRATION OF GLP-1 |
| DOP2006000009A (en) | 2005-01-13 | 2006-08-15 | Arena Pharm Inc | PROCEDURE TO PREPARE ETERES OF PIRAZOLO [3,4-D] PYRIMIDINE |
| WO2006078992A2 (en) | 2005-01-19 | 2006-07-27 | Neurogen Corporation | Heteroaryl substituted piperazinyl-pyridine analogues |
| CA2611446A1 (en) | 2005-06-09 | 2006-12-14 | Banyu Pharmaceutical Co., Ltd. | Npy y2 agonist for use as therapeutic agent for disease accompanied by diarrhea |
| CA2613236A1 (en) | 2005-06-30 | 2007-01-11 | Prosidion Limited | G-protein coupled receptor agonists |
| CN101213192B (en) | 2005-07-01 | 2012-06-06 | Irm责任有限公司 | Pyrimidine-substituted benzimidazole derivatives as protein kinase inhibitors |
| WO2007035355A2 (en) | 2005-09-16 | 2007-03-29 | Arena Pharmaceuticals, Inc. | Modulators of metabolism and the treatment of disorders related thereto |
| TW200745055A (en) | 2005-09-23 | 2007-12-16 | Organon Nv | 4-Phenyl-6-substituted-pyrimidine-2-carbonitrile derivatives |
| AU2006337137B2 (en) | 2005-12-29 | 2012-06-14 | Tersera Therapeutics Llc | Multicyclic amino acid derivatives and methods of their use |
| JP5237115B2 (en) | 2006-01-19 | 2013-07-17 | オーキッド リサーチ ラボラトリーズ リミテッド | New heterocycles |
| PE20071221A1 (en) | 2006-04-11 | 2007-12-14 | Arena Pharm Inc | GPR119 RECEPTOR AGONISTS IN METHODS TO INCREASE BONE MASS AND TO TREAT OSTEOPOROSIS AND OTHER CONDITIONS CHARACTERIZED BY LOW BONE MASS, AND COMBINED THERAPY RELATED TO THESE AGONISTS |
| KR101281962B1 (en) | 2006-04-11 | 2013-07-08 | 아레나 파마슈티칼스, 인크. | Methods of using GPR119 receptor to identify compounds useful for increasing bone mass in an individual |
| TW200811147A (en) | 2006-07-06 | 2008-03-01 | Arena Pharm Inc | Modulators of metabolism and the treatment of disorders related thereto |
| TW200811140A (en) | 2006-07-06 | 2008-03-01 | Arena Pharm Inc | Modulators of metabolism and the treatment of disorders related thereto |
| AU2007291252A1 (en) | 2006-08-30 | 2008-03-06 | Inovacia Ab | Pyridine compounds for treating GPR119 related disorders |
| UA97817C2 (en) | 2006-12-06 | 2012-03-26 | Глаксосмиткляйн Ллк | Heterocyclic derivatives of 4-(methylsulfonyl)phenyl and use thereof |
| JP2010513272A (en) | 2006-12-14 | 2010-04-30 | メルク エンド カムパニー インコーポレーテッド | Acylbipiperidinyl compounds, compositions containing such compounds, and therapeutic methods |
| WO2009038974A1 (en) | 2007-09-20 | 2009-03-26 | Irm Llc | Compounds and compositions as modulators of gpr119 activity |
| KR20100137561A (en) | 2008-04-07 | 2010-12-30 | 아이알엠 엘엘씨 | Compounds and compositions as modulators of gpr119 activity |
| EP2108960A1 (en) | 2008-04-07 | 2009-10-14 | Arena Pharmaceuticals, Inc. | Methods of using A G protein-coupled receptor to identify peptide YY (PYY) secretagogues and compounds useful in the treatment of conditons modulated by PYY |
| WO2010075273A1 (en) | 2008-12-23 | 2010-07-01 | Schering Corporation | Bicyclic heterocycle derivatives and methods of use thereof |
| JP2012513470A (en) | 2008-12-23 | 2012-06-14 | シェーリング コーポレイション | Bicyclic heterocyclic derivatives and methods of use thereof |
| WO2010074271A1 (en) | 2008-12-26 | 2010-07-01 | 武田薬品工業株式会社 | Therapeutic agent for diabetes |
| AR077642A1 (en) | 2009-07-09 | 2011-09-14 | Arena Pharm Inc | METABOLISM MODULATORS AND THE TREATMENT OF DISORDERS RELATED TO THE SAME |
| AR077638A1 (en) | 2009-07-15 | 2011-09-14 | Lilly Co Eli | COMPOSITE OF (METHANOSULPHONYL -PIPERIDIN) - (ALCOXI-ARIL) -TETRAHIDRO- PYRIDINE, PHARMACEUTICAL COMPOSITION THAT INCLUDES IT AND ITS USE TO PREPARE A USEFUL MEDICINAL PRODUCT FOR THE TREATMENT OF DIABETES OR OBESITY |
| WO2011030139A1 (en) | 2009-09-11 | 2011-03-17 | Astrazeneca Ab | 4- (pyrimidin-2-yl) -piperazine and 4- (pyrimidin-2-yl) -piperidine derivatives as gpr119 modulators |
| US20130023494A1 (en) | 2010-04-06 | 2013-01-24 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| EP2619198A1 (en) | 2010-09-22 | 2013-07-31 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| WO2012135570A1 (en) | 2011-04-01 | 2012-10-04 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| US20140066369A1 (en) | 2011-04-19 | 2014-03-06 | Arena Pharmaceuticals, Inc. | Modulators Of The GPR119 Receptor And The Treatment Of Disorders Related Thereto |
| US20140038889A1 (en) | 2011-04-22 | 2014-02-06 | Arena Pharmaceuticals, Inc. | Modulators Of The GPR119 Receptor And The Treatment Of Disorders Related Thereto |
| WO2012145604A1 (en) | 2011-04-22 | 2012-10-26 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| WO2012170702A1 (en) | 2011-06-08 | 2012-12-13 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| WO2013055910A1 (en) | 2011-10-12 | 2013-04-18 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
-
2004
- 2004-01-14 NZ NZ540612A patent/NZ540612A/en not_active IP Right Cessation
- 2004-01-14 SG SG2010011294A patent/SG182004A1/en unknown
- 2004-01-14 PT PT04702248T patent/PT1599468E/en unknown
- 2004-01-14 JP JP2006501019A patent/JP2006516572A/en not_active Withdrawn
- 2004-01-14 AU AU2004205642A patent/AU2004205642C1/en not_active Ceased
- 2004-01-14 CN CN2011104462627A patent/CN102558155A/en active Pending
- 2004-01-14 AT AT04702248T patent/ATE374766T1/en active
- 2004-01-14 EA EA200501132A patent/EA011009B1/en not_active IP Right Cessation
- 2004-01-14 RS YUP-2005/0532A patent/RS20050532A/en unknown
- 2004-01-14 GE GEAP20048939A patent/GEP20084540B/en unknown
- 2004-01-14 KR KR1020107029315A patent/KR20110010824A/en not_active Application Discontinuation
- 2004-01-14 PL PL377847A patent/PL377847A1/en not_active Application Discontinuation
- 2004-01-14 EP EP04702248A patent/EP1599468B1/en not_active Expired - Lifetime
- 2004-01-14 KR KR1020057012965A patent/KR101078098B1/en not_active IP Right Cessation
- 2004-01-14 MX MXPA05007485A patent/MXPA05007485A/en active IP Right Grant
- 2004-01-14 US US10/541,657 patent/US8293751B2/en not_active Expired - Fee Related
- 2004-01-14 BR BR0406761-4A patent/BRPI0406761A/en active Search and Examination
- 2004-01-14 DE DE602004009295T patent/DE602004009295T2/en not_active Expired - Lifetime
- 2004-01-14 CA CA002512899A patent/CA2512899A1/en not_active Abandoned
- 2004-01-14 DK DK04702248T patent/DK1599468T3/en active
- 2004-01-14 ES ES04702248T patent/ES2295816T3/en not_active Expired - Lifetime
- 2004-01-14 WO PCT/US2004/001267 patent/WO2004065380A1/en active Application Filing
-
2005
- 2005-06-08 IL IL169081A patent/IL169081A/en not_active IP Right Cessation
- 2005-07-13 EC EC2005005916A patent/ECSP055916A/en unknown
- 2005-08-03 HR HR20050696A patent/HRP20050696B1/en not_active IP Right Cessation
- 2005-08-05 MA MA28422A patent/MA27707A1/en unknown
- 2005-08-09 CO CO05078853A patent/CO5640119A2/en not_active Application Discontinuation
- 2005-08-11 NO NO20053803A patent/NO20053803L/en not_active Application Discontinuation
- 2005-08-12 IS IS7979A patent/IS2713B/en unknown
- 2005-12-09 HK HK05111332A patent/HK1076815A1/en not_active IP Right Cessation
-
2007
- 2007-12-12 CY CY20071101580T patent/CY1107085T1/en unknown
-
2009
- 2009-11-12 IS IS8859A patent/IS8859A/en unknown
-
2010
- 2010-10-22 JP JP2010238056A patent/JP5566255B2/en not_active Expired - Fee Related
- 2010-11-12 US US12/945,712 patent/US20110082134A1/en not_active Abandoned
- 2010-12-21 AU AU2010257356A patent/AU2010257356A1/en not_active Abandoned
-
2012
- 2012-09-14 US US13/618,592 patent/US8933083B2/en not_active Expired - Fee Related
Patent Citations (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2341925A1 (en) * | 1973-08-20 | 1975-03-06 | Thomae Gmbh Dr K | 2,4, (opt.5-), 6-substd. pyriminidines as antithrombic agents - e.g. 6-methyl-5-nitro-2-piperazino-4-thiomorpolino-pyrimidine |
| DE2831850A1 (en) * | 1978-07-20 | 1980-02-07 | Basf Ag | Diuretic n-carboxy:phenyl:sulphonyl pyrrole derivs. - prepd. from substd. 3-sulphonamido:benzoic acid |
| RU938559C (en) * | 1980-12-12 | 1993-11-30 | Всесоюзный научно-исследовательский химико-фармацевтический институт им.Серго Орджоникидзе | S-derivatives of 5-amyno-6-mercaptopyrimidine possessing antitumoral and cytostatic activity |
| EP0556889A1 (en) * | 1992-02-18 | 1993-08-25 | Duphar International Research B.V | Method of preparing aryl(homo)piperazines |
| EP0667343A1 (en) * | 1994-01-18 | 1995-08-16 | Sandoz Ltd. | Alpha-pyrimidinyl acrylic acid derivatives with fungicidal activity |
| EP0801059A1 (en) * | 1994-11-29 | 1997-10-15 | Dainippon Pharmaceutical Co., Ltd. | Indole derivative |
| WO1996033994A1 (en) * | 1995-04-28 | 1996-10-31 | Nippon Soda Co., Ltd. | Amino-substituted derivatives, process for the preparation thereof, and herbicide |
| WO1996036613A1 (en) * | 1995-05-19 | 1996-11-21 | Nippon Soda Co., Ltd. | Substituted benzoic acid derivatives, process for the production thereof, and herbicides |
| EP0940387A1 (en) * | 1995-10-26 | 1999-09-08 | Tokyo Tanabe Company Limited | Phenylethanolamine compounds useful as beta3 agonist, process for producing the same, and intermediates in the production of the same |
| US5849759A (en) * | 1995-12-08 | 1998-12-15 | Berlex Laboratories, Inc. | Naphthyl-substituted benzimidazole derivatives as anti-coagulants |
| WO1997026252A1 (en) * | 1996-01-19 | 1997-07-24 | Fmc Corporation | Insecticidal n-heterocyclylalkyl- or n-[(polycyclyl)-alkyl]-n'-substituted piperazines |
| US6187777B1 (en) * | 1998-02-06 | 2001-02-13 | Amgen Inc. | Compounds and methods which modulate feeding behavior and related diseases |
| US6414002B1 (en) * | 1999-09-22 | 2002-07-02 | Bristol-Myers Squibb Company | Substituted acid derivatives useful as antidiabetic and antiobesity agents and method |
| WO2001027107A2 (en) * | 1999-10-12 | 2001-04-19 | Bristol-Myers Squibb Company | Heterocyclic sodium/proton exchange inhibitors and method |
| WO2001049677A1 (en) * | 1999-12-30 | 2001-07-12 | H. Lundbeck A/S | Phenylpiperazinyl derivatives |
| WO2001062233A2 (en) * | 2000-02-25 | 2001-08-30 | F. Hoffmann La Roche Ag | Adenosine receptor modulators |
| WO2002002549A1 (en) * | 2000-07-05 | 2002-01-10 | Taisho Pharmaceutical Co., Ltd. | Tetrahydropyridino or piperidino heterocyclic derivatives |
| WO2002006274A1 (en) * | 2000-07-17 | 2002-01-24 | Wyeth | N-(4-sulfonylaryl)cyclylamine-2-hydroxyethylamines as beta-3 adrenergic receptor agonists |
| WO2002098864A1 (en) * | 2001-06-01 | 2002-12-12 | F. Hoffmann-La Roche Ag | Pyrimidine, triazine and pyrazine derivatives as glutamate receptors |
| WO2003002544A1 (en) * | 2001-06-26 | 2003-01-09 | Bristol-Myers Squibb Company | N-heterocyclic inhibitors of tnf-alpha expression |
| WO2003077656A1 (en) * | 2002-03-15 | 2003-09-25 | Ciba Specialty Chemicals Holding Inc. | 4-aminopyrimidines and their use for the antimicrobial treatment of surfaces |
| WO2004000843A1 (en) * | 2002-06-24 | 2003-12-31 | Astrazeneca Ab | NOVEL PURINE- OR PYRROLOL[2,3-d]PYRIMIDINE-2-CARBONITILES FOR TREATING DISEASES ASSOCIATED WITH CYSTEINE PROTEASE ACTIVITY |
| WO2004000819A1 (en) * | 2002-06-24 | 2003-12-31 | Astrazeneca Ab | New use of pyrimidine - or triazine- 2-carbonitiles for treating diseases associated with cysteine protease activity and novel pyrimidine-2-carbonitile derivatives |
| WO2004029204A2 (en) * | 2002-09-27 | 2004-04-08 | Merck & Co., Inc. | Substituted pyrimidines |
Non-Patent Citations (1)
| Title |
|---|
| NORMAN M.H.: "Structure-activity relationships of a series of pyrrolo(3,2-d)pyrimidine derivatives and related compounds as neuropeptide Y5 receptor antagonists", JOURNAL OF MEDICINAL CHEMISTRY, vol. 43, no. 22, 2000, pages 4288 - 4312, XP002285195 * |
Cited By (219)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10202383B2 (en) | 2002-08-21 | 2019-02-12 | Boehringer Ingelheim International Gmbh | 8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions |
| US8664232B2 (en) | 2002-08-21 | 2014-03-04 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | 8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions |
| US9108964B2 (en) | 2002-08-21 | 2015-08-18 | Boehringer Ingelheim International Gmbh | 8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions |
| US9321791B2 (en) | 2002-08-21 | 2016-04-26 | Boehringer Ingelheim International Gmbh | 8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions |
| US9556175B2 (en) | 2002-08-21 | 2017-01-31 | Boehringer Ingelheim International Gmbh | 8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and thier use as pharmaceutical compositions |
| US10023574B2 (en) | 2002-08-21 | 2018-07-17 | Boehringer Ingelheim International Gmbh | 8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions |
| JP2006522143A (en) * | 2003-04-04 | 2006-09-28 | アイアールエム・リミテッド・ライアビリティ・カンパニー | Novel compounds and compositions as protein kinase inhibitors |
| WO2005007647A1 (en) * | 2003-07-11 | 2005-01-27 | Arena Pharmaceuticals, Inc. | Trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto |
| EA010690B1 (en) * | 2003-07-11 | 2008-10-30 | Арена Фармасьютикалз, Инк. | Trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto |
| EP1923390A1 (en) * | 2003-07-11 | 2008-05-21 | Arena Pharmaceuticals, Inc. | Trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto |
| US8697868B2 (en) | 2004-02-18 | 2014-04-15 | Boehringer Ingelheim International Gmbh | 8-[3-amino-piperidin-1-yl]-xanthines, their preparation and their use as pharmaceutical compositions |
| US7335672B2 (en) | 2004-09-13 | 2008-02-26 | Amgen Inc. | Vanilloid receptor ligands and their use in treatments |
| US7777036B2 (en) | 2004-09-20 | 2010-08-17 | Xenon Pharmaceuticals Inc. | Heterocyclic derivatives and their use as therapeutic agents |
| US8026360B2 (en) | 2004-09-20 | 2011-09-27 | Xenon Pharmaceuticals Inc. | Substituted pyridazines as stearoyl-CoA desaturase inhibitors |
| US7919496B2 (en) | 2004-09-20 | 2011-04-05 | Xenon Pharmaceuticals Inc. | Heterocyclic derivatives for the treatment of diseases mediated by stearoyl-CoA desaturase enzymes |
| US8071603B2 (en) | 2004-09-20 | 2011-12-06 | Xenon Pharmaceuticals Inc. | Heterocyclic derivatives and their use as stearoyl-CoA desaturase inhibitors |
| US7829712B2 (en) | 2004-09-20 | 2010-11-09 | Xenon Pharmaceuticals Inc. | Pyridazine derivatives for inhibiting human stearoyl-CoA-desaturase |
| US7767677B2 (en) | 2004-09-20 | 2010-08-03 | Xenon Pharmaceuticals Inc. | Heterocyclic derivatives and their use as stearoyl-CoA desaturase inhibitors |
| EP2316457A1 (en) | 2004-09-20 | 2011-05-04 | Xenon Pharmaceuticals Inc. | Pyridine derivatives for inhibiting human stearoyl-coa-desaturase |
| US7951805B2 (en) | 2004-09-20 | 2011-05-31 | Xenon Pharmaceuticals Inc. | Heterocyclic derivatives and their use as mediators of stearoyl-CoA desaturase |
| US8883805B2 (en) | 2004-11-05 | 2014-11-11 | Boehringer Ingelheim International Gmbh | Process for the preparation of chiral 8-(3-aminopiperidin-1-yl)-xanthines |
| US9751855B2 (en) | 2004-11-05 | 2017-09-05 | Boehringer Ingelheim International Gmbh | Process for the preparation of chiral 8-(3-aminopiperidin-1-yl)-xanthines |
| US9499546B2 (en) | 2004-11-05 | 2016-11-22 | Boehringer Ingelheim International Gmbh | Process for the preparation of chiral 8-(3-aminopiperidin-1-yl)-xanthines |
| CN101123964B (en) * | 2004-12-24 | 2012-11-28 | 普罗西迪恩有限公司 | G-protein coupled receptor(GPR116) agonists and use thereof for treating obesity and diabetes |
| WO2006067531A1 (en) * | 2004-12-24 | 2006-06-29 | Prosidion Ltd | G-protein coupled receptor (gpr116) agonists and use thereof for treating obesity and diabetes |
| JP4916452B2 (en) * | 2004-12-31 | 2012-04-11 | プロシディオン・リミテッド | Derivatives of pyridine, pyrimidine, and pyrazine as GPCR agonists |
| JP2008526724A (en) * | 2004-12-31 | 2008-07-24 | プロシディオン・リミテッド | Derivatives of pyridine, pyrimidine, and pyrazine as GPCR agonists |
| EP2322152A1 (en) * | 2005-01-10 | 2011-05-18 | Arena Pharmaceuticals, Inc. | Method of preparing a composition comprising a GPR119 agonist |
| EP2322157A1 (en) | 2005-01-10 | 2011-05-18 | Arena Pharmaceuticals, Inc. | Composition for the treatment of diabetes and conditions related thereto and for the treatment of conditions ameliorated by increasing a blood GLP-1 level |
| JP2008195733A (en) * | 2005-01-10 | 2008-08-28 | Arena Pharmaceuticals Inc | Combined curing method for diabetes, symptom related to diabetes and symptom to be improved by increase of blood glp-1 level |
| EA011883B1 (en) * | 2005-01-10 | 2009-06-30 | Арена Фармасьютикалз, Инк. | Combination therapy for the treatment of diabetes and conditions related thereto and for the treatment of conditions ameliorated by increasing a blood glp-1 level |
| AU2006205164B2 (en) * | 2005-01-10 | 2011-09-08 | Arena Pharmaceuticals, Inc. | Combination therapy for the treatment of diabetes and conditions related thereto and for the treatment of conditions ameliorated by increasing a blood GLP-1 level |
| JP2012017330A (en) * | 2005-01-10 | 2012-01-26 | Arena Pharmaceuticals Inc | Combination therapy for treating diabetes and condition related to diabetes, and treating condition to be improved with increase in blood glp-1 level |
| EP2322151A2 (en) * | 2005-01-10 | 2011-05-18 | Arena Pharmaceuticals, Inc. | Sustained release formulation comprising a GPR119 agonist and a DPP-IV inhibitor |
| EP2322150A3 (en) * | 2005-01-10 | 2011-11-16 | Arena Pharmaceuticals, Inc. | Method of identifying GLP-1 secretagogues |
| EP1997484A3 (en) * | 2005-01-10 | 2009-02-11 | Arena Pharmaceuticals, Inc. | Method of identifying GLP-1 secretagogues |
| EP2322151A3 (en) * | 2005-01-10 | 2012-01-18 | Arena Pharmaceuticals, Inc. | Sustained release formulation comprising a GPR119 agonist and a DPP-IV inhibitor |
| WO2006076231A3 (en) * | 2005-01-10 | 2007-01-18 | Arena Pharm Inc | Combination therapy for the treatment of diabetes and conditions related thereto and for the treatment of conditions ameliorated by increasing a blood glp-1 level |
| JP2011184458A (en) * | 2005-01-10 | 2011-09-22 | Arena Pharmaceuticals Inc | Combination therapy for treatment of diabetes and conditions related thereto and for treatment of conditions ameliorated by increasing blood glp-1 level |
| EP1808168A1 (en) * | 2005-01-10 | 2007-07-18 | Arena Pharmaceuticals, Inc. | Combination therapy for the treatment of diabetes and conditions related thereto and for the treatment of conditions ameliorated by increasing a blood GLP-1 level |
| EA014156B1 (en) * | 2005-01-10 | 2010-10-29 | Арена Фармасьютикалз, Инк. | Process for preparing 4-(phenoxy-5-methyl-pyrimidin-4-yloxy)piperedine-1-carboxylicacid derivatives and related compounds |
| WO2006076243A1 (en) * | 2005-01-10 | 2006-07-20 | Arena Pharmaceuticals, Inc. | Processes for preparing 4-(phenoxy-5-methyl-pyrimidin-4-yloxy)piperidine-1-carboxylic acid derivatives and related compounds |
| WO2006076231A2 (en) * | 2005-01-10 | 2006-07-20 | Arena Pharmaceuticals, Inc. | Combination therapy for the treatment of diabetes and conditions related thereto and for the treatment of conditions ameliorated by increasing a blood glp-1 level |
| EP2116235A1 (en) | 2005-01-10 | 2009-11-11 | Arena Pharmaceuticals, Inc. | Combination therapy for the treatment of diabetes and conditions related thereto and for the treatment of conditions ameliorated by increasing a blood GLP-1 level |
| JP2008110964A (en) * | 2005-01-10 | 2008-05-15 | Arena Pharmaceuticals Inc | Combination therapy for treatment of diabetes and condition related thereto and for treatment of condition ameliorated by increasing blood glp-1 level |
| KR101016890B1 (en) * | 2005-01-10 | 2011-02-22 | 아레나 파마슈티칼스, 인크. | Combination therapy for the treatment of diabetes and conditions related thereto and for the treatment of conditions ameliorated by increasing a blood glp-1 level |
| EP2322156A1 (en) * | 2005-01-10 | 2011-05-18 | Arena Pharmaceuticals, Inc. | Composition for the treatment of diabetes and conditions related thereto and for the treatment of conditions ameliorated by increasing a blood GLP-1 level |
| WO2007027225A2 (en) | 2005-04-27 | 2007-03-08 | Arena Pharmaceuticals, Inc. | Combination therapy for the treatment of obesity and diabetes and conditions related thereto and for the treatment of conditions ameliorated by increasing a blood glp-1 level |
| US8541457B2 (en) | 2005-06-03 | 2013-09-24 | Xenon Pharmaceuticals Inc. | Aminothiazole derivatives as human stearoyl-CoA desaturase inhibitors |
| US8637530B2 (en) | 2005-07-30 | 2014-01-28 | Boehringer Ingelheim International Gmbh | 8-(3-amino-piperidin-1-yl)-xanthines, their preparation, and their use as pharmaceuticals |
| EP2253311A2 (en) | 2006-04-11 | 2010-11-24 | Arena Pharmaceuticals, Inc. | Use of GPR119 receptor agonists for increasing bone mass and for treating osteoporosis, as well as combination therapy relating thereto |
| WO2007120689A2 (en) | 2006-04-11 | 2007-10-25 | Arena Pharmaceuticals, Inc. | Methods of using gpr119 receptor to identify compounds useful for increasing bone mass in an individual |
| WO2007120702A3 (en) * | 2006-04-11 | 2008-05-22 | Arena Pharm Inc | Use of gpr119 receptor agonists for increasing bone mass and for treating osteoporosis, and combination therapy relating thereto |
| EP2369341A2 (en) * | 2006-04-11 | 2011-09-28 | Arena Pharmaceuticals, Inc. | Methods of using gpr119 receptor to identify compounds useful for increasing bone mass in an individual |
| WO2007120702A2 (en) | 2006-04-11 | 2007-10-25 | Arena Pharmaceuticals, Inc. | Use of gpr119 receptor agonists for increasing bone mass and for treating osteoporosis, and combination therapy relating thereto |
| EP2402750A1 (en) | 2006-04-11 | 2012-01-04 | Arena Pharmaceuticals, Inc. | Methods of using GPR119 receptor to identify compounds useful for increasing bone mass in an individual |
| EP2369341A3 (en) * | 2006-04-11 | 2012-01-04 | Arena Pharmaceuticals, Inc. | Methods of using gpr119 receptor to identify compounds useful for increasing bone mass in an individual |
| CN103536924A (en) * | 2006-04-11 | 2014-01-29 | 艾尼纳制药公司 | Methods of using GPR119 receptor to identify compounds useful for increasing bone mass in an individual |
| EP2253311A3 (en) * | 2006-04-11 | 2011-06-22 | Arena Pharmaceuticals, Inc. | Use of GPR119 receptor agonists for increasing bone mass and for treating osteoporosis, as well as combination therapy relating thereto |
| US9493462B2 (en) | 2006-05-04 | 2016-11-15 | Boehringer Ingelheim International Gmbh | Polymorphs |
| US9266888B2 (en) | 2006-05-04 | 2016-02-23 | Boehringer Ingelheim International Gmbh | Polymorphs |
| US11919903B2 (en) | 2006-05-04 | 2024-03-05 | Boehringer Ingelheim International Gmbh | Polymorphs |
| US11291668B2 (en) | 2006-05-04 | 2022-04-05 | Boehringer Ingelheim International Gmbh | Uses of DPP IV inhibitors |
| US11084819B2 (en) | 2006-05-04 | 2021-08-10 | Boehringer Ingelheim International Gmbh | Polymorphs |
| US9173859B2 (en) | 2006-05-04 | 2015-11-03 | Boehringer Ingelheim International Gmbh | Uses of DPP IV inhibitors |
| US11033552B2 (en) | 2006-05-04 | 2021-06-15 | Boehringer Ingelheim International Gmbh | DPP IV inhibitor formulations |
| US10301313B2 (en) | 2006-05-04 | 2019-05-28 | Boehringer Ingelheim International Gmbh | Polymorphs |
| US10080754B2 (en) | 2006-05-04 | 2018-09-25 | Boehringer Ingelheim International Gmbh | Uses of DPP IV inhibitors |
| US9815837B2 (en) | 2006-05-04 | 2017-11-14 | Boehringer Ingelheim International Gmbh | Polymorphs |
| WO2008017381A1 (en) | 2006-08-08 | 2008-02-14 | Sanofi-Aventis | Arylaminoaryl-alkyl-substituted imidazolidine-2,4-diones, processes for preparing them, medicaments comprising these compounds, and their use |
| WO2008070692A2 (en) | 2006-12-06 | 2008-06-12 | Smithkline Beecham Corporation | Bicyclic compounds and use as antidiabetics |
| EP2325182A1 (en) | 2006-12-06 | 2011-05-25 | Glaxosmithkline LLC | Bicyclic compounds and use as antidiabetics |
| US8399485B2 (en) | 2006-12-14 | 2013-03-19 | Merck Sharp & Dohme Corp. | Acyl bipiperidinyl compounds useful as GPR 119 agonists |
| US9067891B2 (en) | 2007-03-07 | 2015-06-30 | Janssen Pharmaceuticals, Inc. | 1,4-disubstituted 3-cyano-pyridone derivatives and their use as positive allosteric modulators of mGluR2-receptors |
| US7910583B2 (en) | 2007-05-04 | 2011-03-22 | Bristol-Myers Squibb Company | [6,6] and [6,7]-bicyclic GPR119 G protein-coupled receptor agonists |
| US8076322B2 (en) | 2007-05-04 | 2011-12-13 | Bristol-Myers Squibb Company | [6,6] and [6,7]-bicyclic GPR119 G protein-coupled receptor agonists |
| US8513265B2 (en) | 2007-05-04 | 2013-08-20 | Bristol-Myers Squibb Company | [6,6] and [6,7]-bicyclic GPR119 G protein-coupled receptor agonists |
| US8314095B2 (en) | 2007-05-04 | 2012-11-20 | Bristol-Myers Squibb Company | [6,6] and [6,7]-bicyclic GPR119 G protein-coupled receptor agonists |
| US8093257B2 (en) | 2007-05-04 | 2012-01-10 | Bristol-Myers Squibb Company | [6,5]-bicyclic GPR119 G protein-coupled receptor agonists |
| US8476283B2 (en) | 2007-05-04 | 2013-07-02 | Bristol-Myers Squibb Company | [6,5]—bicyclic GPR119 G protein-coupled receptor agonists |
| US8232404B2 (en) | 2007-07-17 | 2012-07-31 | Bristol-Myers Squibb Company | Pyridone GPR119 G protein-coupled receptor agonists |
| US7928230B2 (en) | 2007-07-17 | 2011-04-19 | Bristol-Myers Squibb Company | Method for modulating GPR119 G protein-coupled receptor and selected compounds |
| US8178561B2 (en) | 2007-07-17 | 2012-05-15 | Bristol-Myers Squibb Company | Method for modulating GPR119 G protein-coupled receptor and selected compounds |
| US8513424B2 (en) | 2007-07-17 | 2013-08-20 | Bristol-Myers Squibb Company | Pyridone GPR119 G protein-coupled receptor agonists |
| US8003796B2 (en) | 2007-07-17 | 2011-08-23 | Bristol-Myers Squibb Company | Pyridone GPR119 G protein-coupled receptor agonists |
| WO2009021740A2 (en) | 2007-08-15 | 2009-02-19 | Sanofis-Aventis | Substituted tetrahydronaphthalenes, process for the preparation thereof and the use thereof as medicaments |
| US8551957B2 (en) | 2007-08-16 | 2013-10-08 | Boehringer Ingelheim International Gmbh | Pharmaceutical composition comprising a glucopyranosyl-substituted benzene derivate |
| US11071729B2 (en) | 2007-09-14 | 2021-07-27 | Addex Pharmaceuticals S.A. | 1′,3′-disubstituted-4-phenyl-3,4,5,6-tetrahydro-2H,1′H-[1,4′]bipyridinyl-2′-ones |
| US8153635B2 (en) | 2007-09-20 | 2012-04-10 | Irm Llc | Compounds and compositions as modulators of GPR119 activity |
| US8258156B2 (en) | 2007-09-20 | 2012-09-04 | Irm Llc | Compounds and compositions as modulators of GPR119 activity |
| WO2009055331A2 (en) | 2007-10-22 | 2009-04-30 | Schering Corporation | Bicyclic heterocycle derivatives and their use as modulators of the activity of gpr119 |
| US10973827B2 (en) | 2008-04-03 | 2021-04-13 | Boehringer Ingelheim International Gmbh | DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation |
| US10022379B2 (en) | 2008-04-03 | 2018-07-17 | Boehringer Ingelheim International Gmbh | DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation |
| US9415016B2 (en) | 2008-04-03 | 2016-08-16 | Boehringer Ingelheim International Gmbh | DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation |
| EP2108960A1 (en) | 2008-04-07 | 2009-10-14 | Arena Pharmaceuticals, Inc. | Methods of using A G protein-coupled receptor to identify peptide YY (PYY) secretagogues and compounds useful in the treatment of conditons modulated by PYY |
| WO2009126245A1 (en) * | 2008-04-07 | 2009-10-15 | Arena Pharmaceuticals, Inc. | Methods of using a g protein-coupled receptor to identify peptide yy (pyy) secretagogues and compounds useful in the treatment of conditions modulated by pyy |
| WO2009143049A1 (en) | 2008-05-19 | 2009-11-26 | Schering Corporation | Bicyclic heterocycle derivatives and use thereof as gpr119 modulators |
| WO2010003624A2 (en) | 2008-07-09 | 2010-01-14 | Sanofi-Aventis | Heterocyclic compounds, processes for their preparation, medicaments comprising these compounds, and the use thereof |
| WO2010009195A1 (en) | 2008-07-16 | 2010-01-21 | Schering Corporation | Bicyclic heterocycle derivatives and use thereof as gpr119 modulators |
| US8815876B2 (en) | 2008-07-16 | 2014-08-26 | Merck Sharp & Dohme Corp. | Bicyclic heterocycle derivatives and methods of use thereof |
| US8822480B2 (en) | 2008-07-16 | 2014-09-02 | Merck Sharp & Dohme Corp. | Bicyclic heterocycle derivatives and use thereof as GPR119 modulators |
| US8372837B2 (en) | 2008-07-16 | 2013-02-12 | Bristol-Myers Squibb Company | Pyridone and pyridazone analogues as GPR119 modulators |
| WO2010013849A1 (en) | 2008-08-01 | 2010-02-04 | 日本ケミファ株式会社 | Gpr119 agonist |
| US10034877B2 (en) | 2008-08-06 | 2018-07-31 | Boehringer Ingelheim International Gmbh | Treatment for diabetes in patients inappropriate for metformin therapy |
| US9486526B2 (en) | 2008-08-06 | 2016-11-08 | Boehringer Ingelheim International Gmbh | Treatment for diabetes in patients inappropriate for metformin therapy |
| WO2010029089A3 (en) * | 2008-09-10 | 2010-05-06 | Boehringer Ingelheim International Gmbh | Combination therapy for the treatment of diabetes and related conditions |
| WO2010029089A2 (en) * | 2008-09-10 | 2010-03-18 | Boehringer Ingelheim International Gmbh | Combination therapy for the treatment of diabetes and related conditions |
| US11911388B2 (en) | 2008-10-16 | 2024-02-27 | Boehringer Ingelheim International Gmbh | Treatment for diabetes in patients with insufficient glycemic control despite therapy with an oral or non-oral antidiabetic drug |
| WO2010068601A1 (en) | 2008-12-08 | 2010-06-17 | Sanofi-Aventis | A crystalline heteroaromatic fluoroglycoside hydrate, processes for making, methods of use and pharmaceutical compositions thereof |
| WO2010075271A1 (en) | 2008-12-23 | 2010-07-01 | Schering Corporation | Bicyclic heterocycle derivatives and methods of use thereof |
| US8722882B2 (en) | 2008-12-23 | 2014-05-13 | Merck Sharp & Dohme Corp. | Pyrimidine derivatives as GPCR modulators for use in the treatment of obesity and diabetes |
| WO2010075273A1 (en) | 2008-12-23 | 2010-07-01 | Schering Corporation | Bicyclic heterocycle derivatives and methods of use thereof |
| WO2010075269A1 (en) | 2008-12-23 | 2010-07-01 | Schering Corporation | Pyrimidine derivatives as gpcr modttlators for use in the treatment of obesity and diabetes |
| US9212183B2 (en) | 2008-12-23 | 2015-12-15 | Boehringer Ingelheim International Gmbh | Salt forms of 1-[(4-methyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-(R)-amino-piperidin-1-yl)-xanthine |
| US12115179B2 (en) | 2009-02-13 | 2024-10-15 | Boehringer Ingelheim International Gmbh | Pharmaceutical composition, methods for treating and uses thereof |
| US10406172B2 (en) | 2009-02-13 | 2019-09-10 | Boehringer Ingelheim International Gmbh | Pharmaceutical composition, methods for treating and uses thereof |
| WO2010114957A1 (en) | 2009-04-03 | 2010-10-07 | Schering Corporation | Bicyclic piperidine and piperazine derivatives as gpcr modulators for the treatment of obesity, diabetes and other metabolic disorders |
| WO2010114958A1 (en) | 2009-04-03 | 2010-10-07 | Schering Corporation | Bridged bicyclic heterocycle derivatives and methods of use thereof |
| US8580807B2 (en) | 2009-04-03 | 2013-11-12 | Merck Sharp & Dohme Corp. | Bicyclic piperidine and piperazine derivatives as GPCR modulators for the treatment of obesity, diabetes and other metabolic disorders |
| US10071095B2 (en) | 2009-05-12 | 2018-09-11 | Janssen Pharmaceuticals, Inc. | 1,2,4-triazolo [4,3-A] pyridine derivatives and their use for the treatment of neurological and psychiatric disorders |
| US9737533B2 (en) | 2009-05-12 | 2017-08-22 | Janssen Pharmaceuticals. Inc. | 1,2,4-triazolo [4,3-A] pyridine derivatives and their use for the treatment of prevention of neurological and psychiatric disorders |
| KR101432396B1 (en) | 2009-06-18 | 2014-08-20 | 카딜라 핼쓰캐어 리미티드 | Novel gpr 119 agonists |
| US8772302B2 (en) | 2009-06-18 | 2014-07-08 | Cadila Healthcare Limited | GPR 119 agonists |
| WO2010146605A1 (en) * | 2009-06-18 | 2010-12-23 | Cadila Healthcare Limited | Novel gpr 119 agonists |
| AU2010261341B2 (en) * | 2009-06-18 | 2014-02-06 | Cadila Healthcare Limited | Novel GPR 119 agonists |
| US8481731B2 (en) | 2009-06-24 | 2013-07-09 | Boehringer Ingelheim International Gmbh | Compounds, pharmaceutical composition and methods relating thereto |
| WO2011005929A1 (en) | 2009-07-09 | 2011-01-13 | Arena Pharmaceuticals, Inc. | Piperidine derivative and its use for the treatment of diabets and obesity |
| JP2012533546A (en) * | 2009-07-15 | 2012-12-27 | イーライ リリー アンド カンパニー | GPR119 agonist |
| WO2011023754A1 (en) | 2009-08-26 | 2011-03-03 | Sanofi-Aventis | Novel crystalline heteroaromatic fluoroglycoside hydrates, pharmaceuticals comprising these compounds and their use |
| WO2011025006A1 (en) | 2009-08-31 | 2011-03-03 | 日本ケミファ株式会社 | Gpr119 agonist |
| US20120157443A1 (en) * | 2009-09-04 | 2012-06-21 | Sunesis Pharmaceuticals, Inc. | Bruton's tyrosine kinase inhibitors |
| US10577374B2 (en) | 2009-09-04 | 2020-03-03 | Sunesis Pharmaceuticals, Inc. | Bruton's tyrosine kinase inhibitors |
| US9249146B2 (en) | 2009-09-04 | 2016-02-02 | Biogen Ma Inc. | Bruton'S tyrosine kinase inhibitors |
| US8785440B2 (en) * | 2009-09-04 | 2014-07-22 | Biogen Idec Ma, Inc. | Bruton's tyrosine kinase inhibitors |
| US9790229B2 (en) | 2009-09-04 | 2017-10-17 | Sunesis Pharmaceuticals, Inc. | Bruton's tyrosine kinase inhibitors |
| US20230046457A1 (en) * | 2009-09-04 | 2023-02-16 | Viracta Therapeutics, Inc. | Bruton's tyrosine kinase inhibitors |
| WO2011053688A1 (en) | 2009-10-29 | 2011-05-05 | Schering Corporation | Bridged bicyclic piperidine derivatives and methods of use thereof |
| WO2011062889A1 (en) | 2009-11-23 | 2011-05-26 | Schering Corporation | Pyrimidine ether derivatives and methods of use thereof |
| WO2011062885A1 (en) | 2009-11-23 | 2011-05-26 | Schering Corporation | Fused bicyclic pyrimidine derivatives and methods of use thereof |
| US8912206B2 (en) | 2009-11-23 | 2014-12-16 | Merck Sharp & Dohme Corp. | Pyrimidine ether derivatives and methods of use thereof |
| US9301929B2 (en) | 2009-11-24 | 2016-04-05 | Merck Sharp & Dohme Corp. | Substituted biaryl derivatives and methods of use thereof |
| WO2011066137A1 (en) | 2009-11-24 | 2011-06-03 | Schering Corporation | Substituted biaryl derivatives and methods of use thereof |
| US9457029B2 (en) | 2009-11-27 | 2016-10-04 | Boehringer Ingelheim International Gmbh | Treatment of genotyped diabetic patients with DPP-IV inhibitors such as linagliptin |
| US10092571B2 (en) | 2009-11-27 | 2018-10-09 | Boehringer Ingelheim International Gmbh | Treatment of genotyped diabetic patients with DPP-IV inhibitors such as linagliptin |
| WO2011107494A1 (en) | 2010-03-03 | 2011-09-09 | Sanofi | Novel aromatic glycoside derivatives, medicaments containing said compounds, and the use thereof |
| WO2011113947A1 (en) | 2010-03-18 | 2011-09-22 | Boehringer Ingelheim International Gmbh | Combination of a gpr119 agonist and the dpp-iv inhibitor linagliptin for use in the treatment of diabetes and related conditions |
| WO2011127051A1 (en) | 2010-04-06 | 2011-10-13 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| WO2011127106A1 (en) | 2010-04-08 | 2011-10-13 | Bristol-Myers Squibb Company | Pyrimidinylpiperidinyloxypyridinone analogues as gpr119 modulators |
| US8415367B2 (en) | 2010-04-08 | 2013-04-09 | Bristol-Myers Squibb Company | Pyrimidinylpiperidinyloxypyridinone analogues as GPR119 modulators |
| US9603851B2 (en) | 2010-05-05 | 2017-03-28 | Boehringer Ingelheim International Gmbh | Combination therapy |
| US9186392B2 (en) | 2010-05-05 | 2015-11-17 | Boehringer Ingelheim International Gmbh | Combination therapy |
| US10004747B2 (en) | 2010-05-05 | 2018-06-26 | Boehringer Ingelheim International Gmbh | Combination therapy |
| WO2011140161A1 (en) | 2010-05-06 | 2011-11-10 | Bristol-Myers Squibb Company | Benzofuranyl analogues as gpr119 modulators |
| WO2011140160A1 (en) | 2010-05-06 | 2011-11-10 | Bristol-Myers Squibb Company | Bicyclic heteroaryl compounds as gpr119 modulators |
| WO2011157827A1 (en) | 2010-06-18 | 2011-12-22 | Sanofi | Azolopyridin-3-one derivatives as inhibitors of lipases and phospholipases |
| WO2011161030A1 (en) | 2010-06-21 | 2011-12-29 | Sanofi | Heterocyclic substituted methoxyphenyl derivatives having an oxo group, method for producing same, and use thereof as gpr40 receptor modulators |
| WO2012004269A1 (en) | 2010-07-05 | 2012-01-12 | Sanofi | (2-aryloxy-acetylamino)-phenyl-propionic acid derivatives, method for producing same and use thereof as pharmaceuticals |
| WO2012004270A1 (en) | 2010-07-05 | 2012-01-12 | Sanofi | Spirocyclically substituted 1,3-propane dioxide derivatives, methods for the production thereof and use of the same as medicament |
| WO2012010413A1 (en) | 2010-07-05 | 2012-01-26 | Sanofi | Aryloxy-alkylene substituted hydroxyphenyl hexynoic acids, methods for the production thereof and use of the same as medicament |
| WO2012025811A1 (en) | 2010-08-23 | 2012-03-01 | Lupin Limited | Indolylpyrimidines as modulators of gpr119 |
| EP3323818A1 (en) | 2010-09-22 | 2018-05-23 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| CN103539791A (en) * | 2010-09-22 | 2014-01-29 | 艾尼纳制药公司 | Modulators of the GPR119 receptor and the treatment of disorders related thereto |
| WO2012040279A1 (en) | 2010-09-22 | 2012-03-29 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| KR101871011B1 (en) | 2010-09-22 | 2018-06-25 | 아레나 파마슈티칼스, 인크. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| CN103539791B (en) * | 2010-09-22 | 2017-01-11 | 艾尼纳制药公司 | Modulators of the GPR119 receptor and the treatment of disorders related thereto |
| CN103221410A (en) * | 2010-09-22 | 2013-07-24 | 艾尼纳制药公司 | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| AU2011305525B2 (en) * | 2010-09-22 | 2016-08-18 | Arena Pharmaceuticals, Inc. | Modulators of the GPR119 receptor and the treatment of disorders related thereto |
| US10894787B2 (en) | 2010-09-22 | 2021-01-19 | Arena Pharmaceuticals, Inc. | Modulators of the GPR119 receptor and the treatment of disorders related thereto |
| CN103221410B (en) * | 2010-09-22 | 2017-09-15 | 艾尼纳制药公司 | GPR119 receptor modulators and the treatment to relative obstacle |
| US11911387B2 (en) | 2010-11-15 | 2024-02-27 | Boehringer Ingelheim International Gmbh | Vasoprotective and cardioprotective antidiabetic therapy |
| US9034883B2 (en) | 2010-11-15 | 2015-05-19 | Boehringer Ingelheim International Gmbh | Vasoprotective and cardioprotective antidiabetic therapy |
| WO2012069917A1 (en) | 2010-11-26 | 2012-05-31 | Lupin Limited | Bicyclic gpr119 modulators |
| US9000175B2 (en) | 2010-11-26 | 2015-04-07 | Lupin Limited | Bicyclic GPR119 modulators |
| US10596120B2 (en) | 2011-03-07 | 2020-03-24 | Boehringer Ingelheim International Gmbh | Pharmaceutical compositions |
| US20180185291A1 (en) | 2011-03-07 | 2018-07-05 | Boehringer Ingelheim International Gmbh | Pharmaceutical compositions |
| US11564886B2 (en) | 2011-03-07 | 2023-01-31 | Boehringer Ingelheim International Gmbh | Pharmaceutical compositions |
| WO2012120053A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Branched oxathiazine derivatives, method for the production thereof, use thereof as medicine and drug containing said derivatives and use thereof |
| WO2012120055A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Di- and tri-substituted oxathiazine derivates, method for the production thereof, use thereof as medicine and drug containing said derivatives and use thereof |
| WO2012120056A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Tetrasubstituted oxathiazine derivatives, method for producing them, their use as medicine and drug containing said derivatives and the use thereof |
| WO2012120052A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Oxathiazine derivatives substituted with carbocycles or heterocycles, method for producing same, drugs containing said compounds, and use thereof |
| WO2012120054A1 (en) | 2011-03-08 | 2012-09-13 | Sanofi | Di- and tri-substituted oxathiazine derivates, method for the production thereof, use thereof as medicine and drug containing said derivatives and use thereof |
| WO2012135570A1 (en) | 2011-04-01 | 2012-10-04 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| WO2012145361A1 (en) | 2011-04-19 | 2012-10-26 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| WO2012145604A1 (en) | 2011-04-22 | 2012-10-26 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| WO2012145603A1 (en) | 2011-04-22 | 2012-10-26 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| WO2012170702A1 (en) | 2011-06-08 | 2012-12-13 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| WO2012170867A1 (en) | 2011-06-09 | 2012-12-13 | Rhizen Pharmaceuticals Sa | Novel compounds as modulators of gpr-119 |
| US8962636B2 (en) | 2011-07-15 | 2015-02-24 | Boehringer Ingelheim International Gmbh | Substituted quinazolines, the preparation thereof and the use thereof in pharmaceutical compositions |
| US9199998B2 (en) | 2011-07-15 | 2015-12-01 | Boehringer Ingelheim Internatioal Gmbh | Substituted quinazolines, the preparation thereof and the use thereof in pharmaceutical compositions |
| US8883800B2 (en) | 2011-07-15 | 2014-11-11 | Boehringer Ingelheim International Gmbh | Substituted quinazolines, the preparation thereof and the use thereof in pharmaceutical compositions |
| EP2567959A1 (en) | 2011-09-12 | 2013-03-13 | Sanofi | 6-(4-Hydroxy-phenyl)-3-styryl-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| WO2013037390A1 (en) | 2011-09-12 | 2013-03-21 | Sanofi | 6-(4-hydroxy-phenyl)-3-styryl-1h-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| WO2013045413A1 (en) | 2011-09-27 | 2013-04-04 | Sanofi | 6-(4-hydroxy-phenyl)-3-alkyl-1h-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| WO2013055910A1 (en) | 2011-10-12 | 2013-04-18 | Arena Pharmaceuticals, Inc. | Modulators of the gpr119 receptor and the treatment of disorders related thereto |
| US9555001B2 (en) | 2012-03-07 | 2017-01-31 | Boehringer Ingelheim International Gmbh | Pharmaceutical composition and uses thereof |
| WO2013151982A1 (en) | 2012-04-03 | 2013-10-10 | Arena Pharmaceuticals, Inc. | Methods and compounds useful in treating pruritus, and methods for identifying such compounds |
| US9526730B2 (en) | 2012-05-14 | 2016-12-27 | Boehringer Ingelheim International Gmbh | Use of a DPP-4 inhibitor in podocytes related disorders and/or nephrotic syndrome |
| US10195203B2 (en) | 2012-05-14 | 2019-02-05 | Boehringr Ingelheim International GmbH | Use of a DPP-4 inhibitor in podocytes related disorders and/or nephrotic syndrome |
| US9174965B2 (en) | 2012-05-16 | 2015-11-03 | Bristol-Myers Squibb Company | Pyrimidinylpiperidinyloxypyridone analogues as GPR119 modulators |
| US9713618B2 (en) | 2012-05-24 | 2017-07-25 | Boehringer Ingelheim International Gmbh | Method for modifying food intake and regulating food preference with a DPP-4 inhibitor |
| US10618887B2 (en) | 2012-06-08 | 2020-04-14 | Sunesis Pharmaceuticals, Inc. | Pyrimidinyl tyrosine kinase inhibitors |
| WO2014074668A1 (en) | 2012-11-08 | 2014-05-15 | Arena Pharmaceuticals, Inc. | Modulators of gpr119 and the treatment of disorders related thereto |
| US10584129B2 (en) | 2013-06-04 | 2020-03-10 | Janssen Pharmaceuticals Nv | Substituted 6,7-dihydropyrazolo[1,5-a]pyrazines as negative allosteric modulators of mGluR2 receptors |
| US10106542B2 (en) | 2013-06-04 | 2018-10-23 | Janssen Pharmaceutica Nv | Substituted 6,7-dihydropyrazolo[1,5-a]pyrazines as negative allosteric modulators of mGluR2 receptors |
| US9708315B2 (en) | 2013-09-06 | 2017-07-18 | Janssen Pharmaceutica Nv | 1,2,4-triazolo[4,3-a]pyridine compounds and their use as positive allosteric modulators of MGLUR2 receptors |
| US11369606B2 (en) | 2014-01-21 | 2022-06-28 | Janssen Pharmaceutica Nv | Combinations comprising positive allosteric modulators or orthosteric agonists of metabotropic glutamatergic receptor subtype 2 and their use |
| US12048696B2 (en) | 2014-01-21 | 2024-07-30 | Janssen Pharmaceutica Nv | Combinations comprising positive allosteric modulators or orthosteric agonists of metabotropic glutamatergic receptor subtype 2 and their use |
| US10537573B2 (en) | 2014-01-21 | 2020-01-21 | Janssen Pharmaceutica Nv | Combinations comprising positive allosteric modulators or orthosteric agonists of metabotropic glutamatergic receptor subtype 2 and their use |
| US11103506B2 (en) | 2014-01-21 | 2021-08-31 | Janssen Pharmaceutica Nv | Combinations comprising positive allosteric modulators or orthosteric agonists of metabotropic glutamatergic receptor subtype 2 and their use |
| US9526728B2 (en) | 2014-02-28 | 2016-12-27 | Boehringer Ingelheim International Gmbh | Medical use of a DPP-4 inhibitor |
| KR20150126564A (en) | 2014-05-02 | 2015-11-12 | 현대약품 주식회사 | Novel cyclohexene derivatives, preparation method thereof and pharmaceutical composition for prevention or treatment of the metabolic diseases containing the same as an active ingredient |
| US9758527B2 (en) | 2014-05-02 | 2017-09-12 | Hyundai Pharm Co., Ltd. | Cyclohexene derivative, preparation method therefor, and pharmaceutical composition for preventing or treating metabolic diseases, containing same as active ingredient |
| US11007175B2 (en) | 2015-01-06 | 2021-05-18 | Arena Pharmaceuticals, Inc. | Methods of treating conditions related to the S1P1 receptor |
| US11884626B2 (en) | 2015-06-22 | 2024-01-30 | Arena Pharmaceuticals, Inc. | Crystalline L-arginine salt of (R)-2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclo-penta [b]indol-3-yl)acetic acid(Compound1) for use in S1P1 receptor-associated disorders |
| US10723699B2 (en) | 2015-11-04 | 2020-07-28 | Hyundai Pharm Co., Ltd. | Cyclohexene derivative, preparation method thereof, and pharmaceutical composition for preventing or treating metabolic disease comprising the same as active ingredient |
| US10155000B2 (en) | 2016-06-10 | 2018-12-18 | Boehringer Ingelheim International Gmbh | Medical use of pharmaceutical combination or composition |
| US11174243B2 (en) | 2016-07-21 | 2021-11-16 | Sunesis Pharmaceuticals, Inc. | Succinate forms and compositions of Bruton's tyrosine kinase inhibitors |
| US11534424B2 (en) | 2017-02-16 | 2022-12-27 | Arena Pharmaceuticals, Inc. | Compounds and methods for treatment of primary biliary cholangitis |
| WO2018236896A1 (en) | 2017-06-19 | 2018-12-27 | Arena Pharmaceuticals, Inc. | Compounds and methods for treatment of nafld and nash |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8933083B2 (en) | 1,2,3-trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia | |
| AU2004215344B2 (en) | Phenyl- and pyridylpiperidine-derivatives as modulators of glucose metabolism | |
| JP2006516572A5 (en) | ||
| EP1756084B1 (en) | Substituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto | |
| JP4777244B2 (en) | Trisubstituted aryl and heteroaryl derivatives as metabolic modulators and the prevention and treatment of disorders associated therewith | |
| ZA200505001B (en) | 1,2,3-Trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia | |
| EP1927594A1 (en) | 1,2,3-Trisubstituted aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto such as diabetes and hyperglycemia |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: P-2005/0532 Country of ref document: YU |
|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BW BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE EG ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NA NI NO NZ OM PG PH PL PT RO RU SC SD SE SG SK SL SY TJ TM TN TR TT TZ UA UG US UZ VC VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): BW GH GM KE LS MW MZ SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LU MC NL PT RO SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| WR | Later publication of a revised version of an international search report | ||
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2004205642 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 169081 Country of ref document: IL Ref document number: 540612 Country of ref document: NZ |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 01150/KOLNP/2005 Country of ref document: IN Ref document number: 1150/KOLNP/2005 Country of ref document: IN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2005/05001 Country of ref document: ZA Ref document number: 200505001 Country of ref document: ZA |
|
| WWP | Wipo information: published in national office |
Ref document number: 2004205642 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1200500929 Country of ref document: VN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2512899 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2006501019 Country of ref document: JP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: PA/a/2005/007485 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020057012965 Country of ref document: KR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 377847 Country of ref document: PL Ref document number: 20048022039 Country of ref document: CN Ref document number: 1-2005-501308 Country of ref document: PH |
|
| WWE | Wipo information: entry into national phase |
Ref document number: P20050696A Country of ref document: HR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 05078853 Country of ref document: CO |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2004702248 Country of ref document: EP Ref document number: DZP2005000287 Country of ref document: DZ |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 8939 Country of ref document: GE Ref document number: 200501132 Country of ref document: EA |
|
| WWP | Wipo information: published in national office |
Ref document number: 1020057012965 Country of ref document: KR |
|
| WWP | Wipo information: published in national office |
Ref document number: 2004702248 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: PI0406761 Country of ref document: BR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2006217379 Country of ref document: US Ref document number: 10541657 Country of ref document: US |
|
| WWP | Wipo information: published in national office |
Ref document number: 10541657 Country of ref document: US |
|
| WWG | Wipo information: grant in national office |
Ref document number: 2004702248 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: CR2008-009861 Country of ref document: CR |