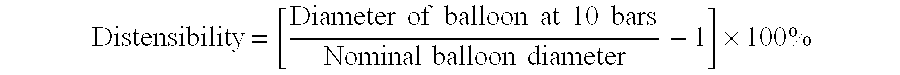US20040097878A1 - Distensible dilatation balloon with elastic stress response and manufacture thereof - Google Patents
Distensible dilatation balloon with elastic stress response and manufacture thereof Download PDFInfo
- Publication number
- US20040097878A1 US20040097878A1 US10/637,575 US63757503A US2004097878A1 US 20040097878 A1 US20040097878 A1 US 20040097878A1 US 63757503 A US63757503 A US 63757503A US 2004097878 A1 US2004097878 A1 US 2004097878A1
- Authority
- US
- United States
- Prior art keywords
- balloon
- parison
- balloons
- temperature
- psi
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 230000003938 response to stress Effects 0.000 title claims abstract description 46
- 238000004519 manufacturing process Methods 0.000 title 1
- 238000000034 method Methods 0.000 claims abstract description 88
- 230000008569 process Effects 0.000 claims abstract description 69
- 230000001954 sterilising effect Effects 0.000 claims abstract description 38
- 229920001400 block copolymer Polymers 0.000 claims description 28
- 229920002635 polyurethane Polymers 0.000 claims description 26
- 239000004814 polyurethane Substances 0.000 claims description 26
- 229920000642 polymer Polymers 0.000 claims description 21
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 claims description 15
- -1 polyethylene terephthalate Polymers 0.000 claims description 11
- 230000003993 interaction Effects 0.000 claims description 9
- 239000000203 mixture Substances 0.000 claims description 7
- 229920006147 copolyamide elastomer Polymers 0.000 claims description 6
- 229920000728 polyester Polymers 0.000 claims description 6
- 229920000139 polyethylene terephthalate Polymers 0.000 claims description 6
- 239000005020 polyethylene terephthalate Substances 0.000 claims description 6
- 230000007423 decrease Effects 0.000 claims description 5
- 238000002844 melting Methods 0.000 claims description 5
- 230000008018 melting Effects 0.000 claims description 5
- 230000005484 gravity Effects 0.000 claims description 4
- 239000004677 Nylon Substances 0.000 claims description 3
- 230000009477 glass transition Effects 0.000 claims description 3
- 238000010438 heat treatment Methods 0.000 claims description 3
- 229920001778 nylon Polymers 0.000 claims description 3
- 239000000463 material Substances 0.000 abstract description 45
- 238000004659 sterilization and disinfection Methods 0.000 abstract description 37
- 230000002411 adverse Effects 0.000 abstract description 17
- 208000031481 Pathologic Constriction Diseases 0.000 description 21
- 230000036262 stenosis Effects 0.000 description 11
- 208000037804 stenosis Diseases 0.000 description 11
- 210000001367 artery Anatomy 0.000 description 8
- 230000003287 optical effect Effects 0.000 description 6
- 230000000704 physical effect Effects 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 239000004698 Polyethylene Substances 0.000 description 5
- 239000012530 fluid Substances 0.000 description 5
- 229920000573 polyethylene Polymers 0.000 description 5
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 229910001873 dinitrogen Inorganic materials 0.000 description 4
- 230000001747 exhibiting effect Effects 0.000 description 4
- RYECOJGRJDOGPP-UHFFFAOYSA-N Ethylurea Chemical compound CCNC(N)=O RYECOJGRJDOGPP-UHFFFAOYSA-N 0.000 description 3
- 238000002399 angioplasty Methods 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- 238000004513 sizing Methods 0.000 description 3
- 210000004204 blood vessel Anatomy 0.000 description 2
- 230000010339 dilation Effects 0.000 description 2
- 229920000554 ionomer Polymers 0.000 description 2
- 229920005862 polyol Polymers 0.000 description 2
- 150000003077 polyols Chemical class 0.000 description 2
- KKEYFWRCBNTPAC-UHFFFAOYSA-L terephthalate(2-) Chemical compound [O-]C(=O)C1=CC=C(C([O-])=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-L 0.000 description 2
- 239000004970 Chain extender Substances 0.000 description 1
- 206010048631 Coronary artery dissection Diseases 0.000 description 1
- 238000005273 aeration Methods 0.000 description 1
- 229920005601 base polymer Polymers 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 230000009172 bursting Effects 0.000 description 1
- 238000007887 coronary angioplasty Methods 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 125000005442 diisocyanate group Chemical group 0.000 description 1
- 238000002224 dissection Methods 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 239000012948 isocyanate Substances 0.000 description 1
- 150000002513 isocyanates Chemical class 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 239000006069 physical mixture Substances 0.000 description 1
- 229920000098 polyolefin Polymers 0.000 description 1
- 229920000915 polyvinyl chloride Polymers 0.000 description 1
- 239000004800 polyvinyl chloride Substances 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 238000012414 sterilization procedure Methods 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 238000003856 thermoforming Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M25/1027—Making of balloon catheters
- A61M25/1029—Production methods of the balloon members, e.g. blow-moulding, extruding, deposition or by wrapping a plurality of layers of balloon material around a mandril
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2/00—Methods or apparatus for disinfecting or sterilising materials or objects other than foodstuffs or contact lenses; Accessories therefor
- A61L2/16—Methods or apparatus for disinfecting or sterilising materials or objects other than foodstuffs or contact lenses; Accessories therefor using chemical substances
- A61L2/20—Gaseous substances, e.g. vapours
- A61L2/206—Ethylene oxide
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L29/00—Materials for catheters, medical tubing, cannulae, or endoscopes or for coating catheters
- A61L29/04—Macromolecular materials
- A61L29/06—Macromolecular materials obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2202/00—Aspects relating to methods or apparatus for disinfecting or sterilising materials or objects
- A61L2202/20—Targets to be treated
- A61L2202/24—Medical instruments, e.g. endoscopes, catheters, sharps
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M2025/0019—Cleaning catheters or the like, e.g. for reuse of the device, for avoiding replacement
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C49/00—Blow-moulding, i.e. blowing a preform or parison to a desired shape within a mould; Apparatus therefor
- B29C49/42—Component parts, details or accessories; Auxiliary operations
- B29C49/46—Component parts, details or accessories; Auxiliary operations characterised by using particular environment or blow fluids other than air
- B29C2049/4602—Blowing fluids
- B29C2049/4605—Blowing fluids containing an inert gas, e.g. helium
- B29C2049/4608—Nitrogen
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C49/00—Blow-moulding, i.e. blowing a preform or parison to a desired shape within a mould; Apparatus therefor
- B29C49/42—Component parts, details or accessories; Auxiliary operations
- B29C49/78—Measuring, controlling or regulating
- B29C49/783—Measuring, controlling or regulating blowing pressure
- B29C2049/7831—Measuring, controlling or regulating blowing pressure characterised by pressure values or ranges
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29L—INDEXING SCHEME ASSOCIATED WITH SUBCLASS B29C, RELATING TO PARTICULAR ARTICLES
- B29L2022/00—Hollow articles
- B29L2022/02—Inflatable articles
- B29L2022/022—Balloons
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29L—INDEXING SCHEME ASSOCIATED WITH SUBCLASS B29C, RELATING TO PARTICULAR ARTICLES
- B29L2031/00—Other particular articles
- B29L2031/753—Medical equipment; Accessories therefor
- B29L2031/7542—Catheters
Definitions
- Distensibility Another important characteristic of balloons in general, and more specifically dilatation balloons, is the distensibility of the finished balloon product.
- the balloons and balloon catheters are then subjected to an aeration step in which the ethylene oxide is allowed to dissipate.
- the novel sterilization process does not adversely affect the improved overall combination of properties exhibited by the balloons of this invention.
- These polymers can be considered to be comprised of polymer chains with individual regions of crystalline and amorphous material and can be referred to as “hard” and “soft” segments respectively.
- the individual polymer chains are able, to a substantial extent, to coil upon themselves and/or around each other in such a way that soft segments are associated with soft segments and hard segments with hard segments, thereby forming separate “domains” approximating soft and hard bodies of polymer, each exhibiting its own physical properties in varying degrees.
- the hard segments are comprised of regions which have significant inter-molecular chain interaction. This provides regions with increased strength and increased elastic stress response. In addition to providing strength, the hard segments are sufficiently rigid to permit the soft segments to stretch and uncoil which provides distensibility.
- the sterilization process cannot, however, be conducted above the heat set temperature since this would relieve the orientation of the polymer chains which was “locked” into place during heat set process.
- the sterilization process appears to be an important factor in determining the final physical characteristics of the balloons and balloon catheters of this invention. Therefore, the novel sterilization process is necessary to ensure a clinically useful and safe finished balloon and balloon catheter with an overall advantageous combination of physical properties (i.e., distensibility, elastic stress response and wall tensile strength) superior to those exhibited by the “compliant” balloons of the prior art.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Anesthesiology (AREA)
- Hematology (AREA)
- Chemical & Material Sciences (AREA)
- Biophysics (AREA)
- Pulmonology (AREA)
- Epidemiology (AREA)
- Child & Adolescent Psychology (AREA)
- Biomedical Technology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Manufacturing & Machinery (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Materials For Medical Uses (AREA)
- Processing And Handling Of Plastics And Other Materials For Molding In General (AREA)
- Treatments Of Macromolecular Shaped Articles (AREA)
Abstract
Balloons and balloon catheters with a superior overall combination of distensibility, elastic stress response and strength. The improved properties of the balloons result from the method or process used to form the balloons, as well as the polymeric materials used in said balloon forming process. Additionally, the enhanced combination of properties of the balloons will not be adversely affected by the novel sterilization process contemplated by this invention.
Description
- Surgical procedures employing balloons and medical devices incorporating those balloons (i.e., balloon catheters) are becoming more common and routine. These procedures, such as angioplasty procedures, are conducted when it becomes necessary to expand or open narrow or obstructed openings in blood vessels and other passageways in the body to increase the flow through the obstructed areas. For example, in an angioplasty procedure, a dilatation balloon catheter is used to enlarge or open an occluded blood vessel which is partially restricted or obstructed due to the existence of a hardened stenosis or buildup within the vessel. This procedure requires that a balloon catheter be inserted into the patient's body and positioned within the vessel so that the balloon, when inflated, will dilate the site of the obstruction or stenosis so that the obstruction or stenosis is minimized, thereby resulting in increased blood flow through the vessel. Often, however, a stenosis requires treatment with multiple balloon inflations. Additionally, many times there are multiple stenoses within the same vessel or artery. Such conditions require that either the same dilatation balloon must be subjected to repeated inflations, or that multiple dilatation balloons must be used to treat an individual stenosis or the multiple stenoses within the same vessel or artery. Additionally, balloons and medical devices incorporating those balloons may also be used to administer drugs to a patient.
- Traditionally, the balloons available to physicians were classified as either “compliant” or “noncompliant”. This classification is based upon the operating characteristics of the individual balloon, which in turn depended upon the process used in forming the balloon, as well as the material used in the balloon forming process. Both types of balloons provide advantageous qualities which were not available from the other.
- A balloon which is classified as “noncompliant” is characterized by the balloon's inability to grow or expand appreciably beyond its rated or nominal diameter. “Noncompliant” balloons are referred to as having minimal distensibility. In balloons currently known in the art (e.g., polyethylene terephthalate), this minimal distensibility results from the strength and rigidity of the molecular chains which make up the base polymer, as well as the orientation and structure of those chains resulting from the balloon formation process. The strength resulting from this highly oriented structure is so great that when the balloon is subjected typical inflation or operating pressures (i.e., about 70 psi to over 200 psi), it will not be stressed above the yield point of the polymeric material.
- The yield point of a material is defined as the stress at which the individual molecular chains move in relation to one another such that when the pressure or stress is relieved, there is permanent deformation of the structure. When a material is subjected to pressure or stress below its yield point, the material will consistently follow the same stress-strain curve when subjected to multiple cycles of applying and relieving the stress or pressure. A material which exhibits the ability to follow the same stress-strain curve during the repeated application and relief of stress is defined as being elastic and as having a high degree of elastic stress response. This elastic behavior is highly desirable in balloons in order to ensure consistent and predictable balloon sizing regardless of the balloon's previous inflation history.
- A balloon which is referred to as being “compliant” is characterized by the balloon's ability to grow or expand beyond its nominal or rated diameter. In balloons currently known in the art (i.e., polyethylene, polyvinylchloride), the balloon's “compliant” nature or distensibility results from the chemical structure of the polymeric material used in the formation of the balloon, as well as the balloon forming process. These polymeric materials have a relatively low yield point. Thus, the inflation pressures used in dilation procedures are typically above the yield point of the materials used to form distensible balloons. A distensible or “compliant” balloon when inflated to normal operating pressures, which are greater than the polymeric material's yield point, is subjected to stress sufficient to permanently realign the individual molecular chains of the polymeric material. The realignment of individual polymer chains permits the balloon to expand beyond its nominal or rated diameter. However, since this realignment is permanent, the balloon will not follow its original stress-strain curve on subsequent inflation-deflation cycles. Therefore, the balloon balloon upon subsequent inflations, will achieve diameters which are greater than the diameters which were originally obtained at any given pressure during the course of the balloon's initial inflation.
- The term “elastic”, as it is used in connection with this invention, refers only to the ability of a material to follow the same stress-strain curve upon the multiple applications of stress. See Beer, F. et al., Mechanics of Materials (McGraw-Hill Book Company 1981), pp. 39-40. Elasticity, however, is not necessarily a function of how distensible a material is. It is possible to have an elastic, non-distensible material or a non-elastic, distensible material. This is best illustrated in FIGS. 1, 2 and 3.
- FIG. 1 represents an elastic, essentially non-distensible material. If this material was used to form a balloon, the balloon would be considered non-distensible because there is very little change in strain (diameter) as the stress applied is increased (inflation pressure). The balloon would be elastic because it follows essentially the same stress-strain (pressure-diameter) curve with the second application of stress (inflation).
- FIG. 2 represents an elastic, distensible material. If this material was used to form a balloon, the balloon would be considered distensible because there is significant change in strain (diameter) as the stress applied is increased (inflation pressure). The balloon would be considered elastic because it follows essentially the same stress-strain (pressure-diameter) curve with the second application of stress (inflation).
- FIG. 3 represents an inelastic, distensible material. Like FIG. 2, FIG. 3 shows a significant change in strain (diameter) and would therefore be considered a distensible balloon material. Unlike FIGS. 1 and 2, however, the same stress-strain (pressure-diameter) curve is not maintained upon the second application of stress (inflation).
- It has been found that the optimal size of a dilatation balloon is about 0.9 to about 1.3 the size of the vessel being treated. See Nichols et al., Importance of Balloon Size in Coronary Angioplasty, J. American College of Cardiology, Vol. 13, 1094 (1989). If an undersized balloon is used, there is a high incidence of significant residual stenosis and a greater need for subsequent dilatation procedures. However, if an oversized balloon is used, there is an increased chance of coronary dissection. Therefore, physicians desire to use a balloon which will closely approximate the size of the occluded vessel or obstructed cavity being treated.
- Because physiological vessels such as arteries are generally tapered, the nominal or rated diameter of balloons commercially available often do not correspond to the size of the vessel being treated. Physicians, therefore, are often faced with the prospect of using an undersized “compliant” balloon which can be expanded beyond its nominal or rated diameter, or an oversized “noncompliant” balloon which will follow the same stress-strain curve during multiple inflations (i.e., is elastic). Thus, physicians can choose from two general types of balloons depending upon whether they require a balloon which grows beyond nominal diameter. They may choose a “noncompliant” balloon if they require a relatively high strength balloon which will not expand much beyond its nominal or rated diameter, or a “compliant” balloon if they require a balloon which is capable of expanding considerably beyond the normal or rated diameter. As will be shown below, each of these properties is advantageous. However, it would be desirable to have to have a “compliant” or distensible balloon which also has the elastic stress response of a “noncompliant” balloon, as well as sufficient strength to be used in dilatation procedures.
- Because physicians using a dilatation balloon do not know prior to the procedure what inflation pressures will be required to dilate a given obstruction or stenosis, it is desirable that the balloon being used have strength capable of withstanding the high inflation pressures typically associated with these procedures (i.e., about 70 to over 200 psi). A high strength dilatation balloon, which is capable of withstanding increased inflation pressure, is safer to use since the chances of the balloon bursting during the procedure are minimized.
- Strength of a balloon is typically quantified by calculating the balloon's wall tensile strength. The overall strength of a balloon can be increased by increasing the balloon's wall thickness. As the wall thickness is increased, the balloon is capable of withstanding higher inflation pressures. However, as the wall thickness of the balloon is increased, the folded profile of the balloon, as well as the balloon's flexibility, may be adversely affected.
-
- Depending upon the material used to form the balloon, the nominal, or rated diameter is achieved typically when the balloon is inflated between to about 5 bars to about 8 bars. The burst pressure is determined at 37° C.
- Since balloons, particularly dilatation balloons, must have the ability to traverse the confines of the obstructed areas to be treated, it is desirable to have a balloon which has a narrow folded profile. This “profile” represents the smallest opening through which the balloon, in its deflated state, may pass. The profile of the balloon depends in large part upon the wall thickness of the finished balloon (i.e., the sterilized dilatation balloon product). Therefore, it is desirable for a finished balloon product to have a folded profile which is as narrow as possible, particularly if the balloon is to be used in an angioplasty procedure.
- Another important characteristic of balloons in general, and more specifically dilatation balloons, is the distensibility of the finished balloon product. Distensibility, also referred to as percent of radial expansion, is typically determined by comparing the nominal or rated diameter of the balloon with the diameter at some arbitrarily selected higher pressure (i.e., 10 bars). The distensibility or percent radial expansion is calculated using the following formula with all measurements taking place at about 37° C.:
- For example, balloons made of polyethylene terephthalate have a low distensibility (i.e., less than about 5% at 200 psi). See for example U.S. Pat. Re. Nos. 32,983 and 33,561 to Levy which discloses balloons formed from polyethylene terephthalate and other polymeric materials.
- It is also desirable that the balloon be elastic or have a high degree of elastic stress response. Elasticity, which also can be referred to as the repeatability of a balloon, is characterized by the ability of the balloon to consistently follow the same stress-strain curve after being subjected to multiple inflations to normal operating or inflation pressures (i.e., about 10 bars or greater). That is, a balloon which has a high degree of elastic stress response will retain the same diameter-pressure relationship and will consistently obtain the same diameter at the same pressure during repeated inflation-deflation cycles. Balloons which have poor elasticity or a low degree of elastic stress response have a tendency to “creep” or “deform” after multiple inflations and fail to return to their nominal or rated diameters after being subjected to multiple inflations at increased pressures.
- A dilatation balloon which has a high degree of elastic stress response is particularly desirable when a physician is treating multiple stenoses within the same artery. If the balloon is “inelastic”, after the first stenosis is dilated at an increased pressure, the physician would not know what the balloon's “new” starting diameter is prior to attempting to dilate subsequent stenoses. If the physician fails to correctly guess the balloon's “new” diameter prior to beginning treatment of another stenosis there is an increased risk of oversizing the balloon which could result in coronary artery dissection or other damage to the vessel. Therefore, to ensure the patient's safety, some physicians elect to remove the balloon catheter from the patient and reintroduce a new sterile balloon catheter prior to attempting to dilate subsequent stenosis within the same vessel. However, this is time-consuming and undesirable for the patient. Additionally, the cost of the individual balloon catheters prohibits the use of multiple balloon catheters when treating multiple stenoses within the same vessel. Thus, to minimize the chance of oversizing the balloon when treating multiple stenoses within the same vessel, a physician may attempt to use a dilatation balloon which is noncompliant. However, as discussed previously, because such a balloon will permit little expansion beyond the balloon's rated or nominal diameter, the physician may not have available a balloon of sufficient size to safely treat the other stenoses within the same vessel.
- Elastic stress response is determined by inflating a balloon to 5 bars at about 37° C. and measuring the balloon's diameter. The balloon is then inflated to a pressure of 10 bars in about 20 seconds and held for an additional 20 seconds at 37° C. The balloon's diameter is then measured. The internal pressure of the balloon is then decreased to 5 bars and the “new” 5 bar diameter of the balloons is determined. For this invention, the elastic stress response or repeatability is calculated using the following equation:
- A balloon with maximum or complete elastic stress response permits the balloon, after being inflated to a pressure of 10 bars, to return to the same diameter it had at 5 bars prior to the inflation to the higher pressure. Such a balloon would have maximum repeatability, or an elastic stress response of 0.00. As the repeatability of the balloon decreases, the elastic stress response decreases and, as defined above, numerically becomes greater than 0.00. For example, balloons formed from polyolefin copolymers in the art have poor repeatability and a relatively low degree of elastic stress response and have a numerical elastic stress response of about 9.
- It would be particularly desirable if a “compliant” balloon was able to possess an adequate degree of distensibility so that the balloon could be inflated to correspond to the size of the vessel being treated, while at the same time being highly elastic to ensure repeatable sizing and a high degree of elastic stress response so that the physician would know the balloon's “new” diameter at all inflation pressures prior to attempting to dilate multiple stenoses within the same vessel. This enhanced combination of properties would allow physicians to conduct dilation procedures in a safer manner in arteries where the physician requires balloon sizing not conveniently provided by “noncompliant” balloon products currently available in the art.
- Another desirable characteristic of a balloon is flexibility. Improved flexibility will permit a balloon to traverse, not only occluded arteries, but also other obstructed or narrow body cavities and openings resulting in minimal damage to the vessel or cavity through which the balloon catheter is being navigated.
- A further desirable property of a dilatation balloon, is the optical clarity of the finished balloon product. Although the optical clarity will not adversely affect a balloon's overall ability to dilate a stenosis or obstruction, most physicians will not use a balloon which has a cloudy appearance. The optical characteristics of a balloon or balloon catheter, therefore, must be taken into account when forming a balloon.
- While the foregoing properties are desirable in balloons, these attributes are typically adversely affected by the sterilization process which all balloons and balloon catheters must be subjected to prior to their use in the human body. For example, when a balloon in the art is exposed to the increased temperature and humidity of a traditional sterilization process (e.g., high humidity, temperature of about 50-60° C., about 12% ethylene oxide and about 88% Freon™ for approximately 12-16 hours) the balloon tends to shrink which causes a corresponding increase in wall thickness. Moreover, this increase in wall thickness will adversely affect the folded profile of the sterilized balloon product. Furthermore, the distensibility of many balloons is adversely affected by the sterilization processes currently used in the art. Therefore, it is also desirable that the sterilization process used to treat balloons and balloon catheters provide adequate sterilization while at the same time not adversely affecting the physical characteristics of the finished balloon or balloon catheter product.
- It has now been found that novel distensible balloons, particularly dilatation balloons, can be formed by processing a polymeric material composed of polymer chains having sufficient regions of molecular structure with inter-molecular chain interaction to ensure the integrity and strength of the structure, as well as sufficient regions which permit sections of the polymer chains to “uncoil” to permit growth. The balloons contemplated by this invention (i) are sufficiently distensible (i.e., about 5 to about 20%) to allow treatment of various sized arteries, (ii) have a high degree of elastic stress response (i.e., less than about 5.00) which permits the physician to treat multiple stenoses within the same artery without having to be concerned with increasing balloon diameter after repeated inflations and (iii) have strength sufficient to treat hardened stenoses (i.e., greater than about 14,000 psi). The balloons formed using the process of this invention will have an overall advantageous combination of these physical properties i.e., distensibility, elastic stress response and tensile strength, superior to those exhibited by the “compliant” balloons currently available. It has also been found that these enhanced properties will not be adversely affected by subjecting the balloons and balloon catheters formed following the method or process of this invention to a novel sterilization process. This novel balloon forming process and novel sterilization process can be used regardless of whether the balloon is coated.
- It is an object of this invention to provide a method or process for producing a balloon, preferably a dilatation balloon, which exhibits an improved overall combination of physical properties, such as distensibility, elastic stress response and strength, superior to those exhibited by “compliant” balloons currently known in the art.
- It is further the object of this invention to provide a novel balloon and a novel balloon catheter in which the balloon exhibits an advantageous overall combination of distensibility, elastic stress response and strength which combination of properties will not be adversely affected by sterilization.
- Still another object of this invention is to provide an improved sterilization procedure which will not adversely affect the distensibility, elastic stress response and strength of the balloons and balloons of the balloon catheters of this invention.
- It is still a further object of this invention to provide a process which will ensure that the balloons formed will have improved optical clarity.
- These objects, as well as others, which will become apparent from the description which follows, are achieved by forming these novel balloons and balloon catheters using the novel process of this invention from certain polymeric materials composed of polymer chains having regions of inter-molecular chain interaction separated by regions in which those individual portions of the polymer chains have the ability to uncoil or stretch. Therefore, the present invention includes (1) novel balloons and balloon catheters which have an improved overall combination of distensibility, elastic stress response and strength, (2) the process or method of forming balloons and balloon catheters from polymeric materials which will result in balloons and balloon catheters exhibiting these improved properties and (3) a novel sterilization process which will not adversely affect these enhanced properties.
- The present invention contemplates balloons characterized by an improved overall combination of distensibility, elastic stress response and wall tensile strength made by the process comprising subjecting a parison, made of a block copolymer having polymer chains with regions of inter-molecular chain interaction separated by regions in which those individual portions of the polymer chains have the ability to stretch or uncoil to at least one axial stretch and at least one radial expansion step. The expanded parison is then subjected to a heat set step to provide the expanded parison and resulting balloon with thermal and dimensional stability. The invention also contemplates a novel sterilization process in which balloons and balloon catheters, after preconditioning, are exposed to ethylene oxide at a temperature of about 40° C. and a relative humidity of about 50-60% for approximately 6 hours. The balloons and balloon catheters are then subjected to an aeration step in which the ethylene oxide is allowed to dissipate. The novel sterilization process does not adversely affect the improved overall combination of properties exhibited by the balloons of this invention.
- It should be understood that the foregoing description of the invention is intended merely to be illustrative and that other embodiments and modifications may be apparent to those skilled in the art without departing from the spirit and scope of the invention.
- The present invention provides for the first time “compliant” balloons, preferably dilatation balloons, which, because of the method or process used to form the balloons, as well as the polymeric materials used in the balloon forming process, produces balloons having a highly desirable combination of distensibility, elastic stress response and strength (i.e., distensibility of about 5 to about 20% and preferably in the range of about 6 to about 17%, elastic stress response of not greater than about 5.00 and preferably in the range of about 0.75 to about 4.00 and wall tensile strength of at least about 14,000 psi, preferably in the range of about 15,000 to about 40,000 psi and most preferably in the range of about 16,000 to about 30,000 psi). The invention also provides a unique method or process using a heat set step in the formation of the balloons of this invention which ensures that the balloons retain their distensibility and strength, and provides balloons with improved optical clarity. Moreover, the invention provides a novel sterilization process which will not adversely affect, to any significant degree, the enhanced combination of properties which are obtained using the novel balloon forming process of this invention.
- The materials which may be used in this novel process or method include polymeric materials having a molecular structure which are composed of individual polymer chains having regions or zones of inter-molecular chain interaction separated by regions or zones in which those individual portions of the polymer chains have the ability to stretch or uncoil. The ability of regions or zones of individual polymer chains to uncoil permits the chains to move upon the application of stress. However because these zones are held in place or secured at either end by zones exhibiting inter-molecular chain interaction, the uncoiled portions return to their original position once the applied stress is removed.
- These polymers can be considered to be comprised of polymer chains with individual regions of crystalline and amorphous material and can be referred to as “hard” and “soft” segments respectively. The individual polymer chains are able, to a substantial extent, to coil upon themselves and/or around each other in such a way that soft segments are associated with soft segments and hard segments with hard segments, thereby forming separate “domains” approximating soft and hard bodies of polymer, each exhibiting its own physical properties in varying degrees. The hard segments are comprised of regions which have significant inter-molecular chain interaction. This provides regions with increased strength and increased elastic stress response. In addition to providing strength, the hard segments are sufficiently rigid to permit the soft segments to stretch and uncoil which provides distensibility.
- The ratio of hard to soft segments and individual chemical structure of the individual segments define the balloon's distensibility, elastic stress response and strength. Therefore, the polymeric material used in accordance with this invention should have hard segments present in an amount sufficient to achieve a high degree of elastic stress response (i.e., not greater than about 5.00) and adequate wall tensile strength (i.e., at least about 14,000 psi), while at the same time having an adequate amount of soft segments to ensure that the balloon is also distensible (i.e., about 5 to about 20%).
- Examples of polymeric materials which have these alternating zones or regions and which may be used in forming the balloons and balloon catheters of this invention include block copolymers, and physical mixtures of different polymers. Examples of block copolymers which may be used include polyester block copolymers, polyamide block copolymers and polyurethane block copolymers. Examples of the mixtures which may be used include mixtures of nylon and polyamide block copolymers and polyethylene terephthalate and polyester block copolymers. The preferred block copolymer which can be used in accordance with the process of this invention is polyurethane block copolymer. This preferred polymer may be made, for example, by a reaction between
- a) an organic diisocyanate;
- b) a polyol; and
- c) at least one chain extender.
- The preferred polyurethanes which can be used in this invention may be varied by using different isocyanates and polyols which will result in different ratios of hard to soft segments as well as different chemical interactions within the individual regions of the polymer.
- An example of the most preferred polyurethane is manufactured by The Dow Chemical Company and marketed under the trade name PELLETHANE 2363-75D. This raw material has a Shore Hardness of about 74 D, a specific gravity of about 1.21, a tensile modulus of about 165,000 psi, a flexural modulus of about 190,000 psi, an ultimate tensile strength of about 6,980 psi and an ultimate elongation of about 250%.
- In accordance with this invention, the balloons are formed from a thin wall parison of a polymeric material, preferably made of a polyurethane block copolymer, which is treated in accordance with the process of this invention. The novel process contemplated by this invention employs a heat set step which will provide a balloon with temperature and dimensional stability. This stability results from the fact that the balloon is heated above the temperature using in the balloon forming process so that the orientation resulting from the processing conditions is “locked” into position.
- The balloons and balloon catheters of this invention may be formed using a mold which can be provided with a heating element. The mold receives a tubular parison made of a polymeric material of the type used in accordance with the present invention. The ends of the parison extend outwardly from the mold and one of the ends is sealed while the other end is affixed to a source of inflation fluid, typically nitrogen gas, under pressure. Clamps or “grippers” are attached to both ends of the parison so that the parison can be drawn apart axially in order to axially stretch the parison while at the same time said parison is capable of being expanded radially or “blown” with the inflation fluid. The radial expansion and axial stretch step or steps may be conducted simultaneously, or depending upon the polymeric material of which the parison is made, following whatever sequence is required to form a balloon. Failure to axial stretch the parison during the balloon forming process will result in result in a balloon which will have an uneven wall thickness and which will exhibit a wall tensile strength lower than the tensile strength obtained when the parison is both radially expanded and axially stretched.
- The polymeric parisons used in this invention are preferably drawn axially and expanded radially simultaneously within the mold. To improve the overall properties of the balloons formed, it is desirable that the parison is axially stretched and blown at temperatures above the glass transition temperature of the polymeric material used. This expansion usually takes place at a temperature between about 80 and about 150° C. depending upon the polymeric material used in the process.
- In accordance with this invention, based upon the polymeric material used, the parison is dimensioned with respect to the intended final configuration of the balloon. It is particularly important that the parison have relatively thin walls. The wall thickness is considered relative to the inside diameter of the parison which has wall thickness-to-inside diameter ratios of less than 0.6 and, preferably between 0.57 and 0.09 or even lower. The use of a parison with such thin walls enables the parison to be stretched radially to a greater and more uniform degree because there is less stress gradient through the wall from the surface of the inside diameter to the surface of the outside diameter. By utilizing a parison which has thin walls, there is less difference in the degree to which the inner and outer surfaces of the tubular parison are stretched.
- The parison is drawn from a starting length L1 to a drawn length L2 which preferably is between about 1.10 to about 6 times the initial length L1. The tubular parison, which has an initial internal diameter ID1 and an outer diameter OD1 is expanded by the inflation fluid emitted under pressure to the parison to an internal diameter ID2 which is preferably about 6 to about 8 times the initial internal diameter ID1 and an outer diameter OD2 which is about equal to or preferably greater than about 3 times the initial outer diameter OD1. The parison is preferably subjected to between 1 and 5 cycles during which the parison is axially stretched and radially expanded with an inflation pressure of between about 100 and about 500 psi. Nitrogen gas is the preferable inflation fluid for the radial expansion step.
- After the desired number of “blow” cycles have been completed, the expanded parison is subjected to a heat set or thermoforming step during which the expanded parison, still subjected to an inflation pressure of about 100 to about 500 psi, is held at a temperature above the temperature at which the balloon was axially stretched and radially expanded, but below the melting temperature of the polymeric material from which the parison was formed. This higher temperature induces crystallization and “freezes” or “locks” the orientation of the polymer chains which resulted from axially stretching and radially expanding the parison. The temperatures which can be used in this heat set step are therefore dependent upon the particular polymeric material used to form the parison and the ultimate properties desired in the balloon product (i.e., distensibility, strength and compliancy). The heat set step ensures that the expanded parison and the resulting balloon will have temperature and dimensional stability. After the heat set step is completed, the mold is cooled to about 37° C. The finished balloon will typically obtain its rated or nominal diameter when inflated to a pressure of about 5 to about 8 bars depending upon the polymeric material used to form the balloon. The balloon thus formed may be removed from the mold, and affixed to a catheter.
- For example, if the parison is formed from the polyurethane marketed by The Dow Chemical Company under the trade name PELLETHANE 2363-75D and axially stretched and radially expanded at a temperature of about 90-100° C., the heat set step would preferably be conducted at about 105-120° C. If this step was conducted at temperatures much above about 120° C., the tensile strength of the resulting polyurethane balloon would decrease significantly. Moreover, if the heat set step was conducted at temperatures significantly higher than 120° C., the distensibility of the resulting polyurethane balloon would also be adversely affected. However, if the heat set was conducted at temperatures below about 100° C., the polyurethane balloons formed would be dimensionally unstable resulting in balloons with uneven wall thicknesses. Additionally, the lower heat set temperature would result in balloons exhibiting physical properties which would more likely be adversely affected during sterilization. Finally, a balloon having a cloudy appearance, a property which physicians find particularly undesirable, would be another consequence of using a low heat set temperature.
- It should be noted that some adjustment in the foregoing axial stretch and radial expansion ratios, as well as the expansion and heat set temperatures may be necessary to take into account the difference in physical properties between the polyurethane block copolymer exemplified above and any other polymeric materials which can be used in accordance with this invention.
- In order to preserve a balloon's distensibility, elastic stress response, wall tensile strength and improved optical clarity, the balloon formed must also be subjected to the novel sterilization process contemplated by the invention. For example, if a sterilization process which is currently available in the art is used (e.g., high relative humidity at about 50-60° C. in the presence of about 12% ethylene oxide and about 88% Freon™ for about 9-16 hours), the elastic stress response, distensibility and the strength of the balloons contemplated by this invention would be adversely affected. When the novel low temperature, low humidity, ethylene oxide sterilization process of this invention is used to sterilize the balloons and balloon catheters of this invention, the elastic stress response, distensibility and strength of the balloons are not adversely affected to any significant degree.
- The novel low temperature, low humidity sterilization process consists of exposing the balloon or balloon catheter to a preconditioning step at temperature about 35 to about 45° C. and a relative humidity of about 55% for about 15 hours. The balloon or balloon catheter is then treated at a temperature of about 35 to about 45° C. and a relative humidity of about 55% with ethylene oxide, preferably in a concentration of about 100%. After being exposed to ethylene oxide for about 6 hours, the products are aerated and kept at a temperature of about 35 to about 45° C. for about 22 hours, in order to permit the ethylene oxide to dissipate. The sterilized balloon products are now ready for human use.
- The sterilization process cannot, however, be conducted above the heat set temperature since this would relieve the orientation of the polymer chains which was “locked” into place during heat set process. The sterilization process appears to be an important factor in determining the final physical characteristics of the balloons and balloon catheters of this invention. Therefore, the novel sterilization process is necessary to ensure a clinically useful and safe finished balloon and balloon catheter with an overall advantageous combination of physical properties (i.e., distensibility, elastic stress response and wall tensile strength) superior to those exhibited by the “compliant” balloons of the prior art.
- A parison was made from the polyurethane manufactured by The Dow Chemical Company and marketed under the trade name PELLETHANE 2363-75D. This material has a Shore Hardness of about 74 D, a specific gravity of about 1.21, a tensile strength of about 165,000 psi, a flexural modulus of about 190,000 psi, an ultimate tensile strength of about 6,980 psi and an ultimate elongation of about 250%. The parison was sealed at one end while the other end was attached to the source of the pressurized inflation fluid, in this example nitrogen gas. Clamps were attached to each end of the parison. The mold was then heated to an operating temperature of about 90-100° C., while the parison was pressurized with nitrogen gas at about 290 psi and held for about 70 seconds.
- The pressure was then relieved and the parison was subjected to a series of radial expansion or “blow” cycles. During each radial expansion or “blow” cycle, the parison was also axially stretched while being pressured at about 290 psi for about 5 seconds. The pressure was then relieved, and the parison was subject to continued axial stretching for about 5 seconds. The parison was then subjected to another expansion cycle. After three expansion or blow cycles, the original outer diameter had increased from 0.035 inches to 0.1181 inches.
- The expanded parison was then pressurized to about 190 psi and was subjected to a heat set step during which the expanded parison was held for about 75 seconds at a temperature of about 110° C. The pressurized balloon was then cooled to about 37° C. for about 30 seconds. The pressure was then relieved and the balloon was held vertically in the mold at about 37° C. for about 120 seconds to minimize balloon curvature. The balloon was released from the clamps and removed from the mold. The balloon, having a nominal or rated diameter of 3.0 mm, displayed an improved overall combination of distensibility, elastic stress response and strength when compared to “compliant” balloons of the art and was ready for attachment to a catheter.
- The balloons formed following the process set forth in Example 1 were placed in a sterilization chamber and kept at a temperature of about 40° C.±3° C. and a relative humidity of about 55% for about 15 hours. The balloons are kept at a temperature of about 40° C.±3° C and were then treated with 100% ethylene oxide. After being exposed to the ethylene oxide for about 6 hours, the balloons were removed from the sterilization chamber and held at a temperature of about 40° C.±3° C. and ambient relative humidity for about 22 hours in order to dissipate the ethylene oxide. At this point, the balloons were sterilized and ready for human use.
- The effect which the novel sterilization process of this invention has on the balloons formed using the balloon forming process contemplated by this invention are demonstrated below. Balloons with a nominal diameter of 3.0 mm were formed from polyurethane following the process described in Example 1. One group of balloons was subjected to the sterilization process contemplated by this invention and described previously in Example 2, sterilization process contemplated by this invention and described previously in Example 2, while the other group of balloons were subjected to a sterilization process currently used in the art.
- In that sterilization process (referred to in this Example as “traditional sterilization process”), the balloons were preconditioned at a temperature of about 43° C. and a relative humidity of about 60% for about 24 hours. The balloons were then treated with about 12% ethylene oxide and 88% Freon™ at a temperature of about 54° C. After being treated with the ethylene oxide mixture for about 9 hours, the balloons are removed from the sterilization chamber and kept at a temperature of about 38° C. for about 22 hours.
- The average wall tensile, burst pressure, elastic stress response and distensibility (i.e., radial expansion) of both sets of balloons were compared below.
Average Wall Average Tensile Burst Average Sterilization Strength Pressure Elastic Stress Average Conditions (psi) (atm) Response Distensibility novel sterilization 16,297 22.0 3.38 9.4% conditions described in Example 2 traditional 14,497 22.6 10.29* 20.2% sterilization process - The following example demonstrates the importance of the heat set step. Three dilatation balloons with a nominal or rated diameter of 3.0 mm, were formed from polyurethane following the process described in Example 1. The average burst pressure, distensibility and wall tensile strength of balloons formed using different heat set temperatures are compared. The burst pressure and distensibility were determined at 37° C.
Heat Set T Temperature Average Wall Tensile Average Burst Average (° C.) Strength (psi) Pressure (atm) Distensibility 160 14,712 12.8 10.26% 132 23,364 20.6 5.81% 118 25,346 22.2 5.96% - The following example demonstrates the improved elastic stress response or “repeatability” which can be obtained by the balloons and balloon catheters formed following the process contemplated by this invention. In this example, dilatation balloons with a nominal or rated diameter of 3.0 mm were formed from polyurethane following the process described in Example 1. A number of polyurethane balloons were sterilized following the process previously described in Example 3 (referred to as “traditional sterilization” in this Example). Another group of polyurethane balloons were sterilized using the novel sterilization contemplated by this invention and previously described in Example 2. The elastic stress response of these polyurethanes balloons were compared with the elastic stress response of other sterilized 3.0 mm balloons known in the art.
Average Average Diameter at Diameter at 5 Bars Averag Initial 5 Bar After A Single Elastic Stress Balloon Inflation Inflation to 10 Bars Response Polyurethane 2.96 3.06 3.38 (sterilization described in Example 2) Polyurethane 2.72 3.00 10.29 (traditional sterilization) Polyethylene 3.02 3.04 0.66 terephthalate Cross-linked 2.98 3.11 4.36 polyethylene Cross-linked 2.93 3.19 8.87 polyolefin-ionomer - The following example demonstrates the improved overall combination of distensibility, elastic stress response and wall tensile strength obtained by forming balloons by using the process of this invention. Balloons with a nominal or rated diameter of 3.0 mm were formed from polyurethane following the process described in Example 1. The average elastic stress response, distensibility and wall tensile strength of those polyurethane balloons are compared with properties of other 3.0 mm balloons of the art.
Average Wall Average Elastic Average Tensile Balloon Stress Response Distensibility Strength (psi) Polyurethane 3.38 9.4% 16,297 Polyethylene 0.66 3.26% 62,081 terephthalate Cross-linked 4.36 9.67% 8,868 polyethylene Cross-linked 8.87 14.64% 6,793 polyolefin-ionomer
Claims (17)
1. A balloon characterized by an improved overall combination of distensibility, elastic stress response and wall tensile strength made by the process comprising:
a. providing a parison of a block copolymer having regions of inter-molecular chain interaction separated by regions in which those individual portions of the polymer chains have the ability to uncoil, said parison having a predetermined original outer diameter, a predetermined wall thickness and a predetermined length;
b. subjecting said parison to at least one axial stretch step and at least one radial expansion step at temperature T1 which is below the melting temperature of said block copolymer to increase the diameter and length of said parison to at least 3 times the original diameter and 2 times the original length and to decrease the original wall thickness to at least 20% of the original wall thickness to form an expanded parison; and
c. heating said expanded parison to a temperature of T2 which is above T1 but below the melting temperature of said block copolymer.
2. The balloon according to claim 1 wherein said radial expansion step is conducted while said parison is simultaneously subjected to said axial stretch step.
3. The balloon according to claim 1 wherein said axial stretch and radial expansion steps are conducted at temperature T1 which is greater than the glass transition temperature of said block copolymer.
4. A balloon according to claim 1 wherein said block copolymer is selected from the group consisting of polyester block copolymers, polyamide block copolymers, polyurethane block copolymers, a mixture of nylon and polyamide block copolymers and a mixture of polyethylene terephthalate and polyester block copolymers.
5. A balloon according to claim 1 wherein said balloon has a distensibility of about 5 to about 20%, an elastic stress response not greater than about 5.00, and a wall tensile strength greater than about 14,000 psi.
6. A balloon according to claim 1 wherein said balloon has a distensibility of about 6 to about 17%, an elastic stress response of about 0.75 to about 4.00 and a wall tensile strength of about 16,000 to about 30,000 psi.
7. A balloon according to claim 1 wherein said block copolymer is a polyurethane having a Shore Hardness of about 74 D, a specific gravity of about 1.21, a tensile modulus of about 165,000 psi, a flexual modulus of about 190,000 psi, an ultimate tensile strength of about 6,980 psi and an ultimate elongation of about 250%.
8. A balloon according to claim 6 wherein T1 is about 90-100° C. and T2 is about 110-120° C.
9. A process of forming a balloon characterized by an improved overall combination of distensibility, elastic stress response and wall tensile strength comprising:
a. providing a parison of a block copolymer having regions of inter-molecular chain interaction separated by regions in which those individual polymer portion of chains have the ability to uncoil, said parison having a predetermined original outer diameter, a predetermined wall thickness and a predetermined length;
b. subjecting said parison to at least one axial stretch step and at least one radial expansion step at temperature T1 which is below the melting temperature of said block copolymer to increase the diameter and length of said parison to at least 3 times the original diameter and 2 times the original length and to decrease the original wall thickness to at least 20% of the original wall thickness to form an expanded parison; and
c. heating said expanded parison to a temperature of T2 which is above T1 but below the melting temperature of said block copolymer.
10. The process according to claim 9 wherein said radial expansion step is conducted while said parison is simultaneously subjected to said axial stretch step.
11. The process according to claim 9 wherein said axial stretch and radial expansion steps are conducted at temperature T1 which is greater than the glass transition temperature of said block copolymer.
12. A process according to claim 9 wherein said block copolymer is selected from the group consisting of polyester block copolymers, polyamide block copolymers, polyurethane block copolymers, a mixture of nylon and polyamide block copolymers and a mixture of polyethylene terephthalate and polyester block copolymers.
13. A process according to claim 9 wherein said balloon formed has a distensibility of about 5 to about 20%, an elastic stress response of not greater than about 5.00 and a wall tensile strength greater than about 14,000 psi.
14. A process according to claim 9 wherein the balloon formed has a distensibility of about 6 to about 17%, an elastic stress response of about 0.75 to about 4.00 and a wall tensile strength of about 16,000 to about 30,000 psi.
15. A process according to claim 9 wherein said block copolymer is a polyurethane having a Shore Hardness of about 74 D, a specific gravity of about 1.21, a tensile modulus of about 165,000 psi, a flexual modulus of about 190,000 psi, an ultimate tensile strength of about 6,980 psi and an ultimate elongation of about 250%.
16. A balloon catheter comprising the balloon of claim 1 .
17. A process for sterilizing balloons and balloon catheters comprising:
a. subjecting said balloons and balloon catheters to a temperature of about to about 45° C. and a relative humidity of about 55% for about 15 hours;
b. treating said balloons and balloon catheters at a temperature of about 35 to about 45° C. and a relative humidity of about 55% with ethylene oxide for about 15 hours; and
c. discontinuing treatment with ethylene oxide and subjecting said balloons and balloon catheters to a temperature of about 35 to about 45° C. for about 22 hours.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/637,575 US20040097878A1 (en) | 1992-09-30 | 2003-08-11 | Distensible dilatation balloon with elastic stress response and manufacture thereof |
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US07/954,750 US5500180A (en) | 1992-09-30 | 1992-09-30 | Method of making a distensible dilatation balloon using a block copolymer |
| US44070095A | 1995-05-15 | 1995-05-15 | |
| US08/883,261 US6210364B1 (en) | 1992-09-30 | 1997-06-26 | Distensible dilatation balloon with elastic stress response |
| US09/192,893 US6283939B1 (en) | 1992-09-30 | 1998-11-16 | Distensible dilatation balloon with elastic stress |
| US09/942,920 US6620381B2 (en) | 1992-09-30 | 2001-08-31 | Sterilization process for a distensible dilatation balloon with elastic stress response |
| US10/637,575 US20040097878A1 (en) | 1992-09-30 | 2003-08-11 | Distensible dilatation balloon with elastic stress response and manufacture thereof |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/942,920 Continuation US6620381B2 (en) | 1992-09-30 | 2001-08-31 | Sterilization process for a distensible dilatation balloon with elastic stress response |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20040097878A1 true US20040097878A1 (en) | 2004-05-20 |
Family
ID=25495874
Family Applications (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US07/954,750 Expired - Lifetime US5500180A (en) | 1992-09-30 | 1992-09-30 | Method of making a distensible dilatation balloon using a block copolymer |
| US08/883,261 Expired - Fee Related US6210364B1 (en) | 1992-09-30 | 1997-06-26 | Distensible dilatation balloon with elastic stress response |
| US09/192,893 Expired - Lifetime US6283939B1 (en) | 1992-09-30 | 1998-11-16 | Distensible dilatation balloon with elastic stress |
| US09/942,920 Expired - Fee Related US6620381B2 (en) | 1992-09-30 | 2001-08-31 | Sterilization process for a distensible dilatation balloon with elastic stress response |
| US10/637,575 Abandoned US20040097878A1 (en) | 1992-09-30 | 2003-08-11 | Distensible dilatation balloon with elastic stress response and manufacture thereof |
Family Applications Before (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US07/954,750 Expired - Lifetime US5500180A (en) | 1992-09-30 | 1992-09-30 | Method of making a distensible dilatation balloon using a block copolymer |
| US08/883,261 Expired - Fee Related US6210364B1 (en) | 1992-09-30 | 1997-06-26 | Distensible dilatation balloon with elastic stress response |
| US09/192,893 Expired - Lifetime US6283939B1 (en) | 1992-09-30 | 1998-11-16 | Distensible dilatation balloon with elastic stress |
| US09/942,920 Expired - Fee Related US6620381B2 (en) | 1992-09-30 | 2001-08-31 | Sterilization process for a distensible dilatation balloon with elastic stress response |
Country Status (5)
| Country | Link |
|---|---|
| US (5) | US5500180A (en) |
| EP (2) | EP0592885B1 (en) |
| JP (1) | JPH06304920A (en) |
| CA (1) | CA2107378A1 (en) |
| DE (2) | DE69334318D1 (en) |
Cited By (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080051819A1 (en) * | 2006-08-25 | 2008-02-28 | Nishith Chasmawala | Apparatus and methods for use of expandable members in surgical applications |
| US7736388B2 (en) | 1999-04-09 | 2010-06-15 | Evalve, Inc. | Fixation devices, systems and methods for engaging tissue |
| US7753923B2 (en) | 1999-04-09 | 2010-07-13 | Evalve, Inc. | Leaflet suturing |
| US7811296B2 (en) | 1999-04-09 | 2010-10-12 | Evalve, Inc. | Fixation devices for variation in engagement of tissue |
| US7981139B2 (en) | 2002-03-01 | 2011-07-19 | Evalve, Inc | Suture anchors and methods of use |
| US7981123B2 (en) | 1997-09-12 | 2011-07-19 | Evalve, Inc. | Surgical device for connecting soft tissue |
| US8029518B2 (en) | 1999-04-09 | 2011-10-04 | Evalve, Inc. | Methods and devices for capturing and fixing leaflets in valve repair |
| US8052592B2 (en) | 2005-09-27 | 2011-11-08 | Evalve, Inc. | Methods and devices for tissue grasping and assessment |
| US8123703B2 (en) | 1999-04-09 | 2012-02-28 | Evalve, Inc. | Steerable access sheath and methods of use |
| US8216256B2 (en) | 1999-04-09 | 2012-07-10 | Evalve, Inc. | Detachment mechanism for implantable fixation devices |
| US8343174B2 (en) | 1999-04-09 | 2013-01-01 | Evalve, Inc. | Locking mechanisms for fixation devices and methods of engaging tissue |
| US8926620B2 (en) | 2006-08-25 | 2015-01-06 | Kyphon Sarl | Apparatus and methods for use of expandable members in surgical applications |
| US10188392B2 (en) | 2014-12-19 | 2019-01-29 | Abbott Cardiovascular Systems, Inc. | Grasping for tissue repair |
| US10238495B2 (en) | 2015-10-09 | 2019-03-26 | Evalve, Inc. | Delivery catheter handle and methods of use |
| US10238494B2 (en) | 2015-06-29 | 2019-03-26 | Evalve, Inc. | Self-aligning radiopaque ring |
| US10314586B2 (en) | 2016-12-13 | 2019-06-11 | Evalve, Inc. | Rotatable device and method for fixing tricuspid valve tissue |
| US10363138B2 (en) | 2016-11-09 | 2019-07-30 | Evalve, Inc. | Devices for adjusting the curvature of cardiac valve structures |
| US10376673B2 (en) | 2015-06-19 | 2019-08-13 | Evalve, Inc. | Catheter guiding system and methods |
| US10390943B2 (en) | 2014-03-17 | 2019-08-27 | Evalve, Inc. | Double orifice device for transcatheter mitral valve replacement |
| US10398553B2 (en) | 2016-11-11 | 2019-09-03 | Evalve, Inc. | Opposing disk device for grasping cardiac valve tissue |
| US10413408B2 (en) | 2015-08-06 | 2019-09-17 | Evalve, Inc. | Delivery catheter systems, methods, and devices |
| US10426616B2 (en) | 2016-11-17 | 2019-10-01 | Evalve, Inc. | Cardiac implant delivery system |
| US10524912B2 (en) | 2015-04-02 | 2020-01-07 | Abbott Cardiovascular Systems, Inc. | Tissue fixation devices and methods |
| US10631871B2 (en) | 2003-05-19 | 2020-04-28 | Evalve, Inc. | Fixation devices, systems and methods for engaging tissue |
| US10667815B2 (en) | 2015-07-21 | 2020-06-02 | Evalve, Inc. | Tissue grasping devices and related methods |
| US10736632B2 (en) | 2016-07-06 | 2020-08-11 | Evalve, Inc. | Methods and devices for valve clip excision |
| US10743876B2 (en) | 2011-09-13 | 2020-08-18 | Abbott Cardiovascular Systems Inc. | System for fixation of leaflets of a heart valve |
| US10779837B2 (en) | 2016-12-08 | 2020-09-22 | Evalve, Inc. | Adjustable arm device for grasping tissues |
| US11065119B2 (en) | 2017-05-12 | 2021-07-20 | Evalve, Inc. | Long arm valve repair clip |
| US11304715B2 (en) | 2004-09-27 | 2022-04-19 | Evalve, Inc. | Methods and devices for tissue grasping and assessment |
| US11653947B2 (en) | 2016-10-05 | 2023-05-23 | Evalve, Inc. | Cardiac valve cutting device |
| US12048624B2 (en) | 2019-07-15 | 2024-07-30 | Evalve, Inc. | Independent proximal element actuation methods |
| US12048448B2 (en) | 2020-05-06 | 2024-07-30 | Evalve, Inc. | Leaflet grasping and cutting device |
| US12121231B2 (en) | 2022-04-18 | 2024-10-22 | Evalve, Inc. | Methods and devices for tissue grasping and assessment |
Families Citing this family (230)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5500180A (en) * | 1992-09-30 | 1996-03-19 | C. R. Bard, Inc. | Method of making a distensible dilatation balloon using a block copolymer |
| US6025044A (en) * | 1993-08-18 | 2000-02-15 | W. L. Gore & Associates, Inc. | Thin-wall polytetrafluoroethylene tube |
| US20030032963A1 (en) | 2001-10-24 | 2003-02-13 | Kyphon Inc. | Devices and methods using an expandable body with internal restraint for compressing cancellous bone |
| US6027486A (en) * | 1996-05-02 | 2000-02-22 | Radiance Medical Systems, Inc. | Interactive angioplasty |
| US5843116A (en) * | 1996-05-02 | 1998-12-01 | Cardiovascular Dynamics, Inc. | Focalized intraluminal balloons |
| US5470313A (en) * | 1994-02-24 | 1995-11-28 | Cardiovascular Dynamics, Inc. | Variable diameter balloon dilatation catheter |
| US5645560A (en) * | 1995-12-15 | 1997-07-08 | Cardiovascular Dynamics, Inc. | Fixed focal balloon for interactive angioplasty and stent implantation |
| US6120523A (en) * | 1994-02-24 | 2000-09-19 | Radiance Medical Systems, Inc. | Focalized intraluminal balloons |
| US5951941A (en) * | 1994-03-02 | 1999-09-14 | Scimed Life Systems, Inc. | Block copolymer elastomer catheter balloons |
| US7163522B1 (en) | 1994-03-02 | 2007-01-16 | Scimed Life Systems, Inc. | Block copolymer elastomer catheter balloons |
| US6171278B1 (en) | 1994-03-02 | 2001-01-09 | Scimed Life Systems, Inc. | Block copolymer elastomer catheter balloons |
| US6406457B1 (en) | 1994-03-02 | 2002-06-18 | Scimed Life Systems, Inc. | Block copolymer elastomer catheter balloons |
| US6146356A (en) * | 1994-03-02 | 2000-11-14 | Scimed Life Systems, Inc. | Block copolymer elastomer catheter balloons |
| US7108826B2 (en) * | 1994-03-02 | 2006-09-19 | Boston Scientific Scimed, Inc. | High compliance, high strength catheter balloons useful for treatment of gastrointestinal lesions |
| US5556383A (en) * | 1994-03-02 | 1996-09-17 | Scimed Lifesystems, Inc. | Block copolymer elastomer catheter balloons |
| US5830182A (en) * | 1994-03-02 | 1998-11-03 | Scimed Life Systems, Inc. | Block copolymer elastomer catheter balloons |
| WO1996012516A1 (en) * | 1994-10-19 | 1996-05-02 | Advanced Cardiovascular Systems, Inc. | High strength dilatation balloons |
| WO1996037240A1 (en) | 1995-05-24 | 1996-11-28 | Schneider (Usa) Inc. | Dilatation balloons containing polyesteretheramide copolymer |
| US5645789A (en) * | 1995-07-20 | 1997-07-08 | Navius Corporation | Distensible pet balloon and method of manufacture |
| JP3400199B2 (en) * | 1995-08-22 | 2003-04-28 | 住友ベークライト株式会社 | Compliant balloon |
| US20060271091A1 (en) * | 1995-09-18 | 2006-11-30 | Campbell Carey V | Balloon catheter device |
| US5868704A (en) * | 1995-09-18 | 1999-02-09 | W. L. Gore & Associates, Inc. | Balloon catheter device |
| US5752934A (en) * | 1995-09-18 | 1998-05-19 | W. L. Gore & Associates, Inc. | Balloon catheter device |
| US6050972A (en) * | 1996-05-20 | 2000-04-18 | Percusurge, Inc. | Guidewire inflation system |
| US6270477B1 (en) * | 1996-05-20 | 2001-08-07 | Percusurge, Inc. | Catheter for emboli containment |
| US20050245894A1 (en) * | 1996-05-20 | 2005-11-03 | Medtronic Vascular, Inc. | Methods and apparatuses for drug delivery to an intravascular occlusion |
| US6022336A (en) | 1996-05-20 | 2000-02-08 | Percusurge, Inc. | Catheter system for emboli containment |
| US20010049517A1 (en) | 1997-03-06 | 2001-12-06 | Gholam-Reza Zadno-Azizi | Method for containing and removing occlusions in the carotid arteries |
| US6652480B1 (en) * | 1997-03-06 | 2003-11-25 | Medtronic Ave., Inc. | Methods for reducing distal embolization |
| US6068623A (en) | 1997-03-06 | 2000-05-30 | Percusurge, Inc. | Hollow medical wires and methods of constructing same |
| EP0906135B1 (en) | 1996-05-20 | 2004-12-29 | Medtronic Percusurge, Inc. | Low profile catheter valve |
| US6152909A (en) * | 1996-05-20 | 2000-11-28 | Percusurge, Inc. | Aspiration system and method |
| US5868705A (en) * | 1996-05-20 | 1999-02-09 | Percusurge Inc | Pre-stretched catheter balloon |
| US6786888B1 (en) | 1996-05-20 | 2004-09-07 | Medtronic Ave, Inc. | Low profile catheter for emboli protection |
| US6325777B1 (en) | 1996-05-20 | 2001-12-04 | Medtronic Percusurge, Inc. | Low profile catheter valve and inflation adaptor |
| CA2261217C (en) | 1996-07-23 | 2008-07-08 | Scimed Life Systems, Inc. | High compliance, high strength catheter balloons useful for treatment of gastrointestinal lesions |
| US7749585B2 (en) * | 1996-10-08 | 2010-07-06 | Alan Zamore | Reduced profile medical balloon element |
| US5871692A (en) * | 1997-01-14 | 1999-02-16 | Steris Corporation | Method and apparatus for cleaning, decontaminating, and sterilizing catheters |
| US6554795B2 (en) | 1997-03-06 | 2003-04-29 | Medtronic Ave, Inc. | Balloon catheter and method of manufacture |
| US6190332B1 (en) | 1998-02-19 | 2001-02-20 | Percusurge, Inc. | Core wire with shapeable tip |
| WO1998038929A1 (en) * | 1997-03-06 | 1998-09-11 | Percusurge, Inc. | Intravascular aspiration system |
| US6849068B1 (en) | 1997-03-06 | 2005-02-01 | Medtronic Ave, Inc. | Aspiration catheter |
| US6355016B1 (en) | 1997-03-06 | 2002-03-12 | Medtronic Percusurge, Inc. | Catheter core wire |
| US20010008661A1 (en) | 1997-05-14 | 2001-07-19 | Eugene J. Jung Jr | Balloon for a dilation catheter and method for manufacturing a balloon |
| CA2232250C (en) * | 1997-05-14 | 2007-06-26 | Navius Corporation | Balloon for a dilation catheter and method for manufacturing a balloon |
| US6306166B1 (en) * | 1997-08-13 | 2001-10-23 | Scimed Life Systems, Inc. | Loading and release of water-insoluble drugs |
| US6358227B1 (en) * | 1997-09-10 | 2002-03-19 | Scimed Life Systems, Inc. | Dilatation catheter balloon made from pen based homopolymer or random copolymer |
| US5976181A (en) | 1997-09-22 | 1999-11-02 | Ave Connaught | Balloon mounted stent and method therefor |
| US5948345A (en) * | 1998-01-05 | 1999-09-07 | Medtronic, Inc. | Method for making medical balloon catheter |
| US6685671B1 (en) * | 1998-01-14 | 2004-02-03 | Sumitomo Bakeleite Co., Ltd. | Balloon catheter for puncturing, medical tube introduction device using the catheter and method for use thereof |
| US6319229B1 (en) | 1998-02-19 | 2001-11-20 | Medtronic Percusurge, Inc. | Balloon catheter and method of manufacture |
| ATE259668T1 (en) | 1998-03-04 | 2004-03-15 | Boston Scient Ltd | COMPOSITION AND METHOD FOR PRODUCING PBT CATHETER BALLOONS |
| US20010001113A1 (en) * | 1998-04-21 | 2001-05-10 | Florencia Lim | Balloon catheter |
| US6287314B1 (en) * | 1998-04-21 | 2001-09-11 | Advanced Cardiovascular Systems, Inc. | Stent deploying catheter system |
| US6719773B1 (en) | 1998-06-01 | 2004-04-13 | Kyphon Inc. | Expandable structures for deployment in interior body regions |
| JP4393706B2 (en) | 1998-06-01 | 2010-01-06 | カイフォン・ソシエテ・ア・レスポンサビリテ・リミテ | Deployable preformed structure for placement within an internal body region |
| US6287506B1 (en) * | 1998-07-09 | 2001-09-11 | Schneider (Usa) Inc. | Method for reducing dilation balloon cone stiffness |
| JP2000217924A (en) * | 1999-02-01 | 2000-08-08 | Kanegafuchi Chem Ind Co Ltd | Extended body for extended catheter and its manufacture |
| WO2000027461A1 (en) * | 1998-11-09 | 2000-05-18 | Datascope Investment Corp. | Intra-aortic balloon catheter having an ultra-thin stretch blow molded balloon membrane |
| US6395208B1 (en) | 1999-01-25 | 2002-05-28 | Atrium Medical Corporation | Method of making an expandable fluoropolymer device |
| US6955661B1 (en) * | 1999-01-25 | 2005-10-18 | Atrium Medical Corporation | Expandable fluoropolymer device for delivery of therapeutic agents and method of making |
| US7637886B2 (en) * | 1999-01-25 | 2009-12-29 | Atrium Medical Corporation | Expandable fluoropolymer device and method of making |
| WO2000051660A1 (en) | 1999-03-05 | 2000-09-08 | Medtronic, Inc. | Polyurethane balloon catheter |
| JP2000279507A (en) * | 1999-03-30 | 2000-10-10 | Nippon Zeon Co Ltd | Balloon catheter with low-temperature blow-molded balloon |
| JP4883433B2 (en) * | 1999-05-16 | 2012-02-22 | 株式会社ワイエス・メディカル | Balloon catheter, method for manufacturing the same, and method for attaching balloon to catheter tube |
| US7892201B1 (en) | 1999-08-27 | 2011-02-22 | Gore Enterprise Holdings, Inc. | Balloon catheter and method of mounting same |
| US6572813B1 (en) | 2000-01-13 | 2003-06-03 | Advanced Cardiovascular Systems, Inc. | Balloon forming process |
| DE10026003A1 (en) * | 2000-05-25 | 2001-12-06 | Bosch Gmbh Robert | stator |
| US6863861B1 (en) | 2000-09-28 | 2005-03-08 | Boston Scientific Scimed, Inc. | Process for forming a medical device balloon |
| US6951674B1 (en) | 2000-11-10 | 2005-10-04 | Scimed Life Systems, Inc. | Blended polyurethane interventional balloon |
| US6673302B2 (en) | 2001-01-24 | 2004-01-06 | Scimed Life Systems, Inc. | Wet processing method for catheter balloons |
| US6946092B1 (en) * | 2001-09-10 | 2005-09-20 | Scimed Life Systems, Inc. | Medical balloon |
| US7005097B2 (en) * | 2002-01-23 | 2006-02-28 | Boston Scientific Scimed, Inc. | Medical devices employing chain extended polymers |
| US7029732B2 (en) * | 2002-02-28 | 2006-04-18 | Boston Scientific Scimed, Inc. | Medical device balloons with improved strength properties and processes for producing same |
| US8425549B2 (en) | 2002-07-23 | 2013-04-23 | Reverse Medical Corporation | Systems and methods for removing obstructive matter from body lumens and treating vascular defects |
| US20040039410A1 (en) * | 2002-08-22 | 2004-02-26 | Brooke Ren | High-strength balloon with tailored softness |
| US8337968B2 (en) | 2002-09-11 | 2012-12-25 | Boston Scientific Scimed, Inc. | Radiation sterilized medical devices comprising radiation sensitive polymers |
| US7226472B2 (en) * | 2002-10-15 | 2007-06-05 | Boston Scientific Scimed, Inc. | Catheter balloon with advantageous cone design |
| DE50209306D1 (en) | 2002-12-31 | 2007-03-08 | Abbott Lab Vascular Entpr Ltd | Catheter with a more flexible area between stem and tip, and method of making the same |
| US20040143283A1 (en) * | 2003-01-17 | 2004-07-22 | Mcgill Scott | Inflation adaptor and method of use |
| US20040260237A1 (en) * | 2003-06-17 | 2004-12-23 | Paul Squadrito | Inflation adaptor with magnetically-assisted loading |
| US20050004594A1 (en) * | 2003-07-02 | 2005-01-06 | Jeffrey Nool | Devices and methods for aspirating from filters |
| US7727442B2 (en) * | 2003-07-10 | 2010-06-01 | Boston Scientific Scimed, Inc. | Medical device tubing with discrete orientation regions |
| EP1508348A1 (en) * | 2003-08-18 | 2005-02-23 | Medtronic Vascular, Inc. | A process for producing a hyper-elastic, high strength dilation balloon made from multi-block copolymers |
| US20050124976A1 (en) * | 2003-12-04 | 2005-06-09 | Devens Douglas A.Jr. | Medical devices |
| US20050127561A1 (en) * | 2003-12-16 | 2005-06-16 | Scimed Life Systems, Inc. | Method of making expandable-collapsible bodies by temperature gradient expansion molding |
| US7601285B2 (en) * | 2003-12-31 | 2009-10-13 | Boston Scientific Scimed, Inc. | Medical device with varying physical properties and method for forming same |
| US7264458B2 (en) * | 2004-01-07 | 2007-09-04 | Boston Scientific Scimed, Inc. | Process and apparatus for forming medical device balloons |
| US7016394B2 (en) * | 2004-04-23 | 2006-03-21 | Ucar Carbon Company Inc. | Male-female electrode joint |
| US20050228428A1 (en) * | 2004-04-07 | 2005-10-13 | Afsar Ali | Balloon catheters and methods for manufacturing balloons for balloon catheters |
| US7892478B2 (en) * | 2004-04-19 | 2011-02-22 | Boston Scientific Scimed, Inc. | Catheter balloon mold form and molding process |
| US7785439B2 (en) * | 2004-09-29 | 2010-08-31 | Abbott Laboratories Vascular Enterprises Limited | Method for connecting a catheter balloon with a catheter shaft of a balloon catheter |
| US7628769B2 (en) * | 2004-05-27 | 2009-12-08 | Abbott Laboratories | Catheter having overlapping stiffening members |
| US7815627B2 (en) | 2004-05-27 | 2010-10-19 | Abbott Laboratories | Catheter having plurality of stiffening members |
| US20070078439A1 (en) * | 2004-05-27 | 2007-04-05 | Axel Grandt | Multiple lumen catheter and method of making same |
| US7785318B2 (en) * | 2004-05-27 | 2010-08-31 | Abbott Laboratories | Catheter having plurality of stiffening members |
| US7794448B2 (en) * | 2004-05-27 | 2010-09-14 | Abbott Laboratories | Multiple lumen catheter and method of making same |
| US7625353B2 (en) * | 2004-05-27 | 2009-12-01 | Abbott Laboratories | Catheter having first and second guidewire tubes and overlapping stiffening members |
| US7658723B2 (en) * | 2004-05-27 | 2010-02-09 | Abbott Laboratories | Catheter having plurality of stiffening members |
| US7527606B2 (en) | 2004-05-27 | 2009-05-05 | Abbott Laboratories | Catheter having main body portion with coil-defined guidewire passage |
| US7635510B2 (en) * | 2004-07-07 | 2009-12-22 | Boston Scientific Scimed, Inc. | High performance balloon catheter/component |
| US20060134357A1 (en) * | 2004-12-16 | 2006-06-22 | Medtronic Vascular, Inc. | Polymer blends for medical balloons |
| US20060135725A1 (en) * | 2004-12-21 | 2006-06-22 | Scimed Life Systems, Inc. | New balloon materials |
| US9586030B2 (en) * | 2004-12-23 | 2017-03-07 | Boston Scientific Scimed, Inc. | Fugitive plasticizer balloon surface treatment for enhanced stent securement |
| US20070009564A1 (en) * | 2005-06-22 | 2007-01-11 | Mcclain James B | Drug/polymer composite materials and methods of making the same |
| US7691082B2 (en) * | 2005-07-01 | 2010-04-06 | Boston Scientific Scimed, Inc. | Medical devices |
| US20090062909A1 (en) | 2005-07-15 | 2009-03-05 | Micell Technologies, Inc. | Stent with polymer coating containing amorphous rapamycin |
| CN105233349B (en) | 2005-07-15 | 2019-06-18 | 胶束技术股份有限公司 | The polymer coating of drug powder comprising controlled morphology |
| US8876763B2 (en) * | 2005-11-01 | 2014-11-04 | Boston Scientific Scimed, Inc. | Composite balloon |
| US7766893B2 (en) * | 2005-12-07 | 2010-08-03 | Boston Scientific Scimed, Inc. | Tapered multi-chamber balloon |
| WO2007075585A2 (en) | 2005-12-16 | 2007-07-05 | Interface Associates, Inc. | Multi-layer balloons for medical applications and methods for manufacturing the same |
| US20070142772A1 (en) * | 2005-12-16 | 2007-06-21 | Medtronic Vascular, Inc. | Dual-Layer Medical Balloon |
| US7828766B2 (en) | 2005-12-20 | 2010-11-09 | Advanced Cardiovascular Systems, Inc. | Non-compliant multilayered balloon for a catheter |
| JP5254805B2 (en) * | 2005-12-23 | 2013-08-07 | シー・アール・バード・インコーポレーテッド | Balloon catheter with a vent hole in the center |
| US8043673B2 (en) | 2006-03-02 | 2011-10-25 | Boston Scientific Scimed, Inc. | Method to make tube-in-tube balloon |
| US20070205539A1 (en) * | 2006-03-03 | 2007-09-06 | Boston Scientific Scimed, Inc. | Balloon mold design |
| US8858855B2 (en) | 2006-04-20 | 2014-10-14 | Boston Scientific Scimed, Inc. | High pressure balloon |
| CA2650590C (en) * | 2006-04-26 | 2018-04-03 | Micell Technologies, Inc. | Coatings containing multiple drugs |
| US7943221B2 (en) * | 2006-05-22 | 2011-05-17 | Boston Scientific Scimed, Inc. | Hinged compliance fiber braid balloon |
| US20070296125A1 (en) * | 2006-06-22 | 2007-12-27 | Joel Colburn | Thin cuff for use with medical tubing and method and apparatus for making the same |
| US8196584B2 (en) | 2006-06-22 | 2012-06-12 | Nellcor Puritan Bennett Llc | Endotracheal cuff and technique for using the same |
| US8434487B2 (en) | 2006-06-22 | 2013-05-07 | Covidien Lp | Endotracheal cuff and technique for using the same |
| US20070295337A1 (en) * | 2006-06-22 | 2007-12-27 | Nelson Donald S | Endotracheal cuff and technique for using the same |
| CN101583384A (en) * | 2006-07-03 | 2009-11-18 | 汉莫堤克股份有限公司 | Manufacture, method, and use of active substance-releasing medical products for permanently keeping blood vessels open |
| US7654264B2 (en) | 2006-07-18 | 2010-02-02 | Nellcor Puritan Bennett Llc | Medical tube including an inflatable cuff having a notched collar |
| US20080097300A1 (en) * | 2006-08-07 | 2008-04-24 | Sherif Eskaros | Catheter balloon with multiple micropleats |
| US9180279B2 (en) | 2006-08-07 | 2015-11-10 | W. L. Gore & Associates, Inc. | Inflatable imbibed polymer devices |
| US7785290B2 (en) * | 2006-08-07 | 2010-08-31 | Gore Enterprise Holdings, Inc. | Non-shortening high angle wrapped balloons |
| US20080140173A1 (en) * | 2006-08-07 | 2008-06-12 | Sherif Eskaros | Non-shortening wrapped balloon |
| US20080125711A1 (en) | 2006-08-07 | 2008-05-29 | Alpini Alfred A | Catheter balloons with integrated non-distensible seals |
| US8460240B2 (en) * | 2006-08-07 | 2013-06-11 | W. L. Gore & Associates, Inc. | Inflatable toroidal-shaped balloons |
| US20080097374A1 (en) * | 2006-08-07 | 2008-04-24 | Korleski Joseph E | Inflatable shaped balloons |
| KR101482671B1 (en) * | 2006-08-21 | 2015-01-14 | 가부시키가이샤 가네카 | Balloon catheter |
| US8609016B2 (en) * | 2006-08-28 | 2013-12-17 | Boston Scientific Scimed, Inc. | Refoldable balloon and method of making and using the same |
| US20080053454A1 (en) * | 2006-09-01 | 2008-03-06 | Nellcor Puritan Bennett Incorporated | Endotracheal tube including a partially inverted cuff collar |
| US8684175B2 (en) | 2006-09-22 | 2014-04-01 | Covidien Lp | Method for shipping and protecting an endotracheal tube with an inflated cuff |
| US8561614B2 (en) * | 2006-09-28 | 2013-10-22 | Covidien Lp | Multi-layer cuffs for medical devices |
| US20080078405A1 (en) * | 2006-09-29 | 2008-04-03 | Crumback Gary L | Self-sizing adjustable endotracheal tube |
| US20080078399A1 (en) | 2006-09-29 | 2008-04-03 | O'neil Michael P | Self-sizing adjustable endotracheal tube |
| US8307830B2 (en) | 2006-09-29 | 2012-11-13 | Nellcor Puritan Bennett Llc | Endotracheal cuff and technique for using the same |
| US7950393B2 (en) * | 2006-09-29 | 2011-05-31 | Nellcor Puritan Bennett Llc | Endotracheal cuff and technique for using the same |
| US20080078401A1 (en) * | 2006-09-29 | 2008-04-03 | Nellcor Puritan Bennett Incorporated | Self-sizing adjustable endotracheal tube |
| US8807136B2 (en) * | 2006-09-29 | 2014-08-19 | Covidien Lp | Self-sizing adjustable endotracheal tube |
| US8088100B2 (en) * | 2006-10-20 | 2012-01-03 | Boston Scientific Scimed, Inc. | Reinforced rewrappable balloon |
| CA2667228C (en) * | 2006-10-23 | 2015-07-14 | Micell Technologies, Inc. | Holder for electrically charging a substrate during coating |
| US8398695B2 (en) * | 2006-11-03 | 2013-03-19 | Boston Scientific Scimed, Inc. | Side branch stenting system using a main vessel constraining side branch access balloon and side branching stent |
| US8414611B2 (en) * | 2006-11-03 | 2013-04-09 | Boston Scientific Scimed, Inc. | Main vessel constraining side-branch access balloon |
| CN101711137B (en) * | 2007-01-08 | 2014-10-22 | 米歇尔技术公司 | Stents having biodegradable layers |
| US11426494B2 (en) | 2007-01-08 | 2022-08-30 | MT Acquisition Holdings LLC | Stents having biodegradable layers |
| US20080171980A1 (en) * | 2007-01-16 | 2008-07-17 | Medtronic Vascular, Inc. | Proximal Shaft for Rapid Exchange Catheter |
| EP2491962A1 (en) * | 2007-01-21 | 2012-08-29 | Hemoteq AG | Medical product for treating closures of bodily passages and preventing reclosures |
| US20080215034A1 (en) * | 2007-03-02 | 2008-09-04 | Jessica Clayton | Endotracheal cuff and technique for using the same |
| US20080210243A1 (en) * | 2007-03-02 | 2008-09-04 | Jessica Clayton | Endotracheal cuff and technique for using the same |
| BRPI0810370A2 (en) * | 2007-04-17 | 2014-10-29 | Micell Technologies Inc | STENT COATED, AND METHOD FOR PREPARING A STENT |
| JP2010527746A (en) | 2007-05-25 | 2010-08-19 | ミセル テクノロジーズ、インコーポレイテッド | Polymer film for medical device coating |
| US9192697B2 (en) | 2007-07-03 | 2015-11-24 | Hemoteq Ag | Balloon catheter for treating stenosis of body passages and for preventing threatening restenosis |
| US8333795B2 (en) * | 2007-08-27 | 2012-12-18 | Boston Scientific Scimed, Inc. | Bulging balloon for bifurcation catheter assembly and methods |
| US20090069878A1 (en) * | 2007-08-27 | 2009-03-12 | Boston Scientific Scimed, Inc. | Bifurcation post-dilatation balloon and methods |
| CN101883605A (en) * | 2007-09-06 | 2010-11-10 | 波士顿科学西美德公司 | Methods and devices for local therapeutic agent delivery to heart valves |
| US8926680B2 (en) | 2007-11-12 | 2015-01-06 | Covidien Lp | Aneurysm neck bridging processes with revascularization systems methods and products thereby |
| US8088140B2 (en) | 2008-05-19 | 2012-01-03 | Mindframe, Inc. | Blood flow restorative and embolus removal methods |
| US8585713B2 (en) | 2007-10-17 | 2013-11-19 | Covidien Lp | Expandable tip assembly for thrombus management |
| US20100022951A1 (en) * | 2008-05-19 | 2010-01-28 | Luce, Forward, Hamilton 7 Scripps, Llp | Detachable hub/luer device and processes |
| US11337714B2 (en) | 2007-10-17 | 2022-05-24 | Covidien Lp | Restoring blood flow and clot removal during acute ischemic stroke |
| US9220522B2 (en) | 2007-10-17 | 2015-12-29 | Covidien Lp | Embolus removal systems with baskets |
| US9198687B2 (en) | 2007-10-17 | 2015-12-01 | Covidien Lp | Acute stroke revascularization/recanalization systems processes and products thereby |
| US10123803B2 (en) | 2007-10-17 | 2018-11-13 | Covidien Lp | Methods of managing neurovascular obstructions |
| US8066757B2 (en) | 2007-10-17 | 2011-11-29 | Mindframe, Inc. | Blood flow restoration and thrombus management methods |
| US20100298928A1 (en) * | 2007-10-19 | 2010-11-25 | Micell Technologies, Inc. | Drug Coated Stents |
| US8750978B2 (en) * | 2007-12-31 | 2014-06-10 | Covidien Lp | System and sensor for early detection of shock or perfusion failure and technique for using the same |
| EP2254485B1 (en) | 2008-02-22 | 2017-08-30 | Covidien LP | Apparatus for flow restoration |
| JP5667559B2 (en) | 2008-03-28 | 2015-02-12 | サーモディクス,インコーポレイティド | Insertable medical device having an elastic matrix with microparticles disposed thereon, and drug delivery method |
| US20090254113A1 (en) * | 2008-04-04 | 2009-10-08 | Medtronic Vascular, Inc. | Dilatation balloon with ridges and methods |
| CN101977650A (en) | 2008-04-11 | 2011-02-16 | 曼德弗雷姆公司 | Monorail neuro-microcatheter for delivery of medical devices to treat stroke, processes and products thereby |
| CN102083397B (en) * | 2008-04-17 | 2013-12-25 | 米歇尔技术公司 | Stents having bioabsorbable layers |
| US20090264822A1 (en) * | 2008-04-21 | 2009-10-22 | Medtronic Vascular, Inc. | Method of Making a Zero-Fold Balloon With Variable Inflation Volume |
| US20090318863A1 (en) * | 2008-06-18 | 2009-12-24 | Boston Scientific Scimed, Inc. | Functional Balloon With Built in Lubricity or Drug Delivery System |
| WO2010009335A1 (en) | 2008-07-17 | 2010-01-21 | Micell Technologies, Inc. | Drug delivery medical device |
| US8834913B2 (en) * | 2008-12-26 | 2014-09-16 | Battelle Memorial Institute | Medical implants and methods of making medical implants |
| US20100241220A1 (en) * | 2009-03-23 | 2010-09-23 | Mcclain James B | Peripheral Stents Having Layers |
| WO2010120552A2 (en) * | 2009-04-01 | 2010-10-21 | Micell Technologies, Inc. | Coated stents |
| EP3366326A1 (en) | 2009-04-17 | 2018-08-29 | Micell Technologies, Inc. | Stents having controlled elution |
| EP2421571A2 (en) * | 2009-04-24 | 2012-02-29 | Boston Scientific Scimed, Inc. | Use of drug polymorphs to achieve controlled drug delivery from a coated medical device |
| CN105214143A (en) * | 2009-04-28 | 2016-01-06 | 苏尔莫迪克斯公司 | For sending the apparatus and method of bioactivator |
| US8590534B2 (en) * | 2009-06-22 | 2013-11-26 | Covidien Lp | Cuff for use with medical tubing and method and apparatus for making the same |
| ES2550634T3 (en) | 2009-07-10 | 2015-11-11 | Boston Scientific Scimed, Inc. | Use of nanocrystals for a drug delivery balloon |
| EP2453834A4 (en) | 2009-07-16 | 2014-04-16 | Micell Technologies Inc | Drug delivery medical device |
| US10080821B2 (en) * | 2009-07-17 | 2018-09-25 | Boston Scientific Scimed, Inc. | Nucleation of drug delivery balloons to provide improved crystal size and density |
| JP2011152181A (en) * | 2010-01-26 | 2011-08-11 | Toray Ind Inc | Balloon catheter |
| WO2011097103A1 (en) * | 2010-02-02 | 2011-08-11 | Micell Technologies, Inc. | Stent and stent delivery system with improved deliverability |
| US8795762B2 (en) | 2010-03-26 | 2014-08-05 | Battelle Memorial Institute | System and method for enhanced electrostatic deposition and surface coatings |
| CA2797110C (en) | 2010-04-22 | 2020-07-21 | Micell Technologies, Inc. | Stents and other devices having extracellular matrix coating |
| EP2569023B1 (en) | 2010-05-10 | 2015-10-21 | SurModics, Inc. | Glycerol ester active agent delivery systems and methods |
| US8927000B2 (en) | 2010-06-30 | 2015-01-06 | Surmodics, Inc. | Lipid coating for medical devices delivering bioactive agent |
| CA2805631C (en) | 2010-07-16 | 2018-07-31 | Micell Technologies, Inc. | Drug delivery medical device |
| US8889211B2 (en) | 2010-09-02 | 2014-11-18 | Boston Scientific Scimed, Inc. | Coating process for drug delivery balloons using heat-induced rewrap memory |
| US8703260B2 (en) | 2010-09-14 | 2014-04-22 | Abbott Cardiovascular Systems Inc. | Catheter balloon and method for forming same |
| WO2012054129A1 (en) | 2010-10-18 | 2012-04-26 | Boston Scientific Scimed, Inc. | Drug eluting medical device utilizing bioadhesives |
| US8177742B1 (en) | 2010-12-23 | 2012-05-15 | Kimberly-Clark Wordwide, Inc. | Inflatable retention system for an enteral feeding device |
| US8668667B2 (en) | 2010-12-30 | 2014-03-11 | Surmodics, Inc. | Double wall catheter for delivering therapeutic agent |
| WO2012092421A2 (en) | 2010-12-30 | 2012-07-05 | Surmodics, Inc. | Composition for intravascular delivery of therapeutic composition |
| US8873900B2 (en) | 2011-04-21 | 2014-10-28 | Medtronic Vascular, Inc. | Balloon catheter with integrated optical sensor for determining balloon diameter |
| US9757497B2 (en) | 2011-05-20 | 2017-09-12 | Surmodics, Inc. | Delivery of coated hydrophobic active agent particles |
| US9861727B2 (en) | 2011-05-20 | 2018-01-09 | Surmodics, Inc. | Delivery of hydrophobic active agent particles |
| US10213529B2 (en) | 2011-05-20 | 2019-02-26 | Surmodics, Inc. | Delivery of coated hydrophobic active agent particles |
| US10464100B2 (en) | 2011-05-31 | 2019-11-05 | Micell Technologies, Inc. | System and process for formation of a time-released, drug-eluting transferable coating |
| US10117972B2 (en) | 2011-07-15 | 2018-11-06 | Micell Technologies, Inc. | Drug delivery medical device |
| US20130030406A1 (en) | 2011-07-26 | 2013-01-31 | Medtronic Vascular, Inc. | Textured Dilatation Balloon and Methods |
| WO2013022458A1 (en) | 2011-08-05 | 2013-02-14 | Boston Scientific Scimed, Inc. | Methods of converting amorphous drug substance into crystalline form |
| WO2013028208A1 (en) | 2011-08-25 | 2013-02-28 | Boston Scientific Scimed, Inc. | Medical device with crystalline drug coating |
| US10188772B2 (en) | 2011-10-18 | 2019-01-29 | Micell Technologies, Inc. | Drug delivery medical device |
| US9555119B2 (en) | 2012-11-05 | 2017-01-31 | Surmodics, Inc. | Composition and method for delivery of hydrophobic active agents |
| US11246963B2 (en) | 2012-11-05 | 2022-02-15 | Surmodics, Inc. | Compositions and methods for delivery of hydrophobic active agents |
| US9132259B2 (en) | 2012-11-19 | 2015-09-15 | Abbott Cardiovascular Systems Inc. | Multilayer balloon for a catheter |
| KR20150143476A (en) | 2013-03-12 | 2015-12-23 | 미셀 테크놀로지즈, 인코포레이티드 | Bioabsorbable biomedical implants |
| WO2014186532A1 (en) | 2013-05-15 | 2014-11-20 | Micell Technologies, Inc. | Bioabsorbable biomedical implants |
| CA2912690C (en) | 2013-05-16 | 2022-05-03 | Surmodics, Inc. | Compositions and methods for delivery of hydrophobic active agents |
| EP3057643A1 (en) * | 2013-10-15 | 2016-08-24 | Boston Scientific Scimed, Inc. | High pressure tear resistant balloon |
| US9668898B2 (en) | 2014-07-24 | 2017-06-06 | Medtronic Vascular, Inc. | Stent delivery system having dynamic deployment and methods of manufacturing same |
| MX2017005408A (en) * | 2014-10-27 | 2017-11-20 | Interface Ass Inc | Methods of manufacturing nested balloons utilizing pressurized constrained annealing. |
| BR112017016342B1 (en) | 2015-01-29 | 2021-05-11 | Surmodics, Inc | drug delivery coating |
| JP6718979B2 (en) | 2016-04-12 | 2020-07-08 | ボストン サイエンティフィック サイムド,インコーポレイテッドBoston Scientific Scimed,Inc. | Medical balloon |
| US10849629B2 (en) | 2016-12-13 | 2020-12-01 | Boston Scientific Scimed, Inc. | Medical balloon |
| WO2018112196A1 (en) | 2016-12-16 | 2018-06-21 | Surmodics, Inc. | Hydrophobic active agent particle coatings and methods for treatment |
| US10898446B2 (en) | 2016-12-20 | 2021-01-26 | Surmodics, Inc. | Delivery of hydrophobic active agents from hydrophilic polyether block amide copolymer surfaces |
| WO2018200661A1 (en) | 2017-04-25 | 2018-11-01 | Boston Scientific Scimed, Inc. | Medical balloon |
| WO2019023185A1 (en) * | 2017-07-26 | 2019-01-31 | Cryterion Medical, Inc. | Method for manufacturing cryogenic balloon for intravascular catheter system |
| CN116407250A (en) | 2017-09-07 | 2023-07-11 | 波士顿科学医学有限公司 | Cryoballoon with greater dimensional adjustability at lower ablation pressures |
| US11653967B2 (en) * | 2018-05-03 | 2023-05-23 | Boston Scientific Scimed, Inc. | System and method for balloon diameter hysteresis compensation |
| EP4288135A1 (en) * | 2021-02-08 | 2023-12-13 | Intratech Medical Ltd. | Spiral-forming balloon for coronary sinus use |
Citations (93)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3625793A (en) * | 1969-09-23 | 1971-12-07 | David S Sheridan | Balloon-type catheters and method of manufacture |
| US3754851A (en) * | 1971-06-01 | 1973-08-28 | Monsanto Co | Apparatus for forming multiaxially oriented containers |
| US4003382A (en) * | 1975-07-25 | 1977-01-18 | Ethicon, Inc. | Retention catheter and method of manufacture |
| US4154244A (en) * | 1977-11-21 | 1979-05-15 | Baxter Travenol Laboratories, Inc. | Balloon-type catheter |
| US4331786A (en) * | 1979-10-02 | 1982-05-25 | Ato Chimie | Moldable and/or extrudable polyether-ester-amide block copolymers |
| US4376834A (en) * | 1981-10-14 | 1983-03-15 | The Upjohn Company | Polyurethane prepared by reaction of an organic polyisocyanate, a chain extender and an isocyanate-reactive material of m.w. 500-20,000 characterized by the use of only 2-25 percent by weight of the latter material |
| US4385089A (en) * | 1977-05-04 | 1983-05-24 | Rhone-Poulenc Industries | Process for preparing biaxially oriented hollow shaped articles from thermoplastic materials |
| US4385635A (en) * | 1980-04-25 | 1983-05-31 | Ruiz Oscar F | Angiographic catheter with soft tip end |
| US4410492A (en) * | 1981-02-13 | 1983-10-18 | Ben Venue Laboratories, Inc. | Sterilizing method incorporating recirculation of chamber atmosphere |
| US4413989A (en) * | 1980-09-08 | 1983-11-08 | Angiomedics Corporation | Expandable occlusion apparatus |
| US4448195A (en) * | 1981-05-08 | 1984-05-15 | Leveen Harry H | Reinforced balloon catheter |
| US4456011A (en) * | 1980-12-22 | 1984-06-26 | Irene Warnecke | Balloon-catheter |
| US4481323A (en) * | 1980-05-07 | 1984-11-06 | Medical Research Associates, Ltd. #2 | Hydrocarbon block copolymer with dispersed polysiloxane |
| US4482518A (en) * | 1981-10-02 | 1984-11-13 | Owens-Illinois, Inc. | Methods for reducing post-mold shrinkage of hollow oriented polyethylene terephthalate containers |
| US4490421A (en) * | 1983-07-05 | 1984-12-25 | E. I. Du Pont De Nemours And Company | Balloon and manufacture thereof |
| US4528343A (en) * | 1981-06-22 | 1985-07-09 | Kanegafuchi Kagaku Kogyo Kabushiki Kaisha | Antithrombogenic elastomer, molded products of the same, and a process for manufacturing the same |
| US4675361A (en) * | 1980-02-29 | 1987-06-23 | Thoratec Laboratories Corp. | Polymer systems suitable for blood-contacting surfaces of a biomedical device, and methods for forming |
| US4722344A (en) * | 1986-05-23 | 1988-02-02 | Critikon, Inc. | Radiopaque polyurethanes and catheters formed therefrom |
| US4737219A (en) * | 1985-02-12 | 1988-04-12 | Becton, Dickinson And Company | Method for bonding polyurethane balloons to multilumen catheters |
| US4786556A (en) * | 1986-03-24 | 1988-11-22 | Becton, Dickinson And Company | Polymeric articles having enhanced antithrombogenic activity |
| US4820349A (en) * | 1987-08-21 | 1989-04-11 | C. R. Bard, Inc. | Dilatation catheter with collapsible outer diameter |
| USRE32983E (en) * | 1983-07-05 | 1989-07-11 | E. I. Du Pont De Nemours And Company | Balloon and manufacture thereof |
| US4848344A (en) * | 1987-11-13 | 1989-07-18 | Cook, Inc. | Balloon guide |
| US4861830A (en) * | 1980-02-29 | 1989-08-29 | Th. Goldschmidt Ag | Polymer systems suitable for blood-contacting surfaces of a biomedical device, and methods for forming |
| US4886506A (en) * | 1986-12-23 | 1989-12-12 | Baxter Travenol Laboratories, Inc. | Soft tip catheter |
| US4898591A (en) * | 1988-08-09 | 1990-02-06 | Mallinckrodt, Inc. | Nylon-PEBA copolymer catheter |
| US4906244A (en) * | 1988-10-04 | 1990-03-06 | Cordis Corporation | Balloons for medical devices and fabrication thereof |
| US4917667A (en) * | 1988-02-11 | 1990-04-17 | Retroperfusion Systems, Inc. | Retroperfusion balloon catheter and method |
| US4937134A (en) * | 1989-04-17 | 1990-06-26 | The Dow Chemical Company | Elastomeric optical interference films |
| US4938676A (en) * | 1988-10-04 | 1990-07-03 | Cordis Corporation | Apparatus for manufacturing balloons for medical devices |
| US4943278A (en) * | 1988-02-29 | 1990-07-24 | Scimed Life Systems, Inc. | Dilatation balloon catheter |
| US4950257A (en) * | 1988-09-15 | 1990-08-21 | Mallinckrodt, Inc. | Catheter introducer with flexible tip |
| US4950239A (en) * | 1988-08-09 | 1990-08-21 | Worldwide Medical Plastics Inc. | Angioplasty balloons and balloon catheters |
| US4952357A (en) * | 1988-08-08 | 1990-08-28 | Scimed Life Systems, Inc. | Method of making a polyimide balloon catheter |
| US4963313A (en) * | 1987-11-30 | 1990-10-16 | Boston Scientific Corporation | Balloon catheter |
| US4994032A (en) * | 1987-12-01 | 1991-02-19 | Terumo Kabushiki Kaisha | Balloon catheter |
| US5017325A (en) * | 1988-10-04 | 1991-05-21 | Cordis Corporation | Stretch-blow molding method for manufacturing balloons for medical devices |
| US5055024A (en) * | 1988-10-04 | 1991-10-08 | Cordis Corporation | Apparatus for manufacturing balloons for medical devices |
| US5087394A (en) * | 1989-11-09 | 1992-02-11 | Scimed Life Systems, Inc. | Method for forming an inflatable balloon for use in a catheter |
| US5108416A (en) * | 1990-02-13 | 1992-04-28 | C. R. Bard, Inc. | Stent introducer system |
| US5108415A (en) * | 1988-10-04 | 1992-04-28 | Cordis Corporation | Balloons for medical devices and fabrication thereof |
| US5156594A (en) * | 1990-08-28 | 1992-10-20 | Scimed Life Systems, Inc. | Balloon catheter with distal guide wire lumen |
| US5156612A (en) * | 1988-10-04 | 1992-10-20 | Cordis Corporation | Balloons for medical devices and fabrication thereof |
| US5169464A (en) * | 1988-05-26 | 1992-12-08 | Family Health International | Method of making a condom by blow extrusion |
| US5192296A (en) * | 1988-08-31 | 1993-03-09 | Meadox Medicals, Inc. | Dilatation catheter |
| US5195969A (en) * | 1991-04-26 | 1993-03-23 | Boston Scientific Corporation | Co-extruded medical balloons and catheter using such balloons |
| US5195989A (en) * | 1990-09-17 | 1993-03-23 | Scimed Life Systems, Inc. | Low profile catheter for increasing lumen size of a blood vessel and guide wire therefor |
| US5195970A (en) * | 1991-04-26 | 1993-03-23 | Gahara William J | Collapsible balloon catheters |
| US5216122A (en) * | 1991-05-21 | 1993-06-01 | Union Carbide Chemicals & Plastics Technology Corporation | Removal of residual ethylene oxide from poly(ethylene oxide) |
| US5217434A (en) * | 1991-10-15 | 1993-06-08 | Scimed Life Systems, Inc. | Innerless dilatation catheter with balloon stretch valve |
| US5223205A (en) * | 1988-10-04 | 1993-06-29 | Cordis Corporation | Method for manufacturing balloons for medical devices |
| US5236659A (en) * | 1988-10-04 | 1993-08-17 | Cordis Corporation | Tailoring expansion properties of balloons for medical devices |
| US5250069A (en) * | 1987-02-27 | 1993-10-05 | Terumo Kabushiki Kaisha | Catheter equipped with expansible member and production method thereof |
| US5254089A (en) * | 1992-04-02 | 1993-10-19 | Boston Scientific Corp. | Medication dispensing balloon catheter |
| US5257974A (en) * | 1992-08-19 | 1993-11-02 | Scimed Life Systems, Inc. | Performance enhancement adaptor for intravascular balloon catheter |
| US5264260A (en) * | 1991-06-20 | 1993-11-23 | Saab Mark A | Dilatation balloon fabricated from low molecular weight polymers |
| US5265622A (en) * | 1990-10-25 | 1993-11-30 | C. R. Bard, Inc. | Guidewire having radially expandable member and method for guiding and advancing a catheter using the same |
| US5281677A (en) * | 1992-09-03 | 1994-01-25 | Becton, Dickinson And Company | Thermoplastic polyurethane blends |
| US5290306A (en) * | 1989-11-29 | 1994-03-01 | Cordis Corporation | Puncture resistant balloon catheter |
| US5295959A (en) * | 1992-03-13 | 1994-03-22 | Medtronic, Inc. | Autoperfusion dilatation catheter having a bonded channel |
| US5295978A (en) * | 1990-12-28 | 1994-03-22 | Union Carbide Chemicals & Plastics Technology Corporation | Biocompatible hydrophilic complexes and process for preparation and use |
| US5300048A (en) * | 1993-05-12 | 1994-04-05 | Sabin Corporation | Flexible, highly radiopaque plastic material catheter |
| US5304134A (en) * | 1992-01-17 | 1994-04-19 | Danforth Biomedical, Inc. | Lubricious yet bondable catheter channel sleeve for over-the-wire catheters |
| US5304340A (en) * | 1991-09-06 | 1994-04-19 | C. R. Bard, Inc. | Method of increasing the tensile strength of a dilatation balloon |
| US5304132A (en) * | 1987-05-06 | 1994-04-19 | Jang G David | Limacon geometry balloon angioplasty catheter systems and method of making same |
| US5304135A (en) * | 1992-08-13 | 1994-04-19 | Cordis Corporation | Axial multi-chamber angioplasty balloon assembly |
| US5306246A (en) * | 1990-11-09 | 1994-04-26 | Boston Scientific Corporation | Balloon for medical catheter |
| US5318532A (en) * | 1989-10-03 | 1994-06-07 | C. R. Bard, Inc. | Multilumen catheter with variable cross-section lumens |
| US5318587A (en) * | 1989-08-25 | 1994-06-07 | C. R. Bard, Inc. | Pleated balloon dilatation catheter and method of use |
| US5328468A (en) * | 1991-10-08 | 1994-07-12 | Terumo Kabushiki Kaisha | Balloon for blood vessel-dilating catheter |
| US5330428A (en) * | 1991-05-14 | 1994-07-19 | Scimed Life Systems, Inc. | Dilatation catheter having a random copolymer balloon |
| US5334160A (en) * | 1992-05-04 | 1994-08-02 | Scimed Life Systems, Inc. | Intravascular catheter with sleeve and method for use thereof |
| US5334146A (en) * | 1990-11-10 | 1994-08-02 | Terumo Kabushiki Kaisha | Catheter balloon having varying wall thickness |
| US5335675A (en) * | 1988-11-15 | 1994-08-09 | Family Health International | Stress-softened elastomeric films, articles, and method and apparatus for making such films and articles |
| US5338295A (en) * | 1991-10-15 | 1994-08-16 | Scimed Life Systems, Inc. | Dilatation catheter with polyimide-encased stainless steel braid proximal shaft |
| US5342305A (en) * | 1992-08-13 | 1994-08-30 | Cordis Corporation | Variable distention angioplasty balloon assembly |
| US5342301A (en) * | 1992-08-13 | 1994-08-30 | Advanced Polymers Incorporated | Multi-lumen balloons and catheters made therewith |
| US5344400A (en) * | 1992-04-06 | 1994-09-06 | Terumo Kabushiki Kaisha | Balloon catheters containing molded polyarylenesulfide material |
| US5344401A (en) * | 1991-12-20 | 1994-09-06 | Interventional Technologies Inc. | Catheter balloon formed from a polymeric composite |
| US5344413A (en) * | 1991-06-28 | 1994-09-06 | C. R. Bard, Inc. | Catheter having a tip connector for rapid catheter exchanges |
| US5348538A (en) * | 1992-09-29 | 1994-09-20 | Scimed Life Systems, Inc. | Shrinking balloon catheter having nonlinear or hybrid compliance curve |
| US5358486A (en) * | 1987-01-09 | 1994-10-25 | C. R. Bard, Inc. | Multiple layer high strength balloon for dilatation catheter |
| US5364357A (en) * | 1989-09-25 | 1994-11-15 | Schneider (Usa) Inc. | Small diameter dilatation catheter having wire reinforced coaxial tubular body |
| US5368566A (en) * | 1992-04-29 | 1994-11-29 | Cardiovascular Dynamics, Inc. | Delivery and temporary stent catheter having a reinforced perfusion lumen |
| US5387199A (en) * | 1992-02-24 | 1995-02-07 | Baxter International Inc. | Polymer blends for torque transmitting catheters |
| US5387225A (en) * | 1988-02-29 | 1995-02-07 | Scimed Life Systems, Inc. | Dilatation catheter with transition member |
| US5395332A (en) * | 1990-08-28 | 1995-03-07 | Scimed Life Systems, Inc. | Intravascualr catheter with distal tip guide wire lumen |
| US5397306A (en) * | 1989-12-20 | 1995-03-14 | Terumo Kabushiki Kaisha | Catheter |
| US5411477A (en) * | 1990-05-11 | 1995-05-02 | Saab; Mark A. | High-strength, thin-walled single piece catheters |
| US5447497A (en) * | 1992-08-06 | 1995-09-05 | Scimed Life Systems, Inc | Balloon catheter having nonlinear compliance curve and method of using |
| US5525388A (en) * | 1992-08-07 | 1996-06-11 | Advanced Cardiovascular Systems, Inc. | Dilatation balloon with constant wall thickness |
| US5556383A (en) * | 1994-03-02 | 1996-09-17 | Scimed Lifesystems, Inc. | Block copolymer elastomer catheter balloons |
| US6210364B1 (en) * | 1992-09-30 | 2001-04-03 | C. R. Bard, Inc. | Distensible dilatation balloon with elastic stress response |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2452227A1 (en) * | 1974-11-04 | 1976-05-06 | Gernot Klaus Brueck | DEVICE FOR RADIATION TREATMENT ON LIVING BODIES |
| US4376334A (en) | 1981-04-27 | 1983-03-15 | Illinois Tool Works Inc. | Method of making terminal clamp assembly |
| US4690844A (en) * | 1984-10-26 | 1987-09-01 | Saudagar Abdul S | Method for parting rubber and products formed thereby, and a method of making a blood vessel |
| EP0439202B1 (en) * | 1989-07-24 | 1993-09-29 | Cordis Corporation | Apparatus and method for manufacturing balloons for medical devices |
| ES2043289T3 (en) * | 1989-09-25 | 1993-12-16 | Schneider Usa Inc | THE EXTRUSION OF MULTIPLE LAYERS AS A PROCEDURE FOR MAKING ANGIOPLASTY BALLS. |
| US5348486A (en) | 1993-08-11 | 1994-09-20 | General Motors Corporation | Heat shielded spark plug boot assembly |
| EP0738168B1 (en) | 1993-10-01 | 2004-01-21 | Boston Scientific Corporation | Medical device balloons containing thermoplastic elastomers |
-
1992
- 1992-09-30 US US07/954,750 patent/US5500180A/en not_active Expired - Lifetime
-
1993
- 1993-09-30 EP EP93115840A patent/EP0592885B1/en not_active Expired - Lifetime
- 1993-09-30 DE DE69334318T patent/DE69334318D1/en not_active Expired - Lifetime
- 1993-09-30 CA CA002107378A patent/CA2107378A1/en not_active Abandoned
- 1993-09-30 EP EP05026246A patent/EP1683539B1/en not_active Expired - Lifetime
- 1993-09-30 DE DE69334289T patent/DE69334289D1/en not_active Expired - Lifetime
- 1993-09-30 JP JP5245007A patent/JPH06304920A/en active Pending
-
1997
- 1997-06-26 US US08/883,261 patent/US6210364B1/en not_active Expired - Fee Related
-
1998
- 1998-11-16 US US09/192,893 patent/US6283939B1/en not_active Expired - Lifetime
-
2001
- 2001-08-31 US US09/942,920 patent/US6620381B2/en not_active Expired - Fee Related
-
2003
- 2003-08-11 US US10/637,575 patent/US20040097878A1/en not_active Abandoned
Patent Citations (99)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3625793A (en) * | 1969-09-23 | 1971-12-07 | David S Sheridan | Balloon-type catheters and method of manufacture |
| US3754851A (en) * | 1971-06-01 | 1973-08-28 | Monsanto Co | Apparatus for forming multiaxially oriented containers |
| US4003382A (en) * | 1975-07-25 | 1977-01-18 | Ethicon, Inc. | Retention catheter and method of manufacture |
| US4385089A (en) * | 1977-05-04 | 1983-05-24 | Rhone-Poulenc Industries | Process for preparing biaxially oriented hollow shaped articles from thermoplastic materials |
| US4154244A (en) * | 1977-11-21 | 1979-05-15 | Baxter Travenol Laboratories, Inc. | Balloon-type catheter |
| US4331786A (en) * | 1979-10-02 | 1982-05-25 | Ato Chimie | Moldable and/or extrudable polyether-ester-amide block copolymers |
| US4861830A (en) * | 1980-02-29 | 1989-08-29 | Th. Goldschmidt Ag | Polymer systems suitable for blood-contacting surfaces of a biomedical device, and methods for forming |
| US4675361A (en) * | 1980-02-29 | 1987-06-23 | Thoratec Laboratories Corp. | Polymer systems suitable for blood-contacting surfaces of a biomedical device, and methods for forming |
| US4385635A (en) * | 1980-04-25 | 1983-05-31 | Ruiz Oscar F | Angiographic catheter with soft tip end |
| US4481323A (en) * | 1980-05-07 | 1984-11-06 | Medical Research Associates, Ltd. #2 | Hydrocarbon block copolymer with dispersed polysiloxane |
| US4413989A (en) * | 1980-09-08 | 1983-11-08 | Angiomedics Corporation | Expandable occlusion apparatus |
| US4456011A (en) * | 1980-12-22 | 1984-06-26 | Irene Warnecke | Balloon-catheter |
| US4410492A (en) * | 1981-02-13 | 1983-10-18 | Ben Venue Laboratories, Inc. | Sterilizing method incorporating recirculation of chamber atmosphere |
| US4448195A (en) * | 1981-05-08 | 1984-05-15 | Leveen Harry H | Reinforced balloon catheter |
| US4528343A (en) * | 1981-06-22 | 1985-07-09 | Kanegafuchi Kagaku Kogyo Kabushiki Kaisha | Antithrombogenic elastomer, molded products of the same, and a process for manufacturing the same |
| US4623347A (en) * | 1981-06-22 | 1986-11-18 | Kanegafuchi Kagaku Kogyo Kabushiki Kaisha | Antithrombogenic elastomer products |
| US4482518A (en) * | 1981-10-02 | 1984-11-13 | Owens-Illinois, Inc. | Methods for reducing post-mold shrinkage of hollow oriented polyethylene terephthalate containers |
| US4376834A (en) * | 1981-10-14 | 1983-03-15 | The Upjohn Company | Polyurethane prepared by reaction of an organic polyisocyanate, a chain extender and an isocyanate-reactive material of m.w. 500-20,000 characterized by the use of only 2-25 percent by weight of the latter material |
| USRE32983E (en) * | 1983-07-05 | 1989-07-11 | E. I. Du Pont De Nemours And Company | Balloon and manufacture thereof |
| US4490421A (en) * | 1983-07-05 | 1984-12-25 | E. I. Du Pont De Nemours And Company | Balloon and manufacture thereof |
| US4737219A (en) * | 1985-02-12 | 1988-04-12 | Becton, Dickinson And Company | Method for bonding polyurethane balloons to multilumen catheters |
| US4786556A (en) * | 1986-03-24 | 1988-11-22 | Becton, Dickinson And Company | Polymeric articles having enhanced antithrombogenic activity |
| US4722344A (en) * | 1986-05-23 | 1988-02-02 | Critikon, Inc. | Radiopaque polyurethanes and catheters formed therefrom |
| US4886506A (en) * | 1986-12-23 | 1989-12-12 | Baxter Travenol Laboratories, Inc. | Soft tip catheter |
| US5358486A (en) * | 1987-01-09 | 1994-10-25 | C. R. Bard, Inc. | Multiple layer high strength balloon for dilatation catheter |
| US5250069A (en) * | 1987-02-27 | 1993-10-05 | Terumo Kabushiki Kaisha | Catheter equipped with expansible member and production method thereof |
| US5304132A (en) * | 1987-05-06 | 1994-04-19 | Jang G David | Limacon geometry balloon angioplasty catheter systems and method of making same |
| US4820349A (en) * | 1987-08-21 | 1989-04-11 | C. R. Bard, Inc. | Dilatation catheter with collapsible outer diameter |
| US4848344A (en) * | 1987-11-13 | 1989-07-18 | Cook, Inc. | Balloon guide |
| US4963313A (en) * | 1987-11-30 | 1990-10-16 | Boston Scientific Corporation | Balloon catheter |
| US4994032A (en) * | 1987-12-01 | 1991-02-19 | Terumo Kabushiki Kaisha | Balloon catheter |
| US4917667A (en) * | 1988-02-11 | 1990-04-17 | Retroperfusion Systems, Inc. | Retroperfusion balloon catheter and method |
| US5387225A (en) * | 1988-02-29 | 1995-02-07 | Scimed Life Systems, Inc. | Dilatation catheter with transition member |
| US4943278A (en) * | 1988-02-29 | 1990-07-24 | Scimed Life Systems, Inc. | Dilatation balloon catheter |
| US5169464A (en) * | 1988-05-26 | 1992-12-08 | Family Health International | Method of making a condom by blow extrusion |
| US4952357A (en) * | 1988-08-08 | 1990-08-28 | Scimed Life Systems, Inc. | Method of making a polyimide balloon catheter |
| US4950239A (en) * | 1988-08-09 | 1990-08-21 | Worldwide Medical Plastics Inc. | Angioplasty balloons and balloon catheters |
| US4898591A (en) * | 1988-08-09 | 1990-02-06 | Mallinckrodt, Inc. | Nylon-PEBA copolymer catheter |
| US5192296A (en) * | 1988-08-31 | 1993-03-09 | Meadox Medicals, Inc. | Dilatation catheter |
| US4950257A (en) * | 1988-09-15 | 1990-08-21 | Mallinckrodt, Inc. | Catheter introducer with flexible tip |
| US5223205A (en) * | 1988-10-04 | 1993-06-29 | Cordis Corporation | Method for manufacturing balloons for medical devices |
| US5017325A (en) * | 1988-10-04 | 1991-05-21 | Cordis Corporation | Stretch-blow molding method for manufacturing balloons for medical devices |
| US5156612A (en) * | 1988-10-04 | 1992-10-20 | Cordis Corporation | Balloons for medical devices and fabrication thereof |
| US4906244A (en) * | 1988-10-04 | 1990-03-06 | Cordis Corporation | Balloons for medical devices and fabrication thereof |
| US5236659A (en) * | 1988-10-04 | 1993-08-17 | Cordis Corporation | Tailoring expansion properties of balloons for medical devices |
| US5108415A (en) * | 1988-10-04 | 1992-04-28 | Cordis Corporation | Balloons for medical devices and fabrication thereof |
| US4938676A (en) * | 1988-10-04 | 1990-07-03 | Cordis Corporation | Apparatus for manufacturing balloons for medical devices |
| US5055024A (en) * | 1988-10-04 | 1991-10-08 | Cordis Corporation | Apparatus for manufacturing balloons for medical devices |
| US5335675A (en) * | 1988-11-15 | 1994-08-09 | Family Health International | Stress-softened elastomeric films, articles, and method and apparatus for making such films and articles |
| US4937134A (en) * | 1989-04-17 | 1990-06-26 | The Dow Chemical Company | Elastomeric optical interference films |
| US5318587A (en) * | 1989-08-25 | 1994-06-07 | C. R. Bard, Inc. | Pleated balloon dilatation catheter and method of use |
| US5364357A (en) * | 1989-09-25 | 1994-11-15 | Schneider (Usa) Inc. | Small diameter dilatation catheter having wire reinforced coaxial tubular body |
| US5318532A (en) * | 1989-10-03 | 1994-06-07 | C. R. Bard, Inc. | Multilumen catheter with variable cross-section lumens |
| US5087394A (en) * | 1989-11-09 | 1992-02-11 | Scimed Life Systems, Inc. | Method for forming an inflatable balloon for use in a catheter |
| US5290306A (en) * | 1989-11-29 | 1994-03-01 | Cordis Corporation | Puncture resistant balloon catheter |
| US5397306A (en) * | 1989-12-20 | 1995-03-14 | Terumo Kabushiki Kaisha | Catheter |
| US5108416A (en) * | 1990-02-13 | 1992-04-28 | C. R. Bard, Inc. | Stent introducer system |
| US5411477A (en) * | 1990-05-11 | 1995-05-02 | Saab; Mark A. | High-strength, thin-walled single piece catheters |
| US5395332A (en) * | 1990-08-28 | 1995-03-07 | Scimed Life Systems, Inc. | Intravascualr catheter with distal tip guide wire lumen |
| US5156594A (en) * | 1990-08-28 | 1992-10-20 | Scimed Life Systems, Inc. | Balloon catheter with distal guide wire lumen |
| US5195989A (en) * | 1990-09-17 | 1993-03-23 | Scimed Life Systems, Inc. | Low profile catheter for increasing lumen size of a blood vessel and guide wire therefor |
| US5265622A (en) * | 1990-10-25 | 1993-11-30 | C. R. Bard, Inc. | Guidewire having radially expandable member and method for guiding and advancing a catheter using the same |
| US5306246A (en) * | 1990-11-09 | 1994-04-26 | Boston Scientific Corporation | Balloon for medical catheter |
| US5334146A (en) * | 1990-11-10 | 1994-08-02 | Terumo Kabushiki Kaisha | Catheter balloon having varying wall thickness |
| US5295978A (en) * | 1990-12-28 | 1994-03-22 | Union Carbide Chemicals & Plastics Technology Corporation | Biocompatible hydrophilic complexes and process for preparation and use |
| US5195970A (en) * | 1991-04-26 | 1993-03-23 | Gahara William J | Collapsible balloon catheters |
| US5195969A (en) * | 1991-04-26 | 1993-03-23 | Boston Scientific Corporation | Co-extruded medical balloons and catheter using such balloons |
| US5330428A (en) * | 1991-05-14 | 1994-07-19 | Scimed Life Systems, Inc. | Dilatation catheter having a random copolymer balloon |
| US5216122A (en) * | 1991-05-21 | 1993-06-01 | Union Carbide Chemicals & Plastics Technology Corporation | Removal of residual ethylene oxide from poly(ethylene oxide) |
| US5264260A (en) * | 1991-06-20 | 1993-11-23 | Saab Mark A | Dilatation balloon fabricated from low molecular weight polymers |
| US5344413A (en) * | 1991-06-28 | 1994-09-06 | C. R. Bard, Inc. | Catheter having a tip connector for rapid catheter exchanges |
| US5304340A (en) * | 1991-09-06 | 1994-04-19 | C. R. Bard, Inc. | Method of increasing the tensile strength of a dilatation balloon |
| US5328468A (en) * | 1991-10-08 | 1994-07-12 | Terumo Kabushiki Kaisha | Balloon for blood vessel-dilating catheter |
| US5338295A (en) * | 1991-10-15 | 1994-08-16 | Scimed Life Systems, Inc. | Dilatation catheter with polyimide-encased stainless steel braid proximal shaft |
| US5217434A (en) * | 1991-10-15 | 1993-06-08 | Scimed Life Systems, Inc. | Innerless dilatation catheter with balloon stretch valve |
| US5344401A (en) * | 1991-12-20 | 1994-09-06 | Interventional Technologies Inc. | Catheter balloon formed from a polymeric composite |
| US5304134A (en) * | 1992-01-17 | 1994-04-19 | Danforth Biomedical, Inc. | Lubricious yet bondable catheter channel sleeve for over-the-wire catheters |
| US5387199A (en) * | 1992-02-24 | 1995-02-07 | Baxter International Inc. | Polymer blends for torque transmitting catheters |
| US5295959A (en) * | 1992-03-13 | 1994-03-22 | Medtronic, Inc. | Autoperfusion dilatation catheter having a bonded channel |
| US5254089A (en) * | 1992-04-02 | 1993-10-19 | Boston Scientific Corp. | Medication dispensing balloon catheter |
| US5344400A (en) * | 1992-04-06 | 1994-09-06 | Terumo Kabushiki Kaisha | Balloon catheters containing molded polyarylenesulfide material |
| US5368566A (en) * | 1992-04-29 | 1994-11-29 | Cardiovascular Dynamics, Inc. | Delivery and temporary stent catheter having a reinforced perfusion lumen |
| US5334160A (en) * | 1992-05-04 | 1994-08-02 | Scimed Life Systems, Inc. | Intravascular catheter with sleeve and method for use thereof |
| US5447497A (en) * | 1992-08-06 | 1995-09-05 | Scimed Life Systems, Inc | Balloon catheter having nonlinear compliance curve and method of using |
| US5525388A (en) * | 1992-08-07 | 1996-06-11 | Advanced Cardiovascular Systems, Inc. | Dilatation balloon with constant wall thickness |
| US5342301A (en) * | 1992-08-13 | 1994-08-30 | Advanced Polymers Incorporated | Multi-lumen balloons and catheters made therewith |
| US5304135A (en) * | 1992-08-13 | 1994-04-19 | Cordis Corporation | Axial multi-chamber angioplasty balloon assembly |
| US5342305A (en) * | 1992-08-13 | 1994-08-30 | Cordis Corporation | Variable distention angioplasty balloon assembly |
| US5257974A (en) * | 1992-08-19 | 1993-11-02 | Scimed Life Systems, Inc. | Performance enhancement adaptor for intravascular balloon catheter |
| US5338300A (en) * | 1992-08-19 | 1994-08-16 | Scimed Life Systems, Inc. | Performance enhancement adaptor for intravascular balloon catheter |
| US5281677A (en) * | 1992-09-03 | 1994-01-25 | Becton, Dickinson And Company | Thermoplastic polyurethane blends |
| US5348538A (en) * | 1992-09-29 | 1994-09-20 | Scimed Life Systems, Inc. | Shrinking balloon catheter having nonlinear or hybrid compliance curve |
| US5403340A (en) * | 1992-09-29 | 1995-04-04 | Scimed Lifesystems Inc. | Shrinking balloon catheter having nonlinear compliance curve |
| US5500181A (en) * | 1992-09-29 | 1996-03-19 | Scimed Life Systems, Inc. | Shrinking balloon catheter having nonlinear compliance curve |
| US6210364B1 (en) * | 1992-09-30 | 2001-04-03 | C. R. Bard, Inc. | Distensible dilatation balloon with elastic stress response |
| US6283939B1 (en) * | 1992-09-30 | 2001-09-04 | Medtronic Ave, Inc. | Distensible dilatation balloon with elastic stress |
| US6620381B2 (en) * | 1992-09-30 | 2003-09-16 | Medtronic Ave, Inc. | Sterilization process for a distensible dilatation balloon with elastic stress response |
| US5300048A (en) * | 1993-05-12 | 1994-04-05 | Sabin Corporation | Flexible, highly radiopaque plastic material catheter |
| US5556383A (en) * | 1994-03-02 | 1996-09-17 | Scimed Lifesystems, Inc. | Block copolymer elastomer catheter balloons |
Cited By (67)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7981123B2 (en) | 1997-09-12 | 2011-07-19 | Evalve, Inc. | Surgical device for connecting soft tissue |
| US9510837B2 (en) | 1997-09-12 | 2016-12-06 | Evalve, Inc. | Surgical device for connecting soft tissue |
| US8740918B2 (en) | 1997-09-12 | 2014-06-03 | Evalve, Inc. | Surgical device for connecting soft tissue |
| US8343174B2 (en) | 1999-04-09 | 2013-01-01 | Evalve, Inc. | Locking mechanisms for fixation devices and methods of engaging tissue |
| US8500761B2 (en) | 1999-04-09 | 2013-08-06 | Abbott Vascular | Fixation devices, systems and methods for engaging tissue |
| US7811296B2 (en) | 1999-04-09 | 2010-10-12 | Evalve, Inc. | Fixation devices for variation in engagement of tissue |
| US7998151B2 (en) | 1999-04-09 | 2011-08-16 | Evalve, Inc. | Leaflet suturing |
| US8029518B2 (en) | 1999-04-09 | 2011-10-04 | Evalve, Inc. | Methods and devices for capturing and fixing leaflets in valve repair |
| US7736388B2 (en) | 1999-04-09 | 2010-06-15 | Evalve, Inc. | Fixation devices, systems and methods for engaging tissue |
| US9510829B2 (en) | 1999-04-09 | 2016-12-06 | Evalve, Inc. | Fixation devices, systems and methods for engaging tissue |
| US8057493B2 (en) | 1999-04-09 | 2011-11-15 | Evalve, Inc. | Fixation devices, systems and methods for engaging tissue |
| US8123703B2 (en) | 1999-04-09 | 2012-02-28 | Evalve, Inc. | Steerable access sheath and methods of use |
| US8187299B2 (en) | 1999-04-09 | 2012-05-29 | Evalve, Inc. | Methods and apparatus for cardiac valve repair |
| US8216256B2 (en) | 1999-04-09 | 2012-07-10 | Evalve, Inc. | Detachment mechanism for implantable fixation devices |
| US9044246B2 (en) | 1999-04-09 | 2015-06-02 | Abbott Vascular Inc. | Methods and devices for capturing and fixing leaflets in valve repair |
| US8409273B2 (en) | 1999-04-09 | 2013-04-02 | Abbott Vascular Inc | Multi-catheter steerable guiding system and methods of use |
| US7753923B2 (en) | 1999-04-09 | 2010-07-13 | Evalve, Inc. | Leaflet suturing |
| US8734505B2 (en) | 1999-04-09 | 2014-05-27 | Evalve, Inc. | Methods and apparatus for cardiac valve repair |
| US8740920B2 (en) | 1999-04-09 | 2014-06-03 | Evalve, Inc. | Fixation devices, systems and methods for engaging tissue |
| US10653427B2 (en) | 2001-06-27 | 2020-05-19 | Evalve, Inc. | Fixation devices, systems and methods for engaging tissue |
| US10624618B2 (en) | 2001-06-27 | 2020-04-21 | Evalve, Inc. | Methods and devices for capturing and fixing leaflets in valve repair |
| US7981139B2 (en) | 2002-03-01 | 2011-07-19 | Evalve, Inc | Suture anchors and methods of use |
| US10828042B2 (en) | 2003-05-19 | 2020-11-10 | Evalve, Inc. | Fixation devices, systems and methods for engaging tissue |
| US10667823B2 (en) | 2003-05-19 | 2020-06-02 | Evalve, Inc. | Fixation devices, systems and methods for engaging tissue |
| US10646229B2 (en) | 2003-05-19 | 2020-05-12 | Evalve, Inc. | Fixation devices, systems and methods for engaging tissue |
| US10631871B2 (en) | 2003-05-19 | 2020-04-28 | Evalve, Inc. | Fixation devices, systems and methods for engaging tissue |
| US11484331B2 (en) | 2004-09-27 | 2022-11-01 | Evalve, Inc. | Methods and devices for tissue grasping and assessment |
| US11304715B2 (en) | 2004-09-27 | 2022-04-19 | Evalve, Inc. | Methods and devices for tissue grasping and assessment |
| US8052592B2 (en) | 2005-09-27 | 2011-11-08 | Evalve, Inc. | Methods and devices for tissue grasping and assessment |
| US8926620B2 (en) | 2006-08-25 | 2015-01-06 | Kyphon Sarl | Apparatus and methods for use of expandable members in surgical applications |
| US20080051819A1 (en) * | 2006-08-25 | 2008-02-28 | Nishith Chasmawala | Apparatus and methods for use of expandable members in surgical applications |
| US8043296B2 (en) | 2006-08-25 | 2011-10-25 | Kyphon Sarl | Apparatus and methods for use of expandable members in surgical applications |
| US12016561B2 (en) | 2011-09-13 | 2024-06-25 | Abbott Cardiovascular Systems Inc. | System for fixation of leaflets of a heart valve |
| US10743876B2 (en) | 2011-09-13 | 2020-08-18 | Abbott Cardiovascular Systems Inc. | System for fixation of leaflets of a heart valve |
| US10792039B2 (en) | 2011-09-13 | 2020-10-06 | Abbott Cardiovascular Systems Inc. | Gripper pusher mechanism for tissue apposition systems |
| US10390943B2 (en) | 2014-03-17 | 2019-08-27 | Evalve, Inc. | Double orifice device for transcatheter mitral valve replacement |
| US11666433B2 (en) | 2014-03-17 | 2023-06-06 | Evalve, Inc. | Double orifice device for transcatheter mitral valve replacement |
| US11109863B2 (en) | 2014-12-19 | 2021-09-07 | Abbott Cardiovascular Systems, Inc. | Grasping for tissue repair |
| US11006956B2 (en) | 2014-12-19 | 2021-05-18 | Abbott Cardiovascular Systems Inc. | Grasping for tissue repair |
| US11229435B2 (en) | 2014-12-19 | 2022-01-25 | Abbott Cardiovascular Systems Inc. | Grasping for tissue repair |
| US10188392B2 (en) | 2014-12-19 | 2019-01-29 | Abbott Cardiovascular Systems, Inc. | Grasping for tissue repair |
| US10893941B2 (en) | 2015-04-02 | 2021-01-19 | Abbott Cardiovascular Systems, Inc. | Tissue fixation devices and methods |
| US10524912B2 (en) | 2015-04-02 | 2020-01-07 | Abbott Cardiovascular Systems, Inc. | Tissue fixation devices and methods |
| US10376673B2 (en) | 2015-06-19 | 2019-08-13 | Evalve, Inc. | Catheter guiding system and methods |
| US10856988B2 (en) | 2015-06-29 | 2020-12-08 | Evalve, Inc. | Self-aligning radiopaque ring |
| US10238494B2 (en) | 2015-06-29 | 2019-03-26 | Evalve, Inc. | Self-aligning radiopaque ring |
| US11759209B2 (en) | 2015-07-21 | 2023-09-19 | Evalve, Inc. | Tissue grasping devices and related methods |
| US11096691B2 (en) | 2015-07-21 | 2021-08-24 | Evalve, Inc. | Tissue grasping devices and related methods |
| US10667815B2 (en) | 2015-07-21 | 2020-06-02 | Evalve, Inc. | Tissue grasping devices and related methods |
| US10413408B2 (en) | 2015-08-06 | 2019-09-17 | Evalve, Inc. | Delivery catheter systems, methods, and devices |
| US10238495B2 (en) | 2015-10-09 | 2019-03-26 | Evalve, Inc. | Delivery catheter handle and methods of use |
| US11109972B2 (en) | 2015-10-09 | 2021-09-07 | Evalve, Inc. | Delivery catheter handle and methods of use |
| US11931263B2 (en) | 2015-10-09 | 2024-03-19 | Evalve, Inc. | Delivery catheter handle and methods of use |
| US10736632B2 (en) | 2016-07-06 | 2020-08-11 | Evalve, Inc. | Methods and devices for valve clip excision |
| US11653947B2 (en) | 2016-10-05 | 2023-05-23 | Evalve, Inc. | Cardiac valve cutting device |
| US11166818B2 (en) | 2016-11-09 | 2021-11-09 | Evalve, Inc. | Devices for adjusting the curvature of cardiac valve structures |
| US10363138B2 (en) | 2016-11-09 | 2019-07-30 | Evalve, Inc. | Devices for adjusting the curvature of cardiac valve structures |
| US10398553B2 (en) | 2016-11-11 | 2019-09-03 | Evalve, Inc. | Opposing disk device for grasping cardiac valve tissue |
| US10426616B2 (en) | 2016-11-17 | 2019-10-01 | Evalve, Inc. | Cardiac implant delivery system |
| US11957358B2 (en) | 2016-12-08 | 2024-04-16 | Evalve, Inc. | Adjustable arm device for grasping tissues |
| US10779837B2 (en) | 2016-12-08 | 2020-09-22 | Evalve, Inc. | Adjustable arm device for grasping tissues |
| US10314586B2 (en) | 2016-12-13 | 2019-06-11 | Evalve, Inc. | Rotatable device and method for fixing tricuspid valve tissue |
| US11406388B2 (en) | 2016-12-13 | 2022-08-09 | Evalve, Inc. | Rotatable device and method for fixing tricuspid valve tissue |
| US11065119B2 (en) | 2017-05-12 | 2021-07-20 | Evalve, Inc. | Long arm valve repair clip |
| US12048624B2 (en) | 2019-07-15 | 2024-07-30 | Evalve, Inc. | Independent proximal element actuation methods |
| US12048448B2 (en) | 2020-05-06 | 2024-07-30 | Evalve, Inc. | Leaflet grasping and cutting device |
| US12121231B2 (en) | 2022-04-18 | 2024-10-22 | Evalve, Inc. | Methods and devices for tissue grasping and assessment |
Also Published As
| Publication number | Publication date |
|---|---|
| US6283939B1 (en) | 2001-09-04 |
| JPH06304920A (en) | 1994-11-01 |
| EP0592885A2 (en) | 1994-04-20 |
| EP1683539B1 (en) | 2010-01-27 |
| DE69334318D1 (en) | 2010-03-18 |
| EP1683539A2 (en) | 2006-07-26 |
| EP0592885A3 (en) | 1995-09-06 |
| US20020053349A1 (en) | 2002-05-09 |
| US5500180A (en) | 1996-03-19 |
| EP1683539A3 (en) | 2008-11-05 |
| DE69334289D1 (en) | 2009-08-13 |
| US6210364B1 (en) | 2001-04-03 |
| CA2107378A1 (en) | 1994-03-31 |
| US6620381B2 (en) | 2003-09-16 |
| EP0592885B1 (en) | 2009-07-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6620381B2 (en) | Sterilization process for a distensible dilatation balloon with elastic stress response | |
| US6406457B1 (en) | Block copolymer elastomer catheter balloons | |
| EP0748232B1 (en) | Block copolymer elastomer catheter balloons | |
| JP3594971B2 (en) | Inflatable balloon containing polyester ether amide copolymer | |
| EP0531117B1 (en) | Method of increasing the tensile strength of a dilatation balloon | |
| EP0355937B1 (en) | Balloon and manufacture thereof | |
| US5830182A (en) | Block copolymer elastomer catheter balloons | |
| US5951941A (en) | Block copolymer elastomer catheter balloons | |
| US9956321B2 (en) | Medical device balloons with improved strength properties and processes for producing same | |
| EP0274411A2 (en) | Thin wall high strength balloon and method of manufacture | |
| USRE32983E (en) | Balloon and manufacture thereof | |
| US20090264822A1 (en) | Method of Making a Zero-Fold Balloon With Variable Inflation Volume | |
| WO1992019440A1 (en) | Improved balloon catheter of low molecular weight pet | |
| US20090254113A1 (en) | Dilatation balloon with ridges and methods | |
| JP2004509712A (en) | Method for manufacturing balloon for medical device | |
| JP3503417B2 (en) | Balloon catheter and method of manufacturing balloon used therein | |
| US7645498B2 (en) | Balloon catheter formed of random copolymerized nylons | |
| CA2184383C (en) | Block copolymer elastomer catheter balloons |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |