KR20200074214A - 암을 치료하는데 사용하기 위한 면역자극 효능작용 항체 - Google Patents
암을 치료하는데 사용하기 위한 면역자극 효능작용 항체 Download PDFInfo
- Publication number
- KR20200074214A KR20200074214A KR1020207015641A KR20207015641A KR20200074214A KR 20200074214 A KR20200074214 A KR 20200074214A KR 1020207015641 A KR1020207015641 A KR 1020207015641A KR 20207015641 A KR20207015641 A KR 20207015641A KR 20200074214 A KR20200074214 A KR 20200074214A
- Authority
- KR
- South Korea
- Prior art keywords
- antibody
- cancer
- agonistic
- receptor
- administered
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 206010028980 Neoplasm Diseases 0.000 title claims abstract description 284
- 201000011510 cancer Diseases 0.000 title claims abstract description 114
- 102000005962 receptors Human genes 0.000 claims abstract description 276
- 108020003175 receptors Proteins 0.000 claims abstract description 276
- 230000001270 agonistic effect Effects 0.000 claims abstract description 247
- 102100022153 Tumor necrosis factor receptor superfamily member 4 Human genes 0.000 claims abstract description 197
- 101710165473 Tumor necrosis factor receptor superfamily member 4 Proteins 0.000 claims abstract description 193
- 238000000034 method Methods 0.000 claims abstract description 190
- 230000003308 immunostimulating effect Effects 0.000 claims abstract description 146
- 238000012544 monitoring process Methods 0.000 claims abstract description 5
- 210000001744 T-lymphocyte Anatomy 0.000 claims description 97
- 238000011282 treatment Methods 0.000 claims description 81
- 230000027455 binding Effects 0.000 claims description 54
- 239000000556 agonist Substances 0.000 claims description 46
- 102100040678 Programmed cell death protein 1 Human genes 0.000 claims description 45
- -1 LIGHT Proteins 0.000 claims description 41
- 229940045513 CTLA4 antagonist Drugs 0.000 claims description 38
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 38
- 108060008683 Tumor Necrosis Factor Receptor Proteins 0.000 claims description 37
- 102000003298 tumor necrosis factor receptor Human genes 0.000 claims description 37
- 201000010099 disease Diseases 0.000 claims description 35
- 101710089372 Programmed cell death protein 1 Proteins 0.000 claims description 31
- 230000001965 increasing effect Effects 0.000 claims description 29
- 230000004614 tumor growth Effects 0.000 claims description 29
- 238000003556 assay Methods 0.000 claims description 28
- 108010074328 Interferon-gamma Proteins 0.000 claims description 25
- 230000001225 therapeutic effect Effects 0.000 claims description 24
- 230000028993 immune response Effects 0.000 claims description 23
- 102100037850 Interferon gamma Human genes 0.000 claims description 22
- 206010025323 Lymphomas Diseases 0.000 claims description 22
- 230000004044 response Effects 0.000 claims description 20
- 108010021064 CTLA-4 Antigen Proteins 0.000 claims description 19
- 206010009944 Colon cancer Diseases 0.000 claims description 19
- 238000011374 additional therapy Methods 0.000 claims description 19
- 206010033128 Ovarian cancer Diseases 0.000 claims description 18
- 108010074708 B7-H1 Antigen Proteins 0.000 claims description 17
- 206010061535 Ovarian neoplasm Diseases 0.000 claims description 17
- 230000002401 inhibitory effect Effects 0.000 claims description 17
- 208000001333 Colorectal Neoplasms Diseases 0.000 claims description 16
- 108091008034 costimulatory receptors Proteins 0.000 claims description 16
- 230000002829 reductive effect Effects 0.000 claims description 16
- 101150013553 CD40 gene Proteins 0.000 claims description 15
- 108010002350 Interleukin-2 Proteins 0.000 claims description 15
- 102100040245 Tumor necrosis factor receptor superfamily member 5 Human genes 0.000 claims description 15
- 230000007423 decrease Effects 0.000 claims description 15
- 239000008194 pharmaceutical composition Substances 0.000 claims description 15
- 102000017578 LAG3 Human genes 0.000 claims description 14
- 101150030213 Lag3 gene Proteins 0.000 claims description 14
- 210000003289 regulatory T cell Anatomy 0.000 claims description 13
- 206010035226 Plasma cell myeloma Diseases 0.000 claims description 12
- 206010005003 Bladder cancer Diseases 0.000 claims description 11
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 claims description 11
- 208000032839 leukemia Diseases 0.000 claims description 11
- 201000005112 urinary bladder cancer Diseases 0.000 claims description 11
- 238000010171 animal model Methods 0.000 claims description 10
- 230000009467 reduction Effects 0.000 claims description 10
- 206010008342 Cervix carcinoma Diseases 0.000 claims description 9
- 101000851376 Homo sapiens Tumor necrosis factor receptor superfamily member 8 Proteins 0.000 claims description 9
- 208000006265 Renal cell carcinoma Diseases 0.000 claims description 9
- 102100036857 Tumor necrosis factor receptor superfamily member 8 Human genes 0.000 claims description 9
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 claims description 9
- 201000010881 cervical cancer Diseases 0.000 claims description 9
- 201000010536 head and neck cancer Diseases 0.000 claims description 9
- 208000014829 head and neck neoplasm Diseases 0.000 claims description 9
- 238000004519 manufacturing process Methods 0.000 claims description 9
- 201000000050 myeloid neoplasm Diseases 0.000 claims description 9
- 230000004936 stimulating effect Effects 0.000 claims description 9
- 210000004881 tumor cell Anatomy 0.000 claims description 9
- 238000001990 intravenous administration Methods 0.000 claims description 8
- 102100025237 T-cell surface antigen CD2 Human genes 0.000 claims description 7
- 208000024313 Testicular Neoplasms Diseases 0.000 claims description 7
- 239000000090 biomarker Substances 0.000 claims description 7
- 208000002154 non-small cell lung carcinoma Diseases 0.000 claims description 7
- 201000003120 testicular cancer Diseases 0.000 claims description 7
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 claims description 7
- 101000795169 Homo sapiens Tumor necrosis factor receptor superfamily member 13C Proteins 0.000 claims description 6
- 102100025390 Integrin beta-2 Human genes 0.000 claims description 6
- 108010061593 Member 14 Tumor Necrosis Factor Receptors Proteins 0.000 claims description 6
- 206010038389 Renal cancer Diseases 0.000 claims description 6
- 206010039491 Sarcoma Diseases 0.000 claims description 6
- 206010057644 Testis cancer Diseases 0.000 claims description 6
- 102100029690 Tumor necrosis factor receptor superfamily member 13C Human genes 0.000 claims description 6
- 102100028785 Tumor necrosis factor receptor superfamily member 14 Human genes 0.000 claims description 6
- 230000001394 metastastic effect Effects 0.000 claims description 6
- 206010061289 metastatic neoplasm Diseases 0.000 claims description 6
- 102100024263 CD160 antigen Human genes 0.000 claims description 5
- 101000761938 Homo sapiens CD160 antigen Proteins 0.000 claims description 5
- 101000935040 Homo sapiens Integrin beta-2 Proteins 0.000 claims description 5
- 101000599852 Homo sapiens Intercellular adhesion molecule 1 Proteins 0.000 claims description 5
- 101000971538 Homo sapiens Killer cell lectin-like receptor subfamily F member 1 Proteins 0.000 claims description 5
- 101001109503 Homo sapiens NKG2-C type II integral membrane protein Proteins 0.000 claims description 5
- 101000633784 Homo sapiens SLAM family member 7 Proteins 0.000 claims description 5
- 101000934346 Homo sapiens T-cell surface antigen CD2 Proteins 0.000 claims description 5
- 102100037877 Intercellular adhesion molecule 1 Human genes 0.000 claims description 5
- 208000008839 Kidney Neoplasms Diseases 0.000 claims description 5
- 102100021458 Killer cell lectin-like receptor subfamily F member 1 Human genes 0.000 claims description 5
- 206010058467 Lung neoplasm malignant Diseases 0.000 claims description 5
- 108010064548 Lymphocyte Function-Associated Antigen-1 Proteins 0.000 claims description 5
- 102100022683 NKG2-C type II integral membrane protein Human genes 0.000 claims description 5
- 102100029198 SLAM family member 7 Human genes 0.000 claims description 5
- 102100027208 T-cell antigen CD7 Human genes 0.000 claims description 5
- 201000010982 kidney cancer Diseases 0.000 claims description 5
- 201000005202 lung cancer Diseases 0.000 claims description 5
- 208000020816 lung neoplasm Diseases 0.000 claims description 5
- 206010060862 Prostate cancer Diseases 0.000 claims description 4
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 4
- 208000005718 Stomach Neoplasms Diseases 0.000 claims description 4
- 208000024770 Thyroid neoplasm Diseases 0.000 claims description 4
- 241000700605 Viruses Species 0.000 claims description 4
- 230000000779 depleting effect Effects 0.000 claims description 4
- 206010017758 gastric cancer Diseases 0.000 claims description 4
- 230000036961 partial effect Effects 0.000 claims description 4
- 201000011549 stomach cancer Diseases 0.000 claims description 4
- 201000002510 thyroid cancer Diseases 0.000 claims description 4
- 206010005949 Bone cancer Diseases 0.000 claims description 3
- 208000018084 Bone neoplasm Diseases 0.000 claims description 3
- 206010006187 Breast cancer Diseases 0.000 claims description 3
- 208000026310 Breast neoplasm Diseases 0.000 claims description 3
- 208000000461 Esophageal Neoplasms Diseases 0.000 claims description 3
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 claims description 3
- 101000914496 Homo sapiens T-cell antigen CD7 Proteins 0.000 claims description 3
- 208000034176 Neoplasms, Germ Cell and Embryonal Diseases 0.000 claims description 3
- 206010030155 Oesophageal carcinoma Diseases 0.000 claims description 3
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims description 3
- 208000000453 Skin Neoplasms Diseases 0.000 claims description 3
- 208000002495 Uterine Neoplasms Diseases 0.000 claims description 3
- 208000029742 colonic neoplasm Diseases 0.000 claims description 3
- 201000004101 esophageal cancer Diseases 0.000 claims description 3
- 201000007270 liver cancer Diseases 0.000 claims description 3
- 208000014018 liver neoplasm Diseases 0.000 claims description 3
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims description 3
- 201000002528 pancreatic cancer Diseases 0.000 claims description 3
- 208000008443 pancreatic carcinoma Diseases 0.000 claims description 3
- 201000000849 skin cancer Diseases 0.000 claims description 3
- 150000003384 small molecules Chemical class 0.000 claims description 3
- 238000011269 treatment regimen Methods 0.000 claims description 3
- 206010046766 uterine cancer Diseases 0.000 claims description 3
- 102000008203 CTLA-4 Antigen Human genes 0.000 claims description 2
- 206010038111 Recurrent cancer Diseases 0.000 claims description 2
- 206010070308 Refractory cancer Diseases 0.000 claims description 2
- 208000025997 central nervous system neoplasm Diseases 0.000 claims description 2
- 201000003115 germ cell cancer Diseases 0.000 claims description 2
- 208000037819 metastatic cancer Diseases 0.000 claims description 2
- 208000011575 metastatic malignant neoplasm Diseases 0.000 claims description 2
- 208000016691 refractory malignant neoplasm Diseases 0.000 claims description 2
- 208000015347 renal cell adenocarcinoma Diseases 0.000 claims description 2
- 102000008096 B7-H1 Antigen Human genes 0.000 claims 1
- 230000003902 lesion Effects 0.000 claims 1
- 230000009870 specific binding Effects 0.000 abstract description 2
- 210000004027 cell Anatomy 0.000 description 85
- 230000000694 effects Effects 0.000 description 65
- 229960003301 nivolumab Drugs 0.000 description 58
- 239000003814 drug Substances 0.000 description 56
- 241000699666 Mus <mouse, genus> Species 0.000 description 55
- 230000014509 gene expression Effects 0.000 description 52
- 241000699670 Mus sp. Species 0.000 description 51
- 239000000427 antigen Substances 0.000 description 50
- 108091007433 antigens Proteins 0.000 description 50
- 102000036639 antigens Human genes 0.000 description 50
- 229940079593 drug Drugs 0.000 description 43
- 239000003795 chemical substances by application Substances 0.000 description 42
- 238000002648 combination therapy Methods 0.000 description 41
- 238000009097 single-agent therapy Methods 0.000 description 40
- 229960005386 ipilimumab Drugs 0.000 description 34
- 230000000259 anti-tumor effect Effects 0.000 description 33
- 239000000203 mixture Substances 0.000 description 33
- 210000004369 blood Anatomy 0.000 description 31
- 239000008280 blood Substances 0.000 description 31
- 230000006044 T cell activation Effects 0.000 description 26
- 239000003446 ligand Substances 0.000 description 26
- 238000002560 therapeutic procedure Methods 0.000 description 25
- 230000037396 body weight Effects 0.000 description 24
- 230000000903 blocking effect Effects 0.000 description 23
- 101000611183 Homo sapiens Tumor necrosis factor Proteins 0.000 description 22
- 102100040247 Tumor necrosis factor Human genes 0.000 description 22
- 230000005764 inhibitory process Effects 0.000 description 22
- 239000000523 sample Substances 0.000 description 22
- 150000001413 amino acids Chemical group 0.000 description 21
- 238000000338 in vitro Methods 0.000 description 21
- 101000611936 Homo sapiens Programmed cell death protein 1 Proteins 0.000 description 20
- 239000005557 antagonist Substances 0.000 description 20
- 102000004127 Cytokines Human genes 0.000 description 19
- 108090000695 Cytokines Proteins 0.000 description 19
- 102100039498 Cytotoxic T-lymphocyte protein 4 Human genes 0.000 description 19
- 102100024216 Programmed cell death 1 ligand 1 Human genes 0.000 description 19
- 150000001875 compounds Chemical class 0.000 description 19
- 239000012636 effector Substances 0.000 description 19
- 230000001404 mediated effect Effects 0.000 description 19
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 18
- 108090000623 proteins and genes Proteins 0.000 description 18
- 210000001266 CD8-positive T-lymphocyte Anatomy 0.000 description 17
- 108060003951 Immunoglobulin Proteins 0.000 description 17
- 102000018358 immunoglobulin Human genes 0.000 description 17
- 101000679851 Homo sapiens Tumor necrosis factor receptor superfamily member 4 Proteins 0.000 description 16
- 101100112922 Candida albicans CDR3 gene Proteins 0.000 description 15
- 241000880493 Leptailurus serval Species 0.000 description 15
- 230000003042 antagnostic effect Effects 0.000 description 15
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 14
- 102000000588 Interleukin-2 Human genes 0.000 description 14
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 14
- 235000001014 amino acid Nutrition 0.000 description 14
- 239000012634 fragment Substances 0.000 description 14
- 230000002093 peripheral effect Effects 0.000 description 14
- 229940024606 amino acid Drugs 0.000 description 13
- 230000006870 function Effects 0.000 description 13
- 235000018102 proteins Nutrition 0.000 description 13
- 102000004169 proteins and genes Human genes 0.000 description 13
- 206010027476 Metastases Diseases 0.000 description 12
- 102000050320 human TNFRSF4 Human genes 0.000 description 12
- 230000009401 metastasis Effects 0.000 description 12
- 210000002966 serum Anatomy 0.000 description 12
- 230000006052 T cell proliferation Effects 0.000 description 11
- YOOAQCZYZHGUAZ-KATARQTJSA-N Thr-Leu-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YOOAQCZYZHGUAZ-KATARQTJSA-N 0.000 description 11
- 238000000684 flow cytometry Methods 0.000 description 11
- 230000028327 secretion Effects 0.000 description 11
- 230000008685 targeting Effects 0.000 description 11
- 238000001802 infusion Methods 0.000 description 10
- 150000003839 salts Chemical class 0.000 description 10
- 241000894007 species Species 0.000 description 10
- 208000024891 symptom Diseases 0.000 description 10
- 238000012360 testing method Methods 0.000 description 10
- 208000003950 B-cell lymphoma Diseases 0.000 description 9
- 108010087819 Fc receptors Proteins 0.000 description 9
- 102000009109 Fc receptors Human genes 0.000 description 9
- OYTPNWYZORARHL-XHNCKOQMSA-N Gln-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)N)N OYTPNWYZORARHL-XHNCKOQMSA-N 0.000 description 9
- 108010065920 Insulin Lispro Proteins 0.000 description 9
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 9
- 102000004473 OX40 Ligand Human genes 0.000 description 9
- 108010042215 OX40 Ligand Proteins 0.000 description 9
- 206010042971 T-cell lymphoma Diseases 0.000 description 9
- 238000004458 analytical method Methods 0.000 description 9
- 230000000875 corresponding effect Effects 0.000 description 9
- 238000010790 dilution Methods 0.000 description 9
- 239000012895 dilution Substances 0.000 description 9
- 230000003828 downregulation Effects 0.000 description 9
- 239000003112 inhibitor Substances 0.000 description 9
- 238000002347 injection Methods 0.000 description 9
- 239000007924 injection Substances 0.000 description 9
- 230000002062 proliferating effect Effects 0.000 description 9
- 230000035755 proliferation Effects 0.000 description 9
- 229920006395 saturated elastomer Polymers 0.000 description 9
- 229940124597 therapeutic agent Drugs 0.000 description 9
- 241000282693 Cercopithecidae Species 0.000 description 8
- SZQCDCKIGWQAQN-FXQIFTODSA-N Cys-Arg-Ala Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(O)=O SZQCDCKIGWQAQN-FXQIFTODSA-N 0.000 description 8
- 238000002965 ELISA Methods 0.000 description 8
- 102000008166 Member 25 Tumor Necrosis Factor Receptors Human genes 0.000 description 8
- 108010060408 Member 25 Tumor Necrosis Factor Receptors Proteins 0.000 description 8
- 229930012538 Paclitaxel Natural products 0.000 description 8
- 102100024213 Programmed cell death 1 ligand 2 Human genes 0.000 description 8
- 208000027585 T-cell non-Hodgkin lymphoma Diseases 0.000 description 8
- 230000004913 activation Effects 0.000 description 8
- 239000002246 antineoplastic agent Substances 0.000 description 8
- 231100000673 dose–response relationship Toxicity 0.000 description 8
- 239000003937 drug carrier Substances 0.000 description 8
- 210000004602 germ cell Anatomy 0.000 description 8
- 230000001506 immunosuppresive effect Effects 0.000 description 8
- 229960001592 paclitaxel Drugs 0.000 description 8
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 8
- 102100027207 CD27 antigen Human genes 0.000 description 7
- 102100032937 CD40 ligand Human genes 0.000 description 7
- 101000914511 Homo sapiens CD27 antigen Proteins 0.000 description 7
- 101000801234 Homo sapiens Tumor necrosis factor receptor superfamily member 18 Proteins 0.000 description 7
- 102100022339 Integrin alpha-L Human genes 0.000 description 7
- 101100407308 Mus musculus Pdcd1lg2 gene Proteins 0.000 description 7
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 7
- 108700030875 Programmed Cell Death 1 Ligand 2 Proteins 0.000 description 7
- QYSFWUIXDFJUDW-DCAQKATOSA-N Ser-Leu-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O QYSFWUIXDFJUDW-DCAQKATOSA-N 0.000 description 7
- 102100033728 Tumor necrosis factor receptor superfamily member 18 Human genes 0.000 description 7
- 102100022205 Tumor necrosis factor receptor superfamily member 21 Human genes 0.000 description 7
- 101710187751 Tumor necrosis factor receptor superfamily member 21 Proteins 0.000 description 7
- 239000004480 active ingredient Substances 0.000 description 7
- 230000008859 change Effects 0.000 description 7
- 238000002512 chemotherapy Methods 0.000 description 7
- 230000002596 correlated effect Effects 0.000 description 7
- 238000004132 cross linking Methods 0.000 description 7
- 238000011161 development Methods 0.000 description 7
- 230000018109 developmental process Effects 0.000 description 7
- 238000010494 dissociation reaction Methods 0.000 description 7
- 230000005593 dissociations Effects 0.000 description 7
- 238000011156 evaluation Methods 0.000 description 7
- XKUKSGPZAADMRA-UHFFFAOYSA-N glycyl-glycyl-glycine Natural products NCC(=O)NCC(=O)NCC(O)=O XKUKSGPZAADMRA-UHFFFAOYSA-N 0.000 description 7
- 210000000987 immune system Anatomy 0.000 description 7
- 229940045207 immuno-oncology agent Drugs 0.000 description 7
- 239000002584 immunological anticancer agent Substances 0.000 description 7
- 238000001727 in vivo Methods 0.000 description 7
- 210000000822 natural killer cell Anatomy 0.000 description 7
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 7
- 108090000765 processed proteins & peptides Proteins 0.000 description 7
- 238000007920 subcutaneous administration Methods 0.000 description 7
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 7
- 108010044292 tryptophyltyrosine Proteins 0.000 description 7
- 102100032912 CD44 antigen Human genes 0.000 description 6
- 101100505161 Caenorhabditis elegans mel-32 gene Proteins 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- 208000002250 Hematologic Neoplasms Diseases 0.000 description 6
- 241000282412 Homo Species 0.000 description 6
- 101000868273 Homo sapiens CD44 antigen Proteins 0.000 description 6
- HYIFFZAQXPUEAU-QWRGUYRKSA-N Leu-Gly-Leu Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC(C)C HYIFFZAQXPUEAU-QWRGUYRKSA-N 0.000 description 6
- SBANPBVRHYIMRR-UHFFFAOYSA-N Leu-Ser-Pro Natural products CC(C)CC(N)C(=O)NC(CO)C(=O)N1CCCC1C(O)=O SBANPBVRHYIMRR-UHFFFAOYSA-N 0.000 description 6
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 6
- FQPDRTDDEZXCEC-SVSWQMSJSA-N Thr-Ile-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O FQPDRTDDEZXCEC-SVSWQMSJSA-N 0.000 description 6
- HTONZBWRYUKUKC-RCWTZXSCSA-N Val-Thr-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O HTONZBWRYUKUKC-RCWTZXSCSA-N 0.000 description 6
- 239000002253 acid Substances 0.000 description 6
- 238000007792 addition Methods 0.000 description 6
- 108010069020 alanyl-prolyl-glycine Proteins 0.000 description 6
- 108010047495 alanylglycine Proteins 0.000 description 6
- 238000009175 antibody therapy Methods 0.000 description 6
- 238000013459 approach Methods 0.000 description 6
- 108010060035 arginylproline Proteins 0.000 description 6
- 210000003719 b-lymphocyte Anatomy 0.000 description 6
- 229940127089 cytotoxic agent Drugs 0.000 description 6
- 230000003111 delayed effect Effects 0.000 description 6
- 230000001419 dependent effect Effects 0.000 description 6
- 238000002474 experimental method Methods 0.000 description 6
- 108010010147 glycylglutamine Proteins 0.000 description 6
- 102000048362 human PDCD1 Human genes 0.000 description 6
- 210000002865 immune cell Anatomy 0.000 description 6
- 108091008042 inhibitory receptors Proteins 0.000 description 6
- 230000037361 pathway Effects 0.000 description 6
- 230000002265 prevention Effects 0.000 description 6
- 108010090894 prolylleucine Proteins 0.000 description 6
- 210000001519 tissue Anatomy 0.000 description 6
- ZDILXFDENZVOTL-BPNCWPANSA-N Ala-Val-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ZDILXFDENZVOTL-BPNCWPANSA-N 0.000 description 5
- JSHWXQIZOCVWIA-ZKWXMUAHSA-N Asp-Ser-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O JSHWXQIZOCVWIA-ZKWXMUAHSA-N 0.000 description 5
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 5
- 102100027581 Forkhead box protein P3 Human genes 0.000 description 5
- FQCILXROGNOZON-YUMQZZPRSA-N Gln-Pro-Gly Chemical compound NC(=O)CC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O FQCILXROGNOZON-YUMQZZPRSA-N 0.000 description 5
- AVZHGSCDKIQZPQ-CIUDSAMLSA-N Glu-Arg-Ala Chemical compound C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCC(O)=O)C(O)=O AVZHGSCDKIQZPQ-CIUDSAMLSA-N 0.000 description 5
- INGJLBQKTRJLFO-UKJIMTQDSA-N Glu-Ile-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H](N)CCC(O)=O INGJLBQKTRJLFO-UKJIMTQDSA-N 0.000 description 5
- PDUHNKAFQXQNLH-ZETCQYMHSA-N Gly-Lys-Gly Chemical compound NCCCC[C@H](NC(=O)CN)C(=O)NCC(O)=O PDUHNKAFQXQNLH-ZETCQYMHSA-N 0.000 description 5
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 description 5
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 5
- 101001117317 Homo sapiens Programmed cell death 1 ligand 1 Proteins 0.000 description 5
- 101000610605 Homo sapiens Tumor necrosis factor receptor superfamily member 10A Proteins 0.000 description 5
- 101000610604 Homo sapiens Tumor necrosis factor receptor superfamily member 10B Proteins 0.000 description 5
- 101000795167 Homo sapiens Tumor necrosis factor receptor superfamily member 13B Proteins 0.000 description 5
- 101000851370 Homo sapiens Tumor necrosis factor receptor superfamily member 9 Proteins 0.000 description 5
- 102100026878 Interleukin-2 receptor subunit alpha Human genes 0.000 description 5
- PMGDADKJMCOXHX-UHFFFAOYSA-N L-Arginyl-L-glutamin-acetat Natural products NC(=N)NCCCC(N)C(=O)NC(CCC(N)=O)C(O)=O PMGDADKJMCOXHX-UHFFFAOYSA-N 0.000 description 5
- FEHQLKKBVJHSEC-SZMVWBNQSA-N Leu-Glu-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC(C)C)C(O)=O)=CNC2=C1 FEHQLKKBVJHSEC-SZMVWBNQSA-N 0.000 description 5
- LCNASHSOFMRYFO-WDCWCFNPSA-N Leu-Thr-Gln Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCC(N)=O LCNASHSOFMRYFO-WDCWCFNPSA-N 0.000 description 5
- FMDHKPRACUXATF-ACZMJKKPSA-N Ser-Gln-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O FMDHKPRACUXATF-ACZMJKKPSA-N 0.000 description 5
- YMTLKLXDFCSCNX-BYPYZUCNSA-N Ser-Gly-Gly Chemical compound OC[C@H](N)C(=O)NCC(=O)NCC(O)=O YMTLKLXDFCSCNX-BYPYZUCNSA-N 0.000 description 5
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 5
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 5
- MBLJBGZWLHTJBH-SZMVWBNQSA-N Trp-Val-Arg Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 MBLJBGZWLHTJBH-SZMVWBNQSA-N 0.000 description 5
- 102100040113 Tumor necrosis factor receptor superfamily member 10A Human genes 0.000 description 5
- 102100040112 Tumor necrosis factor receptor superfamily member 10B Human genes 0.000 description 5
- 102100029675 Tumor necrosis factor receptor superfamily member 13B Human genes 0.000 description 5
- 102100036856 Tumor necrosis factor receptor superfamily member 9 Human genes 0.000 description 5
- QOIKZODVIPOPDD-AVGNSLFASA-N Tyr-Cys-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(O)=O QOIKZODVIPOPDD-AVGNSLFASA-N 0.000 description 5
- PZTZYZUTCPZWJH-FXQIFTODSA-N Val-Ser-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)O)N PZTZYZUTCPZWJH-FXQIFTODSA-N 0.000 description 5
- 230000003213 activating effect Effects 0.000 description 5
- 108010008685 alanyl-glutamyl-aspartic acid Proteins 0.000 description 5
- 230000030741 antigen processing and presentation Effects 0.000 description 5
- 108010008355 arginyl-glutamine Proteins 0.000 description 5
- 108010069205 aspartyl-phenylalanine Proteins 0.000 description 5
- 238000005119 centrifugation Methods 0.000 description 5
- 238000006243 chemical reaction Methods 0.000 description 5
- 238000011284 combination treatment Methods 0.000 description 5
- 230000003247 decreasing effect Effects 0.000 description 5
- 239000006185 dispersion Substances 0.000 description 5
- 238000012377 drug delivery Methods 0.000 description 5
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 5
- 108010067216 glycyl-glycyl-glycine Proteins 0.000 description 5
- 201000005787 hematologic cancer Diseases 0.000 description 5
- 208000024200 hematopoietic and lymphoid system neoplasm Diseases 0.000 description 5
- 102000048776 human CD274 Human genes 0.000 description 5
- 229940072221 immunoglobulins Drugs 0.000 description 5
- 238000009169 immunotherapy Methods 0.000 description 5
- 230000006872 improvement Effects 0.000 description 5
- 238000011534 incubation Methods 0.000 description 5
- 230000003993 interaction Effects 0.000 description 5
- 238000007918 intramuscular administration Methods 0.000 description 5
- 201000011649 lymphoblastic lymphoma Diseases 0.000 description 5
- 230000007246 mechanism Effects 0.000 description 5
- 201000001441 melanoma Diseases 0.000 description 5
- 210000003071 memory t lymphocyte Anatomy 0.000 description 5
- 238000012986 modification Methods 0.000 description 5
- 230000004048 modification Effects 0.000 description 5
- 210000005259 peripheral blood Anatomy 0.000 description 5
- 239000011886 peripheral blood Substances 0.000 description 5
- 102000004196 processed proteins & peptides Human genes 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- 206010041823 squamous cell carcinoma Diseases 0.000 description 5
- 239000006228 supernatant Substances 0.000 description 5
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 5
- 238000005406 washing Methods 0.000 description 5
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 4
- BGFTWECWAICPDG-UHFFFAOYSA-N 2-[bis(4-chlorophenyl)methyl]-4-n-[3-[bis(4-chlorophenyl)methyl]-4-(dimethylamino)phenyl]-1-n,1-n-dimethylbenzene-1,4-diamine Chemical compound C1=C(C(C=2C=CC(Cl)=CC=2)C=2C=CC(Cl)=CC=2)C(N(C)C)=CC=C1NC(C=1)=CC=C(N(C)C)C=1C(C=1C=CC(Cl)=CC=1)C1=CC=C(Cl)C=C1 BGFTWECWAICPDG-UHFFFAOYSA-N 0.000 description 4
- 208000031261 Acute myeloid leukaemia Diseases 0.000 description 4
- 102100022089 Acyl-[acyl-carrier-protein] hydrolase Human genes 0.000 description 4
- WNHNMKOFKCHKKD-BFHQHQDPSA-N Ala-Thr-Gly Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(O)=O WNHNMKOFKCHKKD-BFHQHQDPSA-N 0.000 description 4
- YBZMTKUDWXZLIX-UWVGGRQHSA-N Arg-Leu-Gly Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O YBZMTKUDWXZLIX-UWVGGRQHSA-N 0.000 description 4
- AOHKLEBWKMKITA-IHRRRGAJSA-N Arg-Phe-Ser Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N AOHKLEBWKMKITA-IHRRRGAJSA-N 0.000 description 4
- ANRZCQXIXGDXLR-CWRNSKLLSA-N Asn-Trp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CNC3=CC=CC=C32)NC(=O)[C@H](CC(=O)N)N)C(=O)O ANRZCQXIXGDXLR-CWRNSKLLSA-N 0.000 description 4
- 108010008014 B-Cell Maturation Antigen Proteins 0.000 description 4
- 102000006942 B-Cell Maturation Antigen Human genes 0.000 description 4
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 4
- 101000964894 Bos taurus 14-3-3 protein zeta/delta Proteins 0.000 description 4
- 102100038078 CD276 antigen Human genes 0.000 description 4
- 108010029697 CD40 Ligand Proteins 0.000 description 4
- XMVZMBGFIOQONW-GARJFASQSA-N Cys-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CS)N)C(=O)O XMVZMBGFIOQONW-GARJFASQSA-N 0.000 description 4
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 4
- 102000004190 Enzymes Human genes 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 4
- 229920001917 Ficoll Polymers 0.000 description 4
- JXFLPKSDLDEOQK-JHEQGTHGSA-N Gln-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCC(N)=O JXFLPKSDLDEOQK-JHEQGTHGSA-N 0.000 description 4
- PAQUJCSYVIBPLC-AVGNSLFASA-N Glu-Asp-Phe Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 PAQUJCSYVIBPLC-AVGNSLFASA-N 0.000 description 4
- NVTPVQLIZCOJFK-FOHZUACHSA-N Gly-Thr-Asp Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O NVTPVQLIZCOJFK-FOHZUACHSA-N 0.000 description 4
- 102100034459 Hepatitis A virus cellular receptor 1 Human genes 0.000 description 4
- 101000824278 Homo sapiens Acyl-[acyl-carrier-protein] hydrolase Proteins 0.000 description 4
- 101000889276 Homo sapiens Cytotoxic T-lymphocyte protein 4 Proteins 0.000 description 4
- 101000861452 Homo sapiens Forkhead box protein P3 Proteins 0.000 description 4
- 101000610602 Homo sapiens Tumor necrosis factor receptor superfamily member 10C Proteins 0.000 description 4
- 101000610609 Homo sapiens Tumor necrosis factor receptor superfamily member 10D Proteins 0.000 description 4
- 101000801227 Homo sapiens Tumor necrosis factor receptor superfamily member 19 Proteins 0.000 description 4
- 101000611023 Homo sapiens Tumor necrosis factor receptor superfamily member 6 Proteins 0.000 description 4
- 101000597785 Homo sapiens Tumor necrosis factor receptor superfamily member 6B Proteins 0.000 description 4
- IITVUURPOYGCTD-NAKRPEOUSA-N Ile-Pro-Ala Chemical compound CC[C@H](C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O IITVUURPOYGCTD-NAKRPEOUSA-N 0.000 description 4
- 206010061218 Inflammation Diseases 0.000 description 4
- HXWALXSAVBLTPK-NUTKFTJISA-N Leu-Ala-Trp Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)NC(=O)[C@H](CC(C)C)N HXWALXSAVBLTPK-NUTKFTJISA-N 0.000 description 4
- PVMPDMIKUVNOBD-CIUDSAMLSA-N Leu-Asp-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O PVMPDMIKUVNOBD-CIUDSAMLSA-N 0.000 description 4
- SBANPBVRHYIMRR-GARJFASQSA-N Leu-Ser-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N1CCC[C@@H]1C(=O)O)N SBANPBVRHYIMRR-GARJFASQSA-N 0.000 description 4
- PDIDTSZKKFEDMB-UWVGGRQHSA-N Lys-Pro-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O PDIDTSZKKFEDMB-UWVGGRQHSA-N 0.000 description 4
- UGCIQUYEJIEHKX-GVXVVHGQSA-N Lys-Val-Glu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O UGCIQUYEJIEHKX-GVXVVHGQSA-N 0.000 description 4
- 241000282567 Macaca fascicularis Species 0.000 description 4
- 241001465754 Metazoa Species 0.000 description 4
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 description 4
- KZNQNBZMBZJQJO-UHFFFAOYSA-N N-glycyl-L-proline Natural products NCC(=O)N1CCCC1C(O)=O KZNQNBZMBZJQJO-UHFFFAOYSA-N 0.000 description 4
- 108010032605 Nerve Growth Factor Receptors Proteins 0.000 description 4
- KLYYKKGCPOGDPE-OEAJRASXSA-N Phe-Thr-Leu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O KLYYKKGCPOGDPE-OEAJRASXSA-N 0.000 description 4
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 4
- PRKWBYCXBBSLSK-GUBZILKMSA-N Pro-Ser-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O PRKWBYCXBBSLSK-GUBZILKMSA-N 0.000 description 4
- OBXVZEAMXFSGPU-FXQIFTODSA-N Ser-Asn-Arg Chemical compound C(C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CO)N)CN=C(N)N OBXVZEAMXFSGPU-FXQIFTODSA-N 0.000 description 4
- UIGMAMGZOJVTDN-WHFBIAKZSA-N Ser-Gly-Ser Chemical compound OC[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O UIGMAMGZOJVTDN-WHFBIAKZSA-N 0.000 description 4
- ZIFYDQAFEMIZII-GUBZILKMSA-N Ser-Leu-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O ZIFYDQAFEMIZII-GUBZILKMSA-N 0.000 description 4
- ADJDNJCSPNFFPI-FXQIFTODSA-N Ser-Pro-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CO ADJDNJCSPNFFPI-FXQIFTODSA-N 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- 230000005867 T cell response Effects 0.000 description 4
- MUMGGOZAMZWBJJ-DYKIIFRCSA-N Testostosterone Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 MUMGGOZAMZWBJJ-DYKIIFRCSA-N 0.000 description 4
- BIBYEFRASCNLAA-CDMKHQONSA-N Thr-Phe-Gly Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CC1=CC=CC=C1 BIBYEFRASCNLAA-CDMKHQONSA-N 0.000 description 4
- 102100040115 Tumor necrosis factor receptor superfamily member 10C Human genes 0.000 description 4
- 102100040110 Tumor necrosis factor receptor superfamily member 10D Human genes 0.000 description 4
- 102100033725 Tumor necrosis factor receptor superfamily member 16 Human genes 0.000 description 4
- 102100033760 Tumor necrosis factor receptor superfamily member 19 Human genes 0.000 description 4
- 102100033732 Tumor necrosis factor receptor superfamily member 1A Human genes 0.000 description 4
- 101710187743 Tumor necrosis factor receptor superfamily member 1A Proteins 0.000 description 4
- 102100033733 Tumor necrosis factor receptor superfamily member 1B Human genes 0.000 description 4
- 101710187830 Tumor necrosis factor receptor superfamily member 1B Proteins 0.000 description 4
- 102100035284 Tumor necrosis factor receptor superfamily member 6B Human genes 0.000 description 4
- GAYLGYUVTDMLKC-UWJYBYFXSA-N Tyr-Asp-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 GAYLGYUVTDMLKC-UWJYBYFXSA-N 0.000 description 4
- QUILOGWWLXMSAT-IHRRRGAJSA-N Tyr-Gln-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O QUILOGWWLXMSAT-IHRRRGAJSA-N 0.000 description 4
- AGKDVLSDNSTLFA-UMNHJUIQSA-N Val-Gln-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N1CCC[C@@H]1C(=O)O)N AGKDVLSDNSTLFA-UMNHJUIQSA-N 0.000 description 4
- 238000010521 absorption reaction Methods 0.000 description 4
- 230000009471 action Effects 0.000 description 4
- 210000000612 antigen-presenting cell Anatomy 0.000 description 4
- 239000003963 antioxidant agent Substances 0.000 description 4
- 235000006708 antioxidants Nutrition 0.000 description 4
- 108010043240 arginyl-leucyl-glycine Proteins 0.000 description 4
- 239000011616 biotin Substances 0.000 description 4
- 229960002685 biotin Drugs 0.000 description 4
- 230000010261 cell growth Effects 0.000 description 4
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 4
- 238000011260 co-administration Methods 0.000 description 4
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 4
- HESCAJZNRMSMJG-HGYUPSKWSA-N epothilone A Natural products O=C1[C@H](C)[C@H](O)[C@H](C)CCC[C@H]2O[C@H]2C[C@@H](/C(=C\c2nc(C)sc2)/C)OC(=O)C[C@H](O)C1(C)C HESCAJZNRMSMJG-HGYUPSKWSA-N 0.000 description 4
- 108010078144 glutaminyl-glycine Proteins 0.000 description 4
- 108010050848 glycylleucine Proteins 0.000 description 4
- 230000012010 growth Effects 0.000 description 4
- 238000002513 implantation Methods 0.000 description 4
- 238000010348 incorporation Methods 0.000 description 4
- 230000004054 inflammatory process Effects 0.000 description 4
- 238000007912 intraperitoneal administration Methods 0.000 description 4
- 238000007913 intrathecal administration Methods 0.000 description 4
- 238000002955 isolation Methods 0.000 description 4
- 239000002502 liposome Substances 0.000 description 4
- 210000004185 liver Anatomy 0.000 description 4
- 229950004563 lucatumumab Drugs 0.000 description 4
- 210000002540 macrophage Anatomy 0.000 description 4
- 208000025113 myeloid leukemia Diseases 0.000 description 4
- 230000003285 pharmacodynamic effect Effects 0.000 description 4
- 230000000144 pharmacologic effect Effects 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- 108010029020 prolylglycine Proteins 0.000 description 4
- 230000000069 prophylactic effect Effects 0.000 description 4
- 238000010186 staining Methods 0.000 description 4
- 238000001356 surgical procedure Methods 0.000 description 4
- 108010084932 tryptophyl-proline Proteins 0.000 description 4
- 230000002792 vascular Effects 0.000 description 4
- AADVCYNFEREWOS-UHFFFAOYSA-N (+)-DDM Natural products C=CC=CC(C)C(OC(N)=O)C(C)C(O)C(C)CC(C)=CC(C)C(O)C(C)C=CC(O)CC1OC(=O)C(C)C(O)C1C AADVCYNFEREWOS-UHFFFAOYSA-N 0.000 description 3
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 3
- 102100026423 Adhesion G protein-coupled receptor E5 Human genes 0.000 description 3
- VKKYFICVTYKFIO-CIUDSAMLSA-N Arg-Ala-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCN=C(N)N VKKYFICVTYKFIO-CIUDSAMLSA-N 0.000 description 3
- VUGWHBXPMAHEGZ-SRVKXCTJSA-N Arg-Pro-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCN=C(N)N VUGWHBXPMAHEGZ-SRVKXCTJSA-N 0.000 description 3
- UVTGNSWSRSCPLP-UHFFFAOYSA-N Arg-Tyr Natural products NC(CCNC(=N)N)C(=O)NC(Cc1ccc(O)cc1)C(=O)O UVTGNSWSRSCPLP-UHFFFAOYSA-N 0.000 description 3
- MKJBPDLENBUHQU-CIUDSAMLSA-N Asn-Ser-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O MKJBPDLENBUHQU-CIUDSAMLSA-N 0.000 description 3
- UGXYFDQFLVCDFC-CIUDSAMLSA-N Asn-Ser-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O UGXYFDQFLVCDFC-CIUDSAMLSA-N 0.000 description 3
- JBDLMLZNDRLDIX-HJGDQZAQSA-N Asn-Thr-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O JBDLMLZNDRLDIX-HJGDQZAQSA-N 0.000 description 3
- MNQMTYSEKZHIDF-GCJQMDKQSA-N Asp-Thr-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O MNQMTYSEKZHIDF-GCJQMDKQSA-N 0.000 description 3
- ZUNMTUPRQMWMHX-LSJOCFKGSA-N Asp-Val-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(O)=O ZUNMTUPRQMWMHX-LSJOCFKGSA-N 0.000 description 3
- 108091007065 BIRCs Proteins 0.000 description 3
- 102100038077 CD226 antigen Human genes 0.000 description 3
- 102100025221 CD70 antigen Human genes 0.000 description 3
- KLWPJMFMVPTNCC-UHFFFAOYSA-N Camptothecin Natural products CCC1(O)C(=O)OCC2=C1C=C3C4Nc5ccccc5C=C4CN3C2=O KLWPJMFMVPTNCC-UHFFFAOYSA-N 0.000 description 3
- 201000009030 Carcinoma Diseases 0.000 description 3
- PKNIZMPLMSKROD-BIIVOSGPSA-N Cys-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CS)N PKNIZMPLMSKROD-BIIVOSGPSA-N 0.000 description 3
- AADVCYNFEREWOS-OBRABYBLSA-N Discodermolide Chemical compound C=C\C=C/[C@H](C)[C@H](OC(N)=O)[C@@H](C)[C@H](O)[C@@H](C)C\C(C)=C/[C@H](C)[C@@H](O)[C@@H](C)\C=C/[C@@H](O)C[C@@H]1OC(=O)[C@H](C)[C@@H](O)[C@H]1C AADVCYNFEREWOS-OBRABYBLSA-N 0.000 description 3
- 231100000491 EC50 Toxicity 0.000 description 3
- 206010014733 Endometrial cancer Diseases 0.000 description 3
- 206010014759 Endometrial neoplasm Diseases 0.000 description 3
- 108010021472 Fc gamma receptor IIB Proteins 0.000 description 3
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 3
- ZFBBMCKQSNJZSN-AUTRQRHGSA-N Gln-Val-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZFBBMCKQSNJZSN-AUTRQRHGSA-N 0.000 description 3
- FLQAKQOBSPFGKG-CIUDSAMLSA-N Glu-Cys-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@H](C(O)=O)CCCN=C(N)N FLQAKQOBSPFGKG-CIUDSAMLSA-N 0.000 description 3
- MIIVFRCYJABHTQ-ONGXEEELSA-N Gly-Leu-Val Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O MIIVFRCYJABHTQ-ONGXEEELSA-N 0.000 description 3
- WNZOCXUOGVYYBJ-CDMKHQONSA-N Gly-Phe-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)CN)O WNZOCXUOGVYYBJ-CDMKHQONSA-N 0.000 description 3
- HAOUOFNNJJLVNS-BQBZGAKWSA-N Gly-Pro-Ser Chemical compound NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O HAOUOFNNJJLVNS-BQBZGAKWSA-N 0.000 description 3
- KSOBNUBCYHGUKH-UWVGGRQHSA-N Gly-Val-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)CN KSOBNUBCYHGUKH-UWVGGRQHSA-N 0.000 description 3
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 3
- JBCLFWXMTIKCCB-UHFFFAOYSA-N H-Gly-Phe-OH Natural products NCC(=O)NC(C(O)=O)CC1=CC=CC=C1 JBCLFWXMTIKCCB-UHFFFAOYSA-N 0.000 description 3
- HYWZHNUGAYVEEW-KKUMJFAQSA-N His-Phe-Ser Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CC2=CN=CN2)N HYWZHNUGAYVEEW-KKUMJFAQSA-N 0.000 description 3
- 208000017604 Hodgkin disease Diseases 0.000 description 3
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 3
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 3
- 101000718243 Homo sapiens Adhesion G protein-coupled receptor E5 Proteins 0.000 description 3
- 101000884298 Homo sapiens CD226 antigen Proteins 0.000 description 3
- 101100005713 Homo sapiens CD4 gene Proteins 0.000 description 3
- 101000868215 Homo sapiens CD40 ligand Proteins 0.000 description 3
- 101001068136 Homo sapiens Hepatitis A virus cellular receptor 1 Proteins 0.000 description 3
- 101001046683 Homo sapiens Integrin alpha-L Proteins 0.000 description 3
- 101000831286 Homo sapiens Protein timeless homolog Proteins 0.000 description 3
- 101000752245 Homo sapiens Rho guanine nucleotide exchange factor 5 Proteins 0.000 description 3
- 101000980827 Homo sapiens T-cell surface glycoprotein CD1a Proteins 0.000 description 3
- 101000716149 Homo sapiens T-cell surface glycoprotein CD1b Proteins 0.000 description 3
- 101000716124 Homo sapiens T-cell surface glycoprotein CD1c Proteins 0.000 description 3
- 101000798130 Homo sapiens Tumor necrosis factor receptor superfamily member 11B Proteins 0.000 description 3
- 101000648505 Homo sapiens Tumor necrosis factor receptor superfamily member 12A Proteins 0.000 description 3
- 101000666896 Homo sapiens V-type immunoglobulin domain-containing suppressor of T-cell activation Proteins 0.000 description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 3
- 102100034980 ICOS ligand Human genes 0.000 description 3
- 108010073807 IgG Receptors Proteins 0.000 description 3
- 102000009490 IgG Receptors Human genes 0.000 description 3
- TYYLDKGBCJGJGW-UHFFFAOYSA-N L-tryptophan-L-tyrosine Natural products C=1NC2=CC=CC=C2C=1CC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 TYYLDKGBCJGJGW-UHFFFAOYSA-N 0.000 description 3
- AXZGZMGRBDQTEY-SRVKXCTJSA-N Leu-Gln-Met Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(O)=O AXZGZMGRBDQTEY-SRVKXCTJSA-N 0.000 description 3
- GPICTNQYKHHHTH-GUBZILKMSA-N Leu-Gln-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O GPICTNQYKHHHTH-GUBZILKMSA-N 0.000 description 3
- YOKVEHGYYQEQOP-QWRGUYRKSA-N Leu-Leu-Gly Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O YOKVEHGYYQEQOP-QWRGUYRKSA-N 0.000 description 3
- KIZIOFNVSOSKJI-CIUDSAMLSA-N Leu-Ser-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N KIZIOFNVSOSKJI-CIUDSAMLSA-N 0.000 description 3
- AIMGJYMCTAABEN-GVXVVHGQSA-N Leu-Val-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O AIMGJYMCTAABEN-GVXVVHGQSA-N 0.000 description 3
- 102100029205 Low affinity immunoglobulin gamma Fc region receptor II-b Human genes 0.000 description 3
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 description 3
- GQZMPWBZQALKJO-UWVGGRQHSA-N Lys-Gly-Arg Chemical compound [H]N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O GQZMPWBZQALKJO-UWVGGRQHSA-N 0.000 description 3
- XABXVVSWUVCZST-GVXVVHGQSA-N Lys-Val-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCCN XABXVVSWUVCZST-GVXVVHGQSA-N 0.000 description 3
- 208000025205 Mantle-Cell Lymphoma Diseases 0.000 description 3
- 206010027145 Melanocytic naevus Diseases 0.000 description 3
- 241001529936 Murinae Species 0.000 description 3
- SITLTJHOQZFJGG-UHFFFAOYSA-N N-L-alpha-glutamyl-L-valine Natural products CC(C)C(C(O)=O)NC(=O)C(N)CCC(O)=O SITLTJHOQZFJGG-UHFFFAOYSA-N 0.000 description 3
- 101100342977 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) leu-1 gene Proteins 0.000 description 3
- 208000007256 Nevus Diseases 0.000 description 3
- MCIXMYKSPQUMJG-SRVKXCTJSA-N Phe-Ser-Ser Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O MCIXMYKSPQUMJG-SRVKXCTJSA-N 0.000 description 3
- FGWUALWGCZJQDJ-URLPEUOOSA-N Phe-Thr-Ile Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O FGWUALWGCZJQDJ-URLPEUOOSA-N 0.000 description 3
- XQLBWXHVZVBNJM-FXQIFTODSA-N Pro-Ala-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1 XQLBWXHVZVBNJM-FXQIFTODSA-N 0.000 description 3
- IHCXPSYCHXFXKT-DCAQKATOSA-N Pro-Arg-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O IHCXPSYCHXFXKT-DCAQKATOSA-N 0.000 description 3
- ZSKJPKFTPQCPIH-RCWTZXSCSA-N Pro-Arg-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O ZSKJPKFTPQCPIH-RCWTZXSCSA-N 0.000 description 3
- TUYWCHPXKQTISF-LPEHRKFASA-N Pro-Cys-Pro Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CS)C(=O)N2CCC[C@@H]2C(=O)O TUYWCHPXKQTISF-LPEHRKFASA-N 0.000 description 3
- 101710094000 Programmed cell death 1 ligand 1 Proteins 0.000 description 3
- QEDMOZUJTGEIBF-FXQIFTODSA-N Ser-Arg-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(O)=O QEDMOZUJTGEIBF-FXQIFTODSA-N 0.000 description 3
- PVDTYLHUWAEYGY-CIUDSAMLSA-N Ser-Glu-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O PVDTYLHUWAEYGY-CIUDSAMLSA-N 0.000 description 3
- YUJLIIRMIAGMCQ-CIUDSAMLSA-N Ser-Leu-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YUJLIIRMIAGMCQ-CIUDSAMLSA-N 0.000 description 3
- NNFMANHDYSVNIO-DCAQKATOSA-N Ser-Lys-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O NNFMANHDYSVNIO-DCAQKATOSA-N 0.000 description 3
- RHAPJNVNWDBFQI-BQBZGAKWSA-N Ser-Pro-Gly Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O RHAPJNVNWDBFQI-BQBZGAKWSA-N 0.000 description 3
- OZPDGESCTGGNAD-CIUDSAMLSA-N Ser-Ser-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CO OZPDGESCTGGNAD-CIUDSAMLSA-N 0.000 description 3
- CUXJENOFJXOSOZ-BIIVOSGPSA-N Ser-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CO)N)C(=O)O CUXJENOFJXOSOZ-BIIVOSGPSA-N 0.000 description 3
- 102000008115 Signaling Lymphocytic Activation Molecule Family Member 1 Human genes 0.000 description 3
- 108010074687 Signaling Lymphocytic Activation Molecule Family Member 1 Proteins 0.000 description 3
- 102100024219 T-cell surface glycoprotein CD1a Human genes 0.000 description 3
- 102100025244 T-cell surface glycoprotein CD5 Human genes 0.000 description 3
- BVOVIGCHYNFJBZ-JXUBOQSCSA-N Thr-Leu-Ala Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O BVOVIGCHYNFJBZ-JXUBOQSCSA-N 0.000 description 3
- HOVLHEKTGVIKAP-WDCWCFNPSA-N Thr-Leu-Gln Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O HOVLHEKTGVIKAP-WDCWCFNPSA-N 0.000 description 3
- DXPURPNJDFCKKO-RHYQMDGZSA-N Thr-Lys-Val Chemical compound CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)[C@@H](C)O)C(O)=O DXPURPNJDFCKKO-RHYQMDGZSA-N 0.000 description 3
- 102100032236 Tumor necrosis factor receptor superfamily member 11B Human genes 0.000 description 3
- 102100028786 Tumor necrosis factor receptor superfamily member 12A Human genes 0.000 description 3
- JWHOIHCOHMZSAR-QWRGUYRKSA-N Tyr-Asp-Gly Chemical compound OC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 JWHOIHCOHMZSAR-QWRGUYRKSA-N 0.000 description 3
- MQGGXGKQSVEQHR-KKUMJFAQSA-N Tyr-Ser-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 MQGGXGKQSVEQHR-KKUMJFAQSA-N 0.000 description 3
- MWUYSCVVPVITMW-IGNZVWTISA-N Tyr-Tyr-Ala Chemical compound C([C@@H](C(=O)N[C@@H](C)C(O)=O)NC(=O)[C@@H](N)CC=1C=CC(O)=CC=1)C1=CC=C(O)C=C1 MWUYSCVVPVITMW-IGNZVWTISA-N 0.000 description 3
- 102100038282 V-type immunoglobulin domain-containing suppressor of T-cell activation Human genes 0.000 description 3
- MYLNLEIZWHVENT-VKOGCVSHSA-N Val-Ile-Trp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)NC(=O)[C@H](C(C)C)N MYLNLEIZWHVENT-VKOGCVSHSA-N 0.000 description 3
- 230000002159 abnormal effect Effects 0.000 description 3
- 208000037844 advanced solid tumor Diseases 0.000 description 3
- 230000002411 adverse Effects 0.000 description 3
- 108010086434 alanyl-seryl-glycine Proteins 0.000 description 3
- 239000004037 angiogenesis inhibitor Substances 0.000 description 3
- 230000001093 anti-cancer Effects 0.000 description 3
- 230000001028 anti-proliverative effect Effects 0.000 description 3
- 230000010056 antibody-dependent cellular cytotoxicity Effects 0.000 description 3
- 108010052670 arginyl-glutamyl-glutamic acid Proteins 0.000 description 3
- 108010062796 arginyllysine Proteins 0.000 description 3
- 239000011324 bead Substances 0.000 description 3
- 238000001574 biopsy Methods 0.000 description 3
- 230000008499 blood brain barrier function Effects 0.000 description 3
- 210000001218 blood-brain barrier Anatomy 0.000 description 3
- VSJKWCGYPAHWDS-FQEVSTJZSA-N camptothecin Chemical compound C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-FQEVSTJZSA-N 0.000 description 3
- 229940127093 camptothecin Drugs 0.000 description 3
- 230000030833 cell death Effects 0.000 description 3
- 230000001413 cellular effect Effects 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 230000004540 complement-dependent cytotoxicity Effects 0.000 description 3
- 208000035475 disorder Diseases 0.000 description 3
- 239000002612 dispersion medium Substances 0.000 description 3
- VSJKWCGYPAHWDS-UHFFFAOYSA-N dl-camptothecin Natural products C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)C5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-UHFFFAOYSA-N 0.000 description 3
- 239000002552 dosage form Substances 0.000 description 3
- 210000003162 effector t lymphocyte Anatomy 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 230000002708 enhancing effect Effects 0.000 description 3
- 210000003743 erythrocyte Anatomy 0.000 description 3
- 229960002949 fluorouracil Drugs 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- HPAIKDPJURGQLN-UHFFFAOYSA-N glycyl-L-histidyl-L-phenylalanine Natural products C=1C=CC=CC=1CC(C(O)=O)NC(=O)C(NC(=O)CN)CC1=CN=CN1 HPAIKDPJURGQLN-UHFFFAOYSA-N 0.000 description 3
- 108010019832 glycyl-asparaginyl-glycine Proteins 0.000 description 3
- 229940088597 hormone Drugs 0.000 description 3
- 239000005556 hormone Substances 0.000 description 3
- 210000004408 hybridoma Anatomy 0.000 description 3
- 229960002751 imiquimod Drugs 0.000 description 3
- DOUYETYNHWVLEO-UHFFFAOYSA-N imiquimod Chemical compound C1=CC=CC2=C3N(CC(C)C)C=NC3=C(N)N=C21 DOUYETYNHWVLEO-UHFFFAOYSA-N 0.000 description 3
- 230000005934 immune activation Effects 0.000 description 3
- 229940127121 immunoconjugate Drugs 0.000 description 3
- 230000005847 immunogenicity Effects 0.000 description 3
- 239000007943 implant Substances 0.000 description 3
- 230000006698 induction Effects 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
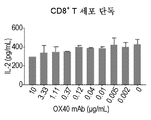
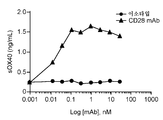
- 230000004073 interleukin-2 production Effects 0.000 description 3
- 239000007951 isotonicity adjuster Substances 0.000 description 3
- 239000000787 lecithin Substances 0.000 description 3
- 235000010445 lecithin Nutrition 0.000 description 3
- 229940067606 lecithin Drugs 0.000 description 3
- 108010073472 leucyl-prolyl-proline Proteins 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 210000004324 lymphatic system Anatomy 0.000 description 3
- 108010064235 lysylglycine Proteins 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 230000002503 metabolic effect Effects 0.000 description 3
- 231100000252 nontoxic Toxicity 0.000 description 3
- 230000003000 nontoxic effect Effects 0.000 description 3
- 230000000771 oncological effect Effects 0.000 description 3
- 210000000056 organ Anatomy 0.000 description 3
- 229960002621 pembrolizumab Drugs 0.000 description 3
- 108010025488 pinealon Proteins 0.000 description 3
- 229920001184 polypeptide Polymers 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 239000003755 preservative agent Substances 0.000 description 3
- 108010093296 prolyl-prolyl-alanine Proteins 0.000 description 3
- 108010087846 prolyl-prolyl-glycine Proteins 0.000 description 3
- 108010070643 prolylglutamic acid Proteins 0.000 description 3
- 238000001959 radiotherapy Methods 0.000 description 3
- 230000000306 recurrent effect Effects 0.000 description 3
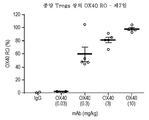
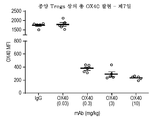
- 230000001105 regulatory effect Effects 0.000 description 3
- 201000006845 reticulosarcoma Diseases 0.000 description 3
- 208000029922 reticulum cell sarcoma Diseases 0.000 description 3
- 229960004641 rituximab Drugs 0.000 description 3
- 238000005070 sampling Methods 0.000 description 3
- 230000035945 sensitivity Effects 0.000 description 3
- 210000003491 skin Anatomy 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 230000000638 stimulation Effects 0.000 description 3
- 238000003860 storage Methods 0.000 description 3
- 229940104230 thymidine Drugs 0.000 description 3
- 238000011200 topical administration Methods 0.000 description 3
- 230000000699 topical effect Effects 0.000 description 3
- 230000001988 toxicity Effects 0.000 description 3
- 231100000419 toxicity Toxicity 0.000 description 3
- 108010035534 tyrosyl-leucyl-alanine Proteins 0.000 description 3
- 108010071635 tyrosyl-prolyl-arginine Proteins 0.000 description 3
- 238000002255 vaccination Methods 0.000 description 3
- 229960005486 vaccine Drugs 0.000 description 3
- 238000010200 validation analysis Methods 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- 230000003442 weekly effect Effects 0.000 description 3
- 108010027345 wheylin-1 peptide Proteins 0.000 description 3
- GJLXVWOMRRWCIB-MERZOTPQSA-N (2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-acetamido-5-(diaminomethylideneamino)pentanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-6-aminohexanoyl]amino]-6-aminohexanoyl]amino]-6-aminohexanoyl]amino]-6-aminohexanoyl]amino]-6-aminohexanoyl]amino]-6-aminohexanoyl]amino]-6-aminohexanamide Chemical compound C([C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(N)=O)C1=CC=C(O)C=C1 GJLXVWOMRRWCIB-MERZOTPQSA-N 0.000 description 2
- IESDGNYHXIOKRW-YXMSTPNBSA-N (2s)-2-[[(2s)-1-[(2s)-6-amino-2-[[(2s,3r)-2-amino-3-hydroxybutanoyl]amino]hexanoyl]pyrrolidine-2-carbonyl]amino]-5-(diaminomethylideneamino)pentanoic acid Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O IESDGNYHXIOKRW-YXMSTPNBSA-N 0.000 description 2
- DIBLBAURNYJYBF-XLXZRNDBSA-N (2s)-2-[[(2s)-2-[[2-[[(2s)-6-amino-2-[[(2s)-2-amino-3-methylbutanoyl]amino]hexanoyl]amino]acetyl]amino]-3-phenylpropanoyl]amino]-3-(4-hydroxyphenyl)propanoic acid Chemical compound C([C@H](NC(=O)CNC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=CC=C1 DIBLBAURNYJYBF-XLXZRNDBSA-N 0.000 description 2
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 description 2
- IHWDSEPNZDYMNF-UHFFFAOYSA-N 1H-indol-2-amine Chemical compound C1=CC=C2NC(N)=CC2=C1 IHWDSEPNZDYMNF-UHFFFAOYSA-N 0.000 description 2
- 102000002627 4-1BB Ligand Human genes 0.000 description 2
- 108010082808 4-1BB Ligand Proteins 0.000 description 2
- 206010069754 Acquired gene mutation Diseases 0.000 description 2
- 206010000830 Acute leukaemia Diseases 0.000 description 2
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 description 2
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 description 2
- 208000036832 Adenocarcinoma of ovary Diseases 0.000 description 2
- LGQPPBQRUBVTIF-JBDRJPRFSA-N Ala-Ala-Ile Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O LGQPPBQRUBVTIF-JBDRJPRFSA-N 0.000 description 2
- WCBVQNZTOKJWJS-ACZMJKKPSA-N Ala-Cys-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(O)=O)C(O)=O WCBVQNZTOKJWJS-ACZMJKKPSA-N 0.000 description 2
- KXEVYGKATAMXJJ-ACZMJKKPSA-N Ala-Glu-Asp Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O KXEVYGKATAMXJJ-ACZMJKKPSA-N 0.000 description 2
- NBTGEURICRTMGL-WHFBIAKZSA-N Ala-Gly-Ser Chemical compound C[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O NBTGEURICRTMGL-WHFBIAKZSA-N 0.000 description 2
- OBVSBEYOMDWLRJ-BFHQHQDPSA-N Ala-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@H](C)N OBVSBEYOMDWLRJ-BFHQHQDPSA-N 0.000 description 2
- FOHXUHGZZKETFI-JBDRJPRFSA-N Ala-Ile-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](C)N FOHXUHGZZKETFI-JBDRJPRFSA-N 0.000 description 2
- FVNAUOZKIPAYNA-BPNCWPANSA-N Ala-Met-Tyr Chemical compound CSCC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 FVNAUOZKIPAYNA-BPNCWPANSA-N 0.000 description 2
- XQNRANMFRPCFFW-GCJQMDKQSA-N Ala-Thr-Asn Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](C)N)O XQNRANMFRPCFFW-GCJQMDKQSA-N 0.000 description 2
- HCBKAOZYACJUEF-XQXXSGGOSA-N Ala-Thr-Gln Chemical compound N[C@@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CCC(N)=O)C(=O)O HCBKAOZYACJUEF-XQXXSGGOSA-N 0.000 description 2
- GIVATXIGCXFQQA-FXQIFTODSA-N Arg-Ala-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCN=C(N)N GIVATXIGCXFQQA-FXQIFTODSA-N 0.000 description 2
- PQWTZSNVWSOFFK-FXQIFTODSA-N Arg-Asp-Asn Chemical compound C(C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N)CN=C(N)N PQWTZSNVWSOFFK-FXQIFTODSA-N 0.000 description 2
- DXQIQUIQYAGRCC-CIUDSAMLSA-N Arg-Asp-Gln Chemical compound C(C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N)CN=C(N)N DXQIQUIQYAGRCC-CIUDSAMLSA-N 0.000 description 2
- ZATRYQNPUHGXCU-DTWKUNHWSA-N Arg-Gly-Pro Chemical compound C1C[C@@H](N(C1)C(=O)CNC(=O)[C@H](CCCN=C(N)N)N)C(=O)O ZATRYQNPUHGXCU-DTWKUNHWSA-N 0.000 description 2
- NMRHDSAOIURTNT-RWMBFGLXSA-N Arg-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N NMRHDSAOIURTNT-RWMBFGLXSA-N 0.000 description 2
- FOQFHANLUJDQEE-GUBZILKMSA-N Arg-Pro-Cys Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CCCN=C(N)N)N)C(=O)N[C@@H](CS)C(=O)O FOQFHANLUJDQEE-GUBZILKMSA-N 0.000 description 2
- DNLQVHBBMPZUGJ-BQBZGAKWSA-N Arg-Ser-Gly Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O DNLQVHBBMPZUGJ-BQBZGAKWSA-N 0.000 description 2
- ZJBUILVYSXQNSW-YTWAJWBKSA-N Arg-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N)O ZJBUILVYSXQNSW-YTWAJWBKSA-N 0.000 description 2
- 102000004452 Arginase Human genes 0.000 description 2
- 108700024123 Arginases Proteins 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- VDCIPFYVCICPEC-FXQIFTODSA-N Asn-Arg-Ala Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(O)=O VDCIPFYVCICPEC-FXQIFTODSA-N 0.000 description 2
- WVCJSDCHTUTONA-FXQIFTODSA-N Asn-Asp-Arg Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O WVCJSDCHTUTONA-FXQIFTODSA-N 0.000 description 2
- FAEFJTCTNZTPHX-ACZMJKKPSA-N Asn-Gln-Ala Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(O)=O FAEFJTCTNZTPHX-ACZMJKKPSA-N 0.000 description 2
- RBOBTTLFPRSXKZ-BZSNNMDCSA-N Asn-Phe-Tyr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O RBOBTTLFPRSXKZ-BZSNNMDCSA-N 0.000 description 2
- BCADFFUQHIMQAA-KKHAAJSZSA-N Asn-Thr-Val Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O BCADFFUQHIMQAA-KKHAAJSZSA-N 0.000 description 2
- LGCVSPFCFXWUEY-IHPCNDPISA-N Asn-Trp-Tyr Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC3=CC=C(C=C3)O)C(=O)O)NC(=O)[C@H](CC(=O)N)N LGCVSPFCFXWUEY-IHPCNDPISA-N 0.000 description 2
- SLHOOKXYTYAJGQ-XVYDVKMFSA-N Asp-Ala-His Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC1=CNC=N1 SLHOOKXYTYAJGQ-XVYDVKMFSA-N 0.000 description 2
- NJIKKGUVGUBICV-ZLUOBGJFSA-N Asp-Ala-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC(O)=O NJIKKGUVGUBICV-ZLUOBGJFSA-N 0.000 description 2
- YNQIDCRRTWGHJD-ZLUOBGJFSA-N Asp-Asn-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CC(O)=O YNQIDCRRTWGHJD-ZLUOBGJFSA-N 0.000 description 2
- VZNOVQKGJQJOCS-SRVKXCTJSA-N Asp-Asp-Tyr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O VZNOVQKGJQJOCS-SRVKXCTJSA-N 0.000 description 2
- HSWYMWGDMPLTTH-FXQIFTODSA-N Asp-Glu-Gln Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O HSWYMWGDMPLTTH-FXQIFTODSA-N 0.000 description 2
- SNDBKTFJWVEVPO-WHFBIAKZSA-N Asp-Gly-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CO)C(O)=O SNDBKTFJWVEVPO-WHFBIAKZSA-N 0.000 description 2
- BKOIIURTQAJHAT-GUBZILKMSA-N Asp-Pro-Pro Chemical compound OC(=O)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 BKOIIURTQAJHAT-GUBZILKMSA-N 0.000 description 2
- VNXQRBXEQXLERQ-CIUDSAMLSA-N Asp-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(=O)O)N VNXQRBXEQXLERQ-CIUDSAMLSA-N 0.000 description 2
- YIDFBWRHIYOYAA-LKXGYXEUSA-N Asp-Ser-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O YIDFBWRHIYOYAA-LKXGYXEUSA-N 0.000 description 2
- IWLZBRTUIVXZJD-OLHMAJIHSA-N Asp-Thr-Asp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O IWLZBRTUIVXZJD-OLHMAJIHSA-N 0.000 description 2
- 208000028564 B-cell non-Hodgkin lymphoma Diseases 0.000 description 2
- 208000032791 BCR-ABL1 positive chronic myelogenous leukemia Diseases 0.000 description 2
- 208000003174 Brain Neoplasms Diseases 0.000 description 2
- 208000011691 Burkitt lymphomas Diseases 0.000 description 2
- 239000004322 Butylated hydroxytoluene Substances 0.000 description 2
- NLZUEZXRPGMBCV-UHFFFAOYSA-N Butylhydroxytoluene Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 NLZUEZXRPGMBCV-UHFFFAOYSA-N 0.000 description 2
- 101710185679 CD276 antigen Proteins 0.000 description 2
- 206010007953 Central nervous system lymphoma Diseases 0.000 description 2
- 241000699800 Cricetinae Species 0.000 description 2
- TVYMKYUSZSVOAG-ZLUOBGJFSA-N Cys-Ala-Ala Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(O)=O TVYMKYUSZSVOAG-ZLUOBGJFSA-N 0.000 description 2
- UPJGYXRAPJWIHD-CIUDSAMLSA-N Cys-Asn-Leu Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O UPJGYXRAPJWIHD-CIUDSAMLSA-N 0.000 description 2
- ZXCAQANTQWBICD-DCAQKATOSA-N Cys-Lys-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CS)N ZXCAQANTQWBICD-DCAQKATOSA-N 0.000 description 2
- FANFRJOFTYCNRG-JYBASQMISA-N Cys-Thr-Trp Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)NC(=O)[C@H](CS)N)O FANFRJOFTYCNRG-JYBASQMISA-N 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 2
- 206010061818 Disease progression Diseases 0.000 description 2
- 102100037354 Ectodysplasin-A Human genes 0.000 description 2
- 108091006020 Fc-tagged proteins Proteins 0.000 description 2
- 201000008808 Fibrosarcoma Diseases 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- JESJDAAGXULQOP-CIUDSAMLSA-N Gln-Arg-Ser Chemical compound C(C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)CN=C(N)N JESJDAAGXULQOP-CIUDSAMLSA-N 0.000 description 2
- UICOTGULOUGGLC-NUMRIWBASA-N Gln-Asp-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)N)N)O UICOTGULOUGGLC-NUMRIWBASA-N 0.000 description 2
- DRDSQGHKTLSNEA-GLLZPBPUSA-N Gln-Glu-Thr Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O DRDSQGHKTLSNEA-GLLZPBPUSA-N 0.000 description 2
- FGYPOQPQTUNESW-IUCAKERBSA-N Gln-Gly-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CCC(=O)N)N FGYPOQPQTUNESW-IUCAKERBSA-N 0.000 description 2
- NSORZJXKUQFEKL-JGVFFNPUSA-N Gln-Gly-Pro Chemical compound C1C[C@@H](N(C1)C(=O)CNC(=O)[C@H](CCC(=O)N)N)C(=O)O NSORZJXKUQFEKL-JGVFFNPUSA-N 0.000 description 2
- MSHXWFKYXJTLEZ-CIUDSAMLSA-N Gln-Met-Asn Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)N)N MSHXWFKYXJTLEZ-CIUDSAMLSA-N 0.000 description 2
- JILRMFFFCHUUTJ-ACZMJKKPSA-N Gln-Ser-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O JILRMFFFCHUUTJ-ACZMJKKPSA-N 0.000 description 2
- OTQSTOXRUBVWAP-NRPADANISA-N Gln-Ser-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O OTQSTOXRUBVWAP-NRPADANISA-N 0.000 description 2
- RSUVOPBMWMTVDI-XEGUGMAKSA-N Glu-Ala-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)C)C(O)=O)=CNC2=C1 RSUVOPBMWMTVDI-XEGUGMAKSA-N 0.000 description 2
- GLWXKFRTOHKGIT-ACZMJKKPSA-N Glu-Asn-Asn Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O GLWXKFRTOHKGIT-ACZMJKKPSA-N 0.000 description 2
- QPRZKNOOOBWXSU-CIUDSAMLSA-N Glu-Asp-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N QPRZKNOOOBWXSU-CIUDSAMLSA-N 0.000 description 2
- OXEMJGCAJFFREE-FXQIFTODSA-N Glu-Gln-Ala Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(O)=O OXEMJGCAJFFREE-FXQIFTODSA-N 0.000 description 2
- HNVFSTLPVJWIDV-CIUDSAMLSA-N Glu-Glu-Gln Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O HNVFSTLPVJWIDV-CIUDSAMLSA-N 0.000 description 2
- KASDBWKLWJKTLJ-GUBZILKMSA-N Glu-Glu-Met Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCSC)C(O)=O KASDBWKLWJKTLJ-GUBZILKMSA-N 0.000 description 2
- XMPAXPSENRSOSV-RYUDHWBXSA-N Glu-Gly-Tyr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O XMPAXPSENRSOSV-RYUDHWBXSA-N 0.000 description 2
- DMYACXMQUABZIQ-NRPADANISA-N Glu-Ser-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O DMYACXMQUABZIQ-NRPADANISA-N 0.000 description 2
- MFYLRRCYBBJYPI-JYJNAYRXSA-N Glu-Tyr-Lys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N)O MFYLRRCYBBJYPI-JYJNAYRXSA-N 0.000 description 2
- YQPFCZVKMUVZIN-AUTRQRHGSA-N Glu-Val-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O YQPFCZVKMUVZIN-AUTRQRHGSA-N 0.000 description 2
- PUUYVMYCMIWHFE-BQBZGAKWSA-N Gly-Ala-Arg Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N PUUYVMYCMIWHFE-BQBZGAKWSA-N 0.000 description 2
- VSVZIEVNUYDAFR-YUMQZZPRSA-N Gly-Ala-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)CN VSVZIEVNUYDAFR-YUMQZZPRSA-N 0.000 description 2
- KRRMJKMGWWXWDW-STQMWFEESA-N Gly-Arg-Phe Chemical compound NC(=N)NCCC[C@H](NC(=O)CN)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 KRRMJKMGWWXWDW-STQMWFEESA-N 0.000 description 2
- GGEJHJIXRBTJPD-BYPYZUCNSA-N Gly-Asn-Gly Chemical compound NCC(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O GGEJHJIXRBTJPD-BYPYZUCNSA-N 0.000 description 2
- XCLCVBYNGXEVDU-WHFBIAKZSA-N Gly-Asn-Ser Chemical compound NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O XCLCVBYNGXEVDU-WHFBIAKZSA-N 0.000 description 2
- GRIRDMVMJJDZKV-RCOVLWMOSA-N Gly-Asn-Val Chemical compound [H]NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O GRIRDMVMJJDZKV-RCOVLWMOSA-N 0.000 description 2
- PMNHJLASAAWELO-FOHZUACHSA-N Gly-Asp-Thr Chemical compound [H]NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O PMNHJLASAAWELO-FOHZUACHSA-N 0.000 description 2
- BYYNJRSNDARRBX-YFKPBYRVSA-N Gly-Gln-Gly Chemical compound NCC(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O BYYNJRSNDARRBX-YFKPBYRVSA-N 0.000 description 2
- PABFFPWEJMEVEC-JGVFFNPUSA-N Gly-Gln-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCC(=O)N)NC(=O)CN)C(=O)O PABFFPWEJMEVEC-JGVFFNPUSA-N 0.000 description 2
- BIRKKBCSAIHDDF-WDSKDSINSA-N Gly-Glu-Cys Chemical compound NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CS)C(O)=O BIRKKBCSAIHDDF-WDSKDSINSA-N 0.000 description 2
- UQJNXZSSGQIPIQ-FBCQKBJTSA-N Gly-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)CN UQJNXZSSGQIPIQ-FBCQKBJTSA-N 0.000 description 2
- SCWYHUQOOFRVHP-MBLNEYKQSA-N Gly-Ile-Thr Chemical compound NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O SCWYHUQOOFRVHP-MBLNEYKQSA-N 0.000 description 2
- IUKIDFVOUHZRAK-QWRGUYRKSA-N Gly-Lys-His Chemical compound NCCCC[C@H](NC(=O)CN)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 IUKIDFVOUHZRAK-QWRGUYRKSA-N 0.000 description 2
- RVGMVLVBDRQVKB-UWVGGRQHSA-N Gly-Met-His Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)CN RVGMVLVBDRQVKB-UWVGGRQHSA-N 0.000 description 2
- NSVOVKWEKGEOQB-LURJTMIESA-N Gly-Pro-Gly Chemical compound NCC(=O)N1CCC[C@H]1C(=O)NCC(O)=O NSVOVKWEKGEOQB-LURJTMIESA-N 0.000 description 2
- JSLVAHYTAJJEQH-QWRGUYRKSA-N Gly-Ser-Phe Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 JSLVAHYTAJJEQH-QWRGUYRKSA-N 0.000 description 2
- ZZWUYQXMIFTIIY-WEDXCCLWSA-N Gly-Thr-Leu Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O ZZWUYQXMIFTIIY-WEDXCCLWSA-N 0.000 description 2
- DNVDEMWIYLVIQU-RCOVLWMOSA-N Gly-Val-Asp Chemical compound NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O DNVDEMWIYLVIQU-RCOVLWMOSA-N 0.000 description 2
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 2
- WMKXFMUJRCEGRP-SRVKXCTJSA-N His-Asn-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)N WMKXFMUJRCEGRP-SRVKXCTJSA-N 0.000 description 2
- MWXBCJKQRQFVOO-DCAQKATOSA-N His-Cys-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CC1=CN=CN1)N MWXBCJKQRQFVOO-DCAQKATOSA-N 0.000 description 2
- TVMNTHXFRSXZGR-IHRRRGAJSA-N His-Lys-Val Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O TVMNTHXFRSXZGR-IHRRRGAJSA-N 0.000 description 2
- 102000003964 Histone deacetylase Human genes 0.000 description 2
- 108090000353 Histone deacetylase Proteins 0.000 description 2
- 101000834898 Homo sapiens Alpha-synuclein Proteins 0.000 description 2
- 101000884279 Homo sapiens CD276 antigen Proteins 0.000 description 2
- 101000934356 Homo sapiens CD70 antigen Proteins 0.000 description 2
- 101000880080 Homo sapiens Ectodysplasin-A Proteins 0.000 description 2
- 101001019455 Homo sapiens ICOS ligand Proteins 0.000 description 2
- 101000652359 Homo sapiens Spermatogenesis-associated protein 2 Proteins 0.000 description 2
- 241000701806 Human papillomavirus Species 0.000 description 2
- HOLOYAZCIHDQNS-YVNDNENWSA-N Ile-Gln-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N HOLOYAZCIHDQNS-YVNDNENWSA-N 0.000 description 2
- NZGTYCMLUGYMCV-XUXIUFHCSA-N Ile-Lys-Arg Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N NZGTYCMLUGYMCV-XUXIUFHCSA-N 0.000 description 2
- 229940076838 Immune checkpoint inhibitor Drugs 0.000 description 2
- 102000037982 Immune checkpoint proteins Human genes 0.000 description 2
- 108091008036 Immune checkpoint proteins Proteins 0.000 description 2
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 2
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 2
- 108700005091 Immunoglobulin Genes Proteins 0.000 description 2
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 2
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 2
- 102000055031 Inhibitor of Apoptosis Proteins Human genes 0.000 description 2
- 102000037984 Inhibitory immune checkpoint proteins Human genes 0.000 description 2
- 108091008026 Inhibitory immune checkpoint proteins Proteins 0.000 description 2
- 102000008070 Interferon-gamma Human genes 0.000 description 2
- 102000015696 Interleukins Human genes 0.000 description 2
- 108010063738 Interleukins Proteins 0.000 description 2
- 208000006404 Large Granular Lymphocytic Leukemia Diseases 0.000 description 2
- FGNQZXKVAZIMCI-CIUDSAMLSA-N Leu-Asp-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CS)C(=O)O)N FGNQZXKVAZIMCI-CIUDSAMLSA-N 0.000 description 2
- CQGSYZCULZMEDE-UHFFFAOYSA-N Leu-Gln-Pro Natural products CC(C)CC(N)C(=O)NC(CCC(N)=O)C(=O)N1CCCC1C(O)=O CQGSYZCULZMEDE-UHFFFAOYSA-N 0.000 description 2
- YFBBUHJJUXXZOF-UWVGGRQHSA-N Leu-Gly-Pro Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N1CCC[C@H]1C(O)=O YFBBUHJJUXXZOF-UWVGGRQHSA-N 0.000 description 2
- HYMLKESRWLZDBR-WEDXCCLWSA-N Leu-Gly-Thr Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O HYMLKESRWLZDBR-WEDXCCLWSA-N 0.000 description 2
- IAJFFZORSWOZPQ-SRVKXCTJSA-N Leu-Leu-Asn Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O IAJFFZORSWOZPQ-SRVKXCTJSA-N 0.000 description 2
- VCHVSKNMTXWIIP-SRVKXCTJSA-N Leu-Lys-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O VCHVSKNMTXWIIP-SRVKXCTJSA-N 0.000 description 2
- XOWMDXHFSBCAKQ-SRVKXCTJSA-N Leu-Ser-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC(C)C XOWMDXHFSBCAKQ-SRVKXCTJSA-N 0.000 description 2
- MVJRBCJCRYGCKV-GVXVVHGQSA-N Leu-Val-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O MVJRBCJCRYGCKV-GVXVVHGQSA-N 0.000 description 2
- YQFZRHYZLARWDY-IHRRRGAJSA-N Leu-Val-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CCCCN YQFZRHYZLARWDY-IHRRRGAJSA-N 0.000 description 2
- 102100029193 Low affinity immunoglobulin gamma Fc region receptor III-A Human genes 0.000 description 2
- 101710099301 Low affinity immunoglobulin gamma Fc region receptor III-A Proteins 0.000 description 2
- MPGHETGWWWUHPY-CIUDSAMLSA-N Lys-Ala-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN MPGHETGWWWUHPY-CIUDSAMLSA-N 0.000 description 2
- UWKNTTJNVSYXPC-CIUDSAMLSA-N Lys-Ala-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN UWKNTTJNVSYXPC-CIUDSAMLSA-N 0.000 description 2
- XNKDCYABMBBEKN-IUCAKERBSA-N Lys-Gly-Gln Chemical compound NCCCC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCC(N)=O XNKDCYABMBBEKN-IUCAKERBSA-N 0.000 description 2
- 102100028198 Macrophage colony-stimulating factor 1 receptor Human genes 0.000 description 2
- 101710150918 Macrophage colony-stimulating factor 1 receptor Proteins 0.000 description 2
- YKWHHKDMBZBMLG-GUBZILKMSA-N Met-Cys-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CCSC)N YKWHHKDMBZBMLG-GUBZILKMSA-N 0.000 description 2
- LBSWWNKMVPAXOI-GUBZILKMSA-N Met-Val-Ser Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O LBSWWNKMVPAXOI-GUBZILKMSA-N 0.000 description 2
- 208000034578 Multiple myelomas Diseases 0.000 description 2
- WUGMRIBZSVSJNP-UHFFFAOYSA-N N-L-alanyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)C(N)C)C(O)=O)=CNC2=C1 WUGMRIBZSVSJNP-UHFFFAOYSA-N 0.000 description 2
- YBAFDPFAUTYYRW-UHFFFAOYSA-N N-L-alpha-glutamyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCC(O)=O YBAFDPFAUTYYRW-UHFFFAOYSA-N 0.000 description 2
- 102000008299 Nitric Oxide Synthase Human genes 0.000 description 2
- 108010021487 Nitric Oxide Synthase Proteins 0.000 description 2
- YGACXVRLDHEXKY-WXRXAMBDSA-N O[C@H](C[C@H]1c2c(cccc2F)-c2cncn12)[C@H]1CC[C@H](O)CC1 Chemical compound O[C@H](C[C@H]1c2c(cccc2F)-c2cncn12)[C@H]1CC[C@H](O)CC1 YGACXVRLDHEXKY-WXRXAMBDSA-N 0.000 description 2
- 108091034117 Oligonucleotide Proteins 0.000 description 2
- 206010061328 Ovarian epithelial cancer Diseases 0.000 description 2
- 229940124060 PD-1 antagonist Drugs 0.000 description 2
- 208000002193 Pain Diseases 0.000 description 2
- 206010057249 Phagocytosis Diseases 0.000 description 2
- HXSUFWQYLPKEHF-IHRRRGAJSA-N Phe-Asn-Arg Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N HXSUFWQYLPKEHF-IHRRRGAJSA-N 0.000 description 2
- BWTKUQPNOMMKMA-FIRPJDEBSA-N Phe-Ile-Phe Chemical compound C([C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 BWTKUQPNOMMKMA-FIRPJDEBSA-N 0.000 description 2
- MSHZERMPZKCODG-ACRUOGEOSA-N Phe-Leu-Phe Chemical compound C([C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 MSHZERMPZKCODG-ACRUOGEOSA-N 0.000 description 2
- KAJLHCWRWDSROH-BZSNNMDCSA-N Phe-Phe-Asp Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC(O)=O)C(O)=O)C1=CC=CC=C1 KAJLHCWRWDSROH-BZSNNMDCSA-N 0.000 description 2
- JDMKQHSHKJHAHR-UHFFFAOYSA-N Phe-Phe-Leu-Tyr Natural products C=1C=C(O)C=CC=1CC(C(O)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)CC1=CC=CC=C1 JDMKQHSHKJHAHR-UHFFFAOYSA-N 0.000 description 2
- YMIZSYUAZJSOFL-SRVKXCTJSA-N Phe-Ser-Asn Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O YMIZSYUAZJSOFL-SRVKXCTJSA-N 0.000 description 2
- QTDBZORPVYTRJU-KKXDTOCCSA-N Phe-Tyr-Ala Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C)C(O)=O QTDBZORPVYTRJU-KKXDTOCCSA-N 0.000 description 2
- GTMSCDVFQLNEOY-BZSNNMDCSA-N Phe-Tyr-Asn Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N GTMSCDVFQLNEOY-BZSNNMDCSA-N 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- VXCHGLYSIOOZIS-GUBZILKMSA-N Pro-Ala-Arg Chemical compound NC(N)=NCCC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1 VXCHGLYSIOOZIS-GUBZILKMSA-N 0.000 description 2
- UTAUEDINXUMHLG-FXQIFTODSA-N Pro-Asp-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@@H]1CCCN1 UTAUEDINXUMHLG-FXQIFTODSA-N 0.000 description 2
- OLTFZQIYCNOBLI-DCAQKATOSA-N Pro-Cys-Lys Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCCN)C(=O)O OLTFZQIYCNOBLI-DCAQKATOSA-N 0.000 description 2
- XJROSHJRQTXWAE-XGEHTFHBSA-N Pro-Cys-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CS)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XJROSHJRQTXWAE-XGEHTFHBSA-N 0.000 description 2
- VPEVBAUSTBWQHN-NHCYSSNCSA-N Pro-Glu-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O VPEVBAUSTBWQHN-NHCYSSNCSA-N 0.000 description 2
- ULIWFCCJIOEHMU-BQBZGAKWSA-N Pro-Gly-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1 ULIWFCCJIOEHMU-BQBZGAKWSA-N 0.000 description 2
- HAAQQNHQZBOWFO-LURJTMIESA-N Pro-Gly-Gly Chemical compound OC(=O)CNC(=O)CNC(=O)[C@@H]1CCCN1 HAAQQNHQZBOWFO-LURJTMIESA-N 0.000 description 2
- LEIKGVHQTKHOLM-IUCAKERBSA-N Pro-Pro-Gly Chemical compound OC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 LEIKGVHQTKHOLM-IUCAKERBSA-N 0.000 description 2
- PCWLNNZTBJTZRN-AVGNSLFASA-N Pro-Pro-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 PCWLNNZTBJTZRN-AVGNSLFASA-N 0.000 description 2
- FDMKYQQYJKYCLV-GUBZILKMSA-N Pro-Pro-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 FDMKYQQYJKYCLV-GUBZILKMSA-N 0.000 description 2
- KWMZPPWYBVZIER-XGEHTFHBSA-N Pro-Ser-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O KWMZPPWYBVZIER-XGEHTFHBSA-N 0.000 description 2
- ZTHYODDOHIVTJV-UHFFFAOYSA-N Propyl gallate Chemical compound CCCOC(=O)C1=CC(O)=C(O)C(O)=C1 ZTHYODDOHIVTJV-UHFFFAOYSA-N 0.000 description 2
- 229940079156 Proteasome inhibitor Drugs 0.000 description 2
- 206010037660 Pyrexia Diseases 0.000 description 2
- WTWGOQRNRFHFQD-JBDRJPRFSA-N Ser-Ala-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O WTWGOQRNRFHFQD-JBDRJPRFSA-N 0.000 description 2
- WDXYVIIVDIDOSX-DCAQKATOSA-N Ser-Arg-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CO)CCCN=C(N)N WDXYVIIVDIDOSX-DCAQKATOSA-N 0.000 description 2
- VGNYHOBZJKWRGI-CIUDSAMLSA-N Ser-Asn-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CO VGNYHOBZJKWRGI-CIUDSAMLSA-N 0.000 description 2
- CTLVSHXLRVEILB-UBHSHLNASA-N Ser-Asn-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CO)N CTLVSHXLRVEILB-UBHSHLNASA-N 0.000 description 2
- GZFAWAQTEYDKII-YUMQZZPRSA-N Ser-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CO GZFAWAQTEYDKII-YUMQZZPRSA-N 0.000 description 2
- GZSZPKSBVAOGIE-CIUDSAMLSA-N Ser-Lys-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O GZSZPKSBVAOGIE-CIUDSAMLSA-N 0.000 description 2
- PPCZVWHJWJFTFN-ZLUOBGJFSA-N Ser-Ser-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O PPCZVWHJWJFTFN-ZLUOBGJFSA-N 0.000 description 2
- OLKICIBQRVSQMA-SRVKXCTJSA-N Ser-Ser-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O OLKICIBQRVSQMA-SRVKXCTJSA-N 0.000 description 2
- NADLKBTYNKUJEP-KATARQTJSA-N Ser-Thr-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O NADLKBTYNKUJEP-KATARQTJSA-N 0.000 description 2
- 108010090804 Streptavidin Proteins 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 208000031673 T-Cell Cutaneous Lymphoma Diseases 0.000 description 2
- 208000026651 T-cell prolymphocytic leukemia Diseases 0.000 description 2
- DWYAUVCQDTZIJI-VZFHVOOUSA-N Thr-Ala-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O DWYAUVCQDTZIJI-VZFHVOOUSA-N 0.000 description 2
- CAJFZCICSVBOJK-SHGPDSBTSA-N Thr-Ala-Thr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O CAJFZCICSVBOJK-SHGPDSBTSA-N 0.000 description 2
- XSLXHSYIVPGEER-KZVJFYERSA-N Thr-Ala-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O XSLXHSYIVPGEER-KZVJFYERSA-N 0.000 description 2
- VASYSJHSMSBTDU-LKXGYXEUSA-N Thr-Asn-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CS)C(=O)O)N)O VASYSJHSMSBTDU-LKXGYXEUSA-N 0.000 description 2
- DSLHSTIUAPKERR-XGEHTFHBSA-N Thr-Cys-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(O)=O DSLHSTIUAPKERR-XGEHTFHBSA-N 0.000 description 2
- DIPIPFHFLPTCLK-LOKLDPHHSA-N Thr-Gln-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N1CCC[C@@H]1C(=O)O)N)O DIPIPFHFLPTCLK-LOKLDPHHSA-N 0.000 description 2
- GKWNLDNXMMLRMC-GLLZPBPUSA-N Thr-Glu-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N)O GKWNLDNXMMLRMC-GLLZPBPUSA-N 0.000 description 2
- SGAOHNPSEPVAFP-ZDLURKLDSA-N Thr-Ser-Gly Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SGAOHNPSEPVAFP-ZDLURKLDSA-N 0.000 description 2
- BEZTUFWTPVOROW-KJEVXHAQSA-N Thr-Tyr-Arg Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N)O BEZTUFWTPVOROW-KJEVXHAQSA-N 0.000 description 2
- OGOYMQWIWHGTGH-KZVJFYERSA-N Thr-Val-Ala Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O OGOYMQWIWHGTGH-KZVJFYERSA-N 0.000 description 2
- AXEJRUGTOJPZKG-XGEHTFHBSA-N Thr-Val-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CS)C(=O)O)N)O AXEJRUGTOJPZKG-XGEHTFHBSA-N 0.000 description 2
- HDQJVXVRGJUDML-UBHSHLNASA-N Trp-Cys-Asn Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(=O)N)C(=O)O)N HDQJVXVRGJUDML-UBHSHLNASA-N 0.000 description 2
- SVGAWGVHFIYAEE-JSGCOSHPSA-N Trp-Gly-Gln Chemical compound C1=CC=C2C(C[C@H](N)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(O)=O)=CNC2=C1 SVGAWGVHFIYAEE-JSGCOSHPSA-N 0.000 description 2
- NLWCSMOXNKBRLC-WDSOQIARSA-N Trp-Lys-Val Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O NLWCSMOXNKBRLC-WDSOQIARSA-N 0.000 description 2
- 102100024587 Tumor necrosis factor ligand superfamily member 15 Human genes 0.000 description 2
- 102100031988 Tumor necrosis factor ligand superfamily member 6 Human genes 0.000 description 2
- SLCSPPCQWUHPPO-JYJNAYRXSA-N Tyr-Glu-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 SLCSPPCQWUHPPO-JYJNAYRXSA-N 0.000 description 2
- KGSDLCMCDFETHU-YESZJQIVSA-N Tyr-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CC2=CC=C(C=C2)O)N)C(=O)O KGSDLCMCDFETHU-YESZJQIVSA-N 0.000 description 2
- UPODKYBYUBTWSV-BZSNNMDCSA-N Tyr-Phe-Cys Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CS)C(O)=O)C1=CC=C(O)C=C1 UPODKYBYUBTWSV-BZSNNMDCSA-N 0.000 description 2
- YYLHVUCSTXXKBS-IHRRRGAJSA-N Tyr-Pro-Ser Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O YYLHVUCSTXXKBS-IHRRRGAJSA-N 0.000 description 2
- RIVVDNTUSRVTQT-IRIUXVKKSA-N Tyr-Thr-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N)O RIVVDNTUSRVTQT-IRIUXVKKSA-N 0.000 description 2
- NUQZCPSZHGIYTA-HKUYNNGSSA-N Tyr-Trp-Gly Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CC3=CC=C(C=C3)O)N NUQZCPSZHGIYTA-HKUYNNGSSA-N 0.000 description 2
- 108010079206 V-Set Domain-Containing T-Cell Activation Inhibitor 1 Proteins 0.000 description 2
- 102100038929 V-set domain-containing T-cell activation inhibitor 1 Human genes 0.000 description 2
- FRUYSSRPJXNRRB-GUBZILKMSA-N Val-Cys-Arg Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N FRUYSSRPJXNRRB-GUBZILKMSA-N 0.000 description 2
- XTDDIVQWDXMRJL-IHRRRGAJSA-N Val-Leu-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](C(C)C)N XTDDIVQWDXMRJL-IHRRRGAJSA-N 0.000 description 2
- SYSWVVCYSXBVJG-RHYQMDGZSA-N Val-Leu-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N)O SYSWVVCYSXBVJG-RHYQMDGZSA-N 0.000 description 2
- KRAHMIJVUPUOTQ-DCAQKATOSA-N Val-Ser-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N KRAHMIJVUPUOTQ-DCAQKATOSA-N 0.000 description 2
- TVGWMCTYUFBXAP-QTKMDUPCSA-N Val-Thr-His Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](C(C)C)N)O TVGWMCTYUFBXAP-QTKMDUPCSA-N 0.000 description 2
- GVNLOVJNNDZUHS-RHYQMDGZSA-N Val-Thr-Lys Chemical compound [H]N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(O)=O GVNLOVJNNDZUHS-RHYQMDGZSA-N 0.000 description 2
- OWFGFHQMSBTKLX-UFYCRDLUSA-N Val-Tyr-Tyr Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)N OWFGFHQMSBTKLX-UFYCRDLUSA-N 0.000 description 2
- ZHWZDZFWBXWPDW-GUBZILKMSA-N Val-Val-Cys Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CS)C(O)=O ZHWZDZFWBXWPDW-GUBZILKMSA-N 0.000 description 2
- LLJLBRRXKZTTRD-GUBZILKMSA-N Val-Val-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(=O)O)N LLJLBRRXKZTTRD-GUBZILKMSA-N 0.000 description 2
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 2
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 2
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 2
- 208000016025 Waldenstroem macroglobulinemia Diseases 0.000 description 2
- 208000033559 Waldenström macroglobulinemia Diseases 0.000 description 2
- 208000019790 abdominal distention Diseases 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 2
- 230000008484 agonism Effects 0.000 description 2
- 108010024078 alanyl-glycyl-serine Proteins 0.000 description 2
- 108010041407 alanylaspartic acid Proteins 0.000 description 2
- 108010087924 alanylproline Proteins 0.000 description 2
- 229940100198 alkylating agent Drugs 0.000 description 2
- 239000002168 alkylating agent Substances 0.000 description 2
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 2
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 2
- 125000000539 amino acid group Chemical group 0.000 description 2
- 239000012491 analyte Substances 0.000 description 2
- 229940121369 angiogenesis inhibitor Drugs 0.000 description 2
- 230000002491 angiogenic effect Effects 0.000 description 2
- 229940045799 anthracyclines and related substance Drugs 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 230000000844 anti-bacterial effect Effects 0.000 description 2
- 230000000340 anti-metabolite Effects 0.000 description 2
- 230000009830 antibody antigen interaction Effects 0.000 description 2
- 239000000611 antibody drug conjugate Substances 0.000 description 2
- 229940049595 antibody-drug conjugate Drugs 0.000 description 2
- 239000003429 antifungal agent Substances 0.000 description 2
- 229940121375 antifungal agent Drugs 0.000 description 2
- 229940100197 antimetabolite Drugs 0.000 description 2
- 239000002256 antimetabolite Substances 0.000 description 2
- 229940034982 antineoplastic agent Drugs 0.000 description 2
- 230000005975 antitumor immune response Effects 0.000 description 2
- 108010009111 arginyl-glycyl-glutamic acid Proteins 0.000 description 2
- 108010091092 arginyl-glycyl-proline Proteins 0.000 description 2
- 108010092854 aspartyllysine Proteins 0.000 description 2
- 108010068265 aspartyltyrosine Proteins 0.000 description 2
- 229940120638 avastin Drugs 0.000 description 2
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 229960000397 bevacizumab Drugs 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 235000020958 biotin Nutrition 0.000 description 2
- 210000001772 blood platelet Anatomy 0.000 description 2
- 210000000988 bone and bone Anatomy 0.000 description 2
- 210000001185 bone marrow Anatomy 0.000 description 2
- 229960001467 bortezomib Drugs 0.000 description 2
- GXJABQQUPOEUTA-RDJZCZTQSA-N bortezomib Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)B(O)O)NC(=O)C=1N=CC=NC=1)C1=CC=CC=C1 GXJABQQUPOEUTA-RDJZCZTQSA-N 0.000 description 2
- 235000010354 butylated hydroxytoluene Nutrition 0.000 description 2
- 229940095259 butylated hydroxytoluene Drugs 0.000 description 2
- 210000004899 c-terminal region Anatomy 0.000 description 2
- 238000011088 calibration curve Methods 0.000 description 2
- 229960004562 carboplatin Drugs 0.000 description 2
- 239000012876 carrier material Substances 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- 239000006285 cell suspension Substances 0.000 description 2
- 229940030156 cell vaccine Drugs 0.000 description 2
- 210000003169 central nervous system Anatomy 0.000 description 2
- 210000003679 cervix uteri Anatomy 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 2
- 229960004316 cisplatin Drugs 0.000 description 2
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 2
- 238000003776 cleavage reaction Methods 0.000 description 2
- 230000000295 complement effect Effects 0.000 description 2
- 230000004154 complement system Effects 0.000 description 2
- 238000013270 controlled release Methods 0.000 description 2
- 201000007241 cutaneous T cell lymphoma Diseases 0.000 description 2
- 108010016616 cysteinylglycine Proteins 0.000 description 2
- 230000016396 cytokine production Effects 0.000 description 2
- 230000009089 cytolysis Effects 0.000 description 2
- 239000002254 cytotoxic agent Substances 0.000 description 2
- 231100000599 cytotoxic agent Toxicity 0.000 description 2
- 230000003013 cytotoxicity Effects 0.000 description 2
- 231100000135 cytotoxicity Toxicity 0.000 description 2
- 229960002806 daclizumab Drugs 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 210000004443 dendritic cell Anatomy 0.000 description 2
- 229940029030 dendritic cell vaccine Drugs 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 230000005750 disease progression Effects 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 230000007783 downstream signaling Effects 0.000 description 2
- 229960004679 doxorubicin Drugs 0.000 description 2
- 229940056913 eftilagimod alfa Drugs 0.000 description 2
- HESCAJZNRMSMJG-KKQRBIROSA-N epothilone A Chemical compound C/C([C@@H]1C[C@@H]2O[C@@H]2CCC[C@@H]([C@@H]([C@@H](C)C(=O)C(C)(C)[C@@H](O)CC(=O)O1)O)C)=C\C1=CSC(C)=N1 HESCAJZNRMSMJG-KKQRBIROSA-N 0.000 description 2
- QXRSDHAAWVKZLJ-PVYNADRNSA-N epothilone B Chemical compound C/C([C@@H]1C[C@@H]2O[C@]2(C)CCC[C@@H]([C@@H]([C@@H](C)C(=O)C(C)(C)[C@@H](O)CC(=O)O1)O)C)=C\C1=CSC(C)=N1 QXRSDHAAWVKZLJ-PVYNADRNSA-N 0.000 description 2
- QAMYWGZHLCQOOJ-WRNBYXCMSA-N eribulin mesylate Chemical compound CS(O)(=O)=O.C([C@H]1CC[C@@H]2O[C@@H]3[C@H]4O[C@@H]5C[C@](O[C@H]4[C@H]2O1)(O[C@@H]53)CC[C@@H]1O[C@H](C(C1)=C)CC1)C(=O)C[C@@H]2[C@@H](OC)[C@@H](C[C@H](O)CN)O[C@H]2C[C@@H]2C(=C)[C@H](C)C[C@H]1O2 QAMYWGZHLCQOOJ-WRNBYXCMSA-N 0.000 description 2
- IJJVMEJXYNJXOJ-UHFFFAOYSA-N fluquinconazole Chemical compound C=1C=C(Cl)C=C(Cl)C=1N1C(=O)C2=CC(F)=CC=C2N=C1N1C=NC=N1 IJJVMEJXYNJXOJ-UHFFFAOYSA-N 0.000 description 2
- 108020001507 fusion proteins Proteins 0.000 description 2
- 102000037865 fusion proteins Human genes 0.000 description 2
- 229950001109 galiximab Drugs 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 2
- 229960005277 gemcitabine Drugs 0.000 description 2
- 208000005017 glioblastoma Diseases 0.000 description 2
- 108010013768 glutamyl-aspartyl-proline Proteins 0.000 description 2
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 2
- 108010049041 glutamylalanine Proteins 0.000 description 2
- 108010000434 glycyl-alanyl-leucine Proteins 0.000 description 2
- 108010050475 glycyl-leucyl-tyrosine Proteins 0.000 description 2
- 108010074027 glycyl-seryl-phenylalanine Proteins 0.000 description 2
- 108010089804 glycyl-threonine Proteins 0.000 description 2
- 210000003128 head Anatomy 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 210000002443 helper t lymphocyte Anatomy 0.000 description 2
- 208000019691 hematopoietic and lymphoid cell neoplasm Diseases 0.000 description 2
- 210000003630 histaminocyte Anatomy 0.000 description 2
- 102000043321 human CTLA4 Human genes 0.000 description 2
- 210000005260 human cell Anatomy 0.000 description 2
- 230000003832 immune regulation Effects 0.000 description 2
- 239000012274 immune-checkpoint protein inhibitor Substances 0.000 description 2
- 238000002991 immunohistochemical analysis Methods 0.000 description 2
- 239000002955 immunomodulating agent Substances 0.000 description 2
- 229960001438 immunostimulant agent Drugs 0.000 description 2
- 239000003022 immunostimulating agent Substances 0.000 description 2
- 238000000099 in vitro assay Methods 0.000 description 2
- 238000005462 in vivo assay Methods 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 230000002757 inflammatory effect Effects 0.000 description 2
- 229960003130 interferon gamma Drugs 0.000 description 2
- 230000000968 intestinal effect Effects 0.000 description 2
- 238000001361 intraarterial administration Methods 0.000 description 2
- 208000037393 large granular lymphocyte leukemia Diseases 0.000 description 2
- 108010044311 leucyl-glycyl-glycine Proteins 0.000 description 2
- 210000000265 leukocyte Anatomy 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 210000004072 lung Anatomy 0.000 description 2
- 210000001165 lymph node Anatomy 0.000 description 2
- 108010017391 lysylvaline Proteins 0.000 description 2
- 230000036210 malignancy Effects 0.000 description 2
- 239000003550 marker Substances 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 230000010534 mechanism of action Effects 0.000 description 2
- 229960004961 mechlorethamine Drugs 0.000 description 2
- HAWPXGHAZFHHAD-UHFFFAOYSA-N mechlorethamine Chemical class ClCCN(C)CCCl HAWPXGHAZFHHAD-UHFFFAOYSA-N 0.000 description 2
- PSGAAPLEWMOORI-PEINSRQWSA-N medroxyprogesterone acetate Chemical compound C([C@@]12C)CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2CC[C@]2(C)[C@@](OC(C)=O)(C(C)=O)CC[C@H]21 PSGAAPLEWMOORI-PEINSRQWSA-N 0.000 description 2
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 2
- 210000001616 monocyte Anatomy 0.000 description 2
- 230000035772 mutation Effects 0.000 description 2
- 201000005962 mycosis fungoides Diseases 0.000 description 2
- 210000003739 neck Anatomy 0.000 description 2
- 210000000440 neutrophil Anatomy 0.000 description 2
- 150000007523 nucleic acids Chemical class 0.000 description 2
- 238000011275 oncology therapy Methods 0.000 description 2
- 230000003204 osmotic effect Effects 0.000 description 2
- 208000013371 ovarian adenocarcinoma Diseases 0.000 description 2
- 210000001672 ovary Anatomy 0.000 description 2
- 201000006588 ovary adenocarcinoma Diseases 0.000 description 2
- 238000007911 parenteral administration Methods 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 230000035699 permeability Effects 0.000 description 2
- 230000008782 phagocytosis Effects 0.000 description 2
- 108010070409 phenylalanyl-glycyl-glycine Proteins 0.000 description 2
- 108010024654 phenylalanyl-prolyl-alanine Proteins 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 229920005862 polyol Polymers 0.000 description 2
- 150000003077 polyols Chemical class 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- 208000016800 primary central nervous system lymphoma Diseases 0.000 description 2
- 208000025638 primary cutaneous T-cell non-Hodgkin lymphoma Diseases 0.000 description 2
- 108010020755 prolyl-glycyl-glycine Proteins 0.000 description 2
- 108010031719 prolyl-serine Proteins 0.000 description 2
- 108010015796 prolylisoleucine Proteins 0.000 description 2
- 239000003207 proteasome inhibitor Substances 0.000 description 2
- 230000005855 radiation Effects 0.000 description 2
- ZAHRKKWIAAJSAO-UHFFFAOYSA-N rapamycin Natural products COCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)C ZAHRKKWIAAJSAO-UHFFFAOYSA-N 0.000 description 2
- 230000008707 rearrangement Effects 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 201000009410 rhabdomyosarcoma Diseases 0.000 description 2
- 230000007017 scission Effects 0.000 description 2
- 238000010187 selection method Methods 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 108010069117 seryl-lysyl-aspartic acid Proteins 0.000 description 2
- 108010026333 seryl-proline Proteins 0.000 description 2
- 230000011664 signaling Effects 0.000 description 2
- 229960002930 sirolimus Drugs 0.000 description 2
- QFJCIRLUMZQUOT-HPLJOQBZSA-N sirolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 QFJCIRLUMZQUOT-HPLJOQBZSA-N 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- GEHJYWRUCIMESM-UHFFFAOYSA-L sodium sulfite Chemical compound [Na+].[Na+].[O-]S([O-])=O GEHJYWRUCIMESM-UHFFFAOYSA-L 0.000 description 2
- 230000037439 somatic mutation Effects 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 230000004083 survival effect Effects 0.000 description 2
- 238000013268 sustained release Methods 0.000 description 2
- 229940126625 tavolimab Drugs 0.000 description 2
- 208000001608 teratocarcinoma Diseases 0.000 description 2
- 229960003604 testosterone Drugs 0.000 description 2
- 230000004797 therapeutic response Effects 0.000 description 2
- 108010061238 threonyl-glycine Proteins 0.000 description 2
- WYWHKKSPHMUBEB-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 2
- 229960005267 tositumomab Drugs 0.000 description 2
- 231100000027 toxicology Toxicity 0.000 description 2
- 231100000041 toxicology testing Toxicity 0.000 description 2
- 230000009261 transgenic effect Effects 0.000 description 2
- 229960000575 trastuzumab Drugs 0.000 description 2
- 229950007217 tremelimumab Drugs 0.000 description 2
- 229950005972 urelumab Drugs 0.000 description 2
- 108010052774 valyl-lysyl-glycyl-phenylalanyl-tyrosine Proteins 0.000 description 2
- 108010003885 valyl-prolyl-glycyl-glycine Proteins 0.000 description 2
- 108010009962 valyltyrosine Proteins 0.000 description 2
- 238000012795 verification Methods 0.000 description 2
- 229940055760 yervoy Drugs 0.000 description 2
- 229960005499 (+)-discodermolide Drugs 0.000 description 1
- FCCNKYGSMOSYPV-DEDISHTHSA-N (-)-Epothilone E Natural products O=C1[C@H](C)[C@H](O)[C@@H](C)CCC[C@H]2O[C@H]2C[C@@H](/C(=C\c2nc(CO)sc2)/C)OC(=O)C[C@H](O)C1(C)C FCCNKYGSMOSYPV-DEDISHTHSA-N 0.000 description 1
- UKIMCRYGLFQEOE-RLHMMOOASA-N (-)-Epothilone F Natural products O=C1[C@H](C)[C@H](O)[C@@H](C)CCC[C@@]2(C)O[C@H]2C[C@@H](/C(=C\c2nc(CO)sc2)/C)OC(=O)C[C@H](O)C1(C)C UKIMCRYGLFQEOE-RLHMMOOASA-N 0.000 description 1
- QIJRTFXNRTXDIP-UHFFFAOYSA-N (1-carboxy-2-sulfanylethyl)azanium;chloride;hydrate Chemical compound O.Cl.SCC(N)C(O)=O QIJRTFXNRTXDIP-UHFFFAOYSA-N 0.000 description 1
- TXXFZHIQMSBVAR-YGFYDDMGSA-N (1r,5s,6s,7r,10s,14s,16s)-14-[(e)-1-(2-ethyl-1,3-thiazol-4-yl)prop-1-en-2-yl]-6,10-dihydroxy-5,7,9,9-tetramethyl-13,17-dioxabicyclo[14.1.0]heptadecane-8,12-dione Chemical compound S1C(CC)=NC(\C=C(/C)[C@H]2OC(=O)C[C@H](O)C(C)(C)C(=O)[C@H](C)[C@@H](O)[C@@H](C)CCC[C@H]3O[C@H]3C2)=C1 TXXFZHIQMSBVAR-YGFYDDMGSA-N 0.000 description 1
- COEXAQSTZUWMRI-STQMWFEESA-N (2s)-1-[2-[[(2s)-2-amino-3-(4-hydroxyphenyl)propanoyl]amino]acetyl]pyrrolidine-2-carboxylic acid Chemical compound C([C@H](N)C(=O)NCC(=O)N1[C@@H](CCC1)C(O)=O)C1=CC=C(O)C=C1 COEXAQSTZUWMRI-STQMWFEESA-N 0.000 description 1
- JPSHPWJJSVEEAX-OWPBQMJCSA-N (2s)-2-amino-4-fluoranylpentanedioic acid Chemical compound OC(=O)[C@@H](N)CC([18F])C(O)=O JPSHPWJJSVEEAX-OWPBQMJCSA-N 0.000 description 1
- MYBGWENAVMIGMM-GIFXNVAJSA-N (2s)-4-(2,5-difluorophenyl)-n-[(3r,4s)-3-fluoro-1-methylpiperidin-4-yl]-2-(hydroxymethyl)-n-methyl-2-phenyl-2,5-dihydro-1h-pyrrole-1-carboxamide Chemical compound N1([C@](C=C(C1)C=1C(=CC=C(F)C=1)F)(CO)C=1C=CC=CC=1)C(=O)N(C)[C@H]1CCN(C)C[C@H]1F MYBGWENAVMIGMM-GIFXNVAJSA-N 0.000 description 1
- ZGEOSZCDHUVWOC-SSHVMUOYSA-N (2s)-6-amino-2-[[(2s)-5-amino-2-[[(2s)-2-[[(2s,3s)-2-[[(2s)-1-[(2s)-2-[[(2s)-2-aminopropanoyl]amino]-3-methylbutanoyl]pyrrolidine-2-carbonyl]amino]-3-methylpentanoyl]amino]propanoyl]amino]-5-oxopentanoyl]amino]hexanoic acid Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](C)N)C(C)C ZGEOSZCDHUVWOC-SSHVMUOYSA-N 0.000 description 1
- OOKIODJYZSVHDO-QMYFOHRPSA-N (2s)-n-tert-butyl-1-[(2s)-1-[(2s)-2-[[(2s)-2-[[(2s)-2-(dimethylamino)-3-methylbutanoyl]amino]-3-methylbutanoyl]-methylamino]-3-methylbutanoyl]pyrrolidine-2-carbonyl]pyrrolidine-2-carboxamide;hydrochloride Chemical compound Cl.CC(C)[C@H](N(C)C)C(=O)N[C@@H](C(C)C)C(=O)N(C)[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(=O)NC(C)(C)C)CCC1 OOKIODJYZSVHDO-QMYFOHRPSA-N 0.000 description 1
- YPBKTZBXSBLTDK-PKNBQFBNSA-N (3e)-3-[(3-bromo-4-fluoroanilino)-nitrosomethylidene]-4-[2-(sulfamoylamino)ethylamino]-1,2,5-oxadiazole Chemical compound NS(=O)(=O)NCCNC1=NON\C1=C(N=O)/NC1=CC=C(F)C(Br)=C1 YPBKTZBXSBLTDK-PKNBQFBNSA-N 0.000 description 1
- ONKCBKDTKZIWHZ-MRWFHJSOSA-N (4r)-4-[[(2r)-6-amino-2-[[(2r)-2-[[4-(aminocarbamothioylamino)benzoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]hexanoyl]amino]-5-[[(2r)-1-amino-6-[bis[2-[[4-[2-(1h-imidazol-5-yl)ethylamino]-4-oxobutanoyl]amino]acetyl]amino]-1-oxohexan-2-yl]amino]-5-oxope Chemical compound C([C@H](C(=O)N[C@H](CCCCN)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@H](CCCCN(C(=O)CNC(=O)CCC(=O)NCCC=1NC=NC=1)C(=O)CNC(=O)CCC(=O)NCCC=1NC=NC=1)C(N)=O)NC(=O)C=1C=CC(NC(=S)NN)=CC=1)C1=CC=C(O)C=C1 ONKCBKDTKZIWHZ-MRWFHJSOSA-N 0.000 description 1
- XAYAKDZVINDZGB-BMVMHAJPSA-N (4s,7r,8s,9s,10e,13z,16s)-4,8-dihydroxy-5,5,7,9,13-pentamethyl-16-[(e)-1-(2-methyl-1,3-thiazol-4-yl)prop-1-en-2-yl]-1-oxacyclohexadeca-10,13-diene-2,6-dione Chemical compound O1C(=O)C[C@H](O)C(C)(C)C(=O)[C@H](C)[C@@H](O)[C@@H](C)\C=C\C\C(C)=C/C[C@H]1C(\C)=C\C1=CSC(C)=N1 XAYAKDZVINDZGB-BMVMHAJPSA-N 0.000 description 1
- FPVKHBSQESCIEP-UHFFFAOYSA-N (8S)-3-(2-deoxy-beta-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol Natural products C1C(O)C(CO)OC1N1C(NC=NCC2O)=C2N=C1 FPVKHBSQESCIEP-UHFFFAOYSA-N 0.000 description 1
- GVJHHUAWPYXKBD-IEOSBIPESA-N (R)-alpha-Tocopherol Natural products OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-IEOSBIPESA-N 0.000 description 1
- FBFJOZZTIXSPPR-UHFFFAOYSA-N 1-(4-aminobutyl)-2-(ethoxymethyl)imidazo[4,5-c]quinolin-4-amine Chemical compound C1=CC=CC2=C(N(C(COCC)=N3)CCCCN)C3=C(N)N=C21 FBFJOZZTIXSPPR-UHFFFAOYSA-N 0.000 description 1
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 1
- GCKMFJBGXUYNAG-UHFFFAOYSA-N 17alpha-methyltestosterone Natural products C1CC2=CC(=O)CCC2(C)C2C1C1CCC(C)(O)C1(C)CC2 GCKMFJBGXUYNAG-UHFFFAOYSA-N 0.000 description 1
- DBPWSSGDRRHUNT-CEGNMAFCSA-N 17α-hydroxyprogesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)C)(O)[C@@]1(C)CC2 DBPWSSGDRRHUNT-CEGNMAFCSA-N 0.000 description 1
- VOXZDWNPVJITMN-ZBRFXRBCSA-N 17β-estradiol Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 VOXZDWNPVJITMN-ZBRFXRBCSA-N 0.000 description 1
- TXQAZWIBPGKHOX-UHFFFAOYSA-N 1H-indol-3-amine Chemical compound C1=CC=C2C(N)=CNC2=C1 TXQAZWIBPGKHOX-UHFFFAOYSA-N 0.000 description 1
- IZZYUABKZYIINT-UHFFFAOYSA-N 2-[3-[(4-cyanophenyl)methyl]indolizin-1-yl]-n-(3-methyl-1,2-thiazol-5-yl)-2-oxoacetamide Chemical compound S1N=C(C)C=C1NC(=O)C(=O)C1=C2C=CC=CN2C(CC=2C=CC(=CC=2)C#N)=C1 IZZYUABKZYIINT-UHFFFAOYSA-N 0.000 description 1
- VNPMDUDIDCXVCH-UHFFFAOYSA-N 2-[4-[2-(2,3-dihydro-1H-inden-2-ylamino)pyrimidin-5-yl]-3-(3-piperazin-1-ylpropyl)pyrazol-1-yl]-1-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)ethanone Chemical compound O=C(CN1C=C(C(CCCN2CCNCC2)=N1)C1=CN=C(NC2CC3=C(C2)C=CC=C3)N=C1)N1CCC2=C(C1)N=NN2 VNPMDUDIDCXVCH-UHFFFAOYSA-N 0.000 description 1
- RTQWWZBSTRGEAV-PKHIMPSTSA-N 2-[[(2s)-2-[bis(carboxymethyl)amino]-3-[4-(methylcarbamoylamino)phenyl]propyl]-[2-[bis(carboxymethyl)amino]propyl]amino]acetic acid Chemical compound CNC(=O)NC1=CC=C(C[C@@H](CN(CC(C)N(CC(O)=O)CC(O)=O)CC(O)=O)N(CC(O)=O)CC(O)=O)C=C1 RTQWWZBSTRGEAV-PKHIMPSTSA-N 0.000 description 1
- FJCDSQATIJKQKA-UHFFFAOYSA-N 2-fluoro-n-[[5-(6-methylpyridin-2-yl)-4-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-1h-imidazol-2-yl]methyl]aniline Chemical compound CC1=CC=CC(C2=C(N=C(CNC=3C(=CC=CC=3)F)N2)C2=CN3N=CN=C3C=C2)=N1 FJCDSQATIJKQKA-UHFFFAOYSA-N 0.000 description 1
- OVSKGTONMLKNPZ-UHFFFAOYSA-N 3-(1-methylindol-3-yl)-4-(1-methyl-6-nitroindol-3-yl)pyrrole-2,5-dione Chemical compound C12=CC=CC=C2N(C)C=C1C1=C(C=2C3=CC=C(C=C3N(C)C=2)[N+]([O-])=O)C(=O)NC1=O OVSKGTONMLKNPZ-UHFFFAOYSA-N 0.000 description 1
- LKKMLIBUAXYLOY-UHFFFAOYSA-N 3-Amino-1-methyl-5H-pyrido[4,3-b]indole Chemical compound N1C2=CC=CC=C2C2=C1C=C(N)N=C2C LKKMLIBUAXYLOY-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- FWBHETKCLVMNFS-UHFFFAOYSA-N 4',6-Diamino-2-phenylindol Chemical compound C1=CC(C(=N)N)=CC=C1C1=CC2=CC=C(C(N)=N)C=C2N1 FWBHETKCLVMNFS-UHFFFAOYSA-N 0.000 description 1
- IDPUKCWIGUEADI-UHFFFAOYSA-N 5-[bis(2-chloroethyl)amino]uracil Chemical compound ClCCN(CCCl)C1=CNC(=O)NC1=O IDPUKCWIGUEADI-UHFFFAOYSA-N 0.000 description 1
- XGTQWEPDCQCNBF-UHFFFAOYSA-N 5-chloro-n-[2-chloro-5-(4-chlorophenyl)sulfonylphenyl]-2-hydroxy-3-iodobenzamide Chemical compound OC1=C(I)C=C(Cl)C=C1C(=O)NC1=CC(S(=O)(=O)C=2C=CC(Cl)=CC=2)=CC=C1Cl XGTQWEPDCQCNBF-UHFFFAOYSA-N 0.000 description 1
- 208000036762 Acute promyelocytic leukaemia Diseases 0.000 description 1
- 102000007471 Adenosine A2A receptor Human genes 0.000 description 1
- 108010085277 Adenosine A2A receptor Proteins 0.000 description 1
- 102100031934 Adhesion G-protein coupled receptor G1 Human genes 0.000 description 1
- 206010001413 Adult T-cell lymphoma/leukaemia Diseases 0.000 description 1
- YYSWCHMLFJLLBJ-ZLUOBGJFSA-N Ala-Ala-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O YYSWCHMLFJLLBJ-ZLUOBGJFSA-N 0.000 description 1
- JAMAWBXXKFGFGX-KZVJFYERSA-N Ala-Arg-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O JAMAWBXXKFGFGX-KZVJFYERSA-N 0.000 description 1
- BUDNAJYVCUHLSV-ZLUOBGJFSA-N Ala-Asp-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O BUDNAJYVCUHLSV-ZLUOBGJFSA-N 0.000 description 1
- BLIMFWGRQKRCGT-YUMQZZPRSA-N Ala-Gly-Lys Chemical compound C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCCCN BLIMFWGRQKRCGT-YUMQZZPRSA-N 0.000 description 1
- HQJKCXHQNUCKMY-GHCJXIJMSA-N Ala-Ile-Asp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](C)N HQJKCXHQNUCKMY-GHCJXIJMSA-N 0.000 description 1
- MNZHHDPWDWQJCQ-YUMQZZPRSA-N Ala-Leu-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O MNZHHDPWDWQJCQ-YUMQZZPRSA-N 0.000 description 1
- OYJCVIGKMXUVKB-GARJFASQSA-N Ala-Leu-Pro Chemical compound C[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@@H]1C(=O)O)N OYJCVIGKMXUVKB-GARJFASQSA-N 0.000 description 1
- RGQCNKIDEQJEBT-CQDKDKBSSA-N Ala-Leu-Tyr Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 RGQCNKIDEQJEBT-CQDKDKBSSA-N 0.000 description 1
- AJBVYEYZVYPFCF-CIUDSAMLSA-N Ala-Lys-Asn Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O AJBVYEYZVYPFCF-CIUDSAMLSA-N 0.000 description 1
- PMQXMXAASGFUDX-SRVKXCTJSA-N Ala-Lys-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@H](C)N)CCCCN PMQXMXAASGFUDX-SRVKXCTJSA-N 0.000 description 1
- YCRAFFCYWOUEOF-DLOVCJGASA-N Ala-Phe-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)C)CC1=CC=CC=C1 YCRAFFCYWOUEOF-DLOVCJGASA-N 0.000 description 1
- DYJJJCHDHLEFDW-FXQIFTODSA-N Ala-Pro-Cys Chemical compound C[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CS)C(=O)O)N DYJJJCHDHLEFDW-FXQIFTODSA-N 0.000 description 1
- IORKCNUBHNIMKY-CIUDSAMLSA-N Ala-Pro-Glu Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O IORKCNUBHNIMKY-CIUDSAMLSA-N 0.000 description 1
- WQLDNOCHHRISMS-NAKRPEOUSA-N Ala-Pro-Ile Chemical compound [H]N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(O)=O WQLDNOCHHRISMS-NAKRPEOUSA-N 0.000 description 1
- XWFWAXPOLRTDFZ-FXQIFTODSA-N Ala-Pro-Ser Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O XWFWAXPOLRTDFZ-FXQIFTODSA-N 0.000 description 1
- HOVPGJUNRLMIOZ-CIUDSAMLSA-N Ala-Ser-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](C)N HOVPGJUNRLMIOZ-CIUDSAMLSA-N 0.000 description 1
- 108700028369 Alleles Proteins 0.000 description 1
- 241000272814 Anser sp. Species 0.000 description 1
- OTOXOKCIIQLMFH-KZVJFYERSA-N Arg-Ala-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCN=C(N)N OTOXOKCIIQLMFH-KZVJFYERSA-N 0.000 description 1
- MFAMTAVAFBPXDC-LPEHRKFASA-N Arg-Asp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCCN=C(N)N)N)C(=O)O MFAMTAVAFBPXDC-LPEHRKFASA-N 0.000 description 1
- NAARDJBSSPUGCF-FXQIFTODSA-N Arg-Cys-Asn Chemical compound C(C[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(=O)N)C(=O)O)N)CN=C(N)N NAARDJBSSPUGCF-FXQIFTODSA-N 0.000 description 1
- AHPWQERCDZTTNB-FXQIFTODSA-N Arg-Cys-Cys Chemical compound C(C[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CS)C(=O)O)N)CN=C(N)N AHPWQERCDZTTNB-FXQIFTODSA-N 0.000 description 1
- UABKKMXFBRQKKI-BJDJZHNGSA-N Arg-Cys-Ser-Arg Chemical compound N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O UABKKMXFBRQKKI-BJDJZHNGSA-N 0.000 description 1
- HPSVTWMFWCHKFN-GARJFASQSA-N Arg-Glu-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCCN=C(N)N)N)C(=O)O HPSVTWMFWCHKFN-GARJFASQSA-N 0.000 description 1
- YQGZIRIYGHNSQO-ZPFDUUQYSA-N Arg-Ile-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N YQGZIRIYGHNSQO-ZPFDUUQYSA-N 0.000 description 1
- UULLJGQFCDXVTQ-CYDGBPFRSA-N Arg-Pro-Ile Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(O)=O UULLJGQFCDXVTQ-CYDGBPFRSA-N 0.000 description 1
- VRTWYUYCJGNFES-CIUDSAMLSA-N Arg-Ser-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O VRTWYUYCJGNFES-CIUDSAMLSA-N 0.000 description 1
- LRPZJPMQGKGHSG-XGEHTFHBSA-N Arg-Ser-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCCN=C(N)N)N)O LRPZJPMQGKGHSG-XGEHTFHBSA-N 0.000 description 1
- QCTOLCVIGRLMQS-HRCADAONSA-N Arg-Tyr-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=C(C=C2)O)NC(=O)[C@H](CCCN=C(N)N)N)C(=O)O QCTOLCVIGRLMQS-HRCADAONSA-N 0.000 description 1
- YNDLOUMBVDVALC-ZLUOBGJFSA-N Asn-Ala-Ala Chemical compound C[C@@H](C(=O)N[C@@H](C)C(=O)O)NC(=O)[C@H](CC(=O)N)N YNDLOUMBVDVALC-ZLUOBGJFSA-N 0.000 description 1
- SLKLLQWZQHXYSV-CIUDSAMLSA-N Asn-Ala-Lys Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(O)=O SLKLLQWZQHXYSV-CIUDSAMLSA-N 0.000 description 1
- KXFCBAHYSLJCCY-ZLUOBGJFSA-N Asn-Asn-Ser Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O KXFCBAHYSLJCCY-ZLUOBGJFSA-N 0.000 description 1
- XVVOVPFMILMHPX-ZLUOBGJFSA-N Asn-Asp-Asp Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O XVVOVPFMILMHPX-ZLUOBGJFSA-N 0.000 description 1
- IYVSIZAXNLOKFQ-BYULHYEWSA-N Asn-Asp-Val Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O IYVSIZAXNLOKFQ-BYULHYEWSA-N 0.000 description 1
- OLVIPTLKNSAYRJ-YUMQZZPRSA-N Asn-Gly-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CC(=O)N)N OLVIPTLKNSAYRJ-YUMQZZPRSA-N 0.000 description 1
- HPBNLFLSSQDFQW-WHFBIAKZSA-N Asn-Ser-Gly Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O HPBNLFLSSQDFQW-WHFBIAKZSA-N 0.000 description 1
- SNYCNNPOFYBCEK-ZLUOBGJFSA-N Asn-Ser-Ser Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O SNYCNNPOFYBCEK-ZLUOBGJFSA-N 0.000 description 1
- AMGQTNHANMRPOE-LKXGYXEUSA-N Asn-Thr-Ser Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O AMGQTNHANMRPOE-LKXGYXEUSA-N 0.000 description 1
- ZAESWDKAMDVHLL-RCOVLWMOSA-N Asn-Val-Gly Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O ZAESWDKAMDVHLL-RCOVLWMOSA-N 0.000 description 1
- XBQSLMACWDXWLJ-GHCJXIJMSA-N Asp-Ala-Ile Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O XBQSLMACWDXWLJ-GHCJXIJMSA-N 0.000 description 1
- MRQQMVZUHXUPEV-IHRRRGAJSA-N Asp-Arg-Phe Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O MRQQMVZUHXUPEV-IHRRRGAJSA-N 0.000 description 1
- ATYWBXGNXZYZGI-ACZMJKKPSA-N Asp-Asn-Gln Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O ATYWBXGNXZYZGI-ACZMJKKPSA-N 0.000 description 1
- SVFOIXMRMLROHO-SRVKXCTJSA-N Asp-Asp-Phe Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 SVFOIXMRMLROHO-SRVKXCTJSA-N 0.000 description 1
- GHODABZPVZMWCE-FXQIFTODSA-N Asp-Glu-Glu Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O GHODABZPVZMWCE-FXQIFTODSA-N 0.000 description 1
- WSGVTKZFVJSJOG-RCOVLWMOSA-N Asp-Gly-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O WSGVTKZFVJSJOG-RCOVLWMOSA-N 0.000 description 1
- ODNWIBOCFGMRTP-SRVKXCTJSA-N Asp-His-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC(O)=O)CC1=CN=CN1 ODNWIBOCFGMRTP-SRVKXCTJSA-N 0.000 description 1
- KTTCQQNRRLCIBC-GHCJXIJMSA-N Asp-Ile-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(O)=O KTTCQQNRRLCIBC-GHCJXIJMSA-N 0.000 description 1
- UZFHNLYQWMGUHU-DCAQKATOSA-N Asp-Lys-Arg Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O UZFHNLYQWMGUHU-DCAQKATOSA-N 0.000 description 1
- DPNWSMBUYCLEDG-CIUDSAMLSA-N Asp-Lys-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O DPNWSMBUYCLEDG-CIUDSAMLSA-N 0.000 description 1
- DONWIPDSZZJHHK-HJGDQZAQSA-N Asp-Lys-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(=O)O)N)O DONWIPDSZZJHHK-HJGDQZAQSA-N 0.000 description 1
- USNJAPJZSGTTPX-XVSYOHENSA-N Asp-Phe-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O USNJAPJZSGTTPX-XVSYOHENSA-N 0.000 description 1
- AHWRSSLYSGLBGD-CIUDSAMLSA-N Asp-Pro-Glu Chemical compound OC(=O)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O AHWRSSLYSGLBGD-CIUDSAMLSA-N 0.000 description 1
- YUELDQUPTAYEGM-XIRDDKMYSA-N Asp-Trp-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)NC(=O)[C@H](CC(=O)O)N YUELDQUPTAYEGM-XIRDDKMYSA-N 0.000 description 1
- SQIARYGNVQWOSB-BZSNNMDCSA-N Asp-Tyr-Phe Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O SQIARYGNVQWOSB-BZSNNMDCSA-N 0.000 description 1
- HTSSXFASOUSJQG-IHPCNDPISA-N Asp-Tyr-Trp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O HTSSXFASOUSJQG-IHPCNDPISA-N 0.000 description 1
- 206010003571 Astrocytoma Diseases 0.000 description 1
- NOWKCMXCCJGMRR-UHFFFAOYSA-N Aziridine Chemical class C1CN1 NOWKCMXCCJGMRR-UHFFFAOYSA-N 0.000 description 1
- 108091008875 B cell receptors Proteins 0.000 description 1
- 102100029822 B- and T-lymphocyte attenuator Human genes 0.000 description 1
- 208000036170 B-Cell Marginal Zone Lymphoma Diseases 0.000 description 1
- 206010003908 B-cell small lymphocytic lymphoma Diseases 0.000 description 1
- MLDQJTXFUGDVEO-UHFFFAOYSA-N BAY-43-9006 Chemical compound C1=NC(C(=O)NC)=CC(OC=2C=CC(NC(=O)NC=3C=C(C(Cl)=CC=3)C(F)(F)F)=CC=2)=C1 MLDQJTXFUGDVEO-UHFFFAOYSA-N 0.000 description 1
- 239000012664 BCL-2-inhibitor Substances 0.000 description 1
- 229940125565 BMS-986016 Drugs 0.000 description 1
- 229940125431 BRAF inhibitor Drugs 0.000 description 1
- 241000193830 Bacillus <bacterium> Species 0.000 description 1
- 229940122035 Bcl-XL inhibitor Drugs 0.000 description 1
- 229940123711 Bcl2 inhibitor Drugs 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 1
- 206010006143 Brain stem glioma Diseases 0.000 description 1
- 206010006272 Breast mass Diseases 0.000 description 1
- 206010006298 Breast pain Diseases 0.000 description 1
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 1
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 1
- 108010046080 CD27 Ligand Proteins 0.000 description 1
- 102100036008 CD48 antigen Human genes 0.000 description 1
- 210000005236 CD8+ effector T cell Anatomy 0.000 description 1
- 101100315624 Caenorhabditis elegans tyr-1 gene Proteins 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- DLGOEMSEDOSKAD-UHFFFAOYSA-N Carmustine Chemical compound ClCCNC(=O)N(N=O)CCCl DLGOEMSEDOSKAD-UHFFFAOYSA-N 0.000 description 1
- 102000014914 Carrier Proteins Human genes 0.000 description 1
- 102000004091 Caspase-8 Human genes 0.000 description 1
- 108090000538 Caspase-8 Proteins 0.000 description 1
- 102000000844 Cell Surface Receptors Human genes 0.000 description 1
- 108010001857 Cell Surface Receptors Proteins 0.000 description 1
- 102000019034 Chemokines Human genes 0.000 description 1
- 108010012236 Chemokines Proteins 0.000 description 1
- 108010019670 Chimeric Antigen Receptors Proteins 0.000 description 1
- 208000010833 Chronic myeloid leukaemia Diseases 0.000 description 1
- 102000008186 Collagen Human genes 0.000 description 1
- 108010035532 Collagen Proteins 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- 206010010774 Constipation Diseases 0.000 description 1
- 206010011224 Cough Diseases 0.000 description 1
- 239000004971 Cross linker Substances 0.000 description 1
- 229930188224 Cryptophycin Natural products 0.000 description 1
- 101710093674 Cyclic nucleotide-gated cation channel beta-1 Proteins 0.000 description 1
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 1
- QLCPDGRAEJSYQM-LPEHRKFASA-N Cys-Arg-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CS)N)C(=O)O QLCPDGRAEJSYQM-LPEHRKFASA-N 0.000 description 1
- NDUSUIGBMZCOIL-ZKWXMUAHSA-N Cys-Asn-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CS)N NDUSUIGBMZCOIL-ZKWXMUAHSA-N 0.000 description 1
- VZKXOWRNJDEGLZ-WHFBIAKZSA-N Cys-Asp-Gly Chemical compound SC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(O)=O VZKXOWRNJDEGLZ-WHFBIAKZSA-N 0.000 description 1
- HIPHJNWPLMUBQQ-ACZMJKKPSA-N Cys-Cys-Gln Chemical compound SC[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@H](C(O)=O)CCC(N)=O HIPHJNWPLMUBQQ-ACZMJKKPSA-N 0.000 description 1
- UFOBYROTHHYVGW-CIUDSAMLSA-N Cys-Cys-His Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC1=CNC=N1)C(O)=O UFOBYROTHHYVGW-CIUDSAMLSA-N 0.000 description 1
- VBPGTULCFGKGTF-ACZMJKKPSA-N Cys-Glu-Asp Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O VBPGTULCFGKGTF-ACZMJKKPSA-N 0.000 description 1
- SKSJPIBFNFPTJB-NKWVEPMBSA-N Cys-Gly-Pro Chemical compound C1C[C@@H](N(C1)C(=O)CNC(=O)[C@H](CS)N)C(=O)O SKSJPIBFNFPTJB-NKWVEPMBSA-N 0.000 description 1
- OZHXXYOHPLLLMI-CIUDSAMLSA-N Cys-Lys-Ala Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O OZHXXYOHPLLLMI-CIUDSAMLSA-N 0.000 description 1
- KSMSFCBQBQPFAD-GUBZILKMSA-N Cys-Pro-Pro Chemical compound SC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 KSMSFCBQBQPFAD-GUBZILKMSA-N 0.000 description 1
- NRVQLLDIJJEIIZ-VZFHVOOUSA-N Cys-Thr-Ala Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C)C(=O)O)NC(=O)[C@H](CS)N)O NRVQLLDIJJEIIZ-VZFHVOOUSA-N 0.000 description 1
- SAEVTQWAYDPXMU-KATARQTJSA-N Cys-Thr-Leu Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O SAEVTQWAYDPXMU-KATARQTJSA-N 0.000 description 1
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 102000003915 DNA Topoisomerases Human genes 0.000 description 1
- 108090000323 DNA Topoisomerases Proteins 0.000 description 1
- 239000012625 DNA intercalator Substances 0.000 description 1
- 239000012626 DNA minor groove binder Substances 0.000 description 1
- 208000019505 Deglutition disease Diseases 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 102100033189 Diablo IAP-binding mitochondrial protein Human genes 0.000 description 1
- 101710101225 Diablo IAP-binding mitochondrial protein Proteins 0.000 description 1
- 206010012735 Diarrhoea Diseases 0.000 description 1
- 208000000059 Dyspnea Diseases 0.000 description 1
- 206010013975 Dyspnoeas Diseases 0.000 description 1
- 108010067668 E 7974 Proteins 0.000 description 1
- 102100025137 Early activation antigen CD69 Human genes 0.000 description 1
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 1
- 208000002460 Enteropathy-Associated T-Cell Lymphoma Diseases 0.000 description 1
- 206010014950 Eosinophilia Diseases 0.000 description 1
- QXRSDHAAWVKZLJ-OXZHEXMSSA-N Epothilone B Natural products O=C1[C@H](C)[C@H](O)[C@@H](C)CCC[C@@]2(C)O[C@H]2C[C@@H](/C(=C\c2nc(C)sc2)/C)OC(=O)C[C@H](O)C1(C)C QXRSDHAAWVKZLJ-OXZHEXMSSA-N 0.000 description 1
- FBHLXXMDOGRQOQ-FCIKGTDHSA-N Epothilone B10 Natural products O=C1[C@H](C)[C@H](O)[C@H](C)CCC[C@@]2(C)O[C@H]2C[C@@H](/C(=C\c2nc(CC)sc2)/C)OC(=O)C[C@H](O)C1(C)C FBHLXXMDOGRQOQ-FCIKGTDHSA-N 0.000 description 1
- BEFZAMRWPCMWFJ-JRBBLYSQSA-N Epothilone C Natural products O=C1[C@H](C)[C@@H](O)[C@@H](C)CCC/C=C\C[C@@H](/C(=C\c2nc(C)sc2)/C)OC(=O)C[C@H](O)C1(C)C BEFZAMRWPCMWFJ-JRBBLYSQSA-N 0.000 description 1
- XOZIUKBZLSUILX-SDMHVBBESA-N Epothilone D Natural products O=C1[C@H](C)[C@@H](O)[C@@H](C)CCC/C(/C)=C/C[C@@H](/C(=C\c2nc(C)sc2)/C)OC(=O)C[C@H](O)C1(C)C XOZIUKBZLSUILX-SDMHVBBESA-N 0.000 description 1
- UKIMCRYGLFQEOE-UHFFFAOYSA-N Epothilone F Natural products O1C(=O)CC(O)C(C)(C)C(=O)C(C)C(O)C(C)CCCC2(C)OC2CC1C(C)=CC1=CSC(CO)=N1 UKIMCRYGLFQEOE-UHFFFAOYSA-N 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 101150089023 FASLG gene Proteins 0.000 description 1
- 108010021468 Fc gamma receptor IIA Proteins 0.000 description 1
- 101710088098 Forkhead box protein P3 Proteins 0.000 description 1
- 108010001498 Galectin 1 Proteins 0.000 description 1
- 102100021736 Galectin-1 Human genes 0.000 description 1
- 102100031351 Galectin-9 Human genes 0.000 description 1
- 101710121810 Galectin-9 Proteins 0.000 description 1
- 241000967808 Garra Species 0.000 description 1
- 208000021309 Germ cell tumor Diseases 0.000 description 1
- 208000032612 Glial tumor Diseases 0.000 description 1
- 201000010915 Glioblastoma multiforme Diseases 0.000 description 1
- 206010018338 Glioma Diseases 0.000 description 1
- DHNWZLGBTPUTQQ-QEJZJMRPSA-N Gln-Asp-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)N)N DHNWZLGBTPUTQQ-QEJZJMRPSA-N 0.000 description 1
- NKCZYEDZTKOFBG-GUBZILKMSA-N Gln-Gln-Arg Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O NKCZYEDZTKOFBG-GUBZILKMSA-N 0.000 description 1
- RBWKVOSARCFSQQ-FXQIFTODSA-N Gln-Gln-Ser Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O RBWKVOSARCFSQQ-FXQIFTODSA-N 0.000 description 1
- HHQCBFGKQDMWSP-GUBZILKMSA-N Gln-Leu-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCC(=O)N)N HHQCBFGKQDMWSP-GUBZILKMSA-N 0.000 description 1
- XZLLTYBONVKGLO-SDDRHHMPSA-N Gln-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(=O)N)N)C(=O)O XZLLTYBONVKGLO-SDDRHHMPSA-N 0.000 description 1
- DRNMNLKUUKKPIA-HTUGSXCWSA-N Gln-Phe-Thr Chemical compound C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)CCC(N)=O)C(O)=O DRNMNLKUUKKPIA-HTUGSXCWSA-N 0.000 description 1
- WBYHRQBKJGEBQJ-CIUDSAMLSA-N Gln-Pro-Cys Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CCC(=O)N)N)C(=O)N[C@@H](CS)C(=O)O WBYHRQBKJGEBQJ-CIUDSAMLSA-N 0.000 description 1
- XKPACHRGOWQHFH-IRIUXVKKSA-N Gln-Thr-Tyr Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O XKPACHRGOWQHFH-IRIUXVKKSA-N 0.000 description 1
- SGVGIVDZLSHSEN-RYUDHWBXSA-N Gln-Tyr-Gly Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)NCC(O)=O SGVGIVDZLSHSEN-RYUDHWBXSA-N 0.000 description 1
- FITIQFSXXBKFFM-NRPADANISA-N Gln-Val-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O FITIQFSXXBKFFM-NRPADANISA-N 0.000 description 1
- CSMHMEATMDCQNY-DZKIICNBSA-N Gln-Val-Tyr Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O CSMHMEATMDCQNY-DZKIICNBSA-N 0.000 description 1
- ITYRYNUZHPNCIK-GUBZILKMSA-N Glu-Ala-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O ITYRYNUZHPNCIK-GUBZILKMSA-N 0.000 description 1
- CVPXINNKRTZBMO-CIUDSAMLSA-N Glu-Arg-Asn Chemical compound C(C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)O)N)CN=C(N)N CVPXINNKRTZBMO-CIUDSAMLSA-N 0.000 description 1
- CKOFNWCLWRYUHK-XHNCKOQMSA-N Glu-Asp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)O)N)C(=O)O CKOFNWCLWRYUHK-XHNCKOQMSA-N 0.000 description 1
- XTZDZAXYPDISRR-MNXVOIDGSA-N Glu-Ile-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N XTZDZAXYPDISRR-MNXVOIDGSA-N 0.000 description 1
- GRHXUHCFENOCOS-ZPFDUUQYSA-N Glu-Ile-Met Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](CCC(=O)O)N GRHXUHCFENOCOS-ZPFDUUQYSA-N 0.000 description 1
- QDMVXRNLOPTPIE-WDCWCFNPSA-N Glu-Lys-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QDMVXRNLOPTPIE-WDCWCFNPSA-N 0.000 description 1
- NNQDRRUXFJYCCJ-NHCYSSNCSA-N Glu-Pro-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(O)=O NNQDRRUXFJYCCJ-NHCYSSNCSA-N 0.000 description 1
- ALMBZBOCGSVSAI-ACZMJKKPSA-N Glu-Ser-Asn Chemical compound C(CC(=O)O)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(=O)N)C(=O)O)N ALMBZBOCGSVSAI-ACZMJKKPSA-N 0.000 description 1
- QOXDAWODGSIDDI-GUBZILKMSA-N Glu-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(=O)O)N QOXDAWODGSIDDI-GUBZILKMSA-N 0.000 description 1
- BPCLDCNZBUYGOD-BPUTZDHNSA-N Glu-Trp-Glu Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)N)C(=O)N[C@@H](CCC(O)=O)C(O)=O)=CNC2=C1 BPCLDCNZBUYGOD-BPUTZDHNSA-N 0.000 description 1
- ZALGPUWUVHOGAE-GVXVVHGQSA-N Glu-Val-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CCC(=O)O)N ZALGPUWUVHOGAE-GVXVVHGQSA-N 0.000 description 1
- RMWAOBGCZZSJHE-UMNHJUIQSA-N Glu-Val-Pro Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)O)N RMWAOBGCZZSJHE-UMNHJUIQSA-N 0.000 description 1
- JXYMPBCYRKWJEE-BQBZGAKWSA-N Gly-Arg-Ala Chemical compound [H]NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(O)=O JXYMPBCYRKWJEE-BQBZGAKWSA-N 0.000 description 1
- CIMULJZTTOBOPN-WHFBIAKZSA-N Gly-Asn-Asn Chemical compound NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O CIMULJZTTOBOPN-WHFBIAKZSA-N 0.000 description 1
- CQZDZKRHFWJXDF-WDSKDSINSA-N Gly-Gln-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CN CQZDZKRHFWJXDF-WDSKDSINSA-N 0.000 description 1
- HQRHFUYMGCHHJS-LURJTMIESA-N Gly-Gly-Arg Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CCCN=C(N)N HQRHFUYMGCHHJS-LURJTMIESA-N 0.000 description 1
- GDOZQTNZPCUARW-YFKPBYRVSA-N Gly-Gly-Glu Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CCC(O)=O GDOZQTNZPCUARW-YFKPBYRVSA-N 0.000 description 1
- HPAIKDPJURGQLN-KBPBESRZSA-N Gly-His-Phe Chemical compound C([C@H](NC(=O)CN)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CNC=N1 HPAIKDPJURGQLN-KBPBESRZSA-N 0.000 description 1
- BHPQOIPBLYJNAW-NGZCFLSTSA-N Gly-Ile-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)CN BHPQOIPBLYJNAW-NGZCFLSTSA-N 0.000 description 1
- PAWIVEIWWYGBAM-YUMQZZPRSA-N Gly-Leu-Ala Chemical compound NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O PAWIVEIWWYGBAM-YUMQZZPRSA-N 0.000 description 1
- CCBIBMKQNXHNIN-ZETCQYMHSA-N Gly-Leu-Gly Chemical compound NCC(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O CCBIBMKQNXHNIN-ZETCQYMHSA-N 0.000 description 1
- FJWSJWACLMTDMI-WPRPVWTQSA-N Gly-Met-Val Chemical compound [H]NCC(=O)N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(O)=O FJWSJWACLMTDMI-WPRPVWTQSA-N 0.000 description 1
- IBYOLNARKHMLBG-WHOFXGATSA-N Gly-Phe-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)CN)CC1=CC=CC=C1 IBYOLNARKHMLBG-WHOFXGATSA-N 0.000 description 1
- QAMMIGULQSIRCD-IRXDYDNUSA-N Gly-Phe-Tyr Chemical compound C([C@H](NC(=O)C[NH3+])C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C([O-])=O)C1=CC=CC=C1 QAMMIGULQSIRCD-IRXDYDNUSA-N 0.000 description 1
- GGLIDLCEPDHEJO-BQBZGAKWSA-N Gly-Pro-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)CN GGLIDLCEPDHEJO-BQBZGAKWSA-N 0.000 description 1
- IXHQLZIWBCQBLQ-STQMWFEESA-N Gly-Pro-Phe Chemical compound NCC(=O)N1CCC[C@H]1C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 IXHQLZIWBCQBLQ-STQMWFEESA-N 0.000 description 1
- SOEGEPHNZOISMT-BYPYZUCNSA-N Gly-Ser-Gly Chemical compound NCC(=O)N[C@@H](CO)C(=O)NCC(O)=O SOEGEPHNZOISMT-BYPYZUCNSA-N 0.000 description 1
- FFJQHWKSGAWSTJ-BFHQHQDPSA-N Gly-Thr-Ala Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O FFJQHWKSGAWSTJ-BFHQHQDPSA-N 0.000 description 1
- CQMFNTVQVLQRLT-JHEQGTHGSA-N Gly-Thr-Gln Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(O)=O CQMFNTVQVLQRLT-JHEQGTHGSA-N 0.000 description 1
- LLWQVJNHMYBLLK-CDMKHQONSA-N Gly-Thr-Phe Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O LLWQVJNHMYBLLK-CDMKHQONSA-N 0.000 description 1
- SFOXOSKVTLDEDM-HOTGVXAUSA-N Gly-Trp-Leu Chemical compound C1=CC=C2C(C[C@@H](C(=O)N[C@@H](CC(C)C)C(O)=O)NC(=O)CN)=CNC2=C1 SFOXOSKVTLDEDM-HOTGVXAUSA-N 0.000 description 1
- OCRQUYDOYKCOQG-IRXDYDNUSA-N Gly-Tyr-Phe Chemical compound C([C@H](NC(=O)CN)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=C(O)C=C1 OCRQUYDOYKCOQG-IRXDYDNUSA-N 0.000 description 1
- 102000019058 Glycogen Synthase Kinase 3 beta Human genes 0.000 description 1
- 108010051975 Glycogen Synthase Kinase 3 beta Proteins 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 102000001398 Granzyme Human genes 0.000 description 1
- 108060005986 Granzyme Proteins 0.000 description 1
- 206010018852 Haematoma Diseases 0.000 description 1
- ZBLLGPUWGCOJNG-UHFFFAOYSA-N Halichondrin B Natural products CC1CC2(CC(C)C3OC4(CC5OC6C(CC5O4)OC7CC8OC9CCC%10OC(CC(C(C9)C8=C)C%11%12CC%13OC%14C(OC%15CCC(CC(=O)OC7C6C)OC%15C%14O%11)C%13O%12)CC%10=C)CC3O2)OC%16OC(CC1%16)C(O)CC(O)CO ZBLLGPUWGCOJNG-UHFFFAOYSA-N 0.000 description 1
- 102000002812 Heat-Shock Proteins Human genes 0.000 description 1
- 108010004889 Heat-Shock Proteins Proteins 0.000 description 1
- 208000000616 Hemoptysis Diseases 0.000 description 1
- 208000032843 Hemorrhage Diseases 0.000 description 1
- 108010007712 Hepatitis A Virus Cellular Receptor 1 Proteins 0.000 description 1
- 102100034458 Hepatitis A virus cellular receptor 2 Human genes 0.000 description 1
- 101710083479 Hepatitis A virus cellular receptor 2 homolog Proteins 0.000 description 1
- MAABHGXCIBEYQR-XVYDVKMFSA-N His-Asn-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC1=CN=CN1)N MAABHGXCIBEYQR-XVYDVKMFSA-N 0.000 description 1
- HIAHVKLTHNOENC-HGNGGELXSA-N His-Glu-Ala Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O HIAHVKLTHNOENC-HGNGGELXSA-N 0.000 description 1
- TTYKEFZRLKQTHH-MELADBBJSA-N His-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CC2=CN=CN2)N)C(=O)O TTYKEFZRLKQTHH-MELADBBJSA-N 0.000 description 1
- CCUSLCQWVMWTIS-IXOXFDKPSA-N His-Thr-Leu Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O CCUSLCQWVMWTIS-IXOXFDKPSA-N 0.000 description 1
- ALPXXNRQBMRCPZ-MEYUZBJRSA-N His-Thr-Phe Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O ALPXXNRQBMRCPZ-MEYUZBJRSA-N 0.000 description 1
- GYXDQXPCPASCNR-NHCYSSNCSA-N His-Val-Asn Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CC1=CN=CN1)N GYXDQXPCPASCNR-NHCYSSNCSA-N 0.000 description 1
- 101000775042 Homo sapiens Adhesion G-protein coupled receptor G1 Proteins 0.000 description 1
- 101000864344 Homo sapiens B- and T-lymphocyte attenuator Proteins 0.000 description 1
- 101000716130 Homo sapiens CD48 antigen Proteins 0.000 description 1
- 101000934374 Homo sapiens Early activation antigen CD69 Proteins 0.000 description 1
- 101001021491 Homo sapiens HERV-H LTR-associating protein 2 Proteins 0.000 description 1
- 101000878602 Homo sapiens Immunoglobulin alpha Fc receptor Proteins 0.000 description 1
- 101001138062 Homo sapiens Leukocyte-associated immunoglobulin-like receptor 1 Proteins 0.000 description 1
- 101000917826 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor II-a Proteins 0.000 description 1
- 101001023379 Homo sapiens Lysosome-associated membrane glycoprotein 1 Proteins 0.000 description 1
- 101000578784 Homo sapiens Melanoma antigen recognized by T-cells 1 Proteins 0.000 description 1
- 101001023712 Homo sapiens Nectin-3 Proteins 0.000 description 1
- 101100407307 Homo sapiens PDCD1LG2 gene Proteins 0.000 description 1
- 101001094545 Homo sapiens Retrotransposon-like protein 1 Proteins 0.000 description 1
- 101000984753 Homo sapiens Serine/threonine-protein kinase B-raf Proteins 0.000 description 1
- 101000831007 Homo sapiens T-cell immunoreceptor with Ig and ITIM domains Proteins 0.000 description 1
- 101000934341 Homo sapiens T-cell surface glycoprotein CD5 Proteins 0.000 description 1
- 101100207070 Homo sapiens TNFSF8 gene Proteins 0.000 description 1
- 101000831567 Homo sapiens Toll-like receptor 2 Proteins 0.000 description 1
- 101000764622 Homo sapiens Transmembrane and immunoglobulin domain-containing protein 2 Proteins 0.000 description 1
- 101000830596 Homo sapiens Tumor necrosis factor ligand superfamily member 15 Proteins 0.000 description 1
- 101000920026 Homo sapiens Tumor necrosis factor receptor superfamily member EDAR Proteins 0.000 description 1
- 208000008454 Hyperhidrosis Diseases 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- 101710093458 ICOS ligand Proteins 0.000 description 1
- 230000005353 IP-10 production Effects 0.000 description 1
- MKWSZEHGHSLNPF-NAKRPEOUSA-N Ile-Ala-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)O)N MKWSZEHGHSLNPF-NAKRPEOUSA-N 0.000 description 1
- FADXGVVLSPPEQY-GHCJXIJMSA-N Ile-Cys-Asn Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(=O)N)C(=O)O)N FADXGVVLSPPEQY-GHCJXIJMSA-N 0.000 description 1
- PPSQSIDMOVPKPI-BJDJZHNGSA-N Ile-Cys-Leu Chemical compound N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(=O)O PPSQSIDMOVPKPI-BJDJZHNGSA-N 0.000 description 1
- HYLIOBDWPQNLKI-HVTMNAMFSA-N Ile-His-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N HYLIOBDWPQNLKI-HVTMNAMFSA-N 0.000 description 1
- JHNJNTMTZHEDLJ-NAKRPEOUSA-N Ile-Ser-Arg Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O JHNJNTMTZHEDLJ-NAKRPEOUSA-N 0.000 description 1
- PELCGFMHLZXWBQ-BJDJZHNGSA-N Ile-Ser-Leu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)O)N PELCGFMHLZXWBQ-BJDJZHNGSA-N 0.000 description 1
- PRTZQMBYUZFSFA-XEGUGMAKSA-N Ile-Tyr-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)NCC(=O)O)N PRTZQMBYUZFSFA-XEGUGMAKSA-N 0.000 description 1
- NXRNRBOKDBIVKQ-CXTHYWKRSA-N Ile-Tyr-Tyr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)N NXRNRBOKDBIVKQ-CXTHYWKRSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 229940126528 Immuno-Oncology Drug Drugs 0.000 description 1
- 206010061598 Immunodeficiency Diseases 0.000 description 1
- 208000029462 Immunodeficiency disease Diseases 0.000 description 1
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 1
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 1
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 description 1
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 description 1
- 102100038005 Immunoglobulin alpha Fc receptor Human genes 0.000 description 1
- 101000668058 Infectious salmon anemia virus (isolate Atlantic salmon/Norway/810/9/99) RNA-directed RNA polymerase catalytic subunit Proteins 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- 102100027670 Islet amyloid polypeptide Human genes 0.000 description 1
- 229940121730 Janus kinase 2 inhibitor Drugs 0.000 description 1
- 206010023126 Jaundice Diseases 0.000 description 1
- 208000007766 Kaposi sarcoma Diseases 0.000 description 1
- SITWEMZOJNKJCH-UHFFFAOYSA-N L-alanine-L-arginine Natural products CC(N)C(=O)NC(C(O)=O)CCCNC(N)=N SITWEMZOJNKJCH-UHFFFAOYSA-N 0.000 description 1
- 239000011786 L-ascorbyl-6-palmitate Substances 0.000 description 1
- QAQJMLQRFWZOBN-LAUBAEHRSA-N L-ascorbyl-6-palmitate Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](O)[C@H]1OC(=O)C(O)=C1O QAQJMLQRFWZOBN-LAUBAEHRSA-N 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- 239000005551 L01XE03 - Erlotinib Substances 0.000 description 1
- 239000005511 L01XE05 - Sorafenib Substances 0.000 description 1
- 206010023804 Large intestine perforation Diseases 0.000 description 1
- QPRQGENIBFLVEB-BJDJZHNGSA-N Leu-Ala-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O QPRQGENIBFLVEB-BJDJZHNGSA-N 0.000 description 1
- KWTVLKBOQATPHJ-SRVKXCTJSA-N Leu-Ala-Lys Chemical compound C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(C)C)N KWTVLKBOQATPHJ-SRVKXCTJSA-N 0.000 description 1
- DQPQTXMIRBUWKO-DCAQKATOSA-N Leu-Ala-Met Chemical compound C[C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](CC(C)C)N DQPQTXMIRBUWKO-DCAQKATOSA-N 0.000 description 1
- UCOCBWDBHCUPQP-DCAQKATOSA-N Leu-Arg-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(O)=O UCOCBWDBHCUPQP-DCAQKATOSA-N 0.000 description 1
- OIARJGNVARWKFP-YUMQZZPRSA-N Leu-Asn-Gly Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O OIARJGNVARWKFP-YUMQZZPRSA-N 0.000 description 1
- TWQIYNGNYNJUFM-NHCYSSNCSA-N Leu-Asn-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O TWQIYNGNYNJUFM-NHCYSSNCSA-N 0.000 description 1
- WIDZHJTYKYBLSR-DCAQKATOSA-N Leu-Glu-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O WIDZHJTYKYBLSR-DCAQKATOSA-N 0.000 description 1
- QJUWBDPGGYVRHY-YUMQZZPRSA-N Leu-Gly-Cys Chemical compound CC(C)C[C@@H](C(=O)NCC(=O)N[C@@H](CS)C(=O)O)N QJUWBDPGGYVRHY-YUMQZZPRSA-N 0.000 description 1
- KGCLIYGPQXUNLO-IUCAKERBSA-N Leu-Gly-Glu Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCC(O)=O KGCLIYGPQXUNLO-IUCAKERBSA-N 0.000 description 1
- VWHGTYCRDRBSFI-ZETCQYMHSA-N Leu-Gly-Gly Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)NCC(O)=O VWHGTYCRDRBSFI-ZETCQYMHSA-N 0.000 description 1
- DDEMUMVXNFPDKC-SRVKXCTJSA-N Leu-His-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](CS)C(=O)O)N DDEMUMVXNFPDKC-SRVKXCTJSA-N 0.000 description 1
- KUIDCYNIEJBZBU-AJNGGQMLSA-N Leu-Ile-Leu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(O)=O KUIDCYNIEJBZBU-AJNGGQMLSA-N 0.000 description 1
- IFMPDNRWZZEZSL-SRVKXCTJSA-N Leu-Leu-Cys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(O)=O IFMPDNRWZZEZSL-SRVKXCTJSA-N 0.000 description 1
- KPYAOIVPJKPIOU-KKUMJFAQSA-N Leu-Lys-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(O)=O KPYAOIVPJKPIOU-KKUMJFAQSA-N 0.000 description 1
- AUNMOHYWTAPQLA-XUXIUFHCSA-N Leu-Met-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O AUNMOHYWTAPQLA-XUXIUFHCSA-N 0.000 description 1
- NJMXCOOEFLMZSR-AVGNSLFASA-N Leu-Met-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(O)=O NJMXCOOEFLMZSR-AVGNSLFASA-N 0.000 description 1
- CHJKEDSZNSONPS-DCAQKATOSA-N Leu-Pro-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O CHJKEDSZNSONPS-DCAQKATOSA-N 0.000 description 1
- LINKCQUOMUDLKN-KATARQTJSA-N Leu-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(C)C)N)O LINKCQUOMUDLKN-KATARQTJSA-N 0.000 description 1
- LJBVRCDPWOJOEK-PPCPHDFISA-N Leu-Thr-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O LJBVRCDPWOJOEK-PPCPHDFISA-N 0.000 description 1
- ILDSIMPXNFWKLH-KATARQTJSA-N Leu-Thr-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O ILDSIMPXNFWKLH-KATARQTJSA-N 0.000 description 1
- AIQWYVFNBNNOLU-RHYQMDGZSA-N Leu-Thr-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O AIQWYVFNBNNOLU-RHYQMDGZSA-N 0.000 description 1
- JGKHAFUAPZCCDU-BZSNNMDCSA-N Leu-Tyr-Leu Chemical compound CC(C)C[C@H]([NH3+])C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C([O-])=O)CC1=CC=C(O)C=C1 JGKHAFUAPZCCDU-BZSNNMDCSA-N 0.000 description 1
- QESXLSQLQHHTIX-RHYQMDGZSA-N Leu-Val-Thr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QESXLSQLQHHTIX-RHYQMDGZSA-N 0.000 description 1
- 102100020943 Leukocyte-associated immunoglobulin-like receptor 1 Human genes 0.000 description 1
- 108010000817 Leuprolide Proteins 0.000 description 1
- GQYIWUVLTXOXAJ-UHFFFAOYSA-N Lomustine Chemical compound ClCCN(N=O)C(=O)NC1CCCCC1 GQYIWUVLTXOXAJ-UHFFFAOYSA-N 0.000 description 1
- 102100029204 Low affinity immunoglobulin gamma Fc region receptor II-a Human genes 0.000 description 1
- 208000028018 Lymphocytic leukaemia Diseases 0.000 description 1
- 206010052178 Lymphocytic lymphoma Diseases 0.000 description 1
- 102000004083 Lymphotoxin-alpha Human genes 0.000 description 1
- 108090000542 Lymphotoxin-alpha Proteins 0.000 description 1
- NTSPQIONFJUMJV-AVGNSLFASA-N Lys-Arg-Val Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(O)=O NTSPQIONFJUMJV-AVGNSLFASA-N 0.000 description 1
- YKIRNDPUWONXQN-GUBZILKMSA-N Lys-Asn-Gln Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N YKIRNDPUWONXQN-GUBZILKMSA-N 0.000 description 1
- DGWXCIORNLWGGG-CIUDSAMLSA-N Lys-Asn-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O DGWXCIORNLWGGG-CIUDSAMLSA-N 0.000 description 1
- KWUKZRFFKPLUPE-HJGDQZAQSA-N Lys-Asp-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O KWUKZRFFKPLUPE-HJGDQZAQSA-N 0.000 description 1
- RZHLIPMZXOEJTL-AVGNSLFASA-N Lys-Gln-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCCCN)N RZHLIPMZXOEJTL-AVGNSLFASA-N 0.000 description 1
- LLSUNJYOSCOOEB-GUBZILKMSA-N Lys-Glu-Asp Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O LLSUNJYOSCOOEB-GUBZILKMSA-N 0.000 description 1
- IMAKMJCBYCSMHM-AVGNSLFASA-N Lys-Glu-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CCCCN IMAKMJCBYCSMHM-AVGNSLFASA-N 0.000 description 1
- ODUQLUADRKMHOZ-JYJNAYRXSA-N Lys-Glu-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCCCN)N)O ODUQLUADRKMHOZ-JYJNAYRXSA-N 0.000 description 1
- NNKLKUUGESXCBS-KBPBESRZSA-N Lys-Gly-Tyr Chemical compound [H]N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O NNKLKUUGESXCBS-KBPBESRZSA-N 0.000 description 1
- AIRZWUMAHCDDHR-KKUMJFAQSA-N Lys-Leu-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O AIRZWUMAHCDDHR-KKUMJFAQSA-N 0.000 description 1
- YTJFXEDRUOQGSP-DCAQKATOSA-N Lys-Pro-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O YTJFXEDRUOQGSP-DCAQKATOSA-N 0.000 description 1
- WQDKIVRHTQYJSN-DCAQKATOSA-N Lys-Ser-Arg Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N WQDKIVRHTQYJSN-DCAQKATOSA-N 0.000 description 1
- CTJUSALVKAWFFU-CIUDSAMLSA-N Lys-Ser-Cys Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N CTJUSALVKAWFFU-CIUDSAMLSA-N 0.000 description 1
- IOQWIOPSKJOEKI-SRVKXCTJSA-N Lys-Ser-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O IOQWIOPSKJOEKI-SRVKXCTJSA-N 0.000 description 1
- DLCAXBGXGOVUCD-PPCPHDFISA-N Lys-Thr-Ile Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O DLCAXBGXGOVUCD-PPCPHDFISA-N 0.000 description 1
- CAVRAQIDHUPECU-UVOCVTCTSA-N Lys-Thr-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O CAVRAQIDHUPECU-UVOCVTCTSA-N 0.000 description 1
- FPQMQEOVSKMVMA-ACRUOGEOSA-N Lys-Tyr-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)NC(=O)[C@H](CCCCN)N)O FPQMQEOVSKMVMA-ACRUOGEOSA-N 0.000 description 1
- HMZPYMSEAALNAE-ULQDDVLXSA-N Lys-Val-Tyr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O HMZPYMSEAALNAE-ULQDDVLXSA-N 0.000 description 1
- 102100035133 Lysosome-associated membrane glycoprotein 1 Human genes 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 208000006662 Mastodynia Diseases 0.000 description 1
- 102100028389 Melanoma antigen recognized by T-cells 1 Human genes 0.000 description 1
- 108010071463 Melanoma-Specific Antigens Proteins 0.000 description 1
- 102000007557 Melanoma-Specific Antigens Human genes 0.000 description 1
- 102000000440 Melanoma-associated antigen Human genes 0.000 description 1
- 108050008953 Melanoma-associated antigen Proteins 0.000 description 1
- 102000012750 Membrane Glycoproteins Human genes 0.000 description 1
- 108010090054 Membrane Glycoproteins Proteins 0.000 description 1
- KQBJYJXPZBNEIK-DCAQKATOSA-N Met-Glu-Arg Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CCCNC(N)=N KQBJYJXPZBNEIK-DCAQKATOSA-N 0.000 description 1
- 206010027480 Metastatic malignant melanoma Diseases 0.000 description 1
- 206010050513 Metastatic renal cell carcinoma Diseases 0.000 description 1
- FQISKWAFAHGMGT-SGJOWKDISA-M Methylprednisolone sodium succinate Chemical compound [Na+].C([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2[C@@H](O)C[C@]2(C)[C@@](O)(C(=O)COC(=O)CCC([O-])=O)CC[C@H]21 FQISKWAFAHGMGT-SGJOWKDISA-M 0.000 description 1
- GCKMFJBGXUYNAG-HLXURNFRSA-N Methyltestosterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@](C)(O)[C@@]1(C)CC2 GCKMFJBGXUYNAG-HLXURNFRSA-N 0.000 description 1
- 108091092878 Microsatellite Proteins 0.000 description 1
- 208000032818 Microsatellite Instability Diseases 0.000 description 1
- 241000699660 Mus musculus Species 0.000 description 1
- 101100519207 Mus musculus Pdcd1 gene Proteins 0.000 description 1
- 101100046559 Mus musculus Tnfrsf12a gene Proteins 0.000 description 1
- 101100153537 Mus musculus Tnfrsf4 gene Proteins 0.000 description 1
- 101100207071 Mus musculus Tnfsf8 gene Proteins 0.000 description 1
- 208000007101 Muscle Cramp Diseases 0.000 description 1
- 208000033761 Myelogenous Chronic BCR-ABL Positive Leukemia Diseases 0.000 description 1
- FBKMWOJEPMPVTQ-UHFFFAOYSA-N N'-(3-bromo-4-fluorophenyl)-N-hydroxy-4-[2-(sulfamoylamino)ethylamino]-1,2,5-oxadiazole-3-carboximidamide Chemical compound NS(=O)(=O)NCCNC1=NON=C1C(=NO)NC1=CC=C(F)C(Br)=C1 FBKMWOJEPMPVTQ-UHFFFAOYSA-N 0.000 description 1
- GUVMFDICMFQHSZ-UHFFFAOYSA-N N-(1-aminoethenyl)-1-[4-[[5-(4-amino-5-methyl-2-oxopyrimidin-1-yl)-3-[[5-(4-amino-5-methyl-2-oxopyrimidin-1-yl)-3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(4-amino-5-methyl-2-oxopyrimidin-1-yl)-3-[[5-(4-amino-5-methyl-2-oxopyrimidin-1-yl)-3-[[5-(4-amino-5-methyl-2-oxopyrimidin-1-yl)-3-[[5-(4-amino-5-methyl-2-oxopyrimidin-1-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[hydroxy-[[3-[hydroxy-[[3-hydroxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy]phosphinothioyl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy]phosphinothioyl]oxyoxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxyoxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxyoxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxyoxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxyoxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxyoxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-5-(2-amino-6-oxo-1H-purin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-5-(2-amino-6-oxo-1H-purin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxyoxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxyoxolan-2-yl]methoxy-hydroxyphosphinothioyl]oxy-5-[[[2-[[[2-[[[5-(2-amino-6-oxo-1H-purin-9-yl)-2-[[[5-(4-amino-2-oxopyrimidin-1-yl)-2-[[hydroxy-[2-(hydroxymethyl)-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-3-yl]oxyphosphinothioyl]oxymethyl]oxolan-3-yl]oxy-hydroxyphosphinothioyl]oxymethyl]oxolan-3-yl]oxy-hydroxyphosphinothioyl]oxymethyl]-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-3-yl]oxy-hydroxyphosphinothioyl]oxymethyl]-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-3-yl]oxy-hydroxyphosphinothioyl]oxymethyl]oxolan-2-yl]-5-methylimidazole-4-carboxamide Chemical compound CC1=C(C(=O)NC(N)=C)N=CN1C1OC(COP(O)(=S)OC2C(OC(C2)N2C(N=C(N)C=C2)=O)COP(O)(=S)OC2C(OC(C2)N2C(NC(=O)C(C)=C2)=O)COP(O)(=S)OC2C(OC(C2)N2C3=C(C(NC(N)=N3)=O)N=C2)COP(O)(=S)OC2C(OC(C2)N2C(N=C(N)C=C2)=O)COP(O)(=S)OC2C(OC(C2)N2C(NC(=O)C(C)=C2)=O)CO)C(OP(O)(=S)OCC2C(CC(O2)N2C(N=C(N)C(C)=C2)=O)OP(O)(=S)OCC2C(CC(O2)N2C(N=C(N)C(C)=C2)=O)OP(O)(=S)OCC2C(CC(O2)N2C(NC(=O)C(C)=C2)=O)OP(O)(=S)OCC2C(CC(O2)N2C(NC(=O)C(C)=C2)=O)OP(O)(=S)OCC2C(CC(O2)N2C3=C(C(NC(N)=N3)=O)N=C2)OP(O)(=S)OCC2C(CC(O2)N2C(NC(=O)C(C)=C2)=O)OP(O)(=S)OCC2C(CC(O2)N2C(N=C(N)C=C2)=O)OP(O)(=S)OCC2C(CC(O2)N2C3=C(C(NC(N)=N3)=O)N=C2)OP(O)(=S)OCC2C(CC(O2)N2C(N=C(N)C(C)=C2)=O)OP(O)(=S)OCC2C(CC(O2)N2C(N=C(N)C(C)=C2)=O)OP(O)(=S)OCC2C(CC(O2)N2C(N=C(N)C(C)=C2)=O)OP(O)(=S)OCC2C(CC(O2)N2C(N=C(N)C(C)=C2)=O)OP(O)(=S)OCC2C(CC(O2)N2C3=C(C(NC(N)=N3)=O)N=C2)OP(O)(=S)OCC2C(CC(O2)N2C(NC(=O)C(C)=C2)=O)OP(O)(=S)OCC2C(CC(O2)N2C(N=C(N)C=C2)=O)OP(O)(=S)OCC2C(CC(O2)N2C3=C(C(NC(N)=N3)=O)N=C2)OP(O)(=S)OCC2C(CC(O2)N2C(NC(=O)C(C)=C2)=O)OP(O)(=S)OCC2C(CC(O2)N2C(NC(=O)C(C)=C2)=O)O)C1 GUVMFDICMFQHSZ-UHFFFAOYSA-N 0.000 description 1
- XZFYRXDAULDNFX-UHFFFAOYSA-N N-L-cysteinyl-L-phenylalanine Natural products SCC(N)C(=O)NC(C(O)=O)CC1=CC=CC=C1 XZFYRXDAULDNFX-UHFFFAOYSA-N 0.000 description 1
- PESQCPHRXOFIPX-UHFFFAOYSA-N N-L-methionyl-L-tyrosine Natural products CSCCC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 PESQCPHRXOFIPX-UHFFFAOYSA-N 0.000 description 1
- XMBSYZWANAQXEV-UHFFFAOYSA-N N-alpha-L-glutamyl-L-phenylalanine Natural products OC(=O)CCC(N)C(=O)NC(C(O)=O)CC1=CC=CC=C1 XMBSYZWANAQXEV-UHFFFAOYSA-N 0.000 description 1
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 description 1
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 1
- 206010028813 Nausea Diseases 0.000 description 1
- 102100035487 Nectin-3 Human genes 0.000 description 1
- 206010061309 Neoplasm progression Diseases 0.000 description 1
- 206010029260 Neuroblastoma Diseases 0.000 description 1
- 208000005890 Neuroma Diseases 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 108091028043 Nucleic acid sequence Proteins 0.000 description 1
- 239000012270 PD-1 inhibitor Substances 0.000 description 1
- 239000012668 PD-1-inhibitor Substances 0.000 description 1
- 208000000821 Parathyroid Neoplasms Diseases 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 208000002471 Penile Neoplasms Diseases 0.000 description 1
- 206010034299 Penile cancer Diseases 0.000 description 1
- 208000027190 Peripheral T-cell lymphomas Diseases 0.000 description 1
- CDNPIRSCAFMMBE-SRVKXCTJSA-N Phe-Asn-Ser Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O CDNPIRSCAFMMBE-SRVKXCTJSA-N 0.000 description 1
- CUMXHKAOHNWRFQ-BZSNNMDCSA-N Phe-Asp-Tyr Chemical compound C([C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=CC=C1 CUMXHKAOHNWRFQ-BZSNNMDCSA-N 0.000 description 1
- OWCLJDXHHZUNEL-IHRRRGAJSA-N Phe-Cys-Val Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(O)=O OWCLJDXHHZUNEL-IHRRRGAJSA-N 0.000 description 1
- CWFGECHCRMGPPT-MXAVVETBSA-N Phe-Ile-Ser Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O CWFGECHCRMGPPT-MXAVVETBSA-N 0.000 description 1
- JLLJTMHNXQTMCK-UBHSHLNASA-N Phe-Pro-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CC1=CC=CC=C1 JLLJTMHNXQTMCK-UBHSHLNASA-N 0.000 description 1
- WWPAHTZOWURIMR-ULQDDVLXSA-N Phe-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CC1=CC=CC=C1 WWPAHTZOWURIMR-ULQDDVLXSA-N 0.000 description 1
- IIEOLPMQYRBZCN-SRVKXCTJSA-N Phe-Ser-Cys Chemical compound N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O IIEOLPMQYRBZCN-SRVKXCTJSA-N 0.000 description 1
- HBXAOEBRGLCLIW-AVGNSLFASA-N Phe-Ser-Gln Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N HBXAOEBRGLCLIW-AVGNSLFASA-N 0.000 description 1
- VGTJSEYTVMAASM-RPTUDFQQSA-N Phe-Thr-Tyr Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O VGTJSEYTVMAASM-RPTUDFQQSA-N 0.000 description 1
- ZYNBEWGJFXTBDU-ACRUOGEOSA-N Phe-Tyr-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](CC2=CC=CC=C2)N ZYNBEWGJFXTBDU-ACRUOGEOSA-N 0.000 description 1
- 208000007913 Pituitary Neoplasms Diseases 0.000 description 1
- 201000005746 Pituitary adenoma Diseases 0.000 description 1
- 206010061538 Pituitary tumour benign Diseases 0.000 description 1
- 208000007452 Plasmacytoma Diseases 0.000 description 1
- 229920002732 Polyanhydride Polymers 0.000 description 1
- 229920000954 Polyglycolide Polymers 0.000 description 1
- 229920001710 Polyorthoester Polymers 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 206010036524 Precursor B-lymphoblastic lymphomas Diseases 0.000 description 1
- 208000009052 Precursor T-Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 1
- 208000017414 Precursor T-cell acute lymphoblastic leukemia Diseases 0.000 description 1
- 206010036711 Primary mediastinal large B-cell lymphomas Diseases 0.000 description 1
- OOLOTUZJUBOMAX-GUBZILKMSA-N Pro-Ala-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O OOLOTUZJUBOMAX-GUBZILKMSA-N 0.000 description 1
- VCYJKOLZYPYGJV-AVGNSLFASA-N Pro-Arg-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O VCYJKOLZYPYGJV-AVGNSLFASA-N 0.000 description 1
- KDIIENQUNVNWHR-JYJNAYRXSA-N Pro-Arg-Phe Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O KDIIENQUNVNWHR-JYJNAYRXSA-N 0.000 description 1
- UPJGUQPLYWTISV-GUBZILKMSA-N Pro-Gln-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O UPJGUQPLYWTISV-GUBZILKMSA-N 0.000 description 1
- UAYHMOIGIQZLFR-NHCYSSNCSA-N Pro-Gln-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O UAYHMOIGIQZLFR-NHCYSSNCSA-N 0.000 description 1
- MGDFPGCFVJFITQ-CIUDSAMLSA-N Pro-Glu-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O MGDFPGCFVJFITQ-CIUDSAMLSA-N 0.000 description 1
- WFHYFCWBLSKEMS-KKUMJFAQSA-N Pro-Glu-Phe Chemical compound N([C@@H](CCC(=O)O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C(=O)[C@@H]1CCCN1 WFHYFCWBLSKEMS-KKUMJFAQSA-N 0.000 description 1
- LGSANCBHSMDFDY-GARJFASQSA-N Pro-Glu-Pro Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CCC(=O)O)C(=O)N2CCC[C@@H]2C(=O)O LGSANCBHSMDFDY-GARJFASQSA-N 0.000 description 1
- UUHXBJHVTVGSKM-BQBZGAKWSA-N Pro-Gly-Asn Chemical compound [H]N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O UUHXBJHVTVGSKM-BQBZGAKWSA-N 0.000 description 1
- VYWNORHENYEQDW-YUMQZZPRSA-N Pro-Gly-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1 VYWNORHENYEQDW-YUMQZZPRSA-N 0.000 description 1
- HAEGAELAYWSUNC-WPRPVWTQSA-N Pro-Gly-Val Chemical compound [H]N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O HAEGAELAYWSUNC-WPRPVWTQSA-N 0.000 description 1
- KLSOMAFWRISSNI-OSUNSFLBSA-N Pro-Ile-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H]1CCCN1 KLSOMAFWRISSNI-OSUNSFLBSA-N 0.000 description 1
- CLJLVCYFABNTHP-DCAQKATOSA-N Pro-Leu-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O CLJLVCYFABNTHP-DCAQKATOSA-N 0.000 description 1
- DRKAXLDECUGLFE-ULQDDVLXSA-N Pro-Leu-Phe Chemical compound CC(C)C[C@H](NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O DRKAXLDECUGLFE-ULQDDVLXSA-N 0.000 description 1
- OFGUOWQVEGTVNU-DCAQKATOSA-N Pro-Lys-Ala Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O OFGUOWQVEGTVNU-DCAQKATOSA-N 0.000 description 1
- ZLXKLMHAMDENIO-DCAQKATOSA-N Pro-Lys-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(O)=O ZLXKLMHAMDENIO-DCAQKATOSA-N 0.000 description 1
- KDBHVPXBQADZKY-GUBZILKMSA-N Pro-Pro-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 KDBHVPXBQADZKY-GUBZILKMSA-N 0.000 description 1
- RCYUBVHMVUHEBM-RCWTZXSCSA-N Pro-Pro-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(O)=O RCYUBVHMVUHEBM-RCWTZXSCSA-N 0.000 description 1
- KBUAPZAZPWNYSW-SRVKXCTJSA-N Pro-Pro-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 KBUAPZAZPWNYSW-SRVKXCTJSA-N 0.000 description 1
- GOMUXSCOIWIJFP-GUBZILKMSA-N Pro-Ser-Arg Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O GOMUXSCOIWIJFP-GUBZILKMSA-N 0.000 description 1
- OWQXAJQZLWHPBH-FXQIFTODSA-N Pro-Ser-Asn Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O OWQXAJQZLWHPBH-FXQIFTODSA-N 0.000 description 1
- GMJDSFYVTAMIBF-FXQIFTODSA-N Pro-Ser-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O GMJDSFYVTAMIBF-FXQIFTODSA-N 0.000 description 1
- SEZGGSHLMROBFX-CIUDSAMLSA-N Pro-Ser-Gln Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O SEZGGSHLMROBFX-CIUDSAMLSA-N 0.000 description 1
- MKGIILKDUGDRRO-FXQIFTODSA-N Pro-Ser-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1 MKGIILKDUGDRRO-FXQIFTODSA-N 0.000 description 1
- HRIXMVRZRGFKNQ-HJGDQZAQSA-N Pro-Thr-Gln Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(O)=O HRIXMVRZRGFKNQ-HJGDQZAQSA-N 0.000 description 1
- GZNYIXWOIUFLGO-ZJDVBMNYSA-N Pro-Thr-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O GZNYIXWOIUFLGO-ZJDVBMNYSA-N 0.000 description 1
- 102000014128 RANK Ligand Human genes 0.000 description 1
- 108010025832 RANK Ligand Proteins 0.000 description 1
- 102000018795 RELT Human genes 0.000 description 1
- 108010052562 RELT Proteins 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 208000015634 Rectal Neoplasms Diseases 0.000 description 1
- 208000007014 Retinitis pigmentosa Diseases 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- BFZKMNSQCNVFGM-UCEYFQQTSA-N Sagopilone Chemical compound O1C(=O)C[C@H](O)C(C)(C)C(=O)[C@H](CC=C)[C@@H](O)[C@@H](C)CCC[C@@]2(C)O[C@H]2C[C@H]1C1=CC=C(SC(C)=N2)C2=C1 BFZKMNSQCNVFGM-UCEYFQQTSA-N 0.000 description 1
- WTUJZHKANPDPIN-CIUDSAMLSA-N Ser-Ala-Lys Chemical compound C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CO)N WTUJZHKANPDPIN-CIUDSAMLSA-N 0.000 description 1
- YQHZVYJAGWMHES-ZLUOBGJFSA-N Ser-Ala-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O YQHZVYJAGWMHES-ZLUOBGJFSA-N 0.000 description 1
- JJKSSJVYOVRJMZ-FXQIFTODSA-N Ser-Arg-Cys Chemical compound C(C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CO)N)CN=C(N)N JJKSSJVYOVRJMZ-FXQIFTODSA-N 0.000 description 1
- QVOGDCQNGLBNCR-FXQIFTODSA-N Ser-Arg-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(O)=O QVOGDCQNGLBNCR-FXQIFTODSA-N 0.000 description 1
- OYEDZGNMSBZCIM-XGEHTFHBSA-N Ser-Arg-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OYEDZGNMSBZCIM-XGEHTFHBSA-N 0.000 description 1
- UBRXAVQWXOWRSJ-ZLUOBGJFSA-N Ser-Asn-Asp Chemical compound C([C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](CO)N)C(=O)N UBRXAVQWXOWRSJ-ZLUOBGJFSA-N 0.000 description 1
- MOVJSUIKUNCVMG-ZLUOBGJFSA-N Ser-Cys-Ser Chemical compound C([C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)O)N)O MOVJSUIKUNCVMG-ZLUOBGJFSA-N 0.000 description 1
- RNMRYWZYFHHOEV-CIUDSAMLSA-N Ser-Gln-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O RNMRYWZYFHHOEV-CIUDSAMLSA-N 0.000 description 1
- CDVFZMOFNJPUDD-ACZMJKKPSA-N Ser-Gln-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O CDVFZMOFNJPUDD-ACZMJKKPSA-N 0.000 description 1
- ZOHGLPQGEHSLPD-FXQIFTODSA-N Ser-Gln-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O ZOHGLPQGEHSLPD-FXQIFTODSA-N 0.000 description 1
- XWCYBVBLJRWOFR-WDSKDSINSA-N Ser-Gln-Gly Chemical compound OC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O XWCYBVBLJRWOFR-WDSKDSINSA-N 0.000 description 1
- VQBCMLMPEWPUTB-ACZMJKKPSA-N Ser-Glu-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O VQBCMLMPEWPUTB-ACZMJKKPSA-N 0.000 description 1
- SFTZWNJFZYOLBD-ZDLURKLDSA-N Ser-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CO SFTZWNJFZYOLBD-ZDLURKLDSA-N 0.000 description 1
- XXXAXOWMBOKTRN-XPUUQOCRSA-N Ser-Gly-Val Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O XXXAXOWMBOKTRN-XPUUQOCRSA-N 0.000 description 1
- DJACUBDEDBZKLQ-KBIXCLLPSA-N Ser-Ile-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(O)=O DJACUBDEDBZKLQ-KBIXCLLPSA-N 0.000 description 1
- JIPVNVNKXJLFJF-BJDJZHNGSA-N Ser-Ile-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CO)N JIPVNVNKXJLFJF-BJDJZHNGSA-N 0.000 description 1
- IUXGJEIKJBYKOO-SRVKXCTJSA-N Ser-Leu-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CO)N IUXGJEIKJBYKOO-SRVKXCTJSA-N 0.000 description 1
- JWOBLHJRDADHLN-KKUMJFAQSA-N Ser-Leu-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O JWOBLHJRDADHLN-KKUMJFAQSA-N 0.000 description 1
- FPCGZYMRFFIYIH-CIUDSAMLSA-N Ser-Lys-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O FPCGZYMRFFIYIH-CIUDSAMLSA-N 0.000 description 1
- HHJFMHQYEAAOBM-ZLUOBGJFSA-N Ser-Ser-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O HHJFMHQYEAAOBM-ZLUOBGJFSA-N 0.000 description 1
- XQJCEKXQUJQNNK-ZLUOBGJFSA-N Ser-Ser-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O XQJCEKXQUJQNNK-ZLUOBGJFSA-N 0.000 description 1
- VGQVAVQWKJLIRM-FXQIFTODSA-N Ser-Ser-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O VGQVAVQWKJLIRM-FXQIFTODSA-N 0.000 description 1
- PCJLFYBAQZQOFE-KATARQTJSA-N Ser-Thr-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CO)N)O PCJLFYBAQZQOFE-KATARQTJSA-N 0.000 description 1
- VLMIUSLQONKLDV-HEIBUPTGSA-N Ser-Thr-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O VLMIUSLQONKLDV-HEIBUPTGSA-N 0.000 description 1
- BDMWLJLPPUCLNV-XGEHTFHBSA-N Ser-Thr-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O BDMWLJLPPUCLNV-XGEHTFHBSA-N 0.000 description 1
- AXKJPUBALUNJEO-UBHSHLNASA-N Ser-Trp-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC(N)=O)C(O)=O AXKJPUBALUNJEO-UBHSHLNASA-N 0.000 description 1
- PIQRHJQWEPWFJG-UWJYBYFXSA-N Ser-Tyr-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C)C(O)=O PIQRHJQWEPWFJG-UWJYBYFXSA-N 0.000 description 1
- GSCVDSBEYVGMJQ-SRVKXCTJSA-N Ser-Tyr-Asp Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](CO)N)O GSCVDSBEYVGMJQ-SRVKXCTJSA-N 0.000 description 1
- HKHCTNFKZXAMIF-KKUMJFAQSA-N Ser-Tyr-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CO)CC1=CC=C(O)C=C1 HKHCTNFKZXAMIF-KKUMJFAQSA-N 0.000 description 1
- OQSQCUWQOIHECT-YJRXYDGGSA-N Ser-Tyr-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OQSQCUWQOIHECT-YJRXYDGGSA-N 0.000 description 1
- IAOHCSQDQDWRQU-GUBZILKMSA-N Ser-Val-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O IAOHCSQDQDWRQU-GUBZILKMSA-N 0.000 description 1
- RCOUFINCYASMDN-GUBZILKMSA-N Ser-Val-Met Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCSC)C(O)=O RCOUFINCYASMDN-GUBZILKMSA-N 0.000 description 1
- HSWXBJCBYSWBPT-GUBZILKMSA-N Ser-Val-Val Chemical compound CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CO)C(C)C)C(O)=O HSWXBJCBYSWBPT-GUBZILKMSA-N 0.000 description 1
- 102100027103 Serine/threonine-protein kinase B-raf Human genes 0.000 description 1
- 206010041067 Small cell lung cancer Diseases 0.000 description 1
- 208000004346 Smoldering Multiple Myeloma Diseases 0.000 description 1
- 208000021712 Soft tissue sarcoma Diseases 0.000 description 1
- 208000005392 Spasm Diseases 0.000 description 1
- 208000000277 Splenic Neoplasms Diseases 0.000 description 1
- 230000020385 T cell costimulation Effects 0.000 description 1
- 208000031672 T-Cell Peripheral Lymphoma Diseases 0.000 description 1
- 208000029052 T-cell acute lymphoblastic leukemia Diseases 0.000 description 1
- 208000037913 T-cell disorder Diseases 0.000 description 1
- 102100039367 T-cell immunoglobulin and mucin domain-containing protein 4 Human genes 0.000 description 1
- 101710174757 T-cell immunoglobulin and mucin domain-containing protein 4 Proteins 0.000 description 1
- 229940126547 T-cell immunoglobulin mucin-3 Drugs 0.000 description 1
- 102100024834 T-cell immunoreceptor with Ig and ITIM domains Human genes 0.000 description 1
- 208000020982 T-lymphoblastic lymphoma Diseases 0.000 description 1
- 108700002718 TACI receptor-IgG Fc fragment fusion Proteins 0.000 description 1
- 229940124613 TLR 7/8 agonist Drugs 0.000 description 1
- 108010014401 TWEAK Receptor Proteins 0.000 description 1
- 102000016946 TWEAK Receptor Human genes 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 229940123237 Taxane Drugs 0.000 description 1
- BPEGJWRSRHCHSN-UHFFFAOYSA-N Temozolomide Chemical compound O=C1N(C)N=NC2=C(C(N)=O)N=CN21 BPEGJWRSRHCHSN-UHFFFAOYSA-N 0.000 description 1
- IGROJMCBGRFRGI-YTLHQDLWSA-N Thr-Ala-Ala Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(O)=O IGROJMCBGRFRGI-YTLHQDLWSA-N 0.000 description 1
- LMMDEZPNUTZJAY-GCJQMDKQSA-N Thr-Asp-Ala Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(O)=O LMMDEZPNUTZJAY-GCJQMDKQSA-N 0.000 description 1
- ASJDFGOPDCVXTG-KATARQTJSA-N Thr-Cys-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(O)=O ASJDFGOPDCVXTG-KATARQTJSA-N 0.000 description 1
- KWQBJOUOSNJDRR-XAVMHZPKSA-N Thr-Cys-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)N1CCC[C@@H]1C(=O)O)N)O KWQBJOUOSNJDRR-XAVMHZPKSA-N 0.000 description 1
- WLDUCKSCDRIVLJ-NUMRIWBASA-N Thr-Gln-Asp Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N)O WLDUCKSCDRIVLJ-NUMRIWBASA-N 0.000 description 1
- UHBPFYOQQPFKQR-JHEQGTHGSA-N Thr-Gln-Gly Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O UHBPFYOQQPFKQR-JHEQGTHGSA-N 0.000 description 1
- VGYBYGQXZJDZJU-XQXXSGGOSA-N Thr-Glu-Ala Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O VGYBYGQXZJDZJU-XQXXSGGOSA-N 0.000 description 1
- QQWNRERCGGZOKG-WEDXCCLWSA-N Thr-Gly-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CC(C)C)C(O)=O QQWNRERCGGZOKG-WEDXCCLWSA-N 0.000 description 1
- FIFDDJFLNVAVMS-RHYQMDGZSA-N Thr-Leu-Met Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(O)=O FIFDDJFLNVAVMS-RHYQMDGZSA-N 0.000 description 1
- NCXVJIQMWSGRHY-KXNHARMFSA-N Thr-Leu-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@@H]1C(=O)O)N)O NCXVJIQMWSGRHY-KXNHARMFSA-N 0.000 description 1
- BDGBHYCAZJPLHX-HJGDQZAQSA-N Thr-Lys-Asn Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O BDGBHYCAZJPLHX-HJGDQZAQSA-N 0.000 description 1
- JLNMFGCJODTXDH-WEDXCCLWSA-N Thr-Lys-Gly Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O JLNMFGCJODTXDH-WEDXCCLWSA-N 0.000 description 1
- LHNNQVXITHUCAB-QTKMDUPCSA-N Thr-Met-His Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N)O LHNNQVXITHUCAB-QTKMDUPCSA-N 0.000 description 1
- HSQXHRIRJSFDOH-URLPEUOOSA-N Thr-Phe-Ile Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O HSQXHRIRJSFDOH-URLPEUOOSA-N 0.000 description 1
- MROIJTGJGIDEEJ-RCWTZXSCSA-N Thr-Pro-Pro Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 MROIJTGJGIDEEJ-RCWTZXSCSA-N 0.000 description 1
- DOBIBIXIHJKVJF-XKBZYTNZSA-N Thr-Ser-Gln Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(N)=O DOBIBIXIHJKVJF-XKBZYTNZSA-N 0.000 description 1
- YRJOLUDFVAUXLI-GSSVUCPTSA-N Thr-Thr-Asp Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC(O)=O YRJOLUDFVAUXLI-GSSVUCPTSA-N 0.000 description 1
- DIHPMRTXPYMDJZ-KAOXEZKKSA-N Thr-Tyr-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N2CCC[C@@H]2C(=O)O)N)O DIHPMRTXPYMDJZ-KAOXEZKKSA-N 0.000 description 1
- LVRFMARKDGGZMX-IZPVPAKOSA-N Thr-Tyr-Thr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)O)C(O)=O)CC1=CC=C(O)C=C1 LVRFMARKDGGZMX-IZPVPAKOSA-N 0.000 description 1
- FYBFTPLPAXZBOY-KKHAAJSZSA-N Thr-Val-Asp Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O FYBFTPLPAXZBOY-KKHAAJSZSA-N 0.000 description 1
- QNXZCKMXHPULME-ZNSHCXBVSA-N Thr-Val-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@@H]1C(=O)O)N)O QNXZCKMXHPULME-ZNSHCXBVSA-N 0.000 description 1
- MNYNCKZAEIAONY-XGEHTFHBSA-N Thr-Val-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O MNYNCKZAEIAONY-XGEHTFHBSA-N 0.000 description 1
- 102100024333 Toll-like receptor 2 Human genes 0.000 description 1
- 102100025946 Transforming growth factor beta activator LRRC32 Human genes 0.000 description 1
- 101710169732 Transforming growth factor beta activator LRRC32 Proteins 0.000 description 1
- 108700019146 Transgenes Proteins 0.000 description 1
- 102100026224 Transmembrane and immunoglobulin domain-containing protein 2 Human genes 0.000 description 1
- UKINEYBQXPMOJO-UBHSHLNASA-N Trp-Asn-Ser Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CO)C(=O)O)N UKINEYBQXPMOJO-UBHSHLNASA-N 0.000 description 1
- DQDXHYIEITXNJY-BPUTZDHNSA-N Trp-Gln-Gln Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N DQDXHYIEITXNJY-BPUTZDHNSA-N 0.000 description 1
- VTHNLRXALGUDBS-BPUTZDHNSA-N Trp-Gln-Glu Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N VTHNLRXALGUDBS-BPUTZDHNSA-N 0.000 description 1
- UHXOYRWHIQZAKV-SZMVWBNQSA-N Trp-Pro-Arg Chemical compound O=C([C@H](CC=1C2=CC=CC=C2NC=1)N)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(O)=O UHXOYRWHIQZAKV-SZMVWBNQSA-N 0.000 description 1
- HHPSUFUXXBOFQY-AQZXSJQPSA-N Trp-Thr-Asn Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)N)O HHPSUFUXXBOFQY-AQZXSJQPSA-N 0.000 description 1
- YXSSXUIBUJGHJY-SFJXLCSZSA-N Trp-Thr-Phe Chemical compound C([C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CC=1C2=CC=CC=C2NC=1)[C@H](O)C)C(O)=O)C1=CC=CC=C1 YXSSXUIBUJGHJY-SFJXLCSZSA-N 0.000 description 1
- PKZIWSHDJYIPRH-JBACZVJFSA-N Trp-Tyr-Gln Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(O)=O PKZIWSHDJYIPRH-JBACZVJFSA-N 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 1
- 102100024584 Tumor necrosis factor ligand superfamily member 12 Human genes 0.000 description 1
- 101710097155 Tumor necrosis factor ligand superfamily member 12 Proteins 0.000 description 1
- 108090000138 Tumor necrosis factor ligand superfamily member 15 Proteins 0.000 description 1
- 108050002568 Tumor necrosis factor ligand superfamily member 6 Proteins 0.000 description 1
- 102100032100 Tumor necrosis factor ligand superfamily member 8 Human genes 0.000 description 1
- 102100022203 Tumor necrosis factor receptor superfamily member 25 Human genes 0.000 description 1
- 102100030810 Tumor necrosis factor receptor superfamily member EDAR Human genes 0.000 description 1
- 206010054094 Tumour necrosis Diseases 0.000 description 1
- 102000007537 Type II DNA Topoisomerases Human genes 0.000 description 1
- 108010046308 Type II DNA Topoisomerases Proteins 0.000 description 1
- BURPTJBFWIOHEY-UWJYBYFXSA-N Tyr-Ala-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 BURPTJBFWIOHEY-UWJYBYFXSA-N 0.000 description 1
- SCCKSNREWHMKOJ-SRVKXCTJSA-N Tyr-Asn-Ser Chemical compound N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O SCCKSNREWHMKOJ-SRVKXCTJSA-N 0.000 description 1
- WZQZUVWEPMGIMM-JYJNAYRXSA-N Tyr-Gln-Lys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCCCN)C(=O)O)N)O WZQZUVWEPMGIMM-JYJNAYRXSA-N 0.000 description 1
- GULIUBBXCYPDJU-CQDKDKBSSA-N Tyr-Leu-Ala Chemical compound [O-]C(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]([NH3+])CC1=CC=C(O)C=C1 GULIUBBXCYPDJU-CQDKDKBSSA-N 0.000 description 1
- HSBZWINKRYZCSQ-KKUMJFAQSA-N Tyr-Lys-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(O)=O HSBZWINKRYZCSQ-KKUMJFAQSA-N 0.000 description 1
- SINRIKQYQJRGDQ-MEYUZBJRSA-N Tyr-Lys-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 SINRIKQYQJRGDQ-MEYUZBJRSA-N 0.000 description 1
- WURLIFOWSMBUAR-SLFFLAALSA-N Tyr-Phe-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=CC=C2)NC(=O)[C@H](CC3=CC=C(C=C3)O)N)C(=O)O WURLIFOWSMBUAR-SLFFLAALSA-N 0.000 description 1
- NHOVZGFNTGMYMI-KKUMJFAQSA-N Tyr-Ser-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 NHOVZGFNTGMYMI-KKUMJFAQSA-N 0.000 description 1
- PWKMJDQXKCENMF-MEYUZBJRSA-N Tyr-Thr-Leu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O PWKMJDQXKCENMF-MEYUZBJRSA-N 0.000 description 1
- 102000003425 Tyrosinase Human genes 0.000 description 1
- 108060008724 Tyrosinase Proteins 0.000 description 1
- 206010046431 Urethral cancer Diseases 0.000 description 1
- 206010046458 Urethral neoplasms Diseases 0.000 description 1
- 208000006593 Urologic Neoplasms Diseases 0.000 description 1
- 206010046788 Uterine haemorrhage Diseases 0.000 description 1
- 108091008605 VEGF receptors Proteins 0.000 description 1
- 201000003761 Vaginal carcinoma Diseases 0.000 description 1
- 206010046910 Vaginal haemorrhage Diseases 0.000 description 1
- AZSHAZJLOZQYAY-FXQIFTODSA-N Val-Ala-Ser Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O AZSHAZJLOZQYAY-FXQIFTODSA-N 0.000 description 1
- COYSIHFOCOMGCF-UHFFFAOYSA-N Val-Arg-Gly Natural products CC(C)C(N)C(=O)NC(C(=O)NCC(O)=O)CCCN=C(N)N COYSIHFOCOMGCF-UHFFFAOYSA-N 0.000 description 1
- VMRFIKXKOFNMHW-GUBZILKMSA-N Val-Arg-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CO)C(=O)O)N VMRFIKXKOFNMHW-GUBZILKMSA-N 0.000 description 1
- GNWUWQAVVJQREM-NHCYSSNCSA-N Val-Asn-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N GNWUWQAVVJQREM-NHCYSSNCSA-N 0.000 description 1
- LIQJSDDOULTANC-QSFUFRPTSA-N Val-Asn-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](C(C)C)N LIQJSDDOULTANC-QSFUFRPTSA-N 0.000 description 1
- JLFKWDAZBRYCGX-ZKWXMUAHSA-N Val-Asn-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CO)C(=O)O)N JLFKWDAZBRYCGX-ZKWXMUAHSA-N 0.000 description 1
- QHDXUYOYTPWCSK-RCOVLWMOSA-N Val-Asp-Gly Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)NCC(=O)O)N QHDXUYOYTPWCSK-RCOVLWMOSA-N 0.000 description 1
- SCBITHMBEJNRHC-LSJOCFKGSA-N Val-Asp-Val Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](C(C)C)C(=O)O)N SCBITHMBEJNRHC-LSJOCFKGSA-N 0.000 description 1
- JXGWQYWDUOWQHA-DZKIICNBSA-N Val-Gln-Phe Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N JXGWQYWDUOWQHA-DZKIICNBSA-N 0.000 description 1
- WDIGUPHXPBMODF-UMNHJUIQSA-N Val-Glu-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N1CCC[C@@H]1C(=O)O)N WDIGUPHXPBMODF-UMNHJUIQSA-N 0.000 description 1
- RKIGNDAHUOOIMJ-BQFCYCMXSA-N Val-Glu-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)C(C)C)C(O)=O)=CNC2=C1 RKIGNDAHUOOIMJ-BQFCYCMXSA-N 0.000 description 1
- UEHRGZCNLSWGHK-DLOVCJGASA-N Val-Glu-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O UEHRGZCNLSWGHK-DLOVCJGASA-N 0.000 description 1
- DJEVQCWNMQOABE-RCOVLWMOSA-N Val-Gly-Asp Chemical compound CC(C)[C@@H](C(=O)NCC(=O)N[C@@H](CC(=O)O)C(=O)O)N DJEVQCWNMQOABE-RCOVLWMOSA-N 0.000 description 1
- LAYSXAOGWHKNED-XPUUQOCRSA-N Val-Gly-Ser Chemical compound CC(C)[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O LAYSXAOGWHKNED-XPUUQOCRSA-N 0.000 description 1
- ZIGZPYJXIWLQFC-QTKMDUPCSA-N Val-His-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](C(C)C)N)O ZIGZPYJXIWLQFC-QTKMDUPCSA-N 0.000 description 1
- WNZSAUMKZQXHNC-UKJIMTQDSA-N Val-Ile-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](C(C)C)N WNZSAUMKZQXHNC-UKJIMTQDSA-N 0.000 description 1
- VHRLUTIMTDOVCG-PEDHHIEDSA-N Val-Ile-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)O)NC(=O)[C@H](C(C)C)N VHRLUTIMTDOVCG-PEDHHIEDSA-N 0.000 description 1
- HGJRMXOWUWVUOA-GVXVVHGQSA-N Val-Leu-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](C(C)C)N HGJRMXOWUWVUOA-GVXVVHGQSA-N 0.000 description 1
- HPANGHISDXDUQY-ULQDDVLXSA-N Val-Lys-Phe Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N HPANGHISDXDUQY-ULQDDVLXSA-N 0.000 description 1
- SBJCTAZFSZXWSR-AVGNSLFASA-N Val-Met-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N SBJCTAZFSZXWSR-AVGNSLFASA-N 0.000 description 1
- VCIYTVOBLZHFSC-XHSDSOJGSA-N Val-Phe-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N2CCC[C@@H]2C(=O)O)N VCIYTVOBLZHFSC-XHSDSOJGSA-N 0.000 description 1
- YTNGABPUXFEOGU-SRVKXCTJSA-N Val-Pro-Arg Chemical compound CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(O)=O YTNGABPUXFEOGU-SRVKXCTJSA-N 0.000 description 1
- VHIZXDZMTDVFGX-DCAQKATOSA-N Val-Ser-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](C(C)C)N VHIZXDZMTDVFGX-DCAQKATOSA-N 0.000 description 1
- ZLNYBMWGPOKSLW-LSJOCFKGSA-N Val-Val-Asp Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O ZLNYBMWGPOKSLW-LSJOCFKGSA-N 0.000 description 1
- 102100033177 Vascular endothelial growth factor receptor 2 Human genes 0.000 description 1
- 206010047700 Vomiting Diseases 0.000 description 1
- 206010047741 Vulval cancer Diseases 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- 210000000683 abdominal cavity Anatomy 0.000 description 1
- 230000003187 abdominal effect Effects 0.000 description 1
- 239000003070 absorption delaying agent Substances 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 230000007059 acute toxicity Effects 0.000 description 1
- 231100000403 acute toxicity Toxicity 0.000 description 1
- 230000002730 additional effect Effects 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 229960005305 adenosine Drugs 0.000 description 1
- 239000002487 adenosine deaminase inhibitor Substances 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 238000009098 adjuvant therapy Methods 0.000 description 1
- 201000005188 adrenal gland cancer Diseases 0.000 description 1
- 208000024447 adrenal gland neoplasm Diseases 0.000 description 1
- 229960000548 alemtuzumab Drugs 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 150000001342 alkaline earth metals Chemical class 0.000 description 1
- 229940045714 alkyl sulfonate alkylating agent Drugs 0.000 description 1
- 150000008052 alkyl sulfonates Chemical class 0.000 description 1
- 230000000735 allogeneic effect Effects 0.000 description 1
- 229940087168 alpha tocopherol Drugs 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- ROBVIMPUHSLWNV-UHFFFAOYSA-N aminoglutethimide Chemical compound C=1C=C(N)C=CC=1C1(CC)CCC(=O)NC1=O ROBVIMPUHSLWNV-UHFFFAOYSA-N 0.000 description 1
- 229960003437 aminoglutethimide Drugs 0.000 description 1
- 208000007502 anemia Diseases 0.000 description 1
- 230000000118 anti-neoplastic effect Effects 0.000 description 1
- 230000010100 anticoagulation Effects 0.000 description 1
- 239000003080 antimitotic agent Substances 0.000 description 1
- 229940045719 antineoplastic alkylating agent nitrosoureas Drugs 0.000 description 1
- 230000004596 appetite loss Effects 0.000 description 1
- 239000008365 aqueous carrier Substances 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 239000010425 asbestos Substances 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 235000010385 ascorbyl palmitate Nutrition 0.000 description 1
- 108010036999 aspartyl-alanyl-histidyl-lysine Proteins 0.000 description 1
- 108010047857 aspartylglycine Proteins 0.000 description 1
- 229950009925 atacicept Drugs 0.000 description 1
- 230000001363 autoimmune Effects 0.000 description 1
- JUHORIMYRDESRB-UHFFFAOYSA-N benzathine Chemical compound C=1C=CC=CC=1CNCCNCC1=CC=CC=C1 JUHORIMYRDESRB-UHFFFAOYSA-N 0.000 description 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 1
- 108010010804 beta2 Heterotrimer Lymphotoxin alpha1 Proteins 0.000 description 1
- 230000001588 bifunctional effect Effects 0.000 description 1
- 108091008324 binding proteins Proteins 0.000 description 1
- 238000010256 biochemical assay Methods 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 229920000249 biocompatible polymer Polymers 0.000 description 1
- 230000008512 biological response Effects 0.000 description 1
- 239000012472 biological sample Substances 0.000 description 1
- 229960000074 biopharmaceutical Drugs 0.000 description 1
- 210000000601 blood cell Anatomy 0.000 description 1
- 230000036765 blood level Effects 0.000 description 1
- 208000027503 bloody stool Diseases 0.000 description 1
- 210000002798 bone marrow cell Anatomy 0.000 description 1
- 210000000481 breast Anatomy 0.000 description 1
- 229960002092 busulfan Drugs 0.000 description 1
- DQXBYHZEEUGOBF-UHFFFAOYSA-N but-3-enoic acid;ethene Chemical compound C=C.OC(=O)CC=C DQXBYHZEEUGOBF-UHFFFAOYSA-N 0.000 description 1
- 235000019282 butylated hydroxyanisole Nutrition 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 230000004611 cancer cell death Effects 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- IQXIUTMSTALSFW-VJFOLWCZSA-N carboplatin paclitaxel Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1.O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 IQXIUTMSTALSFW-VJFOLWCZSA-N 0.000 description 1
- 238000012754 cardiac puncture Methods 0.000 description 1
- 229960005243 carmustine Drugs 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 239000005018 casein Substances 0.000 description 1
- BECPQYXYKAMYBN-UHFFFAOYSA-N casein, tech. Chemical compound NCCCCC(C(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(CC(C)C)N=C(O)C(CCC(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(C(C)O)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(COP(O)(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(N)CC1=CC=CC=C1 BECPQYXYKAMYBN-UHFFFAOYSA-N 0.000 description 1
- 235000021240 caseins Nutrition 0.000 description 1
- 230000020411 cell activation Effects 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 230000032823 cell division Effects 0.000 description 1
- 230000011748 cell maturation Effects 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 238000001516 cell proliferation assay Methods 0.000 description 1
- 230000002490 cerebral effect Effects 0.000 description 1
- 208000019065 cervical carcinoma Diseases 0.000 description 1
- 229960005395 cetuximab Drugs 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- WMRQHSFWMFGIFW-SGNBTFORSA-N chembl1242194 Chemical compound C([C@@]([C@@H]1C[C@H]2C(C)=C[C@H]3[C@@H](O)[C@@H]([C@H]([C@@H]3[C@H]2[C@@H]11)O)C)(C)O2)C[C@H]3OC(=O)C1=C2[C@@H]3C WMRQHSFWMFGIFW-SGNBTFORSA-N 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 239000012829 chemotherapy agent Substances 0.000 description 1
- 238000009104 chemotherapy regimen Methods 0.000 description 1
- 229960004630 chlorambucil Drugs 0.000 description 1
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- VDANGULDQQJODZ-UHFFFAOYSA-N chloroprocaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1Cl VDANGULDQQJODZ-UHFFFAOYSA-N 0.000 description 1
- 229960002023 chloroprocaine Drugs 0.000 description 1
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 1
- 229960001231 choline Drugs 0.000 description 1
- 208000013116 chronic cough Diseases 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 208000024207 chronic leukemia Diseases 0.000 description 1
- 238000011278 co-treatment Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 229920001436 collagen Polymers 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 201000010897 colon adenocarcinoma Diseases 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 108010006226 cryptophycin Proteins 0.000 description 1
- PSNOPSMXOBPNNV-VVCTWANISA-N cryptophycin 1 Chemical compound C1=C(Cl)C(OC)=CC=C1C[C@@H]1C(=O)NC[C@@H](C)C(=O)O[C@@H](CC(C)C)C(=O)O[C@H]([C@H](C)[C@@H]2[C@H](O2)C=2C=CC=CC=2)C/C=C/C(=O)N1 PSNOPSMXOBPNNV-VVCTWANISA-N 0.000 description 1
- 108010083340 cryptophycin 52 Proteins 0.000 description 1
- PSNOPSMXOBPNNV-UHFFFAOYSA-N cryptophycin-327 Natural products C1=C(Cl)C(OC)=CC=C1CC1C(=O)NCC(C)C(=O)OC(CC(C)C)C(=O)OC(C(C)C2C(O2)C=2C=CC=CC=2)CC=CC(=O)N1 PSNOPSMXOBPNNV-UHFFFAOYSA-N 0.000 description 1
- 239000012228 culture supernatant Substances 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 208000035250 cutaneous malignant susceptibility to 1 melanoma Diseases 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- 229960002433 cysteine Drugs 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- 229960001305 cysteine hydrochloride Drugs 0.000 description 1
- 229960000684 cytarabine Drugs 0.000 description 1
- 102000003675 cytokine receptors Human genes 0.000 description 1
- 108010057085 cytokine receptors Proteins 0.000 description 1
- 230000001461 cytolytic effect Effects 0.000 description 1
- 238000004163 cytometry Methods 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 229950007409 dacetuzumab Drugs 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 230000001934 delay Effects 0.000 description 1
- 230000002939 deleterious effect Effects 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 229930184531 desoxyepothilone Natural products 0.000 description 1
- BEFZAMRWPCMWFJ-UHFFFAOYSA-N desoxyepothilone A Natural products O1C(=O)CC(O)C(C)(C)C(=O)C(C)C(O)C(C)CCCC=CCC1C(C)=CC1=CSC(C)=N1 BEFZAMRWPCMWFJ-UHFFFAOYSA-N 0.000 description 1
- XOZIUKBZLSUILX-UHFFFAOYSA-N desoxyepothilone B Natural products O1C(=O)CC(O)C(C)(C)C(=O)C(C)C(O)C(C)CCCC(C)=CCC1C(C)=CC1=CSC(C)=N1 XOZIUKBZLSUILX-UHFFFAOYSA-N 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 150000001991 dicarboxylic acids Chemical class 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- 229940043237 diethanolamine Drugs 0.000 description 1
- RGLYKWWBQGJZGM-ISLYRVAYSA-N diethylstilbestrol Chemical compound C=1C=C(O)C=CC=1C(/CC)=C(\CC)C1=CC=C(O)C=C1 RGLYKWWBQGJZGM-ISLYRVAYSA-N 0.000 description 1
- 229960000452 diethylstilbestrol Drugs 0.000 description 1
- 206010012818 diffuse large B-cell lymphoma Diseases 0.000 description 1
- UGMCXQCYOVCMTB-UHFFFAOYSA-K dihydroxy(stearato)aluminium Chemical compound CCCCCCCCCCCCCCCCCC(=O)O[Al](O)O UGMCXQCYOVCMTB-UHFFFAOYSA-K 0.000 description 1
- MCWXGJITAZMZEV-UHFFFAOYSA-N dimethoate Chemical compound CNC(=O)CSP(=S)(OC)OC MCWXGJITAZMZEV-UHFFFAOYSA-N 0.000 description 1
- FSXRLASFHBWESK-UHFFFAOYSA-N dipeptide phenylalanyl-tyrosine Natural products C=1C=C(O)C=CC=1CC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FSXRLASFHBWESK-UHFFFAOYSA-N 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 208000002173 dizziness Diseases 0.000 description 1
- 229960003668 docetaxel Drugs 0.000 description 1
- 231100000371 dose-limiting toxicity Toxicity 0.000 description 1
- 230000002222 downregulating effect Effects 0.000 description 1
- NOTIQUSPUUHHEH-UXOVVSIBSA-N dromostanolone propionate Chemical compound C([C@@H]1CC2)C(=O)[C@H](C)C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H](OC(=O)CC)[C@@]2(C)CC1 NOTIQUSPUUHHEH-UXOVVSIBSA-N 0.000 description 1
- 229950004683 drostanolone propionate Drugs 0.000 description 1
- 238000007876 drug discovery Methods 0.000 description 1
- 229950009791 durvalumab Drugs 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 230000007937 eating Effects 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- XOPYFXBZMVTEJF-PDACKIITSA-N eleutherobin Chemical compound C(/[C@H]1[C@H](C(=CC[C@@H]1C(C)C)C)C[C@@H]([C@@]1(C)O[C@@]2(C=C1)OC)OC(=O)\C=C\C=1N=CN(C)C=1)=C2\CO[C@@H]1OC[C@@H](O)[C@@H](O)[C@@H]1OC(C)=O XOPYFXBZMVTEJF-PDACKIITSA-N 0.000 description 1
- XOPYFXBZMVTEJF-UHFFFAOYSA-N eleutherobin Natural products C1=CC2(OC)OC1(C)C(OC(=O)C=CC=1N=CN(C)C=1)CC(C(=CCC1C(C)C)C)C1C=C2COC1OCC(O)C(O)C1OC(C)=O XOPYFXBZMVTEJF-UHFFFAOYSA-N 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 210000000750 endocrine system Anatomy 0.000 description 1
- 201000003914 endometrial carcinoma Diseases 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 210000003979 eosinophil Anatomy 0.000 description 1
- 229950006370 epacadostat Drugs 0.000 description 1
- 229930013356 epothilone Natural products 0.000 description 1
- BEFZAMRWPCMWFJ-QJKGZULSSA-N epothilone C Chemical compound O1C(=O)C[C@H](O)C(C)(C)C(=O)[C@H](C)[C@@H](O)[C@@H](C)CCC\C=C/C[C@H]1C(\C)=C\C1=CSC(C)=N1 BEFZAMRWPCMWFJ-QJKGZULSSA-N 0.000 description 1
- XOZIUKBZLSUILX-GIQCAXHBSA-N epothilone D Chemical compound O1C(=O)C[C@H](O)C(C)(C)C(=O)[C@H](C)[C@@H](O)[C@@H](C)CCC\C(C)=C/C[C@H]1C(\C)=C\C1=CSC(C)=N1 XOZIUKBZLSUILX-GIQCAXHBSA-N 0.000 description 1
- FCCNKYGSMOSYPV-UHFFFAOYSA-N epothilone E Natural products O1C(=O)CC(O)C(C)(C)C(=O)C(C)C(O)C(C)CCCC2OC2CC1C(C)=CC1=CSC(CO)=N1 FCCNKYGSMOSYPV-UHFFFAOYSA-N 0.000 description 1
- 150000003883 epothilone derivatives Chemical class 0.000 description 1
- FCCNKYGSMOSYPV-OKOHHBBGSA-N epothilone e Chemical compound C/C([C@@H]1C[C@@H]2O[C@@H]2CCC[C@@H]([C@@H]([C@@H](C)C(=O)C(C)(C)[C@@H](O)CC(=O)O1)O)C)=C\C1=CSC(CO)=N1 FCCNKYGSMOSYPV-OKOHHBBGSA-N 0.000 description 1
- UKIMCRYGLFQEOE-RGJAOAFDSA-N epothilone f Chemical compound C/C([C@@H]1C[C@@H]2O[C@]2(C)CCC[C@@H]([C@@H]([C@@H](C)C(=O)C(C)(C)[C@@H](O)CC(=O)O1)O)C)=C\C1=CSC(CO)=N1 UKIMCRYGLFQEOE-RGJAOAFDSA-N 0.000 description 1
- 229960000439 eribulin mesylate Drugs 0.000 description 1
- AAKJLRGGTJKAMG-UHFFFAOYSA-N erlotinib Chemical compound C=12C=C(OCCOC)C(OCCOC)=CC2=NC=NC=1NC1=CC=CC(C#C)=C1 AAKJLRGGTJKAMG-UHFFFAOYSA-N 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 229960005309 estradiol Drugs 0.000 description 1
- 229930182833 estradiol Natural products 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 1
- 229940093471 ethyl oleate Drugs 0.000 description 1
- 239000005038 ethylene vinyl acetate Substances 0.000 description 1
- 229940012017 ethylenediamine Drugs 0.000 description 1
- 230000005713 exacerbation Effects 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 201000001343 fallopian tube carcinoma Diseases 0.000 description 1
- 238000005206 flow analysis Methods 0.000 description 1
- GIUYCYHIANZCFB-FJFJXFQQSA-N fludarabine phosphate Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@@H]1O GIUYCYHIANZCFB-FJFJXFQQSA-N 0.000 description 1
- 229960005304 fludarabine phosphate Drugs 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- YLRFCQOZQXIBAB-RBZZARIASA-N fluoxymesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1CC[C@](C)(O)[C@@]1(C)C[C@@H]2O YLRFCQOZQXIBAB-RBZZARIASA-N 0.000 description 1
- 229960001751 fluoxymesterone Drugs 0.000 description 1
- 229960002074 flutamide Drugs 0.000 description 1
- MKXKFYHWDHIYRV-UHFFFAOYSA-N flutamide Chemical compound CC(C)C(=O)NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 MKXKFYHWDHIYRV-UHFFFAOYSA-N 0.000 description 1
- 229940014144 folate Drugs 0.000 description 1
- OVBPIULPVIDEAO-LBPRGKRZSA-N folic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-LBPRGKRZSA-N 0.000 description 1
- 235000019152 folic acid Nutrition 0.000 description 1
- 239000011724 folic acid Substances 0.000 description 1
- 239000004052 folic acid antagonist Substances 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 238000007710 freezing Methods 0.000 description 1
- 230000008014 freezing Effects 0.000 description 1
- 230000002538 fungal effect Effects 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 229950000456 galunisertib Drugs 0.000 description 1
- 231100000024 genotoxic Toxicity 0.000 description 1
- 230000001738 genotoxic effect Effects 0.000 description 1
- 238000003881 globally optimized alternating phase rectangular pulse Methods 0.000 description 1
- 108010075431 glycyl-alanyl-phenylalanine Proteins 0.000 description 1
- 108010015792 glycyllysine Proteins 0.000 description 1
- 108010037850 glycylvaline Proteins 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 229950005277 gossypol Drugs 0.000 description 1
- 210000003714 granulocyte Anatomy 0.000 description 1
- 208000035474 group of disease Diseases 0.000 description 1
- 230000009036 growth inhibition Effects 0.000 description 1
- 229940093915 gynecological organic acid Drugs 0.000 description 1
- 230000003394 haemopoietic effect Effects 0.000 description 1
- FXNFULJVOQMBCW-VZBLNRDYSA-N halichondrin b Chemical compound O([C@@H]1[C@@H](C)[C@@H]2O[C@@H]3C[C@@]4(O[C@H]5[C@@H](C)C[C@@]6(C[C@@H]([C@@H]7O[C@@H](C[C@@H]7O6)[C@@H](O)C[C@@H](O)CO)C)O[C@H]5C4)O[C@@H]3C[C@@H]2O[C@H]1C[C@@H]1C(=C)[C@H](C)C[C@@H](O1)CC[C@H]1C(=C)C[C@@H](O1)CC1)C(=O)C[C@H](O2)CC[C@H]3[C@H]2[C@H](O2)[C@@H]4O[C@@H]5C[C@@]21O[C@@H]5[C@@H]4O3 FXNFULJVOQMBCW-VZBLNRDYSA-N 0.000 description 1
- 230000009931 harmful effect Effects 0.000 description 1
- 201000000459 head and neck squamous cell carcinoma Diseases 0.000 description 1
- 239000003481 heat shock protein 90 inhibitor Substances 0.000 description 1
- 208000035861 hematochezia Diseases 0.000 description 1
- 230000002489 hematologic effect Effects 0.000 description 1
- 208000006750 hematuria Diseases 0.000 description 1
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 1
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 1
- 229940022353 herceptin Drugs 0.000 description 1
- 108010092114 histidylphenylalanine Proteins 0.000 description 1
- 229940121372 histone deacetylase inhibitor Drugs 0.000 description 1
- 239000003276 histone deacetylase inhibitor Substances 0.000 description 1
- 238000002868 homogeneous time resolved fluorescence Methods 0.000 description 1
- 230000005745 host immune response Effects 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- 229940071870 hydroiodic acid Drugs 0.000 description 1
- 150000002433 hydrophilic molecules Chemical class 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 229960002899 hydroxyprogesterone Drugs 0.000 description 1
- 230000037315 hyperhidrosis Effects 0.000 description 1
- 230000003463 hyperproliferative effect Effects 0.000 description 1
- 229960001001 ibritumomab tiuxetan Drugs 0.000 description 1
- 230000005965 immune activity Effects 0.000 description 1
- 230000005746 immune checkpoint blockade Effects 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000008088 immune pathway Effects 0.000 description 1
- 102000027596 immune receptors Human genes 0.000 description 1
- 108091008915 immune receptors Proteins 0.000 description 1
- 230000008629 immune suppression Effects 0.000 description 1
- 230000037451 immune surveillance Effects 0.000 description 1
- 230000007813 immunodeficiency Effects 0.000 description 1
- 208000014136 immunodeficiency 16 Diseases 0.000 description 1
- 230000002163 immunogen Effects 0.000 description 1
- 229940027941 immunoglobulin g Drugs 0.000 description 1
- 230000016784 immunoglobulin production Effects 0.000 description 1
- 238000013388 immunohistochemistry analysis Methods 0.000 description 1
- 239000003018 immunosuppressive agent Substances 0.000 description 1
- 229940125721 immunosuppressive agent Drugs 0.000 description 1
- 230000001024 immunotherapeutic effect Effects 0.000 description 1
- 230000001976 improved effect Effects 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 208000015266 indolent plasma cell myeloma Diseases 0.000 description 1
- 239000012678 infectious agent Substances 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 239000007972 injectable composition Substances 0.000 description 1
- 230000015788 innate immune response Effects 0.000 description 1
- 238000011081 inoculation Methods 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000016507 interphase Effects 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 description 1
- 229960004768 irinotecan Drugs 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 108010027338 isoleucylcysteine Proteins 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 229940043355 kinase inhibitor Drugs 0.000 description 1
- 238000009533 lab test Methods 0.000 description 1
- HPJKCIUCZWXJDR-UHFFFAOYSA-N letrozole Chemical compound C1=CC(C#N)=CC=C1C(N1N=CN=C1)C1=CC=C(C#N)C=C1 HPJKCIUCZWXJDR-UHFFFAOYSA-N 0.000 description 1
- 229960003881 letrozole Drugs 0.000 description 1
- 108010034529 leucyl-lysine Proteins 0.000 description 1
- 108010057821 leucylproline Proteins 0.000 description 1
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 1
- 229950011263 lirilumab Drugs 0.000 description 1
- 229960002247 lomustine Drugs 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 208000019017 loss of appetite Diseases 0.000 description 1
- 235000021266 loss of appetite Nutrition 0.000 description 1
- 238000000464 low-speed centrifugation Methods 0.000 description 1
- 201000008443 lung non-squamous non-small cell carcinoma Diseases 0.000 description 1
- 201000010453 lymph node cancer Diseases 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 230000000527 lymphocytic effect Effects 0.000 description 1
- 208000003747 lymphoid leukemia Diseases 0.000 description 1
- 210000003563 lymphoid tissue Anatomy 0.000 description 1
- 108010003700 lysyl aspartic acid Proteins 0.000 description 1
- 108010044348 lysyl-glutamyl-aspartic acid Proteins 0.000 description 1
- 229940124302 mTOR inhibitor Drugs 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 238000009115 maintenance therapy Methods 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 208000026045 malignant tumor of parathyroid gland Diseases 0.000 description 1
- 239000003628 mammalian target of rapamycin inhibitor Substances 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 150000008146 mannosides Chemical class 0.000 description 1
- 201000007924 marginal zone B-cell lymphoma Diseases 0.000 description 1
- 208000021937 marginal zone lymphoma Diseases 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 208000020968 mature T-cell and NK-cell non-Hodgkin lymphoma Diseases 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 229960004616 medroxyprogesterone Drugs 0.000 description 1
- 229960002985 medroxyprogesterone acetate Drugs 0.000 description 1
- RQZAXGRLVPAYTJ-GQFGMJRRSA-N megestrol acetate Chemical compound C1=C(C)C2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(C)=O)(OC(=O)C)[C@@]1(C)CC2 RQZAXGRLVPAYTJ-GQFGMJRRSA-N 0.000 description 1
- 229960004296 megestrol acetate Drugs 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- 229960001428 mercaptopurine Drugs 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 208000021039 metastatic melanoma Diseases 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 229960004584 methylprednisolone Drugs 0.000 description 1
- 229960001566 methyltestosterone Drugs 0.000 description 1
- 239000004530 micro-emulsion Substances 0.000 description 1
- 238000001471 micro-filtration Methods 0.000 description 1
- 238000012737 microarray-based gene expression Methods 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 230000033607 mismatch repair Effects 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 238000012243 multiplex automated genomic engineering Methods 0.000 description 1
- 229960003816 muromonab-cd3 Drugs 0.000 description 1
- 210000000066 myeloid cell Anatomy 0.000 description 1
- PGXYIBJJCLWJST-MUUNZHRXSA-N n-(3-aminopropyl)-n-[(1r)-1-(3-benzyl-7-chloro-4-oxochromen-2-yl)-2-methylpropyl]-4-methylbenzamide Chemical compound NCCCN([C@H](C(C)C)C1=C(C(=O)C2=CC=C(Cl)C=C2O1)CC=1C=CC=CC=1)C(=O)C1=CC=C(C)C=C1 PGXYIBJJCLWJST-MUUNZHRXSA-N 0.000 description 1
- KWQWWUXRGIIBAS-UHFFFAOYSA-N n-[2-(4-hydroxyanilino)pyridin-3-yl]-4-methoxybenzenesulfonamide;hydrochloride Chemical compound Cl.C1=CC(OC)=CC=C1S(=O)(=O)NC1=CC=CN=C1NC1=CC=C(O)C=C1 KWQWWUXRGIIBAS-UHFFFAOYSA-N 0.000 description 1
- 230000008693 nausea Effects 0.000 description 1
- 229950004847 navitoclax Drugs 0.000 description 1
- JLYAXFNOILIKPP-KXQOOQHDSA-N navitoclax Chemical compound C([C@@H](NC1=CC=C(C=C1S(=O)(=O)C(F)(F)F)S(=O)(=O)NC(=O)C1=CC=C(C=C1)N1CCN(CC1)CC1=C(CCC(C1)(C)C)C=1C=CC(Cl)=CC=1)CSC=1C=CC=CC=1)CN1CCOCC1 JLYAXFNOILIKPP-KXQOOQHDSA-N 0.000 description 1
- 230000014399 negative regulation of angiogenesis Effects 0.000 description 1
- 208000025189 neoplasm of testis Diseases 0.000 description 1
- 210000002445 nipple Anatomy 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 230000009871 nonspecific binding Effects 0.000 description 1
- 230000025308 nuclear transport Effects 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 239000004006 olive oil Substances 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 150000002895 organic esters Chemical class 0.000 description 1
- 201000008968 osteosarcoma Diseases 0.000 description 1
- 229940047091 other immunostimulants in atc Drugs 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 239000013610 patient sample Substances 0.000 description 1
- 229950007460 patupilone Drugs 0.000 description 1
- 229940121655 pd-1 inhibitor Drugs 0.000 description 1
- NETARJWZTMGMRM-KJHLVSCNSA-N peloruside A Natural products CC[C@@H](CO)C=C(C)[C@@H]1C[C@H](C[C@H](O)C(C)(C)[C@@]2(O)O[C@@H](C[C@@H](OC)[C@H](O)C(=O)O1)C[C@@H](OC)[C@H]2O)OC NETARJWZTMGMRM-KJHLVSCNSA-N 0.000 description 1
- NETARJWZTMGMRM-JRTPPQMASA-N peloruside A Chemical compound C1[C@H](OC)[C@@H](O)C(=O)O[C@@H](C(\C)=C/[C@@H](CO)CC)C[C@H](OC)C[C@@H](O)C(C)(C)[C@@]2(O)[C@@H](O)[C@@H](OC)C[C@@H]1O2 NETARJWZTMGMRM-JRTPPQMASA-N 0.000 description 1
- FPVKHBSQESCIEP-JQCXWYLXSA-N pentostatin Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC[C@H]2O)=C2N=C1 FPVKHBSQESCIEP-JQCXWYLXSA-N 0.000 description 1
- 229960002340 pentostatin Drugs 0.000 description 1
- 210000000578 peripheral nerve Anatomy 0.000 description 1
- 206010034674 peritonitis Diseases 0.000 description 1
- 108010012581 phenylalanylglutamate Proteins 0.000 description 1
- 108010073025 phenylalanylphenylalanine Proteins 0.000 description 1
- 108010051242 phenylalanylserine Proteins 0.000 description 1
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 1
- 230000001766 physiological effect Effects 0.000 description 1
- 229950010773 pidilizumab Drugs 0.000 description 1
- 208000021310 pituitary gland adenoma Diseases 0.000 description 1
- 210000004180 plasmocyte Anatomy 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 108700002563 poly ICLC Proteins 0.000 description 1
- 229920001200 poly(ethylene-vinyl acetate) Polymers 0.000 description 1
- 229920000747 poly(lactic acid) Polymers 0.000 description 1
- 239000004633 polyglycolic acid Substances 0.000 description 1
- 239000004626 polylactic acid Substances 0.000 description 1
- 208000017805 post-transplant lymphoproliferative disease Diseases 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 229960005205 prednisolone Drugs 0.000 description 1
- OIGNJSKKLXVSLS-VWUMJDOOSA-N prednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OIGNJSKKLXVSLS-VWUMJDOOSA-N 0.000 description 1
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 1
- 229960004618 prednisone Drugs 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 230000003449 preventive effect Effects 0.000 description 1
- 201000006037 primary mediastinal B-cell lymphoma Diseases 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 229960004919 procaine Drugs 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 208000037821 progressive disease Diseases 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 108010077112 prolyl-proline Proteins 0.000 description 1
- 108010053725 prolylvaline Proteins 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 239000000473 propyl gallate Substances 0.000 description 1
- 235000010388 propyl gallate Nutrition 0.000 description 1
- 229940075579 propyl gallate Drugs 0.000 description 1
- 201000005825 prostate adenocarcinoma Diseases 0.000 description 1
- 229940121649 protein inhibitor Drugs 0.000 description 1
- 239000012268 protein inhibitor Substances 0.000 description 1
- 239000003197 protein kinase B inhibitor Substances 0.000 description 1
- 150000003212 purines Chemical class 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 238000002708 random mutagenesis Methods 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 206010038038 rectal cancer Diseases 0.000 description 1
- 201000001275 rectum cancer Diseases 0.000 description 1
- 201000010174 renal carcinoma Diseases 0.000 description 1
- 229950010550 resiquimod Drugs 0.000 description 1
- BXNMTOQRYBFHNZ-UHFFFAOYSA-N resiquimod Chemical compound C1=CC=CC2=C(N(C(COCC)=N3)CC(C)(C)O)C3=C(N)N=C21 BXNMTOQRYBFHNZ-UHFFFAOYSA-N 0.000 description 1
- 229910052895 riebeckite Inorganic materials 0.000 description 1
- 230000036186 satiety Effects 0.000 description 1
- 235000019627 satiety Nutrition 0.000 description 1
- 238000013391 scatchard analysis Methods 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 108010048397 seryl-lysyl-leucine Proteins 0.000 description 1
- 230000001568 sexual effect Effects 0.000 description 1
- 238000004088 simulation Methods 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 229940075439 smac mimetic Drugs 0.000 description 1
- 208000000587 small cell lung carcinoma Diseases 0.000 description 1
- 201000002314 small intestine cancer Diseases 0.000 description 1
- 208000010721 smoldering plasma cell myeloma Diseases 0.000 description 1
- 108010047846 soblidotin Proteins 0.000 description 1
- DZMVCVHATYROOS-ZBFGKEHZSA-N soblidotin Chemical compound CC(C)[C@H](N(C)C)C(=O)N[C@@H](C(C)C)C(=O)N(C)[C@@H]([C@@H](C)CC)[C@H](OC)CC(=O)N1CCC[C@H]1[C@H](OC)[C@@H](C)C(=O)NCCC1=CC=CC=C1 DZMVCVHATYROOS-ZBFGKEHZSA-N 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- WBHQBSYUUJJSRZ-UHFFFAOYSA-M sodium bisulfate Chemical compound [Na+].OS([O-])(=O)=O WBHQBSYUUJJSRZ-UHFFFAOYSA-M 0.000 description 1
- 229910000342 sodium bisulfate Inorganic materials 0.000 description 1
- 229940100996 sodium bisulfate Drugs 0.000 description 1
- HRZFUMHJMZEROT-UHFFFAOYSA-L sodium disulfite Chemical compound [Na+].[Na+].[O-]S(=O)S([O-])(=O)=O HRZFUMHJMZEROT-UHFFFAOYSA-L 0.000 description 1
- 229940001584 sodium metabisulfite Drugs 0.000 description 1
- 235000010262 sodium metabisulphite Nutrition 0.000 description 1
- 229940001482 sodium sulfite Drugs 0.000 description 1
- 235000010265 sodium sulphite Nutrition 0.000 description 1
- 230000000392 somatic effect Effects 0.000 description 1
- 229960003787 sorafenib Drugs 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 208000037959 spinal tumor Diseases 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 201000002471 spleen cancer Diseases 0.000 description 1
- 206010062113 splenic marginal zone lymphoma Diseases 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 238000012414 sterilization procedure Methods 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 239000000021 stimulant Substances 0.000 description 1
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 150000005846 sugar alcohols Polymers 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 108010033090 surfactant protein A receptor Proteins 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 229940120982 tarceva Drugs 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 108010029464 tasidotin Proteins 0.000 description 1
- 229960004964 temozolomide Drugs 0.000 description 1
- ISIJQEHRDSCQIU-UHFFFAOYSA-N tert-butyl 2,7-diazaspiro[4.5]decane-7-carboxylate Chemical compound C1N(C(=O)OC(C)(C)C)CCCC11CNCC1 ISIJQEHRDSCQIU-UHFFFAOYSA-N 0.000 description 1
- 230000002381 testicular Effects 0.000 description 1
- 238000011287 therapeutic dose Methods 0.000 description 1
- 210000001541 thymus gland Anatomy 0.000 description 1
- 208000030901 thyroid gland follicular carcinoma Diseases 0.000 description 1
- 229960003087 tioguanine Drugs 0.000 description 1
- AOBORMOPSGHCAX-DGHZZKTQSA-N tocofersolan Chemical compound OCCOC(=O)CCC(=O)OC1=C(C)C(C)=C2O[C@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C AOBORMOPSGHCAX-DGHZZKTQSA-N 0.000 description 1
- 229960000984 tocofersolan Drugs 0.000 description 1
- 239000003970 toll like receptor agonist Substances 0.000 description 1
- 229940044616 toll-like receptor 7 agonist Drugs 0.000 description 1
- 229940044655 toll-like receptor 9 agonist Drugs 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 231100000816 toxic dose Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 230000002110 toxicologic effect Effects 0.000 description 1
- 231100000759 toxicological effect Toxicity 0.000 description 1
- 239000003053 toxin Substances 0.000 description 1
- 231100000765 toxin Toxicity 0.000 description 1
- 238000011830 transgenic mouse model Methods 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- IUCJMVBFZDHPDX-UHFFFAOYSA-N tretamine Chemical compound C1CN1C1=NC(N2CC2)=NC(N2CC2)=N1 IUCJMVBFZDHPDX-UHFFFAOYSA-N 0.000 description 1
- 229950001353 tretamine Drugs 0.000 description 1
- GFNANZIMVAIWHM-OBYCQNJPSA-N triamcinolone Chemical compound O=C1C=C[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@]([C@H](O)C4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 GFNANZIMVAIWHM-OBYCQNJPSA-N 0.000 description 1
- 229960005294 triamcinolone Drugs 0.000 description 1
- 150000003648 triterpenes Chemical class 0.000 description 1
- 108010080629 tryptophan-leucine Proteins 0.000 description 1
- 108010038745 tryptophylglycine Proteins 0.000 description 1
- 230000005747 tumor angiogenesis Effects 0.000 description 1
- 230000005748 tumor development Effects 0.000 description 1
- 230000005751 tumor progression Effects 0.000 description 1
- 230000005760 tumorsuppression Effects 0.000 description 1
- 230000002100 tumorsuppressive effect Effects 0.000 description 1
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 description 1
- 239000005483 tyrosine kinase inhibitor Substances 0.000 description 1
- 150000004917 tyrosine kinase inhibitor derivatives Chemical class 0.000 description 1
- 108010051110 tyrosyl-lysine Proteins 0.000 description 1
- 230000004222 uncontrolled growth Effects 0.000 description 1
- 241000701161 unidentified adenovirus Species 0.000 description 1
- 230000003827 upregulation Effects 0.000 description 1
- 229960001055 uracil mustard Drugs 0.000 description 1
- 210000001635 urinary tract Anatomy 0.000 description 1
- 238000001291 vacuum drying Methods 0.000 description 1
- 238000009777 vacuum freeze-drying Methods 0.000 description 1
- 108010073969 valyllysine Proteins 0.000 description 1
- 210000005166 vasculature Anatomy 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 230000008673 vomiting Effects 0.000 description 1
- 229940121351 vopratelimab Drugs 0.000 description 1
- 201000004916 vulva carcinoma Diseases 0.000 description 1
- 208000013013 vulvar carcinoma Diseases 0.000 description 1
- 230000003313 weakening effect Effects 0.000 description 1
- 230000004580 weight loss Effects 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 239000002076 α-tocopherol Substances 0.000 description 1
- 235000004835 α-tocopherol Nutrition 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2878—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30, CD40, CD95
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2818—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against CD28 or CD152
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2827—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against B7 molecules, e.g. CD80, CD86
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57484—Immunoassay; Biospecific binding assay; Materials therefor for cancer involving compounds serving as markers for tumor, cancer, neoplasia, e.g. cellular determinants, receptors, heat shock/stress proteins, A-protein, oligosaccharides, metabolites
- G01N33/57492—Immunoassay; Biospecific binding assay; Materials therefor for cancer involving compounds serving as markers for tumor, cancer, neoplasia, e.g. cellular determinants, receptors, heat shock/stress proteins, A-protein, oligosaccharides, metabolites involving compounds localized on the membrane of tumor or cancer cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
- A61K2039/507—Comprising a combination of two or more separate antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/54—Medicinal preparations containing antigens or antibodies characterised by the route of administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/545—Medicinal preparations containing antigens or antibodies characterised by the dose, timing or administration schedule
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/75—Agonist effect on antigen
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/94—Stability, e.g. half-life, pH, temperature or enzyme-resistance
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2500/00—Screening for compounds of potential therapeutic value
- G01N2500/04—Screening involving studying the effect of compounds C directly on molecule A (e.g. C are potential ligands for a receptor A, or potential substrates for an enzyme A)
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/52—Predicting or monitoring the response to treatment, e.g. for selection of therapy based on assay results in personalised medicine; Prognosis
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Engineering & Computer Science (AREA)
- Cell Biology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Oncology (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Food Science & Technology (AREA)
- Microbiology (AREA)
- Analytical Chemistry (AREA)
- Physics & Mathematics (AREA)
- Biotechnology (AREA)
- Pathology (AREA)
- Hospice & Palliative Care (AREA)
- General Physics & Mathematics (AREA)
- Dermatology (AREA)
- Epidemiology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (13)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762580346P | 2017-11-01 | 2017-11-01 | |
| US62/580,346 | 2017-11-01 | ||
| US201762581441P | 2017-11-03 | 2017-11-03 | |
| US62/581,441 | 2017-11-03 | ||
| US201762581905P | 2017-11-06 | 2017-11-06 | |
| US62/581,905 | 2017-11-06 | ||
| US201762583808P | 2017-11-09 | 2017-11-09 | |
| US62/583,808 | 2017-11-09 | ||
| US201862628207P | 2018-02-08 | 2018-02-08 | |
| US62/628,207 | 2018-02-08 | ||
| US201862657616P | 2018-04-13 | 2018-04-13 | |
| US62/657,616 | 2018-04-13 | ||
| PCT/US2018/058704 WO2019089921A1 (en) | 2017-11-01 | 2018-11-01 | Immunostimulatory agonistic antibodies for use in treating cancer |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20200074214A true KR20200074214A (ko) | 2020-06-24 |
Family
ID=64607296
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020207015641A Ceased KR20200074214A (ko) | 2017-11-01 | 2018-11-01 | 암을 치료하는데 사용하기 위한 면역자극 효능작용 항체 |
Country Status (6)
| Country | Link |
|---|---|
| US (2) | US11603410B2 (enExample) |
| EP (1) | EP3704159A1 (enExample) |
| JP (1) | JP2021501801A (enExample) |
| KR (1) | KR20200074214A (enExample) |
| CN (1) | CN111542544A (enExample) |
| WO (1) | WO2019089921A1 (enExample) |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2021501801A (ja) | 2017-11-01 | 2021-01-21 | ブリストル−マイヤーズ スクイブ カンパニーBristol−Myers Squibb Company | 癌の処置に用いるための免疫刺激アゴニスト抗体 |
| US20210277135A1 (en) * | 2018-07-13 | 2021-09-09 | Bristol-Myers Squibb Company | Ox-40 agonist, pd-1 pathway inhibitor and ctla-4 inhibitor combination for use in a method of treating a cancer or a solid tumor |
| CN114641500B (zh) * | 2019-11-21 | 2024-03-29 | 百济神州有限公司 | 使用抗ox40抗体与抗tim3抗体的组合治疗癌症的方法 |
| KR20220103708A (ko) * | 2019-11-21 | 2022-07-22 | 베이진 엘티디 | 항-pd1 또는 항-pdl1 항체와의 병용물 형태로 항-ox40 항체를 사용하는 암 치료의 방법 |
| CN114746446A (zh) * | 2019-11-21 | 2022-07-12 | 百济神州有限公司 | 使用抗ox40抗体与抗tigit抗体组合治疗癌症的方法 |
| CN114729051A (zh) * | 2019-11-21 | 2022-07-08 | 百济神州(北京)生物科技有限公司 | 使用抗ox40抗体与放射组合治疗癌症的方法 |
| US20230022859A1 (en) * | 2019-11-21 | 2023-01-26 | Beigene, Ltd. | Treatment of cancer with anti-ox40 antibodies and multi-kinase inhibitors |
| CA3175413A1 (en) | 2020-04-17 | 2021-10-21 | Yizhen Yang | Anti-ox40 antibody and uses thereof |
| WO2025195496A1 (en) * | 2024-03-21 | 2025-09-25 | Hanx Biopharmaceuticals, (Wuhan) Ltd. | Immunotherapy for ox40 expressing cancer |
Family Cites Families (84)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4475196A (en) | 1981-03-06 | 1984-10-02 | Zor Clair G | Instrument for locating faults in aircraft passenger reading light and attendant call control system |
| US4447233A (en) | 1981-04-10 | 1984-05-08 | Parker-Hannifin Corporation | Medication infusion pump |
| US4439196A (en) | 1982-03-18 | 1984-03-27 | Merck & Co., Inc. | Osmotic drug delivery system |
| US4522811A (en) | 1982-07-08 | 1985-06-11 | Syntex (U.S.A.) Inc. | Serial injection of muramyldipeptides and liposomes enhances the anti-infective activity of muramyldipeptides |
| US4447224A (en) | 1982-09-20 | 1984-05-08 | Infusaid Corporation | Variable flow implantable infusion apparatus |
| US4487603A (en) | 1982-11-26 | 1984-12-11 | Cordis Corporation | Implantable microinfusion pump system |
| US4486194A (en) | 1983-06-08 | 1984-12-04 | James Ferrara | Therapeutic device for administering medicaments through the skin |
| US4596556A (en) | 1985-03-25 | 1986-06-24 | Bioject, Inc. | Hypodermic injection apparatus |
| US5374548A (en) | 1986-05-02 | 1994-12-20 | Genentech, Inc. | Methods and compositions for the attachment of proteins to liposomes using a glycophospholipid anchor |
| MX9203291A (es) | 1985-06-26 | 1992-08-01 | Liposome Co Inc | Metodo para acoplamiento de liposomas. |
| US4790824A (en) | 1987-06-19 | 1988-12-13 | Bioject, Inc. | Non-invasive hypodermic injection device |
| US4941880A (en) | 1987-06-19 | 1990-07-17 | Bioject, Inc. | Pre-filled ampule and non-invasive hypodermic injection device assembly |
| US5108921A (en) | 1989-04-03 | 1992-04-28 | Purdue Research Foundation | Method for enhanced transmembrane transport of exogenous molecules |
| US5312335A (en) | 1989-11-09 | 1994-05-17 | Bioject Inc. | Needleless hypodermic injection device |
| US5064413A (en) | 1989-11-09 | 1991-11-12 | Bioject, Inc. | Needleless hypodermic injection device |
| US5383851A (en) | 1992-07-24 | 1995-01-24 | Bioject Inc. | Needleless hypodermic injection device |
| US5821332A (en) | 1993-11-03 | 1998-10-13 | The Board Of Trustees Of The Leland Stanford Junior University | Receptor on the surface of activated CD4+ T-cells: ACT-4 |
| US6410690B1 (en) | 1995-06-07 | 2002-06-25 | Medarex, Inc. | Therapeutic compounds comprised of anti-Fc receptor antibodies |
| US5922845A (en) | 1996-07-11 | 1999-07-13 | Medarex, Inc. | Therapeutic multispecific compounds comprised of anti-Fcα receptor antibodies |
| WO1998042752A1 (en) | 1997-03-21 | 1998-10-01 | Brigham And Women's Hospital Inc. | Immunotherapeutic ctla-4 binding peptides |
| AU2873999A (en) | 1998-02-24 | 1999-09-06 | Sisters Of Providence In Oregon | Compositions containing an OX-40 receptor binding agent or nucleic acid encoding the same and methods for enhancing antigen-specific immune response |
| WO2000037504A2 (en) | 1998-12-23 | 2000-06-29 | Pfizer Inc. | Human monoclonal antibodies to ctla-4 |
| JP5004390B2 (ja) | 1999-08-23 | 2012-08-22 | デイナ ファーバー キャンサー インスティチュート,インコーポレイテッド | 新規b7−4分子およびその用途 |
| EP1212422B1 (en) | 1999-08-24 | 2007-02-21 | Medarex, Inc. | Human ctla-4 antibodies and their uses |
| PT1234031T (pt) | 1999-11-30 | 2017-06-26 | Mayo Foundation | B7-h1, uma nova molécula imunoregulatória |
| IL149820A0 (en) | 2002-05-23 | 2002-11-10 | Curetech Ltd | Humanized immunomodulatory monoclonal antibodies for the treatment of neoplastic disease or immunodeficiency |
| CA2489004C (en) | 2002-06-13 | 2013-01-08 | Crucell Holland B.V. | Agonistic binding molecules to the human ox40 receptor |
| US7393531B2 (en) * | 2003-01-21 | 2008-07-01 | Arius Research Inc. | Cytotoxicity mediation of cells evidencing surface expression of MCSP |
| EP2053062A1 (en) | 2004-03-24 | 2009-04-29 | Xencor, Inc. | Immunoglobin variants outside the Fc region |
| AU2005307933A1 (en) | 2004-11-23 | 2006-06-01 | Pip Co., Ltd. | Built-in wall water service box |
| CN109485727A (zh) | 2005-05-09 | 2019-03-19 | 小野药品工业株式会社 | 程序性死亡-1(pd-1)的人单克隆抗体及使用抗pd-1抗体来治疗癌症的方法 |
| AU2006244068B9 (en) | 2005-05-10 | 2012-10-25 | Incyte Holdings Corporation | Modulators of indoleamine 2,3-dioxygenase and methods of using the same |
| KR101411165B1 (ko) | 2005-07-01 | 2014-06-25 | 메다렉스, 엘.엘.시. | 예정 사멸 리간드 1 (피디-엘1)에 대한 인간 모노클로날항체 |
| CA2634198C (en) | 2005-12-20 | 2014-06-03 | Incyte Corporation | N-hydroxyamidinoheterocycles as modulators of indoleamine 2,3-dioxygenase |
| CL2007002650A1 (es) | 2006-09-19 | 2008-02-08 | Incyte Corp | Compuestos derivados de heterociclo n-hidroxiamino; composicion farmaceutica, util para tratar cancer, infecciones virales y desordenes neurodegenerativos entre otras. |
| JP5319532B2 (ja) | 2006-09-19 | 2013-10-16 | インサイト・コーポレイション | インドールアミン2,3−ジオキシゲナーゼのモジュレーターとしてのn−ヒドロキシアミジノヘテロサイクル |
| EP1987839A1 (en) | 2007-04-30 | 2008-11-05 | I.N.S.E.R.M. Institut National de la Sante et de la Recherche Medicale | Cytotoxic anti-LAG-3 monoclonal antibody and its use in the treatment or prevention of organ transplant rejection and autoimmune disease |
| US20090028857A1 (en) | 2007-07-23 | 2009-01-29 | Cell Genesys, Inc. | Pd-1 antibodies in combination with a cytokine-secreting cell and methods of use thereof |
| EP2044949A1 (en) | 2007-10-05 | 2009-04-08 | Immutep | Use of recombinant lag-3 or the derivatives thereof for eliciting monocyte immune response |
| CA2932121A1 (en) | 2007-11-30 | 2009-06-11 | Newlink Genetics Corporation | Ido inhibitors |
| EP2262837A4 (en) | 2008-03-12 | 2011-04-06 | Merck Sharp & Dohme | PD-1 BINDING PROTEINS |
| AR072999A1 (es) | 2008-08-11 | 2010-10-06 | Medarex Inc | Anticuerpos humanos que se unen al gen 3 de activacion linfocitaria (lag-3) y los usos de estos |
| WO2010077634A1 (en) | 2008-12-09 | 2010-07-08 | Genentech, Inc. | Anti-pd-l1 antibodies and their use to enhance t-cell function |
| US20110007023A1 (en) | 2009-07-09 | 2011-01-13 | Sony Ericsson Mobile Communications Ab | Display device, touch screen device comprising the display device, mobile device and method for sensing a force on a display device |
| US8722720B2 (en) | 2009-10-28 | 2014-05-13 | Newlink Genetics Corporation | Imidazole derivatives as IDO inhibitors |
| NZ599405A (en) | 2009-11-24 | 2014-09-26 | Medimmune Ltd | Targeted binding agents against b7-h1 |
| PL2949670T3 (pl) | 2009-12-10 | 2019-07-31 | F. Hoffmann-La Roche Ag | Przeciwciała wiążące się preferencyjnie z zewnątrzkomórkową domeną 4 ludzkiego CSF-1R i ich zastosowanie |
| US9150656B2 (en) | 2010-03-04 | 2015-10-06 | Macrogenics, Inc. | Antibodies reactive with B7-H3, immunologically active fragments thereof and uses thereof |
| US9169323B2 (en) | 2010-03-05 | 2015-10-27 | Hoffmann-La Roche Inc. | Antibodies against human CSF-1R |
| US9221910B2 (en) | 2010-03-05 | 2015-12-29 | Hoffmann-La Roche Inc. | Antibodies against human CSF-1R |
| AU2011248083B2 (en) | 2010-05-04 | 2015-09-10 | Five Prime Therapeutics, Inc. | Antibodies that bind CSF1R |
| US8907053B2 (en) | 2010-06-25 | 2014-12-09 | Aurigene Discovery Technologies Limited | Immunosuppression modulating compounds |
| EA029793B1 (ru) | 2010-08-23 | 2018-05-31 | Борд Оф Риджентс, Дзе Юниверсити Оф Техас Систем | Антитела к ох40 и способы их применения |
| MX337040B (es) | 2010-09-09 | 2016-02-09 | Pfizer | Moleculas de union a 4-1bb. |
| NO2694640T3 (enExample) | 2011-04-15 | 2018-03-17 | ||
| PL2699264T3 (pl) | 2011-04-20 | 2018-08-31 | Medimmune, Llc | Przeciwciała i inne cząsteczki wiążące B7-H1 i PD-1 |
| CA2845810C (en) | 2011-08-23 | 2017-03-28 | Board Of Regents, The University Of Texas System | Anti-ox40 antibodies and methods of using the same |
| GB201116092D0 (en) | 2011-09-16 | 2011-11-02 | Bioceros B V | Antibodies and uses thereof |
| EP2785375B1 (en) | 2011-11-28 | 2020-07-22 | Merck Patent GmbH | Anti-pd-l1 antibodies and uses thereof |
| CA2853889A1 (en) | 2011-12-15 | 2013-06-20 | F. Hoffmann-La Roche Ag | Antibodies against human csf-1r and uses thereof |
| BR112014018961A8 (pt) | 2012-02-06 | 2017-07-11 | Genentech Inc | Anticorpo isolado, anticorpo biespecífico, fragmento, ácido nucleico isolado, vetor, célula hospedeira, método de produção de anticorpos, composição farmacêutica, uso do anticorpo, método de tratamento, método de inibição e artigo industrializado |
| AR090263A1 (es) | 2012-03-08 | 2014-10-29 | Hoffmann La Roche | Terapia combinada de anticuerpos contra el csf-1r humano y las utilizaciones de la misma |
| HK1208233A1 (en) | 2012-05-11 | 2016-02-26 | 戊瑞治疗有限公司 | Methods of treating conditions with antibodies that bind colony stimulating factor 1 receptor (csf1r) |
| US9856320B2 (en) | 2012-05-15 | 2018-01-02 | Bristol-Myers Squibb Company | Cancer immunotherapy by disrupting PD-1/PD-L1 signaling |
| AR091649A1 (es) | 2012-07-02 | 2015-02-18 | Bristol Myers Squibb Co | Optimizacion de anticuerpos que se fijan al gen de activacion de linfocitos 3 (lag-3) y sus usos |
| EP2890398A4 (en) | 2012-08-31 | 2016-03-09 | Five Prime Therapeutics Inc | METHODS OF TREATING DISEASES WITH ANTIBODIES THAT BIND COLONY STIMULATING FACTOR RECEIVER 1 (CSF1R) |
| KR20160006168A (ko) | 2013-03-18 | 2016-01-18 | 바이오서오엑스 프로덕스 비.브이. | 인간화 항-cd134(ox40) 항체 및 이의 용도 |
| EP3632934A1 (en) * | 2014-03-31 | 2020-04-08 | F. Hoffmann-La Roche AG | Anti-ox40 antibodies and methods of use |
| RU2016142476A (ru) | 2014-03-31 | 2018-05-07 | Дженентек, Инк. | Комбинированная терапия, включающая антиангиогенезные агенты и агонисты, связывающие ох40 |
| WO2016028810A1 (en) * | 2014-08-18 | 2016-02-25 | Biogen Ma Inc. | Anti-cd40 antibodies and uses thereof |
| TW201619200A (zh) * | 2014-10-10 | 2016-06-01 | 麥迪紐有限責任公司 | 人類化抗-ox40抗體及其用途 |
| CA2966507A1 (en) * | 2014-11-03 | 2016-05-12 | Genentech, Inc. | Methods and biomarkers for predicting efficacy and evaluation of an ox40 agonist treatment |
| MX2017006320A (es) * | 2014-11-17 | 2017-08-10 | Genentech Inc | Terapia combinada que comprende agonistas de unión de ox40 y antagonistas de unión del eje de pd-1. |
| LT3303396T (lt) * | 2015-05-29 | 2023-01-10 | Bristol-Myers Squibb Company | Antikūnai prieš ox40 ir jų panaudojimo būdai |
| KR20180011839A (ko) * | 2015-06-08 | 2018-02-02 | 제넨테크, 인크. | 항-ox40 항체를 이용한 암의 치료 방법 |
| MX2017016502A (es) * | 2015-06-29 | 2018-03-12 | Univ Rockefeller | Anticuerpos contra cd40 con actividad agonista mejorada. |
| WO2017021910A1 (en) * | 2015-08-04 | 2017-02-09 | Glaxosmithkline Intellectual Property Development Limited | Combination treatments and uses and methods thereof |
| CN114380908B (zh) | 2015-10-15 | 2023-03-17 | 苏州丁孚靶点生物技术有限公司 | 抗ox40抗体及其应用 |
| BR112018010172A2 (pt) | 2015-11-19 | 2018-11-21 | Bristol Myers Squibb Co | anticorpos contra receptor de fator de necrose de tumor induzido por glicocorticoide (gitr) e usos dos mesmos |
| EP3383430A4 (en) | 2015-12-02 | 2019-12-18 | Agenus Inc. | ANTIBODIES AND METHOD FOR USE THEREOF |
| EP3383914A4 (en) | 2015-12-02 | 2019-10-30 | Agenus Inc. | ANTI-OX40 ANTIBODIES AND METHOD OF USE THEREOF |
| WO2017096182A1 (en) | 2015-12-03 | 2017-06-08 | Agenus Inc. | Anti-ox40 antibodies and methods of use thereof |
| WO2017134292A1 (en) | 2016-02-04 | 2017-08-10 | Glenmark Pharmaceuticals S.A. | Anti-ox40 antagonistic antibodies for the treatment of atopic dermatitis |
| JP2021501801A (ja) | 2017-11-01 | 2021-01-21 | ブリストル−マイヤーズ スクイブ カンパニーBristol−Myers Squibb Company | 癌の処置に用いるための免疫刺激アゴニスト抗体 |
-
2018
- 2018-11-01 JP JP2020543734A patent/JP2021501801A/ja active Pending
- 2018-11-01 US US16/760,776 patent/US11603410B2/en active Active
- 2018-11-01 CN CN201880085048.3A patent/CN111542544A/zh active Pending
- 2018-11-01 KR KR1020207015641A patent/KR20200074214A/ko not_active Ceased
- 2018-11-01 WO PCT/US2018/058704 patent/WO2019089921A1/en not_active Ceased
- 2018-11-01 EP EP18815061.9A patent/EP3704159A1/en not_active Withdrawn
-
2023
- 2023-03-10 US US18/181,661 patent/US20230357423A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| JP2021501801A (ja) | 2021-01-21 |
| CN111542544A (zh) | 2020-08-14 |
| EP3704159A1 (en) | 2020-09-09 |
| WO2019089921A1 (en) | 2019-05-09 |
| US20230357423A1 (en) | 2023-11-09 |
| US20200369777A1 (en) | 2020-11-26 |
| US11603410B2 (en) | 2023-03-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20230357423A1 (en) | Immunostimulatory agonistic antibodies for use in treating cancer | |
| US20230235060A1 (en) | Neutralization of inhibitory pathways in lymphocytes | |
| US20230111786A1 (en) | Compositions comprising a combination of an anti-lag-3 antibody, a pd-1 pathway inhibitor, and an immunotherapeutic agent | |
| KR102833068B1 (ko) | Pd-1에 대한 항체 분자 및 그의 용도 | |
| EP2699598B1 (en) | Combinations of anti-4-1bb antibodies and adcc-inducing antibodies for the treatment of cancer | |
| US11230598B2 (en) | Antibodies and methods for depleting regulatory bio cells and use in combination with immune checkpoint inhibitors | |
| JP6970099B2 (ja) | ガンの処置のための方法及び医薬組成物 | |
| US12110337B2 (en) | Anti-CD27 antibodies and uses thereof | |
| RU2692248C2 (ru) | Улучшенные способы лечения васкуляризированных злокачественных опухолей | |
| JP2021518140A (ja) | Micaおよび/またはmicbに対する抗体ならびにそれらの使用 | |
| KR20200142542A (ko) | Cd73 길항제 항체 및 pd-1/pd-l1 축 길항제 항체에 의한 항암 조합 요법 | |
| TWI867138B (zh) | 抗tnfr2抗體及其用途 | |
| KR20240099362A (ko) | 혈액암에 대한 lag-3 길항제 요법 | |
| JP2020512357A (ja) | 抗gitr抗体を使用した癌の併用療法 | |
| RU2788092C2 (ru) | Молекулы антител к pd-1 и их применения | |
| HK40110316A (en) | Neutralization of inhibitory pathways in lymphocytes | |
| KR20250005988A (ko) | Kir3dl3 억제제 및 면역 세포 활성화제 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20200601 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20211101 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20240404 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20240614 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20240404 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |