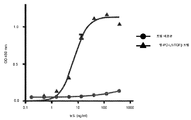
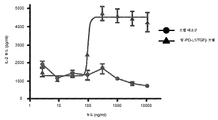
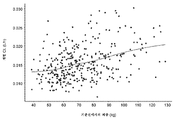
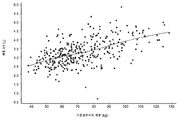
KR20190102059A - 표적화된 TGF-β 억제를 위한 투약 레지멘 및 투여 형태 - Google Patents
표적화된 TGF-β 억제를 위한 투약 레지멘 및 투여 형태 Download PDFInfo
- Publication number
- KR20190102059A KR20190102059A KR1020197022795A KR20197022795A KR20190102059A KR 20190102059 A KR20190102059 A KR 20190102059A KR 1020197022795 A KR1020197022795 A KR 1020197022795A KR 20197022795 A KR20197022795 A KR 20197022795A KR 20190102059 A KR20190102059 A KR 20190102059A
- Authority
- KR
- South Korea
- Prior art keywords
- cancer
- ser
- polypeptide
- thr
- val
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 102000004887 Transforming Growth Factor beta Human genes 0.000 title claims abstract description 237
- 108090001012 Transforming Growth Factor beta Proteins 0.000 title claims abstract description 237
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 title claims abstract description 25
- 230000005764 inhibitory process Effects 0.000 title claims description 15
- 239000002552 dosage form Substances 0.000 title abstract description 5
- 102000004169 proteins and genes Human genes 0.000 claims abstract description 130
- 108090000623 proteins and genes Proteins 0.000 claims abstract description 130
- 108010074708 B7-H1 Antigen Proteins 0.000 claims abstract description 126
- 108090000144 Human Proteins Proteins 0.000 claims abstract description 14
- 102000003839 Human Proteins Human genes 0.000 claims abstract description 14
- 102000008096 B7-H1 Antigen Human genes 0.000 claims abstract 18
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 239
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 209
- 229920001184 polypeptide Polymers 0.000 claims description 205
- 206010028980 Neoplasm Diseases 0.000 claims description 149
- 239000000203 mixture Substances 0.000 claims description 121
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 113
- 238000009472 formulation Methods 0.000 claims description 107
- 238000001990 intravenous administration Methods 0.000 claims description 84
- 238000012377 drug delivery Methods 0.000 claims description 76
- 238000011282 treatment Methods 0.000 claims description 74
- 241000282414 Homo sapiens Species 0.000 claims description 57
- 238000000034 method Methods 0.000 claims description 57
- 201000011510 cancer Diseases 0.000 claims description 54
- 230000027455 binding Effects 0.000 claims description 52
- 208000002154 non-small cell lung carcinoma Diseases 0.000 claims description 45
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 claims description 45
- 206010009944 Colon cancer Diseases 0.000 claims description 42
- 208000001333 Colorectal Neoplasms Diseases 0.000 claims description 38
- 239000012634 fragment Substances 0.000 claims description 35
- 239000012669 liquid formulation Substances 0.000 claims description 32
- 210000004027 cell Anatomy 0.000 claims description 28
- 208000005718 Stomach Neoplasms Diseases 0.000 claims description 26
- 239000000427 antigen Substances 0.000 claims description 26
- 102000036639 antigens Human genes 0.000 claims description 26
- 108091007433 antigens Proteins 0.000 claims description 26
- 206010017758 gastric cancer Diseases 0.000 claims description 26
- 201000011549 stomach cancer Diseases 0.000 claims description 26
- 230000004614 tumor growth Effects 0.000 claims description 24
- 206010041823 squamous cell carcinoma Diseases 0.000 claims description 20
- 201000001441 melanoma Diseases 0.000 claims description 19
- 206010033128 Ovarian cancer Diseases 0.000 claims description 18
- 206010061535 Ovarian neoplasm Diseases 0.000 claims description 18
- 101000712669 Homo sapiens TGF-beta receptor type-2 Proteins 0.000 claims description 17
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims description 17
- 208000009956 adenocarcinoma Diseases 0.000 claims description 17
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims description 17
- 201000002528 pancreatic cancer Diseases 0.000 claims description 17
- 208000008443 pancreatic carcinoma Diseases 0.000 claims description 17
- 208000000453 Skin Neoplasms Diseases 0.000 claims description 16
- 208000005017 glioblastoma Diseases 0.000 claims description 16
- 230000002401 inhibitory effect Effects 0.000 claims description 16
- 201000000849 skin cancer Diseases 0.000 claims description 16
- 102100033455 TGF-beta receptor type-2 Human genes 0.000 claims description 13
- 239000003795 chemical substances by application Substances 0.000 claims description 13
- 206010006187 Breast cancer Diseases 0.000 claims description 11
- 208000026310 Breast neoplasm Diseases 0.000 claims description 11
- 206010060862 Prostate cancer Diseases 0.000 claims description 11
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 11
- 206010008342 Cervix carcinoma Diseases 0.000 claims description 10
- 208000000461 Esophageal Neoplasms Diseases 0.000 claims description 10
- 208000008839 Kidney Neoplasms Diseases 0.000 claims description 10
- 206010027406 Mesothelioma Diseases 0.000 claims description 10
- 206010030155 Oesophageal carcinoma Diseases 0.000 claims description 10
- 206010038389 Renal cancer Diseases 0.000 claims description 10
- 206010039491 Sarcoma Diseases 0.000 claims description 10
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 claims description 10
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 claims description 10
- 201000010881 cervical cancer Diseases 0.000 claims description 10
- 229940124447 delivery agent Drugs 0.000 claims description 10
- 201000004101 esophageal cancer Diseases 0.000 claims description 10
- 201000010982 kidney cancer Diseases 0.000 claims description 10
- 201000007270 liver cancer Diseases 0.000 claims description 10
- 208000014018 liver neoplasm Diseases 0.000 claims description 10
- 201000005112 urinary bladder cancer Diseases 0.000 claims description 10
- 206010005003 Bladder cancer Diseases 0.000 claims description 9
- 206010014733 Endometrial cancer Diseases 0.000 claims description 9
- 206010014759 Endometrial neoplasm Diseases 0.000 claims description 9
- 206010018338 Glioma Diseases 0.000 claims description 9
- 206010025323 Lymphomas Diseases 0.000 claims description 9
- 208000002030 Merkel cell carcinoma Diseases 0.000 claims description 9
- 206010029266 Neuroendocrine carcinoma of the skin Diseases 0.000 claims description 9
- 206010035226 Plasma cell myeloma Diseases 0.000 claims description 9
- 208000024313 Testicular Neoplasms Diseases 0.000 claims description 9
- 206010057644 Testis cancer Diseases 0.000 claims description 9
- 208000024770 Thyroid neoplasm Diseases 0.000 claims description 9
- 208000002495 Uterine Neoplasms Diseases 0.000 claims description 9
- 208000017763 cutaneous neuroendocrine carcinoma Diseases 0.000 claims description 9
- 208000032839 leukemia Diseases 0.000 claims description 9
- 239000012931 lyophilized formulation Substances 0.000 claims description 9
- 201000003120 testicular cancer Diseases 0.000 claims description 9
- 201000002510 thyroid cancer Diseases 0.000 claims description 9
- 206010046766 uterine cancer Diseases 0.000 claims description 9
- 201000008808 Fibrosarcoma Diseases 0.000 claims description 8
- 208000032612 Glial tumor Diseases 0.000 claims description 8
- 208000001894 Nasopharyngeal Neoplasms Diseases 0.000 claims description 8
- 206010061306 Nasopharyngeal cancer Diseases 0.000 claims description 8
- 206010041067 Small cell lung cancer Diseases 0.000 claims description 8
- 210000000270 basal cell Anatomy 0.000 claims description 8
- 201000009036 biliary tract cancer Diseases 0.000 claims description 8
- 208000020790 biliary tract neoplasm Diseases 0.000 claims description 8
- 201000010536 head and neck cancer Diseases 0.000 claims description 8
- 208000014829 head and neck neoplasm Diseases 0.000 claims description 8
- 201000000050 myeloid neoplasm Diseases 0.000 claims description 8
- 201000002120 neuroendocrine carcinoma Diseases 0.000 claims description 8
- 208000000587 small cell lung carcinoma Diseases 0.000 claims description 8
- 201000003793 Myelodysplastic syndrome Diseases 0.000 claims description 7
- 208000037844 advanced solid tumor Diseases 0.000 claims description 6
- 210000003491 skin Anatomy 0.000 claims description 6
- 201000003911 head and neck carcinoma Diseases 0.000 claims description 5
- 230000015572 biosynthetic process Effects 0.000 claims description 4
- 229940071643 prefilled syringe Drugs 0.000 claims description 3
- 208000003200 Adenoma Diseases 0.000 claims description 2
- 206010001233 Adenoma benign Diseases 0.000 claims description 2
- 210000005036 nerve Anatomy 0.000 claims 1
- 208000011580 syndromic disease Diseases 0.000 claims 1
- 230000001588 bifunctional effect Effects 0.000 abstract description 16
- 230000008685 targeting Effects 0.000 abstract description 6
- 235000018102 proteins Nutrition 0.000 description 114
- 102100024216 Programmed cell death 1 ligand 1 Human genes 0.000 description 109
- 230000004044 response Effects 0.000 description 47
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 39
- 201000010099 disease Diseases 0.000 description 35
- 238000004088 simulation Methods 0.000 description 31
- 238000009826 distribution Methods 0.000 description 29
- 235000001014 amino acid Nutrition 0.000 description 28
- 238000002560 therapeutic procedure Methods 0.000 description 26
- 229940024606 amino acid Drugs 0.000 description 24
- 150000001413 amino acids Chemical class 0.000 description 23
- 230000000306 recurrent effect Effects 0.000 description 22
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 20
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 20
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 19
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 17
- -1 ammonium ions Chemical class 0.000 description 17
- 230000037396 body weight Effects 0.000 description 17
- 239000000243 solution Substances 0.000 description 17
- 206010061818 Disease progression Diseases 0.000 description 16
- 230000005750 disease progression Effects 0.000 description 16
- YYSWCHMLFJLLBJ-ZLUOBGJFSA-N Ala-Ala-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O YYSWCHMLFJLLBJ-ZLUOBGJFSA-N 0.000 description 15
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 15
- 210000001744 T-lymphocyte Anatomy 0.000 description 15
- 239000003755 preservative agent Substances 0.000 description 15
- HTONZBWRYUKUKC-RCWTZXSCSA-N Val-Thr-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O HTONZBWRYUKUKC-RCWTZXSCSA-N 0.000 description 14
- 238000013459 approach Methods 0.000 description 14
- 239000000872 buffer Substances 0.000 description 14
- 230000000694 effects Effects 0.000 description 14
- 230000035772 mutation Effects 0.000 description 14
- 102000005962 receptors Human genes 0.000 description 14
- 108020003175 receptors Proteins 0.000 description 14
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 14
- 108010008685 alanyl-glutamyl-aspartic acid Proteins 0.000 description 13
- 238000004458 analytical method Methods 0.000 description 13
- 102000004127 Cytokines Human genes 0.000 description 12
- 108090000695 Cytokines Proteins 0.000 description 12
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 12
- 241000880493 Leptailurus serval Species 0.000 description 12
- 241000699670 Mus sp. Species 0.000 description 12
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 12
- 239000003814 drug Substances 0.000 description 12
- 101100519207 Mus musculus Pdcd1 gene Proteins 0.000 description 11
- UIGMAMGZOJVTDN-WHFBIAKZSA-N Ser-Gly-Ser Chemical compound OC[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O UIGMAMGZOJVTDN-WHFBIAKZSA-N 0.000 description 11
- QYSFWUIXDFJUDW-DCAQKATOSA-N Ser-Leu-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O QYSFWUIXDFJUDW-DCAQKATOSA-N 0.000 description 11
- KBBRNEDOYWMIJP-KYNKHSRBSA-N Thr-Gly-Thr Chemical compound C[C@H]([C@@H](C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)O)N)O KBBRNEDOYWMIJP-KYNKHSRBSA-N 0.000 description 11
- 239000002253 acid Substances 0.000 description 11
- 238000002360 preparation method Methods 0.000 description 11
- 239000008227 sterile water for injection Substances 0.000 description 11
- 210000004881 tumor cell Anatomy 0.000 description 11
- YMTLKLXDFCSCNX-BYPYZUCNSA-N Ser-Gly-Gly Chemical compound OC[C@H](N)C(=O)NCC(=O)NCC(O)=O YMTLKLXDFCSCNX-BYPYZUCNSA-N 0.000 description 10
- XKUKSGPZAADMRA-UHFFFAOYSA-N glycyl-glycyl-glycine Natural products NCC(=O)NCC(=O)NCC(O)=O XKUKSGPZAADMRA-UHFFFAOYSA-N 0.000 description 10
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 10
- 108010026333 seryl-proline Proteins 0.000 description 10
- 230000011664 signaling Effects 0.000 description 10
- 239000011780 sodium chloride Substances 0.000 description 10
- PDUHNKAFQXQNLH-ZETCQYMHSA-N Gly-Lys-Gly Chemical compound NCCCC[C@H](NC(=O)CN)C(=O)NCC(O)=O PDUHNKAFQXQNLH-ZETCQYMHSA-N 0.000 description 9
- 230000000259 anti-tumor effect Effects 0.000 description 9
- 230000008901 benefit Effects 0.000 description 9
- 238000002474 experimental method Methods 0.000 description 9
- 230000006870 function Effects 0.000 description 9
- 108010067216 glycyl-glycyl-glycine Proteins 0.000 description 9
- 238000001802 infusion Methods 0.000 description 9
- 230000000977 initiatory effect Effects 0.000 description 9
- 230000037361 pathway Effects 0.000 description 9
- 108010073969 valyllysine Proteins 0.000 description 9
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 8
- OYTPNWYZORARHL-XHNCKOQMSA-N Gln-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)N)N OYTPNWYZORARHL-XHNCKOQMSA-N 0.000 description 8
- FQCILXROGNOZON-YUMQZZPRSA-N Gln-Pro-Gly Chemical compound NC(=O)CC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O FQCILXROGNOZON-YUMQZZPRSA-N 0.000 description 8
- YQPFCZVKMUVZIN-AUTRQRHGSA-N Glu-Val-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O YQPFCZVKMUVZIN-AUTRQRHGSA-N 0.000 description 8
- MIIVFRCYJABHTQ-ONGXEEELSA-N Gly-Leu-Val Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O MIIVFRCYJABHTQ-ONGXEEELSA-N 0.000 description 8
- WNZOCXUOGVYYBJ-CDMKHQONSA-N Gly-Phe-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)CN)O WNZOCXUOGVYYBJ-CDMKHQONSA-N 0.000 description 8
- 108010065920 Insulin Lispro Proteins 0.000 description 8
- PMGDADKJMCOXHX-UHFFFAOYSA-N L-Arginyl-L-glutamin-acetat Natural products NC(=N)NCCCC(N)C(=O)NC(CCC(N)=O)C(O)=O PMGDADKJMCOXHX-UHFFFAOYSA-N 0.000 description 8
- HYMLKESRWLZDBR-WEDXCCLWSA-N Leu-Gly-Thr Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O HYMLKESRWLZDBR-WEDXCCLWSA-N 0.000 description 8
- KIZIOFNVSOSKJI-CIUDSAMLSA-N Leu-Ser-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N KIZIOFNVSOSKJI-CIUDSAMLSA-N 0.000 description 8
- 229930195725 Mannitol Natural products 0.000 description 8
- PPCZVWHJWJFTFN-ZLUOBGJFSA-N Ser-Ser-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O PPCZVWHJWJFTFN-ZLUOBGJFSA-N 0.000 description 8
- 108010069020 alanyl-prolyl-glycine Proteins 0.000 description 8
- 108010086434 alanyl-seryl-glycine Proteins 0.000 description 8
- 108010008355 arginyl-glutamine Proteins 0.000 description 8
- 239000002585 base Substances 0.000 description 8
- 210000004369 blood Anatomy 0.000 description 8
- 239000008280 blood Substances 0.000 description 8
- 108010001064 glycyl-glycyl-glycyl-glycine Proteins 0.000 description 8
- 108010050848 glycylleucine Proteins 0.000 description 8
- 239000003446 ligand Substances 0.000 description 8
- 239000000594 mannitol Substances 0.000 description 8
- 235000010355 mannitol Nutrition 0.000 description 8
- 206010061289 metastatic neoplasm Diseases 0.000 description 8
- 230000036961 partial effect Effects 0.000 description 8
- 230000036470 plasma concentration Effects 0.000 description 8
- 238000011518 platinum-based chemotherapy Methods 0.000 description 8
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 8
- 229920000053 polysorbate 80 Polymers 0.000 description 8
- 208000037821 progressive disease Diseases 0.000 description 8
- 108010020755 prolyl-glycyl-glycine Proteins 0.000 description 8
- 238000009097 single-agent therapy Methods 0.000 description 8
- 230000001225 therapeutic effect Effects 0.000 description 8
- VKKYFICVTYKFIO-CIUDSAMLSA-N Arg-Ala-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCN=C(N)N VKKYFICVTYKFIO-CIUDSAMLSA-N 0.000 description 7
- WLVLIYYBPPONRJ-GCJQMDKQSA-N Asn-Thr-Ala Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O WLVLIYYBPPONRJ-GCJQMDKQSA-N 0.000 description 7
- MNQMTYSEKZHIDF-GCJQMDKQSA-N Asp-Thr-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O MNQMTYSEKZHIDF-GCJQMDKQSA-N 0.000 description 7
- YNJBLTDKTMKEET-ZLUOBGJFSA-N Cys-Ser-Ser Chemical compound SC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O YNJBLTDKTMKEET-ZLUOBGJFSA-N 0.000 description 7
- FEHQLKKBVJHSEC-SZMVWBNQSA-N Leu-Glu-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC(C)C)C(O)=O)=CNC2=C1 FEHQLKKBVJHSEC-SZMVWBNQSA-N 0.000 description 7
- ZOPISOXXPQNOCO-SVSWQMSJSA-N Ser-Ile-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)O)NC(=O)[C@H](CO)N ZOPISOXXPQNOCO-SVSWQMSJSA-N 0.000 description 7
- MBLJBGZWLHTJBH-SZMVWBNQSA-N Trp-Val-Arg Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 MBLJBGZWLHTJBH-SZMVWBNQSA-N 0.000 description 7
- 239000008365 aqueous carrier Substances 0.000 description 7
- 108010068265 aspartyltyrosine Proteins 0.000 description 7
- 239000000306 component Substances 0.000 description 7
- 230000006240 deamidation Effects 0.000 description 7
- 229940079593 drug Drugs 0.000 description 7
- 229940126534 drug product Drugs 0.000 description 7
- 230000001506 immunosuppresive effect Effects 0.000 description 7
- 230000003902 lesion Effects 0.000 description 7
- 230000001394 metastastic effect Effects 0.000 description 7
- 239000000825 pharmaceutical preparation Substances 0.000 description 7
- 239000004094 surface-active agent Substances 0.000 description 7
- ARHJJAAWNWOACN-FXQIFTODSA-N Ala-Ser-Val Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O ARHJJAAWNWOACN-FXQIFTODSA-N 0.000 description 6
- MKJBPDLENBUHQU-CIUDSAMLSA-N Asn-Ser-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O MKJBPDLENBUHQU-CIUDSAMLSA-N 0.000 description 6
- SOEGEPHNZOISMT-BYPYZUCNSA-N Gly-Ser-Gly Chemical compound NCC(=O)N[C@@H](CO)C(=O)NCC(O)=O SOEGEPHNZOISMT-BYPYZUCNSA-N 0.000 description 6
- AXZGZMGRBDQTEY-SRVKXCTJSA-N Leu-Gln-Met Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(O)=O AXZGZMGRBDQTEY-SRVKXCTJSA-N 0.000 description 6
- QNBVTHNJGCOVFA-AVGNSLFASA-N Leu-Leu-Glu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CCC(O)=O QNBVTHNJGCOVFA-AVGNSLFASA-N 0.000 description 6
- GQZMPWBZQALKJO-UWVGGRQHSA-N Lys-Gly-Arg Chemical compound [H]N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O GQZMPWBZQALKJO-UWVGGRQHSA-N 0.000 description 6
- CTJUSALVKAWFFU-CIUDSAMLSA-N Lys-Ser-Cys Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N CTJUSALVKAWFFU-CIUDSAMLSA-N 0.000 description 6
- YBAFDPFAUTYYRW-UHFFFAOYSA-N N-L-alpha-glutamyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCC(O)=O YBAFDPFAUTYYRW-UHFFFAOYSA-N 0.000 description 6
- FGWUALWGCZJQDJ-URLPEUOOSA-N Phe-Thr-Ile Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O FGWUALWGCZJQDJ-URLPEUOOSA-N 0.000 description 6
- MUJQWSAWLLRJCE-KATARQTJSA-N Ser-Leu-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MUJQWSAWLLRJCE-KATARQTJSA-N 0.000 description 6
- 108091005735 TGF-beta receptors Proteins 0.000 description 6
- MEBDIIKMUUNBSB-RPTUDFQQSA-N Thr-Phe-Tyr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O MEBDIIKMUUNBSB-RPTUDFQQSA-N 0.000 description 6
- SGAOHNPSEPVAFP-ZDLURKLDSA-N Thr-Ser-Gly Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SGAOHNPSEPVAFP-ZDLURKLDSA-N 0.000 description 6
- 102000016715 Transforming Growth Factor beta Receptors Human genes 0.000 description 6
- NUQZCPSZHGIYTA-HKUYNNGSSA-N Tyr-Trp-Gly Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CC3=CC=C(C=C3)O)N NUQZCPSZHGIYTA-HKUYNNGSSA-N 0.000 description 6
- HPANGHISDXDUQY-ULQDDVLXSA-N Val-Lys-Phe Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N HPANGHISDXDUQY-ULQDDVLXSA-N 0.000 description 6
- 108010052670 arginyl-glutamyl-glutamic acid Proteins 0.000 description 6
- 230000000903 blocking effect Effects 0.000 description 6
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 6
- 235000018417 cysteine Nutrition 0.000 description 6
- 108010078144 glutaminyl-glycine Proteins 0.000 description 6
- 238000001727 in vivo Methods 0.000 description 6
- 230000007246 mechanism Effects 0.000 description 6
- 239000008194 pharmaceutical composition Substances 0.000 description 6
- 229920005862 polyol Polymers 0.000 description 6
- 150000003077 polyols Chemical class 0.000 description 6
- 238000011301 standard therapy Methods 0.000 description 6
- KZNICNPSHKQLFF-UHFFFAOYSA-N succinimide Chemical compound O=C1CCC(=O)N1 KZNICNPSHKQLFF-UHFFFAOYSA-N 0.000 description 6
- LKDHUGLXOHYINY-XUXIUFHCSA-N Arg-Ile-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N LKDHUGLXOHYINY-XUXIUFHCSA-N 0.000 description 5
- JJGRJMKUOYXZRA-LPEHRKFASA-N Asn-Arg-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(=O)N)N)C(=O)O JJGRJMKUOYXZRA-LPEHRKFASA-N 0.000 description 5
- KSBHCUSPLWRVEK-ZLUOBGJFSA-N Asn-Asn-Asp Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O KSBHCUSPLWRVEK-ZLUOBGJFSA-N 0.000 description 5
- BUVNWKQBMZLCDW-UGYAYLCHSA-N Asp-Asn-Ile Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O BUVNWKQBMZLCDW-UGYAYLCHSA-N 0.000 description 5
- VAWNQIGQPUOPQW-ACZMJKKPSA-N Asp-Glu-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O VAWNQIGQPUOPQW-ACZMJKKPSA-N 0.000 description 5
- ALMIMUZAWTUNIO-BZSNNMDCSA-N Asp-Tyr-Tyr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ALMIMUZAWTUNIO-BZSNNMDCSA-N 0.000 description 5
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 5
- FCXJJTRGVAZDER-FXQIFTODSA-N Cys-Val-Ala Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O FCXJJTRGVAZDER-FXQIFTODSA-N 0.000 description 5
- RZSLYUUFFVHFRQ-FXQIFTODSA-N Gln-Ala-Glu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O RZSLYUUFFVHFRQ-FXQIFTODSA-N 0.000 description 5
- XJKAKYXMFHUIHT-AUTRQRHGSA-N Gln-Glu-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCC(=O)N)N XJKAKYXMFHUIHT-AUTRQRHGSA-N 0.000 description 5
- HHQCBFGKQDMWSP-GUBZILKMSA-N Gln-Leu-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCC(=O)N)N HHQCBFGKQDMWSP-GUBZILKMSA-N 0.000 description 5
- KUBFPYIMAGXGBT-ACZMJKKPSA-N Gln-Ser-Ala Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O KUBFPYIMAGXGBT-ACZMJKKPSA-N 0.000 description 5
- XMPXVJIDADUOQB-RCOVLWMOSA-N Gly-Gly-Ile Chemical compound CC[C@H](C)[C@@H](C([O-])=O)NC(=O)CNC(=O)C[NH3+] XMPXVJIDADUOQB-RCOVLWMOSA-N 0.000 description 5
- FFJQHWKSGAWSTJ-BFHQHQDPSA-N Gly-Thr-Ala Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O FFJQHWKSGAWSTJ-BFHQHQDPSA-N 0.000 description 5
- ZZWUYQXMIFTIIY-WEDXCCLWSA-N Gly-Thr-Leu Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O ZZWUYQXMIFTIIY-WEDXCCLWSA-N 0.000 description 5
- 241000282412 Homo Species 0.000 description 5
- FGBRXCZYVRFNKQ-MXAVVETBSA-N Ile-Phe-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)O)N FGBRXCZYVRFNKQ-MXAVVETBSA-N 0.000 description 5
- FBGXMKUWQFPHFB-JBDRJPRFSA-N Ile-Ser-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N FBGXMKUWQFPHFB-JBDRJPRFSA-N 0.000 description 5
- HFBCHNRFRYLZNV-GUBZILKMSA-N Leu-Glu-Asp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O HFBCHNRFRYLZNV-GUBZILKMSA-N 0.000 description 5
- ZFNLIDNJUWNIJL-WDCWCFNPSA-N Leu-Glu-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O ZFNLIDNJUWNIJL-WDCWCFNPSA-N 0.000 description 5
- BYPMOIFBQPEWOH-CIUDSAMLSA-N Lys-Asn-Asp Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N BYPMOIFBQPEWOH-CIUDSAMLSA-N 0.000 description 5
- JPYPRVHMKRFTAT-KKUMJFAQSA-N Lys-Phe-Cys Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCCCN)N JPYPRVHMKRFTAT-KKUMJFAQSA-N 0.000 description 5
- SBQDRNOLGSYHQA-YUMQZZPRSA-N Lys-Ser-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SBQDRNOLGSYHQA-YUMQZZPRSA-N 0.000 description 5
- AFVOKRHYSSFPHC-STECZYCISA-N Met-Ile-Tyr Chemical compound CSCC[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 AFVOKRHYSSFPHC-STECZYCISA-N 0.000 description 5
- ODFBIJXEWPWSAN-CYDGBPFRSA-N Met-Ile-Val Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C(C)C)C(O)=O ODFBIJXEWPWSAN-CYDGBPFRSA-N 0.000 description 5
- 206010027476 Metastases Diseases 0.000 description 5
- JMVQDLDPDBXAAX-YUMQZZPRSA-N Pro-Gly-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1 JMVQDLDPDBXAAX-YUMQZZPRSA-N 0.000 description 5
- VYWNORHENYEQDW-YUMQZZPRSA-N Pro-Gly-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1 VYWNORHENYEQDW-YUMQZZPRSA-N 0.000 description 5
- JCLAFVNDBJMLBC-JBDRJPRFSA-N Ser-Ser-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O JCLAFVNDBJMLBC-JBDRJPRFSA-N 0.000 description 5
- 229930006000 Sucrose Natural products 0.000 description 5
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 5
- DIPIPFHFLPTCLK-LOKLDPHHSA-N Thr-Gln-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N1CCC[C@@H]1C(=O)O)N)O DIPIPFHFLPTCLK-LOKLDPHHSA-N 0.000 description 5
- VEIKMWOMUYMMMK-FCLVOEFKSA-N Thr-Phe-Phe Chemical compound C([C@H](NC(=O)[C@@H](N)[C@H](O)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 VEIKMWOMUYMMMK-FCLVOEFKSA-N 0.000 description 5
- YYLHVUCSTXXKBS-IHRRRGAJSA-N Tyr-Pro-Ser Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O YYLHVUCSTXXKBS-IHRRRGAJSA-N 0.000 description 5
- YLHLNFUXDBOAGX-DCAQKATOSA-N Val-Cys-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N YLHLNFUXDBOAGX-DCAQKATOSA-N 0.000 description 5
- JAIZPWVHPQRYOU-ZJDVBMNYSA-N Val-Thr-Thr Chemical compound C[C@H]([C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)O)NC(=O)[C@H](C(C)C)N)O JAIZPWVHPQRYOU-ZJDVBMNYSA-N 0.000 description 5
- OWFGFHQMSBTKLX-UFYCRDLUSA-N Val-Tyr-Tyr Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)N OWFGFHQMSBTKLX-UFYCRDLUSA-N 0.000 description 5
- 150000007513 acids Chemical class 0.000 description 5
- 230000002411 adverse Effects 0.000 description 5
- 108010062796 arginyllysine Proteins 0.000 description 5
- 235000009582 asparagine Nutrition 0.000 description 5
- 229960001230 asparagine Drugs 0.000 description 5
- 108010038633 aspartylglutamate Proteins 0.000 description 5
- 230000001580 bacterial effect Effects 0.000 description 5
- 239000007853 buffer solution Substances 0.000 description 5
- 239000004067 bulking agent Substances 0.000 description 5
- 230000001419 dependent effect Effects 0.000 description 5
- 239000003599 detergent Substances 0.000 description 5
- 231100000673 dose–response relationship Toxicity 0.000 description 5
- 239000012636 effector Substances 0.000 description 5
- 238000004108 freeze drying Methods 0.000 description 5
- 108020001507 fusion proteins Proteins 0.000 description 5
- 102000037865 fusion proteins Human genes 0.000 description 5
- 108010080575 glutamyl-aspartyl-alanine Proteins 0.000 description 5
- 108010027668 glycyl-alanyl-valine Proteins 0.000 description 5
- 108010015792 glycyllysine Proteins 0.000 description 5
- 229910052739 hydrogen Inorganic materials 0.000 description 5
- 108010031424 isoleucyl-prolyl-proline Proteins 0.000 description 5
- 108010044374 isoleucyl-tyrosine Proteins 0.000 description 5
- 108010073472 leucyl-prolyl-proline Proteins 0.000 description 5
- 230000009401 metastasis Effects 0.000 description 5
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 5
- 230000003472 neutralizing effect Effects 0.000 description 5
- 231100000252 nontoxic Toxicity 0.000 description 5
- 230000003000 nontoxic effect Effects 0.000 description 5
- 150000007523 nucleic acids Chemical class 0.000 description 5
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 5
- 108010051242 phenylalanylserine Proteins 0.000 description 5
- 229910052697 platinum Inorganic materials 0.000 description 5
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 5
- 229940068968 polysorbate 80 Drugs 0.000 description 5
- 238000012552 review Methods 0.000 description 5
- 238000005070 sampling Methods 0.000 description 5
- 229910052708 sodium Inorganic materials 0.000 description 5
- 239000011734 sodium Substances 0.000 description 5
- 230000006641 stabilisation Effects 0.000 description 5
- 238000011105 stabilization Methods 0.000 description 5
- 239000005720 sucrose Substances 0.000 description 5
- 208000024891 symptom Diseases 0.000 description 5
- OOSZCNKVJAVHJI-UHFFFAOYSA-N 1-[(4-fluorophenyl)methyl]piperazine Chemical compound C1=CC(F)=CC=C1CN1CCNCC1 OOSZCNKVJAVHJI-UHFFFAOYSA-N 0.000 description 4
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 4
- BUDNAJYVCUHLSV-ZLUOBGJFSA-N Ala-Asp-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O BUDNAJYVCUHLSV-ZLUOBGJFSA-N 0.000 description 4
- IORKCNUBHNIMKY-CIUDSAMLSA-N Ala-Pro-Glu Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O IORKCNUBHNIMKY-CIUDSAMLSA-N 0.000 description 4
- BTRULDJUUVGRNE-DCAQKATOSA-N Ala-Pro-Lys Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O BTRULDJUUVGRNE-DCAQKATOSA-N 0.000 description 4
- UGXYFDQFLVCDFC-CIUDSAMLSA-N Asn-Ser-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O UGXYFDQFLVCDFC-CIUDSAMLSA-N 0.000 description 4
- JBDLMLZNDRLDIX-HJGDQZAQSA-N Asn-Thr-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O JBDLMLZNDRLDIX-HJGDQZAQSA-N 0.000 description 4
- AMGQTNHANMRPOE-LKXGYXEUSA-N Asn-Thr-Ser Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O AMGQTNHANMRPOE-LKXGYXEUSA-N 0.000 description 4
- DPSUVAPLRQDWAO-YDHLFZDLSA-N Asn-Tyr-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](CC(=O)N)N DPSUVAPLRQDWAO-YDHLFZDLSA-N 0.000 description 4
- QOJJMJKTMKNFEF-ZKWXMUAHSA-N Asp-Val-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CC(O)=O QOJJMJKTMKNFEF-ZKWXMUAHSA-N 0.000 description 4
- 108020004414 DNA Proteins 0.000 description 4
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 4
- GPISLLFQNHELLK-DCAQKATOSA-N Gln-Gln-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCC(=O)N)N GPISLLFQNHELLK-DCAQKATOSA-N 0.000 description 4
- AFODTOLGSZQDSL-PEFMBERDSA-N Glu-Asn-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCC(=O)O)N AFODTOLGSZQDSL-PEFMBERDSA-N 0.000 description 4
- BUAKRRKDHSSIKK-IHRRRGAJSA-N Glu-Glu-Tyr Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 BUAKRRKDHSSIKK-IHRRRGAJSA-N 0.000 description 4
- 101001117317 Homo sapiens Programmed cell death 1 ligand 1 Proteins 0.000 description 4
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 4
- ZLFNNVATRMCAKN-ZKWXMUAHSA-N Ile-Ser-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)NCC(=O)O)N ZLFNNVATRMCAKN-ZKWXMUAHSA-N 0.000 description 4
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 4
- 108060003951 Immunoglobulin Proteins 0.000 description 4
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 4
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 4
- ZJZNLRVCZWUONM-JXUBOQSCSA-N Leu-Thr-Ala Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O ZJZNLRVCZWUONM-JXUBOQSCSA-N 0.000 description 4
- RIPJMCFGQHGHNP-RHYQMDGZSA-N Lys-Val-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CCCCN)N)O RIPJMCFGQHGHNP-RHYQMDGZSA-N 0.000 description 4
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 4
- 241001529936 Murinae Species 0.000 description 4
- SITLTJHOQZFJGG-UHFFFAOYSA-N N-L-alpha-glutamyl-L-valine Natural products CC(C)C(C(O)=O)NC(=O)C(N)CCC(O)=O SITLTJHOQZFJGG-UHFFFAOYSA-N 0.000 description 4
- VPEVBAUSTBWQHN-NHCYSSNCSA-N Pro-Glu-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O VPEVBAUSTBWQHN-NHCYSSNCSA-N 0.000 description 4
- UIMCLYYSUCIUJM-UWVGGRQHSA-N Pro-Gly-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1 UIMCLYYSUCIUJM-UWVGGRQHSA-N 0.000 description 4
- QEDMOZUJTGEIBF-FXQIFTODSA-N Ser-Arg-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(O)=O QEDMOZUJTGEIBF-FXQIFTODSA-N 0.000 description 4
- OBXVZEAMXFSGPU-FXQIFTODSA-N Ser-Asn-Arg Chemical compound C(C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CO)N)CN=C(N)N OBXVZEAMXFSGPU-FXQIFTODSA-N 0.000 description 4
- GZFAWAQTEYDKII-YUMQZZPRSA-N Ser-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CO GZFAWAQTEYDKII-YUMQZZPRSA-N 0.000 description 4
- XXXAXOWMBOKTRN-XPUUQOCRSA-N Ser-Gly-Val Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O XXXAXOWMBOKTRN-XPUUQOCRSA-N 0.000 description 4
- YUJLIIRMIAGMCQ-CIUDSAMLSA-N Ser-Leu-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YUJLIIRMIAGMCQ-CIUDSAMLSA-N 0.000 description 4
- RXUOAOOZIWABBW-XGEHTFHBSA-N Ser-Thr-Arg Chemical compound OC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N RXUOAOOZIWABBW-XGEHTFHBSA-N 0.000 description 4
- RTXKJFWHEBTABY-IHPCNDPISA-N Ser-Trp-Tyr Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC3=CC=C(C=C3)O)C(=O)O)NC(=O)[C@H](CO)N RTXKJFWHEBTABY-IHPCNDPISA-N 0.000 description 4
- KZSYAEWQMJEGRZ-RHYQMDGZSA-N Thr-Leu-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O KZSYAEWQMJEGRZ-RHYQMDGZSA-N 0.000 description 4
- OGOYMQWIWHGTGH-KZVJFYERSA-N Thr-Val-Ala Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O OGOYMQWIWHGTGH-KZVJFYERSA-N 0.000 description 4
- FYBFTPLPAXZBOY-KKHAAJSZSA-N Thr-Val-Asp Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O FYBFTPLPAXZBOY-KKHAAJSZSA-N 0.000 description 4
- WQOHKVRQDLNDIL-YJRXYDGGSA-N Tyr-Thr-Ser Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O WQOHKVRQDLNDIL-YJRXYDGGSA-N 0.000 description 4
- PIFJAFRUVWZRKR-QMMMGPOBSA-N Val-Gly-Gly Chemical compound CC(C)[C@H]([NH3+])C(=O)NCC(=O)NCC([O-])=O PIFJAFRUVWZRKR-QMMMGPOBSA-N 0.000 description 4
- CKTMJBPRVQWPHU-JSGCOSHPSA-N Val-Phe-Gly Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)NCC(=O)O)N CKTMJBPRVQWPHU-JSGCOSHPSA-N 0.000 description 4
- HVRRJRMULCPNRO-BZSNNMDCSA-N Val-Trp-Arg Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 HVRRJRMULCPNRO-BZSNNMDCSA-N 0.000 description 4
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 description 4
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 4
- 239000002246 antineoplastic agent Substances 0.000 description 4
- 239000007864 aqueous solution Substances 0.000 description 4
- GUBGYTABKSRVRQ-QUYVBRFLSA-N beta-maltose Chemical compound OC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O GUBGYTABKSRVRQ-QUYVBRFLSA-N 0.000 description 4
- 239000008366 buffered solution Substances 0.000 description 4
- 238000006243 chemical reaction Methods 0.000 description 4
- 238000002512 chemotherapy Methods 0.000 description 4
- DDRJAANPRJIHGJ-UHFFFAOYSA-N creatinine Chemical compound CN1CC(=O)NC1=N DDRJAANPRJIHGJ-UHFFFAOYSA-N 0.000 description 4
- 150000001945 cysteines Chemical class 0.000 description 4
- 238000013461 design Methods 0.000 description 4
- KDQPSPMLNJTZAL-UHFFFAOYSA-L disodium hydrogenphosphate dihydrate Chemical compound O.O.[Na+].[Na+].OP([O-])([O-])=O KDQPSPMLNJTZAL-UHFFFAOYSA-L 0.000 description 4
- 208000035475 disorder Diseases 0.000 description 4
- 210000004602 germ cell Anatomy 0.000 description 4
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 4
- 108010010096 glycyl-glycyl-tyrosine Proteins 0.000 description 4
- 108010089804 glycyl-threonine Proteins 0.000 description 4
- 108010037850 glycylvaline Proteins 0.000 description 4
- 108010085325 histidylproline Proteins 0.000 description 4
- 102000048776 human CD274 Human genes 0.000 description 4
- 230000008629 immune suppression Effects 0.000 description 4
- 102000018358 immunoglobulin Human genes 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 239000000543 intermediate Substances 0.000 description 4
- 108010064235 lysylglycine Proteins 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 238000010172 mouse model Methods 0.000 description 4
- 230000003204 osmotic effect Effects 0.000 description 4
- 230000002093 peripheral effect Effects 0.000 description 4
- 108091033319 polynucleotide Proteins 0.000 description 4
- 102000040430 polynucleotide Human genes 0.000 description 4
- 239000002157 polynucleotide Substances 0.000 description 4
- 230000005855 radiation Effects 0.000 description 4
- 150000003839 salts Chemical class 0.000 description 4
- 210000002966 serum Anatomy 0.000 description 4
- 239000001509 sodium citrate Substances 0.000 description 4
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 4
- 229940074545 sodium dihydrogen phosphate dihydrate Drugs 0.000 description 4
- IESDGNYHXIOKRW-YXMSTPNBSA-N (2s)-2-[[(2s)-1-[(2s)-6-amino-2-[[(2s,3r)-2-amino-3-hydroxybutanoyl]amino]hexanoyl]pyrrolidine-2-carbonyl]amino]-5-(diaminomethylideneamino)pentanoic acid Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O IESDGNYHXIOKRW-YXMSTPNBSA-N 0.000 description 3
- DIBLBAURNYJYBF-XLXZRNDBSA-N (2s)-2-[[(2s)-2-[[2-[[(2s)-6-amino-2-[[(2s)-2-amino-3-methylbutanoyl]amino]hexanoyl]amino]acetyl]amino]-3-phenylpropanoyl]amino]-3-(4-hydroxyphenyl)propanoic acid Chemical compound C([C@H](NC(=O)CNC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=CC=C1 DIBLBAURNYJYBF-XLXZRNDBSA-N 0.000 description 3
- HKZAAJSTFUZYTO-LURJTMIESA-N (2s)-2-[[2-[[2-[[2-[(2-aminoacetyl)amino]acetyl]amino]acetyl]amino]acetyl]amino]-3-hydroxypropanoic acid Chemical compound NCC(=O)NCC(=O)NCC(=O)NCC(=O)N[C@@H](CO)C(O)=O HKZAAJSTFUZYTO-LURJTMIESA-N 0.000 description 3
- OYJCVIGKMXUVKB-GARJFASQSA-N Ala-Leu-Pro Chemical compound C[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@@H]1C(=O)O)N OYJCVIGKMXUVKB-GARJFASQSA-N 0.000 description 3
- WQLDNOCHHRISMS-NAKRPEOUSA-N Ala-Pro-Ile Chemical compound [H]N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(O)=O WQLDNOCHHRISMS-NAKRPEOUSA-N 0.000 description 3
- XWFWAXPOLRTDFZ-FXQIFTODSA-N Ala-Pro-Ser Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O XWFWAXPOLRTDFZ-FXQIFTODSA-N 0.000 description 3
- AOHKLEBWKMKITA-IHRRRGAJSA-N Arg-Phe-Ser Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N AOHKLEBWKMKITA-IHRRRGAJSA-N 0.000 description 3
- SLKLLQWZQHXYSV-CIUDSAMLSA-N Asn-Ala-Lys Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(O)=O SLKLLQWZQHXYSV-CIUDSAMLSA-N 0.000 description 3
- CTQIOCMSIJATNX-WHFBIAKZSA-N Asn-Gly-Ala Chemical compound [H]N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H](C)C(O)=O CTQIOCMSIJATNX-WHFBIAKZSA-N 0.000 description 3
- YRTOMUMWSTUQAX-FXQIFTODSA-N Asn-Pro-Asp Chemical compound NC(=O)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(O)=O YRTOMUMWSTUQAX-FXQIFTODSA-N 0.000 description 3
- LGCVSPFCFXWUEY-IHPCNDPISA-N Asn-Trp-Tyr Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC3=CC=C(C=C3)O)C(=O)O)NC(=O)[C@H](CC(=O)N)N LGCVSPFCFXWUEY-IHPCNDPISA-N 0.000 description 3
- RATOMFTUDRYMKX-ACZMJKKPSA-N Asp-Glu-Cys Chemical compound C(CC(=O)O)[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(=O)O)N RATOMFTUDRYMKX-ACZMJKKPSA-N 0.000 description 3
- SNDBKTFJWVEVPO-WHFBIAKZSA-N Asp-Gly-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CO)C(O)=O SNDBKTFJWVEVPO-WHFBIAKZSA-N 0.000 description 3
- UZFHNLYQWMGUHU-DCAQKATOSA-N Asp-Lys-Arg Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O UZFHNLYQWMGUHU-DCAQKATOSA-N 0.000 description 3
- AHWRSSLYSGLBGD-CIUDSAMLSA-N Asp-Pro-Glu Chemical compound OC(=O)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O AHWRSSLYSGLBGD-CIUDSAMLSA-N 0.000 description 3
- UAXIKORUDGGIGA-DCAQKATOSA-N Asp-Pro-Lys Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CC(=O)O)N)C(=O)N[C@@H](CCCCN)C(=O)O UAXIKORUDGGIGA-DCAQKATOSA-N 0.000 description 3
- SQIARYGNVQWOSB-BZSNNMDCSA-N Asp-Tyr-Phe Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O SQIARYGNVQWOSB-BZSNNMDCSA-N 0.000 description 3
- MLDQJTXFUGDVEO-UHFFFAOYSA-N BAY-43-9006 Chemical compound C1=NC(C(=O)NC)=CC(OC=2C=CC(NC(=O)NC=3C=C(C(Cl)=CC=3)C(F)(F)F)=CC=2)=C1 MLDQJTXFUGDVEO-UHFFFAOYSA-N 0.000 description 3
- UWXFFVQPAMBETM-ZLUOBGJFSA-N Cys-Asp-Asn Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O UWXFFVQPAMBETM-ZLUOBGJFSA-N 0.000 description 3
- MUZAUPFGPMMZSS-GUBZILKMSA-N Cys-Glu-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CS)N MUZAUPFGPMMZSS-GUBZILKMSA-N 0.000 description 3
- ZXCAQANTQWBICD-DCAQKATOSA-N Cys-Lys-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CS)N ZXCAQANTQWBICD-DCAQKATOSA-N 0.000 description 3
- SRZZZTMJARUVPI-JBDRJPRFSA-N Cys-Ser-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CS)N SRZZZTMJARUVPI-JBDRJPRFSA-N 0.000 description 3
- DHNWZLGBTPUTQQ-QEJZJMRPSA-N Gln-Asp-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)N)N DHNWZLGBTPUTQQ-QEJZJMRPSA-N 0.000 description 3
- JXFLPKSDLDEOQK-JHEQGTHGSA-N Gln-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCC(N)=O JXFLPKSDLDEOQK-JHEQGTHGSA-N 0.000 description 3
- XKPACHRGOWQHFH-IRIUXVKKSA-N Gln-Thr-Tyr Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O XKPACHRGOWQHFH-IRIUXVKKSA-N 0.000 description 3
- FITIQFSXXBKFFM-NRPADANISA-N Gln-Val-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O FITIQFSXXBKFFM-NRPADANISA-N 0.000 description 3
- ITYRYNUZHPNCIK-GUBZILKMSA-N Glu-Ala-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O ITYRYNUZHPNCIK-GUBZILKMSA-N 0.000 description 3
- GLWXKFRTOHKGIT-ACZMJKKPSA-N Glu-Asn-Asn Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O GLWXKFRTOHKGIT-ACZMJKKPSA-N 0.000 description 3
- HNVFSTLPVJWIDV-CIUDSAMLSA-N Glu-Glu-Gln Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O HNVFSTLPVJWIDV-CIUDSAMLSA-N 0.000 description 3
- KASDBWKLWJKTLJ-GUBZILKMSA-N Glu-Glu-Met Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCSC)C(O)=O KASDBWKLWJKTLJ-GUBZILKMSA-N 0.000 description 3
- HRBYTAIBKPNZKQ-AVGNSLFASA-N Glu-Lys-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CCC(O)=O HRBYTAIBKPNZKQ-AVGNSLFASA-N 0.000 description 3
- ALMBZBOCGSVSAI-ACZMJKKPSA-N Glu-Ser-Asn Chemical compound C(CC(=O)O)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(=O)N)C(=O)O)N ALMBZBOCGSVSAI-ACZMJKKPSA-N 0.000 description 3
- MFYLRRCYBBJYPI-JYJNAYRXSA-N Glu-Tyr-Lys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N)O MFYLRRCYBBJYPI-JYJNAYRXSA-N 0.000 description 3
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 3
- VSVZIEVNUYDAFR-YUMQZZPRSA-N Gly-Ala-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)CN VSVZIEVNUYDAFR-YUMQZZPRSA-N 0.000 description 3
- KKBWDNZXYLGJEY-UHFFFAOYSA-N Gly-Arg-Pro Natural products NCC(=O)NC(CCNC(=N)N)C(=O)N1CCCC1C(=O)O KKBWDNZXYLGJEY-UHFFFAOYSA-N 0.000 description 3
- GRIRDMVMJJDZKV-RCOVLWMOSA-N Gly-Asn-Val Chemical compound [H]NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O GRIRDMVMJJDZKV-RCOVLWMOSA-N 0.000 description 3
- PABFFPWEJMEVEC-JGVFFNPUSA-N Gly-Gln-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCC(=O)N)NC(=O)CN)C(=O)O PABFFPWEJMEVEC-JGVFFNPUSA-N 0.000 description 3
- HAOUOFNNJJLVNS-BQBZGAKWSA-N Gly-Pro-Ser Chemical compound NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O HAOUOFNNJJLVNS-BQBZGAKWSA-N 0.000 description 3
- 239000004471 Glycine Substances 0.000 description 3
- 229920002683 Glycosaminoglycan Polymers 0.000 description 3
- WMKXFMUJRCEGRP-SRVKXCTJSA-N His-Asn-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)N WMKXFMUJRCEGRP-SRVKXCTJSA-N 0.000 description 3
- WGVPDSNCHDEDBP-KKUMJFAQSA-N His-Asp-Phe Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O WGVPDSNCHDEDBP-KKUMJFAQSA-N 0.000 description 3
- WSXNWASHQNSMRX-GVXVVHGQSA-N His-Val-Gln Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC1=CN=CN1)N WSXNWASHQNSMRX-GVXVVHGQSA-N 0.000 description 3
- MKWSZEHGHSLNPF-NAKRPEOUSA-N Ile-Ala-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)O)N MKWSZEHGHSLNPF-NAKRPEOUSA-N 0.000 description 3
- FADXGVVLSPPEQY-GHCJXIJMSA-N Ile-Cys-Asn Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(=O)N)C(=O)O)N FADXGVVLSPPEQY-GHCJXIJMSA-N 0.000 description 3
- ZUPJCJINYQISSN-XUXIUFHCSA-N Ile-Met-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCCN)C(=O)O)N ZUPJCJINYQISSN-XUXIUFHCSA-N 0.000 description 3
- AYLAAGNJNVZDPY-CYDGBPFRSA-N Ile-Met-Met Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCSC)C(=O)O)N AYLAAGNJNVZDPY-CYDGBPFRSA-N 0.000 description 3
- FQYQMFCIJNWDQZ-CYDGBPFRSA-N Ile-Pro-Pro Chemical compound CC[C@H](C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 FQYQMFCIJNWDQZ-CYDGBPFRSA-N 0.000 description 3
- 108091008028 Immune checkpoint receptors Proteins 0.000 description 3
- 102000037978 Immune checkpoint receptors Human genes 0.000 description 3
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 description 3
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 description 3
- 108010002350 Interleukin-2 Proteins 0.000 description 3
- SENJXOPIZNYLHU-UHFFFAOYSA-N L-leucyl-L-arginine Natural products CC(C)CC(N)C(=O)NC(C(O)=O)CCCN=C(N)N SENJXOPIZNYLHU-UHFFFAOYSA-N 0.000 description 3
- 239000005511 L01XE05 - Sorafenib Substances 0.000 description 3
- OIARJGNVARWKFP-YUMQZZPRSA-N Leu-Asn-Gly Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O OIARJGNVARWKFP-YUMQZZPRSA-N 0.000 description 3
- PVMPDMIKUVNOBD-CIUDSAMLSA-N Leu-Asp-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O PVMPDMIKUVNOBD-CIUDSAMLSA-N 0.000 description 3
- GPICTNQYKHHHTH-GUBZILKMSA-N Leu-Gln-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O GPICTNQYKHHHTH-GUBZILKMSA-N 0.000 description 3
- QJUWBDPGGYVRHY-YUMQZZPRSA-N Leu-Gly-Cys Chemical compound CC(C)C[C@@H](C(=O)NCC(=O)N[C@@H](CS)C(=O)O)N QJUWBDPGGYVRHY-YUMQZZPRSA-N 0.000 description 3
- YOKVEHGYYQEQOP-QWRGUYRKSA-N Leu-Leu-Gly Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O YOKVEHGYYQEQOP-QWRGUYRKSA-N 0.000 description 3
- XVZCXCTYGHPNEM-UHFFFAOYSA-N Leu-Leu-Pro Natural products CC(C)CC(N)C(=O)NC(CC(C)C)C(=O)N1CCCC1C(O)=O XVZCXCTYGHPNEM-UHFFFAOYSA-N 0.000 description 3
- UCXQIIIFOOGYEM-ULQDDVLXSA-N Leu-Pro-Tyr Chemical compound CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 UCXQIIIFOOGYEM-ULQDDVLXSA-N 0.000 description 3
- SBANPBVRHYIMRR-UHFFFAOYSA-N Leu-Ser-Pro Natural products CC(C)CC(N)C(=O)NC(CO)C(=O)N1CCCC1C(O)=O SBANPBVRHYIMRR-UHFFFAOYSA-N 0.000 description 3
- AIQWYVFNBNNOLU-RHYQMDGZSA-N Leu-Thr-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O AIQWYVFNBNNOLU-RHYQMDGZSA-N 0.000 description 3
- YQFZRHYZLARWDY-IHRRRGAJSA-N Leu-Val-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CCCCN YQFZRHYZLARWDY-IHRRRGAJSA-N 0.000 description 3
- XNKDCYABMBBEKN-IUCAKERBSA-N Lys-Gly-Gln Chemical compound NCCCC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCC(N)=O XNKDCYABMBBEKN-IUCAKERBSA-N 0.000 description 3
- WQDKIVRHTQYJSN-DCAQKATOSA-N Lys-Ser-Arg Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N WQDKIVRHTQYJSN-DCAQKATOSA-N 0.000 description 3
- IOQWIOPSKJOEKI-SRVKXCTJSA-N Lys-Ser-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O IOQWIOPSKJOEKI-SRVKXCTJSA-N 0.000 description 3
- MEQLGHAMAUPOSJ-DCAQKATOSA-N Lys-Ser-Val Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O MEQLGHAMAUPOSJ-DCAQKATOSA-N 0.000 description 3
- IEVXCWPVBYCJRZ-IXOXFDKPSA-N Lys-Thr-His Chemical compound NCCCC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 IEVXCWPVBYCJRZ-IXOXFDKPSA-N 0.000 description 3
- DLCAXBGXGOVUCD-PPCPHDFISA-N Lys-Thr-Ile Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O DLCAXBGXGOVUCD-PPCPHDFISA-N 0.000 description 3
- 239000004472 Lysine Substances 0.000 description 3
- 241000282567 Macaca fascicularis Species 0.000 description 3
- RMHHNLKYPOOKQN-FXQIFTODSA-N Met-Cys-Ser Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(O)=O RMHHNLKYPOOKQN-FXQIFTODSA-N 0.000 description 3
- PCTFVQATEGYHJU-FXQIFTODSA-N Met-Ser-Asn Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O PCTFVQATEGYHJU-FXQIFTODSA-N 0.000 description 3
- 241000699666 Mus <mouse, genus> Species 0.000 description 3
- 206010061309 Neoplasm progression Diseases 0.000 description 3
- 101100342977 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) leu-1 gene Proteins 0.000 description 3
- 229940122862 PD-L1 agonist Drugs 0.000 description 3
- MSHZERMPZKCODG-ACRUOGEOSA-N Phe-Leu-Phe Chemical compound C([C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 MSHZERMPZKCODG-ACRUOGEOSA-N 0.000 description 3
- JDMKQHSHKJHAHR-UHFFFAOYSA-N Phe-Phe-Leu-Tyr Natural products C=1C=C(O)C=CC=1CC(C(O)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)CC1=CC=CC=C1 JDMKQHSHKJHAHR-UHFFFAOYSA-N 0.000 description 3
- JLLJTMHNXQTMCK-UBHSHLNASA-N Phe-Pro-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CC1=CC=CC=C1 JLLJTMHNXQTMCK-UBHSHLNASA-N 0.000 description 3
- WWPAHTZOWURIMR-ULQDDVLXSA-N Phe-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CC1=CC=CC=C1 WWPAHTZOWURIMR-ULQDDVLXSA-N 0.000 description 3
- IIEOLPMQYRBZCN-SRVKXCTJSA-N Phe-Ser-Cys Chemical compound N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O IIEOLPMQYRBZCN-SRVKXCTJSA-N 0.000 description 3
- BONHGTUEEPIMPM-AVGNSLFASA-N Phe-Ser-Glu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(O)=O BONHGTUEEPIMPM-AVGNSLFASA-N 0.000 description 3
- MCIXMYKSPQUMJG-SRVKXCTJSA-N Phe-Ser-Ser Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O MCIXMYKSPQUMJG-SRVKXCTJSA-N 0.000 description 3
- IAOZOFPONWDXNT-IXOXFDKPSA-N Phe-Ser-Thr Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O IAOZOFPONWDXNT-IXOXFDKPSA-N 0.000 description 3
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 3
- IHCXPSYCHXFXKT-DCAQKATOSA-N Pro-Arg-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O IHCXPSYCHXFXKT-DCAQKATOSA-N 0.000 description 3
- TUYWCHPXKQTISF-LPEHRKFASA-N Pro-Cys-Pro Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CS)C(=O)N2CCC[C@@H]2C(=O)O TUYWCHPXKQTISF-LPEHRKFASA-N 0.000 description 3
- UAYHMOIGIQZLFR-NHCYSSNCSA-N Pro-Gln-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O UAYHMOIGIQZLFR-NHCYSSNCSA-N 0.000 description 3
- LGSANCBHSMDFDY-GARJFASQSA-N Pro-Glu-Pro Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CCC(=O)O)C(=O)N2CCC[C@@H]2C(=O)O LGSANCBHSMDFDY-GARJFASQSA-N 0.000 description 3
- ZLXKLMHAMDENIO-DCAQKATOSA-N Pro-Lys-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(O)=O ZLXKLMHAMDENIO-DCAQKATOSA-N 0.000 description 3
- YAZNFQUKPUASKB-DCAQKATOSA-N Pro-Lys-Cys Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CS)C(=O)O YAZNFQUKPUASKB-DCAQKATOSA-N 0.000 description 3
- RMODQFBNDDENCP-IHRRRGAJSA-N Pro-Lys-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O RMODQFBNDDENCP-IHRRRGAJSA-N 0.000 description 3
- PCWLNNZTBJTZRN-AVGNSLFASA-N Pro-Pro-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 PCWLNNZTBJTZRN-AVGNSLFASA-N 0.000 description 3
- GOMUXSCOIWIJFP-GUBZILKMSA-N Pro-Ser-Arg Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O GOMUXSCOIWIJFP-GUBZILKMSA-N 0.000 description 3
- OWQXAJQZLWHPBH-FXQIFTODSA-N Pro-Ser-Asn Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O OWQXAJQZLWHPBH-FXQIFTODSA-N 0.000 description 3
- GMJDSFYVTAMIBF-FXQIFTODSA-N Pro-Ser-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O GMJDSFYVTAMIBF-FXQIFTODSA-N 0.000 description 3
- BGWKULMLUIUPKY-BQBZGAKWSA-N Pro-Ser-Gly Chemical compound OC(=O)CNC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1 BGWKULMLUIUPKY-BQBZGAKWSA-N 0.000 description 3
- PRKWBYCXBBSLSK-GUBZILKMSA-N Pro-Ser-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O PRKWBYCXBBSLSK-GUBZILKMSA-N 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 208000003251 Pruritus Diseases 0.000 description 3
- OYEDZGNMSBZCIM-XGEHTFHBSA-N Ser-Arg-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OYEDZGNMSBZCIM-XGEHTFHBSA-N 0.000 description 3
- VGNYHOBZJKWRGI-CIUDSAMLSA-N Ser-Asn-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CO VGNYHOBZJKWRGI-CIUDSAMLSA-N 0.000 description 3
- QPFJSHSJFIYDJZ-GHCJXIJMSA-N Ser-Asp-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CO QPFJSHSJFIYDJZ-GHCJXIJMSA-N 0.000 description 3
- MQQBBLVOUUJKLH-HJPIBITLSA-N Ser-Ile-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O MQQBBLVOUUJKLH-HJPIBITLSA-N 0.000 description 3
- GZSZPKSBVAOGIE-CIUDSAMLSA-N Ser-Lys-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O GZSZPKSBVAOGIE-CIUDSAMLSA-N 0.000 description 3
- FPCGZYMRFFIYIH-CIUDSAMLSA-N Ser-Lys-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O FPCGZYMRFFIYIH-CIUDSAMLSA-N 0.000 description 3
- HHJFMHQYEAAOBM-ZLUOBGJFSA-N Ser-Ser-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O HHJFMHQYEAAOBM-ZLUOBGJFSA-N 0.000 description 3
- XQJCEKXQUJQNNK-ZLUOBGJFSA-N Ser-Ser-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O XQJCEKXQUJQNNK-ZLUOBGJFSA-N 0.000 description 3
- VGQVAVQWKJLIRM-FXQIFTODSA-N Ser-Ser-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O VGQVAVQWKJLIRM-FXQIFTODSA-N 0.000 description 3
- PCJLFYBAQZQOFE-KATARQTJSA-N Ser-Thr-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CO)N)O PCJLFYBAQZQOFE-KATARQTJSA-N 0.000 description 3
- RCOUFINCYASMDN-GUBZILKMSA-N Ser-Val-Met Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCSC)C(O)=O RCOUFINCYASMDN-GUBZILKMSA-N 0.000 description 3
- MFEBUIFJVPNZLO-OLHMAJIHSA-N Thr-Asp-Asn Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O MFEBUIFJVPNZLO-OLHMAJIHSA-N 0.000 description 3
- ASJDFGOPDCVXTG-KATARQTJSA-N Thr-Cys-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(O)=O ASJDFGOPDCVXTG-KATARQTJSA-N 0.000 description 3
- KWQBJOUOSNJDRR-XAVMHZPKSA-N Thr-Cys-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)N1CCC[C@@H]1C(=O)O)N)O KWQBJOUOSNJDRR-XAVMHZPKSA-N 0.000 description 3
- DSLHSTIUAPKERR-XGEHTFHBSA-N Thr-Cys-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(O)=O DSLHSTIUAPKERR-XGEHTFHBSA-N 0.000 description 3
- FIFDDJFLNVAVMS-RHYQMDGZSA-N Thr-Leu-Met Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(O)=O FIFDDJFLNVAVMS-RHYQMDGZSA-N 0.000 description 3
- NCXVJIQMWSGRHY-KXNHARMFSA-N Thr-Leu-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@@H]1C(=O)O)N)O NCXVJIQMWSGRHY-KXNHARMFSA-N 0.000 description 3
- BDGBHYCAZJPLHX-HJGDQZAQSA-N Thr-Lys-Asn Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O BDGBHYCAZJPLHX-HJGDQZAQSA-N 0.000 description 3
- DXPURPNJDFCKKO-RHYQMDGZSA-N Thr-Lys-Val Chemical compound CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)[C@@H](C)O)C(O)=O DXPURPNJDFCKKO-RHYQMDGZSA-N 0.000 description 3
- MROIJTGJGIDEEJ-RCWTZXSCSA-N Thr-Pro-Pro Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 MROIJTGJGIDEEJ-RCWTZXSCSA-N 0.000 description 3
- NQQMWWVVGIXUOX-SVSWQMSJSA-N Thr-Ser-Ile Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O NQQMWWVVGIXUOX-SVSWQMSJSA-N 0.000 description 3
- BEZTUFWTPVOROW-KJEVXHAQSA-N Thr-Tyr-Arg Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N)O BEZTUFWTPVOROW-KJEVXHAQSA-N 0.000 description 3
- UKINEYBQXPMOJO-UBHSHLNASA-N Trp-Asn-Ser Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CO)C(=O)O)N UKINEYBQXPMOJO-UBHSHLNASA-N 0.000 description 3
- DQDXHYIEITXNJY-BPUTZDHNSA-N Trp-Gln-Gln Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N DQDXHYIEITXNJY-BPUTZDHNSA-N 0.000 description 3
- SCCKSNREWHMKOJ-SRVKXCTJSA-N Tyr-Asn-Ser Chemical compound N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O SCCKSNREWHMKOJ-SRVKXCTJSA-N 0.000 description 3
- NSTPFWRAIDTNGH-BZSNNMDCSA-N Tyr-Asn-Tyr Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O NSTPFWRAIDTNGH-BZSNNMDCSA-N 0.000 description 3
- SINRIKQYQJRGDQ-MEYUZBJRSA-N Tyr-Lys-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 SINRIKQYQJRGDQ-MEYUZBJRSA-N 0.000 description 3
- MQGGXGKQSVEQHR-KKUMJFAQSA-N Tyr-Ser-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 MQGGXGKQSVEQHR-KKUMJFAQSA-N 0.000 description 3
- NHOVZGFNTGMYMI-KKUMJFAQSA-N Tyr-Ser-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 NHOVZGFNTGMYMI-KKUMJFAQSA-N 0.000 description 3
- RIVVDNTUSRVTQT-IRIUXVKKSA-N Tyr-Thr-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N)O RIVVDNTUSRVTQT-IRIUXVKKSA-N 0.000 description 3
- GNWUWQAVVJQREM-NHCYSSNCSA-N Val-Asn-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N GNWUWQAVVJQREM-NHCYSSNCSA-N 0.000 description 3
- QHDXUYOYTPWCSK-RCOVLWMOSA-N Val-Asp-Gly Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)NCC(=O)O)N QHDXUYOYTPWCSK-RCOVLWMOSA-N 0.000 description 3
- WDIGUPHXPBMODF-UMNHJUIQSA-N Val-Glu-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N1CCC[C@@H]1C(=O)O)N WDIGUPHXPBMODF-UMNHJUIQSA-N 0.000 description 3
- OQWNEUXPKHIEJO-NRPADANISA-N Val-Glu-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CO)C(=O)O)N OQWNEUXPKHIEJO-NRPADANISA-N 0.000 description 3
- UEHRGZCNLSWGHK-DLOVCJGASA-N Val-Glu-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O UEHRGZCNLSWGHK-DLOVCJGASA-N 0.000 description 3
- ZIGZPYJXIWLQFC-QTKMDUPCSA-N Val-His-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](C(C)C)N)O ZIGZPYJXIWLQFC-QTKMDUPCSA-N 0.000 description 3
- XTDDIVQWDXMRJL-IHRRRGAJSA-N Val-Leu-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](C(C)C)N XTDDIVQWDXMRJL-IHRRRGAJSA-N 0.000 description 3
- SYSWVVCYSXBVJG-RHYQMDGZSA-N Val-Leu-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N)O SYSWVVCYSXBVJG-RHYQMDGZSA-N 0.000 description 3
- KRAHMIJVUPUOTQ-DCAQKATOSA-N Val-Ser-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N KRAHMIJVUPUOTQ-DCAQKATOSA-N 0.000 description 3
- ZLNYBMWGPOKSLW-LSJOCFKGSA-N Val-Val-Asp Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O ZLNYBMWGPOKSLW-LSJOCFKGSA-N 0.000 description 3
- LLJLBRRXKZTTRD-GUBZILKMSA-N Val-Val-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(=O)O)N LLJLBRRXKZTTRD-GUBZILKMSA-N 0.000 description 3
- 239000000556 agonist Substances 0.000 description 3
- 108010005233 alanylglutamic acid Proteins 0.000 description 3
- 125000000217 alkyl group Chemical group 0.000 description 3
- 108010050025 alpha-glutamyltryptophan Proteins 0.000 description 3
- 125000000539 amino acid group Chemical group 0.000 description 3
- 230000033115 angiogenesis Effects 0.000 description 3
- 229940124650 anti-cancer therapies Drugs 0.000 description 3
- 230000005809 anti-tumor immunity Effects 0.000 description 3
- 238000011319 anticancer therapy Methods 0.000 description 3
- 238000003556 assay Methods 0.000 description 3
- 206010003549 asthenia Diseases 0.000 description 3
- 210000002798 bone marrow cell Anatomy 0.000 description 3
- 210000004899 c-terminal region Anatomy 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 239000007979 citrate buffer Substances 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 230000034994 death Effects 0.000 description 3
- 206010061428 decreased appetite Diseases 0.000 description 3
- FSXRLASFHBWESK-UHFFFAOYSA-N dipeptide phenylalanyl-tyrosine Natural products C=1C=C(O)C=CC=1CC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FSXRLASFHBWESK-UHFFFAOYSA-N 0.000 description 3
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 3
- 150000002016 disaccharides Chemical class 0.000 description 3
- 230000017188 evasion or tolerance of host immune response Effects 0.000 description 3
- 230000013595 glycosylation Effects 0.000 description 3
- 238000006206 glycosylation reaction Methods 0.000 description 3
- 108010000434 glycyl-alanyl-leucine Proteins 0.000 description 3
- 108010050475 glycyl-leucyl-tyrosine Proteins 0.000 description 3
- 210000002865 immune cell Anatomy 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 229940042040 innovative drug Drugs 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 108010027338 isoleucylcysteine Proteins 0.000 description 3
- 108010044311 leucyl-glycyl-glycine Proteins 0.000 description 3
- 108010000761 leucylarginine Proteins 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 108010009298 lysylglutamic acid Proteins 0.000 description 3
- 210000002540 macrophage Anatomy 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 238000006386 neutralization reaction Methods 0.000 description 3
- 210000000440 neutrophil Anatomy 0.000 description 3
- 108020004707 nucleic acids Proteins 0.000 description 3
- 102000039446 nucleic acids Human genes 0.000 description 3
- 108010024654 phenylalanyl-prolyl-alanine Proteins 0.000 description 3
- 239000008363 phosphate buffer Substances 0.000 description 3
- 239000002953 phosphate buffered saline Substances 0.000 description 3
- 229920000136 polysorbate Polymers 0.000 description 3
- 229910052700 potassium Inorganic materials 0.000 description 3
- 239000011591 potassium Substances 0.000 description 3
- 230000003389 potentiating effect Effects 0.000 description 3
- 230000000770 proinflammatory effect Effects 0.000 description 3
- 108010070643 prolylglutamic acid Proteins 0.000 description 3
- 108010053725 prolylvaline Proteins 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 210000003289 regulatory T cell Anatomy 0.000 description 3
- 230000003248 secreting effect Effects 0.000 description 3
- 108010048397 seryl-lysyl-leucine Proteins 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 229960003787 sorafenib Drugs 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 229960002317 succinimide Drugs 0.000 description 3
- 235000000346 sugar Nutrition 0.000 description 3
- 238000001356 surgical procedure Methods 0.000 description 3
- 230000002195 synergetic effect Effects 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- 230000014616 translation Effects 0.000 description 3
- 108010036387 trimethionine Proteins 0.000 description 3
- 108010080629 tryptophan-leucine Proteins 0.000 description 3
- 230000005751 tumor progression Effects 0.000 description 3
- 108010052774 valyl-lysyl-glycyl-phenylalanyl-tyrosine Proteins 0.000 description 3
- 108010027345 wheylin-1 peptide Proteins 0.000 description 3
- XVZCXCTYGHPNEM-IHRRRGAJSA-N (2s)-1-[(2s)-2-[[(2s)-2-amino-4-methylpentanoyl]amino]-4-methylpentanoyl]pyrrolidine-2-carboxylic acid Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(O)=O XVZCXCTYGHPNEM-IHRRRGAJSA-N 0.000 description 2
- DIHXSRXTECMMJY-MURFETPASA-N 2-[dimethyl-[(9z,12z)-octadeca-9,12-dienyl]azaniumyl]acetate Chemical group CCCCC\C=C/C\C=C/CCCCCCCC[N+](C)(C)CC([O-])=O DIHXSRXTECMMJY-MURFETPASA-N 0.000 description 2
- RZVAJINKPMORJF-UHFFFAOYSA-N Acetaminophen Chemical compound CC(=O)NC1=CC=C(O)C=C1 RZVAJINKPMORJF-UHFFFAOYSA-N 0.000 description 2
- KIUYPHAMDKDICO-WHFBIAKZSA-N Ala-Asp-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(O)=O KIUYPHAMDKDICO-WHFBIAKZSA-N 0.000 description 2
- BTYTYHBSJKQBQA-GCJQMDKQSA-N Ala-Asp-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](C)N)O BTYTYHBSJKQBQA-GCJQMDKQSA-N 0.000 description 2
- SMCGQGDVTPFXKB-XPUUQOCRSA-N Ala-Gly-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@H](C)N SMCGQGDVTPFXKB-XPUUQOCRSA-N 0.000 description 2
- WZGZDOXCDLLTHE-SYWGBEHUSA-N Ala-Trp-Ile Chemical compound C1=CC=C2C(C[C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(O)=O)NC(=O)[C@H](C)N)=CNC2=C1 WZGZDOXCDLLTHE-SYWGBEHUSA-N 0.000 description 2
- PHQXWZGXKAFWAZ-ZLIFDBKOSA-N Ala-Trp-Lys Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@@H](N)C)C(=O)N[C@@H](CCCCN)C(O)=O)=CNC2=C1 PHQXWZGXKAFWAZ-ZLIFDBKOSA-N 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-M Aminoacetate Chemical compound NCC([O-])=O DHMQDGOQFOQNFH-UHFFFAOYSA-M 0.000 description 2
- JGDGLDNAQJJGJI-AVGNSLFASA-N Arg-Arg-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)N JGDGLDNAQJJGJI-AVGNSLFASA-N 0.000 description 2
- KBBKCNHWCDJPGN-GUBZILKMSA-N Arg-Gln-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O KBBKCNHWCDJPGN-GUBZILKMSA-N 0.000 description 2
- RFXXUWGNVRJTNQ-QXEWZRGKSA-N Arg-Gly-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CCCN=C(N)N)N RFXXUWGNVRJTNQ-QXEWZRGKSA-N 0.000 description 2
- OQCWXQJLCDPRHV-UWVGGRQHSA-N Arg-Gly-Leu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC(C)C)C(O)=O OQCWXQJLCDPRHV-UWVGGRQHSA-N 0.000 description 2
- PYZPXCZNQSEHDT-GUBZILKMSA-N Arg-Met-Asn Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N PYZPXCZNQSEHDT-GUBZILKMSA-N 0.000 description 2
- XSPKAHFVDKRGRL-DCAQKATOSA-N Arg-Pro-Glu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O XSPKAHFVDKRGRL-DCAQKATOSA-N 0.000 description 2
- BECXEHHOZNFFFX-IHRRRGAJSA-N Arg-Ser-Tyr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O BECXEHHOZNFFFX-IHRRRGAJSA-N 0.000 description 2
- QEYJFBMTSMLPKZ-ZKWXMUAHSA-N Asn-Ala-Val Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O QEYJFBMTSMLPKZ-ZKWXMUAHSA-N 0.000 description 2
- APHUDFFMXFYRKP-CIUDSAMLSA-N Asn-Asn-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC(=O)N)N APHUDFFMXFYRKP-CIUDSAMLSA-N 0.000 description 2
- BHQQRVARKXWXPP-ACZMJKKPSA-N Asn-Asp-Glu Chemical compound C(CC(=O)O)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CC(=O)N)N BHQQRVARKXWXPP-ACZMJKKPSA-N 0.000 description 2
- QNJIRRVTOXNGMH-GUBZILKMSA-N Asn-Gln-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CC(N)=O QNJIRRVTOXNGMH-GUBZILKMSA-N 0.000 description 2
- IBLAOXSULLECQZ-IUKAMOBKSA-N Asn-Ile-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H](N)CC(N)=O IBLAOXSULLECQZ-IUKAMOBKSA-N 0.000 description 2
- RCFGLXMZDYNRSC-CIUDSAMLSA-N Asn-Lys-Ala Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O RCFGLXMZDYNRSC-CIUDSAMLSA-N 0.000 description 2
- IDUUACUJKUXKKD-VEVYYDQMSA-N Asn-Pro-Thr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(O)=O IDUUACUJKUXKKD-VEVYYDQMSA-N 0.000 description 2
- UXHYOWXTJLBEPG-GSSVUCPTSA-N Asn-Thr-Thr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O UXHYOWXTJLBEPG-GSSVUCPTSA-N 0.000 description 2
- IXIWEFWRKIUMQX-DCAQKATOSA-N Asp-Arg-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O IXIWEFWRKIUMQX-DCAQKATOSA-N 0.000 description 2
- UQBGYPFHWFZMCD-ZLUOBGJFSA-N Asp-Asn-Asn Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O UQBGYPFHWFZMCD-ZLUOBGJFSA-N 0.000 description 2
- JGDBHIVECJGXJA-FXQIFTODSA-N Asp-Asp-Arg Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O JGDBHIVECJGXJA-FXQIFTODSA-N 0.000 description 2
- PYXXJFRXIYAESU-PCBIJLKTSA-N Asp-Ile-Phe Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O PYXXJFRXIYAESU-PCBIJLKTSA-N 0.000 description 2
- PCJOFZYFFMBZKC-PCBIJLKTSA-N Asp-Phe-Ile Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O PCJOFZYFFMBZKC-PCBIJLKTSA-N 0.000 description 2
- GFYOIYJJMSHLSN-QXEWZRGKSA-N Asp-Val-Arg Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O GFYOIYJJMSHLSN-QXEWZRGKSA-N 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 2
- 206010004593 Bile duct cancer Diseases 0.000 description 2
- BPYKTIZUTYGOLE-IFADSCNNSA-N Bilirubin Chemical compound N1C(=O)C(C)=C(C=C)\C1=C\C1=C(C)C(CCC(O)=O)=C(CC2=C(C(C)=C(\C=C/3C(=C(C=C)C(=O)N\3)C)N2)CCC(O)=O)N1 BPYKTIZUTYGOLE-IFADSCNNSA-N 0.000 description 2
- 101100505161 Caenorhabditis elegans mel-32 gene Proteins 0.000 description 2
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 2
- 206010048610 Cardiotoxicity Diseases 0.000 description 2
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 2
- YASYEJJMZJALEJ-UHFFFAOYSA-N Citric acid monohydrate Chemical compound O.OC(=O)CC(O)(C(O)=O)CC(O)=O YASYEJJMZJALEJ-UHFFFAOYSA-N 0.000 description 2
- VPQZSNQICFCCSO-BJDJZHNGSA-N Cys-Leu-Ile Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O VPQZSNQICFCCSO-BJDJZHNGSA-N 0.000 description 2
- BCWIFCLVCRAIQK-ZLUOBGJFSA-N Cys-Ser-Cys Chemical compound C([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CS)N)O BCWIFCLVCRAIQK-ZLUOBGJFSA-N 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 2
- 108010090461 DFG peptide Proteins 0.000 description 2
- 102100037241 Endoglin Human genes 0.000 description 2
- 108010036395 Endoglin Proteins 0.000 description 2
- CTKXFMQHOOWWEB-UHFFFAOYSA-N Ethylene oxide/propylene oxide copolymer Chemical compound CCCOC(C)COCCO CTKXFMQHOOWWEB-UHFFFAOYSA-N 0.000 description 2
- 208000010201 Exanthema Diseases 0.000 description 2
- ZDJZEGYVKANKED-NRPADANISA-N Gln-Cys-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(O)=O ZDJZEGYVKANKED-NRPADANISA-N 0.000 description 2
- ZEEPYMXTJWIMSN-GUBZILKMSA-N Gln-Lys-Ser Chemical compound NCCCC[C@@H](C(=O)N[C@@H](CO)C(O)=O)NC(=O)[C@@H](N)CCC(N)=O ZEEPYMXTJWIMSN-GUBZILKMSA-N 0.000 description 2
- KLJMRPIBBLTDGE-ACZMJKKPSA-N Glu-Cys-Asn Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(O)=O KLJMRPIBBLTDGE-ACZMJKKPSA-N 0.000 description 2
- RAUDKMVXNOWDLS-WDSKDSINSA-N Glu-Gly-Ser Chemical compound OC(=O)CC[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O RAUDKMVXNOWDLS-WDSKDSINSA-N 0.000 description 2
- FMBWLLMUPXTXFC-SDDRHHMPSA-N Glu-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(=O)O)N)C(=O)O FMBWLLMUPXTXFC-SDDRHHMPSA-N 0.000 description 2
- YHOJJFFTSMWVGR-HJGDQZAQSA-N Glu-Met-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O YHOJJFFTSMWVGR-HJGDQZAQSA-N 0.000 description 2
- CAQXJMUDOLSBPF-SUSMZKCASA-N Glu-Thr-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O CAQXJMUDOLSBPF-SUSMZKCASA-N 0.000 description 2
- UCZXXMREFIETQW-AVGNSLFASA-N Glu-Tyr-Asn Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(O)=O UCZXXMREFIETQW-AVGNSLFASA-N 0.000 description 2
- QRWPTXLWHHTOCO-DZKIICNBSA-N Glu-Val-Tyr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O QRWPTXLWHHTOCO-DZKIICNBSA-N 0.000 description 2
- UGVQELHRNUDMAA-BYPYZUCNSA-N Gly-Ala-Gly Chemical compound [NH3+]CC(=O)N[C@@H](C)C(=O)NCC([O-])=O UGVQELHRNUDMAA-BYPYZUCNSA-N 0.000 description 2
- JRDYDYXZKFNNRQ-XPUUQOCRSA-N Gly-Ala-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)CN JRDYDYXZKFNNRQ-XPUUQOCRSA-N 0.000 description 2
- KRRMJKMGWWXWDW-STQMWFEESA-N Gly-Arg-Phe Chemical compound NC(=N)NCCC[C@H](NC(=O)CN)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 KRRMJKMGWWXWDW-STQMWFEESA-N 0.000 description 2
- VXKCPBPQEKKERH-IUCAKERBSA-N Gly-Arg-Pro Chemical compound NC(N)=NCCC[C@H](NC(=O)CN)C(=O)N1CCC[C@H]1C(O)=O VXKCPBPQEKKERH-IUCAKERBSA-N 0.000 description 2
- QPDUVFSVVAOUHE-XVKPBYJWSA-N Gly-Gln-Val Chemical compound CC(C)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)CN)C(O)=O QPDUVFSVVAOUHE-XVKPBYJWSA-N 0.000 description 2
- JSNNHGHYGYMVCK-XVKPBYJWSA-N Gly-Glu-Val Chemical compound [H]NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O JSNNHGHYGYMVCK-XVKPBYJWSA-N 0.000 description 2
- YWAQATDNEKZFFK-BYPYZUCNSA-N Gly-Gly-Ser Chemical compound NCC(=O)NCC(=O)N[C@@H](CO)C(O)=O YWAQATDNEKZFFK-BYPYZUCNSA-N 0.000 description 2
- SWQALSGKVLYKDT-UHFFFAOYSA-N Gly-Ile-Ala Natural products NCC(=O)NC(C(C)CC)C(=O)NC(C)C(O)=O SWQALSGKVLYKDT-UHFFFAOYSA-N 0.000 description 2
- SCWYHUQOOFRVHP-MBLNEYKQSA-N Gly-Ile-Thr Chemical compound NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O SCWYHUQOOFRVHP-MBLNEYKQSA-N 0.000 description 2
- UHPAZODVFFYEEL-QWRGUYRKSA-N Gly-Leu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)CN UHPAZODVFFYEEL-QWRGUYRKSA-N 0.000 description 2
- GAFKBWKVXNERFA-QWRGUYRKSA-N Gly-Phe-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)CN)CC1=CC=CC=C1 GAFKBWKVXNERFA-QWRGUYRKSA-N 0.000 description 2
- WNGHUXFWEWTKAO-YUMQZZPRSA-N Gly-Ser-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)CN WNGHUXFWEWTKAO-YUMQZZPRSA-N 0.000 description 2
- CUVBTVWFVIIDOC-YEPSODPASA-N Gly-Thr-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)CN CUVBTVWFVIIDOC-YEPSODPASA-N 0.000 description 2
- GWCJMBNBFYBQCV-XPUUQOCRSA-N Gly-Val-Ala Chemical compound NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O GWCJMBNBFYBQCV-XPUUQOCRSA-N 0.000 description 2
- IWXMHXYOACDSIA-PYJNHQTQSA-N His-Ile-Val Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C(C)C)C(O)=O IWXMHXYOACDSIA-PYJNHQTQSA-N 0.000 description 2
- HBGKOLSGLYMWSW-DCAQKATOSA-N His-Pro-Cys Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CC2=CN=CN2)N)C(=O)N[C@@H](CS)C(=O)O HBGKOLSGLYMWSW-DCAQKATOSA-N 0.000 description 2
- QLBXWYXMLHAREM-PYJNHQTQSA-N His-Val-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC1=CN=CN1)N QLBXWYXMLHAREM-PYJNHQTQSA-N 0.000 description 2
- HDOYNXLPTRQLAD-JBDRJPRFSA-N Ile-Ala-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)O)N HDOYNXLPTRQLAD-JBDRJPRFSA-N 0.000 description 2
- LPXHYGGZJOCAFR-MNXVOIDGSA-N Ile-Glu-Leu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CC(C)C)C(=O)O)N LPXHYGGZJOCAFR-MNXVOIDGSA-N 0.000 description 2
- HUORUFRRJHELPD-MNXVOIDGSA-N Ile-Leu-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N HUORUFRRJHELPD-MNXVOIDGSA-N 0.000 description 2
- DSDPLOODKXISDT-XUXIUFHCSA-N Ile-Leu-Val Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O DSDPLOODKXISDT-XUXIUFHCSA-N 0.000 description 2
- YSGBJIQXTIVBHZ-AJNGGQMLSA-N Ile-Lys-Leu Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O YSGBJIQXTIVBHZ-AJNGGQMLSA-N 0.000 description 2
- CAHCWMVNBZJVAW-NAKRPEOUSA-N Ile-Pro-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)O)N CAHCWMVNBZJVAW-NAKRPEOUSA-N 0.000 description 2
- HXIDVIFHRYRXLZ-NAKRPEOUSA-N Ile-Ser-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)O)N HXIDVIFHRYRXLZ-NAKRPEOUSA-N 0.000 description 2
- CNMOKANDJMLAIF-CIQUZCHMSA-N Ile-Thr-Ala Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O CNMOKANDJMLAIF-CIQUZCHMSA-N 0.000 description 2
- NXRNRBOKDBIVKQ-CXTHYWKRSA-N Ile-Tyr-Tyr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)N NXRNRBOKDBIVKQ-CXTHYWKRSA-N 0.000 description 2
- 206010051792 Infusion related reaction Diseases 0.000 description 2
- SITWEMZOJNKJCH-UHFFFAOYSA-N L-alanine-L-arginine Natural products CC(N)C(=O)NC(C(O)=O)CCCNC(N)=N SITWEMZOJNKJCH-UHFFFAOYSA-N 0.000 description 2
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 2
- YOZCKMXHBYKOMQ-IHRRRGAJSA-N Leu-Arg-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)O)N YOZCKMXHBYKOMQ-IHRRRGAJSA-N 0.000 description 2
- MMEDVBWCMGRKKC-GARJFASQSA-N Leu-Asp-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N1CCC[C@@H]1C(=O)O)N MMEDVBWCMGRKKC-GARJFASQSA-N 0.000 description 2
- VQPPIMUZCZCOIL-GUBZILKMSA-N Leu-Gln-Ala Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(O)=O VQPPIMUZCZCOIL-GUBZILKMSA-N 0.000 description 2
- GLBNEGIOFRVRHO-JYJNAYRXSA-N Leu-Gln-Phe Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O GLBNEGIOFRVRHO-JYJNAYRXSA-N 0.000 description 2
- YVKSMSDXKMSIRX-GUBZILKMSA-N Leu-Glu-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O YVKSMSDXKMSIRX-GUBZILKMSA-N 0.000 description 2
- VGPCJSXPPOQPBK-YUMQZZPRSA-N Leu-Gly-Ser Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O VGPCJSXPPOQPBK-YUMQZZPRSA-N 0.000 description 2
- BJWKOATWNQJPSK-SRVKXCTJSA-N Leu-Met-Glu Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N BJWKOATWNQJPSK-SRVKXCTJSA-N 0.000 description 2
- ZDJQVSIPFLMNOX-RHYQMDGZSA-N Leu-Thr-Arg Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N ZDJQVSIPFLMNOX-RHYQMDGZSA-N 0.000 description 2
- AIMGJYMCTAABEN-GVXVVHGQSA-N Leu-Val-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O AIMGJYMCTAABEN-GVXVVHGQSA-N 0.000 description 2
- FMFNIDICDKEMOE-XUXIUFHCSA-N Leu-Val-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O FMFNIDICDKEMOE-XUXIUFHCSA-N 0.000 description 2
- QESXLSQLQHHTIX-RHYQMDGZSA-N Leu-Val-Thr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QESXLSQLQHHTIX-RHYQMDGZSA-N 0.000 description 2
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 2
- WSXTWLJHTLRFLW-SRVKXCTJSA-N Lys-Ala-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(O)=O WSXTWLJHTLRFLW-SRVKXCTJSA-N 0.000 description 2
- NTBFKPBULZGXQL-KKUMJFAQSA-N Lys-Asp-Tyr Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 NTBFKPBULZGXQL-KKUMJFAQSA-N 0.000 description 2
- SFQPJNQDUUYCLA-BJDJZHNGSA-N Lys-Cys-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CCCCN)N SFQPJNQDUUYCLA-BJDJZHNGSA-N 0.000 description 2
- NNCDAORZCMPZPX-GUBZILKMSA-N Lys-Gln-Ser Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CO)C(=O)O)N NNCDAORZCMPZPX-GUBZILKMSA-N 0.000 description 2
- WRODMZBHNNPRLN-SRVKXCTJSA-N Lys-Leu-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O WRODMZBHNNPRLN-SRVKXCTJSA-N 0.000 description 2
- WBSCNDJQPKSPII-KKUMJFAQSA-N Lys-Lys-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(O)=O WBSCNDJQPKSPII-KKUMJFAQSA-N 0.000 description 2
- KVNLHIXLLZBAFQ-RWMBFGLXSA-N Lys-Met-Pro Chemical compound CSCC[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCCCN)N KVNLHIXLLZBAFQ-RWMBFGLXSA-N 0.000 description 2
- AEIIJFBQVGYVEV-YESZJQIVSA-N Lys-Phe-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=CC=C2)NC(=O)[C@H](CCCCN)N)C(=O)O AEIIJFBQVGYVEV-YESZJQIVSA-N 0.000 description 2
- YTJFXEDRUOQGSP-DCAQKATOSA-N Lys-Pro-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O YTJFXEDRUOQGSP-DCAQKATOSA-N 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- FYRUJIJAUPHUNB-IUCAKERBSA-N Met-Gly-Arg Chemical compound CSCC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCCNC(N)=N FYRUJIJAUPHUNB-IUCAKERBSA-N 0.000 description 2
- WPTHAGXMYDRPFD-SRVKXCTJSA-N Met-Lys-Glu Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O WPTHAGXMYDRPFD-SRVKXCTJSA-N 0.000 description 2
- VWWGEKCAPBMIFE-SRVKXCTJSA-N Met-Met-Met Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCSC)C(O)=O VWWGEKCAPBMIFE-SRVKXCTJSA-N 0.000 description 2
- 108091092878 Microsatellite Proteins 0.000 description 2
- PESQCPHRXOFIPX-UHFFFAOYSA-N N-L-methionyl-L-tyrosine Natural products CSCCC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 PESQCPHRXOFIPX-UHFFFAOYSA-N 0.000 description 2
- XMBSYZWANAQXEV-UHFFFAOYSA-N N-alpha-L-glutamyl-L-phenylalanine Natural products OC(=O)CCC(N)C(=O)NC(C(O)=O)CC1=CC=CC=C1 XMBSYZWANAQXEV-UHFFFAOYSA-N 0.000 description 2
- BQVUABVGYYSDCJ-UHFFFAOYSA-N Nalpha-L-Leucyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)C(N)CC(C)C)C(O)=O)=CNC2=C1 BQVUABVGYYSDCJ-UHFFFAOYSA-N 0.000 description 2
- 206010029260 Neuroblastoma Diseases 0.000 description 2
- 108091028043 Nucleic acid sequence Proteins 0.000 description 2
- MFQXSDWKUXTOPZ-DZKIICNBSA-N Phe-Gln-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CC1=CC=CC=C1)N MFQXSDWKUXTOPZ-DZKIICNBSA-N 0.000 description 2
- ZJPGOXWRFNKIQL-JYJNAYRXSA-N Phe-Pro-Pro Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(O)=O)C1=CC=CC=C1 ZJPGOXWRFNKIQL-JYJNAYRXSA-N 0.000 description 2
- XDMMOISUAHXXFD-SRVKXCTJSA-N Phe-Ser-Asp Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O XDMMOISUAHXXFD-SRVKXCTJSA-N 0.000 description 2
- GKRCCTYAGQPMMP-IHRRRGAJSA-N Phe-Ser-Met Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(O)=O GKRCCTYAGQPMMP-IHRRRGAJSA-N 0.000 description 2
- QTDBZORPVYTRJU-KKXDTOCCSA-N Phe-Tyr-Ala Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C)C(O)=O QTDBZORPVYTRJU-KKXDTOCCSA-N 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- 229920001213 Polysorbate 20 Polymers 0.000 description 2
- VDGTVWFMRXVQCT-GUBZILKMSA-N Pro-Glu-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1 VDGTVWFMRXVQCT-GUBZILKMSA-N 0.000 description 2
- CLNJSLSHKJECME-BQBZGAKWSA-N Pro-Gly-Ala Chemical compound OC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H]1CCCN1 CLNJSLSHKJECME-BQBZGAKWSA-N 0.000 description 2
- LPGSNRSLPHRNBW-AVGNSLFASA-N Pro-His-Val Chemical compound C([C@@H](C(=O)N[C@@H](C(C)C)C([O-])=O)NC(=O)[C@H]1[NH2+]CCC1)C1=CN=CN1 LPGSNRSLPHRNBW-AVGNSLFASA-N 0.000 description 2
- OFGUOWQVEGTVNU-DCAQKATOSA-N Pro-Lys-Ala Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O OFGUOWQVEGTVNU-DCAQKATOSA-N 0.000 description 2
- MHHQQZIFLWFZGR-DCAQKATOSA-N Pro-Lys-Ser Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O MHHQQZIFLWFZGR-DCAQKATOSA-N 0.000 description 2
- CGSOWZUPLOKYOR-AVGNSLFASA-N Pro-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 CGSOWZUPLOKYOR-AVGNSLFASA-N 0.000 description 2
- IURWWZYKYPEANQ-HJGDQZAQSA-N Pro-Thr-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(O)=O IURWWZYKYPEANQ-HJGDQZAQSA-N 0.000 description 2
- SHTKRJHDMNSKRM-ULQDDVLXSA-N Pro-Tyr-His Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)N[C@@H](CC3=CN=CN3)C(=O)O SHTKRJHDMNSKRM-ULQDDVLXSA-N 0.000 description 2
- LVVBAKCGXXUHFO-ZLUOBGJFSA-N Ser-Ala-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(O)=O LVVBAKCGXXUHFO-ZLUOBGJFSA-N 0.000 description 2
- JJKSSJVYOVRJMZ-FXQIFTODSA-N Ser-Arg-Cys Chemical compound C(C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CO)N)CN=C(N)N JJKSSJVYOVRJMZ-FXQIFTODSA-N 0.000 description 2
- UCXDHBORXLVBNC-ZLUOBGJFSA-N Ser-Asn-Cys Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(O)=O UCXDHBORXLVBNC-ZLUOBGJFSA-N 0.000 description 2
- OLIJLNWFEQEFDM-SRVKXCTJSA-N Ser-Asp-Phe Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 OLIJLNWFEQEFDM-SRVKXCTJSA-N 0.000 description 2
- TUYBIWUZWJUZDD-ACZMJKKPSA-N Ser-Cys-Gln Chemical compound OC[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@H](C(O)=O)CCC(N)=O TUYBIWUZWJUZDD-ACZMJKKPSA-N 0.000 description 2
- UCOYFSCEIWQYNL-FXQIFTODSA-N Ser-Cys-Met Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCSC)C(O)=O UCOYFSCEIWQYNL-FXQIFTODSA-N 0.000 description 2
- AEGUWTFAQQWVLC-BQBZGAKWSA-N Ser-Gly-Arg Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O AEGUWTFAQQWVLC-BQBZGAKWSA-N 0.000 description 2
- DLPXTCTVNDTYGJ-JBDRJPRFSA-N Ser-Ile-Cys Chemical compound OC[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CS)C(O)=O DLPXTCTVNDTYGJ-JBDRJPRFSA-N 0.000 description 2
- FUMGHWDRRFCKEP-CIUDSAMLSA-N Ser-Leu-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O FUMGHWDRRFCKEP-CIUDSAMLSA-N 0.000 description 2
- VMLONWHIORGALA-SRVKXCTJSA-N Ser-Leu-Leu Chemical compound CC(C)C[C@@H](C([O-])=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]([NH3+])CO VMLONWHIORGALA-SRVKXCTJSA-N 0.000 description 2
- UGGWCAFQPKANMW-FXQIFTODSA-N Ser-Met-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C)C(O)=O UGGWCAFQPKANMW-FXQIFTODSA-N 0.000 description 2
- XGQKSRGHEZNWIS-IHRRRGAJSA-N Ser-Pro-Tyr Chemical compound N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O XGQKSRGHEZNWIS-IHRRRGAJSA-N 0.000 description 2
- CKDXFSPMIDSMGV-GUBZILKMSA-N Ser-Pro-Val Chemical compound [H]N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(O)=O CKDXFSPMIDSMGV-GUBZILKMSA-N 0.000 description 2
- GYDFRTRSSXOZCR-ACZMJKKPSA-N Ser-Ser-Glu Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O GYDFRTRSSXOZCR-ACZMJKKPSA-N 0.000 description 2
- PYTKULIABVRXSC-BWBBJGPYSA-N Ser-Ser-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O PYTKULIABVRXSC-BWBBJGPYSA-N 0.000 description 2
- PLQWGQUNUPMNOD-KKUMJFAQSA-N Ser-Tyr-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(C)C)C(O)=O PLQWGQUNUPMNOD-KKUMJFAQSA-N 0.000 description 2
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 2
- 229940126547 T-cell immunoglobulin mucin-3 Drugs 0.000 description 2
- KZUJCMPVNXOBAF-LKXGYXEUSA-N Thr-Cys-Asp Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(O)=O)C(O)=O KZUJCMPVNXOBAF-LKXGYXEUSA-N 0.000 description 2
- HJOSVGCWOTYJFG-WDCWCFNPSA-N Thr-Glu-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)O)N)O HJOSVGCWOTYJFG-WDCWCFNPSA-N 0.000 description 2
- IMULJHHGAUZZFE-MBLNEYKQSA-N Thr-Gly-Ile Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H]([C@@H](C)CC)C(O)=O IMULJHHGAUZZFE-MBLNEYKQSA-N 0.000 description 2
- YJCVECXVYHZOBK-KNZXXDILSA-N Thr-Ile-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H]([C@@H](C)O)N YJCVECXVYHZOBK-KNZXXDILSA-N 0.000 description 2
- FQPDRTDDEZXCEC-SVSWQMSJSA-N Thr-Ile-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O FQPDRTDDEZXCEC-SVSWQMSJSA-N 0.000 description 2
- VRUFCJZQDACGLH-UVOCVTCTSA-N Thr-Leu-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O VRUFCJZQDACGLH-UVOCVTCTSA-N 0.000 description 2
- STUAPCLEDMKXKL-LKXGYXEUSA-N Thr-Ser-Asn Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O STUAPCLEDMKXKL-LKXGYXEUSA-N 0.000 description 2
- IQPWNQRRAJHOKV-KATARQTJSA-N Thr-Ser-Lys Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCCCN IQPWNQRRAJHOKV-KATARQTJSA-N 0.000 description 2
- LECUEEHKUFYOOV-ZJDVBMNYSA-N Thr-Thr-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@@H](N)[C@@H](C)O LECUEEHKUFYOOV-ZJDVBMNYSA-N 0.000 description 2
- YOPQYBJJNSIQGZ-JNPHEJMOSA-N Thr-Tyr-Tyr Chemical compound C([C@H](NC(=O)[C@@H](N)[C@H](O)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 YOPQYBJJNSIQGZ-JNPHEJMOSA-N 0.000 description 2
- KPMIQCXJDVKWKO-IFFSRLJSSA-N Thr-Val-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O KPMIQCXJDVKWKO-IFFSRLJSSA-N 0.000 description 2
- 206010066901 Treatment failure Diseases 0.000 description 2
- MVHHTXAUJCIOMZ-WDSOQIARSA-N Trp-Arg-Lys Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)O)N MVHHTXAUJCIOMZ-WDSOQIARSA-N 0.000 description 2
- SVGAWGVHFIYAEE-JSGCOSHPSA-N Trp-Gly-Gln Chemical compound C1=CC=C2C(C[C@H](N)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(O)=O)=CNC2=C1 SVGAWGVHFIYAEE-JSGCOSHPSA-N 0.000 description 2
- MKDXQPMIQPTTAW-SIXJUCDHSA-N Trp-Ile-His Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CC2=CNC3=CC=CC=C32)N MKDXQPMIQPTTAW-SIXJUCDHSA-N 0.000 description 2
- ULHASJWZGUEUNN-XIRDDKMYSA-N Trp-Lys-Ser Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O ULHASJWZGUEUNN-XIRDDKMYSA-N 0.000 description 2
- WMIUTJPFHMMUGY-ZFWWWQNUSA-N Trp-Pro-Gly Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CC2=CNC3=CC=CC=C32)N)C(=O)NCC(=O)O WMIUTJPFHMMUGY-ZFWWWQNUSA-N 0.000 description 2
- XOLLWQIBBLBAHQ-WDSOQIARSA-N Trp-Pro-Leu Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(O)=O XOLLWQIBBLBAHQ-WDSOQIARSA-N 0.000 description 2
- QHWMVGCEQAPQDK-UMPQAUOISA-N Trp-Thr-Arg Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)N)O QHWMVGCEQAPQDK-UMPQAUOISA-N 0.000 description 2
- 102000001742 Tumor Suppressor Proteins Human genes 0.000 description 2
- 108010040002 Tumor Suppressor Proteins Proteins 0.000 description 2
- BXJQKVDPRMLGKN-PMVMPFDFSA-N Tyr-Trp-Leu Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 BXJQKVDPRMLGKN-PMVMPFDFSA-N 0.000 description 2
- ZLFHAAGHGQBQQN-GUBZILKMSA-N Val-Ala-Pro Natural products CC(C)[C@H](N)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O ZLFHAAGHGQBQQN-GUBZILKMSA-N 0.000 description 2
- KOPBYUSPXBQIHD-NRPADANISA-N Val-Cys-Glu Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N KOPBYUSPXBQIHD-NRPADANISA-N 0.000 description 2
- CELJCNRXKZPTCX-XPUUQOCRSA-N Val-Gly-Ala Chemical compound CC(C)[C@H](N)C(=O)NCC(=O)N[C@@H](C)C(O)=O CELJCNRXKZPTCX-XPUUQOCRSA-N 0.000 description 2
- UMPVMAYCLYMYGA-ONGXEEELSA-N Val-Leu-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O UMPVMAYCLYMYGA-ONGXEEELSA-N 0.000 description 2
- RFKJNTRMXGCKFE-FHWLQOOXSA-N Val-Leu-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)CC(C)C)C(O)=O)=CNC2=C1 RFKJNTRMXGCKFE-FHWLQOOXSA-N 0.000 description 2
- QRVPEKJBBRYISE-XUXIUFHCSA-N Val-Lys-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C(C)C)N QRVPEKJBBRYISE-XUXIUFHCSA-N 0.000 description 2
- PZTZYZUTCPZWJH-FXQIFTODSA-N Val-Ser-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)O)N PZTZYZUTCPZWJH-FXQIFTODSA-N 0.000 description 2
- TVGWMCTYUFBXAP-QTKMDUPCSA-N Val-Thr-His Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](C(C)C)N)O TVGWMCTYUFBXAP-QTKMDUPCSA-N 0.000 description 2
- LCHZBEUVGAVMKS-RHYQMDGZSA-N Val-Thr-Leu Chemical compound CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)[C@@H](C)O)C(O)=O LCHZBEUVGAVMKS-RHYQMDGZSA-N 0.000 description 2
- 230000002776 aggregation Effects 0.000 description 2
- 238000004220 aggregation Methods 0.000 description 2
- 108010044940 alanylglutamine Proteins 0.000 description 2
- 108010087924 alanylproline Proteins 0.000 description 2
- 230000004075 alteration Effects 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 208000007502 anemia Diseases 0.000 description 2
- 108010077245 asparaginyl-proline Proteins 0.000 description 2
- 229960000074 biopharmaceutical Drugs 0.000 description 2
- 230000036765 blood level Effects 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 210000004413 cardiac myocyte Anatomy 0.000 description 2
- 231100000259 cardiotoxicity Toxicity 0.000 description 2
- YCIMNLLNPGFGHC-UHFFFAOYSA-N catechol Chemical compound OC1=CC=CC=C1O YCIMNLLNPGFGHC-UHFFFAOYSA-N 0.000 description 2
- 238000009104 chemotherapy regimen Methods 0.000 description 2
- 229960004106 citric acid Drugs 0.000 description 2
- 230000000295 complement effect Effects 0.000 description 2
- 229940109239 creatinine Drugs 0.000 description 2
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 2
- 102000003675 cytokine receptors Human genes 0.000 description 2
- 108010057085 cytokine receptors Proteins 0.000 description 2
- 229940127089 cytotoxic agent Drugs 0.000 description 2
- 230000002950 deficient Effects 0.000 description 2
- 230000037430 deletion Effects 0.000 description 2
- 238000012217 deletion Methods 0.000 description 2
- 239000013583 drug formulation Substances 0.000 description 2
- 230000002500 effect on skin Effects 0.000 description 2
- 210000003162 effector t lymphocyte Anatomy 0.000 description 2
- 229920001971 elastomer Polymers 0.000 description 2
- 238000011984 electrochemiluminescence immunoassay Methods 0.000 description 2
- 210000002919 epithelial cell Anatomy 0.000 description 2
- 230000008029 eradication Effects 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 201000005884 exanthem Diseases 0.000 description 2
- 238000000684 flow cytometry Methods 0.000 description 2
- 108010063718 gamma-glutamylaspartic acid Proteins 0.000 description 2
- 108010057083 glutamyl-aspartyl-leucine Proteins 0.000 description 2
- 230000009422 growth inhibiting effect Effects 0.000 description 2
- 230000007062 hydrolysis Effects 0.000 description 2
- 238000006460 hydrolysis reaction Methods 0.000 description 2
- 238000003384 imaging method Methods 0.000 description 2
- 230000005934 immune activation Effects 0.000 description 2
- 238000003018 immunoassay Methods 0.000 description 2
- 229940072221 immunoglobulins Drugs 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 108010057821 leucylproline Proteins 0.000 description 2
- 210000000265 leukocyte Anatomy 0.000 description 2
- 201000005202 lung cancer Diseases 0.000 description 2
- 208000020816 lung neoplasm Diseases 0.000 description 2
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 2
- 108010003700 lysyl aspartic acid Proteins 0.000 description 2
- 108010017391 lysylvaline Proteins 0.000 description 2
- RLSSMJSEOOYNOY-UHFFFAOYSA-N m-cresol Chemical compound CC1=CC=CC(O)=C1 RLSSMJSEOOYNOY-UHFFFAOYSA-N 0.000 description 2
- 230000003211 malignant effect Effects 0.000 description 2
- 208000006178 malignant mesothelioma Diseases 0.000 description 2
- 230000010534 mechanism of action Effects 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 230000033607 mismatch repair Effects 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 210000004738 parenchymal cell Anatomy 0.000 description 2
- AQIXEPGDORPWBJ-UHFFFAOYSA-N pentan-3-ol Chemical compound CCC(O)CC AQIXEPGDORPWBJ-UHFFFAOYSA-N 0.000 description 2
- 230000002085 persistent effect Effects 0.000 description 2
- 229920001983 poloxamer Polymers 0.000 description 2
- 229920001993 poloxamer 188 Polymers 0.000 description 2
- 229940044519 poloxamer 188 Drugs 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 2
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 2
- 229940068977 polysorbate 20 Drugs 0.000 description 2
- 229940068965 polysorbates Drugs 0.000 description 2
- 230000002980 postoperative effect Effects 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 210000001236 prokaryotic cell Anatomy 0.000 description 2
- 108010015796 prolylisoleucine Proteins 0.000 description 2
- VYXXMAGSIYIYGD-NWAYQTQBSA-N propan-2-yl 2-[[[(2R)-1-(6-aminopurin-9-yl)propan-2-yl]oxymethyl-(pyrimidine-4-carbonylamino)phosphoryl]amino]-2-methylpropanoate Chemical group CC(C)OC(=O)C(C)(C)NP(=O)(CO[C@H](C)Cn1cnc2c(N)ncnc12)NC(=O)c1ccncn1 VYXXMAGSIYIYGD-NWAYQTQBSA-N 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 238000001959 radiotherapy Methods 0.000 description 2
- 206010037844 rash Diseases 0.000 description 2
- 238000011084 recovery Methods 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 208000037922 refractory disease Diseases 0.000 description 2
- GHMLBKRAJCXXBS-UHFFFAOYSA-N resorcinol Chemical compound OC1=CC=CC(O)=C1 GHMLBKRAJCXXBS-UHFFFAOYSA-N 0.000 description 2
- 230000037390 scarring Effects 0.000 description 2
- 230000028327 secretion Effects 0.000 description 2
- 108010048818 seryl-histidine Proteins 0.000 description 2
- 108010071207 serylmethionine Proteins 0.000 description 2
- 239000008354 sodium chloride injection Substances 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 230000002269 spontaneous effect Effects 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 230000001629 suppression Effects 0.000 description 2
- 230000009885 systemic effect Effects 0.000 description 2
- 108010071097 threonyl-lysyl-proline Proteins 0.000 description 2
- 108010072986 threonyl-seryl-lysine Proteins 0.000 description 2
- 102000052185 transforming growth factor beta receptor activity proteins Human genes 0.000 description 2
- 108700015056 transforming growth factor beta receptor activity proteins Proteins 0.000 description 2
- 238000002054 transplantation Methods 0.000 description 2
- 108010017949 tyrosyl-glycyl-glycine Proteins 0.000 description 2
- 108010051110 tyrosyl-lysine Proteins 0.000 description 2
- 231100000402 unacceptable toxicity Toxicity 0.000 description 2
- 229960005486 vaccine Drugs 0.000 description 2
- 239000008215 water for injection Substances 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical class OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 1
- AXFMEGAFCUULFV-BLFANLJRSA-N (2s)-2-[[(2s)-1-[(2s,3r)-2-amino-3-methylpentanoyl]pyrrolidine-2-carbonyl]amino]pentanedioic acid Chemical compound CC[C@@H](C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O AXFMEGAFCUULFV-BLFANLJRSA-N 0.000 description 1
- CDKIEBFIMCSCBB-UHFFFAOYSA-N 1-(6,7-dimethoxy-3,4-dihydro-1h-isoquinolin-2-yl)-3-(1-methyl-2-phenylpyrrolo[2,3-b]pyridin-3-yl)prop-2-en-1-one;hydrochloride Chemical compound Cl.C1C=2C=C(OC)C(OC)=CC=2CCN1C(=O)C=CC(C1=CC=CN=C1N1C)=C1C1=CC=CC=C1 CDKIEBFIMCSCBB-UHFFFAOYSA-N 0.000 description 1
- KKMIHKCGXQMFEU-UHFFFAOYSA-N 2-[dimethyl(tetradecyl)azaniumyl]acetate Chemical group CCCCCCCCCCCCCC[N+](C)(C)CC([O-])=O KKMIHKCGXQMFEU-UHFFFAOYSA-N 0.000 description 1
- LMVGXBRDRZOPHA-UHFFFAOYSA-N 2-[dimethyl-[3-(16-methylheptadecanoylamino)propyl]azaniumyl]acetate Chemical compound CC(C)CCCCCCCCCCCCCCC(=O)NCCC[N+](C)(C)CC([O-])=O LMVGXBRDRZOPHA-UHFFFAOYSA-N 0.000 description 1
- TYIOVYZMKITKRO-UHFFFAOYSA-N 2-[hexadecyl(dimethyl)azaniumyl]acetate Chemical compound CCCCCCCCCCCCCCCC[N+](C)(C)CC([O-])=O TYIOVYZMKITKRO-UHFFFAOYSA-N 0.000 description 1
- SNQVCAOGQHOSEN-UHFFFAOYSA-N 2-[methyl(octadecyl)amino]acetic acid Chemical compound CCCCCCCCCCCCCCCCCCN(C)CC(O)=O SNQVCAOGQHOSEN-UHFFFAOYSA-N 0.000 description 1
- SPCKHVPPRJWQRZ-UHFFFAOYSA-N 2-benzhydryloxy-n,n-dimethylethanamine;2-hydroxypropane-1,2,3-tricarboxylic acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O.C=1C=CC=CC=1C(OCCN(C)C)C1=CC=CC=C1 SPCKHVPPRJWQRZ-UHFFFAOYSA-N 0.000 description 1
- WAVYAFBQOXCGSZ-UHFFFAOYSA-N 2-fluoropyrimidine Chemical compound FC1=NC=CC=N1 WAVYAFBQOXCGSZ-UHFFFAOYSA-N 0.000 description 1
- DIROHOMJLWMERM-UHFFFAOYSA-N 3-[dimethyl(octadecyl)azaniumyl]propane-1-sulfonate Chemical compound CCCCCCCCCCCCCCCCCC[N+](C)(C)CCCS([O-])(=O)=O DIROHOMJLWMERM-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 206010001197 Adenocarcinoma of the cervix Diseases 0.000 description 1
- 208000034246 Adenocarcinoma of the cervix uteri Diseases 0.000 description 1
- 208000036764 Adenocarcinoma of the esophagus Diseases 0.000 description 1
- 206010052747 Adenocarcinoma pancreas Diseases 0.000 description 1
- LBJYAILUMSUTAM-ZLUOBGJFSA-N Ala-Asn-Asn Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O LBJYAILUMSUTAM-ZLUOBGJFSA-N 0.000 description 1
- FXKNPWNXPQZLES-ZLUOBGJFSA-N Ala-Asn-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O FXKNPWNXPQZLES-ZLUOBGJFSA-N 0.000 description 1
- NFDVJAKFMXHJEQ-HERUPUMHSA-N Ala-Asp-Trp Chemical compound C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)N NFDVJAKFMXHJEQ-HERUPUMHSA-N 0.000 description 1
- NWVVKQZOVSTDBQ-CIUDSAMLSA-N Ala-Glu-Arg Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O NWVVKQZOVSTDBQ-CIUDSAMLSA-N 0.000 description 1
- KXEVYGKATAMXJJ-ACZMJKKPSA-N Ala-Glu-Asp Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O KXEVYGKATAMXJJ-ACZMJKKPSA-N 0.000 description 1
- KMGOBAQSCKTBGD-DLOVCJGASA-N Ala-His-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@H](C)N)CC1=CN=CN1 KMGOBAQSCKTBGD-DLOVCJGASA-N 0.000 description 1
- UCDOXFBTMLKASE-HERUPUMHSA-N Ala-Ser-Trp Chemical compound C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)N UCDOXFBTMLKASE-HERUPUMHSA-N 0.000 description 1
- OEVCHROQUIVQFZ-YTLHQDLWSA-N Ala-Thr-Ala Chemical compound C[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](C)C(O)=O OEVCHROQUIVQFZ-YTLHQDLWSA-N 0.000 description 1
- CREYEAPXISDKSB-FQPOAREZSA-N Ala-Thr-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O CREYEAPXISDKSB-FQPOAREZSA-N 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- 241000239290 Araneae Species 0.000 description 1
- XVLLUZMFSAYKJV-GUBZILKMSA-N Arg-Asp-Arg Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O XVLLUZMFSAYKJV-GUBZILKMSA-N 0.000 description 1
- PQWTZSNVWSOFFK-FXQIFTODSA-N Arg-Asp-Asn Chemical compound C(C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N)CN=C(N)N PQWTZSNVWSOFFK-FXQIFTODSA-N 0.000 description 1
- OTCJMMRQBVDQRK-DCAQKATOSA-N Arg-Asp-Leu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O OTCJMMRQBVDQRK-DCAQKATOSA-N 0.000 description 1
- ZJEDSBGPBXVBMP-PYJNHQTQSA-N Arg-His-Ile Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O ZJEDSBGPBXVBMP-PYJNHQTQSA-N 0.000 description 1
- CZUHPNLXLWMYMG-UBHSHLNASA-N Arg-Phe-Ala Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](C)C(O)=O)CC1=CC=CC=C1 CZUHPNLXLWMYMG-UBHSHLNASA-N 0.000 description 1
- PRLPSDIHSRITSF-UNQGMJICSA-N Arg-Phe-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O PRLPSDIHSRITSF-UNQGMJICSA-N 0.000 description 1
- ATABBWFGOHKROJ-GUBZILKMSA-N Arg-Pro-Ser Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O ATABBWFGOHKROJ-GUBZILKMSA-N 0.000 description 1
- ASQKVGRCKOFKIU-KZVJFYERSA-N Arg-Thr-Ala Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N)O ASQKVGRCKOFKIU-KZVJFYERSA-N 0.000 description 1
- FOWOZYAWODIRFZ-JYJNAYRXSA-N Arg-Tyr-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](CCCN=C(N)N)N FOWOZYAWODIRFZ-JYJNAYRXSA-N 0.000 description 1
- PSUXEQYPYZLNER-QXEWZRGKSA-N Arg-Val-Asn Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O PSUXEQYPYZLNER-QXEWZRGKSA-N 0.000 description 1
- HUZGPXBILPMCHM-IHRRRGAJSA-N Asn-Arg-Phe Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O HUZGPXBILPMCHM-IHRRRGAJSA-N 0.000 description 1
- MOHUTCNYQLMARY-GUBZILKMSA-N Asn-His-Gln Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC(=O)N)N MOHUTCNYQLMARY-GUBZILKMSA-N 0.000 description 1
- PTSDPWIHOYMRGR-UGYAYLCHSA-N Asn-Ile-Asn Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(O)=O PTSDPWIHOYMRGR-UGYAYLCHSA-N 0.000 description 1
- YVXRYLVELQYAEQ-SRVKXCTJSA-N Asn-Leu-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)N)N YVXRYLVELQYAEQ-SRVKXCTJSA-N 0.000 description 1
- ZUFPUBYQYWCMDB-NUMRIWBASA-N Asn-Thr-Glu Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O ZUFPUBYQYWCMDB-NUMRIWBASA-N 0.000 description 1
- CBHVAFXKOYAHOY-NHCYSSNCSA-N Asn-Val-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O CBHVAFXKOYAHOY-NHCYSSNCSA-N 0.000 description 1
- AXXCUABIFZPKPM-BQBZGAKWSA-N Asp-Arg-Gly Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O AXXCUABIFZPKPM-BQBZGAKWSA-N 0.000 description 1
- XYBJLTKSGFBLCS-QXEWZRGKSA-N Asp-Arg-Val Chemical compound NC(N)=NCCC[C@@H](C(=O)N[C@@H](C(C)C)C(O)=O)NC(=O)[C@@H](N)CC(O)=O XYBJLTKSGFBLCS-QXEWZRGKSA-N 0.000 description 1
- KNMRXHIAVXHCLW-ZLUOBGJFSA-N Asp-Asn-Ser Chemical compound C([C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CO)C(=O)O)N)C(=O)O KNMRXHIAVXHCLW-ZLUOBGJFSA-N 0.000 description 1
- SBHUBSDEZQFJHJ-CIUDSAMLSA-N Asp-Asp-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(O)=O SBHUBSDEZQFJHJ-CIUDSAMLSA-N 0.000 description 1
- OGTCOKZFOJIZFG-CIUDSAMLSA-N Asp-His-Asp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(O)=O)C(O)=O OGTCOKZFOJIZFG-CIUDSAMLSA-N 0.000 description 1
- CYCKJEFVFNRWEZ-UGYAYLCHSA-N Asp-Ile-Asn Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(O)=O CYCKJEFVFNRWEZ-UGYAYLCHSA-N 0.000 description 1
- QNFRBNZGVVKBNJ-PEFMBERDSA-N Asp-Ile-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC(=O)O)N QNFRBNZGVVKBNJ-PEFMBERDSA-N 0.000 description 1
- CJUKAWUWBZCTDQ-SRVKXCTJSA-N Asp-Leu-Lys Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O CJUKAWUWBZCTDQ-SRVKXCTJSA-N 0.000 description 1
- QJHOOKBAHRJPPX-QWRGUYRKSA-N Asp-Phe-Gly Chemical compound OC(=O)C[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CC1=CC=CC=C1 QJHOOKBAHRJPPX-QWRGUYRKSA-N 0.000 description 1
- JSHWXQIZOCVWIA-ZKWXMUAHSA-N Asp-Ser-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O JSHWXQIZOCVWIA-ZKWXMUAHSA-N 0.000 description 1
- JSNWZMFSLIWAHS-HJGDQZAQSA-N Asp-Thr-Leu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)O)NC(=O)[C@H](CC(=O)O)N)O JSNWZMFSLIWAHS-HJGDQZAQSA-N 0.000 description 1
- ITGFVUYOLWBPQW-KKHAAJSZSA-N Asp-Thr-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O ITGFVUYOLWBPQW-KKHAAJSZSA-N 0.000 description 1
- XWKPSMRPIKKDDU-RCOVLWMOSA-N Asp-Val-Gly Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O XWKPSMRPIKKDDU-RCOVLWMOSA-N 0.000 description 1
- JGLWFWXGOINXEA-YDHLFZDLSA-N Asp-Val-Tyr Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 JGLWFWXGOINXEA-YDHLFZDLSA-N 0.000 description 1
- 208000023275 Autoimmune disease Diseases 0.000 description 1
- 102100029822 B- and T-lymphocyte attenuator Human genes 0.000 description 1
- 206010005949 Bone cancer Diseases 0.000 description 1
- 208000018084 Bone neoplasm Diseases 0.000 description 1
- 208000003174 Brain Neoplasms Diseases 0.000 description 1
- 102100038078 CD276 antigen Human genes 0.000 description 1
- 101710185679 CD276 antigen Proteins 0.000 description 1
- 108010021064 CTLA-4 Antigen Proteins 0.000 description 1
- 102000008203 CTLA-4 Antigen Human genes 0.000 description 1
- 229940045513 CTLA4 antagonist Drugs 0.000 description 1
- 101100315624 Caenorhabditis elegans tyr-1 gene Proteins 0.000 description 1
- 241000282832 Camelidae Species 0.000 description 1
- 208000005623 Carcinogenesis Diseases 0.000 description 1
- 201000009030 Carcinoma Diseases 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- AMRLSQGGERHDHJ-FXQIFTODSA-N Cys-Ala-Arg Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O AMRLSQGGERHDHJ-FXQIFTODSA-N 0.000 description 1
- PLBJMUUEGBBHRH-ZLUOBGJFSA-N Cys-Ala-Asn Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(O)=O PLBJMUUEGBBHRH-ZLUOBGJFSA-N 0.000 description 1
- FMDCYTBSPZMPQE-JBDRJPRFSA-N Cys-Ala-Ile Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O FMDCYTBSPZMPQE-JBDRJPRFSA-N 0.000 description 1
- SZQCDCKIGWQAQN-FXQIFTODSA-N Cys-Arg-Ala Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(O)=O SZQCDCKIGWQAQN-FXQIFTODSA-N 0.000 description 1
- YMBAVNPKBWHDAW-CIUDSAMLSA-N Cys-Asp-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CS)N YMBAVNPKBWHDAW-CIUDSAMLSA-N 0.000 description 1
- DYBIDOHFRRUMLW-CIUDSAMLSA-N Cys-Leu-Cys Chemical compound CC(C)C[C@H](NC(=O)[C@@H](N)CS)C(=O)N[C@@H](CS)C(O)=O DYBIDOHFRRUMLW-CIUDSAMLSA-N 0.000 description 1
- LKHMGNHQULEPFY-ACZMJKKPSA-N Cys-Ser-Glu Chemical compound SC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O LKHMGNHQULEPFY-ACZMJKKPSA-N 0.000 description 1
- XSELZJJGSKZZDO-UBHSHLNASA-N Cys-Trp-Asp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](CS)N XSELZJJGSKZZDO-UBHSHLNASA-N 0.000 description 1
- RGHNJXZEOKUKBD-SQOUGZDYSA-M D-gluconate Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O RGHNJXZEOKUKBD-SQOUGZDYSA-M 0.000 description 1
- 201000004624 Dermatitis Diseases 0.000 description 1
- 206010012735 Diarrhoea Diseases 0.000 description 1
- 108060006698 EGF receptor Proteins 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 208000004232 Enteritis Diseases 0.000 description 1
- 102000003951 Erythropoietin Human genes 0.000 description 1
- 108090000394 Erythropoietin Proteins 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 1
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 1
- 201000001342 Fallopian tube cancer Diseases 0.000 description 1
- 208000013452 Fallopian tube neoplasm Diseases 0.000 description 1
- 108010087819 Fc receptors Proteins 0.000 description 1
- 102000009109 Fc receptors Human genes 0.000 description 1
- 108091006020 Fc-tagged proteins Proteins 0.000 description 1
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 description 1
- 208000010412 Glaucoma Diseases 0.000 description 1
- NNQHEEQNPQYPGL-FXQIFTODSA-N Gln-Ala-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(O)=O NNQHEEQNPQYPGL-FXQIFTODSA-N 0.000 description 1
- JHPFPROFOAJRFN-IHRRRGAJSA-N Gln-Glu-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCC(=O)N)N)O JHPFPROFOAJRFN-IHRRRGAJSA-N 0.000 description 1
- SBHVGKBYOQKAEA-SDDRHHMPSA-N Gln-His-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CN=CN2)NC(=O)[C@H](CCC(=O)N)N)C(=O)O SBHVGKBYOQKAEA-SDDRHHMPSA-N 0.000 description 1
- MSHXWFKYXJTLEZ-CIUDSAMLSA-N Gln-Met-Asn Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)N)N MSHXWFKYXJTLEZ-CIUDSAMLSA-N 0.000 description 1
- AQPZYBSRDRZBAG-AVGNSLFASA-N Gln-Phe-Asn Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)N)N AQPZYBSRDRZBAG-AVGNSLFASA-N 0.000 description 1
- BZULIEARJFRINC-IHRRRGAJSA-N Gln-Phe-Glu Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CCC(=O)N)N BZULIEARJFRINC-IHRRRGAJSA-N 0.000 description 1
- JKDBRTNMYXYLHO-JYJNAYRXSA-N Gln-Tyr-Leu Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)CC1=CC=C(O)C=C1 JKDBRTNMYXYLHO-JYJNAYRXSA-N 0.000 description 1
- FHPXTPQBODWBIY-CIUDSAMLSA-N Glu-Ala-Arg Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O FHPXTPQBODWBIY-CIUDSAMLSA-N 0.000 description 1
- OGMQXTXGLDNBSS-FXQIFTODSA-N Glu-Ala-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(O)=O OGMQXTXGLDNBSS-FXQIFTODSA-N 0.000 description 1
- OJGLIOXAKGFFDW-SRVKXCTJSA-N Glu-Arg-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(=O)O)N OJGLIOXAKGFFDW-SRVKXCTJSA-N 0.000 description 1
- LTUVYLVIZHJCOQ-KKUMJFAQSA-N Glu-Arg-Phe Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O LTUVYLVIZHJCOQ-KKUMJFAQSA-N 0.000 description 1
- PAQUJCSYVIBPLC-AVGNSLFASA-N Glu-Asp-Phe Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 PAQUJCSYVIBPLC-AVGNSLFASA-N 0.000 description 1
- VSMQDIVEBXPKRT-QEJZJMRPSA-N Glu-Cys-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CCC(=O)O)N VSMQDIVEBXPKRT-QEJZJMRPSA-N 0.000 description 1
- RFDHKPSHTXZKLL-IHRRRGAJSA-N Glu-Gln-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCC(=O)O)N RFDHKPSHTXZKLL-IHRRRGAJSA-N 0.000 description 1
- ILGFBUGLBSAQQB-GUBZILKMSA-N Glu-Glu-Arg Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O ILGFBUGLBSAQQB-GUBZILKMSA-N 0.000 description 1
- LGYZYFFDELZWRS-DCAQKATOSA-N Glu-Glu-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O LGYZYFFDELZWRS-DCAQKATOSA-N 0.000 description 1
- DVLZZEPUNFEUBW-AVGNSLFASA-N Glu-His-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CCC(=O)O)N DVLZZEPUNFEUBW-AVGNSLFASA-N 0.000 description 1
- YKBUCXNNBYZYAY-MNXVOIDGSA-N Glu-Lys-Ile Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O YKBUCXNNBYZYAY-MNXVOIDGSA-N 0.000 description 1
- DCBSZJJHOTXMHY-DCAQKATOSA-N Glu-Pro-Pro Chemical compound OC(=O)CC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 DCBSZJJHOTXMHY-DCAQKATOSA-N 0.000 description 1
- BXSZPACYCMNKLS-AVGNSLFASA-N Glu-Ser-Phe Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O BXSZPACYCMNKLS-AVGNSLFASA-N 0.000 description 1
- GPSHCSTUYOQPAI-JHEQGTHGSA-N Glu-Thr-Gly Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(O)=O GPSHCSTUYOQPAI-JHEQGTHGSA-N 0.000 description 1
- HVKAAUOFFTUSAA-XDTLVQLUSA-N Glu-Tyr-Ala Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C)C(O)=O HVKAAUOFFTUSAA-XDTLVQLUSA-N 0.000 description 1
- QXUPRMQJDWJDFR-NRPADANISA-N Glu-Val-Ser Chemical compound CC(C)[C@H](NC(=O)[C@@H](N)CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O QXUPRMQJDWJDFR-NRPADANISA-N 0.000 description 1
- JVWPPCWUDRJGAE-YUMQZZPRSA-N Gly-Asn-Leu Chemical compound [H]NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O JVWPPCWUDRJGAE-YUMQZZPRSA-N 0.000 description 1
- VIIBEIQMLJEUJG-LAEOZQHASA-N Gly-Ile-Gln Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(O)=O VIIBEIQMLJEUJG-LAEOZQHASA-N 0.000 description 1
- IKAIKUBBJHFNBZ-LURJTMIESA-N Gly-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)CN IKAIKUBBJHFNBZ-LURJTMIESA-N 0.000 description 1
- VBOBNHSVQKKTOT-YUMQZZPRSA-N Gly-Lys-Ala Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O VBOBNHSVQKKTOT-YUMQZZPRSA-N 0.000 description 1
- FHQRLHFYVZAQHU-IUCAKERBSA-N Gly-Lys-Gln Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O FHQRLHFYVZAQHU-IUCAKERBSA-N 0.000 description 1
- POJJAZJHBGXEGM-YUMQZZPRSA-N Gly-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)CN POJJAZJHBGXEGM-YUMQZZPRSA-N 0.000 description 1
- NVTPVQLIZCOJFK-FOHZUACHSA-N Gly-Thr-Asp Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O NVTPVQLIZCOJFK-FOHZUACHSA-N 0.000 description 1
- XHVONGZZVUUORG-WEDXCCLWSA-N Gly-Thr-Lys Chemical compound NCC(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CCCCN XHVONGZZVUUORG-WEDXCCLWSA-N 0.000 description 1
- SBVMXEZQJVUARN-XPUUQOCRSA-N Gly-Val-Ser Chemical compound NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O SBVMXEZQJVUARN-XPUUQOCRSA-N 0.000 description 1
- 102100034458 Hepatitis A virus cellular receptor 2 Human genes 0.000 description 1
- 101710083479 Hepatitis A virus cellular receptor 2 homolog Proteins 0.000 description 1
- 241000238631 Hexapoda Species 0.000 description 1
- VCDNHBNNPCDBKV-DLOVCJGASA-N His-Ala-Lys Chemical compound C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC1=CN=CN1)N VCDNHBNNPCDBKV-DLOVCJGASA-N 0.000 description 1
- DFHVLUKTTVTCKY-PBCZWWQYSA-N His-Asn-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC1=CN=CN1)N)O DFHVLUKTTVTCKY-PBCZWWQYSA-N 0.000 description 1
- LMMPTUVWHCFTOT-GARJFASQSA-N His-Asp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)O)NC(=O)[C@H](CC2=CN=CN2)N)C(=O)O LMMPTUVWHCFTOT-GARJFASQSA-N 0.000 description 1
- QNILDNVBIARMRK-XVYDVKMFSA-N His-Cys-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CC1=CN=CN1)N QNILDNVBIARMRK-XVYDVKMFSA-N 0.000 description 1
- HVCRQRQPIIRNLY-IUCAKERBSA-N His-Gln-Gly Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)NCC(=O)O)N HVCRQRQPIIRNLY-IUCAKERBSA-N 0.000 description 1
- TVRMJKNELJKNRS-GUBZILKMSA-N His-Glu-Asn Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N TVRMJKNELJKNRS-GUBZILKMSA-N 0.000 description 1
- BPOHQCZZSFBSON-KKUMJFAQSA-N His-Leu-His Chemical compound CC(C)C[C@H](NC(=O)[C@@H](N)Cc1cnc[nH]1)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O BPOHQCZZSFBSON-KKUMJFAQSA-N 0.000 description 1
- VCBWXASUBZIFLQ-IHRRRGAJSA-N His-Pro-Leu Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(O)=O VCBWXASUBZIFLQ-IHRRRGAJSA-N 0.000 description 1
- STGQSBKUYSPPIG-CIUDSAMLSA-N His-Ser-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CN=CN1 STGQSBKUYSPPIG-CIUDSAMLSA-N 0.000 description 1
- UPJODPVSKKWGDQ-KLHWPWHYSA-N His-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC2=CN=CN2)N)O UPJODPVSKKWGDQ-KLHWPWHYSA-N 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 101000864344 Homo sapiens B- and T-lymphocyte attenuator Proteins 0.000 description 1
- 101001138062 Homo sapiens Leukocyte-associated immunoglobulin-like receptor 1 Proteins 0.000 description 1
- 208000037147 Hypercalcaemia Diseases 0.000 description 1
- IIXDMJNYALIKGP-DJFWLOJKSA-N Ile-Asn-His Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N IIXDMJNYALIKGP-DJFWLOJKSA-N 0.000 description 1
- WNQKUUQIVDDAFA-ZPFDUUQYSA-N Ile-Gln-Met Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCSC)C(=O)O)N WNQKUUQIVDDAFA-ZPFDUUQYSA-N 0.000 description 1
- AFERFBZLVUFWRA-HTFCKZLJSA-N Ile-Ile-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CS)C(=O)O)N AFERFBZLVUFWRA-HTFCKZLJSA-N 0.000 description 1
- BBQABUDWDUKJMB-LZXPERKUSA-N Ile-Ile-Ile Chemical compound CC[C@H](C)[C@H]([NH3+])C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C([O-])=O BBQABUDWDUKJMB-LZXPERKUSA-N 0.000 description 1
- DMSVBUWGDLYNLC-IAVJCBSLSA-N Ile-Ile-Phe Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 DMSVBUWGDLYNLC-IAVJCBSLSA-N 0.000 description 1
- NZGTYCMLUGYMCV-XUXIUFHCSA-N Ile-Lys-Arg Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N NZGTYCMLUGYMCV-XUXIUFHCSA-N 0.000 description 1
- JHNJNTMTZHEDLJ-NAKRPEOUSA-N Ile-Ser-Arg Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O JHNJNTMTZHEDLJ-NAKRPEOUSA-N 0.000 description 1
- JCGMFFQQHJQASB-PYJNHQTQSA-N Ile-Val-His Chemical compound N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)O JCGMFFQQHJQASB-PYJNHQTQSA-N 0.000 description 1
- 102000037982 Immune checkpoint proteins Human genes 0.000 description 1
- 108091008036 Immune checkpoint proteins Proteins 0.000 description 1
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 1
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 1
- 102000013463 Immunoglobulin Light Chains Human genes 0.000 description 1
- 108010065825 Immunoglobulin Light Chains Proteins 0.000 description 1
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 1
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 1
- FADYJNXDPBKVCA-UHFFFAOYSA-N L-Phenylalanyl-L-lysin Natural products NCCCCC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FADYJNXDPBKVCA-UHFFFAOYSA-N 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- 102000017578 LAG3 Human genes 0.000 description 1
- 101150030213 Lag3 gene Proteins 0.000 description 1
- 235000017858 Laurus nobilis Nutrition 0.000 description 1
- CZCSUZMIRKFFFA-CIUDSAMLSA-N Leu-Ala-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(O)=O CZCSUZMIRKFFFA-CIUDSAMLSA-N 0.000 description 1
- RRSLQOLASISYTB-CIUDSAMLSA-N Leu-Cys-Asp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(O)=O)C(O)=O RRSLQOLASISYTB-CIUDSAMLSA-N 0.000 description 1
- ZTLGVASZOIKNIX-DCAQKATOSA-N Leu-Gln-Glu Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N ZTLGVASZOIKNIX-DCAQKATOSA-N 0.000 description 1
- CQGSYZCULZMEDE-UHFFFAOYSA-N Leu-Gln-Pro Natural products CC(C)CC(N)C(=O)NC(CCC(N)=O)C(=O)N1CCCC1C(O)=O CQGSYZCULZMEDE-UHFFFAOYSA-N 0.000 description 1
- IWTBYNQNAPECCS-AVGNSLFASA-N Leu-Glu-His Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 IWTBYNQNAPECCS-AVGNSLFASA-N 0.000 description 1
- WQWSMEOYXJTFRU-GUBZILKMSA-N Leu-Glu-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O WQWSMEOYXJTFRU-GUBZILKMSA-N 0.000 description 1
- HVJVUYQWFYMGJS-GVXVVHGQSA-N Leu-Glu-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O HVJVUYQWFYMGJS-GVXVVHGQSA-N 0.000 description 1
- APFJUBGRZGMQFF-QWRGUYRKSA-N Leu-Gly-Lys Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCCCN APFJUBGRZGMQFF-QWRGUYRKSA-N 0.000 description 1
- ZGUMORRUBUCXEH-AVGNSLFASA-N Leu-Lys-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZGUMORRUBUCXEH-AVGNSLFASA-N 0.000 description 1
- REPBGZHJKYWFMJ-KKUMJFAQSA-N Leu-Lys-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N REPBGZHJKYWFMJ-KKUMJFAQSA-N 0.000 description 1
- XOWMDXHFSBCAKQ-SRVKXCTJSA-N Leu-Ser-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC(C)C XOWMDXHFSBCAKQ-SRVKXCTJSA-N 0.000 description 1
- SVBJIZVVYJYGLA-DCAQKATOSA-N Leu-Ser-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O SVBJIZVVYJYGLA-DCAQKATOSA-N 0.000 description 1
- LINKCQUOMUDLKN-KATARQTJSA-N Leu-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(C)C)N)O LINKCQUOMUDLKN-KATARQTJSA-N 0.000 description 1
- LJBVRCDPWOJOEK-PPCPHDFISA-N Leu-Thr-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O LJBVRCDPWOJOEK-PPCPHDFISA-N 0.000 description 1
- JGKHAFUAPZCCDU-BZSNNMDCSA-N Leu-Tyr-Leu Chemical compound CC(C)C[C@H]([NH3+])C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C([O-])=O)CC1=CC=C(O)C=C1 JGKHAFUAPZCCDU-BZSNNMDCSA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 102100020943 Leukocyte-associated immunoglobulin-like receptor 1 Human genes 0.000 description 1
- 206010067125 Liver injury Diseases 0.000 description 1
- IXHKPDJKKCUKHS-GARJFASQSA-N Lys-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCCCN)N IXHKPDJKKCUKHS-GARJFASQSA-N 0.000 description 1
- QUCDKEKDPYISNX-HJGDQZAQSA-N Lys-Asn-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QUCDKEKDPYISNX-HJGDQZAQSA-N 0.000 description 1
- KPJJOZUXFOLGMQ-CIUDSAMLSA-N Lys-Asp-Asn Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N KPJJOZUXFOLGMQ-CIUDSAMLSA-N 0.000 description 1
- OVIVOCSURJYCTM-GUBZILKMSA-N Lys-Asp-Glu Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCC(O)=O OVIVOCSURJYCTM-GUBZILKMSA-N 0.000 description 1
- YFGWNAROEYWGNL-GUBZILKMSA-N Lys-Gln-Asn Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O YFGWNAROEYWGNL-GUBZILKMSA-N 0.000 description 1
- HEWWNLVEWBJBKA-WDCWCFNPSA-N Lys-Gln-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CCCCN HEWWNLVEWBJBKA-WDCWCFNPSA-N 0.000 description 1
- QZONCCHVHCOBSK-YUMQZZPRSA-N Lys-Gly-Asn Chemical compound [H]N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O QZONCCHVHCOBSK-YUMQZZPRSA-N 0.000 description 1
- AIRZWUMAHCDDHR-KKUMJFAQSA-N Lys-Leu-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O AIRZWUMAHCDDHR-KKUMJFAQSA-N 0.000 description 1
- PDIDTSZKKFEDMB-UWVGGRQHSA-N Lys-Pro-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O PDIDTSZKKFEDMB-UWVGGRQHSA-N 0.000 description 1
- DIBZLYZXTSVGLN-CIUDSAMLSA-N Lys-Ser-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O DIBZLYZXTSVGLN-CIUDSAMLSA-N 0.000 description 1
- TVHCDSBMFQYPNA-RHYQMDGZSA-N Lys-Thr-Arg Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O TVHCDSBMFQYPNA-RHYQMDGZSA-N 0.000 description 1
- QVTDVTONTRSQMF-WDCWCFNPSA-N Lys-Thr-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H]([C@H](O)C)NC(=O)[C@@H](N)CCCCN QVTDVTONTRSQMF-WDCWCFNPSA-N 0.000 description 1
- OHXUUQDOBQKSNB-AVGNSLFASA-N Lys-Val-Arg Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O OHXUUQDOBQKSNB-AVGNSLFASA-N 0.000 description 1
- UGCIQUYEJIEHKX-GVXVVHGQSA-N Lys-Val-Glu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O UGCIQUYEJIEHKX-GVXVVHGQSA-N 0.000 description 1
- 208000000172 Medulloblastoma Diseases 0.000 description 1
- 108010061593 Member 14 Tumor Necrosis Factor Receptors Proteins 0.000 description 1
- 102000012220 Member 14 Tumor Necrosis Factor Receptors Human genes 0.000 description 1
- CAODKDAPYGUMLK-FXQIFTODSA-N Met-Asn-Ser Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O CAODKDAPYGUMLK-FXQIFTODSA-N 0.000 description 1
- YYEIFXZOBZVDPH-DCAQKATOSA-N Met-Lys-Asp Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(O)=O YYEIFXZOBZVDPH-DCAQKATOSA-N 0.000 description 1
- SPSSJSICDYYTQN-HJGDQZAQSA-N Met-Thr-Gln Chemical compound CSCC[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCC(N)=O SPSSJSICDYYTQN-HJGDQZAQSA-N 0.000 description 1
- WOGNGBROIHHFAO-JYJNAYRXSA-N Met-Tyr-Met Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CCSC)C(=O)O)N WOGNGBROIHHFAO-JYJNAYRXSA-N 0.000 description 1
- 206010027465 Metastases to skin Diseases 0.000 description 1
- 206010063569 Metastatic squamous cell carcinoma Diseases 0.000 description 1
- 208000032818 Microsatellite Instability Diseases 0.000 description 1
- 241001024304 Mino Species 0.000 description 1
- 101710143111 Mothers against decapentaplegic homolog 3 Proteins 0.000 description 1
- 101000686985 Mouse mammary tumor virus (strain C3H) Protein PR73 Proteins 0.000 description 1
- 208000034578 Multiple myelomas Diseases 0.000 description 1
- 101100288142 Mus musculus Klkb1 gene Proteins 0.000 description 1
- 101100407308 Mus musculus Pdcd1lg2 gene Proteins 0.000 description 1
- 108010066427 N-valyltryptophan Proteins 0.000 description 1
- 206010028813 Nausea Diseases 0.000 description 1
- 101100068676 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) gln-1 gene Proteins 0.000 description 1
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 1
- 206010030137 Oesophageal adenocarcinoma Diseases 0.000 description 1
- 206010061534 Oesophageal squamous cell carcinoma Diseases 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- APJPXSFJBMMOLW-KBPBESRZSA-N Phe-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CC1=CC=CC=C1 APJPXSFJBMMOLW-KBPBESRZSA-N 0.000 description 1
- BIYWZVCPZIFGPY-QWRGUYRKSA-N Phe-Gly-Ser Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)NCC(=O)N[C@@H](CO)C(O)=O BIYWZVCPZIFGPY-QWRGUYRKSA-N 0.000 description 1
- QPVFUAUFEBPIPT-CDMKHQONSA-N Phe-Gly-Thr Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O QPVFUAUFEBPIPT-CDMKHQONSA-N 0.000 description 1
- PMKIMKUGCSVFSV-CQDKDKBSSA-N Phe-His-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CC2=CC=CC=C2)N PMKIMKUGCSVFSV-CQDKDKBSSA-N 0.000 description 1
- MJAYDXWQQUOURZ-JYJNAYRXSA-N Phe-Lys-Gln Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O MJAYDXWQQUOURZ-JYJNAYRXSA-N 0.000 description 1
- ODGNUUUDJONJSC-UFYCRDLUSA-N Phe-Pro-Tyr Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CC2=CC=CC=C2)N)C(=O)N[C@@H](CC3=CC=C(C=C3)O)C(=O)O ODGNUUUDJONJSC-UFYCRDLUSA-N 0.000 description 1
- KLYYKKGCPOGDPE-OEAJRASXSA-N Phe-Thr-Leu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O KLYYKKGCPOGDPE-OEAJRASXSA-N 0.000 description 1
- AOKZOUGUMLBPSS-PMVMPFDFSA-N Phe-Trp-Leu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC(C)C)C(O)=O AOKZOUGUMLBPSS-PMVMPFDFSA-N 0.000 description 1
- CVAUVSOFHJKCHN-BZSNNMDCSA-N Phe-Tyr-Cys Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CS)C(O)=O)C1=CC=CC=C1 CVAUVSOFHJKCHN-BZSNNMDCSA-N 0.000 description 1
- 206010035664 Pneumonia Diseases 0.000 description 1
- RVGRUAULSDPKGF-UHFFFAOYSA-N Poloxamer Chemical compound C1CO1.CC1CO1 RVGRUAULSDPKGF-UHFFFAOYSA-N 0.000 description 1
- 206010049422 Precancerous skin lesion Diseases 0.000 description 1
- KPDRZQUWJKTMBP-DCAQKATOSA-N Pro-Asp-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@@H]1CCCN1 KPDRZQUWJKTMBP-DCAQKATOSA-N 0.000 description 1
- LHALYDBUDCWMDY-CIUDSAMLSA-N Pro-Glu-Ala Chemical compound C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1)C(O)=O LHALYDBUDCWMDY-CIUDSAMLSA-N 0.000 description 1
- MGDFPGCFVJFITQ-CIUDSAMLSA-N Pro-Glu-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O MGDFPGCFVJFITQ-CIUDSAMLSA-N 0.000 description 1
- NAIPAPCKKRCMBL-JYJNAYRXSA-N Pro-Pro-Phe Chemical compound C([C@@H](C(=O)O)NC(=O)[C@H]1N(CCC1)C(=O)[C@H]1NCCC1)C1=CC=CC=C1 NAIPAPCKKRCMBL-JYJNAYRXSA-N 0.000 description 1
- CZCCVJUUWBMISW-FXQIFTODSA-N Pro-Ser-Cys Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O CZCCVJUUWBMISW-FXQIFTODSA-N 0.000 description 1
- FZXSYIPVAFVYBH-KKUMJFAQSA-N Pro-Tyr-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(O)=O)C(O)=O FZXSYIPVAFVYBH-KKUMJFAQSA-N 0.000 description 1
- 108700030875 Programmed Cell Death 1 Ligand 2 Proteins 0.000 description 1
- 101710094000 Programmed cell death 1 ligand 1 Proteins 0.000 description 1
- 102100024213 Programmed cell death 1 ligand 2 Human genes 0.000 description 1
- 102100040678 Programmed cell death protein 1 Human genes 0.000 description 1
- 101710089372 Programmed cell death protein 1 Proteins 0.000 description 1
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 208000006265 Renal cell carcinoma Diseases 0.000 description 1
- 206010063837 Reperfusion injury Diseases 0.000 description 1
- SFZKGGOGCNQPJY-CIUDSAMLSA-N Ser-Asp-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CO)N SFZKGGOGCNQPJY-CIUDSAMLSA-N 0.000 description 1
- BYIROAKULFFTEK-CIUDSAMLSA-N Ser-Asp-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CO BYIROAKULFFTEK-CIUDSAMLSA-N 0.000 description 1
- MOVJSUIKUNCVMG-ZLUOBGJFSA-N Ser-Cys-Ser Chemical compound C([C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)O)N)O MOVJSUIKUNCVMG-ZLUOBGJFSA-N 0.000 description 1
- ULVMNZOKDBHKKI-ACZMJKKPSA-N Ser-Gln-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O ULVMNZOKDBHKKI-ACZMJKKPSA-N 0.000 description 1
- LALNXSXEYFUUDD-GUBZILKMSA-N Ser-Glu-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O LALNXSXEYFUUDD-GUBZILKMSA-N 0.000 description 1
- GJFYFGOEWLDQGW-GUBZILKMSA-N Ser-Leu-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CO)N GJFYFGOEWLDQGW-GUBZILKMSA-N 0.000 description 1
- HDBOEVPDIDDEPC-CIUDSAMLSA-N Ser-Lys-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O HDBOEVPDIDDEPC-CIUDSAMLSA-N 0.000 description 1
- UPLYXVPQLJVWMM-KKUMJFAQSA-N Ser-Phe-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(C)C)C(O)=O UPLYXVPQLJVWMM-KKUMJFAQSA-N 0.000 description 1
- AZWNCEBQZXELEZ-FXQIFTODSA-N Ser-Pro-Ser Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O AZWNCEBQZXELEZ-FXQIFTODSA-N 0.000 description 1
- XZKQVQKUZMAADP-IMJSIDKUSA-N Ser-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(O)=O XZKQVQKUZMAADP-IMJSIDKUSA-N 0.000 description 1
- WLJPJRGQRNCIQS-ZLUOBGJFSA-N Ser-Ser-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O WLJPJRGQRNCIQS-ZLUOBGJFSA-N 0.000 description 1
- OLKICIBQRVSQMA-SRVKXCTJSA-N Ser-Ser-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O OLKICIBQRVSQMA-SRVKXCTJSA-N 0.000 description 1
- OJFFAQFRCVPHNN-JYBASQMISA-N Ser-Thr-Trp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O OJFFAQFRCVPHNN-JYBASQMISA-N 0.000 description 1
- ZKOKTQPHFMRSJP-YJRXYDGGSA-N Ser-Thr-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ZKOKTQPHFMRSJP-YJRXYDGGSA-N 0.000 description 1
- SDFUZKIAHWRUCS-QEJZJMRPSA-N Ser-Trp-Glu Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CO)N SDFUZKIAHWRUCS-QEJZJMRPSA-N 0.000 description 1
- XPVIVVLLLOFBRH-XIRDDKMYSA-N Ser-Trp-Lys Chemical compound NCCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CO)C(O)=O XPVIVVLLLOFBRH-XIRDDKMYSA-N 0.000 description 1
- UBTNVMGPMYDYIU-HJPIBITLSA-N Ser-Tyr-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O UBTNVMGPMYDYIU-HJPIBITLSA-N 0.000 description 1
- 108010003723 Single-Domain Antibodies Proteins 0.000 description 1
- 102000049939 Smad3 Human genes 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- 208000036765 Squamous cell carcinoma of the esophagus Diseases 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 102100039367 T-cell immunoglobulin and mucin domain-containing protein 4 Human genes 0.000 description 1
- 101710174757 T-cell immunoglobulin and mucin domain-containing protein 4 Proteins 0.000 description 1
- 229940123237 Taxane Drugs 0.000 description 1
- BPEGJWRSRHCHSN-UHFFFAOYSA-N Temozolomide Chemical compound O=C1N(C)N=NC2=C(C(N)=O)N=CN21 BPEGJWRSRHCHSN-UHFFFAOYSA-N 0.000 description 1
- GUGOEEXESWIERI-UHFFFAOYSA-N Terfenadine Chemical compound C1=CC(C(C)(C)C)=CC=C1C(O)CCCN1CCC(C(O)(C=2C=CC=CC=2)C=2C=CC=CC=2)CC1 GUGOEEXESWIERI-UHFFFAOYSA-N 0.000 description 1
- 235000005212 Terminalia tomentosa Nutrition 0.000 description 1
- 244000125380 Terminalia tomentosa Species 0.000 description 1
- 208000037063 Thinness Diseases 0.000 description 1
- 201000008982 Thoracic Aortic Aneurysm Diseases 0.000 description 1
- XSLXHSYIVPGEER-KZVJFYERSA-N Thr-Ala-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O XSLXHSYIVPGEER-KZVJFYERSA-N 0.000 description 1
- NAXBBCLCEOTAIG-RHYQMDGZSA-N Thr-Arg-Lys Chemical compound NC(N)=NCCC[C@H](NC(=O)[C@@H](N)[C@H](O)C)C(=O)N[C@@H](CCCCN)C(O)=O NAXBBCLCEOTAIG-RHYQMDGZSA-N 0.000 description 1
- DCLBXIWHLVEPMQ-JRQIVUDYSA-N Thr-Asp-Tyr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 DCLBXIWHLVEPMQ-JRQIVUDYSA-N 0.000 description 1
- ZUUDNCOCILSYAM-KKHAAJSZSA-N Thr-Asp-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O ZUUDNCOCILSYAM-KKHAAJSZSA-N 0.000 description 1
- DGOJNGCGEYOBKN-BWBBJGPYSA-N Thr-Cys-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CS)C(=O)O)N)O DGOJNGCGEYOBKN-BWBBJGPYSA-N 0.000 description 1
- LHEZGZQRLDBSRR-WDCWCFNPSA-N Thr-Glu-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O LHEZGZQRLDBSRR-WDCWCFNPSA-N 0.000 description 1
- MPUMPERGHHJGRP-WEDXCCLWSA-N Thr-Gly-Lys Chemical compound C[C@H]([C@@H](C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)O)N)O MPUMPERGHHJGRP-WEDXCCLWSA-N 0.000 description 1
- GXUWHVZYDAHFSV-FLBSBUHZSA-N Thr-Ile-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O GXUWHVZYDAHFSV-FLBSBUHZSA-N 0.000 description 1
- YOOAQCZYZHGUAZ-KATARQTJSA-N Thr-Leu-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YOOAQCZYZHGUAZ-KATARQTJSA-N 0.000 description 1
- IJVNLNRVDUTWDD-MEYUZBJRSA-N Thr-Leu-Tyr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O IJVNLNRVDUTWDD-MEYUZBJRSA-N 0.000 description 1
- BIBYEFRASCNLAA-CDMKHQONSA-N Thr-Phe-Gly Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CC1=CC=CC=C1 BIBYEFRASCNLAA-CDMKHQONSA-N 0.000 description 1
- DNCUODYZAMHLCV-XGEHTFHBSA-N Thr-Pro-Cys Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CS)C(=O)O)N)O DNCUODYZAMHLCV-XGEHTFHBSA-N 0.000 description 1
- XHWCDRUPDNSDAZ-XKBZYTNZSA-N Thr-Ser-Glu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N)O XHWCDRUPDNSDAZ-XKBZYTNZSA-N 0.000 description 1
- WPSKTVVMQCXPRO-BWBBJGPYSA-N Thr-Ser-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O WPSKTVVMQCXPRO-BWBBJGPYSA-N 0.000 description 1
- XGUAUKUYQHBUNY-SWRJLBSHSA-N Thr-Trp-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCC(O)=O)C(O)=O XGUAUKUYQHBUNY-SWRJLBSHSA-N 0.000 description 1
- 238000008050 Total Bilirubin Reagent Methods 0.000 description 1
- 102000046299 Transforming Growth Factor beta1 Human genes 0.000 description 1
- 102000009618 Transforming Growth Factors Human genes 0.000 description 1
- 108010009583 Transforming Growth Factors Proteins 0.000 description 1
- 101800002279 Transforming growth factor beta-1 Proteins 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- 208000003721 Triple Negative Breast Neoplasms Diseases 0.000 description 1
- DVIIYMVCSUQOJG-QEJZJMRPSA-N Trp-Glu-Asp Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O DVIIYMVCSUQOJG-QEJZJMRPSA-N 0.000 description 1
- XGFGVFMXDXALEV-XIRDDKMYSA-N Trp-Leu-Asn Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)N XGFGVFMXDXALEV-XIRDDKMYSA-N 0.000 description 1
- PKZIWSHDJYIPRH-JBACZVJFSA-N Trp-Tyr-Gln Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(O)=O PKZIWSHDJYIPRH-JBACZVJFSA-N 0.000 description 1
- QYSBJAUCUKHSLU-JYJNAYRXSA-N Tyr-Arg-Val Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(O)=O QYSBJAUCUKHSLU-JYJNAYRXSA-N 0.000 description 1
- QOIKZODVIPOPDD-AVGNSLFASA-N Tyr-Cys-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(O)=O QOIKZODVIPOPDD-AVGNSLFASA-N 0.000 description 1
- BODHJXJNRVRKFA-BZSNNMDCSA-N Tyr-Cys-Tyr Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O BODHJXJNRVRKFA-BZSNNMDCSA-N 0.000 description 1
- QUILOGWWLXMSAT-IHRRRGAJSA-N Tyr-Gln-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O QUILOGWWLXMSAT-IHRRRGAJSA-N 0.000 description 1
- STTVVMWQKDOKAM-YESZJQIVSA-N Tyr-His-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CN=CN2)NC(=O)[C@H](CC3=CC=C(C=C3)O)N)C(=O)O STTVVMWQKDOKAM-YESZJQIVSA-N 0.000 description 1
- SBLZVFCEOCWRLS-BPNCWPANSA-N Tyr-Met-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC1=CC=C(C=C1)O)N SBLZVFCEOCWRLS-BPNCWPANSA-N 0.000 description 1
- VYQQQIRHIFALGE-UWJYBYFXSA-N Tyr-Ser-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 VYQQQIRHIFALGE-UWJYBYFXSA-N 0.000 description 1
- ZPFLBLFITJCBTP-QWRGUYRKSA-N Tyr-Ser-Gly Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(=O)NCC(O)=O ZPFLBLFITJCBTP-QWRGUYRKSA-N 0.000 description 1
- ANHVRCNNGJMJNG-BZSNNMDCSA-N Tyr-Tyr-Cys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)N[C@@H](CS)C(=O)O)N)O ANHVRCNNGJMJNG-BZSNNMDCSA-N 0.000 description 1
- 102000018614 Uromodulin Human genes 0.000 description 1
- 108010027007 Uromodulin Proteins 0.000 description 1
- 108010079206 V-Set Domain-Containing T-Cell Activation Inhibitor 1 Proteins 0.000 description 1
- 102100038929 V-set domain-containing T-cell activation inhibitor 1 Human genes 0.000 description 1
- VJOWWOGRNXRQMF-UVBJJODRSA-N Val-Ala-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N)C(C)C)C(O)=O)=CNC2=C1 VJOWWOGRNXRQMF-UVBJJODRSA-N 0.000 description 1
- KKHRWGYHBZORMQ-NHCYSSNCSA-N Val-Arg-Glu Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N KKHRWGYHBZORMQ-NHCYSSNCSA-N 0.000 description 1
- HZYOWMGWKKRMBZ-BYULHYEWSA-N Val-Asp-Asp Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=O)O)C(=O)O)N HZYOWMGWKKRMBZ-BYULHYEWSA-N 0.000 description 1
- MHAHQDBEIDPFQS-NHCYSSNCSA-N Val-Glu-Met Chemical compound CSCC[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)C(C)C MHAHQDBEIDPFQS-NHCYSSNCSA-N 0.000 description 1
- SYOMXKPPFZRELL-ONGXEEELSA-N Val-Gly-Lys Chemical compound CC(C)[C@@H](C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)O)N SYOMXKPPFZRELL-ONGXEEELSA-N 0.000 description 1
- OPGWZDIYEYJVRX-AVGNSLFASA-N Val-His-Arg Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N OPGWZDIYEYJVRX-AVGNSLFASA-N 0.000 description 1
- OTJMMKPMLUNTQT-AVGNSLFASA-N Val-Leu-Arg Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)[C@H](C(C)C)N OTJMMKPMLUNTQT-AVGNSLFASA-N 0.000 description 1
- LYERIXUFCYVFFX-GVXVVHGQSA-N Val-Leu-Glu Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](C(C)C)N LYERIXUFCYVFFX-GVXVVHGQSA-N 0.000 description 1
- JKHXYJKMNSSFFL-IUCAKERBSA-N Val-Lys Chemical compound CC(C)[C@H](N)C(=O)N[C@H](C(O)=O)CCCCN JKHXYJKMNSSFFL-IUCAKERBSA-N 0.000 description 1
- SSYBNWFXCFNRFN-GUBZILKMSA-N Val-Pro-Ser Chemical compound CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O SSYBNWFXCFNRFN-GUBZILKMSA-N 0.000 description 1
- DEGUERSKQBRZMZ-FXQIFTODSA-N Val-Ser-Ala Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O DEGUERSKQBRZMZ-FXQIFTODSA-N 0.000 description 1
- UJMCYJKPDFQLHX-XGEHTFHBSA-N Val-Ser-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](C(C)C)N)O UJMCYJKPDFQLHX-XGEHTFHBSA-N 0.000 description 1
- 208000033559 Waldenström macroglobulinemia Diseases 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 239000008186 active pharmaceutical agent Substances 0.000 description 1
- 208000036981 active tuberculosis Diseases 0.000 description 1
- 230000003044 adaptive effect Effects 0.000 description 1
- 238000011374 additional therapy Methods 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 201000008395 adenosquamous carcinoma Diseases 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 210000004100 adrenal gland Anatomy 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- 108010070944 alanylhistidine Proteins 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 230000002491 angiogenic effect Effects 0.000 description 1
- 230000001387 anti-histamine Effects 0.000 description 1
- 210000000612 antigen-presenting cell Anatomy 0.000 description 1
- 239000000739 antihistaminic agent Substances 0.000 description 1
- 208000007474 aortic aneurysm Diseases 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 239000013011 aqueous formulation Substances 0.000 description 1
- 108010013835 arginine glutamate Proteins 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 108010069205 aspartyl-phenylalanine Proteins 0.000 description 1
- 108010093581 aspartyl-proline Proteins 0.000 description 1
- 108010047857 aspartylglycine Proteins 0.000 description 1
- 208000010668 atopic eczema Diseases 0.000 description 1
- 230000001363 autoimmune Effects 0.000 description 1
- 230000003385 bacteriostatic effect Effects 0.000 description 1
- 229960000686 benzalkonium chloride Drugs 0.000 description 1
- 229960003872 benzethonium Drugs 0.000 description 1
- UREZNYTWGJKWBI-UHFFFAOYSA-M benzethonium chloride Chemical compound [Cl-].C1=CC(C(C)(C)CC(C)(C)C)=CC=C1OCCOCC[N+](C)(C)CC1=CC=CC=C1 UREZNYTWGJKWBI-UHFFFAOYSA-M 0.000 description 1
- 235000019445 benzyl alcohol Nutrition 0.000 description 1
- 150000003938 benzyl alcohols Chemical class 0.000 description 1
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 1
- 102000012740 beta Adrenergic Receptors Human genes 0.000 description 1
- 108010079452 beta Adrenergic Receptors Proteins 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 229960000397 bevacizumab Drugs 0.000 description 1
- 208000026900 bile duct neoplasm Diseases 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- HUTDDBSSHVOYJR-UHFFFAOYSA-H bis[(2-oxo-1,3,2$l^{5},4$l^{2}-dioxaphosphaplumbetan-2-yl)oxy]lead Chemical compound [Pb+2].[Pb+2].[Pb+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O HUTDDBSSHVOYJR-UHFFFAOYSA-H 0.000 description 1
- 238000006664 bond formation reaction Methods 0.000 description 1
- 210000001185 bone marrow Anatomy 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 230000036952 cancer formation Effects 0.000 description 1
- 238000002619 cancer immunotherapy Methods 0.000 description 1
- 230000004856 capillary permeability Effects 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 150000007942 carboxylates Chemical group 0.000 description 1
- 231100000504 carcinogenesis Toxicity 0.000 description 1
- 208000025188 carcinoma of pharynx Diseases 0.000 description 1
- 230000000747 cardiac effect Effects 0.000 description 1
- 238000000423 cell based assay Methods 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000010001 cellular homeostasis Effects 0.000 description 1
- 201000006662 cervical adenocarcinoma Diseases 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 150000001805 chlorine compounds Chemical class 0.000 description 1
- 208000006990 cholangiocarcinoma Diseases 0.000 description 1
- 229960002303 citric acid monohydrate Drugs 0.000 description 1
- 238000009535 clinical urine test Methods 0.000 description 1
- 238000011260 co-administration Methods 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 208000029742 colonic neoplasm Diseases 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 230000007322 compensatory function Effects 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000002591 computed tomography Methods 0.000 description 1
- 230000008094 contradictory effect Effects 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 230000001054 cortical effect Effects 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 238000012325 curative resection Methods 0.000 description 1
- 208000035250 cutaneous malignant susceptibility to 1 melanoma Diseases 0.000 description 1
- HPXRVTGHNJAIIH-UHFFFAOYSA-N cyclohexanol Chemical compound OC1CCCCC1 HPXRVTGHNJAIIH-UHFFFAOYSA-N 0.000 description 1
- 108010004073 cysteinylcysteine Proteins 0.000 description 1
- 108010016616 cysteinylglycine Proteins 0.000 description 1
- 108010069495 cysteinyltyrosine Proteins 0.000 description 1
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 1
- 238000007405 data analysis Methods 0.000 description 1
- 230000009615 deamination Effects 0.000 description 1
- 238000006481 deamination reaction Methods 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000009547 development abnormality Effects 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 229960000520 diphenhydramine Drugs 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 229940088679 drug related substance Drugs 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 229950009791 durvalumab Drugs 0.000 description 1
- 239000000806 elastomer Substances 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 230000002124 endocrine Effects 0.000 description 1
- 206010015037 epilepsy Diseases 0.000 description 1
- 229940105423 erythropoietin Drugs 0.000 description 1
- 208000028653 esophageal adenocarcinoma Diseases 0.000 description 1
- 208000007276 esophageal squamous cell carcinoma Diseases 0.000 description 1
- 210000003527 eukaryotic cell Anatomy 0.000 description 1
- 210000003020 exocrine pancreas Anatomy 0.000 description 1
- 210000002744 extracellular matrix Anatomy 0.000 description 1
- 208000003457 familial thoracic 1 aortic aneurysm Diseases 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 238000000855 fermentation Methods 0.000 description 1
- 230000004151 fermentation Effects 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 238000007710 freezing Methods 0.000 description 1
- 230000008014 freezing Effects 0.000 description 1
- 201000006585 gastric adenocarcinoma Diseases 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 201000007492 gastroesophageal junction adenocarcinoma Diseases 0.000 description 1
- 229940050410 gluconate Drugs 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 229930182470 glycoside Natural products 0.000 description 1
- 102000035122 glycosylated proteins Human genes 0.000 description 1
- 108091005608 glycosylated proteins Proteins 0.000 description 1
- 108010010147 glycylglutamine Proteins 0.000 description 1
- 230000036449 good health Effects 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 230000009036 growth inhibition Effects 0.000 description 1
- 230000003394 haemopoietic effect Effects 0.000 description 1
- 238000003306 harvesting Methods 0.000 description 1
- 230000002489 hematologic effect Effects 0.000 description 1
- 231100000234 hepatic damage Toxicity 0.000 description 1
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 1
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 1
- 208000029824 high grade glioma Diseases 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 108010025306 histidylleucine Proteins 0.000 description 1
- 108010018006 histidylserine Proteins 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 230000000148 hypercalcaemia Effects 0.000 description 1
- 208000030915 hypercalcemia disease Diseases 0.000 description 1
- 230000002519 immonomodulatory effect Effects 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 229960001438 immunostimulant agent Drugs 0.000 description 1
- 239000003022 immunostimulating agent Substances 0.000 description 1
- 230000003308 immunostimulating effect Effects 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 230000001976 improved effect Effects 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 229960004768 irinotecan Drugs 0.000 description 1
- GURKHSYORGJETM-WAQYZQTGSA-N irinotecan hydrochloride (anhydrous) Chemical compound Cl.C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 GURKHSYORGJETM-WAQYZQTGSA-N 0.000 description 1
- 208000028867 ischemia Diseases 0.000 description 1
- 210000004153 islets of langerhan Anatomy 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 210000002510 keratinocyte Anatomy 0.000 description 1
- 230000003907 kidney function Effects 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 229940043355 kinase inhibitor Drugs 0.000 description 1
- 238000011813 knockout mouse model Methods 0.000 description 1
- 210000000867 larynx Anatomy 0.000 description 1
- 229950010470 lerdelimumab Drugs 0.000 description 1
- 108010034529 leucyl-lysine Proteins 0.000 description 1
- 108010091871 leucylmethionine Proteins 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 230000008818 liver damage Effects 0.000 description 1
- 230000003908 liver function Effects 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 230000007040 lung development Effects 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 239000008176 lyophilized powder Substances 0.000 description 1
- 201000000564 macroglobulinemia Diseases 0.000 description 1
- 230000036210 malignancy Effects 0.000 description 1
- 201000011614 malignant glioma Diseases 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000002483 medication Methods 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 229950005555 metelimumab Drugs 0.000 description 1
- 108010085203 methionylmethionine Proteins 0.000 description 1
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 230000001483 mobilizing effect Effects 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- 208000037891 myocardial injury Diseases 0.000 description 1
- NZXVYLJKFYSEPO-UHFFFAOYSA-N n-[3-(dimethylamino)propyl]-16-methylheptadecanamide Chemical compound CC(C)CCCCCCCCCCCCCCC(=O)NCCCN(C)C NZXVYLJKFYSEPO-UHFFFAOYSA-N 0.000 description 1
- 210000000822 natural killer cell Anatomy 0.000 description 1
- 230000008693 nausea Effects 0.000 description 1
- 230000009826 neoplastic cell growth Effects 0.000 description 1
- 238000006384 oligomerization reaction Methods 0.000 description 1
- 231100000590 oncogenic Toxicity 0.000 description 1
- 230000002246 oncogenic effect Effects 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 210000003101 oviduct Anatomy 0.000 description 1
- DWAFYCQODLXJNR-BNTLRKBRSA-L oxaliplatin Chemical compound O1C(=O)C(=O)O[Pt]11N[C@@H]2CCCC[C@H]2N1 DWAFYCQODLXJNR-BNTLRKBRSA-L 0.000 description 1
- 229960001756 oxaliplatin Drugs 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 201000002094 pancreatic adenocarcinoma Diseases 0.000 description 1
- 201000002628 peritoneum cancer Diseases 0.000 description 1
- 238000002823 phage display Methods 0.000 description 1
- 239000005426 pharmaceutical component Substances 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 210000003800 pharynx Anatomy 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- 108010012581 phenylalanylglutamate Proteins 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 230000026731 phosphorylation Effects 0.000 description 1
- 238000006366 phosphorylation reaction Methods 0.000 description 1
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 1
- 229960000502 poloxamer Drugs 0.000 description 1
- 229950008882 polysorbate Drugs 0.000 description 1
- 238000010837 poor prognosis Methods 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- OXCMYAYHXIHQOA-UHFFFAOYSA-N potassium;[2-butyl-5-chloro-3-[[4-[2-(1,2,4-triaza-3-azanidacyclopenta-1,4-dien-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol Chemical compound [K+].CCCCC1=NC(Cl)=C(CO)N1CC1=CC=C(C=2C(=CC=CC=2)C2=N[N-]N=N2)C=C1 OXCMYAYHXIHQOA-UHFFFAOYSA-N 0.000 description 1
- 201000003725 primary thrombocytopenia Diseases 0.000 description 1
- 238000004393 prognosis Methods 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 108010025826 prolyl-leucyl-arginine Proteins 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 230000001012 protector Effects 0.000 description 1
- 230000000722 protumoral effect Effects 0.000 description 1
- 230000001698 pyrogenic effect Effects 0.000 description 1
- 230000010837 receptor-mediated endocytosis Effects 0.000 description 1
- 210000000664 rectum Anatomy 0.000 description 1
- 230000008672 reprogramming Effects 0.000 description 1
- 238000002271 resection Methods 0.000 description 1
- 201000009410 rhabdomyosarcoma Diseases 0.000 description 1
- 230000001568 sexual effect Effects 0.000 description 1
- 102000035025 signaling receptors Human genes 0.000 description 1
- 108091005475 signaling receptors Proteins 0.000 description 1
- 206010040882 skin lesion Diseases 0.000 description 1
- 231100000444 skin lesion Toxicity 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 229960002668 sodium chloride Drugs 0.000 description 1
- 229940074404 sodium succinate Drugs 0.000 description 1
- ZDQYSKICYIVCPN-UHFFFAOYSA-L sodium succinate (anhydrous) Chemical compound [Na+].[Na+].[O-]C(=O)CCC([O-])=O ZDQYSKICYIVCPN-UHFFFAOYSA-L 0.000 description 1
- HSFQBFMEWSTNOW-UHFFFAOYSA-N sodium;carbanide Chemical group [CH3-].[Na+] HSFQBFMEWSTNOW-UHFFFAOYSA-N 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- SFVFIFLLYFPGHH-UHFFFAOYSA-M stearalkonium chloride Chemical compound [Cl-].CCCCCCCCCCCCCCCCCC[N+](C)(C)CC1=CC=CC=C1 SFVFIFLLYFPGHH-UHFFFAOYSA-M 0.000 description 1
- 230000001954 sterilising effect Effects 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 231100000617 superantigen Toxicity 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 238000011521 systemic chemotherapy Methods 0.000 description 1
- 238000009121 systemic therapy Methods 0.000 description 1
- 238000002626 targeted therapy Methods 0.000 description 1
- 229940104261 taurate Drugs 0.000 description 1
- DKPFODGZWDEEBT-QFIAKTPHSA-N taxane Chemical class C([C@]1(C)CCC[C@@H](C)[C@H]1C1)C[C@H]2[C@H](C)CC[C@@H]1C2(C)C DKPFODGZWDEEBT-QFIAKTPHSA-N 0.000 description 1
- 229960004964 temozolomide Drugs 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 238000011287 therapeutic dose Methods 0.000 description 1
- 108010033670 threonyl-aspartyl-tyrosine Proteins 0.000 description 1
- 206010043554 thrombocytopenia Diseases 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 238000011269 treatment regimen Methods 0.000 description 1
- 208000022679 triple-negative breast carcinoma Diseases 0.000 description 1
- 108010044292 tryptophyltyrosine Proteins 0.000 description 1
- 201000008827 tuberculosis Diseases 0.000 description 1
- 239000000717 tumor promoter Substances 0.000 description 1
- 108010003137 tyrosyltyrosine Proteins 0.000 description 1
- 206010048828 underweight Diseases 0.000 description 1
- 108010009962 valyltyrosine Proteins 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 230000029663 wound healing Effects 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/46—Hybrid immunoglobulins
- C07K16/468—Immunoglobulins having two or more different antigen binding sites, e.g. multifunctional antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61J—CONTAINERS SPECIALLY ADAPTED FOR MEDICAL OR PHARMACEUTICAL PURPOSES; DEVICES OR METHODS SPECIALLY ADAPTED FOR BRINGING PHARMACEUTICAL PRODUCTS INTO PARTICULAR PHYSICAL OR ADMINISTERING FORMS; DEVICES FOR ADMINISTERING FOOD OR MEDICINES ORALLY; BABY COMFORTERS; DEVICES FOR RECEIVING SPITTLE
- A61J1/00—Containers specially adapted for medical or pharmaceutical purposes
- A61J1/05—Containers specially adapted for medical or pharmaceutical purposes for collecting, storing or administering blood, plasma or medical fluids ; Infusion or perfusion containers
- A61J1/10—Bag-type containers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/71—Receptors; Cell surface antigens; Cell surface determinants for growth factors; for growth regulators
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2827—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against B7 molecules, e.g. CD80, CD86
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
- A61K2039/507—Comprising a combination of two or more separate antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/54—Medicinal preparations containing antigens or antibodies characterised by the route of administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/545—Medicinal preparations containing antigens or antibodies characterised by the dose, timing or administration schedule
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/02—Fusion polypeptide containing a localisation/targetting motif containing a signal sequence
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Immunology (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Cell Biology (AREA)
- Toxicology (AREA)
- Zoology (AREA)
- Gastroenterology & Hepatology (AREA)
- Dermatology (AREA)
- Epidemiology (AREA)
- Hematology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicinal Preparation (AREA)
- Endoscopes (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762443698P | 2017-01-07 | 2017-01-07 | |
| US62/443,698 | 2017-01-07 | ||
| US201762581978P | 2017-11-06 | 2017-11-06 | |
| US62/581,978 | 2017-11-06 | ||
| PCT/US2018/012604 WO2018129331A1 (en) | 2017-01-07 | 2018-01-05 | Dosing regimens and dosage forms for targeted tgf-b inhibition |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20190102059A true KR20190102059A (ko) | 2019-09-02 |
Family
ID=62791283
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197022795A Ceased KR20190102059A (ko) | 2017-01-07 | 2018-01-05 | 표적화된 TGF-β 억제를 위한 투약 레지멘 및 투여 형태 |
Country Status (15)
| Country | Link |
|---|---|
| US (1) | US20190330375A1 (enExample) |
| EP (1) | EP3565599A4 (enExample) |
| JP (1) | JP2020514290A (enExample) |
| KR (1) | KR20190102059A (enExample) |
| CN (1) | CN110198738A (enExample) |
| AU (1) | AU2018205233A1 (enExample) |
| BR (1) | BR112019013924A2 (enExample) |
| CA (1) | CA3048646A1 (enExample) |
| CL (1) | CL2019001871A1 (enExample) |
| IL (1) | IL267856A (enExample) |
| MX (1) | MX2019008001A (enExample) |
| PH (1) | PH12019501574A1 (enExample) |
| RU (1) | RU2019124875A (enExample) |
| SG (1) | SG11201906157YA (enExample) |
| WO (1) | WO2018129331A1 (enExample) |
Families Citing this family (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| HK1225740A1 (zh) | 2013-08-22 | 2017-09-15 | Acceleron Pharma Inc. | TGF-β受体II型变体及其用途 |
| EP3331550B1 (en) | 2015-08-04 | 2022-11-02 | Acceleron Pharma Inc. | Composition for treating myeloproliferative disorders |
| SI3628049T1 (sl) | 2017-05-04 | 2023-10-30 | Acceleron Pharma Inc. | Fuzijske beljakovine receptorja TGF-beta tipa II in njihova uporaba |
| AU2019288765A1 (en) * | 2018-06-22 | 2021-01-07 | Merck Patent Gmbh | Dosing regimens for targeted TGF-β inhibition for use in treating biliary tract cancer |
| BR112020026902A2 (pt) * | 2018-07-02 | 2021-03-30 | Merck Patent Gmbh | Terapia combinada com inibição direcionada de tgf-b para o tratamento de câncer de pulmão de células não pequenas avançado |
| SG11202100208VA (en) | 2018-07-09 | 2021-02-25 | Precigen Inc | Fusion constructs and methods of using thereof |
| JP7436477B2 (ja) * | 2018-11-09 | 2024-02-21 | ジエンス ヘンルイ メデイシンカンパニー リミテッド | TGF-β受容体融合タンパク質医薬組成物およびその使用 |
| MX2021009175A (es) | 2019-01-30 | 2021-09-14 | Scholar Rock Inc | Inhibidores especificos del complejo de ltbp de tgf? y usos de los mismos. |
| AU2020290119B2 (en) * | 2019-06-10 | 2024-07-18 | Shandong Boan Biotechnology Co., Ltd. | Bifunctional fusion protein against PDL1 and TGFβ and use thereof |
| CN112574314A (zh) * | 2019-09-30 | 2021-03-30 | 和铂医药(苏州)有限公司 | 一种融合蛋白及其应用 |
| WO2021081321A1 (en) * | 2019-10-24 | 2021-04-29 | Amgen Inc. | Systems and approaches for drug delivery |
| WO2021084124A1 (en) * | 2019-11-01 | 2021-05-06 | Ares Trading S.A. | COMBINED INHIBITION OF PD-1, TGFβ AND ATM TOGETHER WITH RADIOTHERAPY FOR THE TREATMENT OF CANCER |
| EP4054617A4 (en) * | 2019-11-05 | 2023-12-27 | Acceleron Pharma Inc. | Treatments for systemic sclerosis |
| US20230035169A1 (en) * | 2019-12-11 | 2023-02-02 | WuXi Biologics Ireland Limited | BI-FUNCTIONAL ANTIBODY AGAINST PD-L1 AND TGF-Beta |
| CN115135302A (zh) * | 2019-12-20 | 2022-09-30 | 阿雷斯贸易股份有限公司 | IgG:TGFβRII融合蛋白组合物 |
| CN111118064B (zh) * | 2019-12-24 | 2022-10-25 | 华南理工大学 | 一种精子生成障碍动物模型及其制备方法与应用 |
| MX2022008609A (es) | 2020-01-11 | 2022-11-10 | Scholar Rock Inc | Inhibidores de tgf¿ y uso de los mismos. |
| AU2021205440A1 (en) | 2020-01-11 | 2022-09-01 | Scholar Rock,Inc. | TGF-beta inhibitors and use thereof |
| CN115135675A (zh) * | 2020-02-18 | 2022-09-30 | 南京金斯瑞生物科技有限公司 | 融合蛋白及其用途 |
| EP4093779A4 (en) * | 2020-02-25 | 2024-03-06 | Wuxi Biologics Ireland Limited | BIFUNCTIONAL FUSION PROTEIN AND USES THEREOF |
| WO2022011256A1 (en) | 2020-07-10 | 2022-01-13 | Precigen, Inc. | Fusion constructs and methods of using thereof |
| CN116323657B (zh) * | 2020-09-24 | 2023-12-08 | 上海齐鲁制药研究中心有限公司 | 同时靶向PD-L1和TGFβ的双功能分子及其医药用途 |
| US20240148903A1 (en) | 2021-03-08 | 2024-05-09 | Nanjing GenScript Biotech Co., Ltd. | Delivery of antibody by using dual viral vector system |
| WO2022204581A2 (en) | 2021-03-26 | 2022-09-29 | Scholar Rock, Inc. | Tgf-beta inhibitors and use thereof |
| WO2022256723A2 (en) | 2021-06-03 | 2022-12-08 | Scholar Rock, Inc. | Tgf-beta inhibitors and therapeutic use thereof |
| CA3231649A1 (en) * | 2021-11-19 | 2023-05-25 | Cecilia Anna Wilhelmina Geuijen | Multispecific binding moieties comprising pd-1 and tgf-brii binding domains |
| WO2024187051A1 (en) | 2023-03-07 | 2024-09-12 | Scholar Rock, Inc. | Tgf-beta inhibitors for use for treating resistant or unresponsive cancer in patients |
| WO2025240343A1 (en) | 2024-05-13 | 2025-11-20 | Scholar Rock, Inc. | Tgf-beta inhibitors for treating cancer |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5785682A (en) * | 1995-03-22 | 1998-07-28 | Abbott Laboratories | Pre-filled syringe drug delivery system |
| JP6066732B2 (ja) * | 2010-03-05 | 2017-01-25 | ザ・ジョンズ・ホプキンス・ユニバーシティー | 標的免疫調節抗体および融合タンパク質に基づく組成物および方法 |
| US20130110045A1 (en) * | 2011-10-31 | 2013-05-02 | Ming-Yuan Wu | Single use intravenous therapy administering device with needle safety covers |
| PT3489254T (pt) * | 2012-04-30 | 2022-12-30 | Biocon Ltd | Proteínas de fusão direcionadas/imunomoduladoras e seus métodos de fabrico |
| WO2014193898A1 (en) * | 2013-05-31 | 2014-12-04 | Merck Sharp & Dohme Corp. | Combination therapies for cancer |
| SI3105246T1 (sl) * | 2014-02-10 | 2021-11-30 | Merck Patent Gmbh | Ciljana inhibicija TGF BETA |
| CN114920840A (zh) * | 2014-10-14 | 2022-08-19 | 诺华股份有限公司 | 针对pd-l1的抗体分子及其用途 |
| CA2975147C (en) * | 2015-01-31 | 2025-06-10 | The Trustees Of The University Of Pennsylvania | COMPOSITIONS AND METHODS OF ADMINISTRATION OF THERAPEUTIC MOLECULES TO T CELLS |
| EP3277716B1 (en) * | 2015-04-03 | 2020-06-24 | XOMA Technology Ltd. | Treatment of cancer using inhibitors of tgf-beta and pd-1 |
| MX2017014381A (es) * | 2015-05-12 | 2018-03-02 | Genentech Inc | Metodos terapeuticos y diagnosticos para cancer. |
-
2018
- 2018-01-05 WO PCT/US2018/012604 patent/WO2018129331A1/en not_active Ceased
- 2018-01-05 KR KR1020197022795A patent/KR20190102059A/ko not_active Ceased
- 2018-01-05 SG SG11201906157YA patent/SG11201906157YA/en unknown
- 2018-01-05 CN CN201880006085.0A patent/CN110198738A/zh active Pending
- 2018-01-05 BR BR112019013924-9A patent/BR112019013924A2/pt not_active Application Discontinuation
- 2018-01-05 RU RU2019124875A patent/RU2019124875A/ru unknown
- 2018-01-05 EP EP18736497.1A patent/EP3565599A4/en active Pending
- 2018-01-05 MX MX2019008001A patent/MX2019008001A/es unknown
- 2018-01-05 JP JP2019536228A patent/JP2020514290A/ja active Pending
- 2018-01-05 AU AU2018205233A patent/AU2018205233A1/en not_active Abandoned
- 2018-01-05 CA CA3048646A patent/CA3048646A1/en active Pending
-
2019
- 2019-07-02 US US16/460,792 patent/US20190330375A1/en not_active Abandoned
- 2019-07-03 PH PH12019501574A patent/PH12019501574A1/en unknown
- 2019-07-04 IL IL267856A patent/IL267856A/en unknown
- 2019-07-05 CL CL2019001871A patent/CL2019001871A1/es unknown
Also Published As
| Publication number | Publication date |
|---|---|
| CL2019001871A1 (es) | 2019-12-13 |
| RU2019124875A3 (enExample) | 2021-07-08 |
| EP3565599A4 (en) | 2020-07-01 |
| AU2018205233A1 (en) | 2019-07-11 |
| PH12019501574A1 (en) | 2019-11-04 |
| BR112019013924A2 (pt) | 2020-02-11 |
| US20190330375A1 (en) | 2019-10-31 |
| CA3048646A1 (en) | 2018-07-12 |
| SG11201906157YA (en) | 2019-08-27 |
| RU2019124875A (ru) | 2021-02-08 |
| WO2018129331A1 (en) | 2018-07-12 |
| MX2019008001A (es) | 2019-09-09 |
| IL267856A (en) | 2019-09-26 |
| CN110198738A (zh) | 2019-09-03 |
| JP2020514290A (ja) | 2020-05-21 |
| EP3565599A1 (en) | 2019-11-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20190102059A (ko) | 표적화된 TGF-β 억제를 위한 투약 레지멘 및 투여 형태 | |
| AU2019246876B2 (en) | Targeted TGFß Inhibition | |
| KR20230125859A (ko) | 암에 대한 조합 요법 | |
| KR20210020098A (ko) | Iii기 nsclc의 치료 및 치료와 연관된 병리학적 상태의 완화 | |
| US20210061899A1 (en) | Dosing regimens for targeted tgf-b inhibition for use in treating cancer in treatment naïve subjects | |
| US20210115145A1 (en) | Combination therapy with targeted tgf-b inhibition for treatment of advanced non-small cell lung cancer | |
| US20210214446A1 (en) | Dosing regimens for targeted tgf-b inhibition for use in treating biliary tract cancer | |
| HK40050217A (en) | Combination therapy with targeted tgf-b inhibition for treatment of advanced non-small cell lung cancer | |
| HK40042110A (en) | Dosing regimens for targeted tgf-b inhibition for use in treating cancer in treatment naive subjects | |
| HK40012523A (en) | DOSING REGIMENS AND DOSAGE FORMS FOR TARGETED TGF-β INHIBITION | |
| HK40050239A (en) | Treatment of stage iii nsclc and mitigation of pathological conditions associated with the treatment | |
| HK40049078A (en) | Dosing regimens for targeted tgf-β inhibition for use in treating biliary tract cancer | |
| HK40064486A (en) | TARGETED TGFβ INHIBITION |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20190802 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20210105 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20230424 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20231108 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20230424 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |