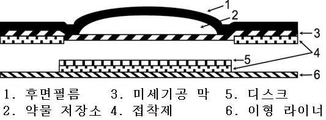
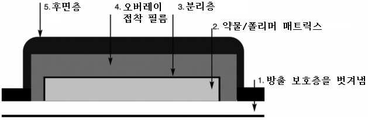
KR20190109399A - 트레프로스티닐 및 이의 염의 피부 및 경피 투여 - Google Patents
트레프로스티닐 및 이의 염의 피부 및 경피 투여 Download PDFInfo
- Publication number
- KR20190109399A KR20190109399A KR1020197018666A KR20197018666A KR20190109399A KR 20190109399 A KR20190109399 A KR 20190109399A KR 1020197018666 A KR1020197018666 A KR 1020197018666A KR 20197018666 A KR20197018666 A KR 20197018666A KR 20190109399 A KR20190109399 A KR 20190109399A
- Authority
- KR
- South Korea
- Prior art keywords
- treprostinil
- drug
- skin
- tds
- patch
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- PAJMKGZZBBTTOY-ZFORQUDYSA-N treprostinil Chemical compound C1=CC=C(OCC(O)=O)C2=C1C[C@@H]1[C@@H](CC[C@@H](O)CCCCC)[C@H](O)C[C@@H]1C2 PAJMKGZZBBTTOY-ZFORQUDYSA-N 0.000 title claims abstract description 205
- 229960005032 treprostinil Drugs 0.000 title claims abstract description 205
- 150000003839 salts Chemical class 0.000 title claims abstract description 143
- 230000002500 effect on skin Effects 0.000 title abstract description 6
- 239000003814 drug Substances 0.000 claims abstract description 356
- 239000000203 mixture Substances 0.000 claims abstract description 106
- 238000000034 method Methods 0.000 claims abstract description 95
- 229940124597 therapeutic agent Drugs 0.000 claims abstract description 76
- 208000002815 pulmonary hypertension Diseases 0.000 claims abstract description 39
- 229940079593 drug Drugs 0.000 claims description 262
- 239000010410 layer Substances 0.000 claims description 118
- 229920000642 polymer Polymers 0.000 claims description 109
- 239000011159 matrix material Substances 0.000 claims description 80
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 79
- -1 fatty acid esters Chemical class 0.000 claims description 77
- 239000012790 adhesive layer Substances 0.000 claims description 62
- 239000000126 substance Substances 0.000 claims description 42
- 150000001875 compounds Chemical class 0.000 claims description 40
- 239000004821 Contact adhesive Substances 0.000 claims description 38
- 230000000699 topical effect Effects 0.000 claims description 36
- 239000012528 membrane Substances 0.000 claims description 30
- 239000003623 enhancer Substances 0.000 claims description 29
- 239000000499 gel Substances 0.000 claims description 27
- 239000007788 liquid Substances 0.000 claims description 21
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 20
- 231100000245 skin permeability Toxicity 0.000 claims description 20
- 238000002604 ultrasonography Methods 0.000 claims description 20
- HIQIXEFWDLTDED-UHFFFAOYSA-N 4-hydroxy-1-piperidin-4-ylpyrrolidin-2-one Chemical compound O=C1CC(O)CN1C1CCNCC1 HIQIXEFWDLTDED-UHFFFAOYSA-N 0.000 claims description 18
- 239000004094 surface-active agent Substances 0.000 claims description 17
- 230000035515 penetration Effects 0.000 claims description 16
- 235000014113 dietary fatty acids Nutrition 0.000 claims description 15
- 239000000194 fatty acid Substances 0.000 claims description 15
- 229930195729 fatty acid Natural products 0.000 claims description 15
- 239000003921 oil Substances 0.000 claims description 15
- 239000007787 solid Substances 0.000 claims description 15
- 230000009885 systemic effect Effects 0.000 claims description 15
- 238000013271 transdermal drug delivery Methods 0.000 claims description 15
- 239000012453 solvate Substances 0.000 claims description 14
- 239000002260 anti-inflammatory agent Substances 0.000 claims description 13
- 229940121363 anti-inflammatory agent Drugs 0.000 claims description 13
- 239000003589 local anesthetic agent Substances 0.000 claims description 13
- 239000003795 chemical substances by application Substances 0.000 claims description 12
- 239000002356 single layer Substances 0.000 claims description 12
- 150000001298 alcohols Chemical class 0.000 claims description 11
- 229940035676 analgesics Drugs 0.000 claims description 11
- 239000000730 antalgic agent Substances 0.000 claims description 11
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 11
- 229960005015 local anesthetics Drugs 0.000 claims description 11
- 238000004520 electroporation Methods 0.000 claims description 10
- 239000005526 vasoconstrictor agent Substances 0.000 claims description 10
- 201000001320 Atherosclerosis Diseases 0.000 claims description 9
- 210000004369 blood Anatomy 0.000 claims description 9
- 239000008280 blood Substances 0.000 claims description 9
- 208000002193 Pain Diseases 0.000 claims description 8
- 238000002679 ablation Methods 0.000 claims description 8
- 239000006071 cream Substances 0.000 claims description 8
- 238000009826 distribution Methods 0.000 claims description 8
- 239000002674 ointment Substances 0.000 claims description 8
- 230000036407 pain Effects 0.000 claims description 8
- 239000006072 paste Substances 0.000 claims description 8
- 229940057950 sodium laureth sulfate Drugs 0.000 claims description 8
- SXHLENDCVBIJFO-UHFFFAOYSA-M sodium;2-[2-(2-dodecoxyethoxy)ethoxy]ethyl sulfate Chemical compound [Na+].CCCCCCCCCCCCOCCOCCOCCOS([O-])(=O)=O SXHLENDCVBIJFO-UHFFFAOYSA-M 0.000 claims description 8
- 150000001491 aromatic compounds Chemical class 0.000 claims description 7
- 201000010099 disease Diseases 0.000 claims description 7
- 239000006210 lotion Substances 0.000 claims description 7
- 239000011859 microparticle Substances 0.000 claims description 7
- YZTJYBJCZXZGCT-UHFFFAOYSA-N phenylpiperazine Chemical compound C1CNCCN1C1=CC=CC=C1 YZTJYBJCZXZGCT-UHFFFAOYSA-N 0.000 claims description 7
- 239000007921 spray Substances 0.000 claims description 7
- 230000002792 vascular Effects 0.000 claims description 7
- 206010019280 Heart failures Diseases 0.000 claims description 6
- 206010064911 Pulmonary arterial hypertension Diseases 0.000 claims description 6
- 229910052783 alkali metal Inorganic materials 0.000 claims description 6
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 claims description 6
- 239000006260 foam Substances 0.000 claims description 6
- 235000015110 jellies Nutrition 0.000 claims description 6
- 238000004519 manufacturing process Methods 0.000 claims description 6
- 230000037317 transdermal delivery Effects 0.000 claims description 6
- 206010007559 Cardiac failure congestive Diseases 0.000 claims description 5
- 208000003790 Foot Ulcer Diseases 0.000 claims description 5
- 208000029523 Interstitial Lung disease Diseases 0.000 claims description 5
- 208000035901 Ischaemic ulcer Diseases 0.000 claims description 5
- 206010040943 Skin Ulcer Diseases 0.000 claims description 5
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 claims description 5
- 239000003146 anticoagulant agent Substances 0.000 claims description 5
- 229940127219 anticoagulant drug Drugs 0.000 claims description 5
- 229940097217 cardiac glycoside Drugs 0.000 claims description 5
- 239000002368 cardiac glycoside Substances 0.000 claims description 5
- 239000002934 diuretic Substances 0.000 claims description 5
- 229940030606 diuretics Drugs 0.000 claims description 5
- 230000002981 neuropathic effect Effects 0.000 claims description 5
- 229930002534 steroid glycoside Natural products 0.000 claims description 5
- 150000008143 steroidal glycosides Chemical class 0.000 claims description 5
- IQKAWAUTOKVMLE-ZSESPEEFSA-M treprostinil sodium Chemical compound [Na+].C1=CC=C(OCC([O-])=O)C2=C1C[C@@H]1[C@@H](CC[C@@H](O)CCCCC)[C@H](O)C[C@@H]1C2 IQKAWAUTOKVMLE-ZSESPEEFSA-M 0.000 claims description 5
- 229960001726 treprostinil sodium Drugs 0.000 claims description 5
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 claims description 5
- UXFQFBNBSPQBJW-UHFFFAOYSA-N 2-amino-2-methylpropane-1,3-diol Chemical compound OCC(N)(C)CO UXFQFBNBSPQBJW-UHFFFAOYSA-N 0.000 claims description 4
- 208000035475 disorder Diseases 0.000 claims description 4
- 239000003937 drug carrier Substances 0.000 claims description 4
- 230000007654 ischemic lesion Effects 0.000 claims description 4
- 210000003734 kidney Anatomy 0.000 claims description 4
- 230000002062 proliferating effect Effects 0.000 claims description 4
- 208000005069 pulmonary fibrosis Diseases 0.000 claims description 4
- 208000032064 Chronic Limb-Threatening Ischemia Diseases 0.000 claims description 3
- 206010062016 Immunosuppression Diseases 0.000 claims description 3
- 206010022562 Intermittent claudication Diseases 0.000 claims description 3
- 206010034576 Peripheral ischaemia Diseases 0.000 claims description 3
- 208000018262 Peripheral vascular disease Diseases 0.000 claims description 3
- 208000006673 asthma Diseases 0.000 claims description 3
- 230000001506 immunosuppresive effect Effects 0.000 claims description 3
- 208000021156 intermittent vascular claudication Diseases 0.000 claims description 3
- 230000007257 malfunction Effects 0.000 claims description 3
- 231100000019 skin ulcer Toxicity 0.000 claims description 3
- 230000003902 lesion Effects 0.000 claims description 2
- 239000000865 liniment Substances 0.000 claims description 2
- 239000002861 polymer material Substances 0.000 claims description 2
- 150000001735 carboxylic acids Chemical group 0.000 claims 1
- 230000010354 integration Effects 0.000 claims 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 claims 1
- 210000003491 skin Anatomy 0.000 description 143
- 235000019441 ethanol Nutrition 0.000 description 42
- 239000000853 adhesive Substances 0.000 description 37
- 230000001070 adhesive effect Effects 0.000 description 32
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 27
- 239000002904 solvent Substances 0.000 description 26
- 239000003961 penetration enhancing agent Substances 0.000 description 24
- 210000000434 stratum corneum Anatomy 0.000 description 24
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 description 23
- 229910052805 deuterium Inorganic materials 0.000 description 23
- 238000009792 diffusion process Methods 0.000 description 22
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 20
- 239000000463 material Substances 0.000 description 18
- 210000002615 epidermis Anatomy 0.000 description 16
- 244000309715 mini pig Species 0.000 description 16
- 229920000058 polyacrylate Polymers 0.000 description 16
- 229920001223 polyethylene glycol Polymers 0.000 description 16
- 239000000739 antihistaminic agent Substances 0.000 description 15
- 238000009472 formulation Methods 0.000 description 15
- 239000004606 Fillers/Extenders Substances 0.000 description 14
- 239000002585 base Substances 0.000 description 14
- 238000010521 absorption reaction Methods 0.000 description 13
- 235000019198 oils Nutrition 0.000 description 13
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 12
- 239000003246 corticosteroid Substances 0.000 description 12
- UQDUPQYQJKYHQI-UHFFFAOYSA-N methyl laurate Chemical compound CCCCCCCCCCCC(=O)OC UQDUPQYQJKYHQI-UHFFFAOYSA-N 0.000 description 12
- 231100000475 skin irritation Toxicity 0.000 description 12
- 230000036556 skin irritation Effects 0.000 description 12
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 11
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 11
- 229920002367 Polyisobutene Polymers 0.000 description 11
- 230000002093 peripheral effect Effects 0.000 description 11
- 239000002202 Polyethylene glycol Substances 0.000 description 10
- 206010040880 Skin irritation Diseases 0.000 description 10
- 229940125715 antihistaminic agent Drugs 0.000 description 10
- 230000008901 benefit Effects 0.000 description 10
- 210000004027 cell Anatomy 0.000 description 10
- 238000004090 dissolution Methods 0.000 description 10
- 230000000694 effects Effects 0.000 description 10
- 150000002148 esters Chemical class 0.000 description 10
- 239000012982 microporous membrane Substances 0.000 description 10
- 230000032258 transport Effects 0.000 description 10
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 9
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 9
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 9
- 239000005642 Oleic acid Substances 0.000 description 9
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 9
- 239000004820 Pressure-sensitive adhesive Substances 0.000 description 9
- 229920002125 Sokalan® Polymers 0.000 description 9
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 9
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 9
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 9
- 229960004063 propylene glycol Drugs 0.000 description 9
- 210000001519 tissue Anatomy 0.000 description 9
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 8
- 239000004698 Polyethylene Substances 0.000 description 8
- 229920002678 cellulose Polymers 0.000 description 8
- 238000012377 drug delivery Methods 0.000 description 8
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 8
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 8
- GLDOVTGHNKAZLK-UHFFFAOYSA-N octadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCO GLDOVTGHNKAZLK-UHFFFAOYSA-N 0.000 description 8
- 229920000573 polyethylene Polymers 0.000 description 8
- 206010015150 Erythema Diseases 0.000 description 7
- GUGOEEXESWIERI-UHFFFAOYSA-N Terfenadine Chemical compound C1=CC(C(C)(C)C)=CC=C1C(O)CCCN1CCC(C(O)(C=2C=CC=CC=2)C=2C=CC=CC=2)CC1 GUGOEEXESWIERI-UHFFFAOYSA-N 0.000 description 7
- 239000002253 acid Substances 0.000 description 7
- 239000003963 antioxidant agent Substances 0.000 description 7
- 235000006708 antioxidants Nutrition 0.000 description 7
- 230000004888 barrier function Effects 0.000 description 7
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 7
- 235000010980 cellulose Nutrition 0.000 description 7
- 229960001334 corticosteroids Drugs 0.000 description 7
- 231100000321 erythema Toxicity 0.000 description 7
- LYCAIKOWRPUZTN-UHFFFAOYSA-N ethylene glycol Natural products OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 7
- 229920001200 poly(ethylene-vinyl acetate) Polymers 0.000 description 7
- 229920000728 polyester Polymers 0.000 description 7
- 238000000634 powder X-ray diffraction Methods 0.000 description 7
- YNDXUCZADRHECN-JNQJZLCISA-N triamcinolone acetonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O YNDXUCZADRHECN-JNQJZLCISA-N 0.000 description 7
- 229960002117 triamcinolone acetonide Drugs 0.000 description 7
- AXTGDCSMTYGJND-UHFFFAOYSA-N 1-dodecylazepan-2-one Chemical compound CCCCCCCCCCCCN1CCCCCC1=O AXTGDCSMTYGJND-UHFFFAOYSA-N 0.000 description 6
- RZRNAYUHWVFMIP-KTKRTIGZSA-N 1-oleoylglycerol Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC(O)CO RZRNAYUHWVFMIP-KTKRTIGZSA-N 0.000 description 6
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 6
- NTYJJOPFIAHURM-UHFFFAOYSA-N Histamine Chemical compound NCCC1=CN=CN1 NTYJJOPFIAHURM-UHFFFAOYSA-N 0.000 description 6
- 206010030113 Oedema Diseases 0.000 description 6
- DQXBYHZEEUGOBF-UHFFFAOYSA-N but-3-enoic acid;ethene Chemical compound C=C.OC(=O)CC=C DQXBYHZEEUGOBF-UHFFFAOYSA-N 0.000 description 6
- 239000001913 cellulose Substances 0.000 description 6
- 239000011248 coating agent Substances 0.000 description 6
- 238000000576 coating method Methods 0.000 description 6
- ZPUCINDJVBIVPJ-LJISPDSOSA-N cocaine Chemical compound O([C@H]1C[C@@H]2CC[C@@H](N2C)[C@H]1C(=O)OC)C(=O)C1=CC=CC=C1 ZPUCINDJVBIVPJ-LJISPDSOSA-N 0.000 description 6
- 210000004207 dermis Anatomy 0.000 description 6
- 239000005038 ethylene vinyl acetate Substances 0.000 description 6
- 230000035699 permeability Effects 0.000 description 6
- 239000004584 polyacrylic acid Substances 0.000 description 6
- 229920001296 polysiloxane Polymers 0.000 description 6
- KAQKFAOMNZTLHT-OZUDYXHBSA-N prostaglandin I2 Chemical compound O1\C(=C/CCCC(O)=O)C[C@@H]2[C@@H](/C=C/[C@@H](O)CCCCC)[C@H](O)C[C@@H]21 KAQKFAOMNZTLHT-OZUDYXHBSA-N 0.000 description 6
- 230000001681 protective effect Effects 0.000 description 6
- 108700004121 sarkosyl Proteins 0.000 description 6
- 229940127291 Calcium channel antagonist Drugs 0.000 description 5
- 206010028980 Neoplasm Diseases 0.000 description 5
- 238000004458 analytical method Methods 0.000 description 5
- 230000001387 anti-histamine Effects 0.000 description 5
- 239000007864 aqueous solution Substances 0.000 description 5
- 239000000480 calcium channel blocker Substances 0.000 description 5
- 238000013270 controlled release Methods 0.000 description 5
- 239000004205 dimethyl polysiloxane Substances 0.000 description 5
- 235000013870 dimethyl polysiloxane Nutrition 0.000 description 5
- 239000003862 glucocorticoid Substances 0.000 description 5
- 229960003921 octisalate Drugs 0.000 description 5
- WCJLCOAEJIHPCW-UHFFFAOYSA-N octyl 2-hydroxybenzoate Chemical compound CCCCCCCCOC(=O)C1=CC=CC=C1O WCJLCOAEJIHPCW-UHFFFAOYSA-N 0.000 description 5
- 230000036470 plasma concentration Effects 0.000 description 5
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 description 5
- 229920000193 polymethacrylate Polymers 0.000 description 5
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 5
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 5
- KSAVQLQVUXSOCR-UHFFFAOYSA-M sodium lauroyl sarcosinate Chemical compound [Na+].CCCCCCCCCCCC(=O)N(C)CC([O-])=O KSAVQLQVUXSOCR-UHFFFAOYSA-M 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- 230000001225 therapeutic effect Effects 0.000 description 5
- BDSYKGHYMJNPAB-LICBFIPMSA-N ulobetasol propionate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H](C)[C@@](C(=O)CCl)(OC(=O)CC)[C@@]2(C)C[C@@H]1O BDSYKGHYMJNPAB-LICBFIPMSA-N 0.000 description 5
- ALSTYHKOOCGGFT-KTKRTIGZSA-N (9Z)-octadecen-1-ol Chemical compound CCCCCCCC\C=C/CCCCCCCCO ALSTYHKOOCGGFT-KTKRTIGZSA-N 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 4
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 4
- QWOJMRHUQHTCJG-UHFFFAOYSA-N CC([CH2-])=O Chemical compound CC([CH2-])=O QWOJMRHUQHTCJG-UHFFFAOYSA-N 0.000 description 4
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 description 4
- 239000001856 Ethyl cellulose Substances 0.000 description 4
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 4
- POPFMWWJOGLOIF-XWCQMRHXSA-N Flurandrenolide Chemical compound C1([C@@H](F)C2)=CC(=O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O POPFMWWJOGLOIF-XWCQMRHXSA-N 0.000 description 4
- 239000000232 Lipid Bilayer Substances 0.000 description 4
- YBGZDTIWKVFICR-JLHYYAGUSA-N Octyl 4-methoxycinnamic acid Chemical compound CCCCC(CC)COC(=O)\C=C\C1=CC=C(OC)C=C1 YBGZDTIWKVFICR-JLHYYAGUSA-N 0.000 description 4
- 229920002565 Polyethylene Glycol 400 Polymers 0.000 description 4
- 239000004743 Polypropylene Substances 0.000 description 4
- 208000014777 Pulmonary venoocclusive disease Diseases 0.000 description 4
- 208000012322 Raynaud phenomenon Diseases 0.000 description 4
- ZZHLYYDVIOPZBE-UHFFFAOYSA-N Trimeprazine Chemical compound C1=CC=C2N(CC(CN(C)C)C)C3=CC=CC=C3SC2=C1 ZZHLYYDVIOPZBE-UHFFFAOYSA-N 0.000 description 4
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 4
- 229960001138 acetylsalicylic acid Drugs 0.000 description 4
- 150000001412 amines Chemical class 0.000 description 4
- BLFLLBZGZJTVJG-UHFFFAOYSA-N benzocaine Chemical compound CCOC(=O)C1=CC=C(N)C=C1 BLFLLBZGZJTVJG-UHFFFAOYSA-N 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 229920001577 copolymer Polymers 0.000 description 4
- 230000003111 delayed effect Effects 0.000 description 4
- XXJWXESWEXIICW-UHFFFAOYSA-N diethylene glycol monoethyl ether Chemical compound CCOCCOCCO XXJWXESWEXIICW-UHFFFAOYSA-N 0.000 description 4
- 229940075557 diethylene glycol monoethyl ether Drugs 0.000 description 4
- 239000003085 diluting agent Substances 0.000 description 4
- 229950010160 dimethocaine Drugs 0.000 description 4
- OWQIUQKMMPDHQQ-UHFFFAOYSA-N dimethocaine Chemical compound CCN(CC)CC(C)(C)COC(=O)C1=CC=C(N)C=C1 OWQIUQKMMPDHQQ-UHFFFAOYSA-N 0.000 description 4
- 239000003995 emulsifying agent Substances 0.000 description 4
- 239000000839 emulsion Substances 0.000 description 4
- 229960001123 epoprostenol Drugs 0.000 description 4
- RRAFCDWBNXTKKO-UHFFFAOYSA-N eugenol Chemical compound COC1=CC(CC=C)=CC=C1O RRAFCDWBNXTKKO-UHFFFAOYSA-N 0.000 description 4
- 150000004665 fatty acids Chemical class 0.000 description 4
- 229960004511 fludroxycortide Drugs 0.000 description 4
- 235000011187 glycerol Nutrition 0.000 description 4
- RZRNAYUHWVFMIP-HXUWFJFHSA-N glycerol monolinoleate Natural products CCCCCCCCC=CCCCCCCCC(=O)OC[C@H](O)CO RZRNAYUHWVFMIP-HXUWFJFHSA-N 0.000 description 4
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 4
- 239000000017 hydrogel Substances 0.000 description 4
- 230000004054 inflammatory process Effects 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 230000007794 irritation Effects 0.000 description 4
- 229910052751 metal Inorganic materials 0.000 description 4
- 239000002184 metal Substances 0.000 description 4
- CSJDCSCTVDEHRN-UHFFFAOYSA-N methane;molecular oxygen Chemical compound C.O=O CSJDCSCTVDEHRN-UHFFFAOYSA-N 0.000 description 4
- 229920000609 methyl cellulose Polymers 0.000 description 4
- 235000010981 methylcellulose Nutrition 0.000 description 4
- 239000001923 methylcellulose Substances 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- GOQYKNQRPGWPLP-UHFFFAOYSA-N n-heptadecyl alcohol Natural products CCCCCCCCCCCCCCCCCO GOQYKNQRPGWPLP-UHFFFAOYSA-N 0.000 description 4
- 229940055577 oleyl alcohol Drugs 0.000 description 4
- XMLQWXUVTXCDDL-UHFFFAOYSA-N oleyl alcohol Natural products CCCCCCC=CCCCCCCCCCCO XMLQWXUVTXCDDL-UHFFFAOYSA-N 0.000 description 4
- 229940068196 placebo Drugs 0.000 description 4
- 239000000902 placebo Substances 0.000 description 4
- 229920001155 polypropylene Polymers 0.000 description 4
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 4
- 239000003755 preservative agent Substances 0.000 description 4
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 4
- HNJBEVLQSNELDL-UHFFFAOYSA-N pyrrolidin-2-one Chemical compound O=C1CCCN1 HNJBEVLQSNELDL-UHFFFAOYSA-N 0.000 description 4
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 4
- 239000011734 sodium Substances 0.000 description 4
- 229940045885 sodium lauroyl sarcosinate Drugs 0.000 description 4
- 229940075560 sodium lauryl sulfoacetate Drugs 0.000 description 4
- 229940067741 sodium octyl sulfate Drugs 0.000 description 4
- UAJTZZNRJCKXJN-UHFFFAOYSA-M sodium;2-dodecoxy-2-oxoethanesulfonate Chemical compound [Na+].CCCCCCCCCCCCOC(=O)CS([O-])(=O)=O UAJTZZNRJCKXJN-UHFFFAOYSA-M 0.000 description 4
- 239000003381 stabilizer Substances 0.000 description 4
- 239000000725 suspension Substances 0.000 description 4
- URAYPUMNDPQOKB-UHFFFAOYSA-N triacetin Chemical compound CC(=O)OCC(OC(C)=O)COC(C)=O URAYPUMNDPQOKB-UHFFFAOYSA-N 0.000 description 4
- 239000000341 volatile oil Substances 0.000 description 4
- RHWRWEUCEXUUAV-ZSESPEEFSA-N 2-[[(1r,2r,3as,9as)-2-hydroxy-1-[(3s)-3-hydroxyoctyl]-2,3,3a,4,9,9a-hexahydro-1h-cyclopenta[g]naphthalen-5-yl]oxy]acetic acid;2-(2-hydroxyethylamino)ethanol Chemical compound OCCNCCO.C1=CC=C(OCC(O)=O)C2=C1C[C@@H]1[C@@H](CC[C@@H](O)CCCCC)[C@H](O)C[C@@H]1C2 RHWRWEUCEXUUAV-ZSESPEEFSA-N 0.000 description 3
- XZIIFPSPUDAGJM-UHFFFAOYSA-N 6-chloro-2-n,2-n-diethylpyrimidine-2,4-diamine Chemical compound CCN(CC)C1=NC(N)=CC(Cl)=N1 XZIIFPSPUDAGJM-UHFFFAOYSA-N 0.000 description 3
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 3
- RZVAJINKPMORJF-UHFFFAOYSA-N Acetaminophen Chemical compound CC(=O)NC1=CC=C(O)C=C1 RZVAJINKPMORJF-UHFFFAOYSA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- 239000004322 Butylated hydroxytoluene Substances 0.000 description 3
- NLZUEZXRPGMBCV-UHFFFAOYSA-N Butylhydroxytoluene Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 NLZUEZXRPGMBCV-UHFFFAOYSA-N 0.000 description 3
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- 108010010803 Gelatin Proteins 0.000 description 3
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 3
- 206010061218 Inflammation Diseases 0.000 description 3
- OCJYIGYOJCODJL-UHFFFAOYSA-N Meclizine Chemical compound CC1=CC=CC(CN2CCN(CC2)C(C=2C=CC=CC=2)C=2C=CC(Cl)=CC=2)=C1 OCJYIGYOJCODJL-UHFFFAOYSA-N 0.000 description 3
- 241001465754 Metazoa Species 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 3
- 229920002556 Polyethylene Glycol 300 Polymers 0.000 description 3
- 229920002562 Polyethylene Glycol 3350 Polymers 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- 210000001015 abdomen Anatomy 0.000 description 3
- 150000007513 acids Chemical class 0.000 description 3
- 230000000202 analgesic effect Effects 0.000 description 3
- 230000003078 antioxidant effect Effects 0.000 description 3
- 229960002537 betamethasone Drugs 0.000 description 3
- UREBDLICKHMUKA-DVTGEIKXSA-N betamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-DVTGEIKXSA-N 0.000 description 3
- 229960001102 betamethasone dipropionate Drugs 0.000 description 3
- CIWBQSYVNNPZIQ-XYWKZLDCSA-N betamethasone dipropionate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)COC(=O)CC)(OC(=O)CC)[C@@]1(C)C[C@@H]2O CIWBQSYVNNPZIQ-XYWKZLDCSA-N 0.000 description 3
- 229960004311 betamethasone valerate Drugs 0.000 description 3
- SNHRLVCMMWUAJD-SUYDQAKGSA-N betamethasone valerate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(OC(=O)CCCC)[C@@]1(C)C[C@@H]2O SNHRLVCMMWUAJD-SUYDQAKGSA-N 0.000 description 3
- 235000010354 butylated hydroxytoluene Nutrition 0.000 description 3
- 229940095259 butylated hydroxytoluene Drugs 0.000 description 3
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 3
- 239000001768 carboxy methyl cellulose Substances 0.000 description 3
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- 150000001768 cations Chemical class 0.000 description 3
- VDANGULDQQJODZ-UHFFFAOYSA-N chloroprocaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1Cl VDANGULDQQJODZ-UHFFFAOYSA-N 0.000 description 3
- 229960002023 chloroprocaine Drugs 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- CBGUOGMQLZIXBE-XGQKBEPLSA-N clobetasol propionate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CCl)(OC(=O)CC)[C@@]1(C)C[C@@H]2O CBGUOGMQLZIXBE-XGQKBEPLSA-N 0.000 description 3
- 229960003920 cocaine Drugs 0.000 description 3
- BMCQMVFGOVHVNG-TUFAYURCSA-N cortisol 17-butyrate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)CO)(OC(=O)CCC)[C@@]1(C)C[C@@H]2O BMCQMVFGOVHVNG-TUFAYURCSA-N 0.000 description 3
- FZCHYNWYXKICIO-FZNHGJLXSA-N cortisol 17-valerate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)CO)(OC(=O)CCCC)[C@@]1(C)C[C@@H]2O FZCHYNWYXKICIO-FZNHGJLXSA-N 0.000 description 3
- 229940111134 coxibs Drugs 0.000 description 3
- 229960002593 desoximetasone Drugs 0.000 description 3
- VWVSBHGCDBMOOT-IIEHVVJPSA-N desoximetasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@H](C(=O)CO)[C@@]1(C)C[C@@H]2O VWVSBHGCDBMOOT-IIEHVVJPSA-N 0.000 description 3
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 3
- 239000002552 dosage form Substances 0.000 description 3
- ODQWQRRAPPTVAG-GZTJUZNOSA-N doxepin Chemical compound C1OC2=CC=CC=C2C(=C/CCN(C)C)/C2=CC=CC=C21 ODQWQRRAPPTVAG-GZTJUZNOSA-N 0.000 description 3
- 229920001971 elastomer Polymers 0.000 description 3
- 235000019325 ethyl cellulose Nutrition 0.000 description 3
- 229920001249 ethyl cellulose Polymers 0.000 description 3
- 239000012530 fluid Substances 0.000 description 3
- GAKMQHDJQHZUTJ-ULHLPKEOSA-N fluocortolone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@@H](C)[C@H](C(=O)CO)[C@@]2(C)C[C@@H]1O GAKMQHDJQHZUTJ-ULHLPKEOSA-N 0.000 description 3
- 210000002683 foot Anatomy 0.000 description 3
- 210000000245 forearm Anatomy 0.000 description 3
- 210000001035 gastrointestinal tract Anatomy 0.000 description 3
- 229920000159 gelatin Polymers 0.000 description 3
- 239000008273 gelatin Substances 0.000 description 3
- 235000019322 gelatine Nutrition 0.000 description 3
- 235000011852 gelatine desserts Nutrition 0.000 description 3
- 150000004676 glycans Chemical class 0.000 description 3
- 229960002475 halometasone Drugs 0.000 description 3
- GGXMRPUKBWXVHE-MIHLVHIWSA-N halometasone Chemical compound C1([C@@H](F)C2)=CC(=O)C(Cl)=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]2(C)C[C@@H]1O GGXMRPUKBWXVHE-MIHLVHIWSA-N 0.000 description 3
- 229960002897 heparin Drugs 0.000 description 3
- 229920000669 heparin Polymers 0.000 description 3
- 210000001624 hip Anatomy 0.000 description 3
- 229960001340 histamine Drugs 0.000 description 3
- 150000004677 hydrates Chemical class 0.000 description 3
- 230000036571 hydration Effects 0.000 description 3
- 238000006703 hydration reaction Methods 0.000 description 3
- 229960001524 hydrocortisone butyrate Drugs 0.000 description 3
- 229910052739 hydrogen Inorganic materials 0.000 description 3
- 239000001257 hydrogen Substances 0.000 description 3
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 3
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 3
- 238000001802 infusion Methods 0.000 description 3
- 238000003780 insertion Methods 0.000 description 3
- 230000037431 insertion Effects 0.000 description 3
- 238000001990 intravenous administration Methods 0.000 description 3
- 239000002650 laminated plastic Substances 0.000 description 3
- 150000002632 lipids Chemical class 0.000 description 3
- 210000004072 lung Anatomy 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 229960001474 meclozine Drugs 0.000 description 3
- INWLQCZOYSRPNW-UHFFFAOYSA-N mepivacaine Chemical compound CN1CCCCC1C(=O)NC1=C(C)C=CC=C1C INWLQCZOYSRPNW-UHFFFAOYSA-N 0.000 description 3
- 229960002409 mepivacaine Drugs 0.000 description 3
- 239000002207 metabolite Substances 0.000 description 3
- 229960002744 mometasone furoate Drugs 0.000 description 3
- WOFMFGQZHJDGCX-ZULDAHANSA-N mometasone furoate Chemical compound O([C@]1([C@@]2(C)C[C@H](O)[C@]3(Cl)[C@@]4(C)C=CC(=O)C=C4CC[C@H]3[C@@H]2C[C@H]1C)C(=O)CCl)C(=O)C1=CC=CO1 WOFMFGQZHJDGCX-ZULDAHANSA-N 0.000 description 3
- 229920003052 natural elastomer Polymers 0.000 description 3
- 229920001194 natural rubber Polymers 0.000 description 3
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 description 3
- 230000000414 obstructive effect Effects 0.000 description 3
- 230000003647 oxidation Effects 0.000 description 3
- 238000007254 oxidation reaction Methods 0.000 description 3
- 239000008194 pharmaceutical composition Substances 0.000 description 3
- 239000002953 phosphate buffered saline Substances 0.000 description 3
- 229950010765 pivalate Drugs 0.000 description 3
- IUGYQRQAERSCNH-UHFFFAOYSA-N pivalic acid Chemical compound CC(C)(C)C(O)=O IUGYQRQAERSCNH-UHFFFAOYSA-N 0.000 description 3
- 239000004014 plasticizer Substances 0.000 description 3
- 229920002401 polyacrylamide Polymers 0.000 description 3
- 229920001282 polysaccharide Polymers 0.000 description 3
- 239000005017 polysaccharide Substances 0.000 description 3
- 229920000136 polysorbate Polymers 0.000 description 3
- 229920002689 polyvinyl acetate Polymers 0.000 description 3
- 239000011118 polyvinyl acetate Substances 0.000 description 3
- 239000011148 porous material Substances 0.000 description 3
- 229960001896 pramocaine Drugs 0.000 description 3
- DQKXQSGTHWVTAD-UHFFFAOYSA-N pramocaine Chemical compound C1=CC(OCCCC)=CC=C1OCCCN1CCOCC1 DQKXQSGTHWVTAD-UHFFFAOYSA-N 0.000 description 3
- 229960004919 procaine Drugs 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 210000001147 pulmonary artery Anatomy 0.000 description 3
- GHBFNMLVSPCDGN-UHFFFAOYSA-N rac-1-monooctanoylglycerol Chemical compound CCCCCCCC(=O)OCC(O)CO GHBFNMLVSPCDGN-UHFFFAOYSA-N 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 238000000926 separation method Methods 0.000 description 3
- 229920005573 silicon-containing polymer Polymers 0.000 description 3
- 229910052708 sodium Inorganic materials 0.000 description 3
- 239000011780 sodium chloride Substances 0.000 description 3
- WFRKJMRGXGWHBM-UHFFFAOYSA-M sodium;octyl sulfate Chemical compound [Na+].CCCCCCCCOS([O-])(=O)=O WFRKJMRGXGWHBM-UHFFFAOYSA-M 0.000 description 3
- 229940035044 sorbitan monolaurate Drugs 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- 230000008961 swelling Effects 0.000 description 3
- 208000024891 symptom Diseases 0.000 description 3
- 229920003051 synthetic elastomer Polymers 0.000 description 3
- 239000005061 synthetic rubber Substances 0.000 description 3
- 230000001839 systemic circulation Effects 0.000 description 3
- 229940037128 systemic glucocorticoids Drugs 0.000 description 3
- 229940052907 telazol Drugs 0.000 description 3
- 229960002372 tetracaine Drugs 0.000 description 3
- GKCBAIGFKIBETG-UHFFFAOYSA-N tetracaine Chemical compound CCCCNC1=CC=C(C(=O)OCCN(C)C)C=C1 GKCBAIGFKIBETG-UHFFFAOYSA-N 0.000 description 3
- 239000003053 toxin Substances 0.000 description 3
- 231100000765 toxin Toxicity 0.000 description 3
- 210000000689 upper leg Anatomy 0.000 description 3
- 235000015112 vegetable and seed oil Nutrition 0.000 description 3
- 239000008158 vegetable oil Substances 0.000 description 3
- 239000003981 vehicle Substances 0.000 description 3
- NOOLISFMXDJSKH-UTLUCORTSA-N (+)-Neomenthol Chemical compound CC(C)[C@@H]1CC[C@@H](C)C[C@@H]1O NOOLISFMXDJSKH-UTLUCORTSA-N 0.000 description 2
- XMGQYMWWDOXHJM-JTQLQIEISA-N (+)-α-limonene Chemical compound CC(=C)[C@@H]1CCC(C)=CC1 XMGQYMWWDOXHJM-JTQLQIEISA-N 0.000 description 2
- KWGRBVOPPLSCSI-WPRPVWTQSA-N (-)-ephedrine Chemical compound CN[C@@H](C)[C@H](O)C1=CC=CC=C1 KWGRBVOPPLSCSI-WPRPVWTQSA-N 0.000 description 2
- GJJFMKBJSRMPLA-HIFRSBDPSA-N (1R,2S)-2-(aminomethyl)-N,N-diethyl-1-phenyl-1-cyclopropanecarboxamide Chemical compound C=1C=CC=CC=1[C@@]1(C(=O)N(CC)CC)C[C@@H]1CN GJJFMKBJSRMPLA-HIFRSBDPSA-N 0.000 description 2
- TVYLLZQTGLZFBW-ZBFHGGJFSA-N (R,R)-tramadol Chemical compound COC1=CC=CC([C@]2(O)[C@H](CCCC2)CN(C)C)=C1 TVYLLZQTGLZFBW-ZBFHGGJFSA-N 0.000 description 2
- ZKMNUMMKYBVTFN-HNNXBMFYSA-N (S)-ropivacaine Chemical compound CCCN1CCCC[C@H]1C(=O)NC1=C(C)C=CC=C1C ZKMNUMMKYBVTFN-HNNXBMFYSA-N 0.000 description 2
- GVJHHUAWPYXKBD-UHFFFAOYSA-N (±)-α-Tocopherol Chemical compound OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 2
- WEEGYLXZBRQIMU-UHFFFAOYSA-N 1,8-cineole Natural products C1CC2CCC1(C)OC2(C)C WEEGYLXZBRQIMU-UHFFFAOYSA-N 0.000 description 2
- KBPLFHHGFOOTCA-UHFFFAOYSA-N 1-Octanol Chemical compound CCCCCCCCO KBPLFHHGFOOTCA-UHFFFAOYSA-N 0.000 description 2
- LEBVLXFERQHONN-UHFFFAOYSA-N 1-butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide Chemical compound CCCCN1CCCCC1C(=O)NC1=C(C)C=CC=C1C LEBVLXFERQHONN-UHFFFAOYSA-N 0.000 description 2
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- CNIIGCLFLJGOGP-UHFFFAOYSA-N 2-(1-naphthalenylmethyl)-4,5-dihydro-1H-imidazole Chemical compound C=1C=CC2=CC=CC=C2C=1CC1=NCCN1 CNIIGCLFLJGOGP-UHFFFAOYSA-N 0.000 description 2
- ZKLPARSLTMPFCP-OAQYLSRUSA-N 2-[2-[4-[(R)-(4-chlorophenyl)-phenylmethyl]-1-piperazinyl]ethoxy]acetic acid Chemical compound C1CN(CCOCC(=O)O)CCN1[C@@H](C=1C=CC(Cl)=CC=1)C1=CC=CC=C1 ZKLPARSLTMPFCP-OAQYLSRUSA-N 0.000 description 2
- ZVTDEEBSWIQAFJ-KHPPLWFESA-N 2-hydroxypropyl (z)-octadec-9-enoate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC(C)O ZVTDEEBSWIQAFJ-KHPPLWFESA-N 0.000 description 2
- SVTBMSDMJJWYQN-UHFFFAOYSA-N 2-methylpentane-2,4-diol Chemical compound CC(O)CC(C)(C)O SVTBMSDMJJWYQN-UHFFFAOYSA-N 0.000 description 2
- OJQMKCBWYCWFPU-UHFFFAOYSA-N ACT-333679 Chemical compound C=1C=CC=CC=1C1=NC(N(CCCCOCC(O)=O)C(C)C)=CN=C1C1=CC=CC=C1 OJQMKCBWYCWFPU-UHFFFAOYSA-N 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 241000416162 Astragalus gummifer Species 0.000 description 2
- MBUVEWMHONZEQD-UHFFFAOYSA-N Azeptin Chemical compound C1CN(C)CCCC1N1C(=O)C2=CC=CC=C2C(CC=2C=CC(Cl)=CC=2)=N1 MBUVEWMHONZEQD-UHFFFAOYSA-N 0.000 description 2
- KUVIULQEHSCUHY-XYWKZLDCSA-N Beclometasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)COC(=O)CC)(OC(=O)CC)[C@@]1(C)C[C@@H]2O KUVIULQEHSCUHY-XYWKZLDCSA-N 0.000 description 2
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 2
- FERIUCNNQQJTOY-UHFFFAOYSA-M Butyrate Chemical compound CCCC([O-])=O FERIUCNNQQJTOY-UHFFFAOYSA-M 0.000 description 2
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Natural products CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 description 2
- 208000024172 Cardiovascular disease Diseases 0.000 description 2
- 240000000467 Carum carvi Species 0.000 description 2
- 235000005747 Carum carvi Nutrition 0.000 description 2
- ZKLPARSLTMPFCP-UHFFFAOYSA-N Cetirizine Chemical compound C1CN(CCOCC(=O)O)CCN1C(C=1C=CC(Cl)=CC=1)C1=CC=CC=C1 ZKLPARSLTMPFCP-UHFFFAOYSA-N 0.000 description 2
- NPBVQXIMTZKSBA-UHFFFAOYSA-N Chavibetol Natural products COC1=CC=C(CC=C)C=C1O NPBVQXIMTZKSBA-UHFFFAOYSA-N 0.000 description 2
- JZUFKLXOESDKRF-UHFFFAOYSA-N Chlorothiazide Chemical compound C1=C(Cl)C(S(=O)(=O)N)=CC2=C1NCNS2(=O)=O JZUFKLXOESDKRF-UHFFFAOYSA-N 0.000 description 2
- 208000026151 Chronic thromboembolic pulmonary hypertension Diseases 0.000 description 2
- NOOLISFMXDJSKH-UHFFFAOYSA-N DL-menthol Natural products CC(C)C1CCC(C)CC1O NOOLISFMXDJSKH-UHFFFAOYSA-N 0.000 description 2
- 206010012434 Dermatitis allergic Diseases 0.000 description 2
- LCGLNKUTAGEVQW-UHFFFAOYSA-N Dimethyl ether Chemical compound COC LCGLNKUTAGEVQW-UHFFFAOYSA-N 0.000 description 2
- 240000002943 Elettaria cardamomum Species 0.000 description 2
- 241000283073 Equus caballus Species 0.000 description 2
- WEEGYLXZBRQIMU-WAAGHKOSSA-N Eucalyptol Chemical compound C1C[C@H]2CC[C@]1(C)OC2(C)C WEEGYLXZBRQIMU-WAAGHKOSSA-N 0.000 description 2
- 239000005770 Eugenol Substances 0.000 description 2
- 206010064503 Excessive skin Diseases 0.000 description 2
- WJOHZNCJWYWUJD-IUGZLZTKSA-N Fluocinonide Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)COC(=O)C)[C@@]2(C)C[C@@H]1O WJOHZNCJWYWUJD-IUGZLZTKSA-N 0.000 description 2
- UGJMXCAKCUNAIE-UHFFFAOYSA-N Gabapentin Chemical compound OC(=O)CC1(CN)CCCCC1 UGJMXCAKCUNAIE-UHFFFAOYSA-N 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical group OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- 229920002907 Guar gum Polymers 0.000 description 2
- 102000003834 Histamine H1 Receptors Human genes 0.000 description 2
- 108090000110 Histamine H1 Receptors Proteins 0.000 description 2
- FOGXJPFPZOHSQS-AYVLZSQQSA-N Hydrocortisone butyrate propionate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)CC)(OC(=O)CCC)[C@@]1(C)C[C@@H]2O FOGXJPFPZOHSQS-AYVLZSQQSA-N 0.000 description 2
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 2
- 206010020772 Hypertension Diseases 0.000 description 2
- HEFNNWSXXWATRW-UHFFFAOYSA-N Ibuprofen Chemical compound CC(C)CC1=CC=C(C(C)C(O)=O)C=C1 HEFNNWSXXWATRW-UHFFFAOYSA-N 0.000 description 2
- 108010076876 Keratins Proteins 0.000 description 2
- 102000011782 Keratins Human genes 0.000 description 2
- ARIWANIATODDMH-UHFFFAOYSA-N Lauric acid monoglyceride Natural products CCCCCCCCCCCC(=O)OCC(O)CO ARIWANIATODDMH-UHFFFAOYSA-N 0.000 description 2
- 235000019501 Lemon oil Nutrition 0.000 description 2
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 description 2
- 208000019693 Lung disease Diseases 0.000 description 2
- 101710122864 Major tegument protein Proteins 0.000 description 2
- FQISKWAFAHGMGT-SGJOWKDISA-M Methylprednisolone sodium succinate Chemical compound [Na+].C([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2[C@@H](O)C[C@]2(C)[C@@](O)(C(=O)COC(=O)CCC([O-])=O)CC[C@H]21 FQISKWAFAHGMGT-SGJOWKDISA-M 0.000 description 2
- BACYUWVYYTXETD-UHFFFAOYSA-N N-Lauroylsarcosine Chemical compound CCCCCCCCCCCC(=O)N(C)CC(O)=O BACYUWVYYTXETD-UHFFFAOYSA-N 0.000 description 2
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 2
- AMQJEAYHLZJPGS-UHFFFAOYSA-N N-Pentanol Chemical compound CCCCCO AMQJEAYHLZJPGS-UHFFFAOYSA-N 0.000 description 2
- BLXXJMDCKKHMKV-UHFFFAOYSA-N Nabumetone Chemical compound C1=C(CCC(C)=O)C=CC2=CC(OC)=CC=C21 BLXXJMDCKKHMKV-UHFFFAOYSA-N 0.000 description 2
- CMWTZPSULFXXJA-UHFFFAOYSA-N Naproxen Natural products C1=C(C(C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-UHFFFAOYSA-N 0.000 description 2
- JAUOIFJMECXRGI-UHFFFAOYSA-N Neoclaritin Chemical compound C=1C(Cl)=CC=C2C=1CCC1=CC=CN=C1C2=C1CCNCC1 JAUOIFJMECXRGI-UHFFFAOYSA-N 0.000 description 2
- 241000283973 Oryctolagus cuniculus Species 0.000 description 2
- 101710148592 PTS system fructose-like EIIA component Proteins 0.000 description 2
- 101710169713 PTS system fructose-specific EIIA component Proteins 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 229920002319 Poly(methyl acrylate) Polymers 0.000 description 2
- 229920002614 Polyether block amide Polymers 0.000 description 2
- 239000004372 Polyvinyl alcohol Substances 0.000 description 2
- 229920001328 Polyvinylidene chloride Polymers 0.000 description 2
- UVMRYBDEERADNV-UHFFFAOYSA-N Pseudoeugenol Natural products COC1=CC(C(C)=C)=CC=C1O UVMRYBDEERADNV-UHFFFAOYSA-N 0.000 description 2
- 208000031467 Pulmonary capillary hemangiomatosis Diseases 0.000 description 2
- KYQCOXFCLRTKLS-UHFFFAOYSA-N Pyrazine Chemical compound C1=CN=CC=N1 KYQCOXFCLRTKLS-UHFFFAOYSA-N 0.000 description 2
- 206010039710 Scleroderma Diseases 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 2
- 241000282887 Suidae Species 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 241000282898 Sus scrofa Species 0.000 description 2
- 201000009594 Systemic Scleroderma Diseases 0.000 description 2
- 206010042953 Systemic sclerosis Diseases 0.000 description 2
- 101710199973 Tail tube protein Proteins 0.000 description 2
- 229920001615 Tragacanth Polymers 0.000 description 2
- XGMPVBXKDAHORN-RBWIMXSLSA-N Triamcinolone diacetate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](OC(C)=O)[C@@](C(=O)COC(=O)C)(O)[C@@]1(C)C[C@@H]2O XGMPVBXKDAHORN-RBWIMXSLSA-N 0.000 description 2
- 102000011016 Type 5 Cyclic Nucleotide Phosphodiesterases Human genes 0.000 description 2
- 108010037581 Type 5 Cyclic Nucleotide Phosphodiesterases Proteins 0.000 description 2
- HUCJFAOMUPXHDK-UHFFFAOYSA-N Xylometazoline Chemical compound CC1=CC(C(C)(C)C)=CC(C)=C1CC1=NCCN1 HUCJFAOMUPXHDK-UHFFFAOYSA-N 0.000 description 2
- 238000005299 abrasion Methods 0.000 description 2
- 150000001242 acetic acid derivatives Chemical class 0.000 description 2
- 150000001252 acrylic acid derivatives Chemical class 0.000 description 2
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 239000002313 adhesive film Substances 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- UCTWMZQNUQWSLP-UHFFFAOYSA-N adrenaline Chemical compound CNCC(O)C1=CC=C(O)C(O)=C1 UCTWMZQNUQWSLP-UHFFFAOYSA-N 0.000 description 2
- 208000017304 adult pulmonary Langerhans cell histiocytosis Diseases 0.000 description 2
- 229960003790 alimemazine Drugs 0.000 description 2
- 150000001340 alkali metals Chemical class 0.000 description 2
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 2
- 150000001342 alkaline earth metals Chemical class 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 229960003099 amcinonide Drugs 0.000 description 2
- ILKJAFIWWBXGDU-MOGDOJJUSA-N amcinonide Chemical compound O([C@@]1([C@H](O2)C[C@@H]3[C@@]1(C[C@H](O)[C@]1(F)[C@@]4(C)C=CC(=O)C=C4CC[C@H]13)C)C(=O)COC(=O)C)C12CCCC1 ILKJAFIWWBXGDU-MOGDOJJUSA-N 0.000 description 2
- 150000001408 amides Chemical class 0.000 description 2
- 230000003444 anaesthetic effect Effects 0.000 description 2
- 239000003945 anionic surfactant Substances 0.000 description 2
- 210000003423 ankle Anatomy 0.000 description 2
- 239000005557 antagonist Substances 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 230000003110 anti-inflammatory effect Effects 0.000 description 2
- 229940111131 antiinflammatory and antirheumatic product propionic acid derivative Drugs 0.000 description 2
- 239000004019 antithrombin Substances 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 229960004574 azelastine Drugs 0.000 description 2
- 229960005274 benzocaine Drugs 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- YWGDOWXRIALTES-NRFANRHFSA-N bepotastine Chemical compound C1CN(CCCC(=O)O)CCC1O[C@H](C=1N=CC=CC=1)C1=CC=C(Cl)C=C1 YWGDOWXRIALTES-NRFANRHFSA-N 0.000 description 2
- 229960002071 bepotastine Drugs 0.000 description 2
- WHGYBXFWUBPSRW-FOUAGVGXSA-N beta-cyclodextrin Chemical compound OC[C@H]([C@H]([C@@H]([C@H]1O)O)O[C@H]2O[C@@H]([C@@H](O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O3)[C@H](O)[C@H]2O)CO)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H]3O[C@@H]1CO WHGYBXFWUBPSRW-FOUAGVGXSA-N 0.000 description 2
- 230000005540 biological transmission Effects 0.000 description 2
- 229920001400 block copolymer Polymers 0.000 description 2
- 210000004204 blood vessel Anatomy 0.000 description 2
- 229960000725 brompheniramine Drugs 0.000 description 2
- ZDIGNSYAACHWNL-UHFFFAOYSA-N brompheniramine Chemical compound C=1C=CC=NC=1C(CCN(C)C)C1=CC=C(Br)C=C1 ZDIGNSYAACHWNL-UHFFFAOYSA-N 0.000 description 2
- 229960003150 bupivacaine Drugs 0.000 description 2
- RYYVLZVUVIJVGH-UHFFFAOYSA-N caffeine Chemical compound CN1C(=O)N(C)C(=O)C2=C1N=CN2C RYYVLZVUVIJVGH-UHFFFAOYSA-N 0.000 description 2
- 239000004202 carbamide Substances 0.000 description 2
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 2
- 235000005300 cardamomo Nutrition 0.000 description 2
- 229940082638 cardiac stimulant phosphodiesterase inhibitors Drugs 0.000 description 2
- ULDHMXUKGWMISQ-UHFFFAOYSA-N carvone Chemical compound CC(=C)C1CC=C(C)C(=O)C1 ULDHMXUKGWMISQ-UHFFFAOYSA-N 0.000 description 2
- 229960000590 celecoxib Drugs 0.000 description 2
- RZEKVGVHFLEQIL-UHFFFAOYSA-N celecoxib Chemical compound C1=CC(C)=CC=C1C1=CC(C(F)(F)F)=NN1C1=CC=C(S(N)(=O)=O)C=C1 RZEKVGVHFLEQIL-UHFFFAOYSA-N 0.000 description 2
- 229960001803 cetirizine Drugs 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 210000000038 chest Anatomy 0.000 description 2
- 229960003291 chlorphenamine Drugs 0.000 description 2
- 230000001684 chronic effect Effects 0.000 description 2
- 229960005233 cineole Drugs 0.000 description 2
- 229960002842 clobetasol Drugs 0.000 description 2
- 229940075614 colloidal silicon dioxide Drugs 0.000 description 2
- 238000004132 cross linking Methods 0.000 description 2
- 230000001186 cumulative effect Effects 0.000 description 2
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 2
- PAFZNILMFXTMIY-UHFFFAOYSA-N cyclohexylamine Chemical compound NC1CCCCC1 PAFZNILMFXTMIY-UHFFFAOYSA-N 0.000 description 2
- 239000003255 cyclooxygenase 2 inhibitor Substances 0.000 description 2
- KWGRBVOPPLSCSI-UHFFFAOYSA-N d-ephedrine Natural products CNC(C)C(O)C1=CC=CC=C1 KWGRBVOPPLSCSI-UHFFFAOYSA-N 0.000 description 2
- 239000000850 decongestant Substances 0.000 description 2
- 229940124581 decongestants Drugs 0.000 description 2
- 229960001271 desloratadine Drugs 0.000 description 2
- 229960003662 desonide Drugs 0.000 description 2
- WBGKWQHBNHJJPZ-LECWWXJVSA-N desonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O WBGKWQHBNHJJPZ-LECWWXJVSA-N 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 206010012601 diabetes mellitus Diseases 0.000 description 2
- 229960001259 diclofenac Drugs 0.000 description 2
- DCOPUUMXTXDBNB-UHFFFAOYSA-N diclofenac Chemical compound OC(=O)CC1=CC=CC=C1NC1=C(Cl)C=CC=C1Cl DCOPUUMXTXDBNB-UHFFFAOYSA-N 0.000 description 2
- BOBLHFUVNSFZPJ-JOYXJVLSSA-N diflorasone diacetate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H](C)[C@@](C(=O)COC(C)=O)(OC(C)=O)[C@@]2(C)C[C@@H]1O BOBLHFUVNSFZPJ-JOYXJVLSSA-N 0.000 description 2
- SMVRDGHCVNAOIN-UHFFFAOYSA-L disodium;1-dodecoxydodecane;sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O.CCCCCCCCCCCCOCCCCCCCCCCCC SMVRDGHCVNAOIN-UHFFFAOYSA-L 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- POULHZVOKOAJMA-UHFFFAOYSA-N dodecanoic acid Chemical compound CCCCCCCCCCCC(O)=O POULHZVOKOAJMA-UHFFFAOYSA-N 0.000 description 2
- 229960001393 dosulepin Drugs 0.000 description 2
- 229960005426 doxepin Drugs 0.000 description 2
- HCFDWZZGGLSKEP-UHFFFAOYSA-N doxylamine Chemical compound C=1C=CC=NC=1C(C)(OCCN(C)C)C1=CC=CC=C1 HCFDWZZGGLSKEP-UHFFFAOYSA-N 0.000 description 2
- 229960005178 doxylamine Drugs 0.000 description 2
- 230000002526 effect on cardiovascular system Effects 0.000 description 2
- 238000001962 electrophoresis Methods 0.000 description 2
- 150000002170 ethers Chemical class 0.000 description 2
- 229960004945 etoricoxib Drugs 0.000 description 2
- MNJVRJDLRVPLFE-UHFFFAOYSA-N etoricoxib Chemical compound C1=NC(C)=CC=C1C1=NC=C(Cl)C=C1C1=CC=C(S(C)(=O)=O)C=C1 MNJVRJDLRVPLFE-UHFFFAOYSA-N 0.000 description 2
- 229960002217 eugenol Drugs 0.000 description 2
- 210000003414 extremity Anatomy 0.000 description 2
- 150000002191 fatty alcohols Chemical class 0.000 description 2
- 229960003592 fexofenadine Drugs 0.000 description 2
- RWTNPBWLLIMQHL-UHFFFAOYSA-N fexofenadine Chemical compound C1=CC(C(C)(C(O)=O)C)=CC=C1C(O)CCCN1CCC(C(O)(C=2C=CC=CC=2)C=2C=CC=CC=2)CC1 RWTNPBWLLIMQHL-UHFFFAOYSA-N 0.000 description 2
- 229960000785 fluocinonide Drugs 0.000 description 2
- WMWTYOKRWGGJOA-CENSZEJFSA-N fluticasone propionate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(OC(=O)CC)[C@@]2(C)C[C@@H]1O WMWTYOKRWGGJOA-CENSZEJFSA-N 0.000 description 2
- 229960000289 fluticasone propionate Drugs 0.000 description 2
- 239000011888 foil Substances 0.000 description 2
- UPBDXRPQPOWRKR-UHFFFAOYSA-N furan-2,5-dione;methoxyethene Chemical compound COC=C.O=C1OC(=O)C=C1 UPBDXRPQPOWRKR-UHFFFAOYSA-N 0.000 description 2
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 description 2
- 239000001087 glyceryl triacetate Substances 0.000 description 2
- 235000013773 glyceryl triacetate Nutrition 0.000 description 2
- 239000000665 guar gum Substances 0.000 description 2
- 235000010417 guar gum Nutrition 0.000 description 2
- 229960002154 guar gum Drugs 0.000 description 2
- 229940115747 halobetasol Drugs 0.000 description 2
- 208000019622 heart disease Diseases 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 2
- OROGSEYTTFOCAN-UHFFFAOYSA-N hydrocodone Natural products C1C(N(CCC234)C)C2C=CC(O)C3OC2=C4C1=CC=C2OC OROGSEYTTFOCAN-UHFFFAOYSA-N 0.000 description 2
- 229960000890 hydrocortisone Drugs 0.000 description 2
- 229960002846 hydrocortisone probutate Drugs 0.000 description 2
- 229960000631 hydrocortisone valerate Drugs 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- 229960001680 ibuprofen Drugs 0.000 description 2
- 239000007943 implant Substances 0.000 description 2
- 238000011065 in-situ storage Methods 0.000 description 2
- CGIGDMFJXJATDK-UHFFFAOYSA-N indomethacin Chemical compound CC1=C(CC(O)=O)C2=CC(OC)=CC=C2N1C(=O)C1=CC=C(Cl)C=C1 CGIGDMFJXJATDK-UHFFFAOYSA-N 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 238000010255 intramuscular injection Methods 0.000 description 2
- 239000007927 intramuscular injection Substances 0.000 description 2
- 239000002563 ionic surfactant Substances 0.000 description 2
- XUGNVMKQXJXZCD-UHFFFAOYSA-N isopropyl palmitate Chemical compound CCCCCCCCCCCCCCCC(=O)OC(C)C XUGNVMKQXJXZCD-UHFFFAOYSA-N 0.000 description 2
- 229940075495 isopropyl palmitate Drugs 0.000 description 2
- 239000008274 jelly Substances 0.000 description 2
- 229960003639 laurocapram Drugs 0.000 description 2
- 239000010501 lemon oil Substances 0.000 description 2
- 229960001508 levocetirizine Drugs 0.000 description 2
- 229960004194 lidocaine Drugs 0.000 description 2
- XMGQYMWWDOXHJM-UHFFFAOYSA-N limonene Chemical compound CC(=C)C1CCC(C)=CC1 XMGQYMWWDOXHJM-UHFFFAOYSA-N 0.000 description 2
- 238000002690 local anesthesia Methods 0.000 description 2
- 229960003088 loratadine Drugs 0.000 description 2
- JCCNYMKQOSZNPW-UHFFFAOYSA-N loratadine Chemical compound C1CN(C(=O)OCC)CCC1=C1C2=NC=CC=C2CCC2=CC(Cl)=CC=C21 JCCNYMKQOSZNPW-UHFFFAOYSA-N 0.000 description 2
- 239000000314 lubricant Substances 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- 229940041616 menthol Drugs 0.000 description 2
- 229960000582 mepyramine Drugs 0.000 description 2
- YECBIJXISLIIDS-UHFFFAOYSA-N mepyramine Chemical compound C1=CC(OC)=CC=C1CN(CCN(C)C)C1=CC=CC=N1 YECBIJXISLIIDS-UHFFFAOYSA-N 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 229960004584 methylprednisolone Drugs 0.000 description 2
- BQJCRHHNABKAKU-KBQPJGBKSA-N morphine Chemical compound O([C@H]1[C@H](C=C[C@H]23)O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O BQJCRHHNABKAKU-KBQPJGBKSA-N 0.000 description 2
- 208000010125 myocardial infarction Diseases 0.000 description 2
- 208000031225 myocardial ischemia Diseases 0.000 description 2
- 229960004270 nabumetone Drugs 0.000 description 2
- 229960002009 naproxen Drugs 0.000 description 2
- CMWTZPSULFXXJA-VIFPVBQESA-N naproxen Chemical compound C1=C([C@H](C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-VIFPVBQESA-N 0.000 description 2
- 210000003739 neck Anatomy 0.000 description 2
- 210000005036 nerve Anatomy 0.000 description 2
- 231100000344 non-irritating Toxicity 0.000 description 2
- 239000002736 nonionic surfactant Substances 0.000 description 2
- SJWFXCIHNDVPSH-UHFFFAOYSA-N octan-2-ol Chemical compound CCCCCCC(C)O SJWFXCIHNDVPSH-UHFFFAOYSA-N 0.000 description 2
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 229960004114 olopatadine Drugs 0.000 description 2
- JBIMVDZLSHOPLA-LSCVHKIXSA-N olopatadine Chemical compound C1OC2=CC=C(CC(O)=O)C=C2C(=C/CCN(C)C)\C2=CC=CC=C21 JBIMVDZLSHOPLA-LSCVHKIXSA-N 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- QVYRGXJJSLMXQH-UHFFFAOYSA-N orphenadrine Chemical compound C=1C=CC=C(C)C=1C(OCCN(C)C)C1=CC=CC=C1 QVYRGXJJSLMXQH-UHFFFAOYSA-N 0.000 description 2
- 229960003941 orphenadrine Drugs 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- WYWIFABBXFUGLM-UHFFFAOYSA-N oxymetazoline Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C)=C1CC1=NCCN1 WYWIFABBXFUGLM-UHFFFAOYSA-N 0.000 description 2
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 2
- XQYZDYMELSJDRZ-UHFFFAOYSA-N papaverine Chemical compound C1=C(OC)C(OC)=CC=C1CC1=NC=CC2=CC(OC)=C(OC)C=C12 XQYZDYMELSJDRZ-UHFFFAOYSA-N 0.000 description 2
- 229960005489 paracetamol Drugs 0.000 description 2
- 230000009057 passive transport Effects 0.000 description 2
- 150000002989 phenols Chemical class 0.000 description 2
- 239000002571 phosphodiesterase inhibitor Substances 0.000 description 2
- 229920002635 polyurethane Polymers 0.000 description 2
- 239000004814 polyurethane Substances 0.000 description 2
- 229920002451 polyvinyl alcohol Polymers 0.000 description 2
- 239000005033 polyvinylidene chloride Substances 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- MVFGUOIZUNYYSO-UHFFFAOYSA-N prilocaine Chemical compound CCCNC(C)C(=O)NC1=CC=CC=C1C MVFGUOIZUNYYSO-UHFFFAOYSA-N 0.000 description 2
- 229960001807 prilocaine Drugs 0.000 description 2
- OJCPSBCUMRIPFL-UHFFFAOYSA-N prolintane Chemical compound C1CCCN1C(CCC)CC1=CC=CC=C1 OJCPSBCUMRIPFL-UHFFFAOYSA-N 0.000 description 2
- 230000002035 prolonged effect Effects 0.000 description 2
- 150000005599 propionic acid derivatives Chemical class 0.000 description 2
- 230000002685 pulmonary effect Effects 0.000 description 2
- 208000037813 pulmonary venous hypertension Diseases 0.000 description 2
- 102000005962 receptors Human genes 0.000 description 2
- 108020003175 receptors Proteins 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 229960001549 ropivacaine Drugs 0.000 description 2
- 239000005060 rubber Substances 0.000 description 2
- 229960004889 salicylic acid Drugs 0.000 description 2
- 150000003902 salicylic acid esters Chemical class 0.000 description 2
- WVYADZUPLLSGPU-UHFFFAOYSA-N salsalate Chemical compound OC(=O)C1=CC=CC=C1OC(=O)C1=CC=CC=C1O WVYADZUPLLSGPU-UHFFFAOYSA-N 0.000 description 2
- 238000007789 sealing Methods 0.000 description 2
- BNRNXUUZRGQAQC-UHFFFAOYSA-N sildenafil Chemical compound CCCC1=NN(C)C(C(N2)=O)=C1N=C2C(C(=CC=1)OCC)=CC=1S(=O)(=O)N1CCN(C)CC1 BNRNXUUZRGQAQC-UHFFFAOYSA-N 0.000 description 2
- 239000013464 silicone adhesive Substances 0.000 description 2
- 229920002379 silicone rubber Polymers 0.000 description 2
- 206010040882 skin lesion Diseases 0.000 description 2
- 231100000444 skin lesion Toxicity 0.000 description 2
- 238000005063 solubilization Methods 0.000 description 2
- 230000007928 solubilization Effects 0.000 description 2
- 239000000021 stimulant Substances 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 229920003048 styrene butadiene rubber Polymers 0.000 description 2
- 150000003462 sulfoxides Chemical class 0.000 description 2
- 229960000894 sulindac Drugs 0.000 description 2
- MLKXDPUZXIRXEP-MFOYZWKCSA-N sulindac Chemical compound CC1=C(CC(O)=O)C2=CC(F)=CC=C2\C1=C/C1=CC=C(S(C)=O)C=C1 MLKXDPUZXIRXEP-MFOYZWKCSA-N 0.000 description 2
- 230000002459 sustained effect Effects 0.000 description 2
- 238000013268 sustained release Methods 0.000 description 2
- 239000012730 sustained-release form Substances 0.000 description 2
- YRCWQPVGYLYSOX-UHFFFAOYSA-N synephrine Chemical compound CNCC(O)C1=CC=C(O)C=C1 YRCWQPVGYLYSOX-UHFFFAOYSA-N 0.000 description 2
- 229960000351 terfenadine Drugs 0.000 description 2
- CFMYXEVWODSLAX-QOZOJKKESA-N tetrodotoxin Chemical compound O([C@@]([C@H]1O)(O)O[C@H]2[C@@]3(O)CO)[C@H]3[C@@H](O)[C@]11[C@H]2[C@@H](O)N=C(N)N1 CFMYXEVWODSLAX-QOZOJKKESA-N 0.000 description 2
- 229950010357 tetrodotoxin Drugs 0.000 description 2
- CFMYXEVWODSLAX-UHFFFAOYSA-N tetrodotoxin Natural products C12C(O)NC(=N)NC2(C2O)C(O)C3C(CO)(O)C1OC2(O)O3 CFMYXEVWODSLAX-UHFFFAOYSA-N 0.000 description 2
- BYJAVTDNIXVSPW-UHFFFAOYSA-N tetryzoline Chemical compound N1CCN=C1C1C2=CC=CC=C2CCC1 BYJAVTDNIXVSPW-UHFFFAOYSA-N 0.000 description 2
- MIMJSJSRRDZIPW-UHFFFAOYSA-N tilmacoxib Chemical compound C=1C=C(S(N)(=O)=O)C(F)=CC=1C=1OC(C)=NC=1C1CCCCC1 MIMJSJSRRDZIPW-UHFFFAOYSA-N 0.000 description 2
- 239000012049 topical pharmaceutical composition Substances 0.000 description 2
- 235000010487 tragacanth Nutrition 0.000 description 2
- 239000000196 tragacanth Substances 0.000 description 2
- 229940116362 tragacanth Drugs 0.000 description 2
- 229960004380 tramadol Drugs 0.000 description 2
- TVYLLZQTGLZFBW-GOEBONIOSA-N tramadol Natural products COC1=CC=CC([C@@]2(O)[C@@H](CCCC2)CN(C)C)=C1 TVYLLZQTGLZFBW-GOEBONIOSA-N 0.000 description 2
- PHTUQLWOUWZIMZ-GZTJUZNOSA-N trans-dothiepin Chemical compound C1SC2=CC=CC=C2C(=C/CCN(C)C)/C2=CC=CC=C21 PHTUQLWOUWZIMZ-GZTJUZNOSA-N 0.000 description 2
- 229960002622 triacetin Drugs 0.000 description 2
- 229960004320 triamcinolone diacetate Drugs 0.000 description 2
- GOZBHBFUQHMKQB-UHFFFAOYSA-N trimecaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=C(C)C=C1C GOZBHBFUQHMKQB-UHFFFAOYSA-N 0.000 description 2
- 229950002569 trimecaine Drugs 0.000 description 2
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 2
- 229950008396 ulobetasol propionate Drugs 0.000 description 2
- 229960002004 valdecoxib Drugs 0.000 description 2
- LNPDTQAFDNKSHK-UHFFFAOYSA-N valdecoxib Chemical compound CC=1ON=C(C=2C=CC=CC=2)C=1C1=CC=C(S(N)(=O)=O)C=C1 LNPDTQAFDNKSHK-UHFFFAOYSA-N 0.000 description 2
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N valeric acid Chemical compound CCCCC(O)=O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 description 2
- 230000024883 vasodilation Effects 0.000 description 2
- 229940124549 vasodilator Drugs 0.000 description 2
- 239000003071 vasodilator agent Substances 0.000 description 2
- 239000001993 wax Substances 0.000 description 2
- 238000005303 weighing Methods 0.000 description 2
- 239000000080 wetting agent Substances 0.000 description 2
- FQTLCLSUCSAZDY-UHFFFAOYSA-N (+) E(S) nerolidol Natural products CC(C)=CCCC(C)=CCCC(C)(O)C=C FQTLCLSUCSAZDY-UHFFFAOYSA-N 0.000 description 1
- NFLGAXVYCFJBMK-RKDXNWHRSA-N (+)-isomenthone Natural products CC(C)[C@H]1CC[C@@H](C)CC1=O NFLGAXVYCFJBMK-RKDXNWHRSA-N 0.000 description 1
- SNICXCGAKADSCV-JTQLQIEISA-N (-)-Nicotine Chemical compound CN1CCC[C@H]1C1=CC=CN=C1 SNICXCGAKADSCV-JTQLQIEISA-N 0.000 description 1
- VLPIATFUUWWMKC-SNVBAGLBSA-N (2r)-1-(2,6-dimethylphenoxy)propan-2-amine Chemical compound C[C@@H](N)COC1=C(C)C=CC=C1C VLPIATFUUWWMKC-SNVBAGLBSA-N 0.000 description 1
- LNAZSHAWQACDHT-XIYTZBAFSA-N (2r,3r,4s,5r,6s)-4,5-dimethoxy-2-(methoxymethyl)-3-[(2s,3r,4s,5r,6r)-3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6r)-4,5,6-trimethoxy-2-(methoxymethyl)oxan-3-yl]oxyoxane Chemical compound CO[C@@H]1[C@@H](OC)[C@H](OC)[C@@H](COC)O[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@H]2[C@@H]([C@@H](OC)[C@H](OC)O[C@@H]2COC)OC)O[C@@H]1COC LNAZSHAWQACDHT-XIYTZBAFSA-N 0.000 description 1
- JESCETIFNOFKEU-SJORKVTESA-N (2s,5r)-5-[4-[(2-fluorophenyl)methoxy]phenyl]pyrrolidine-2-carboxamide Chemical compound N1[C@H](C(=O)N)CC[C@@H]1C(C=C1)=CC=C1OCC1=CC=CC=C1F JESCETIFNOFKEU-SJORKVTESA-N 0.000 description 1
- WRRSFOZOETZUPG-FFHNEAJVSA-N (4r,4ar,7s,7ar,12bs)-9-methoxy-3-methyl-2,4,4a,7,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinoline-7-ol;hydrate Chemical compound O.C([C@H]1[C@H](N(CC[C@@]112)C)C3)=C[C@H](O)[C@@H]1OC1=C2C3=CC=C1OC WRRSFOZOETZUPG-FFHNEAJVSA-N 0.000 description 1
- YYGNTYWPHWGJRM-UHFFFAOYSA-N (6E,10E,14E,18E)-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene Chemical compound CC(C)=CCCC(C)=CCCC(C)=CCCC=C(C)CCC=C(C)CCC=C(C)C YYGNTYWPHWGJRM-UHFFFAOYSA-N 0.000 description 1
- NEBUOXBYNAHKFV-NRFANRHFSA-N (7s)-1'-[[5-(trifluoromethyl)furan-2-yl]methyl]spiro[6h-furo[2,3-f][1,3]benzodioxole-7,3'-indole]-2'-one Chemical compound O1C(C(F)(F)F)=CC=C1CN1C2=CC=CC=C2[C@@]2(C3=CC=4OCOC=4C=C3OC2)C1=O NEBUOXBYNAHKFV-NRFANRHFSA-N 0.000 description 1
- OYHQOLUKZRVURQ-NTGFUMLPSA-N (9Z,12Z)-9,10,12,13-tetratritiooctadeca-9,12-dienoic acid Chemical compound C(CCCCCCC\C(=C(/C\C(=C(/CCCCC)\[3H])\[3H])\[3H])\[3H])(=O)O OYHQOLUKZRVURQ-NTGFUMLPSA-N 0.000 description 1
- ZEUITGRIYCTCEM-KRWDZBQOSA-N (S)-duloxetine Chemical compound C1([C@@H](OC=2C3=CC=CC=C3C=CC=2)CCNC)=CC=CS1 ZEUITGRIYCTCEM-KRWDZBQOSA-N 0.000 description 1
- YNGDWRXWKFWCJY-UHFFFAOYSA-N 1,4-Dihydropyridine Chemical compound C1C=CNC=C1 YNGDWRXWKFWCJY-UHFFFAOYSA-N 0.000 description 1
- WFNAKBGANONZEQ-UHFFFAOYSA-N 1-[(4-chlorophenyl)-phenylmethyl]-4-methylpiperazine Chemical compound C1CN(C)CCN1C(C=1C=CC(Cl)=CC=1)C1=CC=CC=C1 WFNAKBGANONZEQ-UHFFFAOYSA-N 0.000 description 1
- GEHAEMCVKDPMKO-HXUWFJFHSA-N 1-[1-[(2s)-3-(6-chloronaphthalen-2-yl)sulfonyl-2-hydroxypropanoyl]piperidin-4-yl]-1,3-diazinan-2-one Chemical compound O=C([C@@H](CS(=O)(=O)C=1C=C2C=CC(Cl)=CC2=CC=1)O)N(CC1)CCC1N1CCCNC1=O GEHAEMCVKDPMKO-HXUWFJFHSA-N 0.000 description 1
- ARIWANIATODDMH-AWEZNQCLSA-N 1-lauroyl-sn-glycerol Chemical compound CCCCCCCCCCCC(=O)OC[C@@H](O)CO ARIWANIATODDMH-AWEZNQCLSA-N 0.000 description 1
- WECGLUPZRHILCT-GSNKCQISSA-N 1-linoleoyl-sn-glycerol Chemical compound CCCCC\C=C/C\C=C/CCCCCCCC(=O)OC[C@@H](O)CO WECGLUPZRHILCT-GSNKCQISSA-N 0.000 description 1
- VSJJEAXEEBGTGU-UHFFFAOYSA-N 1-methyl-3-[5-(3,4,5-trimethoxyphenyl)thiophen-3-yl]indole Chemical compound COC=1C=C(C=C(C=1OC)OC)C=1SC=C(C=1)C1=CN(C2=CC=CC=C12)C VSJJEAXEEBGTGU-UHFFFAOYSA-N 0.000 description 1
- NZJXADCEESMBPW-UHFFFAOYSA-N 1-methylsulfinyldecane Chemical compound CCCCCCCCCCS(C)=O NZJXADCEESMBPW-UHFFFAOYSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- FUFLCEKSBBHCMO-UHFFFAOYSA-N 11-dehydrocorticosterone Natural products O=C1CCC2(C)C3C(=O)CC(C)(C(CC4)C(=O)CO)C4C3CCC2=C1 FUFLCEKSBBHCMO-UHFFFAOYSA-N 0.000 description 1
- 239000000263 2,3-dihydroxypropyl (Z)-octadec-9-enoate Substances 0.000 description 1
- SKDGWNHUETZZCS-UHFFFAOYSA-N 2,3-ditert-butylphenol Chemical compound CC(C)(C)C1=CC=CC(O)=C1C(C)(C)C SKDGWNHUETZZCS-UHFFFAOYSA-N 0.000 description 1
- NXMZBNYLCVTRGB-UHFFFAOYSA-N 2-(4-ethoxyphenyl)-3-(4-methylsulfonylphenyl)pyrazolo[1,5-b]pyridazine Chemical compound C1=CC(OCC)=CC=C1C1=NN(N=CC=C2)C2=C1C1=CC=C(S(C)(=O)=O)C=C1 NXMZBNYLCVTRGB-UHFFFAOYSA-N 0.000 description 1
- CTPDSKVQLSDPLC-UHFFFAOYSA-N 2-(oxolan-2-ylmethoxy)ethanol Chemical compound OCCOCC1CCCO1 CTPDSKVQLSDPLC-UHFFFAOYSA-N 0.000 description 1
- YFGHCGITMMYXAQ-UHFFFAOYSA-N 2-[(diphenylmethyl)sulfinyl]acetamide Chemical compound C=1C=CC=CC=1C(S(=O)CC(=O)N)C1=CC=CC=C1 YFGHCGITMMYXAQ-UHFFFAOYSA-N 0.000 description 1
- DWHIUNMOTRUVPG-UHFFFAOYSA-N 2-[2-[2-[2-[2-[2-(2-dodecoxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethanol Chemical compound CCCCCCCCCCCCOCCOCCOCCOCCOCCOCCOCCO DWHIUNMOTRUVPG-UHFFFAOYSA-N 0.000 description 1
- JIVPVXMEBJLZRO-CQSZACIVSA-N 2-chloro-5-[(1r)-1-hydroxy-3-oxo-2h-isoindol-1-yl]benzenesulfonamide Chemical compound C1=C(Cl)C(S(=O)(=O)N)=CC([C@@]2(O)C3=CC=CC=C3C(=O)N2)=C1 JIVPVXMEBJLZRO-CQSZACIVSA-N 0.000 description 1
- QPILHXCDZYWYLQ-UHFFFAOYSA-N 2-nonyl-1,3-dioxolane Chemical compound CCCCCCCCCC1OCCO1 QPILHXCDZYWYLQ-UHFFFAOYSA-N 0.000 description 1
- LEACJMVNYZDSKR-UHFFFAOYSA-N 2-octyldodecan-1-ol Chemical compound CCCCCCCCCCC(CO)CCCCCCCC LEACJMVNYZDSKR-UHFFFAOYSA-N 0.000 description 1
- GOLORTLGFDVFDW-UHFFFAOYSA-N 3-(1h-benzimidazol-2-yl)-7-(diethylamino)chromen-2-one Chemical compound C1=CC=C2NC(C3=CC4=CC=C(C=C4OC3=O)N(CC)CC)=NC2=C1 GOLORTLGFDVFDW-UHFFFAOYSA-N 0.000 description 1
- PXQULGMNYRZNIM-UHFFFAOYSA-N 3-anilinopyridine-2-carboxylic acid Chemical class OC(=O)C1=NC=CC=C1NC1=CC=CC=C1 PXQULGMNYRZNIM-UHFFFAOYSA-N 0.000 description 1
- RZRNAYUHWVFMIP-GDCKJWNLSA-N 3-oleoyl-sn-glycerol Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@H](O)CO RZRNAYUHWVFMIP-GDCKJWNLSA-N 0.000 description 1
- QVLJMPBNVQXYEL-UHFFFAOYSA-N 4-O-methylhonokiol Natural products COC1=CC=C(CC=C)C=C1C1=CC=C(O)C(CC=C)=C1 QVLJMPBNVQXYEL-UHFFFAOYSA-N 0.000 description 1
- IJWPAFMIFNSIGD-UHFFFAOYSA-N 4-[3-(3-fluorophenyl)-5,5-dimethyl-4-oxofuran-2-yl]benzenesulfonamide Chemical compound O=C1C(C)(C)OC(C=2C=CC(=CC=2)S(N)(=O)=O)=C1C1=CC=CC(F)=C1 IJWPAFMIFNSIGD-UHFFFAOYSA-N 0.000 description 1
- HBTAOSGHCXUEKI-UHFFFAOYSA-N 4-chloro-n,n-dimethyl-3-nitrobenzenesulfonamide Chemical compound CN(C)S(=O)(=O)C1=CC=C(Cl)C([N+]([O-])=O)=C1 HBTAOSGHCXUEKI-UHFFFAOYSA-N 0.000 description 1
- OQFHJKZVOALSPV-UHFFFAOYSA-N 4-o-methylhonokiol Chemical compound C1=C(CC=C)C(OC)=CC=C1C1=CC(CC=C)=CC=C1O OQFHJKZVOALSPV-UHFFFAOYSA-N 0.000 description 1
- PJJGZPJJTHBVMX-UHFFFAOYSA-N 5,7-Dihydroxyisoflavone Chemical compound C=1C(O)=CC(O)=C(C2=O)C=1OC=C2C1=CC=CC=C1 PJJGZPJJTHBVMX-UHFFFAOYSA-N 0.000 description 1
- NEYKRKVLEWKOBI-UHFFFAOYSA-N 5-[2-ethoxy-5-[4-(2-hydroxyethyl)piperazin-1-yl]sulfonylphenyl]-1-methyl-3-propyl-4h-pyrazolo[4,3-d]pyrimidin-7-one Chemical compound CCCC1=NN(C)C(C(N=2)=O)=C1NC=2C(C(=CC=1)OCC)=CC=1S(=O)(=O)N1CCN(CCO)CC1 NEYKRKVLEWKOBI-UHFFFAOYSA-N 0.000 description 1
- YIEAVVIJPFEHCX-UHFFFAOYSA-N 5-ethyl-2-[5-[4-(2-hydroxyethyl)piperazin-1-yl]sulfonyl-2-propoxyphenyl]-7-propyl-4a,7a-dihydro-3H-pyrrolo[3,2-d]pyrimidin-4-one Chemical compound CCCOC1=CC=C(C=C1C1=NC2C(N(CC)C=C2CCC)C(=O)N1)S(=O)(=O)N1CCN(CCO)CC1 YIEAVVIJPFEHCX-UHFFFAOYSA-N 0.000 description 1
- 229930008281 A03AD01 - Papaverine Natural products 0.000 description 1
- RSWGJHLUYNHPMX-UHFFFAOYSA-N Abietic-Saeure Natural products C12CCC(C(C)C)=CC2=CCC2C1(C)CCCC2(C)C(O)=O RSWGJHLUYNHPMX-UHFFFAOYSA-N 0.000 description 1
- 206010060934 Allergic oedema Diseases 0.000 description 1
- QNZCBYKSOIHPEH-UHFFFAOYSA-N Apixaban Chemical compound C1=CC(OC)=CC=C1N1C(C(=O)N(CC2)C=3C=CC(=CC=3)N3C(CCCC3)=O)=C2C(C(N)=O)=N1 QNZCBYKSOIHPEH-UHFFFAOYSA-N 0.000 description 1
- QTGIAADRBBLJGA-UHFFFAOYSA-N Articaine Chemical compound CCCNC(C)C(=O)NC=1C(C)=CSC=1C(=O)OC QTGIAADRBBLJGA-UHFFFAOYSA-N 0.000 description 1
- 108010027612 Batroxobin Proteins 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- MNIPYSSQXLZQLJ-UHFFFAOYSA-N Biofenac Chemical compound OC(=O)COC(=O)CC1=CC=CC=C1NC1=C(Cl)C=CC=C1Cl MNIPYSSQXLZQLJ-UHFFFAOYSA-N 0.000 description 1
- 102100025422 Bone morphogenetic protein receptor type-2 Human genes 0.000 description 1
- 240000001889 Brahea edulis Species 0.000 description 1
- 241000195940 Bryophyta Species 0.000 description 1
- VOVIALXJUBGFJZ-KWVAZRHASA-N Budesonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(CCC)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O VOVIALXJUBGFJZ-KWVAZRHASA-N 0.000 description 1
- 208000019300 CLIPPERS Diseases 0.000 description 1
- 241000282465 Canis Species 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 239000005973 Carvone Substances 0.000 description 1
- 241000700198 Cavia Species 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- LZZYPRNAOMGNLH-UHFFFAOYSA-M Cetrimonium bromide Chemical compound [Br-].CCCCCCCCCCCCCCCC[N+](C)(C)C LZZYPRNAOMGNLH-UHFFFAOYSA-M 0.000 description 1
- 108091006146 Channels Proteins 0.000 description 1
- HGJXAVROWQLCTP-YABCKIEDSA-N Chebulagic acid Chemical compound O([C@H]1[C@H]2[C@H]3OC(=O)C4=CC(O)=C(O)C(O)=C4C4=C(O)C(O)=C(O)C=C4C(=O)OC[C@@H](O1)[C@H]3OC(=O)[C@@H](CC(O)=O)[C@@H]1[C@@H](C(OC=3C(O)=C(O)C=C(C1=3)C(=O)O2)=O)O)C(=O)C1=CC(O)=C(O)C(O)=C1 HGJXAVROWQLCTP-YABCKIEDSA-N 0.000 description 1
- 229920002052 Chebulagic acid Polymers 0.000 description 1
- 241000219312 Chenopodium Species 0.000 description 1
- 229920001661 Chitosan Polymers 0.000 description 1
- 239000004709 Chlorinated polyethylene Substances 0.000 description 1
- DBAKFASWICGISY-BTJKTKAUSA-N Chlorpheniramine maleate Chemical compound OC(=O)\C=C/C(O)=O.C=1C=CC=NC=1C(CCN(C)C)C1=CC=C(Cl)C=C1 DBAKFASWICGISY-BTJKTKAUSA-N 0.000 description 1
- 108010009685 Cholinergic Receptors Proteins 0.000 description 1
- LUKZNWIVRBCLON-GXOBDPJESA-N Ciclesonide Chemical compound C1([C@H]2O[C@@]3([C@H](O2)C[C@@H]2[C@@]3(C[C@H](O)[C@@H]3[C@@]4(C)C=CC(=O)C=C4CC[C@H]32)C)C(=O)COC(=O)C(C)C)CCCCC1 LUKZNWIVRBCLON-GXOBDPJESA-N 0.000 description 1
- GDLIGKIOYRNHDA-UHFFFAOYSA-N Clomipramine Chemical compound C1CC2=CC=C(Cl)C=C2N(CCCN(C)C)C2=CC=CC=C21 GDLIGKIOYRNHDA-UHFFFAOYSA-N 0.000 description 1
- GJSURZIOUXUGAL-UHFFFAOYSA-N Clonidine Chemical compound ClC1=CC=CC(Cl)=C1NC1=NCCN1 GJSURZIOUXUGAL-UHFFFAOYSA-N 0.000 description 1
- 208000002330 Congenital Heart Defects Diseases 0.000 description 1
- MFYSYFVPBJMHGN-ZPOLXVRWSA-N Cortisone Chemical compound O=C1CC[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 MFYSYFVPBJMHGN-ZPOLXVRWSA-N 0.000 description 1
- MFYSYFVPBJMHGN-UHFFFAOYSA-N Cortisone Natural products O=C1CCC2(C)C3C(=O)CC(C)(C(CC4)(O)C(=O)CO)C4C3CCC2=C1 MFYSYFVPBJMHGN-UHFFFAOYSA-N 0.000 description 1
- ITRJWOMZKQRYTA-RFZYENFJSA-N Cortisone acetate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)C)(O)[C@@]1(C)CC2=O ITRJWOMZKQRYTA-RFZYENFJSA-N 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 229920001651 Cyanoacrylate Polymers 0.000 description 1
- 102000002004 Cytochrome P-450 Enzyme System Human genes 0.000 description 1
- 108010015742 Cytochrome P-450 Enzyme System Proteins 0.000 description 1
- 101710088194 Dehydrogenase Proteins 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 206010052337 Diastolic dysfunction Diseases 0.000 description 1
- BWLUMTFWVZZZND-UHFFFAOYSA-N Dibenzylamine Chemical compound C=1C=CC=CC=1CNCC1=CC=CC=C1 BWLUMTFWVZZZND-UHFFFAOYSA-N 0.000 description 1
- XBPCUCUWBYBCDP-UHFFFAOYSA-N Dicyclohexylamine Chemical compound C1CCCCC1NC1CCCCC1 XBPCUCUWBYBCDP-UHFFFAOYSA-N 0.000 description 1
- HHJIUUAMYGBVSD-YTFFSALGSA-N Diflucortolone valerate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@H](C(=O)COC(=O)CCCC)[C@@]2(C)C[C@@H]1O HHJIUUAMYGBVSD-YTFFSALGSA-N 0.000 description 1
- WDJUZGPOPHTGOT-OAXVISGBSA-N Digitoxin Natural products O([C@H]1[C@@H](C)O[C@@H](O[C@@H]2C[C@@H]3[C@@](C)([C@@H]4[C@H]([C@]5(O)[C@@](C)([C@H](C6=CC(=O)OC6)CC5)CC4)CC3)CC2)C[C@H]1O)[C@H]1O[C@@H](C)[C@H](O[C@H]2O[C@@H](C)[C@@H](O)[C@@H](O)C2)[C@@H](O)C1 WDJUZGPOPHTGOT-OAXVISGBSA-N 0.000 description 1
- SHIBSTMRCDJXLN-UHFFFAOYSA-N Digoxigenin Natural products C1CC(C2C(C3(C)CCC(O)CC3CC2)CC2O)(O)C2(C)C1C1=CC(=O)OC1 SHIBSTMRCDJXLN-UHFFFAOYSA-N 0.000 description 1
- LTMHDMANZUZIPE-AMTYYWEZSA-N Digoxin Natural products O([C@H]1[C@H](C)O[C@H](O[C@@H]2C[C@@H]3[C@@](C)([C@@H]4[C@H]([C@]5(O)[C@](C)([C@H](O)C4)[C@H](C4=CC(=O)OC4)CC5)CC3)CC2)C[C@@H]1O)[C@H]1O[C@H](C)[C@@H](O[C@H]2O[C@@H](C)[C@H](O)[C@@H](O)C2)[C@@H](O)C1 LTMHDMANZUZIPE-AMTYYWEZSA-N 0.000 description 1
- IJVCSMSMFSCRME-KBQPJGBKSA-N Dihydromorphine Chemical compound O([C@H]1[C@H](CC[C@H]23)O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O IJVCSMSMFSCRME-KBQPJGBKSA-N 0.000 description 1
- 229940123688 Direct Factor Xa inhibitor Drugs 0.000 description 1
- 229940123900 Direct thrombin inhibitor Drugs 0.000 description 1
- AJFTZWGGHJXZOB-UHFFFAOYSA-N DuP 697 Chemical compound C1=CC(S(=O)(=O)C)=CC=C1C1=C(C=2C=CC(F)=CC=2)SC(Br)=C1 AJFTZWGGHJXZOB-UHFFFAOYSA-N 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 1
- 102000012085 Endoglin Human genes 0.000 description 1
- 108010036395 Endoglin Proteins 0.000 description 1
- 102000010180 Endothelin receptor Human genes 0.000 description 1
- 108050001739 Endothelin receptor Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 108090000371 Esterases Proteins 0.000 description 1
- OTMSDBZUPAUEDD-UHFFFAOYSA-N Ethane Chemical compound CC OTMSDBZUPAUEDD-UHFFFAOYSA-N 0.000 description 1
- 229920000219 Ethylene vinyl alcohol Polymers 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 241000282324 Felis Species 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 102000009123 Fibrin Human genes 0.000 description 1
- 108010073385 Fibrin Proteins 0.000 description 1
- BWGVNKXGVNDBDI-UHFFFAOYSA-N Fibrin monomer Chemical compound CNC(=O)CNC(=O)CN BWGVNKXGVNDBDI-UHFFFAOYSA-N 0.000 description 1
- GYHNNYVSQQEPJS-UHFFFAOYSA-N Gallium Chemical compound [Ga] GYHNNYVSQQEPJS-UHFFFAOYSA-N 0.000 description 1
- 208000015872 Gaucher disease Diseases 0.000 description 1
- 241000699694 Gerbillinae Species 0.000 description 1
- 108010024636 Glutathione Proteins 0.000 description 1
- 229920000569 Gum karaya Polymers 0.000 description 1
- 208000031886 HIV Infections Diseases 0.000 description 1
- 208000037357 HIV infectious disease Diseases 0.000 description 1
- MUQNGPZZQDCDFT-JNQJZLCISA-N Halcinonide Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H]3OC(C)(C)O[C@@]3(C(=O)CCl)[C@@]1(C)C[C@@H]2O MUQNGPZZQDCDFT-JNQJZLCISA-N 0.000 description 1
- 208000031953 Hereditary hemorrhagic telangiectasia Diseases 0.000 description 1
- 108010007267 Hirudins Proteins 0.000 description 1
- 102000007625 Hirudins Human genes 0.000 description 1
- 101000934635 Homo sapiens Bone morphogenetic protein receptor type-2 Proteins 0.000 description 1
- 101000620009 Homo sapiens Polyunsaturated fatty acid 5-lipoxygenase Proteins 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- 206010021133 Hypoventilation Diseases 0.000 description 1
- 206010021143 Hypoxia Diseases 0.000 description 1
- TZJALUIVHRYQQB-XFDQAQKOSA-N Icariin Natural products O(C)c1ccc(C2=C(O[C@H]3[C@@H](O)[C@H](O)[C@@H](O)[C@H](C)O3)C(=O)c3c(O)cc(O[C@H]4[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O4)c(C/C=C(\C)/C)c3O2)cc1 TZJALUIVHRYQQB-XFDQAQKOSA-N 0.000 description 1
- 208000020875 Idiopathic pulmonary arterial hypertension Diseases 0.000 description 1
- 208000024934 IgG4-related mediastinitis Diseases 0.000 description 1
- 208000032382 Ischaemic stroke Diseases 0.000 description 1
- LPHGQDQBBGAPDZ-UHFFFAOYSA-N Isocaffeine Natural products CN1C(=O)N(C)C(=O)C2=C1N(C)C=N2 LPHGQDQBBGAPDZ-UHFFFAOYSA-N 0.000 description 1
- PWWVAXIEGOYWEE-UHFFFAOYSA-N Isophenergan Chemical compound C1=CC=C2N(CC(C)N(C)C)C3=CC=CC=C3SC2=C1 PWWVAXIEGOYWEE-UHFFFAOYSA-N 0.000 description 1
- YQEZLKZALYSWHR-UHFFFAOYSA-N Ketamine Chemical compound C=1C=CC=C(Cl)C=1C1(NC)CCCCC1=O YQEZLKZALYSWHR-UHFFFAOYSA-N 0.000 description 1
- ZCVMWBYGMWKGHF-UHFFFAOYSA-N Ketotifene Chemical compound C1CN(C)CCC1=C1C2=CC=CC=C2CC(=O)C2=C1C=CS2 ZCVMWBYGMWKGHF-UHFFFAOYSA-N 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- MKXZASYAUGDDCJ-SZMVWBNQSA-N LSM-2525 Chemical compound C1CCC[C@H]2[C@@]3([H])N(C)CC[C@]21C1=CC(OC)=CC=C1C3 MKXZASYAUGDDCJ-SZMVWBNQSA-N 0.000 description 1
- 239000004166 Lanolin Substances 0.000 description 1
- 239000005639 Lauric acid Substances 0.000 description 1
- OYHQOLUKZRVURQ-HZJYTTRNSA-N Linoleic acid Chemical compound CCCCC\C=C/C\C=C/CCCCCCCC(O)=O OYHQOLUKZRVURQ-HZJYTTRNSA-N 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 206010049459 Lymphangioleiomyomatosis Diseases 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- SBDNJUWAMKYJOX-UHFFFAOYSA-N Meclofenamic Acid Chemical compound CC1=CC=C(Cl)C(NC=2C(=CC=CC=2)C(O)=O)=C1Cl SBDNJUWAMKYJOX-UHFFFAOYSA-N 0.000 description 1
- ZRVUJXDFFKFLMG-UHFFFAOYSA-N Meloxicam Chemical compound OC=1C2=CC=CC=C2S(=O)(=O)N(C)C=1C(=O)NC1=NC=C(C)S1 ZRVUJXDFFKFLMG-UHFFFAOYSA-N 0.000 description 1
- NFLGAXVYCFJBMK-UHFFFAOYSA-N Menthone Chemical compound CC(C)C1CCC(C)CC1=O NFLGAXVYCFJBMK-UHFFFAOYSA-N 0.000 description 1
- XADCESSVHJOZHK-UHFFFAOYSA-N Meperidine Chemical compound C=1C=CC=CC=1C1(C(=O)OCC)CCN(C)CC1 XADCESSVHJOZHK-UHFFFAOYSA-N 0.000 description 1
- MWCLLHOVUTZFKS-UHFFFAOYSA-N Methyl cyanoacrylate Chemical compound COC(=O)C(=C)C#N MWCLLHOVUTZFKS-UHFFFAOYSA-N 0.000 description 1
- DUGOZIWVEXMGBE-UHFFFAOYSA-N Methylphenidate Chemical class C=1C=CC=CC=1C(C(=O)OC)C1CCCCN1 DUGOZIWVEXMGBE-UHFFFAOYSA-N 0.000 description 1
- PVLJETXTTWAYEW-UHFFFAOYSA-N Mizolastine Chemical compound N=1C=CC(=O)NC=1N(C)C(CC1)CCN1C1=NC2=CC=CC=C2N1CC1=CC=C(F)C=C1 PVLJETXTTWAYEW-UHFFFAOYSA-N 0.000 description 1
- 239000004909 Moisturizer Substances 0.000 description 1
- 102000010909 Monoamine Oxidase Human genes 0.000 description 1
- 108010062431 Monoamine oxidase Proteins 0.000 description 1
- 101100490437 Mus musculus Acvrl1 gene Proteins 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 208000014767 Myeloproliferative disease Diseases 0.000 description 1
- ICKFFNBDFNZJSX-UHFFFAOYSA-N N'-[(4-chlorophenyl)methyl]-N,N-dimethyl-N'-(2-pyridinyl)ethane-1,2-diamine Chemical compound C=1C=CC=NC=1N(CCN(C)C)CC1=CC=C(Cl)C=C1 ICKFFNBDFNZJSX-UHFFFAOYSA-N 0.000 description 1
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 description 1
- MMOXZBCLCQITDF-UHFFFAOYSA-N N,N-diethyl-m-toluamide Chemical compound CCN(CC)C(=O)C1=CC=CC(C)=C1 MMOXZBCLCQITDF-UHFFFAOYSA-N 0.000 description 1
- IJHNSHDBIRRJRN-UHFFFAOYSA-N N,N-dimethyl-3-phenyl-3-(2-pyridinyl)-1-propanamine Chemical compound C=1C=CC=NC=1C(CCN(C)C)C1=CC=CC=C1 IJHNSHDBIRRJRN-UHFFFAOYSA-N 0.000 description 1
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 1
- JUUFBMODXQKSTD-UHFFFAOYSA-N N-[2-amino-6-[(4-fluorophenyl)methylamino]-3-pyridinyl]carbamic acid ethyl ester Chemical compound N1=C(N)C(NC(=O)OCC)=CC=C1NCC1=CC=C(F)C=C1 JUUFBMODXQKSTD-UHFFFAOYSA-N 0.000 description 1
- HTLZVHNRZJPSMI-UHFFFAOYSA-N N-ethylpiperidine Chemical compound CCN1CCCCC1 HTLZVHNRZJPSMI-UHFFFAOYSA-N 0.000 description 1
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 1
- 229940127523 NMDA Receptor Antagonists Drugs 0.000 description 1
- 238000005481 NMR spectroscopy Methods 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- KTDZCOWXCWUPEO-UHFFFAOYSA-N NS-398 Chemical compound CS(=O)(=O)NC1=CC=C([N+]([O-])=O)C=C1OC1CCCCC1 KTDZCOWXCWUPEO-UHFFFAOYSA-N 0.000 description 1
- RGPDEAGGEXEMMM-UHFFFAOYSA-N Nefopam Chemical compound C12=CC=CC=C2CN(C)CCOC1C1=CC=CC=C1 RGPDEAGGEXEMMM-UHFFFAOYSA-N 0.000 description 1
- PPEKGEBBBBNZKS-UHFFFAOYSA-N Neosaxitoxin Natural products N=C1N(O)C(COC(=O)N)C2N=C(N)NC22C(O)(O)CCN21 PPEKGEBBBBNZKS-UHFFFAOYSA-N 0.000 description 1
- FQTLCLSUCSAZDY-ATGUSINASA-N Nerolidol Chemical compound CC(C)=CCC\C(C)=C\CC[C@](C)(O)C=C FQTLCLSUCSAZDY-ATGUSINASA-N 0.000 description 1
- 208000009905 Neurofibromatoses Diseases 0.000 description 1
- JZFPYUNJRRFVQU-UHFFFAOYSA-N Niflumic acid Chemical compound OC(=O)C1=CC=CN=C1NC1=CC=CC(C(F)(F)F)=C1 JZFPYUNJRRFVQU-UHFFFAOYSA-N 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 229920000459 Nitrile rubber Polymers 0.000 description 1
- 239000000020 Nitrocellulose Substances 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- KYYIDSXMWOZKMP-UHFFFAOYSA-N O-desmethylvenlafaxine Chemical compound C1CCCCC1(O)C(CN(C)C)C1=CC=C(O)C=C1 KYYIDSXMWOZKMP-UHFFFAOYSA-N 0.000 description 1
- LUZWXMGSPXIQHB-UHFFFAOYSA-N OC(=O)CC(C1=CC=C(C=C1)[N+]([O-])=O)C1=C(O)C2=C(OC1=O)C=CC=C2 Chemical compound OC(=O)CC(C1=CC=C(C=C1)[N+]([O-])=O)C1=C(O)C2=C(OC1=O)C=CC=C2 LUZWXMGSPXIQHB-UHFFFAOYSA-N 0.000 description 1
- 206010061876 Obstruction Diseases 0.000 description 1
- 102000004316 Oxidoreductases Human genes 0.000 description 1
- 108090000854 Oxidoreductases Proteins 0.000 description 1
- BRUQQQPBMZOVGD-XFKAJCMBSA-N Oxycodone Chemical compound O=C([C@@H]1O2)CC[C@@]3(O)[C@H]4CC5=CC=C(OC)C2=C5[C@@]13CCN4C BRUQQQPBMZOVGD-XFKAJCMBSA-N 0.000 description 1
- 241000282579 Pan Species 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 208000005764 Peripheral Arterial Disease Diseases 0.000 description 1
- 208000030831 Peripheral arterial occlusive disease Diseases 0.000 description 1
- 239000004264 Petrolatum Substances 0.000 description 1
- PCNDJXKNXGMECE-UHFFFAOYSA-N Phenazine Natural products C1=CC=CC2=NC3=CC=CC=C3N=C21 PCNDJXKNXGMECE-UHFFFAOYSA-N 0.000 description 1
- BHHGXPLMPWCGHP-UHFFFAOYSA-N Phenethylamine Chemical class NCCC1=CC=CC=C1 BHHGXPLMPWCGHP-UHFFFAOYSA-N 0.000 description 1
- ISFHAYSTHMVOJR-UHFFFAOYSA-N Phenindamine Chemical compound C1N(C)CCC(C2=CC=CC=C22)=C1C2C1=CC=CC=C1 ISFHAYSTHMVOJR-UHFFFAOYSA-N 0.000 description 1
- YQKAVWCGQQXBGW-UHFFFAOYSA-N Piperocaine Chemical compound CC1CCCCN1CCCOC(=O)C1=CC=CC=C1 YQKAVWCGQQXBGW-UHFFFAOYSA-N 0.000 description 1
- 229920002845 Poly(methacrylic acid) Polymers 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 239000005062 Polybutadiene Substances 0.000 description 1
- 229920002564 Polyethylene Glycol 3500 Polymers 0.000 description 1
- 108010020346 Polyglutamic Acid Proteins 0.000 description 1
- 102100022364 Polyunsaturated fatty acid 5-lipoxygenase Human genes 0.000 description 1
- 229920002396 Polyurea Polymers 0.000 description 1
- 206010067281 Portopulmonary hypertension Diseases 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- KCLANYCVBBTKTO-UHFFFAOYSA-N Proparacaine Chemical compound CCCOC1=CC=C(C(=O)OCCN(CC)CC)C=C1N KCLANYCVBBTKTO-UHFFFAOYSA-N 0.000 description 1
- RBQOQRRFDPXAGN-UHFFFAOYSA-N Propentofylline Chemical compound CN1C(=O)N(CCCCC(C)=O)C(=O)C2=C1N=CN2CCC RBQOQRRFDPXAGN-UHFFFAOYSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- CAJIGINSTLKQMM-UHFFFAOYSA-N Propoxycaine Chemical compound CCCOC1=CC(N)=CC=C1C(=O)OCCN(CC)CC CAJIGINSTLKQMM-UHFFFAOYSA-N 0.000 description 1
- 229940127314 Prostacyclin Receptor Agonists Drugs 0.000 description 1
- 102100038280 Prostaglandin G/H synthase 2 Human genes 0.000 description 1
- 108050003267 Prostaglandin G/H synthase 2 Proteins 0.000 description 1
- 102000004005 Prostaglandin-endoperoxide synthases Human genes 0.000 description 1
- 108090000459 Prostaglandin-endoperoxide synthases Proteins 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- 208000021068 Pulmonary arterial hypertension associated with portal hypertension Diseases 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 208000003782 Raynaud disease Diseases 0.000 description 1
- 208000001647 Renal Insufficiency Diseases 0.000 description 1
- 206010038743 Restlessness Diseases 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 241000220317 Rosa Species 0.000 description 1
- KHPCPRHQVVSZAH-HUOMCSJISA-N Rosin Natural products O(C/C=C/c1ccccc1)[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 KHPCPRHQVVSZAH-HUOMCSJISA-N 0.000 description 1
- JHBIMJKLBUMNAU-UHFFFAOYSA-N SC-58125 Chemical compound C1=CC(S(=O)(=O)C)=CC=C1N1C(C=2C=CC(F)=CC=2)=CC(C(F)(F)F)=N1 JHBIMJKLBUMNAU-UHFFFAOYSA-N 0.000 description 1
- 229940121991 Serotonin and norepinephrine reuptake inhibitor Drugs 0.000 description 1
- 229920001800 Shellac Polymers 0.000 description 1
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 1
- 102000007637 Soluble Guanylyl Cyclase Human genes 0.000 description 1
- 108010007205 Soluble Guanylyl Cyclase Proteins 0.000 description 1
- NWGKJDSIEKMTRX-AAZCQSIUSA-N Sorbitan monooleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O NWGKJDSIEKMTRX-AAZCQSIUSA-N 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 241000934878 Sterculia Species 0.000 description 1
- 239000002174 Styrene-butadiene Substances 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 206010071436 Systolic dysfunction Diseases 0.000 description 1
- BHEOSNUKNHRBNM-UHFFFAOYSA-N Tetramethylsqualene Natural products CC(=C)C(C)CCC(=C)C(C)CCC(C)=CCCC=C(C)CCC(C)C(=C)CCC(C)C(C)=C BHEOSNUKNHRBNM-UHFFFAOYSA-N 0.000 description 1
- 208000007536 Thrombosis Diseases 0.000 description 1
- 208000034841 Thrombotic Microangiopathies Diseases 0.000 description 1
- 208000024799 Thyroid disease Diseases 0.000 description 1
- 241001521901 Tribulus lanuginosus Species 0.000 description 1
- 229940123445 Tricyclic antidepressant Drugs 0.000 description 1
- PHYFQTYBJUILEZ-UHFFFAOYSA-N Trioleoylglycerol Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC(OC(=O)CCCCCCCC=CCCCCCCCC)COC(=O)CCCCCCCC=CCCCCCCCC PHYFQTYBJUILEZ-UHFFFAOYSA-N 0.000 description 1
- UFLGIAIHIAPJJC-UHFFFAOYSA-N Tripelennamine Chemical compound C=1C=CC=NC=1N(CCN(C)C)CC1=CC=CC=C1 UFLGIAIHIAPJJC-UHFFFAOYSA-N 0.000 description 1
- 208000025865 Ulcer Diseases 0.000 description 1
- SECKRCOLJRRGGV-UHFFFAOYSA-N Vardenafil Chemical compound CCCC1=NC(C)=C(C(N=2)=O)N1NC=2C(C(=CC=1)OCC)=CC=1S(=O)(=O)N1CCN(CC)CC1 SECKRCOLJRRGGV-UHFFFAOYSA-N 0.000 description 1
- 206010047115 Vasculitis Diseases 0.000 description 1
- 206010047139 Vasoconstriction Diseases 0.000 description 1
- QYKIQEUNHZKYBP-UHFFFAOYSA-N Vinyl ether Chemical compound C=COC=C QYKIQEUNHZKYBP-UHFFFAOYSA-N 0.000 description 1
- 229930003427 Vitamin E Natural products 0.000 description 1
- 229920002494 Zein Polymers 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- FJWGYAHXMCUOOM-QHOUIDNNSA-N [(2s,3r,4s,5r,6r)-2-[(2r,3r,4s,5r,6s)-4,5-dinitrooxy-2-(nitrooxymethyl)-6-[(2r,3r,4s,5r,6s)-4,5,6-trinitrooxy-2-(nitrooxymethyl)oxan-3-yl]oxyoxan-3-yl]oxy-3,5-dinitrooxy-6-(nitrooxymethyl)oxan-4-yl] nitrate Chemical compound O([C@@H]1O[C@@H]([C@H]([C@H](O[N+]([O-])=O)[C@H]1O[N+]([O-])=O)O[C@H]1[C@@H]([C@@H](O[N+]([O-])=O)[C@H](O[N+]([O-])=O)[C@@H](CO[N+]([O-])=O)O1)O[N+]([O-])=O)CO[N+](=O)[O-])[C@@H]1[C@@H](CO[N+]([O-])=O)O[C@@H](O[N+]([O-])=O)[C@H](O[N+]([O-])=O)[C@H]1O[N+]([O-])=O FJWGYAHXMCUOOM-QHOUIDNNSA-N 0.000 description 1
- 239000003070 absorption delaying agent Substances 0.000 description 1
- 229960004420 aceclofenac Drugs 0.000 description 1
- 229960002054 acenocoumarol Drugs 0.000 description 1
- 102000034337 acetylcholine receptors Human genes 0.000 description 1
- HWKJSYYYURVNQU-DXJNJSHLSA-N acetyldigoxin Chemical compound C1[C@H](OC(C)=O)[C@H](O)[C@@H](C)O[C@H]1O[C@@H]1[C@@H](C)O[C@@H](O[C@@H]2[C@H](O[C@@H](O[C@@H]3C[C@@H]4[C@]([C@@H]5[C@H]([C@]6(CC[C@@H]([C@@]6(C)[C@H](O)C5)C=5COC(=O)C=5)O)CC4)(C)CC3)C[C@@H]2O)C)C[C@@H]1O HWKJSYYYURVNQU-DXJNJSHLSA-N 0.000 description 1
- 229960003304 acetyldigoxin Drugs 0.000 description 1
- 229960003792 acrivastine Drugs 0.000 description 1
- PWACSDKDOHSSQD-IUTFFREVSA-N acrivastine Chemical compound C1=CC(C)=CC=C1C(\C=1N=C(\C=C\C(O)=O)C=CC=1)=C/CN1CCCC1 PWACSDKDOHSSQD-IUTFFREVSA-N 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 239000002998 adhesive polymer Substances 0.000 description 1
- CGNMLOKEMNBUAI-UHFFFAOYSA-N adrafinil Chemical compound C=1C=CC=CC=1C(S(=O)CC(=O)NO)C1=CC=CC=C1 CGNMLOKEMNBUAI-UHFFFAOYSA-N 0.000 description 1
- 229960002820 adrafinil Drugs 0.000 description 1
- 239000000048 adrenergic agonist Substances 0.000 description 1
- 229940126157 adrenergic receptor agonist Drugs 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 229960000552 alclometasone Drugs 0.000 description 1
- DJHCCTTVDRAMEH-DUUJBDRPSA-N alclometasone dipropionate Chemical compound C([C@H]1Cl)C2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)COC(=O)CC)(OC(=O)CC)[C@@]1(C)C[C@@H]2O DJHCCTTVDRAMEH-DUUJBDRPSA-N 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 150000001335 aliphatic alkanes Chemical class 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- MGVYFNHJWXJYBE-UHFFFAOYSA-N alpha-Acetyl-digoxin Natural products CC1OC(CC(O)C1O)OC2C(O)CC(OC3C(C)OC(CC3OC(=O)C)OC4CCC5(C)C(CCC6C5CCC7(C)C(C(O)CC67O)C8=CC(=O)OC8)C4)OC2C MGVYFNHJWXJYBE-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 229960002414 ambrisentan Drugs 0.000 description 1
- OUJTZYPIHDYQMC-LJQANCHMSA-N ambrisentan Chemical compound O([C@@H](C(OC)(C=1C=CC=CC=1)C=1C=CC=CC=1)C(O)=O)C1=NC(C)=CC(C)=N1 OUJTZYPIHDYQMC-LJQANCHMSA-N 0.000 description 1
- 229940093740 amino acid and derivative Drugs 0.000 description 1
- 229940064734 aminobenzoate Drugs 0.000 description 1
- 229960002980 amitriptyline oxide Drugs 0.000 description 1
- ZPMKQFOGINQDAM-UHFFFAOYSA-N amitriptylinoxide Chemical compound C1CC2=CC=CC=C2C(=CCC[N+](C)([O-])C)C2=CC=CC=C21 ZPMKQFOGINQDAM-UHFFFAOYSA-N 0.000 description 1
- HTIQEAQVCYTUBX-UHFFFAOYSA-N amlodipine Chemical compound CCOC(=O)C1=C(COCCN)NC(C)=C(C(=O)OC)C1C1=CC=CC=C1Cl HTIQEAQVCYTUBX-UHFFFAOYSA-N 0.000 description 1
- 229960000528 amlodipine Drugs 0.000 description 1
- QWGDMFLQWFTERH-UHFFFAOYSA-N amoxapine Chemical compound C12=CC(Cl)=CC=C2OC2=CC=CC=C2N=C1N1CCNCC1 QWGDMFLQWFTERH-UHFFFAOYSA-N 0.000 description 1
- 229960002519 amoxapine Drugs 0.000 description 1
- 230000033115 angiogenesis Effects 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 239000010617 anise oil Substances 0.000 description 1
- 229960002469 antazoline Drugs 0.000 description 1
- REYFJDPCWQRWAA-UHFFFAOYSA-N antazoline Chemical compound N=1CCNC=1CN(C=1C=CC=CC=1)CC1=CC=CC=C1 REYFJDPCWQRWAA-UHFFFAOYSA-N 0.000 description 1
- RWZYAGGXGHYGMB-UHFFFAOYSA-N anthranilic acid Chemical class NC1=CC=CC=C1C(O)=O RWZYAGGXGHYGMB-UHFFFAOYSA-N 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 230000010100 anticoagulation Effects 0.000 description 1
- 229940125681 anticonvulsant agent Drugs 0.000 description 1
- 239000001961 anticonvulsive agent Substances 0.000 description 1
- 239000000935 antidepressant agent Substances 0.000 description 1
- 229940005513 antidepressants Drugs 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 229940127218 antiplatelet drug Drugs 0.000 description 1
- 229960003886 apixaban Drugs 0.000 description 1
- 229950008049 apricoxib Drugs 0.000 description 1
- JTMITOKKUMVWRT-UHFFFAOYSA-N apricoxib Chemical compound C1=CC(OCC)=CC=C1C1=CC(C)=CN1C1=CC=C(S(N)(=O)=O)C=C1 JTMITOKKUMVWRT-UHFFFAOYSA-N 0.000 description 1
- 239000003125 aqueous solvent Substances 0.000 description 1
- BTFJIXJJCSYFAL-UHFFFAOYSA-N arachidyl alcohol Natural products CCCCCCCCCCCCCCCCCCCCO BTFJIXJJCSYFAL-UHFFFAOYSA-N 0.000 description 1
- KXNPVXPOPUZYGB-XYVMCAHJSA-N argatroban Chemical compound OC(=O)[C@H]1C[C@H](C)CCN1C(=O)[C@H](CCCN=C(N)N)NS(=O)(=O)C1=CC=CC2=C1NC[C@H](C)C2 KXNPVXPOPUZYGB-XYVMCAHJSA-N 0.000 description 1
- 229960003856 argatroban Drugs 0.000 description 1
- YFGHCGITMMYXAQ-LJQANCHMSA-N armodafinil Chemical compound C=1C=CC=CC=1C([S@](=O)CC(=O)N)C1=CC=CC=C1 YFGHCGITMMYXAQ-LJQANCHMSA-N 0.000 description 1
- 229960004823 armodafinil Drugs 0.000 description 1
- 230000004872 arterial blood pressure Effects 0.000 description 1
- 229960003831 articaine Drugs 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- GXDALQBWZGODGZ-UHFFFAOYSA-N astemizole Chemical compound C1=CC(OC)=CC=C1CCN1CCC(NC=2N(C3=CC=CC=C3N=2)CC=2C=CC(F)=CC=2)CC1 GXDALQBWZGODGZ-UHFFFAOYSA-N 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- FKQQKMGWCJGUCS-UHFFFAOYSA-N atromentin Chemical compound O=C1C(O)=C(C=2C=CC(O)=CC=2)C(=O)C(O)=C1C1=CC=C(O)C=C1 FKQQKMGWCJGUCS-UHFFFAOYSA-N 0.000 description 1
- AAEDGQBSNHENEM-UHFFFAOYSA-N atromentin Natural products OCC1(O)C2=C(C(=O)C(=C(C2=O)c3ccc(O)cc3)O)c4ccc(O)cc14 AAEDGQBSNHENEM-UHFFFAOYSA-N 0.000 description 1
- 229960000307 avanafil Drugs 0.000 description 1
- WEAJZXNPAWBCOA-INIZCTEOSA-N avanafil Chemical compound C1=C(Cl)C(OC)=CC=C1CNC1=NC(N2[C@@H](CCC2)CO)=NC=C1C(=O)NCC1=NC=CC=N1 WEAJZXNPAWBCOA-INIZCTEOSA-N 0.000 description 1
- 229960000383 azatadine Drugs 0.000 description 1
- SEBMTIQKRHYNIT-UHFFFAOYSA-N azatadine Chemical compound C1CN(C)CCC1=C1C2=NC=CC=C2CCC2=CC=CC=C21 SEBMTIQKRHYNIT-UHFFFAOYSA-N 0.000 description 1
- 229910052788 barium Inorganic materials 0.000 description 1
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 description 1
- 239000003637 basic solution Substances 0.000 description 1
- 229960002210 batroxobin Drugs 0.000 description 1
- 229950000210 beclometasone dipropionate Drugs 0.000 description 1
- 229940092705 beclomethasone Drugs 0.000 description 1
- 235000013871 bee wax Nutrition 0.000 description 1
- 239000012166 beeswax Substances 0.000 description 1
- 229960003515 bendroflumethiazide Drugs 0.000 description 1
- HDWIHXWEUNVBIY-UHFFFAOYSA-N bendroflumethiazidum Chemical compound C1=C(C(F)(F)F)C(S(=O)(=O)N)=CC(S(N2)(=O)=O)=C1NC2CC1=CC=CC=C1 HDWIHXWEUNVBIY-UHFFFAOYSA-N 0.000 description 1
- ZISFCTXLAXIEMV-UHFFFAOYSA-N benzamidenafil Chemical compound C1=C(OC)C(OC)=CC=C1CNC(=O)C1=CC([N+]([O-])=O)=CC=C1NC(C)CO ZISFCTXLAXIEMV-UHFFFAOYSA-N 0.000 description 1
- JUHORIMYRDESRB-UHFFFAOYSA-N benzathine Chemical compound C=1C=CC=CC=1CNCCNCC1=CC=CC=C1 JUHORIMYRDESRB-UHFFFAOYSA-N 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 229960000870 betamethasone benzoate Drugs 0.000 description 1
- SOQJPQZCPBDOMF-YCUXZELOSA-N betamethasone benzoate Chemical compound O([C@]1([C@@]2(C)C[C@H](O)[C@]3(F)[C@@]4(C)C=CC(=O)C=C4CC[C@H]3[C@@H]2C[C@@H]1C)C(=O)CO)C(=O)C1=CC=CC=C1 SOQJPQZCPBDOMF-YCUXZELOSA-N 0.000 description 1
- 229960005354 betamethasone sodium phosphate Drugs 0.000 description 1
- PLCQGRYPOISRTQ-LWCNAHDDSA-L betamethasone sodium phosphate Chemical compound [Na+].[Na+].C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)COP([O-])([O-])=O)(O)[C@@]1(C)C[C@@H]2O PLCQGRYPOISRTQ-LWCNAHDDSA-L 0.000 description 1
- 229950011103 betrixaban Drugs 0.000 description 1
- XHOLNRLADUSQLD-UHFFFAOYSA-N betrixaban Chemical compound C=1C=C(Cl)C=NC=1NC(=O)C1=CC(OC)=CC=C1NC(=O)C1=CC=C(C(=N)N(C)C)C=C1 XHOLNRLADUSQLD-UHFFFAOYSA-N 0.000 description 1
- 229950010365 bicifadine Drugs 0.000 description 1
- OFYVIGTWSQPCLF-NWDGAFQWSA-N bicifadine Chemical compound C1=CC(C)=CC=C1[C@@]1(CNC2)[C@H]2C1 OFYVIGTWSQPCLF-NWDGAFQWSA-N 0.000 description 1
- 229960004314 bilastine Drugs 0.000 description 1
- ACCMWZWAEFYUGZ-UHFFFAOYSA-N bilastine Chemical compound N=1C2=CC=CC=C2N(CCOCC)C=1C(CC1)CCN1CCC1=CC=C(C(C)(C)C(O)=O)C=C1 ACCMWZWAEFYUGZ-UHFFFAOYSA-N 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- OIRCOABEOLEUMC-GEJPAHFPSA-N bivalirudin Chemical compound C([C@@H](C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CC(C)C)C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 OIRCOABEOLEUMC-GEJPAHFPSA-N 0.000 description 1
- 229960001500 bivalirudin Drugs 0.000 description 1
- 108010055460 bivalirudin Proteins 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 230000036772 blood pressure Effects 0.000 description 1
- 238000007664 blowing Methods 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 229960003065 bosentan Drugs 0.000 description 1
- SXTRWVVIEPWAKM-UHFFFAOYSA-N bosentan hydrate Chemical compound O.COC1=CC=CC=C1OC(C(=NC(=N1)C=2N=CC=CN=2)OCCO)=C1NS(=O)(=O)C1=CC=C(C(C)(C)C)C=C1 SXTRWVVIEPWAKM-UHFFFAOYSA-N 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 210000000481 breast Anatomy 0.000 description 1
- 229960003166 bromazine Drugs 0.000 description 1
- NUNIWXHYABYXKF-UHFFFAOYSA-N bromazine Chemical compound C=1C=C(Br)C=CC=1C(OCCN(C)C)C1=CC=CC=C1 NUNIWXHYABYXKF-UHFFFAOYSA-N 0.000 description 1
- 229960003655 bromfenac Drugs 0.000 description 1
- ZBPLOVFIXSTCRZ-UHFFFAOYSA-N bromfenac Chemical compound NC1=C(CC(O)=O)C=CC=C1C(=O)C1=CC=C(Br)C=C1 ZBPLOVFIXSTCRZ-UHFFFAOYSA-N 0.000 description 1
- 229960001705 buclizine Drugs 0.000 description 1
- MOYGZHXDRJNJEP-UHFFFAOYSA-N buclizine Chemical compound C1=CC(C(C)(C)C)=CC=C1CN1CCN(C(C=2C=CC=CC=2)C=2C=CC(Cl)=CC=2)CC1 MOYGZHXDRJNJEP-UHFFFAOYSA-N 0.000 description 1
- 229960004436 budesonide Drugs 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- RMRJXGBAOAMLHD-IHFGGWKQSA-N buprenorphine Chemical compound C([C@]12[C@H]3OC=4C(O)=CC=C(C2=4)C[C@@H]2[C@]11CC[C@]3([C@H](C1)[C@](C)(O)C(C)(C)C)OC)CN2CC1CC1 RMRJXGBAOAMLHD-IHFGGWKQSA-N 0.000 description 1
- 229960001736 buprenorphine Drugs 0.000 description 1
- MTAZNLWOLGHBHU-UHFFFAOYSA-N butadiene-styrene rubber Chemical compound C=CC=C.C=CC1=CC=CC=C1 MTAZNLWOLGHBHU-UHFFFAOYSA-N 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 229920005549 butyl rubber Polymers 0.000 description 1
- 235000019282 butylated hydroxyanisole Nutrition 0.000 description 1
- 229910052792 caesium Inorganic materials 0.000 description 1
- TVFDJXOCXUVLDH-UHFFFAOYSA-N caesium atom Chemical compound [Cs] TVFDJXOCXUVLDH-UHFFFAOYSA-N 0.000 description 1
- 229960001948 caffeine Drugs 0.000 description 1
- VJEONQKOZGKCAK-UHFFFAOYSA-N caffeine Natural products CN1C(=O)N(C)C(=O)C2=C1C=CN2C VJEONQKOZGKCAK-UHFFFAOYSA-N 0.000 description 1
- 244000309466 calf Species 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- FFGPTBGBLSHEPO-UHFFFAOYSA-N carbamazepine Chemical compound C1=CC2=CC=CC=C2N(C(=O)N)C2=CC=CC=C21 FFGPTBGBLSHEPO-UHFFFAOYSA-N 0.000 description 1
- 229960000623 carbamazepine Drugs 0.000 description 1
- 229960000428 carbinoxamine Drugs 0.000 description 1
- OJFSXZCBGQGRNV-UHFFFAOYSA-N carbinoxamine Chemical compound C=1C=CC=NC=1C(OCCN(C)C)C1=CC=C(Cl)C=C1 OJFSXZCBGQGRNV-UHFFFAOYSA-N 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- CREMABGTGYGIQB-UHFFFAOYSA-N carbon carbon Chemical compound C.C CREMABGTGYGIQB-UHFFFAOYSA-N 0.000 description 1
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-N carbonic acid Chemical class OC(O)=O BVKZGUZCCUSVTD-UHFFFAOYSA-N 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 238000007675 cardiac surgery Methods 0.000 description 1
- HHTWOMMSBMNRKP-UHFFFAOYSA-N carvacrol Natural products CC(=C)C1=CC=C(C)C(O)=C1 HHTWOMMSBMNRKP-UHFFFAOYSA-N 0.000 description 1
- RECUKUPTGUEGMW-UHFFFAOYSA-N carvacrol Chemical compound CC(C)C1=CC=C(C)C(O)=C1 RECUKUPTGUEGMW-UHFFFAOYSA-N 0.000 description 1
- 235000007746 carvacrol Nutrition 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 210000003169 central nervous system Anatomy 0.000 description 1
- 201000005667 central retinal vein occlusion Diseases 0.000 description 1
- 229960000541 cetyl alcohol Drugs 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- UGIVASYMZSZAMP-UHFFFAOYSA-N chebulagic acid Natural products OC1C2c3c(OC1=O)c(O)c(O)cc3C(=O)OC4C(OC(=O)c5cc(O)c(O)c(O)c5)OC6COC(=O)c7cc(O)c(O)c(O)c7c8c(O)c(O)c(O)cc8C(=O)OC4C6OC(=O)C2(O)C(=O)O UGIVASYMZSZAMP-UHFFFAOYSA-N 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 229960004831 chlorcyclizine Drugs 0.000 description 1
- HGAZMNJKRQFZKS-UHFFFAOYSA-N chloroethene;ethenyl acetate Chemical class ClC=C.CC(=O)OC=C HGAZMNJKRQFZKS-UHFFFAOYSA-N 0.000 description 1
- 229960001448 chloropyramine Drugs 0.000 description 1
- 229960002155 chlorothiazide Drugs 0.000 description 1
- SOYKEARSMXGVTM-UHFFFAOYSA-N chlorphenamine Chemical compound C=1C=CC=NC=1C(CCN(C)C)C1=CC=C(Cl)C=C1 SOYKEARSMXGVTM-UHFFFAOYSA-N 0.000 description 1
- ZPEIMTDSQAKGNT-UHFFFAOYSA-N chlorpromazine Chemical compound C1=C(Cl)C=C2N(CCCN(C)C)C3=CC=CC=C3SC2=C1 ZPEIMTDSQAKGNT-UHFFFAOYSA-N 0.000 description 1
- 229960001076 chlorpromazine Drugs 0.000 description 1
- 229960001523 chlortalidone Drugs 0.000 description 1
- BHQCQFFYRZLCQQ-OELDTZBJSA-N cholic acid Chemical compound C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(O)=O)C)[C@@]2(C)[C@@H](O)C1 BHQCQFFYRZLCQQ-OELDTZBJSA-N 0.000 description 1
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 1
- 229960001231 choline Drugs 0.000 description 1
- 208000020832 chronic kidney disease Diseases 0.000 description 1
- 208000021930 chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids Diseases 0.000 description 1
- 208000022831 chronic renal failure syndrome Diseases 0.000 description 1
- ARUGKOZUKWAXDS-SEWALLKFSA-N cicaprost Chemical compound C1\C(=C/COCC(O)=O)C[C@@H]2[C@@H](C#C[C@@H](O)[C@@H](C)CC#CCC)[C@H](O)C[C@@H]21 ARUGKOZUKWAXDS-SEWALLKFSA-N 0.000 description 1
- 229950000634 cicaprost Drugs 0.000 description 1
- 229960003728 ciclesonide Drugs 0.000 description 1
- 229950008442 cidoxepin Drugs 0.000 description 1
- WPYWMXNXEZFMAK-UHFFFAOYSA-N cinaciguat Chemical compound C=1C=C(C(O)=O)C=CC=1CN(CCCCC(=O)O)CCC1=CC=CC=C1OCC(C=C1)=CC=C1CCC1=CC=CC=C1 WPYWMXNXEZFMAK-UHFFFAOYSA-N 0.000 description 1
- 229950002128 cinaciguat Drugs 0.000 description 1
- PUFQVTATUTYEAL-UHFFFAOYSA-N cinchocaine Chemical compound C1=CC=CC2=NC(OCCCC)=CC(C(=O)NCCN(CC)CC)=C21 PUFQVTATUTYEAL-UHFFFAOYSA-N 0.000 description 1
- 229940114081 cinnamate Drugs 0.000 description 1
- 230000004087 circulation Effects 0.000 description 1
- YNNUSGIPVFPVBX-NHCUHLMSSA-N clemastine Chemical compound CN1CCC[C@@H]1CCO[C@@](C)(C=1C=CC(Cl)=CC=1)C1=CC=CC=C1 YNNUSGIPVFPVBX-NHCUHLMSSA-N 0.000 description 1
- 229960002881 clemastine Drugs 0.000 description 1
- 229960004703 clobetasol propionate Drugs 0.000 description 1
- 229960001146 clobetasone Drugs 0.000 description 1
- XXIFVOHLGBURIG-OZCCCYNHSA-N clobetasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CCl)(O)[C@@]1(C)CC2=O XXIFVOHLGBURIG-OZCCCYNHSA-N 0.000 description 1
- 229960004606 clomipramine Drugs 0.000 description 1
- 229960002896 clonidine Drugs 0.000 description 1
- CLOMYZFHNHFSIQ-UHFFFAOYSA-N clonixin Chemical compound CC1=C(Cl)C=CC=C1NC1=NC=CC=C1C(O)=O CLOMYZFHNHFSIQ-UHFFFAOYSA-N 0.000 description 1
- 229960001209 clonixin Drugs 0.000 description 1
- 238000011260 co-administration Methods 0.000 description 1
- 229960004126 codeine Drugs 0.000 description 1
- OROGSEYTTFOCAN-DNJOTXNNSA-N codeine Natural products C([C@H]1[C@H](N(CC[C@@]112)C)C3)=C[C@H](O)[C@@H]1OC1=C2C3=CC=C1OC OROGSEYTTFOCAN-DNJOTXNNSA-N 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000013329 compounding Methods 0.000 description 1
- 208000028831 congenital heart disease Diseases 0.000 description 1
- 208000018631 connective tissue disease Diseases 0.000 description 1
- 208000029078 coronary artery disease Diseases 0.000 description 1
- ALEXXDVDDISNDU-JZYPGELDSA-N cortisol 21-acetate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)C)(O)[C@@]1(C)C[C@@H]2O ALEXXDVDDISNDU-JZYPGELDSA-N 0.000 description 1
- 229960004544 cortisone Drugs 0.000 description 1
- 229960003290 cortisone acetate Drugs 0.000 description 1
- 229920006037 cross link polymer Polymers 0.000 description 1
- 239000003431 cross linking reagent Substances 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- UVKZSORBKUEBAZ-UHFFFAOYSA-N cyclizine Chemical compound C1CN(C)CCN1C(C=1C=CC=CC=1)C1=CC=CC=C1 UVKZSORBKUEBAZ-UHFFFAOYSA-N 0.000 description 1
- 229960003564 cyclizine Drugs 0.000 description 1
- 229960004741 cyclomethycaine Drugs 0.000 description 1
- YLRNESBGEGGQBK-UHFFFAOYSA-N cyclomethycaine Chemical compound CC1CCCCN1CCCOC(=O)C(C=C1)=CC=C1OC1CCCCC1 YLRNESBGEGGQBK-UHFFFAOYSA-N 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 229960001140 cyproheptadine Drugs 0.000 description 1
- JJCFRYNCJDLXIK-UHFFFAOYSA-N cyproheptadine Chemical compound C1CN(C)CCC1=C1C2=CC=CC=C2C=CC2=CC=CC=C21 JJCFRYNCJDLXIK-UHFFFAOYSA-N 0.000 description 1
- 229960003850 dabigatran Drugs 0.000 description 1
- YBSJFWOBGCMAKL-UHFFFAOYSA-N dabigatran Chemical compound N=1C2=CC(C(=O)N(CCC(O)=O)C=3N=CC=CC=3)=CC=C2N(C)C=1CNC1=CC=C(C(N)=N)C=C1 YBSJFWOBGCMAKL-UHFFFAOYSA-N 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- IJNIQYINMSGIPS-UHFFFAOYSA-N darexaban Chemical compound C1=CC(OC)=CC=C1C(=O)NC1=CC=CC(O)=C1NC(=O)C1=CC=C(N2CCN(C)CCC2)C=C1 IJNIQYINMSGIPS-UHFFFAOYSA-N 0.000 description 1
- 229950004553 darexaban Drugs 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 229960001623 desvenlafaxine Drugs 0.000 description 1
- 230000009547 development abnormality Effects 0.000 description 1
- 229960003957 dexamethasone Drugs 0.000 description 1
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 1
- VQODGRNSFPNSQE-CXSFZGCWSA-N dexamethasone phosphate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)COP(O)(O)=O)(O)[C@@]1(C)C[C@@H]2O VQODGRNSFPNSQE-CXSFZGCWSA-N 0.000 description 1
- 229960002344 dexamethasone sodium phosphate Drugs 0.000 description 1
- 229960002691 dexbrompheniramine Drugs 0.000 description 1
- ZDIGNSYAACHWNL-HNNXBMFYSA-N dexbrompheniramine Chemical compound C1([C@H](CCN(C)C)C=2N=CC=CC=2)=CC=C(Br)C=C1 ZDIGNSYAACHWNL-HNNXBMFYSA-N 0.000 description 1
- 229960001882 dexchlorpheniramine Drugs 0.000 description 1
- SOYKEARSMXGVTM-HNNXBMFYSA-N dexchlorpheniramine Chemical compound C1([C@H](CCN(C)C)C=2N=CC=CC=2)=CC=C(Cl)C=C1 SOYKEARSMXGVTM-HNNXBMFYSA-N 0.000 description 1
- 229960003428 dexibuprofen Drugs 0.000 description 1
- HEFNNWSXXWATRW-JTQLQIEISA-N dexibuprofen Chemical compound CC(C)CC1=CC=C([C@H](C)C(O)=O)C=C1 HEFNNWSXXWATRW-JTQLQIEISA-N 0.000 description 1
- 229960002783 dexketoprofen Drugs 0.000 description 1
- DKYWVDODHFEZIM-NSHDSACASA-N dexketoprofen Chemical compound OC(=O)[C@@H](C)C1=CC=CC(C(=O)C=2C=CC=CC=2)=C1 DKYWVDODHFEZIM-NSHDSACASA-N 0.000 description 1
- 229960001042 dexmethylphenidate Drugs 0.000 description 1
- DUGOZIWVEXMGBE-CHWSQXEVSA-N dexmethylphenidate Chemical compound C([C@@H]1[C@H](C(=O)OC)C=2C=CC=CC=2)CCCN1 DUGOZIWVEXMGBE-CHWSQXEVSA-N 0.000 description 1
- 229960001985 dextromethorphan Drugs 0.000 description 1
- NIJJYAXOARWZEE-UHFFFAOYSA-N di-n-propyl-acetic acid Natural products CCCC(C(O)=O)CCC NIJJYAXOARWZEE-UHFFFAOYSA-N 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- 229960001673 diethyltoluamide Drugs 0.000 description 1
- 238000000113 differential scanning calorimetry Methods 0.000 description 1
- 229960004154 diflorasone Drugs 0.000 description 1
- 229960002124 diflorasone diacetate Drugs 0.000 description 1
- 229960004091 diflucortolone Drugs 0.000 description 1
- OGPWIDANBSLJPC-RFPWEZLHSA-N diflucortolone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@H](C(=O)CO)[C@@]2(C)C[C@@H]1O OGPWIDANBSLJPC-RFPWEZLHSA-N 0.000 description 1
- 229960003970 diflucortolone valerate Drugs 0.000 description 1
- HUPFGZXOMWLGNK-UHFFFAOYSA-N diflunisal Chemical compound C1=C(O)C(C(=O)O)=CC(C=2C(=CC(F)=CC=2)F)=C1 HUPFGZXOMWLGNK-UHFFFAOYSA-N 0.000 description 1
- 229960000616 diflunisal Drugs 0.000 description 1
- QONQRTHLHBTMGP-UHFFFAOYSA-N digitoxigenin Natural products CC12CCC(C3(CCC(O)CC3CC3)C)C3C11OC1CC2C1=CC(=O)OC1 QONQRTHLHBTMGP-UHFFFAOYSA-N 0.000 description 1
- WDJUZGPOPHTGOT-XUDUSOBPSA-N digitoxin Chemical compound C1[C@H](O)[C@H](O)[C@@H](C)O[C@H]1O[C@@H]1[C@@H](C)O[C@@H](O[C@@H]2[C@H](O[C@@H](O[C@@H]3C[C@@H]4[C@]([C@@H]5[C@H]([C@]6(CC[C@@H]([C@@]6(C)CC5)C=5COC(=O)C=5)O)CC4)(C)CC3)C[C@@H]2O)C)C[C@@H]1O WDJUZGPOPHTGOT-XUDUSOBPSA-N 0.000 description 1
- 229960000648 digitoxin Drugs 0.000 description 1
- SHIBSTMRCDJXLN-KCZCNTNESA-N digoxigenin Chemical compound C1([C@@H]2[C@@]3([C@@](CC2)(O)[C@H]2[C@@H]([C@@]4(C)CC[C@H](O)C[C@H]4CC2)C[C@H]3O)C)=CC(=O)OC1 SHIBSTMRCDJXLN-KCZCNTNESA-N 0.000 description 1
- LTMHDMANZUZIPE-PUGKRICDSA-N digoxin Chemical compound C1[C@H](O)[C@H](O)[C@@H](C)O[C@H]1O[C@@H]1[C@@H](C)O[C@@H](O[C@@H]2[C@H](O[C@@H](O[C@@H]3C[C@@H]4[C@]([C@@H]5[C@H]([C@]6(CC[C@@H]([C@@]6(C)[C@H](O)C5)C=5COC(=O)C=5)O)CC4)(C)CC3)C[C@@H]2O)C)C[C@@H]1O LTMHDMANZUZIPE-PUGKRICDSA-N 0.000 description 1
- 229960005156 digoxin Drugs 0.000 description 1
- LTMHDMANZUZIPE-UHFFFAOYSA-N digoxine Natural products C1C(O)C(O)C(C)OC1OC1C(C)OC(OC2C(OC(OC3CC4C(C5C(C6(CCC(C6(C)C(O)C5)C=5COC(=O)C=5)O)CC4)(C)CC3)CC2O)C)CC1O LTMHDMANZUZIPE-UHFFFAOYSA-N 0.000 description 1
- XYYVYLMBEZUESM-UHFFFAOYSA-N dihydrocodeine Natural products C1C(N(CCC234)C)C2C=CC(=O)C3OC2=C4C1=CC=C2OC XYYVYLMBEZUESM-UHFFFAOYSA-N 0.000 description 1
- 229940031578 diisopropyl adipate Drugs 0.000 description 1
- HSUGRBWQSSZJOP-RTWAWAEBSA-N diltiazem Chemical compound C1=CC(OC)=CC=C1[C@H]1[C@@H](OC(C)=O)C(=O)N(CCN(C)C)C2=CC=CC=C2S1 HSUGRBWQSSZJOP-RTWAWAEBSA-N 0.000 description 1
- 229960004166 diltiazem Drugs 0.000 description 1
- 229960004993 dimenhydrinate Drugs 0.000 description 1
- MZDOIJOUFRQXHC-UHFFFAOYSA-N dimenhydrinate Chemical compound O=C1N(C)C(=O)N(C)C2=NC(Cl)=N[C]21.C=1C=CC=CC=1C(OCCN(C)C)C1=CC=CC=C1 MZDOIJOUFRQXHC-UHFFFAOYSA-N 0.000 description 1
- 229940008099 dimethicone Drugs 0.000 description 1
- 229960001992 dimetindene Drugs 0.000 description 1
- MVMQESMQSYOVGV-UHFFFAOYSA-N dimetindene Chemical compound CN(C)CCC=1CC2=CC=CC=C2C=1C(C)C1=CC=CC=N1 MVMQESMQSYOVGV-UHFFFAOYSA-N 0.000 description 1
- 238000003618 dip coating Methods 0.000 description 1
- SZXQTJUDPRGNJN-UHFFFAOYSA-N dipropylene glycol Chemical compound OCCCOCCCO SZXQTJUDPRGNJN-UHFFFAOYSA-N 0.000 description 1
- 229940113120 dipropylene glycol Drugs 0.000 description 1
- 229960002768 dipyridamole Drugs 0.000 description 1
- IZEKFCXSFNUWAM-UHFFFAOYSA-N dipyridamole Chemical compound C=12N=C(N(CCO)CCO)N=C(N3CCCCC3)C2=NC(N(CCO)CCO)=NC=1N1CCCCC1 IZEKFCXSFNUWAM-UHFFFAOYSA-N 0.000 description 1
- 229940019332 direct factor xa inhibitors Drugs 0.000 description 1
- 208000037765 diseases and disorders Diseases 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- 239000004815 dispersion polymer Substances 0.000 description 1
- DLNKOYKMWOXYQA-UHFFFAOYSA-N dl-pseudophenylpropanolamine Natural products CC(N)C(O)C1=CC=CC=C1 DLNKOYKMWOXYQA-UHFFFAOYSA-N 0.000 description 1
- PRAKJMSDJKAYCZ-UHFFFAOYSA-N dodecahydrosqualene Natural products CC(C)CCCC(C)CCCC(C)CCCCC(C)CCCC(C)CCCC(C)C PRAKJMSDJKAYCZ-UHFFFAOYSA-N 0.000 description 1
- LQZZUXJYWNFBMV-UHFFFAOYSA-N dodecan-1-ol Chemical compound CCCCCCCCCCCCO LQZZUXJYWNFBMV-UHFFFAOYSA-N 0.000 description 1
- LLRANSBEYQZKFY-UHFFFAOYSA-N dodecanoic acid;propane-1,2-diol Chemical compound CC(O)CO.CCCCCCCCCCCC(O)=O LLRANSBEYQZKFY-UHFFFAOYSA-N 0.000 description 1
- QQQMUBLXDAFBRH-UHFFFAOYSA-N dodecyl 2-hydroxypropanoate Chemical compound CCCCCCCCCCCCOC(=O)C(C)O QQQMUBLXDAFBRH-UHFFFAOYSA-N 0.000 description 1
- 229960001850 droxicam Drugs 0.000 description 1
- OEHFRZLKGRKFAS-UHFFFAOYSA-N droxicam Chemical compound C12=CC=CC=C2S(=O)(=O)N(C)C(C2=O)=C1OC(=O)N2C1=CC=CC=N1 OEHFRZLKGRKFAS-UHFFFAOYSA-N 0.000 description 1
- 238000009513 drug distribution Methods 0.000 description 1
- 229960002866 duloxetine Drugs 0.000 description 1
- 229960001971 ebastine Drugs 0.000 description 1
- MJJALKDDGIKVBE-UHFFFAOYSA-N ebastine Chemical compound C1=CC(C(C)(C)C)=CC=C1C(=O)CCCN1CCC(OC(C=2C=CC=CC=2)C=2C=CC=CC=2)CC1 MJJALKDDGIKVBE-UHFFFAOYSA-N 0.000 description 1
- 229960000622 edoxaban Drugs 0.000 description 1
- PSMMNJNZVZZNOI-SJILXJHISA-N edoxaban tosylate hydrate Chemical compound O.CC1=CC=C(S(O)(=O)=O)C=C1.N([C@H]1CC[C@@H](C[C@H]1NC(=O)C=1SC=2CN(C)CCC=2N=1)C(=O)N(C)C)C(=O)C(=O)NC1=CC=C(Cl)C=N1 PSMMNJNZVZZNOI-SJILXJHISA-N 0.000 description 1
- QYDYPVFESGNLHU-UHFFFAOYSA-N elaidic acid methyl ester Natural products CCCCCCCCC=CCCCCCCCC(=O)OC QYDYPVFESGNLHU-UHFFFAOYSA-N 0.000 description 1
- 239000000806 elastomer Substances 0.000 description 1
- 238000005370 electroosmosis Methods 0.000 description 1
- URSRSKSNFPUKGH-UHFFFAOYSA-N embramine Chemical compound C=1C=C(Br)C=CC=1C(C)(OCCN(C)C)C1=CC=CC=C1 URSRSKSNFPUKGH-UHFFFAOYSA-N 0.000 description 1
- 229950000472 embramine Drugs 0.000 description 1
- 239000003974 emollient agent Substances 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 230000003511 endothelial effect Effects 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 229940088598 enzyme Drugs 0.000 description 1
- 229960002179 ephedrine Drugs 0.000 description 1
- RINBGYCKMGDWPY-UHFFFAOYSA-N epitizide Chemical compound C1=C(Cl)C(S(=O)(=O)N)=CC2=C1NC(CSCC(F)(F)F)NS2(=O)=O RINBGYCKMGDWPY-UHFFFAOYSA-N 0.000 description 1
- 229950010350 epitizide Drugs 0.000 description 1
- 229950007830 eribaxaban Drugs 0.000 description 1
- QQBKAVAGLMGMHI-WIYYLYMNSA-N eribaxaban Chemical compound N1([C@H](C[C@H](C1)OC)C(=O)NC=1C(=CC(=CC=1)N1C(C=CC=C1)=O)F)C(=O)NC1=CC=C(Cl)C=C1 QQBKAVAGLMGMHI-WIYYLYMNSA-N 0.000 description 1
- 208000019501 erythrocyte disease Diseases 0.000 description 1
- RONZAEMNMFQXRA-MRXNPFEDSA-N esmirtazapine Chemical compound C1C2=CC=CN=C2N2CCN(C)C[C@@H]2C2=CC=CC=C21 RONZAEMNMFQXRA-MRXNPFEDSA-N 0.000 description 1
- 229950002566 esmirtazapine Drugs 0.000 description 1
- 235000010944 ethyl methyl cellulose Nutrition 0.000 description 1
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 1
- 229940093471 ethyl oleate Drugs 0.000 description 1
- 239000004715 ethylene vinyl alcohol Substances 0.000 description 1
- 229960005293 etodolac Drugs 0.000 description 1
- XFBVBWWRPKNWHW-UHFFFAOYSA-N etodolac Chemical compound C1COC(CC)(CC(O)=O)C2=N[C]3C(CC)=CC=CC3=C21 XFBVBWWRPKNWHW-UHFFFAOYSA-N 0.000 description 1
- 239000010642 eucalyptus oil Substances 0.000 description 1
- 229940044949 eucalyptus oil Drugs 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 229950000484 exisulind Drugs 0.000 description 1
- 238000013265 extended release Methods 0.000 description 1
- 150000002190 fatty acyls Chemical group 0.000 description 1
- 150000002194 fatty esters Chemical class 0.000 description 1
- 150000002195 fatty ethers Chemical class 0.000 description 1
- 229950003499 fibrin Drugs 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000010579 first pass effect Methods 0.000 description 1
- 229930003935 flavonoid Natural products 0.000 description 1
- 150000002215 flavonoids Chemical class 0.000 description 1
- 235000017173 flavonoids Nutrition 0.000 description 1
- 229920005570 flexible polymer Polymers 0.000 description 1
- LPEPZBJOKDYZAD-UHFFFAOYSA-N flufenamic acid Chemical compound OC(=O)C1=CC=CC=C1NC1=CC=CC(C(F)(F)F)=C1 LPEPZBJOKDYZAD-UHFFFAOYSA-N 0.000 description 1
- 229960004369 flufenamic acid Drugs 0.000 description 1
- 229960000676 flunisolide Drugs 0.000 description 1
- 229960001347 fluocinolone acetonide Drugs 0.000 description 1
- FEBLZLNTKCEFIT-VSXGLTOVSA-N fluocinolone acetonide Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O FEBLZLNTKCEFIT-VSXGLTOVSA-N 0.000 description 1
- 229960003973 fluocortolone Drugs 0.000 description 1
- 125000001153 fluoro group Chemical group F* 0.000 description 1
- 229960003667 flupirtine Drugs 0.000 description 1
- 229960003238 fluprednidene Drugs 0.000 description 1
- YVHXHNGGPURVOS-SBTDHBFYSA-N fluprednidene Chemical compound O=C1C=C[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@](C(=C)C4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 YVHXHNGGPURVOS-SBTDHBFYSA-N 0.000 description 1
- 229960002390 flurbiprofen Drugs 0.000 description 1
- SYTBZMRGLBWNTM-UHFFFAOYSA-N flurbiprofen Chemical compound FC1=CC(C(C(O)=O)C)=CC=C1C1=CC=CC=C1 SYTBZMRGLBWNTM-UHFFFAOYSA-N 0.000 description 1
- 229960002714 fluticasone Drugs 0.000 description 1
- MGNNYOODZCAHBA-GQKYHHCASA-N fluticasone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(O)[C@@]2(C)C[C@@H]1O MGNNYOODZCAHBA-GQKYHHCASA-N 0.000 description 1
- 229960001318 fondaparinux Drugs 0.000 description 1
- KANJSNBRCNMZMV-ABRZTLGGSA-N fondaparinux Chemical compound O[C@@H]1[C@@H](NS(O)(=O)=O)[C@@H](OC)O[C@H](COS(O)(=O)=O)[C@H]1O[C@H]1[C@H](OS(O)(=O)=O)[C@@H](O)[C@H](O[C@@H]2[C@@H]([C@@H](OS(O)(=O)=O)[C@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O[C@@H]4[C@@H]([C@@H](O)[C@H](O)[C@@H](COS(O)(=O)=O)O4)NS(O)(=O)=O)[C@H](O3)C(O)=O)O)[C@@H](COS(O)(=O)=O)O2)NS(O)(=O)=O)[C@H](C(O)=O)O1 KANJSNBRCNMZMV-ABRZTLGGSA-N 0.000 description 1
- 210000001061 forehead Anatomy 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 239000012737 fresh medium Substances 0.000 description 1
- 229950011013 funapide Drugs 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 229960002870 gabapentin Drugs 0.000 description 1
- 229910052733 gallium Inorganic materials 0.000 description 1
- WIGCFUFOHFEKBI-UHFFFAOYSA-N gamma-tocopherol Natural products CC(C)CCCC(C)CCCC(C)CCCC1CCC2C(C)C(O)C(C)C(C)C2O1 WIGCFUFOHFEKBI-UHFFFAOYSA-N 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 239000003349 gelling agent Substances 0.000 description 1
- 229930182494 ginsenoside Natural products 0.000 description 1
- 229960003180 glutathione Drugs 0.000 description 1
- 235000003969 glutathione Nutrition 0.000 description 1
- YQEMORVAKMFKLG-UHFFFAOYSA-N glycerine monostearate Natural products CCCCCCCCCCCCCCCCCC(=O)OC(CO)CO YQEMORVAKMFKLG-UHFFFAOYSA-N 0.000 description 1
- SVUQHVRAGMNPLW-UHFFFAOYSA-N glycerol monostearate Natural products CCCCCCCCCCCCCCCCC(=O)OCC(O)CO SVUQHVRAGMNPLW-UHFFFAOYSA-N 0.000 description 1
- 229940087068 glyceryl caprylate Drugs 0.000 description 1
- 125000003976 glyceryl group Chemical group [H]C([*])([H])C(O[H])([H])C(O[H])([H])[H] 0.000 description 1
- 208000007345 glycogen storage disease Diseases 0.000 description 1
- 229940093915 gynecological organic acid Drugs 0.000 description 1
- 229960002383 halcinonide Drugs 0.000 description 1
- 235000015220 hamburgers Nutrition 0.000 description 1
- 210000003128 head Anatomy 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 239000001307 helium Substances 0.000 description 1
- 229910052734 helium Inorganic materials 0.000 description 1
- SWQJXJOGLNCZEY-UHFFFAOYSA-N helium atom Chemical compound [He] SWQJXJOGLNCZEY-UHFFFAOYSA-N 0.000 description 1
- 208000014951 hematologic disease Diseases 0.000 description 1
- 108010005808 hementin Proteins 0.000 description 1
- 208000007475 hemolytic anemia Diseases 0.000 description 1
- 230000002440 hepatic effect Effects 0.000 description 1
- RZXDTJIXPSCHCI-UHFFFAOYSA-N hexa-1,5-diene-2,5-diol Chemical compound OC(=C)CCC(O)=C RZXDTJIXPSCHCI-UHFFFAOYSA-N 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-M hexanoate Chemical compound CCCCCC([O-])=O FUZZWVXGSFPDMH-UHFFFAOYSA-M 0.000 description 1
- 229940051250 hexylene glycol Drugs 0.000 description 1
- 229940006607 hirudin Drugs 0.000 description 1
- WQPDUTSPKFMPDP-OUMQNGNKSA-N hirudin Chemical compound C([C@@H](C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC(OS(O)(=O)=O)=CC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCCN)NC(=O)[C@H]1N(CCC1)C(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H]2CSSC[C@@H](C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(=O)N[C@H](C(NCC(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N2)=O)CSSC1)C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]1NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=2C=CC(O)=CC=2)NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)C(C)C)[C@@H](C)O)CSSC1)C(C)C)[C@@H](C)O)[C@@H](C)O)C1=CC=CC=C1 WQPDUTSPKFMPDP-OUMQNGNKSA-N 0.000 description 1
- 210000003630 histaminocyte Anatomy 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 208000033519 human immunodeficiency virus infectious disease Diseases 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 229960002003 hydrochlorothiazide Drugs 0.000 description 1
- LLPOLZWFYMWNKH-CMKMFDCUSA-N hydrocodone Chemical compound C([C@H]1[C@H](N(CC[C@@]112)C)C3)CC(=O)[C@@H]1OC1=C2C3=CC=C1OC LLPOLZWFYMWNKH-CMKMFDCUSA-N 0.000 description 1
- 229960000240 hydrocodone Drugs 0.000 description 1
- 239000000416 hydrocolloid Substances 0.000 description 1
- 229960001067 hydrocortisone acetate Drugs 0.000 description 1
- 229920001477 hydrophilic polymer Polymers 0.000 description 1
- 150000004679 hydroxides Chemical class 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 1
- 229960000930 hydroxyzine Drugs 0.000 description 1
- ZQDWXGKKHFNSQK-UHFFFAOYSA-N hydroxyzine Chemical compound C1CN(CCOCCO)CCN1C(C=1C=CC(Cl)=CC=1)C1=CC=CC=C1 ZQDWXGKKHFNSQK-UHFFFAOYSA-N 0.000 description 1
- 230000007954 hypoxia Effects 0.000 description 1
- TZJALUIVHRYQQB-XLRXWWTNSA-N icariin Chemical compound C1=CC(OC)=CC=C1C1=C(O[C@H]2[C@@H]([C@H](O)[C@@H](O)[C@H](C)O2)O)C(=O)C2=C(O)C=C(O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O3)O)C(CC=C(C)C)=C2O1 TZJALUIVHRYQQB-XLRXWWTNSA-N 0.000 description 1
- TZJALUIVHRYQQB-UHFFFAOYSA-N icariine Natural products C1=CC(OC)=CC=C1C1=C(OC2C(C(O)C(O)C(C)O2)O)C(=O)C2=C(O)C=C(OC3C(C(O)C(O)C(CO)O3)O)C(CC=C(C)C)=C2O1 TZJALUIVHRYQQB-UHFFFAOYSA-N 0.000 description 1
- 229960002240 iloprost Drugs 0.000 description 1
- HIFJCPQKFCZDDL-ACWOEMLNSA-N iloprost Chemical compound C1\C(=C/CCCC(O)=O)C[C@@H]2[C@@H](/C=C/[C@@H](O)C(C)CC#CC)[C@H](O)C[C@@H]21 HIFJCPQKFCZDDL-ACWOEMLNSA-N 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 229960000905 indomethacin Drugs 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 238000001746 injection moulding Methods 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 238000009434 installation Methods 0.000 description 1
- 239000000543 intermediate Substances 0.000 description 1
- 230000002154 ionophoretic effect Effects 0.000 description 1
- 208000028867 ischemia Diseases 0.000 description 1
- 208000023589 ischemic disease Diseases 0.000 description 1
- WYXXLXHHWYNKJF-UHFFFAOYSA-N isocarvacrol Natural products CC(C)C1=CC=C(O)C(C)=C1 WYXXLXHHWYNKJF-UHFFFAOYSA-N 0.000 description 1
- 229940074928 isopropyl myristate Drugs 0.000 description 1
- JJWLVOIRVHMVIS-UHFFFAOYSA-N isopropylamine Chemical compound CC(C)N JJWLVOIRVHMVIS-UHFFFAOYSA-N 0.000 description 1
- 239000000644 isotonic solution Substances 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 229950002252 isoxicam Drugs 0.000 description 1
- YYUAYBYLJSNDCX-UHFFFAOYSA-N isoxicam Chemical compound OC=1C2=CC=CC=C2S(=O)(=O)N(C)C=1C(=O)NC=1C=C(C)ON=1 YYUAYBYLJSNDCX-UHFFFAOYSA-N 0.000 description 1
- 229940090046 jet injector Drugs 0.000 description 1
- 235000010494 karaya gum Nutrition 0.000 description 1
- 239000000231 karaya gum Substances 0.000 description 1
- 229940039371 karaya gum Drugs 0.000 description 1
- 229960003299 ketamine Drugs 0.000 description 1
- DKYWVDODHFEZIM-UHFFFAOYSA-N ketoprofen Chemical compound OC(=O)C(C)C1=CC=CC(C(=O)C=2C=CC=CC=2)=C1 DKYWVDODHFEZIM-UHFFFAOYSA-N 0.000 description 1
- 229960000991 ketoprofen Drugs 0.000 description 1
- 229960004958 ketotifen Drugs 0.000 description 1
- 201000006370 kidney failure Diseases 0.000 description 1
- 229940039717 lanolin Drugs 0.000 description 1
- 235000019388 lanolin Nutrition 0.000 description 1
- 229940031674 laureth-7 Drugs 0.000 description 1
- 125000000400 lauroyl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- OTQCKZUSUGYWBD-BRHMIFOHSA-N lepirudin Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CS)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O)C(C)C)[C@@H](C)O)[C@@H](C)O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC(C)C)[C@@H](C)O)C1=CC=C(O)C=C1 OTQCKZUSUGYWBD-BRHMIFOHSA-N 0.000 description 1
- 229960004408 lepirudin Drugs 0.000 description 1
- 229950001775 letaxaban Drugs 0.000 description 1
- LEBVLXFERQHONN-INIZCTEOSA-N levobupivacaine Chemical compound CCCCN1CCCC[C@H]1C(=O)NC1=C(C)C=CC=C1C LEBVLXFERQHONN-INIZCTEOSA-N 0.000 description 1
- 229960004288 levobupivacaine Drugs 0.000 description 1
- 229960001120 levocabastine Drugs 0.000 description 1
- ZCGOMHNNNFPNMX-KYTRFIICSA-N levocabastine Chemical compound C1([C@@]2(C(O)=O)CCN(C[C@H]2C)[C@@H]2CC[C@@](CC2)(C#N)C=2C=CC(F)=CC=2)=CC=CC=C1 ZCGOMHNNNFPNMX-KYTRFIICSA-N 0.000 description 1
- 229960000685 levomilnacipran Drugs 0.000 description 1
- UAWXGRJVZSAUSZ-UHFFFAOYSA-N licofelone Chemical compound OC(=O)CC=1N2CC(C)(C)CC2=C(C=2C=CC=CC=2)C=1C1=CC=C(Cl)C=C1 UAWXGRJVZSAUSZ-UHFFFAOYSA-N 0.000 description 1
- 229950003488 licofelone Drugs 0.000 description 1
- 235000001510 limonene Nutrition 0.000 description 1
- 229940087305 limonene Drugs 0.000 description 1
- 235000020778 linoleic acid Nutrition 0.000 description 1
- OYHQOLUKZRVURQ-IXWMQOLASA-N linoleic acid Natural products CCCCC\C=C/C\C=C\CCCCCCCC(O)=O OYHQOLUKZRVURQ-IXWMQOLASA-N 0.000 description 1
- 239000012669 liquid formulation Substances 0.000 description 1
- VOBHXZCDAVEXEY-JSGCOSHPSA-N lisdexamfetamine Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](C)CC1=CC=CC=C1 VOBHXZCDAVEXEY-JSGCOSHPSA-N 0.000 description 1
- 229960001451 lisdexamfetamine Drugs 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- OXROWJKCGCOJDO-JLHYYAGUSA-N lornoxicam Chemical compound O=C1C=2SC(Cl)=CC=2S(=O)(=O)N(C)\C1=C(\O)NC1=CC=CC=N1 OXROWJKCGCOJDO-JLHYYAGUSA-N 0.000 description 1
- 229960002202 lornoxicam Drugs 0.000 description 1
- 239000003055 low molecular weight heparin Substances 0.000 description 1
- 229940127215 low-molecular weight heparin Drugs 0.000 description 1
- 229960002373 loxoprofen Drugs 0.000 description 1
- BAZQYVYVKYOAGO-UHFFFAOYSA-M loxoprofen sodium hydrate Chemical compound O.O.[Na+].C1=CC(C(C([O-])=O)C)=CC=C1CC1C(=O)CCC1 BAZQYVYVKYOAGO-UHFFFAOYSA-M 0.000 description 1
- 229960000994 lumiracoxib Drugs 0.000 description 1
- KHPKQFYUPIUARC-UHFFFAOYSA-N lumiracoxib Chemical compound OC(=O)CC1=CC(C)=CC=C1NC1=C(F)C=CC=C1Cl KHPKQFYUPIUARC-UHFFFAOYSA-N 0.000 description 1
- JGCMEBMXRHSZKX-UHFFFAOYSA-N macitentan Chemical compound C=1C=C(Br)C=CC=1C=1C(NS(=O)(=O)NCCC)=NC=NC=1OCCOC1=NC=C(Br)C=N1 JGCMEBMXRHSZKX-UHFFFAOYSA-N 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- TTZNQDOUNXBMJV-UHFFFAOYSA-N mavacoxib Chemical compound C1=CC(S(=O)(=O)N)=CC=C1N1C(C=2C=CC(F)=CC=2)=CC(C(F)(F)F)=N1 TTZNQDOUNXBMJV-UHFFFAOYSA-N 0.000 description 1
- 229950007241 mavacoxib Drugs 0.000 description 1
- 231100000682 maximum tolerated dose Toxicity 0.000 description 1
- 229960003803 meclofenamic acid Drugs 0.000 description 1
- 229940057917 medium chain triglycerides Drugs 0.000 description 1
- 229960003464 mefenamic acid Drugs 0.000 description 1
- 229960004794 melitracen Drugs 0.000 description 1
- GWWLWDURRGNSRS-UHFFFAOYSA-N melitracen Chemical compound C1=CC=C2C(=CCCN(C)C)C3=CC=CC=C3C(C)(C)C2=C1 GWWLWDURRGNSRS-UHFFFAOYSA-N 0.000 description 1
- 229960001929 meloxicam Drugs 0.000 description 1
- 229930007503 menthone Natural products 0.000 description 1
- 208000030159 metabolic disease Diseases 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 238000006241 metabolic reaction Methods 0.000 description 1
- GKKDCARASOJPNG-UHFFFAOYSA-N metaldehyde Chemical compound CC1OC(C)OC(C)OC(C)O1 GKKDCARASOJPNG-UHFFFAOYSA-N 0.000 description 1
- 239000011140 metalized polyester Substances 0.000 description 1
- MYWUZJCMWCOHBA-VIFPVBQESA-N methamphetamine Chemical class CN[C@@H](C)CC1=CC=CC=C1 MYWUZJCMWCOHBA-VIFPVBQESA-N 0.000 description 1
- NVGOUBIJVPSVSL-UHFFFAOYSA-N methyl 2-(4-aminophenyl)-1-oxo-7-(pyridin-2-ylmethoxy)-4-(3,4,5-trimethoxyphenyl)isoquinoline-3-carboxylate;sulfuric acid Chemical compound OS(O)(=O)=O.C12=CC=C(OCC=3N=CC=CC=3)C=C2C(=O)N(C=2C=CC(N)=CC=2)C(C(=O)OC)=C1C1=CC(OC)=C(OC)C(OC)=C1 NVGOUBIJVPSVSL-UHFFFAOYSA-N 0.000 description 1
- QYDYPVFESGNLHU-KHPPLWFESA-N methyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC QYDYPVFESGNLHU-KHPPLWFESA-N 0.000 description 1
- 229940073769 methyl oleate Drugs 0.000 description 1
- 229920003087 methylethyl cellulose Polymers 0.000 description 1
- AQCHWTWZEMGIFD-UHFFFAOYSA-N metolazone Chemical compound CC1NC2=CC(Cl)=C(S(N)(=O)=O)C=C2C(=O)N1C1=CC=CC=C1C AQCHWTWZEMGIFD-UHFFFAOYSA-N 0.000 description 1
- 229960002817 metolazone Drugs 0.000 description 1
- 229960003404 mexiletine Drugs 0.000 description 1
- 239000013081 microcrystal Substances 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 229960000600 milnacipran Drugs 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 229950002245 mirodenafil Drugs 0.000 description 1
- RONZAEMNMFQXRA-UHFFFAOYSA-N mirtazapine Chemical compound C1C2=CC=CN=C2N2CCN(C)CC2C2=CC=CC=C21 RONZAEMNMFQXRA-UHFFFAOYSA-N 0.000 description 1
- 229960001785 mirtazapine Drugs 0.000 description 1
- 229960001144 mizolastine Drugs 0.000 description 1
- 229960001165 modafinil Drugs 0.000 description 1
- 230000001333 moisturizer Effects 0.000 description 1
- NJTGANWAUPEOAX-UHFFFAOYSA-N molport-023-220-454 Chemical compound OCC(O)CO.OCC(O)CO NJTGANWAUPEOAX-UHFFFAOYSA-N 0.000 description 1
- RZRNAYUHWVFMIP-UHFFFAOYSA-N monoelaidin Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC(O)CO RZRNAYUHWVFMIP-UHFFFAOYSA-N 0.000 description 1
- 229940074096 monoolein Drugs 0.000 description 1
- 229930003658 monoterpene Natural products 0.000 description 1
- 229960005181 morphine Drugs 0.000 description 1
- 235000011929 mousse Nutrition 0.000 description 1
- AJFDBNQQDYLMJN-UHFFFAOYSA-N n,n-diethylacetamide Chemical compound CCN(CC)C(C)=O AJFDBNQQDYLMJN-UHFFFAOYSA-N 0.000 description 1
- IDFANOPDMXWIOP-UHFFFAOYSA-N n,n-dimethylpentan-1-amine Chemical compound CCCCCN(C)C IDFANOPDMXWIOP-UHFFFAOYSA-N 0.000 description 1
- 229960005016 naphazoline Drugs 0.000 description 1
- 239000007923 nasal drop Substances 0.000 description 1
- 229940100662 nasal drops Drugs 0.000 description 1
- 229940097496 nasal spray Drugs 0.000 description 1
- 239000007922 nasal spray Substances 0.000 description 1
- 229960000751 nefopam Drugs 0.000 description 1
- PPEKGEBBBBNZKS-HGRQIUPRSA-N neosaxitoxin Chemical compound N=C1N(O)[C@@H](COC(=O)N)[C@@H]2NC(=N)N[C@@]22C(O)(O)CCN21 PPEKGEBBBBNZKS-HGRQIUPRSA-N 0.000 description 1
- WASNIKZYIWZQIP-AWEZNQCLSA-N nerolidol Natural products CC(=CCCC(=CCC[C@@H](O)C=C)C)C WASNIKZYIWZQIP-AWEZNQCLSA-N 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 201000004931 neurofibromatosis Diseases 0.000 description 1
- 229960002715 nicotine Drugs 0.000 description 1
- SNICXCGAKADSCV-UHFFFAOYSA-N nicotine Natural products CN1CCCC1C1=CC=CN=C1 SNICXCGAKADSCV-UHFFFAOYSA-N 0.000 description 1
- 229960001597 nifedipine Drugs 0.000 description 1
- HYIMSNHJOBLJNT-UHFFFAOYSA-N nifedipine Chemical compound COC(=O)C1=C(C)NC(C)=C(C(=O)OC)C1C1=CC=CC=C1[N+]([O-])=O HYIMSNHJOBLJNT-UHFFFAOYSA-N 0.000 description 1
- 229960000916 niflumic acid Drugs 0.000 description 1
- 229960000965 nimesulide Drugs 0.000 description 1
- HYWYRSMBCFDLJT-UHFFFAOYSA-N nimesulide Chemical compound CS(=O)(=O)NC1=CC=C([N+]([O-])=O)C=C1OC1=CC=CC=C1 HYWYRSMBCFDLJT-UHFFFAOYSA-N 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 150000002825 nitriles Chemical class 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 125000004433 nitrogen atom Chemical group N* 0.000 description 1
- MVPQUSQUURLQKF-MCPDASDXSA-E nonasodium;(2s,3s,4s,5r,6r)-6-[(2r,3r,4s,5r,6r)-6-[(2r,3s,4s,5r,6r)-2-carboxylato-4,5-dimethoxy-6-[(2r,3r,4s,5r,6s)-6-methoxy-4,5-disulfonatooxy-2-(sulfonatooxymethyl)oxan-3-yl]oxyoxan-3-yl]oxy-4,5-disulfonatooxy-2-(sulfonatooxymethyl)oxan-3-yl]oxy-4,5-di Chemical compound [Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[O-]S(=O)(=O)O[C@@H]1[C@@H](OS([O-])(=O)=O)[C@@H](OC)O[C@H](COS([O-])(=O)=O)[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@@H]2[C@@H]([C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3[C@@H]([C@@H](OC)[C@H](O[C@@H]4[C@@H]([C@@H](OC)[C@H](OC)[C@@H](COS([O-])(=O)=O)O4)OC)[C@H](O3)C([O-])=O)OC)[C@@H](COS([O-])(=O)=O)O2)OS([O-])(=O)=O)[C@H](C([O-])=O)O1 MVPQUSQUURLQKF-MCPDASDXSA-E 0.000 description 1
- 229940053973 novocaine Drugs 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- KSCKTBJJRVPGKM-UHFFFAOYSA-N octan-1-olate;titanium(4+) Chemical compound [Ti+4].CCCCCCCC[O-].CCCCCCCC[O-].CCCCCCCC[O-].CCCCCCCC[O-] KSCKTBJJRVPGKM-UHFFFAOYSA-N 0.000 description 1
- WWZKQHOCKIZLMA-UHFFFAOYSA-N octanoic acid Chemical compound CCCCCCCC(O)=O WWZKQHOCKIZLMA-UHFFFAOYSA-N 0.000 description 1
- 229960001679 octinoxate Drugs 0.000 description 1
- YAGMLECKUBJRNO-UHFFFAOYSA-N octyl 4-(dimethylamino)benzoate Chemical compound CCCCCCCCOC(=O)C1=CC=C(N(C)C)C=C1 YAGMLECKUBJRNO-UHFFFAOYSA-N 0.000 description 1
- 239000003883 ointment base Substances 0.000 description 1
- BARWIPMJPCRCTP-UHFFFAOYSA-N oleic acid oleyl ester Natural products CCCCCCCCC=CCCCCCCCCOC(=O)CCCCCCCC=CCCCCCCCC BARWIPMJPCRCTP-UHFFFAOYSA-N 0.000 description 1
- BARWIPMJPCRCTP-CLFAGFIQSA-N oleyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCCOC(=O)CCCCCCC\C=C/CCCCCCCC BARWIPMJPCRCTP-CLFAGFIQSA-N 0.000 description 1
- 229940005483 opioid analgesics Drugs 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- OFPXSFXSNFPTHF-UHFFFAOYSA-N oxaprozin Chemical compound O1C(CCC(=O)O)=NC(C=2C=CC=CC=2)=C1C1=CC=CC=C1 OFPXSFXSNFPTHF-UHFFFAOYSA-N 0.000 description 1
- 229960002739 oxaprozin Drugs 0.000 description 1
- 229960003684 oxedrine Drugs 0.000 description 1
- 229960002085 oxycodone Drugs 0.000 description 1
- 238000002640 oxygen therapy Methods 0.000 description 1
- 229960001528 oxymetazoline Drugs 0.000 description 1
- 229940094443 oxytocics prostaglandins Drugs 0.000 description 1
- 210000000496 pancreas Anatomy 0.000 description 1
- 206010033675 panniculitis Diseases 0.000 description 1
- 229960001789 papaverine Drugs 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 229960004662 parecoxib Drugs 0.000 description 1
- TZRHLKRLEZJVIJ-UHFFFAOYSA-N parecoxib Chemical compound C1=CC(S(=O)(=O)NC(=O)CC)=CC=C1C1=C(C)ON=C1C1=CC=CC=C1 TZRHLKRLEZJVIJ-UHFFFAOYSA-N 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 230000032696 parturition Effects 0.000 description 1
- 229920001277 pectin Polymers 0.000 description 1
- 235000010987 pectin Nutrition 0.000 description 1
- 239000001814 pectin Substances 0.000 description 1
- WCVRQHFDJLLWFE-UHFFFAOYSA-N pentane-1,2-diol Chemical compound CCCC(O)CO WCVRQHFDJLLWFE-UHFFFAOYSA-N 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- 229940021222 peritoneal dialysis isotonic solution Drugs 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 229960000482 pethidine Drugs 0.000 description 1
- 229940066842 petrolatum Drugs 0.000 description 1
- 235000019271 petrolatum Nutrition 0.000 description 1
- 229940124531 pharmaceutical excipient Drugs 0.000 description 1
- 230000003285 pharmacodynamic effect Effects 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 229960003534 phenindamine Drugs 0.000 description 1
- 229960000280 phenindione Drugs 0.000 description 1
- NFBAXHOPROOJAW-UHFFFAOYSA-N phenindione Chemical compound O=C1C2=CC=CC=C2C(=O)C1C1=CC=CC=C1 NFBAXHOPROOJAW-UHFFFAOYSA-N 0.000 description 1
- 229960001190 pheniramine Drugs 0.000 description 1
- 229960004923 phenprocoumon Drugs 0.000 description 1
- DQDAYGNAKTZFIW-UHFFFAOYSA-N phenprocoumon Chemical compound OC=1C2=CC=CC=C2OC(=O)C=1C(CC)C1=CC=CC=C1 DQDAYGNAKTZFIW-UHFFFAOYSA-N 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- 229960001802 phenylephrine Drugs 0.000 description 1
- SONNWYBIRXJNDC-VIFPVBQESA-N phenylephrine Chemical compound CNC[C@H](O)C1=CC=CC(O)=C1 SONNWYBIRXJNDC-VIFPVBQESA-N 0.000 description 1
- 229960000395 phenylpropanolamine Drugs 0.000 description 1
- DLNKOYKMWOXYQA-APPZFPTMSA-N phenylpropanolamine Chemical compound C[C@@H](N)[C@H](O)C1=CC=CC=C1 DLNKOYKMWOXYQA-APPZFPTMSA-N 0.000 description 1
- 229960001526 phenyltoloxamine Drugs 0.000 description 1
- IZRPKIZLIFYYKR-UHFFFAOYSA-N phenyltoloxamine Chemical compound CN(C)CCOC1=CC=CC=C1CC1=CC=CC=C1 IZRPKIZLIFYYKR-UHFFFAOYSA-N 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 150000003014 phosphoric acid esters Chemical class 0.000 description 1
- 125000005498 phthalate group Chemical class 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 229960001045 piperocaine Drugs 0.000 description 1
- 229960002702 piroxicam Drugs 0.000 description 1
- QYSPLQLAKJAUJT-UHFFFAOYSA-N piroxicam Chemical compound OC=1C2=CC=CC=C2S(=O)(=O)N(C)C=1C(=O)NC1=CC=CC=N1 QYSPLQLAKJAUJT-UHFFFAOYSA-N 0.000 description 1
- 230000010118 platelet activation Effects 0.000 description 1
- 239000000106 platelet aggregation inhibitor Substances 0.000 description 1
- 229920001983 poloxamer Polymers 0.000 description 1
- 229920001308 poly(aminoacid) Polymers 0.000 description 1
- 229920001485 poly(butyl acrylate) polymer Polymers 0.000 description 1
- 229920001084 poly(chloroprene) Polymers 0.000 description 1
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 1
- 229920001281 polyalkylene Polymers 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920002857 polybutadiene Polymers 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920006267 polyester film Polymers 0.000 description 1
- 229920000120 polyethyl acrylate Polymers 0.000 description 1
- 229920002643 polyglutamic acid Polymers 0.000 description 1
- 239000004926 polymethyl methacrylate Substances 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 229920001451 polypropylene glycol Polymers 0.000 description 1
- 229950008882 polysorbate Drugs 0.000 description 1
- 229940058401 polytetrafluoroethylene Drugs 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 239000004810 polytetrafluoroethylene Substances 0.000 description 1
- 235000019422 polyvinyl alcohol Nutrition 0.000 description 1
- 239000004800 polyvinyl chloride Substances 0.000 description 1
- 229920000915 polyvinyl chloride Polymers 0.000 description 1
- 208000007232 portal hypertension Diseases 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 239000004036 potassium channel stimulating agent Substances 0.000 description 1
- 229940069328 povidone Drugs 0.000 description 1
- 201000011461 pre-eclampsia Diseases 0.000 description 1
- 229960002794 prednicarbate Drugs 0.000 description 1
- FNPXMHRZILFCKX-KAJVQRHHSA-N prednicarbate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)CC)(OC(=O)OCC)[C@@]1(C)C[C@@H]2O FNPXMHRZILFCKX-KAJVQRHHSA-N 0.000 description 1
- 229960005205 prednisolone Drugs 0.000 description 1
- OIGNJSKKLXVSLS-VWUMJDOOSA-N prednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OIGNJSKKLXVSLS-VWUMJDOOSA-N 0.000 description 1
- 229960004618 prednisone Drugs 0.000 description 1
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 1
- AYXYPKUFHZROOJ-ZETCQYMHSA-N pregabalin Chemical compound CC(C)C[C@H](CN)CC(O)=O AYXYPKUFHZROOJ-ZETCQYMHSA-N 0.000 description 1
- 229960001233 pregabalin Drugs 0.000 description 1
- 238000004321 preservation Methods 0.000 description 1
- 230000037452 priming Effects 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 229960004654 prolintane Drugs 0.000 description 1
- 229960003910 promethazine Drugs 0.000 description 1
- 229960003981 proparacaine Drugs 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 229960002934 propentofylline Drugs 0.000 description 1
- 229950003255 propoxycaine Drugs 0.000 description 1
- JCRIVQIOJSSCQD-UHFFFAOYSA-N propylhexedrine Chemical compound CNC(C)CC1CCCCC1 JCRIVQIOJSSCQD-UHFFFAOYSA-N 0.000 description 1
- 229960000786 propylhexedrine Drugs 0.000 description 1
- 150000003180 prostaglandins Chemical class 0.000 description 1
- 229940127293 prostanoid Drugs 0.000 description 1
- 150000003814 prostanoids Chemical class 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 229960003908 pseudoephedrine Drugs 0.000 description 1
- KWGRBVOPPLSCSI-WCBMZHEXSA-N pseudoephedrine Chemical compound CN[C@@H](C)[C@@H](O)C1=CC=CC=C1 KWGRBVOPPLSCSI-WCBMZHEXSA-N 0.000 description 1
- 230000000506 psychotropic effect Effects 0.000 description 1
- 230000036593 pulmonary vascular resistance Effects 0.000 description 1
- 210000003492 pulmonary vein Anatomy 0.000 description 1
- MIXMJCQRHVAJIO-TZHJZOAOSA-N qk4dys664x Chemical compound O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O MIXMJCQRHVAJIO-TZHJZOAOSA-N 0.000 description 1
- 229960004431 quetiapine Drugs 0.000 description 1
- URKOMYMAXPYINW-UHFFFAOYSA-N quetiapine Chemical compound C1CN(CCOCCO)CCN1C1=NC2=CC=CC=C2SC2=CC=CC=C12 URKOMYMAXPYINW-UHFFFAOYSA-N 0.000 description 1
- 239000000018 receptor agonist Substances 0.000 description 1
- 229940044601 receptor agonist Drugs 0.000 description 1
- 230000008085 renal dysfunction Effects 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 230000029058 respiratory gaseous exchange Effects 0.000 description 1
- 230000004043 responsiveness Effects 0.000 description 1
- 230000002207 retinal effect Effects 0.000 description 1
- 208000004644 retinal vein occlusion Diseases 0.000 description 1
- 229960000529 riociguat Drugs 0.000 description 1
- WXXSNCNJFUAIDG-UHFFFAOYSA-N riociguat Chemical compound N1=C(N)C(N(C)C(=O)OC)=C(N)N=C1C(C1=CC=CN=C11)=NN1CC1=CC=CC=C1F WXXSNCNJFUAIDG-UHFFFAOYSA-N 0.000 description 1
- 229960001148 rivaroxaban Drugs 0.000 description 1
- KGFYHTZWPPHNLQ-AWEZNQCLSA-N rivaroxaban Chemical compound S1C(Cl)=CC=C1C(=O)NC[C@@H]1OC(=O)N(C=2C=CC(=CC=2)N2C(COCC2)=O)C1 KGFYHTZWPPHNLQ-AWEZNQCLSA-N 0.000 description 1
- 229960000371 rofecoxib Drugs 0.000 description 1
- RZJQGNCSTQAWON-UHFFFAOYSA-N rofecoxib Chemical compound C1=CC(S(=O)(=O)C)=CC=C1C1=C(C=2C=CC=CC=2)C(=O)OC1 RZJQGNCSTQAWON-UHFFFAOYSA-N 0.000 description 1
- 229960005328 rupatadine Drugs 0.000 description 1
- WUZYKBABMWJHDL-UHFFFAOYSA-N rupatadine Chemical compound CC1=CN=CC(CN2CCC(CC2)=C2C3=NC=CC=C3CCC3=CC(Cl)=CC=C32)=C1 WUZYKBABMWJHDL-UHFFFAOYSA-N 0.000 description 1
- 229960001860 salicylate Drugs 0.000 description 1
- 229960000953 salsalate Drugs 0.000 description 1
- 201000000306 sarcoidosis Diseases 0.000 description 1
- RPQXVSUAYFXFJA-HGRQIUPRSA-N saxitoxin Chemical compound NC(=O)OC[C@@H]1N=C(N)N2CCC(O)(O)[C@@]22N=C(N)N[C@@H]12 RPQXVSUAYFXFJA-HGRQIUPRSA-N 0.000 description 1
- RPQXVSUAYFXFJA-UHFFFAOYSA-N saxitoxin hydrate Natural products NC(=O)OCC1N=C(N)N2CCC(O)(O)C22NC(N)=NC12 RPQXVSUAYFXFJA-UHFFFAOYSA-N 0.000 description 1
- 230000018040 scab formation Effects 0.000 description 1
- 210000004761 scalp Anatomy 0.000 description 1
- 238000000646 scanning calorimetry Methods 0.000 description 1
- 210000001991 scapula Anatomy 0.000 description 1
- 201000004409 schistosomiasis Diseases 0.000 description 1
- 210000004706 scrotum Anatomy 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 229960003841 selexipag Drugs 0.000 description 1
- QXWZQTURMXZVHJ-UHFFFAOYSA-N selexipag Chemical compound C=1C=CC=CC=1C1=NC(N(CCCCOCC(=O)NS(C)(=O)=O)C(C)C)=CN=C1C1=CC=CC=C1 QXWZQTURMXZVHJ-UHFFFAOYSA-N 0.000 description 1
- 239000003775 serotonin noradrenalin reuptake inhibitor Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 239000008257 shaving cream Substances 0.000 description 1
- 239000004208 shellac Substances 0.000 description 1
- 229940113147 shellac Drugs 0.000 description 1
- ZLGIYFNHBLSMPS-ATJNOEHPSA-N shellac Chemical compound OCCCCCC(O)C(O)CCCCCCCC(O)=O.C1C23[C@H](C(O)=O)CCC2[C@](C)(CO)[C@@H]1C(C(O)=O)=C[C@@H]3O ZLGIYFNHBLSMPS-ATJNOEHPSA-N 0.000 description 1
- 235000013874 shellac Nutrition 0.000 description 1
- UNAANXDKBXWMLN-UHFFFAOYSA-N sibutramine Chemical compound C=1C=C(Cl)C=CC=1C1(C(N(C)C)CC(C)C)CCC1 UNAANXDKBXWMLN-UHFFFAOYSA-N 0.000 description 1
- 229960004425 sibutramine Drugs 0.000 description 1
- 229960003310 sildenafil Drugs 0.000 description 1
- 229920002545 silicone oil Polymers 0.000 description 1
- 239000004945 silicone rubber Substances 0.000 description 1
- 231100000130 skin irritation / corrosion testing Toxicity 0.000 description 1
- NRHMKIHPTBHXPF-TUJRSCDTSA-M sodium cholate Chemical compound [Na+].C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC([O-])=O)C)[C@@]2(C)[C@@H](O)C1 NRHMKIHPTBHXPF-TUJRSCDTSA-M 0.000 description 1
- BTURAGWYSMTVOW-UHFFFAOYSA-M sodium dodecanoate Chemical compound [Na+].CCCCCCCCCCCC([O-])=O BTURAGWYSMTVOW-UHFFFAOYSA-M 0.000 description 1
- 229940082004 sodium laurate Drugs 0.000 description 1
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 1
- AKHNMLFCWUSKQB-UHFFFAOYSA-L sodium thiosulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=S AKHNMLFCWUSKQB-UHFFFAOYSA-L 0.000 description 1
- 235000019345 sodium thiosulphate Nutrition 0.000 description 1
- 238000000527 sonication Methods 0.000 description 1
- 229950004959 sorbitan oleate Drugs 0.000 description 1
- 238000010911 splenectomy Methods 0.000 description 1
- 229940031439 squalene Drugs 0.000 description 1
- TUHBEKDERLKLEC-UHFFFAOYSA-N squalene Natural products CC(=CCCC(=CCCC(=CCCC=C(/C)CCC=C(/C)CC=C(C)C)C)C)C TUHBEKDERLKLEC-UHFFFAOYSA-N 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 239000011115 styrene butadiene Substances 0.000 description 1
- 210000004304 subcutaneous tissue Anatomy 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- MVGSNCBCUWPVDA-MFOYZWKCSA-N sulindac sulfone Chemical compound CC1=C(CC(O)=O)C2=CC(F)=CC=C2\C1=C/C1=CC=C(S(C)(=O)=O)C=C1 MVGSNCBCUWPVDA-MFOYZWKCSA-N 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- 229960000835 tadalafil Drugs 0.000 description 1
- IEHKWSGCTWLXFU-IIBYNOLFSA-N tadalafil Chemical compound C1=C2OCOC2=CC([C@@H]2C3=C([C]4C=CC=CC4=N3)C[C@H]3N2C(=O)CN(C3=O)C)=C1 IEHKWSGCTWLXFU-IIBYNOLFSA-N 0.000 description 1
- KWTWDQCKEHXFFR-SMDDNHRTSA-N tapentadol Chemical compound CN(C)C[C@H](C)[C@@H](CC)C1=CC=CC(O)=C1 KWTWDQCKEHXFFR-SMDDNHRTSA-N 0.000 description 1
- 229960005126 tapentadol Drugs 0.000 description 1
- 229960002871 tenoxicam Drugs 0.000 description 1
- LZNWYQJJBLGYLT-UHFFFAOYSA-N tenoxicam Chemical compound OC=1C=2SC=CC=2S(=O)(=O)N(C)C=1C(=O)NC1=CC=CC=N1 LZNWYQJJBLGYLT-UHFFFAOYSA-N 0.000 description 1
- KKEYFWRCBNTPAC-UHFFFAOYSA-L terephthalate(2-) Chemical compound [O-]C(=O)C1=CC=C(C([O-])=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-L 0.000 description 1
- UWHCKJMYHZGTIT-UHFFFAOYSA-N tetraethylene glycol Chemical compound OCCOCCOCCOCCO UWHCKJMYHZGTIT-UHFFFAOYSA-N 0.000 description 1
- RWRDLPDLKQPQOW-UHFFFAOYSA-N tetrahydropyrrole Substances C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 description 1
- ZQZCOBSUOFHDEE-UHFFFAOYSA-N tetrapropyl silicate Chemical compound CCCO[Si](OCCC)(OCCC)OCCC ZQZCOBSUOFHDEE-UHFFFAOYSA-N 0.000 description 1
- 229960000337 tetryzoline Drugs 0.000 description 1
- 239000003451 thiazide diuretic agent Substances 0.000 description 1
- 239000005458 thiazide-like diuretic Substances 0.000 description 1
- 239000003868 thrombin inhibitor Substances 0.000 description 1
- 230000002537 thrombolytic effect Effects 0.000 description 1
- 208000021510 thyroid gland disease Diseases 0.000 description 1
- 229950001953 tilmacoxib Drugs 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 229930003799 tocopherol Natural products 0.000 description 1
- 239000011732 tocopherol Substances 0.000 description 1
- 235000019149 tocopherols Nutrition 0.000 description 1
- YEZNLOUZAIOMLT-UHFFFAOYSA-N tolfenamic acid Chemical compound CC1=C(Cl)C=CC=C1NC1=CC=CC=C1C(O)=O YEZNLOUZAIOMLT-UHFFFAOYSA-N 0.000 description 1
- 229960002905 tolfenamic acid Drugs 0.000 description 1
- 229960001017 tolmetin Drugs 0.000 description 1
- UPSPUYADGBWSHF-UHFFFAOYSA-N tolmetin Chemical compound C1=CC(C)=CC=C1C(=O)C1=CC=C(CC(O)=O)N1C UPSPUYADGBWSHF-UHFFFAOYSA-N 0.000 description 1
- 229940042129 topical gel Drugs 0.000 description 1
- 229940100617 topical lotion Drugs 0.000 description 1
- 229940041677 topical spray Drugs 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000440 toxicity profile Toxicity 0.000 description 1
- 229960001262 tramazoline Drugs 0.000 description 1
- QQJLHRRUATVHED-UHFFFAOYSA-N tramazoline Chemical compound N1CCN=C1NC1=CC=CC2=C1CCCC2 QQJLHRRUATVHED-UHFFFAOYSA-N 0.000 description 1
- KHPCPRHQVVSZAH-UHFFFAOYSA-N trans-cinnamyl beta-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OCC=CC1=CC=CC=C1 KHPCPRHQVVSZAH-UHFFFAOYSA-N 0.000 description 1
- LLPOLZWFYMWNKH-UHFFFAOYSA-N trans-dihydrocodeinone Natural products C1C(N(CCC234)C)C2CCC(=O)C3OC2=C4C1=CC=C2OC LLPOLZWFYMWNKH-UHFFFAOYSA-N 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 150000003626 triacylglycerols Chemical class 0.000 description 1
- 229960005294 triamcinolone Drugs 0.000 description 1
- GFNANZIMVAIWHM-OBYCQNJPSA-N triamcinolone Chemical compound O=C1C=C[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@]([C@H](O)C4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 GFNANZIMVAIWHM-OBYCQNJPSA-N 0.000 description 1
- 239000003029 tricyclic antidepressant agent Substances 0.000 description 1
- 229960003223 tripelennamine Drugs 0.000 description 1
- 229960001128 triprolidine Drugs 0.000 description 1
- CBEQULMOCCWAQT-WOJGMQOQSA-N triprolidine Chemical compound C1=CC(C)=CC=C1C(\C=1N=CC=CC=1)=C/CN1CCCC1 CBEQULMOCCWAQT-WOJGMQOQSA-N 0.000 description 1
- FQCQGOZEWWPOKI-UHFFFAOYSA-K trisalicylate-choline Chemical compound [Mg+2].C[N+](C)(C)CCO.OC1=CC=CC=C1C([O-])=O.OC1=CC=CC=C1C([O-])=O.OC1=CC=CC=C1C([O-])=O FQCQGOZEWWPOKI-UHFFFAOYSA-K 0.000 description 1
- 229960000281 trometamol Drugs 0.000 description 1
- 229960000438 udenafil Drugs 0.000 description 1
- IYFNEFQTYQPVOC-UHFFFAOYSA-N udenafil Chemical compound C1=C(C=2NC=3C(CCC)=NN(C)C=3C(=O)N=2)C(OCCC)=CC=C1S(=O)(=O)NCCC1CCCN1C IYFNEFQTYQPVOC-UHFFFAOYSA-N 0.000 description 1
- 231100000397 ulcer Toxicity 0.000 description 1
- 229960002249 ulobetasol Drugs 0.000 description 1
- 229940005605 valeric acid Drugs 0.000 description 1
- MSRILKIQRXUYCT-UHFFFAOYSA-M valproate semisodium Chemical compound [Na+].CCCC(C(O)=O)CCC.CCCC(C([O-])=O)CCC MSRILKIQRXUYCT-UHFFFAOYSA-M 0.000 description 1
- 229960000604 valproic acid Drugs 0.000 description 1
- 229960002381 vardenafil Drugs 0.000 description 1
- 230000008728 vascular permeability Effects 0.000 description 1
- 210000005166 vasculature Anatomy 0.000 description 1
- 239000002550 vasoactive agent Substances 0.000 description 1
- 230000025033 vasoconstriction Effects 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- PNVNVHUZROJLTJ-UHFFFAOYSA-N venlafaxine Chemical compound C1=CC(OC)=CC=C1C(CN(C)C)C1(O)CCCCC1 PNVNVHUZROJLTJ-UHFFFAOYSA-N 0.000 description 1
- 229960004688 venlafaxine Drugs 0.000 description 1
- 230000007998 vessel formation Effects 0.000 description 1
- 235000019165 vitamin E Nutrition 0.000 description 1
- 229940046009 vitamin E Drugs 0.000 description 1
- 239000011709 vitamin E Substances 0.000 description 1
- 229940019333 vitamin k antagonists Drugs 0.000 description 1
- 239000000664 voltage gated sodium channel blocking agent Substances 0.000 description 1
- 229960005080 warfarin Drugs 0.000 description 1
- PJVWKTKQMONHTI-UHFFFAOYSA-N warfarin Chemical compound OC=1C2=CC=CC=C2OC(=O)C=1C(CC(=O)C)C1=CC=CC=C1 PJVWKTKQMONHTI-UHFFFAOYSA-N 0.000 description 1
- 229920003169 water-soluble polymer Polymers 0.000 description 1
- 229960000833 xylometazoline Drugs 0.000 description 1
- 229950005371 zaprinast Drugs 0.000 description 1
- REZGGXNDEMKIQB-UHFFFAOYSA-N zaprinast Chemical compound CCCOC1=CC=CC=C1C1=NC(=O)C2=NNNC2=N1 REZGGXNDEMKIQB-UHFFFAOYSA-N 0.000 description 1
- 239000005019 zein Substances 0.000 description 1
- 229940093612 zein Drugs 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- QUEDXNHFTDJVIY-UHFFFAOYSA-N γ-tocopherol Chemical class OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1 QUEDXNHFTDJVIY-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/70—Web, sheet or filament bases ; Films; Fibres of the matrix type containing drug
- A61K9/7023—Transdermal patches and similar drug-containing composite devices, e.g. cataplasms
- A61K9/703—Transdermal patches and similar drug-containing composite devices, e.g. cataplasms characterised by shape or structure; Details concerning release liner or backing; Refillable patches; User-activated patches
- A61K9/7084—Transdermal patches having a drug layer or reservoir, and one or more separate drug-free skin-adhesive layers, e.g. between drug reservoir and skin, or surrounding the drug reservoir; Liquid-filled reservoir patches
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/557—Eicosanoids, e.g. leukotrienes or prostaglandins
- A61K31/5575—Eicosanoids, e.g. leukotrienes or prostaglandins having a cyclopentane, e.g. prostaglandin E2, prostaglandin F2-alpha
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/557—Eicosanoids, e.g. leukotrienes or prostaglandins
- A61K31/5578—Eicosanoids, e.g. leukotrienes or prostaglandins having a pentalene ring system, e.g. carbacyclin, iloprost
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/10—Alcohols; Phenols; Salts thereof, e.g. glycerol; Polyethylene glycols [PEG]; Poloxamers; PEG/POE alkyl ethers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/14—Esters of carboxylic acids, e.g. fatty acid monoglycerides, medium-chain triglycerides, parabens or PEG fatty acid esters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/20—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing sulfur, e.g. dimethyl sulfoxide [DMSO], docusate, sodium lauryl sulfate or aminosulfonic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/22—Heterocyclic compounds, e.g. ascorbic acid, tocopherol or pyrrolidones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0002—Galenical forms characterised by the drug release technique; Application systems commanded by energy
- A61K9/0009—Galenical forms characterised by the drug release technique; Application systems commanded by energy involving or responsive to electricity, magnetism or acoustic waves; Galenical aspects of sonophoresis, iontophoresis, electroporation or electroosmosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0014—Skin, i.e. galenical aspects of topical compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
- A61K9/0021—Intradermal administration, e.g. through microneedle arrays, needleless injectors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/70—Web, sheet or filament bases ; Films; Fibres of the matrix type containing drug
- A61K9/7023—Transdermal patches and similar drug-containing composite devices, e.g. cataplasms
- A61K9/703—Transdermal patches and similar drug-containing composite devices, e.g. cataplasms characterised by shape or structure; Details concerning release liner or backing; Refillable patches; User-activated patches
- A61K9/7092—Transdermal patches having multiple drug layers or reservoirs, e.g. for obtaining a specific release pattern, or for combining different drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/04—Inotropic agents, i.e. stimulants of cardiac contraction; Drugs for heart failure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical & Material Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Epidemiology (AREA)
- Dermatology (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Cardiology (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Heart & Thoracic Surgery (AREA)
- Pulmonology (AREA)
- Immunology (AREA)
- Urology & Nephrology (AREA)
- Hospice & Palliative Care (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Biomedical Technology (AREA)
- Vascular Medicine (AREA)
- Medicinal Preparation (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020237026190A KR20230119035A (ko) | 2016-12-05 | 2017-12-05 | 트레프로스티닐 및 이의 염의 피부 및 경피 투여 |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662430053P | 2016-12-05 | 2016-12-05 | |
| US62/430,053 | 2016-12-05 | ||
| PCT/US2017/064612 WO2018106632A1 (en) | 2016-12-05 | 2017-12-05 | Dermal and transdermal administration of treprostinil and salts thereof |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020237026190A Division KR20230119035A (ko) | 2016-12-05 | 2017-12-05 | 트레프로스티닐 및 이의 염의 피부 및 경피 투여 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20190109399A true KR20190109399A (ko) | 2019-09-25 |
Family
ID=60782377
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197018666A Ceased KR20190109399A (ko) | 2016-12-05 | 2017-12-05 | 트레프로스티닐 및 이의 염의 피부 및 경피 투여 |
| KR1020237026190A Ceased KR20230119035A (ko) | 2016-12-05 | 2017-12-05 | 트레프로스티닐 및 이의 염의 피부 및 경피 투여 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020237026190A Ceased KR20230119035A (ko) | 2016-12-05 | 2017-12-05 | 트레프로스티닐 및 이의 염의 피부 및 경피 투여 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US20190290597A1 (cg-RX-API-DMAC7.html) |
| EP (1) | EP3548010A1 (cg-RX-API-DMAC7.html) |
| JP (2) | JP2020500882A (cg-RX-API-DMAC7.html) |
| KR (2) | KR20190109399A (cg-RX-API-DMAC7.html) |
| CN (1) | CN110381930A (cg-RX-API-DMAC7.html) |
| CA (1) | CA3045917A1 (cg-RX-API-DMAC7.html) |
| IL (1) | IL267046A (cg-RX-API-DMAC7.html) |
| WO (1) | WO2018106632A1 (cg-RX-API-DMAC7.html) |
Families Citing this family (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6542128B2 (ja) | 2013-01-11 | 2019-07-10 | コルセア ファーマ インコーポレイテッド | トレプロスチニルのプロドラッグ |
| US9505737B2 (en) | 2013-01-11 | 2016-11-29 | Corsair Pharma, Inc. | Treprostinil derivative compounds and methods of using same |
| US11607026B2 (en) * | 2014-05-30 | 2023-03-21 | Johnson & Johnson Consumer Inc. | Device for delivery of skin care composition |
| US9643911B2 (en) | 2015-06-17 | 2017-05-09 | Corsair Pharma, Inc. | Treprostinil derivatives and compositions and uses thereof |
| US9394227B1 (en) | 2015-06-17 | 2016-07-19 | Corsair Pharma, Inc. | Treprostinil derivatives and compositions and uses thereof |
| AU2019268407A1 (en) * | 2018-05-14 | 2021-01-07 | Butterworth Building | Oil therapy method and device |
| CN112739679B (zh) * | 2018-09-18 | 2024-03-12 | 伊莱利利公司 | 曲前列环素的特丁胺盐 |
| WO2022038599A1 (en) * | 2020-08-16 | 2022-02-24 | Ofer Spottheim | Medical dressing |
| JP2024094445A (ja) * | 2021-03-17 | 2024-07-10 | 国立大学法人九州大学 | 経皮吸収剤 |
| EP4313007A4 (en) * | 2021-03-31 | 2025-03-12 | Lyfe CHNG, LLC | TRANSDERMAL SYSTEM, FORMULATION AND METHOD FOR THERAPEUTIC ADMINISTRATION OF A PSYCHEDELIC AGENT |
| CN113842377A (zh) * | 2021-08-11 | 2021-12-28 | 杭州师范大学 | 一种经皮递送榄香烯的pdms微针贴片及其制备方法 |
| WO2023085282A1 (ja) * | 2021-11-15 | 2023-05-19 | 東洋インキScホールディングス株式会社 | セレキシパグを有効成分とする貼付剤 |
| WO2023114755A1 (en) * | 2021-12-13 | 2023-06-22 | WELSH Stephen | Adhesive bandage system for medication delivery and indication of procedure location |
| CN118765196A (zh) * | 2022-01-27 | 2024-10-11 | Lts罗曼治疗系统股份公司 | 用于透皮施用赛乐西帕的透皮治疗系统 |
| EP4516297A1 (en) * | 2022-04-29 | 2025-03-05 | Zhaoke Pharmaceutical (Guangzhou) Co., Ltd | Treprostinil soft mist inhalant |
| CA3262150A1 (en) * | 2022-07-15 | 2024-01-18 | Aso Llc | THERAPEUTIC FOOT PAD FOR COUGH TREATMENT |
| CN118178292B (zh) * | 2024-03-07 | 2024-10-01 | 广州博科化妆品有限公司 | 一种含有水蛭素和植物提取物的防脱洗发露及其制备方法 |
Family Cites Families (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3598122A (en) | 1969-04-01 | 1971-08-10 | Alza Corp | Bandage for administering drugs |
| US4144317A (en) | 1975-05-30 | 1979-03-13 | Alza Corporation | Device consisting of copolymer having acetoxy groups for delivering drugs |
| US4262003A (en) | 1975-12-08 | 1981-04-14 | Alza Corporation | Method and therapeutic system for administering scopolamine transdermally |
| US4201211A (en) | 1977-07-12 | 1980-05-06 | Alza Corporation | Therapeutic system for administering clonidine transdermally |
| US4379454A (en) | 1981-02-17 | 1983-04-12 | Alza Corporation | Dosage for coadministering drug and percutaneous absorption enhancer |
| GB9011588D0 (en) * | 1990-05-24 | 1990-07-11 | Wellcome Found | Prostaglandin analogues for use in medicine |
| US5234690A (en) | 1991-08-23 | 1993-08-10 | Cygnus Therapeutic Systems | Transdermal drug delivery device using an unfilled microporous membrane to achieve delayed onset |
| US5797898A (en) | 1996-07-02 | 1998-08-25 | Massachusetts Institute Of Technology | Microchip drug delivery devices |
| US6682757B1 (en) | 2000-11-16 | 2004-01-27 | Euro-Celtique, S.A. | Titratable dosage transdermal delivery system |
| ES2622471T5 (es) | 2003-05-22 | 2020-07-23 | United Therapeutics Corp | Compuestos y procedimientos para la administración de análogos de prostaciclina |
| WO2005009510A2 (en) | 2003-07-23 | 2005-02-03 | The Regents Of The University Of California | Penetration enhancer combinations for transdermal delivery |
| EP1696900B1 (en) * | 2003-12-16 | 2010-07-14 | United Therapeutics Corporation | Use of treprostinil to treat ischemic lesions |
| EP1744739B1 (en) * | 2004-04-12 | 2010-03-31 | United Therapeutics Corporation | Use of treprostinil to treat neuropathic diabetic foot ulcers |
| AU2009231739B2 (en) * | 2008-03-31 | 2015-01-22 | Passport Technologies, Inc. | Permeant delivery system and methods for use thereof |
| US8350079B2 (en) | 2008-05-08 | 2013-01-08 | United Therapeutics Corporation | Treprostinil formulation |
| US20110294815A1 (en) | 2008-06-27 | 2011-12-01 | Harbeson Scott L | Prostacyclin analogs |
| US9327105B2 (en) * | 2010-03-26 | 2016-05-03 | Itrace Biomedical Inc. | Active transdermal drug delivery system and the method thereof |
| CN103193626B (zh) | 2012-01-10 | 2016-05-11 | 上海天伟生物制药有限公司 | 一种前列腺素类似物的晶型及其制备方法和用途 |
| CN103193627B (zh) | 2012-01-10 | 2016-04-20 | 上海天伟生物制药有限公司 | 一种前列腺素类似物的晶型及其制备方法和用途 |
| ES2713988T3 (es) * | 2012-08-01 | 2019-05-24 | United Therapeutics Corp | Tratamiento de hipertensión arterial pulmonar con células progenitoras endoteliales tratadas con prostaciclina |
| US9505737B2 (en) * | 2013-01-11 | 2016-11-29 | Corsair Pharma, Inc. | Treprostinil derivative compounds and methods of using same |
| CN105164098A (zh) | 2013-03-14 | 2015-12-16 | 联合治疗公司 | 曲前列环素的固体形式 |
| US9394227B1 (en) * | 2015-06-17 | 2016-07-19 | Corsair Pharma, Inc. | Treprostinil derivatives and compositions and uses thereof |
-
2017
- 2017-12-05 KR KR1020197018666A patent/KR20190109399A/ko not_active Ceased
- 2017-12-05 CN CN201780084968.9A patent/CN110381930A/zh active Pending
- 2017-12-05 JP JP2019529890A patent/JP2020500882A/ja active Pending
- 2017-12-05 KR KR1020237026190A patent/KR20230119035A/ko not_active Ceased
- 2017-12-05 CA CA3045917A patent/CA3045917A1/en active Pending
- 2017-12-05 WO PCT/US2017/064612 patent/WO2018106632A1/en not_active Ceased
- 2017-12-05 EP EP17818389.3A patent/EP3548010A1/en active Pending
- 2017-12-05 US US16/465,683 patent/US20190290597A1/en not_active Abandoned
-
2019
- 2019-06-02 IL IL267046A patent/IL267046A/en unknown
-
2023
- 2023-07-12 JP JP2023114183A patent/JP2023134620A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| JP2023134620A (ja) | 2023-09-27 |
| US20190290597A1 (en) | 2019-09-26 |
| WO2018106632A1 (en) | 2018-06-14 |
| IL267046A (en) | 2019-07-31 |
| CN110381930A (zh) | 2019-10-25 |
| KR20230119035A (ko) | 2023-08-14 |
| CA3045917A1 (en) | 2018-06-14 |
| EP3548010A1 (en) | 2019-10-09 |
| JP2020500882A (ja) | 2020-01-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2023134620A (ja) | トレプロスチニルおよびその塩の皮膚投与および経皮投与方法 | |
| Ramadon et al. | Enhancement strategies for transdermal drug delivery systems: Current trends and applications | |
| Akhtar et al. | Non-invasive drug delivery technology: Development and current status of transdermal drug delivery devices, techniques and biomedical applications | |
| US5750141A (en) | Administration of vaso-active agent and therapeutic agent | |
| Benson et al. | Topical and transdermal drug delivery: principles and practice | |
| Vitorino et al. | Overcoming the skin permeation barrier: challenges and opportunities | |
| Tiwary et al. | Innovations in transdermal drug delivery: formulations and techniques | |
| ES2452517T3 (es) | Composiciones y métodos para el tratamiento del trastorno de déficit de atención y del trastorno de déficit de atención e hiperactividad con metilfenidato | |
| Tanner et al. | Delivering drugs by the transdermal route: review and comment | |
| AU743516B2 (en) | Topical anesthetic formulation | |
| Ranade | Drug delivery systems. 6. Transdermal drug delivery | |
| Prausnitz et al. | Skin barrier and transdermal drug delivery | |
| AU2009233617B2 (en) | Dermal, transdermal, mucosal or transmucosal ingredient delivery devices | |
| Williams | Topical and transdermal drug delivery | |
| US20140314815A1 (en) | Adhesive solid gel-forming formulations for dermal drug delivery | |
| JP2007520262A (ja) | 経皮または局所薬物輸送に伴う副作用の処置方法 | |
| JP2011505380A (ja) | マイクロニードル孔バイアビリティを向上させるための方法及び組成物 | |
| Pawar et al. | Recent advancements in transdermal drug delivery system | |
| Deulkar et al. | A review on transdermal drug delivery system | |
| Bhowmik et al. | Recent trends in challenges and opportunities in transdermal drug delivery system | |
| US20250205173A1 (en) | Dermal and transdermal administration of treprostinil and salts thereof | |
| Deshwal et al. | Optimization techniques in transdermal drug delivery system | |
| HK40016649A (en) | Dermal and transdermal administration of treprostinil and salts thereof | |
| US12419829B2 (en) | Topical product hands-free applicator drug delivery system and methods of making and using the same | |
| JP2007001938A (ja) | 微細針付き貼付剤 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20190627 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20201109 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20220523 Patent event code: PE09021S01D |
|
| PE0601 | Decision on rejection of patent |
Patent event date: 20230302 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20220523 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |
|
| J201 | Request for trial against refusal decision | ||
| PJ0201 | Trial against decision of rejection |
Patent event date: 20230731 Comment text: Request for Trial against Decision on Refusal Patent event code: PJ02012R01D Patent event date: 20230302 Comment text: Decision to Refuse Application Patent event code: PJ02011S01I Appeal kind category: Appeal against decision to decline refusal Appeal identifier: 2023101001650 Request date: 20230731 |
|
| J301 | Trial decision |
Free format text: TRIAL NUMBER: 2023101001650; TRIAL DECISION FOR APPEAL AGAINST DECISION TO DECLINE REFUSAL REQUESTED 20230731 Effective date: 20240827 |
|
| PJ1301 | Trial decision |
Patent event code: PJ13011S01D Patent event date: 20240827 Comment text: Trial Decision on Objection to Decision on Refusal Appeal kind category: Appeal against decision to decline refusal Request date: 20230731 Decision date: 20240827 Appeal identifier: 2023101001650 |