RU2553989C2 - Способ получения модуляторов регулятора трансмембранной проводимости кистозного фиброза - Google Patents
Способ получения модуляторов регулятора трансмембранной проводимости кистозного фиброза Download PDFInfo
- Publication number
- RU2553989C2 RU2553989C2 RU2012121160/04A RU2012121160A RU2553989C2 RU 2553989 C2 RU2553989 C2 RU 2553989C2 RU 2012121160/04 A RU2012121160/04 A RU 2012121160/04A RU 2012121160 A RU2012121160 A RU 2012121160A RU 2553989 C2 RU2553989 C2 RU 2553989C2
- Authority
- RU
- Russia
- Prior art keywords
- compound
- hcl
- solvent
- cftr
- mixture
- Prior art date
Links
- 0 *C(C1=CNc(cccc2C(F)(F)F)c2C1=O)=O Chemical compound *C(C1=CNc(cccc2C(F)(F)F)c2C1=O)=O 0.000 description 1
- GIFDDEUMMLLKIL-UHFFFAOYSA-N Nc(cc1)c(C(F)(F)F)cc1N1C2CCC1CC2 Chemical compound Nc(cc1)c(C(F)(F)F)cc1N1C2CCC1CC2 GIFDDEUMMLLKIL-UHFFFAOYSA-N 0.000 description 1
- YPPWJGLUMXWZCJ-WSUHUXQVSA-N O=C(C1=CNc2cccc(C(F)(F)F)c2C1=O)N[C@@H]1C(N2C3(CC4)C4C2CC3)=CC1C(F)(F)F Chemical compound O=C(C1=CNc2cccc(C(F)(F)F)c2C1=O)N[C@@H]1C(N2C3(CC4)C4C2CC3)=CC1C(F)(F)F YPPWJGLUMXWZCJ-WSUHUXQVSA-N 0.000 description 1
- WAEYWIDTDWJDJA-UHFFFAOYSA-N OC(C1=CNc(cccc2C(F)(F)F)c2C1=O)=O Chemical compound OC(C1=CNc(cccc2C(F)(F)F)c2C1=O)=O WAEYWIDTDWJDJA-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D215/00—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems
- C07D215/02—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom
- C07D215/16—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D215/48—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen
- C07D215/54—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen attached in position 3
- C07D215/56—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen attached in position 3 with oxygen atoms in position 4
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/08—Bridged systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/407—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil condensed with other heterocyclic ring systems, e.g. ketorolac, physostigmine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/10—Laxatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/18—Drugs for disorders of the alimentary tract or the digestive system for pancreatic disorders, e.g. pancreatic enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/02—Nasal agents, e.g. decongestants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/08—Bronchodilators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/08—Drugs for genital or sexual disorders; Contraceptives for gonadal disorders or for enhancing fertility, e.g. inducers of ovulation or of spermatogenesis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/08—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease
- A61P19/10—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease for osteoporosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/12—Drugs for disorders of the metabolism for electrolyte homeostasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/06—Drugs for disorders of the endocrine system of the anterior pituitary hormones, e.g. TSH, ACTH, FSH, LH, PRL, GH
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/14—Drugs for disorders of the endocrine system of the thyroid hormones, e.g. T3, T4
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/13—Crystalline forms, e.g. polymorphs
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Diabetes (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Pulmonology (AREA)
- Neurology (AREA)
- Hematology (AREA)
- Endocrinology (AREA)
- Physical Education & Sports Medicine (AREA)
- Obesity (AREA)
- Reproductive Health (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Epidemiology (AREA)
- Rheumatology (AREA)
- Urology & Nephrology (AREA)
- Ophthalmology & Optometry (AREA)
- Pregnancy & Childbirth (AREA)
- Emergency Medicine (AREA)
- Psychiatry (AREA)
- Heart & Thoracic Surgery (AREA)
- Hospice & Palliative Care (AREA)
- Psychology (AREA)
- Cardiology (AREA)
- Gynecology & Obstetrics (AREA)
- Gastroenterology & Hepatology (AREA)
- Otolaryngology (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US25463409P | 2009-10-23 | 2009-10-23 | |
| US61/254,634 | 2009-10-23 | ||
| PCT/US2010/053628 WO2011050215A1 (en) | 2009-10-23 | 2010-10-21 | Process for preparing modulators of cystic fibrosis transmembrane conductance regulator |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2012121160A RU2012121160A (ru) | 2013-11-27 |
| RU2553989C2 true RU2553989C2 (ru) | 2015-06-20 |
Family
ID=43530872
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2012121160/04A RU2553989C2 (ru) | 2009-10-23 | 2010-10-21 | Способ получения модуляторов регулятора трансмембранной проводимости кистозного фиброза |
Country Status (16)
| Country | Link |
|---|---|
| US (4) | US8344147B2 (enExample) |
| EP (1) | EP2491018A1 (enExample) |
| JP (1) | JP5789611B2 (enExample) |
| KR (1) | KR20120104554A (enExample) |
| CN (1) | CN102648182A (enExample) |
| AR (1) | AR080065A1 (enExample) |
| AU (1) | AU2010310612A1 (enExample) |
| BR (1) | BR112012009583A2 (enExample) |
| CA (1) | CA2778492A1 (enExample) |
| IL (1) | IL219326A0 (enExample) |
| MX (1) | MX2012004790A (enExample) |
| NZ (1) | NZ600172A (enExample) |
| RU (1) | RU2553989C2 (enExample) |
| TW (1) | TWI475023B (enExample) |
| WO (1) | WO2011050215A1 (enExample) |
| ZA (1) | ZA201202915B (enExample) |
Families Citing this family (47)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ZA200602755B (en) | 2003-09-06 | 2007-06-27 | Vertex Pharma | Modulators of ATP-binding cassette transporters |
| US7977322B2 (en) | 2004-08-20 | 2011-07-12 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| NZ587549A (en) | 2004-06-24 | 2012-10-26 | Vertex Pharma | 6-Amino-indole derivatives and processes for their preparation |
| EP1912983B1 (en) | 2005-08-11 | 2011-06-08 | Vertex Pharmaceuticals, Inc. | Modulators of cystic fibrosis transmembrane conductance regulator |
| KR101331768B1 (ko) * | 2005-11-08 | 2013-11-22 | 버텍스 파마슈티칼스 인코포레이티드 | Atp 결합 카세트 수송체의 헤테로사이클릭 조정제 |
| CA2856037C (en) | 2005-12-28 | 2017-03-07 | Vertex Pharmaceuticals Incorporated | Modulators of atp-binding cassette transporters |
| CA2635581C (en) | 2005-12-28 | 2017-02-28 | Vertex Pharmaceuticals Incorporated | Solid forms of n-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide |
| US7645789B2 (en) * | 2006-04-07 | 2010-01-12 | Vertex Pharmaceuticals Incorporated | Indole derivatives as CFTR modulators |
| USRE50453E1 (en) | 2006-04-07 | 2025-06-10 | Vertex Pharmaceuticals Incorporated | Indole derivatives as CFTR modulators |
| US10022352B2 (en) | 2006-04-07 | 2018-07-17 | Vertex Pharmaceuticals Incorporated | Modulators of ATP-binding cassette transporters |
| PL2674428T3 (pl) | 2006-04-07 | 2017-01-31 | Vertex Pharmaceuticals Incorporated | Modulatory transporterów z kasetą wiążącą ATP |
| US8563573B2 (en) | 2007-11-02 | 2013-10-22 | Vertex Pharmaceuticals Incorporated | Azaindole derivatives as CFTR modulators |
| CA2686838C (en) | 2007-05-09 | 2017-03-14 | Vertex Pharmaceuticals Incorporated | Modulators of cftr |
| AU2008302598B2 (en) | 2007-08-24 | 2014-07-17 | Vertex Pharmaceuticals Incorporated | Isothiazolopyridinones useful for the treatment of (inter alia) Cystic Fibrosis |
| SI2578571T1 (sl) | 2007-11-16 | 2016-01-29 | Vertex Pharmaceuticals Incorporated | Izokinolinski modulatorji prenašalcev z atp-vezavno kaseto |
| HRP20170241T2 (hr) | 2007-12-07 | 2023-03-17 | Vertex Pharmaceuticals Incorporated | Čvrsti oblici 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioksol-5-il)ciklopropankarboksamido)-3-metilpiridin-2-il)benzojeve kiseline |
| US20100036130A1 (en) | 2007-12-07 | 2010-02-11 | Vertex Pharmaceuticals Incorporated | Processes for producing cycloalkylcarboxamido-pyridine benzoic acids |
| AU2008335439A1 (en) * | 2007-12-07 | 2009-06-18 | Vertex Pharmaceuticals Incorporated | Formulations of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid |
| DK2639223T3 (en) | 2007-12-07 | 2017-06-19 | Vertex Pharma | Process for Preparation of Cycloalkylcarboxiamido-pyridine Benzoic Acids |
| NZ736561A (en) | 2008-02-28 | 2018-02-23 | Vertex Pharma | Heteroaryl derivatives as cftr modulators |
| DK2615085T3 (en) | 2008-03-31 | 2015-10-05 | Vertex Pharma | Pyridyl derivatives as CFTR modulators |
| US20100074949A1 (en) | 2008-08-13 | 2010-03-25 | William Rowe | Pharmaceutical composition and administration thereof |
| US12458635B2 (en) | 2008-08-13 | 2025-11-04 | Vertex Pharmaceuticals Incorporated | Pharmaceutical composition and administrations thereof |
| US8716338B2 (en) * | 2008-09-29 | 2014-05-06 | Vertex Pharmaceuticals Incorporated | Dosage units of 3-(6-(1-(2,2-difluorobenzo[D] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid |
| AU2009308241B2 (en) * | 2008-10-23 | 2016-01-07 | Vertex Pharmaceuticals Incorporated | Solid forms of N-(4-(7-azabicyclo[2.2.1]heptan-7-yl)-2-(trifluoromethyl) phenyl)-4-oxo-5-(trifluoromethyl)-1,4-dihydroquinoline-3-carboxamide |
| UA104601C2 (uk) * | 2008-10-23 | 2014-02-25 | Вертекс Фармасьютікалз, Інкорпорейтед | Модулятори регулятора трансмембранної провідності при муковісцидозі |
| PL2408750T3 (pl) | 2009-03-20 | 2016-02-29 | Vertex Pharma | Sposób otrzymywania modulatorów błonowego regulatora przewodnictwa swoistego dla mukowiscydozy |
| EP2477991B1 (en) * | 2009-09-17 | 2015-04-15 | Vertex Pharmaceuticals Incorporated | Process for preparing azabicyclic compounds |
| BR112012009584A2 (pt) * | 2009-10-23 | 2019-09-24 | Vertex Pharma | formas sólidas de n-(4-(7-azabiciclo[2.2.1]heptan-7-il)-2-(trifluorometil)fenil)-4-oxo-5-(trifluorometil)-1,4-di-hidroquinolina-3-carboxamida |
| US8802868B2 (en) | 2010-03-25 | 2014-08-12 | Vertex Pharmaceuticals Incorporated | Solid forms of (R)-1(2,2-difluorobenzo[D][1,3]dioxo1-5-yl)-N-(1-(2,3-dihydroxypropyl-6-fluoro-2-(1-hydroxy-2-methylpropan2-yl)-1H-Indol-5-yl)-Cyclopropanecarboxamide |
| RS54783B1 (sr) | 2010-04-07 | 2016-10-31 | Vertex Pharma | Čvrste forme 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioksol-5-il)ciklopropankarboksiamido)-3-metilpiridin-2-il)benzoeve kiseline |
| DK3150198T3 (da) | 2010-04-07 | 2021-11-01 | Vertex Pharma | Farmaceutiske sammensætninger af 3-(6-(1-(2,2-difluorbenzo[d][1,3]dioxol-5-yl)-cyclopropancarboxamido)-3-methylpyriodin-2-yl)benzoesyre og indgivelse deraf |
| CA2797118C (en) | 2010-04-22 | 2021-03-30 | Vertex Pharmaceuticals Incorporated | Process of producing cycloalkylcarboxamido-indole compounds |
| AR081920A1 (es) * | 2010-05-20 | 2012-10-31 | Vertex Pharma | Procesos de produccion de moduladores del regulador de conductancia transmembrana de fibrosis quistica |
| US8563593B2 (en) | 2010-06-08 | 2013-10-22 | Vertex Pharmaceuticals Incorporated | Formulations of (R)-1-(2,2-difluorobenzo[D] [1,3] dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide |
| MX357328B (es) | 2011-11-08 | 2018-07-05 | Vertex Pharma | Moduladores de trasportadores de casete enlazante de atp. |
| AU2013226076B2 (en) | 2012-02-27 | 2017-11-16 | Vertex Pharmaceuticals Incorporated | Pharmaceutical composition and administration thereof |
| US8674108B2 (en) | 2012-04-20 | 2014-03-18 | Vertex Pharmaceuticals Incorporated | Solid forms of N-[2,4-bis(1,1-dimethylethy)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide |
| EP2872122A1 (en) | 2012-07-16 | 2015-05-20 | Vertex Pharmaceuticals Incorporated | Pharmaceutical compositions of (r)-1-(2,2-diflurorbenzo[d][1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof |
| RU2718044C2 (ru) | 2013-11-12 | 2020-03-30 | Вертекс Фармасьютикалз Инкорпорейтед | Способ получения фармацевтических композиций для лечения опосредованных cftr заболеваний |
| RS62140B1 (sr) | 2014-04-15 | 2021-08-31 | Vertex Pharma | Farmaceutske kompozicije za lečenje bolesti posredovanih transmembranskim regulatorom provodljivosti cistične fibroze |
| KR20170063954A (ko) | 2014-10-07 | 2017-06-08 | 버텍스 파마슈티칼스 인코포레이티드 | 낭성 섬유증 막횡단 전도도 조절자의 조정제의 공-결정 |
| HRP20211194T1 (hr) | 2014-11-18 | 2021-10-29 | Vertex Pharmaceuticals Inc. | Postupak za provođenje testova velike propusnosti putem tekućinske kromatografije visoke djelotvornosti |
| JP7158717B2 (ja) * | 2017-11-22 | 2022-10-24 | 国立大学法人九州工業大学 | 求電子的アジド化剤又はジアゾ化剤 |
| CN109369798B (zh) * | 2018-12-25 | 2020-09-15 | 苏州天马医药集团天吉生物制药有限公司 | 一种合成索玛鲁肽的方法 |
| CR20230120A (es) | 2020-08-07 | 2023-09-01 | Vertex Pharma | Moduladores del regulador de la conductancia transmembrana de la fibrosis quística |
| US12324802B2 (en) | 2020-11-18 | 2025-06-10 | Vertex Pharmaceuticals Incorporated | Modulators of cystic fibrosis transmembrane conductance regulator |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2193558C2 (ru) * | 1997-09-15 | 2002-11-27 | Дзе Проктер Энд Гэмбл Компани | Производные хинолона и фармацевтическая композиция на их основе |
| RU2220960C2 (ru) * | 1997-12-22 | 2004-01-10 | Фармация Энд Апджон Компани | 4-гидрокси-3-хинолинкарбоксамиды и гидразиды, фармацевтическая композиция и способ лечения на их основе |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5304121A (en) | 1990-12-28 | 1994-04-19 | Boston Scientific Corporation | Drug delivery system making use of a hydrogel polymer coating |
| US5886026A (en) | 1993-07-19 | 1999-03-23 | Angiotech Pharmaceuticals Inc. | Anti-angiogenic compositions and methods of use |
| US6099562A (en) | 1996-06-13 | 2000-08-08 | Schneider (Usa) Inc. | Drug coating with topcoat |
| NZ587549A (en) | 2004-06-24 | 2012-10-26 | Vertex Pharma | 6-Amino-indole derivatives and processes for their preparation |
| JP5461405B2 (ja) * | 2007-09-14 | 2014-04-02 | バーテックス ファーマシューティカルズ インコーポレイテッド | 嚢胞性線維症膜コンダクタンス制御因子の調節因子 |
| KR101578235B1 (ko) | 2007-12-10 | 2015-12-16 | 노파르티스 아게 | 유기 화합물 |
| AU2009308241B2 (en) * | 2008-10-23 | 2016-01-07 | Vertex Pharmaceuticals Incorporated | Solid forms of N-(4-(7-azabicyclo[2.2.1]heptan-7-yl)-2-(trifluoromethyl) phenyl)-4-oxo-5-(trifluoromethyl)-1,4-dihydroquinoline-3-carboxamide |
| UA104601C2 (uk) * | 2008-10-23 | 2014-02-25 | Вертекс Фармасьютікалз, Інкорпорейтед | Модулятори регулятора трансмембранної провідності при муковісцидозі |
| BR112012009584A2 (pt) * | 2009-10-23 | 2019-09-24 | Vertex Pharma | formas sólidas de n-(4-(7-azabiciclo[2.2.1]heptan-7-il)-2-(trifluorometil)fenil)-4-oxo-5-(trifluorometil)-1,4-di-hidroquinolina-3-carboxamida |
-
2010
- 2010-10-21 RU RU2012121160/04A patent/RU2553989C2/ru not_active IP Right Cessation
- 2010-10-21 CN CN2010800533805A patent/CN102648182A/zh active Pending
- 2010-10-21 JP JP2012535392A patent/JP5789611B2/ja not_active Expired - Fee Related
- 2010-10-21 US US12/909,750 patent/US8344147B2/en active Active
- 2010-10-21 MX MX2012004790A patent/MX2012004790A/es active IP Right Grant
- 2010-10-21 EP EP10775986A patent/EP2491018A1/en not_active Withdrawn
- 2010-10-21 WO PCT/US2010/053628 patent/WO2011050215A1/en not_active Ceased
- 2010-10-21 NZ NZ600172A patent/NZ600172A/en not_active IP Right Cessation
- 2010-10-21 CA CA2778492A patent/CA2778492A1/en not_active Abandoned
- 2010-10-21 BR BR112012009583A patent/BR112012009583A2/pt not_active IP Right Cessation
- 2010-10-21 AU AU2010310612A patent/AU2010310612A1/en not_active Abandoned
- 2010-10-21 KR KR1020127013136A patent/KR20120104554A/ko not_active Withdrawn
- 2010-10-22 TW TW099136180A patent/TWI475023B/zh not_active IP Right Cessation
- 2010-10-25 AR ARP100103914A patent/AR080065A1/es unknown
-
2012
- 2012-04-20 ZA ZA2012/02915A patent/ZA201202915B/en unknown
- 2012-04-22 IL IL219326A patent/IL219326A0/en unknown
- 2012-11-16 US US13/678,840 patent/US8754222B2/en active Active
-
2014
- 2014-03-07 US US14/200,656 patent/US8884018B2/en active Active
- 2014-07-09 US US14/326,930 patent/US20140336393A1/en not_active Abandoned
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2193558C2 (ru) * | 1997-09-15 | 2002-11-27 | Дзе Проктер Энд Гэмбл Компани | Производные хинолона и фармацевтическая композиция на их основе |
| RU2220960C2 (ru) * | 1997-12-22 | 2004-01-10 | Фармация Энд Апджон Компани | 4-гидрокси-3-хинолинкарбоксамиды и гидразиды, фармацевтическая композиция и способ лечения на их основе |
Also Published As
| Publication number | Publication date |
|---|---|
| CN102648182A (zh) | 2012-08-22 |
| IL219326A0 (en) | 2012-06-28 |
| US8754222B2 (en) | 2014-06-17 |
| JP2013508406A (ja) | 2013-03-07 |
| AU2010310612A1 (en) | 2012-06-14 |
| US8884018B2 (en) | 2014-11-11 |
| WO2011050215A1 (en) | 2011-04-28 |
| MX2012004790A (es) | 2013-02-01 |
| US20110124869A1 (en) | 2011-05-26 |
| TW201120044A (en) | 2011-06-16 |
| RU2012121160A (ru) | 2013-11-27 |
| BR112012009583A2 (pt) | 2015-09-29 |
| US20130072687A1 (en) | 2013-03-21 |
| ZA201202915B (en) | 2013-06-26 |
| TWI475023B (zh) | 2015-03-01 |
| JP5789611B2 (ja) | 2015-10-07 |
| EP2491018A1 (en) | 2012-08-29 |
| KR20120104554A (ko) | 2012-09-21 |
| AR080065A1 (es) | 2012-03-14 |
| US20140336393A1 (en) | 2014-11-13 |
| US8344147B2 (en) | 2013-01-01 |
| AU2010310612A2 (en) | 2012-08-16 |
| US20140187787A1 (en) | 2014-07-03 |
| CA2778492A1 (en) | 2011-04-28 |
| NZ600172A (en) | 2014-07-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2553989C2 (ru) | Способ получения модуляторов регулятора трансмембранной проводимости кистозного фиброза | |
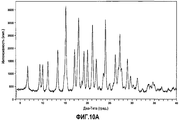
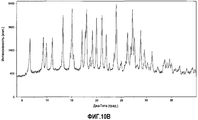
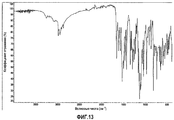
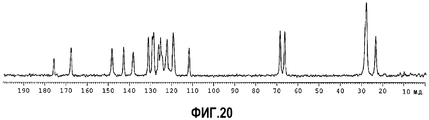
| RU2568608C2 (ru) | Твердые формы n-(4-(7-азабицикло[2.2.1]гептан-7-ил)-2-(трифторметил)фенил)-4-оксо-5-(трифторметил)-1,4-дигидрохинолин-3-карбоксамида | |
| AU2009308241B2 (en) | Solid forms of N-(4-(7-azabicyclo[2.2.1]heptan-7-yl)-2-(trifluoromethyl) phenyl)-4-oxo-5-(trifluoromethyl)-1,4-dihydroquinoline-3-carboxamide | |
| US8674108B2 (en) | Solid forms of N-[2,4-bis(1,1-dimethylethy)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide | |
| HK1173150A (en) | Process for preparing modulators of cystic fibrosis transmembrane conductance regulator | |
| HK1173151B (en) | Solid forms of n-(4-(7-azabicyclo[2.2.1]heptan-7-yl)-2-trifluoromethyl)phenyl)-4-oxo-5-(trifluoromethyl)-1,4-dihydroquinoline-3-carboxamide |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20161022 |