KR20200045446A - 알츠하이머병의 치료를 위한 조성물 및 방법 - Google Patents
알츠하이머병의 치료를 위한 조성물 및 방법 Download PDFInfo
- Publication number
- KR20200045446A KR20200045446A KR1020197037319A KR20197037319A KR20200045446A KR 20200045446 A KR20200045446 A KR 20200045446A KR 1020197037319 A KR1020197037319 A KR 1020197037319A KR 20197037319 A KR20197037319 A KR 20197037319A KR 20200045446 A KR20200045446 A KR 20200045446A
- Authority
- KR
- South Korea
- Prior art keywords
- ptpσ
- therapeutic
- therapeutic agent
- app
- peptide
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 238000000034 method Methods 0.000 title claims abstract description 73
- 208000024827 Alzheimer disease Diseases 0.000 title claims description 38
- 238000011282 treatment Methods 0.000 title claims description 9
- 239000000203 mixture Substances 0.000 title description 15
- 239000003814 drug Substances 0.000 claims abstract description 68
- 229940124597 therapeutic agent Drugs 0.000 claims abstract description 62
- 230000002776 aggregation Effects 0.000 claims abstract description 40
- 238000004220 aggregation Methods 0.000 claims abstract description 40
- 230000007082 Aβ accumulation Effects 0.000 claims abstract description 23
- 230000011664 signaling Effects 0.000 claims abstract description 18
- 230000003197 catalytic effect Effects 0.000 claims abstract description 12
- 230000009467 reduction Effects 0.000 claims abstract description 12
- 230000002401 inhibitory effect Effects 0.000 claims abstract description 11
- 230000005764 inhibitory process Effects 0.000 claims abstract description 10
- 108010044260 Class 2 Receptor-Like Protein Tyrosine Phosphatases Proteins 0.000 claims description 248
- 102000006442 Class 2 Receptor-Like Protein Tyrosine Phosphatases Human genes 0.000 claims description 245
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 172
- 210000004027 cell Anatomy 0.000 claims description 91
- 230000001225 therapeutic effect Effects 0.000 claims description 91
- 150000001413 amino acids Chemical class 0.000 claims description 55
- 210000002569 neuron Anatomy 0.000 claims description 48
- 102000004160 Phosphoric Monoester Hydrolases Human genes 0.000 claims description 35
- 108090000608 Phosphoric Monoester Hydrolases Proteins 0.000 claims description 35
- 230000006870 function Effects 0.000 claims description 26
- 238000006467 substitution reaction Methods 0.000 claims description 16
- 239000003540 gamma secretase inhibitor Substances 0.000 claims description 10
- 238000010521 absorption reaction Methods 0.000 claims description 5
- 229940125759 BACE1 protease inhibitor Drugs 0.000 claims description 4
- 229940125373 Gamma-Secretase Inhibitor Drugs 0.000 claims description 4
- 229940121773 Secretase inhibitor Drugs 0.000 claims description 3
- 101710138741 Receptor-type tyrosine-protein phosphatase F Proteins 0.000 abstract description 54
- 102100039663 Receptor-type tyrosine-protein phosphatase F Human genes 0.000 abstract description 54
- 102000045595 Phosphoprotein Phosphatases Human genes 0.000 abstract description 9
- 108700019535 Phosphoprotein Phosphatases Proteins 0.000 abstract description 9
- 102000004196 processed proteins & peptides Human genes 0.000 description 92
- 241000699670 Mus sp. Species 0.000 description 91
- 108090000623 proteins and genes Proteins 0.000 description 88
- 101710137189 Amyloid-beta A4 protein Proteins 0.000 description 87
- 101710151993 Amyloid-beta precursor protein Proteins 0.000 description 87
- 102100022704 Amyloid-beta precursor protein Human genes 0.000 description 86
- DZHSAHHDTRWUTF-SIQRNXPUSA-N amyloid-beta polypeptide 42 Chemical compound C([C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(O)=O)[C@@H](C)CC)C(C)C)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(C)C)C1=CC=CC=C1 DZHSAHHDTRWUTF-SIQRNXPUSA-N 0.000 description 85
- 210000004556 brain Anatomy 0.000 description 70
- 102000013498 tau Proteins Human genes 0.000 description 66
- 108010026424 tau Proteins Proteins 0.000 description 66
- 102000004169 proteins and genes Human genes 0.000 description 61
- 230000014509 gene expression Effects 0.000 description 60
- 235000018102 proteins Nutrition 0.000 description 59
- 239000013598 vector Substances 0.000 description 59
- 102000002659 Amyloid Precursor Protein Secretases Human genes 0.000 description 49
- 229940024606 amino acid Drugs 0.000 description 49
- 108010043324 Amyloid Precursor Protein Secretases Proteins 0.000 description 47
- 235000001014 amino acid Nutrition 0.000 description 47
- 229920001184 polypeptide Polymers 0.000 description 44
- 230000000694 effects Effects 0.000 description 38
- 150000007523 nucleic acids Chemical class 0.000 description 38
- 108020004414 DNA Proteins 0.000 description 37
- 102000053602 DNA Human genes 0.000 description 37
- 241000699666 Mus <mouse, genus> Species 0.000 description 37
- 241000282414 Homo sapiens Species 0.000 description 32
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 32
- 102000039446 nucleic acids Human genes 0.000 description 31
- 108020004707 nucleic acids Proteins 0.000 description 31
- 239000003795 chemical substances by application Substances 0.000 description 28
- 108700019146 Transgenes Proteins 0.000 description 23
- 230000009261 transgenic effect Effects 0.000 description 23
- 230000027455 binding Effects 0.000 description 22
- 230000002068 genetic effect Effects 0.000 description 20
- 230000032258 transport Effects 0.000 description 20
- 125000003275 alpha amino acid group Chemical group 0.000 description 19
- 230000002950 deficient Effects 0.000 description 19
- 239000000047 product Substances 0.000 description 19
- 210000001519 tissue Anatomy 0.000 description 19
- 230000007812 deficiency Effects 0.000 description 18
- 239000007924 injection Substances 0.000 description 18
- 238000002347 injection Methods 0.000 description 18
- 230000003834 intracellular effect Effects 0.000 description 18
- 238000003776 cleavage reaction Methods 0.000 description 17
- 208000035475 disorder Diseases 0.000 description 16
- 239000012634 fragment Substances 0.000 description 16
- 102000040430 polynucleotide Human genes 0.000 description 16
- 108091033319 polynucleotide Proteins 0.000 description 16
- 239000002157 polynucleotide Substances 0.000 description 16
- 239000000872 buffer Substances 0.000 description 15
- 150000001875 compounds Chemical class 0.000 description 15
- 201000010099 disease Diseases 0.000 description 15
- 239000013603 viral vector Substances 0.000 description 15
- 241001465754 Metazoa Species 0.000 description 13
- 241000699660 Mus musculus Species 0.000 description 13
- 238000000749 co-immunoprecipitation Methods 0.000 description 13
- 210000001947 dentate gyrus Anatomy 0.000 description 13
- 238000002474 experimental method Methods 0.000 description 13
- 238000012545 processing Methods 0.000 description 13
- 238000011830 transgenic mouse model Methods 0.000 description 13
- 108010090849 Amyloid beta-Peptides Proteins 0.000 description 12
- 102000013455 Amyloid beta-Peptides Human genes 0.000 description 12
- ZOHGLPQGEHSLPD-FXQIFTODSA-N Ser-Gln-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O ZOHGLPQGEHSLPD-FXQIFTODSA-N 0.000 description 12
- 238000012360 testing method Methods 0.000 description 12
- 241000701161 unidentified adenovirus Species 0.000 description 12
- 102100021257 Beta-secretase 1 Human genes 0.000 description 11
- 101000894895 Homo sapiens Beta-secretase 1 Proteins 0.000 description 11
- 125000000539 amino acid group Chemical group 0.000 description 11
- 230000009286 beneficial effect Effects 0.000 description 11
- 239000003550 marker Substances 0.000 description 11
- 210000000653 nervous system Anatomy 0.000 description 11
- 102000005962 receptors Human genes 0.000 description 11
- 108020003175 receptors Proteins 0.000 description 11
- 230000002829 reductive effect Effects 0.000 description 11
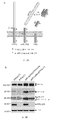
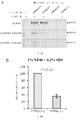
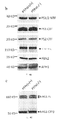
- 238000001262 western blot Methods 0.000 description 11
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 10
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 10
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 10
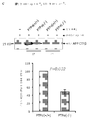
- 238000000692 Student's t-test Methods 0.000 description 10
- 230000007131 anti Alzheimer effect Effects 0.000 description 10
- 210000003169 central nervous system Anatomy 0.000 description 10
- 108010034529 leucyl-lysine Proteins 0.000 description 10
- 230000007017 scission Effects 0.000 description 10
- 235000004400 serine Nutrition 0.000 description 10
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical group C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 9
- ZRHDPZAAWLXXIR-SRVKXCTJSA-N Leu-Lys-Ala Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O ZRHDPZAAWLXXIR-SRVKXCTJSA-N 0.000 description 9
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 9
- ZRPLVTZTKPPSBT-AVGNSLFASA-N Tyr-Glu-Ser Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O ZRPLVTZTKPPSBT-AVGNSLFASA-N 0.000 description 9
- 230000000903 blocking effect Effects 0.000 description 9
- 239000003112 inhibitor Substances 0.000 description 9
- 230000004048 modification Effects 0.000 description 9
- 238000012986 modification Methods 0.000 description 9
- 125000003729 nucleotide group Chemical group 0.000 description 9
- 239000000126 substance Substances 0.000 description 9
- 239000000758 substrate Substances 0.000 description 9
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 8
- -1 Amino, carboxyl Chemical group 0.000 description 8
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 8
- NPROWIBAWYMPAZ-GUDRVLHUSA-N Ile-Asp-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N1CCC[C@@H]1C(=O)O)N NPROWIBAWYMPAZ-GUDRVLHUSA-N 0.000 description 8
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 8
- FKVNLUZHSFCNGY-RVMXOQNASA-N Pro-Ile-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@@H]2CCCN2 FKVNLUZHSFCNGY-RVMXOQNASA-N 0.000 description 8
- 241000700159 Rattus Species 0.000 description 8
- 230000003941 amyloidogenesis Effects 0.000 description 8
- 230000015572 biosynthetic process Effects 0.000 description 8
- 230000007423 decrease Effects 0.000 description 8
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 8
- 210000001320 hippocampus Anatomy 0.000 description 8
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 8
- 235000014304 histidine Nutrition 0.000 description 8
- 238000001727 in vivo Methods 0.000 description 8
- 230000001965 increasing effect Effects 0.000 description 8
- 238000001802 infusion Methods 0.000 description 8
- 230000003993 interaction Effects 0.000 description 8
- 230000035772 mutation Effects 0.000 description 8
- 239000002773 nucleotide Substances 0.000 description 8
- 239000002953 phosphate buffered saline Substances 0.000 description 8
- 108010015796 prolylisoleucine Proteins 0.000 description 8
- 210000000130 stem cell Anatomy 0.000 description 8
- 102100039289 Glial fibrillary acidic protein Human genes 0.000 description 7
- 101710193519 Glial fibrillary acidic protein Proteins 0.000 description 7
- 101000823051 Homo sapiens Amyloid-beta precursor protein Proteins 0.000 description 7
- JDAWAWXGAUZPNJ-ZPFDUUQYSA-N Ile-Glu-Arg Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N JDAWAWXGAUZPNJ-ZPFDUUQYSA-N 0.000 description 7
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 7
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 7
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 7
- 239000004473 Threonine Substances 0.000 description 7
- 210000004899 c-terminal region Anatomy 0.000 description 7
- 238000009472 formulation Methods 0.000 description 7
- 238000001476 gene delivery Methods 0.000 description 7
- 210000005046 glial fibrillary acidic protein Anatomy 0.000 description 7
- 230000001537 neural effect Effects 0.000 description 7
- 210000001428 peripheral nervous system Anatomy 0.000 description 7
- 108010051242 phenylalanylserine Proteins 0.000 description 7
- 229920002477 rna polymer Polymers 0.000 description 7
- 235000008521 threonine Nutrition 0.000 description 7
- KFMBRBPXHVMDFN-UWVGGRQHSA-N Gly-Arg-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)CN)CCCNC(N)=N KFMBRBPXHVMDFN-UWVGGRQHSA-N 0.000 description 6
- 108700003968 Human immunodeficiency virus 1 tat peptide (49-57) Proteins 0.000 description 6
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 6
- BGZCJDGBBUUBHA-KKUMJFAQSA-N Leu-Lys-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O BGZCJDGBBUUBHA-KKUMJFAQSA-N 0.000 description 6
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 6
- 239000004472 Lysine Substances 0.000 description 6
- 108091028043 Nucleic acid sequence Proteins 0.000 description 6
- 230000006933 amyloid-beta aggregation Effects 0.000 description 6
- 206010002022 amyloidosis Diseases 0.000 description 6
- 238000013459 approach Methods 0.000 description 6
- 230000006736 behavioral deficit Effects 0.000 description 6
- 238000010586 diagram Methods 0.000 description 6
- 102000046783 human APP Human genes 0.000 description 6
- 238000003119 immunoblot Methods 0.000 description 6
- 239000003446 ligand Substances 0.000 description 6
- 239000006166 lysate Substances 0.000 description 6
- 235000018977 lysine Nutrition 0.000 description 6
- 230000007246 mechanism Effects 0.000 description 6
- 239000013612 plasmid Substances 0.000 description 6
- 230000008569 process Effects 0.000 description 6
- 230000006886 spatial memory Effects 0.000 description 6
- 238000007920 subcutaneous administration Methods 0.000 description 6
- 239000004094 surface-active agent Substances 0.000 description 6
- 230000008685 targeting Effects 0.000 description 6
- 238000002560 therapeutic procedure Methods 0.000 description 6
- 241000710929 Alphavirus Species 0.000 description 5
- VYLVOMUVLMGCRF-ZLUOBGJFSA-N Asn-Asp-Ser Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O VYLVOMUVLMGCRF-ZLUOBGJFSA-N 0.000 description 5
- 206010012289 Dementia Diseases 0.000 description 5
- KKCUFHUTMKQQCF-SRVKXCTJSA-N Glu-Arg-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O KKCUFHUTMKQQCF-SRVKXCTJSA-N 0.000 description 5
- YCKPUHHMCFSUMD-IUKAMOBKSA-N Ile-Thr-Asp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(=O)O)C(=O)O)N YCKPUHHMCFSUMD-IUKAMOBKSA-N 0.000 description 5
- ONGCSGVHCSAATF-CIUDSAMLSA-N Met-Ala-Glu Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCC(O)=O ONGCSGVHCSAATF-CIUDSAMLSA-N 0.000 description 5
- 241000714177 Murine leukemia virus Species 0.000 description 5
- 102000014736 Notch Human genes 0.000 description 5
- 108010070047 Notch Receptors Proteins 0.000 description 5
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 5
- 241000700605 Viruses Species 0.000 description 5
- 238000003556 assay Methods 0.000 description 5
- 230000033228 biological regulation Effects 0.000 description 5
- 125000002091 cationic group Chemical group 0.000 description 5
- 230000008045 co-localization Effects 0.000 description 5
- 230000006378 damage Effects 0.000 description 5
- 239000000835 fiber Substances 0.000 description 5
- 238000001415 gene therapy Methods 0.000 description 5
- 238000007913 intrathecal administration Methods 0.000 description 5
- 150000002632 lipids Chemical class 0.000 description 5
- 238000004519 manufacturing process Methods 0.000 description 5
- 230000008506 pathogenesis Effects 0.000 description 5
- 238000013518 transcription Methods 0.000 description 5
- 230000035897 transcription Effects 0.000 description 5
- 238000001890 transfection Methods 0.000 description 5
- GSCLWXDNIMNIJE-ZLUOBGJFSA-N Ala-Asp-Asn Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O GSCLWXDNIMNIJE-ZLUOBGJFSA-N 0.000 description 4
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 4
- XSGBIBGAMKTHMY-WHFBIAKZSA-N Asn-Asp-Gly Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(O)=O XSGBIBGAMKTHMY-WHFBIAKZSA-N 0.000 description 4
- 241000219122 Cucurbita Species 0.000 description 4
- 235000009852 Cucurbita pepo Nutrition 0.000 description 4
- 241000283074 Equus asinus Species 0.000 description 4
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Chemical class OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 4
- 239000004471 Glycine Substances 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- 241000713666 Lentivirus Species 0.000 description 4
- QNTJIDXQHWUBKC-BZSNNMDCSA-N Leu-Lys-Phe Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O QNTJIDXQHWUBKC-BZSNNMDCSA-N 0.000 description 4
- MPOHDJKRBLVGCT-CIUDSAMLSA-N Lys-Ala-Asn Chemical compound C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCCCN)N MPOHDJKRBLVGCT-CIUDSAMLSA-N 0.000 description 4
- 241000124008 Mammalia Species 0.000 description 4
- 208000012902 Nervous system disease Diseases 0.000 description 4
- 108090000556 Neuregulin-1 Proteins 0.000 description 4
- 102400000058 Neuregulin-1 Human genes 0.000 description 4
- 241000700584 Simplexvirus Species 0.000 description 4
- 230000032683 aging Effects 0.000 description 4
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 4
- 238000009826 distribution Methods 0.000 description 4
- 230000004927 fusion Effects 0.000 description 4
- 108020001507 fusion proteins Proteins 0.000 description 4
- 102000037865 fusion proteins Human genes 0.000 description 4
- 238000003125 immunofluorescent labeling Methods 0.000 description 4
- 238000001114 immunoprecipitation Methods 0.000 description 4
- 210000004705 lumbosacral region Anatomy 0.000 description 4
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 4
- 210000005036 nerve Anatomy 0.000 description 4
- 230000004770 neurodegeneration Effects 0.000 description 4
- 210000004498 neuroglial cell Anatomy 0.000 description 4
- 210000002475 olfactory pathway Anatomy 0.000 description 4
- 238000012261 overproduction Methods 0.000 description 4
- 238000007911 parenteral administration Methods 0.000 description 4
- 230000001575 pathological effect Effects 0.000 description 4
- 239000000816 peptidomimetic Substances 0.000 description 4
- 239000008194 pharmaceutical composition Substances 0.000 description 4
- 230000026731 phosphorylation Effects 0.000 description 4
- 238000006366 phosphorylation reaction Methods 0.000 description 4
- 230000002797 proteolythic effect Effects 0.000 description 4
- 238000000746 purification Methods 0.000 description 4
- 238000011002 quantification Methods 0.000 description 4
- 230000004044 response Effects 0.000 description 4
- 230000001177 retroviral effect Effects 0.000 description 4
- 239000000523 sample Substances 0.000 description 4
- 230000002269 spontaneous effect Effects 0.000 description 4
- 230000002459 sustained effect Effects 0.000 description 4
- 238000007910 systemic administration Methods 0.000 description 4
- 230000003612 virological effect Effects 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- FWBHETKCLVMNFS-UHFFFAOYSA-N 4',6-Diamino-2-phenylindol Chemical compound C1=CC(C(=N)N)=CC=C1C1=CC2=CC=C(C(N)=N)C=C2N1 FWBHETKCLVMNFS-UHFFFAOYSA-N 0.000 description 3
- 239000005541 ACE inhibitor Substances 0.000 description 3
- LZRNYBIJOSKKRJ-XVYDVKMFSA-N Ala-Asp-His Chemical compound C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N LZRNYBIJOSKKRJ-XVYDVKMFSA-N 0.000 description 3
- 238000010173 Alzheimer-disease mouse model Methods 0.000 description 3
- 102000009091 Amyloidogenic Proteins Human genes 0.000 description 3
- 108010048112 Amyloidogenic Proteins Proteins 0.000 description 3
- JTWOBPNAVBESFW-FXQIFTODSA-N Arg-Cys-Asp Chemical compound C(C[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(=O)O)C(=O)O)N)CN=C(N)N JTWOBPNAVBESFW-FXQIFTODSA-N 0.000 description 3
- UZGFHWIJWPUPOH-IHRRRGAJSA-N Arg-Leu-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N UZGFHWIJWPUPOH-IHRRRGAJSA-N 0.000 description 3
- 239000004475 Arginine Substances 0.000 description 3
- PIWWUBYJNONVTJ-ZLUOBGJFSA-N Asn-Asp-Asn Chemical compound C([C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N)C(=O)N PIWWUBYJNONVTJ-ZLUOBGJFSA-N 0.000 description 3
- 230000007351 Aβ plaque formation Effects 0.000 description 3
- 108091026890 Coding region Proteins 0.000 description 3
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 3
- 238000002965 ELISA Methods 0.000 description 3
- 238000012286 ELISA Assay Methods 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- SYAYROHMAIHWFB-KBIXCLLPSA-N Glu-Ser-Ile Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O SYAYROHMAIHWFB-KBIXCLLPSA-N 0.000 description 3
- RXJFSLQVMGYQEL-IHRRRGAJSA-N Glu-Tyr-Glu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CCC(O)=O)C(O)=O)CC1=CC=C(O)C=C1 RXJFSLQVMGYQEL-IHRRRGAJSA-N 0.000 description 3
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 description 3
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 3
- XIZQPFCRXLUNMK-BZSNNMDCSA-N Lys-Leu-Phe Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)NC(=O)[C@H](CCCCN)N XIZQPFCRXLUNMK-BZSNNMDCSA-N 0.000 description 3
- YBAFDPFAUTYYRW-UHFFFAOYSA-N N-L-alpha-glutamyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCC(O)=O YBAFDPFAUTYYRW-UHFFFAOYSA-N 0.000 description 3
- 229940122116 Nerve growth factor agonist Drugs 0.000 description 3
- 208000028389 Nerve injury Diseases 0.000 description 3
- 208000036110 Neuroinflammatory disease Diseases 0.000 description 3
- 102000035195 Peptidases Human genes 0.000 description 3
- 108091005804 Peptidases Proteins 0.000 description 3
- 229920001213 Polysorbate 20 Polymers 0.000 description 3
- 102000002727 Protein Tyrosine Phosphatase Human genes 0.000 description 3
- 239000012083 RIPA buffer Substances 0.000 description 3
- 241000700161 Rattus rattus Species 0.000 description 3
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 description 3
- 241000711975 Vesicular stomatitis virus Species 0.000 description 3
- 108700005077 Viral Genes Proteins 0.000 description 3
- 208000027418 Wounds and injury Diseases 0.000 description 3
- OIPILFWXSMYKGL-UHFFFAOYSA-N acetylcholine Chemical compound CC(=O)OCC[N+](C)(C)C OIPILFWXSMYKGL-UHFFFAOYSA-N 0.000 description 3
- 229960004373 acetylcholine Drugs 0.000 description 3
- 230000004913 activation Effects 0.000 description 3
- 238000007792 addition Methods 0.000 description 3
- 235000004279 alanine Nutrition 0.000 description 3
- 229960003767 alanine Drugs 0.000 description 3
- 230000009435 amidation Effects 0.000 description 3
- 238000007112 amidation reaction Methods 0.000 description 3
- 230000003942 amyloidogenic effect Effects 0.000 description 3
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 3
- 235000009697 arginine Nutrition 0.000 description 3
- 210000001130 astrocyte Anatomy 0.000 description 3
- 210000003050 axon Anatomy 0.000 description 3
- 230000006399 behavior Effects 0.000 description 3
- 230000003542 behavioural effect Effects 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 239000002439 beta secretase inhibitor Substances 0.000 description 3
- 230000004071 biological effect Effects 0.000 description 3
- 210000000234 capsid Anatomy 0.000 description 3
- 230000015556 catabolic process Effects 0.000 description 3
- 210000000170 cell membrane Anatomy 0.000 description 3
- 230000002490 cerebral effect Effects 0.000 description 3
- 210000004289 cerebral ventricle Anatomy 0.000 description 3
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 230000007547 defect Effects 0.000 description 3
- 238000006731 degradation reaction Methods 0.000 description 3
- 238000012217 deletion Methods 0.000 description 3
- 230000037430 deletion Effects 0.000 description 3
- 210000001787 dendrite Anatomy 0.000 description 3
- 230000004069 differentiation Effects 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 239000003937 drug carrier Substances 0.000 description 3
- 239000003623 enhancer Substances 0.000 description 3
- 239000002532 enzyme inhibitor Substances 0.000 description 3
- 229940125532 enzyme inhibitor Drugs 0.000 description 3
- 238000005194 fractionation Methods 0.000 description 3
- 230000002518 glial effect Effects 0.000 description 3
- 235000013922 glutamic acid Nutrition 0.000 description 3
- 239000004220 glutamic acid Chemical class 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- 230000000971 hippocampal effect Effects 0.000 description 3
- 230000002209 hydrophobic effect Effects 0.000 description 3
- 230000001976 improved effect Effects 0.000 description 3
- 208000014674 injury Diseases 0.000 description 3
- 238000007918 intramuscular administration Methods 0.000 description 3
- 238000001990 intravenous administration Methods 0.000 description 3
- 238000011068 loading method Methods 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 230000001404 mediated effect Effects 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 125000001360 methionine group Chemical group N[C@@H](CCSC)C(=O)* 0.000 description 3
- 238000010369 molecular cloning Methods 0.000 description 3
- 208000015122 neurodegenerative disease Diseases 0.000 description 3
- 230000003959 neuroinflammation Effects 0.000 description 3
- 239000002858 neurotransmitter agent Substances 0.000 description 3
- 230000007170 pathology Effects 0.000 description 3
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 3
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 3
- 230000001323 posttranslational effect Effects 0.000 description 3
- 230000003518 presynaptic effect Effects 0.000 description 3
- 125000006239 protecting group Chemical group 0.000 description 3
- 238000000751 protein extraction Methods 0.000 description 3
- 230000020978 protein processing Effects 0.000 description 3
- 108020000494 protein-tyrosine phosphatase Proteins 0.000 description 3
- 238000007363 ring formation reaction Methods 0.000 description 3
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 3
- 150000003384 small molecules Chemical class 0.000 description 3
- 239000011780 sodium chloride Substances 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 210000000278 spinal cord Anatomy 0.000 description 3
- 238000010186 staining Methods 0.000 description 3
- 239000006228 supernatant Substances 0.000 description 3
- 208000024891 symptom Diseases 0.000 description 3
- 230000000946 synaptic effect Effects 0.000 description 3
- 238000003786 synthesis reaction Methods 0.000 description 3
- 238000012546 transfer Methods 0.000 description 3
- 241001430294 unidentified retrovirus Species 0.000 description 3
- 230000002861 ventricular Effects 0.000 description 3
- UKAUYVFTDYCKQA-UHFFFAOYSA-N -2-Amino-4-hydroxybutanoic acid Natural products OC(=O)C(N)CCO UKAUYVFTDYCKQA-UHFFFAOYSA-N 0.000 description 2
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 2
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 2
- 102000007469 Actins Human genes 0.000 description 2
- 108010085238 Actins Proteins 0.000 description 2
- 108700031308 Antennapedia Homeodomain Proteins 0.000 description 2
- GDVDRMUYICMNFJ-CIUDSAMLSA-N Arg-Cys-Glu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(O)=O)C(O)=O GDVDRMUYICMNFJ-CIUDSAMLSA-N 0.000 description 2
- SARSTIZOZFBDOM-FXQIFTODSA-N Asp-Met-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C)C(O)=O SARSTIZOZFBDOM-FXQIFTODSA-N 0.000 description 2
- QSFHZPQUAAQHAQ-CIUDSAMLSA-N Asp-Ser-Leu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O QSFHZPQUAAQHAQ-CIUDSAMLSA-N 0.000 description 2
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 2
- 238000009020 BCA Protein Assay Kit Methods 0.000 description 2
- 241000283707 Capra Species 0.000 description 2
- 241000702421 Dependoparvovirus Species 0.000 description 2
- 206010061818 Disease progression Diseases 0.000 description 2
- 108091029865 Exogenous DNA Proteins 0.000 description 2
- 241000287828 Gallus gallus Species 0.000 description 2
- WDTAKCUOIKHCTB-NKIYYHGXSA-N Glu-His-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CCC(=O)O)N)O WDTAKCUOIKHCTB-NKIYYHGXSA-N 0.000 description 2
- SLFSYFJKSIVSON-SRVKXCTJSA-N His-Met-Glu Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CC1=CN=CN1)N SLFSYFJKSIVSON-SRVKXCTJSA-N 0.000 description 2
- 241000701044 Human gammaherpesvirus 4 Species 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- HUORUFRRJHELPD-MNXVOIDGSA-N Ile-Leu-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N HUORUFRRJHELPD-MNXVOIDGSA-N 0.000 description 2
- 206010021703 Indifference Diseases 0.000 description 2
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 2
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 2
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 2
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 2
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical class OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 2
- UKAUYVFTDYCKQA-VKHMYHEASA-N L-homoserine Chemical compound OC(=O)[C@@H](N)CCO UKAUYVFTDYCKQA-VKHMYHEASA-N 0.000 description 2
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 2
- 208000026139 Memory disease Diseases 0.000 description 2
- BAVYZALUXZFZLV-UHFFFAOYSA-N Methylamine Chemical compound NC BAVYZALUXZFZLV-UHFFFAOYSA-N 0.000 description 2
- 102000004868 N-Methyl-D-Aspartate Receptors Human genes 0.000 description 2
- 108090001041 N-Methyl-D-Aspartate Receptors Proteins 0.000 description 2
- 229940099433 NMDA receptor antagonist Drugs 0.000 description 2
- 108010025020 Nerve Growth Factor Proteins 0.000 description 2
- 208000007125 Neurotoxicity Syndromes Diseases 0.000 description 2
- 239000000020 Nitrocellulose Substances 0.000 description 2
- 101800001628 Notch 1 intracellular domain Proteins 0.000 description 2
- 102400000552 Notch 1 intracellular domain Human genes 0.000 description 2
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 2
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Natural products OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 2
- HBXAOEBRGLCLIW-AVGNSLFASA-N Phe-Ser-Gln Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N HBXAOEBRGLCLIW-AVGNSLFASA-N 0.000 description 2
- 102100022033 Presenilin-1 Human genes 0.000 description 2
- 239000004365 Protease Substances 0.000 description 2
- 241001428933 Rat coronavirus Species 0.000 description 2
- 230000010799 Receptor Interactions Effects 0.000 description 2
- 108020004511 Recombinant DNA Proteins 0.000 description 2
- 241000710960 Sindbis virus Species 0.000 description 2
- 102000017299 Synapsin-1 Human genes 0.000 description 2
- 108050005241 Synapsin-1 Proteins 0.000 description 2
- FHDLKMFZKRUQCE-HJGDQZAQSA-N Thr-Glu-Arg Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O FHDLKMFZKRUQCE-HJGDQZAQSA-N 0.000 description 2
- 239000007983 Tris buffer Substances 0.000 description 2
- 239000013504 Triton X-100 Substances 0.000 description 2
- 229920004890 Triton X-100 Polymers 0.000 description 2
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 2
- 230000021736 acetylation Effects 0.000 description 2
- 238000006640 acetylation reaction Methods 0.000 description 2
- 230000010933 acylation Effects 0.000 description 2
- 238000005917 acylation reaction Methods 0.000 description 2
- 108010005233 alanylglutamic acid Proteins 0.000 description 2
- 230000004075 alteration Effects 0.000 description 2
- 125000003277 amino group Chemical group 0.000 description 2
- 229910021529 ammonia Inorganic materials 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 239000000427 antigen Substances 0.000 description 2
- 108091007433 antigens Proteins 0.000 description 2
- 102000036639 antigens Human genes 0.000 description 2
- 108010052670 arginyl-glutamyl-glutamic acid Proteins 0.000 description 2
- 235000009582 asparagine Nutrition 0.000 description 2
- 229960001230 asparagine Drugs 0.000 description 2
- 235000003704 aspartic acid Nutrition 0.000 description 2
- CKLJMWTZIZZHCS-REOHCLBHSA-N aspartic acid group Chemical class N[C@@H](CC(=O)O)C(=O)O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 2
- 230000003376 axonal effect Effects 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 2
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 2
- 108091007737 beta-secretases Proteins 0.000 description 2
- 230000001588 bifunctional effect Effects 0.000 description 2
- 210000004900 c-terminal fragment Anatomy 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- MTCMTKNMZCPKLX-UHFFFAOYSA-N chembl359570 Chemical compound N=1OC=2C=C3NC(=O)CC3=CC=2C=1CCC(CC1)CCN1CC1=CC=CC=C1 MTCMTKNMZCPKLX-UHFFFAOYSA-N 0.000 description 2
- 239000000544 cholinesterase inhibitor Substances 0.000 description 2
- 238000010367 cloning Methods 0.000 description 2
- 238000010226 confocal imaging Methods 0.000 description 2
- 230000001276 controlling effect Effects 0.000 description 2
- 238000004132 cross linking Methods 0.000 description 2
- 235000018417 cysteine Nutrition 0.000 description 2
- 231100000433 cytotoxic Toxicity 0.000 description 2
- 230000001472 cytotoxic effect Effects 0.000 description 2
- 230000006735 deficit Effects 0.000 description 2
- 238000000326 densiometry Methods 0.000 description 2
- 238000001739 density measurement Methods 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 238000006471 dimerization reaction Methods 0.000 description 2
- 230000005750 disease progression Effects 0.000 description 2
- ADEBPBSSDYVVLD-UHFFFAOYSA-N donepezil Chemical compound O=C1C=2C=C(OC)C(OC)=CC=2CC1CC(CC1)CCN1CC1=CC=CC=C1 ADEBPBSSDYVVLD-UHFFFAOYSA-N 0.000 description 2
- 210000005110 dorsal hippocampus Anatomy 0.000 description 2
- 238000012377 drug delivery Methods 0.000 description 2
- 230000004064 dysfunction Effects 0.000 description 2
- 238000004520 electroporation Methods 0.000 description 2
- 239000013604 expression vector Substances 0.000 description 2
- 235000019253 formic acid Nutrition 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- ASUTZQLVASHGKV-JDFRZJQESA-N galanthamine Chemical compound O1C(=C23)C(OC)=CC=C2CN(C)CC[C@]23[C@@H]1C[C@@H](O)C=C2 ASUTZQLVASHGKV-JDFRZJQESA-N 0.000 description 2
- BTCSSZJGUNDROE-UHFFFAOYSA-N gamma-aminobutyric acid Chemical compound NCCCC(O)=O BTCSSZJGUNDROE-UHFFFAOYSA-N 0.000 description 2
- 102000038383 gamma-secretases Human genes 0.000 description 2
- 108091007739 gamma-secretases Proteins 0.000 description 2
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 2
- 235000004554 glutamine Nutrition 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 125000000487 histidyl group Chemical group [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C([H])=N1 0.000 description 2
- 239000011539 homogenization buffer Substances 0.000 description 2
- 239000003689 hormone receptor blocking agent Substances 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 238000001361 intraarterial administration Methods 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 238000012417 linear regression Methods 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 210000004379 membrane Anatomy 0.000 description 2
- 108020004999 messenger RNA Proteins 0.000 description 2
- 229930182817 methionine Natural products 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- 230000003278 mimic effect Effects 0.000 description 2
- 239000002052 molecular layer Substances 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- 238000010172 mouse model Methods 0.000 description 2
- 239000003703 n methyl dextro aspartic acid receptor blocking agent Substances 0.000 description 2
- OGZQTTHDGQBLBT-UHFFFAOYSA-N neramexane Chemical compound CC1(C)CC(C)(C)CC(C)(N)C1 OGZQTTHDGQBLBT-UHFFFAOYSA-N 0.000 description 2
- 230000008764 nerve damage Effects 0.000 description 2
- 210000004126 nerve fiber Anatomy 0.000 description 2
- 208000004296 neuralgia Diseases 0.000 description 2
- 210000002241 neurite Anatomy 0.000 description 2
- 230000000626 neurodegenerative effect Effects 0.000 description 2
- 208000021722 neuropathic pain Diseases 0.000 description 2
- 229920001220 nitrocellulos Polymers 0.000 description 2
- 238000010606 normalization Methods 0.000 description 2
- 238000012758 nuclear staining Methods 0.000 description 2
- 229960003104 ornithine Drugs 0.000 description 2
- 230000002018 overexpression Effects 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 230000000750 progressive effect Effects 0.000 description 2
- 210000001176 projection neuron Anatomy 0.000 description 2
- 230000001737 promoting effect Effects 0.000 description 2
- 235000019419 proteases Nutrition 0.000 description 2
- 238000011084 recovery Methods 0.000 description 2
- 230000022532 regulation of transcription, DNA-dependent Effects 0.000 description 2
- 230000010076 replication Effects 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- FSYKKLYZXJSNPZ-UHFFFAOYSA-N sarcosine Chemical compound C[NH2+]CC([O-])=O FSYKKLYZXJSNPZ-UHFFFAOYSA-N 0.000 description 2
- 238000012163 sequencing technique Methods 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 210000003625 skull Anatomy 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 241000894007 species Species 0.000 description 2
- ATHGHQPFGPMSJY-UHFFFAOYSA-N spermidine Chemical compound NCCCCNCCCN ATHGHQPFGPMSJY-UHFFFAOYSA-N 0.000 description 2
- PFNFFQXMRSDOHW-UHFFFAOYSA-N spermine Chemical compound NCCCNCCCCNCCCN PFNFFQXMRSDOHW-UHFFFAOYSA-N 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 230000007470 synaptic degeneration Effects 0.000 description 2
- 238000011191 terminal modification Methods 0.000 description 2
- CWERGRDVMFNCDR-UHFFFAOYSA-N thioglycolic acid Chemical compound OC(=O)CS CWERGRDVMFNCDR-UHFFFAOYSA-N 0.000 description 2
- 238000011200 topical administration Methods 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- 230000009466 transformation Effects 0.000 description 2
- 238000013519 translation Methods 0.000 description 2
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 2
- 238000011144 upstream manufacturing Methods 0.000 description 2
- 239000004474 valine Substances 0.000 description 2
- 229940124648 γ-Secretase Modulator Drugs 0.000 description 2
- VBRJFXSFCYEZMQ-HNNXBMFYSA-N (2s)-2-amino-3-[3-(2-chlorophenyl)-5-(phosphonomethyl)phenyl]propanoic acid Chemical compound OC(=O)[C@@H](N)CC1=CC(CP(O)(O)=O)=CC(C=2C(=CC=CC=2)Cl)=C1 VBRJFXSFCYEZMQ-HNNXBMFYSA-N 0.000 description 1
- UBCHPRBFMUDMNC-UHFFFAOYSA-N 1-(1-adamantyl)ethanamine Chemical compound C1C(C2)CC3CC2CC1(C(N)C)C3 UBCHPRBFMUDMNC-UHFFFAOYSA-N 0.000 description 1
- GGUSQTSTQSHJAH-UHFFFAOYSA-N 1-(4-chlorophenyl)-2-[4-(4-fluorobenzyl)piperidin-1-yl]ethanol Chemical compound C=1C=C(Cl)C=CC=1C(O)CN(CC1)CCC1CC1=CC=C(F)C=C1 GGUSQTSTQSHJAH-UHFFFAOYSA-N 0.000 description 1
- NIRHKQWWUBHGOI-UHFFFAOYSA-N 2-fluoro-5,6-dimethoxy-2,3-dihydroinden-1-one Chemical compound C1=C(OC)C(OC)=CC2=C1C(=O)C(F)C2 NIRHKQWWUBHGOI-UHFFFAOYSA-N 0.000 description 1
- UAIUNKRWKOVEES-UHFFFAOYSA-N 3,3',5,5'-tetramethylbenzidine Chemical compound CC1=C(N)C(C)=CC(C=2C=C(C)C(N)=C(C)C=2)=C1 UAIUNKRWKOVEES-UHFFFAOYSA-N 0.000 description 1
- CWEHWZPCDBRUNO-WLHGVMLRSA-N 3-(1-benzylpiperidin-4-yl)-1-(2,3,4,5-tetrahydro-1h-1-benzazepin-8-yl)propan-1-one;(e)-but-2-enedioic acid Chemical compound OC(=O)\C=C\C(O)=O.C=1C=C2CCCCNC2=CC=1C(=O)CCC(CC1)CCN1CC1=CC=CC=C1 CWEHWZPCDBRUNO-WLHGVMLRSA-N 0.000 description 1
- SNKZJIOFVMKAOJ-UHFFFAOYSA-N 3-Aminopropanesulfonate Chemical compound NCCCS(O)(=O)=O SNKZJIOFVMKAOJ-UHFFFAOYSA-N 0.000 description 1
- BRMWTNUJHUMWMS-UHFFFAOYSA-N 3-Methylhistidine Natural products CN1C=NC(CC(N)C(O)=O)=C1 BRMWTNUJHUMWMS-UHFFFAOYSA-N 0.000 description 1
- 125000006284 3-fluorobenzyl group Chemical group [H]C1=C([H])C(=C([H])C(F)=C1[H])C([H])([H])* 0.000 description 1
- 229940117976 5-hydroxylysine Drugs 0.000 description 1
- ODHCTXKNWHHXJC-VKHMYHEASA-N 5-oxo-L-proline Chemical compound OC(=O)[C@@H]1CCC(=O)N1 ODHCTXKNWHHXJC-VKHMYHEASA-N 0.000 description 1
- SLXKOJJOQWFEFD-UHFFFAOYSA-N 6-aminohexanoic acid Chemical compound NCCCCCC(O)=O SLXKOJJOQWFEFD-UHFFFAOYSA-N 0.000 description 1
- 239000013607 AAV vector Substances 0.000 description 1
- 230000005730 ADP ribosylation Effects 0.000 description 1
- 229940100578 Acetylcholinesterase inhibitor Drugs 0.000 description 1
- GFBLJMHGHAXGNY-ZLUOBGJFSA-N Ala-Asn-Asp Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O GFBLJMHGHAXGNY-ZLUOBGJFSA-N 0.000 description 1
- 208000000044 Amnesia Diseases 0.000 description 1
- 208000037259 Amyloid Plaque Diseases 0.000 description 1
- 240000000662 Anethum graveolens Species 0.000 description 1
- 108010032595 Antibody Binding Sites Proteins 0.000 description 1
- 206010002942 Apathy Diseases 0.000 description 1
- 101100127672 Arabidopsis thaliana LAZY2 gene Proteins 0.000 description 1
- SVHRPCMZTWZROG-DCAQKATOSA-N Arg-Cys-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CCCN=C(N)N)N SVHRPCMZTWZROG-DCAQKATOSA-N 0.000 description 1
- PNQWAUXQDBIJDY-GUBZILKMSA-N Arg-Glu-Glu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O PNQWAUXQDBIJDY-GUBZILKMSA-N 0.000 description 1
- JOADBFCFJGNIKF-GUBZILKMSA-N Arg-Met-Ala Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C)C(O)=O JOADBFCFJGNIKF-GUBZILKMSA-N 0.000 description 1
- XYOVHPDDWCEUDY-CIUDSAMLSA-N Asn-Ala-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O XYOVHPDDWCEUDY-CIUDSAMLSA-N 0.000 description 1
- GMRGSBAMMMVDGG-GUBZILKMSA-N Asn-Arg-Arg Chemical compound C(C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)[C@H](CC(=O)N)N)CN=C(N)N GMRGSBAMMMVDGG-GUBZILKMSA-N 0.000 description 1
- BUVNWKQBMZLCDW-UGYAYLCHSA-N Asp-Asn-Ile Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O BUVNWKQBMZLCDW-UGYAYLCHSA-N 0.000 description 1
- UGKZHCBLMLSANF-CIUDSAMLSA-N Asp-Asn-Leu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O UGKZHCBLMLSANF-CIUDSAMLSA-N 0.000 description 1
- JDHOJQJMWBKHDB-CIUDSAMLSA-N Asp-Asn-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC(=O)O)N JDHOJQJMWBKHDB-CIUDSAMLSA-N 0.000 description 1
- QOVWVLLHMMCFFY-ZLUOBGJFSA-N Asp-Asp-Asn Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O QOVWVLLHMMCFFY-ZLUOBGJFSA-N 0.000 description 1
- QCVXMEHGFUMKCO-YUMQZZPRSA-N Asp-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CC(O)=O QCVXMEHGFUMKCO-YUMQZZPRSA-N 0.000 description 1
- TVIZQBFURPLQDV-DJFWLOJKSA-N Asp-His-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CC(=O)O)N TVIZQBFURPLQDV-DJFWLOJKSA-N 0.000 description 1
- JNNVNVRBYUJYGS-CIUDSAMLSA-N Asp-Leu-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O JNNVNVRBYUJYGS-CIUDSAMLSA-N 0.000 description 1
- 206010003694 Atrophy Diseases 0.000 description 1
- 230000006970 Aβ cytotoxicity Effects 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 108090000715 Brain-derived neurotrophic factor Proteins 0.000 description 1
- 102100037597 Brain-derived neurotrophic factor Human genes 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 108010078791 Carrier Proteins Proteins 0.000 description 1
- 241000700198 Cavia Species 0.000 description 1
- 108091006146 Channels Proteins 0.000 description 1
- 102000003914 Cholinesterases Human genes 0.000 description 1
- 108090000322 Cholinesterases Proteins 0.000 description 1
- QCDFBFJGMNKBDO-UHFFFAOYSA-N Clioquinol Chemical compound C1=CN=C2C(O)=C(I)C=C(Cl)C2=C1 QCDFBFJGMNKBDO-UHFFFAOYSA-N 0.000 description 1
- 101100538048 Colletotrichum orbiculare (strain 104-T / ATCC 96160 / CBS 514.97 / LARS 414 / MAFF 240422) TRM8 gene Proteins 0.000 description 1
- 206010010071 Coma Diseases 0.000 description 1
- 108010069514 Cyclic Peptides Proteins 0.000 description 1
- 102000001189 Cyclic Peptides Human genes 0.000 description 1
- 150000008574 D-amino acids Chemical class 0.000 description 1
- LEVWYRKDKASIDU-QWWZWVQMSA-N D-cystine Chemical compound OC(=O)[C@H](N)CSSC[C@@H](N)C(O)=O LEVWYRKDKASIDU-QWWZWVQMSA-N 0.000 description 1
- 238000000116 DAPI staining Methods 0.000 description 1
- 102000052510 DNA-Binding Proteins Human genes 0.000 description 1
- 101710096438 DNA-binding protein Proteins 0.000 description 1
- 101100516503 Danio rerio neurog1 gene Proteins 0.000 description 1
- 206010059866 Drug resistance Diseases 0.000 description 1
- 206010052804 Drug tolerance Diseases 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 108700024394 Exon Proteins 0.000 description 1
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 1
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 102100037362 Fibronectin Human genes 0.000 description 1
- 108010067306 Fibronectins Proteins 0.000 description 1
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 1
- 108091006027 G proteins Proteins 0.000 description 1
- 102000030782 GTP binding Human genes 0.000 description 1
- 108091000058 GTP-Binding Proteins 0.000 description 1
- 208000032612 Glial tumor Diseases 0.000 description 1
- 206010018338 Glioma Diseases 0.000 description 1
- MPZWMIIOPAPAKE-BQBZGAKWSA-N Glu-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@H](C(O)=O)CCCNC(N)=N MPZWMIIOPAPAKE-BQBZGAKWSA-N 0.000 description 1
- AUTNXSQEVVHSJK-YVNDNENWSA-N Glu-Glu-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O AUTNXSQEVVHSJK-YVNDNENWSA-N 0.000 description 1
- HKTRDWYCAUTRRL-YUMQZZPRSA-N Glu-His Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 HKTRDWYCAUTRRL-YUMQZZPRSA-N 0.000 description 1
- HVYWQYLBVXMXSV-GUBZILKMSA-N Glu-Leu-Ala Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O HVYWQYLBVXMXSV-GUBZILKMSA-N 0.000 description 1
- UERORLSAFUHDGU-AVGNSLFASA-N Glu-Phe-Asn Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)O)N UERORLSAFUHDGU-AVGNSLFASA-N 0.000 description 1
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 description 1
- 101150074178 HNT2 gene Proteins 0.000 description 1
- DQZCEKQPSOBNMJ-NKIYYHGXSA-N His-Thr-Glu Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(O)=O DQZCEKQPSOBNMJ-NKIYYHGXSA-N 0.000 description 1
- 101000891579 Homo sapiens Microtubule-associated protein tau Proteins 0.000 description 1
- 101001087394 Homo sapiens Tyrosine-protein phosphatase non-receptor type 1 Proteins 0.000 description 1
- 108010070875 Human Immunodeficiency Virus tat Gene Products Proteins 0.000 description 1
- 241000700588 Human alphaherpesvirus 1 Species 0.000 description 1
- ZRJBHWIHUMBLCN-SEQYCRGISA-N Huperzine A Natural products N1C(=O)C=CC2=C1C[C@H]1/C(=C/C)[C@]2(N)CC(C)=C1 ZRJBHWIHUMBLCN-SEQYCRGISA-N 0.000 description 1
- PMMYEEVYMWASQN-DMTCNVIQSA-N Hydroxyproline Chemical compound O[C@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-DMTCNVIQSA-N 0.000 description 1
- VSZALHITQINTGC-GHCJXIJMSA-N Ile-Ala-Asp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](CC(=O)O)C(=O)O)N VSZALHITQINTGC-GHCJXIJMSA-N 0.000 description 1
- YKRIXHPEIZUDDY-GMOBBJLQSA-N Ile-Asn-Arg Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N YKRIXHPEIZUDDY-GMOBBJLQSA-N 0.000 description 1
- 206010061598 Immunodeficiency Diseases 0.000 description 1
- 208000029462 Immunodeficiency disease Diseases 0.000 description 1
- 102000016844 Immunoglobulin-like domains Human genes 0.000 description 1
- 108050006430 Immunoglobulin-like domains Proteins 0.000 description 1
- 108091092195 Intron Proteins 0.000 description 1
- YQEZLKZALYSWHR-UHFFFAOYSA-N Ketamine Chemical compound C=1C=CC=C(Cl)C=1C1(NC)CCCCC1=O YQEZLKZALYSWHR-UHFFFAOYSA-N 0.000 description 1
- SNDPXSYFESPGGJ-BYPYZUCNSA-N L-2-aminopentanoic acid Chemical compound CCC[C@H](N)C(O)=O SNDPXSYFESPGGJ-BYPYZUCNSA-N 0.000 description 1
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 1
- RHGKLRLOHDJJDR-BYPYZUCNSA-N L-citrulline Chemical compound NC(=O)NCCC[C@H]([NH3+])C([O-])=O RHGKLRLOHDJJDR-BYPYZUCNSA-N 0.000 description 1
- XIGSAGMEBXLVJJ-YFKPBYRVSA-N L-homocitrulline Chemical compound NC(=O)NCCCC[C@H]([NH3+])C([O-])=O XIGSAGMEBXLVJJ-YFKPBYRVSA-N 0.000 description 1
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- SNDPXSYFESPGGJ-UHFFFAOYSA-N L-norVal-OH Natural products CCCC(N)C(O)=O SNDPXSYFESPGGJ-UHFFFAOYSA-N 0.000 description 1
- LRQKBLKVPFOOQJ-YFKPBYRVSA-N L-norleucine Chemical compound CCCC[C@H]([NH3+])C([O-])=O LRQKBLKVPFOOQJ-YFKPBYRVSA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- WIDZHJTYKYBLSR-DCAQKATOSA-N Leu-Glu-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O WIDZHJTYKYBLSR-DCAQKATOSA-N 0.000 description 1
- AKVBOOKXVAMKSS-GUBZILKMSA-N Leu-Ser-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O AKVBOOKXVAMKSS-GUBZILKMSA-N 0.000 description 1
- 108010000817 Leuprolide Proteins 0.000 description 1
- KEPWSUPUFAPBRF-DKIMLUQUSA-N Lys-Ile-Phe Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O KEPWSUPUFAPBRF-DKIMLUQUSA-N 0.000 description 1
- SKRGVGLIRUGANF-AVGNSLFASA-N Lys-Leu-Glu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O SKRGVGLIRUGANF-AVGNSLFASA-N 0.000 description 1
- 239000007987 MES buffer Substances 0.000 description 1
- 239000007993 MOPS buffer Substances 0.000 description 1
- VHGIWFGJIHTASW-FXQIFTODSA-N Met-Ala-Asp Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC(O)=O VHGIWFGJIHTASW-FXQIFTODSA-N 0.000 description 1
- 102000029749 Microtubule Human genes 0.000 description 1
- 108091022875 Microtubule Proteins 0.000 description 1
- 101100364400 Mus musculus Rtn4r gene Proteins 0.000 description 1
- JDHILDINMRGULE-LURJTMIESA-N N(pros)-methyl-L-histidine Chemical compound CN1C=NC=C1C[C@H](N)C(O)=O JDHILDINMRGULE-LURJTMIESA-N 0.000 description 1
- HOKKHZGPKSLGJE-GSVOUGTGSA-N N-Methyl-D-aspartic acid Chemical compound CN[C@@H](C(O)=O)CC(O)=O HOKKHZGPKSLGJE-GSVOUGTGSA-N 0.000 description 1
- 229940127523 NMDA Receptor Antagonists Drugs 0.000 description 1
- 238000005481 NMR spectroscopy Methods 0.000 description 1
- RHGKLRLOHDJJDR-UHFFFAOYSA-N Ndelta-carbamoyl-DL-ornithine Natural products OC(=O)C(N)CCCNC(N)=O RHGKLRLOHDJJDR-UHFFFAOYSA-N 0.000 description 1
- 102000015336 Nerve Growth Factor Human genes 0.000 description 1
- 102000007072 Nerve Growth Factors Human genes 0.000 description 1
- 208000025966 Neurological disease Diseases 0.000 description 1
- 101100342977 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) leu-1 gene Proteins 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 108700026244 Open Reading Frames Proteins 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 208000002193 Pain Diseases 0.000 description 1
- 229930040373 Paraformaldehyde Natural products 0.000 description 1
- 206010033799 Paralysis Diseases 0.000 description 1
- 208000031481 Pathologic Constriction Diseases 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 229940083963 Peptide antagonist Drugs 0.000 description 1
- 108010043958 Peptoids Proteins 0.000 description 1
- MPGJIHFJCXTVEX-KKUMJFAQSA-N Phe-Arg-Glu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O MPGJIHFJCXTVEX-KKUMJFAQSA-N 0.000 description 1
- 229940122907 Phosphatase inhibitor Drugs 0.000 description 1
- 102000010780 Platelet-Derived Growth Factor Human genes 0.000 description 1
- 108010038512 Platelet-Derived Growth Factor Proteins 0.000 description 1
- 108010036933 Presenilin-1 Proteins 0.000 description 1
- 102000012419 Presenilin-2 Human genes 0.000 description 1
- 108010036908 Presenilin-2 Proteins 0.000 description 1
- 102000015499 Presenilins Human genes 0.000 description 1
- 108010050254 Presenilins Proteins 0.000 description 1
- 229940124158 Protease/peptidase inhibitor Drugs 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 101710149951 Protein Tat Proteins 0.000 description 1
- 206010037660 Pyrexia Diseases 0.000 description 1
- FTALBRSUTCGOEG-UHFFFAOYSA-N Riluzole Chemical compound C1=C(OC(F)(F)F)C=C2SC(N)=NC2=C1 FTALBRSUTCGOEG-UHFFFAOYSA-N 0.000 description 1
- XSVMFMHYUFZWBK-NSHDSACASA-N Rivastigmine Chemical compound CCN(C)C(=O)OC1=CC=CC([C@H](C)N(C)C)=C1 XSVMFMHYUFZWBK-NSHDSACASA-N 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 101100516512 Saccharomyces cerevisiae (strain ATCC 204508 / S288c) NGR1 gene Proteins 0.000 description 1
- 108010077895 Sarcosine Proteins 0.000 description 1
- 229920002684 Sepharose Polymers 0.000 description 1
- ZRJBHWIHUMBLCN-UHFFFAOYSA-N Shuangyiping Natural products N1C(=O)C=CC2=C1CC1C(=CC)C2(N)CC(C)=C1 ZRJBHWIHUMBLCN-UHFFFAOYSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 108700005078 Synthetic Genes Proteins 0.000 description 1
- 101710192266 Tegument protein VP22 Proteins 0.000 description 1
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 1
- 108091023040 Transcription factor Proteins 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- 239000007984 Tris EDTA buffer Substances 0.000 description 1
- 102100033001 Tyrosine-protein phosphatase non-receptor type 1 Human genes 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 241000219977 Vigna Species 0.000 description 1
- 108010003533 Viral Envelope Proteins Proteins 0.000 description 1
- 108010067390 Viral Proteins Proteins 0.000 description 1
- GELXFVQAWNTGPQ-UHFFFAOYSA-N [N].C1=CNC=N1 Chemical compound [N].C1=CNC=N1 GELXFVQAWNTGPQ-UHFFFAOYSA-N 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- MGSKVZWGBWPBTF-UHFFFAOYSA-N aebsf Chemical compound NCCC1=CC=C(S(F)(=O)=O)C=C1 MGSKVZWGBWPBTF-UHFFFAOYSA-N 0.000 description 1
- 239000000556 agonist Substances 0.000 description 1
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 description 1
- 108010041407 alanylaspartic acid Proteins 0.000 description 1
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 1
- DKNWSYNQZKUICI-UHFFFAOYSA-N amantadine Chemical compound C1C(C2)CC3CC2CC1(N)C3 DKNWSYNQZKUICI-UHFFFAOYSA-N 0.000 description 1
- 229960003805 amantadine Drugs 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 150000003862 amino acid derivatives Chemical class 0.000 description 1
- 229960002684 aminocaproic acid Drugs 0.000 description 1
- 108010064397 amyloid beta-protein (1-40) Proteins 0.000 description 1
- 108010064539 amyloid beta-protein (1-42) Proteins 0.000 description 1
- 230000003943 amyloidogenic processing Effects 0.000 description 1
- 210000003484 anatomy Anatomy 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 230000000954 anitussive effect Effects 0.000 description 1
- 230000003042 antagnostic effect Effects 0.000 description 1
- 230000003302 anti-idiotype Effects 0.000 description 1
- 230000000692 anti-sense effect Effects 0.000 description 1
- 230000000890 antigenic effect Effects 0.000 description 1
- 239000003434 antitussive agent Substances 0.000 description 1
- 229940124584 antitussives Drugs 0.000 description 1
- 101150089041 aph-1 gene Proteins 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 210000001742 aqueous humor Anatomy 0.000 description 1
- 239000008135 aqueous vehicle Substances 0.000 description 1
- 108010068380 arginylarginine Proteins 0.000 description 1
- 230000010516 arginylation Effects 0.000 description 1
- 208000037875 astrocytosis Diseases 0.000 description 1
- 230000037444 atrophy Effects 0.000 description 1
- 230000002238 attenuated effect Effects 0.000 description 1
- 125000005605 benzo group Chemical group 0.000 description 1
- 230000003851 biochemical process Effects 0.000 description 1
- OWMVSZAMULFTJU-UHFFFAOYSA-N bis-tris Chemical compound OCCN(CCO)C(CO)(CO)CO OWMVSZAMULFTJU-UHFFFAOYSA-N 0.000 description 1
- 230000008499 blood brain barrier function Effects 0.000 description 1
- 210000001218 blood-brain barrier Anatomy 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 238000006664 bond formation reaction Methods 0.000 description 1
- 230000003925 brain function Effects 0.000 description 1
- 229940077737 brain-derived neurotrophic factor Drugs 0.000 description 1
- 238000006473 carboxylation reaction Methods 0.000 description 1
- 230000001364 causal effect Effects 0.000 description 1
- 239000003518 caustics Substances 0.000 description 1
- 210000005056 cell body Anatomy 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 238000001311 chemical methods and process Methods 0.000 description 1
- 238000007385 chemical modification Methods 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 238000002038 chemiluminescence detection Methods 0.000 description 1
- 125000002668 chloroacetyl group Chemical group ClCC(=O)* 0.000 description 1
- 230000001713 cholinergic effect Effects 0.000 description 1
- 229940048961 cholinesterase Drugs 0.000 description 1
- 230000012085 chronic inflammatory response Effects 0.000 description 1
- 229960002173 citrulline Drugs 0.000 description 1
- 235000013477 citrulline Nutrition 0.000 description 1
- 230000009194 climbing Effects 0.000 description 1
- 229960005228 clioquinol Drugs 0.000 description 1
- 238000011260 co-administration Methods 0.000 description 1
- 208000010877 cognitive disease Diseases 0.000 description 1
- 230000001149 cognitive effect Effects 0.000 description 1
- 230000003931 cognitive performance Effects 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 150000001945 cysteines Chemical class 0.000 description 1
- 229960003067 cystine Drugs 0.000 description 1
- 210000000172 cytosol Anatomy 0.000 description 1
- 230000002354 daily effect Effects 0.000 description 1
- 238000007405 data analysis Methods 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- YSMODUONRAFBET-UHFFFAOYSA-N delta-DL-hydroxylysine Natural products NCC(O)CCC(N)C(O)=O YSMODUONRAFBET-UHFFFAOYSA-N 0.000 description 1
- 230000017858 demethylation Effects 0.000 description 1
- 238000010520 demethylation reaction Methods 0.000 description 1
- 238000004925 denaturation Methods 0.000 description 1
- 230000036425 denaturation Effects 0.000 description 1
- 229960003964 deoxycholic acid Drugs 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 230000003831 deregulation Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 239000012470 diluted sample Substances 0.000 description 1
- 239000000539 dimer Substances 0.000 description 1
- 125000002147 dimethylamino group Chemical group [H]C([H])([H])N(*)C([H])([H])[H] 0.000 description 1
- 231100000676 disease causative agent Toxicity 0.000 description 1
- 206010013395 disorientation Diseases 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- PMMYEEVYMWASQN-UHFFFAOYSA-N dl-hydroxyproline Natural products OC1C[NH2+]C(C([O-])=O)C1 PMMYEEVYMWASQN-UHFFFAOYSA-N 0.000 description 1
- 229960003530 donepezil Drugs 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 230000008482 dysregulation Effects 0.000 description 1
- 238000013399 early diagnosis Methods 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 229950005455 eliprodil Drugs 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 229940088598 enzyme Drugs 0.000 description 1
- 210000002919 epithelial cell Anatomy 0.000 description 1
- YSMODUONRAFBET-UHNVWZDZSA-N erythro-5-hydroxy-L-lysine Chemical compound NC[C@H](O)CC[C@H](N)C(O)=O YSMODUONRAFBET-UHNVWZDZSA-N 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 1
- 125000004494 ethyl ester group Chemical group 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- DEFVIWRASFVYLL-UHFFFAOYSA-N ethylene glycol bis(2-aminoethyl)tetraacetic acid Chemical compound OC(=O)CN(CC(O)=O)CCOCCOCCN(CC(O)=O)CC(O)=O DEFVIWRASFVYLL-UHFFFAOYSA-N 0.000 description 1
- 230000003203 everyday effect Effects 0.000 description 1
- 210000002744 extracellular matrix Anatomy 0.000 description 1
- 239000000284 extract Substances 0.000 description 1
- 238000003804 extraction from natural source Methods 0.000 description 1
- 239000003889 eye drop Substances 0.000 description 1
- 229940012356 eye drops Drugs 0.000 description 1
- 210000000744 eyelid Anatomy 0.000 description 1
- 229960003472 felbamate Drugs 0.000 description 1
- WKGXYQFOCVYPAC-UHFFFAOYSA-N felbamate Chemical compound NC(=O)OCC(COC(N)=O)C1=CC=CC=C1 WKGXYQFOCVYPAC-UHFFFAOYSA-N 0.000 description 1
- ZNOLGFHPUIJIMJ-UHFFFAOYSA-N fenitrothion Chemical group COP(=S)(OC)OC1=CC=C([N+]([O-])=O)C(C)=C1 ZNOLGFHPUIJIMJ-UHFFFAOYSA-N 0.000 description 1
- 210000003754 fetus Anatomy 0.000 description 1
- 150000002211 flavins Chemical class 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 125000002485 formyl group Chemical group [H]C(*)=O 0.000 description 1
- 230000022244 formylation Effects 0.000 description 1
- 238000006170 formylation reaction Methods 0.000 description 1
- 230000008014 freezing Effects 0.000 description 1
- 238000007710 freezing Methods 0.000 description 1
- 229960003980 galantamine Drugs 0.000 description 1
- ASUTZQLVASHGKV-UHFFFAOYSA-N galanthamine hydrochloride Natural products O1C(=C23)C(OC)=CC=C2CN(C)CCC23C1CC(O)C=C2 ASUTZQLVASHGKV-UHFFFAOYSA-N 0.000 description 1
- 229960003692 gamma aminobutyric acid Drugs 0.000 description 1
- 230000006251 gamma-carboxylation Effects 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 230000035784 germination Effects 0.000 description 1
- 125000000291 glutamic acid group Chemical group N[C@@H](CCC(O)=O)C(=O)* 0.000 description 1
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- 230000026781 habituation Effects 0.000 description 1
- 150000003278 haem Chemical group 0.000 description 1
- 230000035876 healing Effects 0.000 description 1
- 230000006801 homologous recombination Effects 0.000 description 1
- 238000002744 homologous recombination Methods 0.000 description 1
- 108091008039 hormone receptors Proteins 0.000 description 1
- 102000057063 human MAPT Human genes 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- ZRJBHWIHUMBLCN-YQEJDHNASA-N huperzine A Chemical compound N1C(=O)C=CC2=C1C[C@H]1\C(=C/C)[C@]2(N)CC(C)=C1 ZRJBHWIHUMBLCN-YQEJDHNASA-N 0.000 description 1
- 238000009396 hybridization Methods 0.000 description 1
- 229940042795 hydrazides for tuberculosis treatment Drugs 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 230000033444 hydroxylation Effects 0.000 description 1
- 238000005805 hydroxylation reaction Methods 0.000 description 1
- 239000002471 hydroxymethylglutaryl coenzyme A reductase inhibitor Substances 0.000 description 1
- 229960002591 hydroxyproline Drugs 0.000 description 1
- 230000000774 hypoallergenic effect Effects 0.000 description 1
- 229950010480 icopezil Drugs 0.000 description 1
- 230000007813 immunodeficiency Effects 0.000 description 1
- 238000003364 immunohistochemistry Methods 0.000 description 1
- 238000012308 immunohistochemistry method Methods 0.000 description 1
- 238000012744 immunostaining Methods 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 230000028709 inflammatory response Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 230000000968 intestinal effect Effects 0.000 description 1
- 230000010189 intracellular transport Effects 0.000 description 1
- 238000007917 intracranial administration Methods 0.000 description 1
- 230000008863 intramolecular interaction Effects 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 238000007914 intraventricular administration Methods 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 230000026045 iodination Effects 0.000 description 1
- 238000006192 iodination reaction Methods 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 239000002563 ionic surfactant Substances 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- RPCVIAXDAUMJJP-PZBABLGHSA-N ispronicline Chemical compound CN[C@@H](C)C\C=C\C1=CN=CC(OC(C)C)=C1 RPCVIAXDAUMJJP-PZBABLGHSA-N 0.000 description 1
- 229950001646 ispronicline Drugs 0.000 description 1
- 229960003299 ketamine Drugs 0.000 description 1
- 210000003140 lateral ventricle Anatomy 0.000 description 1
- 125000001909 leucine group Chemical group [H]N(*)C(C(*)=O)C([H])([H])C(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- GFIJNRVAKGFPGQ-LIJARHBVSA-N leuprolide Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 GFIJNRVAKGFPGQ-LIJARHBVSA-N 0.000 description 1
- 229960004338 leuprorelin Drugs 0.000 description 1
- 239000006193 liquid solution Substances 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 231100000053 low toxicity Toxicity 0.000 description 1
- 238000009593 lumbar puncture Methods 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 238000013507 mapping Methods 0.000 description 1
- BUGYDGFZZOZRHP-UHFFFAOYSA-N memantine Chemical compound C1C(C2)CC3(C)CC1(C)CC2(N)C3 BUGYDGFZZOZRHP-UHFFFAOYSA-N 0.000 description 1
- 229960004640 memantine Drugs 0.000 description 1
- 230000003340 mental effect Effects 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 230000011987 methylation Effects 0.000 description 1
- 238000007069 methylation reaction Methods 0.000 description 1
- 229960001952 metrifonate Drugs 0.000 description 1
- 210000004688 microtubule Anatomy 0.000 description 1
- 102000021160 microtubule binding proteins Human genes 0.000 description 1
- 108091011150 microtubule binding proteins Proteins 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000001823 molecular biology technique Methods 0.000 description 1
- 230000004001 molecular interaction Effects 0.000 description 1
- 230000009456 molecular mechanism Effects 0.000 description 1
- 230000008450 motivation Effects 0.000 description 1
- 210000002161 motor neuron Anatomy 0.000 description 1
- 238000002703 mutagenesis Methods 0.000 description 1
- 231100000350 mutagenesis Toxicity 0.000 description 1
- 230000007498 myristoylation Effects 0.000 description 1
- YCRCWHFFTTVANS-UHFFFAOYSA-N n-(4-acetylpiperazin-1-yl)-4-fluorobenzamide;hydrate Chemical compound O.C1CN(C(=O)C)CCN1NC(=O)C1=CC=C(F)C=C1 YCRCWHFFTTVANS-UHFFFAOYSA-N 0.000 description 1
- SQAZQLMBEHYFJA-BTJKTKAUSA-N n-(benzo[b][1]benzoxepin-5-ylmethyl)-n-methylprop-2-yn-1-amine;(z)-but-2-enedioic acid Chemical compound OC(=O)\C=C/C(O)=O.C#CCN(C)CC1=CC2=CC=CC=C2OC2=CC=CC=C12 SQAZQLMBEHYFJA-BTJKTKAUSA-N 0.000 description 1
- 210000004898 n-terminal fragment Anatomy 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 229960002362 neostigmine Drugs 0.000 description 1
- LULNWZDBKTWDGK-UHFFFAOYSA-M neostigmine bromide Chemical compound [Br-].CN(C)C(=O)OC1=CC=CC([N+](C)(C)C)=C1 LULNWZDBKTWDGK-UHFFFAOYSA-M 0.000 description 1
- 229950004543 neramexane Drugs 0.000 description 1
- 229940053128 nerve growth factor Drugs 0.000 description 1
- 210000005155 neural progenitor cell Anatomy 0.000 description 1
- 230000002314 neuroinflammatory effect Effects 0.000 description 1
- 230000007514 neuronal growth Effects 0.000 description 1
- 230000003961 neuronal insult Effects 0.000 description 1
- 230000007991 neuronal integrity Effects 0.000 description 1
- 230000002981 neuropathic effect Effects 0.000 description 1
- 230000007171 neuropathology Effects 0.000 description 1
- 201000001119 neuropathy Diseases 0.000 description 1
- 230000007823 neuropathy Effects 0.000 description 1
- 231100000189 neurotoxic Toxicity 0.000 description 1
- 230000002887 neurotoxic effect Effects 0.000 description 1
- 239000002581 neurotoxin Substances 0.000 description 1
- 231100000618 neurotoxin Toxicity 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- 239000002736 nonionic surfactant Substances 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 230000030648 nucleus localization Effects 0.000 description 1
- 210000000956 olfactory bulb Anatomy 0.000 description 1
- 210000001706 olfactory mucosa Anatomy 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 229920002866 paraformaldehyde Polymers 0.000 description 1
- YZPOQCQXOSEMAZ-UHFFFAOYSA-N pbt2 Chemical compound ClC1=CC(Cl)=C(O)C2=NC(CN(C)C)=CC=C21 YZPOQCQXOSEMAZ-UHFFFAOYSA-N 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 230000006919 peptide aggregation Effects 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 238000010647 peptide synthesis reaction Methods 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 208000033808 peripheral neuropathy Diseases 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 230000009038 pharmacological inhibition Effects 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 150000003905 phosphatidylinositols Chemical class 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 125000004482 piperidin-4-yl group Chemical group N1CCC(CC1)* 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 230000004481 post-translational protein modification Effects 0.000 description 1
- 244000144977 poultry Species 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 230000013823 prenylation Effects 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 239000001294 propane Substances 0.000 description 1
- 235000019833 protease Nutrition 0.000 description 1
- 238000000159 protein binding assay Methods 0.000 description 1
- 238000000730 protein immunoprecipitation Methods 0.000 description 1
- 238000001742 protein purification Methods 0.000 description 1
- 230000017854 proteolysis Effects 0.000 description 1
- 238000010379 pull-down assay Methods 0.000 description 1
- 239000013014 purified material Substances 0.000 description 1
- 229940043131 pyroglutamate Drugs 0.000 description 1
- 238000004445 quantitative analysis Methods 0.000 description 1
- ZRJBHWIHUMBLCN-BMIGLBTASA-N rac-huperzine A Natural products N1C(=O)C=CC2=C1C[C@@H]1C(=CC)[C@@]2(N)CC(C)=C1 ZRJBHWIHUMBLCN-BMIGLBTASA-N 0.000 description 1
- 230000006340 racemization Effects 0.000 description 1
- 230000007342 reactive astrogliosis Effects 0.000 description 1
- 229940044551 receptor antagonist Drugs 0.000 description 1
- 239000002464 receptor antagonist Substances 0.000 description 1
- 102000027426 receptor tyrosine kinases Human genes 0.000 description 1
- 108091008598 receptor tyrosine kinases Proteins 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 230000000284 resting effect Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 230000002207 retinal effect Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 229960004181 riluzole Drugs 0.000 description 1
- 229960000888 rimantadine Drugs 0.000 description 1
- 229960004136 rivastigmine Drugs 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 229940043230 sarcosine Drugs 0.000 description 1
- 210000004116 schwann cell Anatomy 0.000 description 1
- 210000001044 sensory neuron Anatomy 0.000 description 1
- 238000013207 serial dilution Methods 0.000 description 1
- 238000001542 size-exclusion chromatography Methods 0.000 description 1
- 235000020183 skimmed milk Nutrition 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- FHHPUSMSKHSNKW-SMOYURAASA-M sodium deoxycholate Chemical compound [Na+].C([C@H]1CC2)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC([O-])=O)C)[C@@]2(C)[C@@H](O)C1 FHHPUSMSKHSNKW-SMOYURAASA-M 0.000 description 1
- 238000010532 solid phase synthesis reaction Methods 0.000 description 1
- 230000000392 somatic effect Effects 0.000 description 1
- 210000004092 somatosensory cortex Anatomy 0.000 description 1
- 230000009870 specific binding Effects 0.000 description 1
- 229940063673 spermidine Drugs 0.000 description 1
- 229940063675 spermine Drugs 0.000 description 1
- 208000020431 spinal cord injury Diseases 0.000 description 1
- 230000007480 spreading Effects 0.000 description 1
- 238000003892 spreading Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 230000036262 stenosis Effects 0.000 description 1
- 208000037804 stenosis Diseases 0.000 description 1
- 210000001562 sternum Anatomy 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 230000019635 sulfation Effects 0.000 description 1
- 238000005670 sulfation reaction Methods 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 229960001685 tacrine Drugs 0.000 description 1
- YLJREFDVOIBQDA-UHFFFAOYSA-N tacrine Chemical compound C1=CC=C2C(N)=C(CCCC3)C3=NC2=C1 YLJREFDVOIBQDA-UHFFFAOYSA-N 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 238000010257 thawing Methods 0.000 description 1
- 230000004797 therapeutic response Effects 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 231100000167 toxic agent Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 229960003570 tramiprosate Drugs 0.000 description 1
- 230000002103 transcriptional effect Effects 0.000 description 1
- 230000002463 transducing effect Effects 0.000 description 1
- 238000010361 transduction Methods 0.000 description 1
- 230000026683 transduction Effects 0.000 description 1
- 238000003151 transfection method Methods 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 230000010474 transient expression Effects 0.000 description 1
- 102000035160 transmembrane proteins Human genes 0.000 description 1
- 108091005703 transmembrane proteins Proteins 0.000 description 1
- 238000011269 treatment regimen Methods 0.000 description 1
- NFACJZMKEDPNKN-UHFFFAOYSA-N trichlorfon Chemical compound COP(=O)(OC)C(O)C(Cl)(Cl)Cl NFACJZMKEDPNKN-UHFFFAOYSA-N 0.000 description 1
- 235000002374 tyrosine Nutrition 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 238000010798 ubiquitination Methods 0.000 description 1
- 230000034512 ubiquitination Effects 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 210000002845 virion Anatomy 0.000 description 1
- 230000002747 voluntary effect Effects 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- 238000002424 x-ray crystallography Methods 0.000 description 1
- 229960004664 xaliproden Drugs 0.000 description 1
- WJJYZXPHLSLMGE-UHFFFAOYSA-N xaliproden Chemical compound FC(F)(F)C1=CC=CC(C=2CCN(CCC=3C=C4C=CC=CC4=CC=3)CC=2)=C1 WJJYZXPHLSLMGE-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/1703—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- A61K38/1709—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
- C07K14/4701—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals not used
- C07K14/4711—Alzheimer's disease; Amyloid plaque core protein
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/1703—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- A61K38/1709—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
- A61K38/1716—Amyloid plaque core protein
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/08—Peptides having 5 to 11 amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/10—Peptides having 12 to 20 amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/162—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from virus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/62—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being a protein, peptide or polyamino acid
- A61K47/64—Drug-peptide, drug-protein or drug-polyamino acid conjugates, i.e. the modifying agent being a peptide, protein or polyamino acid which is covalently bonded or complexed to a therapeutically active agent
- A61K47/645—Polycationic or polyanionic oligopeptides, polypeptides or polyamino acids, e.g. polylysine, polyarginine, polyglutamic acid or peptide TAT
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K7/00—Peptides having 5 to 20 amino acids in a fully defined sequence; Derivatives thereof
- C07K7/04—Linear peptides containing only normal peptide links
- C07K7/06—Linear peptides containing only normal peptide links having 5 to 11 amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/46—Hydrolases (3)
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Organic Chemistry (AREA)
- Epidemiology (AREA)
- Gastroenterology & Hepatology (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Immunology (AREA)
- Neurosurgery (AREA)
- Molecular Biology (AREA)
- Zoology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Psychiatry (AREA)
- Hospice & Palliative Care (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Marine Sciences & Fisheries (AREA)
- Toxicology (AREA)
- Virology (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762515272P | 2017-06-05 | 2017-06-05 | |
| US62/515,272 | 2017-06-05 | ||
| PCT/US2018/036116 WO2018226735A1 (en) | 2017-06-05 | 2018-06-05 | Compositions and methods for treating alzheimer's disease |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20200045446A true KR20200045446A (ko) | 2020-05-04 |
Family
ID=64567326
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197037319A Ceased KR20200045446A (ko) | 2017-06-05 | 2018-06-05 | 알츠하이머병의 치료를 위한 조성물 및 방법 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US20200093890A1 (enExample) |
| EP (1) | EP3634979A4 (enExample) |
| JP (1) | JP2020524132A (enExample) |
| KR (1) | KR20200045446A (enExample) |
| CN (1) | CN110945011A (enExample) |
| AU (1) | AU2018281330A1 (enExample) |
| CA (1) | CA3064479A1 (enExample) |
| WO (1) | WO2018226735A1 (enExample) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN120957751A (zh) * | 2023-04-03 | 2025-11-14 | 神经再生制药公司 | 用于神经系统修复的蛋白酪氨酸磷酸酶楔形结构域肽二聚体 |
| WO2025092748A1 (zh) * | 2023-11-01 | 2025-05-08 | 拜西欧斯(北京)生物技术有限公司 | 多肽衍生物、其药物组合物及其用途 |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002083182A2 (en) * | 2001-04-12 | 2002-10-24 | Mcgill University | The effect of receptor protein tyrosine phosphatase sigma (rptp-$g(s)) on neural axon regeneration and synapse modification |
| US7829087B2 (en) * | 2001-07-09 | 2010-11-09 | Elan Pharmaceuticals, Inc. | Methods of treating cognitive impairment |
| WO2008157425A2 (en) * | 2007-06-14 | 2008-12-24 | The Regents Of The University Of California | Compounds for inhibiting protein aggregation, and methods for making and using them |
| CA2764545C (en) * | 2009-06-15 | 2019-05-14 | Katholieke Universiteit Leuven, K.U.Leuven R&D | Bace1 inhibitory antibodies |
| US20130171163A1 (en) * | 2010-08-06 | 2013-07-04 | Marschall Runge | Inhibition of LAR Phosphatase to Enhance Therapeutic Angiogenesis |
| KR102160388B1 (ko) * | 2012-03-19 | 2020-09-28 | 버크 인스티튜트 포 리서치 온 에이징 | App 특이적 bace 억제제(asbi) 및 이의 용도 |
| EP2841086B1 (en) * | 2012-04-09 | 2018-09-26 | Case Western Reserve University | Compositions for use in treating neural injury by inhibiting the activity of lar family phosphatases |
| WO2017197253A2 (en) * | 2016-05-12 | 2017-11-16 | Ohio State Innovation Foundation | Peptides and methods for treating neurodegenerative disorders |
-
2018
- 2018-06-05 CA CA3064479A patent/CA3064479A1/en active Pending
- 2018-06-05 AU AU2018281330A patent/AU2018281330A1/en not_active Abandoned
- 2018-06-05 WO PCT/US2018/036116 patent/WO2018226735A1/en not_active Ceased
- 2018-06-05 EP EP18813399.5A patent/EP3634979A4/en not_active Withdrawn
- 2018-06-05 CN CN201880036972.2A patent/CN110945011A/zh active Pending
- 2018-06-05 JP JP2019565526A patent/JP2020524132A/ja active Pending
- 2018-06-05 US US16/619,317 patent/US20200093890A1/en active Pending
- 2018-06-05 KR KR1020197037319A patent/KR20200045446A/ko not_active Ceased
Also Published As
| Publication number | Publication date |
|---|---|
| EP3634979A4 (en) | 2021-04-14 |
| US20200093890A1 (en) | 2020-03-26 |
| CN110945011A (zh) | 2020-03-31 |
| EP3634979A1 (en) | 2020-04-15 |
| JP2020524132A (ja) | 2020-08-13 |
| WO2018226735A1 (en) | 2018-12-13 |
| AU2018281330A1 (en) | 2019-12-19 |
| CA3064479A1 (en) | 2018-12-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6920324B2 (ja) | 神経細胞の損失予防及び再生の効能を有するペプチド及びこれを含む組成物 | |
| JP2018521014A (ja) | ミスフォールドタンパク質の分解のための組成物及び方法 | |
| US11124553B2 (en) | TDP-43 mitochondrial localization inhibitor for the treatment of neurogenerative disease | |
| US20220104468A1 (en) | Modulation of lc3-associated endocytosis pathway and genetically modified non-human animals as a model of neuroinflammation and neurodegeneration | |
| JP2018525337A (ja) | 軽度認知障害の処置のためのjnkシグナル伝達経路の細胞透過性ペプチド阻害剤の新規使用 | |
| US20180170983A1 (en) | New Use of Cell-Permeable Peptide Inhibitors of the JNK Signal Transduction Pathway for the Treatment of Mild Cognitive Impairment | |
| US10898550B2 (en) | Compositions and methods of treating root avulsion injury | |
| KR20200045446A (ko) | 알츠하이머병의 치료를 위한 조성물 및 방법 | |
| WO2012144972A2 (en) | Methods to inhibit neurodegeneration | |
| Jakobsen et al. | Functional Roles of Pigment Epithelium-Derived Factor in Retinal Degenerative and Vascular Disorders: A Scoping Review | |
| US7790673B2 (en) | Methods and compositions relating to cystatin C | |
| KR102772298B1 (ko) | 미엘린 장애의 치료를 위한 조성물 및 방법 | |
| US20140314790A1 (en) | Caspase 9 inhibition and bri2 peptides for treating dementia | |
| JPWO2007139120A1 (ja) | アミロイドβクリアランス促進剤 | |
| KR101131512B1 (ko) | 퇴행성신경질환 예방 또는 치료용 약제학적 조성물 | |
| EP4471054A2 (en) | C-terminal fragment of apolipoprotein e and application thereof in inhibiting ?-secretase activity | |
| JP5939528B2 (ja) | カルモジュリン様皮膚タンパク質を有効成分として含む医薬組成物 | |
| KR20130003243A (ko) | 세포침투 효율이 향상된 pras40 융합단백질 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20191217 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20210601 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20230728 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20240103 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20230728 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |