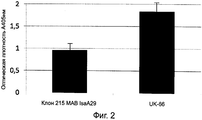
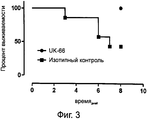
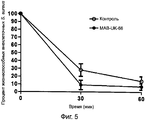
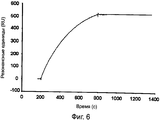
RU2560415C2 - Антитела или их фрагменты, направленные против эпитопа стафилококка золотистого (staphylococcus aureus) - Google Patents
Антитела или их фрагменты, направленные против эпитопа стафилококка золотистого (staphylococcus aureus) Download PDFInfo
- Publication number
- RU2560415C2 RU2560415C2 RU2011146424/10A RU2011146424A RU2560415C2 RU 2560415 C2 RU2560415 C2 RU 2560415C2 RU 2011146424/10 A RU2011146424/10 A RU 2011146424/10A RU 2011146424 A RU2011146424 A RU 2011146424A RU 2560415 C2 RU2560415 C2 RU 2560415C2
- Authority
- RU
- Russia
- Prior art keywords
- antibody
- staphylococcus aureus
- functional fragment
- antibodies
- aureus
- Prior art date
Links
- 239000012634 fragment Substances 0.000 title claims abstract description 71
- 241000191967 Staphylococcus aureus Species 0.000 title claims abstract description 45
- 210000004408 hybridoma Anatomy 0.000 claims abstract description 25
- 208000015181 infectious disease Diseases 0.000 claims abstract description 22
- 241001465754 Metazoa Species 0.000 claims abstract description 21
- 210000004027 cell Anatomy 0.000 claims abstract description 19
- 238000011282 treatment Methods 0.000 claims abstract description 15
- 239000003814 drug Substances 0.000 claims abstract description 14
- 238000000034 method Methods 0.000 claims abstract description 13
- 238000001514 detection method Methods 0.000 claims abstract description 7
- 230000002265 prevention Effects 0.000 claims abstract 5
- RJQXTJLFIWVMTO-TYNCELHUSA-N Methicillin Chemical compound COC1=CC=CC(OC)=C1C(=O)N[C@@H]1C(=O)N2[C@@H](C(O)=O)C(C)(C)S[C@@H]21 RJQXTJLFIWVMTO-TYNCELHUSA-N 0.000 claims description 11
- 229960003085 meticillin Drugs 0.000 claims description 11
- 241000283690 Bos taurus Species 0.000 claims description 10
- 206010040047 Sepsis Diseases 0.000 claims description 9
- 208000004396 mastitis Diseases 0.000 claims description 8
- 210000002381 plasma Anatomy 0.000 claims description 7
- 230000003115 biocidal effect Effects 0.000 claims description 6
- 241001529936 Murinae Species 0.000 claims description 5
- 229940079593 drug Drugs 0.000 claims description 5
- 241000124008 Mammalia Species 0.000 claims description 4
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 3
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 claims description 3
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 claims description 3
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 claims description 3
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 claims description 3
- 241000233866 Fungi Species 0.000 claims description 2
- 241000235342 Saccharomycetes Species 0.000 claims description 2
- 230000009885 systemic effect Effects 0.000 claims description 2
- 230000000699 topical effect Effects 0.000 claims description 2
- 125000003275 alpha amino acid group Chemical group 0.000 claims 2
- 241000282412 Homo Species 0.000 claims 1
- 241000235070 Saccharomyces Species 0.000 claims 1
- 238000011321 prophylaxis Methods 0.000 claims 1
- 230000000694 effects Effects 0.000 abstract description 3
- 201000010099 disease Diseases 0.000 abstract 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 abstract 1
- 239000000126 substance Substances 0.000 abstract 1
- 230000027455 binding Effects 0.000 description 28
- 239000000427 antigen Substances 0.000 description 17
- 102000036639 antigens Human genes 0.000 description 17
- 108091007433 antigens Proteins 0.000 description 17
- 241000894006 Bacteria Species 0.000 description 13
- 241000699670 Mus sp. Species 0.000 description 13
- 238000010494 dissociation reaction Methods 0.000 description 12
- 230000005593 dissociations Effects 0.000 description 12
- 108090000623 proteins and genes Proteins 0.000 description 11
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 10
- 102000004169 proteins and genes Human genes 0.000 description 10
- 150000001413 amino acids Chemical group 0.000 description 9
- 230000003993 interaction Effects 0.000 description 8
- 210000000056 organ Anatomy 0.000 description 6
- 239000011780 sodium chloride Substances 0.000 description 6
- 238000002965 ELISA Methods 0.000 description 5
- 206010057249 Phagocytosis Diseases 0.000 description 5
- 238000005259 measurement Methods 0.000 description 5
- 230000008782 phagocytosis Effects 0.000 description 5
- 239000002953 phosphate buffered saline Substances 0.000 description 5
- 239000002504 physiological saline solution Substances 0.000 description 4
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 3
- 108060003951 Immunoglobulin Proteins 0.000 description 3
- 241000699666 Mus <mouse, genus> Species 0.000 description 3
- 239000003242 anti bacterial agent Substances 0.000 description 3
- 230000037396 body weight Effects 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 230000018109 developmental process Effects 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 102000018358 immunoglobulin Human genes 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 238000011534 incubation Methods 0.000 description 3
- 230000002458 infectious effect Effects 0.000 description 3
- 238000001990 intravenous administration Methods 0.000 description 3
- 239000008267 milk Substances 0.000 description 3
- 210000004080 milk Anatomy 0.000 description 3
- 235000013336 milk Nutrition 0.000 description 3
- 230000003248 secreting effect Effects 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 239000007995 HEPES buffer Substances 0.000 description 2
- 238000011785 NMRI mouse Methods 0.000 description 2
- 108091028043 Nucleic acid sequence Proteins 0.000 description 2
- 108091034117 Oligonucleotide Proteins 0.000 description 2
- 241000191940 Staphylococcus Species 0.000 description 2
- 206010000269 abscess Diseases 0.000 description 2
- 229940088710 antibiotic agent Drugs 0.000 description 2
- 238000011230 antibody-based therapy Methods 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- 230000001332 colony forming effect Effects 0.000 description 2
- 230000009089 cytolysis Effects 0.000 description 2
- 230000001815 facial effect Effects 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 210000000224 granular leucocyte Anatomy 0.000 description 2
- 238000003125 immunofluorescent labeling Methods 0.000 description 2
- 210000000265 leukocyte Anatomy 0.000 description 2
- 238000013507 mapping Methods 0.000 description 2
- 125000000896 monocarboxylic acid group Chemical group 0.000 description 2
- 230000009871 nonspecific binding Effects 0.000 description 2
- 239000002773 nucleotide Substances 0.000 description 2
- 125000003729 nucleotide group Chemical group 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 230000004083 survival effect Effects 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 210000003462 vein Anatomy 0.000 description 2
- 210000002620 vena cava superior Anatomy 0.000 description 2
- IITIZHOBOIBGBW-UHFFFAOYSA-N 3-ethyl-2h-1,3-benzothiazole Chemical compound C1=CC=C2N(CC)CSC2=C1 IITIZHOBOIBGBW-UHFFFAOYSA-N 0.000 description 1
- 101710092462 Alpha-hemolysin Proteins 0.000 description 1
- 101710197219 Alpha-toxin Proteins 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 206010057248 Cell death Diseases 0.000 description 1
- 101710098119 Chaperonin GroEL 2 Proteins 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 206010015548 Euthanasia Diseases 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 102000012966 Growth Differentiation Factor 5 Human genes 0.000 description 1
- 108010090254 Growth Differentiation Factor 5 Proteins 0.000 description 1
- 239000012981 Hank's balanced salt solution Substances 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 1
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 1
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 1
- YQEZLKZALYSWHR-UHFFFAOYSA-N Ketamine Chemical compound C=1C=CC=C(Cl)C=1C1(NC)CCCCC1=O YQEZLKZALYSWHR-UHFFFAOYSA-N 0.000 description 1
- 239000006156 Mannitol salt agar Substances 0.000 description 1
- 101710167839 Morphogenetic protein Proteins 0.000 description 1
- 102000015636 Oligopeptides Human genes 0.000 description 1
- 108010038807 Oligopeptides Proteins 0.000 description 1
- 108700022034 Opsonin Proteins Proteins 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- BELBBZDIHDAJOR-UHFFFAOYSA-N Phenolsulfonephthalein Chemical compound C1=CC(O)=CC=C1C1(C=2C=CC(O)=CC=2)C2=CC=CC=C2S(=O)(=O)O1 BELBBZDIHDAJOR-UHFFFAOYSA-N 0.000 description 1
- 101710124951 Phospholipase C Proteins 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 206010041925 Staphylococcal infections Diseases 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- 101000588258 Taenia solium Paramyosin Proteins 0.000 description 1
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 239000002776 alpha toxin Substances 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 238000003491 array Methods 0.000 description 1
- OHDRQQURAXLVGJ-HLVWOLMTSA-N azane;(2e)-3-ethyl-2-[(e)-(3-ethyl-6-sulfo-1,3-benzothiazol-2-ylidene)hydrazinylidene]-1,3-benzothiazole-6-sulfonic acid Chemical compound [NH4+].[NH4+].S/1C2=CC(S([O-])(=O)=O)=CC=C2N(CC)C\1=N/N=C1/SC2=CC(S([O-])(=O)=O)=CC=C2N1CC OHDRQQURAXLVGJ-HLVWOLMTSA-N 0.000 description 1
- 230000001588 bifunctional effect Effects 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 210000002421 cell wall Anatomy 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 230000009260 cross reactivity Effects 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 1
- 238000012757 fluorescence staining Methods 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 238000003306 harvesting Methods 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 101150026046 iga gene Proteins 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 238000011081 inoculation Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 101150039690 isaA gene Proteins 0.000 description 1
- 229960003299 ketamine Drugs 0.000 description 1
- 231100000518 lethal Toxicity 0.000 description 1
- 230000001665 lethal effect Effects 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 208000015688 methicillin-resistant staphylococcus aureus infectious disease Diseases 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 210000004400 mucous membrane Anatomy 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- 238000007474 nonparametric Mann- Whitney U test Methods 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 230000000242 pagocytic effect Effects 0.000 description 1
- 229960003531 phenolsulfonphthalein Drugs 0.000 description 1
- -1 polyethylene Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 238000013207 serial dilution Methods 0.000 description 1
- JJGWLCLUQNFDIS-GTSONSFRSA-M sodium;1-[6-[5-[(3as,4s,6ar)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoylamino]hexanoyloxy]-2,5-dioxopyrrolidine-3-sulfonate Chemical compound [Na+].O=C1C(S(=O)(=O)[O-])CC(=O)N1OC(=O)CCCCCNC(=O)CCCC[C@H]1[C@H]2NC(=O)N[C@H]2CS1 JJGWLCLUQNFDIS-GTSONSFRSA-M 0.000 description 1
- 238000011895 specific detection Methods 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 210000001913 submandibular gland Anatomy 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
- BPICBUSOMSTKRF-UHFFFAOYSA-N xylazine Chemical compound CC1=CC=CC(C)=C1NC1=NCCCS1 BPICBUSOMSTKRF-UHFFFAOYSA-N 0.000 description 1
- 229960001600 xylazine Drugs 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/573—Immunoassay; Biospecific binding assay; Materials therefor for enzymes or isoenzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/40—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum bacterial
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/14—Drugs for genital or sexual disorders; Contraceptives for lactation disorders, e.g. galactorrhoea
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/12—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria
- C07K16/1267—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria from Gram-positive bacteria
- C07K16/1271—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria from Gram-positive bacteria from Micrococcaceae (F), e.g. Staphylococcus
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/40—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against enzymes
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/569—Immunoassay; Biospecific binding assay; Materials therefor for microorganisms, e.g. protozoa, bacteria, viruses
- G01N33/56911—Bacteria
- G01N33/56938—Staphylococcus
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/77—Internalization into the cell
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/90—Enzymes; Proenzymes
- G01N2333/914—Hydrolases (3)
- G01N2333/924—Hydrolases (3) acting on glycosyl compounds (3.2)
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2469/00—Immunoassays for the detection of microorganisms
- G01N2469/10—Detection of antigens from microorganism in sample from host
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- Immunology (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Microbiology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Epidemiology (AREA)
- Food Science & Technology (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Physics & Mathematics (AREA)
- Biotechnology (AREA)
- Cell Biology (AREA)
- Mycology (AREA)
- Virology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Pregnancy & Childbirth (AREA)
- Endocrinology (AREA)
- Communicable Diseases (AREA)
- Reproductive Health (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US17913309P | 2009-05-18 | 2009-05-18 | |
| US61/179,133 | 2009-05-18 | ||
| PCT/EP2010/056827 WO2010133600A1 (en) | 2009-05-18 | 2010-05-18 | Antibodies or fragments thereof directed against a staphylococcus aureus epitope of isaa or isab |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2011146424A RU2011146424A (ru) | 2013-06-27 |
| RU2560415C2 true RU2560415C2 (ru) | 2015-08-20 |
Family
ID=42316060
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2011146424/10A RU2560415C2 (ru) | 2009-05-18 | 2010-05-18 | Антитела или их фрагменты, направленные против эпитопа стафилококка золотистого (staphylococcus aureus) |
Country Status (13)
| Country | Link |
|---|---|
| US (3) | US8609102B2 (enExample) |
| EP (1) | EP2432804B1 (enExample) |
| JP (2) | JP5882892B2 (enExample) |
| CN (1) | CN102695724B (enExample) |
| AU (1) | AU2010251156C1 (enExample) |
| BR (1) | BRPI1010911A2 (enExample) |
| CA (1) | CA2761886A1 (enExample) |
| ES (1) | ES2537437T3 (enExample) |
| MX (1) | MX2011012248A (enExample) |
| PL (1) | PL2432804T3 (enExample) |
| PT (1) | PT2432804E (enExample) |
| RU (1) | RU2560415C2 (enExample) |
| WO (1) | WO2010133600A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11970527B2 (en) | 2018-07-24 | 2024-04-30 | Medimmune, Llc | Antibody directed against S. aureus clumping factor A (ClfA) |
| RU2818805C2 (ru) * | 2018-07-24 | 2024-05-06 | МЕДИММЬЮН, ЭлЭлСи | АНТИТЕЛО, НАПРАВЛЕННОЕ ПРОТИВ ФАКТОРА СЛИПАНИЯ А (ClfA) S. aureus |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5882892B2 (ja) * | 2009-05-18 | 2016-03-09 | ユリウス・マクシミリアンス−ウニヴェルジテート・ヴュルツブルクJulius Maximilians−Universitaet Wuerzburg | 黄色ブドウ球菌エピトープIsaAまたはIsaBに対する抗体またはそれらのフラグメント |
| WO2013041707A1 (en) | 2011-09-23 | 2013-03-28 | Julius-Maximilians-Universität Würzburg | Peptide or arrangement of peptides forming a staphylococcus aureus epitope binding site |
| US9944694B2 (en) | 2013-12-13 | 2018-04-17 | Rijksuniversiteit Groningen | Antibodies against Staphylococcus aureus and uses therof |
| KR102341805B1 (ko) * | 2019-11-27 | 2021-12-22 | 주식회사 하울바이오 | 황색포도상구균에 특이적으로 반응하는 항-IsaA G4 항체 및 이의 용도 |
| KR102341804B1 (ko) * | 2019-11-27 | 2021-12-22 | 주식회사 하울바이오 | 황색포도상구균을 억제하는 항-IsaA F10 항체 및 이의 용도 |
| US20230357373A1 (en) * | 2019-12-17 | 2023-11-09 | Mie University | Methods and compositions for evaluating and treating fibrosis |
| WO2022250205A1 (ko) * | 2021-05-25 | 2022-12-01 | 주식회사 하울바이오 | 항-IsaA F10 항체의 아토피 피부염 예방 및 개선 용도 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2144190C1 (ru) * | 1998-01-13 | 2000-01-10 | Катханов Али Муратович | Способ диагностики стафилококковой инфекции |
| US6340571B1 (en) * | 1996-03-21 | 2002-01-22 | Bio Merieux | Antibodies specific for Staphylococcus aureus, and use thereof |
| WO2003046007A2 (en) * | 2001-11-22 | 2003-06-05 | Neutec Pharma Plc | Treatment of micro-organism infection |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE4238806A1 (en) * | 1991-11-18 | 1993-05-27 | Dainabot Co | Methicillin-resistant staphylococcus aureus (MRSA)detection method - by detection of MRSA penicillin binding protein (PBP)2 antigen-antibody complex, and synthetic MRSA PBP2 peptides |
| GB0014907D0 (en) * | 2000-06-20 | 2000-08-09 | Univ Sheffield | Antigenic polypeptides |
| KR20070104590A (ko) * | 2005-01-10 | 2007-10-26 | 나비 바이오파마슈티컬즈 | 스타필로코쿠스 아우레우스 감염을 치료하는 방법 |
| JP5882892B2 (ja) * | 2009-05-18 | 2016-03-09 | ユリウス・マクシミリアンス−ウニヴェルジテート・ヴュルツブルクJulius Maximilians−Universitaet Wuerzburg | 黄色ブドウ球菌エピトープIsaAまたはIsaBに対する抗体またはそれらのフラグメント |
-
2010
- 2010-05-18 JP JP2012511263A patent/JP5882892B2/ja not_active Expired - Fee Related
- 2010-05-18 CN CN201080021810.5A patent/CN102695724B/zh not_active Expired - Fee Related
- 2010-05-18 BR BRPI1010911A patent/BRPI1010911A2/pt not_active IP Right Cessation
- 2010-05-18 PL PL10720416T patent/PL2432804T3/pl unknown
- 2010-05-18 WO PCT/EP2010/056827 patent/WO2010133600A1/en not_active Ceased
- 2010-05-18 RU RU2011146424/10A patent/RU2560415C2/ru not_active IP Right Cessation
- 2010-05-18 ES ES10720416.6T patent/ES2537437T3/es active Active
- 2010-05-18 AU AU2010251156A patent/AU2010251156C1/en not_active Ceased
- 2010-05-18 US US13/320,743 patent/US8609102B2/en not_active Expired - Fee Related
- 2010-05-18 PT PT107204166T patent/PT2432804E/pt unknown
- 2010-05-18 CA CA2761886A patent/CA2761886A1/en not_active Abandoned
- 2010-05-18 MX MX2011012248A patent/MX2011012248A/es active IP Right Grant
- 2010-05-18 EP EP10720416.6A patent/EP2432804B1/en not_active Not-in-force
-
2013
- 2013-11-12 US US14/077,927 patent/US9260511B2/en not_active Expired - Fee Related
-
2015
- 2015-10-09 JP JP2015201433A patent/JP2016047828A/ja active Pending
- 2015-12-30 US US14/984,958 patent/US9658230B2/en not_active Expired - Fee Related
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6340571B1 (en) * | 1996-03-21 | 2002-01-22 | Bio Merieux | Antibodies specific for Staphylococcus aureus, and use thereof |
| RU2144190C1 (ru) * | 1998-01-13 | 2000-01-10 | Катханов Али Муратович | Способ диагностики стафилококковой инфекции |
| WO2003046007A2 (en) * | 2001-11-22 | 2003-06-05 | Neutec Pharma Plc | Treatment of micro-organism infection |
Non-Patent Citations (1)
| Title |
|---|
| LORENZ U. et al., Therapeutische EffektivitÄt von monoklonalen AntikÖrpern gegen Staphylococcus aureus in einem Sepsis- und Abszess-Mausmodell, 125. Kongress der Deutschen Gesellschaft fÜr Chirurgie Deutsche Gesellschaft fÜr Chirurgie 22. - 25.04.2008, Berlin. LORENZ U. et al., Human antibody response during sepsis against targets expressed by methicillin resistant Staphylococcus aureus, FEMS Immunology and Medical Microbiology, 2000, Vol. 29, pp. 145-153. * |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11970527B2 (en) | 2018-07-24 | 2024-04-30 | Medimmune, Llc | Antibody directed against S. aureus clumping factor A (ClfA) |
| RU2818805C2 (ru) * | 2018-07-24 | 2024-05-06 | МЕДИММЬЮН, ЭлЭлСи | АНТИТЕЛО, НАПРАВЛЕННОЕ ПРОТИВ ФАКТОРА СЛИПАНИЯ А (ClfA) S. aureus |
Also Published As
| Publication number | Publication date |
|---|---|
| US20120100151A1 (en) | 2012-04-26 |
| JP2016047828A (ja) | 2016-04-07 |
| RU2011146424A (ru) | 2013-06-27 |
| WO2010133600A1 (en) | 2010-11-25 |
| EP2432804B1 (en) | 2015-02-25 |
| PL2432804T3 (pl) | 2015-08-31 |
| US20160109449A1 (en) | 2016-04-21 |
| JP2012527423A (ja) | 2012-11-08 |
| AU2010251156A1 (en) | 2011-11-24 |
| US9658230B2 (en) | 2017-05-23 |
| CA2761886A1 (en) | 2010-11-25 |
| MX2011012248A (es) | 2012-02-29 |
| US9260511B2 (en) | 2016-02-16 |
| EP2432804A1 (en) | 2012-03-28 |
| ES2537437T3 (es) | 2015-06-08 |
| US20140072570A1 (en) | 2014-03-13 |
| AU2010251156C1 (en) | 2015-02-05 |
| US8609102B2 (en) | 2013-12-17 |
| JP5882892B2 (ja) | 2016-03-09 |
| CN102695724A (zh) | 2012-09-26 |
| CN102695724B (zh) | 2016-08-24 |
| PT2432804E (pt) | 2015-06-25 |
| AU2010251156B2 (en) | 2014-10-09 |
| BRPI1010911A2 (pt) | 2019-09-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2560415C2 (ru) | Антитела или их фрагменты, направленные против эпитопа стафилококка золотистого (staphylococcus aureus) | |
| US10906962B2 (en) | Poly-n-acetyl glucosamine (PNAG/dPNAG)-binding peptides and methods of use thereof | |
| JP6064241B2 (ja) | 黄色ブドウ球菌由来のα毒素に対するヒトモノクローナル抗体、及び膿瘍形成の治療又は予防におけるその使用 | |
| US7119172B2 (en) | P. aeruginosa mucoid exopolysaccharide specific binding peptides | |
| AU2014277807B2 (en) | Poly-N-acetyl glucosamine(PNAG/DPNAG)-binding peptides and methods of use thereof | |
| HK1100369B (en) | Poly-n-acetyl glucosamine (pnag/dpnag)-binding peptides and methods of use thereof | |
| HK1171762B (en) | Poly-n-acetyl glucosamine (pnag/dpnag)-binding antibodies | |
| HK1168859A (en) | Poly-n-acetyl glucosamine (pnag/dpnag)-binding peptides and methods of use thereof | |
| HK1169423A (en) | Poly-n-acetyl glucosamine (pnag/dpnag)-binding peptides and methods of use thereof | |
| HK1171762A (en) | Poly-n-acetyl glucosamine (pnag/dpnag)-binding antibodies |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20170519 |