RU2462475C2 - Антитела против вируса гепатита с - Google Patents
Антитела против вируса гепатита с Download PDFInfo
- Publication number
- RU2462475C2 RU2462475C2 RU2010129429/10A RU2010129429A RU2462475C2 RU 2462475 C2 RU2462475 C2 RU 2462475C2 RU 2010129429/10 A RU2010129429/10 A RU 2010129429/10A RU 2010129429 A RU2010129429 A RU 2010129429A RU 2462475 C2 RU2462475 C2 RU 2462475C2
- Authority
- RU
- Russia
- Prior art keywords
- seq
- binding fragment
- hepatitis
- antigen binding
- humanized antibody
- Prior art date
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
- C07K16/10—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses from RNA viruses
- C07K16/1081—Togaviridae, e.g. flavivirus, rubella virus, hog cholera virus
- C07K16/109—Hepatitis C virus; Hepatitis G virus
-
- C07K16/118—
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/569—Immunoassay; Biospecific binding assay; Materials therefor for microorganisms, e.g. protozoa, bacteria, viruses
- G01N33/56983—Viruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/005—Assays involving biological materials from specific organisms or of a specific nature from viruses
- G01N2333/08—RNA viruses
- G01N2333/18—Togaviridae; Flaviviridae
- G01N2333/183—Flaviviridae, e.g. pestivirus, mucosal disease virus, bovine viral diarrhoea virus, classical swine fever virus (hog cholera virus) or border disease virus
- G01N2333/186—Hepatitis C; Hepatitis NANB
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/576—Immunoassay; Biospecific binding assay; Materials therefor for hepatitis
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Virology (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Immunology (AREA)
- Communicable Diseases (AREA)
- Genetics & Genomics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Biomedical Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Oncology (AREA)
- Biochemistry (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- Biophysics (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- General Engineering & Computer Science (AREA)
- Hematology (AREA)
- Physics & Mathematics (AREA)
- Urology & Nephrology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Food Science & Technology (AREA)
- Analytical Chemistry (AREA)
- General Physics & Mathematics (AREA)
- Pathology (AREA)
- Mycology (AREA)
- Epidemiology (AREA)
- Cell Biology (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US606607P | 2007-12-17 | 2007-12-17 | |
| US61/006,066 | 2007-12-17 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2010129429A RU2010129429A (ru) | 2012-01-27 |
| RU2462475C2 true RU2462475C2 (ru) | 2012-09-27 |
Family
ID=40801628
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2010129429/10A RU2462475C2 (ru) | 2007-12-17 | 2008-12-17 | Антитела против вируса гепатита с |
Country Status (18)
| Country | Link |
|---|---|
| US (1) | US9193781B2 (enExample) |
| EP (1) | EP2231704B1 (enExample) |
| JP (1) | JP5763344B2 (enExample) |
| KR (1) | KR20100102163A (enExample) |
| CN (1) | CN101939334B (enExample) |
| AR (1) | AR072039A1 (enExample) |
| AU (1) | AU2008341542B2 (enExample) |
| BR (1) | BRPI0821252A2 (enExample) |
| CA (1) | CA2708740C (enExample) |
| CL (1) | CL2008003784A1 (enExample) |
| IL (1) | IL205795A0 (enExample) |
| MX (1) | MX2010006767A (enExample) |
| NZ (1) | NZ585622A (enExample) |
| PE (1) | PE20091109A1 (enExample) |
| RU (1) | RU2462475C2 (enExample) |
| TW (1) | TW200940090A (enExample) |
| WO (1) | WO2009081285A2 (enExample) |
| ZA (1) | ZA201003509B (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2539770C1 (ru) * | 2013-12-27 | 2015-01-27 | Татьяна Николаевна Власик | Способ нейтрализации вируса гепатита с, полностью человеческое моноклональное антитело против вируса гепатита с (варианты), композиция полностью человеческих моноклональных антител против вируса гепатита с и гибридная мышь/человек клеточная линия - продуцент полностью человеческих моноклональных антител против вируса гепатита с (варианты) |
Families Citing this family (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US12010987B2 (en) | 2004-10-07 | 2024-06-18 | Transmedics, Inc. | Systems and methods for ex-vivo organ care and for using lactate as an indication of donor organ status |
| NZ717789A (en) | 2004-10-07 | 2017-09-29 | Transmedics Inc | Systems and methods for ex-vivo organ care |
| US9078428B2 (en) | 2005-06-28 | 2015-07-14 | Transmedics, Inc. | Systems, methods, compositions and solutions for perfusing an organ |
| US9457179B2 (en) | 2007-03-20 | 2016-10-04 | Transmedics, Inc. | Systems for monitoring and applying electrical currents in an organ perfusion system |
| US9462802B2 (en) | 2008-01-31 | 2016-10-11 | Transmedics, Inc. | Systems and methods for ex vivo lung care |
| US20120027722A1 (en) * | 2008-12-17 | 2012-02-02 | Medical Research Council | Hepatitis c virus combination therapy |
| ES2913104T3 (es) | 2011-04-14 | 2022-05-31 | Transmedics Inc | Solución de cuidado de órganos para perfusión en máquina ex-vivo de pulmones de donantes |
| CN103649316B (zh) | 2011-05-02 | 2017-07-28 | 约翰霍普金斯大学 | 合成的丙型肝炎基因组和制备及使用方法 |
| US20130084301A1 (en) * | 2011-08-30 | 2013-04-04 | Steven Foung | Cluster of Neutralizing Antibodies to Hepatitis C Virus |
| EP2788704B1 (en) * | 2011-12-09 | 2019-03-06 | Applied Materials, Inc. | Heat exchanger for cooling a heating tube and method thereof |
| ITFI20120122A1 (it) * | 2012-06-15 | 2013-12-16 | Kedrion S P A 50 | Anticorpi monoclonali capaci di legare la proteina virale e2 loro preparazione ed uso. |
| CN102928595A (zh) * | 2012-10-12 | 2013-02-13 | 武汉康苑生物医药科技有限公司 | 丙型肝炎病毒抗原抗体时间分辨免疫荧光分析法联合检测及检测试剂盒 |
| WO2014116749A1 (en) | 2013-01-23 | 2014-07-31 | Genentech, Inc. | Anti-hcv antibodies and methods of using thereof |
| RU2685185C2 (ru) | 2013-09-19 | 2019-04-16 | Новавакс, Инк. | Иммуногенные композиции коронавируса ближневосточного респираторного синдрома (mers-cov) и способы |
| CA3185937A1 (en) | 2014-06-02 | 2015-12-10 | Transmedics, Inc. | Ex vivo organ care system |
| GB201415714D0 (en) * | 2014-09-05 | 2014-10-22 | Medical Res Council | Antibodies and antigen binding fragments thereof |
| EP4591895A3 (en) | 2014-12-12 | 2025-11-12 | TransMedics, Inc. | Apparatus and method for organ perfusion |
| AU2016318622B2 (en) | 2015-09-09 | 2021-05-20 | Transmedics, Inc. | Aortic cannula for ex vivo organ care system |
| CN105219780B (zh) * | 2015-10-30 | 2020-06-02 | 中国人民解放军第三军医大学第一附属医院 | 抗多亚基因型hcv抗体基因r3-19及其应用 |
| CN105177017B (zh) * | 2015-10-30 | 2020-06-02 | 中国人民解放军第三军医大学第一附属医院 | 抗多亚基因型hcv抗体基因r4-85及其应用 |
| US10851160B2 (en) * | 2016-03-22 | 2020-12-01 | Institut National De La Sante Et De La Recherche Medicale | Humanized anti-claudin-1 antibodies and uses thereof |
| BR112018074537B1 (pt) | 2016-05-30 | 2023-04-11 | Tevosol, Inc | Método de ventilar um pulmão extraído |
| EP3559038B1 (en) | 2016-12-21 | 2022-12-14 | The United States of America as represented by The Secretary Department of Health and Human Services | Human monoclonal antibodies specific for flt3 and uses thereof |
| US10953089B1 (en) | 2020-01-27 | 2021-03-23 | Novavax, Inc. | Coronavirus vaccine formulations |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006100449A1 (en) * | 2005-03-19 | 2006-09-28 | Medical Research Council | Improvements in or relating to treatment and prevention of viral infections |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU659649B2 (en) * | 1991-06-10 | 1995-05-25 | Lucky Limited | Hepatitis C diagnostics and vaccines |
| ITPN960013A1 (it) * | 1996-02-26 | 1997-08-26 | Marcello Piazza | Farmaco atto a proteggere l'individuo dal contagio di epatite virale, ecc. |
| US20060188511A1 (en) * | 1998-11-05 | 2006-08-24 | Foung Steven K | Prevention and treatment of HCV infection employing antibodies directed against conformational and linear epitopes |
| JP2004524829A (ja) * | 2000-12-01 | 2004-08-19 | アメリカ合衆国 | C型肝炎ウイルスのe2糖タンパク質に特異的なモノクローナル抗体、ならびにc型肝炎の診断、治療、および予防におけるそれらの使用 |
| ITRM20020049A1 (it) * | 2002-01-30 | 2003-07-30 | Biostrands S R L | Frammenti fab di anticorpi monoclonali umani diretti contro la glicoproteina e2 di hcv e dotati di potere neutralizzante in vitro. |
-
2008
- 2008-12-17 WO PCT/IB2008/003952 patent/WO2009081285A2/en not_active Ceased
- 2008-12-17 CN CN200880125928.5A patent/CN101939334B/zh not_active Expired - Fee Related
- 2008-12-17 NZ NZ585622A patent/NZ585622A/xx not_active IP Right Cessation
- 2008-12-17 PE PE2008002086A patent/PE20091109A1/es not_active Application Discontinuation
- 2008-12-17 KR KR1020107015856A patent/KR20100102163A/ko not_active Ceased
- 2008-12-17 CL CL2008003784A patent/CL2008003784A1/es unknown
- 2008-12-17 AU AU2008341542A patent/AU2008341542B2/en not_active Ceased
- 2008-12-17 TW TW097149206A patent/TW200940090A/zh unknown
- 2008-12-17 JP JP2010538953A patent/JP5763344B2/ja not_active Expired - Fee Related
- 2008-12-17 CA CA2708740A patent/CA2708740C/en not_active Expired - Fee Related
- 2008-12-17 MX MX2010006767A patent/MX2010006767A/es not_active Application Discontinuation
- 2008-12-17 BR BRPI0821252-0A patent/BRPI0821252A2/pt not_active IP Right Cessation
- 2008-12-17 US US12/809,050 patent/US9193781B2/en not_active Expired - Fee Related
- 2008-12-17 RU RU2010129429/10A patent/RU2462475C2/ru not_active IP Right Cessation
- 2008-12-17 EP EP08865543.6A patent/EP2231704B1/en not_active Not-in-force
- 2008-12-18 AR ARP080105506A patent/AR072039A1/es not_active Application Discontinuation
-
2010
- 2010-05-16 IL IL205795A patent/IL205795A0/en unknown
- 2010-05-18 ZA ZA2010/03509A patent/ZA201003509B/en unknown
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006100449A1 (en) * | 2005-03-19 | 2006-09-28 | Medical Research Council | Improvements in or relating to treatment and prevention of viral infections |
Non-Patent Citations (1)
| Title |
|---|
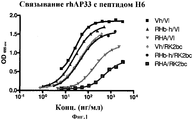
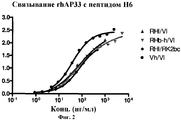
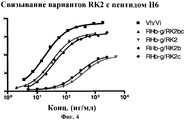
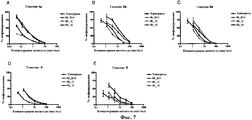
| TARR A.W. et al. Characterization of the hepatitis С virus E2 epitope defined by the broadly neutralizing monoclonal antibody AP33// Hepatology. - 2006 Mar; 43(3), стр.: 592-601. РОЙТ А. и др. Иммунология. - М.: Мир, 200, стр.110-111. * |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2539770C1 (ru) * | 2013-12-27 | 2015-01-27 | Татьяна Николаевна Власик | Способ нейтрализации вируса гепатита с, полностью человеческое моноклональное антитело против вируса гепатита с (варианты), композиция полностью человеческих моноклональных антител против вируса гепатита с и гибридная мышь/человек клеточная линия - продуцент полностью человеческих моноклональных антител против вируса гепатита с (варианты) |
| WO2015099574A1 (ru) * | 2013-12-27 | 2015-07-02 | Татьяна Николаевна ВЛАСИК | Способ нейтрализации вируса гепатита с, полностью человеческое моноклональное антитело против вируса гепатита с (варианты), композиция полностью человеческих моноклональных антител против вируса гепатита с и гибридная мышь/человек клеточная линия-продуцент полностью человеческих моноклональных антител против вируса гепатита с (варианты) |
Also Published As
| Publication number | Publication date |
|---|---|
| US9193781B2 (en) | 2015-11-24 |
| KR20100102163A (ko) | 2010-09-20 |
| MX2010006767A (es) | 2010-10-05 |
| PE20091109A1 (es) | 2009-08-12 |
| EP2231704B1 (en) | 2015-08-12 |
| RU2010129429A (ru) | 2012-01-27 |
| TW200940090A (en) | 2009-10-01 |
| CA2708740C (en) | 2017-07-18 |
| AU2008341542A1 (en) | 2009-07-02 |
| WO2009081285A2 (en) | 2009-07-02 |
| AU2008341542B2 (en) | 2014-12-04 |
| CL2008003784A1 (es) | 2009-08-14 |
| NZ585622A (en) | 2012-10-26 |
| JP2011505867A (ja) | 2011-03-03 |
| CN101939334B (zh) | 2015-01-14 |
| IL205795A0 (en) | 2010-11-30 |
| AR072039A1 (es) | 2010-08-04 |
| JP5763344B2 (ja) | 2015-08-12 |
| HK1144440A1 (en) | 2011-02-18 |
| WO2009081285A3 (en) | 2010-08-26 |
| US20110002926A1 (en) | 2011-01-06 |
| CN101939334A (zh) | 2011-01-05 |
| ZA201003509B (en) | 2011-12-28 |
| EP2231704A2 (en) | 2010-09-29 |
| BRPI0821252A2 (pt) | 2015-06-16 |
| CA2708740A1 (en) | 2009-07-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2462475C2 (ru) | Антитела против вируса гепатита с | |
| JP2011505867A5 (enExample) | ||
| US8513391B2 (en) | Monoclonal antibodies for Ebola and Marburg viruses | |
| HRP20140316T1 (hr) | Humana antitijela protiv virusa hepatitisa c (hcv) i njihova uporaba | |
| RU2019132843A (ru) | Антитело против в7-н3, его антигенсвязывающий фрагмент и их медицинское применение | |
| RU2011105062A (ru) | Нейтрализующие антитела против вируса гриппа а и их использование | |
| RU2010127156A (ru) | Антитела против вируса гриппа и их применение | |
| RU2014151788A (ru) | Молекула, специфически связывающаяся с rsv | |
| RU2016100892A (ru) | Антитела против tweakr и их применение | |
| RU2012153968A (ru) | Композиции и способы лечения инфекций и опухолей | |
| CO6180431A2 (es) | Anticuerpos e inmunoconjugados y sus usos | |
| RU2009144280A (ru) | АНТАГОНИСТЫ CRIg | |
| JP2011527899A5 (enExample) | ||
| RU2014150548A (ru) | Терапевтическое средство от зуда | |
| JP2018520334A5 (enExample) | ||
| RU2014127287A (ru) | Антитела, используемые для пассивной вакцинации против гриппа | |
| RU2020100073A (ru) | Нейтрализующие антитела к вирусу гриппа b и пути их применения | |
| PE20230384A1 (es) | Anticuerpos cd163 o proteinas de union | |
| CN118165106A (zh) | 抗犬il-31单克隆抗体及其应用 | |
| IL277899B1 (en) | Methods and compositions for treating yellow fever | |
| CN114805579B (zh) | 一种抗人ace2蛋白单克隆抗体、核酸分子及应用 | |
| CN118085082A (zh) | 抗猫TNF-α单克隆抗体及其应用 | |
| CN117603346A (zh) | 全人源新型冠状病毒单克隆抗体及其应用 | |
| CN117924481A (zh) | 抗犬il-31单克隆抗体及其应用 | |
| WO2023019174A4 (en) | Antibodies to sars-cov-2 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20121218 |